- 1Department of Burns, The First Affiliated Hospital, Sun Yat-sen University, Guangzhou, China
- 2School of Public Health and Management, Chongqing Medical University, Chongqing, China
- 3University of Hong Kong, Hong Kong Polytechnic University, Kowloon, China
The purpose of this systematic review and meta-analysis is to assess the clinical effectiveness and safety of the medical hydrogel dressings used in skin wounds and therefore to weight the evidence for their clinical application. PubMed/Medline (1980–2019), Cochrane Library (1980–2019), ClinicalTrials.gov, Cochrane CENTRAL, Chinese Journal Full-text Database (CNKI, 1994–2019), and China Biomedy Medicine disc (CBM, 1978–2019), Chinese Scientific Journal Database (VIP, 1989–2019), and Wanfang Database (WFDATA, 1980–2019) were searched to identify relevant clinical trials and studies. Forty-three studies that assessed hydrogel vs. non-hydrogel dressings were identified. Compared to the latter, hydrogel dressings associated with a significantly shortened healing time of degree II burn (superficial and deep) wounds, diabetic foot ulcers, traumatic skin injuries, radioactive skin injuries, dog bites, and body surface ulcers. In addition, hydrogel dressing obviously increased the cure rate of diabetic foot ulcers, surgical wounds, dog bites, and body surface ulcers. Moreover, hydrogel dressing significantly relieved pain in degree II burn (superficial and deep) wounds, traumatic skin injuries, and laser treatment-induced wounds. However, no significant differences obtained between hydrogel and non-hydrogel dressings in the healing time of surgical wounds, the cure rate of inpatients' pressure ulcers, and phlebitis ulcers. This comprehensive systematic review and meta-analysis of the available evidence reveals that the application of hydrogel dressings advances the healing of various wound types and effectively alleviates the pain with no severe adverse reactions. These results strongly indicate that hydrogel products are effective and safe in wound management.
Introduction
Skin is the largest human organ as it reaches almost 10% of the total body mass (Grice et al., 2009) and acts as a key protective barrier against the outside environment. Normally, the human body heal skin injuries via a set of complex and interactive processes that include hemostasis, inflammation, proliferation, and remodeling. However, this healing process can be impaired by various local and systemic factors causing more severe complications and a lower quality of life (Nourian Dehkordi et al., 2019). Plenty of wound care products have been created and developed in the latest decades aimed at promoting wound healing and improving the life quality of the patients afflicted by skin wounds (Metcalfe and Ferguson, 2007; Gil et al., 2013; Chattopadhyay and Raines, 2014; Garg et al., 2015; Xu et al., 2015; Das and Baker, 2016). Therefore, surgeons must specifically select wound treatment products according to the factors impeding wounds healing.
Since the 1960s, wound dressing was considered to play a positive role in wound healing. Wound dressing could establish and maintain an environment apt for wound repair. Winter (1962) were the pioneers of this field by initiating the concept of functional active dressings. According to them, the ideal advanced wound dressing should provide and maintain a moist environment, adequate gaseous exchange, and thermal insulation in the absence of toxic contaminants; it should protect against secondary infections, induce tissue regeneration, relieve wound pain, and promote wound healing quality; finally, it should be elastic, non-antigenic, and allow to manage wound exudate (Purna and Babu, 2000). Considering all the just mentioned factors, hydrogel products have the capacity to act as promising candidates as wound dressings for applications in clinical settings (Qu et al., 2018).
In 1960, Wichterle and Lim prepared the first hydrogels by cross-linking 2-hydroxyethyl methacrylate, thus initiating the application and practice of hydrogels in the biomedical field (Wichterle and Lím, 1960). Hydrogels are extremely hydrophilic. Advanced hydrogel materials are environment-sensitive or stimuli-sensitive, as they start swelling under certain conditions and respond to definite stimuli (Qiu and Park, 2001). They can absorb exudate from the wound surface and promote fibroblast proliferation and cell migration and keratinization. In addition, hydrogels' dense meshes can prevent bacteria from invading the wound while effectively transporting bioactive molecules (such as antibacterial agents and drugs) to the wound surface (Mohan et al., 2007; Tsao et al., 2010; Schwartz et al., 2012; Mao et al., 2017). At the same time, the unique mechanical properties of hydrogels i.e., elasticity and flexibility, allow for their adaptation to different parts of the wound, making them suitable for both wound care and tissue engineering (Huang et al., 2015).
Being a novel category of wet dressings, hydrogel products have been gradually perfected in recent years. Their clinical application has become rather extensive, ranging from dry scab wounds to multiple treatments of skin ulcers, burn wounds, animal bites, bed sores, etc. (Sood et al., 2014). Medicinal hydrogel dressings are endowed with a three-dimensional (3D) crosslinked network structure, which contains three main components, a high-molecular weight compound, propylene glycol, and water. High-molecular weight compounds such as Carboxy Methyl Cellulose (CMC) can double the absorption of wound exudate and necrotic tissue fluid (Roy et al., 2010). Propylene glycol can kill bacteria and prevent bacterial proliferation. In turn, the water in hydrogel dressings can create a relatively moist environment that prevents the wound from drying up (Fan et al., 2014). Therefore, although necrotic tissues in the making go through a slow hydration, the hydrogel dressing ensures a strong absorption of wound exudate. Concurrently, it promotes the debridement of water-soluble materials and absorbs wound carrion to provide a localized moist environment advancing wound healing (Qu et al., 2018). Besides, hydrogels' micro-acidic and hypoxic environment can attract cells involved in wound repair, help inhibit bacterial growth, and promote neoangiogenesis at the wound site (Dong et al., 2016).
Managing wounds through the use of hydrogels has been an accepted practice for decades. At present, many forms of hydrogel and non-hydrogel products are available aimed at managing wounds caused by various injuries. However, the benefits of multiple options also entail many challenges to the clinicians. The purpose of this systematic review and meta-analysis is to assess the clinical effectiveness and safety of the medicinal hydrogel dressings in treating multiple skin wounds compared to non-hydrogel dressings in terms of wound healing time, wound cure rate, pain reduction, and incidence of adverse reactions.
Methods
Systematic Review Eligibility Criteria
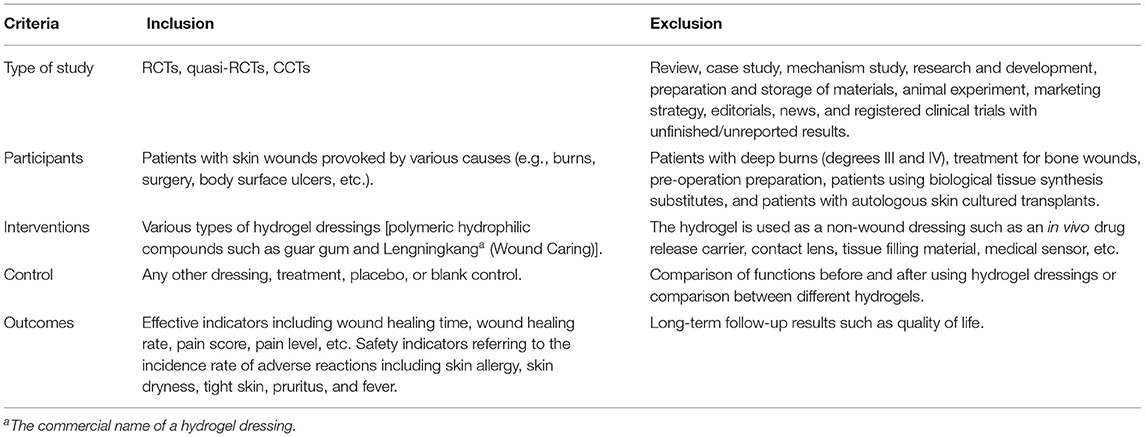
A systematic review was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) guidelines (Shamseer et al., 2015). It was based on the planned Participants, Intervention, Control, Outcome, and Study design (PICOS) elements outlined in Table 1.
Search Strategy
We sought to identify suitable studies by searching the following online databases: PubMed/Medline (1980–2019), Cochrane Library (1980–2019), ClinicalTrials.gov, Cochrane CENTRAL, Chinese Journal Full-text Database (CNKI, 1994–2019), and China Biomedy Medicine disc (CBM, 1978–2019), Chinese Scientific Journal Database (VIP, 1989–2019), and Wanfang Database (WFDATA, 1980–2019). With the combination of subject words and free words, the search terms included two categories: (1) “hydrogel,” “polymeric hydrophilic compound,” “guar gum,” “guar bean,” and “polyvinylpyrrolidone (PVP);” (2) “wound,” “wound surface,” and “burn.” The logical relationship was created with “OR” and “AND,” and the search formula was thereafter developed according to the characteristics of the different databases. The search strategy was improved through a pre-retrieval process. Meanwhile, unpublished studies and conference materials were manually searched, and references of the included literature were also tracked. No language limits were applied.
Study Selection
Two reviewers carried out the preliminary screening by independently reading titles and abstracts to exclude literature that obviously did not conform with the inclusion criteria. As a further screening they read the full texts of the literature that might meet the inclusion criteria. When the two researchers' opinions differed, they consulted and discussed with a third researcher to reach a final decision. During the full-text screening, the information below would be extracted: authors, date of publication, study type, subject characteristics, sample number, loss to or withdrawal from follow-up, intervention measures, and measuring indicators, etc. In case of multiple studies in a single published work, data based on study contents would be extracted as needed. With regard to repeatedly reported studies, only the latest or the most comprehensive one was included.
Quality Evaluation
The quality of the methodology employed by the included studies was evaluated according to the Effective Practice and Organization of Care Group (EPOC) improved scoring standard recommended by The Cochrane Collaboration. The evaluation package included randomization methods, allocation concealment, blinding use, control of loss to follow-up, baseline information, outcome data, etc. Scores of 5–6 were classified as grade A, 2–4 as grade B, and 0–1 as grade C.
Meta-Analysis
Meta-analysis was carried out by using the RevMan5.0 software recommended by The Cochrane Collaboration. Subgroups were divided according to patient (wound) types and types of outcome variables. The relative risk (RR) was taken as the combined effect size for categorical data, while the weighted mean difference (WMD) as the combined effect size for measuring data. Each effect size was shown as 95% CI. The heterogeneity of the study results was tested by χ2 test. When studies showed a statistical homogeneity (P > 0.1, I2 < 50%), a fixed-effect model would be used; otherwise, a random effect model was adopted. For subgroups containing a single study, description, and comparative analysis would be conducted on their results.
Results
Study Selection and Characteristics
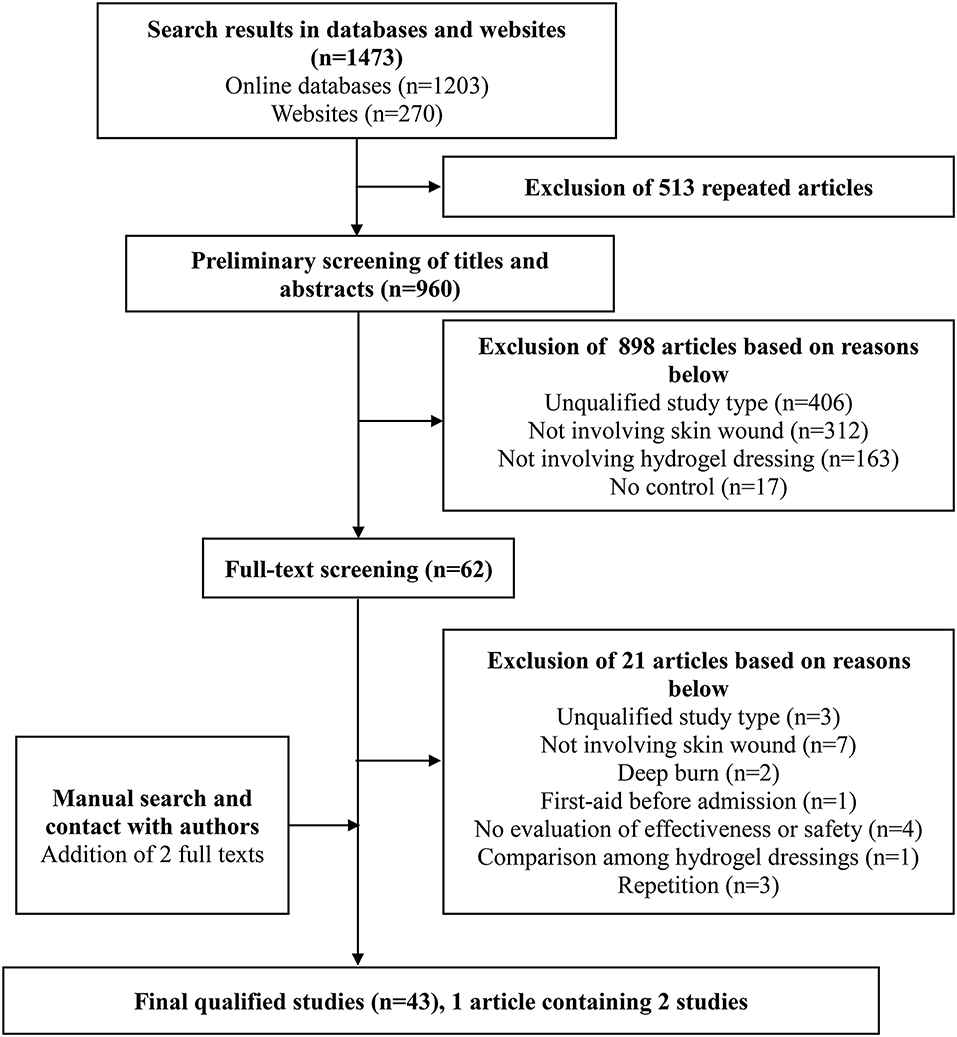
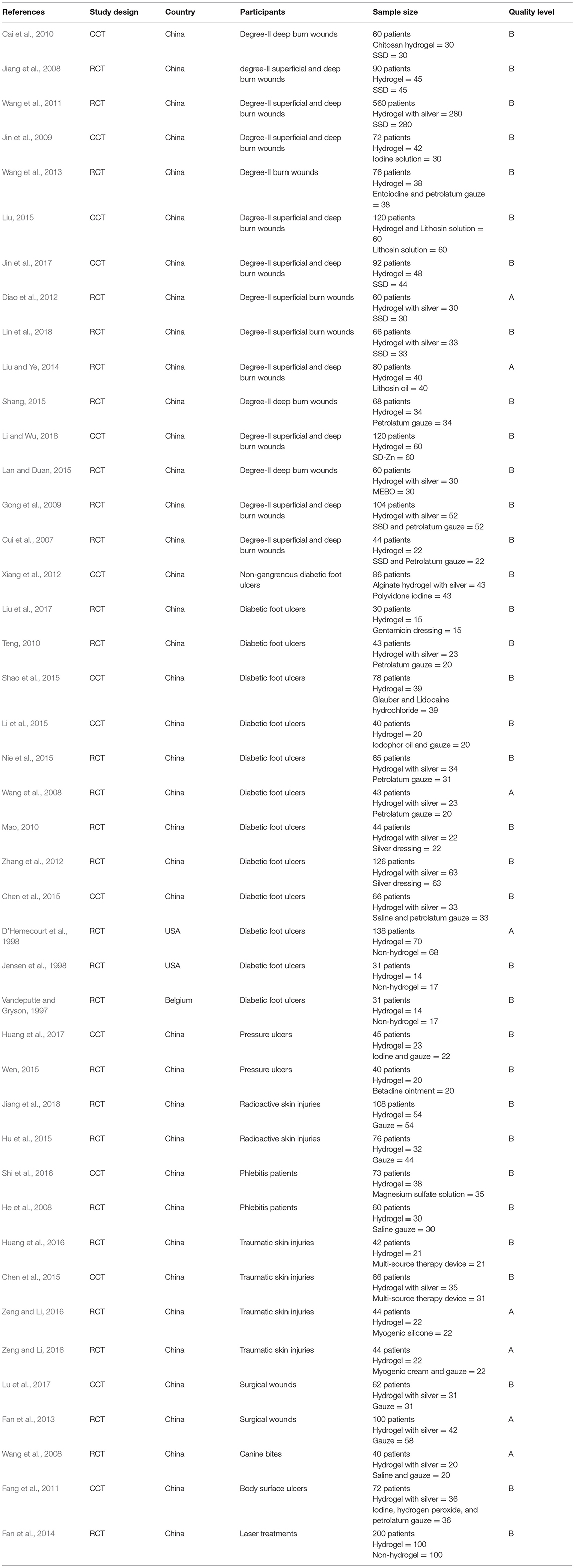
One thousand four hundred and seventy three studies were selected by the preliminary screening. Only 43 studies were kept after screening titles, abstracts, and full-texts (Figure 1), including 29 randomized controlled trials (RCTs) and 14 clinical controlled trials (CCTs) with a total of 3,521 patients. The basic characteristics of the included studies and the results of methodological quality evaluations are shown in Table 2. In all studies, patients' basic situations were comparable between intervention groups and control groups (P > 0.05).
Data Synthesis
Healing Times Comparison of Degree-II Superficial and Deep Burn Wounds
Eleven studies, reported by Cui et al. (2007), Jiang et al. (2008), Gong et al. (2009), Jin et al. (2009), Wang et al. (2011), Diao et al. (2012), Liu and Ye (2014), Liu (2015), Jin et al. (2017), Li and Wu (2018); Lin et al. (2018), compared the healing times of degree-II superficial burn wounds treated with hydrogel dressings or other treatments. There existed a statistical heterogeneity among the study results (P < 0.0001, I2 = 76%). Therefore, the random effect model was applied for meta-synthesis (Figure 2A). The results showed that on average the wound healing time of the hydrogel dressings group was shortened by 2.87 days as compared with the control group and that the difference had a high statistical significance (MD = −2.87, 95% CI: −3.35 to −2.38, P < 0.00001).
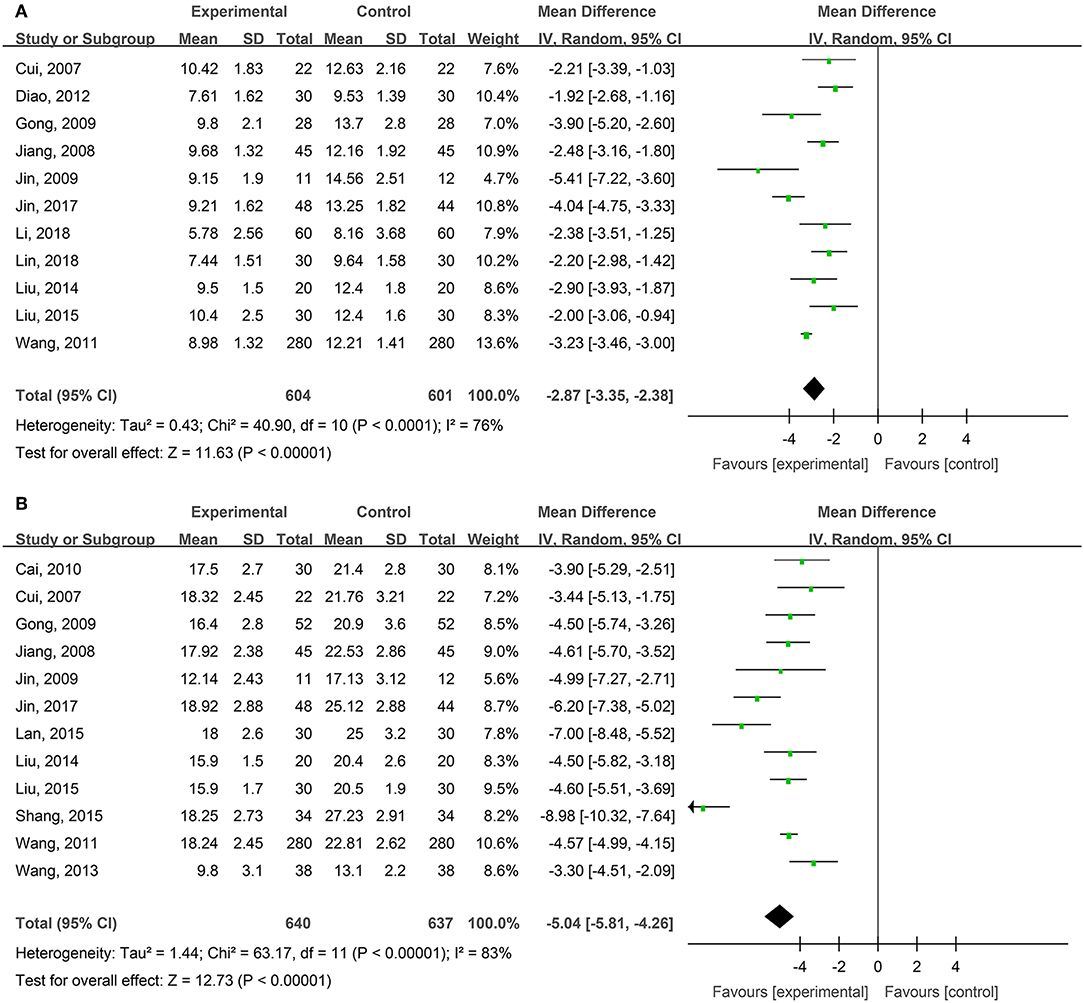
Figure 2. Comparative meta-analysis of the healing times of degree-II superficial (A) and degree-II deep (B) burn wounds.
Twelve studies, reported by Cui et al. (2007), Jiang et al. (2008), Gong et al. (2009), Jin et al. (2009), Cai et al. (2010), Wang et al. (2011), Wang et al. (2013), Liu and Ye (2014), Lan and Duan (2015), Liu (2015), Shang (2015), and Jin et al. (2017), compared the healing times of degree-II deep burn wounds treated with hydrogel dressings or other therapeutics. There existed a statistical heterogeneity among the study results (P < 0.00001, I2 = 83%). Hence, the random effect model was applied for meta-synthesis (Figure 2B). The results revealed that on average the wound healing time of the hydrogel dressings group was shortened by 5.04 days as compared with the control group and that statistically this difference was highly significant (MD = −5.04, 95% CI: −5.81 to −4.26, P < 0.00001).
WHO Pain Ratings of Burn Wounds
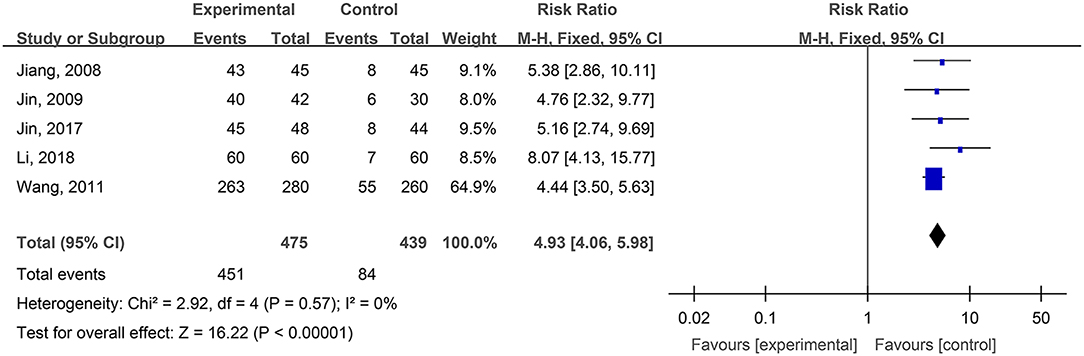
Five studies, reported by Jiang et al. (2008), Jin et al. (2009), Wang et al. (2011), Jin et al. (2017), and Li and Wu (2018), compared the pain ratings difference of burn wounds after treatment with hydrogel dressings or other therapeutic means. There occurred no statistical heterogeneity among the study results (P = 0.57). Consequently, the fixed effect model was applied for meta-synthesis (Figure 3). The results brought to light that patients suffering either grade 0 or grade I pain accounted for a higher proportion among those treated with hydrogel dressings and that statistically the difference was highly significant (OR = 4.93, 95% CI: 4.06–5.98, P < 0.00001).
VAS Pain Scores of Degree-II Superficial and Deep Burn Wounds
Four studies, reported by Diao et al. (2012), Liu and Ye (2014), Liu (2015), and Lin et al. (2018), compared visual analog scale (VAS) pain scores of the burn wounds treated with hydrogel dressings or other therapeutics. There occurred a statistical heterogeneity among the study results (P < 0.00001, I2 = 87%). Accordingly, the random effect model was applied for meta-synthesis (Figure 4A). The results showed that on average the VAS score of the hydrogel dressings group was 3.31 points lower than the control group, and that the difference had a high statistical significance (MD = −3.31, 95% CI: −4.16 to −2.46, P < 0.00001).
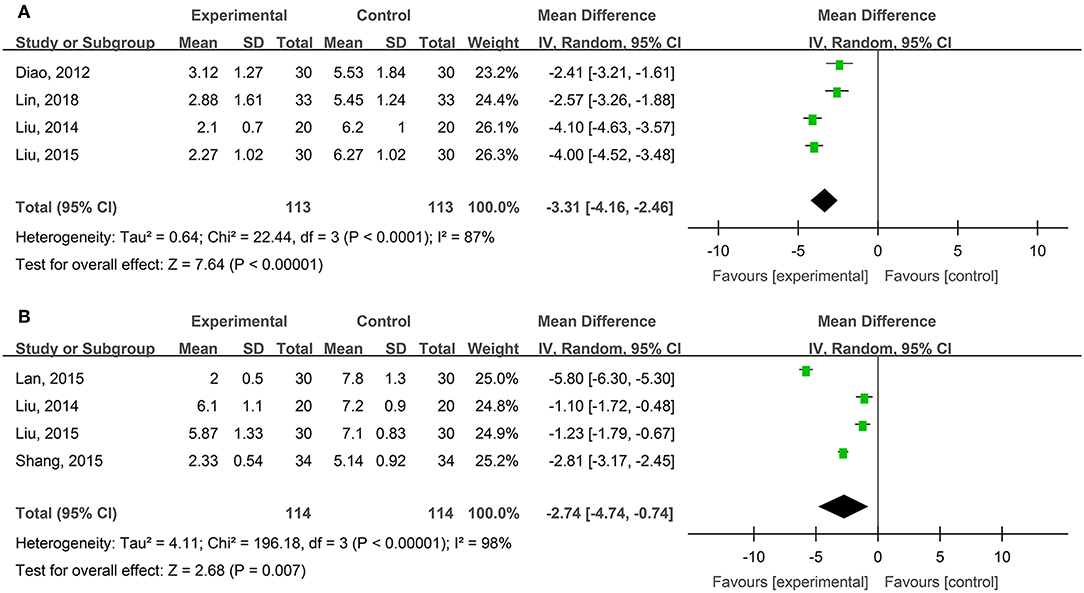
Figure 4. Comparative meta-analysis of VAS pain scores of degree-II superficial (A) and deep (B) burn wounds.
Four studies, reported by Liu and Ye (2014), Lan and Duan (2015), Liu (2015), and Shang (2015), compared VAS pain scores of burn wounds treated with hydrogel dressings or other medicaments. A statistical heterogeneity turned up among the study results (P < 0.00001, I2 = 98%). For that reason, the random effect model was applied for meta-synthesis (Figure 4B). The results made clear that on average the VAS score of the hydrogel dressings group was 2.74 points lower than that of the control group and that the difference was statistically significant (MD = −2.74, 95% CI: −4.74 ~ −0.74, P = 0.007).
Wound Healing Times of Diabetic Foot Ulcers
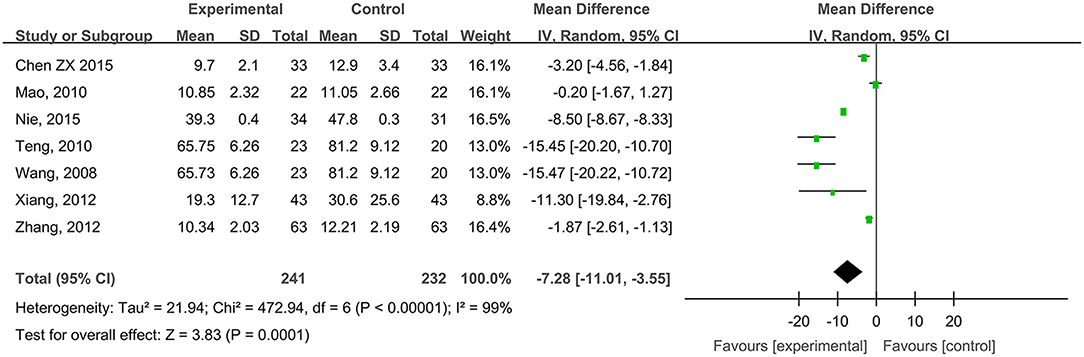
Seven studies, reported by Wang et al. (2008), Mao (2010), Teng (2010), Xiang et al. (2012), Zhang et al. (2012), Chen (2015), and Nie et al. (2015), compared the healing times of diabetic foot ulcer wounds treated with hydrogel dressing or other ministrations. There occurred a statistical heterogeneity among the study results (P < 0.00001, I2 = 99%). Therefore, the random effect model was applied for meta-synthesis (Figure 5). The results made plain that on average the healing time of the hydrogel dressings group was 7.28 days shorter than that of the control group and that the difference had a high statistical significance (MD = −7.28, 95% CI: −11.01 to −3.55, P < 0.0001).
Wound Cure Rates of Diabetic Foot Ulcers
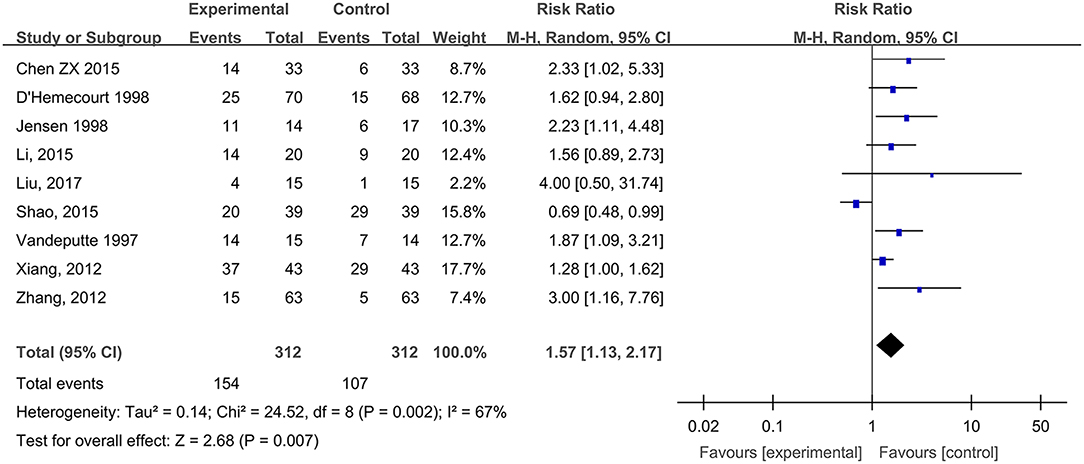
Nine studies, reported by Vandeputte and Gryson (1997), D'Hemecourt et al. (1998), Jensen et al. (1998), Xiang et al. (2012), Zhang et al. (2012), Chen (2015), Li et al. (2015), Shao et al. (2015), and Liu et al. (2017), compared the wound cure rates of diabetic foot ulcers treated with hydrogel dressing or other therapeutics. There existed a statistical heterogeneity among the study results (P = 0.002, I2 = 67%). Hence, the random effect model was applied for meta-synthesis (Figure 6). The results proved that the cure rate of diabetic foot ulcers was higher in the hydrogel dressings group than in the control group and that the difference was statistically significant (RR = 1.57, 95% CI: 1.13–2.17, P = 0.007).
Healing Times of Traumatic Skin Injuries
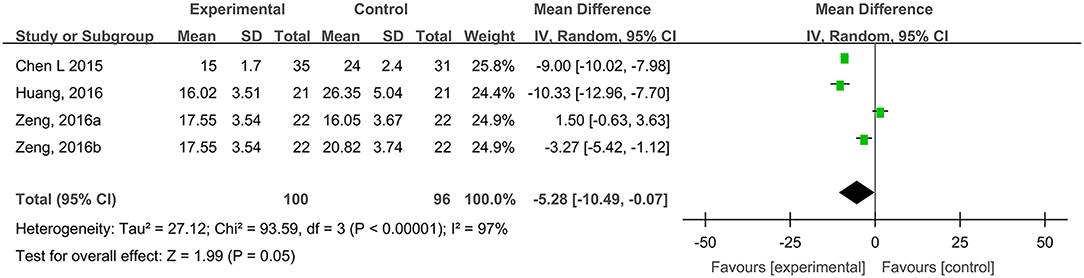
Four studies, reported by Chen et al. (2015), Huang et al. (2016), and Zeng and Li (2016), compared the healing times of traumatic skin injuries treated with hydrogel dressings or other therapeutics. There occurred a statistical heterogeneity among the study results (P < 0.00001, I2 = 97%). Consequently, the random effect model was applied for meta-synthesis (Figure 7). The results revealed that on average the healing time of traumatic skin injuries was 5.28 days shorter in the hydrogel dressing group than in the control group and that the difference reached statistical significance (MD = −5.28, 95% CI: −10.49 to −0.07, P = 0.05).
WHO Pain Ratings of Traumatic Skin Injuries
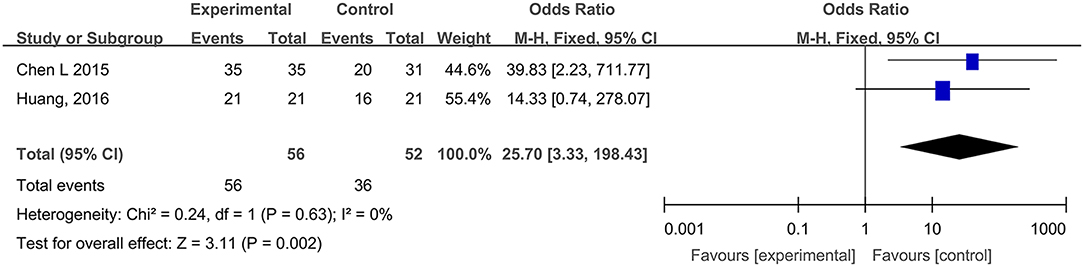
Two studies, reported by Chen et al. (2015) and Huang et al. (2016), compared the WHO pain ratings difference after treatment with hydrogel dressings or other therapeutic interventions. There existed no statistical heterogeneity among the study results (P = 0.63). In consequence, the fixed effect model was applied for meta-synthesis (Figure 8). The results disclosed that patients suffering grade-0 and grade-I pain accounted for a higher proportion than the control group did and that the difference was statistically significant (RR = 25.70, 95% CI: 3.33–198.43, P = 0.002).
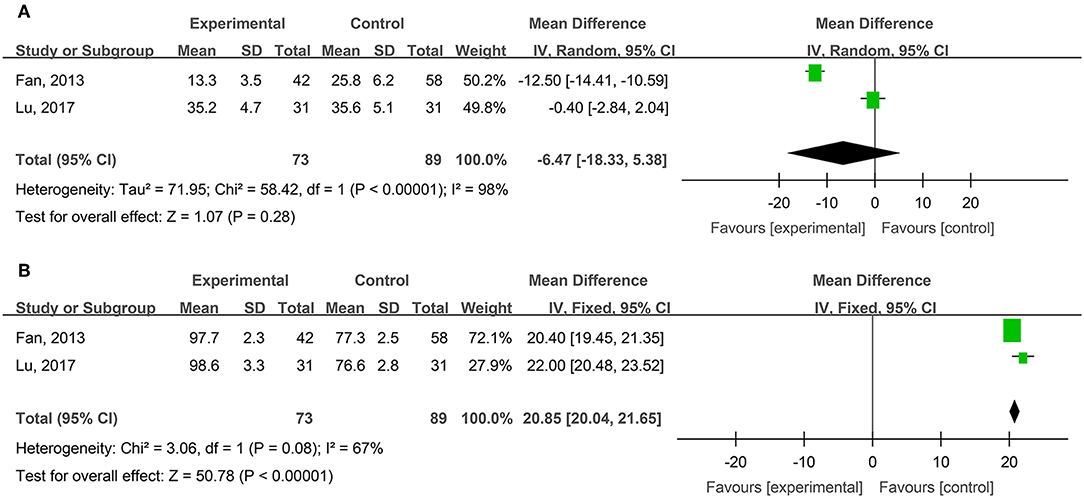
Healing Times and Cure Rates of Surgical Wounds
Two studies, reported by Fan et al. (2013) and Lu et al. (2017), compared the healing times of surgical wounds treated with hydrogel dressing or other ministrations. There existed a statistical heterogeneity among the study results (P < 0.00001, I2 = 98%). Therefore, the random effect model was applied for meta-synthesis (Figure 9A). The results showed that as the healing time of surgical wounds was concerned no statistically significant difference (P = 0.28) intervened between the hydrogel dressings group and the control group.
Two studies, reported by Fan et al. (2013) and Lu et al. (2017), compared the cure rates of surgical wounds medicated with hydrogel dressing or other treatments. There existed no statistical heterogeneity among the study results (P = 0.08). Consequently, the fixed effect model was applied for meta-synthesis (Figure 9B). The results demonstrated that the cure rate of surgical wounds in the hydrogel dressings group was 20.85% higher than in the control group and that statistically the difference was highly significant (MD = 20.85%, 95% CI: 20.04–21.65%, P < 0.00001).
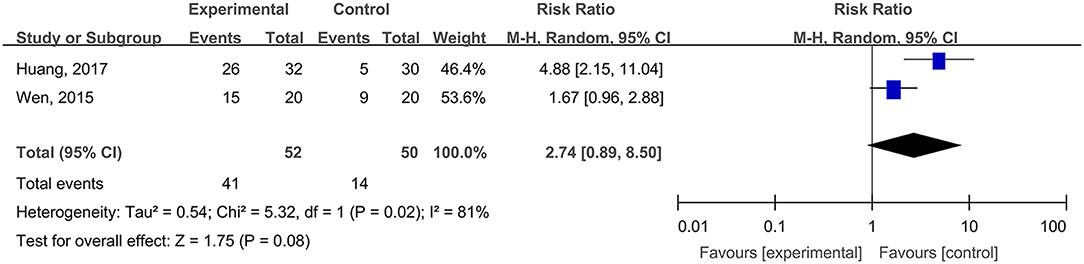
The Cure Rates of Inpatients' Pressure Ulcers
Two studies, reported by Wen (2015) and Huang et al. (2017), compared the cure rates of inpatients' pressure ulcers treated with hydrogel dressings or other therapeutic means. There existed a statistical heterogeneity among the study results (P = 0.002, I2 = 81%). Hence, the random effect model was applied for meta-synthesis (Figure 10). The results revealed that there occurred no statistically significant difference between the hydrogel dressing group and the control group (P = 0.08) in the cure rate of inpatients' pressure ulcers.
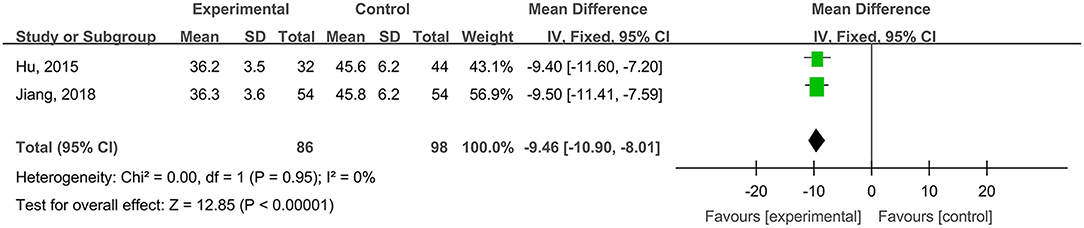
Healing Times of Radioactive Skin Injuries
Two studies, reported by Hu et al. (2015) and Jiang et al. (2018), compared the healing times of radioactive skin injuries treated with hydrogel dressings or other medicaments. There occurred no statistical heterogeneity among the study results (P = 0.95). In consequence, the fixed effect model was applied for meta-synthesis (Figure 11). The results demonstrated that on average the healing time of the hydrogel dressings group was shortened by 9.46 days as compared with that of the control group and that the difference had a high statistical significance (MD = −9.46, 95% CI: −10.90 to −8.01, P < 0.00001).
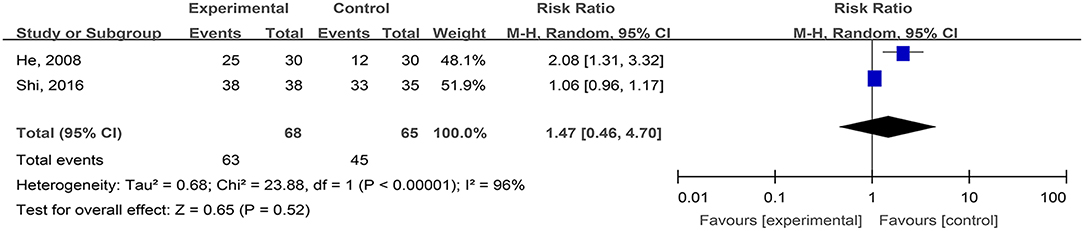
The Cure Rates of Phlebitis Ulcers (Cure and Effectiveness)
Two studies, reported by He et al. (2008) and Shi et al. (2016), compared the cure rates of phlebitis ulcers treated with hydrogel dressings and other ministrations. There existed a statistical heterogeneity among the study results (P < 0.00001, I2 = 96%). Consequently, the random effect model was applied for meta-synthesis (Figure 12). The results indicated that the difference in cure rates between the hydrogel dressings group and the control group of patients with phlebitis ulcers was not statistically significant (P = 0.52).
Dog Bite Wounds, Body Surface Ulcers, and Laser Treatment-Induced Wounds
Only one study, reported by Wang and Teng (2008), compared the cure rates of dog bite wounds treated with hydrogel dressings or saline gauze. The results made known that the healing time of the hydrogel dressings group was 4.0 days shorter than that of controls (t = −16.54, P < 0.001); in addition, the average cure rate of the wounds was 24.8% higher (t = 27.8, P < 0.001) than the controls.
Then again, a single study, reported by Fang et al. (2011), compared the cure rates of body surface ulcers treated with hydrogel dressings or conventional therapy with Iodophor or hydrogen peroxide plus Vaseline gauze. The results revealed that the healing time of the hydrogel dressings group was 18.4 days shorter than that of the controls (t = −5.29, P < 0.001); moreover, the total wound cure rate was also significantly higher than that of the control group (χ2 = 13.78, P < 0.001).
Finally, a lone study, reported by Xin et al. (2014), compared the wound care of patients categorized as hydrogel dressings group and blank control group bearing laser treatment-induced wounds. Concerning VAS scores, as contrasted with the blank control group, the pain score of the hydrogel dressings group was 1.63 lower (t = −6.47, P < 0.001), the burning sensation score was 1.10 lower (t = −8.65, P < 0.001) and the stimulating sensation score was 1.46 lower (t = −10.78, P < 0.001) than the controls.
Data Set of Complaints and Adverse Events
Data Source
Besides the mentioned above Chinese and English databases, a supplementary search was carried out in the State Monitoring System of Drug Adverse Reactions (SMSDAR; http://www.adrs.org.cn/).
Data Synthesis and Analysis
To perform Meta-analyses about the incidence rate of adverse reactions RevMan5.0 software was used and the relative risk was taken as a combined effect size. The heterogeneity of the study results was tested by χ2-test. When the study showed a statistical homogeneity (P > 0.1, I2 < 50%), a fixed effect model was applied, otherwise a random effect model was adopted.
Analysis Result
Three studies, reported by Jin et al. (2009), Diao et al. (2012), and Jin et al. (2017), compared the adverse reaction rates in cases of burn wounds treated with hydrogel dressings or other therapeutics. No statistical heterogeneity was detected among the study results (P = 0.79). Therefore, the random effect model was applied for meta-synthesis (Figure 13). The results disclosed that the incidence rate of adverse reactions—including skin dryness, swelling, pruritus, and fever—was lower in the hydrogel dressings group than in the control group, and that statistically the difference was highly significant (RR = 0.47, 95% CI: 0.33–0.67, P < 0.0001). Other included studies reported no details about patients' adverse reactions.
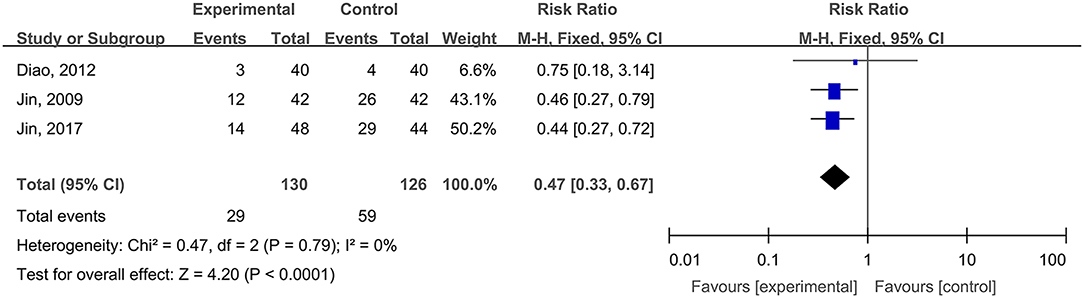
Figure 13. Comparative meta-analysis of the incidence rates of adverse reactions of burn wound-affected patients.
No reports on adverse reactions of using medicinal hydrogels were found in the State Monitoring System of Drug Adverse Reactions (SMSDAR).
Discussion
This study attempted to adopt the Cochrane systematic evaluation and Meta-analysis to assess the effectiveness and safety of hydrogel dressings employed in the management of skin wounds. The results brought to light that the application of medicinal hydrogel dressings can significantly shorten the healing time of skin wounds such as superficial degree-II burns (Figure 2A), deep degree-II burns (Figure 2B), diabetic foot ulcers (Figure 5), traumatic skin injuries (Figure 7), radioactive skin injuries (Figure 11), dog bites (t = −5.29, P < 0.001), and body surface ulcers (t = −5.29, P < 0.001). Hydrogel dressings can also effectively improve the cure rate of diabetic foot ulcers (Figure 6), surgical wounds (Figure 9B), dog bites (t = 27.8, P < 0.001), and body surface ulcers (χ2 = 13.78, P < 0.001). These advantageous effects are likely due to the nearly ideal moist environment that hydrogel dressings provide once applied to skin wounds. This promotes cell viability and physiological functioning and subsequently wound healing. In addition, hydrogel dressings reduce the loss of body fluids while absorbing wound's exudate and advancing autolytic debridement in necrotic wounds and granulating wounds. The hydrogels' swelling property has been proved to decrease the excessive fluid accumulation between the wound surface and the dressing. On the other hand, the hydrogel owns a soft texture and tends to adhere to the wound surface tightly and evenly, which prevents bacterial invasion and reduces soreness as well.
In recent years, with the appearance of new antibiotics and drugs applied to wounds, bactericidal and bacteriostatic substances such as silver ions have been combined with dressings to control local infections and accelerate wound healing. Nanocrystalline silver modulates the inflammatory response through its antimicrobial activity, thereby reducing the infections incidence and leading to an improved wound healing outcome. Furthermore, a faster re-epithelialization occurred in the wounds treated with nanocrystalline silver-coupled dressing rather than with a standard antibiotic solution (Demling and DeSanti, 2002; Nherera et al., 2017).
Study results also indicate that medicinal hydrogels can effectively alleviate the pain and burning and irritating sensations typical of skin wounds. The WHO pain rating of burn wounds (Figure 3) and traumatic skin injuries was significantly lower in the hydrogel dressing treatment studies. In addition, when hydrogel dressings were compared with non-hydrogel treatments, the VAS pain score was obviously lower in superficial degree-II burns (Figure 4A), deep degree-II burns (Figure 4B), and laser treatment-induced wounds. Concurrently, adverse reactions such as wound dryness, swelling, pruritus, and fever were significantly reduced (Figure 13). The benefits brought by hydrogel dressings to wounds might be related to the hydrogel-induced microenvironment that minimizes secondary injuries and alleviates pain by generating a cool feeling and by protecting any exposed peripheral nerve terminals. Our data also indicated that the guar gum-based hydrogel (CQ-01) is safe and can effectively alleviate the intractable pruritus otherwise affecting the patients [the score of Jun Wu scale (JW scale) pruritus rating scale for CQ-01 group was significantly lower than that of the traditional dressing group]. This further supports the clinical antipruritic effect of hydrogel dressings (Wu et al., 2016).
Meta-analysis is an observational study, thus, biases are somehow inevitable (Easterbrook et al., 1991). Among 43 original studies only 8 of them were graded A according to EPOC quality grading, which may potentially prejudice the results. Moreover, some hydrogel dressings were used in combination with other dressings, for example, silver dressings or with Lithosin solution. None of these trials assessed the effects of these combinations. It should be noted that hydrogel dressings are supposed to be applied singly rather than in combination with other therapeutics and that when used in combination their effectiveness and safety cannot be evaluated from individual dressing data. On the other hand, in the result of healing times comparison of burn wounds and others, there existed a statistical heterogeneity among the study results. The main reason for statistical heterogeneity of selected studies is that the sample size of each selected study varies greatly. In addition, clinical heterogeneity may also cause heterogeneity in statistical analysis, such as differences in baseline characteristics and medical conditions of burn patients in various studies, which may affect treatment outcomes.
The limitation of this meta-analysis is that, various dressings were applied in control groups included in this review, such as SSD, Iodine solution, Entoiodine and petrolatum gauze, Lithosin solution and oil, SD-Zn, Petrolatum gauze, Polyvidone iodine, Gentamicin dressing, etc. which may affect the outcomes and potentially add the biases to the study as well.
The main limitation of this review is the potential publication bias in terms of safety assessment of hydrogel dressings. Although we endeavored to collect quite a number of clinical trials by searching both publication databases and SMSDAR, in this systematic review only three studies compared the adverse reactions between hydrogel dressings and other medicinal products. The poor reporting of adverse reactions could be generalizable to the study purpose of clinical trials, which are commonly designed to explore the effectiveness of a dressing in promoting wound healing while they do not focus on the wound site responses to the dressing tested. On the other hand, it is sometimes hard to distinguish an adverse reaction from events related to wound healing.
Conclusions
This evidence-based systematic review and meta-analysis from RCTs and CCTs studies suggests that the use of hydrogel dressings results in a significant decrease in wound healing time, an obvious increase in cure rate, and a satisfying relief of pain as compared to non-hydrogel dressings. All the above-reported results strongly indicate that hydrogel products are effective and safe in wound management. Furthermore, there is a need for high-quality and international multi-center RCTs reporting adverse reactions to help clinicians make informed decisions on the best options for patients suffering from skin wounds.
Data Availability Statement
All datasets generated for this study are included in the article/supplementary material.
Author Contributions
Study design and conception of this manuscript were due to JW and BS. Literature retrieving and studies selection were performed by XL and JL. MY and XW carried out the quality evaluation of the study. Mathematical modeling and meta-analysis were conducted by FZha and FZho. Results analysis and interpretation were done by LZ, HY, and SQ. The manuscript was drafted by LZ and HY. All authors read and approved the final manuscript.
Funding
This work was supported by 100 Talents Program of Sun Yat-Sen University (Y61216), the Three-Three Project of the First Affiliated Hospital, Sun Yat-sen University (Y70214), National Natural Science Foundation of China (NSFC 81601624), China Postdoctoral Science Foundation Grant (2018M631028), and Doctoral Innovation Project of Shenzhen Health and Family Planning System Research Project (201605007).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We would like to thank Prof. Ji Wang and Prof. Xuyang Feng for their useful comments.
Abbreviations
GRADE, Grading of Recommendations Assessment, Development and Evaluation; PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-Analyses; RCT, Randomized Controlled Trial; PICOS, Participants, Intervention, Control, Outcome and Study design; PVP, Polyvinylpyrrolidone; CMC, Carboxy Methyl Cellulose; EPOC, Effective Practice and Organization of Care Group; WMD, Weighted Mean Difference; SSD, Silver Sulphadiazine; VAS, Visual Analog Scale; SMSDAR, State Monitoring System of Drug Adverse Reactions; JW scale, Jun Wu scale.
References
Cai, L., Lv, G., and Chen, J. (2010). Clinical study of photocrosslinked chitosan hydrogel film in the treatment of deep second degree burn wounds. Chin. J. Inj. Repair 5, 61–65.
Chattopadhyay, S., and Raines, R. T. (2014). Review collagen-based biomaterials for wound healing. Biopolymers 101, 821–833. doi: 10.1002/bip.22486
Chen, L., Ning, N., and Chen, H. (2015). Clinical observation of skin wound wet healing treatment. Huaxi Med. 30, 1811–1813.
Chen, Z. X. (2015). Observation on the effect of silver ion dressing combined with hydrogel in the treatment of diabetic foot. Mod. Med. 15, 82–83.
Cui, Z., Liu, L., and Li, J. (2007). Treatment of 42 cases of wounds in burn and plastic donor area with cold Lengningkang dressing. Chin. J. Injury Repair Wound Healing, Electronic Edn. 2, 36–37.
Das, S., and Baker, A. B. (2016). Biomaterials and nanotherapeutics for enhancing skin wound healing. Front. Bioeng. Biotechnol. 4:82. doi: 10.3389/fbioe.2016.00082
Demling, R. H., and DeSanti, M. D. L. (2002). The rate of re-epithelialization across meshed skin grafts is increased with exposure to silver. Burns 28, 264–266. doi: 10.1016/S0305-4179(01)00119-X
D'Hemecourt, P. A., Smiell, J. M., and Karim, M. R. (1998). Sodium carboxymethyl cellulose aqueous-based gel vs becaplermin gel in patients with nonhealing lower extremity diabetic ulcers. Wound 10, 69–75.
Diao, Y., Wang, S., and Chen, M. (2012). Application of hydrogel dressing Ai Kangye silver in the treatment of shallow second degree burn wounds. J. Southeast Univ. 31, 729–731.
Dong, R., Zhao, X., Guo, B., and Ma, P. X. (2016). Self-healing conductive injectable hydrogels with antibacterial activity as cell delivery carrier for cardiac cell therapy. ACS Appl. Mater. Interfaces 8, 17138–17150. doi: 10.1021/acsami.6b04911
Easterbrook, P. J., Berlin, J. A., Gopalan, R., and Matthews, D. R. (1991). Publication bias in clinical research. Lancet337, 867–872. doi: 10.1016/0140-6736(91)90201-Y
Fan, X., Xiao, M., and Wu, Y. (2013). A prospective study of the effect of silver ion combined with hydrogel dressing on postoperative infection wounds. Chin. J. Basic Clin. Med. 20, 102–104.
Fan, Z., Liu, B., Wang, J. Q., Zhang, S., Lin, Q., Gong, P., et al. (2014). A novel wound dressing based on ag/graphene polymer hydrogel: effectively kill bacteria and accelerate wound healing. Adv. Funct. Mater. 24, 3933–3943. doi: 10.1002/adfm.201304202
Fang, Q., Zhang, C., and Cai, X. (2011). Effect of wet therapy on the treatment of surface ulcers. J. Nurs. Train. 26, 2076–2077.
Garg, T., Rath, G., and Goyal, A. K. (2015). Biomaterials-based nanofiber scaffold: targeted and controlled carrier for cell and drug delivery. J. Drug Target. 23, 202–221. doi: 10.3109/1061186X.2014.992899
Gil, E. S., Panilaitis, B., Bellas, E., and Kaplan, D. L. (2013). Functionalized silk biomaterials for wound healing. Adv. Healthc. Mater. 2, 206–217. doi: 10.1002/adhm.201200192
Gong, Z., Yao, J., and Ji, J. (2009). Effects of silver ion dressing combined with hydrogel on wound healing of second-degree burns. J. Clin. Rehabil. Tissue Eng. Res. 13, 8373–8376.
Grice, E. A., Kong, H. H., Conlan, S., Deming, C. B., Davis, J., Young, A. C., et al. (2009). Topographical and temporal diversity of the human skin microbiome. Science 324 1190–1192. doi: 10.1126/science.1171700
He, Q., Wu, G., and Yu, B. (2008). Early results of wound healing hydrogel in the treatment of chronic lower extremity varicose ulcers. Chin. J. Reconstr. Surg. 22, 311–313.
Hu, Y., Xiao, H., and Fu, L. (2015). Study on the effect of new hydrogel dressings on the treatment of wet peeling after radiotherapy. Chin. Nurs. Res. 29, 1635–1637.
Huang, G., Liu, L., and Rongfen, W. (2016). Treatment and nursing observation of 42 cases of acute skin abrasion and contusion. J. Yangtze Univ. 13, 67–68.
Huang, Y., Han, W., and Weng, L. (2017). Application of wet healing therapy in stage III and IV severe pressure ulcers. Modern Clin. Care 16, 46–48.
Huang, Y., Wang, Y., Sun, L., Agrawal, R., and Zhang, M. (2015). Sundew adhesive: a naturally occurring hydrogel. J. R. Soc. Interface 12:12. doi: 10.1098/rsif.2015.0226
Jensen, J. L., Seeley, J., and Gilin, B. (1998). A controlled, randomized comparison of two moist wound healing protocols: carrasyn hydrogel wound dressing and wet-to-moist saline gauze. Adv. Wound Care 11, 323–327.
Jiang, J., Yu, S., and Shen, X. (2008). Treatment of burn wound pain by Lengningkang dressing. Chin. J. Inj. Repair Wound Heal. (Electronic Edition) 3, 200–202.
Jiang, X., Sun, R., and Li, J. (2018). Observation on the effect of hydrogel dressing on radiation-induced skin injury. J. Pract. Clin. Nurs. 3, 91–100.
Jin, A., Cai, L., and Chen, Y. (2017). Comparison of curative effect between hydrogel wound dressing and silver sulfadiazine on bromine burn wounds. Zhejiang Med. J. 39, 1474–1475+1482.
Jin, A., Shi, S., and Lu, J. (2009). “Application of Lengningkang in facial burn wounds,” in Zhejiang Medical Association Burns Surgery Academic Annual Conference Papers (Zhejiang Medical Association).
Lan, Y., and Duan, N. (2015). Observation on the effect of new dressing for debridement and treatment of deep II burn wounds. Contemp. Nurse 5, 116–117.
Li, L., and Wu, B. (2018). Clinical study of early external HD series medical hydrogel wound dressing for small area burn wounds. Chin. J. Damage Restor. 13, 376–377.
Li, P., Chen, Y., and Wo, H. (2015). Clinical efficacy of Sanhuang wet compress hydrogel in the treatment of diabetic foot ulcer wounds. Inner Mongolia Tradition. Chin. Med. 12, 18–19.
Lin, Z., Yang, R., Shubin, R., Yan, L., Zhang, F., Xiong, X., et al. (2018). Analysis of the role of hydrogel dressing in the treatment of superficial second degree burn wounds. J. Gannan Med. College 38, 358–360.
Liu, B. (2015). Comparative Study on the Clinical Efficacy of Wet Protective Dressing for the Treatment of Second Degree Burn Wounds. Ningxia Medical University.
Liu, B., and Ye, L. (2014). Application of hydrogel dressing combined with compound comfrey oil in second degree burn wounds. Chin. J. Aesthetic Med. 23, 1330–1333.
Liu, L., Wang, S., and Cheng, C. (2017). Clinical observation of collagen-loaded nerve growth factor in the treatment of diabetic foot ulcer. J. Armed Police Log. Univ. 26, 421–423.
Lu, L., Xie, C., and Lu, J. (2017). Efficacy evaluation of cefuroxime sodium combined with silver ion and hydrogel dressing for wound healing in patients with postoperative infection. Anti-infected Pharm. 14, 1310–1312.
Mao, C., Xiang, Y., Liu, X., Cui, Z., Yang, X., Yeung, K. W. K., et al. (2017). Photo-inspired antibacterial activity and wound healing acceleration by hydrogel embedded with Ag/Ag@AgCl/ZnO nanostructures. ACS Nano 11, 9010–9021. doi: 10.1021/acsnano.7b03513
Mao, L. (2010). Therapeutic effect of silver ion dressing combined with hydrogel and moist treatment wound dressing on diabetic foot infection. Chin. J. Misdiagn. 10:8348.
Metcalfe, A. D., and Ferguson, M. W. (2007). Tissue engineering of replacement skin: the crossroads of biomaterials, wound healing, embryonic development, stem cells and regeneration. J. R. Soc. Interface 4, 413–437. doi: 10.1098/rsif.2006.0179
Mohan, Y. M., Lee, K., Premkumar, T., and Geckeler, K. E. (2007). Hydrogel networks as nanoreactors: a novel approach to silver nanoparticles for antibacterial applications. Polymer 48, 158–164. doi: 10.1016/j.polymer.2006.10.045
Nherera, L. M., Trueman, P., Roberts, C. D., and Berg, L. (2017). A systematic review and meta-analysis of clinical outcomes associated with nanocrystalline silver use compared to alternative silver delivery systems in the management of superficial and deep partial thickness burns. Burns 43, 939–948. doi: 10.1016/j.burns.2017.01.004
Nie, H., Li, K., and Zhang, Q. (2015). Application of wet dressing in dressing change of elderly diabetic foot ulcer. Inner Mongolia Tradition. Chin. Med. 35, 3582–3584.
Nourian Dehkordi, A., Mirahmadi Babaheydari, F., Chehelgerdi, M., and Raeisi Dehkordi, S. (2019). Skin tissue engineering: wound healing based on stem-cell-based therapeutic strategies. Stem Cell Res. Ther. 10:111. doi: 10.1186/s13287-019-1212-2
Purna, S. K., and Babu, M. (2000). Collagen based dressings–a review. Burns 26, 54–62. doi: 10.1016/S0305-4179(99)00103-5
Qiu, Y., and Park, K. (2001). Environment-sensitive hydrogels for drug delivery. Adv. Drug Deliv. Rev. 53, 321–339. doi: 10.1016/S0169-409X(01)00203-4
Qu, J., Zhao, X., Liang, Y., Zhang, T., Ma, P. X., and Guo, B. (2018). Antibacterial adhesive injectable hydrogels with rapid self-healing, extensibility and compressibility as wound dressing for joints skin wound healing. Biomaterials 183, 185–199. doi: 10.1016/j.biomaterials.2018.08.044
Roy, N., Saha, N., Kitano, T., and Saha, P. (2010). Novel hydrogels of PVP-CMC and their swelling effect on viscoelastic properties. J. Appl. Polym. Sci. 117, 1703–1710. doi: 10.1002/app.32056
Schwartz, V. B., Thetiot, F., Ritz, S., Puetz, S., Choritz, L., Lappas, A., et al. (2012). Antibacterial surface coatings from zinc oxide nanoparticles embedded in poly(N-isopropylacrylamide) hydrogel surface layers. Adv. Funct. Mater. 22, 2376–2386. doi: 10.1002/adfm.201102980
Shamseer, L., Moher, D., Clarke, M., Ghersi, D., Liberati, A., Petticrew, M., et al. (2015). Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015: elaboration and explanation. BMJ 350:g7647. doi: 10.1136/bmj.g7647
Shang, N. (2015). Application of Hydrogel Dressing in Wound Healing After Grinding. Shandong University.
Shao, M., Chen, H., and Chen, Q. (2015). Effects of pain during incision dressing on wound healing in diabetic foot. Chin. J. Modern Nurs. 21, 1522–1524.
Shi, S., LuTing, W., and Wang, Y. (2016). Therapeutic effect of Lengningkang HD-M hydrogel dressing on PICC-associated phlebitis. Hainan Med. J. 27, 2221–2222.
Sood, A., Granick, M. S., and Tomaselli, N. L. (2014). Wound dressings and comparative effectiveness Data. 3, 511–529. doi: 10.1089/wound.2012.0401
Teng, Y. (2010). Evidence-Based Research on Clinical Treatment of Chronic Wounds. Lanzhou: Lanzhou University.
Tsao, C. T., Chang, C. H., Lin, Y. Y., Wu, M. F., Wang, J. L., Han, J. L., et al. (2010). Antibacterial activity and biocompatibility of a chitosan-gamma-poly (glutamic acid) polyelectrolyte complex hydrogel. Carbohydr. Res. 345, 1774–1780. doi: 10.1016/j.carres.2010.06.002
Vandeputte, J., and Gryson, L. (1997). “Diabetic foot infection controlled by immuno-modulating hydrogel containing 65% glycerine. Presentation of a clinical trial,” in 6th European Conference on Advances in Wound Management, Vol. 1997 (Amsterdam), 50–53.
Wang, J., and Teng, Y. (2008). Local treatment of canine bite wound III with silver ion dressing combined with hydrogel: randomized controlled group. Chin. J. Tissue Eng. Res. Clin. Rehab. 12, 2659–2662.
Wang, J., Yan, X., and Teng, Y. (2008). Local treatment of diabetic foot wound with silver ion dressing combined with hydrogel. J. Chongqing Med. Univ. 33, 747–749.
Wang, J., Yan, X., and Teng, Y. (2013). A prospective study of the effect of silver ion combined with hydrogel dressing on postoperative infection wounds. J. Chongqing Med. Univ. 20, 102–104.
Wang, L., Lin, X., and Xiang, X. (2011). Clinical application and safety of Lengningkang dressing in the treatment of burn wounds. China Pharmaceutic. 20, 64–65.
Wen, S. (2015). Nursing experience of hydrogel combined with insulin for the treatment of patients with acne. World's Latest Med. Inform. Abst. 15, 221–226.
Wichterle, O., and Lím, D. (1960). Hydrophilic gels for biological use. Nature 185, 117–118. doi: 10.1038/185117a0
Winter, G. D. (1962). Formation of the scab and the rate of epithelization of superficial wounds in the skin of the young domestic pig. Nature 193, 293–294. doi: 10.1038/193293a0
Wu, J., Xu, R., Zhan, R., Luo, G., Niu, X., Liu, Y., et al. (2016). Effective symptomatic treatment for severe and intractable pruritus associated with severe burn-induced hypertrophic scars: a prospective, multicenter, controlled trial. Burns 42, 1059–1066. doi: 10.1016/j.burns.2015.09.021
Xiang, Y., Zhang, H., and Zou, J. (2012). Clinical study on innovative dressing for the treatment of diabetic foot ulcer. Chin. Pharm. 23, 2063–2064.
Xin, F., Liu, L., and An, Y. (2014). Application of medical hydrogel in laser therapy and multi-center clinical efficacy observation. J. Pract. Dermatol. 7, 193–195.
Xu, R., Luo, G., Xia, H., He, W., Zhao, J., Liu, B., et al. (2015). Novel bilayer wound dressing composed of silicone rubber with particular micropores enhanced wound re-epithelialization and contraction. Biomaterials 40, 1–11. doi: 10.1016/j.biomaterials.2014.10.077
Zeng, Y., and Li, Y. (2016). Application of Shengji ointment combined with silicone dressing in wound care. Contemp. Nurses, 5, 78–80.
Keywords: hydrogel, wound dressing, wound healing, pain relief, meta-analysis, systematic review
Citation: Zhang L, Yin H, Lei X, Lau JNY, Yuan M, Wang X, Zhang F, Zhou F, Qi S, Shu B and Wu J (2019) A Systematic Review and Meta-Analysis of Clinical Effectiveness and Safety of Hydrogel Dressings in the Management of Skin Wounds. Front. Bioeng. Biotechnol. 7:342. doi: 10.3389/fbioe.2019.00342
Received: 28 September 2019; Accepted: 04 November 2019;
Published: 21 November 2019.
Edited by:
Ubaldo Armato, University of Verona, ItalyReviewed by:
Maria Grazia Raucci, Italian National Research Council (CNR), ItalyJingning Huan, Shanghai Jiao Tong University, China
Copyright © 2019 Zhang, Yin, Lei, Lau, Yuan, Wang, Zhang, Zhou, Qi, Shu and Wu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Bin Shu, c2h1YmluMjlAc2luYS5jb20=; Jun Wu, anVud3Vwcm9AMTI2LmNvbQ==
†These authors have contributed equally to this work
 Lijun Zhang
Lijun Zhang Hanxiao Yin1†
Hanxiao Yin1† Xiaoyan Wang
Xiaoyan Wang Fei Zhou
Fei Zhou