- 1US Department of Agriculture, Agricultural Research Service, Beltsville Agricultural Research Center (BARC), Animal Biosciences and Biotechnology Laboratory, Beltsville, MD, United States
- 2AcuFast, Breese, IL, United States
- 3Metabolite Profiling Facility, Bindley Bioscience Center, Purdue University, West Lafayette, IN, United States
Introduction: The swine industry underutilizes cryopreserved boar semen due to poor post-thaw viability and variable fertility outcomes. Current semen evaluation methods are retrospective and insufficient for selecting cryotolerant and fertile sires. The aim of this study was to evaluate multiple reaction monitoring (MRM) profiling as a tool for predicting semen cryotolerance and fertility outcomes.
Methods: Lipidomic and metabolomic analyses using MRM profiling were applied to fresh and post-thaw ejaculates from 16 commercial Duroc boars with known conception rates (CR) from single-sire matings to identify candidate biomarkers predictive of field CR, post-thaw motility loss, and to determine whether CR markers identified in fresh semen persist post-thaw. Boars were classified by their cryotolerance delta score (CDS), which was calculated as the absolute change in motility between arrival at the cryopreservation laboratory and post-thaw, relative to the average loss in motility (low vs. high), and by field CR (low: 75–79%; mid: 80–89%; high: 90–95%).
Results: Distinct lipid and metabolite profiles were associated with each phenotype, revealing 20 candidate markers with an AUC ≥ 0.800 (P < 0.05). Markers predictive of higher post-thaw motility loss included compounds producing MRMs tentatively attributed to saturated long-chain fatty acids and elevated metabolites such as kynurenine (AUC = 0.905). MRMs predictive of < 80% CR were attributed to elevated guanosine (AUC = 0.850) and olealdehyde (AUC = 0.815), whereas > 80% CR showed higher abundance of TG(45:4) (AUC = 0.967) and creatine (AUC = 0.800). Candidate markers for CR were distinct from those associated with motility loss and remained detectable in post-thaw samples.
Discussion: These findings demonstrate that CR and post-thaw motility loss are governed by independent molecular traits and support the development of a multidimensional biomarker-based screening strategy to enhance fertility postthaw. This approach could enable AI centers to improve boar selection and cryopreservation outcomes, ultimately increasing the utility of frozen semen in swine breeding programs.
1 Introduction
With the growing global demand for pork, optimizing genetic progress in swine production has become increasingly critical. Artificial insemination (AI) with fresh, extended semen has been the foundation of genetic progress in swine. However, fresh doses rapidly decline in quality during storage and must be used within 3–5 days of collection, limiting the breeding window, creating bottlenecks, and increasing biosecurity risks (Maes et al., 2008; Schäfer et al., 2017; Alarcón et al., 2021; Hafemeister et al., 2023). Cryopreservation of boar semen offers clear advantages, including long-term storage, reduced transport frequency, and compatibility with technologies such as sex-sorted sperm. Despite decades of refinement, fewer than 1% of artificial inseminations use frozen boar semen, and these are primarily employed to reintroduce elite genetics, heritage breeds, or high-value individuals (Rodríguez-Gil and Estrada, 2013). The primary barrier to adoption lies in the performance gap between frozen–thawed and fresh semen, where only 50–60% of sperm survive the freeze–thaw process, leading to impaired motility, morphology, and membrane function (Rodríguez-Martínez and Peña Vega, 2013; Yeste, 2015, Yeste, 2016). Cryopreservation also induces changes resembling premature capacitation and causes disorder of plasma-membrane lipids, further compromising fertilization capacity, cell signaling, and interaction with the female reproductive tract (Vadnais and Althouse, 2011; Miller, 2024). These impairments ultimately reduce the fertility of the breeding dose and shorten the lifespan of spermatozoa post-thaw compared with fresh semen (Flowers, 1997, Flowers, 2013; Keller and Kerns, 2023).
Another major hurdle for the integration of genetic-advancement methods such as frozen–thawed semen is the lack of readily available tools capable of predicting fertility outcomes before insemination. Evaluation of boar fertility currently requires ≥ 50 single-sire matings (Clark et al., 1989), and routine semen-quality assessment for motility and morphology still allows subfertile boars to enter the breeding herd (Robinson and Buhr, 2005; Minton et al., 2013; Mills et al., 2020). Industry data suggest that approximately 25% of boars achieve conception rates below 80%, highlighting a significant subpopulation that borders or falls below culling thresholds—emphasizing the need for more sensitive selection tools (Shike, 2020). While effective, single-sire matings used for fertility testing can result in negative economic consequences for the sow farm and cannot feasibly be deployed for every boar. Therefore, tools that enable fertility-prediction screening for each sire—and thus predict frozen–thawed breeding-dose fertility—would be highly valuable to the swine industry.
Omics technologies, particularly metabolomics and lipidomics, provide the sensitivity needed to resolve subtle molecular signals likely driving the prevalence of subfertility. Moreover, lipidomic and metabolomic markers are well suited for fertility-biomarker discovery because they capture the molecular phenotype most proximal to physiological conditions. Like fertility traits themselves, the metabolome and lipidome are highly responsive to environmental influences, making them sensitive indicators of reproductive potential (Goodwin, 2004; Watson, 2006; Aldana et al., 2020). In boars, lipidomic and metabolomic profiling of sperm and seminal plasma has revealed associations between energy and lipid metabolism, reproductive performance, capacitation, and cryotolerance (Mateo-Otero et al., 2020; Zhang et al., 2021; Weide et al., 2024; Li et al., 2025) Differentially expressed metabolites linked to sperm function, motility, and membrane integrity are now being mapped across species, breeds, and sample types, suggesting the potential for robust cross-species biomarkers predictive of both fertility and post-thaw semen quality (Menezes et al., 2019; Xu et al., 2023; Du et al., 2024). Recent work by Li et al. (2025) has significantly advanced this area by identifying metabolomic biomarkers of sperm freezability in Duroc, Landrace, and Large White boars. The presence of lipidomic markers of cryotolerance has also been reported in Yorkshire boars (Zhang et al., 2023).
However, traditional exploratory approaches for lipidomic and metabolomic marker discovery are time-consuming, creating a bottleneck for herd-level validation of omic cryotolerance or fertility markers. Multiple reaction monitoring (MRM) profiling, an alternative biomarker-discovery approach, enables high-throughput screening of informative lipid and metabolite features through ion monitoring without full structural elucidation, thereby reducing instrument time and improving diagnostic efficiency (Xie et al., 2021). MRM profiling has already been applied in several fertility-related contexts, including biomarker identification in prepubertal gilts, broiler-breeder roosters, and in distinguishing fertile from subfertile men (Borges et al., 2018; Mills et al., 2021; Bond et al., 2024). In boars, MRM profiling has been used to examine age-related changes in the lipidome and to identify correlations with on-farm fertility measurements such as motility and morphology (Mills et al., 2024). Beyond fertility, MRM profiling has also been extended to metabolic and developmental contexts, including biomarker discovery for polycystic ovary syndrome (PCOS) in women, bovine oocyte and embryo-quality screening, and assessment of perinatal diet effects on gilt reproductive development (Cordeiro et al., 2015; Casey et al., 2018; de Lima et al., 2018; Harlow et al., 2019; Annes et al., 2023; Mills et al., 2023; Reis et al., 2023; de Lima et al., 2024). Taken together, we hypothesized that MRM profiling could elucidate highly informative features capable of predicting boar fertility and cryotolerance traits.
The overarching goal of this work is to develop biologically informed, pre-freeze selection tools that can be deployed within AI centers to optimize selection decisions in the future. Moreover, the ability to predict post-thaw semen quality—including fertility outcomes—would enhance the commercial adoption of frozen–thawed semen and support the broader implementation of reproductive technologies such as sex-sorted semen, thereby improving the global competitiveness of swine-breeding programs. The objective of this study was to evaluate MRM profiling as a tool for predicting semen cryotolerance and fertility outcomes. Specifically, we aimed to (1) identify potential biomarkers predictive of post-thaw motility loss and conception rate in fresh semen, (2) characterize informative features of post-thaw motility loss in post-thaw samples, and (3) assess how cryopreservation alters the abundance of conception-rate markers.
2 Materials and methods
2.1 Animals, sample collection, and conception rate classification
All samples for the study were sourced from ejaculates of 16 sexually mature Duroc boars enrolled in a commercial breeding program during routine collection. Briefly, boars were moved into a collection crate where preputial fluid was removed manually using a double-gloved hand. The sheath area and extended penis were cleaned and dried with a paper towel. The outer glove was removed, and manual collection was completed using the clean glove. The gel and pre-sperm fractions were collected into an inner bag that was detached and discarded prior to collecting the sperm-rich fraction in the collection mug. When collection of the sperm-rich fraction was complete, the collection mug was removed from under the collection area, ensuring only the sperm-rich portion of the ejaculate was collected for cryopreservation. The filter was removed, and the collection bag was closed using a band and sent to the laboratory via pneumatic tube.
Upon arrival at the stud laboratory, a 2 mL aliquot of the sperm-rich fraction was placed into a polypropylene conical tube (Falcon, USA) containing 10 mL of methanol (1:5, v/v). The sample was inverted five times to thoroughly mix the ejaculate and methanol (Mills et al., 2024). Samples were packaged and shipped on cooling packs to Beltsville Agricultural Research Center (BARC) overnight, and frozen upon arrival at −80 °C until lipidomic extraction. The remainder of the ejaculate was evaluated using standard boar stud protocols for semen quality assessment using computer-assisted sperm analysis (CASA). Boars met or exceeded the boar stud’s minimum quality thresholds of 75% motile sperm and 75% normal morphology during routine evaluation. Semen was extended 1:1 (v/v) in AndroStar® Plus (Minitube, Verona, WI, USA) and shipped overnight on cooling packs to the AcuFast cryopreservation laboratory.
Conception rates (CR) for each boar were supported by historical records from single-sire matings produced during routine fertility screening. Sires that fall below 75% CR are considered subfertile and are culled after sufficient data are available (≥50 matings), which is reflected in our cohort (75-95%; ≥ 50: n=13; <50: n=3). With 13–17% of the breeding herd estimated to be subfertile (Minton et al., 2013) and the prevalence of <80% CR boars estimated to be ~25% of the herd (Shike, 2020) identifying and monitoring the remaining 8–12% that border cull thresholds would be valuable. To address this, we stratified boars into three operational bands reflecting herd-level management decisions and the cohort’s conception rate distribution: Low (75–79%; n = 6; “Watch-list”), Mid (80–89%; n=6; “Acceptable”), and High (90–95%; n = 4; “Excellent”). The three individual boars with <50 single-sire matings were distributed equally across CR groups: 47 (79%), 26 (85%), and 42 (95%).
2.2 Cryopreservation and cryotolerance phenotype classification
Extended ejaculates arrived approximately 24 hours post-collection (referred to as check-in) and were evaluated for semen quality estimates including concentration, motility, and morphology. The freezing process was initiated following check-in evaluation. Briefly, semen was extended further to 3:1 (v/v) with AndroStar® Plus (Minitube, Verona, WI, USA) and centrifuged at 10,000 × g at 20 °C for 10 minutes. Seminal plasma was discarded, and the remaining sperm cell pellet was resuspended in a proprietary egg yolk-based cooling extender. Samples were cooled for 1.5 hr to 5 °C and diluted in another proprietary, cryoprotectant-supplemented extender to a final concentration of 450–500 million cells/mL. Motility was evaluated again prior to freezing for quality control purposes, and extended semen was packaged in 0.5 mL straws (IMV Technologies, Osseo, MN, USA) and frozen in a Digitcool programmable freezer (IMV Technologies, Osseo, MN, USA). Straws were submerged into liquid nitrogen and stored for a minimum of 1 day prior to post-thaw semen quality evaluation. A subset of frozen straws was shipped to BARC in a dry shipping container and stored in a liquid nitrogen dewar until thawed for lipidomic and metabolomic sample processing.
The same thawing protocol was used to evaluate post-thaw motility and prepare samples for lipidomic and metabolomic (omic) screening. Briefly, straws were thawed at 38 °C for 30 s in a water bath. Straw contents were deposited into an empty, pre-warmed (38 °C) tube, then extended with 5 mL of AndroStar® Plus and incubated at 38 °C for 20 min. Samples were evaluated for 20-min motility per standard straw quality control protocols, which are used to determine the number of straws for a breeding dose (2 billion motile cells). Samples for omic screening were processed in methanol using the 1:5 (v/v) method described above and stored at −80°C until lipid and metabolite extraction.
Cryotolerance phenotype groups were defined by the relative loss of motility during cryopreservation. A cryotolerance delta score (CDS) was calculated as the absolute change (Δ) in total motility between initial check-in (~24 hours post-collection) and 20 min post-thaw. To account for differences in starting motility, CDS values were normalized to the cohort mean and stratified by standard deviation. Boars with stable or minimally reduced motility post-thaw (Δ ≥ cohort mean; n = 7) were classified as high-CDS, whereas those with larger-than-average reduction in motility (Δ < cohort mean; n = 9) were classified as low-CDS. This approach ensured classification reflected relative cryotolerance performance within the cohort rather than arbitrary cutoffs. Group-level summary statistics for CDS and conception rate are provided in Table 1.
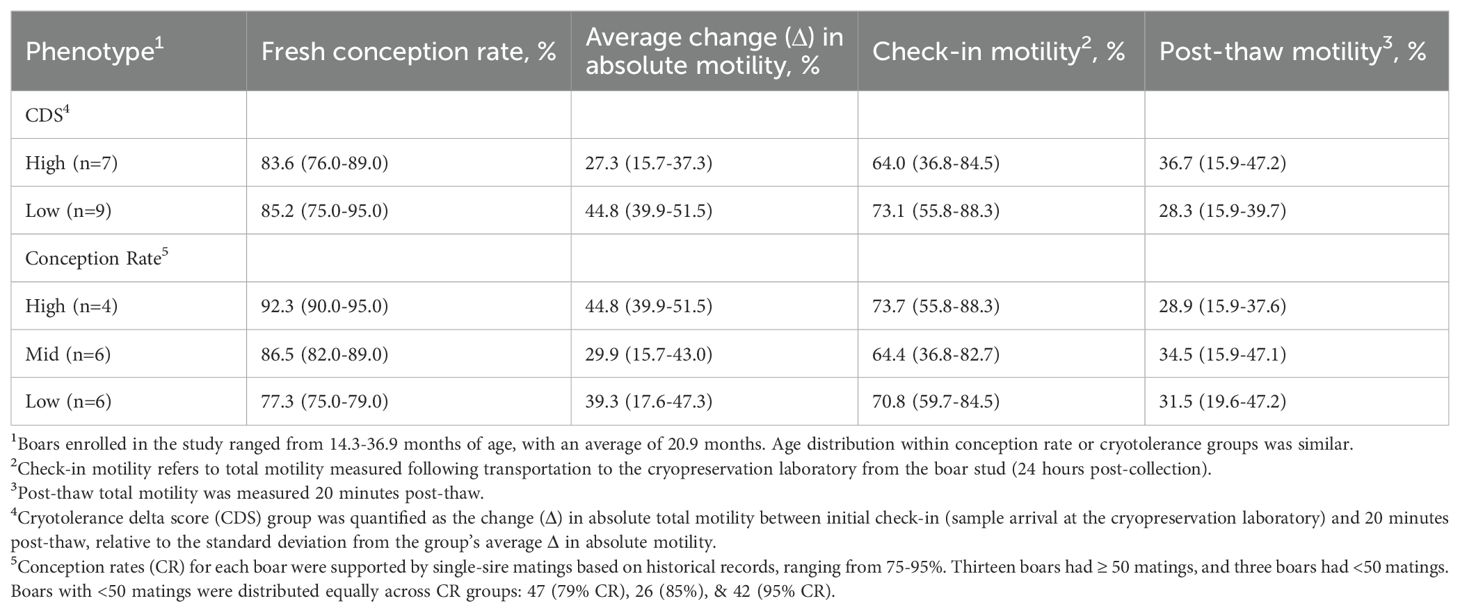
Table 1. Averages and ranges of semen quality parameters, loss in post-thaw motility, and conception rate by cryotolerance delta score (CDS) and conception rate phenotype group.
2.3 Lipid and metabolite extraction
Lipids were extracted from ejaculates using the Bligh and Dyer method (Bligh and Dyer, 1959). Samples were thawed at room temperature, then sonicated for three minutes at 40 kHz in an ultrasonic bath (Bransonic® M2800, Emerson Electric Co., St. Louis, MO, USA). Two hundred microliters of sample were transferred to a new 1.7 mL tube (Axygen®, Corning, New York), and then 550 µL of methanol prepared with 1 mM butylated hydroxytoluene (BHT) and 250 µL of chloroform were added and vortexed for 10 s so that the final concentration of BHT in the solution was 550 nM. Samples were then incubated at 4 °C for 15 min. The addition of 250 µL ultrapure water and 250 µL chloroform induced the formation of two immiscible liquid phases: a lower organic phase (lipids) and an upper polar phase (metabolites). Samples were centrifuged at 10,000 x g for 5 minutes at 4 °C for better separation of the two-phase solution. The lower (lipid) phase was removed and transferred to a new 1.7 mL microcentrifuge tube, followed by transferring the upper (metabolite) phase to a separate 1.7 mL microcentrifuge tube. Samples were dried in a vacuum concentrator for 8 h and stored at −80 °C until ready for MRM profiling.
2.4 MRM profiling of lipids and metabolites
Dried pellets were resuspended in 200 µL of solvent containing acetonitrile, methanol, and 300 mM ammonium acetate (3:6.65:0.35, v/v/v), to yield a final concentration of 10 mM NH4Ac. A further 150× dilution of the sample in solvent was used for direct injection. Eight microliters from each sample were injected into the micro autosampler (G1377A) of a QQQ6410 triple quadrupole mass spectrometer (Agilent Technologies, San Jose, CA) equipped with an ESI ion source. A solvent solution containing acetonitrile with 1% formic acid at 10 µL/min was pumped between injections (CapPump G1376A, Agilent Technologies, San Jose, CA). Pure methanol was injected between samples to remove any remaining lipids from the previous injection.
Multiple reaction monitoring (MRM) profiling was performed in two phases, beginning with a discovery phase that involved screening a large database of MRMs in composite samples (one sample for each experimental group). Samples were pooled in the discovery phase based on CDS phenotype (“high” and “low”) for fresh and post-thaw samples to identify lipid classes of importance prior to individual sample interrogation. MRMs detected in the discovery phase within fresh and post-thaw samples were then used to interrogate individual samples during the screening phase. Samples were screened for the chemical classes acylcarnitine (AC), ceramide (Cer), free fatty acids (FA), phosphatidylcholine (PC), phosphatidylethanolamine (PE), phosphatidylinositol (PI), phosphatidylglycerol (PG), phosphatidylserine (PS), diacylglycerol (DG), and triacylglycerol (TG) using MRM profiling methods previously reported (Reis et al., 2023). Processing of the initial chemical class data was completed using MSConvert20, which converted each set of profiling method data into mzML file format. Signal intensity for ions present in the samples was obtained using an in-house script. Ions with ion count values >30% in at least one of the samples compared with a blank within each profiling method were selected for the screening phase. Due to the large number of MRMs to be screened, the samples were interrogated using four lists of MRMs, which we refer to as methods:
1. Method 1: FA Lipids (Supplementary Table 1)
2. Method 2: PC, Cer, PE, AC, CE, PS, PI, PG Lipids (Supplementary Table 2)
3. Method 3: DG Lipids (Supplementary Table 3)
4. Method 4: TG Lipids (Supplementary Table 4)
Metabolomic analysis was completed during the screening phase and samples were analyzed in both the positive (Supplementary Table 5) and negative (Supplementary Table 6) ion modes. Question marks within metabolite names in the Supplementary Tables indicate unknown structural orientation of the molecule.
2.5 Data processing and statistical analysis
MRM ion pairs were acquired for all methods except for Method 1, where fatty acids did not fragment without derivatization during MRM profiling. As a result, MRMs reported in Method 1 only contain the precursor ion m/z. Tentative lipid class attributions based on associated functional group and biological information were assigned to unidentified MRM ion pairs and precursor ions to increase potential biomarker discovery using lipid maps (http://www.lipidmaps.org/). For data quality purposes, MRM intensities less than 1.3-fold (lipids) or 1.5-fold (metabolites) below the blank were removed. Next, relative ion intensity was calculated by dividing intensity by the sum of intensities of all lipids within a sample by screening method. Relative intensities of MRM ion pairs were uploaded into MetaboAnalyst 6.0 (Pang et al., 2024) and normalized using autoscaling for data visualization, statistical analysis, and biomarker analysis. Statistical analyses included Student’s t-tests to compare cryotolerance phenotypes and one-way ANOVA with Fisher’s post hoc tests to assess differences across conception rate groups. Because conception rate is inherently a continuous variable, Pearson’s correlation analysis was also performed to evaluate the relationship between conception rate and the abundance of informative biomarkers. Data visualizations included hierarchical clustering heat maps and box plots of significant features, with an α-level of 0.05 based on nominal P-values.
Biomarker analyses included receiver operating characteristic (ROC) analysis comparing lower conception rate boars (<80%, n = 6) to all others (n = 10), reflecting the estimated field prevalence where ~25% of boars fall below 80% conception rate (Shike, 2020). This approach enabled us to evaluate whether omics markers could identify borderline boars approaching the cull threshold, thereby demonstrating the sensitivity of the screening approach without requiring phenotype extremes. Predictive performance was visualized in ROC curves and quantified by area under the curve (AUC). The following AUC scale was used to evaluate potential biomarkers: excellent = 0.900–1.000, good = 0.800–0.899, fair = 0.700–0.799, poor = 0.600–0.699, and fail = 0.500–0.599 (Xia et al., 2013). Lipids and metabolites were considered candidate biomarkers if they met either of the following criteria: (1) Pearson’s correlation coefficient (r) ≥ 0.6 with P ≤ 0.05, or (2) area under the ROC curve (AUC) ≥ 0.800 with P ≤ 0.05. Features with AUC ≥ 0.800 and P-values approaching significance (0.05 < P ≤ 0.06) were also retained due to their high discriminatory potential. Tentative attributions that share an MRM are reported with each potential compound separated by a forward slash.
3 Results
3.1 Potential biomarkers of cryotolerance score in fresh semen
Fresh semen profiling distinguished high and low cryotolerance score (CDS) boars. High-CDS samples showed enrichment of select phosphatidylcholines and sphingomyelins, whereas low-CDS boars were characterized by greater abundance of long-chain fatty acids and additional phospholipid and sphingomyelin features. Metabolite analysis revealed enrichment of multiple small molecules in high-CDS semen, including features detected in both positive and negative ion modes. A total of 13 candidate markers for CDS score were identified (AUC ≥ 0.810). Three features with tentative attributions as 1,2-dihydroxyheptadec-16-Yn-4-Yl acetate, heneicosanoic acid, and nonadecanoic acid achieved AUC > 0.900 and had the strongest predictive value (Table 2). Predictive performance and distribution patterns for the top biomarkers identified within screening methods for lipids and metabolites are visualized in Figure 1.
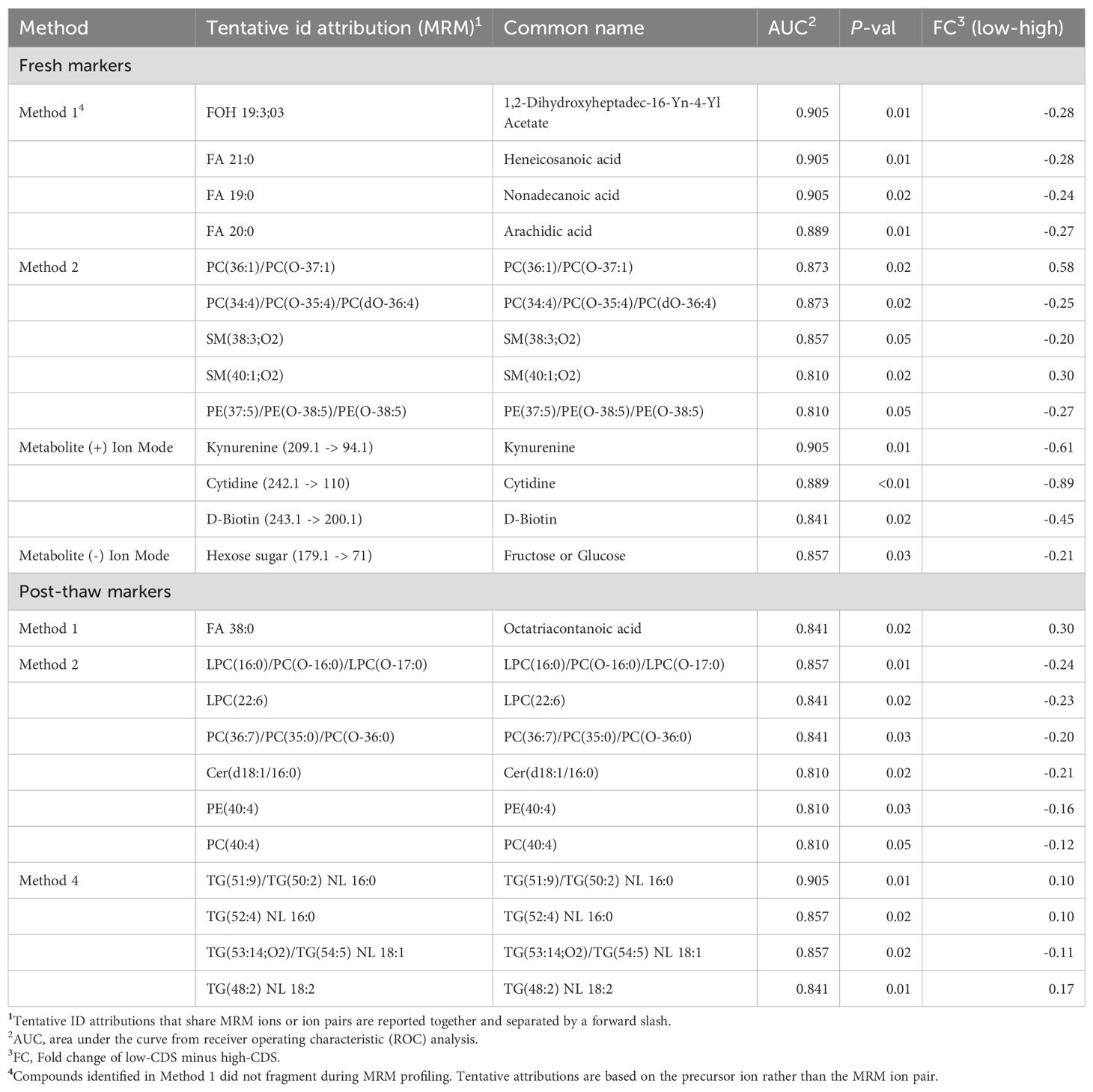
Table 2. Candidate biomarkers associated with cryotolerance delta score (CDS) in fresh and post-thaw semen.
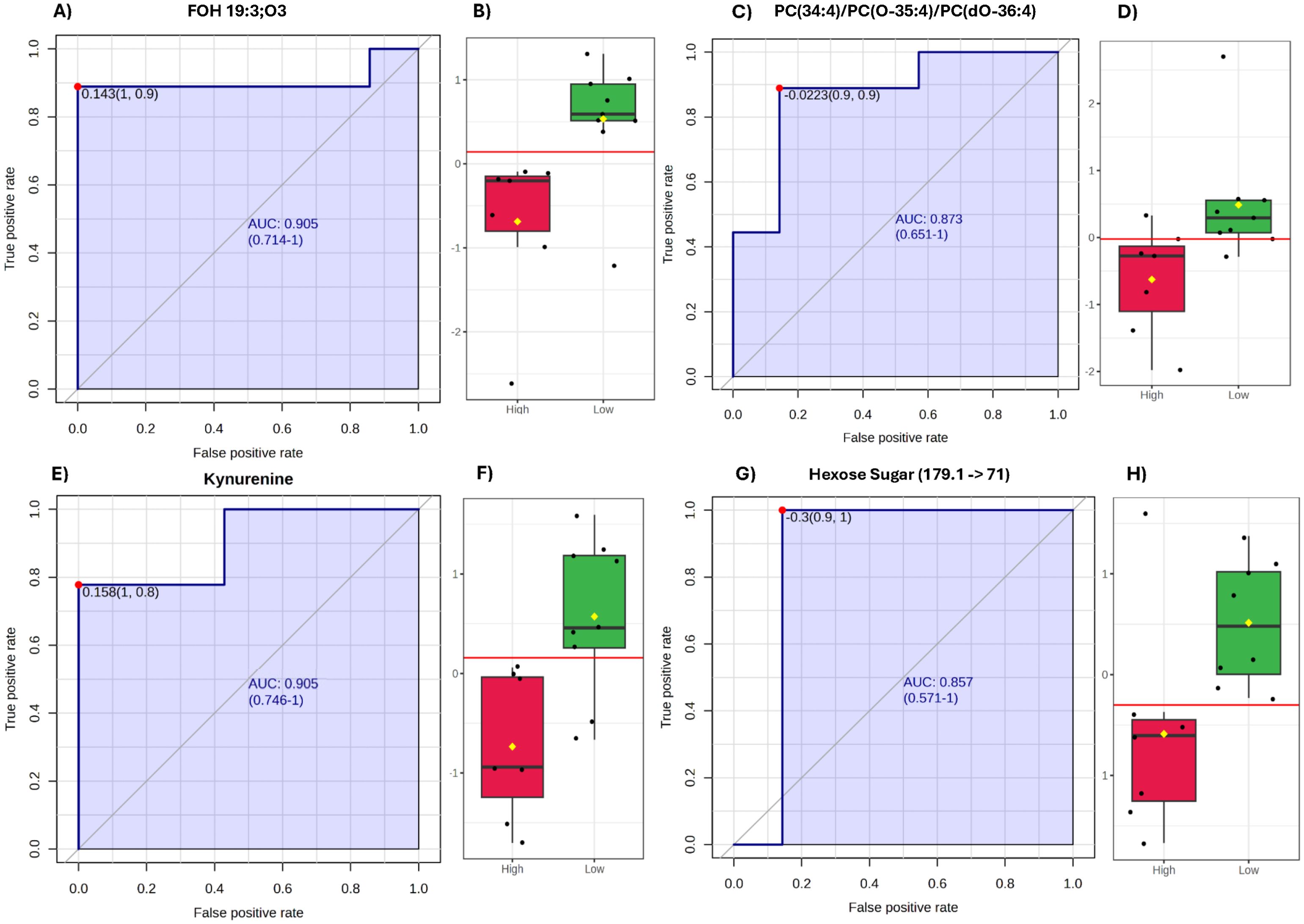
Figure 1. Candidate biomarkers (tentative attribution) predictive of boar sperm cryotolerance delta score (CDS) phenotype. (A, B) FOH 19:3;O3 (Method 1): (A) Receiver operating characteristic (ROC) curve showing the discriminatory capacity of FOH 19:3;O3 between low and high CDS phenotypes (AUC = 0.905). (B) Box plot showing normalized abundance of FOH 19:3;O3 in fresh semen, with significantly higher levels in low-CDS boars (P < 0.01). Yellow diamonds indicate group means. (C, D) PC(34:4)/PC(O-35:4)/PC(dO-36:4) (Method 2): (C) ROC curve demonstrating the discriminative performance of this phosphatidylcholine for classifying boar semen cryotolerance phenotype (AUC = 0.873). Tentative ID attributions that share MRM ions or ion pairs are reported together and separated by a forward slash. (D) Box plot showing normalized lipid abundance, significantly elevated in the low-CDS group (P = 0.02), consistent with impaired membrane dynamics. (E, F) Kynurenine (metabolite, (+) ion mode): (E) ROC curve illustrating strong discriminatory power of kynurenine between high and low cryotolerance groups (AUC = 0.905). (F) Box plot showing normalized kynurenine relative ion intensity, significantly higher in high motility loss (low-CDS) boars (P < 0.01). Yellow diamonds indicate means; red line represents the optimal ROC threshold. (G, H) Hexose sugar (179.1 → 71) (metabolite, (−) ion mode): (G) ROC curve for the hexose sugar (179.1 → 71), a conception rate–associated metabolite with moderate predictive value (AUC = 0.857). The question mark in the metabolite name indicates that structural orientation could be α or β and requires validation with other methods. (H) Box plot comparing normalized abundance across phenotypes, with significantly higher levels in the high motility loss group (P = 0.03), indicating potential disruption in energy metabolism and membrane flexibility.
3.2 Characteristic features of cryotolerance phenotype in post-thaw samples
Because all boars were frozen in the same extender, major differences were not expected; however, post-thaw profiling revealed distinct signatures between CDS phenotypes. High-CDS samples showed enrichment of select fatty acids, such as FA 38:0, and triacylglycerols, whereas low-CDS ejaculates accumulated lysophospholipids, phosphatidylcholines, and ceramides (Table 2). These post-thaw features met biomarker thresholds (AUC ≥ 0.81), while no informative associations were identified for diacylglycerols or metabolites (all AUC < 0.80, P > 0.05; full list provided in Supplementary Table 7).
3.3 Candidate markers predictive of conception rate
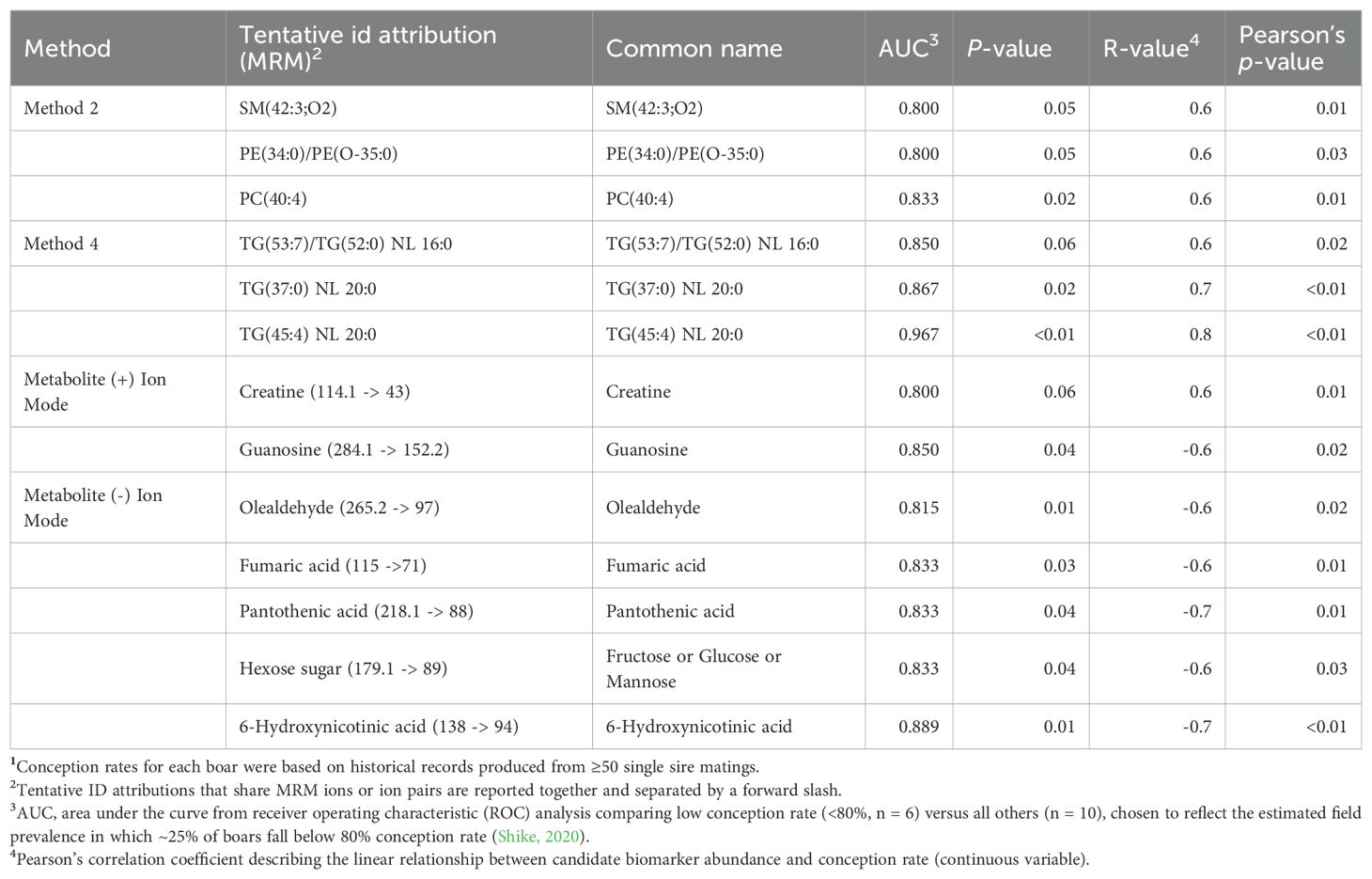
Despite not selecting individuals based on fertility extremes, Pearson’s correlation and ROC analysis identified several features exhibiting strong associations with conception success. Fertility-linked metabolites included both positively and negatively correlated features, while distinct lipid classes also showed strong associations with conception outcomes. Thirteen markers exceeded our candidate biomarker thresholds (|r| ≥ 0.6, AUC ≥ 0.800; Table 3; Figure 2), highlighting their potential to capture fertility variation within commercially relevant ranges. Notably, TG(45:4) NL 20:0 emerged as the most robust conception rate predictor (r = 0.8, AUC = 0.967; Figure 2). These markers effectively stratified boars by fertility outcome but not by post-thaw cryotolerance score class (Tables 2; Table 3).
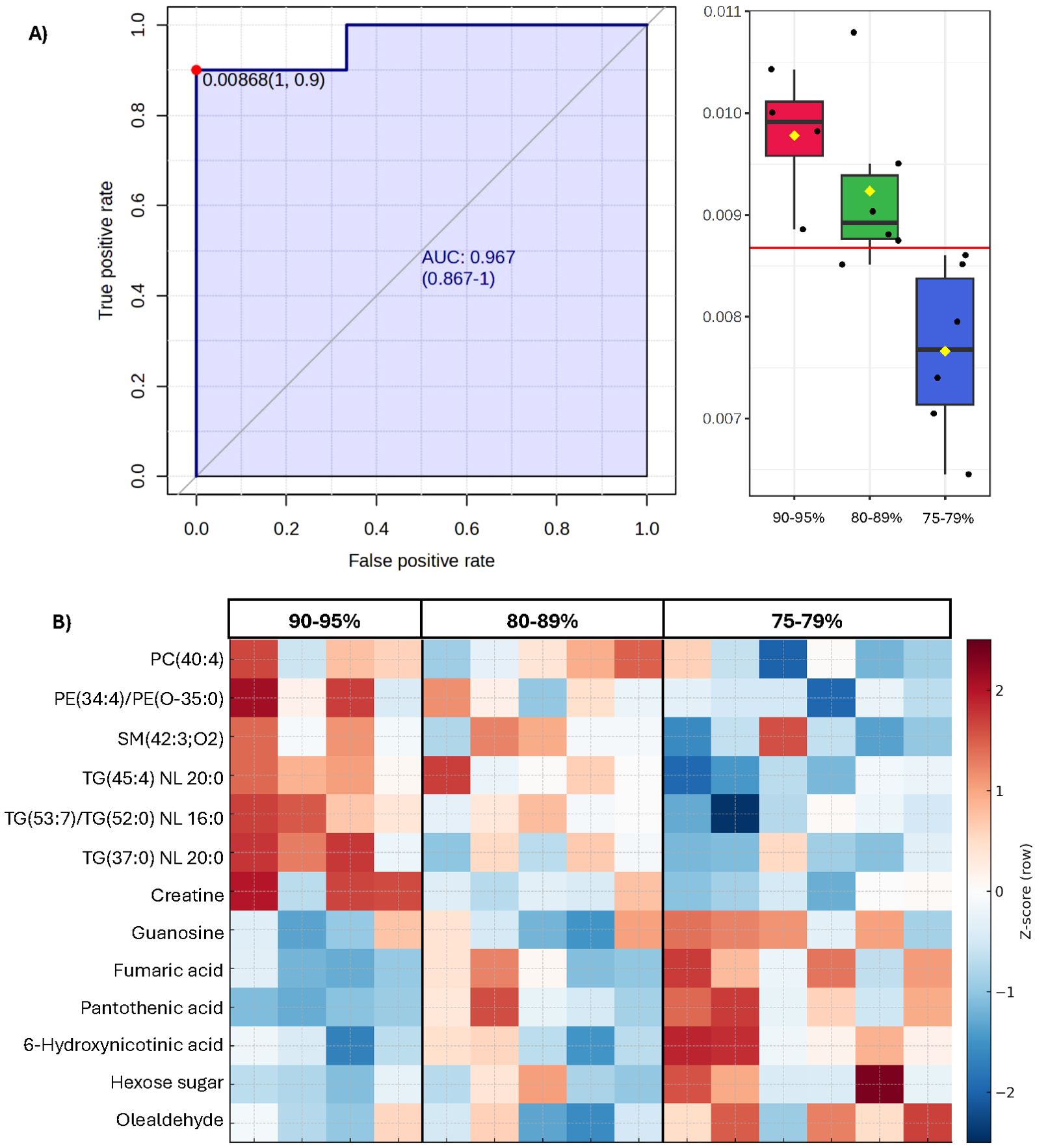
Figure 2. Candidate biomarkers (tentative attribution) predictive of conception rate (CR). (A) ROC curve for the top candidate biomarker for conception rate, TG(45:4) NL 20:0, showing excellent classification of low (75–79%) versus higher conception rate (>80%) boars (AUC = 0.967; P < 0.01). Box plots to the right display relative abundance across conception rate groups (r = 0.8, P < 0.01). Yellow diamonds indicate group means; red line represents the optimal ROC threshold for low versus all others. (B) Heat map of top lipid and metabolite biomarkers identified in Table 3, including PC(40:4), PE(34:4)/PE(O-35:0), SM(42:3;O2), TG(45:4) NL 20:0, TG(37:0) NL 20:0, creatine, guanosine, fumaric acid, pantothenic acid, 6-hydroxynicotinic acid, hexose sugar (179.1 → 89), and olealdehyde. Relative ion intensities were autoscaled, with red indicating higher abundance and blue indicating lower abundance. Distinct molecular profiles stratify boars by conception rate bands (75–79%, 80–89%, 90–95%).
3.4 Persistence of fertility markers post-thaw and relationship with cryotolerance
Important fertility-associated features originally identified in fresh semen persisted post-thaw, but their relative abundance by fresh CR phenotype was not maintained within boar (Figure 3). Several low-CDS boars exhibited higher relative levels of CR-linked lipids such as TG(45:4) NL 20:0. By contrast, some high-CDS boars displayed lower abundance of these same markers post-thaw. These molecular patterns demonstrate the biological independence of post-thaw motility and conception potential, highlighting that post-thaw motility loss is not a complete indicator of frozen-thawed fertility. This further supports integrating multi-trait, pre-thaw screening to identify sires that produce breeding doses that remain both motile and fertile following cryopreservation, thereby optimizing cryotolerance selection. Boars who retained relative abundance of fertility markers pre- and post-thaw, represented as a percentage of their cryotolerance phenotype, are summarized in Figure 4.
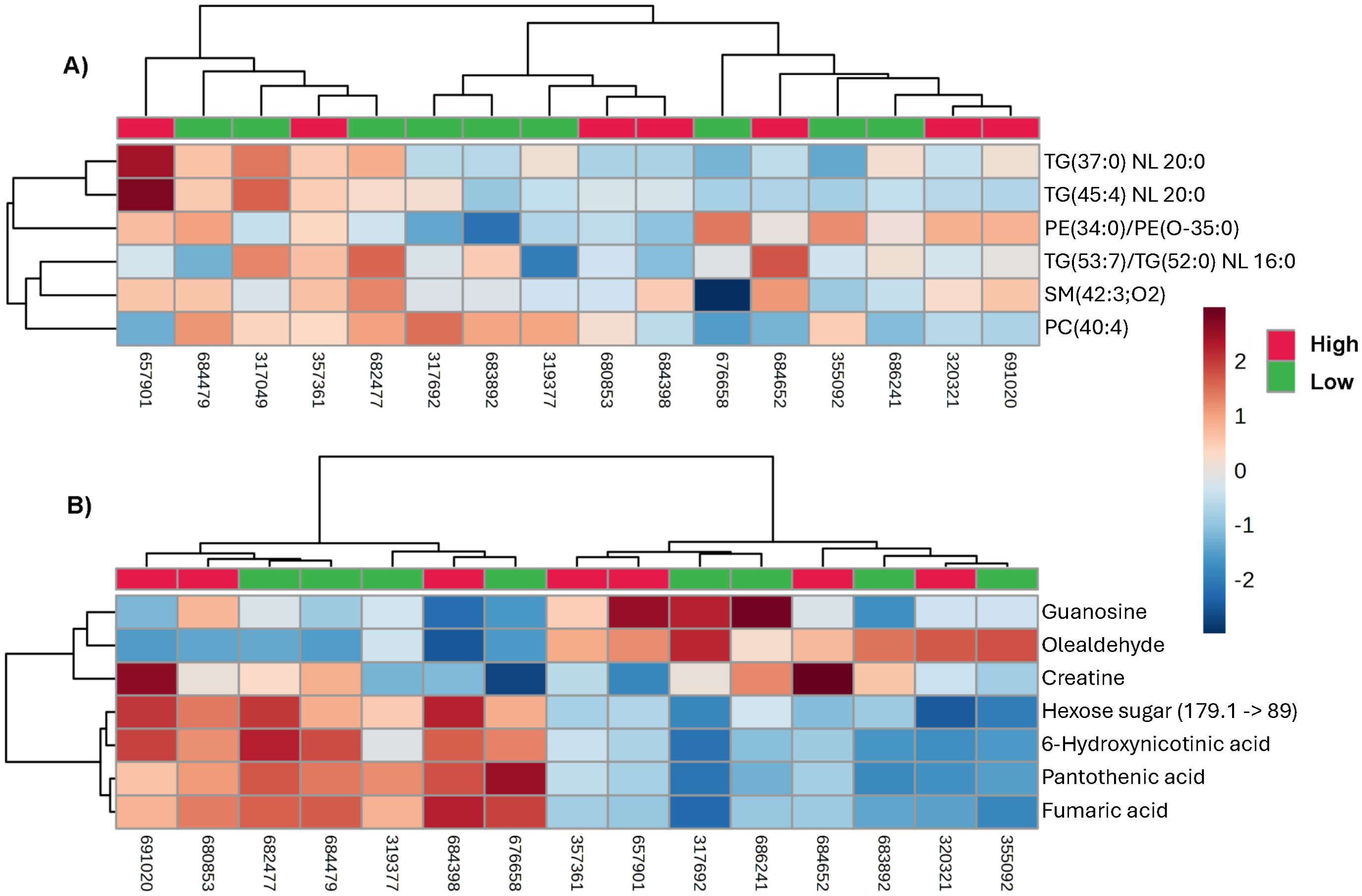
Figure 3. Post-thaw abundance patterns of fertility-associated candidate biomarkers (tentative attribution) across CDS phenotypes. (A) Heat map of lipid candidate biomarkers previously identified in fresh semen as positively associated with conception rate, including triacylglycerol (TG) lipids detected using the neutral loss (NL) of one fatty acid as the product ion of the MRM scan: TG(37:0) NL 20:0, TG(45:4) NL 20:0, PE(34:0)/PE(O-35:0), TG(53:7)/TG(52:0) NL 16:0, SM(42:3;O2), and PC(40:4). Tentative ID attributions that share MRM ions or ion pairs are reported together and separated by a forward slash. (B) Heat map of fertility-linked small-molecule metabolites detected post-thaw, including creatine, olealdehyde, hexose sugar (179.1 → 89), 6-hydroxynicotinic acid, pantothenic acid, and fumaric acid. In both panels, columns represent individual boars, colored by CDS phenotype (top annotation: red = high-CDS, green = low-CDS). Rows represent candidate biomarkers. Values were normalized across samples. Hierarchical clustering of rows and columns was performed using Euclidean distance and average linkage. Several MRMs related to fertility remained highly abundant post-thaw in high motility loss (low-CDS) samples, underscoring the independence of fertility potential from motility loss during the cryopreservation process and supporting the need for pre-thaw biomarker-based screening for multiple desirable traits.
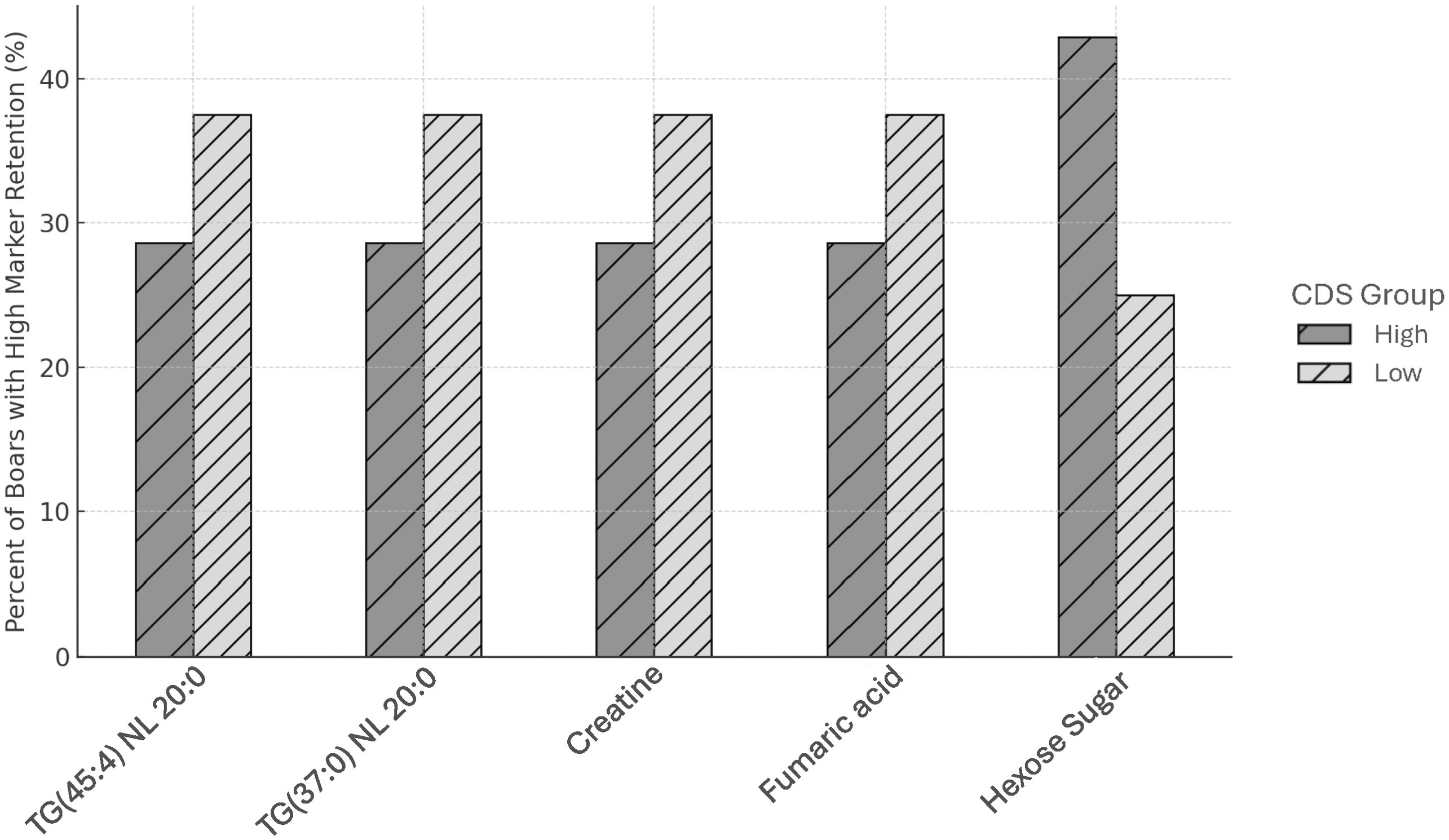
Figure 4. Retention of lipids and metabolites associated with conception rate by CDS group. Bar graph showing the percentage of boars in high and low post-thaw motility loss CDS groups that retain elevated levels of specific biomarkers linked to conception success. Biomarkers include two triacylglycerols [TG(45:4) NL 20:0 and TG(37:0) NL 20:0], creatine, fumaric acid, and hexose sugar (179.1 → 89). A greater proportion of high motility loss (low-CDS) boars exhibit high retention of TGs, creatine, and fumaric acid, whereas low motility loss (high-CDS) boars more frequently retain the hexose sugar. These trends suggest a potential metabolic divergence linked to sperm motility loss and fertility outcomes.
4 Discussion and conclusions
In the present study, our findings support the existence of mechanistically distinct lipidomic and metabolomic signatures for sperm cryotolerance and fertility. However, MRM profiling is an exploratory ion screening tool, and tentative attributions for lipids and metabolites must be validated using approaches such as UHPLC or HRMS to determine final concentration and chemical structure of these small molecules. To help support our exploratory results and biological interpretation, tentative attributions were cross-referenced to previous work that characterized the lipidome and metabolome of boar spermatozoa and seminal plasma. Tentative attributions in the present study are consistent with small molecules previously reported in boar samples characterized with UHPLC-MS, GC-MS, or LC-MS/MS (Am-in et al., 2011; Zhang et al., 2023; Ding et al., 2025; Li et al., 2025).
4.1 Informative lipid features suggest post-thaw motility loss is related to degree of lipid saturation
High motility loss ejaculates (low-CDS) were enriched in saturated fatty acids, including nonadecylic (FA 19:0), arachidic (FA 20:0), and heneicosanoic acid (FA 21:0). Saturated lipids tend to pack tightly in the lipid bilayer, making the plasma membrane more rigid and less flexible to temperature fluctuations (Suo et al., 2024). Because cryotolerance was characterized based on motility in the present study, these findings are consistent with reports that low-motility boars have a higher proportion of saturated fatty acids in fresh ejaculates (Am-in et al., 2011). Lipid profiles of sperm cells from poor freezing Yorkshire boars were enriched in saturated lipids, and their abundance was correlated with weaker plasma membrane integrity, greater mitochondrial dysfunction, and poorer post-thaw motility and viability (Zhang et al., 2023). In contrast, monounsaturated fatty acids appear protective, promoting more flexible membranes; enrichment of these species was a trend in our low motility loss (high-CDS) samples and consistent with reports in Yorkshire boars (Zhang et al., 2023). However, samples enriched with monounsaturated phosphatidylcholines and sphingomyelins were more cryotolerant, indicating that there is a balance required to survive colder temperatures where moderate unsaturation is favored.
Polyunsaturated fatty acids (PUFAs) add further nuance. PUFAs are characteristic of boar spermatozoa and have been associated with fertility in fresh semen (Penny et al., 2000; Rooke et al., 2001). Increased PUFA composition increases plasma membrane fluidity and is typically characteristic of cells more resistant to ice crystal damage (Erickson, 2002). However, while PUFAs aid membrane fusion and function, their high PUFA concentrations also render boar spermatozoa prone to oxidative degradation which is amplified during the cryopreservation process (Liu et al., 2022). Peroxidized lipids disrupt membrane integrity, mitochondrial function, and cell viability—hallmarks of sperm subjected to cold shock and cryopreservation (Hai et al., 2024). Consistent with our findings in saturated fatty acids, Zhang et al. (2023) observed that poor freezing were enriched with PC and PE lipids containing arachidonic acid. Although PUFAs such as α-linolenic acid (18:3), arachidonic acid (ARA 20:4), and docosahexaenoic acid (DHA 22:6) are beneficial for fertility of fresh semen, they may have an opposite effect during cryopreservation (Collodel et al., 2020). Together, these results suggest that cryosurvival depends on a balanced lipid composition: overly rigid membranes resist remodeling, while excessive unsaturation leaves sperm vulnerable to cold-induced peroxidation. Future studies should quantify specific PUFA-containing lipid species in boar spermatozoa and evaluate their inherent risk for cryodamage.
Similar patterns persisted in post-thaw lipidomic signatures. Ejaculates with high motility loss were enriched in polyunsaturated LPCs and PEs, including LPC(22:6), which has been linked to oxidative membrane damage in cattle and roe deer sperm (Fuchs et al., 2007). Cer(d18:1/16:0), a lipid previously linked to increased membrane permeability and induction of apoptosis via mitochondrial pathways (Siskind et al., 2006; Seumois et al., 2007) was also associated with high motility loss in the present study. Seminal plasma extracellular vesicles containing elevated ceramides have been associated with low motility in fresh semen (Ding et al., 2025). Although processing protocols typically minimize seminal plasma inclusion (Vadnais and Althouse, 2011), our findings suggest that vesicle-derived lipids may still influence motility post-thaw and merit further study.
Tentative attributions for other characteristic features in post-thaw samples—such as FA 38:0 and the triacylglycerols—have not been previously identified in boar spermatozoa or seminal plasma. These compounds are likely components of the cryopreservation extender rather than post-thaw modifications of the sperm cell itself. Interestingly, even though all ejaculates were frozen in the same extender, the relative abundance of presumed extender components differed by CDS phenotype. If extender composition varies enough from straw-to-straw to influence cryotolerance and post-thaw sperm metabolism, optimizing extender distribution could represent a new area for improvement.
4.2 Metabolic indicators of oxidative stress differentiate conception rate and motility loss outcomes
Metabolomic profiling revealed that candidate markers for post-thaw motility loss and lower conception rates were associated with elevated metabolites indicative of oxidative stress and inflammation. Kynurenine, a product of tryptophan degradation that reflects oxidative stress and immune activation detrimental to sperm function, was higher in high motility loss ejaculates (Jrad-Lamine et al., 2011, Jrad-Lamine et al., 2013). 6-hydroxynicotinic acid, another metabolite linked to the kynurenine pathway, was inversely correlated with conception rate—but not informative for post-thaw motility loss (AUC<0.6, P>0.1)—suggesting broader dysregulation of NAD metabolism in fresh semen (Alahmar, 2019). Other stress-linked metabolites included cytidine, biotin, olealdehyde, and excess sugar, each consistent with oxidative or metabolic imbalance (Matsuda et al., 1989; Sancho et al., 2006; Pizzimenti et al., 2013; Sprenger et al., 2021; Wang et al., 2025). Notably, guanosine was elevated in lower conception rate ejaculates and is known to be the most susceptible spermatozoal nucleoside to oxidative damage, with direct mechanistic links to male infertility (Kocer et al., 2015; Rashki Ghaleno et al., 2021). In contrast, creatine was the only metabolite positively associated with conception rate. Its role as a substrate for phosphocreatine-driven ATP buffering is well established, and in sperm this pathway is critical for maintaining motility and energy supply during capacitation and fertilization (Ostojic et al., 2022; Wang et al., 2025). When added to insemination media, exogenous creatine improves motility, velocity, and enhances capacitation during in vitro fertilization in mice and swine (Umehara et al., 2018, Umehara et al., 2020). Thus, elevated creatine may reflect an energetically competent metabolic profile that supports fertilization success. While these pathways must be validated in larger cohorts, the recurring theme is that sperm experiencing—or more susceptible to—oxidative or metabolic stress pre-freeze are less resilient to cryopreservation and achieve lower conception rates, although different metabolites appear to underlie each trait.
4.3 Limitations, future directions, and implications
Several limitations must be acknowledged. The sample size (n = 16) and use of a single ejaculate per boar limit generalizability and preclude evaluation of intra-individual consistency. Future studies should include repeated measures, larger cohorts, and multiple breeds to validate biomarkers across a variety of genetic backgrounds. Moreover, field fertility in this study was based on fresh semen; direct fertility trials using cryopreserved semen remain necessary to confirm functional competence. Environmental and nutritional influences on biomarker expression, as well as optimization of extender formulations to preserve favorable lipid profiles, also warrant investigation. Analytical limitations of MRM profiling must also be considered, including reduced fragmentation of underivatized fatty acids and occasional ambiguous attributions. While this underscores the exploratory nature of MRM profiling, reproducible precursor ions and the strong predictive power of identified MRMs justify further targeted validation using high-resolution methods.
This approach addresses key limitations of current workflows that evaluate cryopreservation and conception rate outcomes, which rely heavily on motility and morphology as proxies for reproductive potential. Given the relatively lower abundance of conception rate biomarkers in post-thaw samples from some high-cryotolerance boars, current selection criteria may result in motile breeding doses with relatively less fertility compared to other sires. Moreover, the persistence of fertility markers post-thaw highlights the potential for using molecular screening as an additional quality control layer beyond motility, ensuring that ejaculates not only meet minimum viability thresholds but also retain key fertilization-associated metabolites.
Another important finding was that high-conception boars often overlapped with low cryotolerance phenotypes. For example, polyunsaturated PCs and SMs predicted higher conception rates but were also linked to greater post-thaw motility loss. This decoupling of fertility and cryotolerance observed in lipids and metabolites has practical implications for sire selection, suggesting that optimal candidates for cryopreservation may fall within mid-range conception rates (80–89%), where lipid balance favors both fertility and resilience to freezing. Our findings emphasize the importance of integrated selection systems and strongly support the implementation of a multi-axis evaluation framework for boar semen quality.
4.4 Conclusions
This study delineates distinct lipidomic and metabolomic signatures predictive of post-thaw motility loss and conception success in boars. The molecular dissociation of these two traits provides a framework for reimagining semen quality assessment—not as a unidimensional construct based solely on motility, but as a multidimensional phenotype amenable to precision molecular diagnostics. The deployment of MRM-based biomarker panels in AI centers offers a transformative opportunity to enhance the utility of cryopreserved semen, minimize genetic loss, and improve reproductive efficiency in swine genetic programs.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Ethics statement
Ethical approval was not required for the study involving animals in accordance with the local legislation and institutional requirements because boars were not collected for the sole purpose of this study. Samples were sourced from ejaculates collected from boars enrolled in a commercial breeding program as part of routine collections per standard husbandry protocols.
Author contributions
KM: Conceptualization, Data curation, Formal Analysis, Funding acquisition, Investigation, Methodology, Project administration, Supervision, Visualization, Writing – original draft, Writing – review & editing. AM: Investigation, Project administration, Resources, Writing – review & editing. JB: Conceptualization, Investigation, Project administration, Resources, Writing – review & editing. KW: Conceptualization, Investigation, Project administration, Resources, Writing – review & editing. CF: Data curation, Investigation, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. This research was funded by the in-house USDA-ARS CRIS project number 8042-31000-111-00D to Kayla Mills.
Acknowledgments
All lipidomic and metabolomic data collection and analysis were performed at Purdue University’s Metabolite Profiling Facility in the Bindley Bioscience Center. We are also very appreciative for the generous contributions made by AcuFast of all boar ejaculates used in this study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fanim.2025.1665783/full#supplementary-material
References
Alahmar A. T. (2019). Role of oxidative stress in male infertility: an updated review. J. Hum. Reprod. Sci. 12, 4–18. doi: 10.4103/jhrs.JHRS_150_18
Alarcón L. V., Allepuz A., and Mateu E. (2021). Biosecurity in pig farms: a review. Porcine Health Manage. 7, 5. doi: 10.1186/s40813-020-00181-z
Aldana J., Romero-Otero A., and Cala M. P. (2020). Exploring the lipidome: Current lipid extraction techniques for mass spectrometry analysis. Metabolites 10, 231. doi: 10.3390/metabo10060231
Am-in N., Kirkwood R. N., Techakumphu M., and Tantasuparuk W. (2011). Lipid profiles of sperm and seminal plasma from boars having normal or low sperm motility. Theriogenology 75, 897–903. doi: 10.1016/j.theriogenology.2010.10.032
Annes K., Ferreira C. R., Valente R. S., Marsico T. V., Tannura J. H., da Silveira J. C., et al. (2023). Contribution of lipids to the organelle differential profile of in vitro-produced bovine embryos. Theriogenology 208, 109–118. doi: 10.1016/j.theriogenology.2023.06.005
Bligh E. G. and Dyer W. J. (1959). A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 37, 911–917. 10.1139/o59-099
Bond A., Mills K. M., Ferreira C. R., Harford I., Flack B., Long J. A., et al. (2024). Broiler breeder putative lipid biomarkers associated with sperm mobility. Front. Physiol. 15. doi: 10.3389/fphys.2024.1504557
Borges E. D., Vireque A. A., Berteli T. S., de Lima C. B., Sobreira T. J., Ferreira C. R., et al. (2018). Lipidomics of sperm cells of fertile and sub-fertile men by MRM-profiling. Fertility Sterility 110, e303–e304. doi: 10.1016/j.fertnstert.2018.07.853
Casey T., Harlow K., Ferreira C. R., Sobreira T. J. P., Schinckel A., and Stewart K. (2018). The potential of identifying replacement gilts by screening for lipid biomarkers in reproductive tract swabs taken at weaning. J. Appl. Anim. Res. 46, 667–676. doi: 10.1080/09712119.2017.1384733
Clark L., Schinckel A., Singleton W., Einstein M., and Teclaw R. (1989). Use of farrowing rate as a measure of fertility of boars. J. Am. Veterinary Med. Assoc. 194, 239–243. doi: 10.2460/javma.1989.194.02.239
Collodel G., Castellini C., Lee J. C.-Y., and Signorini C. (2020). Relevance of fatty acids to sperm maturation and quality. Oxid. Med. Cell. Longevity 2020, 7038124. doi: 10.1155/2020/7038124
Cordeiro F. B., Cataldi T. R., do Vale Teixeira da Costa L., de Lima C. B., Stevanato J., Zylbersztejn D. S., et al. (2015). Follicular fluid lipid fingerprinting from women with PCOS and hyper response during IVF treatment. J. Assist. Reprod. Genet. 32, 45–54. doi: 10.1007/s10815-014-0375-0
de Lima C. B., Ferreira C. R., Milazzotto M. P., Sobreira T. J. P., Vireque A. A., and Cooks R. G. (2018). Comprehensive lipid profiling of early stage oocytes and embryos by MRM profiling. J. mass spectrometry: JMS 53, 1247–1252. doi: 10.1002/jms.4301
de Lima C. B., Milazzotto M. P., Vireque A. A., Joaquim D. C., Sobreira T. J. P., and Ferreira C. R. (2024). Impact of extraction methods and transportation conditions on lipid profiles of bovine oocytes. Reprod. Sci. 31, 1948–1957. doi: 10.1007/s43032-024-01524-9
Ding N., Zhang Y., Wang J., Liu J., Zhang J., Zhang C., et al. (2025). Lipidomic and transcriptomic characteristics of boar seminal plasma extracellular vesicles associated with sperm motility. Biochim. Biophys. Acta (BBA) - Mol. Cell Biol. Lipids 1870, 159561. doi: 10.1016/j.bbalip.2024.159561
Du X., Zhang Y., Li D., Han J., Liu Y., Bai L., et al. (2024). Metabolites assay offers potential solution to improve the rooster semen cryopreservation. Theriogenology 221, 9–17. doi: 10.1016/j.theriogenology.2024.03.009
Erickson M. C. (2002). Chemistry and function of phospholipids, Food Lipids (Boca Raton, FL: CRC Press), 60–81.
Flowers W. L. (1997). Management of boars for efficient semen production. J. Reprod. fertility. Supplement 52, 67–78.
Flowers W. (2013). Triennial Reproduction Symposium: sperm characteristics that limit success of fertilization. J. Anim. Sci. 91, 3022–3029. doi: 10.2527/jas.2012-5945
Fuchs B., Müller K., Göritz F., Blottner S., and Schiller J. (2007). Characteristic oxidation products of choline plasmalogens are detectable in cattle and roe deer spermatozoa by MALDI-TOF mass spectrometry. Lipids 42, 991–998. doi: 10.1007/s11745-007-3108-7
Goodwin R. (2004). Tracking Maternal Line Differences National Hog Farmer. Cleveland, OH: Penton Media, Inc.
Hafemeister T., Schulze P., Simmet C., Jung M., Fuchs-Kittowski F., and Schulze M. (2023). Intensity and duration of vibration emissions during shipping as interacting factors on the quality of boar semen extended in beltsville thawing solution. Animals 13, 952. doi: 10.3390/ani13050952
Hai E., Li B., Zhang J., and Zhang J. (2024). Sperm freezing damage: the role of regulated cell death. Cell Death Discov. 10, 239. doi: 10.1038/s41420-024-02013-3
Harlow K., Ferreira C. R., Sobreira T. J. P., Casey T., and Stewart K. (2019). Lipidome profiles of postnatal day 2 vaginal swabs reflect fat composition of gilt’s postnatal diet. PLoS One 14. doi: 10.1371/journal.pone.0215186
Jrad-Lamine A., Henry-Berger J., Damon-Soubeyrand C., Saez F., Kocer A., Janny L., et al. (2013). Indoleamine 2,3-dioxygenase 1 (ido1) is involved in the control of mouse caput epididymis immune environment. PLoS One 8, e66494. doi: 10.1371/journal.pone.0066494
Jrad-Lamine A., Henry-Berger J., Gourbeyre P., Damon-Soubeyrand C., Lenoir A., Combaret L., et al. (2011). Deficient tryptophan catabolism along the kynurenine pathway reveals that the epididymis is in a unique tolerogenic state. J. Biol. Chem. 286, 8030–8042. doi: 10.1074/jbc.M110.172114
Keller A. and Kerns K. (2023). Sperm capacitation as a predictor of boar fertility. Mol. Reprod. Dev. 90, 594–600. doi: 10.1002/mrd.23690
Kocer A., Henry-Berger J., Noblanc A., Champroux A., Pogorelcnik R., Guiton R., et al. (2015). Oxidative DNA damage in mouse sperm chromosomes: Size matters. Free Radical Biol. Med. 89, 993–1002. doi: 10.1016/j.freeradbiomed.2015.10.419
Li M., Bai Y., Zhang J., Wang H., Li J., and Wang W. (2025). Sperm metabolomics identifies freezability markers in Duroc, Landrace, and Large White boars. Theriogenology 240, 117395. doi: 10.1016/j.theriogenology.2025.117395
Liu Y., Cao X., He C., Guo X., Cai H., Aierken A., et al. (2022). Effects of ferroptosis on male reproduction. Int. J. Mol. Sci. 23. doi: 10.3390/ijms23137139
Maes D., Nauwynck H., Rijsselaere T., Mateusen B., Vyt P., de Kruif A., et al. (2008). Diseases in swine transmitted by artificial insemination: an overview. Theriogenology 70, 1337–1345. doi: 10.1016/j.theriogenology.2008.06.018
Mateo-Otero Y., Fernández-López P., Gil-Caballero S., Fernandez-Fuertes B., Bonet S., Barranco I., et al. (2020). (1)H nuclear magnetic resonance of pig seminal plasma reveals intra-ejaculate variation in metabolites. Biomolecules 10. doi: 10.3390/biom10060906
Matsuda Y., Tobari I., Maemori M., and Seki N. (1989). Mechanism of chromosome aberration induction in the mouse egg fertilized with sperm recovered from postmeiotic germ cells treated with methyl methanesulfonate. Mutat. Research/Fundamental Mol. Mech. Mutagenesis 214, 165–180. doi: 10.1016/0027-5107(89)90161-9
Menezes E., Velho A., Santos F., Dinh T., Kaya A., Topper E., et al. (2019). Uncovering sperm metabolome to discover biomarkers for bull fertility. BMC Genomics 20, 1–16. doi: 10.1186/s12864-019-6074-6
Miller D. J. (2024). Sperm in the mammalian female reproductive tract: surfing through the tract to try to beat the odds. Annu. Rev. Anim. Biosci. 12, 301–319. doi: 10.1146/annurev-animal-021022-040629
Mills K. M., Aryal U. K., Sobreira T., Minton A. M., Casey T., and Stewart K. R. (2020). Shotgun proteome analysis of seminal plasma differentiate boars by reproductive performance. Theriogenology 157, 130–139. doi: 10.1016/j.theriogenology.2020.07.013
Mills K. M., Ferreria C. R., Stevens J., Stewart K. R., and Casey T. M. (2021). Biomarkers predictive of long-term fertility found in vaginal lipidome of gilts at weaning. J Anim Sci. 99, skab189. doi: 10.21203/rs.3.rs-210669/v1
Mills K. M., Minton A. M., and Ferreira C. R. (2024). Adapting lipidomic sample processing methods for boars housed in commercial settings. Trans. Anim. Sci. 8, txae139. doi: 10.1093/tas/txae139
Mills K., Sheets J., Teeple K., Mann A., Suarez-Trujillo A., Stewart K., et al. (2023). Low colostrum intake results in potential accumulation of peroxisome lipid substrates in vaginal tissue of 3-week-old gilts. Biol. Open 12, bio060044. doi: 10.1242/bio.060044
Minton A., Johnson A., Werner T., Triemert E., Holden N., Foxcroft G., et al. (2013). Evaluation and economic impact of boar fertility. doi: 10.5555/20133148105
Ostojic S. M., Stea T. H., and Engeset D. (2022). Creatine as a promising component of paternal preconception diet. Nutrients 14. doi: 10.3390/nu14030586
Pang Z., Lu Y., Zhou G., Hui F., Xu L., Viau C., et al. (2024). MetaboAnalyst 6.0: towards a unified platform for metabolomics data processing, analysis and interpretation. Nucleic Acids Res. 52, W398-W406. doi: 10.1093/nar/gkae253
Penny P., Noble R., Maldjian A., and Cerolini S. (2000). Potential role of lipids for the enhancement of boar fertility and fecundity. Pig News Inform. 21, 119-126. doi: 10.5555/20003031339
Pizzimenti S., Ciamporcero E., Daga M., Pettazzoni P., Arcaro A., Cetrangolo G., et al. (2013). Interaction of aldehydes derived from lipid peroxidation and membrane proteins. Front. Physiol. 4, 242. doi: 10.3389/fphys.2013.00242
Rashki Ghaleno L., Alizadeh A., Drevet J. R., Shahverdi A., and Valojerdi M. R. (2021). Oxidation of sperm DNA and male infertility. Antioxidants (Basel) 10. doi: 10.3390/antiox10010097
Reis L. G., Casey T. M., Sobreira T. J. P., Cooper B. R., and Ferreira C. R. (2023). Step-by-step approach to build multiple reaction monitoring (MRM) profiling instrument acquisition methods for class-based lipid exploratory analysis by mass spectrometry. J. Biomol Tech 34. doi: 10.7171/3fc1f5fe.1972c438
Robinson J. A. B. and Buhr M. M. (2005). Impact of genetic selection on management of boar replacement. Theriogenology 63, 668–678. doi: 10.1016/j.theriogenology.2004.09.040
Rodríguez-Gil J. E. and Estrada E. (2013). “Artificial insemination in boar Reproduction,” in Boar Reproduction: Fundamentals and New Biotechnological Trends. Eds. Bonet S., Casas I., Holt W. V., and Yeste M. (Springer Berlin Heidelberg, Berlin, Heidelberg), 589–607.
Rodríguez-Martínez H. and Peña Vega F. (2013). Semen technologies in domestic animal species. Anim. Front. 3, 26–33. doi: 10.2527/af.2013-0030
Rooke J., Shao C., and Speake B. (2001). Effects of feeding tuna oil on the lipid composition of pig spermatozoa and in vitro characteristics of semen. Reproduction-Cambridge- 121, 315–322. doi: 10.1530/rep.0.1210315
Sancho S., Rodríguez-Gil J. E., Pinart E., Briz M., Garcia-Gil N., Badia E., et al. (2006). Effects of exposing boars to different artificial light regimens on semen plasma markers and “in vivo” fertilizing capacity. Theriogenology 65, 317–331. doi: 10.1016/j.theriogenology.2005.05.031
Schäfer J., Waberski D., Jung M., and Schulze M. (2017). Impact of holding and equilibration time on post-thaw quality of shipped boar semen. Anim. Reprod. Sci. 187, 109–115. doi: 10.1016/j.anireprosci.2017.10.014
Seumois G., Fillet M., Gillet L., Faccinetto C., Desmet C., François C., et al. (2007). De novo C16- and C24-ceramide generation contributes to spontaneous neutrophil apoptosis. J. leukocyte Biol. 81, 1477–1486. doi: 10.1189/jlb.0806529
Siskind L. J., Kolesnick R. N., and Colombini M. (2006). Ceramide forms channels in mitochondrial outer membranes at physiologically relevant concentrations. Mitochondrion 6, 118–125. doi: 10.1016/j.mito.2006.03.002
Sprenger H.-G., MacVicar T., Bahat A., Fiedler K. U., Hermans S., Ehrentraut D., et al. (2021). Cellular pyrimidine imbalance triggers mitochondrial DNA–dependent innate immunity. Nat. Metab. 3, 636–650. doi: 10.1038/s42255-021-00385-9
Suo J., Wang J., Zheng Y., Xiao F., Li R., Huang F., et al. (2024). Recent advances in cryotolerance biomarkers for semen preservation in frozen form–A systematic review. PLoS One 19, e0303567. doi: 10.1371/journal.pone.0303567
Umehara T., Kawai T., Goto M., Richards J. S., and Shimada M. (2018). Creatine enhances the duration of sperm capacitation: a novel factor for improving in vitro fertilization with small numbers of sperm. Hum. Reprod. 33, 1117–1129. doi: 10.1093/humrep/dey081
Umehara T., Tsujita N., Goto M., Tonai S., Nakanishi T., Yamashita Y., et al. (2020). Methyl-beta cyclodextrin and creatine work synergistically under hypoxic conditions to improve the fertilization ability of boar ejaculated sperm. Anim. Sci. J. 91, e13493. doi: 10.1111/asj.13493
Vadnais M. L. and Althouse G. C. (2011). Characterization of capacitation, cryoinjury, and the role of seminal plasma in porcine sperm. Theriogenology 76, 1508–1516. doi: 10.1016/j.theriogenology.2011.06.021
Wang Y., Fu X., and Li H. (2025). Mechanisms of oxidative stress-induced sperm dysfunction. Front. Endocrinol. 16. doi: 10.3389/fendo.2025.1520835
Watson A. D. (2006). Lipidomics: a global approach to lipid analysis in biological systems. J. Lipid Res. 47, 2101. doi: 10.1194/jlr.R600022-JLR200
Weide T., Mills K., Shofner I., Breitzman M. W., and Kerns K. (2024). Metabolic shift in porcine spermatozoa during sperm capacitation-induced zinc flux. Int. J. Mol. Sci. 25, 7919. doi: 10.3390/ijms25147919
Xia J., Broadhurst D. I., Wilson M., and Wishart D. S. (2013). Translational biomarker discovery in clinical metabolomics: an introductory tutorial. Metabolomics 9, 280–299. 10.1007/s11306-012-0482-9
Xie Z., Ferreira C. R., Virequ A. A., and Cooks R. G. (2021). Multiple reaction monitoring profiling (MRM profiling): Small molecule exploratory analysis guided by chemical functionality. Chem. Phys. Lipids 235, 105048. doi: 10.1016/j.chemphyslip.2021.105048
Xu B., Bai X., Zhang J., Li B., Zhang Y., Su R., et al. (2023). Metabolomic analysis of seminal plasma to identify goat semen freezability markers. Front. Veterinary Sci. 10, 1132373. doi: 10.3389/fvets.2023.1132373
Yeste M. (2015). Recent advances in boar sperm cryopreservation: state of the art and current perspectives. Reprod. Domest. Anim. 50, 71–79. doi: 10.1111/rda.12569
Yeste M. (2016). Sperm cryopreservation update: Cryodamage, markers, and factors affecting the sperm freezability in pigs. Theriogenology 85, 47–64. doi: 10.1016/j.theriogenology.2015.09.047
Zhang Y., Liang H., Liu Y., Zhao M., Xu Q., Liu Z., et al. (2021). Metabolomic analysis and identification of sperm freezability-related metabolites in boar seminal plasma. Animals 11, 1939. doi: 10.3390/ani11071939
Keywords: boar, cryopreservation, lipidome, biomarker, metabolome
Citation: Mills K, Minton A, Berndtson J, Willenburg K and Ferreira CR (2025) A multi-omics approach identifies candidate biomarkers predictive of boar cryotolerance and conception rate. Front. Anim. Sci. 6:1665783. doi: 10.3389/fanim.2025.1665783
Received: 14 July 2025; Accepted: 14 October 2025;
Published: 06 November 2025.
Edited by:
Muhammet Rasit Ugur, IVF Michigan Fertility Centers, United StatesReviewed by:
Naseer A. Kutchy, University of Alabama at Birmingham, United StatesMartin Ntawubizi, University of Rwanda, Rwanda
Copyright © 2025 Mills, Minton, Berndtson, Willenburg and Ferreira. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Kayla Mills, a2F5bGEubWlsbHNAYWN1ZmFzdHN3aW5lLmNvbQ==
 Kayla Mills
Kayla Mills Amanda Minton2
Amanda Minton2 Christina R. Ferreira
Christina R. Ferreira