- 1Department of Computer Science and Biomedical Informatics, University of Thessaly, Lamia, Greece
- 2EMBL-EBI, Wellcome Genome Campus, Cambridge, United Kingdom
- 3Section of Cell Biology and Biophysics, Department of Biology, School of Sciences, National and Kapodistrian University of Athens, Athens, Greece
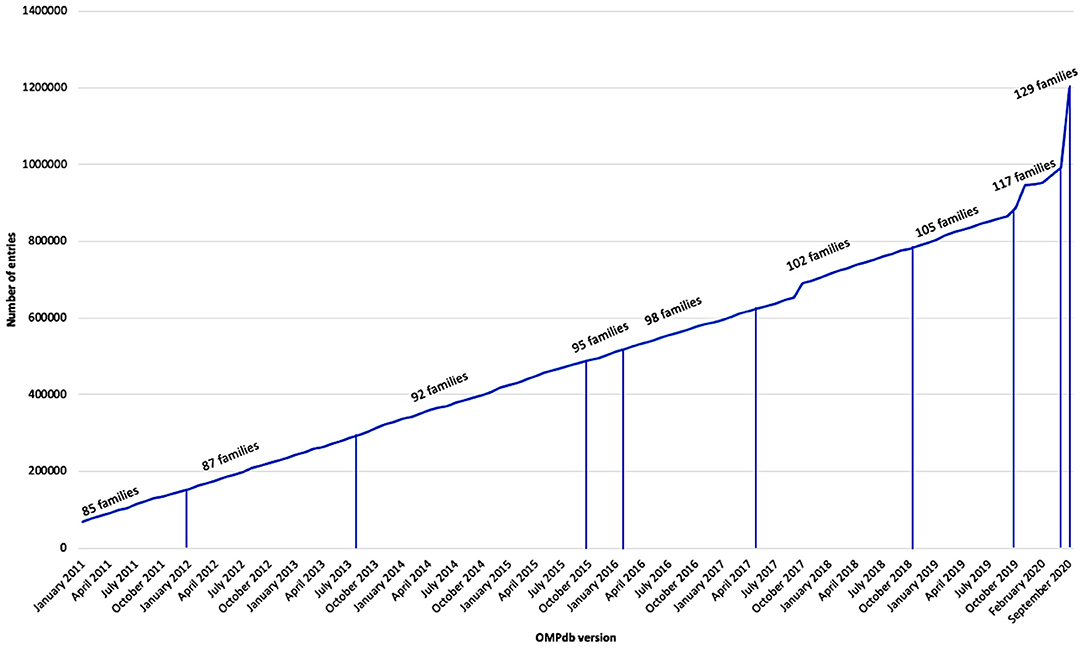
OMPdb (www.ompdb.org) was introduced as a database for β-barrel outer membrane proteins from Gram-negative bacteria in 2011 and then included 69,354 entries classified into 85 families. The database has been updated continuously using a collection of characteristic profile Hidden Markov Models able to discriminate between the different families of prokaryotic transmembrane β-barrels. The number of families has increased ultimately to a total of 129 families in the current, second major version of OMPdb. New additions have been made in parallel with efforts to update existing families and add novel families. Here, we present the upgrade of OMPdb, which from now on aims to become a global repository for all transmembrane β-barrel proteins, both eukaryotic and bacterial.
Introduction
Integral membrane proteins (IMPs) play a vital role in cell tasks and communication. IMPs represent roughly 20–30% of the human genome (von Heijne, 1999). They can be structurally divided into two distinct categories, the α-helical membrane proteins and the β-barrel ones (von Heijne, 1999). While the former are commonly found in the bacterial inner membrane and cell membranes of all eukaryotic cells, the latter are located exclusively in the outer membranes of mitochondria, chloroplasts, and Gram-negative bacteria (Cavalier-Smith, 2000). In contrast to the α-helical proteins, which are the major type of the IMPs, the β-barrel membrane proteins are fewer, comprising <3% of the proteins encoded in bacterial genomes (Casadio et al., 2003) (one order of magnitude lower than that of α-helical membrane proteins). However, the β-barrel membrane proteins participate in crucial biological activities in prokaryotic organisms, as well as in the eukaryotic organelles.
Endosymbiosis of Gram-negative bacteria ancestors with host cells generated eukaryotic organelles, chloroplasts (Kleine et al., 2009) and mitochondria (Gray et al., 1999), whose outer membranes contain pores with β-barrel topology. The eukaryotic β-barrel pores are IMPs and can be classified into three main functional groups. Firstly, the specific diffusion channels group, which consists of the voltage-dependent anion channel (VDAC) (De Pinto et al., 2010), the translocase of the outer mitochondrial membrane (TOM40) (Hill et al., 1998), the outer envelope protein 21 (OEP21) (Hemmler et al., 2006), the outer envelope protein 23 (OEP23) (Goetze et al., 2015), the outer envelope protein (OEP37) (Goetze et al., 2006) and the outer envelope protein 40 (OEP40) (Harsman et al., 2016). Secondly, the non-specific diffusion channels group, that includes only the outer envelope protein 24 (OEP24) (Pohlmeyer et al., 1998). Thirdly, the biogenesis/secretion group, that contains the mitochondrial distribution and morphology protein 10 (MDM10) (Meisinger et al., 2004), the sorting and assembly machinery (SAM50) (Stojanovski et al., 2007), the trigalactosyldiacylglycerol 4 (TGD4) (Wang et al., 2012), the translocon at the outer membrane of chloroplasts 75 (TOC75) (Hinnah et al., 2002) and the outer envelope protein 80 (OEP80) (Gross et al., 2020).
OMPdb is a database of bacterial β-barrel outer membrane proteins (βOMPs). It was launched in 2011 and is until now the most complete collection of Gram-negative bacteria βOMPs. It has been updated continuously using a collection of characteristic profile Hidden Markov Models (pHMMs) as shown in Figure 1.
Up to now, OMPdb (www.ompdb.org) was a repository of only prokaryotic βOMPs and classified them into the following eight functional categories (Tsirigos et al., 2011): receptors, biogenesis/secretion, specific channels, non-specific channels, structural, adhesion, enzymes, and unknown. Specific and non-specific diffusion channels, together with biogenesis/secretion proteins have a crucial role in the bacterial life. Bacterial diffusion channels are the most abundant type of βOMPs in the outer membrane. There are two main classes of bacterial diffusion channels: the non-specific and specific diffusion channels (Schirmer et al., 1995; Koebnik et al., 2000; Schulz, 2002; Nikaido, 2003; Pagès et al., 2008; Wirth et al., 2009; Van Den Berg, 2012). The first class of proteins may have 14 strands, as the outer membrane protein G (OmpG) (Galdiero et al., 2013), 16 strands and permit the flux of hydrophilic substrates smaller than 600 Kda (Ringler and Schulz, 2002) or 26 strands, as the MtrB, the β-barrel member of the outer membrane spanning protein complex, MtrAB (Edwards et al., 2020). The second class members form 12-stranded beta barrels, as in the oligogalacturonate-specific KdgM channel (KdgM) (Wirth et al., 2009), 14-stranded, as the long-chain fatty acid transporter FadL (van den Berg et al., 2004), 16-stranded, like the outer membrane porin B (OprB) (Van Den Berg, 2012) and OprP (Moraes et al., 2007) or 18-stranded, as the outer membrane carboxylate channel (Occ) (Eren et al., 2012; Liu et al., 2012) and maltoporins (Wang et al., 1997; Forst et al., 1998).
In addition to the aforementioned functions of bacterial βOMPs, bacterial adhesion is considered as the first step of biofilm formation and colonization (Dankert et al., 1986). Biofilm, an accumulated biomass of microorganisms, can be harmful to human life where it causes pathogen interaction with host cells (Gristina, 1987; Soto and Hultgren, 1999). Prior to entering inside, pathogenic bacteria adhere to host cells and secrete their products to the host. To this end, bacteria attach to host cells via one of the following three ways: fimbrial (Pili), which is considered the most common proteinous adhesin of Gram-negative bacteria (Soto and Hultgren, 1999) (e.g., Neisseria gonorrhoeae Wall and Kaiser, 1999), non-fimbrial adhesions (autotransporters, like e.g., Haemophilus influenzae Girard and Mourez, 2006), or using other unique nanofibers like Caulobacter crescentus (Corpe, 1972) does. Subsequently, pathogenic bacteria use bacterial secretion as a cellular tool to secrete their virulence factors (predominantly proteins) for invading their host cells. Gram-negative bacteria use eight specific secretion types, with two of them being the most common, i.e., the general secretion (termed the Sec-pathway) and the twin arginine translocation (termed the Tat-pathway Natale et al., 2008; Green and Mecsas, 2016). Despite the high degree of similarity between the targeting signals that direct secretory proteins to their routes, the secretion mechanisms are widely different. The Sec-pathway stimulates the translocation of unfolded proteins, whereupon they fold at the trans-side of the membrane into their native structure. On the contrary, the Tat-pathway catalyzes the translocation of folded proteins (Natale et al., 2008).
To reach their proper location, the bacterial βOMPs are being accelerated by the five units (BamA, -B, -C, -D, and -E) of the β-barrel assembly machine (Bam) (Tomasek and Kahne, 2021). BamA, the core of the machine, forms a 16-stranded β-barrel in addition to a periplasmic domain with five polypeptide translocation-associated (POTRA) motifs. It is thought that these five POTRA motifs interact with the four lipoproteins of the Bam complex (Jansen et al., 2015). The Omp85 superfamily of outer membrane proteins encompasses the translocation machines of bacterial βOMPs (BamA) (Voulhoux et al., 2003), mitochondrial βOMPs (SAM50) (Höhr et al., 2018), and chloroplastic ones (TOC75/OEP80) (Day et al., 2019; Gross et al., 2020).
Lipopolysaccharide (LPS) is substantial for the vitality of most Gram-negative bacteria. It plays critical roles not only in the survival of them in severe environments by composing a biofilm but also in colonizing the infected host and avoiding attacks from the human immune system (Zhang et al., 2013; Whitfield and Trent, 2014). LptD, an integral membrane protein, is considered as one of the largest reported bacterial β-barrel so far. Its 26 stranded β-barrel together with LptE form the LPS complex translocon “barrel and plug” at the outer membrane of the Gram-negative bacteria (Dong et al., 2014; Gu et al., 2015).
All Gram-negative bacteria are always in a crucial need for large substrates, such as vitamin B12 or iron-siderophore complexes. Therefore, the outer membrane of Gram-negative bacteria contains diverse high-molecular-weight proteins called TonB-dependent transporter (TBDTs) (Braun, 1995) (e.g., FhuA Coulton et al., 1986) and FusA (Grinter et al., 2016). Furthermore, βOMPs may act as enzymes like Lipopolysaccharides 3-O-deacylase enzyme PagL (Bishop, 2008). Also, βOMPs maintain the stability of the outer membrane as outer membrane protein (OmpA) (Ringler and Schulz, 2002). Lastly, due to the ever-growing number of sequenced genomes of Gram-negative bacteria, the number of proteins with unknown function is relatively small (Tsirigos et al., 2011).
Apart from the typical βOMPs found in the outer membrane of endosymbiotic organelles (e.g., VDAC Bayrhuber et al., 2008, TOC75 Soll and Schleiff, 2004, and OmpG Galdiero et al., 2013), there are also multimeric transmembrane pores formed either from pathogenic microbes in the host's cells, called Pore-forming Toxins (PFTs) (e.g., Aerolysin, which forms heptameric Iacovache et al., 2016 or non-americ Podobnik et al., 2016 pores and Hemolysin De and Olson, 2011), whose role is not yet clear, or from human cells, called Membrane Attack Complex (MAC), that protect human cells by forming pores in the membrane of pathogenic Gram-negative bacteria (Menny et al., 2018).
Because of the biomedical importance of the βOMPs, several biological databases have been launched to annotate and organize the βOMPs in several ways. The Protein Data Bank of Transmembrane Proteins (PDBTM) (http://pdbtm.enzim.hu) was launched in 2004 as the first comprehensive database for experimentally verified transmembrane proteins either α-helical or β-barrels. The database is being updated continuously using the TMDET algorithm. This algorithm can differentiate between the non-transmembrane proteins and transmembrane proteins based on their 3D coordinates. PDBTM (version 2019-02-22) includes 130 non-redundant βOMPs (Kozma et al., 2013).
TMBB-DB (http://beta-barrel.tulane.edu/) was launched in 2012 as a database for transmembrane βOMPs. TMBB-DB includes around 50,000 predicted transmembrane βOMPs (Freeman and Wimley, 2012). To detect the transmembrane βOMPs, the database uses a compilation of two predictors: the Freeman-Wimley algorithm (Freeman and Wimley, 2010) and SignalP5 (Almagro Armenteros et al., 2019).
The Topology Data Bank of Transmembrane Proteins (TOPDB) (http://topdb.enzim.ttk.mta.hu) was launched in 2008 as a hub for the topology of the 3D solved structure transmembrane proteins. TOPDB was dependent on the retrieved data from the Protein Data Bank (PDB) and PDBTM in parallel with the generated topology prediction by HMMTOP (Tusnády et al., 2008). In 2015, they improved the generated topology data by incorporating high throughput techniques like the sequential positions of N- or O-glycosylations. Further, they developed a new algorithm for collecting the scattered topology from various public databases. In addition, they created a new method for evaluating the reliability of the generated topology data. TOPDB provides now the topology information for 201 βOMPs (Dobson et al., 2015).
The Transporter Classification Database (TCDB) (http://www.tcdb.org) is a database of all the transporter proteins. TCDB provides comprehensive structural, functional and medical information for all the transporter proteins. Moreover, TCDB has a specialized software that has been designed and integrated into the TCDB to focus on the distinctive characteristics of the transporter proteins, and subsequently their biomedical function. TCDB classifies 1260 βOMPs into 93 different families (Saier et al., 2016).
The MemProtMD database (http://memprotmd.bioch.ox.ac.uk) was launched in 2015 acting as a repository for the membrane-embedded protein structures and their lipid interactions. MemProtMD provides the molecular dynamics (MD) simulation results of the protein-lipid interactions for 405 βOMPs (Newport et al., 2019).
The Pfam database (http://pfam.xfam.org/) is a widely used repository for protein families. Pfam classifies the protein sequences into clans, families and domains based on specific pHMM for every family. In Pfam, all the βOMP families are gathered together in one clan called MBB (CL0193). This clan contains 92 families with 1,092,329 protein members (El-Gebali et al., 2019).
Here, we present the upgraded and updated OMPdb, a global repository for all βOMPs with experimentally determined 3D structure or not regardless of their location, function and mechanism of pore formation.
Materials and Methods
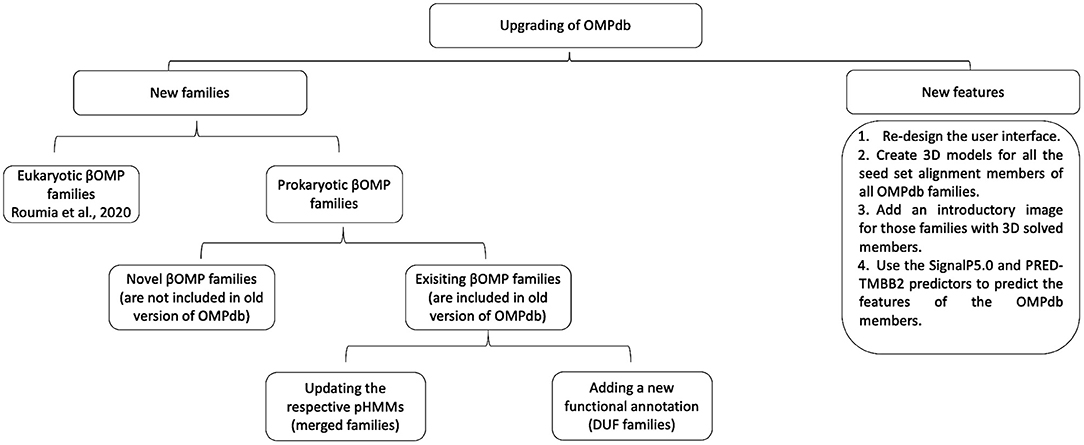
In order to upgrade OMPdb, new features and novel βOMPs families, prokaryotic and eukaryotic ones, are included as shown in Figure 2.
New Families
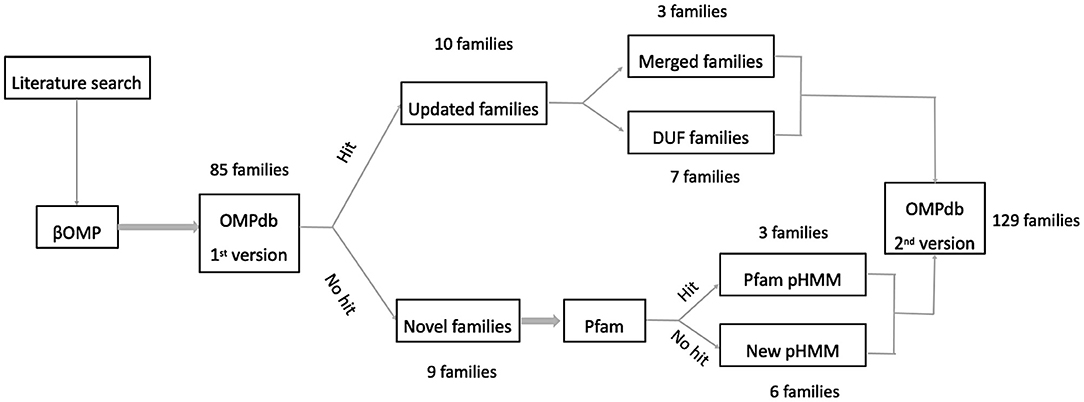
Initially, our efforts were focused on finding as many new transmembrane βOMPs families in the literature as possible. To this end, we based our literature search along two main axes, adding novel transmembrane βOMPs families and updating current transmembrane βOMPs ones, either by adding new functional annotation or by updating their pHMMs as shown in Figure 3.
First, we performed an extensive literature search to find novel families of transmembrane βOMPs in Gram-negative bacteria (not included in OMPdb Tsirigos et al., 2011). Furthermore, we retrieved all the 3D structures of transmembrane βOMPs that were not included in OMPdb after a thorough search in both PDBTM (version 2019-02-22) (Kozma et al., 2013) and MemProtMD (version 13-10-2019) (Newport et al., 2019). Additionally, we tried to characterize and assign function to as many of the Domain of Unknown Function (DUF) families, members of the MBB clan of Pfam version 33.1 May 2020 (El-Gebali et al., 2019), as possible. It is important to mention here that the same procedure was used in both the first OMPdb publication (Tsirigos et al., 2011) and our recent eukaryotic transmembrane βOMPs publication (Roumia et al., 2020) to create new pHMMs for those families without known domains in Pfam. In brief, the β-barrel domain of the experimentally verified protein(s) reported in the respective published articles were used to perform search against the UniProt (The UniProt Consortium, 2019) database with protein BLAST (Altschul et al., 1997). Subsequently, Multiple Sequence Alignments (MSAs) of the best-scoring results for each family were made by Clustal Omega (Li et al., 2015) and these alignments were refined with MUSCLE (Edgar, 2004). Finally, we built the corresponding pHMMs using the refined alignments with HMMER version 3.3 (Eddy, 1998).
For the Autotransporter and TBDTs families, we updated their pHMMs by adding the new members to their respective Pfam MSAs, and then, following the above-mentioned method. For the Omp85 family we built a new pHMM corresponding only to its bacterial members (BamA/TamA) and mitochondrial ones (SAM50). To this end, we searched Uniprot (The UniProt Consortium, 2019) for mitochondrial SAM50 proteins under Omp85 family, merged the SAM50 search results to the BamA/TamA members, and then, followed the aforementioned steps to building the new pHMM.
Finally, we performed a search with our new pHMMs' collection against the reference proteomes, prokaryotic, and eukaryotic, retrieved from the Uniprot database (Release 2020_03) (The UniProt Consortium, 2019).
New Features
As part of the upgrade of OMPdb, we decided to include some new features in the latest version. Providing 3D models for all seed set alignment members for all the families included in the database was the first new feature we added. The models were constructed either via homology modeling using SWISS-MODEL (Biasini et al., 2014) (for the families that contained member(s) with experimentally verified structure) or via de novo modeling using PHYRE2 (Kelley et al., 2015) (for the families without any determined 3D structures). As an additional feature, we now provide, in each entry, information about the signal peptide and the topology of each βOMP using the SignalP5.0 (Almagro Armenteros et al., 2019) and the PRED-TMBB2 (Tsirigos et al., 2016) tool, respectively.
Results
Newly Added Families
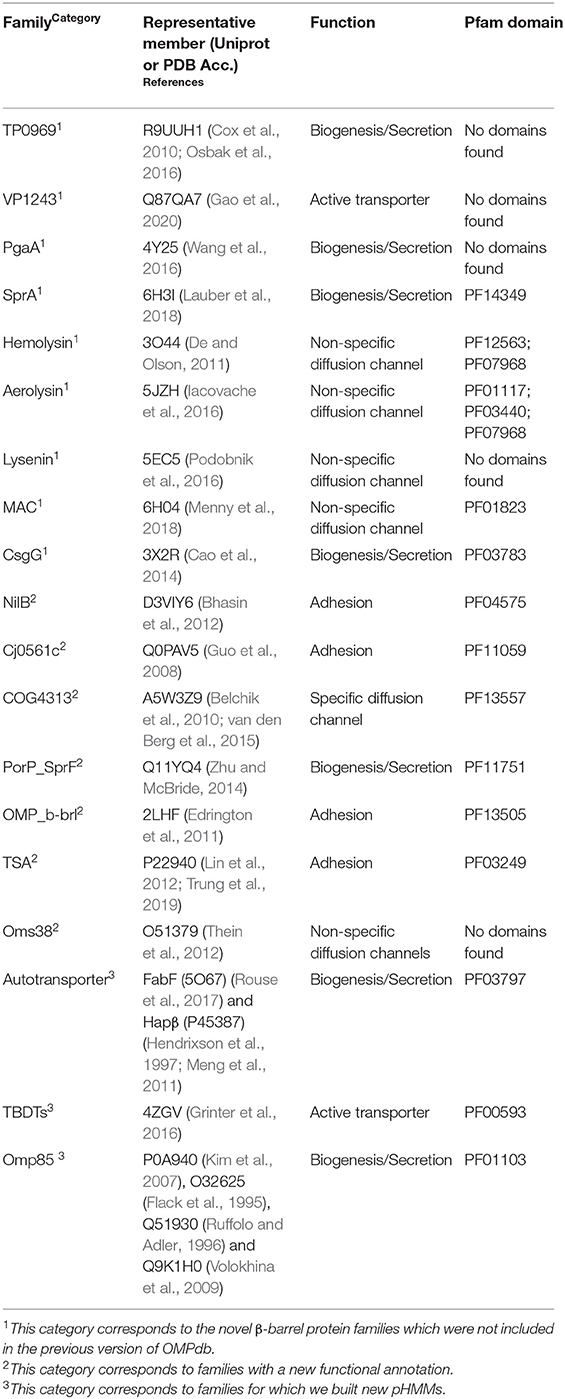
In addition to the 11 new eukaryotic transmembrane βOMPs families (Roumia et al., 2020), we included 19 new or updated prokaryotic βOMPs families presented in Table 1. These families are classified into one of the following three categories: novel βOMPs families not included in the old version of OMPdb, known families with new functional annotation or known families with an updated pHMM. Further, for most of the OMPdb families either updated or not, we added 667 recent publications focusing on their respective function.
Novel Families
This first category includes nine novel families presented below.
(i) TP0969 protein shows structural homology with the TolC protein. TP0969 was predicted as an outer membrane beta barrel by CELLO (Yu et al., 2006), PSORT (Yu et al., 2010), HHomp (Remmert et al., 2009), and PRED-TMBB (Bagos et al., 2004) prediction algorithms and has a signal peptide based on Signal-CF (Chou and Shen, 2007) and SignalP 4.1 (Petersen et al., 2011) prediction algorithms. Furthermore, using mass spectrometry analysis, TP0969 was identified as an outer membrane protein (Cox et al., 2010; Osbak et al., 2016). According to PRED-TMBB2, it forms 12 transmembrane beta strands (Tsirigos et al., 2016).
(ii) Vibrio parahaemolyticus is a significant Gram-negative halophilous pathogen. As a result of the consumption of seafood, it may cause a harmful seafood-borne illness in humans. In addition, it causes a wide range of diseases in aquatic animals (Vora et al., 2005; Kawatsu et al., 2006; Datta et al., 2008). VP1243 is a protective antigen which shows a high effective antimicrobial activity against the mentioned organism. VP1243 is widely distributed and highly conserved among the major Vibrio Species. VP1243 is considered as a hopeful candidate against the Vibrio infections. Western blot analysis revealed that VP1243 is an outer membrane protein (Gao et al., 2020). The first 19 amino acids function as a signal peptide according to SignalP 5.0 (Almagro Armenteros et al., 2019). Further, VP1243 forms 10 transmembrane beta strands based on PRED-TMBB2 prediction (Tsirigos et al., 2016).
(iii) PgaA controls the translocation of de-N-acetylated poly-β-1,6-N-acetyl-d-glucosamine (dPNAG) polymer into the outer membrane of Escherichia coli k-12. dPNAG is crucial for biofilm adhesion and has an important role in the maintenance and development of biofilms integration for various bacterial species. The PgaA β-barrel domain (513–807) consists of 16 antiparallel transmembrane β-sheets with β1 and β16 strands interlocking (Wang et al., 2016).
(iv) The Gram-negative Fibrobacteres-Chlorobi-Bacteroidetes superphylum has a protein export pathway termed the type 9 secretion system (T9SS), which is considered as an important determinant of pathogenicity in serious periodontal disease (Lasica et al., 2017). These bacteroidetes are famous for the quick and unique gliding motility where their cell surface adhesins move on helical tracks (Shrivastava et al., 2016). Cryo-electron microscopy revealed that SprA is the translocon of T9SS. Furthermore, SprA consists of a large transmembrane β-barrel of 36 strands. The SprA barrel has a lateral entry to the external membrane surface because the barrel pore on the extracellular end is closed (Lauber et al., 2018).
In addition to the monomeric βOMPs, we also included the multimeric transmembrane βOMPs to the OMPdb families. There are five such families: Hemolysin, Aerolysin, Lysenin, MAC, and CsgG. The first three families act as PFTs (De and Olson, 2011; Iacovache et al., 2016; Podobnik et al., 2016). The MAC family protects the human cells against bacterial attack (Menny et al., 2018) and the CsgG family is involved in protein secretion (Cao et al., 2014).
PFTs attracted the interest of structural biologists and microbiologists early on due to their ability to act as soluble proteins and transmembrane complexes. PFTs are secreted as water soluble monomers, and later on they connect to the membrane of target cells. There they gather into circular oligomers and enable the insertion into the membrane causing the pore forming and finally the resultant cell death (Anderluh and Lakey, 2008).
(v) Hemolysin, a heptameric porin, is secreted by Vibrio cholerae. The heptamer comprises a ring-like structure with an outer diameter of 135. The channel pore consists of an upper large vestibule formed by β-prism and cytolysin domains, and the stem domains which form a 14-strand β-barrel (De and Olson, 2011).
(vi) Aerolysin is a heptamer where seven monomers form a β-barrel with 14 strands at the outer membrane of Aeromonas hydrophila. The aerolysin pore is more stable due its concentric β-barrel fold (Iacovache et al., 2016).
(vii) Lysenin is a PFT of Eisena fetida and a member of the aerolysin family. It is a non-amer and forms an 18-strand smooth-walled tubular β-barrel attached to the outside by the C-terminal domains. It is a mushroom-like structure which has a transmembrane pore with a central stem built of a long β-barrel. Having a uniform and stable β-barrel through the entire length of protein makes lysenin an exception among PFTs (Podobnik et al., 2016).
(viii) The MAC is a structure formed at the outer membrane of pathogen cells resulting from activation of the host's complement system. It is one of the immune system's first responders. MAC forms damaging transmembrane channels at the cell membrane of pathogens causing cell lysis and death (Charles A Janeway et al., 2001). It is a multiprotein complex, comprised of six polypeptide chains (C5b, C6, C7, C8α, C8β, and C8γ) together with multiple copies of C9 monomer that are arranged in a split-washer configuration, composing a ring in the membrane which permits free flux of molecules in and out of the cell. The cell dies when enough pores are formed (Sharp et al., 2016). Membrane Attack Complex-Perforin (MACPF) domain (PF01823) is a common domain in all C6-C9 (Tschopp et al., 1986). MAC assembly into the membrane starts when C7 connects to C5b6 (a complex formed by C5b and C6) to compose MAC precursor C5b7 (DiScipio et al., 1988). Subsequently, C8 connects irreversibly to the former complex forming the membrane-inserted C5b8 (Steckel et al., 1983). Then, C5b8 binds to 22 C9 monomers to form C5b9 and polymerizes to finish the MAC pore formation (Podack et al., 1982). In the end, the β-barrel pore is composed after transforming the helical bundles in the MACPF domains into transmembrane β-hairpins through an unknown mechanism (Shepard et al., 1998; Shatursky et al., 1999; Rosado et al., 2007). According to the cryo-EM structure, the formed barrel consists of 88 strands (Dudkina et al., 2016).
(ix) In Escherichia coli Xuzhou21, curli subunits are produced and secreted into the outer membrane through the CsgG secretion channel. Curli are a unique group of functional amyloids, which are crucial for host cell adhesion, biofilm formation and colonization of inert surfaces (Barnhart and Chapman, 2006). They are involved in harmful diseases in humans, since they share similar structural and biochemical characteristics with amyloid fibers (Sunde et al., 1997; Moreno-Gonzalez and Soto, 2011). The crystal structure of CsgG showed that it is a symmetric nonameric channel, composed of monomers each having four strands spanning the outer membrane. A 36-stranded β-barrel is formed from nine CsgG monomers. CsgG could perhaps reduce the biofilm formation by controlling Curli secretion and that is why it is studied as a putative antibiotics target (Cao et al., 2014).
Families With Updated Information
From the literature search, we also were able to assign functional annotation to seven families that had unknown function until now.
(i) NilB (DUF560) is a surface-exposed outer membrane protein of Xenorhabdus nematophila. Expression of NilB is suppressed by NilR and growth in nutrient-rich medium. Members of this family exist in diverse bacteria and are common in the genomes of mucosal pathogens. Bioinformatic analyses reported that NilB is the only characterized member of a family of proteins distinguished by a conserved C-terminal domain of unknown function (DUF560) and N-terminal region tetratricopeptide repeats (TPR). Insertion and deletion mutational analyses revealed that NilB forms a β-barrel with 14 transmembrane strands and seven extracellular surface loops, and an N-terminal globular domain. The globular domain and surface loop 6 play a crucial role in the nematode colonization. Epifluorescence microscopy of these mutants revealed that NilB is necessary at early stages of colonization (Bhasin et al., 2012).
(ii) Cj0561c (DUF2860) is a probable membrane fusion protein and contributes to intestinal colonization. Cj0561c is the only characterized member of DUF2860. Cj0561c is encoded as a membrane transporter gene whose transcription is inhibited by CmeR in Campylobacter jejuni (Guo et al., 2008). Based on PRED-TMBB2, Cj0561c is predicted to have 14 beta strands (Tsirigos et al., 2016).
(iii) COG4313 (Phenol_MetA_deg) proteins comprise a huge and widespread family of outer membrane channels and have been involved in the uptake of a set of hydrophobic molecules (van den Berg et al., 2015). The first studied member of COG4313 proteins was the TcpY, an outer membrane protein that eases the uptake of polychlorophenols across the outer membrane of Cupriavidus necator JMP134 (Belchik et al., 2010). Pput2725 is another member of the family and forms a 12-stranded barrel at the outer membrane of Pseudomonas putida with experimentally verified 3D structure. It is suggested that Pput2725 mediates the uptake of hydrophobic aromatic compounds (van den Berg et al., 2015).
(iv) SprP (PorP_SprF) is associated with the outer membrane of Cytophaga hutchinsonii. It is suggested that SprP is involved in protein secretion, a hypothesis made due to its sequence similarity with PorP that is responsible for the secretion of gingipain protease virulence factors in Porphyromonas gingivalis type IX secretion system (T9SS) and SprF that is crucial for the delivery of the gliding motility machinery components to the cell surface (Zhu and McBride, 2014). According to PRED-TMBB2, it is predicted to have 14 transmembrane strands (Tsirigos et al., 2016).
(v) The Outer membrane protein beta-barrel domain (OMP_b-brl) domain is found in a wide range of outer membrane proteins, found mainly in Alphaproteobacteria. The Major Outer Membrane Protein (ompP1) is found in various strains of Coxiella burnetti with typical porin properties. It is heat-modifiable and has a channel activity. ompP1 is predicted to form a β-barrel; Omp3b from Brucella abortus and homologs from various Alphaproteobacteria that are believed to be involved in bacterial surface control and host cell interactions. In Pseudomonas aeruginosa, OprH forms a transmembrane β-barrel protein which consisted of eight strands with four extracellular loops of unequal size. Moreover, in vivo and in vitro biochemical studies revealed that OprH interacts with LPS in P. aeruginosa outer membranes (Varghees et al., 2002; Edrington et al., 2011).
(vi) The Type Specific Antigen (TSA) consists of several antigenic variants in the genera Rickettsia and Orientia that belong to the family Rickettsiaceae. Rickettsiaceae, intracellular bacteria, are the causative agent of the Rickettsioses which are an arthropod-borne zoonoses. The TSA of 56-kilodaltons located on the rickettsial surface is responsible for the variation. Orientia tsutsugamushi are the causative agent of scrub typhus, is an obligate intracellular pathogen. In O. tsutsugamushi, there are three major outer membrane proteins: TSA56, TSA47, and TSA22. The TSA56 is the major outer membrane protein responsible for O. tsutsugamushi adhesion (Lin et al., 2012; Trung et al., 2019).
(vii) Relapsing fever is a worldwide, endemic disease caused by several spirochetal species belonging to the genus Borrelia. Oms38 (outer membrane-spanning protein of 38 kDa) is present in the outer membranes of B. duttonii, B. hermsii, B. recurrentis, and B. turicatae as well. Characterization of Oms38 was performed using the black lipid bilayer method, which demonstrated that Oms38 forms small, water-filled channels of 80 pS in 1 M KCl that did not exhibit voltage-dependent closure. The Oms38 channel is slightly selective for anions and shows a ratio of permeability for cations over anions of 0.41 in KCl. Analysis of the deduced amino acid sequences revealed that Oms38 contains an N-terminal signal sequence which is processed under in vivo conditions. Oms38 is highly conserved within the four studied relapsing fever species, sharing an overall amino acid identity of 58% and with a strong indication for the presence of a ß-barrel formation (Thein et al., 2008). DipA (BB_0418) forms a specific porin for dicarboxylates. It is suggested that DipA plays a crucial role for the flux of specific nutrients toward different Borrelia species (Thein et al., 2012).
Families With Updated pHMMs
During the update of OMPdb, we identified proteins with the same biological function as two pre-existing beta barrel families, namely the Autotransporter (PF03797) and the TBDTs (PF00593) families. Interestingly, the Pfam profiles were unable to detect these proteins, so we updated the respective profiles.
For the Autotransporter family, two new members were added in the seed set, the functional amyloid transporter from Pseudomonas, FabF (PDB: 5O67) (Rouse et al., 2017), and the Haemophilus influenzae Hap β-barrel domain, Hapβ (Uniprot: P45387) (Hendrixson et al., 1997; Meng et al., 2011), and the pHMM was rebuilt. FapF is a new class of secretion system similar to type V secretion in the autotransporter proteins (AT-1). FapF is characterized by a C-terminal, 12 β-stranded, helix-blocked pore in the closed state. In contrast to other autotransporters which completely pass the pore, the N terminus of the truncated FapF structure exits the barrel on the periplasmic side (Rouse et al., 2017). Hapβ is the β-barrel domain of self-associating autotransporters (SAATs). SAATs is a set of virulence factors that enhance bacterial biofilm formation (Klemm et al., 2006). The Haemophilus influenzae Hap SAAT consists of three domains: residues 1–25 correspond to the signal peptide, residues 26-−1036 refer to the Haps called the passenger domain, and residues 1037−1394 form the 14 β-stranded barrel at the C-terminal (Hendrixson et al., 1997; Meng et al., 2011).
TBDTs pHMM was updated by adding the respective members of FusA in the seed set. FusA is used by the phytopathogenic Pectobacterium spp. as a new class of TBDTs. FusA is a 22-stranded transmembrane β-barrel responsible for importing pectocin and ferrodoxin in Pectobacterium spp. (Grinter et al., 2016).
Finally, we created a new pHMM for BamA/TamA and SAM50 families. Previously, BamA/TamA and SAM50, along with TOC75 and OEP80 constituted the Omp85 family (PF01103). Since we were able to create two separated chloroplastic pHMMs for TOC75 and OEP80 (Roumia et al., 2020), we decided to build the new pHHM and now we have the ability to easily distinguish members of the three eukaryotic families (TOC75, OEP80, and SAM50) when searching against eukaryotic proteomes.
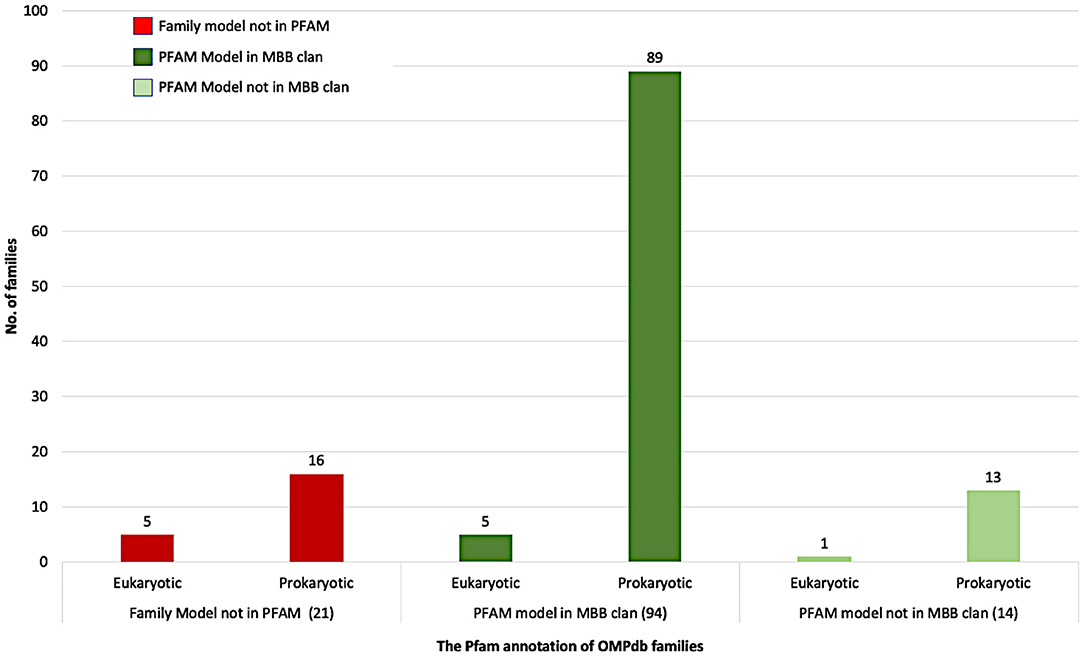
Figure 4 summarizes the Pfam annotation of the 129 families in OMPdb. 72.9% of the families belong to the outer membrane beta-barrel protein superfamily (MBB clan), while 10.8% of the families are in Pfam but not in MBB clan. Interestingly enough, for 16.3% of OMPdb families, there was no information at all in the Pfam database.
Search Using the OMPdb pHMMs' Collection Against Reference Proteomes
In Figure 5, we display the detected proteins by the two pHMM libraries, eukaryotic and prokaryotic, against the retrieved reference proteomes from Uniprot (Release 2020_03). The eukaryotic pHMM library detected at least one protein in 96.97% of the eukaryotic reference proteomes and the number of detected proteins for each family is depicted in Figure 5A. As shown in Figure 5B, the detected prokaryotic proteins were distributed functionally into eight categories. Most of them belong to the receptor category followed by the biogenesis/secretion category, while the enzymes category shows the lowest number of detected proteins.
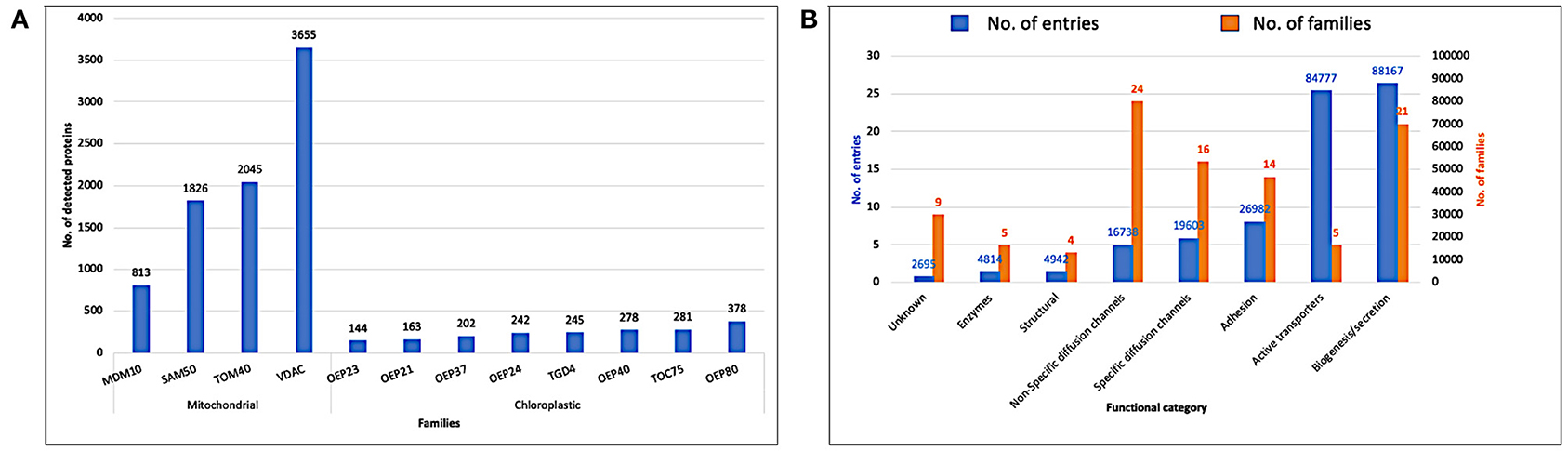
Figure 5. (A) Detected proteins by the eukaryotic pHMM library against eukaryotic reference proteomes (Uniprot release 2020_03). (B) Detected proteins by prokaryotic pHMM library against bacterial reference proteomes (Uniprot release 2020_03).
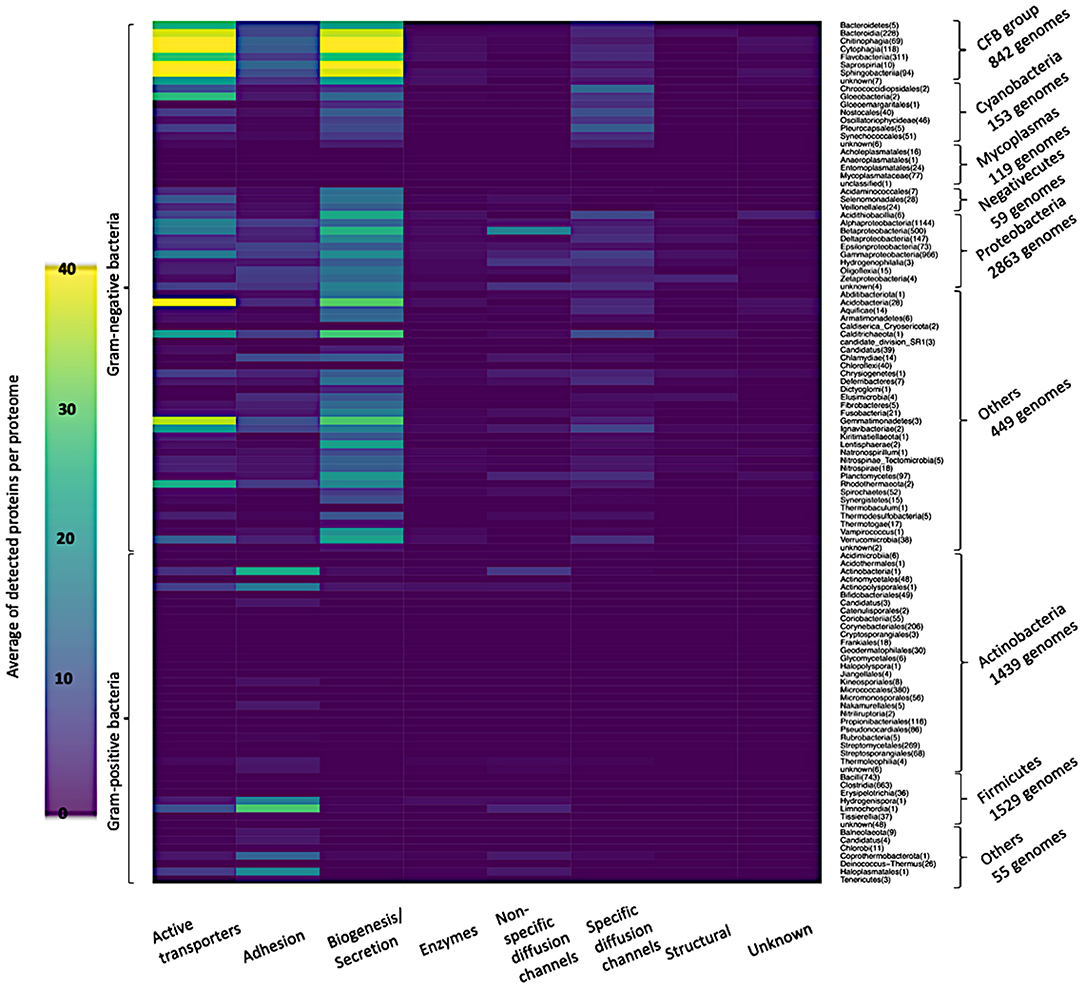
As a next step, we followed the taxonomy to classify the bacterial reference proteomes according to the NCBI Common Taxonomy Tree (Sayers et al., 2009). The bacterial reference proteomes are classified into 16 main taxonomic groups, three groups from Gram-positive bacteria, Actinobacteria, Firmicutes and other bacteria, and the rest 13 groups from Gram-negative bacteria. As shown in Figure 6, Gram-negative bacteria are classified into six main groups: Cytophaga, Fusobacterium, and Bacteroides group (CFB group), Proteobacteria, Cyanobacteria, Mycoplasmas, Negativecutes, and other bacteria. All taxonomic groups that account for <1% of the total reference proteomes in both classes, Gram-positive or Gram-negative bacteria, are classified under “other bacteria.”
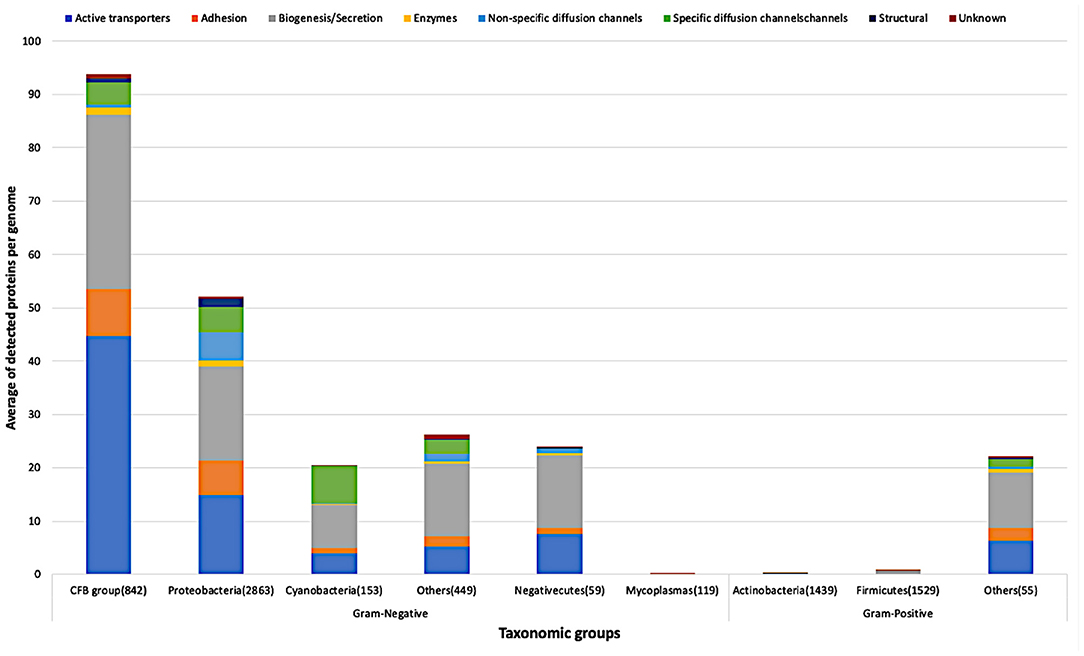
Figure 6. Average of detected functional proteins per genome for the different bacterial taxonomic groups.
CFB group has the highest average of detected proteins followed by the Proteobacteria group of Gram-negative bacteria. In line with what we expected, Actinobacteria and Firmicutes, the two main Gram-positive bacteria taxonomic groups, have few representatives of the above-mentioned functional categories. Nevertheless, it is clear that the Firmicutes group has representatives relatively more than the Actinobacteria group as shown in Figure 6.
Figure 7 shows in detail the average of detected functional proteins for all bacterial taxonomic groups either Gram-negative or Gram-positive ones. Except for the Mycoplasmas group, all Gram-negative bacteria taxonomic groups have a significant number of representatives of the active transporters, biogenesis/secretion, specific diffusion channels, and unknown function categories. For the Cyanobacteria group, the non-specific diffusion channels category is almost absent as shown in Figure 7.
New Features
Beside re-designing the user interface, we now provide structural information through 3D models for all seed set members of each family and this feature gives deep and concise information about the respective family. In total, we provide 3,264 models for families with at least one member with experimentally verified crystal structure and 2,116 models for families without any previous knowledge about their 3D structure. In addition, we provide an introductory structure image structure at the main page of the respective family, for every family including members with experimentally verified 3D. Moreover, for all the OMPdb entries, we present the predicted features of the signal peptide and β-barrel topology using SignalP5 and PRED-TMBB2 predictors.
Discussion
In this work, we presented the new content and updated features of OMPdb, a global hub for all βOMPs using 128 characteristic pHMMs. This collection of pHMMs includes 11 eukaryotic and 117 prokaryotic families in addition to the Treponema pectinovorum ATCC 33768 mompA family (Walker et al., 1997) that lacks a pHMM due to missing known homologues of its representative member.
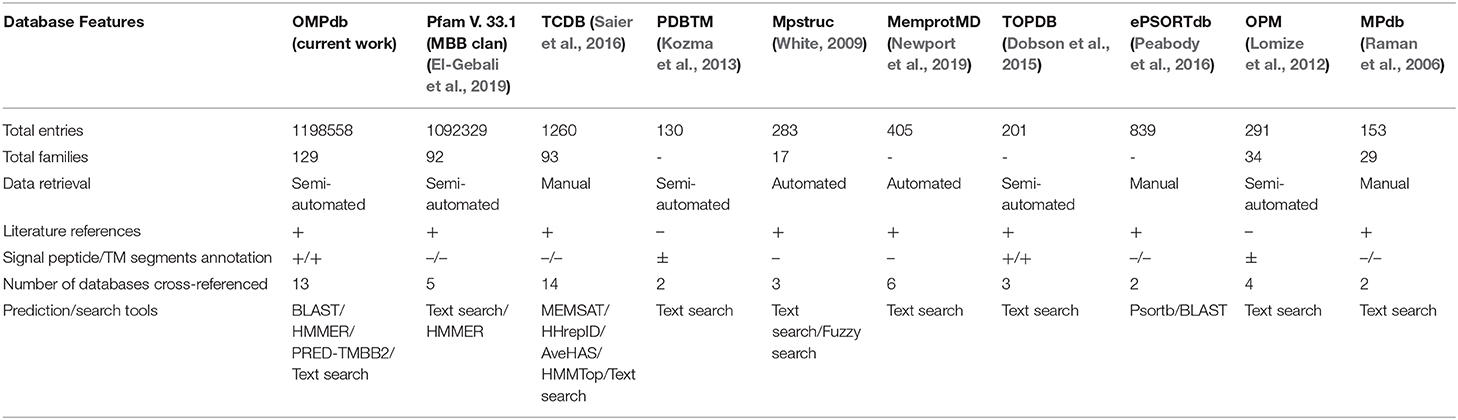
OMPdb in its second version, still has a clear advantage against all the other databases that contain βOMPs. As shown in Table 2, OMPdb is currently the most complete resource for transmembrane β-barrels, since it has the highest number of protein and family entries, provides numerous literature references, offers sequence annotation, cross-references with many related databases and the ability to use search and prediction tools. Due to the discontinuation of TMBETA GENOME (Gromiha et al., 2007), PRNDS (Katta et al., 2004), HHompDB (Remmert et al., 2009), and TMPDB (Ikeda et al., 2003) databases since the first version of OMPdb, we excluded them from the comparison. In contrast, we included in the comparison two new databases, Mpstruc (White, 2009) and MemprotMD (Newport et al., 2019).
Based on whether a family exists in Pfam, and specifically if it is a member of the MBB clan, the OMPdb families can be classified into three categories as shown in Figure 4. The first one includes 94 families belonging to the MBB clan in Pfam. In this category, we updated the pHMMs of MDM10 (PF12519), TBDTs (PF00593) and Autotransporter (PF03797), while the Porin_3 (PF01459) pHMM was replaced with two new pHMMs, VDAC, and TOM40 (Roumia et al., 2020). Furthermore, the pHMM of Omp85 (PF01103) was updated to contain only BamA/TamA and SAM50 proteins due to having two specific pHMMs for TOC75 and OEP80 families (Roumia et al., 2020). The second category refers to 14 families which are in Pfam but not in the MBB clan. Although Hemolysin and SprA families had Pfam pHMMs, we updated their respective pHMMs. For Hemolysin, the respective pHMM (PF12563) did not cover all the transmembrane barrel region, while, in the SprA family (PF14349), the transmembrane barrel part was intermittent. Therefore, we built two novel pHMMs for Hemolysin and SprA families. The third category (families not included in Pfam), consists of 5 eukaryotic (Roumia et al., 2020) and 16 prokaryotic families. Interestingly, for these 21 families, there is no information in Pfam whatsoever and these novel families can only be found in OMPdb.
In OMPdb we focus on offering as much information about the nature of the transmembrane barrel part in each family to the user as possible. To this end, we re-designed the OMPdb user interface to include various new features. The first one is adding an introductory structure image for 44% of the OMPdb families that presents to the user the β-barrel features of these families at one glance.
The 3D structure dictates the biological function of every protein. Knowing the 3D structure of a protein provides a higher level of understanding of its molecular action. This knowledge allows us to create hypotheses on controlling, modifying or affecting on this protein. We now provide a 3D model for each of the seed set members of every OMPdb family. For the families including members with experimentally verified 3D structure (57 families), we constructed the models using homology modeling via the SWISS-MODEL server (Biasini et al., 2014). We provide the used template as the representative structure for each family. We used de novo modeling through PHYRE2 (Kelley et al., 2015) for the rest of the families (71 families).
The nascent βOMPs either eukaryotic (Rapoport, 2007) or prokaryotic (Nouwen et al., 2007) have signal peptides to be recognized by the Sec or Tat pathways. After or during the assembly of the transmembrane proteins, the signal peptides are being cleaved by signal peptidase (SPase). The Tat substrates can be cleaved by SPase I or II while the Sec substrates can be processed by SPase I, II, or III. SignalP5 is the first predictor that provides a deep neural network-based method for predicting the signal peptide cleaved by (Sec/SPI), (Sec/SPII), and (Tat/SPI) (Almagro Armenteros et al., 2019). PRED-TMBB2 is a predictor for the β-barrel topology based on Hidden Markov Models. It excels the best available β-barrel predictor by 7% (Tsirigos et al., 2016) (Tsirigos et al., 2016). Both predictors are therefore used to provide the topological features of each protein.
For the detection of βOMPs in the reference proteomes, we used the collection of OMPdb's pHMMs after excluding all the DUF families (19 families) that do not have any literature information regarding their function (Supplementary Table 1). It is worth noting that the updated Omp85 pHMM was used twice, in both the eukaryotic library to detect the SAM50 members and the prokaryotic library to detect the BamA members.
Very few proteins were detected in the Mycoplasmas group, although they are Gram-negative bacteria, and the reason is the absence of a cell wall in this taxonomic group (Cimolai, 2001) (Figures 6, 7). On the other hand, the Firmicutes group, a group of Gram-positive bacteria, shows a significant number of detected proteins. Firmicutes is a bacterial phylum, in which most of its organisms are Gram-positive bacteria. However, Firmicutes contains a class called Negativecutes, which is classified as Gram-negative bacteria (Sutcliffe, 2010). Interestingly enough, around 51.67% of the detected proteins at the Firmicutes group (1588 proteomes) belong to the 59 Negativecutes proteomes.
Our analysis showed that the biogenesis/secretion category was the largest one in comparison with the other functional categories The Outer membrane Factor family (OMF) comprises 40.5% of all detected proteins. These proteins are involved in the type I secretion pathway (Sec-independent), which is widespread and permits the secretion of proteins of different sizes and functions using uncleaved secretion signal at the C-terminal (Delepelaire, 2004). Around 9% of all detected proteins belong to each of the following families: Secretin, Autotransporter (AT), Porphyromonas gingivalis PorT and Outer Membrane Protein Insertion Porin (OmpIP/Omp85). The members of these four families are involved in type II and type III secretion pathway (Collins et al., 2004), type V secretory pathway (Desvaux et al., 2004), secretion of gingipains from Porphyromonas gingivalis (Nguyen et al., 2009) and the translocation of βOMPs into the outer membrane of Gram-negative bacteria (Noinaj et al., 2015), respectively. As for the active transporters category, it consists of five families (Supplementary Figure 2). It should be noted that the TBDTs family detects around 99.83% of all active transporter proteins. The aforementioned family represents the TBDTs transport system, which is involved in the active transport mechanism of nutrients through the outer membrane of Gram-negative bacteria (Higgs et al., 2002). It is noteworthy that the existence of active transporters, biogenesis/secretion, specific diffusion channels, and non-specific diffusion channels categories in all taxonomic groups of Gram-negative bacteria are in line with the findings of Nikaido (Nikaido, 2003), who revealed that the general part of βOMPs is acting as channels and transporters. The only inconsistency is the absence of non-specific diffusion channels in the Cyanobacteria group. The adhesion to the host cell is the first step bacteria take to establish a successful infection. The bacterial adhesion may occur directly via monomeric adhesins (Soto and Hultgren, 1999) or via highly advanced machines like the type III secretion systems (Autotransporter) (Pizarro-Cerdá and Cossart, 2006), which belongs to the biogenesis/secretion category, or by using special nanofibers (Corpe, 1972). Although there are multiple ways of bacterial adhesion (e.g., autotransporter and special nanofibers), there are 14 families for which their main function is adhesion. The OMP_b-brl forms around 72.74% of the detected adhesion proteins. The representative member of the aforementioned family, OprH, is involved in the stability of the outer membranes of Gram-negative bacteria by interacting with the LPS (Edrington et al., 2011) (Figure 7 and Supplementary Figure 3).
The widespread existence of specific and non-specific diffusion channels in all Gram-negative bacteria reflects the effective role of porins on the bacterial cell life. In addition to acting as passages for the nutrients into the Gram-negative bacteria, porins are affecting the bacterial pathogenicity due to their high prevalence at the bacterial surface structure (Naumann et al., 1999; Achouak et al., 2006; Galdiero et al., 2013; Choi and Lee, 2019) (Supplementary Figures 4, 5). In Cyanobacteria, the absence of the non-specific diffusion channels category reflects the low permeability of the cyanobacterial outer membrane (Figures 6, 7). The reason why the cyanobacterial outer membranes are highly impermeable is to prevent the flux of toxic compounds into the cell and this is an advantage for the cyanobacterial autotrophic lifestyle (Kowata et al., 2017). In the structural category (Supplementary Figure 6), the OmpA family counts around 71.35% of the proteins. This high percentage gives consideration to the critical effect of this domain on the structural strength and shape of bacterial cells (Höltje, 1998), due to the non-covalent interaction between the respective domain with the peptidoglycan layer (Grizot and Buchanan, 2004).
As expected, the enzyme category has the lowest number of detected proteins, because only a limited number of βOMPs act as enzymes (Bishop, 2008). 44.69% of the enzyme proteins belong in the Outer membrane-localized lipid A 3-O-deacylase (PagL) family that is widespread among Gram-negative bacteria (Rutten et al., 2006) (Figure 7, Supplementary Figure 7).
Due to the significant progress that has been made toward the annotation of Gram-negative bacteria genomes, the “unknown” category comprises only around 1.08% of our analysis (Figure 7, Supplementary Figure 8). Around 22% of Pfam proteins belong to DUF families (El-Gebali et al., 2019). Generally, there is an enormous number of proteins with unknown function and the reason is the difficulty of assigning function to proteins, which is a major goal of structural biology, biochemistry and bioinformatics (McKay et al., 2015).
Conclusions
The OMPdb database is a thorough, up-to date and continuously updated βOMPs database. OMPdb, in its second version, contains two different libraries of pHMMs that cover all the kinds of βOMPs. As of September 2020, it has around 1.2 million entries whose domains are classified into 129 different β-barrel families either prokaryotic or eukaryotic ones. OMPdb operates for more than 10 years and will be continuously updated in the future. The database participates in ELIXIR-GR, the Greek National Node of the ESFRI European RI ELIXIR, a distributed infrastructure that will allow the life science research community across Europe to share and store their research data as part of an organized network, whereas, at the same time is in close collaboration with other specialized protein databases (Babbitt et al., 2015; Holliday et al., 2015).
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
Author Contributions
PB conceived the presented idea. PB, KT, and MT conceived and planned the experiments. AR carried out the experiments and wrote the manuscript with support from MT and KT. All authors provided critical feedback and helped shape the research, analysis, and manuscript.
Funding
AR was financially supported as a PhD scholar from a joint program between the ministry of higher education in Egypt and the Greek ministry of foreign affairs (2016/2017). PB, IT, and MT were partially supported by the project ELIXIR-GR: The Greek Research Infrastructure for Data Management and Analysis in Life Sciences (MIS 5002780), which is implemented under the Action Reinforcement of the Research and Innovation Infrastructure funded by the Operational Programme Competitiveness, Entrepreneurship and Innovation (NSRF 2014–2020) and cofinanced by Greece and the European Union (European Regional Development Fund).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fbinf.2021.646581/full#supplementary-material
Abbreviations
βOMPs, β-barrel outer membrane proteins.
References
Achouak, W., Heulin, T., and Pagès, J.-M. (2006). Multiple facets of bacterial porins. FEMS Microbiol. Lett. 199, 1–7. doi: 10.1111/j.1574-6968.2001.tb10642.x
Almagro Armenteros, J. J., Tsirigos, K. D., Sønderby, C. K., Petersen, T. N., Winther, O., Brunak, S., et al. (2019). SignalP 5.0 improves signal peptide predictions using deep neural networks. Nat. Biotechnol. 37, 420–423. doi: 10.1038/s41587-019-0036-z
Altschul, S. F., Madden, T. L., Schäffer, A. A., Zhang, J., Zhang, Z., Miller, W., et al. (1997). Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 25, 3389–3402. doi: 10.1093/nar/25.17.3389
Anderluh, G., and Lakey, J. H. (2008). Disparate proteins use similar architectures to damage membranes. Trends Biochem. Sci. 33, 482–490. doi: 10.1016/j.tibs.2008.07.004
Babbitt, P. C., Bagos, P. G., Bairoch, A., Bateman, A., Chatonnet, A., Chen, M. J., et al. (2015). Creating a specialist protein resource network: a meeting report for the protein bioinformatics and community resources retreat. Database 2015:bav063. doi: 10.1093/database/bav063
Bagos, P. G., Liakopoulos, T. D., Spyropoulos, I. C., and Hamodrakas, S. J. (2004). PRED-TMBB: a web server for predicting the topology of β-barrel outer membrane proteins. Nucleic Acids Res. 32, W400–W404. doi: 10.1093/nar/gkh417
Barnhart, M. M., and Chapman, M. R. (2006). Curli biogenesis and function. Annu. Rev. Microbiol. 60, 131–147. doi: 10.1146/annurev.micro.60.080805.142106
Bayrhuber, M., Meins, T., Habeck, M., Becker, S., Giller, K., Villinger, S., et al. (2008). Structure of the human voltage-dependent anion channel. Proc. Natl. Acad. Sci. U.S.A. 105, 15370–15375. doi: 10.1073/pnas.0808115105
Belchik, S. M., Schaeffer, S. M., Hasenoehrl, S., and Xun, L. (2010). A beta-barrel outer membrane protein facilitates cellular uptake of polychlorophenols in Cupriavidus necator. Biodegradation 21, 431–439. doi: 10.1007/s10532-009-9313-8
Bhasin, A., Chaston, J. M., and Goodrich-Blair, H. (2012). Mutational analyses reveal overall topology and functional regions of NilB, a bacterial outer membrane protein required for host association in a model of animal-microbe mutualism. J. Bacteriol. 194, 1763–1776. doi: 10.1128/JB.06711-11
Biasini, M., Bienert, S., Waterhouse, A., Arnold, K., Studer, G., Schmidt, T., et al. (2014). SWISS-MODEL: modelling protein tertiary and quaternary structure using evolutionary information. Nucleic Acids Res. 42, W252–W258. doi: 10.1093/nar/gku340
Bishop, R. E. (2008). Structural biology of membrane-intrinsic β-barrel enzymes: Sentinels of the bacterial outer membrane. Biochim. Biophys. Acta - Biomembr. 1778, 1881–1896. doi: 10.1016/j.bbamem.2007.07.021
Braun, V. (1995). Energy-coupled transport and signal transduction through the Gram-negative outer membrane via TonB-ExbB-ExbD-dependent receptor proteins. FEMS Microbiol. Rev. 16, 295–307. doi: 10.1111/j.1574-6976.1995.tb00177.x
Cao, B., Zhao, Y., Kou, Y., Ni, D., Zhang, X. C., and Huang, Y. (2014). Structure of the nonameric bacterial amyloid secretion channel. Proc. Natl. Acad. Sci. U.S.A. 111, E5439–E5444. doi: 10.1073/pnas.1411942111
Casadio, R., Fariselli, P., Finocchiaro, G., and Martelli, P. L. (2003). Fishing new proteins in the twilight zone of genomes: the test case of outer membrane proteins in Escherichia coli K12, Escherichia coli O157:H7, and other Gram-negative bacteria. Protein Sci. 12, 1158–1168. doi: 10.1110/ps.0223603
Cavalier-Smith, T. (2000). Membrane heredity and early chloroplast evolution. Trends Plant Sci. 5, 174–182. doi: 10.1016/S1360-1385(00)01598-3
Charles A Janeway, J., Travers, P., Walport, M., and Shlomchik, M. J. (2001). “The complement system and innate immunity,” in Immunobiology Interactive, ed. S. Gibbs (New York, NY: Garland Science), 59–82. Available online at: https://1lib.eu/book/1022278/250ad7?regionChanged=&redirect=29288319 (accessed February 25, 2020).
Choi, U., and Lee, C.-R. (2019). Distinct roles of outer membrane porins in antibiotic resistance and membrane integrity in Escherichia coli. Front. Microbiol. 10:953. doi: 10.3389/fmicb.2019.00953
Chou, K.-C., and Shen, H.-B. (2007). Signal-CF: a subsite-coupled and window-fusing approach for predicting signal peptides. Biochem. Biophys. Res. Commun. 357, 633–640. doi: 10.1016/j.bbrc.2007.03.162
Cimolai, N. (2001). “Mycoplasmas,” in Laboratory Diagnosis of Bacterial Infections, ed N. Cimolai (Boca Raton, FL: CRC Press), 861–892. doi: 10.1201/b14028
Collins, R. F., Frye, S. A., Kitmitto, A., Ford, R. C., Tønjum, T., and Derrick, J. P. (2004). Structure of the Neisseria meningitidis outer membrane PilQ secretin complex at 12 Å resolution. J. Biol. Chem. 279, 39750–39756. doi: 10.1074/jbc.M405971200
Corpe, W. A. (1972). “Microfouling: the role of primary film forming marine bacteria,” in ci.nii.ac.jp, 598–609. Available online at: https://ci.nii.ac.jp/naid/10018002926/ (accessed February 12, 2020).
Coulton, J. W., Mason, P., Cameron, D. R., Carmel, G., Jean, R., and Rode, H. N. (1986). Protein fusions of β-galactosidase to the ferrichrome-iron receptor of Escherichia coli K-12. J. Bacteriol. 165, 181–192. doi: 10.1128/JB.165.1.181-192.1986
Cox, D. L., Luthra, A., Dunham-Ems, S., Desrosiers, D. C., Salazar, J. C., Caimano, M. J., et al. (2010). Surface immunolabeling and consensus computational framework to identify candidate rare outer membrane proteins of Treponema pallidum. Infect. Immun. 78, 5178–5194. doi: 10.1128/IAI.00834-10
Dankert, J., Hogt, A. H., and Feijen, J. (1986). Biomedical polymers: bacterial adhesion, colonization and infection. ci.nii.ac.jp 2, 219–301. Available online at: https://ci.nii.ac.jp/naid/10018453825/ (accessed February 12, 2020).
Datta, S., Janes, M. E., and Simonson, J. G. (2008). Immunomagnetic separation and coagglutination of Vibrio parahaemolyticus with anti-flagellar protein monoclonal antibody. Clin. Vaccine Immunol. 15, 1541–1546. doi: 10.1128/CVI.00141-08
Day, P. M., Inoue, K., and Theg, S. M. (2019). Chloroplast outer membrane β-Barrel proteins use components of the general import apparatus. Plant Cell 31, 1845–1855. doi: 10.1105/tpc.19.00001
De Pinto, V., Messina, A., Lane, D. J. R. R., and Lawen, A. (2010). Voltage-dependent anion-selective channel (VDAC) in the plasma membrane. FEBS Lett. 584, 1793–1799. doi: 10.1016/j.febslet.2010.02.049
De, S., and Olson, R. (2011). Crystal structure of the Vibrio cholerae cytolysin heptamer reveals common features among disparate pore-forming toxins. Proc. Natl. Acad. Sci. U.S.A. 108, 7385–7390. doi: 10.1073/pnas.1017442108
Delepelaire, P. (2004). Type I secretion in gram-negative bacteria. Biochim. Biophys. Acta - Mol. Cell Res. 1694, 149–161. doi: 10.1016/j.bbamcr.2004.05.001
Desvaux, M., Parham, N. J., and Henderson, I. R. (2004). The autotransporter secretion system. Res. Microbiol. 155, 53–60. doi: 10.1016/j.resmic.2003.10.002
DiScipio, R. G., Chakravarti, D. N., Muller-Eberhard, H. J., and Fey, G. H. (1988). The structure of human complement component C7 and the C5b-7 complex. J. Biol. Chem. 263, 549–60. doi: 10.1016/S0021-9258(19)57427-0
Dobson, L., Langó, T., Reményi, I., and Tusnády, G. E. (2015). Expediting topology data gathering for the TOPDB database. Nucleic Acids Res. 43, D283–D289. doi: 10.1093/nar/gku1119
Dong, H., Xiang, Q., Gu, Y., Wang, Z., Paterson, N. G., Stansfeld, P. J., et al. (2014). Structural basis for outer membrane lipopolysaccharide insertion. Nature 511, 52–56. doi: 10.1038/nature13464
Dudkina, N. V., Spicer, B. A., Reboul, C. F., Conroy, P. J., Lukoyanova, N., Elmlund, H., et al. (2016). Structure of the poly-C9 component of the complement membrane attack complex. Nat. Commun. 4:10588. doi: 10.1038/ncomms10588
Eddy, S. R. (1998). Profile hidden Markov models. Bioinformatics 14, 755–63. doi: 10.1093/bioinformatics/14.9.755
Edgar, R. C. (2004). MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 32, 1792–1797. doi: 10.1093/nar/gkh340
Edrington, T. C., Kintz, E., Goldberg, J. B., and Tamm, L. K. (2011). Structural basis for the interaction of lipopolysaccharide with outer membrane protein H (OprH) from Pseudomonas aeruginosa. J. Biol. Chem. 286, 39211–39223. doi: 10.1074/jbc.M111.280933
Edwards, M. J., White, G. F., Butt, J. N., Richardson, D. J., and Clarke, T. A. (2020). The crystal structure of a biological insulated transmembrane molecular wire. Cell 181, 665–673.e10. doi: 10.1016/j.cell.2020.03.032
El-Gebali, S., Mistry, J., Bateman, A., Eddy, S. R., Luciani, A., Potter, S. C., et al. (2019). The Pfam protein families database in 2019. Nucleic Acids Res. 47, D427–D432. doi: 10.1093/nar/gky995
Eren, E., Vijayaraghavan, J., Liu, J., Cheneke, B. R., Touw, D. S., Lepore, B. W., et al. (2012). Substrate specificity within a family of outer membrane carboxylate channels. PLoS Biol. 10:e1001242. doi: 10.1371/journal.pbio.1001242
Flack, F. S., Loosmore, S., Chong, P., and Thomas, W. R. (1995). The sequencing of the 80-kDa D 15 protective surface antigen of Haemophilus influenzae. Gene 156, 97–99. doi: 10.1016/0378-1119(95)00049-C
Forst, D., Welte, W., Wacker, T., and Diederichs, K. (1998). Structure of the sucrose-specific porin ScrY from Salmonella typhimurium and its complex with sucrose. Nat. Struct. Biol. 5, 37–46. doi: 10.1038/nsb0198-37
Freeman, T. C., and Wimley, W. C. (2010). A highly accurate statistical approach for the prediction of transmembrane β-barrels. Bioinformatics 26, 1965–1974. doi: 10.1093/bioinformatics/btq308
Freeman, T. C. J., and Wimley, W. C. (2012). TMBB-DB: a transmembrane β-barrel proteome database. Bioinformatics 28, 2425–2430. doi: 10.1093/bioinformatics/bts478
Galdiero, S., Falanga, A., Cantisani, M., Tarallo, R., Elena Della Pepa, M., D'Oriano, V., et al. (2013). Microbe-host interactions: structure and role of gram-negative bacterial porins. Curr. Protein Pept. Sci. 13, 843–854. doi: 10.2174/138920312804871120
Gao, Z., Ye, C., Zhou, L., Zhang, Y., Ge, Y., Chen, W., et al. (2020). Evaluation of the β-barrel outer membrane protein VP1243 as a candidate antigen for a cross-protective vaccine against Vibrio infections. Microb. Pathog. 147:104419. doi: 10.1016/j.micpath.2020.104419
Girard, V., and Mourez, M. (2006). Adhesion mediated by autotransporters of Gram-negative bacteria: structural and functional features. Res. Microbiol. 157, 407–416. doi: 10.1016/j.resmic.2006.02.001
Goetze, T. A., Patil, M., Jeshen, I., Bölter, B., Grahl, S., and Soll, J. (2015). Oep23 forms an ion channel in the chloroplast outer envelope. BMC Plant Biol. 15:47. doi: 10.1186/s12870-015-0445-1
Goetze, T. A., Philippar, K., Ilkavets, I., Soll, J., and Wagner, R. (2006). OEP37 is a new member of the chloroplast outer membrane ion channels. J. Biol. Chem. 281, 17989–17998. doi: 10.1074/jbc.M600700200
Gray, M. W., Burger, G., and Lang, B. F. (1999). Mitochondrial evolution. Science 283, 1476–1481. doi: 10.1126/science.283.5407.1476
Green, E. R., and Mecsas, J. (2016). Bacterial secretion systems: an overview. Virulence Mech. Bact. Pathog. Fifth Ed. 4, 215–239. doi: 10.1128/9781555819286.ch8
Grinter, R., Josts, I., Mosbahi, K., Roszak, A. W., Cogdell, R. J., Bonvin, A. M. J. J., et al. (2016). Structure of the bacterial plant-ferredoxin receptor FusA. Nat. Commun. 31:13308. doi: 10.1038/ncomms13308
Gristina, A. (1987). Biomaterial-centered infection: microbial adhesion versus tissue integration. Sci. Sciencemag Org. 237, 1588–1595. doi: 10.1126/science.3629258
Grizot, S., and Buchanan, S. K. (2004). Structure of the OmpA-like domain of RmpM from Neisseria meningitidis. Mol. Microbiol. 51, 1027–1037. doi: 10.1111/j.1365-2958.2003.03903.x
Gromiha, M. M., Yabuki, Y., Kundu, S., Suharnan, S., and Suwa, M. (2007). TMBETA-GENOME: database for annotated beta-barrel membrane proteins in genomic sequences. Nucleic Acids Res. 35, D314–D316. doi: 10.1093/nar/gkl805
Gross, L. E., Spies, N., Simm, S., and Schleiff, E. (2020). Toc75-V/OEP80 is processed during translocation into chloroplasts and the membrane embedded form exposes its POTRA domain to the intermembrane space. FEBS Open Bio. 10, 444–454. doi: 10.1002/2211-5463.12791
Gu, Y., Stansfeld, P. J., Zeng, Y., Dong, H., Wang, W., and Dong, C. (2015). Lipopolysaccharide is inserted into the outer membrane through an intramembrane hole, a lumen gate, and the lateral opening of LptD. Structure 23, 496–504. doi: 10.1016/j.str.2015.01.001
Guo, B., Wang, Y., Shi, F., Barton, Y. W., Plummer, P., Reynolds, D. L., et al. (2008). CmeR functions as a pleiotropic regulator and is required for optimal colonization of Campylobacter jejuni in vivo. J. Bacteriol. 190, 1879–1890. doi: 10.1128/JB.01796-07
Harsman, A., Schock, A., Hemmis, B., Wahl, V., Jeshen, I., Bartsch, P., et al. (2016). OEP40, a regulated glucose-permeable β-barrel solute channel in the chloroplast outer envelope membrane. J. Biol. Chem. 291, 17848–17860. doi: 10.1074/jbc.M115.712398
Hemmler, R., Becker, T., Schleiff, E., Bölter, B., Stahl, T., Soll, J., et al. (2006). Molecular properties of Oep21, an ATP-regulated anion-selective solute channel from the outer chloroplast membrane. J. Biol. Chem. 281, 12020–12029. doi: 10.1074/jbc.M513586200
Hendrixson, D. R., De La Morena, M. L., Stathopoulos, C., and St Geme, J. W. III. (1997). Structural determinants of processing and secretion of the Haemophilus influenzae Hap protein. Mol. Microbiol. 26, 505–518. doi: 10.1046/j.1365-2958.1997.5921965.x
Higgs, P. I., Larsen, R. A., and Postle, K. (2002). Quantification of known components of the Escherichia coli TonB energy transduction system: TonB, ExbB, ExbD and FepA. Mol. Microbiol. 44, 271–281. doi: 10.1046/j.1365-2958.2002.02880.x
Hill, K., Model, K., Ryan, M. T., Dietmeier, K., Martint, F., Wagner, R., et al. (1998). Tom40 forms the hydrophilic channel of the mitochondrial import pore for preproteins. Nature 395, 516–521. doi: 10.1038/26780
Hinnah, S. C., Wagner, R., Sveshnikova, N., Harrer, R., and Soll, J. (2002). The chloroplast protein import channel Toc75: pore properties and interaction with transit peptides. Biophys. J. 83, 899–911. doi: 10.1016/S0006-3495(02)75216-8
Höhr, A. I. C., Lindau, C., Wirth, C., Qiu, J., Stroud, D. A., Kutik, S., et al. (2018). Membrane protein insertion through a mitochondrial β-barrel gate. Science 359:eaah6834. doi: 10.1126/science.aah6834
Holliday, G. L., Bairoch, A., Bagos, P. G., Chatonnet, A., Craik, D. J., Finn, R. D., et al. (2015). Key challenges for the creation and maintenance of specialist protein resources. Proteins 83, 1005–1013. doi: 10.1002/prot.24803
Höltje, J.-V. (1998). Growth of the stress-bearing and shape-maintaining murein sacculus of Escherichia coli. Microbiol. Mol. Biol. Rev. 62, 181–203. doi: 10.1128/MMBR.62.1.181-203.1998
Iacovache, I., De Carlo, S., Cirauqui, N., Dal Peraro, M., Van Der Goot, F. G., and Zuber, B. (2016). Cryo-EM structure of aerolysin variants reveals a novel protein fold and the pore-formation process. Nat. Commun. 13:12062. doi: 10.1038/ncomms12062
Ikeda, M., Arai, M., Okuno, T., and Shimizu, T. (2003). TMPDB: a database of experimentally-characterized transmembrane topologies. Nucleic Acids Res. 31, 406–409. doi: 10.1093/nar/gkg020
Jansen, K. B., Baker, S. L., and Sousa, M. C. (2015). Crystal structure of BamB bound to a periplasmic domain fragment of BamA, the central component of the β-barrel assembly machine. J. Biol. Chem. 290, 2126–2136. doi: 10.1074/jbc.M114.584524
Katta, A. V. S. K. M., Marikkannu, R., Basaiawmoit, R. V., and Krishnaswamy, S. (2004). Consensus based validation of membrane porins. In Silico Biol. 4, 549–561.
Kawatsu, K., Ishibashi, M., and Tsukamoto, T. (2006). Development and evaluation of a rapid, simple, and sensitive immunochromatographic assay to detect thermostable direct hemolysin produced by Vibrio parahaemolyticus in enrichment cultures of stool specimens. J. Clin. Microbiol. 44, 1821–1827. doi: 10.1128/JCM.44.5.1821-1827.2006
Kelley, L. A., Mezulis, S., Yates, C. M., Wass, M. N., and Sternberg, M. J. E. (2015). The Phyre2 web portal for protein modeling, prediction and analysis. Nat. Protoc. 10, 845–858. doi: 10.1038/nprot.2015.053
Kim, S., Malinverni, J. C., Sliz, P., Silhavy, T. J., Harrison, S. C., and Kahne, D. (2007). Structure and function of an essential component of the outer membrane protein assembly machine. Science 317, 961–964. doi: 10.1126/science.1143993
Kleine, T., Maier, U. G., and Leister, D. (2009). DNA transfer from organelles to the nucleus: the idiosyncratic genetics of endosymbiosis. Annu. Rev. Plant Biol. 60, 115–138. doi: 10.1146/annurev.arplant.043008.092119
Klemm, P., Vejborg, R. M., and Sherlock, O. (2006). Self-associating autotransporters, SAATs: functional and structural similarities. Int. J. Med. Microbiol. 296, 187–195. doi: 10.1016/j.ijmm.2005.10.002
Koebnik, R., Locher, K. P., and Van Gelder, P. (2000). Structure and function of bacterial outer membrane proteins: barrels in a nutshell. Mol. Microbiol. 37, 239–253. doi: 10.1046/j.1365-2958.2000.01983.x
Kowata, H., Tochigi, S., Takahashi, H., and Kojima, S. (2017). Outer membrane permeability of cyanobacterium Synechocystis sp. strain PCC 6803: studies of passive diffusion of small organic nutrients reveal the absence of classical porins and intrinsically low permeability. J. Bacteriol. 199:e00371-17. doi: 10.1128/JB.00371-17
Kozma, D., Simon, I., and Tusnády, G. E. (2013). PDBTM: Protein data bank of transmembrane proteins after 8 years. Nucleic Acids Res. 41, D524–D529. doi: 10.1093/nar/gks1169
Lasica, A. M., Ksiazek, M., Madej, M., and Potempa, J. (2017). The Type IX Secretion System (T9SS): highlights and recent insights into its structure and function. Front. Cell. Infect. Microbiol. 7:215. doi: 10.3389/fcimb.2017.00215
Lauber, F., Deme, J. C., Lea, S. M., and Berks, B. C. (2018). Type 9 secretion system structures reveal a new protein transport mechanism. Nature 564, 77–82. doi: 10.1038/s41586-018-0693-y
Li, W., Cowley, A., Uludag, M., Gur, T., McWilliam, H., Squizzato, S., et al. (2015). The EMBL-EBI bioinformatics web and programmatic tools framework. Nucleic Acids Res. 43, W580–W584. doi: 10.1093/nar/gkv279
Lin, C.-C., Chou, C.-H., Lin, T.-C., Yang, M.-C., Cho, C.-L., Chang, C.-H., et al. (2012). Molecular characterization of three major outer membrane proteins, TSA56, TSA47 and TSA22, in Orientia tsutsugamushi. Int. J. Mol. Med. 30, 75–84. doi: 10.3892/ijmm.2012.967
Liu, J., Eren, E., Vijayaraghavan, J., Cheneke, B. R., Indic, M., Van Den Berg, B., et al. (2012). OccK channels from Pseudomonas aeruginosa exhibit diverse single-channel electrical signatures but conserved anion selectivity. Biochemistry 51, 2319–2330. doi: 10.1021/bi300066w
Lomize, M. A., Pogozheva, I. D., Joo, H., Mosberg, H. I., and Lomize, A. L. (2012). OPM database and PPM web server: resources for positioning of proteins in membranes. Nucleic Acids Res. 40, D370–D376. doi: 10.1093/nar/gkr703
McKay, T., Hart, K., Horn, A., Kessler, H., Dodge, G., Bardhi, K., et al. (2015). Annotation of proteins of unknown function: initial enzyme results. J. Struct. Funct. Genomics 16, 43–54. doi: 10.1007/s10969-015-9194-5
Meisinger, C., Rissler, M., Chacinska, A., Sanjuán Szklarz, L. K., Milenkovic, D., Kozjak, V., et al. (2004). The mitochondrial morphology protein Mdm10 functions in assembly of the preprotein translocase of the outer membrane. Dev. Cell 7, 61–71. doi: 10.1016/j.devcel.2004.06.003
Meng, G., Spahich, N., Kenjale, R., Waksman, G., and St Geme, J. W. (2011). Crystal structure of the Haemophilus influenzae Hap adhesin reveals an intercellular oligomerization mechanism for bacterial aggregation. EMBO J. 30, 3864–3874. doi: 10.1038/emboj.2011.279
Menny, A., Serna, M., Boyd, C. M., Gardner, S., Joseph, A. P., Morgan, B. P., et al. (2018). CryoEM reveals how the complement membrane attack complex ruptures lipid bilayers. Nat. Commun. 9:5316. doi: 10.1038/s41467-018-07653-5
Moraes, T. F., Bains, M., Hancock, R. E. W., and Strynadka, N. C. J. (2007). An arginine ladder in OprP mediates phosphate-specific transfer across the outer membrane. Nat. Struct. Mol. Biol. 14, 85–87. doi: 10.1038/nsmb1189
Moreno-Gonzalez, I., and Soto, C. (2011). Misfolded protein aggregates: mechanisms, structures and potential for disease transmission. Semin. Cell Dev. Biol. 22, 482–487. doi: 10.1016/j.semcdb.2011.04.002
Natale, P., Brüser, T., and Driessen, A. J. M. (2008). Sec- and Tat-mediated protein secretion across the bacterial cytoplasmic membrane-Distinct translocases and mechanisms. Biochim. Biophys. Acta - Biomembr. 1778, 1735–1756. doi: 10.1016/j.bbamem.2007.07.015
Naumann, M., Rudel, T., and Meyer, T. F. (1999). Host cell interactions and signalling with Neisseria gonorrhoeae. Curr. Opin. Microbiol. 2, 62–70. doi: 10.1016/S1369-5274(99)80011-3
Newport, T. D., Sansom, M. S. P., and Stansfeld, P. J. (2019). The MemProtMD database: a resource for membrane-embedded protein structures and their lipid interactions. Nucleic Acids Res. 47, D390–D397. doi: 10.1093/nar/gky1047
Nguyen, K. A., Zylicz, J., Szczesny, P., Sroka, A., Hunter, N., and Potempa, J. (2009). Verification of a topology model of PorT as an integral outer-membrane protein in Porphyromonas gingivalis. Microbiology 155, 328–337. doi: 10.1099/mic.0.024323-0
Nikaido, H. (2003). Molecular basis of bacterial outer membrane permeability revisited. Microbiol. Mol. Biol. Rev. 67, 593–656. doi: 10.1128/MMBR.67.4.593-656.2003
Noinaj, N., Rollauer, S. E., and Buchanan, S. K. (2015). The β-barrel membrane protein insertase machinery from Gram-negative bacteria. Curr. Opin. Struct. Biol. 31, 35–42. doi: 10.1016/j.sbi.2015.02.012
Nouwen, N., Berrelkamp, G., and Driessen, A. J. M. (2007). Bacterial sec-translocase unfolds and translocates a class of folded protein domains. J. Mol. Biol. 372, 422–433. doi: 10.1016/j.jmb.2007.07.003
Osbak, K. K., Houston, S., Lithgow, K. V., Meehan, C. J., Strouhal, M., Šmajs, D., et al. (2016). Characterizing the syphilis-causing Treponema pallidum ssp. pallidum proteome using complementary mass spectrometry. PLoS Negl. Trop. Dis. 10:e0004988. doi: 10.1371/journal.pntd.0004988
Pagès, J. M., James, C. E., and Winterhalter, M. (2008). The porin and the permeating antibiotic: a selective diffusion barrier in Gram-negative bacteria. Nat. Rev. Microbiol. 6, 893–903. doi: 10.1038/nrmicro1994
Peabody, M. A., Laird, M. R., Vlasschaert, C., Lo, R., and Brinkman, F. S. L. (2016). PSORTdb: expanding the bacteria and archaea protein subcellular localization database to better reflect diversity in cell envelope structures. Nucleic Acids Res. 44, D663–D668. doi: 10.1093/nar/gkv1271
Petersen, T. N., Brunak, S., von Heijne, G., and Nielsen, H. (2011). SignalP 4.0: discriminating signal peptides from transmembrane regions. Nat. Methods 8, 785–786. doi: 10.1038/nmeth.1701
Pizarro-Cerdá, J., and Cossart, P. (2006). Bacterial adhesion and entry into host cells. Cell 124, 715–727. doi: 10.1016/j.cell.2006.02.012
Podack, E., Tschoop, J., and Müller-Eberhard, H. J. (1982). Molecular organization of C9 within the membrane attack complex of complement. induction of circular C9 polymerization by the C5b-8 assembly. J. Exp. Med. 156, 268–282. doi: 10.1084/jem.156.1.268
Podobnik, M., Savory, P., Rojko, N., Kisovec, M., Wood, N., Hambley, R., et al. (2016). Crystal structure of an invertebrate cytolysin pore reveals unique properties and mechanism of assembly. Nat. Commun. 7:11598. doi: 10.1038/ncomms11598
Pohlmeyer, K., Soll, J., Grimm, R., Hill, K., and Wagner, R. (1998). A high-conductance solute channel in the chloroplastic outer envelope from Pea. Plant Cell 10, 1207–1216. doi: 10.1105/tpc.10.7.1207
Raman, P., Cherezov, V., and Caffrey, M. (2006). The membrane protein data bank. Cell. Mol. Life Sci. 63, 36–51. doi: 10.1007/s00018-005-5350-6
Rapoport, T. A. (2007). Protein translocation across the eukaryotic endoplasmic reticulum and bacterial plasma membranes. Nature 450, 663–669. doi: 10.1038/nature06384
Remmert, M., Linke, D., Lupas, A. N., and Söding, J. (2009). HHomp–prediction and classification of outer membrane proteins. Nucleic Acids Res. 37, W446–W451. doi: 10.1093/nar/gkp325
Ringler, P., and Schulz, G. E. (2002). OmpA membrane domain as a tight-binding anchor for lipid bilayers. ChemBioChem 3, 463–466. doi: 10.1002/1439-7633(20020503)3:5<463::AID-CBIC463>3.0.CO;2-P
Rosado, C. J., Buckle, A. M., Law, R. H. P., Butcher, R. E., Kan, W.-T., Bird, C. H., et al. (2007). A common fold mediates vertebrate defense and bacterial attack. Science 317, 1548–1551. doi: 10.1126/science.1144706
Roumia, A. F., Theodoropoulou, M. C., Tsirigos, K. D., Nielsen, H., and Bagos, P. G. (2020). Landscape of eukaryotic transmembrane beta barrel proteins. J. Proteome Res. 19, 1209–1221. doi: 10.1021/acs.jproteome.9b00740
Rouse, S. L., Hawthorne, W. J., Berry, J. L., Chorev, D. S., Ionescu, S. A., Lambert, S., et al. (2017). A new class of hybrid secretion system is employed in Pseudomonas amyloid biogenesis. Nat. Commun. 8:263. doi: 10.1038/s41467-017-00361-6
Ruffolo, C. G., and Adler, B. (1996). Cloning, sequencing, expression, and protective capacity of the oma87 gene encoding the Pasteurella multocida 87-kilodalton outer membrane antigen. Infect. Immun. 64, 3161–3167. doi: 10.1128/IAI.64.8.3161-3167.1996
Rutten, L., Geurtsen, J., Lambert, W., Smolenaers, J. J. M., Bonvin, A. M., De Haan, A., et al. (2006). Crystal structure and catalytic mechanism of the LPS 3-O-deacylase PagL from Pseudomonas aeruginosa. Proc. Natl. Acad. Sci. U.S.A. 103, 7071–7076. doi: 10.1073/pnas.0509392103
Saier, M. H. J., Reddy, V. S., Tsu, B. V., Ahmed, M. S., Li, C., and Moreno-Hagelsieb, G. (2016). The Transporter Classification Database (TCDB): recent advances. Nucleic Acids Res. 44, D372–D379. doi: 10.1093/nar/gkv1103
Sayers, E. W., Barrett, T., Benson, D. A., Bryant, S. H., Canese, K., Chetvernin, V., et al. (2009). Database resources of the national center for biotechnology information. Nucleic Acids Res. 37, D5–D15. doi: 10.1093/nar/gkp382
Schirmer, T., Keller, T. A., Wang, Y. F., and Rosenbusch, J. P. (1995). Structural basis for sugar translocation through maltoporin channels at 3.1 Å resolution. Science 267, 512–514. doi: 10.1126/science.7824948
Schulz, G. E. (2002). The structure of bacterial outer membrane proteins. Biochim. Biophys. Acta 1565, 308–317. doi: 10.1016/S0005-2736(02)00577-1
Sharp, T. H., Koster, A. J., and Gros, P. (2016). Heterogeneous MAC Initiator and pore structures in a lipid bilayer by phase-plate cryo-electron tomography. Cell Rep. 15, 1–8. doi: 10.1016/j.celrep.2016.03.002
Shatursky, O., Heuck, A. P., Shepard, L. A., Rossjohn, J., Parker, M. W., Johnson, A. E., et al. (1999). The mechanism of membrane insertion for a cholesterol-dependent cytolysin: a novel paradigm for pore-forming toxins. Cell 99, 293–299. doi: 10.1016/S0092-8674(00)81660-8
Shepard, L. A., Heuck, A. P., Hamman, B. D., Rossjohn, J., Parker, M. W., Ryan, K. R., et al. (1998). Identification of a membrane-spanning domain of the thiol-activated pore-forming toxin Clostridium perfringens perfringolysin O: an alpha-helical to beta-sheet transition identified by fluorescence spectroscopy. Biochemistry 37, 14563–14574. doi: 10.1021/bi981452f
Shrivastava, A., Roland, T., and Berg, H. C. (2016). The screw-like movement of a gliding bacterium is powered by spiral motion of cell-surface adhesins. Biophys. J. 111, 1008–1013. doi: 10.1016/j.bpj.2016.07.043
Soll, J., and Schleiff, E. (2004). Protein import into chloroplasts. Nat. Rev. Mol. Cell Biol. 9, 222–227. doi: 10.1038/nrm1333
Soto, G. E., and Hultgren, S. J. (1999). MINIREVIEW bacterial adhesins: common themes and variations in architecture and assembly. J. Bacteriol. 181, 1059–1071. doi: 10.1128/JB.181.4.1059-1071.1999
Steckel, E. W., Welbaum, B. E., and Sodetz, J. M. (1983). Evidence of direct insertion of terminal complement proteins into cell membrane bilayers during cytolysis. labeling by a photosensitive membrane probe reveals a major role for the eighth and ninth components. J. Biol. Chem. 258, 4318–24. doi: 10.1016/S0021-9258(18)32625-5
Stojanovski, D., Guiard, B., Kozjak-Pavlovic, V., Pfanner, N., and Meisinger, C. (2007). Alternative function for the mitochondrial SAM complex in biogenesis of α-helical TOM proteins. J. Cell Biol. 179, 881–893. doi: 10.1083/jcb.200706043
Sunde, M., Serpell, L. C., Bartlam, M., Fraser, P. E., Pepys, M. B., and Blake, C. C. F. (1997). Common core structure of amyloid fibrils by synchrotron X-ray diffraction. J. Mol. Biol. 273, 729–739. doi: 10.1006/jmbi.1997.1348
Sutcliffe, I. C. (2010). A phylum level perspective on bacterial cell envelope architecture. Trends Microbiol. 18, 464–470. doi: 10.1016/j.tim.2010.06.005
The UniProt Consortium (2019). UniProt: a worldwide hub of protein knowledge. Nucleic Acids Res. 47, D506–D515. doi: 10.1093/nar/gky1049
Thein, M., Bonde, M., Bunikis, I., Denker, K., Sickmann, A., Bergström, S., et al. (2012). DipA, a pore-forming protein in the outer membrane of Lyme disease spirochetes exhibits specificity for the permeation of dicarboxylates. PLoS ONE 7:e36523. doi: 10.1371/journal.pone.0036523
Thein, M., Bunikis, I., Denker, K., Larsson, C., Cutler, S., Drancourt, M., et al. (2008). Oms38 is the first identified pore-forming protein in the outer membrane of relapsing fever spirochetes. J. Bacteriol. 190, 7035–7042. doi: 10.1128/JB.00818-08
Tomasek, D., and Kahne, D. (2021). The assembly of β-barrel outer membrane proteins. Curr. Opin. Microbiol. 60, 16–23. doi: 10.1016/j.mib.2021.01.009
Trung, N. V., Hoi, L. T., Cuong, D. D., Ha, D. T., Hoa, T. M., Lien, V. N., et al. (2019). Analysis of the 56-kDa type specific antigen gene of Orientia tsutsugamushi from northern Vietnam. PLoS ONE 14:e0221588. doi: 10.1371/journal.pone.0221588
Tschopp, J., Masson, D., and Stanley, K. K. (1986). Structural/functional similarity between proteins involved in complement- and cytotoxic T-lymphocyte-mediated cytolysis. Nature 322, 831–834. doi: 10.1038/322831a0
Tsirigos, K. D., Bagos, P. G., and Hamodrakas, S. J. (2011). OMPdb: a database of {beta}-barrel outer membrane proteins from Gram-negative bacteria. Nucleic Acids Res. 39, D324–D331. doi: 10.1093/nar/gkq863
Tsirigos, K. D., Elofsson, A., and Bagos, P. G. (2016). PRED-TMBB2: improved topology prediction and detection of beta-barrel outer membrane proteins. Bioinformatics 32, i665–i671. doi: 10.1093/bioinformatics/btw444
Tusnády, G. E., Kalmár, L., and Simon, I. (2008). TOPDB: topology data bank of transmembrane proteins. Nucleic Acids Res. 36, D234–D239. doi: 10.1093/nar/gkm751
Van Den Berg, B. (2012). Structural basis for outer membrane sugar uptake in pseudomonads. J. Biol. Chem. 287, 41044–41052. doi: 10.1074/jbc.M112.408518
van den Berg, B., Bhamidimarri, S. P., and Winterhalter, M. (2015). Crystal structure of a COG4313 outer membrane channel. Sci. Rep. 5:11927. doi: 10.1038/srep11927
van den Berg, B., Black, P. N., Clemons, W. M. J., and Rapoport, T. A. (2004). Crystal structure of the long-chain fatty acid transporter FadL. Science 304, 1506–1509. doi: 10.1126/science.1097524
Varghees, S., Kiss, K., Frans, G., Braha, O., and Samuel, J. E. (2002). Cloning and porin activity of the major outer membrane protein P1 from Coxiella burnetii. Infect. Immun. 70, 6741–6750. doi: 10.1128/IAI.70.12.6741-6750.2002
Volokhina, E., Beckers, F., Tommassen, J., and Bos, M. (2009). The beta-barrel outer membrane protein assembly complex of Neisseria Meningitidis. J. Bacteriol. 191, 7074–7085. doi: 10.1128/JB.00737-09
von Heijne, G. (1999). Recent advances in the understanding of membrane protein assembly and structure. Q. Rev. Biophys. 32, 285–307. doi: 10.1017/S0033583500003541
Vora, G. J., Meador, C. E., Bird, M. M., Bopp, C. A., Andreadis, J. D., and Stenger, D. A. (2005). Microarray-based detection of genetic heterogeneity, antimicrobial resistance, and the viable but nonculturable state in human pathogenic Vibrio spp. Proc. Natl. Acad. Sci. U.S.A. 102, 19109–19114. doi: 10.1073/pnas.0505033102
Voulhoux, R., Bos, M. P., Geurtsen, J., Mols, M., and Tommassen, J. (2003). Role of a highly conserved bacterial protein in outer membrane protein assembly. Science 299, 262–5. doi: 10.1126/science.1078973
Walker, S. G., Ebersole, J. L., and Holt, S. C. (1997). Identification, isolation, and characterization of the 42-kilodalton major outer membrane protein (MompA) from Treponema pectinovorum ATCC 33768. J. Bacteriol. 179, 6441–6447. doi: 10.1128/JB.179.20.6441-6447.1997
Wall, D., and Kaiser, D. (1999). Type IV pili and cell motility. Mol. Microbiol. 32, 01–10. doi: 10.1046/j.1365-2958.1999.01339.x
Wang, Y., Pannuri, A. A., Ni, D., Zhou, H., Cao, X., Lu, X., et al. (2016). Structural basis for translocation of a biofilm-supporting exopolysaccharide across the bacterial outer membrane. J. Biol. Chem. 291, 10046–10057. doi: 10.1074/jbc.M115.711762
Wang, Y. F., Dutzler, R., Rizkallah, P. J., Rosenbusch, J. P., and Schirmer, T. (1997). Channel specificity: Structural basis for sugar discrimination and differential flux rates in maltoporin. J. Mol. Biol. 272, 56–63. doi: 10.1006/jmbi.1997.1224
Wang, Z., Xu, C., and Benning, C. (2012). TGD4 involved in endoplasmic reticulum-to-chloroplast lipid trafficking is a phosphatidic acid binding protein. Plant J. 70, 614–623. doi: 10.1111/j.1365-313X.2012.04900.x
White, S. H. (2009). Biophysical dissection of membrane proteins. Nature 459, 344–346. doi: 10.1038/nature08142
Whitfield, C., and Trent, M. S. (2014). Biosynthesis and export of bacterial lipopolysaccharides. Annu. Rev. Biochem. 83, 99–128. doi: 10.1146/annurev-biochem-060713-035600
Wirth, C., Condemine, G., Boiteux, C., Bernèche, S., Schirmer, T., and Peneff, C. M. (2009). NanC crystal structure, a model for outer-membrane channels of the acidic sugar-specific KdgM porin family. J. Mol. Biol. 394, 718–731. doi: 10.1016/j.jmb.2009.09.054
Yu, C.-S., Chen, Y.-C., Lu, C.-H., and Hwang, J.-K. (2006). Prediction of protein subcellular localization. Proteins 64, 643–651. doi: 10.1002/prot.21018
Yu, N. Y., Wagner, J. R., Laird, M. R., Melli, G., Rey, S., Lo, R., et al. (2010). PSORTb 3.0: improved protein subcellular localization prediction with refined localization subcategories and predictive capabilities for all prokaryotes. Bioinformatics 26, 1608–1615. doi: 10.1093/bioinformatics/btq249
Zhang, G., Meredith, T. C., and Kahne, D. (2013). On the essentiality of lipopolysaccharide to Gram-negative bacteria. Curr. Opin. Microbiol. 16, 779–785. doi: 10.1016/j.mib.2013.09.007
Keywords: beta barrel membrane proteins, Gram-negative bacteria, mitochondria, chloroplast, β-barrels, Hidden Markov Model, database, sequence analysis
Citation: Roumia AF, Tsirigos KD, Theodoropoulou MC, Tamposis IA, Hamodrakas SJ and Bagos PG (2021) OMPdb: A Global Hub of Beta-Barrel Outer Membrane Proteins. Front. Bioinform. 1:646581. doi: 10.3389/fbinf.2021.646581
Received: 27 December 2020; Accepted: 18 March 2021;
Published: 09 April 2021.
Edited by:
Michael Gromiha, Indian Institute of Technology Madras, IndiaReviewed by:
Bert Van Den Berg, Newcastle University, United KingdomCastrense Savojardo, University of Bologna, Italy
Joanna Slusky, University of Kansas, United States
Copyright © 2021 Roumia, Tsirigos, Theodoropoulou, Tamposis, Hamodrakas and Bagos. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Pantelis G. Bagos, cGJhZ29zQGNvbXBnZW4ub3Jn
†These authors have contributed equally to this work and share first authorship
 Ahmed F. Roumia
Ahmed F. Roumia Konstantinos D. Tsirigos2†
Konstantinos D. Tsirigos2† Margarita C. Theodoropoulou
Margarita C. Theodoropoulou Ioannis A. Tamposis
Ioannis A. Tamposis Pantelis G. Bagos
Pantelis G. Bagos