- 1Department of Chemical and Pharmaceutical Sciences, University of Trieste, Trieste, Italy
- 2Fondazione Istituto di Ricerca Pediatrica Città della Speranza, Padua, Italy
- 3Sidra Medical and Research Center, Doha, Qatar
- 4Department of Biomedical Engineering, University of Ankara, Ankara, Turkey
- 5Department of Medical Sciences, University of Ferrara, Ferrara, Italy
- 6Maria Cecilia Hospital, GVM Care & Research, Ravenna, Italy
- 7Department of Neurology, Bioengineering, Physical Medicine & Rehabilitation, Center for Neuroengineering and Therapeutics, University of Pennsylvania, Philadelphia, PA, United States
- 8Center for Neurotrauma, Neurodegeneration, and Restoration, Corporal Michael J. Crescenz Veterans Affairs Medical Center, Philadelphia, PA, United States
- 9Stem Cell Institute, University of Ankara, Ankara, Turkey
- 10Department of Biomedical Sciences, University of Padua, Padua, Italy
Cancer is one of the leading causes of death in the world. Therefore, the development of new advanced and targeted strategies in cancer research for early diagnosis and treatment has become essential to improve diagnosis outcomes and reduce therapy side effects. Graphene and more recently, MXene, are the main representatives of the family of two-dimensional (2D) materials and are widely studied as multimodal nanoplatforms for cancer diagnostics and treatment, in particular leveraging their potentialities as photodynamic therapeutic agents. Indeed, due to their irreplaceable physicochemical properties, they are virtuous allies for photodynamic therapy (PDT) in combination with bioimaging, photothermal therapy, as well as drug and gene delivery. In this review, the rapidly progressing literature related to the use of these promising 2D materials for cancer theranostics is described in detail, highlighting all their possible future advances in PDT.
Introduction
Photodynamic therapy (PDT) is a form of phototherapy aimed at achieving cell death via the generation of cytotoxic reactive oxygen species (ROS). Although PDT is still an emerging therapeutic modality, it has already been established as a clinically approved method for the treatment of various malignant diseases, including cancer (Agostinis et al., 2011).
Clinically, PDT is usually used in conjunction with other forms of treatments, such as surgery, radiotherapy (RT), and chemotherapy (CT). Due to its local activation and limited tissue penetration, PDT has relatively low invasiveness, and in many cases, good cosmetic results. Therefore, this therapy is particularly suitable for the treatment of exposed skin and sensitive areas, like the head and neck. Moreover, even though it may induce prolonged periods of skin photosensitivity, during which patients need to avoid light, it lacks the serious adverse events (AE) seen in RT and systemic CT. Surgery represents the first-choice treatment and, for the majority of tumor types, the only curative intervention for early diagnosed cancer. However, since most patients are usually diagnosed at late stages, treatments such CT and RT are then preferred. In case of inoperable disease and failure or refusal of other treatments, PDT can potentially be used as a standalone treatment or in combination with other therapies due to the absence of systemic effects and its ability to preserve the organ function. Furthermore, unlike RT, PDT mechanisms of action allow its use also for repeated treatments.
Currently, there are 563 registered clinical trials for PDT, of which almost 60% are directed against cancer (www.clinicaltrials.gov). Among the drug-device combination products, PDT was the first one approved by the Food and Drug Administration (FDA), being now under investigation in preclinical studies to improve its efficacy and safety (Ferreira Dos Santos et al., 2019). The PDT procedure requires three main components: a photosensitizer (PS), a light source (laser), and molecular tissue oxygen. In the context of cancer treatment, the PS is administered locally or systemically, being accumulated in the tumor site. Subsequently, the patient is locally irradiated with light of a proper wavelength, with the aim to activate the PS in the presence of molecular oxygen (Dolmans et al., 2003).
Following administration, PSs can be internalized by both cancer and normal cells. While healthy tissues can eliminate the PS over time, this is not possible for tumor cells due to the lymphatic inadequacy. The resulting PS retention in tumor tissues, together with the localized activation by irradiation, makes PDT a selective treatment for cancer.
The treatment will result in localized oxidative photodamage, consisting in the oxidation of a large range of cellular biomolecules, including nucleic acids, lipids, and proteins. Consequently, the process will lead to selective cytotoxicity, mainly due to a severe alteration in cell signaling cascades and gene expression regulation. The cellular response to photodamage is closed related to several factors. Due to its characteristics, a PS will usually accumulate toward different cellular organelles (e.g., mitochondria and lysosomes), plasma membrane, Golgi apparatus, or endoplasmic reticulum (ER). Generally, three main mechanisms of photodamage-induced cell death have been described: apoptosis, necrosis and autophagy at the tumor site (Bacellar et al., 2015; Ferreira Dos Santos et al., 2019). This process is also accompanied by the induction of an acute local inflammatory reaction that participates in the removal of dead cells, restoration of normal tissue homeostasis and development of systemic immunity (Henderson et al., 2004; Korbelik, 2006). This ability of PDT to activate multiple cell death pathways enables circumvention of apoptosis-resistance in cancer cells, one of the main problems for anticancer approaches.
The superoxide anions released in type I reactions do not pose particular harm to biological systems directly but contribute to the production of hydrogen peroxide, resulting in lipid peroxidation, ultimately leading to the disruption of cellular membranes. Thanks to the short singlet oxygen (1O2) lifetime of ~40 ns and its short-range action (maximum action radius of about 20 nm), together with the localized PS light-induced activation, PDT is a highly controllable and specific therapy. PS localization can also modulate the subcellular site of action of PDT. Extensive cell damages could also affect apoptotic pathway components, and therefore apoptosis may not be properly executed. Thanks to the autophagy process, cells have the ability to recycle damaged cytoplasmic components and organelles trough the creation of the “autophagosome,” a double membrane structure that after the engulfment of the damaged particles fuses with lysosomes in order to degrade its contents. This autophagic process is not only considered to be a cytoprotective mechanism, being observed also as a cell death mechanism in response to PDT. When the apoptotic mechanism is compromised, cell death mainly occurs through autophagy. This seems to be also correlated with PDT dose, since autophagy can serve as a protective mechanism or initiate the autophagic cell-death, when using low or high doses, respectively (Figures 1A,B). Several preclinical studies have been performed to improve the safety and efficacy of PDT, as well as to extend the number of the different types of diseased tissues that can be treated, thanks to the use of next-generation PSs. The design of second-generation PSs was aimed to develop new agents with higher absorption wavelengths, enabling deeper organs to be targeted thanks to enhanced penetration of light (Lou et al., 2004; Agostinis et al., 2011; Story et al., 2013). Later, the introduction of third-generation PSs allowed improving targeting strategies, such as antibody-directed PS and PS-loaded nanocarriers (Agostinis et al., 2011; Yoon et al., 2013).
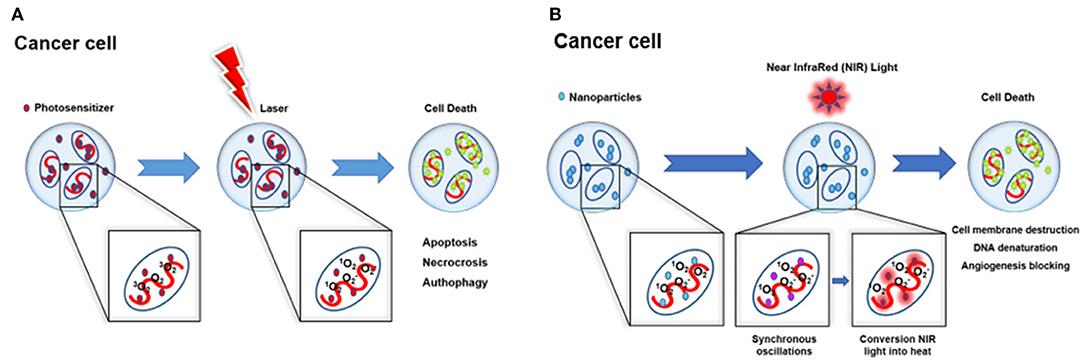
Figure 1. Schematic illustration of phototherapy. (A) PDT mechanisms of action and subsequent induced cell death: apoptosis, necrosis, and autophagy. (B) PTT mechanisms of action and subsequent cell death induced by cell membrane destruction, DNA denaturation, and angiogenesis blocking.
Thanks to the progress of nanotechnology, the improvement of PDT using theranostic two-dimensional (2D) nanomaterials (NMs) is attracting growing attention. Therapeutic strategies were combined with imaging modalities for a theranostic aim in order to monitor the biodistribution of therapeutic agents and to identify and/or localize the tumor mass and its growth (Cho et al., 2013; Wang et al., 2013; Ge et al., 2014; Gollavelli and Ling, 2014; Rong et al., 2014; Kim et al., 2015; Wu et al., 2015; Yan et al., 2015b; Guan et al., 2016; Kalluru et al., 2016; Luo et al., 2016; Gulzar et al., 2018).
Currently, multiple combinations of various therapeutic and diagnostic modalities are adopted to achieve a theranostic effect (Orecchioni et al., 2015; Ji et al., 2019), and can be further improved and expanded thanks to the development of NM-based theranostic nanoplatforms. Among 2D NMs suitable for this purpose, graphene and graphene-based materials (GBMs), including few layer graphene (FLG), graphene oxide (GO), reduced graphene oxide (rGO), nano-graphene oxide (NGO), and graphene quantum dots (GQDs), bring the technological innovations needed to the current societal and industrial challenges (Boukhvalov and Katsnelson, 2008; Park and Ruoff, 2009; Gao et al., 2010; Kuila et al., 2012; Mao et al., 2012; James and Tour, 2013; Quintana et al., 2013; Yang et al., 2013a; Roppolo et al., 2014; Sechi et al., 2014; Servant et al., 2014; Kim et al., 2016; Shin et al., 2016; McManus et al., 2017; Park et al., 2017). Graphene, consisting of a single layer of carbon atoms arranged in a honeycomb structure, exhibits a unique combination of physiochemical properties, including high surface area (2,630 m2 g−1), optimal thermal conductivity (~5,000 Wm K−1), remarkable optical transparency (single layer graphene absorbs ~2.3% of visible light), strong mechanical strength (Young's modulus of ~1 TPa), and room temperature quantum hall effect for electrons and holes (Novoselov et al., 2012). Its 2D plane sp2 hybridization results in delocalized out of plane π bonds providing an outstanding carrier mobility (ranging from ~ 200,000 to ~500,000 cm2 V−1 s−1, in case of suspended graphene or graphene-based field effect transistors, respectively). Due to its characteristics, graphene offers new fascinating perspectives in nanomedicine for the development of new therapeutic delivery approaches, imaging strategies, as well as biosensor-based diagnostic tools (Yang et al., 2013b; Orecchioni et al., 2014, 2015, 2016b; Avitabile et al., 2018; Fadeel et al., 2018b).
Recently, other promising 2D NMs have attracted attention for their possible applications in various fields, including biomedical sciences (Chen et al., 2015; Luo et al., 2018). One of the most recently discovered 2D materials is MXene, which was first introduced by Gogotsi et al. in 2011 (Naguib et al., 2011). Since then, more than 20 species of MXenes have been successfully synthesized, and the structure/properties of more than 70 have been predicted in silico. MXenes are composed of early-transition-metal carbides, carbonitrides, and nitrides with structural formula Mn + 1Xn, where M is an early transition metal, X stands for carbon, nitrogen, or both, and n = 1–3. MXenes are synthesized through selectively etching the A-group element from the precursor ternary-layered carbides of MAX phases, where A represents a group of 12–16 periodic table elements. As a consequence of the selectively etching of the A group with -F containing etchants, such as HF, the resulting MXenes will be characterized by abundant surface-terminating functional groups, e.g., hydroxyl (–OH), oxygen (–O), or fluorine (–F), endowing their hydrophilic nature and allowing their flexible surface modification and functionalization. Thanks to the production scalability, the rich surface chemistry, the metallic conductivity, the excellent mechanical/thermal properties, and ease of processability, MXenes have attracted increasing attention for a number of different applications, such as energy storage (Lukatskaya et al., 2017), electromagnetic interference shielding (Shahzad et al., 2016), electrocatalysts (Seh et al., 2016), electrochemical supercapacitors (Ghidiu et al., 2015), and Li-ion batteries (Er et al., 2014; Anasori et al., 2017), just to name a few.
In recent years, MXenes have also been explored for their applications in biomedicine, especially as building-blocks in nano-biotechnology platforms. From the topological perspective, MXenes share all the advantages of other classes of 2D NMs, stemming from their impressive properties, such as extreme thinness, high surface-to-volume ratio, and mechanical toughness. Additionally, the rich chemistry on the surface of MXenes provides abundant reactive sites for enzyme or drug functionalization, while their volumetric capacitance and metallic conductivity are highly desirable for low-noise and high-fidelity biosensors (Driscoll et al., 2018). MXenes exhibit strong absorption in the near-infrared (NIR) region, both in the first (650–950 nm) and second biological window (1,000–1,350 nm), where the low scattering and energy absorption allow maximum penetration of the radiation through the tissue.
The suitability of GBMs for multiple cancer theranostic applications is due to their unique intrinsic physicochemical properties, making them superior nanotools compared to the existing materials and devices used for this purpose, such as optical transparency, high surface area, easy surface functionalization, and low-cost production. In this contest, the use of GBMs and MXenes has been proposed to enhance PDT efficiency. For example, these promising materials are able to correct some of the limits showed by the conventional PSs required for this medical technique. Those are mainly represented by porphyrin-based molecules, such as Chlorin e6 (Ce6), which are characterized by low solubility, photostability, difficulties in delivery efficiency, and inability to be absorbed in regions where the skin is the most transparent (Detty et al., 2004; Huang, 2005). Besides providing a superior biocompatibility, 2D NMs, and in particular GO, can endow them with higher water dispersibility (Gao et al., 2004; Michalet et al., 2005; Resch-Genger et al., 2008), photostability, cytotoxicity, and ROS-generation efficiency (Ge et al., 2014; Pelin et al., 2018). Other materials, such as GQDs, are able to perform better than conventional PDT agents due to their extremely high 1O2 quantum yield, GQDs (Ge et al., 2014).
Moreover, the particular nanostructure and the large surface area of these 2D NMs facilitate the loading of PSs and other targeting moieties or drugs, allowing a specific release of the treatment and selectivity for cancer cells. Indeed, the presence of the 2D surface characterized by delocalized π electrons and, in particular for GO, the existence of polar functionalities (e.g., epoxide, carbonyl, carboxyl, and hydroxyl groups), allows high drug loading ratios to be reached simply, even of poorly soluble chemotherapeutic drugs, based on electrostatic or hydrophobic interactions and π-π stacking capability, which can even achieve 200 wt% (Augustine et al., 2017). In addition, thanks to the high surface-to-volume ratio, it is possible to reach a superior bio-functionalization, which allows several drugs and molecules to be added, including such fluorescent probes, genes, and targeting moieties to specifically recognize cancer cells, making it possible to achieve their guided and controlled release to the targeted cells.
Furthermore, thanks to the intrinsic NIR absorption properties, GO is a suitable tool for both PDT and photothermal therapy (PTT), obtaining a higher therapeutic efficiency through both in situ production of ROS and tumor ablation under NIR irradiation. Together with PDT, PTT represents an alternative anticancer therapy thanks to the selectivity of the hyperthermic process toward cancer cells, sparing healthy tissues. Irradiation of plasmonic NPs accumulated in the tumor with a light of appropriate wavelength leads the NP conduction band electrons to undergo synchronized oscillations, allowing the conversion of NIR light into heat. There are three mechanisms that lead to cell death: cell membrane damage, denaturation of DNA, and angiogenesis blocking (Figure 1B). The investigation of MXenes as PSs for PDT is still in its infancy and, as of June 2019, most of the published works have reported on different MXene species as photothermal conversion agents (PTAs) for PTT (Lin et al., 2016, 2017; Dai et al., 2017; Han et al., 2018; Feng et al., 2019). Indeed, MXenes show higher photothermal effect compared to GO; thus, they appear particularly suitable as PTA for cancer therapy and imaging (Lin et al., 2016, 2018).
In light of this consideration, in this review, we aim to discuss the current state of the art of PDT in cancer theranostics based on GBMs and MXenes, alone or in combination with other therapies (i.e., PTT and drug delivery). A literature mining protocol was developed to present an overview of the literature in this context, focusing on the different types of models, cancer, functionalization, and combined approaches. A schematic representation of graphene- and MXene-based PDT for cancer theranostic applications is shown in Figure 2. We then have analyzed the future trends in PDT related to graphene and MXene, identifying different knowledge gaps in the field.
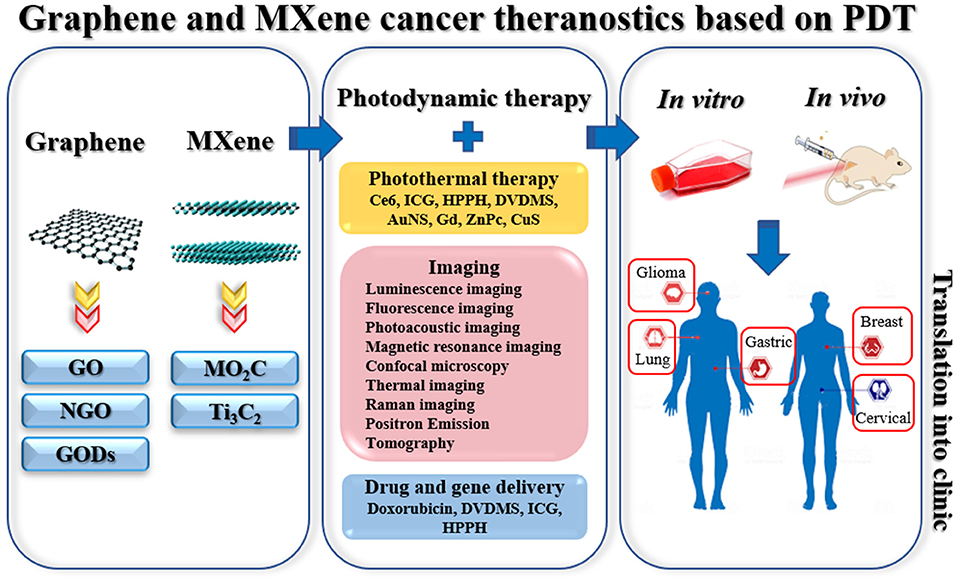
Figure 2. Schematic representation of the current applications in PDT for cancer theranostics based on graphene and MXene. Left panel: representation of graphene and MXene. Middle panel: combined applications with PDT, types of conjugated molecules (for PTT), types of imaging, and examples of conjugated drugs (for drug delivery). Right panel: types of cancer investigated in vitro and in vivo.
Graphene and MXene Literature Surfing
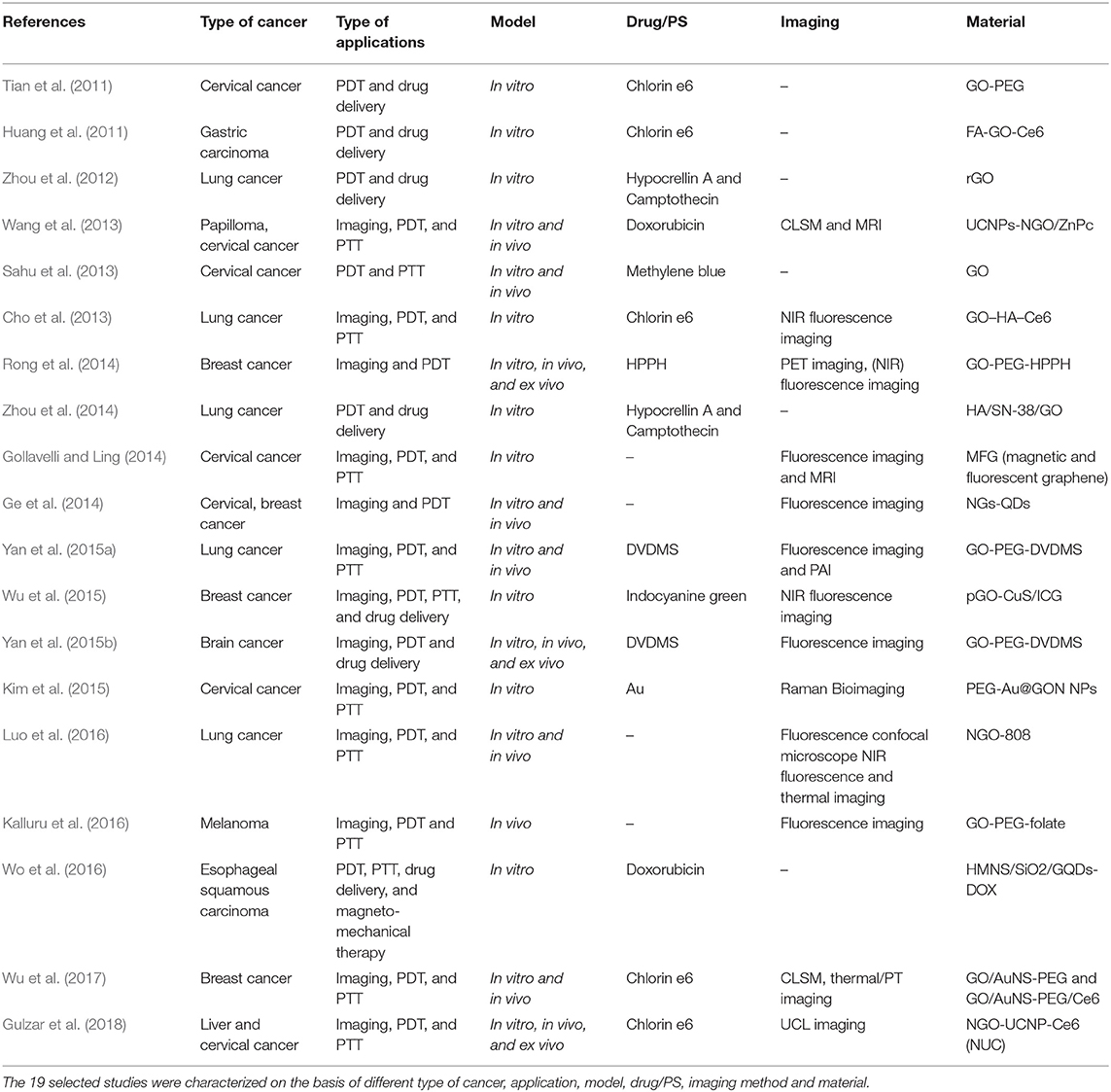
A systematic review of the literature on graphene and MXene, studied in biomedicine as nanotools for cancer theranostic applications based on PDT, was performed with no time restriction, according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines. The electronic databases (PubMed, Scopus, and ToxLine) were used as data sources, via the following keywords in several different combinations: graphene, GBMs, FLG, GO, rGO, NGO, GQDs, MXene, theranostic, and PDT. To help the reader, Table 1 shows all the acronyms used in the text. As an additional tool, high-impact review articles were also considered. The list of reported studies includes all the retrieved publications from 2008 to January 2019. The adopted inclusion criteria were as follows: (1) studies published in English; (2) full text articles; and (3) the use of PDT in combination with other graphene/MXene applications. A total of 20 eligible studies were identified through the literature review for inclusion in the current review, 1 for MXenes and 19 for GBMs. The latter are summarized in Table 2 based on the different types of cancer, combined applications, species of investigation, model, and material used in the respective study. The trend from 2011 to 2018 displays a remarkable growing interest in graphene and GBMs for cancer PDT.
A search on clinical trials was performed using the same criteria; however, although there are currently 325 clinical trials based on PDT for cancer therapy (www.clinicaltrials.gov), none of them involves GBMs or MXenes. This result highlights that the research on 2D nanomaterials for PDT, despite promising results obtained in vitro and in vivo, is still at a very early stage for a clinical translation.
The 19 manuscripts on graphene-based PDT in cancer theranostics were analyzed with respect to type of applications combined with PDT, model used for the study (in vivo or in vitro), and type of cancer studied (Figure 3). Focusing on the association of other cancer theranostic applications, it emerged that PDT was often applied together with one or multiple therapies and imaging modalities: the majority of the studies (58%) concerned the simultaneous application of PDT, imaging, and PTT, drug delivery or other therapies, followed by the combination of PDT with PTT, drug delivery or other therapies (32%), while only 10% of the works used PTD associated with imaging alone (Figure 3A). In particular, PDT was used in combination with different imaging techniques, such as luminescence imaging (CLI), fluorescence imaging, photoacoustic imaging (PAI), magnetic resonance imaging (MRI), confocal microscopy (CLSM), thermal imaging (IRT), Raman imaging, and positron emission tomography (PET). From the analysis of the model used in the works, it emerged that most studies were carried out both in vitro and in vivo (48%), a large number (47%) using only in vitro models consisting of different kinds of cancer cells, while only 5% tested these materials exclusively in vivo (Figure 3B).
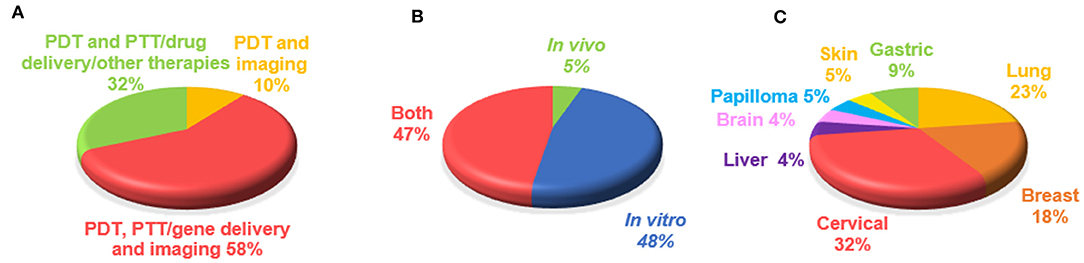
Figure 3. Overview of graphene-based PDT theranostics. Percentages of manuscripts (19 papers) on the basis of (A) type of applications combined with PDT, (B) model used for the study (in vivo or in vitro), (C) type of cancer studied.
Finally, we focused on the different types of tumors studied (Figure 3C), identifying cervical cancer as the most investigated (32% of publications). Indeed, according to the World Health Organization (WHO)1, cervical cancer is the fourth most common type of tumor in women and the eighth most frequently occurring overall; rising with 570,000 new cases in 2018 and representing 6.6% of all female cancers (from world health organization www.who.int). The second largest portion comprises works focusing on lung cancer (23%), followed by publications concerning breast cancer (18%) and gastric cancer (9%). Papers investigating other kinds of tumors, such as skin, brain, liver cancer, and papilloma, make up the remainder (5%).
Progress in Photodynamic Therapy in Graphene- and MXene-mediated Theranostics
GBMs have attracted attention for PDT exploiting their optical loading properties (Avitabile et al., 2018; Viseu et al., 2018). Studies in the field of theranostics started by using GBMs as delivery vehicles for both PS and imaging agent (Sahu et al., 2013; Gollavelli and Ling, 2014), paving the way to the following research for a more detailed exploration of nanotechnology-based PDT in cancer theranostics. Graphene has been shown to adsorb light in the near infrared (NIR) region, allowing its potential application for cancer phototherapy to be evaluated both in vivo and in vitro (Cho et al., 2013; Sahu et al., 2013; Wang et al., 2013; Gollavelli and Ling, 2014; Rong et al., 2014; Kim et al., 2015; Wu et al., 2015, 2017; Yan et al., 2015a; Kalluru et al., 2016; Luo et al., 2016; Wo et al., 2016; Gulzar et al., 2018).
Moreover, it has been demonstrated, both in cell and animal models, that GBMs exhibit several advantages for drug delivery, giving the possibility of high drug loading efficiency, controlled drug release and tumor-targeted drug delivery (Bitounis et al., 2013; Yang et al., 2016; Zhang et al., 2016, 2017). Indeed, different biomolecules, such as DNA, microRNA (miRNA), short interfering RNA (siRNA), and chemotherapeutic drugs can be loaded onto the surface of these materials for gene transfection and drug delivery (Huang et al., 2011; Tian et al., 2011; Zhou et al., 2014; Yan et al., 2015b; Wo et al., 2016; Wu et al., 2017). GBMs and MXenes are also suitable for imaging purposes. In particular, GO-based nanoplatforms show great potential exploitable for imaging purposes, thanks not only to the efficient quenching properties of GO toward several fluorescent moieties, including dyes, quantum dots, and conjugated polymers, but also to its ability to improve their stability, distribution, biocompatibility, and photodynamic efficiency (Yan et al., 2015b). Other materials, such as NGO sheets, have a photoluminescent emission in the visible and infrared regions (Sun et al., 2008). This intrinsic photoluminescence (PL) can be exploited for little background-NIR live cell imaging (Sun et al., 2008). GQDs exhibit multiple properties, ranging from their broad absorption in the visible and NIR light range, their good aqueous dispersibility, deep-red emission, high pH and photo-stability up to their positive biocompatibility. In addition, GQDs display a relevant 1O2 generation yield, beyond 1.3 (almost double compared to the other PDT agents studied in literature). Among the various unique properties, GQDs also present an up-conversion PL (Zhu et al., 2012a,b; Feng et al., 2017), ranging from blue to yellow colors (Li et al., 2013). Due to all their properties, GQDs are able to behave as a multifunctional nanoplatform for the theranostic combination of imaging and highly efficient in vivo PDT (Ge et al., 2014).
For these reasons, scientists attempted to exploit these therapeutic and imaging potentialities of GBMs in cancer theranostics to achieve targeted cancer cell killing as well as less impairment of healthy cells. An example of PDT based on graphene for combined applications in cancer theranostic is reported in Figure 4.
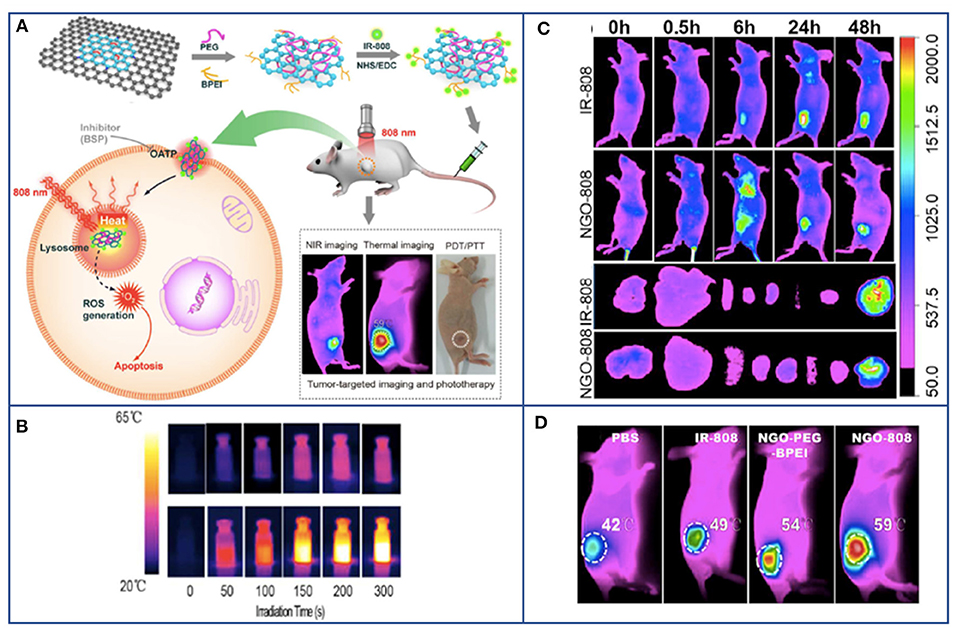
Figure 4. Example of PDT based on graphene for combined and multimodal applications in cancer theranostic. (A) Schematic illustration of NGO-808 preparation and combined A549 tumor xenografts-targeted NIR imaging and synergistic phototherapy (PDT and PTT). (B) Thermal images showing the higher heat generation of NGO-808 (bottom row) compared to that in blank phosphate-buffered saline (upper row) during 5 min 808 nm laser irradiation. (C) In vivo NIR imaging of NGO-808 on A549 tumor xenografts. (D) In vivo combined PDT and PTT on A549 tumor xenografts treated with NGO-808. Adapted with permission from Luo et al. (2016), copyright 2016 American Chemical Society.
Combined Therapy: PDT and Drug Delivery/PTT
The development of outstanding nanoplatforms leveraging PDT and synergistic therapies, based on drug delivery and PTT, is currently being extensively investigated for cancer treatment. In one of the earliest works showing the promise of GBMs in PDT, in 2011, Tian et al. loaded polyethylene glycol (PEG)-functionalized GO with the PS Ce6 via supramolecular π-π stacking (Tian et al., 2011). The material was taken up by cervical cancer cells and resulted in the formation of ROS under light excitation. Anti-cancer activity of the GO-PEG-Ce6-mediated PDT protocol was more pronounced compared to free Ce6. Also, Huang et al. proposed to use GO as a delivery platform for Ce6 (Huang et al., 2011). Like the above studies, Ce6 was loaded onto folic acid targeted GO through π-π stacking and hydrophobic interactions. The system was shown to kill MGC803 gastric cancer cells upon irradiation. Later, Zhou et al. efficiently loaded GO with the PS hypocrellin B (HB) through π-π stacking interaction. They showed that the material was able to generate 1O2 upon irradiation (Zhou et al., 2012). The same group later reported that the efficiency of PS-loaded GO anticancer activity could be even improved through its combination with chemotherapy (Zhou et al., 2014). In particular, in this study, hypocrellin A and 7-ethyl-10-hydroxycamptothecin were co-loaded on GO and the resulting system induced higher cell death in a lung cancer cell line model when exposed to light, demonstrating that chemotherapy and PDT can work synergistically (Zhou et al., 2012).
Later, in another study, functionalized nano-graphene oxide (NGO) was complexed with a PS methylene blue in order to achieve combined PTT/PDT of cancer (Sahu et al., 2013). Due to the pluronic functionalization, material showed great stability in biological fluids. Authors reported that the nanocomplex was efficiently taken up by cancer cells and able to release methylene blue in a pH-dependent manner. Only when exposed to light did the system showed anti-cancer activity in vitro. Following its systemic administration in tumor bearing mice, nanocomplex was shown to accumulate in tumor. When mice were irradiated with NIR light, it caused total ablation of tumor tissue through the combined action of photodynamic and photothermal effects.
These studies suggested the possibility of exploiting the properties of GBMs and MXene to perform an improved PDT in multimodal nanosystems for cancer treatment and paved the way up for future theranostic works.
Theranostics: Imaging and PDT
GBMs can be used not only in PDT protocols and other combined therapies, but also for imaging purpose, allowing the development of new theranostic nanoplatforms for cancer diagnosis and treatment. For example, in 2014, Ge et al. exploited the intrinsic properties of graphene quantum dots (GQDs), such as the broad absorption from the visible to the NIR, high pH- and photo-stability, and biocompatibility for imaging purposes (Ge et al., 2014). In this study, GQDs exhibited a massive 1O2 generation yield, making them efficient multifunctional nanoplatforms for in vivo simultaneous imaging and extremely efficient PDT of different types of cancer, including skin melanoma and tumors located near the skin. During the same year, Rong et al. identified PEG-functionalized GO as a suitable nanoplatform to increase PDT efficacy and improve long-term survival after treatment (Rong et al., 2014). This was obtained mainly thanks to the ability of the GO-based nanotool to serve as a carrier for the PS agent HPPH, increasing its accumulation to the cancer site. In their in vivo studies, the distribution and delivery were traced through fluorescent imaging and positron emission tomography (PET) by the 64Cu radiolabeling of HPPH. Compared to free HPPH, GO-PEG-HPPH enhanced the photodynamic cancer cell killing ability thanks to HPPH's improved tumor delivery.
Theranostics: Imaging, PDT, and Drug Delivery
In a study directed against lung cancer, a novel photo-theranostic platform based on sinoporphyrin sodium (DVDMS) loaded on PEGylated GO was investigated (Yan et al., 2015a). The GO-PEG carrier improved the loaded PS DVDMS fluorescence through intramolecular charge transfer and facilitated tumor accumulation efficiency of DVDMS by enhanced permeability and retention effect. The NIR absorption of GO was enhanced by DVDMS, leading to improved photoacoustic imaging and PTT. The in vivo intravenous injection of low doses of GO-PEG (1 mg/kg) and of DVDMS (2 mg/kg) resulted in 100% tumor eradication.
Theranostics: Imaging, PDT, and PTT
New advances have been made in cancer treatment to establish a targeted protocol that covers the simultaneous application of imaging methods for diagnosis and PTT or PDT for its care. The theranostic progress made by the early studies led to the development of new combined protocols involving graphene nanoplatforms for a simultaneous imaging, PTT, and PDT approach. Wang et al. developed a promising integrated probe for UCL image-guided conjunctional PDT/PTT of cancer (Wang et al., 2013). This multifunctional nanoplatform (UCNPs-NGO/ZnPc) was composed of covalently grafted core–shell structured upconversion nanoparticles (UCNPs) with nanographene oxide (NGO) via bifunctional PEG loaded with phthalocyanine (ZnPc). Authors suggested that this nanoplatform could be used as UCL high contrast imaging probing of cells and whole-body for diagnosis, as well as for PDT causing the formation of cytotoxic 1O2 under light excitation and for PTT, by converting the 808 nm laser energy into thermal energy (Wang et al., 2013). Another promising platform for combined PTT/PDT directed for lung cancer was realized by combining biocompatible HA-conjugated Ce6 with GO (Cho et al., 2013). This dual PTT/PDT enzyme-activatable GO–PS nanoplatform (GO–HA–Ce6) acts as a biologically tunable agent, exploitable for NIR fluorescence imaging and photo-induced cancer therapy (Cho et al., 2013). Another incredible example of graphene-based combined multimodal nanosystem for simultaneous imaging, NIR-induced PTT and PDT was presented by Wu et al. (2017). In this research, all the promising applications of graphene/Au-based nanohybrids have been summed up into one single nanoplatform. They formulated a graphene-Au nanostar hybrid NM (GO/AuNS-PEG) activated by a single wavelength laser-mediated phototheranostic design, based on the loading of Ce6 (GO/AuNS-PEG/Ce6) (Sahu et al., 2013). Gollavelli et al. developed a superparamagnetic graphene-based nanoplatform, so-called MFG-SiNc4, carrying the hydrophobic silicon napthalocyanine bis (trihexylsilyloxide) (SiNc4) PS (Gollavelli and Ling, 2014). The graphene used in the study showed a wide range for NIR absorption (600–1,200 nm). Therefore, the presence of SiNc4, working at any wavelength within this range, facilitated the possibility of single light induced phototherapy. In vitro and in vivo results have shown that the simultaneous dual modal imaging and PTT/PDT abilities of magnetic fluorescent graphene MFGeSiNc4 achieved a significant cell killing efficacy which was a synergistic effect of PDT and PTT. In 2016, Kalluru et al. reported for the first time that NGO showed single-photon excitation wavelength-dependent photoluminescence in the visible and short NIR region, which could be exploitable for in vivo multi-color fluorescence imaging (Kalluru et al., 2016). NGO induced the formation of 1O2 both in vitro and in vivo for combined PDT and PTT in melanoma. When mice with B16F0 melanoma tumors were irradiated with NIR light at ultra-low doses, their average half-life span was improved. In another study, with the introduction of PS (IR-808) on nanoGO, authors were able to combine NIR imaging synergistically with enhanced PDT and PTT (Luo et al., 2016). Tumors were treated with PEG- and PEI-functionalized NGO-808 and irradiated; apoptosis and necrosis occurred, obtaining as result the complete ablation of the tumor. Moreover, no recurrence was observed after 60 days post-irradiation.
In various studies, PEG was combined with the graphene-based nanoplatforms to improve PDT efficacy or the material biodistribution. For example, in 2015, Kim et al. studied the effects of PEGylated graphene-gold nanoparticles (ZnPc-PEG-Au@GON NPs) that, beside possessing a photothermal effect, positively displayed multiple roles as Raman imaging agents, delivery vehicle of ZnPc, and PS for enhanced combined imaging, PTT, and PDT diagnosis and therapy (Kim et al., 2015). A simultaneous and synergistic combination of PDT and PTT was achieved as well as thermal and fluorescence imaging. GO/AuNS-PEG composite demonstrated to produce a high photothermal conversion efficiency due to the graphene and gold nanostars enhanced optical absorbance in the NIR range. The PS-assembled graphene/gold nanostar hybrid completely eliminated the EMT6 xenografted tumors thanks to the synergistic in vivo cancer cell killing of parallel PDT and PTT under a single NIR laser irradiation (660 nm). Indeed, this study further inspires new graphene-Au nanostar hybrid applications as biocompatible nanoplatforms for imaging-guided (fluorescence/thermal/photoacoustic imaging) multimodal breast cancer therapy (PDT/PTT/chemotherapy/sonodynamic therapy) (Wu et al., 2017).
More recently, Zhang et al. (2019) proposed Mo2C MXene ad hoc synthesized in the nanosphere (NSph) topology as a theranostic nanoagent for combined cancer dual therapy (PTT and PDT) and imaging (photoacoustic and computerized tomography). Also in this work, the ROS generation capability was characterized by the modulation of DPBF absorbance at 420 nm under NIR irradiation at 1,064 nm and confirmed by the inhibition of DPBF degradation upon addition of the ROS quencher NaN3. In addition, synergistic PTT and PDT on human liver carcinoma cells (HepG2) in vitro revealed more than 80% of apoptotic cells in a dose-dependent manner, confirming the critical contribution of ROS generation to the efficacy of the Mo2C-mediated PTT/PDT synergistic therapy. In vivo antitumor efficacy tested 14 days post-treatment in HepG2 tumor bearing mice showed complete tumor ablation and lack of regrowth after 10-min NIR exposure in the presence of Mo2C pre-injected into the tumor, whereas control animals, either non-irradiated or irradiated without Mo2C, showed a 4-fold increase in tumor volume. Hematologic, body weight, and post-mortem histological analysis of explanted organ tissue supported the safety of Mo2C NSph as an injectable PTA for cancer theranostics.
Theranostics: Imaging, PDT, Drug Delivery, and PTT
A low number of studies were carried to evaluate the use of GBMs and MXenes for simultaneous imaging, PDT, drug delivery, and PTT. In 2015, a NIR photo-triggered drug delivery system pGO-CuS/IndoCyanine Green (ICG) exhibited high efficacy of photothermal conversion, being a perfect candidate for highly efficient controlled theranostic applications (i.e., bimodal PDT and PTT therapy plus NIR imaging for a broad range of deep-seated cancer tissues) (Wu et al., 2015). This promising nanoplatform displayed optimal stability, high loading efficiency of ICG, good photon energy conversion to heat and significant 1O2 generation yield under NIR laser treatment. It is able, via passive transmembrane pathway, to readily reach the cellular inner cytoplasm as a potent synergic platform for PDT and PTT, killing specifically cancer cells by the appropriate tuning the two NIR light irradiations (Wu et al., 2015). Later, Wo et al. developed a multimodal system which was able to enclose four different synergetic anti-cancer activities: photodynamic toxicity, photothermal damage, chemotherapy, and magnetic field-mediated mechanical stimulation (Wo et al., 2016). The authors formed liposome-stabilized doxorubicin (DOX)-loaded magnetic nanospheres, aimed at enhancing anti-cancer activity through magnetic field-mediated mechanical force and NIR laser irradiation.
Liu et al. (2017) first demonstrated the feasibility of Ti3C2 MXene nanosheets (NSs) as PSs for PDT in a PTT/PDT/chemo-synergistic platform (Figures 5A–C). ROS generation in the presence of Ti3C2 NSs in aqueous suspensions was investigated using 1,3-diphenyli-sobenzofuran (DPBF) as the singlet 1O2 detector. Upon NIR irradiation of Ti3C2 NSs at 808 nm for 10 min, DPBF showed a ~80% decrease in absorbance at 420 nm, consequently revealing the generation of 1O2 (Figure 5D). Similar ROS generation capability, although less pronounced, was observed when Ti3C2 functionalized with DOX was exposed to the same irradiation protocol, thus enabling the development of combined PDT/chemotherapeutics. The exact mechanism of 1O2 generation in Ti3C2 is still unclear and warrants further investigations. The authors attribute it to the energy transfer of photoexcited electrons from Ti3C2 to triplet oxygen (ground state oxygen, 3O2), a mechanism similar to the photodynamic behavior of GQDs (Ge et al., 2014) and black phosphorous (Wang et al., 2015). The localized surface plasmonic resonance (LSPR) effect might also play a role similar to what has been reported for metals, like gold nanoparticles. In these systems, the efficiency of energy transfer is enhanced when the particles are in the aggregated state. Thus, the high surface area of Ti3C2 might be particularly favorable for LSPR. Compared to the individual therapeutic modalities, synergistic PTT/PDT/chemotherapy with Ti3C2-DOX led to significant improvements in therapeutic efficacy and recurrence outcomes against human colon carcinoma (HCT-116) in vivo in tumor-bearing mice. The abundant surface termination in the Ti3C2 NSs also enables specific functionalization to selectively target species in the tumor microenvironment. For example, coating Ti3C2-DOX with hyaluronic acid increased colloidal stability and actively targeted the surface protein CD44+ overexpressed in breast cancer cells.
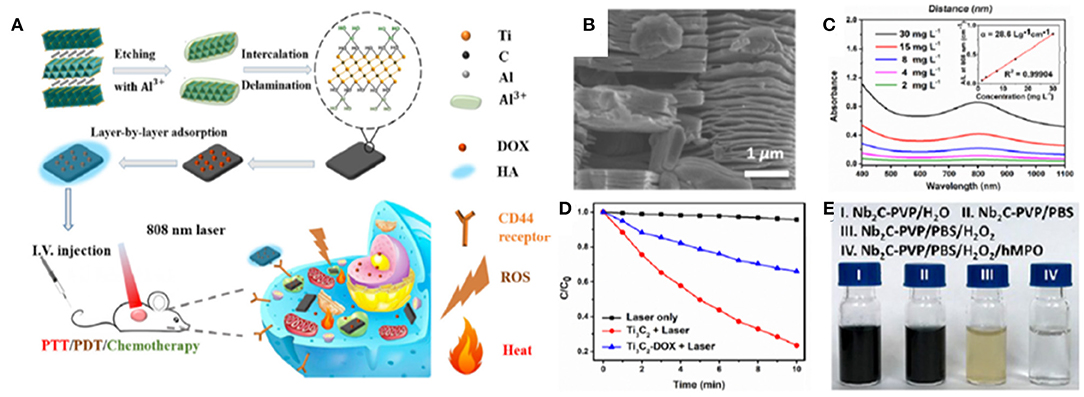
Figure 5. MXenes in cancer PDT. (A) Schematics of a multimodal PTT/PDT/chemotherapy synergistic platform based on Ti3C2 NSs functionalized with DOX. Included is the synthesis if the Ti3C2 NSs from the precursor Ti3AlC2 phase, followed by the exfoliation, intercalation and functionalization steps. (B) Scanning electron microscopy image of exfoliated Ti3C2 NSs. (C) Absorbance spectra and extinction coefficient at varying concentrations of Ti3C2 MXene. (D) ROS generation under NIR irradiation at 808 nm in the presence of Ti3C2 and Ti3C2-DOX NS detected by DBPF absorbance assay. Reproduced with permission from Liu et al. (2017). (E) Biodegradation of Nb2C NS. Reproduced with permission from Lin et al. (2017).
Theranostic Outcomes of the in vivo Studies
By analyzing the in vivo outcomes, it is possible to conclude that all the studies presented very promising results for the use of these materials in cancer theranostics, achieving a significant (Kalluru et al., 2016; Gulzar et al., 2018) or even total cancer ablation without tumor recurrence (Sahu et al., 2013; Wang et al., 2013; Ge et al., 2014; Rong et al., 2014; Yan et al., 2015a,b; Luo et al., 2016; Wu et al., 2017), as shown in Table 3. Among all the materials, GO-based nanoplatforms were the most investigated (5 in vivo studies out of 9), followed by NGO and GQDs (3 and 1 studies out of 9, respectively). Since GO and NGO can act both as a photothermal agent and a delivery carrier for PSs, most the works exploited its theranostic potential for combined PDT and PTT. This strategy led to total cancer ablation between 14 and 21 days.
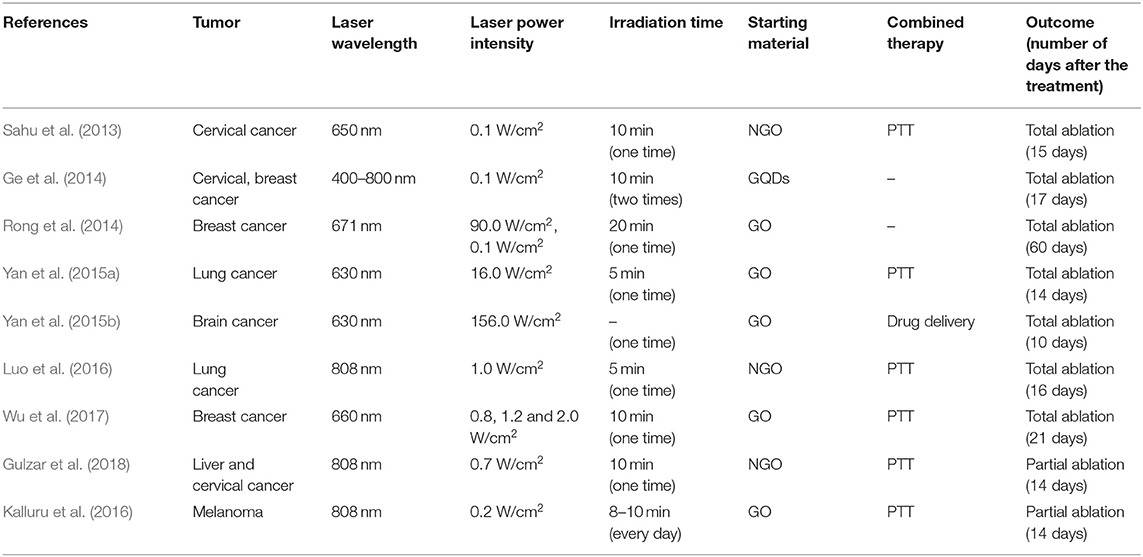
Table 3. Comparison of nanomaterials and laser powers used for PDT-based cancer theranostic applications and relative outcomes in vivo.
Thanks to the to the quantum confinement effect related to their small dimensions, NGO and GQDs possess non-blinking photoluminescence and photostability. Therefore, these materials have been explored mainly for PDT in association with UCL imaging (Luo et al., 2016; Gulzar et al., 2018) or fluorescence imaging (Ge et al., 2014; Luo et al., 2016). In particular, two studies investigated NGO for imaging-guided PDT in combination with PTT (Luo et al., 2016; Gulzar et al., 2018), achieving significant (Gulzar et al., 2018) or even total (Luo et al., 2016) cancer ablation between 14 and 16 days.
Finally, only one study evaluated the suitability of GQDs for PDT-based cancer theranostics based on PDT (Ge et al., 2014). The main advantage of GQDs is represented by their ability to serve as imaging tools and perform better than conventional PDT agents in terms of 1O2 quantum yield. However, since this result is achieved in the visible light region, the suitability of these tools appears to be limited to superficial tumors, such as skin cancer.
Future Perspectives
Following its discovery, graphene has attracted attention of the society in general with several expectations from the public in the context of nanomedicine (Sechi et al., 2014), and the turn of MXene is already on the stage.
Thanks to their chemical, physical and biological properties, graphene and MXene have shown to be powerful tools for PDT in cancer theranostics. Both 2D nanosystems allow the simultaneous application of non-invasive bioimaging and therapeutic strategies that can be associated with PDT, including photothermal therapy, magnetic therapy, and remotely controlled chemotherapy by drug and gene delivery.
The suitability of these promising materials as photothermal agents for tumor therapy and imaging is due to their ability to adsorb light in the NIR region. Moreover, the easy functionalization capability, thanks to their high surface-to-volume ratio, allows the loading of photosensitizer agents on these nanoplatforms, enhancing the targeting and efficiency, resulting in a more localized action, characterized by reduced side effects and improved therapy.
In addition, the combination with chemotherapeutic drugs loaded on these 2D NMs can work synergistically with PDT, leading to an improved anticancer activity. Furthermore, the functionalization with other agents, such as Au, endows them with a high photothermal conversion efficiency that can further enhance their optical absorbance in the NIR range. Beside the therapeutic efficiency of these nanoplatforms, their stability in aqueous matrix can also be improved thanks to their ability to be loaded with hydrophilic molecules, such as PEG. This aspect is of great interest in view of their intravenous administration, since most PSs are hydrophobic and could easily aggregate in biological fluids, with a consequent decrease in their quantum yield and increase in immune responses (Sibani et al., 2008; Kashef et al., 2017).
However, despite the encouraging promising results, there is still an extensive work to be accomplished to further clarify and prove the potentialities of GBM- and MXene-based PDT for cancer theranostics. First of all, for the translation of these 2D materials into clinical application, the assessment of their long-term toxicity is required to fully characterize their safety profile.
The potential widespread use of graphene and MXene-based materials for commercial purposes will favor their interactions with biological and environmental components. Therefore, several studies have been carried out to define the cyto- and bio-compatibility of these nanomaterials in vitro and in vivo (Fadeel et al., 2018a; Lin et al., 2018). These studies, particularly for graphene, state that the toxicity depends on the complex interaction of several physiochemical properties, such as shape, size, functional groups, oxidative state, dispersion state, synthesis methods, exposure times, as well as route and dose of administration. Moreover, graphene can contain several chemical contaminants and impurities coming from synthesis (Liao et al., 2018) and post-synthesis processing steps that can lead to graphene structure disruption and smaller carbonaceous debris production. Therefore, these confounding aspects may elicit variable toxicity responses (Li and Boraschi, 2016).
In this view, various studies have been performed to better understand and predict GBM toxicity and their potential impact on the immune system which governs every aspect of our health, including the way we react to therapies in cancer (Orecchioni et al., 2014, 2016a,b, 2017; Russier et al., 2017; Fadeel et al., 2018b). The obtained results have highlighted the importance of material characterization as a key element for hazard assessment as well as bio and immune compatibility. Therefore, biomedical scientists should not consider graphene as a single material but as a complex class of materials, taking into account the role of physicochemical properties (e.g., lateral dimension, carbon oxygen ratio and number of layers) while assessing the biological effects (Fadeel et al., 2018a). Concerning MXene, preliminary evaluations of Ti3C2 MXene biocompatibility have not evidenced apoptosis or signs of cytotoxicity in vitro in cancer cells (Dai et al., 2017; Lin et al., 2017; Liu et al., 2017; Han et al., 2018) and neurons (Driscoll et al., 2018). Ti3C2 NSs injected in vivo in the blood stream appear to be either excreted in the urine via physiologic renal clearance pathways or retained in the tumor site via the enhanced permeability and retention (EPR) effect, without accumulating in the major organs. Similar findings have been reported for the cyto-biocompatibility and systemic safety of Ta4C3 (Dai et al., 2017; Liu et al., 2018) and Nb2C MXenes (Lin et al., 2017). Despite all these data on MXene biocompatibility, the assessment of its potential toxicity is still at a very early stage. For both types of materials, new studies on their biomedical application should take into consideration general requirements with respect to Minimum Information Reporting in Bio–Nano Experimental Literature (MIRIBEL) and other key considerations on the issue of transparency and reproducibility in nanomedicine, such as that the choice of material physical-chemical characteristics should be tailored for their intended use (Faria et al., 2018; Leong et al., 2019). That consideration is even more valid in the context of cancer theranostics were the starting properties of the material can make the difference in the successful application of new 2D material-based cancer treatment. Another key point is related to the materials' fine characterization and reproducibility, which should be considered in every work based on engineered nanomaterials as a key aspect to avoid hype around the potential translation into clinic (DeLoid et al., 2017).
Beyond the toxicity context and physical-chemical material choice, other knowledge gaps need to be filled to shed light on the actual potentialities of these 2D NMs for cancer theranostics and PDT in particular. Indeed, the understanding of these aspects cannot overlook the elucidation of fundamental mechanisms underlying the ROS generation elicited by these materials, which is at the basis of PDT. Moreover, more efforts should be directed into a deeper understanding of the nanoparticle-tumor interaction, the possibility of a scaled-up synthesis, and the development of regulatory theranostic protocols in order to determine a personalized therapy framework.
Finally, controlling the lifetime of the nanoagents in the body and mitigating the risks related to retention of NMs and their byproducts could significantly advance NM-based theranostic platforms in the translational pipeline (Orecchioni et al., 2015). The complete clearance from the body, along with the biodegradation of 2D nanostructures needs to be assessed for these materials in order to be translated into clinical settings (Andón et al., 2013; Bhattacharya et al., 2013; Farrera et al., 2014; Kurapati et al., 2018; Mukherjee et al., 2018; Martín et al., 2019). Although GBMs can be considered structurally persistent, it has been proved that oxidative enzymes (i.e., peroxidases) are capable of catalyzing the GO degradation in vitro and in vivo (Bai et al., 2014; Kurapati et al., 2018; Mukherjee et al., 2018). Nb2C MXene NSs can be engineered to degrade through an active biodegradation scheme that leverages human myeloperoxidase (hMPO), a free-radical species generating enzyme expressed by neutrophils to carry out their antimicrobial activity (Lin et al., 2017). In the presence of hydrogen peroxide (H2O2), hMPO generates hypochlorous acid and reactive radical intermediates, which degrade polymers and carbon-based materials. The incubation of Nb2C NSs in hMPO and H2O2 enriched medium for 24 h has been reported to cause the complete degradation and disappearance of NSs, thus demonstrating in vitro the feasibility of this enzyme-triggered degradation route for MXenes (Figures 5D,E).
Overall, this review has shown that significant advances in the theranostic use of graphene-based materials and MXenes have been made. However, three main aspects should be carefully taken more into account: (i) the potential not-targeted toxicity, (ii) a choice of physical-chemical material characteristics prior their assessment for cancer therapy, (iii) a fine characterization, and (iv) the assessment of their potential biodegradability. Despite that knowledge gaps in the field still need to be filled, virtuous perspectives for GBMs and MXene were evidenced from over 30 works here analyzed, standing out as the most promising 2D NMs intended to change the patterns of conventional cancer theranostics, guaranteeing new protocols for personalized therapies.
Author Contributions
LD proposed the topic of the review and designed and coordinated the work. LD, AY, and FV wrote the manuscript with the help from AG, LF, and AK. AG, LF, and AK investigated the literature and prepared the figures with the help of LD, AY, and FV. DB and BZ critically revised the manuscript. All authors discussed and revised the manuscript.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors thank the MIUR JTC Graphene 2015 (G-IMMUNOMICS project) and the European Union's HORIZON2020 research and innovation programme under MSCA RISE2016 project Carbo-Immap Grant No. 734381. AY was thankful to the Turkish Academy of Sciences (TUBA) for the financial support under TUBA-GEBIP 2018. FV acknowledges the support from the McCabe Fellow Award and the University of Pennsylvania Research Foundation.
Footnotes
1. ^WHO. Cervical Cancer WHO. Available online at: http://www.who.int/cancer/prevention/diagnosis-screening/cervical-cancer/en/ (accessed July 4, 2019).
References
Agostinis, P., Berg, K., Cengel, K. A., Foster, T. H., Girotti, A. W., Gollnick, S. O., et al. (2011). Photodynamic therapy of cancer: an update. CA Cancer J. Clin. 61, 250–281. doi: 10.3322/caac.20114
Anasori, B., Lukatskaya, M. R., and Gogotsi, Y. (2017). 2D metal carbides and nitrides (MXenes) for energy storage. Nat. Rev. Mater. 2:16098. doi: 10.1038/natrevmats.2016.98
Andón, F. T., Kapralov, A. A., Yanamala, N., Feng, W., Baygan, A., Chambers, B. J., et al. (2013). Biodegradation of single-walled carbon nanotubes by eosinophil peroxidase. Small 9, 2721–2729, 2720. doi: 10.1002/smll.201202508
Augustine, S., Singh, J., Srivastava, M., Sharma, M., Das, A., and Malhotra, B. D. (2017). Recent advances in carbon based nanosystems for cancer theranostics. Biomater. Sci. 5, 901–952. doi: 10.1039/C7BM00008A
Avitabile, E., Bedognetti, D., Ciofani, G., Bianco, A., and Delogu, L. G. (2018). How can nanotechnology help the fight against breast cancer? Nanoscale 10, 11719–11731. doi: 10.1039/C8NR02796J
Bacellar, I. O. L., Tsubone, T. M., Pavani, C., and Baptista, M. S. (2015). Photodynamic efficiency: from molecular photochemistry to cell death. Int. J. Mol. Sci. 16, 20523–20559. doi: 10.3390/ijms160920523
Bai, H., Jiang, W., Kotchey, G. P., Saidi, W. A., Bythell, B. J., Jarvis, J. M., et al. (2014). Insight into the mechanism of graphene oxide degradation via the photo-fenton reaction. J. Phys. Chem. C 118, 10519–10529. doi: 10.1021/jp503413s
Bhattacharya, K., Andón, F. T., El-Sayed, R., and Fadeel, B. (2013). Mechanisms of carbon nanotube-induced toxicity: focus on pulmonary inflammation. Adv. Drug Deliv. Rev. 65, 2087–2097. doi: 10.1016/j.addr.2013.05.012
Bitounis, D., Ali-Boucetta, H., Hong, B. H., Min, D.-H., and Kostarelos, K. (2013). Prospects and challenges of graphene in biomedical applications. Adv. Mater. Weinheim 25, 2258–2268. doi: 10.1002/adma.201203700
Boukhvalov, D. W., and Katsnelson, M. I. (2008). Chemical functionalization of graphene with defects. Nano Lett. 8, 4373–4379. doi: 10.1021/nl802234n
Chen, Y., Tan, C., Zhang, H., and Wang, L. (2015). Two-dimensional graphene analogues for biomedical applications. Chem. Soc. Rev. 44, 2681–2701. doi: 10.1039/C4CS00300D
Cho, Y., Kim, H., and Choi, Y. (2013). A graphene oxide–photosensitizer complex as an enzyme-activatable theranostic agent. Chem. Commun. 49, 1202–1204. doi: 10.1039/c2cc36297j
Dai, C., Chen, Y., Jing, X., Xiang, L., Yang, D., Lin, H., et al. (2017). Two-dimensional tantalum carbide (MXenes) composite nanosheets for multiple imaging-guided photothermal tumor ablation. ACS Nano 11, 12696–12712. doi: 10.1021/acsnano.7b07241
DeLoid, G. M., Cohen, J. M., Pyrgiotakis, G., and Demokritou, P. (2017). Preparation, characterization, and in vitro dosimetry of dispersed, engineered nanomaterials. Nat. Protoc. 12, 355–371. doi: 10.1038/nprot.2016.172
Detty, M. R., Gibson, S. L., and Wagner, S. J. (2004). Current clinical and preclinical photosensitizers for use in photodynamic therapy. J. Med. Chem. 47, 3897–3915. doi: 10.1021/jm040074b
Dolmans, D. E. J. G. J., Fukumura, D., and Jain, R. K. (2003). Photodynamic therapy for cancer. Nat. Rev. Cancer 3:380. doi: 10.1038/nrc1071
Driscoll, N., Richardson, A. G., Maleski, K., Anasori, B., Adewole, O., Lelyukh, P., et al. (2018). Two-dimensional Ti3C2 MXene for high-resolution neural interfaces. ACS Nano 12, 10419–10429. doi: 10.1021/acsnano.8b06014
Er, D., Li, J., Naguib, M., Gogotsi, Y., and Shenoy, V. B. (2014). Ti3C2 MXene as a high capacity electrode material for metal (Li, Na, K, Ca) ion batteries. ACS Appl. Mater. Interfaces 6, 11173–11179. doi: 10.1021/am501144q
Fadeel, B., Bussy, C., Merino, S., Vázquez, E., Flahaut, E., Mouchet, F., et al. (2018a). Safety assessment of graphene-based materials: focus on human health and the environment. ACS Nano 12, 10582–10620. doi: 10.1021/acsnano.8b04758
Fadeel, B., Farcal, L., Hardy, B., Vázquez-Campos, S., Hristozov, D., Marcomini, A., et al. (2018b). Advanced tools for the safety assessment of nanomaterials. Nat. Nanotechnol. 13, 537–543. doi: 10.1038/s41565-018-0185-0
Faria, M., Björnmalm, M., Thurecht, K. J., Kent, S. J., Parton, R. G., Kavallaris, M., et al. (2018). Minimum information reporting in bio–nano experimental literature. Nat. Nanotechnol. 13:777. doi: 10.1038/s41565-018-0246-4
Farrera, C., Bhattacharya, K., Lazzaretto, B., Andón, F. T., Hultenby, K., Kotchey, G. P., et al. (2014). Extracellular entrapment and degradation of single-walled carbon nanotubes. Nanoscale 6, 6974–6983. doi: 10.1039/c3nr06047k
Feng, L., Wu, Y.-X., Zhang, D.-L., Hu, X., Zhang, J., Wang, P., et al. (2017). A near infrared graphene quantum dots-based two-photon nanoprobe for direct bioimaging of endogenous ascorbic acid in living cells. Anal. Chem. 89, 4077–4084. doi: 10.1021/acs.analchem.6b04943
Feng, W., Wang, R., Zhou, Y., Ding, L., Gao, X., Zhou, B., et al. (2019). Ultrathin molybdenum carbide MXene with fast biodegradability for highly efficient theory-oriented photonic tumor hyperthermia. Adv. Funct. Mater. 1901942, 1–15. doi: 10.1002/adfm.201901942
Ferreira Dos Santos, A., Raquel Queiroz De Almeida, D., Ferreira Terra, L., Baptista, M., and Labriola, L. (2019). Photodynamic therapy in cancer treatment–an update review. J. Cancer Metast. Treat. 5:25. doi: 10.20517/2394-4722.2018.83
Gao, X., Cui, Y., Levenson, R. M., Chung, L. W. K., and Nie, S. (2004). In vivo cancer targeting and imaging with semiconductor quantum dots. Nat. Biotechnol. 22, 969–976. doi: 10.1038/nbt994
Gao, X., Jang, J., and Nagase, S. (2010). Hydrazine and thermal reduction of graphene oxide: reaction mechanisms, product structures, and reaction design. J. Phys. Chem. C 114, 832–842. doi: 10.1021/jp909284g
Ge, J., Lan, M., Zhou, B., Liu, W., Guo, L., Wang, H., et al. (2014). A graphene quantum dot photodynamic therapy agent with high singlet oxygen generation. Nat. Commun. 5:4596. doi: 10.1038/ncomms5596
Ghidiu, M., Lukatskaya, M. R., Zhao, M. Q., Gogotsi, Y., and Barsoum, M. W. (2015). Conductive two-dimensional titanium carbide “clay” with high volumetric capacitance. Nature 516, 78–81. doi: 10.1038/nature13970
Gollavelli, G., and Ling, Y. C. (2014). Magnetic and fluorescent graphene for dual modal imaging and single light induced photothermal and photodynamic therapy of cancer cells. Biomaterials 35, 4499–4507. doi: 10.1016/j.biomaterials.2014.02.011
Guan, M., Li, J., Jia, Q., Ge, J., Chen, D., Zhou, Y., et al. (2016). A versatile and clearable nanocarbon theranostic based on carbon dots and gadolinium metallofullerene nanocrystals. Adv. Healthc. Mater. 5, 2283–2294. doi: 10.1002/adhm.201600402
Gulzar, A., Xu, J., Yang, D., Xu, L., He, F., Gai, S., et al. (2018). Nano-graphene oxide-UCNP-Ce6 covalently constructed nanocomposites for NIR-mediated bioimaging and PTT/PDT combinatorial therapy. Dalton Trans. 47, 3931–3939. doi: 10.1039/C7DT04141A
Han, X., Huang, J., Lin, H., Wang, Z., Li, P., and Chen, Y. (2018). 2D ultrathin MXene-based drug-delivery nanoplatform for synergistic photothermal ablation and chemotherapy of cancer. Adv. Healthc. Mater. 306, 1701313–1701394. doi: 10.1002/adhm.201701394
Henderson, B. W., Gollnick, S. O., Snyder, J. W., Busch, T. M., Kousis, P. C., Cheney, R. T., et al. (2004). Choice of oxygen-conserving treatment regimen determines the inflammatory response and outcome of photodynamic therapy of tumors. Cancer Res. 64, 2120–2126. doi: 10.1158/0008-5472.CAN-03-3513
Huang, P., Xu, C., Lin, J., Wang, C., Wang, X., Zhang, C., et al. (2011). Folic acid-conjugated graphene oxide loaded with photosensitizers for targeting photodynamic therapy. Theranostics 1, 240–250. doi: 10.7150/thno/v01p0240
Huang, Z. (2005). A review of progress in clinical photodynamic therapy. Technol. Cancer Res. Treat. 4, 283–293. doi: 10.1177/153303460500400308
James, D. K., and Tour, J. M. (2013). Graphene: powder, flakes, ribbons, and sheets. Acc. Chem. Res. 46, 2307–2318. doi: 10.1021/ar300127r
Ji, D.-K., Ménard-Moyon, C., and Bianco, A. (2019). Physically-triggered nanosystems based on two-dimensional materials for cancer theranostics. Adv. Drug Deliv. Rev. 138, 211–232. doi: 10.1016/j.addr.2018.08.010
Kalluru, P., Vankayala, R., Chiang, C.-S., and Hwang, K. C. (2016). Nano-graphene oxide-mediated in vivo fluorescence imaging and bimodal photodynamic and photothermal destruction of tumors. Biomaterials 95, 1–10. doi: 10.1016/j.biomaterials.2016.04.006
Kashef, N., Huang, Y.-Y., and Hamblin, M. R. (2017). Advances in antimicrobial photodynamic inactivation at the nanoscale. Nanophotonics 6, 853–879. doi: 10.1515/nanoph-2016-0189
Kim, J., Lee, J., Son, D., Choi, M. K., and Kim, D.-H. (2016). Deformable devices with integrated functional nanomaterials for wearable electronics. Nano Converg. 3:4. doi: 10.1186/s40580-016-0062-1
Kim, Y.-K., Na, H.-K., Kim, S., Jang, H., Chang, S.-J., and Min, D.-H. (2015). One-pot synthesis of multifunctional Au@graphene oxide nanocolloid core@shell nanoparticles for Raman bioimaging, photothermal, and photodynamic therapy. Small 11, 2527–2535. doi: 10.1002/smll.201402269
Korbelik, M. (2006). PDT-associated host response and its role in the therapy outcome. Lasers Surg. Med. 38, 500–508. doi: 10.1002/lsm.20337
Kuila, T., Bose, S., Mishra, A., Khanra, P., Kim, N. H., and Lee, J. (2012). Chemical functionalization of graphene and its applications. Prog. Mater. Sci. 57, 1061–1105. doi: 10.1016/j.pmatsci.2012.03.002
Kurapati, R., Mukherjee, S. P., Martín, C., Bepete, G., Vázquez, E., Pénicaud, A., et al. (2018). Degradation of single-layer and few-layer graphene by neutrophil myeloperoxidase. Angew. Chem. Int. Ed. Engl. 57, 11722–11727. doi: 10.1002/anie.201806906
Leong, H. S., Butler, K. S., Brinker, C. J., Azzawi, M., Conlan, S., Dufés, C., et al. (2019). On the issue of transparency and reproducibility in nanomedicine. Nat. Nanotechnol. 14, 629–635. doi: 10.1038/s41565-019-0496-9
Li, L., Wu, G., Yang, G., Peng, J., Zhao, J., and Zhu, J.-J. (2013). Focusing on luminescent graphene quantum dots: current status and future perspectives. Nanoscale 5, 4015–4039. doi: 10.1039/c3nr33849e
Li, Y., and Boraschi, D. (2016). Endotoxin contamination: a key element in the interpretation of nanosafety studies. Nanomedicine (Lond). 11, 269–287. doi: 10.2217/nnm.15.196
Liao, C., Li, Y., and Tjong, S. C. (2018). Graphene nanomaterials: synthesis, biocompatibility, and cytotoxicity. Int. J. Mol. Sci. 19:E3564. doi: 10.3390/ijms19113564
Lin, H., Chen, Y., and Shi, J. (2018). Insights into 2D MXenes for versatile biomedical applications: current advances and challenges ahead. Adv. Sci. 5:1800518. doi: 10.1002/advs.201800518
Lin, H., Gao, S., Dai, C., Chen, Y., and Shi, J. (2017). A two-dimensional biodegradable niobium carbide (MXene) for photothermal tumor eradication in NIR-I and NIR-II biowindows. J. Am. Chem. Soc. 139, 16235–16247. doi: 10.1021/jacs.7b07818
Lin, H., Wang, X., Yu, L., Chen, Y., and Shi, J. (2016). Two-dimensional ultrathin MXene ceramic nanosheets for photothermal conversion. Nano Lett. 17, 384–391. doi: 10.1021/acs.nanolett.6b04339
Liu, G., Zou, J., Tang, Q., Yang, X., Zhang, Y., Zhang, Q., et al. (2017). Surface modified Ti3C2 MXene nanosheets for tumor targeting photothermal/photodynamic/chemo synergistic therapy. ACS Appl. Mater. Interfaces 9, 40077–40086. doi: 10.1021/acsami.7b13421
Liu, Z., Lin, H., Zhao, M., Dai, C., Zhang, S., Peng, W., et al. (2018). 2D superparamagnetic tantalum carbide composite MXenes for efficient breast-cancer theranostics. Theranostics 8, 1648–1664. doi: 10.7150/thno.23369
Lou, P.-J., Jäger, H. R., Jones, L., Theodossy, T., Bown, S. G., and Hopper, C. (2004). Interstitial photodynamic therapy as salvage treatment for recurrent head and neck cancer. Br. J. Cancer 91, 441–446. doi: 10.1038/sj.bjc.6601993
Lukatskaya, M. R., Kota, S., Lin, Z., Zhao, M.-Q., Shpigel, N., Levi, M. D., et al. (2017). Ultra-high-rate pseudocapacitive energy storage in two-dimensional transition metal carbides. Nat. Energy 2:17105. doi: 10.1038/nenergy.2017.105
Luo, S., Yang, Z., Tan, X., Wang, Y., Zeng, Y., Wang, Y., et al. (2016). Multifunctional photosensitizer grafted on polyethylene glycol and polyethylenimine dual-functionalized nanographene oxide for cancer-targeted near-infrared imaging and synergistic phototherapy. ACS Appl. Mater. Interfaces 8, 17176–17186. doi: 10.1021/acsami.6b05383
Luo, Y., Li, Z., Zhu, C., Cai, X., Qu, L., Du, D., et al. (2018). Graphene-like metal-free 2D nanosheets for cancer imaging and theranostics. Trends Biotechnol. 36, 1145–1156. doi: 10.1016/j.tibtech.2018.05.012
Mao, S., Pu, H., and Chen, J. (2012). Graphene oxide and its reduction: modeling and experimental progress. RSC Adv. 2, 2643–2662. doi: 10.1039/c2ra00663d
Martín, C., Kostarelos, K., Prato, M., and Bianco, A. (2019). Biocompatibility and biodegradability of 2D materials: graphene and beyond. Chem. Commun. 55, 5540–5546. doi: 10.1039/C9CC01205B
McManus, D., Vranic, S., Withers, F., Sanchez-Romaguera, V., Macucci, M., Yang, H., et al. (2017). Water-based and biocompatible 2D crystal inks for all-inkjet-printed heterostructures. Nat. Nanotechnol. 12, 343–350. doi: 10.1038/nnano.2016.281
Michalet, X., Pinaud, F. F., Bentolila, L. A., Tsay, J. M., Doose, S., Li, J. J., et al. (2005). Quantum dots for live cells, in vivo imaging, and diagnostics. Science 307, 538–544. doi: 10.1126/science.1104274
Mukherjee, S. P., Gliga, A. R., Lazzaretto, B., Brandner, B., Fielden, M., Vogt, C., et al. (2018). Graphene oxide is degraded by neutrophils and the degradation products are non-genotoxic. Nanoscale 10, 1180–1188. doi: 10.1039/C7NR03552G
Naguib, M., Kurtoglu, M., Presser, V., Lu, J., Niu, J., Heon, M., et al. (2011). Two-dimensional nanocrystals produced by exfoliation of Ti3AlC2. Adv. Mater. 23, 4248–4253. doi: 10.1002/chin.201152200
Novoselov, K. S., Fal′ko, V. I., Colombo, L., Gellert, P. R., Schwab, M. G., and Kim, K. (2012). A roadmap for graphene. Nature 490, 192–200. doi: 10.1038/nature11458
Orecchioni, M., Bedognetti, D., Newman, L., Fuoco, C., Spada, F., Hendrickx, W., et al. (2017). Single-cell mass cytometry and transcriptome profiling reveal the impact of graphene on human immune cells. Nat. Commun. 8:1109. doi: 10.1038/s41467-017-01015-3
Orecchioni, M., Bedognetti, D., Sgarrella, F., Marincola, F. M., Bianco, A., and Delogu, L. G. (2014). Impact of carbon nanotubes and graphene on immune cells. J. Transl. Med. 12:138. doi: 10.1186/1479-5876-12-138
Orecchioni, M., Cabizza, R., Bianco, A., and Delogu, L. G. (2015). Graphene as cancer theranostic tool: progress and future challenges. Theranostics 5, 710–723. doi: 10.7150/thno.11387
Orecchioni, M., Jasim, D. A., Pescatori, M., Manetti, R., Fozza, C., Sgarrella, F., et al. (2016a). Molecular and genomic impact of large and small lateral dimension graphene oxide sheets on human immune cells from healthy donors. Adv. Healthc. Mater. 5, 276–287. doi: 10.1002/adhm.201500606
Orecchioni, M., Ménard-Moyon, C., Delogu, L. G., and Bianco, A. (2016b). Graphene and the immune system: challenges and potentiality. Adv. Drug Deliv. Rev. 105, 163–175. doi: 10.1016/j.addr.2016.05.014
Park, M. V. D. Z., Bleeker, E. A. J., Brand, W., Cassee, F. R., van Elk, M., Gosens, I., et al. (2017). Considerations for safe innovation: the case of graphene. ACS Nano 11, 9574–9593. doi: 10.1021/acsnano.7b04120
Park, S., and Ruoff, R. S. (2009). Chemical methods for the production of graphenes. Nat. Nanotechnol. 4, 217–224. doi: 10.1038/nnano.2009.58
Pelin, M., Fusco, L., Martín, C., Sosa, S., Frontiñán-Rubio, J., Miguel González-Domínguez, J., et al. (2018). Graphene and graphene oxide induce ROS production in human HaCaT skin keratinocytes: the role of xanthine oxidase and NADH dehydrogenase. Nanoscale 10, 11820–11830. doi: 10.1039/C8NR02933D
Quintana, M., Vazquez, E., and Prato, M. (2013). Organic functionalization of graphene in dispersions. Acc. Chem. Res. 46, 138–148. doi: 10.1021/ar300138e
Resch-Genger, U., Grabolle, M., Cavaliere-Jaricot, S., Nitschke, R., and Nann, T. (2008). Quantum dots versus organic dyes as fluorescent labels. Nat. Methods 5, 763–775. doi: 10.1038/nmeth.1248
Rong, P., Yang, K., Srivastan, A., Kiesewetter, D. O., Yue, X., Wang, F., et al. (2014). Photosensitizer loaded nano-graphene for multimodality imaging guided tumor photodynamic therapy. Theranostics 4, 229–239. doi: 10.7150/thno.8070
Roppolo, I., Chiappone, A., Bejtka, K., Celasco, E., Chiodoni, A., Giorgis, F., et al. (2014). A powerful tool for graphene functionalization: Benzophenone mediated UV-grafting. Carbon 77, 226–235. doi: 10.1016/j.carbon.2014.05.025
Russier, J., León, V., Orecchioni, M., Hirata, E., Virdis, P., Fozza, C., et al. (2017). Few-layer graphene kills selectively tumor cells from myelomonocytic leukemia patients. Angew. Chem. Int. Ed. Engl. 56, 3014–3019. doi: 10.1002/anie.201700078
Sahu, A., Choi, W. I., Lee, J. H., and Tae, G. (2013). Graphene oxide mediated delivery of methylene blue for combined photodynamic and photothermal therapy. Biomaterials 34, 6239–6248. doi: 10.1016/j.biomaterials.2013.04.066
Sechi, G., Bedognetti, D., Sgarrella, F., Van Eperen, L., Marincola, F. M., Bianco, A., et al. (2014). The perception of nanotechnology and nanomedicine: a worldwide social media study. Nanomedicine (Lond). 9, 1475–1486. doi: 10.2217/nnm.14.78
Seh, Z. W., Fredrickson, K. D., Anasori, B., Kibsgaard, J., Strickler, A. L., Lukatskaya, M. R., et al. (2016). Two-dimensional molybdenum carbide (MXene) as an efficient electrocatalyst for hydrogen evolution. ACS Energy Lett. 3, 589–594. doi: 10.1021/acsenergylett.6b00247
Servant, A., Bianco, A., Prato, M., and Kostarelos, K. (2014). Graphene for multi-functional synthetic biology: the last “zeitgeist” in nanomedicine. Bioorg. Med. Chem. Lett. 24, 1638–1649. doi: 10.1016/j.bmcl.2014.01.051
Shahzad, F., Alhabeb, M., Hatter, C. B., Anasori, B., Hong, S. M., Koo, C. M., et al. (2016). Electromagnetic interference shielding with 2D transition metal carbides (MXenes). Science 353, 1137–1140. doi: 10.1126/science.aag2421
Shin, S. R., Li, Y.-C., Jang, H. L., Khoshakhlagh, P., Akbari, M., Nasajpour, A., et al. (2016). Graphene-based materials for tissue engineering. Adv. Drug Deliv. Rev. 105, 255–274. doi: 10.1016/j.addr.2016.03.007
Sibani, S. A., McCarron, P. A., Woolfson, A. D., and Donnelly, R. F. (2008). Photosensitiser delivery for photodynamic therapy. Part 2: systemic carrier platforms. Expert Opin. Drug Deliv. 5, 1241–1254. doi: 10.1517/17425240802444673
Story, W., Sultan, A. A., Bottini, G., Vaz, F., Lee, G., and Hopper, C. (2013). Strategies of airway management for head and neck photo-dynamic therapy. Lasers Surg. Med. 45, 370–376. doi: 10.1002/lsm.22149
Sun, X., Liu, Z., Welsher, K., Robinson, J. T., Goodwin, A., Zaric, S., et al. (2008). Nano-graphene oxide for cellular imaging and drug delivery. Nano Res. 1, 203–212. doi: 10.1007/s12274-008-8021-8
Tian, B., Wang, C., Zhang, S., Feng, L., and Liu, Z. (2011). Photothermally enhanced photodynamic therapy delivered by nano-graphene oxide. ACS Nano 5, 7000–7009. doi: 10.1021/nn201560b
Viseu, T., Lopes, C. M., Fernandes, E., Real Oliveira, M. E. C. D., and Lúcio, M. (2018). A systematic review and critical analysis of the role of graphene-based nanomaterials in cancer theranostics. Pharmaceutics 10:282. doi: 10.3390/pharmaceutics10040282
Wang, H., Yang, X., Shao, W., Chen, S., Xie, J., Zhang, X., et al. (2015). Ultrathin black phosphorus nanosheets for efficient singlet oxygen generation. J. Am. Chem. Soc. 137, 11376–11382. doi: 10.1021/jacs.5b06025
Wang, Y., Wang, H., Liu, D., Song, S., Wang, X., and Zhang, H. (2013). Graphene oxide covalently grafted upconversion nanoparticles for combined NIR mediated imaging and photothermal/photodynamic cancer therapy. Biomaterials 34, 7715–7724. doi: 10.1016/j.biomaterials.2013.06.045
Wo, F., Xu, R., Shao, Y., Zhang, Z., Chu, M., Shi, D., et al. (2016). A multimodal system with synergistic effects of magneto-mechanical, photothermal, photodynamic and chemo therapies of cancer in graphene-quantum dot-coated hollow magnetic nanospheres. Theranostics 6, 485–500. doi: 10.7150/thno.13411
Wu, C., Li, D., Wang, L., Guan, X., Tian, Y., Yang, H., et al. (2017). Single wavelength light-mediated, synergistic bimodal cancer photoablation and amplified photothermal performance by graphene/gold nanostar/photosensitizer theranostics. Acta Biomater. 53, 631–642. doi: 10.1016/j.actbio.2017.01.078
Wu, C., Zhu, A., Li, D., Wang, L., Yang, H., Zeng, H., et al. (2015). Photosensitizer-assembled PEGylated graphene-copper sulfide nanohybrids as a synergistic near-infrared phototherapeutic agent. Expert Opin. Drug Deliv. 13, 155–165. doi: 10.1517/17425247.2016.1118049
Yan, X., Hu, H., Lin, J., Jin, A. J., Niu, G., Zhang, S., et al. (2015a). Optical and photoacoustic dual-modality imaging guided synergistic photodynamic/photothermal therapies. Nanoscale 7, 2520–2526. doi: 10.1039/C4NR06868H
Yan, X., Niu, G., Lin, J., Jin, A. J., Hu, H., Tang, Y., et al. (2015b). Enhanced fluorescence imaging guided photodynamic therapy of sinoporphyrin sodium loaded graphene oxide. Biomaterials 42, 94–102. doi: 10.1016/j.biomaterials.2014.11.040
Yang, K., Feng, L., Hong, H., Cai, W., and Liu, Z. (2013a). Preparation and functionalization of graphene nanocomposites for biomedical applications. Nat. Protoc. 8, 2392–2403. doi: 10.1038/nprot.2013.146
Yang, K., Feng, L., and Liu, Z. (2016). Stimuli responsive drug delivery systems based on nano-graphene for cancer therapy. Adv. Drug Deliv. Rev. 105, 228–241. doi: 10.1016/j.addr.2016.05.015
Yang, Y., Asiri, A. M., Tang, Z., Du, D., and Lin, Y. (2013b). Graphene based materials for biomedical applications. Materials Today 16, 365–373. doi: 10.1016/j.mattod.2013.09.004
Yoon, I., Li, J. Z., and Shim, Y. K. (2013). Advance in photosensitizers and light delivery for photodynamic therapy. Clin. Endosc. 46, 7–23. doi: 10.5946/ce.2013.46.1.7
Zhang, B., Wang, Y., and Zhai, G. (2016). Biomedical applications of the graphene-based materials. Mater. Sci. Eng. C Mater. Biol. Appl. 61, 953–964. doi: 10.1016/j.msec.2015.12.073
Zhang, Q., Huang, W., Yang, C., Wang, F., Song, C., Gao, Y., et al. (2019). The theranostic nanoagent Mo2C for multi-modal imaging-guided cancer synergistic phototherapy. Biomater. Sci. 7, 2729–2739. doi: 10.1039/C9BM00239A
Zhang, Q., Wu, Z., Li, N., Pu, Y., Wang, B., Zhang, T., et al. (2017). Advanced review of graphene-based nanomaterials in drug delivery systems: synthesis, modification, toxicity and application. Mater. Sci. Eng. C Mater. Biol. Appl. 77, 1363–1375. doi: 10.1016/j.msec.2017.03.196
Zhou, L., Jiang, H., Wei, S., Ge, X., Zhou, J., and Shen, J. (2012). High-efficiency loading of hypocrellin B on graphene oxide for photodynamic therapy. Carbon 50, 5594–5604. doi: 10.1016/j.carbon.2012.08.013
Zhou, L., Zhou, L., Wei, S., Ge, X., Zhou, J., Jiang, H., et al. (2014). Combination of chemotherapy and photodynamic therapy using graphene oxide as drug delivery system. J. Photochem. Photobiol. B Biol. 135, 7–16. doi: 10.1016/j.jphotobiol.2014.04.010
Zhu, S., Zhang, J., Liu, X., Li, B., Wang, X., Tang, S., et al. (2012a). Graphene quantum dots with controllable surface oxidation, tunable fluorescence and up-conversion emission. RSC Adv. 2, 2717–2720. doi: 10.1039/c2ra20182h
Keywords: photodynamic therapy, theranostics, graphene, MXene, nanomedicine
Citation: Gazzi A, Fusco L, Khan A, Bedognetti D, Zavan B, Vitale F, Yilmazer A and Delogu LG (2019) Photodynamic Therapy Based on Graphene and MXene in Cancer Theranostics. Front. Bioeng. Biotechnol. 7:295. doi: 10.3389/fbioe.2019.00295
Received: 14 July 2019; Accepted: 09 October 2019;
Published: 25 October 2019.
Edited by:
Gianni Ciofani, Italian Institute of Technology, ItalyReviewed by:
Fernando Soto, University of California, San Diego, United StatesStefano Leporatti, Institute of Nanotechnology (NANOTEC), Italy
Pedro Viana Baptista, New University of Lisbon, Portugal
Copyright © 2019 Gazzi, Fusco, Khan, Bedognetti, Zavan, Vitale, Yilmazer and Delogu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Flavia Vitale, dml0YWxlZkBwZW5ubWVkaWNpbmUudXBlbm4uZWR1; Acelya Yilmazer, YXlpbG1hemVyQGFua2FyYS5lZHUudHI=; Lucia Gemma Delogu, bHVjaWFnZW1tYS5kZWxvZ3VAdW5pcGQuaXQ=
 Arianna Gazzi1,2
Arianna Gazzi1,2 Laura Fusco
Laura Fusco Flavia Vitale
Flavia Vitale Lucia Gemma Delogu
Lucia Gemma Delogu