- Department of Neurology, Nanfang Hospital, Southern Medical University, Guangzhou, China
Background and Purpose: This study aims to explore the cause and predictive value of hyperchloremia in critically ill stroke patients.
Materials and Methods: We conducted a retrospective study of a prospectively collected database of adult patients with first-ever acute ischemic stroke (AIS) or intracerebral hemorrhage (ICH) admitted to the neurointensive care unit (NICU) of a university-affiliated hospital, between January 2013 and December 2016. Patients were excluded if admitted beyond 72 h from onset, if they required neurocritical care for less than 72 h, and were treated with hypertonic saline within 72 h or had creatinine clearance less than 15 mL/min.
Results: Of 405 eligible patients, the prevalence of hyperchloremia ([Cl−] ≥ 110 mmol/L) was 8.6% at NICU admission ([Cl−]0) and 17.0% within 72 h ([Cl−]max). Thirty-eight (9.4%) patients had new-onset hyperchloremia and 110 (27.1%) had moderate increase in chloride (Δ[Cl−] ≥ 5 mmol/L; Δ[Cl−] = [Cl−]max − [Cl−]0) in the first 72 h after admission, which were found to be determined by the sequential organ failure assessment score in multivariate logistic regression analysis. Neither total fluid input nor cumulative fluid balance had significant association with such chloride disturbance. New-onset hyperchloremia and every 5 mmol/L increment in Δ[Cl−] were both associated with increased odds of 30-day mortality and 6-month poor outcome, although no independent significance was found in multivariate models.
Conclusion: Hyperchloremia tends to occur in patients more severely affected by AIS and ICH. Although no independent association was found, new-onset hyperchloremia and every 5 mmol/L increment in Δ[Cl−] were related to poorer outcome in critically ill AIS and ICH patients.
Subject terms: clinical studies, intracranial hemorrhage, ischemic stroke, mortality/survival, quality and outcomes.
Introduction
Critically ill acute ischemic stroke (AIS) and intracerebral hemorrhage (ICH) patients account for most admissions to the neurointensive care unit (NICU) (1). These diseases heavily burden the family and society, making treatment and outcome prediction particularly important. Fluid management aiming at maintaining adequate cerebral blood flow and oxygenation plays a crucial role in the management of these patients (2). Because of the propensity to cause brain edema by hypo-osmolar balanced crystalloids, hypertonic saline or normal saline (0.9% saline solution) are more frequently used in brain-injured patients (3). However, concerns have been raised that both hypertonic saline (4, 5) and large-volume infusion of normal saline (6, 7) may raise the risk of hyperchloremia.
Hyperchloremia has been reported to be associated with increased hospital mortality and negative outcome in critically ill patients (8), with severe sepsis and septic shock (9, 10), as well as after surgery and trauma (11, 12). In patients with ICH, a recent study demonstrated higher rates of in-hospital mortality in those who developed moderate hyperchloremia during treatment with continuous intravenous infusion of 3% hypertonic saline, with moderate hyperchloremia independently predicting in-hospital mortality (13). Another study in patients with subarachnoid hemorrhage found a strong association between hyperchloremia and acute kidney injury (AKI) as well as AKI and mortality (14). However, whether routinely used normal saline raises the risk of hyperchloremia in critically ill stroke patients remains largely unknown. Besides, the cause and predictive value of hyperchloremia in these patients requires further evaluation.
In this study, we aimed to identify risk factors that related to the development of hyperchloremia in critically ill patients with AIS and ICH, with particular focus to the amount of normal saline infusion. The influence of hyperchloremia and chloride fluctuation on patient outcome was evaluated as well.
Materials and Methods
Study Design and Participants
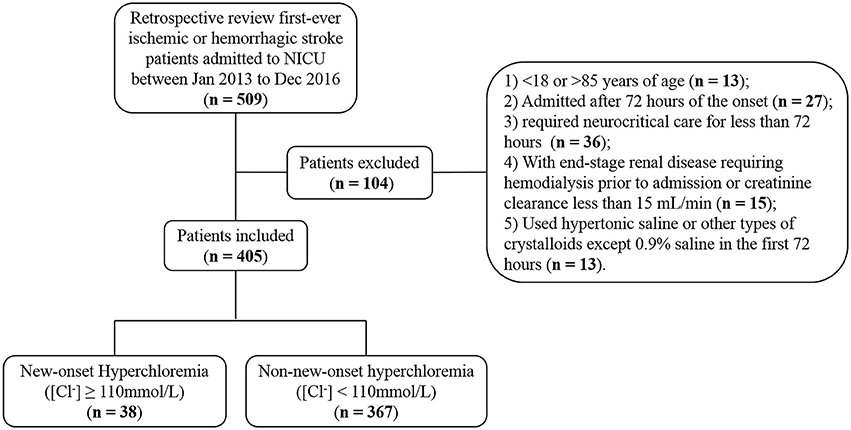
The data that support the findings of this study are available from the corresponding author upon reasonable request. We conducted a retrospective study of a prospectively collected database of adult patients with first-ever AIS or ICH admitted to the NICU of Nanfang Hospital, a university-affiliated academic hospital, between January 2013 and December 2016 (Figure 1). Patients were excluded if they were younger than 18 or older than 85, admitted beyond 72 h of the onset, required neurocritical care for less than 72 h, had premorbid disability [modified Rankin Scale (mRS) > 1] or end-stage renal disease requiring hemodialysis or creatinine clearance less than 15 mL/min. To explore the association between normal saline infusion and hyperchloremia, we also excluded patients who received hypertonic saline (3% or 10%) or other types of crystalloids except 0.9% saline during the first 72 h of NICU admission. The study proposal was approved by the hospital's ethics committee for clinical research. Informed consent was waived by the review board, because this study was observational, retrospective, and all data were fully de-identified.
Study Variables
Electronic medical records were carefully reviewed to collect patient demographics, previous medical history, laboratory values, and ICD-10-based final diagnoses. Since the concentration of serum chloride ([Cl−]) shifts constantly, we defined the [Cl−] at the time of NICU admission as [Cl−]0 (baseline chloride) and the following maximum [Cl−] in the first 72 h as [Cl−]max. Hyperchloremia was defined as [Cl−] ≥ 110 mmol/L (9). The increase in serum chloride (Δ[Cl−] = [Cl−]max − [Cl−]0) was calculated, and Δ[Cl−] ≥ 5 mmol/L was regarded as a moderate increase in chloride (8).
Glasgow Coma Scale (GCS) scores were extracted from the first neurological examination at NICU admission. The total score of Sequential Organ Failure Assessment (SOFA) (15) was obtained according to its corresponding parameters in the first 24 h of NICU admission. The requirement of vasopressor agents and mechanical ventilation within 24 h of NICU admission were also recorded. Total fluid input within the first 72 h was measured, and cumulative fluid balance (CFB) was calculated based on total fluid input minus output during this period. In order to explore the correlation between hyperchloremia and AKI, we used the kidney disease improving global outcomes (KDIGO) as diagnostic criteria of AKI (16).
Study Outcomes
Primary end points were 30-day mortality and 6-month poor outcome, with the latter defined as mRS of 4–6. Information on survival and functional outcome were obtained through telephone review by a trained neurologist blinded to the study data.
Statistical Analysis
Categorical variables were presented as number (%) and were compared using the two-sided chi-square test or Fisher's exact test. Continuous data were presented as mean ± standard deviation (SD) or median [25–75% interquartile range (IQR)] and compared by Student's t-test or Mann–Whitney U test, as appropriate.
To explore risk factors associated with the development of hyperchloremia, patients with normal [Cl−] at the time of NICU admission but who developed hyperchloremia in the first 72 h of NICU stay were defined as new-onset hyperchloremia. The whole study sample was then divided into the new-onset hyperchloremia and non-new-onset hyperchloremia subgroups. Univariate analysis was first performed, and candidate variables that had a p-value less than 0.05 were drawn into multivariate logistic regression model. Candidate variables included demographic data (age, gender), comorbidity (baseline creatinine, diabetes, hypertension, heart failure), indicators of critical illness (AKI, NIHSS, GCS, SOFA, base deficit, vasopressors, mechanical ventilation), and fluid management (total fluid input and CFB in the first 72 h) (9). Only one of the two variables was included in the event of collinearity between variables. The 95% confident intervals (CIs) reported for the logistic regression odds ratios (ORs) were calculated by the Wald estimation. The risk factors of moderate increase in chloride (Δ[Cl−] ≥ 5 mmol/L) were evaluated using similar statistical methods as well.
To evaluate the influence of hyperchloremia on patient outcome, the associations between 30-day mortality or 6-month poor outcome (dependent variables) and (1) [Cl−] at the time of NICU admission ([Cl−]0), (2) maximum [Cl−] at the first 72 h of NICU stay [Cl−]max), (3) Δ[Cl−] (Δ[Cl−] = [Cl−]max − [Cl−]0), (4) new-onset hyperchloremia were examined using logistic regression analysis. The associations between patient outcomes and the independent variables of interest ([Cl−]0, [Cl−]max, Δ[Cl−] and new-onset hyperchloremia) were further examined, separately, in multivariate logistic regression models that adjusted for confounders known to be associated with hospital mortality, as described in the above section. All analyses were performed using SPSS, version 23.0 (SPSS, Chicago, IL). A two-sided p-value less than 0.05 was considered to be statistically significant.
Results
Of 509 patients screened for eligibility, 405 satisfied inclusion and exclusion criteria (Figure 1). Of those, 250 (61.7%) patients were diagnosed with AIS and 155 (38.3%) were ICH. There were 35 (8.6%) patients with hyperchloremia at the time of NICU admission ([Cl−]0 ≥ 110 mmol/L), and the number increased to 69 (17.0%) within 72 h after admission ([Cl−]max ≥ 110 mmol/L). Of note, 38 (9.4%) patients had new-onset hyperchloremia in the first 72 h after NICU admission (Figure 1). The median [Cl−] was 112 mmol/L (111−115 mmol/L) in the new-onset hyperchloremic subgroup and 104 mmol/L (102–107 mmol/L) in the non-new-onset hyperchloremic subgroup. Baseline demographics and clinical characteristics of these two subgroups were summarized in Table 1. The patients with new-onset hyperchloremia had lower GCS, higher NIHSS, and SOFA scores. Moreover, these patients required more vasopressors and mechanical ventilation when compared with those without new-onset hyperchloremia within 72 h of NICU admission. However, neither total fluid input nor CFB had significant association with new-onset hyperchloremia (Table 1).
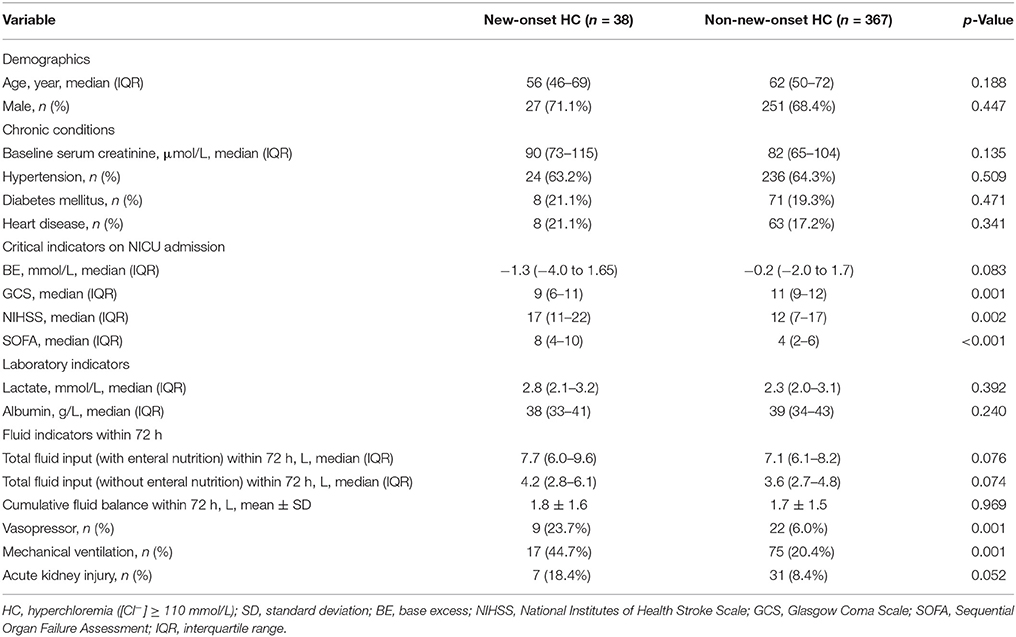
Table 1. Baseline demographics and clinical characteristics stratified by new-onset hyperchloremia ([Cl−] ≥ 110 mmol/L) or not ([Cl−] < 110 mmol/L) within 72 h of NICU admission.
Since GCS is included in SOFA and had collinearity with NIHSS, it was omitted from multivariate logistic analysis. In a multivariate model evaluating risk factors that related to the development of new-onset hyperchloremia, SOFA was identified as the only independent variable that associated with new-onset hyperchloremia (Table 2). Furthermore, SOFA and mechanical ventilation were found to be the risk factors of moderate increase in chloride (Δ[Cl−] ≥ 5 mmol/L) in the first 72 h in both univariate and multivariate logistic analysis (Supplementary Tables S1, S2).
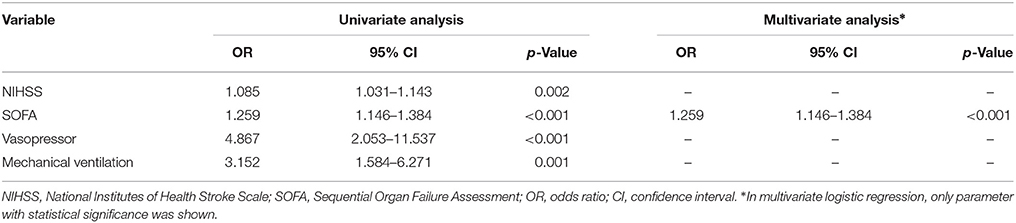
Table 2. Univariate and multivariate logistic regression analysis for risk factors of new-onset hyperchloremia ([Cl−] ≥ 110 mmol/L).
During follow up, 61 (15.1%) patients died within 30 days after admission and 236 (58.3%) patients achieved good outcome at 6 months. Results showed that patients with new-onset hyperchloremia were associated with a 158% increase in odds for 30-day mortality (OR = 2.58; 95% CI, 1.21–5.53; p = 0.015) (Table 3). In addition, each 5 mmol/L increase in [Cl−]0, [Cl−]max, and Δ[Cl−] was associated with a 50, 66, and 55% increase in odds for 30-day mortality, respectively (Table 3). However, none of these changes of chloride showed independent association with 30-day mortality in multivariate logistic analysis, while age, base excess, SOFA, and mechanical ventilation were found to be significant (Table 3). In terms of 6-month functional outcome, new-onset hyperchloremia, [Cl−]max and Δ[Cl−] were all associated with increased risk of poor outcome, with an OR (95% CI) of 3.394 (1.660–6.941), 1.425 (1.205–1.685), and 1.383 (1.143–1.674), respectively (Table 4). Nevertheless, these three indicators were not independently related to 6-month outcome in multivariate models, where age, NIHSS, and SOFA were independent risk factors of poor outcome (Table 4). The detailed statistical results were summarized in Supplementary Tables S3, S4.
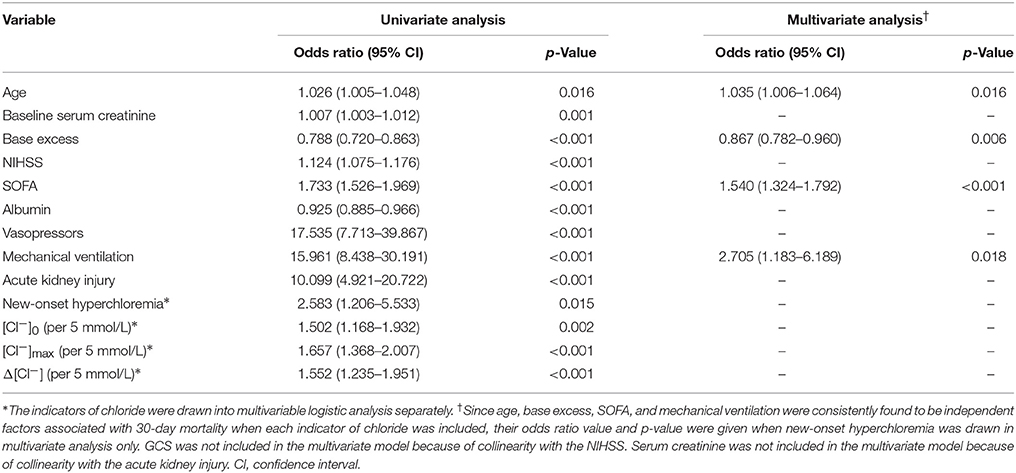
Table 3. Univariate and multivariate logistic regression analysis of risk factors associated with 30-day mortality.
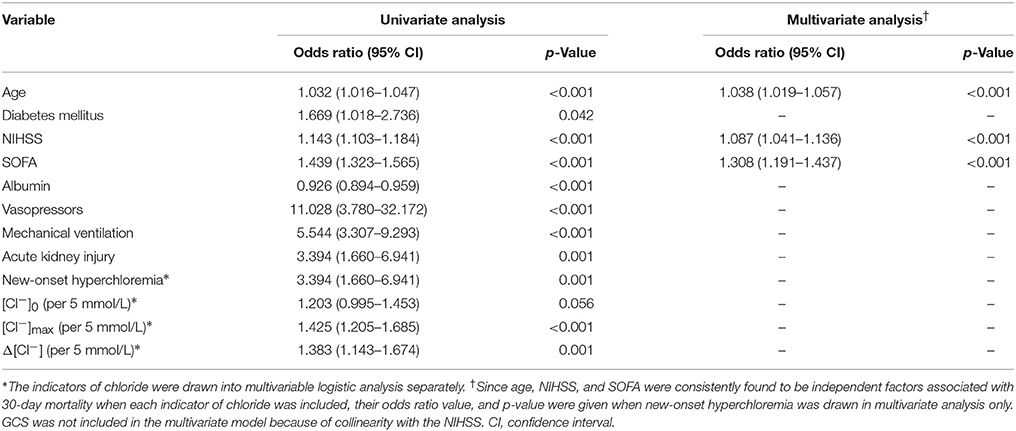
Table 4. Univariate and multivariate logistic regression analysis of risk factors associated with 6-month poor outcome (mRS ≥ 4).
Discussion
In this study, we showed that the prevalence of hyperchloremia in critically ill stroke patients increased from 8.6% at NICU admission to 17.0% in the first 72 h. The SOFA score was identified as an independent risk factor of both new-onset hyperchloremia and moderate increase in chloride (Δ[Cl−] ≥ 5 mmol/L) after NICU admission. For the infusion of normal saline, neither total fluid input nor CFB was significantly related to new-onset hyperchloremia or moderate increase in chloride. Although no independent significance was found in multivariate models, both new-onset hyperchloremia and every 5 mmol/L increment in Δ[Cl−] were associated with increased odds of 30-day mortality or 6-month poor outcome.
Chloride is the most abundant anion in the extracellular fluid, constituting approximately one-third of plasma tonicity, and plays an essential role in various body functions including the regulation of body fluids, electrolyte balance, acid-base status, muscular activity, osmosis, and immunomodulation (17). While the concentration of chloride shifts constantly during hospitalization, chloride receives much less attention than other routinely measured electrolytes until recent reports investigated the influence of hyperchloremia on hospital mortality and AKI (18).
In a retrospective study, hyperchloremia and moderate increase in serum chloride (Δ[Cl−] ≥ 5 mmol/L) were both found to be independently associated with AKI in severe sepsis and septic shock patients (10). (9) revealed that hyperchloremia at 72 h of ICU stay (Cl72) were related to all-cause hospital mortality and every 5 mmol/L increment in Cl72 or Δ[Cl−] was, respectively, associated with a 27 or 37% increase in the odds for hospital mortality in those critically ill septic patients who were already hyperchloremic on admission to the ICU. (19) conducted a respective study on 76,719 hospitalized patients and found that both serum chloride alterations on admission and post-admission serum chloride increase predicted poor outcome. Similar to these studies, we observed that both new-onset hyperchloremia or every 5 mmol/L increment in Δ[Cl−] were associated with increased odds of poor outcome in critically ill stroke patients, although these two variables did not show independent significance in multivariate models. The possible explanation could be that the prevalence of hyperchloremia in our subjects (17.0% in the first 72 h) was much lower than that in other patient groups [65.3% (13) in patients with ICH and treated with hypertonic saline; 40.8% ((10)) and 31.7% (9) in patients with septic shock].
Hyperchloremia may result from disease processes or clinical interventions (18). In the present study, we observed that the main determinant of hyperchloremia in critically ill stroke patients was the disease severity, as reflected by the SOFA score. While previous studies have shown a strong association between hyperchloremia and administration of chloride-rich fluids (18), such as 0.9% saline (20), we did not find a significant association between normal saline infusion and new-onset hyperchloremia or moderate increase in chloride. The plausible explanation was that we only observed the chloride fluctuation within the first 72 h after NICU admission. Extending the observation period might result in different findings. Besides, we have excluded patients who had end-stage renal disease requiring hemodialysis or creatinine clearance less than 15 mL/min at the screening, which may decrease the risk of chloride retention. Finally, compared with the treatment in septic shock, the infused volume of normal saline was much lower in our study subjects, which might have weakened the association between normal saline infusion and chloride fluctuation. Although other balanced crystalloid fluid such Plasma-Lyte (Na, 140 mmol/L; Cl, 98 mmol/L) has been reported to cause less hyperchloremia (21), normal saline remains to be the first choice in most clinical scenarios (22). The encouraging findings from two recent trials that lactated Ringer's solution or Plasma-Lyte A reduced hospital death or adverse kidney events in contrast to saline in critically ill (23) and non-critically ill (24) adult patients may have the potential to change current practice.
Our results should be interpreted with caution. First, the retrospective nature of this study make it susceptible to selection and information bias. Second, we were unable to assess all potentially relevant variables. Still, the candidate variables selected in the present study were mostly in line with two representative studies (9, 14). Third, to explore the correlation between normal saline infusion and chloride disturbance, we have excluded patients who were treated with hypertonic saline within 72 h, which might have underestimated the prevalence of hyperchloremia in our study subjects. Fourth, the amount of chloride add through other chlorinated fluid such as potassium chloride and enteral nutrients were not evaluated. Nevertheless, the contribution of potassium chloride to serum chloride is far less than normal saline, and the supplement of enteral nutrients to patients were strictly conducted according to the operating guidelines (25, 26).
Conclusion
Hyperchloremia is not rare and tends to occur in patients more severely affected by AIS and ICH. While no independent association was found, new-onset hyperchloremia and every 5 mmol/L increment in Δ[Cl−] within 72 h of NICU admission were associated with an increased odds of all cause 30-day mortality and 6-month poor prognosis in critically ill stroke patients. This study indicates that hyperchloremia has clinical importance as an indicator of prognosis in critically ill patients. Prospective study with rigorous design should be help to further explore the casual relationship between hyperchloremia and clinical outcome.
Ethics Statement
This study was approved by the Nanfang Hospital's ethics committee on clinical research. Informed consent was waived by the review board because of the pure observational and retrospective nature of the study.
Author Contributions
SP contributed to study conception and design. KH participated in study conception and design, and helped to draft and revise the manuscript. YH collected data and drafted the manuscript. YW and ZJ performed statistical analysis. SW and ZL participated in patient follow up and helped to revise the manuscript. All authors made substantial contribution, and read and approved the final version of the manuscript.
Funding
This study was supported by the National Key R&D Program of China (2017YFC1307500) and the Outstanding Youths Development Scheme of Nanfang Hospital, Southern Medical University (2016J005).
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors thanked Jun Zhou from Department of Biostatistics, School of Public Health, Southern Medical University, for the assistance in statistical analysis.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fneur.2018.00485/full#supplementary-material
References
1. Su YY, Li X, Li SJ, Luo R, Ding JP, Wang L, et al. Predicting hospital mortality using APACHE II scores in neurocritically ill patients: a prospective study. J Neurol. (2009) 256:1427–33. doi: 10.1007/s00415-009-5129-z
2. van der Jagt M. Fluid management of the neurological patient: a concise review. Crit Care (2016) 20:126. doi: 10.1186/s13054-016-1309-2
3. Lobo DN, Awad S. Should chloride-rich crystalloids remain the mainstay of fluid resuscitation to prevent ‘pre-renal’ acute kidney injury? Con Kidney Int. (2014) 86:1096–105. doi: 10.1038/ki.2014.105
4. Roquilly A, Mahe PJ, Latte DD, Loutrel O, Champin P, Di Falco C, et al. Continuous controlled-infusion of hypertonic saline solution in traumatic brain-injured patients: a 9-year retrospective study. Crit Care (2011) 15:R260. doi: 10.1186/cc10522
5. Jones GM, Bode L, Riha H, Erdman MJ. Safety of continuous peripheral infusion of 3% sodium chloride solution in neurocritical care patients. Am J Crit Care (2016) 26:37–42. doi: 10.4037/ajcc2017439
6. Langer T, Santini A, Scotti E, Van Regenmortel N, Malbrain ML, Caironi P. Intravenous balanced solutions: from physiology to clinical evidence. Anaesthesiol Intensive Ther. (2015) 47:s78–88. doi: 10.5603/AIT.a2015.0079
7. Young P, Bailey M, Beasley R, Henderson S, Mackle D, McArthur C, et al. Effect of a buffered crystalloid solution vs saline on acute kidney injury among patients in the intensive care unit: the SPLIT Randomized Clinical Trial. JAMA (2015) 314:1701–10. doi: 10.1001/jama.2015.12334
8. Van Regenmortel N, Verbrugghe W, Van den Wyngaert T, Jorens PG. Impact of chloride and strong ion difference on ICU and hospital mortality in a mixed intensive care population. Ann Intensive Care (2016) 6:91. doi: 10.1186/s13613-016-0193-x
9. Neyra JA, Canepa-Escaro F, Li X, Manllo J, Adams-Huet B, Yee J, et al. Association of hyperchloremia with hospital mortality in critically ill septic patients. Crit Care Med. (2015) 43:1938–44. doi: 10.1097/CCM.0000000000001161
10. Suetrong B, Pisitsak C, Boyd JH, Russell JA, Walley KR. Hyperchloremia and moderate increase in serum chloride are associated with acute kidney injury in severe sepsis and septic shock patients. Crit Care (2016) 20:315. doi: 10.1186/s13054-016-1499-7
11. Kimura S, Matsumoto S, Muto N, Yamanoi T, Higashi T, Nakamura K, et al. Association of serum chloride concentration with outcomes in postoperative critically ill patients: a retrospective observational study. J Intensive Care (2014) 2:39. doi: 10.1186/2052-0492-2-39
12. Lee JY, Hong TH, Lee KW, Jung MJ, Lee JG, Lee SH. Hyperchloremia is associated with 30-day mortality in major trauma patients: a retrospective observational study. Scand J Trauma Resusc Emerg Med. (2016) 24:117. doi: 10.1186/s13049-016-0311-7
13. Riha HM, Erdman MJ, Vandigo JE, Kimmons LA, Goyal N, Davidson KE, et al. Impact of moderate hyperchloremia on clinical outcomes in intracerebral hemorrhage patients treated with continuous infusion hypertonic saline. Crit Care Med. (2017) 45:e947–53. doi: 10.1097/CCM.0000000000002522
14. Sadan O, Singbartl K, Kandiah PA, Martin KS, Samuels OB. Hyperchloremia is associated with acute kidney injury in patients with subarachnoid hemorrhage. Crit Care Med. (2017) 45:1382–8. doi: 10.1097/CCM.0000000000002497
15. Vincent JL, Moreno R, Takala J, Willatts S, De Mendonça A, Bruining H, et al. The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure. On behalf of the Working Group on Sepsis-Related of the European Society of Intensive Care Medicine. Intensive Care Med. (1996) 22:707–10. doi: 10.1007/BF01709751
16. Kellum JA, Lameire N. Diagnosis, evaluation, and management of acute kidney injury: a KDIGO summary (Part 1). Crit Care (2013) 17:204. doi: 10.1186/cc11454
17. Berend K, van Hulsteijn LH, Gans RO. Chloride: the queen of electrolytes? Eur J Intern Med. (2012) 23:203–11. doi: 10.1016/j.ejim.2011.11.013
18. Yunos NM, Bellomo R, Story D, Kellum J. Bench-to-bedside review: chloride in critical illness. Crit Care (2010) 14:226. doi: 10.1186/cc9052
19. Thongprayoon C, Cheungpasitporn W, Cheng Z, Qian Q. Chloride alterations in hospitalized patients: prevalence and outcome significance. PLoS ONE (2017) 12:e174430. doi: 10.1371/journal.pone.0174430
20. Li H, Sun S, Yap JQ, Chen J, Qian Q. 0.9% saline is neither normal nor physiological. J Zhejiang Univ Sci B (2016) 17:181–7. doi: 10.1631/jzus.B1500201
21. Young JB, Utter GH, Schermer CR, Galante JM, Phan HH, Yang Y, et al. Saline versus Plasma-Lyte A in initial resuscitation of trauma patients: a randomized trial. Ann Surg. (2014) 259:255–62. doi: 10.1097/SLA.0b013e318295feba
22. Cheung WYS, Cheung WK, Lam CH, Chan YW, Chow HC, Cheng KL, et al. Intravenous fluid selection rationales in acute clinical management. World J Emerg Med. (2018) 9:13–9. doi: 10.5847/wjem.j.1920-8642.2018.01.002
23. Semler MW, Self WH, Wanderer JP, Ehrenfeld JM, Wang L, Byrne DW, et al. Balanced crystalloids versus saline in critically ill adults. N Engl J Med. (2018) 378:829–39. doi: 10.1056/NEJMoa1711584
24. Self WH, Semler MW, Wanderer JP, Wang L, Byrne DW, Collins SP, et al. Balanced crystalloids versus saline in noncritically ill adults. N Engl J Med. (2018) 378:819–28. doi: 10.1056/NEJMoa1711586
25. McClave SA, Martindale RG, Vanek VW, McCarthy M, Roberts P, Taylor B, et al. Guidelines for the provision and assessment of nutrition support therapy in the adult critically ill patient: society of Critical Care Medicine (SCCM) and American Society for Parenteral and Enteral Nutrition (A.S.P.E.N.). JPEN J Parenter Enteral Nutr. (2009) 33:277–316. doi: 10.1177/0148607109335234
26. Taylor BE, McClave SA, Martindale RG, Warren MM, Johnson DR, Braunschweig C, et al. Guidelines for the provision and assessment of nutrition support therapy in the adult critically ill patient: society of Critical Care Medicine (SCCM) and American Society for Parenteral and Enteral Nutrition (A.S.P.E.N.). Crit Care Med. (2016) 44:390–438. doi: 10.1097/CCM.0000000000001525
Keywords: hyperchloremia, neurocritical care, mortality, poor prognosis, fluid management
Citation: Huang K, Hu Y, Wu Y, Ji Z, Wang S, Lin Z and Pan S (2018) Hyperchloremia Is Associated With Poorer Outcome in Critically Ill Stroke Patients. Front. Neurol. 9:485. doi: 10.3389/fneur.2018.00485
Received: 06 March 2018; Accepted: 04 June 2018;
Published: 03 July 2018.
Edited by:
Liping Liu, Beijing Tiantan Hospital, Capital Medical University, ChinaReviewed by:
Rick Gill, Hospital of the University of Pennsylvania, United StatesChristoph Stretz, Yale School of Medicine, Yale University, United States
Yuchuan Ding, Wayne State University School of Medicine, United States
Copyright © 2018 Huang, Hu, Wu, Ji, Wang, Lin and Pan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Suyue Pan, cGFuc3V5dWU4MkAxMjYuY29t
†These authors have contributed equally to this work.
 Kaibin Huang
Kaibin Huang Yanhong Hu†
Yanhong Hu† Yongming Wu
Yongming Wu Suyue Pan
Suyue Pan