- 1Department of Radiology, The First Affiliated Hospital of Jinan University, Guangzhou, China
- 2Department of Radiology, Affiliated Hospital of Xiangnan University, Chenzhou, China
- 3Department of Pediatrics, Affiliated Hospital of Xiangnan University, Chenzhou, China
Background: Many studies aimed to evaluate the efficacy and safety of treosulfan-based conditioning regimens for allogeneic hematopoietic cell transplantation (allo-HCT) compared with other regimens, but different outcomes were reported across studies.
Aim: To determine the long-term survival outcomes of treosulfan-based vs. busulfan-based conditioning regimens in myelodysplastic syndrome (MDS)/acute myeloid leukemia (AML) patients.
Methods: PubMed, Embase, and Cochrane library were searched for studies published prior to December 6, 2019. The fixed-effects model was applied for overall survival (OS), leukemia-free survival (LFS), non-relapse mortality (NRM), acute and chronic graft versus host disease (GvHD). Relapse incidence (RI) was pooled by the use of the random-effects model.
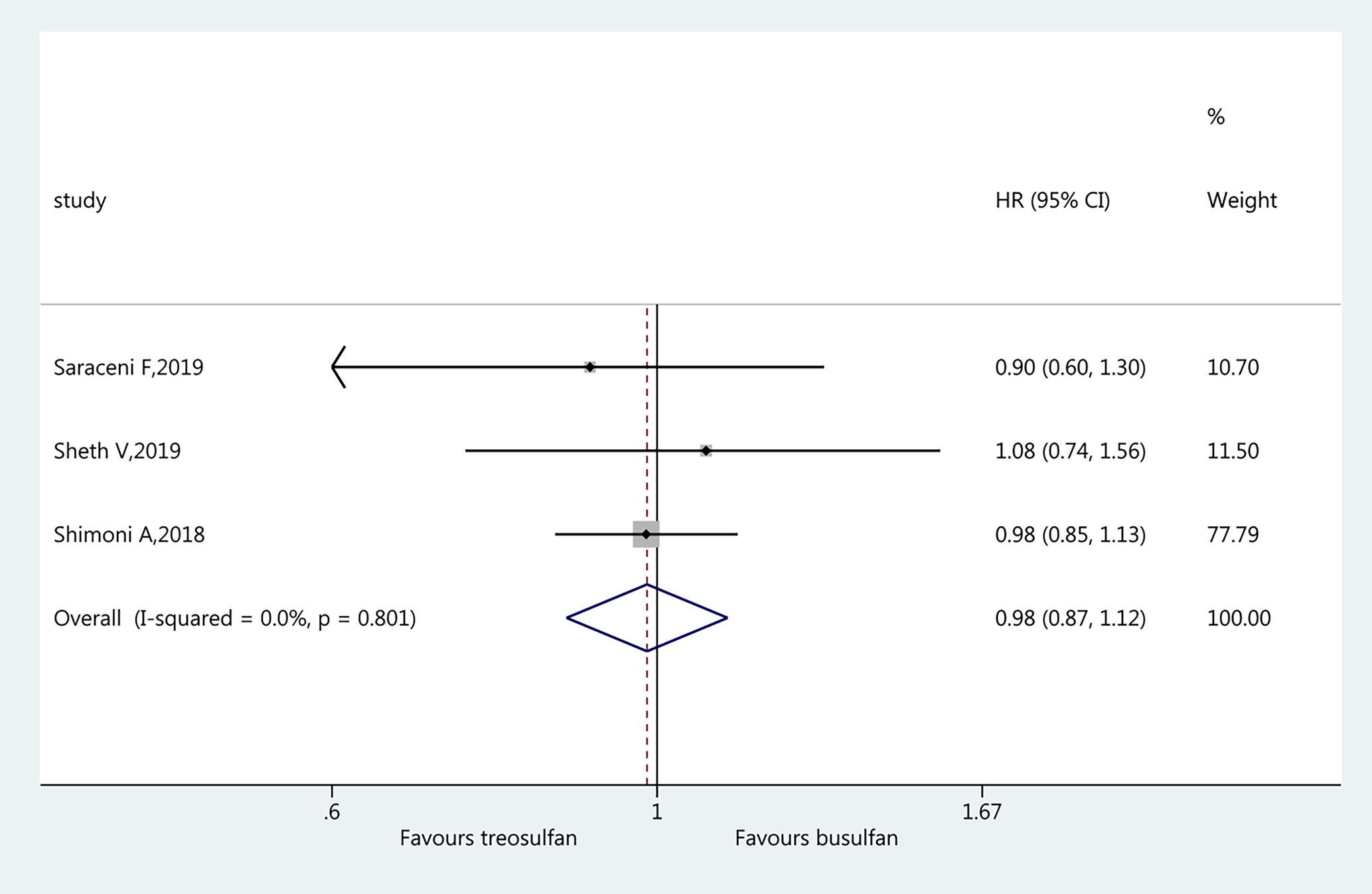
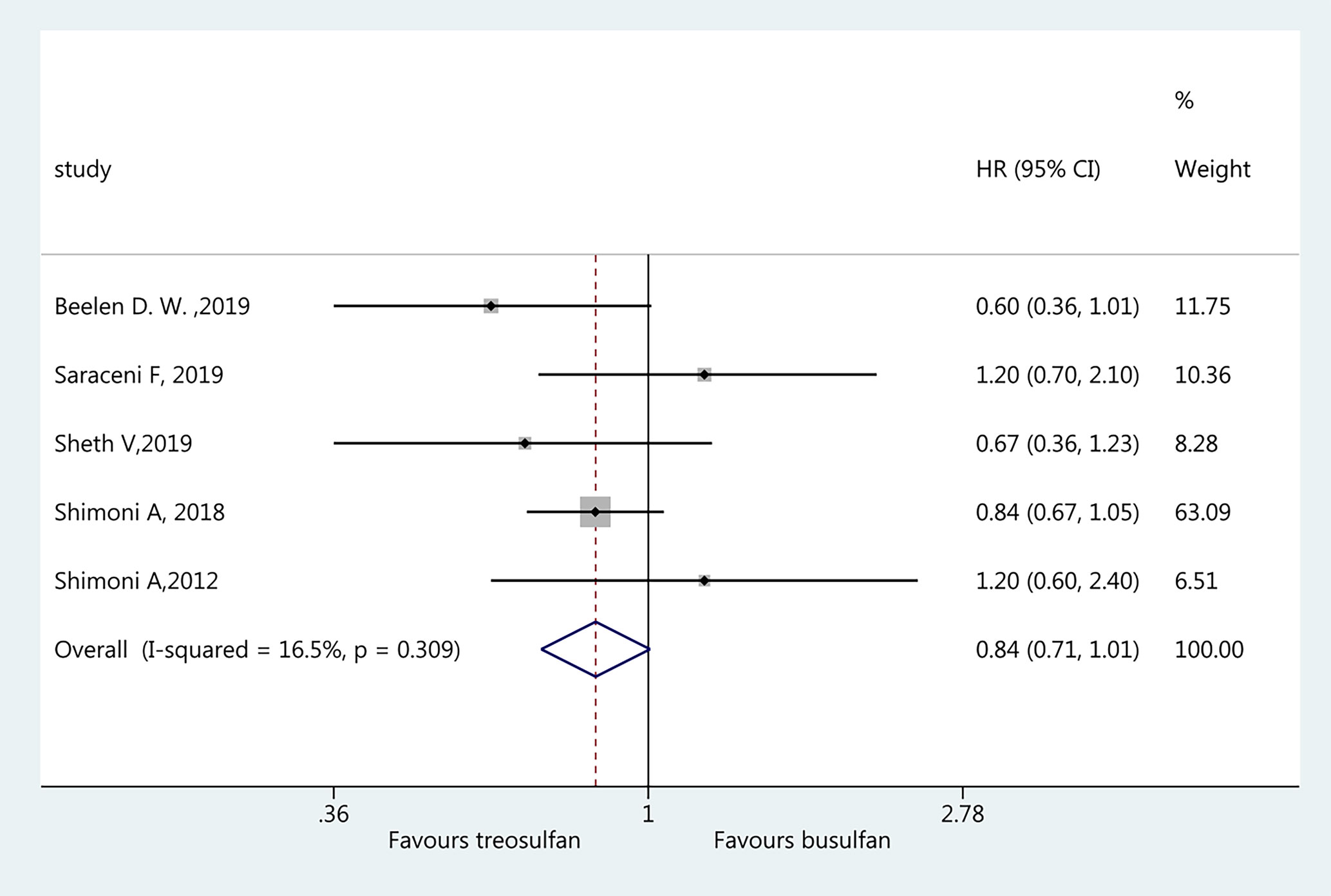
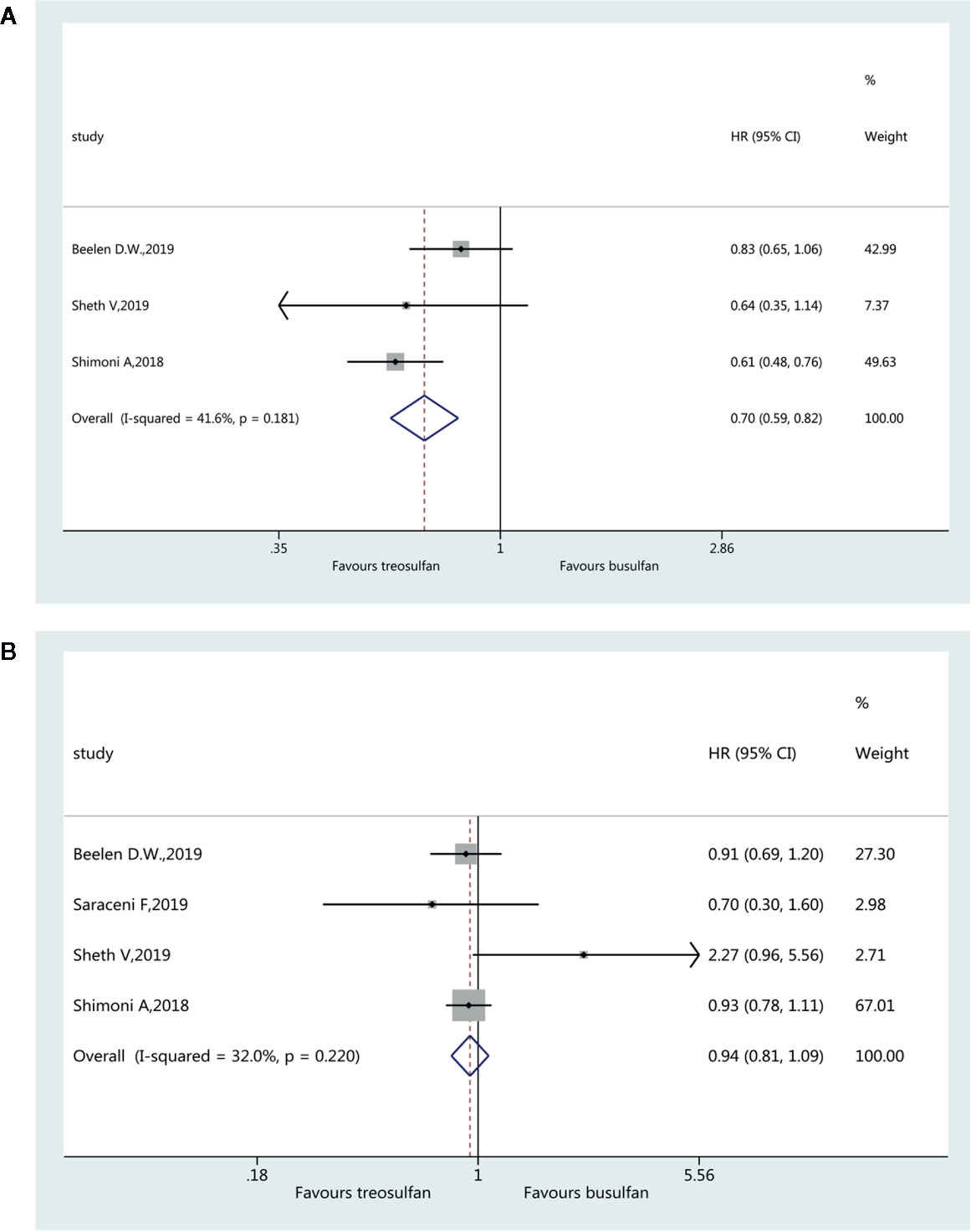
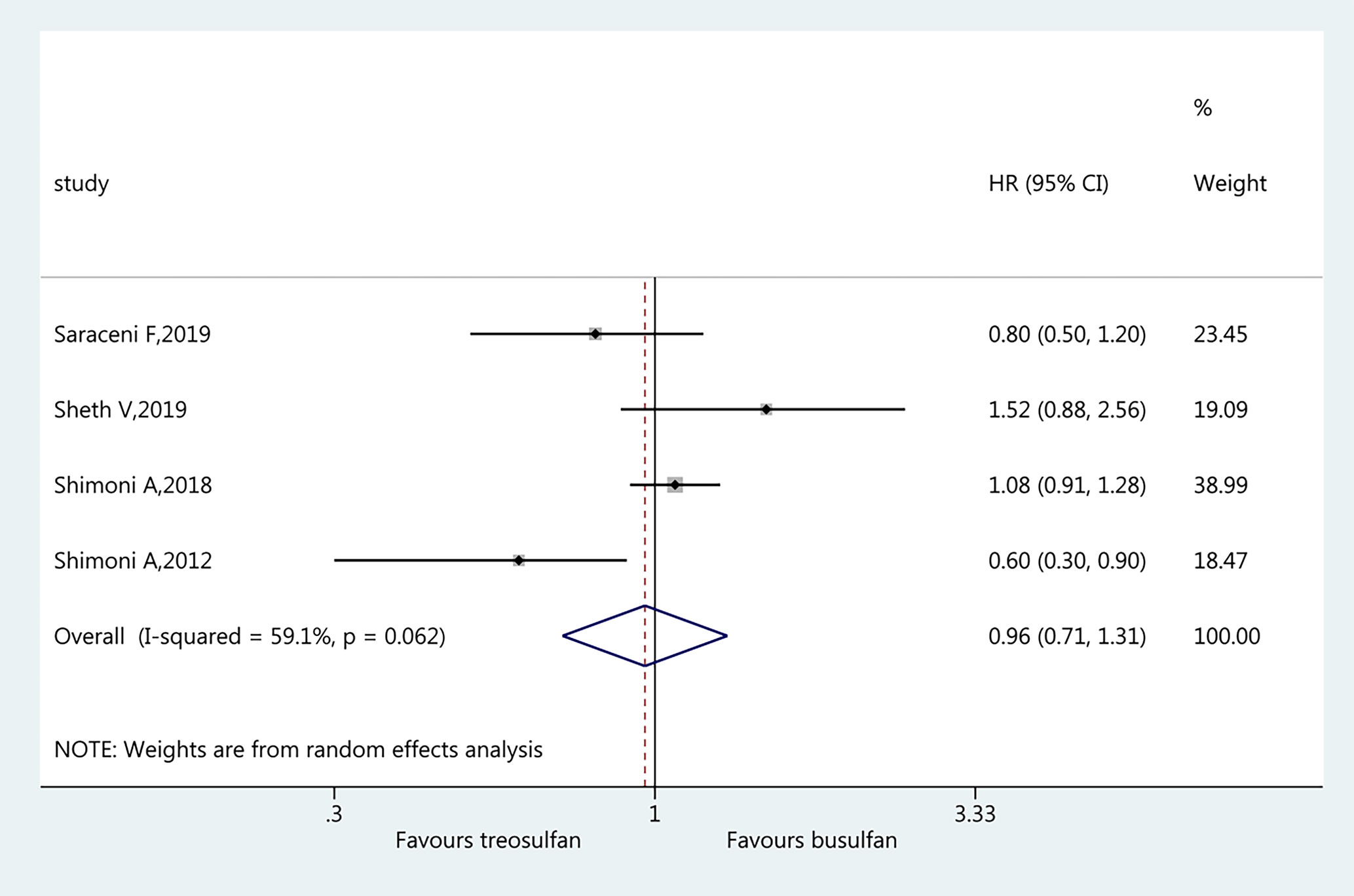
Results: Six studies were included (3,982 patients; range, 57–1,956). The pooled HR for OS favored treosulfan (HR=0.80, 95%CI: 0.71–0.90). There was no significant difference in NRM between the two regimens (HR=0.84, 95%CI=0.71–1.01). There was no significant difference in LFS between the two regimens (HR=0.98, 95%CI=0.87–1.12). Treosulfan-based regimens showed a lower risk of aGvHD (HR=0.70, 95%CI=0.59–0.82), but there was no difference for cGvHD (HR=0.94, 95%CI=0.81–1.09). There was no significant difference in RI between the two regimens (HR=0.96, 95%CI=0.71–1.31). There was no publication bias among these studies.
Conclusion: The current meta-analysis determined that treosulfan-based conditioning regimens could improve the OS in patients with MDS and AML, with lower acute graft-versus-host disease incidence, compared with busulfan-based regimens.
ntroduction
Myelodysplastic syndrome (MDS) is a heterogeneous group of clonal disorders characterized by ineffective hematopoiesis leading to peripheral blood cytopenias and increased risk of transformation to acute myeloid leukemia (1–3). The incidence of MDS in the USA is 4.5 per 100,000 people/year in men and 2.7 per 100,000 people/year in women (4). Acute myeloid leukemia (AML) is a collection of heterogeneous hematopoietic stem cell disorders characterized by incomplete maturation of blood cells and reduced production of normal hematopoietic elements (5, 6). There are an estimated 18,860 new cases of AML and 10,460 deaths from AML in the United States each year (7).
Allogeneic hematopoietic cell transplantation (HCT) offers curative therapy for many patients with MDS or AML. Allo-HSCT is recommend for MDS of intermediate-2- and high-risk as soon as possible, according to The National Comprehensive Cancer Network (NCCN) guidelines (1, 6). Nevertheless, relapse and graft versus host disease (GvHD) are the two main causes of treatment failure (1, 6). Thus the choice of the conditioning regimen is crucial.
Conditioning regimens play important roles in the success of allo-HSCT, and the choice of a regiment depends upon age, disease risk, ECOG status, and remission status at the time of transplantation (8, 9). Conventional myeloablative conditioning regimens (MAC) containing busulfan are among the most widely used for MDS and AML, but they have shortcomings such as toxicity, veno-occlusive diseases, and mortality (8–10). Treosulfan is a recent myelotoxic and immunosuppressive prodrug that does not require enzymatic activation (11, 12). Treosfulfan has strong activity against AML cells (13–15) and has strong immunosuppressive effects and low release of inflammatory cytokines (16). Those characteristics favor the engraftment of the transplanted cells and limit the risk of GvHD (16). Treosulfan-based regimens are considered as effective as conventional MAC, but with lower toxicity and transplant-related mortality (8, 9).
Many studies aimed to evaluate the efficacy and safety of treosulfan-based conditioning regimens compared with other regimens, but different outcomes were reported across studies. No meta-analysis has been published yet as far as we know. Therefore, the purpose of this meta-analysis was to determine the long-term survival outcomes of treosulfan-based conditioning regimens in MDS/AML patients.
Methods
Literature Search
This meta-analysis was performed in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) reporting guidelines. Since no original clinical raw data was collected or utilized, ethical approval was not requested for this meta-analysis.
The study eligibility criteria were: 1) patients: AML/MDS patients older than 18 years (either mentioned in patient eligibility or the minimum age in baseline characteristics was greater than 18); 2) interventions: treosulfan as conditioning regimen before hematopoietic cell transplantation; 3) comparison: busulfan or other conditioning regimens; 4) study types: randomized controlled trials (RCTs), and prospective or retrospective observational cohort studies; and 5) full text published in English. Three recognized electronic databases, PubMed, Embase, and Cochrane library, were searched for studies published prior to December 6, 2019, using the MeSH terms of “Myelodysplastic Syndrome”, “Acute Myeloid Leukemia”, and “treosulfan” combined with relevant key words were used.
Data Extraction and Quality Assessment
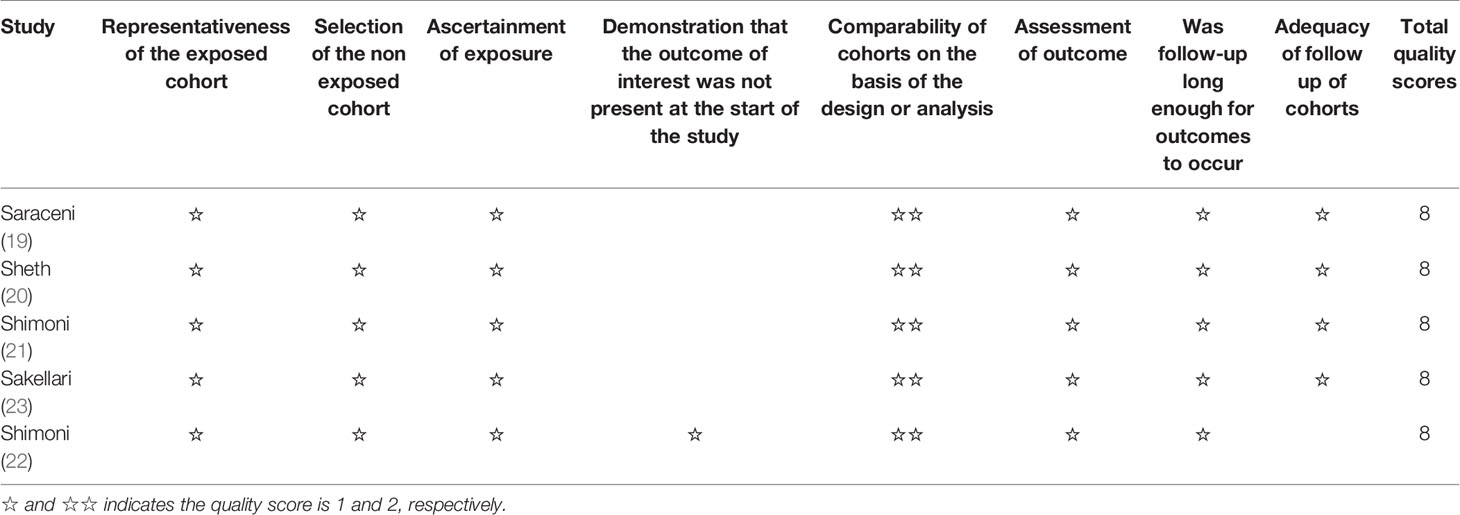
Potentially relevant publications were screened and evaluated by two reviewers double-blindly, with a third reviewer resolving any disagreement. A structured data collection sheet was developed. Two researchers independently extracted the data, including authors, year of publication, country, study design, sample size, age, percentage of males, disease and transplantation characteristics, conditioning regimens characteristics, overall survival (OS), leukemia-free survival (LFS), non-relapse mortality (NRM), acute GvHD (aGvHD), chronic GvHD (cGvHD), and relapse incidence (RI). RCTs were evaluated according to the Cochrane risk bias tool (4). Observational studies were evaluated according to the Newcastle-Ottawa scale (NOS) (17). The NOS assigns a maximum of 9 points for the selection of the control group (4 points), group comparability (two points), and exposures and outcomes (three points).
Statistical Analysis
All analyses were performed using the STATA MP 14.0 software (StataCorp, College Station, Texas, USA). Hazard ratios (HRs) with 95% confidence intervals (CIs) were collected and combined for statistical analysis. Statistical heterogeneity among studies was calculated using the Cochran’s Q test and I2 index, P<0.10 in the Cochran’s Q test and an I2 index >50% indicated high heterogeneity. The random-effects model was used when high heterogeneity was present among studies; otherwise, the fixed-effects model was applied. P-values <0.05 were considered statistically different. Potential publication bias was assessed by funnel plots, Egger’s test, and Begg test.
Results
Study Selection
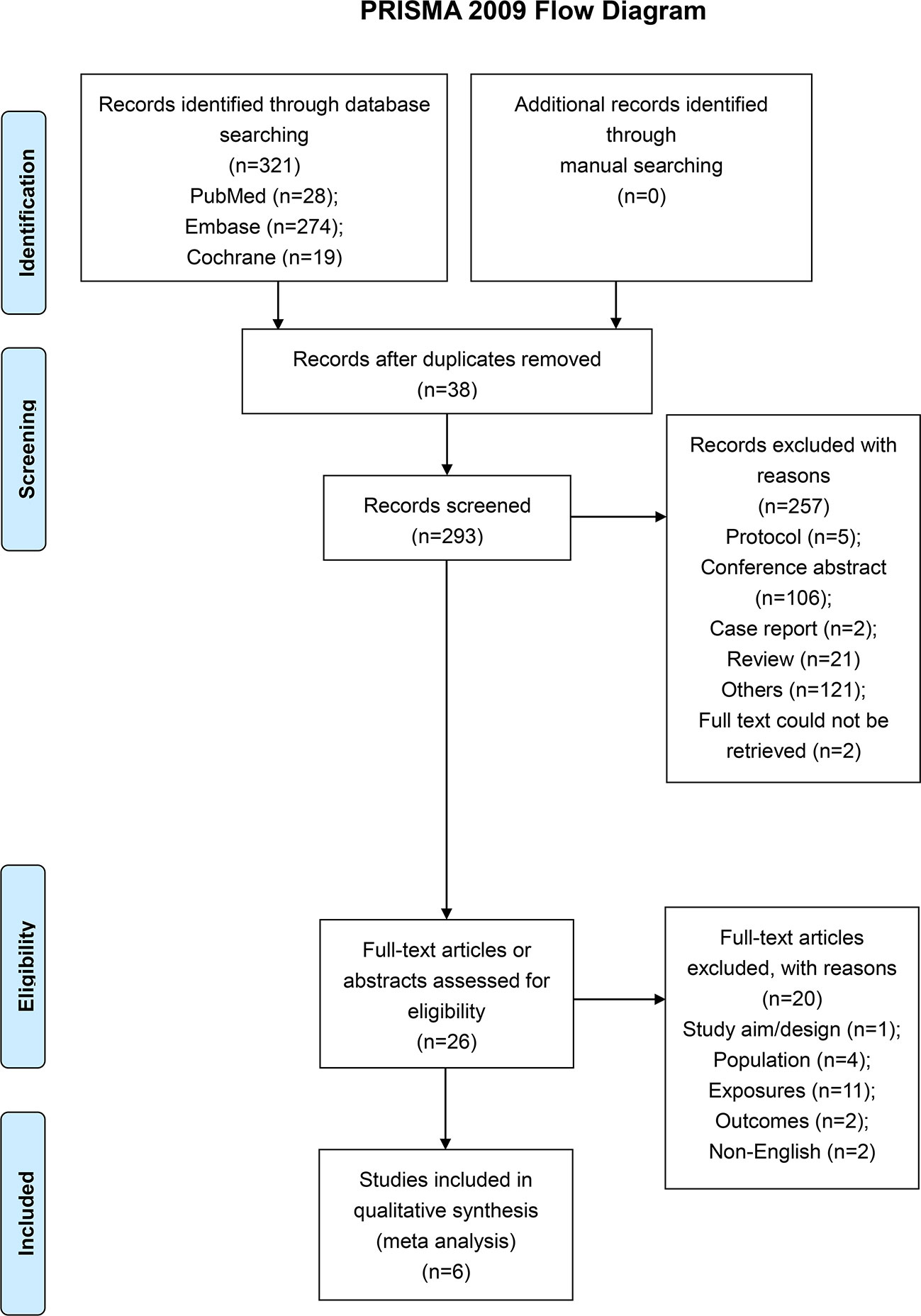
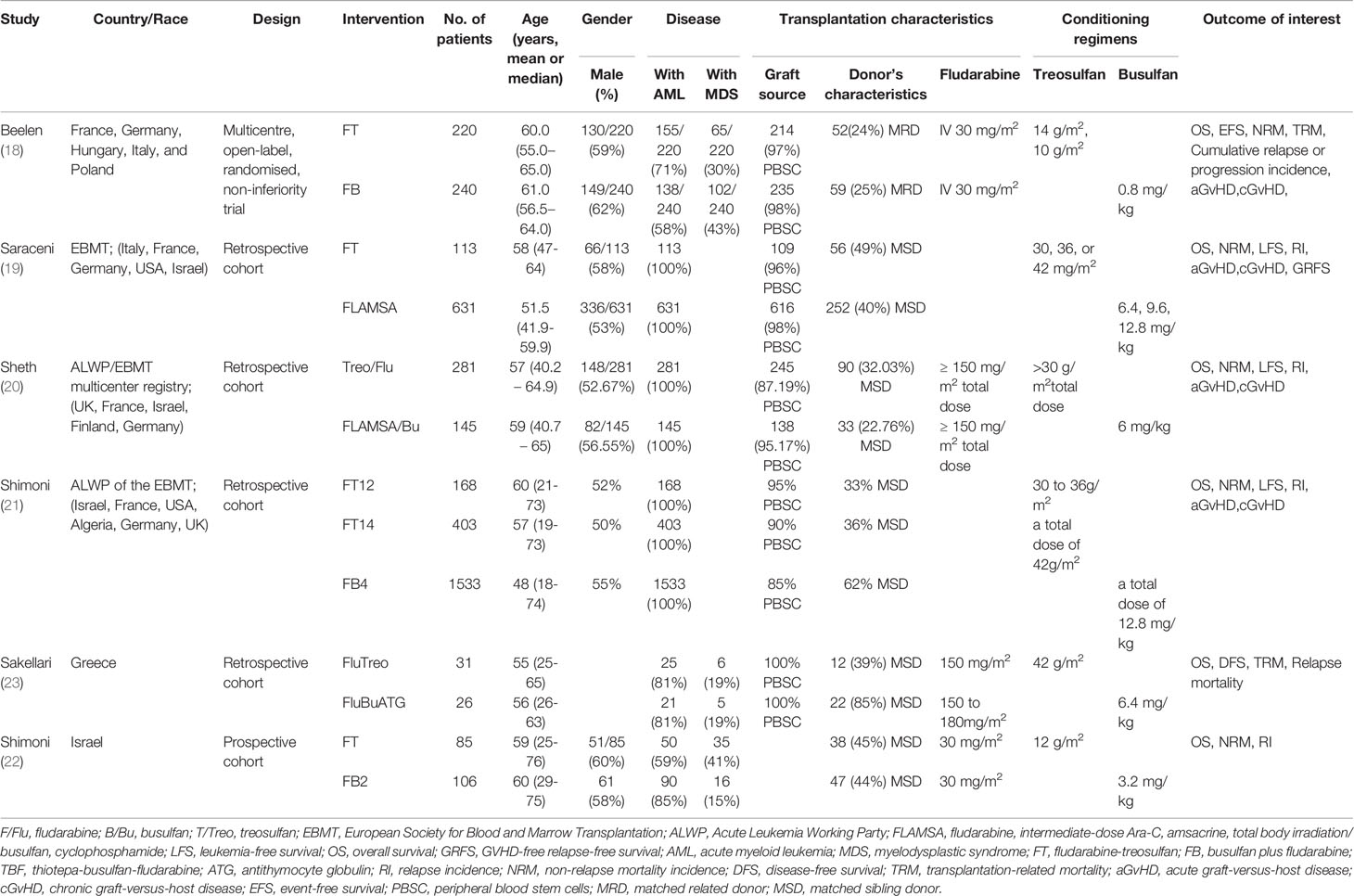
A total of 321 articles were first identified, 38 duplicates were removed, and 293 papers were screened. Twenty-six full-text were assessed for eligibility. Twenty studies were excluded because of study aim/design (n=1), population (n=4), exposures (n=11), outcomes (n=2), and non-English (n=2) (Figure 1 and Supplementary Table 1). Finally, six papers were included (Figure 1). Those six studies (18–23) included 3,982 patients (range, 57-1956) (Table 1). There were five observational studies (19–23) and one randomized trial (18). Tables 2 and 3 presents quality assessment.
Overall Survival
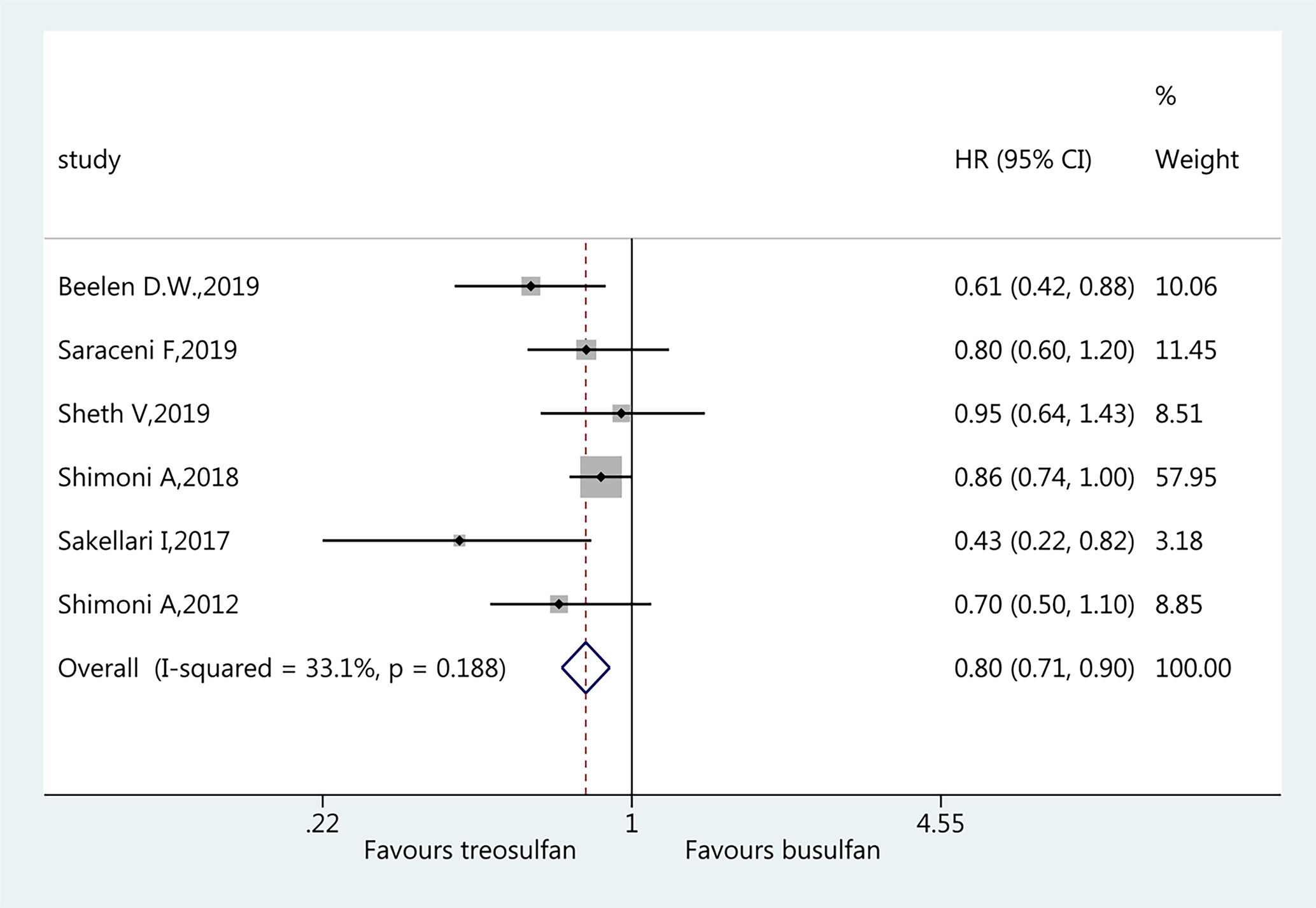
All six studies (18–23) were included for the assessment of OS. The pooled HR favored treosulfan (HR=0.80, 95%CI: 0.71–0.90). No heterogeneity was observed (I2 = 33.1%, P=0.188) (Figure 2).
Non-Relapse Mortality
Five studies (18–22) were included in the assessment of NRM. There was no significant difference in NRM between the two regimens (HR=0.84, 95%CI=0.71–1.01). No heterogeneity was observed (I2 = 16.5%, P=0.309) (Figure 3).
Leukemia-Free Survival
Three studies (19–21) were included in the assessment of LFS. There was no significant difference in LFS between the two regimens (HR=0.98, 95%CI=0.87–1.12). No heterogeneity was observed (I2 = 0%, P=0.801) (Figure 4).
Graft Versus Host Disease
Three studies (19–21) were included for the assessment of aGvHD and favored treosulfan-based regimens (HR=0.70, 95%CI=0.59–0.82). No heterogeneity was observed (I2 = 41.6%, P=0.181) (Figure 5A). Four studies (18–21) were included in the assessment of cGvHD. There was no significant difference in cGvHD between the two regimens (HR=0.94, 95%CI=0.81–1.09). No heterogeneity was observed (I2 = 32.0%, P=0.220) (Figure 5B).
Relapse Incidence
Four studies (19–22) were included in the assessment of RI. There was no significant difference in RI between the two regimens (HR=0.96, 95%CI=0.71–1.31). Heterogeneity was observed (I2 = 59.1%, P=0.062) (Figure 6).
Assessment of Publication Bias
The results of the Begg’s test showed P=0.260 and P=0.806, and the Egger’s test showed P=0.125 and P=0.868 for OS and NRM, respectively, indicating that there was no publication bias among these studies (Supplementary Figures 1, 2).
Discussion
Many studies aimed to evaluate the efficacy and safety of treosulfan-based conditioning regimens for allo-HCT compared with other regimens, but different outcomes were reported across studies. Therefore, this meta-analysis aimed to determine the long-term survival outcomes of treosulfan-based conditioning regimens in MDS/AML patients. The results suggest that treosulfan-based conditioning regimens improve the overall survival in patients with MDS and AML, with lower acute graft-versus-host disease incidence. It adds to the conclusion of the only randomized controlled trial included here (Beelen’s (18)), which showed that treosulfan-based regimens were non-inferior to busulfan when combined with fludarabine. The other five studies that could be included in the meta-analysis were retrospective studies, highlighting the need for additional well-designed randomized controlled trials to compared treosulfan- vs. busulfan-based conditioning regimens in patients MDS/AML, in addition to the results of the meta-analysis itself. This is also the main limitation of the present meta-analysis, and the interpretation of the results should be made with caution.
Conventional MAC containing busulfan are among the most widely used for MDS and AML, but they have shortcomings such as toxicity and transplantation-related mortality (8–10). Treosulfan-based regimens are considered as effective as conventional MAC, but with lower toxicity and transplant-related mortality (8, 9). Accordingly, the present study showed that OS was better with treosulfan-based regimens, and the incidence of aGvHD was lower. Indeed, aGvHD is a severe complication of allo-HSCT and can result in significant morbidity and mortality (24, 25). On the other hand, the occurrence of cGvHD was similar between the two regimens. The traditional busulfan-based regimens are still those being recommended by guidelines (1, 6, 26), but recent data support the use of treosulfan-based regimens because of the suggested better toxicity profile and similar efficacy (8, 9). This similar efficacy has been confirmed in the present meta-analysis, but safety was not directly assessed. Nevertheless, no heterogeneity or publication bias was observed for the main outcomes, suggesting a goods reliability of the results. A number of clinical trials are currently underway and should provide additional insights soon. In the meantime, Nagler et al. (27) reported a low occurrence of veno-occlusive diseases (2%) and deaths (0.4%) with treosulfan, but the lack of a comparator group precluded an actual analysis of safety. Nemecek et al. (28), in a phase II trial without a comparator, showed that the frequencies of aGvHD and cGvHD were 22% and 40%, respectively. Again, such favorable results were also observed in previous studies (29–37).
In addition, NRM, LFS, and RI, which are leukemia-related indicators, were not associated with the use of treosulfan-based regimens. This is supported by a recent report by Nagler et al. (27), who showed that treosulfan-based regimes had a favorable long-term OS and NRM profile, but they did not have a comparator group. A phase II clinical trial, without comparator, showed high OS and LFS and low NRM, also suggesting the clinical benefits of treosulfan in preconditioning regimens (28). Nemecek et al. (38) showed that NRM was 4% at 100 days and 8% at 2 years in patients who received treosulfan/fludarabine preconditioning. A number of non-comparative studies also support those conclusions in various hematologic malignancies, including AML and MDS (29–37). The main reason why those studies could not be included in the present meta-analysis is that they had no comparator group. They do show interesting and even promising results, and the reported NRM, OS, and LFS are similar to those of the studies included in the present meta-analysis, but without a comparator, the exact impact and benefits of treosulfan cannot be determined since its effects might vary among study populations, countries, and supporting treatments, among others.
This meta-analysis has limitations. Only six studies could be included, introducing bias and limiting the generalizability of the results. There was wide variability in the exact regimens and patient characteristics among the studies, leading to significant heterogeneity. Of note, there was only one randomized controlled trial, and the other included studies were retrospective. Such trials are needed to determine the exact benefits of treosulfan-bases regimens for allo-GSCT for patients with MDS or AML.
The present meta-analysis determined that treosulfan-based conditioning regimens improve the OS in patients with MDS and AML, with lower aGvHD incidence.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
Author Contributions
JL conceived and designed the study. SZ and GL performed the literature search, data extraction, quality assessment of the included studies. QC and ZW performed the statistical analysis. JL wrote the paper. JL, SZ, GL, QC, and ZW reviewed and edited the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by The National Natural Science Foundation of China [81571664, 81871323, 81801665]; The National Natural Science Foundation of Guangdong Province [2018B030311024]; The China Postdoctoral Science Foundation [2016M600145]; the Tumor Interventional Clinical Medical Technology Demonstration Base of Hunan Province [2017SK4010]; and the Science and Technology Planning Project of Chenzhou City, Hunan Province [jsyf2017020].
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2020.591363/full#supplementary-material
Supplementary Figure 1 | (A) Sensitivity analysis of overall survival. (B) Sensitivity analysis of non-relapse mortality
Supplementary Figure 2 | (A) Funnel plot of overall survival. (B) Funnel plot of non-relapse mortality.
References
1. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines). Myelodysplastic Syndromes. Version 1.2020. Fort Washington: National Comprehensive Cancer Network (2019).
2. Garcia-Manero G. Myelodysplastic syndromes: 2014 update on diagnosis, risk-stratification, and management. Am J Hematol (2014) 89(1):97–108. doi: 10.1002/ajh.23642
3. Ades L, Itzykson R, Fenaux P. Myelodysplastic syndromes. Lancet (2014) 383(9936):2239–52. doi: 10.1016/S0140-6736(13)61901-7
4. Higgins JP, Montgomery MW. Commentary on “receptivity to weight management interventions among hospitalized obese patients: an untapped opportunity”. South Med J (2013) 106(6):343–4. doi: 10.1097/SMJ.0b013e3182967fef
5. Ferrara F, Schiffer CA. Acute myeloid leukaemia in adults. Lancet (2013) 381(9865):484–95. doi: 10.1016/S0140-6736(12)61727-9
6. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines). Acute Myeloid Leukemia. Version 3.2020. Fort Washington: National Comprehensive Cancer Network (2019).
7. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA: Cancer J Clin (2020) 70(1):7–30. doi: 10.3322/caac.21590
8. Jethava YS, Sica S, Savani B, Socola F, Jagasia M, Mohty M, et al. Conditioning regimens for allogeneic hematopoietic stem cell transplants in acute myeloid leukemia. Bone Marrow Transplant (2017) 52(11):1504–11. doi: 10.1038/bmt.2017.83
9. Gyurkocza B, Sandmaier BM. Conditioning regimens for hematopoietic cell transplantation: one size does not fit all. Blood (2014) 124(3):344–53. doi: 10.1182/blood-2014-02-514778
10. Dikshit N, Gupta P, Sengupta K, Chakraborty J. Treosulfan Based Conditioning Regime for Allogenic HSCT in Resource Poor Setting. Biol Blood Marrow Transplant J Am Soc Blood Marrow Transplant (2019) 25(3 Suppl):S198. doi: 10.1016/j.bbmt.2018.12.790
11. Danylesko I, Shimoni A, Nagler A. Treosulfan-based conditioning before hematopoietic SCT: more than a BU look-alike. Bone Marrow Transplant (2012) 47(1):5–14. doi: 10.1038/bmt.2011.88
12. ten Brink MH, Zwaveling J, Swen JJ, Bredius RG, Lankester AC, Guchelaar HJ. Personalized busulfan and treosulfan conditioning for pediatric stem cell transplantation: the role of pharmacogenetics and pharmacokinetics. Drug Discovery Today (2014) 19(10):1572–86. doi: 10.1016/j.drudis.2014.04.005
13. Munkelt D, Koehl U, Kloess S, Zimmermann SY, Kalaaoui RE, Wehner S, et al. Cytotoxic effects of treosulfan and busulfan against leukemic cells of pediatric patients. Cancer Chemother Pharmacol (2008) 62(5):821–30. doi: 10.1007/s00280-007-0669-3
14. Fichtner I, Becker M, Baumgart J. Antileukaemic activity of treosulfan in xenografted human acute lymphoblastic leukaemias (ALL). Eur J Cancer (2003) 39(6):801–7. doi: 10.1016/S0959-8049(02)00767-0
15. Westerhof GR, Ploemacher RE, Boudewijn A, Blokland I, Dillingh JH, McGown AT, et al. Comparison of different busulfan analogues for depletion of hematopoietic stem cells and promotion of donor-type chimerism in murine bone marrow transplant recipients. Cancer Res (2000) 60(19):5470–8.
16. Sjoo F, Hassan Z, Abedi-Valugerdi M, Griskevicius L, Nilsson C, Remberger M, et al. Myeloablative and immunosuppressive properties of treosulfan in mice. Exp Hematol (2006) 34(1):115–21. doi: 10.1016/j.exphem.2005.09.015
17. Lo CK, Mertz D, Loeb M. Newcastle-Ottawa Scale: comparing reviewers’ to authors’ assessments. BMC Med Res Methodol (2014) 14:45. doi: 10.1186/1471-2288-14-45
18. Beelen DW, Trenschel R, Stelljes M, Groth C, Masszi T, Remenyi P, et al. Treosulfan or busulfan plus fludarabine as conditioning treatment before allogeneic haemopoietic stem cell transplantation for older patients with acute myeloid leukaemia or myelodysplastic syndrome (MC-FludT.14/L): a randomised, non-inferiority, phase 3 trial. Lancet Haematol (2020) 7(1):e28–39. doi: 10.1016/S2352-3026(19)30157-7
19. Saraceni F, Labopin M, Brecht A, Kroger N, Eder M, Tischer J, et al. Fludarabine-treosulfan compared to thiotepa-busulfan-fludarabine or FLAMSA as conditioning regimen for patients with primary refractory or relapsed acute myeloid leukemia: a study from the Acute Leukemia Working Party of the European Society for Blood and Marrow Transplantation (EBMT). J Hematol Oncol (2019) 12(1):44. doi: 10.1186/s13045-019-0727-4
20. Sheth V, Labopin M, Canaani J, Volin L, Brecht A, Ganser A, et al. Comparison of FLAMSA-based reduced intensity conditioning with treosulfan/fludarabine conditioning for patients with acute myeloid leukemia: an ALWP/EBMT analysis. Bone Marrow Transplant (2019) 54(4):531–9. doi: 10.1038/s41409-018-0288-0
21. Shimoni A, Labopin M, Savani B, Hamladji RM, Beelen D, Mufti G, et al. Intravenous Busulfan Compared with Treosulfan-Based Conditioning for Allogeneic Stem Cell Transplantation in Acute Myeloid Leukemia: A Study on Behalf of the Acute Leukemia Working Party of European Society for Blood and Marrow Transplantation. Biol Blood Marrow Transplant J Am Soc Blood Marrow Transplant (2018) 24(4):751–7. doi: 10.1016/j.bbmt.2017.12.776
22. Shimoni A, Shem-Tov N, Volchek Y, Danylesko I, Yerushalmi R, Nagler A. Allo-SCT for AML and MDS with treosulfan compared with BU-based regimens: reduced toxicity vs reduced intensity. Bone Marrow Transplant (2012) 47(10):1274–82. doi: 10.1038/bmt.2012.4
23. Sakellari I, Mallouri D, Gavriilaki E, Batsis I, Kaliou M, Constantinou V, et al. Survival Advantage and Comparable Toxicity in Reduced-Toxicity Treosulfan-Based versus Reduced-Intensity Busulfan-Based Conditioning Regimen in Myelodysplastic Syndrome and Acute Myeloid Leukemia Patients after Allogeneic Hematopoietic Cell Transplantation. Biol Blood Marrow Transplant J Am Soc Blood Marrow Transplant (2017) 23(3):445–51. doi: 10.1016/j.bbmt.2016.11.023
24. Jacobsohn DA, Vogelsang GB. Acute graft versus host disease. Orphanet J Rare Dis (2007) 2:35. doi: 10.1186/1750-1172-2-35
25. Nassereddine S, Rafei H, Elbahesh E, Tabbara I. Acute Graft Versus Host Disease: A Comprehensive Review. Anticancer Res (2017) 37(4):1547–55. doi: 10.21873/anticanres.11483
26. Xu L, Chen H, Chen J, Han M, Huang H, Lai Y, et al. The consensus on indications, conditioning regimen, and donor selection of allogeneic hematopoietic cell transplantation for hematological diseases in China-recommendations from the Chinese Society of Hematology. J Hematol Oncol (2018) 11(1):33. doi: 10.1186/s13045-018-0564-x
27. Nagler A, Labopin M, Beelen D, Ciceri F, Volin L, Shimoni A, et al. Long-term outcome after a treosulfan-based conditioning regimen for patients with acute myeloid leukemia: A report from the Acute Leukemia Working Party of the European Society for Blood and Marrow Transplantation. Cancer (2017) 123(14):2671–9. doi: 10.1002/cncr.30646
28. Nemecek ER, Hilger RA, Adams A, Shaw BE, Kiefer D, Le-Rademacher J, et al. Treosulfan, Fludarabine, and Low-Dose Total Body Irradiation for Children and Young Adults with Acute Myeloid Leukemia or Myelodysplastic Syndrome Undergoing Allogeneic Hematopoietic Cell Transplantation: Prospective Phase II Trial of the Pediatric Blood and Marrow Transplant Consortium. Biol Blood Marrow Transplant J Am Soc Blood Marrow Transplant (2018) 24(8):1651–6. doi: 10.1016/j.bbmt.2018.04.025
29. Casper J, Holowiecki J, Trenschel R, Wandt H, Schaefer-Eckart K, Ruutu T, et al. Allogeneic hematopoietic SCT in patients with AML following treosulfan/fludarabine conditioning. Bone Marrow Transplant (2012) 47(9):1171–7. doi: 10.1038/bmt.2011.242
30. Casper J, Knauf W, Kiefer T, Wolff D, Steiner B, Hammer U, et al. Treosulfan and fludarabine: a new toxicity-reduced conditioning regimen for allogeneic hematopoietic stem cell transplantation. Blood (2004) 103(2):725–31. doi: 10.1182/blood-2002-11-3615
31. Casper J, Wolff D, Knauf W, Blau IW, Ruutu T, Volin L, et al. Allogeneic hematopoietic stem-cell transplantation in patients with hematologic malignancies after dose-escalated treosulfan/fludarabine conditioning. J Clin Oncol Off J Am Soc Clin Oncol (2010) 28(20):3344–51. doi: 10.1200/JCO.2009.23.3429
32. Kroger N, Shimoni A, Zabelina T, Schieder H, Panse J, Ayuk F, et al. Reduced-toxicity conditioning with treosulfan, fludarabine and ATG as preparative regimen for allogeneic stem cell transplantation (alloSCT) in elderly patients with secondary acute myeloid leukemia (sAML) or myelodysplastic syndrome (MDS). Bone Marrow Transplant (2006) 37(4):339–44. doi: 10.1038/sj.bmt.1705259
33. Shimoni A, Hardan I, Shem-Tov N, Rand A, Yerushalmi R, Nagler A. Fludarabine and treosulfan: a novel modified myeloablative regimen for allogeneic hematopoietic stem-cell transplantation with effective antileukemia activity in patients with acute myeloid leukemia and myelodysplastic syndromes. Leukemia Lymphoma (2007) 48(12):2352–9. doi: 10.1080/10428190701671051
34. Ruutu T, Volin L, Beelen DW, Trenschel R, Finke J, Schnitzler M, et al. Reduced-toxicity conditioning with treosulfan and fludarabine in allogeneic hematopoietic stem cell transplantation for myelodysplastic syndromes: final results of an international prospective phase II trial. Haematologica (2011) 96(9):1344–50. doi: 10.3324/haematol.2011.043810
35. Shimoni A, Vago L, Bernardi M, Yerushalmi R, Peccatori J, Greco R, et al. Missing HLA C group 1 ligand in patients with AML and MDS is associated with reduced risk of relapse and better survival after allogeneic stem cell transplantation with fludarabine and treosulfan reduced toxicity conditioning. Am J Hematol (2017) 92(10):1011–9. doi: 10.1002/ajh.24827
36. Yerushalmi R, Shem-Tov N, Danylesko I, Avigdor A, Nagler A, Shimoni A. Fludarabine and treosulfan compared with other reduced-intensity conditioning regimens for allogeneic stem cell transplantation in patients with lymphoid malignancies. Bone Marrow Transplant (2015) 50(12):1526–35. doi: 10.1038/bmt.2015.174
37. Boztug H, Sykora KW, Slatter M, Zecca M, Veys P, Lankester A, et al. European Society for Blood and Marrow Transplantation Analysis of Treosulfan Conditioning Before Hematopoietic Stem Cell Transplantation in Children and Adolescents With Hematological Malignancies. Pediatr Blood Cancer (2016) 63(1):139–48. doi: 10.1002/pbc.25764
38. Nemecek ER, Guthrie KA, Sorror ML, Wood BL, Doney KC, Hilger RA, et al. Conditioning with treosulfan and fludarabine followed by allogeneic hematopoietic cell transplantation for high-risk hematologic malignancies. Biol Blood Marrow Transplant J Am Soc Blood Marrow Transplant (2011) 17(3):341–50. doi: 10.1016/j.bbmt.2010.05.007
Keywords: myelodysplastic syndrome, acute myeloid leukemia, allogeneic hematopoietic cell transplantation, preconditioning regimen, treosulfan, busulfan
Citation: Zhu S, Liu G, Liu J, Chen Q and Wang Z (2020) Long-Term Outcomes of Treosulfan- vs. Busulfan-Based Conditioning Regimen for Patients With Myelodysplastic Syndrome and Acute Myeloid Leukemia Before Hematopoietic Cell Transplantation: A Systematic Review and Meta-Analysis. Front. Oncol. 10:591363. doi: 10.3389/fonc.2020.591363
Received: 04 August 2020; Accepted: 05 October 2020;
Published: 16 December 2020.
Edited by:
Marcos De Lima, Case Western Reserve University, United StatesReviewed by:
Michael Diamantidis, University Hospital of Larissa, GreeceSarah Wall, The Ohio State University, United States
Copyright © 2020 Zhu, Liu, Liu, Chen and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jing Liu, jliu142@outlook.com
†These authors have contributed equally to this work
 Sheng Zhu1,2†
Sheng Zhu1,2†