- 1Institute for Clinical Research and Health Policy Studies, Tufts Medical Center, Boston, MA, United States
- 2Department of Medicine, Tufts University School of Medicine, Boston, MA, United States
- 3The New York Genome Center, New York, NY, United States
- 4Section of Computational Biomedicine, Boston University School of Medicine, Boston, MA, United States
- 5Bioinformatics Program, Boston University, Boston, MA, United States
- 6Department of Biostatistics, Boston University School of Public Health, Boston, MA, United States
- 7Data Intensive Study Center, Tufts University, Medford, MA, United States
Gaussian Graphical Models (GGMs) are a type of network modeling that uses partial correlation rather than correlation for representing complex relationships among multiple variables. The advantage of using partial correlation is to show the relation between two variables after “adjusting” for the effects of other variables and leads to more parsimonious and interpretable models. There are well established procedures to build GGMs from a sample of independent and identical distributed observations. However, many studies include clustered and longitudinal data that result in correlated observations and ignoring this correlation among observations can lead to inflated Type I error. In this paper, we propose a cluster-based bootstrap algorithm to infer GGMs from correlated data. We use extensive simulations of correlated data from family-based studies to show that the proposed bootstrap method does not inflate the Type I error while retaining statistical power compared to alternative solutions when there are sufficient number of clusters. We apply our method to learn the Gaussian Graphic Model that represents complex relations between 47 Polygenic Risk Scores generated using genome-wide genotype data from the Long Life Family Study. By comparing it to the conventional methods that ignore within-cluster correlation, we show that our method controls the Type I error well without power loss.
Introduction
One goal of biomedical research is to understand the network of complex relationships between biological variables and other factors to improve disease diagnosis and prognosis, and to identify drug targets (Vamathevan et al., 2019). The challenge of these analyses is the integration of the effect of multiple variables on more than one outcome of interest, simultaneously, and network modeling is a popular approach to address this task. Correlation networks are often used to model pairwise correlation, and, for example, weighted gene co-expression network analysis (WGCNA) is a popular solution to summarize the effects of multiple molecular features. Gaussian graphical models (GGMs) are a specific type of network modeling that use partial correlation rather than correlation to describe relations between may variables (Becker et al., 2023; Langfelder and Horvath, 2008). The advantage of GGMs is that they show the relation between two variables after “adjusting” for the effects of other variables and are therefore more parsimonious and interpretable. However, the calculation of the partial correlation typically assumes that all the variables are normally distributed (Markowetz and Spang, 2007).
The conventional method for learning a GGM is to perform hypothesis testing of the partial correlations that are derived from the normalized inverse of the variance-covariance matrix of the variables of interest (Whittaker, 2009). This approach roots in the assumptions that the variables follow a multivariate normal distribution, and the sample data consist of independent and identically distributed observations. The assumption of independent observations is violated whenever there is cluster sampling, for example, in family-based studies and several solutions have been proposed to learn GGMs from correlated data. Talluri and Shete adapted the Lasso-penalized maximum likelihood estimator of the precision matrix by incorporating the kinship matrix to account for the correlations introduced by family data (Talluri and Shete, 2014). However, this method requires prior knowledge of the variables’ heritability, which is not always available. Riberiro and Soler further leveraged the properties of family data for learning GGMs that are decomposed into the genetic and environmental networks (Ribeiro and Maria Pavan Soler, 2020). This approach is particularly useful if the goal is to distinguish between genetic and non-genetic contributions to the associations between variables in the model. However, the estimation and inference steps of the partial correlations are time-consuming due to large matrices decompositions and operations. Moreover, both approaches rely on the correct specification of the correlation structure underlying the data and are applicable only within family data framework.
In this work, we propose a cluster-based bootstrap algorithm to learn a GGM from correlated data. This method adapts the family-based bootstrap introduced by Borecki and Province (2008) to test the significance of the partial correlations between the variables and does not need knowledge of the correlations between the observations but only the cluster composition of the data. In addition, this approach is not limited to family-based data. Compared to regression-based methods that are challenged by the complexity of the search for a GGM, the computational complexity of this method remains polynomial. We show through a comprehensive simulation study that our algorithm controls the Type I error well, while retaining good statistical power. We also apply our method in a real-world example to show the impact of ignoring correlated data when building a GGM.
Materials and methods
Methods for learning Gaussian Graphic Models from independent observations
A Gaussian Graphic Model (GGM) is a statistical model that represents properties of marginal and conditional independences of a multivariate Gaussian distribution using an undirected Markov graph (Lauritzen, 1996; Whittaker, 2009). The key rule of an undirected Markov graph is that two variables are conditionally independent given all the other variables in the graph if they are not connected by an edge. Let
Let
where
Figure 1 presents an illustrative GGM depicting the partial correlation network of the four-dimension vector
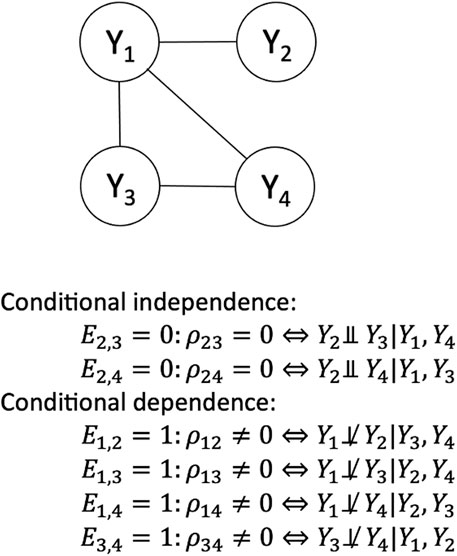
Figure 1. Example of a Gaussian graphical model with 4 vertices and 4 edges. This is a graph associated with vector
We test the null hypothesis of conditional independence of
where
when the sample size
Clustered data issues
The assumption of independent and identically distributed observations is violated in studies with correlated data such as cluster-based sampling or family-based recruitment (Laird, 2004). Subjects within a cluster are correlated due to shared environment components or sharing of genetic factors in family-based studies (Wojczynski et al., 2022). Failure to account for these correlations can lead to false positive results (Cannon et al., 2001).
In the analysis of cluster data, often investigators assume an exchangeable covariance structure, where the correlation of pairs of subjects in the same cluster is constant. Family data is a special type of clustered data in which each family is a cluster unit. The correlation structure of family data can be more complex with correlations between pairs of subjects that depend on their family relationship and shared environment. In genetic studies, the variance of a trait
where
The parameter
where the vector of the environmental components is parameterized as
We next extend the parameterization for the multivariable case in which we assume to have
And we model
where
Bootstrap algorithm on clustered data
The bootstrap method, introduced by Efron (1979), is a widely used resampling technique for statistical inference and hypothesis testing. It involves resampling the data with replacement and then using these samples to estimate the distribution of a statistic of interest. Sherma and Cessie suggested that the bootstrap method could also be used to address issues with correlated data by resampling clusters instead of individuals (Sherman and le Cessie, 1997), and Borecki and Province introduced a family-based bootstrap approach in which the sample units are families and familial relations are ignored in the estimation phase (Borecki and Province, 2008).
Here we propose a generalization of the family-based bootstrap algorithm introduced by Boreki and Province to learn GGMs that account for correlated observations. The steps of the proposed cluster-based bootstrap algorithm are as follows:
Step 1. For
i. Draw
ii. Calculate the sampling variance-covariance matrix
where
iii. For each pair
where
Step 2. Calculate the bootstrap estimate of the standard deviation of
where
Here
Step 3. Test the null hypothesis at level α if
Simulation settings
In the simulation study, we used the method described in the appendix to simulate a multivariable data set with related individuals. We simulated a mixed family structure where half of the families consisted of two parents and one offspring, while the other half consisted of two parents and five offspring. We simulated observations assuming three different numbers of families: 40, 120, and 360 families, resulting in sample sizes of

Figure 2. Graphical representation of the three simulation Markov models used in simulation studies. (a) The independence graph (b) the chain graph (c) the triangle tail graph. Each graph is accompanied by
For each scenario, we learned the GGM structure by using:
• The Fisher’s
• The naïve bootstrap algorithm, where we resampled 50 datasets, and each time sampling 100% individuals with replacement.
• Our proposed cluster-based bootstrap algorithm, in which we resampled 50 and 200 datasets, and for each resample, we drew
We used a level of statistical significance
We repeated this process until
Study cohort
The Long Life Family Study (LLFS) is a family-based study of healthy aging and longevity that recruited over 5,000 family members in approximately 550 families selected for familiar longevity. Participants were enrolled at three American field centers in Boston, Pittsburgh, and New York, along with a European field center based in Denmark (Sebastiani et al., 2009; Wojczynski et al., 2022). Socio-demographic, medical history data, current medications, physical and cognitive function data, and blood samples were collected via in-person visits and phone questionnaires for all subjects at the time of enrollment and during follow-ups (Elo et al., 2013; Newman et al., 2011). Genome-wide genotype data were generated at the Center for Inherited Disease Research (CIDR) using the Illumina Omni 2.5 platform, and genotype calls were cleaned as described in (Bae et al., 2013). The genotype data were imputed with Michigan Imputation Server to the HRC panel (version r1.1 2016) (Das et al., 2016). Genome-wide genotype data are available from dbGaP (dbGaP Study Accession: phs000397.v1.p1). We augmented the genetic data in the LLFS using approximately 3,500 samples that we used as controls in other studies of longevity (Bae et al., 2013). The genotype data are accessible at http://www.illumina.com/documents/icontroldb/document_purpose.pdf. We used these genetic data to calculate the Polygenic Risk Scores (PRS) for 54 health outcomes summarized in Supplementary Table S3. These PRS were calculated as the weighted sum of individual’s genetic variants associated with the corresponding outcome and all the details are described in reference (Gunn et al., 2022).
Implementation and code availability
The code used in this study is available upon request and can also be accessed on GitHub at: https://github.com/QM-DS-Tufts-Medical-Center/GGM-network-Bootstrap.git.
Results
The simulation studies demonstrate that the bootstrap-based approach controls the type I error without losing power
Figures 3, 4 summarizes the results of the FPR for different scenarios and methods. The FPR of the Fisher’s test and the naïve bootstrap algorithm that ignores the family structure increased across all three scenarios as the heritability levels increased, and the inflation rates increased by 2 to 4-fold when the heritability exceeds 0.5. When the number of families was small (
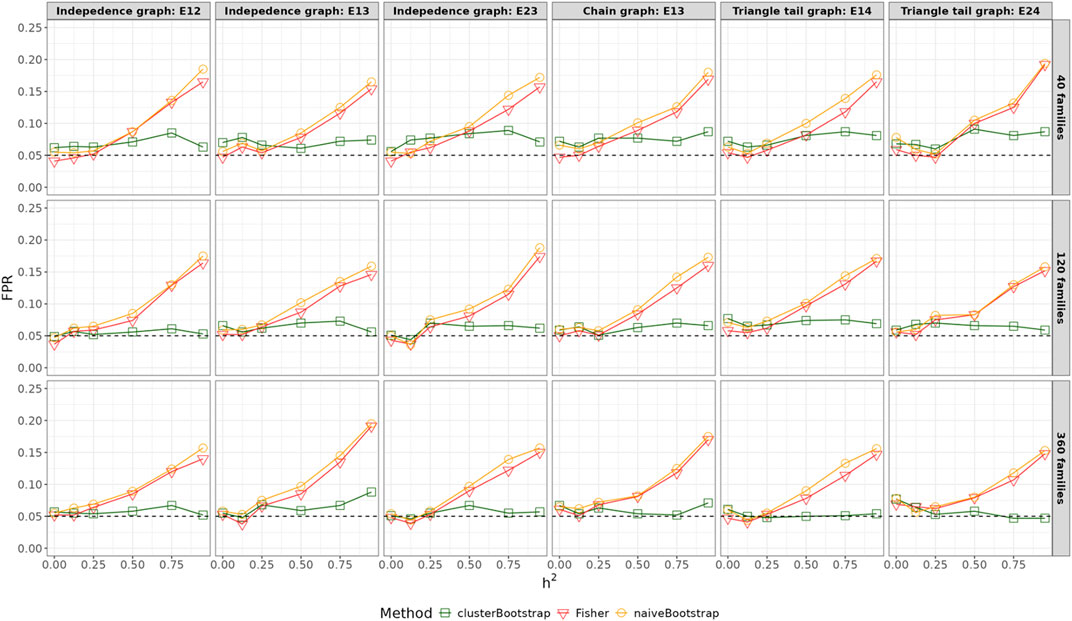
Figure 3. False positive rates across varying heritability for all edges with
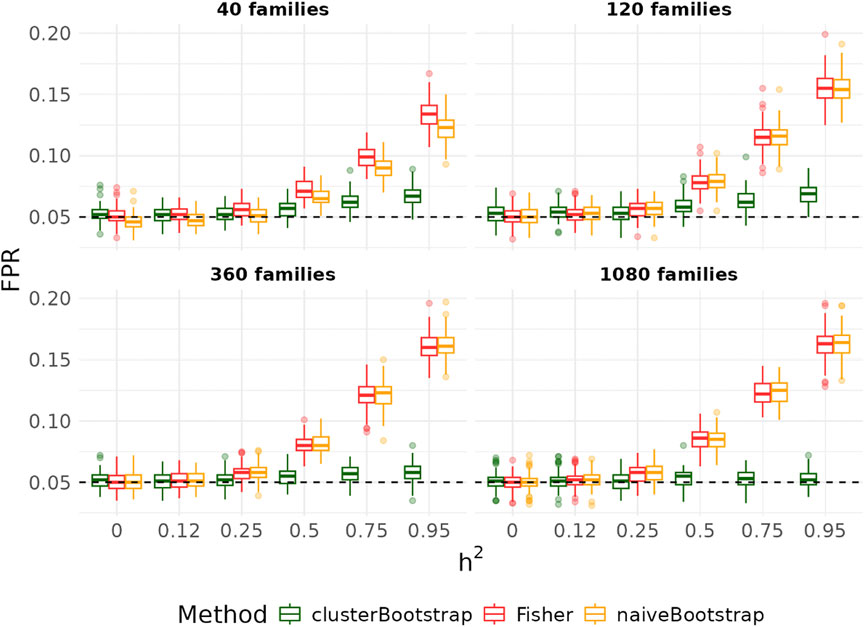
Figure 4. False positive rates across varying heritability for all edges with
Figure 5 summarizes the statistical power of detecting true edges using the Fisher’s test, the naïve bootstrap method and the cluster-based bootstrap algorithm as a function of the heritability in the simple graph settings. As heritability increased, the statistical power decreased particularly with small sample sizes and partial correlations between variables close to 0. However, when the number of families exceeded 360, the power remained consistently above 0.8, irrespective of the heritability values ranging from 0 to 0.95. For smaller family sizes (
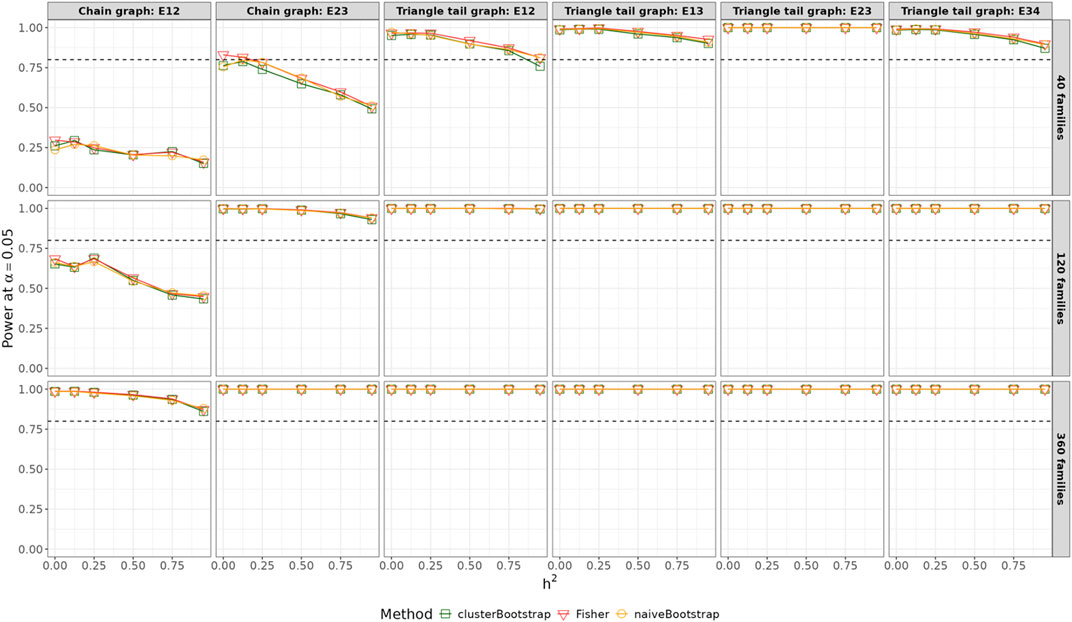
Figure 5. Power across varying heritability for all edges with
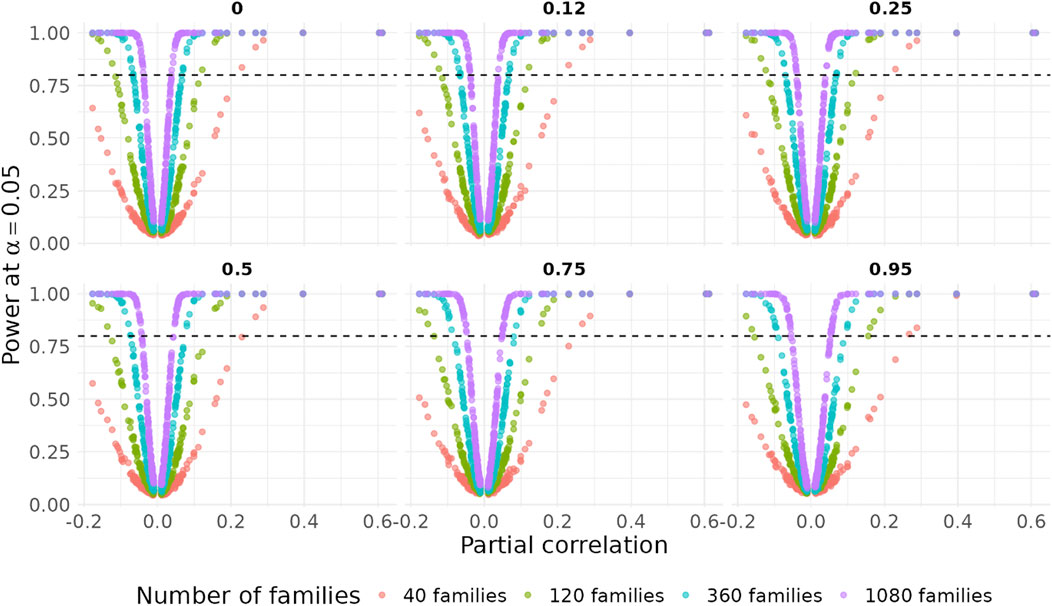
Figure 6. Power across varying heritability for all edges with
Comparing the proposed cluster-based bootstrap algorithm that samples 100% of the clusters with the Fisher’s test and naïve bootstrap method, we observed comparable power across all scenarios and well-controlled the Type I error rate when the number of clusters is sufficiently large. Our results indicated that bootstrapping 50/200 datasets with 100% families resampled yielded the best performance in terms of both power and FPR. However, reducing the proportion of families resampled leads to a decrease in power.
The GGM of polygenic risk scores highlight groups of traits with correlated genetic risks
We applied this new algorithm to characterize the mutual correlations between 47 polygenic risk scores in the LLFS (Gunn et al., 2022; Wojczynski et al., 2022). Polygenic Risk Scores (PRS) for 54 health outcomes using genetic data of 8,190 samples were calculated as described in the methods (Gunn et al., 2022). These PRS reflect the relative genetic risk of developing the outcome in carriers of combinations of risk variants compared to non-carriers. These 54 outcomes include age-related diseases such as Alzheimer’s disease, coronary artery disease, and a variety of other traits related to mental health (e.g., bipolar disorder), and general physiological characteristics as listed in Supplementary Table S3. We further removed two PRS with very skewed distribution (Supplementary Figure S3) and an additional five PRS that had several potential outliers that lie 4 standard deviations away from the means (Supplementary Figure S4). We learned the partial correlation networks of the remaining 47 PRS using three methods: Fisher’s transformation test with independent subsets of the data yielding a sample size of
Figure 7 displays the networks constructed using the three methods. The Fisher’s
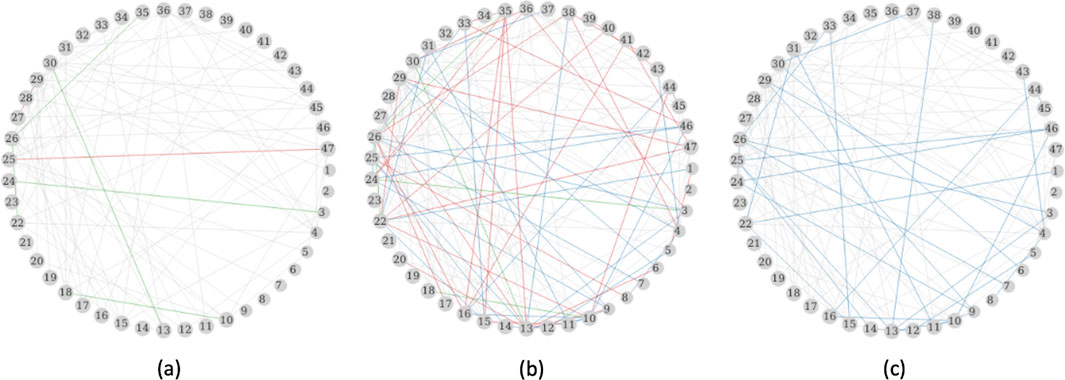
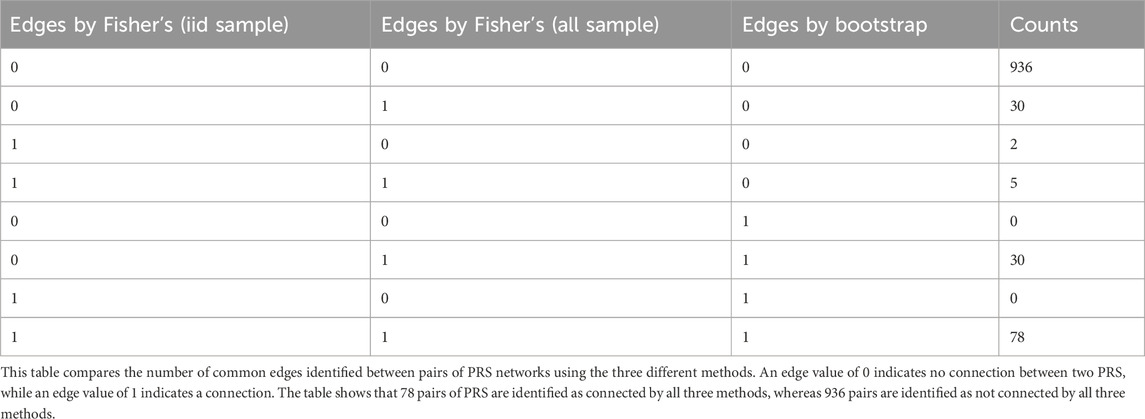
Figure 7. PRS networks inferred with Bonferroni correction. (a) Fisher’s test on sampled iid data, (b) Fisher’s test on all data, and (c) Bootstrap resampling 1,000 datasets and 100% families. Grey edges are edges identified in all three approaches; green edges are intersections by (a) and (b); blue edges are intersections by (b) and (c); red edges are identified only by the corresponding approach. The numbers in each circle is a PRS as listed in Supplementary Table S1.
Evaluation of computation time
We evaluated the computation time of the cluster-based bootstrap algorithm by calculating the CPU time of the resampling steps and the inference steps. We ran the evaluation using a single computer node with 1 core and R version 4.1.1. We sampled 40, 400, and 4,000 families from the LLFS data, and sampled 10, 20, 40 PRS. For each scenario, we obtained the computational time for 50, 200 and 1,000 iterations with 100% of the families resampled each iteration. The algorithm finished in 65 s in the scenario with 40 PRS, 4,000 families and 1,000 iterations as shown in Figure 8. Notably, the resampling step took 52 s that makes up to 81% of the total CPU time.
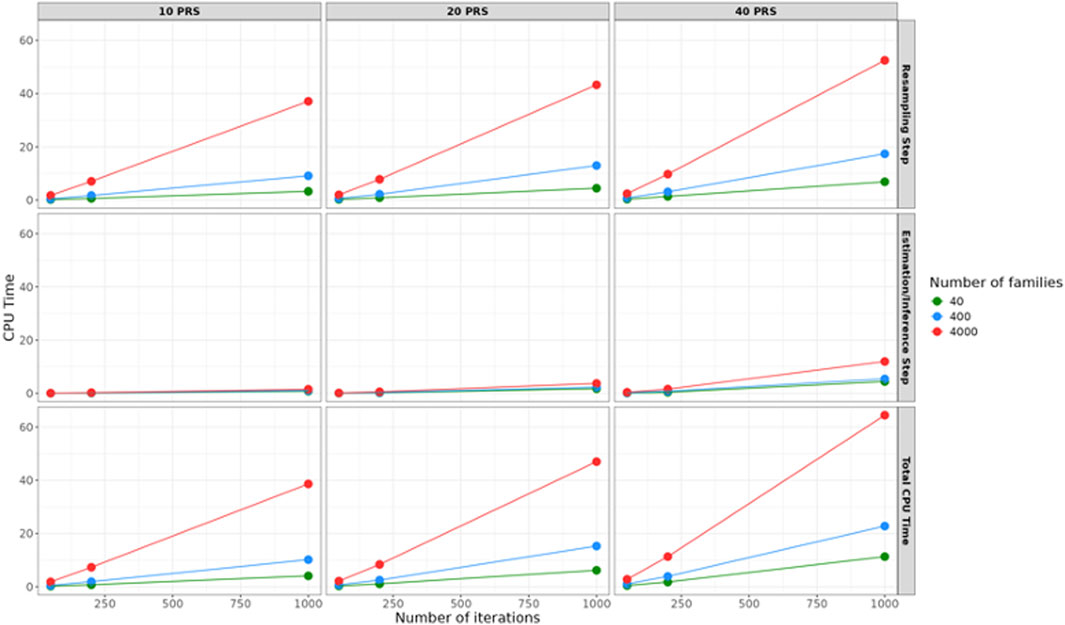
Figure 8. The CPU time (in seconds) for the Bootstrap algorithm. The CPU time increases with the number of families, PRS, and iterations. The figure shows results for 50, 200, and 1,000 iterations. According to the simulation study, more than 50 iterations are generally sufficient. For large numbers of families, selecting 50 to 200 iterations can keep the total CPU time within 15 s.
Discussion
In this study, we introduced a novel cluster-based bootstrap algorithm for learning partial correlation networks from correlated data. We showed in simulated data that this algorithm effectively controls the FPR while maintaining comparable power performance to conventional methods. Although we described the method in the context of family data, the cluster-based bootstrap algorithm can be directly applied to any correlated data setting without explicitly modeling the correlation structure, as it is required in (Ribeiro and Maria Pavan Soler, 2020).
To evaluate our approach, we conducted a comprehensive simulation study that highlighted strengths but also potential limits of this approach. The analysis showed that the algorithm controls the Type I error well without loss of power when both the number of families/clusters and the number of subjects large, such as more than 40, and many subjects. This is consistent with the well-known limitation that the bootstrap estimate of the standard error is not accurate when the number of clusters is small and the test statistics tends to be too liberal (Huang, 2018). This bias leads to a higher FPR regardless of the heritability. Another limitation arises when the sample sizes are smaller than the number of variables, as the test statistic is no longer well-defined. When the heritability of traits is less than 25%, the cluster-based bootstrap algorithm behaves similarly to the Fisher’s test and the naïve bootstrap method that ignores the correlation. However, in omics data analyses, most traits exhibit high heritability, typically exceeding 30%. In such cases, it would be optimal to use the cluster-based bootstrap algorithm if investigators have sufficient computational capacity.
The application of the approach to the PRS analysis shows that correcting for the within-family correlation reduced the number of edges from 143 to 108 and was more powerful than analyzing a subset of independent observations. Through the network analysis, we identified PRS that functioned as central nodes with multiple connections to other PRS. These central nodes include intelligence, ankylosing spondylitis, juvenile idiopathic arthritis, height, heel bone mineral density, and cognitive performance. Our cluster-based method preserved important connections, such as the edge between cognitive performance and FEV1 (Richards et al., 2005), that were missed by Fisher’s test applied to the independent subset. Some of the edges that were detected ignoring the correlation in the observations appeared to be false positive, for example, the edge between the PRS for intelligence and for FEV1. While our method effectively reduces false edges caused by correlated data, the resulting network remains highly connected and challenging to interpret. In future work, we will extend this method to learn sparse networks that yield more interpretable graphs.
The computational efficiency of our algorithm is a function of various factors, including the number of bootstrap iterations, the number of vertices, the number of families/clusters and total number of samples. The number of families impacts the resampling procedure’s runtime, while the number of nodes influences the calculation of partial correlation matrices. Furthermore, the cumulative effect of resampling and partial correlation calculations per iteration significantly contributes to the time needed for constructing Z-scores. A potential improvement to computational efficiency would involve a faster algorithm for calculating the inverse of the variance covariance matrix especially when the number of vertices are very large.
The learning strategy implemented through our proposed algorithms relies on testing multiple null hypotheses
This work has some limitations. For example, we conducted simulation studies using genetically independent traits. It is not straightforward to extend our simulations to genetically correlated traits since the variance-covariance matrix
Conclusion
By displaying conditional dependencies into patterns of edges in a network, GGMs offer a great statistical tool to represent intricate relationships within data in an intuitive manner and could be potentially very useful in the emerging field on multi-omics integration. However, the generation of GGMs from correlated data is a challenging task. We provided a simple method to derive a GGM from correlated data that is computationally efficient and appears to control the FPR without losing statistical power. This approach could increase the use of GGMs in observational study data that often, by design, generate correlated observations.
Data availability statement
The data analyzed in this study is subject to the following licenses/restrictions: The LLFS data are available from dbGaP (dbGaP Study Accession: phs000397.v1.p1). Requests to access the simulated datasets should be directed to emV5dWFuLnNvbmdAdHVmdHNtZWRpY2luZS5vcmc=.
Ethics statement
The studies involving humans were approved by IRB of Washington University at St Louis. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
ZS: Conceptualization, Data curation, Formal Analysis, Investigation, Methodology, Software, Validation, Visualization, Writing – original draft, Writing – review and editing. SG: Data curation, Writing – review and editing. SM: Supervision, Writing – review and editing. GP: Supervision, Writing – review and editing. C-TL: Supervision, Writing – review and editing. KL: Supervision, Writing – review and editing. PS: Conceptualization, Funding acquisition, Investigation, Methodology, Resources, Supervision, Writing – review and editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This research is funded by NIA U19AG063893, UH2AG064704, R01AG061844, U19-AG023122.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fsysb.2025.1589079/full#supplementary-material
References
Almasy, L., and Blangero, J. (1998). Multipoint quantitative-trait linkage analysis in general pedigrees. Am. J. Hum. Genet. 62 (5), 1198–1211. doi:10.1086/301844
Amos, C. I. (1994). Robust variance-components approach for assessing genetic linkage in pedigrees. Am. J. Hum. Genet. 54 (3), 535–543.
Bae, H. T., Sebastiani, P., Sun, J. X., Andersen, S. L., Warwick Daw, E., Terracciano, A., et al. (2013). Genome-wide association study of personality traits in the long Life family study. Front. Genet. 4, 65. doi:10.3389/fgene.2013.00065
Becker, M., Nassar, H., Espinosa, C., Stelzer, I. A., Feyaerts, D., Berson, E., et al. (2023). Large-scale correlation network construction for unraveling the coordination of complex biological systems. Nat. Comput. Sci. 3 (4), 346–359. doi:10.1038/s43588-023-00429-y
Borecki, I. B., and Province, M. A. (2008). Genetic and genomic discovery using family studies. Circulation 118 (10), 1057–1063. doi:10.1161/CIRCULATIONAHA.107.714592
Cannon, M. J., Warner, L., Augusto Taddei, J., and Kleinbaum, D. G. (2001). What can go wrong when you assume that correlated data are independent: an illustration from the evaluation of a childhood health intervention in Brazil. Statistics Med. 20 (9–10), 1461–1467. doi:10.1002/sim.682
Das, S., Forer, L., Schönherr, S., Sidore, C., Locke, A. E., Kwong, A., et al. (2016). Next-generation genotype imputation service and methods. Nat. Genet. 48 (10), 1284–1287. doi:10.1038/ng.3656
Drton, M., and Perlman, M. D. (2007). Multiple testing and error control in Gaussian graphical model selection. Stat. Sci. 22 (3), 430–449. doi:10.1214/088342307000000113
Efron, B. (1979). Computers and the theory of statistics: thinking the unthinkable. SIAM Rev. 21 (4), 460–480. doi:10.1137/1021092
Elo, I. T., Mykyta, L., Sebastiani, P., Christensen, K., Glynn, N. W., and Perls, T. (2013). Age validation in the long Life family study through a linkage to early-life census records. Journals Gerontology - Ser. B Psychol. Sci. Soc. Sci. 68 (4), 580–585. doi:10.1093/geronb/gbt033
Fisher, R. A. A. (1924). The distribution of the partial correlation coefficient. Metron 3, 329–332.
Gunn, S., Wainberg, M., Song, Z., Andersen, S., Boudreau, R., Feitosa, M. F., et al. (2022). Distribution of 54 polygenic risk scores for common diseases in long lived individuals and their offspring. GeroScience 44 (2), 719–729. doi:10.1007/s11357-022-00518-2
Huang, F. L. (2018). Using cluster bootstrapping to analyze nested data with a few clusters. Educ. Psychol. Meas. 78 (2), 297–318. doi:10.1177/0013164416678980
Kolaczyk, E. (2009). Statistical analysis of network data - methods and models. New York: Springer Science+Business Media, LLC, 333–344.
Lange, K. (2022). Mathematical and statistical methods for genetic analysis, 488. New York: Springer.
Langfelder, P., and Horvath, S. (2008). WGCNA: an R package for weighted correlation network analysis. BMC Bioinforma. 9, 559–13. doi:10.1186/1471-2105-9-559
Liu, W. (2013). Gaussian graphical model estimation with false discovery rate control. Ann. Statistics 41 (6), 2948–2978. doi:10.1214/13-AOS1169
Markowetz, F., and Spang, R. (2007). Inferring cellular networks - a review. BMC Bioinforma. 8 (Suppl. 6), S5–S17. doi:10.1186/1471-2105-8-s6-s5
Meinshausen, N., and Bühlmann, P. (2010). Stability selection. J. R. Stat. Soc. Ser. B Stat. Methodol. 72 (4), 417–473. doi:10.1111/j.1467-9868.2010.00740.x
Newman, A. B., Glynn, N. W., Taylor, C. A., Sebastiani, P., Perls, T. T., Mayeux, R., et al. (2011). Health and function of participants in the long Life family study: a comparison with other cohorts. Aging 3 (1), 63–76. doi:10.18632/aging.100242
Ribeiro, A. H., and Maria Pavan Soler, J. (2020). Learning genetic and environmental graphical models from family data. Statistics Med. 39 (18), 2403–2422. doi:10.1002/sim.8545
Richards, M., Strachan, D., Hardy, R., Kuh, D., and Wadsworth, M. (2005). Lung function and cognitive ability in a longitudinal birth cohort study. Psychosom. Med. 67 (4), 602–608. doi:10.1097/01.psy.0000170337.51848.68
Ronald Aylmer, F. (1921). On the “probable error” of a coefficient of correlation deduced from a small sample. Metron 1, 1–32.
Sebastiani, P., Hadley, E. C., Province, M., Christensen, K., Rossi, W., Perls, T. T., et al. (2009). A family longevity selection score: ranking sibships by their longevity, size, and availability for study. Am. J. Epidemiol. 170 (12), 1555–1562. doi:10.1093/aje/kwp309
Sherman, M., and le Cessie, S. (1997). A comparison between bootstrap methods and generalized estimating equations for correlated outcomes in generalized linear models. Commun. Statistics Part B Simul. Comput. 26 (3), 901–925. doi:10.1080/03610919708813417
Talluri, R., and Shete, S. (2014). Gaussian graphical models for phenotypes using pedigree data and exploratory analysis using networks with genetic and nongenetic factors based on genetic analysis workshop 18 data. BMC Proc. 8, S99–5. doi:10.1186/1753-6561-8-S1-S99
Vamathevan, J., Clark, D., Czodrowski, P., Dunham, I., Ferran, E., Lee, G., et al. (2019). Applications of machine learning in drug discovery and development. Nat. Rev. Drug Discov. 18 (6), 463–477. doi:10.1038/s41573-019-0024-5
Whittaker, J. (2009). Graphical models in applied multivariate statistics. Wiley Publishing, 120–194. doi:10.1002/net.3230240213
Wojczynski, M. K., Lin, S. J., Sebastiani, P., Perls, T. T., Lee, J., Kulminski, A., et al. (2022). NIA long Life family study: objectives, design, and heritability of cross-sectional and longitudinal phenotypes. Journals Gerontology - Ser. A Biol. Sci. Med. Sci. 77 (4), 717–727. doi:10.1093/gerona/glab333
Keywords: Gaussian graphical models, corelated data, bootstrap, polygenic risk score, partial correlation
Citation: Song Z, Gunn S, Monti S, Peloso GM, Liu C-T, Lunetta K and Sebastiani P (2025) Learning Gaussian graphical models from correlated data. Front. Syst. Biol. 5:1589079. doi: 10.3389/fsysb.2025.1589079
Received: 06 March 2025; Accepted: 09 June 2025;
Published: 03 July 2025.
Edited by:
Chixiang Chen, University of Maryland, United StatesReviewed by:
Cen Wu, Kansas State University, United StatesHwiyoung Lee, University of Maryland, United States
Copyright © 2025 Song, Gunn, Monti, Peloso, Liu, Lunetta and Sebastiani. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zeyuan Song, emV5dWFuLnNvbmdAdHVmdHNtZWRpY2luZS5vcmc=
 Zeyuan Song
Zeyuan Song Sophia Gunn3
Sophia Gunn3 Stefano Monti
Stefano Monti Gina M. Peloso
Gina M. Peloso Ching-Ti Liu
Ching-Ti Liu Kathryn Lunetta
Kathryn Lunetta Paola Sebastiani
Paola Sebastiani