- 1Laboratoire des Sciences de l'Environnement Marin (LEMAR), Technopole Brest Iroise, Plouzané, France
- 2Department of Marine Sciences, University of Georgia, Athens, Georgia
- 3Department of Earth, Ocean and Ecological Sciences, University of Liverpool, Liverpool, United Kingdom
The concentrations of dissolved copper (Cud), copper-binding ligands, thiourea-type thiols, and humic substances (HSCu) were measured in estuarine waters adjacent to Sapelo Island, Georgia, USA, on a monthly basis from April to December 2014. Here we present the seasonal cycle of copper speciation within the estuary and compare it to the development of an annually occurring bloom of Ammonia Oxidizing Archaea (AOA), which require copper for many enzymes. Two types of complexing ligands (L1 and L2) were found to dominate with mean complex stabilities (log ) of 14.5 and 12.8. Strong complexation resulted in lowering the concentration of free cupric ion (Cu2+) to femtomolar (fM) levels throughout the study and to sub-fM levels during the summer months. A Thaumarchaeota bloom during this period suggests that this organism manages to grow at very low Cu2+ concentrations. Correlation of the concentration of the L1 ligand class with a thiourea-type thiol and the L2 ligand class with HSCu provide an interesting dimension to the identity of the ligand classes. Due to the stronger complex stability, 82–99% of the copper was bound to L1. Thiourea-type thiols typically form Cu(I) species, which would suggest that up to ~90% copper could be present as Cu(I) in this region. In view of the very low concentration of free copper (pCu > 15 at the onset and during the bloom) and a reputedly high requirement for copper, it is likely that the Thaumarchaeota are able to access thiol-bound copper directly.
Introduction
Free Cu2+ is well-known for its toxicity to marine microorganisms (Anderson and Morel, 1978; Sunda and Guillard, 1976). The toxicity threshold varies between species with pM concentrations found to affect cyanobacteria (Brand et al., 1986), well below typical ambient concentrations of dissolved Cu. The speciation of dissolved copper in seawater is usually dominated by organic ligands (Moffett and Dupont, 2007), forming relatively stable complexes with around 99% of the dissolved Cu. The oxidation state of organic copper is generally implied to be Cu(II) at natural pH (Leal and van den Berg, 1998) as inorganic copper(I) is unstable in seawater in spite of stabilization by chloride complexation (Nelson and Mantoura, 1984) and is oxidized to copper(II) in a matter of minutes by dissolved oxygen (Sharma and Millero, 1988). Nevertheless around 10% of the dissolved copper in ocean surface waters has been shown to be Cu(I) (Moffett and Zika, 1988) and potentially up to 80% Cu(I) in estuarine waters (Buerge-Weirich and Sulzberger, 2004). Concentrations of copper complexing organic ligands in seawater and their complex stability constants (log value, based on Cu2+ and L′ and abbreviated here to log ) are typically measured by titrations with copper, using cathodic stripping voltammetry (CSV) and competitive ligand equilibration (CLE-CSV; van den Berg, 1984; Donat et al., 1994). Ocean and coastal waters contain ligands with a large range of complex stabilities, that have for now been sub-divided into at least two distinct ligand classes (L1 and L2), with log 13–16 and log 10–13 (Moffett et al., 1990; Laglera and van den Berg, 2003; Buck and Bruland, 2005; Bundy et al., 2013; Muller and Batchelli, 2013).
Complexed copper is considered less bioavailable and thus less toxic than free Cu2+ (Donat et al., 1994; Moffett et al., 2012; Oldham et al., 2014). Several marine microorganisms have been shown to release copper-binding ligands, such as thiols or phytochelatins, in response to copper (Rijstenbil et al., 1998; Leal et al., 1999; Gordon et al., 2000; Dupont and Ahner, 2005), and are therefore a source of thiols in the water. Thiols are organo-sulfur compounds containing the—SH functional group important for metal detoxification in cell metabolism, forming part of a variety of biogenic sulfur species in the marine environment (Radford-Knoery and Cutter, 1994; Tang et al., 2000). Typical thiols include glutathione (GSH), cysteine and their dimers (e.g., oxidized glutathione, GSSG), as well as larger GSH-cysteine chains (phytochelatins) and mercapto compounds. Reduced sulfur substances (RSS) describe a wider group, which also contains dimethyl sulfide (DMS), thioureas, and thioamides (Laglera and Tovar-Sanchez, 2012). Although, thioureas and thioamides may not technically be considered thiols due to favoring the thione form, we will include them in the discussion of thiols within this paper. Thiols have been shown to occur in estuarine (Dryden et al., 2007), coastal (Tang et al., 2000), and open ocean waters (Le Gall and van den Berg, 1998; Dupont et al., 2006; Swarr et al., 2016), suggesting that they could play a major role in the ocean biogeochemistry of copper. As well as cell exudates, sources of thiols include pore waters (Zhang et al., 2004) and sewage effluents (Dryden et al., 2007). RSS typically form Cu(I) complexes (Leal and van den Berg, 1998; Konigsberger et al., 2015) although Cu(II)-thiolates have been generated artificially (Kitajima et al., 1990). Different thiols bind copper with a range of log values, typically log = 12–14 (Laglera and van den Berg, 2003) in salinities from estuarine to seawater. Unidentified thiols from hydrothermal vents have been measured with log values of 12.5–13.5 (Sander et al., 2007) and other natural ligands, suspected to be unidentified thiol compounds, have been measured with log values of 14–16 (Laglera and van den Berg, 2003).
Humic substances are another source of copper-binding ligands with a complex stability of log = 12–13.5 (Kogut and Voelker, 2001; Whitby and van den Berg, 2015). Humic substances occur in abundance in estuarine and coastal waters (Muller and Batchelli, 2013), accounting for up to 40–60% of DOM (McKnight and Aiken, 1998), with around 4–20% of DOM as humic acid and the majority as fulvics and non-humic material (Sholkovitz, 1976). Humics form 5–25% of dissolved organic carbon (DOC) in the surface ocean (Benner, 2002). The fraction of terrestrial humics which survives estuarine mixing is important for transporting dissolved trace metals, such as copper and iron, to coastal and open ocean waters (Laglera et al., 2011; Misumi et al., 2013; Bundy et al., 2015).
Although, potentially toxic, copper is important in many cellular processes, such as in iron uptake (Peers et al., 2005; Maldonado et al., 2006), even substituting for iron in biochemical pathways of iron-limited Thalassiosira oceanica (Peers and Price, 2006). Ionic copper [as Cu(I) or Cu(II)] is required in enzymatic pathways related to oxidation-reduction reactions such as polyphenol (Arnon, 1949) and ammonia oxidation. Genomes of ammonia-oxidizing archaea (AOA) contain many Cu-dependent metalloenzymes (Amin et al., 2013) with inferred high copper requirements. They differ in this regard from ammonia oxidizing bacteria (AOB), which use Fe-dependent metalloenzymes for many of the same functions. AOA are one of several organisms demonstrated in culture studies to be limited by the availability of copper (Amin et al., 2013), along with methane oxidizing archaea (Glass and Orphan, 2012) and some phytoplankton (Annett et al., 2008; Guo et al., 2010; Walsh et al., 2015). AOA are significant contributors to nitrification and thus to the global nitrogen cycle (Francis et al., 2005; Beman et al., 2010) contributing significantly to nitrous oxide (N2O) fluxes (Santoro and Casciotti, 2011). They are the dominant ammonia oxidizers in the pelagic ocean, accounting for up to 40% of total picoplankton cells in the mesopelagic ocean (Karner et al., 2001) and Antarctic winter populations (Church et al., 2003). Thaumarchaea are responsible for a major fraction of carbon fixation below the euphotic zone (Herndl et al., 2005; Ingalls et al., 2006) and in Antarctic coastal waters during winter (Tolar et al., 2016b).
The main objective of this study was to assess Cu speciation, the ligand identity and possible impact on phytoplankton and AOA growth in estuarine waters. Waters adjacent to Sapelo Island, Georgia, were selected for this study, as they are the location of a regular bloom of Thaumarchaeota, a phylum of the Archaea, which bloom annually from July to September in this region (Hollibaugh et al., 2014).
Methods
Sample Collection
Surface samples were collected monthly from April to December 2014 from 6 stations along the Duplin River, D1–D6 from the RV Salty Dawg, except November, which was not sampled. Additional samples from the Doboy Sound (GCE 4, 5, 6) and the major freshwater end-member of the marsh complex (the Altamaha River, STN 10) were collected at the beginning of the study, in April 2014 (Figure 1). Samples were collected directly into sample bottles (acid-soaked, 2,000 mL amber HDPE, rinsed three times with sample) from a depth of ~20 cm below the surface by reaching overboard and filling the bottle with the mouth pointed upstream while the vessel motored slowly forward. The samples were filtered on the same day as collection through 47 mm diameter, 0.22 μm filters (Millipore, GVWP) held in a Nalgene polycarbonate filtering apparatus, which had been soaked in 0.1 M HCl and rinsed with sample 3 times before use. The filtrate was poured into acid soaked and filtrate-rinsed bottles (500 or 1,000 mL fluorinated polyethylene, FLPE) that were frozen at −80°C immediately after filling to be shipped by courier from GA to the UK for analysis. Once reaching the UK they were stored at −20°C. Upon thawing, samples were swirled gently before use, stored in the dark at 4°C and analyzed within 3 days of defrosting.
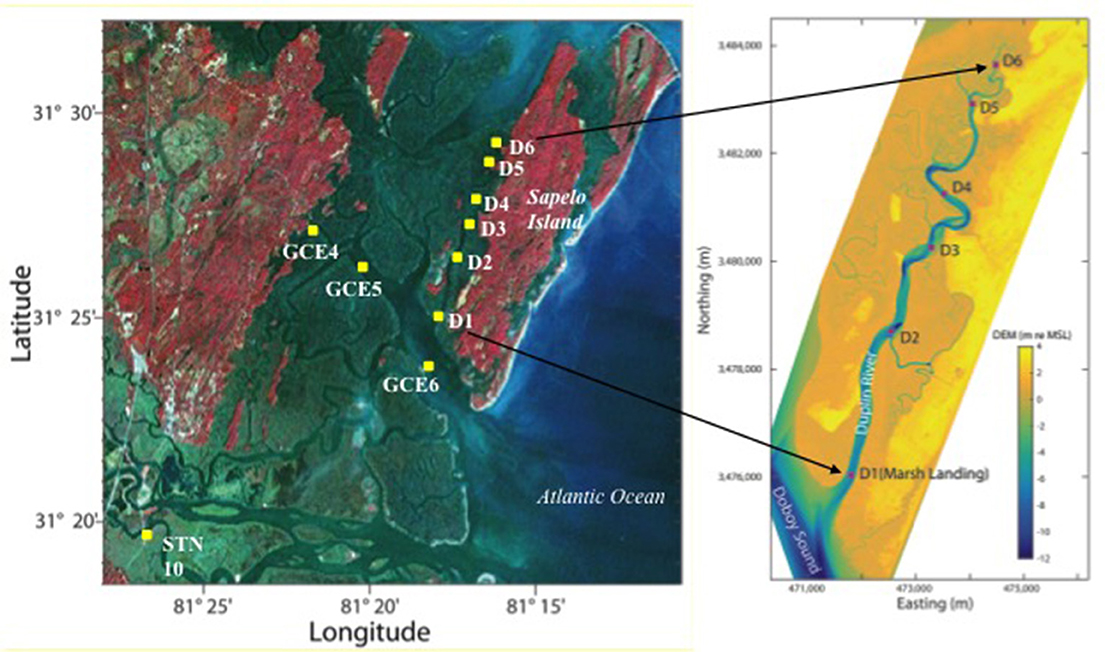
Figure 1. Map of the sampling locations in the estuary of the Duplin River (D1–D6) sampled April–December 2014. Additional stations were regular sampling stations GCE 4–6 in the Doboy Sound and the freshwater end-member (STN 10) in the Altamaha River sampled in April 2014. Elevations in the DEM are in meters relative to mean sea level. DEM plot courtesy of Dr. Daniela Di Iorio/GCE-LTER.
The filters were removed from the filter rig using plastic forceps and placed into Whirl-Pak bags with 1 mL of lysis buffer, then frozen and stored at −80°C until DNA extraction. Additional samples for DNA analysis were collected weekly from a dock at Marsh Landing (31.4179°, −81.2962°; adjacent to Station 1) for another project. Although, efforts were made to collect samples at low tide, there is some variation in the tidal stage at the time of sampling. Samples from July and August were collected during the ebb just before low tide; June, October, and December samples were collected at the lowest point in the tide; samples from April and May were collected at the very beginning of the flood tide and September samples were collected midway through the flood tide.
Equipment and Reagents
The measurements of dissolved copper and complexing ligands were performed by voltammetry as described previously by Whitby and van den Berg (2015). The voltammetric measurements were carried out using a μ-Autolab III potentiostat (Ecochemie, Netherlands) connected to a 663 VA stand (Metrohm) with a hanging mercury drop electrode (HMDE). The set-up included an Ag/AgCl reference electrode with a 3 M KCl salt bridge and a glassy carbon counter electrode, and solutions were stirred with a rotating polytetrafluoroethylene (PTFE) rod. We use a glassy carbon counter electrode as platinum counter electrodes are likely to release platinum ions into solutions (van den Berg et al., 1988). The software was modified to discard 2, instead of the usual 4, drops of mercury between scans to minimize mercury usage. Voltammetric scans used the differential-pulse mode for CSV and the square-wave mode for anodic stripping voltammetry (ASV). The deposition time was between 10 and 30 s for CSV and up to 2.5 min for ASV. Quartz (silica) and PTFE voltammetric cells were cleaned using 0.1 M HCl (trace metal grade) and rinsed with deionized water followed by UV-digested sample before determination. The UV-digestion apparatus contained a high-pressure, 125-W mercury-vapor lamp (van den Berg, 2014), either positioned horizontally above a sample aliquot or surrounded by four 30-mL quartz sample tubes with PTFE caps. The stock borate/ammonia pH buffer (1 M boric acid/0.3 M ammonia) used in all CSV measurements was UV-digested to remove organic matter and contaminating metals were removed by leaving overnight with 100 μM manganese dioxide (MnO2) (van den Berg, 1982) followed by filtration (0.2 μm).
Total Dissolved Copper
Seawater was UV-irradiated (UVSW) in a conditioned quartz voltammetric cell for 45 min and left to cool. The dissolved copper was determined either by CSV in the presence of 20 μM salicylaldoxime (SA) and 0.01 M borate/ammonia pH buffer (pHNBS 8.15) (Campos and van den berg, 1994), or by ASV at pH 2 (June, September, and December samples), or both for inter-comparison. For CSV measurements, the sample was UV-irradiated in the voltammetric cell at the original sample pH, and measured at a deposition potential of −0.15 V, a deposition time of 30 s, and a 1-s potential jump to −1.2 V to desorb any residual organic matter prior to the scan. For ASV measurements, the sample was UV-irradiated and measured at pH 2, using a deposition potential of −0.9 V for 120 or 300 s, followed by 10 s at −1.4 V. Measurements using ASV were found to agree with CSV measurements within the standard deviation of 3 repeat measurements, and additional comparisons on NASS-6 reference material (National Research Council Canada) using ASV were within 5% of the certified value.
Complexing Capacity Titrations
The concentration of copper complexing ligands in each sample was determined by CLE-CSV with ligand competition against SA (Campos and van den berg, 1994). Samples were diluted to 50% for the titrations, to reduce organic interference and lower the concentration range of copper additions, similar to previous studies on estuarine waters (Abualhaija et al., 2015). Dilution was with UV digested sample or, when sample was limited, a mixture of MQ with UV digested Atlantic seawater, combined so as to equal the salinity of the sample being titrated. The starting concentration of Cu in each titration was therefore equal to, or lower than, the concentration of the sample and was accounted for within the calculation. The ligand concentrations were corrected to account for dilution, but the log values were not affected since the salinity was maintained.
For each titration, 80 mL of sample and 80 mL UVSW were transferred to a 250-mL Teflon bottle (Nalgene), and 0.01 M borate buffer and 20 μM SA added. Aliquots of 10 mL seawater, mixed with buffer and SA, were pipetted into 14 25-mL polystyrene (Sterilin) vials with polyethylene lids. The vials were conditioned with UVSW followed by conditioning with diluted sample before initial use. They were rinsed with MQ between different samples but not between titrations of the same sample to minimize de-conditioning. Copper was added to each vial in steps of progressively increasing concentration, typically from 0 to 200 nM. The usual increments were 0, 5, 10, 15, 20, 25, 30, 40, 50, 75, 100, 125, 150, 200 nM Cu. These were then left to equilibrate overnight prior to analysis. The addition of SA in excess prior to the addition of Cu(II) minimized the risk of oxidizing natural thiols by the Cu(II) added during titrations as it kept the concentration of Cu2+ low (Moingt et al., 2010). Similarly, we kept samples in the dark when not in use and equilibrated titrations overnight to minimize exposure to light and reduce the risk of photooxidation (Laglera and van den Berg, 2006). The labile copper concentration (i.e., that which bound with the added SA) in each cell was then determined by CLE-CSV using a 15 s deposition time. The deposition potential was −0.15 V, followed by a 9 s quiescence period at 0 V from where the scan was initiated. No potential jumps were made for the measurement of labile copper. Two fresh copper additions were made at the end of each titration (usually two additions of 50 nM copper) and measured immediately to calibrate the sensitivity of the titration curve, but these were not used in data fitting.
Humic Substances and Thiols
Copper-binding humic substances (HSCu) were determined by CSV at a deposition potential of +0.05 V, after saturation with copper (50–100 nM) in the presence of borate buffer (pHNBS 8.15) (Whitby and van den Berg, 2015), with a deposition time of between 10 and 30 s depending on the concentration. Reference humic acid used for calibrations was Suwannee River humic acid [SRHA, International Humic Substances Society (IHSS) Standard II 2S101H], which was dissolved in MQ water to a concentration of 0.1 g L−1 and stored in the dark at 4°C when not in use. Samples were diluted 90% with UVSW to minimize interference by organic matter and to remain within the linear range (2 mg/L HS in the presence of 50 nM Cu with a 15 s deposition time). A 1-s potential jump from 0 to −0.2 V and back (without stirring) was used to remove possible iodide interference, and scans were initiated from 0 V. A background subtraction was performed on each scan, consisting of the subtraction of a 1-s scan, which provides a flat baseline for more accurate measurement of the HSCu peak and accounts for any diffusion-current from excess inorganic copper. Concentrations of HSCu calibrated on the scale of mg HA L−1 were converted to the nM scale by multiplying with the binding capacity of 18 nmole Cu mg−1 HSCu(Whitby and van den Berg, 2015). The humic standard was used without purification since HSCu measurements were performed in the presence of excess copper.
Stock thiourea (TU) and thioacetamide (TA) (both reagent grade, Fluka) standard solutions were prepared by dissolution in MQ to a concentration of 0.1 M and kept in the dark at 4°C, with dilutions prepared to 10−5 M for thiol measurements. Thiol measurements were modified from existing methods (Laglera and van den Berg, 2003; Laglera and Tovar-Sanchez, 2012) and were made in the presence of borate buffer. The deposition time was between 10 and 30 s depending on the concentration. Measurements were performed without addition of Cu or SA, with a deposition potential of +0.05 V, and a 1-s potential jump to −0.2 V to eliminate iodide interference as in HSCu measurements; although the background subtraction described by Laglera and Tovar-Sanchez (2012) was not employed. Under these conditions we found that thiourea and thioacetamide had the same sensitivity and produced the same thiol concentration in samples, as opposed to differing sensitivities observed when using a deposition potential of −0.1 V (used in earlier work on thiols; Laglera and van den Berg, 2003). Thiol analyses were performed without sample dilution.
Detection Window
At the competing ligand concentration of 20 μM SA, the detection window is centered at an α-coefficient (log αCuSA) of 5.6, strong enough to compete with ligands occurring at 10's of nM with complex stability (log ) of 12–15. We attempted to use a lower detection window to detect weaker ligands (by using 1 and 2 μM SA, log αCuSA ~ 4), but titrations were not successful due to interference from the HSCu peak at around the same potential as the Cu-SA peak (at around −0.2 V), even with sample dilution. Data were interpreted using the van den Berg/Ruzic linearization procedure (Campos and van den berg, 1994) within ProMCC software (Omanovic et al., 2015). Log values are provided on the basis of Cu2+ and L′:
where [L′] is the concentration of L not complexed by Cu. [L′] is affected by side-reactions with major cations and H+, and the K′ values are therefore conditional on the experimental salinity and pH.
Thaumarchaea Quantification
Thaumarchaea abundance was determined by quantitative PCR. Microbial biomass was collected by filtration through 0.22 μm pore size, 47 mm diameter Durapore filters, and frozen at −80°C until processed. Samples of the filtrate were frozen at −80°C for nutrient analysis. Lysozyme, proteinase K, and sodium dodecyl sulfate (SDS) were used to extract deoxyribonucleic acid (DNA) from the filters; the extract was then purified with phenol:chloroform and concentrated by precipitation with ethanol as described previously (Bano and Hollibaugh, 2002). Marine group I Archaea (Thaumarchaeota) 16S rRNA (rrs) and Archaea ammonia monooxygenase subunit A (amoA) genes were quantified using primers ARCHGI334F/ARCHGI554R/TM519AR (Suzuki et al., 2000) and Arch-amoA-for/Arch-amoA-rev (Wuchter et al., 2006), respectively, and protocols described previously (Kalanetra et al., 2009; Tolar et al., 2013) with an iCycler iQ™ Real-Time qPCR detection system (BioRad).
Data Archiving
Copper speciation data collected during this study are archived in the GCE-LTER (http://gce-lter.marsci.uga.edu) data repository under catalog number CHM-OTH-1702, and water quality and qPCR data under catalog number MIC-GCED-1702. Additional data from the study site are available from GCE-LTER (Stations ML, “Marsh Landing”; and GCE10, “Hunt Camp”) and from the Sapelo Island National Estuarine Research Reserve at http://cdmo.baruch.sc.edu/get/export.cfm.
Results
Hydrography
The Duplin River (31.4167°, −81.2974°) is a tidal creek within the Sapelo Island National Estuarine Research Reserve. It separates Sapelo Island from the salt marshes bordering the mainland, but is not a true river. The tidal influence is predominantly from the south-western side where the Duplin River meets Doboy Sound (Figure 1). The Duplin River drains an extensive area of salt marsh through a network of smaller channels. It is also influenced by freshwater inputs from rivers and groundwater from Sapelo Island as well as water draining from the salt marsh at low tides (>2 m mean amplitude). The area is thus a complex network with multiple end-member mixing. Surface water salinity along the Duplin (stations D1–D6) ranged from 13 to 30 during the period studied, with salinity generally lowest in the April samples and highest in September (Supplementary Figure 1A). As well as variation in tide level between sampling months, April experienced the heaviest monthly rainfall, with a major event occurring on the day of sampling (NOAA, 2015). Surface water temperature increased steadily from April to August, with highest surface water temperatures of ~30.4°C, decreasing to ~14°C in December (Supplementary Figure 1B).
Dissolved Copper
The broad salinity range encountered during the study resulted in a wide range of Cud concentrations, since at salinities below 20 the flocculation of Cud and humic substances increases with increasing salinity, although this is followed by little removal above salinity 20 (Sholkovitz, 1976). Cud generally decreased with increasing salinity: from a mean along the Duplin River (stations D1–D6) of 25 nM in April (wide range of Cud: 7.1–65 nM across salinity 13–17) to a mean of 4.5 nM in October (Cud range: 2.9–6.1 nM, at higher salinities of 27–28; Supplementary Figure 2). Increased rainfall, particularly during a major storm event in April, could have played a role in the higher concentrations encountered in April, as intense rainfall events at low tide can cause increased erosion leading to elevated trace metal concentrations (Moskalski et al., 2013; Guan et al., 2015). Cud was relatively constant with a mean of 6.1 nM between July and September (Cud range 3.6–9.6 nM, salinity range 21–30), decreasing in October and increasing by December.
Identifying CSV Peaks
Preliminary CSV measurements of samples from the Duplin River estuary showed the presence of a voltammetric peak at ~−0.5 V (Supplementary Figure 3), corresponding with the peak potential for sulfide and certain thiols (Al-Farawatii and van den Berg, 1997; Laglera and van den Berg, 2003; Laglera and Tovar-Sanchez, 2012). Stability of this peak over multiple repeat scans indicated that it was not sulfide (Al-Farawatii and van den Berg, 1997). We compared cysteine, thiourea, thioacetamide, methanethiol, allylthiourea, and both oxidized and reduced forms of glutathione to the natural peak to determine which would be most suitable as a model thiol compound. We found that thiourea and thioacetamide behaved most similarly to the natural thiol peak in terms of peak shape, position and appearance, and standard additions increased the size of the natural peak, supporting other work on coastal waters where thiourea and thioacetamide were also found to be the best candidates (Al-Farawati and van den Berg, 2001; Laglera and van den Berg, 2003). The voltammetric peak measured for this type of species is that of mercury-bound sulfide (Hg-thiol), and is not due to the reduction of copper stabilized as a copper-bound thiol as for glutathione (Le Gall and van den Berg, 1993) or cysteine (van den Berg et al., 1988), which occur at more positive potentials and have a broader peak appearance. Measurements of the natural thiols were therefore made without any addition of Cu(II), eliminating the risk of thiol oxidation by added Cu(II) (Moingt et al., 2010).
The optimum deposition potential for the natural thiol peak was found to be +0.05 V. At this potential the sensitivity of the analysis for thiourea was found to be the same as that for thioacetamide, with standard additions of either compound giving the same concentration (on the molar scale) compared to differences noted at lower (negative) deposition potentials. The signals from various thiols are known to coalesce into one signal at deposition potentials between +0.02 and +0.07 V (Laglera and Tovar-Sanchez, 2012). Since differences in the sensitivity of thioacetamide and thiourea occur at lower potentials (Laglera and Tovar-Sanchez, 2012), we tested the effect of lowering the deposition potential on the response of thiourea, thioacetamide, and the natural thiol in our samples. Lowering the deposition potential from +0.05 V to −0.1 V caused only a minor decrease (4%) in the sensitivity of the natural thiol but the sensitivity for the thioacetamide dropped by 80%, and the thiourea became undetectable. The difference in the sensitivity behavior of the different model thiols at the electrode compared to the natural thiol peak suggests that the natural thiol may be more similar chemically to thioacetamide than thiourea, but is not identical to either of these, although surface-active materials in the sample (such as HSCu) may affect this analysis. Since both thiourea and thioacetamide gave the same response with standard additions at +0.05 V, this deposition potential was chosen. Despite the deposition potential demonstrating that the natural peak was more similar to thioacetamide rather than to thiourea, thiourea was selected as the standard in order to be comparable to other work, since measurements reported in thiourea (TU) equivalents are generally more common. Since at +0.05 V the same concentration was measured whether using standard additions of thiourea or thioacetamide, the choice of standard had no effect on our measurements of thiol concentrations.
Copper-Binding Ligands
Two ligand classes were detected at all stations across all months, with log values differing by more than a unit between the distinct classes. The ligand concentrations in Duplin River waters ranged from 16 to 51 nM for L1 (72 nM in the Altamaha river end-member) and 30 to 112 nM for L2 (163 nM in the end-member) (Figures 2A,B). The ligand concentrations were inversely related with increasing salinity during individual sampling events (Figures 2A,B) with the seasonal effects imposing scatter on plots of the combined data. The overall variation we observed was therefore a combination of salinity and seasonal effects.
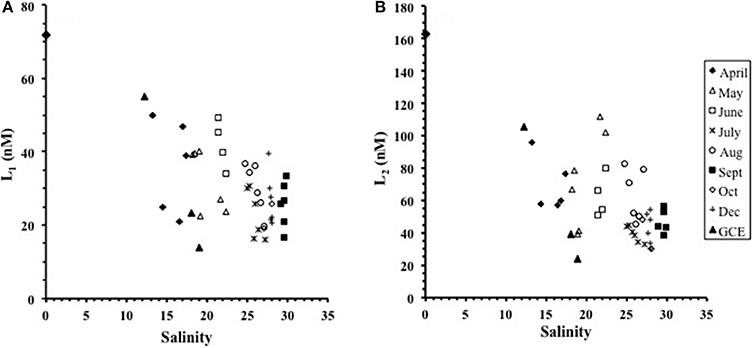
Figure 2. The variation in ligand concentration with salinity for (A) the L1 ligand class and (B) the L2 ligand class. Both L1 and L2 generally decreased with increasing salinity from April to October though the data are affected by localized inputs. The GCE stations and the freshwater end-member (STN 10) sampled in April are included.
The mean log across the studywas 14.5 ± 0.3, with log ranging from 14.0 to 15.2 and varying by a combination of salinity and seasonal effects (Figures 3A,B). The mean value for log was 12.8± 0.4 with log ranging from 12.1 to 13.4 (Figures 3A,B). Fitting the log values as function of salinity to a simple model based on Mg-ion competition:
shows that a decrease in the complex stability with increasing salinity (Figure 3B) can be explained by competition with major cations. Here the complex stability for any given salinity [] was calculated from that at zero salinity by subtracting a side-reaction coefficient for Mg2+ (log αMgL), computed using non-linear data fitting (Figure 3B). Because the concentration of Mg2+ is 5 times greater than that of Ca2+, we assumed that Mg2+ would be the dominant competing cation, but the competition could also include Ca2+ or a combination of the two. Increases in log and log during August–December in the higher salinity (25–30) range (Figure 3B) are not consistent with expectations from the model: the sampling bias toward higher salinity in these months (Supplementary Figure 1) should have given lower log values. The high values we found suggest that different ligands with stronger binding constants were dominant during this period.
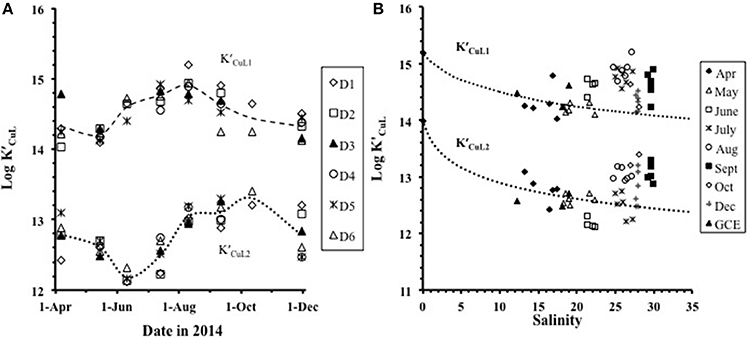
Figure 3. The trend in log and log (A) over the seasonal cycle from April to December and (B) with salinity. The dotted lines in (B) show the modeled trend with salinity for log and log . Plot (B) includes the freshwater end-member station 10 sampled in April.
Composition of the Ligand Classes
Since thiols and humic substances are likely candidates for copper-binding ligands, their concentrations were compared to those of the individual ligand classes (L1 and L2) and their sum, as obtained from titrations. The sum of the concentrations of thiourea-type thiol and humic substances correlated very well with the sum of L1 and L2 (LT) across the seasonal cycle (Figure 4A) with a slope of 0.89 ± 0.08, ρ = 0.82 (where ρ is Spearman's rank correlation coefficient), suggesting they represent the key complexing agents. The concentration of the thiourea-type thiol correlated well with the concentration of L1throughout the study (Figure 4B),with a slope of 1.11 ± 0.13, ρ = 0.85, suggesting L1 is primarily a thiourea-type thiol. The concentration of HSCu correlated well with L2 (Figure 4C), with a slope of 0.85 ± 0.08, ρ = 0.70, suggesting that HSCu is a good model for L2 in these waters. For all three correlations, p < 0.0001, n = 41.
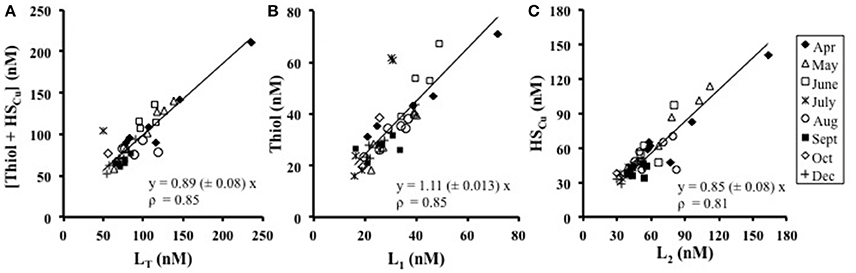
Figure 4. The relationship between the concentrations of (A) [Thiol+HS] against the total ligand (LT), (B) thiourea-type thiols against L1, and (C) HSCu against L2. Thiol measurements are in thiourea equivalents (nM) and HSCu are on the nM scale by multiplying with the binding capacity of 18 (Whitby and van den Berg, 2015). April data includes the freshwater end-member station 10 sampled in April.
Titration of Mixtures of Model Ligands
Mixtures of thiols and humic substances added to UVSW were titrated with copper to evaluate whether we could reproduce titration results from samples using model ligands. Thiourea and SRHA were added to a UV-digested sample of salinity 18.5 (May D5) to a concentration of 10 nM and 1 mg L−1, respectively [equivalent to 18 nanomole (mg HA)−1 Cu-binding capacity; Whitby and van den Berg, 2015). Ligand titrations of the mixture showed the presence of two copper binding ligands: L1 = 10.9 ± 0.6 nM and L2 = 19.1 ± 0.4, in good agreement with the added concentrations of thiourea and humic acid. The data fit for the model ligand titration is shown in Supplementary Figure 5. The stability constant obtained for L2 in the model ligand titration, log = 12.3 ± 0.4, agreed well with log expected for humics in seawater, and is also similar to that for the L2-ligands within the samples. The stability constant for L1 from the model ligand titration, log = 14.9 ± 0.4, was also similar to log of the samples.
Titrations of higher concentrations of thiols (30 nM thiourea) and humic-type ligands (3.5 mg L−1 SRHA) were found to cause the plots of reactive copper vs. added copper to level off at Cu levels >40 nM. This behavior implies electrode saturation by Cu-SA at an unexpectedly low copper level, suggesting a surfactant effect on the electrode of the naturally occurring humic substances or thiol species causing electrode saturation. The same behavior was observed in titrations of UVSW with a higher concentration (50 nM) of thioacetamide (without humic substances). Interestingly, this phenomenon in the synthetic ligand mixtures replicated that seen in titrations of actual samples before dilution to 50%, suggesting that it may be a common problem in titrations of estuarine waters containing high concentrations of thiol-type ligands and humics.
Thaumarchaeota
The abundance of Thaumarchaeota was low and stable through the spring and early summer, then increased dramatically from July, with peak abundance in August at all 6 stations (Figures 5A,B), dissipating by September. The pattern seen in average monthly data (gene copies L−1) from 6 stations along the Duplin River (taken simultaneously with samples used for metal speciation) is consistent with data from weekly samples taken at the mouth of the Duplin River (Marsh Landing, shown in Supplementary Figure 6) and is therefore not an artifact of our sampling regime. Comparison of the time course of Thaumarchaeota abundance to that of [Cu2+] (Figure 5) shows that the Thaumarchaeota abundance started to increase when [Cu2+] decreased, with the bloom occurring during the period of lowest of Cu2+ concentration (Figure 5A). Calculation of the distribution of copper between L1 and L2 showed little variation during the study (Figure 5B) with 90–99% of the copper always bound with L1, suggesting that the concentration of free inorganic copper was the only variable during this period of possible relevance to the bloom of Thaumarchaeota.
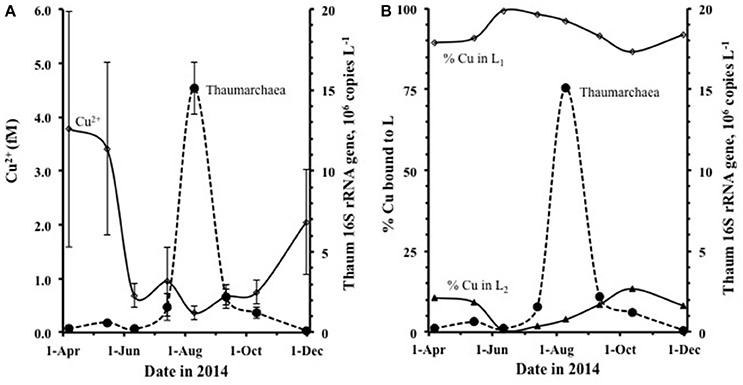
Figure 5. The variation in Thaumarchaea abundance each month sampled compared to (A) the concentration of Cu2+, shown in femtomolar and (B) the % distribution of copper bound to L1 and L2. Each data point shown is the average of 6 separate sample-stations, and the standard deviations are shown.
Discussion
Seasonality in the Data
The salinity in our samples showed a systematic seasonal increase from April to September (Supplementary Figure 1A). Salinity ranged from 13 to 18 in April to 23–27 in August, and 27–31 in subsequent months. The ligand concentrations ranged from 16 to 51 nM (L1) and 30 to 112 nM (L2) over the study. High concentrations of both L1 and L2 in the low-salinity, riverine, end-member (STN 10) demonstrate a contribution of the freshwater source to the relationship between ligand concentration and salinity (Figure 2), with the effect of local freshwater inputs superimposed on this broader scale pattern. The composition of Duplin River water cannot be modeled by conservative mixing between fresh and seawater, as variation in the strength and location of freshwater inputs violates the assumptions of a 2-member mixing model (Smith and Hollibaugh, 1993). Localized inputs on the Duplin River cause major deviations in the salinity plots and, since sampling was conducted over several months, it is not possible to derive a single theoretical dilution line.
Despite the complexity of the system, L1 was generally conservative with salinity except in September and October. In June and September L1 demonstrated large variability between stations of similar salinity, ranging from 34 to 49 nM at Sal 21 to 22, and 16.6 to 33.6 nM at Sal 29.6 to 29.9. These large differences between stations of almost identical salinity indicate that local processes, such as contributions from pore waters or from temporally varying processes linked to biological activity, affected conditions at individual stations. This suggests that as well as a freshwater source, L1 is both produced and utilized along the estuary and, as a result, local concentrations may vary seasonally. This is consistent with the identification of L1 as a thiol-type species, since sediment and pore waters are likely sources of thiols (Kiene et al., 1990; Zhang et al., 2004), which have been shown to diffuse out of sediments in similar shallow water marshes (Chapman et al., 2009).
Comparison of the data (Figure 4C) showed HSCu to correspond with L2 (slope 0.85 ± 0.08). Most of the humic material along the estuary likely originates from terrestrial material delivered to the study area by the Altamaha River, although marine-derived humics may also be present from exchange with coastal waters. The concentration of both L2 and HSCu decreased with increasing salinity in April, October, and December (Supplementary Figure 4), consistent with a dominant terrestrial origin of these humic-type ligands and similar to that found in other estuaries such as that of the Mersey Estuary (Supplementary Figure 4). Between May and September, concentrations of L2 and HSCu either remained constant or increased with increasing salinity >25. This suggests a mid-estuarine source of L2 and HSCu, which could be from increased fluxes of sedimentary organic carbon into the estuary, potentially partly linked to higher bioturbation by fiddler crabs and other invertebrates during summer. Published records show DOC concentrations range from around 3.0 to 9.0 mg/L along the estuary and are not conservative with salinity (Hodson, 2005; Medeiros et al., 2017). Like L2, the concentration of humic substances was generally highest in spring and generally constant from September to December, with elevated concentrations relative to salinity in August.
Potential Issues of the Detection Window and Freezing Temperature
A high detection window was necessary in order to resolve strong ligands in this work because the HSCu peak interfered with the peak for CuSA at lower SA concentrations despite sample dilution. The high detection window may have impacted the detection of weaker ligands if these are out-competed by the added ligand (SA). At the detection window used here (20 μM SA, αCuSA = 105.6) competing ligands are required to bind copper with αCuL >> 103.6 to have an effect of >>1%, and preferably nearer 104 to take variability of the data into account. The L1-type ligands had values for log >14, which means values for αCuL1 > 106 at the 10 nM level, and the L1 species were easily detectable. The L2 type ligands had values for log > 12.1, giving values for αCuL2 > 104.1, sufficiently strong for detection at the 10 nM level. The actual concentrations of L2 were well above the 10 nM level (Supplementary Table 1), and values for log averaged 12.2–13.0, therefore L2 was also readily detected at this high detection window. However, putative (L3) ligands with significantly lower complex stabilities (log < 11) would have caused relatively minor competition against the SA unless at levels of L3 > 10−7 M, therefore these were not detected in this work.
A secondary methodological issue is that samples were frozen at −80°C immediately after filling to be shipped by courier from GA to the UK for analysis. Once reaching the UK they were stored at −20°C. It has been shown that open ocean samples stored at −80°C can provide different results in comparison to samples that were not frozen or that were stored at −20°C (Buck et al., 2012) and this must be considered in relation to the ligand data presented here. Buck et al. (2012) found that samples from the chlorophyll a maximum frozen at −80°C underestimated [L] compared to unfrozen samples, but found the same log Cu2+ (14.56 and 14.51, respectively). They also reported that [L] was overestimated and log , was underestimated if deep-water (3,000 m) samples were frozen, although again this resulted in similar log Cu2+ values (e.g., 13.1, 12.5, and 12.7 for unfrozen, frozen at −20°C and at −80°C, respectively). Since the composition of DOC from estuarine waters is likely more similar to productive surface waters than to old, deep water, we would therefore most likely be underestimating the ligand concentration and overestimating log , resulting in an overestimation (or no change) in our estimate of Cu2+ concentrations.
Complex Stabilities and Possible Competition Effects
The two ligand classes were distinguished on the basis of an average difference in complex stability (log ) of 1.7 log units, with log ranging from 14 to 15.2 (mean 14.5) and log ranging from 12.1 to 14 (mean 12.8). The large ranges for the complex stabilities suggest that either there are more ligands within each group (e.g., substituted thiols and reduced sulfur compounds or different compounds within the humic species), that data are affected by competing reactions, or both. Competition is an aspect that has not been considered experimentally in this work. This competition could be a result of different metal ions binding the same ligand, thus lowering the apparent complex stability. Several metals, like iron, copper, cobalt, and aluminum are known to bind humic substances in seawater and will therefore compete (Yang and van den Berg, 2009; Abualhaija et al., 2015; Whitby and van den Berg, 2015). It is not yet known whether this affects the apparent complex stability significantly. Studies on SRHA standards have demonstrated that the degree of competition between copper and calcium for binding sites in humic substances is weaker than expected (Averett et al., 1994), potentially due to the larger ionic radius of calcium, or because calcium binds more strongly to functional groups containing oxygen, whereas copper forms stronger complexes with sulfur and nitrogen (Nieboer and Richardson, 1980).
Variation in the complex stability could also be due to a seasonal change in the composition of the components contributing to each ligand class; for example, humic material with higher log may be more common later in the year. Similarly, the composition of the compounds contributing to the thiol peak may change, either through variation in the type of compounds released biologically across the season or by modification of the chemical structure of the compounds over time. For example, thiols can be microbially transformed from one type to another: glutathione can be transformed into mercaptoacetate and mercaptoethanol via cysteine and 3-mercaptopyruvate (Kiene et al., 1990). Although, voltammetry can distinguish between some similar thiols (such as glutathione and cysteine) it lacks resolution to conclusively distinguish between other similar compounds (e.g., thiourea and thioacetamide) unless the deposition potential is varied in detail (Laglera et al., 2014). The natural thiols detected here could therefore be a mixture of thiols with similar peaks varying seasonally, and/or include degradation products of localized algal blooms, and include different sulfur species. Furthermore, a small contribution from a low concentration of a very strong ligand such as a chalkophore (Kim et al., 2004) would not be identified but could exaggerate the apparent value for log . Chalkophores are high affinity, copper-complexing agents secreted by specific bacteria forming very strong Cu(I) complexes (Hakemian et al., 2005; El Ghazouani et al., 2012). Chalkophores may be an important ligand in copper complexation, but very little is currently known about the predominance and significance of chalkophores in the marine environment. Models suggest they can outcompete complexation by fulvic-type material (Kraemer et al., 2015). As with any speciation method, CLE-CSV can only detect a certain fraction of the entire pool of ligands, limited by the detection window (Wells et al., 2013; Monticelli and Caprara, 2015), which can span 2–3 orders of magnitude in αCuL (Apte et al., 1988). This, and the fact that ligands with similar log are difficult to resolve within a detection window, mean that unresolved species lead to weighted averages of all ligands within that window (Miller and Bruland, 1997). Therefore, the presence of a very strong ligand at low concentration could exaggerate the values for log even if L1 was mostly comprised of a slightly weaker ligand. This may explain the higher than expected log value for the thiol.
Composition of the Ligand Classes
A study on the Elizabeth River in Virginia measured six different thiol compounds with concentrations varying seasonally (Dryden et al., 2007). They did not test for thiourea or thioacetamide, but found that mercaptosuccinic acid and 2-mercaptoethanol (which we did not test) correlated with the ligand concentration. It is possible that mercaptosuccinic acid could be contributing to the thiol peak observed in our samples, but not 2-mercaptoethanol as its peak potential is more positive and similar to that of cysteine (Casassas et al., 1985). The thiol concentration in the Elizabeth River, particularly mercaptosuccinic acid, was also found to be highest in June similar to our findings, as well as being high in October.
Previous studies in estuarine waters have suggested that thiols likely contribute to the weaker L2 ligand class, which binds 3–23% of copper (Laglera and van den Berg, 2003) with lower log values than those observed here (Luther et al., 1991; Walsh and Ahner, 2013). Our results show the concentration of the natural thiol best correlates with that of the L1 ligand class with a high log , although as discussed this may be the weighted average of thiols plus other very strong ligands present at low concentration. Another study of copper complexation in organic-rich estuarine waters also detected two similar ligand classes with log values of log of 14.9–15.9 (present at a concentration of <4 nM) and log of 11.8–12.7, at concentrations of 50–170 nM (Muller and Batchelli, 2013). Muller and Batchelli (2013) concluded that humic substances made up the stronger ligand class (as opposed to L2 as we suggest) based on the riverine source and estuarine mixing behavior of their L1 ligand class. The L1 and L2 ligand classes in our study were of similar concentration to one another and a reasonable correlation (though weaker) can also be drawn between L1 with humic substances and L2 with the thiol concentration. However, Suwannee River humics (SRHA) have a log 12 (Whitby and van den Berg, 2015), too low to account for L1, and SRHA is likely a good candidate for the humics since the Altamaha and Suwannee rivers are geographically close, drain similar terrestrial environments and are chemically similar (Annett et al., 2008). Furthermore, the results of the model ligand titration suggest that within a mixture of humic acid and a thiourea-type thiol, the concentration of thiourea corresponds to L1 and humics to L2 providing further support for our interpretation of the composition of the ligand classes in the samples.
The correlation of the thiourea-type thiol and HSCu with L1and L2, respectively, suggests that on average thiols could make up ~40% and humics around 60% of the total available copper ligands (LT) measured at this detection window in the estuarine waters around Sapelo Island, potentially in addition to low concentrations of very strong ligands incorporated into L1 or much weaker ligands not detected. The percentage distribution of strong and weaker ligands is similar to that found in the Mersey Estuary where humics were around 69% of total available copper-binding ligands (Abualhaija et al., 2015), whereas thiols have previously been found to account for ~15% of the total ligand in the Elizabeth River (Dryden et al., 2007).
Although, both ligand classes were in excess of copper with the bulk of the ligand pool composed of L2, calculation of the speciation of copper over L1 and L2 showed that the distribution of copper was 82–99% associated with L1(as CuL1). CuL1 generally decreased with increasing salinity across the period studied, whereas the resulting percentage bound to L2 (CuL2) generally increased with increasing salinity, noticeably increasing during the course of the Thaumarchaeota bloom. Due to the presence of the strong L1-ligands, the free cupric ion concentration was also extremely low throughout the study. Concentrations of Cu2+ ranged from 0.9 to 7.5 fM and generally increased with increasing salinity, despite Cud decreasing with increasing salinity. The concentration of Cu2+ in the Duplin River decreased from a mean of 3.8 ± 2.2 fM in April (range 2.6–7.5 fM) to a minimum of 0.4 ± 0.1 fM in August (range 0.2–0.5 fM) during the peak of the Thaumarchaeota bloom (Supplementary Figures 6A,B).
Previous measurements suggest that thiols bind copper as a Cu(I) species (Leal and van den Berg, 1998; Konigsberger et al., 2015; Barman et al., 2016). From our assumption based on the correlation between thiourea-type thiols and L1, an important deduction from this work is that most of the copper in these coastal waters would appear to occur in the reduced form of Cu(I). Copper is thought to occur predominantly as Cu(II) in natural waters containing dissolved oxygen, with around 10% of inorganic copper in surface seawater as Cu(I) due to its stabilization as a chloride species and photochemical effects (Nelson and Mantoura, 1984; Jones et al., 1985; Moffett and Zika, 1988). Our data may suggest that this Cu(I) fraction is much greater, and may in fact dominate the copper chemistry in our samples in the form of a Cu(I)-thiol species. This is consistent with findings along the Scheldt Estuary, where the fraction of Cu(I)/Cutot ranged from 5 to 80%, depending on a combination of salinity (chloride stabilization) and thiol complexation (Buerge-Weirich and Sulzberger, 2004). The tendency for thiol species and Cu(I) to occur in these waters is further enhanced at low concentrations of dissolved oxygen during the summer, which happens regularly at the Marsh Landing site (Hollibaugh et al., 2014) (data from Sapelo Island National Estuarine Research Reserve archived at http://cdmo.baruch.sc.edu/get/export.cfm). It should be noted, however, that recent work using a fluorometric method and direct titration of thiols with Cu(I) (Walsh and Ahner, 2013) has indicated that some Cu(I)-thiol complexes may be considerably weaker than previously suggested by electrochemical techniques (e.g., Leal and van den Berg, 1998), and therefore not stable in natural seawater systems. Further work is necessary to determine the actual significance of Cu(I) organic complexes in oceanic and estuarine regimes.
Implications for Thaumarchaeota
A major finding of our study is that the Thaumarchaeota in the Duplin River were found to grow and even thrive (ammonia oxidation rates >100 nmol L−1 d−1, Tolar et al., 2016a,c) at extremely low Cu2+ concentrations, sub-10−15 M, well below Cu2+ concentrations thought to be limiting ([Cu2+] < 10−12.7 M; (Amin et al., 2013)). During this study there was no obvious limitation of ammonia oxidation as a result of the low Cu2+ concentrations, in contrast to previous reports for Cu2+ concentrations >6 × 10−15 M in the presence of strong copper-binding ligands (Jacquot et al., 2014). Work with the Thaumarchaeote Nitrosopumilus maritimus strain SCM1 has suggested possible copper limitation when [Cu2+] < 0.2 pM (Amin et al., 2013), whereas our work demonstrates that closely related (>99% 16S rRNA similarity) Thaumarchaeota apparently grow well at 1/1,000th this concentration of Cu2+. These comparisons suggest that there is either large variability in the copper requirements between different strains, which seems unlikely given the fundamental roles played by Cu-containing metalloenzymes in AOA (Walker et al., 2010), or that some forms of complexed copper are available to Thaumarchaeota. Although Cu2+ was extremely low, the concentration of Cud was much higher than Cu2+, with mean Cud 6.7 nM in August. During the bloom, up to 99% of copper was bound to the strongest L1 ligand, with log as high as 15.2, suggesting that its dissociation is kinetically slow. It is therefore likely that Thaumarchaeota are indifferent to the low Cu2+ concentration and are able to access the strongly complexed copper directly.
One explanation for the difference observed in natural samples compared to laboratory experiments may be the use of artificial ligands such as EDTA to induce Cu2+ limitation. Thaumarchaeota may not be able to access EDTA-bound Cu(II), however it is plausible that they have mechanisms for obtaining naturally complexed copper [such as thiol-bound Cu(I)]. Coastal and oceanic phytoplankton species have been demonstrated to access Cu bound within strong organic complexes (Guo et al., 2010) and some open ocean phytoplankton can acquire copper complexed with natural and artificial ligands with log up to 15.8 (Semeniuk et al., 2015). A study on San Francisco Bay found copper to be 99% complexed, at log = 12.1, and suggested that this complexation was dominated by the presence of anthropogenic EDTA in those waters (Bedsworth and Sedlak, 1999). Microcosm experiments on a diatom bloom within those samples revealed that this complexed Cu was largely unavailable to the species studied (Beck et al., 2002) despite the relatively low log values. Furthermore, diatoms and coccolithophores demonstrate reduced growth rates when cultured in the presence of EDTA (Muggli and Harrison, 1996), and Amin et al. (2013) report that EDTA concentrations of 11–100 μmol L−1 proved lethal to N. maritimus.
Cu uptake has been shown to be controlled by the oxidation state of the metal and by the metal:ligand ratio, rather than by the concentration of inorganic species of Cu in solution (Semeniuk et al., 2009). The speciation of Cu will affect its availability to microorganisms depending on whether uptake is as Cu(I) or Cu(II), as the uptake may involve a change in oxidation state with associated reaction rates. It is unclear whether Thaumarchaeota are acquiring the thiol-bound copper [as Cu(I)], or the humic-bound copper [as Cu(II)]. Although, in terms of availability it should be easier to access the more weakly bound, L2-complexed copper, we hypothesize that the Thaumarchaeota are accessing the more strongly bound, L1-complexed copper, thought to be thiols, as this is the more abundant fraction. This is consistent with uptake studies showing that addition of Cu(I) ligands enhanced Cu uptake in presence of organically bound Cu(II), suggesting that the mechanism for Cu(II) uptake may even rely on the enzymatic reduction of Cu(II) to Cu(I) (Semeniuk et al., 2015). Cu(II) is reduced to Cu(I) within 2–40 min by Cu(I) binding thiols like glutathione and cysteine (Leal and van den Berg, 1998). Cu-limitation has been demonstrated to increase rates of cell surface reduction of Cu(II) to Cu(I) in Emiliania huxleyi (Walsh et al., 2015). They demonstrated that cysteine can increase the bioavailability of copper to copper-limited cells through the reductive release of Cu(I) from Cu(II) ligands such as EDTA. This mechanism may be relevant in Duplin River waters where free copper is very low but L1-bound copper, thought to be thiol-bound and potentially amounting to up to 99% of the dissolved copper, is relatively abundant.
The mechanism for copper uptake by Thaumarchaeota is not fully understood. The bloom starts when the free Cu′ is already very low (Figure 5A), indicating that the availability of inorganic Cu′, whether as Cu(I) or as Cu(II), is not important. We hypothesize that the copper arrives at the cell as a Cu(I)-thiol species, where there is a direct exchange of copper to Cu-binding groups on the cell wall, allowing active transport of the copper into the cell through a high affinity transporter, as described for E. huxleyi (Walsh et al., 2015). We suspect that the low Cu2+ is not driven directly by the Thaumarchaeota through production of L1, but rather that L1 is released independently, likely by other microorganisms in the water column or from the sediment and pore waters. Other work has demonstrated that the Thaumarchaeote N. maritimus SCM1 did not release strong Cu-binding ligands when under low copper stress (Amin et al., 2013) and the concentration of L1 does not correlate with the onset of the bloom.
Possible Sources of the Ligands
The concentrations of both L1 and L2 were high in the freshwater end-member suggesting a terrestrial or freshwater source for both ligand classes, but both also displayed addition and removal processes along the estuary. Other species that bloom in the area include numerous bacteria (Fallon et al., 1986; Gifford et al., 2011, 2013), diatoms (Williams, 1964; Pomeroy et al., 1981), green algae and euglenoids as well as dinoflagellates (Berman, 1983). The dinoflagellate Amphidinium carterae, more common in coastal regions, has been found to produce very strong ligands (Croot et al., 2000) and dinoflagellates, specifically Kryptoperidinium sp., have been documented to bloom in August in the Duplin River (Berman, 1983). A study on the seasonal cycle of copper speciation within a fjord in Sweden also found stronger ligands in the summer months (log 12.9–14.2) causing a pCu of 11.7–13.8 (Croot, 2003). The L1 ligands measured in the fjord were thought to be related to the seasonal cycle of Synechococcus blooming in the area at this time, and which are known to produce L1 ligands (Moffett et al., 1990; Moffett, 1995). Synechococcus are the second most abundant bacterioplankton fraction in waters offshore of the Duplin River in summer (Lu et al., 2015). As well as water column sources for ligands, likely sources of thiols are benthic microbes and sulfur-containing amino acids within the sediment (Kiene et al., 1990; Chapman et al., 2009), whilst the humics in this type of environment are likely products of relatively recent degradation of local plant matter (Averett et al., 1994) such as fungal breakdown of lignocellulose (Newell, 2001; Buchan et al., 2003). Spartina alterniflora and many of the higher plants in the marsh also release dimethylsulfoniopropionate (DMSP) (Bacic et al., 1998; Kiehn and Morris, 2010), which can add be an additional source of thiols.
Conclusion
Our study indicates that thiols and humic substances are the dominant ligands for copper within the Duplin River estuary, and waters around Sapelo Island. The evidence suggests that around 90% of Cud is complexed to strong L1 ligands, thought to be a thiourea-type thiol based on the correlation with the concentration of L1. Although, this work contributes to our understanding of the identity of the ligand classes, combining this work with additional ligand characterization methods would better identify the nature of the thiols and other compounds dominating Cu complexation in these waters. The good agreement between LT and [thiols + HSCu], as well as the success of the model ligand titration in distinguishing between model compounds as two separate classes, is consistent with previous studies suggesting that thiols and HSCu are the dominant ligand types responsible for controlling copper speciation in estuarine environments and can induce very low concentrations of Cu2+. However, low concentrations of a very strong ligand could be contributing to the high log values we found and influencing the Cu2+ concentration. It is likely that low-oxygen sulfur-containing muds are a source of the thiols and further investigation into this process would add to our understanding of this complex system.
A key finding of this study is that Thaumarchaeota that bloom regularly at the study site do not appear to be limited by low inorganic copper availability and are capable of growth and activity at free Cu2+ concentrations of 0.4 × 10−15 M (pCu 15.4), previously thought to be limiting. It is likely that, since Thaumarchaeota appear to have a high copper requirement, they have developed pathways for obtaining copper from strongly bound species. Based on our data, we hypothesize that the Thaumarchaeota are utilizing complexed copper, possibly as thiol-bound Cu(I). Future studies could focus on Cu availability to natural populations of Thaumarchaeota in a laboratory setting, using natural ligands to induce limitation rather than artificial compounds such as EDTA.
Author Contributions
HW collected samples, prepared all of the data and graphs, and wrote the paper for publication. JH collected samples, provided information, and co-wrote the paper. CvdB supervised and co-wrote the paper.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
HW was supported by a scholarship of the Natural Environmental Research Council (NE/K500975/1). JH thanks NSF OCE 13-35838 for funding this research. We thank Meredith Ross and Qian Liu for their work generating the Thaumarchaea abundance data. We also thank Jacob Shalack, the skipper of the RV Salty Dawg, supported by NSF OCE 12-37140, for his assistance in sample collection. This is publication 1048 from the University of Georgia Marine Institute. CvdB is grateful for on-going support from the University of Liverpool.
Supplementary Material
The Supplementary Material for this article can be found online at: http://journal.frontiersin.org/article/10.3389/fmars.2017.00178/full#supplementary-material
References
Abualhaija, M. M., Whitby, H., and van den Berg, C. M. G. (2015). Competition between copper and iron for humic ligands in estuarine waters. Mar. Chem. 172, 46–56. doi: 10.1016/j.marchem.2015.03.010
Al-Farawatii, R., and van den Berg, C. M. G. (1997). The determination of sulfide in seawater by flow-analysis with voltammetric detection. Mar. Chem. 57, 277–286. doi: 10.1016/S0304-4203(97)00014-5
Al-Farawati, R., and van den Berg, C. M. G. (2001). Thiols in coastal waters of the western North Sea and English Channel. Environ. Sci. Technol. 35, 1902–1911. doi: 10.1021/es000073i
Amin, S. A., Moffett, J. W., Martens-Habbena, W., Jacquot, J. E., Han, Y., Devol, A., et al. (2013). Copper requirements of the ammonia-oxidizing archaeon Nitrosopumilus maritimus SCM1 and implications for nitrification in the marine environment. Limnol. Oceanogr. 58, 2037–2045. doi: 10.4319/lo.2013.58.6.2037
Anderson, D. M., and Morel, F. M. M. (1978). Copper sensitivity of Gonyaulax tamerensis. Limnol. Oceanogr. 23, 283–295. doi: 10.4319/lo.1978.23.2.0283
Annett, A. L., Lapi, S., Ruth, T. J., and Maldonado, M. T. (2008). The effects of Cu and Fe availability on the growth and Cu: C ratios of marine diatoms. Limnol. Oceanogr. 53, 2451–2461. doi: 10.4319/lo.2008.53.6.2451
Apte, S. C., Gardner, M. J., and Ravenscroft, J. E. (1988). An evaluation of voltammetric titration procedures for the determination of trace-metal complexation in natural-waters by use of computer-simulation. Anal. Chim. Acta 212, 1–21. doi: 10.1016/S0003-2670(00)84124-0
Arnon, D. I. (1949). Copper enzymes in isolated chloroplasts. Polyphenoloxidase in Beta vulgaris. Plant Physiol. 24, 1–15. doi: 10.1104/pp.24.1.1
Averett, R. C., Leenheer, J. A., McKnight, D. M., and Thorn, K. A. (1994). Humic Substances in the Suwannee River, Georgia: Interactions, Properties, and Proposed Structures. U.S. Geological Water-Supply Paper 2373, U.S. Geological Survey.
Bacic, M. K., Newell, S. Y., and Yoch, D. C. (1998). Release of dimethylsulfide from dimethylsulfoniopropionate by plant-associated salt marsh fungi. Appl. Environ. Microbiol. 64, 1484–1489.
Bano, N., and Hollibaugh, J. T. (2002). Phylogenetic composition of bacterioplankton assemblages from the Arctic Ocean. Appl. Environ. Microbiol. 68, 505–518. doi: 10.1128/AEM.68.2.505-518.2002
Barman, M. K., Sinha, A. K., and Nembenna, S. (2016). An efficient and recyclable thiourea-supported copper(I) chloride catalyst for azide-alkyne cycloaddition reactions. Green Chem. 18, 2534–2541. doi: 10.1039/C5GC02545A
Beck, N. G., Bruland, K. W., and Rue, E. L. (2002). Short-term biogeochemical influence of a diatom bloom on the nutrient and trace metal concentrations in South San Francisco bay microcosm experiments. Estuaries 25, 1063–1076. doi: 10.1007/BF02692204
Bedsworth, W. W., and Sedlak, D. L. (1999). Sources and environmental fate of strongly complexed nicker in estuarine waters: the role of ethylenediaminetetraacetate. Environ. Sci. Technol. 33, 926–931. doi: 10.1021/es9809556
Beman, J. M., Sachdeva, R., and Fuhrman, J. A. (2010). Population ecology of nitrifying Archaea and Bacteria in the Southern California Bight. Environ. Microbiol. 12, 1282–1292. doi: 10.1111/j.1462-2920.2010.02172.x
Berman, T. (1983). Phosphorus uptake by microplankton in estuarine and coastal shelf waters near Sapelo Island, Georgia, USA. Estuaries 6, 160–166. doi: 10.2307/1351706
Brand, L. E., Sunda, W. G., and Guillard, R. R. L. (1986). Reduction of marine-phytoplankton reproduction rates by copper and Cadmium. J. Exp. Mar. Biol. Ecol. 96, 225–250. doi: 10.1016/0022-0981(86)90205-4
Buchan, A., Newell, S. Y., Butler, M., Biers, E. J., Hollibaugh, J. T., and Moran, M. A. (2003). Dynamics of bacterial and fungal communities on decaying salt marsh grass. Appl. Environ. Microbiol. 69, 6676–6687. doi: 10.1128/AEM.69.11.6676-6687.2003
Buck, K. N., and Bruland, K. W. (2005). Copper speciation in San Francisco Bay: a novel approach using multiple analytical windows. Mar. Chem. 96, 185. doi: 10.1016/j.marchem.2005.01.001
Buck, K. N., Moffett, J., Barbeau, K. A., Bundy, R. M., Kondo, Y., and Wu, J. F. (2012). The organic complexation of iron and copper: an intercomparison of competitive ligand exchange-adsorptive cathodic stripping voltammetry (CLE-ACSV) techniques. Limnol. Oceanogr. Methods 10, 496–515. doi: 10.4319/lom.2012.10.496
Buerge-Weirich, D., and Sulzberger, B. (2004). Formation of Cu(I) in estuarine and marine waters: application of a new solid-phase extraction method to measure Cu(I). Environ. Sci. Technol. 38, 1843–1848. doi: 10.1021/es034845x
Bundy, R. M., Abdulla, H. A. N., Hatcher, P. G., Biller, D. V., Buck, K. N., and Barbeau, K. A. (2015). Iron-binding ligands and humic substances in the San Francisco Bay estuary and estuarine-influenced shelf regions of coastal California. Mar. Chem. 173, 183–194. doi: 10.1016/j.marchem.2014.11.005
Bundy, R. M., Barbeau, K. A., and Buck, K. N. (2013). Sources of strong copper-binding ligands in Antarctic Peninsula surface waters. Deep Sea Res. Part II Top. Stud. Oceanogr. 90, 134–146. doi: 10.1016/j.dsr2.2012.07.023
Campos, M., and van den berg, C. M. G. (1994). Determination of copper complexation in sea-water by cathodic stripping voltammetry and ligand competition with salicylaldoxime. Anal. Chim. Acta 284, 481–496. doi: 10.1016/0003-2670(94)85055-0
Casassas, E., Arino, C., and Esteban, M. (1985). Cathodic stripping voltammetry of 2-mercaptoethanol. Anal. Chim. Acta 176, 113–119. doi: 10.1016/S0003-2670(00)81638-4
Chapman, C. S., Capodaglio, G., Turetta, C., and van den Berg, C. M. G. (2009). Benthic fluxes of copper, complexing ligands and thiol compounds in shallow lagoon waters. Mar. Environ. Res. 67, 17–24. doi: 10.1016/j.marenvres.2008.07.010
Church, M. J., Delong, E. F., Ducklow, H. W., Karner, M. B., Preston, C. M., and Karl, D. M. (2003). Abundance and distribution of planktonic Archaea and Bacteria in the waters west of the Antarctic Peninsula. Limnol. Oceanogr. 48, 1893–1902. doi: 10.4319/lo.2003.48.5.1893
Croot, P. L. (2003). Seasonal cycle of copper speciation in Gullmar Fjord, Sweden. Limnol. Oceanogr. 48, 764–776. doi: 10.4319/lo.2003.48.2.0764
Croot, P. L., Moffett, J. W., and Brand, L. E. (2000). Production of extracellular Cu complexing ligands by eucaryotic phytoplankton in response to Cu stress. Limnol. Oceanogr. 45, 619–627. doi: 10.4319/lo.2000.45.3.0619
Donat, J. R., Lao, K. A., and Bruland, K. W. (1994). Speciation of dissolved copper and nickel in South San-Francisco Bay - a multimethod approach. Anal. Chim. Acta 284, 547–571. doi: 10.1016/0003-2670(94)85061-5
Dryden, C. L., Gordon, A. S., and Donat, J. R. (2007). Seasonal survey of copper-complexing ligands and thiol compounds in a heavily utilized, urban estuary: Elizabeth River, Virginia. Mar. Chem. 103, 276–288. doi: 10.1016/j.marchem.2006.09.003
Dupont, C. L., and Ahner, B. A. (2005). Effects of copper, cadmium, and zinc on the production and exudation of thiols by Emiliania huxleyi. Limnol. Oceanogr. 50, 508–515. doi: 10.4319/lo.2005.50.2.0508
Dupont, C. L., Moffett, J. W., Bidigare, R. R., and Ahner, B. A. (2006). Distributions of dissolved and particulate biogenic thiols in the subartic Pacific Ocean. Deep Sea Res. Part I Oceanogr. Res. Pap. 53, 1961–1974. doi: 10.1016/j.dsr.2006.09.003
El Ghazouani, A., Basle, A., Gray, J., Graham, D. W., Firbank, S. J., and Dennison, C. (2012). Variations in methanobactin structure influences copper utilization by methane-oxidizing bacteria. Proc. Natl. Acad. Sci. U.S.A. 109, 8400–8404. doi: 10.1073/pnas.1112921109
Fallon, R. D., Newell, S. Y., Sherr, B. F., and Sherr, E. B. (1986). Factors affecting bacerial biomass and growth in the Duplin River estuary and coastal Atlantic Ocean. Ifremer Actes de Colloques 3, 137–145.
Francis, C. A., Roberts, K. J., Beman, J. M., Santoro, A. E., and Oakley, B. B. (2005). Ubiquity and diversity of ammonia-oxidizing archaea in water columns and sediments of the ocean. Proc. Natl. Acad. Sci. U.S.A. 102, 14683–14688. doi: 10.1073/pnas.0506625102
Gifford, S. M., Sharma, S., Booth, M., and Moran, M. A. (2013). Expression patterns reveal niche diversification in a marine microbial assemblage. ISME J. 7, 281–298. doi: 10.1038/ismej.2012.96
Gifford, S. M., Sharma, S., Rinta-Kanto, J. M., and Moran, M. A. (2011). Quantitative analysis of a deeply sequenced marine microbial metatranscriptome. ISME J. 5, 461–472. doi: 10.1038/ismej.2010.141
Glass, J. B., and Orphan, V. J. (2012). Trace metal requirements for microbial enzymes involved in the production and consumption of methane and nitrous oxide. Front. Microbiol. 3:61. doi: 10.3389/fmicb.2012.00061
Gordon, A. S., Donat, J. R., Kango, R. A., Dyer, B. J., and Stuart, L. M. (2000). Dissolved copper-complexing ligands in cultures of marine bacteria and estuarine water. Mar. Chem. 70, 149–160. doi: 10.1016/S0304-4203(00)00019-0
Guan, J., Yan, B., Zhu, H., Wang, L., Lu, D., and Cheng, L. (2015). Flux characteristics of total dissolved iron and its species during extreme rainfall event in the midstream of the Heilongjiang river. J. Environ. Sci. 30, 74–80. doi: 10.1016/j.jes.2014.10.009
Guo, J., Annett, A. L., Taylor, R. L., Lapi, S., Ruth, T. J., and Maldonado, M. T. (2010). Copper-uptake kinetics of coastal and oceanic diatoms. J. Phycol. 46, 1218–1228. doi: 10.1111/j.1529-8817.2010.00911.x
Hakemian, A. S., Tinberg, C. E., Kondapalli, K. C., Telser, J., Hoffman, B. M., Stemmler, T. L., et al. (2005). The copper chelator methanobactin from Methylosinus trichosporium OB3b binds copper(I). J. Am. Chem. Soc. 127, 17142–17143. doi: 10.1021/ja0558140
Herndl, G. J., Reinthaler, T., Teira, E., van Aken, H., Veth, C., Pernthaler, A., et al. (2005). Contribution of Archaea to total prokaryotic production in the deep Atlantic Ocean. Appl. Environ. Microbiol. 71, 2303–2309. doi: 10.1128/AEM.71.5.2303-2309.2005
Hodson, R. E. (2005). December 2003 Surface Water Dissolved Organic Carbon Concentrations at Ten Georgia Coastal Ecosystems LTER Sampling Sites. Georgia Coastal Ecosystems LTER Data Catalog.
Hollibaugh, J. T., Gifford, S. M., Moran, M. A., Ross, M. J., Sharma, S., and Tolar, B. B. (2014). Seasonal variation in the metratranscriptomes of a Thaumarchaeota population from SE USA coastal waters. ISME J. 8, 685–698. doi: 10.1038/ismej.2013.171
Ingalls, A. E., Shah, S. R., Hansman, R. L., Aluwihare, L. I., Santos, G. M., Druffel, E. R. M., et al. (2006). Quantifying archaeal community autotrophy in the mesopelagic ocean using natural radiocarbon. Proc. Natl. Acad. Sci. U.S.A. 103, 6442–6447. doi: 10.1073/pnas.0510157103
Jacquot, J. E., Horak, R. E. A., Amin, S. A., Devol, A. H., Ingalls, A. E., Armbrust, E. V., et al. (2014). Assessment of the potential for copper limitation of ammonia oxidation by Archaea in a dynamic estuary. Mar. Chem. 162, 37–49. doi: 10.1016/j.marchem.2014.02.002
Jones, G. J., Waite, T. D., and Smith, J. D. (1985). Light-dependent reduction of copper(II) and its effect on cell-mediated, thiol-dependent superoxide production. Biochem. Biophys. Res. Commun. 128, 1031–1036. doi: 10.1016/0006-291X(85)90151-2
Kalanetra, K. M., Bano, N., and Hollibaugh, J. T. (2009). Ammonia-oxidizing Archaea in the Arctic Ocean and Antarctic coastal waters. Environ. Microbiol. 11, 2434–2445. doi: 10.1111/j.1462-2920.2009.01974.x
Karner, M. B., Delong, E. F., and Karl, D. M. (2001). Archaeal dominance in the mesopelagic zone of the Pacific Ocean. Nature 409, 507–510. doi: 10.1038/35054051
Kiehn, W. M., and Morris, J. T. (2010). Variability in dimethylsulfoniopropionate (DMSP) concentrations in Spartina alterniflora and the effect on Littoraria irrorata. Mar. Ecol. Prog. Ser. 406, 47–55. doi: 10.3354/meps08548
Kiene, R. P., Malloy, K. D., and Taylor, B. F. (1990). Sulfur-containing amino-acids as precursors of thiols in anoxic coastal sediments. Appl. Environ. Microbiol. 56, 156–161.
Kim, H. J., Graham, D. W., Dispirito, A. A., Alterman, M. A., Galeva, N., Larive, C. K., et al. (2004). Methanobactin, a copper-acquisition compound from methane-oxidizing bacteria. Science 305, 1612–1615. doi: 10.1126/science.1098322
Kitajima, N., Fujisawa, K., and Morooka, Y. (1990). Formation and characterization of a mononuclear (acylperoxo)copper(II) complex. Inorg. Chem. 29, 357–358. doi: 10.1021/ic00328a001
Kogut, M. B., and Voelker, B. M. (2001). Strong copper-binding behavior of terrestrial humic substances in seawater. Environ. Sci. Technol. 35, 1149–1156. doi: 10.1021/es0014584
Konigsberger, L. C., Konigsberger, E., Hefter, G., and May, P. M. (2015). Formation constants of copper(I) complexes with cysteine, penicillamine and glutathione: implications for copper speciation in the human eye. Dalton Trans. 44, 20413–20425. doi: 10.1039/C5DT02129D
Kraemer, S. M., Duckworth, O. W., Harrington, J. M., and Schenkeveld, W. D. C. (2015). Metallophores and trace metal biogeochemistry. Aquat. Geochem. 21, 159–195. doi: 10.1007/s10498-014-9246-7
Laglera, L. M., Battaglia, G., and van den Berg, C. M. G. (2011). Effect of humic substances on the iron speciation in natural waters by CLE/CSV. Mar. Chem. 127, 134–143. doi: 10.1016/j.marchem.2011.09.003
Laglera, L. M., Downes, J., Tovar-Sanchez, A., and Monticelli, D. (2014). Cathodic pseudopolarography: a new tool for the identification and quantification of cysteine, cystine and other low molecular weight thiols in seawater. Anal. Chim. Acta 836, 24–33. doi: 10.1016/j.aca.2014.05.026
Laglera, L. M., and Tovar-Sanchez, A. (2012). Direct recognition and quantification by voltammetry of thiol/thioamide mixes in seawater. Talanta 89, 496–504. doi: 10.1016/j.talanta.2011.12.075
Laglera, L. M., and van den Berg, C. M. G. (2003). Copper complexation by thiol compounds in estuarine waters. Mar. Chem. 82, 71–89. doi: 10.1016/S0304-4203(03)00053-7
Laglera, L. M., and van den Berg, C. M. G. (2006). Photochemical oxidation of thiols and copper complexing ligands in estuarine waters. Mar. Chem. 101, 130–140. doi: 10.1016/j.marchem.2006.01.006
Leal, M. F. C., and van den Berg, C. M. G. (1998). Evidence for strong copper(I) complexation by organic ligands in seawater. Aquat. Geochem. 4, 49–75. doi: 10.1023/A:1009653002399
Leal, M. F. C., Vasconcelos, M., and van den Berg, C. M. G. (1999). Copper-induced release of complexing ligands similar to thiols by Emiliania huxleyi in seawater cultures. Limnol. Oceanogr. 44, 1750–1762. doi: 10.4319/lo.1999.44.7.1750
Le Gall, A.-C., and van den Berg, C. M. G. (1993). Cathodic stripping voltammetry of glutathione in natural waters. Analyst 118, 1411–1415. doi: 10.1039/an9931801411
Le Gall, A.-C., and van den Berg, C. M. G. (1998). Folic acid and glutathione in the water column of the North East Atlantic. Deep Sea Res. Part I Oceanogr. Res. Pap. 45, 1903–1918. doi: 10.1016/S0967-0637(98)00042-9
Lu, X. X., Sun, S. L., Zhang, Y. Q., Hollibaugh, J. T., and Mou, X. Z. (2015). Temporal and vertical distributions of bacterioplankton at the Gray's Reef National Marine Sanctuary. Appl. Environ. Microbiol. 81, 910–917. doi: 10.1128/AEM.02802-14
Luther, G. W., Church, T. M., and Powell, D. (1991). Sulfur speciation and sufide oxidation in the water column of the black sea. Deep Sea Res. Part A Oceanogr. Res. Pap. 38, S1121–S1137.
Maldonado, M. T., Allen, A. E., Chong, J. S., Lin, K., Leus, D., Karpenko, N., et al. (2006). Copper-dependent iron transport in coastal and oceanic diatoms. Limnol. Oceanogr. 51, 1729–1743. doi: 10.4319/lo.2006.51.4.1729
McKnight, D. M., and Aiken, G. R. (1998). “Sources and age of aquatic humus,” in Aquatic Humic Substances: Ecology and Biogeochemistry, eds D. Hessen and L. Tranvik (Berlin: Springer-Verlag), 9–39.
Medeiros, P. M., Babcock-Adams, L., Seidel, M., Castelao, R. M., Di Lorio, D., Hollibaugh, J. T., et al. (2017). Export of terrigenous dissolved organic matter across a broad continental shelf. Limnol. Oceanogr. doi: 10.1002/lno.10528. [Epub ahead of print].
Miller, L. A., and Bruland, K. W. (1997). Competitive equilibration techniques for determining transition metal speciation in natural waters: Evaluation using model data. Anal. Chim. Acta 343, 161–181. doi: 10.1016/S0003-2670(96)00565-X
Misumi, K., Lindsay, K., Moore, J. K., Doney, S. C., Tsumune, D., and Yoshida, Y. (2013). Humic substances may control dissolved iron distributions in the global ocean: Implications from numerical simulations. Global Biogeochem. Cycles 27, 450–462. doi: 10.1002/gbc.20039
Moffett, J. W. (1995). Temporal and spatial variability of copper complexation by strong chelators in the sargasso-sea. Deep Sea Res. Part I Oceanogr. Res. Pap. 42, 1273–1295. doi: 10.1016/0967-0637(95)00060-J
Moffett, J. W., and Dupont, C. (2007). Cu complexation by organic ligands in the sub-arctic NW Pacific and Bering Sea. Deep Sea Res. Part I Oceanogr. Res. Pap. 54, 586–595. doi: 10.1016/j.dsr.2006.12.013
Moffett, J. W., Tuit, C. B., and Ward, B. B. (2012). Chelator-induced inhibition of copper metalloenzymes in denitrifying bacteria. Limnol. Oceanogr. 57, 272–280. doi: 10.4319/lo.2012.57.1.0272
Moffett, J. W., and Zika, R. G. (1988). Measurement of copper(I) in surface waters of the sub-tropical Atlantic and Gulf of Mexico. Geochim. Cosmochim. Acta 52, 1849–1857. doi: 10.1016/0016-7037(88)90008-7
Moffett, J. W., Zika, R. G., and Brand, L. E. (1990). Distribution and potential sources and sinks of copper chelators in the Sargasso Sea. Deep Sea Res. Part A Oceanogr. Res. Pap. 37, 27–36. doi: 10.1016/0198-0149(90)90027-S
Moingt, M., Bressac, M., Belanger, D., and Amyot, M. (2010). Role of ultra-violet radiation, mercury and copper on the stability of dissolved glutathione in natural and artificial freshwater and saltwater. Chemosphere 80, 1314–1320. doi: 10.1016/j.chemosphere.2010.06.041
Monticelli, D., and Caprara, S. (2015). Voltammetric tools for trace element speciation in fresh waters: methodologies, outcomes and future perspectives. Environ. Chem. 12, 683–705. doi: 10.1071/EN14233
Moskalski, S. M., Torres, R., Bizimis, M., Goni, M., Bergamaschi, B., and Fleck, J. (2013). Low-tide rainfall effects on metal content of suspended sediment in the Sacramento-San Joaquin Delta. Cont. Shelf Res. 56, 39–55. doi: 10.1016/j.csr.2013.02.001
Muggli, D. L., and Harrison, P. J. (1996). EDTA suppresses the growth of oceanic phytoplankton from the Northeast Subarctic Pacific. J. Exp. Mar. Biol. Ecol. 205, 221–227. doi: 10.1016/S0022-0981(96)02611-1
Muller, F. L. L., and Batchelli, S. (2013). Copper binding by terrestrial versus marine organic ligands in the coastal plume of River Thurso, North Scotland. Estuar. Coast. Shelf Sci. 133, 137–146. doi: 10.1016/j.ecss.2013.08.024
NOAA (2015). Old Tower, Sapelo Island, Doboy Sound, GA (US) Stationid: 8675622. Tides and Currents, NOAA.gov. Available online at: http://www.tidesandcurrents.noaa.gov/noaatidepredictions/viewDailyPredictions.jsp?bmon=12&bday=15&byear=2015&timelength=daily&timeZone=2&dataUnits=0&datum=MLLW&timeUnits=2&interval=highlow&format=Submit&Stationid=8675622
Nelson, A., and Mantoura, R. F. C. (1984). Voltammetry of copper species in estuarine waters. Part I. Electrochemistry of copper species in chloride media. J. Electroanal. Chem. 164, 237–252. doi: 10.1016/S0022-0728(84)80209-0
Newell, S. Y. (2001). Multiyear patterns of fungal biomass dynamics and productivity within naturally decaying smooth cordgrass shoots. Limnol. Oceanogr. 46, 573–583. doi: 10.4319/lo.2001.46.3.0573
Nieboer, E., and Richardson, D. H. S. (1980). The replacement of the non-descript term heavy-metals by a biologically and chemically significant classification of metal-ions. Environ. Poll. B Chem. Phys. 1, 3–26. doi: 10.1016/0143-148X(80)90017-8
Oldham, V. E., Swenson, M. M., and Buck, K. N. (2014). Spatial variability of total dissolved copper and copper speciation in the inshore waters of Bermuda. Mar. Pollut. Bull. 79, 314–320. doi: 10.1016/j.marpolbul.2013.12.016
Omanovic, D., Gamier, C., and Pizeta, I. (2015). ProMCC: an all-in-one tool for trace metal complexation studies. Mar. Chem. 173, 25–39. doi: 10.1016/j.marchem.2014.10.011
Peers, G., and Price, N. M. (2006). Copper-containing plastocyanin used for electron transport by an oceanic diatom. Nature 441, 341–344. doi: 10.1038/nature04630
Peers, G., Quesnel, S. A., and Price, N. M. (2005). Copper requirements for iron acquisition and growth of coastal and oceanic diatoms. Limnol. Oceanogr. 50, 1149–1158. doi: 10.4319/lo.2005.50.4.1149
Pomeroy, L. R., Wiegert, R. G., and Wiebe, W. J. (1981). The Ecology of a Salt Marsh. New York, NY: Springer-Verlag New York Inc.
Radford-Knoery, J., and Cutter, G. A. (1994). Biogeochemistry of dissolved hydrogen sulfide species and carbonyl sulfide in the western North Atlantic Ocean. Geochim. Cosmochim. Acta 58, 5421–5431. doi: 10.1016/0016-7037(94)90239-9
Rijstenbil, J. W., Haritonidis, S., Malea, P., Seferlis, M., and Wijnholds, J. A. (1998). Thiol pools and glutathione redox ratios as possible indicators of copper toxicity in the green macroalgae Enteromorpha spp. from the scheldt estuary (SW Netherlands, Belgium) and Thermaikos Gulf (Greece, N Aegean Sea). Hydrobiologia 385, 171–181. doi: 10.1023/A:1003502428466
Sander, S. G., Koschinsky, A., Massoth, G., Stott, M., and Hunter, K. A. (2007). Organic complexation of copper in deep-sea hydrothermal vent systems. Environ. Chem. 4, 81–89. doi: 10.1071/EN06086
Santoro, A. E., and Casciotti, K. L. (2011). Enrichment and characterization of ammonia-oxidizing archaea from the open ocean: phylogeny, physiology and stable isotope fractionation. ISME J. 5, 1796–1808. doi: 10.1038/ismej.2011.58
Semeniuk, D. M., Bundy, R. M., Payne, C. D., Barbeau, K. A., and Maldonado, M. T. (2015). Acquisition of organically complexed copper by marine phytoplankton and bacteria in the northeast subarctic Pacific Ocean. Mar. Chem. 173, 222–233. doi: 10.1016/j.marchem.2015.01.005
Semeniuk, D. M., Cullen, J. T., Johnson, W. K., Gagnon, K., Ruth, T. J., and Maldonado, M. T. (2009). Plankton copper requirements and uptake in the subarctic Northeast Pacific Ocean. Deep Sea Res. Part I Oceanogr. Res. Pap. 56, 1130–1142. doi: 10.1016/j.dsr.2009.03.003
Sharma, V. K., and Millero, F. J. (1988). Effect of ionic interactions on the rates of oxidation of Cu(I) with O2 in natural waters. Mar. Chem. 25, 141–161. doi: 10.1016/0304-4203(88)90061-8
Sholkovitz, E. R. (1976). Flocculation of dissolved organic and inorganic matter during mixing of river water and seawater. Geochim. Cosmochim. Acta 40, 831–845. doi: 10.1016/0016-7037(76)90035-1
Smith, S. V., and Hollibaugh, J. T. (1993). Coastal metabolism and the ocanic organic-carbon balance. Rev. Geophys. 31, 75–89. doi: 10.1029/92RG02584
Sunda, W. G., and Guillard, R. R. L. (1976). Relationship between cupric ion activity and toxicity of copper to phytoplankton. J. Mar. Res. 34, 511–529.
Suzuki, M. T., Taylor, L. T., and Delong, E. F. (2000). Quantitative analysis of small-subunit rRNA genes in mixed microbial populations via 5'-nuclease assays. Appl. Environ. Microbiol. 66, 4605–4614. doi: 10.1128/AEM.66.11.4605-4614.2000
Swarr, G. J., Kading, T., Lamborg, C. H., Hammerschmidt, C. R., and Bowman, K. L. (2016). Dissolved low-molecular weight thiol concentrations from the U.S. GEOTRACES North Atlantic Ocean zonal transect. Deep Sea Res. Part I Oceanogr. Res. Pap. 116, 77–87. doi: 10.1016/j.dsr.2016.06.003
Tang, D. G., Hung, C. C., Warnken, K. W., and Santschi, P. H. (2000). The distribution of biogenic thiols in surface waters of Galveston Bay. Limnol. Oceanogr. 45, 1289–1297. doi: 10.4319/lo.2000.45.6.1289
Tolar, B. B., King, G. M., and Hollibaugh, J. T. (2013). An analysis of thaumarchaeota populations from the northern gulf of Mexico. Front. Microbiol. 4:72. doi: 10.3389/fmicb.2013.00072
Tolar, B. B., Powers, L. C., Miller, W. L., Wallsgrove, N. J., Popp, B. N., and Hollibaugh, J. T. (2016a). Ammonia oxidation in the ocean can be inhibited by nanomolar concentrations of hydrogen peroxide. Front. Mar. Sci. 3:237. doi: 10.3389/fmars.2016.00237
Tolar, B. B., Ross, M. J., Wallsgrove, N. J., Liu, Q., Aluwihare, L. I., Popp, B. N., et al. (2016b). Contribution of ammonia oxidation to chemoautotrophy in Antarctic coastal waters. ISME J. 10, 2605–2619. doi: 10.1038/ismej.2016.61
Tolar, B. B., Wallsgrove, N. J., Popp, B. N., and Hollibaugh, J. T. (2016c). Oxidation of urea-derived nitrogen by Thaumarchaeota-dominated marine nitrifying communities. Environ. Microbiol. doi: 10.1111/1462-2920.13457. [Epub ahead of print].
van den Berg, C. M. (2014). UV Digestion Apparatus. Available online at: http://pcwww.liv.ac.uk/~sn35/Site/UV_digestion_apparatus.html/journalabbrev 2014
van den Berg, C. M. G. (1982). Determination of copper complexation with natural organic ligands in seawater by equilibration with MnO2 II. Experimental procedures and application to surface seawater. Mar. Chem. 11, 323–342. doi: 10.1016/0304-4203(82)90029-9
van den Berg, C. M. G. (1984). Determination of the complexing capacity and conditional stability constants of complexes of copper(II) with natural organic ligands in seawater by cathodic stripping voltammetry of copper-catechol complex ions. Mar. Chem. 15, 1–18. doi: 10.1016/0304-4203(84)90035-5
van den Berg, C. M. G., Househam, B. C., and Riley, J. P. (1988). Determination of cystine and cysteine in seawater using cathodic stripping voltammetry in the presence of Cu(II). J. Electroanal. Chem. 239, 137–148. doi: 10.1016/0022-0728(88)80275-4
Walker, C. B., De La Torre, J. R., Klotz, M. G., Urakawa, H., Pinel, N., Arp, D. J., et al. (2010). Nitrosopumilus maritimus genome reveals unique mechanisms for nitrification and autotrophy in globally distributed marine crenarchaea. Proc. Natl. Acad. Sci. U.S.A. 107, 8818–8823. doi: 10.1073/pnas.0913533107
Walsh, M. J., and Ahner, B. A. (2013). Determination of stability constants of Cu(I), Cd(II) & Zn(II) complexes with thiols using fluorescent probes. J. Inorg. Biochem. 128, 112–123. doi: 10.1016/j.jinorgbio.2013.07.012
Walsh, M. J., Goodnow, S. D., Vezeau, G. E., Richter, L. V., and Ahner, B. A. (2015). Cysteine enhances bioavailability of copper to marine phytoplankton. Environ. Sci. Technol. 49, 12145–12152. doi: 10.1021/acs.est.5b02112
Wells, M., Buck, K. N., and Sander, S. G. (2013). New approach to analysis of voltammetric ligand titration data improves understanding of metal speciation in natural waters. Limnol. Oceanogr. Methods 11, 450–465. doi: 10.4319/lom.2013.11.450
Whitby, H., and van den Berg, C. M. G. (2015). Evidence for copper-binding humic substances in seawater. Mar. Chem. 173, 282–290. doi: 10.1016/j.marchem.2014.09.011
Williams, R. B. (1964). Division rates of salt-marsh diatoms in relation to salinity + cell-size. Ecology 45, 877–880. doi: 10.2307/1934940
Wuchter, C., Abbas, B., Coolen, M. J. L., Herfort, L., VAN Bleijswijk, J., Timmers, P., et al. (2006). Archaeal nitrification in the ocean. Proc. Natl. Acad. Sci. U.S.A. 103, 12317–12322. doi: 10.1073/pnas.0600756103
Yang, R., and van den Berg, C. M. (2009). Metal complexation by humic substances in seawater. Environ. Sci. Technol. 43, 7192–7197. doi: 10.1021/es900173w
Keywords: copper speciation, cathodic stripping voltammetry, humic substances, thiols, thiourea, organic ligands, thaumarchaeota, ammonia-oxidizing archaea
Citation: Whitby H, Hollibaugh JT and van den Berg CMG (2017) Chemical Speciation of Copper in a Salt Marsh Estuary and Bioavailability to Thaumarchaeota. Front. Mar. Sci. 4:178. doi: 10.3389/fmars.2017.00178
Received: 30 January 2017; Accepted: 22 May 2017;
Published: 13 June 2017.
Edited by:
Kristen Nicolle Buck, University of South Florida, United StatesReviewed by:
Jochen Nuester, Bigelow Laboratory for Ocean Sciences, United StatesMar Nieto-Cid, Consejo Superior de Investigaciones Científicas (CSIC), Spain
Katherine Barbeau, University of California, San Diego, United States
Copyright © 2017 Whitby, Hollibaugh and van den Berg. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hannah Whitby, aGFubmFoLndoaXRieUBsaXZlLmNvLnVr
 Hannah Whitby
Hannah Whitby James T. Hollibaugh
James T. Hollibaugh Constant M. G. van den Berg
Constant M. G. van den Berg