- 1Department of Neurology, Faculty of Medicine, Ain Shams University, Cairo, Egypt
- 2Faculty of Medicine, Okasha Institute of Psychiatry, Ain Shams University, Cairo, Egypt
- 3Faculty of Medicine, Ain Shams University, Cairo, Egypt
Objective: The prevalence of non-motor symptoms (NMSs) and their impact on health-related quality of life (HRQoL) in Parkinson’s disease (PD) has been reported inconsistently among different populations. In this study, we aimed to investigate the NMSs and HRQoL profiles and their correlation in Egyptian PD patients, using a culturally adapted Arabic version of the 39-item Parkinson’s disease questionnaire (PDQ-39).
Methods: Ninety-seven PD patients were rated using the unified Parkinson’s disease rating scale (UPDRS), the non-motor symptoms scales (NMSS), Beck depression inventory (BDI), and the Arabic version of PDQ-39. We used the Spearman’s rank correlation and multiple linear regression analyses to evaluate the relationship between NMSs domains and HRQoL dimensions.
Results: Fatigue/sleep (91.3%) and mood/cognitive disturbances (87%) were the most frequently and severely affected NMSS domains. Other common NMSs included urinary (75.9%), memory/attention (72.4%), gastrointestinal (67.8%), and cardiovascular problems (64.8%). The total NMSS scores were positively correlated with UPDRS I, II, and III scores. Depression was prevalent in 76.7% of PD patients. Moreover, all enrolled PD patients reported impairment in different HRQoL dimensions, especially mobility (98.9%), activities of daily living (97.8%), and emotional well-being (95.5%). The summary index of PDQ-39 was correlated to the total NMSS, UPDRS-I, UPDRS-II Off, UPDRS-III (Off and On states), and BDI scores.
Conclusion: This study showed the high prevalence of NMSs and the value of NMSS and BDI scores as predictors of HRQoL in Egyptian PD patients. Therefore, characterizing the NMSs profile is essential for tailoring management strategies for PD patients.
Introduction
Parkinson’s disease (PD) is the second most common neurodegenerative disorder worldwide, caused by degeneration of dopaminergic neurons in the substantia nigra, and affects approximately 1–3% of the elderly population (≥60 years) (1). Health-related quality of life (HRQoL) is a multidimensional, self-reported measure of the disease impact on the patients’ lives. Improving the HRQoL is the aim of care in chronic diseases, especially PD in which HRQoL is determined by motor, non-motor symptoms (NMSs), and other social factors (2).
The NMSs consist of autonomic dysfunction, sensory symptoms, neuropsychiatric disturbances, sleep problems, fatigue, and gastrointestinal (GIT) disorders. Their impact on HRQoL was reported to be greater than the motor symptoms of PD (3). However, the prevalence of NMSs and their influence on HRQoL in PD patients had been shown to vary among different countries and cultures (4–8). For example, constipation and cognitive deficit were highly prevalent in Asian (especially Chinese) patients (8), compared with Western countries. Moreover, another study showed that bodily discomfort and stigma were the most impaired HRQoL domains among Chinese PD patients, in contrast to other populations (9). These differences could be attributed to several demographic, genetic, and clinical variations in PD patients (10). Moreover, different NMS profiles were identified, resulting in the proposal of NMS subtypes of PD (11). Therefore, identifying the prevalent NMS subtype in various settings will help to personalize the management of PD patients (12).
Despite the high prevalence and genetic variability of PD in Egyptian and Arab populations (13–15), only few studies investigated the NMSs profile in Egyptian PD patients and none—to the best of our knowledge—explored their value as predictors of HRQoL (16, 17). The prevalence of PD among the Egyptian population is the highest compared with other Arabic countries, which may be due to genetic or environmental factors (14, 18). Providing a culturally adapted Arabic version of the 39-item Parkinson’s disease questionnaire (PDQ-39) would facilitate epidemiological studies and improve patients’ care.
Therefore, this study aimed to explore the NMS and HRQoL profiles and their correlations in Egyptian PD patients. Furthermore, it provides a culturally adapted Arabic version of the PDQ-39 scale.
Materials and Methods
Patients
Ninety-seven patients diagnosed with idiopathic PD were recruited from the movement disorders outpatient clinic at Ain Shams University Hospitals (Cairo, Egypt) between 2013 and 2017. The recruited patients were diagnosed according to the British Parkinson’s disease Society Brain Bank criteria (19). Patients with atypical or secondary Parkinsonism and those with other comorbid chronic diseases that could affect PD NMSs and/or HRQoL were excluded. Moreover, PD patients who refused or could not complete questionnaires, e.g., due to severe cognitive impairment or being on antidepressants were excluded. The Ethical Committee at the Faculty of Medicine, Ain Shams University approved the study protocol. All subjects gave written informed consent according to the Declaration of Helsinki (1975, as revised in 2008).
Outcome Measurement
All subjects were evaluated using the unified Parkinson’s disease rating scale (UPDRS), Hoehn and Yahr scale (H&Y), and Schwab and England scale (S&E) in medication “off” and “on” states by a movement disorders expert. Different UPDRS subscales were estimated, including cognition (UPDRS-I), activities of daily living (ADL, UPDRS-II), motor (UPDRS-III), and total UPDRS scores. Moreover, depression was assessed using the Beck depression inventory (BDI) (20).
The NMSs were assessed in all patients, using the non-motor symptoms scale (NMSS) (21), which consists of 30 items, grouped in nine domains [cardiovascular (CVS), sleep/fatigue, mood/cognition, perception/hallucinations, memory/attention, GIT, urinary, sexual, and miscellaneous symptoms]. The frequency of each NMS (item) was calculated (item score ≥ 1), and the summary index for each domain was estimated (the sum of included items divided by the maximum possible score then multiplied by 100) to allow crude comparisons between the severity of different domains.
The HRQoL of PD patients was measured using a culturally adapted Arabic version of the Parkinson’s disease questionnaire (PDQ-39) (22), which is formed of 39 items (ranged from 0 = never to 4 = always) that are grouped into eight dimensions. These dimensions include mobility (1–10, 10 items), ADL (11–16, 6 items), emotional well-being (17–22, 6 items), stigma (23–26, 4 items), social support (27–29, 3 items), cognition (30–33, 4 items), communication (34–36, 3 items), and bodily discomfort (37–39, 3 items). Following answering the questionnaire, a summary index for each dimension (subscales) was calculated by dividing the sum of included items by the maximum possible score then multiplying by 100. Then, the total score [PDQ-39 summary index (PDQ-39 SI)] was calculated by summation of the eight dimensions’ scores divided by 8. Consequently, lower scores reflect better HRQoL (23). The frequency of impairment of each dimension to any degree (score > 0) was also estimated.
PDQ-39 Cultural (Same Language) Adaptation
After a written agreement from the scale provider (Isis outcomes), the Arabic version of PDQ-39 (Tunisia) was culturally adapted and changed to an Egyptian version to avoid some words that were not familiar with the Egyptian culture. According to the standard methodology (24), two Egyptian native speakers reviewed the Tunisian Arabic version and identified terms that were unfamiliar separately. The comments of the two reviewers were revised by a third Egyptian individual to confirm their acceptability and cultural relevance. Finally, back-translation of revised items from the Egyptian Arabic version to English was performed to ensure accuracy, followed by a cognitive debriefing with 10 Egyptian patients to confirm that the new version is well understood.
Statistical Analyses
Statistical analyses were performed using the SPSS (version 23 for windows). Categorical data, such as the frequency of NMSs, were described using frequencies and percentages, while continuous data were expressed as mean and standard deviation (SD) values. The comparison between two independent variables was made using the independent t-test, while the Spearman’s rank correlation coefficient was used to evaluate the association between PDQ-39 dimensions, NMSS domains, and other variables. Moreover, we used the multiple linear regression analysis to determine the predictors of PDQ-SI. The level of statistical significance (p-value) was set at 0.05.
Results
Clinical and Demographic Characteristics of PD Patients
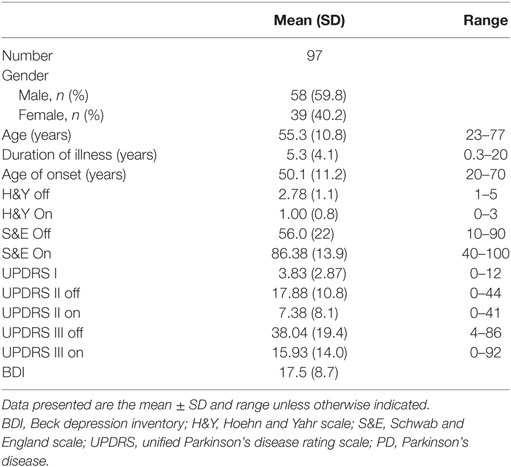
Ninety-seven PD [58 males (59.8%) and 39 females (40.2%)] patients were enrolled in this cross-sectional study (mean age at enrollment: 55.3 ± 10.8 years and mean age of disease onset: 50.1 ± 11.2 years). The mean disease staging in the Off state (H&Y Off scale) was 2.8 ± 1.1 (stage 1: 6.4%; 1.5: 12.8%; 2: 13.8%, 2.5: 11.7; 3: 34%; 4: 12.8%; 5: 8.5%), while the mean motor severity in the Off state (UPDRS III Off) was 38.04 ± 19.37. The prevalence of depression was 76.7% in the enrolled cohort (Table 1). Females had younger age at study enrollment (p = 0.007) and at disease onset (p = 0.004), as well as worse S&E Off scores (p = 0.01); however, males and females had comparable disease duration, staging, and UPDRS scores.
NMSs Prevalence and Correlations
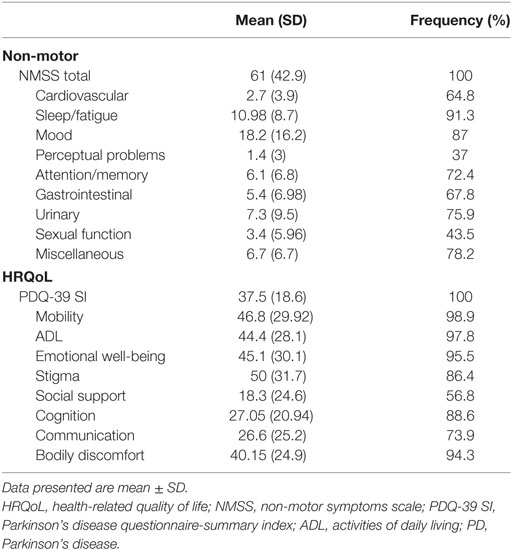
All patients (100%) suffered from one or more NMSs. The most common and severely affected domains were sleep/fatigue (91.3%), mood/cognition (87%), miscellaneous symptoms (78.2%), urinary symptoms (75.9%), and memory/attention impairment (72.4%) (Table 2). Fatigue was the most frequent NMS (81.8%), followed by mood symptoms (sadness 75%, nervousness 69.6%, and lack of motivation 68.5%), forgetfulness (65.5%), and urinary symptoms (nocturia and urgency 58.6%) (Figure 1). No gender differences were detected except that females had worse miscellaneous domain scores (p = 0.018, females experienced more pain and sweating) and higher BDI scores (p = 0.012).
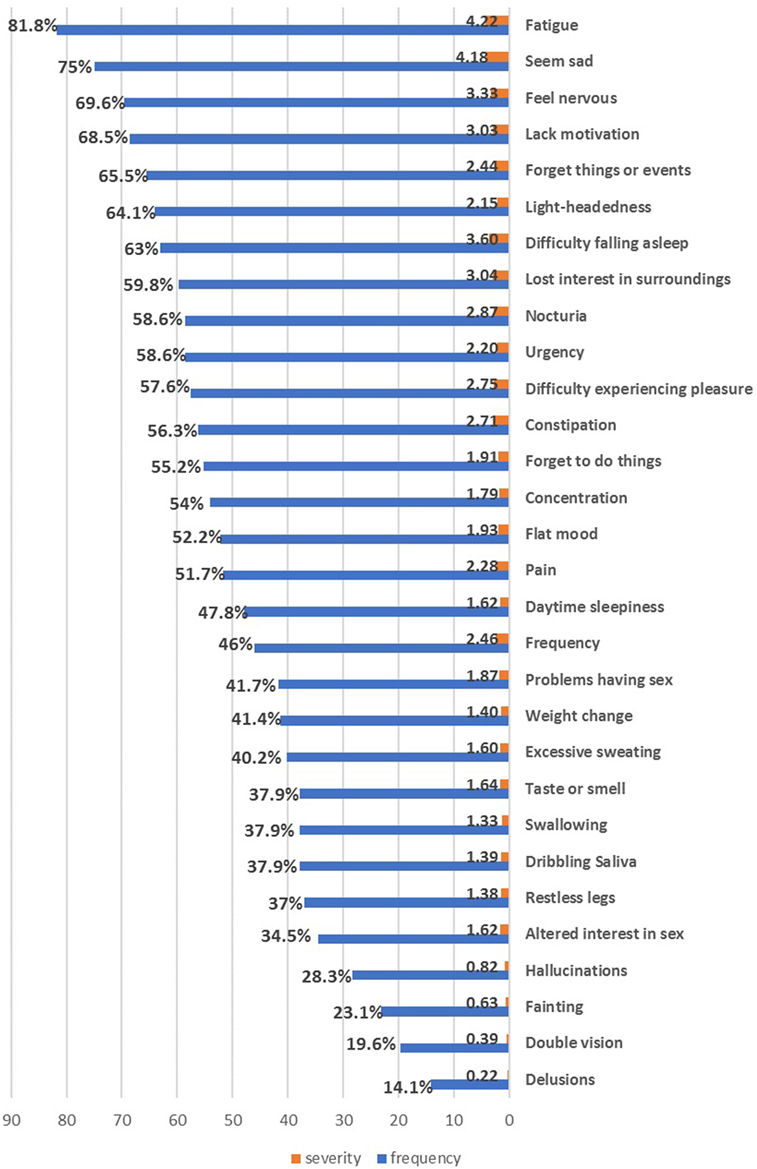
Figure 1. The frequency and severity of individual non-motor symptoms in the enrolled Parkinson’s disease patients.
The total NMSS score was significantly correlated with S&E Off, UPDRS I, UPDRS II (Off & On), UPDRS III (Off & On), and BDI scores, but not with age, duration of the disease, or H&Y staging (r = 0.204, p = 0.06). Most of NMSs domains (except sexual and urinary domains) were correlated with UPDRS-I and II Off scores (Table A in Supplementary Material).
Quality of Life State and Correlations
All enrolled PD patients had impaired HRQoL with comparable values (scores between 40 and 50) and frequency (88.6–98.9%) in most dimensions, except for social support, cognition, and communication (had lower scores, indicating better HRQoL aspects) (Table 2). About 86% of PD patients felt stigmatized to some degree, while 56.8% of the cohort reported incomplete social support. Females showed significantly more impaired (higher values) PDQ-SI, mobility, ADL, and bodily discomfort scores (p = 0.016, 0.001, 0.003, and 0.047, respectively).
The PDQ-SI score was inversely correlated with the patients’ age at enrollment (r = −0.220, p = 0.04), age of disease onset (r = −0.272, p = 0.01) and S&E daily activity scores (Off & On scores) (r = −0.483 and −0.361, p < 0.01, and 0.01, respectively) (Table B in Supplementary Material). This means that younger age at enrollment or disease onset and less independence in daily activity were associated with more impaired HRQoL. In addition, the PDQ-SI was positively correlated with female gender, H&Y (Off and On) disease stage (r = 0.318 and 0.298, p = 0.003 and 0.006, respectively), UPDRS-I and UPDRS-II Off (p < 0.001), UPDRS-III (Off and On) scores (Table 3). Thus, HRQoL impairment was associated with more impaired cognition, daily activity, and “Off and On” motor features. The disease duration did not correlate with PDQ-SI; however, it was correlated with ADL and Stigma dimensions (r = 0.226, 0.229; p = 0.030, 0.036, respectively).
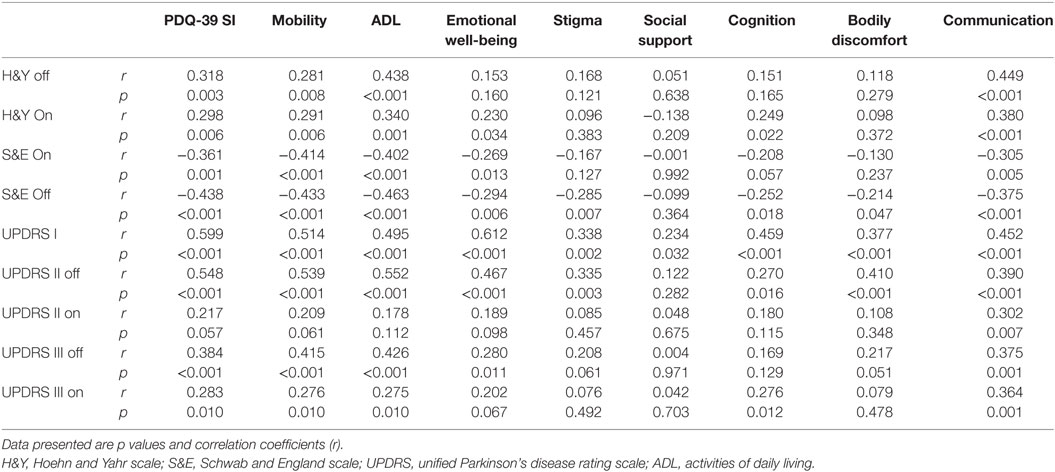
Table 3. Correlations between quality of life domains and disease stage, patient disability and UPDRS subscores.
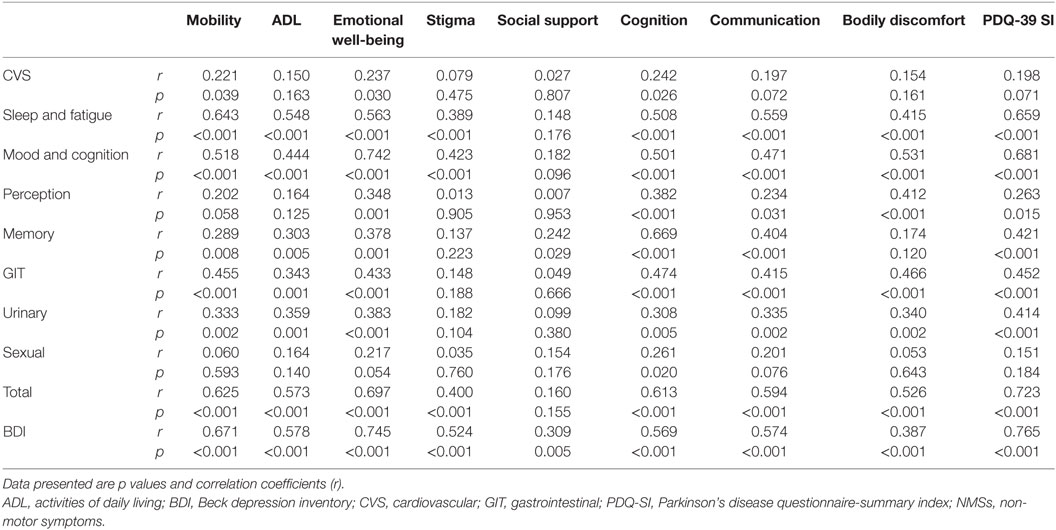
Moreover, PDQ-SI was strongly correlated to the total and most of NMS subscales’ scores (except CVS and sexual domains) and BDI score (p < 0.001). The total NMSS score was strongly correlated to PDQ-39 dimensions (p < 0.001), except social support. Likewise, most of the PDQ-39 dimensions had a significant correlation with most of NMSS domains, except social support (Table 4). In summary, the more severe and frequent NMSs are, the more impaired HRQoL becomes in PD patients.
Multiple linear regression analysis was performed to determine the predictors of the PDQ-SI from different variables including age at enrollment and disease onset, gender, disease duration, UPDDRS I, II, III, BDI, H&Y, S&E, and total NMSS scores. Depression (BDI) and the total NMSS score explained 50.5% of the PDQ-SI. Depression was the primary predictor (adjusted R2 0.497, p < 0.0001). On removing BDI score from the model, the total NMSS score and UPDRS-I (cognition) were the independent predictors of HRQoL and explained 53.7% of patients’ HRQoL. The total NMSS score was the primary predictor (adjusted R2: 0.480, p < 0.0001).
Discussion
This study explored the non-motor and HRQoL profiles of PD patients in an African country with a high prevalence of PD. It confirmed the marked impact of NMSs and depression on HRQoL impairment. Moreover, it provides a culturally adapted Arabic version of the PDQ-39 to promote further research and care of Arabic PD patients. To the best of our knowledge, this is the first study to investigate the HRQoL and its association with NMSs in Egyptian PD patients.
Our study showed a high prevalence of NMSs in Egyptian PD patients (100%). Fatigue, mood disturbances (sadness, nervousness, and lack of motivation), and memory impairment were the most frequent NMSs. Likewise, the most severe and frequently affected domains were sleep/fatigue and mood/cognition domains. Gender differences were minimal and restricted to miscellaneous symptoms. A similar high prevalence of NMSs and frequency of NMS domains were reported in Upper Egypt by Khedr and colleagues (16). This NMS profile in the Egyptian patients is more consistent with the depression/anxiety PD subtype (11).
Previous studies have identified differences in NMS profile between different countries and races. Reports from different Asian countries identified GIT symptoms, especially constipation as the most frequent NMS (8). Nocturia was the most frequent NMS, followed by fatigue and dribbling of saliva in a multicenter European study (3). Cognition, sleep followed by urinary symptoms were the most common NMS in an Estonian cohort (25), while fatigue followed by urinary symptoms were more common in the Spanish population (26). These differences could be attributed to several demographic, genetic, and clinical variations in enrolled patients (age, the age of onset, disease duration, and severity), as well as different assessment questionnaires (10, 12).
The different dimensions of HRQoL were impaired in all patients and markedly correlated to NMSS total score and depression severity. Moreover, it was associated with motor severity, disease staging, and impaired daily activity in Off and On states. However, regression analysis confirmed the independent predicting effect of NMSs for HRQoL. This is consistent with the findings of former studies in different populations in which the total NMSS score was the main predictor of QoL. Mood/cognition and sleep/fatigue had the strongest correlation with PDQ-SI in accordance with prior studies (3, 7, 27).
Neuropsychiatric symptoms, especially depression, were reported as independent determinants of HRQoL (28). In consistence with previous studies (25, 26, 29), depression was recognized as the main independent predictor of HRQoL. This could be explained by the high prevalence of depression (76.7%) and the strong correlation between depression and all HRQoL dimensions, several NMS domains and total NMSS scores. Consequently, depression affects HRQoL directly and indirectly through its impact on the NMSs, especially emotional well-being and cognition (28). In agreement with several previous studies, depression was recognized as the main predictor of poor HRQoL in PD patients (29, 30). Antonini et al. reported improvement in mobility and ADL after depression treatment, indicating that mood problems affect how PD patients perceive their motor functions (31).
Intriguingly, 86.4% of PD patients felt stigmatized, while 56.8% of the cohort reported incomplete social support. Stigma was associated with younger age of enrollment, younger age of disease onset, disease severity, impaired daily activities and cognition, as well as depression. This is consistent with prior studies (32, 33). Furthermore, it was correlated with fatigue/memory, mood/cognition domains, and total NMSS scores. Stigma is an important determinant of HRQoL, related to cultural and social factors (32, 34). Consequently, the identification and management of this feature are crucial for the improvement of HRQoL of PD patients.
Although this study provides a comprehensive assessment of the impact of different NMS domains on HRQoL for the first time in Egyptian patients, it has some limitations. The relatively small sample size and the fact that the majority of enrolled patients came from a low socioeconomic class restrict the generalizability of our findings. Moreover, as a clinic-based study [plus the relatively short disease duration (5.3 years)], patients with advanced stages and cognitive impairment were under-represented. Furthermore, some NMSs are prevalent in the elderly population without PD; therefore, a case–control study would elucidate whether the investigated NMSs are PD related.
In conclusion, this study demonstrated the high prevalence of NMSs in Egyptian PD patients and the significant impact of NMSs and depression on HRQoL. Thus, the assessment and management of NMSs are as important as the motor aspects of PD. Future larger studies can use our culturally adapted questionnaire to evaluate the correlation between NMSs domains and HRQoL dimensions in patients with advanced PD.
Ethics Statement
The ethical committee of the faculty of medicine, Ain Shams University has approved this study. The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national and institutional committees on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008. All subjects gave written informed consent according to the Declaration of Helsinki.
Author Contributions
All authors made substantial contributions, revised the manuscript, and approved the final version. AS, HE, SA, and ME: conception and design of the study. AS, AB, MH, MK, and EH: data collection and analysis. AS, AB, AA, and HE: first draft writing. All authors: revision of the manuscript.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank Ms Enas Shalash, MSc in written and simultaneous translation, for her help in the cultural adaptation of the PDQ-39.
Supplementary Material
The Supplementary Material for this article can be found online at https://www.frontiersin.org/articles/10.3389/fneur.2018.00357/full#supplementary-material.
Abbreviations
ADL, activities of daily living; BDI, Beck depression inventory; HRQoL, health-related quality of life; H&Y, Hoehn and Yahr scale; NMSs, non-motor symptoms; NMSS, non-motor symptoms scale; PD, Parkinson’s disease; PDQ-SI, Parkinson’s disease questionnaire-summary index; S&E, Schwab and England scale; UPDRS, unified Parkinson’s disease rating scale.
References
1. Hirsch L, Jette N, Frolkis A, Steeves T, Pringsheim T. The incidence of Parkinson’s disease: a systematic review and meta-analysis. Neuroepidemiology (2016) 46:292–300. doi:10.1159/000445751
2. Martinez-Martin P. What is quality of life and how do we measure it? Relevance to Parkinson’s disease and movement disorders. Mov Disord (2017) 32:382–92. doi:10.1002/mds.26885
3. Martinez-Martin P, Rodriguez-Blazquez C, Kurtis MM, Chaudhuri KR. The impact of non-motor symptoms on health-related quality of life of patients with Parkinson’s disease. Mov Disord (2011) 26:399–406. doi:10.1002/mds.23462
4. Barone P, Antonini A, Colosimo C, Marconi R, Morgante L, Avarello TP, et al. The PRIAMO study: a multicenter assessment of nonmotor symptoms and their impact on quality of life in Parkinson’s disease. Mov Disord (2009) 24:1641–9. doi:10.1002/mds.22643
5. Dotchin CL, Jusabani A, Walker RW. Non-motor symptoms in a prevalent population with Parkinson’s disease in Tanzania. Parkinsonism Relat Disord (2009) 15:457–60. doi:10.1016/j.parkreldis.2008.11.013
6. Leonardi M, Raggi A, Pagani M, Carella F, Soliveri P, Albanese A, et al. Relationships between disability, quality of life and prevalence of nonmotor symptoms in Parkinson’s disease. Parkinsonism Relat Disord (2012) 18:35–9. doi:10.1016/j.parkreldis.2011.08.011
7. Song W, Guo X, Chen K, Chen X, Cao B, Wei Q, et al. The impact of non-motor symptoms on the health-related quality of life of Parkinson’s disease patients from Southwest China. Parkinsonism Relat Disord (2014) 20:149–52. doi:10.1016/j.parkreldis.2013.10.005
8. Yu RL, Wu RM, Chan AY, Mok V, Wu YR, Tilley BC, et al. Cross-cultural differences of the non-motor symptoms studied by the traditional Chinese version of the International Parkinson and Movement Disorder Society-Unified Parkinson’s Disease Rating Scale. Mov Disord Clin Pract (2017) 4:68–77. doi:10.1002/mdc3.12349
9. Zhao YJ, Tan LC, Lau PN, Au WL, Li SC, Luo N. Factors affecting health-related quality of life amongst Asian patients with Parkinson’s disease. Eur J Neurol (2008) 15:737–42. doi:10.1111/j.1468-1331.2008.02178.x
10. Konno T, Al-Shaikh RH, Deutschlander AB, Uitti RJ. Biomarkers of nonmotor symptoms in Parkinson’s disease. Int Rev Neurobiol (2017) 133:259–89. doi:10.1016/bs.irn.2017.05.020
11. Sauerbier A, Jenner P, Todorova A, Chaudhuri KR. Non motor subtypes and Parkinson’s disease. Parkinsonism Relat Disord (2016) 22(Suppl 1):S41–6. doi:10.1016/j.parkreldis.2015.09.027
12. Titova N, Chaudhuri KR. Personalized medicine and nonmotor symptoms in Parkinson’s disease. Int Rev Neurobiol (2017) 134:1257–81. doi:10.1016/bs.irn.2017.05.015
13. Al-Kurdi AA, Mubaidin AM, Bohlega S. Problems of Parkinson’s disease in the Arab world. Neurosciences (Riyadh) (2001) 6:188–91.
14. Lesage S, Dürr A, Tazir M, Lohmann E, Leutenegger A-L, Janin S, et al. LRRK2 G2019S as a cause of Parkinson’s disease in North African Arabs. N Engl J Med (2006) 354:422–3. doi:10.1056/NEJMc055540
15. Benamer HT, De Silva R, Siddiqui KA, Grosset DG. Parkinson’s disease in Arabs: a systematic review. Mov Disord (2008) 23:1205–10. doi:10.1002/mds.22041
16. Khedr EM, El Fetoh NA, Khalifa H, Ahmed MA, El Beh KM. Prevalence of non motor features in a cohort of Parkinson’s disease patients. Clin Neurol Neurosurg (2013) 115:673–7. doi:10.1016/j.clineuro.2012.07.032
17. Khedr EM, Fawi G, Abbas MA, Mohammed TA, El-Fetoh NA, Attar GA, et al. Prevalence of parkinsonism and Parkinson’s disease in Qena governorate/Egypt: a cross-sectional community-based survey. Neurol Res (2015) 37:607–18. doi:10.1179/1743132815Y.0000000020
18. Ahmed H, Abushouk AI, Gabr M, Negida A, Abdel-Daim MM. Parkinson’s disease and pesticides: a meta-analysis of disease connection and genetic alterations. Biomed Pharmacother (2017) 90:638–49. doi:10.1016/j.biopha.2017.03.100
19. Hughes AJ, Daniel SE, Kilford L, Lees AJ. Accuracy of clinical diagnosis of idiopathic Parkinson’s disease: a clinico-pathological study of 100 cases. J Neurol Neurosurg Psychiatry (1992) 55:181–4. doi:10.1136/jnnp.55.3.181
20. Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J. An inventory for measuring depression. Arch Gen Psychiatry (1961) 4:561–71. doi:10.1001/archpsyc.1961.01710120031004
21. Chaudhuri KR, Martinez-Martin P, Brown RG, Sethi K, Stocchi F, Odin P, et al. The metric properties of a novel non-motor symptoms scale for Parkinson’s disease: results from an international pilot study. Mov Disord (2007) 22:1901–11. doi:10.1002/mds.21596
22. Peto V, Jenkinson C, Fitzpatrick R. PDQ-39: a review of the development, validation and application of a Parkinson’s disease quality of life questionnaire and its associated measures. J Neurol (1998) 245(Suppl 1):S10–4. doi:10.1007/PL00007730
23. Martinez-Martin P, Jeukens-Visser M, Lyons KE, Rodriguez-Blazquez C, Selai C, Siderowf A, et al. Health-related quality-of-life scales in Parkinson’s disease: critique and recommendations. Mov Disord (2011) 26:2371–80. doi:10.1002/mds.23834
24. Wild D, Grove A, Martin M, Eremenco S, Mcelroy S, Verjee-Lorenz A, et al. Principles of Good practice for the translation and cultural adaptation process for patient-reported outcomes (PRO) measures: report of the ISPOR Task Force for Translation and Cultural Adaptation. Value Health (2005) 8:94–104. doi:10.1111/j.1524-4733.2005.04054.x
25. Kadastik-Eerme L, Rosenthal M, Paju T, Muldmaa M, Taba P. Health-related quality of life in Parkinson’s disease: a cross-sectional study focusing on non-motor symptoms. Health Qual Life Outcomes (2015) 13:83. doi:10.1186/s12955-015-0281-x
26. Santos-Garcia D, de la Fuente-Fernandez R. Impact of non-motor symptoms on the quality of life of patients with Parkinson’s disease: some questions beyond research findings. J Neurol Sci (2013) 335:239. doi:10.1016/j.jns.2013.09.019
27. Scaravilli T, Gasparoli E, Rinaldi F, Polesello G, Bracco F. Health-related quality of life and sleep disorders in Parkinson’s disease. Neurol Sci (2003) 24:209–10. doi:10.1007/s10072-003-0134-y
28. Balestrino R, Martinez-Martin P. Neuropsychiatric symptoms, behavioural disorders, and quality of life in Parkinson’s disease. J Neurol Sci (2017) 373:173–8. doi:10.1016/j.jns.2016.12.060
29. Gallagher DA, Lees AJ, Schrag A. What are the most important nonmotor symptoms in patients with Parkinson’s disease and are we missing them? Mov Disord (2010) 25:2493–500. doi:10.1002/mds.23394
30. Sławek J, Derejko M, Lass P. Factors affecting the quality of life of patients with idiopathic Parkinson’s disease – a crosssectional study in an outpatient clinic attendees. Parkinsonism Relat Disord (2005) 11:465–8. doi:10.1016/j.parkreldis.2005.04.006
31. Antonini A, Tesei S, Zecchinelli A, Barone P, De Gaspari D, Canesi M, et al. Randomized study of sertraline and low-dose amitriptyline in patients with Parkinson’s disease and depression: effect on quality of life. Mov Disord (2006) 21:1119–22. doi:10.1002/mds.20895
32. Ma HI, Saint-Hilaire M, Thomas CA, Tickle-Degnen L. Stigma as a key determinant of health-related quality of life in Parkinson’s disease. Qual Life Res (2016) 25:3037–45. doi:10.1007/s11136-016-1329-z
33. Tu XJ, Hwang WJ, Ma HI, Chang LH, Hsu SP. Determinants of generic and specific health-related quality of life in patients with Parkinson’s disease. PLoS One (2017) 12:e0178896. doi:10.1371/journal.pone.0178896
Keywords: 39-item Parkinson’s disease questionnaire, Arabic, Egypt, non-motor, Parkinson’s disease, quality of life
Citation: Shalash AS, Hamid E, Elrassas HH, Bedair AS, Abushouk AI, Khamis M, Hashim M, Ahmed NS, Ashour S and Elbalkimy M (2018) Non-Motor Symptoms as Predictors of Quality of Life in Egyptian Patients With Parkinson’s Disease: A Cross-Sectional Study Using a Culturally Adapted 39-Item Parkinson’s Disease Questionnaire. Front. Neurol. 9:357. doi: 10.3389/fneur.2018.00357
Received: 22 February 2018; Accepted: 02 May 2018;
Published: 24 May 2018
Edited by:
Stefania Mondello, Università degli Studi di Messina, ItalyReviewed by:
Rodolfo Gabriel Gatto, University of Illinois at Chicago, United StatesE. Lezi, Duke University, United States
Copyright: © 2018 Shalash, Hamid, Elrassas, Bedair, Abushouk, Khamis, Hashim, Ahmed, Ashour and Elbalkimy. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ali S. Shalash, YWxpX25ldXJvQHlhaG9vLmNvbQ==, ZHJhbGlfc2hhbGFzaEBtZWQuYXN1LmVkdS5lZw==
 Ali S. Shalash
Ali S. Shalash Eman Hamid1
Eman Hamid1 Hanan Hani Elrassas
Hanan Hani Elrassas Ahmed Safwat Bedair
Ahmed Safwat Bedair Abdelrahman Ibrahim Abushouk
Abdelrahman Ibrahim Abushouk Samia Ashour
Samia Ashour