Abstract
Arboviruses such as yellow fever, dengue, chikungunya and zika are transmitted mainly by the mosquito vector Aedes aegypti. Especially in the tropics, inefficacy of mosquito control causes arboviruses outbreaks every year, affecting the general population with debilitating effects in infected individuals. Several strategies have been tried to control the proliferation of A. aegypti using physical, biological, and chemical control measures. Other methods are currently under research and development, amongst which the use of nanotechnology has attracted a lot of attention of the researchers in relation to the production of more effective repellents and larvicides with less toxicity, and development of rapid sensors for the detection of virus infections. In this review, the utilization of nano-based formulations on control and diagnosis of mosquito-borne diseases were discussed. We also emphasizes the need for future research for broad commercialization of nano-based formulations in world market aiming a positive impact on public health.
Introduction
Rampant growth of human population has led to major challenges of sustainable food production and disease control in the twenty-first century (Roni et al., 2015). Arthropods are vectors of some deadly diseases, which can lead to epidemics or pandemics (Murugan et al., 2015). On top of the list are mosquitoes (Diptera: Culicidae) that are a cause of a major concern around the world because they can act as vectors of a variety of harmful pathogens and parasites (Benelli, 2016; Benelli and Mehlhorn, 2016). Aedes aegypti and Aedes albopictus are the most important global vectors of arboviruses, such as dengue, yellow fever, chikungunya, and zika viruses (Durán et al., 2016). Arboviruses have long been treated as neglected diseases around the world. However, in recent years there have been a number of epidemics caused by arboviruses - such as dengue, chikungunya, yellow fever and unprecedented zika (Wilder-Smith et al., 2017). The main factors contributing to these outbreaks have been considered to be urbanization, modernization and increased international mobility of the general population (Tavares et al., 2018).
Control of arboviruses is difficult due to many factors, such as lack of effective vaccines for most of the arboviruses, lack of antiviral drugs, insecticide resistance in the vectors such as Aedes species and failure of vector control strategies that would decrease human-vector contact (Batool et al., 2018). In this scenario, development of new approaches to rapidly detect, and control dissemination of arboviruses are a priority and a public health imperative. In this regard, the importance of nanobiotechnology has been gradually realized as an emerging technology of the future due to exceptional new benefits (Suganya et al., 2017). In the vector control applications, nanoparticles could be applied for: (a) the development of new drugs, with higher activity, decreased toxicity and sustained release; (b) development of new repellent formulations based on natural or synthetic compounds; (c) control of vectors by the use of nanoparticles with repellent, insecticidal or larvicidal activities (Magro et al., 2019); and (d) development of biosensors that can rapidly detect and diagnose the mosquito transmitted viral diseases (Durán et al., 2016; Benelli et al., 2017; Nicolini et al., 2017a). Due to the lack of specific drugs for viral diseases, nanobiotechnology has appeared as an important new breakthrough, which could be potentially used for treatment of patients infected with arboviruses. VivaGel® is a poly-L-lysine dendrimer-based formulation, which has shown efficient antiviral activity against zika virus (ZIKV) (Starpharma, 2016). Recently, a number of reviews have been published on the contribution of nanotechnology to control arboviruses epidemics.
The developments in the area of nanomedicines is also promising new treatments for different diseases, improving the efficacy and bioavailability of drugs, with controlled release formulations that require optimal doses and consequently lesser adverse effects. This review discusses the current status of nanobiotechnology relevant to the control of arbovirus mosquito vectors, and highlights how it provides key tools for exploring new perspectives in the treatment of arboviruses.
Nanotechnology for Arbovirus Detection and Control
Several strategies have been applied to prevent proliferation of Aedes species using physical, biological, and chemical control approaches. Other methods under research and development, are also being studied, including the use of nanotechnology to produce repellent and larvicidal formulations that are more efficacious and less toxic. The development of nanotechnology-based sensors for rapid viral detection has also attracted the attention of scientific community (Figure 1).
Figure 1
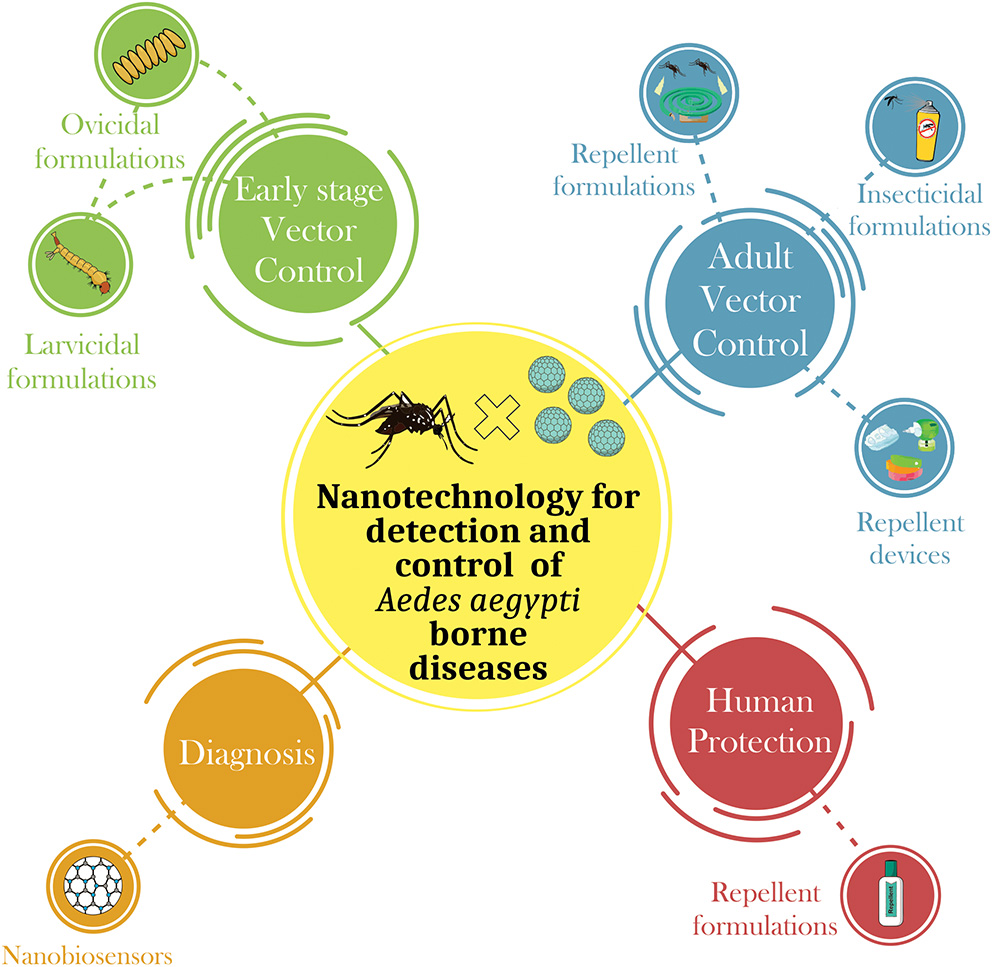
Summary of the main applications of nanotechnology in the control of Aedes aegypti-borne diseases. There are four main areas where nanotechnolgy can be connected: (i) Early stage vector control—The nanodevices for the control of Aedes aegypti can be developed in order to control this pest in its different stages of life. Many studies have developed nanodevices to control the early stages of mosquitoes, i.e., products that exhibit ovicidal and/or larvicidal activity; (ii) Adult vector control—adult insect control is also highly studied form of control through the development of nanoformulations that exhibit larvicidal and/or repellent activity; (iii) Human protection—following the recent epidemic of arboviruses transmitted by Aedes aegypti, many studies have focused on the development of forms of immunization of humans through the development of nanovaccines, and (iv) Diagnosis—the development of nanobiosensors that quickly detect the presence of arboviruses in the host, thus expediting the decision for the most effective treatment.
Biosensors
Rapid diagnosis of important arboviruses-borne diseases such as dengue, chikungunya, zika, and yellow fever is essential in order to reduce and avoid further dissemination of the infections within the general population (Patterson et al., 2016). The WHO has emphasized the importance of developing point-of-care (POC) tests that are ASSURED (Affordable, Sensitive, Specific, User-friendly, Robust and rapid, Equipment-free, and Deliverable) (Pashchenko et al., 2018). An ideal technique for the on-site detection of arboviruses should have these characteristics and enable early detection of the disease. Fast and timely diagnosis is crucial for the confirmation of viral infection so that it can be followed by clinical treatment, and necessary measures can be put in place for monitoring and protection of public health (Rashid and Yusof, 2018). Currently, diagnosis of the infections caused by arboviruses in the genus Flavivirus (family Flaviviridae) is often late, ineffective, and dependent on the clinical symptoms. The final decision on the infection generally requires a long waiting time, collection of samples from suspected patients (blood, urine, or saliva), transportation and preservation, and laboratory procedures by trained health staff.
Given such difficulties in the early detection of an impending epidemic of a viral infection, such as dengue, chikungunya, zika, and yellow fever (Nakata and Röst, 2015), there has been an urgent need for the improvement of existing tools and development of new biosensing technologies that are rapid, effective, and applicable in terms of real-time diagnosis. Biosensors are biologically-selective analytical devices that are able to recognize analytes in a complex sample matrix without the need for lengthy sample treatments. The biologically-selective part of biosensors enables them to produce highly specific responses by means of a transduction system that acts to convert the biological recognition into a quantifiable electrical signal (Figure 2). For the detection of arboviruses, the system must be able to identify low concentrations in complex sample matrices, such as blood, saliva, urine, and serum, without pretreatment or with minimal sample preparation.
Figure 2
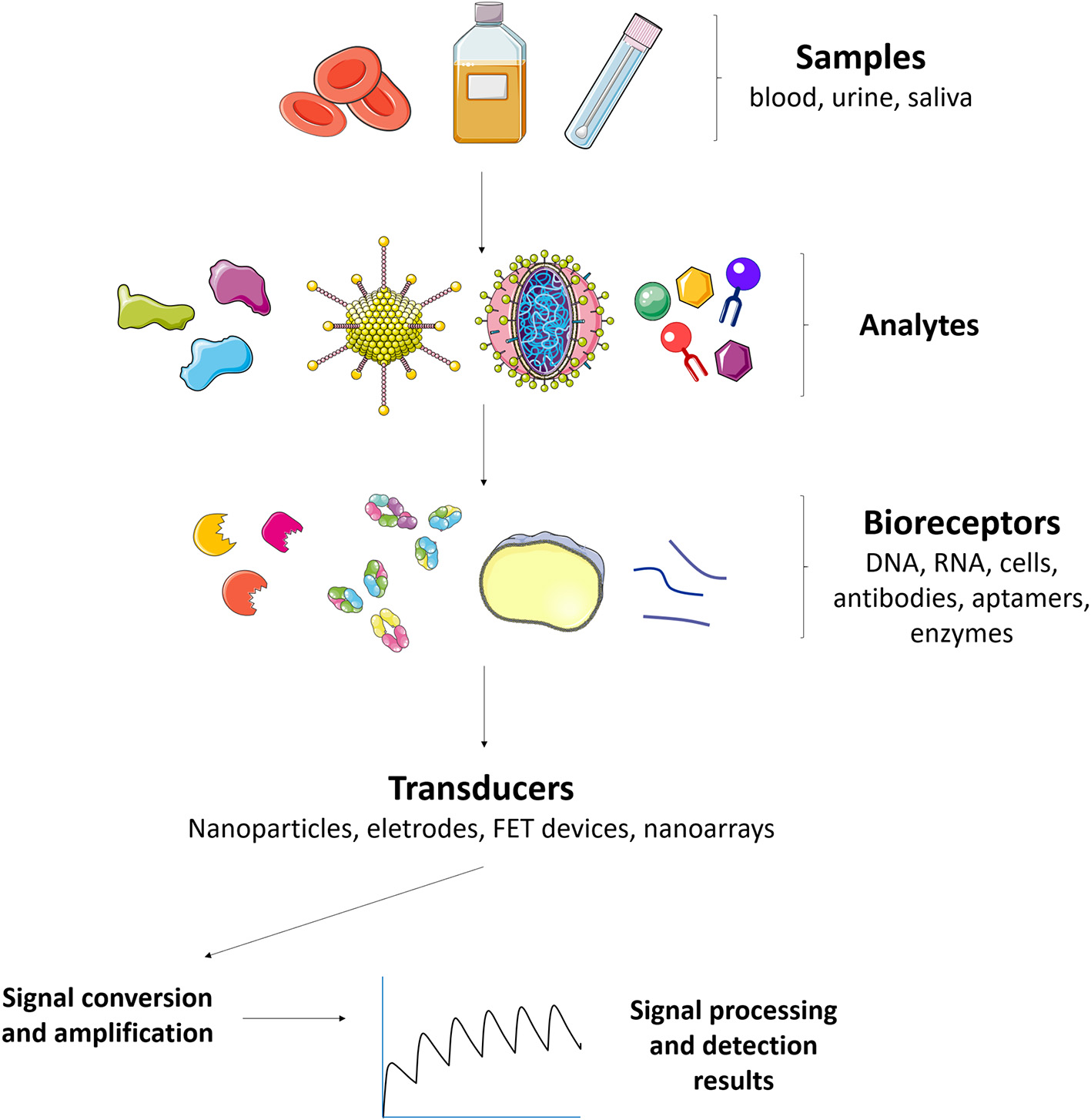
Major constituents of a biosensor for arboviruses detection and control. Different kinds of samples (blood, urine, saliva) can be used to detect analytes such as NS1 antigen and IgM antidengue, zika, and/or chikungunya antibodies. The analytes can interact with bioreceptors (aptamers, gold nanoparticles, glycan, fluorophores, enzymes, viral antigens, nucleic acids, and polyclonal antibodies for instance) that are connected to transducers and the signal converted and amplified in order to monitor the arboviruses.
The great advantage of a biosensor is that the bioreceptor interacts specifically with an analyte molecule. The specific interaction causes one or more physicochemical changes (production of ions, colored moieties, electrons, gases, heat, mass, or light) (Sethi, 1994). These responses can then be amplified and transformed into easily interpretable results. For the control and diagnosis of an endemic disease, an ideal biosensor device should be able to detect the arbovirus during all stages of infection, so the device must be designed to carry more than just one bio-receptor (multiplex sensing). Such features can be achieved using lateral-flow assays (LFAs) and lateral flow immunoassay (LFIA).
Several recent studies have used mainly LFAs as the basis for the construction of ASSURED biosensing devices. These assays can be performed using microfluidic technologies such as paper-based miniaturized devices that combine several recognition steps in a small area for naked-eye detection and quantification of compounds in complex mixtures, with the sample being collected on a test device and the results being displayed in real time (Koczula and Gallotta, 2016). Glucose, urine, and pregnancy test strips are examples of LFA devices, where the fluid containing the sample (blood, saliva, serum, etc.) moves by capillary action through various stages were antibodies and conjugated labels (nanoparticles, for instance) can interact and react to the fluid and, finally, show (sandwich assays) or not (competitive assays) a colored line at the test line position. More detailed information about LFAs can be found at (Sajid et al., 2015; Koczula and Gallotta, 2016; Carrell et al., 2019). Figure 3 provides a scheme of biosensor based on LFA where a colorimetric lateral flow biosensor (LFB) for the visual detection of dengue-1 RNA using dextrin-capped gold nanoparticle (AuNP) as label can be seen.
Figure 3
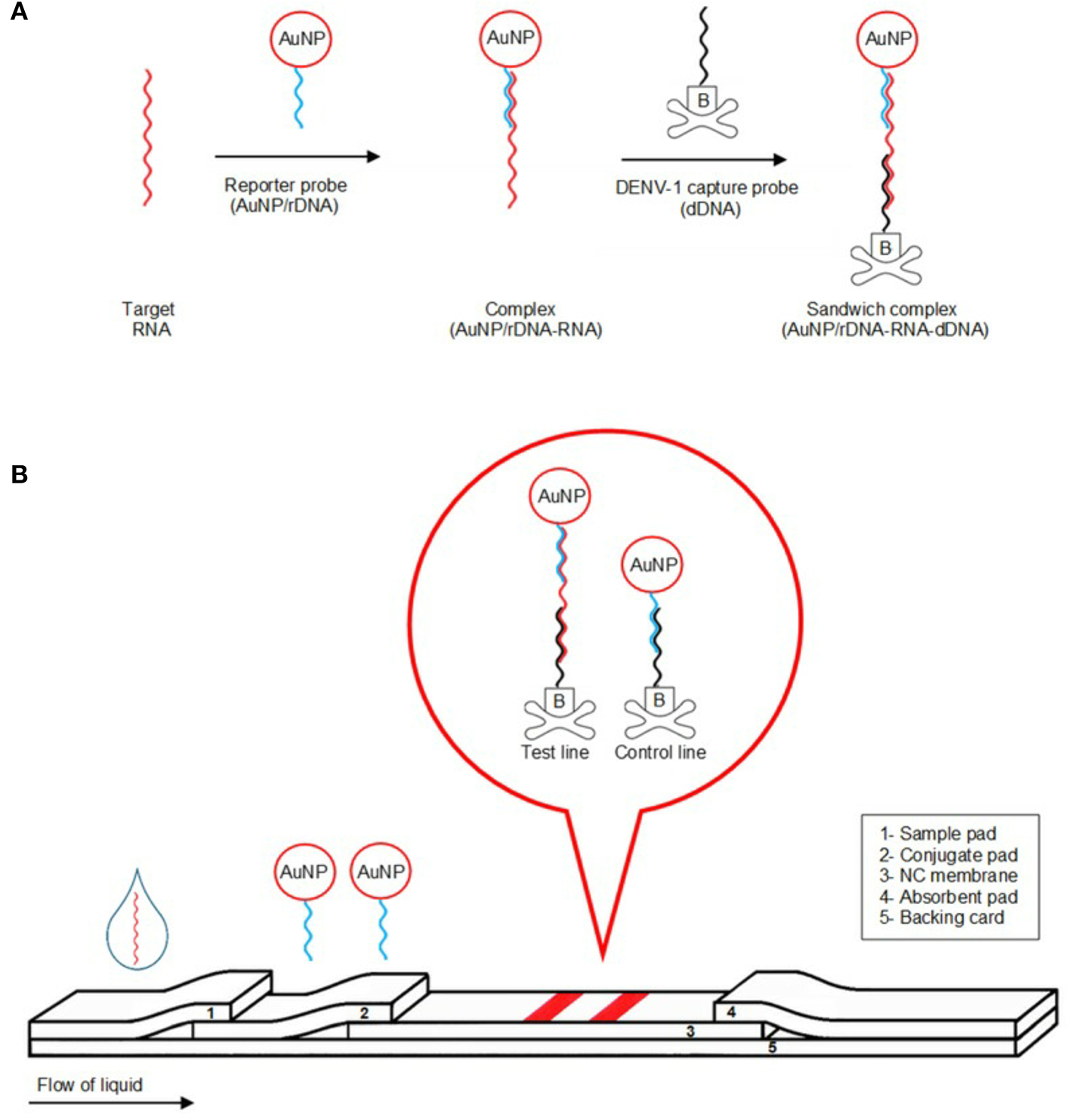
Illustration of detection mechanism of the detection of dengue-1 RNA using dextrin-capped AuNP as label in a POC device. (A) Formation of AuNP/rDNA-RNA-dDNA sandwich complex. (B) Schematic of visual detection. Reprinted with permission from Yrad et al. (2019). Visual detection of dengue-1 RNA using gold nanoparticle-based lateral flow biosensor. Diagnostics 9:74.
The detection of dengue and yellow fever has been performed with a platform for multiplexed pathogen detection employing multi-colored silver nanoplates (Yen et al., 2015) as demonstrated by Yen et al. (2015). In this study, the authors showed that the color of test lines could differentiate among different bioreceptors, with the analyses being performed in various ways, including the use of mobile phone applications. Current improvements in LFA technology are associated with the use of nanotechnological tools, such as lab-on-a-chip devices and nanoparticles that change color when aggregated. These improvements have significantly enhanced diagnosis sensitivity and selectivity.
Nawaz et al. (2018) reported a novel method for the detection, classification, and antibody screening of dengue virus, based on electrochemical impedance spectroscopy (EIS), involving protein recognition by means of a self-assembly process based on polymer matrix composites. However, as mentioned previously, it is very important to be able to achieve early detection of arbovirus infection but the biosensors had generally been designed to detect NS-type proteins that are only produced from the fifth day of infection onwards (Nawaz et al., 2018). Omar et al. (2018) overcame this limitation by designing an optical sensor based on the surface plasmon resonance phenomenon, which was applied to the diagnosis of dengue virus structural E-protein that forms the coat of the host virus itself. This protein can be detected earlier, at the start of the immune system response to the infection (Omar et al., 2018).
Figure 4 shows the schematic immobilization of IgM in gold/Fe-MPA-NCCCTAB (3-mercaptopropionicacid - nanocellulose crystalline/hexadecyltrimethylammonium bromide, respectively)/EDC-N-hydroxysuccinimide (NHS) for early detection of dengue virus E-protein using surface plasmon resonance explored by Omar et al. (2018). By introducing IgM immobilized Fe-MPA-NCC-CTAB/EDC-NHS on a gold surface, it is possible to determine the E-protein concentration in a range of 0.0001–10 nM. The sensitivity found by optical sensor in contact with DENV is 39.96° nM−1 (Omar et al., 2018).
Figure 4
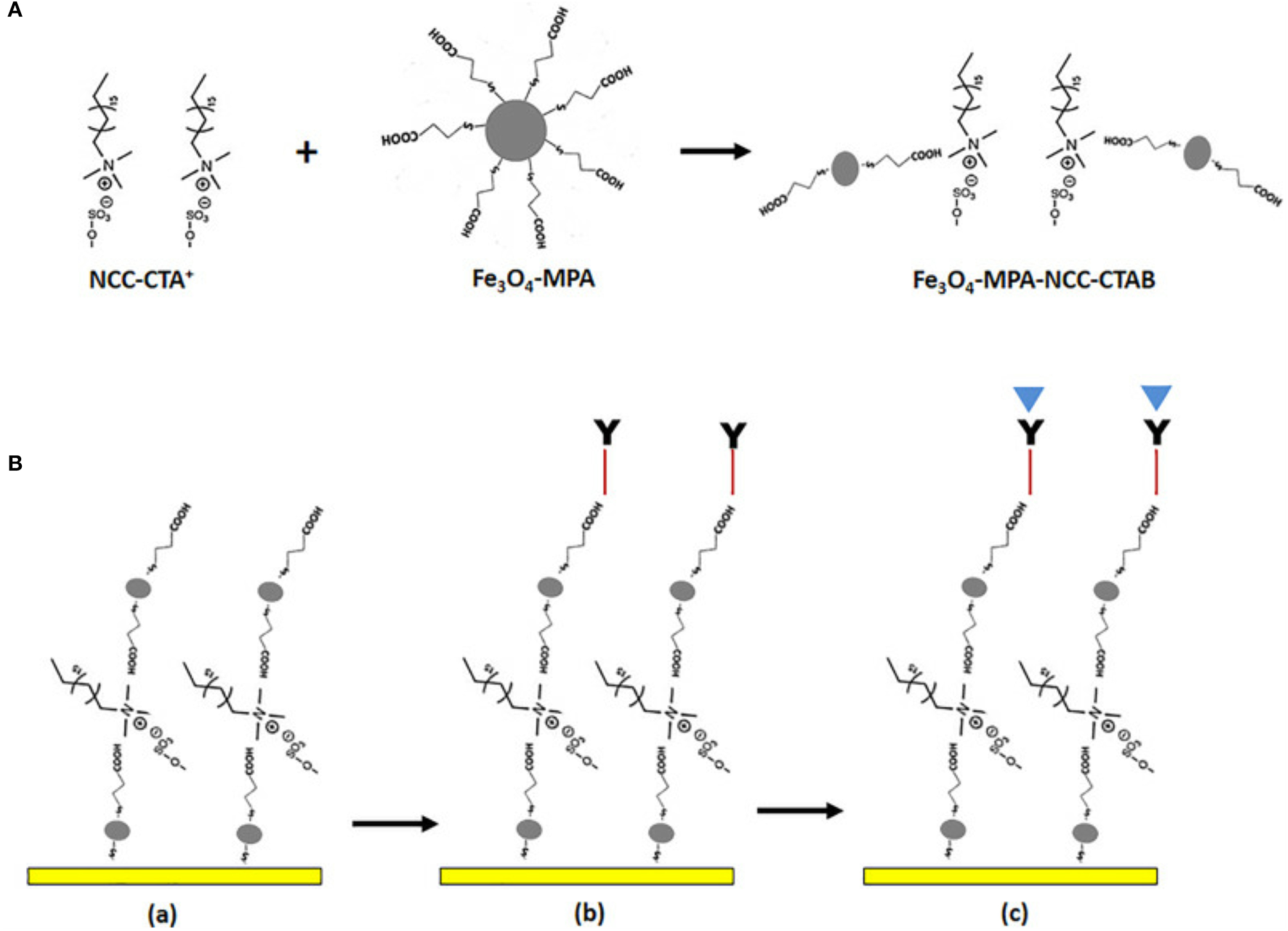
(A) Illustration of possible mechanism for the preparation of Fe-MPA-NCC-CTAB composite aiming the dengue virus recognition (B) The sensor functionalization. (a) Surface activation of gold/Fe-MPA-NCC-CTAB layer, (b) Immobilize the IgM antibody via EDC-NHS cross-linker, (d) Injection of dengue E-protein solution. Reprinted with permission from Omar et al. (2018). Development of an optical sensor based on surface plasmon resonance phenomenon for diagnosis of dengue virus E-protein, Sensing and Bio-Sensing Research 20, 16–21.
Vinayagam et al. (2018) reported the recognition of serotype-specific DENV employing multicolor triangular silver nanoparticles (TAg), which has the potential to be a powerful diagnosis technique that is able to differentiate between various serotypes. The color responses were established on the interaction of a TAg-DNA probe with a specific strand, resulting in the creation of a network association between the DNA probe and the dengue virus RNA, according to serotype. This was the first report of DNA conjugated to triangular silver nanoparticles, based on the pH reduction method. This biosensor has not yet been tested using real samples from infected patients, although the proposed technology appears to be very promising for use in clinical POC diagnostic testing (Vinayagam et al., 2018).
Other available methodologies and R&D developments for the improvement and development of biosensing systems for the detection of arboviruses are shown in Table 1.
Table 1
| Biosensor type | Disease | LOD* | Detection speed | Detection medium | References |
|---|---|---|---|---|---|
| Electrochemical biosensor based on surface imprinted polymers | zika | 2 × 10−4 PFU.mL−1 |
– | Buffer | Tancharoen et al., 2019 |
| 2 × 10−3-5 × 10−2 PFU.mL−1 | Serum | ||||
| Magneto-enzyme LFIA combining super-paramagnetic nanoparticles | DENV-1 | 0.25 ng.mL−1 | 90 min | Serum | Thanh et al., 2019 |
| DENV-2 | 0.1 ng.mL−1 | ||||
| DENV-3 | 0.25 ng.mL−1 | ||||
| DENV-4 | 1.0 ng.mL−1 | ||||
| Nitrogen-doped porous carbon-based fluorescence sensor | zika RNA | 0.23 nM | 40–80 min | Saliva | Li et al., 2019 |
| Paper-plastic microfluidic hybrid chip integrated with a lateral flow immunoassay | Dengue | 84.66 ng.mL−1 | <2 min | Spiked buffer | Yuzon et al., 2019 |
| Trapezoidal SiNWs array fabricated by AFM-LAO | Dengue | 2.22 fM | Real-time | DNA | Yusoh et al., 2019 |
| Aptamer–gold nanoparticle conjugates | zika | 10 ng | Real-time | A. aegypti salivary gland extract | Bosak et al., 2019 |
| Acrylic-based genosensor (DNA biosensor) | Dengue (DEN-2) | 1.21 × 10−16 M | 30 min | Blood | Mazlan et al., 2019 |
| Urine | |||||
| Saliva | |||||
| Gold nanoparticle-based lateral flow biosensor | Dengue | 0.01 μM | 20 min | Synthetic dengue-1 target | Yrad et al., 2019 |
| 1.2 × 104 PFU.mL−1 |
Pooled Human Sera | ||||
| Fluorescent lateral flow immunoassay | zika (NS1) | 0.045 ng.mL−1 | 20 min | Buffer | Rong et al., 2019 |
| 0.15 ng.mL−1 | Serum | ||||
| Localized surface plasmon resonance immunosensors | Dengue (4 serotypes) | 107 TCID50.mL−1 | <5 min | – | Basso et al., 2018 |
| Two-dimensional MoS2 nanosheets-based disposable biosensor (electrochemical detection) | Chikungunya | 3.4 nmol.L−1 | <60 min | PBS serum | Singhal et al., 2018 |
| Paper-based DNA biosensor using gold shell-coated magnetic nanocubes | Chikungunya | 0.1 nmol.L−1 | – | PBS serum | Singhal et al., 2018 |
| Laser-cut microfluidic device made of glass-fiber paper | Non-structural 1 (NS1) viral protein and specific IgM | 25 ng.mL−1 | <10 min | Blood and plasma | Theillet et al., 2018 |
| Graphene-based biosensor employing precise immobilized monoclonal antibody | zika | 450 pmol.L−1 | 5 min | – | Afsahi et al., 2018 |
| Electrochemical immunosensor | Dengue | 0.3 ng.mL−1 | – | Serum | Nawaz et al., 2018 |
| Reverse-transcription LAMP coupled with reverse dot blot | zika | <2 × 103 (6 RNA copies per reaction) | Between 3 and 10 min | Saliva | Sabalza et al., 2018 |
| Multiplex tools with target-specific fluorescently tagged strand displaceable probes with RT-LAMP | Dengue | ~1.22 PFU equivalent viral RNAs | 30 min | Urine and plasma | Yaren et al., 2017 |
| zika | ~0.71 PFU equivalent viral RNAs | ||||
| Chikungunya | ~38 copies of viral RNA | ||||
| Electrochemical stand with electrospun semi-conducting manganese (III) oxide (Mn2O3) nanofibers for DNA hybridization detection | Dengue | 120 × 10−21 mol.L−1 | – | Spiked serum | Tripathy et al., 2017 |
| Electrochemical capacitive sensing | Dengue | 0.5 ng.mL−1 | – | Serum | Cecchetto et al., 2017 |
| zika | |||||
| Chikungunya | |||||
| Coupling of reverse-transcription loop-mediated isothermal amplification (RT-LAMP) with the quenching of unincorporated amplification signal reporters (QUASR) technique | Dengue | 103.4 copies.μL−1 | <40 min | Blood, urine, and saliva | Priye et al., 2017 |
| zika | 105-102 PFU equivalent.mL−1 (104-101 copies per rxn) 108-103 PFU.mL−1 |
7–15 min <40 min |
|||
| Chikungunya | |||||
| Surface-enhanced Raman spectroscopy (SERS)-based sandwich immunoassays (LFA) |
zika (NS1) | 0.72 ng.mL−1 | 20 min | Serum | Sánchez-Purrà et al., 2017 |
| Dengue | 7.67 ng.mL−1 | ||||
| Bead-based immunofluorescence assay on a microfluidic dielectrophoresis platform | Dengue | 104 PFU.mL−1 | 5 min | – | Iswardy et al., 2017 |
| Optical caustic plasmonic light scattering sensor | Dengue | 50 pg.mL−1 | 15 min | Serum | García et al., 2017 |
| Carbon nanotube-based chemiresistor functionalized with heparin | Yellow fever | 8.4 × 102 TCID50.mL−1 |
10 min | – | Wasik et al., 2017 |
| Dengue | |||||
| Multiplexed assay on a nanostructured plasmonic gold (pGOLD) platform | zika (NS1) | 0.33 IgG level | 120 min | Serum | Zhang et al., 2017 |
| 0.30 IgA level | |||||
| Reverse transcription strand invasion-based amplification (RT-SIBA) with fluorescence detection | zika | 5,000 copies.mL−1 | <30 min | Lysis buffer | Eboigbodin et al., 2016 |
| Detection using isothermal amplification, AC susceptometry, and magnetic nanoparticles | zika virus oligonucleotide | 1 aM | 27 min | Serum | Tian et al., 2016 |
| Reverse-transcription loop-mediated isothermal amplification (RT-LAMP) | zika | 50–100 PFU.mL−1 | 40 min | Saliva | Song et al., 2016 |
| Lateral flow assay using multicolored silver nanoparticles | Dengue | 150 ng.mL−1 | – | Blood | Yen et al., 2015 |
| Carbon nanotube-ink printed electrode | Dengue | 12 ng.mL−1 | – | Serum | Dias et al., 2013 |
| Optical DNA biosensor based on square-planar ethyl piperidine substituted nickel (II) salphen complex | Dengue | 0.2 mol.L−1 | 30–120 min | Saliva and urine | Ariffin et al., 2013 |
| Microfluidic system combined with microvalves and micropumps for rapid DNA hybridization using shuttle flow | Dengue (4 serotypes) | 100 pmol.L−1 | 90 s | – | Huang et al., 2010 |
| Microfluidic chip that accomplish DNA/RNA amplification, sample injection, and separation of nucleic acid products | Dengue | – | <5 min | – | Huang et al., 2010 |
| Sensor-based microchip employing a magnetic bead bioassay platform | Dengue (antidengue virus IgG) | 100 pg.mL−1 | – | – | Aytur et al., 2006 |
Main biosensors developed for the detection of arboviruses, together with their operational principles and applications.
Limit of detection.
The recent increase in the cases of epidemic infections worldwide (Nicolini et al., 2017b) has also been matched by an increase in the available commercial biosensing devices and diagnostic methodologies. An excellent example is the invention designed by Kaushik and Nair (2018). The device is based on a modeling and electrochemical immunosensing approach for the detection of zika virus and offers an extremely low detection limit (picomolar). The biosensor is shaped to be worn on an individual's skin for infection screening. It matches the analyte measurement to the baseline in order to determine if an infection is present. After signal interpretation, the result is transmitted as an alert message. The device can be classified as a point-of-care biosensor, since it complies with the ASSURED features as proposed by the WHO.
The Ulisse Biomed SRL product portfolio includes a biosensor for the determination of an infection, and possible associated infections, caused by several viral pathogens, including zika, dengue, and chikungunya. The biosensor designed by Braga et al. provides a method for obtaining qualitative and quantitative data related to viral infections. This specific biosensor consists of an antigen (biologically active responsive element) bound covalently to the surface of one or more carbon nanotubes and/or metal nanoparticles present on part of a microelectrode surface, together with an electrically conducting part where a transduction system (sensor) converts the biochemical response into an electric signal (Braga et al., 2015).
Table 2 lists some of the published patents related to the improvement of biosensing tools and emerging new technologies for more efficient detection of analytes related to dengue, chikungunya, zika, and yellow fever.
Table 2
| Country | Company | Product technology | |||
|---|---|---|---|---|---|
| Year | Patent number | References | |||
| United States | Mikrogen, GmbH | Method for the immunological diagnosis of a sample with a potential infection with an arbovirus and test kits suitable for this purpose | 2019 | US2019/0227065A1 | Soutschek et al., 2019 |
| United States | The USA, as represented by the Secretary, Dept. Of health and human services | Compositions and methods for the diagnosis and treatment of zika virus infection | 2017 | WO2018152496A1 | Akahata and Ueno, 2017 |
| United States | University of Central Florida Research Foundation, Inc. (UCFRF) | A payload reservoir comprising an insect attractant or insect food source; and (b) A detector conjugate comprising a gold nanoparticle conjugated to a specific detector molecule that binds specifically to a protein present in the saliva of a specific insect to be detected | 2018 | US20180231550A | Willenberg and Seal, 2018 |
| United States | The Hong Kong polytechnic university | Microarray design of hybrid upconversion nanoparticles on a nanoporous anodized alumina membrane heterogeneous assay for simultaneous detection of multiple oligonucleotides | 2018 | US2018/0246084 A1 | Hao et al., 2018 |
| United States | Sympano, Inc. | Nano-field electrical Sensor for Biomarkes and other targets analytes by determining impedance in bodily fluid on nanoporous membrane | US2018/0067107 A1 | Barrett et al., 2018 | |
| China/ United States | Ulisse Biomed SRL | Biosensors for the detection of infection and associated maladies | 2017 | CN106461667A US20170212116A1 |
Braga et al., 2015 |
| United States | DexCom, Inc. | Transcutaneous analyte sensor for transcutaneous measurement of glucose in a host | 2018 | US7654956B2 | Brister et al., 2012 |
| Spain | Universidad complutense de Madrid | Biosensor for the detection of nucleic acids | 2017 | ES2580138B2 | Cabarcos et al., 2017 |
| United States | University of Ottawa, University of Malaya | Long-range surface plasmon-polariton biosensor | 2018 | WO2018090125A1 | Berini and Wong, 2018 |
| United States | Florida International University | Electrochemical sensing device based on nano-devices for fast zika Virus detection | 2018 | US10012645B2 | Kaushik and Nair, 2018 |
| United States | San Diego State University (SDSU) Foundation | Cell-based devices for track small molecules that restrain enzymes | 2018 | US10006077B2 | Wolkowicz, 2018 |
| United States | Eccrine Systems, Inc. | Biosensing device aimed to be used in humans skin to track an infection by one or more antigens | 2018 | WO2018026931A1 | Beech et al., 2018 |
| United States | Aviana molecular technologies, LLC | Multiplex acoustical biosensor with higher sensitivity | 2011 | US20110136262A1 | Ragavan et al., 2011 |
| United States | Purdue research foundation | Electrochemical biosensor for RNA and DNA sensing | 2017 | US20170107565A1 | Marinero-Caceres et al., 2017 |
| United States | Aviana molecular technologies, LLC | Biocoated piezoelectric biosensor platform for point-of-care diagnostic use | 2015 | US20150111765A1 | Laury-Kleintop and Rutner, 2015 |
| France/ United States |
Cornell Research Foundation, Inc. | Microfluidic biosensor and methods of use | 2005 | WO2005084404A2 | Baeumner et al., 2005 |
| Australia/ United States |
Lifeprint Australia Pty Ltd. | Auto-feedback loop biosensor—signal amplification auto-feedback loop for the detection of a target analyte in a sample | 2009 | WO2009152566A1 | Fletcher and Milligan, 2009 |
| United States | UT-Battelle, LLC | Biosensor which has multiple functions and broad spectrum and methods of utilization | 2004 | USOO6743581B1 | Vo-Dinh, 2004 |
Patents related to the detection and control of arboviruses.
The advancement of technology can show how to improve the ways to detect arboviruses with nano based-tools. However, just a few publications on the use of nano-tools has been clearly demonstrated. Nanosensors have the potential to be allies in the early detection of Aedes aegypti-borne diseases by offering novel approaches to achieve sensitive, specific, and stable recognition in complex matrices in quick or real-time diagnostics. Although some variations in synthesis protocols can still be tested to improve the productivity and efficiency of nanomaterials for diagnostic applications, more research on the way lab-to-practical nanodiagnostics is needed. Not only more research must be done on nanosensors for A. aegypit-borne diseases, but the upscale of the applicability is urgently necessary, as shown by the few patents filed for this purpose (Table 2). One of the ways for this accomplishment is to narrow the scientific boundaries between disciplines such as chemistry, sociology, bio, and nanotechnology and information technology. A good example that results from this new multidisciplinary approach is the smartphones-based POC devices, as previously cited. They are a real and promising novel tool for flaviviruses detection without complex instruments, since, in a simple way, the blood sample can be analyzed under 40 min (Priye et al., 2017; Rong et al., 2019). Despite the advances, still, there is an urgent need for proper and precise use of nanosensors in hospitals, field, and to prevent these potential health risk diseases.
Insect Repellents
The development of new nanotechnology-based formulations for the encapsulation of natural and synthetic repellents is an important strategy for obtaining systems that are more effective and have fewer undesirable impacts. These sustained-release formulations provide controlled or slow release of active agents into the environment, increasing the duration of action and reducing human exposure to the agent (for example, by permeation through the skin). Encapsulation also protects the active agent against premature degradation caused by the effects of light, temperature, oxidation, and humidity, among others (Tavares et al., 2018). Numerous matrices (synthetic and natural) can be used for the preparation of carrier systems, including polymers, proteins, lipids, polysaccharides, and others. It should be noted that the main desirable characteristics of such matrices are biocompatibility and biodegradability, as well as low cost (Barradas et al., 2016).
Gomes et al. encapsulated DEET in polymeric nanospheres, resulting in particles with an average diameter of 114 ± 37 nm, low polydispersion index, and stability as a function of time. The sustained release of nanoencapsulated DEET provided repellency for over 9 h, which was longer than obtained using free DEET. The results showed that the release mechanism was temperature dependent, which the authors highlighted as having great potential, since the release rate could be adjusted by alteration of temperature (Gomes et al., 2018).
Silva et al. (2019) encapsulated essential oils of Piper aduncum L. and Piper hispidinervum in gelatin nanoparticles and evaluated effect against Aedes aegypti Linn. Results showed a high encapsulation efficiency of the EOs (around 80%), average size around 100–200 nm, zeta potential around−40 mV. Both encapsulated EOs reached lethal dosages within 24 h of exposure and total mortality of the tested pests (Silva et al., 2019).
Forgearin et al. prepared and characterized permethrin-loaded lipid nanocapsules and tested their application as repellents in clothes. The formulations presented a mean particle diameter of 201 ± 4 nm, with a monomodal size distribution and permethrin content of 4.6 ± 0.1 mg/mL. It was observed that even after washing and with the action of temperature, the polyester fabrics containing the nanoparticles had higher concentrations of permethrin, compared to those containing only the free compound. The results showed that the innovative repellent spray composed of the nanoparticle formulation was useful for the impregnation of clothes and was promising for the protection of an individual against insects (Forgearini et al., 2016).
Werdin González et al. prepared and characterized polymeric nanoparticles (composed of PEG and chitosan) for the encapsulation of essential oils (geranium and bergamot). Evaluation was also made of the acute and residual larvicidal activities of the formulations against Culex pipiens. Physicochemical characterization showed that the PEG nanoparticles containing the essential oils had a mean size of <255 nm and provided encapsulation efficiencies between 68 and 77%, while the chitosan nanoparticles presented a mean size of <535 nm and encapsulation efficiencies between 22 and 38%. Both systems showed high larvicidal activity (acute and residual), with the chitosan-based formulations having the best effects. These findings demonstrated the potential of polymeric nanoparticles containing essential oils for use as eco-friendly larvicidal products (Werdin González et al., 2017).
Silva et al. studied the encapsulation of the essential oils of Piper aduncum L. and Piper hispidinervum C. in gelatin nanoparticles, with evaluation of the biological effects against Aedes aegypti. The encapsulation efficiencies exceeded 80%, and the particles were spherical, monodispersed, and smaller than 100 nm in size. Both of the encapsulated essential oils provided lethal effects within 24 h of exposure, with Aedes aegypti mortality greater than 80% (Silva et al., 2019).
It should be noted that the search for new formulations has also led to patenting. For example, patent BR1020180168665 describes the preparation and characterization of nanostructured lipid carriers (NLCs) and nanoemulsions containing citronella and neem oil, for the control of insects such as Aedes aegypti. The results showed that both the nanoemulsions and the NLCs loaded with the essential oils caused 100% mortality of A. aegypti larvae during the first day of exposure, while the NLCs without essential oil induced 100% mortality after 10 days of exposure. Both carriers showed satisfactory efficacy in the control of A. aegypti larvae (Fraceto et al., 2018).
The patent WO2017143421A1 describes the invention of cosmetic formulations for use as topical insect repellents, employing polymeric micelles, nanoemulsions, and solid lipid nanoparticles containing active repellent substances. Nanoencapsulation techniques were used to produce systems consisting of nanostructures with stable hydrophobic and hydrophilic chains. The formulations presented sustained release of the active substances, consequently providing long duration of action of the repellents, together with greater safety (Paula et al., 2017).
The patent US20190160016A1 present an invention to a nanoparticle composition comprising one or more β-triketones selected from Leptospermum scoparium botanical extract. The prepared system presented high insecticidal activity against ecotoparasites, and according to the inventors can also be applied to repel insects like Aedes aegypti (Thomas, 2019)
Table 3 presents other studies concerning the use of formulations based on micro/nanotechnology for the encapsulation of compounds (natural and synthetic) presenting repellent activity.
Table 3
| Systems | Matrices | Active agents | Main characteristics | References |
|---|---|---|---|---|
| Polymeric nanoparticles | Poly(ethylene glycol) (PEG) | Diethylphenylacetamide (DEPA) | Diameter: 149 ± 1.06 nm; Properties: 5-fold decrease of median lethal indices (LC50), compared to free DEPA | Balaji et al., 2017 |
| Polymeric nanospheres | Poly(n-butyl methacrylate-co-methyl methacrylate) | N,N-diethyl-m-toluamide (DEET) | Diameter: 114 ± 37 nm. Properties: release rate of the encapsulated DEET provides repellency for over 9 h and is and more controlled when compared to the free DEET |
Gomes et al., 2018 |
| Nanoemulsion | Polaxamer 407 | Ethyl butylacetylaminopropionate (IR3535) | Diameter: ± 200 nm; Properties: Nanoemulsion less retained by the epidermis and not toxic to the cells |
Pinto et al., 2017 |
| Gel/Nanoparticle | Chitosan | Zanthoxylum acanthopodium essential oil (ZA EO) | Encapsulation efficiency of 96.64%; Properties: Reduction in essential oil permeation in in vitro membrane study and mosquito repellent activity against Aedes aegypti with protection time of 2 h | Sharma, 2019 |
| Polymeric nanoparticles | Polyethylene glycol | Quercetin | Diameter: 124.0 ± 1.1 nm; Properties: Stability at 4°C, affected larval Aedes aegypti development, less toxic than non-encapsulated quercetin toward C. vulgaris (green alga) | Pessoa et al., 2018 |
| Polymeric micelles | PEG and PLGA | Pyrethrins | Diameter: 140–320 nm. Properties: Protection against ultraviolet degradation (at 26°C) and high larvicidal activity against Culex pipiens pallens | Zhang et al., 2018 |
| Polymeric microparticles | Gum arabic | Essential oils and DEET | Diameter: 1–68 mm; Properties: Spherical shapes and cotton fabric impregnated with system presentend better insect repellency, compared to DEET | Eyupoglu et al., 2018 |
| Nanoparticles | Chitosan | Siparuna guianensis essential oil | Diameter: 268 ± 3.4 nm; Encapsulation efficiency 84.8–88.0% Properties: 100% mortality during the first week and provides Against Aedes aegypti mosquito larvae |
Ferreira et al., 2019 |
| Polymeric nanoparticles | Polaxamer 407 | Eugenol, 1,8-cineole, geraniol, linalool, carvacrol, α-terpineol, citronellol, thymol, and menthol | Diameter: Around 40 nm; Properties: Mortalities ranging from 30 to 60% against insects with linalool and 1,8-cineole being most effective | Lucia et al., 2017 |
| Polymeric microparticles | Cellulose | N,N-diethyl-m-toluamide (DEET) | Encapsulation efficiency of 98%. Properties: A significant reduction in release rate of DEET |
Kadam et al., 2019 |
| Inclusion complexes | β-Cyclodextrin | Lippia gracilis essential oil | Inclusion complex formation by kneading and co-evaporation with essential oil content ~15%; Properties: LC50 of 33 ppm toward Aedes aegypti larvae and inclusion complex was not harmful to non-target organisms |
Galvão et al., 2018 |
| Polymeric micropartices | Carboxy-methylcellulose (CMC) | Essential oils (Alpinia galanga, Citrus grandis, and C. aurantifolia), and DEET | Diameter: 4–200 μm. Properties: The same period of repellent activity for essential oil encapsulated in comparison with microencapsulated DEET. Extended duration of repellent activity (between 1 and 2 h) compared with commercial formulations | Misni et al., 2017 |
| Nanoemulsion | Tween 80 | Vitex negundo L. essential oil | Diameter: <200 nm; Properties: Nanoemulsion with higher larvicidal activity (Aedes aegypti) compared with only essential oil; | Balasubramani et al., 2017 |
| Nanoemulsion | Tween 80 | Ocimum sanctum essential oil | Diameter: 50–300 nm; Properties: Nanoemulsion with potential insecticidal effect against Aedes aegypti and C. quinquefaciatus adults | Ramar et al., 2017 |
| Nanofibrous | Cellulose | Citriodiol (CD) | Properties: Nanofibrous presented more prolonged repellency (34 days) than monolithic ones in experiments using Aedes aegipty | Muñoz et al., 2019 |
Formulations based on modified release systems for the encapsulation of active agents with insect repellent properties.
DEET is currently considered a “gold standard” due to its outstanding protection against mosquitoes and other biting insects. It is the most common active ingredient in all commercially available repellents and is used as a comparative for other substances (Khater et al., 2019). However, due to indiscriminate use has suffered resistance effects, leading to loss of formulations effectiveness. In addition, due to its toxicity has raised health and environmental concerns Thus, the search for natural alternative repellents as well as new molecules is necessary. This is the case of compound IR3535, one of the newest products with odorless and non-toxic characteristics, which is recommended for children over 6 months of age and pregnant women (Benelli et al., 2018a).
It is in this scenario of innovation and search for new solutions that nanotechnology applies. It is becoming increasingly necessary to develop formulations that increase the repellent's longevity by controlling delivery and evaporation rate. In addition, the different applications forms such as sprays, creams, lotions, aerosols, oils, adhesives, protective clothing, treated nets, among others, is very important in order to ensure options for people living in endemic areas (Tavares et al., 2018; Agnihotri et al., 2019). Thus, encapsulation in micro/nanoparticles, cyclodextrins, micelles, hydrogels among others constitutes an approach to modify the physicochemical properties of encapsulated repellents. When applied in topical formulations or in personal protective clothes, for example, they have been shown to be more effective in increasing repellency time and also in reducing dermal absorption, improving the safety profiles of these products (Ahmed et al., 2019; Osanloo et al., 2019). Innovative nanotechnology-based formulations should be followed by safety and efficacy studies, as this will increase consumer confidence in this new formulations (Hameed et al., 2019).
Larvicidal and Ovicidal Nanoparticles
The green or biological synthesis of nanoparticles, also called biogenic synthesis, can offer advantages over the classical nanotechnological techniques currently employed. The new synthesis methods are economical, fast, and less expensive, and are performed at ambient temperature and pressure. In contrast, standard physical and chemical techniques typically involve high energy consumption, due to the need for high pressures and temperatures (Benelli et al., 2018b). In addition, potentially harmful reagents and solvent are not used in green synthesis methods, because the reducing and stabilizing agents are substituted by molecules produced by living organisms (Kumar et al., 2015). These agents can be extracted from bacteria, fungi, yeasts, algae, or plants (Sintubin et al., 2012). The technique can be used to produce nanoparticles composed of metals, metal oxides, silica, and carbon (Benelli et al., 2016).
Recent studies have reported the synthesis of various nanoparticles using different natural extracts, with demonstration of the larvicidal, ovicidal, and mosquiticidal activities of these nanoparticles. Udayabhanu et al. observed the larvicidal effect of titanium dioxide nanoparticles (TiO2 NPs) synthesized using an aqueous extract of Euphorbia hirta leaves against the larvae of Aedes aegypti (LC50 = 13.2 mg/L) and Culex quinquefasciatus (LC50 = 6.89 mg/L) (Udayabhanu et al., 2018).
Another study proposed the green synthesis of Ag NPs for use as an environmentally-friendly alternative to pyrethroid and carbamate larvicides. Silver nanoparticles synthesized from extracts of the Quisqualis indica plant showed high toxicity against the vectors of filariasis, zika virus, and malaria. In addition, toxicity tests employing three non-target organisms indicated low toxicity of these systems (Govindarajan et al., 2016).
The patent databases include inventions that describe methods of green synthesis of metal nanoparticles and metal oxides, with broad applications in consumer products, medicines, pharmaceuticals, and other biomedical products (Hoag et al., 2011; Liu et al., 2013; Yujia et al., 2015; Awad et al., 2016). There have been several reports concerning vector control using biogenic nanoparticles (Table 4), indicating the promising potential of these nanoparticles that are both environmentally-friendly and highly effective for vector control.
Table 4
| Name | Action | Organism species |
LC50/ LC90 in
A. aegypti |
Treatment time | References |
|---|---|---|---|---|---|
| AgNP | Larvicidal property against fourth instar larvae of Aedes aegypti | Apple extract | AgNPs -T 15.76/27.7 ppm AgNPs -RT 29.81/42.3 ppm |
24 h | Ali et al., 2017 |
| AgNP | Ovicidal activity against Aedes aegypti | Bauhinea acumiata leaf powder aqueous extract | 27.19/52.32 μg.mL−1 | 24 h | Alharbi et al., 2018 |
| AgNP | Larvicidal activity against Aedes aegypti, Anopheles stephensi, and Culex quinquefasciatus | Leaf extracts of Leucas aspera and Hyptis suaveolens | 4.02/11.22 mg.mL−1 | 24 h | Elumalai et al., 2017 |
| AgNP | Ovicidal activity against A. aegypti eggs | Holarrhena antidysenterica bark extract | 5.53/12.01 ppm | 72 h | Kumar et al., 2018 |
| ZnONP | Larvicidal and ovicidal activities against Aedes aegypti | Scadoxus multiflorus leaf powder aqueous extract | 34.04/78.06 ppm | 24 h | Al-Dhabi and Valan Arasu, 2018 |
| ZnONP | Larvicidal activity against of Aedes aegypti (4th instar) | Extract of the seaweed Ulva lactuca | 22.38/41.94 μg.mL−1 | 24 h | Ishwarya et al., 2018 |
| ZnONP | Larvicidal activity against fourth instar of Aedes aegypti | Pedalium murex seed extract | 34.88/64.56 μg.mL−1 | 24 h | Ishwarya et al., 2017 |
| AgNP | Larvicidal property against Anopheles stephen and A. aegypti | Belosynapsi Kewensis leaf extract | 84.2/117.3 ppm | 24 h | Bhuvaneswari et al., 2016 |
| AgNP | Potential larvicidal activity against larvae of Aedes aegypti (3rd instar), Anopheles stephensi, and Culex quinquefasciatus | Aqueous leaf extract of Heliotropium indicum | 72.72/126.86 μg.mL−1 | 24 h | Veerakumar et al., 2014 |
| AgNP | Larvicidal activity against third and larvae of Aedes aegypti (4th instar) | Leaf extract of Derris trifoliata | 3rd instar: 7.0/17.76 mg.mL−1 4th instar: 5.87/12.11 mg.mL−1 |
24 h | Kumar et al., 2017 |
| AgNP | Larvicidal activity against 1st−4th instar larvae dengue vector | 3,5 di-t-butyl-4 hidroxyanisole isolated from Cynodon dactylon leaf | 1st−4th instar: 2.5; 2.78; 3.02; 3.05/8.28; 7.47;8.13;8.74 μg.mL−1 | 24 h | Ramanibai and Velayutham, 2016 |
| AgNP | Larvicidal and pupicidal against Aedes aegypti and Anopheles stephensi | Aqueous leaf filtrate from Artemisia nilagirica | 1st−4th instar: 0.46; 0.35; 0.33; 0.21% Pupa: 0.16% LC90: N/A |
24 h | Nalini et al., 2017 |
Biogenic nanoparticles tested for the control of disease vectors.
N/A- data not provided by the autor; LC50, Lethal Concentration that kills 50% of the exposed larvae; LC90, Lethal Concentration that kills 90% of the exposed larvae; AgNPs—T, prepared by heating; AgNPs—RT, prepared by non-heating.
Gaps, Obstacles, and Conclusions
The potential application of nano-based formulations in the field of arboviruses management was investigated by analyzing the number of publications in the last ten years. The results revealed that around 1000 articles were published worldwide, which an exponential trend in publication numbers after 2016, mainly for researches related to zika and chikungunya. Overall, many publications have shown that nanotechnology has led to rapid advancements in the development of pesticides, repellents, drug delivery system and diagnostic devices for arboviruses management. However, the clinical translational of nano-based formulations has some bottlenecks that hinders the broad acceptance and commercialization of these products (Hua et al., 2018; Soares et al., 2018). Also, it's already known, that nanomaterials, independently of their composition and method of production, have new properties, which are not observed by their bulk materials (Laux et al., 2018). These novel properties have brought innovative solutions due their flexibleness, responsiveness and possibility of functionalization, the last one, which is very useful for drug targeting delivery (Jeevanandam et al., 2018). However, the behavior, fate, bioavailability nanomaterials in the environment and toxicity of non-target organisms should be better understood the possible environmental impacts (Dinda, 2018).
Much debate still exists regarding the legislation of nanomaterials, which is in an early stage of development. In addition, there is a lack of a clear definition of nanomaterials, lack of standard methods for assessment of pharmacology, toxicology and efficacy evaluation of nano-based formulations and lack of worldwide network for gathering and sharing pertinent information. According to Schnell-Inderst et al. (2018) 12% of the documents related to the test of medical devices are written by academics and the regulators write the rest. In addition, there is a low number of experts in nanomaterials in regulatory agencies, resulting in the delayed development of these documents. Another issue is that FDA use the traditional regulatory frameworks to approve products, which contains nanomaterials (Jones et al., 2019). Nano-based formulations are evaluated by FDA using case-by-case approach, through combination product framework in order to determine regulatory framework will be used. Three main challenges should be addressed by FDA to boost the market of nano-based formulations: (i) development of a regulatory framework specific to nanomaterials; (ii) development of methods capable of characterize and quantify the toxicological impacts of nanomaterials, and (iii) deal with public acceptance and understanding through awareness programs and product labeling (Hua et al., 2018; Jones et al., 2019).
Also, economic issues can limiting the broad commercialization of nano-based formulations (Ventola, 2017; Jones et al., 2019). In comparison to conventional therapies, the production of a nano-based formulations required higher initial investments that will only be worth it from a business perspective if brings good opportunities for the pharmaceutical company, justifying their investment of R&D sector and reduce substantially health care costs (Benelli et al., 2017; Hua et al., 2018). Future research should explore the possibility of (i) determine the mechanism of action of nano-based formulations; (ii) understand the behavior and interaction of nanomaterial in complex biological matrix, such as human body; (iii) evaluate the possible toxicity and residual effects of nanomaterials in both target and non-target organisms; (iv) development of a international legislation; (v) create a standard definition of nanotechnology and nanomaterials, and (vi) standardized methods for establishing the risk-benefit of nanomaterials, in order to create large scale production of nanodevices to fight against of arboviruses as suggested in literature (Parisi et al., 2015; Kah et al., 2018a,b).
As conclusion, as indicated in this review, it is important to learn and appreciate the great potential offered by nanotechnology in relation to the development of new and more efficient tools and products. In this regard, it is extremely important that ongoing research involves all sectors: academia, industries, research centers, and government agencies, in order to turning this technology into a sustainable commercial reality and to create alternatives for detection and control of Aedes aegypti-borne diseases.
Statements
Author contributions
EC, JO, and LF proposed the structure of the manuscript. EC, JO, DA, CR, CB, VM, and RM wrote the manuscript. EC and LF revised the manuscript. All authors read and approved the final version of the manuscript.
Acknowledgments
The authors are grateful for financial support provided by MCTIC-CNPq/MEC-CAPES/MS-Decit/FNDCT N° 14/2016–Prevenção e Combate ao vírus Zika—Processo CNPq: 440350/2016-6 e Processo CAPES: 88881.130834/2016-01. The authors thank the São Paulo State Research Foundation (FAPESP) for scholarships awarded to EC (#2017/24402-1), DA (#2018/14743-0), and CR (2018/02404-5).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1
Afsahi S. Lerner M. B. Goldstein J. M. Lee J. Tang X. Bagarozzi D. A. et al . (2018). Novel graphene-based biosensor for early detection of Zika virus infection. Biosens. Bioelectron.100, 85–88. 10.1016/j.bios.2017.08.051
2
Agnihotri A. Wazed Ali S. Das A. Alagirusamy R. (2019). “11 - Insect-repellent textiles using green and sustainable approaches,” in The Impact and Prospects of Green Chemistry for Textile Technology The Textile Institute Book Series, eds IslamS.ButolaB. S. (Woodhead Publishing), 307–325. 10.1016/B978-0-08-102491-1.00011-3
3
Ahmed T. Hyder M. Z. Liaqat I. Scholz M. (2019). Climatic conditions: conventional and nanotechnology-based methods for the control of mosquito vectors causing human health issues. Int. J. Environ. Res. Public Health16:3165. 10.3390/ijerph16173165
4
Akahata W. Ueno R. (2017). Zika Virus Virus Like Particle. Available online at: https://patents.google.com/patent/WO2017150683A1/en (accessed November 14, 2018).
5
Al-Dhabi N. A. Valan Arasu M. (2018). Environmentally-friendly green approach for the production of zinc oxide nanoparticles and their anti-fungal, ovicidal, and larvicidal properties. Nanomaterials8:500. 10.3390/nano8070500
6
Alharbi N. S. Govindarajan M. Kadaikunnan S. Khaled J. M. Almanaa T. N. Alyahya S. A. et al . (2018). Nanosilver crystals capped with Bauhinia acuminata phytochemicals as new antimicrobials and mosquito larvicides. J. Trace Elem. Med. Biol.50, 146–153. 10.1016/j.jtemb.2018.06.016
7
Ali Z. A. Roslan M. A. Yahya R. Wan Sulaiman W. Y. Puteh R. (2017). Eco-friendly synthesis of silver nanoparticles and its larvicidal property against fourth instar larvae of Aedes aegypti. IET Nanobiotechnol.11, 152–156. 10.1049/iet-nbt.2015.0123
8
Ariffin S. A. B. Adam T. Hashim U. Faridah Sfaridah S. Zamri I. Uda M. N. A. (2013). Plant diseases detection using nanowire as biosensor transducer. Adv. Mat. Res.832, 113–117. 10.4028/www.scientific.net/AMR.832.113
9
Awad M. A. G. Hendi A. A. Wagealla M. A. E. Ortashi K. M. O. Virk P. (2016). Synthesis of Nanoparticles of Metals and Metal Oxides Using Plant Seed Extract. Available online at: https://patents.google.com/patent/US9428399B1/en?q=green+sinthesis&q=%22silver+nanoparticle%22&oq=green+sinthesis++%22silver+nanoparticle%22+ (accessed October 18, 2018).
10
Aytur T. Foley J. Anwar M. Boser B. Harris E. Beatty P. R. (2006). A novel magnetic bead bioassay platform using a microchip-based sensor for infectious disease diagnosis. J. Immunol. Methods314, 21–29. 10.1016/j.jim.2006.05.006
11
Baeumner A. J. Kwakye S. D. S. Goral V. N. Zaytseva N. V. (2005). Microfluidic Biosensor and Methods of Use. Available online at: https://patents.google.com/patent/WO2005084404A2/en?oq=WO2005084404A2 (accessed November 14, 2018).
12
Balaji A. P. B. Ashu A. Manigandan S. Sastry T. P. Mukherjee A. Chandrasekaran N. (2017). Polymeric nanoencapsulation of insect repellent: Evaluation of its bioefficacy on Culex quinquefasciatus mosquito population and effective impregnation onto cotton fabrics for insect repellent clothing. J. King Saud University29, 517–527. 10.1016/j.jksus.2016.12.005
13
Balasubramani S. Rajendhiran T. Moola A. K. Diana R. K. B. (2017). Development of nanoemulsion from Vitex negundo L. essential oil and their efficacy of antioxidant, antimicrobial and larvicidal activities (Aedes aegypti L.). Environ. Sci. Pollution Res.24, 15125–15133. 10.1007/s11356-017-9118-y
14
Barradas T. Perdiz Senna J. Ricci Júnior E. Regina Elias Mansur C. (2016). Polymer-based drug delivery systems applied to insects repellents devices: a review. Curr. Drug Deliv.13, 221–235. 10.2174/1567201813666151207110515
15
Barrett T. W. Gautam S. Mitra R. Thorne J. (2018). Nano-Field Electrical Sensor. U.S. Patent No 20180067107A1. Available online at: https://patents.google.com/patent/US20180067107A1/en?q=Nano-field&q=electrical&q=Sensor&q=Biomarkes&q=targets&q=analytes&q=determining&q=impedance&q=bodily+fluid&q=nanoporous+membrane&oq=Nano-field+electrical+Sensor+for+Biomarkes+and+other+targets+analytes+by+determining+impedance+in+bodily+fluid+on+nanoporous+membrane (accessed February 12, 2020).
16
Basso C. R. Tozato C. C. Crulhas B. P. Castro G. R. Junior J. P. A. Pedrosa V. A. (2018). An easy way to detect dengue virus using nanoparticle-antibody conjugates. Virology513, 85–90. 10.1016/j.virol.2017.10.001
17
Batool K. Alam I. Wu S. Liu W. Zhao G. Chen M. et al . (2018). Transcriptomic analysis of aedes aegypti in response to mosquitocidal Bacillus thuringiensis LLP29 toxin. Sci. Rep.8:12650. 10.1038/s41598-018-30741-x
18
Beech R. WHITE E. Katchman B. (2018). Biosensor Disease and Infection Screening. Available online at: https://patents.google.com/patent/WO2018026931A1/en?oq=WO2018026931A1 (accessed November 14, 2018).
19
Benelli G. (2016). Plant-mediated biosynthesis of nanoparticles as an emerging tool against mosquitoes of medical and veterinary importance: a review. Parasitol. Res.115, 23–34. 10.1007/s00436-015-4800-9
20
Benelli G. Caselli A. Canale A. (2017). Nanoparticles for mosquito control: challenges and constraints. J. King Saud Univers.29, 424–435. 10.1016/j.jksus.2016.08.006
21
Benelli G. Jeffries C. L. Walker T. (2016). Biological control of mosquito vectors: past, present, and future. Insects7:52. 10.3390/insects7040052
22
Benelli G. Maggi F. Pavela R. Murugan K. Govindarajan M. Vaseeharan B. et al . (2018a). Mosquito control with green nanopesticides: towards the one health approach? A review of non-target effects. Environ. Sci. Pollut. Res.25, 10184–10206.
23
Benelli G. Maggi F. Pavela R. Murugan K. Govindarajan M. Vaseeharan B. et al . (2018b). Mosquito control with green nanopesticides: towards the one health approach? A review of non-target effects. Environ. Sci. Pollut. Res. Int.25, 10184–10206. 10.1007/s11356-017-9752-4
24
Benelli G. Mehlhorn H. (2016). Declining malaria, rising of dengue and Zika virus: insights for mosquito vector control. Parasitol. Res.115, 1747–1754. 10.1007/s00436-016-4971-z
25
Berini P. S. J. Wong W. R. (2018). Long-Range Surface Plasmon-Polariton Biosensor. Available online at: https://patents.google.com/patent/WO2018090125A1/fr?oq=WO2018090125A1+[Accessed+November+14%2c+2018 (accessed January 15, 2020).
26
Bhuvaneswari R. Xavier R. J. Arumugam M. (2016). Larvicidal property of green synthesized silver nanoparticles against vector mosquitoes (Anopheles stephensi and Aedes aegypti). J. King Saud Univers. Sci.28, 318–323. 10.1016/j.jksus.2015.10.006
27
Bosak A. Saraf N. Willenberg A. Kwan M. W. C. Alto B. W. Jackson G. W. et al (2019). Aptamer-gold nanoparticle conjugates for the colorimetric detection of arboviruses and vector mosquito species. RSC Adv.9, 23752–23763. 10.1039/C9RA02089F
28
Braga L. Marini B. Ippodrino R. (2015). Biosensors for the Detection of Infection and Associated Maladies. Available online at: https://patents.google.com/patent/WO2015114506A3/en (accessed November 14, 2018).
29
Brister M. Neale P. V. Saint S. Petisce J. R. McGee T. F. Codd D. S. et al . (2012). Transcutaneous Analyte Sensor. Available online at: https://patents.google.com/patent/US8229534B2/en (accessed November 14, 2018).
30
Cabarcos E. J. L. González D. M. Laurenti M. Cristóbal P. A. Retama J. R. (2017). Biosensor for the Detection of Nucleic Acids, Preparation Method and Use. Available online at: https://patents.google.com/patent/ES2580138A1/en (accessed November 14, 2018).
31
Carrell C. Kava A. Nguyen M. Menger R. Munshi Z. Call Z. et al . (2019). Beyond the lateral flow assay: a review of paper-based microfluidics. Microelectron. Eng.206, 45–54. 10.1016/j.mee.2018.12.002
32
Cecchetto J. Fernandes F. C. B. Lopes R. Bueno P. R. (2017). The capacitive sensing of NS1 Flavivirus biomarker. Biosens. Bioelectron.87, 949–956. 10.1016/j.bios.2016.08.097
33
Dias A. C. M. S. Gomes-Filho S. L. R. Silva M. M. S. Dutra R. F. (2013). A sensor tip based on carbon nanotube-ink printed electrode for the dengue virus NS1 protein. Biosens. Bioelectron.44, 216–221. 10.1016/j.bios.2012.12.033
34
Dinda S. C. (2018). “Bioaccumulation and toxic profiling of nanostructured particles and materials,” in Unraveling the Safety Profile of Nanoscale Particles and Materials - From Biomedical to Environmental Applications, eds de Castro GomesA. F. (University of Minho). 10.5772/intechopen.74802
35
Durán N. Islan G. A. Durán M. Castro G. R. (2016). Nanobiotechnology Solutions against Aedes aegypti. J. Braz. Chem. Soc.27, 1139–1149. 10.5935/0103-5053.20160122
36
Eboigbodin K. E. Brummer M. Ojalehto T. Hoser M. (2016). Rapid molecular diagnostic test for Zika virus with low demands on sample preparation and instrumentation. Diagn. Microbiol. Infect. Dis.86, 369–371. 10.1016/j.diagmicrobio.2016.08.027
37
Elumalai D. Hemavathi M. Deepaa C. V. Kaleena P. K. (2017). Evaluation of phytosynthesised silver nanoparticles from leaf extracts of Leucas aspera and Hyptis suaveolens and their larvicidal activity against malaria, dengue and filariasis vectors. Parasite Epidemiol. Control2, 15–26. 10.1016/j.parepi.2017.09.001
38
Eyupoglu S. Kut D. Girisgin A. O. Eyupoglu C. Ozuicli M. Dayioglu H. et al . (2018). Investigation of the bee-repellent properties of cotton fabrics treated with microencapsulated essential oils. Textile Res. J.89, 1417–1435. 10.1177/0040517518773370
39
Ferreira T. P. Haddi K. Corrêa R. F. T. Zapata V. L. B. Piau T. B. Souza L. F. N. et al . (2019). Prolonged mosquitocidal activity of Siparuna guianensis essential oil encapsulated in chitosan nanoparticles. PLoS Negl. Trop. Dis.13:e0007624. 10.1371/journal.pntd.0007624
40
Fletcher S. J. Milligan A. S. (2009). Auto-Feedback Loop Biosensor. Available online at: https://patents.google.com/patent/WO2009152566A1/en?oq=WO2009152566A1 (accessed November 14, 2018).
41
Forgearini J. C. Michalowski C. B. Assumpção E. Pohlmann A. R. Guterres S. S. (2016). Development of an insect repellent spray for textile based on permethrin-loaded lipid-core nanocapsules. J. Nanosci. Nanotechnol.16, 1301–1309. 10.1166/jnn.2016.11665
42
Fraceto L. F. Barbosa M. C. M. Oliveira J. L. Campos E. V. R. (2018). Sistemas Nanoestruturados E Seu Uso. Available online at: https://patents.google.com/patent/WO2017143421A1/pt?oq=repelentes+aedes+aegypti (accessed February 12, 2020).
43
Galvão J. G. Cerpe P. Santos D. A. Gonsalves J. K. Santos A. J. Nunes R. K. et al . (2018). Lippia gracilis essential oil in β-cyclodextrin inclusion complexes: an environmentally safe formulation to control Aedes aegypti larvae: Lippia gracilis essential oil in β-cyclodextrin inclusion complexes. Pest Manag. Sci.75, 452–459. 10.1002/ps.5138
44
García A. A. Franco L. S. Pirez-Gomez M. A. Pech-Pacheco J. L. Mendez-Galvan J. F. Machain-Williams C. et al . (2017). Feasibility study of an optical caustic plasmonic light scattering sensor for human serum anti-dengue protein E antibody detection. Diagnostics7:47. 10.3390/diagnostics7030047
45
Gomes G. M. Bigon J. P. Montoro F. E. Lona L. M. F. (2018). Encapsulation of N,N-diethyl- meta-toluamide (DEET) via miniemulsion polymerization for temperature controlled release: encapsulation of N,N-diethyl- meta-toluamide (DEET) via miniemulsion polymerization for temperature controlled release. J. Appl. Polym. Sci.139:47139. 10.1002/app.47139
46
Govindarajan M. Vijayan P. Kadaikunnan S. Alharbi N. S. Benelli G. (2016). One-pot biogenic fabrication of silver nanocrystals using Quisqualis indica: effectiveness on malaria and Zika virus mosquito vectors, and impact on non-target aquatic organisms. J. Photochem. Photobiol. Biol.162, 646–655. 10.1016/j.jphotobiol.2016.07.036
47
Hameed A. Fatima G. R. Malik K. Muqadas A. Fazal-ur-Rehman M. (2019). Scope of nanotechnology in cosmetics: dermatology and skin care products. J. Med. Chem. Sci.2, 9–16. 10.26655/jmchemsci.2019.6.2
48
Hao J. Yang M. Tsang M.-K. Ye W. (2018). Heterogeneous Microarray Based Hybrid Upconversion Nanoprobe/Nanoporous Membrane System. U.S. Patent No 20180246084. Available online at: https://patents.google.com/patent/US20180246084A1/en?oq=US20180246084+-+Heterogeneous+microarray+based+hybrid+upconversion+nanoprobe%2fnanoporous+membrane+system (accessed February 12, 2020).
49
Hoag G. E. Collins J. B. Varma R. S. Nadagouda M. N. (2011). Green Synthesis of Nanometals Using Plant Extracts and Use Thereof. Available online at: https://patents.google.com/patent/US8057682B2/en?q=green+sinthesis&q=%22silver+nanoparticle%22&oq=green+sinthesis++%22silver+nanoparticle%22+ (accessed October 18, 2018).
50
Hua S. de Matos M. B. C. Metselaar J. M. Storm G. (2018). Current trends and challenges in the clinical translation of nanoparticulate nanomedicines: pathways for translational development and commercialization. Front. Pharmacol.9:14. 10.3389/fphar.2018.00790
51
Huang S. Li C. Lin B. Qin J. (2010). Microvalve and micropump controlled shuttle flow microfluidic device for rapid DNA hybridization. Lab Chip10, 2925–2931. 10.1039/c005227b
52
Ishwarya R. Vaseeharan B. Anuradha R. Rekha R. Govindarajan M. Alharbi N. S. et al . (2017). Eco-friendly fabrication of Ag nanostructures using the seed extract of Pedalium murex, an ancient Indian medicinal plant: histopathological effects on the Zika virus vector Aedes aegypti and inhibition of biofilm-forming pathogenic bacteria. J. Photochem. Photobiol. B Biol.174, 133–143. 10.1016/j.jphotobiol.2017.07.026
53
Ishwarya R. Vaseeharan B. Kalyani S. Banumathi B. Govindarajan M. Alharbi N. S. et al . (2018). Facile green synthesis of zinc oxide nanoparticles using Ulva lactuca seaweed extract and evaluation of their photocatalytic, antibiofilm and insecticidal activity. J. Photochem. Photobiol. Biol.178, 249–258. 10.1016/j.jphotobiol.2017.11.006
54
Iswardy E. Tsai T.-C. Cheng I.-F. Ho T.-C. Perng G. C. Chang H.-C. (2017). A bead-based immunofluorescence-assay on a microfluidic dielectrophoresis platform for rapid dengue virus detection. Biosens. Bioelectron.95, 174–180. 10.1016/j.bios.2017.04.011
55
Jeevanandam J. Barhoum A. Chan Y. S. Dufresne A. Danquah M. K. (2018). Review on nanoparticles and nanostructured materials: history, sources, toxicity and regulations. Beilstein J. Nanotechnol.9, 1050–1074. 10.3762/bjnano.9.98
56
Jones A. A. D. Mi G. Webster T. J. (2019). A status report on FDA approval of medical devices containing nanostructured materials. Trends Biotechnol.37, 117–120. 10.1016/j.tibtech.2018.06.003
57
Kadam S. L. Yadav P. Bhutkar S. Patil V. D. Shukla P. G. Shanmuganathan K. (2019). Sustained release insect repellent microcapsules using modified cellulose nanofibers (mCNF) as pickering emulsifier. Colloids Surfaces A.582:123883. 10.1016/j.colsurfa.2019.123883
58
Kah M. Kookana R. S. Gogos A. Bucheli T. D. (2018a). A critical evaluation of nanopesticides and nanofertilizers against their conventional analogues. Nat. Nanotech13, 677–684. 10.1038/s41565-018-0131-1
59
Kah M. Walch H. Hofmann T. (2018b). Environmental fate of nanopesticides: durability, sorption and photodegradation of nanoformulated clothianidin. Environ. Sci.5, 882–889. 10.1039/C8EN00038G
60
Kaushik A. Nair M. (2018). Rapid Zika Virus Detection Using Nano-Enabled Electrochemical Sensing System. Available online at: https://patents.google.com/patent/US20180059099A1/en (accessed November 14, 2018).
61
Khater H. F. Selim A. M. Abouelella G. A. Abouelella N. A. Murugan K. Vaz N. P. et al . (2019). “Commercial mosquito repellents and their safety concerns,” in Malaria, eds KasengaF. H. (Malawi Adventist University). 10.5772/intechopen.87436
62
Koczula K. M. Gallotta A. (2016). Lateral flow assays. Essays Biochem.60, 111–120. 10.1042/EBC20150012
63
Kumar D. Kumar G. Agrawal V. (2018). Green synthesis of silver nanoparticles using Holarrhena antidysenterica (L.) Wall.bark extract and their larvicidal activity against dengue and filariasis vectors. Parasitol. Res.117, 377–389. 10.1007/s00436-017-5711-8
64
Kumar S. Lather V. Pandita D. (2015). Green synthesis of therapeutic nanoparticles: an expanding horizon. Nanomedicine10, 2451–2471. 10.2217/nnm.15.112
65
Kumar V. A. Ammani K. Jobina R. Subhaswaraj P. Siddhardha B. (2017). Photo-induced and phytomediated synthesis of silver nanoparticles using Derris trifoliata leaf extract and its larvicidal activity against Aedes aegypti. J. Photochem. Photobiol. B Biol.171, 1–8. 10.1016/j.jphotobiol.2017.04.022
66
Laury-Kleintop L. Rutner H. (2015). Biocoated Piezoelectric Biosensor Platform for Point-Of-Care Diagnostic Use. Available online at: https://patents.google.com/patent/US20150111765A1/en?oq=US20150111765A1 (accessed November 14, 2018).
67
Laux P. Tentschert J. Riebeling C. Braeuning A. Creutzenberg O. Epp A. et al . (2018). Nanomaterials: certain aspects of application, risk assessment and risk communication. Arch. Toxicol.92, 121–141. 10.1007/s00204-017-2144-1
68
Li J. Yang K. Wu Z. Li X. Duan Q. (2019). Nitrogen-doped porous carbon-based fluorescence sensor for the detection of ZIKV RNA sequences: fluorescence image analysis. Talanta205:120091. 10.1016/j.talanta.2019.06.091
69
Liu S. Liu L. Tan X. Meng B. (2013). Environmentally-Friendly Synthetic Method for Metal Nanoparticle. Available online at: https://patents.google.com/patent/CN103071808A/en?q=green&q=synthesis&q=nanoparticle&oq = +green+synthesis+nanoparticle (accessed October 18, 2018).
70
Lucia A. Toloza A. C. Guzmán E. Ortega F. Rubio R. G. (2017). Novel polymeric micelles for insect pest control: encapsulation of essential oil monoterpenes inside a triblock copolymer shell for head lice control. PeerJ5:e3171. 10.7717/peerj.3171
71
Magro M. Bramuzzo S. Baratella D. Ugolotti J. Zoppellaro G. Chemello G. et al . (2019). Self-assembly of chlorin-e6 on γ-Fe2O3 nanoparticles: application for larvicidal activity against Aedes aegypti. J. Photochem. Photobiol.194, 21–31. 10.1016/j.jphotobiol.2019.03.004
72
Marinero-Caceres E. E. Kuhn R. J. Stanciu L. A. Jin S.-A. (2017). Functionalized Particles for Label-Free DNA Impedimetric Biosensor for DNA and RNA Sensing. Available online at: https://patents.google.com/patent/US20170107565A1/en?oq=US+2017%2f0107565+A1 (accessed November 14, 2018).
73
Mazlan N.-F. Tan L. L. Karim N. H. A. Heng L. Y. Jamaluddin N. D. Yusof N. Y. M. et al . (2019). Acrylic-based genosensor utilizing metal salphen labeling approach for reflectometric dengue virus detection. Talanta198, 358–370. 10.1016/j.talanta.2019.02.036
74
Misni N. Nor Z. M. Ahmad R. (2017). Repellent effect of microencapsulated essential oil in lotion formulation against mosquito bites. J. Vector Borne Dis.54, 44–53.
75
Muñoz V. Buffa F. Molinari F. Hermida L. G. García J. J. Abraham G. A. (2019). Electrospun ethylcellulose-based nanofibrous mats with insect-repellent activity. Mater. Lett.253, 289–292. 10.1016/j.matlet.2019.06.091
76
Murugan K. Dinesh D. Paulpandi M. Althbyani A. D. M. Subramaniam J. Madhiyazhagan P. et al . (2015). Nanoparticles in the fight against mosquito-borne diseases: bioactivity of Bruguiera cylindrica-synthesized nanoparticles against dengue virus DEN-2 (in vitro) and its mosquito vector Aedes aegypti (Diptera: Culicidae). Parasitol. Res.114, 4349–4361. 10.1007/s00436-015-4676-8
77
Nakata Y. Röst G. (2015). Global analysis for spread of infectious diseases via transportation networks. J. Math. Biol.70, 1411–1456. 10.1007/s00285-014-0801-z
78
Nalini M. Lena M. Sumathi P. Sundaravadivelan C. (2017). Effect of phyto-synthesized silver nanoparticles on developmental stages of malaria vector, Anopheles stephensi and dengue vector, Aedes aegypti. Egyptian J. Basic Appl. Sci.4, 212–218. 10.1016/j.ejbas.2017.04.005
79
Nawaz M. H. Hayat A. Catanante G. Latif U. Marty J. L. (2018). Development of a portable and disposable NS1 based electrochemical immunosensor for early diagnosis of dengue virus. Anal. Chim. Acta1026, 1–7. 10.1016/j.aca.2018.04.032
80
Nicolini A. M. McCracken K. E. Yoon J.-Y. (2017a). Future developments in biosensors for field-ready Zika virus diagnostics. J. Biol. Eng.11:7.
81
Nicolini A. M. McCracken K. E. Yoon J.-Y. (2017b). Future developments in biosensors for field-ready Zika virus diagnostics. J. Biol. Eng.11:7. 10.1186/s13036-016-0046-z
82
Omar N. A. S. Fen Y. W. Abdullah J. Chik C. E. N. Mahdi M. A. (2018). Development of an optical sensor based on surface plasmon resonance phenomenon for diagnosis of dengue virus E-protein. Sens. Bio-Sensing Res.20, 16–21. 10.1016/j.sbsr.2018.06.001
83
Osanloo M. Arish J. Sereshti H. (2019). Developed methods for the preparation of electrospun nanofibers containing plant-derived oil or essential oil: a systematic review. Polym. Bull.10.1007/s00289-019-03042-0. [Epub ahead of print].
84
Parisi C. Vigani M. Rodríguez-Cerezo E. (2015). Agricultural nanotechnologies: what are the current possibilities?Nano Today10, 124–127. 10.1016/j.nantod.2014.09.009
85
Pashchenko O. Shelby T. Banerjee T. Santra S. (2018). A comparison of optical, electrochemical, magnetic, and colorimetric point-of-care biosensors for infectious disease diagnosis. ACS Infect. Dis.4, 1162–1178. 10.1021/acsinfecdis.8b00023
86
Patterson J. Sammon M. Garg M. (2016). Dengue, Zika and Chikungunya: emerging arboviruses in the new world. West. J. Emerg. Med.17, 671–679. 10.5811/westjem.2016.9.30904
87
Paula C. A. D. Dias A. M. R. Heffliger R. A. (2017). Sistema Nanométrico De Liberação Prolongada De Ativos Cosméticos e/ou Repelentes. Available online at: https://patents.google.com/patent/WO2017143421A1/pt?oq=WO2017143421A1 (accessed November 14, 2018).
88
Pessoa L. Z. da Duarte J. L. Ferreira R. M. dos Oliveira A. E. M. Cruz R. A. S. Faustino S. M. M. et al . (2018). Nanosuspension of quercetin: preparation, characterization and effects against Aedes aegypti larvae. Revista Brasileira de Farmacog.28, 618–625. 10.1016/j.bjp.2018.07.003
89
Pinto I. C. Cerqueira-Coutinho C. S. Santos E. P. Carmo F. A. Ricci-Junior E. (2017). Development and characterization of repellent formulations based on nanostructured hydrogels. Drug Dev. Ind. Pharm.43, 67–73. 10.1080/03639045.2016.1220564
90
Priye A. Bird S. W. Light Y. K. Ball C. S. Negrete O. A. Meagher R. J. (2017). A smartphone-based diagnostic platform for rapid detection of Zika, chikungunya, and dengue viruses. Sci. Rep.7:44778. 10.1038/srep44778
91
Ragavan V. V. Roy A. Rutner H. (2011). Integrated Microchip Sensor System for Detection of Infectious Agents. Available online at: https://patents.google.com/patent/US20110136262A1/en?oq=US+2011%2f0136262+A1 (accessed November 14, 2018).
92
Ramanibai R. Velayutham K. (2016). Synthesis of silver nanoparticles using 3,5-di-t-butyl-4-hydroxyanisole from Cynodon dactylon against Aedes aegypti and Culex quinquefasciatus. J. Asia Pac. Entomol.19, 603–609. 10.1016/j.aspen.2016.06.007
93
Ramar M. Manonmani P. Arumugam P. Kannan S. K. Erusan R. R. Murugan K. (2017). Nano-insecticidal formulations from essential oil (Ocimum sanctum) and fabricated in filter paper on adult of Aedes aegypti and Culex quinquefasciatus. J. Entomol. Zool. Stud.5, 1769–1774.
94
Rashid J. I. A. Yusof N. A. (2018). Laboratory diagnosis and potential application of nucleic acid biosensor approach for early detection of dengue virus infections. Biosci. Biotechnol. Res. Asia15, 245–255. 10.13005/bbra/2628
95
Rong Z. Wang Q. Sun N. Jia X. Wang K. Xiao R. et al . (2019). Smartphone-based fluorescent lateral flow immunoassay platform for highly sensitive point-of-care detection of Zika virus nonstructural protein 1. Anal. Chim. Acta1055, 140–147. 10.1016/j.aca.2018.12.043
96
Roni M. Murugan K. Panneerselvam C. Subramaniam J. Nicoletti M. Madhiyazhagan P. et al . (2015). Characterization and biotoxicity of Hypnea musciformis-synthesized silver nanoparticles as potential eco-friendly control tool against Aedes aegypti and Plutella xylostella. Ecotoxicol. Environ. Saf.121, 31–38. 10.1016/j.ecoenv.2015.07.005
97
Sabalza M. Yasmin R. Barber C. A. Castro T. Malamud D. Kim B. J. et al . (2018). Detection of Zika virus using reverse-transcription LAMP coupled with reverse dot blot analysis in saliva. PLoS ONE13:e0192398. 10.1371/journal.pone.0192398
98
Sajid M. Ilyas M. Basheer C. Tariq M. Daud M. Baig N. et al . (2015). Impact of nanoparticles on human and environment: review of toxicity factors, exposures, control strategies, and future prospects. Environ. Sci. Pollut Res. Int.22, 4122–4143. 10.1007/s11356-014-3994-1
99
Sánchez-Purrà M. Carré-Camps M. de Puig H. Bosch I. Gehrke L. Hamad-Schifferli K. (2017). Surface-enhanced raman spectroscopy-based sandwich immunoassays for multiplexed detection of Zika and Dengue viral biomarkers. ACS Infect Dis.3, 767–776. 10.1021/acsinfecdis.7b00110
100
Schnell-Inderst P. Hunger T. Conrads-Frank A. Arvandi M. Siebert U. (2018). Recommendations for primary studies evaluating therapeutic medical devices were identified and systematically reported through reviewing existing guidance. J. Clin. Epidemiol.94, 46–58. 10.1016/j.jclinepi.2017.10.007
101
Sethi R. S. (1994). Transducer aspects of biosensors. Biosens. Bioelectr.9, 243–264. 10.1016/0956-5663(94)80127-4
102
Sharma D. H. K. (2019). Formulation and evaluation of controlled release herbal mosquito repellent gel containing encapsulated essential oils obtained from natural sources indigenous to Northeast India. Asian J. Pharmaceut.13. 10.22377/ajp.v13i01.3005
103
Silva L. S. Mar J. M. Azevedo S. G. Rabelo M. S. Bezerra J. A. Campelo P. H. et al . (2019). Encapsulation of Piper aduncum and Piper hispidinervum essential oils in gelatin nanoparticles: a possible sustainable control tool of Aedes aegypti, Tetranychus urticae and Cerataphis lataniae. J. Sci. Food Agric.99, 685–695. 10.1002/jsfa.9233
104
Singhal C. Dubey A. Mathur A. Pundir C. S. Narang J. (2018). Paper based DNA biosensor for detection of chikungunya virus using gold shells coated magnetic nanocubes. Proc. Biochem.74, 35–42. 10.1016/j.procbio.2018.08.020
105
Sintubin L. Verstraete W. Boon N. (2012). Biologically produced nanosilver: current state and future perspectives. Biotechnol. Bioeng.109, 2422–2436. 10.1002/bit.24570
106
Soares S. Sousa J. Pais A. Vitorino C. (2018). Nanomedicine: principles, properties, and regulatory issues. Front. Chem. 6:360. 10.3389/fchem.2018.00360
107
Song J. Mauk M. G. Hackett B. A. Cherry S. Bau H. H. Liu C. (2016). Instrument-free point-of-care molecular detection of zika virus. Anal. Chem.88, 7289–7294. 10.1021/acs.analchem.6b01632
108
Soutschek E. Boecher O. Noelting C. (2019). Method for the Immunological Diagnosis of a Sample with a Potential Infection with an Arbovirus and Test Kits Suitable for This Purpose. U.S. Patent No 20190227065. Available online at: http://www.freepatentsonline.com/y2019/0227065.html (accessed February 12, 2020).
109
Starpharma (2016). Starpharma|VivaGel® active against Zika virus. Starpharma. Available online at: https://www.starpharma.com/news/281 (accessed December 31, 2019).
110
Suganya P. Vaseeharan B. Vijayakumar S. Balan B. Govindarajan M. Alharbi N. S. et al . (2017). Biopolymer zein-coated gold nanoparticles: synthesis, antibacterial potential, toxicity and histopathological effects against the Zika virus vector Aedes aegypti. J. Photochem. Photobiol.173, 404–411. 10.1016/j.jphotobiol.2017.06.004
111
Tancharoen C. Sukjee W. Thepparit C. Jaimipuk T. Auewarakul P. Thitithanyanont A. et al . (2019). Electrochemical biosensor based on surface imprinting for Zika virus detection in serum. ACS Sens.4, 69–75. 10.1021/acssensors.8b00885
112
Tavares M. da Silva M. R. M. de Oliveira de Siqueira L. B. Rodrigues R. A. S. Bodjolle-d'Almeida L. dos Santos E. P. et al . (2018). Trends in insect repellent formulations: a review. Int. J. Pharm.539, 190–209. 10.1016/j.ijpharm.2018.01.046
113
Thanh B. T. Van Sau N. Ju H. Bashir M. J. K. Jun H. K. Phan T. B. et al . (2019). Immobilization of protein a on monodisperse magnetic nanoparticles for biomedical applications. J. Nanomater. 2019:2182471. 10.1155/2019/2182471
114
Theillet G. Rubens A. Foucault F. Dalbon P. Rozand C. Leparc-Goffart I. et al . (2018). Laser-cut paper-based device for the detection of dengue non-structural NS1 protein and specific IgM in human samples. Arch. Virol.163, 1757–1767. 10.1007/s00705-018-3776-z
115
Thomas J. (2019). Anti-Parasitic Compositions and Methods. Available online at: https://patents.google.com/patent/US20190160016A1/en (accessed January 6, 2020).
116
Tian B. Qiu Z. Ma J. Zardán Gómez de la Torre T. Johansson C. Svedlindh P. et al . (2016). Attomolar Zika virus oligonucleotide detection based on loop-mediated isothermal amplification and AC susceptometry. Biosens. Bioelectron.86, 420–425. 10.1016/j.bios.2016.06.085
117
Tripathy S. Krishna Vanjari S. R. Singh V. Swaminathan S. Singh S. G. (2017). Electrospun manganese (III) oxide nanofiber based electrochemical DNA-nanobiosensor for zeptomolar detection of dengue consensus primer. Biosens. Bioelectr.90, 378–387. 10.1016/j.bios.2016.12.008
118
Udayabhanu J. Kannan V. Tiwari M. Natesan G. Giovanni B. Perumal V. (2018). Nanotitania crystals induced efficient photocatalytic color degradation, antimicrobial and larvicidal activity. J. Photochem. Photobiol. B Biol.178, 496–504. 10.1016/j.jphotobiol.2017.12.005
119
Veerakumar K. Govindarajan M. Rajeswary M. Muthukumaran U. (2014). Mosquito larvicidal properties of silver nanoparticles synthesized using Heliotropium indicum (Boraginaceae) against Aedes aegypti, Anopheles stephensi, and Culex quinquefasciatus (Diptera: Culicidae). Parasitol. Res.113, 2363–2373. 10.1007/s00436-014-3895-8
120
Ventola C. L. (2017). Progress in nanomedicine: approved and investigational nanodrugs. P T42, 742–755.
121
Vinayagam S. Rajaiah P. Mukherjee A. Natarajan C. (2018). DNA-triangular silver nanoparticles nanoprobe for the detection of dengue virus distinguishing serotype. Spectrochim. Acta A Mol. Biomol. Spectrosc.202, 346–351. 10.1016/j.saa.2018.05.047
122
Vo-Dinh T. (2004). Multifunctional and Multispectral Biosensor Devices and Methods of Use. Available online at: https://patents.google.com/patent/US6743581B1/en (accessed November 14, 2018).
123
Wasik D. Mulchandani A. Yates M. V. (2017). A heparin-functionalized carbon nanotube-based affinity biosensor for dengue virus. Biosens. Bioelectron.91, 811–816. 10.1016/j.bios.2017.01.017
124
Werdin González J. O. Jesser E. N. Yeguerman C. A. Ferrero A. A. Fernández Band B. (2017). Polymer nanoparticles containing essential oils: new options for mosquito control. Environ. Sci. Pollut. Res.24, 17006–17015. 10.1007/s11356-017-9327-4
125
Wilder-Smith A. Gubler D. J. Weaver S. C. Monath T. P. Heymann D. L. Scott T. W. (2017). Epidemic arboviral diseases: priorities for research and public health. Lancet Infect. Dis.17:e101–e106. 10.1016/S1473-3099(16)30518-7
126
Willenberg B. J. Seal S. (2018). Passive Insect Surveillance Sensor Device. Available online at: https://patents.google.com/patent/WO2017027677A1/en?q=Passive&q=Insect&q=Surveillance&q=Sensor&q=Device&oq=+Passive+Insect+Surveillance+Sensor+Device (accessed February 11, 2020).
127
Wolkowicz R. (2018). Compositions and Cell-Based Methods for Monitoring the Activity of a Dengue Virus Protease. Available online at: https://patents.google.com/patent/US10006077B2/en?oq=US10006077B2 (accessed November 14, 2018).
128
Yaren O. Alto B. W. Gangodkar P. V. Ranade S. R. Patil K. N. Bradley K. M. et al . (2017). Point of sampling detection of Zika virus within a multiplexed kit capable of detecting dengue and chikungunya. BMC Infect. Dis.17:293. 10.1186/s12879-017-2382-0
129
Yen C.-W. de Puig H. Tam J. Gómez-Márquez J. Bosch I. Hamad-Schifferli K. et al . (2015). Multicolored silver nanoparticles for multiplexed disease diagnostics: distinguishing dengue, yellow fever, and ebola viruses. Lab Chip15, 1638–1641. 10.1039/C5LC00055F
130
Yrad F. M. Castañares J. M. Alocilja E. C. (2019). Visual detection of dengue-1 RNA using gold nanoparticle-based lateral flow biosensor. Diagnostics9:74. 10.3390/diagnostics9030074
131
Yujia G, H. Wu Y. Chi D. (2015). Green Synthesis Method of Gold Nanoparticles. Available online at: https://patents.google.com/patent/CN105057692A/en?q=green&q=synthesis&q=nanoparticle&oq = +green+synthesis+nanoparticle (accessed October 18, 2018).
132
Yusoh S. N. Yaacob K. A. Alias N. N. (2019). Trapezoidal SiNWs array fabricated by AFM-LAO for Dengue virus DNA oligomer detection. Mater. Res. Express6:115005. 10.1088/2053-1591/ab3571
133
Yuzon Ma. K. Kim J.-H. Kim S. (2019). A novel paper-plastic microfluidic hybrid chip integrated with a lateral flow immunoassay for dengue nonstructural protein 1 antigen detection. BioChip J.13, 277–287. 10.1007/s13206-019-3305-5
134
Zhang B. Pinsky B. A. Ananta J. S. Zhao S. Arulkumar S. Wan H. et al . (2017). Diagnosis of Zika virus infection on a nanotechnology platform. Nat. Med.23, 548–550. 10.1038/nm.4302
135
Zhang Y. Chen W. Jing M. Liu S. Feng J. Wu H. et al . (2018). Self-assembled mixed micelle loaded with natural pyrethrins as an intelligent nano-insecticide with a novel temperature-responsive release mode. Chem. Eng. J.361, 1381–1391. 10.1016/j.cej.2018.10.132
Summary
Keywords
Aedes aegypti , vector control, biosensors, larvicides, arboviruses, nanobiotechnology
Citation
Campos EVR, de Oliveira JL, Abrantes DC, Rogério CB, Bueno C, Miranda VR, Monteiro RA and Fraceto LF (2020) Recent Developments in Nanotechnology for Detection and Control of Aedes aegypti-Borne Diseases. Front. Bioeng. Biotechnol. 8:102. doi: 10.3389/fbioe.2020.00102
Received
27 November 2019
Accepted
03 February 2020
Published
20 February 2020
Volume
8 - 2020
Edited by
Eden Morales-Narváez, Centro de Investigaciones en Optica, Mexico
Reviewed by
Abdelrahim H. A. Hassan, Beni-Suef University, Egypt; Jie Liu, South China Agricultural University, China
Updates
Copyright
© 2020 Campos, de Oliveira, Abrantes, Rogério, Bueno, Miranda, Monteiro and Fraceto.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Leonardo Fernandes Fraceto leonardo.fraceto@unesp.br
This article was submitted to Nanobiotechnology, a section of the journal Frontiers in Bioengineering and Biotechnology
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.