- 1Environmental Technology Division, CSIR-Institute of Himalayan Bioresource Technology (Council of Scientific and Industrial Research), Palampur, Himachal, Pradesh, India
- 2Academy of Scientific and Innovative Research (AcSIR), Ghaziabad, India
- 3Centre for High Altitude Biology (CeHAB), Research Centre of CSIR-IHBT, Lahaul and Spiti, Himachal Pradesh, India
Investigating thermal tolerance is vital in understanding how plant species would respond to future global temperature increase with greater rates in alpine areas, especially in the Himalaya, a biodiversity hotspot. In this study, we investigated the leaf thermal tolerance of 52 species from the Himalayan alpine region, to assess 1) the response of alpine species to different temperatures (28°C, 33°C, 38°C, 43°C, 48°C, 53°C, and 58°C), 2) the dependence of thermal tolerance (T50) on various eco-physiological leaf traits, and 3) variation in thermal tolerance among different growth forms. We found the thermal tolerance of various species to be in the range of 44.9°C to 65.9°C, the highest in graminoids (53.8°C ± 8.2°C), followed by forbs (49.8°C ± 2.2°C) and rosettes (48.7°C ± 2.4°C). We observed a significant positive correlation between T50 and leaf traits such as leaf mass per area and leaf dry matter content. We also determined thermal safety margins (TSMs), which ranged from 20.3°C (Malva neglecta) to 40.5°C (Calamagrostis emodensis) for most of the species, with a few species under 20°C, except for Rosularia alpestris at 9.7°C. Our results suggest that alpine species from the Western Himalaya, with elevated T50 and wide TSMs, are currently within their thermal safety range and are not as susceptible to temperature rise in the near future, compared to species from tropical and subtropical eco-regions, provided these are able to modulate their leaf temperature within theired ran specifige of tolerance.
1 Introduction
Global temperature has been on the rise, and it will continue to rise further in the future (McCulloch et al., 2024). With increase in global temperatures and sequential changes in climate patterns, it is expected that heat stress would threaten numerous plant species to the extent that it may lead to regional elimination of the already vulnerable species, which are operating at their maximum temperature thresholds (Sastry and Barua, 2017; O’sullivan et al., 2017; Feeley et al., 2020; Slot et al., 2021). A species’ ability to tolerate temperature is an essential trait of ecological importance, as this decides and governs their performance and distribution, thereby influencing the composition of communities and the functioning of ecosystems (Deutsch et al., 2008; Araújo et al., 2013; Khaliq et al., 2014; Feeley et al., 2020; Lancaster and Humphreys, 2020; Ahrens et al., 2021). While the consequences of global temperature warming have been evident in global mountain ecosystems, the effects are more prominent at higher elevations, such as in the alpine region, where low temperature-adapted native species could gradually become more vulnerable to the warming of the alpine environment (Dangles et al., 2017; Cuesta et al., 2020; Sklenář et al., 2023). Thus, it is of utmost importance to understand the thermal tolerance of alpine plants in evaluating their susceptibility to global warming, considering the current scenario of rapid rise in temperatures and changing climate patterns (O’sullivan et al., 2017; Sklenář et al., 2023; Hansen et al., 2025).
Most of the studies on the thermal tolerance of plants have been conducted in tropical and subtropical eco-regions, such as in the tropical forests of Brazil (Doughty and Goulden, 2008), rainforests of lowland tropical Peruvian Amazon (O’sullivan et al., 2017), Fairchild Tropical Botanic Garden in Coral Gables (Florida, USA) (Perez and Feeley, 2020), and tropical forests of Panama (Slot et al., 2021), or mostly focused on tropical tree species from the south-eastern Amazon region (Tiwari et al., 2021), specific tropical species such as Ficus insipida (Krause et al., 2010), tropical montane tree species from Rwanda (Africa) (Tarvainen et al., 2022), and various tropical tree species from Western Ghats, India (Sastry and Barua, 2017; Javad et al., 2025). These studies suggest that species occurring in these eco-regions are at greater peril, as they are already performing at their maximum thermal thresholds. In contrast, studies on the thermal tolerance of alpine species are limited. Such studies include investigation of thermal tolerance on specific species (such as Loiseleuria procumbens, Soldanella pusilla, Rhododendron ferrugineum, Senecio incanus, Ranunculus glacialis, and Pterocephalus lasiospermus), tropical alpine paramo species, and some alpine glacier foreland species from the Alps (Braun et al., 2002; Marcante et al., 2014; Buchner et al., 2015; Perera-Castro et al., 2018; Leon-Garcia and Lasso, 2019), and focused investigations on Erysimum scoparium, an evergreen shrub (González-Rodríguez et al., 2021). These studies have suggested that an increase in global temperature may pose a risk to alpine plant species occurring in these regions. However, studies on the thermal tolerance of plants to high temperatures from the Himalayan alpine region are lacking. The Himalaya, situated in the tropical latitudes, is reported to be warming at a much higher rate than the global average (Dimri et al., 2022) and also due to elevation-dependent warming; the higher elevations are reported to be warming at higher rates (Krishnan et al., 2019), which could place alpine vegetation communities at greater risks (Pepin et al., 2019). The Himalaya is home to numerous plant species, among which several are endemic, and so it is considered a very important biotic realm in the world (Wambulwa et al., 2021). Therefore, it is essential that we study the thresholds of the thermal tolerance of Himalayan alpine species, which would facilitate the assessment and broadening of our understanding of the impact that global warming could pose to species in the alpine region.
Leaf temperature is principally influenced by the ambient temperature; however, it is also modulated by transpiration and leaf traits (Lambers et al., 1998; Defraeye et al., 2013; Scheffers et al., 2016). Structural leaf traits [such as leaf mass per area (LMA), leaf dry matter content (LDMC) and specific leaf area (SLA) (Jones, 1992; Loveys et al., 2002; Curtis et al., 2012; Sastry and Barua, 2017)], morphological traits (Leigh et al., 2017; Monteiro et al., 2016), and stomatal conductance (Jones, 1992; Manzi et al., 2025) regulate leaf-to-air temperature difference; these traits can also determine the levels and intensity of the temperature tolerance of plants. For example, in warm and arid climates, a leaf’s dimension modulates leaf-to-air temperature difference, whereas species having increased structural leaf investment with smaller leaves, or species with bigger leaves and low stomatal conductance, showed a higher tolerance to increased temperatures (Leigh et al., 2017; Sastry and Barua, 2017; Manzi et al., 2025). Additionally, a plant’s growth form also defines its level of temperature tolerance; rosette, cushion, and stunted growth forms are reported to have considerably higher levels of tolerance to increased temperature than the erect herbaceous plants and forbs (Buchner and Neuner, 2003; Leon-Garcia and Lasso, 2019). Furthermore, leaf habits, such as evergreen and deciduous, also influence leaf temperature tolerance (Zhang et al., 2012). Thus, it is apparent from all these studies that thermal tolerance in plants is greatly regulated and maintained by leaf traits and growth forms, and the latter need to be ascertained for understanding the inherent dependencies.
At present, our knowledge of the thermal tolerance of plants to higher temperatures and the relationship between leaf traits and leaf temperature is mostly from tropical regions and predominantly of tree species. The few studies conducted on the thermal tolerance of alpine plants suggest that future increases in temperature in these eco-regions may pose a risk to their existence (Larcher et al., 1998; Marcante et al., 2014; Buchner et al., 2015; Perera-Castro et al., 2018; Leon-Garcia and Lasso, 2019; González-Rodríguez et al., 2021). Alpine regions are primarily characterized by a decline in atmospheric pressure and, consequently, a regime of low temperatures and increased solar radiation intensities (Körner, 2007). Even though a low temperature regime persists in the alpine region, the possible damages to plants due to high temperatures at mid-day times during clear summer days are also a factual risk (Gauslaa, 1984; Neuner et al., 1999; Perera-Castro et al., 2018; González-Rodríguez et al., 2021; Sklenář et al., 2023). Moreover, on a daily basis, huge temperature fluctuations with sub-zero night temperatures are a characteristic of tropical alpine regions such as the Himalaya, and a plant’s ability to endure this fluctuating environmental condition is the main feature in shaping the composition and structure of plant communities that occur along the elevation gradient (Rundel et al., 1994). Further, the limited ability of tropical alpine plants to acclimate and survive the cold winter temperatures may also limit the occurrence of tropical alpine species in hotter climate regions, which will therefore make them highly susceptible to an increase in temperature (Sklenar et al., 2023).
Alpine environments are reported to be much influenced by erratic weather patterns, especially the precipitation pattern combined with frequent drought and heat waves (Choler, 2023). The ability to tolerate these erratic weather patterns must be a key feature for the survival of species found in this region. Therefore, we hypothesized that 1) thermal tolerance in species from colder environments will be greater, as species occurring in alpine regions may possess high thermal upper limits and be secured by a wide thermal safety margin to withstand higher temperatures physiologically than what is expected by their climatic niches (Leon-Garcia and Lasso, 2019). 2) Species having costlier leaf investments, such as high LMA, greater leaf thickness (LT), and LDMC, will also influence thermal tolerance (Sastry and Barua, 2017; Monteiro et al., 2016). 3) Species with shorter growth forms, such as rosettes, will have higher thermal tolerance, as they grow close to the ground surface and therefore are exposed to a warmer conditions (Squeo et al., 1991; Sklenář et al., 2016; Leon-Garcia and Lasso, 2019).
Thus, we conducted a study with the aim of evaluating thermal tolerance and thermal safety margins (TSMs) of alpine species belonging to different growth forms and their relationship with leaf functional traits. We selected three different sites from the Himalayan alpine region with a significant amount of climatic variation. The present study was thus undertaken with the following objectives: 1) to estimate the variation in thermal tolerance among various species and their growth forms and to evaluate the relationship between thermal tolerance and growth forms, 2) to elucidate the relationship between thermal tolerance and leaf traits, and 3) to study the variation in TSMs among various species to evaluate as to how vulnerable the Himalayan alpine species are to future temperature increase. We evaluated TSM as follows: TSM=T50 − Tleaf.
2 Materials and methods
2.1 Study area
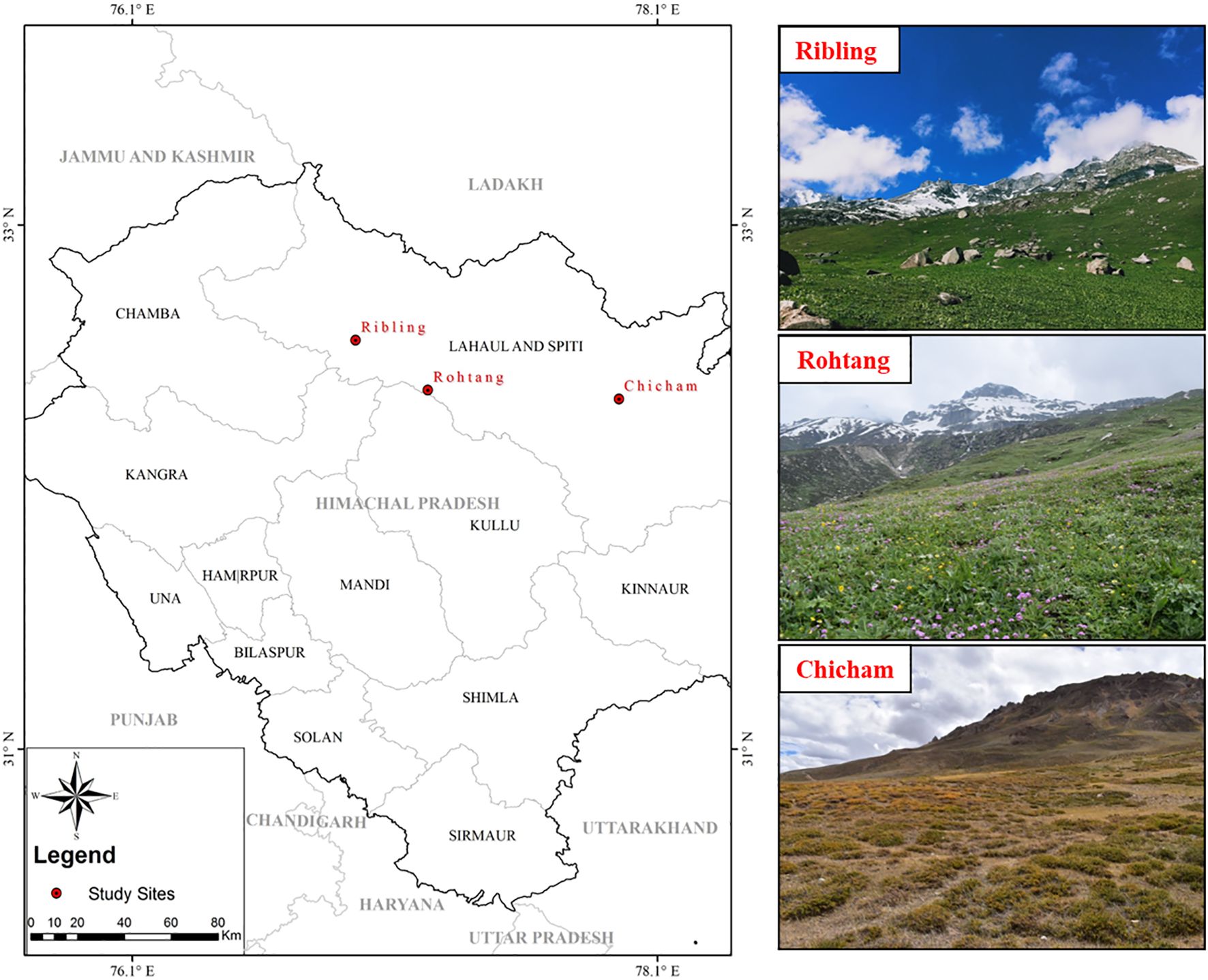
The present study was conducted in the Western Himalaya Region at three sites: Ribling (32°34′19″N 76°58′27″E) and Chicham (32°20′46″N 77°59′06″E), both of which lie on the southern slopes, and Rohtang (32°22′48″N 77°15′05″E), which lies on the northern slope. The study sites are depicted in the map (Figure 1), and their description is provided in Supplementary Table S1. The region spanning these sites is rich and diverse in vegetation with high climatic variation, wherein one can find mountain slopes that receive more sunlight (southern slopes) and are drier and warmer, in contrast with the northern slopes, which are cold and humid (Måren et al., 2015). Further, the alpine region of the Himalaya is unique compared to other eco-regions across the globe, as the Himalaya is situated in the tropical latitudes, presenting exceptionally high elevations, thereby a rarefied atmosphere and high radiation in addition to a low temperature regime. This uniqueness also endows this eco-region to harbor a rich diversity of plants (with diverse growth forms and life-forms) and many endemics. The recently reported “elevational dependent warming” posits a higher temperature increase in the alpine region of the Hindu Kush Himalaya–Tibet Plateau, compared to other eco-regions (Dimri and Allen, 2020).
2.2 Study species
A total of 52 alpine plant species occurring at three distant sites were selected for the present study. The plant species were selected as representative of various growth forms (e.g., graminoid, forb, rosette, and dwarf shrub) and are common and abundantly found in the study area. A list of these species is provided in Supplementary Table S2 along with brief details. The study was carried out in 2022 and 2023 during the peak of the growing season from mid-June to mid-August.
2.3 Sampling and estimation of leaf eco-physiological traits
Mature disease-free and completely opened sun-facing leaves from several individuals (n=7) of various species were sampled to evaluate T50, Tcrit, and leaf functional traits. At least five to 10 leaves per plant were collected from each of the species. Sampled leaves were placed in a sealed plastic bag that contained a wad of rolled wet tissue to maintain moisture levels, which was then placed into an ice box containing ice packs to be transported to the lab. Fresh weight (FW) was recorded for the leaf samples collected. Thereafter, the saturated weight (SW) was determined after keeping the samples in a moist paper towel for 12 hours at 4°C. Also, the leaves were scanned using a digital scanner (imageCLASS D520, Canon Inc., Tokyo, Japan) to measure leaf area (LA) using the ImageJ software (version 1.47v). The samples were then put in properly labeled paper bags and kept in a hot air oven for 72 hours at 65°C to dry. The leaves were reweighed after drying to measure dry weight (DW) (Ryser et al., 2008; Pérez-Harguindeguy et al., 2013). LMA was estimated as the dry weight per unit area of leaf discs that were cut using a circular puncher to make an area of 0.8 cm2. SLA was estimated as the inverse ratio of LMA. LDMC was measured as a ratio of dry weight to saturated weight, and RWC was estimated as (FW − DW)/(SW − DW) × 100 (Rathore et al., 2018).
2.4 Estimation of thermal tolerance (T50)
To measure T50, leaf discs (0.8 cm2) were cut using a circular puncher from disease-free leaves (n=7) collected from seven individuals per species (n=1 leaf/individual), while whole leaflets were used for species that had succulent or very small leaves. From each leaf, leaf discs (or whole leaf, if succulent) (n=7 for each temperature) were placed onto muslin cloth with multiple layers to support the adaxial surface to avoid anaerobiosis and to cover the abaxial surface with a single layer. These wrapped discs were then placed inside a Ziploc pouch and subjected to specific preset temperatures (28°C, 33°C, 38°C, 43°C, 48°C, 53°C, and 58°C), utilizing a water bath (JBN18 NOVA, Grant Instruments, Ltd., Cambridge, UK) for T50 estimation, following Krause et al. (2010) and Leon-Garcia and Lasso (2019). The mean growing season temperature (MGT) recorded at Ribling (one of the three study sites) was 22.7°C from July to August for the years 2016–2019. Therefore, our initial treatment temperature was + 5°C from the mean growing season temperature, and an increment of 5°C above this level was maintained throughout our temperature treatment. Each temperature treatment was maintained for 15 minutes (Leon-Garcia and Lasso, 2019). After the treatment, leaf discs were incubated in the dark for 24 hours at room temperature (25°C–28°C) by placing the discs on a wet tissue towel in a Petri dish (Leon-Garcia and Lasso, 2019). T50 and Tcrit were estimated by observing a decrease in Fv/Fm ratio on dark-incubated leaf discs (n=7 discs/species), following Krause et al. (2010). The latter was measured using a portable fluorometer (FluorPen FP 110, Photon Systems Instruments, Czechia). A logistic curve was fitted to the change in Fv/Fm against temperature for all the studied species, and T50 and Tcrit, the temperatures at which Fv/Fm is reduced to 50% and 15%, respectively, were estimated. The R package “fitplc”, employing the Weibull model and a confidence interval 95%, was used to fit the curves, and T50 and Tcrit were determined from these curves. The argument “Kmax” was modified to match the control mean Fv/Fm for all the species. From the curve, T50 and Tcrit were determined at the temperature when the Fv/Fm value was 50% and 15%, respectively, of the upper asymptote.
2.5 Thermal safety margin
The difference between T50 and mean air temperature is often considered as the TSM, where it is assumed that air and leaf temperatures are equal (Sastry and Barua, 2017; O’sullivan et al., 2017). TSM was calculated as the difference between the optimal leaf temperature of a day (Tleaf) and T50 (Leon-Garcia and Lasso, 2019), assuming that the temperature of the leaf normally surpasses the temperature of the air (Krause et al., 2010; O’sullivan et al., 2017). Tleaf (Supplementary Table S4) was calculated from thermal images taken using a portable thermal imaging camera (FLIR T650sc, FLIR Systems AB, Täby, Sweden) on five to seven healthy sun-exposed leaves per plant, of seven plants per species, and at least three images of the same leaf were taken; thermal imaging was undertaken only during the sunny days and between 10:00 am and 12:00 pm local time. The thermal images obtained were analyzed using the ResearchIR software (FLIR Systems AB, Sweden).
2.6 Statistical analysis
The means and standard deviation were calculated for all of the estimated parameters. The Shapiro–Wilk and Levene’s tests were used to check for the assumptions of normal distribution and homogeneity of variances before data analysis. To investigate the variation in T50 among all the studied species, we inspected the differences in Fv/Fm across all the 52 studied species using one-way ANOVA. Additionally, post-hoc multiple comparisons were performed using Tukey’s test to infer the differences among the species. The correlation among leaf traits was assessed using Spearman’s rank correlation coefficient, utilizing a species-level “means of values” dataset. Spearman’s coefficient applies to data distributions that are not normal, is not affected by outliers in the dataset, and is more robust compared to the conventional Pearson’s correlation. Spearman’s rank correlation analysis was performed using the “corrplot”, “datasets”, and “PerformanceAnalytics” packages to examine the relationship between T50 and leaf traits. For this, the estimates of T50, Tcrit, Tleaf, and leaf traits were used. The variation in T50 among various growth forms was analyzed using a linear mixed-effects (LME) model with “Type III analysis of variance” using “Satterthwaite’s method” (Bates et al., 2015; Kuznetsova et al., 2017). The “growth form” was considered as a fixed effect and “species” as a random factor. The LME model was applied with the “lme4” and “lmerTest” packages. All the statistical analyses were performed in R (Ver 4.4.3, R Development Core Team, 2025).
3 Results
3.1 Thermal tolerance (T50) of photosystem II
The response of chlorophyll a fluorescence Fv/Fm after dark adaptation in temperatures ranging from 28°C to 43°C revealed a slight to no change. A sudden decline in Fv/Fm was observed only after 48°C for most of the species. Further, a drastic decline in Fv/Fm was observed only after 53°C for a few of the species, such as Bistorta affinis, Geum elatum, Hyoscyamus niger, Lindelofia longiflora, Mentha longifolium, Picrorhiza kurroa, Polygonum sp., Ranunculus hirtellus, Rosularia alpestris, Rumex acetosa, Rumex nepalensis, and Sibbaldia cuneata (see Supplementary Table S3). Furthermore, for most of the species, Fv/Fm was reduced to zero at 58°C. The temperature treatment logistic curves fitted to obtain T50, Tcrit, and the response of Fv/Fm at various temperatures for all the species are provided in Supplementary Figure S1, and the mean values of Fv/Fm recorded at different temperatures are provided in Supplementary Table S3. The species such as Calamagrostis emodensis, B. affinis, and R. acetosa showed the highest T50 at 65.9°C, 54.4°C, and 53.7°C, respectively. The species that showed a lower T50 were Malva neglecta (46°C) and Plantago himalaica (44.9°C). The T50 of C. emodensis (graminoid) was found to be considerably higher than that of other species. Tcrit for all the studied species was observed to range from 35.67°C to 50.86°C. The variation in T50 among species was statistically significant (F=2,371; p<0.001), belonging to various growth forms such as, between forbs and rosettes, between rosettes and graminoid, and between rosettes and dwarf shrubs. The values of T50 and Tcrit for all the study species are provided in Supplementary Table S4.
3.2 Leaf eco-physiological traits and their relationship with T50, Tcrit, and Tleaf
A significant positive correlation was observed between T50, leaf mass area (LMA), and LDMC, but there was no significant correlation of T50 with other traits such as SLA, relative water content (RWC), and LT. A significant positive correlation was also observed between T50 and Tcrit, and between Tcrit and Tleaf. The results of Spearman’s correlation are depicted in Figure 2, and mean values of all the studied leaf traits are given in Supplementary Table S5.
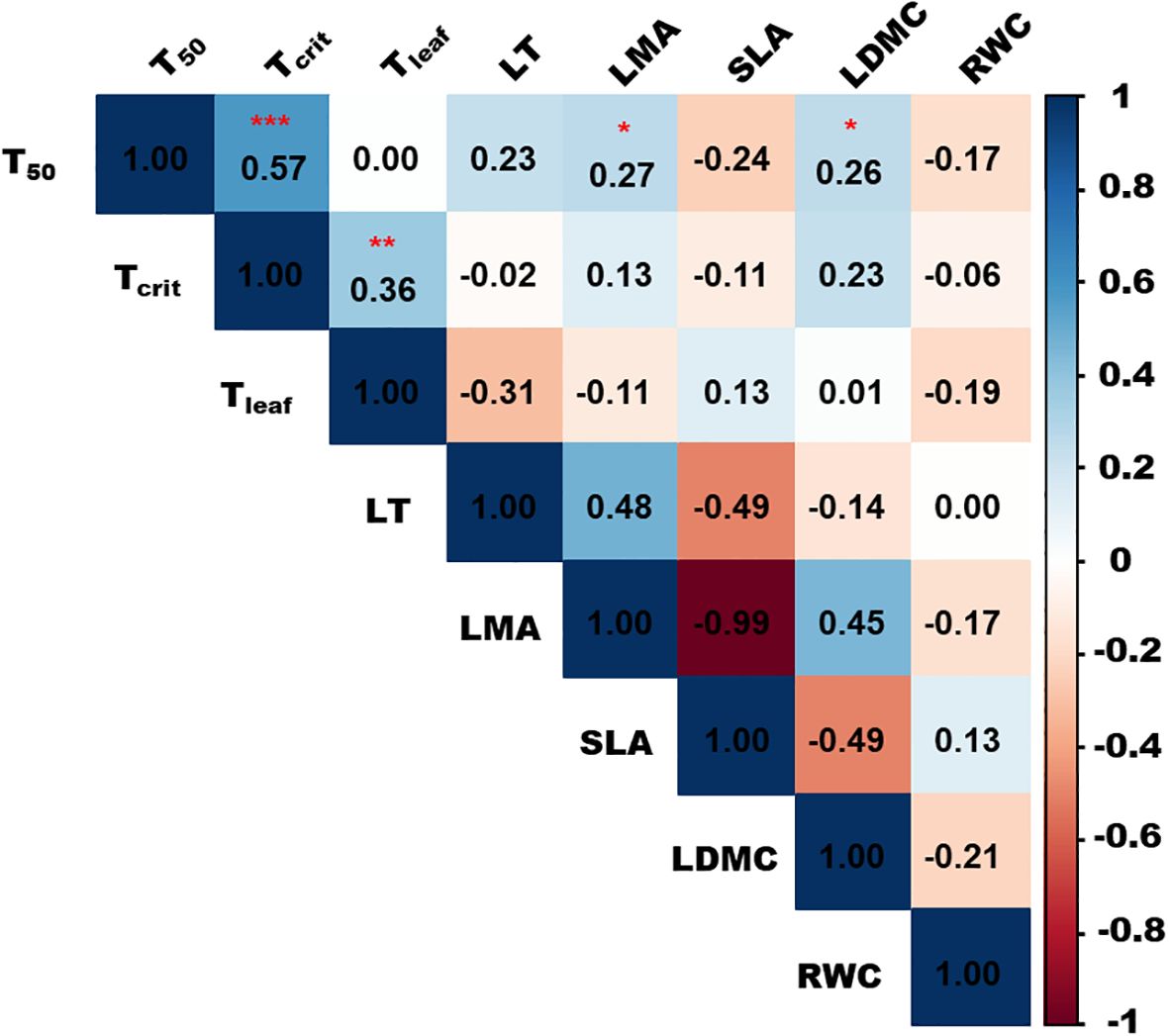
Figure 2. Correlation analysis between T50, Tleaf, Tcrit, and studied leaf traits. The colors represent correlation coefficients, indicating the strength and magnitude of the correlation. The blue color shows positive correlations, pink color shows negative correlations, and white color shows no significant relationship. "*" represents p < 0.05, "**" represents p < 0.01 and "***" represents p < 0.001.
3.3 Relationship between growth form and T50
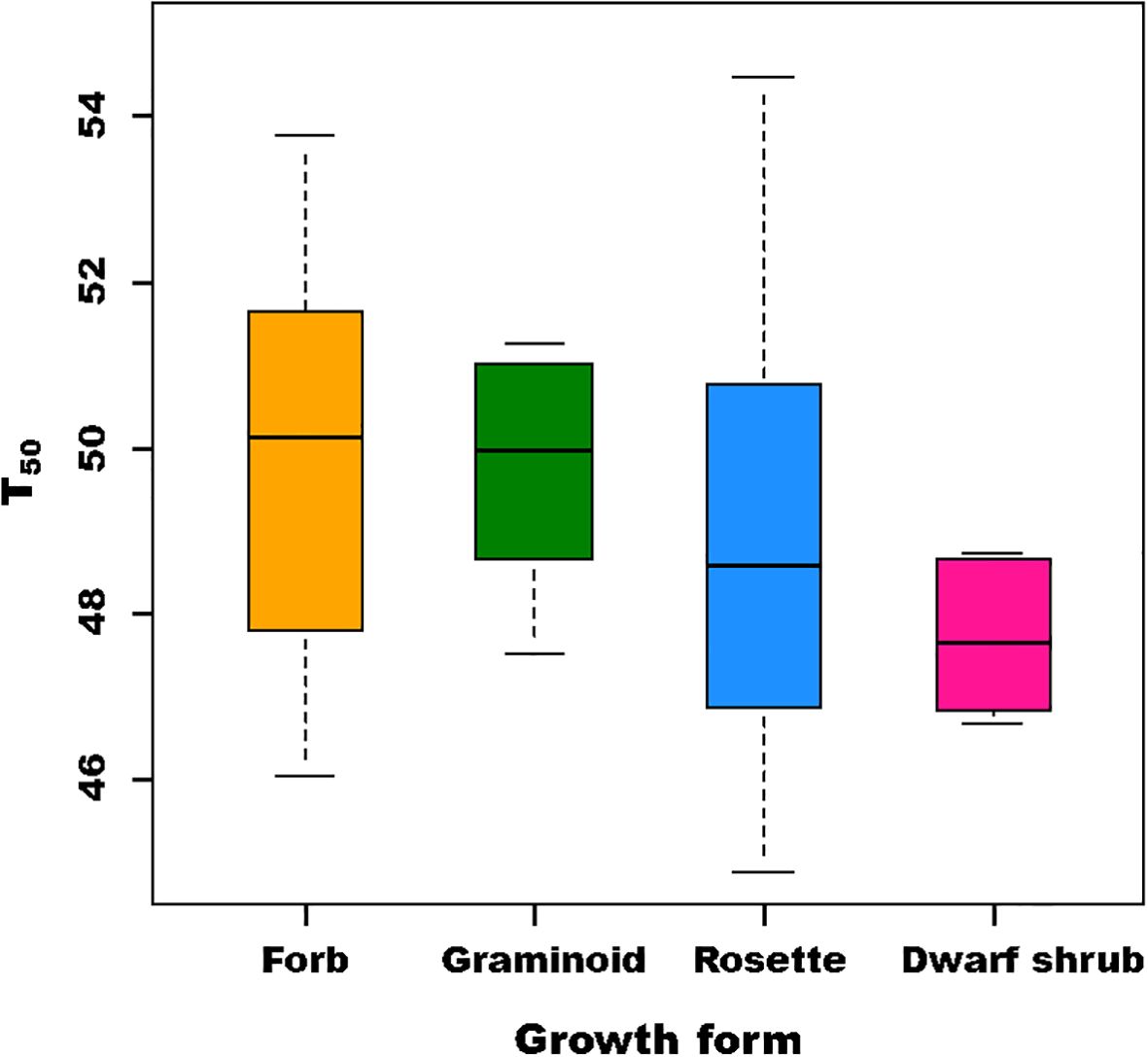
The T50 of the studied species was found to range from 44.9°C to 65.9°C (Supplementary Table S4). The highest T50 was observed for a graminoid (C. emodensis) at 65.9°C, followed by a rosette at 54.4°C (Bistorta affinis), a forb at 54°C (R. acetosa), and a dwarf shrub (Rhododendron anthopogon) at 49°C. Overall, the highest thermal tolerance was observed for graminoid (53.8°C ± 8.2°C) and forb (49.8°C ± 2.2°C), followed by rosette (48.7°C ± 2.4°C) and dwarf shrub (47.7°C ± 0.96°C). Since one of the species had an unusually high value of T50, which may have impacted the statistical analysis, the data in the LME model were analyzed after both including and excluding the species, with “growth form” as a fixed effect and “species” as a random effect. Thus, the variation in T50 was not observed to be significantly different among the various growth forms (Figure 3 and Supplementary Table S6).
3.4 Thermal safety margin
The thermal safety margins of all the studied species were found to be much higher than their leaf temperature (Tleaf) (see Supplementary Table S4) recorded through thermal imaging during the study period. TSMs ranged from 20°C to 40.5°C for most of the species. A few species, such as Epilobium royleanum, Potentilla cuneata, Psychrogeton andryaloides, and Taraxacum officinale (at Ribling), had TSMs below 20°C, with R. alpestris having the lowest TSM at 9.7°C (see Supplementary Table S4). Therefore, it can be inferred that, currently, our studied species are not enduring temperatures close to their critical temperature (Tcrit or T50) (see Figure 4).
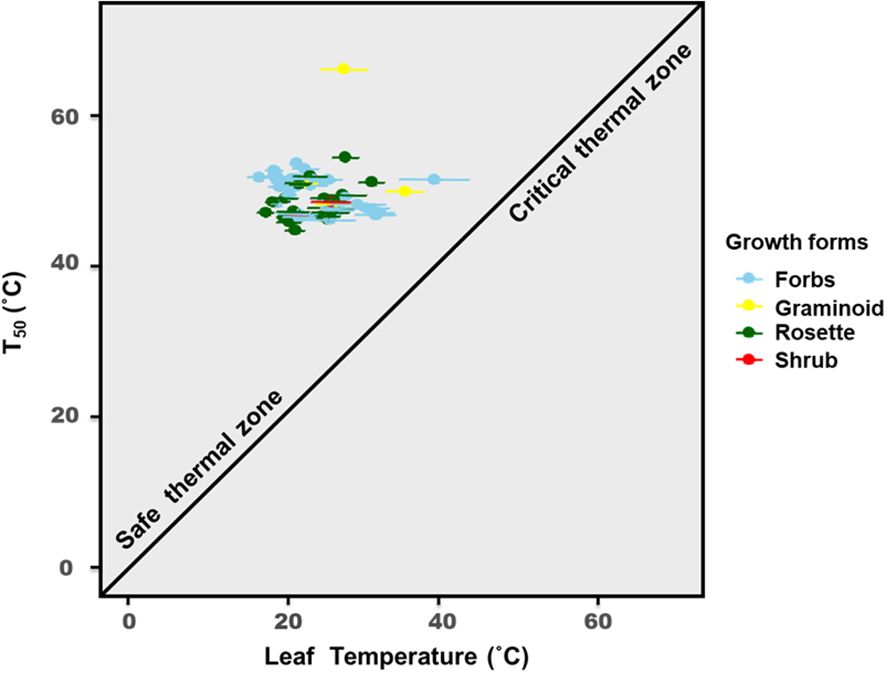
Figure 4. Graphical representation of thermal susceptibility for the 52 studied species. The black bold line represents the zone where thermal tolerance and leaf temperature are equal; also, the line shows the partition between the safe thermal zone and critical thermal zone, where T50 is higher than the leaf temperature recorded during the day of sampling. The colors represent the species’ growth forms. Lines in each point are the error bars depicting standard deviation for both T50 (°C) and leaf temperature (°C).
4 Discussion
The aim of our study was to estimate the critical temperature (Tcrit), thermal tolerance (T50), and TSM of alpine plant species from the Himalaya. We also evaluated whether there is any relationship between T50 and Tcrit with Tleaf, LMA and other leaf traits, and whether T50 varies among various species and across different growth forms. We found the T50 of all the studied species to be higher than the current ambient air temperature recorded at the study sites (Supplementary Table S4). Similar studies conducted in other eco-regions have also reported such observations (Perera-Castro et al., 2018; Leon-Garcia and Lasso, 2019). Also, for most of the species, TSMs were found to be well above 20°C, which suggests that at present, these species may have a sufficient buffer from experiencing thermal damage caused by future warming conditions in the alpine region of the Himalaya, corroborating our first hypothesis. A high marginal difference between the T50 values of the studied species implies that species in these regions are, at present, not susceptible to an increase in temperature and are surviving within their thermal protection region. Further, the difference in thermal tolerance among species was non-random, wherein a significant positive correlation was observed with important leaf functional traits.
T50 describes the injury to photosystem II (PS II), which is an irrevocable event (Braun et al., 2002; Krause et al., 2010), as PS II is more sensitive to high temperatures than PS I (Berry and Bjorkman, 1980; Havaux, 1993). In the present study, we observed T50 to range from 44.9°C to 53.7°C for most of the studied species, which is similar to reports from other alpine regions such as the Alps and the temperate eco-regions (Neuner et al., 2000; Braun et al., 2002; Körner, 2003; Leon-Garcia and Lasso, 2019; González-Rodríguez et al., 2021; Sklenar et al., 2023). For some species, such as B. affinis, C. emodensis, G. elatum, H. niger, L. longiflora, M. longifolium, P. kurroa, Polygonum sp., R. hirtellus, R. alpestris, R. acetosa, R. nepalensis, and S. cuneata, we observed a dramatic change in Fv/Fm at 53°C. Apart from T50, we found Tcrit to also vary greatly among the studied species and to range between 35.67°C and 50.86°C. Perez and Feeley (2020) also reported somewhat similar values of Tcrit for tropical tree species, varying between 37°C and 48°C. T50 and Tcrit describe different amplitudes of thermal tolerance and suggest different extents of thermal damage caused to the leaf. The T50 describes the damage of the leaf at 50%, and Tcrit indicates tissue damage at the initial stage, which is at 15% (O’sullivan et al., 2017; Perez and Feeley, 2020). Therefore, when we calculate the TSM using either T50 or Tcrit values and subtract it from leaf temperature or air temperature (O’sullivan et al., 2017; Sastry and Barua, 2017; Leon-Garcia and Lasso, 2019; Perez and Feeley, 2020), we can understand how close the species are performing to their thermal threshold.
Further, we found T50 to have a positive correlation with leaf traits such as LMA and LDMC, which support our second hypothesis that species having increased leaf structural investment will have increased tolerance toward high temperature, as also suggested by various studies (Curtis et al., 2012; Monteiro et al., 2016; Sastry and Barua, 2017; Leigh et al., 2017). LMA is a key trait in explaining the resource acquisition strategies in “rapid-slow” leaf economic spectrums (Wright et al., 2004; Reich, 2014). As reported by Sastry and Barua (2017) and Zhang et al. (2012), T50 shows a positive relationship with species having higher LMA (evergreen species) and a negative relationship with species having lower LMA (deciduous species). This suggests that the species with lower LMA will be more vulnerable to rising temperatures. The increase in LMA is also reported to be associated with high tolerance to cold temperatures (González-Zurdo et al., 2016; Zhang et al., 2020). The correlation between T50 and LMA could be explained by the assertion that lower LMA indicates a high photosynthesis rate, which is accompanied by high conductance and transpiration (Brodribb et al., 2007; Reich, 2014), as leaf temperature is regulated by transpiration (Lambers et al., 1998; Defraeye et al., 2013; Scheffers et al., 2016) and stomatal conductance (Jones, 1992). Conversely, higher LMA and LDMC are linked with greater investment in leaf structure to generate robust tissues to enable such species to be more stress-tolerant, hence providing a higher capability to tolerate higher temperatures (Wright et al., 2004).
Thus, it is apparent that species having leaves that possess resilience toward stressful environments are not as susceptible to high heat stress, which otherwise may have caused impairment to their photosynthetic apparatus. Furthermore, the relationship between T50 and LMA may not be the same across all species, although we found a positive correlation between LMA and T50. A study conducted on woody species from around the world by O’sullivan et al. (2017) found no significant relationship between thermal tolerance and LMA. Therefore, further studies on various species from different regions and spanning a wide environmental gradient are required to better understand the relationship between thermal tolerance and leaf traits to accurately decipher whether these could be region- or species-specific. Furthermore, the observed values of Tleaf were found to be positively correlated with Tcrit, but not with T50, unlike that reported by Perez and Feeley (2020). A recent study by Cox et al. (2025) also reported a positive correlation between Tcrit and Tleaf; the latter relates to leaf size and leaf thickness and also has a key role in leaf energy balance and in transpiration during heatwaves (Curtis et al., 2012; Leigh et al., 2012; Drake et al., 2018), which may explain its relationship with Tcrit. Also, Cox et al. (2025) observed that variation in Tcrit was related to Tleaf, which suggests that regulating leaf temperature by plants is a vital process in avoiding thermal damage.
We documented that the T50 of Himalayan alpine species varied across different growth forms; however, the differences among them were not found to be significant, unlike those suggested by Leon-Garcia and Lasso (2019). They reported that, among alpine plants, the rosette growth form was the most tolerant to high temperature compared to the other three growth forms: grasses, forbs, and shrubs. In our study, we had two species of dwarf shrubs, four graminoids, 18 rosettes, and 34 forbs. Although the relationship between T50 and growth forms showed non-significant results when analyzed using LME, there were important insights from the analysis. Moreover, we had undertaken the LME model considering C. emodensis (graminoid), which had the highest T50 (65.9°C); when we included it in the LME analysis, we observed p=0.02 (statistically significant), and when we excluded it, p=0.3 (non-significant). Notwithstanding this, we observed that rosette growth forms had a high T50 ranging from 44.9°C to 54.4°C, yet it was not the highest among the four growth forms studied. Graminoids had the highest T50, ranging from 48°C to 65.9°C, which was followed by forbs (46.3°C to 53.7°C) and dwarf shrubs (46.7°C to 48.5°C). A similar finding of high thermal tolerance in grasses was reported by Leon-Garcia and Lasso (2019). A study by Sklenář et al. (2023) observed that growth forms such as shrubs and rosette-like growth habits have high resilience to high temperatures. This finding partially supports our third hypothesis that much shorter growth forms, such as rosettes, will have higher thermal tolerance, similar to the observation made by Buchner and Neuner (2003) and Leon-Garcia and Lasso (2019). From our study, it can be inferred that rosettes, graminoids, and other forbs had somewhat equivalent thermal tolerance, slightly higher than that of dwarf shrubs. Aside from high heat tolerance in rosette growth forms, these are also reported to have high tolerance toward cold temperature (Squeo et al., 1991). The reason behind rosette forms being highly tolerant to both temperature extremes may be because they have the ability to decouple the plant body temperature from the surroundings (Körner and Larcher, 1988). This in turn offsets an increase in leaf temperature from an increase in ambient air temperature (Salisbury and Spomer, 1964; Körner and Cochrane, 1983), which may also explain the need for higher tolerance to high temperature (Meinzer and Goldstein, 1985). In addition, the high T50 of graminoids may possibly lie in their morphological characters, such as tillers and root systems. Xu and Huang (2001), in the case of Agrostis palustris Huds, suggested that a higher density of tillers may result in an increased rate of photosynthesis at the canopy level with better light interception and, consequently, a higher carbohydrate accumulation (Xu and Huang, 2001). A denser root system allows better nutrient and water uptake during thermal stress and also allows transpirational cooling using water from the soil surface (Engelke et al., 1985). Our results also showed that grasses had high LDMC, which is a trait that supports high thermal tolerance.
High T50 and variation in T50 among the growth forms (although not significant) may be an indication that growth forms coupled with traits that support high heat tolerance, traits other than those discussed here, such as hairiness, dense pubescence, or insulating features of leaf structure, may also influence thermal tolerance, as most of our species were found to have different leaf structures. For example, species such as Anaphalis nepalensis, Arnebia euchroma, Picris hieracioides, Potentilla argyrophylla, P. andryaloides, S. cuneata, and Verbascum thapsus have either sparsely or densely (tomentose) hairy leaves. Furthermore, the ambient humidity conditions could also be one of the factors influencing the thermal tolerances of species. Buchner and Neuner (2003) reported that species found in low-humidity areas showed higher tolerance to high temperature, whereas species occurring in high-humidity conditions showed a lower thermal tolerance. In our study, two species, viz., P. argyrophylla and T. officinale, both of which are common in Ribling and Rohtang, the sites representing low and high relative humidity, respectively, we found the T50 of these two species to be 1°C to 3°C higher in the low-humidity site compared to the high-humidity site. However, this finding was not consistent for other species, such as L. longiflora, R. nepalensis, and R. acetosa, which are also common in our study sites, which may be due to the ambient temperature at that particular point in time when we collected the data (see Supplementary Tables S1, S7), as ambient temperature is a main contributor in determining leaf temperature (Defraeye et al., 2013; Curtis et al., 2016). The T50 values that we have observed in our study on alpine plants of the Himalaya do align with reports from other alpine regions, such as temperate alpine, tropical alpine, and the Alps. The T50 ranging from 45°C to 57°C in these various studies and our present study implies that alpine plants have high thermal tolerance to a wide range of temperatures, which may be the reason that these plants are able to survive low threshold of cold night temperatures to high peaks of daytime temperature (Neuner et al., 1999; Neuner et al., 2000; Braun et al., 2002; Körner, 2003; Leon-Garcia and Lasso, 2019; González-Rodríguez et al., 2021; Sklenar et al., 2023).
We observed wide TSMs ranging from 20°C to 40.5°C for most of our study species. A study by Leon-Garcia and Lasso (2019) also reported wide TSMs ranging from 12.1°C to 30°C in 21 Andean tropical alpine plants. In contrast, a study by Perez and Feeley (2020) found modest TSMs between 6°C and 14°C, suggesting that species from this tropical region may be vulnerable to a rise in temperature in the future. Furthermore, there are other reports from tropical to subtropical to temperate regions (Curtis et al., 2016; O’sullivan et al., 2017; Sastry and Barua, 2017; Kitudom et al., 2022) that suggested narrower TSMs of some species. These findings imply that species in these regions (tropical, subtropical, and temperate) could be more susceptible to future warming, as these species already exist near their maximum thermal threshold. Moreover, thermal tolerance is considered to be time-dependent (Sutcliffe, 1977), and extended periods of exposure to high temperatures will amplify the risk of damage regardless of the ability to withstand high temperatures (Buchner and Neuner, 2003). In tropical tree species of the Amazon, Kullberg et al. (2024) observed that although these Amazon species were able to acclimatize and increase their T50 with an increase in mean growing season temperature, acclimatization was, however, inadequate in consistently maintaining the TSMs in accordance with an increase in mean growing season temperature. This ultimately resulted in the decline of leaf health and thus the performance of trees, which suggests that continuous exposure to temperature stress will have a negative effect in the long run, even on species having wider TSMs. The RCP 2.6, RCP 4.5, RCP 6.0, and RCP 8.5 climate change scenarios predicts rise in global mean temperature by 1°C to 1.6°C, 2°C to 3°C, 3°C to 4°C, and 3°C to 5.1°C respectively, and these also indicate an increase in global temperature from 0.4°C to 2.6°C and 0.3°C to 4.8°C by the middle of 21st and end of 21st century (De Pryck, 2021). Additionally, a recent study by Hansen et al. (2025) reported that the rate of increase in temperature has dramatically increased above 50%. They also observed that just in the last couple of years, the increase in global temperature was recorded at 0.4°C, whereas during the 1970–2010 period, it was reported at 0.18°C. Moreover, higher elevations are reported to be warming up at an alarming rate because of elevation-dependent warming (Krishnan et al., 2019; Dimri et al., 2022). Therefore, even though the alpine species having wide TSMs at present are safe and nowhere near their maximum thermal thresholds, the threat of global warming to the alpine plant community is real, and the consequences are imminent. Further, the present study was conducted on mature plants and during the peak growing season, but studies on the thermal tolerance of young plants, considering the water stress conditions, need to be undertaken. The temperature near the soil surface is greater during the spring season when the snow melts, and the emerging new plantlets would be at greater risk of thermal damage. The ability to withstand, survive, and grow will ultimately depend on the inherent thermal tolerance of the species (Marcante et al., 2014). Drought- or water-stressed conditions are reported to increase thermal tolerance in tropical tree species (Sastry et al., 2018); however, these species are also reported to be performing at their maximum thermal threshold (Sastry and Barua, 2017). Therefore, such species could be more at risk due to the temperature increase, as global warming will be accompanied by erratic weather patterns (Choler, 2023). In addition, a rise in temperature will lead to a decline in soil water accessibility due to evaporation, which will affect the plant–water relations (Wahid et al., 2007; Beaumont et al., 2011). Therefore, studies focusing on the thermal tolerance of young plants and targeted toward drought effects will help in assessing the vulnerability of Himalayan alpine plants to future climate change scenarios. However, at present, our finding demonstrates that alpine species from the Himalayan alpine region have the ability to tolerate high temperatures, suggesting that future warming may not essentially lead to range shifting (Lenoir et al., 2010), or the probable annihilation of the Himalayan alpine species, that is, if high temperature tolerance is the deciding factor in the fate of alpine plants in a warming world.
5 Conclusion
Our study reveals that the upper thermal threshold of species from the Himalayan alpine region at present is not near their critical point, as the thermal safety margins of most of the species were well above 20°C. T50 was also found to be higher, ranging from 44.9°C to 65.9°C, and showed a positive correlation with key leaf traits such as LMA and LDMC. The studied species had a wide range of TSMs and high T50, which suggests that these species at present may not be adversely affected by a possible 1°C to 2°C rise in future temperatures. The significance of key leaf traits and associated mechanisms involved in imparting a relatively high tolerance to higher temperatures requires comprehensive and thorough studies. Such studies should include various species from different eco-regions spanning across the globe to estimate the level of their thermal tolerance and the threats of rising global temperatures on plants and their communities.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
Author contributions
NH: Conceptualization, Writing – review & editing, Data curation, Formal Analysis, Validation, Investigation, Methodology, Writing – original draft. AC: Conceptualization, Formal Analysis, Validation, Writing – review & editing, Data curation, Supervision.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. This study was supported by a financial grant from the Council of Scientific and Industrial Research, India (Project entitled "Bioprospecting, conservation and sustainable resource generation of high altitude bioresources at Centre for High Altitude Biology (CeHAB)"; Code:MLP 0205).
Acknowledgments
The authors wish to thank the Director, CSIR-IHBT Palampur, for providing the necessary facilities. Mr. Om Parkash is acknowledged for his help in plant identification. Ms. Nandita Mehta, Ms. Sita Kumari, Mr. Lakhbeer Singh, Mr. Bittu Thakur, Mr. Manish Kumar Sharma, and Mr Anupam Bhatt are acknowledged for their help during data analysis. Mr. Girja Nand and the staff of CeHAB are acknowledged for their unconditional help while carrying out field work and lab work at Ribling.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphgy.2025.1652412/full#supplementary-material
References
Ahrens C. W., Challis A., Byrne M., Leigh A., Nicotra A. B., Tissue D., et al. (2021). Repeated extreme heatwaves result in higher leaf thermal tolerances and greater safety margins. New Phytol. 232, 1212–1225. doi: 10.1111/nph.17640
Araújo M. B., Ferri-Yáñez F., Bozinovic F., Marquet P. A., Valladares F., and Chown S. L. (2013). Heat freezes niche evolution. Ecol. Lett. 16, 1206–1219. doi: 10.1111/ele.12155
Bates D., Mächler M., Bolker B., and Walker S. (2015). Fitting linear mixed effects models using lme4. J. Stat. Softw. 6, 1–48. doi: 10.18637/jss.v067.i01
Beaumont L. J., Pitman A., Perkins S., Zimmermann N. E., Yoccoz N. G., and Thuiller W. (2011). Impacts of climate change on the world’s most exceptional ecoregions. Proc. Natl. Acad. Sci. 108, 2306–2311. doi: 10.1073/pnas.1007217108
Berry J. and Bjorkman O. (1980). Photosynthetic response and adaptation to temperature in higher plants. Annu. Rev. Plant Physiol. 31, 491–543. doi: 10.1146/annurev.pp.31.060180.002423
Braun V., Buchner O., and Neuner G. (2002). Thermotolerance of photosystem 2 of three alpine plant species under field conditions. Photosynthetica 40, 587–595. doi: 10.1023/A:1024312304995
Brodribb T. J., Feild T. S., and Jordan G. J. (2007). Leaf maximum photosynthetic rate and venation are linked by hydraulics. Plant Physiol. 144, 1890–1898. doi: 10.1104/pp.107.101352
Buchner O. and Neuner G. (2003). Variability of heat tolerance in alpine plant species measured at different altitudes. Arctic Antarctic Alpine Res. 35, 411–420. doi: 10.1657/1523-0430(2003)035[0411:VOHTIA]2.0.CO;2
Buchner O., Stoll M., Karadar M., Kranner I., and Neuner G. (2015). Application of heat stress in situ demonstrates a protective role of irradiation on photosynthetic performance in alpine plants. Plant Cell Environ. 38, 812–826. doi: 10.1111/pce.12455
Choler P. (2023). Above-treeline ecosystems facing drought: lessons from the 2022 European summer heat wave. Biogeosciences 20, 4259–4272. doi: 10.5194/bg-20-4259-2023
R Development Core Team (2025). R: A language and environment for statistical computing (Vienna, Austria: R Foundation for Statistical Computing). Available online at: https://www.R-project.org/. doi: 10.1111/pce.13133R
Cox D., Marchin R. M., Ellsworth D. S., Wujeska-Klause A., Ossola A., Crous K. Y., et al. (2025). Thermal safety margins and peak leaf temperatures predict vulnerability of diverse plant species to an experimental heatwave. Plant Cell Environ. doi: 10.18637/jss.v067.i01
Cuesta F., Tovar C., Llambí L. D., Gosling W. D., Halloy S., Carilla J., et al. (2020). Thermal niche traits of high alpine plant species and communities across the tropical Andes and their vulnerability to global warming. J. Biogeogr. 47, 408–420. doi: 10.1111/jbi.13759
Curtis E. M., Gollan J., Murray B. R., and Leigh A. (2016). Native microhabitats better predict tolerance to warming than latitudinal macro-climatic variables in arid-zone plants. J. Biogeogr. 43, 1156–1165. doi: 10.1111/jbi.12713
Curtis E. M., Leigh A., and Rayburg S. (2012). Relationships among leaf traits of Australian arid zone plants: alternative modes of thermal protection. Aust. J. Bot. 60, 471–483. doi: 10.1071/BT11284
Dangles O., Rabatel A., Kraemer M., Zeballos G., Soruco A., Jacobsen D., et al. (2017). Ecosystem sentinels for climate change? Evidence of wetland cover changes over the last 30 years in the tropical Andes. PloS One 12, e0175814. doi: 10.1371/journal.pone.0175814
Defraeye T., Verboven P., Ho Q. T., and Nicolai B. (2013). Convective heat and mass exchange predictions at leaf surfaces: Applications, methods and perspectives. Comput. Electron. Agric. 96, 180–201. doi: 10.1016/j.compag.2013.05.008
De Pryck K. (2021). Intergovernmental expert consensus in the making: the case of the summary for policy makers of the IPCC 2014 Synthesis Report. Global Environ. Politics 21, 108–129. doi: 10.1162/glep_a_00574
Deutsch C. A., Tewksbury J. J., Huey R. B., Sheldon K. S., Ghalambor C. K., Haak D. C., et al. (2008). Impacts of climate warming on terrestrial ectotherms across latitude. Proc. Natl. Acad. Sci. U.S.A. 105, 6668–6672. doi: 10.1073/pnas.0709472105
Dimri A. P. and Allen S. (2020). Himalayan climate interaction. Front. Environ. Sci. 8, 96. doi: 10.3389/fenvs.2020.00096
Dimri A. P., Palazzi E., and Daloz A. S. (2022). Elevation dependent precipitation and temperature changes over Indian Himalayan region. Climate Dynamics 59, 1–21. doi: 10.1007/s00382-021-06113-z
Doughty C. E. and Goulden M. L. (2008). Are tropical forests near a high temperature threshold? J. Geophys. Res: Biogeosciences 113, G00B07. doi: 10.1029/2007JG000632
Drake J. E., Tjoelker M. G., Vårhammar A., Medlyn B. E., Reich P. B., Leigh A., et al. (2018). Trees tolerate an extreme heatwave via sustained transpirational cooling and increased leaf thermal tolerance. Global Change Biol. 24, 2390–2402. doi: 10.1111/gcb.14037
Engelke M. C., Kenna M. P., and Leman V. G. (1985). Quality turf in the natural environmentenhanced through genetic improvement. US Golf Assn Sect Rec 23, 5–7.
Feeley K., Martinez-Villa J., Perez T., Silva Duque A., Triviño Gonzalez D., and Duque A. (2020). The thermal tolerances, distributions, and performances of tropical montane tree species. Front. Forests Global Change 3. doi: 10.3389/ffgc.2020.00025
Gauslaa Y. (1984). Heat resistance and energy budget in different Scandinavian plants. Ecography 7, 5–6.
González-Rodríguez Á.M., Pérez-Martín E. M., Brito P., and Fernández-Marín B. (2021). Unexpected vulnerability to high temperature in the mediterranean alpine shrub Erysimum scoparium (Brouss. ex Willd.) Wettst. Plants 10, 379. doi: 10.3390/plants10020379
González-Zurdo P., Escudero A., Babiano J., García-Ciudad A., and Mediavilla S. (2016). Costs of leaf reinforcement in response to winter cold in evergreen species. Tree Physiol. 36, 273–286. doi: 10.1093/treephys/tpv134
Hansen J. E., Kharecha P., Sato M., Tselioudis G., Kelly J., Bauer S. E., et al. (2025). Global warming has accelerated: are the United Nations and the public well-informed? Environment: Sci. Policy Sustain. Dev. 67, 6–44. doi: 10.1080/00139157.2025.2434494
Havaux M. (1993). Characterization of thermal damage to the photosynthetic electron transport system in potato leaves. Plant Sci. 94, 19–33. doi: 10.1016/0168-9452(93)90003-I
Javad A., Premugh V., Tiwari R., Bandaru P., Sunny R., Hegde B., et al. (2025). Leaf temperatures in an Indian tropical forest exceed physiological limits but durations of exposures are currently not sufficient to cause lasting damage. Global Change Biol. 31, e70069. doi: 10.1111/gcb.70069
Jones H. G. (1992). Plants and microclimate: a quantitative approach to environmental plant physiology. 2, 428. Cambridge University Press.
Khaliq I., Hof C., Prinzinger R., Böhning-Gaese K., and Pfenninger M. (2014). Global variation in thermal tolerances and vulnerability of endotherms to climate change. Proc. R. Soc. B: Biol. Sci. 281, 20141097. doi: 10.1098/rspb.2014.1097
Kitudom N., Fauset S., Zhou Y., Fan Z., Li M., He M., et al. (2022). Thermal safety margins of plant leaves across biomes under a heatwave. Sci. Total Environ. 806, 150416. doi: 10.1016/j.scitotenv.2021.150416
Körner C. (2003). The alpine life zone. In Alpine plant life: Functional plant ecology of high mountain ecosystems 9–20. Berlin, Heidelberg: Springer Berlin Heidelberg.
Körner C. and Cochrane P. (1983). Influence of plant physiognomy on leaf temperature on clear midsummer days in the Snowy Mountains, south-eastern Australia. Acta Oecol., Oecol. Plant. 4, 117–124.
Körner C. and Larcher W. (1988). Plant life in cold climates. In Symposia of the Society for Experimental Biology 42, 25–57. The Company of Biologists Ltd.
Krause G. H., Winter K., Krause B., Jahns P., García M., Aranda J., et al. (2010). High-temperature tolerance of a tropical tree, Ficus insipida: methodological reassessment and climate change considerations. Funct. Plant Biol. 37, 890–900. doi: 10.1071/FP10034
Krishnan R., Shrestha A. B., Ren G., Rajbhandari R., Saeed S., Sanjay J., et al. (2019). “Unravelling climate change in the Hindu Kush Himalaya: rapid warming in the mountains and increasing extremes,” in The Hindu Kush Himalaya assessment: Mountains, climate change, sustainability and people (Springer International Publishing, Cham), 57–97. doi: 10.1007/978-3-319-92288-1_3
Kullberg A. T., Coombs L., Soria Ahuanari R. D., Fortier R. P., and Feeley K. J. (2024). Leaf thermal safety margins decline at hotter temperatures in a natural warming ‘experiment’in the Amazon. New Phytol. 241, 1447–1463. doi: 10.1111/nph.19413
Kuznetsova A., Brockhoff P. B., and Christensen R. H. (2017). lmerTest package: tests in linear mixed effects models. J. Stat. software 82, 1–26. doi: 10.18637/jss.v082.i13
Lambers H., Chapin F. S. III, and Pons T. L. (1998). Leaf energy budgets: effects of radiation and temperature. In Plant Physiological Ecology, 210–229. New York, NY: Springer New York. doi: 10.1007/978-1-4757-2855-2_4
Lancaster L. T. and Humphreys A. M. (2020). Global variation in the thermal tolerances of plants. Proc. Natl. Acad. Sci. 117, 13580–13587. doi: 10.1073/pnas.1918162117
Larcher W., Wagner J., and Lütz C. (1998). The effect of heat on photosynthesis, dark respiration and cellular ultrastructure of the arctic-alpine psychrophyte Ranunculus glacialis. Photosynthetica 34, 219–232. doi: 10.1023/A:1006840623763
Leigh A., Sevanto S., Ball M. C., Close J. D., Ellsworth D. S., Knight C. A., et al. (2012). Do thick leaves avoid thermal damage in critically low wind speeds? New Phytol. 194, 477–487. doi: 10.1111/j.1469-8137.2012.04058.x
Leigh A., Sevanto S., Close J. D., and Nicotra A. B. (2017). The influence of leaf size and shape on leaf thermal dynamics: does theory hold up under natural conditions? Plant Cell Environ. 40, 237–248. doi: 10.1111/pce.12857
Lenoir J., Gégout J. C., Guisan A., Vittoz P., Wohlgemuth T., Zimmermann N. E., et al. (2010). Going against the flow: potential mechanisms for unexpected downslope range shifts in a warming climate. Ecography 33, 295–303. doi: 10.1111/j.1600-0587.2010.06279.x
Leon-Garcia I. V. and Lasso E. (2019). High heat tolerance in plants from the Andean highlands: Implications for paramos in a warmer world. PloS One 14, e0224218. doi: 10.1371/journal.pone.0224218
Lin H., Chen Y., Zhang H., Fu P., and Fan Z. (2017). Stronger cooling effects of transpiration and leaf physical traits of plants from a hot dry habitat than from a hot wet habitat. Funct. Ecol. 31, 2202–2211. doi: 10.1111/1365-2435.12923
Loveys B. R., Scheurwater I., Pons T. L., Fitter A. H., and Atkin O. K. (2002). Growth temperature influences the underlying components of relative growth rate: an investigation using inherently fast-and slow-growing plant species. Plant Cell Environ. 25, 975–988. doi: 10.1046/j.1365-3040.2002.00879.x
Manzi O. J. L., Mujawamariya M., Tarvainen L., Ziegler C., Andersson M. X., Dusenge M. E., et al. (2025). Photosynthetic heat tolerance partially acclimates to growth temperature in tropical montane tree species. Plant Cell Environ. 48, 7848–7861. doi: 10.1111/pce.70079
Marcante S., Erschbamer B., Buchner O., and Neuner G. (2014). Heat tolerance of early developmental stages of glacier foreland species in the growth chamber and in the field. Plant Ecol. 215, 747–758. doi: 10.1007/s11258-014-0361-8
Måren I. E., Karki S., Prajapati C., Yadav R. K., and Shrestha B. B. (2015). Facing north or south: Does slope aspect impact forest stand characteristics and soil properties in a semiarid trans-Himalayan valley? J. Arid Environ. 121, 112–123. doi: 10.1016/j.jaridenv.2015.06.004
McCulloch M. T., Winter A., Sherman C. E., and Trotter J. A. (2024). 300 years of sclerosponge thermometry shows global warming has exceeded 1.5 C. Nat. Climate Change 14, 171–177. doi: 10.1038/s41558-023-01919-7
Meinzer F. and Goldstein G. (1985). Some consequences of leaf pubescence in the Andean giant rosette plant Espeletia timotensis. Ecology 66, 512–520. doi: 10.2307/1940399
Monteiro M. V., Blanuša T., Verhoef A., Hadley P., and Cameron R. W. (2016). Relative importance of transpiration rate and leaf morphological traits for the regulation of leaf temperature. Aust. J. Bot. 64, 32–44. doi: 10.1071/BT15198
Neuner G., Braun V., Buchner O., and Taschler D. (1999). Leaf rosette closure in the alpine rock species Saxifraga paniculata Mill.: significance for survival of drought and heat under high irradiation. Plant Cell Environ. 22, 1539–1548. doi: 10.1046/j.1365-3040.1999.00508.x
Neuner G., Buchner O., and Braun V. (2000). Short-term changes in heat tolerance in the alpine cushion plant Silene acaulis ssp. excapa [All.] J. Braun at different altitudes. Plant Biol. 2, 677–683. doi: 10.1055/s-2000-16635
O’sullivan O. S., Heskel M. A., Reich P. B., Tjoelker M. G., Weerasinghe L. K., Penillard A., et al. (2017). Thermal limits of leaf metabolism across biomes. Global Change Biol. 23, 209–223. doi: 10.1111/gcb.13477
Pepin N., Deng H., Zhang H., Zhang F., Kang S., and Yao T. (2019). An examination of temperature trends at high elevations across the Tibetan Plateau: the use of MODIS LST to understand patterns of elevation-dependent warming. J. Geophys. Res. Atmos. 124, 5738–5756. doi: 10.1029/2018JD029798
Perera-Castro A. V., Brito P., and González-Rodríguez A. M. (2018). Changes in thermic limits and acclimation assessment for an alpine plant by chlorophyll fluorescence analysis: F v/F m vs. R fd. Photosynthetica 56, 527–536. doi: 10.1007/s11099-017-0691-6
Perez T. M. and Feeley K. J. (2020). Photosynthetic heat tolerances and extreme leaf temperatures. Funct. Ecol. 34, 2236–2245. doi: 10.1111/1365-2435.13658
Perez-Harguindeguy N., Diaz S., Garnier E., Lavorel S., Poorter H., Jaureguiberry P., et al. (2013). New handbook for standardised measurement of plant functional traits worldwide. Aust. J. Bot. 64, 715–716. doi: 10.1071/BT12225
Rathore N., Thakur D., and Chawla A. (2018). Seasonal variations coupled with elevation gradient drives significant changes in eco-physiological and biogeochemical traits of a high altitude evergreen broadleaf shrub, Rhododendron anthopogon. Plant Physiol. Biochem. 132, 708–719. doi: 10.1016/j.plaphy.2018.08.009
Reich P. B. (2014). The world-wide ‘fast–slow’plant economics spectrum: a traits manifesto. J. Ecol. 102, 275–301. doi: 10.1111/1365-2745.12211
Rundel P. W., Smith A. P., and Meinzer F. C. (Eds.). (1994). Tropical alpine environments: plant form and function. (New York: Cambridge University Press).
Ryser P., Bernardi J., and Merla A. (2008). Determination of leaf fresh mass after storage between moist paper towels: Constraints and reliability of the method. J. Exp. Bot. 59, 2461–2467. doi: 10.1093/jxb/ern120
Salisbury F. B. and Spomer G. G. (1964). Leaf temperatures of alpine plants in the field. Planta 60, 497–505.
Sastry A. and Barua D. (2017). Leaf thermotolerance in tropical trees from a seasonally dry climate varies along the slow-fast resource acquisition spectrum. Sci. Rep. 7, 11246. doi: 10.1038/s41598-017-11343-5
Sastry A., Guha A., and Barua D. (2018). Leaf thermotolerance in dry tropical forest tree species: relationships with leaf traits and effects of drought. AoB Plants 10, plx070. doi: 10.1093/aobpla/plx070
Scheffers B. R., Edwards D. P., Macdonald S. L., Senior R. A., Andriamahohatra L. R., Roslan N., et al. (2016). Extreme thermal heterogeneity in structurally complex tropical rain forests. Biotropica 49, 35–44. doi: 10.1111/btp.12355
Sklenář P., Jaramillo R., Wojtasiak S. S., Meneses R. I., Muriel P., and Klimeš A. (2023). Thermal tolerance of tropical and temperate alpine plants suggests that ‘mountain passes are not higher in the tropics’. Global Ecol. Biogeogr. 32, 1073–1086. doi: 10.1111/geb.13678
Sklenář P., Kučerová A., Macková J., and Romoleroux K. (2016). Temperature microclimates of plants in a tropical alpine environment: How much does growth form matter? Arctic Antarctic Alpine Res. 48, 61–78. doi: 10.1657/AAAR0014-084
Slot M., Cala D., Aranda J., Virgo A., Michaletz S. T., and Winter K. (2021). Leaf heat tolerance of 147 tropical forest species varies with elevation and leaf functional traits, but not with phylogeny. Plant Cell Environ. 44, 2414–2427. doi: 10.1111/pce.14060
Squeo F. A., Rada F., Azócar A., and Goldstein G. (1991). Freezing tolerance and avoidance in high tropical Andean plants: is it equally represented in species with different plant height? Oecologia 86, 378–382. doi: 10.1007/BF00317604
Sutcliffe J. (1977). Plants and temperature. Institute of biology's studies in biology 86. Edward Arnold Ltd.
Tarvainen L., Wittemann M., Mujawamariya M., Manishimwe A., Zibera E., Ntirugulirwa B., et al. (2022). Handling the heat–photosynthetic thermal stress in tropical trees. New Phytol. 233, 236–250. doi: 10.1111/nph.17809
Tiwari R., Gloor E., da Cruz W. J. A., Schwantes Marimon B., Marimon-Junior B. H., Reis S. M., et al. (2021). Photosynthetic quantum efficiency in south-eastern Amazonian trees may be already affected by climate change. Plant Cell Environ. 44, 2428–2439. doi: 10.1111/pce.13770
Wahid A., Gelani S., Ashraf M., and Foolad M. R. (2007). Heat tolerance in plants: an overview. Environ. Exp. Bot. 61, 199–223. doi: 10.1016/j.envexpbot.2007.05.011
Wambulwa M. C., Milne R., Wu Z. Y., Spicer R. A., Provan J., Luo Y. H., et al. (2021). Spatiotemporal maintenance of flora in the Himalaya biodiversity hotspot: Current knowledge and future perspectives. Ecol. Evol. 11, 10794–10812. doi: 10.1002/ece3.7906
Wright I. J., Reich P. B., Westoby M., Ackerly D. D., Baruch Z., Bongers F., et al. (2004). The worldwide leaf economics spectrum. Nature 428, 821–827. doi: 10.1038/nature02403
Xu Q. and Huang B. (2001). Morphological and physiological characteristics associated with heat tolerance in creeping bentgrass. Crop Sci. 41, 127–133. doi: 10.2135/cropsci2001.411127x
Zhang J. L., Poorter L., Hao G. Y., and Cao K. F. (2012). Photosynthetic thermotolerance of woody savanna species in China is correlated with leaf life span. Ann. Bot. 110, 1027–1033. doi: 10.1093/aob/mcs172
Keywords: thermal tolerance, alpine species, plant functional traits, growth forms, thermal safety margins, global climate change
Citation: Hopak NE and Chawla A (2025) Key eco-physiological leaf traits suggest a moderate to high level of thermal tolerance of alpine plants in the Western Himalaya. Front. Plant Physiol. 3:1652412. doi: 10.3389/fphgy.2025.1652412
Received: 23 June 2025; Accepted: 25 September 2025;
Published: 22 October 2025.
Edited by:
Rishikesh Bhalerao, Swedish University of Agricultural Sciences, SwedenReviewed by:
Ketong Yang, Tianjin University, ChinaOlivier Jean Leonce Manzi, University of Gothenburg, Sweden
Pallavi Sati, Hemwati Nandan Bahuguna Garhwal University, India
Copyright © 2025 Hopak and Chawla. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Amit Chawla, YW1pdGNoYXdsYTIxQGdtYWlsLmNvbQ==
 Nang Elennie Hopak
Nang Elennie Hopak Amit Chawla
Amit Chawla