- Vanda Pharmaceuticals Inc., Washington, CA, United States
Background: Most travelers experience Jet Lag Disorder (JLD) symptoms due to misalignment of their circadian rhythms with respect to the new time zone. We assessed the efficacy and safety of tasimelteon (HETLIOZ®) in healthy participants using a laboratory model of JLD induced by an 8-h phase advance of the sleep-wake cycle (JET8 Study). We hypothesized that tasimelteon treatment in participants experiencing JLD would cause increased sleep time, increased next-day alertness, and reduced next-day sleepiness.
Methods: We undertook a randomized, double-blind, placebo-controlled trial in 12 US clinical research sleep centers. We screened healthy adults ages 18–73 years, who were eligible for the randomization phase of JET8 if they typically went to bed between 21:00 and 01:00, slept between 7 and 9 h each night, and slept at a consistent bedtime. We used block randomization stratified by site to assign participants (1:1) to receive a single oral dose of tasimelteon (20 mg) or placebo 30 min before their 8-h phase-advanced bedtime. The primary endpoint was Total Sleep Time in the first 2/3 of the night (TST2/3), which was measured by polysomnography during the 8-h sleep episode, and assessed in the intent-to-treat population. The trial is completed and registered with ClinicalTrials.gov, NCT03373201.
Results: Between October 16, 2017 and January 17, 2018, we screened 607 healthy participants for JET8, of whom 320 (53%) were assigned to receive tasimelteon (n = 160) or placebo (n = 160). Tasimelteon treatment increased TST2/3 (primary endpoint) by 60.3 min (95%CI 44.0 to 76.7, P < 0.0001) and whole night TST by 85.5 min (95% CI 64.3 to 106.6, P < 0.0001), improved next day alertness, next day sleepiness, and shortened latency to persistent sleep by −15.1 min (95% CI −26.2 to −4.0, P = 0.0081).
Conclusion: A single dose of tasimelteon improves the primary symptoms of JLD, including nighttime insomnia and next day functioning among participants in a laboratory model of JLD simulating eastward trans-meridian travel by inducing an 8-h phase advance of the sleep-wake cycle.
Introduction
Jet Lag Disorder (JLD), also known as Jet Lag and Circadian Rhythm Sleep-Wake Disorder—Jet Lag Type, is a Circadian Rhythm Sleep-Wake Disorder (CRSWD) characterized by a mismatch between the timing of an individual's endogenous circadian cycle and the sleep and wake patterns required following a rapid change in time zone. After traveling across two or more time zones, a traveler's endogenous circadian clock becomes misaligned with the destination's local time zone (1, 2). The mismatch between a traveler's endogenous circadian cycle and their extrinsic 24-h light-dark cycle may cause them to experience symptoms of JLD due to a misaligned circadian rhythm with respect to the new time zone. The essential features of JLD, according to the International Classification of Sleep Disorders Third Edition, are night-time insomnia and daytime sleepiness when the sleep-wake schedule is shifted in the new time zone (1). Other symptoms that may be associated with JLD include impairment of daytime functioning and malaise (3). Excessive daytime sleepiness and circadian misalignment can lead to impaired concentration, attention, performance, and alertness (4). JLD can also be associated with gastrointestinal symptoms, headache, fatigue, general malaise, decreased appetite, indigestion, and menstrual symptoms in women, though these are not the cardinal features of the disorder (5).
The endogenous circadian clock drives an intrinsic 24-h rhythm in humans that regulates hormone levels, body temperature, metabolism, and the sleep-wake cycle. The circadian timing system (CTS) is composed of a hypothalamic circadian pacemaker located in the suprachiasmatic nuclei (SCN), an array of SCN outputs, and a system of molecular clocks in peripheral tissues (6). The light-dark cycle is the major environmental time cue and most powerful synchronizer (“zeitgeber”) of the CTS to the Earth's 24-h day. Light sensed by intrinsically photosensitive Retinal Ganglion Cells (ipRGCs) provides information on the daily light-dark cycle to the SCN via the retinohypothalamic tract (RHT). Through this input, the SCN is synchronized (entrained) to a 24-h light-dark cycle. The SCN also functions as the master body clock by entraining the body's peripheral clocks to a 24-h rhythm through endocrine, neuronal, and physiological signals that regulate cyclic levels of melatonin, cortisol, and core body temperature (7, 8). Through the synchronization of peripheral clocks, the SCN governs the optimal timing of key physiological processes, including those of the cardiovascular system, metabolism, immune regulation, and the rest-activity and sleep-wake cycles (9–11).
Sleep disruption is the primary symptom of JLD, and can be especially severe when traveling in an eastward direction, which requires a phase advance of both sleep-wake timing and circadian rhythmicity (5, 12). Following eastward travel, the first two thirds of the night is usually very disrupted, as that portion of the night overlaps with the traveler's wake-maintenance zone (13). Further, a phase advance of longer than 7 h can induce an “antidromic” response, wherein the individual will start delaying to the new phase instead of advancing (for instance, delaying 16-h instead of advancing by 8-h) (12). Due to the phase advance associated with eastward flights across 3–8 time zones, travelers have difficulty going to sleep at the new bedtime, whereas after westward flights across 3–8 time zones, travelers have difficulty remaining awake in the evening and have early morning awakenings in the new time zone (13, 14). Additionally, depending on the number of hours of the phase advance and speed to physiologically adjust to the new time zone, the main effect can be on sleep latency and may only affect the first night or two of the trip (15). For instance, west coast to east coast US travel results in a 3-h phase advance, which is equivalent to being required to sleep 3 h before an individual's usual bedtime. This would result in difficulty falling asleep, but after the first 3 h, the individual would be attempting to sleep during their typical sleep cycle. Additionally, for this phase advance, the effect may only be experienced on the first night, with adaptation to the new time zone on subsequent nights, as demonstrated in a previous study (15).
The hormone melatonin has a robust endogenous circadian secretory profile that peaks during night hours. Misaligned melatonin rhythms are associated with CRSWD including Non-24-H Sleep-Wake Disorder (Non-24), Delayed Sleep-Wake Phase Disorder, and JLD. Two types of G-protein-coupled receptors for melatonin, MT1 and MT2, are located in the SCN. Melatonin exerts sleep-promoting effects, although the exact mechanism is not clear. It has been demonstrated that activation of the MT1 receptor by melatonin suppresses firing of SCN neurons (16), which may interfere with the wake-promoting signal. It has also been suggested that the sleep-promoting effects may result from melatonin's ability to induce hypothermia (17).
Tasimelteon is a Dual Melatonin Receptor Agonist (DMRA) that has been shown to regulate the timing of melatonin secretion when administered to participants in advance of the usual dim light onset of melatonin (18). We have previously shown that tasimelteon can entrain the circadian clock of individuals who are not entrained to the 24-h day and have Non-24 by administering tasimelteon every night 30 min before the target bedtime (18). We have further shown that administration of tasimelteon advances the circadian clock during a 5-h phase advance (15). It has also been reported that exogenous melatonin administration can entrain and shift the circadian clock in humans (19–21). Tasimelteon has not previously been tested to phase advance individuals more than 5 h, and there are no phase response curve data available for tasimelteon. However, phase response curve data for melatonin suggests that the maximal resetting effect of melatonin for inducing a phase advance occurs at about 3-h before dim light melatonin onset (DLMO) timing (about 5-h before the start of the scheduled sleep episode) and can advance the endogenous circadian phase by about 1-h after one night (21). Little is known about the impact of administering a melatonin-receptor agonist following a phase advance in sleep timing of more than 5-h.
Here we present the results of the JET8 study, a phase III clinical trial to determine the efficacy and safety of a single oral dose of tasimelteon in healthy participants in a laboratory simulation of JLD associated with an 8-h phase advance of sleep-wake timing.
Methods
Study Design
The study used a randomized, double-blind, placebo-controlled, parallel design and was approved by institutional review boards, either Chesapeake IRB or BioMed IRB, at all sleep centers. Screening consisted of a clinical site visit to evaluate eligibility followed by a screening interval lasting from 1 to 4 weeks. Evaluation, which consisted of a clinical site overnight stay, followed successful completion of the screening procedure (Figure 2). Additionally, the end of Daylight Saving Time (DST) occurred during the study. Study sites were instructed not to schedule any participants for evaluation for the day before, or the week following, November 4, 2017. This was done to allow participants to naturally adjust to the 1-h phase delay induced by DST. Twelve US study sites with single-bed suites were utilized for the evaluation visit. Suites were free of time cues such as light and sound.
Participants
Participants recruited were men and women aged 18 to 73 years, in good health (determined by medical and psychiatric history, physical examination, electrocardiography, serum chemistry, hematology, urinalysis, and urine toxicology), who were not at high risk for common sleep disorders including obstructive sleep apnea and chronic insomnia, as assessed using validated questionnaires. Study participants provided written informed consent before any screening procedures began.
At the inpatient screening visit, participants self-reported their habitual bedtime and self-determined target bedtime. Participants were excluded at screening if their habitual and target bedtimes were not between 21:00 and 01:00, or if their average habitual total reported sleep durations were less than 7-h, or more than 9-h. After completion of the screening visit, participants were required to maintain a consistent sleep-wake schedule for at least 1 week prior to the start of the study. Participants were instructed to adhere to their self-selected target bedtime for the duration of screening. Participants were eligible for randomization if they met their target bedtime, ± 30 min, for the three nights prior to the inpatient evaluation visit, and at least five of the seven nights before the evaluation visit. Exclusions also occurred if participants did not have an average sleep episode duration of at least 6.7-h and no more than 9-h for the seven nights before evaluation. For the last three nights prior to the inpatient evaluation visit, the participant must have had at least 7-h and no more than 10-h, of self-reported total sleep time for each night.
Randomization and Masking
Randomization was performed through an interactive web response system (IWRS). When each participant arrived at the evaluation visit, the investigator or designee utilized the IWRS system to distribute study medication from the capsule-containing bottle. Participants were randomly assigned to either tasimelteon (20 mg) or placebo, in a 1:1 ratio. All capsules were size 1, opaque, hard gelatin, and the color was dark blue with two white bars printed on both the cap and body of the capsule. Placebo was provided in size and appearance identical to those containing tasimelteon. An unblinded, third-party statistician prepared the randomization scheme. Randomization was stratified by study site.
Procedures
During screening, assessments taken included the Karolinska Sleepiness Scale (KSS), a Visual Analog Scale (VAS), the Morningness-Eveningness Questionnaire (MEQ), and a post-sleep questionnaire (PSQ). The KSS and VAS were subjective measures of next day functioning and alertness. The KSS queried participants as to how sleepy they felt on a 9-point scale with 1 being extremely awake and 9 being extremely sleepy/fighting to stay awake. The VAS was a self-rated scale to assess sleepiness. Participants marked along a 100 mm line to represent their current state of sleepiness, 0 being very sleepy and 100 being very alert. The PSQ was used as a subjective assessment of wakefulness after sleep onset (WASO), sleep latency, total sleep time (TST), number of nocturnal awakenings, and overall sleep quality. Overall sleep quality was reported on a scale from 1 to 5, corresponding to “poor,” “fair,” “average,” “good,” or “excellent.”
Daily electronic diaries and wrist actigraphy were recorded to ensure compliance with eligibility parameters during outpatient screening. These eligibility parameters were used to ensure that the participants were not acutely or chronically sleep deprived in the days preceding the inpatient overnight phase advance of sleep-wake timing.
For the 8-h sleep-wake phase advance, each participants' bedtime was advanced by 8-h compared to the target bedtime established during screening (Figure 3). A single oral dose administration of medication, tasimelteon or placebo, occurred 30 min (± 5 min) prior to lights off. Tasimelteon and placebo were manufactured by Patheon Pharmaceuticals Inc. in Cincinnati, Ohio, US. Participants' sleep duration, timing, and architecture were monitored objectively by polysomnography (PSG), and centrally scored in 30-s epochs. Polysomnographic data were analyzed for the single inpatient 8-h sleep episode during the evaluation visit.
During evaluation, assessments taken included the KSS, VAS, and PSQ. The KSS and VAS were each administered four times following the dose administration after awakening. The first KSS and VAS assessments were conducted 90 min (± 15 min) after wake time, and the following three KSS and VAS assessments were conducted once every 2-h, over the following 6-h. The PSQ was administered once, approximately 30 min (± 15 min) after the end of the bedrest episode, following the 8-h phase advance.
Outcomes
The primary outcome measure was total sleep time in the first two-thirds of the night (TST2/3) following an 8-h phase advance of the sleep episode. TST2/3 was selected as the primary endpoint for multiple reasons. The first two-thirds of the night are maximally overlapped with the previous circadian day, including the circadian wake maintenance zone (13), when the output of the central circadian pacemaker is most likely to interfere with sleep. Further, many individuals habitually sleep less than 8 h per night and therefore wakefulness in the final third of an 8-h interval may represent the normal morning after a sufficient night of sleep.
Secondary objective outcomes measured by PSG included TST, WASO, and latency to persistent sleep (LPS). WASO was defined as the time spent awake in the interval between onset of persistent sleep and the end of the bedrest episode. LPS was defined as the length of time elapsed between the start of the bedrest episode and onset of persistent sleep. Subjective secondary outcome measures included the next day residual effects of tasimelteon, as measured by individual and average KSS scores, individual and average VAS scores, and the PSQ. Average Night 1 KSS and Average Night 1 VAS combined all four KSS and VAS assessments collected after dose administration. These primary and secondary outcomes were centrally assessed across all study centers.
Safety assessments included regular monitoring and recording of adverse events; monitoring of hematology, serum chemistry, and urinalysis values; monitoring of vital signs; performance of physical examinations; and performance of electrocardiograms. Safety assessments were performed at the inpatient screening and evaluation visits, and any supplementary unscheduled visits.
Statistical Analysis
For hypothesis testing, a sample size of 150 individuals per treatment group was estimated to provide ~3% power to detect a 30-min difference in TST2/3 following an 8-h phase advance bedtime between the treatment group and placebo based on a two-tailed t-test with α = 0.05 (15) and a standard deviation (SD) of 75 min, which was estimated from a previous study comparing doses of tasimelteon and placebo in healthy volunteers in a 5-h phase advance model (15). Additionally, this sample size provided at least 85% power to detect a difference of 28 min (SD 80) in TST between the treatment group and placebo.
The intent-to-treat (ITT) population was defined as all individuals randomized into the study who received a dose of study drug and had complete PSG data. The difference between treatment groups was summarized by the difference between the least squared means (LS) and the 95% confidence interval (95% CI). Hypotheses tested were declared statistically significant if the calculated P-values were ≤ 0.05. All primary and secondary objective outcome measures between treatment groups were analyzed by analysis of variance (ANOVA). The Wilcoxon Rank-Sum test of TST2/3 was performed as a sensitivity analysis. All primary and secondary endpoints were pre-specified in the statistical analysis plan. All analyses and tabulations were performed using SAS® version 9.3 or higher. This study is registered with clinicaltrials.gov under NCT03373201.
Results
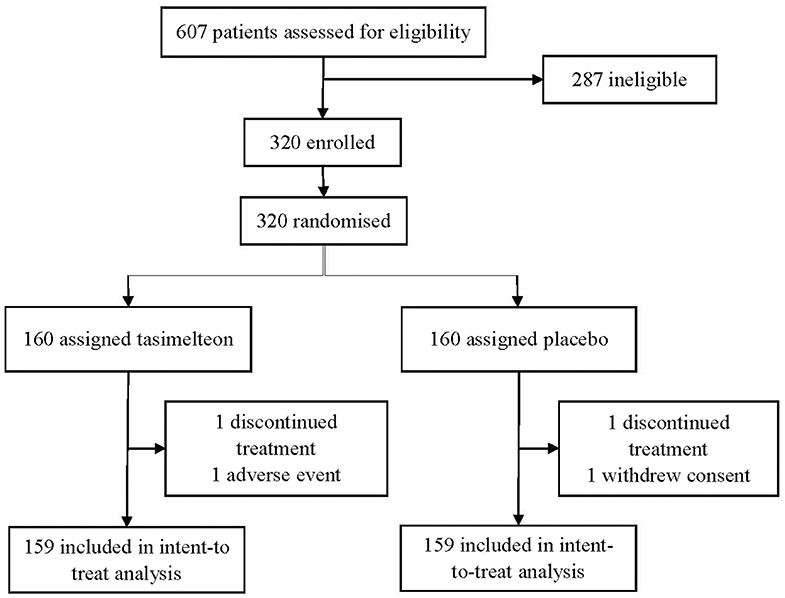
Between October 16, 2017 and January 17, 2018 a total of 607 participants were assessed for eligibility in the JET8 study. 320 (52.7%) participants met the inclusion criteria and were enrolled. Following randomization, one participant (0.3%) in the placebo group withdrew consent and one participant (0.3%) in the tasimelteon group discontinued due to a serious adverse event determined to be unrelated to therapy. 318 individuals completed the study and were included in the ITT population analysis (Figure 1).
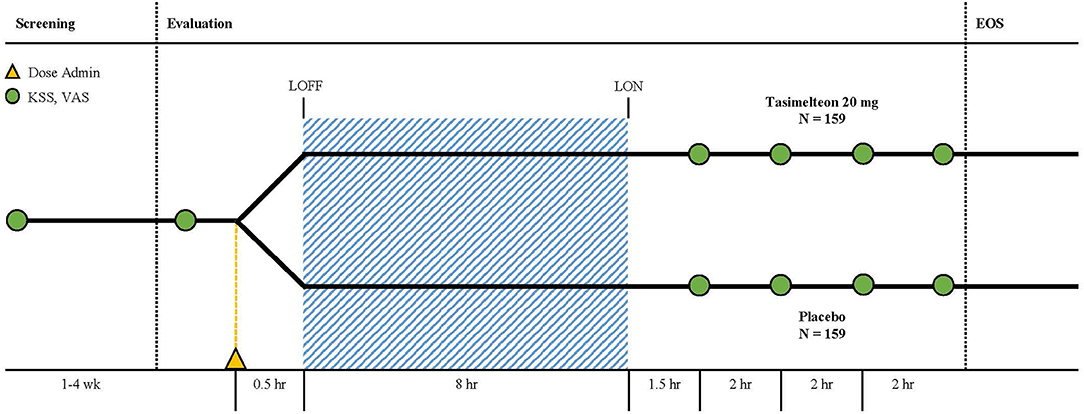
Figure 1. The study consisted of a 1–4 week screening period followed by an evaluation visit where patients were dosed with tasimelteon 20 mg or placebo 30-min prior to their pre-determined 8-h phase advance bedtime. Polysomnography was performed during the 8-h sleeping period. Karolinska Sleepiness Scale (KSS) and Visual Analog Scale (VAS) assessments were completed once during screening, once before the phase advance, and four times after waking.
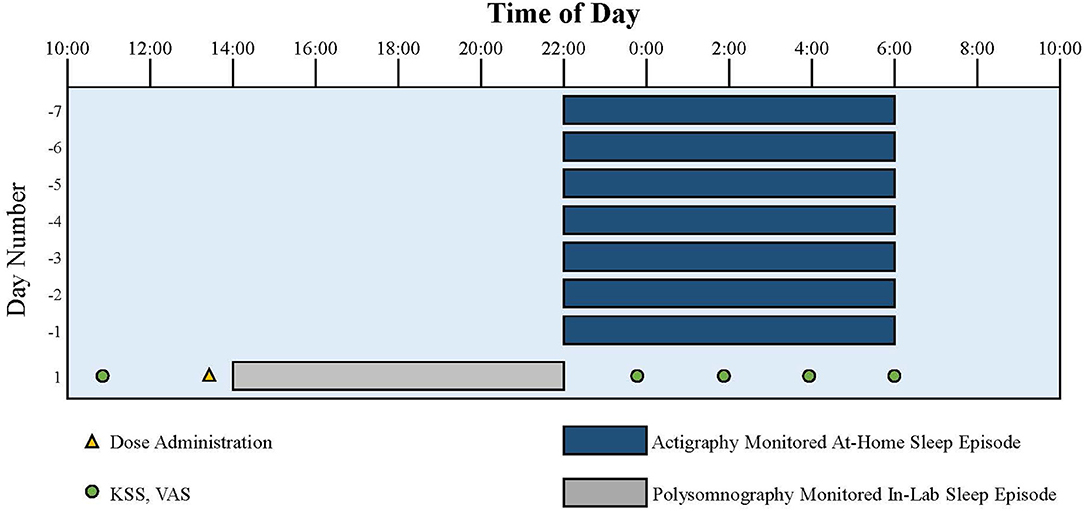
Figure 2. Representation of the study protocol for an individual with a usual target bedtime of 22:00. Dark blue bars indicate at-home sleep monitored with actigraphy and photometry. Gray bar indicates in-lab sleep monitored with polysomnography following the 8-hour phase advance imposed to model the change of sleep timing required after a trip from Los Angeles to London. Yellow triangle indicates dose administration. Green circles indicate KSS and VAS assessments.
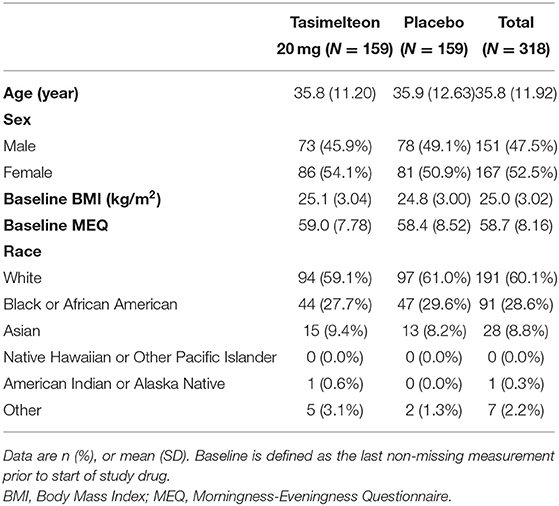
Baseline demographics were similar between tasimelteon and placebo groups. Of the 318 ITT participants, 151 (47.5%) were male. The average age was 35.8 (SD 11.92), and average BMI was 25.0 (SD 3.02). There was no significant difference in the baseline MEQ scores between the groups (Table 1). The racial breakdown was 191 (60.1%) White, 91 (28.6%) Black or African American, 28 (8.8%) Asian, 0 (0.0%) Native Hawaiian or Other Pacific Islander, 1 (0.3%) American Indian or Alaska Native, and 7 (2.2%) Other (Table 1).
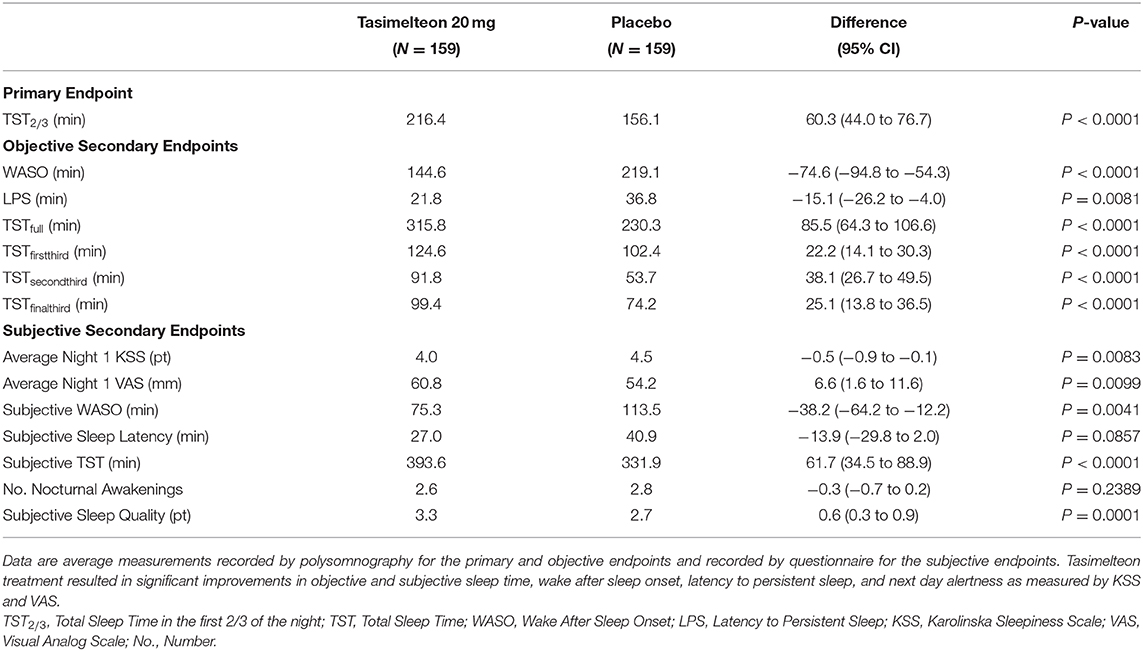
Tasimelteon treatment resulted in increased TST2/3 by 60.3 min (95% CI 44.0 to 76.7, P < 0.0001) in the first two-thirds of the night (Table 2).
Over the full 8-h sleep episode, TST increased by 85.5 min (95% CI 64.3 to 106.6, P < 0.0001) for the tasimelteon group compared to the placebo group. WASO was reduced by 74.6 min (95% CI −94.8 to −54.3, P < 0.0001) for the tasimelteon group compared to the placebo group. LPS was decreased by 15.1 min (95% CI −26.2 to −4.0, P = 0.0081) for the tasimelteon group compared to the placebo group (Table 2). Additionally, for each individual third of the night, average TST was significantly increased in the tasimelteon group compared to the placebo group. Average TST was increased for the tasimelteon group compared to the placebo group in the first third of the night (from minute 0 to 160) by 22.2 min (95% CI 14.1 to 30.3, P < 0.0001), in the second third of the night (from minute 161 to 320) by 38.1 min (95% CI 26.7 to 49.5, P < 0.0001), and in the final third of the night (from minute 321 to 480) by 25.1 min (95% CI 13.8 to 36.5, P < 0.0001) (Table 2). For each hour of the night, average TST was also significantly increased for the tasimelteon group compared to the placebo group (Figure 4).
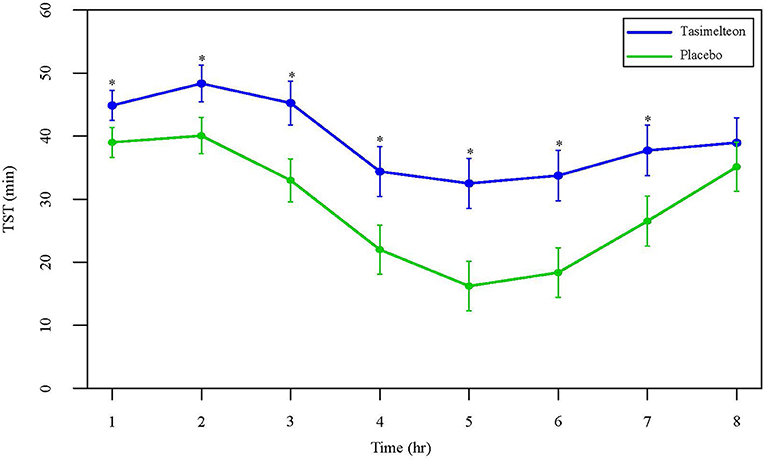
Figure 4. Data are LS Mean (95% CI) for average TST by hour between tasimelteon and placebo. TST during the 8-h phase advance demonstrated a statistically significant increase in the tasimelteon group compared to placebo for hours 1–7. *P < 0.001.
Secondary outcome measures of subjective sleep efficacy were assessed by subject reported measures including the KSS, VAS, and PSQ. Average Night 1 KSS was decreased for the tasimelteon group by −0.5 pts (95% CI −0.9 to −0.1, P = 0.0083) compared to the placebo group (Table 2). Average Night 1 VAS was increased for the tasimelteon group by 6.6 mm (95% CI 1.6 to 11.6, P = 0.0099) compared to the placebo group (Table 2). KSS and VAS administered at each time point are shown in Figure 5. Subjective measures assessed by the PSQ including subjective WASO, subjective TST, and overall sleep quality were significantly improved for the tasimelteon group. Subjective WASO was reduced by −38.2 min (95% CI −64.2 to −12.2, P = 0.0041) for the tasimelteon group compared to the placebo group. Subjective TST was increased by 61.7 min (95% CI 34.5 to 88.9, P < 0.0001) for the tasimelteon group compared to the placebo group. Overall sleep quality increased by 0.6 pts (95% CI 0.3 to 0.9, P = 0.0001) in the tasimelteon group compared to the placebo group. Trends in subjective sleep latency and the subjective number of nocturnal awakenings were also observed (Table 2).
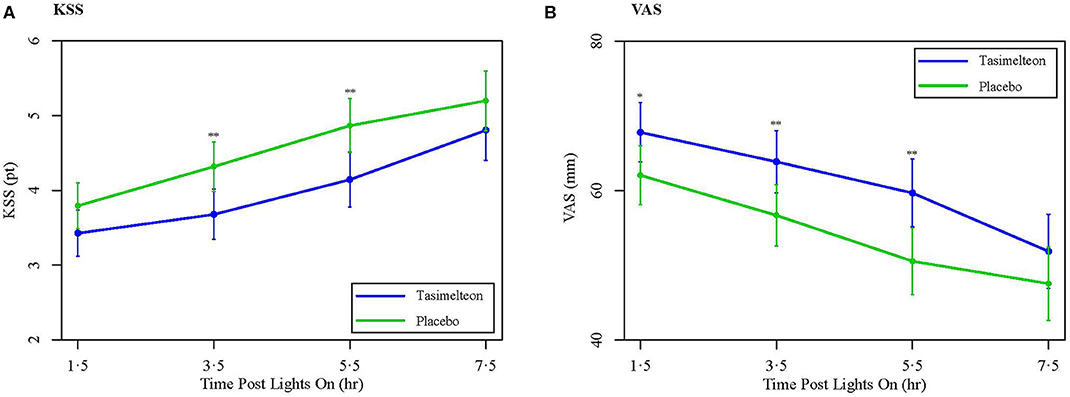
Figure 5. (A) Data are LS Mean (95% CI) for average Night 1 KSS values between tasimelteon and placebo. KSS values, 1 pt being extremely awake and 9 pts being extremely sleepy/fighting to stay awake, demonstrated a statistically significant decrease following the 8-h phase advance in the tasimelteon group compared to the placebo group for 3.5 and 5.5 h post-lights on. **P < 0.01. (B) Data are LS Mean (95% CI) for average Night 1 VAS values between tasimelteon and placebo. VAS values, 0 mm being very sleepy and 100 mm being very alert, demonstrated a statistically significant increase following the 8-h phase advance in the tasimelteon group compared to the placebo group for 1.5, 3.5, and 5.5 h post-lights on. **P < 0.01, *P < 0.05.
A pre-specified responder analysis of participants on tasimelteon and placebo showed that 11.3% of participants taking placebo achieved a sleep efficiency of ≥ 85% during the phase advance night vs. 32.7% of participants taking tasimelteon achieving this sleep efficiency (P < 0.0001).
The safety analysis included data from 320 participants (tasimelteon n = 160, placebo n = 160) who received one treatment dose after randomization. Discontinuation due to treatment emergent adverse events was insignificant between treatment groups. The most common treatment emergent adverse event reported in the JET8 study was headache (8[5%] tasimelteon vs. 4[2.5%] placebo). One serious adverse event was reported but was determined by the Principal Investigator to be unrelated to study drug.
Discussion
Treatment with 20 mg tasimelteon achieved clinically meaningful improvements in objective and subjective measures of sleep duration and sleep quality for participants exposed to an 8-h phase advance, in a model of the phase shift induced when travelers fly eastward across eight time zones. The effects of tasimelteon were observed on the first night of treatment. Participants treated with tasimelteon not only fell asleep an average of 15.1 min faster, but also slept 85.5 min longer than placebo during the 8-h sleep episode scheduled at a time equivalent to the circadian challenge induced by a flight from Los Angeles to London. Participants taking tasimelteon reported lower sleepiness scores and higher alertness scores the day after the phase advance, compared to placebo. Participants taking tasimelteon, self-aware of the improvement, also reported that they fell asleep faster, spent less time awake during the night, slept longer, and rated their overall sleep quality higher as compared to individuals taking placebo.
Tasimelteon treatment also resulted in increased alertness and decreased sleepiness the day following the phase advance, as measured by the VAS and the KSS, which has been validated against objective EEG measures of sleepiness and objective measures of neurobehavioral performance (22). These improvements in next-day functioning are clinically important given the safety consequences of reduced alertness and sleepiness among individuals experiencing JLD resulting in occupational hazards and motor vehicle accidents. The symptoms of JLD can be severe in up to a quarter of patients. Further, the fatigue and cognitive effects can result in significant errors when performance is critical, such as when operating machinery or being a member of the flight crew. Although it is well-known that sleep deprivation causes significant morbidity and mortality annually as a result of performance impairment, fatigue during times of eastward JLD itself has been implicated as the causative agent of several airplane accidents (14, 23, 24).
There is currently no treatment for JLD approved by the US Food and Drug Administration (FDA) or the European Medicines Agency (EMA). The American Academy of Sleep Medicine (AASM) recommends the use of light therapy, slowly changing sleep-wake schedule timing in advance of a trip, and the nutritional supplement melatonin, although an appropriate dosage form has not been determined (25). Caffeine may counteract jet lag-induced sleepiness but may also disrupt night-time sleep (25). The AASM acknowledges the use of hypnotic sleeping pills as a rational treatment for JLD; however, they mention hypnotics are not necessary, have risks of adverse effects like global amnesia, and should be used on a short-term basis (25). Travelers who follow even the above recommendations continue to report symptoms of JLD.
It is estimated that about two-thirds of people who complete eastward, transmeridian travel across three or more time zones experience at least moderate JLD, and almost one-third experience severe symptoms of JLD (24, 26). In 2018, 93 million US citizens traveled internationally. Outbound overseas travel from the US totaled 41.8 million travelers (27). Of the millions of travelers who cross time zones each year, 80% report disrupted sleep during the scheduled sleep episode in the new time zone (28).
Although previous studies demonstrated that treatment with oral melatonin after a phase advance may reduce sleep onset latency and improve sleep quality (19, 20), increasingly studies are focusing on synthetic Dual Melatonin Receptor Agonists, as they may have a more stable pharmacokinetic profile, may be more potent, and may produce a greater effect than melatonin (5, 29). Prior studies with tasimelteon, which has selective agonist activity at the MT1 and MT2 receptors (15, 30), have demonstrated that when taken on a fixed time schedule, can entrain the circadian clock of blind individuals with Non-24 to the 24-h day (18). The central circadian pacemaker regulates the circadian rhythms of hormones, many aspects of physiology, metabolism, and behavior, including melatonin and cortisol, and synchronizes them with the 24-h day (6, 7). For this study, we hypothesized that tasimelteon would provide an alternative 24-h time cue necessary to resynchronize the CTS to the newly imposed 24-h day.
Tasimelteon has been shown to have different affinities for the MT1 and MT2 receptors, with a 2 to 4-fold higher affinity for the MT2 receptor than the MT1 receptor, and a 60% higher affinity for the MT2 receptor than melatonin itself, but about equal affinity to melatonin to the MT1 receptor (16). This is notable since the MT2 receptor is responsible for mediating circadian phase shifting (16). The differential affinity between tasimelteon and melatonin at the MT2 receptor may in part account for both the failure of melatonin to show benefit in a large JLD study and the success of tasimelteon in this model for JLD (26).
The robust results observed in this study confirm that participants who took tasimelteon had longer and better quality sleep. Lastly, we found that the morning following tasimelteon dosing, participants experienced fewer symptoms of JLD, as they reported feeling both more alert and less sleepy.
We believe that the underlying mechanism involves tasimelteon replacing the light-dark cycle as the time cue that synchronizes the CTS to the 24-h day. If the CTS of an individual with JLD can be re-synchronized to the new 24-h light-dark cycle, we predict that the master body clock will be able to regulate the secretion of hormones like melatonin and cortisol in phase with the new time zone, thus allowing an individual to feel alert during the day and sleepy at night.
While there are inter-individual differences in susceptibility to JLD, responder analysis demonstrates that only 11% of participants achieved a high (≥85%) sleep efficiency when treated with placebo during an 8-h sleep phase advance, whereas a third (32.7%) of participants reach that high sleep efficiency when treated with tasimelteon following the 8-h sleep phase advance (P < 0.0001). Moreover, inter-individual differences in chronotype cannot account for this robust effect of tasimelteon, as the average MEQ score of the participants in the group treated with placebo was comparable to that of participants in the group treated with tasimelteon (Table 1).
In this study, tasimelteon was safe and well-tolerated. This clinical trial demonstrates that tasimelteon is effective for treating the symptoms of JLD following a shift in the timing of the sleep-wake cycle, comparable to that required following eastward travel across eight time zones.
Current therapies available for the treatment of JLD have not been validated and do not fundamentally address the underlying circadian dysfunction. These include sedative hypnotics such as zolpidem and eszopiclone, and wake promoting agents such as modafinil. In the largest (N = 257) double-blind trial to evaluate the efficacy of melatonin for the treatment of JLD, 0.5 and 5 mg of melatonin were reported to be ineffective (26). Similarly, treatment of healthy participants with 1, 4, or 8 mg of ramelteon failed to improve sleep (31). Moreover, 26% of over-the-counter formulations of melatonin, which is weakly regulated by the FDA as a food supplement and not as a drug, have been reported to be contaminated with serotonin, putting users at risk for serotonin syndrome (32).
Potential limitations of the study include that Jet Lag was induced by an immediate phase advance of the sleep-wake cycle in a sleep clinic, rather than jet travel in the eastward direction. However, the model in the JET8 study helps eliminate confounders unrelated to JLD, which is fundamentally a circadian dysfunction unrelated to the actual travel in a jet plane. Further, this potential limitation was tested in another study with tasimelteon, the JET study, wherein participants flew from the US to Europe. The JET study met its primary endpoint (source: in publication), demonstrating that tasimelteon is effective to treat JLD in a model that uses transatlantic flights. Additionally, JET8 participants were studied and dosed for one night, as opposed to multiple night dosing. However, JLD may affect individuals for only one night, depending on the degree of phase advance, the length of the trip, and the speed at which the individual adapts to the new time zone. Finally, the JET8 study protocol results in a “first night effect,” in which a new sleeping environment induces insomnia. One potential limitation therefore is that a sleep study in a clinic may be at least partially causing first night effect-induced insomnia in addition to phase advance insomnia. However, given that following transmeridian travel many individuals will be sleeping in an unfamiliar environment such as a hotel, the present study also successfully simulates that aspect as well. Further, comparison of the differences in results between this study, which employed an 8-h phase advance, and the JET5 study, which employed a 5-h phase advance, demonstrates that a difference in the number of time zones advanced results in significant differences in JLD symptoms with and without treatment (15).
In summary, the results of the JET8 study demonstrate effectiveness of tasimelteon in treating the symptoms of JLD as shown in this model for the disorder. The magnitude of the total benefit over placebo is significant and clinically meaningful. The results of the study strongly suggest that tasimelteon may be an effective therapeutic tool in the treatment of individuals with JLD.
Data Availability Statement
All datasets presented in this study are included in the article/supplementary material.
Ethics Statement
The studies involving human participants were reviewed and approved by the Institutional Review Boards of both Chesapeake and BioMed. The patients/participants provided their written informed consent to participate in this study. This clinical trial is registered at: HTTP://clinicaltrials.gov/, under TRN: NCT0337320.
Author Contributions
CP, GB, CX, and MP contributed to the study concept and design. JW, CX, CP, and MP developed the statistical analysis plan. CP wrote the report in collaboration with MK, JB, VP, LP, and MM. CP revised the report with participation from all authors. All authors reviewed and approved the report before submission.
Funding
The funder of the study designed the study, performed data analysis, data interpretation, and writing of the report. The corresponding authors had full access to all of the data in the study and had final responsibility for the decision to submit for publication.
Conflict of Interest
CP, MK, JB, MM, VP, LP, GB, JW, CX, and MP are employees of Vanda Pharmaceuticals. The clinical trial was sponsored by Vanda Pharmaceuticals.
Acknowledgments
We thank the volunteers, without whom the project would not be conducted. We thank the investigators and staff of the study sites. We thank Charles A. Czeisler for his strategic advice, and colleagues at Vanda Pharmaceuticals for their trial support.
References
1. American Academy of Sleep Medicine (AASM). International Classification of Sleep Disorders – Third Edition (ICSD-3). 3rd ed. Darien, IL: American Academy of Sleep Medicine (2014).
2. Weingarten JA, Collop NA. Air travel: effects of sleep deprivation and jet lag. Chest. (2013) 144:1394–401. doi: 10.1378/chest.12-2963
3. Auger RR, Burgess HJ, Emens JS, Deriy LV, Thomas SM, Sharkey KM. Clinical practice guideline for the treatment of intrinsic circadian rhythm sleep-wake disorders: Advanced SleepWake Phase Disorder (ASWPD), Delayed Sleep-Wake Phase Disorder (DSWPD), Non-24-Hour Sleep-Wake Rhythm Disorder (N24SWD), and irregular sleep-wa. J Clin Sleep Med. (2015) 11:1199–236. doi: 10.5664/jcsm.5100
4. Lockley SW, Arendt J, Skene DJ. Visual impairment and circadian rhythm disorders. Neurosci Biobehav Psychol. (2007) 9:428–37. doi: 10.1016/B978-0-12-809324-5.01285-2
5. Waterhouse J, Reilly T, Atkinson G, Edwards B. Jet lag: trends and coping strategies. Lancet. (2007) 369:1117–29. doi: 10.1016/S0140-6736(07)60529-7
6. Czeisler CA, Klerman EB. Circadian and sleep-dependent regulation of hormone release in humans. Recent ProgHormRes. (1999) 54:97–132.
7. Czeisler CA, Khalsa SBS. The Human Circadian Timing System and Sleep-Wake Regulation. In: Kryger MH, Roth T, Dement WC, editors. Principles and Practice of Sleep Medicine. Philadelphia, PA: W.B. Saunders Company (2000). p. 353–75.
8. Berson DM, Dunn FA, Takao M. Phototransduction by retinal ganglion cells that set the circadian clock. Science. (2002) 295:1070–3. doi: 10.1126/science.1067262
9. Scheer FAJL, Hilton MF, Mantzoros CS, Shea SA. Adverse metabolic and cardiovascular consequences of circadian misalignment. Proc Natl Acad SciUSA. (2009) 106:4453–8. doi: 10.1073/pnas.0808180106
10. Reutrakul S, Hood MM, Crowley SJ, Morgan MK, Teodori M, Knutson KL, et al. Chronotype is independently associated with glycemic control in type 2 diabetes. Diabetes Care. (2013) 36:2523–9. doi: 10.2337/dc12-2697
11. Nguyen KD, Fentress S, Qiu Y, Yun K, Cox JS, Chawla A. Circadian gene bmal1 regulates diurnal oscillations of ly6chi inflammatory monocytes. Science. (2013) 341:1483–8. doi: 10.1126/science.1240636
12. Arendt J. Approaches to the pharmacological management of jet lag. Drugs. (2018) 2018:1419–31. doi: 10.1007/s40265-018-0973-8
13. Shekleton JA, Rajaratnam SM, Gooley JJ, Van Reen E, Czeisler CA, Lockley SW. Improved neurobehavioral performance during the wake maintenance zone. J Clin Sleep Med. (2013) 9:353–62. doi: 10.5664/jcsm.2588
14. Waterhouse J, Reilly T, Atkinson G. Jet-lag. Lancet. (1997) 350:1611–6. doi: 10.1016/S0140-6736(97)07569-7
15. Rajaratnam SM, Polymeropoulos MH, Fisher DM, Roth T, Scott C, Birznieks G, et al. Melatonin agonist tasimelteon (VEC-162) for transient insomnia after sleep-time shift: two randomised controlled multicentre trials. Lancet. (2009) 373:482–91. doi: 10.1016/S0140-6736(08)61812-7
16. Liu C, Weaver DR, Jin X, Shearman LP, Pieschl RL, Gribkoff VK, Reppert SM. Molecular dissection of two distinct actions of melatonin on the suprachiasmatic circadian clock. Neuron. (1997) 19:91–102. doi: 10.1016/s0896-6273(00)80350-5
17. Van Den Heuvel CJ, Kennaway DJ, Dawson D. Thermoregulatory and soporific effects of very low dose melatonin injection. Am J Physiol. (1999) 276(2 Pt 1):E249–54. doi: 10.1152/ajpendo.1999.276.2.E249
18. Lockley SW, Dressman MA, Licamele L, Xiao C, Fisher DM, Flynn-Evans EE, et al. Tasimelteon for non-24-hour sleep-wake disorder in totally blind people (SET and RESET): two multicentre, randomised, double-masked, placebo-controlled phase 3 trials. Lancet. (2015) 386:1754–64. doi: 10.1016/S0140-6736(15)60031-9
19. Arendt J, Skene D. Melatonin as a chronobiotic. Sleep Med Rev. (2005) 9:25–39. doi: 10.1016/j.smrv.2004.05.002
20. Beaumont M, Batejat D, Pierard C, Van Beers P, Denis J, Coste O, et al. Caffeine or melatonin effects on sleep and sleepiness after rapid eastward transmeridian travel. J Appl Physiol. (2004) 96:50–8. doi: 10.1152/japplphysiol.00940.2002
21. Lewy AJ, Bauer VK, Ahmed S, Thomas KH, Cutler NL, Singer CM, et al. The human phase response curve (PRC) to melatonin is about 12 hours out of phase with the PRC to light. ChronobiolInt. (1998) 15:71–83. doi: 10.3109/07420529808998671
22. Kaida K, Takahashi M, Åkerstedt T, Nakata A, Otsuka Y, Haratani T, Fukasawa K. Validation of the Karolinska sleepiness scale against performance and EEG variables. Clin Neurophysiol. (2006) 117:1574–81. doi: 10.1016/j.clinph.2006.03.011
23. Samel A. Wegmann HM. Vejvoda M. Jet lag and sleepiness in aircrew. J Sleep Res. (1995) 4:30–6. doi: 10.1111/j.1365-2869.1995.tb00223.x
24. Becker T, Penzel T, Fietze I. A new German Charite Jet Lag Scale for jet lag symptoms and application. Ergonomics. (2015) 58:811–21. doi: 10.1080/00140139.2014.982209
25. Morgenthaler TI, Lee-Chiong T, Alessi C, Friedman L, Aurora RN, Boehlecke B, et al. Practice parameters for the clinical evaluation and treatment of circadian rhythm sleep disorders. An American Academy of Sleep Medicine report. Sleep. (2007) 30:1445–59. doi: 10.1093/sleep/30.11.1445
26. Spitzer RL, Terman M, Williams JBW, Terman JS, Malt UF, Singer F, et al. Jet lag: Clinical features, validation of a new syndrome-specific scale, and lack of response to melatonin in a randomized, double-blind trial. Am J Psychiatry. (1999) 156:1392–6. doi: 10.1176/ajp.156.9.1392
27. U.S.Department of Commerce Office of National Travel and Tourism ITA. TI News: An Information Service From the National Travel & Tourism Office (NTTO) [Internet]. (2019). Available online at: https://travel.trade.gov/tinews/archive/tinews2019/20190402.asp (accessed April 5, 2019).
28. Travnickova-Bendova Z, Cermakian N, Reppert SM, Sassone-Corsi P. Bimodal regulation of mPeriod promoters by CREB-dependent signaling and CLOCK/BMAL1 activity. ProcNatlAcadSci USA. (2002) 99:7728–33. doi: 10.1073/pnas.102075599
29. Srinivasan V, Singh J, Pandi-Perumal SR, Brown GM, Spence DW, Cardinali DP. Jet lag, circadian rhythm sleep disturbances, and depression: the role of melatonin and its analogs. AdvTher. (2010) 27:796–813. doi: 10.1007/s12325-010-0065-y
30. Lavedan C, Forsberg M, Gentile AJ. Tasimelteon: A selective and unique receptor binding profile. Neuropharmacology. (2015) 91:142–7. doi: 10.1016/j.neuropharm.2014.12.004
31. Zee PC, Wang-weigand S, Wright KP, Peng X, Roth T. Effects of ramelteon on insomnia symptoms induced by rapid, eastward travel. Sleep Med. (2010) 11:525–33. doi: 10.1016/j.sleep.2010.03.010
Keywords: jet lag disorder, circadian rhythm, tasimelteon, sleep, circadian, Hetlioz, jet lag
Citation: Polymeropoulos CM, Mohrman MA, Keefe MS, Brzezynski JL, Wang J, Prokosch LS, Polymeropoulos VM, Xiao C, Birznieks G and Polymeropoulos MH (2020) Efficacy of Tasimelteon (HETLIOZ®) in the Treatment of Jet Lag Disorder Evaluated in an 8-h Phase Advance Model; a Multicenter, Randomized, Double-Blind, Placebo-Controlled Trial. Front. Neurol. 11:611. doi: 10.3389/fneur.2020.00611
Received: 04 April 2020; Accepted: 25 May 2020;
Published: 09 July 2020.
Edited by:
Ahmed S. BaHammam, King Saud University, Saudi ArabiaReviewed by:
Yoshiaki Yamaguchi, Kyoto University, JapanIngo Fietze, Charité – Universitätsmedizin Berlin, Germany
Copyright © 2020 Polymeropoulos, Mohrman, Keefe, Brzezynski, Wang, Prokosch, Polymeropoulos, Xiao, Birznieks and Polymeropoulos. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Christos M. Polymeropoulos, Y2hyaXN0b3MucG9seW1lcm9wb3Vsb3NAdmFuZGFwaGFybWEuY29t
 Christos M. Polymeropoulos
Christos M. Polymeropoulos Michael A. Mohrman
Michael A. Mohrman Madison S. Keefe
Madison S. Keefe Jennifer L. Brzezynski
Jennifer L. Brzezynski Jingyuan Wang
Jingyuan Wang Lydia S. Prokosch
Lydia S. Prokosch Vasilios M. Polymeropoulos
Vasilios M. Polymeropoulos Changfu Xiao
Changfu Xiao Mihael H. Polymeropoulos
Mihael H. Polymeropoulos