- 1Department of Clinical and Biological Sciences, University of Turin, Turin, Italy
- 2Neuroscience Institute Cavalieri Ottolenghi, University of Turin, Turin, Italy
Peripheral nerve injury treatment is a relevant problem because of nerve lesion high incidence and because of unsatisfactory regeneration after severe injuries, thus resulting in a reduced patient’s life quality. To repair severe nerve injuries characterized by substance loss and to improve the regeneration outcome at both motor and sensory level, different strategies have been investigated. Although autograft remains the gold standard technique, a growing number of research articles concerning nerve conduit use has been reported in the last years. Nerve conduits aim to overcome autograft disadvantages, but they must satisfy some requirements to be suitable for nerve repair. A universal ideal conduit does not exist, since conduit properties have to be evaluated case by case; nevertheless, because of their high biocompatibility and biodegradability, natural-based biomaterials have great potentiality to be used to produce nerve guides. Although they share many characteristics with synthetic biomaterials, natural-based biomaterials should also be preferable because of their extraction sources; indeed, these biomaterials are obtained from different renewable sources or food waste, thus reducing environmental impact and enhancing sustainability in comparison to synthetic ones. This review reports the strengths and weaknesses of natural-based biomaterials used for manufacturing peripheral nerve conduits, analyzing the interactions between natural-based biomaterials and biological environment. Particular attention was paid to the description of the preclinical outcome of nerve regeneration in injury repaired with the different natural-based conduits.
Introduction
Peripheral nerve repair outcome after an injury is often poor, indeed it has been estimated that only 3% of patients recover sensibility while the motor function is recovered by less than 25% of patients (Houshyar et al., 2019).
Peripheral nerve repair and the consequent recovery of sensory and/or motor function is a great challenge for both researchers in biomedical sciences and bioengineering, but also for clinicians. Peripheral nerve injuries can be repaired through surgical techniques, however, when a nerve injury with loss of substance (>5 mm) occurs, its repair involves the use of grafts of different origin, among which nerve guidance conduits (NGC) (Meek and Coert, 2008) which reduce myofibroblast infiltration and scar tissue formation, and physically support regenerating nerves (Lundborg, 2000).
In the last 10 years, a progressive increase in the number of publications concerning the use of new artificial conduits to promote peripheral nerve regeneration has been reported, demonstrating a greater interest in this field by researchers and clinicians. Indeed, there is an increase in the number of publications dealing with conduits for nerve regeneration over the years: a PubMed search, using the string “conduit OR tube OR (“Tissue Scaffolds”[Mesh]) AND “Nerve Regeneration”[Mesh],” delivered 409 results for the period 2000–2009 and 989 results for the period 2010–2019.
The use of conduits to bridge a nerve gap has shown promising results, but clinical applications are limited to the reconstruction of short nerve lesions (Wang and Sakiyama-Elbert, 2019). Indeed, different artificial nerve conduits are commercially available and approved by the FDA (US Food and Drug Administration) (Kehoe et al., 2012), but no implant is approved and available for 3.0cm or longer nerve defect, the injury length usually considered critical. The most frequently used FDA-approved natural-based biomaterials are collagen (NeuraGen®, Neuroflex®, NeuroMatrix®, RevolNerv®) and chitosan (Reaxon®), as recently accurately reviewed by Kornfeld et al. (2018). All marketed and FDA approved nerve conduits demonstrate satisfying recovery, but with some side effects or regeneration failure (Kornfeld et al., 2018). Therefore, further researches are necessary to develop new conduits that lead to better outcomes.
Clinical trials in the field of peripheral nerve regeneration must be preceded by long-lasting preclinical research, which usually starts with the manufacturing of a biomaterial with precise characteristics (Archibald et al., 1991; Hashimoto et al., 2002; Ahmed et al., 2003; Jansen et al., 2004; Sundback et al., 2004; Yang et al., 2007a; Xie et al., 2008; He et al., 2009). When finalized, this biomaterial is primarily tested on in vitro models to assess its biocompatibility, cytotoxicity, genotoxicity, the absence of toxic degradation products, the interaction between cells and the biomaterial, its degradation rate, cell proliferation upon the biomaterial and so on. In vitro assays are more easily reproducible and standardized, they also allow to reduce the number of animals used for in vivo experiments, following the “3R” philosophy (Reduce, Replace, and Refine), as declared in the document “Recognition and Alleviation of Pain in Laboratory Animals,” edited by the National Research Council (National Research Council (Us) Committee on Recognition, and Alleviation of Pain in Laboratory Animals., 2010).
Preliminary in vitro tests are usually performed before proceeding to in vivo tests on different animal models. For short gaps up to 15 mm mice and rats can be used; for longer defects, experimental models such as rabbit, pig, sheep, cat, dog or primate are generally adopted (Angius et al., 2012; Kornfeld et al., 2018). Most of in vivo models used to study biomaterials for NGCs are rats since this model is a compromise between mice, which allow performing nerve length-limited injuries, and larger animal models, the maintenance of which is more demanding and expensive, thus suggesting that they are more useful to test biomaterials in an advanced stage of NGC development. Indeed, if in vivo testing on rat fails, it is not recommended to test NGCs on larger animals.
In vivo, biomaterials are used as nerve guidance conduit (tubular conduit) or as conduit internal filler. In vivo tests allow to quantitatively assess nerve regeneration (i.e., myelin thickness, axon density and number, g-ratio and so on), functional recovery, evaluation of target re-innervation (Navarro, 2016), interactions between biomaterials and surrounding environment or interaction with re-growing tissues. In vivo tests must be performed to confirm the biocompatibility of the biomaterial, the non-toxicity of its degradation products, the scar formation and the absence of adverse immune response. Indeed, through in vitro assays, tissue reaction cannot be evaluated, in terms of local and systemic responses, because vascularization, extracellular matrix formation and oxygen supply are missing. Moreover, in vivo studies allow to evaluate long-term effects on the biomaterial under biological conditions: before clinical trial design, tests to evaluate the implanted biomaterial degradation rate must be carried out. When a device overcomes all preclinical tests, it is ready to be included in a clinical trial. Nevertheless, it is important to remember that animal models represent an approximation of human physiological and pathological processes (Talac et al., 2004; Angius et al., 2012; Kaplan et al., 2015). Moreover, it should also be kept in mind that not always clinical trials reach their completion or lead to satisfactory results. On the contrary, most of them fail during recruitment phases (stringency of inclusion/exclusion criteria) or in the follow-up phases (Carvalho et al., 2019). Thus, the commercial release of a device is a long-lasting, tiresome and expensive process. It is also important to remember that, beyond the research costs, biomaterial production and its release have costs; therefore, devices are often expensive and, for this reason, they have a reduced utilization in clinical practice.
In this review, the main characteristics of the ideal nerve conduit are summarized and discussed and the strengths and weaknesses of natural-based biomaterials used for nerve conduit manufacturing are reported. In particular, the description of preclinical outcomes of nerve regeneration after injury repair with natural-based conduits and the interactions between the biomaterial and regenerating tissue are the aim of this review.
Biomaterial Characteristics to Be Used as a Nerve Conduit
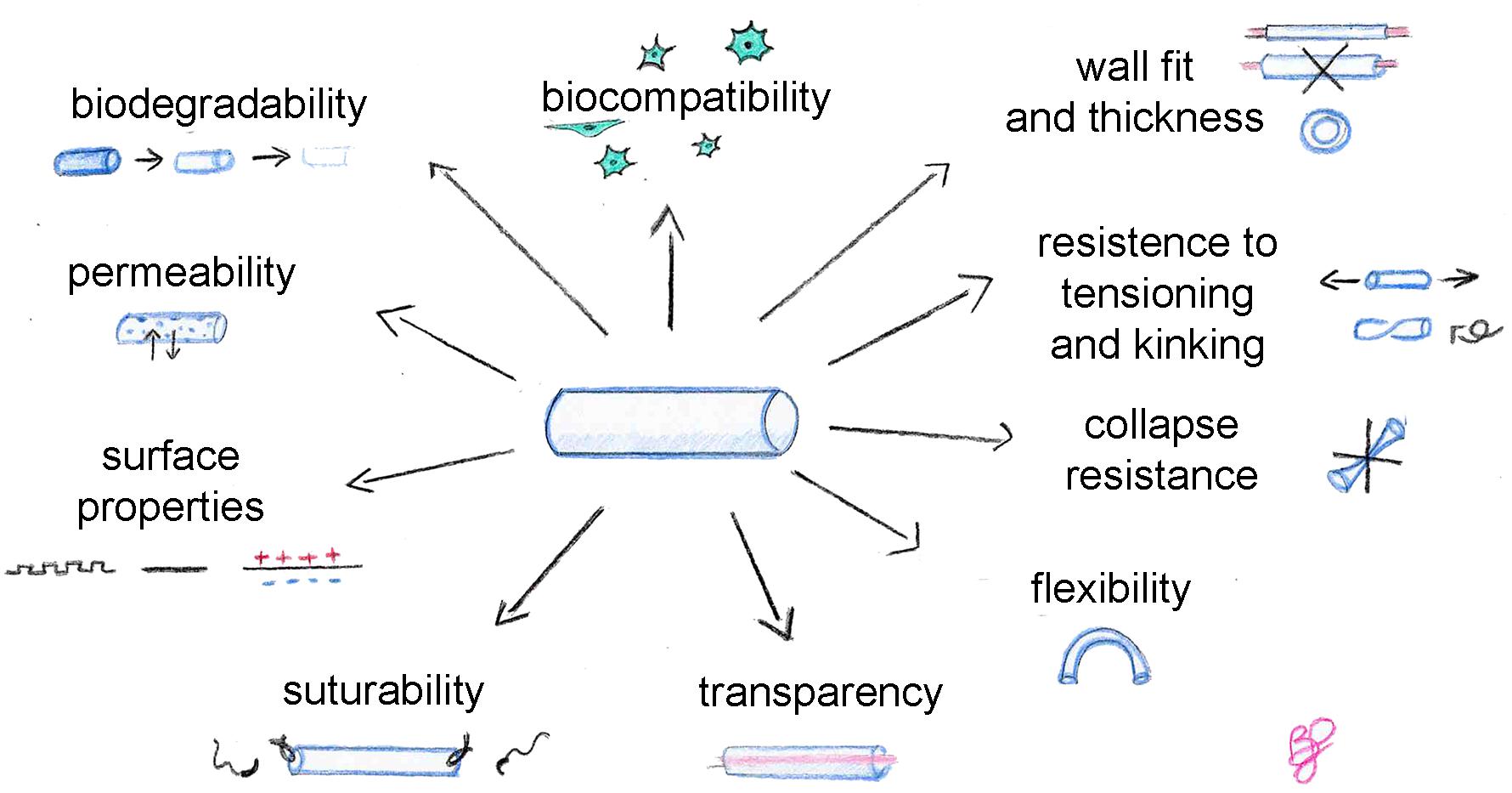
An ideal biomaterial, suitable for tissue engineering but also for nerve conduit production, must satisfy some requirements and find the right balance between different properties (Figure 1), such as biocompatibility, biodegradability, permeability, adequate biomechanical and surface properties. In addition, these specific physical features are recommended for conduits: flexibility, resistance to collapse and resistance to tension, adequate wall thickness, specific conduit diameter, and suturability. Transparency is not necessary, but it is appreciated by clinicians during surgeries.
Biocompatibility is assessed taking into account three parameters: (i) blood compatibility: the biomaterial in contact with the blood must not induce hemolysis or coagulation which can lead to thrombi formation; (ii) histocompatibility: the biomaterial must not induce side effects on the surrounding tissues, including scar formation, inflammation and any immune system response; (iii) mechanical compatibility: the biomaterial must present mechanical properties similar to the host tissue to avoid a local increase of tissue, reducing tissues natural/physiological mobility (Gu et al., 2011; Pinho et al., 2016).
Since passive mechanical forces influence the neuronal healing processes, it must be considered that a defined amount of mechanical stimulus helps nerve regeneration (Pfister et al., 2006; Loverde et al., 2011; Loverde and Pfister, 2015), but an exaggerated stimulus has opposite effects. In particular, strain forces transferred by the inner layer of the NGCs to the neuronal cell membranes must be lower than a certain level of strain considering that the mean strain resistance of cell membranes of sensory neurons [∼3,000 Pascal ([Pa)] and motor neurons (∼500 Pa) are different (Koch et al., 2012; Spedden et al., 2012; Moore and Staii, 2018) and that a higher mechanical force can damage the cell membranes activating cell apoptosis pathways. Also, the outer layer must mimic the resistance of the connective structures of the peripheral nerves which have a mean resistance of 580,000 Pa (Borschel et al., 2003; Giannessi et al., 2019).
The degradation kinetics of the substrate must be compatible with the regeneration timing of the specific tissue for which it is used. Ideally, the scaffold should be integrated into the surrounding tissue and gradually be replaced by the extracellular matrix and by the cells to restore the functionality of the tissue (Bačáková et al., 2004; Deng et al., 2008).
Conduit degradation should accommodate nerve regeneration timing: slow enough to maintain its strength and shape to guide and support axonal growth and not too quick to allow scar tissue formation around the re-growing axons between the two stumps (Chrząszcz et al., 2018; Houshyar et al., 2019). For example, too fast degradation of the conduit can cause an inflammatory response inducing a suboptimal nerve regeneration (Yu et al., 2011). Ideally, conduits should be fully resorbed when nerve repair is completed. Thus, the ideal degradation for a conduit should be faster at the proximal stump and gradually decreasing over distance with a slow degradation rate near the distal end (Reid et al., 2013).
Optimal degradation timing depends on defect length: longer nerve gaps require biomaterials with a longer degradation time, such as poly 3-hydroxybutyric acid (PHB), which is particularly appropriate for long-gap nerve injury repair. For a nerve gap of 10 mm, the axonal phase of nerve regeneration occurs around the third week of regeneration (Belkas et al., 2004; Carvalho et al., 2019). So, the conduit should ideally be significantly degraded after this phase, to mitigate entrapment-like symptoms and to abolish secondary surgeries required to remove the conduit, both are conditions commonly observed by using non-biodegradable conduits. Moreover, an ideal conduit should be semipermeable. Permeability is necessary for cell viability as it promotes the gas and nutrients exchanges and waste removal consisting of metabolites produced by the cells themselves (Ijkema-Paassen et al., 2004; de Ruiter et al., 2009; Liu and Hsu, 2020). Thus, an ideal scaffold for biomedical applications must supply a sufficient permeability, which also influences the ability to form fibrin matrices, useful during tissue regeneration processes to guide axons regeneration (She et al., 2008; Gu et al., 2011). Indeed, it has been shown that during the early stage of nerve regeneration, nerve wall permeability increases, and a similar behavior should be mimicked by the conduit (Rodríguez et al., 1999).
Conduit permeability increases with pore size (O’Brien et al., 2005): nerve conduits with large pores better support axonal growth in comparison with those displaying smaller pores (Clements et al., 2016; Liu et al., 2018). The optimal pore size range was 10–20 μm (Goulart et al., 2016), nevertheless, it should be higher than 4 μm and lower than 30 μm, thus allowing nutrients inflow and at the same time preventing extracellular matrix fibroblast incoming and growth factor outflow (Du and Jia, 2019).
In regards fibroblast ingrowth in the conduit during regeneration, it is needed to consider that different fibroblast types colonize the conduit: external fibroblast influx needs to be avoided, while nerve fibroblast migration from the two nerve stumps seems to have a positive role, as suggested by our recent paper (Fornasari et al., 2020). Nevertheless, the suggested 10–20 μm diameter seems too high, as it is known that cells can pass through smaller diameter pores (as shown in the Transwell migration assay, where cells pass through 8 μm pores), thus suggesting that probably <8 μm would be a better choice to allow nutrient exchange without undesired cell migration. An adequate permeability is important also to guarantee an adequate neurotrophic factor inflow inside the conduit, which is necessary for longer conduits since one common problem is the lack of neurotrophic factors required to support nerve regeneration. These characteristics should be tuned through conduit production methods.
A semipermeable nerve conduit is more effective to promote nerve regeneration when compared with low permeable or impermeable conduits (Rodríguez et al., 1999; Vleggeert-Lankamp et al., 2007; Dodla and Bellamkonda, 2008; Chiono and Tonda-Turo, 2015). Moreover, permeability is influenced by the hydrophilic properties of the material; moderate hydrophilicity of the material allows a better cell adhesion, compared to very high hydrophilicity or hydrophobicity (Liu et al., 2007).
Porosity affects the biomechanical/physical properties of the biomaterial, such as flexibility, resistance to collapse and tension, which are very important features considering the different biological and mechanical insults to which the conduit is subjected after in vivo implantation (Belkas et al., 2005). An adequate balance between conduit flexibility and stiffness should be achieved, as flexibility is a crucial requirement in clinical practice (de Ruiter et al., 2009; Gu et al., 2011). Indeed, digital nerve defects are the most frequent nerve injuries and flexible tubes are required for joint movements (Rasappan et al., 2018). Nevertheless, a certain mechanical resistance is required to avoid conduit collapse which could compromise nerve regeneration, since longer conduits are more subject to collapse or kinking. The collapse and/or kinking of the implant might compromise nerve regeneration too, by leading to nerve compression and ischemia (Pawelec et al., 2019).
Flexibility allows avoiding mechanical injuries of the surrounding tissues and the regenerating axons. Moreover, high rigidity of the implant may lead to nerve stump escape from the tube lumen and complicate the suturing processes of the device during implantation, indeed a not excessive rigidity allows an easy suturing procedure. Furthermore, the conduit should be resistant to tension to avoid tears from nerve tensioning during movements.
Surface properties, including surface functionalization, are not fundamental but should also be taken into account to enhance conduit performance and are important for the interaction between scaffold and cells (Ratner and Bryant, 2004). Surface chemistry alteration is an effective strategy to promote cell adhesion and neurite outgrowth (Oh et al., 2013; Sarker et al., 2018).
A large surface area promotes cell adhesion and proliferation, an oriented surface influences cell behavior (Ahmed et al., 2003; Jiang et al., 2010). Macro (>10 μm), micro (between 0.5 and 10 μm) and nano (<0.5 μm) roughness can determine a different adhesion pattern (Rajab et al., 2017; Carvalho et al., 2019). Nanostructured conduit internal surfaces could improve cell adhesion, while microstructured surfaces are useful to target regrowing axons toward the target organ. Thus, an internal microstructured surface could be useful for conduits to repair long defects, where neurotrophic factor diffusion is not enough to reach regrowing axons (Wang et al., 2001; Sun and Downes, 2009). The conduit surface is generally modified from smooth to rough in three ways: by adding grooves, holes or raised parts (Sun and Downes, 2009; Tonda-Turo et al., 2011a; Gnavi et al., 2015). Differences in nanoroughness of the same biomaterial can affect the NGC wettability (Yang et al., 2005; Sun and Downes, 2009); moreover, nanometric roughness positively influences cell adhesion as it mimics the extracellular matrix structure (Marshall et al., 2010) and enhances cell growth and cytoskeleton elongation (Huang et al., 2015). Microstructured scaffolds might be useful to mimic bigger structures, such as the bands of Büngner, encouraging axonal regrowth during peripheral nerve regeneration (Bozkurt et al., 2012; Gnavi et al., 2015). Thanks to several manufacturing processes like laser surface texturing, chemical substances, plasma treatment, electrospinning or use of a mold, changes in roughness can be made for most of the available biomaterials (Kim et al., 2016; Berkovitch et al., 2018; Chen et al., 2019).
Furthermore, surface charges can enhance the biological response in vivo. The electrical charge or conductivity of a material influences cell colonization; cells adhere to negatively charged substrates because of the presence of positive charges on the cell membrane surface (Lesný et al., 2006; Bacakova et al., 2011). Moreover, neurite outgrowth can be increased by electrically conducting polymers (Schmidt et al., 1997; Dadsetan et al., 2009; Jing et al., 2018).
Nerve regeneration outcome is also influenced by the nerve guide diameter, which has to match with the dimension of the proximal and distal stumps of the injured nerve. Conduits not fitting well with the nerve stumps might compromise nerve regeneration: smaller diameters might lead to chronic nerve compression, while a conduit with too large diameter allows the incoming of undesired cells and fibrous tissue formation, in addition to adverse growth factors outflow (Daly et al., 2012; Isaacs et al., 2014; Yi et al., 2019). Thanks to the physical and chemical properties of the biomaterials described, internal conduits diameter can be quite easily modified through conduit processing methods.
Also, the wall thickness must be considered during nerve guide design, because as reported by Ducker and Hayes there is a strong correlation between conduit wall thickness and neuroma formation (Ducker and Hayes, 1968). Wall thickness influences different characteristics such as permeability, but also mechanical properties like the flexibility and solidity of the conduit, thus it should be considered during the conduit design processes.
Conduit walls more than 0.8 mm thick reduce axonal growth (Rutkowski and Heath, 2002), and this reduction is attributed to permeability and porosity reduction, which are important for nerve regeneration. Indeed, in silico experiments suggest that a 0.6 mm wall thickness, with an 80% porosity, can be considered optimal for nerve regeneration (Kokai et al., 2009; Tonda-Turo et al., 2011a; Chiono and Tonda-Turo, 2015). Data obtained from in vitro and in vivo experiments on rat sciatic nerve injury model demonstrated that a wall thickness lower than 0.6 mm or higher than 0.8 mm is suboptimal and leads to controversial results (Rutkowski and Heath, 2002; Stang et al., 2009; Tonda-Turo et al., 2011a; Chiono and Tonda-Turo, 2015; Teuschl et al., 2015; Wang et al., 2017; Jahromi et al., 2020). Nevertheless, further studies are necessary to identify the more adequate thickness, since in literature conflictual in vivo results were published.
Wall thickness is also important since it is one of the parameters influencing conduit suturability. An ideal conduit could be easy to suture; the nerve guides must allow the suture needle to pass through the wall avoiding the escaping of the nerve stumps from the conduit lumen, but also they must be strong enough to allow the suture to bind the proximal and distal nerve stumps without detaching if subjected to tension forces during movement (Nectow et al., 2012).
Finally, transparency is a characteristic appreciated by surgeons because it allows the optimal positioning of nerve stump ends during nerve repair surgery. This characteristic is also useful during preclinical assays since it allows to directly observe in situ if nerve regeneration occurs (Gu et al., 2014).
Natural-Based vs. Synthetic Biomaterials
Biomaterials of natural origin are suitable for tissue engineering as they provide adhesion molecules, cell binding sites and are compatible with surrounding tissues. Because of their large availability, large quantities of natural-based biomaterials are accessible at reasonable prices (Dalamagkas et al., 2016), but their purification is less standardized in comparison to synthetic biomaterials. Thus, the problem with such natural biomaterials is the difficulty to find an easily accessible purified source for large-scale production. Nevertheless, some biopolymers overcome this problem, such as PHB, which is synthesized by bacteria in bioreactors (Koller, 2018), and chitosan, which is extracted from chitin, an abundant polysaccharide derived from shellfish waste. Indeed, it has been estimated that more than 10,000 tons are produced by shellfish waste each year: quantities, which could provide enough material to cover market demands for tissue engineering (Hamed et al., 2016).
Natural biomaterial advantages derive from the fact that, after a proper purification, these biomaterials generally do not determine unexpected or unwelcome immune-mediated responses. They provide better biocompatibility in comparison to synthetic ones: usually, these biomaterials are biodegradable and integrate with the surrounding tissues. Their degradation products, compared to synthetic ones, are less cytotoxic and more biocompatible as well as more easily degraded and metabolized by the host tissues (Stoppel et al., 2015).
Natural polymers, thanks to their excellent biocompatibility and bioactive properties, allow better interactions between the scaffold and the tissue, which improve cell adhesion, proliferation and tissue regeneration (Arslantunali et al., 2014; de Queiroz Antonino et al., 2017).
However, natural-based biomaterials present some limitations, such as the need for extensive purification or chemical heterogeneity, which leads to variable mechanical properties like degradation rate. Besides, natural polymers usually possess poor mechanical properties and batch-to-batch variability which have restricted their widespread use (Schmidt and Leach, 2003).
On the other hand, synthetic biomaterials own more tunable mechanical properties, which can be obtained through small changes during the manufacturing process (Dalamagkas et al., 2016). This characteristic, which determines synthetic biomaterial high reproducibility, together with the easier access to large-scale production, makes them an appealing source. For many years the lack of binding sites and the reduced biocompatibility of synthetic biomaterials were great concerns; anyhow, currently, tissue engineering goes beyond the problem through the design of scaffolds enriched with nanostructured surface topography, which offer binding sites to cells (Singh et al., 2014). Scaffold surface topographical modifications influence cell growth, migration and adhesion by affecting actin cytoskeleton reorganization, focal adhesion formation and distribution and lamellipodia and filopodia formation (Mattila and Lappalainen, 2008; Harvey et al., 2013; Rahmany and Van Dyke, 2013; Zhao et al., 2020). Moreover, it has been demonstrated that topographic cues improve axonal growth and could minimize atrophy of innervated organ distal to the lesion resulting in nerve functional recovery (Chiono and Tonda-Turo, 2015).
Natural-Based Biomaterials
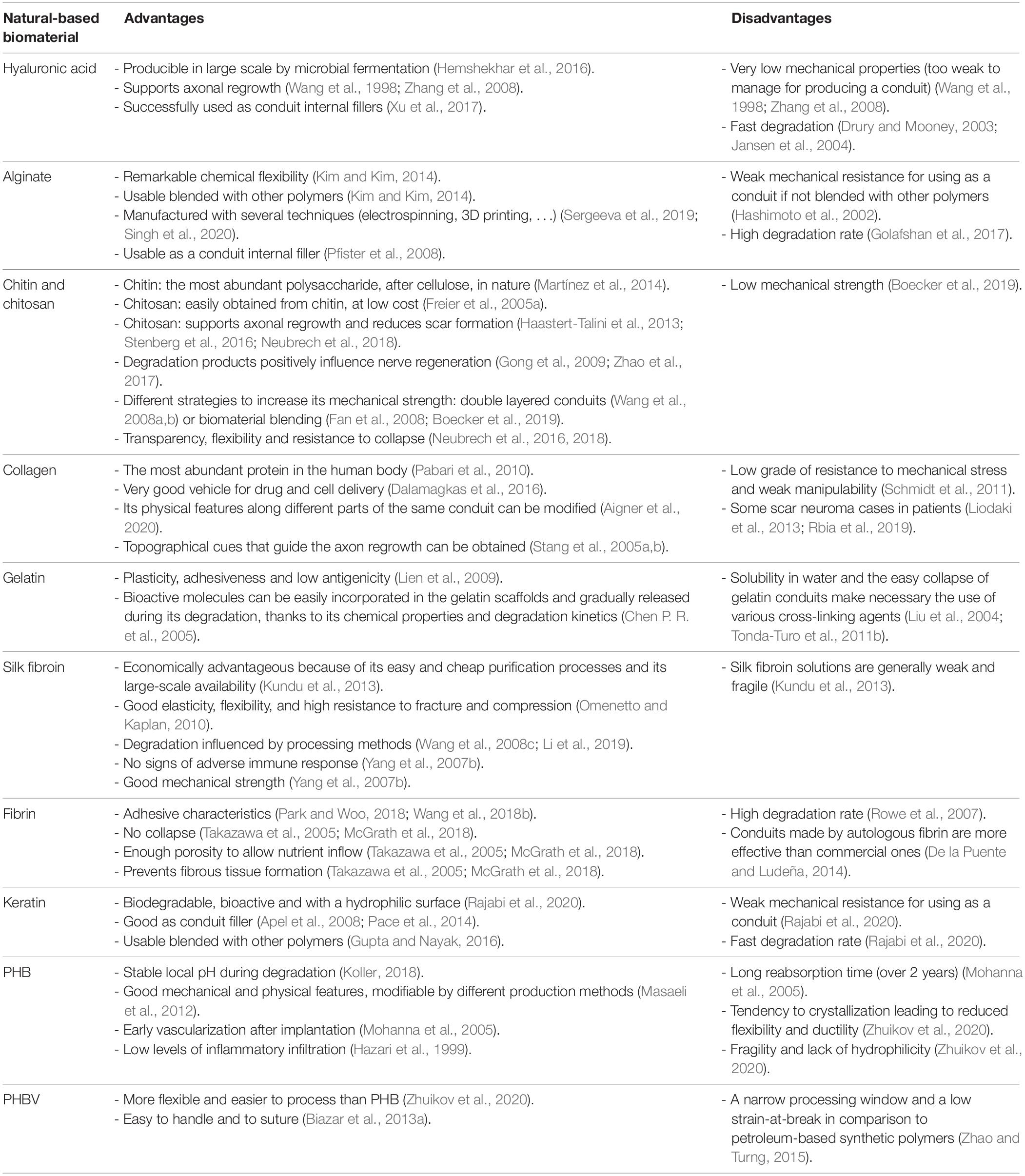
The most studied natural-based biomaterials used to support nerve regeneration (Table 1) are polysaccharides (hyaluronic acid, alginate, chitin and chitosan), proteins (collagen, gelatin, silk fibroin, fibrin, and keratin) and polyesters derived from natural sources [poly (3-hydroxybutyric acid) and poly (3-hydroxybutyric acid-co-3-hydroxyvaleric acid)] (Arslantunali et al., 2014; Raza et al., 2020). Nevertheless, hyaluronic acid, alginate and keratin, discussed in the following paragraphs, do not possess enough mechanical strength to be used alone to produce a NGC, but can be used as internal fillers for nerve conduits with successful results.
Polysaccharides
Hyaluronic Acid
Hyaluronic acid (HA) is a glycosaminoglycan which composes the extracellular matrix and, interacting with other extracellular molecules, is involved in the regulation of different cellular processes (Hemshekhar et al., 2016). To obtain a large amount of HA and to avoid animal-derived pathogen risks, HA could be produced in large-scale by microbial fermentation. Mechanical properties and degradation of HA can be tuned through chemical and physical processes of crosslinking with divinyl sulfone followed by freezing and lyophilization to create a porous structure (Ortuno-Lizarán et al., 2016; Vilariño-Feltrer et al., 2016). Moreover, HA can be solved with sodium chloride and directly poured into a porous sponge (Li et al., 2018; Entekhabi et al., 2020) or suspended in physiological saline solution to obtain a suitable viscosity useful to produce hydrogel fillers for NGCs (Li et al., 2018).
Hyaluronic acid is characterized by non-adhesive cue, is biocompatible and supports axonal regrowth (Wang et al., 1998; Zhang et al., 2008), but it owns very low mechanical properties. Indeed, even if blended with other biomaterials such as chitosan, it is too weak to manage (Li et al., 2018). In vivo it is degraded by hyaluronidases, which are widely diffuse in the organism, determining its fast degradation (Drury and Mooney, 2003).
It has also been reported that a nerve conduit based on an esterified HA derivative showed a fast degradation, not compatible with the timing necessary to support nerve regeneration, and the formation of fibrous tissue and a substantial cell ingrowth was observed (Jansen et al., 2004).
These characteristics make HA unsuitable as a conduit for nerve regeneration, but for its characteristics is very suitable as conduit internal filler, mostly in hydrogel form (Xu et al., 2017).
Alginate
Alginate is commonly extracted from brown seaweed and thanks to its biocompatibility is widely used in biomedical applications (Shen et al., 2005). The alginate is composed of mannuronic acid and guluronic acid which confer remarkable chemical flexibility compared to other degradable biocompatible materials with a notable resemblance to the mammalian extracellular matrix structure (Kim and Kim, 2014). It is easily modifiable via chemical reactions [e.g., alginate dialdehyde formed by periodate oxidation of sodium alginate) (Dranseikiene et al., 2020) and physical crosslinking using Ca ions, maintaining a negligible inflammatory response (Sun and Tan, 2013)].
This biomaterial can promote nerve regeneration but has a weak mechanical resistance, insufficient to bear physiological loading conditions and the high degradation rate justifies the use of alginate blended with other polymers (Hashimoto et al., 2002; Omidian et al., 2006; Kim and Kim, 2014; Shen and Hsieh, 2014; Golafshan et al., 2017), hybridized by incorporating nanofillers (Homaeigohar et al., 2019) or both (Chen et al., 2019). Alginate blending with biomaterials of natural origins could be a successful strategy: the research performed by Pfister et al. (2007a) demonstrated the effectiveness of a blend conduit consisting of alginate and chitosan to support nerve regeneration for short nerve gaps. This conduit possesses a good permeability and an adequate mechanical strength, which also allows an easy suturing, and promotes cell adhesion thanks to its hydrophilic nature (Pfister et al., 2007a).
The physical properties of alginate make it suitable to be manufactured with several techniques such as magnetic templating (Singh et al., 2020), electrospinning (Kim and Kim, 2014; Chen et al., 2018; Kuznetsov et al., 2018), microfluidics (Costantini et al., 2016; Hu Y. et al., 2017), gas foaming, emulsion freeze drying, 3D printing (You et al., 2017), and hard templating on vaterite CaCO3 crystals (Sergeeva et al., 2019).
Alginate is also used successfully as a conduit internal filler for growth factor delivery in nerve regeneration (Ohta et al., 2004; Pfister et al., 2007b, 2008) and for molecule controlled release at various pH values (Augst et al., 2006; Gao et al., 2009; Jeon et al., 2009). In particular, alginate as internal filler leaves no residue 4 months after surgery in a cat model of 50 mm gap sciatic nerve injury repaired with tubulation (Sufan et al., 2001) and is also able to promote specifically adhesion and proliferation of nerve cells in rats with 10 mm sciatic nerve gap 7 weeks after surgery (Askarzadeh et al., 2020).
Chitin and Chitosan
Chitin is a linear homopolymer of N-acetyl-D-glucosamine, belonging to the glycosaminoglycan family; in nature, it is the most abundant polysaccharide, after cellulose, and it is obtained from arthropod exoskeleton (Martínez et al., 2014).
Crustacean shells, due to their abundance, are considered the main source for chitin isolation; indeed, chitin derived from their wastes represents around half of shellfish total weight. Chitin can be extracted with biological (microbial) or chemical methods (Ramírez et al., 2010; Hamed et al., 2016).
Chitin is mainly used in its partial deacetylated form, chitosan, in many different fields ranging from the food industry and agriculture (Boonlertnirun et al., 2008) through pharmaceutics (Varshosaz, 2006; Mitsou et al., 2020) to regenerative medicine (e.g., biomedical patches, artificial skin and orthopedic tissue engineering, nerve conduits) (Shapira et al., 2016; Porpiglia et al., 2018; Sandri et al., 2019; Ryu et al., 2020; Tao et al., 2020a). Chitosan can be easily obtained from chitin, at low cost, through alkaline hydrolysis (Freier et al., 2005a).
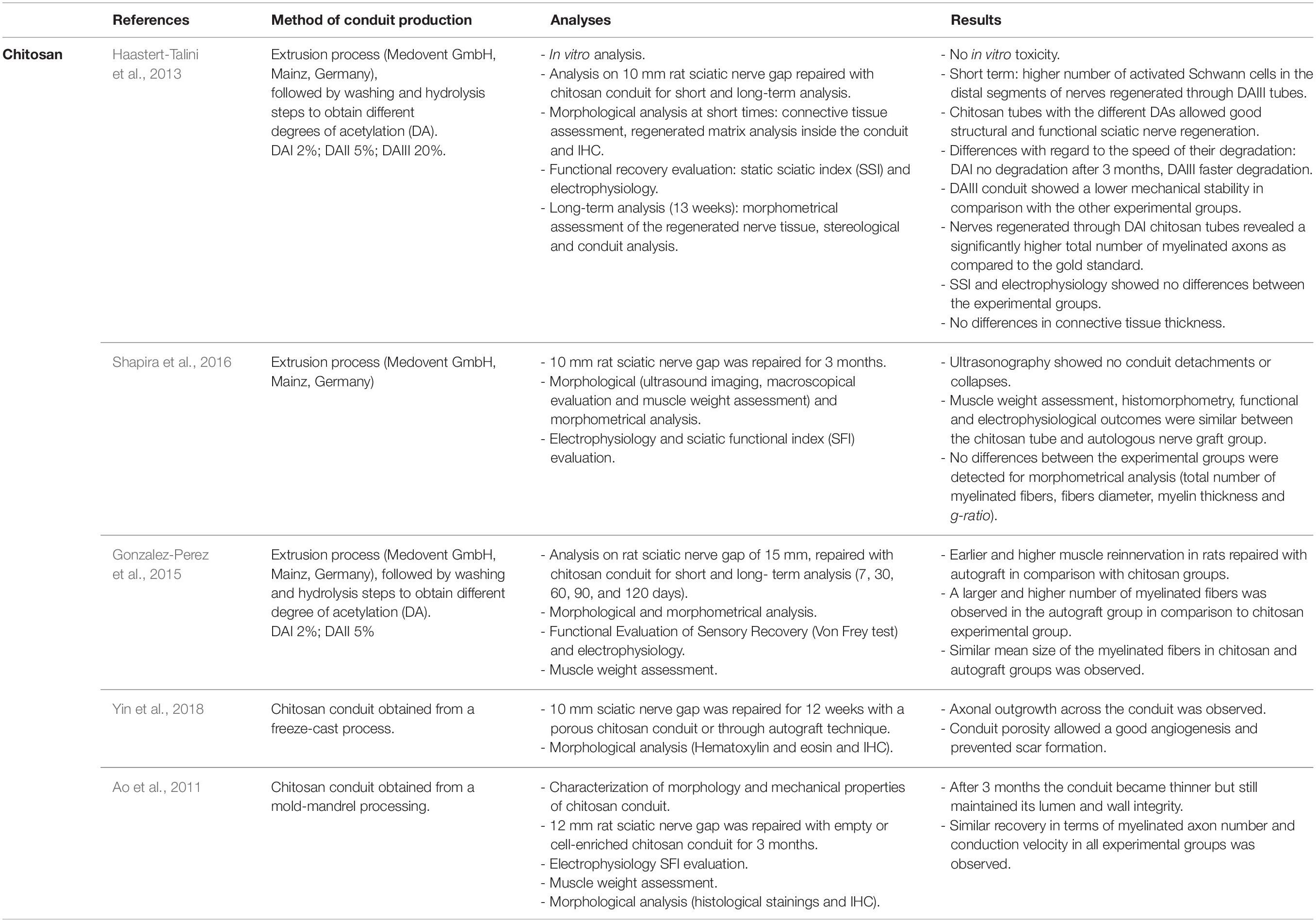
Chitosan is widely used to support peripheral nerve regeneration and its effectiveness has been investigated in several studies (Table 2). It possesses many suitable characteristics to be used in this field: it is biocompatible (Yuan et al., 2004; Freier et al., 2005a; Simões et al., 2011), it can support axon regrowth (Haastert-Talini et al., 2013; Stenberg et al., 2016) and reduce scar tissue formation (Neubrech et al., 2018). Moreover, it is a re-absorbable biomaterial whose degradation products (including chito-oligosaccharides) positively influence nerve regeneration (Gong et al., 2009; Zhao et al., 2017). Furthermore, chitosan can be processed through many fabrication technologies (Ruini et al., 2015; Tonda-Turo et al., 2017b) to produce NGCs both as external wall material and cell-based therapies as internal filler (Boido et al., 2019; Tonda-Turo et al., 2020). One factor limiting chitosan use as a nerve guide is its low mechanical strength; nevertheless, Freier et al. (2005b) demonstrated that modifying the chitosan acetylation degree, its mechanical stability can be improved and conduits with different acetylation degree were also tested in vivo (Haastert-Talini et al., 2013). Other strategies can be used either to increase chitosan mechanical strength, either to avoid the guide collapse, such as double layered conduit production (Wang et al., 2008a, b) or chitosan blending with biomaterials presenting a high mechanical force (Fan et al., 2008; Boecker et al., 2019). Indeed, chitosan has been extensively studied in vivo, in combination with different synthetic polymers, successfully bridging nerve defects (Jiao et al., 2009; Ding et al., 2010). For example, Xie et al. (2008) tested with positive results, a chitosan-polylactic acid (PLA) blend, taking advantage of PLA mechanical properties and of chitosan biocompatibility.
Chitosan is an attractive material for nerve guide manufacturing because of its versatility, and its surface texture can also be easily modified to better support axonal regrowth (Shuai et al., 2013; Wrobel et al., 2014).
In recent years, various studies on chitosan-made tubes have demonstrated chitosan conduit efficacy in inducing nerve regeneration for bridging peripheral nerve defects of 8 mm or more in rat models (Ao et al., 2011; Haastert-Talini et al., 2013; Gonzalez-Perez et al., 2015; Shapira et al., 2016; Ronchi et al., 2018; Yin et al., 2018; Crosio et al., 2019). 8 mm median nerve defects were also immediately or delayed repaired with chitosan conduits to test if chitosan guide enrichment with muscle fibers, used as internal filler, better promote nerve regeneration; morphometrical and functional analysis demonstrated that nerve regeneration was obtained in both experimental groups with similar results, suggesting that for short gaps the use of hollow chitosan guide is sufficient to obtain nerve regeneration (Ronchi et al., 2018; Crosio et al., 2019). Another study investigated nerve regeneration in rat sciatic nerves 3 months after 10 mm nerve repair with chitosan conduits with three different deacetylation degrees (Haastert-Talini et al., 2013) or with autografts, showing no significant differences among all the experimental groups at functional, biomolecular and morphological levels. Similar results were obtained by Shapira et al. (2016) which observed, 3 months after 10 mm nerve gap repair, that electrophysiological, morphological, morphometrical outcomes were comparable between nerve repair through autograft or chitosan tube.
Similar experimental conditions (10 mm gap and 3 months post-operative) were used to test nerve regeneration inside a freeze-cast, double-layered chitosan tube, where authors observed that the conduit porosity allows good angiogenesis and prevents scar formation. Nevertheless, this study needs further analysis to be completed (Yin et al., 2018).
Experimental studies of nerve gaps longer than 10 mm repaired with chitosan tubes showed that chitosan conduit permits nerve regeneration, even if lower than those of nerve repaired through the autologous graft. Gonzalez-Perez et al. (2015) tested chitosan tubes with two different deacetylation levels on 15 mm nerve defects; 4 months after the repair they obtained a similar number of myelinated fibers and similar functional and electrophysiological results. As additional experimental group, they tested nerve regeneration in a silicon tube where nerve regeneration was not observed. Also, Ao et al. (2011) observed for the critical nerve gap length of 12 mm nerve regeneration inside a hollow chitosan conduit, although only cell enriched experimental groups showed morphological and functional results similar to those achieved by the autograft group.
Different clinical trials on chitosan based-conduits were planned during these years, and in 2015 a chitosan nerve conduit, Reaxon®, was commercialized; it allows to bridge nerve gaps up to 26 mm. Transparency, flexibility and resistance to collapse are amongst the advantages of the Reaxon® conduit. In clinical practice, it has been shown that the use of this conduit for hand nerve injury repair with end-to-end technique positively influences sensory and motor recovery in patients treated with this conduit (Neubrech et al., 2016, 2018). In order to optimize the regeneration process, manufacturers suggest using the Reaxon® Nerve Guide in anatomical sites able to maintain it moistened and to avoid its drying and its consequent stiffening.
Proteins
Collagen
The main reason why collagen has been so commonly used to create nerve conduits in the last three decades (Eppley and Delfino, 1988; Pabari et al., 2010) is that it is the most abundant protein in the human body (Pabari et al., 2010). Collagen is a major component of the extracellular matrix and of the peripheral nervous system envelope. It can be isolated from many biological tissues (e.g., tendon, skin, and bone). Collagen is a very good vehicle for drug and cell delivery (Dalamagkas et al., 2016) and it is possible to modify its physical features along different parts of the same conduit (Aigner et al., 2020). The different binding domains on it give the possibility to create topographical cues that guide the axon regrowth (Stang et al., 2005a, b) facilitating cell adhesion, survival and migration (Alberti et al., 2014; Sulong et al., 2014; Drobnik et al., 2017a, b). Many researchers have shown that collagen has biological properties superior to other materials available in the market for nerve scaffold fabrication and it is also effective over a nerve gap distance of at least 15 mm (Archibald et al., 1991). 10 mm long hollow conduits reported better results in rat nerve regeneration and muscle re-innervation if compared to collagen polyglycolic acid (PGA)-filled conduits (Saltzman et al., 2019). A conduit made by mixing types I and III collagen filled with collagen filaments was not inferior to autologous graft in treating 30 mm nerve defects, with 80% of patients reporting sensory recovery after 12 months (Saeki et al., 2018). There are few collagen nerve conduits approved by the Food and Drug Administration (FDA) and Conformity Europe (CE). NeuraGen®, a nerve conduit made of type I bovine collagen derived from the Achilles tendon (Di Summa et al., 2014), reported overlapped results compared to allograft 12 months post-surgery in patients with the reconstruction of a digital nerve gap inferior to 2.5 cm (Rbia et al., 2019). However, some scar neuroma cases, with and without foreign bodies, imposed conduit removal (Liodaki et al., 2013; Rbia et al., 2019). Maybe due to the tube high stiffness and high cost, it is not so popular among surgeons (Arslantunali et al., 2014; Sedaghati et al., 2014). RevolNerv®, a porcine skin-derived collagen type I based-conduit, showed good clinical outcomes and the absence of post-surgical neuromas in 163 patients that underwent wrapping direct end-to-end sutures (Thomsen and Schlur, 2013) and comparable results with uncoated direct sutures for palmar digital nerves were observed (Arnaout et al., 2014). Neuroflex® and NeuroMatrix®, respectively, made in collagen types I and III, were approved in 2001, but clinical and preclinical researches are still lacking (Meek and Coert, 2008; Stang et al., 2009; Chrząszcz et al., 2018). No clinical and preclinical researches have shown immune response due to allogenic origin of collagen NGCs (Archibald et al., 1995; Boeckstyns et al., 2013; Chrząszcz et al., 2018). Indeed, the high purification process by which those NGCs are manufactured is able to remove all impurities that can trigger an immune response (Wong et al., 2014; Maurer et al., 2018). Because collagen has such powerful clinical effects, but low grade of resistance to mechanical stress and weak manipulability (Schmidt et al., 2011), future researches will be necessary to tackle these problems. Indeed, collagen could also be blended with other biomaterials like silk fibroin or chitosan, that are able to increase dramatically its mechanical resistance (Huang et al., 2011; Teuschl et al., 2015; Xu et al., 2016).
Gelatin
Gelatin is obtained by the thermal denaturation of collagen. Animal-derived gelatin has been widely investigated for medical applications, for its biocompatibility, its plasticity and adhesiveness. Gelatin is less expensive than collagen and has relatively low antigenicity compared to its precursor (Lien et al., 2009). Nevertheless, its solubility in water and the easy collapse of gelatin conduits make necessary the use of various cross-linking agents, resulting in the alteration of its mechanical and physical properties with a controlled degradation rate (Liu et al., 2004; Tonda-Turo et al., 2011b) (Table 3).
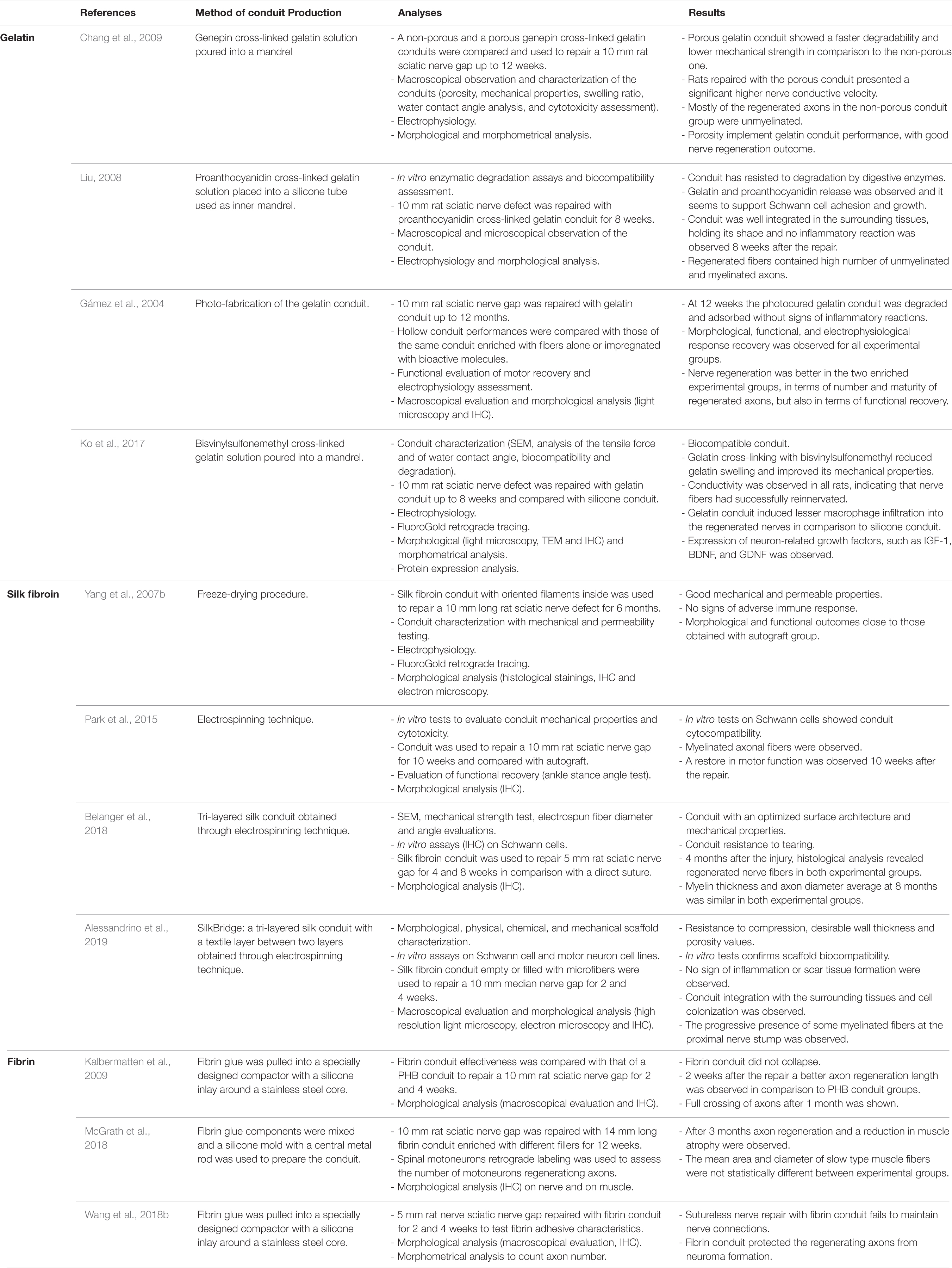
Among gelatin crosslinkers, genipin has been often used in tissue engineering applications. Genepin has low cytotoxicity and it is of natural origin, it can be obtained from geniposide isolated from the fruits of Gardenia jasminoides ELLIS (Cenni et al., 2000; Sung et al., 2001). Different gelatin-based conduits were produced using it as cross-linker. Chen Y. S. et al. (2005) used a genepin cross-linked gelatin conduit to repair a 10 mm rat sciatic nerve defect for 8 weeks confirming conduit biocompatibility, but with controversial results: nerve regeneration inside the conduit was not compared with a positive control group as autograft and they observed that after 8 weeks most of the regenerated axons were not myelinated. The authors also showed that the biomaterial starts to degrade 6 weeks from the repair and after 8 weeks the fragmentation is clear (Chen Y. S. et al., 2005). Four years later, the same authors showed that an increased conduit porosity can implement its performance, with good regeneration in the rat model, giving real perspective for future studies in longer defects (Chang et al., 2009).
Another natural cross-linking agent, proanthocyanidin, which is usually used as an antioxidant, was used to stabilize a gelatin conduit, reducing its degradation rate. The proanthocyanidin cross-linked gelatin conduit presents a rough outer surface and was used to repair a 10 mm nerve gap and the regeneration was assessed 8 weeks after the repair (Liu, 2008). The conduit was tested for its biocompatibility and degradation: gelatin and proanthocyanidin release was observed and it seems to support Schwann cell adhesion and growth. After 8 weeks in vivo, the conduit was well integrated into the surrounding tissues, holding its shape intact and no inflammatory reaction was observed. Histological and electrophysiological measurements were assessed and the regenerated units containing unmyelinated and myelinated axons were abundant.
Gámez et al. (2003) prepared a biodegradable gelatin-based nerve conduit using photoreactive gelatin able to be cross-linked upon visible-light irradiation. The conduit 15 mm long was implanted between the proximal and distal stump of a 10 mm rat sciatic nerve gap up to 12 months (Gámez et al., 2003). The photo-fabrication of the gelatin conduit allowed to obtain a conduit with controlled features such as length, wall thickness and inner diameter. The hollow conduit performances were compared with those of the same conduit enriched with fibers alone or impregnated with bioactive molecules (Gámez et al., 2004). Despite nerve regeneration was better in the two enriched experimental groups, morphological, functional, and electrophysiological response recovery were observed for all experimental groups.
Recently, the biocompatibility of a gelatin conduit cross-linked with bisvinylsulfonemethyl was assessed. Nerve regeneration at 8 weeks in a 10 mm rat sciatic nerve defect repaired through this conduit was similar to that obtained using a silicone guide previously tested. Gelatin cross-linking with bisvinylsulfonemethyl reduces gelatin swelling and improves its mechanical properties; moreover, unlike other gelatin conduits, this nerve guide is transparent (Ko et al., 2017).
A different study exploited gelatin proper characteristics to be used as protection around a rat sciatic nerve repaired with end-to-end technique. This scaffold possessed low mechanical strength: it collapsed and adhered to the repaired nerve, like a sheath. It was observed that the use of this scaffold around the nerve reduces scar formation, slows down inflammatory cell arrival, and shows better results in comparison to end-to-end repaired nerve unprotected by gelatin (Tao et al., 2017). To avoid gelatin tube collapse, recently a gelatin based-tube was stabilized with titanium micro-rods (Uranues et al., 2020). This conduit was tested for 8 weeks in mini-pig models to repair 6 mm sciatic nerve gaps and results similar to those obtained with a direct coaptation of the same gap were obtained at morphological, electrophysiological and functional level. Nevertheless, despite the conduit does not generate any immune response, titanium is synthetic and not biodegradable, thus reducing the attractiveness toward a gelatin conduit which is otherwise of natural origin and biodegradable.
Indeed, due to its weak mechanical strength and its rapid degradation, most of the gelatin-based scaffolds have been prepared using gelatin combined with other biomaterials of different origins, such as chitosan (Nie et al., 2014) or bioglasses used to produce conduits (Koudehi et al., 2014); or poly-L-lactic acid (PLLA) (Binan et al., 2014) and polycaprolactone (PCL) (Vatankhah et al., 2014) to obtain electrospun mats. Thanks to its chemical properties and its degradation kinetics, the gelatin scaffold can easily incorporate bioactive molecules which can be gradually released during scaffold degradation (Chen P. R. et al., 2005).
Silk Fibroin
Silk proteins are synthesized in the silk glands of arthropods like spiders and silkworms, and during metamorphosis it is spun in fibers. The main source of silk fibroin for human applications is silkworm, in particular Bombyx mori, which is extensively used in the textile industry and allows obtaining a high silk yield in comparison to spiders. Moreover, spider silk use is restricted for the less silk yield, for their cannibalistic behavior and their heterogeneity in nature (Pérez-Rigueiro et al., 2005; Omenetto and Kaplan, 2010; Radtke, 2016). Silk fibroin use is economically advantageous, in comparison to other natural-based biomaterials, because of its easy and cheap purification processes and its large-scale availability exploiting silk industry infrastructures (Kundu et al., 2013).
Silk fibroin possesses characteristics that suggest its use in biomedical applications: it contains repeated aminoacidic sequences, which determine the formation of a high number of β-sheets, which confer good mechanical properties (i.e., elasticity, flexibility, and high resistance to fracture and compression) and influence its degradation. Silk is biodegradable and it is slowly degraded by proteolytic enzymes (Altman et al., 2003; Yang et al., 2007a; Cao and Wang, 2009; Keten et al., 2010); its degradation is also influenced by silk processing methods: silk with a high crystal content ensures a low degradation rate (Wang et al., 2008c; Li et al., 2019). Nevertheless, these characteristics are attributed to native silk fibers, while silk derived from silk fibroin solutions are generally weak and fragile.
Silk fibroin biocompatibility is well established as silkworm-derived silk is widely used in clinical practice for surgical sutures (Cao and Wang, 2009). The absence of cytotoxicity was demonstrated both in vitro and in vivo. Studies on dorsal root ganglia, primary cultures of Schwann cells and embryonic rat hippocampal neurons showed no deleterious effects (Yang et al., 2007a; Tang et al., 2009; Zhao et al., 2013).
Nerve conduits made by silk fibroin obtained from both spiders and silkworms were able to bridge peripheral nerve gaps and to guide nerve regeneration. Although different studies confirmed that spider silk conduits promote nerve regeneration (Allmeling et al., 2008; Radtke et al., 2011; Huang et al., 2012), here we focused our attention on Bombyx mori derived silk fibroin, since it is the most available source. Silk conduits with different characteristics were tested over time with success (Table 3). Yang et al. (2007b) developed a silk fibroin conduit with oriented filaments inside which gives results close to those obtained with autograft in bridging a 10 mm rat sciatic nerve gap. This conduit is biocompatible, showing no signs of adverse immune response 6 months after the implantation, it is porous and its pore size is appropriate to allow nutrient exchange and to block unwanted cell inflow; it also possesses good mechanical strength, it is resistant to the surrounding muscular contraction and maintains its original shape. The conduit starts to be degraded 6 months after implantation.
Despite Yang’s conduit was obtained through freeze-drying processing, Park et al. (2015) produced a porous silk conduit through the electrospinning technique. This guide possesses mechanical characteristics similar to the freeze-drying conduit, but its wall is thinner and the conduit production method is easier. The electrospun conduit was used to bridge 10 mm nerve defects and a restore in motor function was observed 10 weeks after the repair in a rat model.
Multilayered silk conduits were produced and tested to obtain suitable characteristics for a nerve regeneration comparable with those obtained with gold standard repair techniques. Belanger et al. (2018) developed a tri-layered silk conduit, composed by two perpendicular layers of aligned fibers outside and one inner layer of random fibers, and compared its performance in nerve repair to a direct suture to repair 5 mm sciatic nerve defect. The device structure increases its inner surface area, improving axonal regrowth in terms of myelin thickness and axon diameter 8 weeks after the repair, even if no significant differences are observed in comparison to end-to-end repaired nerves. Nevertheless, conduit use is favorable since it reduces nerve tensioning during regeneration in comparison to end-to-end repair technique; moreover, this conduit is also easy to suture and possesses an optimized surface architecture and good mechanical properties.
Another tri-layered silk scaffold, used to repair a 10 mm gap and compared with autograft, consists of a textile layer interposed between two electrospun layers with controlled wall thickness and porosity (Alessandrino et al., 2019). In vitro assays carried out with Schwann cell and motor neuron cell lines demonstrated its biocompatibility, which was also confirmed by in vivo experiments: no inflammatory response and scar formation around the implant were observed 2 weeks after the repair. This short-term study showed early cellular colonization and a progressive axonal regrowth.
Since native silk confers good tensile and mechanical properties to conduits, and conduits produced with fibroin solutions do not possess the same characteristics, silk fibroin obtained from fibroin solutions could be blended with different biomaterials to reach adequate mechanical properties (Wang and Cai, 2010; Xu et al., 2016). Blending silk fibroin with synthetic biomaterial could also be a strategy to improve synthetic conduit performance and biocompatibility, since silk fibroin could stimulate fibroblast proliferation and VEGF secretion, which results in improved angiogenesis inside the conduit, proven to positively influence nerve regeneration (Wang et al., 2018a). Silk fibroin could also be blended with natural biomaterial such as collagen, indeed a silk fibroin-collagen nerve conduit, on which adipose-derived stem cells were co-cultured with Schwann cells, showed good results with nerve regeneration acceleration (Xu et al., 2016).
Fibrin
Fibrin is a fibrillar protein formed during blood clotting. Fibrin is mainly involved in hemostasis, but it plays a role in wound healing by forming a temporary matrix (Park and Woo, 2018).
Due to its role in hemostasis, it is widely used in clinical practice as a surgical glue (Albala and Lawson, 2006) and it has also been used to join nerve stumps with successful results (Ornelas et al., 2006a, b). Fibrin glue is biocompatible, and can be obtained from donors or can be acquired by different companies for clinical practice (Le Guéhennec et al., 2004), nevertheless, it can be obtained from the patient since it has been demonstrated that cell survival is better on autologous fibrin scaffolds (De la Puente and Ludeña, 2014).
The mechanical properties of this biomaterial are easily and highly tunable varying fibrin concentration and processing temperatures (Bruekers et al., 2016). However, since fibrin degradation rate is high, antifibrinolytic agents are necessary to prevent the conduit lysis (Rowe et al., 2007).
As well as for its high biocompatibility, fibrin can be used as a scaffold in tissue engineering thanks to its versatility, indeed its dissolving and coagulation characteristics can be modified by changing the dilution (Bensaïd et al., 2003; Fang et al., 2004). Thanks to fibrin adhesive characteristics, this type of conduit could be not sutured; nevertheless, a recent study demonstrated that sutureless nerve repair with fibrin conduit fails to maintain nerve connections due to poor mechanical stretch resistance (Wang et al., 2018b).
Different fibrin conduits were tested over time with success (Table 3). Kalbermatten et al. (2009) demonstrated the effectiveness in rat sciatic nerve regeneration of a conduit made by fibrin glue to repair 10 mm defects. Also, the fibrin conduit demonstrated a better axon regeneration length in comparison with PHB conduits 2 weeks after the repair and after 28 days axon full crossing was observed. This conduit does not collapse, supports axonal sprouting and is porous, allowing nutrient inflow, but at the same time its surface prevents fibrous tissue formation (Takazawa et al., 2005). Recently, the same fibrin conduit 14 mm long, filled with fibrin matrix or fibrin matrix containing human mesenchymal stem cells, was used to repair a 10 mm gap in rat sciatic nerve and after 3 months axon regeneration and a reduction in muscle atrophy were observed (McGrath et al., 2018), confirming its effectiveness in nerve repair promotion.
Keratin
The use of keratin as a biomaterial was the focus of several researches in the last four decades. “Hard” keratin proteins can be isolated from protective tissues such as hooves, nails and hairs, that contain structural proteins that are more resistant than “soft” keratin derived from cytoskeletal elements found in epithelial tissues (Hill et al., 2010). Human hair is a highly ornate superstructure composed of fibers that are self-assembled in the follicle, that are regulated by more than 30 cytokines and growth factors (Lyons et al., 1990; Jones et al., 1991; Hardy, 1992; Blessing et al., 1993; Stenn et al., 1994; Motonobu et al., 2013).
Keratin was demonstrated to be biocompatible, biodegradable, bioactive and it possesses a hydrophilic surface, which is absent in many synthetic polymers. Nevertheless, the practical use of keratin-based biomaterials is limited due to its poor physical and mechanical properties (fast degradation rate and low molecular weight) that can be improved using various cross-linking agents (Rajabi et al., 2020). Keratin itself has too weak mechanical resistance to be used alone as a nerve conduit, indeed it is largely used as conduit filler.
Because of its neuroinductive capability (Apel et al., 2008; Pace et al., 2013), human keratin has proven to be effective in promoting nerve regeneration in short gaps (5–15 mm) if used as hydrogel-filler for conduits in mouse (Apel et al., 2008; Sierpinski et al., 2008), rat (Lin et al., 2012; Pace et al., 2013), rabbit (Hu et al., 2003; Hill et al., 2011), and macaque models (Pace et al., 2014).
Besides, Gupta and Nayak used keratin as a protein source for scaffold fabrication and they succeeded to produce a keratin/alginate scaffold for tissue engineering applications. This scaffold was never used in vivo for peripheral nerve repair assessment, even if in vitro experiments reported promising results (Gupta and Nayak, 2016).
Polyesters
Polyesters are natural biodegradable biopolymers, which can be obtained from renewable resources; among them the polyhydroxyalkanoates (PHA) family is the most successful one in tissue engineering applications (Anjum et al., 2016; Basnett et al., 2017). PHAs, which possess properties similar to the conventional petrochemical polymers, are usually produced by bacterial and archaeal fermentation in conditions of nutrient depletion or excess of carbon sources and are accumulated within their cytoplasm (Chen, 2010; Rodriguez-Contreras, 2019). PHA family members differ widely in their structure and properties depending on the type of microorganism, biosynthesis conditions, and on which carbon source was used during the production process (Licciardello et al., 2019; Mozejko-Ciesielska et al., 2019).
The specific advantage of PHA is its stable local pH during degradation, which improves its biocompatibility in comparison to other biomaterials used for medical devices (Koller, 2018). Nevertheless, PHA production has high costs, which could be reduced by different strategies, which include the development of recombinant microorganisms able to produce large PHA amounts, as recently reviewed by Zheng et al. (2020).
Poly (3-hydroxybutyric acid) and poly (3-hydroxybutyric acid-co-3-hydroxyvaleric acid) are the most widely studied members of this family. These polymers can be easily processed to obtain conduits using different production methods such as extrusion, electrospinning and rolling sheets on a mandrel (Arslantunali et al., 2014) and several preclinical studies on nerve regeneration using these PHA-based conduits were carried out with successful results (Table 4).
Poly (3-Hydroxybutyric Acid) (PHB)
Poly (3-hydroxybutyric acid) is the main polymer of the PHA family (Zhuikov et al., 2020). It is an innovative product of natural origin, obtained from bacterial fermentation, with potential characteristics including biodegradability and biocompatibility. Its promising applications as a biomaterial in different fields of tissue engineering (Wu et al., 2009; Bagdadi et al., 2018) and of peripheral nerve regeneration have been documented (Young et al., 2002). PHB conduits possess good mechanical and physical features, which can be modified by different production methods (Masaeli et al., 2012). Moreover, they are biodegradable, even if reabsorption time is long (over 2 years) and show early vascularization after implantation (Mohanna et al., 2005). PHB stability could be a disadvantage for short-term applications, but it is useful for long-term nerve reconstructions.
The PHB has been used as an alternative to direct epineural repair (Åberg et al., 2009) and to bridge short and long nerve defects. PHB conduits have been successfully used to repair a 10 mm gap in rat sciatic nerves up to 30 days; low levels of inflammatory infiltration were detected, macrophage levels were similar to those of rat nerves repaired with autograft and a good axon regrowth was observed. No failure in nerve regeneration was observed at all (Hazari et al., 1999).
For long nerve gaps, PHB conduits were tested on rabbits. Using the rabbit common peroneal nerve model, empty PHB conduits effectiveness was assessed across 2, 3, and 4 cm nerve defects up to 63 days. This study demonstrated good nerve regeneration across long gaps (Young et al., 2002). Nevertheless, regeneration through a hollow guide for long gaps can be enhanced through the addition of trophic factors, such as the glial growth factor/neuregulin1 (GGF/NRG1), which was resuspended in an alginate hydrogel used to bridge 2–4 cm gaps in rabbit common peroneal nerve with successful results both in short- and long-term (120 days) experimental conditions (Mohanna et al., 2003, 2005). These data suggest that PHB nerve conduits are suitable for long-gap nerve injury repair.
Due to their success in nerve regeneration, PHB conduits had been also used to compare fibrin glue conduits (Kalbermatten et al., 2009) and afterward the same authors also used a PHB conduit to evaluate the potentiality of an internal filler composed by a fibrin matrix loaded with Schwann cells or differentiated mesenchymal stem cells to repair 10 mm gaps in rat (Kalbermatten et al., 2008b). As an alternative to PHB nerve guides, PHB strips seeded with Schwann cells were used to bridge a 10 mm sciatic nerve gap in rats, since strips can provide a direct contact among the biomaterials, cells and surrounding tissues. PHB strips seem to support the early stages of nerve regeneration and show a fast regeneration 2 weeks after the repair (Kalbermatten et al., 2008a). More recently, the same authors tested PHB strips seeded with Schwann cells or with adipose-derived stem cells in a long-term study (12 months). Animals treated with enriched strips showed a better functional recovery in comparison with control animals treated without cells, with a higher number of axons reaching the distal stump and reduced muscle atrophy (Schaakxs et al., 2017).
Since chitosan reduced PHB crystallization, these biomaterials were successfully used in combination to produce aligned fibers by electrospinning technique to produce scaffolds for nerve tissue engineering (Karimi et al., 2018). Synthetic biomaterials such as polycaprolactone were also successfully used to be blended with PHB, to produce nerve guides with requested characteristics (Hinüber et al., 2014).
Poly (3-Hydroxybutyrate-Co-hydroxyvalerate) (PHBV)
Since PHB has some disadvantages, such as fragility, lack of hydrophilicity and tendency to crystallize, characteristics which reduce flexibility and ductility of the conduit, the copolymer PHBV was made to compensate these disadvantages of PHB in order to improve the physic-chemical properties of that biomaterial (Zhuikov et al., 2020). In this way, mechanical properties can be modulated by co-polymerization of PHB with hydroxyvalerate to form PHBV biopolymer (Koller, 2018), considered more flexible and easier to process, even if it presents some disadvantages such as a narrow processing window and a low strain-at-break in comparison to petroleum-based synthetic polymers (Zhao and Turng, 2015).
Biazar et al. (2013c) used a PHBV fibrous tubular conduit produced by electrospinning technique to repair a 30 mm gap in rat sciatic nerve with satisfactory results within 4 months, with skeletal muscle reinnervation and a myelinated nerve fiber diameter distribution similar to that of the autograft experimental group. Beyond its high porosity (about 95%) and biocompatibility, this conduit is easy to handle and to suture; it is flexible and can also be bent up to 180° and then it restores its original shape. The histomorphological study and behavioral tests confirmed and strongly supported the use of this conduit to repair 30 mm nerve gaps (Biazar et al., 2013b). The same experiments performed with PHBV fibrous conduit were replicated with the introduction of Schwann cells inside the conduit, confirming its effectiveness (Biazar and Heidari Keshel, 2013; Biazar et al., 2013a).
PHBV efficacy in nerve repair was also tested through the production of a PHBV conduit made by micropatterned wafers (Karimi et al., 2014). The authors observed, in rat sciatic nerve, sensory and motor function recovery 4 months after a 30 mm gap repair with a PHVB conduit enriched or not with Schwann cells.
Also aligned nanofibrous PHBV-based conduits were tested to repair nerve injuries. Hu F. et al. (2017) repaired 10 mm rat sciatic nerve defect up to 10 weeks, with aligned electrospun nanofibres conduit alone or enriched with adipose-derived mesenchymal stem cells (ASCs) or FGF2-miR-218-induced ASCs, demonstrating that FGF2-miR-218 induction approach combined with the presence of the PHBV scaffold improves nerve regeneration. In another study, the PHBV biopolymer was derived from Alcaligenes eutrophus using a nitrogen-rich medium and an excess of carbon source. A conduit composed of PHBV oriented nanofibers, used to repair a 10 mm nerve gap, was obtained by electrospinning technique and, to prevent a collapse, the conduit was filled with a drop of 1% agarose (Demirbilek et al., 2015). Nevertheless, the PHBV tendency to collapse was not observed in the other studies reported in this paragraph, even in presence of longer gaps. Functional and morphological analyses were performed 2 and 4 months after the repair (functional recovery was also tested after 1 month) and it was demonstrated that nerve regeneration is similar to that observed with autograft, even if the autograft group presents a better and faster regeneration in comparison to the PHBV conduit group (Demirbilek et al., 2015). Interestingly, regenerating nerves of the PHBV conduit group were more vascularized then those of the autograft group 8 weeks after the repair; the presence of blood vessels should sustain nerve regeneration by allowing nutrient inflow.
A nanofibrous PHBV conduit cross-linked with chitosan was obtained and showed its suitable physical, mechanical, and structural properties to promote nerve regeneration after a 10 mm gap repair, up to 4 months. The conduit was also enriched with Schwann cells, which adhere well to the fibrous scaffold (Biazar and Keshel, 2013).
Future Trends in the Design of NGCs Using Natural-Based Biomaterials
Conventional processing technologies associated with surface modification approaches have been successfully applied in the NGC development so far (Chiono and Tonda-Turo, 2015). Natural-based biopolymers were processed mainly through the casting of porous membranes and subsequent wrapping, electrospinning and dip-molding technologies to obtain channels. However, these methods do not allow to obtain some suitable mechanical characteristics or to obtain complex geometry as well as to encapsulate biological cues such as growth factors and nerve supporting cells into the conduit to enhance the regeneration process. Recently, non-conventional technologies have raised interest as an alternative to process natural-based polymers to form NGCs (Johnson and Jia, 2016). Among others, 3D bioprinting offers unique features to allow customized geometry via clinical imaging of damaged nerve including biological cues within the fabrication process enhancing the effectiveness of the biological stimuli after in vivo implantation. Maiti and Díaz (2018) have recently revised the use of bio-inks as forming materials for the development of NGCs, highlighting the superiority of natural-based biopolymers compared to synthetic ones. Furthermore, recent results reported the possibility to produce nerve guidance channels as well as lumen loading cells and the establishment of growth factor gradients along the length of a nerve guide using 3D bioprinting (Petcu et al., 2018). On the other hand, recent innovations on biomaterials design are being directed toward the use of modified natural polymers to confer improved mechanical properties (Noè et al., 2020; Tonda-Turo et al., 2020). The use of methacrylate natural polymers is currently wildly applied to form complex geometries using additive manufacturing. Among others, methacrylated gelatin (GelMA), thanks to its biocompatibility and mechanical tunability, has been applied in many fields of tissue engineering and NGC having multiple channel geometries have been produced through 3D bioprinting of GelMA (Ye et al., 2020). 3D printed GelMA hydrogels have been tested as nerve conduits showing promising results. The high hydrophilicity of this biomaterial allows the incorporation of nanoparticles (Tao et al., 2019; Xu et al., 2019) or platelets (Tao et al., 2020b) to increase peripheral nerve regeneration and functional recovery.
Conclusion
Conduits obtained from natural-based biomaterials share many characteristics with synthetic ones, which are also suitable for their use in peripheral nerve regeneration. Nevertheless, the advantage of natural biopolymers is not only their higher biocompatibility, but more relevant is the fact that these polymers can be extracted by different renewable sources and some of them can be obtained from wasted food. Currently, renewable source use is of fundamental interest for the world populations to enhance sustainability, environmental protection and to preserve human well-being. Even the patient itself could be the source of the biomaterial for the conduit production, like in the case of fibrin and keratin, thus reducing the risk of rejection and increasing cell survival and nerve regeneration.
Another important aspect to consider is that natural-based biomaterials are bioactive. In the literature the attention is mainly focused on the bioactivity effect on nerve regeneration, suggesting that the release of neurotrophic factors or the presence of bioactive binding sites inside a conduit positively influences nerve regeneration. Synthetic materials hold the promise that their properties can easily be tuned and controlled; however, they lack sites for specific proteins to bind and cells to interact with, so there is insufficient integration with the native tissue. Thus, different strategies are used by researchers to improve conduit performance, such as the introduction of internal filler hydrogels or cells releasing neurotrophic factors or the direct modification of the conduit. Some conduits are directly modified to obtain a covalent attachment of biochemical cues such as proteins, peptide sequences, and growth factors to improve their bioactivity. Natural materials have the advantage of conferring the needed biological sites for proteins to bind and biological cues for cell behavior to be controlled. This bioactivity is less under control in comparison to that of synthetic biomaterials, nevertheless, in most cases, as previously reported, the release of molecules, such as chito-oligosaccharides (Gong et al., 2009; Zhao et al., 2017), improves nerve regeneration without interfering with other biological processes. In the literature, most of preclinical studies pay more attention to nerve regeneration inside the conduit and biomaterial local effects in the implantation site rather than on possible systemic consequences. Indeed, few studies consider the systemic effect of the biomaterials, which could be a strategy to address the problems of the non-controlled multi-bioactivity of the different biopolymers. Nevertheless, researchers tend to consider as a good characteristic the presence of bioactive sites on natural biopolymers, rather than consider it as a problem. Furthermore, some authors believe that a natural biomaterial, such as silk, requires to be modified through different strategies, to increase its bioactivity (Magaz et al., 2018).
As well as biomaterials in general, natural-based biomaterials described in this manuscript present advantages and disadvantages (Table 1), and some of them are more suitable for being used as nerve conduits or as internal filler of the conduits. To overcome natural biomaterial limitations, a successful strategy is biomaterial blending. The combination of natural-based biomaterials with synthetic ones can enhance the poor mechanical characteristics of the natural ones, while thanks to their higher biocompatibility, natural biomaterial blending reduces inflammatory response induced by synthetic materials (Nectow et al., 2012). Also, natural-based biomaterial blending could be a successful strategy since it could allow using natural biomaterial commonly used as an internal filler (as alginate) to obtain conduits, exploiting the mechanical strength of other ones like chitosan (Pfister et al., 2007a).
Finally, it appears increasingly clear that a universal ideal conduit is difficult to be produced since conduit properties have to be evaluated case by case according to the gap length, but also on the implant anatomical district. Also, for conduit wall fitting nerve diameter should be taken into account and nerve elasticity changes depending on nerve size (∼21,188 Pa for a pig sciatic nerve and ∼10,910,000 Pa for a human median nerve) and on how distal the nerve lesion is from the spinal cord (Stouthandel et al., 2020). Then, it must be considered that when Young’s modulus is reported in the validation studies of a biomaterial, only partial information is given referring to the NGC outer layer mechanical behavior, a characteristic which can be modified by the biomaterial dryness (De Masi et al., 2019) and by the concentration and type of fillers that generally increase the NGC stiffness (Ryan et al., 2017; Tonda-Turo et al., 2017a; Vijayavenkataraman et al., 2019).
In addition, in preclinical studies other information about conduit manufacturing and some details about conduit characteristics are often missing, such as wall thickness or porosity, which could be useful to understand how nerve regeneration is influenced by conduit properties. Furthermore, the conduit properties often are not described with quantitative parameters that could allow standardization of criteria and protocols.
Moreover, even if some characteristics of an ideal conduit are well outlined, such as biocompatibility, other more complex like conduit degradation or wall thickness, which influences conduit permeability and porosity, are non-solved required properties, which need further investigations. Consequently, despite conduits showed to efficiently support nerve regeneration and are often successfully used, nerve autografts continue to be the gold standard technique for repairing long nerve defects, thus highlighting the need to develop more effective alternatives.
Author Contributions
BF, GG, and SR organized the manuscript. BF prepared the figures. BF and GC wrote different sections of the manuscript. All authors contributed to manuscript revision, and read and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thanks Chiara Tonda Turo for helpful suggestion for the paragraph on “Future trends in the design of NGCs using natural-based biomaterials” and for critical reading of the manuscript.
References
Åberg, M., Ljungberg, C., Edin, E., Millqvist, H., Nordh, E., Theorin, A., et al. (2009). Clinical evaluation of a resorbable wrap-around implant as an alternative to nerve repair: a prospective, assessor-blinded, randomised clinical study of sensory, motor and functional recovery after peripheral nerve repair. J. Plast. Reconstr. Aesthetic Surg. 62, 1503–1509. doi: 10.1016/j.bjps.2008.06.041
Ahmed, Z., Underwood, S., and Brown, R. A. (2003). Nerve guide material made from fibronectin: assessment of in vitro properties. Tissue Eng. 9, 219–231. doi: 10.1089/107632703764664693
Aigner, T. B., Haynl, C., Salehi, S., O’Connor, A., and Scheibel, T. (2020). Nerve guidance conduit design based on self-rolling tubes. Mater. Today Biol. 5:100042. doi: 10.1016/j.mtbio.2020.100042
Albala, D. M., and Lawson, J. H. (2006). Recent clinical and investigational applications of fibrin sealant in selected surgical specialties. J. Am. Coll. Surg. 202, 685–697. doi: 10.1016/j.jamcollsurg.2005.11.027
Alberti, K. A., Hopkins, A. M., Tang-Schomer, M. D., Kaplan, D. L., and Xu, Q. (2014). The behavior of neuronal cells on tendon-derived collagen sheets as potential substrates for nerve regeneration. Biomaterials 35, 3551–3557. doi: 10.1016/j.biomaterials.2013.12.082
Alessandrino, A., Fregnan, F., Biagiotti, M., Muratori, L., Bassani, G. A., Ronchi, G., et al. (2019). SilkBridgeTM: a novel biomimetic and biocompatible silk-based nerve conduit. Biomater. Sci. 7, 4112–4130. doi: 10.1039/c9bm00783k
Allmeling, C., Jokuszies, A., Reimers, K., Kall, S., Choi, C. Y., Brandes, G., et al. (2008). Spider silk fibres in artificial nerve constructs promote peripheral nerve regeneration. Cell Prolif. 41, 408–420. doi: 10.1111/j.1365-2184.2008.00534.x
Altman, G. H., Diaz, F., Jakuba, C., Calabro, T., Horan, R. L., Chen, J., et al. (2003). Silk-based biomaterials. Biomaterials 24, 401–416. doi: 10.1016/S0142-9612(02)00353-8
Angius, D., Wang, H., Spinner, R. J., Gutierrez-Cotto, Y., Yaszemski, M. J., and Windebank, A. J. (2012). A systematic review of animal models used to study nerve regeneration in tissue-engineered scaffolds. Biomaterials 33, 8034–8039. doi: 10.1016/j.biomaterials.2012.07.056
Anjum, A., Zuber, M., Zia, K. M., Noreen, A., Anjum, M. N., and Tabasum, S. (2016). Microbial production of polyhydroxyalkanoates (PHAs) and its copolymers: a review of recent advancements. Int. J. Biol. Macromol. 89, 161–174. doi: 10.1016/j.ijbiomac.2016.04.069
Ao, Q., Fung, C. K., Yat-Ping Tsui, A., Cai, S., Zuo, H. C., Chan, Y. S., et al. (2011). The regeneration of transected sciatic nerves of adult rats using chitosan nerve conduits seeded with bone marrow stromal cell-derived Schwann cells. Biomaterials 32, 787–796. doi: 10.1016/j.biomaterials.2010.09.046
Apel, P. J., Garrett, J. P., Sierpinski, P., Ma, J., Atala, A., Smith, T. L., et al. (2008). Peripheral nerve regeneration using a keratin-based scaffold: long-term functional and histological outcomes in a mouse model. J. Hand Surg. Am. 33, 1541–1547. doi: 10.1016/j.jhsa.2008.05.034
Archibald, S. J., Krarup, C., Shefner, J., Li, S. -T., and Madison, R. D. (1991). A collagen-based nerve guide conduit for peripheral nerve repair: an electrophysiological study of nerve regeneration in rodents and nonhuman primates. J. Comp. Neurol. 306, 685–696. doi: 10.1002/cne.903060410
Archibald, S. J., Shefner, J., Krarup, C., and Madison, R. D. (1995). Monkey median nerve repaired by nerve graft or collagen nerve guide tube. J. Neurosci. 15, 4109–4123. doi: 10.1523/jneurosci.15-05-04109.1995
Arnaout, A., Fontaine, C., and Chantelot, C. (2014). Sensory recovery after primary repair of palmar digital nerves using a Revolnerv collagen conduit: a prospective series of 27 cases. Chir. Main 33, 279–285. doi: 10.1016/j.main.2014.05.002
Arslantunali, D., Dursun, T., Yucel, D., Hasirci, N., and Hasirci, V. (2014). Peripheral nerve conduits: technology update. Med. Dev. Evid. Res. 7, 405–424. doi: 10.2147/MDER.S59124
Askarzadeh, N., Nazarpak, M. H., Mansoori, K., Farokhi, M., Gholami, M., Mohammadi, J., et al. (2020). Bilayer cylindrical conduit consisting of electrospun polycaprolactone nanofibers and dsc cross-linked sodium alginate hydrogel to bridge peripheral nerve gaps. Macromol. Biosci. 2000149, 1–15. doi: 10.1002/mabi.202000149
Augst, A. D., Kong, H. J., and Mooney, D. J. (2006). Alginate hydrogels as biomaterials. Macromol. Biosci. 6, 623–633. doi: 10.1002/mabi.200600069
Bacakova, L., Filova, E., Parizek, M., Ruml, T., and Svorcik, V. (2011). Modulation of cell adhesion, proliferation and differentiation on materials designed for body implants. Biotechnol. Adv. 29, 739–767. doi: 10.1016/j.biotechadv.2011.06.004
Bačáková, L., Filová, E., Rypáček, F., Švorčík, V., and Starý, V. (2004). Cell adhesion on artificial materials for tissue engineering. Physiol. Res. 53, S35–S45.
Bagdadi, A. V., Safari, M., Dubey, P., Basnett, P., Sofokleous, P., Humphrey, E., et al. (2018). Poly(3-hydroxyoctanoate), a promising new material for cardiac tissue engineering. J. Tissue Eng. Regen. Med. 12, e495–e512. doi: 10.1002/term.2318
Basnett, P., Lukasiewicz, B., Marcello, E., Gura, H. K., Knowles, J. C., and Roy, I. (2017). Production of a novel medium chain length poly(3-hydroxyalkanoate) using unprocessed biodiesel waste and its evaluation as a tissue engineering scaffold. Microb. Biotechnol. 10, 1384–1399. doi: 10.1111/1751-7915.12782
Belanger, K., Schlatter, G., Hébraud, A., Marin, F., Testelin, S., Dakpé, S., et al. (2018). A multi-layered nerve guidance conduit design adapted to facilitate surgical implantation. Heal. Sci. Rep. 1:e86. doi: 10.1002/hsr2.86
Belkas, J. S., Munro, C. A., Shoichet, M. S., Johnston, M., and Midha, R. (2005). Long-term in vivo biomechanical properties and biocompatibility of poly(2-hydroxyethyl methacrylate-co-methyl methacrylate) nerve conduits. Biomaterials 26, 1741–1749. doi: 10.1016/j.biomaterials.2004.05.031
Belkas, J. S., Shoichet, M. S., and Midha, R. (2004). Peripheral nerve regeneration through guidance tubes. Neurol. Res. 26, 151–160. doi: 10.1179/016164104225013798
Bensaïd, W., Triffitt, J. T., Blanchat, C., Oudina, K., Sedel, L., and Petite, H. (2003). A biodegradable fibrin scaffold for mesenchymal stem cell transplantation. Biomaterials 24, 2497–2502. doi: 10.1016/S0142-9612(02)00618-X
Berkovitch, Y., Cohen, T., Peled, E., Schmidhammer, R., Florian, H., Teuschl, A. H., et al. (2018). Hydrogel composition and laser micropatterning to regulate sciatic nerve regeneration. J. Tissue Eng. Regen. Med 12, 1049-1061. doi: 10.1002/term.2606
Biazar, E., and Heidari Keshel, S. (2013). A nanofibrous PHBV tube with Schwann cell as artificial nerve graft contributing to Rat sciatic nerve regeneration across a 30-mm defect bridge. Cell Commun. Adhes 20, 41-49. doi: 10.3109/15419061.2013.774378
Biazar, E., and Keshel, S. H. (2013). Chitosan-cross-linked nanofibrous PHBV nerve guide for rat sciatic nerve regeneration across a defect bridge. ASAIO J. 59, 651–659. doi: 10.1097/MAT.0b013e3182a79151
Biazar, E., Keshel, S. H., and Pouya, M. (2013a). Behavioral evaluation of regenerated rat sciatic nerve by a nanofibrous PHBV conduit filled with Schwann cells as artificial nerve graft. Cell Commun. Adhes. 20, 93-103. doi: 10.3109/15419061.2013.833191
Biazar, E., Keshel, S. H., and Pouya, M. (2013b). Efficacy of nanofibrous conduits in repair of long-segment sciatic nerve defects. Neural Regen. Res. 8, 2501–2509. doi: 10.3969/j.issn.1673-5374.2013.27.001
Biazar, E., Keshel, S. H., Pouya, M., Rad, H., Nava, M. O., Azarbakhsh, M., et al. (2013c). Nanofibrous nerve conduits for repair of 30-mm-long sciatic nerve defects. Neural Regen. Res. 8, 2266–2274. doi: 10.3969/j.issn.1673-5374.2013.24.006
Binan, L., Tendey, C., De Crescenzo, G., El Ayoubi, R., Ajji, A., and Jolicoeur, M. (2014). Differentiation of neuronal stem cells into motor neurons using electrospun poly-l-lactic acid/gelatin scaffold. Biomaterials 35, 664–674. doi: 10.1016/j.biomaterials.2013.09.097
Blessing, M., Nanney, L. B., King, L. E., Jones, C. M., and Hogan, B. L. M. (1993). Transgenic mice as a model to study the role of TGF-β-related moleules in hair follicles. Genes Dev. 7, 204-215. doi: 10.1101/gad.7.2.204
Boecker, A., Daeschler, S. C., Kneser, U., and Harhaus, L. (2019). Relevance and recent developments of chitosan in peripheral nerve surgery. Front. Cell. Neurosci. 13:104. doi: 10.3389/fncel.2019.00104
Boeckstyns, M. E. H., Sørensen, A. I., Viñeta, J. F., Rosén, B., Navarro, X., Archibald, S. J., et al. (2013). Collagen conduit versus microsurgical neurorrhaphy: 2-year follow-up of a prospective, blinded clinical and electrophysiological multicenter randomized, controlled trial. J. Hand Surg. Am. 38, 2405-2411. doi: 10.1016/j.jhsa.2013.09.038
Boido, M., Ghibaudi, M., Gentile, P., Favaro, E., Fusaro, R., and Tonda-Turo, C. (2019). Chitosan-based hydrogel to support the paracrine activity of mesenchymal stem cells in spinal cord injury treatment. Sci. Rep. 9:6402. doi: 10.1038/s41598-019-42848-w
Boonlertnirun, S., Boonraung, C., and Suvanasara, R. (2008). Application of Chitosan in Rice Production. Available online at: http://www.ojs.materialsconnex.com/index.php/jmmm/article/view/310 (accessed March 29, 2020).
Borschel, G. H., Kia, K. F., Kuzon, W. M., and Dennis, R. G. (2003). Mechanical properties of acellular peripheral nerve. J. Surg. Res. 114, 133-139. doi: 10.1016/S0022-4804(03)00255-5
Bozkurt, A., Lassner, F., O’Dey, D., Deumens, R., Böcker, A., Schwendt, T., et al. (2012). The role of microstructured and interconnected pore channels in a collagen-based nerve guide on axonal regeneration in peripheral nerves. Biomaterials 33, 1363–1375. doi: 10.1016/j.biomaterials.2011.10.069
Bruekers, S. M. C., Jaspers, M., Hendriks, J. M. A., Kurniawan, N. A., Koenderink, G. H., Kouwer, P. H. J., et al. (2016). Fibrin-fiber architecture influences cell spreading and differentiation. Cell Adhes. Migr. 10, 495-504. doi: 10.1080/19336918.2016.1151607
Cao, Y., and Wang, B. (2009). Biodegradation of silk biomaterials. Int. J. Mol. Sci. 10, 1514–1524. doi: 10.3390/ijms10041514
Carvalho, C. R., Oliveira, J. M., and Reis, R. L. (2019). Modern trends for peripheral nerve repair and regeneration: beyond the hollow nerve guidance conduit. Front. Bioeng. Biotechnol. 7:337. doi: 10.3389/fbioe.2019.00337
Cenni, E., Ciapetti, G., Stea, S., Corradini, A., and Carozzi, F. (2000). Biocompatibility and performance in vitro of a hemostatic gelatin sponge. J. Biomater. Sci. Polym. Ed. 11, 685–699. doi: 10.1163/156856200743959
Chang, J.-Y., Ho, T.-Y., Lee, H.-C., Lai, Y.-L., Lu, M.-C., Yao, C.-H., et al. (2009). Highly permeable genipin-cross-linked gelatin conduits enhance peripheral nerve regeneration. Artif. Org. 33, 1075–1085. doi: 10.1111/j.1525-1594.2009.00818.x
Chen, G.-Q. (2010). “Plastics Completely Synthesized by Bacteria: Polyhydroxyalkanoates,” in. Berlin: Springer, 17–37. doi: 10.1007/978-3-642-03287-5_2
Chen, H., Xie, S., Yang, Y., Zhang, J., and Zhang, Z. (2018). Multiscale regeneration scaffold in vitro and in vivo. J. Biomed. Mater. Res. Part B Appl. Biomater. 106, 1218-1225. doi: 10.1002/jbm.b.33926
Chen, P. R., Chen, M. H., Lin, F. H., and Su, W. Y. (2005). Release characteristics and bioactivity of gelatin-tricalcium phosphate membranes covalently immobilized with nerve growth factors. Biomaterials 26, 6579–6587. doi: 10.1016/j.biomaterials.2005.03.037
Chen, S., Zhao, Y., Yan, X., Zhang, L., Li, G., and Yang, Y. (2019). PAM/GO/gel/SA composite hydrogel conduit with bioactivity for repairing peripheral nerve injury. J. Biomed. Mater. Res. Part A. 107, 1273-1283. doi: 10.1002/jbm.a.36637
Chen, Y. S., Chang, J. Y., Cheng, C. Y., Tsai, F. J., Yao, C. H., and Liu, B. S. (2005). An in vivo evaluation of a biodegradable genipin-cross-linked gelatin peripheral nerve guide conduit material. Biomaterials 26, 3911–3918. doi: 10.1016/j.biomaterials.2004.09.060
Chiono, V., and Tonda-Turo, C. (2015). Trends in the design of nerve guidance channels in peripheral nerve tissue engineering. Prog. Neurobiol. 131, 87–104. doi: 10.1016/j.pneurobio.2015.06.001
Chrząszcz, P., Derbisz, K., Suszyński, K., Miodoński, J., Trybulski, R., Lewin-Kowalik, J., et al. (2018). Application of peripheral nerve conduits in clinical practice: a literature review. Neurol. Neurochir. Pol. 52, 427–435. doi: 10.1016/j.pjnns.2018.06.003
Clements, B. A., Bushman, J., Murthy, N. S., Ezra, M., Pastore, C. M., and Kohn, J. (2016). Design of barrier coatings on kink-resistant peripheral nerve conduits. J. Tissue Eng. 7, 2041731416629471. doi: 10.1177/2041731416629471
Costantini, M., Colosi, C., Mozetic, P., Jaroszewicz, J., Tosato, A., Rainer, A., et al. (2016). Correlation between porous texture and cell seeding efficiency of gas foaming and microfluidic foaming scaffolds. Mater. Sci. Eng. C 62, 668-677. doi: 10.1016/j.msec.2016.02.010
Crosio, A., Fornasari, B., Gambarotta, G., Geuna, S., Raimondo, S., Battiston, B., et al. (2019). Chitosan tubes enriched with fresh skeletal muscle fibers for delayed repair of peripheral nerve defects. Neural Regen. Res. 14:1079. doi: 10.4103/1673-5374.250628
Dadsetan, M., Knight, A. M., Lu, L., Windebank, A. J., and Yaszemski, M. J. (2009). Stimulation of neurite outgrowth using positively charged hydrogels. Biomaterials 30, 3874–3881. doi: 10.1016/j.biomaterials.2009.04.018
Dalamagkas, K., Tsintou, M., and Seifalian, A. (2016). Advances in peripheral nervous system regenerative therapeutic strategies: a biomaterials approach. Mater. Sci. Eng. C. Mater. Biol. Appl. 65, 425–432. doi: 10.1016/j.msec.2016.04.048
Daly, W. T., Yao, L., Abu-rub, M. T., O’Connell, C., Zeugolis, D. I., Windebank, A. J., et al. (2012). The effect of intraluminal contact mediated guidance signals on axonal mismatch during peripheral nerve repair. Biomaterials 33, 6660–6671. doi: 10.1016/j.biomaterials.2012.06.002
De la Puente, P., and Ludeña, D. (2014). Cell culture in autologous fibrin scaffolds for applications in tissue engineering. Exp. Cell Res. 322, 1-11. doi: 10.1016/j.yexcr.2013.12.017
De Masi, A., Tonazzini, I., Masciullo, C., Mezzena, R., Chiellini, F., Puppi, D., et al. (2019). Chitosan films for regenerative medicine: fabrication methods and mechanical characterization of nanostructured chitosan films. Biophys. Rev. 11, 807-815. doi: 10.1007/s12551-019-00591-6
de Queiroz Antonino, R. S. C. M., Lia Fook, B. R. P., de Oliveira Lima, V. A., de Farias Rached, R. Í, Lima, E. P. N., da Silva Lima, R. J., et al. (2017). Preparation and Characterization of Chitosan Obtained from Shells of Shrimp (Litopenaeus vannamei Boone). Mar. Drugs 15:141. doi: 10.3390/md15050141
de Ruiter, G. C. W., Malessy, M. J. A., Yaszemski, M. J., Windebank, A. J., and Spinner, R. J. (2009). Designing ideal conduits for peripheral nerve repair. Neurosurg. Focus 26, 1–9. doi: 10.3171/FOC.2009.26.2.E5
Demirbilek, M., Sakar, M., Karahaliloglu, Z., Erdal, E., Yalcin, E., Bozkurt, G., et al. (2015). Aligned bacterial PHBV nanofi brous conduit for peripheral nerve regeneration. Artif. Cells, Nanomed. Biotechnol. 43, 243-251. doi: 10.3109/21691401.2013.875033
Deng, M., Chen, G., Burkley, D., Zhou, J., Jamiolkowski, D., Xu, Y., et al. (2008). A study on in vitro degradation behavior of a poly(glycolide-co-L-lactide) monofilament. Acta Biomater. 4, 1382–1391. doi: 10.1016/j.actbio.2008.03.011
Di Summa, P. G., Kingham, P. J., Campisi, C. C., Raffoul, W., and Kalbermatten, D. F. (2014). Collagen (NeuraGen) nerve conduits and stem cells for peripheral nerve gap repair. Neurosci. Lett. 572, 26-31. doi: 10.1016/j.neulet.2014.04.029
Ding, F., Wu, J., Yang, Y., Hu, W., Zhu, Q., Tang, X., et al. (2010). Use of tissue-engineered nerve grafts consisting of a chitosan/poly(lactic- co-glycolic acid)-based scaffold included with bone marrow mesenchymal cells for bridging 50-mm dog sciatic nerve gaps. Tissue Eng. Part A 16, 3779–3790. doi: 10.1089/ten.tea.2010.0299
Dodla, M. C., and Bellamkonda, R. V. (2008). Differences between the effect of anisotropic and isotropic laminin and nerve growth factor presenting scaffolds on nerve regeneration across long peripheral nerve gaps. Biomaterials 29, 33–46. doi: 10.1016/j.biomaterials.2007.08.045
Dranseikiene, D., Schrüfer, S., Schubert, D. W., Reakasame, S., and Boccaccini, A. R. (2020). Cell-laden alginate dialdehyde–gelatin hydrogels formed in 3D printed sacrificial gel. J. Mater. Sci. Mater. Med. 31, 1–5. doi: 10.1007/s10856-020-06369-7
Drobnik, J., Pietrucha, K., Kudzin, M., Mader, K., Szymañski, J., and Szczepanowska, A. (2017a). Comparison of various types of collagenous scaffolds applied for embryonic nerve cell culture. Biologicals 46, 74-80. doi: 10.1016/j.biologicals.2017.01.001
Drobnik, J., Pietrucha, K., Piera, L., Szymañski, J., and Szczepanowska, A. (2017b). Collagenous scaffolds supplemented with hyaluronic acid and chondroitin sulfate used for wound fibroblast and embryonic nerve cell culture. Adv. Clin. Exp. Med. 26, 223-230. doi: 10.17219/acem/62835
Drury, J. L., and Mooney, D. J. (2003). Hydrogels for tissue engineering: scaffold design variables and applications. Biomaterials 24, 4337–4351. doi: 10.1016/S0142-9612(03)00340-5
Du, J., and Jia, X. (2019). Engineering nerve guidance conduits with three-dimenisonal bioprinting technology for long gap peripheral nerve regeneration. Neural Regen. Res. 14:2073. doi: 10.4103/1673-5374.262580
Ducker, T. B., and Hayes, G. J. (1968). Experimental improvements in the use of Silastic cuff for peripheral nerve repair. J. Neurosurg. 28, 582–587. doi: 10.3171/jns.1968.28.6.0582
Entekhabi, E., Haghbin Nazarpak, M., Shafieian, M., Mohammadi, H., Firouzi, M., and Hassannejad, Z. (2020). Fabrication and in vitro evaluation of 3D composite scaffold based on collagen/hyaluronic acid sponge and electrospun PCL nanofibers for peripheral nerve regeneration. J. Biomed. Mater. Res. Part A 2020, 1–13. doi: 10.1002/jbm.a.37023
Eppley, B. L., and Delfino, J. J. (1988). Collagen tube repair of the mandibular nerve: a preliminary investigation in the rat. J. Oral Maxillofac. Surg. 46, 41-47. doi: 10.1016/0278-2391(88)90298-4
Fan, W., Gu, J., Hu, W., Deng, A., Ma, Y., Liu, J., et al. (2008). Repairing a 35-mm-long median nerve defect with a chitosan/PGA artificial nerve graft in the human: a case study. Microsurgery 28, 238–242. doi: 10.1002/micr.20488
Fang, H., Peng, S., Chen, A., Li, F., Ren, K., and Hu, N. (2004). Biocompatibility studies on fibrin glue cultured with bone marrow mesenchymal stem cells in vitro. J. Huazhong Univ. Sci. Technolog. Med. Sci. 24, 272–274. doi: 10.1007/bf02832010
Fornasari, B. E., El Soury, M., Nato, G., Fucini, A., Carta, G., Ronchi, G., et al. (2020). Fibroblasts colonizing nerve conduits express high levels of soluble neuregulin1, a factor promoting schwann cell dedifferentiation. Cells 9:1366. doi: 10.3390/cells9061366
Freier, T., Koh, H. S., Kazazian, K., and Shoichet, M. S. (2005a). Controlling cell adhesion and degradation of chitosan films by N-acetylation. Biomaterials 26, 5872–5878. doi: 10.1016/j.biomaterials.2005.02.033
Freier, T., Montenegro, R., Koh, H. S., and Shoichet, M. S. (2005b). Chitin-based tubes for tissue engineering in the nervous system. Biomaterials 26, 4624–4632. doi: 10.1016/j.biomaterials.2004.11.040
Gámez, E., Goto, Y., Nagata, K., Iwaki, T., Sasaki, T., and Matsuda, T. (2004). Photofabricated gelatin-based nerve conduits: nerve tissue regeneration potentials. Cell Transplant. 13, 549–564. doi: 10.3727/000000004783983639
Gámez, E., Ikezaki, K., Fukui, M., and Matsuda, T. (2003). Photoconstructs of nerve guidance prosthesis using photoreactive gelatin as a scaffold. Cell Transplant. 12, 481–490. doi: 10.3727/000000003108747046
Gao, C., Liu, M., Chen, J., and Zhang, X. (2009). Preparation and controlled degradation of oxidized sodium alginate hydrogel. Polym. Degrad. Stab. 94, 1405-1410. doi: 10.1016/j.polymdegradstab.2009.05.011
Giannessi, E., Stornelli, M. R., Coli, A., and Sergi, P. N. (2019). A quantitative investigation on the peripheral nerve response within the small strain range. Appl. Sci 9:1115. doi: 10.3390/app9061115
Gnavi, S., Fornasari, B. E., Tonda-Turo, C., Ciardelli, G., Zanetti, M., Geuna, S., et al. (2015). The influence of electrospun fibre size on Schwann cell behaviour and axonal outgrowth. Mater. Sci. Eng. C. Mater. Biol. Appl. 48, 620–631. doi: 10.1016/j.msec.2014.12.055
Golafshan, N., Kharaziha, M., and Fathi, M. (2017). Tough and conductive hybrid graphene-PVA: alginate fibrous scaffolds for engineering neural construct. Carbon N.Y. 111, 752-763. doi: 10.1016/j.carbon.2016.10.042
Gong, Y., Gong, L., Gu, X., and Ding, F. (2009). Chitooligosaccharides promote peripheral nerve regeneration in a rabbit common peroneal nerve crush injury model. Microsurgery 29, 650–656. doi: 10.1002/micr.20686
Gonzalez-Perez, F., Cobianchi, S., Geuna, S., Barwig, C., Freier, T., Udina, E., et al. (2015). Tubulization with chitosan guides for the repair of long gap peripheral nerve injury in the rat. Microsurgery 35, 300–308. doi: 10.1002/micr.22362
Goulart, C. O., Pereira Lopes, F. R., Monte, Z. O., Dantas, S. V., Souto, A., Oliveira, J. T., et al. (2016). Evaluation of biodegradable polymer conduits - poly(l-lactic acid) - for guiding sciatic nerve regeneration in mice. Methods 99, 28–36. doi: 10.1016/j.ymeth.2015.09.008
Gu, X., Ding, F., and Williams, D. F. (2014). Neural tissue engineering options for peripheral nerve regeneration. Biomaterials 34, 6143-6156. doi: 10.1016/j.biomaterials.2014.04.064
Gu, X., Ding, F., Yang, Y., and Liu, J. (2011). Construction of tissue engineered nerve grafts and their application in peripheral nerve regeneration. Prog. Neurobiol. 93, 204–230. doi: 10.1016/j.pneurobio.2010.11.002
Gupta, P., and Nayak, K. K. (2016). Optimization of keratin/alginate scaffold using RSM and its characterization for tissue engineering. Int. J. Biol. Macromol. 85, 141–149. doi: 10.1016/j.ijbiomac.2015.12.010
Haastert-Talini, K., Geuna, S., Dahlin, L. B., Meyer, C., Stenberg, L., Freier, T., et al. (2013). Chitosan tubes of varying degrees of acetylation for bridging peripheral nerve defects. Biomaterials 34, 9886–9904. doi: 10.1016/j.biomaterials.2013.08.074
Hamed, I., Özogul, F., and Regenstein, J. M. (2016). Industrial applications of crustacean by-products (chitin, chitosan, and chitooligosaccharides): a review. Trends Food Sci. Technol. 48, 40–50. doi: 10.1016/j.tifs.2015.11.007
Hardy, M. H. (1992). The secret life of the hair follicle. Trends Genet. 8, 55-61. doi: 10.1016/0168-9525(92)90350-D
Harvey, A. G., Hill, E. W., and Bayat, A. (2013). Designing implant surface topography for improved biocompatibility. Exp. Rev. Med. Dev. 10, 257–267. doi: 10.1586/erd.12.82
Hashimoto, T., Suzuki, Y., Kitada, M., Kataoka, K., Wu, S., Suzuki, K., et al. (2002). Peripheral nerve regeneration through alginate gel: analysis of early outgrowth and late increase in diameter of regenerating axons. Exp. Brain Res. 146, 356-358. doi: 10.1007/s00221-002-1173-y
Hazari, A., Wiberg, M., Johansson-Rudén, G., Green, C., and Terenghi, G. (1999). A resorbable nerve conduit as an alternative to nerve autograft in nerve gap repair. Br. J. Plast. Surg. 52, 653-657. doi: 10.1054/bjps.1999.3184
He, Q., Zhang, T., Yang, Y., and Ding, F. (2009). In vitro biocompatibility of chitosan-based materials to primary culture of hippocampal neurons. J. Mater. Sci. Mater. Med. 20, 1457-1466. doi: 10.1007/s10856-009-3702-8
Hemshekhar, M., Thushara, R. M., Chandranayaka, S., Sherman, L. S., Kemparaju, K., and Girish, K. S. (2016). Emerging roles of hyaluronic acid bioscaffolds in tissue engineering and regenerative medicine. Int. J. Biol. Macromol. 86, 917–928. doi: 10.1016/j.ijbiomac.2016.02.032
Hill, P., Brantley, H., and Van Dyke, M. (2010). Some properties of keratin biomaterials: kerateines. Biomaterials 31, 585-593. doi: 10.1016/j.biomaterials.2009.09.076
Hill, P. S., Apel, P. J., Barnwell, J., Smith, T., Koman, L. A., Atala, A., et al. (2011). Repair of peripheral nerve defects in rabbits using keratin hydrogel scaffolds. Tissue Eng. Part A 17, 1499-1505. doi: 10.1089/ten.tea.2010.0184
Hinüber, C., Chwalek, K., Pan-Montojo, F. J., Nitschke, M., Vogel, R., Brünig, H., et al. (2014). Hierarchically structured nerve guidance channels based on poly-3-hydroxybutyrate enhance oriented axonal outgrowth. Acta Biomater. 10, 2086–2095. doi: 10.1016/j.actbio.2013.12.053
Homaeigohar, S., Tsai, T. Y., Young, T. H., Yang, H. J., and Ji, Y. R. (2019). An electroactive alginate hydrogel nanocomposite reinforced by functionalized graphite nanofilaments for neural tissue engineering. Carbohydr. Polym 224:115112. doi: 10.1016/j.carbpol.2019.115112
Houshyar, S., Bhattacharyya, A., and Shanks, R. (2019). Peripheral nerve conduit: materials and Structures. ACS Chem. Neurosci. 10, 3349–3365. doi: 10.1021/acschemneuro.9b00203
Hu, F., Zhang, X., Liu, H., Xu, P., Doulathunnisa, Teng, G., et al. (2017). Neuronally differentiated adipose-derived stem cells and aligned PHBV nanofiber nerve scaffolds promote sciatic nerve regeneration. Biochem. Biophys. Res. Commun. 489, 171-178. doi: 10.1016/j.bbrc.2017.05.119
Hu, Q. L., Piao, Y. J., and Zou, F. (2003). Expression and distribution of NGF and p75 during rabbit tibial nerve repair induced by human hair keratin conduits. Di Yi Jun Yi Da Xue Xue Bao 23, 929-932.
Hu, Y., Mao, A. S., Desai, R. M., Wang, H., Weitz, D. A., and Mooney, D. J. (2017). Controlled self-assembly of alginate microgels by rapidly binding molecule pairs. Lab. Chip. 17, 2481-2490. doi: 10.1039/c7lc00500h
Huang, C., Chen, R., Ke, Q., Morsi, Y., Zhang, K., and Mo, X. (2011). Electrospun collagen-chitosan-TPU nanofibrous scaffolds for tissue engineered tubular grafts. Coll. Surf. B Biointerf. 82, 307-315. doi: 10.1016/j.colsurfb.2010.09.002
Huang, C., Ouyang, Y., Niu, H., He, N., Ke, Q., Jin, X., et al. (2015). Nerve guidance conduits from aligned nanofibers: improvement of nerve regeneration through longitudinal nanogrooves on a fiber surface. ACS Appl. Mater. Interf. 7, 7189–7196. doi: 10.1021/am509227t
Huang, W., Begum, R., Barber, T., Ibba, V., Tee, N. C. H., Hussain, M., et al. (2012). Regenerative potential of silk conduits in repair of peripheral nerve injury in adult rats. Biomaterials 33, 59–71. doi: 10.1016/j.biomaterials.2011.09.030
Ijkema-Paassen, J., Jansen, K., Gramsbergen, A., and Meek, M. F. (2004). Transection of peripheral nerves, bridging strategies and effect evaluation. Biomaterials 25, 1583–1592. doi: 10.1016/S0142-9612(03)00504-0
Isaacs, J., Mallu, S., Yan, W., and Little, B. (2014). Consequences of oversizing: nerve-to-nerve tube diameter mismatch. J. Bone Jt. Surg. Am. Vol 95, 2144-2151. doi: 10.2106/JBJS.M.01420
Jahromi, H. K., Farzin, A., Hasanzadeh, E., Barough, S. E., Mahmoodi, N., Najafabadi, M. R. H., et al. (2020). Enhanced sciatic nerve regeneration by poly-L-lactic acid/multi-wall carbon nanotube neural guidance conduit containing Schwann cells and curcumin encapsulated chitosan nanoparticles in rat. Mater. Sci. Eng. C 109:110564. doi: 10.1016/j.msec.2019.110564
Jansen, K., Van Der Werff, J. F. A., Van Wachem, P. B., Nicolai, J. P. A., De Leij, L. F. M. H., and Van Luyn, M. J. A. (2004). A hyaluronan-based nerve guide: in vitro cytotoxicity, subcutaneous tissue reactions, and degradation in the rat. Biomaterials 25, 483–489. doi: 10.1016/S0142-9612(03)00544-1
Jeon, O., Bouhadir, K. H., Mansour, J. M., and Alsberg, E. (2009). Photocrosslinked alginate hydrogels with tunable biodegradation rates and mechanical properties. Biomaterials 30, 2724-2734. doi: 10.1016/j.biomaterials.2009.01.034
Jiang, X., Lim, S. H., Mao Hai-Quan, H. Q., and Chew, S. Y. (2010). Current applications and future perspectives of artificial nerve conduits. Exp. Neurol. 223, 86–101. doi: 10.1016/j.expneurol.2009.09.009
Jiao, H., Yao, J., Yang, Y., Chen, X., Lin, W., Li, Y., et al. (2009). Chitosan/polyglycolic acid nerve grafts for axon regeneration from prolonged axotomized neurons to chronically denervated segments. Biomaterials 30, 5004–5018. doi: 10.1016/j.biomaterials.2009.05.059
Jing, W., Ao, Q., Wang, L., Huang, Z., Cai, Q., Chen, G., et al. (2018). Constructing conductive conduit with conductive fibrous infilling for peripheral nerve regeneration. Chem. Eng. J. 345, 566–577. doi: 10.1016/j.cej.2018.04.044
Johnson, B. N., and Jia, X. (2016). 3D printed nerve guidance channels: computer-aided control of geometry, physical cues, biological supplements and gradients. Neural Regen. Res. 11, 1568–1569. doi: 10.4103/1673-5374.193230
Jones, C. M., Lyons, K. M., and Hogan, B. L. M. (1991). Involvement of Bone Morphogenetic Protein-4 (BMP-4) and Vgr-1 in morphogenesis and neurogenesis in the mouse. Development 111, 531-542.
Kalbermatten, D. F., Erba, P., Mahay, D., Wiberg, M., Pierer, G., and Terenghi, G. (2008a). Schwann cell strip for peripheral nerve repair. J. Hand Surg. Eur. 33, 587–594. doi: 10.1177/1753193408090755
Kalbermatten, D. F., Kingham, P. J., Mahay, D., Mantovani, C., Pettersson, J., Raffoul, W., et al. (2008b). Fibrin matrix for suspension of regenerative cells in an artificial nerve conduit. J. Plast. Reconstr. Aesthetic Surg. 61, 669-675. doi: 10.1016/j.bjps.2007.12.015
Kalbermatten, D. F., Pettersson, J., Kingham, P. J., Pierer, G., Wiberg, M., and Terenghi, G. (2009). New fibrin conduit for peripheral nerve repair. J. Reconstr. Microsurg. 25, 27–33.
Kaplan, H. M., Mishra, P., and Kohn, J. (2015). The overwhelming use of rat models in nerve regeneration research may compromise designs of nerve guidance conduits for humans. J. Mater. Sci. Mater. Med. 26, 1–5. doi: 10.1007/s10856-015-5558-4
Karimi, A., Karbasi, S., Razavi, S., and Zargar, E. N. (2018). Poly(hydroxybutyrate)/chitosan Aligned Electrospun Scaffold as a Novel Substrate for Nerve Tissue Engineering. Adv. Biomed. Res. 7:44. doi: 10.4103/abr.abr_277_16
Karimi, M., Biazar, E., Keshel, S. H., Ronaghi, A., Doostmohamadpour, J., Janfada, A., et al. (2014). Rat sciatic nerve reconstruction across a 30 mm defect bridged by an oriented porous PHBV tube with schwann cell as artificial nerve graft. ASAIO J. 60, 224-233. doi: 10.1097/MAT.0000000000000044
Kehoe, S., Zhang, X. F., and Boyd, D. (2012). FDA approved guidance conduits and wraps for peripheral nerve injury: a review of materials and efficacy. Injury 43, 553–572. doi: 10.1016/j.injury.2010.12.030
Keten, S., Xu, Z., Ihle, B., and Buehler, M. J. (2010). Nanoconfinement controls stiffness, strength and mechanical toughness of B -sheet crystals in silk. Nat. Mater. 9, 359–367. doi: 10.1038/nmat2704
Kim, J. I., Hwang, T. I., Aguilar, L. E., Park, C. H., and Kim, C. S. (2016). A controlled design of aligned and random nanofibers for 3D Bi-functionalized nerve conduits fabricated via a novel electrospinning set-up. Sci. Rep. 6:23761. doi: 10.1038/srep23761
Kim, M. S., and Kim, G. (2014). Three-dimensional electrospun polycaprolactone (PCL)/alginate hybrid composite scaffolds. Carbohydr. Polym. 114, 213-221. doi: 10.1016/j.carbpol.2014.08.008
Ko, C. H., Shie, M. Y., Lin, J. H., Chen, Y. W., Yao, C. H., and Chen, Y. S. (2017). Biodegradable bisvinyl sulfonemethyl-crosslinked gelatin conduit promotes regeneration after peripheral nerve injury in adult rats. Sci. Rep. 7:17489. doi: 10.1038/s41598-017-17792-2
Koch, D., Rosoff, W. J., Jiang, J., Geller, H. M., and Urbach, J. S. (2012). Strength in the periphery: growth cone biomechanics and substrate rigidity response in peripheral and central nervous system neurons. Biophys. J. 102, 452-460. doi: 10.1016/j.bpj.2011.12.025
Kokai, L. E., Lin, Y. C., Oyster, N. M., and Marra, K. G. (2009). Diffusion of soluble factors through degradable polymer nerve guides: controlling manufacturing parameters. Acta Biomater. 5, 2540–2550. doi: 10.1016/j.actbio.2009.03.009
Koller, M. (2018). Biodegradable and Biocompatible Polyhydroxy-alkanoates (PHA): auspicious microbial macromolecules for pharmaceutical and therapeutic applications. Molecules 23:362. doi: 10.3390/molecules23020362
Kornfeld, T., Vogt, P. M., and Radtke, C. (2018). Nerve grafting for peripheral nerve injuries with extended defect sizes. Wiener Medizinische Wochenschrift 169, 240–251. doi: 10.1007/s10354-018-0675-6
Koudehi, M. F., Fooladi, A. A. I., Mansoori, K., Jamalpoor, Z., Amiri, A., and Nourani, M. R. (2014). Preparation and evaluation of novel nano-bioglass/gelatin conduit for peripheral nerve regeneration. J. Mater. Sci. Mater. Med. 25, 363–373. doi: 10.1007/s10856-013-5076-1
Kundu, B., Rajkhowa, R., Kundu, S. C., and Wang, X. (2013). Silk fibroin biomaterials for tissue regenerations. Adv. Drug Deliv. Rev. 65, 457–470. doi: 10.1016/j.addr.2012.09.043
Kuznetsov, K. A., Stepanova, A. O., Kvon, R. I., Douglas, T. E. L., Kuznetsov, N. A., Chernonosova, V. S., et al. (2018). Electrospun produced 3D matrices for covering of vascular stents: paclitaxel release depending on fiber structure and composition of the external environment. Materials 11:2176. doi: 10.3390/ma11112176
Le Guéhennec, L., Layrolle, P., Daculsi, G., Redl, H., Pandit, A., and Czernuszka, J. (2004). A review of bioceramics and fibrin sealant. Eur. Cells Mater. 8, 1–11. doi: 10.22203/eCM.v008a01
Lesný, P., Přádný, M., Jendelová, P., Michálek, J., Vacík, J., and Syková, E. (2006). Macroporous hydrogels based on 2-hydroxyethyl methacrylate. Part 4: growth of rat bone marrow stromal cells in three-dimensional hydrogels with positive and negative surface charges and in polyelectrolyte complexes. J. Mater. Sci. Mater. Med. 17, 829–833. doi: 10.1007/s10856-006-9842-1
Li, R., Liu, H., Huang, H., Bi, W., Yan, R., Tan, X., et al. (2018). Chitosan conduit combined with hyaluronic acid prevent sciatic nerve scar in a rat model of peripheral nerve crush injury. Mol. Med. Rep. 17, 4360–4368. doi: 10.3892/mmr.2018.8388
Li, X., Wang, L., Li, L., Luo, Z., Yan, S., Zhang, Q., et al. (2019). Water-stable silk fibroin nerve conduits with tunable degradation prepared by a mild freezing-induced assembly. Polym. Degrad. Stab. 164, 61–68. doi: 10.1016/j.polymdegradstab.2019.04.006
Licciardello, G., Catara, A. F., and Catara, V. (2019). Production of polyhydroxyalkanoates and extracellular products using Pseudomonas corrugata and P. mediterranea: a review. Bioengineering 6:105. doi: 10.3390/bioengineering6040105
Lien, S.-M., Ko, L.-Y., and Huang, T.-J. (2009). Effect of pore size on ECM secretion and cell growth in gelatin scaffold for articular cartilage tissue engineering. Acta Biomater. 5, 670–679. doi: 10.1016/j.actbio.2008.09.020
Lin, Y. C., Ramadan, M., Van Dyke, M., Kokai, L. E., Philips, B. J., Rubin, J. P., et al. (2012). Keratin gel filler for peripheral nerve repair in a rodent sciatic nerve injury model. Plast. Reconstr. Surg. 129, 67-78. doi: 10.1097/PRS.0b013e3182268ae0
Liodaki, E., Bos, I., Lohmeyer, J. A., Senyaman, O., Mauss, K. L., Siemers, F., et al. (2013). Removal of collagen nerve conduits (NeuraGen) after unsuccessful implantation: focus on histological findings. J. Reconstr. Microsurg. 29, 517-522. doi: 10.1055/s-0033-1348033
Liu, B.-S. (2008). Fabrication and evaluation of a biodegradable proanthocyanidin-crosslinked gelatin conduit in peripheral nerve repair. J. Biomed. Mater. Res. Part A 87A, 1092–1102. doi: 10.1002/jbm.a.31916
Liu, B.-S., Yao, C.-H., Hsu, S.-H., Yeh, T.-S., Chen, Y.-S., and Kao, S.-T. (2004). A novel use of genipin-fixed gelatin as extracellular matrix for peripheral nerve regeneration. J. Biomater. Appl. 19, 21–34. doi: 10.1177/0885328204042544
Liu, D., Mi, D., Zhang, T., Zhang, Y., Yan, J., Wang, Y., et al. (2018). Tubulation repair mitigates misdirection of regenerating motor axons across a sciatic nerve gap in rats. Sci. Rep. 8:3443. doi: 10.1038/s41598-018-21652-y
Liu, X., Lim, J. Y., Donahue, H. J., Dhurjati, R., Mastro, A. M., and Vogler, E. A. (2007). Influence of substratum surface chemistry/energy and topography on the human fetal osteoblastic cell line hFOB 1.19: phenotypic and genotypic responses observed in vitro. Biomaterials 28, 4535–4550. doi: 10.1016/j.biomaterials.2007.06.016
Liu, Y., and Hsu, S. (2020). Biomaterials and neural regeneration. Neural Regen. Res. 15:1243. doi: 10.4103/1673-5374.272573
Loverde, J. R., Ozoka, V. C., Aquino, R., Lin, L., and Pfister, B. J. (2011). Live imaging of axon stretch growth in embryonic and adult neurons. J. Neurotr. 28, 2389-2403. doi: 10.1089/neu.2010.1598
Loverde, J. R., and Pfister, B. J. (2015). Developmental axon stretch stimulates neuron growth while maintaining normal electrical activity, intracellular calcium flux, and somatic morphology. Front. Cell. Neurosci. 9:308. doi: 10.3389/fncel.2015.00308
Lundborg, G. (2000). A 25-year perspective of peripheral nerve surgery: evolving neuroscientific concepts and clinical significance. J. Hand Surg. Am. 25, 391–414. doi: 10.1053/jhsu.2000.4165
Lyons, K. M., Pelton, R. W., and Hogan, B. L. M. (1990). Organogenesis and pattern formation in the mouse: RNA distribution patterns suggest a role for bone morphogenetic protein-2A (BMP-2A). Development 109, 833-844. doi: 10.1016/0168-9525(90)90274-a
Magaz, A., Faroni, A., Gough, J. E., Reid, A. J., Li, X., and Blaker, J. J. (2018). Bioactive silk-based nerve guidance conduits for augmenting peripheral nerve repair. Adv. Healthc. Mater. 7:e1800308. doi: 10.1002/adhm.201800308
Maiti, B., and Díaz, D. D. (2018). 3D printed polymeric hydrogels for nerve regeneration. Polymers 10:1041. doi: 10.3390/POLYM10091041
Marshall, S. J., Bayne, S. C., Baier, R., Tomsia, A. P., and Marshall, G. W. (2010). A review of adhesion science. Dent. Mater. 26, e11–e16. doi: 10.1016/j.dental.2009.11.157
Martínez, J. P., Falomir, M. P., and Gozalbo, D. (2014). “Chitin: A Structural Biopolysaccharide with Multiple Applications,” in eLS. Chichester: John Wiley & Sons, Ltd, doi: 10.1002/9780470015902.a0000694.pub3
Masaeli, E., Morshed, M., Rasekhian, P., Karbasi, S., Karbalaie, K., Karamali, F., et al. (2012). Does the tissue engineering architecture of poly(3-hydroxybutyrate) scaffold affects cell-material interactions? J. Biomed. Mater. Res. A 100, 1907–1918. doi: 10.1002/jbm.a.34131
Mattila, P. K., and Lappalainen, P. (2008). Filopodia: molecular architecture and cellular functions. Nat. Rev. Mol. Cell Biol. 9, 446–454. doi: 10.1038/nrm2406
Maurer, T., Stoffel, M. H., Belyaev, Y., Stiefel, N. G., Vidondo, B., Kuker, S., et al. (2018). Structural characterization of four different naturally occurring porcine collagen membranes suitable for medical applications. PLoS One 13:e0205027. doi: 10.1371/journal.pone.0205027
McGrath, A. M., Brohlin, M., Wiberg, R., Kingham, P. J., Novikov, L. N., Wiberg, M., et al. (2018). Long-term effects of fibrin conduit with human mesenchymal stem cells and immunosuppression after peripheral nerve repair in a xenogenic model. Cell Med. 10:215517901876032. doi: 10.1177/2155179018760327
Meek, M. F., and Coert, J. H. (2008). US Food and Drug Administration/Conformit Europe- approved absorbable nerve conduits for clinical repair of peripheral and cranial nerves. Ann. Plast. Surg. 60, 466–472. doi: 10.1097/SAP.0b013e31804d441c
Mitsou, E., Pletsa, V., Sotiroudis, G. T., Panine, P., Zoumpanioti, M., and Xenakis, A. (2020). Development of a microemulsion for encapsulation and delivery of gallic acid, The role of chitosan. Coll. Surf. B Biointerf. 190:110974. doi: 10.1016/j.colsurfb.2020.110974
Mohanna, P. N., Terenghi, G., and Wiberg, M. (2005). Composite PHB-GGF conduit for long nerve gap repair: a long-term evaluation. Scand. J. Plast. Reconstr. Surg. Hand Surg. 39, 129–137. doi: 10.1080/02844310510006295
Mohanna, P. N., Young, R. C., Wiberg, M., and Terenghi, G. (2003). A composite pol-hydroxybutyrate-glial growth factor conduit for long nerve gap repairs. J. Anat 203, 553-565. doi: 10.1046/j.1469-7580.2003.00243.x
Moore, P., and Staii, C. (2018). Cytoskeletal Dynamics of Neurons Measured by Combined Fluorescence and Atomic Force Microscopy. MRS Adv. 4, 1-6. doi: 10.1557/adv.2018.101
Mozejko-Ciesielska, J., Marciniak, P., and Szacherska, K. (2019). Polyhydroxyalkanoates synthesized by Aeromonas species: trends and challenges. Polymers 11:1328. doi: 10.3390/polym11081328
National Research Council (Us) Committee on Recognition, and Alleviation of Pain in Laboratory Animals. (2010). Recognition, and Alleviation of Pain in Laboratory Animals. Washington, DC: National Academies Press, doi: 10.17226/12526
Navarro, X. (2016). Functional evaluation of peripheral nerve regeneration and target reinnervation in animal models: a critical overview. Eur. J. Neurosci. 43, 271–286. doi: 10.1111/ejn.13033
Nectow, A. R., Marra, K. G., and Kaplan, D. L. (2012). Biomaterials for the development of peripheral nerve guidance conduits. Tissue Eng. Part B Rev. 18, 40–50. doi: 10.1089/ten.teb.2011.0240
Neubrech, F., Heider, S., Harhaus, L., Bickert, B., Kneser, U., and Kremer, T. (2016). Chitosan nerve tube for primary repair of traumatic sensory nerve lesions of the hand without a gap: study protocol for a randomized controlled trial. Trials 17:48. doi: 10.1186/s13063-015-1148-5
Neubrech, F., Sauerbier, M., Moll, W., Seegmüller, J., Heider, S., Harhaus, L., et al. (2018). Enhancing the outcome of traumatic sensory nerve lesions of the hand by additional use of a chitosan nerve tube in primary nerve repair: a randomized controlled bicentric trial. Plast. Reconstr. Surg. 142, 415–424. doi: 10.1097/PRS.0000000000004574
Nie, X., Deng, M., Yang, M., Liu, L., Zhang, Y., and Wen, X. (2014). Axonal regeneration and remyelination evaluation of chitosan/gelatin-based nerve guide combined with transforming growth factor-β1 and schwann cells. Cell Biochem. Biophys. 68, 163–172. doi: 10.1007/s12013-013-9683-8
Noè, C., Tonda-Turo, C., Chiappone, A., Sangermano, M., and Hakkarainen, M. (2020). Light processable starch hydrogels. Polymers 12:1359. doi: 10.3390/polym12061359
O’Brien, F. J., Harley, B. A., Yannas, I. V., and Gibson, L. J. (2005). The effect of pore size on cell adhesion in collagen-GAG scaffolds. Biomaterials 26, 433–441. doi: 10.1016/j.biomaterials.2004.02.052
Oh, S. H., Kim, J. R., Kwon, G. B., Namgung, U., Song, K. S., and Lee, J. H. (2013). Effect of surface pore structure of nerve guide conduit on peripheral nerve regeneration. Tissue Eng. Part C Methods 19, 233–243. doi: 10.1089/ten.tec.2012.0221
Ohta, M., Suzuki, Y., Chou, H., Ishikawa, N., Suzuki, S., Tanihara, M., et al. (2004). Novel heparin/alginate gel combined with basic fibroblast growth factor promotes nerve regeneration in rat sciatic nerve. J. Biomed. Mater. Res. 71A, 661–668. doi: 10.1002/jbm.a.30194
Omenetto, F. G., and Kaplan, D. L. (2010). New opportunities for an ancient material. Science 329, 528–531. doi: 10.1126/science.1188936
Omidian, H., Rocca, J. G., and Park, K. (2006). Elastic, superporous hydrogel hybrids of polyacrylamide and sodium alginate. Macromol. Biosci. 6, 703-710. doi: 10.1002/mabi.200600062
Ornelas, L., Padilla, L., Di Silvio, M., Schalch, P., Esperante, S., López Infante, R., et al. (2006a).). Fibrin glue: an alternative technique for nerve coaptation - Part I. Wave amplitude, conduction velocity, and plantar-length factors. J. Reconstr. Microsurg. 22, 119–122.
Ornelas, L., Padilla, L., Di Silvio, M., Schalch, P., Esperante, S., López Infante, R., et al. (2006b). Fibrin glue: an alternative technique for nerve coaptation - Part II. Nerve regeneration and histomorphometric assessment. J. Reconstr. Microsurg. 22, 123–128.
Ortuno-Lizarán, I., Vilariño-Feltrer, G., Martínez-Ramos, C., Monleón Pradas, M., and Vallés-Lluch, A. (2016). Influence of synthesis parameters on hyaluronic acid hydrogels intended as nerve conduits. Biofabrication 8:045011. doi: 10.1088/1758-5090/8/4/045011
Pabari, A., Yang, S. Y., Seifalian, A. M., and Mosahebi, A. (2010). Modern surgical management of peripheral nerve gap. J. Plast. Reconstr. Aesthetic Surg. 63, 1941-1948. doi: 10.1016/j.bjps.2009.12.010
Pace, L. A., Plate, J. F., Mannava, S., Barnwell, J. C., Koman, L. A., Li, Z., et al. (2014). A human hair keratin hydrogel scaffold enhances median nerve regeneration in nonhuman primates: an electrophysiological and histological study. Tissue Eng. Part A 20, 507–517. doi: 10.1089/ten.tea.2013.0084
Pace, L. A., Plate, J. F., Smith, T. L., and Van Dyke, M. E. (2013). The effect of human hair keratin hydrogel on early cellular response tosciatic nerve injury in a rat model. Biomaterials 34, 5907-5914. doi: 10.1016/j.biomaterials.2013.04.024
Park, C. H., and Woo, K. M. (2018). “Fibrin-Based Biomaterial Applications In Tissue Engineering and Regenerative Medicine,” in Advances in Experimental Medicine and Biology. New York, NY: Springer, 253–261. doi: 10.1007/978-981-13-0445-3_16
Park, S. Y., Ki, C. S., Park, Y. H., Lee, K. G., Kang, S. W., Kweon, H. Y., et al. (2015). Functional recovery guided by an electrospun silk fibroin conduit after sciatic nerve injury in rats. J. Tissue Eng. Regen. Med. 9, 66–76. doi: 10.1002/term.1615
Pawelec, K. M., Hix, J., Shapiro, E. M., and Sakamoto, J. (2019). The mechanics of scaling-up multichannel scaffold technology for clinical nerve repair. J. Mech. Behav. Biomed. Mater. 91, 247-254. doi: 10.1016/j.jmbbm.2018.12.016
Pérez-Rigueiro, J., Elices, M., Plaza, G., Real, J. I., and Guinea, G. V. (2005). The effect of spinning forces on spider silk properties. J. Exp. Biol. 208, 2633–2639. doi: 10.1242/jeb.01701
Petcu, E., Midha, R., McColl, E., Popa-Wagner, A., Chirila, T., and Dalton, P. (2018). 3D printing strategies for peripheral nerve regeneration. Biofabrication 10:032001.
Pfister, B. J., Bonislawski, D. P., Smith, D. H., and Cohen, A. S. (2006). Stretch-grown axons retain the ability to transmit active electrical signals. FEBS Lett. 580, 3525-3531. doi: 10.1016/j.febslet.2006.05.030
Pfister, L. A., Alther, E., Papaloïzos, M., Merkle, H. P., and Gander, B. (2008). Controlled nerve growth factor release from multi-ply alginate/chitosan-based nerve conduits. Eur. J. Pharm. Biopharm. 69, 563–572. doi: 10.1016/j.ejpb.2008.01.014
Pfister, L. A., Papaloïzos, M., Merkle, H. P., and Gander, B. (2007a). Hydrogel nerve conduits produced from alginate/chitosan complexes. J. Biomed. Mater. Res. Part A 80A, 932–937. doi: 10.1002/jbm.a.31052
Pfister, L. A., Papaloïzos, M., Merkle, H. P., and Gander, B. (2007b). Nerve conduits and growth factor delivery in peripheral nerve repair. J. Peripher. Nerv. Syst. 12, 65–82. doi: 10.1111/j.1529-8027.2007.00125.x
Pinho, A. C., Fonseca, A. C., Serra, A. C., Santos, J. D., and Coelho, J. F. J. (2016). Peripheral nerve regeneration: current status and new strategies using polymeric materials. Adv. Healthc. Mater. 5, 2732–2744. doi: 10.1002/adhm.201600236
Porpiglia, F., Bertolo, R., Fiori, C., Manfredi, M., De Cillis, S., and Geuna, S. (2018). Chitosan membranes applied on the prostatic neurovascular bundles after nerve-sparing robot-assisted radical prostatectomy: a phase II study. BJU Int. 121, 472–478. doi: 10.1111/bju.13959
Radtke, C. (2016). Natural occurring silks and their analogues as materials for nerve conduits. Int. J. Mol. Sci. 17, 1754. doi: 10.3390/ijms17101754
Radtke, C., Allmeling, C., Waldmann, K. H., Reimers, K., Thies, K., Schenk, H. C., et al. (2011). Spider silk constructs enhance axonal regeneration and remyelination in long nerve defects in sheep. PLoS One 6:e16990. doi: 10.1371/journal.pone.0016990
Rahmany, M. B., and Van Dyke, M. (2013). Biomimetic approaches to modulate cellular adhesion in biomaterials: a review. Acta Biomater. 9, 5431–5437. doi: 10.1016/j.actbio.2012.11.019
Rajab, F. H., Liauw, C. M., Benson, P. S., Li, L., and Whitehead, K. A. (2017). Production of hybrid macro/micro/nano surface structures on Ti6Al4V surfaces by picosecond laser surface texturing and their antifouling characteristics. Coll. Surf. B Biointerf. 160, 688–696. doi: 10.1016/j.colsurfb.2017.10.008
Rajabi, M., Ali, A., McConnell, M., and Cabral, J. (2020). Keratinous materials: structures and functions in biomedical applications. Mater. Sci. Eng. C 110:110612. doi: 10.1016/j.msec.2019.110612
Ramírez, M. Á, Rodríguez, A. T., Alfonso, L., and Peniche, C. (2010). Chitin and its Derivatives As Biopolymers With Potential Agricultural Applications. Available online at: https://pdfs.semanticscholar.org/244b/07bf7f95cdfe78e0d3760dff8c8180c66050.pdf (accessed March 29, 2020).
Rasappan, K., Rajaratnam, V., and Wong, Y. R. (2018). Conduit-based nerve repairs provide greater resistance to tension compared with primary repairs: a biomechanical analysis on large animal samples. Plast. Reconstr. Surg. - Glob. Open 6:e1981. doi: 10.1097/GOX.0000000000001981
Ratner, B. D., and Bryant, S. J. (2004). Biomaterials: where we have been and where we are going. Annu. Rev. Biomed. Eng. 6, 41–75. doi: 10.1146/annurev.bioeng.6.040803.140027
Raza, C., Riaz, H. A., Anjum, R., and Shakeel, N. A. (2020). Repair strategies for injured peripheral nerve: review. Life Sci. 243:117308. doi: 10.1016/j.lfs.2020.117308
Rbia, N., Bulstra, L. F., Saffari, T. M., Hovius, S. E. R., and Shin, A. Y. (2019). Collagen nerve conduits and processed nerve allografts for the reconstruction of digital nerve gaps: a single-institution case series and review of the literature. World Neurosurg. 127, e1176-e1184. doi: 10.1016/j.wneu.2019.04.087
Reid, A. J., de Luca, A. C., Faroni, A., Downes, S., Sun, M., Terenghi, G., et al. (2013). Long term peripheral nerve regeneration using a novel PCL nerve conduit. Neurosci. Lett. 544, 125–130. doi: 10.1016/j.neulet.2013.04.001
Rodríguez, F. J., Gómez, N., Perego, G., and Navarro, X. (1999). Highly permeable polylactide-caprolactone nerve guides enhance peripheral nerve regeneration through long gaps. Biomaterials 20, 1489–1500. doi: 10.1016/S0142-9612(99)00055-1
Rodriguez-Contreras, A. (2019). Recent advances in the use of polyhydroyalkanoates in biomedicine. Bioengineering 6:82. doi: 10.3390/bioengineering6030082
Ronchi, G., Fornasari, B. E., Crosio, A., Budau, C. A., Tos, P., Perroteau, I., et al. (2018). Chitosan tubes enriched with fresh skeletal muscle fibers for primary nerve repair. Biomed. Res. Int. 2018:9175248. doi: 10.1155/2018/9175248
Rowe, S. L., Lee, S. Y., and Stegemann, J. P. (2007). Influence of thrombin concentration on the mechanical and morphological properties of cell-seeded fibrin hydrogels. Acta Biomater. 3, 59-67. doi: 10.1016/j.actbio.2006.08.006
Ruini, F., Tonda-Turo, C., Chiono, V., and Ciardelli, G. (2015). Chitosan membranes for tissue engineering: comparison of different crosslinkers. Biomed. Mater. 10:065002. doi: 10.1088/1748-6041/10/6/065002
Rutkowski, G. E., and Heath, C. A. (2002). Development of a bioartificial nerve graft. II. nerve regeneration in vitro. Biotechnol. Prog. 18, 373–379. doi: 10.1021/bp020280h
Ryan, A. J., Lackington, W. A., Hibbitts, A. J., Matheson, A., Alekseeva, T., Stejskalova, A., et al. (2017). A physicochemically optimized and neuroconductive biphasic nerve guidance conduit for peripheral nerve repair. Adv. Healthc. Mater. 6:1700954. doi: 10.1002/adhm.201700954
Ryu, J. H., Choi, J. S., Park, E., Eom, M. R., Jo, S., Lee, M. S., et al. (2020). Chitosan oral patches inspired by mussel adhesion. J. Control. Release 317, 57–66. doi: 10.1016/j.jconrel.2019.11.006
Saeki, M., Tanaka, K., Imatani, J., Okamoto, H., Watanabe, K., Nakamura, T., et al. (2018). Efficacy and safety of novel collagen conduits filled with collagen filaments to treat patients with peripheral nerve injury: a multicenter, controlled, open-label clinical trial. Injury 49, 766–774. doi: 10.1016/j.injury.2018.03.011
Saltzman, E. B., Villa, J. C., Doty, S. B., Feinberg, J. H., Lee, S. K., and Wolfe, S. W. (2019). A comparison between two collagen nerve conduits and nerve autograft: a rat model of motor nerve regeneration. J. Hand Surg. Am. 44, 700.e1–700.e9. doi: 10.1016/j.jhsa.2018.10.008
Sandri, G., Rossi, S., Bonferoni, M. C., Miele, D., Faccendini, A., Del Favero, E., et al. (2019). Chitosan/glycosaminoglycan scaffolds for skin reparation. Carbohydr. Polym. 220, 219–227. doi: 10.1016/j.carbpol.2019.05.069
Sarker, M. D., Naghieh, S., McInnes, A. D., Schreyer, D. J., and Chen, X. (2018). Regeneration of peripheral nerves by nerve guidance conduits: influence of design, biopolymers, cells, growth factors, and physical stimuli. Prog. Neurobiol. 171, 125–150. doi: 10.1016/j.pneurobio.2018.07.002
Schaakxs, D., Kalbermatten, D. F., Pralong, E., Raffoul, W., Wiberg, M., and Kingham, P. J. (2017). Poly-3-hydroxybutyrate strips seeded with regenerative cells are effective promoters of peripheral nerve repair. J. Tissue Eng. Regen. Med. 11, 812–821. doi: 10.1002/term.1980
Schmidt, C. E., and Leach, J. B. (2003). Neural Tissue Engineering: Strategies for Repair and Regeneration. Annu. Rev. Biomed. Eng. 5, 293–347. doi: 10.1146/annurev.bioeng.5.011303.120731
Schmidt, C. E., Shastri, V. R., Vacanti, J. P., and Langer, R. (1997). Stimulation of neurite outgrowth using an electrically conducting polymer. Proc. Natl. Acad. Sci. U.S.A. 94, 8948–8953. doi: 10.1073/pnas.94.17.8948
Schmidt, T., Stachon, S., MacK, A., Rohde, M., and Just, L. (2011). Evaluation of a thin and mechanically stable collagen cell carrier. Tissue Eng. Part C Methods 17, 1161–1170. doi: 10.1089/ten.tec.2011.0201
Sedaghati, T., Jell, G., and Seifalian, A. (2014). Investigation of Schwann cell behaviour on RGD-functionalised bioabsorbable nanocomposite for peripheral nerve regeneration. N. Biotechnol. 31, 203-213. doi: 10.1016/j.nbt.2014.01.002
Sergeeva, A., Vikulina, A. S., and Volodkin, D. (2019). Porous alginate scaffolds assembled using vaterite CaCO3 crystals. Micromachines 10:357. doi: 10.3390/mi10060357
Shapira, Y., Tolmasov, M., Nissan, M., Reider, E., Koren, A., Biron, T., et al. (2016). Comparison of results between chitosan hollow tube and autologous nerve graft in reconstruction of peripheral nerve defect: an experimental study. Microsurgery 36, 664–671. doi: 10.1002/micr.22418
She, Z., Jin, C., Huang, Z., Zhang, B., Feng, Q., and Xu, Y. (2008). Silk fibroin/chitosan scaffold: Preparation, characterization, and culture with HepG2 cell. J. Mater. Sci. Mater. Med. 19, 3545–3553. doi: 10.1007/s10856-008-3526-y
Shen, F., Li, A. A., Cornelius, R. M., Cirone, P., Childs, R. F., Brash, J. L., et al. (2005). Biological properties of photocrosslinked alginate microcapsules. J. Biomed. Mater. Res. Part B Appl. Biomater. 75B, 425–434. doi: 10.1002/jbm.b.30323
Shen, W., and Hsieh, Y. L. (2014). Biocompatible sodium alginate fibers by aqueous processing and physical crosslinking. Carbohydr. Polym 102, 893-900. doi: 10.1016/j.carbpol.2013.10.066
Shuai, H. H., Yang, C. Y., Harn, H. I. C., York, R. L., Liao, T. C., Chen, W. S., et al. (2013). Using surfaces to modulate the morphology and structure of attached cells-a case of cancer cells on chitosan membranes. Chem. Sci. 4, 3058-3067. doi: 10.1039/c3sc50533b
Sierpinski, P., Garrett, J., Ma, J., Apel, P., Klorig, D., Smith, T., et al. (2008). The use of keratin biomaterials derived from human hair for the promotion of rapid regeneration of peripheral nerves. Biomaterials 29, 118–128. doi: 10.1016/j.biomaterials.2007.08.023
Simões, M. J., Gärtner, A., Shirosaki, Y., Gil da Costa, R. M., Cortez, P. P., Gartnër, F., et al. (2011). In vitro and in vivo chitosan membranes testing for peripheral nerve reconstruction. Acta Med. Port. 24, 43–52. doi: 10.20344/amp.344
Singh, D., Singh, D., Zo, S., and Han, S. S. (2014). Nano-biomimetics for nano/micro tissue regeneration. J. Biomed. Nanotechnol. 10, 3141–3161. doi: 10.1166/jbn.2014.1941
Singh, I., Lacko, C. S., Zhao, Z., Schmidt, C. E., and Rinaldi, C. (2020). Preparation and evaluation of microfluidic magnetic alginate microparticles for magnetically templated hydrogels. J. Colloid Interface Sci. 561, 647-658. doi: 10.1016/j.jcis.2019.11.040
Spedden, E., White, J. D., Naumova, E. N., Kaplan, D. L., and Staii, C. (2012). Elasticity maps of living neurons measured by combined fluorescence and atomic force microscopy. Biophys. J. 103, 868-877. doi: 10.1016/j.bpj.2012.08.005
Stang, F., Fansa, H., Wolf, G., and Keilhoff, G. (2005a). Collagen nerve conduits - Assessment of biocompatibility and axonal regeneration. Biomed. Mater. Eng 15, 3-15.
Stang, F., Fansa, H., Wolf, G., Reppin, M., and Keilhoff, G. (2005b). Structural parameters of collagen nerve grafts influence peripheral nerve regeneration. Biomaterials 26, 3083-3091. doi: 10.1016/j.biomaterials.2004.07.060
Stang, F., Keilhoff, G., and Fansa, H. (2009). Biocompatibility of different nerve tubes. Materials 26, 3083-3091. doi: 10.3390/ma2041480
Stenberg, L., Kodama, A., Lindwall-Blom, C., and Dahlin, L. B. (2016). Nerve regeneration in chitosan conduits and in autologous nerve grafts in healthy and in type 2 diabetic Goto-Kakizaki rats. Eur. J. Neurosci. 43, 463–473. doi: 10.1111/ejn.13068
Stenn, K. S., Prouty, S. M., and Seiberg, M. (1994). Molecules of the cycling hair follicle - a tabulated review. J. Dermatol. Sci. 7, S109-S124. doi: 10.1016/0923-1811(94)90042-6
Stoppel, W. L., Ghezzi, C. E., McNamara, S. L., Black, L. D. I. I. I., and Kaplan, D. L. (2015). Clinical applications of naturally derived biopolymer-based scaffolds for regenerative medicine. Ann. Biomed. Eng. 43, 657–680. doi: 10.1007/s10439-014-1206-2
Stouthandel, M. E. J., Vanhove, C., Devriendt, W., De Bock, S., Debbaut, C., Vangestel, C., et al. (2020). Biomechanical comparison of Thiel embalmed and fresh frozen nerve tissue. Anat. Sci. Int. 95, 399-407. doi: 10.1007/s12565-020-00535-1
Sufan, W., Suzuki, Y., Tanihara, M., Ohnishi, K., Suzuki, K., Endo, K., et al. (2001). Sciatic nerve regeneration through alginate with tubulation or nontubulation repair in cat. J. Neurotrauma 18, 329-338. doi: 10.1089/08977150151070991
Sulong, A. F., Fiassan, N. H., Hwei, N. M., Lokanathan, Y., Naicker, A. S., Abdullah, S., et al. (2014). Collagen-Coated Polylactic-Glycolic Acid (PLGA) seeded with neural-differentiated human mesenchymal stem cells as a potential nerve conduit. Adv. Clin. Exp. Med. 23, 353-362. doi: 10.17219/acem/37125
Sun, J., and Tan, H. (2013). Alginate-based biomaterials for regenerative medicine applications. Materials 6, 1285-1309. doi: 10.3390/ma6041285
Sun, M., and Downes, S. (2009). Physicochemical characterisation of novel ultra-thin biodegradable scaffolds for peripheral nerve repair. J. Mater. Sci. Mater. Med. 20, 1181–1192. doi: 10.1007/s10856-008-3671-3
Sundback, C. A., Shyu, J. Y., Wu, A. J., Sheahan, T. P., Wang, Y., Faquin, W. C., et al. (2004). In vitro and in vivo biocompatibility analysis of poly (glycerol sebacate) as a potential nerve guide material. Mater. Res. Soc. Symp. Proc. 26, 5454-5464.
Sung, H., Liang, I., Chen, C., Huang, R., and Liang, H. (2001). Stability of a biological tissue fixed with a naturally occurring crosslinking agent (genipin). J. Biomed. Mater. Res 55, 538–546.
Takazawa, R., Yamato, M., Kageyama, Y., Okano, T., and Kihara, K. (2005). Mesothelial cell sheets cultured on fibrin gel prevent adhesion formation in an intestinal hernia model. Tissue Eng. 11, 618–625. doi: 10.1089/ten.2005.11.618
Talac, R., Friedman, J. A., Moore, M. J., Lu, L., Jabbari, E., Windebank, A. J., et al. (2004). Animal models of spinal cord injury for evaluation of tissue engineering treatment strategies. Biomaterials 25, 1505–1510. doi: 10.1016/S0142-9612(03)00497-6
Tang, X., Ding, F., Yang, Y., Hu, N., Wu, H., and Gu, X. (2009). Evaluation on in vitro biocompatibility of silk fibroin-based biomaterials with primarily cultured hippocampal neurons. J. Biomed. Mater. Res. Part A 91A, 166–174. doi: 10.1002/jbm.a.32212
Tao, F., Cheng, Y., Shi, X., Zheng, H., Du, Y., Xiang, W., et al. (2020a). Applications of chitin and chitosan nanofibers in bone regenerative engineering. Carbohydr. Polym. 230:115658. doi: 10.1016/j.carbpol.2019.115658
Tao, J., Hu, Y., Wang, S., Zhang, J., Liu, X., Gou, Z., et al. (2017). A 3D-engineered porous conduit for peripheral nerve repair. Sci. Rep. 7, 1–13. doi: 10.1038/srep46038
Tao, J., Liu, H., Wu, W., Zhang, J., Liu, S., Zhang, J., et al. (2020b). 3D-printed nerve conduits with live platelets for effective peripheral nerve repair. Adv. Funct. Mater. 2004272. doi: 10.1002/adfm.202004272
Tao, J., Zhang, J., Du, T., Xu, X., Deng, X., Chen, S., et al. (2019). Rapid 3D printing of functional nanoparticle-enhanced conduits for effective nerve repair. Acta Biomater. 90, 49–59. doi: 10.1016/j.actbio.2019.03.047
Teuschl, A. H., Schuh, C., Halbweis, R., Pajer, K., Márton, G., Hopf, R., et al. (2015). A new preparation method for anisotropic silk fibroin nerve guidance conduits and its evaluation in vitro and in a rat sciatic nerve defect model. Tissue Eng. Part C Methods 21, 945-957. doi: 10.1089/ten.tec.2014.0606
Thomsen, L., and Schlur, C. (2013). Incidence of painful neuroma after end-to-end nerve suture wrapped into a collagen conduit, A prospective study of 185cases. Chir. Main 32, 335-340. doi: 10.1016/j.main.2013.07.001
Tonda-Turo, C., Audisio, C., Gnavi, S., Chiono, V., Gentile, P., Raimondo, S., et al. (2011a). Porous poly(îμ-caprolactone) nerve guide filled with porous gelatin matrix for nerve tissue engineering. Adv. Eng. Mater. 13, B151-B164. doi: 10.1002/adem.201080099
Tonda-Turo, C., Carmagnola, I., Chiappone, A., Feng, Z., Ciardelli, G., Hakkarainen, M., et al. (2020). Photocurable chitosan as bioink for cellularized therapies towards personalized scaffold architecture. Bioprinting 18:e00082. doi: 10.1016/j.bprint.2020.e00082
Tonda-Turo, C., Gentile, P., Saracino, S., Chiono, V., Nandagiri, V. K., Muzio, G., et al. (2011b). Comparative analysis of gelatin scaffolds crosslinked by genipin and silane coupling agent. Int. J. Biol. Macromol. 49, 700–706. doi: 10.1016/j.ijbiomac.2011.07.002
Tonda-Turo, C., Gnavi, S., Ruini, F., Gambarotta, G., Gioffredi, E., Chiono, V., et al. (2017a). Development and characterization of novel agar and gelatin injectable hydrogel as filler for peripheral nerve guidance channels. J. Tissue Eng. Regen. Med 11, 197-208. doi: 10.1002/term.1902
Tonda-Turo, C., Ruini, F., Ramella, M., Boccafoschi, F., Gentile, P., Gioffredi, E., et al. (2017b). Non-covalently crosslinked chitosan nanofibrous mats prepared by electrospinning as substrates for soft tissue regeneration. Carbohydr. Polym. 162, 82–92. doi: 10.1016/j.carbpol.2017.01.050
Uranues, S., Bretthauer, G., Tomasch, G., Rafolt, D., Nagele-Moser, D., Berghold, A., et al. (2020). A new synthetic conduit for the treatment of peripheral nerve injuries. World J. Surg. 44, 3373-3382. doi: 10.1007/s00268-020-05620-0
Varshosaz, J. (2006). Development of bioadhesive chitosan gels for topical delivery of lidocaine. Sci. Pharm. 74, 209–232. doi: 10.3797/scipharm.2006.74.209
Vatankhah, E., Semnani, D., Prabhakaran, M. P., Tadayon, M., Razavi, S., and Ramakrishna, S. (2014). Artificial neural network for modeling the elastic modulus of electrospun polycaprolactone/gelatin scaffolds. Acta Biomater. 10, 709–721. doi: 10.1016/j.actbio.2013.09.015
Vijayavenkataraman, S., Kannan, S., Cao, T., Fuh, J. Y. H., Sriram, G., and Lu, W. F. (2019). 3D-Printed PCL/PPy conductive scaffolds as three-dimensional porous nerve guide conduits (NGCs) for peripheral nerve injury repair. Front. Bioeng. Biotechnol. 7:266. doi: 10.3389/fbioe.2019.00266
Vilariño-Feltrer, G., Martínez-Ramos, C., Monleón-De-La-Fuente, A., Vallés-Lluch, A., Moratal, D., Barcia Albacar, J. A., et al. (2016). Schwann-cell cylinders grown inside hyaluronic-acid tubular scaffolds with gradient porosity. Acta Biomater. 30, 199–211. doi: 10.1016/j.actbio.2015.10.040
Vleggeert-Lankamp, C. L. A. M., de Ruiter, G. C. W., Wolfs, J. F. C., Pêgo, A. P., van den Berg, R. J., Feirabend, H. K. P., et al. (2007). Pores in synthetic nerve conduits are beneficial to regeneration. J. Biomed. Mater. Res. Part A 80A, 965–982. doi: 10.1002/jbm.a.30941
Wang, C., Jia, Y., Yang, W., Zhang, C., Zhang, K., and Chai, Y. (2018a). Silk fibroin enhances peripheral nerve regeneration by improving vascularization within nerve conduits. J. Biomed. Mater. Res. Part A 106, 2070–2077. doi: 10.1002/jbm.a.36390
Wang, G. W., Yang, H., Wu, W. F., Zhang, P., and Wang, J. Y. (2017). Design and optimization of a biodegradable porous zein conduit using microtubes as a guide for rat sciatic nerve defect repair. Biomaterials 131, 145-159. doi: 10.1016/j.biomaterials.2017.03.038
Wang, K., Nemeth, I. R., Seckel, B. R., Chakalis-Haley, D. P., Swann, D. A., Kuo, J., et al. (1998). Hyaluronic acid enhances peripheral nerve regeneration in vivo. Microsurgery 18, 270–275.
Wang, S., and Cai, L. (2010). Polymers for Fabricating Nerve Conduits. Int. J. Polym. Sci. 2010. doi: 10.1155/2010/138686
Wang, S., Wan, A. C. A., Xu, X., Gao, S., Mao, H. Q., Leong, K. W., et al. (2001). A new nerve guide conduit material composed of a biodegradable poly(phosphoester). Biomaterials 22, 1157–1169. doi: 10.1016/S0142-9612(00)00356-2
Wang, W., Degrugillier, L., Tremp, M., Prautsch, K., Sottaz, L., Schaefer, D. J., et al. (2018b). Nerve repair with fibrin nerve conduit and modified suture placement. Anat. Rec. 301, 1690–1696. doi: 10.1002/ar.23921
Wang, W., Itoh, S., Matsuda, A., Aizawa, T., Demura, M., Ichinose, S., et al. (2008a). Enhanced nerve regeneration through a bilayered chitosan tube: the effect of introduction of glycine spacer into the CYIGSR sequence. J. Biomed. Mater. Res. Part A 85A, 919–928. doi: 10.1002/jbm.a.31522
Wang, W., Itoh, S., Matsuda, A., Ichinose, S., Shinomiya, K., Hata, Y., et al. (2008b). Influences of mechanical properties and permeability on chitosan nano/microfiber mesh tubes as a scaffold for nerve regeneration. J. Biomed. Mater. Res. Part A 84A, 557–566. doi: 10.1002/jbm.a.31536
Wang, Y., Rudym, D. D., Walsh, A., Abrahamsen, L., Kim, H. J., Kim, H. S., et al. (2008c). In vivo degradation of three-dimensional silk fibroin scaffolds. Biomaterials 29, 3415–3428. doi: 10.1016/j.biomaterials.2008.05.002
Wang, Z. Z., and Sakiyama-Elbert, S. E. (2019). Matrices, scaffolds & carriers for cell delivery in nerve regeneration. Exp. Neurol. 319:112837. doi: 10.1016/j.expneurol.2018.09.020
Wong, F. S. Y., Chan, B. P., and Lo, A. C. Y. (2014). Carriers in cell-based therapies for neurological disorders. Int. J. Mol. Sci. 15, 10669-10723. doi: 10.3390/ijms150610669
Wrobel, S., Serra, S. C., Ribeiro-Samy, S., Sousa, N., Heimann, C., Barwig, C., et al. (2014). In Vitro evaluation of cell-seeded chitosan films for peripheral nerve tissue engineering. Tissue Eng. Part A 20, 2339–2349. doi: 10.1089/ten.tea.2013.0621
Wu, Q., Wang, Y., and Chen, G.-Q. (2009). Medical Application of Microbial Biopolyesters Polyhydroxyalkanoates. Artif. Cells Blood Substitutes, Biotechnol. 37, 1–12. doi: 10.1080/10731190802664429
Xie, F., Li, Q. F., Gu, B., Liu, K., and Shen, G. X. (2008). In vitro and in vivo evaluation of a biodegradable chitosan-PLA composite peripheral nerve guide conduit material. Microsurgery 28, 471–479. doi: 10.1002/micr.20514
Xu, H., Zhang, L., Bao, Y., Yan, X., Yin, Y., Li, Y., et al. (2017). Preparation and characterization of injectable chitosan–hyaluronic acid hydrogels for nerve growth factor sustained release. J. Bioact. Compat. Polym. 32, 146–162. doi: 10.1177/0883911516662068
Xu, X., Tao, J., Wang, S., Yang, L., Zhang, J., Zhang, J., et al. (2019). 3D printing of nerve conduits with nanoparticle-encapsulated RGFP966. Appl. Mater. Today 16, 247–256. doi: 10.1016/j.apmt.2019.05.014
Xu, Y., Zhang, Z., Chen, X., Li, R., Li, D., and Feng, S. (2016). A silk fibroin/collagen nerve scaffold seeded with a co-culture of schwann cells and adipose-derived stem cells for sciatic nerve regeneration. PLoS One 11:e0147184. doi: 10.1371/journal.pone.0147184
Yang, F., Murugan, R., Wang, S., and Ramakrishna, S. (2005). Electrospinning of nano/micro scale poly(l-lactic acid) aligned fibers and their potential in neural tissue engineering. Biomaterials 26, 2603–2610. doi: 10.1016/j.biomaterials.2004.06.051
Yang, Y., Chen, X., Ding, F., Zhang, P., Liu, J., and Gu, X. (2007a). Biocompatibility evaluation of silk fibroin with peripheral nerve tissues and cells in vitro. Biomaterials 28, 1643-1652. doi: 10.1016/j.biomaterials.2006.12.004
Yang, Y., Ding, F., Wu, J., Hu, W., Liu, W., Liu, J., et al. (2007b). Development and evaluation of silk fibroin-based nerve grafts used for peripheral nerve regeneration. Biomaterials 28, 5526–5535. doi: 10.1016/j.biomaterials.2007.09.001
Ye, W., Li, H., Yu, K., Xie, C., Wang, P., Zheng, Y., et al. (2020). 3D printing of gelatin methacrylate-based nerve guidance conduits with multiple channels. Mater. Des. 192:108757. doi: 10.1016/j.matdes.2020.108757
Yi, S., Xu, L., and Gu, X. (2019). Scaffolds for peripheral nerve repair and reconstruction. Exp. Neurol. 319, 112761. doi: 10.1016/j.expneurol.2018.05.016
Yin, K., Divakar, P., Hong, J., Moodie, K. L., Rosen, J. M., Sundback, C. A., et al. (2018). Freeze-cast porous chitosan conduit for peripheral nerve repair. Mater. Res. Soc. 3, 1677–1683. doi: 10.1557/adv.2018.194
You, F., Wu, X., and Chen, X. (2017). 3D printing of porous alginate/gelatin hydrogel scaffolds and their mechanical property characterization. Int. J. Polym. Mater. Polym. Biomater. 66, 299-306. doi: 10.1080/00914037.2016.1201830
Young, R. C., Terenghi, G., and Wiberg, M. (2002). Poly-3-hydroxybutyrate (PHB): a resorbable conduit for long-gap repair in peripheral nerves. Br. J. Plast. Surg. 55, 235–240. doi: 10.1054/bjps.2002.3798
Yu, W., Zhao, W., Zhu, C., Zhang, X., Ye, D., Zhang, W., et al. (2011). Sciatic nerve regeneration in rats by a promising electrospun collagen/poly(ε-caprolactone) nerve conduit with tailored degradation rate. BMC Neurosci 12:68. doi: 10.1186/1471-2202-12-68
Yuan, Y., Zhang, P., Yang, Y., Wang, X., and Gu, X. (2004). The interaction of Schwann cells with chitosan membranes and fibers in vitro. Biomaterials 25, 4273–4278. doi: 10.1016/j.biomaterials.2003.11.029
Zhang, H., Wei, Y. T., Tsang, K. S., Sun, C. R., Li, J., Huang, H., et al. (2008). Implantation of neural stem cells embedded in hyaluronic acid and collagen composite conduit promotes regeneration in a rabbit facial nerve injury model. J. Transl. Med. 6:67. doi: 10.1186/1479-5876-6-67
Zhao, H., and Turng, L. S. (2015). Mechanical performance of microcellular injection molded biocomposites from green plastics: PLA and PHBV. Biocomposites 2015, 141–160. doi: 10.1016/B978-1-78242-373-7.00015-9
Zhao, X., Jin, L., Shi, H., Tong, W., Gorin, D., Kotelevtsev, Y., et al. (2020). Recent advances of designing dynamic surfaces to regulate cell adhesion. Colloids Interface Sci. Commun. 35:100249. doi: 10.1016/j.colcom.2020.100249
Zhao, Y., Wang, Y., Gong, J., Yang, L., Niu, C., Ni, X., et al. (2017). Chitosan degradation products facilitate peripheral nerve regeneration by improving macrophage-constructed microenvironments. Biomaterials 134, 64–77. doi: 10.1016/j.biomaterials.2017.02.026
Zhao, Y., Zhao, W., Yu, S., Guo, Y., Gu, X., and Yang, Y. (2013). Biocompatibility evaluation of electrospun silk fibroin nanofibrous mats with primarily cultured rat hippocampal neurons. Biomed. Mater. Eng. 23, 545–554. doi: 10.3233/BME-130775
Zheng, Y., Chen, J. C., Ma, Y. M., and Chen, G. Q. (2020). Engineering biosynthesis of polyhydroxyalkanoates (PHA) for diversity and cost reduction. Metab. Eng. 58, 82-93. doi: 10.1016/j.ymben.2019.07.004
Zhuikov, V. A., Zhuikova, Y. V., Makhina, T. K., Myshkina, V. L., Rusakov, A., Useinov, A., et al. (2020). Comparative structure-property characterization of Poly(3-Hydroxybutyrate-Co-3-Hydroxyvalerate)s films under hydrolytic and enzymatic degradation: finding a transition point in 3-hydroxyvalerate content. Polymers 12:728. doi: 10.3390/polym12030728
Keywords: biopolymer, tissue engineering, peripheral nerve repair, nerve guidance conduit, natural biomaterial
Citation: Fornasari BE, Carta G, Gambarotta G and Raimondo S (2020) Natural-Based Biomaterials for Peripheral Nerve Injury Repair. Front. Bioeng. Biotechnol. 8:554257. doi: 10.3389/fbioe.2020.554257
Received: 21 April 2020; Accepted: 23 September 2020;
Published: 16 October 2020.
Edited by:
Ana Marina Ferreira-Duarte, Newcastle University, United KingdomReviewed by:
Stephanie K. Seidlits, University of California, Los Angeles, United StatesMaling Gou, Sichuan University, China
Copyright © 2020 Fornasari, Carta, Gambarotta and Raimondo. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Stefania Raimondo, c3RlZmFuaWEucmFpbW9uZG9AdW5pdG8uaXQ=
 Benedetta E. Fornasari
Benedetta E. Fornasari Giacomo Carta
Giacomo Carta Giovanna Gambarotta
Giovanna Gambarotta Stefania Raimondo
Stefania Raimondo