- 1Molecular Allergology, Paul-Ehrlich-Institut, Langen, Germany
- 2Department of Biosciences, Paris Lodron University of Salzburg, Salzburg, Austria
- 3Allergy and Immunology Section, CSIR-Institute of Genomics and Integrative Biology, New Delhi, India
- 4Department of Dermatology, University Medical Center of the Johannes Gutenberg-University Mainz, Mainz, Germany
- 5Batch Control and Allergen Analysis, Paul-Ehrlich-Institut, Langen, Germany
- 6Clinical Immunology Service in Hospital Militar Dr. Carlos Arvelo, Caracas, Venezuela
- 7Acquired Immunodeficiency Research Center, Isfahan University of Medical Sciences, Isfahan, Iran
- 8Department of Internal Medicine, Yonsei University College of Medicine, Seoul, South Korea
- 9Thermo Fisher Scientific, Immunodiagnostics, Uppsala, Sweden
Background: Manifestation of respiratory allergy to American cockroach (Periplaneta americana) is prominent in the subtropical and tropical areas. However, co-existing perennial indoor inhalant allergies frequently compromise clinical diagnosis of cockroach allergy, and the analysis of sensitization pattern is limited by the lack of Periplaneta allergens widely available for component-resolved diagnostics (CRD).
Objective: To evaluate a collection of previously described recombinant Periplaneta allergens for CRD in cockroach allergy.
Methods: A panel of nine recombinant Periplaneta allergens (Per a 1–5, 7–10) was generated, purified, and subjected to physicochemical characterization by applying circular dichroism (CD) spectroscopy, dynamic light scattering (DLS), amino acid (AA) analysis, and mass spectrometry (MS). Patients (n = 117) from India, Korea, Venezuela, and Iran, reporting perennial respiratory indoor allergies with IgE sensitization to cockroach (P. americana and/or Blattella germanica), were included. The sensitization profile was monitored by the experimental ImmunoCAP testing.
Results: ImmunoCAP testing confirmed IgE sensitization to Periplaneta and/or Blattella extract in 98 of 117 patients (r = 0.95). Five out of 117 patients were sensitized to only one of the two cockroach species. Within the whole study group, the prevalence of sensitization to individual allergens varied from 4% (Per a 2) to 50% (Per a 9), with the highest IgE values to Per a 9. Patients from four countries displayed different sensitization profiles at which Per a 3 and Per a 9 were identified as major allergens in India and Korea. Periplaneta-derived lipocalin and myosin light chain were characterized as new minor allergens, designated as Per a 4 and Per a 8. Periplaneta extract showed higher diagnostic sensitivity than all individual components combined, suggesting the existence of allergens yet to be discovered.
Conclusion: Utilization of a panel of purified Periplaneta allergens revealed highly heterogeneous sensitization patterns and allowed the classification of lipocalin and myosin light chain from Periplaneta as new minor allergens.
Introduction
The most common indoor aeroallergens are house dust and storage mites, pet dander, mold, and cockroaches (1). German cockroach (Blattella germanica), American cockroach (Periplaneta americana), Oriental cockroach (Blatta orientalis), brown-banded cockroach (Supella longipalpa), and smoky brown cockroach (Periplaneta fuliginosa) have been reported to cause allergic asthma morbidity worldwide (2). Cockroach allergy is predominantly caused by Blattella and Periplaneta in temperate and (sub)tropical areas and is a global health problem due to the increasing infestation of cockroaches in human housing environments (3). Cockroach allergens are present in saliva, fecal particles, spermatophores, shredded skin, and desiccated remains of insect bodies (2). In studies including children and adults, presented to hospital, the prevalence of cockroach allergy ranges from 17 to 41% in the United States, and 60–80% of inner-city children with asthma were sensitized to cockroach. It has been suggested that exposure to cockroach allergens appears to have a greater effect on asthma morbidity than dust mite or pet allergens, in particular among inner-city children (2).
Allergic sensitization to cockroach is frequently investigated by skin testing using crude extracts and by the measurement of specific IgE to cockroach allergens (2). However, the usage of allergen extracts possesses some limitations. Commercial or self-prepared allergen extracts at most are standardized based on in-house assays, causing the lack of comparability between different manufacturers. Moreover, the lack of immune-dominant allergen(s) and the complex patterns of IgE responses to cockroach extracts have made it difficult to produce standardized cockroach allergen extracts (4). Therefore, one approach to overcome these drawbacks is the utilization of purified allergens for component-resolved diagnostics (CRD).
Whereas, allergic sensitization to B. germanica allergens has been investigated in detail, data referring to Periplaneta allergens are less available. However, the tested cockroach species frequently is not indicated in the respective reports. Of note, species-specific sensitization to Periplaneta and Blattella has been reported, and in one study, only 68% of Blattella-reactive patients showed sensitization to Periplaneta (5). Vice versa, in China, 25.7% of allergic patients were sensitized to Periplaneta allergens, whereas only 18.7% were sensitized to Blattella allergens (6), indicating allergenic differences between these cockroach species or different diagnostic sensitivity of the extracts used. Although homologous allergens have been described for Periplaneta and Blattella, the presence of different allergenic components between the species needs to be considered. So far, among 13 suggested Periplaneta allergens, 11 have been officially recognized by the WHO/IUIS Allergen Nomenclature Sub-Committee (7, 8). Per a 1 (25–45 kDa, midgut microvilli-like protein) (9–11), Per a 2 (42 kDa, aspartic protease-like protein) (12), Per a 3 (46–79 kDa, homologue of arylphorin and insect hemocyanin) (13, 14), Per a 5 (23 kDa, glutathione S-transferase) (15–17), Per a 6 (18 kDa, troponin C) (18), Per a 7 (33 kDa, tropomyosin) (19, 20), Per a 9 (43 kDa, arginine kinase) (21, 22), Per a 10 (28 kDa, serine protease) (23), Per a 11 (55 kDa, α-amylase) (24), Per a 12 (45 kDa, chitinase) (24), and Per a 13 (36 kDa, glyceraldehyde-3-phosphate dehydrogenase) (24). Although lipocalin (Acc.No. AY792948) (25, 26) and myosin light chain (Acc.No. JQ279816, unpublished) have been suggested as potential Periplaneta allergens and have been proposed as Per a 4 and Per a 8, they are less well-characterized.
So far, studies addressing the sensitizing capacity of Periplaneta allergens were mainly performed with selected allergens rather than by CRD using the panel of Periplaneta allergens. Of note, data on the prevalence of IgE binding to target allergens are highly variable between different studies (7). An ELISA-based CRD study conducted in Taiwan, enrolling Blattella-sensitized patients with persistent asthma and including recombinant Per a 1 through Per a 7 and Per a 9, revealed a reactivity with Per a 2 and Per a 9 to be associated with severe asthma and allergic rhinitis, respectively (12). Taken together, the reports on the contribution of individual Periplaneta allergens in cockroach allergy are not consistent. It is worth noting that study results on the clinical significance of individual Periplaneta allergens may vary substantially depending on the quality of purified allergens used for antibody detection, patient inclusion criteria, co-exposure to cross-reactive allergens from other sources, and the geographical area where the study is conducted.
The present study aimed to investigate the molecular sensitization profile in a substantial number of cockroach-sensitized patients recruited in four different countries, all with perennial respiratory indoor allergy. For the purpose of CRD, a set of nine recombinant Periplaneta allergens were produced and characterized by uniform methods.
Materials and Methods
Patients and Patient's Sera
For CRD, patients (n = 117) reporting perennial respiratory indoor allergy with confirmed IgE sensitization to P. americana and/or B. germanica were included in the study. Patients' sensitization to cockroach was monitored by IgE-ELISA (India, n = 35) and IgE-immunoblotting (Iran, n = 30) using the self-prepared Periplaneta extract, by SPT using Blattella extract (ALK, Round Rock, TX, United States) (Venezuela, n = 25), and Blattella extract ImmunoCAP (i6, Thermo Fisher Scientific, Uppsala, Sweden) (Korea, n = 27), respectively. Screening of Periplaneta allergen expression and purification was done using sera from cockroach-allergic patients (Germany, n = 1; Plasmalab, Everett, WA, United States, n = 6). Ethical approval was obtained from IHEC (no. CLP 0019, CSIR-IGIB) and Isfahan University of Medical Sciences and Health Services (no. 295264).
Preparation of P. americana Extract
Periplaneta americana extract was produced as described in the Supplementary Material. In brief, proteins were extracted from lyophilized powder of defatted whole-body Periplaneta cockroaches using phosphate-buffered saline (PBS), and the protein fractions were gained after subsequent centrifugation and filtration steps.
Generation and Physicochemical Characterization of Recombinant Periplaneta Allergens
Detailed steps for the preparation of recombinant Per a allergens are depicted in the Supplementary Material. For cDNA cloning, published GenBank amino acid (AA) sequences were used as template, and signal peptides were excluded from the sequence. Five out of the nine proteins were produced as non-tagged proteins. Per a 2 and Per a 3 contain a C-terminal Histag, whereas Per a 5 and Per a 10 comprise a N-terminal Histag for purification. All proteins were expressed in Escherichia coli and purified by multiple chromatographic steps. Final protein concentration and purity was checked by bicinchoninic acid (BCA) (Sigma-Aldrich, United States), amino acid analysis (AAA), and sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS–PAGE). Additionally, mass spectrometry (MS) (intact masses and tryptic digestion) analysis was performed to confirm the identity of recombinant proteins. To determine the structural integrity and the aggregation status of the proteins, circular dichroism (CD), and dynamic light scattering (DLS) were carried out. Recombinant expression of individual allergens and analytical methods are described in the Supplementary Material.
IgE Immunoblot and CRD
Immunoblotting was performed to (1) demonstrate IgE sensitization of Iranian patients recruited by clinical history (not shown) and (2) to assess the IgE sensitization pattern for selected patients lacking detectable IgE binding to purified Periplaneta allergens. Briefly, P. americana extract (50 μg/cm) was applied to SDS–PAGE, transferred to nitrocellulose membrane (0.2 μm, Amersham Protean, GE Healthcare, Freiburg, Germany) by semi-dry blotting, and visualized by Ponceau S staining (Sigma-Aldrich, Munich, Germany). After blocking with 0.3% Tween-20 in Tris-buffered saline (TBS, 50 mM Tris, 150 mM NaCl, pH 7.4), the membrane was incubated overnight with patient's serum (500 μl/strip, diluted 1:10 in TBS, 0.05% Tween-20, 0.1% BSA), and bound IgE was detected as described elsewhere (27).
Specific IgE values to Periplaneta (i206) and Blattella extract (i6) were determined by ImmunoCAP tests (Thermo Fisher Scientific) and to single recombinant Periplaneta allergens by experimental ImmunoCAP tests as previously described (28). Assay background of each test was assessed and cutoff level for positivity adapted as required (0.35 kUA/L for Per a 2, Per a 5, and Per a 10 and 0.10 kUA/L for all other allergens).
Results
Generation of Recombinant Periplaneta Allergens
All recombinant Periplaneta allergens were expressed in E. coli as non-fusion as well as N- or C-terminal Histag proteins. After multistep chromatography, purified proteins were characterized by uniform physicochemical methods as indicated in Supplementary Table 1. Purity and apparent molecular weight of all proteins were assessed by SDS–PAGE performed under reducing conditions (Figure 1).
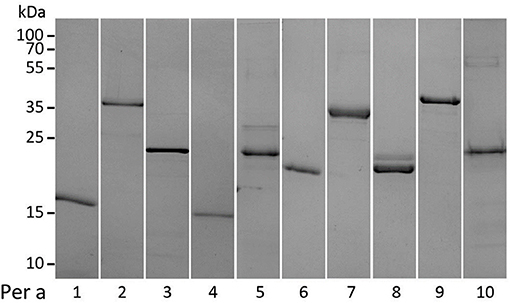
Figure 1. SDS–PAGE and Coomassie staining of recombinant Periplaneta allergens, Per a 1 to Per a 10 (1-10), under reducing conditions.
The identity of all nine recombinant Periplaneta proteins included in the study was confirmed by MS analysis, by either intact mass analysis or in-solution/in-gel digestion and AA analysis. The identity of Per a 6 could not be confirmed, and it was therefore excluded from the study. Of note, the MS analysis revealed that a high proportion of Per a 8 had a truncation at the N-terminus of the protein, with only 10% being present as the full-length protein (Supplementary Table 1). The truncation observed for Per a 8 did not affect the secondary structure of the protein. CD spectroscopy confirmed that most of the allergens show structural integrity or were at least partially folded (Supplementary Table 1). Analysis by DLS revealed that some recombinant allergens tended to partially aggregate; however Per a 4, Per a 8, and Per a 9 were determined as mostly monomeric proteins.
IgE Sensitization to Periplaneta and Blattella Allergens
Periplaneta- and Blattella-specific IgE was analyzed by ImmunoCAP testing in all 117 serum samples (Figure 2), showing an overall interspecies correlation of r = 0.95 and r = 0.96–0.98 for individual patient collectives. In vitro IgE reactivity to Blattella and Periplaneta was confirmed in 83% (97/117) and 81% (95/117) of the patients, respectively. Four subjects were sensitized to Blattella but not to Periplaneta, and one was sensitized to Periplaneta but not to Blattella. In total, 98 of 117 patients reacted to one or both of the cockroach species (Figure 2). Although patients were preselected by sensitization to cockroach by ELISA, SPT, and immunoblotting, 19/117 sera (comprising 6/35 from India, 3/25 from Venezuela, 10/30 from Iran) were tested negative to both species by ImmunoCAP.
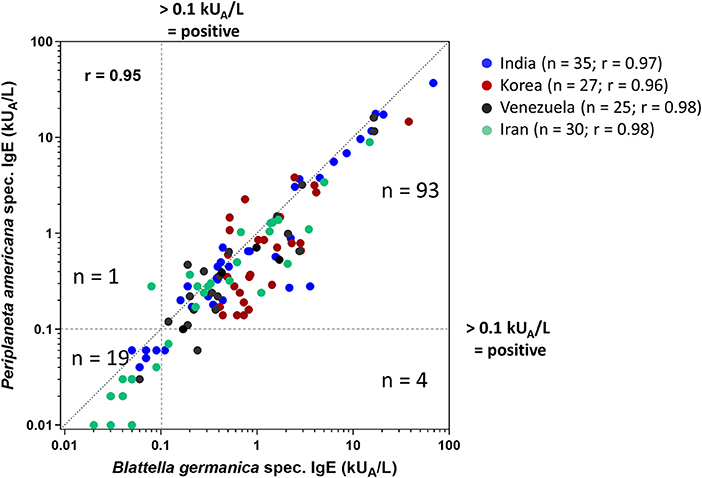
Figure 2. Comparison of Periplaneta (i206)- and Blattella (i6)-specific IgE values in 117 patients (India, n = 35; Korea, n = 27; Venezuela, n = 25; Iran, n = 30) reporting cockroach allergy by ImmunoCAP testing (cutoff 0.1 kUA/L); r-values are indicated.
Component-resolved Diagnosis of Periplaneta Allergy by ImmunoCAP Testing
In total, nine recombinant Periplaneta allergens (Per a 1 to Per a 10, except Per a 6) were included for CRD by experimental ImmunoCAP testing. Since the preparation of Per a 2, Per a 5, and Per a 10 displayed a tendency of unspecific IgE binding, the threshold for test positivity was set to 0.35 kUA/L for these allergens (Figure 3B). Other Periplaneta allergens were evaluated with a cutoff of 0.1 kUA/L (Figure 3A). Although no major allergen could be identified, Per a 3 and Per a 9 appeared as the most important in this study. The frequencies of sensitization to the different allergens in the entire study population were as follows: Per a 1: 16% (16/98), Per a 3: 41% (40/98), Per a 4: 18% (18/98), Per a 7: 28% (27/98), Per a 8: 24% (23/98), and Per a 9: 50% (49/98) (Figure 3A). Per a 4 and Per a 8 were characterized as minor allergens in comparison with other Periplaneta allergens. The frequency of IgE recognition of Per a 2, Per a 5, and Per a 10 was 4, 19, and 21%, respectively (Figure 3B).
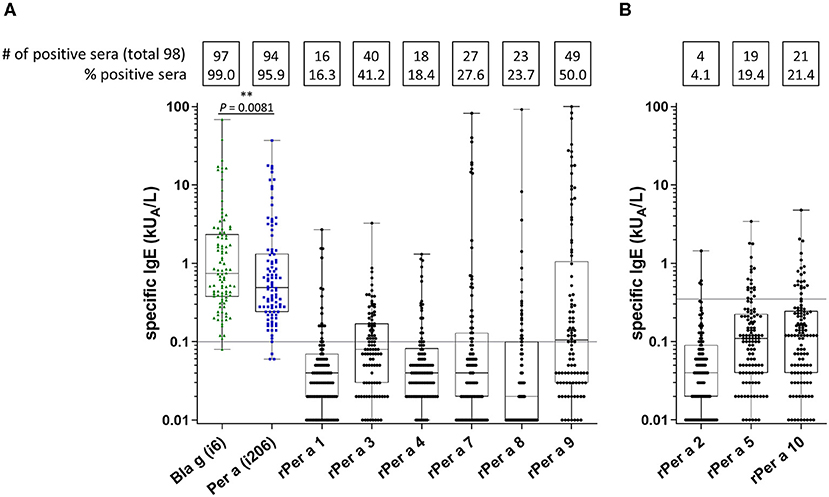
Figure 3. CRD of cockroach allergy by experimental ImmunoCAP testing including cockroach-allergic patients preselected by positive Periplaneta- or Blattella-specific ImmunoCAP values (n = 98). Specific IgE values for Periplaneta and Blattella extracts and Per a 1, Per a 3, Per a 4, Per a 7, Per a 8, and Per a 9 (cutoff 0.1 kUA/L) (A), as well as for Per a 2, Per a 5, and Per a 10 (cutoff 0.35 kUA/L) (B) are depicted. (paired t-test).
The sensitization pattern within each patient group was diverse and not comparable between the different patient collectives (Figure 4). Analysis of individual patient groups from different geographical areas revealed the highest prevalence of IgE reactivity to Per a 3 (59 and 67%) and Per a 9 (57 and 67%) in Indian and Korean patients. Per a 9 was the most prominent (45%) allergen also among the Iranian patients, whereas patients from Venezuela reacted predominantly to Per a 7 (31%) (Figure 4). Per a 4 and Per a 8 were identified as minor allergens in patients from almost all investigated geographies. However, the prevalence of sensitization to these components was the lowest among subjects from Venezuela. Patients from India and Korea showed low frequencies of IgE reactivity to Per a 2, Per a 5, and Per a 10 (Figure 4) (India: 3% to Per a 2, 28% each to Per a 5 and 10; Korea: 11% to Per a 2, 44% to Per a 5 and 48% to Per a 10). Notably, none of the Venezuelan or Iranian patients were sensitized to any of these three proteins (Figure 4).
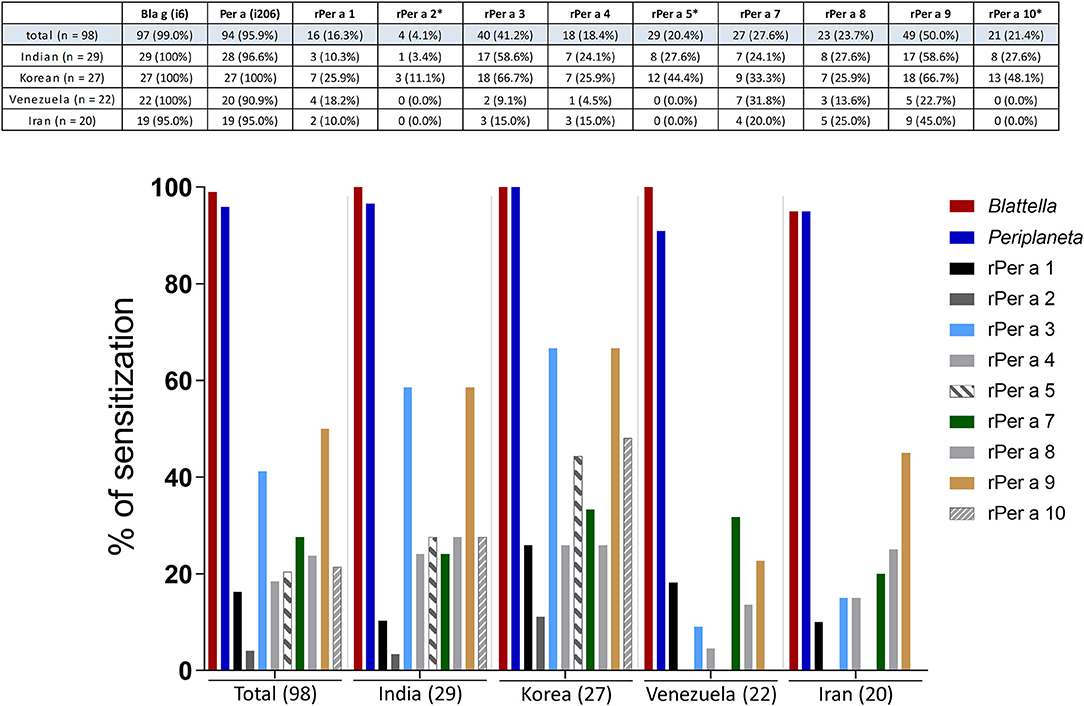
Figure 4. Comparison of IgE antibody levels to Periplaneta (i206), Blattella (i6), and purified recombinant Periplaneta allergens in respective patient collectives from India (n = 29), Korea (n = 27), Venezuela (n = 22), and Iran (n = 20). Prevalence (%) of IgE sensitization to Per a 1, Per a 3, Per a 4, Per a 7, Per a 8, and Per a 9 (cutoff of 0.1 kUA/L) and Per a 2*, Per a 5*, and Per a 10* (cutoff of 0.35 kUA/L) are shown.
Of the 95 Periplaneta ImmunoCAP-positive patients, 28 (29%) did not react with any of the purified allergens tested (Supplementary Table 2). This discrepancy was most prominent in serum samples from Iran 9/19 (47%), but less frequent in samples from India 6/28 (21%), Korea 7/27 (26%), and Venezuela 6/21 (29%). It is tempting to speculate that these patients are sensitized to other Periplaneta proteins or isoforms not reflected by the present CRD panel. In contrast, only 4% (5/117) of the patients showed IgE sensitization (with IgE levels close above the cutoff) to one or more of the purified allergen(s) despite a negative test result with Blattella and Periplaneta ImmunoCAP.
IgE Immune Reactivity of Periplaneta Extract
Sera of cockroach-sensitized patients (n = 7) from Venezuela either lacking any IgE reactivity (VR03, VR06, VR10, VR20) or showing very low IgE values of 0.12 kUA/L (VR07, VR12) to purified allergens were further analyzed by immunoblotting using Periplaneta extract (Figure 5). Two sera (Sal and PL6) were applied as positive controls. IgE binding to putative novel and yet not identified Periplaneta proteins was demonstrated for six out of seven sera, showing IgE binding to distinct high molecular weight (HMW) proteins and proteins with an apparent molecular weight of 16 kDa (VR3, VR12), 22 kDa (VR12), and 40 kDa (VR6, VR9, and VR20) not verified so far.
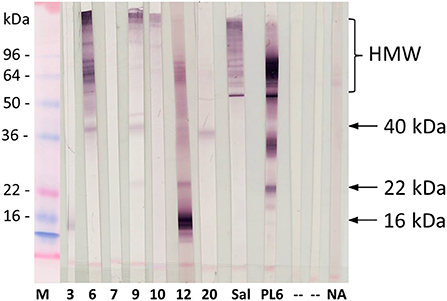
Figure 5. IgE-reactivity pattern of cockroach-reactive patients from Venezuela (n = 7, VR3, VR6, VR7, VR9, VR10, VR12, VR20) showing negative ImmunoCAP results for recombinant Periplaneta allergens, Germany (Sal), and pooled sera from cockroach-sensitized patients (PL6) with Periplaneta extract. M, marker; P, Ponceau S, staining; NA, non-atopic control; “-”, secondary antibody control. Arrows indicate the putative yet not identified allergens.
However, the accumulation of both proteins, lipocalin and myosin light chain, in Periplaneta extract was confirmed by MS/MS analysis. Peptides derived from both allergens showed a sequence coverage of 15 and 29% (for Per a 4) and 34 and 59% (for Per a 8), in two extracts analyzed independently (Supplementary Figure 3).
Discussion
Cockroaches are an important source of indoor aeroallergens. However, allergens from American cockroach (P. americana) are less investigated in comparison with German cockroach (B. germanica) allergens, and reports on the involvement of individual Periplaneta allergens in allergy related morbidity are inconsistent. Notably, clinically relevant sensitization to Blattella across Europe was reported in the GA2LEN skin test study (29). In addition, sensitization to different cockroach species has been reported in South Italy (30), demonstrating positive RAST results to Periplaneta in four of 15 cockroaches (mixed species) SPT-positive patients (31). However, in Italian children, sensitization was substantially higher to Periplaneta than to Blattella (32). So far, the molecular sensitization profile to Periplaneta allergens was not addressed in these studies, except one study describing reactivity to Per a 7 (tropomyosin) in 41% of Periplaneta-sensitized indoor allergic patients from Marseille (19). All these studies implicate that cockroach allergen exposure and sensitization are a global health problem that will likely increase along with global warming. This prompted us to investigate the involvement of Periplaneta allergens in the sensitization profile to cockroach.
In the present study, using different patient collectives, a substantial panel of recombinant Periplaneta allergens, including yet less well-characterized allergens Per a 4 and Per a 8, was utilized for CRD employing a uniform analytical methodology. The identity of all purified Periplaneta allergens used in the study was confirmed by MS. We attempted to produce Per a 6 but were unable to confirm its identity. Three known Periplaneta allergens Per a 11 to Per a 13 were not included in the study since they were not yet been identified at the time the study was initiated.
A total of 117 patients from four countries with subtropical or tropical climates, with perennial respiratory indoor allergy, and evidence of IgE sensitization to cockroach (evaluated by in vitro and in vivo methods), were included in the study. Of note, the limitation of the study is the lack of consistent inclusion criteria in terms of clinical cross-reactivity (co-sensitization with cross-reactive mite and insect allergens), and the application of different IgE detection methods in different cohorts, which can lead to a bias of the preselected patients. Cockroach-specific IgE was re-evaluated and confirmed in 81% and 83% of all sera by commercial ImmunoCAP testing, using Periplaneta or Blattella extracts, respectively. Whereas, sera from Korean patients were preselected by positive ImmunoCAP results, IgE sensitization of patients from Venezuela, India, and Iran was assessed by either skin testing, ELISA, or immunoblotting, showing a correlation with Periplaneta-positive ImmunoCAP testing between 63 and 80%. Discrepancy between results from ImmunoCAP and other test systems might be due to various applications of non-standardized and self-prepared extracts, as well as the different diagnostic sensitivity of each test system.
ImmunoCAP testing showed an almost similar diagnostic sensitivity for Blattella and Periplaneta extract and levels of IgE to the two cockroach species were strongly correlated (r = 0.95). Only one patient with low Periplaneta-specific IgE but negative Blattella-specific IgE values was detected. Vice versa, four patients with reactivity to Blattella, but without sensitization to Periplaneta, were identified. Finally, the diagnostic sensitivity using recombinant allergens was increased for five patients who did not react with any of the two extracts. The overall concordant results are likely explained by the presence of conserved and cross-reactive allergens in Periplaneta and Blattella, consistent with the official recognition of allergens from at least seven groups identified in both species. However, only partial IgE cross-reactivity between members of homologous groups has been reported (2). Slight variability of IgE-binding capacity between the species might be due to distinct expression levels of certain allergens in cockroaches, a variable presence of allergens in the applied extracts, or the abundance of species-specific allergens. In line with this, group 4 and 8 allergens were already denominated according to the IUIS allergen nomenclature database for Blattella but not for Periplaneta, whereas group 10 and 13 allergens have so far not been identified in Blattella. In the present study, we produced recombinant Periplaneta proteins homologous to Bla g 4 and Bla g 8 and included them in the panel of allergens used for CRD.
Component-resolved diagnostics was performed with patients (n = 98) reporting perennial respiratory indoor allergies and IgE sensitization to Blattella or Periplaneta, confirmed by ImmunoCAP testing as a uniform methodology across the entire population. Results showed only 68% (67/98) of ImmunoCAP-positive patients to react with any of the nine recombinant Periplaneta allergens tested. However, a study addressing CRD of Blattella allergy suggested a panel of only four allergens (Bla g 1, Bla g 2, Bla g 4, and Bla g 5) to be sufficient to identify 95% of cockroach-allergic patients in the United States (33). In another study, all patients with airway allergy and positive Blattella ImmunoCAP values were reported to react with at least one of Per a 1 to 7 and Per a 9, tested by ELISA (12). In contrast, our study is in agreement with other reports demonstrating that a set of several recombinant Blattella allergens fall short of reflecting the IgE reactivity of corresponding natural Blattella allergen extract (34, 35). By applying a panel of allergens, only 49% and 62% of cockroach-allergic patients were tested positive either by skin testing (35) or by in vitro assays (34), respectively. Both studies are in accordance with our present results showing a higher diagnostic sensitivity of the natural extract in comparison with the applied recombinant allergens. The higher diagnostic value of cockroach extract in comparison with the panel of purified allergens can be explained by missing allergens which need to be included in CRD, structural modification of recombinant allergens affecting the IgE reactivity (e.g., mediated by posttranslational modifications, or partial aggregation of recombinants). Furthermore, the presence of IgE-reactive natural variants/isoforms that are not reflected by the selected recombinant allergens could play a role in the lower diagnostic sensitivity of the CRD.
The present study demonstrated a heterogeneous pattern of IgE sensitization to Periplaneta allergens in different patient collectives. The IgE-reactivity profiles could be in part due to the exposure and IgE responses to abundant cross-reactive homologous allergens. For all Periplaneta allergens except Per a 1, homologous groups and potential cross-reactive allergens have been described among invertebrates other than cockroaches (1). In agreement with a previous notion by Pomes et al. (1), we did not observe any dominant and thus major Periplaneta allergen considering the whole study collective. Importantly, the analysis of the individual geographical different cohort revealed Per a 3 and Per a 9 as major allergens (with IgE prevalence ≥50% in the respective patient group) in India and Korea. This, together with the occurrence of extract-positive subjects without IgE to any of the components included in this study, indicates the need to identify and evaluate additional Periplaneta allergens in each cohort.
In the present study, lipocalin and myosin light chain were classified for the first time as minor Periplaneta allergens in all investigated cohorts with an overall frequency of IgE sensitization of 18 and 24%, respectively. Both proteins were accepted by the WHO/IUIS Allergen Nomenclature Sub-Committee as Per a 4 and Per a 8. Sensitization to recombinant Per a 4 was most prevalent in patients from Korea (26%), and sensitization to recombinant Per a 8 was most prevalent in Indian patients (28%). Whether the IgE-binding capacity of recombinant allergens is reflected by the natural counterparts remains open. However, our study provided evidence that the prominence of allergens is different in the respective patient collective. The results are in agreement with a limited number of studies describing molecular sensitization profiles to Periplaneta allergens and showing a heterogeneous clinical significance of single allergens. In line with this, the prevalence of IgE sensitization of 5–100% for Per a 1, 63% for Per a 2, 26–95% for Per a 3, 30–70% for Per a 5 (16), 13–54% for Per a 7, 80–100% for Per a 9, 82% for Per a 10, 83% for Per a 11, and 64% for Per a 12 has been reported (7). A retrospective study of 118 cockroach-sensitized subjects from inner-city environments in the United States revealed that only 13% were sensitized to Per a 7 (34). In contrast, Per a 7 was found to be the dominant allergen among 55 cockroach-allergic patients in Brazil, with a prevalence of IgE reactivity of 42% (35).
Our data suggested the involvement of yet unidentified Periplaneta allergens (including isoforms and variants) and/or a potential important role of Per a 6, Per a 11 to Per a 13, which were not tested in the current study. Moreover, 2D-immunoblotting and subsequent MS analysis using pooled sera, not reactive to any of the tested recombinant Per a allergens, revealed IgE binding to 12- and 16-kDa proteins, in addition to a 40-kDa band which might correspond to Per a 11 to Per a 13 (data not shown). In addition, the role of protein glycosylation in the immunogenicity of cockroach allergy (2) needs to be considered. Insects express immunogenic core α-1,3 carbohydrate structures with additional −1,6 fucosylation (36), and glycan modification of Blattella allergens has been described (37, 38). Although reports on cross-reactive carbohydrate determinants (CCD) of cockroach allergens are limited, and routine diagnosis lacks insect-specific glycan moieties, it is tempting to speculate that a substantial number of cockroach-sensitized patients are reactive to glycan structures which in part may explain our observed gap in diagnostic sensitivity between natural cockroach extracts and a panel of recombinant, non-glycosylated allergens.
In summary, we show that levels of IgE antibodies to Periplaneta and Blattella are strongly correlating and that natural Periplaneta extract displays higher diagnostic sensitivity than a panel of nine recombinant Periplaneta allergens. For reasons that are elusive, patient collectives from different countries showed heterogeneous sensitization profiles. Notably, major allergens could be identified only for individual cohorts, while Per a 4 and Per a 8 were identified as minor allergens. Further improvement of Periplaneta CRD requires exploration of the role of isoforms, glycan determinants, and additional yet unknown allergens, which are likely classified already in other insects.
Data Availability Statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/Supplementary Material.
Ethics Statement
The studies involving human participants were reviewed and approved by Ethical approval was obtained from IHEC (no. CLP 0019, CSIR-IGIB) and Isfahan university of Medical Sciences and Health services (no. 295264). Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
Author Contributions
JS, RR, RS, SSa, FT, J-WP, and NA recruited and characterized the patients. Recombinant Per a 1, Per a 2, and Pera 3 were prepared and characterized by SE and IP. Per a 4, Per a 7, Per a 8, and Per a 9 by AW and AJ. Per a 5, Per a 6, and Per a 10 by SSh. Protein and MS analysis were performed by BS, BK, SW, GG and PB. Experimental ImmunoCAPs were established and performed by JL and FFü. NA, FFe, and SV were responsible for the study. GG, AW, and SSc coordinated the study and wrote the manuscript. All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
This study was funded by ERAnet New INDIGO Project GENALL DBT2-038. The authors wish to thank Ulrica Olsson for preparing experimental ImmunoCAP tests and Elke Haberkorn for excellent technical support and conducting the ImmunoCAP measurements.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/falgy.2021.691627/full#supplementary-material
References
1. Pomés A, Chapman MD, Wünschmann S. Indoor allergens and allergic respiratory disease. Curr Allergy Asthma Rep. (2016) 16:43. doi: 10.1007/s11882-016-0622-9
2. Do DC, Zhao Y, Gao P. Cockroach allergen exposure and risk of asthma. Allergy. (2016) 71:463–74. doi: 10.1111/all.12827
3. Nasirian H. Infestation of cockroaches (Insecta: Blattaria) in the human dwelling environments: a systematic review and meta-analysis. Acta Trop. (2017) 167:86–98. doi: 10.1016/j.actatropica.2016.12.019
5. Tsai JJ, Kao MH, Wu CH. Hypersensitivity of bronchial asthmatics to cockroach in Taiwan. Comparative study between American and German cockroaches. Int Arch Allergy Immunol. (1998) 117:180–6. doi: 10.1159/000024008
6. Sun B-Q, Lai X-X, Gjesing B, Spangfort MD, Zhong N-S. Prevalence of sensitivity to cockroach allergens and IgE cross-reactivity between cockroach and house dust mite allergens in Chinese patients with allergic rhinitis and asthma. Chin Med J. (2010) 123:3540–4. doi: 10.3760/cma.j.issn.0366-6999.2010.24.007
7. Pomés A, Mueller GA, Randall TA, Chapman MD, Arruda LK. New insights into cockroach allergens. Curr Allergy Asthma Rep. (2017) 17:25. doi: 10.1007/s11882-017-0694-1
8. Sookrung N, Tungtrongchitr A, Chaicumpa W. Cockroaches: allergens, component-resolved diagnosis (CRD) and component-resolved immunotherapy. Curr Protein Peptide Sci. (2020) 21:124–41. doi: 10.2174/1389203720666190731144043
9. Wu C-H, Wang NM, Lee M-F, Kao C-Y, Luo S-F. Cloning of the American cockroach Cr-PII allergens: evidence for the existence of cross-reactive allergens between species. J Allergy Clin Immunol. (1998) 101:832–40. doi: 10.1016/S0091-6749(98)70312-4
10. Melén E, Pomés A, Vailes LD, Arruda L, Chapman MD. Molecular cloning of Per a 1 and definition of the cross-reactive Group 1 cockroach allergens. J Allergy Clin Immunol. (1999) 103:859–64. doi: 10.1016/s0091-6749(99)70430-6
11. Yang C-Y, Wu J-D, Wu C-H. Sequence analysis of the first complete cDNA clone encoding an American cockroach Per a 1 allergen. Biochim Biophys Acta Gene Struct Express. (2000) 1517:153–8. doi: 10.1016/s0167-4781(00)00235-9
12. Lee M-F, Song P-P, Hwang G-Y, Lin S-J, Chen Y-H. Sensitization to Per a 2 of the American cockroach correlates with more clinical severity among airway allergic patients in Taiwan. Ann Allergy Asthma Immunol. (2012) 108:243–8. doi: 10.1016/j.anai.2012.01.014
13. Wu CH, Lee MF, Liao SC, Luo SF. Sequencing analysis of cDNA clones encoding the American cockroach Cr-PI allergens. J Biol Chem. (1996) 271:17937–43. doi: 10.1074/jbc.271.30.17937
14. Wu CH, Lee MF, Wang NM, Luo SF. Sequencing and immunochemical characterization of the American cockroach Per a 3 (Cr-PI) isoallergenic variants. Mol Immunol. (1997) 34:1–8. doi: 10.1016/s0161-5890(97)00009-6
15. Sookrung N, Reamtong O, Poolphol R, Indrawattana N, Seesuay W, Saelim N, et al. Glutathione S-transferase (GST) of American cockroach, Periplaneta americana: classes, isoforms, and allergenicity. Sci Rep. (2018) 8:484. doi: 10.1038/s41598-017-18759-z
16. Wei J-F, Yang H, Li D, Gao P, He S. Preparation and identification of Per a 5 as a novel American cockroach allergen. Mediat Inflamm. (2014) 2014:1–10. doi: 10.1155/2014/591468
17. Sharma S, Arora B, Gaur SN, Arora N. Bioinformatic and immunological investigation of Per a 5 (delta class GST) allergen from Periplaneta americana. Mol Immunol. (2021) 132:93–101. doi: 10.1016/j.molimm.2021.01.026
18. Hindley J, Wunschmann S, Satinover S, Woodfolk J, Chew F, Chapman M, et al. Bla g 6: a troponin C allergen from Blattella germanica with IgE binding calcium dependence. J Allergy Clin Immunol. (2006) 117:1389–95. doi: 10.1016/j.jaci.2006.02.017
19. Asturias JA, Gómez-Bayón N, Arilla MC, Martínez A, Palacios R, Sánchez-Gascón F, et al. Molecular characterization of American cockroach tropomyosin (Periplaneta americana allergen 7), a cross-reactive allergen. J Immunol. (1999) 162:4342–8.
20. Santos AB, Chapman MD, Aalberse RC, Vailes LD, Ferriani VP, Oliver C, et al. Cockroach allergens and asthma in Brazil: identification of tropomyosin as a major allergen with potential cross-reactivity with mite and shrimp allergens. J Allergy Clin Immunol. (1999) 104:329–37. doi: 10.1016/s0091-6749(99)70375-1
21. Sookrung N, Chaicumpa W, Tungtrongchitr A, Vichyanond P, Bunnag C, Ramasoota P, et al. Periplaneta americana arginine kinase as a major cockroach allergen among Thai patients with major cockroach allergies. Environ Health Perspect. (2006) 114:875–80. doi: 10.1289/ehp.8650
22. Yang H, Chen H, Jin M, Xie H, He S, Wei J-F. Molecular cloning, expression, IgE binding activities and in silico epitope prediction of Per a 9 allergens of the American cockroach. Int J Mol Med. (2016) 38:1795–805. doi: 10.3892/ijmm.2016.2793
23. Sudha VT, Arora N, Gaur SN, Pasha S, Singh BP. Identification of a serine protease as a major allergen (Per a 10) of Periplaneta americana. Allergy. (2008) 63:768–76. doi: 10.1111/j.1398-9995.2007.01602.x
24. Fang Y, Long C, Bai X, Liu W, Rong M, Lai R, et al. Two new types of allergens from the cockroach, Periplaneta americana. Allergy. (2015) 70:1674–8. doi: 10.1111/all.12766
25. Tan YW, Chan SL, Ong TC, Le Yit Y, Tiong YS, Chew FT, et al. Structures of two major allergens, Bla g 4 and Per a 4, from cockroaches and their IgE binding epitopes. J Biol Chem. (2009) 284:3148–57. doi: 10.1074/jbc.M807209200
26. Guang-Li W, Wei L, Ling-Yun L, Song-Quan W. Cloning and purification of Per a 4, a gene encoding a Periplaneta americana allergen, and preparation of its monoclonal antibodies. Ann Clin Lab Sci. (2018) 48:323–7.
27. Wangorsch A, Jamin A, Lidholm J, Gräni N, Lang C, Ballmer-Weber B, et al. Identification and implication of an allergenic PR-10 protein from walnut in birch pollen associated walnut allergy. Mol Nutr Food Res. (2017) 61(4). doi: 10.1002/mnfr.201600902
28. Marknell DeWitt A, Niederberger V, Lehtonen P, Spitzauer S, Sperr WR, Valent P, et al. Molecular and immunological characterization of a novel timothy grass (Phleum pratense) pollen allergen, Phl p 11. Clin Exp Allergy. (2002) 32:1329–40. doi: 10.1046/j.1365-2222.2002.01467.x
29. Burbach GJ, Heinzerling LM, Edenharter G, Bachert C, Bindslev-Jensen C, Bonini S, et al. GA 2 LEN skin test study II: clinical relevance of inhalant allergen sensitizations in Europe. Allergy. (2009) 64:1507–15. doi: 10.1111/j.1398-9995.2009.02089.x
30. Liccardi G, Baldi G, Ciccarelli A, Cutajar M, D'Amato M, Gargano D, et al. Sensitization to cockroach allergens in the urban atopic populations living in Campania district (southern Italy). A multicenter study. Eur Ann Allergy Clin Immunol. (2014) 46:12–6.
31. Liccardi G, Salzillo A, Piccolo A, Russo M, D'Amato M, Stanziola A, et al. Has sensitization to cockroach allergens changed during the last 17 years in the urban atopic population living in Naples (Southern Italy)? J Investig Allergol Clin Immunol. (2013) 23:57–9.
32. Peruzzi M, Luca M de, Novembre E, Martino M de, Vierucci A. Incidence of cockroach allergy in atopic Italian children. Ann Allergy Asthma Immunol. (1999) 83:167–71. doi: 10.1016/S1081-1206(10)62631-2
33. Arruda LK, Barbosa MC, Santos AB, Moreno AS, Chapman MD, Pomés A. Recombinant allergens for diagnosis of cockroach allergy. Curr Allergy Asthma Rep. (2014) 14:1–20. doi: 10.1007/s11882-014-0428-6
34. Satinover SM, Reefer AJ, Pomes A, Chapman MD, Platts-Mills TA, Woodfolk JA. Specific IgE and IgG antibody-binding patterns to recombinant cockroach allergens. J Allergy Clin Immunol. (2005) 115:803–9. doi: 10.1016/j.jaci.2005.01.018
35. Barbosa MC, Santos AB, Ferriani VP, Pomés A, Chapman MD, Arruda L. Efficacy of recombinant allergens for diagnosis of cockroach allergy in patients with asthma and/or rhinitis. Int Arch Allergy Immunol. (2013) 161:213–9. doi: 10.1159/000346318
36. Altmann F. Coping with cross-reactive carbohydrate determinants in allergy diagnosis. Allergo J Int. (2016) 25:98–105. doi: 10.1007/s40629-016-0115-3
37. Glesner J, Wünschmann S, Li M, Gustchina A, Wlodawer A, Himly M, et al. Mechanisms of allergen-antibody interaction of cockroach allergen Bla g 2 with monoclonal antibodies that inhibit IgE antibody binding. PLoS ONE. (2011) 6:e22223. doi: 10.1371/journal.pone.0022223
Keywords: cockroach allergy, American cockroach, Blattella germanica, component-resolved diagnosis, Periplaneta americana (Insecta)
Citation: Wangorsch A, Jamin A, Eichhorn S, Pablos I, Sharma S, Schweidler B, Kastner B, Wildner S, Saloga J, Führer F, Reyna Orozco RR, Sherkat R, Sadeghi S, Teifoori F, Park J-W, Briza P, Vieths S, Ferreira F, Arora N, Lidholm J, Gadermaier G and Scheurer S (2021) Component-Resolved Diagnosis of American Cockroach (Periplaneta americana) Allergy in Patients From Different Geographical Areas. Front. Allergy 2:691627. doi: 10.3389/falgy.2021.691627
Received: 06 April 2021; Accepted: 03 June 2021;
Published: 29 June 2021.
Edited by:
Alain Jacquet, Chulalongkorn University, ThailandReviewed by:
Andreas L. Lopata, James Cook University, AustraliaAnchalee Tungtrongchitr, Mahidol University, Thailand
Copyright © 2021 Wangorsch, Jamin, Eichhorn, Pablos, Sharma, Schweidler, Kastner, Wildner, Saloga, Führer, Reyna Orozco, Sherkat, Sadeghi, Teifoori, Park, Briza, Vieths, Ferreira, Arora, Lidholm, Gadermaier and Scheurer. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Stephan Scheurer, c3RlcGhhbi5zY2hldXJlckBwZWkuZGU=
 Andrea Wangorsch
Andrea Wangorsch Annette Jamin1
Annette Jamin1 Swati Sharma
Swati Sharma Stefan Vieths
Stefan Vieths Fatima Ferreira
Fatima Ferreira Naveen Arora
Naveen Arora Gabriele Gadermaier
Gabriele Gadermaier Stephan Scheurer
Stephan Scheurer