Abstract
Introduction:
Nutritional status influences developmental processes, including sexual maturation. While the effects of macronutrients on reproductive development are well studied, the role of specific amino acid composition in ovarian and follicular development in birds remains less explored. Here, we investigated the impact of dietary restriction and amino acid supplementation on growth and reproductive development of Japanese quail (Coturnix japonica).
Methods:
Birds were assigned to five dietary treatments: control (ad libitum), 20% dietary restriction (DR20), and DR20 supplemented with methionine (DR20+Met), leucine (DR20+Leu) or both (DR20+Leu+Met) at levels 20% above the recommended nutrient content.
Results and discussion:
Dietary restriction reduced body mass, ovary mass, ovary index, and total antioxidant capacity without affecting hierarchical follicle counts or size. However, supplementation with either amino acid improved these parameters from dietary restriction to the control levels. Furthermore, methionine supplementation alone or combination of methionine and leucine significantly increased follicle numbers, whereas leucine alone had no effect on hierarchical follicle numbers. Our findings underscore the importance of amino acids in mitigating the adverse effects of dietary restriction on growth, reproduction, and oxidative balance in birds at the onset of reproductive maturation.
1 Introduction
Sexual maturation is a critical life stage characterized by metabolic, reproductive, and physiological changes. During this period, sufficient nutrient intake is vital to meet the increased energy demands of growth and reproduction, enabling animals to reach adult size and sustain reproductive functions (Ruffino et al., 2014; Milenkaya et al., 2015). Consequently, the development of reproductive structures is largely driven by nutritional availability (Whelan et al., 2021; Korver, 2023).
In birds, reproductive maturation is a tightly regulated process marked by rapid somatic growth and ovarian and follicular development, both of which require substantial metabolic investment (Taghipour-Shahbandi et al., 2024). A key indicator of ovarian maturation is the high ovary index (Abdul-Rahman et al., 2018), which is a measure of ovary development relative to body mass in females (Johnson, 2014, 2015). Ovarian maturation involves follicle differentiation and the recruitment of small follicles into preovulatory hierarchical follicles. These hierarchical follicles, ranging from the largest (F1) to the smallest (F5 up to F6), along with nonhierarchical follicles such as small yellow follicles (SYF), large white follicles (LWF) and small white follicles (SWF) undergo rapid growth by incorporating yolk-containing nutrients such as lipoproteins (Navara et al., 2023; Song et al., 2023). The metabolic demands of ovarian development are substantial, particularly during the rapid follicular growth and yolk accumulation of both hierarchical follicles up to 5 or 6 (F1 largest to F5 smallest) and nonhierarchical, including small yellow follicles (SYF), large white follicles (LWF), and small white follicles (SWF), impose significant physiological costs.
Nutritional deficiencies caused by environmental challenges force organisms to prioritize energy allocation for survival over reproduction, consequently delaying sexual maturation and reducing reproductive output (Huber, 2018; Afrouziyeh et al., 2021). The low energy allocation leads to slower growth and limits fat deposits, which is crucial for reproductive development (Zuidhof et al., 2014; Van Der Klein et al., 2018). In birds, feed restriction lowers reproductive success by limiting the clutch size, offspring quality and delaying the development of reproductive organs (Hargitai et al., 2022; Lu et al., 2021, 2023; Reda et al., 2024).
Reduced feed intake is directly linked to lower dietary antioxidant levels, which can decrease antioxidant capacity and increase susceptibility to oxidative stress (Hargitai et al., 2022). Oxidative stress can negatively impact various cellular functions essential for growth and reproductive success (Oke et al., 2024). Oxidative stress occurs when the production of reactive oxygen species (ROS) exceeds the body’s ability to neutralize them through its antioxidant defense system, leading to damage to macromolecules such as DNA, protein, and lipids (Juan et al., 2021). This imbalance is particularly harmful during periods of rapid growth and sexual maturation (Wiersma et al., 2004; Romero-Haro et al., 2016).
Plasma total antioxidant capacity (TAC) is a biomarker for the body’s ability to counteract ROS during growth and reproductive maturation (Oke et al., 2024). The antioxidant defense system includes both endogenous enzymes (e.g. glutathione peroxidase, superoxide dismutase) and dietary antioxidants (e.g. vitamins, tocopherols, carotenoids, tocotrienols) (Surai, 2020; Desbruslais and Wealleans, 2022). A significant proportion of antioxidants is obtained from dietary sources, and their availability is influenced by protein intake (Alan and McWilliams, 2013; Mavrommatis et al., 2021). Low dietary protein disrupts antioxidant activity by altering energy balance, leading to oxidative stress (Tarry-Adkins et al., 2010; Maniam and Morris, 2012). Although feed deprivation reduces antioxidant defense systems, its precise effects on plasma TAC remain unclear. However, oxidative stress is known to reduce reproductive performance, resulting in economic losses (Bacou et al., 2021).
Among the macronutrients in animal diets, proteins receive particular attention because they provide essential amino acids required for biological functions, whereas carbohydrates and lipids primarily serve as energy sources (Beski et al., 2015). Methionine and leucine, in particular, are critical for cell and tissue formation, growth, reproduction and antioxidant regulation. We recently showed that supplementing Japanese quail eggs with 2% leucine or methionine significantly increased post-hatch body weight and had a programmatic effect on somatic growth. The effect of leucine was detectable as early as day 5, while methionine had an impact from day 7 onward, with both continuing to enhance growth until day 21 (Ndunguru et al., 2024a, b). Similarly, a study on ducks showed that dietary methionine supplementation improved egg weight, the body mass of one-day-old ducklings and total antioxidant capacity (Ruan et al., 2018). In pigeons, elevated serum levels of leucine and methionine were associated with increased body mass, ovary mass, oviduct mass and egg white during the egg-laying cycles (Ren et al., 2021).
Despite the findings in other avian species, the effects of dietary interventions on physiological processes of growth and sexual maturation in Japanese quail remain unclear. In particular, the effects of dietary restriction and targeted nutrient supplementation on ovarian development and antioxidant capacity in sexually maturing Japanese quail remain unexplored. This represents a significant gap in our knowledge regarding how dietary restriction and specific nutrients influence growth, ovarian follicular development and how the activity of plasma total antioxidant capacity is affected by diet, as well as its implication in reproductive maturation. This study aimed to examine the effects of feed restriction on ovarian follicle development and plasma antioxidant status (TAC) during sexual maturation in quail. Additionally, we investigated whether methionine and leucine supplementation could counteract the effects of feed restriction.
We hypothesized that feed restriction would reduce ovarian mass, follicle number, and TAC, while methionine and leucine supplementation would restore these parameters to levels comparable to unrestricted controls.
2 Materials and methods
2.1 Experimental animal, housing, experimental design and measurements
In this experiment, Japanese quails were reared in the animal house of the Institute of Animal Science, Biotechnology and Environmental Science, University of Debrecen. The basal (control) grower feed was prepared according to the National Research Council (National Research Council (NRC), 1994) recommendation for quails on a corn-wheat-soybean meal basis. At the age of 5 weeks, we selected 40 female quails with similar body mass and kept them in individual cages for one week to acclimate them to the experimental conditions, with each group consisting of 8 birds (n = 8). The cages were housed within the same controlled environment, maintaining a temperature of 23–25°C and a relative humidity of 60–65%. During the acclimation period, we allowed free access to breeder feed and water. Individual body mass changes were recorded weekly, while daily feed intake was measured with an accuracy of 0.01 g to determine the average daily feed requirement per bird for the duration of the experiment. We began the two-week experimental period when the quails reached six weeks of age. We randomly allocated individuals into five experimental groups (8 birds per group, housed individually): ad libitum as a control feed, a 20% restriction (DR20) (i.e. 80% of the average individual feed intake), a 20% feed restriction supplemented with either methionine, leucine or both, 20% above the recommended nutrient levels (DR20+Met, DR20+Leu and DR20+Leu+Met, respectively; Table 1). The 20% dietary restriction rate was determined from our recent report that showed a moderate reduction in body mass and reproductive parameters such as egg laying probability, without compromising the egg size, compared to 30% and 40% dietary restrictions, which severely reduced the body mass, egg size and egg number (Reda et al., 2024).
Table 1
| Ingredients % | Treatments | ||||
|---|---|---|---|---|---|
| Control | DR20 | DR20+Met | DR20+Leu | DR20+Met+Leu | |
| Corn | 25.00 | 25.00 | 25.00 | 25.00 | 25.00 |
| Wheat | 10.10 | 10.10 | 10.00 | 10.00 | 10.00 |
| Wheat bran | 10.00 | 10.00 | 9.96 | 9.16 | 9.02 |
| Pea | 13.02 | 13.02 | 12.90 | 12.39 | 12.30 |
| Soybean meal | 24.86 | 24.86 | 24.96 | 25.48 | 25.57 |
| Sunflower oil | 9.07 | 9.07 | 9.12 | 9.27 | 9.30 |
| Limestone | 5.83 | 5.83 | 5.83 | 5.82 | 5.82 |
| MCP | 0.96 | 0.96 | 0.96 | 0.97 | 0.97 |
| L-Lys | 0 | 0 | 0 | 0 | 0 |
| DL-Met | 0.19 | 0.19 | 0.30 | 0.19 | 0.30 |
| L-Thr | 0.07 | 0.07 | 0.07 | 0.07 | 0.07 |
| L-Leu | 0.01 | 0.01 | 0.01 | 0.39 | 0.39 |
| L-Ile | 0.005 | 0.005 | 0.005 | 0.19 | 0.19 |
| L-Val | 0.005 | 0.005 | 0.005 | 0.19 | 0.19 |
| Salt | 0.38 | 0.38 | 0.38 | 0.38 | 0.38 |
| Premixturea | 0.50 | 0.50 | 0.50 | 0.50 | 0.50 |
| Calculated nutrient content | |||||
| ME, MJ/kg | 12.13 | 12.13 | 12.13 | 12.13 | 12.13 |
| Crude protein, % | 18.00 | 18.00 | 18.00 | 18.00 | 18.00 |
| Lys, % | 1.01 | 1.01 | 1.01 | 1.02 | 1.02 |
| Met % | 0.45 | 0.45 | 0.56 | 0.45 | 0.56 |
| Thr, % | 0.74 | 0.74 | 0.74 | 0.74 | 0.74 |
| Trp, % | 0.22 | 0.22 | 0.22 | 0.22 | 0.22 |
| Leu, % | 1.42 | 1.42 | 1.42 | 1.78 | 1.78 |
| Ile, % | 0.77 | 0.77 | 0.77 | 0.95 | 0.95 |
| Val, % | 0.87 | 0.87 | 0.87 | 1.05 | 1.05 |
| Leu/Ile, ratio | 1.84 | 1.84 | 1.84 | 1.87 | 1.87 |
| Leu/Val ratio | 1.63 | 1.63 | 1.63 | 1.69 | 1.69 |
| Arg, % | 1.26 | 1.26 | 1.26 | 1.26 | 1.26 |
| Ca, % | 2.50 | 2.50 | 2.50 | 2.50 | 2.50 |
| P, % | 0.61 | 0.61 | 0.61 | 0.60 | 0.60 |
| non phytate P, % | 0.35 | 0.35 | 0.35 | 0.35 | 0.35 |
| Na, % | 0.15 | 0.15 | 0.15 | 0.15 | 0.15 |
Composition and nutrient content of the experimental feeds.
Control, Ad libitum feeding; DR20, 20% dietary restriction; DR20+Met, 20% dietary restriction supplementation with 20% methionine; DR20+Leu, 20% dietary feed restriction supplementation with 20% leucine; DR20+Met+Leu, 20% dietary restriction supplementation with 20% methionine and 20 leucine. Leucine was supplemented together with isoleucine and valine as BCAA in a ratio of 2:1:1, respectively. aThe premix provided the following per kilogram of complete diet: 5000 NE vitamin A, 1000 NE vitamin D3, 24.5 mg/kg vitamin E, 1 mg vitamin K3, 0.75 mg vitamin B1, 2.5 mg vitamin B2, 6 mg Ca-d-Pantothetane, 2 mg vitamin B6, 10 ug vitamin B12, 55 µg biotin, 12.5 mg niacin, 0.3 mg folic acid, 1500 mg choline chloride, 66 mg Zn, 9.6 mg Cu, 48.1 mg Fe, 66 mg Mn, 0.9 mg I, 0.21 mg Se, 60 µg Co.
2.2 Measurements
At the end of the experimental period (8 weeks old), we measured live body mass using a digital scale with 0.01 g accuracy (Avantor, Radnor, PA, USA). Blood samples were collected into the capillary tubes by punching the wing vein and transferred to the Eppendorf tubes. Then, blood samples were immediately centrifuged for 10 minutes at 10000g, the plasma was separated and stored at –80°C for further laboratory analysis. After blood collection, the birds were sacrificed by cervical dislocation and collecting ovaries, then measured ovary mass using a digital balance (0.01 g accuracy) and first (F1), second (F2) and third (F3) hierarchical follicle mass (0.01 g accuracy), follicle diameter to the nearest mm using a digital vernier calliper (0.1 mm accuracy) and follicle number. Each treatment group consisted of 8 birds (n = 8); however, the number of each hierarchical follicle (F1, F2 and F3) analyzed were not necessarily equal to the number of birds in each group, as birds can have different numbers of hierarchical follicles at a given time (Ma et al., 2024) (Figure 1). In cases where multiple follicles were at similar development stages, they were all considered in the analysis rather than limiting the count to one follicle per bird. To account for the variability of the follicle number, we identified a type of follicle based on the follicle mass and diameter as described (Arora and Samples, 2011; Sreesujatha et al., 2016). To determine the reproductive investment, we computed the female ovary index of each bird in the experimental group (Kimario et al., 2020; Jiang et al., 2022). The ovary index is a proportional ovary mass expressed as a percentage of total body mass calculated using the formula described previously (Jiang et al., 2022).
Figure 1
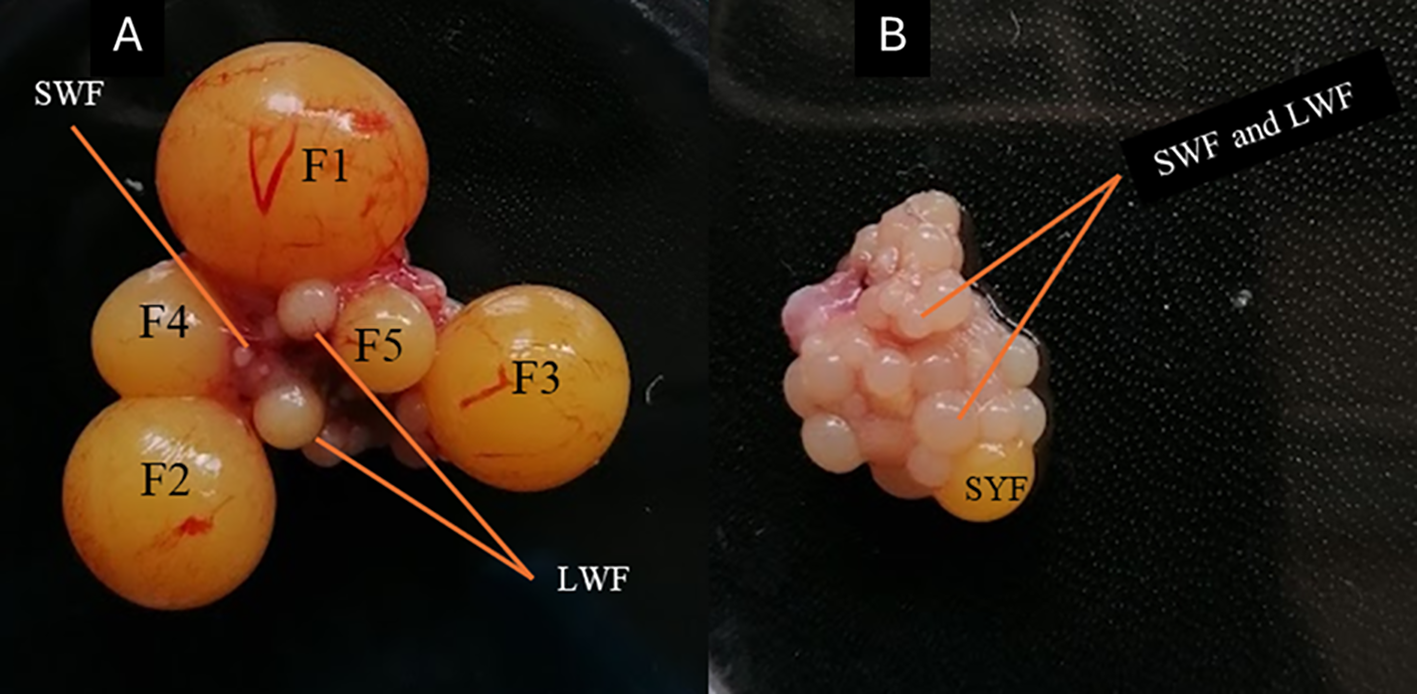
Representative image of the follicles of Japanese quails at 8 weeks old; (A) Showing the hierarchy of follicles, F1-largest follicle to F5-smallest follicle (B) Without developed hierarchical follicles. LWF-large white follicles, SWF-small white follicles, SYF-small yellow follicles of Japanese quails at 8 weeks.
2.3 Total antioxidant capacity analyses
The total antioxidant capacity (TAC) of quail plasma samples (n = 7, for each group) was analyzed using the Antioxidant Assay Kit (Sigma-Aldrich, Merck, KGaA, Darmstadt, Germany) following the manufacturer’s instructions. The absorbance of standards and samples was measured in duplicate at 570 nm using a microplate reader (Synergy HT Multi-Mode Microplate Reader, BioTek Instruments Inc., Winooski, USA). The concentrations of the TAC were calculated as Trolox equivalent antioxidant capacity (TEAC) and expressed in millimole Trolox equivalent per volume (mmol Trolox equivalents/L).
2.4 Statistical analyses
All statistical analyses were performed using R in the RStudio version 4.3.3 ‘Angel Food Cake’ (http://www.r-project.org/). Graphs (images) were visualized using the ggplot function provided by the ‘ggplot2’ package version 3.4.3 (Vaida and Blanchard, 2005). We used corrected Akaike’s information criterion (AICc) for small sample sizes for model selection (Guthery et al., 2003). We follow a forward model selection procedure beginning with a null model. Other variables were then added at a time as fixed effect and compared using AICc to identify the appropriate model. Accordingly, we compared the linear additive model, and linear interaction model by observing and selecting the lower AIC when analyzing ovary mass, ovary index and follicles. We analyzed the effects of amino acid supplementation on top of dietary restriction on final body mass, growth rate, number of hierarchical follicles and TAC with the function lm using a linear model (Searle, 1997). To analyze the effects of treatment on final body mass, we employed linear model while controlling for the initial body mass. Similarly, we used linear additive model analyze the effects of treatment on ovary mass, ovary index, follicle mass and follicle diameter while controlling for the initial body mass. To analyze the effects of dietary restriction and amino acid supplementation on ovary mass, ovary index, hierarchical follicle mass and diameter as response variables, we used a linear additive model while controlling for the final body mass (Searle et al., 1980). A one-way Analysis of Variance (ANOVA) was used to assess the statistical significance among the treatment groups on final body mass, growth rate, number of ovaries and TAC. A two-way ANOVA was used to determine the statistical difference among the treatment groups on ovary mass, ovary index, hierarchical follicle mass, follicle diameter, and follicle number. The Tukey multiple comparison test was used to compare the means between the treatment groups at p < 0.05.
3 Results
3.1 Body mass and growth rate
The initial body mass was similar among the treatment groups (F4,33 = 0.287, p = 0.884, Table 2). Dietary restriction treatment significantly reduced the final body mass (t = 3.989, 95% CI: 11.16, 69.42, p < 0.003, Table 2). However, supplementation of leucine, methionine and their interaction on top of restricted feeding improved the final body mass similar to the control group (DR20+Leu: t = 2.088, 95% CI: −8.33, 51.97, p = 0.249; DR20+Met: t = 2.171, 95% CI: −7.204, 51.05, p = 0.216; DR20+Leu+Met: t = −1.980, 95% CI: −9.46, 50.85, p = 0.298, Table 2). The multiple comparisons showed no significant differences between amino acid supplemented and the restricted groups in final body mass (p > 0.05 for all, Table 2). Growth rate was affected by dietary treatments (Table 2). The dietary restriction reduced growth rate compared to the control group (t = 6.161, 95% CI: 1.46, 4.03, p < 0.001; Table 2). Supplementing methionine or leucine individually or in combination with a restricted diet slightly reduced restriction effects on growth rate, but remained below the control group (DR20+Met: t = 4.394, 95% CI: 0.67, 3.25, p = 0.001; DR20+Leu: t = 4.501, 95% CI: 0.76, 3.41, p < 0.001; DR20+Leu+Met: t = 3.143, 95% CI: 0.12, 2.781, p = 0.027, Table 2). All other pairwise comparisons were not significant (p > 0.05 for all, Table 2).
Table 2
| Parameters | Treatments | P-value | ||||
|---|---|---|---|---|---|---|
| Control | DR20 | DR20+Leu | DR20+Met | DR20+Leu+Met | ||
| Initial body mass (g) | 233.98 ± 7.78 | 232.14 ± 10.04 | 241.24 ± 5.99 | 239.48 ± 5.25 | 233.59 ± 5.61 | 0.884 |
| Final body mass (g) | 265.94 ± 9.34a | 225.65 ± 7.15b | 244.11 ± 6.97ab | 244.01 ± 5.47ab | 245.24 ± 5.95ab | 0.009 |
| Growth rate (g/day) | 2.28 ± 0.38a | −0.46 ± 0.38c | 0.21 ± 0.37bc | 0.32 ± 0.15bc | 0.83 ± 0.22bc | <0.001 |
Comparative effects of amino acid supplementation on initial body mass, final body mass, change in body mass and growth rate in Japanese quails reared on restricted feeding.
Values are presented in mean ± standard error of the mean (SE) from sample size n = 8 for each treatment group. Means lacking a common superscript in a row differ significantly (p < 0.05). Control:- Ad libitum feeding; DR20:- 20% dietary restriction; DR20+Met:- 20% dietary restriction supplementation with 20% methionine; DR20+Leu: 20% dietary restriction supplementation with 20% leucine; DR20+Met+Leu: 20% dietary restriction supplementation with 20% methionine and 20% leucine.
3.2 Ovary mass and index
Dietary restriction significantly reduced ovary mass compared to the controls (t = 2.624, 95% CI: 0.25, 5.32, p = 0.013; Figure 2A). The supplementation of leucine (either alone or in combination with methionine) significantly increased ovary mass relative to the restricted group (DR20+Leu: t = −2.913, 95% CI: −5.80, −0.02, p = 0.047, DR20+Leu+Met: t = −2.939, 95% CI: −5.83, −0.05, p = 0.045) and improved ovary mass from dietary restricted similar to control levels (DR20+Leu: t = −0.378, 95% CI: −3.266, 2.51, p = 0.995, DR20+Leu+Met: t = −0.404, 95% CI: −3.29, 2.48, p = 0.994, Figure 2A). Dietary restriction reduced the ovary index compared to the control group (DR20: t = −1.761, 95% CI: 0.45, 1.84, p = 0.04; Figure 2B). The supplementation of leucine either alone or combined with methionine, tended to increase ovary index compared to the restricted group (DR20+Leu: t = −2.652, 95% CI: −2.27, 0.10, p = 0.085, DR20+Met: t = −1.224, 95% CI: −1.63, 0.66, p = 0.738, DR20+Leu+Met: t = −2.614, 95% CI: −2.26, 0.11, p = 0.092), bringing it to a level statistically similar to the control group (DR20+Leu: t = −0.951, 95% CI: −1.58, 0.80, p = 0.875; DR20+Met: t = −0.537, 95% CI: −0.93, 1.36, p = 0.383; DR20+Leu+Met: t = −0.913, 95% CI: −1.161, 81, p = 0.889, Figure 2B).Hierarchical follicle mass, diameter, and follicle counts.
Figure 2
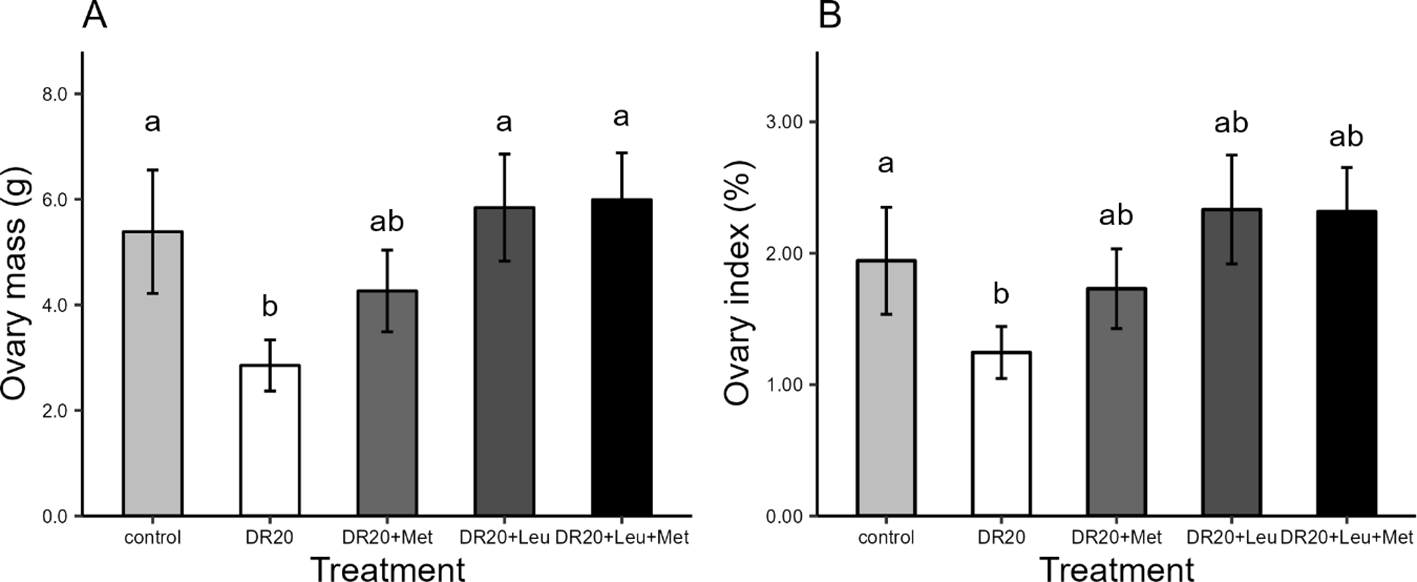
Effects of dietary treatments on (A) Ovary mass (B) Ovary index of Japanese quails at the age of 8 weeks. Control: ad libitum feeding; DR20: 20% dietary feed restriction; DR20+Met: 20% dietary restriction supplemented with 20% methionine; DR20+Leu: 20% dietary restriction supplemented with 20% leucine; and DR20+Met+Leu: 20% dietary feed restriction supplemented with both 20% methionine and 20% leucine. Bars represent the mean ± standard error (SE) from 8 birds in each treatment group. Different letters above bars indicate statistically significant differences among groups (p < 0.05). Dietary restriction reduced ovary mass and index, but supplementation with leucine and methionine improved both to control levels.
Dietary restriction had no significant effect on the mass or diameter of F2 and F3 hierarchical follicles compared to the control group (p > 0.05 for all treatments; Table 3), but decreased F1 diameter (p = 0.035). Supplementation with leucine, methionine, or their combination did not significantly alter follicle mass relative to the restricted group (p > 0.05 for all treatments; Table 3) and diameter of F2 F3 follicles compared to the restricted group (p > 0.05 for all). However, a combination of methionine and leucine significantly increased the diameter of F1 follicles compared to the restricted group (p = 0.033). The average number of hierarchical follicles (F1, F2, and F3) responded significantly to dietary treatment (Figure 3). Dietary restriction and leucine supplementation alone did not significantly differ from the control group (DR20: t = −0.913, p = 0.885; DR20+Leu: t = 0.00, p = 1.000). In contrast, methionine supplementation, either alone or combined with leucine, increased follicle numbers compared to the restricted group (DR20+Met: t = −8.216, p < 0.001; DR20+Leu+Met: t = −6.390, p < 0.001, Figure 3). Furthermore, both the methionine-supplemented and combined supplementation groups had significantly more follicles than the leucine-only group (DR20+Met: t = −7.303, p < 0.001, DR20+Met+Leu: t = −9.129, p < 0.001, Figure 3). However, the two methionine-supplemented groups had a similar number of follicles (t = 1.826, p = 0.411, Figure 3).
Table 3
| Variables | Follicles | Treatments | |||||
|---|---|---|---|---|---|---|---|
| Control | DR20 | DR20+Met | DR20+Leu | DR20+Leu+Met | P-value | ||
| Follicle mass(g) | F1 | 2.690 ± 0.10 (n=4) | 1.996 ± 0.07 (n=8) | 2.157 ± 0.25 (n=5) | 2.462 ± 0.18 (n=5) | 2.548 ± 0.26 (n=7) | 0.115 |
| F2 | 1.610 ± 0.09 (n=5) | 1.122 ± 0.11 (n=5) | 1.033 ± 0.24 (n=7) | 1.464 ± 0.19 (n=8) | 1.590 ± 0.24 (n=8) | 0.064 | |
| F3 | 0.575 ± 0.9 (n=5) | 0.356 ± 0.07 (n=5) | 0.351 ± 0.11 (n=8) | 0.640 ± 0.04 (n=4) | 0.589 ± 0.18 (n=8) | 0.333 | |
| Follicle diameter (mm) | F1 | 18.240 ± 0.34a (n=4) | 14.874 ± 0.23b (n=8) | 16.181 ± 0.92ab (n=5) | 17.420 ± 0.67ab (n=5) | 17.696 ± 0.74a (n=7) | 0.011 |
| F2 | 14.895 ± 0.42 (n=5) | 12.996 ± 0.28 (n=5) | 12.190 ± 1.07 (n=7) | 14.170 ± 0.71 (n=8) | 14.622 ± 0.83 (n=8) | 0.071 | |
| F3 | 9.920 ± 0.55 (n=5) | 8.858 ± 0.13 (n=5) | 8.644 ± 0.99 (n=8) | 10.853 ± 0.30 (n=4) | 10.660 ± 0.72 (n=8) | 0.169 | |
Comparative effects of amino acid supplementation on follicle mass and follicle diameter of Japanese quails at 8-week age during dietary restriction.
Values are presented in mean ± standard error of the mean (SE). Means lacking a common superscript in a row differ significantly (p < 0.05). Control, Ad libitum feeding; DR20, 20% dietary restriction; DR20+Met, 20% dietary restriction supplementation with 20% methionine; DR20+Leu, 20% dietary restriction supplementation with 20% leucine; DR20+Met+Leu, 20% dietary restriction supplementation with 20% methionine and 20% leucine.
Figure 3

The effects of dietary treatments on average number of hierarchical follicles in each treatment of Japanese quails at the age of 8 weeks. Control: Ad libitum feeding; DR20: 20% dietary restriction; DR20+Met: 20% dietary restriction supplemented with 20% methionine, DR20+Leu:20% dietary restriction supplemented with 20% leucine and DR20+Met+Leu: 20% dietary restriction supplemented with 20% methionine and 20% leucine. Bars represent the mean ± standard error (SE) from 8 birds in each treatment group. Different letters above bars indicate statistically significant differences among groups (p < 0.05). Methionine supplementation increased follicle numbers compared to the control and restricted group. The highest count was in the combined supplementation of methionine and leucine.
3.3 Plasma total antioxidant capacity
Dietary treatments affected total antioxidant capacity levels similarly to body mass (Figure 4). Dietary restriction significantly reduced antioxidant capacity compared to the control group (t = 2.838, 95% CI: 0.02, 1.46, p = 0.009; Figure 4). However, supplementation with methionine, leucine individually or their combination improved the total antioxidant capacity levels similar to the control group (DR20+Leu: t = −1.297, 95% CI: −0.46, 1.17, p = 0.573; DR20+Met: t = −1.741, 95% CI: −0.47, 1.02, p = 0.323; DR20+Leu+Met: = −1.094, 95% CI: −0.34, 1.20, p = 0.696; Figure 4). In contrast, none of the amino acid supplementation within a restricted diet showed a significant difference from the restricted group (p > 0.05 for all, Figure 4).
Figure 4
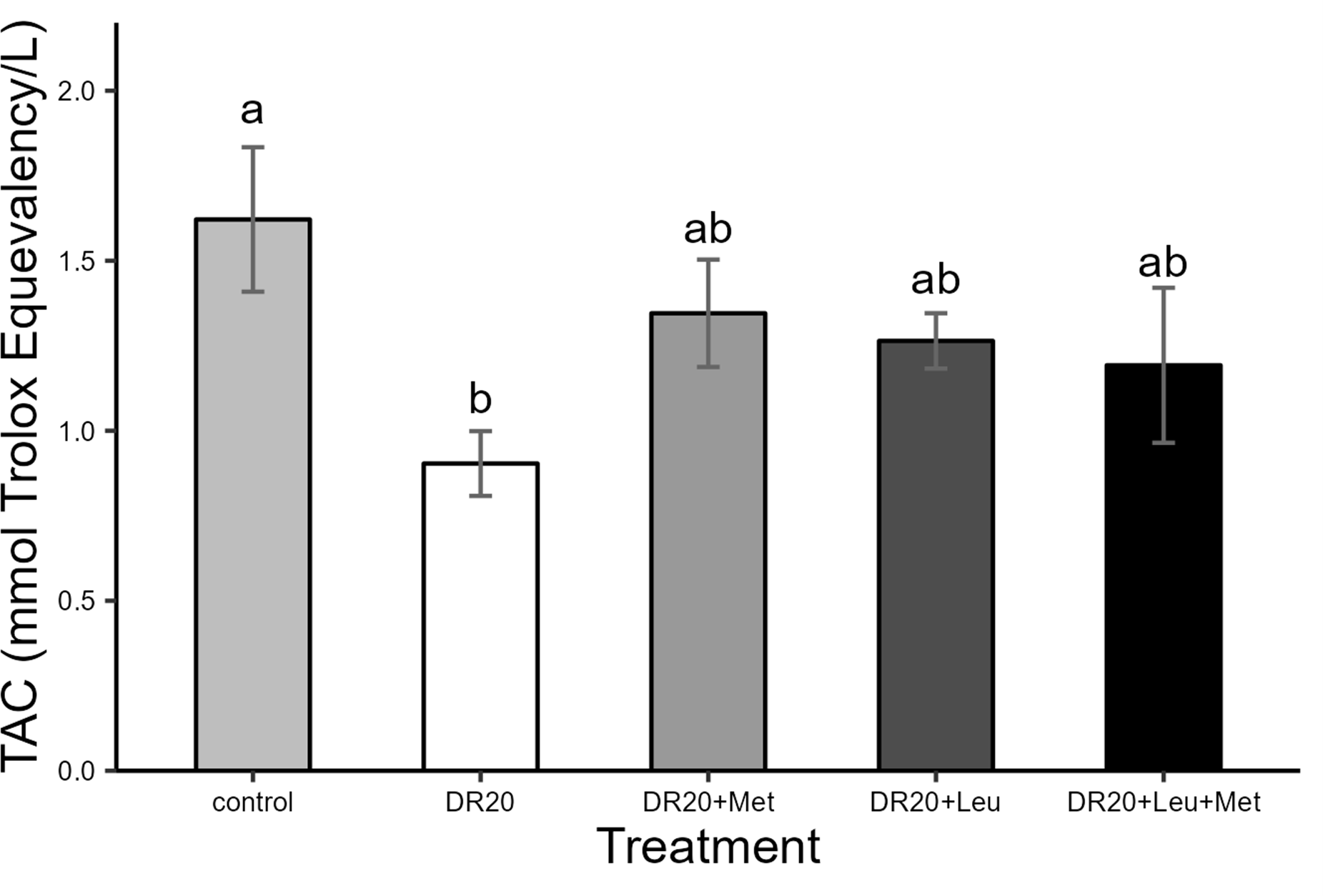
Effects of dietary treatments on total antioxidant capacity (TAC) in Japanese quails at the age of 8 weeks. Control: Ad libitum feeding; DR20: 20% dietary restriction; DR20+Met: 20% dietary restriction supplemented with 20% methionine, DR20+Leu:20% dietary restriction supplemented with 20% leucine and DR20+Met+Leu: 20% dietary restriction supplemented with 20% methionine and 20% leucine. Bars represent the mean ± standard error of the mean (SE) from the sample size (n = 7 for each group). Different letters above bars indicate statistically significant differences among groups. (p < 0.05). Supplementation with leucine and methionine alone was effective in improving the TAC levels of the dietary-restricted group to levels comparable to those of the control.
3.4 Association between somatic and reproductive variables and plasma total antioxidant capacity
Regardless of the treatment, all parameters were significantly correlated with plasma total antioxidant capacity (TAC) (final body mass: t = 2.859, 95% CI: 3.09, 20.77, R2 = 0.214, p < 0.01; growth rate: t = 2.817, 95% CI: 43.49, 356.65, R2 = 0.209; p = 0.0148; ovary mass: t = 2.285, 95% CI: 0.01, 0.13, R2 = 0.148, p = 0.029; Figure 5), except ovary index which showed marginal correlation (t = 1.997, 95% CI: −0.004, 0.345, R2 = 0.148, p = 0.055). There was no significant correlation between dietary treatment and follicle measurements, mass, and diameter (p > 0.05 for all).
Figure 5
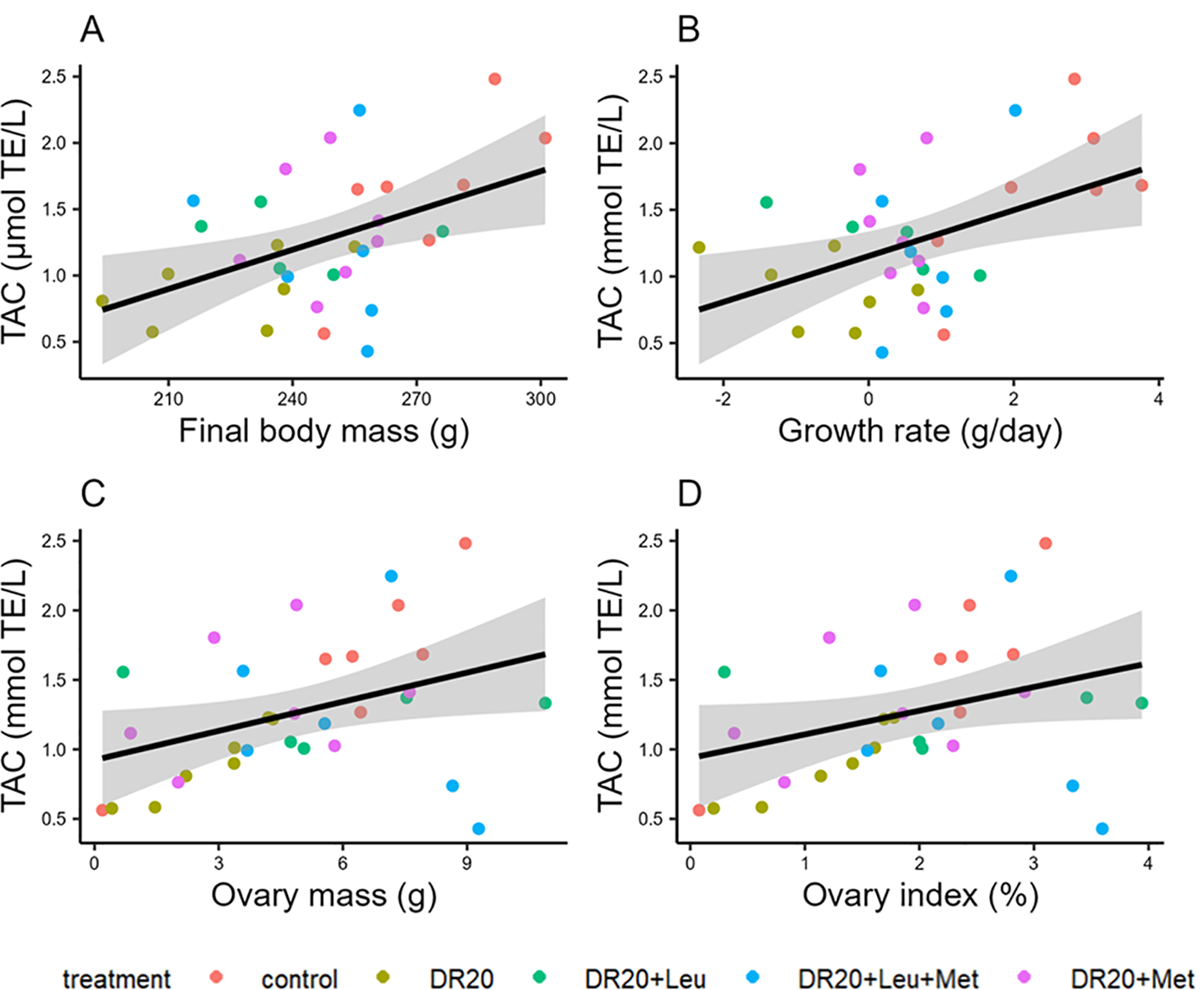
The association between total antioxidant capacity (TAC) with (A) final body mass, (B) growth rate, (C) ovary mass and (D) ovary index. Colors indicate the treatments. Abbreviations: millimole Trolox equivalent per volume (mmol TE/L).
4 Discussion
Growth and sexual maturation are essential stages in animals as they both determine the timing of reproduction, which has a pivotal contribution to fitness. Several factors, such as environmental conditions (e.g. light intensity, temperature), age, genetics, nutrition, and body condition, influence the growth and onset of sexual maturation in avian species (Reddish et al., 2003; Widowski et al., 2022). Nutritional status, in particular, plays a significant role in enhancing growth and development, with long-lasting consequences on reproduction (Renema et al., 2007; Yin et al., 2023). Nutritional challenges may induce developmental stress, impacting reproductive investment and the development of reproductive organs at the onset of sexual maturation (Lu et al., 2021; Hargitai et al., 2022). However, the importance of specific nutrients, such as amino acids, in shaping avian growth patterns and influencing reproductive maturation has recently received increased attention (Alagawany et al., 2021; Ndunguru et al., 2024a, b).
In this study, we used dietary restrictions to represent a nutritional challenge and supplemented amino acids during the restriction, expecting amino acid supplementation could mitigate the effects of dietary restriction. We found that 20% dietary restriction reduced body mass and growth rate compared to the ad libitum individuals (Table 2). These results indicate that nutrient status can positively influence the body mass increase and the development of reproductive structures. We have recently shown in 8-week-old Japanese quails that 20% feed restriction led to moderate weight loss accompanied by reduced egg mass, egg number and laying probability (Reda et al., 2024). Consistently, the lowest body weight gain was observed from two weeks 12 hours daily feed-restricted chicken compared to that of the ad libitum control (Gobane et al., 2021), similar to other studies applying 20% (Van Der Klein et al., 2018; Lu et al., 2021) or 10% feed restriction (Urdaneta-Rincon and Leeson, 2002).
However, contrasting results have also been reported. For example, a 20% dietary restriction in broiler chickens did not affect body mass (Butzen et al., 2013), while an alternating feed restriction combined with ad libitum refeeding even increased body mass (Tesfaye et al., 2009). Similarly, another study showed that 30% feed restriction from 14 to 21 days of age in chickens increased body mass gain by 7% to 15% up to slaughter age indicating the compensatory effects, but the final body mass decreased in males by 10% and in females by 16% compared to ad libitum-fed chickens (Tůmová et al., 2022). At an early age (1 to 4 or 8 days), gradient feed restriction (10% and 20%) in chicken showed reduced body mass during the short restriction period compared to ad libitum with pronounced growth retardation observed in 20% feed restriction (Lippens et al., 2000). Lippens et al. reported that, on reaching 42 days, normal feeding resulted in a similar body mass to that of those subjected to 10% feed restriction, compared to ad libitum. However, a 20% feed restriction resulted in birds having significantly lower body mass, with males being heavier than females at 42 days old (Lippens et al., 2000). Another study showed that one week 12 hours daily of feed restriction compensated and reached the same body mass as the ad libitum birds at weeks 5 and 6 of age (Gobane et al., 2021). These contradictory results show that to differences in bird species/strains, age or restriction timing, sex-specific influence on feed restriction (Lippens et al., 2000), and severity of restriction may influence the restriction effects. Supplementing dietary-restricted individuals with methionine and leucine individually or in combination reduced the effects of dietary restriction, slightly increased body mass, and improved growth rate to similar to the control group (Table 2). Essential amino acids are well-known for their role in protein synthesis and are vital for supporting growth (Zhang et al., 2020). Previously, we showed in Japanese quails that embryonic supplementation of methionine or leucine increased postnatal growth and development (Ndunguru et al., 2024a, b). The finding suggests that leucine and methionine supplementation counteracted the growth limitations associated with dietary restrictions by enhancing protein synthesis. The increasing final body mass and growth rate collaborate with the previous report demonstrating the anabolic effects of the specific essential amino acids in animals (Berrazaga et al., 2019). Furthermore, methionine and leucine are critical in supporting the metabolism that regulates protein synthesis and energy balance, which explains the growth-promoting effects (Babazadeh and Ahmadi Simab, 2022; Rehman et al., 2023). Supplementing these amino acids could be a strategy to improve growth performance in nutritionally challenged individuals.
Dietary restriction during sexual maturation decreased ovary mass and ovary index. Nutritional status regulates the critical, highly demanding sexual maturation stage by influencing the development of reproductive organs and improving their function (Rizzoto et al., 2019; Yin et al., 2023). The growth and development of sexual organs occur rapidly closer to the sexual maturation stage (Cui et al., 2020; Shi et al., 2020). Adequate nutrient and energy intake is essential for gonadal development, which is important for synthesizing the reproductive hormones prior to sexual maturation (Yang et al., 2024). Restricted diets are known to reduce ovary development and reproductive performance in birds (Lu et al., 2023; Pan et al., 2014; Renema et al., 1995; Yin et al., 2023, but see Bruggeman et al., 1999; McGovern et al., 1997). Our study revealed that supplementing leucine and methionine or their combination within a restricted diet improved ovary growth by increasing ovary mass compared to the dietary-restricted group. Similarly, the ovary index increased to the level of the control group, but did not significantly differ from the dietary restricted group. Previous studies have shown that amino acid supplementation, such as methionine and valine, improved oviduct and follicle development in quails and chickens (Bunchasak and Silapasorn, 2005; Hanafy and Attia, 2018), a key indicator of reproductive organ development and maturation (Jiang et al., 2022; Li et al., 2022). The observed increase in ovary mass and index following amino acid supplementation suggests a compensatory reproductive investment that mitigates the effects of dietary restriction. The reproductive performance of laying Japanese quail and chicken is greatly influenced by the availability of essential nutrients in their diet and the appropriate development of their ovaries (Ma et al., 2020; Santana et al., 2025). Providing a balanced and nutritious diet during sexual is vital for maximizing their reproductive efficiency. A critical component of poultry nutrition is formulating diets that achieve optimal amino acid ratios (Adhikari et al., 2025). Methionine, the first-limiting amino acid in birds, serves as a sulfur donor and plays essential roles in protein synthesis, cellular differentiation and proliferation (Zhang et al., 2020). In laying Japanese quail, methionine supplementation did not increase follicle number but increased follicle diameter (Santana et al., 2025).
Dietary restriction did not affect the number or size (weight and diameter) of the hierarchical follicles, except F1 follicle diameter, which decreased with dietary restriction. In poultry, selection for rapid growth has been linked to increased follicle numbers (Mfoundou et al., 2021), while nutritional status plays a key role in follicular development (Eitan and Soller, 2009). Overfeeding during sexual maturation results in excessive follicular growth and ovulatory disruption, whereas moderate feed restriction may help regulate follicle development (Renema et al., 1995; Diaz and Anthony, 2013). However, findings are inconsistent, with some studies reporting fewer yellow follicles under dietary restriction (Lu et al., 2023; Gholami-Soltanmoradi et al., 2024).
In our study, methionine supplementation, alone or with leucine, increased the number of follicles without altering the follicle size, whereas leucine alone did not. While the supplementing combination of methionine and leucine increased F1 follicle diameter. A critical component of poultry nutrition is formulating diets that achieve optimal amino acid ratios (Adhikari et al., 2025). Methionine, the first-limiting amino acid in birds, serves as a sulfur donor and plays essential roles in protein synthesis, and cellular differentiation and proliferation (Zhang et al., 2020). In laying Japanese quail, methionine supplementation did not increase follicle number but increased follicle diameter (Santana et al., 2025). These results suggest that different amino acids may differentially influence ovarian follicular differentiation and growth, possibly through distinct metabolic pathways. For instance, methionine plays a role in primordial germ cell commitment via the methionine cycle and epigenetic modifications (Zhang et al., 2020), while leucine supports cell proliferation and metabolism through the mTOR pathway (Correia et al., 2022). These results suggest and highlight potential nutrient-specific effects on hierarchical follicular development, though further research is needed to clarify the underlying mechanisms.
Feed restriction reduced the plasma total antioxidant capacity, but supplementation with leucine, methionine or their combination ameliorated the negative effects of dietary restriction, leading to values statistically comparable to control levels. Both amino acids have a more substantial oxidative capacity with a significant function in providing oxidative energy through the regulation of protein metabolism (Zhang et al., 2024). Oxidative stress negatively impacts ovarian and follicular development in poultry (Wang et al., 2021), and increased antioxidant activity may help mitigate oxidative stress during growth and sexual maturation (Oke et al., 2024). Regardless of the dietary treatment, the plasma total antioxidant capacity levels correlated with most measured parameters except follicles, suggesting that birds maintained the ability to neutralize free radicals and other reactive species molecules despite an energetic deficit. Nevertheless, methionine and leucine together provided the greatest antioxidant boost, highlighting their potential to mitigate nutritional stress. Methionine serves as a precursor to cysteine, which is a rate-limiting substrate for the synthesis of glutathione (GSH) (Lugata et al., 2022). Glutathione is one of the most potent intracellular antioxidants that play a central role in maintaining a redox balance and protecting the cell from oxidative damage (Hassanpour et al., 2024). The methionine intake has shown an increased hepatic and systemic GSH level in broiler chicken, enhancing the total antioxidant capacity (Hassanpour et al., 2024), while methionine restriction reduced the GSH levels (Tamanna et al., 2019). In addition, leucine modulates the mTOR pathway, which is involved in cellular antioxidant responses (Rehman et al., 2023). Nutritional activation of mTOR promotes mitochondrial biogenesis and elevates the antioxidant defenses such as superoxide dismutase (SOD), contributing to the improve total antioxidant activity (Tsang et al., 2018). The mechanisms may underline the correlation observed in the present and warrant further investigation.
5 Conclusion
The study highlights the critical role of dietary composition, particularly amino acids, in mitigating the effects of dietary restriction on growth and reproductive maturation. Supplementing amino acids along with a restricted diet mitigated the adverse effects of dietary restriction, improving growth and ovarian development during sexual maturation. While dietary restriction alone did not affect hierarchical follicle numbers, methionine supplementation increased follicle counts, suggesting its specific role in follicular differentiation. These findings emphasize the importance of optimizing dietary composition to manage developmental stress and enhance reproductive potential in poultry. Future research could explore the broader implications of amino acid balance across different avian species and investigate underlying metabolic and endocrine mechanisms shaping reproductive maturation under nutritional constraints.
Statements
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Ethics statement
Experiments were approved by the Institutional Committee of Animal Welfare permit number 5/2021/DEMAB. We carried out the trial at the experimental farm of the University of Debrecen, Farm and Regional Research Institute, Kismacs, Hungary. The study was conducted in accordance with the local legislation and institutional requirements.
Author contributions
SN: Formal analysis, Writing – review & editing, Conceptualization, Methodology, Writing – original draft, Visualization, Investigation, Validation, Data curation. GR: Conceptualization, Writing – review & editing, Methodology, Formal analysis. BC: Writing – review & editing, Methodology, Resources. GG: Writing – review & editing, Methodology, Resources. RK: Writing – review & editing, Methodology. CS: Resources, Methodology, Writing – review & editing. ÁL: Formal analysis, Resources, Visualization, Data curation, Project administration, Validation, Methodology, Writing – review & editing, Funding acquisition, Supervision, Conceptualization, Investigation. LC: Validation, Writing – review & editing, Funding acquisition, Resources, Supervision, Project administration, Conceptualization.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. The National Development Research and Innovation Office (OTKA K139021) funded the study to Ádám Z. Lendvai and Levente Czeglédi. Sawadi F. Ndunguru and Gebrehaweria K. Reda received a Dissertation grant from the Tempus Public Foundation for Ph.D. studies. We greatly appreciate the support from the University of Debrecen Program for Scientific Publication.
Acknowledgments
We express our sincere gratitude to Danny Wadday, Doha Khalifeh and Eszter Czeglédi for their support and participation during sample collection. We also thank the farm managers and staff at the Institute of Agricultural Research and Educational Farm of the University of Debrecen for their logistic support during the research period.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fanim.2025.1622877/full#supplementary-material
References
1
Abdul-RahmanI. I.JeffcoateI.ObeseF. Y. (2018). Age-related changes in the gross anatomy of the reproductive organs, and associated steroid hormone profiles in male and female Guinea fowls (Numida meleagris). Veterinary Anim. Sci.6, 41–49. doi: 10.1016/j.vas.2018.07.003
2
AdhikariR.RochellS. J.KriseldiR.SilvaM.GreinerL.WilliamsC.et al. (2025). Recent advances in protein and amino acid nutritional dynamics in relation to performance, health, welfare, and cost of production. Poultry Sci.104, 104852. doi: 10.1016/j.psj.2025.104852
3
AfrouziyehM.ZukiwskyN. M.ZuidhofM. J. (2021). Timing of growth affected broiler breeder feeding motivation and reproductive traits. Poultry Sci.100, 101375. doi: 10.1016/j.psj.2021.101375
4
AlagawanyM.ElnesrS. S.FaragM. R.TiwariR.YatooMohd.I.et al. (2021). Nutritional significance of amino acids, vitamins and minerals as nutraceuticals in poultry production and health – a comprehensive review. Veterinary Q.41, 1–29. doi: 10.1080/01652176.2020.1857887
5
AlanR. R.McWilliamsS. R. (2013). Oxidative stress, circulating antioxidants, and dietary preferences in songbirds. Comp. Biochem. Physiol. Part B: Biochem. Mol. Biol.164, 185–193. doi: 10.1016/j.cbpb.2012.12.005
6
AroraK. L.SamplesO. (2011). Role of body weight on reproductive and physiological traits in Japanese quail layers (Coturnix japonica). Int. J. Poultry Sci.10, 640–643. doi: 10.3923/ijps.2011.640.643
7
BabazadehD.Ahmadi SimabP. (2022). Methionine in poultry nutrition: A review. J. World’s Poultry Sci.1, 1–11. doi: 10.58803/jwps.v1i1.1
8
BacouE.WalkC.RiderS.LittaG.Perez-CalvoE. (2021). Dietary oxidative distress: A review of nutritional challenges as models for poultry, swine and fish. Antioxidants10, 525. doi: 10.3390/antiox10040525
9
BerrazagaI.MicardV.GueugneauM.WalrandS. (2019). The role of the anabolic properties of plant- versus animal-based protein sources in supporting muscle mass maintenance: A critical review. Nutrients11, 1825. doi: 10.3390/nu11081825
10
BeskiS. S. M.SwickR. A.IjiP. A. (2015). Specialized protein products in broiler chicken nutrition: A review. Anim. Nutr.1, 47–53. doi: 10.1016/j.aninu.2015.05.005
11
BruggemanV.OnagbesanO.D’HondtE.BuysN.SafiM.VanmontfortD.et al. (1999). Effects of timing and duration of feed restriction during rearing on reproductive characteristics in broiler breeder females. Poultry Sci.78, 1424–1434. doi: 10.1093/ps/78.10.1424
12
BunchasakC.SilapasornT. (2005). Effects of adding methionine in low-protein diet on production performance, reproductive organs and chemical liver composition of laying hens under tropical conditions. Int. J. Poultry Sci.4, 301–308. doi: 10.3923/ijps.2005.301.308
13
ButzenF. M.RibeiroA. M. L.VieiraM. M.KesslerA. M.DadaltJ. C.DellaM. P. (2013). Early feed restriction in broilers. I–Performance, body fraction weights, and meat quality. J. Appl. Poultry Res.22, 251–259. doi: 10.3382/japr.2012-00639
14
CorreiaB.SousaM. I.BrancoA. F.RodriguesA. S.Ramalho-SantosJ. (2022). Leucine and arginine availability modulate mouse embryonic stem cell proliferation and metabolism. Int. J. Mol. Sci.23, 14286. doi: 10.3390/ijms232214286
15
CuiZ.AmevorF. K.FengQ.KangX.SongW.ZhuQ.et al. (2020). Sexual maturity promotes yolk precursor synthesis and follicle development in hens via liver-blood-ovary signal axis. Animals10, 2348. doi: 10.3390/ani10122348
16
DesbruslaisA.WealleansA. (2022). Oxidation in poultry feed: impact on the bird and the efficacy of dietary antioxidant mitigation strategies. Poultry1, 246–277. doi: 10.3390/poultry1040022
17
DiazF. J.AnthonyK. (2013). Feed restriction inhibits early follicular development in young broiler-breeder hens. Anim. Reprod.10, 79–87.
18
EitanY.SollerM. (2009). Problems associated with broiler breeder entry into lay: A review and hypothesis. World’s Poultry Sci. J.65, 641–648. doi: 10.1017/S0043933909000452
19
Gholami-SoltanmoradiM.SolkaM.MarchewkaJ.SeidaviA.CooperR. G.DadashbeikiM.et al. (2024). Feeding frequency can affect the morphology of reproductive tract in broiler breeder hens. Poultry Sci.103, 104307. doi: 10.1016/j.psj.2024.104307
20
GobaneZ.GoniS.ChikwandaD.ZhouL. (2021). The effect of quantitative feed restriction duration on growth performance and carcass characteristics of broiler chickens. Open J. Anim. Sci.11, 635–645. doi: 10.4236/ojas.2021.114043
21
GutheryF. S.BurnhamK. P.AndersonD. R. (2003). Model selection and multimodel inference: A practical information-theoretic approach. J. Wildlife Manage.67, 655. doi: 10.2307/3802723
22
HanafyA. M.AttiaF. A. M. (2018). Productive and reproductive responses of breeder Japanese quails to different dietary crude protein and l-valine levels. Egyptian Poultry Sci. J.38, 735–753. doi: 10.21608/epsj.2018.17102
23
HargitaiR.BorossN.TóthZ.LendvaiÁ.Z. (2022). Food restriction delays breeding and affects insulin-like growth factor-1, oxidative damage and haematocrit value before egg-laying in female canaries. J. Avian Biol.2022, e02866. doi: 10.1111/jav.02866
24
HassanpourH.NasiriL.FallahA. A.AhmadipourB.KaewduangtaW. (2024). Effect of dietary methionine and its analogs on oxidant/antioxidant status of blood and liver in broilers under normal condition or environmental stress: A systematic review and meta-analysis. J. Agric. Food Res.18, 101523. doi: 10.1016/j.jafr.2024.101523
25
HuberK. (2018). Invited review: Resource allocation mismatch as pathway to disproportionate growth in farm animals – prerequisite for a disturbed health. Animal12, 528–536. doi: 10.1017/S1751731117002051
26
JiangD.ZhouX.XuY.LiufuS.FuX.XuD.et al. (2022). Effects of stocking density on ovarian development and maturation during the rearing period in Shan-ma ducks. Poultry Sci.101, 101809. doi: 10.1016/j.psj.2022.101809
27
JohnsonA. L. (2014). The avian ovary and follicle development: Some comparative and practical insights. Turkish J. Veterinary Anim. Sci.38, 660–669. doi: 10.3906/vet-1405-6
28
JohnsonA. L. (2015). Ovarian follicle selection and granulosa cell differentiation. Poultry Sci.94, 781–785. doi: 10.3382/ps/peu008
29
JuanC. A.Pérez de la LastraJ. M.PlouF. J.Pérez-LebeñaE. (2021). The chemistry of reactive oxygen species (ROS) revisited: outlining their role in biological macromolecules (DNA, lipids and proteins) and induced pathologies. Int. J. Mol. Sci.22, 4642. doi: 10.3390/ijms22094642
30
KimarioE. P.JohnJ. R.PratapH. B. (2020). Gonadosomatic index infers the breeding season of the House Crow Corvus splendens in Dar es Salaam, Tanzania. Scopus: Journal of East African Ornithology40 (1), 1–6.
31
KorverD. R. (2023). Review: Current challenges in poultry nutrition, health, and welfare. Animal17, 100755. doi: 10.1016/j.animal.2023.100755
32
LiH.HouY.ChenJ.WuH.HuangL.HuJ.et al. (2022). Dietary naringin supplementation on laying performance and antioxidant capacity of Three-Yellow breeder hens during the late laying period. Poultry Sci.101, 102023. doi: 10.1016/j.psj.2022.102023
33
LippensM.RoomG.De GrooteG.DecuypereE. (2000). Early and temporary quantitative food restriction of broiler chickens. 1. Effects on performance characteristics, mortality and meat quality. Br. Poultry Sci.41, 343–354. doi: 10.1080/713654926
34
LuJ.LiY. F.QuL.MaM.YangX. D.ShenM. M.et al. (2021). Effects of energy-restricted feeding during rearing on sexual maturation and reproductive performance of Rugao layer breeders. Poultry Sci.100, 101225. doi: 10.1016/j.psj.2021.101225
35
LuJ.WangQ.WangK. H.MaM.WangX. G.GuoJ.et al. (2023). Effects of energy restriction during growing phase on the productive performance of Hyline Brown laying hens aged 6 to 72 wk. Poultry Sci.102, 102942. doi: 10.1016/j.psj.2023.102942
36
LugataJ. K.OrtegaA. D. S. V.SzabóC. (2022). The role of methionine supplementation on oxidative stress and antioxidant status of poultry-A review. Agriculture12, 1701. doi: 10.1016/j.psj.2023.103250
37
MaY.ChengB.ZhouS.WangY.JingY.LengL.et al. (2024). Comparative analyses of laying performance and follicular development characteristics between fat and lean broiler lines. Poultry Sci.103 (1), 103250. doi: 10.1016/j.psj.2023.103250
38
MaY.ZhouS.LinX.ZengW.MiY.ZhangC. (2020). Effect of dietary N-carbamylglutamate on development of ovarian follicles via enhanced angiogenesis in the chicken. Poultry Sci.99, 578–589. doi: 10.3382/ps/pez545
39
ManiamJ.MorrisM. J. (2012). The link between stress and feeding behaviour. Neuropharmacology63, 97–110. doi: 10.1016/j.neuropharm.2012.04.017
40
MavrommatisA.GiamouriE.MyrtsiE. D.EvergetisE.FilippiK.PapapostolouH.et al. (2021). Antioxidant status of broiler chickens fed diets supplemented with vinification by-products: A valorization approach. Antioxidants10, 1250. doi: 10.3390/antiox10081250
41
McGovernR. H.RenemaR. A.RobinsonF. E. (1997). Increased feed allocation does not stimulate increased ovarian development or increased egg output in 54-week-old broiler breeder hens. Can. J. Anim. Sci.77, 177–179. doi: 10.4141/A96-089
42
MfoundouJ. D. L.GuoY. J.LiuM. M.RanX. R.FuD. H.YanZ. Q.et al. (2021). The morphological and histological study of chicken left ovary during growth and development among Hy-line brown layers of different ages. Poultry Sci.100, 101191. doi: 10.1016/j.psj.2021.101191
43
MilenkayaO.CatlinD. H.LeggeS.WaltersJ. R. (2015). Body condition indices predict reproductive success but not survival in a sedentary, tropical bird. PloS One10, e0136582. doi: 10.1371/journal.pone.0136582
44
NavaraK. J.GradenK.MendonçaM. T. (2023). Dietary yolk supplementation decreases rates of yolk deposition in Japanese quail. J. Exp. Zoology Part A: Ecol. Integr. Physiol.339, 63–73. doi: 10.1002/jez.2653
45
NdunguruS. F.RedaG. K.CsernusB.KnopR.GulyásG.SzabóC.et al. (2024a). Embryonic methionine triggers post-natal developmental programming in Japanese quail. J. Comp. Physiol. B194, 179–189. doi: 10.1007/s00360-024-01542-8
46
NdunguruS. F.RedaG. K.CsernusB.KnopR.LugataJ. K.SzabóC.et al. (2024b). Embryonic Leucine Promotes Early Postnatal Growth via mTOR Signalling in Japanese Quails. Animals14, 2596. doi: 10.3390/ani14172596
47
National Research Council (NRC) (1994). Nutrient Requirements of Poultry: Ninth Revised Edition. (Washington, DC: National Academy Press). doi: 10.17226/2114
48
OkeO. E.AkosileO. A.OniA. I.OpowoyeI. O.IsholaC. A.AdebiyiJ. O.et al. (2024). Oxidative stress in poultry production. Poultry Sci.103, 104003. doi: 10.1016/j.psj.2024.104003
49
PanY.-E.LiuZ.-C.ChangC.-J.HuangY.-F.LaiC.-Y.WalzemR. L.et al. (2014). Feed restriction ameliorates metabolic dysregulation and improves reproductive performance of meat-type country chickens. Anim. Reprod. Sci.151, 229–236. doi: 10.1016/j.anireprosci.2014.10.003
50
RedaG. K.NdunguruS. F.CsernusB.GulyásG.KnopR.SzabóC.et al. (2024). Dietary restriction and life-history trade-offs: Insights into mTOR pathway regulation and reproductive investment in Japanese quail. J. Exp. Biol.227, jeb247064. doi: 10.1242/jeb.247064
51
ReddishJ.NestorK.LilburnM. (2003). Effect of selection for growth on onset of sexual maturity in randombred and growth-selected lines of Japanese quail. Poultry Sci.82, 187–191. doi: 10.1093/ps/82.2.187
52
RehmanS. U.AliR.ZhangH.ZafarM. H.WangM. (2023). Research progress in the role and mechanism of Leucine in regulating animal growth and development. Front. Physiol.14. doi: 10.3389/fphys.2023.1252089
53
RenY.LiX.HanG.WangM.XiM.ShenJ.et al. (2021). Dynamic variations in serum amino acid and the related gene expression in liver, ovary, and oviduct of pigeon during one egg-laying cycle. Poultry Sci.100, 101184. doi: 10.1016/j.psj.2021.101184
54
RenemaR. A.RobinsonF. E.MelnychukV. L.HardinR. T.BagleyL. G.EmmersonD. A.et al. (1995). The use of feed restriction for improving reproductive traits in male-line large white Turkey hens. Poultry Sci.74, 102–120. doi: 10.3382/ps.0740102
55
RenemaR. A.RobinsonF. E.ZuidhofM. J. (2007). Reproductive efficiency and metabolism of female broiler breeders as affected by genotype, feed allocation, and age at photostimulation. 2. Sexual maturation. Poultry Sci.86, 2267–2277. doi: 10.1093/ps/86.10.2267
56
RizzotoG.SekharD.ThundathilJ. C.ChelikaniP. K.KastelicJ. P. (2019). Calorie restriction modulates reproductive development and energy balance in pre-pubertal male rats. Nutrients11, 1993. doi: 10.3390/nu11091993
57
Romero-HaroA. A.SorciG.Alonso-AlvarezC. (2016). The oxidative cost of reproduction depends on early development oxidative stress and sex in a bird species. Proc. R. Soc. B: Biol. Sci.283, 20160842. doi: 10.1098/rspb.2016.0842
58
RuanD.FouadA. M.FanQ.XiaW.WangS.ChenW.et al. (2018). Effects of dietary methionine on productivity, reproductive performance, antioxidant capacity, ovalbumin and antioxidant-related gene expression in laying duck breeders. Br. J. Nutr.119, 121–130. doi: 10.1017/S0007114517003397
59
RuffinoL.SaloP.KoivistoE.BanksP. B.KorpimäkiE. (2014). Reproductive responses of birds to experimental food supplementation: A meta-analysis. Front. Zoology11, 80. doi: 10.1186/s12983-014-0080-y
60
SantanaT. P.Da SilvaA. C. F.BastosM. S.Dos Santos ConceiçãoJ.De Souza KhatlabA.GasparinoE.et al. (2025). Methionine supplementation of maternal diet improves hatching traits, initial development, and performance in Japanese quail fed different levels of methionine during growth. Anim. Sci. J.96, e70044. doi: 10.1111/asj.70044
61
SearleS. R. (1997). Linear models. 1st ed (New York, USA: Wiley and Sons Inc.). doi: 10.1002/9781118491782
62
SearleS. R.SpeedF. M.MillikenG. A. (1980). Population marginal means in the linear model: an alternative to least squares means. Am. Statistician34, 216–221. doi: 10.1080/00031305.1980.10483031
63
ShiL.SunY.XuH.LiuY.LiY.HuangZ.et al. (2020). Effect of age at photostimulation on sexual maturation and egg-laying performance of layer breeders. Poultry Sci.99, 812–819. doi: 10.1016/j.psj.2019.12.027
64
SongX.WangD.ZhouY.SunY.AoX.HaoR.et al. (2023). Yolk precursor synthesis and deposition in hierarchical follicles and effect on egg production performance of hens. Poultry Sci.102, 102756. doi: 10.1016/j.psj.2023.102756
65
SreesujathaR. M.JeyakumarS.KunduA.BalasundaramC. (2016). Use of transcutaneous ultrasonography to characterize ovarian status, size distribution, and hierarchical status of follicles in Japanese quail (Coturnix coturnix japonica). Theriogenology86, 1231–1239. doi: 10.1016/j.theriogenology.2016.04.026
66
SuraiP. F. (2020). Antioxidants in poultry nutrition and reproduction: an update. Antioxidants9, 105. doi: 10.3390/antiox9020105
67
Taghipour-ShahbandiM.ZhandiM.Ansari-PirsaraeiZ.YousefiA. R. (2024). Exploration of age-related changes in reproductive parameters of female Japanese quail (Coturnix japonica). Poultry Sci.103, 104499. doi: 10.1016/j.psj.2024.104499
68
TamannaN.KroekerK.BraunK.BanhS.TrebergJ. R. (2019). The effect of short-term methionine restriction on glutathione synthetic capacity and antioxidant responses at the whole tissue and mitochondrial level in the rat liver. Exp. Gerontology127, 110712. doi: 10.1016/j.exger.2019.110712
69
Tarry-AdkinsJ. L.ChenJ.JonesR. H.SmithN. H.OzanneS. E. (2010). Poor maternal nutrition leads to alterations in oxidative stress, antioxidant defense capacity, and markers of fibrosis in rat islets: Potential underlying mechanisms for development of the diabetic phenotype in later life. FASEB J.24, 2762–2771. doi: 10.1096/fj.10-156075
70
TesfayeE.TamirB.HaileA.DessieT. (2009). Effects of feed restriction on production and reproductive performance of Rhode Island red pullets. Afr. J. Agric. Res.4, 642–648.
71
TsangC. K.ChenM.ChengX.QiY.ChenY.DasI.et al. (2018). SOD1 phosphorylation by mTORC1 couples nutrient sensing and redox regulation. Mol. Cell70, 502–515.e8. doi: 10.1016/j.molcel.2018.03.029
72
TůmováE.ChodováD.VolekZ.EbeidT. A.KettaM.SkřivanováV. (2022). A comparative study on the effect of quantitative feed restriction in males and females of broiler chickens, rabbits and nutrias. I. Performance and carcass composition. Czech J. Anim. Sci.67, 47–54. doi: 10.17221/185/2021-CJAS
73
Urdaneta-RinconM.LeesonS. (2002). Quantitative and qualitative feed restriction on growth characteristics of male broiler chickens. Poultry Sci.81, 679–688. doi: 10.1093/ps/81.5.679
74
VaidaF.BlanchardS. (2005). Conditional Akaike information for mixed-effects models. Biometrika92, 351–370. doi: 10.1093/biomet/92.2.351
75
Van Der KleinS. A. S.BédécarratsG. Y.RobinsonF. E.ZuidhofM. J. (2018). Early photostimulation at the recommended body weight reduced broiler breeder performance. Poultry Sci.97, 3736–3745. doi: 10.3382/ps/pey215
76
WangY.GuoZ.MengJ.ChenX.YangZ.YangH.et al. (2021). Effect of supplementary methionine on feather growth and related indicators of pigeon squabs. Braz. J. Poultry Sci.23, eRBCA–2021-1468. doi: 10.1590/1806-9061-2021-1468
77
WhelanS.HatchS. A.Benowitz-FredericksZ. M.ParenteauC.ChastelO.ElliottK. H. (2021). The effects of food supply on reproductive hormones and timing of reproduction in an income-breeding seabird. Hormones Behav.127, 104874. doi: 10.1016/j.yhbeh.2020.104874
78
WidowskiT. M.CooleyL.HendriksenS.PeixotoM. R. L. V. (2022). Maternal age and maternal environment affect egg composition, yolk testosterone, offspring growth and behaviour in laying hens. Sci. Rep.12, 1828. doi: 10.1038/s41598-022-05491-6
79
WiersmaP.SelmanC.SpeakmanJ. R.VerhulstS. (2004). Birds sacrifice oxidative protection for reproduction. Proc. R. Soc. London. Ser. B: Biol. Sci.271, S360–S363. doi: 10.1098/rsbl.2004.0171
80
YangW.LangX.SongD.XuH.ZhangC.GuoL.et al. (2024). Comparative analysis of reproductive hormones, serum biochemical indexes and ovarian metabolites in Muscovy breeder duck at different laying stages. Poultry Sci.103, 104370. doi: 10.1016/j.psj.2024.104370
81
YinL.ChenQ.HuangQ.WangX.ZhangD.LinZ.et al. (2023). Physiological role of dietary energy in the sexual maturity: Clues of body size, gonad development, and serum biochemical parameters of Chinese indigenous chicken. Poultry Sci.102, 103157. doi: 10.1016/j.psj.2023.103157
82
ZhangM.IwataS.HajimeM.OhkuboN.TodorokiY.MiyataH.et al. (2020). Methionine commits cells to differentiate into plasmablasts through epigenetic regulation of BTB and CNC homolog 2 by the methyltransferase EZH 2. Arthritis Rheumatol.72, 1143–1153. doi: 10.1002/art.41208
83
ZhangW.LiuJ.ZhouY.LiuS.WuJ.JiangH.et al. (2024). Signaling pathways and regulatory networks in quail skeletal muscle development: Insights from whole transcriptome sequencing. Poultry Sci.103, 103603. doi: 10.1016/j.psj.2024.103603
84
ZuidhofM. J.SchneiderB. L.CarneyV. L.KorverD. R.RobinsonF. E. (2014). Growth, efficiency, and yield of commercial broilers from 1957, 1978, and 2005. Poultry Sci.93, 2970–2982. doi: 10.3382/ps.2014-04291
Summary
Keywords
amino acids, dietary restriction, Japanese quail, ovary follicles, total antioxidant capacity
Citation
Ndunguru SF, Reda GK, Csernus B, Gulyás G, Knop R, Szabó C, Lendvai ÁZ and Czeglédi L (2025) Amino acid supplementation supports growth and reproductive development under dietary restriction. Front. Anim. Sci. 6:1622877. doi: 10.3389/fanim.2025.1622877
Received
04 May 2025
Accepted
09 June 2025
Published
04 July 2025
Volume
6 - 2025
Edited by
Assar Ali Shah, Jiangsu University, China
Reviewed by
Yuwen Dong, University of Pennsylvania, United States
Muhammad Tayyab Khan, University of Veterinary and Animal Sciences, Pakistan
Updates
Copyright
© 2025 Ndunguru, Reda, Csernus, Gulyás, Knop, Szabó, Lendvai and Czeglédi.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Sawadi F. Ndunguru, sawadindunguru@gmail.com
†These authors have contributed equally to this work
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.