Abstract
Introduction:
Functional food products are widely available in the market and have several benefits, such as high protein and low calorie content, for addressing obesity. However, the regulation of product content, which results in weight maintenance, needs to be described. This study aimed to explore methods to assess the influence of anti-obesity food bars made from soybeans on antioxidants in functional food products that are widely available in the market.
Methods:
These methods include network pharmacology screening, bioactive gene analysis, interaction network development, phytochemical screening, molecular docking, and antioxidant activity testing.
Results:
Screening revealed that the main bioactivities of the soybean food bars were glycitein and 6″-Omalonylglycitin, which have a high affinity for molecular docking. The food bar methanol and ethanol extracts had higher ES50 values (1.30 and 2.00 mg/mL, respectively) than genistein (0.13 mg/mL), indicating weak antioxidant activity. Therefore, the ethanol and methanol extracts of the soybean food bar exhibited weak antioxidant activity.
Conclusion:
This study suggests that soybean-based food bars may have potential anti-obesity relevance through predicted interactions with leptin signaling proteins, network pharmacology analysis, and measurable antioxidant activity.
1 Introduction
Obesity is a common problem because it can lead to several diseases that culminate in death. According to WHO data from 2017, more than 4 million people die of obesity annually. Moreover, since 1975, the number of obese children and adolescents has quadrupled from 4% to 18% in 2016, and since 1980, the number of obese adults has increased by more than 200% (WHO, 2022). Obesity causes cognitive dysfunction by triggering mechanisms that accelerate reactive oxygen species (ROS) production. ROS induce oxidative stress in cells and tissues, causing DNA mutations that can increase the risk of developing obesity. Owing to the seriousness of the obesity problem, many groups have started innovations that simply address overweight individuals. Phytochemicals have the potential to overcome obesity; therefore, they are added to processed foods known as functional foods (Konstantinidi and Koutelidakis, 2019; Saad et al., 2021; Sandner et al., 2020; Patil et al., 2025).
Functional foods contain active substances that have been proven to provide physiological health benefits. The expected physiological function of functional foods is to reduce body weight by optimizing the fiber and antioxidant content. Natural antioxidants can modulate oxidative stress caused by ROS, improve immune function in obesity, and stabilize and simultaneously reduce body weight and body mass index (Bonomini, 2023; Fekete et al., 2025; Ham and Joung, 2021). The bioactive compounds found in plants, such as isoflavones, can stimulate the process of lipolysis, regulate adipogenesis and apoptosis in adiposity cells, and act as antioxidants that counteract free radicals resulting from high amounts of oxidative stress in obese people (Saad et al., 2021; Kim, 2022; Sohn et al., 2021; Younis et al., 2025).
Several functional food products have become popular and have generated excellent profits. Previous research has shown that in 2019, the global functional food market was valued at USD 177.770 million, and the compounded annual growth rate (CAGR) of the functional food market is expected to increase by 6.7% by 2027 compared to 2021 (Domínguez Díaz et al., 2020; Fuso et al., 2023; Ojwach et al., 2022; Szakos et al., 2022). The functional food market in 2025 based on region with the highest percentage is occupied by Asia Pacific at 47.5%, then followed respectively by North America (23.8%), Europe (15.8), rest of the world (12.9) (Doshi, 2025). Despite the vast functional food development and market, the mechanism of action of products to address overweight has not yet been determined.
Isoflavones are found in leguminous plants, particularly soybeans (Kim, 2022; Messina et al., 2025). As bioactive compounds, isoflavones have antioxidant activity (Saad et al., 2021; Sohn et al., 2021; Kim et al., 2020). The structure and function of plant-derived isoflavones resemble those of the hormone estrogen, which is a phytoestrogen. Isoflavones with the most potent estrogenic activity include genistein, daidzein, and glycitein (Kim, 2022; Chiang et al., 2016; Choi et al., 2020; Intharuksa et al., 2025). This study aimed to explore and predict the potential of soybean food bar extracts as functional foods with anti-obesity properties using network pharmacology, molecular docking, and antioxidant activity measurements.
2 Materials and methods
2.1 Identification and screening of bioactive
Bioactive compounds in soybeans were obtained from the KNApSAcK database (http://www.knapsackfamily.com/) using the query “Glycine max” (Liu et al., 2017). The KNApSAcK database contains 63,723 metabolite entries, 159,101 metabolite-species pairs, and 24,749 species entries. OB and DL parameters were screened for all soybean bioactives using the Traditional Chinese Medicine Systems Pharmacology (TCMSP) database platform (https://old.tcmsp-e.com/index.php). The TCMSP database uses data from 29.384 compounds and 3,311 targets that can be used as sources of analysis (Ru et al., 2014; Zhao et al., 2022; Wang S. et al., 2021). Bioactive compounds in food bars with OB ≥ 30% and DL ≥ 0.18 were selected (Tao et al., 2020; Wang K. et al., 2021; Han et al., 2024).
2.2 Identification of component-target gene network-protein targets
Basic compound information was obtained from the PubChem database (https://pubchem.ncbi.nlm.nih.gov/) (Kim et al., 2021). Bioactive protein targets were identified using the STITCH database (http://stitch.embl.de), which contains 9.643.763 proteins from 2.031 organisms (Chen et al., 2023; Szklarczyk et al., 2021). Proteins were extracted using UniProt Knowledgebase (UniProtKB), which contains 54.247.468 sequence items (www.uniprot.org) (Zhang et al., 2020; Consortium et al., 2025).
2.3 Obesity-associated targets prediction and construction of PPI network
GeneCards (https://www.genecards.org/) and STRING (https://string-db.org/cgi/input.pl) was used to identify obesity-associated genes (Safran et al., 2021; Szklarczyk et al., 2023; Zhou et al., 2022). The query used to obtain related targets was “Oxidative Stress”, “antioxidant”, “ROS”, and “obesity”. PPI network interactions were performed using the INPUT database (http://cbcb.cdutcm.edu.cn/INPUT) (Chen et al., 2023).
2.4 GO and KEGG pathway enrichment analysis
GO and KEGG analyses use the INPUT database (http://cbcb.cdutcm.edu.cn/INPUT) and STRING (https://string-db.org/cgi/input.pl) to process and visualize data from the Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) (Chen et al., 2023; Szklarczyk et al., 2023). GO analysis identified biological processes (BP), molecular functions (MF), and cellular components (CC) (Liao et al., 2019).
2.5 Component–target molecular docking
The 3D structure of the target protein LEP (PDB ID: 1AX8) was obtained from the RCSB PDB database (https://www.rcsb.org/) and UniProt Knowledgebase (www.uniprot.org). The target protein selected for docking was leptin (LEP, PDB ID: 1AX8), a key adipokine that regulates energy balance, lipolysis, and satiety signaling, making it biologically relevant for anti-obesity mechanisms. LEP was identified as one of the top-ranked hub genes in the network pharmacology analysis, indicating its central role in the soybean bioactive compound–target interaction network. The protein was prepared using Pymol 2.2.5 and saved in PDB file format. Hydrogen atoms were added, and the protein was protonated at physiological pH 7.4 (Saxton et al., 2023; Ren et al., 2025).
The bioactive ligands were obtained from the PubChem database (https://pubchem.ncbi.nlm.nih.gov/) to download a three-dimensional (3D) structure with the SDF file type. Open Babel software (PyRx 0.8) was used to change the file type from SDF to MOL2 for molecular docking analysis using AutoDock Vina (PyRx 0.8). The resulting output had the best affinity, with the most negative value, and was visualized using PyMOL software (Kondapuram et al., 2021). Lorcaserin was used as a comparator in this study. Lorcaserin was used as a redocking control to validate grid parameters and pocket definition (Bohula et al., 2018; Singh and Singh, 2020; Wagner et al., 2023; Tuccinardi et al., 2019).
2.6 Phytochemical profiling and antioxidant activity assays
2.6.1 Material
DPPH (≥95% purity, Sigma-Aldrich, United States), genistein (≥98% purity, Sigma-Aldrich, United States), and ferric chloride FeCl3.6H2O 5% (Merck, Germany) were used as reagents. Analytical grade methanol (80%) and ethanol (70%) (Merck, Germany) were used as solvents for sample preparation and dilution.
2.6.2 Thin layer chromatography (TLC) assay
Sample preparation and extraction were performed as previously described (Borges et al., 2020). The soybean food bar powder was air-dried at 40 °C until a constant weight was reached before extraction to correct for the moisture content. A 5 g sample of the soybean food bar was extracted by ultrasonication for 30 min at room temperature (25 °C ± 1 °C), carried out three times using 70% ethanol (50 mL) and 80% methanol (50 mL). The solvent-to-sample ratio was maintained at 1:10 (w/v) for all extraction cycles. The residue from the first extraction was extracted again using a new solvent of the same volume and type. The extraction process was carried out until the obtained dregs did not form a green color when dropped with FeCl3 solution, and the extracted solution was clear (the residual fraction still contained flavonoids). This ensured that the extraction process was optimal and that the active substances in the sample were completely extracted (Hayat et al., 2020; Zhang et al., 2019). The filtrate resulting from the extraction was combined and concentrated at 40 °C–50 °C in a water bath until the remaining volume reached ±25 mL (Fahad et al., 2021). Each evaporated extract was mixed with methanol to a total volume of 25 mL, and a liquid extract of 200 mg/mL was obtained. The samples were stored at 4 °C until further analysis to prevent the degradation of phenolic and isoflavone compounds (Shawky and Sallam, 2017).
Qualitative testing of flavonoids was performed using 5% FeCl3. Isoflavone testing was carried out using TLC silica gel 60 F254 aluminum plates (Merck, 0.25 mm thickness). Samples (5 μL) were spotted on silica gel 60 F254 aluminum plates (Merck, 0.25 mm) and developed in the mobile phase (ethyl acetate:methanol:distilled water:acetic acid, 100:20:16:1, v/v/v/v). The chamber was pre-saturated for 15 min (Shawky and Sallam, 2017; Pobłocka-Olech et al., 2018). Genistein was used as a marker compound for qualitative standardization of the extracts through TLC analysis.
2.6.3 Antioxidant activity
The ethanol liquid extract was prepared with a concentration series of 1.75, 1.5, 1.25, 1.0, 0.75, 0.5, and 0.25 mg/mL in methanol, and methanol aqueous extracts were prepared with concentrations of 0.5, 1.0, 1.5, 2.0, 2.5, 3.0, and 3.5 mg/mL in methanol. Genistein was used as a positive control at concentrations of 0.160, 0.140, 0.120, 0.100, 0.080, 0.060, and 0.040 mg/mL. The negative control was a 0.15 mM DPPH solution (Shawky and sallam, 2017). The absorbance was measured using a UV-Visible spectrophotometer (Pharmaspec UV 1700 SHIMADZU) equipped with a 1 cm quartz cuvette at a wavelength of 517 nm over a time range of 0–120 min (Kim et al., 2020). The mixture was incubated in the dark for 30 min at a controlled room temperature (25 °C ± 1 °C).
The absorbance of the remaining DPPH compounds that did not react with the test compounds was calculated as the percentage of free radical scavengers using Equation 1.
Ac is the negative control absorbance, and As is the sample absorbance (Fahad et al., 2021). The antioxidant activity was evaluated using the DPPH radical scavenging assay, and the ES50 (Effective Scavenging 50%) value was calculated as the extract concentration (mg/mL) required to scavenge 50% of DPPH radicals under the experimental conditions. The ES50 concept is analogous to IC50 but emphasizes scavenging efficiency over inhibition. All results are expressed as mg/mL for consistency.
3 Results and discussion
3.1 Bioactive compounds of the soybean food bar
Three hundred and forty-six bioactive compounds were identified from the soybean using the query “glycine max.” The main bioactive compounds in soybean-based food bars are glycitein and 6″-O-malonylglycitin. The use of bioactive compounds with oral bioavailability (OB) and drug-likeness (DL) values facilitates the optimization of product formulations. This parameter is related to absorption and bioavailability in the gastrointestinal tract (Oliveira et al., 2022; Wang et al., 2023; Bekele and Admassu Emire, 2023; Yu et al., 2018). Glycitein and 6″-O-malonylglycitin compounds have OB and DL criteria that meet the requirements with values seen in Table 1. The complete list of soybean bioactive compounds, predicted targets, and enriched pathways is available in Supplementary Material S1 (Supplementary Tables S1–S4).
TABLE 1
| Mol ID | Bioactive | OB% | DL |
|---|---|---|---|
| MOL008400 | Glycitein | 50.48 | 0.24 |
| MOL011691 | 6″-O-malonylglycitin | 30.40 | 0.81 |
Main bioactive of soybean-based food bar.
3.2 Construction of soybean–bioactive–target network
The bioactive relationship in the soybean food bar has a bond with the target, as shown in Figure 1, and information related to the compound is shown in Table 2. Network Pharmacology analysis using the STITCH database obtained ties between Glycitein (MOL008400) and six genes with scores of MMP13 (0.800), JUN (0.700), MAPK1 (0.700), MAPK3 (0.700), SP1 (0.700), and CDK4 (0.700). One gene was linked to 6″-O-malonylglycitin (MOL011691), with an SUB1 score (0.428). Genes linked to bioactives in the STITCH database were associated with oxidative stress, antioxidants, and obesity, according to analyses using GeneCards and STRING (Zhang et al., 2022).
FIGURE 1
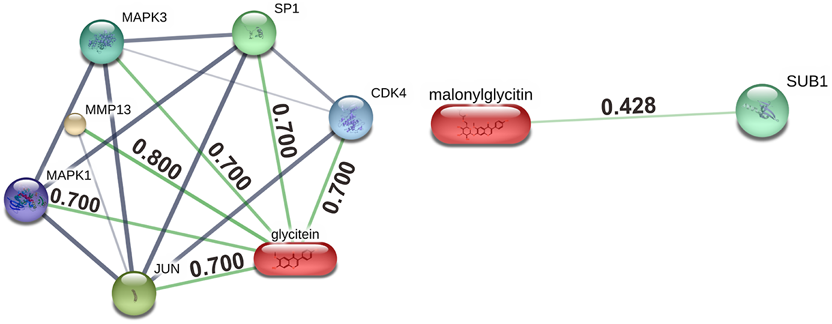
Bioactive-target network of soybean food bars.
TABLE 2
| CAS | Compound | SMILES |
|---|---|---|
| 40,957-83-3 | Glycitein | COC1 = C(C=C2C(=C1)C (=O)C (=CO2)C3 = CC = C(C=C3)O)O |
| 137,705-39-6 | 6″-O-malonylglycitin | COC1 = C(C=C2C(=C1)C (=O)C (=CO2)C3 = CC = C(C=C3)O)OC4C(C(C(C(O4) COC(=O)CC(=O)O)O)O)O |
Basic information on soybean bioactive food bars.
3.3 Prediction results of obesity targets and the construction of the protein-protein interactions (PPI) network
Targets identified from the query “obesity” were 66 target genes analyzed using the STRING database. Gene targets related to obesity are shown in Figure 2A. The core targets that functioned as hub genes were sequenced based on their degree. The degree values of these genes were as follows: MC4R (Ham and Joung, 2021), CEP290 (Ham and Joung, 2021), BBS1 (Kim, 2022), BBS5 (Kim, 2022), LEPR (Kim, 2022), BBS4 (Kim, 2022), INS (Kim, 2022), BBS2 (Kim, 2022), POMC (Kim, 2022), and LEP (Kim, 2022). The PPI network, which was analyzed using the INPUT database, is shown in Figure 2B.
FIGURE 2
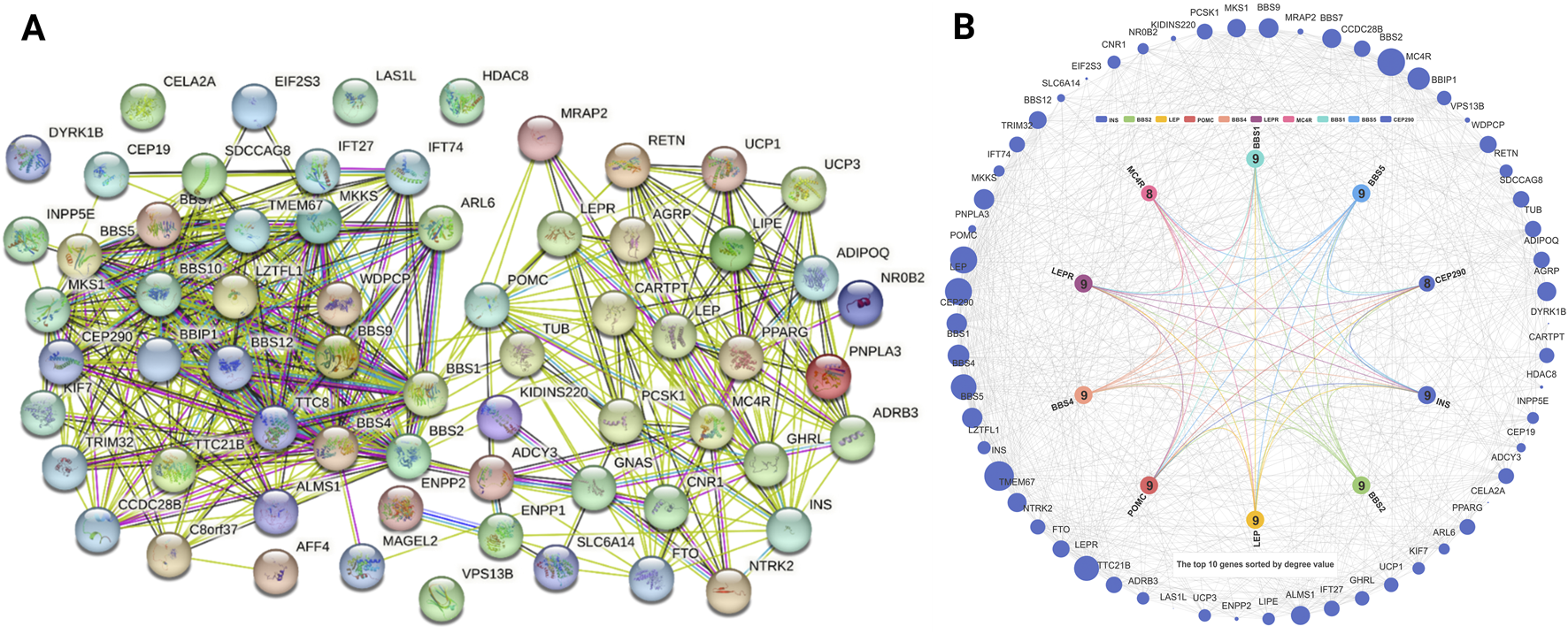
(A) Target genes associated with obesity and (B) The PPI network of 66 target genes related to obesity.
3.4 Molecular docking
The results of molecular docking analysis showed that the bioactive glycitein and 6″-O-malonylglycitin had a stronger affinity than lorcaserin (control), with binding affinity values of −7.1, −6.7, and −5.2 kcal/mol, respectively. The molecular docking results are shown in Figure 3. The binding pocket was defined around the co-crystallized ligand site with grid box dimensions of 37.2360 × 42.9117 × 46.8780 Å, centered at coordinates (X:58.8463 Y: 31.2328 Z: 5.8357). As a positive control, lorcaserin, a clinically approved anti-obesity drug known to interact with leptin pathways, was redocked into the same pocket to validate the docking protocol performance. The root-mean-square deviation (RMSD) between thredockeded and crystallographic ligands was <2.0 Å, confirming docking reliability.
FIGURE 3
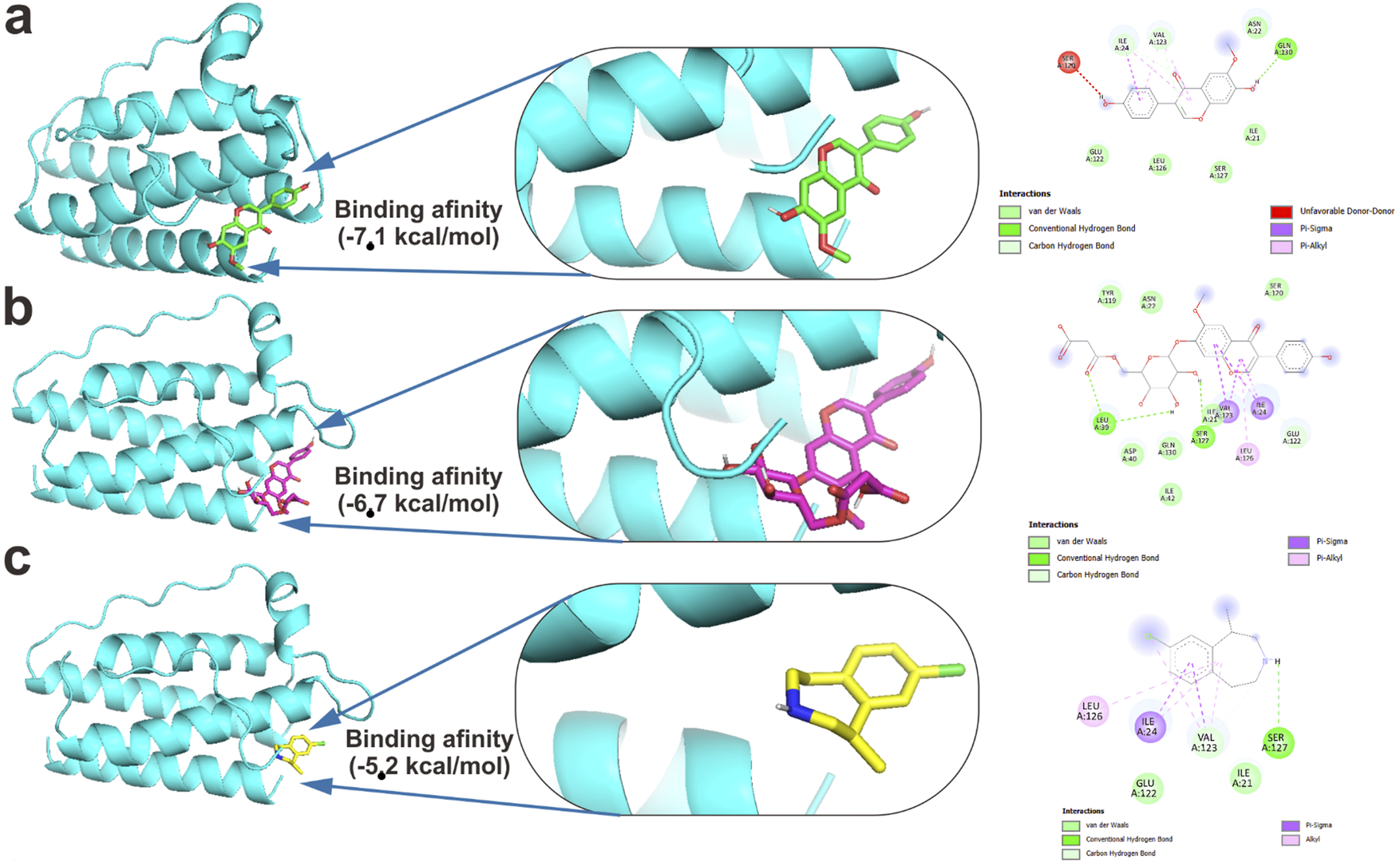
Molecular docking interactions of soybean isoflavones with LEP (PDB ID: 1AX8). (A) Glycitein, (B) 6“-O-malonylglycitin, and (C) Lorcaserin (control) after redocking validation (RMSD < 2.0 Å) showing anti-obesity conformations within the defined pocket.
Network pharmacology and molecular docking analyses suggested possible interactions of daidzein and genistein with leptin signaling proteins, providing a hypothesis for their potential anti-obesity relevance, which requires validation through cellular and in vivo experiments.
3.5 Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) enrichment analysis
The top genes in the GO analysis were analyzed using several parameters by classifying the top 10 significantly enriched terms and the top five pathways-gene networks. The highest aspect of the CC was the basal ciliary body. The highest MF aspect was DNA-binding transcription factor-binding activity. The highest BP aspect was cilium organization. The KEGG enrichment pathway related to obesity targets soybean-based food bars, including the regulation of lipolysis in adipocytes, AMPK signaling pathway, adipocytokine signaling pathway, thermogenesis, and non-alcoholic fatty liver disease. The results of this analysis are shown in Figure 4.
FIGURE 4
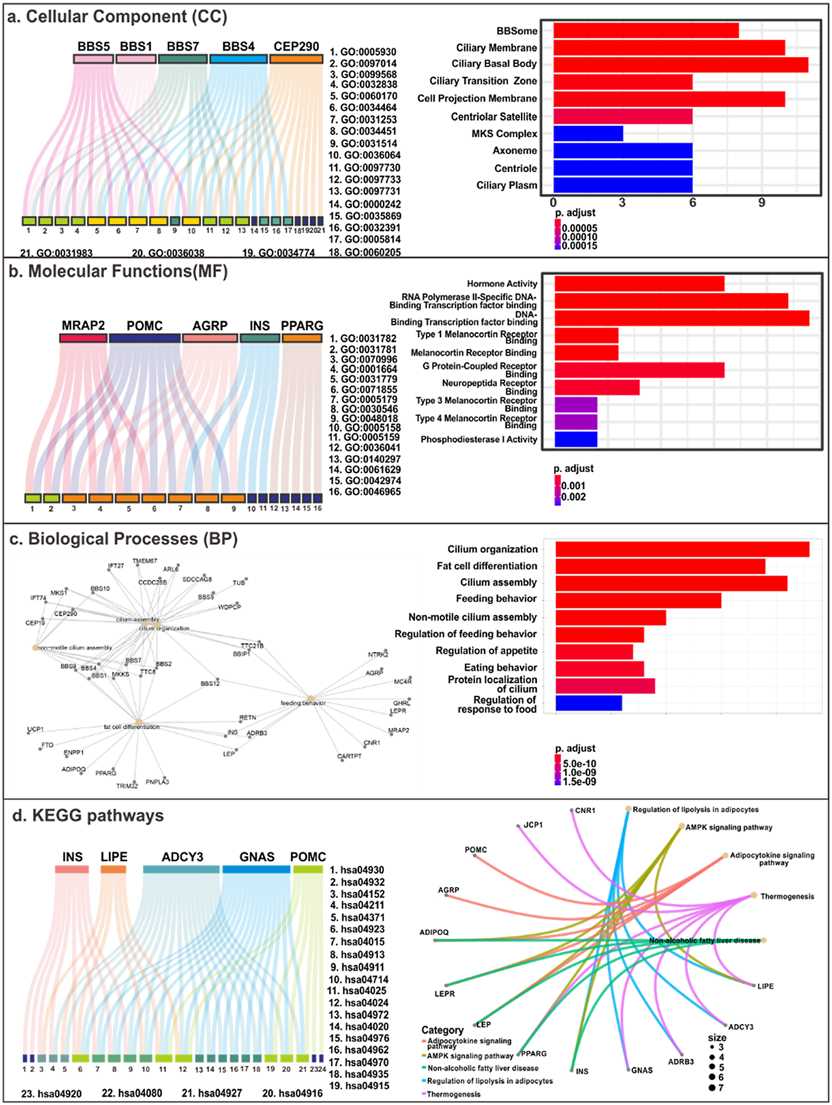
GO and KEGG enrichment analysis.
3.6 Antioxidant activity
The free radical test is often used in the 2,2-diphenyl-1-picrylhydrazyl (DPPH) method because it can provide a profile of the antioxidant capabilities of the tested compounds, providing valid, accurate, sensitive, highly reproducible, easy-to-use, and inexpensive results (Fuso et al., 2023; Lu et al., 2015). DPPH is a dark-purple artificial free radical compound with an unstable nitrogen atom. The DPPH method involves the donation of electron atoms from antioxidant compounds to DPPH compounds, which neutralizes the free radical properties of DPPH compounds, as indicated by a decrease in the intensity of the purple color of DPPH (Fuso et al., 2023). Measurement of antioxidant activity using the DPPH method is based on the fact that the compounds contained in soybeans have more than one OH group, and the OH group in the flavonoid structure is in the B-ring position, thereby increasing the accuracy of the test (Platzer et al., 2021; Liu et al., 2023; Gulcin and Alwasel, 2023).
Ultrasonication with 80% methanol and 70% ethanol prevents secondary metabolite degradation (changes in the structure and composition of isoflavones) and produces a high yield. This method can be used to produce secondary metabolites with diverse biological and pharmacological properties. Isoflavonoids are extracted from soybeans using a 70% ethanol-water mixture and an 80% methanol-water mixture because isoflavonoids are present as aglycones or free forms and mostly as glycosides (Blicharski and Oniszczuk, 2017; Gbedo et al., 2025).
The use of 70% ethanol and 80% methanol was due to the presence of isoflavone compounds in the form of glycosides with hydroxy groups, which are hydrophilic and mostly soluble in hydroalcoholic solvents. Therefore, solvents mixed with water have been used to optimize the extraction process (Kim et al., 2020; Choi et al., 2020). The aim of using two different types of solvents was to compare whether there were differences in the levels of antioxidant activity as scavengers of DPPH free radicals in different solvents, but both types of semi-polar solvents (Gao et al., 2021; Abd Elhamid et al., 2022). Methanol and ethanol can also be used to extract isoflavones. Previous research has found differences in the yield of isoflavones produced, and the two solvents have different capabilities depending on temperature, pressure, flow rate, and sample particle size. This study produced the highest yield of isoflavones in 70% ethanol, based on ES50 data, compared to methanol. The mechanism that explains this difference in ability is the ultrasonication method in the extraction process, which causes increased mass transfer and disruption of the cells. Soybeans are more easily dissolved in 70% ethanol solvent (Blicharski and Oniszczuk, 2017; Fahmi et al., 2014; Mahrous et al., 2025).
The results of the qualitative test of the soybean-based food product samples with the addition of FeCl3 were positive. The color is formed as a result of the reaction of polyphenolic compounds, which have an O2 atom with a lone pair of electrons donated to Fe3+ by an empty orbital to form a polyphenolic Fe3+ complex, which is a green covalent coordination bond (Shawky and Sallam, 2017; Wasihun et al., 2023).
The use of a mobile phase (ethyl acetate, methanol, distilled water, and acetic acid) with polarity variations is optimal for glycosidic isoflavones contained in soybeans because it produces perfect separation in the stationary phase (Puri and Panda, 2015; Patil and Kumar, 2025). The elution results were detected using UV 254 light, and the densitometer is shown in Figure 5. The results of the analysis showed that the sample spots were not very visible; however, when detected with a densitometer, there was a sample analyte in an area parallel to the genistein standard, such that the AUC value of the analyte was so small that it was not visible under UV light 254. This result was attributed to the minimal number of genistein compounds present in the samples. The Rf value of genistein was 0.81, whereas those of the ethanol and methanol extracts were 0.82. The presence of sample analytes in areas parallel to the genistein standard indicated that the ethanol and methanol extracts of food bar products from soybeans contained genistein.
FIGURE 5
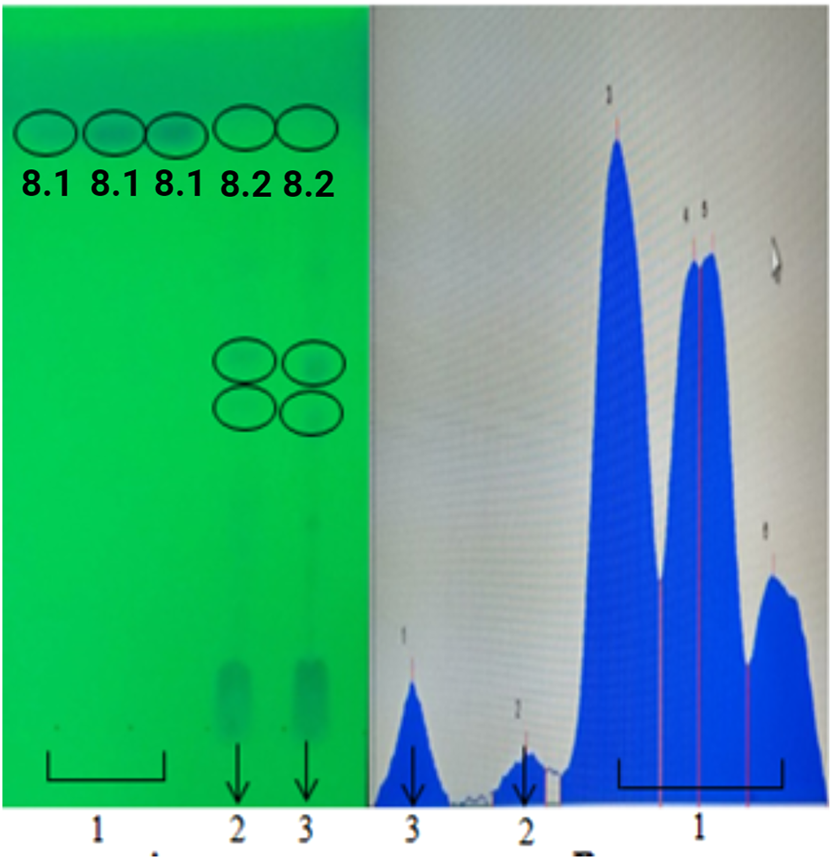
TLC results of qualitative analysis of isoflavones at UV 254 nm (A) and densitometric scan results (B). (1) genistein standard, (2) soybean food bar ethanol extract, and (3) soybean food bar methanol extract..
Antioxidant activity tests were performed at several stages using the DPPH assay. The initial testing stage determined the operating time (OT) for optimal and stable absorbance measurements. The reaction between DPPH and ethanol and methanol extracts of food bar products made from soybeans and genistein was optimal. OT was determined at a wavelength of 517 nm within the time range of 0–120 min (Kim et al., 2020). The results of determining the operating time showed that the genistein absorbance was stable at 30–36 min, ethanol extract of food bar products made from soybean at 31–35 min, and methanol extract of food bar products made from soybean at 34–40 min after the addition of DPPH.
The second stage involved measuring the maximum wavelength to determine the wavelength at which the maximum absorbance occurred. The maximum wavelength measurement used a negative control solution of DPPH, because the principle of the DPPH method is to measure the absorbance of the remaining DPPH solution, which does not react with antioxidant compounds from genistein, ethanol extract, or methanol extract of food bar products made from soybeans. Maximum wavelength measurements were performed in the 400–600 nm range. The measurement results showed that the maximum absorbance wavelength of the 0.15 mM DPPH negative control with genistein and ethanol extracts of food bar products made from soybean was 516 nm, and the maximum wavelength of the methanol extract was 515.60 nm. The wavelength obtained was based on the maximum DPPH wavelength theory, which ranges from to 515–520 nm (Kim et al., 2020).
The maximum absorbance wavelengths of the ethanol and methanol extracts of food bar products made from soybean and genistein without adding 0.15 mM DPPH solution were also measured to ensure that the absorbance measured was the correct absorbance for DPPH compounds. The ethanol and methanol extracts of food bar products made from soybeans and genistein showed maximum absorbance at 262 nm in band I and 343 nm in band II. The maximum wavelength was different from that of DPPH. This indicates that the measured absorbance is the actual absorbance of the residual DPPH, which does not react with the ethanol and methanol extracts of food bar products made from soybeans or genistein. The theory also reinforces this that the maximum absorption of band I isoflavones is in the wavelength range of 245–275 nm, and band II is in the range of 310–330 nm (Kim et al., 2020; Choi et al., 2020; Avior et al., 2013).
Measurement of antioxidant ability using parameters and percentage of free radical scavengers and ES50. The percentage of free radical scavengers was calculated by reducing the absorbance of the negative control to that of the test compound or genistein, which reacted with 0.15 mM DPPH. The ES50 value describes the concentration of the test compound that exhibits 50 free radical scavenging activity. The ES50 value was obtained from the results of the linear regression of the concentration of the test compound with the percentage of free radical scavengers to obtain a linear regression equation, which was then used to enter the value of 50 in the y-parameter to obtain the concentration value (x) as the ES50 value of the test compound and genistein. The free radical scavenging percentages of DPPH and ES50 for the test compounds and genistein are shown in Tables 3–5, respectively.
TABLE 3
| No. | % Free radical scavenging ethanol extract (mg/mL) | Linear regression equations | ES50 (mg/mL) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| 0.25 | 0.50 | 0.75 | 1.00 | 1.25 | 1.50 | 1.75 | |||
| 1 | 21.14 | 28.66 | 35.54 | 43.97 | 50.32 | 54.86 | 59.53 | y = 26.05x + 15.95 | 1.31 |
| 2 | 21.27 | 28.66 | 35.67 | 44.62 | 49.94 | 55.64 | 59.92 | y = 26.31x + 15.93 | 1.29 |
| 3 | 21.79 | 27.63 | 35.93 | 44.63 | 50.45 | 55.12 | 60.83 | y = 26.66x + 15.68 | 1.29 |
| 4 | 22.18 | 28.15 | 36.19 | 44.23 | 49.68 | 55.77 | 59.66 | y = 25.88x + 16.38 | 1.30 |
| 5 | 21.79 | 27.89 | 36.32 | 44.10 | 50.45 | 55.12 | 60.44 | y = 26.36x + 15.94 | 1.29 |
| Average | 1.30 | ||||||||
| SD | 0.07 | ||||||||
| CV | 0.77% | ||||||||
| Percent value of inhibition | Weak | ||||||||
Percentage values of DPPH radical scavengers and ES50 values of the ethanol extract.
TABLE 4
| No. | % Free radical scavenging methanol extract (mg/mL) | Linear regression equations | ES50 (mg/mL) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| 0.5 | 1.0 | 1.5 | 2.0 | 2.5 | 3.0 | 3.5 | |||
| 1 | 19.95 | 25.81 | 47.63 | 56.36 | 62.59 | 66.71 | 71.70 | y = 18.00x + 14.10 | 1.99 |
| 2 | 19.58 | 27.68 | 47.51 | 54.11 | 62.72 | 67.71 | 70.82 | y = 17.78x + 14.44 | 2.00 |
| 3 | 21.32 | 25.69 | 47.01 | 54.11 | 63.47 | 65.96 | 71.45 | y = 17.67x + 14.51 | 2.00 |
| 4 | 19.45 | 26.06 | 47.13 | 55.99 | 62.97 | 66.96 | 71.45 | y = 18.11x + 13.76 | 2.00 |
| 5 | 16.21 | 26.56 | 47.13 | 55.74 | 62.72 | 67.96 | 71.45 | y = 18.86x + 11.95 | 2.01 |
| Average | 2.00 | ||||||||
| SD | 0.007 | ||||||||
| CV | 0.35% | ||||||||
| Percent value of inhibition | Weak | ||||||||
Percentage values of DPPH radical scavengers and ES50 values of the methanol extract.
TABLE 5
| No. | % Free radical scavenging genistein (μg/mL) | Linear regression equations | ES50 (mg/mL) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| 40 | 60 | 80 | 100 | 120 | 140 | 160 | |||
| 1 | 14.22 | 21.34 | 30.30 | 38.41 | 46.80 | 53.34 | 61.31 | y = 0.396x – 1.641 | 0.13041 |
| 2 | 14.37 | 21.48 | 30.01 | 38.69 | 47.23 | 53.49 | 61.45 | y = 0.397x – 1.625 | 0.13004 |
| 3 | 14.08 | 21.62 | 29.87 | 38.55 | 47.08 | 53.49 | 61.59 | y = 0.399x – 1.867 | 0.12999 |
| 4 | 14.22 | 21.62 | 30.30 | 38.41 | 47.08 | 53.34 | 61.17 | y = 0.394x – 1.456 | 0.13060 |
| 5 | 14.51 | 21.76 | 30.16 | 38.26 | 46.94 | 53.63 | 61.31 | y = 0.394x – 1.368 | 0.13038 |
| Average | 0.13028 | ||||||||
| SD | 0.00026 | ||||||||
| CV | 0.2% | ||||||||
| Percent value of inhibition | Moderate | ||||||||
Percent value of DPPH radical scavengers and ES50 values of genistein.
The data in Tables 3–5 show that the higher the concentration of the test compound, the greater the percentage of free radical scavengers, because of the better capture of DPPH free radicals by the test compound and genistein. The average ES50 values of genistein, ethanol extract, and methanol extract of food bar products made from soybeans were 0.13, 1.30, and 2.00 mg/mL, respectively. This value indicates the free radical scavenging activity in the order from largest to smallest, namely, genistein, ethanol extract, and methanol extract, in soy-based food bar products. However, genistein has a moderate antioxidant potential based on its antioxidant strength. Ethanol and methanol extracts of food bar products made from soybeans have very weak potency. These data indicate that the extract had a measurable but relatively weaker scavenging capacity than genistein. Because the extract represents a complex mixture and the values were not normalized to the total phenolic content or active compound concentration, the comparison in this study was interpreted qualitatively rather than quantitatively.
The results of the analysis of the antioxidant activity of soybean-based food bar products as diet companion foods to help overcome obesity can be considered weak because they contain low levels of isoflavones. The genistein positive control antioxidant activity test results showed an ES50 value of 0.13 mg/mL, which means that it has moderate potential as an antioxidant. There is a direct relationship between antioxidant activity and the mechanism by which obesity is overcome. The stronger the antioxidant potential of a compound, the greater its potential to overcome obesity. This is based on an antioxidant mechanism that neutralizes free radicals resulting from lipid metabolism in the form of lipolysis in patients with obesity. The stronger the antioxidant activity, the greater the number of free radicals that can be neutralized (Kim et al., 2020; Khutami et al., 2022; Lee et al., 2022; Nakai et al., 2020). The very low DPPH scavenging activity observed suggests that the antioxidant contribution of the soybean extract is measurable but limited in this study. Such activity may contribute indirectly to metabolic regulation and should be confirmed using complementary assays.
4 Conclusion
The network pharmacology-based soybean food bar contains the main ingredients glycitein and 6″-O-malonylglycitin, which are related to anti-obesity genes. Bioactive glycitein and 6″-O-malonylglycitin have a stronger affinity than lorcaserin for binding to the LEP protein. Soybean-based food bar products have weak antioxidant potential for scavenging DPPH free radicals. These results showed a significant difference in the ES50 values between the two extracts and between each extract and genistein. The current findings suggest that soybean-based food bars may interact with leptin-related signaling pathways, indicating their potential relevance in obesity regulation. Molecular docking and network pharmacology analyses indicated that daidzein and genistein may be associated with leptin signaling and energy metabolism, supporting their predicted biological relevance. These findings are hypothesis-generating and provide a predictive framework for future cellular and in vivo investigations of the anti-obesity potential of soybean isoflavones as functional food ingredients.
5 Limitations and future perspectives
The present study provides predictive insights into the antioxidant and anti-obesity effects of soybean food bars using an integrated network pharmacology and molecular docking approach. However, the current findings are limited by the lack of normalization to the total phenolic content or quantified active compound concentrations within the extract. Future analytical work should determine the total phenolic content (expressed as mg gallic acid equivalent per gram of extract) and quantify the major isoflavones, such as genistein and daidzein, using validated chromatographic techniques. Such normalization will enable a more accurate comparison of antioxidant and biological potency between crude extracts and reference compounds.
Furthermore, as the current predictions are based only on in silico and in vitro chemical assays, these results remain hypothesis-generating. To substantiate the predicted bioactivities, subsequent studies should include cellular-level evaluations and in vivo investigations focusing on leptin-related pathways, adipogenesis regulation, and oxidative stress modulation. Future studies will provide essential mechanistic validation of the anti-obesity potential of soybean isoflavones as functional food bioactives.
Statements
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found below: https://pubchem.ncbi.nlm.nih.gov/, 5317750 https://pubchem.ncbi.nlm.nih.gov/, 172642065 http://www.wwpdb.org/, 1AX8.
Author contributions
TR: Conceptualization, Investigation, Methodology, Software, Validation, Writing – original draft, Writing – review and editing, Visualization. FH: Writing – review and editing, Conceptualization, Data curation, Formal Analysis, Funding acquisition, Methodology, Project administration, Resources, Writing – original draft. FN: Writing – review and editing, Supervision. SA: Writing – review and editing, Investigation. RN: Writing – review and editing, Investigation. AM: Writing – review and editing, Supervision.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Acknowledgments
We sincerely thank the esteemed Tantan Institute for their invaluable contributions to our research. Their expert insights and profound input in Network Pharmacology, Molecular Docking, and Nutrition have been instrumental in enriching the depth and quality of our study.
Conflict of interest
Author AM was employed by Guangzhou HC Pharmaceutical Co., Ltd.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fntpr.2025.1694933/full#supplementary-material
References
1
Abd Elhamid M. A. Mandour A. S. Ismail T. A. Al-Zohairy A. M. Almowallad S. Alqahtani L. S. et al (2022). Powerful antioxidants and cytotoxic activities of the methanol extracts from eight soybean cultivars. Molecules27 (9), 2895. 10.3390/molecules27092895
2
Avior Y. Bomze D. Ramon O. Nahmias Y. (2013). Flavonoids as dietary regulators of nuclear receptor activity. Food Funct.4 (6), 831–844. 10.1039/c3fo60063g
3
Bekele D. W. Admassu Emire S. (2023). Formulation optimization and characterization of functional Kemesha. Heliyon9 (10), e20829. 10.1016/j.heliyon.2023.e20829
4
Blicharski T. Oniszczuk A. (2017). Extraction methods for the isolation of isoflavonoids from plant material. Open Chem.15 (1), 34–45. 10.1515/chem-2017-0005
5
Bohula E. A. Wiviott S. D. McGuire D. K. Inzucchi S. E. Kuder J. Im K. et al (2018). Cardiovascular safety of lorcaserin in overweight or Obese patients. N. Engl. J. Med.379 (12), 1107–1117. 10.1056/nejmoa1808721
6
Bonomini F. (2023). Antioxidants and obesity. Int. J. Mol. Sci.24, 12832. 10.3390/ijms241612832
7
Borges A. José H. Homem V. Simões M. (2020). Comparison of techniques and solvents on the antimicrobial and antioxidant potential of extracts from acacia dealbata and olea europaea. Antibiotics9 (2), 48. 10.3390/antibiotics9020048
8
Chen W. Liu X. Zhang S. Chen S. (2023). Artificial intelligence for drug discovery: resources, methods, and applications. Mol. Ther. Nucleic Acids31, 691–702. 10.1016/j.omtn.2023.02.019
9
Chiang C. M. Wang D. S. Chang T. S. (2016). Improving free radical scavenging activity of soy isoflavone glycosides daidzin and genistin by 3′-hydroxylation using recombinant Escherichia coli. Molecules21 (12), 1723. 10.3390/molecules21121723
10
Choi Y. M. Yoon H. Lee S. Ko H. C. Shin M. J. Lee M. C. et al (2020). Isoflavones, anthocyanins, phenolic content, and antioxidant activities of black soybeans (Glycine max (L.) Merrill) as affected by seed weight. Sci. Rep.10 (1), 19960–13. 10.1038/s41598-020-76985-4
11
Consortium T. U. Martin M. J. Orchard S. Magrane M. Adesina A. Ahmad S. et al (2025). UniProt: the Universal protein knowledgebase in 2025. Nucleic Acids Res.53 (D1), D609–D617. 10.1093/nar/gkae1010
12
Domínguez Díaz L. Fernández-Ruiz V. Cámara M. (2020). An international regulatory review of food health-related claims in functional food products labeling. J. Funct. Foods68 (December 2019), 103896. 10.1016/j.jff.2020.103896
13
Doshi Y. (2025). Functional food market analysis and forecast: 2025-2032.
14
Fahad F. I. Barua N. Shafiqul Islam M. Al Jawad Sayem S. Barua K. Uddin M. J. et al (2021). Investigation of the pharmacological properties of lepidagathis hyalina nees through experimental approaches. Life11 (3), 180–16. 10.3390/life11030180
15
Fahmi R. Khodaiyan F. Pourahmad R. Emam-Djomeh Z. (2014). Effect of ultrasound assisted extraction upon the Genistin and Daidzin contents of resultant soymilk. J. Food Sci. Technol.51 (10), 2857–2861. 10.1007/s13197-012-0744-6
16
Fekete M. Lehoczki A. Kryczyk-Poprawa A. Zábó V. Varga J. T. Bálint M. et al (2025). Functional foods in modern nutrition science: mechanisms, evidence, and public health implications. Nutrients17 (13), 2153. 10.3390/nu17132153
17
Fuso A. Dejonghe W. Cauwenberghs L. Rosso G. Rosso F. Manera I. et al (2023). DPPH radical scavenging activity of xylo-oligosaccharides mixtures of controlled composition: a step forward in understanding structure–activity relationship. J. Funct. Foods101 (November 2022), 105417. 10.1016/j.jff.2023.105417
18
Gao Z. Wang C. Li Z. (2021). Effect of ethanol extract of black soybean coat on physicochemical properties and biological activities of chitosan packaging film. Food Sci. Biotechnol.30 (10), 1369–1381. 10.1007/s10068-021-00968-y
19
Gbedo C. Arnaud E. Servent A. Fontana A. Ollier L. Strub C. (2025). Impact of cooking and Rhizopus oligosporus fermentation on antinutritional factors and isoflavones in soybeans. Food Bioproc Tech.18, 9927–9943. 10.1007/s11947-025-04015-0
20
Gulcin İ. Alwasel S. H. (2023). DPPH radical scavenging assay. Processes11 (8), 2248. 10.3390/pr11082248
21
Ham D. Joung H. (2021). Understanding the associations between dietary antioxidants and obesity. J. Obes. Metab. Syndr.29 (3), 163–165. 10.7570/jomes20070
22
Han B. Luo J. Xu B. (2024). Revealing molecular mechanisms of the bioactive saponins from edible root of Platycodon grandiflorum in combating obesity. Plants13 (8), 1123. 10.3390/plants13081123
23
Hayat J. Akodad M. Moumen A. Baghour M. Skalli A. Ezrari S. et al (2020). Phytochemical screening, polyphenols, flavonoids and tannin content, antioxidant activities and FTIR characterization of Marrubium vulgare L. from 2 different localities of Northeast of Morocco. Heliyon6 (11), e05609. 10.1016/j.heliyon.2020.e05609
24
Intharuksa A. Arunotayanun W. Na Takuathung M. Chaichit S. Prasansuklab A. Chaikhong K. et al (2025). Daidzein and genistein: natural phytoestrogens with potential applications in hormone replacement therapy. Int. J. Mol. Sci.26 (14), 6973. 10.3390/ijms26146973
25
Khutami C. Sumiwi S. A. Khairul Ikram N. K. Muchtaridi M. (2022). The effects of antioxidants from natural products on obesity, dyslipidemia, diabetes and their molecular signaling mechanism. Int. J. Mol. Sci.23 (4), 2056. 10.3390/ijms23042056
26
Kim I. S. (2022). Current perspectives on the beneficial effects of soybean isoflavones and their metabolites on plants. Food Sci. Biotechnol.31 (5), 515–526. 10.1007/s10068-022-01070-7
27
Kim M. Im S. Cho Y. K. Choi C. Son Y. Kwon D. et al (2020). Anti-obesity effects of soybean embryo extract and enzymatically-modified isoquercitrin. Biomolecules10 (10), 1394–14. 10.3390/biom10101394
28
Kim S. Chen J. Cheng T. Gindulyte A. He J. He S. et al (2021). PubChem in 2021: new data content and improved web interfaces. Nucleic Acids Res.49 (D1), D1388–D1395. 10.1093/nar/gkaa971
29
Kondapuram S. K. Sarvagalla S. Coumar M. S. (2021). “Chapter 22 - docking-based virtual screening using PyRx tool: autophagy target Vps34 as a case Study. In: coumar MSBTMD for CADD,” in Molecular docking for computer-aided drug design (Academic Press), 463–477. Available online at: https://www.sciencedirect.com/science/article/pii/B9780128223123000199.
30
Konstantinidi M. Koutelidakis A. E. (2019). Functional foods and bioactive compounds: a review of its possible role on weight management and obesity’s metabolic consequences. Medicines6 (3), 94. 10.3390/medicines6030094
31
Lee H. B. Lee A. Y. Jang Y. Kwon Y. H. (2022). Soy isoflavone ameliorated the alterations in circulating adipokines and microRNAs of mice fed a high-fat diet. Food Funct.13 (23), 12268–12277. 10.1039/d2fo02106d
32
Liao Y. Wang J. Jaehnig E. J. Shi Z. Zhang B. (2019). WebGestalt 2019: gene set analysis toolkit with revamped UIs and APIs. Nucleic Acids Res.47 (W1), W199–W205. 10.1093/nar/gkz401
33
Liu K. Abdullah A. A. Huang M. Nishioka T. Altaf-Ul-Amin M. Kanaya S. (2017). Novel approach to classify plants based on metabolite-content similarity. Biomed. Res. Int.2017, 1–12. 10.1155/2017/5296729
34
Liu W. T. Huang C. L. Liu R. Yang T. C. Lee C. L. Tsao R. et al (2023). Changes in isoflavone profile, antioxidant activity, and phenolic contents in Taiwanese and Canadian soybeans during tempeh processing. LWT15, 186. 10.1016/j.lwt.2023.115207
35
Lu T. M. Ko H. H. Ng L. T. Hsieh Y. P. (2015). Free-radical-scavenging, antityrosinase, and cellular melanogenesis inhibitory activities of synthetic isoflavones. Chem. Biodivers.12 (6), 963–979. 10.1002/cbdv.201400208
36
Mahrous R. S. R. Fattah B. A. Rahman Y. A. Sallam S. M. Shawky E. (2025). Green and efficient extraction of soybean (Glycine max (L.) Merr.) isoflavones using natural deep eutectic solvents-based ultrasound-assisted extraction. Food Bioproc Tech.10.1007/s11947-025-04031-0
37
Messina M. Barnes S. Setchell K. D. (2025). Perspective: Isoflavones—Intriguing molecules but much remains to be learned about these soybean constituents. Adv. Nutr.16 (5), 100418. 10.1016/j.advnut.2025.100418
38
Nakai S. Fujita M. Kamei Y. (2020). Health promotion effects of soy isoflavones. J. Nutr. Sci. Vitaminol. (Tokyo)66 (6), 502–507. 10.3177/jnsv.66.502
39
Ojwach J. Adetunji A. I. Mutanda T. Mukaratirwa S. (2022). Oligosaccharides production from coprophilous fungi: an emerging functional food with potential health-promoting properties. Biotechnol. Rep.33 (November 2021), e00702. 10.1016/j.btre.2022.e00702
40
Oliveira N. Cádiz-Gurrea M. de la L. Silva A. M. Macedo C. Rodrigues F. Costa P. (2022). Development and optimization of a topical formulation with castanea sativa shells extract based on the concept “quality by design.”. Sustain. Switz.14 (1). 10.3390/su14010129
41
Patil P. Kumar P. (2025). Exploring kudzu: extraction, quantification, and health impacts of bioactive compounds. Fitoterapia, 182. 10.1016/j.fitote.2025.106453
42
Patil B. S. Patil J. K. Chaudhari H. S. Patil B. S. (2025). Oxidative stress, inflammation, and obesity: insights into mechanism and therapeutic targets. MDPI AG, 6.
43
Platzer M. Kiese S. Herfellner T. Schweiggert-Weisz U. Miesbauer O. Eisner P. (2021). Common trends and differences in antioxidant activity analysis of phenolic substances using single electron transfer based assays. Molecules26 (5), 1244. 10.3390/molecules26051244
44
Pobłocka-Olech L. Migas P. Krauze-Baranowska M. (2018). TLC determination of some flavanones in the buds of different genus Populus species and hybrids. Acta Pharm.68 (2), 199–210. 10.2478/acph-2018-0018
45
Puri A. Panda B. P. (2015). Simultaneous estimation of glycosidic isoflavones in fermented and unfermented soybeans by TLC-densitometric method. J. Chromatogr. Sci.53 (2), 338–344. 10.1093/chromsci/bmu045
46
Ren S. Meng F. Zeng J. Zhang X. Meng D. Zhang W. et al (2025). Leptin/PPARγ interaction mediates obesity-driven Th17 differentiation in rheumatoid arthritis. Biochimica Biophysica Acta (BBA) - Mol. Basis Dis.1872, 168069. 10.1016/j.bbadis.2025.168069
47
Ru J. Li P. Wang J. Zhou W. Li B. Huang C. et al (2014). TCMSP: a database of systems pharmacology for drug discovery from herbal medicines. J. Cheminform6 (1), 13–16. 10.1186/1758-2946-6-13
48
Saad B. Ghareeb B. Kmail A. (2021). Metabolic and epigenetics action mechanisms of antiobesity medicinal plants and phytochemicals. Evidence-Based Complementary Altern. Med.2021, 1–19. 10.1155/2021/9995903
49
Safran M. Rosen N. Twik M. BarShir R. Stein T. I. Dahary D. et al (2021). The GeneCards suite. Practical guide to life science databases, 27–56.
50
Sandner G. König A. Wallner M. Weghuber J. (2020). Functional foods - dietary or herbal products on obesity: application of selected bioactive compounds to target lipid metabolism. Curr. Opin. Food Sci.34, 9–20. 10.1016/j.cofs.2020.09.011
51
Saxton R. A. Caveney N. A. Moya-Garzon M. D. Householder K. D. Rodriguez G. E. Burdsall K. A. et al (2023). Structural insights into the mechanism of leptin receptor activation. Nat. Commun.14 (1), 1797. 10.1038/s41467-023-37169-6
52
Shawky E. Sallam S. M. (2017). Simultaneous determination of soyasaponins and isoflavones in soy (Glycine max L.) products by HPTLC-densitometry-Multiple detection. J. Chromatogr. Sci.55 (10), 1059–1065. 10.1093/chromsci/bmx062
53
Singh A. K. Singh R. (2020). Efficacy and safety of lorcaserin in obesity: a systematic review and meta-analysis of randomized controlled trials. Expert Rev. Clin. Pharmacol.13 (2), 183–190. 10.1080/17512433.2020.1703109
54
Sohn S. I. Pandian S. Oh Y. J. Kang H. J. Cho W. S. Cho Y. S. (2021). Metabolic engineering of isoflavones: an updated overview. Front. Plant Sci.12 (June), 670103–670117. 10.3389/fpls.2021.670103
55
Szakos D. Ózsvári L. Kasza G. (2022). Health-related nutritional preferences of older adults: a segmentation study for functional food development. J. Funct. Foods92 (March), 105065. 10.1016/j.jff.2022.105065
56
Szklarczyk D. Gable A. L. Nastou K. C. Lyon D. Kirsch R. Pyysalo S. et al (2021). The STRING database in 2021: customizable protein–protein networks, and functional characterization of user-uploaded gene/measurement sets. Nucleic Acids Res.49, D605–D612. 10.1093/nar/gkaa1074
57
Szklarczyk D. Kirsch R. Koutrouli M. Nastou K. Mehryary F. Hachilif R. et al (2023). The STRING database in 2023: protein–protein association networks and functional enrichment analyses for any sequenced genome of interest. Nucleic Acids Res.51 (D1), D638–D646. 10.1093/nar/gkac1000
58
Tao Q. Du J. Li X. Zeng J. Tan B. Xu J. et al (2020). Network pharmacology and molecular docking analysis on molecular targets and mechanisms of Huashi Baidu formula in the treatment of COVID-19. Drug Dev. Ind. Pharm.0 (0), 1345–1353. 10.1080/03639045.2020.1788070
59
Tuccinardi D. Farr O. M. Upadhyay J. Oussaada S. M. Mathew H. Paschou S. A. et al (2019). Lorcaserin treatment decreases body weight and reduces cardiometabolic risk factors in obese adults: a six-month, randomized, placebo-controlled, double-blind clinical trial. Diabetes Obes. Metab.21 (6), 1487–1492. 10.1111/dom.13655
60
Wagner S. Brierley D. I. Leeson-Payne A. Jiang W. Chianese R. Lam B. Y. H. et al (2023). Obesity medication lorcaserin activates brainstem GLP-1 neurons to reduce food intake and augments GLP-1 receptor agonist induced appetite suppression. Mol. Metab.68 (December 2022), 101665. 10.1016/j.molmet.2022.101665
61
Wang S. Chang Y. Liu B. Chen H. Sun B. Zhang N. (2021). Characterization of the key aroma-active compounds in yongchuan douchi (Fermented soybean) by application of the sensomics approach. Molecules26 (10), 3048–14. 10.3390/molecules26103048
62
Wang K. Li K. Chen Y. Wei G. Yu H. Li Y. et al (2021). Computational network pharmacology–based strategy to capture key functional components and decode the mechanism of chai-hu-shu-gan-san in treating depression. Front. Pharmacol.12 (November), 782060–17. 10.3389/fphar.2021.782060
63
Wang S. Cheng Y. Wang J. Ding M. Fan Z. (2023). Antioxidant activity, formulation, optimization and characterization of an oil-in-water nanoemulsion loaded with lingonberry (Vaccinium vitis-idaea L.) leaves polyphenol extract. Foods12 (23), 4256. 10.3390/foods12234256
64
Wasihun Y. Alekaw Habteweld H. Dires Ayenew K. (2023). Antibacterial activity and phytochemical components of leaf extract of Calpurnia aurea. Sci. Rep.13 (1), 9767. 10.1038/s41598-023-36837-3
65
WHO (2022). World health Organization obesity. Available online at: https://www.who.int/health-topics/obesity#tab=tab_1.
66
Younis M. I. Tlay R. H. Altemimi A. B. Ruan Z. Abedelmaksoud T. G. (2025). Synergistic effects of bioactive compounds on human adiposity mechanisms of fat loss and fat accumulation. Food Sci. Nutr.13 (10), e71061. 10.1002/fsn3.71061
67
Yu L. Jin W. Li X. Zhang Y. (2018). Optimization of bioactive ingredient extraction from Chinese herbal medicine glycyrrhiza glabra: a comparative study of three optimization models. Evidence-Based Complementary Altern. Med.2018, 6391414. 10.1155/2018/6391414
68
Zhang X. Wang X. Wang M. Cao J. Xiao J. Wang Q. (2019). Effects of different pretreatments on flavonoids and antioxidant activity of Dryopteris erythrosora leave. PLoS One.14 (1), e0200174–17. 10.1371/journal.pone.0200174
69
Zhang M. Yuan Y. Zhou W. Qin Y. Xu K. Men J. et al (2020). Network pharmacology analysis of chaihu Lizhong Tang treating non-alcoholic fatty liver disease. Comput. Biol. Chem.86 (January), 107248. 10.1016/j.compbiolchem.2020.107248
70
Zhang Z. Zheng Y. Bian X. Wang M. Chou J. Liu H. et al (2022). Identification of key genes and pathways associated with oxidative stress in periodontitis. Oxid. Med. Cell Longev.2022, 9728172. 10.1155/2022/9728172
71
Zhao S. Kanno Y. Li W. (2022). Molecular mechanism of the effect of gegen qinlian decoction on type 2 diabetes mellitus based on network pharmacology and molecular docking. Pharmacol. Res. - Mod. Chin. Med.3 (March), 100107. 10.1016/j.prmcm.2022.100107
72
Zhou Y. Zhang Y. Lian X. Li F. Wang C. Zhu F. et al (2022). Therapeutic target database update 2022: facilitating drug discovery with enriched comparative data of targeted agents. Nucleic Acids Res.50 (1), D1398–D1407. 10.1093/nar/gkab953
Summary
Keywords
computational pharmacy, comorbidity, flavonoids, obesity, oxidative stress, soy, metabolic syndrome, weight management
Citation
Rustandi T, Hayati F, Nugroho F, Amalia SP, Nastasya R and Mahal A (2025) Network pharmacology-based strategy and phytochemical screening of antioxidants suggesting the potential anti-obesity relevance of soybean food bars. Front. Nat. Prod. 4:1694933. doi: 10.3389/fntpr.2025.1694933
Received
29 August 2025
Revised
17 October 2025
Accepted
24 October 2025
Published
17 November 2025
Volume
4 - 2025
Edited by
Usama Ramadan Abdelmohsen, Deraya University, Egypt
Reviewed by
Omnia Abdelhafez, Deraya University, Egypt
Nourhan Hisham Shady, Deraya University, Egypt
Updates
Copyright
© 2025 Rustandi, Hayati, Nugroho, Amalia, Nastasya and Mahal.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Tedi Rustandi, tedirustandi26@gmail.com; Fakhriah Hayati, fakhriahhayati@gmail.com
†These authors have contributed equally to this work and share first authorship
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.