- 1Department of General Surgery, Chun'an First People's Hospital (Zhejiang Provincial People's Hospital Chun'an Branch), Hangzhou, China
- 2Key Laboratory of Tumor Molecular Diagnosis and Individualized Medicine of Zhejiang Province, People's Hospital of Hangzhou Medical College, Zhejiang Provincial People's Hospital, Hangzhou, China
- 3Clinical Research Institute, Zhejiang Provincial People's Hospital of Hangzhou Medical College, People's Hospital, Hangzhou, China
- 4Department of Traumatology, Tiantai People's Hospital of Zhejiang Province (Tiantai Branch of Zhejiang People's Hospital), Taizhou, China
The applications of hydrogels in biomedical field has been since multiple decades. Discoveries in biology and chemistry render this platform endowed with much engineering potentials and growing continuously. Novel approaches in constructing these materials have led to the production of complex hybrid hydrogels systems that can incorporate both natural and synthetic polymers and other functional moieties for mediated cell response, tunable release kinetic profiles, thus they are used and research for diverse biomedical applications. Recent advancement in this field has established promising techniques for the development of biorelevant materials for construction of hybrid hydrogels with potential applications in the delivery of cancer therapeutics, drug discovery, and re-generative medicines. In this review, recent trends in advanced hybrid hydrogels systems incorporating nano/microstructures, their synthesis, and their potential applications in tissue engineering and anticancer drug delivery has been discussed. Examples of some new approaches including click reactions implementation, 3D printing, and photopatterning for the development of these materials has been briefly discussed. In addition, the application of biomolecules and motifs for desired outcomes, and tailoring of their transport and kinetic behavior for achieving desired outcomes in hybrid nanogels has also been reviewed.
Introduction
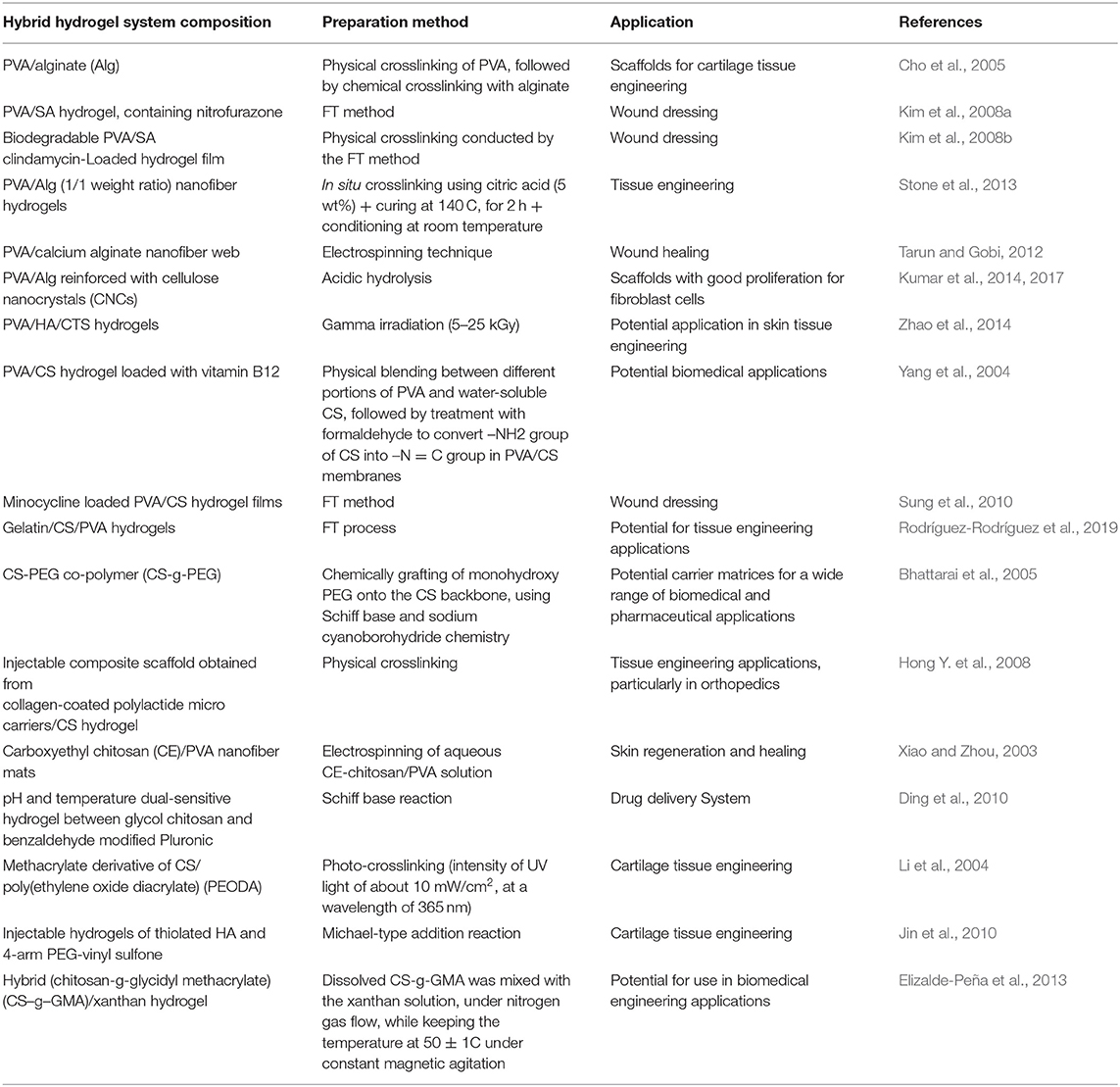
The use of nanotechnology in medicine, known as nanomedicine, offers several exciting possibilities in healthcare. The pharmacological properties such as circulating half-life and the solubility of drug molecules can be dramatically enhanced by employing nanoscale delivery vehicles (Shi et al., 2010). Nanotechnology has played a crucial role in the development of drug delivery systems since liposomes were first identified in the 1960s and adopted as protein and drug carriers for the treatment of various diseases (Bangham and Horne, 1964). As delivery vehicles, a range of inorganic/organic nanomaterials and other similar devices have been used to set up efficient therapeutic modalities (Figure 1). To date, the Food and Drug Administration (FDA) has licensed more than two dozen therapeutic products based on nanotechnology for clinical usage while some more are in clinical trial stages (Wagner et al., 2006; Zhang et al., 2008; Davis et al., 2010). Of these substances, most follow non-target delivery mechanisms (e.g., polymers and liposomes) and are thus known to be nanotherapeutics of the first generation (Riehemann et al., 2009).
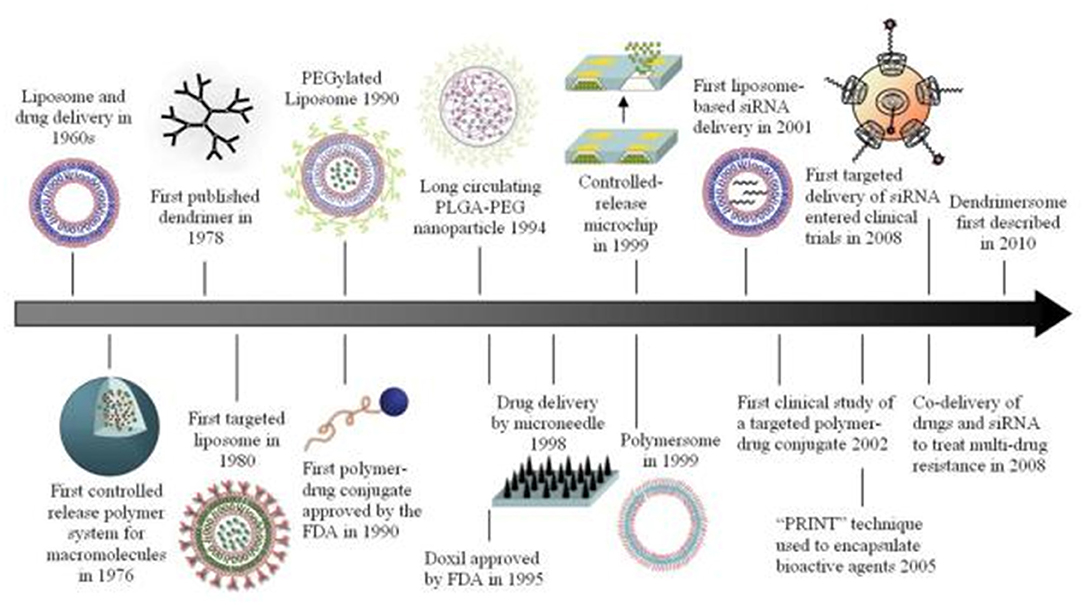
Figure 1. Different types of inorganic/organic nanomaterials and similar other devices that have been used as carrier systems for drugs or other bioactive substances. Reproduced with permission from Shi et al. (2010). Copyright (2010) American Chemical Society.
Nanosystems of the first generation offers several benefits relative to traditional drug delivery. In general, they can improve therapeutic effectiveness by extending drug half-life, increasing hydrophobic drug solubility, decreasing potential immunogenicity, and/or releasing drugs in a prolonged or stimulated manner. Furthermore, because of the enhanced permeability and retention (EPR) effect in specific tissues such as tumors, nanoscale particles can tend to grow passively (Maeda, 2010). In addition to these clinically efficient nanosystems, nanotechnologies have been used to facilitate new therapies and to create “hybrid” and “smart” nanosystems for next-generation biomedical applications (Shi et al., 2010).
Hydrogels are networks of hydrophilic polymer that can retain, expand, and carry large volumes of liquid in the simplest form (Shi et al., 2010). Hydrogels are innately well-suited for biological applications with their elevated permeability, and water content as well as their structural and viscoelasticity resemblance to the cell membrane. As demonstrated by Wichterle and Lim in 1960, these main features make them appealing for biomedical use as well as continue to exist as hubs for tissue engineering and drug delivery (Bangham and Horne, 1964). Enhanced material versatility and function have been facilitated by chemical advances that enable the integration of molecules that can guide cellular activities. Moreover, it also enables the hydrogel system to be activated under time control, as well as allow the incorporation of transporters such as nanoparticles and microstructured domains into the hydrogel network.
Hydrogels typically comprise polar or functional groups with specific charges that give them hydrophilicity, water absorption ability, and, therefore, swell up in a particular medium with improved vulnerability to stimuli, etc. (Durmaz and Okay, 2000; Ekici and Saraydin, 2004). The concept of Hybrid Hydrogels remains debatable. They either are described as complicated, consisting of hundreds of physically or chemically cross-linked nanogels (Zhang et al., 2018), or they relate to structures made up of various nanoparticles and/or polymers, such as magnetic, carbonaceous, and plasmonic nanoparticles. It has also been defined as a system comprising of building blocks that are functionally, morphologically, and chemically different from at least two groups related by physical or chemical means. It might involve biologically active polymers, such as pro-polysaccharides and/or polysaccharides hybridization can occur at microscopic or molecular levels depending on the size and role of the basic components (Jia and Kiick, 2009; Molina et al., 2015).
Each medical approach provides for the specific selection of a combination of composite material to match the required functional and structural properties with a new profile that must be effectively generated by an advanced polymer device (Myung et al., 2008). The combination of protein and other polymers is one of the most important examples. Conjugation or polymerization (click chemistry) with synthetic polymers may lead to such combinations, resulting in vitro and in vivo compatible hybrid hydrogels for application in drug delivery studies, wound healing and tissue engineering applications (Jonker et al., 2012; Lau and Kiick, 2015), or the extraction of growth factors from the extracellular environment (Maisani et al., 2017). Hybrid hydrogels are typically heterogeneous and are important to ensure adequate cell organization, cell-cell interactions, and adhesion for medical purposes (Yang et al., 2016; Joseph et al., 2018; Patel et al., 2018).
Owing to their versatile properties, hybrid hydrogels have got increasing scientific interest for diverse applications in biomedical fields. Recent advancements in hybrid hydrogels have led to their potential applications in the delivery of cancer therapeutics, and re-generative medicines. In this review, recent trends in advanced hybrid hydrogels systems incorporating nano/microstructures, their synthesis, and their potential applications in tissue engineering and anticancer drug delivery are discussed. Examples of some new approaches including click reactions implementation, 3D printing, and photopatterning for the development of these materials, the application of biomolecules and motifs for desired outcomes are reviewed.
Hybrid Nanogels
Subsequently formed hybrid nanogels are highly crosslinked nano-sized systems (Dorwal, 2012; Yadav et al., 2017) having a size <100 nm (Bencherif et al., 2009) and a non-fluid colloidal/polymer network which incorporates the characteristics of both nanomaterials and hydrogels. Their nanoscale offers a broad bioconjugation surface area, a prolonged circulation duration in the blood, and the potential to be actively or passively focused at the specified area of action such as tumor sites (Molina et al., 2015). Smart hybrid hydrogels/nanogels have the capability to efficiently respond via changes in their refractive index, volume, and hydrophobicity/hydrophilicity, etc. to biomedically relevant changes such as temperature, glucose, pH, electrical field, magnetic field, light, ionic force/concentration, redox environment, chemicals, or specific biomarkers, etc. Micro-and nano-sized hydrogels are quicker than their macroscopic or bulk alternatives in responding to environmental changes that can be used more effectively in biomedical and sensing applications (Sahiner et al., 2006).
Multifunctional Hybrid Nanogels
In the field of medicine/nanomedicine, multifunctional hybrid nanogels have identified uses of consistent optical sensing of specified stimuli in complex samples such as bioreactor fluids, blood and intracellular imaging, leading to the clarification of complex biological processes, the development of innovative diagnostics, and clinical applications (Wu and Zhou, 2010).
Hybrid Polymer Nanogel/Hydrogels
Hybrid polymer nanogel/hydrogels include interpenetrated networks (IPNs) and core-shell particles. The core-shell strategy is especially useful for targeting therapy, while the interpenetration allows the development of multi responsive nanogels and the control of the drug release profile (Lohani et al., 2014).
Physical Hydrogels
Physical hydrogels result by ionic and physical interactions, such as hydrogen bonds, coordination bonds, electrostatic and hydrophobic interactions in certain conditions and physico-chemical interactions (stereo-complexation, charge condensation, or supramolecular chemistry) (Hoare and Kohane, 2008). By changing the temperature, pH, ionic strength, or solvent composition, they mostly form a homogeneous solution and re-gel when they return to their initial conditions, being reversible gels, generally unstable and mechanically weak (Ebara et al., 2014). Physical hydrogels are not all being reversible gels with physical stimuli, such as Chitosan/GP gel. The physical cross-links are also formed by crystallization (Amini and Nair, 2012), between amphiphilic block and graft copolymers (Jin, 2012), and protein interactions (Augst et al., 2006). Physically crosslinked hydrogels show stimuli-responsiveness and self-healing properties, but their mechanical strength is low and they often exhibit plastic flow (Czarnecki et al., 2016).
Chemically or Covalently Crosslinked Hydrogels
Chemically or covalently crosslinked hydrogels with a permanently fixed shape at rest, exhibit a low fracture toughness and extensibility. Therefore, it is preferred to create both physically and covalently crosslinking hydrogels (Sun et al., 2012; Lin et al., 2015), resulting doubly-crosslinked hybrid gels that combine all mentioned properties (Narita et al., 2013). Many double network (DN) hydrogels prepared by double chemically crosslinking or by hybrid physical/chemical crosslinking imply crosslinking agents, but they present toxicity which is an important disadvantage (Vasile et al., 2020). Some derivatives of DN hydrogel, not hydrogel itself are toxic and the toxicity of such system is due to the use of crosslinking agents. Designing a new generation of DN gels comprising two non-covalent associated networks is a promising technique.
Kondo and coworkers (Kondo et al., 2015) prepared a dually-crosslinked polymer gel with a very homogeneous network architecture, using a tetra-arm star-shaped poly(ethylene glycol) (PEG), PEG and poly(dimethylsiloxane) (PDMS) building blocks linked by orthogonal cross-coupling, The obtained network from hydrophilic and hydrophobic components regularly and uniformly distributed is non-covalent hydrophobic association whose strength is tuned by the molar ratio of the hydrophilic PEG and the hydrophobic PDMS segments (Sletten and Bertozzi, 2009).
Self-Assembling Hybrid Hydrogels
Self-assembling hybrid hydrogels containing peptides provide the desired biological functionality and biodegradability, are able to mimic biological structures and materials having direct biomedical applications, namely as carriers for drug and cell delivery (e.g., incorporation of bioactive sequences from natural proteins). To control mechanical, biocompatibility and degradation properties, the peptides are combined with polymeric networks (Kopeček and Yang, 2012; Rodriguez et al., 2016) by chemical modification, covalently linking or non-covalent interactions between peptides and polymers (Baker and Chen, 2012).
Hybrid hydrogels self-assembled from graft copolymers via formation of coiled coil antiparallel heterodimers was also demonstrated, based on HPMA copolymers backbone and a pair of oppositely charged peptide grafts. The formation of these hybrid hydrogels was reversible (Yang et al., 2006). ADNA/poly(lactic-co-glycolic acid) (PLGA) hybrid hydrogel (HDNA) was prepared for water-insoluble ophthalmic therapeutic delivery of dexamethasone and it may be applied in treatment of various eye diseases (Ren et al., 2019).
Methods of Preparation of Polymeric Hybrid Hydrogels
Routes for Hybrid Hydrogels
Crosslinking approaches may include: (i) physical crosslinking (employing frequent freezing/thawing cycles leading to cryogels) through the complex process of coacervation, H-bonding, or ion interaction; (ii) grafting or chemical crosslinking by co-polymerization, polymerization, and chemical conversion (via employing crosslinking agents such as glutaraldehyde, glyoxal, borates, etc., and (iii) grafting or crosslinking through irradiations (gamma radiation or electron beam, based on the dose of the irradiations). The characteristics of hydrogels can be determined by various parameters, such as the structure, the shape of the cross-link, the final density, and the development of polymers, whereas environmental factors (such as ion power, pH, temperature, etc.) can be regulated for physical hydrogels.
Chemically cross-linked gels are prepared by reverse microemulsion, irradiation (ultraviolet, high-energy radiation via employing electron beam, or gamma), emulsion, radical polymerization/crosslinking, inverse mini-emulsion, heating, photolithographic chemical reactions via cross-linker as di-sulfide cross-linking, ionic, click chemistry (Thiol-ene couplings, Azide-alkyne cyclo-addition reactions, Tetrazine-norbornene chemistry, and Diels-Alder reactions), Schiff base crosslinking with a huge ensemble of reactions including Nucleophile addition and Michaelis-Arbuzov reaction (Gulrez et al., 2011), Michael type reactions and enzymatic cross-linking (Khademhosseini and Langer, 2007). For hydrogel preparation, both physical and chemical cross-linking methods are used (Budama-Kilinc et al., 2018).
The use of living/controlled radical polymerization techniques like iodine-mediated polymerization (RITP) degenerative chain transfer polymerization, reversible polymerization of addition-fragmentation chain transfer and catalytic atom (group) transfer radical polymerization (ATRP) is a transition toward the development of complex structures with a significant degree of versatility and configurational range (Barner-Kowollik, 2008). A new technique of hybrid hydrogel synthesis is required for the non-covalent binding of genetically engineered coiled-coil protein motifs to the hydrophilic synthetic HPMA copolymer backbone. The coiled-coil domains' self-assembly provided the physical crosslinking (Wang et al., 2001).
Chemical Modifications
Chemical changes include a variety of ligands that can be used for the delivery of targeted drugs, stimulation of release of the drug, or the preparation of complex materials. Lim et al. have documented the cross-linking of the hybrid network and the conjugation of proteins to the gel backbone as a functional protein immobilization platform (Lim et al., 2019).
Functionalization
Surface functionalization of hybrid hydrogels/nanogels can also be accomplished with specific ligands for achieving targeted therapy and reducing the toxicity (Sierra-Martin and Fernandez-Barbero, 2015). This type of functionalization is also critical for the development of various types of micro/macro/nanogel morphologies, such as hallow, core-and-shell, multilayer microgels, hairy microgels (Sanson and Rieger, 2010), etc.
Stealth Functionalization
A non-secondary requirement is needed for hybrid nanosystems/nanogels for biomedical purposes and drug delivery, as their biocompatibility is required both to minimize the organism's immune or inflammatory responses and to increase the supply of blood, biodistribution, and bioavailability of the transported drugs. Hybrid nanogels must be specifically aimed and explicitly constructed to accomplish this requirement. A very large range of architectures emerges from their functionalization, modification, and decoration (Kabanov and Vinogradov, 2009). Similarly, their modification can also be achieved via their conjugation with both inorganic (Karg, 2016) and organic (Wu and Wang, 2016) forms of nanostructures and nanoparticles. Hybrid nanogel reveal diverse morphologies depending on both the assembly techniques and the particle form, each element either being core or shell, of varying architecture and size (Eslami et al., 2019). These different morphologies can be achieved by physical crosslinking or chemical reactions, depending on ionic interactions, hydrogen bonds as well as other intermolecular bonds. Appropriate biocompatibility and surface decoration are, therefore, important parameters that greatly influence the biodistribution, along with the components, the surface charge, and the interaction of the ligands. Numerous covert functionalization, such as polyethylene glycols or chitosan, manipulate hydrophilic polymeric chains have been used for stealth purpose.
PEGylation
The process of PEGylation is carried out for increasing lifespan of circulation of nanostructures, thus leading to improved bioavailability of the drugs loaded in these nanostructures (Grover et al., 2013). A protein corona is developed around the antifouling PEG functionalization following this modification (Foster, 2010). It would create a restricted zone around the nanoparticles and reduce the enclosure of plasma proteins and the subsequent absorption of macrophages. PEGylation depends on a variety of factors including the hydrophilic characteristics of the PEG chains as well as molecular weight in 2000-13,000 Da range.
New Approaches for the Development of Hybrid Hydrogels
Current advances in contemporary polymer chemical transformations have made it possible to develop advanced material systems. In general, “click reactions” (Thiol-norbornene click reaction, and Thiol-maleimide Michael addition) are highly helpful as they are orthogonal to many natural chemical functions, have few by-products, and can be tuned to demonstrate reversible and dynamically correct relationships with the resulting thioether succinimide linkage (DeForest and Anseth, 2011; Koshy et al., 2016). They are especially useful for the development of hydrogels with tunable viscoelasticity, high biocompatibility, and most significantly, for their capability to transport, protect and sensitively release their loaded therapeutic contents in the surrounding tissue (triggered by changes in pH or by the action biological substrates) (Baldwin and Kiick, 2011). For instance, hyaluronic acid hydrogels based on copper(I)-catalyzed azide-alkene cycloaddition (CuAAC) have been employed as cell scaffolds as well as repositories for the drugs (Crescenzi et al., 2007). In addition, the toxic catalysts employed in copper-catalyzed reactions can be eliminated or reduced with copper-free click chemistries like those used in azide-alkyne cycloaddition (Jiang et al., 2015), in radical-mediated thiol-ene/yne chemistry (Zhu et al., 2015), the Diels-Alder reaction (Fan et al., 2015), tetrazole- alkene photo-click chemistry (Fan et al., 2013), and the oxime reaction (Mukherjee et al., 2015).
Although hydrogels have key properties that make them ideal for biomedical applications such as tissues engineering and drug delivery (Jia and Kiick, 2009; Slaughter et al., 2009), it is still important to optimize their mechanical power, degradation/ release kinetics, as well as the bioactivities of loaded therapeutic contents. There are major heterogeneity and network defects in typical synthetic hydrogels such as phase-separated regions, entanglements, and chain ends which have a dramatic effect on mechanical properties and modify the biological activity and diffusion rates of active molecules (Jia and Kiick, 2009). Hybrid hydrogels are, however, designed to solve the problems associated with the development of current formulations. Further, they also extend a variety of applications from medical (spatial-temporally regulated profiles of drug release) (Chivers and Smith, 2017; Wang et al., 2018) to optoelectronics and magnetic materials (high-tech applications such as the manufacture of high-charge organic electronic devices having excellent properties related charge transport) (Babu et al., 2014; Amabilino et al., 2017). Hybrid hydrogels are made up of building blocks that are chemically, functionally, and morphologically different and may include biologically active peptides, proteins, or micro/ nanostructures that are interconnected by chemical or physical means. In general, peptides and proteins that are integrated into networks react with synthetic polymers through conjugation or polymerization (click chemistry) approaches to generate in vitro (cells proliferation, differentiation and migration studies) and in vivo (tissue engineering, drug delivery, and wound healing) compatible hybrid hydrogels (Jonker et al., 2012). The encapsulation of micro/ nanostructures within the hydrogels can be accomplished through chemical or physical encapsulation to improve the yield of mechanical reinforcement and the controlled delivery of cargo (Lau and Kiick, 2015) or sequestration of growth factors from the surrounding setting (Maisani et al., 2017). In addition, the heterogeneity of hybrid hydrogels will enhance cells organization, adhesion, and interactions between cells to generate tissue constructs having enhanced electroactivity, mechanical integrity, and cell organization (Shin et al., 2013; Xavier et al., 2015).
Release Mechanisms of Bioactive Molecules From Hybrid Hydrogels
In a hydrogel network, the inclusion of micron-sized domains or particles enables improved structural integrity and can eventually serves as a tool for guiding cell behavior. Cells are known to show enough sensitivity to material properties such as size, modulus, pore, and elasticity, so it is of great interest to use these cell-modulating material properties for developing materials that facilitate growth, singling, proliferation, migration, and expression of cells (Vikingsson et al., 2015).
They are usually isotropic materials, despite various excellent properties of hydrogels for drug release, which can only experience uniform contraction and volumetric expansion in response to stimuli (Jeon et al., 2017) in contrast to different human tissues. Hybrid hydrogels that contain domains of micron-sized have been designed for maintaining cellular development, promoting microstructure-mediated cell migration, and release of their loaded bioactive agents in a regulated way under the influence of naturally occurring microscale structural motifs.
Micron-sized particles, including nanoparticles, can be integrated into a hydrogel network covalently or non-covalently to generate enhanced mechanical strength, release bioactive molecules, and facilitate a range of anticipated cellular responses. In general, the release of the loaded therapeutic substances from hybrid hydrogels can happen via one or more of three mechanisms: (1) The burst or abrupt release that occurs as result of dissolution of the drugs absorbed on the surface of hydrogel; (2) release of the drugs due to their diffusion via the micro-particle or hydrogel matrix; and (3) release of the drugs due the hydrogel degradation (Mahmoudian and Ganji, 2017). How the hydrogel releases the drug is often essential to achieve desirable therapeutic outcomes, and the required duration of drug availability (short term vs. long term) and its release profile (continuous vs. pulsatile) depend on the specific application. When the drug is exhausted, the hydrogel should be designed to either degrade to avoid surgical removal, or to be re-used by drug refilling. The degradation of the hydrogel may also need to be tailored to coordinate with tissue regeneration. Besides the general requirements, there exist other application-based requirements. For example, in the treatment of skin wounds, hydrogels are placed on dynamic surfaces to which they need to be adhesive and conform, while being tough enough to tolerate the surface movement (for e.g., strain of knee bending up to 50%) and deformation derived from the environment (for e.g., compression and scratching) (Burczak et al., 1994; Kong et al., 2004; Mahmoudian and Ganji, 2017). Hydrogels consist of a cross-linked polymer network, and open spaces (that is, meshes) between polymer chains; the meshes allow for liquid and small solute diffusion. Typical mesh sizes reported for hydrogels range from 5-100 nm, (Garcia and Kiick, 2019) and a number of approaches exist to determine the mesh size. Mesh size also play a key role in controlling diffusion and release of the bioactives from hydrogel system (Burczak et al., 1994). Another strategy to control the release of drug molecules initially entrapped in a hydrogel is to regulate network degradation. The mesh size increases as the network degrades, allowing drugs to diffuse out of the hydrogel. Degradation can occur in the polymer backbone or at the cross-links, and is typically mediated by hydrolysis (Kong et al., 2004; Boontheekul et al., 2005; O'Shea et al., 2015) or enzyme activity. An alternate strategy to release entrapped drugs is the controlled swelling of hydrogels. As a hydrogel swells, the mesh size increases. The extent of swelling of a hydrogel is a balance between forces that constrain network deformation and the osmosis that leads to water absorption (Brannon-Peppas and Peppas, 1991; Hong W. et al., 2008). The swelling behavior can be sensitive to various external conditions, including temperature (Hirokawa and Tanaka, 1984), glucose (Kokufata et al., 1991; Obaidat and Park, 1997), pH (Zhang et al., 2015), ionic strength (Ohmine and Tanaka, 1982), light (Yan et al., 2012), and electric fields (Murdan, 2003). These cues have been widely exploited in drug delivery. A final approach to release entrapped drug molecules is to mechanically deform the network, as this can both increase the mesh size by changing the network structure and trigger convective flow within the network (Huebsch et al., 2014). This strategy can generate pulsatile release patterns with fine control over the magnitude of the instantaneous release rate (Huebsch et al., 2014) using purely mechanical deformation, or using ultrasound and magnetic field-induced deformations.
These bioactive molecule release mechanisms include handles that can be modulated to affect transport; particle composition and polymer network can therefore be controlled to regulate mesh size and chemical degradation rates, and bio-responsive domains can be integrated to allow cell-responsive actions (Rehmann et al., 2017; Lau et al., 2018).
For instance, when vancomycin hydrochloride HMPC nanoparticles were included in a hydrogen based on chitosan/glycerophosphate, there was a noticeable effect of prolonging the drug release due to the diffusion of the transport mechanism (Mahmoudian and Ganji, 2017). In this situation, the drug release is slowed by the need to be diffused into both the hydrogel network and the microparticles. In another study, the drug release from carbohydrate based injectable hydrogel containing anionically functionalized microgels [poly(NIPAM functionalized with acrylic acid)] was studied (Sivakumaran et al., 2011). The release of loaded bupivacaine was regulated in this system not only by diffusive contributions, such as the hydrogel crosslinking density but also by the drug's partitioning affinity between the microgel phases and the bulk, which permitted minimization of the initial rapid release. In a study, a series of hydrogels made of self-assembling peptides, five graphene derivatives (GDs), and three different gelling b-sheet-forming with various surface chemistries were studied. The mechanical properties of hydro-gels were examined with the inclusion of GDs in a peptide nanofiber matrix for investigating the molecular interactions between the filler and the matrix (Wychowaniec et al., 2018). The effects of coupled hydrophobic interactions and electrostatic and the consequent outcomes of these interactions on the module were evaluated by changing the surface chemistry of the GDs and the peptides physicochemical properties. If both hydrophobic interactions and long-range electrostatic interactions are favorable, G0 increases, and the relative strength of each contribution determines the outcome of G0 when the interactions compete (Wychowaniec et al., 2018).
However, because of geometry, the capability to modulate the drug release from and into micro-domains is limited. Diffusion is restricted with increased particle diameter because spheres have a decreased surface area to volume ratio (Bjørge et al., 2018). Particles with diverse geometries can be created to compensate for this limitation, allowing for maximum surface area and a correspondingly increased range of possible drug release rates. One of such examples is the possibility to improve the release rate of encapsulated Bovine serum albumin (BSA) by using spheroid particles created by squeezing chitosan droplets between two super-amphiphobic surfaces, accompanied by UV-crosslinking. The encapsulation of BSA was accomplished in the particles and a more rapid release from spheroids than from spheres was observed (Bjørge et al., 2018).
The advantages of anisotropic microparticles such as ellipsoids, rods, spheroids, and discs have also shown to apply to hemodynamic environments, with improved marginalization, wall contact, and adhesion suggested by both experimental studies and theoretical modeling (Sen Gupta, 2016). The inclusion of spheroid particles has shown to enhance both the cell viability and diffusion (Bjørge et al., 2018). Increased cell viability and diffusion and the possible hemodynamic transport advantages from anisotropic particles indicate effectiveness for the use of complex anisotropic particles in hybrid hydrogels. Moreover, the non-circular micro-patterned regions in hybrid hydrogels have also revealed an increased absorption of toxin, thus resulting in the detoxification capability of these products (Chen et al., 2017). Hemolytic studies have revealed that star-shaped canals result in a higher detoxification efficiency in comparison to circular canals. This can be attributed to multiple surface plane, increased surface perimeter, and planar area (Chen et al., 2017).
Recent Trends in the Incorporation of Nano/Microstructures in Hybrid Hydrogels
Several methods have been used to generate hydrogels that involve micronized domains such as emulsion stabilization, photopatterning, and liquid-liquid phase separation, rather than the manufacturing and eventual microparticles incorporation in hydrogels (Garcia and Kiick, 2019). Although all these techniques can create desirable microstructures with different degrees of control, the phase separation has specific advantages due to the one-step manufacture, specificity of the actions, and tunability (Lau et al., 2018). These methods are widely employed to create microstructured resilin-like polypeptide (RLP)-PEG hydrogels. The separation of liquid-liquid phase of RLP and PEG functionalized with acrylate combined by UV-crosslinking is employed for generating RLP-rich hydrogel microdomains distributed in a PEG hydrogel matrix (Lau et al., 2018). High cell viability and direct cell localization around RLP-rich domains are enabled by RLP-PEG hydrogels. By choosing the time point at which the phase-separating solutions are crosslinked after mixing, the domain size can be easily tuned, and the selection of LLPS conditions can provide bicontinuous networks when crosslinks occur during the initial phases of spinodal decomposition (Lau et al., 2018). Although this approach is easily used in situ and provides tunability in terms of domain size, there is a restricted range of structures.
Methods like 3D printing and photopatterning are widely used to create hydrogels with detailed and precise microstructures. Techniques like these, however, are resolution-limited and labor-intensive, which limits the versatility of manufacturing and production quality (Nawroth et al., 2018). Techniques, such as such as laser-mediated UV photo-patterning, are less time-consuming and highly precise, and currently being investigated to overcome these limitations (Duan et al., 2019). As an example, photopatterning of riboflavin-50 phosphate gelatin hydrogels as a non-toxic UV photosensitizer has allowed faster development of micropatterned substrates with less variability and spatial resolution compared to conventional photolithographic and micromolding techniques (Nawroth et al., 2018). In combination with crosslinking and casting of polymeric materials, these techniques could be used to produce hybrid hydrogels with domains having a fine tunability. Micro-patterned gels could also be assembled for generating multilayered heterogeneous materials with embedded high-resolution microchannels that enable perfusion (Attalla et al., 2018). The outcome is a hollow microchannel hydrogel (150 mm-1 mm) that enables faster cell viability when evaluated over a 7-day duration by using silicon carbide as an adhesive to promote strong binding between micro-patterned collagen and alginate hybrid hydrogel films (Attalla et al., 2018).
Applications of Hybrid Hydrogels
Hybrid Hydrogels in Anticancer Treatment
Cancer stands on top of the deadliest diseases worldwide. About 9.6 million people died of this disease around the world according to 2018 report (Bray, 2018). Surgery, radiotherapy, chemotherapy, and immunotherapy are the cancer treatment strategies applied clinically now a day. Though, chemotherapy seems effective up to some extent, full application of this strategy is hindered by the adverse anticancer drugs' reactions, drug tolerance, low therapeutic index, and effecting other tissues alongside target tissues (Senapati et al., 2018). Hydrogels, being comprised of excessive water components and a cross-linked polymer network endowed with extraordinary biocompatibility, less cytotoxicity, and excellent drug-loading capacities (Li and Mooney, 2016). Thus, they offer numerous desirable characteristics to be used for anticancer drug delivery and have been the research interest for application in cancer drug delivery in recent years (Fang et al., 2018; Wu Q. et al., 2019). The main drawbacks of conventional anticancer delivery methods include frequent administration via intravenous route and systemic distribution. To address these issues, different approaches for fabrication and construction of hybrid hydrogel systems have been practiced in recent years. The subsequent section highlights some of the recent examples used for construction of hybrid hydrogels-based systems for anticancer drug delivery application.
Wu et al. recently have reported manganese dioxide nanosheet hybrid hydrogel and used for an anti-tumor application. Their method of hybrid hydrogel preparation was feasible and convenient, and showed good biocompatibility and is expected to be applied in clinics. They initially prepared a single-sheet manganese dioxide nanosheet layer using simple mechanical stripping mode. Subsequently they crosslinked caffeic acid modified chitosan via an in-situ oxidation mode on the surface of the nanosheet and thus obtained the manganese dioxide nanosheet hybrid hydrogel. The resultant hydrogel showed good injectability potential due to the presence of excessive hydrogen bonds and π- π interactions in the hydrogel. In addition, the hydrogel showed good tissue adhesion because of the presence of phenolic hydroxyl groups of the caffeic acid in the system. As the tumor microenvironment possess high concentration of hydrogen peroxide (H2O2), the manganese dioxide nanosheet hybrid hydrogel catalyzed the H2O2 decomposition and generated oxygen, thus the tumors' cells hypoxic condition was overcome, sensitivity of cancer cells to anticancer drugs was improved, and the melanoma cells growth was effectively inhibited by combining with the emerging photothermal treatment (Wu M. et al., 2019).
Metal organic frame-works (MOFs) have also been encapsulated in hydrogel system to produce hybrid system. In an example, Lian et al. produced such a system and used for the detection of Mitoxantrone Figure 2. Their MOFs of sandwiched mixed matrix membrane (MMM) based framework-hydrogel hybrid system showed excellent mitoxantrone detection performance with sensitivity up to ppb-level (13.4 ppb) and good serum selectivity among other anticancer drugs. Their flexible MMM based system could be employed for point-of-care testing bioactive drugs in biological medium. It was concluded that the MMM can identify mitoxantrone among other different anticancer drugs or their metabolites/ derivatives with nearly similar chemical structures, thus, can be successfully employed for point-of care testing of drugs in biological medium (Lian et al., 2020).
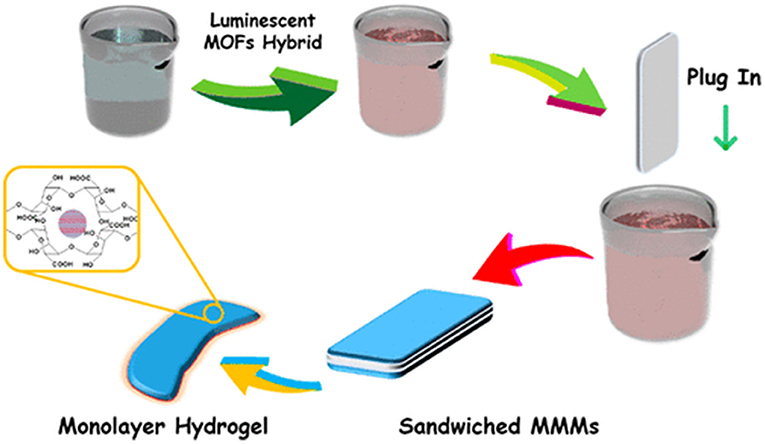
Figure 2. A sandwich of Mixed Matrix Membrane (MMM) on the basis of hybrid hydrogel containing luminescent MOF. Reproduced with permission from Lian et al. (2020). Copyright (2020) American Chemical Society.
In another example, Javanbakht et al. developed an anticancer drug (5-fluorouracil; 5-FU) encapsulated porous Zn-based MOFs (MOF-5) and embedded in CM-cellulose (CM-cellulose) biopolymer to produce 5-FU@MOF-5 bio nanocomposite hybrid hydrogel bead system coated with CMC. The 5-FU was encapsulated in Zn-based MOFs (MOF-5) via immersion of the MFOs in the drug solution. The pH sensitive biopolymer of CMC was used for the protection and carrying of 5-FU encapsulated MOF-5 nano-hybrid through the digestive tract. The drug release in simulated gastric environment showed that the MOFs containing hybrid hydrogel possessed a sustained release profile and showed noticeable toxicity against HeLa cells in the MTT assay. Their findings showed that the prepared hydrogel beads system could be used as a potential anticancer drug delivery system for 5-FU targeting in colon cancer (Javanbakht et al., 2020).
Schneible et al. constructed a hybrid hydrogel-based system comprised of modified graphene oxide (GO) nanoparticles that were embedded in Max8 peptide hydrogel for controlled kinetics and molar ratios release of anticancer drugs i.e., gemcitabine (GEM) and doxorubicin (DOX). Initially, they modified GO nanoparticles (tGO) for affording high loading sustained release of DOX (19% over 72 h and 31.4% over 4 wk). They also utilized Molecular dynamics (MD) simulations to model the DOX loading mechanism as a function of surface modification. A Max8 based hydrogel was prepared in parallel for the release of GEM with rapid release kinetics and achieved a 10 times higher molar ratio to DOX. These DOX/tGO nanoparticles were then embedded in a hydrogel matrix of GEM/Max8. The developed hybrid system was was also tested against a triple negative breast cancer (TNBC) cell line (MDA-MB-231). The results of their study showed that the composite formulation synergistically enhanced the anticancer effect as compared to the administration of DOXGEM combination in solution form (Schneible et al., 2020).
A Prussian blue (PB) nanoparticles' embedded nanozyme-hydrogel (hPB-gellan) based hybrid injectable hydrogel system was developed by Hao et al. The biomimetic cascade bioreactor was developed for combining antitumor therapy via providing long-term delivery and spatiotemporally-controlled of anticancer agents. The hybrid nanozyme-hydrogel (hPB-gellan) was doped with PB nanoparticles through nanoprecipitation method in the gellan matrix of polysaccharide. The resultant PB nanoparticles were of 10 nm size and displayed dual functions of photothermal agent and as a nanozyme for the decomposition of H2O2 into oxygen. Due to its self-recovery and shear-thinning properties, the developed hybrid hydrogel was administered locally into tumors and showed resistance against body metabolism and clearance for long-term. In-vivo, the antitumor effects of this system showed great elimination of tumors in combined photothermal and starvation therapy (GOD/hPB-gellan + NIR) group with 99.7% tumor suppression rate after 22 days treatment. The enhanced anticancer effect of this system was believed to be due to the NIR-triggered hyperthermia attack and GOD-mediated holding attack starvation from the catalytic bioreactor of the hybrid system. Keeping in view the advantageous properties of biocompatibility, easy manipulation in treatment, and simple synthetic approach, this hybrid hydrogel has great potential for clinics (Hao et al., 2020).
A hybrid Hydrogel system was developed by Capanema et al. composed of CMC-Silver nanoparticles-Doxorubicin for Antibacterial and Anticancer effects. The hybrid hydrogel system was made of silver nanoparticles (AgNPs) embedded in the doxorubicin (DOX)- conjugated CMC polymer crosslinked networks. A green synthetic approach was used for the preparation of this system using a one-pot reduction of Ag+ by CMC polymer in situ that also worked as capping ligand, followed by DOX conjugation electrostatically in aqueous media. The prepared nano sized conjugates were then crosslinked with citric acid (CA) chemically under mild temperature and pH environments. The prepared hybrid system showed tuned intracellular DOX kinetics in-vitro, suggesting synergistic effects for killing melanoma cancer cells with AgNPs. In addition, hybrid system also showed good antimicrobial potentials against different Gram-positive and Gram-negative bacteria. Thus, an innovative hybrid hydrogels platform was produced with anticancer and antibacterial properties and could be successfully applied as a weapon against skin cancer (Capanema et al., 2019).
Paclitaxel (PTX) loaded Quantum dots (QDs) embedded polypeptide hybrid hydrogel system has been developed by Jin et al., The injectable multifunctional hydrogel was prepared for sustained chemo-photothermal cancer therapy. Based on an engineered coiled-coil polypeptide, paclitaxel (PTX) and Ag2S quantum dots (QDs) were designed for chemo-photothermal therapy in a sustained profile. Oil-sol. Initially, hydrogel was prepared with engineered polypeptide (PC10A) and then PTX and Ag2S QDs were loaded into the nanogel via ultrasonic treatment to prepare PC10A/Ag2S QD/PTX nanogel system. Then, PC10A/Ag2S QD/PTX multifunctional hydrogels were constructed by dissolving PC10A/Ag2S QD/PTX nanogels into the previously prepared PC10A hydrogel. The PC10A/Ag2S QD/PTX hydrogel was also suitable for direct injection to the tumor site. In-vitro and in-vivo toxicity studies showed that prepared hybrid hydrogel system was extremely biocompatible. The combined therapy effectively suppressed the SKOV3 ovarian carcinoma tumor cells growth in comparison with chemotherapy and single near-IR photothermal therapy. Moreover, the system was real-time monitored for in-vivo degradation using photoacoustic imaging near-IR fluorescence imaging. Their results showed that the prepared multifunctional injectable PC10A/Ag2S QD/PTX hydrogel had the potential for use as theranostic system for sustained cancer treatments (Jin et al., 2019).
Gangrade and Mandal prepared a silk based nano hybrid hydrogel system for targeted, localized, and on-demand anticancer drugs' delivery. The hybrid formulation contained (DOX)-loaded folic acid functionalized single-walled carbon nanotubes (SWCNTFA/ DOX) and a blend of two varieties of silk protein. A sustained and slow release of DOX over 14-day study was observed (Figure 3). The DOX release was assessed in different parameters i.e., rate of silk degradation, concentration of the SWCNTFA/ DOX payload, pH of the released medium, and temperature of incubation. DOX release was stimulated with exposure to intermittent near-IR light of the hybrid gel system. Folic acid receptor-positive (FR +ve) cancer cells active targeting of SWCNT-FA/DOX was observed in in-vitro studies. Being viscoelastic in nature, the silk hydrogel was easy to inject to the target site. Thus, their prepared silk hybrid hydrogel system could allow its intra-tumoral or near- tumoral implantation, where it will serve as anticancer drug loaded nanoparticles depot (Gangrade and Mandal, 2019).
Figure 3. Design and development of injectable multifunctional hydrogel based on carbon nanotube impregnated silk for on-demand targeted anticancer drug delivery. Reproduced with permission from Gangrade and Mandal (2019). Copyright (2019) American Chemical Society.
Similarly, an injectable hydrogel-encapsulating PTX-loaded red blood cell (RBC) membrane nanoparticles (PRNP-gel) was designed by Qian et al. The (PRNP-gel) system was constructed using temperature -induced phase transition of polyethylene-glycol (PEG) modified bovine serum albumin (PEG-BSA). The prepared PRNP were of spherical shape with nearly 133 nm diameter and negative zeta potential. The system drug loading efficiency was found 85% with loading content of 22%. The in-situ gelation took 12 min when the gel precursor was injected subcutaneously or incubated at 37°C. A sustained release profile of the drug was shown by the in-situ-forming hydrogel and total PTX release after 6 days was around 30%. The PRNP-gel showed excellent in-vivo and in-vitro biodegradability and cytocompatibility. The nanoparticle-hydrogel hybrid system was used for local chemotherapy as a drug carrier to reduce the systemic toxicity and enhance therapeutic concentrations at tumor site. In peritoneal dissemination and subcutaneous xenograft model, the in-vivo anticancer evaluation of this system showed that it possessed excellent tumor growth suppression potential after a single injection (Figure 4). Thus, the prepared injectable hydrogel platform could potentially be used as a promising system for local delivery of anticancer drugs (Qian et al., 2019).
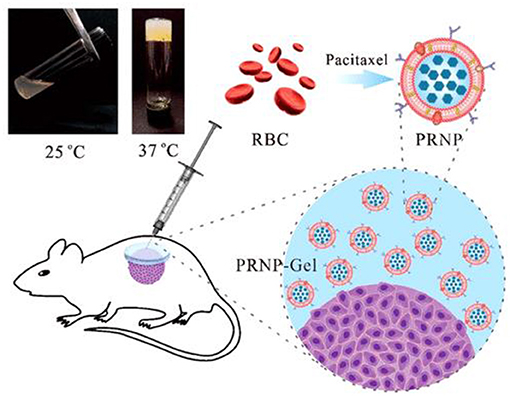
Figure 4. Injectable albumin hydrogel hybridized with paclitaxel-loaded red blood cell membrane nanoparticles for the treatment of gastric cancer with peritoneal metastasis. Reproduced with permission from Qian et al. (2019). Copyright (2019) American Chemical Society.
Hybrid Hydrogels in Tissue Engineering
Hydrogels stands the most attracting candidates among different investigated tissue engineering scaffolds because of their natural extracellular matrix (ECM) structural resemblance, innate biocompatibility, higher water contents, tunable viscoelasticity and high permeability for essential nutrients and oxygen (Lee and Mooney, 2001). The physical properties of hydrogels can easily be manipulated in response to changes in environment and cellular activities (Gil and Hudson, 2004; Kopeček, 2007). Chemically or physically crosslinked hydrogel systems can be constructed in the living cells, thus allow its in situ encapsulation for tissue engineering (Sakai et al., 2009; Xing et al., 2011). Different types of hydrogel materials have been used for tissue engineering applications (Raschip et al., 2007, 2013) including reconstituted ECM components or natural proteins and carbohydrates (Beamish et al., 2010; Hejčl et al., 2010; Zustiak et al., 2013), self-assembling peptides (Jiang et al., 2008; Deshmukh et al., 2010), and synthetic hydrogels (Schmedlen et al., 2002; Higuchi et al., 2006; Ossipov et al., 2007). Though, they show unique properties that them as nanostructured scaffolds for tissue engineering. However, several limitations are associated with single-component hydrogels owing to the low versatility of single component used in their formulations. To achieve multi-component hybrid hydrogel-based systems, various approaches have been applied. In an example, Amiryaghoubi et al. developed thermo-sensitive injectable hydrogel system composed of poly (N-isopropylacrylamide) (PNIPAAm) based copolymer/graphene oxide (GO) composite with different chitosan feed ratio through physical and chemical crosslinking. This hybrid hydrogel system was used for the differentiation and proliferation of the human dental pulp stem cells (hDPSCs) to the osteoblasts. The copolymer/GO composite was prepared in the presence of GO using free-radical copolymerization of (Nisopropylacrylamide) (NIPAAm), itaconic acid (IA) and maleic anhydride-modified PEG. It was ultimately used for the construction of hydrogels system. The hydrogel enhanced the deposition of minerals and the activity of alkaline phosphatase (ALP), mainly attributed to the oxygen and amine-containing functional groups of CS and GO. The hydrogel also upregulated the expression of the osteocalcin and Runt-related transcription factor 2 in the hDPSCs cultivated in both osteogenic and normal media and also promoted the absorption of osteogenic inducer. Thus it was proposed to be a constructing scaffold in bone tissue engineering for the transplantation of hDPSCs (Amiryaghoubi et al., 2020).
Recently, Lee et al. synthesized a hyaluronate-alginate hybrid (HAH) hydrogel by introduction of alginate to hyaluronate backbone with different molecular weights (700–2,500 kDa) and HAH hydrogels were designed in the presence of calcium ions. As the molecular weight of the hyaluronate were increased in the HAH polymer, an increase in the storage shear moduli of the hydrogels was observed. Further modification of the HAH hydrogels with histidine-alanine-valine (HAV) and arginine-glycine- aspartic acid (RGD) peptides were induced for enhancing cell-cell interactions and cell-matrix respectively. With an increase in storage shear moduli of the gel, the differentiation (chondrogenic) of ATDC5 cells encapsulated in the HAH hydrogels were increased in-vitro and in-vivo. Such approach of viscoelastic properties regulation of hydrogels via polymers with varying molecular weights at same crosslinking degree could prove beneficial in different cartilage regeneration and other tissue engineering applications (Lee et al., 2020).
Modifying the ECM based hydrogels properties by conjugation with different synthetic polymers is an emerging strategy for the design of hybrid hydrogels for different tissue engineering applications. In a study, Raj et al. conjugated poly(ethylene glycol) diacrylate (PEGDA) a synthetic polymer with porcine cholecyst derived ECM (C-ECM) (1% wt/vol) at various concentrations (0.2 - 2% wt/vol) and prepared a biosynthetic hydrogel with enhanced physico-mechanical properties for application in skeletal muscle tissue engineering. N-hydroxy succinimide was used for C-ECM functionalization with acrylate groups and it was then conjugated with PEGDA in presence of ammonium persulfate and ascorbic acid through free radical polymerization process. The hydrogel formulation containing PEGDA (0.2 and 0.5% wt/vol) were found suitable for proliferation and growth of skeletal myoblasts and were non-toxic. Their study showed a process for modulation of ECM hydrogels properties via conjugation with bioinert polymers for application in skeletal muscle tissue engineering (Raj et al., 2020).
Samanipour et al. developed both covalently and physically conjugated nanocage-laden hydrogels between the gelatin methacryloyl (GelMA) hydrogel matrix and surface of the nanocage. They used Ferritin and its empty-core equivalent apoferritin as nanocages that easily incorporated into a GelMA hydrogel via physical bonding (Figure 5). Apoferritin and Ferritin were modified chemically to present the methacryloyl groups for the fabrication of covalently conjugated nanocage-laden GelMA hydrogels. The covalently conjugated FerMA- and ApoMA-GelMA hydrogels showed better ability for tunable mechanical properties as compared to those systems synthesized via direct ferritin and apoferritin dispersion in GelMA hydrogels with physical bonding. Moreover, a cumulative release test of small molecules on fluorescein isothiocyanate (FITC) encapsulated apoferritin and ApoMA incorporated GelMA hydrogels by pH stimulus was performed. The nanocage incorporated hydrogels showed excellent potential for tissue engineering and drug delivery (Samanipour et al., 2019).
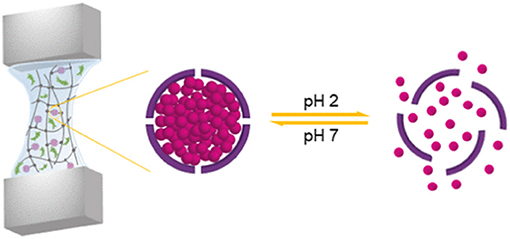
Figure 5. Ferritin nanocage conjugated hybrid hydrogel for tissue engineering and drug delivery applications. reproduced with permission from Samanipour et al. (2019). Copyright (2019) American Chemical Society.
Chen et al. prepared poly(e-caprolactone) (PCL)- meniscus extracellular matrix (MECM) PCL-MECM-Based Hydrogel Hybrid for Meniscal Fibrochondrocytes loading and used for whole Meniscus regeneration in a Rabbit Meniscectomy Model. They first investigated five concentration (0, 0.5, 1, 2, and 4%) of MECM-based hydrogel for the matrix-forming phenotype of meniscal fibrochondrocytes (MFCs) and promoting cell proliferation and found that 2% strongly enhanced chondrogenic marker mRNA expression and cell proliferation. In addition, the 2% system showed highest glycosaminoglycan (GAG) and collagen production on 14th day. They, finally constructed a hybrid scaffold via 3D printing a wedge-shaped PCL scaffold backbone, followed by injection with the optimized MECM-based hydrogel (2%) that served as cell delivery system. The hybrid PCL-hydrogel scaffold yielded near to native meniscus biomechanical properties. The PCL-hydrogel, PCL scaffold, and MFCs-loaded hybrid scaffold (PCL-hydrogel-MFCs) were finally implanted in the knee joints of rabbits that underwent total medial meniscectomy (Figure 6). After six months, the PCL-hydrogel-MFCs group animals showed extremely better gross appearance and cartilage protection than the other two groups. Thus, the prepared MFCs seeded PCLMECM-based hydrogel hybrid could successfully be used for whole meniscus regeneration, and the cell loaded PCL-MECM-based hydrogel hybrid could be used as a promising technique for regeneration of meniscus in future (Chen et al., 2019).
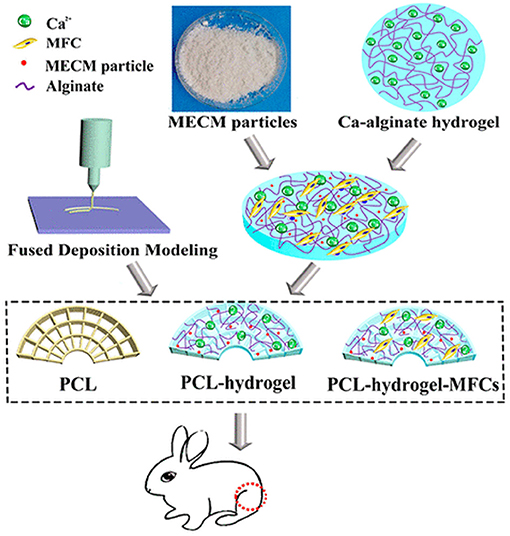
Figure 6. PCL-MECM-Based hydrogel hybrid scaffolds and meniscal fibrochondrocytes promote whole meniscus regeneration in a rabbit meniscectomy model. Reproduced with permission from Chen et al. (2019). Copyright (2019) American Chemical Society.
Zhang et. al has suggested and designed recently a potent hybrid hydrogel method i.e., “peptide-/drug-directed self-assembly” as shown in Figure 7. The hybrid hydrogel system was synthesized using PEG-based Fmoc-FF peptide hybrid polyurethane, in which curcumin was encapsulated via self-assembly with Fmoc-FF peptide through π-π stacking. Curcumin loading efficiency was improved to 3.3 wt% with sustained release profile from the matrix. Moreover, the mechanical properties of the hydrogel were improved with curcumin loading and became nearly similar to that of the natural soft tissues. In addition, the hybrid hydrogel system was injectable and possessed self-healing potential due to reversible and noncovalent Fmoc-FF peptide/curcumin co-assembly. The hydrogels improved cutaneous wound healing in full-thickness skin defected model in-vivo. This new peptide-/drug-directed self-assembly of hybrid hydrogel could potentially be used as a promising system for biomedical application and in tissue engineering (Zhang et al., 2019).
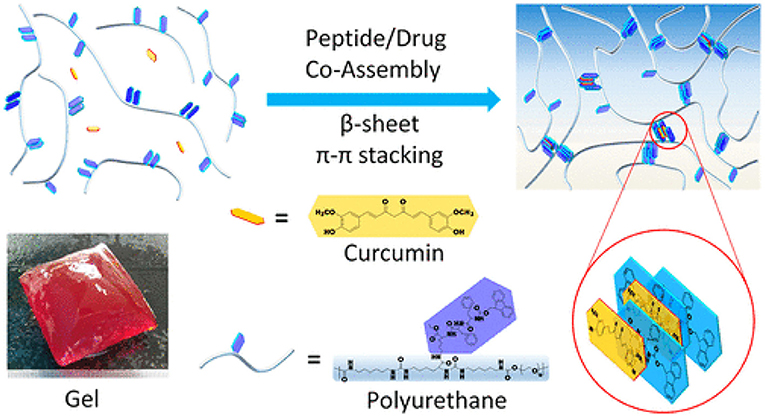
Figure 7. Peptide-/Drug-Directed self-assembly of hybrid polyurethane hydrogels for wound healing. Reproduced with permission from (Zhang et al., 2019). Copyright (2019) American Chemical Society.
The application of hybrid hydrogel systems with biomedical applications is summarized in Table 1 whereas its status in preclinical and clinical phase in Table 2 below.
Conclusions and Future Perspectives
Hydrogels containing nano/microstructures allow the design and development of hybrid hydrogels having multiple functionalities for diverse biomedical applications. The inclusion of particles and domains lead to stimuli-responsive material behavior, targeted drug therapy, tuned cellular response, and improved physical and mechanical properties. Hybrid hydrogels are currently used extensively for targeted cancer chemotherapy as well as tissue engineering applications. However, hybrid hydrogels based pharmaceutical preparations have yet entered the clinical phase. As the research regarding hybrid hydrogels is still at infancy, the development of new systems is going to be inspired by biological motifs and structures that closely resemble the environment, for which they are intended. New research for the facile synthesis of hybrid hydrogels via one-step easy methods will be influential to the field as new biomaterials with tunable properties can be prepared for the targeted and desired applications. As the research and innovations continue for preparation of such hybrid hydrogels, advances in biomedical, chemical, and materials engineering will follow.
Author Contributions
M-HC, X-YC, and L-QF wrote and collected the data. L-QF and W-LD arranged the data and designed the Manuscript. XY, X-ZM, and P-YH revised the manuscript, design, and supervised the whole study. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by the Zhejiang Provincial Science and Technology Projects (Grant No. LGF19H160024 to M-HC and LGF20H180007 to L-QF), National Natural Science Foundation of China (Grant No. 81672430 to X-ZM), Zhejiang Provincial Medical and Healthy Science and Technology Projects (Grant No. 2019KY248 to P-YH).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
Alles, N., Soysa, N. S., Hussain, M. A., Tomomatsu, N., Saito, H., Baron, R., et al. (2009). Polysaccharide nanogel delivery of a TNF-α and RANKL antagonist peptide allows systemic prevention of bone loss. Eur. J. Pharm. Sci. 37, 83–88. doi: 10.1016/j.ejps.2009.01.002
Amabilino, D. B., Smith, D. K., and Steed, J. W. (2017). Supramolecular materials. Chem. Soc. Rev. 46, 2404–2420. doi: 10.1039/C7CS00163K
Amini, A. A., and Nair, L. S. (2012). Injectable hydrogels for bone and cartilage repair. Biomed. Mater. 7:024105. doi: 10.1088/1748-6041/7/2/024105
Amiryaghoubi, N., Pesyan, N. N., Fathi, M., and Omidi, Y. (2020). Injectable thermosensitive hybrid hydrogel containing graphene oxide and chitosan as dental pulp stem cells scaffold for bone tissue engineering. Int. J. Biol. Macromol. 162, 1338–1357. doi: 10.1016/j.ijbiomac.2020.06.138
Attalla, R., Ling, C. S., and Selvaganapathy, P. R. (2018). Silicon carbide nanoparticles as an effective bioadhesive to bond collagen containing composite gel layers for tissue engineering applications. J. Adv. Healthc. Mater. 7:1701385. doi: 10.1002/adhm.201701385
Augst, A. D., Kong, H. J., and Mooney, D. J. (2006). Alginate hydrogels as biomaterials. Macromol. Biosci. 6, 623–633. doi: 10.1002/mabi.200600069
Babu, S. S., Praveen, V. K., and Ajayaghosh, A. (2014). Functional π-gelators and their applications. Chem. Rev. 114, 1973–2129. doi: 10.1021/cr400195e
Baker, B. M., and Chen, C. S. (2012). Deconstructing the third dimension–how 3D culture microenvironments alter cellular cues. J. Cell Sci. 125, 3015–3024. doi: 10.1242/jcs.079509
Baldwin, A. D., and Kiick, K. L. (2011). Tunable degradation of maleimide–thiol adducts in reducing environments. Bioconjug. Chem. 22, 1946–1953. doi: 10.1021/bc200148v
Bangham, A. D., and Horne, R. (1964). Negative staining of phospholipids and their structural modification by surface-active agents as observed in the electron microscope. J. Mol. Biol. 8, 660–668. doi: 10.1016/S0022-2836(64)80115-7
Beamish, J. A., Zhu, J., Kottke-Marchant, K., and Marchant, R. E. (2010). The effects of monoacrylated poly (ethylene glycol) on the properties of poly (ethylene glycol) diacrylate hydrogels used for tissue engineering. J. Biomed. Mater. Res. Part A 92, 441–450. doi: 10.1002/jbm.a.32353
Bencherif, S. A., Siegwart, D. J., Srinivasan, A., Horkay, F., Hollinger, J. O., Washburn, N. R., et al. (2009). Nanostructured hybrid hydrogels prepared by a combination of atom transfer radical polymerization and free radical polymerization. Biomater. Sci. 30, 5270–5278. doi: 10.1016/j.biomaterials.2009.06.011
Bhattarai, N., Ramay, H. R., Gunn, J., Matsen, F. A., and Zhang, M. (2005). PEG-grafted chitosan as an injectable thermosensitive hydrogel for sustained protein release. J. Control. Release 103, 609–624. doi: 10.1016/j.jconrel.2004.12.019
Bjørge, I. M., Costa, A. M., Silva, A. S., Vidal, J. P., Nóbrega, J. M., and Mano, J. F. (2018). Tuneable spheroidal hydrogel particles for cell and drug encapsulation. J. Soft matter. 14, 5622–5627. doi: 10.1039/C8SM00921J
Boontheekul, T., Kong, H.-J., and Mooney, D. J. (2005). Controlling alginate gel degradation utilizing partial oxidation and bimodal molecular weight distribution. Biomaterials 26, 2455–2465. doi: 10.1016/j.biomaterials.2004.06.044
Brannon-Peppas, L., and Peppas, N. A. (1991). Equilibrium swelling behavior of pH-sensitive hydrogels. Chem. Eng. Sci. 46, 715–722. doi: 10.1016/0009-2509(91)80177-Z
Bray, F. (2018). Ferlay j, Soerjomataram I, Siegel RL, Torre LA and jemal A: Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 68, 394–424. doi: 10.3322/caac.21492
Budama-Kilinc, Y., Cakir-Koc, R., Aslan, B., Özkan, B., Mutlu, H., and Üstün, E. (2018). “Hydrogels in regenerative medicine,” in Biomaterials in Regenerative Medicine, eds L. A. Dobrzanski (London: IntechOpen Limited), 277–301. doi: 10.5772/intechopen.70409
Burczak, K., Fujisato, T., Hatada, M., and Ikada, Y. (1994). Protein permeation through poly (vinyl alcohol) hydrogel membranes. Biomaterials 15, 231–238. doi: 10.1016/0142-9612(94)90072-8
Capanema, N. S., Carvalho, I. C., Mansur, A. A., Carvalho, S. M., Lage, A. P., and Mansur, H. S. (2019). Hybrid hydrogel composed of carboxymethylcellulose–silver nanoparticles–doxorubicin for anticancer and antibacterial therapies against melanoma skin cancer cells. ACS Appl. Nano Mater. 2, 7393–7408. doi: 10.1021/acsanm.9b01924
Chen, M., Feng, Z., Guo, W., Yang, D., Gao, S., Li, Y., et al. (2019). PCL-MECM-based hydrogel hybrid scaffolds and meniscal fibrochondrocytes promote whole meniscus regeneration in a rabbit meniscectomy model. ACS Appl. Mater. Interfaces 11, 41626–41639. doi: 10.1021/acsami.9b13611
Chen, M. S., Zhang, Y., and Zhang, L. (2017). Fabrication and characterization of a 3D bioprinted nanoparticle-hydrogel hybrid device for biomimetic detoxification. Nanoscale 9, 14506–14511. doi: 10.1039/C7NR05322C
Chivers, P. R., and Smith, D. K. (2017). Spatially-resolved soft materials for controlled release–hybrid hydrogels combining a robust photo-activated polymer gel with an interactive supramolecular gel. Chem. Sci. 8, 7218–7227. doi: 10.1039/C7SC02210G
Cho, S. H., Oh, S. H., and Lee, J. H. (2005). Fabrication and characterization of porous alginate/polyvinyl alcohol hybrid scaffolds for 3D cell culture. J. Biomater. Sci. 16, 933–947. doi: 10.1163/1568562054414658
Crescenzi, V., Cornelio, L., Di Meo, C., Nardecchia, S., and Lamanna, R. (2007). Novel hydrogels via click chemistry: synthesis and potential biomedical applications. Biomacromolecules 8, 1844–1850. doi: 10.1021/bm0700800
Czarnecki, S., Rossow, T., and Seiffert, S. (2016). Hybrid polymer-network hydrogels with tunable mechanical response. Polymers 8:82. doi: 10.3390/polym8030082
Davis, M. E., Chen, Z., and Shin, D. M. (2010). Nanoparticle therapeutics: an emerging treatment modality for cancer. Nanosci. Technol. 239–50. doi: 10.1142/9789814287005_0025
DeForest, C. A., and Anseth, K. S. (2011). Cytocompatible click-based hydrogels with dynamically tunable properties through orthogonal photoconjugation and photocleavage reactions. Nat. Chem. 3, 925–931. doi: 10.1038/nchem.1174
Deshmukh, M., Singh, Y., Gunaseelan, S., Gao, D., Stein, S., and Sinko, P. J. (2010). Biodegradable poly (ethylene glycol) hydrogels based on a self-elimination degradation mechanism. Biomaterials 31, 6675–6684. doi: 10.1016/j.biomaterials.2010.05.021
Ding, C., Zhao, L., Liu, F., Cheng, J., Gu, J., Dan, S., et al. (2010). Dually responsive injectable hydrogel prepared by in situ cross-linking of glycol chitosan and benzaldehyde-capped PEO-PPO-PEO. Biomacromolecules 11, 1043–1051. doi: 10.1021/bm1000179
Dorwal, D. (2012). Nanogels as novel and versatile pharmaceuticals. Int. J. Pharm. Pharm. Sci. 4, 67–74.
Duan, B., Xu, C., Das, S., Chen, J. M., and Butcher, J. T. (2019). Spatial regulation of valve interstitial cell phenotypes within three-dimensional micropatterned hydrogels. ACS Biomater. Sci. 5, 1416–1425. doi: 10.1021/acsbiomaterials.8b01280
Durmaz, S., and Okay, O. (2000). Acrylamide/2-acrylamido-2-methylpropane sulfonic acid sodium salt-based hydrogels: synthesis and characterization. Polymers 41, 3693–3704. doi: 10.1016/S0032-3861(99)00558-3
Ebara, M., Kotsuchibashi, Y., Narain, R., Idota, N., Kim, Y.-J., Hoffman, J. M., et al. (2014). Smart Biomaterials. Tsukuba: Springer.
Ekici, S., and Saraydin, D. (2004). Synthesis, characterization and evaluation of IPN hydrogels for antibiotic release. Drug Deliv. 11, 381–388. doi: 10.1080/10717540490884804
Elizalde-Peña, E., Zarate-Triviño, D., Nuño-Donlucas, S., Medina-Torres, L., Gough, J., Sanchez, I., et al. (2013). Synthesis and characterization of a hybrid (chitosan-g-glycidyl methacrylate)–xanthan hydrogel. J. Biomater. Sci. 24, 1426–1442. doi: 10.1080/09205063.2013.763526
Eslami, P., Rossi, F., and Fedeli, S. (2019). Hybrid nanogels: stealth and biocompatible structures for drug delivery applications. Pharmaceutics 11:71. doi: 10.3390/pharmaceutics11020071
Fan, M., Ma, Y., Zhang, Z., Mao, J., Tan, H., Hu, X., et al. (2015). Biodegradable hyaluronic acid hydrogels to control release of dexamethasone through aqueous Diels–Alder chemistry for adipose tissue engineering. Mater. Sci. 56, 311–317. doi: 10.1016/j.msec.2015.04.004
Fan, Y., Deng, C., Cheng, R., Meng, F., and Zhong, Z. (2013). In situ forming hydrogels via catalyst-free and bioorthogonal “tetrazole–alkene” photo-click chemistry. Biomacromolecules 14, 2814–2821. doi: 10.1021/bm400637s
Fang, Y., Tan, J., Lim, S., and Soh, S. (2018). Rupturing cancer cells by the expansion of functionalized stimuli-responsive hydrogels. NPG Asia Mater. 10:e465. doi: 10.1038/am.2017.232
Foster, L. J. R. (2010). PEGylation and bioPEGylation of polyhydroxyalkanoates: synthesis, characterisation and applications. InTech 2010, 243–256. doi: 10.5772/10265
Gangrade, A., and Mandal, B. B. (2019). Injectable carbon nanotube impregnated silk based multifunctional hydrogel for localized targeted and on-demand anticancer drug delivery. ACS Biomater. Sci. Eng. 5, 2365–2381. doi: 10.1021/acsbiomaterials.9b00416
Garcia, C. G., and Kiick, K. L. (2019). Methods for producing microstructured hydrogels for targeted applications in biology. Acta Biomater. 84:34–48. doi: 10.1016/j.actbio.2018.11.028
Gil, E. S., and Hudson, S. M. (2004). Stimuli-reponsive polymers and their bioconjugates. Prog. Polym. Sci. 29, 1173–1222. doi: 10.1016/j.progpolymsci.2004.08.003
Grover, G. N., Rao, N., and Christman, K. L. (2013). Myocardial matrix–polyethylene glycol hybrid hydrogels for tissue engineering. Nanotechnology 25:014011. doi: 10.1088/0957-4484/25/1/014011
Gulrez, S. K., Al-Assaf, S., and Phillips, G. O. (2011). “Hydrogels: methods of preparation, characterisation and applications,” in Progress in Molecular Environmental Bioengineering-From Analysis Modeling to Technology Applications. London: IntechOpen Limited, 117–1150.
Hao, Y., Liu, Y., Wu, Y., Tao, N., Lou, D., Li, J., et al. (2020). robust hybrid nanozyme@ hydrogel platform as a biomimetic cascade bioreactor for combination antitumor therapy. Biomater. Sci. 8, 1830–1839. doi: 10.1039/C9BM01837A
Hasegawa, U., Sawada, S.-,i, Shimizu, T., Kishida, T., Otsuji, E., Mazda, O., et al. (2009). Raspberry-like assembly of cross-linked nanogels for protein delivery. J. Control. Release 140, 312–317. doi: 10.1016/j.jconrel.2009.06.025
Hashimoto, Y., Mukai, S.-,a, Sawada, S.-,i, Sasaki, Y., and Akiyoshi, K. (2015). Nanogel tectonic porous gel loading biologics, nanocarriers, and cells for advanced scaffold. Biomaterials 37, 107–115. doi: 10.1016/j.biomaterials.2014.10.045
Hejčl, A., Šed, ý J., Kapcalová, M, Toro, D. A., Amemori, T., Lesn,ý, P., et al. (2010). HPMA-RGD hydrogels seeded with mesenchymal stem cells improve functional outcome in chronic spinal cord injury. Stem Cells Dev. 19, 1535–1546. doi: 10.1089/scd.2009.0378
Higuchi, A., Aoki, N., Yamamoto, T., Miyazaki, T., Fukushima, H., Tak, T. M., et al. (2006). Temperature-induced cell detachment on immobilized pluronic surface. J. Biomed. Mater. Res. Part A 79, 380–392. doi: 10.1002/jbm.a.30773
Hirokawa, Y., and Tanaka, T, (eds). (1984). “Volume phase transition in a non-ionic gel,” in AIP Conference Proceedings: American Institute of Physics. (Maryland).
Hoare, T. R., and Kohane, D. S. (2008). Hydrogels in drug delivery: progress and challenges. Polymers 49, 1993–2007. doi: 10.1016/j.polymer.2008.01.027
Hong, W., Zhao, X., Zhou, J., and Suo, Z. (2008). A theory of coupled diffusion and large deformation in polymeric gels. J. Mech. 56, 1779–1793. doi: 10.1016/j.jmps.2007.11.010
Hong, Y., Gong, Y., Gao, C., and Shen, J. (2008). Collagen-coated polylactide microcarriers/chitosan hydrogel composite: injectable scaffold for cartilage regeneration. J. Biomed. Mater. Res. Part A 85, 628–637. doi: 10.1002/jbm.a.31603
Huebsch, N., Kearney, C. J., Zhao, X., Kim, J., Cezar, C. A., Suo, Z., et al. (2014). Ultrasound-triggered disruption and self-healing of reversibly cross-linked hydrogels for drug delivery and enhanced chemotherapy. Proc. Natl. Acad. Sci. U.S.A. 111, 9762–9767. doi: 10.1073/pnas.1405469111
Javanbakht, S., Hemmati, A., Namazi, H., and Heydari, A. (2020). Carboxymethylcellulose-coated 5-fluorouracil@ MOF-5 nano-hybrid as a bio-nanocomposite carrier for the anticancer oral delivery. Int. J. Biol. Macromol. 155:876–882. doi: 10.1016/j.ijbiomac.2019.12.007
Jeon, S.-J., Hauser, A. W., and Hayward, R. C. (2017). Shape-morphing materials from stimuli-responsive hydrogel hybrids. Acc. Chem. Res. 50, 161–169. doi: 10.1021/acs.accounts.6b00570
Jia, X., and Kiick, K. L. (2009). Hybrid multicomponent hydrogels for tissue engineering. Macromol. Biosci. 9, 140–156. doi: 10.1002/mabi.200800284
Jiang, H., Qin, S., Dong, H., Lei, Q., Su, X., Zhuo, R., et al. (2015). An injectable and fast-degradable poly (ethylene glycol) hydrogel fabricated via bioorthogonal strain-promoted azide–alkyne cycloaddition click chemistry. Soft Matter. 11, 6029–6036. doi: 10.1039/C5SM00508F
Jiang, Z., Hao, J., You, Y., Liu, Y., Wang, Z., and Deng, X. (2008). Biodegradable and thermoreversible hydrogels of poly (ethylene glycol)-poly (ε-caprolactone-co-glycolide)-poly (ethylene glycol) aqueous solutions. J. Biomed. Mater. Res. Part A 87, 45–51. doi: 10.1002/jbm.a.31699
Jin, R. (2012). In-situ forming biomimetic hydrogels for tissue regeneration. Biomedicine 2, 35–58. doi: 10.5772/38852
Jin, R., Teixeira, L. M., Krouwels, A., Dijkstra, P. J., Van Blitterswijk, C., Karperien, M., et al. (2010). Synthesis and characterization of hyaluronic acid–poly (ethylene glycol) hydrogels via michael addition: an injectable biomaterial for cartilage repair. Acta Biomater. 6, 1968–1977. doi: 10.1016/j.actbio.2009.12.024
Jin, R., Yang, X., Zhao, D., Hou, X., Li, C., Song, X., et al. (2019). An injectable hybrid hydrogel based on a genetically engineered polypeptide for second near-infrared fluorescence/photoacoustic imaging-monitored sustained chemo-photothermal therapy. Nanoscale 11, 16080–16091. doi: 10.1039/C9NR04630E
Jonker, A. M., Löwik, D. W., and van Hest, J. C. (2012). Peptide-and protein-based hydrogels. Chemistry of Materials. 24, 759–773. doi: 10.1021/cm202640w
Joseph, C. A., McCarthy, C. W., Tyo, A. G., Hubbard, K. R., Fisher, H. C., Altscheffel, J. A., et al. (2018). Development of an injectable nitric oxide releasing poly (ethylene) glycol-fibrin adhesive hydrogel. ACS Biomater. Sci. 5, 959–969. doi: 10.1021/acsbiomaterials.8b01331
Kabanov, A. V., and Vinogradov, S. V. (2009). Nanogels as pharmaceutical carriers: finite networks of infinite capabilities. Angewandte Chemie. 48, 5418–5429. doi: 10.1002/anie.200900441
Kageyama, S., Kitano, S., Hirayama, M., Nagata, Y., Imai, H., Shiraishi, T., et al. (2008). Humoral immune responses in patients vaccinated with 1–146 HER2 protein complexed with cholesteryl pullulan nanogel. Cancer Sci. 99, 601–607. doi: 10.1111/j.1349-7006.2007.00705.x
Karg, M. (2016). Functional materials design through hydrogel encapsulation of inorganic nanoparticles: recent developments and challenges. Macromol. Chem. Phys. 217, 242–255. doi: 10.1002/macp.201500334
Kawabata, R., Wada, H., Isobe, M., Saika, T., Sato, S., Uenaka, A., et al. (2007). Antibody response against NY-ESO-1 in CHP-NY-ESO-1 vaccinated patients. Int. J. Cancer 120, 2178–2184. doi: 10.1002/ijc.22583
Khademhosseini, A., and Langer, R. (2007). Microengineered hydrogels for tissue engineering. Biomaterials 28, 5087–5092. doi: 10.1016/j.biomaterials.2007.07.021
Kim, J. O., Choi, J. Y., Park, J. K., Kim, J. H., Jin, S. G., Chang, S. W., et al. (2008b). Development of clindamycin-loaded wound dressing with polyvinyl alcohol and sodium alginate. Biol. Pharma. Bull. 31, 2277–2282. doi: 10.1248/bpb.31.2277
Kim, J. O., Park, J. K., Kim, J. H., Jin, S. G., Yong, C. S., Li, D. X., et al. (2008a). Development of polyvinyl alcohol–sodium alginate gel-matrix-based wound dressing system containing nitrofurazone. Int. J. Pharm. 359, 79–86. doi: 10.1016/j.ijpharm.2008.03.021
Kokufata, E., Zhang, Y.-Q., and Tanaka, T. (1991). Saccharide-sensitive phase transition of a lectin-loaded gel. Nature 351, 302–304. doi: 10.1038/351302a0
Kondo, S., Hiroi, T., Han, Y. S., Kim, T. H., Shibayama, M., Chung, U. I., et al. (2015). Reliable hydrogel with mechanical “fuse link” in an aqueous environment. Adv. Mater. 27, 7407–7411. doi: 10.1002/adma.201503130
Kong, H. J., Kaigler, D., Kim, K., and Mooney, D. J. (2004). Controlling rigidity and degradation of alginate hydrogels via molecular weight distribution. Biomacromolecules 5, 1720–1727. doi: 10.1021/bm049879r
Kopeček, J. (2007). Hydrogel biomaterials: a smart future? Biomaterials 28, 5185–5192. doi: 10.1016/j.biomaterials.2007.07.044
Kopeček, J., and Yang, J. (2012). Smart self-assembled hybrid hydrogel biomaterials. Angew. Chem. 51, 7396–7417. doi: 10.1002/anie.201201040
Koshy, S. T., Desai, R. M., Joly, P., Li, J., Bagrodia, R. K., Lewin, S. A., et al. (2016). Click-crosslinked injectable gelatin hydrogels. Adv. Healthc. Mater. 5, 541–547. doi: 10.1002/adhm.201500757
Kumar, A., Lee, Y., Kim, D., Rao, K. M., Kim, J., Park, S., et al. (2017). Effect of crosslinking functionality on microstructure, mechanical properties, and in vitro cytocompatibility of cellulose nanocrystals reinforced poly (vinyl alcohol)/sodium alginate hybrid scaffolds. Int. J. Biol. Macromol. 95, 962–973. doi: 10.1016/j.ijbiomac.2016.10.085
Kumar, A., Negi, Y. S., Choudhary, V., and Bhardwaj, N. K. (2014). Characterization of cellulose nanocrystals produced by acid-hydrolysis from sugarcane bagasse as agro-waste. J. Mater. Phys. Chem. 2, 1–8. doi: 10.12691/jmpc-2-1-1
Lau, H. K., and Kiick, K. L. (2015). Opportunities for multicomponent hybrid hydrogels in biomedical applications. Biomacromolecules 16, 28–42. doi: 10.1021/bm501361c
Lau, H. K., Paul, A., Sidhu, I., Li, L., Sabanayagam, C. R., Parekh, S. H., et al. (2018). Microstructured elastomer-PEG hydrogels via kinetic capture of aqueous liquid–liquid phase separation. Adv. Sci. 5:1701010. doi: 10.1002/advs.201701010
Lee, H. J., Seo, Y., Kim, H. S., Lee, J. W., and Lee, K. Y. (2020). Regulation of the viscoelastic properties of hyaluronate–alginate hybrid hydrogel as an injectable for chondrocyte delivery. ACS Omega 5, 15567–15575. doi: 10.1021/acsomega.0c01763
Lee, K. Y., and Mooney, D. J. (2001). Hydrogels for tissue engineering. Chem. Rev. 101, 1869–1880. doi: 10.1021/cr000108x
Li, J., and Mooney, D. J. (2016). Designing hydrogels for controlled drug delivery. Nat. Rev. Mater. 1, 1–17. doi: 10.1038/natrevmats.2016.71
Li, Q., Williams, C. G., Sun, D. D., Wang, J., Leong, K., and Elisseeff, J. H. (2004). Photocrosslinkable polysaccharides based on chondroitin sulfate. J. Biomed. Mater. Res. Part A 68, 28–33. doi: 10.1002/jbm.a.20007
Lian, X., Zhang, Y., Wang, J., and Yan, B. (2020). Antineoplastic mitoxantrone monitor: a sandwiched mixed matrix membrane (MMM) based on a luminescent MOF–hydrogel hybrid. Inorg. Chem. 59, 10304–10310. doi: 10.1021/acs.inorgchem.0c01451
Lim, S., Jung, G. A., Muckom, R. J., Glover, D. J., and Clark, D. S. (2019). Engineering bioorthogonal protein–polymer hybrid hydrogel as a functional protein immobilization platform. Chem. Commun. 55, 806–809. doi: 10.1039/C8CC08720B
Lin, P., Ma, S., Wang, X., and Zhou, F. (2015). Molecularly engineered dual-crosslinked hydrogel with ultrahigh mechanical strength, toughness, and good self-recovery. Adv. Mater. 27, 2054–2059. doi: 10.1002/adma.201405022
Lohani, A., Singh, G., Bhattacharya, S. S., and Verma, A. (2014). Interpenetrating polymer networks as innovative drug delivery systems. Drug Deliv. 2014:583612. doi: 10.1155/2014/583612
Maeda, H. (2010). Tumor-selective delivery of macromolecular drugs via the EPR effect: background and future prospects. Bioconjug. Chem. 21, 797–802. doi: 10.1021/bc100070g
Mahmoudian, M., and Ganji, F. (2017). Vancomycin-loaded HPMC microparticles embedded within injectable thermosensitive chitosan hydrogels. Progr. Biomater. 6, 49–56. doi: 10.1007/s40204-017-0066-x
Maisani, M., Pezzoli, D., Chassande, O., and Mantovani, D. (2017). Cellularizing hydrogel-based scaffolds to repair bone tissue: How to create a physiologically relevant micro-environment? J. Tissue Eng. 8:2041731417712073. doi: 10.1177/2041731417712073
Molina, M., Asadian-Birjand, M., Balach, J., Bergueiro, J., Miceli, E., and Calderón, M. (2015). Stimuli-responsive nanogel composites and their application in nanomedicine. Chem. Soc. Rev. 44, 6161–6186. doi: 10.1039/C5CS00199D
Mukherjee, S., Hill, M. R., and Sumerlin, B. S. (2015). Self-healing hydrogels containing reversible oxime crosslinks. Soft Matter. 11, 6152–6161. doi: 10.1039/C5SM00865D
Murdan, S. (2003). Electro-responsive drug delivery from hydrogels. J. Control. Release 92, 1–17. doi: 10.1016/S0168-3659(03)00303-1
Myung, D., Waters, D., Wiseman, M., Duhamel, P. E., Noolandi, J., Ta, C. N., et al. (2008). Progress in the development of interpenetrating polymer network hydrogels. Polym. Adv. Technol. 19, 647–657. doi: 10.1002/pat.1134
Narita, T., Mayumi, K., Ducouret, G., and Hébraud, P. (2013). Viscoelastic properties of poly (vinyl alcohol) hydrogels having permanent and transient cross-links studied by microrheology, classical rheometry, and dynamic light scattering. Macromolecules. 46, 4174–4183. doi: 10.1021/ma400600f
Nawroth, J. C., Scudder, L. L., Halvorson, R. T., Tresback, J., Ferrier, J. P. Jr, Sheehy, S. P., et al. (2018). Automated fabrication of photopatterned gelatin hydrogels for organ-on-chips applications. Biofabrication 10:025004. doi: 10.1088/1758-5090/aa96de
Obaidat, A. A., and Park, K. (1997). Characterization of protein release through glucose-sensitive hydrogel membranes. Biomaterials 18, 801–806. doi: 10.1016/S0142-9612(96)00198-6
Ohmine, I., and Tanaka, T. (1982). Salt effects on the phase transition of ionic gels. J. Chem. Phys. 77, 5725–5729. doi: 10.1063/1.443780
O'Shea, T. M., Aimetti, A. A., Kim, E., Yesilyurt, V., and Langer, R. (2015). Synthesis and characterization of a library of in-situ curing, nonswelling ethoxylated polyol thiol-ene hydrogels for tailorable macromolecule delivery. Adv. Mater. 27, 65–72. doi: 10.1002/adma.201403724
Ossipov, D. A., Brännvall, K., Forsberg-Nilsson, K., and Hilborn, J. (2007). Formation of the first injectable poly (vinyl alcohol) hydrogel by mixing of functional PVA precursors. J. Appl. Polym. Sci. 106, 60–70. doi: 10.1002/app.26455
Patel, D., Sharma, S., Screen, H. R., and Bryant, S. J. (2018). Effects of cell adhesion motif, fiber stiffness, and cyclic strain on tenocyte gene expression in a tendon mimetic fiber composite hydrogel. Biochem. Biophys. Res. Commun. 499, 642–647. doi: 10.1016/j.bbrc.2018.03.203
Qian, H., Qian, K., Cai, J., Yang, Y., Zhu, L., and Liu, B. (2019). Therapy for gastric cancer with peritoneal metastasis using injectable albumin hydrogel hybridized with paclitaxel-loaded red blood cell membrane nanoparticles. ACS Biomater. Sci. Eng. 5, 1100–1112. doi: 10.1021/acsbiomaterials.8b01557
Raj, R., Sobhan, P. K., Pratheesh, K. V., and Anilkumar, T. V. (2020). A cholecystic extracellular matrix-based hybrid hydrogel for skeletal muscle tissue engineering. J. Biomed. Mater. Res. Part A 108, 1922–1933. doi: 10.1002/jbm.a.36955
Raschip, I. E., Hitruc, G. E., Vasile, C., and Popescu, M.-C. (2013). Effect of the lignin type on the morphology and thermal properties of the xanthan/lignin hydrogels. Int. J. Biol. Macromol. 54, 230–237. doi: 10.1016/j.ijbiomac.2012.12.036
Raschip, I. E., Vasile, C., Ciolacu, D., and Cazacu, G. (2007). Semi-interpenetrating polymer networks containing polysaccharides. I Xanthan/Lignin networks. High Perform. Polym. 19, 603–620. doi: 10.1177/0954008307081202
Rehmann, M. S., Skeens, K. M., Kharkar, P. M., Ford, E. M., Maverakis, E., Lee, K. H., et al. (2017). Tuning and predicting mesh size and protein release from step growth hydrogels. Biomacromolecules 18, 3131–3142. doi: 10.1021/acs.biomac.7b00781
Ren, N., Sun, R., Xia, K., Zhang, Q., Li, W., Wang, F., et al. (2019). DNA-based hybrid hydrogels sustain water-insoluble ophthalmic therapeutic delivery against allergic conjunctivitis. ACS Appl. Mater. Interfaces 11, 26704–26710. doi: 10.1021/acsami.9b08652
Riehemann, K., Schneider, S. W., Luger, T. A., Godin, B., Ferrari, M., and Fuchs, H. (2009). Nanomedicine—challenge and perspectives. Angew. Chem. 48, 872–897. doi: 10.1002/anie.200802585
Rodriguez, L. M. D. L., Hemar, Y., Cornish, J., and Brimble, M. A. (2016). Structure–mechanical property correlations of hydrogel forming β-sheet peptides. Chem. Soc. Rev. 45, 4797–4824. doi: 10.1039/C5CS00941C
Rodríguez-Rodríguez, R., García-Carvajal, Z., Jiménez-Palomar, I., Jiménez-Avalos, J., and Espinosa-Andrews, H. (2019). Development of gelatin/chitosan/PVA hydrogels: Thermal stability, water state, viscoelasticity, and cytotoxicity assays. J. Appl. Polym. Sci. 136:47149. doi: 10.1002/app.47149
Sahiner, N., Godbey, W., McPherson, G. L., and John, V. T. (2006). Microgel, nanogel and hydrogel–hydrogel semi-IPN composites for biomedical applications: synthesis and characterization. Colloid Polymer Sci. 284, 1121–1129. doi: 10.1007/s00396-006-1489-4
Sakai, S., Hirose, K., Taguchi, K., Ogushi, Y., and Kawakami, K. (2009). An injectable, in situ enzymatically gellable, gelatin derivative for drug delivery and tissue engineering. Biomaterials 30, 3371–3377. doi: 10.1016/j.biomaterials.2009.03.030
Samanipour, R., Wang, T., Werb, M., Hassannezhad, H., Ledesma Rangel, J. M., Hoorfar, M., et al. (2019). Ferritin nanocage conjugated hybrid hydrogel for tissue engineering and drug delivery applications. ACS Biomater. Sci. Eng. 6, 277–287. doi: 10.1021/acsbiomaterials.9b01482
Sanson, N., and Rieger, J. (2010). Synthesis of nanogels/microgels by conventional and controlled radical crosslinking copolymerization. Polym. Chem. 1, 965–977. doi: 10.1039/c0py00010h
Schmedlen, R. H., Masters, K. S., and West, J. L. (2002). Photocrosslinkable polyvinyl alcohol hydrogels that can be modified with cell adhesion peptides for use in tissue engineering. Biomaterials. 23, 4325–4332. doi: 10.1016/S0142-9612(02)00177-1
Schneible, J. D., Shi, K., Young, A. T., Ramesh, S., He, N., Dowdey, C. E., et al. (2020). Modified gaphene oxide (GO) particles in peptide hydrogels: a hybrid system enabling scheduled delivery of synergistic combinations of chemotherapeutics. J. Mater. Chem. B 8, 3852–3868. doi: 10.1039/D0TB00064G
Sen Gupta, A. (2016). Role of particle size, shape, and stiffness in design of intravascular drug delivery systems: insights from computations, experiments, and nature. Nanomed. Nanobiotechnol. 8, 255–270. doi: 10.1002/wnan.1362
Senapati, S., Mahanta, A. K., Kumar, S., and Maiti, P. (2018). Controlled drug delivery vehicles for cancer treatment and their performance. Sig. Transduct. Target. Ther. 3, 1–19. doi: 10.1038/s41392-017-0004-3
Seo, S., Lee, C.-S., Jung, Y.-S., and Na, K. (2012). Thermo-sensitivity and triggered drug release of polysaccharide nanogels derived from pullulan-g-poly (L-lactide) copolymers. Carbohydr. Polym. 87, 1105–1111. doi: 10.1016/j.carbpol.2011.08.061
Shi, J., Votruba, A. R., Farokhzad, O. C., and Langer, R. (2010). Nanotechnology in drug delivery and tissue engineering: from discovery to applications. Nano Lett. 10, 3223–3230. doi: 10.1021/nl102184c
Shin, S. R., Jung, S. M., Zalabany, M., Kim, K., Zorlutuna, P., Kim, S. B., et al. (2013). Carbon-nanotube-embedded hydrogel sheets for engineering cardiac constructs and bioactuators. ACS Nano. 7, 2369–2380. doi: 10.1021/nn305559j
Shingel, K. I., Di Stabile, L., Marty, J. P., and Faure, M. P. (2006). Inflammatory inert poly (ethylene glycol)–protein wound dressing improves healing responses in partial-and full-thickness wounds. Int. Wound J. 3, 332–342. doi: 10.1111/j.1742-481X.2006.00262.x
Sierra-Martin, B., and Fernandez-Barbero, A. (2015). Multifunctional hybrid nanogels for theranostic applications. J. Soft Matter. 11, 8205–8216. doi: 10.1039/C5SM01789K
Sivakumaran, D., Maitland, D., and Hoare, T. (2011). Injectable microgel-hydrogel composites for prolonged small-molecule drug delivery. Biomacromolecules 12, 4112–4120. doi: 10.1021/bm201170h
Slaughter, B. V., Khurshid, S. S., Fisher, O. Z., Khademhosseini, A., and Peppas, N. A. (2009). Hydrogels in regenerative medicine. Adv. Mater. 21, 3307–3329. doi: 10.1002/adma.200802106
Sletten, E. M., and Bertozzi, C. R. (2009). Bioorthogonal chemistry: fishing for selectivity in a sea of functionality. Angew. Chem. 48, 6974–6998. doi: 10.1002/anie.200900942
Stone, S. A., Gosavi, P., Athauda, T. J., and Ozer, R. R. (2013). In situ citric acid crosslinking of alginate/polyvinyl alcohol electrospun nanofibers. Mater. Lett. 112:32–35. doi: 10.1016/j.matlet.2013.08.100
Sun, J.-Y., Zhao, X., Illeperuma, W. R., Chaudhuri, O., Oh, K. H., Mooney, D. J., et al. (2012). Highly stretchable and tough hydrogels. Nature 489, 133–136. doi: 10.1038/nature11409
Sung, J. H., Hwang, M.-R., Kim, J. O., Lee, J. H., Kim, Y. I., Kim, J. H., et al. (2010). Gel characterisation and in vivo evaluation of minocycline-loaded wound dressing with enhanced wound healing using polyvinyl alcohol and chitosan. Int. J. Pharm. 392, 232–240. doi: 10.1016/j.ijpharm.2010.03.024
Tarun, K., and Gobi, N. (2012). Calcium alginate/PVA blended nano fibre matrix for wound dressing. Indian J. Fibre Text. Res. 37.
Uenaka, A., Wada, H., Isobe, M., Saika, T., Tsuji, K., Sato, E., et al. (2007). T cell immunomonitoring and tumor responses in patients immunized with a complex of cholesterol-bearing hydrophobized pullulan (CHP) and NY-ESO-1 protein. Cancer Immun. Arch. 7:9.
Vasile, C., Pamfil, D., Stoleru, E., and Baican, M. (2020). New developments in medical applications of hybrid hydrogels containing natural polymers. Molecules 25:1539. doi: 10.3390/molecules25071539
Vikingsson, L., Claessens, B., Gómez-Tejedor, J. A., Ferrer, G. G., and Ribelles, J. G. (2015). Relationship between micro-porosity, water permeability and mechanical behavior in scaffolds for cartilage engineering. J. Mech. Behav. Biomed. Mater. 48, 60–69. doi: 10.1016/j.jmbbm.2015.03.021
Wagner, V., Dullaart, A., Bock, A.-K., and Zweck, A. (2006). The emerging nanomedicine landscape. Nat. Biotechnol. 24, 1211–1217. doi: 10.1038/nbt1006-1211
Wang, C., Kopecek, J., and Stewart, R. J. (2001). Hybrid hydrogels cross-linked by genetically engineered coiled-coil block proteins. Biomacromolecules 2, 912–920. doi: 10.1021/bm0155322
Wang, Y., Zhang, S., and Benoit, D. S. (2018). Degradable poly (ethylene glycol)(PEG)-based hydrogels for spatiotemporal control of siRNA/nanoparticle delivery. J. Control. Release 287, 58–66. doi: 10.1016/j.jconrel.2018.08.002
Wu, H.-Q., and Wang, C.-C. (2016). Biodegradable smart nanogels: a new platform for targeting drug delivery and biomedical diagnostics. Langmuir 32, 6211–6225. doi: 10.1021/acs.langmuir.6b00842
Wu, M., Hou, P., Dong, L., Cai, L., Chen, Z., Zhao, M., et al. (2019). Manganese dioxide nanosheets: from preparation to biomedical applications. Int. J. Nanomed. 14:4781. doi: 10.2147/IJN.S207666
Wu, Q., He, Z., Wang, X., Zhang, Q., Wei, Q., Ma, S., et al. (2019). Cascade enzymes within self-assembled hybrid nanogel mimicked neutrophil lysosomes for singlet oxygen elevated cancer therapy. Nat. Commun. 10:240. doi: 10.1038/s41467-018-08234-2
Wu, W., and Zhou, S. (2010). Hybrid micro-/nanogels for optical sensing and intracellular imaging. Nano Rev. 1:5730. doi: 10.3402/nano.v1i0.5730
Wychowaniec, J. K., Iliut, M., Zhou, M., Moffat, J., Elsawy, M. A., Pinheiro, W. A., et al. (2018). Designing peptide/graphene hybrid hydrogels through fine-tuning of molecular interactions. Biomacromolecules 19, 2731–2741. doi: 10.1021/acs.biomac.8b00333
Xavier, J. R., Thakur, T., Desai, P., Jaiswal, M. K., Sears, N., Cosgriff-Hernandez, E., et al. (2015). Bioactive nanoengineered hydrogels for bone tissue engineering: a growth-factor-free approach. ACS Nano 9, 3109–3118. doi: 10.1021/nn507488s
Xiao, C., and Zhou, G. (2003). Synthesis and properties of degradable poly (vinyl alcohol) hydrogel. Polym. Degradat. Stabil. 81, 297–301. doi: 10.1016/S0141-3910(03)00100-9
Xing, Y., Cheng, E., Yang, Y., Chen, P., Zhang, T., Sun, Y., et al. (2011). Self-assembled DNA hydrogels with designable thermal and enzymatic responsiveness. Adv. Mater. 23, 1117–1121. doi: 10.1002/adma.201003343
Yadav, H., Al Halabi, N., and Alsalloum, G. (2017). Nanogels as novel drug delivery systems-a review. J. Pharmacol. Pharma. Res. 1.
Yan, B., Boyer, J.-C., Habault, D., Branda, N. R., and Zhao, Y. (2012). Near infrared light triggered release of biomacromolecules from hydrogels loaded with upconversion nanoparticles. J. Am. Chem. Soc. 134, 16558–16561. doi: 10.1021/ja308876j
Yang, G., Lin, H., Rothrauff, B. B., Yu, S., and Tuan, R. S. (2016). Multilayered polycaprolactone/gelatin fiber-hydrogel composite for tendon tissue engineering. Acta Biomater. 35, 68–76. doi: 10.1016/j.actbio.2016.03.004
Yang, J., Xu, C., Wang, C., and Kopecek, J. (2006). Refolding hydrogels self-assembled from N-(2-hydroxypropyl) methacrylamide graft copolymers by antiparallel coiled-coil formation. Biomacromolecules7, 1187–1195. doi: 10.1021/bm051002k
Yang, J. M., Su, W. Y., and Yang, M. C. (2004). Evaluation of chitosan/PVA blended hydrogel membranes. J. Memb. Sci. 236, 39–51. doi: 10.1016/j.memsci.2004.02.005
Zhang, F., Hu, C., Kong, Q., Luo, R., and Wang, Y. (2019). Peptide-/drug-directed self-assembly of hybrid polyurethane hydrogels for wound healing. ACS Appl. Mater. Interfaces 11, 37147–37155. doi: 10.1021/acsami.9b13708
Zhang, L., Gu, F., Chan, J., Wang, A., Langer, R., and Farokhzad, O. (2008). Nanoparticles in medicine: therapeutic applications and developments. Clin. Pharmacol. Therap. 83, 761–769. doi: 10.1038/sj.clpt.6100400
Zhang, S., Bellinger, A. M., Glettig, D. L., Barman, R., Lee, Y.-A. L., Zhu, J., et al. (2015). A pH-responsive supramolecular polymer gel as an enteric elastomer for use in gastric devices. Nat. Mater. 14, 1065–1071. doi: 10.1038/nmat4355
Zhang, T., Yang, R., Yang, S., Guan, J., Zhang, D., Ma, Y., et al. (2018). Research progress of self-assembled nanogel and hybrid hydrogel systems based on pullulan derivatives. Drug Deliv. 25, 278–292. doi: 10.1080/10717544.2018.1425776
Zhao, L., Gwon, H.-J., Lim, Y.-M., Nho, Y.-C., and Kim, S. Y. (2014). Hyaluronic acid/chondroitin sulfate-based hydrogel prepared by gamma irradiation technique. Carbohydr. Polym. 102:598–605. doi: 10.1016/j.carbpol.2013.11.048
Zhu, W., Xiong, L., Wang, H., Zha, G., Du, H., Li, X., et al. (2015). Sustained drug release from an ultrathin hydrogel film. Polym. Chem. 6, 7097–7099. doi: 10.1039/C5PY01204J
Keywords: hybrid hydrogels, design and development, biomedical applications, cancer drugs, tissue engineering
Citation: Cai M-H, Chen X-Y, Fu L-Q, Du W-L, Yang X, Mou X-Z and Hu P-Y (2021) Design and Development of Hybrid Hydrogels for Biomedical Applications: Recent Trends in Anticancer Drug Delivery and Tissue Engineering. Front. Bioeng. Biotechnol. 9:630943. doi: 10.3389/fbioe.2021.630943
Received: 18 November 2020; Accepted: 11 January 2021;
Published: 17 February 2021.
Edited by:
Wei Li, University of Helsinki, FinlandReviewed by:
Jingjing Wu, Massachusetts Institute of Technology, United StatesPatcharakamon Nooeaid, Srinakharinwirot University, Thailand
Copyright © 2021 Cai, Chen, Fu, Du, Yang, Mou and Hu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiao-Zhou Mou, bW91eHpAemp1LmVkdS5jbg==; Pei-Yang Hu, aHB5OTRAc2luYS5jb20=
†These authors have contributed equally to this work
 Mao-Hua Cai1†
Mao-Hua Cai1† Xiao-Zhou Mou
Xiao-Zhou Mou