- 1Independent Research Pharmacist, Cardiff, United Kingdom
- 2The Demodex Project®, Cardiff Medicentre, Cardiff, United Kingdom
Demodex eyelash mites are increasingly associated with eye and skin inflammation in humans, and cause demodectic mange in mammals. Informal accounts of symptom improvement and reduced need for anti-allergy medicines, when Demodex reproduction is prevented, indicate a further role linking Demodex to rhinitis, asthma and dermatitis. Their mobility, allergenic debris and consequential immunological impact may also explain progression of allergies in the “allergic march”. Being photophobic and nocturnal, Demodex folliculorum shelter, feed, and sleep in eyelash follicles during daylight. Coston (1967) speculated that Demodex emerge to mate during darkness and observed that medicated ointments rubbed into the eyelid margins at bedtime treated Demodex blepharitis effectively, presumably by preventing mating. Sixteen cases are described retrospectively whereby interested volunteers adopted Coston's technique, using unmedicated petroleum jelly. To break the lifecycle, a minimum 28-day course was advised, though concordance varied. Fourteen people reported relief from a surprising range of symptoms including not only dry eye and blepharitis but also rhinitis, asthma, angioedema and seborrhoeic dermatitis. Analysis of GP prescribing data in three volunteers allowed comparison of five-year periods immediately before and after starting continuous treatment. Mean yearly issues of anti-allergy and antimicrobial medicines reduced from 15.6 (range 8–25) to 1.8 (range 0–4), representing an 88.5% decrease for Volunteer 1 and from 5.8 (range 3–9) and 14.2 issues (range 9–24) to zero for both Volunteer 2 and Volunteer 13 respectively, representing 100% reductions in prescribing. Exacerbations of acne and dermatitis in two cases illustrate possible Demodex involvement in common dermatoses. This account is limited by its informal and retrospective nature in a disparate cohort, without assessment of Demodex levels. These preliminary observations support the hypothesis that Demodex allergens may trigger facial, ocular and respiratory inflammation and that reducing mite count with petroleum jelly improves symptoms. Formal clinical trials are needed to test this hypothesis.
1 Introduction – Demodex features, role in eye and skin disease, testing and treatment
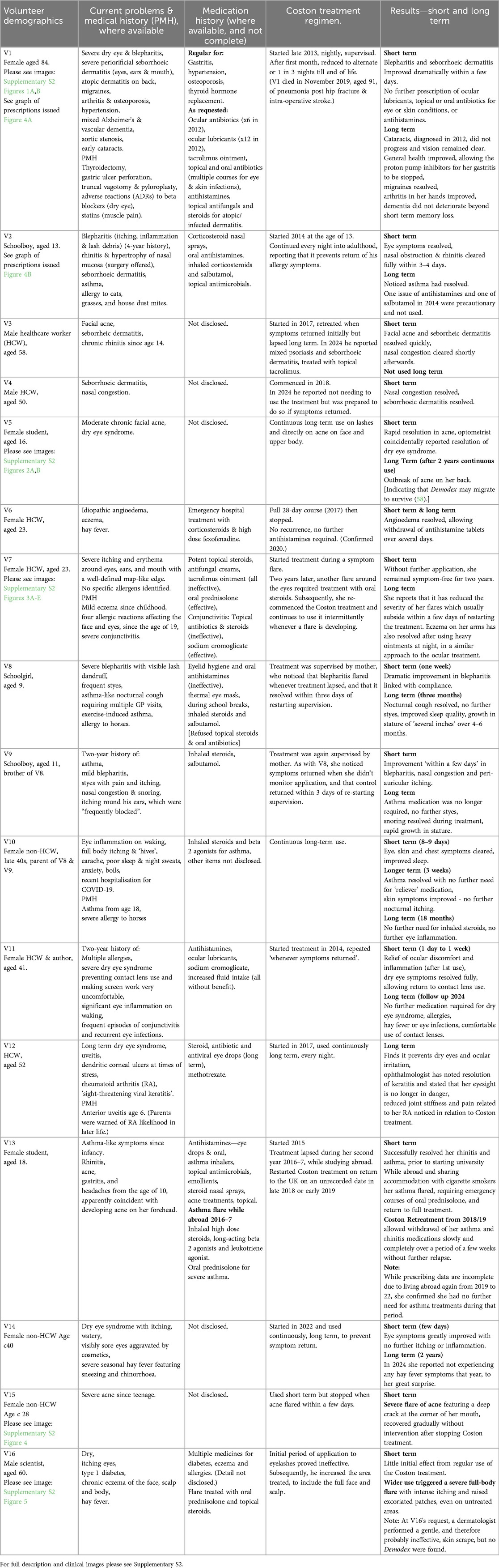
Classed as arachnids, Demodex (Figure 1) are eight-legged arthropods closely related to spiders and scorpions. Their subclass, the “Acari” includes ticks and other mites, including house dust mites (HDMs), with their well-recognised allergenic potential, “chiggers”, or “harvest mites”, and scabies (1) all of which impact human health (2). Demodex are stated to be the most common parasite in human skin and eyes (3), generally assumed to be referring to the eye adnexa. They are also the most highly evolved microorganisms within the human microbiome (4), possibly pre-dating or evolving alongside early humans (5). Transmission is predominantly vertical through close maternal contact (6, 7).
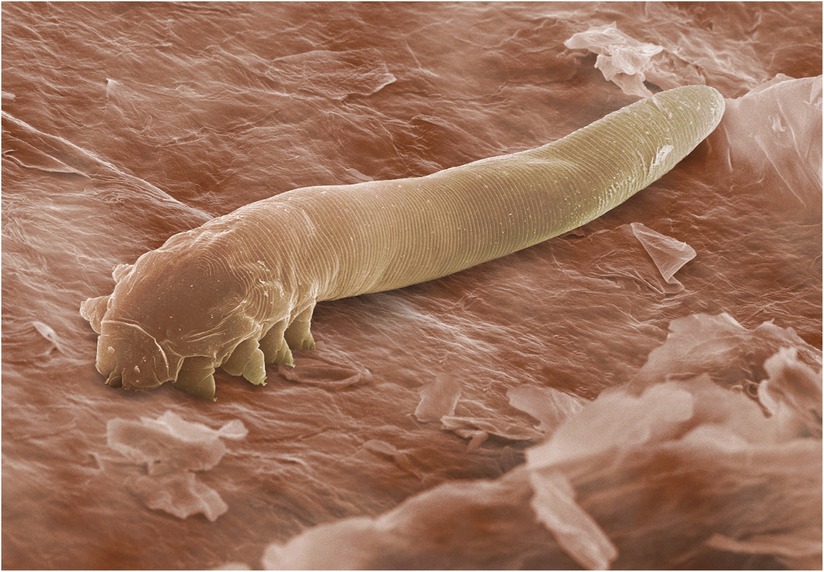
Figure 1. Scanning electron micrograph of an adult Demodex folliculorum. ©Power & Syred, Science Photo Library.
Two closely related species are found in humans: worm-shaped Demodex folliculorum which group together in eyelash and hair follicles (Figure 2) and their shorter, solitary cousins, Demodex brevis1. The latter live deep in dermal sebaceous glands, and in the lipid-secreting meibomian glands on the inner eyelid margin (3).
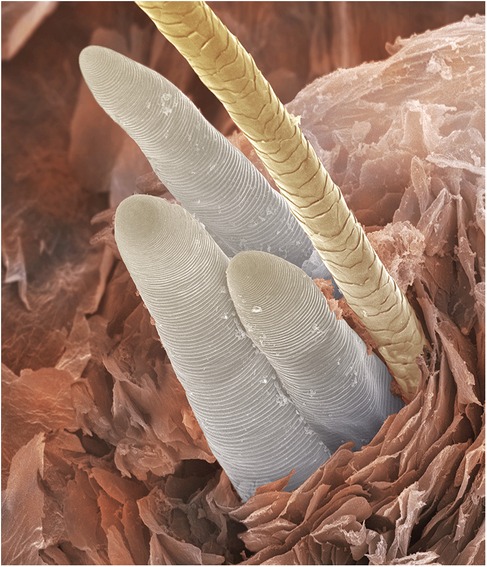
Figure 2. Scanning electron micrograph showing Demodex tails protruding from a hair follicle. ©Power & Syred, Science Photo Library.
Many detailed reviews on the features, behaviour and means of eradicating Demodex mites have been published over the years, (3, 8–24). This article describes the effects of using a technique thought to reduce ocular Demodex and how this has led to a hypothesis which proposes a causative role for Demodex in respiratory and dermatological inflammation.
Demodex are known to cause demodectic mange in dogs and other mammals (25, 26) and are associated with chronic inflammatory skin conditions in humans (4), including rosacea (27–29), acne (29–31), seborrhoeic dermatitis (29) pityriasis, peri-oral dermatitis, facial pigmentation and scalp eruptions (3). “Primary demodicosis” usually affects the elderly, typically appearing round the mouth, whereas the more diffuse 'secondary' form affects the face and trunk, often in younger people (4). Demodex infestation increases with age, from 84% of the population at age 60, to 100% in people over 70 years (32). They proliferate when immunity is compromised (4, 18), during puberty, and at other times of increased production of sebum (26), which is a key nutrient (6). This possibly explains exacerbation of Demodex-related skin conditions in adolescents and at times of stress.
Demodex have highly developed mechanisms for evading immune attack (33–35), but they also evade suspicion in clinical and research settings, being found on healthy people and animals as well as those suffering significant illness. Direct causation of illness is consequently difficult to establish (26). Demodex also have many potential mechanisms for causing tissue damage and immunological reactions (3, 14, 26), such as release of digestive enzymes2 and allergenic Der f proteins (36) and their role as vectors for other microbes3.
In eye disease, Demodex infestation is associated with blepharitis (18, 37, 38), dry eye syndrome4 (18, 39, 40), meibomian gland disease (MGD) (3, 41) keratoconjunctivitis sicca (27, 42–44), conjunctivitis, keratitis and pterygium, or “Surfer's Eye” (3). They are also found in higher density in patients with basal cell carcinoma of the eyelid (45).
Blepharitis is a common inflammatory eye condition across all ages and all ethnic groups (46), predominantly developing in middle age but also affecting children. It occurs in 37% of ophthalmology cases and 47% of optometrist cases and is classed as either “anterior”, for eyelash follicle involvement, “posterior” for meibomian gland blockade, or “mixed”5. Symptoms include itching, redness, flaking, and crusting of the eyelids. Progression may involve damage to the eyelid margins and cornea through increased vascularity and ulceration leading to photophobia, blurred vision and sight loss. Burning sensations and irritation are significant problems, often accompanied by increased lachrymation and erythema (4).
While a connection is not always considered, accepted signs of Demodex infestation mirror symptoms of blepharitis, namely cylindrical dandruff, scaly or waxy debris on the eyelashes, eyelid erythema and increased eyelid vascularity (42). However, lash epilation has also revealed Demodex infestation in a blepharitis patient with unilateral fine follicular scaling (47) and in blepharo-keratoconjunctivitis patients with clean lashes (42). Distension or “pouting” of eyelash follicles has also been reported as a pathognomonic sign (22); eyelash loss (madarosis) and inward turning of the lashes (trichiasis) causing corneal abrasion may also be seen (3). Individuals with Demodex are more likely to report itching (39, 48) and ocular discomfort, which correlates closely with infestation levels (48, 49). While lash epilation for microscopy has been used in many studies, eyelash rotation (50) and the “lateral eyelash tension technique” (51) have been found to achieve higher counts, allowing mites to be counted in situ, without removal of lashes. These techniques require only a slit lamp, good tweezers, and a steady hand.
Various treatments have been suggested for eradicating ocular Demodex and treating blepharitis though no formal consensus on the best option has been reached (52). The NICE Clinical Knowledge Summary for blepharitis5 does not include Demodex as a potential cause but recommends eyelid hygiene, warm compresses and eyelid massage as symptomatic treatments. Oral and topical antimicrobials are allowed where necessary. NICE also advises that a cure is generally not possible, though evidence for the efficacy of a 3-month course of topical ivermectin 1.0% cream in treating ocular demodicosis has been shown (22). Lotilaner is now available as eye drops for Demodex blepharitis. Previously it was exclusively a veterinary medicine for killing ticks, mites and fleas at any stage of the lifecycle (53).
In 1967 Tullos D. Coston published a detailed review of the lifecycle and behaviour of Demodex mites and appraised potential treatments for Demodex blepharitis (10). Many would be considered noxious by today's standards, but he noticed that ointments spread in the eyelashes overnight were particularly effective. He also suspected that Demodex emerge from eyelash follicles during darkness to copulate. Our first volunteer had suffered for more than thirty years with debilitating dry eye disease and blepharitis6. She agreed to try using Vaseline®, applied in this way at night, to physically prevent mite reproduction. The surprising results are summarised below and described fully in Supplementary S2.
It was later discovered that the same technique is also used by a very small number of clinicians, in conjunction with tea tree oil wipes, heated eye masks, or as monotherapy for blepharitis, MGD and dry eye symptoms. A GP has reported success with the technique for Demodex blepharitis (54), and LJ Geisse has described using it for “hundreds of patients” over a three-year period7, although not in the context of reducing Demodex. There appear to be no other reports of Vaseline®, known generically as “petroleum jelly”, “white soft paraffin” or “petrolatum”, being used in this very specific way at bedtime. It is used for Phthirus pubis, or “crab lice”, applied four times a day when ocular infestation occurs (55).
A link between Demodex proteins, rhinitis and sinusitis has already been proposed8 and high levels of gravid Demodex have been reported in nasal discharge (56). Both support the likelihood that Demodex and their associated proteins will infiltrate the respiratory and gastrointestinal systems.
2 Methods – collection of cases and application of petroleum jelly to trap Demodex
Upon learning of this topic, personal contacts of the author often express a wish to try the treatment for themselves. Between 2013 and 2023, sixteen individuals, many being healthcare professionals, volunteered to try Coston's technique. Initially, their use was for dry eye symptoms or blepharitis but later progressed, as experience increased, to include allergic eye conditions, rhinitis, and asthma. All were given verbal advice to follow a simple routine immediately before going to sleep in a dark room. Instructions comprised careful handwashing then taking a large pea-sized amount of petroleum jelly with a fingertip and gently working it into the base of their eyelashes along the margins of their firmly closed eyelids. The aim of creating a glutinous environment within the eyelashes to trap mites as they emerge at night to mate was explained. Applied in this way, the petroleum jelly does not get inside the lids or contact the ocular surface and may be removed the following morning. Advice to use the treatment for at least 28 consecutive nights was given, to exceed the Demodex lifecycle of 14–21 days (9, 57). Except for Volunteer 15, everyone was aware of the treatment rationale. Retrospectively, every volunteer, or their legal guardian, emailed consent for their case reports and photographs to be published.
3 Case reports – volunteers with dry eye syndrome, blepharitis, rhinitis, allergies, acne & asthma
Please see Table 1 for a summary of medical history, use of the Coston Treatment and outcomes. For full description and clinical images, please see Supplementary S2.
4 Results – resolution of symptoms and reduction in GP prescribing
Table 2 describes the conditions relieved or exacerbated in the volunteers following use of petroleum jelly to reduce ocular Demodex.
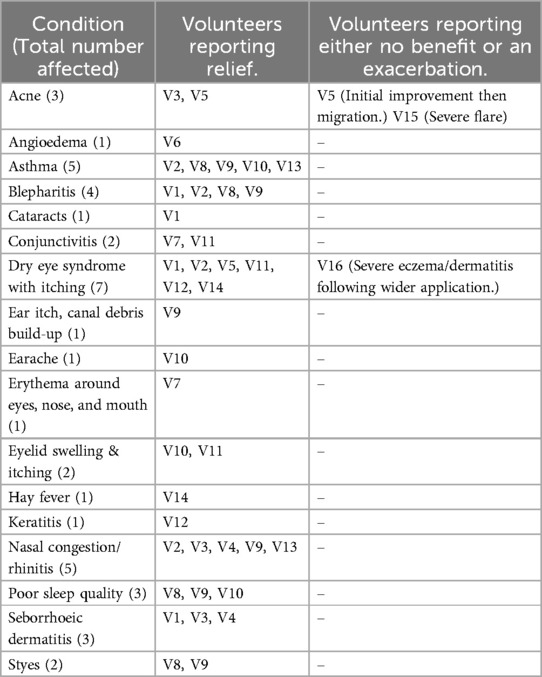
Table 2. Conditions relieved or exacerbated in the volunteers following use of petroleum jelly to reduce ocular demodex.
The self-administered Coston technique, using petroleum jelly, was initially proposed for dry eye symptoms or blepharitis. All cases of dry eye or blepharitis resolved except one, V16, who used the treatment more widely and suffered a severe full body flare of his pre-existing dermatitis. Four further volunteers with a history of severe allergic ocular symptoms, including one with recurrent angioedema (V6, V7, V10, V11), also reported full resolution, as did all five people who had suffered chronic nasal congestion (V2, V3, V4, V9 & V13). In the five volunteers who had asthma as a comorbidity to eye symptoms (V2, V8, V9, V10 & V13), the improvement was an unexpected observation, while V13 later restarted the petroleum jelly in a successful concerted attempt to resolve her asthma and stop her medication.
When the volunteers used the treatment they experienced resolution of blepharitis, dry eye, ocular allergies and infections, keratitis, hay fever and respiratory inflammation in the form of rhinitis and asthma. Other incidental findings included resolution of seborrhoeic dermatitis in three volunteers (V1, V3 & V4), relief of earache and ear itch in one volunteer each (V9, V10), and deeper sleep, reported in three members of the same family (V8, V9, V10). V14 reported absence of hay fever symptoms in the second year of treatment, V1's cataracts did not develop over the following seven years and V12's resolution of keratitis was also unexpected.
A reduced need for prescribed and purchased medicines was reported in at least 11 volunteers. This was verified in three volunteers by analysis of their GP prescribing data. For V1, ocular lubricants, topical immunosuppressants, topical and oral antibiotics, antihistamines and, subsequently, medication for gastritis were no longer requested. For V2 and V13, medications no longer needed included nasal steroids and asthma treatments.
The volunteers generally described their improvements as total; no one reported mild or moderate improvement for any condition. Volunteers found that symptoms correlated closely with use of the treatment, and that their symptoms returned if compliance was poor. V6, who achieved 28 nights of continuous treatment for her angioedema, was able to withdraw her high-dose antihistamines without relapse, and without needing further treatment.
These results are surprising, considering the simple physical technique involving an inert substance. While these cases do not provide direct evidence of reduction in Demodex levels, the reported correlation between treatment and symptom resolution indicates a probable association. Improvement in eye and skin conditions could be attributed to a lubricant, emollient, or placebo effect, or to spontaneous fluctuations. However, resolution of nasal congestion and asthma is harder to explain. It points strongly to these conditions being caused or exacerbated by inflammatory proteins, including some identified as Der f house dust mite-type allergens (36), leeching from debris left by ocular Demodex.
5 Hypothesis – Demodex allergens may cause respiratory inflammation
When Demodex mites disintegrate in the eye, inflammatory Der f proteins and other allergenic debris will drain into the nasal passages via lachrymal fluid and be inhaled. This may trigger inflammatory reactions including rhinitis and asthma. Established links between ocular and respiratory inflammation may therefore be explained.
Eradicating ocular Demodex with petroleum jelly, applied at night to impede mating, may provide a new approach to first-line management of dry eye, blepharitis, rhinitis, asthma and other allergic conditions, improving quality of life and reducing medication needs, prescribing costs and clinic time.
6 Discussion
An association between eye inflammation in the form of “allergic conjunctivitis” with rhinitis and asthma has been described by Sánchez-Hernández et al. (59). They found that allergic conjunctivitis occurred in 88.3% of patients with rhinitis and in 38.8% of patients with asthma. Paediatric results were higher at 93% and 47% respectively. Asthma and rhinitis correlated with conjunctivitis in severity and duration, demonstrating the “allergic march” and supporting the “one airway, one disease” hypothesis that asthma onset follows allergic conjunctivitis and rhinitis (59). Awareness that ocular demodicosis may be misdiagnosed as allergic conjunctivitis, viral keratitis or other inflammatory eye conditions (42) increases the relevance of this information and strengthens the case for a shared underlying aetiology between eye inflammation and allergic respiratory conditions.
The resolution in seborrhoeic dermatitis and changes in acne and atopic dermatitis were all unexpected, though all three conditions have already been associated with Demodex (29) and association between Demodex and these and other skin conditions including rosacea and pityriasis folliculorum, appears to be gaining recognition (29, 60).
Three volunteers (V1, V3, V4) who had coincidental seborrhoeic dermatitis used petroleum jelly only expecting to treat their eye or nasal symptoms. The jelly was applied only in the periocular region, yet facial seborrhoeic dermatitis resolved in all three volunteers, alongside some improvement in acne in V3. This suggests that the “allergic march” may start with ocular Demodex. Their mobility and 'site specificity', particularly in the sebaceous periorificial regions (4, 10), make it plausible that Demodex could migrate from the ocular area to trigger skin inflammation. Concomitantly their allergenic debris would pervade the respiratory tract as the Demodex population rises, offering a mechanism whereby dermatitis, rhinitis and asthma are related. As Demodex are transmitted primarily through close maternal contact (5), familial traits in atopic diseases may be explained. We should also consider that once allergenic proteins enter the nasal passages they will be swallowed, perhaps explaining the link between eye inflammation and inflammatory bowel diseases (61, 62), as noted by Burrill Crohn in 1925 (63) and possibly as seen in V1.
Formal measurement of Demodex levels and their debris in the skin and respiratory tract before and after the Coston treatment could strengthen the evidence for Demodex causing seborrhoeic dermatitis, acneiform conditions, atopic dermatitis and other associated dermatoses. It may also inform the current debate on where the “allergic march” originates (64). Whether treating these skin conditions conversely reduces ocular inflammation was not elucidated in these volunteers, but merits investigation.
6.1 Risk of exacerbation in acne and atopic dermatitis
Skin flares are well-known features of acne and atopic dermatitis, often in response to identifiable environmental changes, including temperature and psychological stress though the reactivity of ectoparasites is not yet commonly considered as a potential mechanism9. As petroleum jelly is inert, the eruptions of acne in V15 and dermatitis in V16, which developed in untreated areas, are more easily explained as a “threat response” rather than a pharmacological adverse reaction. It demonstrates that an environmental threat may cause Demodex to react en masse or to migrate to a safer location, and that a “hornets” nest approach” to treatment may be prudent. A role for Demodex in atopic dermatitis is now recognised (65, 66), and the clinical parallels between canine demodicosis and some human skin conditions are striking10.
6.2 Demodex and acne
The exacerbation of acne in V15 aligns with research supporting a causal link between Demodex and acne (29–31). Viewed in cross-section, Figure 3 shows canine Demodex or “mange mites” tightly packed in a sebaceous gland. This also reminds us that acne treatments sometimes cause an initial exacerbation before improvement prevails, such as is seen with oral isotretinoin11. As isotretinoin is thought to reduce sebum production, the initial flare may denote a panic reaction by Demodex to the dwindling supply of nutrients (67). “Acneiform dermatitis”, a reported side effect of topical steroids12, may also be explained in terms of Demodex proliferation (26) due to local immunosuppression (33, 68–70) though signs may not be apparent while the medication is being used. The contraindication of immunosuppressants where infection is present12 might wisely be considered in cases where demodicosis has not been ruled out.
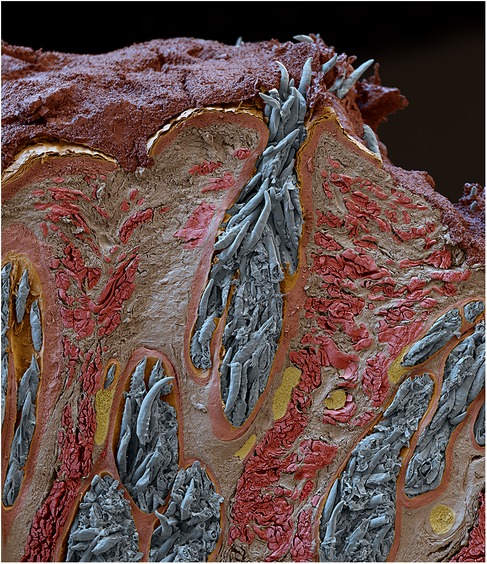
Figure 3. Scanning electron micrograph of a sebaceous gland from a dog, showing heavy Demodex infestation © Eye of science, science photo library.
Caution therefore seems warranted when considering the Coston treatment for eye or respiratory symptoms in patients with significant skin disease and protocols for treating Demodex-related conditions should include measures for preventing or managing any initial exacerbation.
6.3 Clues for Demodex presence in the skin
Ectoparasites may be unpredictable due to the range of subtypes and their levels of reactivity. Melatonin increases mobility and reproduction in invertebrates (7), so nocturnal or early morning symptoms may be a feature. Skin symptoms include itching, burning, rash (39), scaling, dryness, lichenification (25), and acneiform outbreaks (31). Sequencing of mitochondrial DNA has shown D. folliculorum to be more metabolically active and likely to cause type I allergic reactions such as erythema and itching. By comparison, D. brevis focus more on producing enzymes for digesting chitin, host skin and serum molecules to facilitate penetration deep into the skin13 (20, 36). Therefore, while itching may be a strong clue to Demodex presence, absence of itching or negative skin surface tests should not exclude ectoparasites from a differential diagnosis. While cracking and fissure formation seem not to have been directly related to Demodex presence, lipid-loss and lichenification are likely to cause dryness and loss of elasticity, possibly leading to the cracking and flaking seen in eczema, ichthyosis and cheilitis.
Flares and periods of remission without identifiable reason, or where an environmental factor can be pinpointed, may be further signs of ectoparasite presence14. Fluctuations may be related to the tendency of Demodex to synchronise their life cycles (14). Coordinated shedding of allergens at regular intervals during moulting (9, 14) and mass rupture on death (16), are likely to amplify the allergenic impact.
Many parasites must migrate to specific sites or tissues within the host. Such “site specificity” is a common feature in many parasitic infections; for sexually reproducing parasites it facilitates finding a mate (58). Localisation of a skin condition is therefore another clue that ectoparasites may be present, and lesions with a well-defined map-like edge, as seen in the images of Volunteers V1 and V715 may denote such colonisation16, as may changes in melanisation17 (71). It seems likely that a threat to survival would cause Demodex to move to another area, perhaps explaining the spread of symptoms seen with Volunteers V15 & V16. The potential benefit in making a timely and correct diagnosis cannot be overstated18.
Medicinal treatments for human demodicosis have been reviewed by Lam et al. (19, 72) and include both oral and topical preparations of metronidazole, ivermectin, and topical preparations of permethrin, lindane, benzyl benzoate and crotamiton. Treatment with oral ivermectin is given weekly and may be needed for six weeks or more, depending on Demodex density (73). This allows eggs and larvae, which are resistant to ivermectin, to mature and become susceptible to treatment.
6.4 Demodex & the immune system
Demodex mites have colonized mammalian hair follicles and sebaceous glands for millions of years; their presence being generally tolerated by the host immune system (74). The balance between asymptomatic colonization and pathogenic infestation is likely to be determined by both host and Demodex factors (35). Accounts of the complex interactions between Demodex and their host's immunological defence mechanisms are already available (26, 33, 35, 70, 72, 74–77). Comparison between human and canine demodicosis (35, 76) reveals a similar ability of Demodex to trigger and evade innate and adaptive immune responses in both humans and dogs (35), possibly even suppressing the innate host response to evade expulsion (70).
Inflammatory responses are triggered when Demodex cause mechanical damage by using their claws and piercing mouthparts to penetrate the dermis, destroying epithelial cells and ingesting the contents (11, 12, 72). Perifollicular inflammation may be the result of this process occurring inside hair follicles (13, 70). Extrafollicular mites and their debris, containing chitin (29), crystalline waste products, and microbes for which they may be vectors19 (78–80) can trigger the inflammation cascade via the toll-like receptor 2 (TLR2) innate immunity pathway (35, 72) or stimulate a granulomatous foreign body reaction. Proteins obtained from bacteria carried by Demodex have also been observed to trigger inflammatory reactions, causing neutrophils to migrate, degranulate and release cytokines (81).
Mechanical damage to the eye adnexa includes blockage of meibomian glands (72) increasing the risk of evaporative dry eye symptoms (82). Digestive proteases and lipases released by Demodex20 may trigger host protease-activated receptors, cause anti-microbial peptides to be secreted and upregulate pro-inflammatory cytokines (72). In people with dry eye syndrome, lachrymal proteases are known to damage the ocular surface (82), warranting investigation for correlation with Demodex presence.
Keratinocytes and sebocytes both feature type 2 toll-like receptors which span the cell membrane. When they detect Demodex chitin it triggers an innate immune response (74, 77). This is thought to be the main mechanism for controlling mite population (74). Increased TLR2 production has been identified in papulopustular rosacea, which is strongly associated with Demodex presence (12); changes in production and distribution of TLR2 are also seen in atopic dermatitis, contact dermatitis and psoriasis (83), which are currently rarely ascribed to Demodex. However, Demodex may secrete bioactive molecules that affect TLR2 receptor expression (74) as a means of countering this phenomenon.
It has been shown that type 2 innate immunity reduces skin inflammation caused by Demodex. Decreased type 2 cytokine expression is observed in patients with Demodex-associated rhinophyma, affecting follicles of the nose, while activation of group 2 innate lymphoid cells (ILC2s), interleukin-13 (IL-13), and its receptor, IL-4Ra-IL-13Ra1, limit proliferation and spread of mites (75). Conversely, an absence of type 2 cytokines allows overgrowth of Demodex, with lymphocytic infiltrates, marked ILC2 activation and the development of inflammatory symptoms (75). Th2 cytokines have been implicated in asthma due to their role in the complex process of immunoglobulin E (IgE) production, and activation of mast cells and eosinophils (84).
The carbohydrate-like Tn antigen expressed by Demodex can modulate the secretion of pro-inflammatory mediators such as IL-8 and tumour necrosis factor (TNF)-α from the pilosebaceous unit of the host, which interferes with the innate immune response of the host to facilitate the invasion and population expansion of Demodex (29, 70). Acaricidal treatment decreases the antigenic load and reverses T-cell exhaustion, leading to a clinical cure (74).
An increased rate of lymphocyte apoptosis has been found to relate to Demodex density with the functional activity of leucocytes being significantly lower in infested individuals. This local immunosuppression attributed to Demodex may facilitate their survival in host skin. Secondary immunosuppression may also trigger demodicosis following corticosteroid use, cytostatic therapy or immunodeficiency disease (33). Analysis of the Demodex transcriptome has confirmed that nine “Der f” HDM allergens are highly expressed in D. folliculorum, and a link with erythema, papules, itching, and other symptoms of type I allergic reactions has already been proposed (36). However, the lack of a skin-prick test for Demodex allergy currently prevents screening for Demodex allergy and assessment of cross-reactivity between Demodex and HDM allergens.
The Retzingers' Acari Hypothesis III (66) proposed that, in atopic dermatitis and related conditions, the immune response is targeting infestation by “vector active acarians” and their dietary elements, thus stimulating production of IgE as part of the “atopic march”. This theory is developed in their Acari Hypothesis IV which proposes that mites and ticks are major causes of allergy through their “pathogenic payload” and that humans, monkeys and apes have evolved the eccrine system of sweat glands to deter infestation (85).
During infestation with Demodex, and in conditions where Demodex have been implicated, IgE is frequently elevated. IgE concentrations correlate with severity of atopic dermatitis (86) and are raised in patients with allergic asthma and rhinitis, who are allergic to HDMs (87). IgE has been found to be increased in patients with papulopustular rosacea compared with control groups in line with Demodex infestation (88) and in immunocompetent mice infested with Demodex, where significantly increased IgE concentrations fell after treatment with imidacloprid-moxidectin, a veterinary antiparasitic treatment (89).
High numbers of Demodex have been reported to induce proinflammatory cytokine secretion, whereas lower numbers did not (74). The phenomenon of life-cycle synchronicity (14) is likely to amplify any such reaction. In advanced disease in dogs, significantly elevated expression of TLR2, transforming growth factor (TGF)-β, and IL-10 and reduced expression of TLR6 have been found in the peripheral blood mononuclear cells (PBMCs) (90). It is thought that overexpression of the TLR2 gene might be responsible for Demodex-induced clinical manifestations, while down regulation of TLR4 and TLR6 gene expression and induction of systemic TGF-β and IL-10 could be strategies used by Demodex mites to avoid immune attack (34, 90). The phenomenon of T-cell exhaustion, which can be seen in advanced disease, features low IL-2 levels alongside high IL-10 and TGF-β production by lymphocytes, as described in other viral and parasitic diseases (16, 29, 74).
6.5 Safety and merits of the Coston treatment, using petroleum jelly
6.5.1 Patient acceptability and potential effect on quality of life, in adults and children
The Coston Treatment is only applied at night, and the jelly is wiped or washed away on waking. All volunteers, including the children, readily accepted the treatment if the rationale was explained. Rapid relief of troublesome symptoms provided ongoing incentive. Full-scale clinical trials, which include a process for counting Demodex, would provide a more accurate assessment of safety, efficacy, and patient acceptability. The potential impact on patients' quality of life cannot be overstated. Even among our volunteers were cases where utter misery was alleviated. The impact on a child of having her mother bring an eye bag into school every day, or an academic not being able to work at a computer screen, the stigma and discomfort of skin conditions, and the impact and risks from respiratory inflammation, are clear too. If a cheap product which can be supplied without prescription can be shown to reduce ocular and respiratory inflammation, it could prove helpful in low-income countries where such treatments are most needed. Whether it would deter other eye-seeking parasites also merits investigation.
6.5.2 Reduction in GP prescribing of allergy-related medicines and antibiotics
Subject to further assessment, the Coston technique, using petroleum jelly, is potentially a simple, cheap and effective first step in the treatment of dry eye and blepharitis. Volunteers 1–14 all reported needing fewer anti-allergy or dry eye treatments which provides a key economic incentive for further investigation. Retrospective analysis of prescribing data for Volunteers 1 and 2 and 13 is shown in Figure 4. These graphs portray almost total eradication of the need for ocular lubricants, antihistamines, nasal sprays and in V2 and V13 for asthma medication too; a phenomenon reinforced by anecdotal accounts from other volunteers. The role of Demodex as a vector for other micro-organisms (78) may explain why no further antibiotics were prescribed for these three volunteers, and merits further research.
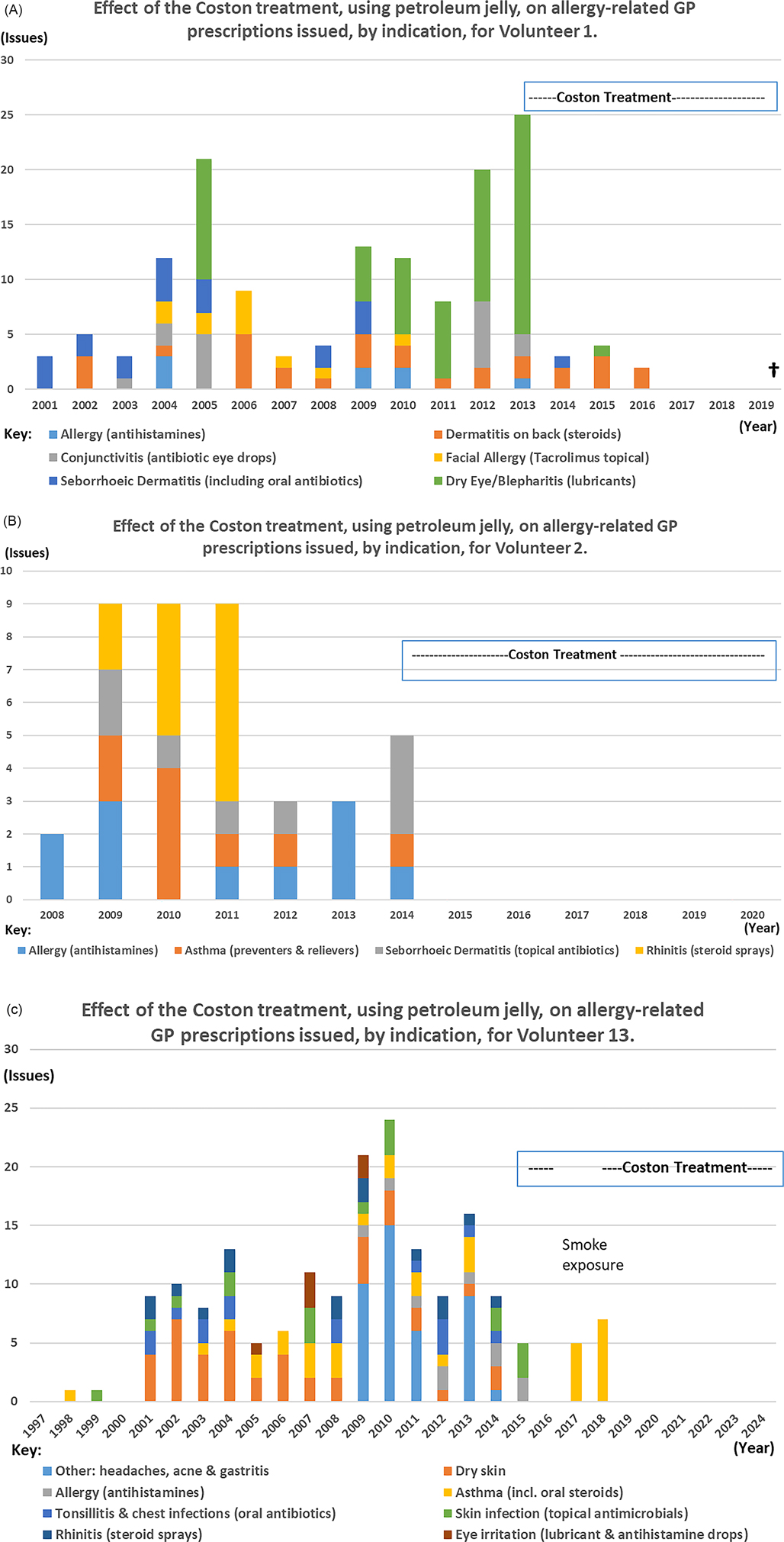
Figure 4. Graphs to show the impact of the Coston technique on the number of allergy-related items issued by the GP for Volunteers 1, 2 and 13 for the years for which data were available. Years of birth for V1, V2 and V13 were 1928, 2000 and 1997 respectively. (A) Effect of Coston treatment, using petroleum jelly, on allergy-related GP prescriptions issued, by indication, for Volunteer 1. (B) Effect of Coston treatment, using petroleum jelly, on allergy-related GP prescriptions issued, by indication, for Volunteer 2. (C) Effect of Coston treatment, using petroleum jelly, on allergy-related GP prescriptions issued, by indication, for Volunteer 13.
Comparing the five-year periods immediately before and after starting continuous treatment, the mean number of anti-allergy and antimicrobial medicines prescribed per year for Volunteer 1, reduced from 15.6 (range 8–25) to 1.8 issues (range 0–4), representing an 88.5% decrease in prescribing. For Volunteers 2 and 13, mean issues fell from 5.8 (range 3–9) and 14.2 (range 9–24) respectively, to zero, representing 100% reductions in relevant prescribing for both people.
6.5.3 Pharmaceutical considerations in the use of petroleum jelly
Petroleum jelly, a by-product of the oil industry, is a purified mixture of mineral oils and natural waxes. It has low allergenic potential and does not require preservatives due to the absence of water. As an inert vehicle for drug delivery in many ophthalmic preparations it comes into contact with the cornea and protects it (91). Available all over the world at minimal cost, it can be supplied without a prescription. Due to the physical action and complete lack of any active substance, it poses no risk of causing antimicrobial resistance and will not provide any sustenance for lipid-loving creatures such as Demodex. The cases described show that physical entrapment to prevent Demodex from mating could be a useful way to eradicate them from the eyelashes, allowing pharmacological agents to be reserved for more serious conditions. Like many cosmetic products applied externally to the eyelid margins of closed eyes, it does not need to be sterile though good handwashing before application is prudent. A patient advice sheet should be provided to explain the rationale for treatment, and how to apply it.
Despite the names 'soft paraffin', “petrolatum”, or “petroleum jelly”, the manufacturer's hazard assessment states that it is “non-flammable”21. All pharmaceutical emollients are required to carry a flammability warning, whether they contain paraffin derivatives, plant oils or animal fats, due to the reported accelerant action of emollients in general on the ignition and speed at which fabrics burn22. This warning appears not to be required for soft paraffin in its pure form, which is described as an “occlusive” rather than an “emollient”, so the packaging does not carry a flammability warning.
7 Suggestions for further research
Results from our volunteers, collected retrospectively and informally over several years, appear to reveal previously overlooked potential for Demodex to cause harm and suggest that their characteristics should be much more widely studied.
Trials on the safety and effectiveness of Coston's technique using petroleum jelly in blepharitis, dry eye syndrome, and other eye conditions linked to Demodex presence, seem warranted. Manipulation methods such as eyelash twirling (50) or lateral tension (51) may be the most accessible and acceptable techniques for counting mites, though PCR (92) or ELISA23 may also be feasible.
If the Coston technique can be shown to reduce Demodex count, a larger study should investigate possible wider clinical and economic benefits, including impact on prescription requests for medications for allergies, rhinitis, asthma and on quality of life. Other key outcomes may include reductions in surgery for nasal congestion and antibiotic use for skin and eye infections, with all the associated implications for microbial resistance. Whether a course of treatment before ocular surgery reduces post-operative inflammation or consequential dry eye symptoms, also merits investigation.
Alongside formal research, alerting clinicians to signs of ectoparasite activity may prove beneficial across a range of medical specialities. Closer links with arachnologists and acarologists, entomologists and veterinary ectoparasitologists would expedite our learning and help develop and normalise testing procedures. Use of the Coston petroleum jelly treatment as a deterrent for other eye-seeking parasites in low-income countries may also merit consideration.
Testing for ectoparasite presence and clinical trials of antiparasitic agents in a range of skin conditions would seem prudent. These should include atopic dermatitis, acneiform conditions, urticaria, angioedema, psoriasis, vitiligo, melanomas, and other conditions characterised by clear delineation with inflammation or pigmentation changes. Testing for Demodex 18s rRNA in recalcitrant sinusitis and in lung aspirates of patients with severe asthma or multi-resistant pneumonias could inform treatment, giving a positive impact on patient outcomes, reducing expenditure on medicines and hospital services, and help in the battle against antimicrobial resistance.
8 Falsifiers which could disprove the hypothesis
The hypotheses proposed would be falsified if the following research outcomes were established:
1. A suitably powered trial using a verified means of counting Demodex in eye lash follicles would find no significant reduction in Demodex count after a one-month course of petroleum jelly, correctly applied every night.
2. A longer-term trial in asthma and rhinitis patients would show no difference in symptoms or any reduction in use of symptomatic treatments between a group treated to reduce ocular Demodex and an untreated group.
3. Demodex proteins and debris would never show allergenic potential in a ’skin prick' test in atopic patients, if one should become available.
4. PCR testing for Demodex species in the nasal secretions of rhinitis patients, or in sputum samples from asthma and pneumonia patients, would always be negative.
5. Verified skin tests such as the standardised skin surface biopsy (93), PCR for Demodex 18s rRNA (65, 92), standard biopsy or deep skin scrape (94), or confocal microscopy (95) performed by fully trained individuals would find no evidence of Demodex in patients with dermatitis or acneiform conditions and symptoms would not respond to topical or oral antiparasitic treatments.
9 Limitations
This report is a retrospective account of unexpected clinical changes which occurred when a group of individuals, linked only by social contact with the author, used the treatment informally, in line with their individual preferences. Their demographics ranged widely in terms of age (9–84 years) and underlying health status. There was no access to specialist assessment of Demodex levels before or after treatment, which would be required in a clinical trial. With one exception, volunteers did not adhere to the advice to apply the petroleum jelly for 28 days. Some used shorter courses when required and some opted to use it long term. Formal trials would help to determine the most appropriate duration of treatment for specific indications and the likelihood of relapse.
10 Conclusions & summary
Applying petroleum jelly to the eyelash roots at night may reduce ocular Demodex levels by immobilising them when they roam at night to mate. This simple technique is based on Coston's observations in 1967 (10) that ointments in general were particularly effective if applied in this way. However, the technique is seldom used. The self-application by fascinated volunteers, accumulated over a 10-year period, has demonstrated potential effectiveness for dry eye disease and blepharitis, and therefore merits further investigation. Unexpected improvements in ocular allergies including angioedema, periocular and seborrhoeic dermatitis and acne were also seen, plus a striking resolution in rhinitis and asthma. Volunteers also described wider benefits to their wellbeing; a child no longer needed to use a heated “eye bag” in school, and an author returned to using her laptop and wearing contact lenses. Reduction in nocturnal coughing and a mother's perception of improved sleep quality is also described. The use of petroleum jelly in this way appeared acceptable to children and the elderly alike.
Demodex levels were not measured in these volunteers because their experiences were reported retrospectively, so a direct association between mite levels and symptom severity has not been established. However, the treatment had good clinical effect which the volunteers reported as correlating closely with changes in their compliance.
The unexpected outcomes in our 16 volunteers point to a mechanism whereby Demodex debris and allergenic proteins, including HDM-type “Der f” allergens, are transported via lachrymal fluid into nasal passages. Inhalation may then cause further inflammatory conditions including rhinitis and asthma. This is proposed as a hypothesis, and initial research methods to test and to falsify this hypothesis have been suggested. The reduction in GP prescribing for allergic and inflammatory conditions including dry eye, blepharitis, rhinitis and asthma, and of antimicrobials for eye and skin infections, if confirmed by further research, may provide an economical way to reduce prescribing and clinic costs. It may also help in ongoing campaigns to reduce antimicrobial use by physically removing a vector. Counselling to explain the technique and the rationale for treatment seems important, ideally supported by provision of a patient advice sheet.
The two cases where moderate acne and dermatitis flared and spread may demonstrate the phenomenon of parasites reacting adversely to an environmental threat to their survival. They also highlight the reported, but apparently not well-recognised, links between acne, dermatitis and Demodex (29–31, 65, 66). Clinical strategies to reduce the risk of a flare may need to be devised. Routine testing and increased clinical awareness of the signs of parasite presence may reduce the risk of dermatological or respiratory demodicosis being overlooked.
In addition to the already-established links between Demodex and several ocular and skin conditions, the unexpected benefits reported by the volunteers may also provide clues for a wider pathogenic role for Demodex and their debris in causing systemic inflammation. This may not be limited to the respiratory system because entry into the gastrointestinal tract seems equally likely. If these connections are confirmed, we may start to recognise a “Demodex Syndrome” where inflammatory conditions can be related to Demodex presence, particularly where an environmental factor, including variation in available nutrients, steroid hormones or immune response, can be identified.
Use of nightly petroleum jelly for dry eye and blepharitis has the advantage of being cheap, potentially very effective, and accessible without prescription, even in low-income countries without risk of causing resistance. It may also reduce use of steroids or other immunosuppressants which are generally contraindicated if an infestation or infection is suspected. The favourable risk-benefit ratio of this physical method may allow empirical treatment as the relationship between Demodex and ocular discomfort gains recognition. Further studies are required to confirm the impact of Coston's technique, using nightly petroleum jelly, on Demodex count and ocular symptoms. Wider potential benefits in allergic conditions including rhinitis and asthma, and other measures of health and wellbeing, such as GP prescribing rates and quality of life, remain to be assessed.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
This paper describes a case-series of individuals, reported retrospectively, who elected to treat themselves for Demodex in pursuit of their own research. Each volunteer, or their legal guardian, has provided written consent to publish their experiences, to use their images, and, where applicable, to access their GP prescribing data.
Author contributions
DS-F: Conceptualization, Data curation, Formal analysis, Writing – original draft, Writing – review & editing.
Funding
The author declares that no financial support was received for the research and/or publication of this article.
Acknowledgments
The author would like to thank James Coulson, Professor of Clinical Pharmacology, Therapeutics & Toxicology, Cardiff University, for his detailed feedback on this paper, advice and mentorship, Colin Dayan, Professor of Clinical Diabetes and Metabolism, Cardiff University School of Medicine, and David Linden, Professor of Translational Neuroscience, and Scientific Director of the Mental Health and Neuroscience Research institute, Maastricht University, for their feedback, advice and mentorship, Miranda Whitten, Molecular Entomologist & Honorary Senior Lecturer, Swansea University Medical School, for information, advice and signposting to entomological papers, James Wolffsohn, Professor and Dean of Optometry, Aston University, for his encouragement, advice and general feedback on this manuscript, Alex Müntz, Professor & Head of Institute of Optometry, University of Applied Sciences and Arts, Northwestern Switzerland for his encouragement and advice, Rosemary Soper, Cardiff and Vale UHB Librarian, and her successors, for access to medical journals and numerous literature searches over many years, the sixteen volunteers for their interest in the subject, and their invaluable feedback, without which this paper would not have been conceived, and her family, colleagues and friends for their interest, encouragement and support.
Conflict of interest
The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. Trademarks have been registered for The Demodex Project® and for Parasplat®, because a bespoke product with full instructions for application would simplify patient consultations, prescribing and ‘over the counter’ sales. This would be marketed in a not-for-profit or charitable context with the hope of raising funds for future Demodex research.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Author disclaimer
The views and ideas expressed in this article and any appendices are those of the author and not of her employer. All content presented is for informational and educational use only, and for the purpose of stimulating research; no part is intended or implied to be a substitute for professional medical advice, diagnosis, or treatment. It is assumed that healthcare professionals will use their clinical judgement, knowledge and expertise when deciding whether it is appropriate to use any part of this paper to influence their practice. The use of petroleum jelly as described in the text has not yet been subjected to formal assessment of safety and efficacy in clinical trials. Patients should always seek the advice of their physician or other qualified healthcare provider if they have questions or concerns and should not stop taking their current medication because of anything they have read in any part of this paper or any appendix to it. To the extent legally permissible, the author, people acknowledged, and Cardiff and Vale University Health Board disclaim any responsibility and any liability whatsoever for damages arising out of any clinical decisions based on use, inappropriate use or disregard of the information provided herein.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/falgy.2025.1576102/full#supplementary-material
Footnotes
1. ^See Supplementary S1 Figure 1A
2. ^Please see Supplementary S1A for more information on digestive enzymes and pre-oral digestion.
3. ^Please see Supplementary S1B for more information on vector status.
4. ^Lowery RS. Ed. Law SK, Rapuano CJ, Dahl AA. Adult Blepharitis. Medscape. https://emedicine.medscape.com/article/1211763-overview Updated 17/8/23, accessed 3/1/25.
5. ^Blepharitis: NICE Clinical Knowledge Summary. https://cks.nice.org.uk/blepharitis#!scenario. Updated September 2024, accessed 3/1/25.
6. ^Please see Supplementary S2, figures 1A & 1B for images of Volunteer 1
7. ^Geisse LJ. The Vaseline Routine. Am. Ac. of Ophthalmol. Eye Net Magazine. July 2023 (Letter). https://www.aao.org/eyenet/article/letters-28
8. ^Busby TL. Anti-demodectic active agents and topical compositions for the treatment of demodicosis in humans and animals. (2016) US 20160287566 A1 (Patent application) https://patents.justia.com/patent/20160287566
9. ^Please see Supplementary S1C on environmental reactivity for further information.
10. ^For more information on veterinary parallels with human dermatoses please see Supplementary S1D
11. ^Isotretinoin soft capsules 20mg (Roaccutane). Summary of Product Characteristics. Updated 31/10/23 accessed 4/1/25 https://www.medicines.org.uk/emc/product/6470/smpc#UNDESIRABLE_EFFECTS
12. ^Mometasone 0.1% Ointment Summary of Product Characteristics https://www.medicines.org.uk/emc/product/9994/smpc#gref Updated 3/1/25, accessed 5/1/25
13. ^Please see Supplementary S1A for more detail.
14. ^Please see Supplementary S1C for more detail.
15. ^Images may be found in Supplementary S2 Figures 1A, 3C),
16. ^With thanks to Dr Miranda Whitten, Molecular Entomologist, Swansea University, for this information (personal communication December 2020).
17. ^Please see Supplementary 1E on arthropods and melanin.
18. ^For more information on diagnosis, and the possible impact of missing a diagnosis, please see Supplementary S1F & 1G
19. ^For more detail on the vector status of Demodex please see Supplementary S1B.
20. ^For more detail on enzymes and pre-oral digestion please see Supplementary S1A.
21. ^Unilever Vaseline Petroleum Jelly Cosmetic Product Information Sheet. Updated 20/1/20, obtained from Unilever 14/7/23. Previous (2010) version available at https://safety365.sevron.co.uk/substances/accessSDS/SDS-20928-56a50fd4966712.91522705
22. ^Emollients and risk of severe and fatal burns: new resources available - GOV.UK MHRA update 20 May 2021 to Drug Safety Update (2020) 14 (1): 6. Accessed 5/1/25.
23. ^Lubna F. Diagnosis of canine demodicosis by ELISA. (2016 Doctoral dissertation, PVNR TVU). https://krishikosh.egranth.ac.in/items/2604ec3f-ad74-4d53-ba57-7d33bbbef3e0
References
1. Platts-Mills TAE, Adkinson NF, Bochner BS, Busse WW, Holgate ST, Lemanske R, et al. Middleton’s Allergy Principles and Practice. 7th edn. Philadelphia: Mosby Elsevier (2009). p. 539–52.
2. Linn C, O’Malley A, Khatri K, Wright EM, Sebagh D, Grbić M, et al. Microscopic menaces: the impact of mites on human health. Int J Mol Sc. (2024) 25(7):3675. doi: 10.3390/ijms25073675
3. Luo X, Li J, Chen C, Tseng S, Liang L. Ocular demodicosis as a potential cause of ocular surface inflammation. Cornea. (2017) 36:S9–S14. doi: 10.1097/ico.0000000000001361
4. Chen W, Plewig G. Human demodicosis: revisit and a proposed classification. B J Dermatol. (2014) 170(6):1219–25. doi: 10.1111/bjd.12850
5. Palopoli MF, Fergus DJ, Minot S, Pei DT, Simison BW, Fernandez-Silva I, et al. Global divergence among human follicle mites. P Natl Ac Sci U S A. (2015) 112(52):15958–63. doi: 10.1073/pnas.1512609112
6. Jacob S, VanDaele MA, Brown JN. Treatment of Demodex-associated inflammatory skin conditions: a systematic review. Dermatol Ther. (2019) 32:e13103. doi: 10.1111/dth.13103
7. Smith G, Manzano-Marín A, Reyes-Prieto M, Sofia Antunes CSR, Ashworth V, Goselle ON, et al. Human follicular mites: ectoparasites becoming symbionts. Mol Biol Evol. (2022) 39:6.msac125. doi: 10.1093/molbev/msac125
8. Hirst AS. Studies on Acari. London: Brit. Mus. Nat. Hist. (1919). doi: 10.5555/19191000262 (Abstract only seen).
9. Spickett SG. Studies on Demodex folliculorum simon (1842). I. Life history. Parasitology. (1961) 51:181–92. doi: 10.1017/s003118200006858x
10. Coston TO. Demodex folliculorum blepharitis. Trans Am Ophth Soc. (1967) 65:362–92. Available online at: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1310279/pdf/taos00034-0371.pdf
11. Desch C, Nutting W. Demodex folliculorum (simon) and D. brevis (akbulatova) of man: redescription and re-evaluation. J Parasitol. (1972) 58(1):169–77. doi: 10.2307/3278267
12. Forton FMN. Papulopustular rosacea, skin immunity and Demodex: pityriasis folliculorum as a missing link. JEADV. (2012) 26:19–28. doi: 10.1111/j.1468-3083.2011.04310.x
13. Rather PA, Hassan I. Human Demodex mite: the versatile mite of dermatological importance. Ind J Dermatol. (2014) 59(1):60–6. doi: 10.4103/0019-5154.123498
14. Nicholls SG, Oakley CL, Tan A, Vote BJ. Demodex species in human ocular disease: new clinicopathological aspects. Int Ophthalmol. (2017) 37:303–12. doi: 10.1007/s10792-016-0249-9
15. Litwin D, Chen W, Dzika E, Korycinska J. Human permanent ectoparasites; recent advances on biology and clinical significance of Demodex mites: narrative review article. Iran J Parasitol. (2017) 12(1):12–21.28747952
16. Fromstein SR, Harthan JS, Patel J, Opitz DL. Demodex blepharitis: clinical perspectives. Clin Optom. (2018) 10:57–63. doi: 10.2147/OPTO.S142708
17. Navel V, Mulliez A, Benoist d’Azy C, Baker JS, Malecase J, Chiambaretta F, et al. Efficacy of treatments for Demodex blepharitis: a systematic review and meta-analysis. Ocul Surf. (2019) 17(4):655–69. doi: 10.1016/j.jtos.2019.06.004
18. Zhang AC, Müntz A, Wang MTM, Craig JP, Downie LE. Ocular Demodex: a systematic review of the clinical literature. Ophthal Physl Opt. (2020) 40:389–432. doi: 10.1111/opo.12691
19. Lam NSK, Long XX, Li X, Yang L, Griffin RC, Doery JCG. Comparison of the efficacy of tea tree (Melaleuca alternifolia) oil with other current pharmacological management in human demodicosis: a systematic review. Parasitology. (2020) 147(14):1587–613. doi: 10.1017/S003118202000150X
20. Bitton E, Aumond S. Demodex and eye disease: a review. Clin Exp Optom. (2021) 104(3):285–94. doi: 10.1111/cxo.13123
21. Sharma N, Martin E, Pearce EI, Hagan S. A delphi approach to establishing consensus on best practice for the diagnosis and treatment of Demodex blepharitis. Cont Lens Anterior Eye. (2023) 47(1):102080. doi: 10.1016/j.clae.2023.102080
22. Smith M, Wolffsohn JS, Chiang JCB. Topical ivermectin 1.0% cream in the treatment of ocular demodicosis. Contact Lens and Anterior Eye. (2023) 47(1):102099. doi: 10.1016/j.clae.2023.102099
23. Li J, Wei E, Reisinger A, French LE, Clanner-Engelshofen BM, Reinholz M. Comparison of different anti-Demodex strategies: a systematic review and meta-analysis. Dermatology. (2023) 239(1):12–31. doi: 10.1159/000526296
24. Chatterjee S, Gupta J, Srinivas SP, Rao SK. Demodex and the eye – a review. Indian J Ophthalmol. (2025) 73(1):10–8. doi: 10.4103/IJO.IJO_1591_24
25. Foley R, Kelly P, Gatault S, Powell F. Demodex: a skin resident in man and his best friend. JEADV. (2021) 35(1):62–72. doi: 10.1111/jdv.16461
26. Elston CA, Elston DM. Demodex mites. Clin Dermatol. (2014) 32(6):739–43. doi: 10.1016/j.clindermatol.2014.02.012
27. Rousta ST. Pediatric blepharokeratoconjunctivitis: is there a ‘right’ treatment? Curr Opin Ophthalmol. (2017) 28(5):449–53. UI: 28696955. doi: 10.1097/ICU.0000000000000399
28. Chang YS, Huang YC. Role of Demodex mite infestation in rosacea: a systematic review and meta-analysis. J Am Acad Dermatol. (2017) 77:441–7. doi: 10.1016/j.jaad.2017.03.040
29. Karabay EA, Cerman AA. Demodex folliculorum infestations in common facial dermatoses: acne vulgaris, rosacea, seborrheic dermatitis. An Bras Dermatol. (2020) 95:187–93. doi: 10.1016/j.abd.2019.08.023
30. Zhao YE, Hu L, Wu LP, Ma JX-J. A meta-analysis of association between acne vulgaris and Demodex infestation. Zhejiang Univ Sci B. (2012) 13(3):192–202. doi: 10.1631/jzus.b1100285
31. Akçınar UG, Ünal E, Al FD. Demodex spp. as a possible aetiopathogenic factor of acne and relation with acne severity and type. Adv Dermatol Allergol. (2018) 35(2):174–81. doi: 10.5114/ada.2018.75239
32. Post CF, Juhlin E. Demodex folliculorum and blepharitis. Arch Dermatol. (1963) 88(3):298–302. doi: 10.1001/archderm.1963.01590210056008
33. Akilov OE, Mumcuoglu KY. Immune response in demodicosis. JEADV. (2004) 18:440–4. doi: 10.1111/j.1468-3083.2004.00964.x
34. Kumari P, Nigam R, Choudhury S, Singh SK, Yadav B, Kumar D, et al. Demodex canis targets TLRs to evade host immunity and induce canine demodicosis. Parasite Immunol. (2018) 40(3):e12509. doi: 10.1111/pim.12509
35. Gazi U, Taylan-Ozkan A, Mumcuoglu KY. Immune mechanisms in human and canine demodicosis: a review. Parasite Immunol. (2019) 41:e12673. doi: 10.1111/pim.12673
36. Hu L, Zhao Y, Niu D, Gong X, Yang R. de novo transcriptome sequencing and differential gene expression analysis of two parasitic human Demodex species. Parasitol Res. (2019) 118:3223–35. doi: 10.1007/s00436-019-06461-0
37. Nemet AY, Vinker S, Kaiserman I. Associated morbidity of blepharitis. Ophthalmology. (2011) 118(6):1062–8. doi: 10.1016/j.ophtha.2010.10.015
38. Zhao Y, Wu L, Hu L, Xu J. Association of blepharitis with Demodex: a meta-analysis. Ophthal Epidemiol. (2012) 19(2):95–102. doi: 10.3109/09286586.2011.642052
39. Cheng AM, Hwang J, Dermer H, Galor A. Prevalence of ocular demodicosis in an older population and its association with symptoms and signs of dry eye. Cornea. (2021) 40(8):995–1001. doi: 10.1097/ICO.0000000000002542
40. Ayyildiz T, Sezgin FM. The effect of ocular Demodex colonization on schirmer test and OSDI scores in newly diagnosed dry eye patients. Eye Contact Lens. (2020) 46(1):S39–41. UI: 31393313. doi: 10.1097/icl.0000000000000640
41. Zhang X-B, Ding Y-H, He W. The association between Demodex infestation and ocular surface manifestations in meibomian gland dysfunction. Int J Ophthalmol. (2018) 11:589–92. doi: 10.18240/ijo.2018.04.08
42. Patel NV, Mathur U, Gandhi A, Singh M. Demodex blepharokeratoconjunctivitis affecting young patients: a case series. Ind J Ophthalmol. (2020) 68(5):745–9. doi: 10.4103/ijo.ijo_1402_19
43. Pant OP, Hao JL, Zhou DD, Lu CW. Tectonic keratoplasty using femtosecond laser lenticule in pediatric patients with corneal perforation secondary to blepharokeratoconjunctivitis: a case report and literature review. J Int Med Res. (2019) 47(5):2312–20. doi: 10.1177/0300060519841163
44. Daniel MC, O’Gallagher M, Hingorani M, Dahlmann-Noor A, Tuft S. Challenges in the management of pediatric blepharokeratoconjunctivitis/ocular rosacea. Expert Rev Ophthalmol. (2016) 11(4):299–309. doi: 10.1080/17469899.2016.1209408
45. Erbagci Z, Erbagci I, Erkilic S. High incidence of demodicidosis in eyelid basal cell carcinomas. Int J Dermatol. (2003) 42:567–71. doi: 10.1046/j.1365-4362.2003.01928.x
46. Lindsley K, Matsumura S, Hatef E, Akpek EK. Interventions for chronic blepharitis. Cochrane DB Sys Rev. (2012) 5:CD005556. doi: 10.1002/14651858.CD005556.pub2
47. Tatu AL, Cristea VC. Unilateral blepharitis with fine follicular scaling. J Cutan Med Surg. (2017) 21(5):442. doi: 10.1177/1203475417711124
48. Udomwech L, Phasuk N. Multiple eyelid signs are suggestive of Demodex infestation. Clin Ophthalmol. (2021) 15:671–8. doi: 10.2147/OPTH.S298099
49. Lee SH, Chun YS, Kim JH, Kim ES, Kim JC. The relationship between Demodex and ocular discomfort. Invest Ophth Vis Sci. (2010) 51(6):2906–11. doi: 10.1167/iovs.09-4850
50. Murphy O, O’Dwyer V, Lloyd-McKernan A. The clinical use of eyelash manipulation in the diagnosis of Demodex folliculorum blepharitis. Eye Contact Lens. (2020) 46:S33–8. doi: 10.1097/icl.0000000000000608
51. Müntz A, Purslow C, Wolffsohn JS, Craig JP. Improved Demodex diagnosis in the clinical setting using a novel in situ technique. Cont Lens Anterio. (2020) 43(4):345–9. doi: 10.1016/j.clae.2019.11.009
52. Ayres BD, Donnenfeld E, Farid M, Gaddie IB, Gupta PK, Holland E, et al. Clinical diagnosis and management of Demodex blepharitis: the Demodex expert panel on treatment and eyelid health (DEPTH). Eye. (2023) 37:3249–55. doi: 10.1038/s41433-023-02500-4
53. Zeid MA, Elrosasy A, Abbas AW, Elganady A, Rhab AE, Serhan HA. Efficacy and safety of lotilaner ophthalmic solution 0.25% in the treatment of Demodex blepharitis: a systematic review and meta-analysis. Ocul Immunol Inflamm. (2024) 32(10):2494–505. doi: 10.1080/09273948.2024.2309552
55. Bhatt A. Ocular Infections. Feigin and Cherry’s Textbook of Pediatric Infectious Diseases. 8th ed. Philadelphia, PA: Elsevier (2019). p. 578–97.
56. Attar NR. Demodex folliculorum in nasal discharge: a case report of yet unknown significance. Glob J Oto. (2018) 18(2):555984. doi: 10.19080/GJO.2018.18.555984
57. Rufli T, Mumcuoglu Y. The hair follicle mites Demodex folliculorum and Demodex brevis: biology and medical importance. Dermatology. (1981) 162(1):1. doi: 10.1159/000250228
58. Loker ES, Hofkin BV. Parasitology, A Conceptual Approach. New York/London: Garland Science, Taylor & Francis Group (2015). ISBN 978-0-8153-4473-5. p. 93–8.
59. Sánchez-Hernández MC, Dordal MT, Navarro AM, Dávila I, Fernández-Parra B, Colás C, et al. Severity and duration of allergic conjunctivitis: are they associated with severity and duration of allergic rhinitis and asthma? Eur Ann Aller Clin Immunol. (2022) 54(6):277. doi: 10.23822/EurAnnACI.1764-1489.231
60. Chioveanu FG, Niculet E, Torlac C, Busila C, Tatu AL. Beyond the surface: understanding Demodex and its link to blepharitis and facial dermatoses. Clin Ophthalmol. (2024) 18:1801–10. doi: 10.2147/OPTH.S440199
61. Yilmaz S, Aydemir E, Maden A, Unsal B. The prevalence of ocular involvement in patients with inflammatory bowel disease. Int J Colorectal Dis. (2007) 22:1027–30. doi: 10.1007/s00384-007-0275-1
62. Rodriguez Duran M, O’Keefe GAD. Ocular extraintestinal manifestations and treatments in patients with inflammatory bowel disease. Front. Ophthalmol. (2024) 3:1257068. doi: 10.3389/fopht.2023.1257068
64. Dharmage SC, Lowe AJ, Matheson MC, Burgess JA, Allen KJ, Abramson MJ. Atopic dermatitis and the atopic march revisited. Allergy. (2014) 69(1):17–27. doi: 10.1111/all.12268
65. Edslev SM, Andersen PS, Agner T, Saunte DML, Ingham AC, Johannesen TB, et al. Identification of cutaneous fungi and mites in adult atopic dermatitis: analysis by targeted 18S rRNA amplicon sequencing. BMC Microbiol. (2021) 21:72. doi: 10.1186/s12866-021-02139-9
66. Retzinger AC, Retzinger GS. The Acari hypothesis, III: atopic dermatitis. Pathogens. (2022) 11(10):1083. doi: 10.3390/pathogens11101083
67. Chakmakchi AMJ, Alatas ET, Yurekli A, Aydoğdu CT, Demir Pektas S. Therapeutic modulation of Demodex density via isotretinoin: insights from a prospective dermatological investigation. J Cosmet Dermatol. (2025) 24(6):e70249. doi: 10.1111/jocd.70249
68. Keles H, Yuksel EP, Aydin F, Senturk N. Pre-treatment and post-treatment Demodex densities in patients under immunosuppressive treatments. Medicina (B Aires). (2020) 56(3):107. doi: 10.3390/medicina56030107
69. Pahuja S, Puranik C, Jelliti B, Khairallah M, Sangwan VS. Parasitic infections of the external eye. Ocul Immunol Inflamm. (2013) 21(4):292–9. doi: 10.3109/09273948.2013.770889
70. Moran EM, Foley R, Powell FC. Demodex and rosacea revisited. Clin Dermatol. (2017) 35(2):195–200. doi: 10.1016/j.clindermatol.2016.10.014
71. Whitten MMA, Coates CJ. Re-evaluation of insect melanogenesis research: views from the dark side. Pigm Cell Melanoma R. (2017) 30:386–401. doi: 10.1111/pcmr.12590
72. Lam N, Long X, Griffin R, Doery J, Lu F. Human demodicidosis and the current treatment options. Hong Kong J Dermatol. (2018) 26:10–7.
73. Paichitrojjana A, Chalermchai T. Evaluating the efficacy of oral ivermectin on clinical symptoms and Demodex densities in patients with demodicosis. Drug Des Dev Ther. (2024) 18:5299–306. doi: 10.2147/DDDT.S498938
74. Ferrer L, Ravera I, Silbermayr K. Immunology and pathogenesis of canine demodicosis. Vet Dermatol. (2014) 25(5):427-e6. doi: 10.1111/vde.12136
75. Ricardo-Gonzalez RR, Kotas ME, O’Leary CE, Singh K, Damsky W, Liao C, et al. Innate type 2 immunity controls hair follicle commensalism by Demodex mites. Immunity. (2022) 55(10):1891–908. doi: 10.1016/j.immuni.2022.08.001
76. Nooraei S, Mohseni Z. Comparison of canine and human immune system response to demodicosis. Authorea [Preprint]. (2024). doi: 10.22541/au.171919493.36798843/v1
77. Lacey N, Russell-Hallinan A, Zouboulis CC, Powell FC. Demodex mites modulate sebocyte immune reaction: possible role in the pathogenesis of rosacea. Brit J Dermatol. (2018) 179(2):420–30. doi: 10.1111/bjd.16540
78. English FP, Iwamoto T, Darrell RW, DeVoe AG. The vector potential of Demodex folliculorum. Arch Ophthalmol. (1970) 84(1):83–5. doi: 10.1001/archopht.1970.00990040085020
79. Liu J, Sheha H, Tseng S. Pathogenic role of Demodex mites in blepharitis. Curr Opin Allergy Cl. (2010) 10(5):505–10. doi: 10.1097/ACI.0b013e32833df9f4
80. Tatu AL, Nwabudike LC. Reply to: Kubiak K et al. Endosymbiosis and its significance in dermatology. JEADV. (2018) 32(9):e346–7. doi: 10.1111/jdv.14921
81. O’Reilly N, Bergin D, Reeves EP, McElvaney NG, Kavanagh K. Demodex-associated bacterial proteins induce neutrophil activation. Br J Dermatol. (2012) 166(4):753–60. doi: 10.1111/j.1365-2133.2011.10746.x
82. Wolffsohn JW, Arita R, Chalmers R, Djalilian A, Dogru M, Dumbleton K, et al. TFOS DEWS II diagnostic methodology report. Ocul Surf. (2017) 15(3):539–74. ISSN 1542-0124. doi: 10.1016/j.jtos.2017.05.001
83. Panzer R, Blobel C, Fölster-Holst R, Proksch E. TLR 2 and TLR 4 expression in atopic dermatitis, contact dermatitis and psoriasis. Exp Dermatol. (2014) 23(5):364–6. doi: 10.1111/exd.12383
84. Wills-Karp M. Interleukin-13 in asthma pathogenesis. Immunol Rev. (2004) 202:175–90. doi: 10.1111/j.0105-2896.2004.00215.x
85. Retzinger AC, Retzinger GS. The Acari hypothesis, IV: revisiting the role of hygiene in allergy. Front Allergy. (2024) 5:1–7. doi: 10.3389/falgy.2024.1415124
86. Fatima K, Khan Q-ud-D, Wara N-ul-, Ali A, Javaid S, Ejaz H. Correlation of the severity of atopic dermatitis and serum IgE levels. IJBR. (2025) 3(3):153–7. doi: 10.70749/ijbr.v3i3.802
87. d’Alessandro M, Bergantini L, Perrone A, Cameli P, Beltrami V, Alderighi L, et al. House dust mite allergy and the der p1 conundrum: a literature review and case series. Allergies. (2021) 1(2):108–14. doi: 10.3390/allergies1020008
88. Abd-El-Al AM, Bayoumy AM, Abou Salem EA. A study on Demodex folliculorum in rosacea. J Egypt Soc Parasitol. (1997) 1:183–95.
89. Morris MG, Ricart Arbona RJ, Daniels K, Gardner R, Easthausen I, Boteler WL, et al. Mite burden and immunophenotypic response to Demodex musculi in Swiss Webster, BALB/c, C57BL/6, and NSG mice. Comp Med. (2020) 70(4):336–48. doi: 10.30802/AALAS-CM-19-000097
90. Soman SP, Singh SK, Kumari P, Choudhury S, Singh A, Kanwal S, et al. Quantification of immuno-regulatory cytokine and toll-like receptors gene expression in dogs with generalized demodicosis. Vet Parasitol. (2020) 280:109063. doi: 10.1016/j.vetpar.2020.109063
91. Kamrani P, Hedrick J, Marks JG, Zaenglein AL. Petroleum jelly: a comprehensive review of its history, uses, and safety. J Am Acad Dermatol. (2024) 90(4):807–13. doi: 10.1016/j.jaad.2023.06.010
92. Kasetsuwan N, Kositphipat K, Busayarat M, Threekhan P, Preativatanyou K, Phumee A, et al. Prevalence of ocular demodicosis among patients at tertiary care center, Bangkok, Thailand. Int J Ophthalmol. (2017) 10(1):122–7. doi: 10.18240/ijo.2017.01.20
93. Aytekin S, Yaşar S, Göktay F, Güneş P. Spontaneous fluorescence of Demodex in the dark. JEADV. (2016) 30:320–38. doi: 10.1111/jdv.12776
94. Mueller RS, Rosenkrantz W, Bensignor E, Karas-Tezcza J, Paterson T, Shipstone MA. Diagnosis and treatment of demodicosis in dogs and cats. Clinical consensus guidelines of the world association for veterinary dermatology. Vet Dermatol. (2020) 31:4–e2. doi: 10.1111/vde.12806
Keywords: Demodex, blepharitis, asthma, rhinitis, allergy, dermatitis, prescribing, petroleum jelly
Citation: Senior-Fletcher DE (2025) Reducing ocular Demodex using petroleum jelly may alleviate dry eye syndrome, blepharitis, facial dermatoses, ocular and respiratory allergies, and decrease associated prescribing: a hypothesis. Front. Allergy 6:1576102. doi: 10.3389/falgy.2025.1576102
Received: 13 February 2025; Accepted: 9 July 2025;
Published: 20 August 2025.
Edited by:
Lizette M Lorenz, North Carolina State University, United StatesReviewed by:
Cristine Secco Rosario, Federal University of Paraná, BrazilAlin Laurentiu Tatu, Dunarea de Jos University, Romania
Copyright: © 2025 Senior-Fletcher. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Diana E. Senior-Fletcher, aW5mb0B0aGVkZW1vZGV4cHJvamVjdC5vcmcudWs=
 Diana E. Senior-Fletcher
Diana E. Senior-Fletcher