- 1CAB International (CABI), Nairobi, Kenya
- 2Department of Crop Inspection and Certification, Ministry of Agriculture, Animal Industry and Fisheries, Entebbe, Uganda
- 3Crop and Animal Production, Faculty of Agriculture and Environmental Sciences, Mountains of the Moon University, Fort Portal, Uganda
- 4College of Agricultural & Environmental Sciences, Makerere University, Kampala, Uganda
- 5Department of Crop Sciences, Faculty of Agriculture and Animal Sciences, Busitema University, Tororo, Uganda
- 6Alliance of Bioversity International and The International Center for Tropical Agriculture (CIAT), c/o National Agricultural Research Laboratories, Kampala, Uganda
- 7Department of Crop Protection, Ministry of Agriculture, Animal Industry and Fisheries, Entebbe, Uganda
- 8Crops and Natural Resources, Ngetta Zonal Agricultural Research and Development Institute, Lira, Uganda
- 9Department of Crop Science and Production, Kabale University, Kabale, Uganda
- 10Horticulture and Oil palm Program, National Crops Resources Research Institute, Kampala, Uganda
- 11Phytosanitary and Biosecurity, Kenya Plant Health Inspectorate Service (KEPHIS), Nairobi, Kenya
- 12Faculty of Agriculture, Kyambogo University, Kampala, Uganda
- 13College of Natural Sciences, Makerere University, Kampala, Uganda
In recent years, various invasive species have been introduced to sub-Saharan Africa, partly due to insufficient information about potential invasions, which has led countries to respond reactively rather than proactively. This information can be gathered through horizon scanning. Using the CABI Horizon Scanning Tool, 9,071 pest species have been identified as unreported in Uganda. A subset of 1,517 was prioritised for rapid risk assessment based on guidelines that evaluated the likelihood of entry and establishment, the magnitude of socio-economic and environmental impact, as well as potential pathways of introduction. These pest species included 357 arthropods, 130 bacteria, 74 chromista, 417 fungi, 19 molluscs, 124 nematodes, nine protists, and 387 viruses and viroids, of which 360 of the 1,517 were reported as invasive. Vectors and vectored organisms were also assessed to determine their associated risk. Management actions were recommended for 618 species, which included 160 arthropods, 70 bacteria, 30 chromista, 174 fungi, six molluscs, 41 nematodes, three protists, eight viroids, and 126 viruses. These pest species either attained an overall risk score of 54 and above or a lower score for specific pest species. The actions included targeted surveillance, regulation supported by pest risk analysis, contingency planning, publicity, management by the industry, and research. This information is vital for risk monitoring and management and can be utilised by countries in the East African Region.
Introduction
The agricultural sector is vital to the economic development of many countries in sub-Saharan Africa (SSA), contributing an average of 25% to the Gross Domestic Product and supporting over 80% of rural populations (Osabohien et al., 2019). In Uganda, this sector, which is overseen by the Ministry of Agriculture, Animal Industry and Fisheries (MAAIF), accounts for approximately 24% of GDP, 35% of export earnings, and employs about 68% of the labour force. This sector comprises various sub-sectors, including crops, fish, forestry, livestock, and poultry (MAAIF, 2025). The main forestry trees include eucalyptus (Eucalyptus spp.), Musizi (Maesopsis eminii), Mvule (Milicia excelsa), pines (Pinus spp.), and the umbrella tree (Maesopsis eminii) (Katende et al., 1995; Sseremba et al., 2011). The 2024 national population and housing census indicated that 61% of the households in the agricultural sector engaged in crop production, 37% in livestock production, 16% in tree production, and 11% in aquaculture (UBOS, 2024).
The Agricultural Sector Strategic Plan (ASSP) developed by the MAAIF prioritised only 12 commodities, including crops such as banana (Musa spp.), cassava (Manihot esculenta), cocoa (Theobroma cacao), coffee (Coffea arabica and Coffea canephora), common bean (Phaseolus vulgaris), cotton (Gossypium hirsutum), maize (Zea mays), oil palm (Elaeis guineensis), potatoes (Solanum tuberosum), rice (Oryza sativa), tea (Camellia sinensis), oil seeds, fruits and vegetables, as well as dairy, fish, and livestock (meat) (MAAIF, 2025). However, the strategic plan has prioritised four commodities, including cocoa, cotton, oil seeds, and oil palm. The oil seeds include groundnuts (Arachis hypogaea), sesame (Sesamum indicum), soybean (Glycine max), and sunflower (Helianthus annuus) (MAAIF, 2025). Coffee is Uganda’s leading export crop, followed by cotton and tea. The 2024 national population and housing census indicated that coffee was produced by 1.7 M smallholders and constituted 22% of the total products exported and earned Uganda USD 1.4 bn, while 250,000 households produced cotton, earning Uganda USD 13.7 M in raw cotton exports (UBOS, 2024).
However, the sector is at a crossroads due to land degradation, largely driven by human-mediated activities, declining soil fertility resulting from poor farming practices, and the scourge of pests1 (Bekunda et al., 2010; Nkonya et al., 2016; Stewart et al., 2020; Slayi et al., 2024). Recent decades have witnessed increased outbreaks of endemic pests linked to a warming planet and indiscriminate use of pesticides, as well as heightened invasions by transboundary pests, particularly locusts, and new incursions of non-native pest species that have become invasive2 (De Groote et al., 2020; Prasanna et al., 2022).
Invasive pest species reported in SSA in the recent past include maize chlorotic mottle virus (MCMoV) (Wangai et al., 2012), Fusarium oxysporum f.sp. cubense Tropical Race 4 (FoC TR4) (Butler, 2013), tomato leaf miner (Pthorimaea absoluta) (Guimapi et al., 2016), papaya mealybug (Paracoccus marginatus) (Macharia et al., 2017), fall armyworm (Spodoptera frugiperda) (De Groote et al., 2020), potato cyst nematodes (Globodera rostochiensis and Globodera pallida) (Mburu et al., 2020), golden apple snail (Pomacea canaliculata) (Buddie et al., 2021), and avocado sunblotch viroid (ASBVd) (Kibwage et al., 2023). The maize lethal necrosis disease condition, first reported in East Africa in 2013, is caused by MCMoV when it infects cereals, especially maize, with any of the Johnsongrass mosaic virus, maize dwarf mosaic virus, sugarcane mosaic virus, or wheat streak mosaic virus (Louie, 1980; Adams et al., 2013; Stewart et al., 2017).
The impact of invasive pests on biodiversity, economies, and livelihoods, as well as the consequences of their management, is well documented. For instance, their effects on biodiversity and the production of unsafe food, partly blamed on the indiscriminate and excessive use of pesticides (Aktar et al., 2009; Al-Zyoud, 2014; Md Meftaul et al., 2020); the loss of trade opportunities because some invasive species are classified as A13 quarantine pests (Papadopoulos et al., 2024); and the reduction in profit margins due to increased management costs which has a direct effect on rising food prices and loss of jobs along the agricultural value chain (McAusland and Costello, 2004; Bradshaw et al., 2024). Unlike endemic pests, for which management options are available, and good farming practices can be enforced to limit new lethal outbreaks, prevention remains the most cost-effective management strategy for invasive pests (Mack et al., 2000; Saul et al., 2017; Venette et al., 2021).
However, with porous borders and weak border biosecurity systems in most countries in SSA, prevention is often limited or impossible (Obah-Akpowoghaha et al., 2020; Dorjee et al., 2021). This situation is exacerbated by a lack of information regarding potential invasions and the limited capacity to prevent them, which confines these countries to reactive strategies only (Mulema et al., 2022). The information gap can be bridged through horizon scanning to enable risk identification and prioritisation, followed by enhancing capacity in risk monitoring and management to tackle the challenges associated with preventing invasions (Sutherland et al., 2008; Kendig et al., 2022; Kenis et al., 2022). Horizon scanning involves the systematic search for potential biological invasions and assessing their possible impacts on the economy, society, and environment, considering potential opportunities for mitigating these effects (Sutherland et al., 2008, 2010, 2020; Roy et al., 2014).
Horizon scanning has been utilised to prioritise pests that may require further action to prevent their introduction or spread once they have been introduced at the country and regional levels (Roy et al., 2014; Gallardo et al., 2016; Bayón and Vilà, 2019; Kenis et al., 2022). Consequently, a horizon scanning study was conducted in Uganda to prioritise non-native pests that have not yet been reported but could be introduced and become invasive, thus threatening agriculture, biodiversity, forestry, the economy, and ultimately livelihoods (Mack et al., 2000; Day et al., 2017; Pratt et al., 2017; Adams et al., 2018; Graziosi et al., 2020; Greenfield, 2020). Previously, horizon scanning relied on extensive desk research by a panel of experts to gather information about invasive species, followed by risk assessment (Bayón and Vilà, 2019; Seymour et al., 2020; Dawson et al., 2023; Lieurance et al., 2023). However, CABI’s Horizon Scanning Tool was used in this study. The Horizon Scanning Tool uses information in datasheets available in the CABI Compendium to identify pests that are not yet known to occur in the country at risk. The Tool has been used to identify and prioritise invasive pests for which preventive action is required in Ghana, Kenya, the United States of America, and Zambia (Kendig et al., 2022; Kenis et al., 2022; Mulema et al., 2022, 2024; Lieurance et al., 2023).
Materials and methods
Assessment team
A team of 29 Subject Matter Experts (SMEs) convened in March 2024 in Fort Portal, Uganda, to conduct a horizon scanning exercise. They were drawn from academia (including the Universities of Busitema, Kabale, Kyambogo, Makerere, and the Mountains of the Moon); the National Research System (which comprises institutions under the National Agricultural Research Organisation, NARO); a department within the Ministry of Agriculture, Animal Industry and Fisheries (Department of Crop Protection); a regulatory agency (National Plant Protection Organisation (NPPO) of Uganda, the Department of Crop Inspection and Certification, DCIC); and international research organisations (the Alliance of Bioversity International and CIAT and CABI). The SMEs represented various disciplines, including bacteriology, entomology, mycology, nematology, and virology and demonstrated substantial experience in policy and phytosanitary matters.
Identification of non-native plant pests
Utilising the premium version of the Horizon Scanning Tool, a preliminary selection of plant pests not yet reported in Uganda was conducted. The parameters set in the tool included identifying the area at risk (Uganda) and selecting regions from which non-native pests are likely to be introduced (source areas). The source areas included all geographical regions in Africa, Asia, Europe, North America, Oceania, and South America. All parameters except for the type of organism were left open. The pest organisms considered included arthropods, bacteria (including phytoplasma), chromista, fungi, molluscs, protists, plant parasitic nematodes, and viruses (including viroids). Following the scan, a list was downloaded in Excel (.xlsx) format for further analysis.
Prioritisation of non-native plant pests for rapid risk assessment
The initial list was reviewed to eliminate pests represented only by their genera. Three criteria were then employed to prioritise pests for rapid risk assessment. First, the pest had to infect crops and trees cultivated in the area at risk (Uganda). Second, it had to be a vector and also on the horizon scanning list. Third, while invasiveness was not considered for bacteria, chromista, fungi, molluscs, nematodes, or protists, it was a criterion for selecting the arthropods due to their sheer numbers. Thus, invasive arthropods that affect the host (value chains) were thus prioritised. Following the assessment of bacteria, fungi, nematodes, phytoplasma, viroids, and viruses, arthropod vectors that are part of the horizon scanning output were incorporated into the list of already prioritised arthropods. Throughout the prioritisation, the SMEs also excluded pests known to occur in Uganda. The final list was subjected to a rapid risk assessment.
Description of the scoring system for rapid risk assessment
The risk-scoring guidelines employed were based on the descriptions provided by Roy et al. (2019) but have been modified in the works of Kenis et al., 2022 and Mulema et al., 2022. Roy et al. (2019) evaluated the likelihood of introduction (entry), establishment, spread, and the potential negative impact on biodiversity and ecosystem services. However, this study assessed the likelihood of introduction (entry), establishment (including aspects of spread), and potential socio-economic and environmental (biodiversity) impacts. A five-point scoring system detailed in Supplementary File S1 was utilised for the four parameters (entry, establishment, socio-economic, and biodiversity impact).
Likelihood of entry
A score of one indicated that the organism was absent from Africa and unlikely to be found in an imported commodity; two indicated absence from Africa but likely to be infrequently imported on a commodity; three was ascribed to three scenarios, namely present in Africa (not in countries neighbouring Uganda) and spread slowly; or absent from Africa but shown to spread rapidly across several continents, or often associated to a commodity commonly imported, or frequently intercepted in Uganda; four indicated presence in Africa (not in neighbouring countries) and rapid spread or presence in a neighbouring country with slow spread; and five indicated presence in a neighbouring country (Democratic Republic of the Congo (DR Congo), Kenya, Rwanda, South Sudan, and Tanzania) with rapid spread.
Pathway for introduction
An alien species may enter a new geographical or political region through three primary mechanisms that comprise six pathways. A pathway refers to any means that facilitates the entry and spread of a pest (IPPC Secretariat, 2021). The three mechanisms include the importation of a commodity (three pathways: contaminant, escape, and release), the arrival of a transport vector (one pathway: stowaway), and the natural spread from a neighbouring region (two pathways: corridor and unaided) (Hulme et al., 2008). However, for this study, only three pathways were considered: contaminant (CO), stowaway (ST), and unaided (UN).
Likelihood of establishment
A score of one indicated that Uganda was climatically unsuitable, or that host plants were not present; two meant that only a few areas in Uganda were climatically suitable, or that host plants were rare; three indicated that large regions of Uganda were climatically suitable, but host plants were rare; or that only a few areas in Uganda were climatically suitable, yet the host plants were at least moderately abundant; four signified that large regions of Uganda were climatically suitable, but the host plants were moderately abundant; and five denoted that large areas in Uganda were climatically suitable, but the host plants were very abundant.
Assessment of potential socio-economic impact
A score of one indicated that the alien species do not attack plants that are cultivated or utilised; two, that the alien species damage plants that are only occasionally cultivated or utilised; three, that the alien species damage plants that are regularly cultivated or utilised but without threatening the cultivation, utilisation, or trade of this crop; four, that the alien species has the potential to threaten, at least locally, the cultivation of a plant that is regularly cultivated or utilised, or to periodically attack a crop that is key to the economy of Uganda without threatening the latter; and five, that the alien species has the potential to threaten, at least locally, a crop that is key to the economy of Uganda.
Assessment of the potential impact on biodiversity
A score of one indicated that the alien species would not affect any native species; two, that the alien species would affect individuals of a native species without affecting its population level; three, that the alien species has the potential to lower the population levels of a native species; four, that the alien species has the potential to eradicate a native species locally or to affect populations of a protected or keystone species; and five, that the alien species has the potential to eliminate a native species or to eradicate a keystone species locally. Native species refers to species within their natural range, including shifts in range occurring without human intervention and includes animals, plants or any other organisms, including pathogens.
Scoring of the prioritised species
The SMEs were grouped according to their specialities, forming five groups, each with a minimum of three members. They included Group One (arthropods), Group Two (bacteriology, covering bacteria, protists, and phytoplasma), Group Three (mycology, encompassing chromista and fungi), Group Four (nematology, which also assessed molluscs), and Group Five (virology, addressing viruses and viroids). Due to the large number of arthropods requiring assessment, two subgroups were created. The scores for each species attribute were discussed comprehensively, and any discrepancies were resolved until a consensus was reached prior to confirming the final score. As detailed in Blackburn et al. (2014), a low, medium, and high confidence rating was indicated for each of the four scored attributes, the likely pathway of arrival, and the overall risk score. Appropriate groups assessed vectors and vectored organisms.
Determining risk
Risk is a function of the probability (likelihood) of an event occurring and the impact tied to that probability. Consequently, the overall risk score was derived from the following formula:
Likelihood of entry x likelihood of establishment x (magnitude of socio-economic impact + magnitude of impact on biodiversity)
The highest expected overall risk score was 250, while the lowest stood at two.
Actions for management
An overall score of 54 was considered the cut-off for recommending an appropriate management action. This score was chosen as it is only achievable for species with a score of three in all assessable attributes or more than three in at least three parameters. A score of three or higher indicated a situation with an increased likelihood of entry, establishment, and greater impact (socio-economic or biodiversity). Scores below three were regarded as low risk since they signified a reduced likelihood of entry, establishment, and impact; a score of three was viewed as moderate, whereas scores above three (four and five) suggested a heightened likelihood of entry, establishment, and impact (as opposed to the lower scores of one and two). A no-action recommendation was made for species that recorded an overall risk score below 54. In contrast, a management action was advised for all pests that attained an overall risk score above 54. These actions included detection surveillance for pests reported in a neighbouring country to establish pest status in Uganda, regulation supported by Pest Risk Analysis (PRA4), contingency planning for preparedness, raising awareness, industry initiatives to prevent pest introduction and management after introduction, and research.
Detection surveillance was also considered if the assessment team confirmed a previous interception or for a pest affecting a host commodity frequently imported from a country where the pest is endemic. Pest Risk Analysis is key in providing guidelines to manage pathways and limit the introduction of quarantine pests. However, in certain special cases, action was considered for some species that recorded an overall risk score below 54. These included species currently regarded by trading partners as high-risk A1 or A25 quarantine pests, such as the subspecies of Xylella fastidiosa. A no-action recommendation was also suggested for some species that recorded an overall risk score above 54. This applied to species for which the main pathway of introduction was deemed implausible. This category comprised pests transmitted by vectors rather than through seed (seed-borne6 or seed-transmitted7), yet the vectors were unlikely to be introduced with a viable pathogenic organism. Others in this category included soil-borne pests that were not likely to be associated with the commodity. A good example would be soil-borne fungi that are not seed-transmitted. Furthermore, a no-action recommendation was also suggested when insufficient information was available for the assessment of a particular species or for vectors of assessed species and pests vectored by assessed species already reported in Uganda.
Results
Generation of the list for assessment
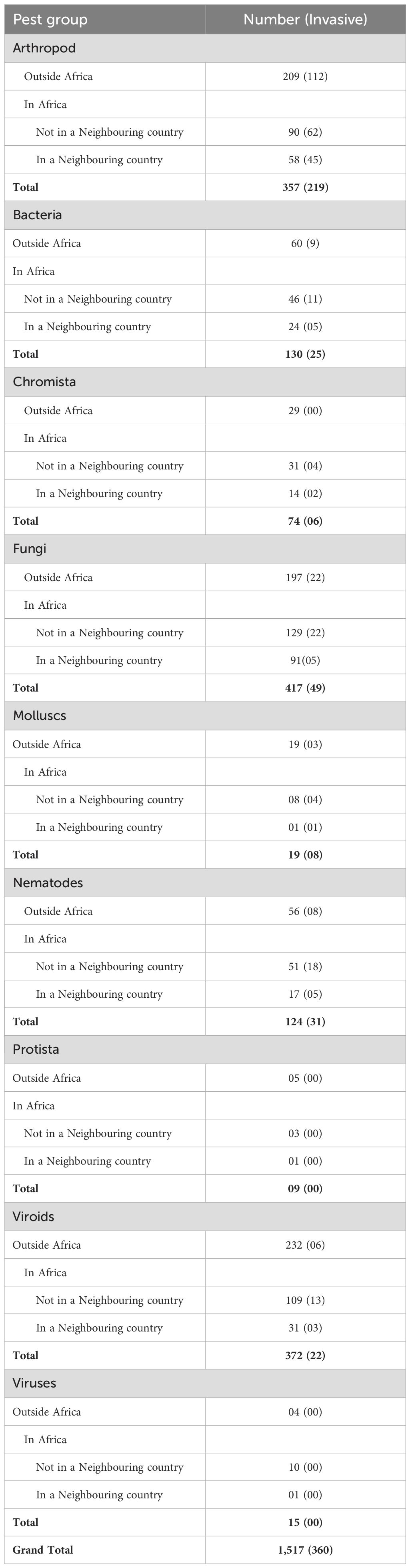
The global horizon scan identified 9,071 species not yet reported as present in Uganda. These species comprised 5,866 arthropods, 321 bacteria, 167 chromista, 1,684 fungi, 33 molluscs, 270 nematodes, 12 protists, and 718 viruses and viroids. The initial output from the Horizon Scanning Tool was subsequently filtered to include only species with complete names, resulting in 8,398 species that comprised 5,551 arthropods, 284 bacteria, 160 chromista, 1,569 fungi, 26 molluscs, 183 nematodes, 10 protists, and 645 viruses and viroids. The species reported as invasive and affecting crops and trees grown in Uganda were selected for a further detailed rapid risk assessment, resulting in a total of 1,517 species, of which 360 (24%) had been documented as invasive. These 1,517 species comprised 357 arthropods (Supplementary File S2), 130 bacteria (Supplementary File S3), 74 chromista (Supplementary File S4), 417 fungi (Supplementary File S5), 19 molluscs (Supplementary File S6), 124 nematodes (Supplementary File S7), nine protists (Supplementary File S8), and 387 viruses and viroids (Supplementary File S9). The 357 arthropods comprised 219 invasive species and 138 vectors that were part of the horizon scanning output. All vectors (arthropods, fungi, nematodes, plants and protists) and vectored species (bacteria, fungi, nematodes, protists, viruses and viroids) were subjected to a rapid risk assessment (Supplementary File S10). All data has been consolidated in Supplementary File S11.
Assessment of arthropods
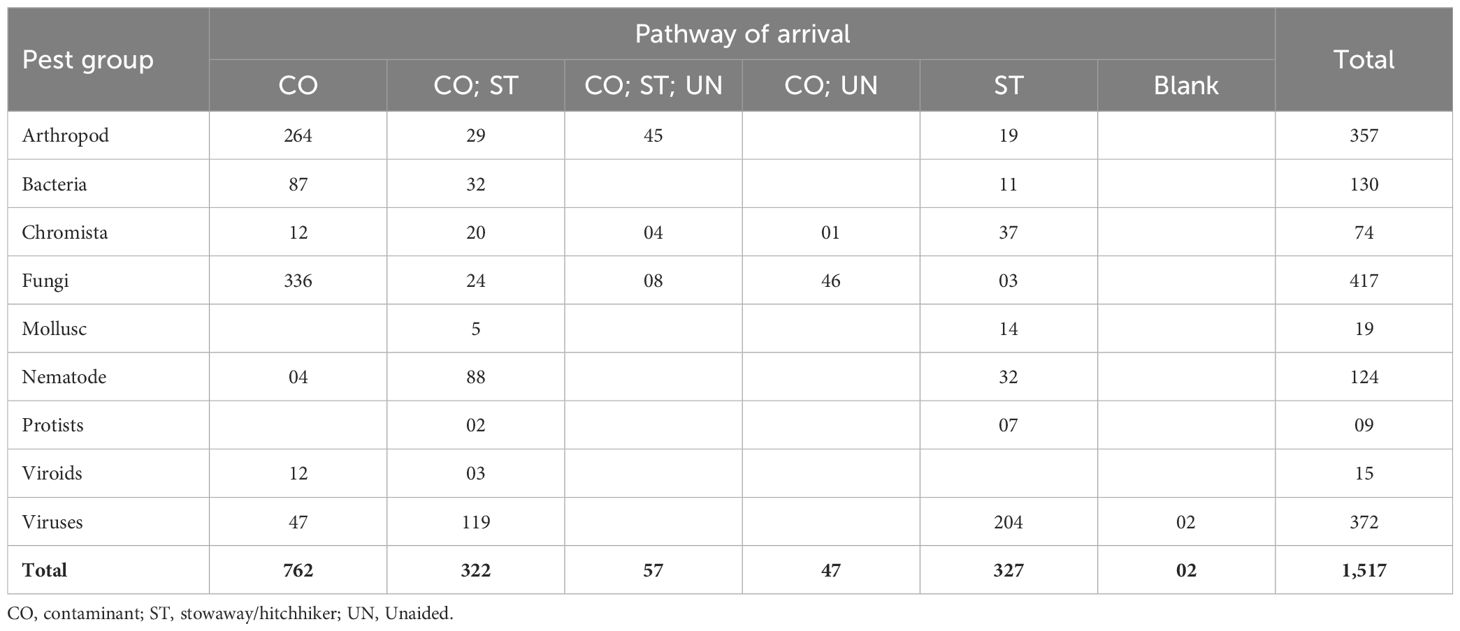
The 357 arthropods prioritised for rapid risk assessment included 219 species (62%) documented as invasive. Of the 357 species, 148 (42%) had been reported in Africa, a proportion of which (38%; n=58 of 147) was present in a neighbouring country (Table 1). Most arthropods were likely to be introduced exclusively as contaminants (74%, n=264 of 357), while the least (5%, n=19 of 357) could be introduced solely as stowaways. The remaining arthropods (21%; n=73 of 357) could be introduced through more than one pathway, of which 38% (n=28 of 73) could be introduced as contaminants or stowaways and 62% (n=45) as contaminants, stowaways or unaided (Table 2). The highest overall risk score was 175 recorded for 11 species, which included Aphis spiraecola, Metopolophium dirhodum, Metopolophium festucae, Prays citri, Pseudaulacaspis pentagona, Rhopalosiphum padi, Rhyzopertha dominica, Thrips palmi, Trialeurodes vaporariorum, Xylosandrus crassiusculus, and Xylosandrus morigerus while the lowest was two recorded for Myzus ornatus, Nearctaphis bakeri. The 351 assessed species also comprised 156 (44%) that vectored other assessed species. All data from this assessment is presented in Supplementary File S2.
Assessment of bacteria
The 130 species of bacteria prioritised for rapid risk assessment included 25 species (19%) documented as invasive. A proportion of 54% (n=70 of 130) had been reported in Africa, of which 24 species (34%) were reported as present in a neighbouring country (Table 2). Most assessed bacteria were likely to be introduced exclusively as contaminants (67%, n=87 of 130), while the least (9%, n=11 of 130) could be introduced solely as stowaways. The remaining 25% (n=32 of 130) could be introduced through multiple pathways, specifically contaminants or stowaways (Table 2). The highest overall risk score was 175 recorded for Xanthomonas vasicola pv. vasculorum, while the lowest was eight recorded for four species, including Strawberry lethal decline phytoplasma; Strawberry witches’ broom phytoplasma, Xanthomonas translucens pv. arrhenatheri, and Xanthomonas translucens pv. graminis. Twenty-seven (21%) of the assessed species were vectored, all of which were phytoplasmas, except for Dickeya zeae, Pantoea ananatis, Pantoea stewartii subsp. stewartii, Spiroplasma citri, Spiroplasma kunkelii, Xylella fastidiosa subsp. fastidiosa (xff), Xylella fastidiosa subsp. multiplex (xfm), and Xylella fastidiosa subsp. pauca (xfp). Xylella fastidioda has the most known and potential vectors. All data from this assessment is presented in Supplementary File S3.
Assessment of chromista
The 74 assessed Chromista (oomycetes) comprised six species documented as invasive. Most (61%, n=45 of 74) of the species had been reported in Africa, 14 of which (31%, n=14 of 45) had been reported in a neighbouring country (Table 1). Half (50%, n=37 of 74) of the species were likely to be introduced exclusively as stowaways, while 16% (n=12 of 74) as contaminants. Others (34%; n=25 of 74) could be introduced through multiple pathways; for instance, 20 as contaminants or stowaways; one (Pythium aphanidermatum) as a contaminant or unaided; and four (Peronospora farinosa f.sp. betae, Phytophthora hibernalis, Phytophthora phaseoli and Trachysphaera fructigena) through all three pathways (contaminant, stowaway, and unaided) (Table 2). The highest overall risk score was 125, recorded for Phytophthora nicotianae and Pythium aphanidermatum, while the lowest was six, recorded for seven species, including Aphanomyces raphani, Peronospora dianthicola, Phytophthora gloverana, Phytophthora pisi, Pythium mamillatum, Pythium sulcatum, and Pythium uncinulatum. All data from this assessment is presented in Supplementary File S4.
Assessment of fungi
The 417 fungi prioritised for assessment included 49 (12%) documented as invasive. More than half (53%, n=220) had been reported in Africa, with 41% (n=91 of 220) reported in a neighbouring country (Table 1). Eight in every ten (81%, n=336 of 417) of the assessed species could be introduced solely as contaminants, whereas approximately one in every ten (1%, n=3 of 417) could be introduced as stowaways. The remaining assessed species could be introduced through multiple pathways. For instance, 6% (n=24 of 417) could be introduced as either contaminants or stowaways, 11% (n=46 of 417) as contaminants or unaided, and 2% (n=8 of 417) through all three pathways (contaminants, stowaways, and unaided) (Table 2). The highest overall risk score was 125, noted for 17% (n=69 of 417) of the species, while the lowest score was 25, recorded for 28% (n=118 of 417) of the species. All data from this assessment is presented in Supplementary File S5.
Assessment of molluscs
The 19 molluscs prioritised for assessment included eight species documented as invasive, representing 42%. Nine (47%) of the assessed molluscs had been reported in Africa, but only one (P. canaliculata) had been reported in a neighbouring country (Kenya) (Table 1). Species documented in Africa included Arion hortensis, Bradybaena similaris, Cornu aspersum, Deroceras laeve, Deroceras reticulatum, Lehmannia valentiana, Limax maximus, Pomacea canaliculata, and Rumina decollata. Fourteen (74%) of the assessed molluscs could be introduced exclusively as stowaways, while the remaining five (26%) could be introduced as contaminants or stowaways (Table 2). The highest overall risk score was 96, recorded for P. canaliculata, whereas the lowest was eight, recorded for Arion ater. All data from this assessment is presented in Supplementary File S6.
Assessment of nematodes
The 124 prioritised parasitic plant nematodes included 31 (25%) species documented as invasive. Sixty-eight species, representing 58%, had been reported in Africa (Table 1). One-quarter (25%; n=17 of 68) of the species had been reported in a neighbouring country (Table 1). Four (3%) of the species, including Achlysiella williamsi, Anguina tritici, Aphasmatylenchus straturatus, and Ditylenchus gigas, could be introduced exclusively as contaminants, 32 (26%) exclusively as stowaways, while the majority (71%; n=88 of 124) either as contaminants or stowaways) (Table 2). The highest overall risk score was 150, recorded for Meloidogyne enterolobii, while the lowest was six, recorded for Rotylenchus paravitis. Four of the nematodes in this category could be vectored and included Bursaphelenchus fungivorus, Bursaphelenchus mucronatus, Bursaphelenchus xylophilus, and Rhadinaphelenchus cocophilus. Thirteen of the assessed nematodes, representing 10%, are known vectors for bacteria and viruses. These include Anguina agrostis, A. tritici, Longidorus elongatus, Paratrichodorus minor, Paratrichodorus pachydermus, Paratrichodorus porosus, Trichodorus primitivus, Trichodorus similis, Trichodorus viruliferus, Xiphinema americanum, Xiphinema diversicaudatum, Xiphinema rivesi, and Zygotylenchus guevarai. All data from this assessment is presented in Supplementary File S7.
Assessment of protists
The nine protists prioritised for assessment included four species reported in Africa, with only one (Spongospora subterranea) documented in a neighbouring country (Table 1). The other three species reported in Africa were Plasmodiophora brassicae, Polymyxa betae, and Polymyxa graminis. Seven (78%) protists could be introduced exclusively as stowaways, while the remaining two (22%), Ligniera vasculorum and Spongospora subterranea could enter through two pathways (contaminants or stowaways) (Table 2). The highest overall risk score was 90, attributed to Polymyxa graminis, while the lowest was 15, assigned to three species, Physarum cinereum, Phytomonas leptovasorum, and Phytomonas staheli. All data from this assessment is presented in Supplementary File S8.
Assessment of viruses and viroids
The 387 virus and viroid species prioritised for assessment included 15 (4%) viroids and 372 (96%) viruses (Table 1). Among these, 22 had been reported as invasive, all being viruses (Table 1). A proportion of 73% (n=11 of 15) of the viroids and 38% (n=140 of 372) of the viruses had been reported in Africa. Of the 11 viroids documented in Africa, one (avocado sunblotch viroid) was reported in a neighbouring country (Kenya) (Kibwage et al., 2023), while the 140 viruses included 31 reported in a neighbouring country (Table 1). The majority (80%; n=12 of 15) of viroids were likely to be introduced as contaminants, whereas the least (20%; n=3 of 15) were likely to be introduced as contaminants or stowaways (Table 2). For viruses, the majority (55%; n=204 of 372) of viruses were likely to be exclusively introduced as stowaways, while the least was likely to be exclusively introduced as contaminants (Table 2). A proportion of 32% (n=119 of 372) were likely to be introduced either as contaminants or stowaways (Table 2). For two of the viruses, Desmodium yellow mottle virus and pepper chlorotic spot virus, the main mechanism of spread could not be determined from the available information. The highest overall risk score for viroids was 120, recorded for tomato apical stunt viroid, while the lowest was 30, recorded for tomato chlorotic dwarf viroid. The highest overall risk score for viruses was 175, recorded for barley yellow dwarf virus, while the lowest was six, scored for 24 viruses. All data from this assessment is presented in Supplementary File S9.
Assessment of vectored organisms
The total number of vectored species was 174, which included 26 bacteria, four fungi, three nematodes, one protist, 139 viruses, and one viroid. Eleven species were not assessed as they had already been reported as present in Uganda. These included Candidatus Phytoplasma aurantifolia, Ceratocystis fimbriata, citrus tristeza virus, cowpea aphid-borne mosaic virus, cucumber mosaic virus, Impatiens necrotic spot virus, maize chlorotic mottle virus, potato spindle tuber viroid, potato virus X, Ralstonia solanacearum, and tomato spotted wilt virus. The 163 species that were assessed comprised 24 bacteria, three fungi, three nematodes, one protist, and 132 viruses. Of the 163 species, 33% (n=54) were reported in Africa, with 32% (n=17 of 54) reported in a neighbouring country.
The arthropods vectored the majority of the pathogenic organisms. However, additional vectors included nematodes from the genera Longidorus, Paratrichodorus, Trichodorus, and Xiphinema; fungi from the Euwallacea genus; and protists in the genera Polymyxa and Spongospora. The highest overall risk score was 150, recorded for nine species, which included alfalfa mosaic virus, barley yellow dwarf viruses, Candidatus Liberibacter solanacearum, Candidatus Phytoplasma asteris, maize dwarf mosaic virus, Pectobacterium carotovorum, potato virus Y, Streptomyces scabiei, and tomato chlorosis virus. The lowest score was six recorded for nine species, which included broad bean true mosaic virus, lucerne transient streak virus, parsnip mosaic virus, rubus yellow net virus, ryegrass mosaic virus, strawberry latent C virus, strawberry mottle virus, tulip breaking virus, and wheat yellow leaf virus. All data from this assessment is presented in the relevant sheet in Supplementary File S10.
Assessment of vector species
The identified vector species totalled 323; however, 35 species were eliminated as they are reportedly present in Uganda, leaving only 288 for assessment. Of the 288 species, 28% (n=80) had been reported in Africa, with 29% (n=23 of 80) reported in a neighbouring country. The 288 species comprised 265 (92%) arthropods, three fungi, four plants, and three protists. The fungi belonged to the genus Olpidium (O. bornovanus, O. brassicae, and O. radicale); nematodes were from the genera Longidorus, Paratrichodorus, Trichodorus, and Xiphinema; the protists from Polymyxa (P. betae and P. graminis) and Spongospora (S. subterranea); and the plants were from Cuscuta (C. ceanothi, C. europaea, C. reflexa, and C. subinclusa). The highest overall risk score was 175 recorded for six species, which included Aphis spiraecola, M. dirhodum, Metopolophium festucae, R. padi, T. palmi, and Trialeurodes vaporariorum. The lowest overall risk score was two recorded for 21 species. Most of the arthropods were Hemiptera, specifically from the family Cicadellidae, followed by the Aphididae. All data from this assessment is presented in the relevant sheet in Supplementary File S10.
Actions for management
No-action
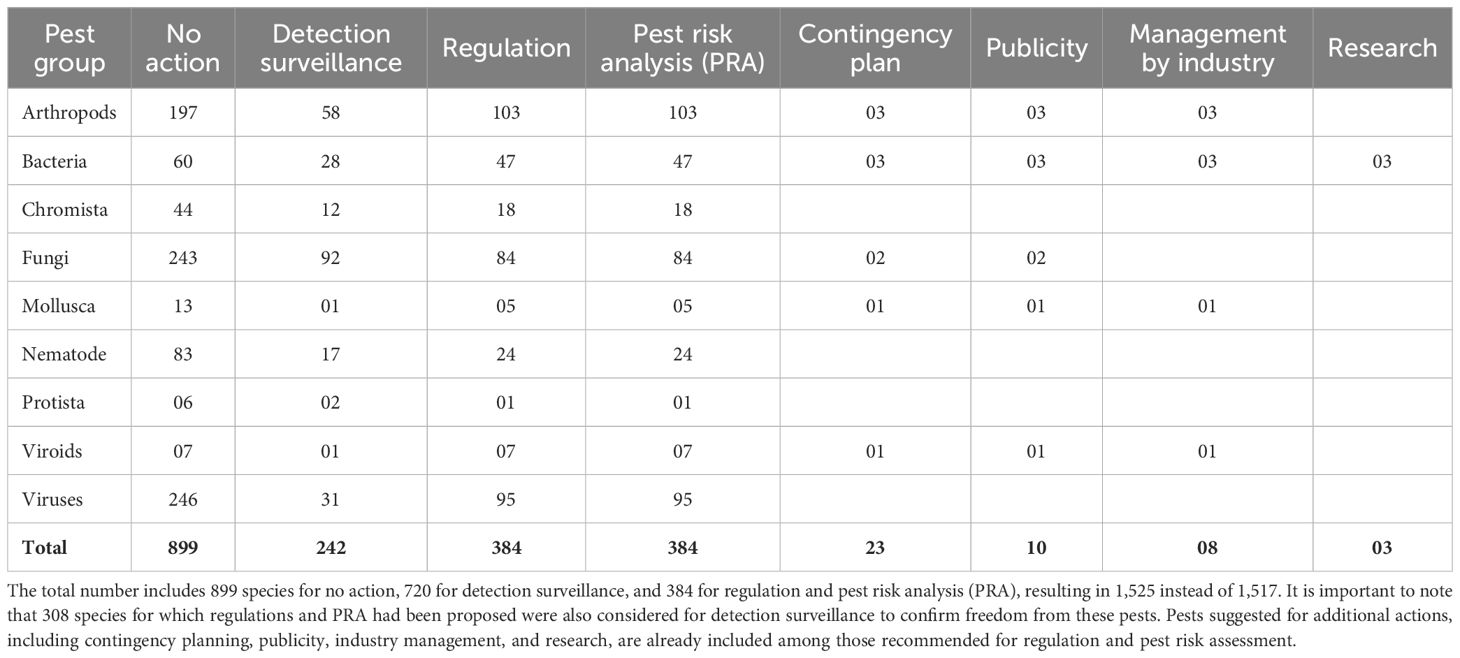
Of the 1,517 assessed species, 59% (n=899) were not considered for any action at this time, although the risk associated with certain pests could be monitored (Table 3). This category included 874 pest species, which represents 97% (N=899) that recorded an overall risk score below 54. The remaining 3% (n=25 of 899) recorded an overall risk score above 54. A no-action was recommended for 61% (n=107 of 174) of the assessed vectored species. These 107 species included 12 reported in Uganda and one species (Angiostrongylus cantonensis) known to affect humans but not plants. The 12 species are Candidatus Phytoplasma aurantifolia, Ralstonia solanacearum, Ceratocystis fimbriata, citrus tristeza virus, cowpea aphid-borne mosaic virus, cucumber mosaic virus, Impatiens necrotic spot virus, maize chlorotic mottle virus, potato spindle tuber viroid, potato virus S, potato virus X, and tomato spotted wilt virus. All data from this assessment is presented in Supplementary File S11. A no-action was similarly suggested for 72% (n=231 of 323) of the assessed vector species. They included 35 species known to occur in Uganda. All data from this assessment is presented in Supplementary File S11.
Detection surveillance
For the remaining 618 species (41%; N=1,517) requiring action, detection surveillance was recommended for 39% (n=242) of these species. Among these 242 species, 2% (n=6) were not reported as present in Africa, while 98% (n=236) were reported as present. Of the 236 species, 8 (3%) were not reported in a neighbouring country, whereas 228 (97%) were reported in a neighbouring country. The six species include Dickeya oryzae, Ips acuminatus, Pectobacterium parvum, Pectobacterium peruviense, Pectobacterium polaris, and Pectobacterium punjabense, while the eight species were Bemisia tabaci (MEAM1), FoC TR4, Magnaporthe oryzae Triticum pathotype, Oligonychus perseae, Pectobacterium parmentieri, Plasmodiophora brassicae, Sogatella furcifera, and T. palmi. Of the 67 of 174 species (39%) for which an action was suggested for the vectored species, detection surveillance was advised for 18 species (Supplementary File S11). Additionally, for 92 of 323 species for which an action was suggested for the vector species, detection surveillance was recommended for 25 species (Supplementary File S11). The complete list of pests (n=256) suggested for surveillance in Uganda is presented in Supplementary File S12.
Regulation and pest risk analysis
For the 618 (37%; N=1,517) assessed species requiring action, regulation, and PRA were considered for 384 species, accounting for 62%. Among these 384 species, 381 recorded an overall score of 54 or above, while three recorded only 35. The three species were Pectobacterium peruviense, Pectobacterium polaris, and Pectobacterium punjabense. Of the 323 assessed vector species, regulation and PRA were recommended for 66 (20%) species (relevant sheet in Supplementary File S10), while of the 174 organisms vectored by assessed species, the same action was advised for 49 (28%) species (relevant sheet in Supplementary File S10).
Supplementary File S12 (relevant sheet) provides a comprehensive reconciled list of non-native pest species (n=411) for which regulation and PRA are recommended. The majority (76%; n=314) of the species were reported in Africa. While the majority recorded an overall risk score of 54 and above, 11 species recorded a lower overall risk score. These included Lepyronia quadrangularis, Macugonalia cavifrons, Macugonalia leucomelas, Molomea consolida, Oragua discoidula, Parathona gratiosa, P. peruviense, P. polaris, P. punjabense, Plesiommata mollicella, and Tapajosa rubromarginata. Some of the pest species, although with an overall risk score above 54, are not seed- but vector-transmitted; hence, the likelihood of introduction is limited. However, the decision for regulation or non-regulation is the mandate of the NPPO. These include Pantoea stewartii subsp. stewartii, rice dwarf virus, rice gall dwarf virus, rice grassy stunt virus, rice hoja blanca virus, rice ragged stunt virus, rice stripe necrosis virus, rice tungro bacilliform virus, and rice tungro spherical virus, among others. Notably, these rice viruses have not yet been reported anywhere in Africa.
Except for Nephotettix nigropictus (Cameroon), Polymyxa graminis (Burkina Faso, Côte d’Ivoire, Mali, Niger, and Senegal), and Sogatella furcifera (Egypt), for which regulation was suggested, the other vectors of the aforementioned rice viruses including Nephotettix cincticeps, Nephotettix malayanus, Nephotettix parvus, Nephotettix virescens, Nilaparvata bakeri, Nilaparvata lugens, Nilaparvata muiri, Recilia dorsalis, Unkanodes albifascia, and Unkanodes sapporonus have not been reported as present anywhere in Africa. These vectors are primarily found in Asia, where these viruses are endemic.
Suggested additional actions
These include developing contingency plans, raising awareness (publicity) about the species, management by the industry, and conducting research to generate further phytosanitary evidence to support either regulation or deregulation. A contingency plan was suggested for 26 pest species, which include seven arthropods, five bacteria, four fungi, one mollusc, eight nematodes, and one viroid. For 28 species, including three arthropods, 19 bacteria, two fungi, one mollusc, one viroid, and one virus, raising awareness was proposed. The industry plays a crucial role in preventing the introduction of new pests. Eight pests, consisting of three arthropods, three bacteria, one mollusc, and one viroid, were highlighted, for which raising awareness in the short term is advised. Phytosanitary research is essential in refining phytosanitary decisions, and research has been proposed for three bacterial species, all part of the Xylella genus. The recommendations for additional actions are not intended to be exhaustive. All data from this assessment is presented in Supplementary File S12.
Summary of pests affecting key value chains
Considering the crops prioritised by the ASSP, examples of pests for urgent prevention of introduction have been highlighted (Table 4). For banana, they include, for instance, Banana mild mosaic virus; Ceroplastes stellifer, FoC TR4; cassava, cassava witches’ broom, Nipaecoccus nipae, Schistocerca nitens; citrus, Brevipalpus lewisi, Citrus cachexia viroid, and Xfp; cocoa, Phenacoccus hargreavesi, Phytophthora heveae, and Spodoptera litura; coffee, S. litura, Xff, and Xfp; common bean, bean pod mottle virus, Curtobacterium flaccumfaciens pv. flaccumfaciens, and Southern bean mosaic virus; cotton, Meloidogyne acronea, S. litura, and Tobacco streak virus; maize, Heterodera zeae, Maize bushy stunt phytoplasma, and Peronosclerospora sacchari; potatoes, Clavibacter sepedonicus, Dickeya dadantii, and Dickeya solani; rice, Alphanucleorhabdovirus oryzae, Ditylenchus angustus, and Hoplolaimus indicus; tea, P. porosus, Pseudococcus viburni, and S. litura.
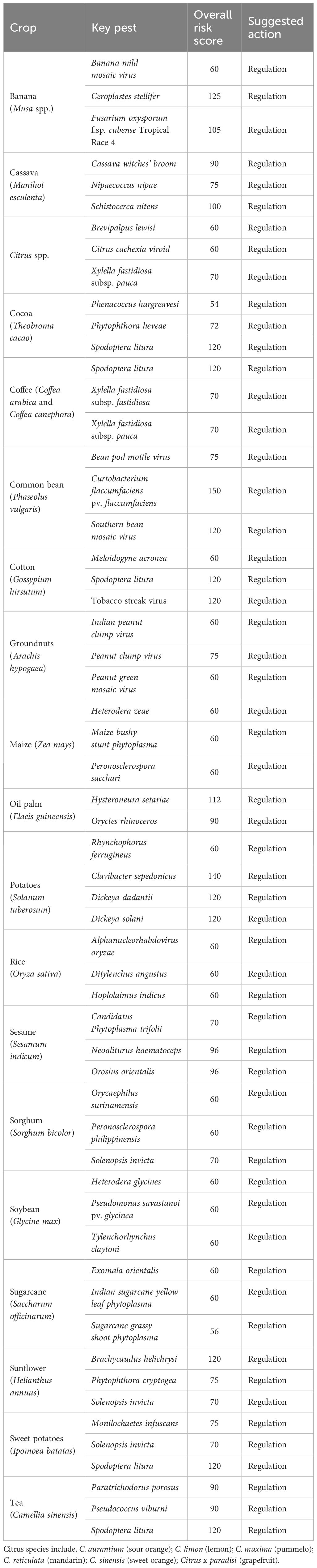
Table 4. Major crops produced in Uganda and potential invasive pests that can cause significant economic damages and losses.
Other key crops include sorghum, for which Oryzaephilus surinamensis, Peronosclerospora philippinensis, Solenopsis invicta have been prioritised; sugarcane, Exomala orientalis, Indian sugarcane yellow leaf phytoplasma, Sugarcane grassy shoot phytoplasma; and sweetpotato, Monilochaetes infuscans, S. invicta, and S. litura. For the oil crops, they include Indian peanut clump virus, Peanut clump virus, Peanut green mosaic virus, prioritised for groundnuts; oil palm, Hysteroneura setariae, Oryctes rhinoceros, and Rhynchophorus ferrugineus; sesame, Candidatus Phytoplasma trifolii, Neoaliturus haematoceps, and Orosius orientalis; soybean, Heterodera glycines, Pseudomonas savastanoi pv. glycinea, and Tylenchorhynchus claytoni; sunflower, Brachycaudus helichrysi, Phytophthora cryptogea, and S. invicta. Some of these pests have been highlighted for regulation in other countries. They include C. sepedonicus, C. flaccumfaciens pv. flaccumfaciens, D. solani, and FoC TR4 (EFSA PLH Panel et al., 2018, 2019, 2022).
Discussion
The CABI Horizon Scanning Tool generated a list of pests not yet reported in Uganda. Using a predetermined prioritisation criterion, a number of pests were selected and subjected to a rapid risk assessment based on four attributes: the likelihood of entry (introduction) and establishment (including components of spread), alongside the magnitude of socio-economic and environmental (biodiversity) impacts (Kenis et al., 2022; Mulema et al., 2022, 2024). Furthermore, three pathways (contaminant, stowaway, and unaided) through which the pests could be introduced were assessed. The contaminant pathway, relevant to seed-borne and seed-transmitted pests, was considered more plausible for species within and beyond Africa (Denancé and Grimault, 2022); however, the stowaway pathway, applicable to vector- and soil-borne pests (Montagnani et al., 2022), was deemed more plausible for species reported in a neighbouring country.
The overall risk score was employed to rank species based on their potential threat to Uganda’s economy and biodiversity. The information supporting the assessments was gathered from CABI Compendium enhanced datasheets, reviewed and published resources (journals and reviews), EPPO datasheets, grey8 literature, and SME opinions. Although they did not influence the overall risk score, the likely pathway of arrival and associated confidence levels directed discussions regarding the possibility of entry and establishment. Following the assessment, management actions to inform phytosanitary measures were recommended for a subset of pests, guided by the minimum overall risk score achieved. These actions are proposed to ensure adequate prevention and preparedness for new incursions.
Incursions occur in three main stages, transport, establishment, and spread. Following these stages, introduced species may have an impact that leads to significant damage to agriculture, biodiversity, the economy, and, ultimately, livelihoods (Hulme, 2009; Roy et al., 2024). The primary rationale for advocating regulation supported by PRA is to identify probable pathways through which potentially invasive species may be introduced, allowing for their restriction (Simberloff et al., 2013). This serves as the foundation for prevention, which represents the most cost-effective management strategy. Prevention necessitates investment in border biosecurity, surveillance, quarantine, and research to better understand species behaviour (MacLeod and Lloyd, 2020; Turner et al., 2021). This understanding is vital for establishing or refining phytosanitary measures. In this study, surveillance was primarily focussed on two categories of pests. The first category pertains to pests reported in neighbouring countries. In accordance with Article VII, Sections g and j of the International Plant Protection Convention (IPPC), it is crucial to determine the status of these pests in Uganda before implementing regulations. The second category encompasses pests identified by the East African Region as posing high phytosanitary concern, necessitating the confirmation of freedom from the pest, followed by the establishment of mechanisms to prevent or minimise their introduction. The prevention of pest incursions is largely overseen by the NPPO (DCIC), collaborating with various stakeholders in the Plant Health System.
Once an incursion is confirmed, the management objective shifts from prevention to eradication, as the invasive species has already spread (Roy et al., 2024). Eradication requires an effective early detection and rapid response system (Reaser et al., 2020). It is achievable in the early stages of establishment when the distribution and abundance of the invasive species are low. The first step is to determine how far the invasive species has spread in order to establish its boundaries. This can be achieved through a delimiting surveillance exercise (Liu et al., 2021). Once the boundaries are established, the species may be eradicated by utilising low-risk pesticides, physical methods, and/or biological control products (Venette et al., 2021). Although eradication is more costly than prevention, the economic benefits of eradication far outweigh the associated costs in the long term. Furthermore, for an effective early detection and rapid response system, eradication necessitates a well-structured preparedness mechanism guided by clear contingency plans (Roy et al., 2024). A contingency plan aims to ensure a rapid and effective response to an outbreak of a pest that has been assessed as likely to cause significant economic and/or environmental impact. Some pests for which a contingency plan is necessary have been suggested.
Eradication may not be feasible, either because the incursion was not detected early enough, and the pest has already become widely spread or because the species can spread rapidly on its own. Therefore, the priority is to halt further spread through containment, similar to what occurred with S. frugiperda (De Groote et al., 2020) and P. absoluta (Desneux et al., 2011). Containment involves implementing measures to eliminate outlying (satellite) infestations and prevent spread beyond the boundaries of core infestations (infections that are too large and well-established to eradicate). It may also involve regulatory actions, such as restricting the movement of planting materials and/or produce that could transport the pest from one area to another (Kansiime et al., 2017). Additionally, containment may require ongoing pest control activities to maintain pest-free areas or areas of low pest prevalence. Once an invasive species becomes too widespread and abundant, both eradication and containment efforts become impractical. There is no economic justification for attempting to establish pest-free zones or areas of low pest prevalence once a pest has disseminated extensively (Kansiime et al., 2017). The primary responsibility for managing a widespread pest lies with the farmer, who must implement management strategies to mitigate the pest’s destructive impact on plant populations. They receive support from extension service providers who offer practical guidance on effective management options. The main objective of horizon scanning is to ensure that the introduction of pests capable of easily establishing and spreading is effectively hindered.
Conclusion
The horizon scanning study has identified high-risk invasive pests that could threaten Uganda’s agriculture, biodiversity, forestry, and livelihoods. This information is vital for preventing the introduction of new pests, contingency planning for preparedness and early detection to ensure the eradication of new incursions or suppressing their spread through containment. This will only be achievable if Uganda establishes an effective risk monitoring, early detection, and rapid response system. Creating a pest risk register will facilitate risk monitoring and guide risk management. Furthermore, DCIC should devise a strategy for risk communication and a code of conduct for key stakeholders to minimise the introduction of highly damaging pests identified in this study. The considerable number of pests reported in neighbouring countries illustrates an information gap that must be addressed to limit the imposition of phytosanitary measures on pests that could be present in Uganda. The suggested actions aim to prevent the introduction, establishment, and spread of quarantine pests or to generate, through research, the information necessary to guide phytosanitary decisions. However, DCIC might struggle to implement these actions due to limited resources (both human and infrastructural). Consequently, DCIC must forge strategic working relationships with key actors in the Plant Health System, including academia, extension delivery organisations (public and private), research institutions (public, private, and international), and regional NPPOs. This study also emphasises the need for a regional rather than a country-specific approach, as some high-risk pests are common across countries, as observed in studies from Ghana, Kenya, and Zambia.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Author contributions
JM: Methodology, Formal analysis, Validation, Visualization, Funding acquisition, Writing – original draft, Writing – review & editing, Conceptualization, Resources. CN: Visualization, Validation, Formal analysis, Writing – review & editing. JK: Writing – review & editing, Formal analysis, Visualization, Validation. GT: Validation, Formal analysis, Visualization, Writing – review & editing. RA: Writing – review & editing, Visualization, Formal analysis, Validation. MC: Visualization, Formal analysis, Validation, Writing – review & editing. CG: Formal analysis, Writing – review & editing, Validation, Visualization. FK: Validation, Formal analysis, Visualization, Writing – review & editing. BK: Formal analysis, Writing – review & editing, Visualization, Validation. DKu: Validation, Formal analysis, Writing – review & editing, Visualization. BM: Validation, Writing – review & editing, Visualization, Formal analysis. JBM: Writing – review & editing, Validation, Visualization, Formal analysis. YM: Validation, Writing – review & editing, Formal analysis, Visualization. HM: Writing – review & editing, Validation, Formal analysis, Visualization. FM: Visualization, Formal analysis, Validation, Writing – review & editing. EN: Formal analysis, Visualization, Writing – review & editing, Validation. IRa: Visualization, Validation, Formal analysis, Writing – review & editing. GS: Writing – review & editing, Formal analysis, Validation, Visualization. AS: Validation, Visualization, Formal analysis, Writing – review & editing. BT: Visualization, Formal analysis, Writing – review & editing, Validation. SO: Validation, Writing – review & editing, Visualization, Formal analysis. IO: Writing – review & editing, Formal analysis, Visualization, Validation. CAli: Formal analysis, Visualization, Validation, Writing – review & editing. CAlo: Validation, Visualization, Formal analysis, Writing – review & editing, Funding acquisition. VT: Visualization, Formal analysis, Validation, Writing – review & editing. DKa: Visualization, Validation, Writing – review & editing, Formal analysis. PM: Validation, Visualization, Writing – review & editing, Formal analysis. MOr: Formal analysis, Visualization, Validation, Writing – review & editing. SA: Writing – review & editing, Formal analysis, Visualization, Validation. MA: Visualization, Formal analysis, Writing – review & editing, Validation. IRw: Validation, Formal analysis, Visualization, Writing – review & editing. JT: Writing – review & editing, Validation, Formal analysis, Visualization. HT: Validation, Formal analysis, Writing – review & editing, Visualization. EO: Formal analysis, Visualization, Writing – review & editing, Validation. MOc: Visualization, Writing – review & editing, Validation, Formal analysis.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. The study was conducted as part of CABI’s PlantwisePlus Programme, which is funded by the UK Foreign, Commonwealth and Development Office (FCDO), Netherlands Directorate-General for International Cooperation (DGIS), Swiss Agency for Development and Cooperation (SDC) and the European Commission (DG INTPA). CABI is an international intergovernmental organisation and gratefully acknowledges the core financial support from our member countries (and lead organisations), including the United Kingdom (Foreign, Commonwealth and Development Office), China (Chinese Ministry of Agriculture and Rural Affairs), Australia (Australian Centre for International Agricultural Research), Canada (Agriculture and Agri-Food Canada), the Netherlands (Directorate-General for International Cooperation), Switzerland (Swiss Agency for Development and Cooperation), and Ireland (Irish Aid, International Fund for Agricultural Development-IFAD). For details, see http://www.cabi.org/about-cabi/who-we-work-with/key-donors.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fagro.2025.1601845/full#supplementary-material.
Footnotes
- ^ The term “pest” is used within the context of the International Plant Protection Convention (IPPC) and refers to any species, strain, or biotype of plant, animal, or pathogenic agent injurious to plants or plant products (International Standards for Phytosanitary Measures (ISPM) Number 5). Pathogenic agents include bacteria, fungi, oomycetes, phytoplasma, viroid and virus while animals may include arthropods, molluscs, and nematodes (IPPC Secretariat, 2021a).
- ^ A species whose introduction outside its natural past or present distribution by the human agency directly or indirectly threatens biological diversity.
- ^ A pest that is absent in the area at risk (country or region), although it might be present in that country and region's immediate neighbours.
- ^ PRA is a process that uses scientific, economic, and biological evidence to determine if an organism is a pest, if it should be regulated, and what phytosanitary measures should be taken against it.
- ^ A pest that is present in a country or region, but which is not widely distributed and is subject to official control.
- ^ A pest carried by seeds externally or internally that may or may not be transmitted to plants growing from these seeds and cause their infestation.
- ^ A pest is a seed-borne pest transmitted via seeds directly to plants growing from these seeds and causing their infestation.
- ^ Grey literature included blogs, conference papers and proceedings, disease reports, dissertations and theses, government documents, newspaper articles, PRA reports on specific pests, and working papers.
References
Adams I. P., Miano D. W., Kinyua Z. M., Wangai A., Kimani E., Phiri N., et al. (2013). Use of next-generation sequencing for the identification and characterization of Maize chlorotic mottle virus and Sugarcane mosaic virus causing maize lethal necrosis in Kenya. Plant Pathol. 62, 741–749. doi: 10.1111/j.1365-3059.2012.02690.x
Adams V. M., Douglas M. M., Jackson S. E., Scheepers K., Kool J. T., and Setterfield S. A. (2018). Conserving biodiversity and Indigenous bush tucker: Practical application of the strategic foresight framework to invasive alien species management planning. Conserv. Lett. 11, e12441. doi: 10.1111/conl.124412
Aktar M. W., Sengupta D., and Chowdhury A. (2009). Impact of pesticides use in agriculture: their benefits and hazards. Interdiscip. Toxicol. 2, 1–12. doi: 10.2478/v10102-009-0001-7
Al-Zyoud F. A. (2014). Indiscriminate use and improper application of pesticides by Jordanian vegetable and fruit farmers. Egypt. J. Agric. Sci. 65, 344–359. doi: 10.21608/ejarc.2014.214013
Bayón Á. and Vilà M. (2019). Horizon scanning to identify invasion risk of ornamental plants marketed in Spain. NeoBiota 52, 47–86. doi: 10.3897/neobiota.52.38113
Bekunda M., Sanginga N., and Woomer P. L. (2010). “Restoring soil fertility in sub-Sahara Africa,” in Advances in agronomy. Ed. Sparks D. L. (Cambridge, Massachusetts: Academic Press), 183–236. doi: 10.1016/S0065-2113(10)08004-1
Blackburn T. M., Essl F., Evans T., Hulme P. E., Jeschke J. M., Kühn I., et al. (2014). A unified classification of alien species based on the magnitude of their environmental impacts. PloS Biol. 12, e1001850. doi: 10.1371/journal.pbio.1001850
Bradshaw C. J. A., Hulme P. E., Hudgins E. J., Leung B., Kourantidou M., Courtois P., et al. (2024). Damage costs from invasive species exceed management expenditure in nations experiencing lower economic activity. Ecol. Econ. 220, 108166. doi: 10.1016/j.ecolecon.2024.108166
Buddie A. G., Rwomushana I., Offord L. C., Kibet S., Makale F., Djeddour D., et al. (2021). First report of the invasive snail Pomacea canaliculata in Kenya. CABI Agric. Biosci. 2, 11. doi: 10.1186/s43170-021-00032-z
Dawson W., Peyton J. M., Pescott O. L., Adriaens T., Cottier-Cook E. J., Frohlich D. S., et al. (2023). Horizon scanning for potential invasive non-native species across the United Kingdom Overseas Territories. Conserv. Lett. 16, e12928. doi: 10.1111/conl.12928
Day R., Abrahams P., Bateman M., Beale T., Clottey V., Cock M., et al. (2017). Fall armyworm: Impacts and implications for Africa. Outlooks Pest Manage. 28, 196–201. doi: 10.1564/v28_oct_02
De Groote H., Kimenju S. C., Munyua B., Palmas S., Kassie M., and Bruce A. (2020). Spread and impact of fall armyworm (Spodoptera frugiperda J.E. Smith) in maize production areas of Kenya. Agric. Ecosyst. Environ. 292, 106804. doi: 10.1016/j.agee.2019.106804
Denancé N. and Grimault V. (2022). Seed pathway for pest dissemination: The ISTA Reference Pest List, a bibliographic resource in non-vegetable crops. EPPO Bull. 52, 434–445. doi: 10.1111/epp.12834
Desneux N., Luna M. G., Guillemaud T., and Urbaneja A. (2011). The invasive South American tomato pinworm, Tuta absoluta, continues to spread in Afro-Eurasia and beyond: the new threat to tomato world production. J. Pest Sci. 84, 403–408. doi: 10.1007/s10340-011-0398-6
Dorjee, Johnson S. B., Buckmaster A. J., and Downey P. O. (2021). Developing a hybrid weed risk assessment system for countries with open and porous borders: insights from Bhutan. Biol. Invasions 23, 2945–2959. doi: 10.1007/s10530-021-02552-1
EFSA PLH Panel, Bragard C., Baptista P., Chatzivassiliou E., Di Serio F., Gonthier P., et al. (2022). Pest categorisation of Fusarium oxysporum f. sp. cubense Tropical Race 4. EFSA J. 20, e07092. doi: 10.2903/j.efsa.2022.7092
EFSA PLH Panel, Bragard C., Dehnen-Schmutz K., Di Serio F., Gonthier P., Jaques Miret J. A., et al. (2019). Pest categorisation of Clavibacter sepedonicus. EFSA J. 17, e05670. doi: 10.2903/j.efsa.2019.5670
EFSA PLH Panel, Jeger M., Bragard C., Caffier D., Candresse T., Chatzivassiliou E., et al. (2018). Pest categorisation of Curtobacterium flaccumfaciens pv. flaccumfaciens. EFSA J. 16, e05299. doi: 10.2903/j.efsa.2018.5299
Gallardo B., Zieritz A., Adriaens T., Bellard C., Boets P., Britton J. R., et al. (2016). Trans-national horizon scanning for invasive non-native species: a case study in western Europe. Biol. Invasions 18, 17–30. doi: 10.1007/s10530-015-0986-0
Graziosi I., Tembo M., Kuate J., and Muchugi A. (2020). Pests and diseases of trees in Africa: A growing continental emergency. Plants People Planet 2, 14–28. doi: 10.1002/ppp3.31
Greenfield P. (2020). Increase in invasive species poses dramatic threat to biodiversity – report (London, United Kingdom: The Guardian). Available online at: https://www.theguardian.com/environment/2020/jul/15/increase-in-invasive-species-poses-dramatic-threat-to-biodiversity-report-aoe.
Guimapi R. Y. A., Mohamed S. A., Okeyo G. O., Ndjomatchoua F. T., Ekesi S., and Tonnang H. E. Z. (2016). Modeling the risk of invasion and spread of Tuta absoluta in Africa. Ecol. Complex. 28, 77–93. doi: 10.1016/j.ecocom.2016.08.001
Hulme P. E. (2009). Trade, transport and trouble: managing invasive species pathways in an era of globalization. J. Appl. Ecol. 46, 10–18. doi: 10.1111/j.1365-2664.2008.01600.x
Hulme P. E., Bacher S., Kenis M., Klotz S., Kühn I., Minchin D., et al. (2008). Grasping at the routes of biological invasions: a framework for integrating pathways into policy. J. Appl. Ecol. 45, 403–414. doi: 10.1111/j.1365-2664.2007.01442.x
IPPC Secretariat (2021). International standards for phytosanitary measures (ISPM), publication no. 5: glossary of phytosanitary terms (Rome, Italy: Food and Agriculture Organization of the United Nations).
Kansiime M., Mulema J., Karanja D., Romney D., and Day R. (2017). Crop pests and disease management in Uganda: status and investment needs (Rome, Italy: Platform for Agricultural Risk Management (PARM), International Fund for Agricultural Development (IFAD).
Katende A. B., Birnie A., and Tengnas B. (1995). Useful trees and shrubs for Uganda: identification, propagation and management for agricultural and pastoral communities (Nairobi, Kenya: Regional Soil Conservation Unit).
Kendig A. E., Canavan S., Anderson P. J., Flory S. L., Gettys L. A., Gordon D. R., et al. (2022). Scanning the horizon for invasive plant threats using a data-driven approach. NeoBiota 74, 129–154. doi: 10.3897/neobiota.74.83312
Kenis M., Agboyi L. K., Adu-Acheampong R., Ansong M., Arthur S., Attipoe P. T., et al. (2022). Horizon scanning for prioritising invasive alien species with potential to threaten agriculture and biodiversity in Ghana. NeoBiota 71, 129–148. doi: 10.3897/neobiota.71.72577
Kibwage P., Avedi E., Kipkoech H., Muthomi E., Oronje M., Macharia I., et al. (2023). First report of Avocado sunblotch viroid in avocado in Kenya. New Dis. Rep. 48, e12212. doi: 10.1002/ndr2.12212
Lieurance D., Canavan S., Behringer D. C., Kendig A. E., Minteer C. R., Reisinger L. S., et al. (2023). Identifying invasive species threats, pathways, and impacts to improve biosecurity. Ecosphere 14, e4711. doi: 10.1002/ecs2.4711
Liu Y., Wang P., Thomas M. L., Zheng D., and McKirdy S. J. (2021). Cost-effective surveillance of invasive species using info-gap theory. Sci. Rep. 11, 22828. doi: 10.1038/s41598-021-02299-8
MAAIF (2025). Agricultural sector potential in Uganda (Entebbe, Uganda: Ministry of Agriculture, Animal Industry and Fisheries (MAAIF). Available online at: https://www.agriculture.go.ug/agricultural-sector-potential/.
Macharia I., Kimani E., Koome F., Kosiom T., Heya H., Otipa M., et al. (2017). First report and distribution of the papaya mealybug, Paracoccus marginatus, in Kenya. J. Agric. Urban Entomol. 33, 142–150. doi: 10.3954/jaue17-02.1
Mack R. N., Simberloff D., Mark Lonsdale W., Evans H., Clout M., and Bazzaz F. A. (2000). Biotic invasions: causes, epidemiology, global consequences, and control. Ecol. Appl. 10, 689–710. doi: 10.1890/1051-0761(2000)010[0689:BICEGC]2.0.CO;2
MacLeod A. and Lloyd S. (2020). The emergence of prioritisation systems to inform plant health biosecurity policy decisions. Emerg. Top. Life Sci. 4, 463–471. doi: 10.1042/ETLS20200341
Mburu H., Cortada L., Haukeland S., Ronno W., Nyongesa M., Kinyua Z., et al. (2020). Potato cyst nematodes: A new threat to potato production in East Africa. Front. Plant Sci. 11. doi: 10.3389/fpls.2020.00670
McAusland C. and Costello C. (2004). Avoiding invasives: trade-related policies for controlling unintentional exotic species introductions. J. Environ. Econ. Manage. 48, 954–977. doi: 10.1016/j.jeem.2003.11.002
Md Meftaul I., Venkateswarlu K., Dharmarajan R., Annamalai P., and Megharaj M. (2020). Pesticides in the urban environment: A potential threat that knocks at the door. Sci. Total Environ. 711, 134612. doi: 10.1016/j.scitotenv.2019.134612
Montagnani C., Gentili R., Brundu G., Caronni S., and Citterio S. (2022). Accidental introduction and spread of top invasive alien plants in the European Union through human-mediated agricultural pathways: What should we expect? Agronomy 12 (2), 423. doi: 10.3390/agronomy12020423
Mulema J., Day R., Nunda W., Akutse K. S., Bruce A. Y., Gachamba S., et al. (2022). Prioritization of invasive alien species with the potential to threaten agriculture and biodiversity in Kenya through horizon scanning. Biol. Invasions. 24 (9), 2933–2949. doi: 10.1007/s10530-022-02824-4
Mulema J., Phiri S., Bbebe N., Chandipo R., Chijikwa M., Chimutingiza H., et al. (2024). Rapid risk assessment of plant pathogenic bacteria and protists likely to threaten agriculture, biodiversity and forestry in Zambia. NeoBiota 91, 145–178. doi: 10.3897/neobiota.91.113801
Nkonya E., Johnson T., Kwon H. Y., and Kato E. (2016). “Economics of land degradation in Sub-Saharan Africa,” in Economics of land degradation and improvement – A global assessment for sustainable development. Eds. Nkonya E., Mirzabaev A., and von Braun J. (Springer International Publishing, Cham), 215–259. doi: 10.1007/978-3-319-19168-3_9
Obah-Akpowoghaha N. G., Ojakorotu V., and Tarro M. L. (2020). Porous borders and the challenge of national integration in Africa. J. Afr. Foreign Aff. 7, 89–111. doi: 10.31920/2056-5658/2020/v7n3a5
Osabohien R., Matthew O., Gershon O., Ogunbiyi T., and Nwosu E. (2019). Agriculture development, employment generation and poverty reduction in West Africa. Open Agric. J. 13, 82–89. doi: 10.2174/1874331501913010082
Papadopoulos N. T., De Meyer M., Terblanche J. S., and Kriticos D. J. (2024). Fruit flies: challenges and opportunities to stem the tide of global invasions. Annu. Rev. Entomol. 69, 355–373. doi: 10.1146/annurev-ento-022723-103200
Prasanna B. M., Carvajal-Yepes M., Kumar P. L., Kawarazuka N., Liu Y., Mulema A. A., et al. (2022). Sustainable management of transboundary pests requires holistic and inclusive solutions. Food Secur. 14, 1449–1457. doi: 10.1007/s12571-022-01301-z
Pratt C. F., Constantine K. L., and Murphy S. T. (2017). Economic impacts of invasive alien species on African smallholder livelihoods. Glob. Food Secur. 14, 31–37. doi: 10.1016/j.gfs.2017.01.011
Reaser J. K., Burgiel S. W., Kirkey J., Brantley K. A., Veatch S. D., and Burgos-Rodríguez J. (2020). The early detection of and rapid response (EDRR) to invasive species: a conceptual framework and federal capacities assessment. Biol. Invasions 22, 1–19. doi: 10.1007/s10530-019-02156-w
Roy H. E., Bacher S., Essl F., Adriaens T., Aldridge D. C., Bishop J. D. D., et al. (2019). Developing a list of invasive alien species likely to threaten biodiversity and ecosystems in the European Union. Glob. Change Biol. 25, 1032–1048. doi: 10.1111/gcb.14527
Roy H. E., Pauchard A., Stoett P., Renard Truong T., Lipinskaya T., and Vicente J. R. (2024). “IPBES invasive alien species assessment,” in Chapter 1. Introducing biological invasions and the IPBES thematic assessment of invasive alien species and their control. Bonn, Germany. doi: 10.5281/zenodo.10677041
Roy H. E., Peyton J., Aldridge D. C., Bantock T., Blackburn T. M., Britton R., et al. (2014). Horizon scanning for invasive alien species with the potential to threaten biodiversity in Great Britain. Glob. Change Biol. 20, 3859–3871. doi: 10.1111/gcb.12603
Saul W.-C., Roy H. E., Booy O., Carnevali L., Chen H.-J., Genovesi P., et al. (2017). Assessing patterns in introduction pathways of alien species by linking major invasion data bases. J. Appl. Ecol. 54, 657–669. doi: 10.1111/1365-2664.12819
Seymour C. L., Gillson L., Child M. F., Tolley K. A., Curie J. C., da Silva J. M., et al. (2020). Horizon scanning for South African biodiversity: A need for social engagement as well as science. Ambio 49, 1211–1221. doi: 10.1007/s13280-019-01252-4
Simberloff D., Martin J.-L., Genovesi P., Maris V., Wardle D. A., Aronson J., et al. (2013). Impacts of biological invasions: what’s what and the way forward. Trends Ecol. Evol. 28, 58–66. doi: 10.1016/j.tree.2012.07.013
Slayi M., Zhou L., Dzvene A. R., and Mpanyaro Z. (2024). Drivers and consequences of land degradation on livestock productivity in Sub-Saharan Africa: A systematic literature review. Land 13 (9), 1402. doi: 10.3390/land13091402
Sseremba O. E., Kaboggoza J. R. S., Ziraba N. Y., Mugabi P., Banana A. Y., Zziwa A., et al. (2011). Timber management practices and timber species used by small scale furniture workshops in Uganda. Maderas Cienc. Tecnol. 13, 347–358. doi: 10.4067/S0718-221X2011000300010
Stewart Z. P., Pierzynski G. M., Middendorf B. J., and Prasad P. V. V. (2020). Approaches to improve soil fertility in sub-Saharan Africa. J. Exp. Bot. 71, 632–641. doi: 10.1093/jxb/erz446
Stewart L. R., Willie K., Wijeratne S., Redinbaugh M. G., Massawe D., Niblett C. L., et al. (2017). Johnsongrass mosaic virus contributes to maize lethal necrosis in East Africa. Plant Dis. 101, 1455–1462. doi: 10.1094/pdis-01-17-0136-re
Sutherland W. J., Albon S. D., Allison H., Armstrong-Brown S., Bailey M. J., Brereton T., et al. (2010). Review: The identification of priority policy options for UK nature conservation. J. Appl. Ecol. 47, 955–965. doi: 10.1111/j.1365-2664.2010.01863.x
Sutherland W. J., Bailey M. J., Bainbridge I. P., Brereton T., Dick J. T. A., Drewitt J., et al. (2008). Future novel threats and opportunities facing UK biodiversity identified by horizon scanning. J. Appl. Ecol. 45, 821–833. doi: 10.1111/j.1365-2664.2008.01474.x
Sutherland W. J., Dias M. P., Dicks L. V., Doran H., Entwistle A. C., Fleishman E., et al. (2020). A horizon scan of emerging global biological conservation issues for 2020. Trends Ecol. Evol. 35, 81–90. doi: 10.1016/j.tree.2019.10.010
Turner R. M., Brockerhoff E. G., Bertelsmeier C., Blake R. E., Caton B., James A., et al. (2021). Worldwide border interceptions provide a window into human-mediated global insect movement. Ecol. Appl. 31, e02412. doi: 10.1002/eap.2412
UBOS (2024). Uganda bureau of statistics (UBOS) 2024: the national population and housing census 2024 - final report - volume I (Kampala, Uganda: Uganda Bureau of Statistics). Available online at: https://nwoya.go.ug/sites/default/files/National-Population-and-Housing-Census-2024-Final-Report-Volume-1-Main.pdf (Accessed June 25, 2025).
Venette R. C., Gordon D. R., Juzwik J., Koch F. H., Liebhold A. M., Peterson R. K. D., et al. (2021). “Early intervention strategies for invasive species management: connections between risk assessment, prevention efforts, eradication, and other rapid responses,” in Invasive species in forests and rangelands of the United States: A comprehensive science synthesis for the United States forest sector. Eds. Poland T. M., Patel-Weynand T., Finch D. M., Miniat C. F., Hayes D. C., and Lopez V. M. (Springer International Publishing, Cham), 111–131. doi: 10.1007/978-3-030-45367-1_6
Keywords: invasive alien species, horizon scanning, rapid risk assessment, pest risk identification, pest risk analysis, pest risk management
Citation: Mulema J, Nankinga C, Kagorora JPK, Tusiime G, Amayo R, Chemonges M, Gumisiriya C, Kato F, Kigongo BM, Kutunga D, Mudde B, Muhumuza JB, Mukasa Y, Musiimenta H, Muzira F, Namasa EJ, Ramathan I, Sebutare G, Ssamula A, Tukahirwa B, Opio SM, Obare IJ, Aliamo C, Alokit C, Tumuhaise V, Karanja D, Mwambu P, Oronje M, Athman SY, Akiri M, Rwomushana I, Tugume J, Talwana H, Onkendi E and Ochwo M (2025) Prioritising non-native pest species to inform plant health biosecurity policy decisions and to safeguard agriculture, forestry, biodiversity, and livelihoods in Uganda. Front. Agron. 7:1601845. doi: 10.3389/fagro.2025.1601845
Received: 28 March 2025; Accepted: 10 July 2025;
Published: 07 August 2025.
Edited by:
Emmanouil Roditakis, Hellenic Mediterranean University, GreeceReviewed by:
Luis F. Aristizabal, Consultant, Kailua-Kona, United StatesPhumudzo Patrick Tshikhudo, University of South Africa, South Africa
Copyright © 2025 Mulema, Nankinga, Kagorora, Tusiime, Amayo, Chemonges, Gumisiriya, Kato, Kigongo, Kutunga, Mudde, Muhumuza, Mukasa, Musiimenta, Muzira, Namasa, Ramathan, Sebutare, Ssamula, Tukahirwa, Opio, Obare, Aliamo, Alokit, Tumuhaise, Karanja, Mwambu, Oronje, Athman, Akiri, Rwomushana, Tugume, Talwana, Onkendi and Ochwo. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Joseph Mulema, ai5tdWxlbWFAY2FiaS5vcmc=
†ORCID: Joseph Mulema, orcid.org/0000-0002-8738-1306
Robert Amayo, orcid.org/0000-0001-8343-6261
Martin Chemonges, orcid.org/0000-0002-0781-5523
Costa Gumisiriya, orcid.org/0009-0007-9132-2935
Barnabas Mudde, orcid.org/0000-0001-7999-1537
Morris Akiri, orcid.org/0009-0002-0515-3551
Ivan Rwomushana, orcid.org/0000-0001-5840-8058
Shahasi Yusuf Athman, orcid.org/0000-0002-2510-4469
Mildred Ochwo, orcid.org/0000-0001-7474-2857
 Joseph Mulema
Joseph Mulema Caroline Nankinga2
Caroline Nankinga2 Geoffrey Tusiime
Geoffrey Tusiime Emmanuel John Namasa
Emmanuel John Namasa Gilbert Sebutare
Gilbert Sebutare Joab Tugume
Joab Tugume Mildred Ochwo
Mildred Ochwo