- 1Institute of Neurology, Huashan Hospital, Fudan University, Shanghai, China
- 2National Clinical Research Center for Aging and Medicine, Huashan Hospital, Fudan University, Shanghai, China
- 3Department of Cardiology, Huashan Hospital, Fudan University, Shanghai, China
- 4Department of Biostatistics, School of Public Health, Fudan University, Shanghai, China
- 5Key Laboratory of Public Health Safety of Ministry of Education, Shanghai, China
- 6JC School of Public Health and Primary Care, The Chinese University of Hong Kong, Hong Kong, Hong Kong
Background: To explore the association between blood pressure and cognition in older participants in the Shanghai Aging Study.
Methods: Data were drawn from 3,327 participants at the baseline of Shanghai Aging Study. History of hypertension was inquired and confirmed from participants' medical records. Systolic blood pressure (SBP) and diastolic blood pressure (DBP) were measured by research nurses in the early morning. Participants were diagnosed with “cognitive normal,” “mild cognitive impairment (MCI),” or “dementia” by neurologists using DSM-IV and Petersen criteria. Multivariate logistic regression was used to evaluate the association between history of hypertension, duration of hypertension, SBP, DBP, or classification of blood pressure and cognitive function. Generalized linear model was used to assess the relation between duration of hypertension, SBP, or DBP and Mini Mental State Examination (MMSE).
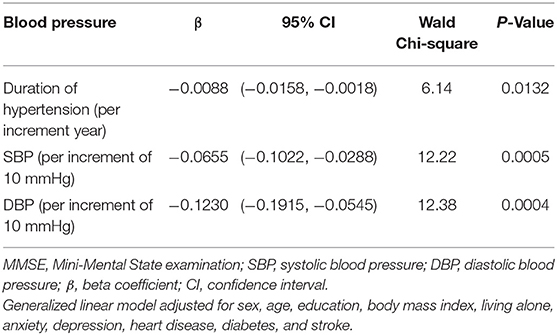
Results: A significantly higher proportion of hypertension [78 (76.5%)] was found in participants with dementia than in those with MCI [347 (59.3%)] and cognitive normal [1,350 (51.1%)] (P < 0.0001). Participants with dementia had significantly higher SBP [157.6 (26.1) mmHg] than those with MCI [149.0 (23.7) mmHg] and cognitive normal [143.7 (22.6) mmHg] (P < 0.0001). After adjusting for sex, age, education, living alone, body mass index, anxiety, depression, heart disease, diabetes, and stroke, the likelihood of having dementia was positively associated with history of hypertension (OR = 2.10; 95% CI: 1.22, 3.61), duration of hypertension (OR = 1.02 per increment year; 95% CI: 1.01, 1.04), higher SBP (OR = 1.14 per increment of 10 mmHg; 95% CI: 1.04, 1.25), higher DBP (OR = 1.22 per increment of 10 mmHg; 95% CI: 1.02, 1.45), moderate hypertension (OR = 2.09; 95% CI: 1.10, 3.99), or severe hypertension (OR = 2.45; 95% CI: 1.20, 4.99). The MMSE score was inversely correlated to duration of hypertension (β = −0.0088 per increment year; 95% CI: −0.0158, −0.0018, P = 0.0132), SBP (β = −0.0655 per increment of 10 mmHg; 95% CI: −0.1022, −0.0288, P = 0.0005), and DBP (β = −0.1230 per increment of 10 mmHg; 95% CI: −0.1915, −0.0545, P = 0.0004).
Conclusion: Our results suggest that hypertension and high blood pressure may be potential risk factors for dementia. Blood pressure management for the elderly may be important for maintaining cognitive vitality.
Introduction
Cognitive impairment has a great impact on disability and mortality among the elderly, while it also reduces the quality of life for both patients and their caregivers. Approximately, there have been 24.3 million prevalent dementia cases, with 4.6 million new cases worldwide every year (1). China had 9.2 million cases of dementia in 2010 (2), and the case number will rise to the world's top by 2025 (1).
Hypertension is a highly prevalent condition, occurring in one-third of the world's adults and in two-thirds of adults over 65 years of age (3, 4). Both hypertension and dementia are age-related comorbidities which may induce considerable disabilities (1, 5–7). Some epidemiological studies showed that hypertension is an important risk factor of dementia (8, 9), which was evident from the positive relationship between blood pressure at midlife and the subsequently higher risk of cognitive impairment or dementia late in life (10–12); however, some other studies provided contradictory evidence that low blood pressure was a risk factor for dementia and cognitive decline (13–15). Until now, the relationship between hypertension and dementia or cognitive decline has been inconsistent, and mixed findings have been reported from cross-sectional and longitudinal studies (6, 16–18).
Epidemiological dementia research in China is still far behind developed countries. Only two epidemiological studies examined the relation between hypertension and cognitive impairment in the elderly in China, but it was inconclusive because of small sample size and simple neuropsychological assessments (19, 20). The Shanghai Aging Study intended to identify the prevalence and the incidence of dementia and mild cognitive impairment (MCI) among a cohort of old adults in an urban community in Shanghai, China (21). We, therefore, intend to explore the association between blood pressure and cognition in this cohort by analyzing the baseline data of the Shanghai Aging Study.
Materials and Methods
Recruitment of Participants
From January 2010 to December 2012, the Shanghai Aging Study recruited 3,836 permanent residents aged ≥50 years in the Jingansi community, Shanghai. Participants were excluded if they were (1) residing in nursing homes or other institutions; (2) suffering from severe schizophrenia or mental retardation, based on the data abstracted from their medical record or diagnosed by neurologists; or (3) suffering from severe vision, hearing, or verbal impairment and could not participate in the neuropsychological evaluation. Detailed process of the participant recruitment has been published elsewhere (21). In this study, we used the dataset of 3,327 participants aged 60–85 years.
This study was approved by the Medical Ethics Committee of Huashan Hospital, Fudan University, Shanghai, China. All the participants and/or their legal guardians provided their written informed consent to participate in the study.
Demographic Characteristics and Medical History
Participants were interviewed face-to-face by neurologists and research nurses to collect information on their demographic characteristics, including age, sex, and education. The participants' weight and height were measured and used to calculate the body mass index (BMI: the weight in kilograms divided by the square of the height in meters). We also obtained lifestyle factors, such as living alone, cigarette smoking, and alcohol drinking. Their history of chronic diseases, such as diabetes, stroke, and heart disease (including coronary artery disease and arrhythmia), was inquired and confirmed from their medical records.
Blood Pressure Measurement
From 7:30 to 8:00 a.m., after at least 5 min of rest in a seated position blood pressure was measured twice, with a standard sized cuff placed on the right arm at heart level of the seated subject, by trained research nurses using a validated and calibrated digital electronic tensiometer (M4; OMRON Corp., Kyoto, Japan) (22). We recorded the blood pressure as the mean of two measurements of systolic blood pressure (SBP) and diastolic blood pressure (DBP).
Hypertension and Classification of Blood Pressure
Hypertension was defined as the reported physician-diagnosed hypertension, with or without treatment. Duration of hypertension was also inquired and confirmed from the participants' medical records (19, 23). According to the 2010 Chinese guidelines for the management of hypertension, blood pressure was classified into four categories: severe hypertension: SBP ≥180 mmHg or DBP ≥110 mmHg; moderate hypertension: SBP of 160–179 mmHg or DBP of 100–109 mmHg; mild hypertension: SBP of 140–159 mmHg or DBP of 90–99 mmHg; and normal blood pressure: SBP < 140 mmHg and DBP < 90 mmHg (23).
Neuropsychological Assessments
Cognitive function of each participant was assessed by a neuropsychological test battery, which covers domains of global cognition, executive function, spatial construction function, memory, attention, and language. The battery contained (1) Mini Mental State Examination (MMSE); (2) Conflicting Instructions Task (Go/No Go Task); (3) Stick Test; (4) Modified Common Objects Sorting Test; (5) Auditory Verbal Learning Test; (6) Modified Fuld Object Memory Evaluation; (7) Trail-making test A&B; and (8) RMB (Chinese currency) test. Neuropsychological tests were administered by study psychometrists according to the different education levels of each participant. Test 1, 2, 3, 4, 5, and 7 in the battery were used in participants with education ≥6 years; test 1, 2, 3, 4, 6, and 8 were used in those with education < 6 years. All tests were conducted in Chinese within 90 min. A pilot validation study was conducted for each cognitive measure using corrections for gender, age, and years of education in a community of healthy elderly people, and the normative data and the detailed description of these tests were reported elsewhere (24, 25).
Neurological Exams
Neurologists examined each participant for their reflexes and motor responses. They also administered the Center for Epidemiologic Studies Depression Scale (CES-D) (26) and the Zung Self-Rating Anxiety Scale (SAS) (27) to evaluate whether each participant met the criteria of having a major depression (CES-D ≥ 16) or anxiety (SAS > 44) episode within the past week. Neurologists also administered the Clinical Dementia Rating (CDR) (28, 29) and Activities of Daily Living (ADL) (30) scale to obtain information on cognitive complaints and activities of daily living.
Consensus Diagnoses
After each clinical assessment, two study neurologists, one neuropsychologist, and one neuroepidemiologist reviewed the functional, medical, neurological, psychiatric, and neuropsychological data and reached a consensus regarding the presence or absence of dementia using DSM-IV criteria (31). Only those who were not diagnosed with dementia were considered for the diagnosis of MCI, which was defined according to Petersen's criteria (32). Diagnostic procedures were reported elsewhere (21). Based on the consensus diagnoses, the subjects were classified into three groups with dementia, MCI, and cognitive normal.
APOE Genotype Assessment
DNA was extracted from blood or saliva collected from the study participants. Apolipoprotein E (APOE) genotyping was conducted by the TaqmanSNP method (33). The presence of at least one ε4 allele was defined as being APOE-ε4 positive.
Statistical Analyses
Continuous variables were expressed as the mean ± standard deviation (SD) or median (25%, 75%), and categorical variables were expressed as frequencies (%). The analysis of variance (ANOVA) and Kruskal-Wallis test were used to compare the continuous variables; the Cochran-Mantel-Haenszel Chi-squared test was used to compare the categorical variables. Multivariate logistic regression model was used to detect the association between history of hypertension, duration of hypertension, SBP, DBP, or blood pressure categories and different clinical cognitive diagnoses, which were adjusted for confounders. Measurement of the association was presented as odds ratio (OR) and 95% confidence interval (CI). Generalized linear model was used to evaluate the relation between duration of hypertension, SBP, or DBP and MMSE, which were adjusted for confounders. Systolic and diastolic blood pressures were regarded as continuous variables and were expressed in units of 10 mmHg (original blood pressure value divided by 10) in the multivariate logistic regression model and in the generalized linear model.
All the P-values and 95% CIs were estimated in two-tailed tests. Differences were considered to be statistically significant at P < 0.05. The data analysis was conducted using SAS 9.4 (SAS Institute Inc., Cary, NC, USA).
Results
Demographic, Lifestyles, and Medical History of the Participants
Among the 3,327 participants, 1,519 (45.7%) were males. The mean age of all the participants was 70.1 (SD 7.2) years, and the mean year of education was 11.7 (SD 4.2) years. Five hundred and eighty-five (17.6%) participants were diagnosed as MCI, while 102 (3.1%) were diagnosed as dementia. Age, education, MMSE score, alcohol drinking, APOE-ε4 allele, history of heart disease, diabetes, stroke, anxiety, and depression were found to be significantly different across groups with different diagnosis of cognition (Table 1).
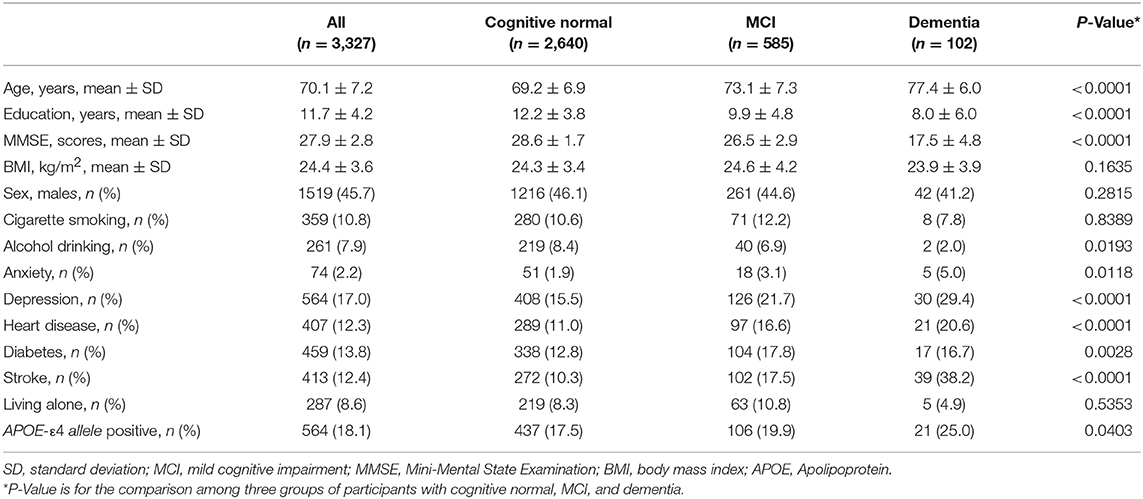
Table 1. Demographic, lifestyles, and medical history of the participants with cognitive normal, MCI, and dementia.
Hypertension and Blood Pressure of Groups With Different Cognition
One thousand seven hundred and seventy-five (53.4%) participants were suffering from hypertension. Participants with dementia had a significantly higher proportion of having hypertension (76.5%) than those with MCI (59.3%) and cognitive normal (51.1%) (P < 0.0001). Participants with dementia had significantly longer duration of hypertension (median 10 years) than those with MCI (median 4 years) and cognitive normal (median 1 year) (P < 0.0001). Participants with dementia had significantly higher SBP [157.6 mmHg (SD 26.1)] than those with MCI [149.0 mmHg (SD 23.7)] and cognitive normal [143.7 mmHg (SD 22.6)] (P < 0.0001). The distribution of blood pressure category was significantly different across the three groups (P < 0.001). More moderate and severe hypertension (47.0%) was seen in participants with dementia than those with MCI (33.4%) and cognitive normal (23.5%) (P < 0.001; Table 2).
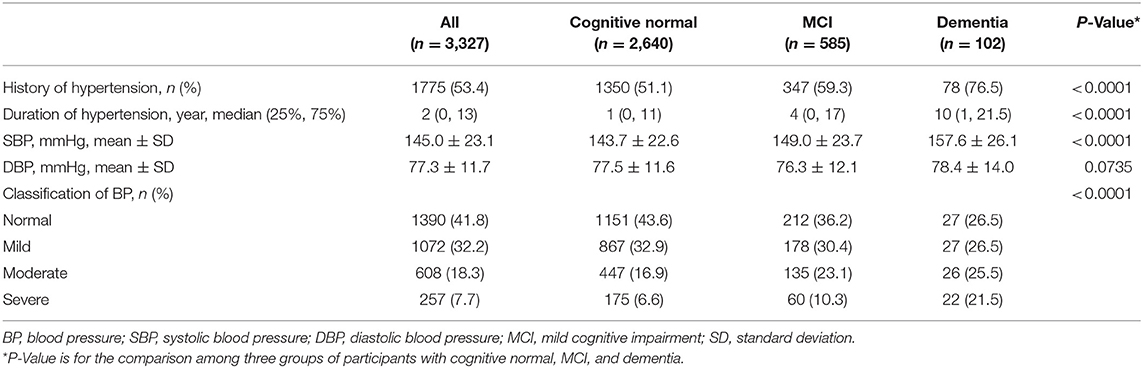
Table 2. Hypertension and blood pressure of the participants with cognitive normal, MCI, and dementia.
Association Between Blood Pressure and MMSE
After adjusting for age, sex, education, BMI, living alone, anxiety, depression, heart disease, diabetes, and stroke in the generalized linear model, the decline in MMSE score was significantly correlated with duration of hypertension (β = −0.0088 per increment year; 95% CI: −0.0158, −0.0018; P = 0.0132), SBP (β = −0.0655 per increment of 10 mmHg: 95% CI: −0.1022, −0.0288; P = 0.0005), and DBP (β = −0.1230 per increment of 10 mmHg; 95% CI: −0.1915, −0.0545; P = 0.0004; Table 3).
Association Between Hypertension, Blood Pressure, and Cognition
After adjusting for age, sex, education, BMI, living alone, anxiety, depression, heart disease, diabetes, and stroke, the likelihood of having dementia was positively associated with history of hypertension (OR = 2.10; 95% CI: 1.22, 3.61), duration of hypertension (OR = 1.02 per increment year; 95% CI: 1.01,1.04), higher SBP (OR = 1.14 per increment of 10 mmHg; 95% CI: 1.04, 1.25), higher DBP (OR = 1.22 per increment of 10 mmHg; 95% CI: 1.02, 1.45), moderate hypertension (OR = 2.09; 95% CI: 1.10, 3.99), or severe hypertension (OR = 2.45; 95% CI: 1.20, 4.99), but it was not associated with mild hypertension (OR = 1.31; 95% CI: 0.70, 2.45). There was no significant association between MCI and hypertension or blood pressure (Table 4).
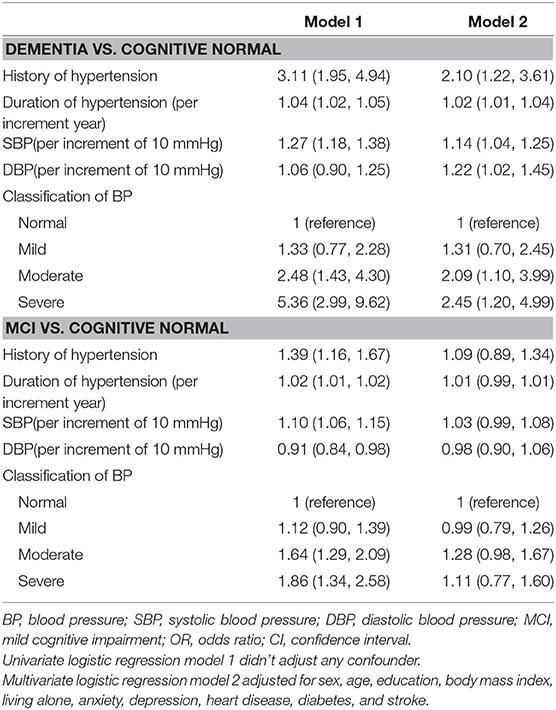
Table 4. Adjusted odds ratios for hypertension and blood pressure among participants with dementia vs. cognitive normal, and with MCI vs. cognitive normal [OR (95% CI)].
Discussion
Our study indicated that history of hypertension, duration of hypertension, and high blood pressure were positively associated with dementia among older Chinese people living in an urban community. The MMSE score, which represented the global cognition, was inversely correlated to duration of hypertension, SBP, and DBP. One advantage of the current study was the reliable diagnosis, which was conducted by neurologists with consensus diagnosis at one of the top institutions of neurology in China. Other advantages included the population-based study design with a large sample and the adjustment for confounders, such as sociodemographic characteristics, health behaviors, and medical conditions.
Result from our study was consistent with most of the previous studies which suggested that blood pressure and hypertension are key risk factors for cognitive impairment. In a cross-sectional epidemiological study with 19,836 participants in India/America, an increment of 10 mmHg in DBP was associated with a 7% (95% CI: 1–14%; P = 0.0275) higher odds of cognitive impairment based on 6-Item Screener. No independent association was identified between impaired cognitive status and SBP (OR = 1.02; 95% CI: 0.99–1.06) or pulse pressure (OR = 0.99; 95% CI: 0.95–1.04) (34). However, some studies showed inverse association. A cross-national epidemiological study was conducted in India and America with 4,810 subjects of 55 years and older; in Ballabgarh, India, for every 10 mmHg increase in SBP there was a 10% reduction in cognitive impairment based on a set of cognitive tests (OR = 0.90; 95% CI: 0.83–0.97), and there was a 13% reduction in cognitive impairment (OR = 0.87; 95% CI: 0.76–0.99) with every 10 mmHg increase in DBP; in the Monongahela Valley, America, a similar association between DBP and cognitive impairment was observed, but it did not remain significant after adjustment for confounders (OR = 0.83; 95% CI: 0.65–1.06) (35).
Few studies were reported in the Chinese population. In a cross-sectional study with 1,799 participants (aged 40–85) in a rural area of Xi'an, stratified multivariate analysis revealed a positive relation between SBP (when regarded as continuous data) and cognitive impairment (MMSE) in patients aged 40–49 years (OR = 1.35 per 10 mmHg; 95% CI: 1.04–1.75; P = 0.025) and 50–59 years (OR = 1.19 per 10 mmHg; 95% CI: 1.03–1.37; P = 0.019). The analysis turned out to be insignificant for patients aged 60–69 years (OR = 0.88 per 10 mmHg; 95% CI: 0.73–1.06; P = 0.171) and ≥70 years (OR = 0.93 per 10 mmHg; 95% CI: 0.77–1.11; P = 0.416). Results similar to those obtained for SBP were obtained for DBP, mean arterial blood pressure, and high blood pressure as well (20). In a prospective observational study with four rural counties in China, 2,000 rural Chinese people aged 65 years and older (median age 70, range 65–92) participated in a baseline evaluation. Two and a half years after baseline the evaluation, a follow-up evaluation of 1,737 subjects was conducted. Cognitive decline based on a set of cognitive tests was derived as the difference between baseline and follow-up scores. Untreated hypertension was associated with greater cognitive decline in this Chinese cohort (19).
The possible mechanism explaining the effect of hypertension on cognitive impairment is not yet clear. A number of autopsy studies (36, 37) have shown that the probability of dementia manifestation for a given level of Alzheimer's disease (AD) pathology is increased by the presence of cerebrovascular pathology, which is strongly linked to hypertension (38–45). Animal studies suggest that cerebral ischemia may be involved in the initiation of AD through upregulation of amyloid precursor protein gene expression (46–48), promotion of amyloid precursor protein cleavage into beta-amyloid peptides (49), or reduction in beta-amyloid peptide clearance (50). Uncontrolled hypertension appears to predict the level of neurofibrillary tangles and neuritic plaques (pathologic indicators of AD) in the brain (51–53), which could be a direct effect of hypertension on AD pathology. Blood pressure may be related to AD initiation or progression through mechanisms that involve beta-amyloid peptides (54), which aggregate to form neuritic plaques.
Some limitations existed in our study. Firstly, we failed to draw conclusions about causal relationship between hypertension and cognitive impairment, especially in the case of the disease that is both age-related and associated with a biased participation rate, from this cross-sectional study design. Secondly, we have adjusted as many potential confounders as possible in the logistic regression model, but we still could not exclude the possible influence of uncollected confounders, such as life experience (e.g., interests, hobbies, leisure activities) and ones' innate intelligence, which could also reflect cognitive reserve. Thirdly, spot blood pressure measurement might not represent the whole situation of blood pressure level. But for each participant, we measured the blood pressure at the same time in the early morning; therefore, it is better than those measured at other time points. Finally, our study site lies in the urban center of Shanghai, and the participants had higher education than most of the others in China. Therefore, the results could not be generalized to the whole Chinese population.
Our results suggest that hypertension and high blood pressure may be potential risk factors for dementia. Blood pressure management for the elderly may be important for maintaining cognitive vitality. The association between blood pressure and risk of cognitive impairment needs to be further studied and validated by prospective studies with longer follow-up time in older population.
Author Contributions
This work was conceptualized by DD, YS, and ZH and all of them approved the protocol. Data collection was done by DD, QZ, QG, XL, and LZ. Statistical analysis was undertaken by XL, WD, and JL. XL, YS, LT, and DD prepared the manuscript. YS and DD are the guarantors of this paper.
Funding
This project was funded by Shanghai Brain-Intelligence Project from STCSM [grant number 16JC1420500], Scientific Research Project from STCSM [grant number 17411950701, 17411950106], the Natural Science Foundation and Major Basic Research Program of Shanghai [grant number 16JC1420100], and the National Natural Science Foundation of China [grant number 81773513].
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors thank Dr. Minhua Shao for her technical assistance in the APOE genotype assays; Zhaolan Ding, Meihua Jin, Meirong Chen, Zeya Wang, Meizheng Shi, Jingping Ye, Meiping He, Lanfang Yu, Deping Chen, Fusheng Gong, Meili Shi, Wenying Zhou, Shumin Chen, Xiudi Xu, Meiling Huang, Linghua Ding, Wenfan Zhu, Zhi Zhou, Xiaoying Liu, Fuqin Gao, Peng Gong, Lin Lu, Meng Wang, Ting Zhang, Yaru Guo, Xiaoli Jin, Shiqi Li, Qiongyi Xu, and Yiping Wang for their efforts in conducting the study; and all the participants for their cooperation.
References
1. Ferri CP, Prince M, Brayne C, Brodaty H, Fratiglioni L, Ganguli M, et al. Global prevalence of dementia: a Delphi consensus study. Lancet (2005) 366:2112–7. doi: 10.1016/S0140-6736(05)67889-0
2. Chan KY, Wang W, Wu JJ, Liu L, Theodoratou E, Car J, et al. Epidemiology of Alzheimer's disease and other forms of dementia in China, 1990-2010: a systematic review and analysis. Lancet (2013) 381:2016–23. doi: 10.1016/S0140-6736(13)60221-4
3. Mills KT, Bundy JD, Kelly TN, Reed JE, Kearney PM, Reynolds K, et al. Global disparities of hypertension prevalence and control: a systematic analysis of population-based studies from 90 countries. Circulation (2016) 134:441–50. doi: 10.1161/CIRCULATIONAHA.115.018912
4. Kearney PM, Whelton M, Reynolds K, Muntner P, Whelton PK, He J. Global burden of hypertension: analysis of worldwide data. Lancet (2005) 365:217–23. doi: 10.1016/S0140-6736(05)17741-1
5. Wimo A, Winblad B, Jonsson L. An estimate of the total worldwide societal costs of dementia in 2005. Alzheimers Dement. (2007) 3:81–91. doi: 10.1016/j.jalz.2007.02.001
6. Duron E, Hanon O. Vascular risk factors, cognitive decline, and dementia. Vasc Health Risk Manag. (2008) 4:363–81. doi: 10.2147/VHRM.S1839
7. Addo J, Smeeth L, Leon DA. Hypertension in sub-saharan Africa: a systematic review. Hypertension (2007) 50:1012–8. doi: 10.1161/HYPERTENSIONAHA.107.093336
8. Gregg EW, Yaffe K, Cauley JA, Rolka DB, Blackwell TL, Narayan KM, et al. Is diabetes associated with cognitive impairment and cognitive decline among older women? Study of Osteoporotic Fractures Research Group. Arch Intern Med. (2000) 160:174–80. doi: 10.1001/archinte.160.2.174
9. Ryglewicz D, Rodo M, Kunicki PK, Bednarska-Makaruk M, Graban A, Lojkowska W, et al. Plasma antioxidant activity and vascular dementia. J Neurol Sci. (2002) 203–204:195–7. doi: 10.1016/S0022-510X(02)00290-3
10. Launer LJ, Masaki K, Petrovitch H, Foley D, Havlik RJ. The association between midlife blood pressure levels and late-life cognitive function. The Honolulu-Asia aging study. JAMA (1995) 274:1846–51. doi: 10.1001/jama.274.23.1846
11. Skoog I, Lernfelt B, Landahl S, Palmertz B, Andreasson LA, Nilsson L, et al. 15-year longitudinal study of blood pressure and dementia. Lancet (1996) 347:1141–5. doi: 10.1016/S0140-6736(96)90608-X
12. Kivipelto M, Helkala EL, Laakso MP, Hanninen T, Hallikainen M, Alhainen K, et al. Apolipoprotein E epsilon4 allele, elevated midlife total cholesterol level, and high midlife systolic blood pressure are independent risk factors for late-life Alzheimer disease. Ann Intern Med. (2002) 137:149–55. doi: 10.7326/0003-4819-137-3-200208060-00006
13. Qiu C, von Strauss E, Fastbom J, Winblad B, Fratiglioni L. Low blood pressure and risk of dementia in the Kungsholmen project:a 6-year follow-up study. Arch Neurol. (2003) 60:223–8. doi: 10.1001/archneur.60.2.223
14. Guo Z, Viitanen M, Fratiglioni L, Winblad B. Low blood pressure and dementia in elderly people: the Kungsholmen project. BMJ (1996) 312:805–8.
15. Guo Z, Fratiglioni L, Winblad B, Viitanen M. Blood pressure and performance on the Mini-Mental State Examination in the very old. Cross-sectional and longitudinal data from the Kungsholmen Project. Am J Epidemiol. (1997) 145:1106–13.
16. Hanon O, Forette F. Treatment of hypertension and prevention of dementia. Alzheimers Dement. (2005) 1:30–7. doi: 10.1016/j.jalz.2005.06.022
17. Seux ML, Thijs L, Forette F, Staessen JA, Birkenhager WH, Bulpitt CJ, et al. Correlates of cognitive status of old patients with isolated systolic hypertension: the Syst-Eur Vascular Dementia Project. J Hypertens (1998) 16:963–9. doi: 10.1097/00004872-199816070-00009
18. Scherr PA, Hebert LE, Smith LA, Evans DA. Relation of blood pressure to cognitive function in the elderly. Am J Epidemiol. (1991) 134:1303–15.
19. Gao S, Jin Y, Unverzagt FW, Liang C, Hall KS, Ma F, et al. Hypertension and cognitive decline in rural elderly Chinese. J Am Geriatr Soc. (2009) 57:1051–7. doi: 10.1111/j.1532-5415.2009.02267.x
20. Shang S, Li P, Deng M, Jiang Y, Chen C, Qu Q. The age-dependent relationship between blood pressure and cognitive impairment: a cross-sectional study in a rural Area of Xi'an, China. PLoS ONE (2016) 11:e159485. doi: 10.1371/journal.pone.0159485
21. Ding D, Zhao Q, Guo Q, Meng H, Wang B, Yu P, et al. The Shanghai Aging Study: study design, baseline characteristics, and prevalence of dementia. Neuroepidemiology (2014) 43:114–22. doi: 10.1159/000366163
22. Alperovitch A, Blachier M, Soumare A, Ritchie K, Dartigues JF, Richard-Harston S, et al. Blood pressure variability and risk of dementia in an elderly cohort, the Three-City Study. Alzheimers Dement. (2014) 10:S330–7. doi: 10.1016/j.jalz.2013.05.1777
23. Writing Group of 2010 Chinese Guidelines for the Management of Hypertension. 2010 Chinese guidelines for the management of hypertension. Chin J Cardiol. (2011) 39:7. doi: 10.3760/cma.j.issn.0253-3758.2011.07.002
24. Zhang MY, Katzman R, Salmon D, Jin H, Cai GJ, Wang ZY, et al. The prevalence of dementia and Alzheimer's disease in Shanghai, China: impact of age, gender, and education. Ann Neurol. (1990) 27:428–37. doi: 10.1002/ana.410270412
25. Ding D, Zhao Q, Guo Q, Meng H, Wang B, Luo J, et al. Prevalence of mild cognitive impairment in an urban community in China: a cross-sectional analysis of the Shanghai Aging Study. Alzheimers Dement. (2015) 11:300–9. doi: 10.1016/j.jalz.2013.11.002
26. Zhang J, Norvilitis JM. Measuring Chinese psychological well-being with Western developed instruments. J Pers Assess. (2002) 79:492–511. doi: 10.1207/S15327752JPA7903_06
27. Zung WW. A rating instrument for anxiety disorders. Psychosomatics (1971) 12:371–9. doi: 10.1016/S0033-3182(71)71479-0
28. Morris JC. The Clinical Dementia Rating (CDR): current version and scoring rules. Neurology (1993) 43:2412–4. doi: 10.1212/WNL.43.11.2412-a
29. Lim WS, Chong MS, Sahadevan S. Utility of the clinical dementia rating in Asian populations. Clin Med Res. (2007) 5:61–70. doi: 10.3121/cmr.2007.693
30. Lawton MP, Brody EM. Assessment of older people: self-maintaining and instrumental activities of daily living. Gerontologist (1969) 9:179–86. doi: 10.1093/geront/9.3_Part_1.179
31. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 4th Edn. Washington, DC: American Psychiatric Association (1994). p. 143–7.
32. Petersen RC. Mild cognitive impairment as a diagnostic entity. J Intern Med. (2004) 256:183–94. doi: 10.1111/j.1365-2796.2004.01388.x
33. Smirnov DA, Morley M, Shin E, Spielman RS, Cheung VG. Genetic analysis of radiation-induced changes in human gene expression. Nature (2009) 459:587–91. doi: 10.1038/nature07940
34. Tsivgoulis G, Alexandrov AV, Wadley VG, Unverzagt FW, Go RC, Moy CS, et al. Association of higher diastolic blood pressure levels with cognitive impairment. Neurology (2009) 73:589–95. doi: 10.1212/WNL.0b013e3181b38969
35. Pandav R, Dodge HH, DeKosky ST, Ganguli M. Blood pressure and cognitive impairment in India and the United States: a cross-national epidemiological study. Arch Neurol. (2003) 60:1123–8. doi: 10.1001/archneur.60.8.1123
36. Schneider JA, Wilson RS, Bienias JL, Evans DA, Bennett DA. Cerebral infarctions and the likelihood of dementia from Alzheimer disease pathology. Neurology (2004) 62:1148–55. doi: 10.1212/01.WNL.0000118211.78503.F5
37. Snowdon DA, Greiner LH, Mortimer JA, Riley KP, Greiner PA, Markesbery WR. Brain infarction and the clinical expression of Alzheimer disease. The Nun Study. JAMA (1997) 277:813–7. doi: 10.1001/jama.1997.03550020045023
38. de Leeuw FE, de Groot JC, Oudkerk M, Witteman JC, Hofman A, van Gijn J, et al. Hypertension and cerebral white matter lesions in a prospective cohort study. Brain (2002) 125:765–72. doi: 10.1093/brain/awf077
39. Viswanathan A, Chabriat H. Cerebral microhemorrhage. Stroke (2006) 37:550–5. doi: 10.1161/01.STR.0000199847.96188.12
40. Kazui S, Levi CR, Jones EF, Quang L, Calafiore P, Donnan GA. Risk factors for lacunar stroke: a case-control transesophageal echocardiographic study. Neurology (2000) 54:1385–7. doi: 10.1212/WNL.54.6.1385
41. Veldink JH, Scheltens P, Jonker C, Launer LJ. Progression of cerebral white matter hyperintensities on MRI is related to diastolic blood pressure. Neurology (1998) 51:319–20. doi: 10.1212/WNL.51.1.319
42. Longstreth WJ, Bernick C, Manolio TA, Bryan N, Jungreis CA, Price TR. Lacunar infarcts defined by magnetic resonance imaging of 3660 elderly people: the Cardiovascular Health Study. Arch Neurol. (1998) 55:1217–25. doi: 10.1001/archneur.55.9.1217
43. Prabhakaran S, Wright CB, Yoshita M, Delapaz R, Brown T, DeCarli C, et al. Prevalence and determinants of subclinical brain infarction: the Northern Manhattan Study. Neurology (2008) 70:425–30. doi: 10.1212/01.wnl.0000277521.66947.e5
44. Vermeer SE, Koudstaal PJ, Oudkerk M, Hofman A, Breteler MM. Prevalence and risk factors of silent brain infarcts in the population-based Rotterdam Scan Study. Stroke (2002) 33:21–5. doi: 10.1161/hs0102.101629
45. Vernooij MW, van der Lugt A, Ikram MA, Wielopolski PA, Niessen WJ, Hofman A, et al. Prevalence and risk factors of cerebral microbleeds: the Rotterdam Scan Study. Neurology (2008) 70:1208–14. doi: 10.1212/01.wnl.0000307750.41970.d9
46. Shi J, Yang SH, Stubley L, Day AL, Simpkins JW. Hypoperfusion induces overexpression of beta-amyloid precursor protein mRNA in a focal ischemic rodent model. Brain Res. (2000) 853:1–4. doi: 10.1016/S0006-8993(99)02113-7
47. Jin K, Mao XO, Eshoo MW, Nagayama T, Minami M, Simon RP, et al. Microarray analysis of hippocampal gene expression in global cerebral ischemia. Ann Neurol. (2001) 50:93–103. doi: 10.1002/ana.1073
48. Nihashi T, Inao S, Kajita Y, Kawai T, Sugimoto T, Niwa M, et al. Expression and distribution of beta amyloid precursor protein and beta amyloid peptide in reactive astrocytes after transient middle cerebral artery occlusion. Acta Neurochir. (Wien) (2001) 143:287–95. doi: 10.1007/s007010170109
49. Saido TC, Yokota M, Maruyama K, Yamao-Harigaya W, Tani E, Ihara Y, et al. Spatial resolution of the primary beta-amyloidogenic process induced in postischemic hippocampus. J Biol Chem. (1994) 269:15253–7.
50. Weller RO, Yow HY, Preston SD, Mazanti I, Nicoll JA. Cerebrovascular disease is a major factor in the failure of elimination of Abeta from the aging human brain: implications for therapy of Alzheimer's disease. Ann N Y Acad Sci. (2002) 977:162–8. doi: 10.1111/j.1749-6632.2002.tb04812.x
51. Petrovitch H, White LR, Izmirilian G, Ross GW, Havlik RJ, Markesbery W, et al. Midlife blood pressure and neuritic plaques, neurofibrillary tangles, and brain weight at death: the HAAS. Honolulu-Asia aging Study. Neurobiol Aging (2000) 21:57–62. doi: 10.1016/S0197-4580(00)00106-8
52. Hoffman LB, Schmeidler J, Lesser GT, Beeri MS, Purohit DP, Grossman HT, et al. Less Alzheimer disease neuropathology in medicated hypertensive than nonhypertensive persons. Neurology (2009) 72:1720–6. doi: 10.1212/01.wnl.0000345881.82856.d5
53. Sparks DL, Scheff SW, Liu H, Landers TM, Coyne CM, Hunsaker JR. Increased incidence of neurofibrillary tangles (NFT) in non-demented individuals with hypertension. J Neurol Sci. (1995) 131:162–9. doi: 10.1016/0022-510X(95)00105-B
Keywords: cognitive function, dementia, mild cognitive impairment, hypertension, blood pressure, community-based study
Citation: Liang X, Shan Y, Ding D, Zhao Q, Guo Q, Zheng L, Deng W, Luo J, Tse LA and Hong Z (2018) Hypertension and High Blood Pressure Are Associated With Dementia Among Chinese Dwelling Elderly: The Shanghai Aging Study. Front. Neurol. 9:664. doi: 10.3389/fneur.2018.00664
Received: 29 March 2018; Accepted: 25 July 2018;
Published: 04 September 2018.
Edited by:
Ingrid van der Mei, Menzies Institute for Medical Research, AustraliaReviewed by:
Christophe Tzourio, Université de Bordeaux, FranceSteve Simpson Jr, University of Melbourne, Australia
Copyright © 2018 Liang, Shan, Ding, Zhao, Guo, Zheng, Deng, Luo, Tse and Hong. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ying Shan, c2hhbnlpbmdsaUB5YWhvby5jb20=
Ding Ding, ZGluZ2RpbmdAaHVhc2hhbi5vcmcuY24=
 Xiaoniu Liang
Xiaoniu Liang Ying Shan2,3*
Ying Shan2,3* Ding Ding
Ding Ding Qihao Guo
Qihao Guo Wei Deng
Wei Deng