- 1Second Department of Neurology, AHEPA University Hospital, Aristotle University of Thessaloniki, Thessaloniki, Greece
- 2Second Department of Neurology, Attikon Hospital, National and Kapodistrian University of Athens, Athens, Greece
- 3Polytechnic School, University of Western Macedonia, Kozani, Greece
Introduction: Percutaneous closure of patent foramen ovale (PFO) in selected patients with cryptogenic cerebrovascular ischemic events (CEs) decreases the risk of recurrent stroke; however, optimal patient selection criteria are still under investigation. Candidates for PFO closure are usually selected from the pool of CE patients with a high risk of Paradoxical Embolism (RoPE) score. The RoPE score calculates the probability that PFO is causally related to stroke, based on PFO prevalence in patients with CE compared with that in healthy subjects. The latter has been set at 25% based on the average of autopsy and transesophageal echocardiography (TEE) studies.
Methods: We conducted a comprehensive review of studies investigating PFO prevalence in general population and in patients with CE and non-CE using autopsy, TEE, transcranial Doppler (TCD) or transthoracic echocardiography (TTE). Studies were excluded if they (1) reported data from referred subjects with underlying cerebrovascular disease or (2) did not specify etiologically the events.
Results: In healthy/control subjects, PFO prevalence was 24.2% (1,872/7,747) in autopsy studies, 23.7% (325/1,369) in TEE, 31.3% (111/355) in TCD, and 14.7% (186/1,267) in TTE studies. All diagnostic modalities included PFO prevalence was higher in CE compared with healthy/control population [odds ratio (OR) = 3.1, 95% confidence interval (CI) = 2.5–3.8] and compared with non-CE (OR = 2.3, 95% CI = 2.0–2.6). In patients with CE, PFO prevalence in the young compared to the old was higher when the diagnostic modality was TEE (48.9 vs. 27.3%, p < 0.0001, OR = 2.6 with 95% CI = 2.0–3.3) or TCD (58.1 vs. 41%, OR = 1.9, 95% CI = 1.6–2.5), but not TTE (53.3 vs. 37.5%, p = 0.16). Regarding non-CE, PFO prevalence in the young compared to the old was higher when the diagnostic modality was TEE (20 vs. 12.9%, OR = 1.7, 95% CI = 1.0–2.8) but not TTE (10.4 vs. 7.8%, p = 0.75) or TCD (22.8 vs. 20.1%, p = 0.56).
Conclusions: Given the limitations of autopsy and TEE studies, there is good reason not to take a fixed 25% PFO prevalence for granted. The estimation of degree of causality may be underestimated or overestimated in populations with PFO prevalence significantly lower or higher than the established. Given the high sensitivity, non-invasive nature, low cost, and repeatability of TCD, future large-scale TCD-based studies should investigate potential heterogeneity in PFO prevalence in different healthy racial/ethnic populations.
Introduction
In 1564, the Italian anatomist and surgeon Leonardo Botallo claimed in his publication “De catarrho commentarius” that he had discovered a “duct,” which connected the right with the left atrium. He called it the “vena arteriarum nutria,” which is nowadays known as foramen ovale or foramen Botalli (1). Three centuries later, Julius Cohnheim, a German professor of pathology, was the first to describe a case of fatal paradoxical embolism through a patent foramen ovale (PFO) to the middle cerebral artery (2). In 1880, Moritz Litten documented a second case of paradoxical embolism to the lower extremity (2). Patency of the foramen ovale is normal during fetal life allowing blood from the inferior vena cava to pass from the right to the left atrium, bypassing the lungs. At birth, pulmonary blood flow increases greatly because right heart pressure and pulmonary vascular resistance drop as pulmonary arterioles open in reaction to oxygen filling the alveoli. Left atrial pressure is increased resulting in functional closure of the foramen ovale. Anatomic closure occurs later in infancy in the majority of population, but sometimes the closure is incomplete and remains as PFO (3, 4).
Despite a thorough investigation, the etiology of cerebrovascular ischemic events remains undetermined in almost 10–40% of cases (5). Numerous case-control studies showed that PFO prevalence is remarkably high in patients with cryptogenic strokes (CSs) compared to the healthy population. It is considered that a part of these strokes may be attributed to paradoxical embolism or in situ thrombus formation in a PFO niche; therefore, PFO closure may be effective in secondary stroke prevention. The first three randomized controlled trials (RCTs) that addressed this issue (CLOSURE I, RESPECT, PC Trial) (6–8) failed to show superiority of PFO closure vs. best medical treatment (9). Despite the negative results, the suspicion that PFO was etiologically related with CS was strong. Four years later, three new RCTs (CLOSE, Gore REDUCE, DEFENSE-PFO) (10–12) and the extended follow-up results of the RESPECT trial (13) showed superiority of PFO closure compared to antiplatelet agents in appropriately selected patients using specific devices (14). Nevertheless, the optimal candidates for PFO closure are still not precisely known. The Risk of Paradoxical Embolism (RoPE) score (15) has been developed to facilitate the selection of CS patients who might benefit from PFO closure. The RoPE score applies Bayes' theorem and calculates the probability that PFO is causally related to stroke [PFO attributable fraction (PFOAF)], with higher scores implying greater possibility that a PFO is etiologically associated with a CS. Calculations are based on PFO prevalence in patients with CS compared with that in healthy subjects. The latter is considered to be 25% and the former is estimated at 40%, based on the RoPE database of 3,674 patients with CS (15). However, PFO prevalence in non-selected populations varies widely, and PFOAF may be “inflated” or “deflated,” depending on numbers.
Therefore, we conducted a comprehensive critical review of the available epidemiological data on PFO prevalence in the general population and in stroke (cryptogenic and non-cryptogenic) stratified by diagnostic modality [autopsy, transthoracic (TTE) and transesophageal echocardiography (TEE), transcranial Doppler (TCD)] and by age (young vs. old). We provide a critical appraisal of each PFO screening modality, and we underscore methodological downsides of individual epidemiological studies that have impacted on the estimation of PFO prevalence in the general population and in distinct stroke patient subgroups and hitherto have been uncommented on.
Methods
We performed a detailed search in MEDLINE, SCOPUS, Cochrane Library, and Google scholar up to November 1, 2019, using the following terms in combination: “patent foramen ovale,” “PFO,” “right-to-left-shunt,” “prevalence of patent foramen ovale,” “prevalence of PFO,” “frequency of PFO,” “cryptogenic stroke,” “cryptogenic stroke and patent foramen ovale,” “autopsy studies and patent foramen ovale,” “transthoracic echocardiography and patent foramen ovale,” “transesophageal echocardiography and patent foramen ovale,” “transcranial Doppler and patent foramen ovale,” “PFO and cerebrovascular ischemic events,” “PFO and migraine.” We also searched the reference lists of all relevant articles. Both English and foreign language articles were reviewed. We included case-control, population-based, and cohort studies that examined PFO prevalence in patients with cerebrovascular ischemic events (cryptogenic or of known cause) and in the general population (healthy population or patients with diseases other than cerebrovascular disease), using autopsy or a validated ultrasound diagnostic modality (TEE, TTE, TCD). Patent foramen ovale documentation per diagnostic modality was as follows: (1) autopsy studies were conducted in patients with a cause of death other than cerebrovascular disease, and foramen ovale patency was demonstrated via a probe or a pencil; (2) in most TEE and TTE studies, investigations were evaluated by two different cardiologists and considered positive if one to five microbubbles were detected after the use of gelatin or saline contrast within three to five heart cycles after opacification of the right atrium, at rest and during Valsalva maneuver; (3) TCD examinations were also evaluated by one or two neurologists and considered positive if one to three microembolic signals were detected within 15–40 s after the injection of gelatin or saline contrast, at rest and during Valsalva maneuver.
Studies were included if (1) they reported data from a general population or from subjects of all ages without known cerebrovascular disease, who were referred for PFO detection; (2) they specified the etiologic type of ischemic cerebrovascular event as cryptogenic (CE) vs. event of known cause (non-CE); (3) they reported PFO prevalence in patients with transient ischemic attacks (TIAs) and stroke as a single group. In studies that separately reported PFO prevalence in patients with TIAs and stroke, only data from the latter were included in the analysis. Furthermore, we included data from studies in migraineurs that reported PFO prevalence in a non-migraineur population arm. Studies were excluded if (1) they reported data from subjects with an underlying cerebrovascular disease, who were referred for PFO detection; (2) they did not specify the type of ischemic cerebrovascular event. For duplicate studies, we included only the updated article with the most informative data. We did not include review articles of previously included studies unless new data were reported. The extracted information was stratified and analyzed by diagnostic modality (autopsy, TEE, TTE, TCD), health status (healthy population/controls vs. stroke), CS status (yes vs. no), and age (young vs. old per authors' definition). Patent foramen ovale prevalence between different age and diagnostic modality subgroups was compared using the χ2 test. For the included studies, we calculated odds ratios (ORs) for PFO prevalence in CE compared with healthy/control population and also compared with non-CE, individually and cumulatively, stratified by diagnostic modality.
Results
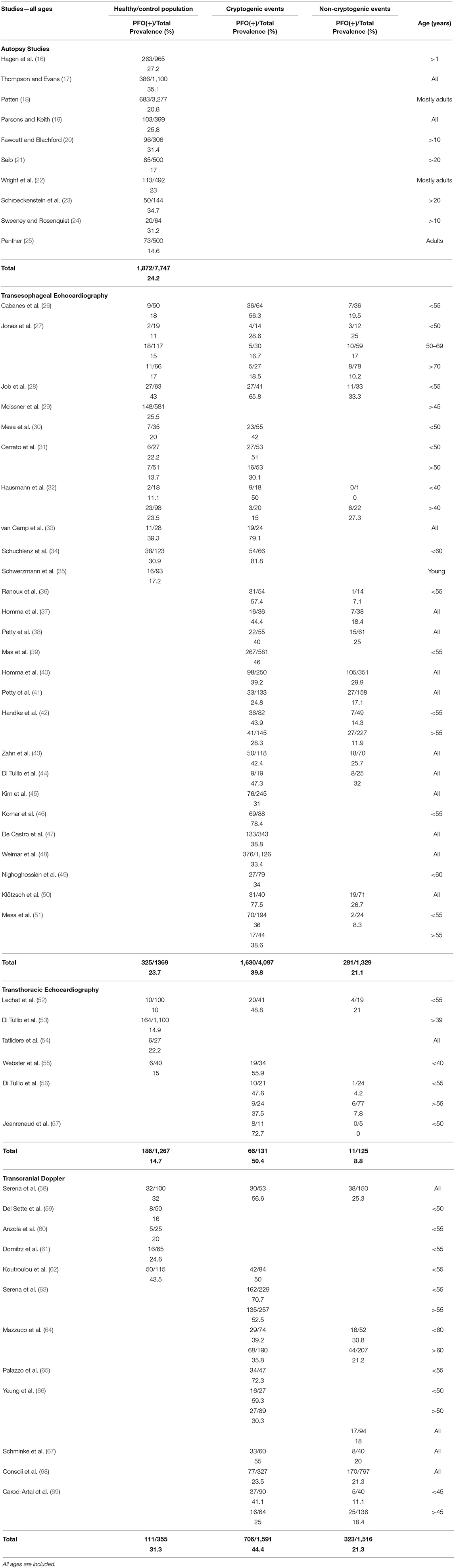
Our search resulted in 1,032 studies, which were individually assessed. We identified 66 relevant articles, of which 54 were finally included in our review (Table 1) (16–69). We found 10 autopsy studies with 7,747 subjects (16–25). Patent foramen ovale was documented in 1,872 of them [24.2%, 95% confidence interval (CI) = 23.2–25.1]. We included 26 TEE studies in total (26–51). One study (29) was exclusively conducted on a healthy population. One study was conducted on a healthy population compared with migraineurs with aura (35). Twenty-four studies reported data from patients with cerebrovascular ischemic events (CE or non-CE), of which four studies also included TIAs (10–20% of the total events) (2, 31, 48, 49). Three studies also investigated a healthy population (27, 28, 34), and five studies also investigated control patients who underwent TEE for reasons other than ischemic cerebrovascular events (26, 30–33). Cumulatively, PFO was documented in 325 of 1,369 (23.7, 95% CI = 21.6–26.1) healthy subjects/controls, in 1,630 of 4,097 (39.8, 95% CI = 38.3–41.3) patients with CE and in 281 of 1,329 (21.1, 95% CI = 19.0–23.4) patients with non-CE. We included six TTE studies (52–57). One study was exclusively conducted on a healthy population (53). One study was conducted on a healthy population compared with migraineurs (54). Four studies (52, 55–57) reported data from patients with cerebrovascular ischemic events, of which one study also included TIAs in unknown percentage (55). One study (55) also investigated a healthy population, and one study (52) also investigated patients without cerebrovascular events who underwent TTE as a preparation for posterior fossa surgery. Cumulatively, PFO was documented in 186 of 1,267 (14.7, 95% CI = 12.8–16.7) healthy subjects/controls, in 66 of 131 (50.4, 95% CI = 41.9–58.8) patients with CE, and in 11 of 125 (8.8, 95% CI = 4.8–15.2) patients with non-CE. In our review, we included 12 TCD studies (58–69). Three studies were conducted in migraineurs compared to a healthy population (59–61), and nine studies reported data from patients with cerebrovascular events, of which five studies (63–67) also included TIAs (20–75% of the total events). Two studies also investigated a healthy population (58, 62). Cumulatively, PFO was documented in 111 of 355 (31.3, 95% CI = 26.7–36.3) healthy subjects/controls, in 706 of 1,591 (44.4, 95% CI = 41.9–46.8) patients with CE, and in 323 of 1,516 (21.3, 95% CI = 19.3–23.4) patients with non-CE.
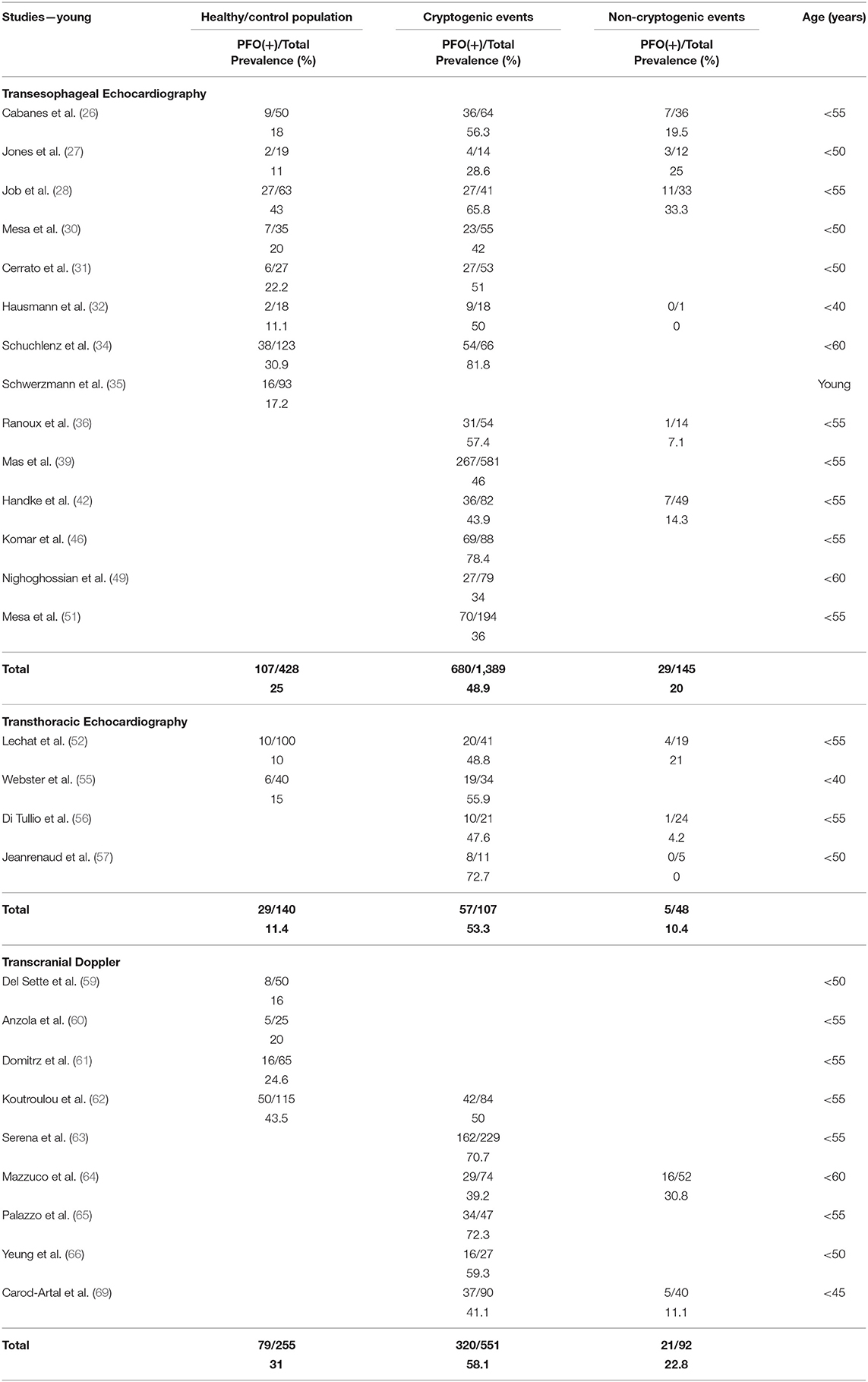
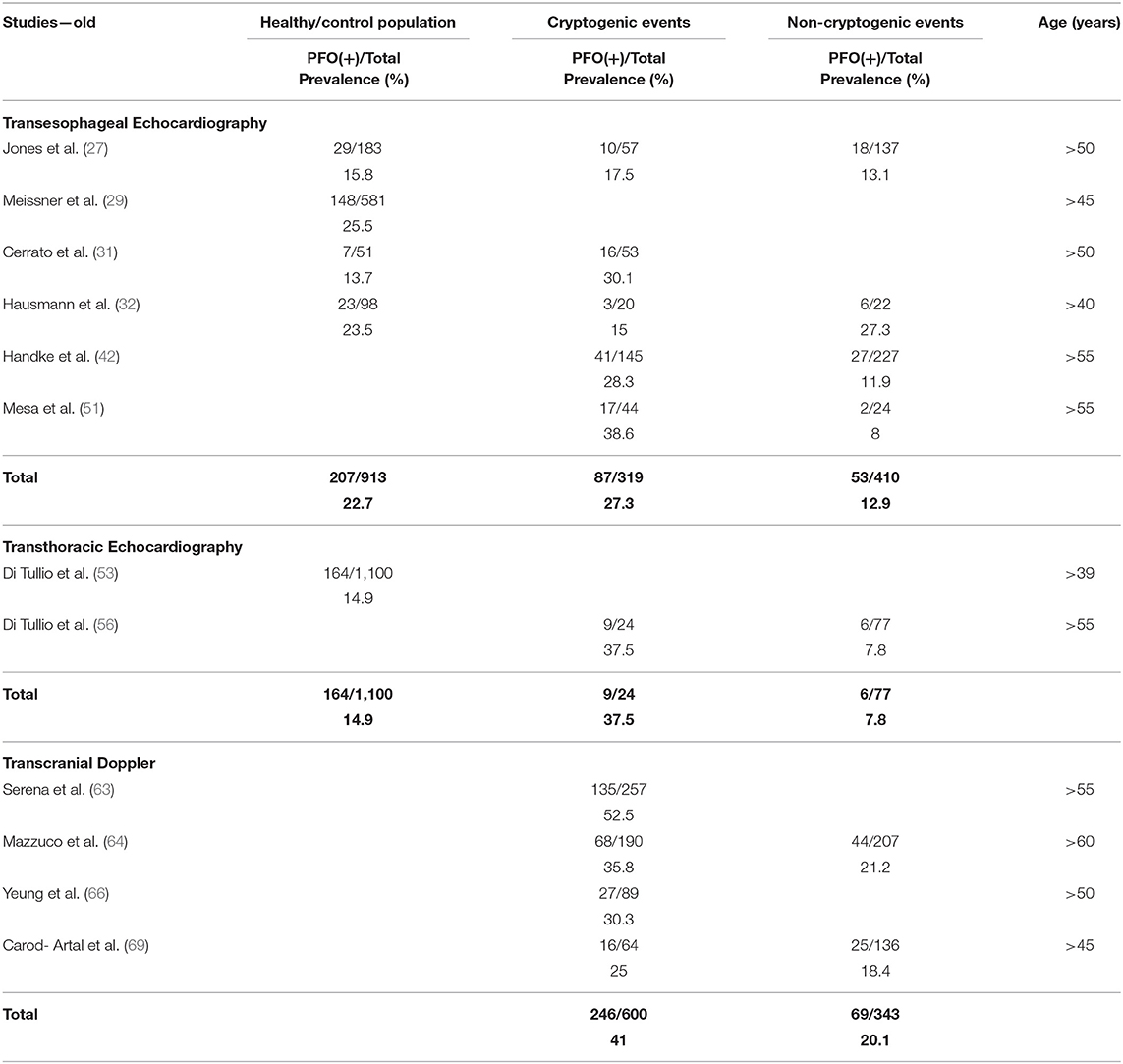
Tables 2, 3 present the results of our review in young and old subjects, respectively. The age cutoff per individual study ranged between 40 and 60 years. In healthy/control population, there was no difference of PFO prevalence between the young and the old age groups, when the diagnostic modality was TEE (25 vs. 22.7%, p = 0.35) or TTE (11.4 vs. 14.9%, p = 0.07). Concerning TCD, a comparison was not possible because data were not available for the old age group. In patients with CE, PFO prevalence in the young compared to the old age group was higher when the diagnostic modality was TEE (48.9 vs. 27.3%, p < 0.0001, OR = 2.6 with 95% CI = 2.0–3.3) or TCD (58.1 vs. 41%, p < 0.0001, OR = 1.9 with 95% CI = 1.6–2.5), but not TTE (53.3 vs. 37.5%, p = 0.16). Finally, in patients with non-CE, PFO prevalence in the young compared to the old age group was higher when the diagnostic modality was TEE (20.0 vs. 12.9%, p = 0.04, OR = 1.7 with 95% CI = 1.0–2.8) but not TTE (10.4 vs. 7.8%, p = 0.75) or TCD (22.8 vs. 20.1%, p = 0.56).
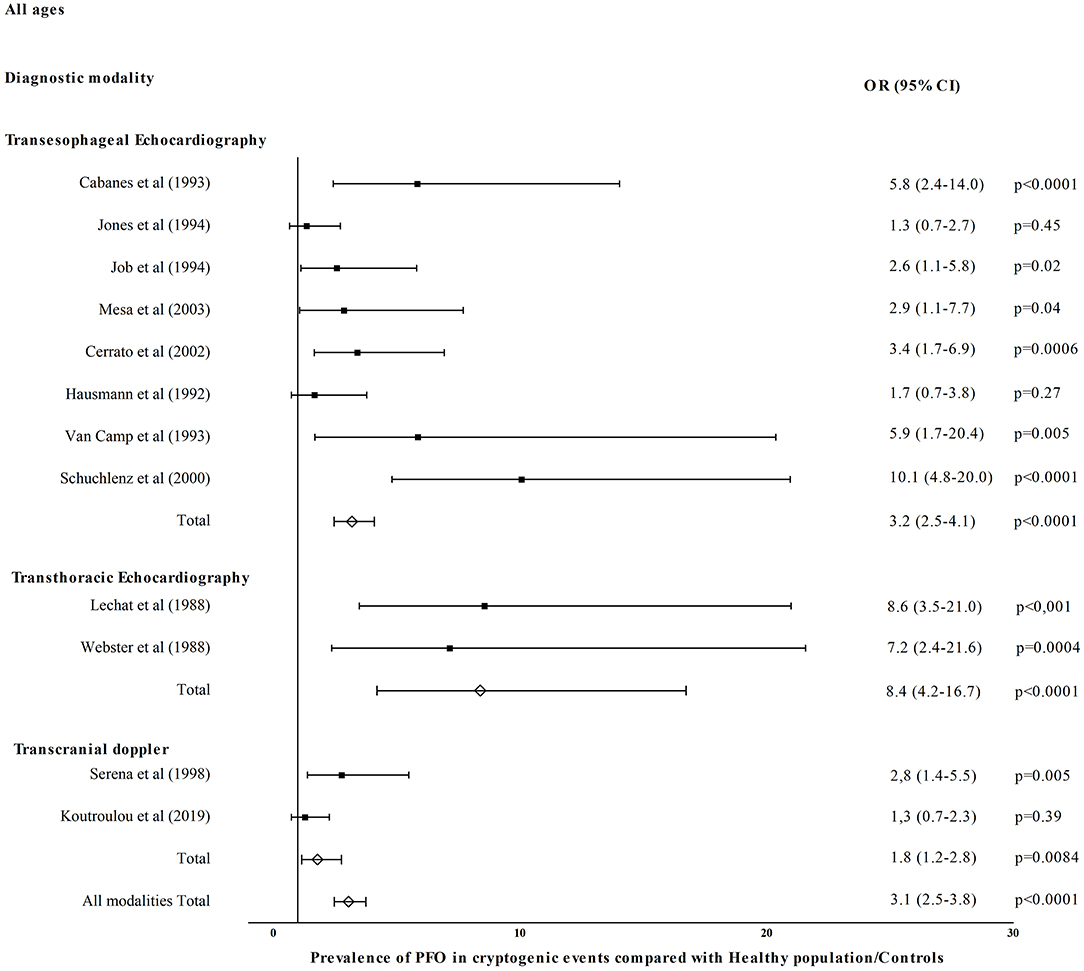
Figure 1 shows OR for PFO prevalence in CE compared with healthy/control population for eight TEE studies (26–28, 30–34), two TTE studies (52, 55), and two TCD studies (58, 62). Patent foramen ovale prevalence was higher in CE in TEE (OR = 3.2, 95% CI = 2.5–4.1, p < 0.0001), TTE (OR = 8.4, 95% CI = 4.2–16.7, p < 0.0001), and TCD studies (OR = 1.8, 95% CI = 1.2–2.8, p = 0.008). All diagnostic modalities included PFO prevalence was higher in CE compared with healthy/control population (OR = 3.1, 95% CI = 2.5–3.8, p < 0.0001). Figure 2 shows OR for PFO prevalence in CE compared with non-CE for 14 TEE studies (26–28, 32, 36–38, 40–44, 50, 51), three TTE studies (52, 56, 57), and six TCD studies (58, 64, 66–69). Patent foramen ovale prevalence was higher in patients with CE in TEE (OR = 2.4, 95% CI = 2.0–2.8, p < 0.0001), TTE (OR = 9.7, 95% CI = 4.7–20.3, p < 0.0001), and TCD studies (OR = 1.9, 95% CI = 1.6–2.3, p < 0.0001). All diagnostic modalities included, PFO prevalence was higher in CE compared with non-CE (OR = 2.3, 95% CI = 2.0–2.6, p < 0.0001).
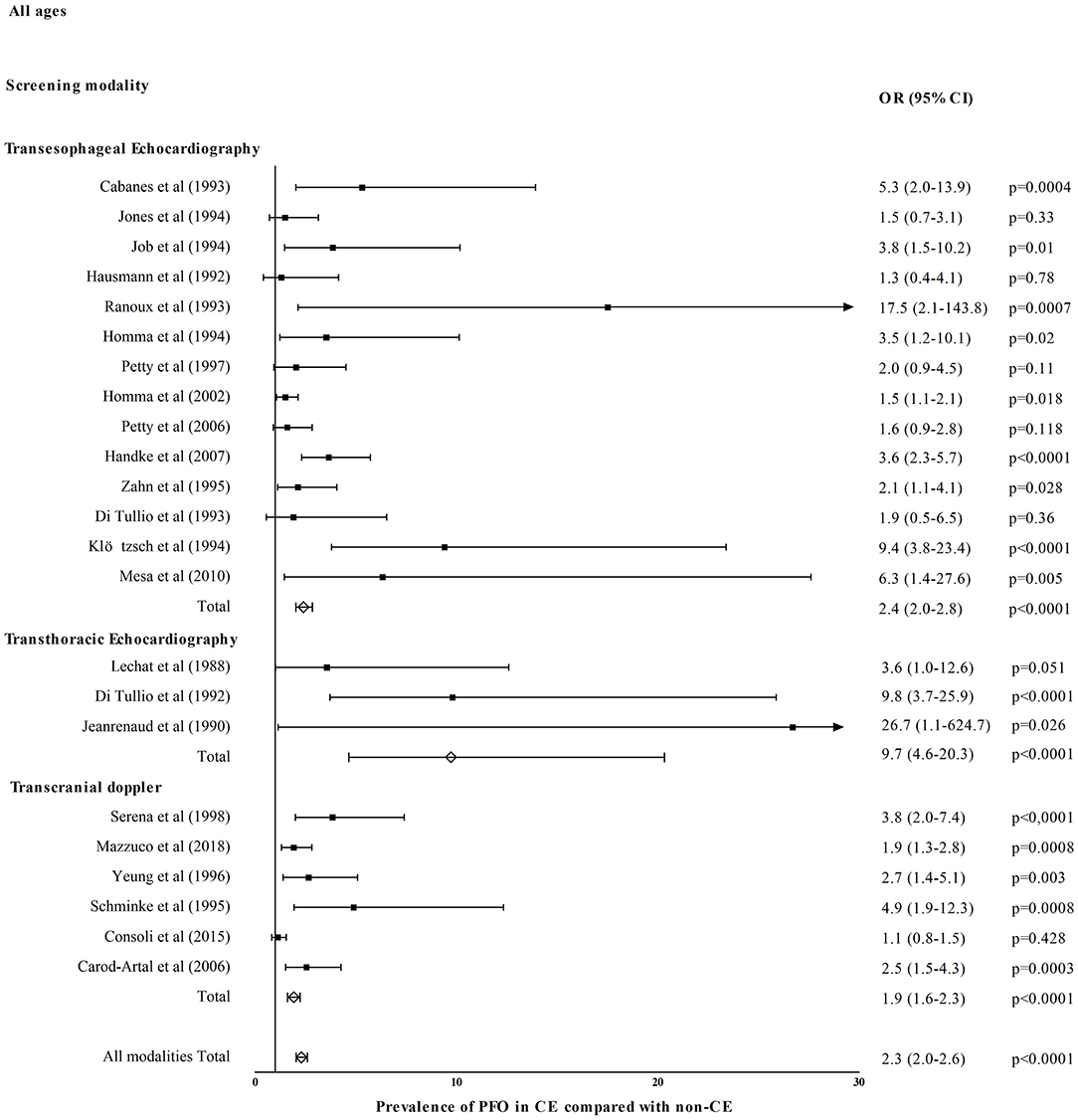
Figure 2. Prevalence of PFO in cryptogenic events (CE) compared with events of known cause (non-CE).
Discussion
Patent foramen ovale is not rare in the general population, but its detection has increasingly gained interest during the last two centuries, especially after its association with paradoxical embolism. Until late twentieth century, PFO detection relied exclusively on autopsy studies owing to lack of accurate in vivo diagnostic methods. However, even the more recent and better conducted studies admitted inherent limitations such as the use of formalin-fixed and not fresh specimens (16). The latter could have limited the detection of small-to-medium interatrial patency due to shrinkage of the fixed fibroelastic elements of the foramen ovale. Further possible disadvantages included the use of probes that could identify PFOs only larger than 1 mm and the inclusion of children. Interestingly, Hagen et al. (16) observed that PFO incidence was higher in younger subjects; conversely, PFO size was bigger in older subjects. They hypothesized that the former may be attributed to the increasing incidence of spontaneous anatomic closure of relatively small PFOs with advancing age, caused by age-related fibroelastic thickening of the valve of fossa ovalis. Consequently, relatively larger PFOs remain in late adult life, and their size may undergo further modification by stretching (16).
The development of echocardiography (initially TTE and later TEE) during the second half of the twentieth century provided the first in vivo diagnostic tools for PFO. A second breakthrough in PFO detection happened after the development of TCD by Aaslid et al. (70) in 1982. Etiologic classification systems of ischemic stroke consider PFO as a medium-to-low or uncertain-risk emboligenic cardiac source (71, 72). Accordingly, the latest RCTs (10–12) documented spectacular superiority of percutaneous PFO closure only in carefully selected patients with CSs over best medical treatment, hence the need to detect reliably PFO in CS sufferers with the three available ultrasound modalities. Hitherto, TEE is considered the “gold standard” for the documentation of PFO (73, 74). A meta-analysis comparing TTE with TEE as a reference in 3,067 patients (75) evidenced the low sensitivity (45.1%) but very high specificity (99.6%) of TTE for PFO detection. The former can be attributed to several technical limitations: (1) atrial structures are located in the far ultrasound beam field and are subjected to acoustic interference by the chest wall; (2) during right-to-left shunt (RLS) provoking maneuvers, there is considerable lung interference, interrupting continuous imaging of the atria; (3) there is limited ability to document increased right-to-left atrial pressure gradient by visualizing movement of the septum toward the left atrium (73). Consequently, TTE even when performed with contrast agent and RLS provoking maneuvers is a poor screening tool for PFO: a negative examination should not rule out PFO presence, particularly if clinical suspicion is high.
The potentially causal relationship of PFO with some of cryptogenic ischemic events of the brain led vascular neurologists to incorporate contrast TCD in their routine workup for CS for more than 20 years, especially after the standardization of the technical protocol for the detection and quantification of RLS (76). Transcranial Doppler lacks direct visualization of atrial structures and documents RLS regardless of the subjacent pathology: PFO or (rarely) pulmonary arteriovenous malformations (PAVMs). However, it is the only diagnostic modality that (1) proves the emboligenic potential of RLS to the target organ (brain) and (2) quantifies the burden of embolism (number of microembolic signals corresponding to microbubbles) to the recipient (brain) and not to the source (left atrium). Furthermore, TCD is non-invasive, safe, and easily repeatable with low cost, and patients are alert and able to perform effective and calibrated Valsalva maneuvers. The latter may have significant impact on shunt quantification (77) and represents a major limitation of TEE because patients tend to perform ineffective Valsalva maneuvers owing to poor cooperation under sedation, dysphagia, or to the presence of the TEE probe in their esophagus. Additionally, TEE has certain esophagus-related contraindications (varices, diverticula, strictures, Barrett esophagus, Mallory-Weiss tear, important hemorrhagic risk) and may have rare but severe complications (aspiration, esophageal bleeding, or perforation).
Meta-analyses comparing TCD with TEE (75, 78) concluded that TCD has excellent diagnostic accuracy and should be used as a first-choice screening tool for PFO in patients with CS, reserving TEE to provide complementary anatomic details that may influence treatment decisions (PFO morphology, presence of atrial septum aneurysm). An updated meta-analysis of 2,751 patients by the authors of the European position paper on the management of patients with PFO (79) reconfirmed the excellent accuracy of TCD compared with TEE (sensitivity of 94%, specificity of 92%, area under the receiver operating characteristic curve of 0.97). Although TEE has been considered as the “gold standard” for PFO detection, there is good evidence to think that TEE is a standard of uncertain validity. Most of the studies that compared the two modalities did not verify the origin of presumed false-positive TCD results. Frequently, the latter were arbitrarily attributed to possible PAVMs, an entity considered particularly rare with a prevalence of 1 in 2,600 (80). Furthermore, PAVMs may sometimes be misinterpreted by TEE as well (78). A meta-analysis of 164 patients comparing TEE with autopsy, cardiac surgery, and/or catheterization as the gold standard showed a sensitivity of 89.2% and specificity of 91.4% to detect PFO and concluded that TEE should be complemented by highly sensitive screening tests, namely, TCD (81). Estimation of the degree of RLS in all patients undergoing cardiac catheterization for PFO closure could be used as an alternative gold standard and could be compared with preprocedural TEE and TCD data. The superior sensitivity of TCD has also been demonstrated in a study (82) where TEE failed to document RLS in 15% of patients with CS, and of those, 40% had large RLSs. Therefore, “false-positive” TCD results may, in fact, represent true PFOs that are missed because of TEE limitations, and a negative TEE should not negate the need for a complementary TCD investigation.
According to our review, PFO prevalence in the general population across all ages was roughly 24% in autopsy and TEE studies. As expected, this percentage was much smaller in TTE studies (15%), whereas in the highly sensitive TCD studies, PFO prevalence was higher (~31%). The results were similar with small differences when subjects were stratified into young and old age groups. The results should be viewed under the limitations of the relatively small size (355 subjects) of healthy population in TCD studies and of the absence of TCD data in the old age group. Future TCD studies should focus on elderly general population and provide evidence regarding the differential PFO prevalence and magnitude of RLS with increasing age, as suggested by autopsy studies. Furthermore, in three of five TCD studies that estimated PFO prevalence (59–61), the healthy population comprised non-migraineurs, resulting in prevalence as low as 16% (59). Because migraineurs constitute 10–15% (83) of the general population and migraineurs are more likely to have a PFO (84), future studies on PFO prevalence in the general population should not exclude migraineurs.
Of note is the considerable variability in PFO prevalence among studies that used the same diagnostic modality. In autopsy studies, PFO prevalence ranged from 14.6 to 35.1%, in TEE studies from 11 to 43%, in TTE from 10 to 22.2%, and in TCD studies from 16 to 43.5%. The heterogeneous results could be attributed to (1) selection bias because in most ultrasound-based studies the reported “healthy population” consisted of patients who underwent an examination for a reason other than cerebrovascular event, and PFO detection was not the primary endpoint; (2) technical differences in PFO detection and RLS quantification; (3) different PFO prevalence in discrete ethnic/racial populations. Hitherto, the latter issue has not been addressed, and a “fixed” 25% (mainly based on autopsy and TEE studies) has been established as PFO prevalence across the general population and has been used for the calculation of PFOAF (15). However, given the limitations of autopsy and TEE studies, there is good reason not to take this percentage for granted. Interestingly, a recent TCD study conducted in a national population that comprised healthy Greek adults younger than 55 years and included subjects with migraine without aura (~10% of the total population) found much higher PFO prevalence (43.5%) compared to previous TCD studies in other populations (62). Interest in optimal patient selection for PFO closure or possibly for long-term anticoagulation with direct oral anticoagulants (85) remains keen and the RoPE score may be useful in guiding patient management; albeit it lacks large external validation studies, and it is heavily age weighted. Therefore, the estimation of degree of causality (PFOAF) may be underestimated or overestimated in ethnic/racial populations with PFO prevalence significantly lower or higher than the established 25%.
Although this review is not systematic and does not include meta-analytic methodology, it has the advantage of including only studies with a clear etiologic classification of stroke (cryptogenic vs. non-cryptogenic). We excluded studies with a vague definition of CS or studies that included “pseudo” CSs, and we excluded data from patients with TIAs. Transient ischemic attacks are a “soft” and overused diagnosis, and TIA definition has evolved over the years from time-specific to tissue-specific (86). Reversible deficits, particularly in the elderly, may be caused by amyloid angiopathy, an easily missed diagnosis unless blood-sensitive magnetic resonance imaging sequences are performed (87). Accordingly, all recent successful PFO closure trials did not include patients with TIAs (10–12).
In our review, PFO prevalence was nearly 2-fold in CE compared with non-CE (OR ranging widely from 1.1 to 17.5 in individual studies) in accordance with previous random-effects meta-analyses that established the strong association between CS and PFO with OR in the order of 2.9 (88, 89). This marked difference persisted regardless of age confirming a meta-analysis in older patients with OR in the order of 2.5 (64). However, young patients with CE had higher PFO prevalence compared to older patients reflecting the stronger association of CE with PFO in younger ages (88, 89). Concerning non-CE, PFO prevalence across the board and particularly in older patients was numerically lower than in the general population possibly owing to the decreasing frequency and less implication of PFO in stroke mechanisms with increasing age (16). We showed that PFO prevalence across all ages was ~3-fold in CE compared with healthy population/controls with OR ranging from 1.3 to 10.1. This is in accordance with random-effects OR from previous meta-analyses ranging from 2.1 to 2.9 (88, 89). The above association is mainly driven by TEE and TTE studies, whereas only two TCD studies compared PFO prevalence in CE with a relatively small non-selected general population of 215 subjects in total (58, 62). Given the high sensitivity, non-invasive nature, low cost, and repeatability of TCD, future large-scale TCD-based studies should investigate potential heterogeneity in PFO prevalence in different healthy racial/ethnic populations. The latter may have important implications in individualizing PFO-associated stroke risk assessment and management in the forthcoming era of precision medicine.
Author Contributions
IK and TK data acquisition, data analysis and interpretation, drafting of the manuscript. GT data interpretation, critical revision for important intellectual content. DT data acquisition, data analysis, and interpretation. DK and NG critical revision for important intellectual content.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. Alexi-Meskishvili V, Böttcher W. The first closure of the persistent ductus arteriosus. Ann Thorac Surg. (2010) 90:349–56. doi: 10.1016/j.athoracsur.2010.04.036
2. Lippmann H, Rafferty T. Patent foramen ovale and paradoxical embolization: a historical perspective. Yale J Biol Med. (1993) 66:11–7.
3. Hara H, Virmani R, Ladich E, Mackey-Bojack S, Titus J, Reisman M, et al. Patent foramen ovale: current pathology, pathophysiology, and clinical status. J Am Coll Cardiol. (2005) 46:1768–76. doi: 10.1016/j.jacc.2005.08.038
4. Kutty S, Sengupta P, Khandheria B. Patent foramen ovale: the known and the to be known. J Am Coll Cardiol. (2012) 59:1665–71. doi: 10.1016/j.jacc.2011.09.085
6. Furlan A, Reisman M, Massaro J, Mauri L, Adams H, Albers G, et al. Closure or medical therapy for cryptogenic stroke with patent foramen ovale. N Engl J Med. (2012) 366:991–9. doi: 10.1056/NEJMoa1009639
7. Carroll J, Saver J, Thaler D, Smalling R, Berry S, MacDonald L, et al. Closure of patent foramen ovale versus medical therapy after cryptogenic stroke. N Engl J Med. (2013) 368:1092–100. doi: 10.1056/NEJMoa1301440
8. Meier B, Kalesan B, Mattle H, Khattab A, Hildick-Smith D, Dudek D, et al. Percutaneous closure of patent foramen ovale in cryptogenic embolism. N Engl J Med. (2013) 368:1083–91. doi: 10.1056/NEJMoa1211716
9. Katsanos A, Spence D, Bogiatzi C, Parissis J, Giannopoulos S, Frogoudaki A, et al. Recurrent stroke and patent foramen ovale: a systematic review and meta-analysis. Stroke. (2014) 45:3352–9. doi: 10.1161/STROKEAHA.114.007109
10. Mas JL, Derumeaux G, Guillon B, Massardier E, Hosseini H, Mechtouff L, et al. Patent foramen ovale closure or anticoagulation vs. antiplatelets after stroke. N Engl J Med. (2017) 377:1011–21. doi: 10.1056/NEJMoa1705915
11. Sondergaard L, Kasner S, Rhodes J, Andersen G, Iversen H, Nielsen-Kudsk J, et al. Patent foramen ovale closure or antiplatelet therapy for cryptogenic stroke. N Engl J Med. (2017) 377:1033–42. doi: 10.1056/NEJMoa1707404
12. Lee P, Song J-K, Kim J, Heo R, Lee S, Kim D-H, et al. Cryptogenic stroke and high-risk patent foramen ovale: the DEFENSE- PFO trial. J Am Coll Cardiol. (2018) 71:2335–42. doi: 10.1016/j.jacc.2018.02.046
13. Saver J, Carroll J, Thaler D, Smalling R, MacDonald L, Marks D, et al. Long- term outcomes of patent foramen ovale closure or medical therapy after stroke. N Engl J Med. (2017) 377:1022–32. doi: 10.1056/NEJMoa1610057
14. Tsivgoulis G, Katsanos A, Mavridis D, Frogoudaki A, Vrettou A-R, Ikonomidis I, et al. Percutaneous patent foramen ovale closure for secondary stroke prevention: network meta-analysis. Neurology. (2018) 91:e8–18. doi: 10.1212/WNL.0000000000005739
15. Kent D, Ruthazer R, Weinmar C, Mas J-L, Serena J, Homma S, et al. An index to identify stroke- related vs incidental patent foramen ovale in cryptogenic stroke. Neurology. (2013) 81:619–25. doi: 10.1212/WNL.0b013e3182a08d59
16. Hagen P, Scholz D, Edwards W. Incidence and size of patent foramen ovale during the first 10 decades of life: an autopsy study of 965 normal hearts. Mayo Clin Proc. (1984) 59:17–20. doi: 10.1016/S0025-6196(12)60336-X
19. Parsons FG, Keith A. Seventh report of the committee of collective investigation of the Anatomical Society o Great Britain and Ireland, for the year 1896–1897. J Anat Physiol. (1897) 32:164–86.
20. Fawcett E, Blachford JV. The frequency of an opening between the right and left auricles at the seat of the fetal foramen ovale. J Anat Physiol. (1900) 35:67–70.
21. Seib GA. Incidence of the patent foramen ovale cordis in adult American whites and American negroes. Am J Anat. (1934) 55:511–25. doi: 10.1002/aja.1000550306
22. Wright RR, Anson BJ, Cleveland HC. The vestigial valves and the interatrial foramen ovale of the adult human heart. Anat Rec. (1948) 100:331–5. doi: 10.1002/ar.1091000305
23. Schroeckenstein RF, Wasenda GJ, Edwards JE. Valvular competent patent foramen ovale in adults. Minn Med. (1972) 55:11–3.
24. Sweeney LJ, Rosenquist GC. The normal anatomy of the atrial septum in the human heart. Am Heart J. (1979) 98:194–9. doi: 10.1016/0002-8703(79)90221-7
25. Penther P. Patent foramen ovale: an anatomical study: a propos of 500 consecutive autopsies. Arch Mal Coeur Vaiss. (1994) 87:15–21.
26. Cabanes L, Mas JL, Cohen A, Amarenco P, Cabanes PA, Oubary P, et al. Atrial septal aneurysm and patent foramen ovale as risk factors for cryptogenic stroke in patients less than 55 years of age: a study using transesophageal echocardiography. Stroke. (1993) 24:1865–73. doi: 10.1161/01.STR.24.12.1865
27. Jones E, Calafiore P, Donnan G, Tonkin A. Evidence that patent foramen ovale is not a risk factor for cerebral ischemia in the elderly. Am J Cardiol. (1994) 74:596–9. doi: 10.1016/0002-9149(94)90750-1
28. Job F, Ringelstein EB, Grafen Y, Flachskampf F, Doherty C, Stockmanns A, et al. Comparison of transcranial contrast doppler sonography and transesophageal contrast echocardiography for the detection of patent foramen ovale in young stroke patients. Am J Cardiol. (1994) 74:381–4. doi: 10.1016/0002-9149(94)90407-3
29. Meissner I, Whisnant JP, Khandheria BK, Spittell PC, O'Fallon WM, Pascoe RD, et al. Prevalence of potential risk factors for stroke assessed by transesophageal echocardiography and carotid ultrasonography: the SPARC study. Mayo Clin Proc. (1999) 74:862–9. doi: 10.4065/74.9.862
30. Mesa D, Franco M, Suárez de Lezo J, Muñoz J, Rus C, Delgado M, et al. Prevalence of patent foramen ovale in young patients with cryptogenic stroke. Rev Esp Cardiol. (2003) 56:662–8. doi: 10.1157/13049647
31. Cerrato P, Imperiale D, Priano L, Mangiardi L, Morello M, Marson AM, et al. Transoesophageal echocardiography in patients without arterial and major cardiac sources of embolism: difference between stroke subtypes. Cerebrovasc Dis. (2002) 13:174–83. doi: 10.1159/000047772
32. Hausmann D, Műgge A, Becht I, Daniel W. Diagnosis of patent foramen ovale by transesophageal echocardiography and association with cerebral and peripheral embolic events. Am J Cardiol. (1992) 70:668–72. doi: 10.1016/0002-9149(92)90210-P
33. van Camp G, Schulze D, Cosyns B, Vandenbossche JL. Relation between patent foramen ovale and unexplained stroke. Am J Cardiol. (1993) 71:596–8. doi: 10.1016/0002-9149(93)90518-H
34. Schuchlenz H, Weihs W, Horner S, Quehenberger F. The association between the diameter of a patent foramen ovale and the risk of embolic cerebrovascular events. Am J Med. (2000) 109:456–62. doi: 10.1016/S0002-9343(00)00530-1
35. Schwerzmann M, Nedeltchev K, Lagger F, Mattle HP, Windecker S, Meier B, et al. Prevalence and size of directly detected patent foramen ovale in migraine with aura. Neurology. (2005) 65:1415–8. doi: 10.1212/01.wnl.0000179800.73706.20
36. Ranoux D, Cohen A, Cabanes L, Amarenco P, Bousser MG, Mas JL. Patent foramen ovale: is stroke due to paradoxical embolism? Stroke. (1993) 24:31–4. doi: 10.1161/01.STR.24.1.31
37. Homma S, Di Tullio MR, Sacco RL, Mihalatos D, Li Mandri G, Mohr JP. Characteristics of patent foramen ovale associated with cryptogenic stroke: a biplane transesophageal echocardiography study. Stroke. (1994) 25:582–6. doi: 10.1161/01.STR.25.3.582
38. Petty GW, Khandheria BK, Chu CP, Sicks JD, Whisnant JP. Patent foramen ovale in patients with cerebral infarction. A transesophageal echocardiographic study. Arch Neurol. (1997) 54:819–22. doi: 10.1001/archneur.1997.00550190013008
39. Mas J-L, Arquizan C, Lamy C, Zuber M, Cabanes L, Derumeaux G, et al. Recurrent cerebrovascular events associated with patent foramen ovale, atrial septal aneurysm, or both. N Engl J Med. (2001) 345:1740–6. doi: 10.1056/NEJMoa011503
40. Homma S, Sacco RL, Di Tullio MR, Sciacca RR, Mohr JP. Effect of medical treatment in stroke patients with patent foramen ovale: patent foramen ovale in cryptogenic stroke study. Circulation. (2002) 105:2625–31. doi: 10.1161/01.CIR.0000017498.88393.44
41. Petty GW, Khandheria BK, Meissner I, Whisnant JP, Rocca WA, Christianson TJ, et al. Population-based study of the relationship between patent foramen ovale and cerebrovascular ischemic events. Mayo Clin Proc. (2006) 81:602–8. doi: 10.4065/81.5.602
42. Handke M, Harloff A, Olschewski M, Hetzel A, Geibel A. Patent foramen ovale and cryptogenic stroke in older patients. N Engl J Med. (2007) 357:2262–8. doi: 10.1056/NEJMoa071422
43. Zahn R, Lehmkuhl S, Lotter R, Zander M, Senges J. Cardiac sources of cerebral ischemic events with special regard to a patent foramen ovale. Herz Kreislauf . (1995) 27:279–84.
44. Di Tullio M, Sacco RL, Venketasubramanian N, Sherman D, Mohr JP, Homma S. Comparison of diagnostic techniques for the detection of a patent foramen ovale in stroke patients. Stroke. (1993) 24:1020–4. doi: 10.1161/01.STR.24.7.1020
45. Kim BJ, Kim NY, Kang DW, Kim JS, Kwon SU. Provoked right-to- left shunt in patent foramen ovale associates with ischemic stroke in posterior circulation. Stroke. (2014) 45:3707–10. doi: 10.1161/STROKEAHA.114.007453
46. Komar M, Olszowska M, Przewlocki T, Podolec J, Stepniewski J, Sobien B, et al. Transcranial doppler ultrasonography should it be the first choice for persistent foramen ovale screening? Cardiovasc Ultrasound. (2014) 12:16. doi: 10.1186/1476-7120-12-16
47. De Castro S, Papetti F, Di Angelantonio E, Razmovska B, Truscelli G, Tuderti U, et al. Feasibility and clinical utility of transesophageal echocardiography in the acute phase of cerebral ischemia. Am J Cardiol. (2010) 106:1339–44. doi: 10.1016/j.amjcard.2010.06.066
48. Weimar C, Holle DN, Benemann J, Schmid E, Schminke U, Haberl RL, et al. Current management and risk of recurrent stroke in cerebrovascular patients with right-to-left cardiac shunt. Cerebrovasc Dis. (2009) 28:349–56. doi: 10.1159/000229553
49. Nighoghossian N, Perinetti M, Barthelet M, Adeleine P, Trouillas P. Potential cardioembolic sources of stroke in patients less than 60 years of age. Eur Heart J. (1996) 17:590–4. doi: 10.1093/oxfordjournals.eurheartj.a014913
50. Klötzsch C, Janssen G, Berlit P. Transesophageal echocardiography and contrast- TCD in the detection of a patent foramen ovale: experiences with 111 patients. Neurology. (1994) 44:1603–6. doi: 10.1212/WNL.44.9.1603
51. Mesa D, Ruiz M, Delgado M, Suárez de Lezo J, Pan M, Tejero I, et al. Prevalence of patent foramen ovale determined by transesophageal echocardiography in patients with cryptogenic stroke aged 55 years or older. Same as younger patients? Rev Esp Cardiol. (2010) 63:315–22. doi: 10.1016/S1885-5857(10)70064-5
52. Lechat P, Mas JL, Lascault G, Loron P, Theard M, Klimczc M, et al. Prevalence of patent foramen ovale in patients with stroke. N Engl J Med. (1988) 318:1148–52. doi: 10.1056/NEJM198805053181802
53. Di Tullio MR, Sacco RL, Sciacca RR, Jin Z, Homma S. Patent foramen ovale and the risk of ischemic stroke in a multiethnic population. J Am Coll Cardiol. (2007) 49:797–802. doi: 10.1016/j.jacc.2006.08.063
54. Tatlidede AD, Oflazoglu B, Çelik SE, Anadol Ü, Forta H. Prevalence of patent foramen ovale in patients with migraine. Agri. (2007) 19:39–42.
55. Webster MW, Chancellor AM, Smith HJ, Swift DL, Sharpe DN, Bass NM, et al. Patent foramen ovale in young stroke patients. Lancet. (1988) 2:11–2. doi: 10.1016/S0140-6736(88)92944-3
56. Di Tullio M, Sacco RL, Gopal A, Mohr JP, Homma S. Patent foramen ovale as a risk factor for cryptogenic stroke. Ann Intern Med. (1992) 117:461–5. doi: 10.7326/0003-4819-117-6-461
57. Jeanrenaud X, Bogousslavsky J, Payot M, Regli F, Kappenberger L. Patent foramen ovale and cerebral infarct in young patients. Schweiz Med Wochenschr. (1990) 120:823–9.
58. Serena J, Segura T, Perez-Ayuso MJ, Bassaganyas J, Molins A, Dávalos A. The need to quantify right-to-left shunt in acute ischemic stroke: a case- control study. Stroke. (1998) 29:1322–8. doi: 10.1161/01.STR.29.7.1322
59. Del Sette M, Angeli S, Leandri M, Ferriero G, Bruzzone GL, Finocchi C, et al. Migraine with aura and right-to-left shunt on transcranial doppler: a case- control study. Cerebrovasc Dis. (1998) 8:327–30. doi: 10.1159/000015875
60. Anzola GP, Magoni M, Guindani M, Rozzini L, Dalla Volta G. Potential source of cerebral embolism in migraine with aura: a transcranial Doppler study. Neurology. (1999) 52:1622–5. doi: 10.1212/WNL.52.8.1622
61. Domitrz I, Mieszkowski J, Kaminska A. Relationship between migraine and patent foramen ovale: a study of 121 patients with migraine. Headache. (2007) 47:1311–8. doi: 10.1111/j.1526-4610.2006.00724.x
62. Koutroulou I, Tsivgoulis G, Tsalikakis D, Karacostas D, Grigoriadis N, Karapanayiotides T. Prevalence of Right-to-left Cardiac Shunt in the Greek Population is High and Impacts on the Interpretation of the Risk of Paradoxical Embolism (RoPE) Score. In: International Stroke Conference. Honolulu (2019). p. 50(suppl):TMP88 doi: 10.1161/str.50.suppl_1.TMP88
63. Serena J, Marti-Fàbregas J, Santamarina E, Rodríguez JJ, Perez-Ayuso MJ, Masjuan J, et al. Recurrent stroke and massive right-to-left shunt: results from the prospective Spanish multicenter (CODICIA) study. Stroke. (2008) 39:3131–6. doi: 10.1161/STROKEAHA.108.521427
64. Mazzucco S, Li L, Binney L, Rothwell PM, Oxford Vascular Study Phenotyped Cohort. Prevalence of patent foramen ovale in cryptogenic transient ischaemic attack and non-disabling stroke at older ages: a population-based study, systematic review, and meta-analysis. Lancet Neurol. (2018) 17:609–17. doi: 10.1016/S1474-4422(18)30167-4
65. Palazzo P, Ingrand P, Agius P, Belhadj Chaidi R, Neau JP. Transcranial doppler to detect right-to-left shunt in cryptogenic acute ischemic stroke. Brain Behav. (2019) 9:e01091. doi: 10.1002/brb3.1091
66. Yeung M, Khan KA, Shuaib A. Transcranial Doppler ultrasonography in the detection of venous to arterial shunting in acute stroke and transient ischaemic attacks. J Neurol Neurosurg Psychiatry. (1996) 61:445–9. doi: 10.1136/jnnp.61.5.445
67. Schminke U, Ries S, Daffertshofer M, Staedt U, Hennerici M. Patent foramen ovale: a potential source of cerebral embolism? Cerebrovasc Dis. (1995) 5:133–8. doi: 10.1159/000107838
68. Consoli D, Paciaroni M, Galati F, Aguggia M, Melis M, Malferrari G, et al. Prevalence of patent foramen ovale in ischaemic stroke in Italy: Results of SISIFO study. Cerebrovasc Dis. (2015) 39:162–9. doi: 10.1159/000375152
69. Carod-Artal FJ, da Silveira Ribeiro L, Braga H, Kummer W, Mesquita HM, Vargas AP. Prevalence of patent foramen ovale in migraine patients with and without aura compared with stroke patients. A transcranial Doppler study. Cephalalgia. (2006) 26:934–9. doi: 10.1111/j.1468-2982.2006.01156.x
70. Aaslid R, Markwalder TM, Nornes H. Noninvansive transcranial doppler ultrasound recording of flow velocity in basal cerebral arteries. J Neurosurg. (1982) 57:769–74. doi: 10.3171/jns.1982.57.6.0769
71. Adams HP Jr, Bendixen BH, Kappelle LJ, Biller J, Love BB, Gordon DL, et al. Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. Stroke. (1993) 24:35–41. doi: 10.1161/01.STR.24.1.35
72. Ay H, Benner T, Arsava EM, Furie KL, Singhal AB, Jensen MB, et al. A computerized algorithm for etiologic classification of ischemic stroke: the causative classification of stroke system. Stroke. (2007) 38:2979–84. doi: 10.1161/STROKEAHA.107.490896
73. Pearson AC, Labovitz AJ, Tatineni A, Gomez CR. Superiority of transesophageal echocardiography in detecting cardiac source of embolism in patients with cerebral ischemia of uncertain etiology. J Am Coll Cardiol. (1991) 17:66–72. doi: 10.1016/0735-1097(91)90705-E
74. de Belder MA, Tourikis L, Leech G, Camm AJ. Risk of patent foramen ovale for thromboembolic events in all age groups. Am J Cardiol. (1992) 69:1316–20. doi: 10.1016/0002-9149(92)91228-V
75. Katsanos AH, Psaltopoulou T, Sergentanis TN, Frogoudaki A, Vrettou AR, Ikonomidis I, et al. Transcranial Doppler versus transthoracic echocardiography for the detection of patent foramen ovale in patients with cryptogenic cerebral ischemia: a systematic review and diagnostic test accuracy meta-analysis. Ann Neurol. (2016) 79:625–35. doi: 10.1002/ana.24609
76. Jauss M, Zanette E. Detection of right-to-left shunt with ultrasound contrast agent and transcranial doppler sonography. Cerebrovasc Dis. (2000) 10:490–6. doi: 10.1159/000016119
77. Devuyst G, Piechowski-Józwiak B, Karapanayiotides T, Fitting JW, Kémeny V, Hirt L, et al. Controlled contrast transcranial doppler and arterial blood gas analysis to quantify shunt through patent foramen ovale. Stroke. (2004) 35:859–63. doi: 10.1161/01.STR.0000119384.28376.EB
78. Mojadidi MK, Roberts SC, Winoker JS, Romero J, Goodman-Meza D, Gevorgyan R, et al. Accuracy of transcranial doppler for the diagnosis of intracardiac right-to-left shunt: A bivariate meta- analysis of prospective studies. J Am Coll Cardiol Img. (2014) 7:236–50. doi: 10.1016/j.jcmg.2013.12.011
79. Pristipino C, Sievert H, D' Ascenzo F, Mas JL, Meier B, Scacciatella P, et al. European position paper on the management of patients with patent foramen ovale. General approach and left circulation thromboembolism. Eurointervention. (2019) 14:1389–402. doi: 10.4244/EIJ-D-18-00622
80. Shovlin CL. Pulmonary arteriovenous malformations. Am J Respir Crit Care Med. (2014) 190:1217–28. doi: 10.1164/rccm.201407-1254CI
81. Mojadidi KM, Bogush N, Caceres JD, Msaouel P, Tobis JM. Diagnostic accuracy of transesophageal echocardiography for the detection of patent foramen ovale: a meta- analysis. Echocardiography. (2014) 31:752–8. doi: 10.1111/echo.12462
82. Tobe J, Bogiatzi C, Munoz C, Tamayo A, Spence JD. Transcranial doppler is complementary to echocardiography for detection and risk stratification of patent foramen ovale. Can J Cardiol. (2016) 32:986.e9–16. doi: 10.1016/j.cjca.2015.12.009
83. Woldeamanuel Y, Cowan R. Migraine affects 1 in 10 people worldwide featuring recent rise: a systematic review and meta-analysis of community-based studies involving 6 million participants. J Neurol Sci. (2017) 372:307–15. doi: 10.1016/j.jns.2016.11.071
84. Schwedt TJ, Demaerschalk BM, Dodick DW. Patent foramen ovale and migraine: a quantitative systematic review. Cephalalgia. (2008) 28:531–40. doi: 10.1111/j.1468-2982.2008.01554.x
85. Kasner SE, Swaminathan B, Lavados P, Sharma M, Muir K, Veltkamp R, et al. Rivaroxaban or aspirin for patent foramen ovale and embolic stroke of undetermined source: a prespecified subgroup analysis from the NAVIGATE ESUS trial. Lancet Neurol. (2018) 17:1053–60. doi: 10.1016/s1474-4422(18)30319-3
86. Albers GW, Caplan LR, Easton JD, Fayad PB, Mohr JP, Saver JL, et al. Transient ischemic attack. Proposal for a new definition. N Engl J Med. (2002) 347:1713–6. doi: 10.1056/NEJMsb020987
87. Charidimou A, Baron JC, Werring DJ. Transient focal neurological episodes, cerebral amyloid angiopathy, and intracerebral hemorrhage risk: looking beyond TIAs. Int J Stroke. (2013) 8:105–8. doi: 10.1111/ijs.12035
88. Overell JR, Bone I, Lees KR. Interatrial septal abnormalities and stroke. A meta-analysis of case- control studies. Neurology. (2000) 55:1172–9. doi: 10.1212/WNL.55.8.1172
Keywords: PFO, epidemiology, stroke, TCD, review
Citation: Koutroulou I, Tsivgoulis G, Tsalikakis D, Karacostas D, Grigoriadis N and Karapanayiotides T (2020) Epidemiology of Patent Foramen Ovale in General Population and in Stroke Patients: A Narrative Review. Front. Neurol. 11:281. doi: 10.3389/fneur.2020.00281
Received: 05 February 2020; Accepted: 25 March 2020;
Published: 28 April 2020.
Edited by:
Vincent Thijs, University of Melbourne, AustraliaReviewed by:
Michael V. Mazya, Karolinska University Hospital, SwedenAndrea Morotti, Neurological Institute Foundation Casimiro Mondino (IRCCS), Italy
Copyright © 2020 Koutroulou, Tsivgoulis, Tsalikakis, Karacostas, Grigoriadis and Karapanayiotides. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Theodoros Karapanayiotides, dGthcmFwYW5heWlvdGlkZXNAYXV0aC5ncg==
 Ioanna Koutroulou
Ioanna Koutroulou Georgios Tsivgoulis
Georgios Tsivgoulis Dimitrios Tsalikakis3
Dimitrios Tsalikakis3 Nikolaos Grigoriadis
Nikolaos Grigoriadis Theodoros Karapanayiotides
Theodoros Karapanayiotides