- 1Department of Agricultural Biology, Colorado State University, Fort Collins, CO, United States
- 2Graduate Degree Program in Ecology, Colorado State University, Fort Collins, CO, United States
Trap cropping involves the use of plant species or genotypes to attract pest insects away from the main crop to avoid pest damage. In this study, we evaluated the potential of using winter triticale (x Triticosecale) as a trap crop for the wheat stem sawfly (Cephus cinctus Norton), an economically devastating pest of wheat (Triticum aestivum L.). The wheat stem sawfly larvae consume parenchyma tissue within the wheat stem and cut the stem at the base causing it to lodge. Triticale is, on average taller and has a larger stem diameter than winter wheat. These traits are considered attractive to adult females when choosing hosts for oviposition. We conducted a two-year field study of one winter wheat and one winter triticale genotype combination for its potential as a trap crop. To complement the field study, we grew three genotypes of winter triticale and one winter wheat genotype in cone-tainers and infested them in the field. The cone-tainer and field studies revealed that the chosen winter triticale genotypes were not more attractive than the winter wheat genotypes for adult wheat stem sawflies. The field study also evaluated the average larval position in the stem and found the average position was variable between sampling dates in both crops. Thus, determining the precise timing of field swathing could destroy significant portions of larval populations. Future research should focus on genotype selection to establish triticale-wheat cultivar combinations to create a push-pull system.
Introduction
Trap crops are used to attract insect pests away from the crop of interest. Typically, plants are planted in a small stand near the crop of interest and are destroyed or treated with pesticides once pest densities are high (Hokkanen and Jokioinen, 1991). Some common methods of trap cropping include: perimeter planting where the trap crop is planted around the border of the main crop; sequential planting where the trap crop is planted earlier than the main crop; multiple planting where several other trap crops are used; and push-pull, where the trap crop is more attractive than the main crop (Hokkanen and Jokioinen, 1991; Shelton and Badenes-Perez, 2006). Using a trap crop can increase yield and an overall reduction in pesticide use (Hokkanen and Jokioinen, 1991). Some examples of effective trap crops include using sunflowers as a trap crop for Lygus pratensis in Chinese cotton fields (Zhang et al., 2020). Cotton fields bordered by sunflower had 45% less damage to bolls and 28% less damage to leaves than fields not bordered by sunflower. Blue Hubbard squash as a trap crop for striped cucumber beetle, Acalymma vittatum F., in butternut squash reduced insecticide use by 94% in North American butternut squash fields (Cavanagh et al., 2009). Also, trap crops can increase insect diversity and promote natural enemies (Tiwari et al., 2020). For example, sweet alyssum, Lobularia maritima, improved predator abundance for green peach aphid, Myzus persicae, in radish in Nepal (Tiwari et al., 2020). Some trap crops may enhance pest control by producing semiochemicals (Shelton and Badenes-Perez, 2006), making the trap crop more attractive to pests (Khan et al., 2016). Trap cropping can be improved through various manipulations, mainly using sex and aggregation pheromones (Hokkanen and Jokioinen, 1991). Yet, very few trap crop schemes have been widely adopted (Shelton and Badenes-Perez, 2006). Successful implementation often depends on the retention of the pest on the trap crop, the suitability of the system, and a minimal increase in supplemental management strategies (Holden et al., 2012; Sharma et al., 2019). A major pest of wheat (Triticum aestivum L.) known as the wheat stem sawfly (Hymenoptera: Cephidae: Cephus cinctus Norton), is considered to have life history attributes, such as low fecundity and a short adult lifespan, that make it suitable to be managed using trap crops (Morrill et al., 2001).
Grain-yield loss from the wheat stem sawfly exceeds $350 million annually in the northern Great Plains of North America (Beres et al., 2011b). Its life cycle makes it particularly difficult to control using conventional methods. The adult wheat stem sawfly lay their eggs within the stem of their host, and as the larvae develop, they consume parenchyma tissue (Ainslie, 1920). Toward the end of the season, the last instar larva creates a hibernaculum (stub) by cutting at the base of the stem, causing the seed head to fall to the ground. Cut stems are difficult to harvest and are easily blown over (Ainslie, 1920). Current management methods include biological control and solid stem genotypes (Delaney et al., 2010; Beres et al., 2011b; Rand et al., 2012; Peairs et al., 2014). A comprehensive review of wheat stem sawfly biology and management practices is reported by Beres et al. (2011b). Previous work dating back to 1922 suggests trap crops can control wheat stem sawflies (Criddle, 1922). In particular, winter wheat in Montana is potentially used as a trap crop for wheat stem sawflies in spring wheat cropping systems (Morrill et al., 2001; Beres et al., 2009; Buteler et al., 2010). The early planting of winter wheat attracted wheat stem sawfly adults from neighboring fallow wheat stubble and served as a sink for eggs. Later, the trap crop could be harvested or swathed to destroy larva developing within the stems (Morrill et al., 2001). Studies suggest stem height, stem solidness, and semiochemicals play a role in attractiveness and retention rates of winter wheat to wheat stem sawfly (Morrill et al., 2001; Beres et al., 2009; Buteler et al., 2010; Buteler and Weaver, 2012). A pheromone found to attract adult females of wheat stem sawfly could be introduced into trap strips to improve the attractiveness of trap crops. However, the two pheromones currently described are not sex discriminant (Bartelt et al., 2002; Cossé et al., 2002).
Since most wheat grown in Colorado is winter wheat, we focused on assessing the retention, attractiveness, and host suitability of winter triticale (x Triticosecale Wittmack), a cross between wheat and rye (Secale cereale, Will.), as a trap crop for the wheat stem sawfly in a winter wheat cropping system. Winter triticale was chosen for this study as it has tall stems, good grain and forage yield potential, early maturity, and desirable forage quality (Oettler, 2005; Estrada-Campuzano et al., 2012; Randhawa et al., 2015), making this plant attractive to wheat farmers who also feed cattle (Harper et al., 2017; Coblentz et al., 2018). Previous studies found rye to be a poor host (Criddle, 1922) which may play a part in the effectiveness of triticale as a trap crop.
In the current study, we evaluated the attractiveness and retention of wheat and triticale over two years using both cone-tainer and field experiments. The cone-tainer experiment was designed to assess three triticale genotypes as suitable hosts for the wheat stem sawfly compared to a highly attractive winter wheat genotype. The field experiments included one genotype of triticale compared to wheat and pheromone traps (Cossé et al., 2002) to assess if the pheromones could increase attraction and retention in either wheat or triticale plots.
Methods
Cone-Tainer Experiment to Test Host Preference and Performance on Triticale vs. Wheat
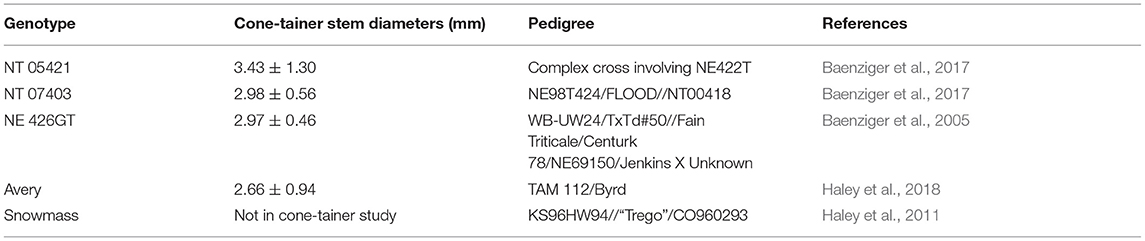
In 2019 and 2020, we compared three winter triticale genotypes; “NT 05421,” “NT 07403,” and “NE 426GT” to “Avery,” a winter wheat genotype known to be highly attractive to wheat stem sawfly [Colorado State University Extension (CSU), 2020] (Table 1) and was the second most popular variety to be grown in Colorado in 2020 (Pachl et al., 2020). All three triticale genotypes are grown predominantly for forage. We germinated seeds on 5 x 10 mm germination blotter paper (Anchor Paper Co. St. Paul, MN) with 5 ml of tap water. Seeds were kept at an average temperature of 24°C for three days. Germinated seeds were then vernalized by placing them in a cold room and held at 4°C for seven weeks. After vernalization, a single seedling was planted in a cone-tainer (Stuewe & Sons SC10U UV-stabilized cones: 3.8 cm diameter x 20.9 cm depth, 164 ml volume) with a soil mixture of 7 parts soil 2 parts perlite, and a cotton ball at the bottom to prevent soil loss. Due to changes in our soil distributor, we used Metro Mix (Sungro, Agawam, MA) in 2019 and 20B/30V (Lambert, Québec, Canada) in 2020. Both brands used had comparable compositions of sphagnum peat moss and bark.
Plants were grown in a greenhouse at Colorado State University with supplemental light [430W HPS (High-Pressure Sodium) fixtures—P.L. Light Systems, bulbs—GE Lucalox lu400 series, 400W]. The greenhouse had a 16L: 8D photoperiod and the day: night temperature of 23:18°C. Plastic trays were placed under 42 cone-tainers, and all trays were bottom watered as needed. Germination and planting dates were staggered over two weeks in 2019 and three weeks in 2020. We did this as peak sawfly flight is highly variable across years and wanted to ensure uniform maturity of plants. In each tray, cone-tainers of triticale were planted in alternating rows to Avery, and trays were placed randomly in the greenhouse. One genotype of triticale was planted per tray. Plants were fertilized once a week, beginning one week after initial planting, with 300 ml 15-16-17 Peters General Purpose Fertilizer (J. R. Peters, Allentown, PA) at 296 ppm in an aqueous solution. To promote primary stem growth, we cut and removed secondary tillers leaving only the primary tiller. This was conducted 10 days before infestation so plants could recover from injury. In 2019, once plants started to tiller, tillers were cut twice two weeks apart. In 2020, we cut tillers once, three weeks after planting.
Wheat Stem Sawfly Infestation and Data Collection
We transported wheat plants to the field for infestation once plants reached Zadoks growth stage 32–75 (Zadoks et al., 1974). The wheat stem sawfly requires a stem for larval development; hence we considered Zadoks 32 (when two to three nodes were visible) to Zadoks 75 (ripening of kernels) as the appropriate stages for oviposition (Criddle, 1923). This range was chosen due to large varietal differences in maturity. We only brought plants out on calm sunny days, conditions favorable for adult sawfly activity. We measured infestation pressure by sweeping 100 times using 180-degree pendulum sweeps with a standard 38 cm diameter sweep net. All infestations occurred on a field of infested wheat stubble bordering growing wheat at Wickstrom Farms, near Orchard, CO. Trays were placed 10 meters apart, and trays were set 5 meters apart within rows.
In 2019, cone-tainer trays of a single planting date were placed in the field for 24 h on May 24 and for 1 h 30 min on May 30. In 2020, trays were placed in the field for 2 h on May 22 and June 5 and for 4 h on May 29. Exposure times were variable based on variable adult infestation pressures and accounted for during statistical analysis. Plants in each round of exposure were from the same planting date. All exposures started at 9 a.m. Before plants were brought back to the greenhouse, sawfly adults were removed, and plants left outside for 2–3 h and checked again to ensure no foraging females were brought inside. Once we brought the trays back to the lab, half of the plants were dissected to examine stems for eggs, while the other half remained in the greenhouse to allow larvae to mature. While examining stems for eggs, we recorded stem diameter using a digital caliper (Pittsburgh, Model 47257) by measuring the first visible node's width, the stem's maturity on the Zadoks scale, and the number of eggs present.
Thirty days after infestation, the remaining stems were dissected to assess wheat stem sawfly presence. All stems were examined on the same day. We considered a stem infested if there were frass and/or larvae present in the stem. If larvae were present, they were weighed, and pictures of the head capsule were taken. Using ImageJ (Rueden et al., 2017), the length of the head capsule was measured. Body measurements were recorded as a proxy to compare growth and development among genotypes, as suggested by Kumar and Venkatesan (2019). We noted if stems had been cut to form hibernaculum chambers. Assessing whether a stem was cut or not enables us to determine the development time of the larvae when compared to other genotypes since the creation of a hibernaculum occurs during the last larval instar (Ainslie, 1920). In 2019, we only dissected plants with more than one visible node. To collect more precise measurements on stem development in 2020, we recorded the Zadoks growth stage of the stem during dissections. We only considered plants at Zadoks growth stages higher than 32 (plants with more than two nodes) for further analysis. If there were multiple stems in a cone-tainer we dissected the oldest stem based on the Zadoks growth stage.
Statistical Analysis for Cone-Tainer Plants
Analysis of data was conducted using R (R Core Team, 2019, Version 3.6.2) and R packages lme4 (Bates et al., 2015), ggplot2 (Wickham, 2016), and emmeans (Lenth, 2022). We used a linear mixed model with the infestation date as a random effect and year, Zadoks growth stage, genotype, and stem diameter as fixed effects to analyze the number of eggs within a stem. We included year, Zadoks growth stage, and stem diameter in the model, all known confounding variables for oviposition rates. Analysis of stem diameters was conducted using a linear model with genotype and year as independent variables.
We classified stem infestation data as binomial. To do so we categorized stems as being infested or not infested when frass was present or not. This is based on the life history of the wheat stem sawfly where often only one larva can survive to maturity per stem (Ainslie, 1920) and was also the case in the collected data. The proportion of stems infested was calculated as the number of infested stems divided by the total number of stems sampled. Using a generalized linear mixed model (GLMM), these data were evaluated with a binomial error distribution and a logit link function. In the GLMM model, infestation date was a random effect, and genotype was a fixed effect. We also analyzed the number of stems where a hibernaculum (stub) was formed. The number of stubs per genotype was also analyzed using a generalized linear model with a binomial error distribution and a logit link function with genotype and year as independent variables. Larval head length and weight were analyzed using a linear mixed model with infestation date as a random effect and genotype as a fixed effect.
Field Experiment to Test Effects of Planting a Triticale Trap Crop on Wheat Infestation
A winter wheat field bordering a heavily infested wheat stubble field near Orchard, CO was chosen for this experiment in 2019 and 2020. Fields in 2019 and 2020, were different but <2 km apart. Plots were planted at 67.25 kilograms/hectare of seed with a 25 cm row spacing along one edge border of the focal crop next to a wheat fallow field (Figure 1). No insecticides were applied to the field in both years. The treatments were winter wheat “Snowmass,” or winter triticale NT 05421. Snowmass was also the cultivar planted in the main field. Two treatments were planted in a Randomized Complete Block Design, replicated 12 times, so each block contained two plots of each treatment. Plots were 1/26 the length of the field edge (31 m each) to allow for a buffer plot to be planted on either end (24 plots + 2 buffer plots). In 2019, only we placed pheromone traps in half of the winter wheat and triticale plots. We used a single pheromone lure consisting of 9-acetyloxynonanal (Chemtica, San Jose, Costa Rica). This pheromone is considered attractive to wheat stem sawfly (Cossé et al., 2002).

Figure 1. Field plot design in 2019, showing blocks 1 and 2 as examples. We had a total of 12 replications and two buffer plots. Plots were 31 m each. In 2020 pheromone was not added to plots but field design was otherwise unchanged.
Plots were visited weekly to accomplish the following in 2019 and 2020: (1) 10 sweeps using 180-degree pendulum sweeps with a standard 38 cm diameter sweep net were taken from the center of each plot to determine in-plot wheat stem sawfly abundance and (2) 10 tillers were taken from the middle of each plot and dissected to determine larval position over time. In 2019, pheromones were placed at the height of 1 m on a Rebell® sticky trap (Andermatt Biocontrol Suisse AG, Grossdietwil, Switzerland) in the center of the plots with pheromone treatment. Pheromones and sticky traps were replaced weekly. The sticky trap and pheromone portion of the study were not continued in 2020, since adult sawfly captured in traps, despite being present for a week, was often only a fraction caught in the sweep samples in a single day. Processing of the sticky traps was also more time consuming than sweep trap samples. Additionally, there were no statistical differences between plots with pheromones present and not present. The larval position was calculated by measuring the larval distance from root crown. On sample date 5/22/2020, 100 sweeps were conducted per plot so sweep totals were divided by 10. The collaborating farmer swathed plots on (6/26/2019, 6/15/2020) at the height of 15–20 cm. Then 100 wheat stems were collected from the remaining stubble in each plot approximately one month later (7/20/2019, 7/20/2020) from each plot. To determine the final infestation levels, we dissected stems to look for signs of infestation, as described above. To determine infestation of the main crop we collected and dissected 100 stems of stubble from the main field wheat adjacent to the plots (9/11/2019, 9/21/2020).
Statistical Analysis for Field Experiment
We conducted separate analyses for 2019 and 2020, as the experimental design included a pheromone in 2019 but not in 2020. We used a linear model with crop type (wheat or triticale) and date sampled as independent variables for sweep net data and larval position. To examine the effects of pheromone presence on sweep samples, we used a linear model with treatment and date sampled as independent variables and the number of adults caught in sweeps as dependent variables. To analyze infestation of the plots and main field wheat adjacent plots we used a linear model with crop type and with or without pheromone treatment in 2019 and crop type in 2020 as independent variables.
Results
Cone-Tainer Experiment
Triticale varieties NT 07403, NE 426GT, and NT 05421 had larger stem diameters than Avery. The average stem diameter in 2019 and 2020 for the genotypes tested are presented in Table 1. There were differences in stem diameters between genotypes [F(3,425) = 20.867, P = < 0.0001]. NT 05421 had the largest stem diameter when compared to the other genotypes tested (NT 07403: P = 0.001, NE 426GT: P = <0.047, Avery: P = <0.0001). Avery stem diameters were smaller than all triticale genotypes (NT 07403, NE 426GT, NT 05421: P = < 0.0001). NT 07403 and NE 426GT stem diameters were similar (P = 0.699). NE 426GT had more eggs inside stems than NT 07403 (P = 0.0126) (Figure 2). NT 07403, NE 426GT, NT 05421, and Avery did not have different egg counts (Figure 2). Pairwise comparisons and estimated marginal means, provided in Supplementary Figure 1.
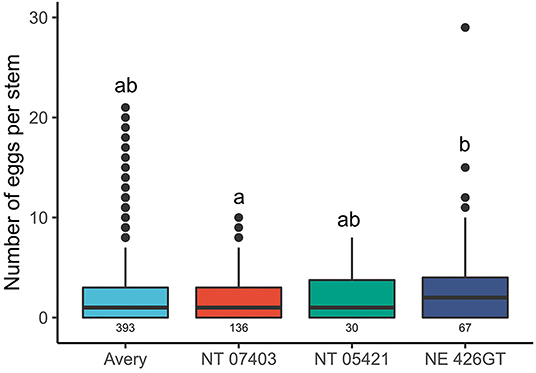
Figure 2. Effects of triticale genotypes and wheat on the number of wheat stem sawfly eggs found stems. Sample size is represented by the numbers beneath each boxplot (total stems cut). The boxes represent the 25th and 75th percentiles; the horizontal line in each box indicates the median. The whiskers signify maximum and minimum values and circles indicate outliers. Letters indicate significant pairwise differences (P < 0.05, Tukey HSD corrected post hoc test). Pairwise comparisons and estimated marginal means, provided in Supplementary Figure 1.
The number of adult sawflies found in sweep nets for each infestation date varied across dates. In 2019, 767 sawflies were captured on May 24 and 1,148 on May 30. In 2020, we collected 145 sawflies on May 22, 54 sawflies on May 29, and 4 sawflies on June 5.
Avery stems had the highest probability of infestation, higher than NT 0521 (P = < 0.0001) and NE 42GT (P = < 0.0001). Avery had a similar probability of infestation when compared to NT 07403 (P = 0.933) (Figure 3A). Avery stems had the highest probability of being cut when compared with NT 7403 (P = 0.010). No other genotypes differed in the number of stems cut (Figure 3B).
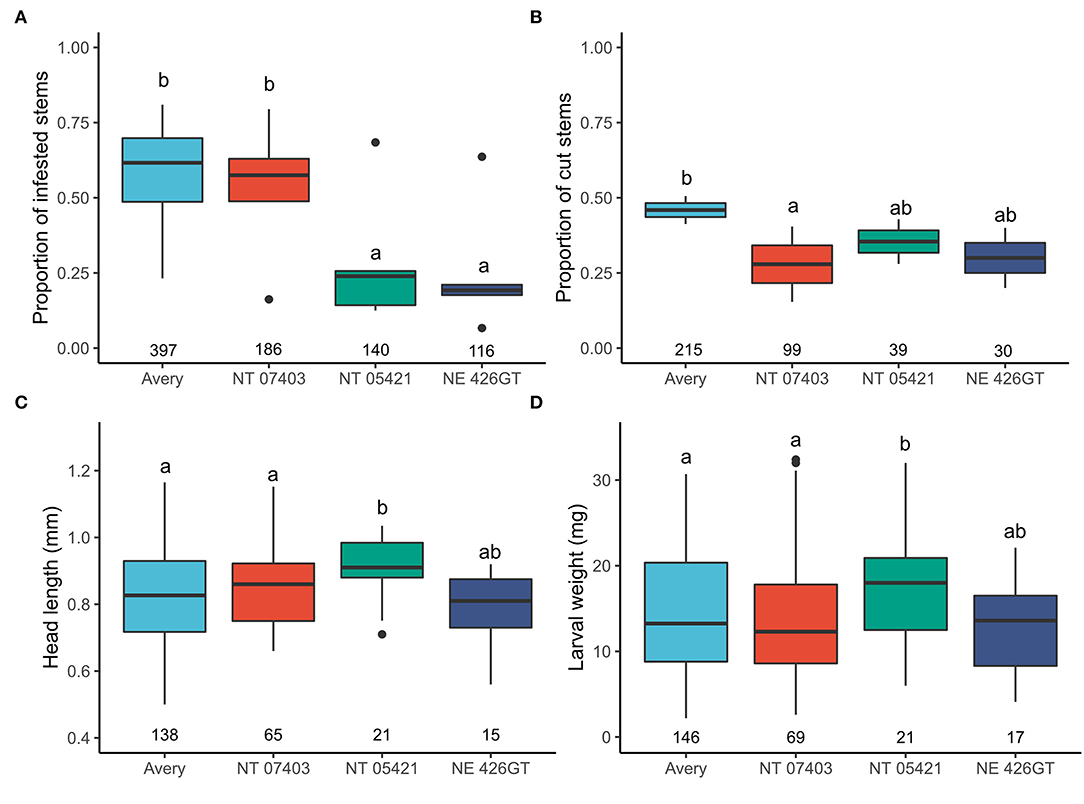
Figure 3. (A) Effects of triticale genotypes and wheat on the proportion of stems infested with wheat stem sawfly, (B) on the proportion of stems that contained frass and were cut by the wheat stem sawfly, (C) on wheat stem sawfly larval weight, (D) on wheat stem sawfly larval head capsule length. N is represented by the numbers beneath each boxplot (total stems cut). Letters indicate significant pairwise differences (P < 0.05, Tukey HSD corrected post hoc test). Pairwise comparisons and estimated marginal means, provided in Supplementary Figure 1.
Larvae in NT 05421 had larger head capsule lengths and body weights than Avery (head: P = <0.0001, weight P = 0.019) and NT 07403 (P = 0.026, P = 0.047) (Figures 3C,D). Pairwise comparisons and estimated marginal means, provided in Supplementary Figure 2.
Field Experiment
In 2019, adult abundance in plots with and without pheromones was similar [F(125) = 0.018, P = 0.892]. More wheat stem sawfly adults were caught in sweeps in wheat plots than triticale plots on all dates in 2019 (P = 0.003) and 2020 (P = < 0.001) (Figure 4). In 2019 and 2020, larval positions were similar in triticale and wheat stems. However, in 2019, larvae were higher in position in triticale and wheat when sampled on July 16th compared to July 10th (P = 0.039) (Figure 5). In 2020, larvae were in a higher position in the stem on May 29th than on July 5th (P = 0.003) or July 11th (P = 0.003) for both triticale and wheat (Figure 5).
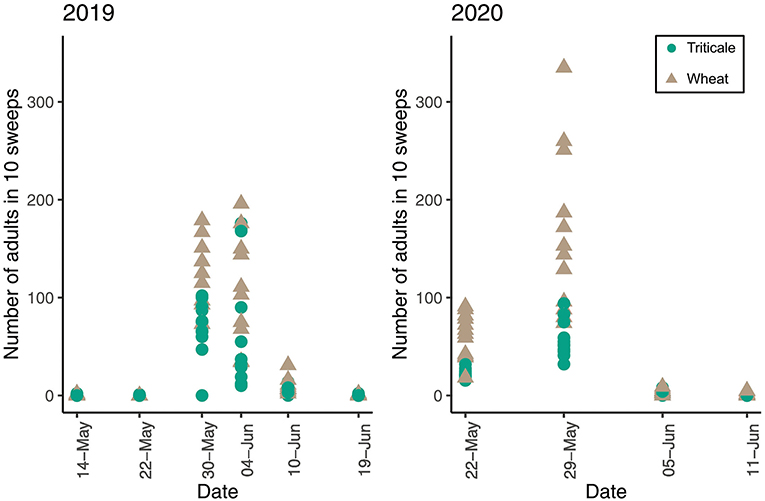
Figure 4. Number of adult wheat stem sawfly caught in 10 sweeps in triticale and wheat plots during the 2019 and 2020 trap crop field trial. Points represent adults caught in plots.
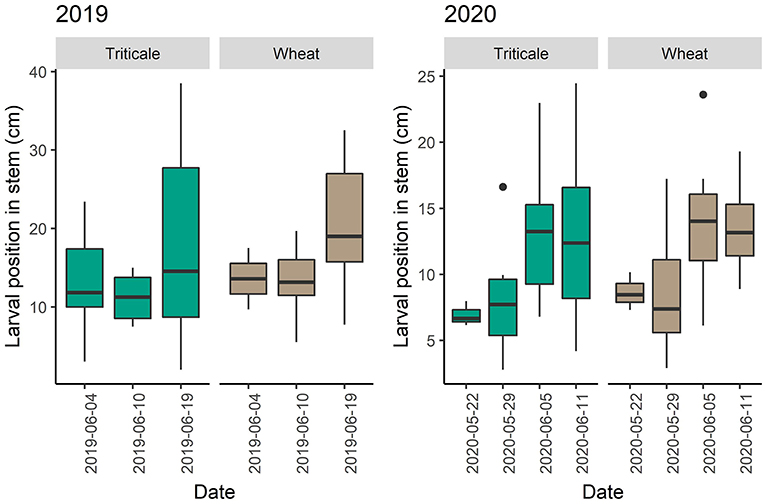
Figure 5. Changes in wheat stem sawfly larval position from the roots across dates and type of crop (wheat or triticale). Boxplots showing average larval position in the stem during collection dates in 2019 and 2020.
Stubble infestations in wheat and triticale plots were similar in both years [2019: F(1,21) = 0.334, P = 0.569, 2020: F(1,22) = 0.744, P = 0.397]. In 2019, the presence of pheromone traps did not affect infestation rates [F(1,21) = 2.079, P = 0.164]. The infestation rates of the wheat adjacent to either crop was similar in both years [2019: F(1,21) = 1.403, P = 0.249, 2020: F(1,22) = 4.013, P = 0.0576] (Figure 6).
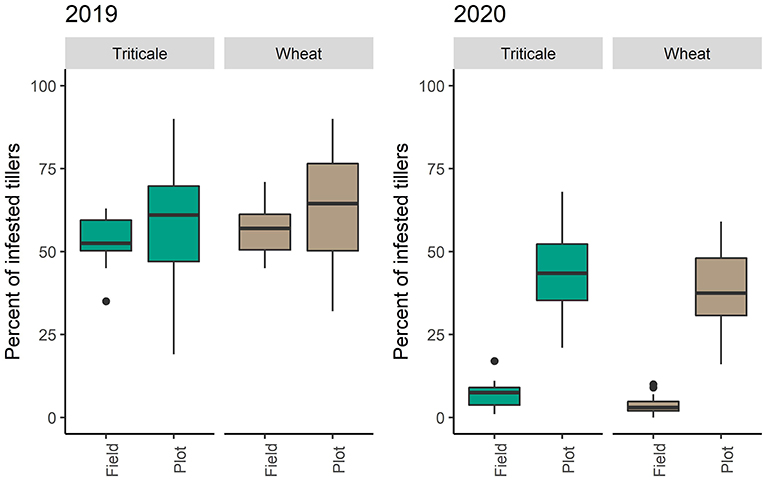
Figure 6. Differences in the number of infested stems between trial plots and adjacent wheat for 2019 and 2020.
Discussion
The winter triticale genotypes were as attractive as the winter wheat based on cone-tainer and field studies. Over the two years of this experiment, the field and greenhouse-reared plants were exposed to differing pest abundance. Still, infestation in triticale was consistently similar to that observed in Avery in the cone-tainer study and Snowmass in the field. There were slight differences in wheat stem sawfly attraction when looking at eggs laid to the three triticale genotypes in the cone-tainer study, which suggest using more attractive triticale varieties could improve the effectiveness of trap cropping, particularly when combined with less attractive wheat cultivars. Further exploration into using attractive triticale genotypes paired with less attractive wheat genotypes could potentially find triticale useful in a push-pull system.
Early studies in wheat stem sawfly trap crops found smooth brome, Bromus inermis, and fall rye, Secale cereale, could be effective for management (Criddle, 1922). Smooth brome was found to mature earlier than spring and winter wheat and have low larval survival and higher parasitism rates. Similarly, fall rye was harvested early in the summer, killing larvae before stubs could be formed (Criddle, 1922).
More recent studies suggest using certain winter wheat cultivars can be effective trap crops to spring wheat due to earlier maturities and taller stems (Morrill et al., 2001; Buteler et al., 2009; Weaver et al., 2009; Buteler and Weaver, 2012). For example, the spring wheat variety Reeder was preferred for oviposition over Conan during choice tests which may be due to greater amounts of attractive chemicals being emitted (Weaver et al., 2009). Our study found more adult sawflies in sweeps on wheat plots compared to triticale, which might suggest Snowmass is more attractive to sawflies than NT 05421 in the field. The addition of the sawfly pheromone did not have any effect on the number of adults captured. Attractiveness may have differed, but infestations of stubble collected in plots and adjacent wheat did not differ between wheat and triticale. Previous work examining winter wheat trap strip widths of 3, 6, 10 m and 0.6 km in length found no relation between wheat stem sawfly infestation and width of trap strip (Morrill et al., 2001).
In the cone-tainer experiment, we found sawfly larvae were much larger when developing in triticale stems when compared to wheat. Using the cone-tainer method, we could directly compare larvae on each planting date as we knew the exact date of infestation. Since we measured all stems for infestation on the same day, we could measure which larva in each genotype created stubs or not. We found more stubs in Avery than in NT 0743, suggesting differences in larval maturity. This is unlikely due to stem maturity as all uncut stems of both Avery and NT 0743 had an average Zadok's growth stage of 75. Slower larval maturity could be helpful when considering the timing of swathing to destroy larval populations, possibly due to larger stem diameters.
Swathing has long been proposed as a method of control for wheat stem sawfly (Holmes and Peterson, 1965; Wallace and McNeal, 1966; Holmes, 1978). However, swathing is more expensive, labor-intensive and should only be used when a crop is heavily infested (Nansen et al., 2005; Beres et al., 2011a). Thus, it is often suggested to swath only field perimeters since the highest infestations occur at field edges (Weaver et al., 2005). We observed the average larval position moving up and down throughout the growing season in the field experiment. Often sawfly larvae are thought to only move toward the base of the stem during development (Ainslie, 1920). The average position in the stem varied between years in both wheat and triticale plots. We also saw much lower infestation rates in the adjacent wheat plots in 2020 than in 2019. In 2020, the experimental field was swathed earlier in the season than in 2019, which may have removed larvae higher up in stems. Timing of swathing has been shown to affect sawfly survival, and the yield trade-off is the loss to wheat stem sawfly or early swathing (Holmes and Peterson, 1965). However, since triticale is often used for forage early swathing may be less deterring for farmers.
A recent review on using trap crops for cereal crop management suggests lack of adoption may be due to increased management inputs, cost efficiency, and lack of trap crops that can meet broad-acre cereal crop production (Sharma et al., 2019). While triticale is a suitable host for the wheat stem sawfly, the combinations of genotypes chosen for this study would not comprise an effective trap crop system for wheat stem sawfly. Triticale's desirable forage yield and quality could still be helpful for farms also growing forage when field edges are swathed (Oettler, 2005; Estrada-Campuzano et al., 2012; Randhawa et al., 2015). Genotype selection will play an essential role in creating an effective wheat-triticale push-pull trap cropping system. Based on our results, an effective triticale trap crop used for forage would have a large stem diameter, similar maturity to the main crop at adult sawfly flight, high infestation, slow larval development, and reducing cutting. The main crop should be planted with a solid stem genotype or a genotype known to be less attractive than the triticale variety. Future research should examine combinations of triticale and wheat genotypes for their effectiveness as trap crops.
Data Availability Statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found below: 10.6084/m9.figshare.16606427.
Author Contributions
EP, DC, FP, and PN contributed to conception and design of the study. FP, PN, and PO supervised and co-directed the research. EP performed experimental work, data analyses, and wrote the first draft of the manuscript. All authors contributed to manuscript revision, read, and approved the submitted version.
Funding
This research was supported by the Colorado Wheat Administrative Committee, the Colorado Wheat Research Foundation, and the Colorado Agricultural Experiment Station Project 646.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We thank the members of the CSU insectary for collecting and processing samples, the Wickstrom family for the use of their field, and the Franklin Graybill Statistical Laboratory at Colorado State University for statistical consulting.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fagro.2022.779013/full#supplementary-material
References
Ainslie, C. N. (1920). The western grass-stem sawfly. U. S. Dep. Agric. Bull. 841, 1–27. doi: 10.5962/bhl.title.108176
Baenziger, P. S., Jannink, J.-L., and Gibson, L. R. (2005). Registration of ‘NE426GT' winter triticale. Crop Sci. 45, 796–797. doi: 10.2135/cropsci2005.0796
Baenziger, P. S., Vogel, K., Mitchell, R., Wegulo, S., Regassa, T., Santra, D., et al. (2017). Release of seven winter triticale lines. University of Nebraska-Lincoln Department of Agronomy and Horticulture (UNL) and United States Department of Agriculture (USDA). Available online at: https://agronomy.unl.edu/Baenziger/2017-03-09_Approval-of-Triticale-Release.pdf
Bartelt, R. J., Cossé, A. A., Petroski, R. J., and Weaver, D. K. (2002). Cuticular hydrocarbons and novel alkenediol diacetates from wheat stem sawfly (Cephus cinctus): natural oxidation to pheromone components. J. Chem. Ecol. 28, 385–405. doi: 10.1023/A:1017994410538
Bates, D., Machler, M., Bolker, B. M., and Walker, S. C. (2015). Fitting linear mixed-effects models using lme4. J. Stat. Softw. 67, 1–48. doi: 10.18637/jss.v067.i01
Beres, B. L., Cárcamo, H. A., and Bremer, E. (2009). Evaluation of alternative planting strategies to reduce wheat stem sawfly (Hymenoptera: Cephidae) damage to spring wheat in the Northern Great Plains. J. Econ. Entomol. 102, 2137–2145. doi: 10.1603/029.102.0617
Beres, B. L., Cárcamo, H. A., Weaver, D. K., Dosdall, L. M., Evenden, M. L., Hill, B. D., et al. (2011a). Integrating the building blocks of agronomy and biocontrol into an IPM strategy for wheat stem sawfly. Prairie Soils Crop. J. 4, 54–65. doi: 10.7939/r3-wkca-sw57
Beres, B. L., Dosdall, L. M., Weaver, D. K., Cárcamo, H. A., and Spaner, D. M. (2011b). Biology and integrated management of wheat stem sawfly and the need for continuing research. Can. Entomol. 143, 105–125. doi: 10.4039/n10-056
Buteler, M., and Weaver, D. K. (2012). Host selection by the wheat stem sawfly in winter wheat and the role of semiochemicals mediating oviposition preference. Entomol. Exp. Appl. 143, 138–147. doi: 10.1111/j.1570-7458.2012.01237.x
Buteler, M., Weaver, D. K., Bruckner, P. L., Carlson, G. R., Berg, J. E., and Lamb, P. F. (2010). Using agronomic traits and semiochemical production in winter wheat cultivars to identify suitable trap crops for the wheat stem sawfly. Can. Entomol. 142, 222–233. doi: 10.4039/n09-072
Buteler, M., Weaver, D. K., and Peterson, R. K. D. (2009). Oviposition behavior of the wheat stem sawfly when encountering plants infested with cryptic conspecifics. Environ. Entomol. 38, 1707–1715. doi: 10.1603/022.038.0624
Cavanagh, A., Hazzard, R., Adler, L. S., and Boucher, J. (2009). Using trap crops for control of Acalymma vittatum (Coleoptera: Chrysomelidae) reduces insecticide use in butternut squash. J. Econ. Entomol. 102, 1101–1107. doi: 10.1603/029.102.0331
Coblentz, W. K., Akins, M. S., Kalscheur, K. F., Brink, G. E., and Cavadini, J. S. (2018). Effects of growth stage and growing degree day accumulations on triticale forages: 1. Dry matter yield, nutritive value, and in vitro dry matter disappearance. J. Dairy Sci. 101, 8965–8985. doi: 10.3168/jds.2018-14868
Colorado State University Extension (CSU). (2020). 2020 Dryland Winter Wheat Variety Performance Trial at Orchard. Fort Collins, CO: Colorado State University Extension (CSU).
Cossé, A. A., Bartelt, R. J., Weaver, D. K., and Zilkowski, B. W. (2002). Pheromone components of the wheat stem sawfly: Identification, electrophysiology, and field bioassay. J. Chem. Ecol. 28, 407–423. doi: 10.1023/A:1017946527376
Criddle, N. (1923). The life habits of Cephus cinctus Nort. in Manitoba. Can. Entomol. 55, 1–4. doi: 10.4039/Ent551-1
Delaney, K. J., Weaver, D. K., and Peterson, R. K. D. (2010). Photosynthesis and yield reductions from wheat stem sawfly (Hymenoptera: Cephidae): interactions with wheat solidness, water stress, and phosphorus deficiency. J. Econ. Entomol. 103, 516–524. doi: 10.1603/EC09229
Estrada-Campuzano, G., Slafer, G. A., and Miralles, D. J. (2012). Differences in yield, biomass and their components between triticale and wheat grown under contrasting water and nitrogen environments. F. Crop. Res. 128, 167–179. doi: 10.1016/j.fcr.2012.01.003
Haley, S. D., Johnson, J. J., Peairs, F. B., Stromberger, J. A., Heaton, E. E., Seifert, S. A., et al. (2011). Registration of ‘Snowmass' wheat. J. Plant Regist. 5, 87–90. doi: 10.3198/jpr2010.03.0175crc
Haley, S. D., Johnson, J. J., Peairs, F. B., Stromberger, J. A., Hudson-Arns, E. E., Seifert, S. A., et al. (2018). Registration of ‘Avery' hard red winter wheat. J. Plant Regist. 12, 362–366. doi: 10.3198/jpr2017.11.0080crc
Harper, M. T., Oh, J., Giallongo, F., Roth, G. W., and Hristov, A. N. (2017). Inclusion of wheat and triticale silage in the diet of lactating dairy cows. J. Dairy Sci. 100, 6151–6163. doi: 10.3168/jds.2017-12553
Hokkanen, H. M. T., and Jokioinen, F. (1991). Trap cropping in pest management. Annu. Rev. Entomol. 36, 119–138. doi: 10.1146/annurev.en.36.010191.001003
Holden, M. H., Ellner, S. P., Lee, D. H., Nyrop, J. P., and Sanderson, J. P. (2012). Designing an effective trap cropping strategy: the effects of attraction, retention and plant spatial distribution. J. Appl. Ecol. 49, 715–722. doi: 10.1111/j.1365-2664.2012.02137.x
Holmes, N. D., and Peterson, L. K. (1965). Swathing wheat and survival of wheat stem sawfly. Can. J. Plant Sci. 45, 579–581. doi: 10.4141/cjps65-109
Khan, Z., Midega, C. A. O., Hooper, A., and Pickett, J. (2016). Push-pull: chemical ecology-based integrated pest management technology. J. Chem. Ecol. 42, 689–697. doi: 10.1007/s10886-016-0730-y
Kumar, A., and Venkatesan, C. (2019). Experimental Techniques in Host-Plant Resistance, 1st Edn. Singapore: Spring Nature Singapore Pte Ltd. doi: 10.1007/978-981-13-2652-3
Lenth, R. V. (2022). emmeans: Estimated Marginal Means, aka Least-Squares Means. R package version 1.7.2. Available online at: https://CRAN.R-project.org/package=emmeans
Morrill, W. L., Weaver, D. K., and Johnson, G. D. J. (2001). Trap strip and field border modification for management of the wheat stem sawfly (Hymenoptera: Cephidae). J. Entomol. Sci. 36, 34–45. doi: 10.18474/0749-8004-36.1.34
Nansen, C., Payton, M. E., Runyon, J. B., Weaver, D. K., Morrill, W. L., and Sing, S. E. (2005). Preharvest sampling plan for larvae of the wheat stem sawfly, Cephus cinctus (Hymenoptera: Cephidae), in winter wheat fields. Can. Entomol. 137, 602–614. doi: 10.4039/n04-087
Oettler, G. (2005). The fortune of a botanical curiosity - Triticale: past, present and future. J. Agric. Sci. 143, 329–346. doi: 10.1017/S0021859605005290
Pachl, C., Ott, R., and Meyer, B. (2020). Colorado Winter Wheat Varieties 2020 Crop. United States Department of Agriculture, Lakewood, United States. Available online at: https://www.nass.usda.gov/Statistics_by_State/Colorado/Publications/_Releases/2020/CO-Winter-Wheat-Varieties-02262020.pdf
Peairs, F. B., Rudolph, J. B., Randolph, T. L., and Cockrell, D. M. (2014). 2014 Colorado Field Crop Insect Management Research and Demonstration Trials. Colorado State University, Agricultural Experiment Station.
R Core Team. (2019). R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. Available online at: https://www.R-project.org/
Rand, T. A., Waters, D. K., Shanower, T. G., and Berzonsky, W. A. (2012). Effects of genotypic variation in stem solidity on parasitism of a grass-mining insect. Basic Appl. Ecol. 13, 250–259. doi: 10.1016/j.baae.2012.03.005
Randhawa, H. S., Bona, L., and Graf, R. J. (2015). Triticale Breeding—Progress and Prospect. Triticale. Springer. 281–317. doi: 10.1007/978-3-319-22551-7_2
Rueden, C. T., Schindelin, J., Hiner, M. C., DeZonia, B. E., Walter, A. E., Arena, E. T., et al. (2017). ImageJ2: ImageJ for the next generation of scientific image data. BMC Bioinformatics 18, 1–26. doi: 10.1186/s12859-017-1934-z
Sharma, A., Shrestha, G., and Reddy, G. V. P. (2019). Trap crops: how far we are from using them in cereal crops? Ann. Entomol. Soc. Am. 112, 330–339. doi: 10.1093/aesa/say047
Shelton, A. M., and Badenes-Perez, F. R. (2006). Concepts and applications of trap cropping in pest management. Annu. Rev. Entomol. 51, 285–308. doi: 10.1146/annurev.ento.51.110104.150959
Tiwari, S., Sharma, S., and Wratten, S. D. (2020). Flowering alyssum (Lobularia maritima) promote arthropod diversity and biological control of Myzus persicae. J. Asia. Pac. Entomol. 23, 634–640. doi: 10.1016/j.aspen.2020.05.002
Wallace, L. E., and McNeal, F. H. (1966). Stem sawflies of economic importance in grain crops in the United States. USDA Tech. Bull. 1350, 1–50.
Weaver, D. K., Buteler, M., Hofland, M. L., Runyon, J. B., Nansen, C., Talbert, L. E., et al. (2009). Cultivar preferences of ovipositing wheat stem sawflies as influenced by the amount of volatile attractant. J. Econ. Entomol. 102, 1009–1017. doi: 10.1603/029.102.0320
Weaver, D. K., Nansen, C., Runyon, J. B., Sing, S. E., and Morrill, W. L. (2005). Spatial distributions of Cephus cinctus Norton (Hymenoptera: Cephidae) and its braconid parasitoids in Montana wheat fields. Biol. Control 34, 1–11. doi: 10.1016/j.biocontrol.2005.04.001
Zadoks, J. C., Chang, T. T., and Konzak, C. F. (1974). A decimal code for the growth stages of cereals. Weed Res. 14, 415–421. doi: 10.1111/j.1365-3180.1974.tb01084.x
Keywords: wheat, wheat stem sawfly, triticale, trap crop, integrated pest management (IPM), host preference
Citation: Peirce ES, Cockrell DM, Ode PJ, Peairs FB and Nachappa P (2022) Triticale as a Potential Trap Crop for the Wheat Stem Sawfly (Hymenoptera: Cephidae) in Winter Wheat. Front. Agron. 4:779013. doi: 10.3389/fagro.2022.779013
Received: 17 September 2021; Accepted: 23 March 2022;
Published: 18 April 2022.
Edited by:
Charles Midega, International Centre of Insect Physiology and Ecology (ICIPE), KenyaReviewed by:
Jozsef Kiss, Szent István University, HungaryBrian L. Beres, Agriculture and Agri-Food Canada, Canada
Copyright © 2022 Peirce, Cockrell, Ode, Peairs and Nachappa. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Erika S. Peirce, ZXJpa2EucGVpcmNlQGNvbG9zdGF0ZS5lZHU=
 Erika S. Peirce
Erika S. Peirce Darren M. Cockrell1
Darren M. Cockrell1 Paul J. Ode
Paul J. Ode Punya Nachappa
Punya Nachappa