- 1Department of Surgical, Medical, Molecular Pathology and Critical Care, University of Pisa, Pisa, Italy
- 2Department of Experimental and Clinical Medicine, University of Pisa, Pisa, Italy
- 3Department of Pharmacy, University of Pisa, Pisa, Italy
Over the last two decades, the study of the relationship between nature and nurture in shaping human behavior has encountered a renewed interest. Behavioral genetics showed that distinct polymorphisms of genes that code for proteins that control neurotransmitter metabolic and synaptic function are associated with individual vulnerability to aversive experiences, such as stressful and traumatic life events, and may result in an increased risk of developing psychopathologies associated with violence. On the other hand, recent studies indicate that experiencing aversive events modulates gene expression by introducing stable changes to DNA without modifying its sequence, a mechanism known as “epigenetics”. For example, experiencing adversities during periods of maximal sensitivity to the environment, such as prenatal life, infancy and early adolescence, may introduce lasting epigenetic marks in genes that affect maturational processes in brain, thus favoring the emergence of dysfunctional behaviors, including exaggerate aggression in adulthood. The present review discusses data from recent research, both in humans and animals, concerning the epigenetic regulation of four genes belonging to the neuroendocrine, serotonergic and oxytocinergic pathways—Nuclear receptor subfamily 3-group C-member 1 (NR3C1), oxytocin receptor (OXTR), solute carrier-family 6 member 4 (SLC6A4) and monoamine oxidase A (MAOA)—and their role in modulating vulnerability to proactive and reactive aggressive behavior. Behavioral genetics and epigenetics are shedding a new light on the fine interaction between genes and environment, by providing a novel tool to understand the molecular events that underlie aggression. Overall, the findings from these studies carry important implications not only for neuroscience, but also for social sciences, including ethics, philosophy and law.
New Frontiers in Epigenetic Research
Studies both in animals (Mosaferi et al., 2015) and humans (McEwen et al., 2012) indicate that the environment, mostly during prenatal stage and infancy, impact significantly on neural development, as several critical periods with lasting consequences on behavior have been documented (Stiles and Jernigan, 2010). Moreover, rodent studies have found that adolescent expression of 5-HT1B receptors has a direct impact on later patterns of aggressive behavior (Nautiyal et al., 2015). Therefore, the flexibility of neural programing during critical periods seems to be a significant mediator of long-lasting effects on behavior (Morrone, 2010). In particular, adversities experienced during prenatal life and infancy interfere with the normal processes of cell proliferation and differentiation leading to altered neural circuits that may result in cognitive and emotional deficits. These alterations have been associated with both proactive (e.g., children callous-unemotional traits) and reactive aggressive behavior (e.g., children externalizing disorder spectrum) that may anticipate Antisocial Personality Disorder (Frick and White, 2008; Buchmann et al., 2014; Mann et al., 2015; Rosell and Siever, 2015).
Aggression, throughout evolution, serves an important role in the survival of a species (Darwin, 1859, 1871). Being aggressive gives the best chances for survival and reproduction (Veroude et al., 2016). This is true for all mammalian species, including human. However, when excessive, the consequences of aggressive acts can be maladaptive (Takahashi and Miczek, 2014; Waltes et al., 2016).
Experiencing repeated aversive life events or protracted stress during pregnancy, especially during the first trimester of gestation, results in increased risk of physically-aggressive tendencies, delinquency and conduct disorder, both in early childhood and adolescence (Kvalevaag et al., 2014; Van den Bergh et al., 2017). During the first trimester, the neuroectoderm develops and becomes the source of neural progenitor cells, as well as the foundation of the neural tube (Stiles and Jernigan, 2010). Similar outcomes are predictable by postnatal traumas. The risk of aggressive behavior in childhood is particularly high in infants neglected during their first 2 years of life, when the brain doubles its volume and a massive synaptogenesis occurs (Knickmeyer et al., 2008; Tau and Peterson, 2010). Neglecting to provide early-life basic physical needs and emotional support as a parent can later lead to higher scores of aggression in childhood, measured by the Child Behavior Checklist (Kotch et al., 2008). Moreover, recurrent experiences of emotional abuse or witnessing violence throughout childhood predict physical aggressive behavior in adulthood (Sansone et al., 2012).
Studies in animals support the implication of prenatal and childhood adversities in the origin of aggressive behavior. In juvenile and adult male rats, for example, an increased number of physical attacks toward inoffensive peers and females have been predicted by repeated maternal separation in the first 2 weeks of life or by post-weaning social isolation (Haller et al., 2014). In rats, both prenatal and early postnatal stressors, like physical restraint during pregnancy or repeated maternal separation after birth, interfere with normal cell proliferation and differentiation and with dendritic formation, leading to altered neural circuits that may result in exaggerate aggressive behaviors (Lukas et al., 2010; de Souza et al., 2013). These early aversive experiences affect the functioning of many biochemical pathways (e.g., vasopressin, oxytocin, serotonin and cortisol pathways) that play a crucial role for the development of social skills and for the response to stress; the persistence of these alterations predisposes juvenile rats to excessive offensive play-fighting and then, as adults, to high levels of offensive attacks toward peers (Veenema et al., 2006; Veenema and Neumann, 2009; Veenema, 2009; Lukas et al., 2010; de Souza et al., 2013; Haller et al., 2014).
In addition to prenatal and early postnatal life, adolescence also represents a time-window particularly sensitive to external/environmental events, as in this period the brain concludes its maturation process (Morrone, 2010). As demonstrated by rats, during this period of life, a massive reorganization occurs in specific brain areas—hippocampus, cortex and amygdala—whose morphological and functional alterations have been linked to violence in humans (Isgor et al., 2004; Morrison et al., 2014); an increased amygdala volume, for example, has been observed in incarcerated criminals (Schiffer et al., 2011). Furthermore, peripubertal exposure of rats to fear-inducing stressors, such as the presence or the smell of a predator, predicts the expression of aggressive behavior later in adulthood (Cordero et al., 2013; Márquez et al., 2013).
It has been known for some time that genetic variants, which regulate aminergic signaling in brain, modulate vulnerability to aversive environmental factors resulting in different behavioral phenotypes (for a review see Iofrida et al., 2014; Veroude et al., 2016). More recently, it has emerged that environmental factors stably affect gene expression by producing specific signals to DNA, chromatin and mRNA that do not modify the nucleotide sequence (Bale, 2015). This phenomenon, known as epigenetics, probably mediates the long-lasting effects of aversive experiences on brain and behavior, through the generation of new trajectories of neuronal development (Morrison et al., 2014; Bale, 2015).
The Epigenetic Mechanisms: An Overview
The main epigenetic changes playing an active role in gene expression regulation are represented by DNA methylation, post-translational histone modifications and post-transcriptional regulation by microRNAs (miRNAs; Dolinoy et al., 2007; Chhabra, 2015).
DNA methylation is carried out by three active isoforms of the DNA methyltransferase family (DNMT-1, -3a and -3b), which are ubiquitous nuclear enzymes, able to transfer residues of methyl groups from the S-adenosylmethionine (SAM) to unmethylated cytosines, preferably cytosine-guanine dinucleotides (CpGs; Chiang et al., 1996). Most CpGs are grouped in specific loci of the genome, the CpG islands, which are located into promoters, exons and, to a lower extent, introns (Schwartz et al., 2009; Gelfman et al., 2013). DNMTs inhibit DNA transcription by blocking the interactions among DNA, RNA polymerase II and transcription factors, by promoting the heterochromatin formation and by interfering with the splicing process (Maunakea et al., 2013). DNMT-1, also called maintenance methyltransferase, methylates the newly replicated strand of DNA by copying the methylation patterns from the parent strand. Its role is to preserve the correct DNA methylation pattern during mitosis in daughter cells (Bird, 2002). DNMT-3a and -3b perform de novo methylation of unmethylated CpGs and produce new DNA methylation marks. The de novo methylation mainly occurs in the early embryonic cells and, not surprisingly, both enzymes are highly expressed in these cells (Okano et al., 1999).
Post-translational histone modifications are covalent modifications of the amino-terminal tails of the histones including acetylation, phosphorylation, methylation and ubiquitylation. Such modifications influence the interaction between DNA and histones, thus modifying the chromatin compacting state (Bannister and Kouzarides, 2011). Histone acetylation is mediated by the histone acetyltransferase (HAT) enzymes that cause chromatin decondensation by transferring acetyl groups from the acetyl-Coenzyme A to lysine residues within the amino-terminal tails of nucleosomal histones. The addition of acetyl groups neutralizes the positive charge of lysines, weakening the interaction between histones and DNA, thus making DNA accessible to the transcriptional machinery (Bannister and Kouzarides, 2011). At the opposite, the histone deacetylases (HDACs) remove acetyl groups from lysine residues to restore their positive charge. Histone deacetylation allows histones to tightly bind DNA, thus favoring a more compact configuration of chromatin and a consequent inhibition of transcription (Lombardi et al., 2011). Histone phosphorylation predominantly occurs on threonine, tyrosine and serine residues and is mediated by kinases that transfer a phosphate group from ATP to the hydroxyl group of the target amino-acid side chain. The addition of phosphate groups negatively charges histones, thus weakening their interaction with DNA. The histone dephosphorylation is catalyzed by phosphatases (Bannister and Kouzarides, 2011). Histone methylation takes place on the side chains of lysines and arginines, within the histone tails (Kouzarides, 2007). Histone Lysine Methyltransferase (HKMT) and Protein Arginine Methyltransferase (PRMT) are the enzymes that catalyze the transfer of a methyl group from SAM to lysine and arginine residues, respectively (Bannister and Kouzarides, 2011). Both lysine and arginine methylations act either as activators or as repressors for transcription (Kouzarides, 2007). Lysine residues are de-methylated by both the lysine-specific demethylase 1 (LSD1) and the jumonji domain 2 protein (JMJD2), whereas the jumonji domain 6 protein (JMJD6) de-methylates the arginine residues (Bannister and Kouzarides, 2011). Histone ubiquitylation consists of a binding between histone lysine residues and ubiquitin, through the sequential action of three enzymes: E1-activating, E2-conjugating and E3-ligating enzymes. Also this histone modification can be either activatory or repressive for transcription. Ubiquitin is removed by specific isopeptidases named de-ubiquitin enzymes (Bannister and Kouzarides, 2011).
miRNAs are untranslated transcripts that originate from MIR genes, located in clusters within the introns of other genes (Bhat et al., 2016). MIR genes are transcribed by RNApol II or III in long primary transcripts called pri-miRNAs that undergo extensive processing to generate mature double-stranded miRNAs. One strand is complementary to the 3’untraslated region of the target mRNA, where it binds, thus blocking gene expression either temporarily, through the mRNA translational repression, or permanently, through the mRNA cleavage (Issler and Chen, 2015).
The above-described chromatin modifications are extremely dynamic and subjected to continuous changes in response to external stimuli. As such, these molecular processes are vulnerable to modifications before and after childbirth, rendering gene expression plastic throughout mammalian life. Thus, they represent promising targets for behavioral treatment strategies. Talking about aggressive behavior predisposition, however, a role has been described to date only for DNA methylation and histone acetylation, whose scientific evidence in literature is reviewed below.
Genes Whose Epigenetic Marks Are Involved in Human Aggressive Behavior
Nuclear Receptor Subfamily 3-Group C-Member 1 (Glucocorticoid Receptor; NR3C1)
Nuclear receptor subfamily 3-group C-member 1 (NR3C1) encodes for a nuclear glucocorticoid receptor that interacts with cortisol to control the functioning of the hypothalamic-pituitary-adrenocortical (HPA) axis via a negative feedback that ultimately inhibits cortisol release (Kino and Chrousos, 2002).
According to a meta-analysis published in 2009 (Hawes et al., 2009), a great amount of data shows that cortisol is reduced in antisocial behavior. Low basal levels of blood cortisol, for example, have been associated with externalizing behavior in childhood (Alink et al., 2008) and adolescence (Shoal et al., 2003; Shirtcliff et al., 2005). In adolescence, low plasma concentration of cortisol has been negatively correlated also to low self-control (Shoal et al., 2003), delinquent behavior and proactive and reactive aggression (Poustka et al., 2010). Interestingly, a history of child abuse and neglect predicted lower HPA activity and higher trait and state aggression in adults, suggesting that the HPA hypo-activity may be a mediator between environment and long-lasting aggressive behavior (Gowin et al., 2013). A recent study confirmed this hypothesis; specifically, the hypo-methylation of NR3C1, which translates into augmented inhibitory control of HPA axis, has been shown to be induced by early adverse family environment, and to represent a risk factor for aggressive externalizing behavior in adolescence (Heinrich et al., 2015). As these epigenetic changes are produced during infancy when brain development is maximal, they persist well beyond in life (Radtke et al., 2011). They significantly impact neurodevelopment and predispose to behavioral alterations, including impaired stress response and poor self-regulation (Conradt et al., 2013), which concur in predisposing to aggressive behavior.
Oxytocin Receptor (OXTR)
Oxytocin is a hypothalamic hormone, also known as the “social neuropeptide”, that regulates complex social behaviors by promoting attachment and facilitating social interactions (Meyer-Lindenberg et al., 2011). Impaired functioning of the oxytocinergic system has been observed in rodents with aggressive behavior (Lubin et al., 2003; McMurray et al., 2008), and a lower oxytocin concentration in the central nervous system represents a predisposing factor to human aggressive behavior (Lee et al., 2009; Jokinen et al., 2012).
Social environment induces changes in the oxytocinergic system, especially during the early postnatal period and the infancy (Veenema, 2012). Oxytocin secretion (measured in saliva and whole blood), for instance, is stimulated in infants and children by maternal care (Wismer Fries et al., 2005; Tsuji et al., 2015), while childhood maltreatments, especially emotional abuses, result in lower levels of oxytocin in the cerebral spinal fluid of adults (Heim et al., 2009). Similarly, a lower expression of the oxytocin receptor (OXTR) has been detected in rodents and macaques poorly nurtured (Francis et al., 2000; Baker et al., 2017).
DNA methylation of OXTR is an important mechanism linking aversive experiences to susceptibility to abnormal behavior in adulthood (Veenema, 2012; Unternaehrer et al., 2015; Ziegler et al., 2016). A history of repeated early abuses and traumatic experiences, in fact, has been correlated to increased OXTR methylation in depressed and anxious adults (Smearman et al., 2016; Gowin et al., 2017).
OXTR methylation is affected by negative events also before birth. In particular, newborns from women who suffered from drug addiction, psychopathy or showed criminal behaviors during pregnancy, carried hyper-methylated OXTR and had an increased probability of developing callous-unemotional traits (Cecil et al., 2014), indicative of stable and severe aggressive behavior (Frick and White, 2008).
Serotonin Pathway
Serotonin plays a key role in most of psychiatric conditions and in antisocial/aggressive personality (Nutt, 2008; Seo et al., 2008). Brain serotonin concentration is regulated by serotonin transporter solute carrier-family 6 member 4 (SLC6A4) that controls its reuptake from the synaptic cleft, and by monoamine oxidase A (MAOA) that catabolizes serotonin (Shih et al., 1999).
Hypo-functioning of serotonin neurotransmission has been linked to higher risk of aggressive behaviors (Davidson et al., 2000). For instance, the brain expression of SLC6A4 is reduced in aberrant impulsive-aggressive individuals (Frankle et al., 2005). Consistently, early aversive experiences exert epigenetic regulation of SLC6A4 with implications in the development of such conditions (Provencal and Binder, 2015). Childhood stress, e.g., bullying victimization by peers, increased the saliva methylation of SLC6A4 promoter from age 5 to age 10 (Ouellet-Morin et al., 2013). Moreover, as observed in females, being physically (including sexually) abused by parents from childhood to adolescence predicts, in adulthood, both an increased SLC6A4 methylation in peripheral white cells (Beach et al., 2010) and a higher risk of developing long-lasting antisocial personality disorders (Beach et al., 2011, 2013). An in vivo study in males found a similar link between physical abuses experienced in childhood and SLC6A4 hyper-methylation in peripheral lymphocytes correlating with low brain (orbitofrontal cortex) synthesis of serotonin (Wang et al., 2012). These data suggest that SLC6A4 is silenced by early stressors as a protective mechanism aimed at the potentiation of the serotonergic neurotransmission; however, a long-lasting hyper-methylation results in lower cortical thickness (Park et al., 2015; Won et al., 2016) and alters amygdala reactivity (Nikolova et al., 2014), thus probably predisposing to aggressive behavior. For example, adolescents that have been raised in low socioeconomic status show higher methylation of SLC6A4 in peripheral lymphocytes and higher amygdala activation in response to fearful faces (Swartz et al., 2017). As far as the orbitofrontal cortex concerns, an increased activity of this brain area predicted aggressive responses to angry faces (Beyer et al., 2015); moreover, morphological asymmetry of this area has been associated with higher scores at the Lifetime History of Aggression, and Buss-Perry Aggression scales (Antonucci et al., 2006).
Finally, in a rat model of pathological aggression, the exposure to peripubertal stress affected the connectivity between amygdala and orbitofrontal cortex accompanied by a parallel increase of MAOA expression in the frontal cortex in adulthood. Interestingly, an increased H3 acetylation of MAOA was observed in the prefrontal cortex suggesting that the aversive experience has induced a stable epigenetic regulation of the transcription of this gene (Márquez et al., 2013).
Conclusion
In recent years, neuroscientific research has focused more and more on the biological mechanisms that predispose to behavioral disorders as a consequence of the exposure to aversive environments. Specific genetic variants, in interaction with negative environmental experiences during prenatal life, childhood and adolescence, have been shown to affect the development of long-lasting aggressive behavior and psychiatric disorders in adulthood, with significant social, legal and moral implications (Rigoni et al., 2010; Sartori et al., 2011; Jones et al., 2013; Roth, 2013; Iofrida et al., 2014; Rota et al., 2016; Pellegrini et al., 2017). As a matter of fact, recent studies suggest that the same genetic variants that increase the risk of aggressive behavior in combination with a negative environment, may actually act as plasticity variants, making the brain more sensitive also to positive environmental inputs, resulting in increased prosocial behavior (Belsky et al., 2009; Simons et al., 2011; Iofrida et al., 2014).
Aggression actually represents an evolutionary important behavior fostered by stressful life events, fundamental to deal with life threating situations and to preserve one’s own life (Stiles and Jernigan, 2010). However, if exaggerate and uncontrolled, it represents a pathological condition characterizing externalizing behavior, conduct disorders, callous-unemotional traits and psychopathy (Beach et al., 2011; Kumsta et al., 2013; Cecil et al., 2014; Heinrich et al., 2015; Kundakovic et al., 2015).
Over the last few years, the epigenetic mechanisms underlying human aggressive behavior have been attracting a growing interest, as they provide a fascinating and reliable explanation of the gene-environment interplay that modulates human violent behavior. Epigenetics, indeed, plays a central role in the adaptation of the human organism to the changing environment. This concept emerged first from studies conducted in monozygotic twins, which showed that different phenotypes may originate from identical genotypes due to epigenetic changes (Poulsen et al., 2007). These differences progressively increase as twins become older, along with the diversification of their lifestyles and living environments (Fraga et al., 2005).
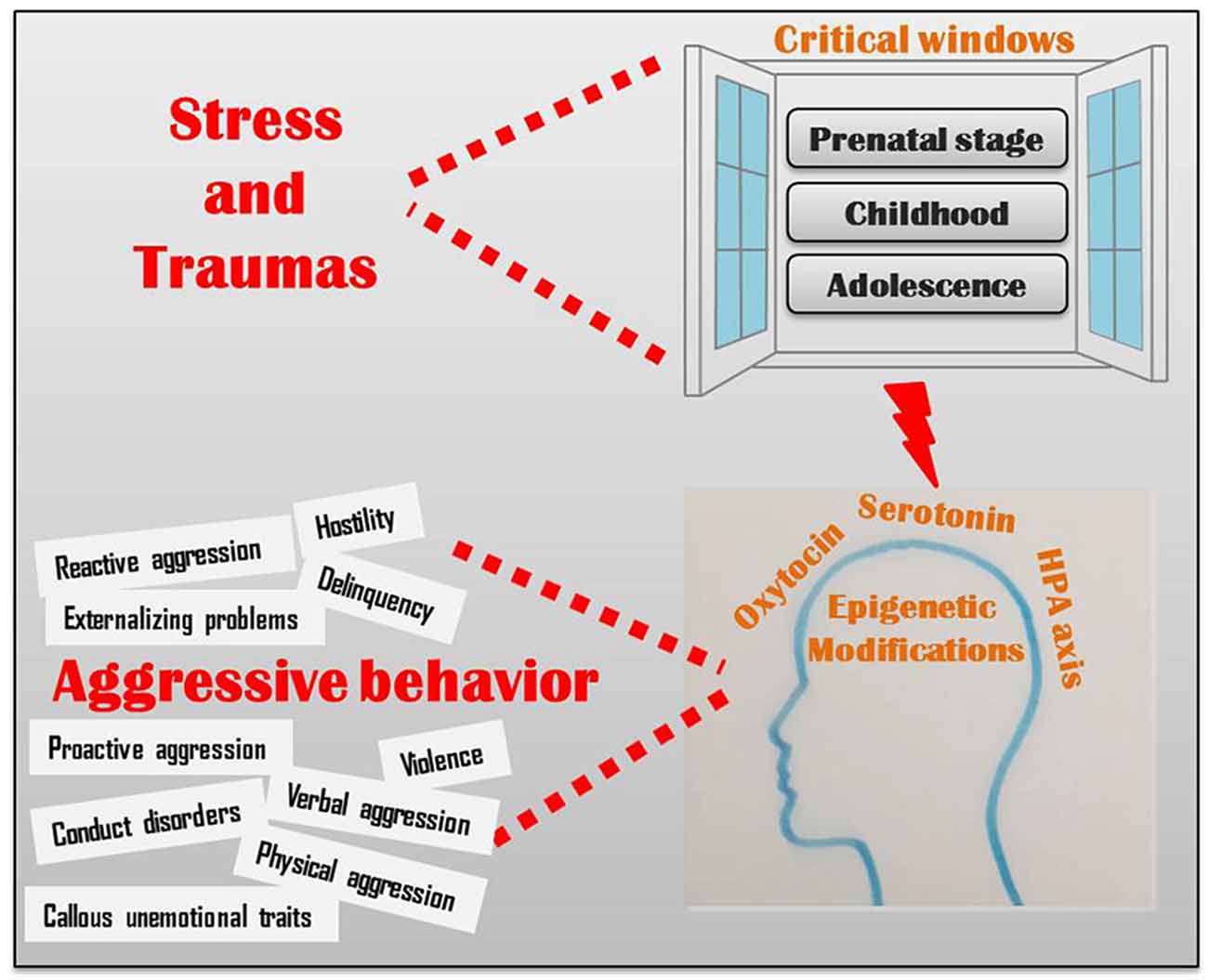
Although the existence of a genetic blueprint underlying brain development is undeniable, the epigenetic control of biological pathways, including the neuroendocrine, serotonergic and oxytocinergic pathways, significantly mediates the behavioral responses to the environment (Figure 1; Veenema, 2012; Waltes et al., 2016). Epigenetic changes in these pathways may alter brain morphology and functioning in areas that hold a crucial role in cognitive and emotional processes underlying aggression (Conradt et al., 2013; Ouellet-Morin et al., 2013; Suri et al., 2013; Booij et al., 2015; Puglia et al., 2015; Gowin et al., 2017). Recent data indicate that these epigenetic marks may be, in some extent, reversed by the exposure to an enriched environment therapy; for example, massage therapy significantly reduced aggressive behavior in children and adolescence (Diego et al., 2002; Garner et al., 2008), probably by epigenetic mechanisms (McCreary and Metz, 2016). Alternatively, it is possible to intervene by a pharmacological therapy, as shown in rats: treating aggressive adult rats that had experienced peripubertal stress with a MAOA inhibitor reversed their aberrant behavior (Márquez et al., 2013).
In conclusion, epigenetics is shedding a new light on the fine interaction between nature and nurture, by providing a novel tool to understand the molecular events that underlie the relationship among genes, brain, environment and behavior. Altogether, the results of the studies that we briefly discussed in the present article, clearly indicate that, when it comes to (human) behavior, nature and nurture are not to be regarded as two distinct and separate factors, contrary to the alternating predominance of either one that has been proposed in different historic phases (Levitt, 2013; Moore, 2016). Indeed, distinct genetic backgrounds differentially modulate the individual susceptibility to the environment and at the same time various environmental conditions differentially affect gene expression, in an intimate and fascinating manner that scientists have now begun to disentangle. The findings from this research pave the way to a novel approach to the understanding of human behavior, with important implications also for social sciences, including philosophy, ethics and law. Unveiling the molecular mechanisms that regulate the expression of human behavior will provide a solid scientific basis to what philosophy already sensed since its dawn, suffice it to mention what the great Plato wrote over 25 centuries ago: “No one is willingly evil, but one can become evil for a bad disposition in his body and for a training without a true education; this is hideous for everyone and happens against his will” (Timeus, 86e).
Author Contributions
SPalumbo, VM and CI searched and reviewed the scientific literature; all the authors discussed the findings from the literature; SPalumbo and VM drafted the manuscript; SPellegrini conceived the work and revised the manuscript.
Funding
This work was supported by a Grant from Fondazione Gio.I.A, Pisa (Italy) and by Fondazione Cassa di Risparmio di Lucca (Grant 2016-2017).
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
Alink, L. R., van Ijzendoorn, M. H., Bakermans-Kranenburg, M. J., Mesman, J., Juffer, F., and Koot, H. M. (2008). Cortisol and externalizing behavior in children and adolescents: mixed meta-analytic evidence for the inverse relation of basal cortisol and cortisol reactivity with externalizing behavior. Dev. Psychobiol. 50, 427–450. doi: 10.1002/dev.20300
Antonucci, A. S., Gansler, D. A., Tan, S., Bhadelia, R., Patz, S., and Fulwiler, C. (2006). Orbitofrontal correlates of aggression and impulsivity in psychiatric patients. Psychiatry Res. 147, 213–220. doi: 10.1016/j.pscychresns.2005.05.016
Baker, M., Lindell, S. G., Driscoll, C. A., Zhou, Z., Yuan, Q., Schwandt, M. L., et al. (2017). Early rearing history influences oxytocin receptor epigenetic regulation in rhesus macaques. Proc. Natl. Acad. Sci. U S A 114, 11769–11774. doi: 10.1073/pnas.1706206114
Bale, T. L. (2015). Epigenetic and transgenerational reprogramming of brain development. Nat. Rev. Neurosci. 16, 332–344. doi: 10.1038/nrn3818
Bannister, A. J., and Kouzarides, T. (2011). Regulation of chromatin by histone modifications. Cell Res. 21, 381–395. doi: 10.1038/cr.2011.22
Booij, L., Tremblay, R. E., Szyf, M., and Benkelfat, C. (2015). Genetic and early environmental influences on the serotonin system: consequences for brain development and risk for psychopathology. J. Psychiatry Neurosci. 40, 5–18. doi: 10.1503/jpn.140099
Beach, S. R., Brody, G. H., Lei, M. K., Gibbons, F. X., Gerrard, M., Simons, R. L., et al. (2013). Impact of child sex abuse on adult psychopathology: a genetically and epigenetically informed investigation. J. Fam. Psychol. 27, 3–11. doi: 10.1037/a0031459
Beach, S. R., Brody, G. H., Todorov, A. A., Gunter, T. D., and Philibert, R. A. (2010). Methylation at SLC6A4 is linked to family history of child abuse: an examination of the Iowa Adoptee sample. Am. J. Med. Genet. B Neuropsychiatry Genet. 153B, 710–713. doi: 10.1002/ajmg.b.31028
Beach, S. R., Brody, G. H., Todorov, A. A., Gunter, T. D., and Philibert, R. A. (2011). Methylation at 5HTT mediates the impact of child sex abuse on women’s antisocial behavior: an examination of the Iowa adoptee sample. Psychosom. Med. 73, 83–87. doi: 10.1097/PSY.0b013e3181fdd074
Belsky, J., Jonassaint, C., Pluess, M., Stanton, M., Brummett, B., and Williams, R. (2009). Vulnerability genes or plasticity genes? Mol. Psychiatry 14, 746–754. doi: 10.1038/mp.2009.44
Beyer, F., Münte, T. F., Göttlich, M., and Krämer, U. M. (2015). Orbitofrontal cortex reactivity to angry facial expression in a social interaction correlates with aggressive behavior. Cereb. Cortex 25, 3057–3063. doi: 10.1093/cercor/bhu101
Bhat, S. S., Jarmolowski, A., and Szweykowska-Kulińska, Z. (2016). MicroRNA biogenesis: epigenetic modifications as another layer of complexity in the microRNA expression regulation. Acta Biochim. Pol. 63, 717–723. doi: 10.18388/abp.2016_1370
Bird, A. (2002). DNA methylation patterns and epigenetic memory. Genes Dev. 16, 6–21. doi: 10.1101/gad.947102
Buchmann, A., Hohmann, S., Brandeis, D., Banaschewski, T., and Poustka, L. (2014). Aggression in children and adolescents. Curr. Top. Behav. Neurosci. 17, 421–442. doi: 10.1007/7854_2013_261
Cecil, C. A., Lysenko, L. J., Jaffee, S. R., Pingault, J. B., Smith, R. G., Relton, C. L., et al. (2014). Environmental risk, Oxytocin Receptor Gene (OXTR) methylation and youth callous-unemotional traits: a 13-year longitudinal study. Mol. Psychiatry 19, 1071–1077. doi: 10.1038/mp.2014.95
Chhabra, R. (2015). miRNA and methylation: a multifaceted liaison. Chembiochem 16, 195–203. doi: 10.1002/cbic.201402449
Chiang, P. K., Gordon, R. K., Tal, J., Zeng, G. C., Doctor, B. P., Pardhasaradhi, K., et al. (1996). S-Adenosylmethionine and methylation. FASEB J. 10, 471–480. doi: 10.1096/fasebj.10.4.8647346
Conradt, E., Lester, B. M., Appleton, A. A., Armstrong, D. A., and Marsit, C. J. (2013). The roles of DNA methylation of NR3C1 and 11β-HSD2 and exposure to maternal mood disorder in utero on newborn neurobehavior. Epigenetics 8, 1321–1329. doi: 10.4161/epi.26634
Cordero, M. I., Ansermet, F., and Sandi, C. (2013). Long-term programming of enhanced aggression by peripuberty stress in female rats. Psychoneuroendocrinology 38, 2758–2769. doi: 10.1016/j.psyneuen.2013.07.005
Davidson, R. J., Putnam, K. M., and Larson, C. L. (2000). Dysfunction in the neural circuitry of emotion regulation—a possible prelude to violence. Science 289, 591–594. doi: 10.1126/science.289.5479.591
de Souza, M. A., Centenaro, L. A., Menegotto, P. R., Henriques, T. P., Bonini, J., Achaval, M., et al. (2013). Prenatal stress produces social behavior deficits and alters the number of oxytocin and vasopressin neurons in adult rats. Neurochem. Res. 38, 1479–1489. doi: 10.1007/s11064-013-1049-5
Diego, M. A., Field, T., Hernandez-Reif, M., Shaw, J. A., Rothe, E. M., Castellanos, D., et al. (2002). Aggressive adolescents benefit from massage therapy. Adolescence 37, 597–607.
Dolinoy, D. C., Weidman, J. R., and Jirtle, R. L. (2007). Epigenetic gene regulation: linking early developmental environment to adult disease. Reprod. Toxicol. 23, 297–307. doi: 10.1016/j.reprotox.2006.08.012
Fraga, M. F., Ballestar, E., Paz, M. F., Ropero, S., Setien, F., Ballestar, M. L., et al. (2005). Epigenetic differences arise during the lifetime of monozygotic twins. Proc. Natl. Acad. Sci. U S A 102, 10604–10609. doi: 10.1073/pnas.0500398102
Francis, D. D., Champagne, F. C., and Meaney, M. J. (2000). Variations in maternal behaviour are associated with differences in oxytocin receptor levels in the rat. J. Neuroendocrinol. 12, 1145–1148. doi: 10.1046/j.1365-2826.2000.00599.x
Frankle, W. G., Lombardo, I., New, A. S., Goodman, M., Talbot, P. S., Huang, Y., et al. (2005). Brain serotonin transporter distribution in subjects with impulsive aggressivity: a positron emission study with [11C]McN 5652. Am. J. Psychiatry 162, 915–923. doi: 10.1176/appi.ajp.162.5.915
Frick, P. J., and White, S. F. (2008). Research review: the importance of callous-unmemotional traits for developmental models of aggressive and antisocial behavior. J. Child Psychol. Psychiatry 49, 359–375. doi: 10.1111/j.1469-7610.2007.01862.x
Garner, B., Phillips, L. J., Schmidt, H. M., Markulev, C., O’Connor, J., Wood, S. J., et al. (2008). Pilot study evaluating the effect of massage therapy on stress, anxiety and aggression in a young adult psychiatric inpatient unit. Aust. N Z J. Psychiatry 42, 414–422. doi: 10.1080/00048670801961131
Gelfman, S., Cohen, N., Yearim, A., and Ast, G. (2013). DNA-methylation effect on cotranscriptional splicing is dependent on GC architecture of the exon-intron structure. Genome Res. 23, 789–799. doi: 10.1101/gr.143503.112
Gowin, J. L., Green, C. E., Alcorn, J. L. III., Swann, A. C., Moeller, F. G., and Lane, S. D. (2013). The role of cortisol and psychopathy in the cycle of violence. Psychopharmacology 227, 661–672. doi: 10.1007/s00213-013-2992-1
Gowin, J. P., Zhou, Q. Q., Booij, L., Boivin, M., Côté, S. M., Hébert, M., et al. (2017). Associations among oxytocin receptor gene (OXTR) DNA methylation in adulthood, exposure to early life adversity and childhood trajectories of anxiousness. Sci. Rep. 7:7446. doi: 10.1038/s41598-017-07950-x
Haller, J., Harold, G., Sandi, C., and Neumann, I. D. (2014). Effects of adverse early-life events on aggression and anti-social behaviours in animals and humans. J. Neuroendocrinol. 26, 724–738. doi: 10.1111/jne.12182
Hawes, D. J., Brennan, J., and Dadds, M. R. (2009). Cortisol, callous-unemotional traits, and pathways to antisocial behavior. Curr. Opin. Psychiatry 22, 357–362. doi: 10.1097/YCO.0b013e32832bfa6d
Heim, C., Young, L. J., Newport, D. J., Mletzko, T., Miller, A. H., and Nemeroff, C. B. (2009). Lower CSF oxytocin concentrations in women with a history of childhood abuse. Mol. Psychiatry 14, 954–958. doi: 10.1038/mp.2008.112
Heinrich, A., Buchmann, A. F., Zohsel, K., Dukal, H., Frank, J., Treutlein, J., et al. (2015). Alterations of glucocorticoid receptor gene methylation in externalizing disorders during childhood and adolescence. Behav. Genet. 45, 529–536. doi: 10.1007/s10519-015-9721-y
Iofrida, C., Palumbo, S., and Pellegrini, S. (2014). Molecular genetics and antisocial behavior: where do we stand? Exp. Biol. Med. 239, 1514–1523. doi: 10.1177/1535370214529508
Isgor, C., Kabbaj, M., Akil, H., and Watson, S. J. (2004). Delayed effects of chronic variable stress during peripubertal-juvenile period on hippocampal morphology and on cognitive and stress axis functions in rats. Hippocampus 14, 636–648. doi: 10.1002/hipo.10207
Issler, O., and Chen, A. (2015). Determining the role of microRNAs in psychiatric disorders. Nat. Rev. Neurosci. 16, 201–212. doi: 10.1038/nrn3879
Jokinen, J., Chatzittofis, A., Hellström, C., Nordström, P., Uvnäs-Moberg, K., and Asberg, M. (2012). Low CSF oxytocin reflects high intent in suicide attempters. Psychoneuroendocrinology 37, 482–490. doi: 10.1016/j.psyneuen.2011.07.016
Jones, O. D., Marois, R., Farah, M. J., and Greely, H. T. (2013). Law and neuroscience. J. Neurosci. 33, 17624–17630. doi: 10.1523/JNEUROSCI.3254-13.2013
Kino, T., and Chrousos, G. P. (2002). Tissue-specific glucocorticoid resistance-hypersensitivity syndromes: multifactorial states of clinical importance. J. Allergy Clin. Immunol. 109, 609–613. doi: 10.1067/mai.2002.123708
Knickmeyer, R. C., Gouttard, S., Kang, C., Evans, D., Wilber, K., Smith, J. K., et al. (2008). A structural MRI study of human brain development from birth to 2 years. J. Neurosci. 28, 12176–12182. doi: 10.1523/JNEUROSCI.3479-08.2008
Kotch, J. B., Lewis, T., Hussey, J. M., English, D., Thompson, R., Litrownik, A. J., et al. (2008). Importance of early neglect for childhood aggression. Pediatrics 121, 725–731. doi: 10.1542/peds.2006-3622
Kouzarides, T. (2007). Chromatin modifications and their function. Cell 128, 693–705. doi: 10.1016/j.cell.2007.02.005
Kumsta, R., Hummel, E., Chen, F. S., and Heinrichs, M. (2013). Epigenetic regulation of the oxytocin receptor gene: implications for behavioral neuroscience. Front. Neurosci. 7:83. doi: 10.3389/fnins.2013.00083
Kundakovic, M., Gudsnuk, K., Herbstman, J. B., Tang, D., Perera, F. P., and Champagne, F. A. (2015). DNA methylation of BDNF as a biomarker of early-life adversity. Proc. Natl. Acad. Sci. U S A 112, 6807–6813. doi: 10.1073/pnas.1408355111
Kvalevaag, A. L., Ramchandani, P. G., Hove, O., Eberhard-Gran, M., Assmus, J., Havik, O. E., et al. (2014). Does paternal mental health in pregnancy predict physically aggressive behavior in children? Eur. Child Adolesc. Psychiatry 23, 993–1002. doi: 10.1007/s00787-014-0587-y
Lee, R., Ferris, C., Van de Kar, L. D., and Coccaro, E. F. (2009). Cerebrospinal fluid oxytocin, life history of aggression and personality disorder. Psychoneuroendocrinology 34, 1567–1573. doi: 10.1016/j.psyneuen.2009.06.002
Levitt, M. (2013). Perceptions of nature, nurture and behaviour. Life Sci. Soc. Policy 9:13. doi: 10.1186/2195-7819-9-13
Lombardi, P. M., Cole, K. E., Dowling, D. P., and Christianson, D. W. (2011). Structure, mechanism, and inhibition of histone deacetylases and related metalloenzymes. Curr. Opin. Struct. Biol. 21, 735–743. doi: 10.1016/j.sbi.2011.08.004
Lubin, D. A., Elliott, J. C., Black, M. C., and Johns, J. M. (2003). An oxytocin antagonist infused into the central nucleus of the amygdala increases maternal aggressive behavior. Behav. Neurosci. 117, 195–201. doi: 10.1037/0735-7044.117.2.195
Lukas, M., Bredewold, R., Neumann, I. D., and Veenema, A. H. (2010). Maternal separation interferes with developmental changes in brain vasopressin and oxytocin receptor binding in male rats. Neuropharmacology 58, 78–87. doi: 10.1016/j.neuropharm.2009.06.020
Mann, F. D., Briley, D. A., Tucker-Drob, E. M., and Harden, K. P. (2015). A behavioral genetic analysis of callous-unemotional traits and Big Five personality in adolescence. J. Abnorm. Psychol. 124, 982–993. doi: 10.1037/abn0000099
Márquez, C., Poirier, G. L., Cordero, M. I., Larsen, M. H., Groner, A., Marquis, J., et al. (2013). Peripuberty stress leads to abnormal aggression, altered amygdala and orbitofrontal reactivity and increased prefrontal MAOA gene expression. Transl. Psychiatry 3:e216. doi: 10.1038/tp.2012.144
Maunakea, A. K., Chepelev, I., Cui, K., and Zhao, K. (2013). Intragenic DNA methylation modulates alternative splicing by recruiting MeCP2 to promote exon recognition. Cell Res. 23, 1256–1269. doi: 10.1038/cr.2013.110
McCreary, J. K., and Metz, G. A. S. (2016). Environmental enrichment as an intervention for adverse health outcomes of prenatal stress. Environ. Epigenet. 2:dvw013. doi: 10.1093/eep/dvw013
McEwen, B. S., Eiland, L., Hunter, R. G., and Miller, M. M. (2012). Stress and anxiety: structural plasticity and epigenetic regulation as a consequence of stress. Neuropharmacology 62, 3–12. doi: 10.1016/j.neuropharm.2011.07.014
McMurray, M. S., Joyner, P. W., Middleton, C. W., Jarrett, T. M., Elliott, D. L., Black, M. A., et al. (2008). Intergenerational effects of cocaine on maternal aggressive behavior and brain oxytocin in rat dams. Stress 11, 398–410. doi: 10.1080/10253890701850239
Meyer-Lindenberg, A., Domes, G., Kirsch, P., and Heinrichs, M. (2011). Oxytocin and vasopressin in the human brain: social neuropeptides for translational medicine. Nat. Rev. Neurosci. 12, 524–538. doi: 10.1038/nrn3044
Moore, D. S. (2016). Behavioral epigenetics. Wiley Interdiscip. Rev. Syst. Biol. Med. 9:e1333. doi: 10.1002/wsbm.1333
Morrison, K. E., Rodgers, A. B., Morgan, C. P., and Bale, T. L. (2014). Epigenetic mechanisms in pubertal brain maturation. Neuroscience 264, 17–24. doi: 10.1016/j.neuroscience.2013.11.014
Morrone, M. C. (2010). Brain development: critical periods for cross-sensory plasticity. Curr. Biol. 20, R934–R936. doi: 10.1016/j.cub.2010.09.052
Mosaferi, B., Babri, S., Ebrahimi, H., and Mohaddes, G. (2015). Enduring effects of post-weaning rearing condition on depressive- and anxiety-like behaviors and motor activity in male rats. Physiol. Behav. 142, 131–136. doi: 10.1016/j.physbeh.2015.02.015
Nautiyal, K. M., Tanaka, K. F., Barr, M. M., Tritschler, L., Le Dantec, Y., David, D. J., et al. (2015). Distinct circuits underlie the effects of 5-HT1B receptors on aggression and impulsivity. Neuron 86, 813–826. doi: 10.1016/j.neuron.2015.03.041
Nikolova, Y. S., Koenen, K. C., Galea, S., Wang, C. M., Seney, M. L., Sibille, E., et al. (2014). Beyond genotype: serotonin transporter epigenetic modification predicts human brain function. Nat. Neurosci. 17, 1153–1155. doi: 10.1038/nn.3778
Nutt, D. J. (2008). Relationship of neurotransmitters to the symptoms of major depressive disorder. J. Clin. Psychiatry 69, 4–7.
Okano, M., Bell, D. W., Haber, D. A., and Li, E. (1999). DNA methyltransferases Dnmt3a and Dnmt3b are essential for de novo methylation and mammalian development. Cell 99, 247–257. doi: 10.1016/s0092-8674(00)81656-6
Ouellet-Morin, I., Wong, C. C., Danese, A., Pariante, C. M., Papadopoulos, A. S., Mill, J., et al. (2013). Increased serotonin transporter gene (SERT) DNA methylation is associated with bullying victimization and blunted cortisol response to stress in childhood: a longitudinal study of discordant monozygotic twins. Psychol. Med. 43, 1813–1823. doi: 10.1017/s0033291712002784
Park, S., Lee, J. M., Kim, J. W., Cho, D. Y., Yun, H. J., Han, D. H., et al. (2015). Associations between serotonin transporter gene (SLC6A4) methylation and clinical characteristics and cortical thickness in children with ADHD. Psychol. Med. 45, 3009–3017. doi: 10.1017/S003329171500094X
Pellegrini, S., Palumbo, S., Iofrida, C., Melissari, E., Rota, G., Mariotti, V., et al. (2017). Genetically-driven enhancement of dopaminergic transmission affects moral acceptability in females but not in males: a pilot study. Front. Behav. Neurosci. 11:156. doi: 10.3389/fnbeh.2017.00156
Poulsen, P., Esteller, M., Vaag, A., and Fraga, M. F. (2007). The epigenetic basis of twin discordance in age-related diseases. Pediatr. Res. 61, 38R–42R. doi: 10.1203/pdr.0b013e31803c7b98
Poustka, L., Maras, A., Hohm, E., Fellinger, J., Holtmann, M., Banaschewski, T., et al. (2010). Negative association between plasma cortisol levels and aggression in a high-risk community sample of adolescents. J. Neur Transm. 117, 621–627. doi: 10.1007/s00702-010-0386-7
Provencal, N., and Binder, E. B. (2015). The neurobiological effects of stress as contributors to psychiatric disorders: focus on epigenetics. Curr. Opin. Neurobiol. 30, 31–37. doi: 10.1016/j.conb.2014.08.007
Puglia, M. H., Lillard, T. S., Morris, J. P., and Connelly, J. J. (2015). Epigenetic modification of the oxytocin receptor gene influences the perception of anger and fear in the human brain. Proc. Natl. Acad. Sci. U S A 112, 3308–3313. doi: 10.1073/pnas.1422096112
Radtke, K. M., Ruf, M., Gunter, H. M., Dohrmann, K., Schauer, M., Meyer, A., et al. (2011). Transgenerational impact of intimate partner violence on methylation in the promoter of the glucocorticoid receptor. Transl. Psychiatry 1:e21. doi: 10.1038/tp.2011.21
Rigoni, D., Pellegrini, S., Mariotti, V., Cozza, A., Mechelli, A., Ferrara, S. D., et al. (2010). How neuroscience and behavioral genetics improve psychiatric assessment: report on a violent murder case. Front. Behav. Neurosci. 4:160. doi: 10.3389/fnbeh.2010.00160
Rosell, D. R., and Siever, L. J. (2015). The neurobiology of aggression and violence. CNS Spectr. 20, 254–279. doi: 10.1017/S109285291500019X
Rota, G., Palumbo, S., Lattanzi, N., Manfrinati, A., Sarlo, M., Lotto, L., et al. (2016). Harm aversion explains utilitarian choices in moral decision-making in males but not in females. Arch. Ital. Biol. 154, 50–58. doi: 10.12871/00039829201622
Roth, T. L. (2013). Epigenetic mechanisms in the development of behavior: advances, challenges, and future promises of a new field. Dev. Psychopathol. 25, 1279–1291. doi: 10.1017/s0954579413000618
Sansone, R. A., Leung, J. S., and Wiederman, M. W. (2012). Five forms of childhood trauma: relationships with aggressive behavior in adulthood. Prim. Care Companion CNS Disord. 14:PCC.12m01353. doi: 10.4088/pcc.12m01353
Sartori, G., Pellegrini, S., and Mechelli, A. (2011). Forensic neurosciences: from basic research to applications and pitfalls. Curr. Opin. Neurol. 24, 371–377. doi: 10.1097/WCO.0b013e3283489754
Schiffer, B., Müller, B. W., Scherbaum, N., Hodgins, S., Forsting, M., Wiltfang, J., et al. (2011). Disentangling structural brain alterations associated with violent behavior from those associated with substance use disorders. Arch. Gen. Psychiatry 68, 1039–1049. doi: 10.1001/archgenpsychiatry.2011.61
Schwartz, S., Meshorer, E., and Ast, G. (2009). Chromatin organization marks exon-intron structure. Nat. Struct. Mol. Biol. 16, 990–995. doi: 10.1038/nsmb.1659
Seo, D., Patrick, C. J., and Kennealy, P. J. (2008). Role of serotonin and dopamine system interactions in the neurobiology of impulsive aggression and its comorbidity with other clinical disorders. Aggress. Violent Behav. 13, 383–395. doi: 10.1016/j.avb.2008.06.003
Shih, J. C., Chen, K., and Ridd, M. J. (1999). Monoamine oxidase: from genes to behavior. Annu. Rev. Neurosci. 22, 197–217. doi: 10.1146/annurev.neuro.22.1.197
Shirtcliff, E. A., Granger, D. A., Booth, A., and Johnson, D. (2005). Low salivary cortisol levels and externalizing behavior problems in youth. Dev. Psychopathol. 17, 167–184. doi: 10.1017/s0954579405050091
Shoal, G. D., Giancola, P. R., and Kirillova, G. P. (2003). Salivary cortisol, personality, and aggressive behavior in adolescent boys: a 5-year longitudinal study. J. Am. Acad. Child Adolesc. Psychiatry 42, 1101–1107. doi: 10.1097/01.chi.0000070246.24125.6d
Simons, R. L., Lei, M. K., Beach, S. R., Brody, G. H., Philibert, R. A., and Gibbons, F. X. (2011). Social environmental variation, plasticity genes, and aggression: evidence for the differential susceptibility hypothesis. Am. Sociol. Rev. 76, 833–912. doi: 10.1177/0003122411427580
Smearman, E. L., Almli, L. M., Conneely, K. N., Brody, G. H., Sales, J. M., Bradley, B., et al. (2016). Oxytocin receptor genetic and epigenetic variations: association with child abuse and adult psychiatric symptoms. Child. Dev. 87, 122–134. doi: 10.1111/cdev.12493
Stiles, J., and Jernigan, T. L. (2010). The basics of brain development. Neuropsychol. Rev. 20, 327–348. doi: 10.1007/s11065-010-9148-4
Suri, D., Veenit, V., Sarkar, A., Thiagarajan, D., Kumar, A., Nestler, E. J., et al. (2013). Early stress evokes age-dependent biphasic changes in hippocampal neurogenesis, BDNF expression, and cognition. Biol. Psychiatry 73, 658–666. doi: 10.1016/j.biopsych.2012.10.023
Swartz, J. R., Hariri, A. R., and Williamson, D. E. (2017). An epigenetic mechanism links socioeconomic status to changes in depression-related brain function in high-risk adolescents. Mol. Psychiatry 22, 209–214. doi: 10.1038/mp.2016.82
Takahashi, A., and Miczek, K. A. (2014). Neurogenetics of aggressive behavior—studies in rodents. Curr. Top. Behav. Neurosci. 17, 3–44. doi: 10.1007/7854_2013_263
Tau, G. Z., and Peterson, B. S. (2010). Normal development of brain circuits. Neuropsychopharmacology 35, 147–168. doi: 10.1038/npp.2009.115
Tsuji, S., Yuhi, T., Furuhara, K., Ohta, S., Shimizu, Y., and Higashida, H. (2015). Salivary oxytocin concentrations in seven boys with autism spectrum disorder received massage from their mothers: a pilot study. Front. Psychiatry 6:58. doi: 10.3389/fpsyt.2015.00058
Unternaehrer, E., Meyer, A. H., Burkhardt, S. C., Dempster, E., Staehli, S., Theill, N., et al. (2015). Childhood maternal care is associated with DNA methylation of the genes for brain-derived neurotrophic factor (BDNF) and oxytocin receptor (OXTR) in peripheral blood cells in adult men and women. Stress 18, 451–461. doi: 10.3109/10253890.2015.1038992
Van den Bergh, B. R. H., van den Heuvel, I., Lahti, M., Braeken, M., de Rooij, S. R., Entringer, S., et al. (2017). Prenatal developmental origins of behavior and mental health: the influence of maternal stress in pregnancy. Neurosci. Biobehav. Rev. doi: 10.1016/j.neubiorev.2017.07.003 [Epub ahead of print].
Veenema, A. H. (2009). Early life stress, the development of aggression and neuroendocrine and neurobiological correlates: what can we learn from animal models? Front. Neuroendocrinol. 30, 497–518. doi: 10.1016/j.yfrne.2009.03.003
Veenema, A. H. (2012). Toward understanding how early-life social experiences alter oxytocin- and vasopressin-regulated social behaviors. Horm. Behav. 61, 304–312. doi: 10.1016/j.yhbeh.2011.12.002
Veenema, A. H., Blume, A., Niederle, D., Buwalda, B., and Neumann, I. D. (2006). Effects of early life stress on adult male aggression and hypothalamic vasopressin and serotonin. Eur. J. Neurosci. 24, 1711–1720. doi: 10.1111/j.1460-9568.2006.05045.x
Veenema, A. H., and Neumann, I. D. (2009). Maternal separation enhances offensive play-fighting, basal corticosterone and hypothalamic vasopressin mRNA expression in juvenile male rats. Psychoneuroendocrinology 34, 463–467. doi: 10.1016/j.psyneuen.2008.10.017
Veroude, K., Zhang-James, Y., Fernàndez-Castillo, N., Bakker, M. J., Cormand, B., and Faraone, S. V. (2016). Genetics of aggressive behavior: an overview. Am. J. Med. Genet. B Neuropsychiatr. Genet. 171, 3–43. doi: 10.1002/ajmg.b.32364
Waltes, R., Chiocchetti, A. G., and Freitag, C. M. (2016). The neurobiological basis of human aggression: a review on genetic and epigenetic mechanisms. Am. J. Med. Genet. B Neuropsychiatr. Genet. 171, 650–675. doi: 10.1002/ajmg.b.32388
Wang, D., Szyf, M., Benkelfat, C., Provençal, N., Turecki, G., Caramaschi, D., et al. (2012). Peripheral SLC6A4 DNA methylation is associated with in vivo measures of human brain serotonin synthesis and childhood physical aggression. PLoS One 7:e39501. doi: 10.1371/journal.pone.0039501
Wismer Fries, A. B., Ziegler, T. E., Kurian, J. R., Jacoris, S., and Pollak, S. D. (2005). Early experience in humans is associated with changes in neuropeptides critical for regulating social behavior. Proc. Natl. Acad. Sci. U S A 102, 17237–17240. doi: 10.1073/pnas.0504767102
Won, E., Choi, S., Kang, J., Kim, A., Han, K. M., Chang, H. S., et al. (2016). Association between reduced white matter integrity in the corpus callosum and serotonin transporter gene DNA methylation in medication-naive patients with major depressive disorder. Transl. Psychiatry 6:e866. doi: 10.1038/tp.2016.137
Keywords: epigenetics, aversive environment, aggressive behavior, NR3C1, OXTR, SLC6A4, MAOA
Citation: Palumbo S, Mariotti V, Iofrida C and Pellegrini S (2018) Genes and Aggressive Behavior: Epigenetic Mechanisms Underlying Individual Susceptibility to Aversive Environments. Front. Behav. Neurosci. 12:117. doi: 10.3389/fnbeh.2018.00117
Received: 09 January 2018; Accepted: 28 May 2018;
Published: 13 June 2018.
Edited by:
Aki Takahashi, University of Tsukuba, JapanReviewed by:
Bauke Buwalda, University of Groningen, NetherlandsHerb E. Covington, Tufts University, United States
Copyright © 2018 Palumbo, Mariotti, Iofrida and Pellegrini. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Silvia Pellegrini, c2lsdmlhLnBlbGxlZ3JpbmlAbWVkLnVuaXBpLml0
 Sara Palumbo
Sara Palumbo Veronica Mariotti
Veronica Mariotti Caterina Iofrida
Caterina Iofrida Silvia Pellegrini
Silvia Pellegrini