- 1Department of Medical Ultrasonics, The Third Affiliated Hospital of Sun Yat-sen University, Guangdong, China
- 2Department of Ultrasound, Xiamen Chinese Medical Hospital, Fujian, China
- 3Department of Ultrasound, The First Hospital of Jilin University, Jilin, China
- 4Department of Ultrasound, West China Hospital, Sichuan, China
- 5Department of Ultrasonography, Shengli Clinical Medical College of Fujian Medical University, Fujian, China
Background and Aim: To evaluate the clinical effect of ultrasound (US)-guided percutaneous thermal ablation of hepatic focal nodular hyperplasia (FNH).
Methods: A retrospective analysis of the clinical data of patients undergoing US-guided percutaneous thermal ablation of FNH from November 2008 to August 2021 at five medical centers in China was conducted.
Results: A total of 53 patients were included (26 males and 27 females). The mean age was 35.1 ± 10.8 years. Sixty-five lesions (46 solitary cases and 7 cases with multiple lesions) were included, 70.8% (46/65) of which were located in the right liver lobe. The mean tumor length was 2.9 ± 1.5 cm. All patients successfully completed the ablation treatment. Immediate postoperative imaging showed that the primary technical success rate was 94.3% (50/53). Two patients underwent ablation 3 and 6 months after the primary ablation, and the secondary technical success rate was 100% (2/2). The incidence of complications was 3.8% (2/53). Imaging follow-up was conducted regularly after ablation, and no residual lesion enlargement or tumor recurrence was observed during the follow-up period. The technique efficacy rate was 98.1% (52/53).
Conclusion: US-guided percutaneous thermal ablation is a safe and effective treatment for FNH of the liver.
Introduction
Focal nodular hyperplasia (FNH) is the second most common benign liver tumor in adults, with a prevalence of 0.3–3% in the general population (Oldhafer et al., 2020; Zhu et al., 2021). The exact mechanism of FNH is still unclear, but most scholars agree that nonneoplastic hyperplasia of hepatic parenchyma, which can be caused by anomalous aortic vascularization, secondary thrombosis, reactive hyperplasia after hepatocellular injury caused by vasculitis, or abnormal blood perfusion, may develop into FNH (Towbin et al., 2011; Franchi-Abella et al., 2013; Zarfati et al., 2020; Yao et al., 2021). Since FNH is usually asymptomatic and there have been no reports of malignant progression of this disease, conservative observation should be considered first. However, clinical treatment should be considered if the diagnosis is unclear, the patient has symptoms or the lesion becomes enlarged during follow-up (Perrakis et al., 2017; Jung et al., 2019).
Surgical resection and transarterial embolization (TAE) are the common treatment methods for FNH. Surgical resection has consistently been considered the preferred treatment, but it may cause greater damage and has a moderate incidence of complications and postoperative mortality (Virgilio and Cavallini, 2018), which may cause concerns for some patients. TAE can be applied to patients who are ineligible for resection and in cases where it is desirable to preserve the normal liver parenchyma. It is also commonly used to reduce the volume of lesions and control pain before surgery (Amesur et al., 2009; Arts et al., 2010; Virgilio and Cavallini, 2018), but TAE also has the risk of residual (Zhang et al., 2017) and increased radiation exposure.
Thermal ablation, a minimally invasive approach, has been widely applied in the treatment of small hepatocellular carcinoma and other solid tumors. Its advantage lies in its curative effect, minimal invasion and lighter economic burden. Theoretically, thermal ablation can also induce curative effects in FNH patients, but there have been few reports (Hedayati et al., 2010; Yao et al., 2021) about using ablation for treating FNH patients.
Therefore, this study aims to analyze the efficacy of US-guided thermal ablation for FNH by assessing clinical data of FNH patients from five medical centers.
Materials and Methods
Patients
The study was approved by the Ethical Review Board of the Third Affiliated Hospital of Sun Yat-sen University. We performed a retrospective analysis of patient data using uniform data tables in five medical centers in China. The inclusion criteria for the study were as follows: 1) a diagnosis of FNH confirmed by pathological biopsy, or typical imaging characteristics (Figures 1–3) shown by contrast-enhanced ultrasound (CEUS) and hepatic magnetic resonance imaging (MRI); and 2) application of US-guided percutaneous thermal ablation (Figure 4A). The exclusion criteria were as follows: 1) application of thermal ablation combined with other treatments, such as surgical resection or TAE, on lesions diagnosed as FNH; and 2) lack of postoperative follow-up. The basic information included age, sex, and the number, location, and size of the lesions, and biopsy results, and information related to the ablation procedure was collected.
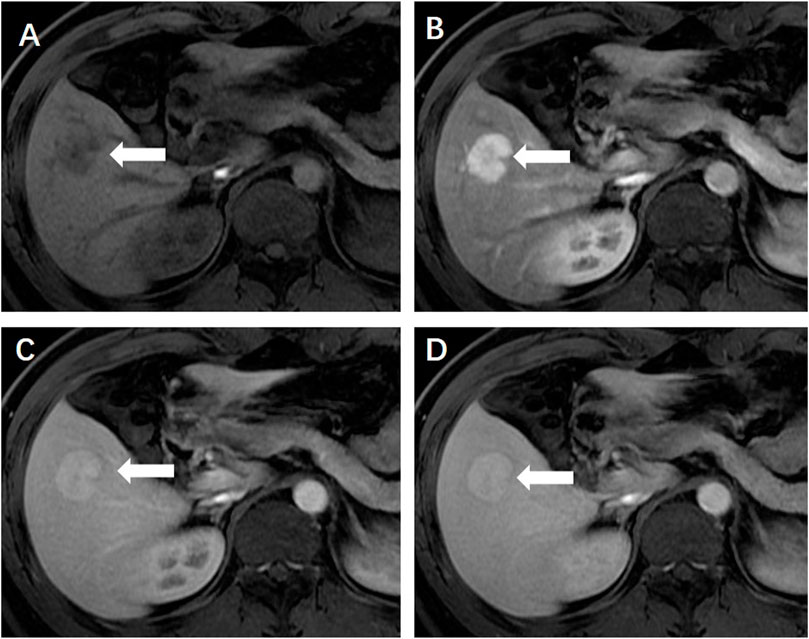
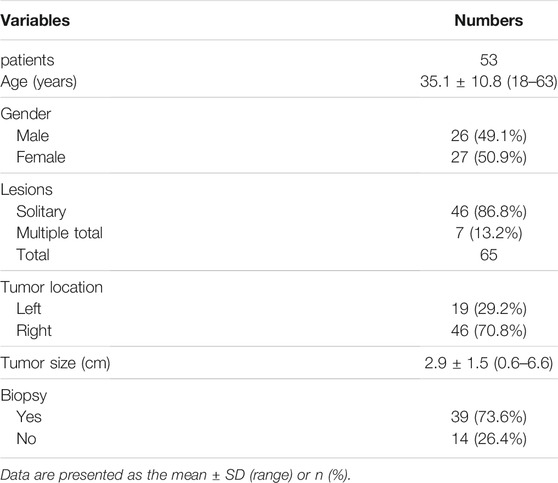
FIGURE 1. (A) Enhanced magnetic resonance imaging revealed a solid focal liver lesion (←) which showed hyperenhancement in the arterial phase (B) and isointensity in the portal venous (C) and delayed phase (D).
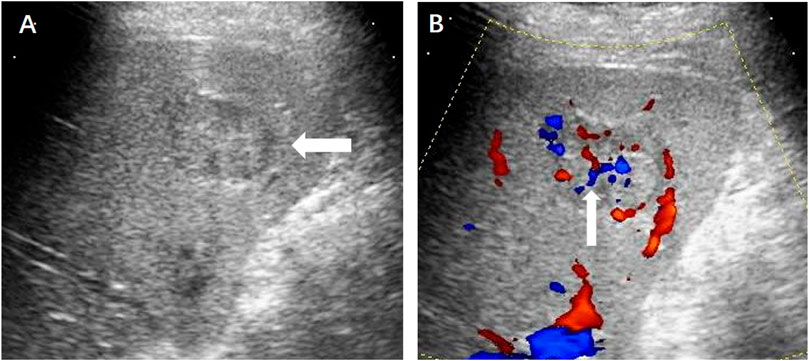
FIGURE 2. (A) Ultrasound revealed a hypoechoic hepatic lesion (←). (B) The lesion had a characteristic radiant blood flow (↑) shown by Color Doppler ultrasound.
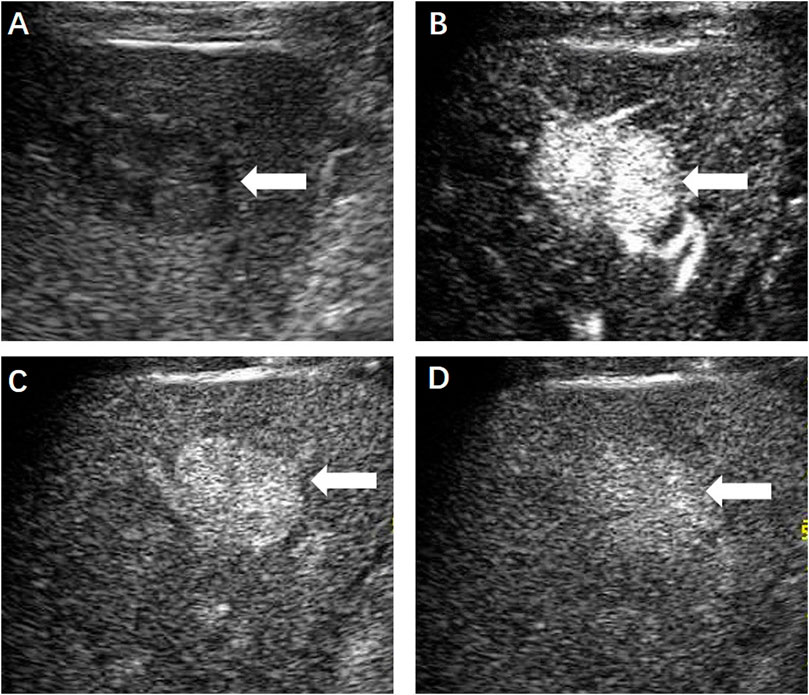
FIGURE 3. (A) Contrast-enhanced ultrasound displayed a hypoechoic hepatic lesion (←) which showed obvious hyperenhancement in the arterial phase (B) and slightly enhancement in the portal (C) and delayed phase (D).
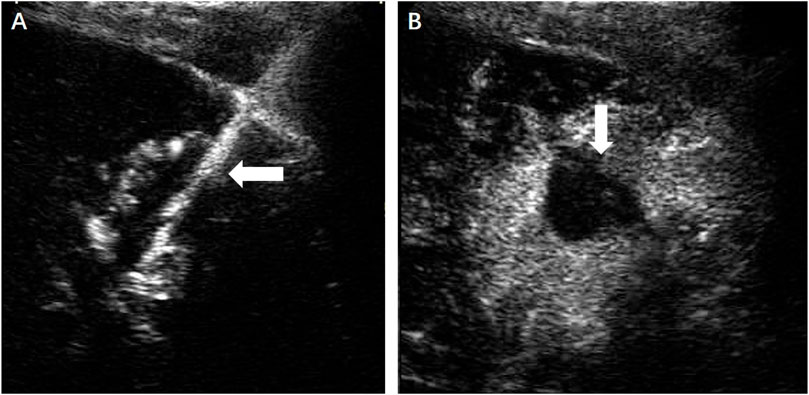
FIGURE 4. (A) Under the guidance of contrast-enhanced ultrasound, the microwave antenna (←) was inserted directly to the lesion. (B) No enhancement of the ablation zone (↓) could be seen in the arterial phase.
Assessment of Therapeutic Efficacy and Follow-Up
The efficacy of thermal ablation was evaluated by US, computed tomography (CT) or MRI. Technical success was defined as the absence of blood supply in the ablation zone assessed by CEUS immediately after the treatment (Figure 4B) and the first postoperative day. After discharge, imaging follow-up was conducted regularly among all the patients until September 2021. Technique efficacy was defined based on a lack of enhancements of the primary lesion seen on enhanced-CT or MRI (Figure 5) at least 1 month after ablation.
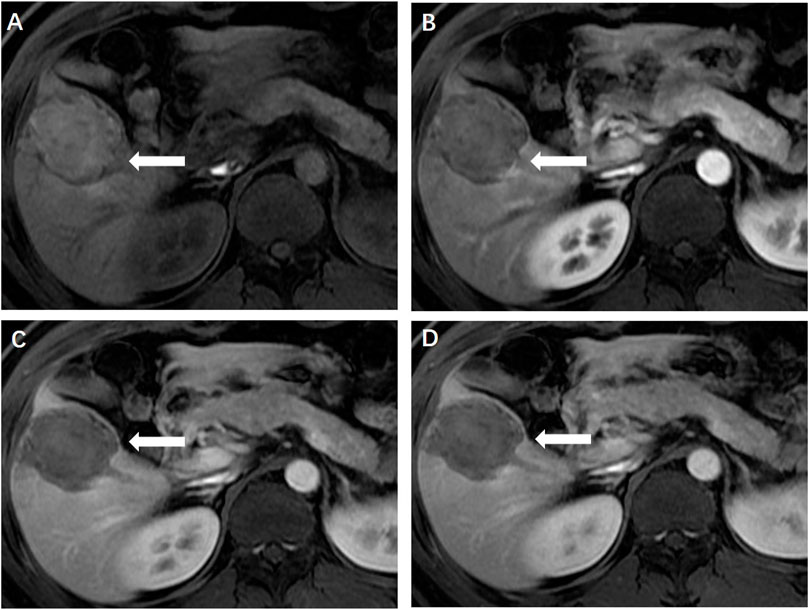
FIGURE 5. (A) Magnetic resonance imaging 1 month later showed the ablation zone was isointensity (←) and had no enhancement in the arterial (B), portal venous (C) and delayed phase (D), indicating completely ablation of the lesion.
Results
Patient Profiles
A total of 53 patients were enrolled between November 2008 and August 2021. The baseline characteristics of the patients are presented in Table 1.
Operation and Outcomes
All the patients underwent US-guided percutaneous thermal ablation, including 31 patients who received microwave ablation (MWA), 20 patients who received radiofrequency ablation (RFA) and two patients who received RFA combined with MWA. Artificial ascites (15/53), one-lung ventilation (2/53) and laparoscopy (3/53) were used for assistance. All patients completed the ablation treatment successfully. Immediate postoperative CEUS showed that the ablation zone covered the initial lesions in 50 cases, which indicated that the primary technical success rate was 94.3% (50/53). Partial residual lesions were seen in three patients, two of whom underwent secondary ablation 3 and 6 months after the first procedure. The secondary technical success rate was 100% (2/2).
Complications
The incidence of complications was 3.8% (2/53). One patient had intraoperative needle tract bleeding, which could be controlled by ablation. The other patient developed acute renal failure. After proper specialized treatment, the patient was discharged 1 month later. The rest of the patients did not experience severe complications. Some of the patients (24.5%, 13/53) had minor adverse reactions such as pain, slightly elevated aminotransferase levels and discomfort in the abdomen after ablation, which spontaneously resolved in a short time.
Follow-Up
After ablation, all the patients were regularly followed up with imaging tests including US, CT or MRI. The residual part of the lesion in one patient showed no obvious changes on 1-month CEUS and 3-month MRI, so the patient did not undergo secondary ablation. The rest of the patients showed no evident residual lesions or tumor recurrence during the follow-up period. The technique efficacy rate was 98.1% (52/53). Furthermore, long-term complications related to ablation did not occur in any patient.
Discussion
Thermal ablation, including RFA and MWA, is a major radical method for hepatocellular carcinoma and liver metastasis. A retrospective cohort study (Wang et al., 2016) of 221 patients analyzed the efficacy of MWA for liver cancer, with 90.95% of patients achieving initial complete ablation at the technical evaluation 1 month after surgery. You et al. used thermal ablation to treat 85 liver tumors, and the technical success rate was 100% (You et al., 2021). One instance of a residual lesion was found by CECT/CEMRI 1 month after surgery, and the technique efficacy rate was 98.8%. Technically successful ablation can provide 50–70% 5-year overall survival for very early and early HCC patients (Meloni et al., 2021). FNH is characterized as benign in nature but has abundant arterial blood supply, compared with liver cancer. Technically, thermal ablation can also be considered when clinical treatment is indicated in FNH patients. Hedayati et al. reported the first case of FNH treated with RFA in 2010 (Hedayati et al., 2010). The patient was a 21-year-old female with a history of progressive right upper abdominal pain. The size of the lesion was 2.2 cm and RFA was completed under the guidance of CT. Although a small nodular area of rim enhancement was noted on the follow-up CT 2 months later, the patient’s symptoms were significantly relieved. Overall, the treatment was considered successful. In our study, all patients were treated with US-guided percutaneous thermal ablation with a primary technical success rate of 93.9%, and two patients also achieved technical success after secondary ablation. Only one patient decided not to undergo secondary ablation. Most lesions were discovered accidentally, and only a few patients had obvious symptoms, which were significantly relieved after thermal ablation. During the follow-up period, there were no clear residual lesions or tumor recurrence in any patients except in one patient who did not achieve technical success after the first ablation. The overall technique efficacy rate was 98.1% (52/53). Our results suggest that, similar to the situation in liver cancer, thermal ablation for FNH can also achieve curative effects. In addition, our study is the first retrospective analysis of multicenter US-guided thermal ablation for FNH patients, and the results also provide a theoretical basis for prospective studies in the future.
For patients with unresectable lesions or who are ineligible for surgery, TAE is an alternative treatment. In 2013, Birn et al. reported a total of 17 lesions in 12 patients with FNH treated with TAE (Birn et al., 2013). The symptoms were completely relieved in seven patients and partially relieved in five patients after embolization. Only five of the 17 lesions were completely embolized based on a comparison with the lesion appearance on preoperative imaging. Virgilio et al. summarized 17 studies on the use of TAE in the treatment of FNH (Virgilio and Cavallini, 2018). A total of 128 patients received effective treatment, and each patient received TAE at least once. Local recurrence was found in only one of the treated patients during 54 months of follow-up. Although TAE is an effective treatment for FNH, there is a risk of increased radiation exposure in some specific patients, such as children. Our team reported a case of a 9-year-old girl who was diagnosed with FNH and treated by thermal ablation (Yao et al., 2021). The maximum diameter of the lesion was 2.9 cm, and it was treated by US-guided microwave ablation. Both the immediate postoperative CEUS and 1-month MRI after ablation showed that the lesion had been completely ablated. Similarly, in this study, 97.9% of the patients achieved complete remission of symptoms after one or two ablations and no recurrence of lesions was found during the current follow-up period. These findings indicated that thermal ablation, which is also a minimally invasive technique, can achieve therapeutic effects comparable to TAE and a lower residual lesion rate. Moreover, US-guided percutaneous thermal ablation has the advantage of no radiation exposure and may be more suitable for children or patients who want to reduce the risk of radiation exposure.
In terms of safety, complications of surgical resection mainly include intraoperative and postoperative bleeding, biliary fistula, intestinal obstruction, etc., with a mortality rate of approximately 2%. Although it is currently the preferred treatment for FNH, some scholars (Virgilio and Cavallini, 2018) believe that surgical resection-related complications and mortality are serious and unacceptable for the treatment of benign diseases. The common complications of thermal ablation for liver cancer mainly include subcapsular hematoma, abdominal skin burn and pleural effusion (Spiliotis et al., 2021). These complications may also occur during FNH treatment. However, in this study, only two patients had complications. One patient was found to have needle tract bleeding during the operation, and timely treatment with ablation stopped the bleeding. The other patient developed acute renal failure after surgery, which was relieved by specialized treatment. The rest of the patients did not develop severe complications. Some patients had postoperative adverse reactions, such as wound pain, mildly elevated aminotransferase levels, and discomfort in the upper abdomen, but these symptoms spontaneously resolved in a short time. Similar to thermal ablation, TAE is a relatively safe treatment. Postembolization syndrome, which is characterized by fever, loss of appetite, abdominal pain, low-grade fever, or nausea, is a common complication of TAE, but the symptoms are transient and self-limited (Virgilio and Cavallini, 2018). There is no literature comparing the safety of TAE and thermal ablation. The results of this study indicate that thermal ablation is a safe treatment with less trauma and a lower complication rate than surgical resection. Compared with that of TAE, the safety of thermal ablation is not significantly lower.
However, there are some limitations in this study. 1) Our study is a retrospective study, and only 53 cases were included. If thermal ablation is to be widely applied in the clinical treatment of FNH patients, more cases are needed to prove its effectiveness and safety. 2) This study only included patients treated with thermal ablation and failed to compare them with patients treated with surgical resection or TAE; as such, the results are not sufficiently convincing.
In general, US-guided percutaneous thermal ablation is an effective and safe treatment, and can be used as a radical treatment for FNH patients with appropriate treatment indications.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.
Author Contributions
SW and KL were responsible for design of the study. XY and KL drafted, interpretated and reviewed the manuscript. JC, QL, DZ and SW performed the data analysis. All authors read and approved the final manuscript.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors, and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Amesur, N., Hammond, J. S., Zajko, A. B., Geller, D. A., and Gamblin, T. C. (2009). Management of Unresectable Symptomatic Focal Nodular Hyperplasia with Arterial Embolization. J. Vasc. Interv. Radiol. 20 (4), 543–547. doi:10.1016/j.jvir.2009.01.001
Arts, C. H. P., van Hillegersberg, R., de Kort, G. A. P., and Moll, F. L. (2010). Inferior Caval Vein Thrombosis Owing to Compression of Focal Nodular Hyperplasia: Surgical Resection after Shrinkage by Hepatic Artery Embolization. Vascular 18 (1), 53–58. doi:10.2310/6670.2009.00030
Birn, J., Williams, T. R., Croteau, D., Schwartz, S., Sturza, S., and Getzen, T. (2013). Transarterial Embolization of Symptomatic Focal Nodular Hyperplasia. J. Vasc. Interv. Radiol. 24 (11), 1647–1655. doi:10.1016/j.jvir.2013.07.012
Franchi-Abella, S., Branchereau, S., Paulette, B., and Bioulac-Sage, P. (2013). Benign Hepatocellular Tumors in Children: Focal Nodular Hyperplasia and Hepatocellular Adenoma. Int. J. Hepatol. 2013, 215064–215111. doi:10.1155/2013/215064
Hedayati, P., VanSonnenberg, E., Shamos, R., Gillespie, T., and McMullen, W. (2010). Treatment of Symptomatic Focal Nodular Hyperplasia with Percutaneous Radiofrequency Ablation. J. Vasc. Interv. Radiol. 21 (4), 582–585. doi:10.1016/j.jvir.2009.12.385
Jung, J.-M., Hwang, S., Kim, K.-H., Ahn, C.-S., Moon, D.-B., Ha, T.-Y., et al. (2019). Surgical Indications for Focal Nodular Hyperplasia of the Liver: Single-Center Experience of 48 Adult Cases. Ann. Hepatobiliary Pancreat. Surg. 23 (1), 8. doi:10.14701/ahbps.2019.23.1.8
Meloni, M. F., Francica, G., Chiang, J., Coltorti, A., Danzi, R., and Laeseke, P. F. (2021). Use of Contrast‐Enhanced Ultrasound in Ablation Therapy of HCC: Planning, Guiding, and Assessing Treatment Response. J. Ultrasound Med. 40 (5), 879–894. doi:10.1002/jum.15471
Oldhafer, K. J., Habbel, V., Horling, K., Makridis, G., and Wagner, K. C. (2020). Benign Liver Tumors. Visc. Med. 36 (4), 292–303. doi:10.1159/000509145
Perrakis, A., Vassos, N., Grützmann, R., and Croner, R. S. (2017). What Is Changing in Indications and Treatment of Focal Nodular Hyperplasia of the Liver. Is There Any Place for Surgery? Ann. Hepatol. 16 (3), 333–341. doi:10.5604/16652681.1235475
Spiliotis, A. E., Gäbelein, G., Holländer, S., Scherber, P., Glanemann, M., and Patel, B. (2021). Microwave Ablation Compared with Radiofrequency Ablation for the Treatment of Liver Cancer: A Systematic Review and Meta-Analysis. Radiol. Oncol. 55 (3), 247–258. doi:10.2478/raon-2021-0030
Towbin, A. J., Luo, G. G., Yin, H., and Mo, J. Q. (2011). Focal Nodular Hyperplasia in Children, Adolescents, and Young Adults. Pediatr. Radiol. 41 (3), 341–349. doi:10.1007/s00247-010-1839-8
Virgilio, E., and Cavallini, M. (2018). Managing Focal Nodular Hyperplasia of the Liver: Surgery or Minimally-Invasive Approaches? A Review of the Preferable Treatment Options. Anticancer Res. 38 (1), 33–36. doi:10.21873/anticanres.12188
Wang, T., Lu, X., Chi, J., Ding, M., Zhang, Y., Tang, X., et al. (2016). Microwave Ablation of Hepatocellular Carcinoma as First-Line Treatment: Long Term Outcomes and Prognostic Factors in 221 Patients. Sci. Rep-Uk 6 (1). doi:10.1038/srep32728
Yao, Z., Zeng, Q., Yu, X., Lin, S., Jiang, S., Ma, D., et al. (2021). Case Report: Ultrasound-Guided Percutaneous Microwave Ablation of Focal Nodular Hyperplasia in a 9-Year-Old Girl. Front. Pediatr. 9, 710779. doi:10.3389/fped.2021.710779
You, Y., Zhang, M., Li, K., Zeng, Q., Luo, L., Long, Y., et al. (2021). Feasibility of 3D US/CEUS-US/CEUS Fusion Imaging-Based Ablation Planning in Liver Tumors: a Retrospective Study. Abdom. Radiol. 46 (6), 2865–2874. doi:10.1007/s00261-020-02909-5
Zarfati, A., Chambers, G., Pio, L., Guerin, F., Fouquet, V., Franchi-Abella, S., et al. (2020). Management of Focal Nodular Hyperplasia of the Liver: Experience of 50 Pediatric Patients in a Tertiary Center. J. Pediatr. Surg. 55 (9), 1885–1891. doi:10.1016/j.jpedsurg.2020.01.009
Zhang, G., Wang, M., Duan, F., Yuan, K., Li, K., Yan, J., et al. (2017). Transarterial Embolization with Bleomycin for Symptomatic Hepatic Focal Nodular Hyperplasia. Diagn. Interv. Radiol. 23 (1), 66–70. doi:10.5152/dir.2016.16061
Keywords: focal nodular hyperplasia, ultrasonography, radiofrequency ablation, microwave ablation, thermal ablation
Citation: Yu X, Chang J, Zhang D, Lu Q, Wu S and Li K (2022) Ultrasound-Guided Percutaneous Thermal Ablation of Hepatic Focal Nodular Hyperplasia——A Multicenter Retrospective Study. Front. Bioeng. Biotechnol. 9:826926. doi: 10.3389/fbioe.2021.826926
Received: 01 December 2021; Accepted: 20 December 2021;
Published: 06 January 2022.
Edited by:
Yuce Li, Sungkyunkwan University, South KoreaReviewed by:
Shixian Lv, Peking University, ChinaJunchao Wei, Nanchang University, China
Jinjin Chen, Tufts University, United States
Copyright © 2022 Yu, Chang, Zhang, Lu, Wu and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Kai Li, bGlrYWlAbWFpbC5zeXN1LmVkdS5jbg==; Songsong Wu, NDY1MjY5ODk4QHFxLmNvbQ==
†These authors have contributed equally to this work and share first authorship
 Xuan Yu
Xuan Yu Jiandong Chang2†
Jiandong Chang2† Dezhi Zhang
Dezhi Zhang Qiang Lu
Qiang Lu Kai Li
Kai Li