- 1Department of Physics, Faculty of Science and Arts, Inonu University, Malatya, Türkiye
- 2Department of Scientific Research Projects, Sivas University of Science and Technology, Sivas, Türkiye
- 3Nanotechnology Application and Research Center, Niğde Ömer Halisdemir University, Niğde, Türkiye
- 4Department of Physics, Faculty of Science and Arts, Niğde Ömer Halisdemir University, Niğde, Türkiye
Nanoporous platinum (Pt) films are synthesized at room temperature using a straightforward and cost-effective dealloying technique. This method is suitable for producing various nanoporous materials for diverse applications. Copper (Cu) atoms in the PtCu alloy films were selectively dissolved in a nitric acid solution, at varying times, to obtain nanoporous films. PtCu alloy thin films were then deposited onto a glass substrate utilizing the magnetron co-sputtering method with approximately 50 nm thickness. After 20 h dealloying in the acid solution, the residual Cu content in the alloy was less than 1% (atomic rate), and a regular nanoporous Pt structure was observed. The hydrogen detection properties of the nanoporous Pt films thus produced were investigated at various temperatures within a concentration range between 10 ppm and 5% hydrogen. The results demonstrated that a very high sensor response of 64 was obtained for the first exposure to 1% hydrogen at 150 °C, but the nanoporous Pt sensor resistance did not return to the baseline resistance. To utilize this nanoporous Pt film as a reversible hydrogen sensor, the film must be pre-exposed to hydrogen. After pre-exposure, the sensor response of the as-prepared nanoporous Pt was approximately 4.5, resulting from exposure to 1% hydrogen at 150 °C, and the limit of detection was lower than 10 ppm. Data regarding the mechanism of the nanoporous Pt sensor device were clarified through surface scattering. The main contributions of this research are that sensing nanoporous film has a high surface-to-volume ratio, the sensor exhibited a very high initial response (∼64) to 1% hydrogen at 150 °C, the sensor mechanism is governed by surface scattering, and pre-exposure to hydrogen is needed for reversible sensing operation in practical usage.
1 Introduction
Hydrogen is widely used in various industrial sectors, including semiconductor technologies, transportation, food, and chemistry (Abe et al., 2019; Ball et al., 2015). Recently, hydrogen fuel-cell-powered vehicles have been introduced by major automotive manufacturers such as BMW, Toyota, Honda, and Hyundai. However, it is crucial to take precautions against a small gas leak when using hydrogen, which is flammable at low temperatures, explosive when present in the air at a level of 4%, odorless, and invisible to the eye. Therefore, producing hydrogen sensors with a wide detection range, sensitive response, and easy installation is a prerequisite for minimizing the risks associated with working with hydrogen (Rane et al., 2015). Considering sensor performance (measuring concentration range, accuracy, response time, life time, and power consumption), metallic resistive hydrogen sensors are one of the best alternatives (Hubert et al., 2011; Sisman et al., 2024). Pt and Pd, which are more preferred as sensing materials in resistive hydrogen sensors, are also used as contact-doping elements and as decorative elements in/on sensitive layers in different types of sensors (Kilinc, 2013; Penner, 2017; Koo et al., 2020; Sahoo and Kale, 2021). Research on platinum (Pt), one of the sensing materials utilized in metallic resistive hydrogen sensors, has been comparatively limited compared to palladium (Pd)—approximately 20 research articles focus on Pt-based resistive hydrogen sensors (Kilinc and Erkovan, 2023). Pt and Pt alloys have been used in resistive hydrogen sensor applications in nanostructure forms such as thin film (Cai et al., 2023; Patel et al., 1999; Tanaka et al., 2018; Kilinc et al., 2022; Erkovan et al., 2022; Sennik et al., 2016), bilayered films (Hassan et al., 2016), core-shell nanoparticle layers (Rajoua et al., 2018; Uddin et al., 2016), nanoporous film (Sener et al., 2023; Abburi and Yeh, 2012), and nanowires (Cao et al., 2019; Ding et al., 2017; Yoo et al., 2015; Yang et al., 2012). For example, Patel et al. (1999) investigated the hydrogen sensing properties of Pt films with 35 Å thickness. These films were prepared using electron beam evaporation on a SiO2 substrate, depending on the temperature and hydrogen concentrations. An oxygen ratio of 5% in nitrogen was used as the carrier gas; the electrical resistance of the Pt film declined in the hydrogen environment, and the sensitivity of the Pt film was enhanced with temperature (Patel et al., 1999). Yang et al. (2012) produced Pd and Pt nanowires by the electrodeposition method and compared the hydrogen sensing of a single Pt nanowire with Pd nanowire and found a decline in the electrical resistance of the Pt nanowire when exposed to hydrogen. They determined a decrease in the electrical resistance of the Pt nanostructures in the hydrogen environment with the surface scattering phenomenon. Nevertheless, the increase in the electrical resistance of Pt nanostructures in the hydrogen medium has been explained through grain boundary electron scattering (Rajoua et al., 2018), defect-dominated electron scattering (Cao et al., 2019), and PtHx formation (Abburi and Yeh, 2012).
Nanoporous Pt structure films have been produced by coating Pt films on anodic aluminum oxide (AAO) nanotemplates and investigating their hydrogen sensing properties (Sener et al., 2023). The resistance of the nanoporous Pt film decreased in a hydrogen atmosphere, yielding approximately 13% sensitivity to 1% hydrogen at room temperature (Sener et al., 2023). Abburi and Yeh (2012) produced nanoporous Pt film structures from a PtCu alloy using dealloying and investigated their hydrogen sensing properties, which depend on pore size and temperature. They reported a sensitivity of 3.5% for a nanoporous Pt film with a pore size of 35 nm. Conversely, they attributed the observed increase in the resistance of the nanoporous Pt film in a hydrogen atmosphere to the formation of PtHx. In this study, although nanoporous Pt films were fabricated using a similar method, a decrease in the resistance of the films was observed in the hydrogen environment. To the best of our knowledge, the highest sensitivity reported among Pt-based resistive hydrogen sensors was achieved upon the film’s initial exposure to hydrogen. The hydrogen sensing properties of nanoporous Pt films were systematically investigated in terms of operating temperature, hydrogen concentration, and annealing temperature. Furthermore, the sensing mechanism of the nanoporous Pt film sensors was examined and discussed.
This study reports a highly sensitive nanoporous platinum (Pt) film hydrogen sensor prepared through a straightforward dealloying of magnetron co-sputtered PtCu thin films. The sensor exhibited a very high initial response (∼64) to 1% hydrogen at 150 °C—the highest value ever reported for Pt-based resistive hydrogen sensors. In contrast to earlier reports, the sensor mechanism is governed by surface scattering, leading to a resistance decrease upon exposure to hydrogen. A new pre-hydrogenation process is also presented for a reversible sensing operation, which facilitates practical usability.
2 Materials and methods
The preliminary results were presented at a conference (9th International Electronic Conference on Sensors and Applications) and published in its proceedings (Sener et al., 2022). Platinum–copper (PtCu) alloy films, approximately 50 nm thick, were coated onto a microscope glass slide using the magnetron co-sputtering method with an atomic ratio of 15:85, respectively. All metallic film depositions were performed utilizing a NANOVAK 400 physical vapor deposition (PVD) system configured with dual sputtering and dual thermal evaporation sources. The glass was used as the substrate material, with a series of meticulous cleaning processes being employed prior to PtCu coating. The cleaning regimen began with subjecting the substrates to a sequence of solvents: acetone, isopropyl alcohol, and ultra-pure water (18 MΩ). Each solvent was applied for 10 min, 10 min, and 10 min, within an ultrasonic bath. Subsequent to this cleansing, the substrates were meticulously dried using a stream of nitrogen (N2) gas. In the final stage of the cleaning process, an ultrasonic plasma cleaner was engaged to effectively eliminate any residual contaminants. Magnetron sputtering was used to initiate the film growth process, forming a film with a thickness of 50 nm. After a base pressure of 1.6x10−6 Torr was obtained in the PVD chamber, approximately 3 sccm ultra-pure argon (Ar, purity: 99,999%) gas was injected into the chamber to maintain a growth pressure of 3 mTorr. Then, co-deposition was achieved using a radio frequency (RF) and direct current (DC) power level of 50 Watts with Cu and Pt targets, respectively. PtCu alloy films were dealloyed in 1 M nitric acid (HNO3) solution for various times (from 15 min to 20 h) to obtain nanoporous Pt films. The fabrication of nanoporous Pt using dealloying is schematically illustrated in Supplementary Figure S1a. The nanoporous Pt films, which were dealloyed for 5 h, were annealed at temperatures of 200–300 °C to determine the annealing effect on structure and hydrogen sensing. Scanning electron microscopy (SEM, Zeiss LEO-EVO 40), field emission scanning electron microscopy (FESEM, Zeiss - Gemini 300), energy-dispersive X-ray spectroscopy (EDX) attached to the FESEM or SEM, X-ray photoelectron spectroscopy (XPS, Specs-Flex), and an X-ray diffraction (XRD, Rigaku - D/Max 2,200) spectrum were used to clarify the morphologies, crystal structures, composition of the nanoporous Pt and PtCu alloy films, and electronic structures.
Before determining the hydrogen sensing properties, two silver (Ag) contact electrodes were deposited onto the nanoporous Pt films via thermal evaporation with a shadow mask. Supplementary Figure S1b shows a schematic diagram of the measurement setup for a resistive gas sensor device. Continuous measurement of nanoporous Pt’s electrical resistance was performed using a two-point probe configuration connected to a Keithley 2700 multimeter, while the atmosphere within a purpose-built measurement cell was systematically altered. The measurement cell consisted of a flow-type aluminum chamber that incorporated an integrated heatable sample holder. The precise regulation of hydrogen gas concentration, ranging from 10 ppm to 5%, was achieved by utilizing two mass flow controllers (Alicat). A Lakeshore 335 temperature controller facilitated the precise adjustment of temperature between 25 °C and 150 °C. All measurement data were logged using a LabVIEW-based data acquisition system connected to a personal computer via a general purpose interface bus (GPIB).
3 Results and discussion
3.1 Structural characterization
Figure 1 presents the FESEM images of the as-prepared PtCu alloy thin film and the nanoporous Pt films obtained by dealloying the alloy in a 1 M HNO3 solution for durations of 1 to 20 h. Dealloying is a critical process in the fabrication of the nanoporous platinum (Pt) films herein. Following the deposition of PtCu alloy thin films via magnetron co-sputtering, dealloying was achieved by soaking the films in a 1 M nitric acid (HNO3) solution for varying periods of time. This involved the selective dissolution of less noble copper (Cu) atoms in the alloy to create a three-dimensional, bicontinuous nanoporous Pt network. The resulting structure has a high surface-to-volume ratio and interconnected porosity at the nanoscale. These properties are essential for enhancing gas–solid interactions and achieving hydrogen adsorption. The dealloying process also provides an easy, room-temperature, scalable route to create functional nanostructured films without relying on templates or lithographic patterning, rendering it extremely viable for sensor applications. The as-prepared PtCu alloy films have a large-grained structure with little porosity (Figure 1a). During the dealloying of PtCu films with HNO3 solution for 1, 5, and 20 h, nanoporous film was observed, and the porosity increased with increasing time (Figures 1b–d, respectively). A more detailed time-dependent surface examination was performed by SEM, and SEM images at 80KX magnification are provided in Supplementary Figure S2. Based on the SEM and FESEM results (Supplementary Figure S2b–d), it is observed that the amount of abrasion on the surface gradually increased when the dealloying time of the PtCu alloy film in HNO3 solution was increased up to 2 h. On the other hand, when the PtCu alloy film was dealloyed in HNO3 solution for 5 h or longer, the surface morphology of the nanopores appeared largely unchanged, with the porosity remaining approximately constant. This consistency is evident in Figures 1c, d and also in Supplementary Figures S2e, f.
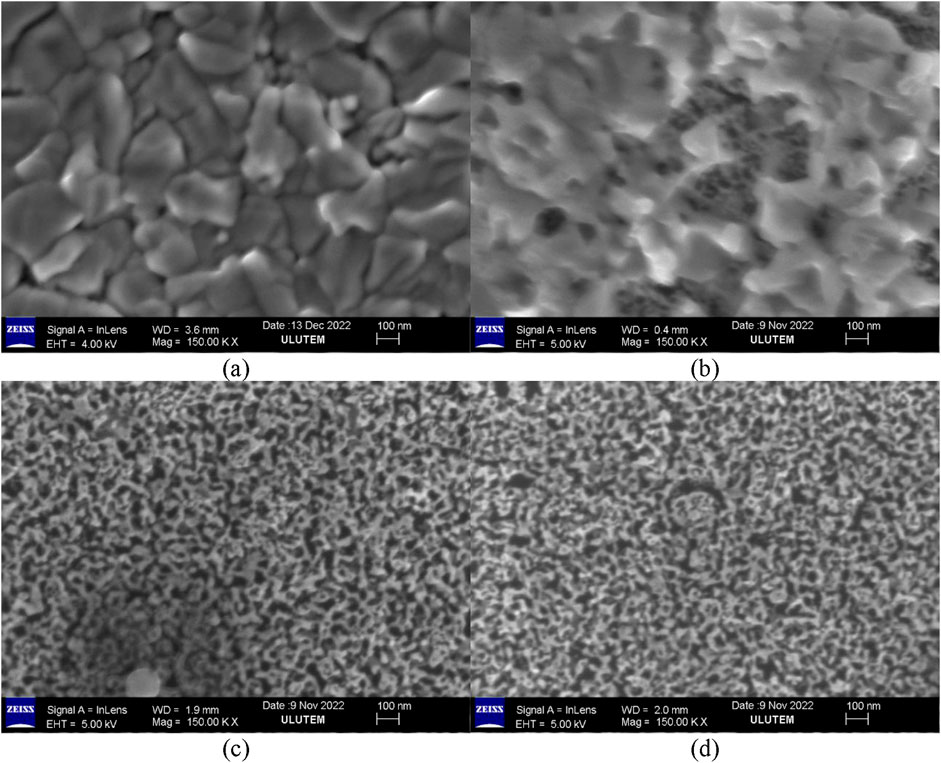
Figure 1. FESEM images of the as-prepared PtCu alloy thin film (a) and nanoporous Pt produced by dealloying in 1 M HNO3 solution at different times (b): 1 h; (c) 5 h; (d) 20 h.
The EDX results of the as-prepared PtCu alloy thin film (a) and nanoporous film produced by dealloying in 1 M HNO3 solution at different times (b: 1 h, c: 2 h, d: 20 h) are presented in Figure 2. Peaks of Si, Ca, Na, Mg, Al, O, Cu, and Pt elements were detected in the samples prepared on the glass substrate according to the EDX spectrums. The observed peaks corresponding to Ca, Na, Mg, Al, and Si elements were attributed to the inherent material composition of the microscopic glass substrate. Conversely, the Cu and Pt peaks originated from the deposited PtCu alloy coating. The atomic ratio of the PtCu alloy was calculated from the EDX spectrum by considering only the percentages of Cu and Pt and was noted to be 15.9:84.1 for the as-prepared PtCu alloy thin film. According to Figure 2b, the copper ratio in the alloy decreased to approximately 56% for the sample that was dealloyed in a HNO3 solution for 1 h. In PtCu, alloys dealloyed in a 1 M HNO3 solution for 5 and 20 h; there were no Cu peaks in the EDX spectrums (Figures 2c, d). This can be attributed to the possibility that either no Cu atoms remain in the structure or that the residual Cu concentration is below the detection limit of the EDX instrument. More detailed analyses conducted using X-ray photoelectron spectroscopy (XPS) support the latter explanation, indicating that Cu residues persist even in the film dealloyed for 20 h. Moreover, this observation supports the fact that the surface of nanoporous Pt, which had been dealloyed for 5 h or more, was the same in SEM and FESEM images.
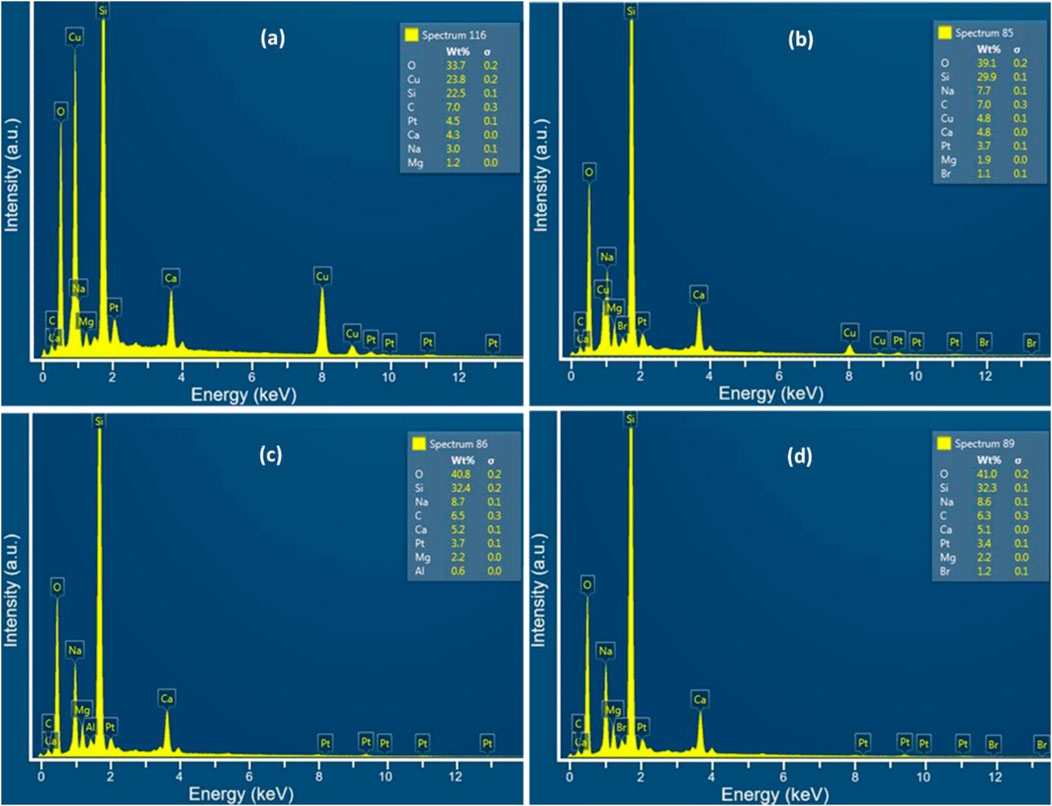
Figure 2. EDX analysis of the as-prepared PtCu alloy thin film (a) and nanoporous films produced by etching in 1 M HNO3 solution at various times (b): 1 h; (c) 5 h; (d) 20 h.
The X-ray diffraction (XRD) graph of the PtCu alloy film and Pt thin film is given in Figure 3a. When the diffraction pattern was examined, it was determined that the diffraction peaks at 39.9 °, 46.3 °, 67.6 °, and 81.5 ° of the Pt film belonged to the planes (111), (200), (220), and (311), respectively, and these peaks reveal Pt film in face-centered cubic (fcc) structure. As shown in Figure 3a, the diffraction peaks of the PtCu alloy exhibit a shift toward higher angles. This could be ascribed to the shrinkage in the lattice structure resulting from the replacement of small Cu atoms with larger Pt atoms (Kang et al., 2015). Similar shifts in diffraction peaks have been observed in previous studies of various PtCu alloy nanostructures, including nanoparticles, nanocrystals, and nanoclusters (Jiang et al., 2013; Oezaslan et al., 2011; Toshima and Wang, 1994). The diffraction peak at 42.2 ° corresponded to the (111) lattice plane, which was reported for PtCu3 (1:3) alloy nanoparticles (Weihua et al., 2006). In our case, a diffraction peak at 43.2 ° corresponding to the (111) lattice plane for the PtCu alloy film was obtained. This indicates a Cu atom proportion of over 75% in the alloy. XPS survey spectra for nanoporous Pt fabricated by dealloying PtCu alloy film in a 1 M HNO3 solution for 20 h are shown in Supplementary Figure S3. We can clearly observe Cu, Pt, C, Si, and O peaks here. The XPS spectra in the Pt 4f and Cu 2p regions for the nanoporous Pt film that were observed by dealloying the PtCu alloy film in the 1 M HNO3 solution for 20 h are presented in Figures 3b, c, respectively. The Pt region XPS spectra have two peaks at approximately 70.8 eV and 72 eV, corresponding to the Pt 4f7/2 core level of metallic Pto and Pt2+ (PtO), respectively. Additionally, two peaks at approximately 74.2 eV and 76.4 eV correspond to the Pt 4f5/2 core level of Pto and Pt2+ (Figure 3b). The Cu XPS spectra show that the Cu 2p3/2 peak contains two peaks at 932.4 eV and 934.1 eV, corresponding to a small metallic Cuo and Cu2+ (CuO or Cu(OH)2) (Figure 3c). Similar Cu+2 and Pt+2 oxide peaks were found for PtCu nanodendrites (Du et al., 2018), core-shell-structured nanoporous PtCu (Qiu et al., 2015b), nanoporous PtCu bimetallic microwire (Qiu et al., 2015a), and nanoporous Pt prepared by dealloying PtCu thin film (Giarratano et al., 2018). As a result, approximately 0.7% Cu remained in the Pt nanoporous films obtained by dealloying PtCu alloy in acid solution for 20 h. In PtCu alloys dealloyed in 1 M HNO3 solution for both 5 and 20 h, no Cu peaks were observed in the EDX spectra (Figures 2c,d). This indicates that the residual Cu content likely falls below the detection limit of the EDX technique. XPS analysis confirmed this, revealing that approximately 0.7% Cu was still present in the 20-h dealloyed film, thereby demonstrating the superior sensitivity of XPS for trace element analysis. There are also small amounts of PtO and CuO oxide structures in the nanoporous film.
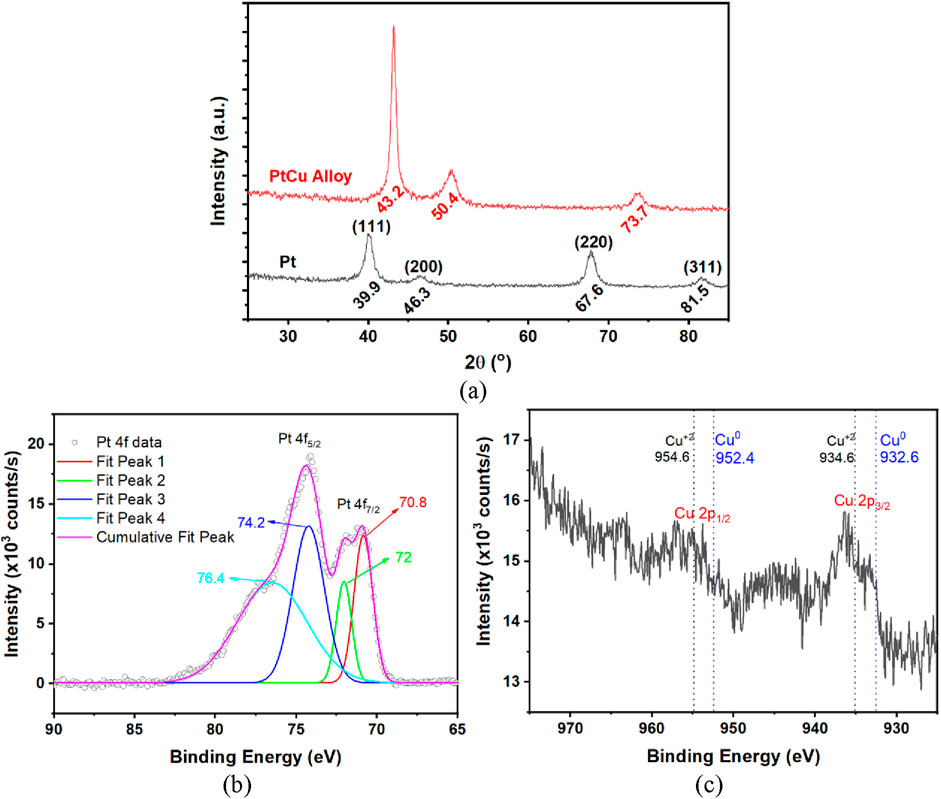
Figure 3. XRD pattern of PtCu alloy and Pt thin films (a), XPS Pt 4f region (b), and Cu 2p region (c) spectrum of nanoporous Pt prepared by dealloying PtCu alloy film in 1 M HNO3 solution for 20 h.
3.2 Hydrogen sensing
Nanoporous Pt film prepared by dealloying PtCu alloy film in the 1 M HNO3 solution for 5 h was chosen to investigate hydrogen-gas-sensing properties and examine the annealing effect on the hydrogen-sensing properties. Nanoporous Pt samples were annealed at 200 °C and 300 °C. Figure 4a shows the resistance change of the as-prepared nanoporous Pt film during exposure to four times 10,000 ppm (1%) hydrogen and dry air cleaning at 50 °C. In the initial instance of hydrogen exposure to the nanoporous Pt sensor device, a decrease in resistance from 321 Ω to 196 Ω was observed. Then, although the measurement cell was purged with dry air for 2 h, the resistance increased slowly and did not recover to the baseline resistance. The resistance decreased when exposed to 10,000 ppm hydrogen for the second, third, and fourth times, but the resistance change was small compared to the first time. There was no recovery during the dry air cleaning. A similar behavior was obtained for Pt nanoporous structures annealed at 200 °C and 300 °C. Figure 4b presents the resistance versus time profile of the nanoporous Pt films annealed at 200 °C and 300 °C, subsequently exposed to dry air and 10,000 ppm hydrogen at 50 °C. In order to desorb hydrogen from the Pt nanoporous material, the devices were heated to 160 °C, kept at this temperature for 1 h, and then cooled to room temperature under a dry air flow. Even so, the resistance value did not recover to the first baseline value. This can be attributed, not to one reason but to a combination of the following reasons: the Cu/CuO remaining in the nanoporous Pt structure, the reduction of PtO in the nanoporous structure to metallic Pt, the hydrogens diffusing into the nanopores forming chemical bonds with the structure, and disordered nanoporous structure with factors such as defects, lattice expansion-contraction, and strain. Hakamada et al. (2011) prepared nanoporous Pt with a dealloying method and investigated high pressure hydrogen absorption/desorption properties. They found abnormal hydrogen absorption/desorption features and explained this by the relaxation/preservation of lattice strain in Pt hydride using first-principles calculations. In addition, Maya et al. (1999) prepared a few µm thick platinum dioxide (PtO2) films using the RF magnetron sputtering method and investigated the reduction properties of these films under 4% hydrogen flow or the electrochemical reduction features at room temperature. They observed the reduction of the PtO2 film to a metallic Pt film through exposure to hydrogen or by electrochemical means. On the other hand, the baseline resistances of the as-prepared, 200 °C, and 300 °C annealed nanoporous Pt were 321 Ω, 274 Ω, and 233 Ω, respectively (Figures 4a,b). The decrease in the baseline resistance of nanoporous Pt with increasing annealing temperature could be explained by the structural reorganization of the nanoporous material. Figures 4c, d reveal the sensor response of as-prepared, 200 °C, and 300 °C annealed nanoporous Pt, which was calculated using Equation 1:
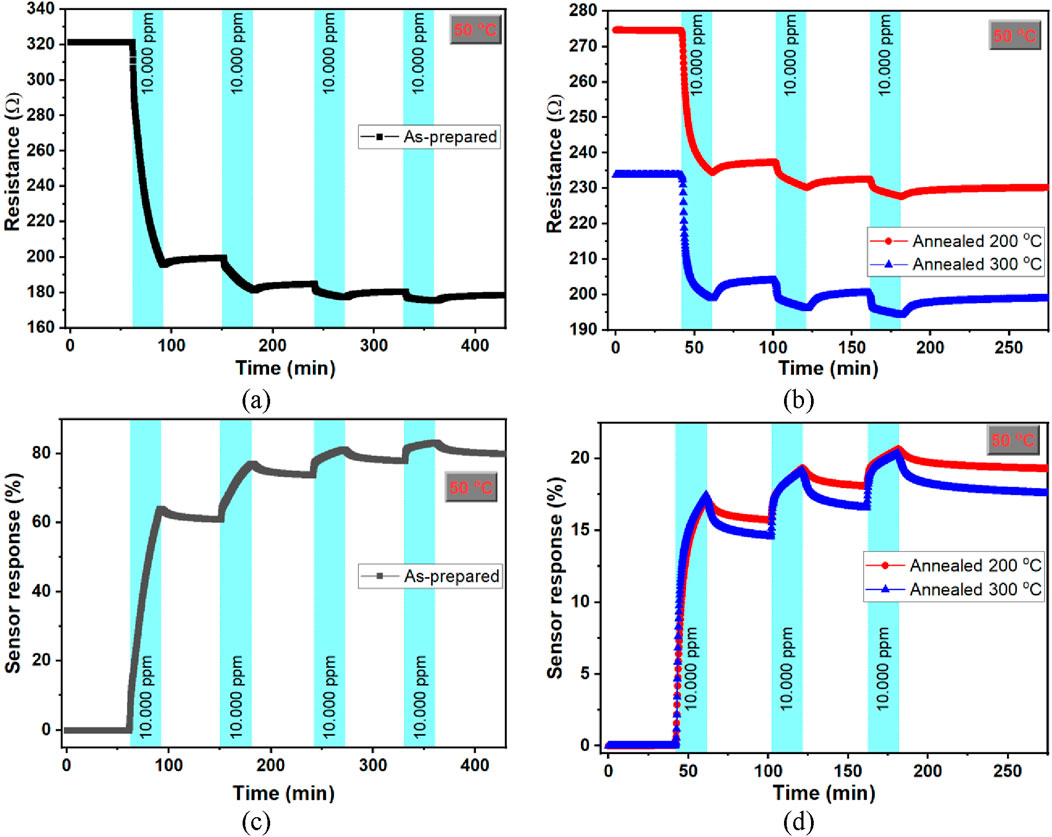
Figure 4. Resistance and sensor response versus time graph for as-prepared (a,c) and annealed nanoporous Pt film at 200–300 °C (b,d) exposed to 10,000 ppm (1%) hydrogen at 50 °C.
where
Figure 5a illustrates the variation in resistance of as-prepared, 200 °C, and 300 °C annealed nanoporous Pt films upon exposure to varying hydrogen concentrations ranging from 1,000 ppm to 3% at an operating temperature of 150 °C. Upon exposure to 1,000 ppm hydrogen, a reduction in the electrical resistance of the nanoporous Pt films was observed. Subsequently, the resistance exhibited a slow, incremental increase during the purging process with high-purity dry air. These trends were consistently reproduced for the remaining hydrogen concentrations examined. Notably, an increase in hydrogen concentration resulted in a corresponding amplification of the resistance change observed in the nanoporous Pt films. The reactions occurring on the nanoporous Pt film surface in different atmospheres are as follows (Gland et al., 1982; Sachs et al., 2001):
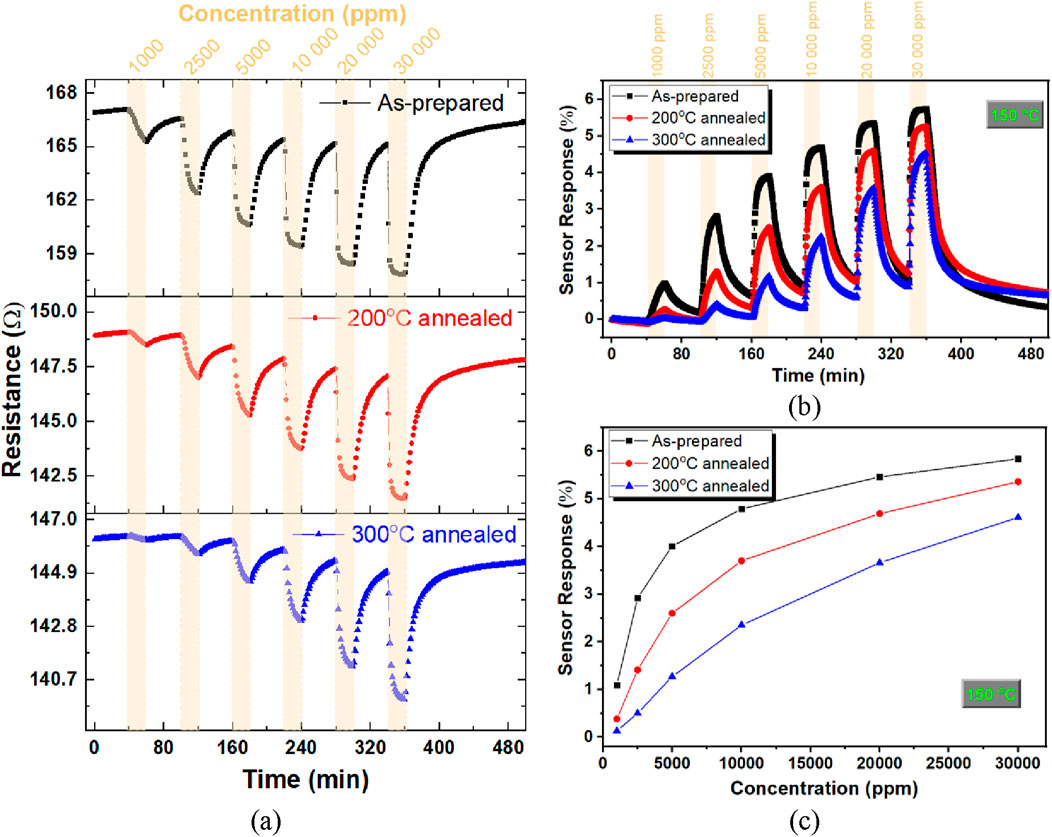
Figure 5. Resistance (a), and sensor response (b) versus time graphs, and sensor response as a function of concentration chart (c) of the nanoporous Pt films depending on annealing exposed to hydrogen concentration (1000 ppm–3%) at 150 °C.
The proposed mechanism for hydrogen sensing is as follows. Under a dry air flow, the surface of the nanoporous Pt film becomes saturated with adsorbed oxygen species (chemical reaction in Equation 2), which influences its electrical resistance. Upon exposure of the nanoporous Pt film to hydrogen, a displacement reaction occurred in which hydrogen atoms interacted with and displaced adsorbed oxygen atoms (chemical reaction in Equation 3), leading to a reduction in electron surface scattering. Therefore, the decrease in the resistance of the nanoporous Pt film can be determined by the surface scattering phenomenon. During the recovery in dry air, oxygen molecules interacted with the adsorbed hydrogen, displacing hydrogen atoms and creating a reversible reaction cycle (chemical reaction in Equation 4). Parallel trends in hydrogen sensing have been noted concerning Pt nanowires (Yang et al., 2012; Yoo et al., 2015; Ding et al., 2017), nanoporous Pt on AAO nanotemplate (Sener et al., 2023), and Pt thin films (Sennik et al., 2016; Patel et al., 1999; Tanaka et al., 2018). In addition, the annealing effect on the baseline resistance and the sensor response of nanoporous Pt thin films are similar to the results before pre-exposure to 4% hydrogen, as mentioned in the previous paragraph. Figure 5b shows the sensor response versus time graph for the as-prepared, 200 °C, and 300 °C annealed nanoporous Pt films exposed to various hydrogen concentrations at 150 °C. It has been established that the sensor responses of all nanoporous Pt films exhibited an increase in response as the concentration was enhanced. The as-prepared nanoporous Pt film showed the highest sensor response in the measured concentration and temperature intervals. Figure 5c shows the sensor response as a function of the concentration curve for the as-prepared, 200 °C, and 300 °C annealed nanoporous Pt at 150 °C. For example, a wide hydrogen concentration range from 10 ppm to 50,000 ppm tests was carried out for the as-prepared nanoporous Pt film as given in the preliminary results that presented at a conference and published in its proceedings (Sener et al., 2022). Therefore, the limit of detection for the as-prepared nanoporous Pt film was lower than 10 ppm.
Figure 6a presents the temperature-dependent sensor response for the as-prepared, 200 °C, and 300 °C annealed nanoporous Pt at 150 °C exposed to 10,000 ppm hydrogen. The sensor response of all nanoporous sensor devices increased with temperature. Figure 6b shows the repeatability of the as-prepared, 200 °C, and 300 °C annealed nanoporous Pt at 150 °C exposed to 11 times 10,000 ppm hydrogen. The sensor response variation was below 10% for 11 consecutive 10,000 ppm hydrogen injections for approximately 7 h, and the average sensor responses were obtained as 4.55, 3.39, and 2.51 for the as-prepared, 200 °C, and 300 °C annealed nanoporous Pt, respectively.
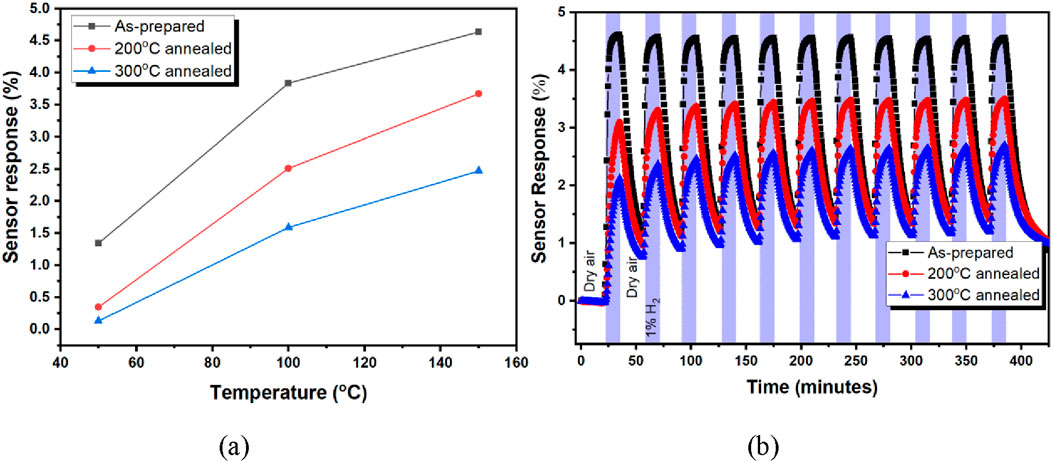
Figure 6. Temperature-dependent sensor response (a) and repeatability (b) of the as prepared, 200 °C, and 300 °C annealed nanoporous Pt at 150 °C exposed to 10,000 ppm hydrogen.
4 Conclusion
In the present study, a novel avenue for hydrogen sensing was unveiled through the fabrication of nanoporous platinum (Pt) films using the dealloying technique. Employing the magnetron co-sputtering approach, platinum–copper (PtCu) alloy films approximately 50 nm thick were meticulously deposited onto glass substrates. The central step involved the strategic immersion of PtCu alloy films in a 1 M nitric acid solution for varying durations for the subsequent transformation into nanoporous Pt films. Remarkably, after a 5-h and more immersion period, an intriguing outcome surfaced—the almost complete removal of Cu from the alloy matrix, resulting in a nanoporous Pt film distinguished by a well-defined and uniform pore structure. The primary objective of the study was to assess the performance of the nanoporous Pt film as a hydrogen sensor across a broad range of concentrations and temperatures. A key finding emerged as the film demonstrated an impressive sensor response of approximately 4.5 when subjected to 10,000 ppm hydrogen concentration. Furthermore, the underlying sensing mechanism of the nanoporous Pt film was attributed to the surface scattering phenomenon, whereby hydrogen interaction induces changes in electrical conductivity, leading to measurable alterations in resistance. The culmination of this research highlights the remarkable promise of nanoporous Pt films as a robust candidate for hydrogen sensing applications. Nanoporous Pt film sensors could potentially be used in leakage detection, breath hydrogen analysis, and transformer oil degradation.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors without undue reservation.
Author contributions
MS: Data curation, Methodology, Writing – original draft, Investigation, Visualization, Validation, Writing – review and editing, Conceptualization, Formal Analysis. AA: Data curation, Writing – original draft, Investigation, Conceptualization, Writing – review and editing, Validation, Methodology, Formal Analysis. RZ: Formal Analysis, Conceptualization, Methodology, Funding acquisition, Writing – review and editing, Supervision, Writing – original draft, Resources. NK: Formal Analysis, Writing – review and editing, Methodology, Validation, Project administration, Supervision, Conceptualization, Software, Funding acquisition, Writing – original draft, Resources.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This study was supported by the Scientific and Technological Research Council of Türkiye (TUBITAK) with a project number of 121M681.
Acknowledgments
The authors would like to thank TUBITAK for financial support.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnano.2025.1599264/full#supplementary-material
References
Abburi, A., and Yeh, W. J. (2012). Temperature and pore size dependence on the sensitivity of a hydrogen sensor based on nanoporous platinum thin films. IEEE Sensors J. 12, 2625–2629. doi:10.1109/jsen.2012.2199298
Abe, J. O., Popoola, A., Ajenifuja, E., and Popoola, O. M. (2019). Hydrogen energy, economy and storage: review and recommendation. Int. J. hydrogen energy 44, 15072–15086. doi:10.1016/j.ijhydene.2019.04.068
Ball, M., Basile, A., and Veziroglu, T. N. (2015). Compendium of hydrogen energy: hydrogen use, safety and the hydrogen economy. Amsterdam: Woodhead Publishing.
Cai, T., Zhang, H., Xing, H., Xia, X., Zhang, Z., Zhang, K., et al. (2023). Pd-Pt alloy-based hydrogen sensors based on patterned sapphire substrate for wide-range hydrogen detection. J. Electron. Mater. 52, 4959–4970. doi:10.1007/s11664-023-10418-6
Cao, F., Zhao, P. F., Wang, Z., Zhang, X. H., Zheng, H., Wang, J. B., et al. (2019). An ultrasensitive and ultraselective hydrogen sensor based on defect-dominated electron scattering in Pt nanowire arrays. Adv. Mater. Interfaces 6, 1801304. doi:10.1002/admi.201801304
Ding, M. N., Liu, Y., Wang, G. M., Zhao, Z. P., Yin, A. X., He, Q. Y., et al. (2017). Highly sensitive chemical detection with tunable sensitivity and selectivity from ultrathin platinum nanowires. Small 13, 1602969. doi:10.1002/smll.201602969
Du, Y., Ni, K., Zhai, Q., Yun, Y., Xu, Y., Sheng, H., et al. (2018). Facile air oxidative induced dealloying of hierarchical branched PtCu nanodendrites with enhanced activity for hydrogen evolution. Appl. Catal. A General 557, 72–78. doi:10.1016/j.apcata.2018.03.014
Erkovan, M., Deger, C., Cardoso, S., and Kilinc, N. (2022). Hydrogen-sensing properties of ultrathin Pt-Co alloy films. Chemosensors 10, 512. doi:10.3390/chemosensors10120512
Giarratano, F., Arzac, G., Godinho, V., Hufschmidt, D., De Haro, M. J., Montes, O., et al. (2018). Nanoporous Pt-based catalysts prepared by chemical dealloying of magnetron-sputtered Pt-Cu thin films for the catalytic combustion of hydrogen. Appl. Catal. B Environ. 235, 168–176. doi:10.1016/j.apcatb.2018.04.064
Gland, J. L., Fisher, G. B., and Kollin, E. B. (1982). The hydrogen oxygen reaction over the Pt(111) surface - transient titration of adsorbed oxygen with hydrogen. J. Catal. 77, 263–278. doi:10.1016/0021-9517(82)90167-1
Hakamada, M., Furukawa, T., Yamamoto, T., Takahashi, M., and Mabuchi, M. (2011). Abnormal hydrogen absorption/desorption properties of nanoporous Pt with large lattice strains. Mater. Trans. 52, 806–809. doi:10.2320/matertrans.m2010403
Hassan, K., Uddin, A. S. M. I., and Chung, G. S. (2016). Fast-response hydrogen sensors based on discrete Pt/Pd bimetallic ultra-thin films. Sensors Actuators B-Chemical 234, 435–445. doi:10.1016/j.snb.2016.05.013
Hubert, T., Boon-Brett, L., Black, G., and Banach, U. (2011). Hydrogen sensors - a review. Sensors Actuators B-Chemical 157, 329–352. doi:10.1016/j.snb.2011.04.070
Jiang, Y., Jia, Y., Zhang, J., Zhang, L., Huang, H., Xie, Z., et al. (2013). Underpotential deposition-induced synthesis of composition-tunable Pt Cu nanocrystals and their catalytic properties. Chemistry–A Eur. J. 19, 3119–3124. doi:10.1002/chem.201203729
Kang, W., Li, R., Wei, D., Xu, S., Wei, S., and Li, H. (2015). CTAB-Reduced synthesis of urchin-like Pt–Cu alloy nanostructures and catalysis study towards the methanol oxidation reaction. RSC Adv. 5, 94210–94215. doi:10.1039/c5ra20464j
Kilinc, N. (2013). Resistive hydrogen sensors based on nanostructured metals and metal alloys. Nanosci. Nanotechnol. Lett. 5, 825–841. doi:10.1166/nnl.2013.1653
Kilinc, N., and Erkovan, M. J. (2023). Nanostructured platinum and platinum alloy-based resistive hydrogen sensors: a review. 48, 18, doi:10.3390/csac2023-14912
Kilinc, N., Sanduvac, S., and Erkovan, M. (2022). Platinum-nickel alloy thin films for low concentration hydrogen sensor application. J. Alloys Compd. 892, 162237. doi:10.1016/j.jallcom.2021.162237
Kilinc, N., Cardoso, S., and Erkovan, M. (2024). Rare Earth material for hydrogen gas sensing: PtGd alloy thin films as a promising frontier. Nanomaterials 14, 1098. doi:10.3390/nano14131098
Koo, W. T., Cho, H. J., Kim, D. H., Kim, Y. H., Shin, H., Penner, R. M., et al. (2020). Chemiresistive hydrogen sensors: fundamentals, recent advances, and challenges. Acs Nano 14, 14284–14322. doi:10.1021/acsnano.0c05307
Maya, L., Brown, G. M., and Thundat, T. (1999). Porous platinum electrodes derived from the reduction of sputtered platinum dioxide films. J. Appl. Electrochem. 29, 881–886. doi:10.1023/a:1003581715968
Oezaslan, M., Hasché, F., and Strasser, P. (2011). In situ observation of bimetallic alloy nanoparticle formation and growth using high-temperature XRD. Chem. Mater. 23, 2159–2165. doi:10.1021/cm103661q
Patel, S. V., Gland, J. L., and Schwank, J. W. (1999). Film structure and conductometric hydrogen-gas-sensing characteristics of ultrathin platinum films. Langmuir 15, 3307–3311. doi:10.1021/la9809426
Penner, R. M. (2017). A nose for hydrogen gas: fast, sensitive H-2 sensors using electrodeposited nanomaterials. Accounts Chem. Res. 50, 1902–1910. doi:10.1021/acs.accounts.7b00163
Qiu, H.-J., Shen, X., Wang, J., Hirata, A., Fujita, T., Wang, Y., et al. (2015a). Aligned nanoporous Pt–Cu bimetallic microwires with high catalytic activity toward methanol electrooxidation. Acs Catal. 5, 3779–3785. doi:10.1021/acscatal.5b00073
Qiu, H.-J., Xu, H., Li, X., Wang, J., and Wang, Y. (2015b). Core–shell-structured nanoporous PtCu with high Cu content and enhanced catalytic performance. J. Mater. Chem. A 3, 7939–7944. doi:10.1039/c5ta00020c
Rajoua, K., Baklouti, L., and Favier, F. (2018). Platinum for hydrogen sensing: surface and grain boundary scattering antagonistic effects in Pt@Au core-shell nanoparticle assemblies prepared using a langmuir-blodgett method. Phys. Chem. Chem. Phys. 20, 383–394. doi:10.1039/c7cp06645g
Rane, S., Arbuj, S., Rane, S., and Gosavi, S. (2015). Hydrogen sensing characteristics of Pt–SnO 2 nano-structured composite thin films. J. Mater. Sci. Mater. Electron. 26, 3707–3716. doi:10.1007/s10854-015-2889-3
Sachs, C., Hildebrand, M., Völkening, S., Wintterlin, J., and Ertl, G. (2001). Spatiotemporal self-organization in a surface reaction: from the atomic to the mesoscopic scale. Science 293, 1635–1638. doi:10.1126/science.1062883
Sahoo, T., and Kale, P. (2021). Work function-based metal-oxide-semiconductor hydrogen sensor and its functionality: a review. Adv. Mater. Interfaces 8, 2100649. doi:10.1002/admi.202100649
Sener, M., Altuntepe, A., Zan, R., and Kilinc, N. (2022). Fabrication of nanoporous platinum films with dealloying method for hydrogen sensor application. Eng. Proc. 27, 25. doi:10.3390/ecsa-9-13317
Sener, M., Sisman, O., and Kilinc, N. (2023). AAO-assisted nanoporous platinum films for hydrogen sensor application. Catalysts 13, 459. doi:10.3390/catal13030459
Sennik, E., Urdem, S., Erkovan, M., and Kilinc, N. (2016). Sputtered platinum thin films for resistive hydrogen sensor application. Mater. Lett. 177, 104–107. doi:10.1016/j.matlet.2016.04.134
Sisman, O., Erkovan, M., and Kilinc, N. (2024). Hydrogen sensors for safety applications. Towards Hydrogen Infrastruct., 275–314. doi:10.1016/b978-0-323-95553-9.00061-3
Tanaka, T., Hoshino, S., Takahashi, T., and Uchida, K. (2018). Nanoscale Pt thin film sensor for accurate detection of ppm level hydrogen in air at high humidity. Sensors Actuators B-Chemical 258, 913–919. doi:10.1016/j.snb.2017.11.115
Toshima, N., and Wang, Y. (1994). Preparation and catalysis of novel colloidal dispersions of copper/noble metal bimetallic clusters. Langmuir 10, 4574–4580. doi:10.1021/la00024a031
Uddin, A. S. M. I., Yaqoob, U., Hassan, K., and Chung, G. S. (2016). Effects of Pt shell thickness on self-assembly monolayer pd@Pt core-shell nanocrystals based hydrogen sensing. Int. J. Hydrogen Energy 41, 15399–15410. doi:10.1016/j.ijhydene.2016.06.138
Weihua, W., Xuelin, T., Kai, C., and Gengyu, C. (2006). Synthesis and characterization of Pt–Cu bimetallic alloy nanoparticles by reverse micelles method. Colloids Surfaces A Physicochem. Eng. Aspects 273, 35–42. doi:10.1016/j.colsurfa.2005.07.029
Yang, F., Donavan, K. C., Kung, S. C., and Penner, R. M. (2012). The surface scattering-based detection of hydrogen in air using a platinum nanowire. Nano Lett. 12, 2924–2930. doi:10.1021/nl300602m
Keywords: nanoporous, platinum, dealloying method, co-sputtering, hydrogen sensor, resistive sensor
Citation: Sener M, Altuntepe A, Zan R and Kilinc N (2025) Dealloyed nanoporous platinum films: synthesis, characterization, and hydrogen sensing properties. Front. Nanotechnol. 7:1599264. doi: 10.3389/fnano.2025.1599264
Received: 24 March 2025; Accepted: 23 July 2025;
Published: 22 August 2025.
Edited by:
Cristina Satriano, University of Catania, ItalyReviewed by:
Tiziana Polichetti, Energy and Sustainable Economic Development (ENEA), ItalyJagriti Behal, Sri Sai University, India
Copyright © 2025 Sener, Altuntepe, Zan and Kilinc. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Necmettin Kilinc, bmVjbWV0dGlua2lsaW5jQGdtYWlsLmNvbQ==, bmVjbWV0dGluLmtpbGluY0Bpbm9udS5lZHUudHI=
 Melike Sener1
Melike Sener1 Ali Altuntepe
Ali Altuntepe Necmettin Kilinc
Necmettin Kilinc