- 1Department of Dermatology, University of Texas MD Anderson Cancer Center, Houston, TX, United States
- 2Department of Dermatology, University of Texas Health Science Center- Houston, Houston, TX, United States
- 3Department of Internal Medicine, HCA Houston Healthcare West, Houston, TX, United States
- 4Department of Internal Medicine, Texas Health Presbyterian Hospital, Dallas, TX, United States
Immune checkpoint inhibitors (ICIs) have revolutionized cancer therapy. Since the approval of ipilimumab in 2011, a total of nine ICIs have gained indications for various solid and hematologic malignancies. The expanding use of ICIs in oncology underscores the need for diagnosis and treatment expertise in immune related adverse events (irAE). Cutaneous toxicities are the earliest and most common irAE in this class of therapy. In addition to the more frequent reactions including vitiligo, lichenoid dermatitis, psoriasiform dermatitis, other less common skin toxicities including bullous dermatoses, neutrophilic dermatoses, and autoimmune dermato-rheumatologic diseases have been reported. Even though less than 3% of cutaneous irAEs (irCAEs) are classified as grade 3 or higher events, irCAEs can greatly impact quality of life. Appropriate management of irCAEs is critical to avoid unwarranted interruptions or discontinuation of lifesaving immunotherapy.
Introduction
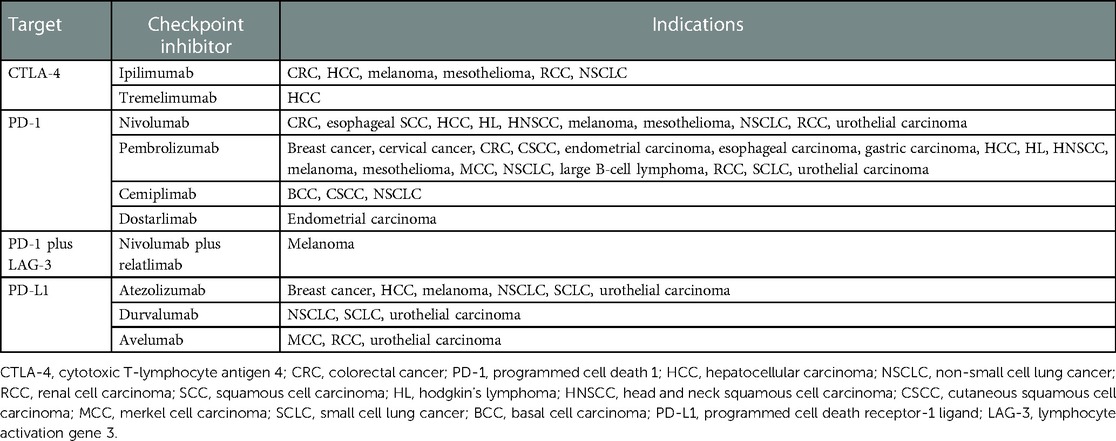
Immune checkpoint inhibitors (ICIs) are monoclonal antibodies that enhance T-lymphocyte response by targeting cytotoxic T-lymphocyte antigen 4 (CTLA-4), programmed cell death 1 (PD-1), and programmed cell death ligand 1 (PD-L1). Ipilimumab was the first ICI approved in 2011 for advanced melanoma. Since then, nine checkpoint inhibitors have approved indications for various solid and hematologic malignancies (Table 1) (1–3).
Antibodies to the PD-1/PD-L1 or CTLA-4 axis lift the constitutional inhibitory immune response. This enhances anti-tumor lymphocyte activity, but also contributes to immune related adverse events (irAEs) in over a third of patients (4). While any organ system is susceptible, cutaneous irAEs (irCAEs) are among the first and most frequent to develop, affecting 30%–60% of patients (5–8). Appropriate diagnosis and management of irCAEs is associated with reduced use of immunosuppressive agents and continuation of lifesaving immunotherapy (8). As the approved clinical use of ICIs broadens, recognition and appropriate management of dermatological toxicities becomes increasingly important. In this article we provide an updated review of the clinical spectrum and management of irCAEs.
Epidemiology
IrCAEs are not dose dependent and occur irrespective of underlying malignancy (6), although melanoma and renal cell carcinoma appear to portend a greater risk (4). Patient characteristics such as cytokine profiles and human leukocyte antigens may also predict irCAEs (7).
CTLA-4 inhibitors are traditionally associated with a higher incidence of irCAEs compared to PD-1/PD-L1 inhibitors (1, 5, 7, 9), 43%–45% vs. 18%–34%, respectively (5, 10). True incidence of irCAEs overall is difficult to ascertain, as outside of clinical trials, mild toxicities may be underreported. Additionally, the frequency of specific dermatoses may be confounded by nonspecific rash terminology used in ICI trials (11).
IrCAEs manifest earlier than other irAEs, usually within 3–6 weeks and 5–9 weeks after initiation of ipilimumab and PD-1/PD-L1 inhibitors, respectively (7). Most irCAEs are low-grade with less than 3% progressing to grade 3 or 4 reactions per Common Terminology Criteria for Adverse Events (CTCAE) v.5 grading (7).
Combination immunotherapy with CTLA-4 and PD-(L)1 inhibitors is increasingly utilized. This improves therapeutic efficacy but also increases the incidence of all and high-grade irAEs (1, 9, 12), including irCAEs (59%–72%) (7). Grade 3–4 irCAEs occur in 2%–3% of patients receiving monotherapy compared to 4%–10% of patients on combination regimens (6).
Pathogenesis
Checkpoints maintain immune homeostasis and self-tolerance. PD-1 is expressed by T-cells, B-cells, natural killer cells, and tumor-infiltrating lymphocytes (2). Antigen-presenting cells and nonimmune cells, including tumor cells, express ligands PD-L1 and PD-L2 (3, 7, 9). The binding of PD-L1/PD-L2 to PD-1 prevents lymphocyte activation against self-antigens but it inadvertently enables tumor evasion (1, 2, 5). PD-(L)1 inhibitors disrupt this interaction, thereby promoting T-cell activity.
CTLA-4, which is expressed on activated T-cells, inhibits T-cell activation when bound to co-stimulatory molecule CD28 (1, 7). CTLA-4 inhibitors, ipilimumab and tremelimumab interfere with this inhibitory signal and allow for unopposed T-lymphocyte activation.
Investigative targets for immune inhibitor pathways include lymphocyte-activation gene-3 (LAG3), T-cell immunoglobulin and mucin domain-3 (TIM3), V-domain Ig suppressor of T-cell activation (VISTA), and B and T lymphocyte attenuator (BTLA) (9). Relatlimab, a LAG3 inhibitor, was recently approved in combination with nivolumab for advanced melanoma (3).
While enhancing anti-tumor activity, the pharmacological blockade of CTLA-4 and PD-1/PD-L1 promotes autoimmunity via activation of tissue-resident immune cells (5, 13). IrAEs can also arise from cross-reactivity between tumor cells and self-antigens on normal tissue (5, 13). There is evidence to suggest that photodamaged skin is more susceptible to irCAEs (5, 14). Ultraviolet-induced cellular injury and subsequent release of self-antigens creates a pro-inflammatory milieu where autoreactive T-cells are already primed before ICI exposure (5, 13). Though further studies are needed, oral nicotinamide may help delay the onset of irCAEs (14).
Cutaneous adverse events
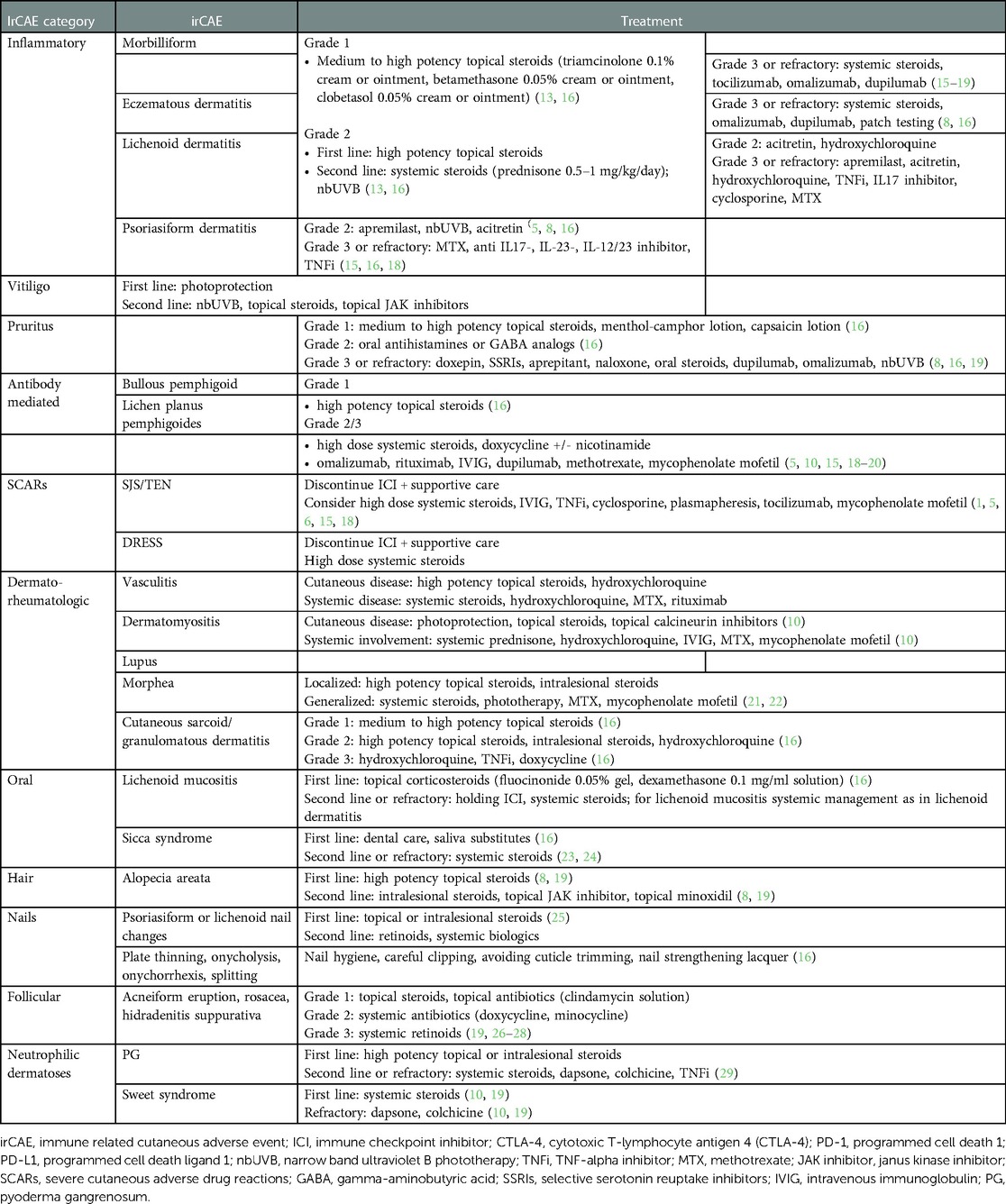
The most common irCAEs include pruritus, vitiligo, morbilliform drug, eczematous, lichenoid, and psoriasiform eruptions. Most irCAEs are mild and can be managed without discontinuation of immunotherapy (7, 15). Table 2 includes a summary of management of mucocutaneous adverse events in the setting of ICIs. Grade 1–2 eruptions can typically be managed with topical steroids, bland emollients, and oral antihistamines, whereas high-grade eruptions require more specific management based on the rash type (13, 16).
Morbilliform/maculopapular eruptions
Morbilliform eruptions are the most frequent irCAE seen in 49%–68% of patients on CTLA-4 inhibitors and 20% of patients on PD-1/PD-L1 inhibitors (1, 10, 17). Onset occurs in the first 3–6 weeks after ICI initiation (1, 7, 17). Clinical findings include variably pruritic coalescing macules and papules. Histopathological examination reveals interface changes and a perivascular/periadnexal lymphocytic infiltrate with or without eosinophils (7, 18). Morbilliform rashes are usually low-grade and self-limited within 2–3 months (1, 5, 15). For high grade rash with intractable pruritus, prednisone 0.5–1 mg/kg/day can be given and tapered once the eruption improves to grade 1 (16, 18, 19). Steroid-sparing alternatives (SSAs) including narrowband ultraviolet light B (nbUVB), tocilizumab (anti-IL6R monoclonal antibody), dupilumab (anti-IL4Ralpha antibody), or omalizumab (anti-IgE monoclonal antibody) (15, 18) can be considered for severe presentations and chronic management.
Pruritus
Pruritus, with or without rash affects 25%–36% of patients on CTLA-4 immunotherapy and up to 47% of patients on combination ICI (5, 6). Less than 3% of patients develop refractory grade 3 pruritus (5, 8). Onset occurs in the first 3–10 weeks of therapy (8). Basic laboratory workup is recommended for recalcitrant cases and should include eosinophil counts, serum IgE, renal, and hepatic prolife (8, 16, 19). Additionally, nonbullous phase of bullous pemphigoid (BP) should be considered and ruled out with skin biopsy and serologies (19).
Considerations for recalcitrant pruritus include biologics such as dupilumab and omalizumab, aprepitant (neurokinin 1 receptor agonist), low dose naltrexone, and systemic steroids (1, 8, 16, 19).
Lichenoid eruptions
Lichenoid dermatitis is well documented with PD-1/PD-L1 inhibitors and less reported with ipilimumab therapy (1, 7, 8, 10). Most lichenoid reactions are mild with only 2% being grade 3 or higher (6). Pruritus is common and clinical presentation is heterogeneous ranging from flat-topped purple papules of classic lichen planus to a more morbilliform appearing rash (7, 8). Other variants include inverse, erosive, and hypertrophic lichen planus (LP) (5, 8). Genitalia, oral mucosa, and nails can be affected. Bullous lichen planus pemphigoides (LPP), pityriasis lichenoides chronica, and lichen sclerosus have been reported (7). Histopathology shows lichenoid infiltrate at the dermo-epidermal junction, basal vaculoar degeneration, and Civatte bodies (7, 8). Compared to idiopathic (LP), spongiosis is a feature seen more in ICI-induced lichenoid eruptions (1, 15, 17). Additionally, the inflammatory infiltrate is more closely related to the immune cell composition seen in acute graft-vs.-host-disease than to idiopathic LP (30).
For high-grade rashes, methotrexate, tumor necrosis alpha (TNF-alpha) inhibitors, cyclosporine, IL17 inhibitors, and apremilast have been used (8, 10, 17, 19). Importantly, lichenoid reactions may persist after immunotherapy discontinuation (19).
Psoriasiform eruptions
Psoriasis secondary to anti-PD-1/PD-L1 therapy has an incidence of 12% and it occurs de novo or as exacerbation of pre-existing disease (1, 5, 6, 8, 15). De novo psoriasis is seen within 5–12 weeks of ICI initiation (8). Exacerbation of pre-existing disease occurs earlier and can affect up to 80% of patients (1, 17, 19). Plaque psoriasis is the most common but other subtypes including palmoplantar, pustular, erythrodermic, and inverse have been reported (5, 6, 8, 18). Importantly, psoriasiform reactions can be associated with psoriatic arthritis and uveitis (5, 6, 18). Histopathology shows epidermal acanthosis, parakeratosis, hypogranulosis with variable spongiosis (7, 18).
Grade 3 or higher eruptions require holding ICIs and consideration for methotrexate or systemic biologics, including IL17, IL23, IL12/23 antibodies, or TNF-alpha inhibitors (15, 18). In patients with active malignancy, broad immunosuppressants should be avoided and more targeted regimens pursued. TNF- and IL12/23-inhibitors have a higher risk of infection than newer biologics (10). IL23 and IL17 antibodies selectively inhibit Th17 axis cytokines, are minimally immunosuppressive, and have a rapid onset of action (1). However, IL17 inhibitors may exacerbate colitis as an irAE from ICIs (17). Though targeted SSAs are preferred, the effect of biologics approved in primary psoriasis has not been widely evaluated in oncology patients on immunotherapy (10, 19).
Eczematous reactions
Eczematous dermatitis affects up to 20% of patients on PD-1/PD-L1 inhibitors and up to 68% of patients on anti-CTLA-4 agents (8). It has an early onset within 6 weeks of therapy (8). Clinical findings include ill-defined coalescing erythematous patches and papules with secondary skin changes and flexural predominance (5). Main histopathological features include epidermal spongiosis and minimal lymphocyte exocytosis with perivascular, lymphocytic infiltrate with eosinophils (7, 8).
For recalcitrant grade 3 rash, advanced therapy with dupilumab or omalizumab can be pursued (8).
Vitiligo
Vitiligo is mostly reported in patients with metastatic melanoma (1, 5, 7, 8). In skin of color patients, it can have a significant psychosocial impact, particularly in cosmetically sensitive areas. In contrast to intrinsic vitiligo, it affects sun exposed surfaces more commonly and is less likely to koebnerize (1, 5). Poliosis can be seen at the same time (8). Onset is delayed, usually months after ICI initiation (8). Strict photoprotection is recommended. Though treatment is not required, topical steroids, topical janus kinase inhibitors, and nbUVB can be considered (31) Vitiligo usually persists after ICI discontinuation (1, 8).
Immunobullous reactions
Bullous pemphigoid (BP) is well-documented in the setting of PD-1/PD-L1 inhibitors, with an incidence of 1%–5% (8). Onset can be delayed to months, making it one of the longer latency irCAEs (6, 7, 18, 19). ICI-induced BP presents similarly to intrinsic disease with tense bullae overlying erythematous plaques, accompanied or preceded by intractable pruritus (5, 15). Nonbullous manifestations are not uncommon and include pruritic urticarial papules and plaques. In contrast to idiopathic BP, mucosal involvement is common, up to 40% (8, 18).
As in classic BP, histopathology demonstrates subepidermal clefting with eosinophils. DIF shows linear deposits of IgG and C3 at the epidermal site or roof of the split (7, 8, 18). Antibodies against BP180 and less frequently BP230 are often elevated (8, 18).
BP may lead to ICI discontinuation in up to 70% of patients and can often be more resistant to treatment (30). As even small body surface area (BSA) involvement can be significant, high potency topical steroids are often used in conjunction with systemic therapies. First line systemic agents include oral steroids and doxycycline with or without niacinamide. An SSA should be started at presentation for grade 2 or higher eruptions to avoid ICI interruption and reliance on systemic steroids (20). Omalizumab, dupilumab, rituximab, intravenous immunoglobulin (IVIG), methotrexate can be used for grade 2–3 disease (5, 10, 15, 18–20). Importantly, BP can persist after discontinuation of ICIs emphasizing the need for selecting SSAs (1, 8).
LPP has overlapping features of BP and lichen planus. It has been reported mainly in the setting of nivolumab and pembrolizumab (32–34). Clinical features are heterogeneous with papules, plaques, erosions, and bullae on trunk and extremities. Oral involvement is common with one case series showing 50% (33). Histopathologic features include subepidermal blister with lichenoid or vaculoar interface dermatitis. DIF demonstrates linear IgG and C3 along the basement membrane zone. Serologies are positive for BP180 antibodies, while antibodies to BP230 have not been reported thus far (33–37).
Other less frequently reported blistering diseases include pemphigus vulgaris, mucous membrane pemphigoid, linear IgA bullous dermatosis, and dermatitis herpetiformis (6, 8, 18).
Severe cutaneous adverse reactions (SCARs)
True Stevens Johnson syndrome and toxic epidermal necrolysis (SJS/TEN) from CTLA-4 and PD-1/PD-L1 inhibitors are rare but they portend poor prognosis with mortality rates of 10% for SJS and 50% for TEN (6, 18). Clinical manifestations include fever, malaise, and diffuse erythema progressing to flaccid bullae with epidermal detachment. Mucosal involvement affects the conjunctivae, genitalia, oral cavity, the respiratory, and gastrointestinal tract (5, 18). Skin biopsy reveals full thickness epidermal necrosis with a sparse dermal infiltrate (7, 15, 18).
In addition to discontinuing the culprit medication, supportive care, including ophthalmologic, gynecologic and urologic evaluation is critical. Admission to an intensive care or burn unit is recommended for extensive involvement. High dose steroids (prednisone or methylprednisolone 1 mg/kg/day) are usually administered and tapered once re-epithelialization occurs (16, 18). Intravenous immunoglobulin (IVIG), cyclosporine, tocilizumab, TNF-inhibitors, plasmapheresis, mycophenolate mofetil can also be used to halt progression (1, 5, 6, 15, 18).
PD-1/PD-L1 inhibitors can be associated with SJS/TEN-like eruptions, which unlike true SJS/TEN, evolve over weeks from milder morbilliform rashes (10, 18, 19). Thus it is crucial for patients with morbilliform eruptions to be monitored for red-flag symptoms, such as targetoid lesions or bullae that could indicate progression to a SCAR. SJS/TEN-like eruptions can also occur de novo weeks to months after ICI initiation (18). These reactions are milder than true SJS/TEN with less eye involvement and less denuded skin. Careful ICI rechallenge can be considered for SJS/TEN-like eruptions, if patients lack an alternative anti-cancer therapy (18). For true SJS/TEN, however, rechallenge is contraindicated (13, 18).
Drug reaction with eosinophilia and systemic symptoms (DRESS) to ICIs is rare and it presents with fever, generalized morbilliform eruption, and concurrent systemic irAEs such as hepatitis, colitis, azotemia (18). Management includes discontinuation of ICIs and high dose steroids tapered slowly over 6–8 weeks. SSAs such as TNF-inhibitors, tocilizumab, or dupilumab may be considered (18).
Acute generalized exanthematous pustulosis (AGEP) to ICIs is extremely rare. Pembrolizumab, ipilimumab, nivolumab, and atezolizumab have been implicated (18). AGEP presents within 48 h, with edema, erythema, and sterile pustules. Resolution is rapid after culprit withdrawal. Topical and systemic steroids (0.5–1 mg/kg/day) can be used and re-challenge can be considered (18).
Dermatologic-rheumatologic diseases
Underlying connective tissue disease is not a contraindication to ICIs, though 50% of patients experience an exacerbation (19). Only a minority (20%–30%), however, will experience severe enough flares to warrant ICI discontinuation (13, 19, 23, 38).
Dermatomyositis has been reported to CTLA-4 and PD-1 inhibitors, though given the paraneoplastic association, exact incidence is unclear (17, 19, 39).
ICI-induced systemic lupus and lupus-like eruptions, including subacute cutaneous, bullous, and chilblain lupus have been reported in patients receiving PD-1 inhibitors or combination ICI (19, 24, 40). Interestingly, ICI-induced lupus is not female predominant and lupus autoantibodies are absent (24).
Scleroderma, eosinophilic fasciitis, and morphea (including localized plaque and generalized) have been linked to PD-1/PD-L1 inhibitors (8, 21, 22, 40–42). Underlying systemic sclerosis portends a risk for disease exacerbation including worsening skin thickening and renal crisis (43).
Vasculitis irAEs are rare, <1%, and have been reported from anti-PD1 or combination immunotherapy (40). The predominant types include giant cell arteritis, aortitis, or central nervous system vasculitis (23, 40). Reported smaller vessels vasculitides include leukocytoklastic vasculitis, type III cryoglobulinemia, granulomatosis with polyangiits and eosinophilic granulomatosis with polyangiitis (EGPA) (19, 23, 44–46). Evaluation for systemic involvement is required and often systemic steroids are initiated (19). Severe involvement may require plasma exchange or rituximab (24). Mepolizumab (IL5 antibody) was used in a case of EGPA from nivolumab (46).
Granulomatous reactions, including sarcoidosis or sarcoid-like reactions have been associated with anti-CTLA-4 and anti-PD-1/PD-L1 therapy with onset anywhere from two weeks to two years (6, 8, 9). Cutaneous sarcoidosis is seen more frequently in melanoma patients on ipilimumab (9). Other organs including the eyes, lymph nodes, and lungs can be affected (9, 17, 19, 40). ICI-induced sarcoid appears to have a mild course and in many cases discontinuation of ICIs may not be indicated (47).
Hair and nail toxicities
Alopecia areata or universalis, poliosis, changes in hair texture, and less commonly hair repigmentation have been reported, though hair toxicities to ICIs are less frequent than with conventional chemotherapy (8–10, 19, 48–50). Eosinophilic folliculitis after nivolumab leading to scarring alopecia was described in one report (51). Though ICI-induced alopecia is rare (1% for PD-1/PD-L1 inhibitors and 5% for CTLA-4 inhibitors), it can greatly impact quality of life (8, 49, 50, 52). Severe disease may warrant systemic immunomodulators such as JAK inhibitors, though a risk-benefit analysis must be performed to avoid interference with immunotherapy. Some cases may spontaneously resolve (49).
Lichen planus and psoriasis of the nail can present with or without cutaneous disease (16, 25). Non-specific nail toxicities include plate thinning, onycholysis, onychorrhexis, and splitting (8, 16). Diffuse onychodystrophy and paronychia were reported with nivolumab (53). Two cases of clinical onycholysis presenting histologically with lichenoid changes were described, also in the setting of nivolumab (54).
Oral mucosal toxicities
Compared to cytotoxic chemotherapy, the prevalence of oral toxicity is lower, affecting up to 7% of patients on PD-1/PD-L1 inhibitors (8, 52, 55). Lichenoid reactions followed by xerostomia are the most common (11, 48). Clinical findings include reticulated white patches, erythema, erosions, or gingival desquamation (8, 56). A case of lichenoid granulomatous stomatitis to nivolumab was reported, clinically mimicking lichenoid mucositis (57). Extensive involvement or recalcitrant symptoms may necessitate holding of ICI and systemic management as per cutaneous lichenoid eruptions.
One case of immune-mediated glossitis was described in association with pembrolizumab, subsequently improving on oral prednisone (58).
Anti-PD-1/PD-L1 induced sicca syndrome with xerostomia and parotid enlargement can mimic Sjogren's. SSA/SSB-antibodies, however, are absent (23, 24).
Neutrophilic dermatoses
Sweet syndrome has been reported with ipilimumab in patients with melanoma (7, 17, 19) and less commonly with PD-1/PD-L1 inhibitors (7, 17, 19). Pyoderma gangrenosum (PG) has been infrequently associated with ipilimumab in 2 cases and pembrolizumab in one (7, 17, 19, 29).
Follicular reactions
Follicular eruptions are less frequent and later in onset than with targeted therapy, nonetheless papulopustular rosacea, acneiform rash, and hidradenitis have all been reported, mainly to anti-PD-1 agents (19, 26–28).
Grading criteria
Grading follows the CTCAE, which focuses on BSA involvement (59). The severity of irCAEs should not be merely based on BSA but rather on symptoms and specific dermatosis. For instance, a morbilliform eruption with >30% BSA can be managed conservatively without ICI discontinuation in contrast to SJS with <5% BSA (10). A modified grading criteria produced by the American Society of Clinical Oncology focuses on symptoms and quality of life and appears more applicable to irCAEs (60) (Supplementary Tables S1, 9 S2).
Summary
In general, irCAEs indicate a positive anti-tumor response (11, 12, 61). This has been well documented with vitiligo, which is predictive of response, progression-free survival, and overall survival in patients with metastatic melanoma (1, 7, 9, 12, 13). Progression free survival was seen to be higher in patients who experienced flares of psoriasis (18). Though the data is limited, alopecia was also found to have a positive tumor response (49).
Most irCAEs can be managed without discontinuation of ICIs. For high-grade eruptions that necessitate high doses of systemic steroids or other immunosuppressant, choice of therapy must be carefully evaluated to avoid dampening anti-tumor response (13, 18). There is increasing support for prioritizing targeted, non-steroidal immunomodulators with less T-cell impact (13, 18). However, there is lack of data on the effect of SSA, such as biologics, on immunotherapy underscoring the need for further studies in this area of oncodermatology.
Author contributions
Substantial contributions to the conception or design of the work; or the acquisition, analysis, or interpretation of data for the work: FM, ABP, PVK, AB, WM. Drafting the work or revising it critically for important intellectual content: FM, ABP. Provide approval for publication of the content: FM, ABP. Agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved: ABP. All authors contributed to the article and approved the submitted version.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/falgy.2023.1147513/full#supplementary-material.
References
1. Chen CH, Yu HS, Yu S. Cutaneous adverse events associated with immune checkpoint inhibitors: a review article. Curr Oncol. (2022) 29(4):2871–86. doi: 10.3390/curroncol29040234
2. Costa B, Vale N. Dostarlimab: a review. Biomolecules. (2022) 12(8):1031. doi: 10.3390/biom12081031
3. Paik J. Nivolumab plus relatlimab: first approval. Drugs. (2022) 82(8):925–31. doi: 10.1007/s40265-022-01723-1
4. Wongvibulsin S, Pahalyants V, Kalinich M, Murphy W, Yu K, Wang F, et al. Epidemiology and risk factors for the development of cutaneous toxicities in patients treated with immune-checkpoint inhibitors: a United States population-level analysis. J Am Acad Dermatol. (2022) 86(3):563–72. doi: 10.1016/j.jaad.2021.03.094
5. Quach HT, Johnson DB, LeBoeuf NR, Zwerner JP, Dewan AK. Cutaneous adverse events caused by immune checkpoint inhibitors. J Am Acad Dermatol. (2021) 85(4):956–66. doi: 10.1016/j.jaad.2020.09.054
6. Bhardwaj M, Chiu MN, Pilkhwal Sah S. Adverse cutaneous toxicities by PD-1/PD-L1 immune checkpoint inhibitors: pathogenesis, treatment, and surveillance. Cutan Ocul Toxicol. (2022) 41(1):73–90. doi: 10.1080/15569527.2022.2034842
7. Ellis SR, Vierra AT, Millsop JW, Lacouture ME, Kiuru M. Dermatologic toxicities to immune checkpoint inhibitor therapy: a review of histopathologic features. J Am Acad Dermatol. (2020) 83(4):1130–43. doi: 10.1016/j.jaad.2020.04.105
8. Apalla Z, Rapoport B, Sibaud V. Dermatologic immune-related adverse events: the toxicity spectrum and recommendations for management. Int J Womens Dermatol. (2021) 7(5Part A):625–35. doi: 10.1016/j.ijwd.2021.10.005
9. Gault A, Anderson AE, Plummer R, Stewart C, Pratt AG, Rajan N. Cutaneous immune-related adverse events in patients with melanoma treated with checkpoint inhibitors. Br J Dermatol. (2021) 185(2):263–71. doi: 10.1111/bjd.19750
10. Muntyanu A, Netchiporouk E, Gerstein W, Gniadecki R, Litvinov IV. Cutaneous immune-related adverse events (irAEs) to immune checkpoint inhibitors: a dermatology perspective on management. J Cutan Med Surg. (2021) 25(1):59–76. doi: 10.1177/1203475420943260
11. Le TK, Kaul S, Cappelli LC, Naidoo J, Semenov YR, Kwatra SG. Cutaneous adverse events of immune checkpoint inhibitor therapy: incidence and types of reactive dermatoses. J Dermatolog Treat. (2022) 33(3):1691–5. doi: 10.1080/09546634.2021.1898529
12. Patel AB, Farooq S, Welborn M, Amaria R, Chon SY, Diab A, et al. Cutaneous adverse events in 155 patients with metastatic melanoma consecutively treated with anti-CTLA4 and anti-PD1 combination immunotherapy: incidence, management, and clinical benefit. Cancer. (2022) 128(5):975–83. doi: 10.1002/cncr.34004
13. Park BC, Jung S, Chen ST, Dewan AK, Johnson DB. Challenging dermatologic considerations associated with immune checkpoint inhibitors. Am J Clin Dermatol. (2022) 23(5):707–17. doi: 10.1007/s40257-022-00706-y
14. De Giorgi V, Colombo J, Trane L, Silvestri F, Venturi F, Zuccaro B, et al. Cutaneous immune-related adverse events and photodamaged skin in patients with metastatic melanoma: could nicotinamide be useful? Clin Exp Dermatol. (2022) 47(8):1558–60. doi: 10.1111/ced.15215
15. Nadelmann ER, Yeh JE, Chen ST. Management of cutaneous immune-related adverse events in patients with cancer treated with immune checkpoint inhibitors: a systematic review. JAMA Oncol. (2022) 8(1):130–8. doi: 10.1001/jamaoncol.2021.4318
16. Patel AB, Lacouture ME. Mucocutaneous toxicities associated with immune checkpoint inhibitors. In: Mockenhaupt M, Atkins MB, Waltham, MA: UpToDate Inc. Available at: https://www.uptodate.com/contents/mucocutaneous-toxicities-associated-with-immune-checkpoint-inhibitors (Accessed January 1, 2023).
17. Sollena P, Cappilli S, Federico F, Schinzari G, Tortora G, Peris K. “Skin rashes” and immunotherapy in melanoma: distinct dermatologic adverse events and implications for therapeutic management. Hum Vaccin Immunother. (2022) 18(3):1889449. doi: 10.1080/21645515.2021.1889449
18. Kuo AM, Markova A. High grade dermatologic adverse events associated with immune checkpoint blockade for cancer. Front Med. (2022) 9:898790. doi: 10.3389/fmed.2022.898790
19. Malviya N, Tattersall IW, Leventhal J, Alloo A. Cutaneous immune-related adverse events to checkpoint inhibitors. Clin Dermatol. (2020) 38(6):660–78. doi: 10.1016/j.clindermatol.2020.06.011
20. Bur D, Patel AB, Nelson K, Huen A, Pacha O, Phillips R, et al. A retrospective case series of 20 patients with immunotherapy-induced bullous pemphigoid with emphasis on management outcomes. J Am Acad Dermatol. (2022) 87(6):1394–5. doi: 10.1016/j.jaad.2022.08.001
21. Langan EA, Budner K, Zillikens D, Terheyden P. Generalized morphoea in the setting of combined immune checkpoint inhibitor therapy for metastatic melanoma: a case report. Medicine. (2021) 100(16):e25513. doi: 10.1097/MD.0000000000025513
22. Acar A, Oraloglu G, Yaman B, Karaarslan I. Nivolumab-induced plaque morphea in a malign melanoma patient. J Cosmet Dermatol. (2021) 20(8):2645–7. doi: 10.1111/jocd.13914
23. Zhong H, Zhou J, Xu D, Zeng X. Rheumatic immune-related adverse events induced by immune checkpoint inhibitors. Asia Pac J Clin Oncol. (2021) 17(3):178–85. doi: 10.1111/ajco.13346
24. Shen P, Deng X, Hu Z, Chen Z, Huang Y, Wang K, et al. Rheumatic manifestations and diseases from immune checkpoint inhibitors in cancer immunotherapy. Front Med. (2021) 8:762247. doi: 10.3389/fmed.2021.762247
25. Wetzel ML, Rubin AI, Hanania H, Patel AB. Treatment recommendations for nail unit toxicities secondary to targeted cancer therapy based on collective experience and evidence-based literature review. J Am Acad Dermatol. (2022) 87(1):180–3. doi: 10.1016/j.jaad.2021.07.022
26. O'Connor C, Power D, Gleeson C, Heffron C. Pembrolizumab-induced follicular eruption and response to isotretinoin. Immunotherapy. (2021). doi: 10.2217/imt-2021-0001. [Epub ahead of print]
27. Maillard A, Pastor D, Merat R. Anti-PD-1-induced hidradenitis suppurativa. Dermatopathology. (2021) 8(1):37–9. doi: 10.3390/dermatopathology8010007
28. Freites-Martinez A, Nikolaou V, Lallas K, Carrera C, Sollena P, Apalla Z, et al. Clinical characterization and treatment outcomes of follicular cutaneous immune-related adverse events caused by immune checkpoint inhibitors: a multicenter retrospective study. J Am Acad Dermatol. (2022):S0190–9622(22)02769-4. doi: 10.1016/j.jaad.2022.08.063
29. Tsibris H, Lian C, Ho A. Pembrolizumab-associated pyoderma gangrenosum in a patient with metastatic squamous cell carcinoma. Dermatol Online J. (2021) 27(4):13030/qt4hs6n388. doi: 10.5070/D3274053158
30. Almodovar Cruz GE, Kaunitz G, Stein JE, Sander I, Hollmann T, Cottrell TR, et al. Immune cell subsets in interface cutaneous immune-related adverse events associated with anti-PD-1 therapy resemble acute graft versus host disease more than lichen planus. J Cutan Pathol. (2022) 49(8):701–8. doi: 10.1111/cup.14242
31. Karri PV, Tahseen D, Patel AB. Treatment of checkpoint inhibitor-induced vitiligo in a patient with metastatic renal cell cancer. Dermatitis. (2021) 32(4):e68–9. doi: 10.1097/DER.0000000000000670
32. Shah RR, Bhate C, Hernandez A, Ho CH. Lichen planus pemphigoides: a unique form of bullous and lichenoid eruptions secondary to nivolumab. Dermatol Ther. (2022) 35(5):e15432. doi: 10.1111/dth.15432
33. Boyle MM, Ashi S, Puiu T, Reimer D, Sokumbi O, Soltani K, et al. Lichen planus pemphigoides associated with PD-1 and PD-L1 inhibitors: a case series and review of the literature. Am J Dermatopathol. (2022) 44(5):360–7. doi: 10.1097/DAD.0000000000002139
34. Mueller KA, Cordisco MR, Scott GA, Plovanich ME. A case of severe nivolumab-induced lichen planus pemphigoides in a child with metastatic spitzoid melanoma. Pediatr Dermatol. (2023) 40(1):154–6. doi: 10.1111/pde.15097
35. Yoshida S, Shiraishi K, Yatsuzuka K, Mori H, Koga H, Ishii N, et al. Lichen planus pemphigoides with antibodies against the BP18° C-terminal domain induced by pembrolizumab in a melanoma patient. J Dermatol. (2021) 48(9):e449–51. doi: 10.1111/1346-8138.16006
36. Qian J, Kubicki SL, Curry JL, Jahan-Tigh R, Benjamin R, Heberton M, et al. Pembrolizumab-induced rash in a patient with angiosarcoma. JAAD Case Rep. (2022) 29:21–4. doi: 10.1016/j.jdcr.2022.08.030
37. Wat M, Mollanazar NK, Ellebrecht CT, Forrestel A, Elenitsas R, Chu EY. Lichen-planus-pemphigoides-like reaction to PD-1 checkpoint blockade. J Cutan Pathol. (2022) 49(11):978–87. doi: 10.1111/cup.14299
38. Gremese E, Alivernini S, Ferraccioli ES, Ferraccioli G. Checkpoint inhibitors (CPI) and autoimmune chronic inflammatory diseases (ACIDs): tolerance and loss of tolerance in the occurrence of immuno-rheumatologic manifestations. Clin Immunol. (2020) 214:108395. doi: 10.1016/j.clim.2020.108395
39. Messer A, Drozd B, Glitza IC, Lu H, Patel AB. Dermatomyositis associated with nivolumab therapy for melanoma: a case report and review of the literature. Dermatol Online J. (2020) 26(8):13030/qt4c21b068. doi: 10.5070/D3268049887
40. Abdel-Wahab N, Suarez-Almazor ME. Frequency and distribution of various rheumatic disorders associated with checkpoint inhibitor therapy. Rheumatology. (2019) 58(Suppl 7):vii40–8. doi: 10.1093/rheumatology/kez297
41. Fattore D, Battista T, De Lucia M, Annunziata MC, Fabbrocini G. Scleroderma-like syndrome in the setting of pembrolizumab therapy for non-small cell lung cancer: diagnosis and dermatologic management. Case Rep Dermatol. (2022) 14(2):225–9. doi: 10.1159/000525887
42. Martel J, Cho WC, Runge JS, Patel AB, Tayar J, Woodman K, et al. Durvalumab associated generalized morphea with overlapping vitiligo. JAAD Case Rep. (2022) 30:83–6. doi: 10.1016/j.jdcr.2022.10.007
43. Terrier B, Humbert S, Preta LH, Delage L, Razanamaheri J, Laurent-Roussel S, et al. Risk of scleroderma according to the type of immune checkpoint inhibitors. Autoimmun Rev. (2020) 19(8):102596. doi: 10.1016/j.autrev.2020.102596
44. Kefas J, Harwood C, Lewis MJ, Szlosarek P. Small vessel vasculitis and dry gangrene secondary to combined CTLA-4 and PD-1 blockade in malignant mesothelioma. BMC Rheumatol. (2022) 6(1):10. doi: 10.1186/s41927-021-00238-8
45. Nagaoka-Takatori A, Ishii M, Hayama K, Obinata D, Yamaguchi K, Takahashi S, et al. A case of IgA vasculitis during nivolumab therapy for renal cell carcinoma. Clin Cosmet Investig Dermatol. (2021) 14:1885–8. doi: 10.2147/CCID.S343876
46. Harada M, Naoi H, Yasuda K, Ito Y, Kagoo N, Kutoba T, et al. Programmed cell death-1 blockade in kidney carcinoma may induce eosinophilic granulomatosis with polyangiitis: a case report. BMC Pulm Med. (2021) 21(1):6. doi: 10.1186/s12890-020-01375-5
47. Chanson N, Ramos-Casals M, Pundole X, Suijkerbuijk K, E Silva MJDB, Lidar M, et al. Immune checkpoint inhibitor-associated sarcoidosis: a usually benign disease that does not require immunotherapy discontinuation. Eur J Cancer. (2021) 158:208–16. doi: 10.1016/j.ejca.2021.05.041
48. Lacouture M, Sibaud V. Toxic side effects of targeted therapies and immunotherapies affecting the skin, oral mucosa, hair, and nails. Am J Clin Dermatol. (2018) 19(Suppl 1):31–9. doi: 10.1007/s40257-018-0384-3
49. Antoury L, Maloney NJ, Bach DQ, Goh C, Cheng K. Alopecia areata as an immune-related adverse event of immune checkpoint inhibitors: a review. Dermatol Ther. (2020) 33(6):e14171. doi: 10.1111/dth.14171
50. Kim KH, Sim WY, Lew BL. Nivolumab-induced alopecia areata: a case report and literature review. Ann Dermatol. (2021) 33(3):284–8. doi: 10.5021/ad.2021.33.3.284
51. Rossi A, Magri F, Caro G, Federico A, Fortuna MC, Soda G, et al. Eosinophilic folliculitis of the scalp associated with PD-1/PDL1 inhibitors. J Cosmet Dermatol. (2020) 19(12):3367–70. doi: 10.1111/jocd.13388
52. Yang W, Li S, Yang Q. Risk of dermatologic and mucosal adverse events associated with PD-1/PD-L1 inhibitors in cancer patients: a meta-analysis of randomized controlled trials. Medicine. (2019) 98(20):e15731. doi: 10.1097/MD.0000000000015731
53. Zahoor F, Ahmed N, Afzal G. Onychopathy induced by nivolumab: a targeted immunotherapy. Cureus. (2022) 14(7):e26950. doi: 10.7759/cureus.26950
54. van Damme C, Sibaud V, André J, Richert B, Berlingin E. Anti-programmed cell death protein 1-induced lichenoid changes of the nail unit: histopathologic description. JAAD Case Rep. (2021) 10:110–2. doi: 10.1016/j.jdcr.2021.02.016
55. Peña-Cardelles JF, Salgado-Peralvo AO, Garrido-Martínez P, Cebrián-Carretero JL, Pozo-Kreilinger JJ, Moro-Rodríguez JE. Oral mucositis. Is it present in the immunotherapy of the immune checkpoint PD1/PD-L1 against oral cancer? A systematic review. Med Oral Patol Oral Cir Bucal. (2021) 26(4):e494–501. doi: 10.4317/medoral.24353
56. Shazib MA, Woo SB, Sroussi H, Carvo I, Treister N, Farag A, et al. Oral immune-related adverse events associated with PD-1 inhibitor therapy: a case series. Oral Dis. (2020) 26(2):325–33. doi: 10.1111/odi.13218
57. Gouveris P, Georgakopoulou EA, Grigoraki A, Zouki DN, Kardara VE, Ioannou S, et al. Nivolumab-induced lichenoid granulomatous stomatitis in a patient with advanced melanoma: a case report. Mol Clin Oncol. (2022) 16(4):79. doi: 10.3892/mco.2022.2512
58. Alias A, Hall JA, Kulkarni P, Gowan AC. Pembrolizumab-induced immune-mediated glossitis. Cureus. (2022) 14(1):e21708. doi: 10.7759/cureus.21708
59. U.S. Department of Health and Human Services. Common Terminology Criteria for Adverse Events (CTCAE) Version 5.0 (2017). Retrieved at: https://ctep.cancer.gov/protocoldevelopment/electronic_applications/docs/ctcae_v5_quick_reference_5×7.pdf
60. Patel AB, Pacha O. Skin reactions to immune checkpoint inhibitors. Adv Exp Med Biol. (2020) 1244:235–46. doi: 10.1007/978-3-030-41008-7_11
61. Tang K, Seo J, Tiu BC, Le TK, Pahalyants V, Raval NS, et al. Association of cutaneous immune-related adverse events with increased survival in patients treated with anti-programmed cell death 1 and anti-programmed cell death ligand 1 therapy. JAMA Dermatol. (2022) 158(2):189–93. doi: 10.1001/jamadermatol.2021.5476
Keywords: dermatitis, immune checkpoint inhibitor, pruritus, drug rash, anti CTLA-4, anti PD-1, anti PD-L1, immunotherapy
Citation: Muhaj F, Karri PV, Moody W, Brown A and Patel AB (2023) Mucocutaneous adverse events to immune checkpoint inhibitors. Front. Allergy 4:1147513. doi: 10.3389/falgy.2023.1147513
Received: 18 January 2023; Accepted: 15 February 2023;
Published: 2 March 2023.
Edited by:
Alexander Batista Duharte, Maimonides Biomedical Research Institute of Cordoba (IMIBIC), SpainReviewed by:
Yoshihiro Noguchi, Gifu Pharmaceutical University, Japan© 2023 Muhaj, Karri, Moody, Brown and Patel. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Anisha B. Patel QXBhdGVsMTFAbWRhbmRlcnNvbi5vcmc=
Specialty Section: This article was submitted to Drug, Venom & Anaphylaxis, a section of the journal Frontiers in Allergy
 Fiorinda Muhaj1
Fiorinda Muhaj1 Anisha B. Patel
Anisha B. Patel