- 1Department of Biomedical Engineering, Carnegie Mellon University, Pittsburgh, PA, United States
- 2Department of Materials Science and Engineering, Carnegie Mellon University, Pittsburgh, PA, United States
Biophysical properties of the extracellular environment dynamically regulate cellular fates. In this review, we highlight silk, an indispensable polymeric biomaterial, owing to its unique mechanical properties, bioactive component sequestration, degradability, well-defined architectures, and biocompatibility that can regulate temporospatial biochemical and biophysical responses. We explore how the materiobiology of silks, both mulberry and non-mulberry based, affect cell behaviors including cell adhesion, cell proliferation, cell migration, and cell differentiation. Keeping in mind the novel biophysical properties of silk in film, fiber, or sponge forms, coupled with facile chemical decoration, and its ability to match functional requirements for specific tissues, we survey the influence of composition, mechanical properties, topography, and 3D geometry in unlocking the body’s inherent regenerative potential.
Introduction
To form and regenerate tissues, cells attain a staggering amount of molecular information from their microenvironment; where the extracellular matrix (ECM) is not only a “guiding” element for cells, but also highly responsive to cellular behavior (Place et al., 2009). The goal of tissue engineering is to provide cues that stimulate these extraordinary native processes to engineer lost or damaged tissue. Toward this goal, instructive and dynamic features in scaffolds (Li et al., 2017) can be used to drive the body’s intrinsic organizational potential and self-repair abilities.
Biomaterials are never truly inert, being at best biotolerable. The cell-substrate interface serves as more than just a boundary separating the host and material; instead, it introduces physical and chemical cues for cellular adhesion and the subsequent induction of tissue generation or rejection (Biggs et al., 2010). Chemical constituents have been the focus of biomaterial design for several years, but there is increasing recognition of the significance of other material features such as mechanical properties, topology, and 3D geometry in directing cellular behavior (Cukierman et al., 2001; Stevens and George, 2005; Curtis et al., 2006). The ECM’s stiffness can independently dictate differentiation into cells as functionally divergent as bone and nerve (Pelham and Wang, 1997; Engler et al., 2006). The topography and hydrophilicity of biomaterials can enable cellular adhesion even in the absence of cell adhesion peptides (Woo et al., 2003). Dynamic tuning of material properties including availability of cellular adhesion sequences (Kloxin et al., 2009), mechanical characteristics (Guvendiren and Burdick, 2012) and ECM degradability (Khetan et al., 2013) can induce changes in cellular behavior. Such emerging dynamic biomaterial chemistries can provide a “give and take” between cells and materials (Murphy et al., 2014). This concept has been termed “materiobiology” and was introduced by Li et al. (2017) to describe the influence of materials on biological functions at different cellular levels.
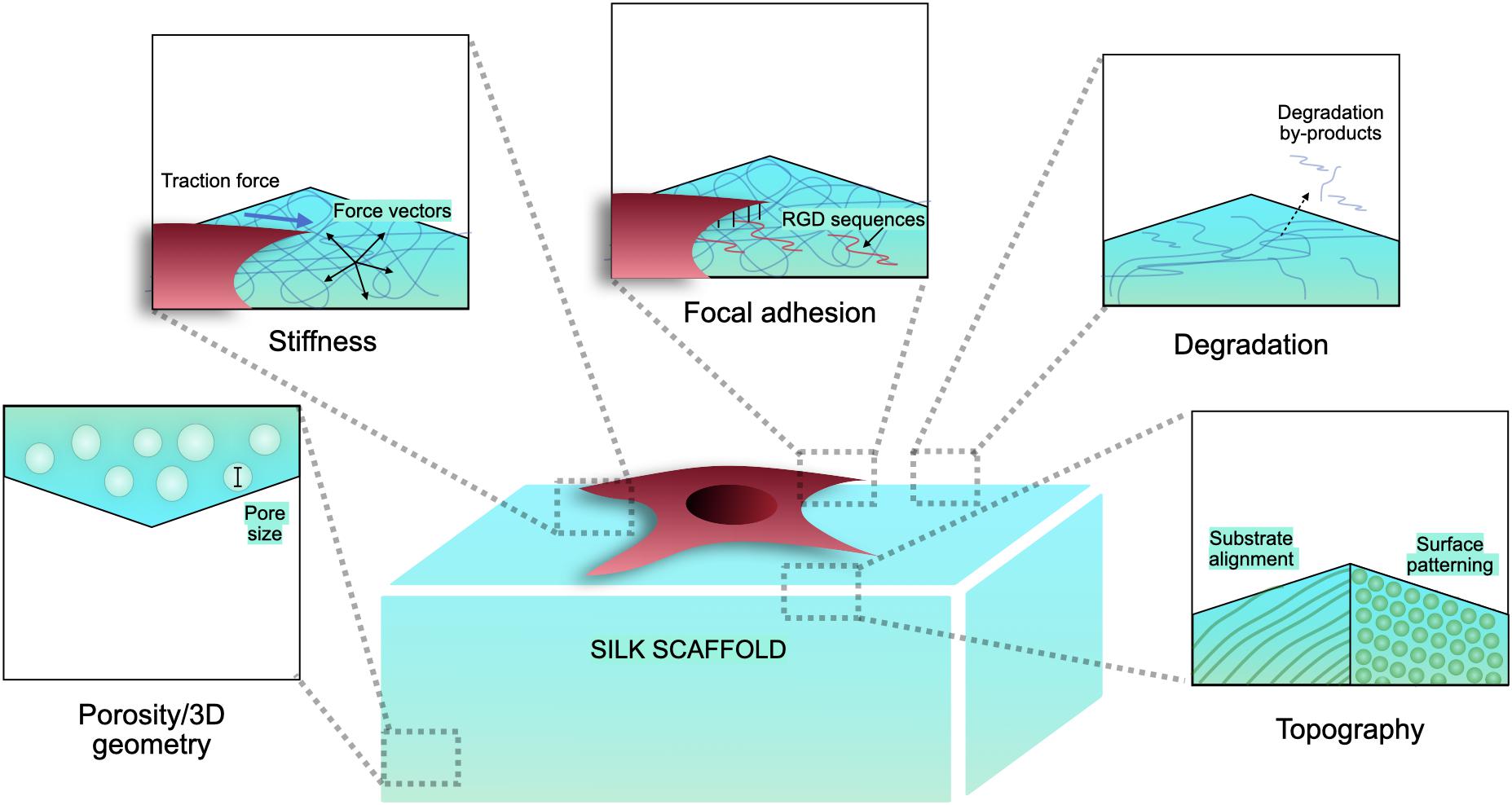
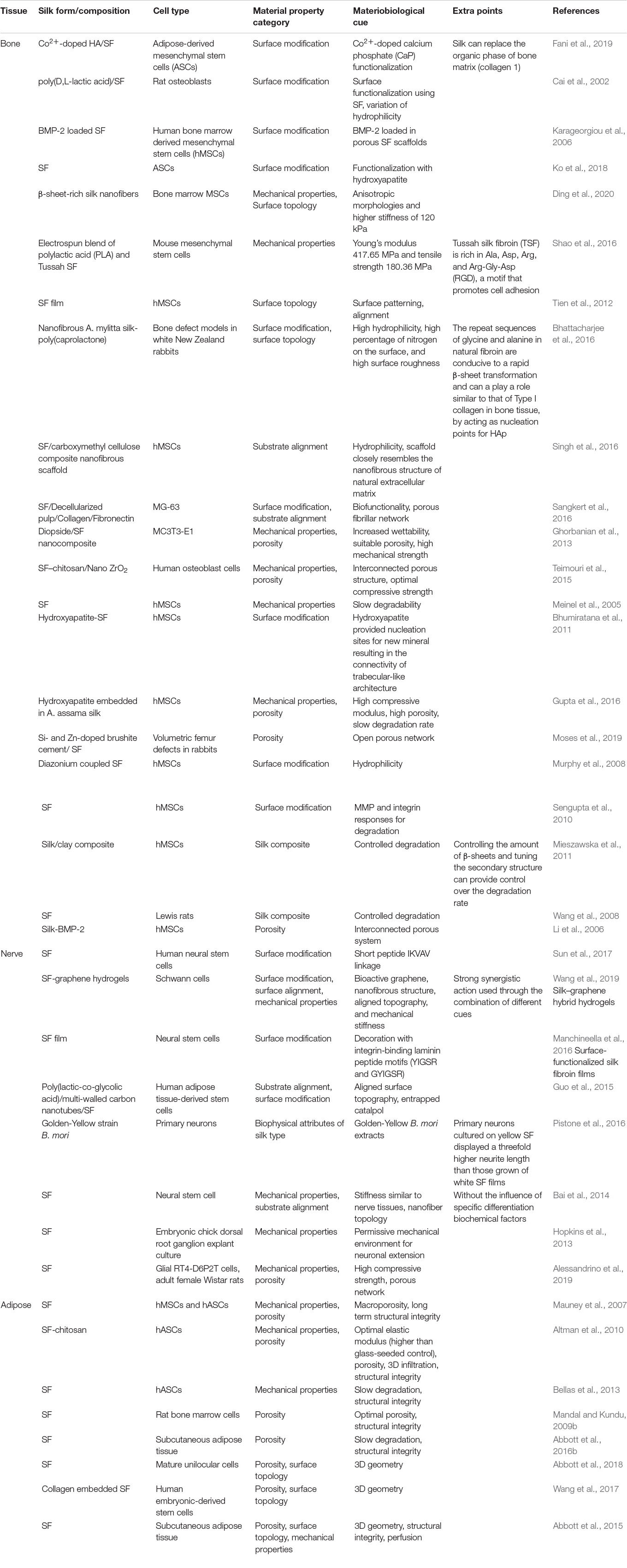
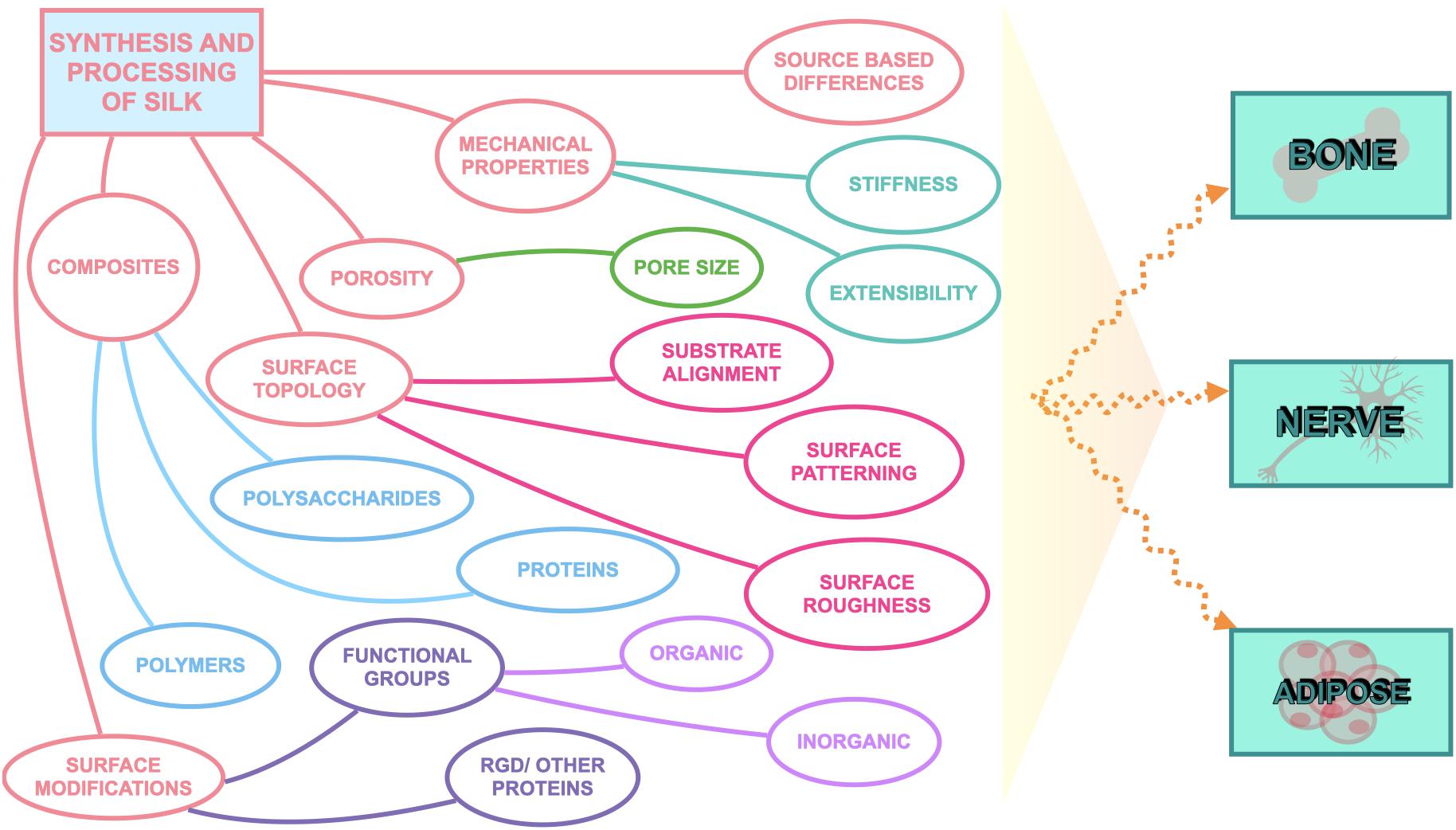
Silk has emerged as a natural biomaterial that can govern, and perhaps even trigger, specific stem cell differentiation based on its intrinsic toughness, mechanical strength, biocompatibility, molecular tunability, topography, geometry, chemical functionality, degradability, and degradation by-products (Figure 1; Altman et al., 2003; Karageorgiou et al., 2006; Pritchard et al., 2011). Silks are spun into fibrous polymers by certain lepidopteral larvae like silkworms, spiders, scorpions, and flies. Silk fibroin (SF), a fibrous protein derived from B. mori, will be the primary focus of this review given its extensive use in tissue regeneration (Kaplan et al., 1993; Altman et al., 2003). On the basis of feeding habitats, silkworm-based silk can be broadly classified as mulberry (B. mori from Bombycidae family) and non-mulberry (Saturniidae family) (Kaplan et al., 1993). While silks vary in structure, composition, and features based on their source, silks are characterized by highly repetitive primary sequences that contribute to homogeneity in their β-sheet secondary structure. In contrast with globular proteins, the β-sheet structure of silk displays superior mechanical properties that are highly tunable. For instance, silk scaffolds can withstand high compressive loads without failure for bone tissue engineering applications (Cunniff et al., 1994). Whereas for ligaments, the high tensile strength of silk biomaterials can be used to reinstate knee function (Altman et al., 2002). This review discusses the materiobiology of silk, highlighting its ECM-mimicking potential and application in stimulating tissue regeneration (Table 1) by influencing cellular adhesion, proliferation, migration, and differentiation. Specifically, materiobiology design considerations will be addressed for tailoring cellular fate: topology (alignment, patterning, roughness), surface modifications, composites, mechanical properties, and material source (Figure 2).
Tailoring Silk Biomaterials to Control Cellular Fate
Biomaterial Surface Topology
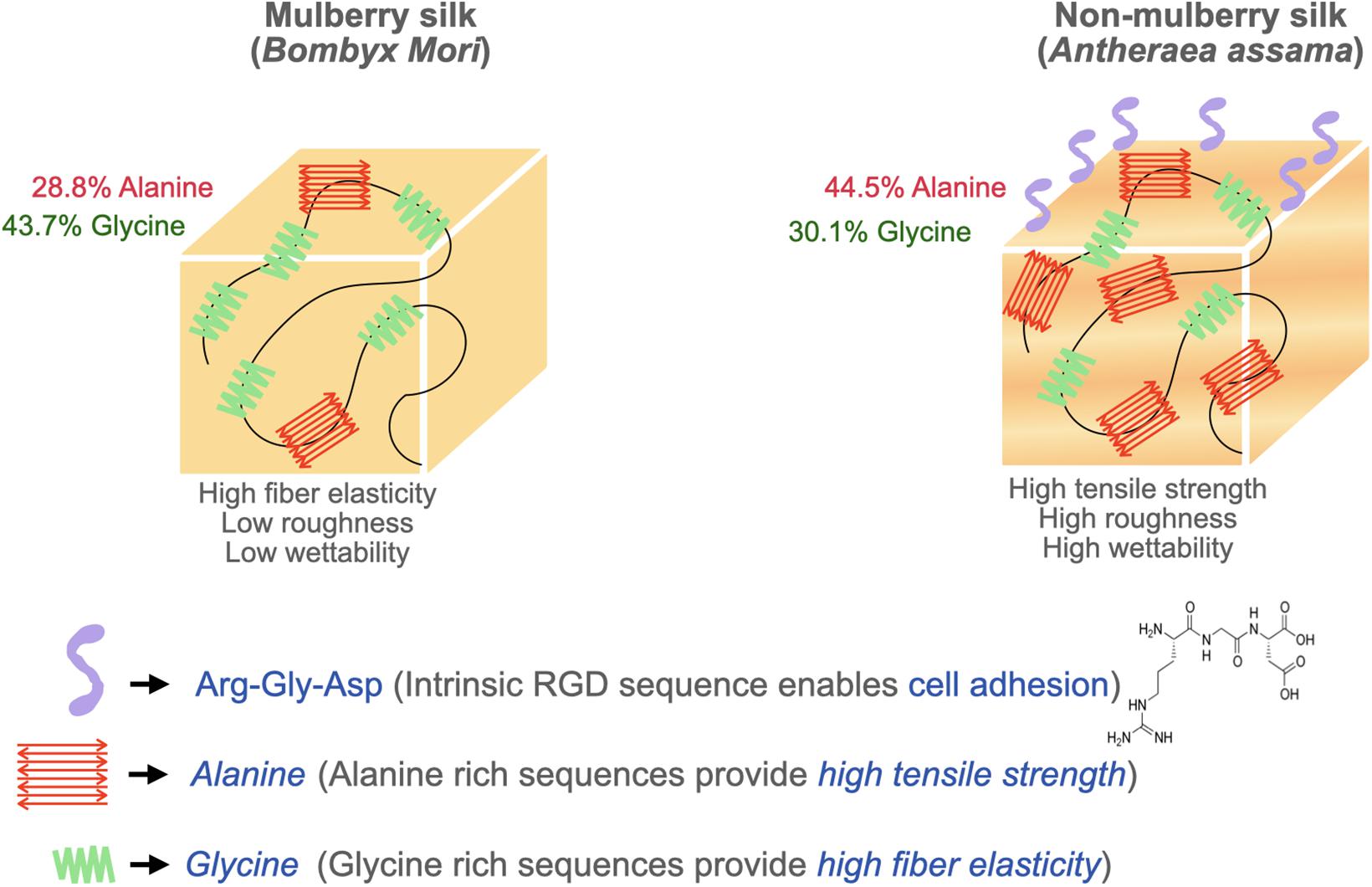
Surface topography plays a crucial role in the regulation of cell adhesion (Guilak et al., 2009; Wong et al., 2013). Cell behaviors are regulated by nanotopography typically via the variation of cellular spatiotemporal dynamics and the sensing behaviors of intracellular mechanosensors (Chen et al., 2014). Silk proteins retrieved from different sources possess unique amino acids leading to varying chemistry, roughness, mechanical properties, and wettability (Figure 3). To compare the cell-substrate interface of different silk sources, vascular cells were cultured on mulberry B. mori and non-mulberry A. assama silk films (Gupta et al., 2019). After culturing vascular cells on a range of engineered silk films with different surface patterns, it was determined that A. assama films favored endothelial cell growth regardless of substrate alignment. In contrast, smooth muscle cells required unidirectional alignment to develop a contractile phenotype that was observed for both silk sources. This study highlights the important synergistic interaction between cell type, surface topography, and physicochemical properties of silk biomaterials for dictating cell fates (Gupta et al., 2019).
Substrate Alignment and Surface Patterning
Unlike flat surfaces, nanofibrous substrates can upregulate integrin expression to promote cell adhesions (Bottino et al., 2011). By harnessing substrate alignment at the nano- and micro-scale, silk materials can be designed to tailor cellular fates. For example, the desirability of silk fibers with a narrow distribution of widths offer comparable morphological cues from individual fibers to support collective cell development (Zhu et al., 2015). In a separate study, soft lithography was used to surface pattern SF to evaluate the effect of surface morphology on cell proliferation, orientation, and ECM alignment on corneal fibroblasts (Gil et al., 2010). Interestingly, the depth of the grooves was found to have greater impact on the cell orientation compared to the width (Patil et al., 2020). Patterning silk has also been harnessed to design “co-culture” systems that provide spatial control over homo- and hetero-typic cellular interactions at the micron level (Battiston et al., 2014). For instance, corneal stroma and corneal epithelium have been cocultured in one system using micropatterned silk films (Gosselin et al., 2018). Additionally, surface patterning can be utilized to regulate cellular proliferation. For instance, Schwann cells and PC12 cells proliferate more on aligned silk hydrogels. Additionally, the cells grow along the oriented layers, display elongated shapes, and have a significantly narrow angular distribution (Wang et al., 2019).
It should be noted that the extent and direction of influence of nano-patterning on cell proliferation varies with different cell types. Contradictory studies have shown that surface patterning can have opposite effects on endothelial cell proliferation (Lu et al., 2008; Moffa et al., 2014; Gupta et al., 2019). A plausible explanation for this discrepancy might suggest this phenomenon is related to the size, depth, and peak distance of grooves. Furthermore, when culturing smooth muscle cells on aligned silk films, a suppression of cell proliferation rate is typically observed, a feature observed during phenotype transition from synthetic (high proliferation index) to contractile (low proliferation index) (Beamish et al., 2010).
Substrate alignment also plays a crucial role in forming optimal niches for guiding stem cell differentiation toward neuronal and osteogenic lineages. For example, SF nanofibrous matrices with aligned structures were used to guide nerve cell regeneration (Dinis, 2014). In another study, secretion of nerve regeneration factors was observed on aligned silk-graphene composite hydrogels confirming the stimulating effect of aligned structures for stimulating a neuronal phenotype (Wang et al., 2019). Furthermore, another report investigated neuron differentiation and found that laminin-coated electrospun aligned SF mats showed an increase in neuron differentiation compared to the non-aligned control groups (Li et al., 2019). In another study, nanofiber-graphene composite scaffolds which not only induced cell neurites to arrange along the fiber direction, but also promoted the growth of cells with significant expression of neuronal marker β3-tubulin (Qing et al., 2018). Additionally, patterned SF films supported osteogenic differentiation along with lamellar cellular alignment and matrix deposition in a spatially controlled manner (Tien et al., 2012). Therefore, alignment and patterning of silk scaffolds can be considered to tune cellular differentiation.
Surface Roughness
The cellular response to silk’s surface roughness is controversial due to inconsistent methodologies across studies. One study indicated that surfaces with a moderate roughness (10–45 nm) with a nearly Brownian fractal dimension (∼2.5) promote maximum cell proliferation rates (Gentile et al., 2010). It has also been reported that cells experience higher proliferation only in a range of critical roughness (Anselme et al., 2000). Furthermore, it was reported a positive influence of surface roughness in the range of 1–7 nm on cell proliferation in the case of non-mulberry silk. However, a range of surface roughness beyond the “critical range” can result in lower cell proliferation in mulberry silk. This may be partly attributable to the absence of RGD sequences in mulberry silk (Mandal et al., 2010). On the other hand, other studies have reported insignificant differences in proliferation on varying surface roughness and eliminated its influence as a factor on cell proliferation during endothelial cell culture on silk films (Gupta et al., 2019). Such paradoxical behavior is attributable to the undefined aspects of surface roughness and requires more specific definitions that consider different nanostructure profiles, irregular features, grooves, and broadness of peaks.
Surface Modifications
The origin of silk’s tunable biophysical properties and mechanical strength comes from its inherent ability to self-assemble into hydrophobic crystalline β-sheets; however, high β-sheet content materials result in inefficient cellular adhesion (Zhou et al., 2001; Cai et al., 2002). To combat this cellular interaction while maintaining desirable bulk material properties, surface modification of reactive amino acid residues on SF has been explored to achieve the surface attachment of small molecules, polymers, growth factors, cell binding ligands and ECM to improve cell adhesion and hydrophilic interactions on silk (Li et al., 2012).
SF has reactive carboxyl and amino groups in its side chains that can be conjugated to different functional groups. For example, plasma immersion ion implantation (PIII) treatments have been used to covalently bind proteins to the SF surface and improve cellular interactions (Kondyurin et al., 2018). Another simple surface modification technique involves plasma etching for grafting poly acrylic acid (pAAc) and poly hydroxyethylmethacrylate on regenerated SF films. The carboxyl functional groups on pAAc-grafted SFs can be further conjugated to other polymers and dyes like rhodamine. Furthermore, this technique can be used to tune the properties of SFs from low cell adhesion (unmodified SF) to high cell adhesion (pAAc–SF) and back to low cell adhesion (PEG–SF). Moreover, they achieved spatial control over the cellular adhesion property of the SF (Dhyani and Singh, 2014). A different study proposed a method to enhance bone matrix formation and hydroxyapatite (HA) mineralization by introducing carboxyl groups onto SF fibers. The abundance of polar and negatively charged groups also played a significant role in altering the protein assembly process and providing chemical handles for further modification (Zheng et al., 2016).
It has also been demonstrated that amine- and carboxyl- functionalized nanocomposite scaffolds can direct differentiation of human adipose derived stem cells toward osteogenic and chondrogenic lineages, respectively (Griffin et al., 2017). Silk films can be modified with carboxylic groups and phosphate groups using graft polymerization to control cellular differentiation (Patil and Singh, 2018). Silk films with carboxylic groups induced chondrogenic differentiation of human mesenchymal stem cell (hMSC), whereas those with phosphate groups induced osteogenic differentiation. Furthermore, grafting of these functional groups on silk simultaneously can provide spatiotemporal control over differentiation on the same surface (Patil et al., 2020).
Surface functionalization can also be used to mimic ECM specific signaling. Surface biofunctionalization with cell binding RGD peptides has been used to enhance cell proliferation through an integrin-mediated process (Rajendran et al., 2013; Gupta et al., 2019). Another ECM mimetic example is the decoration of SF films with integrin-binding laminin peptide motifs. These materials provide a rational design to mimic the functions of high molecular weight laminin proteins while also circumventing the high costs and stringent handling conditions that are linked with using whole laminin proteins (Manchineella et al., 2016; Li et al., 2019).
Surface modifications with inorganic compositions also have roles in regulating cellular processes. For example, a calcium phosphate-coated surface enhances cell proliferation, as compared to an uncoated one (Yang et al., 2010). Hydroxyapatite (HAp) functionalization is used to enhance the osteoconductive biological signals associated with osteogenesis and mineralization of stem cells (Ko et al., 2018). Cobalt (Co2+) has also been used as a dopant with silk owing to its unique ability to stimulate neovascularization. Co2+ when used with HA/SF scaffold, makes a great candidate for inducing angiogenesis and bone formation in vitro and in vivo (Fani et al., 2019). Therefore, inorganic elements can also be utilized in silk scaffolds to guide cellular differentiation.
Silk Composites
SF can be amalgamated or cross-linked with proteins, polysaccharide, polymers, and other functional materials to make composites with the advantages of both materials (Patil et al., 2020). Cell adherence on silk is often hampered as a consequence of its hydrophobicity. For instance, silk fibers are stronger and stiffer while collagen fibers provide surface adhesion molecules. In this composite approach, the incorporation of silk is known to enhance the mechanical properties of collagen-silk composite fibers (compared to collagen alone) making it tunable for different applications. In silk-dominant collagen composites, after a delay in initial attachment, cells proliferate at a similar rate as that of cells on collagen-dominant composites (Zhu et al., 2015). In another study, enhanced cell adhesion and proliferation of nerve cells was observed on silk nanofibres through the introduction of exfoliated graphene sheets forming active cues to optimize cyto-responses (Wang et al., 2019). Genetic engineering is also utilized to functionally fuse ECM motifs to silk proteins. In one example, a motif from fibronectin was used to synthesize fibronectin-silk with the ability to self-assemble into networks of microfibers under physiological-like conditions to improve cell proliferation (Johansson et al., 2019). Therefore, depending on the application, composites can be considered to enhance cellular adhesion and proliferation.
Silk scaffolds can also be loaded with differentiation-inducing growth factors. For example, a silk microsphere/scaffold was developed with a concentration gradient to release multiple growth factors in a spatially controlled manner (Wang et al., 2009). In another study, SF nanohydroxyapatite scaffold were synthesized to enable sequential and sustained release of stromal cell derived factor-1 (Shen et al., 2016). Silk can also be used to reinforce nanoparticles. In one study, a water- dispersible HA nanoparticles was fabricated with SF nanofibers to create a scaffold with programmable sustained release of BMP-2 (Ding et al., 2016). Despite the widespread use of growth factors-loaded scaffolds, it is important to note that growth factors have short half-lives, lasting only a few minutes, and the doses that are reportedly efficient in vitro may not yield similar results in vivo.
Mechanical Properties
Stiffer matrices induce tensional forces, causing the cell-matrix adhesion proteins to trigger a mechanotransductive pathway (Sridharan et al., 2009). This interaction between cellular and substrate mechanical modulus is a principal component of the reciprocal relation between cell and matrix (van Helvert et al., 2018). Silk demonstrates exceptional mechanical properties, including high tensile strength and extensibility, making it one of the toughest known materials (Shao and Vollrath, 2002; Becker et al., 2003). The exceptional strength of silkworm and spider silks, exceeding that of steel, arises from β-sheet nanocrystals that universally consist of highly conserved poly-(Gly-Ala) and poly-Ala domains. Despite the key molecular interactions in β-sheet nanocrystals being hydrogen bonds, size effects can be exploited to create bioinspired materials with tunable mechanical properties. In fact, silk has been tailored for applications as soft as the brain to as stiff as bone (Abbott et al., 2016a). However, reports suggest that cells spread better on stiffer silk substrates as opposed to those with low rigidity (Gupta et al., 2019). A plausible explanation for this behavior may be the imbalance between cell traction forces and corresponding ECM response, a crucial parameter for assembly of cell-matrix complexes and cell spreading. For instance, a study reported that the fine tuning of silk scaffold’s stiffness induces different endothelial migration and aggregation (Lu et al., 2019), suggesting a sensitive dependence of cell migration on mechanical cues.
SF is primarily utilized in its crystalline β-sheet form for tissue engineering applications. While higher β-sheet content reduces cellular adhesion and proliferation properties, it possesses superior mechanical strength (Manchineella et al., 2016). Non-mulberry silk films are mechanically stiffer and exhibited higher tensile modulus compared to mulberry silk films. The higher tensile strength and elongation of non-mulberry silk is attributable to the presence of distinct polyalanine stretches in its native structure, resulting in higher concentrations of antiparallel β-sheet structures compared to mulberry silk (Lefèvre et al., 2007). Therefore, silk source should be considered to tailor cellular outcomes. Physical interactions between cells and the stiffness and elasticity of the scaffold can influence stem cell behavior (Rowlands et al., 2008; Guilak et al., 2009). Stem cells have a tendency to differentiate into specific lineages when cultured on scaffolds with an elasticity similar to that of native tissue. The substrate’s elasticity affects the intracellular signaling through mechanotransducers such as Rho kinase and focal adhesion kinase which play a significant role in determining the stem cell lineage (Shih et al., 2011). This concept has been exploited to design tough anisotropic silk nanofiber hydrogels with different stiffnesses (52 and 120 kPa) for bone regeneration. This study demonstrated a higher expression of osteogenic genes on the stiffer hydrogels (120 kPa) revealing that a higher stiffness provides strong cues to control cell behaviors and osteogenic differentiation (Ding et al., 2020). Another study synthesized composite fibers using dragline silk with collagen at various ratios to examine the effect of mechanical properties on stem cell differentiation. The ultimate tensile strength and elasticity of the composite fibers increased with silk ratio while there was a slight reduction in stretchability (Ko et al., 2018). Their study concluded that the incorporation of silk proteins to collagen dramatically increased the matrix stability against excessive fiber swelling and shape deformation in cell culture medium. Matrices containing 15 wt% silk in collagen (CS15) and 30 wt% silk in collagen (CS30) were found to induce a level of neural differentiation comparable to that of pure collagen. In particular, CS15 matrix induced the highest extent of cell polarization and promoted the development of extended 1D neural filaments strictly in-line with the aligned fibers (Ko et al., 2018). In an effort to utilize different mechanical cues, another report used oxidized silk scaffolds to achieve a very high compressive modulus demonstrating the effect of matrix stiffness on fostering hMSC osteogenesis (35 times higher than their non-oxidized counterparts) (Zheng et al., 2016). The contrasting effects of different matrix stiffness and elasticity indicate the strong association of differentiation with mechanical properties.
Porosity
Three dimensional porous silk scaffolds are used to provide structure and biomechanical cues for seeded cells until they are organized into a functional tissue (Zmora et al., 2002). Within these scaffolds, cell proliferation, migration, and differentiation are directly governed by size and porosity. To optimize pore microstructures and connectivity silk scaffolds can be fabricated with different porogens or freeze-dry regimes (Rockwood et al., 2011). Bigger pore sizes are generally linked to enhanced cell proliferation and migration (Murphy et al., 2010); however, 50–75 μm pores showed better cell proliferation than 75–100 μm pores in human primary dermal fibroblasts (Mandal and Kundu, 2009a). The effect of pore size on cellular fate is cell line specific. For bone marrow stromal cells expressing BMP7, a SF scaffold pore size between 100 and 300 μm resulted in enhanced cell proliferation and ECM production over smaller pore sizes (Zhang et al., 2010). For chondrocytes, however, the opposite has been observed with smaller SF scaffold pore sizes (90–250 μm) providing the best environment for adhesion and proliferation (Han et al., 2015). Therefore, porosity is a critical parameter that is cell type specific and should be optimized for each tissue engineered application.
Conclusion
In summary, the biophysical properties of silk can be used to control cellular fate, including cellular adhesion, proliferation, migration, and differentiation. Beyond the inherent properties of silk, which can be patterned and aligned, the ability of silk to be combined with other functional materials or undergo relatively simple surface modifications can enhance or tune its biophysical influence on cells. Moreover, the exceptional mechanical properties of silk make it well-suited for a variety of tissue engineering applications. Finally, cellular behavior is critically regulated by silk type owing to a strong dependence on the availability of RGD sequences, hydrophilicity, and mechanical properties.
Author Contributions
DK, MD, and RA performed the conception or design of the work, revised it critically for important intellectual content, provided approval for publication of the content, and agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. DK drafted the work. All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
Abbott, R. D., Borowsky, F. E., Alonzo, C. A., Zieba, A., Georgakoudi, I., and Kaplan, D. L. (2018). Variability in responses observed in human white adipose tissue models. J. Tissue Eng. Regen. Med. 12, 840–847. doi: 10.1002/term.2572
Abbott, R. D., Kimmerling, E. P., Cairns, D. M., and Kaplan, D. L. (2016a). Silk as a biomaterial to support long-term three-dimensional tissue cultures. ACS Appl. Mater. Interfaces 8, 21861–21868. doi: 10.1021/acsami.5b12114
Abbott, R. D., Raja, W. K., Wang, R. Y., Stinson, J. A., Glettig, D. L., Burke, K. A., et al. (2015). Long term perfusion system supporting adipogenesis. Methods 84, 84–89. doi: 10.1016/j.ymeth.2015.03.022
Abbott, R. D., Wang, R. Y., Reagan, M. R., Chen, Y., Borowsky, F. E., Zieba, A., et al. (2016b). The use of silk as a scaffold for mature, sustainable unilocular adipose 3D tissue engineered systems. Adv. Healthcare Mater. 5, 1667–1677. doi: 10.1002/adhm.201600211
Alessandrino, A., Fregnan, F., Biagiotti, M., Muratori, L., Bassani, G. A., Ronchi, G., et al. (2019). SilkBridgeTM: a novel biomimetic and biocompatible silk-based nerve conduit. Biomater. Sci. 7, 4112–4130. doi: 10.1039/C9BM00783K
Altman, A. M., Gupta, V., Ríos, C. N., Alt, E. U., and Mathur, A. B. (2010). Adhesion, migration and mechanics of human adipose-tissue-derived stem cells on silk fibroin–chitosan matrix. Acta Biomater. 6, 1388–1397. doi: 10.1016/j.actbio.2009.10.034
Altman, G. H., Diaz, F., Jakuba, C., Calabro, T., Horan, R. L., Lu, H., et al. (2003). Silk-based biomaterials. Biomaterials 24, 401–416. doi: 10.1016/S0142-9612(02)00353-8
Altman, G. H., Horan, R. L., Lu, H. H., Moreau, J., Martin, I., Richmond, J. C., et al. (2002). Silk matrix for tissue engineered anterior cruciate ligaments. Biomaterials 23, 4131–4141. doi: 10.1016/s0142-9612(02)00156-4
Anselme, K., Linez, P., Bigerelle, M., Le Maguer, D., Le Maguer, A., Hardouin, P., et al. (2000). The relative influence of the topography and chemistry of TiAl6V4 surfaces on osteoblastic cell behaviour. Biomaterials 21, 1567–1577. doi: 10.1016/s0142-9612(00)00042-9
Bai, S., Zhang, W., Lu, Q., Ma, Q., Kaplan, D. L., and Zhu, H. (2014). Silk nanofiber hydrogels with tunable modulus to regulate nerve stem cell fate. J. Mater. Chem. B 2, 6590–6600. doi: 10.1039/C4TB00878B
Battiston, K. G., Cheung, J. W. C., Jain, D., and Santerre, J. P. (2014). Biomaterials in co-culture systems: towards optimizing tissue integration and cell signaling within scaffolds. Biomaterials 35, 4465–4476. doi: 10.1016/j.biomaterials.2014.02.023
Beamish, J. A., He, P., Kottke-Marchant, K., and Marchant, R. E. (2010). Molecular regulation of contractile smooth muscle cell phenotype: implications for vascular tissue engineering. Tissue Eng. Part B Rev. 16, 467–491. doi: 10.1089/ten.teb.2009.0630
Becker, N., Oroudjev, E., Mutz, S., Cleveland, J. P., Hansma, P. K., Hayashi, C. Y., et al. (2003). Molecular nanosprings in spider capture-silk threads. Nat. Mater. 2, 278–283. doi: 10.1038/nmat858
Bellas, E., Marra, K. G., and Kaplan, D. L. (2013). Sustainable three-dimensional tissue model of human adipose tissue. Tissue Eng. Part C Methods 19, 745–754. doi: 10.1089/ten.TEC.2012.0620
Bhattacharjee, P., Naskar, D., Maiti, T. K., Bhattacharya, D., Das, P., Nandi, S. K., et al. (2016). Potential of non-mulberry silk protein fibroin blended and grafted poly(ϵ-caprolactone) nanofibrous matrices for in vivo bone regeneration. Coll. Surf. B Biointerf. 143, 431–439. doi: 10.1016/j.colsurfb.2016.03.058
Bhumiratana, S., Grayson, W. L., Castaneda, A., Rockwood, D. N., Gil, E. S., Kaplan, D. L., et al. (2011). Nucleation and growth of mineralized bone matrix on silk-hydroxyapatite composite scaffolds. Biomaterials 32, 2812–2820. doi: 10.1016/j.biomaterials.2010.12.058
Biggs, M. J. P., Richards, R. G., and Dalby, M. J. (2010). Nanotopographical modification: a regulator of cellular function through focal adhesions. Nanomedicine 6, 619–633. doi: 10.1016/j.nano.2010.01.009
Bottino, M. C., Thomas, V., and Janowski, G. M. (2011). A novel spatially designed and functionally graded electrospun membrane for periodontal regeneration. Acta Biomater. 7, 216–224. doi: 10.1016/j.actbio.2010.08.019
Cai, K., Yao, K., Lin, S., Yang, Z., Li, X., Xie, H., et al. (2002). Poly(D,L-lactic acid) surfaces modified by silk fibroin: effects on the culture of osteoblast in vitro. Biomaterials 23, 1153–1160. doi: 10.1016/s0142-9612(01)00230-7
Chen, W., Shao, Y., Li, X., Zhao, G., and Fu, J. (2014). Nanotopographical surfaces for stem cell fate control: engineering mechanobiology from the bottom. Nano Today 9, 759–784. doi: 10.1016/j.nantod.2014.12.002
Cukierman, E., Pankov, R., Stevens, D. R., and Yamada, K. M. (2001). Taking cell-matrix adhesions to the third dimension. Science 294, 1708–1712. doi: 10.1126/science.1064829
Cunniff, P. M., Fossey, S. A., Auerbach, M. A., Song, J. W., Kaplan, D. L., Wade Adams, W., et al. (1994). Mechanical and thermal properties of dragline silk from the spider Nephila clavipes. Polymers Adv. Technol. 5, 401–410. doi: 10.1002/pat.1994.220050801
Curtis, A. S. G., Dalby, M., and Gadegaard, N. (2006). Cell signaling arising from nanotopography: implications for nanomedical devices. Nanomedicine (Lond) 1, 67–72. doi: 10.2217/17435889.1.1.67
Dhyani, V., and Singh, N. (2014). Controlling the cell adhesion property of silk films by graft polymerization. ACS Appl. Mater. Interfaces 6, 5005–5011. doi: 10.1021/am4060595
Ding, Z., Fan, Z., Huang, X., Lu, Q., Xu, W., and Kaplan, D. L. (2016). Silk–Hydroxyapatite nanoscale scaffolds with programmable growth factor delivery for bone repair. ACS Appl. Mater. Interfaces 8, 24463–24470. doi: 10.1021/acsami.6b08180
Ding, Z., Lu, G., Cheng, W., Xu, G., Zuo, B., Lu, Q., et al. (2020). Tough anisotropic silk nanofiber hydrogels with osteoinductive capacity. ACS Biomater. Sci. Eng. 6, 2357–2367. doi: 10.1021/acsbiomaterials.0c00143
Dinis, T. M. (2014). Complementary effects of two growth factors in multifunctionalized silk nanofibers for nerve reconstruction. PLoS One 9:e109770.
Engler, A. J., Sen, S., Sweeney, H. L., and Discher, D. E. (2006). Matrix elasticity directs stem cell lineage specification. Cell 126, 677–689. doi: 10.1016/j.cell.2006.06.044
Fani, N., Farokhi, M., Azami, M., Kamali, A., Bakhshaiesh, N. L., Ebrahimi-Barough, S., et al. (2019). Endothelial and osteoblast differentiation of adipose-derived mesenchymal stem cells using a cobalt-doped CaP/Silk Fibroin Scaffold. ACS Biomater. Sci. Eng. 5, 2134–2146. doi: 10.1021/acsbiomaterials.8b01372
Gentile, F., Tirinato, L., Battista, E., Causa, F., Liberale, C., di Fabrizio, E. M., et al. (2010). Cells preferentially grow on rough substrates. Biomaterials 31, 7205–7212. doi: 10.1016/j.biomaterials.2010.06.016
Ghorbanian, L., Emadi, R., Razavi, S. M., Shin, H., and Teimouri, A. (2013). Fabrication and characterization of novel diopside/silk fibroin nanocomposite scaffolds for potential application in maxillofacial bone regeneration. Int. J. Biol. Macromol. 58, 275–280. doi: 10.1016/j.ijbiomac.2013.04.004
Gil, E. S., Park, S. H., Marchant, J., Omenetto, F., and Kaplan, D. L. (2010). Response of human corneal fibroblasts on silk film surface patterns. Macromol. Biosci. 10, 664–673. doi: 10.1002/mabi.200900452
Gosselin, E. A., Torregrosa, T., Ghezzi, C. E., Mendelsohn, A. C., Gomes, R., Funderburgh, J. L., et al. (2018). Multi-layered silk film coculture system for human corneal epithelial and stromal stem cells. J. Tissue Eng. Regen. Med. 12, 285–295. doi: 10.1002/term.2499
Griffin, M. F., Ibrahim, A., Seifalian, A. M., Butler, P. E. M., Kalaskar, D. M., and Ferretti, P. (2017). Chemical group-dependent plasma polymerisation preferentially directs adipose stem cell differentiation towards osteogenic or chondrogenic lineages. Acta Biomater. 50, 450–461. doi: 10.1016/j.actbio.2016.12.016
Guilak, F., Cohen, D. M., Estes, B. T., Gimble, J. M., Liedtke, W., and Chen, C. S. (2009). Control of stem cell fate by physical interactions with the extracellular matrix. Cell Stem. Cell. 5, 17–26. doi: 10.1016/j.stem.2009.06.016
Guo, J.-H., Liu, Y., Lv, Z. J., Wei, W. J., Guan, X., Guan, Q. L., et al. (2015). Potential neurogenesis of human adipose-derived stem cells on electrospun catalpol-loaded composite nanofibrous scaffolds. Ann. Biomed. Eng. 43, 2597–2608. doi: 10.1007/s10439-015-1311-x
Gupta, P., Adhikary, M., Kumar, J. C. M. M., Bhardwaj, N., and Mandal, B. B. (2016). Biomimetic, osteoconductive non-mulberry silk fiber reinforced tricomposite scaffolds for bone tissue engineering. ACS Appl. Mater. Interfaces 8, 30797–30810. doi: 10.1021/acsami.6b11366
Gupta, P., Moses, J. C., and Mandal, B. B. (2019). Surface patterning and innate physicochemical attributes of silk films concomitantly govern vascular cell dynamics. ACS Biomater. Sci. Eng. 5, 933–949. doi: 10.1021/acsbiomaterials.8b01194
Guvendiren, M., and Burdick, J. A. (2012). Stiffening hydrogels to probe short- and long-term cellular responses to dynamic mechanics. Nat. Commun. 3:1. doi: 10.1038/ncomms1792
Han, K.-S., Song, J. E., Tripathy, N., Kim, H., Moon, B. M., Park, C. H., et al. (2015). Effect of pore sizes of silk scaffolds for cartilage tissue engineering. Macromol. Res. 23, 1091–1097. doi: 10.1007/s13233-015-3156-4
Hopkins, A. M., Laporte, L. D., Tortelli, F., Spedden, E., Staii, C., Atherton, T. J., et al. (2013). Silk hydrogels as soft substrates for neural tissue engineering. Adv. Funct. Mater. 23, 5140–5149. doi: 10.1002/adfm.201300435
Johansson, U., Widhe, M., Shalaly, N. D., Arregui, I. L., Nilebäck, L., Tasiopoulos, C. P., et al. (2019). Assembly of functionalized silk together with cells to obtain proliferative 3D cultures integrated in a network of ECM-like microfibers. Sci. Rep. 9:6291. doi: 10.1038/s41598-019-42541-y
Kaplan, D., Adams, W. W., Farmer, B., and Viney, C. (1993). “Silk: biology, structure, properties, and genetics,” in Silk Polymers, eds D. Kaplan, W. Wade Adams, B. Farmer, and C. Viney (Washington, DC: American Chemical Society), 2–16. doi: 10.1021/bk-1994-0544.ch001
Karageorgiou, V., Tomkins, M., Fajardo, R., Meinel, L., Snyder, B., Wade, K., et al. (2006). Porous silk fibroin 3-D scaffolds for delivery of bone morphogenetic protein-2 in vitro and in vivo. J. Biomed. Mater. Res. A 78, 324–334. doi: 10.1002/jbm.a.30728
Khetan, S., Guvendiren, M., Legant, W. R., Cohen, D. M., Chen, C. S., and Burdick, J. A. (2013). Degradation-mediated cellular traction directs stem cell fate in covalently crosslinked three-dimensional hydrogels. Nat. Mater. 12:5. doi: 10.1038/nmat3586
Kloxin, A. M., Kasko, A. M., Salinas, C. N., and Anseth, K. S. (2009). Photodegradable hydrogels for dynamic tuning of physical and chemical properties. Science 324, 59–63. doi: 10.1126/science.1169494
Ko, E., Lee, J. S., Kim, H., Yang, S. Y., Yang, D., Yang, K., et al. (2018). Electrospun silk fibroin nanofibrous scaffolds with two-stage hydroxyapatite functionalization for enhancing the osteogenic differentiation of human adipose-derived mesenchymal stem cells. ACS Appl. Mater. Interfaces 10, 7614–7625. doi: 10.1021/acsami.7b03328
Kondyurin, A., Lau, K., Tang, F., Akhavan, B., Chrzanowski, W., Lord, M. S., et al. (2018). Plasma ion implantation of silk biomaterials enabling direct covalent immobilization of bioactive agents for enhanced cellular responses. ACS Appl. Mater. Interfaces 10, 17605–17616. doi: 10.1021/acsami.8b03182
Lefèvre, T., Rousseau, M.-E., and Pézolet, M. (2007). Protein secondary structure and orientation in silk as revealed by raman spectromicroscopy. Biophys. J. 92, 2885–2895. doi: 10.1529/biophysj.106.100339
Li, C., Vepari, C., Jin, H.-J., Kim, H. J., and Kaplan, D. L. (2006). Electrospun silk-BMP-2 scaffolds for bone tissue engineering. Biomaterials 27, 3115–3124. doi: 10.1016/j.biomaterials.2006.01.022
Li, G., Chen, K., You, D., Xia, M., Li, W., Fan, S., et al. (2019). Laminin-Coated electrospun regenerated silk fibroin mats promote neural progenitor cell proliferation, differentiation, and survival in vitro. Front. Bioeng. Biotechnol 7:190. doi: 10.3389/fbioe.2019.00190
Li, G., Liu, H., Li, T., and Wang, J. (2012). Surface modification and functionalization of silk fibroin fibers/fabric toward high performance applications. Mater. Sci. Eng. C 32, 627–636. doi: 10.1016/j.msec.2011.12.013
Li, Y., Xiao, Y., and Liu, C. (2017). The horizon of materiobiology: a perspective on material-guided cell behaviors and tissue engineering. Chem. Rev. 117, 4376–4421. doi: 10.1021/acs.chemrev.6b00654
Lu, J., Rao, M. P., MacDonald, N. C., Khang, D., and Webster, T. J. (2008). Improved endothelial cell adhesion and proliferation on patterned titanium surfaces with rationally designed, micrometer to nanometer features. Acta Biomater. 4, 192–201. doi: 10.1016/j.actbio.2007.07.008
Lu, X., Ding, Z., Xu, F., Lu, Q., and Kaplan, D. L. (2019). Subtle regulation of scaffold stiffness for the optimized control of cell behavior. ACS Appl. Bio Mater. 2, 3108–3119. doi: 10.1021/acsabm.9b00445
Manchineella, S., Thrivikraman, G., Basu, B., and Govindaraju, T. (2016). Surface-functionalized silk fibroin films as a platform to guide neuron-like differentiation of human mesenchymal stem cells. ACS Appl. Mater. Interfaces 8, 22849–22859. doi: 10.1021/acsami.6b06403
Mandal, B. B., Das, S., Choudhury, K., and Kundu, S. C. (2010). Implication of silk film RGD availability and surface roughness on cytoskeletal organization and proliferation of primary rat bone marrow cells. Tissue Eng. Part A 16, 2391–2403. doi: 10.1089/ten.tea.2009.0206
Mandal, B. B., and Kundu, S. C. (2009b). Osteogenic and adipogenic differentiation of rat bone marrow cells on non-mulberry and mulberry silk gland fibroin 3D scaffolds. Biomaterials 30, 5019–5030. doi: 10.1016/j.biomaterials.2009.05.064
Mandal, B. B., and Kundu, S. C. (2009a). Cell proliferation and migration in silk fibroin 3D scaffolds. Biomaterials 30, 2956–2965. doi: 10.1016/j.biomaterials.2009.02.006
Mauney, J. R., Nguyen, T., Gillen, K., Kirker-Head, C., Gimble, J. M., and Kaplan, D. L. (2007). Engineering adipose-like tissue in vitro and in vivo utilizing human bone marrow and adipose-derived mesenchymal stem cells with silk fibroin 3D scaffolds. Biomaterials 28, 5280–5290. doi: 10.1016/j.biomaterials.2007.08.017
Meinel, L., Fajardo, R., Hofmann, S., Langer, R., Chen, J., Snyder, B., et al. (2005). Silk implants for the healing of critical size bone defects. Bone 37, 688–698. doi: 10.1016/j.bone.2005.06.010
Mieszawska, A. J., Llamas, J. G., Vaiana, C. A., Kadakia, M. P., Naik, R. R., and Kaplan, D. L. (2011). Clay-enriched silk biomaterials for bone formation. Acta Biomater. 7, 3036–3041. doi: 10.1016/j.actbio.2011.04.016
Moffa, M., Sciancalepore, A. G., Passione, L. G., and Pisignano, D. (2014). Combined nano- and micro-scale topographic cues for engineered vascular constructs by electrospinning and imprinted micro-patterns. Small 10, 2439–2450. doi: 10.1002/smll.201303179
Moses, J. C., Dey, M., Devi, K. B., Roy, M., Nandi, S. K., and Mandal, B. B. (2019). Synergistic effects of silicon/zinc doped brushite and silk scaffolding in augmenting the osteogenic and angiogenic potential of composite biomimetic bone grafts. ACS Biomater. Sci. Eng. 5, 1462–1475. doi: 10.1021/acsbiomaterials.8b01350
Murphy, A. R., St John, P., and Kaplan, D. L. (2008). Modification of silk fibroin using diazonium coupling chemistry and the effects on hMSC proliferation and differentiation. Biomaterials 29, 2829–2838. doi: 10.1016/j.biomaterials.2008.03.039
Murphy, C. M., Haugh, M. G., and O’Brien, F. J. (2010). The effect of mean pore size on cell attachment, proliferation and migration in collagen-glycosaminoglycan scaffolds for bone tissue engineering. Biomaterials 31, 461–466. doi: 10.1016/j.biomaterials.2009.09.063
Murphy, W. L., McDevitt, T. C., and Engler, A. J. (2014). Materials as stem cell regulators. Nat. Mater. 13, 547–557. doi: 10.1038/nmat3937
Patil, S., Dhyani, V., Kaur, T., and Singh, N. (2020). Spatiotemporal control over cell proliferation and differentiation for tissue engineering and regenerative medicine applications using silk fibroin scaffolds. ACS Appl. Bio Mater. 3, 3476–3493. doi: 10.1021/acsabm.0c00305
Patil, S., and Singh, N. (2018). Spatially controlled functional group grafting of silk films to induce osteogenic and chondrogenic differentiation of human mesenchymal stem cells. Mater. Sci. Eng. C 91, 796–805. doi: 10.1016/j.msec.2018.06.008
Pelham, R. J., and Wang, Y. (1997). Cell locomotion and focal adhesions are regulated by substrate flexibility. PNAS 94, 13661–13665. doi: 10.1073/pnas.94.25.13661
Pistone, A., Sagnella, A., Chieco, C., Bertazza, G., Varchi, G., Formaggio, F., et al. (2016). Silk fibroin film from golden-yellow Bombyx mori is a biocomposite that contains lutein and promotes axonal growth of primary neurons. Biopolymers 105, 287–299. doi: 10.1002/bip.22806
Place, E. S., Evans, N. D., and Stevens, M. M. (2009). Complexity in biomaterials for tissue engineering. Nat. Mater. 8, 457–470. doi: 10.1038/nmat2441
Pritchard, E. M., Valentin, T., Boison, D., and Kaplan, D. L. (2011). Incorporation of proteinase inhibitors into silk-based delivery devices for enhanced control of degradation and drug release. Biomaterials 32, 909–918. doi: 10.1016/j.biomaterials.2010.09.055
Qing, H., Jin, G., Zhao, G., Huang, G., Ma, Y., Zhang, X., et al. (2018). Heterostructured Silk-nanofiber-reduced graphene oxide composite scaffold for SH-SY5Y cell alignment and differentiation. ACS Appl. Mater. Interfaces 10, 39228–39237. doi: 10.1021/acsami.8b12562
Rajendran, P., Rengarajan, T., Thangavel, J., Nishigaki, Y., Sakthisekaran, D., Sethi, G., et al. (2013). The vascular endothelium and human diseases. Int. J. Biol. Sci. 9, 1057–1069. doi: 10.7150/ijbs.7502
Rockwood, D. N., Preda, R. C., Yücel, T., Wang, X., Lovett, M. L., and Kaplan, D. L. (2011). Materials fabrication from Bombyx mori silk fibroin. Nat. Protoc. 6:10. doi: 10.1038/nprot.2011.379
Rowlands, A. S., George, P. A., and Cooper-White, J. J. (2008). Directing osteogenic and myogenic differentiation of MSCs: interplay of stiffness and adhesive ligand presentation. Am. J. Physiol. Cell Physiol. 295, C1037–C1044. doi: 10.1152/ajpcell.67.2008
Sangkert, S., Kamonmattayakul, S., Chai, W. L., and Meesane, J. (2016). A biofunctional-modified silk fibroin scaffold with mimic reconstructed extracellular matrix of decellularized pulp/collagen/fibronectin for bone tissue engineering in alveolar bone resorption. Mater. Lett. 166, 30–34. doi: 10.1016/j.matlet.2015.12.032
Sengupta, S., Park, S. H., Seok, G. E., Patel, A., Numata, K., Lu, C. L., et al. (2010). Quantifying osteogenic cell degradation of silk biomaterials. Biomacromolecules 11, 3592–3599. doi: 10.1021/bm101054q
Shao, W., He, J., Han, Q., Sang, F., Wang, Q., Chen, L., et al. (2016). A biomimetic multilayer nanofiber fabric fabricated by electrospinning and textile technology from polylactic acid and Tussah silk fibroin as a scaffold for bone tissue engineering. Mater. Sci. Eng. C 67, 599–610. doi: 10.1016/j.msec.2016.05.081
Shao, Z., and Vollrath, F. (2002). Surprising strength of silkworm silk. Nature 418:6899. doi: 10.1038/418741a
Shen, X., Zhang, Y., Gu, Y., Xu, Y., Liu, Y., Li, B., et al. (2016). Sequential and sustained release of SDF-1 and BMP-2 from silk fibroin-nanohydroxyapatite scaffold for the enhancement of bone regeneration. Biomaterials 106, 205–216. doi: 10.1016/j.biomaterials.2016.08.023
Shih, Y.-R. V., Tseng, K.-F., Lai, H.-Y., Lin, C.-H., and Lee, O. K. (2011). Matrix stiffness regulation of integrin-mediated mechanotransduction during osteogenic differentiation of human mesenchymal stem cells. J. Bone Miner. Res. 26, 730–738. doi: 10.1002/jbmr.278
Singh, B. N., Panda, N. N., Mund, R., and Pramanik, K. (2016). Carboxymethyl cellulose enables silk fibroin nanofibrous scaffold with enhanced biomimetic potential for bone tissue engineering application. Carbohydr. Polymers 151, 335–347. doi: 10.1016/j.carbpol.2016.05.088
Sridharan, I., Kim, T., and Wang, R. (2009). Adapting collagen/CNT matrix in directing hESC differentiation. Biochem. Biophys. Res. Commun. 381, 508–512. doi: 10.1016/j.bbrc.2009.02.072
Stevens, M. M., and George, J. H. (2005). Exploring and engineering the cell surface interface. Science 310, 1135–1138. doi: 10.1126/science.1106587
Sun, W., Incitti, T., Migliaresi, C., Quattrone, A., Casarosa, S., and Motta, A. (2017). Viability and neuronal differentiation of neural stem cells encapsulated in silk fibroin hydrogel functionalized with an IKVAV peptide. J. Tissue Eng. Regen. Med. 11, 1532–1541. doi: 10.1002/term.2053
Teimouri, A., Ebrahimi, R., Emadi, R., Beni, B. H., and Chermahini, A. N. (2015). Nano-composite of silk fibroin–chitosan/Nano ZrO2 for tissue engineering applications: fabrication and morphology. Int. J. Biol. Macromol. 76, 292–302. doi: 10.1016/j.ijbiomac.2015.02.023
Tien, L. W., Gil, E. S., Park, S.-H., Mandal, B. B., and Kaplan, D. L. (2012). Patterned silk film scaffolds for aligned lamellar bone tissue engineering. Macromol. Biosci. 12, 1671–1679. doi: 10.1002/mabi.201200193
van Helvert, S., Storm, C., and Friedl, P. (2018). Mechanoreciprocity in cell migration. Nat. Cell Biol. 20, 8–20. doi: 10.1038/s41556-017-0012-0
Wang, L., Song, D., Zhang, X., Ding, Z., Kong, X., Lu, Q., et al. (2019). Silk–graphene hybrid hydrogels with multiple cues to induce nerve cell behavior. ACS Biomater. Sci. Eng. 5, 613–622. doi: 10.1021/acsbiomaterials.8b01481
Wang, R. Y., Abbott, R. D., Zieba, A., Borowsky, F. E., and Kaplan, D. L. (2017). Development of a three-dimensional adipose tissue model for studying embryonic exposures to obesogenic chemicals. Ann. Biomed. Eng. 45, 1807–1818. doi: 10.1007/s10439-016-1752-x
Wang, X., Wenk, E., Zhang, X., Meinel, L., Vunjak-Novakovic, G., and Kaplan, D. L. (2009). Growth factor gradients via microsphere delivery in biopolymer scaffolds for osteochondral tissue engineering. J. Control. Release 134, 81–90. doi: 10.1016/j.jconrel.2008.10.021
Wang, Y., Rudym, D. D., Walsh, A., Abrahamsen, L., Kim, H. J., Kim, H. S., et al. (2008). In vivo degradation of three-dimensional silk fibroin scaffolds. Biomaterials 29, 3415–3428. doi: 10.1016/j.biomaterials.2008.05.002
Wong, H. M., Zhao, Y., Tam, V., Wu, S., Chu, P. K., Zheng, Y., et al. (2013). In vivo stimulation of bone formation by aluminum and oxygen plasma surface-modified magnesium implants. Biomaterials 34, 9863–9876. doi: 10.1016/j.biomaterials.2013.08.052
Woo, K. M., Chen, V. J., and Ma, P. X. (2003). Nano-fibrous scaffolding architecture selectively enhances protein adsorption contributing to cell attachment. J. Biomed. Mater. Res. A 67, 531–537. doi: 10.1002/jbm.a.10098
Yang, L., Hedhammar, M., Blom, T., Leifer, K., Johansson, J., Habibovic, P., et al. (2010). Biomimetic calcium phosphate coatings on recombinant spider silk fibres. Biomed. Mater. 5:045002. doi: 10.1088/1748-6041/5/4/045002
Zhang, Y., Fan, W., Ma, Z., Wu, C., Fanga, W., Liu, G., et al. (2010). The effects of pore architecture in silk fibroin scaffolds on the growth and differentiation of mesenchymal stem cells expressing BMP7. Acta Biomater. 6, 3021–3028. doi: 10.1016/j.actbio.2010.02.030
Zheng, K., Chen, Y., Huang, W., Lin, Y., Kaplan, D. L., and Fan, Y. (2016). Chemically functionalized silk for human bone marrow-derived mesenchymal stem cells proliferation and differentiation. ACS Appl. Mater. Interfaces 8, 14406–14413. doi: 10.1021/acsami.6b03518
Zhou, C. Z., Confalonieri, F., Jacquet, M., Perasso, R., Li, Z. G., and Janin, J. (2001). Silk fibroin: structural implications of a remarkable amino acid sequence. Proteins 44, 119–122. doi: 10.1002/prot.1078
Zhu, B., Li, W., Lewis, R. V., Segre, C. U., and Wang, R. (2015). E-Spun composite fibers of collagen and dragline silk protein: fiber mechanics, biocompatibility, and application in stem cell differentiation. Biomacromolecules 16, 202–213. doi: 10.1021/bm501403f
Keywords: silk, biomaterials, tissue engineering, materiobiology, cellular behaviors
Citation: Kochhar D, DeBari MK and Abbott RD (2021) The Materiobiology of Silk: Exploring the Biophysical Influence of Silk Biomaterials on Directing Cellular Behaviors. Front. Bioeng. Biotechnol. 9:697981. doi: 10.3389/fbioe.2021.697981
Received: 20 April 2021; Accepted: 31 May 2021;
Published: 22 June 2021.
Edited by:
Antonella Motta, University of Trento, ItalyReviewed by:
Ahmed El-Fiqi, Dankook University, South KoreaJennifer Patterson, Instituto IMDEA Materiales, Spain
Copyright © 2021 Kochhar, DeBari and Abbott. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Rosalyn D. Abbott, cmFiYm90dEBhbmRyZXcuY211LmVkdQ==
 Dakshi Kochhar
Dakshi Kochhar Megan K. DeBari
Megan K. DeBari Rosalyn D. Abbott
Rosalyn D. Abbott