- Division of Poultry Nutrition, Health and Physiology, Indian Council of Agricultural Research (ICAR)-Directorate of Poultry Research, Hyderabad, Telangana, India
Introduction: Antimicrobial growth promoters (AGPs) have traditionally been used in chicken production to improve performance. However, such products may promote the development and spread of antimicrobial resistance genes (ARGs), which is of huge societal concern. The evaluation of newer feed additives as an alternative to AGPs for broiler chickens in terms of their effect on gut microbes and resistome profile besides effectiveness under challenging housing systems like deep litter systems under tropical conditions is urgently needed.
Methods: We investigated the comparative efficacy of phytase super dosing (P, phytase at 2,000 FTY/kg feed) alone and its combinations with other feed additives, namely phytase plus butyrate (PB, phytase 2,000 FTU/kg feed plus encapsulated sodium butyrate 500g/t feed), phytase plus essential oil (EO) (PE, phytase 2,000 FTU/kg feed plus encapsulated essential oil 400g/t feed), and phytase plus probiotic Bacillus subtilis (PBS, phytase 2,000 FTU/kg feed plus B. subtilis 400g/t feed) as alternatives to AGPs alongside two control groups, namely an AGP, bacitracin methylene disalicylate, (B, 500 g/t feed) and negative control (C), on growth, immunity, feed efficiency, and survivability under a deep litter system of rearing. We also compared the gut microbiome and resistome profile in the bestperforming treatment group and control groups.
Results: At day 35, broilers fed the phytase alone or in combination with butyrate, EO, or B. subtilis had significantly higher cumulative performance in terms of feed efficiency than those fed B or C with the best feed efficiency recorded in the PE group. Overall, the cumulative average mortality rates were 4.57%, 4.0%, 5.14%, 6.28%, 2.86%, and 1.71% in the C, B, P, PB, PE, and PBS groups, respectively. The supplementation of phytase at a super dosing level plus encapsulated EO resulted in a significant increase in the relative abundance of beneficial bacteria in the gut compared to the controls. The density of ARG or mobile genetic elements was not influenced by treatments.
Discussion: The study showed that phytase super dosing alone delivered better feed efficiency in broiler chickens compared to the control or AGP, and encapsulated EO or B. subtilis plus phytase super dosing can reduce the mortality rate.
1 Introduction
Among various livestock species, chickens are among the most efficient converters of feed into high-quality protein sources for human nutrition. High feed efficiency, faster growth, and lesser disease incidence are prerequisites to maintaining profitability in a highly competitive poultry production system. To achieve faster growth, optimal feed efficiency, and reduced pathogen contamination in poultry products, maintaining a healthy gut is of paramount importance. Poor gut health in broilers has been associated with gut wall morphology changes, systemic infection, and malabsorption. It has been reported that more than 50% of the therapeutic use of antibiotics in broilers in the European Union is to control intestinal pathologies (Persoons et al., 2012). Traditionally, antibiotic growth promoters are used to improve gut health and feed efficiency in broiler chickens. However, there is increasing societal concern about applying antibiotics in feed as growth promoters in chickens as the use of antibiotics are giving rise to antimicrobial resistance (AMR) at a faster rate than the rate at which newer antibiotics are being developed. Prolonged use of low doses of antibiotics as growth promoters in food animals has been shown to favor the selection of resistance to more than one class of antimicrobials in the bacterial community residing in the gut (Paul et al., 2022). Bacteria can acquire resistance to antibiotics through different mechanisms such as the development of antibiotic efflux, modification of antibiotic targets through genetic mutations or posttranslational modifications of the target protein, synthesis of antibiotic hydrolyzing enzymes, and reduced expression of porin proteins (Jian et al., 2021). Non-dividing microorganisms in the stationary phase exposed to sublethal concentrations of antibiotics were shown to develop antibiotic resistance through adaptive mutation, directed mutation, or selection-induced mutation (Tello et al., 2012; Pulingam et al., 2022). The selection pressure exerted by carbapenem antibiotics favored the emergence of mutations in genes that regulate the expression of porins (Baroud et al., 2013). Thus, prolonged use of antibiotics as growth promoters may promote the emergence and dissemination of antimicrobial resistance genes (ARGs) to microbial communities in humans or the environment besides generating drug residues in animal products. Consequently, the development of new feed additives as an alternative to antibiotic growth promoters (AGPs) has emerged as a priority area in animal production (Abd El-Hack et al., 2022). Different types of alternatives to AGPs are being marketed but most of them lack consistency, often require high doses, and often are not cost-effective (Demir et al., 2005; Lillehoj and Liu, 2018). Among various alternatives to AGPs, encapsulated essential oils, encapsulated organic acid, especially butyrate, and some probiotics have been reported to improve the performance of broiler chicken to a comparable or better level than that of AGP more or less consistently. Some studies showed that phytase may also enhance the performance of broiler chickens and yield similar growth performance and economic efficiency to those of AGP (Zinc bacitracin)-supplemented groups (Attia et al., 2015). Super dosing of phytase, where phytase is given at a higher than a normal dose, is a recent concept in non-ruminant nutrition that allows the breakdown of lower phytate esters, allowing better utilization of protein, energy, and minerals and facilitating the production of myo-inositol with a growth-promoting effect (Fernandes et al., 2019). More and more farmers are adopting phytase supplementation in broiler chickens. However, limited research has been undertaken to evaluate the effect of phytase super dosing alone and the potential benefits, if any, of its combination with other commonly used alternatives to antibiotic growth promoters on performance, immunity, survivability, gut microbiome, or resistome profile. The effects of AGPs or alternatives to AGP supplementation on performance are often influenced by environmental hygienic conditions or stressors. The majority of broiler chickens in tropical countries are reared in a deep litter system. Toxic gases (ammonia and hydrogen sulfide) together with stressors such as heat, humidity, or coccidiosis in deep litter housing systems pose a threat to chickens’ health by dampening the adaptive immune response and promoting inflammation (Hofmann et al., 2020). The objective of this study was to evaluate the comparative efficacy of phytase super dosing alone and in combinations with other alternatives to antibiotic growth promoters, such as encapsulated essential oil, encapsulated butyric acid, and Bacillus subtilis (probiotic), on growth, immunity, feed efficiency, and survivability under a deep litter system of rearing and also to compare gut microbiome and resistome profile in the best-performing treatment group and control groups.
2 Materials and methods
2.1 Feed additives
2.1.1 Preparation of immobilized essential oil microspheres
In the present study, a composite feed additive developed in our lab in an earlier study, containing cinnamon oil, thyme oil, and carvacrol immobilized in a matrix of calcium alginate and sunflower oil for targeted delivery of the EO blend in the intestines of chickens, was utilized to study its effects on broiler chickens. An explanation of the method of preparation and storage of the additive has already been described in an earlier publication (Paul et al., 2023). Briefly, a 2% (w/v) sodium alginate solution was prepared, and essential oils and sunflower oil were added to the alginate solution and mixed well by stirring. The mixture was then extruded through a syringe into a 0.1 M calcium chloride solution, and beads were allowed to remain there for 30 min to harden. Calcium chloride was decanted, and the microsphere beads were rinsed twice in distilled water and then air dried. The average recovery of essential oils and sunflower oil was above 80%. The air-dried microspheres (0.85–1.25 mm in diameter) of the composite additive (EO) dissolved in gastric pH within 5 min on shaking but remained intact at a pH range of 6–8.
2.1.2 Other additives
A commercial phytase enzyme was purchased from BASF India Ltd, Mumbai, India, and was used in some of the experimental groups as specified later.
A commercial probiotic (B.subtilis, 20 billion colonies forming units/g) was purchased from Zytex, Mumbai, India, and was used in one experimental group.
Coated sodium butyrate was procured from a commercial feed-additive company (InduceAcid-Buty, CP Group, Henan, PR China) and had 30% sodium butyrate activity. It was also used in one experimental group.
An antibiotic growth promoter, namely, bacitracin methylene disalicylate (BMD), purchased from Zoetis India Ltd (Mumbai, India), was also used as a positive control in one experimental group at the dose level recommended by its manufacturer (500 g/ton feed).
2.2 Birds, experimental groups, and experimental design
A total of 1,800 newly hatched chicks obtained from a commercial hatchery (Venkateswara Hatcheries Pvt Ltd., Hyderabad 500001, India) were randomly distributed into six experimental groups in a completely randomized design. Each experimental group was allotted 12 replicated pens with 25 chicks in each replicate. The number of birds and replicates was decided based on a target of achieving a statistical power of 0.8 with a significance level of 0.05 in detecting a minimum treatment difference of approximately 50 g body weight (BW) considering the variability and statistical power analysis of our previous studies (Demetro et al., 2013). Birds were maintained in floor pens in an open-sided house with a floor space of 1.1 ft2 per bird (as per the recommendations of the breeder agency). The minimum and maximum temperatures and relative humidity in the house during the experiment were 14.3°C–36.5°C and 28%–82%, respectively. The ammonia concentration in the house was 10.54–14.22 ppm. Brooding was done at a temperature range of 30°C –33°C for up to 21 days with the help of incandescent bulbs. All of the groups were offered the same basal diet. The negative control (C) group’s feed was not supplemented with any AGP or alternatives to AGP feed additive. The five other groups were supplemented with one of the five additives, namely, BMD (B, 500 g/t feed, as recommended by the manufacturer), phytase (P, 2,000 FTU/kg feed), phytase plus butyrate (PB, phytase 2,000 FTU/kg feed plus encapsulated sodium butyrate 500g/t feed), phytase plus essential oil (PE, phytase 2,000 FTU/kg feed plus encapsulated essential oil 400g/t feed), and phytase plus probiotic B. subtilis (PBS, phytase 2,000 FTU/kg feed plus B. subtilis 400g/t feed).
2.3 Feeding regimen and performance
The chickens were fed their diets ad libitum as per the feeding standards recommended by the supplier of the chicken strain. A three-phase feeding regime (pre-starter: 1 to 14 days, starter: 15 to 28 days, and finisher: 29 to 42 days) was followed. Maize-soybean meal-based control diets (CD) were provided ad libitum from 1–42 days. Metabolizable energy (ME) and crude protein levels in the pre-starter, starter, and finisher diets were 2,950, 3,050, and 3,150 kcal/kg, and 22.5%, 21.5%, and 19%, respectively. A detailed description of diet composition has been presented in the Supplementary Material (Supplementary Table S1). The range of minimum and maximum temperatures in the house during the experiment was 19.3°C to 36.16°C. The birds were wing-tagged, weighed, and vaccinated with the Marek’s disease vaccine on arrival. Brooding was provided with incandescent bulbs and additional heat was provided with coal during the initial 3 weeks of age by providing approximately 37oC at bird level during the first week. Subsequently, the temperature was gradually reduced to the ambient temperature at day 21, after which the birds were reared at the ambient temperature. The feed was offered and made available in the feeders placed in each pen. A weighed quantity of feed was offered daily and leftover feed was weighted at weekly intervals. The feed for each phase was prepared in a single batch and stored in a container with a lid for daily use. All of the birds were provided with ad libitum access to the drinking water through separate drinkers in each pen. BW was recorded at weekly intervals for each pen and a per bird average weight was calculated for each pen. The birds were vaccinated with the Newcastle disease (LaSota strain) vaccine on the 5th and 28th day and with the infectious bursal disease vaccine on the 10th and 16th day. Mortality was recorded daily, and the pen number, the wing band number, and the body weight of the dead birds were recorded. The feed conversion ratio (FCR) was adjusted for mortality. Cages were placed distant from each other to avoid fecal contamination between pens.
2.4 Chemical analysis of proximate principles
The CP (Procedure 4.2.03, using the Kjeldahl method after acid hydrolysis), fat (Procedure 4.8.01, after extraction with petroleum ether using the Soxhlet method), and ash (Procedure 4.8.03, by igniting at 550°C for 3 h in a muffle furnace) contents of the feed were determined using AOAC procedures (AOAC, 1990). The Ca levels were determined using an atomic absorption spectrophotometer according to the manufacturer’s instructions (AAnalyst 400, PerkinElmer, and Shelton, CT, USA). The phosphorus (P) levels were estimated using a colorimetric procedure as described previously (Paul et al., 2023).
2.5 Immunity parameters
2.5.1 Hemagglutination inhibition assay
The hemagglutination inhibition (HI) test was carried out on serum samples collected from randomly selected birds (10/group) at the 5th week of age. The Newcastle disease virus (NDV) antigen was prepared according to the method described by Beard et al. (1975). Briefly, the NDV LaSota strain was grown in specific pathogen-free embryonated chicken eggs, which were incubated at 37°C for 4 days. The allantoic fluids from infected eggs were then clarified by centrifugation at 1,500 × g for 30 min. Next, the virus was inactivated overnight at 37°C with 0.1% (v/v) formalin, aggregated with 10% (w/v) polyethylene glycol at 6,000 × g for 2 h at 4°C, and then precipitated by centrifugation at 8,000 × g for 30 min. The pellet was subsequently resuspended in 1/20 volume of 0.01 M phosphate-buffered saline (PBS), pH 7.4, after which the inactivated NDV antigen was titrated using a microtiter hemagglutination (HA) test with chicken red blood cells (RBCs) and stored at −70°C until being used as an NDV antigen in an HI test. The HI titer of the NDV antigen (LaSota virus stock) was adjusted by dilution to contain 4 units of hemagglutination activity. The highest dilution of serum samples that inhibited agglutination of chicken RBCs by NDV was considered the HI titer.
2.5.2 Cutaneous basophil hypersensitivity test
The cell-mediated immune (CMI) response was assessed during the 5th week of age by a cutaneous basophilic hypersensitivity test using phytohemagglutinin lectin from Phaseolus vulgaris (PHAP). Birds (6/group) were injected intra-dermally on the leg toe web with 0.1 mg PHAP in 0.1 ml PBS. The thickness was measured using a thickness gauge before and 24 h after injecting the PHAP. The CMI response was considered as the difference in the thickness before and after injecting with PHAP. The preinjection thickness was used as a control instead of injecting phosphate-buffered saline in the other leg toe as suggested by Smits et al. (1999).
2.6 Carcass traits
Carcass traits, including ready-to-cook yield (RTC) and relative weights of breast meat, liver, and abdominal fat, were recorded by slaughtering two birds per replicate at day 36. The weights of these portions were expressed as g per kg of pre-slaughter live weight of the respective bird.
2.7 Caecal content sample collection
On the 36th day of age, 10 healthy chickens were selected (one per pen) per group, and euthanized by cervical dislocation. The caecal contents were opened using sterile scissors, and the luminal contents of the caeca were collected into sterile storage vials. For every g of caecal content, 5 mL 1X phosphate-buffered saline was added and mixed by vortexing to produce a homogenate. The homogenized caecal content was immediately stored in a freezer at −20°C, transported to the laboratory, and stored at −80°C.
2.8 DNA extraction
DNA was extracted from the homogenized caecal contents of individual chickens following the bead beating plus column method described previously (Yu and Morrison, 2004) using the DNA purification columns from the QIAamp Fast DNA Stool Mini kit (QIAGEN GmbH, Hilden, Germany). DNA concentration and quality were assessed using a Qubit 3.0 fluorometer (ThermoFisher Scientific, Waltham, MA, USA, <ns/>Q33238) and also by gel electrophoresis. DNA was stored in a freezer at −20°C until further processing.
2.9 Shotgun metagenomic sequencing, sequence processing, and analysis
For shotgun metagenomic sequencing, 18 DNA samples (six individual birds slaughtered at 36 days of age) were sampled per group and each bird from a separate pen was selected randomly. QC-passed DNA samples (100 ng DNA per sample) were fragmented and tagged with sequencing adapters using the TruSeq Nano Library preparation kit (Illumina, San Diego, CA, United States, <ns/>20015964), and the extracted DNA samples resulted in libraries of the appropriate size and concentration to be sequenced. Shotgun metagenomic sequencing was performed using the NovaSeq 6000 sequencer (Illumina) at 150 bp in paired-end mode. Following sequencing, all reads were assessed for quality parameters and were subjected to trimming, adapter removal, and host genome sequence removal [using Bowtie (Langmead and Salzberg, 2012), SAM tools (Danecek et al., 2021), and BED tools (Aaron et al., 2010) against Gallus (taxonomy is 9031) and pair-end read merging using the Fastp program (version 0.23.1; Chen et al., 2018)]. The Kaiju webserver (v1.9.0) was used in default mode for the taxonomic assignment of sequences. Kaiju translates reads into amino acid sequences and then compares them to a reference database containing bacterial, fungal, viral, and microbial eukaryotic protein sequences using the Burrows–Wheeler algorithm and assigns each read to a taxon in the NCBI taxonomy. By using protein-level classification, Kaiju has been shown to achieve a higher sensitivity compared with methods based on nucleotide comparison (Menzel et al., 2016). The Kaiju output was converted to a OTU table and taxonomy table using a Python script (Paul et al., 2022).
The OTU table and consensus taxonomy files along with the metadata file were converted into a phyloseq object and analyzed using R (R Core Team, 2020). OTUs classified as taxons other than bacteria were removed. For alpha diversity and rarefaction analysis, the data were rarified to an even depth (at 19,715 sequences per sample). For the analyses of differential abundance, the creation of stacked bar plots and upset diagram data were filtered for the low-count and low-variance OTUs. OTUs with < 5 members or appearing in <2 samples were removed and the data were normalized using the cumulative sum scaling method.
Beta diversity profiling and significance testing were carried out at the OTU level using non-metric multidimensional scaling (NMDS) ordination based on different distance methods, such as Bray–Curtis dissimilarities, Jaccard index, and Jensen–Shannon diversion, using statistical methods, such as permutational multivariate analysis of variance (PERMANOVA) and homogeneity of group dispersion (PERMDISP) using the MicrobiomeAnalyst web server (version 2.0) (https://www.microbiomeanalyst.ca/) after disabling default settings for data filtering for the low count and low variances but data were rarefied to minimum library size before analysis.
Differential abundance was analyzed using MetagenomeSeq(fitZiG) followed by the Benjamini–Hochberg false discovery rate (FDR) correction for multiple comparisons for the detection of significant differences. OTUs showing significant differences in abundance among groups on MetagenomeSeq(fitZiG) analysis were further compared pairwise using the non-parametric Mann–Whitney U test (Wilcoxon rank sum test) as implemented in SPSS (SPSS, 2008). The random forest analysis options available in MicrobiomeAnalyst were also utilized to identify important OTU-defining groups. Taxonomic assignments (for the top 24 taxa) were presented as stacked bar plots from normalized relative percent abundance data. Bubble plots of OTUs significantly differing in abundance among groups in the MetagenomeSeq(fitZiG) analysis were created using R.
For the estimation of the abundance of AMR genes, GROOT (version 1.12), a tool to profile antimicrobial resistance genes in the metagenome (resistome profiler software), and the “groot-db” version (a pre-clustered database derived from ResFinder, ARG-annot, and CARD databases) of the databases provided with the software were used (Rowe and Winn, 2018). The authors claim that GROOT is both faster and more accurate than contemporary tools such as ARGS-OAP and AMRPlusPlus (Rowe and Winn, 2018). For the estimation of the abundance of mobile genetic elements, the mobileOG database (Brown et al., 2022) and a mobile genetic element annotation pipeline mobileOG-pl.v. kynite (https://mobileogdb.flsi.cloud.vt.edu/) were used (https://github.com/clb21565/mobileOG-db/tree/main/mobileOG-pl).
2.10 Statistical analysis
The performance variable, slaughter, and digestibility data were analyzed by one-way analysis of variance (ANOVA) using the general linear model univariate procedure available in SPSS (SPSS, 2008). At the detection of an overall significant difference, specific differences between pairs of means were tested using Duncan’s multiple range test (DMRT) at a p-value < 0.05. In this study, for post hoc analysis, DMRT was chosen over another popular test, i.e., Tukey’s HSD, as DMRT has higher power (higher probability of detecting differences when they exist) than Tukey’s HSD. However, DMRT is known to have a lower ability to control for a type I (family-wise) error. We have considered DMRT only on a significant overall F test to overcome the effect of a large type I error probability (Chew, 1976). DMRT is commonly used in poultry nutrition as even a lesser difference in body weight (as low as 50 g) or feed efficiency has substantial commercial value. For the shotgun sequence data, at the detection of a significant difference in the overall abundance between groups in the MaAsLin2 analysis, followed by Benjamini–Hochberg FDR correction of p-values for multiple comparisons, groups were compared pairwise using the non-parametric Mann–Whitney U-test (Wilcoxon rank-sum test), as implemented in SPSS. Alpha diversity metrics were compared at the OTU level using the non-parametric Wilcoxon test, as implemented in the MicrobiotaProcess library in R (https://github.com/YuLab-SMU/MicrobiotaProcess/). Beta diversity profiling and significance testing were carried out using NMDS ordination based on different distance methods, using statistical methods, such as PERMANOVA and homogeneity of group dispersion of permutational analysis of multivariate dispersions (PERMDISPs).
Data on mobile genetic elements and AMR genes were normalized to per million reads and tested for normality (Shapiro–Wilk test and univariate normal plots) and equal variance (Levene’s test). For statistical analysis, normalized data on mobile genetic elements were statistically analyzed using the non-parametric Kruskal-Wallis test, as implemented in SPSS.
3 Results
Details of the composition of experimental diets are provided in Supplementary Table 1.
3.1 Effects on performance, slaughter variables, and immunity parameters
The results indicated that body weight gain (BWG) and feed intake (FI) were not affected by the dietary treatments. However, the feed conversion ratio (FCR) was affected on both days 21 and 35 (Table 1). The feed conversion ratio in the C group was significantly better (a lower value of FCR is considered better in terms of production efficiency, as it signifies that less feed was needed to produce a kilogram of body weight gain) than the B group at day 21 but was comparable at day 35. The inclusion of the enzyme alone did not improve FCR compared to the C at 21 d. However, the combination of phytase with the butyrate or EO significantly (p < 0.05) improved the FCR compared to C (PE vs. C: p < 0.001) or B (PE vs. B: p < 0.001) at day 21. At day 35, broilers fed the phytase alone or in combination with butyrate, EO, or B.subtilis had significantly (p < 0.05) higher cumulative performance in terms of feed efficiency than those fed the B or C with the best feed efficiency recorded in the phytase plus EO (PE) group.
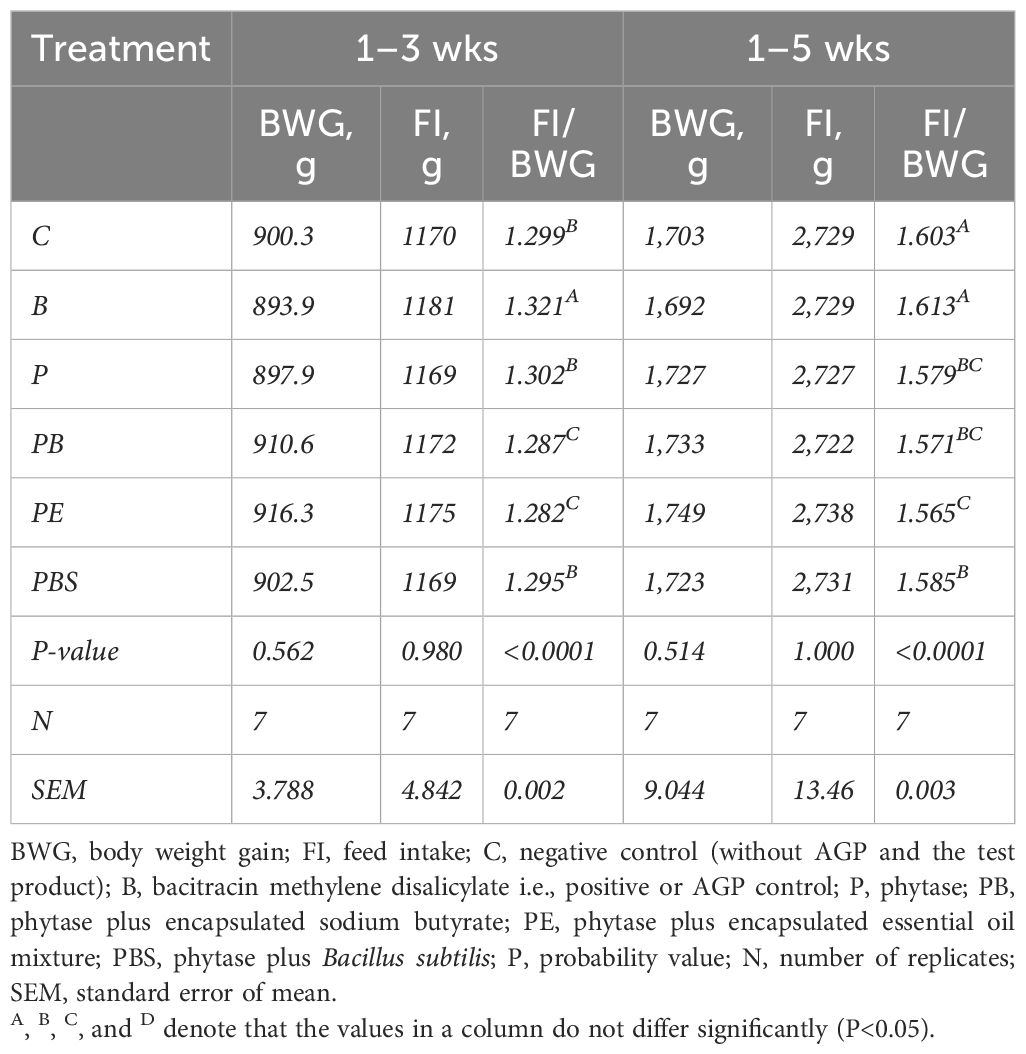
Table 1. Performance of broilers fed a combination of neutraceuticals as potential alternatives to antibiotics (BMD).
All the slaughter variables tested, except the abdominal fat content, were not significantly (P > 0.05) affected by the treatments employed in the current study (Supplementary Table S2). The relative weights of abdominal fat in PE, PBS, and B groups were significantly lower than the control or remaining groups. The immune responses (both HI and CMI) were not affected by the inclusion of either AGPs or the alternatives tested in the current study.
Cumulative mortality rates were relatively lower in the PE (2.86%) and PBS (1.71%) groups compared to other groups (4.57%, 4.0%, 5.14%, and 6.28% in the groups C, B, P, and PB, respectively).
Humoral immune response in terms of ND titer (log 2 values) was comparable among the groups (p = 0.696). Cell-mediated immune response (mm increase in skin thickness in response to PHAP) was also comparable among the groups (p = 0.241).
3.2 Microbiome sequencing
The shotgun sequencing generated approximately 1.4 billion raw reads from the caecal content of 18 chickens with a range of 50.2 to 138.2 million raw paired-end reads per sample. After merging paired-end reads, filtering low-quality sequences, and host sequence removal, the average number of quality-controlled sequences per sample was 47,009 (range, 20,593–65,368). From the shotgun data, a total of 1,509 phylotype OTUs were detected.
3.3 Taxonomic assignment
At the class level, Clostridia was the most dominant class in all the groups (Figure 1). The proportion of Bacteroidia was lower in the control group, higher in the B group, and intermediate in the PE group. Whereas, the proportion of Deltaproteobacteria was lower in the PE group compared to the remaining groups.
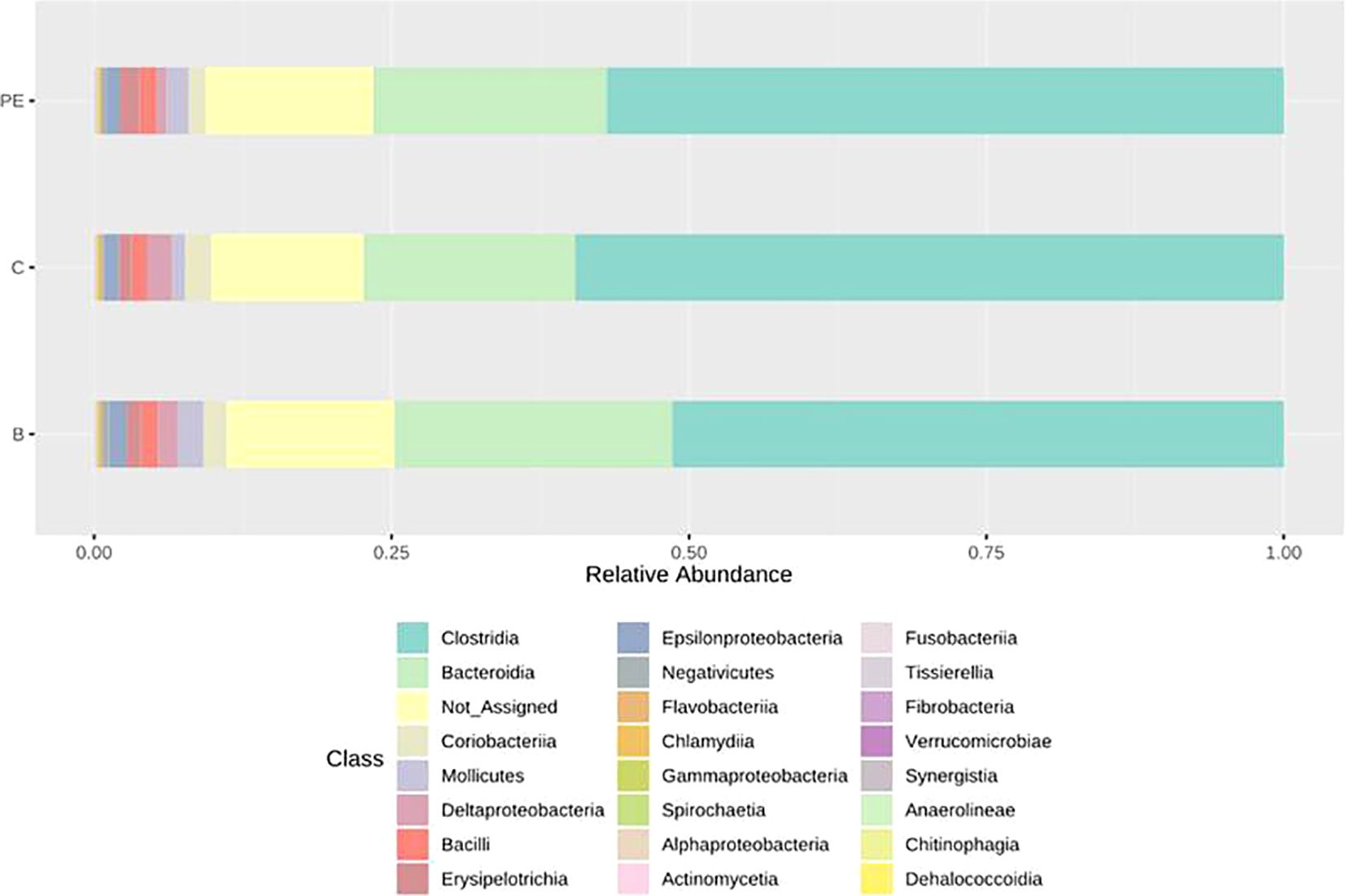
Figure 1. Bar plots of the average normalized relative abundance of the 24 most abundant bacterial taxa at the class level found in different groups.
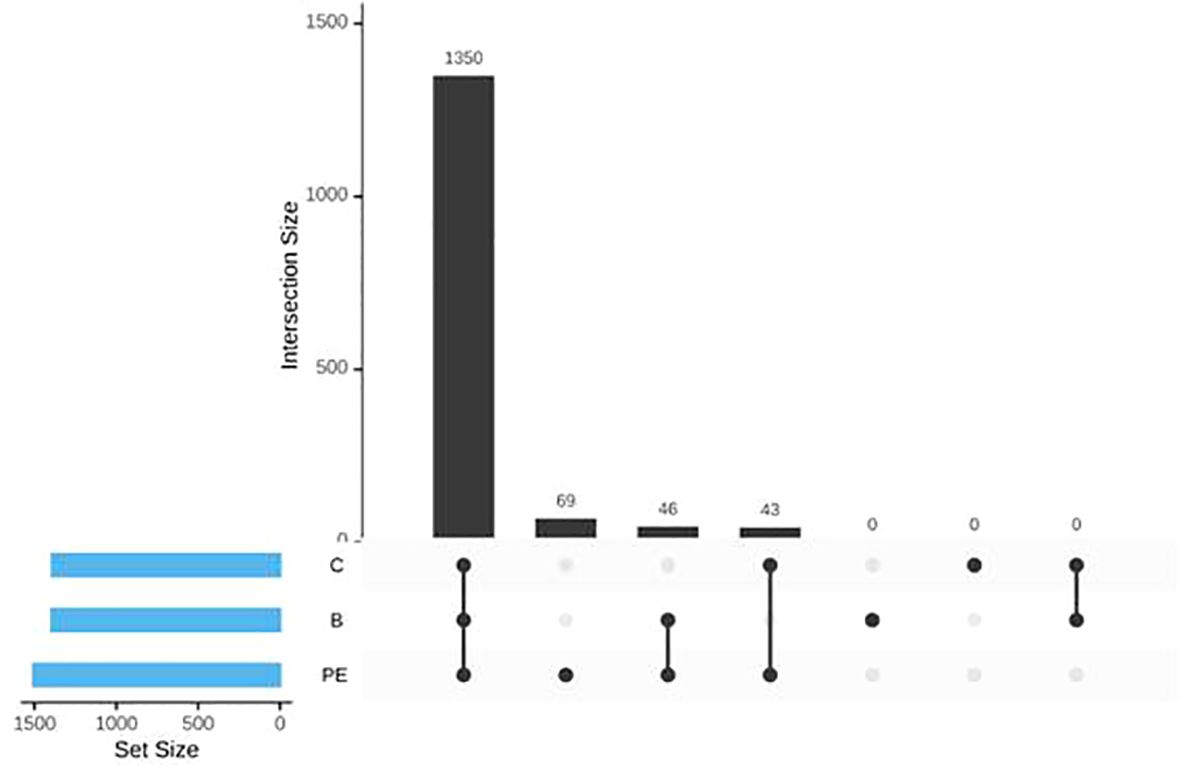
The UpSet diagram indicated a high level of overlap of non-rare OTUs (OTUs with at least five members and occurring in at least two samples) among different groups where 1,350 OTUs were detected in all the groups. A total of 69 OTUs were detected only in the PE group (Figure 2). A total of 46 OTUs were absent in the control group but present in the other two groups. In contrast, a total of 43 OTUs were not detected in the B group but were present in both the C and PE groups.
3.4 Alpha diversity and rarefaction analysis
Alpha diversity metrics, namely the Shannon index, Simpson’s index, and Fisher index, did not differ significantly (p-values: 0.311, 0.266, and 0.358, respectively) among the groups (Supplementary Figure S1). Richness estimators, such as observed richness, Chao1, and ACE, also did not differ significantly (p-values: 0.581 and 0.433, respectively) among the groups.
The rarefaction curve indicates the relationship between the number of sequences and the number of taxonomic OTUs detected, and the steeper the slope, the higher the diversity. The rarefaction curve (Figure 3) approached the asymptotic level for each group, indicating the availability of sufficient reads to represent each microbial community. The PE group had relatively higher diversity compared to other groups.
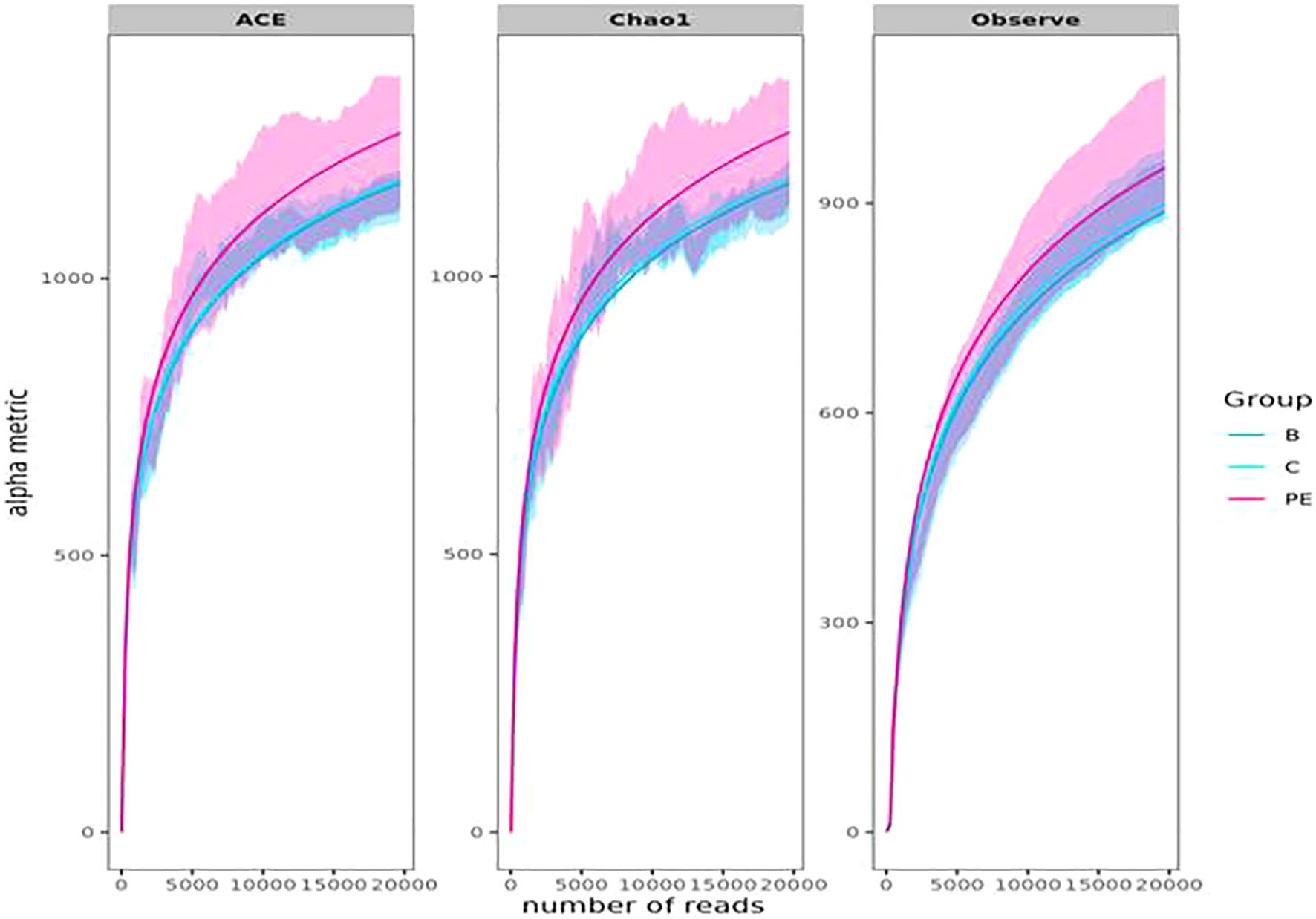
Figure 3. Rarefaction curves based on ACE, Chao1, and observed OTUs. Bacterial sequences were rarefied to the minimum library size without data filtering for rare OTUs.
3.5 Microbial beta diversity
Beta diversity was visualized using NMDS and the plot obtained through NMDS ordination using Bray–Curtis metrics is presented in Supplementary Figure S2. The beta diversity plot depicts the partitioning of biological diversity among groups along a gradient, i.e., the number of species shared between the two groups. Beta diversity ordination resulted in clear visual separation of samples due to groups but there was a high degree of overlap among the groups. The PERMANOVA test performed using different beta diversity metrics showed no significant differences (R-square:0.11286; p-value:0.562 on the Bray–Curtis index) in community structure among different groups. The beta dispersion values (PERMDISP) were non-significant (p-value:0.262 on the Bray–Curtis index) for all groups, indicating a homogeneous dispersion among groups.
3.6 Differential abundances of bacteria
MetagenomeSeq (fitZiG) analysis of CSS normalized sequences with FDR correction indicated that 18 OTUs differed significantly (the FDR-corrected p-values ranged from 0.03807 to 2.26E-06) in abundance among groups (Figure 4). Pairwise comparison among groups using the Mann–Whitney U test showed that the abundance of some of the OTUs belonging to the species Subdoligranulum variable, phylum Firmicutes, and order Clostridiales were significantly lower in the B group than in the control group. The abundance of some of the OTUs belonging to the species Bernesiella viscericola, order Clostridiales, and uncultured species level group Clostridium 2812 was significantly higher in the PE group compared to the control group. The abundance of an OTU belonging to the species Subdoligranulum variable and another OTU belonging to the species Barnesiella visericola were significantly higher in the PE group compared to the B group. The abundances of some of the OTUs belonging to the species Parabacteroides johnsonii and uncultured species level groups Clostridium 2621and Coralococcus CAG 1435 tended to be higher (P<0.1) in the B group compared to the control group. The abundances of one OTU belonging to the order Clostridiales and another OTU belonging to the uncultured species level group Clostridium 2812 tended to differ between the B and PE groups.
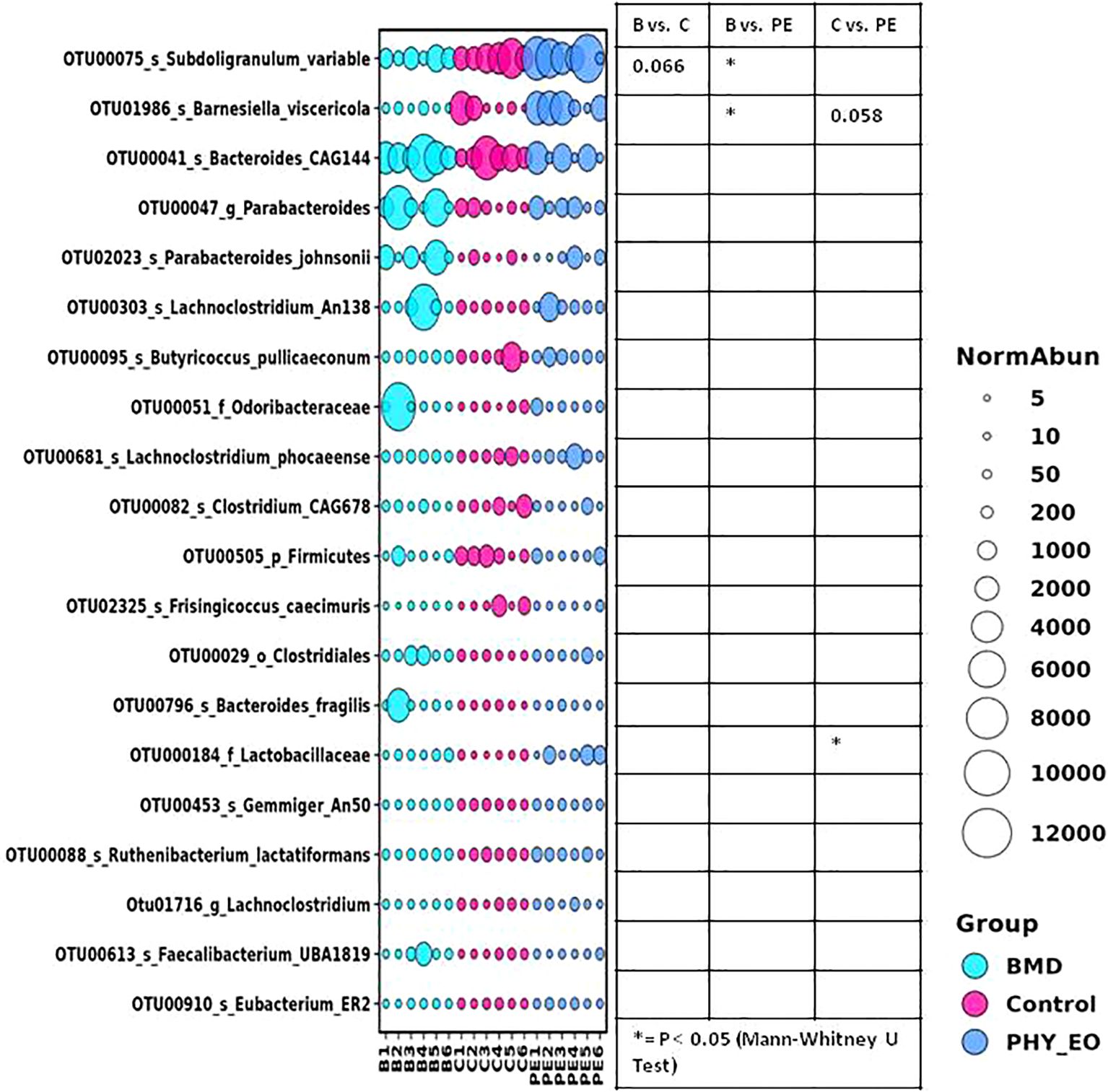
Figure 4. Differential abundance of gut microbiota in different groups at the OTU level [bubble plot of OTUs differing significantly in abundance among groups in the MetagenomeSeq (fitZiG) analysis]. The size of the bubbles in the bubble plot indicates the normalized (cumulative sum scaling) abundance of each OTU.
The group-specific biomarkers based on random forest algorithm analysis indicated that some OTUs belonging to the species Lachnoclostridium phylofermentans, species Clostridium Bornimense, genus Roseburia, and species Galacturonibacter soehngenii had higher values of mean decrease in accuracy compared to other OTUs, indicating the higher importance of these taxa in defining bacterial community structure difference among different groups (Figure 5). Other important OTUs that contributed to the difference in community structure were affiliated with the genus Clostridium, phylum Tenericutes, species Anaerovirgula multivorans, and phylum Firmicutes.
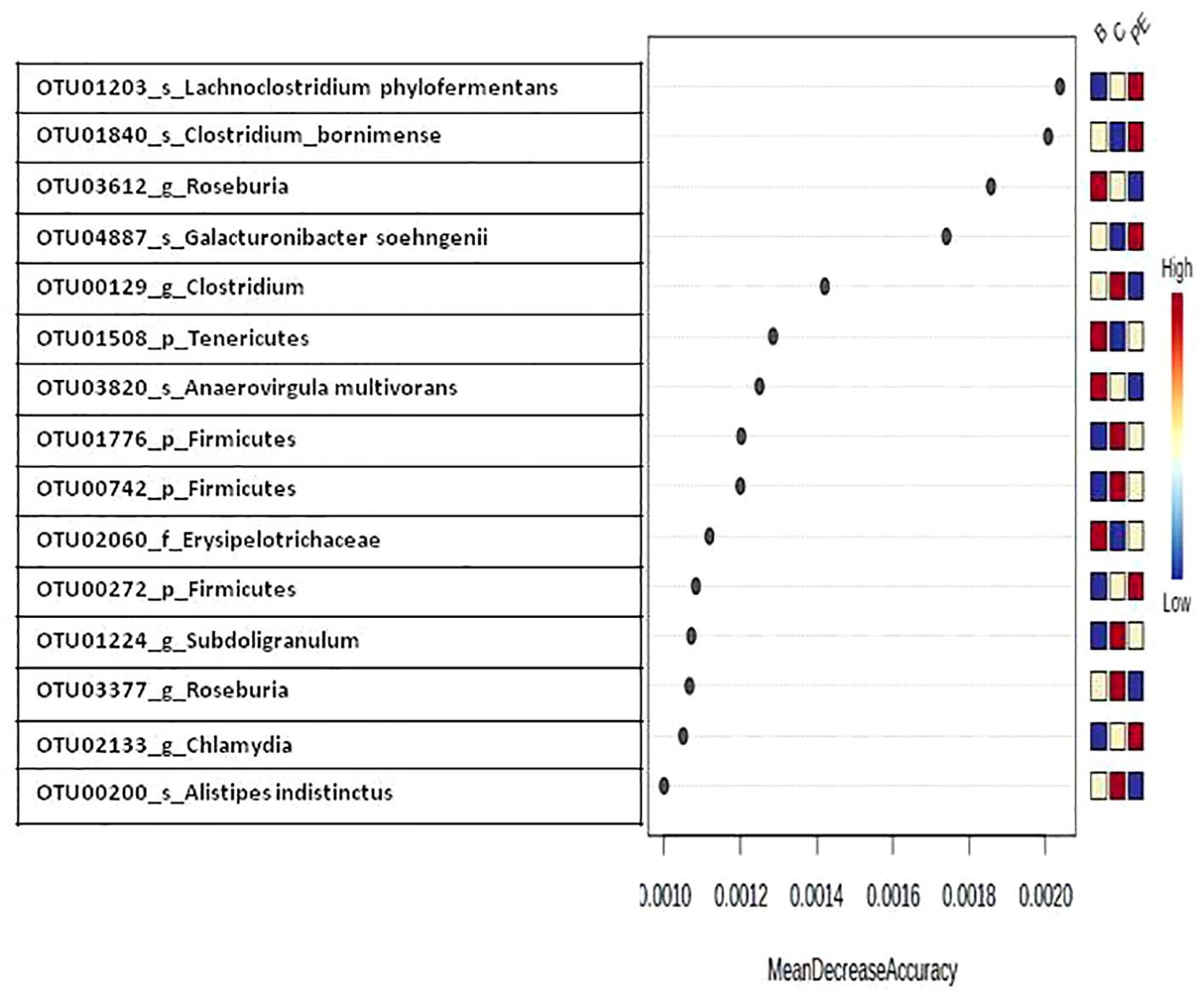
Figure 5. Random forest variable importance plot of caecal bacteria at the OTU level. Mean decrease in accuracy is the measure of the performance of the model without each category. A higher value indicates the importance of that taxa in predicting group. Removal of that taxa causes the model to lose accuracy in prediction.
3.7 Effects of treatments on the density of antimicrobial resistance genes
In the caecal metagenomic DNA, ARGs belonging to four antimicrobial classes were detected, namely, aminoglycosides, extended-spectrum beta-lactamase, Tetracycline, and macrolides-lincosamide-streptogramin class. However, the densities of ARGs expressed as reads per million reads did not differ significantly (p-values: 0.169 to 0.581) among the groups (Table 2).

Table 2. Effect of treatments on the density of antimicrobial resistance genes in the caecal microbiome of chickens.
3.8 Effects of treatments on the density of mobile genomic elements in the caecal microbiome of chickens
In the caecal metagenomic DNA, mobile genetic elements belonging to six classes were detected, namely, virulence factor, plasmid, phage, insertion sequence, integrative genetic elements, and multiple types with a relatively higher density of virulence factor, phage, and integrative genetic elements than other classes. However, the densities of ARGs expressed as reads per million reads did not differ significantly (p-values: 0.097 to 0.633) among the groups (Table 3).
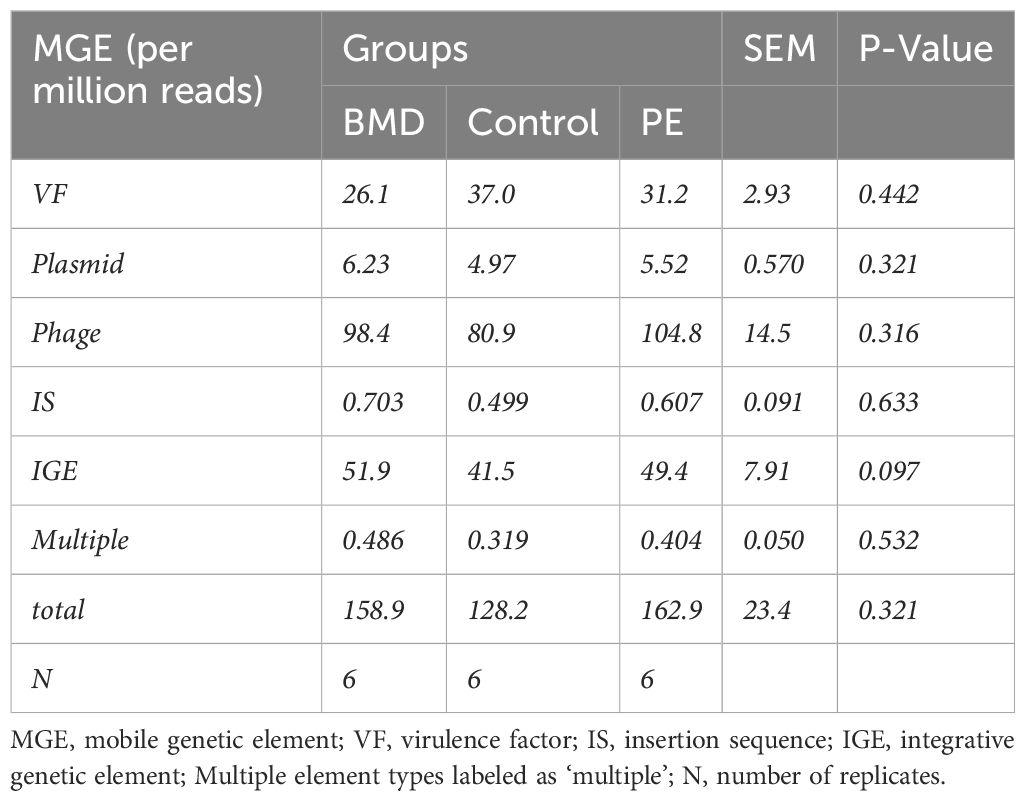
Table 3. Effect of treatments on the density of mobile genetic elements in the caecal microbiome of chickens.
4 Discussion
The AMR associated with the feeding of AGPs represents a significant global public health concern for poultry production. The use of a routine dose of phytase (up to 500 units per kg diet) was originally proposed as an animal feed additive to enhance P utilization in animal feed. Phytase super dosing is a recent concept where high doses of the enzyme in non-ruminants have been shown to have growth-promoting effects (Fernandes et al., 2019). Phytase super dosing facilitates the complete degradation of phytic acid and facilitates myo-inositol synthesis, playing a role in cell growth and improving the digestibility of amino acids, energy, and minerals. This results in better animal performance, exceeding improvement estimated only based on nutrient release values (Cowieson et al., 2015). Phytase supplementation has been shown to increase beneficial fibrolytic, lactic acid, and butyrate-producing bacterial genera in pigs due to a reduction in P availability in the hindgut (Metzler-Zebeli et al., 2020). Short-chain fatty acids such as butyrate produced by gut bacteria have various beneficial effects on host health, including energy metabolism, immune function, and gut barrier integrity (Donohoe et al., 2011; Morrison and Preston, 2016).
However, reports on the effect of phytase super dosing and its combination with other alternatives to antibiotic growth promoters on performance and gut microbiome or resistome profile of chickens reared in deep litter systems under tropical conditions could not be found.
Encapsulated organic acids, especially butyric acid, are considered promising antibiotic alternatives in non-ruminants. Dietary coated organic acid supplementation in poultry improves nutrient digestibility, increases populations of beneficial microflora (Lactobacillus spp.), and reduces harmful bacteria (C. perfringens, E.coli, and Salmonella spp.) in the gut (Dai et al., 2021).
EOs have also been used as a promising in-feed antibiotic alternative due to their natural occurrence, low toxicity, residue-free properties, and antioxidant, anti-bacterial, and anti-inflammatory activities. Studies on poultry have revealed that supplementing with EOs could improve growth performance, modulate gut microbial compositions, and alleviate the negative effects of gut pathogens (Yin et al., 2017; Liu et al., 2018; Paul et al., 2023). Cinnamon oil, thyme oil, and carvacrol have been shown to have antimicrobial properties primarily due to their ability to disrupt cell membranes, inhibiting cell division, and affecting metabolic processes either individually or synergistically (Reyes-Jurado et al., 2016). Further, essential oils have also been shown to have antioxidant and immunomodulatory effects, besides the ability to upregulate genes related to the endocrine and metabolic function of chickens. Probiotics are known to promote growth rate, exclude pathogens from the gut, and also provide vitamin B complex and other nutrients and hence are considered an effective alternative to antibiotic growth promoters. Among a large number of microorganisms serving as probiotics in poultry production, spore-forming bacteria such as B. subtilis are gaining popularity due to their tolerance and survival under hostile gastrointestinal environments. Dietary supplementation of B. subtilis significantly improved performance, decreased mortality rates, increased the density of beneficial bacteria, and reduced the density of pathogens in the gut of poultry (Qiu et al., 2021).
In the present study, the AGPs did not modify the growth rate or feed efficiency significantly. However, phytase super dosing (2,000 unit per kg diet) alone or combinations of phytase super dosing and encapsulated butyrate, encapsulated essential oils, or probiotic B. subtilis significantly improved FE compared to the AGP and negative control groups, and the highest FE was observed in the phytase plus encapsulated essential oil group. However, the addition of encapsulated butyrate, encapsulated EO, or B. subtilis did not increase FE significantly compared to the phytase super dosing alone.
The phytase plus EO group had a significantly better FE compared to the phytase plus B. subtilis group.
Previously, Attia et al. (2015) showed that phytase at a traditional dose may enhance the performance of broiler chickens and yield similar growth performance and economic efficiency to those of AGP (Zinc bacitracin)-supplemented groups. Bello et al. (2022) observed that phytase super dosing in a deep litter system improved growth performance in broiler chickens fed a diet without added inorganic phosphorus. Ennis et al. (2020) reported that super dosing with phytase improved the broiler growth performance with a better feed conversion ratio of 4 and 3 points during the 28-day and 44-day periods, respectively, in a floor-rearing system. Our results are in concordance with these studies.
The mortality percentage was the lowest in the phytase super dosing plus B. subtilis group, closely followed by the phytase super dosing plus EO group.
The effects of AGP supplementation on performance response have remained inconsistent and are often influenced by environmental hygiene conditions or stressors. Previously, Casewell et al. (2003) observed that antibiotics were ineffective in improving the growth performance of chickens housed in a clean environment. In a previous study, we observed that supplementation with AGPs did not exert a growth-promoting effect, except for the 1–21 d period in one of the three experimental feeding trials (Paul et al., 2022). In a meta-analysis involving 174 scientific articles on broiler chickens, Cardinal et al. (2019) reported that higher weight gain and a lower feed conversion ratio were observed in the AGP-fed groups during the initial phase (1 to 21 d) and the total period (1 to 42 d) with no difference in the final phase (22 to 42 d). A reduction in the effectiveness of AGPs over the last three decades due to increasing levels of antimicrobial resistance (Laxminarayan et al., 2015) may also be a potential contributory factor to the observed ineffectiveness of the AGP in the present study.
The present study indicated that supplementation of phytase or its combinations with EO, sodium butyrate, or the probiotic did not influence cellular immunity (PHAP response) or humoral immunity (HI titer against NDV). In contrast, we reported that an immobilized blend of EO significantly increased cellular immunity in broiler chickens when reared in a clean environment in battery brooder cages (Paul et al., 2023). El-Shall et al. (2020) also reported that supplementation with a commercial EO mixture showed an immune-stimulating response to vaccines in chickens. Previously, dietary supplementation of B. subtilis was shown to improve the immune response of broiler chickens (Wang et al., 2022). Similarly, sodium butyrate supplementation has been shown to improve cell-mediated and humoral immunity in chickens (Sikandar et al., 2017).
However, the mortality rate was much lower in the PBS and PE groups compared to the other groups, indicating the practical beneficial effects of these additives, the possible reasons for which cannot be explained based on a few immunity parameters alone. EOs and probiotics might have influenced mortality through factors other than immunity, such as anti-inflammatory, antioxidant, antimicrobial, or pathogen exclusion effects.
Ammonia emission is strongly affected by manure management and therefore ammonia concentrations are generally higher in litter-based housing types (David et al., 2015). H2S likewise results from the degradation of liquid manure under anaerobic conditions. Concentrations in poultry production vary between 0 to 9 ppm in floor-based and 0 to 0.2 ppm in cage systems (Guarrasi et al., 2015). Compared to fresh air conditions, exposure to NH3 between 26 and 60 ppm decreased antibody titers after NDV vaccination and total serum concentrations of IgY, IgM, and IgA (Wang et al., 2010). H2S also exhibits profound effects on the immune system. Similarly to NH3, 20 or 30 ppm H2S activated inflammatory responses due to an increase in the pro-inflammatory cytokines (Hu et al., 2018). Toxic gases and stressors such as heat, humidity, or coccidiosis in a deep litter housing system pose a threat to chickens’ health by dampening the adaptive immune response and promoting inflammation (Hofmann et al., 2020). Hence, the effects of additives on immune responses reported based on studies carried out in a clean environment may differ from what we observed in the deep-litter system. In deep litter systems, managing ammonia and hydrogen sulfide requires control of moisture, ventilation, and manure management, which often tend to be suboptimal under practical circumstances. The feed additives might help in achieving better performance under stressful setups such as deep litter systems through modulation of the gut environment.
In the chicken gut, there are a variety of potentially pathogenic bacteria such as E.coli, C. perfringens, Salmonella spp., Campylobacter, and Enterococcus, which affect the intestinal epithelium, cause systemic infection in the host, reduce the digestibility of feed, and causes performance loss and mortality. They are also causative agents for zoonotic diseases and may carry and transmit antimicrobial resistance and mobile genetic elements. However, chicken guts harbor a variety of beneficial bacteria that have health-promoting effects such as competitive exclusion of pathogens, improving intestinal barrier and immunity, and production of bacteriocins against pathogens. A detailed analysis of the changes in the population density of gut microbes, antimicrobial resistance genes, and mobile genetic elements can give detailed insight into the gut-health-promoting potential and potential to reduce the spread of antimicrobial resistance of such additives.
In this study, we compared gut microbiome, resistome, and mobile genetic element profiles of the top-performing treatment group, negative control (basal diet), and the AGP control group. The absence of significant differences in the density of ARGs or mobile genetic elements with the use of various alternatives to AGP feed additives suggests that they are unlikely to promote the development and spread of resistant bacteria and this can ease safety concerns related to the use of alternatives to antibiotic growth promoters. This study also provides an insight into the level of abundance of ARGs and mobile genetic elements in chickens reared in deep litter and tropical conditions in a comprehensive manner, which can serve as baseline data and may help in developing tools for surveillance of such elements in chicken farm environments in the future.
Taxonomic assignment data indicated that Clostridia was the most abundant class, followed by Bacteroidia in all three groups, which is in agreement with our previous reports (Paul et al., 2022, Paul et al., 2023). The average proportion of Bacteroidia was higher in the PE and B groups compared to the control. The taxonomic analysis did not indicate a substantial abundance of E.coli or any other known pathogenic bacteria (abundance being <0.1%). The UpSet diagram indicated that the majority of the OTUs were common to all the groups and that only a few OTUs were unique to the PE group. Rarefaction analysis indicated that the PE group had a relatively higher diversity compared to the other groups. The alpha and beta diversity metrics did not differ significantly among the groups. Previously, some studies indicated that AGPs have no significant effects on alpha diversity metrics (Proctor and Phillips, 2019), however, some studies indicated a significant increase (Crisol-Martinez et al., 2017) or decrease (Choi et al., 2018) in alpha diversity metrics. Previously, we observed that AGP, yeast, and EO supplementation resulted in a significant effect on alpha and beta diversity metrics in the gut microbiome of chickens reared in cages (Paul et al., 2023).
Differential abundance analysis using MetagenomeSeq (fitZiG) followed by FDR correction identified 18 OTUs differing significantly between at least one pair of the groups. The supplementation of phytase at the super-dose level plus encapsulated EO resulted in a significant increase in the relative abundance of bacteria belonging to the species Barnesiella viscericola, order Clostridiales, and uncultured Clostridium compared to the control. Barnesiella is considered a beneficial gut microbe that plays a vital role in promoting health. The introduction of Barnesiella into the gastrointestinal tract was shown to prevent the colonization of vancomycin-resistant Enterococcus faecium (Ubeda et al., 2013). Barnesiella has also been shown to regulate the immune system and control inflammation (Daillere et al., 2016). The supplementation with the AGP (BMD) significantly increased the abundance of Subdoligranulum variable, phylum Firmicutes, and order Clostridiales. Subdoligranulum variable was shown to produce butyrate (Holmstrom et al., 2004), a short-chain fatty acid known for its gut health potential (Van Hul et al., 2020).
Random forest analysis identified microbes, such as Lachnoclostridium phylofermentans, species Clostridium Bornimense, genus Roseburia, and species Galacturonibacter soehngenii, as having higher contributions in defining differences in microbial community structure among the B, C, and PE groups. The PE group had a higher density of Lachnoclostridium phylofermentans, Clostridium Bornimense, and Galacturonibacter soehngenii compared to the C or B groups. Lachnoclostridium phylofermentans is a fiber-fermenting bacteria that plays a crucial role in gut health and immune function through the production of beneficial metabolites such as short-chain fatty acids (Zaplana et al., 2024). Clostridium bornimese is an anaerobic bacteria with an ability to produce short-chain fatty acids and thus may play a role in gut health but research on its potential role as a probiotic is limited. Galacturonibacter soehngenii is a strict anaerobe belonging to the Lachnospiraceae family and is capable of producing butyrate from various sugars, lactate, and acetate and is considered a probiotic candidate for humans (Gilijamse et al., 2020). Notably, the density of the genus Roseburia was higher in the B group compared to the C or PE group. Roseburia spp. plays an important role in gut health and the maturation of the immune system, primarily through the production of butyrate (La Rosa et al., 2019).
Calculations using the performance, mortality, feed intake, and cost of additives from the experimental data show a cost-benefit ratio of 1:3, 1:7.9, and 1:8 for phytase alone, phytase plus encapsulated EO, and phytase plus B. subtilis, respectively and no benefit in BMD or phytase plus butyrate groups over the control group.
In conclusion, the data presented herein demonstrate that phytase super dosing alone can improve the performance of broiler chickens compared with the control or AGP. The addition of encapsulated butyrate, encapsulated EO, or B. subtilis did not increase FE significantly compared to the phytase super-dosing alone, although some numeric improvement was evident; however, the mortality rate was lower in the phytase plus B. subtilis and phytase plus encapsulated EO groups compared to the other groups. The densities of some of the beneficial microbes were higher in the phytase plus EO group and BMD group compared to the control. The density of ARGs or mobile genetic elements was not influenced by the addition of the AGP or the phytase plus encapsulated EO. Thus, phytase super dosing alone or in combination with encapsulated EO or B. subtilis can be used as an effective alternative to antibiotic growth promoters for broiler chickens reared in deep litter systems under tropical conditions. This study will contribute to global efforts to reduce AMR and develop strategies for sustainable poultry production practices.
Data availability statement
The original contributions presented in the study are publicly available. This data can be found here: NCBI BioProject PRJNA1177199.
Ethics statement
The study protocol was approved by the Institute Animal Ethics Committee of ICAR-Directorate of Poultry Research vide its approval number IAEC/DPR/20/8 dated 20.11.2020. The study was conducted in accordance with the local legislation and institutional requirements.
Author contributions
SP: Conceptualization, Data curation, Investigation, Methodology, Project administration, Visualization, Writing – original draft. SR: Conceptualization, Data curation, Investigation, Supervision, Writing – review & editing. RC: Writing – review & editing. MR: Writing – review & editing. BP: Data curation, Investigation, Writing – review & editing. SY: Data curation, Investigation, Writing – review & editing. AK: Data curation, Investigation, Writing – review & editing. GN: Data curation, Formal Analysis, Investigation, Methodology, Writing – review & editing. VK: Data curation, Formal Analysis, Investigation, Writing – review & editing. PK: Data curation, Investigation, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This research work was partly supported by the Department of Biotechnology, Government of India under an India-UK collaborative research project entitled, ‘Chicken or egg: drivers of antimicrobial resistance in poultry in India’ (Grant no: BT/IN/Indo-UK/AMR/05/NH/2018–19 dated 10.9.2018).
Acknowledgments
The authors gratefully acknowledge the healthcare support for the experimental birds provided by the health section of ICAR-DPR.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fanim.2025.1539543/full#supplementary-material
Supplementary Figure 1 | Alpha diversity across samples. Differences in alpha diversity metrics of microbial diversity and richness in the gut microbiota of chickens in top-performing and control groups are shown as violin plots.
Supplementary Figure 2 | Beta diversity among top-performing and control groups. The beta diversity plot was visualized using non-metric multidimensional scaling-based ordination at the OTU level using Bray–Curtis metrics.
Supplementary Table 1 | Ingredient and nutrient composition of basal diets (common for all groups).
Supplementary Table 2 | Effect of treatments on slaughter variables and immune responses in broiler chickens.
References
Aaron R., Quinlan I., Hall M. (2010). BEDTools: a flexible suite of utilities for comparing genomic features. Bioinform. 26, 841–842. doi: 10.1093/bioinformatics/btq033
Abd El-Hack M. E., El-Saadony M. T., Salem H. M., El-Tahan A. M., Soliman M. M., Youssef G. B., et al. (2022). Alternatives to antibiotics for organic poultry production: Types, modes of action and impacts on bird’s health and production. Poult. Sci. 101, 101696. doi: 10.1016/j.psj.2022.101696
AOAC (1990). Official methods of analysis. 15th ed (Washington, DC, USA: Association of Official Analytical Chemists).
Attia Y. A., Bovera F., Abd El-Hamid A. E., Tag El-Din A. E., Al-Harthi M. A., El-Shafy A. S. (2015). Effect of zinc bacitracin and phytase on growth performance, nutrient digestibility, carcass and meat traits of broilers. J. Anim. Phys. Anim. Nutr. 100, 486–491.
Baroud M., Dandache I., Araj G. F., Wakim R., Kanj S., Kanafani Z., et al. (2013). Underlying mechanisms of carbapenem resistance in extended-spectrum β-lactamase-producing Klebsiella pneumoniae and Escherichia coli isolates at a tertiary care centre in Lebanon: role of OXA-48 and NDM-1 carbapenemases. Int. J. Antimicrob Agents. 41, 75–79. doi: 10.1016/j.ijantimicag.2012.08.010
Beard C. W., Hopkins S. R., Hammond J. (1975). Preparation of Newcastle disease virus hemagglutination-inhibition test antigen. Avian Dis. 19, 692–699. doi: 10.2307/1589182
Bello A., Dersjant-Li Y., van Eerden E., Kwakernaak C., Marchal L. (2022). Supplementation of an all-plant-based inorganic phosphate-free diet with a novel phytase maintained tibia ash and performance in broilers under a commercial production setting. J. Appl. Poult. Res. 31, 100253. doi: 10.1016/j.japr.2022.100253
Brown C. L., Mullet J., Hindi F., Stoll J. E., Gupta S., Choi M., et al. (2022). mobileOG-db: a manually curated database of protein families mediating the life cycle of bacterial mobile genetic elements. Appl. Environ. Microbiol. 88, e00991–e00922. doi: 10.1128/aem.00991-22
Cardinal K. M., Kipper M., Andretta I., MaChado L. R. A. (2019). Withdrawal of antibiotic growth promoters from broiler diets: performance indexes and economic impact. Poult Sci. 98, 6659–6667. doi: 10.3382/ps/pez536
Casewell M., Friis C., Marco E., McMullin P., Phillips I. (2003). The European ban on growth-promoting antibiotics and emerging consequences for human and animal health. J. Antimicrob. Chemother. 52, 159–161. doi: 10.1093/jac/dkg313
Chen S., Zhou Y., Chen Y., Gu J. (2018). fastp: an ultra-fast all-in-one FASTQ preprocessor. Bioinform. 34, i884–i890. doi: 10.1093/bioinformatics/bty560
Choi J. H., Lee K., Kim D. W., Kil D. Y., Kim G. B., Cha C. J. (2018). Influence of dietary avilamycin on ileal and cecal microbiota in broiler chickens. Poult. Sci. 97, 970–979. doi: 10.3382/ps/pex360
Cowieson A. J., Aureli R., Guggenbuhl P., Fru-Nji F. (2015). Possible involvement of myo-inositol in the physiological response of broilers to high doses of microbial phytase. Anim. Prod. Sci. 55, 710–719. doi: 10.1071/AN14044
Crisol-Martinez E., Stanley D., Geier M. S., Hughes R. J., Moore R. J. (2017). Understanding the mechanisms of zinc bacitracin and avilamycin on animal production: Linking gut microbiota and growth performance in chickens. Appl. Microbiol. Biotechnol. 101, 4547–4559. doi: 10.1007/s00253-017-8193-9
Dai D., Qiu K., Zhang H., Wu S., Han Y., Wu Y., et al. (2021). Organic acids as alternatives for antibiotic growth promoters alter the intestinal structure and microbiota and improve the growth performance in broilers. Front. Microbiol. 11, 114. doi: 10.3389/fmicb.2020.618144
Daillere R., Vétizou M., Waldschmitt N., Yamazaki T., Isnard C., Poirier-Colame V., et al. (2016). Enterococcus hirae and Barnesiella intestinihominis Facilitate Cyclophosphamide-Induced Therapeutic Immunomodulatory Effects. Immunity. 45, 931–943. doi: 10.1016/j.immuni.2016.09.009
Danecek P., Bonfield J. K., Liddle J., Marshall J., Ohan V., Pollard M. O., et al. (2021). Twelve years of SAMtools and BCFtools. Gigascience 10, giab008. doi: 10.1093/gigascience/giab008
David B., Mejdell C., Michel V., Lund V., Moe R. O. (2015). Air Quality in Alternative Housing Systems may have an Impact on Laying Hen Welfare. Part II-Ammonia. Animals. 5, 886–896. doi: 10.3390/ani5030389
Demetro C. G. B., Menten J. F. M., Leandro R. A., Brien C. (2013). Experimental power considerations-justifying replication for animal care and use committees. Poultry Sci. 92, 2490–2497.
Demir E., Sarica Ş., Özcan M. A., Suiçmez M. (2005). The use of natural feed additives as alternative to an antibiotic growth promoter in broiler diets. Arch.Geflügelk. 69, (3). S. 110–116. doi: 10.1016/S0003-9098(25)00580-6
Donohoe D. R., Garge N., Zhang X., Sun W., O’Connell T. M., Bunger M. K., et al. (2011). The microbiome and butyrate regulate energy metabolism and autophagy in the mammalian colon. Cell Metab. 13, 517–526. doi: 10.1016/j.cmet.2011.02.018
El-Shall N. A., Shewita R. S., Abd El-Hack M. E., AlKahtane A., Alarifi S., Alkahtani S., et al. (2020). Effect of essential oils on the immune response to some viral vaccines in broiler chickens, with special reference to Newcastle disease virus. Poult Sci. 99, 2944–2954. doi: 10.1016/j.psj.2020.03.008
Ennis C. E., Jackson M., Gutierrez O., Cantley S., Wamsley K. G. S. (2020). Phytase and carbohydrase inclusion strategies to explore synergy within low-energy diets to optimize 56-day male broiler performance and processing. J. Appl. Poult. Res. 29, 1045–1067. doi: 10.1016/j.japr.2020.09.013
Fernandes J. I. M., Horn D., Ronconi E. J., Buzim R., Lima F. K., Pazdiora D. A. (2019). Effect of phytase super dosing on digestibility and bone integrity of broilers. J. Appl. Poult. Res. 28, 390–398. doi: 10.3382/japr/pfz001
Gilijamse P. W., Hartstra A., Levin E., Wortelboer K., Serlie M. J., Ackermans M. T., et al. (2020). Treatment with Anaerobutyricum soehngenii: a pilot study of safety and dose–response effects on glucose metabolism in human subjects with metabolic syndrome. NPJ Biofilms Microbiomes 6, 16. doi: 10.1038/s41522-020-0127-0
Guarrasi J., Trask C., Kirychuk S. A. (2015). systematic review of occupational exposure to hydrogen sulfide in livestock operations. J. Agromedicine. 20, 225–236. doi: 10.1080/1059924X.2015.1009667
Hofmann T., Schmucker S. S., Bessei W., Grashorn M., Stefanski V. (2020). Impact of housing environment on the immune system in chickens: A review. Anim. (Basel). 10, 1138. doi: 10.3390/ani10071138
Holmstrom K., Collins M. D., Moller T., Falsen E., Lawson P. A. (2004). Subdoligranulum variabile gen. nov., sp. nov. from human feces. Anaerobe. 10, 197–203.
Hu X., Chi Q., Wang D., Chi X., Teng X., Li S. (2018). Hydrogen sulfide inhalation-induced immune damage is involved in oxidative stress, inflammation, apoptosis and the Th1/Th2 imbalance in broiler bursa of Fabricius. Ecotoxicol. Environ. Saf. 164, 201–209. doi: 10.1016/j.ecoenv.2018.08.029
Jian Z., Zeng L., Xu T., Sun S., Yan S., Yang L., et al. (2021). Antibiotic resistance genes in bacteria: occurrence, spread, and control. J. Basic Microbiol. 61, 1049–1070. doi: 10.1002/jobm.202100201
Langmead B., Salzberg S. (2012). Fast gapped-read alignment with Bowtie 2. Nat. Methods 9, 357–359. doi: 10.1038/nmeth.1923
La Rosa S. L., Leth M. L., Michalak L., Hansen M. E., Pudlo N. A., Glowacki R., et al. (2019). The human gut Firmicute Roseburia intestinalis is a primary degrader of dietary β-mannans. Nat. Commun. 10, 905. doi: 10.1038/s41467-019-08812-y
Laxminarayan R., Van Boeckel T., Teillant A. (2015). The economic costs of withdrawing antimicrobial growth promoters from the livestock sector in OECD food, agriculture and fisheries papers (OECD Publishing, Paris, France). doi: 10.1787/5js64kst5wvl-en
Lillehoj H., Liu Y. (2018). Calsamiglia, S. et al. Phytochemicals as antibiotic alternatives to promote growth and enhance host health. Vet Res. 49, 76. doi: 10.1186/s13567-018-0562-6
Liu S. D., Song M. H., Yun W., Lee J. H., Lee C. H., Kwak W. G., et al. (2018). Effects of oral administration of different dosages of carvacrol essential oils on intestinal barrier function in broilers. J. Anim. Physiol. Anim. Nutr. (Berl). 102, 1257–1265. doi: 10.1111/jpn.2018.102.issue-5
Menzel P., Ng K., Krogh A. (2016). Fast and sensitive taxonomic classification for metagenomics with Kaiju. Nat.Commun. 7, 11257. doi: 10.1038/ncomms11257
Metzler-Zebeli B. U., Klinsoda J., Vötterl J. C., Verhovsek D. (2020). Maturational changes alter effects of dietary phytase supplementation on the fecal microbiome in fattening pigs. Microorganisms 8, 1073. doi: 10.3390/microorganisms8071073
Morrison D. J., Preston T. (2016). Formation of short chain fatty acids by the gut microbiota and their impact on human metabolism. Gut Microbes. 7, 189–200. doi: 10.1080/19490976.2015.1134082
Paul S. S., Rao S. V. R., Chatterjee R. N., Raju M. V. L. N., Mahato A. K., Prakash B., et al. (2023). An immobilized form of a blend of essential oils improves the density of beneficial bacteria, in addition to suppressing pathogens in the gut and also improves the performance of chicken breeding. Microorganisms 11.1960doi: 10.3390/microorganisms11081960
Paul S. S., Rao S. V. R., Hegde N., Williams N. J., Chatterjee R. N., Raju M. V. L. N., et al. (2022). Effects of dietary antimicrobial growth promoters on performance parameters and abundance and diversity of broiler chicken gut microbiome and selection of antibiotic resistance genes. Front. Microbiol. 13, 905050. doi: 10.3389/fmicb.2022.905050
Persoons D., Dewulf J., Smet A., Herman L., Heyndrickx M., Martel A., et al. (2012). Antimicrobial use in Belgian broiler production. Prev. Vet. Med. 105, 320–325. doi: 10.1016/j.prevetmed.2012.02.020
Proctor A., Phillips G. J. (2019). Differential effects of bacitracin methylene disalicylate (BMD) on the distal colon and cecal microbiota of young broiler chickens. Front. Vet. Sci. 6. doi: 10.3389/fvets.2019.00114
Pulingam T., Parumasivam T., Gazzali A. M., Sulaiman A. M., Chee J. Y., Lakshmanan M., et al. (2022). Antimicrobial resistance: Prevalence, economic burden, mechanisms of resistance and strategies to overcome. Eur. J. Pharm. Sci. 170, n106103doi: 10.1016/j.ejps.2021.106103
Qiu K., Li C. L., Wang J., Qi G. H., Gao J., Zhang H. J., et al. (2021). Effects of dietary supplementation with bacillus subtilis, as an alternative to antibiotics, on growth performance, serum immunity, and intestinal health in broiler chickens. Front. Nutr. 8. doi: 10.3389/fnut.2021.786878
R Core Team (2020). R: A language and environment for statistical computing (Vienna, Austria: R Foundation for Statistical Computing). Available at: https://www.R-project.org/ (Accessed January 12, 2024).
Reyes-Jurado F., López-Malo A., Palou E. (2016). Antimicrobial activity of individual and combined essential oils against foodborne pathogenic bacteria. J. Food Prot. 79, 309–315. doi: 10.4315/0362-028X.JFP-15-392
Rowe W. P. M., Winn M. D. (2018). Indexed variation graphs for efficient and accurate resistome profiling. Bioinform. 34, 3601–3608. doi: 10.1093/bioinformatics/bty387
Sikandar A., Zaneb H., Younus M., Masood S., Aslam A., Khattak F., et al. (2017). Effect of sodium butyrate on performance, immune status, microarchitecture of small intestinal mucosa and lymphoid organs in broiler chickens. Asian-Australas. J. Anim. Sci. 30, 690–699. doi: 10.5713/ajas.16.0824
Smits J. E., Bortolotti G. R., Tella J. L. (1999). Simplifying the phytohaemagglutinin skin –testing technique in studies of avian immunocompetence. Funct. Ecol. 13, 567–572. doi: 10.1046/j.1365-2435.1999.00338.x
SPSS (2008). SPSS inc. Released SPSS statistics for windows, version 17.0 (Chicago, IL, USA: SPSS Inc).
Tello A., Austin B., Telfer T. C. (2012). Selective pressure of antibiotic pollution on bacteria of importance to public health. Environ. Health Perspect. 120, 1100–1106. doi: 10.1289/ehp.1104650
Ubeda C., Bucci V., Caballero S., Djukovic A., Toussaint N. C., Equinda M., et al. (2013). Intestinal microbiota containing Barnesiella species cures vancomycin-resistant Enterococcus faecium colonization. Infect. Immun. 81, 965–973. doi: 10.1128/IAI.01197-12
Van Hul M., Le Roy T., Prifti E., Dao M. C., Paquot A., Zucker J. D., et al. (2020). From correlation to causality: the case of Subdoligranulum. Gut Microbes. 12, 1–13.
Wang J., Ishfaq M., Miao Y., Liu Z., Hao M., Wang C., et al. (2022). Dietary administration of Bacillus subtilis KC1 improves growth performance, immune response, heat stress tolerance, and disease resistance of broiler chickens. Poult. Sci. 101, 101693. doi: 10.1016/j.psj.2021.101693
Wang Y. M., Meng Q. P., Guo Y. M., Wang Y. Z., Wang Z., Yao Z. L., et al. (2010). Effect of atmospheric ammonia on growth performance and immunological response of broiler chickens. J. Anim. Vet. Adv. 9, 2802–2806. doi: 10.3923/javaa.2010.2802.2806
Yin D., Du E., Yuan J., Gao J., Wang Y. L., Aggrey S. E., et al. (2017). Supplemental thymol and carvacrol increases ileum Lactobacillus population and reduces effect of necrotic enteritis caused by Clostridium perfringes in chickens. Sci. Rep. 7, 1–11. doi: 10.1038/s41598-017-07420-4
Yu Z., Morrison M. (2004). Improve extraction of PCR-quality community DNA from digesta and facaes samples. Biotecniques 36, 808–812. doi: 10.2144/04365ST04
Keywords: phytase super dosing, gut microbiome, antimicrobial resistance, antibiotic growth promoter, broiler chicken
Citation: Paul SS, Rao SVR, Chatterjee RN, Raju MVLN, Prakash B, Yadav SP, Kannan A, Reddy GN, Kumar V and Kumar PSP (2025) Evaluation of phytase super dosing and its combinations with other additives as an alternative to antibiotic growth promoters in broiler chickens reared in a deep litter system. Front. Anim. Sci. 6:1539543. doi: 10.3389/fanim.2025.1539543
Received: 04 December 2024; Accepted: 07 April 2025;
Published: 02 May 2025.
Edited by:
Avishek Biswas, Central Institute for Research on Cattle, IndiaReviewed by:
Ravikanthreddy Poonooru, University of Missouri, United StatesRangsun Charoensook, Naresuan University, Thailand
Copyright © 2025 Paul, Rao, Chatterjee, Raju, Prakash, Yadav, Kannan, Reddy, Kumar and Kumar. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shyam Sundar Paul, c3NwYXVsY2lyYkBnbWFpbC5jb20=
 Shyam Sundar Paul
Shyam Sundar Paul Savaram Venkata Rama Rao
Savaram Venkata Rama Rao Rudra Nath Chatterjee
Rudra Nath Chatterjee Mantena Venkata Lakshmi Narasimha Raju
Mantena Venkata Lakshmi Narasimha Raju Godumagadda Narender Reddy
Godumagadda Narender Reddy Vikas Kumar
Vikas Kumar Prakki Santosh Phani Kumar
Prakki Santosh Phani Kumar