- 1Astral Systems, Bristol, United Kingdom
- 2HH Wills Physics Laboratory, Interface Analysis Centre, University of Bristol, Bristol, United Kingdom
- 3AWE Nuclear Security Technologies, Reading, United Kingdom
Nuclear forensic science aims to correlate measurable parameters to the processing history of nuclear materials to support law enforcement investigations. Controlled studies on elemental fractionation with processing are valued on materials of known provenance to validate methods and signatures. There is need to understand how useful current applied techniques are when applied to thorium materials. In this study, we discuss the potential nuclear forensic signatures in thorium materials and report an academic study processing a monazite ore of known provenance through a historic industrial process to thorium dioxide. The measurements traced a variety of ‘fingerprint’ material properties and impurities through the processing route. It was shown that radiometric methods, relative rare earth element abundances, impurities, radiochronometry and microscopy were useful for characterising the material.
Introduction
Nuclear forensic science aims to support law enforcement investigations into nuclear and radiological materials found outside of regulatory control (Mayer et al., 2013; Varga et al., 2022). The state of practice is widely reported, applying a multidisciplinary and iterative approach to defensibly measure parameters of interest from collected sample materials whilst maintaining quality control (Taylor et al., 2020). Typically, non-destructive assay of the material will be applied, followed by traditional forensics, and wet chemistry techniques depending on investigative priorities. Analytical techniques may be used to answer specific questions or be used to provide data to compare between exhibits, places, historical records and inform assessments.
The nuclear forensic analysis of thorium materials is underreported compared to literature reports for pre- and post-irradiation uranium and plutonium materials (Higginson et al., 2022; Rim et al., 2017; Keegan et al., 2016). Because thorium has low specific activity per unit mass and it is fertile rather than fissile, it is an indirect use material as defined by the International Atomic Energy Agency (IAEA) (IAEA, 2005). There are well established uses for thorium material in industry, generally for its high melting point, density, physical and materials properties. These uses include gas mantles, mixed oxide fuel, welding rod coatings, ceramics, arc lamps, and as a dopant in glass lenses and within premium alloys (IAEA, 2005). There are reported incidents involving thorium materials outside of regulatory control and one reported nuclear forensic case study in the literature (Moody and Grant, 1999). The limited literature to date drives the aim of this work to complete a series of studies to investigate thorium material processing for nuclear forensics. Herein we report a laboratory scale recreation and analysis of a processing pathway historically used in the UK to process bulk ore to thorium dioxide product to understand the application of current techniques.
In this study an ore was provided by the British Geological Survey (BGS) and was originally sourced from Kerala (referred to as Travancore at the time of sample collection) in SW India. Through archival study, this is believed to be one of the mining sites which provided material for the initial UK test reactor experiments (Jamrack, 2025). We applied a series of analytical techniques to evaluate the measurable materials parameters as the material was processed utilizing tools available in an academic research laboratory. These measurements include physical characteristics, Rare Earth Element (REE) ratios, gamma and alpha spectrometry, trace impurities by application of Inductively Coupled Plasma Optical Emission Spectroscopy (ICP-OES) data.
Introduction to the Th fuel cycle and potential nuclear forensic signatures
At present, there is a renaissance in research scale activities involving thorium-containing materials and the development and scale up of thorium fuel cycles in certain countries worldwide (Ault et al., 2017; Evitts et al., 2021). Several nations also have a legacy of Th processing going back to the start of the nuclear era, operating as large-scale producers of thorium materials for prototype thorium reactors, along with international sellers ultilising various process technologies operated at a smaller scale for industrial applications (IAEA, 2005). Thorium is typically recovered from ore by a series of chemical processes to produce an initial ore concentrate. This material is then further purified to a final form of metal, oxide, ceramic or salt. These materials may also then be blended with other actinides as mixed oxide samples and/or be produced as a fuel for nuclear applications.
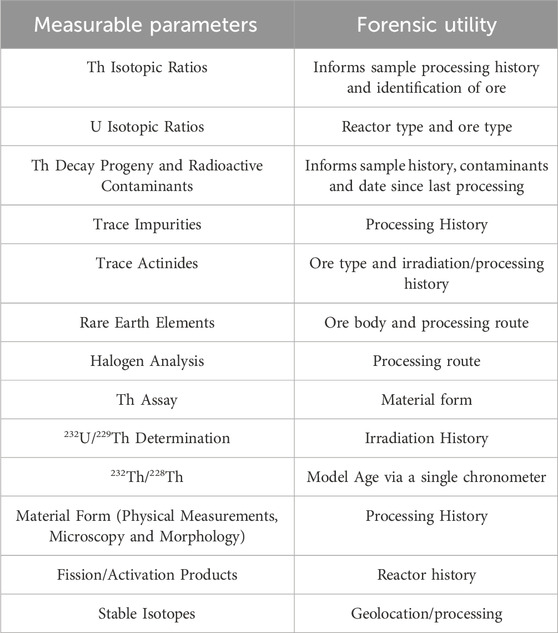
It is important to initially review the potential nuclear forensic signatures in thorium materials and the analytical methods available in standard laboratories to be applied. There are reported methods for the analytical radiochemistry and analysis of thorium-containing materials that can be applied for nuclear forensics and fuel development purposes (Shen et al., 2002; Hyde, 1959; Kayzar-Boggs et al., 2021). There is also academic measurement interest, for example, in earth and planetary sciences (Allègre et al., 1986). There are several relevant thorium isotopic and assay reference materials that can be used to underpin measurement traceability to validate analytical methods and ensure defensibility of results (Holmes, 2001; Essex et al., 2018). Studies on the impact of isotopic signatures in thorium/uranium mixed fuels are a recent highlight to understand post irradiation signatures of interest compared to standard nuclear forensic approaches (Evitts et al., 2021). Recent work has also explored the radio-chronometric analysis of thorium decay systems for age dating of processed nuclear materials within decades of the last date of purification or irradiation (Kayzar-Boggs et al., 2021).
Nuclear forensic science needs to consider and understand what measurable parameters are possible for thorium materials as they are processed and used for industrial applications (Ault et al., 2017; IAEA, 2005). The validation of these measurements with materials of known provenance can underpin the science basis to support investigative questions. There are inherent progeny species in a nuclear material, as well as species generated from neutron irradiation and purification of fissile and fertile materials in irradiation facilities. There are also potential contaminant species residual from the initial ore or introduced by anthropogenic processing activities. The relative quantities of contaminants measured in a sample can be indicative of the chemical techniques used to process the material.
The historic use of thorium in nuclear fuels means there are well defined compositional (elemental) specifications as well as archive analytical data that can be drawn upon to inform a forensic analysis of any intercepted illicit thorium materials. A few of these analytes (e.g., Th, U, P, S, REE) could be major or minor impurities requiring different analytical methods depending on the stage in the fuel cycle. Based on the reported specifications for thorium oxide in reactor fuel, example impurities include U, Cd, Li, B, Ca, Fe, Si, Na, Ce, Eu, Gd, Ho, Lu, Tb, Al, Ti, Ba, Tl, In, Rb, Cl, Mn, Nb, N, Cu, Cr, Mg, Ni, Ga and Mo (Ade et al., 2014; Oecd, 2001). Specifications for thorium purified from monazite and other uranium bearing ores are also available to derive a list of analytes to measure in early fuel cycle materials (Tuovinen et al., 2015). The geological analysis of thorium related ores such as monazite, bastnasite, xenotime, and thorite also offers a toolkit to apply to early fuel cycle materials for comparative purposes. There are documented studies that have investigated isotopic variations between and within ore bodies, including both stable (e.g., Sr) and rare earth isotopes (e.g., Nd), as well as studies reporting processes for the extraction of thorium from ores (Chen et al., 2017; Hetherington et al., 2010).
These impurity analytes can be measured by standard elemental analysis, high precision mass spectrometry and halogen analysis techniques such as ion chromatography on purified samples containing the analyte. Careful consideration of the development of methods for the digestion, removal of analytical interferences (spectral, radiometric, isobaric), isotope dilution and separation of elemental impurities from feasible matrices is required. Alpha decay of the major thorium isotopes and radioactivity of the thorium decay chain also enable the application of standard radiometric techniques to thorium materials. Once confidence in radiochemistry is established, it is feasible to leverage the extant capability in nuclear forensic laboratories and validate measurements (Taylor et al., 2020).
A radiochemical perspective of Thorium signatures is also of value. This is because every thorium-containing ore body has a differing geological formation history; the Th isotopic profile and trace metal abundance should be uniquely characteristic and therefore traceable in unprocessed Th ores in a similar manner to U ore concentrates. Geolocation techniques would also be relevant based on stable isotope ratios (e.g., Sr and Nd isotopes) (Bernhard, 2021; Moody et al., 2005).
The processing of thorium compounds to metallic and molten salt forms is another area for investigation. The use of chemical reduction, casting, heat treatment and machining processes could impart additional measurable parameters of interest, for example, the use of sintering agents (e.g., Ca, Nb, Mg) and cladding/matrix materials (e.g., Al, F, Cl) in fuel production. Post irradiation, the change in isotopic composition of the material, such as the minor Th isotopes (e.g., 229Th) and trace actinides (232U) and fission/activation products, provide a means for determining whether the material was recovered and repurified after utilisation for nuclear power generation, or as an irradiation target. Other elemental constituents of a Th sample can provide clues about separation techniques used in the preparation of the material (e.g., P from use of TBP in reprocessing vs. ore processing), as well as the time that the sample was last purified. There are reported examples of the use of the 228Th/232Th ratio (via 228Ra/224Ra) to analyse for chemical purification and produce model ages that are relevant to nuclear forensics for a sample before equilibrium is re-established after 60 years (Kayzar-Boggs et al., 2021). A summary is provided in Table 1 as a guide for practitioners:
Methodology, results and discussion
This study aimed to enhance prior exploratory studies of the nuclear forensic analysis of thorium materials. The first aim was to process a well characterized ore material through to a refined thoria following a reported legacy processing route from the UK (Jamrack, 2025). We will discuss this process and the initial characterization of a material processed by this route, working at the several hundred-gram laboratory scale.
A generalized outline of the flowsheet for monazite processing is presented for clarity, followed by specific details on the steps followed in this work:
• Ore mining: The first step is to mine the monazite (a thorium-containing phosphate mineral) from the earth. Extraction can be achieved in various ways, such as open-pit mining or underground mining.
• Ore preparation: Monazite ore is typically extracted from heavy mineral sands (Jamrack, 2025). The ore is first ground into a fine powder to increase its surface area and then subjected to physical separation techniques like gravity separation, magnetic separation, or electrostatic separation to concentrate the monazite away from the other accessory minerals.
• Monazite acid digestion/leaching: The concentrated monazite is treated with a strong acid, usually H2SO4 or HCl, under high temperature and pressure. This process, known as cracking, breaks down the monazite mineral structure, releasing REEs, thorium, and uranium into the acidic liquor for subsequent selective chemical treatments.
• Alkali leaching: The cracked monazite is sometimes then subjected to alkali leaching to ensure complete dissolution of the thorium, uranium, and rare earth elements. This pH reversal step usually involves treatment with a solution of concentrated sodium hydroxide or other suitable alkali reagents.
• Rare earth element separation: The resulting solution is subjected to a series of precipitation, solvent extraction or ion exchange steps to separate and purify the rare earth elements, matrix elements and uranium from thorium. The REEs are typically separated into individual elements or groups of elements with similar chemical properties. Historic processes such as those re-investigated here, applied continued cycle precipitations to purify the thorium from the coexisting matrix elements, exploiting the difference in solubility of the various different compounds of thorium, uranium and REE.
• Product recovery: the thorium, uranium and REE product streams are further purified depending on the intended application. The material then converted into either an oxide, nitrate, fluoride or metal.
The process investigated here is a legacy process using repeat cycle precipitations (Jamrack, 2025). The process aligns with the above generalized scheme but with some specific differences:
1. The scheme targets pure rare earth element streams in addition to pure thorium.
2. Monazite sands are ground and homogenized for sample separation, magnetically separated and then the residual material is digested using NaOH at 200°C and the insoluble residue containing thorium is removed for further processing.
3. The thorium phosphate containing residue is then dissolved in sulphuric acid at ambient conditions, and the thorium and REE oxalates are precipitated from solution using oxalic acid. The product is washed with water.
4. The causticisation of the oxalate product using sodium hydroxide ahead of re-dissolution is the next step to reduce the phosphate content of the thorium product using sodium hydroxide as the reagent.
5. The thorium oxalate is dissolved again using sulphuric acid, followed by addition of ammonium hydroxide to generate thorium hydroxide.
6. The thorium hydroxide is then dissolved in sulphuric acid and thorium thiosulphate is precipitated using Na2S2O3. This precipitation cycle removes REE as sulphates and phosphates. The REE is reduced to 0.1% content from around 10%.
7. The thorium sulphate product is recrystallized then dissolved and precipitated as thorium hydroxide using sodium hydroxide. This material is then thermally decomposed at 350°C to produce a thorium dioxide product.
Characterisation of the starting ore material
The ore material was provided by the BGS and this material was processed on the >500 g scale relative to the fume hood limits in the radiological laboratory. The material was originally sourced from Kerala (referred to as Travancore at the time of sample collection) in SW India. This ore was initially characterized using Scanning Electron Microscopy (SEM), Gamma Spectroscopy and X-ray Fluorescence (XRF) spectroscopy.
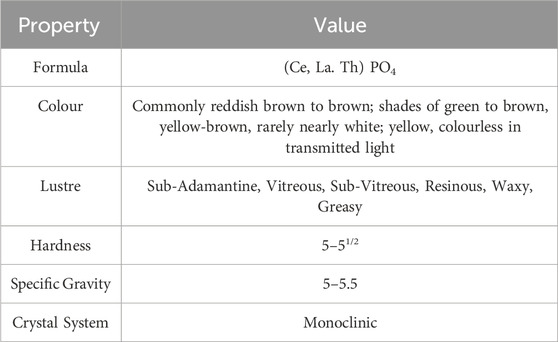
The measured thorium content was 6.1 weight percent (wt%) of the precursor monazite sand (using XRF). The properties of the sample (Table 2) and the information provided by the BGS suggests that it has already undergone some mechanical processing and mineral separation and that it was a high-grade ore.
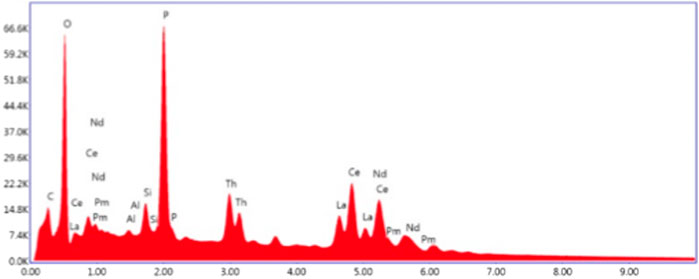
Under the optical microscope the monazite sand was black/brown to pale yellow in colour, showing a vitreous sheen. The sand grains were well rounded, well sorted and, despite colour differences, were determined to have very similar elemental makeup across and within the grains. This was semi-quantitatively confirmed by SEM-EDX in the initial nondestructive assay and by XRF (Figures 1–3). No biological contamination of the sample was found in any of the analyses. For these reasons, we believe that this monazite sand was pre-sorted by sieving (due to consistent grain sizes), washing and magnetic separation (ascertained by testing with a strong magnetic field) before it was sent to the UK from India and archived. The grains show signs of mechanical abrasion but no signs of chemical pitting from any in situ leaching. The material is readily digested when exposed to strong acids or alkalis. The monazite quickly breaks down and becomes translucent, taking on a pale yellow-to-white appearance, but does not dissolve entirely without both an alkali and acid step as would be expected from monazite ore dissolution procedures detailed above.
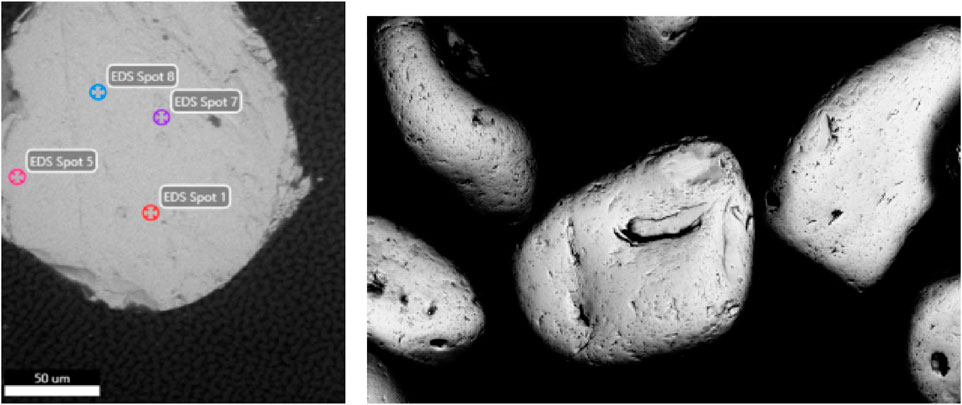
Figure 2. Electron micrograph of representative polished monazite sand grains, showing EDS spot locations (left) and Monazite sand grains, ×662 magnification, under Backscatted Electron (BSE). Using BSE imaging mode enables a sample to be quickly visually assessed to see if there is substantial Z-number variation across a mass of sample material.
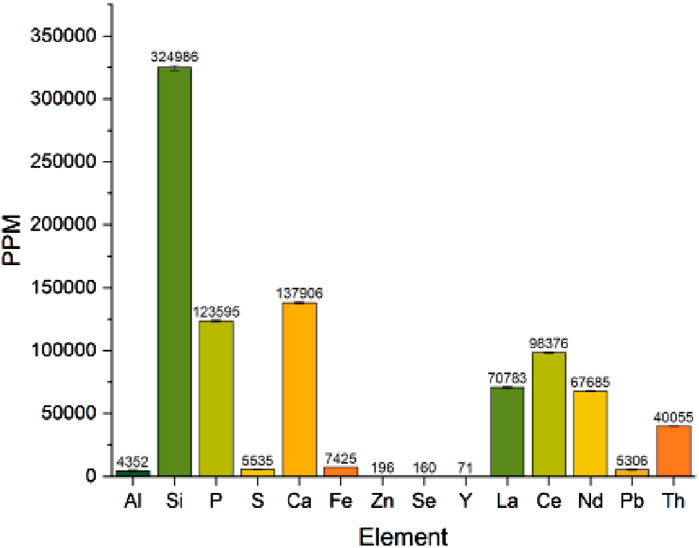
The uranium and lead concentrations in the sample were close to the detection limit of the EDS instrument on the SEM, but decay product isotopes of both elements were readily measured using gamma spectroscopy. Although lanthanum and neodymium concentrations in the sample are similar in magnitude (approximately 70,700 ± 640 ppm and 67,600 ± 538 ppm, respectively using benchtop XRF–Figure 4) we are able to quantify the material composition semi-quantitatively without the requirement to chemically digest the material. The isotopes and gamma-emitting decay products in the material were determined to be consistent with natural thorium. More comprehensive characterisation may also then be applied to the ore using advanced measurement techniques as discussed in the introductory section.
The isotopic and trace elemental analysis of the dissolved material, including the analysis of REE is consistent with those reported from southern India (Anitha et al., 2020; Veerasamy et al., 2021). The quantities of these lanthanides and absence of the heavy lanthanides is a differentiator to other ores reported. The results were normalized as described elsewhere and compared as part of the process stages detailed above from ore to oxide (Figure 5) (Nakamura, 1974). The abundance and relative concentrations of the light rare earth elements (La-Sm) were determined to be consistent with reported materials, with detection limits (sub-ppm) measured for the REE from Eu-Lu with the exception of Gd, Dy and Er. The isotopic actinide composition of the material was determined to be consistent with natural uranium and thorium (determined by gamma spectrometry and mass spectrometry against isotopic standards for uranium) (Higginson et al., 2021). This isotopic signature remained consistent throughout purification of the material to an oxide using mass spectrometry so further discussion on this is not included, but this would be indicative of an early fuel cycle material prior to irradiation and could be investigated further in future work utilizing high precision isotope mass spectrometry.
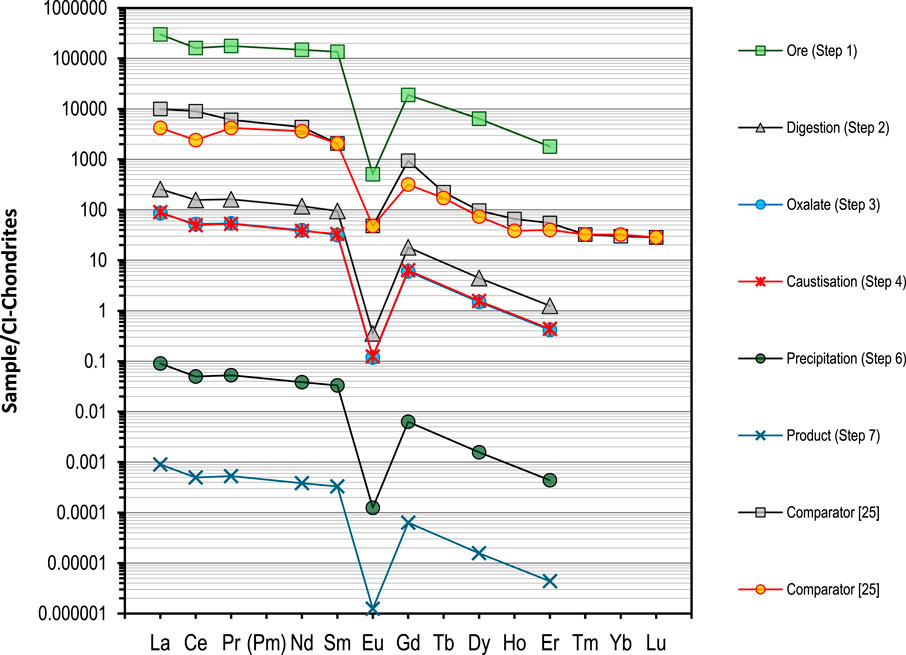
Figure 5. REE spider diagram normalized to Cl-chrondrite and compared against example data in (Veerasamy et al., 2021). Data is also consistent with monazite data reported by studies on comparison and provenance of ores with respect to REE patterns from other thorium minerals (Tay et al., 2020).
The sample monazite materials were dissolved without the requirement for comminution of the material. A glove bag was used to contain dispersible materials prior to dissolution to maintain sample integrity. The monazite particulate was then mixed with a sodium hydroxide (60% v/v) and heated, with stirring at 120°C–140°C. Sodium hydroxide reacts readily with the monazite under these conditions. The phosphate group in the monazite is transformed into soluble phosphates, while the metal ions (thorium, uranium, and REEs) react to form insoluble hydroxides. The mixture was then allowed to settle and was filtered. The soluble phosphates remain in the supernatant, which was filtered off, leaving behind a solid residue composed of metal hydroxides of thorium, uranium, and REEs. This solid residue was then ready for further processing steps. In batch studies, >500 g of monazite was added to 2 L of 60% NaOH at 120°C–140°C and mixed with a coated stirring bar at 1–200 rpm. To ensure that the mixture never dried out, water was added at regular intervals. A standard borosilicate glass beaker of an appropriate size was used to contain the process mixture. Filtration was performed through relatively coarse whatman filter papers. This phase worked best at high concentrations of NaOH and temperatures over 200°C. To ensure complete dissolution of the solids, the mixture was left to react for an entire week, although, in higher temperature and higher-pressure industrial processes, this process can be completed more rapidly.
The reaction slurry was then filtered at 65°C and the solid product retained. The product of this process is an insoluble residue (including Th). This stage was not designed to dissolve any of the thorium but instead, produce a fine “silica slurry”; most of the initial phosphate is dissolved and removed during the filtering of this stage and is not measured in high concentrations in the arising solid residues. The analysis of the insoluble residue material agreed well with predicted material measurable propetries. The material was measured by High Resolution Gamma Spectrometry (HRGS) and was still confirmed to contain U and Th (U/Th = 0.75). The radioactive decay series is perturbed from secular equilibrium and Ac, Pb, Ra, Tl, Pb and Bi were readily detectable by HRGS indicating the carry-through of many of the decay daughter isotopes into the residue. Subsamples of the material were processed for analysis and the 228Ra/232Th model age was calculated as 0.32 years (U = 10% k = 2, reference date: 26/10/2022) in agreement with the known timeline of materials processing within a year. The material was shown to contain the same ratios of REE as the precursor monazite (Figure 5), with 1–100 ppm La-Eu in the measured diluent solutions. The REE/chrondite normalised data is quite specific as the ratios remain consistent (preserved) and the absence of the heavier REE is different in other ore materials reported (Tay et al., 2020). The Th assay was determined to be 47±5% by alpha spectrometry and Inductively Coupled Plasma–Optical Emission Spectroscopy (ICP-OES). Notable impurities in the solution by ICP-OES were Al (580 ppm), Fe (10 ppm) and Si (200 ppm). Elements Eu, Tb, Cr, Cu, Ti, Ni, Mg, Ba, Ca, Ba, Sn and other trace impurities screened via semi-quantitative analysis were all determined to be at concetrations below the detection limit of the analytical techniques employed.
The material was then dissolved in sulphuric acid at 120°C, followed by filtration to remove insolubles. HRGS measured decay progeny from 228Ac, 214Pb, 212Bi, 208Tl. The relative REE ratios remain consistent, but were observed to have reduced in total concetration by approximately 5 times. The model age of the material was calculated to be 0.34 years (U = 10% k = 2, reference date: 26/10/2022). Trace imputities such as Al (220 ppm) and Fe (2 ppm) were also reduced in abundance by this processing step by measurement using XRF.
In the next processing step, oxalate was used to selectively precipitate out thorium and REEs from the solution. This final compound was dissolved and measured. The results yielded consistent analytical results from the prior processing step. The REE content of the final solid was below 1 ppm, Al was measured in the sample (280 ppm±10% k = 2) and the model age of the material was determined to be (0.21 years U = 10% k = 2, reference date: 26/10/2022). The total cumulative uranium and REE content of the material was reduced to <10 ppm.
In the next stage, the product is further purified by dissolution and re-precipitation. The first stage is required to remove group I-II sulphates and the resulting precipitated thorium hydroxide product was calcined to remove the hydrogen and produce a thorium dioxide product. This final material was consistent with material measured in the previous processing steps using the available methods. The material contained minimal trace impurities below 10 ppm including REE, U and other notable impurities listed above (e.g., Mo, Fe) with the exception of Al (220 ppm ± 10% k = 2). At this stage, sensitive mass spectrometry methods are be needed to measure the residual REE pattern in the final material along with other trace-level impurities.
In the recreation and analysis of the monazite ore to thorium dioxide the following areas were demonstrated and developed:
• The use of nondestructive analysis tools, such as HRGS, optical microscopy, SEM and XRF were demonstrated to be able to quickly characterize the starting material ahead of processing and should serve as useful tools in the future for rapid screening of unknown samples.
• Upon processing the monazite material by the archive flowsheet, the isotopic profile and REE pattern were shown to be retained. The Th/U ratio was shown to increase with purification as the thorium as it is concentrated by the process.
• The U, REE and P content were shown to reduce upon processing to thoria. Key steps in this process are identified.
• Anthropogenic and process impurities were removed throughout the process to ppm levels (Fe, Al, Na, S, Si, B) and were quantified either by use of ICP-OES, XRF and SEM-EDX in gross levels. At specific steps it was possible to measure enhanced process impurities added (e.g., Na, Si) from the processing of the samples in labware with mineral acids or the use of sodium hydroxide.
• The final product material was low in impurities relevant to reported ‘nuclear grade’ specifications for fuel cycle applications
• Due to the removal of radium in the samples with purification, measurements using the Ra decay progeny and subsequent model ages produced were shown to be within the timeline of the purification of the material.
• In this limited study, no impurities of note >1 ppm were observed in the final product, specific to the cycle precipitation process with respect to other benchmark routes to purify monazite ores to thoria using liquid-liquid extraction and exchange chromatography (P, S, Na). It is unclear therefore if many trace elemental impurities would be useful signatures to distinguish between high separation factor purification approaches.
• Further measurements of the samples by highly sensitive mass spectrometry techniques for isotopes and impurities below ppm concentration levels would be beneficial to help define whether light REE ratios are maintained throughout the entire process. Additionally, archival measurement of real material from the same process would provide a useful comparison of impurities but also a useful test for radiochronometry measurements.
Experimental
A detailed description of the process is given in the methodology, results and discussion section, as it is essential to the narrative of the investigation. Additional experimental information is given here for completeness.
Samples of monazite were provided by the BGS as described above. The above section also describes the process flow in detail, recreating the flowsheet. All reagents and chemicals used were analytical grade unless stated otherwise (Fisher scientific UK). Whatman filter papers and standard laboratory glassware and hot plates were used for these experiments.
Microscopy characterization of the samples was completed using a Zeiss Axioscope and Axiocam 7 for light microscopy and a Zeiss FEG-SEM and a Zeiss EVO MA 10 SEM for electron micrscopy and EDX analysis. An Olympus Vanta C-series pXRF was used for the elemental characterization of the bulk samples.
The radiometric measurements of samples were completed by dissolution of the sample into a controlled 5 mL geometry in 0.5 M HCl and measurement against traceable multi gamma standards and traceable thorium and radium activity standards (National Physical Laboratory, Teddington, UK). Prior publications describe the setup of the HRGS, ICP-OES and Alpha Spectrometry systems (Higginson et al., 2021; Gilligan et al., 2024; Skinner and Knight, 2016). Radiochemistry methods followed prior reported techniques using chloride media to separate Th from impurities (Moody and Grant, 1999). Radiochronometry measurements were carried out following the procedures reported in (Ault et al., 2017), using Ra separation onto resolve filters and alpha spectrometry measurements of radium alongside measurement of activity certified 226-Ra. Measurement of thorium and radium isotopes by alpha spectrometry and sample aliquot mass were used to calculate model ages using ENDSF nuclear data for the decay pairs (Koide et al., 1973).
Conclusion
This work aims to begin a series of complementary investigations into the nuclear forensic signatures of thorium fuel cycle materials. We have reported a study to process monazite ore material at the laboratory bench scale to produce a refined thoria product, using a historic UK process. The processing route was proven to be successful, producing a thoria (ThO2) product material of high purity. Measurement of the material using the available techniques traced a variety of ‘fingerprint’ material properties and impurities through the multi-stage processing route. It was shown, as predicted, that radiometric methods, relative REE abundances, isotopes, radiochronometry and SEM were useful for characterising the material. The REE pattern was retained and diluted throughout processing of the material to an oxide. Impurities which were measured to appear during processing aligned with the chemistry and reagents used, as well as being added by interactions of the acid and alkali reagents with the conventional laboratory equipment (e.g., borosilicate glass, Fe, Al).
As the forensic signatures here are relevant to early fuel cycle processing, depending on the step (1–7) the material purity increases with each stage with thorium content increasing. As the materials used are acid and bases, the buildup and removal of Na/S in the material is not greatly useful except to differentiate between digestion approach. We identified key stages which meaningfully impact the measurable parameters being the sulphate precipitation which removes most initial phosphorous (major element) from the product at an early stage and the oxalate (Th and RRE) and thiosulphate (Th) precipitation which reduces phosphate content further and REE content to <0.1% of the thorium content. Measurement of exemplar archive materials are planned for future work to compare to this current laboratory scale study as well as additional measurements applying sensitive mass spectrometry techniques with ultra-trace level measurement capabilities. It is also important to continue trials to process the material through alternate flowsheets (e.g., using ion exchange and not precipitation processes) to compare materials between different processing routes.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author contributions
EH: Conceptualization, Data curation, Formal Analysis, Investigation, Methodology, Project administration, Software, Validation, Visualization, Writing – original draft, Writing – review and editing. MH: Conceptualization, Data curation, Formal Analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review and editing. PK: Funding acquisition, Methodology, Project administration, Resources, Supervision, Writing – original draft, Writing – review and editing. TS: Investigation, Project administration, Supervision, Writing – original draft, Writing – review and editing. TM: Data curation, Investigation, Methodology, Project administration, Resources, Supervision, Writing – original draft, Writing – review and editing. CG: Formal Analysis, Writing – original draft, Writing – review and editing. KK: Funding acquisition, Writing – original draft, Writing – review and editing. SC: Writing – original draft, Writing – review and editing. CB: Writing – original draft, Writing – review and editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. AWE Nuclear Security Technologies and the University of Bristol are thanked for supporting this work with funding.
Acknowledgments
Roy Awbery is thanked for his advice and support to this project from the inception.
Conflict of interest
Author EH was employed by Astral Systems. Authors MH, PK, CG, KK, SC, and CB were employed by AWE Nuclear Security Technologies.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The authors declare that this study received funding from AWE Nuclear Security Technologies. The funder had the following involvement in the study: AWE supervisory staff were industrial supervisors and worked on the aims and design of the project, as well as providing experimental analysis of samples produced in the work and archive reports on the historical process.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Ade, B., Worrall, A., and Powers, J. (2014). Safety and regulatory issues of the thorium fuel cycle.
Allègre, C. J., Dupré, B., and Lewin, E. (1986). Thorium/uranium ratio of the Earth. Chem. Geol. 56 (Issues 3–4), 219–227. doi:10.1016/0009-2541(86)90005-7
Anitha, J. K., Rejith, R. G., and Sundararajan, M. (2020). Monazite chemistry and its distribution along the coast of Neendakara–Kayamkulam belt, Kerala, India. SN Appl. Sci. 2, 812. doi:10.1007/s42452-020-2594-6
Ault, T., Krahn, S., and Croff, A. (2017). Thorium fuel cycle research and literature: trends and insights from eight decades of diverse projects and evolving priorities. Ann. Nucl. Energy 110, 726–738. doi:10.1016/j.anucene.2017.06.026
Bernhard, S. (2021). Monazite microstructures and their interpretation in petrochronology. Front. Earth Sci. 9, 668566. doi:10.3389/feart.2021.668566
Chen, W., Honghui, H., Bai, T., and Jiang, S. (2017). Geochemistry of monazite within carbonatite related REE deposits. Resources 6, 51. doi:10.3390/resources6040051
Essex, R. M., Mann, J. L., Williams, R. W., Kinman, W. S., Hubert, A., Bennett, M. E., et al. (2018). A new thorium-229 reference material. Appl. Radiat. Isotopes 134, 23–31. doi:10.1016/j.apradiso.2017.07.050
Evitts, L. J., Gilbert, M. R., Middleburgh, S. C., Lee, W. E., and Dahlfors, M. (2021). Utilizing neutronics modelling to predict changing Pu ratios in UO2 in the presence of Th. Prog. Nucl. Energy 137, 103762. doi:10.1016/j.pnucene.2021.103762
Gilligan, C. R. D., Stokes, T. C., Goodwin, M. A., John, D., Wroe-Brown, J., Higginson, M., et al. (2024). Determination of the absolute intensity of the 1205 keV γ-ray emission from 91Y. Appl. Radiat. Isotopes 205, 111172. doi:10.1016/j.apradiso.2023.111172
Hetherington, C. J., Harlov, D. E., and Budzyń, B. (2010). Experimental metasomatism of monazite and xenotime: mineral stability, REE mobility and fluid composition. Mineralogy Petrology 99, 165–184. doi:10.1007/s00710-010-0110-1
Higginson, M., Dawkins, B., Shaw, T., Taylor, F., Ingman, L., Dunn, S., et al. (2021). Development of assay, isotopic and trace actinide measurements on solid plutonium and uranium samples by high resolution gamma spectrometry. J. Radioanal. Nucl. Chem. 330, 901–911. doi:10.1007/s10967-021-08043-w
Higginson, M. A., Kayzar-Boggs, T. M., Chen, C. Y., Cross, S. T. J., Denton, J. S., Dunne, J. A., et al. (2022). Establishing discordance as a radiochronometric signature for nuclear forensic investigations: a multi-laboratory intercomparison exercise. J. Radioanal. Nucl. Chem. 331, 4799–4815. doi:10.1007/s10967-022-08428-5
Holmes, L. (2001). Standard reference materials for thorium analysis. Radiat. Prot. Dosim. 97 (2), 199–202. doi:10.1093/oxfordjournals.rpd.a006661
Jamrack, W. D. (2025). The production of pure thoria from monazite. Thorium Limited Report AB 7/4273, 1955 Public Record.
Kayzar-Boggs, T. M., Kinman, W. S., Bostick, D. A., Cardon, A., Foley, R. R., Hexel, C. R., et al. (2021). Exploring the use of thorium isotope compositions and concentrations as nuclear forensic signatures for uranium ore concentrates. J. Radioanal. Nucl. Chem. 327, 877–889. doi:10.1007/s10967-020-07534-6
Keegan, E., Kristo, M. J., Toole, K., Kips, R., and Young, E. (2016). Nuclear forensics: scientific analysis supporting law enforcement and nuclear security investigations. Chemistry 88 (3), 1496–1505. doi:10.1021/acs.analchem.5b02915
Koide, M., Bruland, K. W., and Goldberg, E. D. (1973). Th-228/Th-232 and Pb-210 geochronologies in marine and lake sediments. Geochimica Cosmochimica Acta 37 (5), 1171–1187. doi:10.1016/0016-7037(73)90054-9
Mayer, K., Wallenius, M., and Varga, Z. (2013). Nuclear forensic science: correlating measurable material parameters to the history of nuclear material. Chem. Rev. 113 (2), 884–900. doi:10.1021/cr300273f
Moody, K. J., and Grant, P. M. (1999). Nuclear forensic analysis of thorium. J. Radioanal. Nucl. Chem. 241, 157–167. doi:10.1007/BF02347304
Moody, K. J., Grant, P. M., and Hutcheon, I. D. (2005). Nuclear forensic analysis. 1st ed. CRC Press. doi:10.1201/9780203507803
Nakamura, N. (1974). Determination of REE, Ba, Fe, Mg, Na and K in carbonaceous and ordinary chondrites. Geochimica Cosmochimica Acta 38, 757–775. doi:10.1016/0016-7037(74)90149-5
Rim, J. H., Kuhn, K. J., Tandon, L., Xu, N., Porterfield, D. R., Worley, C. G., et al. (2017). Determination of origin and intended use of plutonium metal using nuclear forensic techniques. Forensic Sci. Int. 273, 1–9. doi:10.1016/j.forsciint.2017.01.014
Shen, C.-C., Edwards, R. L., Cheng, H., Dorale, J. A., Thomas, R. B., Bradley Moran, S., et al. (2002). Uranium and thorium isotopic and concentration measurements by magnetic sector inductively coupled plasma mass spectrometry. Chem. Geol. 185 (Issues 3–4), 165–178. doi:10.1016/S0009-2541(01)00404-1
Skinner, M., and Knight, D. (2016). The behaviour of selected fission products and actinides on UTEVA® resin. J. Radioanal. Nucl. Chem. 307, 2549–2555. doi:10.1007/s10967-016-4706-8
Tay, S. L., Scott, J. M., Palmer, M. C., Reid, M. R., and Stirling, C. H. (2020). Occurrence, geochemistry and provenance of REE-bearing minerals in marine placers on the West Coast of the South Island, New Zealand. N. Z. J. Geol. Geophys. 64(1), 89–106. doi:10.1080/00288306.2020.1736585
Taylor, F., Higginson, M. A., Marsden, O., and Schwantes, J. (2020). State of practice and emerging application of analytical techniques of nuclear forensic analysis: highlights from the 5th collaborative materials exercise of the nuclear forensics international technical working group (ITWG). J. Radioanal. Nucl. Chem. 323, 415–430. doi:10.1007/s10967-019-06950-7
Tuovinen, H., Vesterbacka, D., Pohjolainen, E., Read, D., Solatie, D., and Lehto, J. (2015). A comparison of analytical methods for determining uranium and thorium in ores and mill tailings. J. Geochem. Explor. 148, 174–180. doi:10.1016/j.gexplo.2014.09.004
Varga, Z., Wallenius, M., Krachler, M., Rauff-Nisthar, N., Fongaro, L., Knott, A., et al. (2022). Trends and perspectives in nuclear forensic science. TrAC Trends Anal. Chem. 146, 116503. doi:10.1016/j.trac.2021.116503
Veerasamy, N., Murugan, R., Kasar, S., Inoue, K., Kavasi, N., Balakrishnan, S., et al. (2021). Geochemical characterization of monazite sands based on rare earth elements, thorium and uranium from a natural high background radiation area in Tamil Nadu, India. J. Environ. Radioact. 232, 106565. doi:10.1016/j.jenvrad.2021.106565
Keywords: nuclear forensic analysis, thorium fuel cycle, nuclear forensic signatures, actinide analysis, thorium analysis
Citation: Holland E, Higginson MA, Kaye P, Scott TB, Martin T, Gilligan CRD, Kennedy K, Cross S and Brook C (2025) Nuclear forensic analysis of thorium materials: recreation of a legacy processing method. Front. Nucl. Eng. 4:1634367. doi: 10.3389/fnuen.2025.1634367
Received: 24 May 2025; Accepted: 26 July 2025;
Published: 23 September 2025.
Edited by:
Rebecca Abergel, University of California, Berkeley, United StatesReviewed by:
Stefan Neumeier, Helmholtz Association of German Research Centres (HZ), GermanyJeremy Inglis, Los Alamos National Laboratory (DOE), United States
Copyright © 2025 Holland, Higginson, Kaye, Scott, Martin, Gilligan, Kennedy, Cross and Brook. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Matthew A. Higginson, bWF0dGhldy5oaWdnaW5zb25AYXdlLmNvLnVr
 Erin Holland1,2
Erin Holland1,2 Matthew A. Higginson
Matthew A. Higginson Thomas B. Scott
Thomas B. Scott