- 1Experimental Psychology, Helmholtz Institute, Utrecht University, Utrecht, Netherlands
- 2Department of Psychiatry, University Medical Center Utrecht, Utrecht, Netherlands
Autism spectrum disorder (ASD) is a heterogeneous neurodevelopmental disorder characterized by social impairments and restricted, repetitive behaviors. Treatment of ASD is notoriously difficult and might benefit from identification of underlying mechanisms that overlap with those disturbed in other developmental disorders, for which treatment options are more obvious. One example of the latter is attention-deficit hyperactivity disorder (ADHD), given the efficacy of especially stimulants in treatment of ADHD. Deficiencies in catecholaminergic systems [dopamine (DA), norepinephrine (NE)] in ADHD are obvious targets for stimulant treatment. Recent findings suggest that dysfunction in catecholaminergic systems may also be a factor in at least a subgroup of ASD. In this review we scrutinize the evidence for catecholaminergic mechanisms underlying ASD symptoms, and also include in this analysis a third classic ascending arousing system, the acetylcholinergic (ACh) network. We complement this with a comprehensive review of DA-, NE-, and ACh-targeted interventions in ASD, and an exploratory search for potential treatment-response predictors (biomarkers) in ASD, genetically or otherwise. Based on this review and analysis we propose that (1) stimulant treatment may be a viable option for an ASD subcategory, possibly defined by genetic subtyping; (2) cerebellar dysfunction is pronounced for a relatively small ADHD subgroup but much more common in ASD and in both cases may point toward NE- or ACh-directed intervention; (3) deficiency of the cortical salience network is sizable in subgroups of both disorders, and biomarkers such as eye blink rate and pupillometric data may predict the efficacy of targeting this underlying deficiency via DA, NE, or ACh in both ASD and ADHD.
Introduction
Autism spectrum disorder (ASD) is a neurodevelopmental disorder characterized by behavioral (including social) deficits (American Psychiatric Association, 2013). In the DSM-5 the two foremost criteria for ASD, often referred to as the core deficits, are social deficits and restricted and repetitive behaviors. In addition to these core deficits, individuals with ASD suffer from a wide range of impairments. Examples of such impairments are deficits in learning (Klinger et al., 2007; Schreibman et al., 2015), executive function (Craig et al., 2016), language (Tager-Flusberg, 2006; Edgar et al., 2015), aggression (Kanne and Mazurek, 2011), hyperactivity (Lecavalier, 2006), sleeping (Malow et al., 2006; Deserno et al., 2019), and sensory processing (Marco et al., 2011). Many view ASD as a very heterogeneous disorder, partly due to the great variability in the presentation as well as severity of symptoms (Masi et al., 2017). While high-functioning groups can – at least in part – effectively participate in society, low-functioning ASD groups show higher severity of symptoms (e.g., no verbal communication) and cannot function independently (Lord et al., 2004; Whitby and Mancil, 2009; Baghdadli et al., 2012; Masi et al., 2017). Due to the great heterogeneity of ASD in addition to the complex nature of social communication and interaction, it is hard to identify effective biological/pharmacological treatments for ASD. To date, there are no effective medications that treat the core deficits of ASD and many treatments tend to focus on other symptoms such as irritability (Coury, 2010).
As to the underlying neurobiological correlates, ASD has been linked to impaired function or structure of prefrontal cortex (PFC; Carper and Courchesne, 2005; Stoner et al., 2014; Zhu et al., 2015), amygdala (Baron-Cohen et al., 2000), cerebellum (Becker and Stoodley, 2013; Hampson and Blatt, 2015), insula (Uddin and Menon, 2009; Kosaka et al., 2010), basal ganglia (Nayate et al., 2005), as well as numerous other brain regions (Stoner et al., 2014). Others have linked the dysfunction of broader systems or networks to ASD. Examples are the broken mirror neuron hypothesis and the dysfunction of the salience network (SaN; Ramachandran and Oberman, 2006; Uddin and Menon, 2009). However, neither of these hypotheses have been able to consistently account for the wide range of underlying neurobiological correlates of ASD [i.e., see Southgate and Hamilton (2008) for a criticism on the broken mirror neuron hypothesis; Müller, 2007]. Thus, the focus on only one or a group of neural structures has not led to a unifying hypothesis of ASD, as no consistent pathology has emerged for the disorder – no genetic or neurobiological factors are very consistently present in ASD, nor are they specific when compared to other psychiatric disorders (Müller, 2007; Amaral et al., 2008; de la Torre-Ubieta et al., 2016). Müller (2007) suggests that ASD should be viewed as a distributed disorder in which most — if not all — brain networks are affected. Therefore, examining connectivity between and within brain regions and neural networks could yield a more comprehensive picture of the neurobiological deficits underlying ASD.
Connectivity within well-defined networks in relation to ASD has been an area of great interest (e.g., Mizuno et al., 2006; Kleinhans et al., 2008; Monk et al., 2009; Anderson et al., 2011; Wass, 2011; Nair et al., 2013; Uddin et al., 2013; Kana et al., 2014; Nomi and Uddin, 2015; Duan et al., 2017). Sridharan et al. (2008) suggest that the SaN is responsible for switching between the activation of the default-mode network (DMN) and executive control networks (ECN; Seeley et al., 2007; Menon and Uddin, 2010). Whereas the DMN is often regarded as the resting-state network that activates when no task is being performed, the ECN activates during the performance of tasks (Seeley et al., 2007; Sridharan et al., 2008; Toro et al., 2008; Raichle, 2015). Some authors suggest that the anterior insula, an important structure in the SaN, is aberrantly connected in ASD (Uddin and Menon, 2009; Ebisch et al., 2011; Guo et al., 2019). Similar ideas have been proposed for the DMN and ECN (Murdaugh et al., 2012; Moseley et al., 2015; Farrant and Uddin, 2016). Nevertheless, results from over- and under-connectivity investigations are — likely in part due to methodological problems and the heterogeneity of ASD cohorts — inconsistent and reproducibility has been limited (Müller et al., 2011).
Understanding neural connectivity is an important first step in identifying how the brain effectuates important functions. However, a connection diagram of the brain should indeed be seen as a first step, and not as the final answer to comprehending how nervous systems realize function. One potential complication, as evidenced in animal studies, is that connectivity is dynamic and can change over time (Brezina, 2010; Marder, 2012; Bargmann and Marder, 2013). Such dynamic changes over time occur across several years in interaction with the environment (Lawrence et al., 2019), but also within a matter of (milli)seconds (Marder, 2012). Almost all current human ASD studies have assessed connectivity as being static within a single functional magnetic resonance imaging (fMRI) scanning session, while such connections are likely dynamic (Brezina, 2010; Marder, 2012; Falahpour et al., 2016). One study did consider these dynamic changes when studying individuals with ASD. Using fMRI, Falahpour et al. (2016) compared conventional ‘static’ scanning procedures with ‘dynamic’ scanning procedures that take into account physiological change during the scanning period (Allen et al., 2014). Using the static procedure, previous findings were replicated: the ASD group showed aberrant connectivity in the DMN. In contrast, the dynamic scanning procedure showed that the ASD group not differ from control subjects in peak connectivity but did show greater intraindividual variability of functional connectivity during the scanning period. Thus, connections in ASD are not ‘broken’ but show higher levels of intraindividual variability across time (Falahpour et al., 2016; London, 2018). Variability of connectivity, as opposed to under- or over-connectivity, may contribute to the pathology of ASD. It is therefore important to consider what drives the variability of connectivity. One obvious perspective is the role of the classic ascending modulating neurotransmitter systems, or neuromodulatory systems.
Neuromodulation is the altering of activity within a neural network by electrical, mechanical, or chemical means (Krames et al., 2009).1 Nervous systems have a wide range of such neuromodulatory systems (Brezina, 2010; Marder, 2012). This review focuses on the three classic neuromodulatory systems: NE from the locus coeruleus (LC), DA from the ventral tegmental area (VTA) and the substantia nigra pars compacta (SNc), and acetylcholine (ACh) from the nucleus basalis of Meynert (NBM; Andeìn et al., 1966; Foote and Morrison, 1987; McCormick, 1989, 1992; Mesulam, 2000; Aston-Jones and Cohen, 2005a; Stahl, 2008). These ascending projections have widespread potent and sustained–or more transient (Aston-Jones and Cohen, 2005b; e.g., Aston-Jones and Cohen, 2005a; Schutte et al., 2020)–modulatory effects on neurotransmission in the forebrain (Andeìn et al., 1966; Foote and Morrison, 1987; McCormick, 1989, 1992; Robbins, 2000; Stahl, 2008; Marder, 2012). In addition, focusing on these three systems in relation to ASD also provides a window on potential underlying mechanisms that overlap with those disturbed in other developmental disorders, for which treatment options are more obvious. The prime example is attention-deficit hyperactivity disorder (ADHD), given the efficacy of especially stimulants in treatment of ADHD (Wilens, 2008; Huss et al., 2017).
Indeed, neuromodulatory neurotransmitters have been of particular interest in relation to attention-deficit hyperactivity disorder (ADHD; Robbins, 2000; Robbins and Arnsten, 2009; del Campo et al., 2011). ADHD is a neurodevelopmental disorder characterized by hyperactivity, impulsivity and inattention (American Psychiatric Association, 2013). Although ASD and ADHD differ (i.e., age of onset and typical introverted vs. extraverted profiles), the disorders do share many similarities. For example, ASD and ADHD show overlap in genetic risk factors (Ronald et al., 2008; Niklasson et al., 2009; Geschwind, 2011) and there is a high level of comorbidity between the two disorders (∼41–78% of ASD individuals experience ADHD symptoms; Clark et al., 1999; Simonoff et al., 2008; Murray, 2010; Rommelse et al., 2011; Antshel et al., 2013; van Steensel et al., 2013; Stevens et al., 2016). Both disorders also have a higher prevalence in boys than girls (Barkley, 2006; Bruchmüller et al., 2012; American Psychiatric Association, 2013; Loomes et al., 2017). Additionally, ASD and ADHD groups show impaired social cognition and suffer comparable deficits in executive functioning (Sinzig et al., 2008; Caillies et al., 2014; Mazza et al., 2014; Bora and Pantelis, 2016; Craig et al., 2016). Moreover, accumulating evidence suggests a considerable overlap in neural correlates between the two disorders. For example, ASD and ADHD show comparable diffusion tract results, levels of gyrification and white matter structure (Aoki et al., 2017; Kushki et al., 2019; Gharehgazlou et al., 2022).
Dysfunction of the DA and NE systems has often been suggested as the neurobiological mechanism underlying ADHD (e.g., Robbins and Arnsten, 2009; del Campo et al., 2011; Xing et al., 2016; Faraone, 2018). For example, genes coding important proteins for catecholaminergic neuromodulation have often been implicated in the disorder and current ADHD medication such as methylphenidate (MPH) targets (by blocking reuptake transporters) DA and NE (Stahl, 2013; Huss et al., 2017; Maia et al., 2017; Faraone, 2018; Myer et al., 2018; Faraone and Larsson, 2019). When compared to ASD, similar findings have been reported in regard to connectivity in ADHD: intraindividual variability of connectivity seems to be affected (Wang R. et al., 2015; Wang et al., 2018). More specifically, the DMN seems to show lower intraindividual variability in connectivity, while the rest of the brain shows higher intraindividual variability in connectivity (de Lacy and Calhoun, 2018). In a case study, Salgado et al. (2007) describe a patient with a brainstem lesion who showed ADHD symptoms (e.g., inattention). These symptoms could be ameliorated by MPH (Salgado et al., 2007). This illustrates the important role of the brainstem catecholamines in ADHD symptoms and how ADHD medication can help restore neuromodulation (see Johnston et al., 2014).
If ASD and ADHD are characterized by similar neuromodulatory disturbances, targeting the same neurotransmitter systems as current ADHD medication may prove helpful for the treatment of ASD. To illustrate more generally, such off-label application of stimulants has also proven valuable in other contexts such as subpopulations within major depressive disorder (Candy et al., 2008; Corp et al., 2014). In this review, catecholaminergic and cholinergic neuromodulation will be examined in relation to ASD and ADHD – with a focus on the SaN, cerebellum and genetics (to be further introduced later). These neuromodulatory systems will be described in detail and related to the symptomatology of ASD and ADHD. Subsequently, human trials with pharmacological agents targeting the catecholaminergic and cholinergic systems in ASD will be discussed. This will provide insight into which pharmacological agents could help the treatment of ASD and whether such treatments could be similar to the current pharmacological treatment of ADHD. Furthermore, this approach allows for personalizing treatments based on the underlying (dys)functioning of neuromodulatory systems, which is highly compatible with the now established Research Domain Criteria (RDoC) approach (see Pacheco et al., 2022).
The locus coeruleus-norepinephrine neuromodulatory system
Connectivity of the locus coeruleus
The LC is a bilateral nucleus in the brainstem containing ∼60,000 NE neurons that project throughout almost the entirety of the brain (Andeìn et al., 1966; Mouton et al., 1994; Aston-Jones and Cohen, 2005a; Szabadi, 2013; Aston-Jones and Waterhouse, 2016). Classically, the LC-NE system was thought to influence the level of arousal and was suggested to be important in the modulation of whole brain states (Jouvet, 1969; Berridge and Waterhouse, 2003). The widespread projections of the system are in line with such an idea. However, recent evidence shows that individual neurons in LC have idiosyncratic projections (Chandler et al., 2014; Kebschull et al., 2016). Furthermore, ensembles of LC-NE neurons can signal specific neural sites (reviewed in Totah et al., 2018; Chandler et al., 2019). This challenges the classical idea that the LC-NE system projects homogenously across neural sites and suggests that the system may be more flexible than previously thought.
Research in rodents has shown that ACC and prefrontal areas receive the most abundant LC-NE projections (Schwarz et al., 2015). Uniquely, insular cortex receives input from both LC-NE neurons and prefrontal NE neurons (Robertson et al., 2013). Using viral-genetic tracing methods, Schwarz et al. (2015) were able to elucidate afferent projections to LC. Afferent projections come from many sites including cortical areas and amygdala. Notably, Purkinje cells (PC) in the cerebellum provide a considerable fraction of the input into LC and do not project to any LC-NE output sites (Schwarz et al., 2015). In turn, LC seems to modulate cerebellar associative synaptic plasticity at climbing fiber-PC synapses (Carey and Regehr, 2009). This indicates reciprocal neural actions between LC and cerebellum.
Norepinephrinergic neuromodulation in ASD and ADHD
In an extensive review, London (2018) described how the LC-NE system may be involved in the symptomatology of ASD (also see Mehler and Purpura, 2009). Although mostly based on indirect evidence, LC-NE system dysfunction has the potential to explain many symptoms and impairments reported in ASD and ADHD. Potential aberrant LC-NE system functioning in ASD can explain deficits in learning, attention, language, sensory processing and emotional functioning, but can also explain symptoms linked to the autonomic nervous system and sleeping problems (London, 2018). Individuals with ADHD show all of the impairments listed above, although these impairments differ substantially in severity (Cohen et al., 2000; Bruce et al., 2006; Geurts and Embrechts, 2008; Tonhajzerova et al., 2009; Konofal et al., 2010; Ghanizadeh, 2011; Musser et al., 2011; Craig et al., 2016; Gregory et al., 2017; Chevrier et al., 2019). The impairments mentioned above can be linked to the LC-NE system (London, 2018), which likely functions aberrantly in ADHD (Darcq and Kieffer, 2015; Xing et al., 2016; Faraone, 2018; Faraone and Larsson, 2019). Thus, potential impaired LC-NE system functioning can explain a wide range of symptoms and impairments that are reported in both ASD and ADHD. It is important to establish if, and in what way, the LC-NE system functions differently in ASD and ADHD when compared to the healthy population so that pharmacological targets may be identified.
Molecular genetics
Genetically, some genes have been identified that may influence the development of the LC-NE system, which may be affected in ASD and ADHD. Dopamine β-hydroxylase (DBH) is an enzyme that catalyzes the conversion of DA into NE (Kaufman and Friedman, 1965; Stahl, 2013). Alterations of DBH, the gene coding DBH, can lead to a relative increase in DA levels and a relative decrease in NE levels, which is highly relevant for LC-NE functioning. DBH seems to play a role in ADHD (Wigg et al., 2002; Zhang et al., 2004; Tong et al., 2015; but see Inkster et al., 2004). For example, DBH gene variant rs129882 was found to be associated with ADHD in a large sample (Tong et al., 2015). In ASD, DBH has also been implicated and maternal levels of DBH seem to play a role (Robinson et al., 2001; Jones et al., 2004; Yrigollen et al., 2008; Jwaid et al., 2020). Furthermore, in an ASD sample, DBH was associated with both ASD and ADHD behaviors (Barrie et al., 2018). Another potentially relevant NE-related gene is SLC6A2, which codes for the NE reuptake transporter. Although some studies report associations between SLC6A2 and ADHD (Bobb et al., 2005; Sengupta et al., 2012; Hawi et al., 2013), others report no such association (McEvoy et al., 2002; de Luca et al., 2004). Sengupta et al. (2012) suggest that sex, subtypes of ADHD phenotypes and specific haplotype blocks of the SLC6A2 gene are important factors to consider (see Zhu et al., 2004). Other evidence shows that SLC6A2 is important in identifying whether MPH can be an effective treatment for ADHD. Specifically, two polymorphisms, rs28386840 and rs5569, were found to be associated with decreased MPH efficacy in ADHD (Yang et al., 2004; Myer et al., 2018). The one study examining a relation between SLC6A2 and ASD did not reveal a significant association (Park et al., 2014). Other work has also pointed toward epigenetic factors during early development in combination with LC-NE that may affect ASD symptom onset and severity (Mehler and Purpura, 2009). More specifically, it has been suggested that fever during this early period may restore LC-NE dysfunction, which in turn is thought to lead to decreased severity of ASD symptomatology.
Purkinje cells and cerebellum
As stated, PCs in the cerebellum have substantial projections to the LC (Schwarz et al., 2015). Notably, the lower total number of PCs or decreased PC density, are some of the most replicable neurobiological findings in ASD (∼75% of ASD subjects show dysfunction/decreased number of PCs; Fatemi et al., 2002; Whitney et al., 2009; Passarelli et al., 2013; Hampson and Blatt, 2015). Some evidence indicates that PC numbers are also decreased in ADHD, but more research is necessary (Rout et al., 2012; Passarelli et al., 2013). As Rout et al. (2012) note, decreased PC numbers may only be observed in an ADHD subtype with cerebellar dysfunction as revealed from more global neuroimaging assessment (see Durston et al., 2011). Currently, the genetic underpinnings of PCs in ASD are still beginning to be understood (Hampson and Blatt, 2015). One study by Rout et al. (2012) reported increased serum levels of antibodies against glutamic acid decarboxylase 65 (GAD65) in ASD and ADHD groups when compared to control subjects (GAD65-antibodies were not present in any of the healthy subjects). GAD65 is important in γ-aminobutyric acid (GABA) synthesis. Serum from the ASD and ADHD groups was subsequently applied to mouse cerebellum, where the antibodies reacted with PCs. Application of these antibodies ultimately resulted in PC death (Mitoma et al., 2003; Rout et al., 2012). Increased antibodies against GAD65 may therefore contribute to PC dysfunction in ASD and ADHD, but research in larger samples is required. Aberrant afferent PC projections in ASD and perhaps ADHD to LC may lead to LC-NE dysfunction. However, the reverse is also conceivable. As stated, the LC modulates cerebellar associative synaptic plasticity, which influences PC firing (Carey and Regehr, 2009). Therefore, anomalous LC-NE functioning can lead to disturbed PC activity. The last option is that the reciprocal projections between LC and cerebellum in both directions are anomalous, which would also indicate LC-NE and PC/cerebellar dysfunction.
Functional networks
Among its widespread projections, LC innervates two important nodes of the SaN: ACC and insular cortex (Seeley et al., 2007; Robertson et al., 2013; Schwarz et al., 2015). Neuromodulation may play an important role in SaN regulation since this network shows a high level of dynamic changes over time when compared to other networks (Chen et al., 2016). Dysfunction of SaN has been suggested to underlie ASD and has been implicated in ADHD (Uddin and Menon, 2009; Aboitiz et al., 2014; Sidlauskaite et al., 2016). Interestingly, when compared to ECN, DMN and SaN, similar functions have been proposed for the LC-NE system (Aston-Jones and Cohen, 2005a,b). According to Adaptive Gain Theory, the LC has two modes. A phasic mode in which phasic activity is relatively high and tonic activity is moderate and a tonic mode which shows the reverse pattern. When in phasic mode, metabolic resources are used to process task-relevant stimuli, and this improves current task performance. In contrast, when in tonic mode, metabolic resources are no longer used to focus on the task and is linked to distractibility. Importantly, in tonic mode, resources are used to identify salient stimuli in the environment (Aston-Jones and Cohen, 2005a,b; Aston-Jones and Waterhouse, 2016). Whereas ECN and LC’s phasic mode are important during task performance, DMN and SaN can be linked to LC’s tonic mode. Furthermore, Zerbi et al. (2019) reported altered levels of intrinsic connectivity within the DMN and SaN in rodents after LC activation. These data further implicate the role of the LC-NE system in network modulatory effects.
Altogether, accumulating evidence from genetic, network and cellular studies is emerging for aberrant functioning of the LC-NE system in ASD and ADHD. LC-NE dysfunction may explain numerous symptoms observed in both disorders and NE could therefore be a potential pharmacological target for ameliorating such symptoms. However, note that some of the presented evidence is somewhat indirect and more direct evidence is needed to precisely identify in what way the LC-NE system functions aberrantly in ASD and ADHD. One possibility is that mutual connections between LC and cerebellar PCs are instrumental in this respect, pointing to a possible efficacy of NE-directed treatment in a majority of ASD, and perhaps a minority of the ADHD population.
The dopaminergic neuromodulatory system
Connectivity of VTA and SNc
The DArgic neuromodulatory system has two important DArgic nuclei in the brainstem: the SNc and VTA (Mesulam, 2000; Beier et al., 2015). It is important to consider the differences between these two nuclei. Firstly, the SNc and VTA have different efferent and afferent projections (Watabe-Uchida et al., 2012). The SNc mainly innervates dorsal striatum (DS; Lerner et al., 2015; Morales and Margolis, 2017). Moreover, evidence shows that the medial SNc and lateral SNc have independent efferent pathways to medial and lateral DS, respectively (Lerner et al., 2015). In turn, lateral DS was found to project to SNc (Watabe-Uchida et al., 2012; Lerner et al., 2015). VTA was found to project to nucleus accumbens (NAc), PFC and amygdala (di Michele et al., 2005; Chandler et al., 2013; Beier et al., 2015, 2019; Morales and Margolis, 2017). Afferent projections to VTA come from many areas including NAc, amygdala and ventral pallidum (Watabe-Uchida et al., 2012; Beier et al., 2015, 2019).
Dopaminergic neuromodulation in ASD and ADHD
Recently, the DA system has also been implicated in ASD. Pavãl (2017) posits that two DArgic pathways play an important role in the core deficits of ASD: the mesocorticolimbic (MCL) pathway and the nigrostriatal (NS) pathway. VTA projections to PFC and NAc make up the MCL-pathway. This pathway is important in reward processing and shows hypoactivation in ASD (Dichter et al., 2012b; reviewed in Pellissier et al., 2018). In ASD, the value of social rewards is thought to be greatly reduced, which results in a lack of social motivation (Dawson et al., 2005; Chevallier et al., 2012). Social motivation theory suggests that a lack of social motivation is the underlying cause of many social impairments in ASD (Chevallier et al., 2012; Dichter et al., 2012a). Thus, aberrant MCL-pathway functioning is thought to underlie the social impairments of ASD (Pavãl, 2017). However, note that others posit that dysfunction of the Social Brain Network (i.e., inferior frontal gyrus, amygdala and fusiform face area) can explain impaired social motivation (Misra, 2014), which would imply that social motivation can be impaired without MCL-pathway dysfunction. The NS-pathway consists of SNc projections to DS. This pathway plays an important role in motor aspects of goal-directed behavior (Lewis et al., 2007; Howe and Dombeck, 2016). For example, increased sensitivity of striatal and cerebellar DA D2 receptors underlies motor impairments in Parkinson’s disease (Rinne et al., 1990). Both optogenetic and pharmacological stimulation of the DA D1 receptor in the NS-pathway results in repetitive and stereotyped autistic-like behavior in mice (Lee et al., 2018). These data implicate the NS-pathway in the core behavioral deficit of ASD (Horev et al., 2011; Pavãl, 2017; Lee et al., 2018). However, accumulating evidence opposes the functional distinction between the MCL- and NS-pathways (Rossi et al., 2013; Ilango et al., 2014; Haber, 2016). For example, rodents will self-administer stimulation to the SNc, implying that this structure plays a role in reward, although not a part of the MCL-pathway (Ilango et al., 2014). Nonetheless, even if the pathways are not functionally distinct, evidence suggests that DArgic dysfunction may underlie the core symptoms of ASD.
It is generally accepted that dysfunction of the DA system plays a vital role in ADHD pathology (Levy and Swanson, 2001; Faraone et al., 2005; Tripp and Wickens, 2009; Faraone and Mick, 2010; Purper-Ouakil et al., 2011; Faraone, 2018). DA dysfunction in ADHD has been linked to deficits in executive functions and learning (Swanson, 2003; Silvetti et al., 2013) but also to inattention, hyperactivity and impulsivity (Swanson, 2003; Genro et al., 2010). The development of SN and VTA connectivity has been shown to be disturbed in ADHD (Tomasi and Volkow, 2012). Furthermore, dysfunction of the MCL-pathway has been associated with ADHD-behaviors (Stark et al., 2011) and the NS-pathway has been linked to hyperactivity in the disorder (Genro et al., 2010). These data indicate dysfunction of the MCL- and NS-pathways in ASD and ADHD.
Molecular genetics
Irregularities in genes that are involved in the development of the DA system have been identified in ASD and ADHD. SLC6A3 is a gene that codes for the DA reuptake transporter, which has a high density in striatum and NAc (Brooks, 2016; Salatino-Oliveira et al., 2018). This gene has often been implicated in ADHD (Gizer et al., 2009; Faraone et al., 2014; Faraone and Larsson, 2019). For example, a meta-analysis reported a relation between a polymorphism of the SLC6A3 and increased DA transporter presence in striatal areas as determined by positron emission topography (PET; Faraone et al., 2014). This polymorphism has also been linked to ADHD in adults (Franke et al., 2010). With respect to ASD, although some contradictory results regarding SLC6A3 involvement have been reported (e.g., Makkonen et al., 2008), most studies indicate that SLC6A3 is associated with the disorder (Hamilton et al., 2013; Nguyen et al., 2014). Genes coding the different DA receptors in relation to ASD and ADHD have also been investigated. Strong evidence suggests DRD4 and DRD5 are associated with ADHD, while weaker evidence suggests an association between ADHD and receptor genes DRD1 and DRD2 (Gizer et al., 2009). Moreover, multiple studies report DRD3 to have no association with ADHD (Faraone et al., 2005; Genro et al., 2010). In ASD, many DA receptor genes have been implicated. DRD1, DRD2, DRD3, DRD4, and DRD5 have all been associated with the disorder (Hettinger, 2009; Gadow et al., 2010; Taurines et al., 2011; Staal et al., 2012; Nguyen et al., 2014). Furthermore, associations between polymorphisms in DRD3, DRD4 and repetitive behaviors in ASD have been reported (Gadow et al., 2010; Staal et al., 2012). This directly links DA dysfunction to ASD symptomatology. Taurines et al. (2011) compared the mRNA expression of DRD4 and DRD5 between ASD, ADHD and control groups. The data showed lower DRD4-mRNA in ASD and ADHD groups. Moreover, the ASD group showed lower DRD5-mRNA when compared to the ADHD and control groups (Taurines et al., 2011). Additionally, recent work has underlined the potential importance of epigenetic factors in ADHD (Pineda-Cirera et al., 2019). Methylation of multiple genes (ARTN, PIDD1, and C2orf82) were found to be linked to expression of these genes, mainly in cerebellum, subcortical regions and frontal cortex, and this was predictive of ADHD. Importantly, these genes have been implicated in fetal and postnatal neurodevelopment including NAc, pointing toward involvement of epigenetic factors in the dysfunction of the DA system in ADHD (Pineda-Cirera et al., 2019). Altogether, genetic factors seem to contribute to dysfunction of the DA system in both ASD and ADHD. Although identified genetic loci in the DA system are shared between the two disorders, there are also important differences such as the role of DRD3.
Functional networks
As discussed, the SaN has been implicated in ASD and ADHD symptomatology. The SN has been shown to be connected with the SaN in humans using diffusion tensor imaging (Zhang et al., 2017). Additionally, functional connectivity between SN, VTA and SaN nodes has been reported in an fMRI study (Seeley et al., 2007). In an extensive review, the importance of the DA system in SaN functioning has been described (Peters et al., 2016). The authors suggest that cortico-striatal-thalamic loop circuits can regulate SaN functioning (Alexander et al., 1986; Peters et al., 2016; McCutcheon et al., 2019). More specifically, VTA and rostral SN pars reticularis innervate thalamic and striatal areas, which subsequently modulate cortical nodes of the SaN (del Campo et al., 2011; Haber, 2016; Peters et al., 2016). In line with this, deep brain stimulation of NAc and SN was found to boost activity in cortical SaN nodes (Alexander et al., 1986; Peters et al., 2016). In turn, a PET study showed that repetitive transcranial magnetic stimulation of dorsal lateral PFC increased DArgic transmission in caudate nucleus and thalamus (Strafella et al., 2001). This shows that SaN nodes and subcortical areas can reciprocally activate one another (Alexander et al., 1986; Peters et al., 2016). Functionally, the DA system and SaN have been proposed to have similar roles. Contemporary studies suggest that DArgic modulation not only influences reward learning and goal-directed behavior, but also plays a role in directing attention toward salient stimuli in the environment (Horvitz, 2000; Koob and Volkow, 2010; Kroemer et al., 2014; Peters et al., 2016). Together, this illustrates the importance of the DA system in SaN functioning.
In sum, dysfunction of the DA system likely contributes to the symptomatology in ASD and ADHD. Whereas there is strong evidence for the relation between DA dysfunction and ADHD, such evidence is relatively still emerging for ASD. Nevertheless, genetic evidence strongly implicates DA dysfunction in both disorders as DBH, SLC6A3 and DA receptor genes have been implicated in ASD and ADHD. Moreover, the influence of the DA system on the SaN can help explain symptomatology in ASD and ADHD. Importantly, the DA system can be targeted by available pharmacological treatments, which could improve the future treatment of ASD. Further research should focus on elucidating exactly how the DA system is altered in ASD and ADHD, so that specific targets within the system may be identified.
The nucleus basalis-acetylcholine neuromodulatory system
Connectivity of the nucleus basalis
The NBM is an important cholinergic nucleus positioned in the basal forebrain and has widespread projections across the brain (Mesulam et al., 1983; Mesulam and Geula, 1988; McCormick, 1989; Mesulam, 2000; Liu et al., 2015). There are two types of cholinergic receptors: nicotinic (nAChR) and muscarinic receptors (mAChR; Jensen et al., 2005; Stahl, 2013; Fuenzalida et al., 2016). Comparable to the LC-NE system, the cholinergic system has classically been thought to modulate arousal (Szerb, 1967; Phillis, 1968; Aston-Jones and Cohen, 2005a). More recently, this system has been implicated in memory and attentional functions (Mesulam, 2000; Arnold et al., 2002; Liu et al., 2015; Mueller et al., 2017), but also in cognitive flexibility and social communication (Ragozzino et al., 1998; Karvat and Kimchi, 2014; Wang L. et al., 2015; Liu et al., 2018). No viral-tracing genetic studies have been performed to precisely examine the topography of the NBM. Nonetheless, the connections of the NBM have been examined, primarily in animal studies. NBM has widespread cortical projections, which are relatively more ventral when compared to the DA and NE systems (Mesulam et al., 1983; Mesulam and Geula, 1988; Kenemans and Ramsey, 2013; Liu et al., 2015). Furthermore, NBM has been shown to be connected to frontal areas and visual cortex (Nagasaka et al., 2017; Huppeì-Gourgues et al., 2018). Additionally, connections between the NBM and ventral striatal areas have been described (Shu et al., 2019). Also, NBM and amygdala have reciprocal projections to one another (Woolf and Butcher, 1982; Mesulam et al., 1983; Mesulam, 2000; Aitta-aho et al., 2018).
Cholinergic neuromodulation in ASD and ADHD
Cholinergic neuromodulation is also altered in ASD. Cholinergic neurons in the NBM have been shown to be altered in size, number and structure in ASD (Kemper and Bauman, 1998). Moreover, decreased levels of the ACh precursor choline have been reported in ASD (Sokol et al., 2002; Friedman et al., 2006). Furthermore, epibatidine - a marker for α4β2-nAChRs (Jensen et al., 2005) – shows altered binding in frontal, parietal, striatal and cerebellar regions in ASD groups (Perry et al., 2001; Lee et al., 2002; Martin-Ruiz et al., 2004; Ray et al., 2005; Deutsch et al., 2010; Mukaetova-Ladinska et al., 2010; Marotta et al., 2020). Notably, decreased cerebellar binding of α4-nAChRs may lead to PC loss (Lee et al., 2002). Additionally, multiple preclinical ASD mouse models appear to suffer cholinergic deficits (Artoni et al., 2019; Marotta et al., 2020). For example, the BTBR mouse model for ASD is an inbred mouse strain that is characterized by a deletion within the Itpr3 gene (Meyza and Blanchard, 2017). BTBR mice show repetitive behaviors, impairments in social communication and aberrant nicotinic cholinergic neurotransmission (Wang L. et al., 2015). Nicotine administration in BTBR mice ameliorated these characteristic ASD-like behaviors (Wang L. et al., 2015). Another study in BTBR mice reported similar effects when donepezil – an acetylcholinesterase inhibitor - was administered (Karvat and Kimchi, 2014). Although mAChRs have not been investigated extensively in relation to ASD, one study did report a 30% decrease in M1 receptor binding in ASD (Perry et al., 2001; Lee et al., 2002; Mukaetova-Ladinska et al., 2010). More research is necessary to understand potential dysfunction of the muscarinic system in ASD.
The cholinergic system has also been implicated in ADHD. Compared to healthy subjects, there is a larger percentage of smokers among the ADHD population, accompanied with lower percentages of quitting smoking (Pomerleau et al., 1995; Moolchan et al., 2000). This increase of consuming nicotine-containing substances could be a form of self-medication among ADHD individuals (Levin and Rezvani, 2002; but this could also be due to elevated impulsivity in ADHD, see Sousa et al., 2011) – similar ideas have been proposed for schizophrenia (Kumari and Postma, 2005). As discussed briefly, individuals with ADHD show a wide range of impairments such as decreased inhibition, impulsivity and executive functioning. Impaired cholinergic neurotransmission has been suggested to play a role in these cognitive deficits in ADHD (Potter et al., 2006, 2014). For example, one placebo-controlled study showed that nicotine administration improved performance on the Stroop task and also decreased stop-signal reaction time (SSRT) in ADHD subjects (Potter and Newhouse, 2004). Logemann et al. (2014) could not replicate the nicotinic effect on SSRT in healthy subjects, but strong responders to nicotine did show an enhanced stop-P3 electroencephalogram component when nicotine was administered (Kenemans, 2015). The nicotinic system has also been suggested to be involved in impulsivity, further underlining the importance of the cholinergic system in ADHD (Ohmura et al., 2012). Similar to ASD, mAChRs have not yet been thoroughly investigated in relation to ADHD. Nonetheless, mAChRs do appear to have decreased binding capacity in ADHD (Johansson et al., 2013). Together, more research is needed to elucidate the role of mAChRs in ADHD.
Molecular genetics
Based on an analysis of genome-wide association study data, cholinergic receptor genes have been identified as candidate genes in ASD (Lee et al., 2012). Specifically, CHRNA7 [coding the α7-nAChR (Jensen et al., 2005)] has been associated with ASD, as has been CHRFAM7A (Bacchelli et al., 2015) – an exclusively human and highly polymorphic hybrid gene consisting of a duplicated portion of CHRNA7 fused to exons A-E of FAM7A (Gault et al., 1998; Wall et al., 2009; Pinto et al., 2010; Casey et al., 2012; Huguet et al., 2013; Bacchelli et al., 2015; Gillentine and Schaaf, 2015; Gillentine et al., 2017). CHRNA7 has also been implicated in ADHD (Gillentine and Schaaf, 2015; Sinkus et al., 2015; Valbonesi et al., 2015; Gillentine et al., 2017; Baccarin et al., 2020), but not all studies have not replicated this finding (Kent et al., 2001; Faraone et al., 2005; Ross, 2012). CHRFAM7A has also been associated with ADHD (Williams et al., 2012; Baccarin et al., 2020), but replication is required to confirm this association. Another gene, CHRNA4, codes for the α4-nAChR and has been identified as a candidate gene for ASD, but this gene seems to only play a role in specific cases (i.e., combinations with other genes or pathologies; Moessner et al., 2007; Wall et al., 2009; Oikonomakis et al., 2016). Conversely, evidence for the involvement of CHRNA4 in ADHD is strong, as multiple studies have reported an association of ADHD with the gene (Todd et al., 2003; Lee et al., 2008; Wallis et al., 2009; Faraone and Mick, 2010; Mastronardi et al., 2016). Moreover, CHRNA4 has been linked to attentional problems and to SaN functioning (Todd et al., 2003; Sadaghiani et al., 2017).
Functional networks
The cholinergic system may play a role in SaN functioning. Striatum, ACC and insula have the highest density of α4β2-nAChRs in the brain as determined by PET (Gallezot et al., 2005; Picard et al., 2013). As stated, these areas are crucial SaN nodes (Seeley et al., 2007; Peters et al., 2016). Sadaghiani et al. (2017) reported that CHRNA4 polymorphism rs1044396 increased activity in ACC, insula and anterior prefrontal areas. Of note, rs1044396 showed no activity-altering effects in DMN and ECN (Sadaghiani et al., 2017). As mentioned previously, the cholinergic system seems to be involved in inhibiting responses (Logemann et al., 2014; Kenemans, 2015). Nicotine administration improves performance on stopping tasks in ADHD (Potter and Newhouse, 2004; Potter et al., 2006). Interestingly, Peters et al. (2016) reported that stopping and SaN show a remarkable overlap in activated brain areas. Kenemans and Ramsey (2013) posit that the cholinergic system is involved in salience detection (Furey et al., 2008). For example, when physostigmine, an acetylcholinesterase inhibitor, is administered, visual cortex showed enhanced activity to the first (and novel) trial, but not to subsequent trials (Furey et al., 2000). Furthermore, amygdala has also been suggested to be involved in salience detection (Sander et al., 2003; Kenemans and Ramsey, 2013). Amygdala is innervated by both the NBM as well as non-NBM cholinergic nuclei in the brainstem (Aitta-aho et al., 2018). In turn, amygdala innervates multiple neuromodulatory nuclei such as NBM, LC, VTA and SNc (Cardinal et al., 2002; Watabe-Uchida et al., 2012; Beier et al., 2015, 2019; Schwarz et al., 2015). These connections of the amygdala allow this structure to modulate activity of multiple neuromodulatory nuclei which modulate SaN functioning.
In sum, cholinergic neurotransmission is affected by genetic and molecular factors in both ASD and ADHD. The direct genetic and molecular influences of nAChRs within the SaN as well as the indirect influences via the amygdala make the cholinergic system crucial for proper SaN functioning. Therefore, dysfunction of the cholinergic system may underlie SaN dysfunction in ASD and ADHD. Dysfunction in salience detection can explain a myriad of symptoms in these disorders and normalizing this system may be a viable target for pharmacological interventions.
Pharmacological interventions in ASD
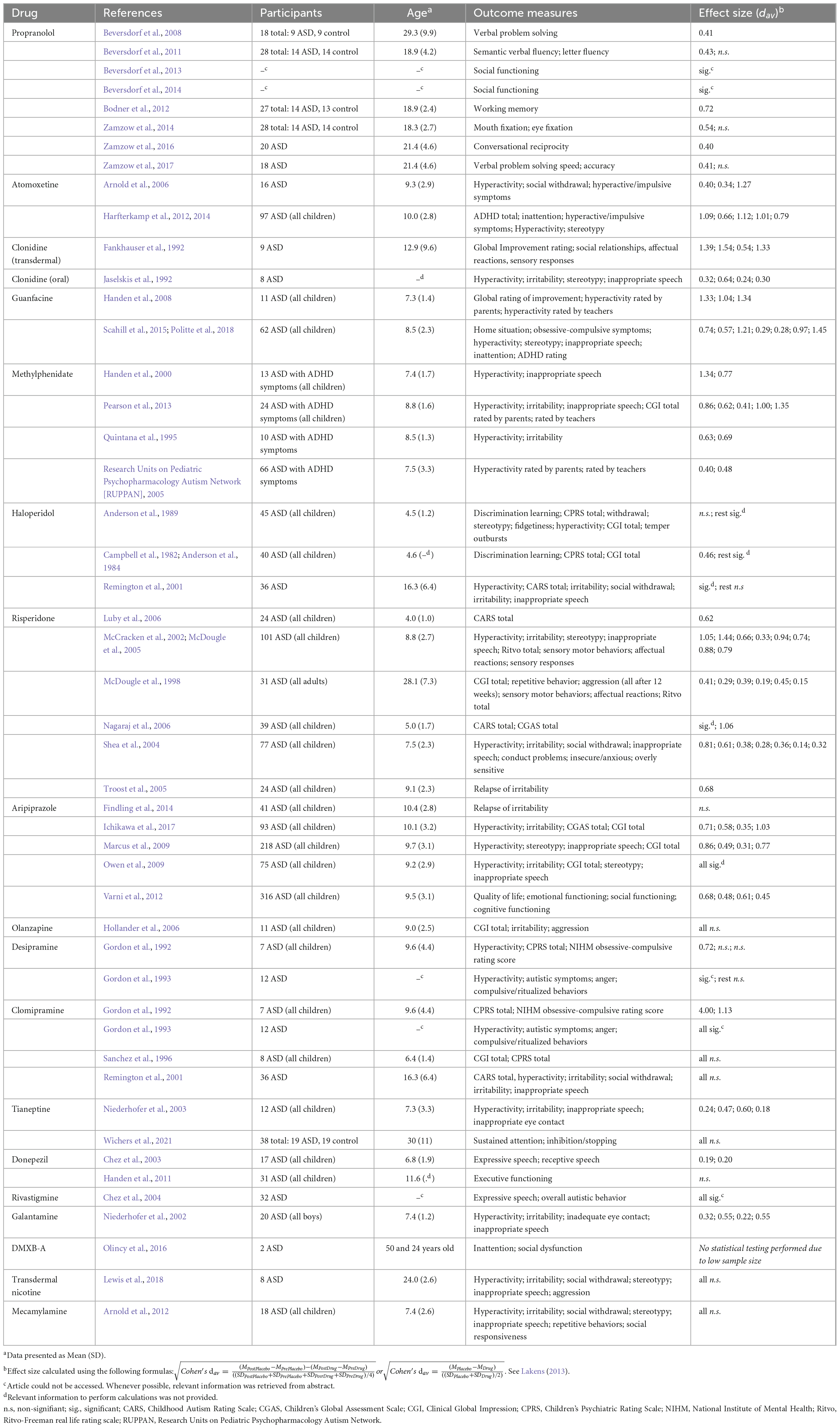
As reviewed, the catecholaminergic and cholinergic systems play a role in ASD and ADHD symptomatology. Pharmacological interventions can target these systems which may help alleviate symptoms (Stahl, 2013). Currently, the atypical antipsychotics risperidone and aripiprazole are the only FDA approved medications for ASD and these drugs are generally prescribed to treat irritability (Bonnot and Holzer, 2012). In practice however, other off-label medications such as stimulants are prescribed to treat ASD (Murray et al., 2014). It is important to consider how prescribed medications affect ASD symptomatology and if these medications are tolerable within the ASD population. Table 1 shows all current randomized and placebo-controlled pharmacological trials in human ASD subjects. Only drugs targeting the catecholaminergic and cholinergic–and sometimes serotonergic–systems are considered.
Norepinephrinergic interventions in ASD
Propranolol
Propranolol blocks NE β1 and β2 receptors in both the central and autonomic nervous systems (Mansur et al., 1998; Meyer and Quenzer, 2013). Multiple placebo-controlled studies have reported improvements in ASD, including normalization of facial scanning, enhanced (non-)verbal communication and improved social functioning (Beversdorf et al., 2013, 2014; Zamzow et al., 2014, 2016, 2017). Notably, one study showed that the effect of propranolol on verbal problem solving speed was mediated by heart rate variability and baseline anxiety measures (Zamzow et al., 2017). London et al. (2020) reported propranolol to lower aggressive and self-injurious behaviors, but the study was not placebo controlled. Additionally, while in some studies anxiety was unaffected, others reported a decrease in anxiety (Sagar-Ouriaghli et al., 2018). Furthermore, in one study propranolol administration boosted functional connectivity in the DMN in both control and ASD groups, while another study reports increased functional connectivity in language areas in the left hemisphere in ASD (Narayanan et al., 2010; Hegarty et al., 2017). Intraindividual variability of functional connectivity was not assessed in these studies. Although studies have investigated the effects of propranolol on specific symptoms of ASD (i.e., facial scanning and language), the core deficits (i.e., social impairments and repetitive and restricted behavior) have not often been assessed (but see Beversdorf et al., 2013; Beversdorf et al., 2014). Additionally, most propranolol trials have small sample sizes and do not directly assess outcome measures that are clinically relevant, such as irritability, ADHD symptomatology and stereotypical behavior. Since most of these studies were not full clinical trials but instead single dose pharmacological studies (Beversdorf et al., 2013, 2014; Zamzow et al., 2014, 2016, 2017), it is necessary to conduct double-blind placebo controlled trials to properly assess the possible effects of propranolol on the core deficits of ASD and to assess propranolol’s potential effects in clinical treatments.
Atomoxetine
Atomoxetine is a selective norepinephrine reuptake inhibitor, effectively enhancing NErgic transmission (Ettinger, 2011). Multiple studies have investigated whether atomoxetine can ameliorate ADHD symptoms in ASD groups. All currently reviewed studies report beneficial effects of atomoxetine on hyperactivity, inattention and impulsive behavior (Arnold et al., 2006; Harfterkamp et al., 2012, 2013, 2014). One limitation of the current ASD atomoxetine trials is that most studies have investigated the effect of atomoxetine on ADHD symptoms in ASD, but ASD symptoms have often not been assessed (but see Harfterkamp et al., 2014). However, in a non-placebo controlled trial, Jou et al. (2005) report an improvement of general behavior (e.g., less deviant) after atomoxetine treatment when compared to before starting the treatment. Additionally, one trial reported that atomoxetine can decrease stereotypical behaviors in ASD (Harfterkamp et al., 2014). In conclusion, although atomoxetine seems to alleviate ADHD symptoms in ASD, it remains unclear whether atomoxetine can aid in the treatment of ASD symptomatology.
Clonidine and guanfacine
Clonidine and guanfacine are α2 receptor agonists and operate mainly presynaptically (on autoreceptors; Meyer and Quenzer, 2013). Two double-blind placebo-controlled trials have assessed the potential therapeutic effects of clonidine – a net NE antagonist – in ASD. One trial showed that oral clonidine may help treat hyperactivity, irritability, stereotypy and inappropriate speech (Jaselskis et al., 1992). Another study, using transdermal clonidine, administration reported beneficial effects on global improvement, social relationships, affectual reactions and sensory responses (Fankhauser et al., 1992). These trials seem promising for clonidine as a treatment option, but they are limited by their small sample sizes, as well as the side effect profile of the drug (i.e., irritability and sedation).
Lastly, α2 receptor agonist guanfacine has recently been investigated in ASD, in part due to the recent interest in extended-release guanfacine and ADHD trials investigating the drug. Guanfacine seems to have a myriad of positive effects in ASD. The two existing trials report beneficial effects on hyperactivity, stereotypy, inappropriate speech, inattention, ADHD-behaviors, global ratings of improvement, compulsive behaviors as well as an improved home situation (Handen et al., 2008; Scahill et al., 2015; Politte et al., 2018). Adverse effects were limited (i.e., drowsiness) and tolerability was generally high (Scahill et al., 2015; Politte et al., 2018). It is important to note that both clonidine and guanfacine can show net NE agonistic or antagonistic effects based on the dose (Svensson et al., 1975). Future studies - and potentially clinicians – should take this dose-dependent effect into account since this may affect efficacy and tolerability of these substances.
Dopaminergic interventions: Stimulants and (a)typical antipsychotics in ASD
Stimulants
Individuals with ADHD are commonly prescribed stimulants such as MPH or amphetamines (Murray et al., 2014), which both block DA and NE reuptake, along with some other agonistic mechanisms for especially amphetamines. MPH also reportedly lowers ADHD symptoms such as hyperactivity, inattention and impulsivity in ASD (e.g., Quintana et al., 1995; Pearson et al., 2013). Nonetheless, individuals with ASD have a lower chance of responding to MPH and suffer from adverse effects more often than ADHD individuals (Handen et al., 2000; Research Units on Pediatric Psychopharmacology Autism Network [RUPPAN], 2005). McCracken et al. (2014) showed that efficacy and tolerability of MPH in ASD is mediated by genes: DRD1, ADRA2, COMT, DRD3, DRD4, SLC6A3, SLC6A4, DRD2, and DRD3. Similar genes mediate the efficacy of MPH in ADHD, as SLC6A3, DRD4, and COMT have been implicated (Myer et al., 2018). Although MPH increased social skills and the clinical global impression in ASD in some studies, a meta-analysis showed that MPH does not significantly affect the core deficits of ASD (Sturman et al., 2017). However, this could be due to the low number of studies assessing the core deficits or genetic mediation of efficacy and tolerability of MPH (McCracken et al., 2014).
Antipsychotics
Haloperidol is a typical antipsychotic that mainly antagonizes DArgic neurotransmission by blocking the DA D2 receptor. Administration of haloperidol was found to decrease hyperactivity in two reports (Anderson et al., 1989; Remington et al., 2001). Furthermore, compared to placebo haloperidol beneficially impacted global impression and children’s psychiatry rating scale scores in two studies (Campbell et al., 1982; Anderson et al., 1984; Remington et al., 2001). Inconsistent results are found when examining discrimination learning. One trial reports a significant, albeit modest, increase in discrimination learning (Campbell et al., 1982; Anderson et al., 1984), while another trial reports no such effects (Anderson et al., 1984). Although, in one study haloperidol decreased ASD symptoms such as withdrawal and stereotypy (Anderson et al., 1989), these effects were not replicated in a later trial (Remington et al., 2001).
The atypical antipsychotics risperidone and aripiprazole seem to share therapeutic effects in ASD, along with partial overlap in their pharmacodynamic profiles (5-HT2A antagonism for both, but partial D2 agonism for aripiprazole versus D2 (as well as α1) antagonism for risperidone). Both drugs decrease hyperactivity and irritability and both drugs seem to benefit the clinical global impression (Aman et al., 2010; Cohen et al., 2013; Table 1). Additionally, the drugs have overlapping side effects such as weight change, altered appetite, drowsiness and sedation. A trial comparing risperidone to aripiprazole in ASD revealed that the efficacy and tolerability are comparable (Ghanizadeh et al., 2014). Nonetheless, differences between the effects of risperidone and aripiprazole are also reported. While risperidone has been found to reduce aggression and autistic behaviors (McDougle et al., 1998; Luby et al., 2006; Nagaraj et al., 2006), aripiprazole improved quality of life (Varni et al., 2012). Although these atypical antipsychotics may reduce overall autistic behaviors in some studies, adverse effects are often problematic and these medications are not tolerable in many individuals (Orsolini et al., 2016). Genetic studies may help identify genes that mediate efficacy and tolerability of atypical antipsychotics in the treatment of ASD. Note that one trial investigating another atypical antipsychotic, olanzapine, showed no significant therapeutic effects (Hollander et al., 2006). Lastly, none of the reviewed studies have assessed the effects of MPH or typical antipsychotics on the DA MCL- and NS- pathways in ASD. Investigating such effects in ASD may provide insight into the potential therapeutic effects of these agents on the core deficits of the disorder (Pavãl, 2017).
Cholinergic interventions and tricyclic antidepressants in ASD
Cholinergic agents
Drugs targeting the cholinergic system in ASD have not been extensively researched. Many of these agents enhance cholinergic transmission by inactivating the acetylcholine-esterase enzyme (donepezil, rivastigmine) or by additional direct agonism of the nicotine receptor (galantamine; Stahl, 2008). The most researched cholinergic drug in relation to the disorder is donepezil. Donepezil reportedly increased both receptive and expressive speech, but did not influence executive functioning or overall autistic behaviors when compared to placebo (Chez et al., 2003; Handen et al., 2011). One placebo-controlled galantamine study has been conducted which reports improvements in hyperactivity, irritability, eye-contact and inappropriate speech (Niederhofer et al., 2002; also see Ghaleiha et al., 2014 where galantamine was added to the existing prescribed medication). Rivastigmine seems to improve expressive speech and overall autistic behaviors, but not enough research has been conducted to properly assess how these agents may benefit future treatment (Chez et al., 2004). A relatively new drug DMXB-A, an α7-nAChR agonist, improved neurocognition in schizophrenia (Olincy et al., 2006). In a sample of only two adults with ASD, the drug decreased inattention and decreased social dysfunction in one of these subjects (Olincy et al., 2016). Transdermal nicotine administration showed no significant effects in a placebo-controlled trial (Lewis et al., 2018; Table 1). The nicotinic antagonist mecamylamine also showed no significant effects in a placebo-controlled trial (Arnold et al., 2012; Table 1). As stated, cholinergic drugs have only been scarcely researched in relation to ASD. Double-blind placebo-controlled studies are necessary to understand how these agents can potentially aid future treatment of ASD.
Tricyclic antidepressants
Tricyclic antidepressants (TCAs) enhance especially NE transmission, but also 5-HT transmission; in both cases this is effectuated through reuptake blocking and thought to underly the antidepressant effect. Of note, they also block muscarinic ACh receptors which accounts for most of their undesired side effects (Kenemans, 2017). TCAs have also been investigated in relation to ASD. Across two trials, desipramine seemed to decrease hyperactivity but compulsive and autistic behaviors remained unaffected (Gordon et al., 1992, 1993). In contrast, the same trials showed that clomipramine did decrease ASD symptoms, compulsive behaviors and also hyperactivity (Gordon et al., 1992, 1993). However, other clomipramine trials did not replicate such effects (Sanchez et al., 1996; Remington et al., 2001). Another TCA, tianeptine, seems to decrease hyperactivity, irritability, inappropriate speech and inappropriate eye contact (Niederhofer et al., 2003). A recent fMRI study showed that tianeptine did not behaviorally affect sustained attention or inhibition/stopping in ASD, but the drug did normalize ECN activation during tasks (Wichers et al., 2021). Overall, although some trials report beneficial effects of TCAs in ASD, most trials show little to no significant therapeutic effects of this class of medications.
Discussion
As reviewed, dysfunction of catecholaminergic and cholinergic neuromodulation play a role in the symptomatology of ASD and ADHD (Faraone et al., 2005; Potter et al., 2006; Faraone and Mick, 2010; Pavãl, 2017; London, 2018). Importantly, the dysfunction of these neuromodulatory systems seem to share similarities - as well as differences - between both disorders at the level of genetics, functional networks and at the cellular level that may provide guidance for the development of biological/pharmacological treatment options.
Accumulating evidence suggests overlapping genetic contributions to potential dysfunction of the catecholaminergic and cholinergic systems in ASD and ADHD (Faraone et al., 2005; Nguyen et al., 2014; Bacchelli et al., 2015; Faraone and Larsson, 2019). However, note that many of the reported studies concerning the genetics of the catecholaminergic and cholinergic systems have limited effects. This in turn may be directly related to the effectiveness of either NE- or DA- or ACh-directed interventions in either population. Therefore, more specific, personalizing biomarkers may be useful in designing intervention strategies especially in relation to ASD. One further clue is that there are also differences in the genetic associations (DRD3, specific for ASD, and SLC6A2, specific for ADHD).
One further difference between ASD and ADHD in the dysfunction of neuromodulatory systems is the role of PCs. PC functioning is affected by especially by NE and ACh neuromodulatory systems, and has been implicated in ASD pathology, but less strongly in ADHD (Rout et al., 2012). Therefore, targeting PCs may be suitable for ASD, and a specific ADHD subgroup (see Durston et al., 2011). Given the role of the NE and ACh systems in PC function, it seems useful to further investigate NE- and ACh-targeting agents as effective treatments in ASD. Trials so far have yielded promising results, especially for the NE-reuptake inhibitor atomoxetine, for α2 agonist guanfacine and for ACh-esterase inhibitors. As said, a similar strategy could be beneficial for a subgroup within the ADHD population. Personalizing or biomarking this subgroup could perhaps be effectuated using specific assessments for cerebellar dysfunction. For ASD, especially drugs targeting the cholinergic system have been under-examined, while accumulating neurobiological evidence suggests that these drugs can potentially help treatment (Lippiello, 2006; Mukaetova-Ladinska et al., 2010). Furthermore the efficacy of propranolol in ASD on verbal problem solving seems to be mediated by heart rate variability and baseline anxiety measures, further underlining the need for personalizing treatments (Zamzow et al., 2017; Beversdorf, 2020). Additionally, future pharmacological trials should consider the core deficits of ASD, as these deficits are currently not often assessed. Future studies should also investigate the neurobiological effects of these agents in ASD patients, as preliminarily done for propranolol and tianeptine (Narayanan et al., 2010; Hegarty et al., 2017; Wichers et al., 2021). For example, the intraindividual variability of connectivity can be assessed to see how pharmacological agents may influence this potential underlying mechanism of ASD pathology (Falahpour et al., 2016).
As for stimulants, MPH is a generally effective medication in ADHD, but only few studies have assessed its effects on core ASD deficits. The efficacy and tolerability of MPH are mediated by genetic factors in both ASD and ADHD groups (McCracken et al., 2014; Myer et al., 2018). As mentioned, while some MPH trials show some improvements in ASD symptomatology, a meta-analysis showed that MPH does not significantly influence the core deficits of ASD (Sturman et al., 2017). However, as supported by the genetic mediation studies (McCracken et al., 2014; Myer et al., 2018), MPH may only be effective in specific ASD subgroups. One clue is the association between MPH effectiveness and varieties of the DRD3 and SLC6A2 genes, in both ASD and ADHD populations. Other biomarkers may also be predictive in ASD. For example, pupillometry measures have been used in ASD to identify dysfunction of the LC-NE and NBM-ACh systems (Lynch, 2018; Artoni et al., 2019; de Vries et al., 2021). In addition, future research could investigate the value of eye blink rate to assess DA functioning in ASD (Jongkees and Colzato, 2016). Alternatively subgroups could be created based on cognitive functioning (i.e., attention or working memory), which has proved useful in identifying and predicting ADHD severity as well as its prognosis (Musser and Raiker, 2019; Pacheco et al., 2022). Pupillometry, eye blink rate and cognitive functioning, in combination with gene studies, may help identify which pharmacological treatment may be most effective for a specific individual.
In line with this personalization of treatments, adopting the RDoC approach may prove useful (Pacheco et al., 2022). Classifying disorders not categorically, but instead on a continuous scale based on traits (or other biomarkers) may prove more useful in investigating and understanding these disorders. Such an approach has already been found to be fruitful in both cognitive as well as imaging studies (Aoki et al., 2017; Kushki et al., 2019; Musser and Raiker, 2019; Gharehgazlou et al., 2022; Pacheco et al., 2022). In these studies the continuous approach could help predict severity of symptoms as well as neural underpinning more effectively than when adopting the classical categorical approach (Aoki et al., 2017; Kushki et al., 2019; Musser and Raiker, 2019; Gharehgazlou et al., 2022; Pacheco et al., 2022). Future research should incorporate this approach by at least including a continuous measure of ASD/ADHD traits.
The current review has some limitations. First, not all potentially important neuromodulatory substances have been examined. Although not considered here, the histaminergic, serotoninergic and opioid systems have been implicated in ASD pathology (Eissa et al., 2018; Pellissier et al., 2018). Also, the current review has examined the neuromodulatory systems as more or less independent, while these systems interact. For example, the dorsal raphe nuclei project to VTA, and VTA projects to the LC (Beier et al., 2015; Morales and Margolis, 2017). Moreover, NE reuptake transporters also clear DA from synapses in frontal areas (Madras et al., 2005). This illustrates how these systems are connected to one another and that the interactions between these systems should be investigated further (Marder, 2012).
Altogether, DA, NE, and ACh are important systems in ASD and ADHD symptomatology. Although the dysfunction of these systems show overlap between the disorders, there are also differences such as PC/cerebellar functioning and the involvement of specific genes (DRD3, SLC6A2). Current medications prescribed for ADHD are effective in ADHD groups, but there is currently not enough evidence to suggest that these medications can consistently and effectively alleviate core symptoms in ASD groups. NErgic agents, MPH, and cholinergic agents should be investigated further using double-blind placebo-controlled trials to confirm the potential therapeutic value of these agents and to assess their neurobiological effects in ASD. Lastly, biomarkers such as pupillometry, eye blink rate, cognitive functioning and genetics should be investigated in relation to ASD to identify which medications may be most effective for a specific individual.
Author contributions
DK wrote the original draft of the manuscript. PD and JK provided the critical comments. DK and JK revised the manuscript. All authors contributed to the article and approved the submitted version.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Footnotes
- ^ Although some contemporary work uses the term neuromodulation in relation to transcranial magnetic stimulation, transcranial direct-current stimulation or implants to modulate neural activity (e.g., Sokhadze et al., 2016; Nobusako et al., 2017; Krames et al., 2018), here we use this term in relation to neurotransmitters in the brain.
References
Aboitiz, F., Ossandón, T., Zamorano, F., Palma, B., and Carrasco, X. (2014). Irrelevant stimulus processing in ADHD: Catecholamine dynamics and attentional networks. Front. Psychol. 5:183. doi: 10.3389/fpsyg.2014.00183
Aitta-aho, T., Hay, Y., Phillips, B., Saksida, L., Bussey, T., Paulsen, O., et al. (2018). Basal forebrain and brainstem cholinergic neurons differentially impact amygdala circuits and learning-related behavior. Curr. Biol. 28, 2557–2569.e4. doi: 10.1016/j.cub.2018.06.064
Alexander, G., DeLong, M., and Strick, P. (1986). Parallel organization of functionally segregated circuits linking basal ganglia and cortex. Annu. Rev. Neurosci. 9, 357–381. doi: 10.1146/annurev.ne.09.030186.002041
Allen, E. A., Damaraju, E., Plis, S. M., Erhardt, E. B., Eichele, T., and Calhoun, V. D. (2014). Tracking whole-brain connectivity dynamics in the resting state. Cereb. Cortex 24, 663–676. doi: 10.1093/cercor/bhs352
Aman, M. G., Kasper, W., Manos, G., Mathew, S., Marcus, R., Owen, R., et al. (2010). Line-item analysis of the aberrant behavior checklist: Results from two studies of aripiprazole in the treatment of irritability associated with autistic disorder. J. Child Adolesc. Psychopharmacol. 20, 415–422. doi: 10.1089/cap.2009.0120
Amaral, D., Schumann, C., and Nordahl, C. (2008). Neuroanatomy of autism. Trends Neurosci. 31, 137–145. doi: 10.1016/j.tins.2007.12.005
American Psychiatric Association (2013). Diagnostic and statistical manual of mental disorders (DSM-5§). Washington, DC: American Psychiatric Publishing, Inc.
Andeìn, N. E., Dahlström, A., Fuxe, K., Larsson, K., Olson, L., and Ungerstedt, U. (1966). Ascending monoamine neurons to the telencephalon and diencephalon. Acta Physiol. Scand. 67, 313–326. doi: 10.1111/j.1748-1716.1966.tb03318.x
Anderson, J. S., Druzgal, T. J., Froehlich, A., DuBray, M. B., Lange, N., Alexander, A. L., et al. (2011). Decreased interhemispheric functional connectivity in autism. Cereb. Cortex 21, 1134–1146. doi: 10.1093/cercor/bhq190
Anderson, L. T., Campbell, M., Adams, P., Small, A. M., Perry, R., and Shell, J. (1989). The effects of haloperidol on discrimination learning and behavioral symptoms in autistic children. J. Autism Dev. Disord. 19, 227–239. doi: 10.1007/BF02211843
Anderson, L. T., Campbell, M., Grega, D. M., Perry, R., Small, A. M., and Green, W. H. (1984). Haloperidol in the treatment of infantile autism: Effects on learning and behavioral symptoms. Am. J. Psychiatry 141, 1195–1202. doi: 10.1176/ajp.141.10.1195
Antshel, K., Zhang-James, Y., and Faraone, S. (2013). The comorbidity of ADHD and autism spectrum disorder. Expert Rev. Neurother. 13, 1117–1128. doi: 10.1586/14737175.2013.840417
Aoki, Y., Yoncheva, Y., Chen, B., Nath, T., Sharp, D., Lazar, M., et al. (2017). Association of white matter structure with autism spectrum disorder and attention-deficit/hyperactivity disorder. JAMA Psychiatry 74, 1120–1128. doi: 10.1001/jamapsychiatry.2017.2573
Arnold, H. M., Burk, J. A., Hodgson, E. M., Sarter, M., and Bruno, J. P. (2002). Differential cortical acetylcholine release in rats performing a sustained attention task versus behavioral control tasks that do not explicitly tax attention. Neuroscience 114, 451–460. doi: 10.1016/S0306-4522(02)00292-0
Arnold, L. E., Aman, M. G., Cook, A. M., Witwer, A. N., Hall, K. L., Thompson, S., et al. (2006). Atomoxetine for hyperactivity in autism spectrum disorders: Placebo-controlled crossover pilot trial. J. Am. Acad. Child Adolesc. Psychiatry 45, 1196–1205. doi: 10.1097/01.chi.0000231976.28719.2a
Arnold, L. E., Aman, M. G., Hollway, J., Hurt, E., Bates, B., Li, X., et al. (2012). Placebo-controlled pilot trial of mecamylamine for treatment of autism spectrum disorders. J. Child Adolesc. Psychopharmacol. 22, 198–205. doi: 10.1089/cap.2011.0056
Artoni, P., Piffer, A., Vinci, V., LeBlanc, J., Nelson, C. A., Hensch, T. K., et al. (2019). Deep learning of spontaneous arousal fluctuations detects early cholinergic defects across neurodevelopmental mouse models and patients. Proc. Natl. Acad. Sci. U.S.A. 177, 23298–23303. doi: 10.1073/pnas.1820847116
Aston-Jones, G., and Cohen, J. (2005a). An integrative theory of locus coeruleus-norpeinephrine function: Adaptive gain and optimal performance. Annu. Rev. Neurosci. 28, 403–450. doi: 10.1146/annurev.neuro.28.061604.135709
Aston-Jones, G., and Cohen, J. (2005b). Adaptive gain and the role of the locus coeruleus–norepinephrine system in optimal performance. J. Comp. Neurol. 493, 99–110. doi: 10.1002/cne.20723
Aston-Jones, G., and Waterhouse, B. (2016). Locus coeruleus: From global projection system to adaptive regulation of behavior. Brain Res. 1645, 75–78. doi: 10.1016/j.brainres.2016.03.001
Baccarin, M., Picinelli, C., Tomaiuolo, P., Castronovo, P., Costa, A., Verdecchia, M., et al. (2020). Appropriateness of array-CGH in the ADHD clinics: A comparative study. Genes Brain Behav. 19:e12651. doi: 10.1111/gbb.12651
Bacchelli, E., Battaglia, A., Cameli, C., Lomartire, S., Tancredi, R., Thomson, S., et al. (2015). Analysis of CHRNA7 rare variants in autism spectrum disorder susceptibility. Am. J. Med. Genet. A 167, 715–723. doi: 10.1002/ajmg.a.36847
Baghdadli, A., Assouline, B., Sonieì, S., Pernon, E., Darrou, C., Michelon, C., et al. (2012). Developmental trajectories of adaptive behaviors from early childhood to adolescence in a cohort of 152 children with autism spectrum disorders. J. Autism Dev. Disord. 42, 1314–1325. doi: 10.1007/s10803-011-1357-z
Bargmann, C., and Marder, E. (2013). From the connectome to brain function. Nat. Methods 10, 483–490. doi: 10.1038/nmeth.2451
Barkley, R. (2006). “Attention-deficit/hyperactivity disorder,” in Behavioral and emotional disorders in adolescents: Nature, assessment, and treatment, eds D. A. Wolfe and E. J. Mash (New York, NY: Guilford Publications), 91–152.
Baron-Cohen, S., Ring, H. A., Bullmore, E. T., Wheelwright, S., Ashwin, C., and Williams, S. C. (2000). The amygdala theory of autism. Neurosci. Biobehav. Rev. 24, 355–364. doi: 10.1016/S0149-7634(00)00011-7
Barrie, E. S., Pinsonneault, J. K., Sadee, W., Hollway, J. A., Handen, B. L., Smith, T., et al. (2018). Testing genetic modifiers of behavior and response to atomoxetine in autism spectrum disorder with ADHD. J. Dev. Phys. Disabil. 30, 355–371. doi: 10.1007/s10882-018-9590-4
Becker, E., and Stoodley, C. (2013). “Autism spectrum disorder and the cerebellum,” in International review of neurobiology, ed. G. Konopka (Cambridge, MA: Academic Press), 1–34.
Beier, K. T., Steinberg, E. E., DeLoach, K. E., Xie, S., Miyamichi, K., Schwarz, L., et al. (2015). Circuit architecture of VTA dopamine neurons revealed by systematic input-output mapping. Cell 162, 622–634. doi: 10.1016/j.cell.2015.07.015
Beier, K., Gao, X., Xie, S., DeLoach, K., Malenka, R., and Luo, L. (2019). Topological organization of ventral tegmental area connectivity revealed by viral-genetic dissection of input-output relations. Cell Rep. 26, 159–167.e6. doi: 10.1016/j.celrep.2018.12.040
Berridge, C., and Waterhouse, B. (2003). The locus coeruleus–noradrenergic system: Modulation of behavioral state and state-dependent cognitive processes. Brain Res. Rev. 42, 33–84. doi: 10.1016/S0165-0173(03)00143-7
Beversdorf, D. Q. (2020). The role of the noradrenergic system in autism spectrum disorders, implications for treatment. Semin. Pediatr. Neurol. 35:100834. doi: 10.1016/j.spen.2020.100834
Beversdorf, D. Q., Carpenter, A. L., Miller, R. F., Cios, J. S., and Hillier, A. (2008). Effect of propranolol on verbal problem solving in autism spectrum disorder. Neurocase 14, 378–383. doi: 10.1080/13554790802368661
Beversdorf, D. Q., Saklayen, S., Higgins, K. F., Bodner, K. E., Kanne, S. M., and Christ, S. E. (2011). Effect of propranolol on word fluency in autism. Cogn. Behav. Neurol. 24, 11–17. doi: 10.1097/WNN.0b013e318204d20e
Beversdorf, D., Ferguson, B., Reznicek, E., Lewis, M., Christ, S., and Stichter, J. (2013). Effects of propranolol on social functioning in autism spectrum disorder. Neurology 80:S18.002.
Beversdorf, D., Zamzow, R., Ferguson, B., Martin, T., Lewis, M., and Stichter, J. (2014). Predictors of response to propranolol for social functioning in autism spectrum disorder. Neurology 82, I4–I1.
Bobb, A. J., Addington, A. M., Sidransky, E., Gornick, M. C., Lerch, J. P., Greenstein, D. K., et al. (2005). Support for association between ADHD and two candidate genes: NET1 and DRD1. Am. J. Med. Genet. B Neuropsychiatr. Genet. 134B, 67–72. doi: 10.1002/ajmg.b.30142
Bodner, K., Beversdorf, D., Saklayen, S., and Christ, S. (2012). Noradrenergic moderation of working memory impairments in adults with autism spectrum disorder. J. Int. Neuropsychol. Soc. 18, 556–564. doi: 10.1017/S1355617712000070
Bonnot, O., and Holzer, L. (2012). Use of antipsychotics in child and adolescent. Neuropsychiatr. Enfance Adolesc. 60, 12–19. doi: 10.1016/j.neurenf.2011.07.003
Bora, E., and Pantelis, C. (2016). Meta-analysis of social cognition in attention-deficit/hyperactivity disorder (ADHD): Comparison with healthy controls and autistic spectrum disorder. Psychol. Med. 46, 699–716. doi: 10.1017/S0033291715002573
Brezina, V. (2010). Beyond the wiring diagram: Signalling through complex neuromodulator networks. Philos. Trans. R. Soc. B Biol. Sci. 365, 2363–2374. doi: 10.1098/rstb.2010.0105
Brooks, D. (2016). Molecular imaging of dopamine transporters. Ageing Res. Rev. 30, 114–121. doi: 10.1016/j.arr.2015.12.009
Bruce, B., Thernlund, G., and Nettelbladt, U. (2006). ADHD and language impairment. Eur. Child Adolesc. Psychiatry 15, 52–60. doi: 10.1007/s00787-006-0508-9
Bruchmüller, K., Margraf, J., and Schneider, S. (2012). Is ADHD diagnosed in accord with diagnostic criteria? Overdiagnosis and influence of client gender on diagnosis. J. Consult. Clin. Psychol. 80, 128–138. doi: 10.1037/a0026582
Caillies, S., Bertot, V., Motte, J., Raynaud, C., and Abely, M. (2014). Social cognition in ADHD: Irony understanding and recursive theory of mind. Res. Dev. Disabil. 35, 3191–3198. doi: 10.1016/j.ridd.2014.08.002
Campbell, M., Anderson, L. T., Small, A. M., Perry, R., Green, W. H., and Caplan, R. (1982). The effects of haloperidol on learning and behavior in autistic children. J. Autism Dev. Disord. 12, 167–175. doi: 10.1007/BF01531306
Candy, B., Jones, L., Williams, R., Tookman, A., and King, M. (2008). Psychostimulants for depression. Cochrane Database Syst. Rev. 2:CD006722. doi: 10.1002/14651858.CD006722.pub2
Cardinal, R., Parkinson, J., Hall, J., and Everitt, B. (2002). Emotion and motivation: The role of the amygdala, ventral striatum, and prefrontal cortex. Neurosci. Biobehav. Rev. 26, 321–352. doi: 10.1016/S0149-7634(02)00007-6
Carey, M., and Regehr, W. (2009). Noradrenergic control of associative synaptic plasticity by selective modulation of instructive signals. Neuron 62, 112–122. doi: 10.1016/j.neuron.2009.02.022
Carper, R., and Courchesne, E. (2005). Localized enlargement of the frontal cortex in early autism. Biol. Psychiatry 57, 126–133. doi: 10.1016/j.biopsych.2004.11.005
Casey, J. P., Magalhaes, T., Conroy, J. M., Regan, R., Shah, N., Anney, R., et al. (2012). A novel approach of homozygous haplotype sharing identifies candidate genes in autism spectrum disorder. Hum. Genet. 131, 565–579. doi: 10.1007/s00439-011-1094-6
Chandler, D. J., Jensen, P., McCall, J. G., Pickering, A. E., Schwarz, L. A., and Totah, N. K. (2019). Redefining noradrenergic neuromodulation of behavior: Impacts of a modular locus coeruleus architecture. J. Neurosci. 39, 8239–8249. doi: 10.1523/JNEUROSCI.1164-19.2019
Chandler, D., Gao, W., and Waterhouse, B. (2014). Heterogeneous organization of the locus coeruleus projections to prefrontal and motor cortices. Proc. Natl. Acad. Sci. U.S.A. 111, 6816–6821. doi: 10.1073/pnas.1320827111
Chandler, D., Lamperski, C., and Waterhouse, B. (2013). Identification and distribution of projections from monoaminergic and cholinergic nuclei to functionally differentiated subregions of prefrontal cortex. Brain Res. 1522, 38–58. doi: 10.1016/j.brainres.2013.04.057
Chen, T., Cai, W., Ryali, S., Supekar, K., and Menon, V. (2016). Distinct global brain dynamics and spatiotemporal organization of the salience network. PLoS. Biol. 14:e100249. doi: 10.1371/journal.pbio.1002469
Chevallier, C., Kohls, G., Troiani, V., Brodkin, E. S., and Schultz, R. T. (2012). The social motivation theory of autism. Trends Cogn. Sci. 16, 231–239. doi: 10.1016/j.tics.2012.02.007
Chevrier, A., Bhaijiwala, M., Lipszyc, J., Cheyne, D., Graham, S., and Schachar, R. (2019). Disrupted reinforcement learning during post-error slowing in ADHD. PLoS One 14:e0206780. doi: 10.1371/journal.pone.0206780
Chez, M. G., Aimonovitch, M., Buchanan, T., Mrazek, S., and Tremb, R. J. (2004). Treating autistic spectrum disorders in children: Utility of the cholinesterase inhibitor rivastigmine tartrate. J. Child Neurol. 19, 165–169.
Chez, M., Buchanan, T., Becker, M., Kessler, J., Aimonovitch, M., Mrazek, S., et al. (2003). Donepezil hydrochloride: A double-blind study in autistic children. J. Pediatr. Neurol. 1, 83–88. doi: 10.1055/s-0035-1557175
Clark, T., Feehan, C., Tinline, C., and Vostanis, P. (1999). Autistic symptoms in children with attention deficit-hyperactivity disorder. Eur. Child Adolesc. Psychiatry 8, 50–55. doi: 10.1007/s007870050083
Cohen, D., Raffin, M., Canitano, R., Bodeau, N., Bonnot, O., Périsse, D., et al. (2013). Risperidone or aripiprazole in children and adolescents with Autism and/or intellectual disability: A Bayesian meta-analysis of efficacy and secondary effects. Res. Autism Spectr. Disord. 7, 167–175. doi: 10.1016/j.rasd.2012.08.001
Cohen, N. J., Vallance, D. D., Barwick, M., Im, N., Menna, R., Horodezky, N. B., et al. (2000). The interface between ADHD and language impairment: An examination of language, achievement, and cognitive processing. J. Child Psychol. Psychiatry 41, 353–362. doi: 10.1111/1469-7610.00619
Corp, S., Gitlin, M., and Altshuler, L. (2014). A review of the use of stimulants and stimulant alternatives in treating bipolar depression and major depressive disorder. J. Clin. Psychiatry 75, 1010–1018. doi: 10.4088/JCP.13r08851
Coury, D. (2010). Medical treatment of autism spectrum disorders. Curr. Opin. Neurol. 23, 131–136. doi: 10.1097/WCO.0b013e32833722fa
Craig, F., Margari, F., Legrottaglie, A. R., Palumbi, R., de Giambattista, C, and Margari, L. (2016). A review of executive function deficits in autism spectrum disorder and attention-deficit/hyperactivity disorder. Neuropsychiatr. Dis. Treat. 12, 1191–1202. doi: 10.2147/NDT.S104620
Darcq, E., and Kieffer, B. (2015). PI3K signaling in the locus coeruleus: A new molecular pathway for ADHD research. EMBO Mol. Med. 7, 859–861. doi: 10.15252/emmm.201505266
Dawson, G., Webb, S., and McPartland, J. (2005). Understanding the nature of face processing impairment in autism: Insights from behavioral and electrophysiological studies. Dev. Neuropsychol. 27, 403–424. doi: 10.1207/s15326942dn2703_6
de la Torre-Ubieta, L., Won, H., Stein, J., and Geschwind, D. (2016). Advancing the understanding of autism disease mechanisms through genetics. Nat. Med. 22, 345–361. doi: 10.1038/nm.4071
de Lacy, N., and Calhoun, V. (2018). Dynamic connectivity and the effects of maturation in youth with attention deficit hyperactivity disorder. Netw. Neurosci. 3, 195–216. doi: 10.1162/netn_a_00063
de Luca, V., Muglia, P., Jain, U., and Kennedy, J. (2004). No evidence of linkage or association between the norepinephrine transporter (NET) gene MnlI polymorphism and adult ADHD. Am. J. Med. Genet. B Neuropsychiatr. Genet. 124B, 38–40. doi: 10.1002/ajmg.b.20075
de Vries, L., Fouquaet, I., Boets, B., Naulaers, G., and Steyaert, J. (2021). Autism spectrum disorder and pupillometry: A systematic review and meta-analysis. Neurosci. Biobehav. Rev. 120, 479–508. doi: 10.1016/j.neubiorev.2020.09.032
del Campo, N., Chamberlain, S., Sahakian, B., and Robbins, T. (2011). The roles of dopamine and noradrenaline in the pathophysiology and treatment of attention-deficit/hyperactivity disorder. Biol. Psychiatry 69, e145–e157. doi: 10.1016/j.biopsych.2011.02.036
Deserno, M., Borsboom, D., Begeer, S., Agelink van Rentergem, J., Mataw, K., and Geurts, H. (2019). Sleep determines quality of life in autistic adults: A longitudinal study. Autism Res. 12, 794–801. doi: 10.1002/aur.2103
Deutsch, S. I., Urbano, M. R., Neumann, S. A., Burket, J. A., and Katz, E. (2010). Cholinergic abnormalities in autism: Is there a rationale for selective nicotinic agonist interventions? Clin. Neuropharmacol. 33, 114–120. doi: 10.1097/WNF.0b013e3181d6f7ad
di Michele, F., Prichep, L., John, E., and Chabot, R. (2005). The neurophysiology of attention-deficit/hyperactivity disorder. Int. J. Psychophysiol. 58, 81–93. doi: 10.1016/j.ijpsycho.2005.03.011
Dichter, G. S., Felder, J. N., Green, S. R., Rittenberg, A. M., Sasson, N. J., and Bodfish, J. W. (2012b). Reward circuitry function in autism spectrum disorders. Soc. Cogn. Affect. Neurosci. 7, 160–172. doi: 10.1093/scan/nsq095
Dichter, G. S., Damiano, C., and Allen, J. (2012a). Reward circuitry dysfunction in psychiatric and neurodevelopmental disorders and genetic syndromes: Animal models and clinical findings. J. Neurodev. Disord. 4:19. doi: 10.1186/1866-1955-4-19
Duan, X., Chen, H., He, C., Long, Z., Guo, X., Zhou, Y., et al. (2017). Resting-state functional under-connectivity within and between large-scale cortical networks across three low-frequency bands in adolescents with autism. Prog. Neuropsychopharmacol. Biol. Psychiatry 79, 434–441. doi: 10.1016/j.pnpbp.2017.07.027
Durston, S., van Belle, J., and de Zeeuw, P. (2011). Differentiating frontostriatal and fronto-cerebellar circuits in attention-deficit/hyperactivity disorder. Biol. Psychiatry 69, 1178–1184. doi: 10.1016/j.biopsych.2010.07.037
Ebisch, S. J., Gallese, V., Willems, R. M., Mantini, D., Groen, W. B., Romani, G. L., et al. (2011). Altered intrinsic functional connectivity of anterior and posterior insula regions in high-functioning participants with autism spectrum disorder. Hum. Brain Mapp. 32, 1013–1028. doi: 10.1002/hbm.21085
Edgar, J. C., Khan, S. Y., Blaskey, L., Chow, V. Y., Rey, M., Gaetz, W., et al. (2015). Neuromagnetic oscillations predict evoked-response latency delays and core language deficits in autism spectrum disorders. J. Autism Dev. Disord. 45, 395–405. doi: 10.1007/s10803-013-1904-x
Eissa, N., Al-Houqani, M., Sadeq, A., Ojha, S. K., Sasse, A., and Sadek, B. (2018). Current enlightenment about etiology and pharmacological treatment of autism spectrum disorder. Front. Neurosci. 12:304. doi: 10.3389/fnins.2018.00304
Falahpour, M., Thompson, W., Abbott, A., Jahedi, A., Mulvey, M., Datko, M., et al. (2016). Underconnected, but not broken? Dynamic functional connectivity MRI shows underconnectivity in autism is linked to increased intra-individual variability across time. Brain Connect. 6, 403–414. doi: 10.1089/brain.2015.0389
Fankhauser, M. P., Karumanchi, V. C., German, M. L., Yates, A., and Karumanchi, S. D. (1992). A double-blind, placebo-controlled study of the efficacy of transdermal clonidine in autism. J. Clin. Psychiatry 53, 77–82.
Faraone, S. (2018). The pharmacology of amphetamine and methylphenidate: Relevance to the neurobiology of attention-deficit/hyperactivity disorder and other psychiatric comorbidities. Neurosci. Biobehav. Rev. 87, 255–270. doi: 10.1016/j.neubiorev.2018.02.001
Faraone, S. V., Perlis, R. H., Doyle, A. E., Smoller, J. W., Goralnick, J. J., Holmgren, M. A., et al. (2005). Molecular genetics of attention-deficit/hyperactivity disorder. Biol. Psychiatry 57, 1313–1323. doi: 10.1016/j.biopsych.2004.11.024
Faraone, S. V., Spencer, T. J., Madras, B. K., Zhang-James, Y., and Biederman, J. (2014). Functional effects of dopamine transporter gene genotypes on in vivo dopamine transporter functioning: A meta-analysis. Mol. Psychiatry 19, 880–889. doi: 10.1038/mp.2013.126
Faraone, S., and Larsson, H. (2019). Genetics of attention deficit hyperactivity disorder. Mol. Psychiatry 24, 562–575. doi: 10.1038/s41380-018-0070-0
Faraone, S., and Mick, E. (2010). Molecular Genetics of attention deficit hyperactivity disorder. Psychiatr. Clin. 33, 159–180. doi: 10.1016/j.psc.2009.12.004
Farrant, K., and Uddin, L. (2016). Atypical developmental of dorsal and ventral attention networks in autism. Dev. Sci. 19, 550–563. doi: 10.1111/desc.12359
Fatemi, S. H., Halt, A. R., Realmuto, G., Earle, J., Kist, D. A., Thuras, P., et al. (2002). Purkinje cell size is reduced in cerebellum of patients with autism. Cell Mol. Neurobiol. 22, 171–175. doi: 10.1023/A:1019861721160
Findling, R. L., Mankoski, R., Timko, K., Lears, K., McCartney, T., McQuade, R. D., et al. (2014). A randomized controlled trial investigating the safety and efficacy of aripiprazole in the long-term maintenance treatment of pediatricpatients with irritability associated with autistic disorder. J. Clin. Psychiatry 75, 22–30. doi: 10.4088/jcp.13m08500
Foote, S., and Morrison, J. (1987). Extrathalamic modulation of cortical function. Annu. Rev. Neurosci. 10, 67–95. doi: 10.1146/annurev.ne.10.030187.000435
Franke, B., Vasquez, A. A., Johansson, S., Hoogman, M., Romanos, J., Boreatti-Hümmer, A., et al. (2010). Multicenter analysis of the SLC6A3/DAT1 VNTR haplotype in persistent ADHD suggests differential involvement of the gene in childhood and persistent ADHD. Neuropsychopharmacology 35, 656–664. doi: 10.1038/npp.2009.170
Friedman, S. D., Shaw, D. W., Artru, A. A., Dawson, G., Petropoulos, H., and Dager, S. R. (2006). Gray and white matter brain chemistry in young children with autism. Arch. Gen. Psychiatry 63, 786–794. doi: 10.1001/archpsyc.63.7.786
Fuenzalida, M., Peìrez, M., and Arias, H. R. (2016). Role of nicotinic and muscarinic receptors on synaptic plasticity and neurological diseases. Curr. Pharm. Des. 22, 2004–2014.
Furey, M., Pietrini, P., and Haxby, J. (2000). Cholinergic enhancement and increased selectivity of perceptual processing during working memory. Science 290, 2315–2319. doi: 10.1126/science.290.5500.2315
Furey, M., Pietrini, P., Haxby, J., and Drevets, W. (2008). Selective effects of cholinergic modulation ontask performance during selective attention. Neuropsychopharmacology 33, 913–923. doi: 10.1038/sj.npp.1301461
Gadow, K. D., Devincent, C. J., Olvet, D. M., Pisarevskaya, V., and Hatchwell, E. (2010). Association of DRD4 polymorphism with severity of oppositional defiant disorder, separation anxiety disorder and repetitive behaviors in children with autism spectrum disorder. Eur. J. Neurosci. 32, 1058–1065. doi: 10.1111/j.1460-9568.2010.07382.x
Gallezot, J., Bottlaender, M., Greìgoire, M., Roumenov, D., Deverre, J., Coulon, C., et al. (2005). In vivo imaging of human cerebral nicotinic acetylcholine receptors with 2-18F-fluoro-A-85380 and PET. J. Nucl. Med. 46, 240–247.
Gault, J., Robinson, M., Berger, R., Drebing, C., Logel, J., Hopkins, J., et al. (1998). Genomic organization and partial duplication of the human α7 neuronal nicotinic acetylcholine receptor gene (CHRNA7). Genomics 52, 173–185. doi: 10.1006/geno.1998.5363
Genro, J., Kieling, C., Rohde, L., and Hutz, M. (2010). Attention-deficit/hyperactivity disorder and the dopaminergic hypotheses. Expert Rev. Neurother. 10, 587–601. doi: 10.1586/ern.10.17
Geschwind, D. (2011). Genetics of autism spectrum disorders. Trends Cogn. Sci. 15, 409–416. doi: 10.1016/j.tics.2011.07.003
Geurts, H., and Embrechts, M. (2008). Language profiles in ASD, SLI, and ADHD. J. Autism Dev. Disord. 38:1931. doi: 10.1007/s10803-008-0587-1
Ghaleiha, A., Ghyasvand, M., Mohammadi, M., Farokhnia, M., Yadegari, N., Tabrizi, M., et al. (2014). Galantamine efficacy and tolerability as an augmentative therapy in autistic children: A randomized, double-blind, placebo-controlled trial. J. Psychopharmacol. (Oxf) 28, 677–685. doi: 10.1177/0269881113508830
Ghanizadeh, A. (2011). Sensory processing problems in children with ADHD, a systematic review. Psychiatry Invest. 8, 89–94. doi: 10.4306/pi.2011.8.2.89
Ghanizadeh, A., Sahraeizadeh, A., and Berk, M. (2014). A head-to-head comparison of aripiprazole and risperidone for safety and treating autistic disorders, a randomized double blind clinical trial. Child Psychiatry Hum. Dev. 45, 185–192. doi: 10.1007/s10578-013-0390-x
Gharehgazlou, A., Vandewouw, M., Ziolkowski, J., Wong, J., Crosbie, J., Schachar, R., et al. (2022). Cortical gyrification morphology in ASD and ADHD: Implication for further similarities or disorder-specific features? Cereb. Cortex 32, 2332–2342. doi: 10.1093/cercor/bhab326
Gillentine, M. A., Berry, L. N., Goin-Kochel, R. P., Ali, M. A., Ge, J., Guffey, D., et al. (2017). The cognitive and behavioral phenotypes of individuals with CHRNA7 duplications. J. Autism Dev. Disord. 47, 549–562. doi: 10.1007/s10803-016-2961-8
Gillentine, M., and Schaaf, C. (2015). The human clinical phenotypes of altered CHRNA7 copy number. Biochem. Pharmacol. 97, 352–362. doi: 10.1016/j.bcp.2015.06.012
Gizer, I., Ficks, C., and Waldman, I. (2009). Candidate gene studies of ADHD: A meta-analytic review. Hum. Genet. 126, 51–90. doi: 10.1007/s00439-009-0694-x
Gordon, C. T., Rapoport, J. L., Hamburger, S. D., State, R. C., and Mannheim, G. B. (1992). Differential response of seven subjects with autistic disorder to clomipramine and desipramine. Am. J. Psychiatry 149, 363–366. doi: 10.1176/ajp.149.3.363
Gordon, C. T., State, R. C., Nelson, J. E., Hamburger, S. D., and Rapoport, J. L. (1993). A double-blind comparison of clomipramine, desipramine, and placebo in the treatment of autistic disorder. Arch. Gen. Psychiatry 50, 441–447. doi: 10.1001/archpsyc.1993.01820180039004
Gregory, A. M., Agnew-Blais, J., Matthews, T., Moffitt, T., and Arseneault, L. (2017). Associations between ADHD and sleep quality: Longitudinal analyses from a nationally-representative cohort of twins. J. Clin. Child Adolesc. Psychol. 46, 284–294. doi: 10.1080/15374416.2016.1183499
Guo, X., Duan, X., Suckling, J., Chen, H., Liao, W., Cui, Q., et al. (2019). Partially impaired functional connectivity states between right anterior insula and default mode network in autism spectrum disorder. Hum. Brain Mapp. 40, 1264–1275. doi: 10.1002/hbm.24447
Hamilton, P. J., Campbell, N. G., Sharma, S., Erreger, K., Hansen, F. H., Saunders, C., et al. (2013). De novo mutation in the dopamine transporter gene associates dopamine dysfunction with autism spectrum disorder. Mol. Psychiatry 18, 1315–1323. doi: 10.1038/mp.2013.102
Hampson, D., and Blatt, G. (2015). Autism spectrum disorders and neuropathology of the cerebellum. Front. Neurosci. 9:420. doi: 10.3389/fnins.2015.00420
Handen, B. L., Johnson, C. R., McAuliffe-Bellin, S., Murray, P. J., and Hardan, A. Y. (2011). Safety and efficacy of donepezil in children and adolescents with autism: Neuropsychological measures. J. Child Adolesc. Psychopharmacol. 21, 43–50. doi: 10.1089/cap.2010.0024
Handen, B., Johnson, C., and Lubetsky, M. (2000). Efficacy of methylphenidate among children with autism and symptoms of attention-deficit hyperactivity disorder. J. Autism Dev. Disord. 30, 245–255. doi: 10.1023/a:1005548619694
Handen, B., Sahl, R., and Hardan, A. (2008). Guanfacine in children with autism and/or intellectual disabilities. J. Dev. Behav. Pediatr. 29, 303–308. doi: 10.1097/DBP.0b013e3181739b9d
Harfterkamp, M., Buitelaar, J. K., Minderaa, R. B., van de Loo-Neus, G., van der Gaag, R., and Hoekstra, P. J. (2013). Long-term treatment with atomoxetine for attention-deficit/hyperactivity disorder symptoms in children and adolescents with autism spectrum disorder: An open-label extension study. J. Child Adolesc. Psychopharmacol. 23, 194–199. doi: 10.1089/cap.2012.0012
Harfterkamp, M., Buitelaar, J. K., Minderaa, R. B., van de Loo-Neus, G., van der Gaag, R., and Hoekstra, P. J. (2014). Atomoxetine in autism spectrum disorder: No effects on social functioning; some beneficial effects on stereotyped behaviors, inappropriate speech, and fear of change. J. Child Adolesc. Psychopharmacol. 24, 481–485. doi: 10.1089/cap.2014.0026
Harfterkamp, M., van de Loo-Neus, G, Minderaa, R. B., van der Gaag, R., Escobar, R., Schacht, A., et al. (2012). A randomized double-blind study of atomoxetine versus placebo for attention-deficit/hyperactivity disorder symptoms in children with autism spectrum disorder. J. Am. Acad. Child Adolesc. Psychiatry 51, 733–741. doi: 10.1016/j.jaac.2012.04.011
Hawi, Z., Matthews, N., Barry, E., Kirley, A., Wagner, J., Wallace, R. H., et al. (2013). A high density linkage disequilibrium mapping in 14 noradrenergic genes: Evidence of association between SLC6A2, ADRA1B and ADHD. Psychopharmacology (Berl) 225, 895–902. doi: 10.1007/s00213-012-2875-x
Hegarty, J. P. II, Ferguson, B. J., Zamzow, R. M., Rohowetz, L. J., Johnson, J. D., Christ, S. E., et al. (2017). Beta-adrenergic antagonism modulates functional connectivity in the default mode network of individuals with and without autism spectrum disorder. Brain Imaging Behav. 11, 1278–1289. doi: 10.1007/s11682-016-9604-8
Hettinger, J. (2009). The role of dopamine-related genes in autism spectrum disorders: Evidence for specific genes and risk for ASD in families with affected males. PH D. thesis. Kingston, ON: Queen’s University.
Hollander, E., Wasserman, S., Swanson, E. N., Chaplin, W., Schapiro, M. L., Zagursky, K., et al. (2006). A double-blind placebo-controlled pilot study of olanzapine in childhood/adolescent pervasive developmental disorder. J. Child Adolesc. Psychopharmacol. 16, 541–548. doi: 10.1089/cap.2006.16.541
Horev, G., Ellegood, J., Lerch, J. P., Son, Y. E., Muthuswamy, L., Vogel, H., et al. (2011). Dosage-dependent phenotypes in models of 16p11.2 lesions found in autism. Proc. Natl. Acad. Sci. U.S.A. 108, 17076–17081. doi: 10.1073/pnas.1114042108
Horvitz, J. (2000). Mesolimbocortical and nigrostriatal dopamine responses to salient non-reward events. Neuroscience 96, 651–656. doi: 10.1016/S0306-4522(00)00019-1
Howe, M., and Dombeck, D. (2016). Rapid signaling in distinct dopaminergic axons during locomotion and reward. Nature 535, 505–510. doi: 10.1038/nature18942
Huguet, G., Ey, E., and Bourgeron, T. (2013). The genetic landscapes of autism spectrum disorders. Annu. Rev. Genomics Hum. Genet. 14, 191–213. doi: 10.1146/annurev-genom-091212-153431
Huppeì-Gourgues, F., Jegouic, K., and Vaucher, E. (2018). Topographic organization of cholinergic innervation from the basal forebrain to the visual cortex in the rat. Front. Neural Circuits 12:19. doi: 10.3389/fncir.2018.00019
Huss, M., Duhan, P., Gandhi, P., Chen, C., Spannhuth, C., and Kumar, V. (2017). Methylphenidate dose optimization for ADHD treatment: Review of safety, efficacy, and clinical necessity. Neuropsychiatr. Dis. Treat. 13, 1741–1751. doi: 10.2147/NDT.S130444
Ichikawa, H., Mikami, K., Okada, T., Yamashita, Y., Ishizaki, Y., Tomoda, A., et al. (2017). Aripiprazole in the treatment of irritability in children and adolescents with autism spectrum disorder in Japan: A randomized, double-blind, placebo-controlled study. Child Psychiatry Hum. Dev. 48, 796–806. doi: 10.1007/s10578-016-0704-x
Ilango, A., Kesner, A. J., Keller, K. L., Stuber, G. D., Bonci, A., and Ikemoto, S. (2014). Similar roles of substantia nigra and ventral tegmental dopamine neurons in reward and aversion. J. Neurosci. 34, 817–822. doi: 10.1523/JNEUROSCI.1703-13.2014
Inkster, B., Muglia, P., Jain, U., and Kennedy, J. (2004). Linkage disequilibrium analysis of the dopamine beta-hydroxylase gene in persistent attention deficit hyperactivity disorder. Psychiatr. Genet. 14, 117–120. doi: 10.1097/01.ypg.0000107932.32051.1c
Jaselskis, C., Cook, E., and Fletcher, K. (1992). Clonidine treatment of hyperactive and impulsive children with autistic disorder. J. Clin. Psychopharmacol. 12, 322–327. doi: 10.1097/00004714-199210000-00005
Jensen, A., Frølund, B., Liljefors, T., and Krogsgaard-Larsen, P. (2005). Neuronal nicotinic acetylcholine receptors: Structural revelations, target identifications, and therapeutic inspirations. J. Med. Chem. 48, 4705–4745. doi: 10.1021/jm040219e
Johansson, J., Landgren, M., Fernell, E., Lewander, T., and Venizelos, N. (2013). Decreased binding capacity (Bmax) of muscarinic acetylcholine receptors in fibroblasts from boys with attention-deficit/hyperactivity disorder (ADHD). Atten. Defic. Hyperact. Disord. 5, 267–271. doi: 10.1007/s12402-013-0103-0
Johnston, B. A., Mwangi, B., Matthews, K., Coghill, D., Konrad, K., and Steele, J. D. (2014). Brainstem abnormalities in attention deficit hyperactivity disorder support high accuracy individual diagnostic classification. Hum. Brain Mapp. 35, 5179–5189. doi: 10.1002/hbm.22542
Jones, M., Palmour, R., Zwaigenbaum, L., and Szatmari, P. (2004). Modifier effects in autism at the MAO-A and DBH loci. Am. J. Med. Genet. Part B Neuropsychiatr. Genet. 126B, 58–65. doi: 10.1002/ajmg.b.20172
Jongkees, B., and Colzato, L. (2016). Spontaneous eye blink rate as predictor of dopamine-related cognitive function–a review. Neurosci. Biobehav. Rev. 71, 58–82. doi: 10.1016/j.neubiorev.2016.08.020
Jou, R., Handen, B., and Hardan, A. (2005). Retrospective assessment of atomoxetine in children and adolescents with pervasive developmental disorders. J. Child Adolesc. Psychopharmacol. 15, 325–330. doi: 10.1089/cap.2005.15.325
Jouvet, M. (1969). Biogenic amines and the states of sleep. Science 163, 32–41. doi: 10.1126/science.163.3862.32
Jwaid, A., Muslem, Z., and Dawood, E. (2020). Measurement the level of dopamine beta hydroxylase enzyme in autistic Iraqi children. Prensa Med. Argent. 106, 1–7.
Kana, R., Uddin, L., Kenet, T., Chugani, D., and Müller, R. (2014). Brain connectivity in autism. Front. Hum. Neurosci. 8:349. doi: 10.3389/fnhum.2014.00349
Kanne, S., and Mazurek, M. (2011). Aggression in children and adolescents with ASD: Prevalence and risk factors. J. Autism Dev. Disord. 41, 926–937. doi: 10.1007/s10803-010-1118-4
Karvat, G., and Kimchi, T. (2014). Acetylcholine elevation relieves cognitive rigidity and social deficiency in a mouse model of autism. Neuropsychopharmacology 39, 831–840. doi: 10.1038/npp.2013.274
Kebschull, J. M., Silva, P. G., Reid, A. P., Peikon, I. D., Albeanu, D. F., and Zador, A. M. (2016). High-throughput mapping of single-neuron projections by sequencing of barcoded RNA. Neuron 91, 975–987. doi: 10.1016/j.neuron.2016.07.036
Kemper, T., and Bauman, M. (1998). Neuropathology of infantile autism. J. Neuropathol. Exp. Neurol. 57, 645–652. doi: 10.1097/00005072-199807000-00001
Kenemans, J. (2015). Specific proactive and generic reactive inhibition. Neurosci. Biobehav. Rev. 56, 115–126. doi: 10.1016/j.neubiorev.2015.06.011
Kenemans, J. L. (2017). Psychopharmacology: The effects of drugs and medication on human brain and behavior. Amsterdam: Boom.
Kenemans, L., and Ramsey, N. (2013). Psychology in the brain: Integrative cognitive neuroscience. London: Palgrave Macmillan.
Kent, L., Green, E., Holmes, J., Thapar, A., Gill, M., Hawi, Z., et al. (2001). No association between CHRNA7 microsatellite markers and attention-deficit hyperactivity disorder. Am. J. Med. Genet. 105, 686–689. doi: 10.1002/ajmg.1553
Kleinhans, N. M., Richards, T., Sterling, L., Stegbauer, K. C., Mahurin, R., Johnson, L. C., et al. (2008). Abnormal functional connectivity in autism spectrum disorders during face processing. Brain 131, 1000–1012. doi: 10.1093/brain/awm334
Klinger, L., Klinger, M., and Pohlig, R. (2007). “Implicit learning impairments in autism spectrum disorders: Implications for treatment,” in New developments in autism: The future is today, 1st Edn, eds C. Vizcaino, J. Perez, M. Comi, and P. Gonzalez (London: Jessica Kingsley Publishers), 76–103.
Konofal, E., Lecendreux, M., and Cortese, S. (2010). Sleep and ADHD. Sleep Med. 11, 652–658. doi: 10.1016/j.sleep.2010.02.012
Koob, G., and Volkow, N. (2010). Neurocircuitry of addiction. Neuropsychopharmacology 35, 217–238. doi: 10.1038/npp.2009.110
Kosaka, H., Omori, M., Munesue, T., Ishitobi, M., Matsumura, Y., Takahashi, T., et al. (2010). Smaller insula and inferior frontal volumes in young adults with pervasive developmental disorders. Neuroimage 50, 1357–1363. doi: 10.1016/j.neuroimage.2010.01.085
Krames, E., Hunter Peckham, P., Rezai, A., and Aboelsaad, F. (2009). “What is neuromodulation?,” in Neuromodulation, eds E. Krames, P. Peckham, and A. Rezai (San Diego, CA: Academic Press), 3–8.
Krames, E., Peckham, P., and Rezai, A. (2018). Neuromodulation: Comprehensive textbook of principles, technologies, and therapies. Cambridge, MA: Academic Press.
Kroemer, N. B., Guevara, A., Teodorescu, I. C., Wuttig, F., Kobiella, A., and Smolka, M. N. (2014). Balancing reward and work: Anticipatory brain activation in NAcc and VTA predict effort differentially. Neuroimage 102, 510–519. doi: 10.1016/j.neuroimage.2014.07.060
Kumari, V., and Postma, P. (2005). Nicotine use in schizophrenia: The self medication hypotheses. Neurosci. Biobehav. Rev. 29, 1021–1034. doi: 10.1016/j.neubiorev.2005.02.006
Kushki, A., Anagnostou, E., Hammill, C., Duez, P., Brian, J., Iaboni, A., et al. (2019). Examining overlap and homogeneity in ASD, ADHD, and OCD: A data-driven, diagnosis-agnostic approach. Transl. Psychiatry 9:318. doi: 10.1038/s41398-019-0631-2
Lakens, D. (2013). Calculating and reporting effect sizes to facilitate cumulative science: A practical primer for t-tests and ANOVAs. Front. Psychol. 4:863. doi: 10.3389/fpsyg.2013.00863
Lawrence, K., Hernandez, L., Bookheimer, S., and Dapretto, M. (2019). Atypical longitudinal development of functional connectivity in adolescents with autism spectrum disorder. Autism Res. 12, 53–65. doi: 10.1002/aur.1971
Lecavalier, L. (2006). Behavioral and emotional problems in young people with pervasive developmental disorders: Relative prevalence, effects of subject characteristics, and empirical classification. J. Autism Dev. Disord. 36, 1101–1114. doi: 10.1007/s10803-006-0147-5
Lee, J., Laurin, N., Crosbie, J., Ickowicz, A., Pathare, T., Malone, M., et al. (2008). Association study of the nicotinic acetylcholine receptor α4 subunit gene, CHRNA4, in attention-deficit hyperactivity disorder. Genes Brain Behav. 7, 53–60. doi: 10.1111/j.1601-183X.2007.00325.x
Lee, M., Martin-Ruiz, C., Graham, A., Court, J., Jaros, E., Perry, R., et al. (2002). Nicotinic receptor abnormalities in the cerebellar cortex in autism. Brain 125, 1483–1495. doi: 10.1093/brain/awf160
Lee, T., Raygada, M., and Rennert, O. (2012). Integrative gene network analysis provides novel regulatory relationships, genetic contributions and susceptible targets in autism spectrum disorders. Gene 496, 88–96. doi: 10.1016/j.gene.2012.01.020
Lee, Y., Kim, H., Kim, J., Park, J., Choi, J., Lee, J., et al. (2018). Excessive D1 dopamine receptor activation in the dorsal striatum promotes autistic-like behaviors. Mol. Neurobiol. 55, 5658–5671. doi: 10.1007/s12035-017-0770-5
Lerner, T. N., Shilyansky, C., Davidson, T. J., Evans, K. E., Beier, K. T., Zalocusky, K. A., et al. (2015). Intact-brain analyses reveal distinct information carried by SNc dopamine subcircuits. Cell 162, 635–647. doi: 10.1016/j.cell.2015.07.014
Levin, E., and Rezvani, A. (2002). Nicotinic treatment for cognitive dysfunction. Curr. Drug Targets CNS Neurol. Disord. 1, 423–431. doi: 10.2174/1568007023339102
Levy, F., and Swanson, J. (2001). Timing, space and ADHD: The dopamine theory revisited. Aust. N. Z. J. Psychiatry 35, 504–511. doi: 10.1046/j.1440-1614.2001.00923.x
Lewis, A. S., Schalkwyk, G. I., Lopez, M. O., Volkmar, F. R., Picciotto, M. R., and Sukhodolsky, D. G. (2018). An exploratory trial of transdermal nicotine for aggression and irritability in adults with autism spectrum disorder. J. Autism Dev. Disord. 48, 2748–2757. doi: 10.1007/s10803-018-3536-7
Lewis, M., Tanimura, Y., Lee, L., and Bodfish, J. (2007). Animal models of restricted repetitive behavior in autism. Behav. Brain Res. 176, 66–74. doi: 10.1016/j.bbr.2006.08.023
Lippiello, P. (2006). Nicotinic cholinergic antagonists: A novel approach for the treatment of autism. Med. Hypotheses 66, 985–990. doi: 10.1016/j.mehy.2005.11.015
Liu, A., Chang, R., Pearce, R., and Gentleman, S. (2015). Nucleus basalis of Meynert revisited: Anatomy, history and differential involvement in Alzheimer’s and Parkinson’s disease. Acta Neuropathol. (Berl) 129, 527–540. doi: 10.1007/s00401-015-1392-5
Liu, R., Crawford, J., Callahan, P. M., Terry, A. V., Constantinidis, C., and Blake, D. T. (2018). Intermittent stimulation in the nucleus basalis of Meynert improves sustained attention in rhesus monkeys. Neuropharmacology 137, 202–210. doi: 10.1016/j.neuropharm.2018.04.026
Logemann, H., Böcker, K., Deschamps, P., Kemner, C., and Kenemans, J. (2014). The effect of enhancing cholinergic neurotransmission by nicotine on EEG indices of inhibition in the human brain. Pharmacol. Biochem. Behav. 122, 89–96. doi: 10.1016/j.pbb.2014.03.019
London, E. (2018). Neuromodulation and a reconceptualization of autism spectrum disorders: Using the locus coeruleus functioning as an exemplar. Front. Neurol. 9:1120. doi: 10.3389/fneur.2018.01120
London, E., Yoo, J., Fethke, E. D., and Zimmerman-Bier, B. (2020). The safety and effectiveness of high-dose propranolol as a treatment for challenging behaviors in individuals with autism spectrum disorders. J. Clin. Psychopharmacol. 40, 122–129. doi: 10.1097/JCP.0000000000001175
Loomes, R., Hull, L., and Mandy, W. (2017). What is the male-to-female ratio in autism spectrum disorder? A systematic review and meta-analysis. J. Am. Acad. Child Adolesc. Psychiatry 56, 466–474. doi: 10.1016/j.jaac.2017.03.013
Lord, C., Risi, S., and Pickles, A. (2004). “Trajectory of language development in autistic spectrum disorders,” in Developmental language disorders: From phenotypes to etiologies, eds M. L. Rice and S. F. Warren (Mahwah, NJ: Lawrence Erlbaum Associates Publishers), 7–29.
Luby, J., Mrakotsky, C., Stalets, M. M., Belden, A., Heffelfinger, A., Williams, M., et al. (2006). Risperidone in preschool children with autistic spectrum disorders: An investigation of safety and efficacy. J. Child Adolesc. Psychopharmacol. 16, 575–587. doi: 10.1089/cap.2006.16.575
Lynch, G. (2018). Using pupillometry to assess the atypical pupillary light reflex and LC-NE system in ASD. Behav. Sci. 8:108. doi: 10.3390/bs8110108
Madras, B., Miller, G., and Fischman, A. (2005). The dopamine transporter and attention-deficit/hyperactivity disorder. Biol. Psychiatry 57, 1397–1409. doi: 10.1016/j.biopsych.2004.10.011
Maia, C. R., Cortese, S., Caye, A., Deakin, T. K., Polanczyk, G. V., Polanczyk, C. A., et al. (2017). Long-term efficacy of methylphenidate immediate-release for the treatment of childhood ADHD: A systematic review and meta-analysis. J. Atten. Disord. 21, 3–13. doi: 10.1177/1087054714559643
Makkonen, I., Riikonen, R., Kokki, H., Airaksinen, M. M., and Kuikka, J. T. (2008). Serotonin and dopamine transporter binding in children with autism determined by SPECT. Dev. Med. Child Neurol. 50, 593–597. doi: 10.1111/j.1469-8749.2008.03027.x
Malow, B. A., Marzec, M. L., McGrew, S. G., Wang, L., Henderson, L. M., and Stone, W. L. (2006). Characterizing sleep in children with autism spectrum disorders: A multidimensional approach. Sleep 29, 1563–1571. doi: 10.1093/sleep/29.12.1563
Mansur, A. P., Avakian, S. D., Paula, R. S., Donzella, H., Santos, S. R., and Ramires, J. A. (1998). Pharmacokinetics and pharmacodynamics of propranolol in hypertensive patients after sublingual administration: Systemic availability. Braz. J. Med. Biol. Res. 31, 691–696. doi: 10.1590/S0100-879X1998000500014
Marco, E., Hinkley, L., Hill, S., and Nagarajan, S. (2011). Sensory processing in autism: A review of neurophysiologic findings. Pediatr. Res. 69, 48–54. doi: 10.1203/PDR.0b013e3182130c54
Marcus, R. N., Owen, R., Kamen, L., Manos, G., McQuade, R. D., Carson, W. H., et al. (2009). A placebo-controlled, fixed-dose study of aripiprazole in children and adolescents with irritability associated with autistic disorder. J. Am. Acad. Child Adolesc. Psychiatry 48, 1110–1119. doi: 10.1097/CHI.0b013e3181b76658
Marder, E. (2012). Neuromodulation of neuronal circuits: Back to the future. Neuron 76, 1–11. doi: 10.1016/j.neuron.2012.09.010
Marotta, R., Risoleo, M. C., Messina, G., Parisi, L., Carotenuto, M., Vetri, L., et al. (2020). The neurochemistry of autism. Brain Sci. 10:163. doi: 10.3390/brainsci10030163
Martin-Ruiz, C. M., Lee, M., Perry, R. H., Baumann, M., Court, J. A., and Perry, E. K. (2004). Molecular analysis of nicotinic receptor expression in autism. Mol. Brain Res. 123, 81–90. doi: 10.1016/j.molbrainres.2004.01.003
Masi, A., DeMayo, M., Glozier, N., and Guastella, A. (2017). An overview of autism spectrum disorder, heterogeneity and treatment options. Neurosci. Bull. 33, 183–193. doi: 10.1007/s12264-017-0100-y
Mastronardi, C. A., Pillai, E., Pineda, D. A., Martinez, A. F., Lopera, F., Velez, J. I., et al. (2016). Linkage and association analysis of ADHD endophenotypes in extended and multigenerational pedigrees from a genetic isolate. Mol. Psychiatry 21, 1434–1440. doi: 10.1038/mp.2015.172
Mazza, M., Pino, M. C., Mariano, M., Tempesta, D., Ferrara, M., Berardis, D. D., et al. (2014). Affective and cognitive empathy in adolescents with autism spectrum disorder. Front. Hum. Neurosci. 8:791. doi: 10.3389/fnhum.2014.00791
McCormick, D. (1989). Cholinergic and noradrenergic modulation of thalamocortical processing. Trends Neurosci. 12, 215–221. doi: 10.1016/0166-2236(89)90125-2
McCormick, D. (1992). Neurotransmitter actions in the thalamus and cerebral cortex and their role in neuromodulation of thalamocortical activity. Prog. Neurobiol. 39, 337–388. doi: 10.1016/0301-0082(92)90012-4
McCracken, J. T., Badashova, K. K., Posey, D. J., Aman, M. G., Scahill, L., Tierney, E., et al. (2014). Positive effects of methylphenidate on hyperactivity are moderated by monoaminergic gene variants in children with autism spectrum disorders. Pharmacogenomics J. 14, 295–302. doi: 10.1038/tpj.2013.23
McCracken, J. T., McGough, J., Shah, B., Cronin, P., Hong, D., Aman, M. G., et al. (2002). Risperidone in children with autism and serious behavioral problems. N. Engl. J. Med. 347, 314–321. doi: 10.1056/NEJMoa013171
McCutcheon, R. A., Nour, M. M., Dahoun, T., Jauhar, S., Pepper, F., Expert, P., et al. (2019). Mesolimbic dopamine function is related to salience network connectivity: An integrative positron emission tomography and magnetic resonance study. Biol. Psychiatry 85, 368–378. doi: 10.1016/j.biopsych.2018.09.010
McDougle, C. J., Holmes, J. P., Carlson, D. C., Pelton, G. H., Cohen, D. J., and Price, L. H. (1998). A double-blind, placebo-controlled study of risperidone in adults with autistic disorder and other pervasive developmental disorders. Arch. Gen. Psychiatry 55, 633–641. doi: 10.1001/archpsyc.55.7.633
McDougle, C. J., Scahill, L., Aman, M. G., McCracken, J. T., Tierney, E., Davies, M., et al. (2005). Risperidone for the core symptom domains of autism: Results from the study by the autism network of the research units on pediatric psychopharmacology. Am. J. Psychiatry 162, 1142–1148. doi: 10.1176/appi.ajp.162.6.1142
McEvoy, B., Hawi, Z., Fitzgerald, M., and Gill, M. (2002). No evidence of linkage or association between the norepinephrine transporter (NET) gene polymorphisms and ADHD in the Irish population. Am. J. Med. Genet. 114, 665–666. doi: 10.1002/ajmg.10416
Mehler, M. F., and Purpura, D. P. (2009). Autism, fever, epigenetics and the locus coeruleus. Brain Res. Rev. 59, 388–392. doi: 10.1016/j.brainresrev.2008.11.001
Menon, V., and Uddin, L. (2010). Saliency, switching, attention and control: A network model of insula function. Brain Struct. Funct. 214, 655–667. doi: 10.1007/s00429-010-0262-0
Mesulam, M. (2000). “Behavioral neuroanatomy: Large-scale networks, association cortex, frontal syndromes, the limbic system, and hemispheric specializations,” in Principles of behavioral and cognitive neurology, 2nd Edn, ed. M. Mesulam (New York, NY: Oxford University Press), 1–120.
Mesulam, M., and Geula, C. (1988). Nucleus basalis (Ch4) and cortical cholinergic innervation in the human brain: Observations based on the distribution of acetylcholinesterase and choline acetyltransferase. J. Comp. Neurol. 275, 216–240. doi: 10.1002/cne.902750205
Mesulam, M., Mufson, E., Levey, A., and Wainer, B. (1983). Cholinergic innervation of cortex by the basal forebrain: Cytochemistry and cortical connections of the septal area, diagonal band nuclei, nucleus basalis (Substantia innominata), and hypothalamus in the rhesus monkey. J. Comp. Neurol. 214, 170–197. doi: 10.1002/cne.902140206
Meyer, J. S., and Quenzer, L. F. (2013). Psychopharmacology: Drugs, the brain, and behavior, 2nd Edn. Sunderland: Sinauer.
Meyza, K., and Blanchard, D. (2017). The BTBR mouse model of idiopathic autism–current view on mechanisms. Neurosci. Biobehav. Rev. 76, 99–110. doi: 10.1016/j.neubiorev.2016.12.037
Misra, V. (2014). The social brain network and autism. Ann. Neurosci. 21, 69–73. doi: 10.5214/ans.0972.7531.210208
Mitoma, H., Ishida, K., Shizuka-Ikeda, M., and Mizusawa, H. (2003). Dual impairment of GABAA- and GABAB-receptor-mediated synaptic responses by autoantibodies to glutamic acid decarboxylase. J. Neurol. Sci. 208, 51–56. doi: 10.1016/S0022-510X(02)00423-9
Mizuno, A., Villalobos, M. E., Davies, M. M., Dahl, B. C., and Müller, R. (2006). Partially enhanced thalamocortical functional connectivity in autism. Brain Res. 1104, 160–174. doi: 10.1016/j.brainres.2006.05.064
Moessner, R., Marshall, C. R., Sutcliffe, J. S., Skaug, J., Pinto, D., Vincent, J., et al. (2007). Contribution of SHANK3 mutations to autism spectrum disorder. Am. J. Hum. Genet. 81, 1289–1297. doi: 10.1086/522590
Monk, C., Peltier, S., Wiggins, J., Weng, S., Carrasco, M., Risi, S., et al. (2009). Abnormalities of intrinsic functional connectivity in autism spectrum disorders. Neuroimage 47, 764–772. doi: 10.1016/j.neuroimage.2009.04.069
Moolchan, E., Ernst, M., and Henningfield, J. (2000). A review of tobacco smoking in adolescents: Treatment implications. J. Am. Acad. Child Adolesc. Psychiatry 39, 682–693. doi: 10.1097/00004583-200006000-00006
Morales, M., and Margolis, E. (2017). Ventral tegmental area: Cellular heterogeneity, connectivity and behaviour. Nat. Rev. Neurosci. 18, 73–85. doi: 10.1038/nrn.2016.165
Moseley, R. L., Ypma, R. J., Holt, R. J., Floris, D., Chura, L. R., Spencer, M. D., et al. (2015). Whole-brain functional hypoconnectivity as an endophenotype of autism in adolescents. Neuroimage Clin. 9, 140–152. doi: 10.1016/j.nicl.2015.07.015
Mouton, P., Pakkenberg, B., Gundersen, H., and Price, D. (1994). Absolute number and size of pigmented locus coeruleus neurons in young and aged individuals. J. Chem. Neuroanat. 7, 185–190. doi: 10.1016/0891-0618(94)90028-0
Mueller, A., Hong, D., Shepard, S., and Moore, T. (2017). Linking ADHD to the neural circuitry of attention. Trends Cogn. Sci. 21, 474–488. doi: 10.1016/j.tics.2017.03.009
Mukaetova-Ladinska, E., Westwood, J., and Perry, E. (2010). “Cholinergic component of autism spectrum disorder,” in The neurochemical basis of autism: From molecules to minicolumns, ed. G. Blatt (Boston, MA: Springer), 129–161.
Müller, R. (2007). The study of autism as a distributed disorder. Ment. Retard. Dev. Disabil. Res. Rev. 13, 85–95. doi: 10.1002/mrdd.20141
Müller, R., Shih, P., Keehn, B., Deyoe, J., Leyden, K., and Shukla, D. (2011). Underconnected, but how? A survey of functional connectivity MRI studies in autism spectrum disorders. Cereb. Cortex 21, 2233–2243. doi: 10.1093/cercor/bhq296
Murdaugh, D. L., Shinkareva, S. V., Deshpande, H. R., Wang, J., Pennick, M. R., and Kana, R. K. (2012). Differential deactivation during mentalizing and classification of autism based on default mode network connectivity. PLoS One 7:e50064. doi: 10.1371/journal.pone.0050064
Murray, M. J. (2010). Attention-deficit/hyperactivity disorder in the context of autism spectrum disorders. Curr. Psychiatry Rep. 12, 382–388. doi: 10.1007/s11920-010-0145-3
Murray, M. L., Hsia, Y., Glaser, K., Simonoff, E., Murphy, D. G., Asherson, P. J., et al. (2014). Pharmacological treatments prescribed to people with autism spectrum disorder (ASD) in primary health care. Psychopharmacology (Berl) 231, 1011–1021. doi: 10.1007/s00213-013-3140-7
Musser, E. D., Backs, R. W., Schmitt, C. F., Ablow, J. C., Measelle, J. R., and Nigg, J. T. (2011). Emotion regulation via the autonomic nervous system in children with attention-deficit/hyperactivity disorder (ADHD). J. Abnorm. Child Psychol. 39, 841–852. doi: 10.1007/s10802-011-9499-1
Musser, E., and Raiker, J. (2019). Attention-deficit/hyperactivity disorder: An integrated developmental psychopathology and research domain criteria (RDoC) approach. Compr. Psychiatry 90, 65–72. doi: 10.1016/j.comppsych.2018.12.016
Myer, N., Boland, J., and Faraone, S. (2018). Pharmacogenetics predictors of methylphenidate efficacy in childhood ADHD. Mol. Psychiatry 23, 1929–1936. doi: 10.1038/mp.2017.234
Nagaraj, R., Singhi, P., and Malhi, P. (2006). Risperidone in children with autism: Randomized, placebo-controlled, double-blind study. J. Child Neurol. 21, 450–455. doi: 10.1177/08830738060210060801
Nagasaka, K., Watanabe, Y., and Takashima, I. (2017). Topographical projections from the nucleus basalis magnocellularis (Meynert) to the frontal cortex: A voltage-sensitive dye imaging study in rats. Brain Stimul. 10, 977–980. doi: 10.1016/j.brs.2017.06.008
Nair, A., Treiber, J. M., Shukla, D. K., Shih, P., and Müller, R. (2013). Impaired thalamocortical connectivity in autism spectrum disorder: A study of functional and anatomical connectivity. Brain 136, 1942–1955. doi: 10.1093/brain/awt079
Narayanan, A., White, C. A., Saklayen, S., Scaduto, M. J., Carpenter, A. L., Abduljalil, A., et al. (2010). Effect of propranolol on functional connectivity in autism spectrum disorder–a pilot study. Brain Imaging Behav. 4, 189–197. doi: 10.1007/s11682-010-9098-8
Nayate, A., Bradshaw, J., and Rinehart, N. (2005). Autism and Asperger’s disorder: Are they movement disorders involving the cerebellum and/or basal ganglia? Brain Res. Bull. 67, 327–334. doi: 10.1016/j.brainresbull.2005.07.011
Nguyen, M., Roth, A., Kyzar, E. J., Poudel, M. K., Wong, K., Stewart, A. M., et al. (2014). Decoding the contribution of dopaminergic genes and pathways to autism spectrum disorder (ASD). Neurochem. Int. 66, 15–26. doi: 10.1016/j.neuint.2014.01.002
Niederhofer, H., Staffen, W., and Mair, A. (2002). Galantamine may be effective in treating autistic disorder. BMJ 325, 1422–1422.
Niederhofer, H., Staffen, W., and Mair, A. (2003). Tianeptine: A novel strategy of psychopharmacological treatment of children with autistic disorder. Hum. Psychopharmacol. 18, 389–393. doi: 10.1002/hup.491
Niklasson, L., Rasmussen, P., Oskarsdoìttir, S., and Gillberg, C. (2009). Autism, ADHD, mental retardation and behavior problems in 100 individuals with 22q11 deletion syndrome. Res. Dev. Disabil. 30, 763–773. doi: 10.1016/j.ridd.2008.10.007
Nobusako, S., Nishi, Y., Nishi, Y., Shuto, T., Asano, D., Osumi, M., et al. (2017). Transcranial direct current stimulation of the temporoparietal junction and inferior frontal cortex improves imitation-inhibition and perspective-taking with no effect on the autism-spectrum quotient score. Front. Behav. Neurosci. 11:84. doi: 10.3389/fnbeh.2017.00084
Nomi, J., and Uddin, L. (2015). Developmental changes in large-scale network connectivity in autism. Neuroimage Clin. 7, 732–741. doi: 10.1016/j.nicl.2015.02.024
Ohmura, Y., Tsutsui-Kimura, I., and Yoshioka, M. (2012). Impulsive behavior and nicotinic acetylcholine receptors. J. Pharmacol. Sci. 118, 413–422. doi: 10.1254/jphs.11R06CR
Oikonomakis, V., Kosma, K., Mitrakos, A., Sofocleous, C., Pervanidou, P., Syrmou, A., et al. (2016). Recurrent copy number variations as risk factors for autism spectrum disorders: Analysis of the clinical implications. Clin. Genet. 89, 708–718. doi: 10.1111/cge.12740
Olincy, A., Blakeley-Smith, A., Johnson, L., Kem, W. R., and Freedman, R. (2016). Brief report: Initial trial of alpha7-nicotinic receptor stimulation in two adult patients with autism spectrum disorder. J. Autism Dev. Disord. 46, 3812–3817. doi: 10.1007/s10803-016-2890-6
Olincy, A., Harris, J. G., Johnson, L. L., Pender, V., Kongs, S., Allensworth, D., et al. (2006). Proof-of-concept trial of an alpha7 nicotinic agonist in schizophrenia. Arch. Gen. Psychiatry 63, 630–638. doi: 10.1001/archpsyc.63.6.630
Orsolini, L., Tomasetti, C., Valchera, A., Vecchiotti, R., Matarazzo, I., Vellante, F., et al. (2016). An update of safety of clinically used atypical antipsychotics. Expert Opin. Drug Saf. 15, 1329–1347. doi: 10.1080/14740338.2016.1201475
Owen, R., Sikich, L., Marcus, R., Corey-Lisle, P., Manos, G., and McQuade, R. (2009). Aripiprazole in the treatment of irritability in children and adolescents with autistic disorder. Pediatrics 124, 1533–1540. doi: 10.1542/peds.2008-3782
Pacheco, J., Garvey, M., Sarampote, C., Cohen, E., Murphy, E., and Friedman-Hill, S. (2022). Annual research review: The contributions of the RDoC research framework on understanding the neurodevelopmental origins, progression and treatment of mental illnesses. J. Child Psychol. Psychiatry 63, 360–376. doi: 10.1111/jcpp.13543
Park, S., Park, J., Cho, S., Kim, B., Shin, M., Kim, J., et al. (2014). No association of the norepinephrine transporter gene (SLC6A2) and cognitive and behavioural phenotypes of patients with autism spectrum disorder. Eur. Arch. Psychiatry Clin. Neurosci. 264, 507–515. doi: 10.1007/s00406-013-0480-6
Passarelli, F., Donfrancesco, R., Nativio, P., Pascale, E., Trani, M. D., Patti, A. M., et al. (2013). Anti-Purkinje cell antibody as a biological marker in attention deficit/hyperactivity disorder: A pilot study. J. Neuroimmunol. 258, 67–70. doi: 10.1016/j.jneuroim.2013.02.018
Pavãl, D. (2017). A dopamine hypothesis of autism spectrum disorder. Dev. Neurosci. 39, 355–360. doi: 10.1159/000478725
Pearson, D. A., Santos, C. W., Aman, M. G., Arnold, L. E., Casat, C. D., Mansour, R., et al. (2013). Effects of extended release methylphenidate treatment on ratings of attention-deficit/hyperactivity disorder (ADHD) and associated behavior in children with autism spectrum disorders and ADHD symptoms. J. Child Adolesc. Psychopharmacol. 23, 337–351. doi: 10.1089/cap.2012.0096
Pellissier, L., Gandiìa, J., Laboute, T., Becker, J., and Le Merrer, J. (2018). μ opioid receptor, social behaviour and autism spectrum disorder: Reward matters. Br. J. Pharmacol. 175, 2750–2769. doi: 10.1111/bph.13808
Perry, E. K., Lee, M. L., Martin-Ruiz, C. M., Court, J. A., Volsen, S. G., Merrit, J., et al. (2001). Cholinergic activity in autism: Abnormalities in the cerebral cortex and basal forebrain. Am. J. Psychiatry 158, 1058–1066. doi: 10.1176/appi.ajp.158.7.1058
Peters, S., Dunlop, K., and Downar, J. (2016). Cortico-striatal-thalamic loop circuits of the salience network: A central pathway in psychiatric disease and treatment. Front. Syst. Neurosci. 10:104. doi: 10.3389/fnsys.2016.00104
Phillis, J. (1968). Acetylcholine release from the cerebral cortex: Its role in cortical arousal. Brain Res. 7, 378–389. doi: 10.1016/0006-8993(68)90004-8
Picard, F., Sadaghiani, S., Leroy, C., Courvoisier, D. S., Maroy, R., and Bottlaender, M. (2013). High density of nicotinic receptors in the cingulo-insular network. Neuroimage 79, 42–51. doi: 10.1016/j.neuroimage.2013.04.074
Pineda-Cirera, L., Shivalikanjli, A., Cabana-Domínguez, J., Demontis, D., Rajagopal, V., Børglum, A., et al. (2019). Exploring genetic variation that influences brain methylation in attention-deficit/hyperactivity disorder–translational psychiatry. Transl. Psychiatry 9:242. doi: 10.1038/s41398-019-0574-7
Pinto, D., Pagnamenta, A. T., Klei, L., Anney, R., Merico, D., Regan, R., et al. (2010). Functional impact of global rare copy number variation in autism spectrum disorders. Nature 466, 368–372. doi: 10.1038/nature09146
Politte, L. C., Scahill, L., Figueroa, J., McCracken, J. T., King, B., and McDougle, C. J. (2018). A randomized, placebo-controlled trial of extended-release guanfacine in children with autism spectrum disorder and ADHD symptoms: An analysis of secondary outcome measures. Neuropsychopharmacology 43, 1772–1778. doi: 10.1038/s41386-018-0039-3
Pomerleau, O., Downey, K., Stelson, F., and Pomerleau, C. (1995). Cigarette smoking in adult patients diagnosed with attention deficit hyperactivity disorder. J. Subst. Abuse 7, 373–378. doi: 10.1016/0899-3289(95)90030-6
Potter, A., and Newhouse, P. (2004). Effects of acute nicotine administration on behavioral inhibition in adolescents with attention-deficit/hyperactivity disorder. Psychopharmacology (Berl) 176, 183–194. doi: 10.1007/s00213-004-1874-y
Potter, A., Newhouse, P., and Bucci, D. (2006). Central nicotinic cholinergic systems: A role in the cognitive dysfunction in attention-deficit/hyperactivity disorder? Behav. Brain Res. 175, 201–211. doi: 10.1016/j.bbr.2006.09.015
Potter, A., Schaubhut, G., and Shipman, M. (2014). Targeting the nicotinic cholinergic system to treat attention-deficit/hyperactivity disorder: Rationale and progress to date. CNS Drugs 28, 1103–1113. doi: 10.1007/s40263-014-0208-9
Purper-Ouakil, D., Ramoz, N., Lepagnol-Bestel, A., Gorwood, P., and Simonneau, M. (2011). Neurobiology of attention deficit/hyperactivity disorder. Pediatr. Res. 69, 69–76. doi: 10.1203/PDR.0b013e318212b40f
Quintana, H., Birmaher, B., Stedge, D., Lennon, S., Freed, J., Bridge, J., et al. (1995). Use of methylphenidate in the treatment of children with autistic disorder. J. Autism Dev. Disord. 25, 283–294. doi: 10.1007/BF02179289
Ragozzino, M. E., Pal, S. N., Unick, K., Stefani, M. R., and Gold, P. E. (1998). Modulation of hippocampal acetylcholine release and spontaneous alternation scores by intrahippocampal glucose injections. J. Neurosci. 18, 1595–1601. doi: 10.1523/JNEUROSCI.18-04-01595.1998
Raichle, M. (2015). The Brain’s default mode network. Annu. Rev. Neurosci. 38, 433–447. doi: 10.1146/annurev-neuro-071013-014030
Ray, M. A., Graham, A. J., Lee, M., Perry, R. H., Court, J. A., and Perry, E. K. (2005). Neuronal nicotinic acetylcholine receptor subunits inautism: An immunohistochemical investigation in the thalamus. Neurobiol. Dis. 19, 366–377. doi: 10.1016/j.nbd.2005.01.017
Remington, G., Sloman, L., Konstantareas, M., Parker, K., and Gow, R. (2001). Clomipramine versus haloperidol in the treatment of autistic disorder: A double-blind, placebo-controlled, crossover study. J. Clin. Psychopharmacol. 21, 440–444. doi: 10.1097/00004714-200108000-00012
Research Units on Pediatric Psychopharmacology Autism Network [RUPPAN] (2005). Randomized, controlled, crossover trial of methylphenidate in pervasive developmental disorders with hyperactivity. Arch. Gen. Psychiatry 62, 1266–1274. doi: 10.1001/archpsyc.62.11.1266
Rinne, U. K., Laihinen, A., Rinne, J. O., Någren, K., Bergman, J., and Ruotsalainen, U. (1990). Positron emission tomography demonstrates dopamine D2 receptor supersensitivity in the striatum of patients with early Parkinson’s disease. Mov. Disord. 5, 55–59. doi: 10.1002/mds.870050114
Robbins, T. (2000). Chemical neuromodulation of frontal-executive functions in humans and other animals. Exp. Brain Res. 133, 130–138. doi: 10.1007/s002210000407
Robbins, T., and Arnsten, A. (2009). The neuropsychopharmacology of Fronto-executive function: Monoaminergic modulation. Annu. Rev. Neurosci. 32, 267–287. doi: 10.1146/annurev.neuro.051508.135535
Robertson, S., Plummer, N., de Marchena, J., and Jensen, P. (2013). Developmental origins of central norepinephrine neuron diversity. Nat. Neurosci. 16, 1016–1023. doi: 10.1038/nn.3458
Robinson, P. D., Schutz, C. K., Macciardi, F., White, B. N., and Holden, J. J. (2001). Genetically determined low maternal serum dopamine β-hydroxylase levels and the etiology of autism spectrum disorders. Am. J. Med. Genet. 100, 30–36. doi: 10.1002/ajmg.1187
Rommelse, N. N., Geurts, H. M., Franke, B., Buitelaar, J. K., and Hartman, C. A. (2011). A review on cognitive and brain endophenotypes that may be common in autism spectrum disorder and attention-deficit/hyperactivity disorder and facilitate the search for pleiotropic genes. Neurosci. Biobehav. Rev. 35, 1363–1396. doi: 10.1016/j.neubiorev.2011.02.015
Ronald, A., Simonoff, E., Kuntsi, J., Asherson, P., and Plomin, R. (2008). Evidence for overlapping genetic influences on autistic and ADHD behaviours in a community twin sample. J. Child Psychol. Psychiatry 49, 535–542. doi: 10.1111/j.1469-7610.2007.01857.x
Ross, R. (2012). Advances in the genetics of ADHD. Am. J. Psychiatry 169, 115–117. doi: 10.1176/appi.ajp.2011.11111647
Rossi, M. A., Sukharnikova, T., Hayrapetyan, V. Y., Yang, L., and Yin, H. H. (2013). Operant self-stimulation of dopamine neurons in the substantia nigra. PLoS One 8:e65799. doi: 10.1371/journal.pone.0065799
Rout, U., Mungan, N., and Dhossche, D. (2012). Presence of GAD65 autoantibodies in the serum of children with autism or ADHD. Eur. Child Adolesc. Psychiatry 21, 141–147. doi: 10.1007/s00787-012-0245-1
Sadaghiani, S., Ng, B., Altmann, A., Poline, J., Banaschewski, T., Bokde, A. L., et al. (2017). Overdominant effect of a CHRNA4 polymorphism on cingulo-opercular network activity and cognitive control. J. Neurosci. 37, 9657–9666. doi: 10.1523/JNEUROSCI.0991-17.2017
Sagar-Ouriaghli, I., Lievesley, K., and Santosh, P. (2018). Propranolol for treating emotional, behavioural, autonomic dysregulation in children and adolescents with autism spectrum disorders. J. Psychopharmacol. (Oxf) 32, 641–653. doi: 10.1177/0269881118756245
Salatino-Oliveira, A., Rohde, L., and Hutz, M. (2018). The dopamine transporter role in psychiatric phenotypes. Am. J. Med. Genet. B Neuropsychiatr. Genet. 177, 211–231. doi: 10.1002/ajmg.b.32578
Salgado, J. V., Costa-Silva, M., Malloy-Diniz, L. F., Siqueira, J. M., and Teixeira, A. L. (2007). Prefrontal cognitive dysfunction following brainstem lesion. Clin. Neurol. Neurosurg. 109, 379–382. doi: 10.1016/j.clineuro.2007.01.002
Sanchez, L. E., Campbell, M., Small, A. M., Cueva, J. E., Armenteros, J. L., and Adams, P. B. (1996). A pilot study of clomipramine in young autistic children. J. Am. Acad. Child Adolesc. Psychiatry 35, 537–544. doi: 10.1097/00004583-199604000-00021
Sander, D., Grafman, J., and Zalla, T. (2003). The human amygdala: An evolved system for relevance detection. Rev. Neurosci. 14, 303–316. doi: 10.1515/revneuro.2003.14.4.303
Scahill, L., McCracken, J. T., King, B. H., Rockhill, C., Shah, B., Politte, L., et al. (2015). Extended-release guanfacine for hyperactivity in children with autism spectrum disorder. Am. J. Psychiatry 172, 1197–1206. doi: 10.1176/appi.ajp.2015.15010055
Schreibman, L., Dawson, G., Stahmer, A. C., Landa, R., Rogers, S. J., McGee, G. G., et al. (2015). Naturalistic developmental behavioral interventions: Empirically validated treatments for autism spectrum disorder. J. Autism Dev. Disord. 45, 2411–2428. doi: 10.1007/s10803-015-2407-8
Schutte, I., Deschamps, P., van Harten, P., and Kenemans, J. (2020). Dopaminergic and noradrenergic manipulation of anticipatory reward and probability event-related potentials. Psychopharmacology (Berl) 237, 2019–2030. doi: 10.1007/s00213-020-05515-x
Schwarz, L. A., Miyamichi, K., Gao, X. J., Beier, K. T., Weissbourd, B., DeLoach, K. E., et al. (2015). Viral-genetic tracing of the input–output organization of a central noradrenaline circuit. Nature 524, 88–92. doi: 10.1038/nature14600
Seeley, W. W., Menon, V., Schatzberg, A. F., Keller, J., Glover, G. H., Kenna, H., et al. (2007). Dissociable intrinsic connectivity networks for salience processing and executive control. J. Neurosci. 27, 2349–2356. doi: 10.1523/JNEUROSCI.5587-06.2007
Sengupta, S. M., Grizenko, N., Thakur, G. A., Bellingham, J., DeGuzman, R., Robinson, S., et al. (2012). Differential association between the norepinephrine transporter gene and ADHD: Role of sex and subtype. J. Psychiatry Neurosci. 37, 129–137. doi: 10.1503/jpn.110073
Shea, S., Turgay, A., Carroll, A., Schulz, M., Orlik, H., Smith, I., et al. (2004). Risperidone in the treatment of disruptive behavioral symptoms in children with autistic and other pervasive developmental disorders. Pediatrics 114, e634–e641. doi: 10.1542/peds.2003-0264-F
Shu, S. Y., Jiang, G., Zheng, Z., Ma, L., Wang, B., Zeng, Q., et al. (2019). A new neural pathway from the ventral striatum to the nucleus basalis of Meynert with functional implication to learning and memory. Mol. Neurobiol. 56, 7222–7233. doi: 10.1007/s12035-019-1588-0
Sidlauskaite, J., Sonuga-Barke, E., Roeyers, H., and Wiersema, J. (2016). Altered intrinsic organisation of brain networks implicated in attentional processes in adult attention-deficit/hyperactivity disorder: A resting state study of attention, default mode and salience network connectivity. Eur. Arch. Psychiatry Clin. Neurosci. 266, 349–357. doi: 10.1007/s00406-015-0630-0
Silvetti, M., Wiersema, J., Sonuga-Barke, E., and Verguts, T. (2013). Deficient reinforcement learning in medial frontal cortex as a model of dopamine-related motivational deficits in ADHD. Neural Netw. 46, 199–209. doi: 10.1016/j.neunet.2013.05.008
Simonoff, E., Pickles, A., Charman, T., Chandler, S., Loucas, T., and Baird, G. (2008). Psychiatric disorders in children with autism spectrum disorders: Prevalence, comorbidity, and associated factors in a population-derived sample. J. Am. Acad. Child Adolesc. Psychiatry 47, 921–929. doi: 10.1097/CHI.0b013e318179964f
Sinkus, M. L., Graw, S., Freedman, R., Ross, R. G., Lester, H. A., and Leonard, S. (2015). The human CHRNA7 and CHRFAM7A genes: A review of the genetics, regulation, and function. Neuropharmacology 96, 274–288. doi: 10.1016/j.neuropharm.2015.02.006
Sinzig, J., Bruning, N., Morsch, D., and Lehmkuhl, G. (2008). Attention profiles in autistic children with and without comorbid hyperactivity and attention problems. Acta Neuropsychiatr. 20, 207–215. doi: 10.1111/j.1601-5215.2008.00292.x
Sokhadze, E., Casanova, M., El-Baz, A., Farag, H., Li, X., and Wang, Y. (2016). TMS-based neuromodulation of evoked and induced gamma oscillations and event-related potentials in children with autism. NeuroRegulation 3:101. doi: 10.15540/nr.3.3.101
Sokol, D., Dunn, D., Edwards-Brown, M., and Feinberg, J. (2002). Hydrogen proton magnetic resonance spectroscopy in autism: Preliminary evidence of elevated choline/creatine ratio. J. Child Neurol. 17, 245–249. doi: 10.1177/088307380201700401
Sousa, N. O., Grevet, E. H., Salgado, C. A., Silva, K. L., Victor, M. M., Karam, R. G., et al. (2011). Smoking and ADHD: An evaluation of self medication and behavioral disinhibition models based on comorbidity and personality patterns. J. Psychiatr Res. 45, 829–834. doi: 10.1016/j.jpsychires.2010.10.012
Southgate, V., and Hamilton, A. F. (2008). Unbroken mirrors: Challenging a theory of autism. Trends Cogn. Sci. 12, 225–229. doi: 10.1016/j.tics.2008.03.005
Sridharan, D., Levitin, D., and Menon, V. (2008). A critical role for the right Fronto-insular cortex inswitching between central-executive and default-mode networks. Proc. Natl. Acad. Sci. U.S.A. 105, 12569–12574. doi: 10.1073/pnas.0800005105
Staal, W., de Krom, M., and de Jonge, M. (2012). Brief report: The dopamine-3-receptor gene (DRD3) is associated with specific repetitive behavior in autism spectrum disorder (ASD). J. Autism Dev. Disord. 42, 885–888. doi: 10.1007/s10803-011-1312-z
Stahl, S. (2008). Stahl’s essential psychopharmacology: Neuroscientific basis and practical application, 3rd Edn. Cambridge: Cambridge University Press.
Stahl, S. (2013). Stahl’s essential psychopharmacology: Neuroscientific basis and practical applications, 4th Edn. Cambridge: Cambridge University Press.
Stark, R., Bauer, E., Merz, C. J., Zimmermann, M., Reuter, M., Plichta, M. M., et al. (2011). ADHD related behaviors are associated with brain activation in the reward system. Neuropsychologia 49, 426–434. doi: 10.1016/j.neuropsychologia.2010.12.012
Stevens, T., Peng, L., and Barnard-Brak, L. (2016). The comorbidity of ADHD in children diagnosed with autism spectrum disorder. Res. Autism Spectr. Disord. 31, 11–18. doi: 10.1016/j.rasd.2016.07.003
Stoner, R., Chow, M. L., Boyle, M. P., Sunkin, S. M., Mouton, P. R., Roy, S., et al. (2014). Patches of disorganization in the neocortex of children with autism. N. Engl. J. Med. 370, 1209–1219. doi: 10.1056/NEJMoa1307491
Strafella, A., Paus, T., Barrett, J., and Dagher, A. (2001). Repetitive transcranial magnetic stimulation of the human prefrontal cortex induces dopamine release in the caudate nucleus. J. Neurosci. 21, RC157–RC157. doi: 10.1523/JNEUROSCI.21-15-j0003.2001
Sturman, N., Deckx, L., and van Driel, M. (2017). Methylphenidate for children and adolescents with autism spectrum disorder. Cochrane Database Syst. Rev. 11, 1465–1858. doi: 10.1002/14651858.CD011144.pub2
Svensson, T., Bunney, B., and Aghajanian, G. (1975). Inhibition of both noradrenergic and serotonergic neurons in brain by the α-adrenergic agonist clonidine. Brain Res. 92, 291–306. doi: 10.1016/0006-8993(75)90276-0
Szabadi, E. (2013). Functional neuroanatomy of the central noradrenergic system. J. Psychopharmacol. (Oxf) 27, 659–693. doi: 10.1177/0269881113490326
Szerb, J. (1967). Cortical acetylcholine release and electroencephalographic arousal. J. Physiol. 192, 329–343. doi: 10.1113/jphysiol.1967.sp008303
Tager-Flusberg, H. (2006). Defining language phenotypes in autism. Clin. Neurosci. Res. 6, 219–224. doi: 10.1016/j.cnr.2006.06.007
Taurines, R., Grünblatt, E., Schecklmann, M., Schwenck, C., Albantakis, L., Reefschläger, L., et al. (2011). Altered mRNA expression of monoaminergic candidate genes in the blood of children with attention deficit hyperactivity disorder and autism spectrum disorder. World J. Biol. Psychiatry 12, 104–108. doi: 10.3109/15622975.2011.600297
Todd, R., Lobos, E., Sun, L., and Neuman, R. (2003). Mutational analysis of the nicotinic acetylcholine receptor alpha 4 subunit gene in attention deficit/hyperactivity disorder: Evidence for association of an intronic polymorphism with attention problems. Mol. Psychiatry 8, 103–108. doi: 10.1038/sj.mp.4001257
Tomasi, D., and Volkow, N. (2012). Abnormal functional connectivity in children with attention- deficit/hyperactivity disorder. Biol. Psychiatry 71, 443–450. doi: 10.1016/j.biopsych.2011.11.003
Tong, J., McKinley, L., Cummins, T. D., Johnson, B., Matthews, N., Vance, A., et al. (2015). Identification and functional characterisation of a novel dopamine beta hydroxylase gene variant associated with attention deficit hyperactivity disorder. World J. Biol. Psychiatry 16, 610–618. doi: 10.3109/15622975.2015.1036771
Tonhajzerova, I., Ondrejka, I., Adamik, P., Hruby, R., Javorka, M., Trunkvalterova, Z., et al. (2009). Changes in the cardiac autonomic regulation in children with attention deficit hyperactivity disorder (ADHD). Indian J. Med. Res. 130, 44–50.
Toro, R., Fox, P., and Paus, T. (2008). Functional coactivation map of the human brain. Cereb. Cortex 18, 2553–2559. doi: 10.1093/cercor/bhn014
Totah, N., Neves, R., Panzeri, S., Logothetis, N., and Eschenko, O. (2018). The locus coeruleus is a complex and differentiated neuromodulatory system. Neuron 99, 1055–1068.e6. doi: 10.1016/j.neuron.2018.07.037
Tripp, G., and Wickens, J. (2009). Neurobiology of ADHD. Neuropharmacology 57, 579–589. doi: 10.1016/j.neuropharm.2009.07.026
Troost, P. W., Lahuis, B. E., Steenhuis, M., Ketelaars, C. E., Buitelaar, J. K., van Engeland, H, et al. (2005). Long-term effects of risperidone in children with autism spectrum disorders: A placebo discontinuation study. J. Am. Acad. Child Adolesc. Psychiatry 44, 1137–1144. doi: 10.1097/01.chi.0000177055.11229.76
Uddin, L., and Menon, V. (2009). The anterior insula in autism: Under-connected and under-examined. Neurosci. Biobehav. Rev. 33, 1198–1203. doi: 10.1016/j.neubiorev.2009.06.002
Uddin, L., Supekar, K., and Menon, V. (2013). Reconceptualizing functional brain connectivity in autism from a developmental perspective. Front. Hum. Neurosci. 7:458. doi: 10.3389/fnhum.2013.00458
Valbonesi, S., Magri, C., Traversa, M., Faraone, S. V., Cattaneo, A., Milanesi, E., et al. (2015). Copy number variants in attention-deficit hyperactive disorder: Identification of the 15q13 deletion and its functional role. Psychiatr. Genet. 25, 59–70. doi: 10.1097/YPG.0000000000000056
van Steensel, F., Bögels, S., and de Bruin, E. (2013). Psychiatric comorbidity in children with autism spectrum disorders: A comparison with children with ADHD. J. Child Fam. Stud. 22, 368–376. doi: 10.1007/s10826-012-9587-z
Varni, J. W., Handen, B. L., Corey-Lisle, P. K., Guo, Z., Manos, G., Ammerman, D. K., et al. (2012). Effect of aripiprazole 2 to 15 mg/d on health-related quality of life in the treatment of irritability associated with autistic disorder in children: A post hoc analysis of two controlled trials. Clin. Ther. 34, 980–992. doi: 10.1016/j.clinthera.2012.02.023
Wall, D. P., Esteban, F. J., Deluca, T. F., Huyck, M., Monaghan, T., Mendizabal, N. V., et al. (2009). Comparative analysis of neurological disorders focuses genome-wide search for autism genes. Genomics 93, 120–129. doi: 10.1016/j.ygeno.2008.09.015
Wallis, D., Arcos-Burgos, M., Jain, M., Castellanos, F. X., Palacio, J. D., Pineda, D., et al. (2009). Polymorphisms in the neural nicotinic acetylcholine receptor α4 subunit (CHRNA4) are associated with ADHD in a genetic isolate. Atten. Defic. Hyperact. Disord. 1, 19–24. doi: 10.1007/s12402-009-0003-5
Wang, L., Almeida, L. E., Spornick, N. A., Kenyon, N., Kamimura, S., Khaibullina, A., et al. (2015). Modulation of social deficits and repetitive behaviors in a mouse model of autism: The role of the nicotinic cholinergic system. Psychopharmacology (Berl) 232, 4303–4316. doi: 10.1007/s00213-015-4058-z
Wang, R., Lin, P., and Wu, Y. (2015). “Exploring dynamic temporal-topological structure of brain network within ADHD,” in Advances in cognitive neurodynamics (IV), ed. H. Liljenström (Dordrecht: Springer), 93–98.
Wang, X., Jiao, Y., and Li, L. (2018). Identifying individuals with attention deficit hyperactivity disorder based on temporal variability of dynamic functional connectivity. Sci. Rep. 8:11789. doi: 10.1038/s41598-018-30308-w
Wass, S. (2011). Distortions and disconnections: Disrupted brain connectivity in autism. Brain Cogn. 75, 18–28. doi: 10.1016/j.bandc.2010.10.005
Watabe-Uchida, M., Zhu, L., Ogawa, S. K., Vamanrao, A., and Uchida, N. (2012). Whole-brain mapping of direct inputs to midbrain dopamine neurons. Neuron 74, 858–873. doi: 10.1016/j.neuron.2012.03.017
Whitby, P., and Mancil, G. (2009). Academic achievement profiles of children with high functioningautism and asperger syndrome: A review of the literature. Educ. Train. Dev. Disabil. 44, 551–560.
Whitney, E. R., Kemper, T. L., Rosene, D. L., Bauman, M. L., and Blatt, G. J. (2009). Density of cerebellar basket and stellate cells in autism: Evidence for a late developmental loss of Purkinje cells. J. Neurosci. Res. 87, 2245–2254. doi: 10.1002/jnr.22056
Wichers, R. H., Findon, J. L., Jelsma, A., Giampietro, V., Stoencheva, V., Robertson, D. M., et al. (2021). Modulation of atypical brain activation during executive functioning in autism: A pharmacological MRI study of tianeptine. Mol. Autism 12:14. doi: 10.1186/s13229-021-00422-0
Wigg, K., Zai, G., Schachar, R., Tannock, R., Roberts, W., Malone, M., et al. (2002). Attention deficit hyperactivity disorder and the gene for dopamine beta-hydroxylase. Am. J. Psychiatry 159, 1046–1048. doi: 10.1176/appi.ajp.159.6.1046
Wilens, T. (2008). Effects of methylphenidate on the catecholaminergic system in attention- deficit/hyperactivity disorder. J. Clin. Psychopharmacol. 28:S46. doi: 10.1097/JCP.0b013e318173312f
Williams, N. M., Franke, B., Mick, E., Anney, R. J., Freitag, C. M., Gill, M., et al. (2012). Genome-wide analysis of copy number variants in attention deficit hyperactivity disorder: The role of rare variants and duplications at 15q13.3. Am. J. Psychiatry 169, 195–204. doi: 10.1176/appi.ajp.2011.11060822
Woolf, N., and Butcher, L. (1982). Cholinergic projections to the basolateral amygdala: A combined Evans Blue and acetylcholinesterase analysis. Brain Res. Bull. 8, 751–763. doi: 10.1016/0361-9230(82)90102-2
Xing, B., Li, Y., and Gao, W. (2016). Norepinephrine versus dopamine and their interaction in modulating synaptic function in the prefrontal cortex. Brain Res. 1641, 217–233. doi: 10.1016/j.brainres.2016.01.005
Yang, L., Wang, Y., Li, J., and Faraone, S. (2004). Association of norepinephrine transporter gene with methylphenidate response. J. Am. Acad. Child Adolesc. Psychiatry 43, 1154–1158. doi: 10.1097/01.chi.0000131134.63368.46
Yrigollen, C. M., Han, S. S., Kochetkova, A., Babitz, T., Chang, J. T., Volkmar, F. R., et al. (2008). Genes controlling affiliative behavior as candidate genes for autism. Biol. Psychiatry 63, 911–916. doi: 10.1016/j.biopsych.2007.11.015
Zamzow, R. M., Christ, S. E., Saklayen, S. S., Moffitt, A. J., Bodner, K. E., Higgins, K. F., et al. (2014). Effect of propranolol on facial scanning in autism spectrum disorder: A preliminary investigation. J. Clin. Exp. Neuropsychol. 36, 431–445. doi: 10.1080/13803395.2014.904844
Zamzow, R. M., Ferguson, B. J., Ragsdale, A. S., Lewis, M. L., and Beversdorf, D. Q. (2017). Effects of acute beta-adrenergic antagonism on verbal problem solving in autism spectrum disorder and exploration of treatment response markers. J. Clin. Exp. Neuropsychol. 39, 596–606. doi: 10.1080/13803395.2016.1252724
Zamzow, R. M., Ferguson, B. J., Stichter, J. P., Porges, E. C., Ragsdale, A. S., Lewis, M. L., et al. (2016). Effects of propranolol on conversational reciprocity in autism spectrum disorder: A pilot, double-blind, single-dose psychopharmacological challenge study. Psychopharmacology (Berl) 233, 1171–1178. doi: 10.1007/s00213-015-4199-0
Zerbi, V., Floriou-Servou, A., Markicevic, M., Vermeiren, Y., Sturman, O., Privitera, M., et al. (2019). Rapid reconfiguration of the functional connectome after chemogenetic locus coeruleus activation. Neuron 103, 702–718.e5. doi: 10.1016/j.neuron.2019.05.034
Zhang, H., Wang, Y., Li, J., Wang, B., and Yang, L. (2004). Association of dopamine beta-hydroxylase polymorphism with attention deficit hyperactivity disorder in children. J. Peking Univ. Health Sci. 36, 290–293.
Zhang, Y., Larcher, K., Misic, B., and Dagher, A. (2017). Anatomical and functional organization of the human substantia nigra and its connections. Elife 6:e26653. doi: 10.7554/eLife.26653
Zhu, H., Li, J., Fan, Y., Li, X., Huang, D., and He, S. (2015). Atypical prefrontal cortical responses to joint/non-joint attention in children with autism spectrum disorder (ASD): A functional near-infrared spectroscopy study. Biomed. Opt. Express 6, 690–701. doi: 10.1364/BOE.6.000690
Keywords: dopamine, norepinephrine, acetylcholine, cerebellum, Purkinje cells, genetics, salience network, biomarkers
Citation: Koevoet D, Deschamps PKH and Kenemans JL (2023) Catecholaminergic and cholinergic neuromodulation in autism spectrum disorder: A comparison to attention-deficit hyperactivity disorder. Front. Neurosci. 16:1078586. doi: 10.3389/fnins.2022.1078586
Received: 05 September 2022; Accepted: 15 December 2022;
Published: 06 January 2023.
Edited by:
Juan J. Canales, Victoria University of Wellington, New ZealandReviewed by:
Yuyang Luo, Massachusetts Eye and Ear Infirmary and Harvard Medical School, United StatesDavid Quentin Beversdorf, University of Missouri, United States
Copyright © 2023 Koevoet, Deschamps and Kenemans. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Damian Koevoet,  damiankoevoet@gmail.com
damiankoevoet@gmail.com
 Damian Koevoet
Damian Koevoet P. K. H. Deschamps
P. K. H. Deschamps J. L. Kenemans
J. L. Kenemans