- 1Cancer Research Center, Nantong Tumor Hospital, Nantong, China
- 2Cancer Research Institute, The Affiliated Tumor Hospital of Nantong University, Nantong, China
- 3Cancer Research Center, Nantong, China
Nanozymes with phosphatase-like activity are a class of artificial nano-catalysts that mimic the catalytic functions of natural phosphatases. They have attracted widespread attention in the biomedical field in recent years owing to their superior stability, controllability, and low cost. Nanozyme demonstrates unique potential in regulating physiological phosphorus metabolism, intervening in disease-related signaling pathways, detecting disease biomarkers, and treating conditions such as tumors and inflammation. Despite significant progress in nanozyme technology for mimicking natural enzymes in recent years, the research field of phosphatase-mimicking nanozymes remains in a relatively preliminary stage. The types and mechanisms of nanozymes with phosphatase-like activity that have been systematically explored remain quite limited. With the deepening understanding of the role of phosphatases in complex biological processes including disease signal regulation, metabolic disorders, and bone disease pathogenesis, there is an urgent need to develop novel nano-materials that simulate phosphatases with more precise functions to meet the catalytic demands of diverse biological environments. This review focuses on recent research advancements in phosphatase-like nanozymes, with a critical summary of different types of nanomaterials. It aims to provide a theoretical basis and technical reference for the development of phosphatase-mimicking nanozymes with high catalytic activity, excellent biocompatibility, and targeted properties. By analyzing the key challenges and research gaps currently facing the field, we aim to provide new ideas for its continued development and accelerate its practical translation in precision medicine.
1 Introduction
Phosphatases are a class of hydrolases that catalyze substrate dephosphorylation reactions. They remove phosphate groups from proteins or other molecules and, together with protein kinases, constitute the core mechanism for regulating protein phosphorylation modification. This mechanism plays a key role in maintaining cell signaling, metabolic homeostasis, cell cycle regulation, gene expression, cellular differentiation, and other life processes (Kaplan, 1972; Stanford and Bottini, 2023).
Abnormal phosphatase function is closely associated with the occurrence and development of many diseases. Specifically, upregulation or downregulation of phosphatase activity leads to imbalance of signaling pathways, interfering with basic physiological processes such as cell proliferation, differentiation, and apoptosis, thereby inducing pathological states. For example, deletion or mutation of PTEN (a protein tyrosine phosphatase) is common in various solid and hematological tumors: its inactivation causes sustained activation of the PI3K/AKT pathway, promoting cell survival and tumor growth (Cheng et al., 2024). Additionally, certain phosphatases (e.g., PP2A) are functionally impaired in neurodegenerative diseases like Alzheimer’s disease, affecting neuronal microtubule stability and the removal of phosphorylated proteins involved in aberrant Tau protein aggregation (Sardoiwala et al., 2024). Phosphatase abnormalities are also strongly linked to metabolic and immunologic diseases such as type 2 diabetes and systemic lupus erythematosus (Hosen et al., 2024). Thus, phosphatases serve not only as important targets for studying disease molecular mechanisms but also provide potential intervention pathways for clinical diagnosis and targeted therapy.
Although natural phosphatases perform crucial functions under physiological conditions, they exhibit limitations in basic research and clinical applications (Wang et al., 2016; Gao and Yan, 2016; Zhang et al., 2021; Huang et al., 2019; Liang et al., 2019; Wu et al., 2019; Jiang et al., 2019). First, natural phosphatases typically possess high substrate specificity and complex regulatory mechanisms, which limit their applicability in multi-target regulation or broad-spectrum dephosphorylation studies (Lai et al., 2022). Second, many natural phosphatases depend on specific intracellular environments (e.g., pH, metal ions, cofactors) to maintain stability and catalytic activity; their functions are readily compromised when removed from physiological conditions. Additionally, the prevalence of conformational polymorphism and multiple splice variants in phosphatases results in highly organized expression and function, thereby increasing the difficulty of recombinant expression and structure–function studies in vitro (Sayegh et al., 2016). More importantly, certain natural phosphatases may exert dual or even opposing biological effects in different disease states. For instance, they might inhibit tumor growth in some contexts while promoting tumor progression in others, thereby limiting their controllability and safety as drug targets (Gesmundo et al., 2019; Li et al., 2015). Consequently, strategies for modifying, artificially designing, or selectively regulating natural phosphatases have emerged (Wu et al., 2019; Di et al., 2022).
Nanomaterials exhibit significant advantages in the biomedical field due to their unique physicochemical properties. Firstly, they possess a high specific surface area and adjustable size, enabling efficient drug loading and sustained release, which enhance the bioavailability and targeting of drugs. Secondly, their excellent surface modifiability allows functional coupling with various biomolecules (e.g., antibodies, peptides, nucleic acids), thereby enabling precise recognition and treatment of specific cells or tissues. Thirdly, certain nanomaterials (such as gold nanoparticles, quantum dots, and magnetic nanoparticles) exhibit excellent optical, electrical, or magnetic properties, making them applicable in high-sensitivity imaging, diagnostic, and therapeutic integrated platforms. Additionally, nanomaterials can overcome biological barriers of traditional drugs (e.g., the blood–brain barrier), thereby improving treatment efficiency for complex diseases (such as brain tumors and neurodegenerative diseases).
With the rapid advancement of nanotechnology and nanomaterials, Scrimin et al. in 2004 utilized triazole-functionalized gold nanoparticles as catalysts in transphosphorylation reactions, discovering that this nanomaterial could catalyze the hydrolysis of phosphate esters (Manea et al., 2004). They coined the term “Nanozyme” for this novel catalyst. Since then, “Nanozyme” has officially become a new nomenclature in the field of artificial enzymes, marking the entry of artificial enzyme research into a new era of rapid progress. Following the 2007 discovery by Chinese scientists that Fe3O4 nanoparticles exhibit peroxidase-like activity and the integration of enzyme research methods into nanotechnology, numerous novel artificial enzymes (nanozymes) based on nanomaterials have been developed and reported (Gao et al., 2007).
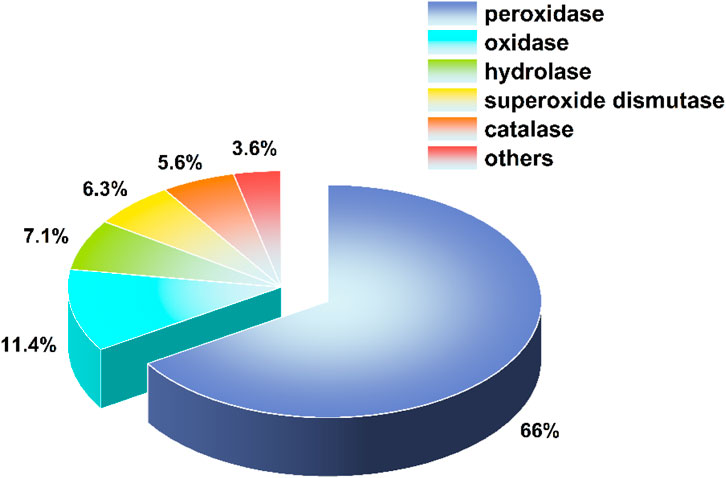
Phosphatases, as a class of hydrolytic enzymes, play a crucial role in the normal functioning of biological processes such as blood glucose regulation and energy transfer. Nanomaterials that mimic phosphatases are emerging as novel functional materials in disease treatment, owing to their ability to stably exhibit dephosphorylation catalytic activity under non-physiological conditions. Such nanomaterials enable precise therapy by regulating abnormally activated signaling pathways. In tumors, they can simulate dephosphorylation to inhibit pro-proliferative pathways (e.g., AKT and ERK), inducing cell apoptosis (Cheng et al., 2024). In neurodegenerative diseases, they can target abnormally phosphorylated proteins (such as Tau protein) to alleviate neurotoxicity and pathological progression (Hau-Ting et al., 2024). In chronic inflammation or autoimmune diseases, regulating the phosphorylation status of key signaling molecules (e.g., NF-κB or MAPK) inhibits inflammatory factor release, thereby reducing tissue damage (Guo et al., 2024). Furthermore, in metabolic disorders, phosphatase-mimicking nanomaterials are expected to improve insulin sensitivity by modulating insulin signaling pathways. In summary, nano-phosphatases, characterized by their stability, high efficiency, and strong designability, provide a new strategy for targeted intervention in complex diseases, demonstrating broad clinical application prospects. Current nanozyme research is predominantly focused on pseudo-oxidoreductases, with only a small fraction dedicated to pseudo-hydrolases and other types of nanozymes (Figure 1) (Wu et al., 2019; Wang et al., 2022). To date, only a very limited number of nanozymes with phosphatase-like activity have been explored. Thus, there is an urgent need to guide the design of advanced nanozymes with high stability and phosphatase-like activity by reviewing recent reports on phosphatase-mimicking nanozymes (Wu et al., 2023).
2 Design strategies for phosphatase-mimicking nanomaterials
Although research on phosphatase-like nanozymes remains in its infancy, several representative phosphatase-mimicking nanomaterials have already been developed. Based on their composition, they can be primarily classified into two categories: metal oxides and metal-organic frameworks (MOFs) (Jiang et al., 2020).
2.1 Metal oxide-type phosphatase nanozymes
Given the presence of two Zn2+ ions in the active site of natural phosphatases, which play a crucial role in enzyme catalytic kinetics, initial attempts to develop artificial phosphatase-like enzymes focused on metal ions (Liu et al., 2005). Among these, the lanthanide Ce3+/Ce4+ is notable for its exceptional ability to hydrolyze phosphate esters. Surface-functionalized oxidase nanoenzymes play a crucial role in the synthesis of novel and highly efficient nanomaterials, mainly manifested in the enhancement of catalytic activity: by mimicking the active center of natural oxidases, they effectively catalyze the removal of reactive oxygen species (such as ·OH, H2O2) or substrate oxidation/phosphorylation reactions. Compared with traditional materials, their catalytic efficiency is significantly improved, and they can still maintain stability even under extreme pH or temperature conditions. Currently, research on phosphatase-mimicking nanomaterials primarily focuses on Ce-based or Zr-based nanomaterials, particularly cerium oxide nanoparticles (Figure 2A) (Tan et al., 2022; Kuchma et al., 2010; Manto et al., 2017; Huang et al., 2021). Ce is regarded as one of the most promising metals due to its unique valence characteristics and catalytic activity similar to that of phosphatases. In recent years, many cerium-based nanomaterials have attracted significant research interest because they can catalyze dephosphorylation reactions. Inorganic nanomaterials have the advantages of low cost, good stability, and simple synthesis. These cerium-based nanomaterials overcome the inherent defects of natural enzymes and show great potential as substitutes for natural phosphatases. In addition, other factors, such as end caps, surface area, and main exposed plane, also affect the catalytic performance of cerium-based nanoenzymes. Compared with natural phosphatases, cerium-based nanoenzymes have stronger resistance to interference. Although the catalytic efficiency and selectivity of nano-sized cerium dioxide are still not satisfactory at present, it is still a promising substitute for natural phosphatases and has broad application prospects. CeO2 nanomaterials have been found to exhibit various types of mimetic enzyme activities. In addition to their commonly observed redox enzyme activities, they also display phosphatase-like activity. Furthermore, their enzymatic activity is closely related to factors such as surface valence states, the transformation between Ce3+ and Ce4+, and the presence of oxygen vacancies on the material’s surface (Xiao et al., 2022). A series of porous cerium dioxide nanorods (PN-CeO2) synthesized by Yao et al. are considered potential tools for bioanalysis and biomolecule applications (Yao et al., 2018). Based on the electrostatic attraction between negatively charged bacteria and positively charged nanoparticles, metal and metal oxide nanoparticles bind to the bacterial cell wall. This interaction not only inhibits bacterial growth but also induces the production of reactive oxygen species (ROS), leading to cell death. It is worth noting that the fibrous CeO2 nanomaterials synthesized by Kato et al. exhibited higher phosphatase-like activity than the polyhedral and cubic forms (Figure 2B) (Kato et al., 2020). This characteristic not only significantly influences antibacterial applications, but the material’s high antigen-antibody binding ability also endows it with great potential in biosensors and biological catalysis. A type of ultra-small and well-dispersed CeO2 nanoparticles synthesized by Xiong et al. has been demonstrated to disrupt cellular homeostasis through their phosphatase-like activity, thereby promoting oxidative stress and ferroptosis (Figure 2C) (Xiong et al., 2022). Ferroptosis is a regulated cell death (RCD) form regulated by ions (Fe2+), characterized by abnormal accumulation of lipid peroxides. The core of its molecular mechanism lies in the loss of activity of glutathione peroxidase 4 (GPX4) or the dysfunction of the intracellular antioxidant defense system (such as the cysteine-glutamate-glutathione antioxidant axis), which leads to the uncontrolled accumulation of lipid ROS, ultimately causing oxidative damage to the cell membrane lipid layer and cell death. Through this activity, CeO2 continuously catalyzes the dephosphorylation of NADPH and its synthetic precursor glucose-6-phosphate, thereby inhibiting the biosynthesis of glutathione and cancer cells’ resistance to ferroptosis. This indirectly suppresses the self-repair mechanisms of cells weakened by oxidative stress, which is exacerbated by enhanced glutathione supply and ferroptosis resistance. This finding provides a new perspective for regulating ferroptosis in cancer cells using non-redox nanomaterials and broadens the application of phosphatase-mimicking nanomaterials in early disease intervention.

Figure 2. Structural diagram of metal oxide-based phosphatase nanozymes. (A) The research has found that the acidic cerium species on the octahedral (111) surface can selectively catalyze the dephosphorylation reaction of the substrate, while the electron-rich counterparts on the cubic (100) surface only promote the oxidation reaction of the substrate. (B) Schematic diagram of the synthesis of cerium oxide fiber particles with high phosphatase-like activity. (C) Diagrammatic representation of the NADP (H)-mediated glutathione regeneration pathway in ferroptosis suppression. (D) Schematic illustration of the ZrO2/CeO2/polyacrylic acid nanocomposites as an alkaline phosphatase mimetic enzyme for the detection of methyl parathion. Reprinted with permission (A) (Tan et al., 2022), (B) (Kato et al., 2020), (C) (Xiong et al., 2022) and (D) (Wu et al., 2021).
Zirconium-based nanomaterials also play a significant role in phosphatase-mimicking nanozymes, such as in the detection of phosphorus-containing drugs (Figure 2D) (Wu et al., 2023; Wu et al., 2021). Notably, Hu et al. first discovered that ZrO2 nanoparticles exhibit phosphatase-like activity using the fluorescent substrate 4-methylumbelliferyl phosphate and evaluated this activity using natural enzyme research methods (Hu et al., 2020). Furthermore, the catalytic interactions of ZrO2 nanoparticles with biologically relevant molecules were systematically evaluated. The results demonstrated that ZrO2 nanoparticles exhibit phosphatase-like activity, effectively catalyzing the hydrolytic dephosphorylation of adenosine triphosphate (ATP) and O-phospho-L-tyrosine. In contrast, no detectable interaction was observed between ZrO2 nanoparticles and DNA strands, indicating their substrate selectivity. These findings provide critical insights into the biochemical reactivity and potential biomedical applications of ZrO2 nanoparticles. Although its phosphatase activity is not as strong as that of CeO2, ZrO2 is more suitable for large-scale development and application due to the greater abundance of the Zr element on Earth compared to currently studied Ce-based nanozymes.
Therefore, functionalizing the surfaces of oxide particles to mimic the catalytic microenvironment of natural phosphatase active sites is crucial and requires further in-depth exploration. Future research should focus on investigating the surface functionalization of oxide-based phosphatase nanozymes to develop more efficient phosphatase nanomaterials.
2.2 MOF-like phosphatase nanozyme
MOFs (Metal-Organic Frameworks) are crystalline porous materials formed by the self-assembly of metal centers and organic ligands. They are characterized by a high specific surface area, tunable porosity, and uniformly distributed active sites within the crystalline porous structure (Xu et al., 2019). The metal (oxide) clusters at the nodes of these frameworks have a unit structure remarkably similar to that of natural enzymes, making them ideal catalytic active sites (Mian et al., 2020). For instance, Mian et al. identified the zinc-based metal–organic framework MFU-4L as a potential hydrolytic catalyst, attributing its activity to the presence of Zn (II)–OH moieties located at the framework nodes. These functional groups closely mimic the structural characteristics of the active sites in carbonic anhydrase (CA), a zinc-containing metalloenzyme renowned for its remarkable efficiency in catalyzing the hydrolysis of phosphate esters. Therefore, by selecting suitable metal ions and organic linkers, it is possible to design nanomaterials with enzyme-like activity, which have advantages over natural enzymes, such as a simpler preparation process, easy storage, and high tolerance to temperature and pH. Currently, commonly used metal elements in MOF materials that simulate phosphatase include Zr, Hf, Ce, and Zn (Cavka et al., 2008; Katz et al., 2014). For instance, Gu et al. reported on the phosphatase catalytic activity of UiO-66(Ce), where the abundant Ce(III)/Ce(IV) coupling sites enable UiO-66(Ce) to selectively catalyze the dephosphorylation hydrolysis of the phosphate ester substrates ATP and ADP under physiological conditions, effectively removing the phosphate groups from ATP and ADP through hydrolysis (Stanford and Bottini, 2023). This discovery has important guiding significance for reducing thrombosis in blood-contacting biomedical devices and for regulating various life activities related to ATP/ADP. Wu et al. first explored the phosphatase-like activity of the Zr-based MOF material MIP-202(Zr). MIP-202(Zr), as an environmentally friendly α-amino acid-based zirconium metal-organic framework (Zr-MOF), demonstrates remarkable green chemical characteristics in its synthesis process: using biogenic L-aspartic acid (L-Asp) as the organic ligand and water as the sole solvent. This study for the first time reveals the phosphatase mimicking activity of MIP-202(Zr) and successfully applies it to the specific detection of phosphate groups in drug molecules. The material synthesis adopts the hydrothermal method as a sustainable strategy, using ZrCl4 as the metal precursor and constructing a stable framework structure with 12 coordination Zr6 (μ3-O)4 (μ3-OH)4 secondary building units (SBUs) through self-assembly with L-Asp. Unlike other reported Zr-based MOF materials, this material acts as a phosphatase-mimicking green nanozyme for phosphatase-like reactions. Moreover, its phosphatase-like activity can be inhibited by phosphorus-containing drugs, eliminating the need for organic solvents and toxic ligands. This broadens the application of phosphatase-mimicking nanozymes in clinical pharmacy (Wu et al., 2023).
2.3 Other types of phosphatase nanomaterials
In addition to the aforementioned materials used for constructing phosphatase-like nanozymes, other material types include active molecules (e.g., amino acids, deferoxamine, and insulin) (Me et al., 2012; Arad et al., 2022; Qiu et al., 2019), metal (ion)-modified carbon-based materials (such as carbon quantum dots and activated carbon frameworks) (Figure 3A) (Wei et al., 2019; Du et al., 2020; Cao et al., 2018; Liu et al., 2019), and graphene (Figure 3B) (Li et al., 2021; Ma et al., 2018). However, single-component materials often struggle to achieve catalytic activity; most are composite materials. The main reasons why single-component materials perform poorly as catalysts are related to their structural characteristics, the uniformity and stability of the active sites. Specifically: 1) Inhomogeneity of active sites: Single-component materials usually have no carriers, resulting in uneven distribution of active sites. This limits the activity and selectivity of the catalyst because catalytic reactions depend on specific active sites. In multi-component catalysts, the interaction between the active component and the carrier can provide more active sites, which may be more uniform, thereby improving catalytic efficiency. 2) Stability issues: During catalytic processes, single-component materials may be more prone to agglomeration or form larger aggregates, which reduces the number of active sites and lowers the stability of the catalyst. Additionally, single-component materials may lack the protective effect provided by the carrier, making them more susceptible to failure in high-temperature or corrosive environments. 3) Adsorption problems of reactants and products: Single-component materials may have strong adsorption capabilities for specific reactants or products, which may lead to catalyst poisoning or carbon deposition, further reducing their performance. For example, in graphene-based phosphatase nanozyme composites, graphene acts as a carrier with a large specific surface area but lacks the ability to catalyze phosphate ester hydrolysis. The actual hydrolytic function is performed by the loaded metal nanomaterials. Nevertheless, these auxiliary materials play important roles in enhancing adsorption affinity, improving the stability of catalytic active sites, and promoting substrate mass transfer.

Figure 3. Structural schematic of other types of phosphatase nanomaterials. (A) The unique properties of CeCD as a model phosphatase and the IFE sensing strategy provide an ideal platform for monitoring the catalytic hydrolysis of phosphates. (B) By constructing catalytic active sites, a biomimetic catalyst was prepared to effectively degrade certain organic phosphorus nerve agent analogues (such as paraoxon and diphosphenol). Reprinted with permission: (A) (Du et al., 2020) and (B) (Ma et al., 2018).
Research on non-metallic materials related to phosphatase-mimicking nanomaterials is also a noteworthy direction. Investigating and developing the phosphatase-like activity of non-metallic materials not only expands the variety of nanomaterials but also deepens understanding of the mechanisms by which nanomaterials catalyze phosphate ester hydrolysis. Lei et al. developed a novel type of phosphatase nanozyme—boron nanosheets—based on their high phosphatase-like activity and, for the first time, applied them to the hydrolytic conversion of anticancer prodrugs, opening a new direction for the biological applications of boron nanosheets (Lei et al., 2024). In this study, the catalytic rate of boron nanosheets is as high as 17 times that of the known phosphatase-mimicking nanozymes. By introducing polyols and Lewis bases, the catalytic activity of boron nanosheets was attributed to the Lewis acidity of the B atoms in the boron nanosheets. This discovery provides a new approach for the development of hydrolytic enzyme-mimicking nanozymes.
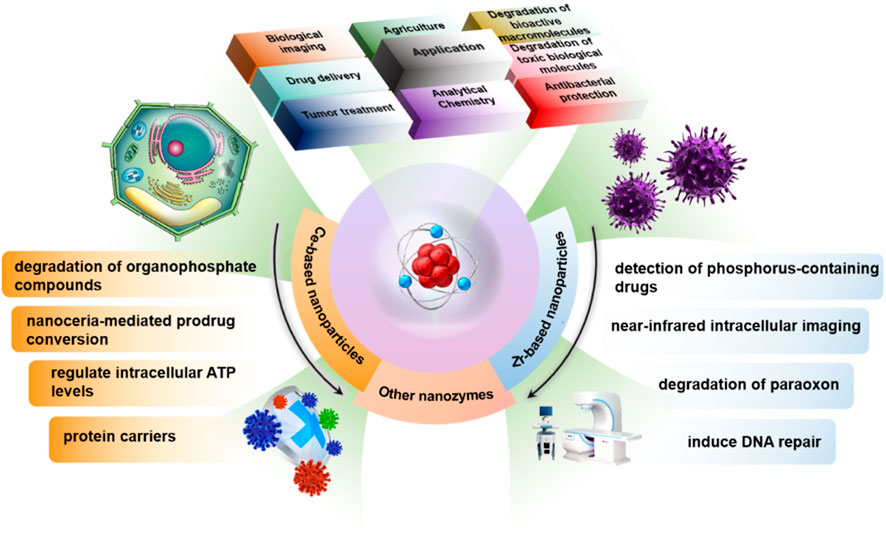
3 Application areas in biomedical science
Phosphate ester substances are widely present in living organisms, and their phosphorylation and dephosphorylation constitute central life processes. In recent years, phosphatase-mimicking nanomaterials have demonstrated great potential in diverse biomedical applications, encompassing tumor therapy (Cao et al., 2018), prevention of allergic diseases (Lin et al., 2021), antibacterial protection (Liu et al., 2019; Khulbe et al., 2020), degradation of toxic biomolecules (Gao et al., 2022), degradation of bioactive macromolecules (Sokolov et al., 2020; Li et al., 2014; Cheng et al., 2012), and multiple directions including biological imaging (Hu et al., 2020) and drug delivery (Walther et al., 2021) (Figure 4). Leveraging their simulated phosphatase hydrolytic activity, nanozymes not only regulate key phosphate metabolic processes within cells but also provide new tools for precise intervention in pathological signaling pathways. For instance, Qu et al. prepared a porous carbon composite material loaded with cerium oxide nanoparticles using Ce-MOF as a precursor via a high-temperature pyrolysis strategy, demonstrating excellent hydrolytic capacity for phosphate esters (Cao et al., 2018). This material efficiently consumes ATP in tumor cells, achieving energy deprivation akin to “starvation therapy,” thereby enhancing chemotherapy sensitivity and treatment efficacy.
To address the limited efficacy of traditional small-molecule mast cell stabilizers in the long-term prevention of allergic diseases, Lin et al. developed a phosphatase-mimicking nano-stabilizer (PMNS) based on cerium dioxide nanoparticles (CeNPs) for the effective prevention of allergic diseases (Lin et al., 2021). This nanomaterial utilizes the regenerable oxygen vacancies on the surface of CeNPs as catalytic active sites, exhibiting continuous and stable phosphatase-mimetic activity. Upon allergen stimulation, it can effectively mediate the dephosphorylation process of phosphoproteins within mast cells. By dynamically regulating the phosphorylation signal cascade in mast cells, PMNS can inhibit the occurrence of degranulation reactions, thereby blocking the pathological responses triggered by the release of downstream allergic mediators (e.g., histamine, cytokines). Ultimately, this enables proactive intervention and prevention of allergic diseases.
Notably, Liu et al. successfully synthesized a metal-organic framework/cerium-based nanozyme with dual enzyme-like biological activities and applied it to the efficient removal of bacterial biofilms (Liu et al., 2019). On the one hand, this system endows the material with catalytic activity similar to that of deoxyribonuclease (DNase) by attaching cerium complexes to the surface of the MOF, effectively hydrolyzing extracellular DNA (eDNA) and thus destroying the structural foundation of the biofilm. On the other hand, doping with gold (Au) elements enhances its peroxidase-like activity, enabling in-situ oxidation and killing of bacteria encapsulated within the biofilm. This synergistic mechanism not only decomposes the biofilm but also effectively prevents bacterial re-proliferation and biofilm recurrence, demonstrating broad prospects for anti-infection applications. Additionally, a polymer-coated cerium dioxide nanozyme developed by Mugesh et al. exhibits phospholipase-like activity, which specifically disrupts the phospholipid layer of bacterial cell membranes, demonstrating significant antibacterial activity against both Gram-positive and Gram-negative bacteria (Khulbe et al., 2020).
In addition to their use in traditional dephosphorylation reactions, phosphatase-mimicking nanomaterials have also demonstrated extensive potential in multiple other fields of biological analysis and clinical applications. For instance, Li et al. successfully synthesized a rare earth-based nanomaterial, GdF3, and were the first to apply it to the enrichment of phosphopeptides (Li et al., 2014). The research team used density functional theory (DFT) calculations to explore the potential mechanisms underlying the dephosphorylation process involving GdF3 and its hydrolysis product GdPO4. This work not only reports the first application of GdF3 in the recognition and capture of phosphopeptides but also provides a theoretical basis and inspiration for understanding the molecular mechanisms of catalytic dephosphorylation reactions mediated by rare earth nanomaterials.
Furthermore, the porous rare earth phosphate microspheres developed by Cheng et al. show great potential as efficient phosphopeptide affinity probes (Cheng et al., 2012). This study innovatively employed the ion-exchange method to prepare monodisperse REPO4 (RE = Yb, Gd, Y) hollow microspheres with unique spike-like morphology. Through comprehensive material characterization (including XRD, EDS, FTIR, SEM, TEM, and N2 adsorption-desorption tests), it was confirmed that this material possesses a regular hollow structure, extremely high specific surface area, and excellent monodispersity. Particularly noteworthy is that these nanostructured materials possess a high specific surface area and controllable pore diameters, which can significantly enhance the specificity of recognition for target phosphopeptides. Meanwhile, they can effectively eliminate the “shadow effect” that may be caused by deep pores, thereby improving the sensitivity and signal-to-noise ratio of mass spectrometry (MS) detection. This provides a powerful tool for the efficient separation and precise qualitative analysis of phosphopeptides in complex samples.
In the field of drug delivery and transformation, the phosphatase activity of nanozymes also exhibits significant application potential. Walther et al. reported the use of cerium dioxide nanoparticles as biomimetic phosphatases, which can catalyze the conversion of phosphoester prodrugs into active therapeutic molecules in complex physiological environments (Walther et al., 2021). This study not only verified that the nanozymes maintained stable phosphatase-like activity in vivo but also emphasized the close integration of substrate spectrum research and functional design, providing a new direction for the development of controllable drug release systems and targeted therapeutic strategies.
In summary, phosphatase-mimetic nanomaterials are continuously expanding their functional frontiers in biological systems, providing innovative platforms for key fields such as disease treatment, precise diagnosis, and gene editing. They are expected to become a critical driving force in the integrated development of nanomedicine and catalytic therapy.
4 Challenges and future prospects
In recent years, phosphatase-mimicking enzymes have demonstrated significant potential in disease treatment, particularly in regulating abnormal phosphorylation signaling, intervening in tumor microenvironments, and treating neurodegenerative diseases, with preliminary achievements made. However, the effective translation from laboratory research to clinical application still faces several key scientific and technological challenges that urgently require in-depth investigation and breakthroughs.
First, current research on metal-based phosphatase nanomaterials remains focused on lanthanide element systems, while the potential of other metals in terms of biocompatibility and catalytic performance has not been systematically explored. In disease treatment—especially in in vivo applications with extremely high biosafety requirements—developing new metallic or non-metallic alternative materials with superior biocompatibility is of great significance. Additionally, existing studies have shown that non-metal-based nanozymes generally exhibit low activity; thus, it is urgent to introduce externally controllable factors (e.g., photothermal effects, electrical stimulation, or magnetic responsiveness) to endow them with controllable, efficient, and targeted catalytic capabilities. This is crucial for precisely regulating abnormal phosphorylation levels in pathological tissues, improving treatment selectivity, and reducing side effects. Furthermore, phosphatase nanomaterials still have limitations in the specific recognition of pathological substrates. Currently, there is a lack of systematic strategies to enhance their catalytic selectivity in complex pathological environments. By constructing mimics of natural enzyme active sites, adjusting the microenvironment at the catalytic interface, or modifying surface functional groups, it is expected that their targeted catalytic capacity in tumors, inflammation, or neurological diseases can be significantly enhanced, thereby achieving more precise therapeutic interventions. In conclusion, although phosphatase-like nanozymes have shown promising applications in in vitro models, their practical application in clinical disease diagnosis and treatment remains rather limited. Future efforts should focus on enhancing their integration with multifunctional nanoplatforms (such as drug delivery systems, responsive hydrogels, and bioimaging probes) to promote their in-depth development in precision therapy, combination treatment, and integrated intelligent diagnosis and treatment.
To summarize, phosphatase-mimicking nanozymes, as emerging biocatalytic materials, hold broad development prospects in disease treatment. However, to truly realize their clinical potential, continuous efforts are needed in material innovation, clarification of catalytic mechanisms, enhancement of selectivity, and integrated applications. This will ultimately provide more efficient, safe, and intelligent treatment solutions for major diseases.
5 Conclusion
Phosphatases play an indispensable catalytic role in physiological processes such as cell signaling, energy metabolism, and bone mineralization. Their central position in biocatalytic systems and phosphate metabolism networks makes phosphatase-mimicking systems one of the important directions in current nanozyme research. This article reviews the research status and applications of phosphatase-mimicking nanozymes.
Natural phosphatases can generally be classified into three categories based on the types of phosphate ester bonds in their substrates: phosphomonoesterase, phosphodiesterase, and phosphotriesterase. Among them, alkaline phosphatase (ALP) is a nonspecific phosphomonoesterase widely distributed in various organisms except higher plants, and it possesses the ability to catalyze the hydrolysis of various phosphomonoester substrates (Chen et al., 2018a). ALP primarily participates in the transfer and hydrolysis of phosphate groups in the body, playing a crucial role in maintaining phosphate homeostasis inside and outside cells, regulating calcium-phosphorus metabolism, and bone tissue mineralization (Chen et al., 2018b). In biomedicine, ALP is widely used in various immunoassay technologies due to its high catalytic efficiency and broad substrate adaptability. It particularly serves as an enzyme label in conventional immunodiagnostic methods like enzyme-linked immunosorbent assay (ELISA) and Western blot, performing signal amplification and targeted detection functions (Zhao et al., 2019). Additionally, the ATP phosphorylation/dephosphorylation cycle is a core mechanism for maintaining cellular energy metabolism, while abnormal changes in phosphatase activity are often closely associated with various pathological conditions. Clinical studies have confirmed that ALP level fluctuations can serve as important biomarkers for diseases such as prostate-related disorders (Prensner et al., 2012), liver dysfunction, and bone metabolism disorders (PIOVESAN and González, 2023). As a key enzyme involved in critical physiological processes (e.g., cell signaling, metabolic regulation, and bone mineralization), alkaline phosphatase exhibits specific activity variations during the onset and progression of multiple diseases, making it widely recognized as a potential biomarker of significant clinical value (Zhao et al., 2025; Wang et al., 2025; Tampieri et al., 2025). However, despite the broad application prospects of natural phosphatases in diagnosis and therapy, their inherent limitations severely restrict practical application expansion. These include issues such as catalytic activity being significantly affected by environmental conditions, poor stability, high separation-purification costs, and activity loss during long-term storage or repeated use.
In recent years, to overcome the aforementioned bottlenecks, researchers have turned their focus to nanozymes with phosphatase-like activity. Owing to their highly tunable physicochemical structures, excellent thermodynamic stability, pH tolerance, and favorable scalability with cost-control advantages, these materials demonstrate significant potential as substitutes for natural enzymes across various fields. Compared with natural phosphatases, phosphatase-mimicking nanozymes not only exhibit stronger environmental adaptability and storage stability but also enable further optimization of catalytic performance through surface engineering and structural regulation, significantly expanding their application prospects in complex biological systems. In the realm of disease therapy, nanozymes have been widely applied to regulate redox homeostasis in the body, eliminate excessive ROS, catalyze localized reactions at disease sites to generate cytotoxic free radicals, or intervene in metabolic pathways by mimicking natural enzyme functions. This enables intervention and treatment for various pathological conditions, including tumors, infections, inflammatory diseases, and neurodegenerative disorders. These advancements not only provide a theoretical basis for expanding the functional roles of nanozymes in disease treatment but also offer novel research strategies for designing hydrolytic enzyme-mimicking nanozymes, including those analogous to phosphatases.
Despite the broad application prospects of phosphatase-mimicking nanozymes in biomedical fields such as disease diagnosis and treatment, systematic and in-depth theoretical support for their in vivo action mechanisms is currently lacking, and a comprehensive and persuasive mechanistic framework remains to be established. In particular, issues related to substrate recognition non-specificity and their impact on targeted catalytic reactions have not been fully elucidated. Key questions regarding the specificity of catalytic reactions of phosphatase-like nanozymes in complex biological systems, substrate selectivity, and their actual contribution to overall therapeutic effects require further clarification. Moreover, the potential toxicity of nanomaterials in biological organisms remains a significant limiting factor in clinical translation. There is currently a lack of systematic and rigorous experimental validation for the metabolic pathways, biodistribution, physiological degradation, and interference of phosphatase-like nanomaterials with key in vivo metabolic pathways. In particular, the potential effects of long-term exposure on tissue homeostasis, immune responses, and cell signal transduction have not been thoroughly assessed. Therefore, future research urgently needs to strengthen the systematic analysis of the biological behavior of phosphatase-mimicking nanomaterials from a mechanistic perspective. This should be combined with high spatiotemporal resolution in situ characterization techniques, advanced in vivo tracking methods, and multi-omics analysis strategies to deeply reveal their dynamic responses and biocompatibility in physiological environments. This is of great significance for establishing a more rigorous and reliable theoretical framework, guiding their safe and efficient biomedical applications, and promoting their clinical translation in precision medicine.
Author contributions
YC: Formal Analysis, Investigation, Validation, Writing – original draft. SZ: Formal Analysis, Investigation, Conceptualization, Supervision, Writing – review and editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Arad, E., Yosefi, G., Kolusheva, S., Bitton, R., Rapaport, H., and Jelinek, R. (2022). Native glucagon amyloids catalyze key metabolic reactions. ACS Nano 16 (8), 12889–12899. doi:10.1021/acsnano.2c05166
Cao, F., Zhang, Y., Sun, Y., Wang, Z., Zhang, L., Huang, Y., et al. (2018). Ultrasmall nanozymes isolated within porous carbonaceous frameworks for synergistic cancer therapy: enhanced oxidative damage and reduced energy supply. Chem. Mater. 30 (21), 7831–7839. doi:10.1021/acs.chemmater.8b03348
Cavka, J. H., Jakobsen, S., Olsbye, U., Guillou, N., Lamberti, C., Bordiga, S., et al. (2008). A new zirconium inorganic building brick forming metal organic frameworks with exceptional stability. J. Am. Chem. Soc. 130 (42), 13850–13851. doi:10.1021/ja8057953
Chen, C., Yuan, Q., Ni, P., Jiang, Y., Zhao, Z., and Lu, Y. (2018a). Fluorescence assay for alkaline phosphatase based on ATP hydrolysis-triggered dissociation of cerium coordination polymer nanoparticles. Analyst 143 (16), 3821–3828. doi:10.1039/c8an00787j
Chen, C., Zhao, J., Lu, Y., Sun, J., and Yang, X. (2018b). Fluorescence immunoassay based on the phosphate-triggered fluorescence turn-on detection of alkaline phosphatase. Anal. Chem. 90 (5), 3505–3511. doi:10.1021/acs.analchem.7b05325
Cheng, G., Zhang, J. L., Liu, Y. L., Sun, D., and Ni, J. (2012). Monodisperse REPO4 (RE= Yb, Gd, Y) hollow microspheres covered with nanothorns as affinity probes for selectively capturing and labeling phosphopeptides. Chem. A Eur. J. 18 (7), 2014–2020. doi:10.1002/chem.201103328
Cheng, J., Yu, H., Zhang, Z. F., Jiang, H. X., Wu, P., Wang, Z. G., et al. (2024). Mxene-bpV plays a neuroprotective role in cerebral ischemia-reperfusion injury by activating the Akt and promoting the M2 microglial polarization signaling pathways. J. Mater. Sci. Mater. Med. 35 (1), 42. doi:10.1007/s10856-024-06811-0
Di, Y., Zhang, E., Yang, Z., Shen, Q., Fu, X., Song, G., et al. (2022). Selective fluorescence imaging of cancer cells based on ROS-Triggered intracellular cross-linking of artificial enzyme. Angew. Chem. Int. Ed. Engl. 61 (14), e202116457. doi:10.1002/ange.202116457
Du, J., Qi, S., Chen, J., Yang, Y., Fan, T., Zhang, P., et al. (2020). Fabrication of highly active phosphatase-like fluorescent cerium-doped carbon dots for in situ monitoring the hydrolysis of phosphate diesters. RSC Adv. 10 (68), 41551–41559. doi:10.1039/d0ra07429b
Gao, L., and Yan, X. J. S. C. L. S. (2016). Nanozymes: an emerging field bridging nanotechnology and biology. Sci. China Life Sci. 59 (4), 400–402. doi:10.1007/s11427-016-5044-3
Gao, L., Zhuang, J., Nie, L., Zhang, J., Zhang, Y., Gu, N., et al. (2007). Intrinsic peroxidase-like activity of ferromagnetic nanoparticles. Nat. Nanotechnol. 2 (9), 577–583. doi:10.1038/nnano.2007.260
Gao, M., Liua, X., Wang, Z., Wang, H., Asset, T., Wu, D., et al. (2022). Engineering catalytic dephosphorylation reaction for endotoxin inactivation. Nano Today, 44, 101456. doi:10.1016/j.nantod.2022.101456
Gesmundo, I., Di Blasio, L., Banfi, D., Villanova, T., Fanciulli, A., Favaro, E., et al. (2019). Proton pump inhibitors promote the growth of androgen-sensitive prostate cancer cells through ErbB2, ERK1/2, PI3K/Akt, GSK-3β signaling and inhibition of cellular prostatic acid phosphatase. Cancer Lett. 449, 252–262. doi:10.1016/j.canlet.2019.02.028
Guo, Y., Ashrafizadeh, M., Tambuwala, M. M., Ren, J., Orive, G., and Yu, G. (2024). P-glycoprotein (P-gp)-driven cancer drug resistance: biological profile, non-coding RNAs, drugs and nanomodulators. Drug Discov. Today 29 (11), 104161. doi:10.1016/j.drudis.2024.104161
Hau-Ting, W. J., Cai-Syaun Wu, M., Chiang, C. K., Huang, P., Gong, T., Yong, K., et al. (2024). Enhanced photodynamic therapy for neurodegenerative diseases: development of Azobenzene-Spiropyran@Gold nanoparticles for controlled singlet oxygen generation. Chem. Weinheim der Bergstrasse, Ger. 30 (62), e202402479. doi:10.1002/chem.202402479
Hosen, M. E., Rahman, M. A., Rahman, M. S., Akash, S., Khalekuzzaman, M., Alsahli, A. A., et al. (2024). Synthesis of silver nanoparticles using Camellia sinensis leaf extract: promising particles for the treatment of cancer and diabetes. Chem. & Biodivers. 21 (3), e202301661. doi:10.1002/cbdv.202301661
Hu, X., Huang, T., Liao, H., Hu, L., and Wang, M. (2020). The phosphatase-like activity of zirconium oxide nanoparticles and their application in near-infrared intracellular imaging. J. Mater. Chem. B 8 (20), 4428–4433. doi:10.1039/d0tb00450b
Huang, Y., Ren, J., and Qu, X. J. C. R. (2019). Nanozymes: classification, catalytic mechanisms, activity regulation, and applications. Chem. Rev. 119 (6), 4357–4412. doi:10.1021/acs.chemrev.8b00672
Huang, T., Hu, X., Wang, M., Wu, Y., Hu, L., and Xia, Z. (2021). Ionic liquid-assisted chemiluminescent immunoassay of prostate specific antigen using nanoceria as an alkaline phosphatase-like nanozyme label. Chem. Commun. 57 (24), 3054–3057. doi:10.1039/d1cc00155h
Jiang, D., Ni, D., Rosenkrans, Z. T., Huang, P., Yan, X., and Cai, W. (2019). Nanozyme: new horizons for responsive biomedical applications. Chem. Soc. Rev. 48 (14), 3683–3704. doi:10.1039/c8cs00718g
Jiang, L., Sun, Y., Chen, Y., and Nan, P. (2020). From DNA to nerve agents–the biomimetic catalysts for the hydrolysis of phosphate esters. ChemistrySelect 5 (30), 9492–9516. doi:10.1002/slct.202001947
Kaplan, M. M. J. G. (1972). Alkaline phosphatase. Gastroenterology 62 (3), 452–468. doi:10.1016/s0016-5085(72)80154-9
Kato, K., Lee, S., and Nagata, F. J. A. P. T. (2020). Catalytic performance of ceria fibers with phosphatase-like activity and their application as protein carriers. Adv. Powder Technol. 31 (7), 2880–2889. doi:10.1016/j.apt.2020.05.016
Katz, M. J., Mondloch, J. E., Totten, R. K., Park, J. K., Nguyen, S. T., Farha, O. K., et al. (2014). Simple and compelling biomimetic metal–organic framework catalyst for the degradation of nerve agent simulants. Angew. Chem. Int. Ed. Engl. 126 (2), 507–511. doi:10.1002/ange.201307520
Khulbe, K., Karmakar, K., Ghosh, S., Chandra, K., Chakravortty, D., and Mugesh, G. (2020). Nanoceria-based phospholipase-mimetic cell membrane disruptive antibiofilm agents. ACS Appl. Bio Mater. 3 (7), 4316–4328. doi:10.1021/acsabm.0c00363
Kuchma, M. H., Komanski, C. B., Colon, J., Teblum, A., Masunov, A. E., Alvarado, B., et al. (2020). Phosphate ester hydrolysis of biologically relevant molecules by cerium oxide nanoparticles. 6 (6): 738–744. doi:10.1016/j.nano.2010.05.004
Lai, W., Guo, J., Wang, Y., Lin, Y., Ye, S., Zhuang, J., et al. (2022). Enzyme-controllable just-in-time production system of copper hexacyanoferrate nanoparticles with oxidase-mimicking activity for highly sensitive colorimetric immunoassay. Talanta 247, 123546. doi:10.1016/j.talanta.2022.123546
Lei, Y., Zhao, Q., Huang, Z., Huang, Y., Wang, M., Hu, L., et al. (2024). Boron nanosheets as a phosphatase mimicking nanozyme with ultrahigh catalytic activity for prodrug-based cancer therapy. Chem. Commun. 60 (26), 3523–3526. doi:10.1039/d3cc05616c
Li, L.-P., Liu, J.-Z., Xu, L.-N., Li, Z., Bai, Y., Xiao, Y. L., et al. (2014). GdF 3 as a promising phosphopeptide affinity probe and dephospho-labelling medium: experiments and theoretical explanation. Chem. Commun. 50 (78), 11572–11575. doi:10.1039/c4cc04090b
Li, M., Lai, P., Chou, Y., Chi, A. P., Mi, Y. Z., Khoo, K. H., et al. (2015). Protein tyrosine phosphatase PTPN3 inhibits lung cancer cell proliferation and migration by promoting EGFR endocytic degradation. Oncogene 34 (29), 3791–3803. doi:10.1038/onc.2014.312
Li, Y., Yan, J., Yu, D., Lei, P., Shen, W., Zhong, M., et al. (2021). Hydrolysis of organophosphorus agents catalyzed by cobalt nanoparticles supported on three-dimensional nitrogen-doped graphene. Inorg. Chem. 60 (23), 17635–17640. doi:10.1021/acs.inorgchem.1c02217
Liang, M., and Nanozymes, Y. X. J. A. O. C. R. (2019). From new concepts, mechanisms, and standards to applications. 52(8): 2190–2200.
Lin, P., Cao, M., Xia, F., Liao, H., Sun, H., Wang, Q., et al. (2021). A phosphatase-mimetic nano-stabilizer of mast cells for long-term prevention of allergic disease. Adv. Sci. (Weinh). 8 (8), 2004115. doi:10.1002/advs.202004115
Liu, T., Neverov, A. A., Tsang, J. S., and Brown, R. S. (2005). Mechanistic studies of La3+- and Zn2+-catalyzed methanolysis of aryl phosphate and phosphorothioate triesters. Development of artificial phosphotriesterase systems. Dev. Artif. phosphotriesterase Syst. 3 (8), 1525–1533. doi:10.1039/b502569a
Liu, Z., Wang, F., Ren, J., and Qu, X. (2019). A series of MOF/Ce-based nanozymes with dual enzyme-like activity disrupting biofilms and hindering recolonization of bacteria. Biomaterials 208, 21–31. doi:10.1016/j.biomaterials.2019.04.007
Ma, X., Zhang, L., Xia, M., Zhang, X., and Zhang, Y. (2018). Catalytic degradation of organophosphorous nerve agent simulants by polymer beads@ graphene oxide with organophosphorus hydrolase-like activity based on rational design of functional bimetallic nuclear ligand, 355, 65–73.
Manea, F., Houillon, F., Pasquato, L., and Scrimin, P. (2004). Nanozymes: gold-nanoparticle-based transphosphorylation catalysts. catalysts 43, 6165–6169. doi:10.1002/anie.200460649
Manto, M. J., Xie, P., and Wang, C. J. A. C. (2017). Catalytic dephosphorylation using ceria nanocrystals. ACS Catal. 7 (3), 1931–1938. doi:10.1021/acscatal.6b03472
Medeiros, M., Orth, E. S., Manfredi, A. M., Pavez, P., Micke, G. A., Kirby, A. J., et al. (2012). Dephosphorylation reactions of mono-di-and triesters of 2, 4-dinitrophenyl phosphate with deferoxamine and benzohydroxamic acid. J. Org. Chem. 77 (23), 10907–10913. doi:10.1021/jo302374q
Mian, M. R., Islamoglu, T., Afrin, U., Goswami, S., Cao, R., Kirlikovali, K. O., et al. (2020). Catalytic degradation of an organophosphorus agent at Zn–OH sites in a metal–organic framework. Chem. Mater. 32 (16), 6998–7004. doi:10.1021/acs.chemmater.0c02373
Piovesan, D., and González, A. (2023). Editorial: Highlights in rehabilitation for musculoskeletal conditions 2021/22. Front. Rehabil. Sci. 4, 1130142. doi:10.3389/fresc.2023.1130142
Prensner, J. R., Rubin, M. A., Wei, J. T., and Chinnaiyan, A. M. (2012). Beyond PSA: the next generation of prostate cancer biomarkers. Sci. Transl. Med. 4 (127), 127rv3–127rv123. doi:10.1126/scitranslmed.3003180
Qiu, L., Lv, P., Zhao, C., Feng, X., Fang, G., Liu, J., et al. (2019). Electrochemical detection of organophosphorus pesticides based on amino acids conjugated nanoenzyme modified electrodes. 286: 386–393. doi:10.1016/j.snb.2019.02.007
Sardoiwala, M. N., Biswal, L., and Choudhury, S. R. (2024). Immunomodulator-derived nanoparticles induce neuroprotection and regulatory T cell action to alleviate parkinsonism. ACS Appl. Mater. & interfaces 16 (30), 38880–38892. doi:10.1021/acsami.3c18226
Sayegh, R. S., Tamaki, F. K., Marana, S. R., Salinas, R. K., and Arantes, G. M. (2016). Conformational flexibility of the complete catalytic domain of Cdc25B phosphatases. Proteins. 84 (11), 1567–1575. doi:10.1002/prot.25100
Sokolov, M. R., Enakieva, Y. Y., Yapryntsev, A. D., Shiryaev, A. A., Zvyagina, A. I., and Kalinina, M. A. (2020). Intercalation of porphyrin-based SURMOF in layered Eu (III) hydroxide: an approach toward symbimetic hybrid materials. Adv. Funct. Mater. 30 (27), 2000681. doi:10.1002/adfm.202000681
Stanford, S. M., and Bottini, N. J. N. R. D. D. (2023). Targeting protein phosphatases in cancer immunotherapy and autoimmune disorders. Nat. Rev. Drug Discov. 22 (4), 273–294. doi:10.1038/s41573-022-00618-w
Tampieri, A., Tavoni, M., Vicidomini, T., Inam, H., Restivo, E., Visai, L., et al. (2025). Injectable bioactive scaffold able to stimulate oral bone regeneration on demand. J. Mater. Sci. Mater. Med. 36 (1), 31. doi:10.1007/s10856-025-06879-2
Tan, Z., Wang, Y., Zhang, J., Zhang, Z., Man Wong, S. S., Zhang, S., et al. (2022). Shape regulation of CeO2 nanozymes boosts reaction specificity and activity. Eur. J. Inorg. Chem. 2022 (20), e202200202. doi:10.1002/ejic.202200202
Walther, R., Huynh, T. H., Monge, P., Fruergaard, A. S., Mamakhel, A., and Zelikin, A. N. (2021). Ceria nanozyme and phosphate prodrugs: drug synthesis through enzyme mimicry. ACS Appl. Mater. Interfaces 13 (22), 25685–25693. doi:10.1021/acsami.1c03890
Wang, X., Hu, Y., and Wei, H. J. I. C. F. (2016). Nanozymes in bionanotechnology: from sensing to therapeutics and beyond. Inorg. Chem. Front. 3 (1), 41–60. doi:10.1039/c5qi00240k
Wang, X., Liu, Y., Liu, J., Qu, J., Huang, J., Tan, R., et al. (2022). A bifunctional electrochemical sensor for simultaneous determination of electroactive and non-electroactive analytes: a universal yet very effective platform serving therapeutic drug monitoring. Biosens. Bioelectron., 208: 114233, doi:10.1016/j.bios.2022.114233
Wang, B., Tao, M., Zhu, W., Li, J., and Hai, Z. (2025). In situ self-assembled probe for antioxidant and anti-inflammatory therapy of inflammatory bowel disease. ACS Appl. bio Mater. 8 (5), 4285–4293. doi:10.1021/acsabm.5c00394
Wei, J., Yang, Y., Dong, J., Wang, S., and Li, P. (2019). Fluorometric determination of pesticides and organophosphates using nanoceria as a phosphatase mimic and an inner filter effect on carbon nanodots. 186, 1–9.
Wu, J., Wang, X., Wang, Q., Lou, Z., Li, S., Zhu, Y., et al. (2019). Nanomaterials with enzyme-like characteristics (nanozymes): next-generation artificial enzymes (II). Chem. Soc. Rev. 48 (4), 1004–1076. doi:10.1039/c8cs00457a
Wu, X., Wei, J., Wu, C., Lv, G., and Wu, L. (2019). ZrO2/CeO2/polyacrylic acid nanocomposites with alkaline phosphatase-like activity for sensing. Spectrochim. Acta. A Mol. Biomol. Spectrosc. 263: 120165, doi:10.1016/j.saa.2021.120165
Wu, Y., Huang, T., Luo, Y., Dai, L., Wang, M., Xia, Z., et al. (2023). Zirconium-amino acid framework as a green phosphatase-like nanozyme for the selective detection of phosphate-containing drugs. Chem. Commun. 59 (8), 1098–1101. doi:10.1039/d2cc06606h
Xiao, G., Li, H., Zhao, Y., Wei, H., Li, J., and Su, H. (2022). Nanoceria-based artificial nanozymes: review of materials and applications. ACS Appl. Nano Mater. 5 (10), 14147–14170. doi:10.1021/acsanm.2c03009
Xiong, Y., Su, L., Ye, F., and Zhao, S. (2022). Inhibition of NADP (H) supply by highly active phosphatase-like ceria nanozymes to boost oxidative stress and ferroptosis. Mater. Today Chem. 23, 100672. doi:10.1016/j.mtchem.2021.100672
Xu, M., Feng, L., Yan, L.-N., Meng, S. S., Yuan, S., He, M. J., et al. (2019). Discovery of precise pH-controlled biomimetic catalysts: defective zirconium metal–organic frameworks as alkaline phosphatase mimics. Nanoscale 11 (23), 11270–11278. doi:10.1039/c9nr02962a
Yao, T., Tian, Z., Zhang, Y., and Qu, Y. (2018). Phosphatase-like activity of porous nanorods of CeO2 for the highly stabilized dephosphorylation under interferences. ACS Appl. Mater. Interfaces 11 (1), 195–201. doi:10.1021/acsami.8b17086
Zhang, R., Yan, X., and Fan, K. J. A. O. M. R. (2021). Nanozymes inspired by natural enzymes. Acc. Mater. Res. 2 (7), 534–547. doi:10.1021/accountsmr.1c00074
Zhao, D., Li, J., Peng, C., Zhu, S., Sun, J., and Yang, X. (2019). Fluorescence immunoassay based on the alkaline phosphatase triggered in situ fluorogenic reaction of o-phenylenediamine and ascorbic acid. Anal. Chem. 91 (4), 2978–2984. doi:10.1021/acs.analchem.8b05203
Keywords: nanozymes, phosphatase, biomedical, nanomaterials, disease treatment
Citation: Cao Y and Zhu S (2025) Nanozymes with phosphatase-like activity. Front. Nanotechnol. 7:1645583. doi: 10.3389/fnano.2025.1645583
Received: 13 June 2025; Accepted: 12 August 2025;
Published: 26 August 2025.
Edited by:
Jie He, University of Connecticut, United StatesReviewed by:
Zichao Wei, Saint-Gobain Research North America, United StatesManthan Sarkar, University of Connecticut, United States
Copyright © 2025 Cao and Zhu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shudong Zhu, MTEyNTUzNzA4MEBxcS5jb20=
 Yu Cao1,2,3
Yu Cao1,2,3 Shudong Zhu
Shudong Zhu