- 1Neuroimaging Research Unit, Division of Neuroscience, Institute of Experimental Neurology, IRCCS San Raffaele Scientific Institute, Milan, Italy
- 2Neurology Unit, IRCCS San Raffaele Scientific Institute, Milan, Italy
- 3Vita-Salute San Raffaele University, Milan, Italy
Chronic migraine is a highly disabling disease with a great impact on socioeconomic functioning and quality of life of migraine patients. Chronic migraine usually evolves from episodic migraine that gradually increases in attack frequency, supporting the view of migraine as a spectrum disorder. Pathophysiological mechanisms responsible for migraine chronification are not fully understood. Likewise episodic migraine, chronic migraine patients show widespread functional and structural alterations of cortical and subcortical pain-related brain areas. However, chronic migraine patients experience a more pronounced dysfunction of the pain inhibitory network and an increased sensitization of the central pain pathways, which might explain the higher susceptibility to migraine attacks. Imaging studies have highlighted that brain regions with a key role in migraine attack generation, like the pons and hypothalamus, might also be involved in migraine chronification. Whether brain alterations are biomarkers that predispose migraine patients to chronification or reflect adaptive or maladaptive responses to the increasing headache frequency is still a matter of debate. The central mechanisms of action of chronic migraine preventive treatments and imaging biomarkers that could predict patients' treatment response have also been explored. In this new era of migraine treatments, a better understanding of chronic migraine pathophysiology will pave the way for the development of new improved treatments specifically designed for chronic migraine patients.
Introduction
Chronic migraine is a highly disabling disease. Relative to episodic migraine, patients with chronic migraine have greater headache-related impact on socioeconomic functioning and worse quality of life (1). According to the International Classification of Headache Disorders (2), chronic migraine is defined as at least 15 days of headache occurring each month, including at least 8 days a month of headache attacks with migrainous features, for more than 3 months. The prevalence of chronic migraine is around 1–2% in the general population. Chronic migraine usually evolves from episodic migraine that gradually increases in attack frequency, with an annual progression rate of about 3% (3, 4). The main risk factors for transition are female sex, low educational status, baseline high attack frequency, obesity, stressful life events, snoring, ineffective acute treatments, and overuse of acute migraine medications (5, 6). At least 50% of patients with chronic migraine regularly overuse one or more drugs usually taken for acute migraine treatment, thus fulfilling the diagnosis of chronic migraine with medication overuse (2, 7, 8). Compared to episodic migraine patients, patients with chronic migraine are more likely to have psychiatric comorbidities, like depression and anxiety, respiratory and cardiovascular diseases (1). Chronic migraine is a dynamic state, with patients moving in and out of the chronic condition. About 26% of patients with chronic migraine remit to the episodic form within 2 years (9).
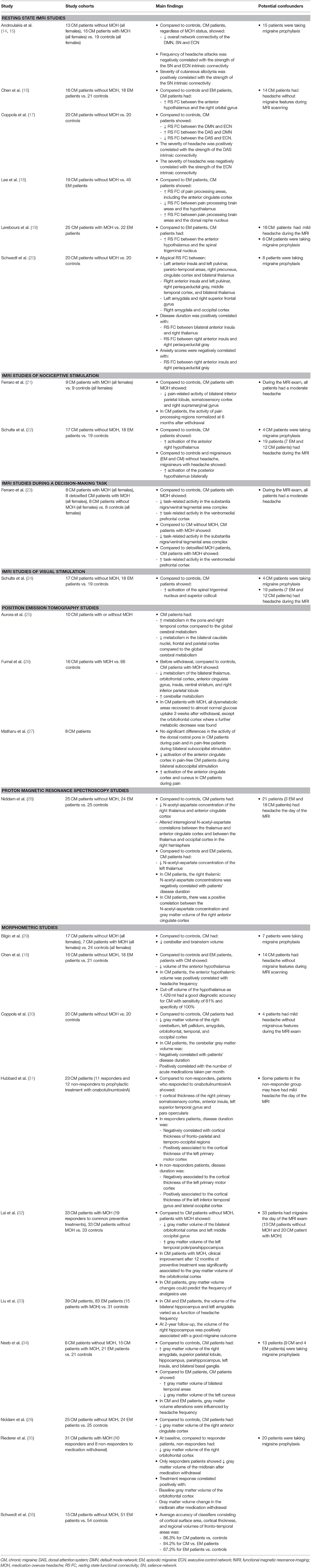
Pathophysiological mechanisms responsible for migraine chronification are not fully understood. Patients with chronic migraine might have a lower sensory threshold and an increased susceptibility to migraine attacks (3). Central and peripheral sensitization processes can contribute to the pathophysiology of chronic migraine. Of note, compared to episodic migraine patients, patients with chronic migraine have higher plasma levels of vasoactive neuropeptides, such as the calcitonin gene related peptide and vasoactive intestinal peptide, thus suggesting an altered activity of the trigeminal and cranial autonomic system (3, 10, 11). Dysfunction of cortical and subcortical brain areas involved in pain processing, such as the thalamus, hypothalamus, somatosensory and anterior cingulate cortex, might also have a pivotal role in migraine transformation. There is evidence that an altered balance between the facilitatory and inhibitory activity of pain-related brain regions might contribute to the development of symptoms commonly reported by chronic migraine patients, like cutaneous allodynia (12, 13). Our understanding of the pathophysiology of chronic migraine has improved considerably with a series of imaging studies, which have provided insights into the function and structure of human brain networks that could be involved in migraine chronification. This review will focus the attention on neuroimaging studies in patients with chronic migraine, highlighting the evidence behind the involvement of key brain areas, such as the pons and hypothalamus, and the pain network in migraine chronification. Table 1 summarizes the main findings of neuroimaging studies in chronic migraine patients.
Imaging the Pain Network in Chronic Migraine
Pain experience is a complex process involving sensory, affective, and cognitive brain networks. Similar to previous findings in episodic migraine patients (37), an altered functional recruitment of brain areas involved in the sensory-discriminative and affective aspects of pain, including the insula, prefrontal, anterior cingulate and somatosensory cortex, has been demonstrated in chronic migraine patients (20). Maladaptive functional activation of brain networks involved in attentive and executive functions, such as the executive control, default mode and dorsal attention network, have also been revealed in patients with chronic migraine. Thus, suggesting that the reaction to painful stimuli, preparation of responses, and allocation of attentional resources to pain are impaired in chronic migraine patients (14, 15, 17). Whether cognitive symptoms, particularly deficits in attention and executive functions, might influence the functional activity of brain cognitive networks has never been investigated. A comprehensive neuropsychological assessment should be included in future studies. The salience network has a key role in defining the saliency of incoming painful stimuli. In chronic migraine patients, the presence of cutaneous allodynia was associated to an increased activity of the salience network. These findings support a possible involvement of the salience network in central sensitization (14).
Several studies (29, 30, 34) demonstrated that chronic migraine is also associated with morphometric alterations of brain areas known to be involved in pain modulation and in the different aspects of pain processing. Regions of increased and decreased gray matter volume, including the brainstem, cerebellum, basal ganglia, amygdala, frontal, temporal and occipital areas, have been found in chronic migraine patients compared to controls (29, 30, 34).
Whether these functional and structural alterations are the consequence of the recurrence of headache attacks or might predispose to chronic migraine is still a matter of debate. Some studies demonstrated functional (14, 20) and structural (30, 33, 34) plasticity of nociceptive brain areas that are linked to the headache attack frequency and disease duration. Repetitive headache attacks can remodel the pain network, thus increasing the susceptibility to the onset of further attacks and leading to chronic central sensitization. On the other hand, other investigations did not confirm such correlation (29).
Dynamic functional (21, 26) and structural (32, 35) changes in pain processing structures were also revealed in chronic migraine patients with medication overuse. Interestingly, imaging alterations of the thalamus, insula, anterior cingulate, and parietal cortex reverted after medication withdrawal, probably reflecting the consequences rather than the causes of medication overuse in these patients. While alterations of mesocorticolimbic dopaminergic areas, such as the ventral tegmental area (23) and orbitofrontal cortex (26), persisted following detoxification, suggesting that these findings might represent a brain trait that predisposes certain migraine patients to the development of medication overuse.
Quantitative MRI techniques have shown increased iron deposition in the periaqueductal gray, red nucleus, and basal ganglia in migraine patients and patients with chronic daily headache (38–40). A higher risk to have iron deposition was associated to higher attack frequency or longer disease duration, suggesting a causal relationship between migraine and these abnormalities (38). The observed association between repeated migraine attacks and increased iron accumulation in the brainstem and deep gray matter nuclei involved in central pain processing support the possibility that migraine has cumulative effects on brain structure and homeostasis. However, a follow-up study did not find any significant progression of iron accumulation over 9 years (41).
A further unanswered question is whether neuroimaging alterations are common to episodic and chronic migraine patients or are specifically involved in migraine chronification. There is evidence showing a more pronounced dysfunction of the pain inhibitory (18) and thalamocortical (28) pathway in chronic than episodic migraine. Using resting state functional MRI, Lee and coworkers (18) have shown an increased functional connectivity of pain processing brain areas, especially the anterior cingulate cortex, in chronic migraine patients compared to patients with episodic migraine (18). Reduced N-acetyl-aspartate concentration in the thalamus and anterior cingulate cortex has been found in chronic migraine patients, but not in patients with episodic migraine (28). Interestingly, the interregional correlations of N-acetyl-aspartate levels between the thalamus and the anterior cingulate cortex shifted from positive in controls to negative in chronic migraine patients. Thus, suggesting that neuronal reorganization in the thalamocortical pathway might contribute to migraine chronification.
Chronic migraine is also associated to more extensive brain structural alterations. Schwedt and colleagues (36) reported that alterations of cortical thickness, cortical surface area and regional volumes of fronto-temporal brain areas could discriminate chronic migraine patients from controls and from patients with episodic migraine with an accuracy of 86 and 84%, respectively. While, the accuracy for discriminating episodic migraine patients from controls was of only 67%. A greater iron accumulation was found in chronic migraine patients compared to patients with episodic migraine (39, 40). Larger volume of iron deposits could identify chronic migraine with a sensitivity ranging from 80 to 93% and a specificity ranging from 71 to 97% (40). The increased iron levels in the anti-nociceptive network in chronic migraine patients might constitute a physiologic response to repeated activation of nuclei involved in central pain processing, which may play a role in the chronification of migraine.
In migraine patients, the perception of the headache pain can be exacerbated by the exposure of lights. There is evidence showing that photic signals coming from the retina can converge on thalamic trigeminovascular neurons that project to cortical areas involved in the processing of pain and visual perception. Thus, supporting the link between the visual and trigeminal pain processing system (42). Interestingly, compared to controls and episodic migraine patients, chronic migraine patients showed and increased activity of the spinal trigeminal nucleus and superior colliculi during visual stimulation with a rotating checkerboard. The increased trigeminal activation during visual stimulation was significantly influenced by the experience of headache. These findings corroborate the crosslink between the visual and trigeminal systems and demonstrate a more pronounced sensitization of these two pathways in patients with chronic migraine (24).
Imaging the Migraine “Generators” in Chronic Migraine
Although our understanding of the pathophysiology of migraine has progressed over the last years, where exactly migraine attacks originate is still an unresolved question. Several studies demonstrated a selective activation of the dorsal pons during spontaneous (43, 44) and nitroglycerin-triggered (45) migraine attacks, which persisted after complete pain-resolution due to triptan administration (44), in patients with episodic migraine. Thus, leading the authors to hypothesize that this brainstem region might represent the so-called migraine “generator.” An increased cerebral metabolism in the pons has also been described in patients with chronic migraine during and outside the headache phase (25, 27). Similar to episodic migraine, the dysfunctional activation of this brainstem region did not change after electrical suboccipital stimulation, supporting the key role of this region in migraine attack generation as well as in migraine chronification (27).
Recent MRI studies have pointed the attention to the role of the hypothalamus in migraine attack generation. Positron emission tomography (46) and functional MRI (43) studies revealed increased hypothalamic activity before and during the headache phase of the migraine attack in episodic migraine patients. An altered functional coupling between the hypothalamus and the spinal trigeminal nucleus during the precital phase and between the hypothalamus and the pons during the ictal phase have also been demonstrated (43). These findings suggest that the hypothalamus-brainstem network might be the real driver of migraine attacks.
Different regions of the hypothalamus seem be involved in the onset of the migraine attack and in migraine chronification. Chronic migraine patients with and without medication overuse showed a selective increased activity of the anterior hypothalamus during trigeminal painful stimulation (22) and in a rest condition (16, 19), compared to controls and patients with episodic migraine. Thus, suggesting that the anterior hypothalamus plays a crucial role in the pathophysiology of chronic migraine. While, the most posterior hypothalamic part was specifically linked to the acute headache phase of the migraine attack (22).
Relative to episodic migraine, an increased activation of the hypothalamus seems to facilitate the recruitment of cortical areas involved in pain processing in chronic migraine patients (18).
In conjunction with functional alterations, structural plasticity of the anterior hypothalamus has been demonstrated in patients with chronic migraine. A hypothalamic volume lower than 1.43 ml had a good diagnostic accuracy for chronic migraine with sensitivity of 81% and specificity of 100% (16).
Imaging Biomarkers of Treatment Response in Chronic Migraine
Imaging techniques can provide new insights into the central mechanisms of action of treatments commonly used for chronic migraine. A positron emission tomography study (27) showed significant functional modulation of brain regions involved in the affective aspects of pain, including the anterior cingulate cortex and cuneus, during bilateral electrical suboccipital stimulation in a small cohort of chronic migraine patients.
Now that new mechanism-based treatments specific for migraine are available a better prediction of treatment response might facilitate the selection of the most appropriate treatment for each patient. Chronic migraine patients who responded to OnabotulinumtoxinA treatment, as evidenced by reversal from a chronic to an episodic state, had distinct patterns of morphometric and functional alterations in pain processing areas compared to those patients who did not respond (31). The identification of the brain pathways that are involved in disease reversal or progression can lead to a better understanding of the mechanisms underlying the migraine chronification. All of this is critical to discovering new treatments that prevent or slow the progression to chronic migraine. Imaging biomarkers that could predict patients' treatment response have also been identified in chronic migraine patients with medication overuse. Rieder and coworkers (35) showed significant volumetric changes in the midbrain after the withdrawal of acute headache medications only in those patients who responded to the treatment. Moreover, decreased gray matter volume of the orbitofrontal cortex predicted a negative response to detoxification.
Conclusions
Significant advances in our understanding of chronic migraine pathophysiology have been made over the last years. Neuroimaging findings support the view that migraine is a spectrum disorder, with clinical and pathophysiological features that can progress over time. Chronic and episodic migraine share similar functional and structural alterations in brain regions implicated in the generation of the migraine attack and in pain processing. However, chronic migraine patients experience a more pronounced dysfunction of the pain inhibitory network and an increased sensitization of the central pain pathways, which might explain the higher susceptibility to migraine attacks. Whether brain alterations are biomarkers that predispose migraine patients to chronification or reflect adaptive or maladaptive responses to the increasing headache frequency is still debated. Longitudinal studies including large sample size of patients with episodic and chronic migraine are warranted. Future studies combining multimodal data, such as functional MRI, structural MRI and electroencephalographic data, might help us to achieve a better understanding of chronic migraine pathophysiology. In the future, imaging patterns that predict whether an episodic migraine patient will evolve to a chronic form should be identified. This might lead to an early prevention of migraine transformation. In this new era of migraine treatments, a better understanding of chronic migraine pathophysiology will pave the way for the development of new improved treatments specifically designed for chronic migraine patients.
Author Contributions
MF and RM contributed to the study concept and drafting/revising the manuscript.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. Buse DC, Manack A, Serrano D, Turkel C, Lipton RB. Sociodemographic and comorbidity profiles of chronic migraine and episodic migraine sufferers. J Neurol Neurosurg Psychiatr. (2010) 81:428–32. doi: 10.1136/jnnp.2009.192492
2. Headache Classification Committee of the International Headache Society (IHS). The international classification of headache disorders, 3rd Edn. Cephalalgia. (2018) 38:1–211. doi: 10.1177/0333102417738202
3. May A, Schulte LH. Chronic migraine: risk factors, mechanisms and treatment. Nat Rev Neurol. (2016) 12:455–64. doi: 10.1038/nrneurol.2016.93
4. Scher AI, Stewart WF, Ricci JA, Lipton RB. Factors associated with the onset and remission of chronic daily headache in a population-based study. Pain. (2003) 106:81–9. doi: 10.1016/S0304-3959(03)00293-8
5. Lipton RB. Tracing transformation: chronic migraine classification, progression, and epidemiology. Neurology. (2009) 72(5 Suppl):S3–7. doi: 10.1212/WNL.0b013e3181974b19
6. Katsarava Z, Schneeweiss S, Kurth T, Kroener U, Fritsche G, Eikermann A, et al. Incidence and predictors for chronicity of headache in patients with episodic migraine. Neurology. (2004) 62:788–90. doi: 10.1212/01.WNL.0000113747.18760.D2
7. Schwedt TJ, Chong CD. Medication overuse headache: pathophysiological insights from structural and functional brain MRI research. Headache. (2017) 57:1173–8. doi: 10.1111/head.13037
8. Westergaard ML, Glumer C, Hansen EH, Jensen RH. Prevalence of chronic headache with and without medication overuse: associations with socioeconomic position and physical and mental health status. Pain. (2014) 155:2005–13. doi: 10.1016/j.pain.2014.07.002
9. Manack A, Buse DC, Serrano D, Turkel CC, Lipton RB. Rates, predictors, and consequences of remission from chronic migraine to episodic migraine. Neurology. (2011) 76:711–8. doi: 10.1212/WNL.0b013e31820d8af2
10. Cernuda-Morollon E, Martinez-Camblor P, Alvarez R, Larrosa D, Ramon C, Pascual J. Increased VIP levels in peripheral blood outside migraine attacks as a potential biomarker of cranial parasympathetic activation in chronic migraine. Cephalalgia. (2015) 35:310–6. doi: 10.1177/0333102414535111
11. Cernuda-Morollon E, Larrosa D, Ramon C, Vega J, Martinez-Camblor P, Pascual J. Interictal increase of CGRP levels in peripheral blood as a biomarker for chronic migraine. Neurology. (2013) 81:1191–6. doi: 10.1212/WNL.0b013e3182a6cb72
12. Aurora SK, Brin MF. Chronic migraine: an update on physiology, imaging, and the mechanism of action of two available pharmacologic therapies. Headache. (2017) 57:109–25. doi: 10.1111/head.12999
13. Bigal ME, Ashina S, Burstein R, Reed ML, Buse D, Serrano D, et al. Prevalence and characteristics of allodynia in headache sufferers: a population study. Neurology. (2008) 70:1525–33. doi: 10.1212/01.wnl.0000310645.31020.b1
14. Androulakis XM, Krebs K, Peterlin BL, Zhang T, Maleki N, Sen S, et al. Modulation of intrinsic resting-state fMRI networks in women with chronic migraine. Neurology. (2017) 89:163–9. doi: 10.1212/WNL.0000000000004089
15. Androulakis XM, Krebs KA, Jenkins C, Maleki N, Finkel AG, Rorden C, et al. Central executive and default mode network intranet work functional connectivity patterns in chronic migraine. J Neurol Disord. (2018) 6:393. doi: 10.4172/2329-6895.1000393
16. Chen Z, Chen X, Liu M, Ma L, Yu S. Volume of hypothalamus as a diagnostic biomarker of chronic migraine. Front Neurol. (2019) 10:606. doi: 10.3389/fneur.2019.00606
17. Coppola G, Di Renzo A, Petolicchio B, Tinelli E, Di Lorenzo C, Parisi V, et al. Aberrant interactions of cortical networks in chronic migraine: a resting-state fMRI study. Neurology. (2019) 92:e2550–e8. doi: 10.1212/WNL.0000000000007577
18. Lee MJ, Park BY, Cho S, Kim ST, Park H, Chung CS. Increased connectivity of pain matrix in chronic migraine: a resting-state functional MRI study. J Headache Pain. (2019) 20:29. doi: 10.1186/s10194-019-0986-z
19. Lerebours F, Boulanouar K, Barege M, Denuelle M, Bonneville F, Payoux P, et al. Functional connectivity of hypothalamus in chronic migraine with medication overuse. Cephalalgia. (2019) 39:892–9. doi: 10.1177/0333102419833087
20. Schwedt TJ, Schlaggar BL, Mar S, Nolan T, Coalson RS, Nardos B, et al. Atypical resting-state functional connectivity of affective pain regions in chronic migraine. Headache. (2013) 53:737–51. doi: 10.1111/head.12081
21. Ferraro S, Grazzi L, Mandelli ML, Aquino D, Di Fiore D, Usai S, et al. Pain processing in medication overuse headache: a functional magnetic resonance imaging (fMRI) study. Pain Med. (2012) 13:255–62. doi: 10.1111/j.1526-4637.2011.01183.x
22. Schulte LH, Allers A, May A. Hypothalamus as a mediator of chronic migraine: evidence from high-resolution fMRI. Neurology. (2017) 88:2011–6. doi: 10.1212/WNL.0000000000003963
23. Ferraro S, Grazzi L, Muffatti R, Nava S, Ghielmetti F, Bertolino N, et al. In medication-overuse headache, FMRI shows long-lasting dysfunction in midbrain areas. Headache. (2012) 52:1520–34. doi: 10.1111/j.1526-4610.2012.02276.x
24. Schulte LH, Allers A, May A. Visual stimulation leads to activation of the nociceptive trigeminal nucleus in chronic migraine. Neurology. (2018) 90:e1973–e8. doi: 10.1212/WNL.0000000000005622
25. Aurora SK, Barrodale PM, Tipton RL, Khodavirdi A. Brainstem dysfunction in chronic migraine as evidenced by neurophysiological and positron emission tomography studies. Headache. (2007) 47:996–1003; discussion 4–7. doi: 10.1111/j.1526-4610.2007.00853.x
26. Fumal A, Laureys S, Di Clemente L, Boly M, Bohotin V, Vandenheede M, et al. Orbitofrontal cortex involvement in chronic analgesic-overuse headache evolving from episodic migraine. Brain. (2006) 129(Pt 2):543–50. doi: 10.1093/brain/awh691
27. Matharu MS, Bartsch T, Ward N, Frackowiak RS, Weiner R, Goadsby PJ. Central neuromodulation in chronic migraine patients with suboccipital stimulators: a PET study. Brain. (2004) 127(Pt 1):220–30. doi: 10.1093/brain/awh022
28. Niddam DM, Lai KL, Tsai SY, Lin YR, Chen WT, Fuh JL, et al. Neurochemical changes in the medial wall of the brain in chronic migraine. Brain. (2018) 141:377–90. doi: 10.1093/brain/awx331
29. Bilgic B, Kocaman G, Arslan AB, Noyan H, Sherifov R, Alkan A, et al. Volumetric differences suggest involvement of cerebellum and brainstem in chronic migraine. Cephalalgia. (2016) 36:301–8. doi: 10.1177/0333102415588328
30. Coppola G, Petolicchio B, Di Renzo A, Tinelli E, Di Lorenzo C, Parisi V, et al. Cerebral gray matter volume in patients with chronic migraine: correlations with clinical features. J Headache Pain. (2017) 18:115. doi: 10.1186/s10194-017-0825-z
31. Hubbard CS, Becerra L, Smith JH, DeLange JM, Smith RM, Black DF, et al. Brain changes in responders vs. non-responders in chronic migraine: markers of disease reversal. Front Hum Neurosci. (2016) 10:497. doi: 10.3389/fnhum.2016.00497
32. Lai TH, Chou KH, Fuh JL, Lee PL, Kung YC, Lin CP, et al. Gray matter changes related to medication overuse in patients with chronic migraine. Cephalalgia. (2016) 36:1324–33. doi: 10.1177/0333102416630593
33. Liu HY, Chou KH, Lee PL, Fuh JL, Niddam DM, Lai KL, et al. Hippocampus and amygdala volume in relation to migraine frequency and prognosis. Cephalalgia. (2017) 37:1329–1336. doi: 10.1177/0333102416678624
34. Neeb L, Bastian K, Villringer K, Israel H, Reuter U, Fiebach JB. Structural gray matter alterations in chronic migraine: implications for a progressive disease? Headache. (2017) 57:400–16. doi: 10.1111/head.13012
35. Riederer F, Gantenbein AR, Marti M, Luechinger R, Kollias S, Sandor PS. Decrease of gray matter volume in the midbrain is associated with treatment response in medication-overuse headache: possible influence of orbitofrontal cortex. J Neurosci. (2013) 33:15343–9. doi: 10.1523/JNEUROSCI.3804-12.2013
36. Schwedt TJ, Chong CD, Wu T, Gaw N, Fu Y, Li J. Accurate classification of chronic migraine via brain magnetic resonance imaging. Headache. (2015) 55:762–77. doi: 10.1111/head.12584
37. Messina R, Filippi M, Goadsby PJ. Recent advances in headache neuroimaging. Curr Opin Neurol. (2018) 31:379–85. doi: 10.1097/WCO.0000000000000573
38. Welch KM, Nagesh V, Aurora SK, Gelman N. Periaqueductal gray matter dysfunction in migraine: cause or the burden of illness? Headache. (2001) 41:629–37. doi: 10.1046/j.1526-4610.2001.041007629.x
39. Tepper SJ, Lowe MJ, Beall E, Phillips MD, Liu K, Stillman MJ, et al. Iron deposition in pain-regulatory nuclei in episodic migraine and chronic daily headache by MRI. Headache. (2012) 52:236–43. doi: 10.1111/j.1526-4610.2011.02056.x
40. Dominguez C, Lopez A, Ramos-Cabrer P, Vieites-Prado A, Perez-Mato M, Villalba C, et al. Iron deposition in periaqueductal gray matter as a potential biomarker for chronic migraine. Neurology. (2019) 92:e1076–e85. doi: 10.1212/WNL.0000000000007047
41. Palm-Meinders IH, Koppen H, Terwindt GM, Launer LJ, van Buchem MA, Ferrari MD, et al. Iron in deep brain nuclei in migraine? CAMERA follow-up MRI findings. Cephalalgia. (2017) 37:795–800. doi: 10.1177/0333102416668654
42. Noseda R, Burstein R. Migraine pathophysiology: anatomy of the trigeminovascular pathway and associated neurological symptoms, CSD, sensitization and modulation of pain. Pain. (2013) 154(Suppl 1):S44–S53. doi: 10.1016/j.pain.2013.07.021
43. Schulte LH, May A. The migraine generator revisited: continuous scanning of the migraine cycle over 30 days and three spontaneous attacks. Brain. (2016) 139(Pt 7):1987–93. doi: 10.1093/brain/aww097
44. Weiller C, May A, Limmroth V, Juptner M, Kaube H, Schayck RV, et al. Brain stem activation in spontaneous human migraine attacks. Nat Med. (1995) 1:658–60. doi: 10.1038/nm0795-658
45. Maniyar FH, Sprenger T, Monteith T, Schankin C, Goadsby PJ. Brain activations in the premonitory phase of nitroglycerin-triggered migraine attacks. Brain. (2014) 137(Pt 1):232–41. doi: 10.1093/brain/awt320
Keywords: chronic migraine, neuroimaging, chronification, pain network, biomarkers
Citation: Filippi M and Messina R (2020) The Chronic Migraine Brain: What Have We Learned From Neuroimaging? Front. Neurol. 10:1356. doi: 10.3389/fneur.2019.01356
Received: 25 September 2019; Accepted: 09 December 2019;
Published: 09 January 2020.
Edited by:
Sabina Cevoli, IRCCS Institute of Neurological Sciences of Bologna (ISNB), ItalyReviewed by:
Gianluca Coppola, Sapienza University of Rome, ItalyFilippo Brighina, University of Palermo, Italy
Copyright © 2020 Filippi and Messina. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Massimo Filippi, ZmlsaXBwaS5tYXNzaW1vQGhzci5pdA==
 Massimo Filippi
Massimo Filippi Roberta Messina
Roberta Messina