- Department of Agricultural Economics and Animal Production, University of Limpopo, Sovenga, Limpopo, South Africa
Broiler chickens are economically, nutritionally, and culturally very important. An experiment was conducted to determine the effect of dietary threonine level on the growth performance of slow-growing 50- to 91-day-old male Boschveld chickens. A total of 75 chickens were used in a complete randomized design comprising five dietary treatments, with three replicates and five chickens per replicate. The dietary treatments had similar nutrient contents but different threonine levels: 4.0, 7.5, 8.0, 8.5, and 9.0 g/kg dry matter (DM). The collected data were subjected to one-way analysis of variance using SAS version 9.4. A quadratic equation was used to determine the dietary threonine levels for the optimal performance of the chickens. The threonine levels affected (p<0.05) the feed intake, body weight gain, feed conversion ratio, metabolizable energy intake, nitrogen retention, abdominal fat weight, and the meat crude protein and threonine levels of the chickens. The body weight gain, feed conversion ratio, metabolizable energy intake, nitrogen retention, abdominal fat weight, and the meat crude protein and threonine levels of the chickens were optimized at dietary threonine levels of 6.12, 6.82, 5.71, 6.0, 6.9, 5.9, and 5.7 g/kg DM, respectively. However, the feed intake of the chickens decreased (p<0.05) when the dietary threonine levels were increased. The dietary threonine levels did not affect (p<0.05) the pH values of chicken meat. However, the threonine level in the diet affected (p<0.05) the chicken meat color. The chicken meat lightness and the hue angle had a positive relationship (p<0.05) with the dietary threonine levels, while the meat redness, yellowness, and chroma had a negative relationship (p<0.05) with the dietary threonine levels. It was concluded that the production parameters of male Boschveld chickens were optimized at different dietary threonine levels.
Introduction
One of the major challenges in the poultry industry faced by developing countries, such as those in Africa, is the feed cost due to the high prices of protein and energy sources (Abbas, 2013). Indigenous chicken breeds are extremely important, particularly in developing countries, as they are a major source of protein for the people. However, there are limited data on the dietary nutrient levels for the optimal performance of slow-growing indigenous chicken breeds (Manyelo et al., 2020; Ng’ambi et al., 2017). NRC (1994) suggested lower dietary nutrient levels for the optimal performance of slow-growing chicken breeds compared with those of fast-growing broiler chickens. Slow-growing indigenous female Venda chickens had lower dietary threonine levels for optimal performance than fast-growing broiler chickens (Ng’ambi et al., 2017). The authors also observed that the threonine requirements for optimal performance were dependent on the chicken parameters, i.e., feed intake, feed conversion ratio (FCR), growth rate, and carcass traits, among others. Threonine is an essential amino acid in chickens as their body is not capable of synthesizing threonine de novo (Pokoo-Aikins et al., 2022; Lehninger et al., 2000; NRC, 1994). Thus, dietary threonine levels are important for the optimal performance of broiler chickens as deficiency of this amino acid may result in increased FCR, decreased weight gain and nitrogen retention, low carcass characteristics, poor intestinal morphology, weak immunity, and slow functional development (Qaisrani et al., 2018; Manyelo and Ng’ambi, 2024). Chickens fed a diet deficient in threonine perform poorly (Kidd et al., 2004; Dozier et al., 2000). Threonine is known to be the most important amino acid for the maintenance of the intestinal mucosa as it is used in the synthesis of mucin that covers the surface area of the mucosa (Ahmed et al., 2020). According to Debnath et al. (2020), threonine is also known to maintain the intestinal strength and growth in addition to providing energy for normal intestinal functions. An increase in supplemental threonine levels has been reported to provide sufficient concentrations for the high turnover of mucosal tissues (Rezaeipour et al., 2012). Increasing the dietary l-threonine improved the morphological aspects of the small intestine of chickens, resulting in a greater development rate compared with chickens fed threonine-deficient diets, which exhibited a decreased nutrient absorption in the digestive tract (Wasman, 2022). Increases in the number of heterophils are an essential measure of immune system function, and threonine increases the globulin levels in the blood and lymphocytes (Azzam et al., 2011). There are no data in the literature on the threonine levels in the diet for optimal performance of the slow-growing indigenous Boschveld chicken breed found in South Africa. The objective of this study was to determine the threonine levels in a high-protein diet for the optimal performance of slow-growing male Boschveld chickens aged between 50 and 91 days.
Materials and methods
Diets, design, and procedures
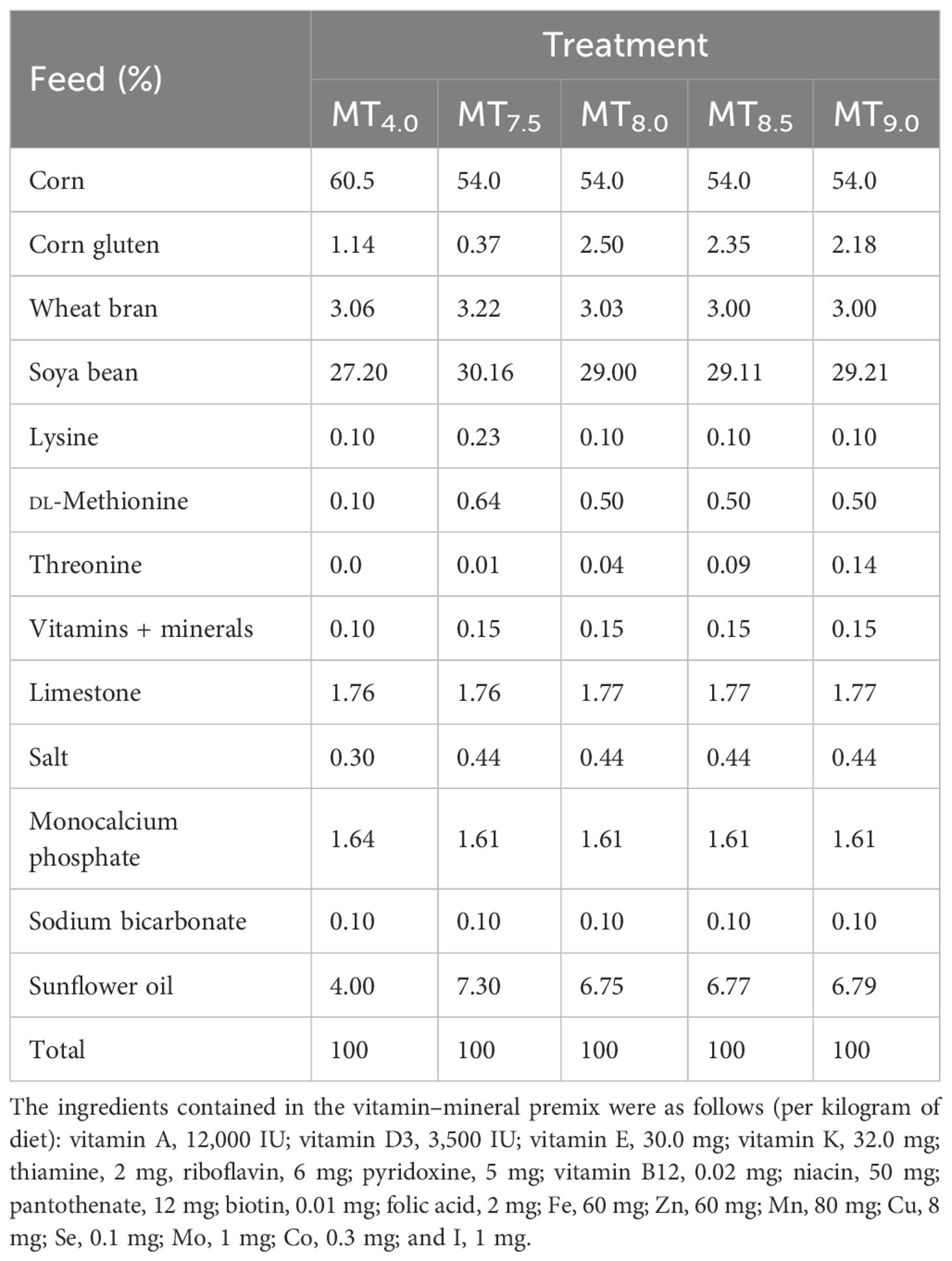
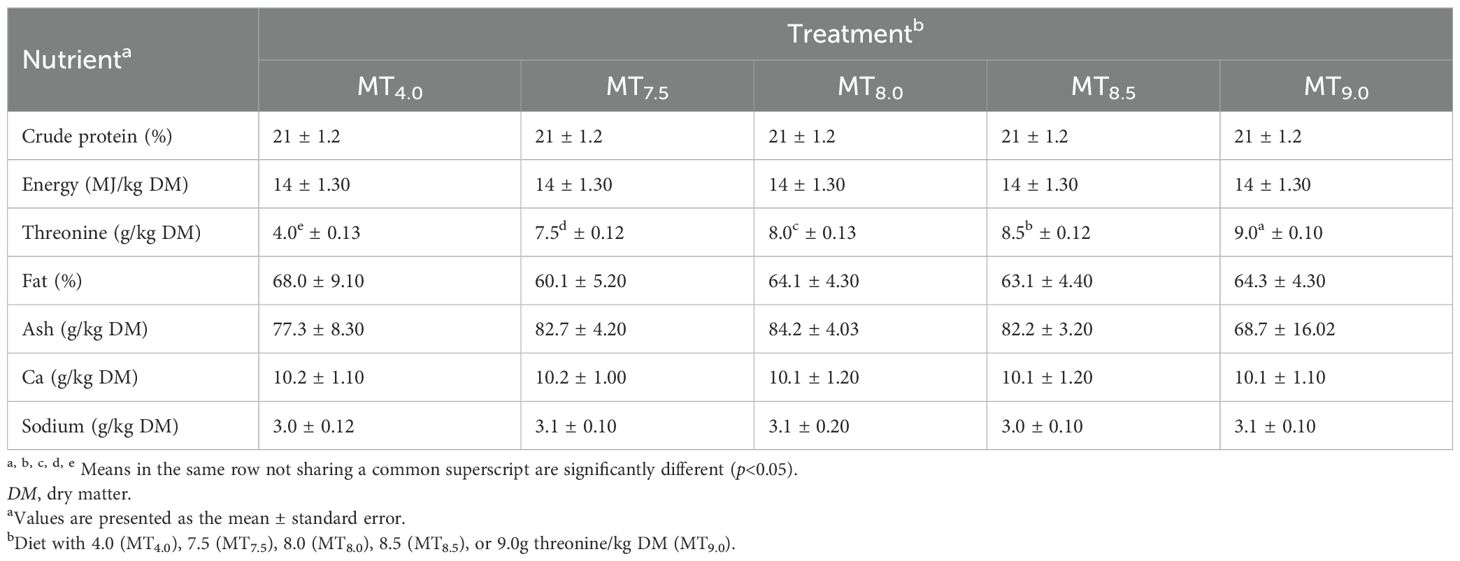
This experiment was performed at the University of Limpopo (−27.55° S and 24.77° E). The study was approved by the University of Limpopo Animal Research Ethics Committee (AREC/20/2023:PG). This study determined the threonine levels for the optimal performance of 50- to 91-day-old male Boschveld chickens. A total of 75 male chickens weighing an average of 600 ± 10g were allocated into five treatment groups, with three replicates and five chickens per replicate (5 × 3 × 5 = 75 chickens), in a completely randomized design (Okpanachi et al., 2015; Matius et al., 2022). The diets were isocaloric (14 MJ/kg dry matter, DM) and isonitrogenous [210g crude protein (CP)/kg DM] but with threonine levels of 4.0 (MT4.0), 7.5 (MT7.5), 8.0 (MT8), 8.5 (MT8.5), or 9.0 g/kg DM (MT9.0) (Tables 1, 2). The chickens had been raised on a diet with 220g CP/kg DM and 12 MJ/kg DM before the commencement of the experiment. The chickens were raised in a well-ventilated house with 15 m2 × 2 m2 pens of wire mesh. The house temperature was maintained between 23°C and 30°C. Light was provided for 20h to ensure adequate growth and development of the chickens (National Audit of Continence Care, 2010). Feed and water were available all the time.
Data collection
The live weights of the chickens were taken at the beginning of the experiment and weekly thereafter. The daily diet intakes were determined. The daily live weight gains and feed efficiencies were calculated. Apparent nutrient digestibility measurements were carried out when the chickens were between 84 and 91 days old. Two birds were randomly selected from each replicate, placed in metabolic cages with dimensions of 60cm × 50cm with a wire mesh bottom of 1cm × 1cm, and were used for digestibility determination. The fecal matter was collected from day 4 until day 7. The feces were weighed, dried at 70°C in an oven for 48h, and then weighed to determine nutrient digestibility. Thereafter, digestibility was calculated using the following formula:
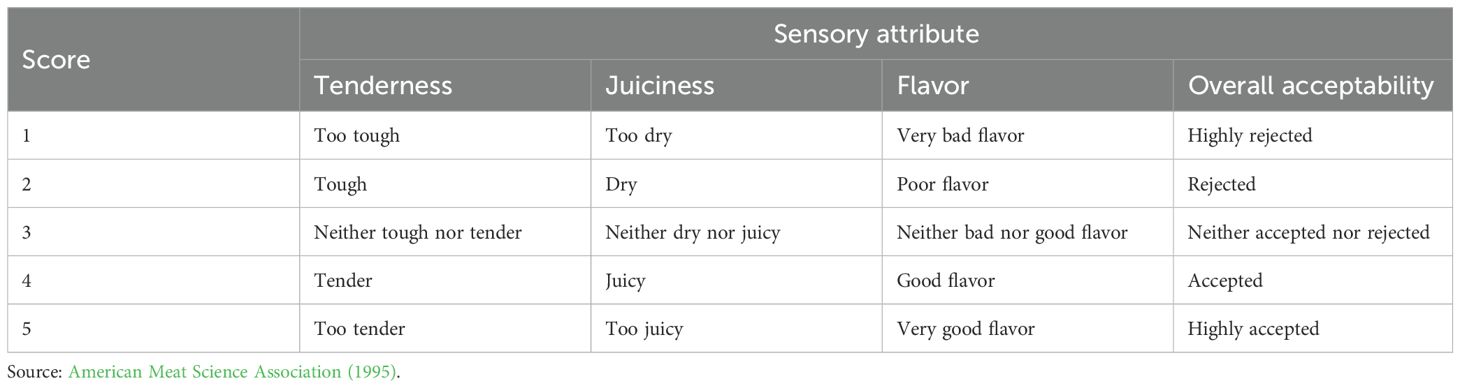
At the age of 91 days, three chickens from each replicate were slaughtered using the cervical dislocation method and, within 10 s after dislocation, the birds were killed (AVMA, 2020). Breast meat samples were analyzed for nutrient contents (AOAC, 2012), cooking loss (McDonald et al., 2010), and sensory attributes (American Meat Science Association, 1995) (Table 3).
An electronic scale was used to measure the dressed carcass and carcass part weights of the chickens. The digestive organ weight and length were measured using an electric scale and a measuring tape, respectively. A digital pH meter (BASIC 20 pH Meter; Crison, Barcelona, Spain) was used to measure the digesta and meat pH values. Meat color was determined with the use of the Hunter-Lab test system, where L* is the lightness, a* is the redness, b* is the yellowness, and h is the hue angle (angular position of a color on the color wheel). Shear force values of the meat were determined using the Warner–Bratzler shear force (Novakovi and Tomaševi, 2017).
Chemical analysis
Samples of feeds and feces were analyzed for nutrient contents (AOAC, 2012). The threonine in the feeds was determined using ion exchange chromatography (HPLC, University of Limpopo, South Africa). The dietary apparent metabolizable energy (ME) content was calculated as described by McDonald et al. (2010).
Data analysis
The collected data were analyzed using the general linear model (GLM) procedure of the statistical analysis of variance (SAS, 2020), version 9.4. Where significant treatment effects were detected, the means were separated using Duncan’s test for multiple comparisons (p<0.05). The statistical model used was as follows:
where Yij is the performance response of the chickens, μ is the overall mean, Ti is the effect of dietary threonine level, and eij is the random error.
The following quadratic equation was used to determine the threonine level in the diet for optimal performance (SAS, 2020):
where Y is the optimal performance, a is the intercept, b is the coefficient of the equation, x is the dietary threonine level, −b1/2b2 is the threonine level for optimal response, and e is the error.
A linear regression equation (SAS, 2020) was used to determine the relationship between dietary threonine level and chicken performance.
where Y is the chicken performance, a is the intercept, b is the coefficient of the linear equation, and x is the threonine level in the diet.
Results
The treatments had similar nutrients, except for threonine (Table 4). The dietary threonine level affected the feed intake, the live weight gain, the feed efficiency, the ME intake, and the N retention of 50- to 90-day-old slow-growing male Boschveld chickens (p<0.05) (Table 5). The quadratic equations indicated that the live weight gain, the FCR, the ME intake, and the N retention of the chickens were optimized at dietary threonine levels of 6.12 (r2 = 0.634), 6.82 (r2 = 0.521), 5.71 (r2 = 0.619), and 6.0 g/kg DM (r2 = 0.41), respectively. However, the feed intake of the chickens decreased as the dietary threonine level increased (Y=4.30 + 157.90x, r2 = 0.358, p<0.05).
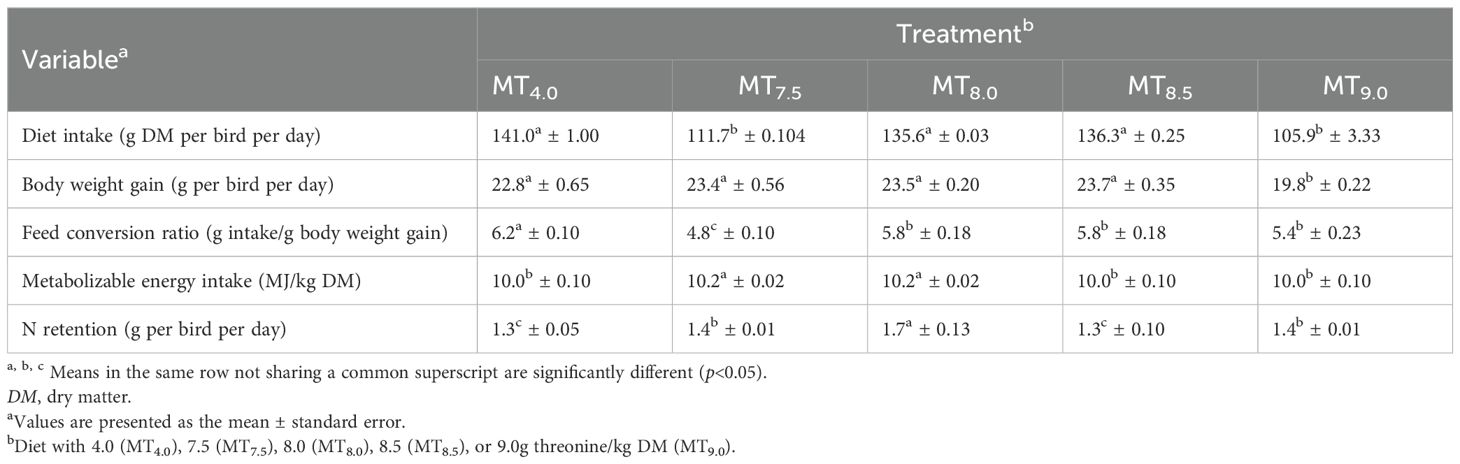
Table 5. Effect of the dietary threonine level on the performance of slow-growing male Boschveld chickens aged 50–91 days.
The dietary threonine level did not affect the gut organ digesta pH and the length of slow-growing chickens aged 91 days (p > 0.05) (Table 6). Similarly, the weights of the gizzard, ileum, duodenum, cecum, and large intestine of the chickens were not affected by the threonine level in the diet (p > 0.05). However, the weights of the crop and jejunum of the chickens were affected by the threonine level in the diet (p<0.05). The weights of the crop (Y = −12.40 + 10.00x + −0.84x2, r2 = 0.831) and jejunum (Y=14.88 + 2.92x + −0.26x2, r2 = 0.238) of Boschveld chickens were optimized at 6.0g threonine/kg DM diet.
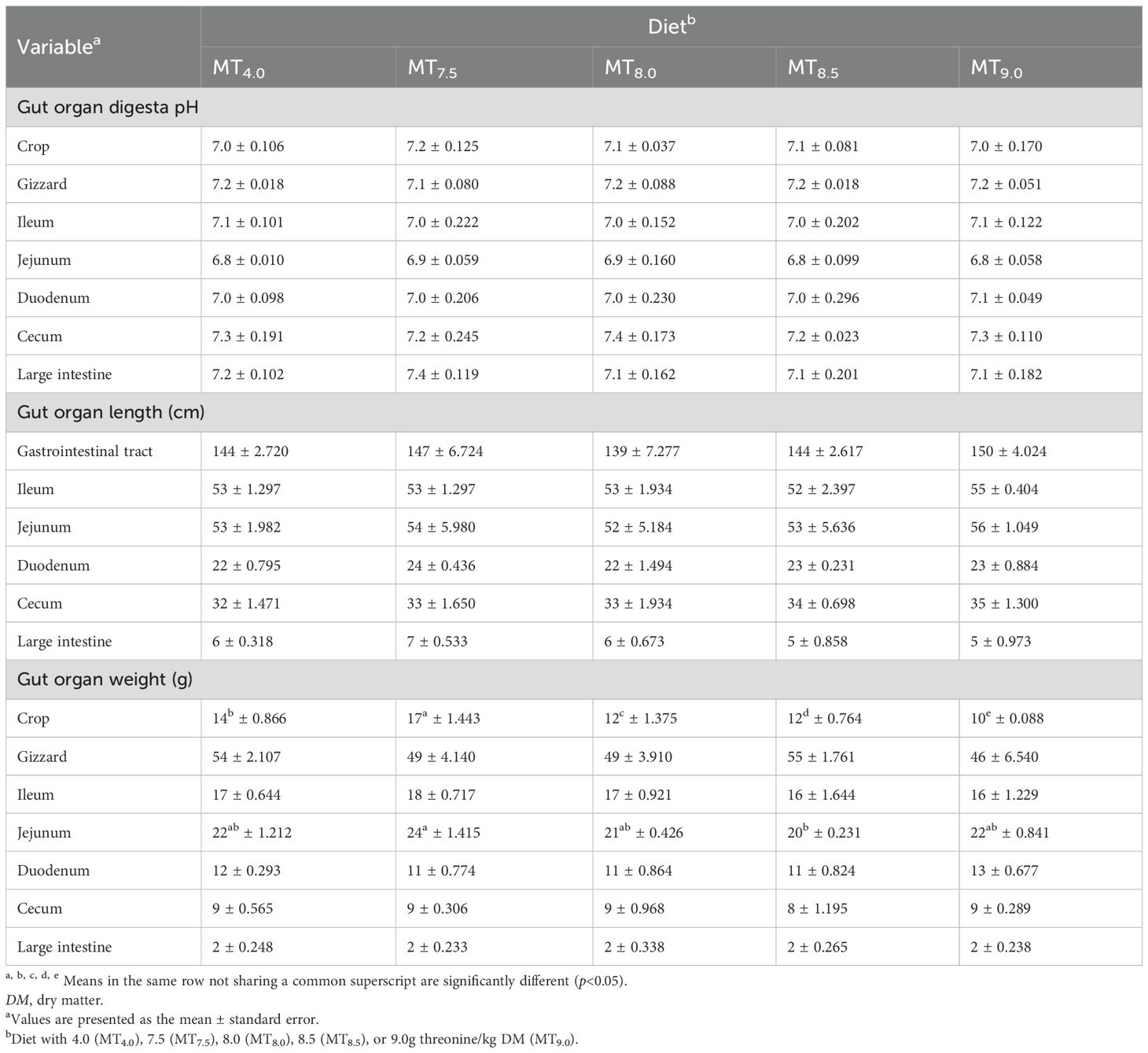
Table 6. Effect of threonine level in the diet on the gut organ pH, length, and weight of 91-day-old slow-growing indigenous male Boschveld chickens.
The weights of the carcass, thigh, drumstick, and breast of the slow-growing 91-day-old male Boschveld chickens were affected (p<0.05) by the threonine level in the diet (Table 7). Positive relationships were observed between the threonine level in the diet and the carcass (r2 = 0.947), thigh (r2 = 0.870), drumstick (r2 = 0.801), and breast weights (r2 = 0.078) of the chickens (Table 8). The abdominal fat weight of Boschveld chickens was optimized at a threonine level of 6.9 g/kg DM diet (Y = −12.21 + 8.66x + −0.63x2, r2 = 0.396). Different dietary threonine levels of 5.9 (Y=47.982 + 7.040x − 0.590x2, r2 = 0.827) and 5.7 g/kg DM (Y=1.988 + 0.340x − 0.030x2, r2 = 0.745) optimized the meat CP and threonine contents, respectively.
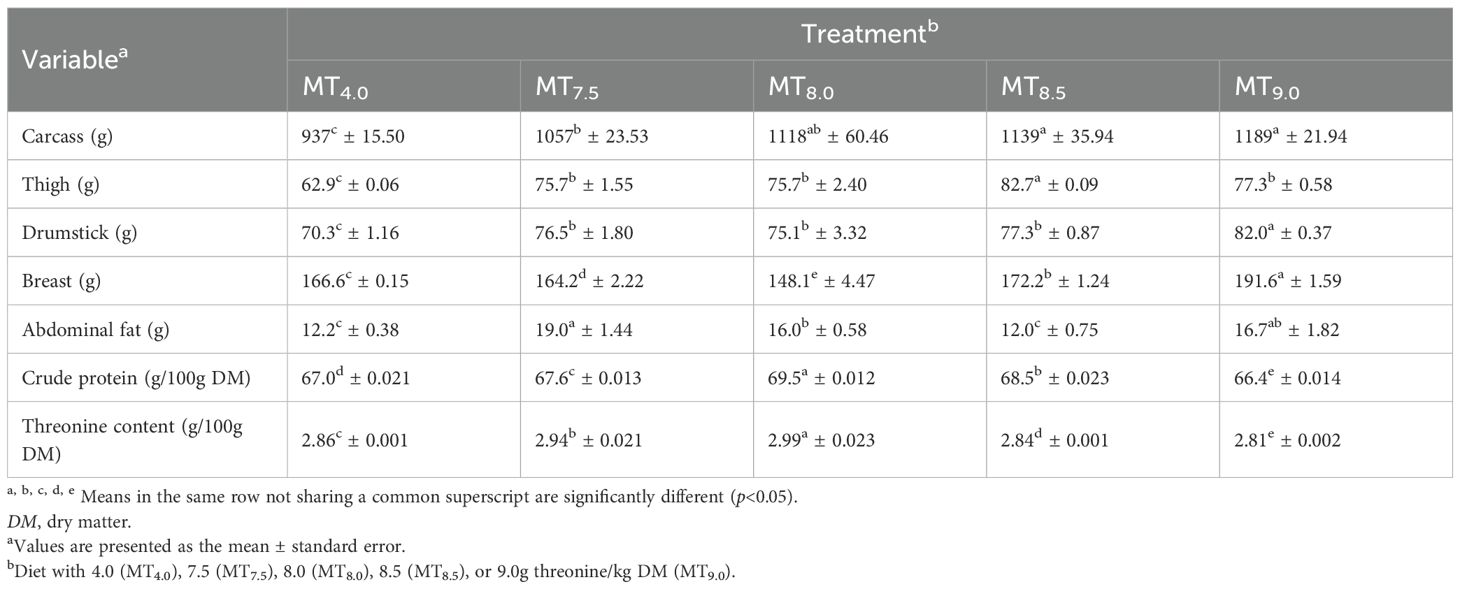
Table 7. Effect of threonine level in the diet on the meat part weights of 91-day-old slow-growing male Boschveld chickens.
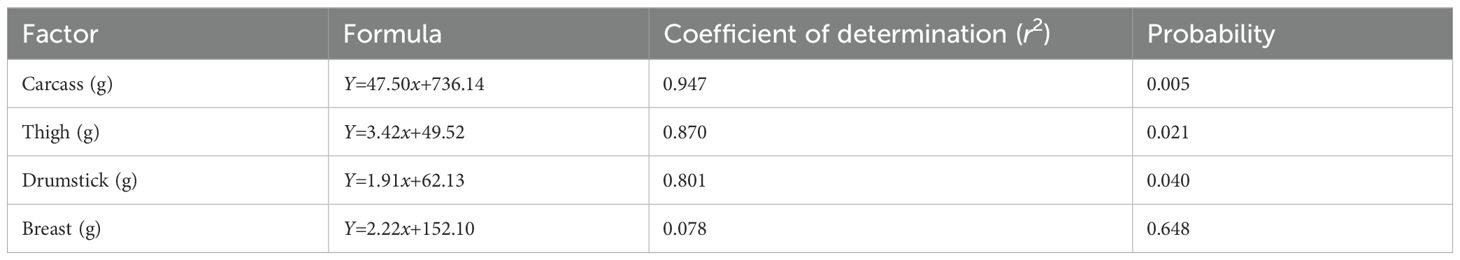
Table 8. Relationship between the threonine level in the diet and the meat part weights of 91-day-old slow-growing male Boschveld chickens.
The dietary threonine level did not affect Boschveld chicken meat pH values (p > 0.05) (Table 9). However, the chicken meat color was affected (p<0.05) by the dietary threonine level. Negative relationships were observed between the dietary threonine levels and the meat redness, yellowness, and chroma (p<0.05), while positive relationships were observed between the dietary threonine levels and the meat lightness and hue angle (p<0.05) (Table 10). The meat juiciness, flavor, overall acceptability, and cooking loss values of Boschveld chickens were not affected by the threonine level in the diet (p > 0.05) (Table 11). However, the meat tenderness increased with an increase in dietary threonine level (Y=0.29x+1.02, r2 = 0.799, p<0.05).
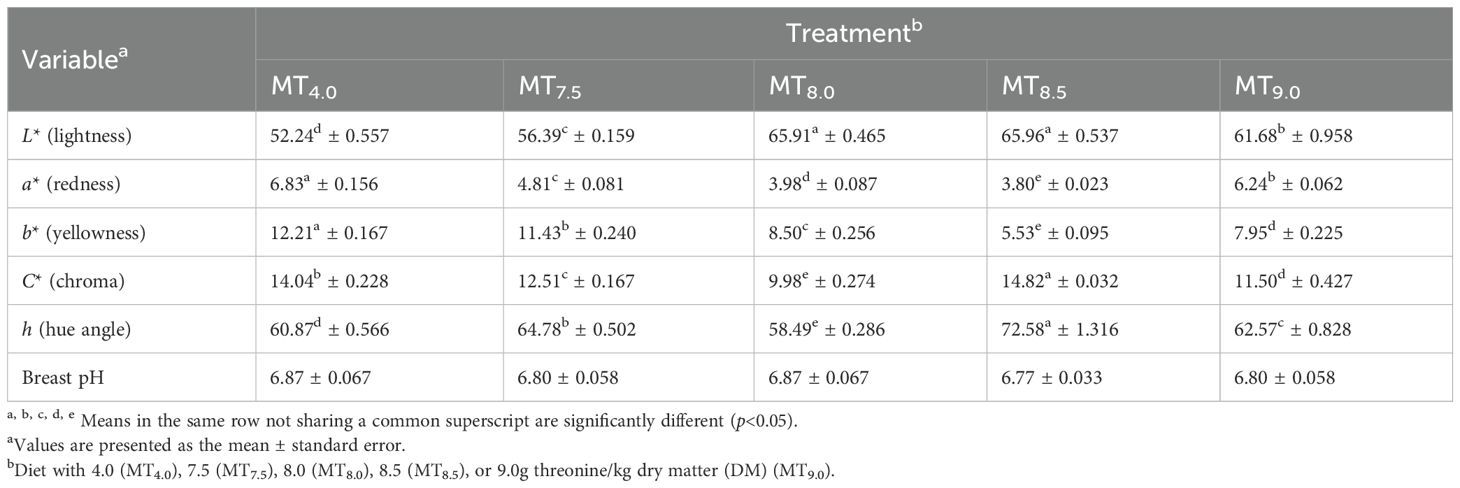
Table 9. Effect of the dietary threonine level on the breast color and pH values of 91-day-old slow-growing male Boschveld chickens.
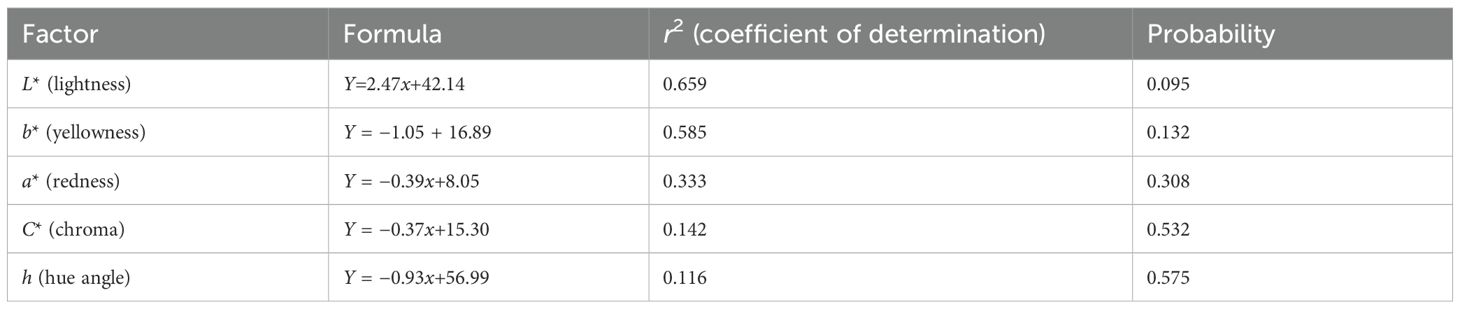
Table 10. Relationship between the dietary threonine level and breast color of 91-day-old slow-growing male Boschveld chickens.
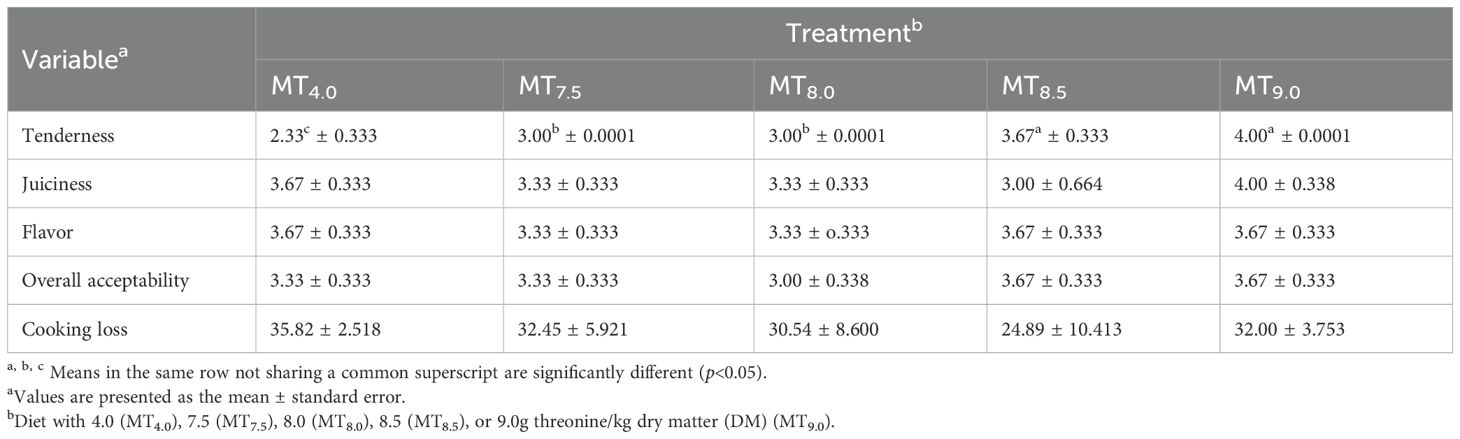
Table 11. Effect of the dietary threonine level on the meat sensory attributes and cooking loss of 91-day-old slow-growing male Boschveld chickens.
Discussion
The diets used in this study had similar nutrient levels except for dietary threonine, which ranged from 4.0 to 9.0 g/kg DM. The body weight gain, the FCR, the ME intake, the nitrogen retention, the abdominal fat weight, and the meat CP and threonine levels of the chickens were optimized at dietary threonine levels of 6.12, 6.82, 5.71, 6.0, 6.9, 5.9, and 5.7 g/kg DM, respectively, possibly indicating different threonine requirements for the different chicken production parameters (Ciftci and Ceylan, 2024; Ng’ambi et al., 2017; NRC, 1994). Ng’ambi et al. (2017) reported that 6.1, 6.4, 5.7, and 6.1g threonine/kg DM diet optimized the body weight gain, the feed efficiency, the ME intake, and the nitrogen retention of slow-growing 50- to 91-day-old Venda chickens, respectively. The present results indicate that the voluntary feed intake of male Boschveld chickens decreased with an increase in the dietary threonine level, possibly indicating that the dietary threonine level for optimal voluntary feed intake was lower than the dietary threonine levels used. However, Ng’ambi et al. (2017) reported that the optimal feed intake for Venda chickens was achieved at a dietary threonine level of 6.1 g/kg DM. On the other hand, Najafi et al. (2017) reported increased feed intake by 5.1%, increased body weight gain by 6.4%, and better FCR when chickens were fed diets supplemented with threonine levels up to 0.93%. Kheiri and Alibeyghi (2017) reported that broilers fed diets containing 0.9% threonine had 1.1% increased feed intake, 3.2% higher body weight gain, and 1.7% better FCR. Min et al. (2017) reported that broiler chickens fed a diet containing 0.75% threonine had 1.6% higher feed intake, 6% higher average daily gain, and 4.2% better FCR. The differences in the results between the studies may be due to the different diets and chicken genotypes used (NRC, 1994).
In this study, the threonine level in the diet did not affect the gut organ digesta pH and length of the 91-day-old male Boschveld chickens, possibly indicating that the dietary threonine levels for optimal gut organ digesta pH and length were lower than or equal to the levels used in the experiment. Ng’ambi et al. (2017) reported no dietary threonine level effect on the digesta pH values of Venda chickens. A dietary threonine level of 8.0 g/kg DM was recommended for the optimal gut morphology of 22- to 42-day-old fast-growing broiler chickens (NRC, 1994). Jamroz (2005) observed that longer intestines improved the feed digestibility in broiler chickens. The weights of the gizzard, ileum, duodenum, cecum, and large intestine of male Boschveld chickens were not affected by the threonine levels in the diet; however, the weights of the crop and jejunum were affected by the dietary threonine levels. A threonine level of 6 g/kg DM diet optimized both the crop and jejunum weights of male Boschveld chickens. However, Rezaeipour et al. (2012) reported that dietary threonine levels did not affect the gut organ weights of broiler chickens.
Threonine is important for protein synthesis in chickens (Lehninger et al., 2000; NRC, 1994). Increasing the dietary threonine levels improved the carcass, thigh, drumstick, and breast weights of 50- to 91-day-old male Boschveld chickens, possibly indicating that the threonine levels used were lower than the optimal requirements for these production variables. However, Ng’ambi et al. (2017) observed that 6.2g threonine/kg DM diet optimized the carcass and breast weights of indigenous Venda chickens, which is lower than the dietary threonine levels of 7.5, 8.0, 8.5, and 9.0 g/kg DM used in the present study. Al-Hayani (2017); Kidd et al. (2004), and NRC (1994) reported 6.8, 6.5, and 8.6g threonine/kg DM diet, respectively, for optimal breast weights of 22- to 42-day-old broiler chickens. Dietary threonine levels of 5.9, 5.7, and 6.9 g/kg DM optimized the meat CP content, threonine content, and abdominal fats in male Boschveld chicken, respectively, possibly indicating different threonine requirements for the different chicken production parameters (Ng’ambi et al., 2017; NRC, 1994). The improved meat CP and threonine contents due to the increased threonine levels in the diet did not affect the meat juiciness, flavor, overall acceptability, or the cooking loss of Boschveld chicken. Ng’ambi et al. (2017) also observed that dietary threonine levels of 4–8 g/kg DM did not have any effect on the meat sensory attributes of slow-growing female Venda chickens. However, dietary amino acids affected the meat sensory attributes in chickens (Lawrie, 2006). The meat pH values of Boschveld chickens were not affected by the threonine levels in the diet, possibly indicating that the dietary threonine levels for optimal meat pH values were equal to or lower than the levels used in the present study. Schiavone et al. (2017) reported that the dietary threonine level had no effect on the meat pH values of broiler chickens. The meat color of Boschveld chickens was affected by the dietary threonine level. Negative relationships were observed between the dietary threonine levels and the meat redness, yellowness, and chroma, while positive relationships were observed between the threonine level in the diet and the lightness and hue angle of the meat. Zadeh et al. (2019) reported that 6.7 and 8.1g threonine/kg DM in the diet resulted in similar quail meat colors. Alaa El- Din et al. (2019) reported that the color of fresh meat and its stability can vary widely among species and cuts from the same animal due to differences in the anatomical, physiological function, and biochemical processes of the muscles where the meat is obtained from.
Conclusion and recommendations
The dietary threonine levels used in the present study affected the growth performance of 50- to 91-day-old male Boschveld chickens. The live weight gain, the feed efficiency, the ME intake, the nitrogen retention, and the abdominal fat weight and threonine levels of the chickens were optimized at dietary threonine levels of 6.14, 6.82, 5.71, 6.0, 6.9, and 5.9 g/kg DM, respectively, possibly indicating that the threonine requirements for optimal performance of the chickens are dependent on the particular production variables of interest, i.e., feed intake, FCR, body weight gain, and carcass characteristics, among others. It is recommended that when formulating diets for indigenous Boschveld chickens, the threonine levels should be based on the parameters of interest and breed as broiler chicken lines are constantly being improved through efficient breeding. In conclusion, more research is required to explore the biological and physiological reasons behind this.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The animal study was approved by University of Limpopo Animal Research and Ethics Committee. The study was conducted in accordance with the local legislation and institutional requirements.
Author contributions
TS: Data curation, Project administration, Visualization, Writing – original draft, Writing – review & editing, Formal analysis. JN: Data curation, Formal analysis, Software, Writing – original draft, Writing – review & editing. TM: Conceptualization, Data curation, Investigation, Methodology, Project administration, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. This work was supported by the National Research Foundation (MND210714622580).
Acknowledgments
University of Limpopo Livestock Unit workers are acknowledged for their help.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found onlineat: https://www.frontiersin.org/articles/10.3389/fanim.2025.1572249/full#supplementary-material
References
Abbas T. E. (2013). The use of Moringa oleifera in poultry diets. Turkish J. Veterinary Anim. Sci. 37, 492–496. doi: 10.3906/vet-1211-40
Ahmed I., Qaisrani S. N., Azam F., Pasha T. N., Bibi F., Naveed S., et al. (2020). Interactive effects of threonine levels and protein source on growth performance and carcass traits, gut morphology, ileal digestibility of protein and amino acids, and immunity in broilers. Poultry Sci. 99, 280–289. doi: 10.3382/ps/pez488
Alaa El- Din B., Morton J., Bhat Z. F., and Ling-Ming K. (2019). “Meat colour: factors affecting colour stability.” in Encyclopedia of Food Chemistry Edition: Volume 2, (Academic Press, ELSEVIER PUBLICATIONS). doi: 10.1016/B978-0-12-814026-0.21665-X
Al-Hayani W. K. (2017). Effect of threonine supplementation on broiler chicken productivity traits. Int. J. Poultry Sci. 16, 160–168. doi: 10.3923/ijps.2017.160.168
American Meat Science Association (1995). Research Guidelines for Cookery, Sensory Evaluation and Instrumental Measurements of Fresh Meat (Chicago, IL., USA: American Meat Science Association), 47, ISBN: ISBN-13: 978-0887000188.
AOAC (2012). Official Methods of Analysis. 19th edition (North Frederic Avenue, USA: AOAC International).
AVMA (2020). Guidelines for the Euthanasia of Animals (Schaumburg II: Animal Veterinary Association).
Azzam M. M. M., Dong X. Y., Xie P., Wang C., and Zou T. (2011). The effect of supplemental L-threonine on laying performance, serum free amino acids and immune function of laying hens under high-temperature and high-humidity environmental climates. J. Appl. Poultry. Res. 20, 361–370. doi: 10.3382/japr.2010-00308
Ciftci I. and Ceylan N. (2024). Effects of dietary threonine and crude protein on growth performance, carcase and meat composition of broiler chickens. Br. Poultry Sci. 45, 280–289. doi: 10.1080/00071660410001715894
Debnath B. C., Biswas P., Roy B., and Chatterjee P. N. (2020). Threonine supplementation on gut histomorphometry and mucin biology of broilers. Indian J. Anim. Health 59, 206–209. doi: 10.36062/ijah.59.2SPL.2020.206-209
Dozier W., Moran E., and Kidd M. (2000). Threonine requirement of broiler males from 42 to 56 days in a summer environment. J. Appl. Poultry Res. 9, 496–500. doi: 10.1093/japr/9.4.496
Jamroz D. (2005). “Comparative characteristics of gastrointestinal tract development and digestibility of nutrients in young chickens, ducks, and geese,” in Proceedings of the 15th European Symposium on Poultry Nutrition, Balatonfured, Hungary, September 25-29. (Balatonfured, Hungary: World’s Poultry Science Association), 74–85.
Kheiri F. and Alibeyghi M. (2017). Effect of different levels of lysine and threonine on carcass characteristics, intestinal microflora and growth performance of broiler chicks. Ital. J. Anim. Sci. 16, 580–587. doi: 10.1080/1828051X.2017.1302824
Kidd M. T., Corzo A., Hoehler D., Kerr B. J., Barber S. J., and Branton S. L. (2004). Threonine needs of broiler chickens with different growth rates. Poultry Sci. 83, 1368–1375. doi: 10.1093/ps/83.8.1368
Lehninger A. L., Nelson D. L., and Cox M. M. (2000). Principles of Biochemistry. 2nd edition (New York: Worth Publishers Incorporation).
Manyelo T. G. and Ng’ambi J. W. (2024). “An updated review of optimal threonine requirements in broiler and indigenous slow-growing chickens,” in Modern Technology and Traditional Husbandry of Broiler Farming. Agricultural Sciences. (London, United Kingdom: IntechOpen). doi: 10.5772/intechopen.1005832
Manyelo T. G., Selaledi L., Hassan Z. M., and Mabelebele M. (2020). Local chicken breeds of Africa: Their description, uses and conservation methods. Animals 10, 2257. doi: 10.3390/ani10122257
Matius N. B., Ni Ketut S. R., and Ni Made Y. (2022). The effect of giving sorghum on the growth of super village chickens age 3–10 week. Sustain. Environ. Agric. Sci. 6, 81–87. doi: 10.22225/seas.6.2.4974.81-87
McDonald P., Edwards R. A., Greenhalgh J. F. D., and Morgan C. A. (2010). Animal Nutrition. Sixth Edition (London: Pearson ed Education Limited).
Min Y., Liu S., Qu Z., Meng G., and Gao Y. (2017). Effects of dietary threonine levels on growth performance, serum biochemical indexes, antioxidant capacities, and gut morphology in broiler chickens. Poultry Sci. 96, 1290–1297. doi: 10.3382/ps/pew393
Najafi R., Ahmar R., and Tazehkand G. (2017). Effect of different dietary threonine levels on optimal growth performance and intestinal morphology in 1–14 days old Ross 308 broilers. Braz. J. Poultry Sci. 19, 59–66. doi: 10.1590/1806-9061-2016-0327
National Audit of Continence Care (2010).NACC full organisational and clinical report. Available online at: http://www.nafis.go.ke/livestock/poultry-chicken/general-information/feeds-and-feeding/ (Accessed August 14, 2024).
Ng’ambi J. W., Ramuthaga N., and Brown D. (2017). Effect of dietary threonine level on productivity and carcass characteristics of indigenous Venda chickens. Indian J. Anim. Res. 10, 18805. doi: 10.18805/ijar.v0iOF.9188
Novakovi S. and Tomaševi I. (2017). A comparison between Warner-Bratzler shear force measurement and texture profile analysis of meat and meat products: a review. IOP Conf. Ser.: Earth Environ. Sci. 85 (1), 012063.
NRC (1994). Nutrient Requirements of Poultry. 9th Revised Edition (Washington, DC: National Academic Press).
Okpanachi U., Boyi P. U., Egbu C. F., and Oyibo A. (2015). Performance and internal organ characteristics of broiler chickens fed urea-treated and untreated rice milling waste. Int. J. Anim. Biol. 1, 130–135.
Pokoo-Aikins A., Timmons J. R., Min B. R., Lee W. R., Mwangi S. N., McDonough C. M., et al. (2022). Effects of varying levels of dietary DL-methionine supplementation on breast meat quality of male and female broilers. Poultry 1, 40–53. doi: 10.3390/poultry1010005
Qaisrani S. N., Ahmed I., Azam F., Bibi F., Saima P. T. N., and Azam F. (2018). Threonine in broiler diets - An updated review. Ann. Anim. Sci. 18, 1-23. doi: 10.2478/aoas-2018-0020
Rezaeipour V., Fonani H., and Irani M. (2012). Effects of dietary L-threonine and Saccharomyces cerevisiae on performance, intestinal morphology, and immune response of broiler chickens. South Afr. J. Anim. Sci. 42, 267–272. doi: 10.4314/sajas.v42i3.8
SAS (2020). Statistical Analysis System, User’s Guide. Statistical. Version 9.4 th ed (Cary, North Carolina: SAS. Inst. Inc).
Schiavone A., Cullere M., De Marco M., Meneguz M., Biasato I., Bergagna S., et al. (2017). Partial or total replacement of soybean oil by black soldier fly larvae (Hermetia illucens L.) fat in broiler diets: Effect on growth performances, feed-choice, blood traits, carcass characteristics and meat quality. Ital. J. Anim. Sci. 16, 93–100.
Wasman P. H. (2022). Effect of L-threonine supplementation to diet on some productive and physiological of traits broiler chickens under heat stress conditions. Diyala Agric. Sci. J. 14, 47–53. doi: 10.52951/dasj.22140106
Keywords: threonine, chickens, growth, gut, carcass
Citation: Sekgotodi TM, Ng’ambi JW and Manyelo TG (2025) Effect of threonine level in a high-protein diet on the growth performance and carcass characteristics of 50- to 91-day-old slow-growing indigenous male Boschveld chickens. Front. Anim. Sci. 6:1572249. doi: 10.3389/fanim.2025.1572249
Received: 06 February 2025; Accepted: 30 September 2025;
Published: 21 October 2025.
Edited by:
Anna Stępniowska, University of Life Sciences of Lublin, PolandReviewed by:
Chompunut Lumsangkul, National Chung Hsing University, TaiwanCharlotte Paës, École d’Ingénieurs de PURPAN, France
Copyright © 2025 Sekgotodi, Ng’ambi and Manyelo. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Tlou Grace Manyelo, Z3JhY2UubWFueWVsb0B1bC5hYy56YQ==
 Tebogo Mack Sekgotodi
Tebogo Mack Sekgotodi Tlou Grace Manyelo
Tlou Grace Manyelo