Abstract
Plants used in traditional medicine represent an important source of new compounds. Hallea ledermannii (H. ledermannii) (Krause) Verdc. (Rubiaceae), Gossypium barbadense (G. barbadense) (Malvaceae), Pycnanthus angolensis (P. angolensis) (Myristicaceae), Drypetes gossweileri (D. gossweileri) S. Moore (Euphorbiaceae) and Scyphocephalium ochocoa (S. ochocoa) Warb (Myristicaceae) are five plants widely used in traditional Gabonese medicine as antimicrobials. However, little is known about the active compounds associated with their biological activities. Based on botanical studies and the claims of traditional healers regarding the antimicrobial effects of these plants, a study to evaluate the antimicrobial activity and phyto-chemical profile of aqueous extracts of three plants (bark of H. ledermannii, G. barbadense root bark and P. angolensis bark) and methanolic extracts of two plants (S. ochocoa stem bark and D. gossweileri root bark). Under the guidance of LC-MS detection, identified twenty seven (27) potentially active compounds. Eight (8) of these belong to the quinovic acid-type triterpenoid sap-onins identified in the aqueous extract of H. ledermannii, eleven (11) are dibenzofurans, chroman and stigmasterol detected in the methanolic extract of S. ochocoa and eight (8) compounds in the methanolic extract of D. gossweileri are friedelin, drypemolundein B and gossweilone, to name but a few. In parallel, the five extracts were tested on reference bacterial strains: Staphylococcus aureus ATCC 25923, Pseudomonas aeruginosa ATCC 278533, Salmonella thyphi ATCC 13311, Klebsiella pneumoniae ATCC 700603, Shigella flexneri ATCC 24570. Three of these extracts (aqueous extract of H. ledermannii and two methanolic extracts of S. ochocoa stem bark and D. gossweileri root bark) showed moderate activity against Staphylococcus aureus ATCC 25923, with inhibition zones of 12.3 ± 0.5 mm, 10.1 ± 0.5 mm and 7,6 ± 0 mm respectively. In addition, we assessed the toxicity of the three extracts that showed antimicrobial activity using an invertebrate model, Galleria mellonella (GM). We found that the LD varied according to the concentration of the plant material. The aqueous extract of H. ledermannii and the methanolic extracts of S. ochocoa and D. gossweileri were not toxic to G. mellonella. The LD50s (mg/mL) obtained were 93.2 mg/mL [717.2 g/kg body weight (bw)] and 100 mg/mL (762.3 g/kg bw), 95.4 mg/mL [721.1 g/kg body weight (bw)].
Introduction
Natural products derived from plants, alongside synthetic products based on combinatorial chemistry and genomics, remain an essential source for the discovery of new compounds (Newman and Cragg, 2007). In sub-Saharan Africa, particularly Gabon, the practices of traditional healers have contributed to the collection of ethnobotanical data and suspicions of bioactivity of several plants in humans, thus offering advantages and assets for the implementation of genuine discovery of compounds targeted against tropical infectious diseases (Karaman et al., 2003; Kokoska et al., 2002). The emergence of multi-resistant bacterial pathogens and the increase in antimicrobial resistance drive the need for extensive and sustained investigations towards discovering new antimicrobials. The collection of plants used by traditional healers, testing for antimicrobial properties of the plant extracts, chemical characterization of molecules and evaluation of cytotoxicity are the main steps of discovering new compounds derived from natural products. These steps are often challenging, especially when generating comprehensive and stringent preclinical toxicity data that will lead to first use in humans (Ignasiak and Maxwell, 2017). The invertebrate model Galleria mellonella (GM) (Ogungbemi and van Gestel, 2018; Megaw et al., 2015) is technically easy to transfer from one laboratory to another. It is being investigated as a potential biological alternative to the rat and mouse models (Allegra et al., 2018; Moya-Anderico et al., 2021).
We carried out a study of extracts from five plants; namely Hallea ledermannii (Krause) Verdc. Bark (Rubiaceae), Scyphocephalium ochocoa Warb stem bark (Myristicaceae), Gossypium barbadense root bark (Malvaceae), Pycnanthus angolensis bark (Myristicaceae) and Drypetes gossweileri S. Moore root bark (Euphorbiaceae) (List, 2010). They have been found in the swampy riverine forests and rainforests of Africa and are used by healers for a variety of ailments, including skin and microbial infections, dysentery, pain and as a febrifuge (Burkill, 1997), abscesses, anaemia, general fatigue, infections and respiratory tract infections including tuberculosis (Tchouya et al., 2015; Ngouela et al., 2003).
Here, we report the results of our investigations to identify the compounds that induced antimicrobial effects the plant extracts using Liquid Chromatography-Electrospray Ionization-Mass Spectrometry (LC-ESI-MS) guided method consisting on the characterization of specialised metabolites contained in the plant extracts. To fine-tune the identification of the compounds, ultra-high-performance liquid chromatography-high resolution mass spectrometry (UHPLC/HRMS-MS) analyses were carried out on the three active extracts for quinovic acid-type triterpenoid saponins, dibenzofurans, chroman and stigmasterol constituting the main family of compounds in these extracts. The toxicity profiles of the identified compounds using the GM as a model were analysed. To our knowledge, this study is the first study to present a comprehensive characterisation of these three extracts.
Materials and methods
Plant material
Specimens of H. ledermannii (Rubiaceae) G. barbadense (Malvaceae), P. angolensis (Myristicaceae), D. gossweileri (Putranjivaceae) and S. ochocoa (Myristicaceae) referenced as HL_01, GB_01, PA_01, DG_01 and SO 02, respectively were made kindly available to us the National Institut de Pharmacopée et de Médecine Traditionnelle (IPHAMETRA) Gabon Libreville. Botanical identification was confirmed by Prof. Paul Henri Bourobou Bourou and Mr. Raoul Niangadouma of the Institut de Pharmacopée et de Médecine Traditionnelle (IPHAMETRA). The dried plant material obtained was then pulverized using a Retsch1 MM 400 ball mill. (Monitoring and Control Pty Ltd., Haans, Germany). at a frequency of 30.0 Hz for 120 min frequency of 30.0 Hz for 120 s to obtain fine brown powders. To ensure particle size consistency, these powders were sieved using a 500 mm mesh (Endcotts Filters Ltd., London, United Kingdom).
Extraction of secondary metabolites
The resulting powders of H. ledermannii (245 g) G. barbadense (205 g), P. angolensis (147 g), D. gossweileri (345 g) and S. ochocoa (305 g) respectively were extracted by maceration with various solvents. The first extraction was performed in hexane, followed by ethyl acetate, dichloromethane, methanol and water. Extraction with each solvent was carried out at room temperature in the laboratory for 24 h. The extracts obtained were filtered using filter paper and then evaporated using a rotary evaporator. Only aqueous extracts were freeze-dried.
Data dependent LC-HRMS2 analyses
HPLC-QTOF-HRES analyses were achieved by coupling the LC system to a hybrid quadrupole time-of-flight mass spectrometer Agilent 6,530 (Agilent Technologies, Massy, France) equipped with an ESI source, operating in positive ion mode. Source parameters were set as follows: capillary temperature at 320°C, source voltage at 3500 V, and sheath gas flow rate at 10 L.min−1. The divert valve was set to waste for the first 3 min. MS scans were operated in full-scan mode from m/z 100 to 1700 (0.1 s scan time) with a mass resolution of 11,000 at m/z 922. A MS1 scan was followed by MS2 scans of the three most intense ions above an absolute threshold of 5,000 counts. The selected parent ions were fragmented with one collision energy fixed at 45 eV and an isolation window of 1.3 amu. The calibration solution containined two internal reference masses (purine, C5H4N4, m/z 121.050873, and HP-921 [hexakis-(1H,1H,3H-tetrafluoropentoxy) phosphazene], C18H18O6N3P3F24, m/z 922.0098). A permanent MS/MS exclusion list criteria was set to prevent oversampling of the internal calibrant. LC-UV and MS data acquisition and processing were performed using MassHunter Workstation software (Agilent Technolo-gies, Massy, France).
In order to clarify the concordance of the raw formulae obtained with the MS
2spectra and the compounds present in the databases with a view to dereplication, we used the identification certainty rules proposed by
Dormann et al. (2012)These rules indicate a degree of confidence for the identification of compounds, divided into 5 levels (1–5). They are based on the agreement of several parameters:
• retention time (the authors recommend co-injection);
• precise mass measurement (HR-MS; identical crude formulae, matching isotopic masses);
• matching MS/MS and UV spectra. Level 1 corresponds to reliable identification of a compound.
For this level, the MS and MS2 spectra and the retention time are compared with a physically available reference substance, which is not our case. Level 2 is proposed for satisfactory comparison with data in a database (level 2a), or for satisfactory interpretation of MS and MS/MS data (level 2b). For this level, the retention times between an analyte and a reference are not necessarily concordant, indicating a similarity (sometimes relative in structural terms) but not allowing the identity of an experimental match to be affirmed with certainty. The other levels (3, 4 and 5) are not described in detail. The use of this classification is indicative.
Antimicrobial assays
Bacteria strains
In this study, the following reference strains of bacteria: Pseudomonas aeruginosa ATCC 278533, Salmonella thyphi ATCC 13311, Klebsiella pneumoniae ATCC 700603, Shigella flexneri ATCC 24570 were used as gram negative and as gram positive Staphylococcus aureus ATCC 25923 and clinical isolate of methicillin-resistant Staphylococcus aureus (MRSA) were used. The reference bacteria were purchased from American Type Culture Collection (ATCC). These germs were brought to room tempera-ture and then suspended in Brain Heart Infusion nutrient broth (Oxoid Ltd., Wade Road, Basingstoke, Hants, RG24 8PW, United Kingdom) in order to revive them. 10 μL of each reference bacterium are added to 1 mL of BHI then incubated at 37°C overnight.
Preparationn of bacteria
After incubation, bacterial suspensions containing gram negative bacteria (Pseudomonas aeruginosa ATCC 278533, S. thyphi ATCC 13311, K. pneumoniae ATCC 700603), were cultured on MacConkey agar (Oxoid Ltd., Wade Road, Basingstoke, Hants, RG24 8PW, United Kingdom), the gram-negative bacterium S. flexneri ATCC 24570 was inoculated on Hektoen enteric agar (Becton, Dickinson and company Spark, MD 21152 United States). The gram-positive bacteria staphylococcus aureus was inoculated on Columbia blood agar (Oxoid Ltd., Wade Road, Basingstoke, Hants, RG24 8PW, United Kingdom); in order to obtain pure colonies.
Antimicrobial activity evaluation of plant extracts by Kirby Bauer method
Preparation of inoculum
Kirby Bauer’s method (1, 2) was used to assess the antimicrobial activities of plant extracts. A pure colony of identical morphology of each bacterium is suspended in 4 mL of physiological water (NaCl). The turbidity of this bacterial suspension is measured at 0.5 McFarland, this corresponds to 1.5 × 108 CFU (Karaman et al., 2003).
Challenge of plant extracts with bacteria
Bacterial solution was collected with a clean cotton swab and dipped into Mueller Hinton agar (Oxoid Ltd., Wade Road, Basingstoke, Hants, RG24 8PW, United Kingdom), in which the antimicrobial tests were performed. The agar was dried for 5 min. We made 6 mm disks from filter paper. These disks were individually soaked with 5 µL of each plant extract at an initial concentration of 1 mg/mL. The soaked discs were then placed on Petri dishes containing Mueller Hinton medium. Petri dishes were then incubated at 37°C overnight. The zone of inhibition was measured in millimeters using a ruler.
Assessment of acute toxicity using the Galleria mellonella model
Cytotoxicity was evaluated on the G. mellonella (GM) larvae. GM larvae were obtained from the Medical University of Vienna, Clinical Department of Infections and Tropical Medicine Control Center 3P Währinger Gürtel 18–20, 1090 Vienna. They were stored at 15°C before use. Dead larvae and larvae with dark spots or presenting signs of melanization were removed. A selection of larvae with masses between 0.2 and 0.5 g were chosen for testing. For each concentration, three groups of six (6) larvae were tested. Using a PBS solution, three extract concentrations were obtained: 20 mg/mL; 60 mg/mL and 100 mg/mL. Injection of 20µL of each substance was performed using a 0.3mL Terumo® Myjector® U-100 sy-ringe. Injections were made below the last prominent left leg. A group of 6 larvae injected with sterile PBS was considered as a control. All larvae were incubated at 37°C in the dark. Larval mortality was monitored every 24 h for 120 h. An individual was considered dead when it failed to reorient itself or respond to various stimuli. The 50% lethal dose (LD50) was determined by taking the average weight of a group of 15 larvae from the formula:
Figure
Results
LC-MS based analysis of the three active extracts
LC-MS analysis in this study was only carried out on the three plants that showed potential activity. In order to rapidly identify the compounds potentially responsible for this activity, aqueous extracts of H. ledermannii bark, and D. gossweileri root bark and methanolic extracts of S. ochocoa stem bark were rigorously analyzed by LC-HRMS/MS in positive and negative ionization mode, using data-dependent acquisition.
A Total Ion Chromatogram (TIC) of each crude extract showing peaks corresponding to a chemical compound in the extract is presented in (Figure 1). A manual inspection of the resulted MS/MS spectra led to the putative identification of chemical constituents of the crude extracts (Figures 2–4). The names of the putatively identified compounds are recorded in (Tables 1–3). Known compounds were annotated using search in the most recent version of the dictionary of natural products database (Natural Products database, 2023; Scifinder, 2023; PubChem, 2023).
FIGURE 1

A total ion chromatogram (TIC) in positive and negative ion mode of the aqueous extract and methanolic extract of the three active extracts, obtained on an Agilent 6530 Q/ToF (Scan rang). [(A) ESI TIC Scan crude extract of H. ledermannii; (B) ESI TIC Scan crude extract of S. ochocoa and (C) ESI TIC Scan crude extract of D. gossweileri].
FIGURE 2
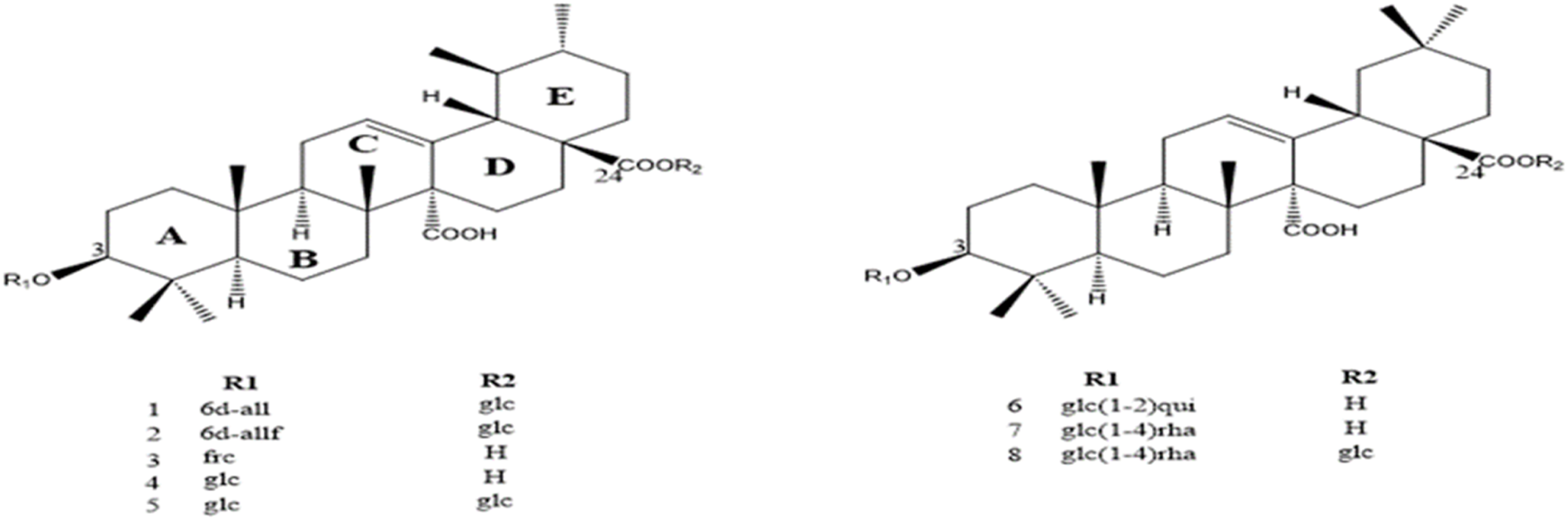
Structure of the compounds isolated from the bark of Hallea ledermannii (Krause) Verdc. (Rubiaceae) (6 d-all, 6-deoxy-β-D allopyranosyl; 6 d-allf, 6-deoxy-β-D allofuranosyl; Frc, fucopyranoside, glc, glucopyranoside, H, hydrogène; glc (1–2) rha, glucopyranosyl-(1→4) rhamnopyranoside; glc (1–2) rha, glucopyranosyl quinovic acid).
FIGURE 3
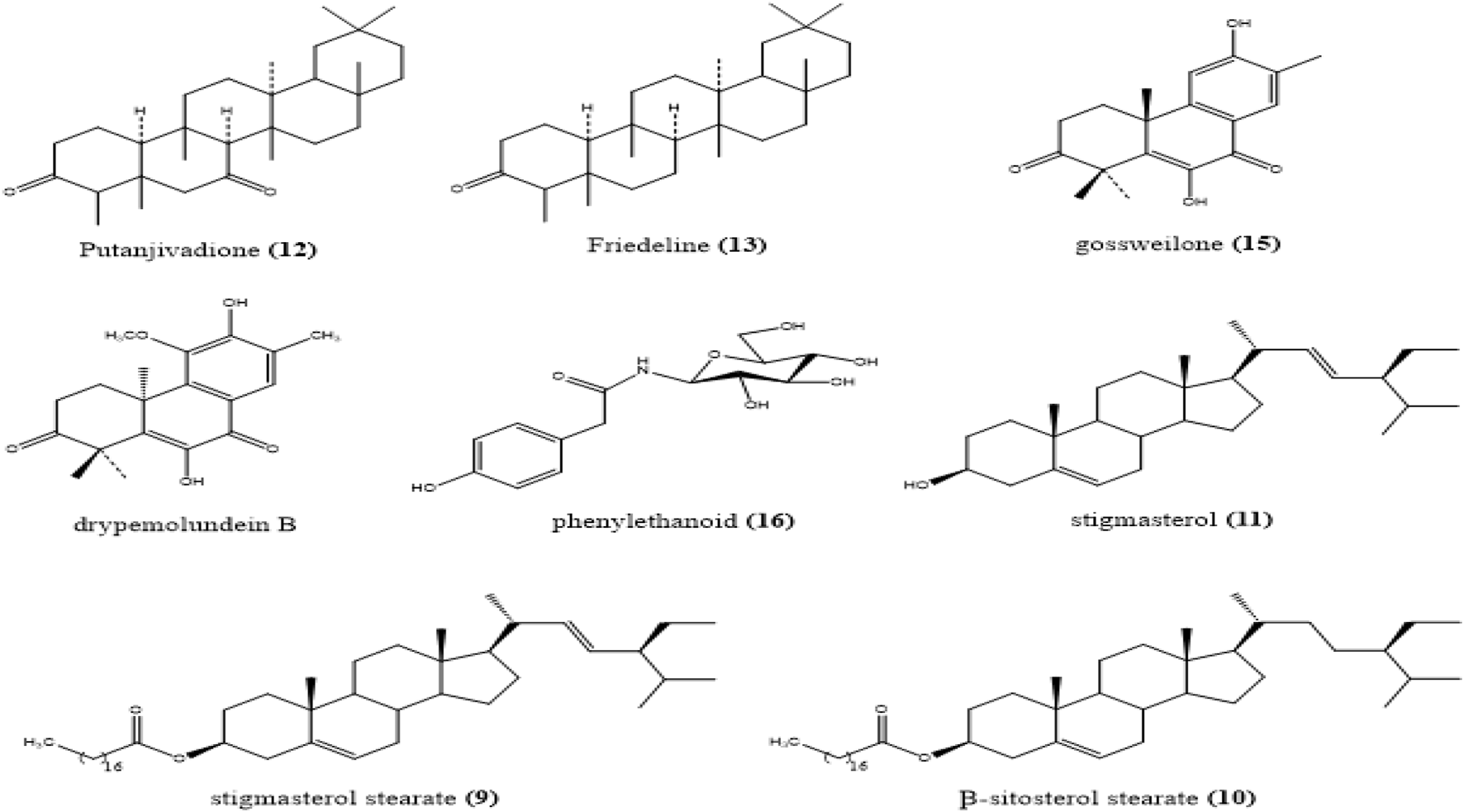
Structure of the compounds isolated from the root bark of Drypetes gossweileri.
FIGURE 4
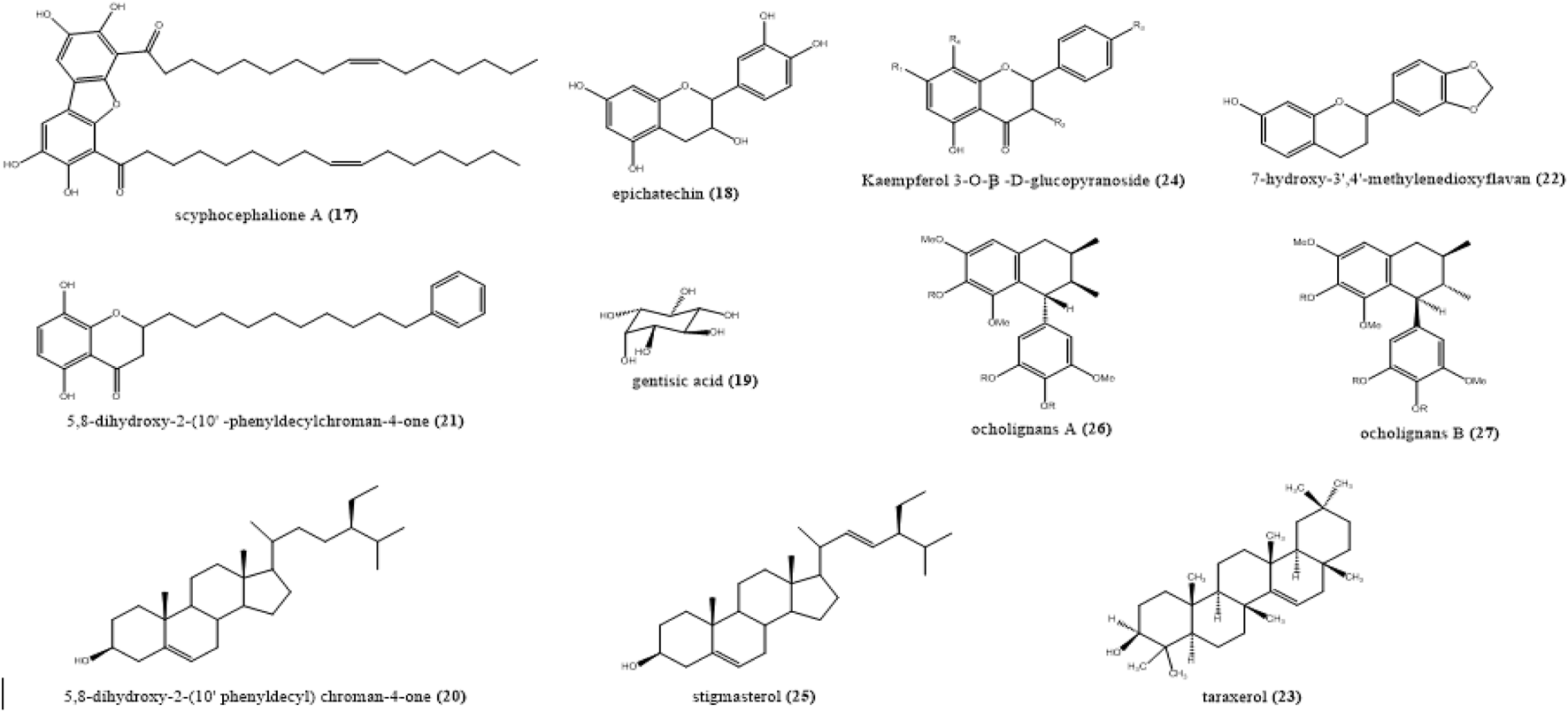
Structure of the compounds isolated from the stem bark of Scyphocephalium ochocoa Warb (Myristicaceae).
TABLE 1
| Peak | RT (min) | m/z [M-H]- | Molecular formulas [M] | Ions tentative identification | Confidence level |
|---|---|---|---|---|---|
| 1 | 25.76 | 793.4377 | C42H65O14 | cincholic acid-3-O-β-D-glucopyranosyl-(1→4)-β-D-quinovopyranoside | 3 |
| 2 | 31.79 | 955.4903 | C48H76O19 | quinovic acid-3 β-O-6-deoxy-β-D-allofuranosyl-28-O-β-D-glucopyranoside | 3 |
| 3 | 29.85 | 779.4219 | C41H64O14 | cincholic acid-3-O-β-D-glucopyranosyl-(1→4)-α-L-rhamnopyranoside | 3 |
| 4 | 32.45 | 672. 4240 | C39H60O9 | quinovic acid-3 β-O-6-deoxy-β-D-allopyranosyl-28-O-β-D glucopyranoside | 3 |
| 5 | 33.27 | 795.1209 | C42H66O14 | cincholic acid-3-O-β-D-glucopyranosyl-(1→4)-α- L-rhamnopyranosyl-28-β-D-glucopyranoside | 3 |
| 6 | 35.62 | 793.4377 | C42H65O14 | quinovic acid-3-O-β-D-fucopyranoside | 3 |
| 7 | 34.20 | 793.4380 | C41H66O11 | quinovic acid-28-O-β-D-glucopyranoside | 3 |
| 8 | 37.81 | 648.8219 | C36H56O10 | quinovic acid-3-O-β-D quinovopyranoside | 3 |
Identification of compound from the aqueous extract of H. ledermannii bark by LC−ESI-MS in the negative ion mode.
TABLE 2
| Peak | RT (min) | m/z [M-H]+ | Molecular formulas [M] | Ions tentative identification | Confidence level |
|---|---|---|---|---|---|
| 9 | 32.756 | 680.302 | C44H64O7 | stigmasterol stearate | 3 |
| 10 | 33.798 | 654.319 | C15H14O6 | β-sitosterol stearate | 3 |
| 11 | 35.806 | 414.313 | C7H6O6 | stigmasterol | 3 |
| 12 | 27.467 | 442.345 | C30H50O2 | putanjivadione | 3 |
| 13 | 37.254 | 426.302 | C30H50O | friedelin | 3 |
| 14 | 29.823 | 456.7152 | C30H48O3 | drypemolundein B | 3 |
| 15 | 26.798 | 299.145 | C18H20O4 | gossweilone | 3 |
| 16 | 35.665 | 448.4321 | C21H20O11 | phenylethanoid | 3 |
Tentative identification of compound from the methanolic extract of D. gossweileri stem bark by LC−ESI-MS in the positive ion mode.
TABLE 3
| Peak | RT (min) | m/z [M-H]+ | Molecular formulas [M] | Ions tentative identification | Confidence level |
|---|---|---|---|---|---|
| 17 | 4.356 | 703.4563 | C44H64O7 | scyphocephalione A | 3 |
| 18 | 3.798 | 290.2712 | C15H14O6 | epicatechin | 3 |
| 19 | 4.506 | 154.1380 | C7H6O6 | gentisic acid | 3 |
| 20 | 4.867 | 396.2292 | C25H32O4 | 5,8-dihydroxy-2-(10′-phenyldecyl)chroman-4-one | 3 |
| 21 | 5.854 | 414.7119 | C29H50O | β-sitosterol | 3 |
| 22 | 5.223 | 270.2919 | C16H14O4 | 7-hydroxy-3′,4′-methylenedioxyflavan | 3 |
| 23 | 5.198 | 426.7240 | C30H50O | taraxerol | 3 |
| 24 | 5.965 | 448.4321 | C21H20O11 | kaempferol 3-O-β -D-glucopyranoside | 3 |
| 25 | 6.456 | 412.690 | C29H48O | Stigmasterol | 3 |
| 26 | 1. 854 | 373.1630 | C29H48O | Ocholignans A | 3 |
| 27 | 2.342 | 373.1630 | C29H48O | Ocholignans B | 3 |
Tentative identification of compound from the methanolic extract of S. ochocoa stem bark by LC−ESI-MS in the positive ion mode.
The total ion chromatogram (TIC) of the aqueous extract of H. ledermannii bark (A) showed eight distinct peaks (ranging from a retention time (tR) of 28.7–37.5 min) (Figure 1). A comparison between the MS data obtained and those published for compounds previously isolated from the bark of H. ledermannii was carried out for dereplication purposes (Figure 2). The peak 1, 2, 6 and 7 showed an ion at m/z 793.4382 [M-H]-, m/z 955. 4903 [M-H]-, m/z 793.4377 [M-H]- and m/z 793.4380 [M-H]- corresponding to the molecular formula C42H66O14, C48H76O19, C42H65O14, and C41H66O11 respectively. While peaks 3, 4, 5 and 8 showed ions at m/z 779.4219 [M-H]-, m/z 672.4240 [M-H]-, m/z 795.1209 [M-H]-, m/z 648.8219 [M-H]-, corresponding to the molecular formula C41H64O14, C39H60O9, C42H66O14 and C36H56O10 respectively.
The base peak chromatogram of the HPLC/HRMS analysis presented in Figure 1 on the extract obtained from the root bark of D. gossweileri (C) detected (8) main chromatographic peaks in the [M + H]+ ionic mode. Manual inspection of the MS/MS spectra obtained led to the putative identification of the chemical constituents of the extract. The 8 compouds are identified (Figure 4): stigmasterol stearate (9), β-sitosterol stearate (10) and β-sitosterol (11) at m/z 680.302 [M + H]+, m/z 654.319 [M + H]+ and m/z 414.313 [M + H]+, respectively. As for the triterpenoids, putanjivadione an ion m/z 442.345 (Calcd for C30H50O2) (12), friedelin (13) with an ion of m/z 426.302 (Calcd for C30H50O) and drypemolundein B (14) with an ion m/z: 456.7152. (Calcd for C30H48O3). Diterpenes were identified, namely Gossweilone (15) with an ion m/z 299.145 (Calcd for C18H20O4).
Using the same procedure, with the methanolic extract of S. ochocoa stem bark (C), several compounds were putatively identified, namely dibenzofurans such as scyphocephalus (17)m/z 703. 4563 [M + H]+ corresponding to the molecular formula C44H64O7; 5,8-dihydroxy-2-(10′-phenyldecyl) chroman-4-one (20)m/z 396. 2292 [M + H]+ C25H32O4; a sterol such as stigmasterol (25)m/z 412.690 [M + H]+ C29H48O; 7-hydroxy-3′,4′-methylenedioxyflavan (22)m/z 270.2919 [M + H]+ C16H14O4) and 2, 7′-cyclolignans as ocholignans A (26) corresponding to the m/z 375. 1630, molecular formula C21H26O6 [M + H]+ (Table 3).
Biological activity of the five (5) crude extracts
The 5 crude extracts obtained from the plants studied were tested biologically. Growth inhibition was observed on methicillin-resistant Staphylococcus aureus (MRSA). For 3 of them, namely the aqueous extracts of H. ledermannii and the methanolic extract of D. gossweileri and S. ochocoa with inhibition zones of 12.3 ± 0.5 mm, 7,6 ± 0 mm and 10.1 ± 0.5 mm respectively (Table 4). The other two extracts were found to be non-susceptible.
TABLE 4
| Inhibition zone diameters (mm) | ||||||
|---|---|---|---|---|---|---|
| Gram-negative | Gram-positive | |||||
| Plants | Extracts | Ps. aeruginosa ATCC 278533 | Sal. Thyphi ATCC 13311 | Kl. Pneumoniae ATCC 700603 | Shigella flexneri | Staph. Aureus ATCC 25923 |
| H. ledermannii (bark) | Aqueous | — | — | — | — | 12.3 ± 0.5 mm |
| D. gossweileri (root bark) | Methanolic | — | — | — | — | 7.6 ± 0 mm |
| S. ochocoa (Stem bark) | Methanolic | — | — | — | — | 10.1 ± 0.5 mm |
| P. angolensis (bark) | Aqueous | — | -— | — | — | — |
| G. barbadense (root bark) | Aqueous | — | — | — | — | — |
| Positive control | Ceftriazone | 22 | 39 | 18 | 29 | 22 |
| Negative control | DMSO | — | — | — | — | — |
Susceptibility of gram-positive and gram-negative bacteria against aqueous extract of H. ledermannii, G. barbadense, P. angolensis and the methanolic extract of S. ochocoao and D. gossweilerileri.
Following this analysis, we assessed the toxicity of the three extracts using the invertebrate model, G. mellonella (GM). We found that the LD varied with the concentration of the plant material (Table 5). The aqueous extract of H. ledermannii, and the methanolic extract of D. gossweileri, S. ochocoa were non toxic to GM. The LD50 (mg/mL) obtained were 93.2 mg/mL [717.2 g/kg body weight (bw)], 95.4 mg/mL (721.1 g/kg bw) and 100 mg/mL (762.3 g/kg bw) respectively.
TABLE 5
| Plants | Parts used | Extracts | LD50 | |
|---|---|---|---|---|
| (mg/mL) | (g/Kg of bw) | |||
| H. ledermannii | Bark | Aqueous | 93.2 ± 1 | 717.2 ± 1 |
| D. gossweileri | Root bark | Methanolic | 95.4 ± 0.5 | 721.1 ± 05 |
| S. ochocoa | Stem bark | Methanolic | 100 ± 0 | 762.3 ± 0 |
LD50 values for the aqueous extract of H. ledermannii, D. gossweileri and S. ochocoa methanolic tested on Galleria mellonella model.
Discussion
Due to the prevalence of multi-resistant bacteria and their low sensitivity to antibiotics, all the extracts were tested for their antibacterial activity. They were tested at a concentration of 250 ug/mL against four reference strains, namely Pseudomonas aeruginosa ATCC 278533, S. thyphi ATCC 13311, K. pneumoniae ATCC 700603, S. flexneri ATCC 24570 were used as gram negative and as gram positive Staphylococcus aureus ATCC 25923. The positive control was ceftriaxone and the negative control was DMSO. Staphylococcus aureus ATCC 25923 was the only gram-positive bacterium tested. This bacterium was only sensitive to three of the five extracts. The aqueous extracts of H. ledermannii, with a zone of inhibition of 12 mm, was the extract that showed the highest antibacterial activity. This result is similar to that found by Adesegun et al. (2012). Who evaluated the activity of leaf extract of H. ledermannii. The methanolic extract of S. ochocoao, with a zone of inhibition of 10 mm. This is almost half the zone of inhibition of the antibiotic ceftriaxone used as a positive control. This result is similar to that obtained by Tchouya et al. (2015) and Foundikou et al. (2018). And the methanolic extract of D. gossweilerii, was moderately active with a zone of inhibition of 7 mm. This result is similar to a DCM extract of D. gossweileri bark showed antibacterial activity with MIC values of 0.25–1.00 mg/mL against Staphylococcus aureus (Wansi et al., 2016). The results showed that none of the extracts showed activity against gram-negative bacteria (Table 4).
The toxicity test was carried out on the three plants with antimicrobial activity: the aqueous extract of H. ledermannii, the methanolic extract of S. ochocoa and the methanolic extract of D. gossweilerii. This test was carried out over 4 days and in triplicate. Although the differences were not statistically significant, the mortality rates obtained with the 100 mg/mL concentrations were visually higher than those obtained with the 20 and 60 mg/mL concentrations (SI). The results showed that the LD50 of the aqueous extract of H. ledermannii 93.241 mg/mL (717.2 g/kg) was slightly lower than that of the methanolic extract of D. gossweilerii 95.41 mg/mL (721.1 g/kg) and S. ochocoa 100 mg/mL (762.3 g/kg). The compounds contained in the aqueous extract of H. ledermannii are therefore more toxic. However, compared to other medicinal plant extracts, the toxicity of the aqueous extract of H. ledermannii and the methanolic extract of S. ochocoa and D. gossweilerii is very low. In deed, for example, the hydroethanol extract of Citratus officinalis, whose toxicity was as-sessed on the G. mellonella model, was 4.87 mg/mL (90 g/kg larval mass) (Mbarga et al., 2021). Extracts of total alkaloids from the plant Artemisia annua showed a toxicity of 200 g/kg; the isolated anti-plasmodial molecules were judged to be non-toxic to humans (Sunmonu and Afolayan, 2013). Com-paring the results of the studies cited with our own, the toxicity of the extracts studied is much lower than that of Artemisia annua, leading to the conclusion that the various molecules that could be extracted from it would be harmless to humans.
Many structurally diverse secondary metabolites have already been isolated from H. ledermannii. In our study, Using the molecular formula obtained by MS/MS only one result corresponding to the quinovic acid triterpenoid saponin compounds previously described from the stem bark of Hallea ledermannii (Table 1) (Koffi et al., 2023; Kang et al., 2003). On the basis of these results, we can assume that quinovic acid-type triterpenoid saponins could be one of the compounds responsible for the antibacterial activity of this extract.
Manual inspection of the MS/MS spectra of methanolic extract of D. gossweileri stem bark led to the putative identification of the chemical constituents of the extract; A total of 8 compounds were identified. These metabolites belong to families of natural compounds, namely steroids, diterpenes and triterpenoids. Among the steroids, stigmasterol stearate, β-sitosterol stearate and β-sitosterol, were putatively identified. Compounds, 10 and 11 were previously isolated by Matochko (2010) and Ata et al., in 2011. We have the putanjivadione, friedelin and drypemolundein B. All these compounds had been described by Ngouela et al. (2003); Wandji et al. (2003) or by Wandji et al. (2000) in D. gossweileri stem bark extracts (Ngouela et al., 2003; Wandji et al., 2000). Diterpenes were identified, namely Gossweilone. This compound had already been isolated previously by Ngouela et al. (2003) in methanolic extract of D. gossweileri stem bark.
All compounds have been previously described in S. ochocoaI (Foundikou et al., 2018). In another study, ocholignan A was found to possess significant in vitro antibacterial activity against methicillin-resistant Gram-positive Staphylococcus aureus ATCC 33591 and Staphylococcus aureus 78–13607A, with a MIC of 16 lg/mL, respectively (Hu et al., 2005). Based on these results, we can speculate that cyclolignans, like ocholignans, could be one of the compounds responsible for the antimicrobial activity of the extract.
Conclusion
To the best of our knowledge, our work reports the chemical and biochemical study of five plants used in traditional medicine in Gabon. First of all, it was shown that three of the five plants, namely: H. ledermannii, D. gossweileri (Putranjivaceae) and S. ochocoa can biosynthesise antimicrobial compounds. In addition, using the LCMS/MS data base approach, we were able to easily identify (27) compounds likely to be responsible for the biological activity observed, namely quinovic acid-type triterpenoid saponins detected in the stem bark of Hallea ledermannii; steroids such as stigmasterol stearate, β-sitosterol stearate, β-sitosterol, putanjivadione, friedelin and drypemolundein B in extracts of D. gossweileri root bark extracts and in the ethanolic. Extract of S. ochocoa Scyphocephalus, chroman 5,8-dihydroxy-2-(10 phenyldecyl)chroman-4-one methylenedieth-er 7-hydroxy-3′,4′-methylenedioxyflavan 6 and 2, 7-cyclolignans as ocholignans A. Our study highlighted that the three extracts can produce various compounds with antimicro-bial activity against Staphylococcus aureus, providing important documentation of the an-tibacterial activity of these compounds. In addition, the aqueous extract of the bark of H. ledermannii and the methanolic extract of the stem bark of S. ochocoaI and D. gossweileri root bark extracts were found to be non-cytotoxic on the G. mellonella model.
Statements
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding authors.
Author contributions
EON’n: Conceptualization, Data curation, Formal Analysis, Investigation, Methodology, Software, Supervision, Validation, Visualization, Writing–original draft. PE: Investigation, Methodology, Writing–review and editing. JE: Methodology, Software, Writing–review and editing. AB: Investigation, Writing–review and editing. RK: Conceptualization, Investigation, Methodology, Project administration, Writing–review and editing. MN’n: Writing–review and editing. FO: Writing–review and editing. BL: Conceptualization, Funding acquisition, Investigation, Resources, Writing–review and editing. PK: Conceptualization, Funding acquisition, Project administration, Resources, Visualization, Writing–review and editing. SA: Funding acquisition, Project administration, Resources, Supervision, Writing–review and editing, Conceptualization, Methodology, Validation.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This project/publication was supported in part by Africa-UniNet, financed by the Austrian Federal Ministry of Education, Science and Research (BMBWF) and implemented by OeAD.
Acknowledgments
The authors would also like to thank Mehdi Beniddir, Professeur des universités en pharmacognosie Université Paris-Saclay, UFR de Pharmacie for obtaining and analyzing the spectral data by HPLC-QTOF-HRES, Professor Paul Henri Bourobou Bourou and Mr Raoul Niangadouma of the Institut de Pharmacopée et de Médecine Traditionnelle (IPHAMETRA) for their help in the botanical identification of the plants studied.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1
Adesegun S. A. Anyika N. E. Adekoya T. O. Essien S. G. (2012). Antibacterial and antioxidant investigations of Hallea ledermannii leaf extract. Indian J. Sci. Technol.5 (1), 1885–1887.
2
Allegra E. Titball R. W. Carter J. Champion O. L. (2018). Galleria mellonella larvae allow the discrimination of toxic and non-toxic chemicals. Chemosphere198, 469–472. 10.1016/j.chemosphere.2018.01.175
3
Burkill S. (1997). Student empowerment through group work : a case study. J. Geogr. High. Educ.21 (1), 89–94. 10.1080/03098269708725412
4
Dormann C. F. Schymanski S. J. Cabral J. Chuine I. Graham C. Hartig F. et al (2012). Correlation and process in species distribution models: bridging a dichotomy. J. Biogeogr.39 (12), 2119–2131. 10.1111/j.1365-2699.2011.02659.x
5
Foundikou H. Mbiantcha M. Bankeu Kezetas J. Tchouankeu J. C. Shaheen F. Choudhary M. I. et al (2018). Two new alkylresorcinol derivatives from the leaves of Scyphocephalium ochocoa. Z. für Naturforsch. B73 (6), 381–388. 10.1515/znb-2018-0008
6
Hu J.-F. Garo E. Yoo H.-D. Cremin P. A. Goering M. G. O’Neil-Johnson M. et al (2005). Cyclolignans from Scyphocephalium ochocoa via high-throughput natural product chemistry methods. Phytochemistry66 (9), 1077–1082. 10.1016/j.phytochem.2005.03.014
7
Ignasiak K. Maxwell A. (2017). Galleria mellonella (greater wax moth) larvae as a model for antibiotic susceptibility testing and acute toxicity trials. BMC Res. notes10, 428–8. 10.1186/s13104-017-2757-8
8
Kang W.-Y. Wang J.-S. Yang X.-S. Hao X.-J. (2003). Triterpenoid saponins from luculia pincia hook. Chin. J. Chem.21 (11), 1501–1505. 10.1002/cjoc.20030211122
9
Karaman I. Şahin F. Güllüce M. Öǧütçü H. Şengül M. Adıgüzel A. (2003). Antimicrobial activity of aqueous and methanol extracts of Juniperus oxycedrus L. J. Ethnopharmacol.85 (2-3), 231–235. 10.1016/s0378-8741(03)00006-0
10
Koffi J.-M. K. Yao-Kouassi P. A. Magid A. A. Akissi Z. L. E. Martinez A. Sayagh C. et al (2023). New triterpenoid saponins from the stem bark of Hallea ledermannii. Tetrahedron Lett.116, 154335. 10.1016/j.tetlet.2022.154335
11
Kokoska L. Polesny Z. Rada V. Nepovim A. Vanek T. (2002). Screening of some Siberian medicinal plants for antimicrobial activity. J. Ethnopharmacol.82 (1), 51–53. 10.1016/s0378-8741(02)00143-5
12
List P. (2010). The plant list. Version.
13
Matochko W. (2010). Identification of acetylcholinesterase inhibiting natural products from buxus natalensis and Drypetes gossweileri. Available at: https://mspace.lib.umanitoba.ca/handle/1993/4237.
14
Mbarga M. J. A. Podoprigora I. V. Anyutoulou K. L. D. (2021). Galleria mellonella (greater wax moth) as an eco-friendly in vivo approach for the assessment of the acute toxicity of medicinal plants: application to some plants from Cameroon. Open Veterinary J.11 (4), 651–661. 10.5455/ovj.2021.v11.i4.15
15
Megaw J. Thompson T. P. Lafferty R. A. Gilmore B. F. (2015). Galleria mellonella as a novel in vivo model for assessment of the toxicity of 1-alkyl-3-methylimidazolium chloride ionic liquids. Chemosphere139, 197–201. 10.1016/j.chemosphere.2015.06.026
16
Moya-Anderico L. Vukomanovic M. del Mar Cendra M. Segura-Feliu M. Gil V. Del Río J. A. et al (2021). Utility of Galleria mellonella larvae for evaluating nanoparticle toxicology. Chemosphere266, 129235. 10.1016/j.chemosphere.2020.129235
17
Natural Products database (2023). Natural Products databaseAvailable at: http://dnp.chemnetbase.com accessed April 7, 2023.
18
Newman D. J. Cragg G. M. (2007). Natural products as sources of new drugs over the last 25 years. J. Nat. Prod.70 (3), 461–477. 10.1021/np068054v
19
Ngouela S. Ngoupayo J. Noungoue D. T. Tsamo E. Connolly J. D. (2003). Gossweilone: a new podocarpane derivative from the stem bark of Drypetes gossweileri (Euphorbiaceae). Bull. Chem. Soc. Ethiop.17 (2). 10.4314/bcse.v17i2.61672
20
Ogungbemi A. O. van Gestel C. A. (2018). Extrapolation of imidacloprid toxicity between soils by exposing Folsomia candida in soil pore water. Ecotoxicology27, 1107–1115. 10.1007/s10646-018-1965-x
21
Pubchem Pubchem Available at: https://pubchem.ncbi.nlm.nih.gov/compound, accessed April 7, 2023.
22
Scifinder (2023). Scifinder. Available at: http://scifinder.cas.org accessed April 7, 2023.
23
Sunmonu T. O. Afolayan A. J. (2013). Evaluation of antidiabetic activity and associated toxicity of Artemisia afra aqueous extract in wistar rats. Evidence-Based Complementary Altern. Med.2013, 1–8. 10.1155/2013/929074
24
Tchouya G. R. F. Souza A. Tchouankeu J. C. Yala J.-F. Boukandou M. Foundikou H. et al (2015). Ethnopharmacological surveys and pharmacological studies of plants used in traditional medicine in the treatment of HIV/AIDS opportunistic diseases in Gabon. J. Ethnopharmacol.162, 306–316. 10.1016/j.jep.2014.12.052
25
Wandji J. Wansi J. D. Fuendjiep V. Dagne E. Mulholland D. A. Tillequin F. et al (2000). Sesquiterpene lactone and friedelane derivative from Drypetes molunduana. Phytochemistry54 (8), 811–815. 10.1016/s0031-9422(00)00040-6
26
Wandji J. Tillequin T. Mulholland D. A. Tsabang N. Seguin E. Verite P. et al (2003). Pentacyclic triterpenoid and saponins from Gambeya boukokoensis. Phytochemistry64 (4), 845–849. 10.1016/S0031-9422(03)00495-3
27
Wansi J. D. Wandji J. Sewald N. Nahar L. Martin C. Sarker S. D. (2016). Phytochemistry and pharmacology of the genus Drypetes: a review. J. Ethnopharmacol.190, 328–353. 10.1016/j.jep.2016.06.060
Summary
Keywords
LC-MS based analysis, antimicrobial compounds, cytotoxic activity, traditional Gabonese medicine, LC-MS
Citation
Otogo N’nang E, Essone PN, Ella Ndong J, Boueya A, Kokou K, Kriz R, N’nengué MA, Ovono Abessolo F, Lell B, Kremsner PG and Agnandji ST (2025) LC-MS based analysis reveal antimicrobial compounds from Gabonese pharmacopoeia: chemical characterisation and cytotoxicity evaluation. Front. Nat. Produc. 3:1478361. doi: 10.3389/fntpr.2024.1478361
Received
09 August 2024
Accepted
11 November 2024
Published
09 January 2025
Volume
3 - 2024
Edited by
Olumayokun Olajide, University of Huddersfield, United Kingdom
Reviewed by
Hiba Riyadh Al-Abodi, College of Science University of Al-Qadisiyah, Iraq
Ankanahalli N. Nanjaraj Urs, Washington University in St. Louis, United States
Updates
Copyright
© 2025 Otogo N’nang, Essone, Ella Ndong, Boueya, Kokou, Kriz, N’nengué, Ovono Abessolo, Lell, Kremsner and Agnandji.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Elvis Otogo N’nang, elvisotogonnang@gmail.com; Selidji T. Agnandji, agnandjis@cermel.org
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.