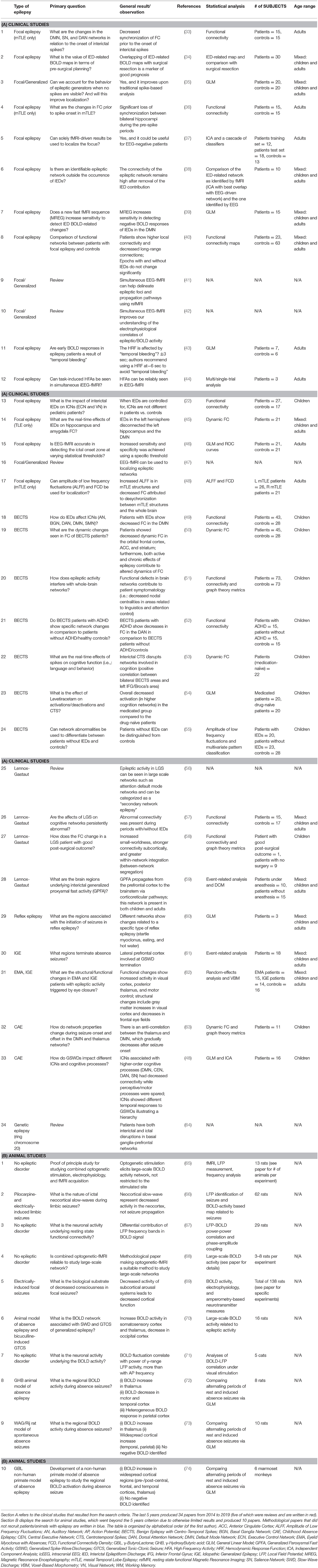
- 1EEG and Epilepsy Unit, Neurology Department, University Hospitals and Faculty of Medicine of Geneva, Geneva, Switzerland
- 2Neurology Clinic, University Hospitals and Faculty of Medicine of Geneva, Geneva, Switzerland
Brain functions do not arise from isolated brain regions, but from interactions in widespread networks necessary for both normal and pathological conditions. These Intrinsic Connectivity Networks (ICNs) support cognitive processes such as language, memory, or executive functions, but can be disrupted by epileptic activity. Simultaneous EEG-fMRI can help explore the hemodynamic changes associated with focal or generalized epileptic discharges, thus providing information about both transient and non-transient impairment of cognitive networks related to spatio-temporal overlap with epileptic activity. In the following review, we discuss the importance of interictal discharges and their impact on cognition in different epilepsy syndromes. We explore the cognitive impact of interictal activity in both animal models and human connectivity networks in order to confirm that this effect could have a possible clinical impact for prescribing medication and characterizing post-surgical outcome. Future work is needed to further investigate electrophysiological changes, such as amplitude/latency of single evoked responses or spontaneous epileptic activity in either scalp or intracranial EEG and determine its relative change in hemodynamic response with subsequent network modifications.
Introduction
Epilepsy cannot be reduced solely to the dysfunction of the seizure onset zone (SOZ), as more widespread abnormalities can be seen, resulting in heterogeneous deficits across cognitive domains (1–6). This supports the view that epilepsy is a network disease associated with complex cognitive deficits (7–11). While these cognitive deficiencies are increasingly recognized as important co-morbidities of epileptic disorders, they are still insufficiently understood and investigated. These deficits can also affect cortical regions that are remote from the epileptogenic zone. For instance, patients with temporal lobe epilepsy can suffer from frontal lobe dysfunction (executive functions) (12, 13). Conversely, patients with frontal lobe epilepsy can suffer from medial temporal lobe dysfunction (memory encoding) (14).
Epileptic Activity Can Dynamically Affect Cognition
Different hypotheses have tried to explain these deficits. A disruptive role of interictal epileptic discharges (IEDs) during ongoing physiological activity has been shown even if these discharges do not result in clinical signs of a seizure; the occurrence of IEDs can therefore be related to transient cognitive impairment (15–18). Previous studies based on intracranial EEG have investigated how epileptic activity can alter normal cognitive processing through large-scale network disruption (16–18); however, due to the low spatial sampling of electrophysiological recordings, it is often challenging to map these networks without prior assumptions on the relevant brain regions to be recorded. Although intracranial EEG has high temporal and spatial resolutions, it has a low spatial sampling, thus preventing this tool to be used alone to investigate large-scale networks.
Interactions Between Epileptic Activity and Cognitive Networks
Cognition engages large-scale brain networks (19–21). Resting-state fMRI (rsfMRI) investigates synchronous activity between regions in the absence of an explicit task and can be subdivided into Intrinsic Connectivity Networks (ICNs) (22). The spatial organization of ICNs has been consistent with relevant cognitive tasks, however with subtle variations (23). As such, previous studies have implied that cognitive networks remain dynamically active even during periods of rest (24, 25). The effect of interictal activity could explain part of the nature of cognitive dysfunction in patients with epilepsy. So far, studies have mostly focused on the cognitive disturbances associated with the occurrence of IEDs (15–18). However, the interactions between detailed spatio-temporal aspects of epileptic activity and changes in ICNs and task-related cognitive networks have not been greatly explored. Therefore, the current review will discuss the current applications of EEG-fMRI in relation to cognition in both human and animal studies.
EEG-fMRI
The simultaneous recording of EEG and fMRI allows for data acquisition with high spatio-temporal resolution, thereby making it possible to map hemodynamic changes related to interictal epileptic activity (26, 27). EEG-fMRI is classically used to estimate the localization of the epileptogenic zone in the context of pre-surgical investigation of epilepsies (28–31), and only a few studies have used EEG-fMRI to investigate the direct effect of epileptic activity on cognition (22, 32).
Methods
For this review, we performed a comprehensive literature search on the Medline PubMed database of all original research articles to date (July 2019) within the last 5 years (see Figure 1) with the keywords: (1) “epilepsy” AND “cognitive OR cognition” AND “EEG-fMRI”, (2) “epilepsy” AND “cognitive OR cognition” AND “EEG AND fMRI AND simultaneous.” However, due to the restrictive parameters, we only received one paper as a result in animal studies; therefore the parameters were extended to become more permissive by excluding the “cognitive OR cognition” criteria and expanding the timeline. Articles were excluded from the review if they were case studies or not in English. Some of the resulting papers (see Table 1) were methods-based, and were therefore summarized in the review, but not explained in detail as the purpose was to explore the role of EEG-fMRI in cognition. In the following sections, we discuss the role of EEG-fMRI in investigating the interaction between epileptic discharges and cognitive networks.
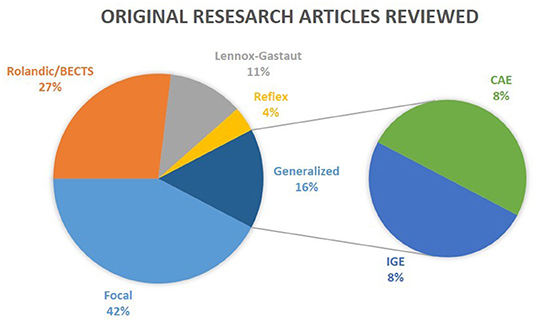
Figure 1. Epilepsy types reviewed. The results of the review on clinical studies are separated into five distinct categories: Rolandic/BECTS, Lennox-Gastaut, Reflex, Focal, and Generalized. CAE and IGE patients are considered subgroups of Generalized epilepsy. Reviews were not taken into consideration in this illustration.
EEG-fMRI in Animal Models of Epilepsy
The use of combined EEG-fMRI in animal models, and in particular animal models of epilepsy, comes with two major benefits: first, it allows us to control for more parameters than in human research, thus providing more insights into the biological substrates of the BOLD signal, as illustrated by studies using optogenetic tools (65). Second, it gives access to the epileptic network (10, 11, 75), as it offers the opportunity to sample multiple brain regions related to the activity of the epileptic focus, with much higher spatial and temporal resolution in comparison to studies in humans (76).
BOLD signal analysis can highlight the network recruited during epileptic seizures. Different studies on animal models of generalized seizures (70, 72–74) have shown that the increase in BOLD activity is heterogeneous, and involves specifically thalamo-cortical circuits. These results are in line with the hypothesis that generalized seizures actually represent rapidly-propagating seizures with bilateral onset (77). Thus, fMRI signal can be used to map the network related to one particular “pre-identified” neural activity.
The inverse approach, i.e., to use BOLD signal to identify regions of interest and then guide electrophysiological recordings, is also a powerful tool, as shown in an elegant study in a rat model of temporal lobe epilepsy (69). In this study, the authors investigated the mechanisms of loss of consciousness using EEG-fMRI together with choline amperometry recordings. In short, they found that during focal limbic seizures, BOLD signal increases in the hippocampus [as expected (66)] and also decreases in cortical areas. This result was associated with a decreased firing of cholinergic neurons, but not non-cholinergic neurons, in the subcortical arousal system of the brainstem (69). This could explain, at least in part, the alteration of arousal during focal seizure. Very brief or partial arousal impairment could play an important role in transient cognitive impairments. Therefore, BOLD-guided electrophysiology provides a complementary tool to investigate the perturbation of brain networks during seizures. Aside from consciousness, EEG-fMRI studies of cognition in animals have remained scarce thus far (67, 71).
EEG-fMRI in the Study of Cognition in Humans
Previous studies have commented on the relationship between cognition and ICNs extracted from traditional resting state fMRI, especially in relation to patients with epilepsy (78–80). ICNs can be ascribed to specific functions, such as self-awareness, attention, cognitive control, or perceptions such as visual, auditory, or motor (81–83). There is some spatial overlap between these networks in both patients and healthy controls; however the abnormal modulation of activity between these networks can be indicative of a patient's clinical syndrome.
Over the last 5 years there has been a substantial increase in the use of EEG-fMRI, especially for pre-surgical evaluations for patients with epilepsy (7, 35, 37, 41, 46, 47). However, the effects of IEDs on cognitive networks were not often explored until recently. Following pioneering work relating IED-correlated decreases in Default Mode Network activity in temporal lobe epilepsy (84) and generalized epilepsy (85), recent works have shown the possible impact of interictal activity on several ICNs in focal epilepsy in adults (33, 36, 45), focal epilepsy in children (22), children with idiopathic focal epilepsy [Benign Epilepsy with Centro-temporal Spikes (BECTS)] (53, 55, 86), epileptic encephalopathy (56–59), as well as generalized epilepsies (61, 64), including Childhood Absence Epilepsy (CAE) (87), and even reflex epilepsies (60). The majority of recent EEG-fMRI studies who evaluate the interaction between interictal discharges, ICNs, and their relationship to neuropsychological outcome have been in BECTS patients; these studies found a negative correlation between cognitive functioning and Functional Connectivity (FC). Nevertheless, though patients with epilepsy are a heterogeneous population, all groups show a widespread influence of interictal activity on ICNs; as ICNs have previously been related to cognitive function, this strengthens the notion that IEDs have a definitive impact on cognitive functioning.
IEDs and Cognitive Performance
There are two ways to study the impact of IEDs on cognitive processing. One is to compare cognitive processing between patients with different IED occurrences (or other IED parameters such as: duration, or periods before vs. after onset of IEDs). Some evidence suggests that IEDs can be a marker of poor cognitive prognosis (88, 89) and their treatment could improve behavior in children (90). IED burden also plays a role, as shown by the fact that a diurnal occurrence of IEDs >10% of EEG duration is correlated with poorer information processing speed, verbal memory and visuo-motor integration in children (91).
Another way to probe the mechanisms through which IEDs perturb cognitive functions is to ask whether or not the occurrence of a single IED can directly affect brain processing. Indeed, IEDs could affect normal cognitive processing through transient disruption of brain networks, a paradigm known as transitory cognitive impairment (TCI) (92). Aarts et al. (93) showed that the occurrence of IEDs in patients with different kinds of epilepsy affected performance during a cognitive task, and further showed that left-sided IEDs tended to elicit errors in the verbal task and right-sided IEDs in the non-verbal task. Kleen et al. and Ung et al. added a level of complexity by showing that the laterality of the IEDs relative to the epileptic focus determined the existence of abnormal processing. It is interesting to observe that cognitive processing in turn can also modulate IED frequency (17, 94). An increase of temporal IEDs was indeed observed during cognitive tasks involving temporal structures (94), suggesting that increases in physiological activity might also favor the recruitment of local pathological networks. This further entangles the relationship between epileptic and physiological activity.
IEDs and Cognitive Networks
These studies highlight the fact that consideration of IEDs has to be integrated with network imaging to understand how IEDs affect brain processing. This was investigated in a patient with idiopathic generalized epilepsy using EEG-fMRI during a memory task, which showed that IEDs perturb the brain network recruited by the task (95). Furthermore, recent studies have found that IEDs interfere with whole brain networks (49, 51), and indeed a recent review found a consensus between studies in both BECTS and CAE patients confirming the significant impact of IEDs on FC measurements (96).
If IEDs and sub-clinical features affect ICNs and therefore the underlying cognitive attributes, the next step is to understand when and how these changes occur. To answer the first question, both Burianová et al. (33) and Faizo et al. (36) explored connectivity prior to IED onset in TLE patients to determine the temporal extent at which connectivity is altered. Regardless of the presence of IEDs, both studies showed patients with abnormal connectivity networks. Burianová et al. (33) demonstrated decreases in functional connectivity (FC) in prefrontal cortices and increases in subcortical areas such as the thalamus (33). However, FC changes were also found prior to IED onset in hippocampal areas (36), thus corroborating the evidence suggesting decreases in FC between the hippocampus and PFC in TLE patients (28). They also found reduced connectivity of the DMN, which occurred prior to IED periods, while reduced connectivity of the salience network occurred during IED periods, relating to behavioral changes in consciousness and attention. Changes in connectivity seen prior to IEDs are particularly interesting as pre-IED hemodynamic changes have also been seen when studying the hemodynamic response function using deconvolution (43, 97, 98). Though the origin of this phenomenon is still unknown, it certainly reflects the existence of pre-IED specific neuronal activity. It would be interesting for future studies to explore the variability of HRF change in this context.
Transient Effects of IEDs On Epileptic and Cognitive Networks
Differences in connectivity measures remain in the absence of IED activity and this implies a separation between “transient” and “non-transient” effects. This can be seen in both adults and children. The connectivity pattern obtained from IED-correlated fMRI analysis is largely preserved in the absence of IEDs (38, 40).
Regarding cognition, Shamshiri et al. (22) found connectivity differences in cognitive networks (related to attention) in a group of children with focal epilepsy compared to controls. However, no evidence remained for non-transient differences in network connectivity between patients and controls, after accounting for IED effects (see Figure 2). These results were also consistent with a MEG study in children with focal epilepsy patients by Ibrahim et al. (99), but are inconsistent with those studies mentioned above (33, 36), possibly due to differences between adult and pediatric populations and their respective variability in plasticity and disease duration (99). Instead, for BECTS patients, several studies reported decreases in functional connectivity regardless of the presence of IEDs (50, 51, 86, 100). These patients showed decreased FC in the inferior frontal gyrus, anterior cingulate cortex, and the striatum, which have previously been related to cognitive control (86). This is particularly interesting as patients with BECTS often display behavioral difficulties and language delays (53). However, the effect of medication should also be taken under consideration when determining differences in functional connectivity. Indeed studies in BECTS patients have shown decreased connectivity in higher order functioning cognitive networks of drug naïve patients in comparison to medicated patients (54). The investigation of the difference between transient vs. non-transient changes in connectivity could benefit from simultaneous EEG-fMRI recordings and accounting for the age-related influence on long-term connectivity changes.
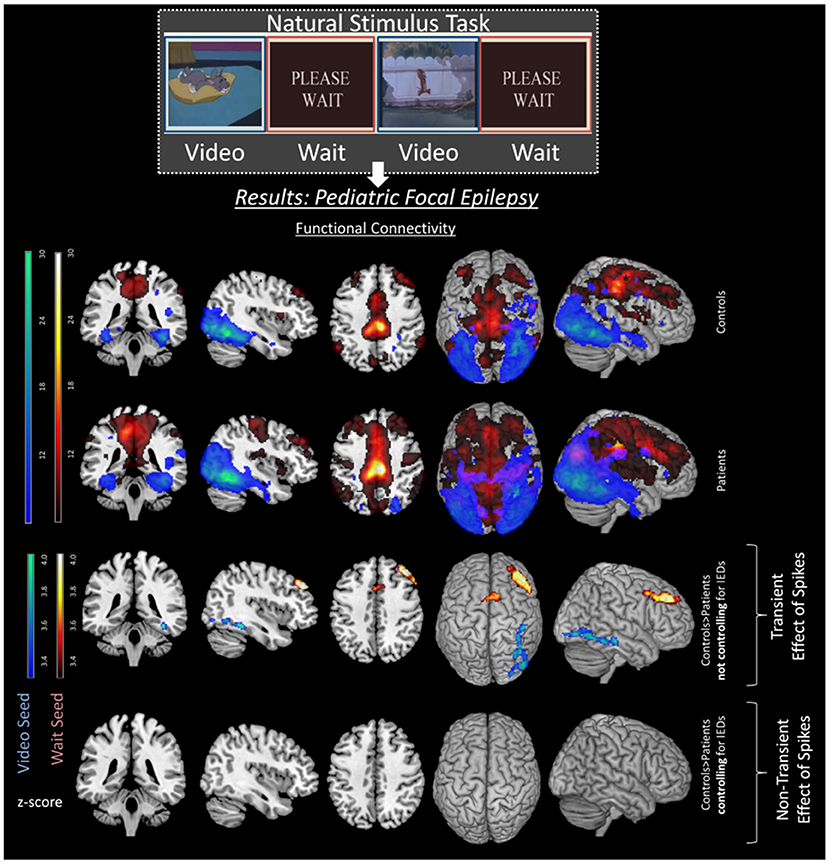
Figure 2. Transient effects of IEDs in pediatric focal epilepsy patients. Image with permission Shamshiri et al. (22) illustrating the effects of spikes on FC networks of a resting state task. Differences between controls (top row) and patients (second row) can be seen in the third row. These differences are including both transient and long-term effects of spikes as spikes are not controlled for in the analysis. However, once the transient effects of spikes are accounted for, the group differences disappear (fourth row), emphasizing the effect of IEDs on ICNs.
Spatial Considerations of IEDs
It is not only the temporal dynamics of interictal activity that are interesting, but also where these events occur. Indeed the spatial pattern can have an influence on which cognitive domain is predominantly affected. For example, in TLE patients the laterality of IED activity can preferentially damage certain cognitive abilities, such that left temporal IEDs were associated with disconnections to the hippocampus and the Default Mode Network (DMN) while right temporal IEDs were co-activated with the reward-emotion network, which could be involved in forced normalization (a condition in which patients show psychiatric degradation when the IEDs disappear under treatment) (45).
In contrast to local IEDs, such as those seen in TLE patients, generalized (bilateral synchronous) epileptic activity can have a more global effect on ICNs. CAE patients also have widespread GSWD-related decreases found in DMN, DAN, central executive, and salience networks (87). Also, in ring chromosome 20 syndrome, which is a rare and severe form of generalized epilepsy, increases in slow wave rhythm were related to decreases in activity of the DMN and Dorsal Attention Network (DAN) (64). However, the clinical meaning of this slow-wave activity, and whether it supplies transient or long-term effects on cognition, is still under debate. Patients with Lennox-Gastaut syndrome suffer from diffuse cognitive impairment and present widespread, often “generalized” epileptiform activity. In this group, there is no difference in network behavior between fMRI periods affected or unaffected by discharges (101). This pattern is in favor of a more chronic and enduring impairment in this condition, as reflected by the associated encephalopathy. Therefore, generalized epilepsies also show widespread decreases in ICNs especially corresponding to higher order cognitive processes (64, 87).
Perspectives
The study of IED-related effects on cognitive networks may be difficult in many patients, given the lack of frequent IEDs to model. Other approaches to model pathologic activity using EEG topographies (31, 34) or other EEG features such as decomposition using Independent Component Analysis (102) may offer alternative markers of epileptic activity to correlate with cognitive network alterations.
Simultaneous intracranial EEG and fMRI would allow to better map fMRI network alterations correlated to intracranial pathological EEG activity. Such recordings (103, 104) focused on the mapping of epileptic network (32) and the coupling between neuronal activity and hemodynamic changes, which is related to the fundamental assumptions underlying fMRI studies. These fMRI studies take advantage of the relationship between neuronal activity (mainly post-synaptic potentials) and deoxyhemoglobin concentration (42) to show the focal changes related to the epileptogenic zone, and reveal distant BOLD modulations related to the interictal epileptic network (104). Simultaneous recordings of intracranial EEG and scalp EEG could also uncover new non-invasive markers of epileptic activity that are currently undetectable on scalp EEG but could nevertheless affect cognitive processing. Such markers could be used to refine EEG-fMRI analysis (105, 106).
The possibility to inform fMRI analysis using EEG-derived brain activity offers several perspectives to study the spatio-temporal aspects of cognitive networks, at rest or engaged in specific tasks, in a more selective way than using fMRI, EEG or MEG alone. The characteristics of task-related EEG evoked responses (amplitude, latency) can be included in the fMRI analysis to model and map the network involved in such responses, such as attention and error monitoring (107, 108) and therefore also study interactions with epileptic activity. EEG measures of arousal (e.g., drowsiness or sleep) could also be valuable to study alterations of cognitive networks. Changes in arousal have a significant effect on fMRI connectivity patterns than can even be used to monitor drowsiness during scanning (109). This could be particularly relevant when studying patients with epilepsy vs. controls when drowsiness could show group differences, notably related to drug-induced sedation, sleep deprivation or scanner related anxiety. Antiepileptic drugs affect fMRI brain networks in healthy controls (110) and the effect other drugs, such as donepezil and memantine in the field of dementia, have also been documented (111). This contribution of medication is hard to disentangle from the effect of disease, notably due to the high variability of drug regimes in patient groups and the difficulty to recruit drug naïve patients. EEG markers of medication, such as beta activity or increased drowsiness could be used to try to model this effect in the analysis.
Conversely, fMRI offers the possibility of high spatial resolution to localize cortical and subcortical brain regions at a whole brain scale that are involved in EEG patterns and therefore make it superior in this regard to source localization and connectivity measures based on EEG or MEG alone. Also, taking advantage of the combined high temporal and spatial resolution of EEG and fMRI, EEG connectivity analysis describing directed connections and dynamic aspects (high temporal resolution) could be based on spatial networks revealed by fMRI (whole brain, high spatial resolution) to enhance network characterization.
Future studies could also address the relationship between IEDs and brain rhythms (11, 17), and how this disrupts normal cognitive processing, which are known to rely on specific brain oscillatory activity (19, 20, 112–114).
Conclusion
Overall, the temporal and spatial effects of epileptic activity and medication can all influence changes in ICNs and cognitive functioning. Although there has been an increase in interest regarding EEG-fMRI and the effects of epileptic activity on ICNs, as reflected by the number of results from our search (see Figure 1, and Table 1), there is still much to learn about how to use this information to understand the long-term impact of interictal activity and cognition and improve the decision making regarding the therapy of patients with epilepsy. Globally, there are differences between focal/non-focal epilepsies, especially in regards to which ICNs or task-related networks are more sensitive to IEDs and how the epileptogenic network influenced the findings. Nevertheless all groups show a widespread influence of interictal activity but also some IED-independent alterations.
Author Contributions
ES researched, wrote, and reviewed all work pertaining to human subjects. LS researched, wrote, and reviewed all work pertaining to animal models. SV edited all work that was reviewed in this article.
Funding
The study was funded by Swiss National Science Foundation (grants 169198 and Sinergia 170873) and part of this work was performed within the FLAG-ERA/JTC 2017 SCALES project.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. Hilger E, Zimprich F, Pataraia E, Aull-Watschinger S, Jung R, Baumgartner C, et al. Psychoses in epilepsy: a comparison of postictal and interictal psychoses. Epilepsy Behav. (2016) 60:58–62. doi: 10.1016/j.yebeh.2016.04.005
2. Lin J, Mula M, Hermann B. Uncovering the lifespan neurobehavioral comorbidities of epilepsy. Lancet. (2012) 38:1180–92. doi: 10.1016/S0140-6736(12)61455-X
3. Oyegbile TO, Dow C, Jones J, Bell B, Rutecki P, Sheth R. The nature and course of neuropsychological morbidity in chronic temporal lobe epilepsy. Neurology. (2004) 62:1736–42. doi: 10.1212/01.WNL.0000125186.04867.34
4. Savage N. Epidemiology: the complexities of epilepsy. Nature. (2014) 511:S2–3. doi: 10.1038/511S2a
5. Schoenfeld J, Seidenberg M, Woodard A, Hecox K, Inglese C, Mack K, et al. Neuropsychological and behavioral status of children with complex partial seizures. Dev Med Child Neurol. (1999) 41:724–31. doi: 10.1017/S0012162299001486
6. Hermann B, Seidenberg M, Jones J. The neurobehavioural comorbidities of epilepsy: can a natural history be developed? Lancet Neurol. (2008) 7:151–60. doi: 10.1016/S1474-4422(08)70018-8
7. Centeno M, Carmichael DW. Network connectivity in epilepsy: resting state fMRI and EEG-fMRI contributions. Front Neurol. (2014) 5:93. doi: 10.3389/fneur.2014.00093
8. Laufs H, Rodionov R, Thornton R, Duncan JS, Lemieux L, Tagliazucchi E. Altered FMRI connectivity dynamics in temporal lobe epilepsy might explain seizure semiology. Front Neurol. (2014) 5:175. doi: 10.3389/fneur.2014.00175
9. Pittau F, Mégevand P, Sheybani L, Abela E, Grouiller F, Spinelli L, et al. Mapping epileptic activity: sources or networks for the clinicians? Front Neurol. (2014) 5:218. doi: 10.3389/fneur.2014.00218
10. Sheybani L, Birot G, Contestabile A, Seeck M, Kiss JZ, Schaller K, et al. Electrophysiological evidence for the development of a self-sustained large-scale epileptic network in the kainate mouse model of temporal lobe epilepsy. J Neurosci. (2018) 38:3776–91. doi: 10.1523/JNEUROSCI.2193-17.2018
11. Sheybani L, van Mierlo P, Birot G, Michel CM, Quairiaux C. Large-Scale 3–5 Hz oscillation constrains the expression of neocortical fast ripples in a mouse model of mesial temporal lobe epilepsy. eNeuro. (2019) 6:ENEURO.0494–18.2019. doi: 10.1523/ENEURO.0494-18.2019
12. Stretton J, Pope RA, Winston GP, Sidhu MK, Symms M, Duncan JS, et al. Temporal lobe epilepsy and affective disorders: the role of the subgenual anterior cingulate cortex. J Neurol Neurosurg Psychiatry. (2015) 86:144–51. doi: 10.1136/jnnp-2013-306966
13. Keller SS, Baker G, Downes JJ, Roberts N. Quantitative MRI of the prefrontal cortex and executive function in patients with temporal lobe epilepsy. Epilepsy Behav. (2009) 15:186–95. doi: 10.1016/j.yebeh.2009.03.005
14. Centeno M, Vollmar C, O'Muircheartaigh J, Stretton J, Bonelli SB, Symms MR, et al. Memory in frontal lobe epilepsy: an fMRI study. Epilepsia. (2012) 53:1756–64. doi: 10.1111/j.1528-1167.2012.03570.x
15. Horak PC, Meisenhelter S, Song Y, Testorf ME, Kahana MJ, Viles WD, et al. Interictal epileptiform discharges impair word recall in multiple brain areas. Epilepsia. (2017) 58:373–80. doi: 10.1111/epi.13633
16. Kleen JK, Scott RC, Holmes GL, Roberts DW, Rundle MM, Testorf M, et al. Hippocampal interictal epileptiform activity disrupts cognition in humans. Neurology. (2013) 81:18–24. doi: 10.1212/WNL.0b013e318297ee50
17. Matsumoto JY, Stead M, Kucewicz MT, Matsumoto AJ, Peters PA, Brinkmann BH, et al. Network oscillations modulate interictal epileptiform spike rate during human memory. Brain. (2013) 136:2444–56. doi: 10.1093/brain/awt159
18. Ung H, Cazares C, Nanivadekar A, Kini L, Wagenaar J, Becker D, et al. Interictal epileptiform activity outside the seizure onset zone impacts cognition. Brain. (2017) 18:2157–68. doi: 10.1093/brain/awx143
19. Backus AR, Schoffelen JM, Szebényi S, Hanslmayr S, Doeller CF. Hippocampal-prefrontal theta oscillations support memory integration. Curr Biol. (2016) 26:450–7. doi: 10.1016/j.cub.2015.12.048
20. Spellman T, Rigotti M, Ahmari SE, Fusi S, Gogos JA, Gordon JA. Hippocampal-prefrontal input supports spatial encoding in working memory. Nature. (2015) 522:309–14. doi: 10.1038/nature14445
21. Wacongne C, Labyt E, van Wassenhove V, Bekinschtein T, Naccache L, Dehaene S. Evidence for a hierarchy of predictions and prediction errors in human cortex. Proc Natl Acad Sci USA. (2011) 108:20754–9. doi: 10.1073/pnas.1117807108
22. Shamshiri EA, Tierney TM, Centeno M, St. Pier K, Pressler RM, Sharp DJ, et al. Interictal activity is an important contributor to abnormal intrinsic network connectivity in paediatric focal epilepsy. Hum Brain Mapp. (2017) 236:221–36. doi: 10.1002/hbm.23356
23. Doucet GE, He X, Sperling MR, Sharan A, Tracy JI. From “rest” to language task: task activation selects and prunes from broader resting-state network. Hum Brain Mapp. (2017) 38:2540–52. doi: 10.1002/hbm.23539
24. Fox MD, Snyder AZ, Vincent JL, Corbetta M, Van Essen DC, Raichle ME. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc Natl Acad Sci USA. (2005) 102:9673–8. doi: 10.1073/pnas.0504136102
25. Smith SJM. EEG in the diagnosis, classification, and management of patients with epilepsy. J Neurol Neurosurg Psychiatry. (2005) 76(Suppl. 2):ii2–7. doi: 10.1136/jnnp.2005.069245
26. Ives JR, Warach S, Schmitt F, Edelman RR, Schomer DL. Monitoring the patient's EEG during echo planar MRI. Electroencephalogr Clin Neurophysiol. (1993) 87:417–20. doi: 10.1016/0013-4694(93)90156-P
27. Lemieux L, Salek-Haddadi A, Josephs O, Allen P, Toms N, Scott C, et al. Event-related fMRI with simultaneous and continuous EEG: description of the method and initial case report. Neuroimage. (2001) 14:780–7. doi: 10.1006/nimg.2001.0853
28. Pittau F, Grova C, Moeller F, Dubeau F, Gotman J. Patterns of altered functional connectivity in mesial temporal lobe epilepsy. Epilepsia. (2012) 53:1013–23. doi: 10.1111/j.1528-1167.2012.03464.x
29. Thornton RC, Rodionov R, Laufs H, Vulliemoz S, Vaudano A, Carmichael D, et al. Imaging hemodynamic changes related to seizures: comparison of EEG-based general linear model, independent component analysis of fMRI and intracranial EEG. Neuroimage. (2010) 53:196–205. doi: 10.1016/j.neuroimage.2010.05.064
30. van Houdt PJ, de Munck JC, Leijten FSS, Huiskamp GJM, Colon AJ, Boon PAJM, et al. EEG-fMRI correlation patterns in the presurgical evaluation of focal epilepsy: a comparison with electrocorticographic data and surgical outcome measures. Neuroimage. (2013) 75:246–56. doi: 10.1016/j.neuroimage.2013.02.033
31. Grouiller F, Thornton RC, Groening K, Spinelli L, Duncan JS, Schaller K, et al. With or without spikes: localization of focal epileptic activity by simultaneous electroencephalography and functional magnetic resonance imaging. Brain. (2011) 134:2867–86. doi: 10.1093/brain/awr156
32. Chaudhary UJ, Duncan JS, Lemieux L. Mapping hemodynamic correlates of seizures using fMRI: a review. Hum Brain Mapp. (2013) 34:447–66. doi: 10.1002/hbm.21448
33. Burianová H, Faizo NL, Gray M, Hocking J, Galloway G, Reutens D. Altered functional connectivity in mesial temporal lobe epilepsy. Epilepsy Res. (2017) 137:45–52. doi: 10.1016/j.eplepsyres.2017.09.001
34. Coan AC, Chaudhary UJ, Grouiller F, Campos BM, Perani S, De Ciantis A, et al. EEG-fMRI in the presurgical evaluation of temporal lobe epilepsy. J Neurol Neurosurg Psychiatry. (2016) 87:642–9. doi: 10.1136/jnnp-2015-310401
35. Ebrahimzadeh E, Soltanian-Zadeh H, Araabi BN, Fesharaki SSH, Habibabadi JM. Component-related BOLD response to localize epileptic focus using simultaneous EEG-fMRI recordings at 3T. J Neurosci Methods. (2019) 322:34–49. doi: 10.1016/j.jneumeth.2019.04.010
36. Faizo NL, Burianová H, Gray M, Hocking J, Galloway G, Reutens D. Identification of pre-spike network in patients with mesial temporal lobe epilepsy. Front Neurol. (2014) 5:222. doi: 10.3389/fneur.2014.00222
37. Hunyadi B, Tousseyn S, Dupont P, Van Huffel S, De Vos M, Van Paesschen W. A prospective fMRI-based technique for localising the epileptogenic zone in presurgical evaluation of epilepsy. Neuroimage. (2015) 113:329–39. doi: 10.1016/j.neuroimage.2015.03.011
38. Iannotti GR, Grouiller F, Centeno M, Carmichael DW, Abela E, Wiest R, et al. Epileptic networks are strongly connected with and without the effects of interictal discharges. Epilepsia. (2016) 57:1086–96. doi: 10.1111/epi.13400
39. Jacobs J, Menzel A, Ramantani G, Körbl K, Assländer J, Schulze-Bonhage A, et al. Negative BOLD in default-mode structures measured with EEG-MREG is larger in temporal than extra-temporal epileptic spikes. Front Neurosci. (2014) 8:335. doi: 10.3389/fnins.2014.00335
40. Luo C, An D, Yao D, Gotman J. Patient-specific connectivity pattern of epileptic network in frontal lobe epilepsy. NeuroImage Clin. (2014) 4:668–75. doi: 10.1016/j.nicl.2014.04.006
41. Maesawa S, Bagarinao E, Fujii M, Futamura M, Wakabayashi T. Use of network analysis to establish neurosurgical parameters in gliomas and epilepsy. Neurol Med Chir (Tokyo). (2016) 56:158–69. doi: 10.2176/nmc.ra.2015-0302
42. Murta T, Leite M, Carmichael DW, Figueiredo P, Lemieux L. Electrophysiological correlates of the BOLD signal for EEG-informed fMRI. Hum Brain Mapp. (2015) 36:391–414. doi: 10.1002/hbm.22623
43. Rollings DT, Assecondi S, Ostwald D, Porcaro C, McCorry D, Bagary M, et al. Early hemodynamic changes observed in patients with epilepsy, in a visual experiment and in simulations. Clin Neurophysiol. (2016) 127:245–53. doi: 10.1016/j.clinph.2015.07.008
44. Saignavongs M, Ciumas C, Petton M, Bouet R, Boulogne S, Rheims S, et al. Neural activity elicited by a cognitive task can be detected in single-trials with simultaneous intracerebral EEG-fMRI recordings. Int J Neural Syst. (2017) 27:1750001. doi: 10.1142/S0129065717500010
45. Tong X, An D, Xiao F, Lei D, Niu R, Li W, et al. Real-time effects of interictal spikes on hippocampus and amygdala functional connectivity in unilateral temporal lobe epilepsy: an EEG-fMRI study. Epilepsia. (2019) 60:246–54. doi: 10.1111/epi.14646
46. Tousseyn S, Dupont P, Goffin K, Sunaert S, Van Paesschen W. Sensitivity and specificity of interictal EEG-fMRI for detecting the ictal onset zone at different statistical thresholds. Front Neurol. (2014) 5:131. doi: 10.3389/fneur.2014.00131
47. van Graan LA, Lemieux L, Chaudhary UJ. Methods and utility of EEG-fMRI in epilepsy. Quant Imaging Med Surg. (2015) 5:300–12. doi: 10.3978/j.issn.2223-4292.2015.02.04
48. Zhang Z, Xu Q, Liao W, Wang Z, Li Q, Yang F, et al. Pathological uncoupling between amplitude and connectivity of brain fluctuations in epilepsy. Hum Brain Mapp. (2015) 36:2756–66. doi: 10.1002/hbm.22805
49. Li R, Ji GJ, Yu Y, Yu Y, Ding MP, Tang YL, et al. Epileptic discharge related functional connectivity within and between networks in benign epilepsy with centrotemporal spikes. Int J Neural Syst. (2017) 27:1750018. doi: 10.1142/S0129065717500186
50. Li R, Liao W, Yu Y, Chen H, Guo X, Tang YL, et al. Differential patterns of dynamic functional connectivity variability of striato–cortical circuitry in children with benign epilepsy with centrotemporal spikes. Hum Brain Mapp. (2018) 39:1207–17. doi: 10.1002/hbm.23910
51. Xiao F, Lei D, An D, Li L, Chen S, Chen F, et al. Functional brain connectome and sensorimotor networks in rolandic epilepsy. Epilepsy Res. (2015) 113:113–25. doi: 10.1016/j.eplepsyres.2015.03.015
52. Xiao F, Li L, An D, Lei D, Tang Y, Yang T, et al. Altered attention networks in benign childhood epilepsy with centrotemporal spikes (BECTS): a resting-state fMRI study. Epilepsy Behav. (2015) 45:234–41. doi: 10.1016/j.yebeh.2015.01.016
53. Xiao F, An D, Lei D, Li L, Chen S, Wu X, et al. Real-time effects of centrotemporal spikes on cognition in rolandic epilepsy. J Neurol. (2016) 86:544–51. doi: 10.1212/WNL.0000000000002358
54. Zhang Q, Yang F, Hu Z, Xu Q, Bernhardt BC, Quan W, et al. Antiepileptic drug of levetiracetam decreases centrotemporal spike-associated activation in rolandic epilepsy. Front Neurosci. (2018) 12:1–9. doi: 10.3389/fnins.2018.00796
55. Zhu Y, Yu Y, Shinkareva SV, Ji GJ, Wang J, Wang ZJ, et al. Intrinsic brain activity as a diagnostic biomarker in children with benign epilepsy with centrotemporal spikes. Hum Brain Mapp. (2015) 36:3878–89. doi: 10.1002/hbm.22884
56. Archer JS, Warren AE, Jackson GD, Abbott DF. Conceptualizing lennox–gastaut syndrome as a secondary network epilepsy. Front Neurol. (2014) 5:225. doi: 10.3389/fneur.2014.00225
57. Warren AE, Abbott DF, Vaughan DN, Jackson GD, Archer JS. Abnormal cognitive network interactions in Lennox-Gastaut syndrome: a potential mechanism of epileptic encephalopathy. Epilepsia. (2016) 57:812–22. doi: 10.1111/epi.13342
58. Warren AEL, Harvey AS, Abbott DF, Vogrin SJ, Bailey C, Davidson A, et al. Cognitive network reorganization following surgical control of seizures in Lennox-Gastaut syndrome. Epilepsia. (2017) 58:e75–81. doi: 10.1111/epi.13720
59. Warren AEL, Harvey AS, Vogrin SJ, Bailey C, Davidson A, Jackson GD, et al. The epileptic network of Lennox-Gastaut syndrome. Neurology. (2019) 93:e215–26. doi: 10.1212/WNL.0000000000007775
60. Sandhya M, Bharath RD, Panda R, Chandra SR, Kumar N, George L, et al. Understanding the pathophysiology of reflex epilepsy using simultaneous EEG-fMRI. Epileptic Disord. (2014) 16:19–29. doi: 10.1684/epd.2014.0632
61. Benuzzi F, Ballotta D, Mirandola L, Ruggieri A, Vaudano AE, Zucchelli M, et al. An EEG-fMRI study on the termination of generalized spike-and-wave discharges in absence epilepsy. PLoS ONE. (2015) 10:e0130943. doi: 10.1371/journal.pone.0130943
62. Vaudano AE, Ruggieri A, Tondelli M, Avanzini P, Benuzzi F, Gessaroli G, et al. The visual system in eyelid myoclonia with absences. Ann Neurol. (2014) 76:412–27. doi: 10.1002/ana.24236
63. Liao W, Zhang Z, Mantini D, Xu Q, Ji GJ, Zhang H, et al. Dynamical intrinsic functional architecture of the brain during absence seizures. Brain Struct Funct. (2014) 219:2001–15. doi: 10.1007/s00429-013-0619-2
64. Vaudano AE, Ruggieri A, Vignoli A, Canevini MP, Meletti S. Emerging neuroimaging contribution to the diagnosis and management of the ring chromosome 20 syndrome. Epilepsy Behav. (2015) 45:155–63. doi: 10.1016/j.yebeh.2015.02.002
65. Duffy BA, Choy MK, Chuapoco MR, Madsen M, Lee JH. MRI compatible optrodes for simultaneous LFP and optogenetic fMRI investigation of seizure-like afterdischarges. Neuroimage. (2015) 123:173–84. doi: 10.1016/j.neuroimage.2015.07.038
66. Englot DJ, Mishra AM, Mansuripur PK, Herman P, Hyder F, Blumenfeld H. Remote effects of focal hippocampal seizures on the rat neocortex. J Neurosci. (2008) 28:9066–81. doi: 10.1523/JNEUROSCI.2014-08.2008
67. Jaime S, Gu H, Sadacca BF, Stein EA, Cavazos JE, Yang Y, et al. Delta rhythm orchestrates the neural activity underlying the resting state BOLD signal via phase-amplitude coupling. Cereb Cortex. (2019) 29:119–33. doi: 10.1093/cercor/bhx310
68. Lee JH, Durand R, Gradinaru V, Zhang F, Goshen I, Kim DS, et al. Global and local fMRI signals driven by neurons defined optogenetically by type and wiring. Nature. (2010) 465:788–92. doi: 10.1038/nature09108
69. Motelow JE, Li W, Zhan Q, Mishra AM, Sachdev RN, Liu G, et al. Decreased subcortical cholinergic arousal in focal seizures. Neuron. (2015) 85:233–6. doi: 10.1016/j.neuron.2014.12.058
70. Nersesyan H, Hyder F, Rothman DL, Blumenfeld H. Dynamic fMRI and EEG recordings during spike-wave seizures and generalized tonic-clonic seizures in WAG/Rij rats. J Cereb Blood Flow Metab. (2004) 24:589–99. doi: 10.1097/01.WCB.0000117688.98763.23
71. Niessing J, Ebisch B, Schmidt KE, Niessing M, Singer W, Galuske RAW. Hemodynamic signals correlate tightly with synchronized gamma oscillations. Science. (2005) 309:948–51. doi: 10.1126/science.1110948
72. Tenney JR, King JA, Duong TQ, Ferris CF. Corticothalamic activation during experimental absence seizures in Rats: a functional MRI assessment. Epilepsia. (2003) 44:1133–40. doi: 10.1046/j.1528-1157.2003.61002.x
73. Tenney JR, Duong TQ, King JA, Ferris CF. fMRI of brain activation in a genetic rat model of absence seizures. Epilepsia. (2004) 45:576–82. doi: 10.1111/j.0013-9580.2004.39303.x
74. Tenney JR, Marshall PC, King JA, Ferris CF. FMRI of generalized absence status epilepticus in conscious marmoset monkeys reveals corticothalamic activation. Epilepsia. (2004) 45:1240–7. doi: 10.1111/j.0013-9580.2004.21504.x
75. Pittau F, Grouiller F, Spinelli L, Seeck M, Michel CM, Vulliemoz S. The role of functional neuroimaging in pre-surgical epilepsy evaluation. Front Neurol. (2014) 5:31. doi: 10.3389/fneur.2014.00031
76. Lee JH. Informing brain connectivity with optogenetic functional magnetic resonance imaging. Neuroimage. (2012) 62:2244–9. doi: 10.1016/j.neuroimage.2012.01.116
77. Meeren HKM, Pijn JPM, Van Luijtelaar ELJM, Coenen AML, Lopes da Silva FH. Cortical focus drives widespread corticothalamic networks during spontaneous absence seizures in rats. J Neurosci. (2002) 22:1480–95. doi: 10.1523/JNEUROSCI.22-04-01480.2002
78. Yang T, Luo C, Li Q, Guo Z, Liu L, Gong Q, et al. Altered resting-state connectivity during interictal generalized spike-wave discharges in drug-naïve childhood absence epilepsy. Hum Brain Mapp. (2013) 34:1761–7. doi: 10.1002/hbm.22025
79. Zhang Z, Lu G, Zhong Y, Tan Q, Yang Z, Liao W, et al. Impaired attention network in temporal lobe epilepsy: a resting FMRI study. Neurosci Lett. (2009) 458:97–101. doi: 10.1016/j.neulet.2009.04.040
80. Vlooswijk MC, Jansen JF, de Krom MC, Majoie HJ, Hofman PA, Backes WH, et al. Functional MRI in chronic epilepsy: associations with cognitive impairment. Lancet Neurol. (2010) 9:1018–27. doi: 10.1016/S1474-4422(10)70180-0
81. Fox MD, Raichle ME. Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nat Rev Neurosci. (2007) 8:700–11. doi: 10.1038/nrn2201
82. Mantini D, Perrucci MG, Del Gratta C, Romani GL, Corbetta M. Electrophysiological signatures of resting state networks in the human brain. Proc Natl Acad Sci USA. (2007) 104:13170–5. doi: 10.1073/pnas.0700668104
83. Sadaghiani S, Poline JB, Kleinschmidt A, D'Esposito M. Ongoing dynamics in large-scale functional connectivity predict perception. Proc Natl Acad Sci USA. (2015) 112:8463–8. doi: 10.1073/pnas.1420687112
84. Laufs H, Hamandi K, Salek-Haddadi A, Kleinschmidt AK, Duncan JS, Lemieux L. Temporal lobe interictal epileptic discharges affect cerebral activity in “default mode” brain regions. Hum Brain Mapp. (2007) 28:1023–32. doi: 10.1002/hbm.20323
85. Gotman J, Grova C, Bagshaw A, Kobayashi E, Aghakhani Y, Dubeau F. Generalized epileptic discharges show thalamocortical activation and suspension of the default state of the brain. Proc Natl Acad Sci USA. (2005) 102:15236–40. doi: 10.1073/pnas.0504935102
86. Li R, Wang L, Chen H, Guo X, Liao W, Tang YL, et al. Abnormal dynamics of functional connectivity density in children with benign epilepsy with centrotemporal spikes. Brain Imaging Behav. (2018) 1–10.
87. Zhang Z, Liao W, Wang Z, Xu Q, Yang F, Mantini D, et al. Epileptic discharges specifically affect intrinsic connectivity networks during absence seizures. J Neurol Sci. (2014) 336:138–45. doi: 10.1016/j.jns.2013.10.024
88. Holmes GL, Lenck-Santini PP. Role of interictal epileptiform abnormalities in cognitive impairment. Epilepsy Behav. (2006) 8:504–15. doi: 10.1016/j.yebeh.2005.11.014
89. Khan OI, Zhao Q, Miller F, Holmes GL. Interictal spikes in developing rats cause long-standing cognitive deficits. Neurobiol Dis. (2010) 39:362–71. doi: 10.1016/j.nbd.2010.05.002
90. Pressler RM, Robinson RO, Wilson GA, Binnie CD. Treatment of interictal epileptiform discharges can improve behavior in children with behavioral problems and epilepsy. J Pediatr. (2005) 146:112–7. doi: 10.1016/j.jpeds.2004.08.084
91. Ebus S, Arends J, Hendriksen J, van der Horst E, de la Parra N, Hendriksen R, et al. Cognitive effects of interictal epileptiform discharges in children. Eur J Paediatr Neurol. (2012) 16:697–706. doi: 10.1016/j.ejpn.2012.05.010
92. Binnie CD. Cognitive impairment during epileptiform discharges: is it ever justifiable to treat the EEG? Lancet Neurol. (2003) 2:725–30. doi: 10.1016/S1474-4422(03)00584-2
93. Aarts JH, Binnie CD, Smit AM, Wilkins AJ. Selective cognitive impairment during focal and generalized epileptiform EEG activity. Brain. (1984) 107(Pt 1):293–308. doi: 10.1093/brain/107.1.293
94. Vivekananda U, Bush D, Bisby JA, Diehl B, Jha A, Nachev P, et al. Spatial and episodic memory tasks promote temporal lobe interictal spikes. Ann Neurol. (2019) 86:304–9. doi: 10.1002/ana.25519
95. Chaudhary UJ, Centeno M, Carmichael DW, Vollmar C, Rodionov R, Bonelli S, et al. Imaging the interaction: epileptic discharges, working memory, and behavior. Hum Brain Mapp. (2013) 34:2910–7. doi: 10.1002/hbm.22115
96. Bear JJ, Chapman KE, Tregellas JR. The epileptic network and cognition: what functional connectivity is teaching us about the childhood epilepsies. Epilepsia. (2019) 60:1491–507. doi: 10.1111/epi.16098
97. Jacobs J, LeVan P, Moeller F, Boor R, Stephani U, Gotman J, et al. Hemodynamic changes preceding the interictal EEG spike in patients with focal epilepsy investigated using simultaneous EEG-fMRI. Neuroimage. (2009) 45:1220–31. doi: 10.1016/j.neuroimage.2009.01.014
98. Lu Y, Bagshaw AP, Grova C, Kobayashi E, Dubeau F, Gotman J. Using voxel-specific hemodynamic response function in EEG-fMRI data analysis. Neuroimage. (2006) 32:238–47. doi: 10.1016/j.neuroimage.2005.11.040
99. Ibrahim GM, Cassel D, Morgan BR, Smith ML, Otsubo H, Ochi A, et al. Resilience of developing brain networks to interictal epileptiform discharges is associated with cognitive outcome. Brain. (2014) 137:2690–702. doi: 10.1093/brain/awu214
100. Ji GJ, Yu Y, Miao HH, Wang ZJ, Tang YL, Liao W. Decreased network efficiency in benign epilepsy with centrotemporal spikes. Radiology. (2017) 283:186–94. doi: 10.1148/radiol.2016160422
101. Warren AEL, Abbott DF, Jackson GD, Archer JS. Thalamocortical functional connectivity in Lennox–Gastaut syndrome is abnormally enhanced in executive-control and default-mode networks. Epilepsia. (2017) 58:2085–97. doi: 10.1111/epi.13932
102. Jann K, Kottlow M, Dierks T, Boesch C, Koenig T. Topographic electrophysiological signatures of fMRI resting state networks. PLoS ONE. (2010) 5:e12945. doi: 10.1371/journal.pone.0012945
103. Carmichael DW, Vulliemoz S, Rodionov R, Thornton JS, McEvoy AW, Lemieux L. Simultaneous intracranial EEG-fMRI in humans: protocol considerations and data quality. Neuroimage. (2012) 63:301–9. doi: 10.1016/j.neuroimage.2012.05.056
104. Vulliemoz S, Carmichael DW, Rosenkranz K, Diehl B, Rodionov R, Walker MC, et al. Simultaneous intracranial EEG and fMRI of interictal epileptic discharges in humans. Neuroimage. (2011) 54:182–90. doi: 10.1016/j.neuroimage.2010.08.004
105. Ramantani G, Dümpelmann M, Koessler L, Brandt A, Cosandier-Rimélé D, Zentner J, et al. Simultaneous subdural and scalp EEG correlates of frontal lobe epileptic sources. Epilepsia. (2014) 55:278–88. doi: 10.1111/epi.12512
106. Koessler L, Cecchin T, Colnat-Coulbois S, Vignal JP, Jonas J, Vespignani H, et al. Catching the invisible: mesial temporal source contribution to simultaneous EEG and SEEG recordings. Brain Topogr. (2014) 28:5–20. doi: 10.1007/s10548-014-0417-z
107. Debener S, Ullsperger M, Siegel M, Engel AK. Single-trial EEG – fMRI reveals the dynamics of cognitive function. Trends Cogn Sci. (2006) 10:558–63. doi: 10.1016/j.tics.2006.09.010
108. Kyathanahally SP, Wang Y, Calhoun VD. Investigation of true high frequency electrical substrates of fMRI-based resting state networks using parallel independent component analysis of simultaneous EEG / fMRI data. Front Neuroinform. (2017) 11:1–12. doi: 10.3389/fninf.2017.00074
109. Tagliazucchi E, Laufs H. Decoding wakefulness levels from typical fMRI resting-state data reveals reliable drifts between wakefulness and sleep. Neuron. (2014) 82:695–708. doi: 10.1016/j.neuron.2014.03.020
110. Wandschneider B, Burdett J, Townsend L, Hill A, Thompson PJ, Duncan JS, et al. Effect of topiramate and zonisamide on fMRI cognitive networks. Neurology. (2017) 88:1165–71. doi: 10.1212/WNL.0000000000003736
111. Wirsich J, Rey M, Guye M, Bénar C, Lanteaume L, Ridley B, et al. Brain networks are independently modulated by donepezil, sleep, and sleep deprivation. Brain Topogr. (2018) 31:380–91. doi: 10.1007/s10548-017-0608-5
112. Fuentemilla L, Penny WD, Cashdollar N, Bunzeck N, Düzel E. Theta-coupled periodic replay in working memory. Curr Biol. (2010) 20:606–12. doi: 10.1016/j.cub.2010.01.057
113. Sadaghiani S, Kleinschmidt A. Brain networks and α-oscillations: structural and functional foundations of cognitive control. Trends Cogn Sci. (2016) 20:805–17. doi: 10.1016/j.tics.2016.09.004
Keywords: EEG-fMRI, epilepsy, review, neuroimaging, interictal epileptiform discharge
Citation: Shamshiri EA, Sheybani L and Vulliemoz S (2019) The Role of EEG-fMRI in Studying Cognitive Network Alterations in Epilepsy. Front. Neurol. 10:1033. doi: 10.3389/fneur.2019.01033
Received: 09 July 2019; Accepted: 11 September 2019;
Published: 24 September 2019.
Edited by:
Britta Wandschneider, University College London, United KingdomReviewed by:
Yann Quidé, UNSW, AustraliaBo Gao, Affiliated Hospital of Guizhou Medical University, China
Copyright © 2019 Shamshiri, Sheybani and Vulliemoz. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Elhum A. Shamshiri, RWxodW0uU2hhbXNoaXJpQHVuaWdlLmNo
†These authors have contributed equally to this work
 Elhum A. Shamshiri
Elhum A. Shamshiri Laurent Sheybani
Laurent Sheybani Serge Vulliemoz
Serge Vulliemoz