- USDA Regulatory Science Center of Excellence, University of Arkansas at Pine Bluff, Pine Bluff, AR, United States
The increasing application of engineered nanoparticles (ENPs) in agriculture for enhanced crop production and protection has raised significant concerns about their environmental fate and potential toxicity. This review examines how particle size, surface coating, and aging influence the transport and toxicity of nanoparticles in agricultural ecosystems. Smaller nanoparticles exhibit greater mobility and reactivity, often leading to increased plant uptake and potential phytotoxic effects, including reduced germination, root inhibition, and oxidative stress. Surface coatings, such as polyethylene glycol (PEG) or natural organic matter, play a crucial role in modulating nanoparticle behavior by stabilizing dispersion, altering bioavailability, and mitigating toxicity. As nanoparticles age in the environment, processes like sulfidation, oxidation, and biotransformation modify their physicochemical properties, often reducing their toxicity but complicating their long-term environmental behavior. The interaction of these variables with soil properties, microbial communities, and plant systems underscores the complexity of nanoparticle dynamics in agricultural settings. While laboratory studies have provided valuable insights, long-term field data and assessments under realistic agrarian conditions remain limited. A better understanding of these factors is essential for predicting environmental impacts and guiding the development of safer and more sustainable nanotechnologies in agriculture. The increasing use of nanoparticles (NPs) in various industrial and consumer applications has led to their inevitable release into agricultural ecosystems. This review article explores the environmental fate, transport, and toxicity of NPs in agroecosystems, emphasizing how particle size, surface coating, and aging influence their interactions with soil, water, plants, and microorganisms. Mechanistic insights, recent findings, and knowledge gaps are discussed to inform safer nanoparticle design and sustainable agricultural practices.
Introduction
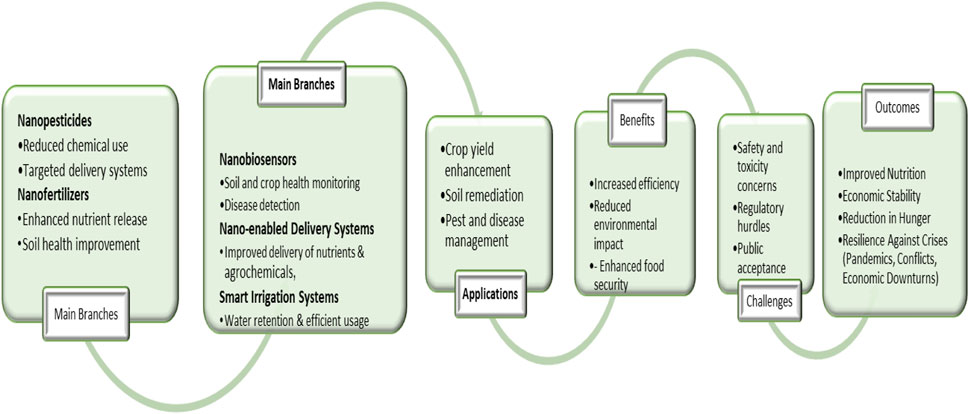
Nanotechnology has revolutionized many sectors, including agriculture, where nanoparticles are used for targeted delivery of agrochemicals, nano-fertilizers, and pest control agents. However, the unintentional release of engineered nanoparticles (ENPs) poses potential risks to soil health, crop productivity, and food safety. Understanding the behavior of NPs in the complex agricultural environment is essential. It has emerged as a transformative force in agriculture, offering innovative solutions to longstanding challenges related to crop productivity, nutrient use efficiency, pest control, and environmental sustainability. ENPs typically defined as materials with at least one dimension under 100 nm, are increasingly incorporated into agrochemical formulations such as nano-fertilizers, nano-pesticides, and soil conditioners due to their enhanced reactivity, targeted delivery potential, and controlled release properties (Bouhadi et al., 2025; Kah et al., 2018; Servin et al., 2015). However, as their application expands, so does the concern about their unintended introduction into agricultural ecosystems through diverse pathways, including irrigation with contaminated water, nanoparticle-laden biosolids, atmospheric deposition, and direct incorporation into soil or plant systems (Figure 1). Once in the environment, ENPs interact dynamically with soil components, plant roots, microbial communities, and water sources, triggering complex transport and transformation behaviors that are often governed by three principal factors: particle size, surface coating, and environmental aging (Wang et al., 2023; Ma and Wang, 2010; Shiv et al., 2024). Particle size fundamentally influences nanoparticle mobility, uptake, and toxicity, as smaller particles possess a greater surface area-to-volume ratio and tend to exhibit higher chemical reactivity and bioavailability, facilitating their translocation across biological membranes and into edible plant tissues (Nawaz, 2025; Rico et al., 2011; Servin et al., 2015). Surface coatings intentionally added during synthesis to stabilize nanoparticles modulate their physicochemical interactions with the environment by altering surface charge, hydrophobicity, and aggregation behavior; standard coatings like polyethylene glycol (PEG), citrate, and natural organic matter have been shown to either mitigate or enhance toxicity depending on their composition and environmental context (Wikipedia, 2025; Keller et al., 2013; Ahmed et al., 2021). Meanwhile, ecological aging, encompassing a suite of physical, chemical, and biological processes, transforms ENPs over time through sulfidation, oxidation-reduction, dissolution, and microbial interaction (Mondejar-Lopez et al., 2024). These transformations can reduce or amplify the toxicity of ENPs, impact on their bioavailability, and generate new nano-species or by-products whose environmental and toxicological profiles are not well understood (Shiv et al., 2024; Shao et al., 2022; Lowry et al., 2012).
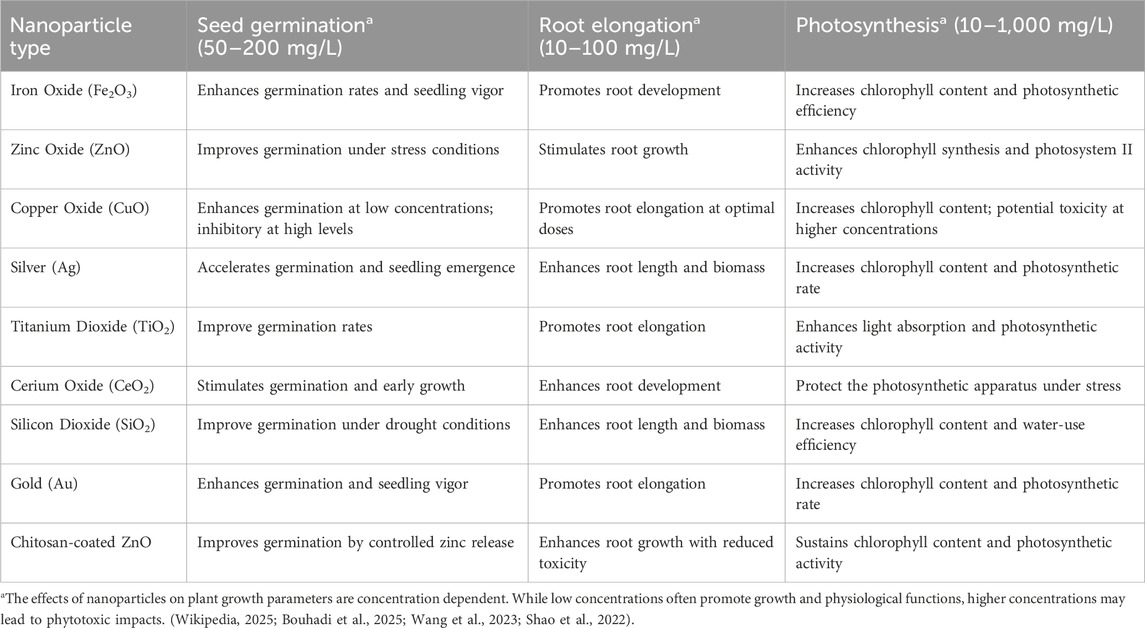
The toxicity of ENPs to plants varies by nanoparticle type and environmental concentration, with observed effects ranging from inhibited seed germination, root elongation, and photosynthesis, to increased oxidative stress and altered nutrient uptake (Bouhadi et al., 2025; Singh et al., 2021; Ma et al., 2010; Dimkpa et al., 2012). Moreover, ENPs have been shown to disrupt soil microbial communities, suppress enzymatic activities crucial to nutrient cycling, and impair beneficial plant-microbe symbioses, thereby threatening soil health and fertility (Nawaz, 2025; Ge et al., 2011; Raliya et al., 2015). While laboratory studies have contributed significantly to our understanding of nanoparticle behavior and effects, they often fail to replicate the complexity of real agricultural environments shaped by variable soil chemistry, climate conditions, and co-exposure to other agrochemicals. Long-term field studies remain sparse, and significant knowledge gaps persist regarding the chronic effects of low-level ENP exposure, the cumulative impact of mixed nanoparticle systems, and their interactions with climate-induced stressors. As such, we must advance our mechanistic understanding of how particle size, surface modifications, and aging processes influence ENP transport and toxicity across trophic levels and over time (Figure 2). This knowledge is essential not only for the accurate risk assessment and regulation of nanotechnology in agriculture but also for guiding the design of next-generation ENPs that are both effective and environmentally benign, aligning technological innovation with the principles of ecological safety and sustainable food production (Wikipedia, 2025; Bundschuh et al., 2018; Rico et al., 2011).
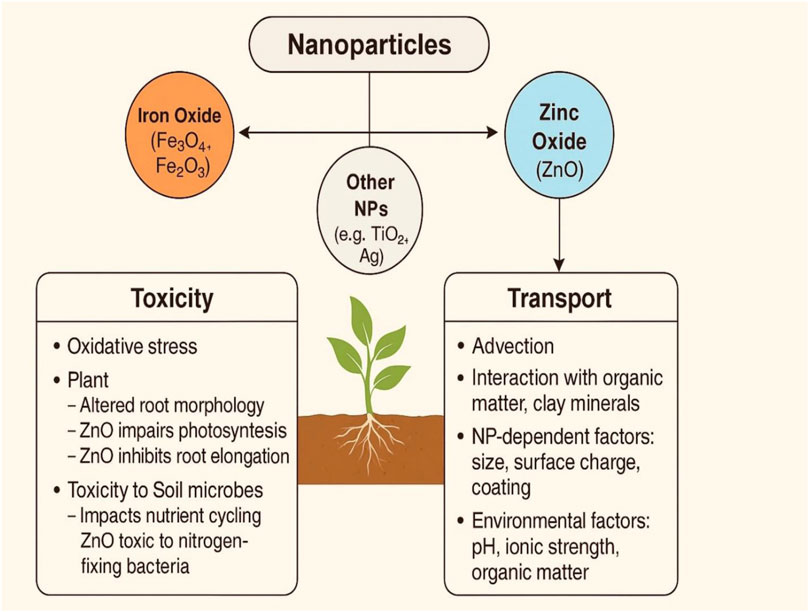
The toxicity and transport of nanoparticles (NPs) in agricultural ecosystems are increasingly scrutinized due to their widespread use and potential environmental impacts. Among the most studied NPs are iron oxide (Fe3O4 or Fe2O3) and zinc oxide (ZnO), both of which are widely applied in fertilizers, pesticides, and soil amendments due to their nutrient-delivering and antimicrobial properties. However, their interactions with soil biota, plants, and water systems raise concerns. While generally considered less toxic, iron oxide nanoparticles (IONPs) can induce oxidative stress and alter root morphology and microbial community structure at higher concentrations (Kumar et al., 2021). They tend to aggregate in soil, reducing mobility but posing risks through prolonged exposure and accumulation. In contrast, ZnO nanoparticles are more soluble and mobile, and their dissolution releases Zn2+ ions, which can disrupt plant physiological processes, impair photosynthesis, and inhibit root elongation (Dimkpa et al., 2012). Moreover, ZnO NPs have shown significant toxicity toward beneficial soil microbes, including nitrogen-fixing bacteria and mycorrhizal fungi, impacting nutrient cycling and plant health (Raliya and Tarafdar, 2013). Transport mechanisms of these NPs in soil involve advection, diffusion, and interaction with organic matter and clay minerals. The size, surface charge, and coating of nanoparticles greatly influence their mobility and bioavailability. For instance, smaller particles with high surface area are more likely to penetrate plant root systems and enter the food chain (Servin et al., 2015). Environmental factors such as pH, ionic strength, and the presence of natural organic matter further modulate NP behavior, enhancing their dispersion or promoting aggregation. Other NPs like titanium dioxide (TiO2) and silver (Ag) nanoparticles also exhibit varying degrees of phytotoxicity and persistence, with TiO2 typically showing low toxicity but potential for accumulation, and Ag NPs being notably toxic to both plants and microbes even at low concentrations (Wang et al., 2016). Hence, while nanoparticles offer innovative tools for sustainable agriculture, their unintended ecotoxicological effects and complex transport dynamics warrant careful risk assessment and regulation to avoid long-term environmental consequences (Schlagenhauf et al., 2015).
Classification and common types of nanoparticles in agriculture
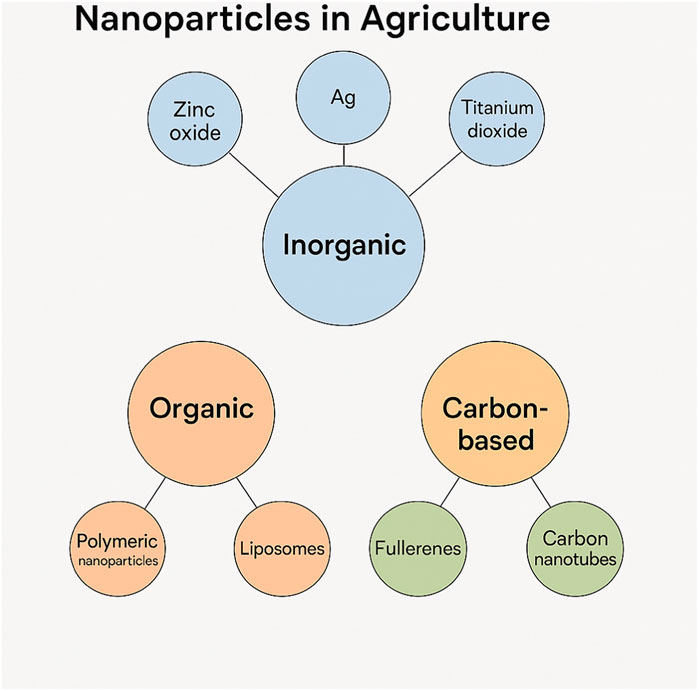
Nanoparticles (NPs) have become increasingly prevalent in agriculture due to their unique properties, such as increased surface area, reactivity, and the ability to interact with plant systems, soil, and pests. Their application in agriculture spans various uses, from enhancing plant growth and soil fertility to controlling pests and diseases. The classification of nanoparticles used in agriculture is based on their size, material composition, and intended functional role in agricultural applications. Understanding the different types of nanoparticles and their classifications is essential to assess their effectiveness, environmental impact, and potential toxicity. Nanoparticles can be classified in several ways, but the most common categorizations are based on their material composition, size, and shape (Figure 3).
Material composition
Nanoparticles can be categorized based on the materials from which they are made. The key categories include:
(a) Metal-based nanoparticles: These include nanoparticles composed of metals like silver (AgNPs), copper (CuNPs), zinc oxide (ZnO NPs), and titanium dioxide (TiO2 NPs). Metal-based nanoparticles are widely used in agriculture for their antimicrobial properties, as they can help control plant diseases and protect crops from pathogens (Raha and Ahmaruzzaman, 2022). They are also used in nanofertilizers for enhanced nutrient delivery.
(b) Carbon-based nanoparticles: This class includes materials like carbon nanotubes (CNTs), graphene oxide (GO), and fullerenes. These nanoparticles are gaining attention due to their high surface area, conductivity, and ability to improve plant growth, nutrient uptake, and stress resistance (Mukherjee et al., 2016). Carbon-based nanoparticles also exhibit potential as carriers for pesticide delivery, enabling more targeted treatments.
(c) Polymeric nanoparticles: These are made from biocompatible and biodegradable polymers such as poly (lactic acid) (PLA), polycaprolactone (PCL), or chitosan. They are used in controlled release systems for fertilizers and pesticides, offering advantages in reducing nutrient loss, enhancing efficiency, and minimizing environmental contamination (Kumari and Yadav, 2014).
(d) Ceramic nanoparticles: These include silica-based nanoparticles (SiO2 NPs) that are often used in plant growth promotion. They can enhance soil structure and water retention. SiO2 nanoparticles can also improve the delivery of plant nutrients and help plants resist abiotic stressors like drought and salinity (Bhat et al., 2021).
Size-based classification
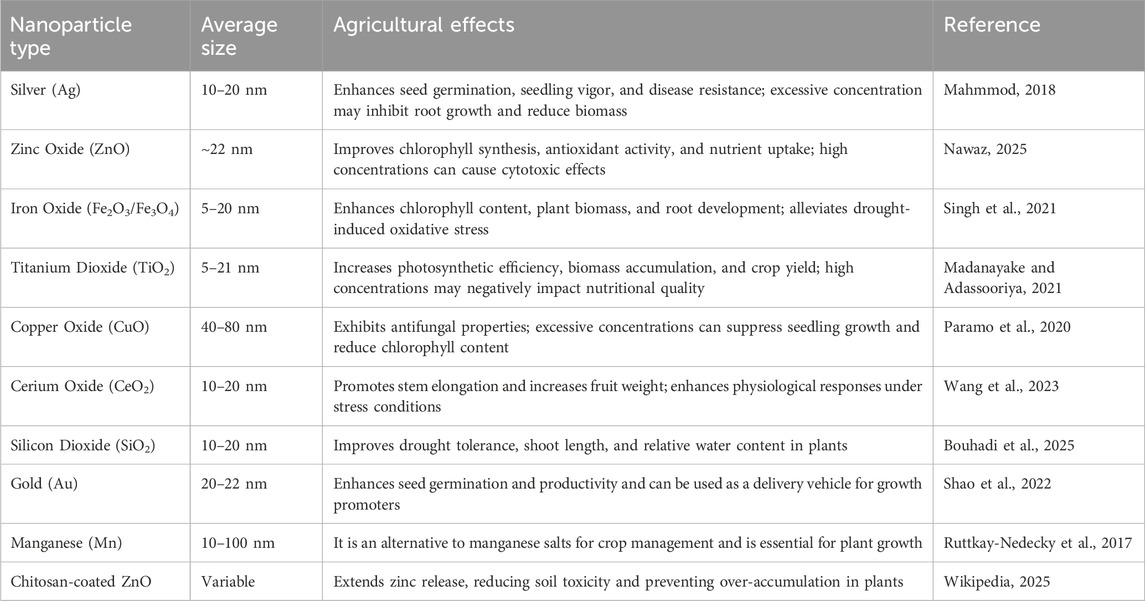
Nanoparticles are defined by size, typically in the 1–100 nm range. The size of nanoparticles greatly influences their reactivity, bioavailability, and interaction with biological systems (Table 1).
(a) Nanoscale particles (<100 nm): These particles exhibit unique properties such as high surface-to-volume ratios, which can enhance their reactivity and interaction with plant cells. These particles are often used for improving nutrient uptake, controlling pests, or delivering active agents like herbicides and insecticides.
(b) Submicron particles (100 nm–1 µm): These particles are larger than typical nanoparticles but maintain specific nanomaterial properties. Their use in agriculture is less common, but they are often employed in slow-release systems for fertilizers or agrochemicals.
Shape-based classification
Nanoparticles can also be classified according to shape, affecting their function and interactions with biological systems.
(a) Spherical nanoparticles: These are the most common form of nanoparticles and are often used in nanofertilizer or pesticide formulations due to their ability to be easily synthesized and controlled in size.
(b) Rod-shaped nanoparticles: Rods or nanorods have higher surface areas and may enhance their interaction with plant roots, making them ideal for specific agricultural applications, such as pest control and nutrient delivery systems.
(c) Nanosheets: These are flat, two-dimensional nanoparticles, and materials like graphene oxide and molybdenum disulfide fall into this category. They are being explored to enhance soil fertility and plant growth and improve stress tolerance (Zhang et al., 2008).
Common types of nanoparticles used in agriculture
Silver nanoparticles (AgNPs)
Silver nanoparticles are among agriculture’s most studied and widely applied nanoparticles (Table 1). Known for their strong antimicrobial properties, AgNPs are used in agricultural applications to control diseases and fungi that affect crops. Their ability to release silver ions makes them effective against various pathogens, including bacteria and fungi. AgNPs are also utilized in soil treatments to promote plant growth by enhancing soil microbial health (Rai et al., 2009). However, their potential toxicity to non-target organisms, such as soil microbes and aquatic microorganisms, necessitates caution in their use.
Zinc oxide nanoparticles (ZnO NPs)
Zinc oxide nanoparticles are commonly used in agriculture for their antimicrobial properties and as a source of zinc, an essential micronutrient for plants. ZnO NPs can be used in nano fertilizers to enhance plant growth and crop yield. They also improve plant resistance to environmental stresses like UV radiation (Raha and Ahmaruzzaman, 2022). Additionally, ZnO NPs have been used sustainably to control pests and pathogens, reducing the need for chemical pesticides. However, their accumulation in soil and potential long-term effects on plant health and soil microorganisms require further investigation.
Titanium dioxide nanoparticles (TiO2 NPs)
Titanium dioxide nanoparticles are used in agricultural practices primarily for their photocatalytic properties, which can help break down organic pollutants and pesticides in the environment. TiO2 NPs are also employed as additives in fertilizers, as they can enhance the uptake of nutrients by plants, promote plant growth, and help plants cope with oxidative stress caused by environmental factors like drought and pollution (Jampilek and Kralova, 2015).
Carbon nanotubes (CNTs)
Carbon nanotubes (CNTs) are extensively used in agriculture to improve the delivery of nutrients and pesticides to plants. Due to their high surface area and ability to be functionalized, CNTs can be used to deliver fertilizers, herbicides, and fungicides in a controlled manner. They also have potential applications in enhancing soil structure, water retention, and the remediation of contaminated soils. However, concerns about the toxicity of CNTs to soil organisms and their potential persistence in the environment remain (Laux et al., 2018).
Chitosan nanoparticles
Chitosan nanoparticles are derived from chitin, a biopolymer found in the exoskeleton of crustaceans. Chitosan-based nanoparticles are biodegradable and eco-friendly, making them ideal for agricultural applications. These nanoparticles are used to deliver pesticides, fungicides, and fertilizers. Additionally, chitosan nanoparticles can enhance plant growth, improve soil health, and protect against plant diseases by inducing systemic resistance (Kurczewska 2023).
Transport mechanisms in soil and water in relation to nanoparticles in agriculture
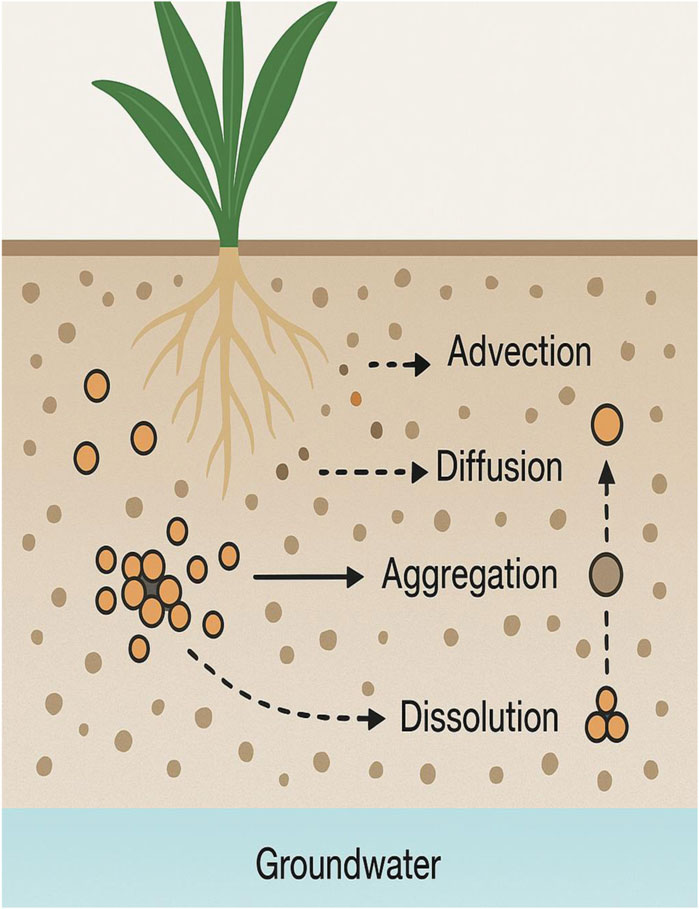
Nanoparticles enter soil through irrigation, biosolids, or atmospheric deposition. Their mobility is influenced by physicochemical properties and environmental conditions (Figure 4). The Key Transport Factors are Particle Size (smaller particles exhibit greater mobility), Surface Coating (alters surface charge and interaction with soil colloids), and Soil pH and Organic Matter (affect aggregation and adsorption).
Transport mechanisms
The transport of engineered nanoparticles (ENPs) in soil and water systems is critical in determining their environmental fate, bioavailability, and potential risks to agroecosystems. Understanding these mechanisms is essential for assessing exposure pathways, predicting accumulation in plant tissues, and formulating regulations for safe nanoparticle application in agriculture. Once introduced into the environment through nano-enabled fertilizers, pesticides, biosolid amendments, or irrigation with nanoparticle-containing water, ENPs undergo complex physical and chemical interactions with soil and water matrices. These interactions influence whether nanoparticles remain mobile or become immobilized, whether they transform, and to what extent they are taken up by plants or leach into groundwater. The primary transport mechanisms governing nanoparticle mobility in soil and water include advection, diffusion, dispersion, filtration, aggregation, sedimentation, dissolution, and interaction with soil biota and organic matter (Ahmed et al., 2021; Hotze et al., 2010). In saturated and unsaturated soil conditions, advection, the bulk movement of water carrying ENPs, is a dominant process, particularly for smaller, stable nanoparticles that resist aggregation. As water moves through soil pores, it transports dispersed nanoparticles. The rate and extent of advective transport are influenced by soil texture, structure, water content, and hydraulic conductivity. Diffusion, driven by concentration gradients, plays a more limited role but becomes significant in stagnant or low-flow environments where Brownian motion governs nanoparticle displacement (Nowack and Bucheli, 2007). Mechanical dispersion of nanoparticles due to velocity variations within the soil pore network also contributes to the spatial distribution of nanoparticles, particularly in heterogeneous soil systems. Once within the soil matrix, nanoparticles may interact with solid surfaces, leading to filtration and retention. Physical straining occurs when nanoparticles become trapped in small soil pores, especially if their size approaches or exceeds the pore throat diameter. Electrostatic interactions between particle surfaces and charged mineral or organic soil components also contribute to attachment and immobilization (Ghosh et al., 2008; Kumari and Yadav, 2014).
Factors influencing transport
A key factor influencing ENP transport is aggregation. Due to van der Waals attractions, inorganic nanoparticles such as TiO2, ZnO, and Ag can aggregate, reducing mobility by forming larger agglomerates that sediment or become physically filtered.
Aggregation
Aggregation is influenced by particle surface charge (zeta potential), ionic strength, pH, and the presence of multivalent cations (e.g., Ca2+, Mg2+), which can compress the electrical double layer and promote particle-particle contact (El Badawy et al., 2011). Conversely, surface coatings such as polyethylene glycol (PEG), natural organic matter (NOM), or surfactants can sterically stabilize nanoparticles and maintain colloidal stability, enhancing their transport through soils (Zhang et al., 2008). Dissolution of metal-based ENPs, such as ZnO or Ag nanoparticles, releases ionic species (e.g., Zn2+, Ag+) that may have different mobility and toxicity profiles than the original particles. Dissolved ions can leach readily into groundwater or adsorb onto soil particles, where they may enter plant root systems or microbial communities (Ahmed et al., 2021), nanoparticle interactions with soil constituents, including clays, oxides, organic matter, and biota, significantly impact transport. Clay minerals and iron/aluminum oxides, due to their high surface area and charge density, can strongly adsorb nanoparticles or facilitate heteroaggregation (Vural Kaymaz et al., 2023). Soil organic matter (SOM), including humic and fulvic acids, can act as stabilizing agents and aggregation inducers, depending on their concentration and binding affinity. SOM may coat ENPs, altering their surface properties and influencing mobility, reactivity, and uptake (Diegoli et al., 2008). Biological factors, including microbial exudates, root secretions, and extracellular polymeric substances (EPS), can also bind or transform nanoparticles, affecting their movement and bioavailability. For instance, microbial reduction or oxidation may alter nanoparticle valency or generate reactive intermediates, influencing their solubility and interactions with other soil components (Maurer-Jones et al., 2013). In aquatic environments, including irrigation water, drainage systems, and groundwater, the transport of ENPs is governed by similar principles but under different physicochemical conditions. Hydrodynamic forces, colloidal stability, and interactions with suspended solids and dissolved organic matter (DOM) are crucial. Nanoparticles in water may remain suspended as colloids, aggregate and settle, or adsorb to sediment surfaces. The presence of DOM can significantly enhance or hinder ENP transport depending on whether it stabilizes or bridges particles (Skjolding et al., 2016). ENPs that remain suspended can travel considerable distances from their application sites, raising concerns about off-target contamination of water bodies and aquatic ecosystems.
Aging
The aging of nanoparticles in soil and water adds another layer of complexity to their transport behavior. Aging involves chemical transformations such as oxidation (e.g., of Fe or Cu nanoparticles), sulfidation (e.g., of Ag nanoparticles in the presence of sulfides), and interaction with natural organic matter or microbial metabolites. These processes alter the size, solubility, surface charge, and aggregation state of ENPs, often reducing their mobility and toxicity—but sometimes forming new reactive or persistent forms (Bolan et al., 2024). For example, sulfidated Ag nanoparticles exhibit lower solubility and mobility than pristine Ag NPs, resulting in reduced leaching potential but possibly longer soil persistence. Overall, the transport of ENPs in soil and water is not governed by a single mechanism but is a result of a complex interplay between particle-specific properties (size, shape, surface chemistry), environmental factors (pH, ionic strength, soil texture, SOM content), and biological interactions. These mechanisms ultimately determine the exposure of plants and soil organisms to nanoparticles, influencing their ecological and toxicological outcomes. Therefore, accurately predicting the environmental behavior of ENPs requires integrative models that incorporate multi-scale processes, experimental validation under field-relevant conditions, and long-term monitoring to assess risks associated with their agricultural use.
Toxicity to plants and soil microorganisms
The toxicity of nanoparticles (NPs) to plants and soil microorganisms is closely linked to their concentration, as these materials can exhibit either beneficial or detrimental effects depending on the dose and exposure conditions. At low concentrations, specific nanoparticles such as zinc oxide (ZnO), iron oxide (Fe2O3), and titanium dioxide (TiO2) may enhance plant growth by improving photosynthesis, nutrient uptake, and stress tolerance. However, at higher concentrations, these same nanoparticles can become phytotoxic, leading to oxidative stress, inhibition of seed germination, stunted root elongation, and reduced chlorophyll content (Rastogi et al., 2017; Hsueh et al., 2015). Similarly, soil microbial communities are susceptible to nanoparticle exposure. For instance, silver nanoparticles (AgNPs), even at relatively low levels, can disrupt microbial biomass, inhibit nitrogen-fixing bacteria, and alter the structure and function of key microbial populations responsible for nutrient cycling (Ge et al., 2011; Chhipa, 2017). The dose-dependent nature of nanoparticle toxicity underscores the importance of assessing environmental concentrations and application practices to balance their agricultural benefits while minimizing ecological risks.
Plant toxicity from nanoparticle transport in agricultural systems
The increasing use of engineered nanoparticles (ENPs) in agriculture, for fertilizers, pesticides, and soil conditioners, has raised significant concerns regarding their potential phytotoxic effects due to their transport through soil and water systems (Table 2). Once applied to agricultural fields, plant roots can take up nanoparticles either passively via apoplastic pathways or through endocytosis and carrier-mediated transport mechanisms, depending on particle size, charge, and surface modifications (Rico et al., 2011). As they move within the plant vascular system, particularly via xylem and phloem, ENPs can accumulate in various tissues, including roots, stems, leaves, and even edible parts. Their internalization may disrupt cellular processes by generating reactive oxygen species (ROS), interfering with enzymatic activities, altering nutrient uptake, and causing oxidative stress, DNA damage, and membrane dysfunction (Ma et al., 2010). For instance, silver nanoparticles (AgNPs) cause lipid peroxidation and protein degradation in plants like rice and wheat, leading to stunted growth and chlorosis (Abbas et al., 2020). Similarly, zinc oxide (ZnO) and titanium dioxide (TiO2) nanoparticles, while often considered less toxic, have demonstrated root elongation inhibition and reduced biomass in several plant species due to their accumulation in root tissues and subsequent disturbance of hormonal signaling pathways (Jampilek and Kralova, 2015). The degree of toxicity is strongly influenced by the physicochemical properties of the nanoparticles, particularly size (smaller particles are more easily taken up), surface coating (which can either mitigate or amplify toxicity), and aging processes (such as sulfidation or oxidation) that alter nanoparticle reactivity and bioavailability (Shiv et al., 2024). Moreover, soil properties like pH, organic matter content, and microbial activity modulate nanoparticle behavior, either enhancing or reducing their uptake by plants. For example, natural organic matter (NOM) may coat nanoparticles, reducing their surface reactivity and toxicity. At the same time, acidic soils can increase the solubility and ion release from metal-based nanoparticles, intensifying their phytotoxic effects (Khodakovskaya et al., 2013). The long-term exposure of crops to ENPs also raises concerns about potential bioaccumulation and trophic transfer in food chains. Given these risks, comprehensive understanding and risk assessment of ENP-plant interactions under realistic field conditions are urgently needed to guide the sustainable application of nanotechnology in agriculture.
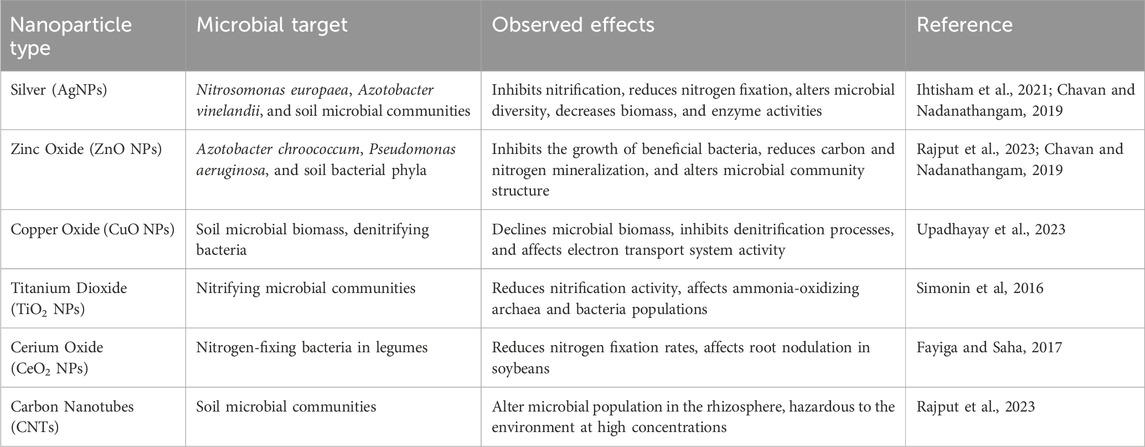
Microbial toxicity from nanoparticle transport in agricultural systems
The transport of engineered nanoparticles (ENPs) in agricultural soils poses significant risks to microbial communities that are fundamental to soil health, nutrient cycling, and plant productivity (Table 3). As nanoparticles migrate through the soil matrix via water flow, diffusion, or root exudate-driven gradients, they inevitably meet soil microorganisms, including bacteria, fungi, and archaea. These interactions can result in microbial toxicity, with consequences ranging from altered metabolic activity and enzyme inhibition to cell membrane disruption and death (Kahru and Dubourguier, 2010). The extent and mechanism of toxicity depend heavily on nanoparticle properties such as composition, size, shape, surface charge, and coating. Metal-based nanoparticles like silver (AgNPs), copper oxide (CuO NPs), and zinc oxide (ZnO NPs) are particularly potent due to their capacity to release toxic metal ions (e.g., Ag+, Cu2+, Zn2+), generate reactive oxygen species (ROS), and interact directly with microbial membranes, leading to oxidative stress and DNA damage (Auffan et al., 2009; Ge et al., 2011). For example, AgNPs have been shown to reduce the abundance of key nitrogen-fixing and nitrifying bacteria, such as Rhizobium and Nitrosomonas, thereby impairing nitrogen cycling in soil ecosystems (Shah and Belozerova, 2009). Similarly, ZnO nanoparticles can inhibit microbial respiration and enzymatic activity, such as dehydrogenase and urease, critical for organic matter decomposition and nutrient turnover (Parada et al., 2019).
The environmental fate and bioavailability of nanoparticles further influence microbial toxicity. In soils rich in organic matter or clay minerals, ENPs may become immobilized or undergo surface transformations (e.g., sulfidation, oxidation), which can mitigate their toxic effects by reducing ion release and reactivity (Cornelis et al., 2014). However, in sandy or low-organic soils, ENPs remain more bioavailable and mobile, increasing their contact with microbial cells. Surface coatings (e.g., natural organic matter, polymers, or surfactants) can also modulate microbial responses by shielding toxic surfaces or enhancing uptake (Simonin and Richaume, 2015). Long-term exposure to sublethal concentrations of ENPs may disrupt microbial diversity and function, even without immediate lethality, by shifting community composition toward more resistant species or reducing overall functional redundancy. Such changes may compromise ecosystem services, including decomposition, nutrient mineralization, and plant-microbe symbiosis. For instance, mycorrhizal fungi and plant growth-promoting rhizobacteria (PGPR) may be particularly sensitive to nanoparticle-induced stress, potentially weakening plant resilience and growth (Liu et al., 2023; Diegoli et al., 2008). Moreover, nanoparticle exposure can induce microbial resistance mechanisms, such as efflux pumps and extracellular polymeric substances (EPS) secretion, which may have broader implications for environmental antimicrobial resistance. Given the vital role of microbes in sustaining soil fertility and ecosystem balance, understanding the nuanced effects of nanoparticle transport and transformation on microbial communities is crucial for developing sustainable nanotechnology applications in agriculture.
Influence of particle size on nanoparticle transport in agricultural systems
Particle size is one of the most critical factors governing the transport, fate, and bioavailability of nanoparticles (NPs) in agricultural environments, influencing their interactions with soil particles, water, plants, and microorganisms. Smaller nanoparticles, typically those below 100 nm, exhibit higher mobility in soil and water systems due to their lower gravitational settling rates, reduced aggregation potential, and enhanced Brownian motion, facilitating their penetration through soil pores and root tissues (Lowry et al., 2012). Their increased specific surface area also enhances reactivity with soil constituents, such as organic matter, clay minerals, and microbial biofilms, potentially altering their environmental charge and stability (Navarro et al., 2008). For example, smaller silver nanoparticles (<20 nm) have been shown to move more freely through sandy loam soils compared to their larger counterparts, raising concerns about their deeper leaching into groundwater and uptake by plants (Cornelis et al., 2014). In contrast, larger particles are more prone to aggregation, sedimentation, and retention in the upper soil layers. They are often immobilized by strong interactions with soil colloids and organic macromolecules, limiting their mobility and ecological impact (Keller et al., 2013).
In plant uptake, smaller nanoparticles more readily pass through the root epidermis and endodermal layers, especially via apoplectic transport, and translocate to aerial tissues through xylem and phloem pathways. Studies have shown that plants such as Arabidopsis thaliana and rice can absorb and accumulate smaller TiO2 and ZnO nanoparticles in roots, stems, and leaves, leading to phytotoxic effects, including reduced biomass and oxidative stress (Laux et al., 2018). Additionally, particle size affects the rate and extent of dissolution, particularly in metal-based nanoparticles. Smaller particles have greater surface curvature and higher free energy, resulting in increased dissolution rates and ion release—factors that enhance toxicity toward soil microbes and plants (Auffan et al., 2009). This size-dependent dissolution is especially significant for ZnO and CuO nanoparticles, where released Zn2+ and Cu2+ ions contribute substantially to their toxicological profiles in the rhizosphere. Furthermore, the influence of size extends to the interaction of NPs with microbial communities; smaller particles can more easily penetrate microbial membranes or adhere to cell surfaces, disrupting cellular integrity and metabolism (Ge et al., 2011). However, the behavior of nanoparticles is highly context-dependent, influenced by soil texture, moisture, ionic strength, and organic matter content, which can either promote or inhibit transport depending on particle size. For instance, even small nanoparticles may be immobilized in clay-rich soil through surface adsorption or aggregation with clay platelets. Understanding and controlling nanoparticle size is essential for predicting their environmental behavior and developing safer nanomaterials for sustainable agriculture. The following graph depicts the inverse relationship between particle size and plant toxicity level (Figure 5). As the particle size increases, the toxicity level decreases, a general trend observed in many nanoparticle-plant interactions. Smaller particles tend to be more toxic due to their higher reactivity and ability to penetrate plant tissues more easily.
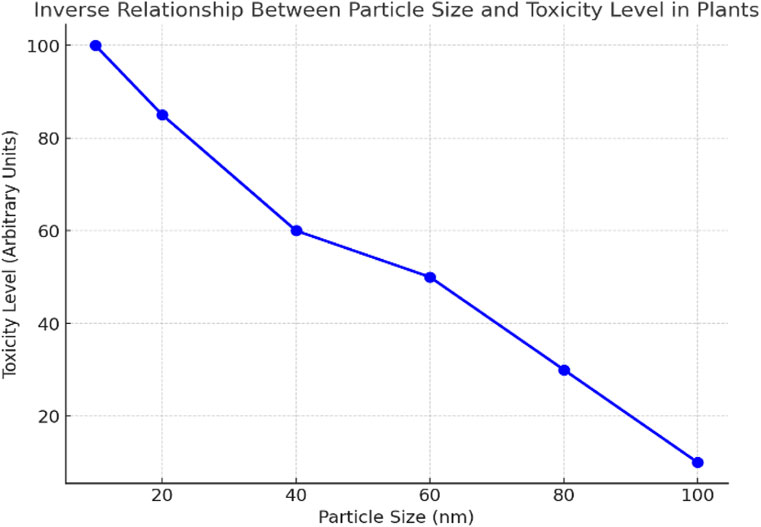
Figure 5. Inverse relationship between particle size and plant toxicity level. [Data were compiled from available literature, including studies by Ge et al. (2011), Rastogi et al. (2017), Chhipa (2017), Yadav (2025), Bouhadi et al. (2025), Wang et al. (2023), Shao et al. (2022), and additional information sourced from Wikipedia (2025)].
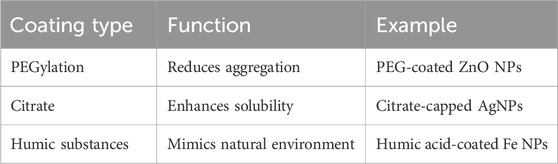
Role of surface coatings in agricultural nanoparticles
Surface coatings play a pivotal role in modulating the behavior, stability, and toxicity of engineered nanoparticles (ENPs) used in agriculture (Table 4). The surface modification of nanoparticles with organic or inorganic coatings can significantly influence their interactions with plants, soil, and microorganisms, affecting their overall environmental impact. Coatings, such as polymers, surfactants, or natural substances like humic acids and proteins, can improve nanoparticle dispersion in aqueous environments, preventing agglomeration and ensuring more uniform distribution in soil and plant tissues (Vural Kaymaz et al., 2023). For example, polyethylene glycol (PEG) coatings can enhance the solubility and bioavailability of nanoparticles, facilitating their uptake by plant roots and improving nutrient delivery (Djanaguiraman et al., 2024). Moreover, surface coatings can reduce the toxicity of nanoparticles by altering their surface charge and reactivity. Coating with biocompatible materials can decrease the release of toxic ions from metal-based nanoparticles, such as silver or copper, thereby reducing oxidative stress and DNA damage in plants and soil microorganisms (Auffan et al., 2009). Additionally, surface coatings can enhance the stability of nanoparticles under environmental conditions, preventing their aggregation and ensuring their persistence in agricultural systems. However, the type of coating, its thickness, and its chemical composition are critical factors that determine the efficacy of the nanoparticles for farm applications. While specific coatings may reduce toxicity and enhance nanoparticle uptake, others may increase their environmental persistence, leading to potential long-term ecological effects (Keller et al., 2013). Thus, optimizing surface coatings is essential for balancing the benefits of nanoparticle use in agriculture by minimizing potential risks to plant health, soil fertility, and environmental sustainability. Surface coatings (e.g., polymers, natural organic matter) can modulate nanoparticle behavior, stabilize against aggregation, reduce or enhance toxicity, and alter plant uptake.
Aging and transformation of agricultural nanoparticles in soil
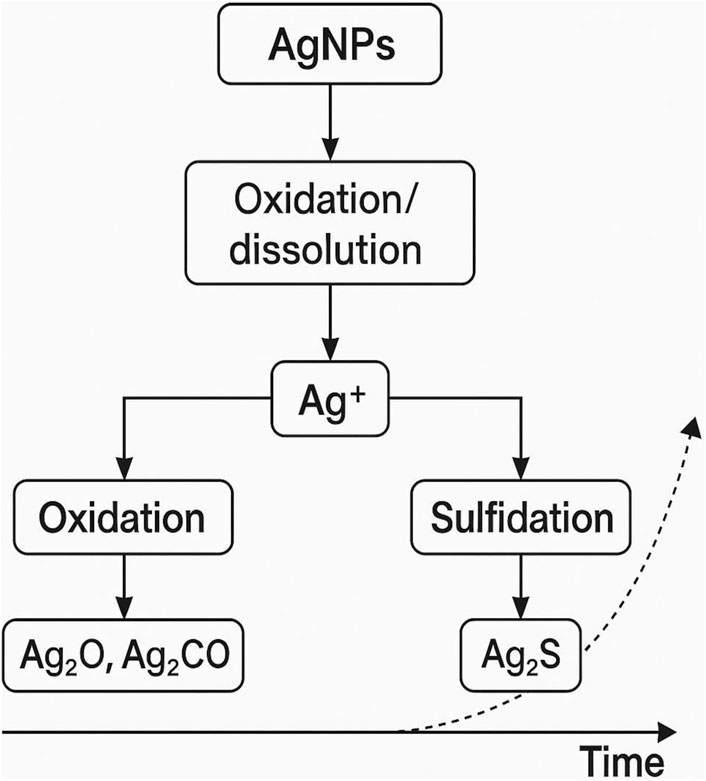
Nanoparticles undergo physical, chemical, and biological transformations: Sulfidation- AgNPs convert to Ag2S, reducing toxicity, Oxidation/Reduction- Alters surface reactivity, and Biotransformation Microbial interactions lead to new nano-species (Figure 6). The aging and transformation of engineered nanoparticles (ENPs) in soil are critical factors that influence their environmental behavior, bioavailability, and potential toxicity in agricultural systems. Upon their introduction into soil, nanoparticles undergo various physical, chemical, and biological processes that alter their size, shape, surface properties, and reactivity over time. These transformations are influenced by a range of soil characteristics, such as pH, organic matter content, ionic strength, and microbial activity, all of which can modify the fate and impact of nanoparticles on plants and soil ecosystems (Scown et al., 2010). Aging refers to the gradual changes in nanoparticle characteristics as they interact with soil components and environmental factors, such as water and temperature fluctuations. For example, metal-based nanoparticles like silver (AgNPs) and copper oxide (CuO NPs) are prone to oxidation or sulfidation in the presence of sulfur-containing compounds and oxygen, which can result in the formation of less toxic metal sulfides or oxides (Bystrzejewska-Piotrowska et al., 2009). This process can reduce their reactivity and toxicity, potentially mitigating their impact on soil microorganisms and plants. On the other hand, some transformations can increase nanoparticle toxicity, such as the dissolution of specific metal nanoparticles into more bioavailable ionic forms, which may be more harmful to plants and soil biota (Auffan et al., 2009; Yang et al., 2014).
The transformation of nanoparticles in soil also includes processes such as aggregation and adsorption to soil particles or organic matter, which can influence their mobility and persistence. Smaller nanoparticles aggregate more readily, forming smaller particles that are less mobile and more likely to be retained in the upper soil layers (Lecoanet et al., 2004). This aggregation can reduce the risk of nanoparticles leaching into groundwater but may increase their potential for interaction with plant roots and soil microorganisms. Organic matter, such as humic substances and root exudates, can also coat nanoparticles, affecting their stability and uptake by plants. These interactions are not only influenced by the physicochemical properties of nanoparticles but also by the microbial activity in the soil. Soil microbes can facilitate the transformation of nanoparticles by producing extracellular enzymes and metabolites that alter the surface characteristics of nanoparticles or induce their dissolution (Maurer-Jones et al., 2013). Additionally, the presence of soil microorganisms can mediate the bioavailability of nanoparticles through the formation of biofilms, which can either enhance or limit nanoparticle uptake by plants depending on the microbial composition and the nature of the nanoparticle surface. The aging and transformation of nanoparticles in soil have significant implications for their environmental impact, particularly in the context of agricultural sustainability. For example, over time, some nanoparticles may lose their toxic properties, reducing their potential to harm plant health and soil ecosystems. However, the persistence of transformed nanoparticles in the soil matrix, especially in the case of highly stable metal oxides or insoluble sulfides, may pose long-term ecological risks, particularly if they are taken up by plants or consumed by soil organisms. Therefore, understanding the dynamics of nanoparticle aging and transformation in soil is essential for assessing the risks and benefits of using nanoparticles in agriculture.
Knowledge gaps and future directions
The use of engineered nanoparticles (ENPs) in agricultural systems has garnered significant attention due to their potential to improve crop productivity, soil fertility, and pest control. However, the increasing application of nanoparticles in agriculture has raised concerns regarding their environmental fate, mobility, toxicity, and overall impact on agricultural ecosystems. Despite significant progress in understanding the role of particle size, surface coatings, and aging processes in nanoparticle behavior, several critical knowledge gaps remain that must be addressed to better predict and manage the risks associated with their use. These knowledge gaps pertain to both the fundamental scientific understanding of nanoparticle transformations in soil and the practical implications of their environmental and ecological effects.
One of the most pressing knowledge gaps is the need for a more comprehensive understanding of the long-term fate and behavior of nanoparticles in soils under field conditions. While laboratory-based studies provide valuable insights into the initial interactions between nanoparticles and soil components, they often fail to account for the complexity of real-world agricultural environments. Soil composition, texture, moisture content, pH, and microbial activity all influence the transport, transformation, and bioavailability of nanoparticles, yet the interactions between these factors are often poorly understood. For example, although smaller nanoparticles are typically more mobile and bioavailable, they may undergo aggregation or transformation over time, potentially reducing their toxicity but complicating their long-term effects. The influence of aging and surface transformations (e.g., oxidation, sulfidation, or adsorption to organic matter) on nanoparticle toxicity and transport remains poorly characterized, and further research is needed to determine how these processes alter the bioavailability of nanoparticles to plants and soil organisms (Keller et al., 2013). Surface coating is a crucial determinant of nanoparticle behavior and toxicity, yet the role of different coatings in modulating nanoparticle fate is still not fully understood. While some surface coatings (such as polyethylene glycol or polyvinyl alcohol) can enhance nanoparticle stability and reduce toxicity by preventing aggregation or shielding reactive surfaces, others may promote nanoparticle uptake by plants or microorganisms, thereby increasing their bioavailability and potential toxicity (Simonin and Richaume, 2015). Furthermore, coatings made from natural materials, such as humic substances, may interact with soil colloids or microbial biofilms, influencing both nanoparticle transport and toxicity in ways that are not yet fully elucidated. There is also a need to explore the effects of surface coatings on the long-term persistence of nanoparticles in soils, as well as their degradation and release of potentially toxic ions. Future research should aim to develop a better understanding of the interactions between coatings, nanoparticles, and the soil matrix, as well as how these interactions change over time as nanoparticles age and transform in the soil environment.
Another major knowledge gap lies in understanding the effects of nanoparticle aging on their toxicity and transport in agricultural ecosystems. While much is known about the immediate interactions between nanoparticles and plant or microbial systems, the long-term impacts of aging processes on nanoparticle toxicity remain largely unexplored. Aging processes such as aggregation, oxidation, and dissolution can alter the physical and chemical properties of nanoparticles, potentially reducing or increasing their toxicity depending on the transformation processes involved. For example, the aging of silver nanoparticles (AgNPs) in soil may lead to the formation of silver sulfide (Ag2S), which is less toxic to plants and soil microorganisms compared to the uncoated AgNPs (Abbas et al., 2020). Conversely, the dissolution of nanoparticles such as zinc oxide (ZnO) or copper oxide (CuO) can release metal ions into the soil, increasing the toxicity of these nanoparticles over time (Parada et al., 2019). Understanding how nanoparticles transform and age in soils, and how these transformations impact their ecological effects, will be crucial for evaluating the long-term risks and benefits of nanoparticle use in agriculture.
The integration of nanoscale materials into agricultural systems also calls for better models to predict the transport and fate of nanoparticles in the environment. Current models of nanoparticle behavior in soil often fail to fully integrate the complexity of nanoparticle interactions with soil matrices and biological systems, making it difficult to predict the potential ecological consequences of nanoparticle exposure. The development of more accurate models that incorporate the effects of particle size, surface coating, aging, and environmental conditions will be essential for risk assessment and management of ENPs in agriculture. These models should account for the dynamic and heterogeneous nature of soils and agricultural environments, as well as the interactions between nanoparticles and the biota that inhabit these ecosystems. Moreover, field-based studies that examine the real-world transport and fate of nanoparticles under diverse soil and climate conditions are necessary to validate the assumptions made in laboratory-based models and ensure their applicability in agricultural practice. In addition to scientific advancements, future research should focus on developing strategies for minimizing the potential risks associated with the use of nanoparticles in agriculture. One promising avenue is the development of “green” nanoparticles, which are synthesized using sustainable methods and incorporate biodegradable or non-toxic materials that reduce the risk of long-term environmental contamination. For example, the use of biopolymers, natural surfactants, or plant-derived materials such as nanoparticle coatings could offer an environmentally friendly alternative to traditional synthetic coatings (Mondéjar-López et al., 2024). Furthermore, precision agriculture techniques, such as targeted delivery systems for nanoparticles, could help reduce the environmental impact of nanoparticle applications by ensuring that they are delivered only to the areas of the soil or plant where they are needed most, thereby minimizing the risk of unintended exposure to non-target organisms.
In conclusion, while significant progress has been made in understanding the toxicity, transport, and transformation of nanoparticles in agricultural ecosystems, substantial knowledge gaps remain that must be addressed in future research. To effectively manage the risks and maximize the benefits of nanotechnology in agriculture, we must deepen our understanding of how particle size, surface coatings, and aging processes influence nanoparticle behavior in soils and their impact on plant and microbial health. Interdisciplinary research that combines material science, environmental science, and agricultural practices will be critical for addressing these challenges and ensuring the sustainable use of nanoparticles in agriculture.
Conclusion
Multiple factors, including particle size, surface coating, and aging processes, influence nanoparticles’ toxicity and transport in agricultural ecosystems. As nanotechnology continues to expand its role in agriculture, understanding the interactions between engineered nanoparticles (ENPs) and the environment become increasingly critical for ensuring their safe and practical application. Nanoparticle size determines their mobility, bioavailability, and potential toxicity. Smaller nanoparticles are typically more mobile and can penetrate plant tissues more readily, increasing their potential for toxicity. However, surface coatings can modify these properties, enhance the stability of nanoparticles, reduce toxicity, and improve their interaction with soil and plants. Coatings such as organic polymers or natural compounds can prevent aggregation and improve nanoparticle dispersion, thereby influencing their transport and uptake by plants. At the same time, these coatings can alter the environmental fate of nanoparticles, either mitigating or exacerbating their toxic effects depending on the materials used and the environmental conditions. The aging of nanoparticles in soil introduces additional complexities in their behavior and environmental impact. Over time, nanoparticles such as aggregation, oxidation, or dissolution may transform, which can alter their toxicity and mobility. For example, metal-based nanoparticles may oxidize or form fewer toxic compounds over time, reducing their potential to harm plants and soil organisms. However, in some cases, aging processes may release more bioavailable and toxic ions, complicating risk assessment. Despite considerable advancements in understanding the behavior of nanoparticles under laboratory conditions, significant knowledge gaps remain regarding their long-term effects in real-world agricultural systems. Future research should address these gaps by exploring the interactions between nanoparticles and various soil components, plant systems, and microorganisms under diverse environmental conditions. Furthermore, while the potential benefits of nanoparticles in agriculture, such as enhanced nutrient delivery and pest control, are promising, their widespread use necessitates careful consideration of their environmental and ecological risks. A more comprehensive understanding of nanoparticle transformations in soil and the development of more accurate models of nanoparticle transport and fate will be essential for informed decision-making in agricultural nanotechnology. Moreover, using environmentally benign materials for surface coatings and adopting precision farming techniques could help mitigate potential risks while optimizing the benefits of nanoparticles in farm practices. Thus, balancing the promise of nanotechnology with environmental stewardship will require ongoing research, interdisciplinary collaboration, and a commitment to sustainable agricultural practices.
Implications
Understanding nanoparticles’ dissolution, toxicity, and transport in agricultural ecosystems has significant implications for environmental sustainability, food safety, and agricultural productivity. Variations in particle size, surface coatings, and aging processes can dramatically influence nanoparticle behavior, affecting their mobility through soil, plant uptake, and potential accumulation in food crops. Smaller particles, for example, may dissolve more readily and move farther through the soil, increasing exposure potential and bioavailability. Surface modifications can alter reactivity and toxicity, impacting soil microbiota and plant health. Furthermore, aging in natural environments may change the particles’ physicochemical properties, influencing long-term environmental fate. These factors underscore the need for precise risk assessments and the development of safer nanomaterials tailored for agricultural use, ensuring that the benefits of nanotechnology do not come at the expense of ecological and human health.
Author contributions
SI: Funding acquisition, Validation, Writing – review and editing, Conceptualization, Investigation, Supervision, Methodology, Resources, Software, Formal Analysis, Writing – original draft, Project administration, Data curation, Visualization.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by a grant from the United States Department of Agriculture – National Institute of Food and Agriculture (USDA-NIFA CBG), Award No. 11011117 [GR100153], titled “Dissolution, Toxicity and Transport of Nanoparticles in Agricultural Ecosystems: Impacts of Particle Size, Surface Coating and Aging”.
Conflict of interest
The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that Generative AI was used in the creation of this manuscript. ChatGPT was used to improve some figures: https://chatgpt.com.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Abbas, Q., Yousaf, B., Amina, A. M. U., Munir, M. A. M., El-Naggar, A., Rinklebe, J., et al. (2020). Transformation pathways and fate of engineered nanoparticles (ENPs) in distinct interactive environmental compartments: a review. Environ. Int. 138, 105646. doi:10.1016/j.envint.2020.105646
Ahmed, B., Rizvi, A., Ali, K., Lee, J., Zaidi, A., Khan, M. S., et al. (2021). Nanoparticles in the soil–plant system: a review. Environ. Chem. Lett. 19, 1545–1609. doi:10.1007/s10311-020-01138-y
Auffan, M., Rose, J., Bottero, J. Y., Lowry, G. V., Jolivet, J. P., and Wiesner, M. R. (2009). Towards a definition of inorganic nanoparticles from an environmental, health and safety perspective. Nat. Nanotechnol. 4 (10), 634–641. doi:10.1038/nnano.2009.242
Bhat, J. A., Rajora, N., Raturi, G., Sharma, S., Dhiman, P., Sanand, S., et al. (2021). Silicon nanoparticles (SiNPs) in sustainable agriculture: major emphasis on the practicality, efficacy and concerns. Nanoscale. Adv. 3 (14), 4019–4028. doi:10.1039/d1na00233c
Bolan, S., Sharma, S., Mukherjee, S., Zhou, P., Mandal, J., Srivastava, P., et al. (2024). The distribution, fate, and environmental impacts of food additive nanomaterials in soil and aquatic ecosystems. Sci. Total Environ. 916, 170013. doi:10.1016/j.scitotenv.2024.170013
Bouhadi, M., Javed, Q., Jakubus, M., Elkouali, M., Fougrach, H., Ansar, A., et al. (2025). Nanoparticles for sustainable agriculture: assessment of benefits and risks. Agronomy 15 (5), 1131. doi:10.3390/agronomy15051131
Bundschuh, M., Filser, J., Lüderwald, S., McKee, M. S., Metreveli, G., Schaumann, G. E., et al. (2018). Nanoparticles in the environment: where do we come from, where do we go to? Environ. Sci. Eur. 30, 6. doi:10.1186/s12302-018-0132-6
Bystrzejewska-Piotrowska, G., Golimowski, J., and Urban, P. L. (2009). Nanoparticles: their potential toxicity, waste and environmental management. Waste. Manag. 29 (9), 2587–2595. doi:10.1016/j.wasman.2009.04.001
Chavan, S., and Nadanathangam, V. (2019). Effects of nanoparticles on plant growth-promoting bacteria in Indian agricultural soil. Agronomy 9 (3), 140. doi:10.3390/agronomy9030140
Chhipa, H. (2017). Nanofertilizers and nanopesticides for agriculture. Environ. Chem. Lett. 15, 15–22. doi:10.1007/s10311-016-0600-4
Cornelis, G., Hund-Rinke, K., Kuhlbusch, T., van den Brink, N., and Nickel, C. (2014). Fate and bioavailability of engineered nanoparticles in soils: a review. Crit. Rev. Environ. Sci. Technol. 44 (24), 2720–2764. doi:10.1080/10643389.2013.829767
Diegoli, S., Manciulea, A. L., Begum, S., Jones, I. P., Lead, J. R., and Preece, J. A. (2008). Interaction between manufactured gold nanoparticles and naturally occurring organic macromolecules. Sci. Total Environ. 373 (2-3), 51–61. doi:10.1016/j.scitotenv.2008.04.023
Dimkpa, C. O., McLean, J. E., Latta, D. E., Manangón, E., Britt, D. W., Johnson, W. P., et al. (2012). CuO and ZnO nanoparticles: phytotoxicity, metal speciation, and induction of oxidative stress in sand-grown wheat. J. Nanoparticle. Res. 14 (9), 1125–15. doi:10.1007/s11051-012-1125-9
Djanaguiraman, M., Anbazhagan, V., Dhankher, O. P., and Prasad, P. V. V. (2024). Uptake, translocation, toxicity, and impact of nanoparticles on plant physiological processes. Plants (Basel). 13 (22), 3137. doi:10.3390/plants13223137
El Badawy, A. M., Silva, R. G., Morris, B., Scheckel, K. G., Suidan, M. T., and Tolaymat, T. M. (2011). Surface charge-dependent toxicity of silver nanoparticles. Environ. Sci. Technol. 45 (1), 283–287. doi:10.1021/es1034188
Fayiga, A. O., and Saha, U. K. (2017). Nanoparticles in biosolids: effect on soil health and crop growth. Ann. Environ. Sci. Toxicol. 2 (1), 059–067. doi:10.17352/aest.000013
Ge, Y., Schimel, J. P., and Holden, P. A. (2011). Evidence for negative effects of TiO2 and ZnO nanoparticles on soil bacterial communities. Environ. Sci. Technol. 45 (2), 1659–1664. doi:10.1021/es103040t
Ghosh, S., Mashayekhi, H., Pan, B., and Xing, B. (2008). Colloidal behavior of aluminum oxide nanoparticles as affected by pH and natural organic matter. Langmuir 24 (21), 12385–12391. doi:10.1021/la802015f
Hotze, E. M., Phenrat, T., and Lowry, G. V. (2010). Nanoparticle aggregation: challenges to understanding transport and reactivity in the environment. J. Environ. Qual. 39 (6), 1909–1924. doi:10.2134/jeq2009.0462
Hsueh, Y. H., Ke, W. J., Hsieh, C. T., Lin, K. S., Tzou, D. Y., and Chiang, C. L. (2015). ZnO nanoparticles affect Bacillus subtilis cell growth and biofilm formation. PLoS One 10 (6), e0128457. doi:10.1371/journal.pone.0128457
Ihtisham, M., Noori, A., Yadav, S., Sarraf, M., Kumari, P., Brestic, M., et al. (2021). Silver nanoparticle’s toxicological effects and phytoremediation. Nanomaterials 11 (9), 2164. doi:10.3390/nano11092164
Jampilek, J., and Kralova, K. (2015). Application of nanotechnology in agriculture and food industry, its prospects and risks. Ecol. Chem. Eng. S. 22(3): 321–361. doi:10.1515/eces-2015-0018
Kah, M., Kookana, R. S., Gogos, A., and Bucheli, T. D. (2018). A critical evaluation of nanopesticides and nanofertilizers against their conventional analogues. Nat. Nanotechnol. 13 (8), 677–684. doi:10.1038/s41565-018-0131-1
Kahru, A., and Dubourguier, H. C. (2010). From ecotoxicology to nanoecotoxicology. Toxicology 269 (2–3), 105–119. doi:10.1016/j.tox.2009.08.016
Keller, A. A., McFerran, S., Lazareva, A., and Suh, S. (2013). Global life cycle releases of engineered nanomaterials. J. Nanopart. Res. 15, 1692. doi:10.1007/s11051-013-1692-4
Khodakovskaya, M. V., Kim, B. S., Kim, J. N., Alimohammadi, M., Dervishi, E., Mustafa, T., et al. (2013). Carbon nanotubes as plant growth regulators: effects on tomato growth, reproductive system, and soil microbial community. Small 9 (1), 115–123. doi:10.1002/smll.201201225
Kumar, V., Sharma, A., Kaur, R., and Yin, S. (2021). Iron oxide nanoparticles impact on growth, oxidative stress, and enzymatic antioxidant system in plants: a review. Environ. Nanotechnol. Monit. Manag. 15, 100408. doi:10.1016/j.enmm.2020.100408
Kumari, A., and Yadav, S. K. (2014). Nanotechnology in agri-food sector. Crit. Rev. Food. Sci. Nutr. 54 (8), 975–984. doi:10.1080/10408398.2011.621095
Kurczewska, J. (2023). Chitosan-based nanoparticles with optimized parameters for targeted delivery of a specific anticancer drug-A comprehensive review. Pharmaceutics 15 (2), 503. doi:10.3390/pharmaceutics15020503
Laux, P., Riebeling, C., Booth, A. M., Brain, J. D., Brunner, J., Cerrillo, C., et al. (2018). Challenges in characterizing the environmental fate and effects of carbon nanotubes and inorganic nanomaterials in aquatic systems. Environ. Sci. Nano. 5, 48–63. doi:10.1039/C7EN00594F
Lecoanet, H. F., Bottero, J. Y., and Wiesner, M. R. (2004). Laboratory assessment of the mobility of nanomaterials in porous media. Environ. Sci. Technol. 38 (19), 5164–5169. doi:10.1021/es0352303
Liu, Z., Faizan, M., Zheng, L., Cui, L., Han, C., Chen, H., et al. (2023). Nanoparticles enhance plant resistance to abiotic stresses: a bibliometric statistic. Agronomy 13 (3), 729. doi:10.3390/agronomy13030729
Lowry, G. V., Espinasse, B. P., Badireddy, A. R., Richardson, C. J., Reinsch, B. C., Bryant, L. D., et al. (2012). Long-term transformation and fate of manufactured Ag nanoparticles in a simulated large-scale freshwater emergent wetland. Environ. Sci. Technol. 46 (13), 7027–7036. doi:10.1021/es204608d
Ma, X., Geisler-Lee, J., Deng, Y., and Kolmakov, A. (2010). Interactions between engineered nanoparticles (ENPs) and plants: phytotoxicity, uptake and accumulation. Sci. Total Environ. 408 (16), 3053–3061. doi:10.1016/j.scitotenv.2010.03.031
Madanayake, N. H., and Adassooriya, N. M. (2021). Phytotoxicity of nanomaterials in agriculture. Open. Biotechnol. J. 15, 109–118. doi:10.2174/1874070702115010109
Mahmmod, A. (2018). Brief overview of the application of silver nano[particles to improve growth of crop plants]. IET. Nanobiotechnol. 12 (6), 701–705. doi:10.1049/iet-nbt.2017.0273
Maurer-Jones, M. A., Gunsolus, I. L., Murphy, C. J., and Haynes, C. L. (2013). Toxicity of engineered nanoparticles in the environment. Anal. Chem. 85 (6), 3036–3049. doi:10.1021/ac303636s
Mondéjar-López, M., García-Simarro, M. P., Navarro-Simarro, P., Gómez-Gómez, L., Ahrazem, O., and Niza, E. (2024). A review on the encapsulation of “eco-friendly” compounds in natural polymer-based nanoparticles as next generation nano-agrochemicals for sustainable agriculture and crop management. Int. J. Biol. Macromol. 180 (3), 136030. doi:10.1016/j.ijbiomac.2024.136030
Mukherjee, A., Majumdar, S., Servin, A. D., Pagano, L., Dhankher, O. P., and White, J. C. (2016). Carbon nanomaterials in agriculture: a critical review. Front. Plant Sci. 7, 172. doi:10.3389/fpls.2016.00172
Navarro, E., Baun, A., Behra, R., Hartmann, N. B., Filser, J., Miao, A. J., et al. (2008). Environmental behavior and ecotoxicity of engineered nanoparticles to algae, plants, and fungi. Ecotoxicology 17 (5), 372–386. doi:10.1007/s10646-008-0214-0
Nawaz, A. (2025). Nanoparticles and sustainable agriculture. New York, NY: Cultivation. Available online at: https://cultivationag.com/nanoparticles-and-sustainable-agriculture/ (Accessed June 11, 2025).
Nowack, B., and Bucheli, T. D. (2007). Occurrence, behavior and effects of nanoparticles in the environment. Environ. Pollut. 150 (1), 5–22. doi:10.1016/j.envpol.2007.06.006
Parada, J., Rubilar, O., Fernández-Baldo, M. A., Bertolino, F. A., Durán, N., Seabra, A. B., et al. (2019). The nanotechnology among US: are metal and metal oxides nanoparticles a nano or mega risk for soil microbial communities? Crit. Rev. Biotechnol. 39 (2), 157–172. doi:10.1080/07388551.2018.1523865
Paramo, L. A., Feregrino-Pérez, A. A., Guevara, R., Mendoza, S., and Karen, E. (2020). Nanoparticles in agroindustry: applications, toxicity, challenges, and trends. Nanomaterials 10 (9), 1654. doi:10.3390/nano10091654
Raha, S., and Ahmaruzzaman, M. (2022). ZnO nanostructured materials and their potential applications: progress, challenges and perspectives. Nanoscale. Adv. 4 (8), 1868–1925. doi:10.1039/D1NA00880C
Rai, M., Yadav, A., and Gade, A. (2009). Silver nanoparticles as a new generation of antimicrobials. Biotechnol. Adv. 27 (1), 76–83. doi:10.1016/j.biotechadv.2008.09.002
Rajput, V. D., Kumari, A., Upadhyay, S. K., Minkina, T., Mandzhieva, S., Ranjan, A., et al. (2023). Can nanomaterials improve the soil microbiome and crop productivity? Agriculture 13 (2), 231. doi:10.3390/agriculture13020231
Raliya, R., Nair, R., Chavalmane, S., Wang, W. N., and Biswas, P. (2015). Mechanistic evaluation of translocation and physiological impact of titanium dioxide and zinc oxide nanoparticles on the tomato (Solanum lycopersicum L.) plant. Metallomics 7 (12), 1584–1594. doi:10.1039/c5mt00168d
Raliya, R., and Tarafdar, J. C. (2013). ZnO nanoparticle biosynthesis and its effect on phosphorous-mobilizing enzyme secretion and gum contents in clusterbean (Cyamopsis tetragonoloba L.). Agric. Res. 2 (1), 48–57. doi:10.1007/s40003-012-0049-z
Rastogi, A., Zivcak, M., Sytar, O., Kalaji, H. M., He, X., Mbarki, S., et al. (2017). Impact of metal and metal oxide nanoparticles on plant: a critical review. Front. Chem. 5, 78. doi:10.3389/fchem.2017.00078
Rico, C. M., Majumdar, S., Duarte-Gardea, M., Peralta-Videa, J. R., and Gardea-Torresdey, J. L. (2011). Interaction of nanoparticles with edible plants and their possible implications in the food chain. J. Agric. Food. Chem. 59 (8), 3485–3498. doi:10.1021/jf104517j
Ruttkay-Nedecky, B., Krystofova, O., Nejdl, L., and Adam, V. (2017). Nanoparticles based on essential metals and their phytotoxicity. J. Nanobiotechnol. 15, 33. doi:10.1186/s12951-017-0268-3
Schlagenhauf, L., Buerki-Thurnherr, T., Kuo, Y.-Y., Wichser, A., Nüesch, F., Wick, P., et al. (2015). Carbon nanotubes released from an epoxy-based nanocomposite: quantification and particle toxicity. Environ. Sci. Technol. 49 (17), 10616–10623. doi:10.1021/acs.est.5b02750
Scown, T. M., van Aerle, R., and Tyler, C. R. (2010). Review: do engineered nanoparticles pose a significant threat to the aquatic environment? Crit. Rev. Toxicol. 40 (7), 653–670. doi:10.3109/10408444.2010.494174
Servin, A., Elmer, W., Mukherjee, A., De la Torre-Roche, R., Hamdi, H., White, J. C., et al. (2015). A review of the use of engineered nanomaterials to suppress plant disease and enhance crop yield. J. Nanoparticle Res. 17 (2), 92. doi:10.1007/s11051-015-2907-7
Shah, V., and Belozerova, I. (2009). Influence of metal nanoparticles on the soil microbial community and germination of lettuce seeds. Water. Air. Soil. Pollut. 197 (1–4), 143–148. doi:10.1007/s11270-008-9797-6
Shao, C., Zhao, H., and Wang, P. (2022). Recent development in functional nanomaterials for sustainable and smart agricultural chemical technologies. Nano. Converg. 9 (1), 11. doi:10.1186/s40580-022-00302-0
Simonin, M., Martins, J. M., Uzu, G., Vince, E., and Richaume, A. (2016). Combined study of titanium dioxide nanoparticle transport and toxicity on microbial nitrifying communities under single and repeated exposures in soil columns. Environ. Sci. Technol. 50 (19), 10693–10699. doi:10.1021/acs.est.6b02415
Simonin, M., and Richaume, A. (2015). Impact of engineered nanoparticles on the activity, abundance, and diversity of soil microbial communities: a review. Environ. Sci. Pollut. Res. 22, 13710–13723. doi:10.1007/s11356-015-4171-x
Singh, R. P., Handa, R., and Manchanda, G. (2021). Nanoparticles in sustainable agriculture: an emerging opportunity. J. Control. Release. 329, 1234–1248. doi:10.1016/j.jconrel.2020.10.051
Skjolding, L. M., Sørensen, S. N., Hartmann, N. B., Hjorth, R., Hansen, S. F., and Baun, A. (2016). Aquatic ecotoxicity testing of nanoparticles-the quest to disclose nanoparticle effects. Angew. Chem. Int. Ed. Engl. 55 (49), 15224–15239. doi:10.1002/anie.201604964
Upadhayay, V. K., Chitara, M. K., Mishra, D., Jha, M. N., Jaiswal, A., Kumari, G., et al. (2023). Synergistic impact of nanomaterials and plant probiotics in agriculture: a tale of two-way strategy for long-term sustainability. Front. Microbiol. 14, 1133968. doi:10.3389/fmicb.2023.1133968
Vural Kaymaz, S., Nobar, H. M., Sarıgül, H., Soylukan, C., Akyüz, L., and Yüce, M. (2023). Nanomaterial surface modification toolkit: principles, components, recipes, and applications. Adv. Colloid. Interface. Sci. 322, 103035. doi:10.1016/j.cis.2023.103035
Wang, P., Lombi, E., Zhao, F.-J., and Kopittke, P. M. (2016). Nanotechnology: a new opportunity in plant sciences. Trends. Plan.t Sci. 21 (8), 699–712. doi:10.1016/j.tplants.2016.04.005
Wang, X., Xie, H., Wang, P., and Yin, H. (2023). Nanoparticles in plants: uptake, transport and physiological activity in leaf and root. Mater. 16 (8), 3097. doi:10.3390/ma16083097
Wikipedia (2025). Nanotechnology in agriculture. San Francisco, California: Wikipedia. Available online at: https://en.wikipedia.org/wiki/Nanotechnology_in_agriculture.
Yadav, M. (2025). Nanoparticle-facilitated targeted nutrient delivery in plants: breakthroughs and mechanistic insights. Plant. Nano. Biol. 12, 100156. doi:10.1016/j.plana.2025.100156
Yang, Y., Wang, Y., Westerhoff, P., Hristovski, K., Jin, V. L., Johnson, M. V., et al. (2014). Metal and nanoparticle occurrence in biosolid-amended soils. Sci. Total. Environ. 485-486, 441–449. doi:10.1016/j.scitotenv.2014.03.122
Keywords: nanoparticles, toxicity, transport, agriculture, surface coating, aging, environmental impact
Citation: Islam S (2025) Toxicity and transport of nanoparticles in agriculture: effects of size, coating, and aging. Front. Nanotechnol. 7:1622228. doi: 10.3389/fnano.2025.1622228
Received: 02 May 2025; Accepted: 06 June 2025;
Published: 24 June 2025.
Edited by:
Amitava Mukherjee, VIT University, IndiaReviewed by:
Ilika Ghosh, Max Planck Florida Institute for Neuroscience (MPFI), United StatesBaisista Saha, KIIT University, India
Copyright © 2025 Islam. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shahidul Islam, aXNsYW1zQHVhcGIuZWR1
 Shahidul Islam
Shahidul Islam