Abstract
Pigs are social, hierarchical animals. Frequent mixing and regrouping of unfamiliar animals are common production practices that result in disruption and re-establishment of hierarchies. Little research has focused on the emotional component of this experience. Reward downshift paradigms have been investigated as a promising method for evaluating the affective state of animals. Therefore, we sought to evaluate responses to reward downshift as a method for evaluating the affective states of high vs. low-ranking sows. Pigs of either high (n = 8) or low (n = 9) social hierarchy (based on feed order) were enrolled. Pigs were initially trained to approach and consume a sweet sports drink (Gatorade, 5.8% sugar). The reward was then downshifted to a 1:10 diluted solution (0.58% sugar), and the approach latency, volume consumed, and peak force applied to the reward container were measured for each subject over a 3 min test for four consecutive days. Pigs of high social standing reacted to the downshift by decreasing their consumption both initially and over subsequent test days as well as slowing their approach to the reward over time. Low ranking sows also reduced their immediate consumption but did not show changes over the subsequent test days following the downshift. The reward appears to be valuable to low-rank animals regardless of its quality, potentially indicating lower reward expectations, value in the non-competitive access to a resource, or the stress-buffering action of the reward, possibly reflective of a relative negative affective state. Our findings suggest that reward valuation can be promising tool for the assessment of an animal’s affective states, but further research will be needed to fully understand its utility.
Introduction
Pigs are social animals that establish a strong hierarchy from birth (Scipioni et al., 2009). Common farm practices such as the frequent mixing of animals in a restricted space requires re-establishment of hierarchies (Verdon et al., 2015) and can lead to aggression, injury, increased stress levels and decreased reproductive performance. In group housed sows, hierarchies often revolve around access to limited valuable resources such as feed, preferential lying areas (Turner and Edwards, 2004), and enrichment (Elmore et al., 2011) where low-ranking individuals generally have more difficulty accessing these resources (O’Connell et al., 2003; Elmore et al., 2011). Higher ranking sows typically have higher feed consumption, more frequent visits to feeders (Ochoteco-Asensio et al., 2024), higher weight gains (Verdon et al., 2016), and enhanced immune response and disease resistance than their low-ranking counterparts (Hessing et al., 1994; Tuchscherer et al., 1998; da Fonseca de Oliveira et al., 2023). In comparison, low-ranking individuals sustain more injuries and are more fearful of novelty (O’Connell et al., 2004).
Research on the link between social hierarchy and welfare has focused largely on physiological and resource-based indicators such as stress hormone concentrations and feed access. However, these measures may not fully reflect the welfare impact of social rank. For example, while cortisol and catecholamine concentrations generally increase in times of social stress, this response occurs independently of hierarchy status (de Groot et al., 2001), suggesting these indicators do not differentiate the experience of subordinate and dominant individuals. Other studies have found no impact of social hierarchy on cortisol levels or growth performance (O’Connell et al., 2003; da Fonseca de Oliveira et al., 2023), although differences in feeder visits were observed (da Fonseca de Oliveira et al., 2023).
While physiological and production measures provide valuable information, they do not convey the affective states of the animals (i.e. the emotional component of their experience), which is a key component of their welfare (Fraser, 2008). We propose integrating measures of affect in the study of social hierarchy to complement our understanding of its effects on welfare.
Given the limitations of physiological measures, behavioral paradigms such as successive negative contrast (SNC) provide an alternative approach to assess affective states in animals. SNC is a behavioral disruption that occurs as a result of a sudden downshift in the quantity or quality of an anticipated reward (Papini, 2006). Initially, SNC research focused predominantly on neurological fundamentals in laboratory rodents (Flaherty et al., 1986; Mitchell and Flaherty, 1998; Mitchell and Flaherty, 2005). Because SNC responses have been found to vary depending on the underlying affective state of an animal, SNC tests have been developed as a promising method for evaluating welfare (Burman et al., 2008; Ellis et al., 2020). In general, behavioral and physiological responses to SNC tests indicate that reward downshift leads to increased frustration and aggression (Papini and Dudley, 1997; Dzik et al., 2024). For example, facial expression in dogs were found to differ depending on whether they were in a context of anticipation of a high-value food reward or denied access to a reward that led to frustration (Bremhorst et al., 2019), and lambs displayed increased heart rates and performed an operant task more frequently after the reward was downshifted (Greiveldinger et al., 2011). Previous research investigating frustration states in pigs has been conducted (Dantzer et al., 1980; Dantzer et al., 1987; Lewis, 1999). However, to our knowledge, frustration responses to SNC paradigms have been applied in pigs to assess their welfare only once (Luo et al., 2020) and have never been investigated in the context of social hierarchy.
The objective of this study was therefore to investigate responses to a reward downshift as a method for evaluating the affective state of high vs. low ranking sows. Successive negative contrast paradigms typically investigate the appetitive and consummatory phases of reward acquisition as measured through approach latency (appetitive phase) and amount of reward consumed (consummatory phase). However, given that aggression towards conspecifics in pigs (Arnone and Dantzer, 1980; Dantzer et al., 1980) and self-reported frustration and aggression towards a button box in humans (Yu et al., 2014) have been documented as a result of reward extinction, we sought to integrate an additional component investigating the force applied to the reward container (Ede et al., 2023). We hypothesized that low-ranking sows would exhibit a stronger response to a reward downshift compared to higher ranking sows (i.e. higher approach latency, lower reward consumption and higher force applied), indicating a more negative affective state.
Methods
All experimental animal procedures were approved by the University of Pennsylvania’s Institutional Animal Care and Use Committee (Protocol #804656). This study was conducted at the University of Pennsylvania Swine Teaching and Research Center in Kennett Square, Pennsylvania, USA between October 2023 and April 2024.
Animals
All experimental animals were housed in a 359 m2 group pen with approximately 115 individuals (including non-experimental animals). The barn temperature was set at 17 °C and was continuously ventilated with exhaust fans. The group pen featured two straw pits (8.5 x 4.3 m) and several chew toys provided for enrichment (Green Natural Rubber Luna 142, EasyFix, Galway, Ireland). Herd health was monitored daily by farm staff to ensure sufficient feed consumption and that no sows were injured or lame. Thirty female Norsvin/Topigs were initially enrolled and divided in two groups: High and Low hierarchy (n = 15 per group). Based on (Mitchell et al., 2012) we aimed for a minimum of 8 subjects per group. We used feed order as a proxy for social hierarchy as a strong relationship was previously found between feed order and various measures of social rank (O’Connell et al., 2003; Lanthony et al., 2022). Two Electronic Sow Feeders (Schauer Compident Electronic Sow Feeding system, Prambachkirchen, Austria) were installed in the group pen, and recorded the order in which animals entered the feed system every day (average daily number of sows feeding on a feeder = 65.3 ± 3.4). Three experimental replicates of 10 sows each were carried out. For each replicate, social hierarchy was determined based on the average feed order over the seven days preceding enrollment. The five sows with the highest feed order averages were classified as high hierarchy, while the five with the lowest feed order averages were classified as low hierarchy (average ± SD feed order: high = 4.8 ± 1.5, low = 58.7 ± 3.8). To avoid late gestation sows from dropping out of the 2 weeks-long trial due to giving birth, an animal’s expected farrowing date had to be at least 3 weeks later than the start of the trial for inclusion. Thirteen sows were excluded from the study for the following reasons: ten did not fulfill the approach criteria (see following section), two were lame and one had a high-stress reaction out of the home pen. The final number of subjects included in analyses was 8 high-ranking and 9 low-ranking pigs.
Protocol
The trial took place over 2 weeks (with two days of rest) and was divided in two main phases: high value reward (Frost Glacier Freeze Thirst Quencher Sports Drink [5.8% sugar], Gatorade, Chicago, USA, based on (Neary et al., 2024)), and lower value reward (1:10 diluted to 0.58% sugar, based on (Figueroa et al., 2015)). We opted for a downshift in reward quality rather than quantity for two reasons: (i) to allow ad libitum consumption during tests, hence avoiding a ceiling effect, and (ii) reward quality was suggested as more motivating than reward quantity in dogs (Riemer et al., 2018). Protocol summary is presented in Table 1.
Table 1
| Day | Session | Reward | Test location | Test duration |
|---|---|---|---|---|
| 1 | Habituation 1 | High (Gatorade) | Home pen | ~1 min. |
| 2 | Habituation 2 | High (Gatorade) | Experimental arena | 10 min. |
| 3 | Pre-shift 1 | High (Gatorade) | Experimental arena | 3 min. |
| 4 | Pre-shift 2 | High (Gatorade) | Experimental arena | 3 min. |
| 5 | Pre-shift 3 | High (Gatorade) | Experimental arena | 3 min. |
| 8 | Pre-shift 4 | High (Gatorade) | Experimental arena | 3 min. |
| 9 | Post-shift 1 | Low (1:10 dilution) | Experimental arena | 3 min. |
| 10 | Post-shift 2 | Low (1:10 dilution) | Experimental arena | 3 min. |
| 11 | Post-shift 3 | Low (1:10 dilution) | Experimental arena | 3 min. |
| 12 | Post-shift 4 | Low (1:10 dilution) | Experimental arena | 3 min. |
Summary for the reward downshift protocol.
Pigs were trained to approach a high value reward (Gatorade), which was reduced in quality by diluting it by a factor of 10 after 4 pre-shift sessions.
On the first day (Habituation 1), pigs were initially exposed to the reward to avoid potential effects of food neophobia. Experimenters entered the home pen carrying a waterer (Weaver Livestock, Ohio, USA) containing the high value reward, and gently encouraged experimental animals to sample the solution by bringing the waterer to their snouts. On the second day (Habituation 2), pigs were led individually to the experimental apparatus for 10 min. The apparatus was a 5.2 x 2.1 m arena with the waterer containing the ad libitum reward mounted on a gate opposite to the entrance (Figure 1). It was in the same building as the group pen, allowing acoustic and olfactory (but not visual) contact with animals from the home pen during testing sessions.
Figure 1
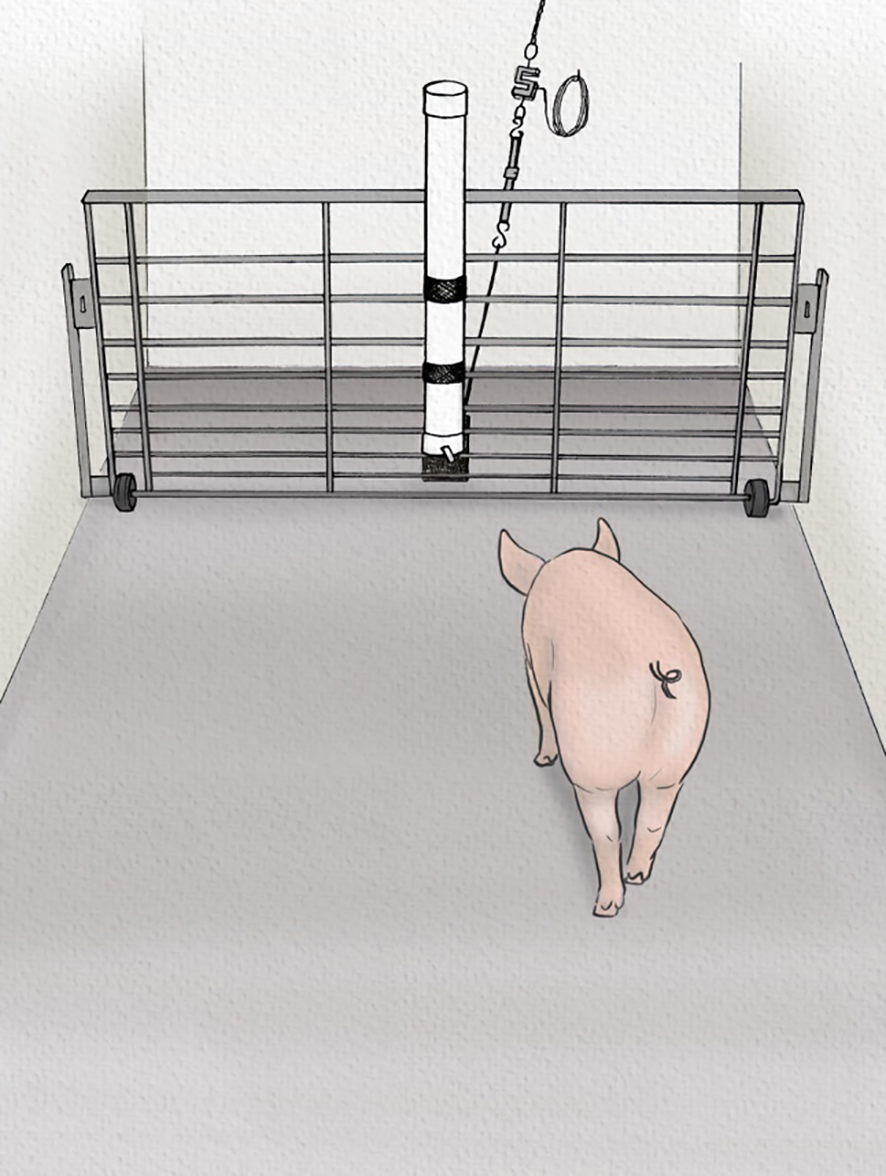
Sows were trained in an arena to approach a waterer containing a high reward (sports drink), which was then switched to a low reward (1:10 diluted sports drink). The waterer was attached to a load cell measuring the amount of force applied. Illustration by Ann Sanderson (independent illustrator).
After these two habituations, pigs were trained to approach the high value reward in 4 sessions (Pre-shifts 1-4). During Pre-shifts, animals were led individually and left in the experimental pen for 3 min (based on (Luo et al., 2020), increased to 3 min because of the larger reward). A line of grain (~2 m) was sprinkled in front of the waterer to encourage its exploration. To avoid enrolling animals excessively impacted by social isolation or not interested enough by the reward, pigs who had not spontaneously (i.e. without any help) approached the waterer by the fourth Pre-shift were excluded from the trial.
In sessions following Pre-shift 4 (Post-shift 1-4), the reward in the waterer was down-shifted to a 1:10 dilution of the initial reward. After Post-shift 4, animals were weighed (all: 246.5 ± 46.6 kg, high-rank: 280.5 ± 42.7 kg, low-rank: 216.5 ± 23.1 kg) and re-integrated to routine farm management.
Measurements
All measurements were collected in the experimental apparatus described above which contained a watering device consisting of a standard pig water nipple mounted on a liquid reservoir (Figure 1). The measurements included the following assessments.
Latency to approach reward
Latency to approach reward was measured as the time elapsed from the moment the animal entered the experimental apparatus until it made mouth or tongue contact with the waterer's nipple. If a subject did not approach the waterer within 3 min, latency was recorded as 181 s (3 occurrences).
Consumption of reward
Consumption of reward captured the volume drank by the sow over the 3 min test period and was measured as the difference between the initial reservoir volume at the start of the trial minus the ending volume.
Force applied to reward container
Force applied to reward container was measured via a load cell (S-beam, LC103B-2K, Omega, Norwalk, USA) attached to the waterer by a rope and ratchet strap to collect tension and compression applied by the sow to the waterer. Data was recorded at a 2 Hz rate on a data logger (OM-CP-BRIDGE-101A, Omega) and extracted via the OM-CP Data Logger Software (v4.2.25.7). To account for the variation in baseline tension on the waterer for each session, raw force data from the load cell for each animal and session was converted to net force by subtracting the median force of the 60 seconds before they entered the arena. The peak net force recorded was then extracted for each animal and session.
Statistical analysis
Data was analyzed in two phases. First, we examined the initial response of high and low hierarchy animals to the downshift, and second we analyzed the sows’ extended responses to the downshift across Post-shift sessions 1 through 4.
Latency to approach reward
Latency to approach the reward was converted into a binary variable classifying sows’ response as either “Fast approach” (< 33.4s) or “No fast approach” (> 33.4s). The 1.5 interquartile range method (Tukey, 1977) was used to define this threshold due to poor model residuals when using raw latency data (see Supplementary Figure 1 for more information). For the initial response, a chi-square test was used to compare the number of “Fast approaches” between Post-session 1 and Post-session 2 since latency during Post1 still reflected the expectation of a high reward, as they had not yet sampled the downshifted reward. For the extended response, latency data was analyzed using a binomial linear mixed model (Bates et al., 2015; R Core Team, 2021). The model employed “Fast approach” (yes/no) as the outcome variable, the interaction between hierarchy and session as a fixed effect, and animal ID as a random effect. Results are presented as odds-ratio.
Consumption of the reward and force applied to reward container
Consumption and peak force were analyzed using paired t-tests to compare outcome values during Post-session 1 vs. baseline (defined below). For the extended responses to the downshift, consumption and peak force data were analyzed using linear mixed models taking respective baselines and the interaction between session and hierarchy as fixed effects and animal ID as a random effect. All model residuals were examined visually. Consumption data was square root transformed, and peak force data was log transformed to better fit normality and homoscedasticity assumptions. P-values for mixed models were obtained with the lmerTest package (Bates et al., 2015). Results are presented with 95% Confidence intervals (CI), and significance threshold was set at P = 0.05. Appropriate baselines for reward consumption and peak force applied were determined comparing the fitness of models using different values for baseline (i.e. last pre-shift session only, average of last two pre-shift sessions, average of last three or average of all pre-shift sessions). Pooling of baseline values were conducted as no difference between pre-shift sessions were found (see Supplementary Figure 2, Table 1). The mean of all pre-shift sessions was used as baseline for reward consumption, and the mean of the last three pre-shift sessions was used for peak force. These choices improved model fit, but did not influence the significance of fixed effects (see Supplementary Table 2 for details).
Results
Sows from high and low social rank did not differ in their approach to the reward (estimate difference = -0.01, z = 1.9e-31, CI = [-0.34, 0.31], P = 1), their consumption of reward (estimate difference = -1.0e-5, t = -9.9e-5, CI = [-0.22, 0.22], P = 0.99), or force they applied on the reward container (estimate difference = 8.7e-3, t = 0.04, CI = [-0.46, 0.47], P = 0.97) under baseline conditions when exposed to the high quality reward. However, following the downshift to the lower reward solution, individual sow responses were variable but differences emerged between high and low hierarchy animals (Figure 2) in both their initial and extended responses to the diluted reward (Table 2).
Figure 2
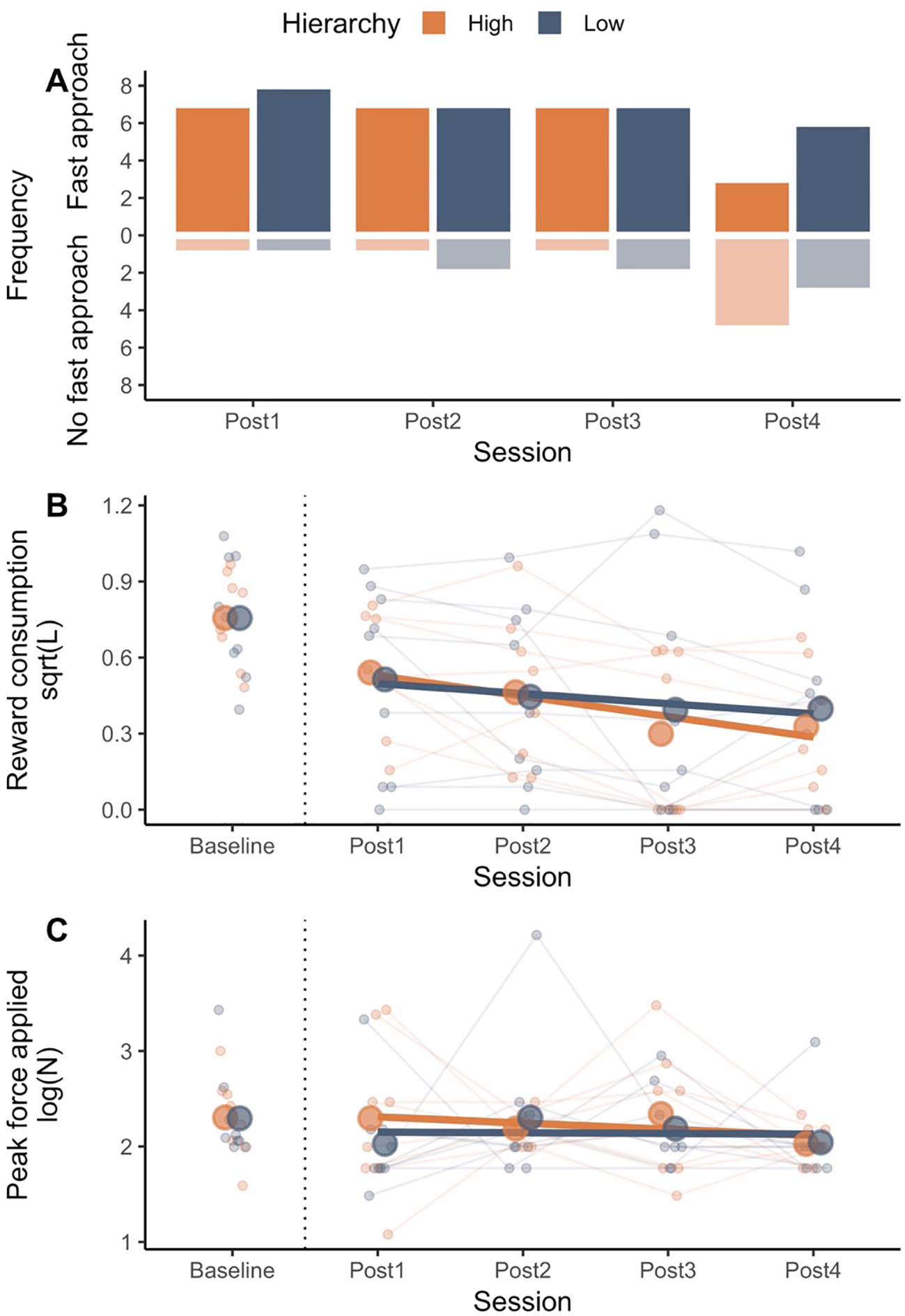
Sows of high and low social hierarchy (estimated by feed ranks) were offered a high reward (sports drink) during baseline. The reward was then downshifted (1:10 diluted sports drink), and offered over 4 test days (Sessions Post1 to Post4). Approach to the reward [converted to binary outcome, panel (A)], amount of reward consumed [square root transformed, panel (B)] and peak force applied to the container [log-transformed, (C)] are presented. Approach latency during Post1 was used as the approach baseline as animals were still expecting a high reward during the first session after the downshift.
Table 2
| Hierarchy | Initial response | Extended response | ||
|---|---|---|---|---|
| High | Low | High | Low | |
| Fast approach | X2 = 0 P = 1 | X2 = 0.07 P = 0.80 | [0.05, 0.97] P = 0.046 | [0.28, 1.14] P = 0.09 |
| Consumption | [-0.33, -0.10] P = 0.004 | [-0.46, -0.03] P = 0.033 | [-0.13, -0.03] P = 0.002 | [-0.09, 0.004] P = 0.08 |
| Peak force | [-0.70, 0.69] P = 0.98 | [-0.56, 0.03] P = 0.07 | [-0.16, 0.10] P = 0.67 | [-0.17, 0.09] P = 0.56 |
Detailed results of the responses of pigs (High vs. Low social hierarchy) to a downshift in reward quality (sports drink to 1:10 diluted sports drink).
Initial responses (baseline vs. first post-shift session) and extended responses (evolution over the post-shift sessions) are presented. Numbers in brackets are 95% confidence intervals (Odds ratios for fast approach, square root transformed for consumption, log-transformed for peak force); bolded values are significant p-values (<0.05).
Latency to approach reward
No differences in the frequency of fast approach by high or low hierarchy animals was observed between the first and second session following the downshift (P > 0.05, Table 2). With prolonged exposure to the low-reward solution, only high-ranking sows showed a significant decrease in fast approaches across sessions (OR estimate = 0.21, t = -2.0, CI = [0.05, 0.97], P = 0.046, Figure 2) with a noted change in behavior during the final session.
Consumption of the reward
Sows of both high and low hierarchies displayed a decrease in consumption of the reward during their first exposure to the low reward solution compared to their baselines (High hierarchy: estimate = -0.21, t = -4.3, CI = [-0.33, -0.10], P = 0.004. Low hierarchy: estimate = -0.24, t = -2.6, CI = [-0.46, -0.03], P = 0.033, Table 2). Only high hierarchy animals continued to reduce significantly their consumption of the diluted reward throughout the remaining test sessions (estimate = -0.08, t = -3.2, CI = [-0.13, -0.03], P = 0.002, Figure 2).
Force applied to reward container
High or low hierarchy sows failed to show any differences in the peak force applied to the container for either their initial or extended responses to the reward downshift (Table 2; Figure 2).
Discussion
Following a downshift in reward quality, pigs of high social rank showed a decrease in their frequency of fast approach over days, as well as an initial and extended decline in reward consumption. Pigs of low social rank only displayed an initial decrease in consumption and maintained their extended anticipatory and consummatory responses after the downshift. These results are in opposition with our predictions, which hypothesized the low-rank animals to be in a negative state leading to an increased sensitivity to a reward downshift.
Apparent contradictory outcomes in reward motivation are surprising but not without precedent in the literature. A paradoxical effect was found in a study on rodent cage enrichment where rats housed in a barren environment were faster to approach a reward than rats housed in an enriched cage (Mitchell et al., 2012). Authors suggested that taking part in the experimental paradigm was itself an enrichment for the barren housed rats, which induced some level of positive affect. In the present study, all animals were housed in the same environment, so perhaps the opportunity for non-competitive access to a palatable reward was a positive event for pigs of low social status regardless of reward quality. This interpretation remains speculative as post-shift reward access did not appear to be reinforcing for low-rank individuals (i.e. no overall trends towards increased reward consumption and decreased approach latency were observed).
In another example, hens exposed to a negative stressor (social isolation) were faster to approach a reward than control hens (Hernandez et al., 2015). Authors noted that release from a negative condition could constitute a positive event: in our case, the temporary release from the social stress of the pen during experimental trials could have constituted enough of a positive event to explain the low ranking animals’ maintenance of approach and consumption of the low-quality reward. Alternatively, being in a negative affective state might increase the reward’s incentive value (Hernandez et al., 2015).
Interestingly, rodent studies on the relationship between stress and the incentive value of sucrose are in accordance with our results: a sweet solution was found to dampen the stress response to restraint (Ulrich-Lai et al., 2010), and rats exposed to a variety of negative events (overnight illumination, cage tilt, confinement, soiled cage…) displayed an increase in operant performance for a sucrose reward (Willner et al., 1998). In humans, depressive mood induced by music led to higher cravings for chocolate (Willner et al., 1998), and a positive association has been reported between sugar intake and depressive symptoms (Li et al., 2023). Although the directionality of the relationship between sugar consumption and depression in humans is still unclear, a reciprocal link has been suggested between obesity and depressive symptoms (Luppino et al., 2010). The idea that stress increased the reward’s value should be interpreted cautiously as we observed no difference in reward consumption during baseline. However, this absence of difference could be attributed to a ceiling effect caused by the use of the high-reward.
Authors have also noted diverging effects of negative states on reward sensitivity. Rats exposed to social defeat over 5 weeks displayed a decrease in preference for a sucrose solution (Rygula et al., 2005), akin to anhedonia (i.e. a loss of interest for pleasurable events). Conversely, animals in a negative state were suggested to display an increased interest in rewards as a way to compensate for their negative state with positive experiences (van der Harst and Spruijt, 2007), as illustrated by barren-housed rats showing increased anticipation to a sucrose reward in comparison to enriched animals (van der Harst et al., 2003). Perhaps our results highlight a similar mechanism where low-rank animals were more inclined to attempt to offset the negative experience of poor social status with the positive experience of a reward.
Another interpretation for the apparent increased sensitivity in reward downshift from the high-rank pigs is that dominant individuals were the ones experiencing more severe stress. This seems unlikely as most of the literature points to low-rank individuals being subjected to stressful conditions such as agonistic behaviors, compromised access to resources and worsened performance (O’Connell et al., 2003; Verdon et al., 2016). Nevertheless, dominant pigs have been found to be more disrupted by social stress in their immune function compared to subordinates (de Groot et al., 2001), and an elevated adrenocortical stress response has been reported in humans of high social position (Hellhammer et al., 1997). A review of human literature highlighted that the relationship between social rank and stress is influenced not only by an individual’s position in a hierarchy, but also highly impacted by the stability of their status and sense of control (Sherman and Mehta, 2020). To our knowledge, the relationship between these components have not been explored in pigs but are likely to be significant in studies of social stress.
Another way to interpret our results is through the lens of cost-benefit behavioral adjustments based on home-pen expectations. High-rank sows, accustomed to consistent and preferential access to high-value feed, likely had higher expectations of the reward. Their decreased interest in the low reward reflected a rational adjustment in response to their failed expectations. In contrast, low-rank animals maintained a prolonged interest in low-reward across the post-shift sessions likely associated with generalized lower expectations arising from challenges to accessing high-value feed in the home pen. As a result, low-rank individuals prioritized securing any available resource regardless of quality, given their experience of competition from dominant individuals.
An alternative (or complementary) explanation could be that low-rank animals failed to update their expectations in their extended response. Such deficit in updating processes (‘Bayesian blindness’) has been linked to psychological disorders in humans and proposed as a potential marker of poor welfare in animals (Lecorps and Weary, 2024). Unfortunately, this area remains under-researched and is unlikely to be the sole mechanism as low-rank individuals did not show a failure to update their expectations in their initial response. Immediately after the downshift, low-rank sows reduced their reward consumption (like high-rank) without delaying their approach latency.
These findings align with previous work suggesting the dissociation of anticipatory (i.e., ‘wanting’) and consummatory (i.e., ‘liking’) responses to reward devaluation. For example, rats experiencing a reduction in reward quality (32% to 4% sucrose solution) showed a lower reward consumption than unshifted individuals, but no reliable differences in approach to the reward were found. Additionally, the authors noted a general lack of correlation between anticipatory and consummatory measures (Flaherty and Caprio, 1976).
Overall our findings support the broader idea that the response to a reward is shaped by distinct psychological components of anticipation and consumption (Berridge et al., 2009). However, the implications of this distinction for welfare research remain largely unexplored.
In addition to the common anticipatory and consummatory responses measured in reward downshift paradigms, we attempted to integrate a complementary outcome reflecting the vigor of the response. We had hypothesized that pigs would react in an aggressive way towards the reward container after the downshift, but we did not observe this effect as the peak force applied on the container was similar before and after the downshift. Our prediction was based on previous results noting that pigs became aggressive towards an unfamiliar conspecific when subjected to a reward extinction (Arnone and Dantzer, 1980). Interestingly, this aggression was not observed if the conspecific was familiar. Perhaps pigs confronted with a frustrating situation are more likely to direct their aggression towards an unfamiliar element of their environment (conspecific or other) which they identify as the cause of frustration. This could explain our negative force results as no novel element was introduced during the downshift. Alternatively, perhaps our paradigm did not induce an emotional frustration response entailing an aggressive component, but rather reflected a behavioral adjustment to reward devaluation. Perhaps more simply, our novel contraption to measure force did not have the sensitivity to detect a treatment effect for our low sample size.
Other limits to our paradigm are important to note. First, one-third of our subjects were excluded for not approaching the reward during the pre-shift phase; this selection bias implies our results are not representative of pigs who were either not interested by the reward, or too disturbed by the social isolation associated with the test. However, this selection was deliberate: if pigs did not approach the reward after 2 habituations and 4 pre-tests, it was a strong indication that they did not want to participate. Instead of prolonging the training phase to presumably increase enrollment, we opted to focus our experimental design on animal agency (Englund and Cronin, 2023) over participation. A potential refinement of our training paradigm could be preliminary preference tests to identify valued rewards for each subject. Additionally, as previously noted in calves, pigs’ temperament could be taken into account in their training to reduce drop-outs (Webb et al., 2015).
The second limit was the timing of the test in relation to mixing. We waited at least one week for animals to be in the group before enrolling them. This allowed us to test individuals with a relatively stable hierarchical order, but arguably did not reflect the most stressful period of hierarchy establishment: aggression, injuries and cortisol levels have been reported to be at their peak within 1 or 2 days after mixing, but to subside in the following days (Meese and Ewbank, 1973; Tuchscherer et al., 1998; Hemsworth et al., 2013; Verdon et al., 2016).
One final point is the link between parity, bodyweight and social rank. As expected, low-rank animals were lighter and lower parity than high-rank ones (Supplementary Table 3); and bodyweight has been correlated and used as a predictor of social status (Bus et al., 2021; Lanthony et al., 2022). Based on (Lanthony et al., 2022), we chose to focus solely on feed order as a proxy measure of hierarchical status, however, balancing for parity and bodyweight within hierarchical groups - although challenging - could minimize its potential effect on results.
From a practical perspective, our findings that low-rank individuals maintain their approach and consumption of low-quality rewards are of note for both research and routine care. In behavioral studies using rewards, the influence of social rank should be considered in experimental design and statistical modelling. In farm settings, access to high-fiber, lower-palatability feedstuffs such as straw has the potential to increase welfare, particularly of the low-ranking sows. Dominant sows might see value in this alternative feed, perhaps because it helps with satiety, hence reducing competition for primary feed access. Alternatively, if not valued by the dominant sows, this research suggests that lower-ranking sows might still find it rewarding. Where competition for feed access can be detrimental for health and welfare, providing additional sources of feed could help ensure proper nutrition for low-rank individuals with limited competition.
Conclusion
Pigs of high social rank displayed initial and extended responses to a reward downshift, whereas pigs of low social standing maintained their approach and consumption of the reward after its decrease in quality. We suggest low-rank pigs valued the reward regardless of its quality, potentially reflecting negative affect. We invite further investigation into the impact of social hierarchies on affective states, and note the importance of rank stability and animals’ perceived agency in future research on social stress.
Statements
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Ethics statement
The animal study was approved by University of Pennsylvania’s Institutional Animal Care and Use Committee. The study was conducted in accordance with the local legislation and institutional requirements.
Author contributions
TE: Conceptualization, Investigation, Writing – original draft, Writing – review & editing. SI: Conceptualization, Investigation, Writing – original draft, Writing – review & editing. TP: Conceptualization, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by the Marie A. Moore Fund for Welfare Research.
Acknowledgments
We would like to thank the Swine Teaching and Research Center farm staff Lori Stone, Jillian Mosley and Hanna Lee for their routine care of the animals during the study. We also thank Natchanon Dumniem for his help.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fanim.2025.1570586/full#supplementary-material
References
1
ArnoneM.DantzerR. (1980). Does frustration induce aggression in pigs? Appl. Anim. Ethology6, 351–362. doi: 10.1016/0304-3762(80)90135-2
2
BatesD.MächlerM.BolkerB.WalkerS. (2015). Fitting linear mixed-effects models using lme4. J. Stat. Soft67, 1–48. doi: 10.18637/jss.v067.i01
3
BerridgeK. C.RobinsonT. E.AldridgeJ. W. (2009). Dissecting components of reward: ‘liking’, ‘wanting’, and learning. Curr. Opin. Pharmacol.9, 65–73. doi: 10.1016/j.coph.2008.12.014
4
BremhorstA.SutterN. A.WürbelH.MillsD. S.RiemerS. (2019). Differences in facial expressions during positive anticipation and frustration in dogs awaiting a reward. Sci. Rep.9, 19312. doi: 10.1038/s41598-019-55714-6
5
BurmanO. H. P.ParkerR. M. A.PaulE. S.MendlM. (2008). Sensitivity to reward loss as an indicator of animal emotion and welfare. Biol. Lett.4, 330–333. doi: 10.1098/rsbl.2008.0113
6
BusJ. D.BoumansI. J. M. M.WebbL. E.BokkersE. A. M. (2021). The potential of feeding patterns to assess generic welfare in growing-finishing pigs. Appl. Anim. Behav. Sci.241, 105383. doi: 10.1016/j.applanim.2021.105383
7
da Fonseca de OliveiraA. C.WebberS. H.Ramayo-CaldasY.DalmauA.CostaL. B. (2023). Hierarchy establishment in growing finishing pigs: impacts on behavior, growth performance, and physiological parameters. Animals13, 292. doi: 10.3390/ani13020292
8
DantzerR.ArnoneM.MormedeP. (1980). Effects of frustration on behaviour and plasma corticosteroid levels in pigs. Physiol. Behav.24, 1–4. doi: 10.1016/0031-9384(80)90005-0
9
DantzerR.GonyouH. W.CurtisS. E.KelleyK. W. (1987). Changes in serum cortisol reveal functional differences in frustration-induced chain chewing in pigs. Physiol. Behav.39, 775–777. doi: 10.1016/0031-9384(87)90265-4
10
de GrootJ.RuisM. A. W.ScholtenJ. W.KoolhaasJ. M.BoersmaW. J. A. (2001). Long-term effects of social stress on antiviral immunity in pigs. Physiol. Behav.73, 145–158. doi: 10.1016/S0031-9384(01)00472-3
11
DzikM. V.CarballoF.CavalliC.IglesiasM.FaragóT.KubinyiE.et al. (2024). What if the reward is not as yummy? Study of the effects of successive negative contrast in domestic dogs in two different tasks. J. Veterinary Behav.72, 18–27. doi: 10.1016/j.jveb.2023.12.009
12
EdeT.von KeyserlingkM. A. G.WearyD. M. (2023). Exploring the effect of pain on response to reward loss in calves. Sci. Rep.13, 15403. doi: 10.1038/s41598-023-42740-8
13
EllisS. L. H.RiemerS.ThompsonH.BurmanO. H. P. (2020). Assessing the external validity of successive negative contrast – implications for animal welfare. J. Appl. Anim. Welfare Sci.23, 54–61. doi: 10.1080/10888705.2019.1572509
14
ElmoreM. R. P.GarnerJ. P.JohnsonA. K.KirkdenR. D.RichertB. T.PajorE. A. (2011). Getting around social status: Motivation and enrichment use of dominant and subordinate sows in a group setting. Appl. Anim Behav. Sci.133, 154–163. doi: 10.1016/j.applanim.2011.05.017
15
EnglundM. D.CroninK. A. (2023). Choice, control, and animal welfare: definitions and essential inquiries to advance animal welfare science. Front. Veterinary Sci.10. doi: 10.3389/fvets.2023.1250251
16
FigueroaJ.Solà-OriolD.MantecaX.PérezJ. F.DwyerD. M. (2015). Anhedonia in pigs? Effects of social stress and restraint stress on sucrose preference. Physiol. Behav.151, 509–515. doi: 10.1016/j.physbeh.2015.08.027
17
FlahertyC. F.CaprioM. (1976). The dissociation of instrumental and consummatory measures of contrast. Am. J. Psychol.89, 485–498. doi: 10.2307/1421620
18
FlahertyC. F.GrigsonP. S.RowanG. A. (1986). Chlordiazepoxide and the determinants of negative contrast. Anim. Learn. Behav.14, 315–321. doi: 10.3758/BF03200073
19
FraserD. (2008). Understanding animal welfare. Acta Vet Scand.50, S1. doi: 10.1186/1751-0147-50-S1-S1
20
GreiveldingerL.VeissierI.BoissyA. (2011). The ability of lambs to form expectations and the emotional consequences of a discrepancy from their expectations. Psychoneuroendocrinology36, 806–815. doi: 10.1016/j.psyneuen.2010.11.002
21
HellhammerD. H.BuchtalJ.GutberletI.KirschbaumC. (1997). Social hierarchy and adrenocortical stress reactivity in men. Psychoneuroendocrinology22, 643–650. doi: 10.1016/S0306-4530(97)00063-2
22
HemsworthP. H.RiceM.NashJ.GiriK.ButlerK. L.TilbrookA. J.et al. (2013). Effects of group size and floor space allowance on grouped sows: Aggression, stress, skin injuries, and reproductive performance. J. Anim. Sci.91, 4953–4964. doi: 10.2527/jas.2012-5807
23
HernandezC. E.HinchG.LeaJ.FergusonD.LeeC. (2015). Acute stress enhances sensitivity to a highly attractive food reward without affecting judgement bias in laying hens. Appl. Anim. Behav. Sci.163, 135–143. doi: 10.1016/j.applanim.2014.12.002
24
HessingM. J. C.ScheepensC. J. M.SchoutenW. G. P.TielenM. J. M.WiepkemaP. R. (1994). Social rank and disease susceptibility in pigs. Veterinary Immunol. Immunopathology43, 373–387. doi: 10.1016/0165-2427(94)90158-9
25
LanthonyM.DanglotM.ŠpinkaM.TalletC. (2022). Dominance hierarchy in groups of pregnant sows: Characteristics and identification of related indicators. Appl. Anim. Behav. Sci.254, 105683. doi: 10.1016/j.applanim.2022.105683
26
LecorpsB.WearyD. (2024). Animal affect, welfare and the Bayesian brain. Anim. Welfare33, e39. doi: 10.1017/awf.2024.44
27
LewisN. J. (1999). Frustration of goal-directed behaviour in swine. Appl. Anim. Behav. Sci.64, 19–29. doi: 10.1016/S0168-1591(99)00025-8
28
LiP.YinF.ZhaoY.LiuY.ZhangR.WangJ.et al. (2023). Total sugar intake is associated with higher prevalence of depressive symptoms in obese adults. Front. Public Health10. doi: 10.3389/fpubh.2022.1069162
29
LuoL.ReimertI.GraatE. A. M.SmeetsS.KempB.BolhuisJ. E. (2020). Effects of early life and current housing on sensitivity to reward loss in a successive negative contrast test in pigs. Anim Cognit.23, 121–130. doi: 10.1007/s10071-019-01322-w
30
LuppinoF. S.de WitL. M.BouvyP. F.StijnenT.CuijpersP.PenninxB. W. J. H.et al. (2010). Overweight, obesity, and depression: A systematic review and meta-analysis of longitudinal studies. Arch. Gen. Psychiatry67, 220–229. doi: 10.1001/archgenpsychiatry.2010.2
31
MeeseG. B.EwbankR. (1973). The establishment and nature of the dominance hierarchy in the domesticated pig. Anim. Behav.21, 326–334. doi: 10.1016/S0003-3472(73)80074-0
32
MitchellC.FlahertyC. (1998). Temporal dynamics of corticosterone elevation in successive negative contrast. Physiol. Behav.64, 287–292. doi: 10.1016/S0031-9384(98)00072-9
33
MitchellC. P.FlahertyC. F. (2005). Differential effects of removing the glucose or saccharin components of a glucose–saccharin mixture in a successive negative contrast paradigm. Physiol. Behav.84, 579–583. doi: 10.1016/j.physbeh.2005.02.005
34
MitchellE. N.MarstonH. M.NuttD. J.RobinsonE. S. J. (2012). Evaluation of an operant successive negative contrast task as a method to study affective state in rodents. Behav. Brain Res.234, 155–160. doi: 10.1016/j.bbr.2012.06.016
35
NearyJ. M.AliA. B. A.JacobsL. (2024). Application of an attention bias test after surgical castration in piglets. Livestock Sci.290, 105612. doi: 10.1016/j.livsci.2024.105612
36
O’ConnellN. E.BeattieV. E.MossB. W. (2003). Influence of social status on the welfare of sows in static and dynamic groups. Anim. Welfare12, 239–249. doi: 10.1017/S0962728600025665
37
O’ConnellN. E.BeattieV. E.MossB. W. (2004). Influence of social status on the welfare of growing pigs housed in barren and enriched environments. Anim. Welfare13, 425–431. doi: 10.1017/S0962728600028682
38
Ochoteco-AsensioJ.ZigovskiG.Batista CostaL.Rio-LópezR.Clavell-SansalvadorA.Ramayo-CaldasY.et al. (2024). Effect on feeding behaviour and growing of being a dominant or subordinate growing pig and its relationship with the faecal microbiota. Animals14, 1906. doi: 10.3390/ani14131906
39
PapiniM. R. (2006). Role of surprising nonreward in associative learning. Japanese J. Anim. Psychol.56, 35–54. doi: 10.2502/janip.56.35
40
PapiniM. R.DudleyR. T. (1997). Consequences of surprising reward omissions. Rev. Gen. Psychol.1, 175–197. doi: 10.1037/1089-2680.1.2.175
41
R Core Team (2021). R: A language and environment for statistical computing. Available online at: https://www.R-project.org/ (Accessed Juanuary 25, 2022).
42
RiemerS.EllisS. L. H.ThompsonH.BurmanO. H. P. (2018). Reinforcer effectiveness in dogs—The influence of quantity and quality. Appl. Anim. Behav. Sci.206, 87–93. doi: 10.1016/j.applanim.2018.05.016
43
RygulaR.AbumariaN.FlüggeG.FuchsE.RütherE.Havemann-ReineckeU. (2005). Anhedonia and motivational deficits in rats: Impact of chronic social stress. Behav. Brain Res.162, 127–134. doi: 10.1016/j.bbr.2005.03.009
44
ScipioniR.MartelliG.Antonella VolpelliL. (2009). Assessment of welfare in pigs. Ital. J. Anim. Sci.8, 117–137. doi: 10.4081/ijas.2009.s1.117
45
ShermanG. D.MehtaP. H. (2020). Stress, cortisol, and social hierarchy. Curr. Opin. Psychol.33, 227–232. doi: 10.1016/j.copsyc.2019.09.013
46
TuchschererM.PuppeB.TuchschererA.KanitzE. (1998). Effects of social status after mixing on immune, metabolic, and endocrine responses in pigs. Physiol. Behav.64, 353–360. doi: 10.1016/S0031-9384(98)00084-5
47
TukeyJ. W. (1977). Exploratory data analysis (Reading, Massachusetts: Addison-Wesley Publishing Company). Available online at: http://www.gbv.de/dms/bowker/toc/9780201076165.pdf (Accessed March 4, 2025).
48
TurnerS. P.EdwardsS. A. (2004). Housing immature domestic pigs in large social groups: implications for social organisation in a hierarchical society. Appl. Anim. Behav. Sci.87, 239–253. doi: 10.1016/j.applanim.2004.01.010
49
Ulrich-LaiY. M.ChristiansenA. M.OstranderM. M.JonesA. A.JonesK. R.ChoiD. C.et al. (2010). Pleasurable behaviors reduce stress via brain reward pathways. Proc. Natl. Acad. Sci.107, 20529–20534. doi: 10.1073/pnas.1007740107
50
van der HarstJ. E.BaarsA.-M.SpruijtB. M. (2003). Standard housed rats are more sensitive to rewards than enriched housed rats as reflected by their anticipatory behaviour. Behav. Brain Res.142, 151–156. doi: 10.1016/S0166-4328(02)00403-5
51
van der HarstJ. E.SpruijtB. M. (2007). Tools to measure and improve animal welfare: Reward-related behaviour. Anim. Welfare16, 67–73. doi: 10.1017/S0962728600031742
52
VerdonM.HansenC. F.RaultJ.-L.JongmanE.HansenL. U.PlushK.et al. (2015). Effects of group housing on sow welfare: A review. J. Anim. Sci.93, 1999–2017. doi: 10.2527/jas.2014-8742
53
VerdonM.MorrisonR. S.RiceM.HemsworthP. H. (2016). Individual variation in sow aggressive behavior and its relationship with sow welfare. J. Anim. Sci.94, 1203–1214. doi: 10.2527/jas.2015-0006
54
WebbL. E.van ReenenC. G.JensenM. B.SchmittO.BokkersE. A. M. (2015). Does temperament affect learning in calves? Appl. Anim. Behav. Sci.165, 33–39. doi: 10.1016/j.applanim.2015.01.013
55
WillnerP.BentonD.BrownE.CheetaS.DaviesG.MorganJ.et al. (1998). Depression” increases “craving” for sweet rewards in animal and human models of depression and craving. Psychopharmacology136, 272–283. doi: 10.1007/s002130050566
56
YuR.MobbsD.SeymourB.RoweJ. B.CalderA. J. (2014). The neural signature of escalating frustration in humans. Cortex54, 165–178. doi: 10.1016/j.cortex.2014.02.013
Summary
Keywords
animal welfare, animal emotions, affective states, successive negative contrast, SNC, swine, frustration, social rank
Citation
Ede T, Ibach S and Parsons TD (2025) Social hierarchy impacts response to reward downshift in sows. Front. Anim. Sci. 6:1570586. doi: 10.3389/fanim.2025.1570586
Received
03 February 2025
Accepted
17 April 2025
Published
13 May 2025
Volume
6 - 2025
Edited by
Leonie Jacobs, Virginia Tech, United States
Reviewed by
Javiera Calderón-Amor, Austral University of Chile, Chile
Lu Luo, Nanjing Agricultural University, China
Updates
Copyright
© 2025 Ede, Ibach and Parsons.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Thomas Ede, tede@vet.upenn.edu
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.