- 1Department of Animal Science, Federal University of Viçosa, Viçosa, Minas Gerais, Brazil
- 2Department of Animal Bioscience, University of Guelph, Guelph, ON, Canada
- 3Cargill Animal Nutrition, Campinas, São Paulo, Brazil
- 4Evonik Brasil Ltda, São Paulo, Brazil
- 5Department of Animal Science, Federal University of Lavras, Lavras, Minas Gerais, Brazil
Guanidinoacetic acid (GAA) is a precursor of creatine and is an arginine-sparing compound that may improve energy metabolism and muscle growth. Its potential in beef cow–calf systems, however, is still poorly understood. This study evaluated the effects of supplementing pregnant cows with GAA during late gestation on muscle development and adipogenesis in beef calves. A total of 24 pregnant Brahman cows carrying male or female fetuses received either a control diet or a diet supplemented with 0.2% GAA from day 180 to day 270 of gestation. Cows were weighed at the beginning and at the end of the trial to assess body weight (BW), and daily feed intake was recorded. Blood was collected on day 227 of gestation for plasma amino acid profiling, and the carcass traits were assessed via ultrasound. At 45 days of age, muscle biopsies were collected for mRNA expression and protein abundance. All statistical analyses were performed in SAS Studio using a mixed model including the fixed effects of treatment and offspring sex. In cows, GAA supplementation did not affect the BW, average daily gain, or feed intake (p > 0.05), but increased the plasma arginine, citrulline, and ornithine levels (p ≤ 0.02) and the final ribeye area (p = 0.01). The calves from GAA-supplemented cows exhibited increased p-Akt/Akt (p = 0.03) and p-mTOR/mTOR (p < 0.01) ratios, with treatment × sex interactions (p = 0.02). The MYOD1 mRNA expression was upregulated (p = 0.01), whereas MYOG remained unchanged (p = 0.14). The PAX7 protein tended to be higher (p = 0.07) and PAX3 reduced (p = 0.01) in GAA calves. No differences were detected for the adipogenic markers. These findings suggest that maternal GAA supplementation can stimulate muscle development in beef calves without altering intramuscular adipogenesis, indicating a potential strategy to enhance muscle growth programming in cow–calf production systems.
1 Introduction
Pregnancy is a nutritionally demanding process, requiring substantial amounts of ATP to support conceptus development through biosynthesis, nutrient transport, metabolism, and tissue remodeling (Aldoretta and Hay, 1995; Johnson et al., 2023; Seo et al., 2021). In beef cattle production, maternal nutrition directly influences fetal skeletal muscle development and ultimately affects the growth efficiency and long-term productivity of the offspring (Costa et al., 2021; Santos et al., 2022; Barcelos et al., 2022). Maternal supplementation with protein and energy may result in different outcomes on postnatal performance by modulating the energy metabolism and muscle growth (Shokrollahi et al., 2024; Kladt et al., 2025; Shokrollahi et al., 2025) and by increasing the intramuscular fat deposition (Marquez et al., 2017; Nascimento et al., 2024; Sanglard et al., 2023; Carvalho et al., 2022). Moreover, nutrition strategies or feed additives that enhance placental vascularization may increase fetal nutrient availability, promoting a more efficient fetal development and an improved offspring performance after birth (Reynolds et al., 2023).
Guanidinoacetic acid (GAA) is derived from amino acids and functions as a direct precursor of creatine, which is an essential molecule in the energy synthesis of the cell (Ostojic, 2015). In metabolically active tissues such as the skeletal muscle, the creatine (Cr)–creatine kinase (CK)–phosphocreatine (PCr) system plays a crucial role in buffering and shuttling ATP. The uterus, placenta, and fetus can synthesize creatine and contribute to its compartmentalization in fetal fluids, which serve as nutrient reservoirs for fetal development (Sah et al., 2025). Studies have shown the dynamic expression of the Cr–CK–PCr system at the maternal–placental interface, with increased creatine biosynthesis and ATP transport activity during critical phases such as implantation (Seo et al., 2021; Guingand et al., 2024). Although creatine supplementation in late-gestation sows did not alter the Cr–CK–PCr system, the detection of its key components in the endometrium and fetal muscle highlights the importance of this system in meeting the elevated energy demands during late gestation and in supporting fetal development (Lopez et al., 2025).
Due to its thermal instability and high production cost, creatine has not been widely adopted in animal nutrition as a feed additive (Baker, 2009). GAA, on the other hand, has been widely studied not only as a creatine precursor but also as an efficient arginine-sparing molecule (Liu et al., 2015; Sousa et al., 2024; Yan et al., 2021). Compared with creatine, it presents higher bioavailability due to the presence of multiple transporters, effectively elevates hepatic and muscular creatine, shows good thermal stability and lower cost (Khajali et al., 2020; Giraldi et al., 2024), and has been proven safe in monogastric species, with no concerns for consumer safety under approved conditions of use (EFSA, 2022). Arginine is an essential amino acid particularly relevant in fetal development due to its involvement in multiple metabolic processes, such as cellular signaling and protein synthesis (Wu and Morris, 1998; Morris, 2007).
Although the fetal origins of muscle development and energy metabolism are increasingly recognized, the role of maternal GAA supplementation in these processes remains only partially understood. Sousa et al. (2024), in a companion study conducted within the same experimental framework, demonstrated that maternal GAA supplementation can enhance the placental blood flow through nitric oxide (NO) pathways and highlighted its biochemical capacity as an arginine-sparing compound. While these findings provide important mechanistic insights, they were focused on maternal and placental adaptations and did not address downstream effects on fetal muscle tissue. Therefore, the present study uniquely investigates how maternal GAA supplementation during late gestation influences early skeletal muscle development and intramuscular adipogenesis in beef calves. This study expands on these previous findings by directly evaluating postnatal skeletal muscle and adipogenic pathways, providing novel insights into how maternal GAA supplementation during late gestation may influence offspring growth and metabolic programming.
2 Materials and methods
2.1 Animals, treatments, and experimental design
All animal care and handling procedures were previously approved by the Animal Care and Use Committee of the Department of Animal Science at the Universidade Federal de Viçosa, Viçosa, Minas Gerais, Brazil (protocol 04/2022).
The methodology used in this study has been previously described by Sousa et al. (2024). A total of 24 pregnant Brahman cows (532 ± 15.1 kg) carrying male (n = 12) or female (n = 12) fetuses were used. Briefly, cows were subjected to a fixed-time artificial insemination protocol with four attempts using semen from the same sire. Therefore, four groups of cows were established based on the days of gestation. After pregnancy confirmation, the cows were managed as a single herd on Marandu palisade grass (Urochloa brizantha). On day 170 of gestation, the cows were housed individually for a 10-day adaptation period and fed a basal diet ad libitum (Sousa et al., 2024). From day 180 to day 270, the cows received either a control (CON) diet or a diet supplemented with 0.2% GAA (dry matter basis) (GuanAMINO®; Evonik Operations GmbH, Hanau-Wolfgang, Germany). GAA (GuanAMINO®; Evonik Operations GmbH, Hanau-Wolfgang, Germany) was incorporated into the mineral mixture (Probeef Reprodução Seca; Cargill Nutrição Animal, Itapira, SP, Brazil). On day 270, the cows were moved to pasture for calving. Postpartum, the cow–calf pairs remained as a single herd until weaning (210 days). From day 90 onward, calves had access to creep-feeding (5.0 g/kg body weight, BW). The composition of the supplement provided through the creep-feeding system is shown in Table 1.
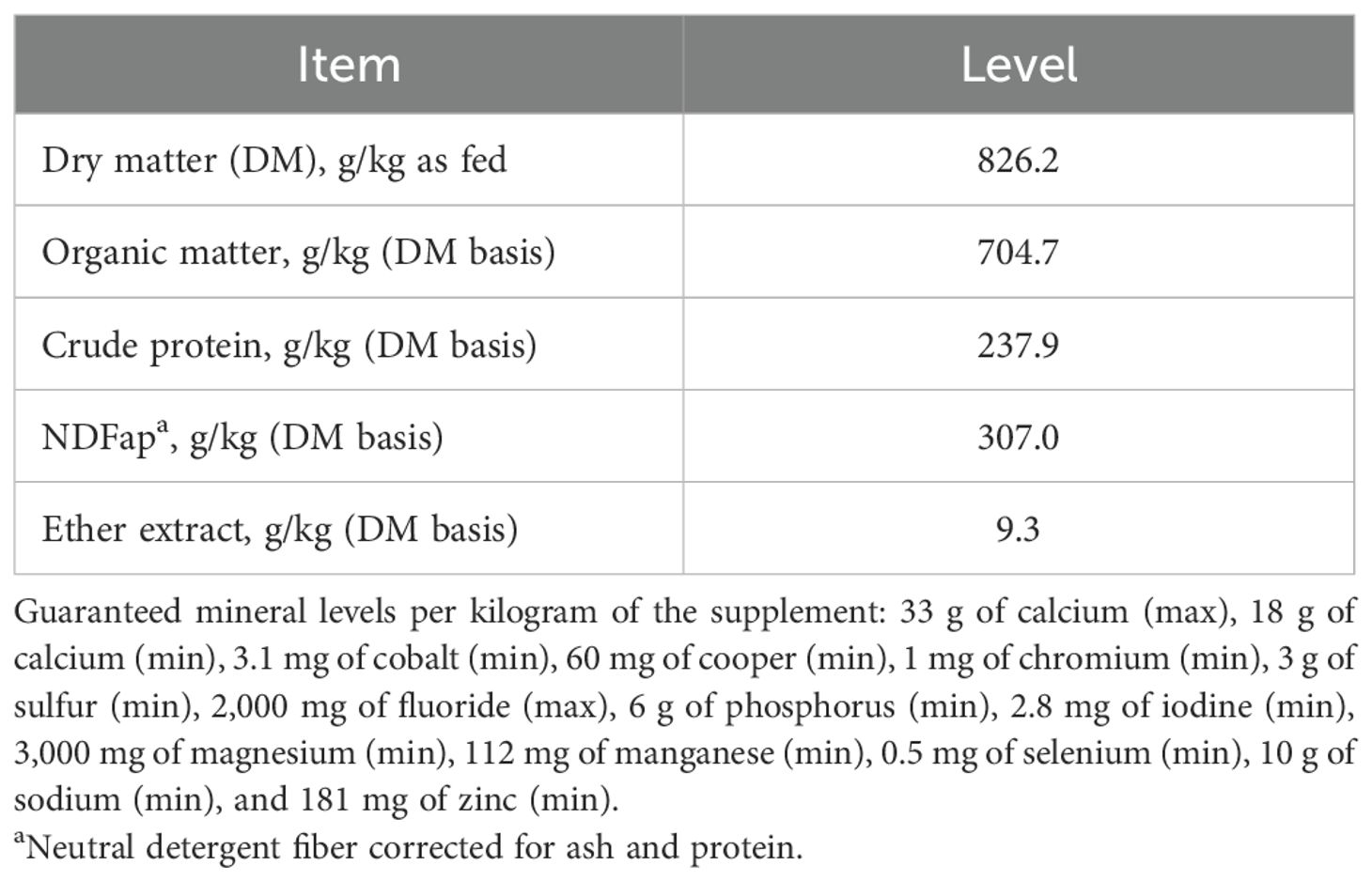
Table 1. Nutrient composition of the supplement (Probeef® Bambini Creep) provided to the calves through the creep-feeding system.
2.2 Cows’ performance and metabolic evaluation
The cows were weighed at the beginning and at the end of the experiment, following a 16-h fasting period, to determine the initial body weight (IBW) and the final body weight (FBW) and to calculate the average daily gain (ADG) during the experimental period.
The voluntary feed intake of each cow was individually recorded daily throughout the experimental period. Concentrate samples were collected separately for each production batch, while forage and orts samples were collected daily and stored at −20°C for subsequent nutrient composition analysis.
On day 227 of gestation, blood samples were taken via jugular venipuncture into vacuum tubes (Vacuplast® Collect Time, Cotia, SP, Brazil) containing sodium heparin for analysis of the plasma amino acid profile and immediately frozen (−20°C) for subsequent analysis. Plasma amino acids, such as arginine, citrulline, and ornithine, were quantified by high-performance liquid chromatography (Xevo™ TQD; Waters Corporation, Milford, MA, USA).
Carcass measurements of the cows were evaluated at the beginning and at the end of the experiment via ultrasound (Aloka SSD500II; Mitaka, Tokyo, Japan) using a 3.5-MHz linear probe. The initial and final ribeye areas (IREA and FREA, respectively) were measured at the Longissimus lumborum (12th–13th ribs). Images were analyzed using BioSoft Toolbox® II for Beef (Biotronics, Ames, IA, USA).
2.3 Calf skeletal muscle sampling
At 45 days of age, Longissimus lumborum muscle samples (located between the 12th and 13th ribs) were biopsied from the calves. Briefly, the area was cleaned with 70% ethanol, and then the incision was made 10 min after local anesthesia (2% lidocaine). The muscle sample was washed with sterile saline solution (0.9% NaCl). The muscle samples were immediately snap-frozen and powdered on liquid nitrogen using a pre-cold mortar and pestle. The samples were stored at −80°C until mRNA and protein expression analyses.
2.4 Total RNA extraction and mRNA expression analyses
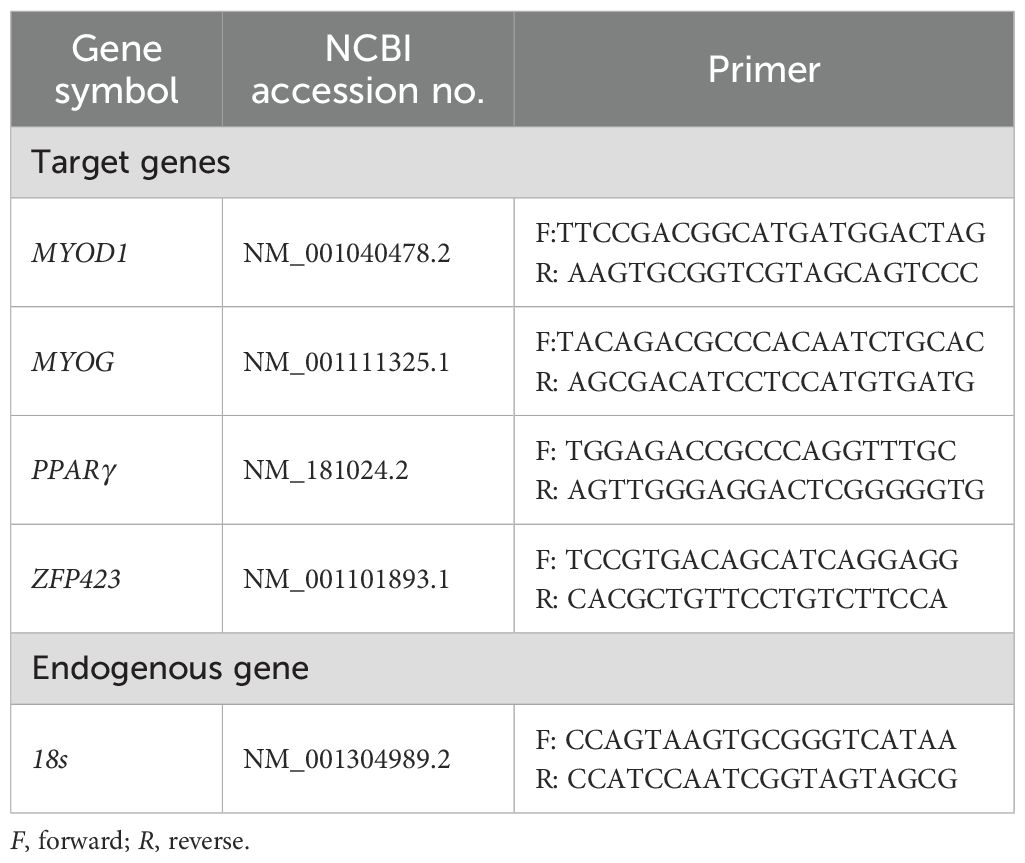
Total RNA was extracted from 0.1 g of tissue using TRIzol® (Invitrogen™, Thermo Fisher Scientific®, Hillsboro, OR, USA) following the manufacturer’s recommendations. The total RNA was quantified with a NanoDrop spectrophotometer (Thermo Fisher Scientific®, Waltham, MA, USA), ensuring an optimal 260:280 ratio between 1.8 and 2.0, and the integrity was assessed in a 1% agarose gel. The RNA samples were reverse-transcribed into cDNA using the High-Capacity cDNA Reverse Transcription Kit (Applied Biosystems, Foster City, CA, USA). The primers (Table 2) for the amplification of the target and endogenous genes were designed using PrimerQuest Software (PrimerQuest–design qPCR assays | IDT; idtdna.com) with sequences obtained from GenBank (GenBank Overview; nih.gov). Real-time quantitative PCR was performed in the thermal cycler QuantStudio 3 (Applied Biosystems, Foster City, CA, USA) using the SYBR Green detection method (Applied Biosystems, Foster City, CA, USA) and the SYBR™ Green PCR Master Mix (Invitrogen™, Thermo Fisher Scientific®, Hillsboro, OR, USA). The results of gene expression were calculated according to the methods described by Steibel et al. (2009).
2.5 Protein extraction and Western blotting analyses
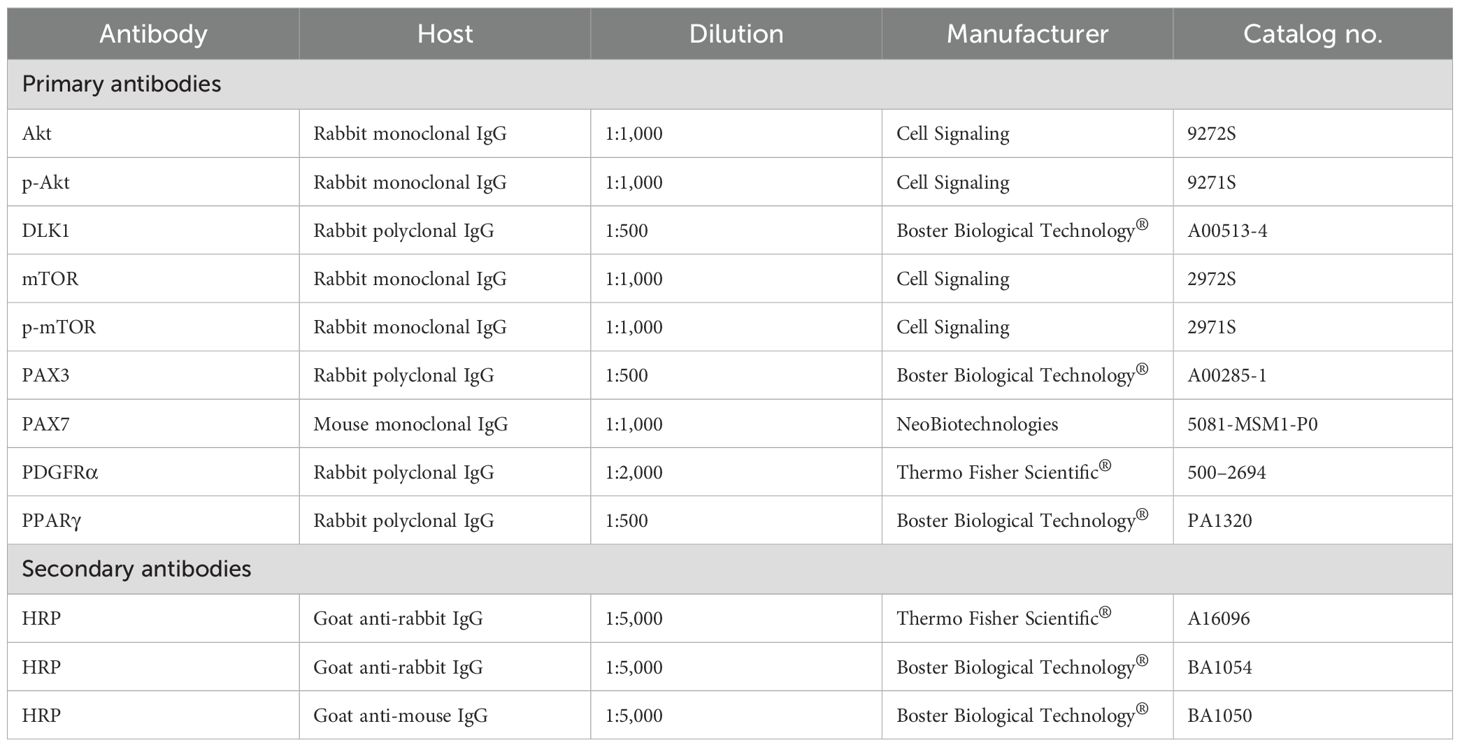
Total protein was extracted from 0.1 g of tissue in 1 ml of lysis buffer [10 mM of Tris–HCl (pH 7.6), 150 mM of NaCl, 1% of Triton X-100, 0.5% sodium deoxycholate, 1% sodium dodecyl sulfate (SDS), and 1% of protease inhibitor cocktail mammalian cells and tissues] (Sigma-Aldrich®, St. Louis, MO, USA), and the lysate was sonicated. The total protein content was estimated using the Bradford protein assay (Bio-Rad, Hercules, CA, USA), aliquoted, and stored at −80°C. The proteins were separated using a 10% SDS-PAGE gel loaded with 40 μg of protein per sample, transferred into a 0.45-μm nitrocellulose membrane (Bio-Rad, Hercules, CA, USA) using a semi-dry Trans-Blot Turbo Transfer System (Bio-Rad, Hercules, CA, USA), and blocked for 1 h at room temperature with 3% bovine serum albumin (BSA) (Sigma Aldrich®, St. Louis, MO, USA) in 1× Tris-buffered saline (TBS1×; 50 mM Tris–HCl, pH 7.5, 150 mM NaCl) (Sigma Aldrich®, St. Louis, MO, USA). Subsequently, the membranes were incubated for 12 h at 4°C with primary antibodies (Table 3) diluted in blocking solution. After 12 h of incubation, the membranes were washed three times for 5 min with Tris-buffered saline and 0.1% Tween® (TBST) and incubated with a secondary antibody (Table 3) diluted in blocking solution for 1 h at room temperature. The membranes were then washed with TBST three times for 7 min, revealed with the ECL Plus Western Blotting Detection System (GE HealthCare, Buckinghamshire, UK), and the images generated with the ChemiDoc XRS+ System (Bio-Rad, Hercules, CA, USA) and evaluated using Image Lab Software (Bio-Rad, Hercules, CA, USA). For internal control, two reference samples from the same tissue and experiment were loaded on each gel. The internal control that had a greater band intensity (expressed in optical densitometry units) was used to normalize the remaining samples as described by Cruzen et al. (2014).
2.6 Statistical analysis
The protein abundance data were analyzed using a mixed model that included the fixed effects of maternal treatment (CON or GAA) and sex and the random effect of gestation group. The variables were assessed for homoscedasticity of the error variances between treatments using Levene’s test. Prior to the final analysis, the residuals were evaluated for normality. For each analysis, data points were removed one at a time until absolute Studentized residuals were lower than 3 and had non-significant (p > 0.01) Shapiro–Wilk’s test for normality (only one data point was excluded in the analysis of PPARγ protein abundance). Expected means were generated from the final models and separated using Tukey’s test when significant (p < 0.05).
Prior to the analysis of the RT-PCR data, the values were adjusted () for their respective primer efficiency and back-transformed to the scale. Afterward, the data were analyzed according to the linear mixed model below, following the strategy proposed by Steibel et al. (2009), where the data on the target and endogenous control genes are analyzed simultaneously. Treatment and sex effects were assessed through orthogonal contrasts, as in Steibel et al. (2009). Expected were computed as , such that the differences between the levels of treatment and sex were estimated as and then used to compute the gene ratio expression (GRE) as:
Estimates of GREs were separated using contrasts when significant (p < 0.05).
When pertinent, the IBW and IREA were used as covariates. If the effect of these variables was found to be non-significant, the model was reparameterized by excluding them. All analyses were performed in SAS Studio 3.81 (Enterprise Edition, SAS Institute Inc., Cary, NC, USA).
3 Results
3.1 Maternal performance and metabolism
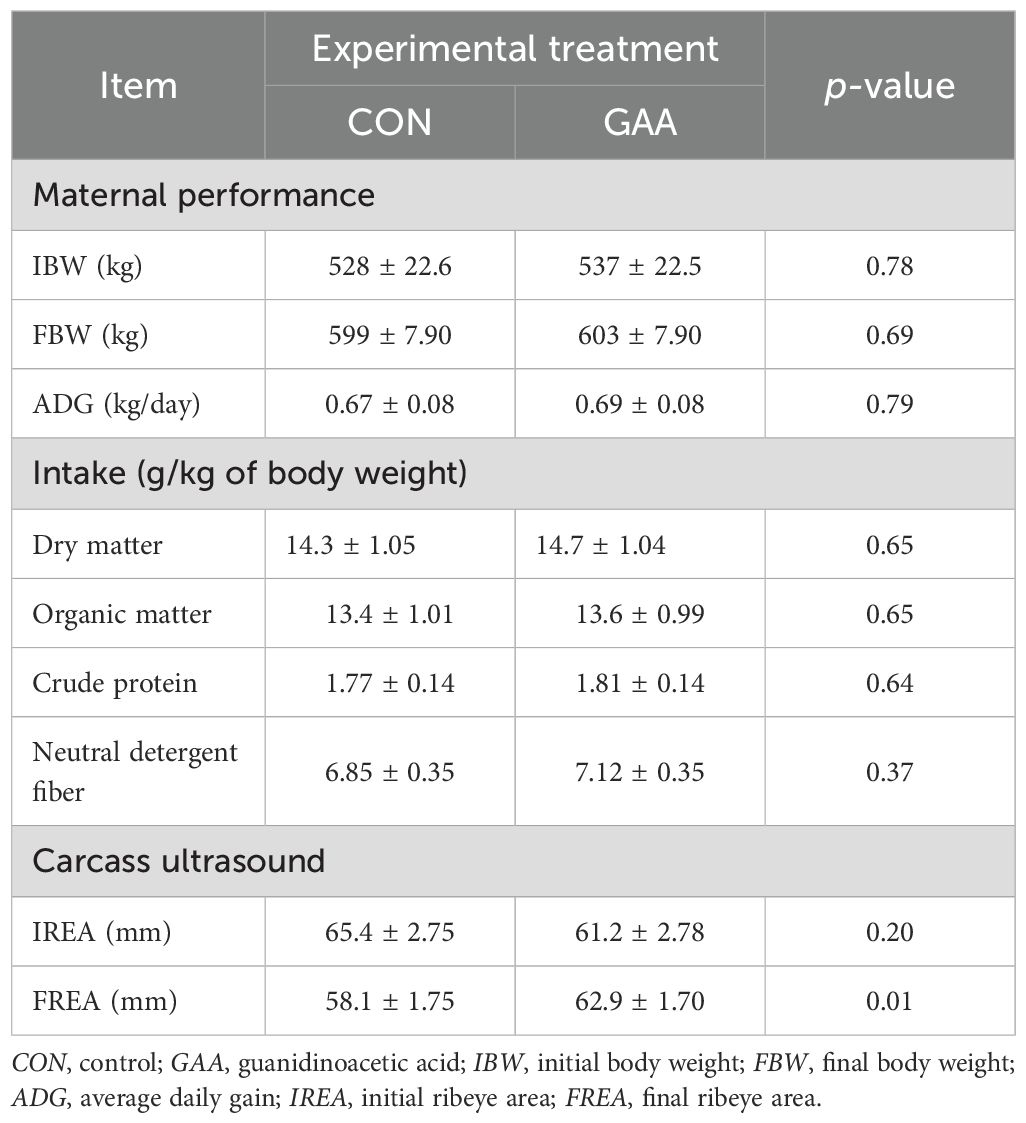
The complete results on the performance of cows were thoroughly discussed by Sousa et al. (2024). In brief, the IBW (p = 0.78) and the FBW (p = 0.69) did not differ between treatments, nor did the ADG (in kilograms per day) during the experimental period (p = 0.79) (Table 4).
In addition, no differences were observed in the voluntary intake (in grams per kilogram of BW) of dry matter (p = 0.65), organic matter (p = 0.65), crude protein (p = 0.64), or neutral detergent fiber (p = 0.37) (Table 4).
The plasma concentrations of arginine (p = 0.01), citrulline (p = 0.02), and ornithine (p = 0.01) were greater in GAA cows compared with the CON (Figure 1).
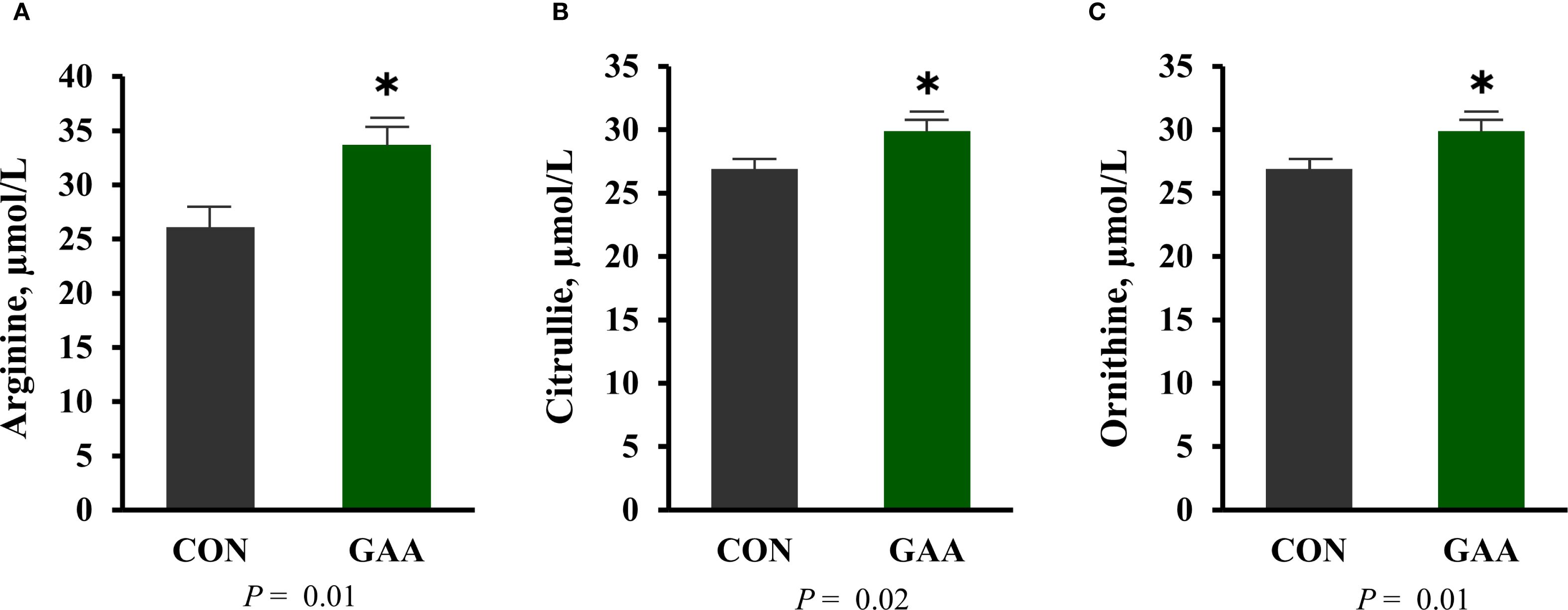
Figure 1. (A–C) Least squares mean ± SEM of the maternal plasma concentration (in micromoles per liter) of arginine (A), citrulline (B), and ornithine (C). Differences (*) were considered when p ≤ 0.05.
Regarding the carcass ultrasound measurements (in millimeters), no differences were observed in the IREA (p = 0.20) between groups. However, the cows from the GAA group showed greater FREA (p = 0.01) compared with the CON group (Table 4).
3.2 Protein abundance and mRNA expression of the muscle development markers in offspring
Calves born to GAA-supplemented cows exhibited a greater p-Akt/Akt ratio compared with those in the CON group (p = 0.03). A treatment × sex interaction was observed (p = 0.02), where the GAA-supplemented male calves had a higher p-Akt/Akt ratio than the CON males, while no differences were detected between the female calves from both groups (Figure 2).
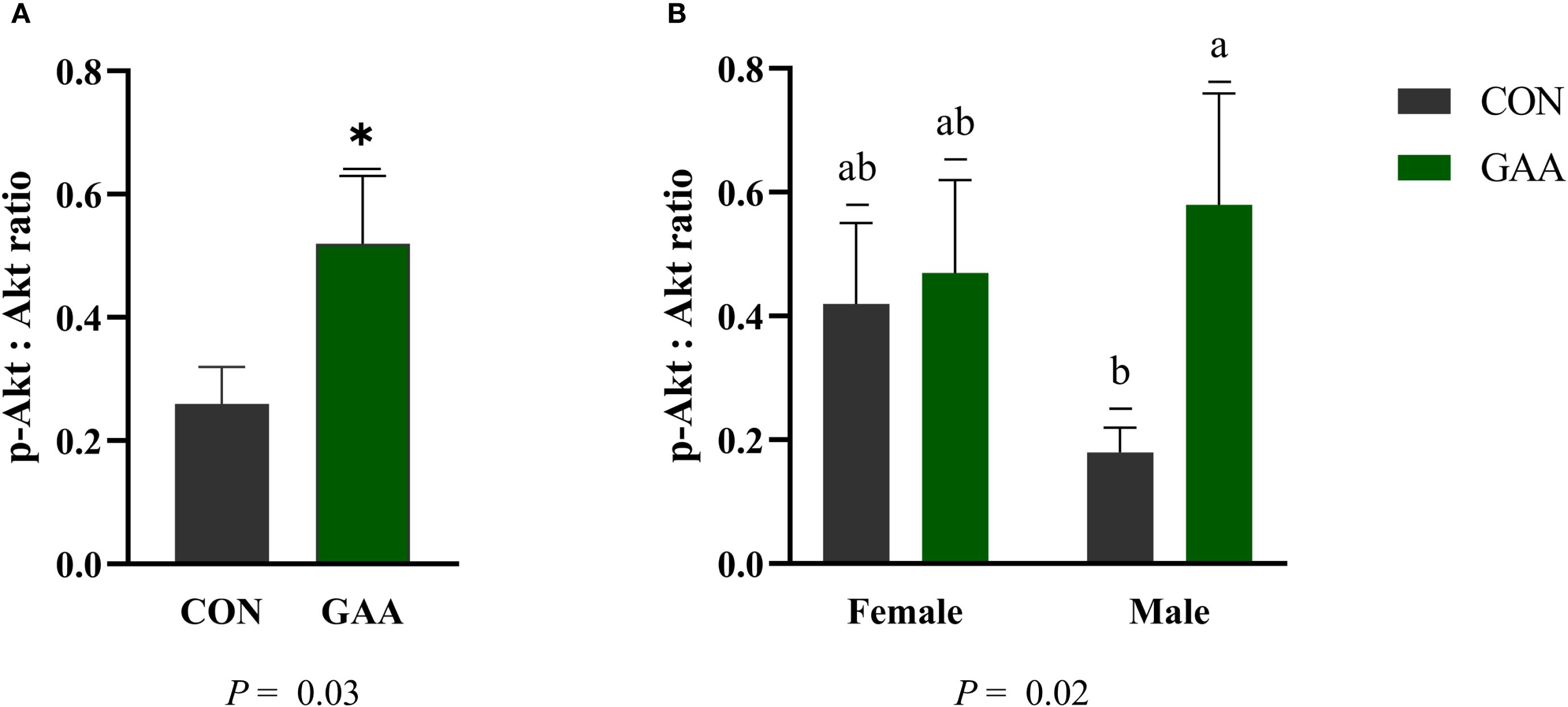
Figure 2. (A) Least squares mean ± SEM of the protein abundance of the phosphoprotein kinase B (p-Akt)/protein kinase B (Akt) ratio in the skeletal muscle of calves born to beef cows supplemented with guanidinoacetic acid (GAA) (n = 12) or the control (CON) (n = 12) at late gestation. Differences (*) were considered when p ≤ 0.05. (B) Least squares mean ± SEM of the protein abundance of the p-Akt/Akt ratio in the skeletal muscle of calves born to beef cows supplemented with GAA or CON at late gestation, showing the interaction between treatment and sex. Differences (A, B) were considered when p ≤ 0.05.
Similarly, the phospho-mechanistic target of rapamycin (p-mTOR)/mechanistic target of rapamycin (mTOR) ratio was higher in calves from the GAA-supplemented cows than in those from the CON group (p = 0.004). A significant treatment × sex interaction was found (p = 0.02), with the GAA-supplemented female calves showing a greater p-mTOR/mTOR ratio than the CON females, whereas no differences were observed among males (Figure 3).
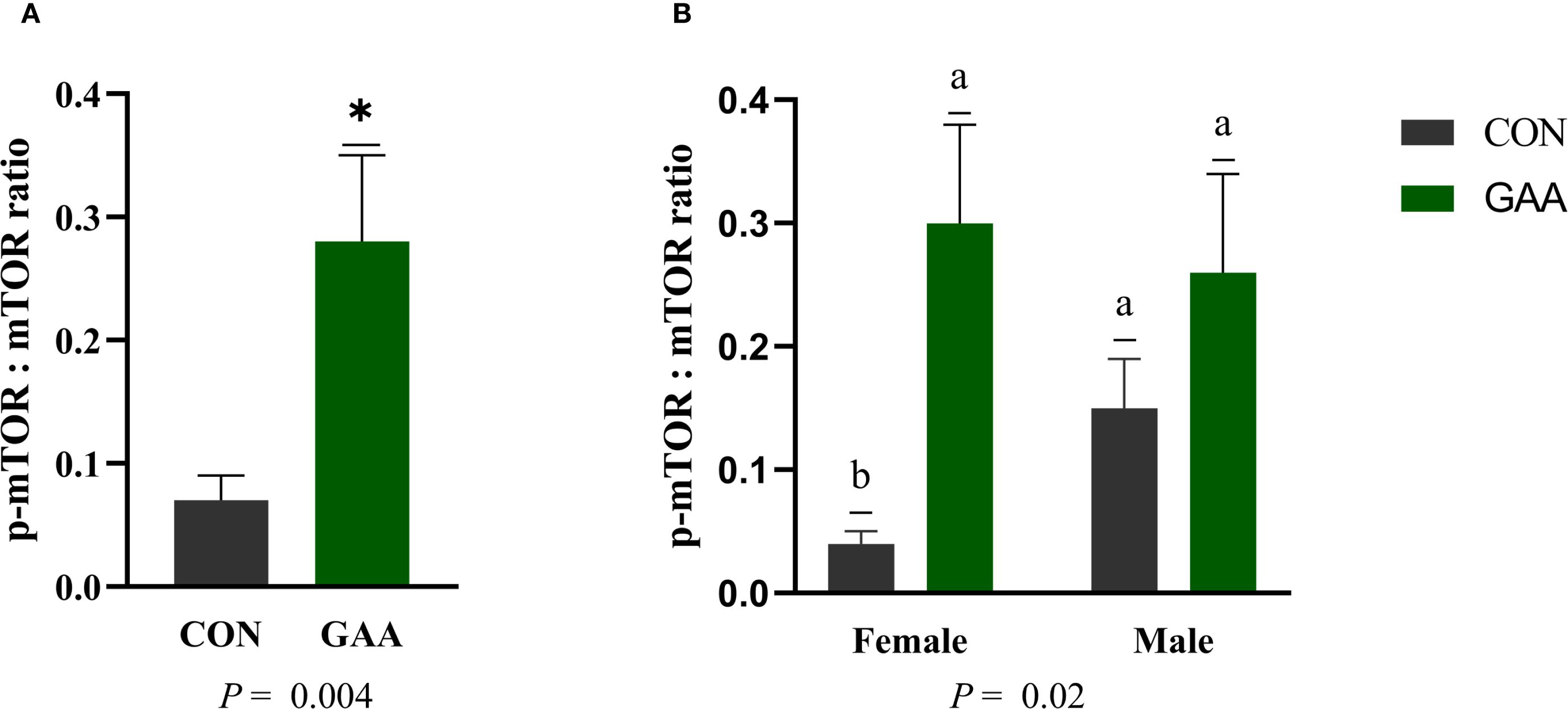
Figure 3. (A) Least squares mean ± SEM of the protein abundance of the phospho-mechanistic target of rapamycin kinase (p-mTOR)/mechanistic target of rapamycin kinase (mTOR) ratio in the skeletal muscle of calves born to beef cows supplemented with guanidinoacetic acid (GAA) (n = 12) or the control (CON) (n = 12) at late gestation. Differences (*) were considered when p ≤ 0.05. (B) Least squares mean ± SEM of the protein abundance of the p-mTOR/mTOR ratio in the skeletal muscle of calves born to beef cows supplemented with GAA or CON at late gestation, showing the interaction between treatment and sex. Differences (A, B) were considered when p ≤ 0.05.
The protein abundance of PAX3 was greater in CON calves compared with GAA calves (p = 0.01). A treatment × sex interaction was observed (p = 0.02), where CON males exhibited higher PAX3 abundance than GAA males, but no differences were observed among females. A tendency for greater PAX7 abundance was found in GAA calves compared with CON calves (p = 0.07), with no treatment × sex interaction detected (Figure 4).
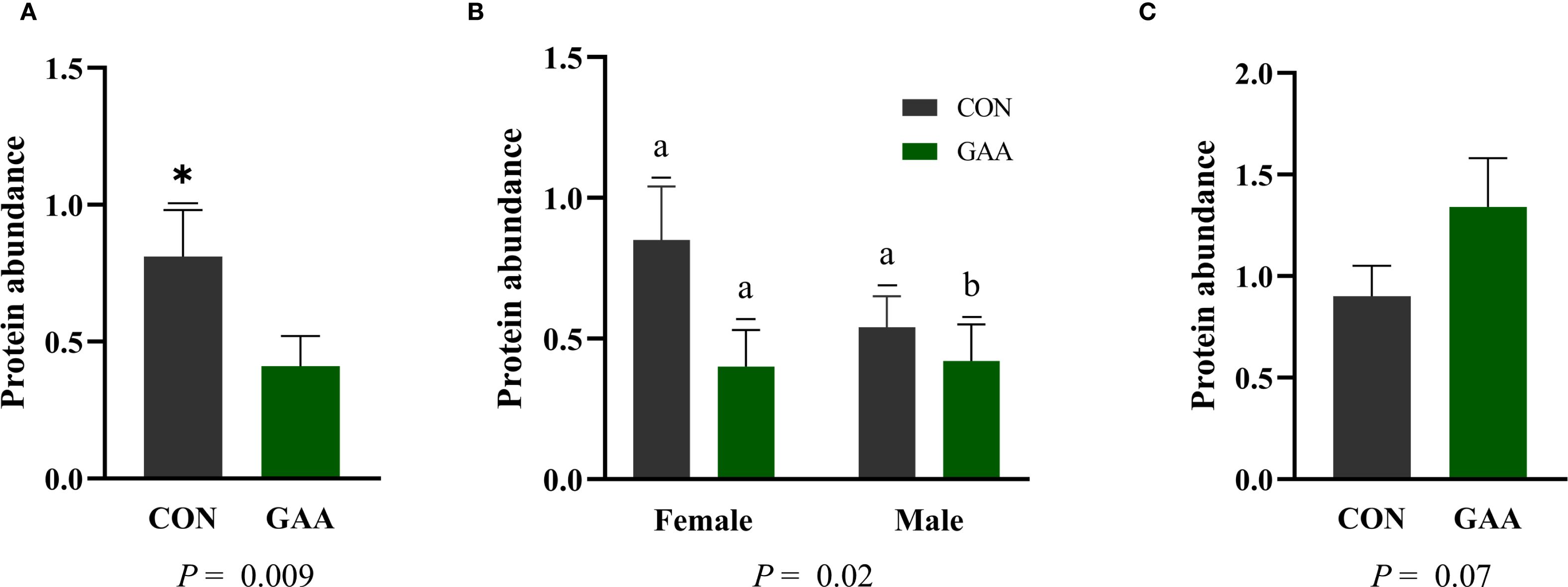
Figure 4. (A) Least squares mean ± SEM of the protein abundance of paired box 3 (PAX3) in the skeletal muscle of calves born to beef cows supplemented guanidinoacetic acid (GAA) (n = 12) or the control (CON) (n = 12) at late gestation. Differences (*) were considered when p ≤ 0.05. (B) Least squares mean ± SEM of the protein abundance of PAX3 in the skeletal muscle of calves born to beef cows supplemented with guanidinoacetic acid (GAA) (n = 12) or control (CON) (n = 12) at late gestation, showing the interaction between treatment and sex. Differences (A, B) were considered when p ≤ 0.05. (C) Least squares mean ± SEM of the protein abundance of paired box 7 (PAX7) in the skeletal muscle of calves born to beef cows supplemented with GAA (n = 12) or CON (n = 12) at late gestation. Differences were considered when p ≤ 0.05.
Regarding mRNA expression, the expression of MYOD1 was significantly higher in GAA calves than in CON calves (p = 0.01), whereas the expression of MYOG did not differ between groups (p = 0.14) (Figure 5).
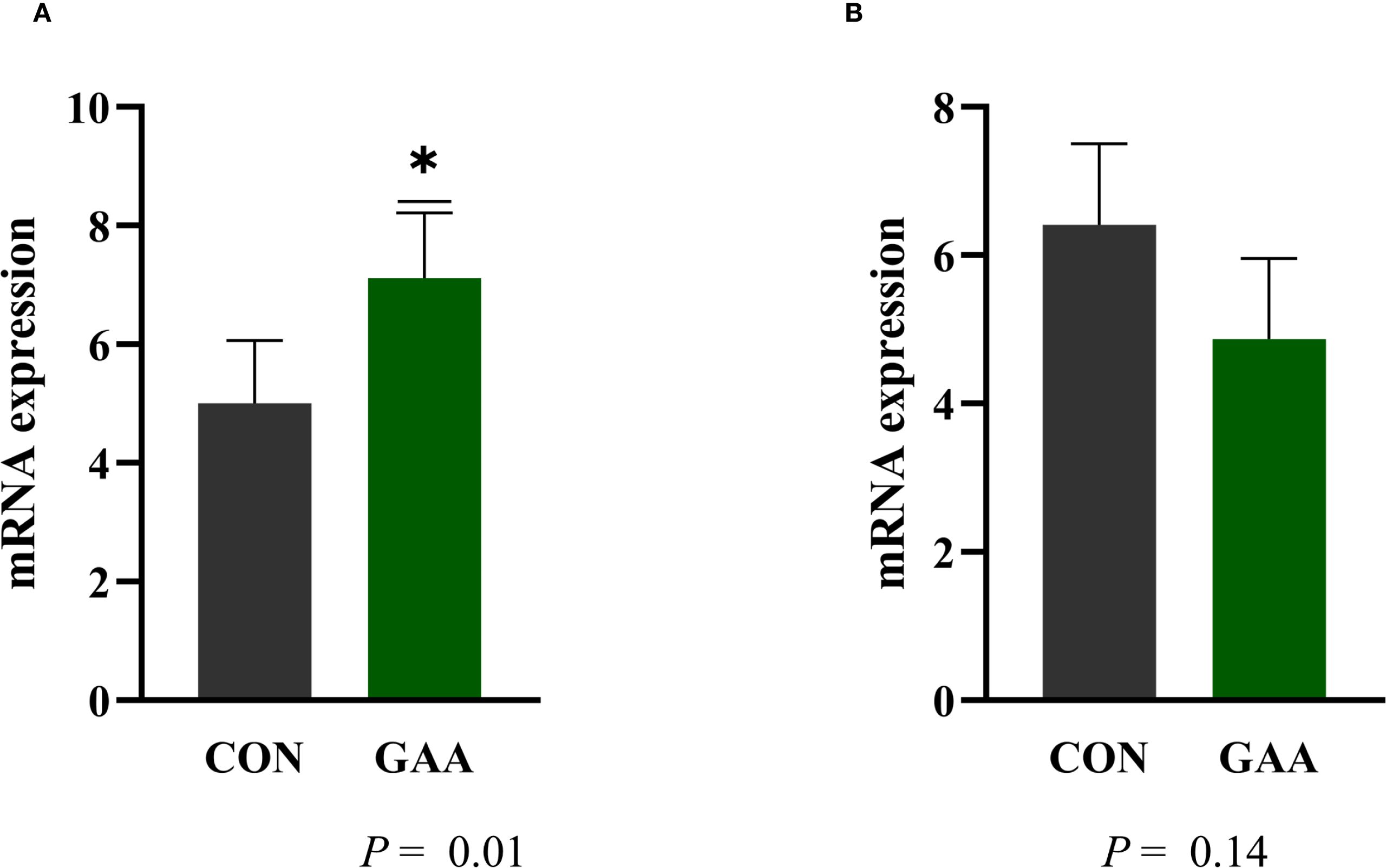
Figure 5. Least squares mean ± SEM of the mRNA expression of myogenic differentiation 1 (MYOD1) (A) and myogenin (MYOG) (B) in the skeletal muscle of calves born to beef cows supplemented with guanidinoacetic acid (GAA) (n = 12) or the control (CON) (n = 12) at late gestation. Differences (*) were considered when p ≤ 0.05.
3.3 Protein abundance and mRNA expression of the fibroadipogenic and adipogenic markers in the skeletal muscle of calves
No differences were observed between the GAA and CON groups in the protein abundance of DLK1 (p = 0.10), PPARγ (p = 0.10), or PDGFRα (p = 0.29) (Table 5).
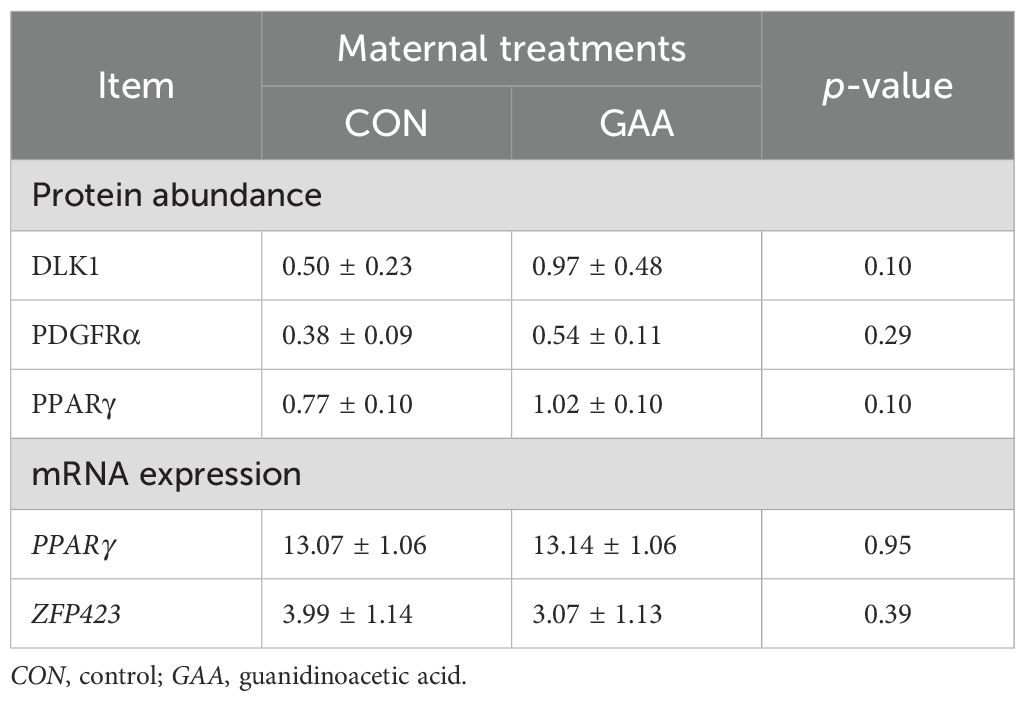
Table 5. Least squares mean ± SEM of the protein abundance and mRNA expression of the fibroadipogenic and adipogenic markers in the skeletal muscle of the offspring per treatment.
Similarly, the mRNA expression of PPARγ (p = 0.95) and ZFP423 (p = 0.39) did not differ between groups (Table 5).
4 Discussion
Given the energy demands on gestation and the central role of creatine in cellular energy buffering, strategies that modulate creatine metabolism, such as GAA supplementation, have been extensively studied in different species and production phases (Yan et al., 2021; Esser et al., 2018). In our study, cows that received GAA supplementation during late gestation exhibited greater ribeye area compared with the non-supplemented cows, despite no differences in the ADG or dry matter intake between treatments. This finding suggests that GAA supplementation may have attenuated the extent of maternal tissue mobilization typically observed during late gestation, possibly by enhancing the efficiency of energy metabolism and reducing the metabolic burden on maternal reserves. Despite the observed changes, it must be noted that, ruminal degradation may have limited the systemic availability of dietary GAA, as only 47%–49% is estimated to escape the rumen intact (Speer et al., 2020). This constraint has been highlighted in studies employing post-ruminal GAA infusion, which demonstrated that bypassing ruminal degradation significantly enhances the creatine supply in cattle (Ardalan et al., 2020). These findings underscore the importance of the delivery method in determining the physiological response to GAA supplementation. It is noteworthy that, in the present study, the serum and urinary creatine concentrations remained unchanged (Sousa et al., 2024). Previous research has shown that newly synthesized creatine is rapidly taken up by high-demand tissues such as the muscle and the brain, with the plasma creatine levels typically returning to baseline within 1–3 h post-synthesis or supplementation (Wyss and Kaddurah-Daouk, 2000; Brosnan and Brosnan, 2007). In addition, the kidneys reabsorb a significant portion of filtered creatine, limiting urinary excretion unless the tissue stores are saturated (Asiriwardhana et al., 2024). Given that the cows in this study were at late gestation, a stage marked by substantially elevated energy and nutrient demands due to the exponential growth of the fetuses, it is plausible that any increase in the systemic creatine supply was rapidly utilized by maternal and fetal tissues to support the intensified metabolic activity. This heightened demand may help explain the absence of detectable changes in the blood and urinary creatine concentrations following GAA supplementation. Although creatine was likely rapidly utilized to meet the heightened metabolic demands of late gestation, the concurrent increases in plasma arginine, ornithine, and citrulline suggest a metabolic adaptation favoring efficient resource allocation. These elevations indicate a reduced need for arginine as a substrate for endogenous GAA synthesis, as supported by the observed downregulation of hepatic AGAT activity (Sousa et al., 2024). Moreover, the greater plasma ornithine and citrulline concentrations may have enhanced arginine regeneration through the urea cycle, thereby supporting higher systemic arginine availability.
Citrulline also serves as a precursor for de novo arginine synthesis and participates in the arginine–NO pathway, which directly supports NO production. The elevated plasma arginine and citrulline concentrations observed in the GAA-supplemented cows may have contributed to the increased NO synthesis, as confirmed by the higher serum NO in GAA cows (Sousa et al., 2024). As NO is a crucial vasodilator that enhances placental blood flow (Morris, 2007; Elmetwally et al., 2022), its increase may have supported the greater placental vascularization observed in GAA cows (Sousa et al., 2024). This increased placental blood flow may enhance the nutrient delivery to the fetus, with glucose being the primary substrate transferred (Baumann et al., 2002).
In previous studies, arginine supplementation not only increased the plasma insulin concentrations but also activated the IGF-1/insulin/Akt/mTOR signaling pathway, which is the main pathway involved in protein synthesis and muscle growth (Dou et al., 2023; Yao et al., 2008). This mechanistic link provides a potential explanation for the greater activation of Akt and mTOR observed in calves born to GAA-supplemented cows. Two primary mechanisms may underline this effect: firstly, the increased nutrient availability, potentially driven by the higher creatine concentrations derived from GAA, which could elevate the levels of IGF-1, a key upstream regulator of Akt (Liu et al., 2021; Li et al., 2022); secondly, the greater availability of arginine itself, which can directly activate mTOR in a NO-dependent manner (Wang et al., 2018a). Nevertheless, the lack of direct measurements of IGF-1 or insulin, as well as the downstream markers of energy metabolism such as mitochondrial function and ATP content, represents a limitation of the present study.
Beyond nutrient availability, intrinsic fetal factors such as sex and the developmental stage appear to modulate the responsiveness of fetal tissues to maternal supplementation strategies. Earlier studies have reported sex-related differences in muscle development, with male fetuses displaying greater myogenic gene expression (Gionbelli et al., 2018) and enhanced muscle growth during the same developmental window (Barcelos et al., 2022). More recently, Sah et al. (2025) provided evidence that the creatine biosynthesis pathway and its regulatory enzymes are modulated in a sex- and stage-specific manner, with female offspring exhibiting higher expression of creatine transporters and biosynthetic enzymes in distinct tissues and gestational stages. These findings reinforce the need to investigate whether maternal GAA supplementation interacts with fetal sex to modulate muscle energy metabolism.
In the present study, sex-specific treatment interactions were also observed, with Akt activation being more pronounced in GAA males and mTOR activation higher in GAA females. However, these findings cannot be fully explained by the current knowledge on sex-related differences in muscle development and creatine metabolism. Evidence from placental and fetal biology supports the existence of sexual dimorphism in mTOR regulation, but the mechanistic basis remains poorly understood. For instance, Akhaphong et al. (2021) reported sex-dependent differences in a genetic model with placental mTOR deletion, although the underlying regulatory pathways were not clarified. Similarly, Sedlmeier et al. (2021) showed sexually dimorphic expression of placental mTOR and amino acid transporters in response to maternal diet, but without a definitive mechanistic explanation. Beetch and Alejandro (2021) also emphasized the need for mechanistic studies to clarify how placental mTOR perturbations drive sexually dimorphic metabolic outcomes. Collectively, these findings indicate that sex-specific regulation of mTOR signaling is evident, but incompletely understood, with epigenetic regulation remaining a plausible contributor. Thus, while our study revealed distinct sex-specific interactions for Akt and mTOR, the mechanistic basis of these observations could not be determined and must be acknowledged as a limitation.
Due to the key role of the Akt/mTOR signaling pathway in regulating satellite cell function and myogenesis (Zhang et al., 2015), we evaluated whether maternal GAA supplementation, in addition to altering the energy metabolism in calf skeletal muscle, would also influence cell commitment toward the myogenic lineage. In myogenesis, PAX3 primarily governs early skeletal muscle formation in the embryo, whereas PAX7 takes precedence in postnatal muscle growth and adult muscle progenitor proliferation to a more differentiated state (Buckingham and Relaix, 2015). Moreover, MYOD1, an early myogenic regulatory factor, is crucial for committing progenitor cells to the myogenic lineage and stimulating myoblast proliferation (Jennings et al., 2016; Berkes and Tapscott, 2005). Previous findings have shown that GAA supplementation enhances myogenic differentiation and muscle growth by increasing the expression of MYOD1, which facilitates myoblast expansion and early differentiation (Wang et al., 2018b; Yan et al., 2021). A study conducted with broiler embryos supplemented in ovo with creatine pyruvate showed increased expression of MYOD1 and PAX7 (Zhao et al., 2017). In our study, however, we observed a tendency of elevated PAX7 protein abundance in the GAA group, in addition to a greater expression of MYOD1, indicating a potentially expanded satellite cell pool, which is essential for postnatal muscle growth and regeneration. Taken together, increased activation of the Akt/mTOR pathway, particularly with sex-specific patterns, and the upregulation of the satellite (PAX7) and myogenic (MYOD1) marker indicate a potential enhancement of the myogenic commitment and expansion phase.
Considering that maternal supplementation during late gestation can improve maternal body condition and the uterine environment, as well as stimulate the fibroadipogenic progenitor cells and promote adipogenic differentiation in the offspring (Du et al., 2013; Duarte et al., 2014; Du et al., 2015, 2023; Du, 2023), and given that GAA functions as a direct precursor of creatine, a key molecule for ATP production, together with our observation of the higher final REA in GAA-supplemented cows, we hypothesized that maternal GAA supplementation during this period could potentially influence the commitment of fibroadipogenic progenitor cells toward mature adipocytes. Nevertheless, no significant differences were observed in the expression of the key fibroadipogenic markers DLK1, PPARγ, PDGFRα, and ZFP423 between calves born to GAA-supplemented dams and those in the CON group. This lack of detectable effect may reflect the prioritization of the Cr–CK–PCr system in meeting the high energy demands of maternal and fetal tissues during late gestation (Lopez et al., 2025). Consequently, GAA appears to act more efficiently as an arginine-sparing strategy, enhancing the downstream pathways such as Akt/mTOR signaling and NO-mediated metabolism rather than directly modulating the classical adipogenic signaling in fetal skeletal muscle.
5 Conclusions
Maternal GAA supplementation during late gestation in beef cows may reduce the mobilization of maternal reserves, as well as enhance placental function, through the sparing of arginine for NO synthesis and improved vascularization. These systemic changes were accompanied by the activation of Akt/mTOR signaling and the upregulation of key myogenic markers (MYOD1 and PAX7) in the offspring, indicating evidence of early molecular responses that suggest a potential enhancement in muscle growth programming.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The animal study was approved by Animal Care and Use Committee of the Department of Animal Science at the Universidade Federal de Viçosa. The study was conducted in accordance with the local legislation and institutional requirements.
Author contributions
LK: Data curation, Formal Analysis, Investigation, Methodology, Writing – original draft, Writing – review & editing. TC: Investigation, Methodology, Writing – original draft, Writing – review & editing. LS: Conceptualization, Investigation, Methodology, Writing – original draft, Writing – review & editing. MS: Investigation, Methodology, Writing – original draft, Writing – review & editing. JV: Writing – original draft, Writing – review & editing. LR: Investigation, Methodology, Writing – original draft, Writing – review & editing. LM: Investigation, Methodology, Writing – original draft, Writing – review & editing. WS: Data curation, Investigation, Methodology, Visualization, Writing – original draft, Writing – review & editing. PP: Conceptualization, Investigation, Writing – original draft, Writing – review & editing. TR: Conceptualization, Investigation, Methodology, Writing – original draft, Writing – review & editing. CS: Investigation, Methodology, Project administration, Writing – original draft, Writing – review & editing. MG: Conceptualization, Investigation, Methodology, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. MD: Conceptualization, Data curation, Formal Analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. We thank Cargill Animal Nutrition and Evonik (grant no. 044-2022), NSERC (grant no. 401862), CNPq – Conselho Nacional de Desenvolvimento Científico e Tecnológico (grant no. 313858/2021-7; grant no. 173980/2023-0), CAPES – Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (grant no. 001), and FAPEMIG (Grant# APQ-02902-24), for the funding support. The funders were not involved in the study design, collection, analysis, interpretation of data, the writing of this article, or the decision to submit it for publication.
Conflict of interest
Author TR was employed by the company Evonik Brasil Ltda. Author PP was employed by company Cargill Animal Nutrition.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fanim.2025.1671346/full#supplementary-material
References
Akhaphong B., Baumann D. C., Beetch M., Lockridge A. D., Jo S., Wong A., et al. (2021). Placental mTOR complex 1 regulates fetal programming of obesity and insulin resistance in mice. JCI Insight 6, e149271. doi: 10.1172/jci.insight.149271
Aldoretta P. W. and Hay W. W. (1995). Metabolic substrates for fetal energy metabolism and growth. Clin. Perinatol. 22, 15–36. doi: 10.1016/S0095-5108(18)30299-9
Ardalan M., Batista E. D., and Titgemeyer E. C. (2020). Effect of post-ruminal guanidinoacetic acid supplementation on creatine synthesis and plasma homocysteine concentrations in cattle. J. Anim. Sci. 98, skaa072. doi: 10.1093/jas/skaa072
Asiriwardhana M. U., Dinesh O. C., Brunton J. A., and Bertolo R. F. (2024). Dietary methionine enhances portal appearance of guanidinoacetate and synthesis of creatine in Yucatan miniature piglets. J. Nutr. 154, 1571–1581. doi: 10.1016/j.tjnut.2024.03.017
Baker D. H. (2009). Advances in protein-amino acid nutrition of poultry. Amino Acids 37, 29–41. doi: 10.1007/s00726-008-0198-3
Barcelos S. S., Nascimento K. B., Silva T. E., Mezzomo R., Alves K. S., Souza Duarte M., et al. (2022). The effects of prenatal diet on calf performance and perspectives for fetal programming studies: A meta-analytical investigation. Animals 12, 2145. doi: 10.3390/ani12162145
Baumann M. U., Deborde S., and Illsley N. P. (2002). Placental glucose transfer and fetal growth. Endocrine 19, 13–22. doi: 10.1385/ENDO:19:1:13
Beetch M. and Alejandro E. U. (2021). Placental mTOR Signaling and Sexual Dimorphism in Metabolic Health across the Lifespan of Offspring. Children 8, 970. doi: 10.3390/children8110970
Berkes C. A. and Tapscott S. J. (2005). MyoD and the transcriptional control of myogenesis. Semin. Cell Dev. Biol. 16, 585–595. doi: 10.1016/j.semcdb.2005.07.006
Brosnan J. T. and Brosnan M. E. (2007). Creatine: endogenous metabolite, dietary, and therapeutic supplement. Annu. Rev. Nutr. 27, 241–261. doi: 10.1146/annurev.nutr.27.061406.093621
Buckingham M. and Relaix F. (2015). PAX3 and PAX7 as upstream regulators of myogenesis. Semin. Cell Dev. Biol. 44, 115–125. doi: 10.1016/j.semcdb.2015.09.017
Carvalho E. B., Costa T. C., Sanglard L. P., Nascimento K. B., Meneses J. A. M., Galvão M. C., et al. (2022). Transcriptome profile in the skeletal muscle of cattle progeny as a function of maternal protein supplementation during mid-gestation. Livest. Sci. 263, 104995. doi: 10.1016/j.livsci.2022.104995
Costa T. C., Gionbelli M. P., and Duarte M. S. (2021). Fetal programming in ruminant animals: Understanding the skeletal muscle development to improve the quality of meat. Anim. Front. 12, 440–447. doi: 10.1093/af/vfab061
Cruzen S. M., Paulino P. V. R., Lonergan S. M., and Huff-Lonergan E. (2014). Postmortem proteolysis in three muscles from growing and mature beef cattle. Meat Sci. 96, 854–861. doi: 10.1016/j.meatsci.2013.09.021
Dou L., Sun L., Liu C., Su L., Chen X., Yang Z., et al. (2023). Effect of dietary arginine supplementation on protein synthesis, meat quality and flavor in growing lambs. Meat Sci. 204, 109291. doi: 10.1016/j.meatsci.2023.109291
Du M. (2023). Prenatal development of muscle and adipose and connective tissues and its impact on meat quality. Meat Muscle Biol. 7, 16230–16231. doi: 10.22175/mmb.16230
Du M., Huang Y., Das A. K., Yang Q., Duarte M. S., Dodson M. V., et al. (2013). Manipulating mesenchymal progenitor cell differentiation to optimize performance and carcass value of beef cattle. J. Anim. Sci. 91, 1419–1427. doi: 10.2527/jas2012-5670
Du M., Wang B., Fu X., Yang Q., and Zhu M. J. (2015). Fetal programming in meat production. Meat Sci. 109, 40–47. doi: 10.1016/j.meatsci.2015.04.010
Duarte M. S., Paulino P. V. R., Nascimento C. S., Botelho M. E., Martins T. S., Filho S. C. V., et al. (2014). Maternal overnutrition enhances mRNA expression of adipogenic markers and collagen deposition in skeletal muscle of beef cattle fetuses. J. Anim. Sci. 92, 3846–3854. doi: 10.2527/jas.2014-7568
EFSA Panel on Additives and Products or Substances used in Animal Feed (FEEDAP), Bampidis V., Azimonti G., Bastos M. D. L., Christensen H., Dusemund B., et al. (2022). Safety and efficacy of a feed additive consisting of guanidinoacetic acid for all animal species (Alzchem Trostberg GmbH). EFSA J. 20, e07269. doi: 10.2903/j.efsa.2022.7269
Elmetwally M. A., Li X., Johnson G. A., Burghardt R. C., Herring C. M., Kramer A. C., et al. (2022). Dietary supplementation with L-arginine between days 14 and 25 of gestation enhances NO and polyamine syntheses and the expression of angiogenic proteins in porcine placentae. Amino Acids 54, 193–204. doi: 10.1007/s00726-021-03097-2
Esser A. F. G., Taniguti T. L., Silva A. M., Vanroo E., Kaneko I. N., Santos T. C., et al. (2018). Efeito da suplementação de ácido guanidinoacético e arginina em dietas vegetais para frangos de corte sobre o desempenho, rendimento de carcaça e qualidade de carne. Semin. Cienc. Agrar. 39, 1307–1318. doi: 10.5433/1679-0359.2018v39n3p1307
Gionbelli T. R. S., Veloso C. M., Rotta P. P., Valadares Filho S. C., C. Carvalho B., Marcondes M. I., et al. (2018). Foetal development of skeletal muscle in bovines as a function of maternal nutrition, foetal sex and gestational age. J. Anim. Physiol. Anim. Nutr. 102, 545–556. doi: 10.1111/jpn.12786
Giraldi G. C., Wolschick G. J., Signor M. H., Lago R. V. P., de Souza Muniz A. L., Draszevski T. M. R., et al. (2024). Effects of dietary guanidinoacetic acid on the performance, rumen fermentation, metabolism, and meat of confined steers. Animals 14, 2617. doi: 10.3390/ani14172617
Guingand D. L., Palmer K. R., Callahan D. L., Snow R. J., Davies-Tuck M. L., and Ellery S. J. (2024). Creatine and pregnancy outcomes: a prospective cohort study of creatine metabolism in low-risk pregnant females. Am. J. Clin. Nutr. 119, 838–849. doi: 10.1016/j.ajcnut.2023.11.006
Jennings T. D., Gonda M. G., Underwood K. R., Wertz-Lutz A. E., and Blair A. D. (2016). The influence of maternal nutrition on expression of genes responsible for adipogenesis and myogenesis in the bovine fetus. Animal 10, 1697–1705. doi: 10.1017/S1751731116000665
Johnson G. A., Seo H., Bazer F. W., Wu G., Kramer A. C., McLendon B. A., et al. (2023). Metabolic pathways utilized by the porcine conceptus, uterus, and placenta. Mol. Reprod. Dev. 90, 673–683. doi: 10.1002/mrd.23570
Khajali F., Lemme A., and Rademacher-Heilshorn M. (2020). Guanidinoacetic acid as a feed supplement for poultry. World’s Poultry. Sci. J. 76, 270–291. doi: 10.1080/00439339.2020.1716651
Kladt L. V., Jiang M., Li Z., Veloso C. M., Hare K. S., Silva W., et al. (2025). Maternal metabolizable energy intake during late gestation affect energy metabolism of the skeletal muscle of beef offspring. J. Anim. Sci. 103, skaf203. doi: 10.1093/jas/skaf203
Li X., Liu X., Song P., Zhao J., Zhang J., and Zhao J. (2022). Skeletal muscle mass, meat quality and antioxidant status in growing lambs supplemented with guanidinoacetic acid. Meat Sci. 192, 108906. doi: 10.1016/j.meatsci.2022.108906
Liu Y. J., Chen J. Z., Wang D. H., Wu M. J., Zheng C., Wu Z. Z., et al. (2021). Effects of guanidinoacetic acid and coated folic acid supplementation on growth performance, nutrient digestion and hepatic gene expression in Angus bulls. Br. J. Nutr. 126, 510–517. doi: 10.1017/S0007114520004341
Liu Y., Li J. L., Li Y. J., Gao T., Zhang L., Gao F., et al. (2015). Effects of dietary supplementation of guanidinoacetic acid and combination of guanidinoacetic acid and betaine on postmortem glycolysis and meat quality of finishing pigs. Anim. Feed Sci. Technol. 205, 82–89. doi: 10.1016/j.anifeedsci.2015.03.010
Lopez A. N., Olivarez M. A., Stenhouse C., Moses R. M., Newton M. G., Sah N., et al. (2025). Effects of dietary supplementation of creatine on fetal development in gilts at d 60 and d 90 of gestation. J. Anim. Sci. Biotechnol. 16, 1–12. doi: 10.1186/s40104-025-01166-0
Marquez D. C., Paulino M. F., Rennó L. N., Villadiego F. C., Ortega R. M., Moreno D. S., et al. (2017). Supplementation of grazing beef cows during gestation as a strategy to improve skeletal muscle development of the offspring. Animal 11, 2184–2192. doi: 10.1017/S1751731117000982
Morris S. M. (2007). Arginine metabolism: Boundaries of our knowledge. J. Nutr. 137, 1602S–1609S. doi: 10.1093/jn/137.6.1602S
Nascimento K. B., Galvão M. C., Meneses J. A. M., Ramírez-Zamudio G. D., Pereira D. G., Paulino P. V. R., et al. (2024). Maternal protein supplementation during mid-gestation improves offspring performance and metabolism in beef cows. J. Anim. Sci. 102, 1–17. doi: 10.1093/jas/skae058
Ostojic S. M. (2015). Advanced physiological roles of guanidinoacetic acid. Eur. J. Nutr. 54, 1211–1215. doi: 10.1007/s00394-015-1050-7
Reynolds L. P., Dahlen C. R., Ward A. K., Crouse M. S., Borowicz P. P., Davila-Ruiz B. J., et al. (2023). Role of the placenta in developmental programming: Observations from models using large animals. Anim. Reprod. Sci. 257, 107322. doi: 10.1016/j.anireprosci.2023.107322
Sah N., Stenhouse C., Halloran K. M., Moses R. M., Newton M. G., Seo H., et al. (2025). Effect of gestational age and fetal sex on metabolism of creatine by uteri, placentae, and fetuses of pigs. Biol. Reprod. 112, 728–742. doi: 10.1093/biolre/ioaf015
Sanglard L. M. P., Sampaio C. B., Duarte M. S., Marquez D. C., Silva W. M., Santos M. M., et al. (2023). Impact of maternal protein supplementation during mid or late gestation on skeletal muscle energy metabolism of beef calves. Cienc. Rural 53, 1–11. doi: 10.1590/0103-8478cr20210917
Santos M. M., Costa T. C., Ramírez-Zamudio G. D., Nascimento K. B., Gionbelli M. P., and Duarte M. S. (2022). Prenatal origins of productivity and quality of beef. Rev. Bras. Zootec. 51, e20220061. doi: 10.37496/rbz5120220061
Sedlmeier E. M., Meyer D. M., Stecher L., Sailer M., Daniel H., Hauner H., et al. (2021). Fetal sex modulates placental microRNA expression, potential microRNA-mRNA interactions, and levels of amino acid transporter expression and substrates: INFAT study subpopulation analysis of n-3 LCPUFA intervention during pregnancy and associations with offspring body composition. BMC Mol. Cell Biol. 22, 15. doi: 10.1186/s12860-021-00345-x
Seo H., Johnson G. A., Bazer F. W., Wu G., McLendon B. A., and Kramer A. C. (2021). “Cell-specific expression of enzymes for serine biosynthesis and glutaminolysis in farm animals,” in Amino acids in nutrition and health: amino acids in the nutrition of companion, zoo and farm animals (Springer International Publishing, Cham), 17–28. doi: 10.1007/978-3-030-54462-1_2
Shokrollahi B., Park M., Baek Y. C., Jin S., Jang G. S., Moon S. J., et al. (2024). Differential gene expression in neonatal calf muscle tissues from Hanwoo cows overfed during mid to late pregnancy period. Sci. Rep. 14, 23298. doi: 10.1038/s41598-024-74976-3
Shokrollahi B., Park M., Jang G.-S., Jin S., Moon S.-J., Um K.-H., et al. (2025). Maternal overnutrition in beef cattle: effects on fetal programming, metabolic health, and postnatal outcomes. Biology 14, 645. doi: 10.3390/biology14060645
Sousa L. C. O., Matos E. M. A., Santos M. M., Detmann E., Sampaio C. B., Sancler-Silva Y. F. R., et al. (2024). Dietary guanidinoacetic acid as arginine spare molecule for beef cows at late gestation: Effects on cow’s performance and metabolism, and offspring growth and development. Anim. Feed Sci. Technol. 315, 1–11. doi: 10.1016/j.anifeedsci.2024.116047
Speer H., Pearl K., and Titgemeyer E. (2020). Relative bioavailability of guanidinoacetic acid delivered ruminally or abomasally to cattle. J. Anim. Sci. 98, skaa282. doi: 10.1093/jas/skaa282
Steibel J. P., Poletto R., Coussens P. M., and Rosa G. J. M. (2009). A powerful and flexible linear mixed model framework for the analysis of relative quantification RT-PCR data. Genomics 94, 146–152. doi: 10.1016/j.ygeno.2009.04.008
Wang R., Jiao H., Zhao J., Wang X., and Lin H. (2018a). L-arginine enhances protein synthesis by phosphorylating MTOR (Thr 2446) in a nitric oxide-dependent manner in C2C12 cells. Oxid. Med. Cell. Longev. 2018, 7569127. doi: 10.1155/2018/7569127
Wang Y., Ma J., Qiu W., Zhang J., Feng S., Zhou X., et al. (2018b). Guanidinoacetic acid regulates myogenic differentiation and muscle growth through miR-133a-3p and miR-1a-3p co-mediated Akt/MTOR/S6K signaling pathway. Int. J. Mol. Sci. 19, 2837. doi: 10.3390/ijms19092837
Wu G. and Morris S. M. (1998). Arginine metabolism: Nitric oxide and beyond. Biochem. J. 336, 1–17. doi: 10.1042/bj3360001
Wyss M. and Kaddurah-Daouk R. (2000). Creatine and creatinine metabolism. Physiol. Rev. 80, 1107–1213. doi: 10.1152/physrev.2000.80.3.1107
Yan Z., Yan Z., Liu S., Yin Y., Yang T., and Chen Q. (2021). Regulative mechanism of guanidinoacetic acid on skeletal muscle development and its application prospects in animal husbandry: A review. Front. Nutr. 8. doi: 10.3389/fnut.2021.714567
Yao K., Yin Y. L., Chu W., Liu Z., Deng D., Li T., et al. (2008). Dietary arginine supplementation increases MTOR signaling activity in skeletal muscle of neonatal pigs. J. Nutr. 138, 867–872. doi: 10.1093/jn/138.5.867
Zhang P., Liang X., Shan T., Jiang Q., Deng C., Zheng R., et al. (2015). mTOR is necessary for proper satellite cell activity and skeletal muscle regeneration. Biochem. Biophys. Res.Commun. 463, 102–108. doi: 10.1016/j.bbrc.2015.05.032
Keywords: arginine, creatine, fetal programming, myogenesis, beef cattle
Citation: Kladt LV, Costa TC, Sousa LCO, Santos MM, Varizi JKC, Rodrigues LS, Motta LJM, Silva W, Paulino PVR, Resende TL, Sampaio CB, Gionbelli MP and Duarte MS (2025) Effects of maternal supplementation of guanidinoacetic acid on skeletal muscle growth and metabolism in beef offspring. Front. Anim. Sci. 6:1671346. doi: 10.3389/fanim.2025.1671346
Received: 22 July 2025; Accepted: 22 September 2025;
Published: 10 October 2025.
Edited by:
Susan Kay Duckett, Clemson University, United StatesReviewed by:
Borhan Shokrollahi, Islamic Azad University, IranSimeng Yi, China Agricultural University, China
Copyright © 2025 Kladt, Costa, Sousa, Santos, Varizi, Rodrigues, Motta, Silva, Paulino, Resende, Sampaio, Gionbelli and Duarte. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Marcio S. Duarte, bWR1YXJ0ZUB1b2d1ZWxwaC5jYQ==; bWFyY2lvLmR1YXJ0ZUB1ZnYuYnI=
 Luiza V. Kladt
Luiza V. Kladt Thaís C. Costa1
Thaís C. Costa1 Mateus P. Gionbelli
Mateus P. Gionbelli Marcio S. Duarte
Marcio S. Duarte