- 1Cancer Center, Dongguan Engineering Research Center for Innovative Boron Drugs and Novel Radioimmune Drugs, The 10th Affiliated Hospital of Southern Medical University, Southern Medical University, Guangzhou, China
- 2Guangdong Engineering Research Center of Boron Neutron Therapy and Application in Malignant Tumors, The 10th Affiliated Hospital of Southern Medical University, Southern Medical University, Guangzhou, China
In clinical practice, the management of most non-surgically resectable solid tumors necessitates a multidisciplinary treatment approach. Optimal solutions involve the integration of local and systemic treatments, such as targeted immunotherapy and chemotherapy. Micron-sized radioactive microspheres or particles have gained widespread application in the localized treatment of various organ tumors, encompassing liver cancer, lung cancer, tongue cancer, pancreatic cancer, head and neck cancer, ovarian cancer, bone cancer, among others. As such, the design and development of novel multifunctional radioactive microspheres constitute a crucial foundation for achieving effective local treatment in liver cancer and other cancer types. This article critically reviews the current developmental landscape, identifies challenges, and explores opportunities in the field of radioactive internal irradiation microspheres in recent years. The insights provided serve as a valuable reference for selecting and determining the developmental direction of clinical brachytherapy treatment carriers.
1 Introduction
Radioactive microspheres have emerged as a groundbreaking platform for locoregional cancer therapy, particularly in the treatment of unresectable solid tumors (Wu et al., 2023; Yang et al., 2023; Sun et al., 2025). By delivering high-dose radiation directly to tumor vasculature via intra-arterial administration, these microspheres enable precise tumor ablation while sparing surrounding healthy tissues—a principle central to brachytherapy (Filippi et al., 2020; Dong et al., 2024). Over the past decades, advancements in material science, radiochemistry, and interventional oncology have propelled radioactive microspheres into clinical practice, offering a minimally invasive alternative to conventional radiotherapy and systemic therapies for hepatocellular carcinoma (HCC), metastatic liver tumors, and other malignancies (Sun et al., 2025; Gupta et al., 2024).
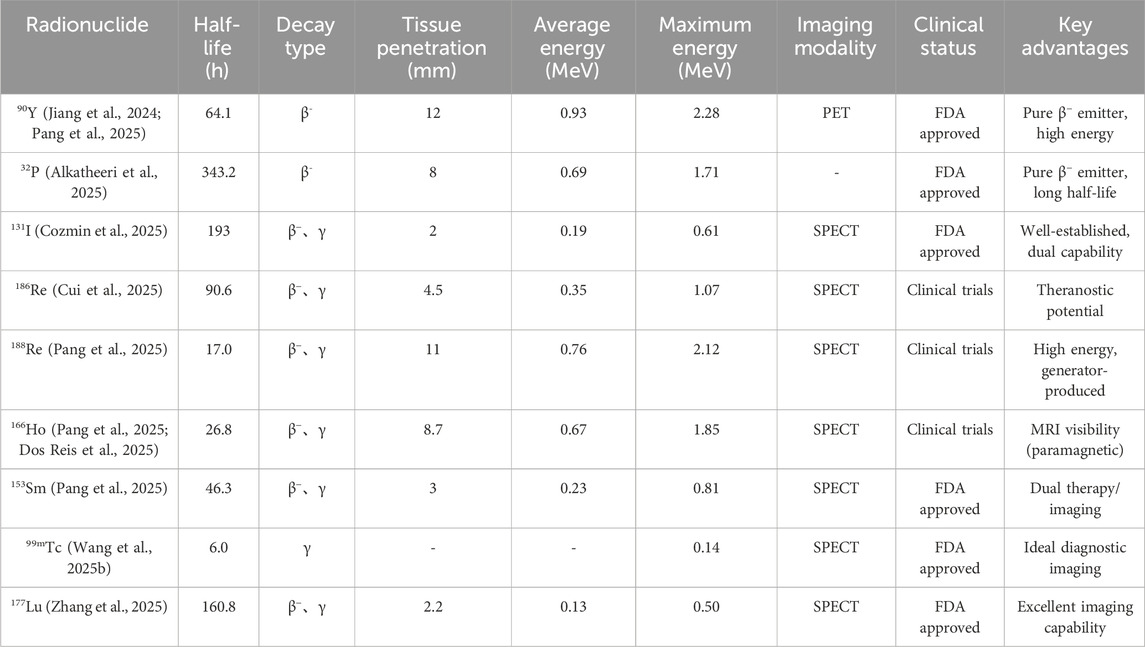
The therapeutic efficacy of radioactive microspheres hinges on two critical components: the microsphere carrier and the encapsulated radionuclide (Wang L. et al., 2025; Pang et al., 2025). An ideal microsphere must exhibit 1) mechanical robustness to withstand vascular transport (Arranja et al., 2018); 2) chemical stability to prevent radionuclide leaching (Gupta et al., 2024); 3) biocompatibility to minimize systemic toxicity (Kühnel et al., 2022); and 4) tunable degradation kinetics for repeated treatments if required (Drescher et al., 2020a). Among radionuclides, β-emitters such as yttrium-90 (90Y) and holmium-166 (166Ho) dominate clinical use due to their moderate tissue penetration (8–12 mm) and favorable half-lives (Drescher et al., 2020b; Westcott et al., 2016; Morsink et al., 2024). Notably, 166Ho and lutetium-177 (177Lu) also emit γ-rays, permitting single-photon emission computed tomography (SPECT) imaging for real-time dosimetry—a feature absent in pure β-emitters like 90Y, which relies on suboptimal bremsstrahlung imaging (Cunha et al., 2023; Xiao et al., 2023).
Despite their promise, clinical translation faces persistent challenges. First, radionuclide leakage—observed in early-generation resin-based 90Y microspheres—can lead to off-target radiation exposure (Jiang et al., 2024). Second, imaging limitations complicate post-treatment verification; for instance, 90Y’s bremsstrahlung emissions produce low-resolution SPECT images (Debebe et al., 2019; Zade et al., 2013). Third, dose heterogeneity arises from uneven microsphere distribution in tumor vasculature, necessitating improved predictive dosimetry models (Wu X. et al., 2022). Recent innovations aim to address these issues: 1) Material engineering: Glass microspheres sintered with 90Y2O3 exhibit superior 90Y retention compared to resin counterparts (Gallio et al., 2016; Filippi et al., 2018). 2) Multimodal microspheres: Designs incorporating photothermal agents (e.g., 131I-polydopamine microspheres) or immunomodulators enable combinatorial therapy (Li et al., 2018; Fiedler et al., 2018; Ehlerding et al., 2018). 3) Advanced imaging: Microspheres co-loaded with 99mTc or 166Ho allow SPECT/MRI-guided interventions (Son et al., 2021; Stella et al., 2020).
This review synthesizes the evolution of radioactive microspheres, from their physicochemical design to clinical applications, and highlights unresolved challenges in nuclear safety, imaging, and scalability. By critically evaluating recent preclinical and clinical data, we aim to outline a roadmap for next-generation theranostic microspheres in precision oncology.
2 Advances in the research of radioactive microspheres for the treatment of liver cancer
Brachytherapy using radioactive microspheres has emerged as a promising approach for unresectable liver cancer and other solid tumors (Wang L. et al., 2025; Arranja et al., 2018; Nuzulia et al., 2024). Since the pioneering work with 198Au colloids, various radionuclides—including 90Y, 32P, 131I, 188Re, 166Ho, 153Sm, and 177Lu—have been developed to optimize therapeutic efficacy and diagnostic compatibility (Figure 1; Table 1). (Souza et al., 2022; Gulec et al., 2022; Abadie et al., 2021) Key challenges persist, such as radionuclide leakage, carrier biocompatibility, and precise tumor targeting (Pang et al., 2025). Recent advancements focus on multifunctional designs combining radiotherapy with imaging modalities and adjunct therapies (chemotherapy, photothermal) (Wu et al., 2023; Xiao et al., 2023; Bellendorf et al., 2024). This section comprehensively reviews the evolution, limitations, and innovations in radioactive microspheres, highlighting their clinical translation and future directions for hepatocellular carcinoma and other malignancies treatment.
Figure 1. Properties of radioactive microspheres (Sun et al., 2025; Chen. et al., 2025).
2.1 90Y microsphere
Yttrium-90 (90Y) is a beta-emitting radioisotope used for brachytherapy in liver cancer treatment due to its 12 mm tissue penetration range (Dong et al., 2024; Christie et al., 2011; Talebi and Rajabi, 2022). Early 90Y resin microspheres faced discontinuation due to significant 90Y leaching and adverse effects. Subsequent efforts focused on mitigating leaching by incorporating stable 90Y into Yttrium oxide (Y2O3) combined with alumina and silica, followed by high-temperature melting and neutron bombardment to create 90Y glass microspheres. This process reduced toxicity associated with 90Y leakage.
The Affiliated Zhongshan Hospital of Fudan University developed 90Y glass microspheres (30–50 μm) with a density of 3.27 g/mL, which require further clinical validation (Yan et al., 1993). High density can hinder injection, and limited microsphere count may inadequately irradiate large tumors. Resin microspheres, with their lower density, offer easier injection. To address 90Y leaching from resin microspheres, an alkali precipitation step was added post-ion exchange (Lau et al., 1994). This reduced the leakage rate while maintaining safety, enabling its usage for tumors with large blood supply. However, some leaching persisted, necessitating glucose or contrast agents for dispersion (Westcott et al., 2016). Metal ions in tumor tissue contributed to leakage rates of 1/1000 to 4/1000. Amthauer et al. detected radioactivity in urine from patients treated with 90Y resin microspheres, potentially from reversible ion exchange (Grosser et al., 2018). Commercial options include SIR-Spheres® (polymer) and TheraSphere® (glass), containing 1.2 million spheres per vial at 3 GBq activity (2500 GBq/microsphere). Glass microspheres are denser than blood and resin microspheres three times as much as blood (Baino et al., 2021). Zhen et al.'s pooled analysis of 16 studies demonstrates that TARE with 90Y microspheres achieves median overall survival of 14.3 months (95% CI: 11.9-17.1) in unresectable ICC (Intrahepatic Cholangiocarcinoma), with disease control rates of 77.2% (RECIST criteria: 11.5% partial response, 61.5% stable disease). Both glass (14.0 months OS) and resin (14.3 months OS) microspheres show comparable efficacy, with predominantly mild adverse events requiring no intervention (Zhen et al., 2019).
2.2 32P microspheres
Phosphorus-32 (32P) is a pure beta-emitting radionuclide characterized by a half-life of 14.3 days and a maximum tissue penetration radius of 8 mm (Order et al., 1996; Rahman et al., 2020). These properties render 32P particularly suitable for long-distance transport and prolonged internal radiation therapy. Wallner et al. were the first to report the application of 32P colloidal chromic phosphate bound to albumin particles for brachytherapy of unresectable pancreatic tumors. However, the small particle size of 32P colloidal chromic phosphate and the high interstitial pressure of tumors may cause radioactive toxicity side effects when injected, no subsequent clinical reports using this method were seen. Masakazu Kawashita et al. from Kyoto University mixed Y2O3, Al2O3, and SiO2 and melted them at 1600°C to produce rectangular glass pieces material with a size of 1 cm in length and width, then bombarded red phosphorus with an electron beam to produce phosphorus ions (P+) and implanted them into the glass material to produce P+ rich glass material, which was finally bombarded by a high-flux neutron reactor to obtain 32P/90Y/88Y/91Y/154Eu glass microspheres (Kawashita et al., 1999). Therefore, Masakazu Kawashita from Kyoto University also successfully prepared YPO4 and Y2O3 microspheres with diameters ranging from 20 to 30 μm using a high-frequency induction heating plasma method (Kawashita, 2002). How to stably bind 32P onto microsphere carriers is an urgent problem that needs solving before applying 32P microspheres in clinical liver cancer brachytherapy treatments. Recent clinical studies demonstrate promising outcomes for 32P in pancreatic cancer treatment. A propensity-score weighted analysis of 104 LAPC patients showed that combining chemotherapy with EUS-guided 32P implantation significantly improved outcomes versus standard therapy (chemotherapy ± chemoradiotherapy). The combination therapy group exhibited 189 days longer restricted mean survival time (527.2 vs. 338.0 days), 168.6 days longer local progression-free survival, and 23.9% higher downstaging probability within 30 months. These results highlight 32P’s potential when combined with systemic therapy for localized tumor control (Lim et al., 2025).
2.3 131I microsphere
Iodine-131 (131I) is a diagnostic and therapeutic radionuclide (half-life: 8.04 days) that emits both β-rays (99%) and γ-rays (1%). SPECT imaging can be performed, and its maximum tissue range radius is 2 mm (Larson et al., 2015; Li Y. et al., 2025). In 1992, Li et al. labeled gelatin microspheres with 131I and combined them with chemotherapeutic drugs to treat nine patients with unresectable liver cancer (Chen et al., 1992). However, the microspheres caused severe ectopic embolism, leading to one fatality due to systemic 131I leakage. Chen et al. synthesized biodegradable 131I/125I dual-labeled gelatin microspheres for rabbit liver embolization and metabolism studies using the chloramine T labeling method, and found that a small amount of 131I/125I would be released into the blood along with the degradation of gelatin microspheres as well as taken up by the thyroid gland (Ma et al., 2012). In 2016, Li et al. prepared 131I-gelatin-chitosan microspheres by reverse emulsion crosslinking method and chloramine T labeling method to overcome the problem of glass microspheres being non-degradable and unable to repeat brachytherapy treatment (Cai et al., 2016). Four years later, Li et al. synthesized a novel biodegradable chitosan-collagen composite microsphere labeled with ideal sedimentation efficiency, good in vitro and in vivo stability (Pang et al., 2020). Zhang et al. prepared a kind of silk protein microsphere labeled with 131I (131I-SFMs) for brachytherapy treatment of rat liver tumors by emulsification crosslinking method combined with chloramine T labeling method (Wu M. R. et al., 2022). Song et al. developed a type of poly (lactic acid) microsphere coated with copper sulfide nanoparticles and paclitaxel labeled with 131I (131I-HCuSNPs-MS-PTX), which can be used for SPECT and photoacoustic dual-modal imaging, as well as effective chemotherapy, radioembolization therapy, and photothermal therapy (Liu et al., 2018). Clinical studies of 131I SPECT/CT in differentiated thyroid cancer (DTC) demonstrate its prognostic value for lymph node metastases (LNM). A retrospective analysis of 942 DTC patients revealed that those without LNM achieved complete response (CR) faster (median 9.4 months vs. 44 months with LNM, HR 2.2) and had better progression-free survival (PFS, HR 0.46). Patients with enlarged 131I-negative LNM showed the longest time to CR (24 months), while those with small LNM had PFS comparable to LNM-negative cases. Treatment strategies should be individualized, with reoperation preferred for enlarged LNM (13.5 months to CR) and second radioiodine therapy for small LNM (better PFS, HR 4.0) (Heinrich et al., 2025).
Recent advancements in 131I microspheres focus on multifunctional designs. Sun et al. developed dual-functional 131I-PDA@PVA microspheres combining radioembolization and photothermal therapy (Sun et al., 2025). These microspheres exhibited high stability (76.5% retention in serum), excellent elasticity, and synergistic tumor inhibition (10% cell viability) via β/γ radiation and NIR-triggered hyperthermia (ΔT > 20°C). SPECT/CT enabled real-time tracking, demonstrating clinical potential for HCC theranostics.
2.4 186Re/188Re microsphere
Rhenium-186 (186Re) and Rhenium-188 (188Re) (half-lives: 3.8 days and 0.71 days, respectively) are β- and γ-emitting radionuclides suitable for SPECT imaging with tissue range radii are 4.5 mm and 11 mm (Häfeli et al., 2007; Pourhabib et al., 2019). In 1998, Ehrhardt et al. prepared glass microspheres enriched with 186Re and 188Re by mixed ReO2 powder with glass frits and calcined them at 1050°C, then bombarded them with a neutron reactor (Conzone et al., 1998). However, the in vitro stability was poor. Hafeli et al. prepared a biodegradable 186Re/188Re polylactic acid microsphere by neutron reactor bombardment method (Häfeli et al., 1999; Häfeli et al., 2001). Subsequently, Hafeli et al. prepared 186Re/188Re fibrin glue with high adhesion strength in moist tissue by bombarding Re-enriched fibrinogen with a low-flux neutron reactor for intratumoral irradiation therapy (Häfeli et al., 2007). Mostafa et al. prepared a polylactic acid microsphere loaded with 188Re sulfide colloid nanoparticles, with a microsphere diameter range of 13–48 μm and a188Re labeling efficiency of up to 99% (Jamre et al., 2018). Lee et al. prepared a type of 188Re microsphere by mixing 188Re (OH2)3 (CO)3+, which was formed by the interaction of amino borane reduction of 188ReO4 and carbon monoxide, with human serum albumin microspheres, with a diameter of 13–48 μm, for the treatment of in situ hepatocellular carcinoma in rats (Ni et al., 2015), Saatchi et al. mixed polylactic acid containing dimethylpyridine amine with polycaprolactone as the dispersed phase and used microfluidic technology to prepare a type of mixed biodegradable microsphere for chelating labeling of 188Re (De La Vega et al., 2019). A Phase 1 trial (NCT01906385) evaluated 186Re-nanoliposomes (186RNL) delivered via convection-enhanced delivery in 21 recurrent glioblastoma patients. The maximum tolerated dose was not reached (up to 22.3 mCi), with most adverse events unrelated to treatment. Median overall survival was 11 months (17 months for patients receiving >100 Gy tumor absorbed dose vs. 6 months for <100 Gy), exceeding standard care outcomes. Disease control was achieved in 61.9% of patients (57.1% stable disease, 4.8% partial response), demonstrating 186Re’s potential for targeted brain tumor therapy (Brenner et al., 2025).
2.5 166Ho microsphere
Holmium-166 (166Ho) possesses a half-life of 1.1 days, a maximum tissue range of 8.7 mm, and emits both β-rays and γ-rays (81 keV, 62%), making it a diagnostic-therapeutic nuclide for SPECT and magnetic resonance dual-modal imaging (Kühnel et al., 2022; Seevinck et al., 2012; Vente et al., 2014). Nijsen et al. prepared polylactic acid microspheres by solvent evaporation method, then added non-radioactive acetylacetone complexed 165Ho compound (165Ho-acetylacetone) to bind with polylactic acid microspheres (Nijsen et al., 1999; Nijsen et al., 2001). They filtered out microspheres with a particle size range of 20-50 μm through a filter screen and finally obtained 166Ho-acetylacetone-polylactic acid microspheres by bombarding them with a neutron reactor for 6 h. Under Good Manufacturing Practice (GMP) guidelines, Nijsen et al. optimized the evaporation temperature, sieving, and raw material selection in the solvent evaporation process to achieve gram-scale microsphere production. (Zielhuis et al., 2006). To improve the specific activity and 166Ho stability of 166Ho microspheres in vivo, Nijsen et al. formed two kinds of inorganic-like 166Ho microspheres (166HoPO4 microspheres and 166Ho(OH)3 microspheres) by ion exchange and neutron reactor irradiation of solid acetylacetone holmium microspheres with NaH2PO4 or NaOH (Arranja et al., 2020). A prospective phase 2 study (NCT03379844) in 31 HCC patients demonstrated the clinical utility of 166Ho-microspheres radioembolization. Hepatobiliary scintigraphy revealed significant reductions in treated liver volume (17%, p = 0.0027) and function (16%, p = 0.0017), with cirrhotic patients showing limited functional compensation (10% decrease vs. 0% in non-cirrhotics). The technique effectively maintained liver function in Child Pugh ≤ B7 patients (median MELD 9), with hepatic clearance rates correlating with biochemical markers (bilirubin, albumin, ALT; p < 0.05), supporting its safety profile in selected HCC populations (Reinders et al., 2025).
2.6 153Sm microsphere
Samarium-153 (153Sm) is a radioactive nuclide that can release both β-rays with a maximum energy of 810 keV and γ-rays (103 keV), making it suitable for clinical SPECT imaging and radiotherapy (Wang et al., 2020; Bayouth et al., 1994; Wong et al., 2023). Bai et al. prepared a novel 153Sm-labeled biodegradable polylactide microsphere and studied its stability in rabbits. Yeong et al. labeled 152Sm on negatively charged acrylic microspheres by electrostatic adsorption then treated them with sodium carbonate solution for alkaline precipitation, and finally irradiated them with a neutron reactor for 6 h to obtain 153Sm microspheres (Wong et al., 2019). Subsequently, Yeong et al. used a solid-phase-oil-water solvent evaporation method to prepare 152Sm2O3 microspheres, which were then irradiated with a neutron reactor to obtain 153Sm microspheres with an average particle size of 33 μm, and no longer-lived radioactive nuclides or elemental impurities were found (Tan et al., 2022). Ying et al. also developed a biodegradable 152Sm-acetylacetone-polylactide microsphere for intra-arterial radioembolization therapy of liver tumors (Wong et al., 2020). Recent advances in 153Sm microspheres demonstrate their potential for combined chemo-radioembolization. Researchers successfully developed sulphonated polystyrene microspheres (31.95 ± 0.26 μm) co-loaded with [153Sm]Sm2O3 (2.82 ± 0.6 GBq/g) and doxorubicin (55.6% ± 1.1% encapsulation efficiency). These theranostic microspheres showed excellent radionuclide retention (>99% over 300 h) in physiological conditions with no detectable impurities after neutron activation. The formulation meets key requirements for intraarterial liver cancer treatment, offering simultaneous chemotherapy and radiotherapy delivery while maintaining optimal physicochemical properties for clinical translation (Wong et al., 2025).
2.7 Other radioactive microspheres
Ytterbium-175 (175Yb) is a radioactive isotope for studying in vivo biodistribution. In addition, Lutetium-177 (177Lu) is the metal nuclide for clinical diagnosis and treatment of diseases (O'Neill et al., 2020; Jamre et al., 2019). Shamsaei et al. designed and developed a novel biodegradable 175Yb-labeled polylactide microsphere for intratumoral irradiation radiotherapy embolization (Jamre et al., 2019). Gao et al. prepared a177Lu silica microsphere that can be used for SPECT imaging by directly physically mixing 177LuCl3 solution with mesoporous silica microspheres and then performing alkali precipitation, and directly used it for intratumoral injection therapy of tumor-bearing mice and achieved good anti-tumor efficacy (Wu X. et al., 2022).
Recent advancements in 177Lu-based therapies include the development of injectable 3D hollow porous granular hydrogels (177Lu-3D-HPGH) for precise brachytherapy (Xu et al., 2023). Synthesized via microfluidics and UV cross-linking, these hydrogels, developed by Xu et al., demonstrate high radiolabeling efficiency (97.85%), uniform tumor distribution, and robust anti-tumor efficacy in preclinical models, offering a promising theranostic platform for HCC treatment. Zhao et al. engineered 177Lu-PCMs using radiation-induced graft polymerization (Zhao et al., 2024). These phosphocholine-modified microspheres demonstrated ultra-stable Lu coordination (DFT-confirmed chelation), mechanical robustness (117.2 μm size), and precise tumor targeting in rabbit VX2 models. SPECT/CT-guided intra-arterial brachytherapy achieved complete tumor regression without ectopic leakage, highlighting translational potential for image-guided HCC treatment.
Innovative radio-immunotherapy approaches have emerged. Yang et al. created 177Lu-labeled alginate microspheres co-loaded with IDO1 inhibitor Indoximod (Yang et al., 2023). The 2 μm microspheres achieved >90% labeling efficiency, suppressed kynurenine pathways, and enhanced CD8+ T-cell infiltration. Combined with αPD-L1, they inhibited distal tumors in H22 models via DC maturation and Treg downregulation, showcasing a promising immunomodulatory platform.
In summary, most traditional radioactive microspheres are prepared by “cold” microspheres using methods such as melt spraying, solvent evaporation, inverse emulsion cross-linking, and alkali precipitation. Finally, they all need to be bombarded by high-flux neutron reactors to activate the “cold” microspheres into radioactive microspheres.
3 Future prospect of clinical application of radioactive microspheres
Compared with conventional therapies, radioactive microspheres offer distinct advantages and limitations. Versus TACE, microspheres provide more sustained radiation exposure (weeks vs. days) with better tumor penetration but require specialized nuclear facilities (Nuzulia et al., 2024; Welling et al., 2023). Relative to systemic therapies (sorafenib, lenvatinib), they demonstrate higher local control rates with fewer systemic side effects, though lack distant disease control (Nuzulia et al., 2024).
90Y microspheres are representative example of radioactive microspheres to analyze a series of clinical problems. The range of TACE and brachytherapy will no longer be limited to HCC with the development of technology. For instance, in bone cancer treatment, radionuclide-doped hydroxyapatite microspheres serve as bone graft scaffolds, where beta emitters deliver localized high-dose radiation to kill cancer cells. And hydroxyapatite microspheres can also promote bone tissue growth and regeneration as scaffolds (Nuzulia et al., 2024). Whether it is innovation based on material or radioactive elements, the area of application and the route of treatment will be greatly developed in the future. By the delivery of efficient drug and targeted delivery, a lot of non-radioactive microspheres have been combined many immunotherapies, such as boron neutron capture therapy (BNCT) and photodynamic therapy (PDT).
Emerging technologies like SHIFT (Superstable Homogeneous Iodinated Formulation) are reshaping brachytherapy. Chen et al. developed radiolipiodol via CO2 supercritical fluid, achieving ≥99% labeling efficiency and > 2-week tumor retention in preclinical models (Chen et al., 2025). Clinical trials confirmed minimal 18F leakage (T/N ratio ≥50), offering a paradigm shift in stable nuclide encapsulation for precision HCC therapy. Regulatory pathways for novel agents (e.g., 177Lu) face challenges: FDA requires standardized dosimetry protocols, while EMA mandates comparative efficacy data. Centralized 177Lu production could reduce costs versus decentralized 90Y systems. Practical adoption hinges on streamlining supply chains and establishing clear criteria for combination therapies (Pang et al., 2025).
Looking forward, radioactive microspheres can potentially treat cancers efficiently through three key research directions: 1) Personalized dosimetry optimization using AI-based tumor perfusion analysis to predict microsphere distribution patterns (Dong et al., 2025); 2) Development of “smart” microspheres with stimuli-responsive drug release (pH, enzyme, or temperature-activated) for precision combination therapies (Li J. et al., 2025); and 3) Standardization of radio-immunotherapy protocols combining PD-1/PD-L1 inhibitors with radionuclides to enhance abscopal effects (Li et al., 2024). Critical technological gaps remain in real-time intraprocedural dosimetry systems and scalable manufacturing of multifunctional microspheres. The integration of theranostic radionuclides with advanced biomaterials may enable truly personalized treatment regimens based on tumor molecular profiling. These innovations could revolutionize locoregional cancer therapy while maintaining manageable toxicity profiles.
4 Conclusion
Radioactive microspheres have emerged as a pivotal modality in the locoregional treatment of non-surgically resectable solid tumors, particularly in hepatocellular carcinoma and other malignancies. This review delineates the evolution, challenges, and future directions of these innovative therapeutic agents. The principle of delivering high-dose radiation directly to tumor vasculature allows for effective tumor ablation while preserving surrounding healthy tissues, a core aspect of brachytherapy. Key advancements in material science and radiochemistry have enabled the design of multifunctional microspheres that combine therapeutic and imaging capabilities. For instance, radionuclides such as 90Y and 166Ho have shown clinical promise due to their optimal tissue penetration and compatibility with imaging modalities like SPECT. Nevertheless, challenges such as radionuclide leakage, imaging limitations, and dose heterogeneity remain, necessitating enhanced predictive dosimetry models and improved material formulations. Recent innovations include engineered glass microspheres that mitigate leakage and designs integrating targeted drug delivery systems, such as radio-immunotherapy approaches. As technological advancements continue, the landscape of radioactive microspheres is set to expand significantly, fostering new applications beyond traditional cancer therapies and enhancing the precision of locoregional management strategies. Thus, ongoing research into optimizing these therapeutic platforms is crucial for advancing the field of precision oncology.
Author contributions
XJ: Investigation, Writing – original draft, Writing – review and editing. LC: Investigation, Writing – review and editing. XX: Conceptualization, Supervision, Writing – review and editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This review was supported by the Guangdong Province Basic and Applied Basic Research Fund Special Project (Regional Cultivation Project) (No. 2023A1515140032) and the Open Fund of the China Spallation Neutron Source Songshan Lake Scientific City (No. KFKT2023A02).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Abadie, P., Hesse, M., Louppe, A., Lhommel, R., Walrand, S., and Jamar, F. (2021). Microspheres used in liver radioembolization: from conception to clinical effects. Molecules 26 (13), 3966. doi:10.3390/molecules26133966
Alkatheeri, A., Salih, S., Kamil, N., Alnuaimi, S., Abuzar, M., and Abdelrahman, S. S. (2025). Nano-radiopharmaceuticals in Colon cancer: current applications, challenges, and future directions. Pharm. (Basel) 18 (2), 257. doi:10.3390/ph18020257
Arranja, A. G., Hennink, W. E., Chassagne, C., Denkova, A. G., and Nijsen, J. F. W. (2020). Preparation and characterization of inorganic radioactive holmium-166 microspheres for internal radionuclide therapy. Mater Sci. Eng. C Mater Biol. Appl. 106, 110244. doi:10.1016/j.msec.2019.110244
Arranja, A. G., Hennink, W. E., Denkova, A. G., Hendrikx, R. W. A., and Nijsen, J. F. W. (2018). Radioactive holmium phosphate microspheres for cancer treatment. Int. J. Pharm. 548 (1), 73–81. doi:10.1016/j.ijpharm.2018.06.036
Baino, F., Fiume, E., Ciavattini, S., Kargozar, S., Borges, R., Genova, L. A., et al. (2021). Biomedical radioactive glasses for brachytherapy. Mater. (Basel) 14 (5), 1131. doi:10.3390/ma14051131
Bayouth, J. E., Macey, D. J., Kasi, L. P., and Fossella, F. V. (1994). Dosimetry and toxicity of samarium-153-EDTMP administered for bone pain due to skeletal metastases. J. Nucl. Med. 35 (1), 63–69.
Bellendorf, A., Mader, N., Mueller, S. P., Ezziddin, S., Bockisch, A., Grafe, H., et al. (2024). Safety and efficacy of selective internal radionuclide therapy with (90)Y glass microspheres in patients with progressive hepatocellular carcinoma after the failure of repeated transarterial chemoembolization. Pharm. (Basel) 17 (1), 101. doi:10.3390/ph17010101
Brenner, A. J., Patel, T., Bao, A., Phillips, W. T., Michalek, J. E., Youssef, M., et al. (2025). Convection enhanced delivery of rhenium ((186)Re) obisbemeda ((186)RNL) in recurrent glioma: a multicenter, single arm, phase 1 clinical trial. Nat. Commun. 16 (1), 2079. doi:10.1038/s41467-025-57263-1
Cai, H. W., Pang, F. W., Zhang, W. J., Peng, F. Y., and Li, L. (2016). Preparation of 131I-Collagen-Chitosan microspheres for interventional radionuclide therapy. J. Nucl. Med. 57, 2.
Chen, H., Xiong, Y., Teng, M., Li, Y., Zhang, D., Ren, Y., et al. (2025). A preclinical and first-in-human study of superstable homogeneous radiolipiodol for revolutionizing interventional diagnosis and treatment of hepatocellular carcinoma. Acta Pharm. Sin. B. doi:10.1016/j.apsb.2025.02.021
Chen, X., Wu, Y., Zhong, D., Li, L., Tan, T., Xie, X., et al. (1992). Hepatic carcinoma treated by hepatic arterial embolization using 131I and chemotherapeutic agent gelatin microspheres: report of 9 cases. Hua Xi Yi Ke Da Xue Xue Bao 23 (4), 420–423.
Christie, J. K., Malik, J., and Tilocca, A. (2011). Bioactive glasses as potential radioisotope vectors for in situ cancer therapy: investigating the structural effects of yttrium. Phys. Chem. Chem. Phys. 13 (39), 17749–17755. doi:10.1039/c1cp21764j
Conzone, S. D., Häfeli, U. O., Day, D. E., and Ehrhardt, G. J. (1998). Preparation and properties of radioactive rhenium glass microspheres intended for in vivo radioembolization therapy. J. Biomed. Mater Res. 42 (4), 617–625. doi:10.1002/(sici)1097-4636(19981215)42:4<617::aid-jbm19>3.0.co;2-4
Cozmin, M., Lungu, I. I., Cernei, R., Marin, G. A., Duceac, L. D., Calin, G., et al. (2025). Harnessing radionuclides: unveiling the promising role of radiopharmaceuticals in cancer theranostics and palliative care. Curr. Radiopharm. 18, 159–173. doi:10.2174/0118744710337420250102054532
Cui, M. H., Zhu, M. M., Tang, D. S., Cao, Z., Xiao, H. H., and Shang, K. (2025). Nanotheranostics with radionuclides for cancer diagnosis and therapy. Adv. Funct. Mater. 35 (23), 35. doi:10.1002/adfm.202423445
Cunha, L., Baete, K., Leijen, C., and Jamar, F. (2023). Main challenges in radiation protection with emerging radionuclide therapies. Q. J. Nucl. Med. Mol. Imaging 67 (1), 14–28. doi:10.23736/s1824-4785.22.03502-6
Debebe, S. A., Adjouadi, M., Gulec, S. A., Franquiz, J., and McGoron, A. J. (2019). (90) Y SPECT/CT quantitative study and comparison of uptake with pretreatment (99 M) Tc-MAA SPECT/CT in radiomicrosphere therapy. J. Appl. Clin. Med. Phys. 20 (2), 30–42. doi:10.1002/acm2.12512
De La Vega, J. C., Esquinas, P. L., Rodríguez-Rodríguez, C., Bokharaei, M., Moskalev, I., Liu, D., et al. (2019). Radioembolization of hepatocellular carcinoma with Built-In dosimetry: first in vivo results with uniformly-sized, biodegradable microspheres labeled with (188)Re. Theranostics 9 (3), 868–883. doi:10.7150/thno.29381
Dong, Y., Ren, M., Tu, J., and Chen, Y. (2025). Artificial intelligence-enabled microsphere imaging immunosensor based on magnetic metal-organic frameworks-assisted sample pretreatment for detecting aflatoxin B(1) in peanuts. J. Hazard Mater 493, 138410. doi:10.1016/j.jhazmat.2025.138410
Dong, Y., Yin, L., Huang, J., Hu, D., Sun, J., Zhang, Z., et al. (2024). (99m)Tc/(90)Y radiolabeled biodegradable gel microspheres for lung shutting fraction assessment and radioembolization in hepatocellular carcinoma theranostics. Mater Today Bio 29, 101367. doi:10.1016/j.mtbio.2024.101367
Dos Reis, S. R. R., Gomes-da-Silva, N. C., Alencar, L. M. R., de Menezes, A. S., de Menezes, F. D., Golokhvast, K. S., et al. (2025). Eco-friendly approaches in oncology: developing holmium ((166)Ho) glass microspheres for hepatocellular radioembolization. ACS Omega 10 (22), 22426–22433. doi:10.1021/acsomega.4c08734
Drescher, R., Kühnel, C., Seifert, P., Gühne, F., and Freesmeyer, M. (2020b). Renal and intestinal excretion of (90)Y and (166)Ho after transarterial radioembolization of liver tumors. AJR Am. J. Roentgenol. 214 (5), 1158–1164. doi:10.2214/ajr.19.22049
Drescher, R., Seifert, P., Gühne, F., Kühnel, C., Aschenbach, R., and Freesmeyer, M. (2020a). Transarterial radioembolization with Yttrium-90 glass microspheres: distribution of residual activity and flow dynamics during administration. J. Vasc. Interv. Radiol. 31 (9), 1467–1474. doi:10.1016/j.jvir.2020.02.002
Ehlerding, E. B., Lacognata, S., Jiang, D., Ferreira, C. A., Goel, S., Hernandez, R., et al. (2018). Targeting angiogenesis for radioimmunotherapy with a (177)Lu-labeled antibody. Eur. J. Nucl. Med. Mol. Imaging 45 (1), 123–131. doi:10.1007/s00259-017-3793-2
Fiedler, L., Kellner, M., Gosewisch, A., Oos, R., Böning, G., Lindner, S., et al. (2018). Evaluation of (177)Lu[Lu]-CHX-A″-DTPA-6A10 fab as a radioimmunotherapy agent targeting carbonic anhydrase XII. Nucl. Med. Biol. 60, 55–62. doi:10.1016/j.nucmedbio.2018.02.004
Filippi, L., Cianni, R., Schillaci, O., and Bagni, O. (2020). Molecular and metabolic imaging of hepatic neuroendocrine tumors following radioembolization with 90Y-microspheres. Curr. Med. Imaging 16 (5), 545–552. doi:10.2174/1573405615666190114150038
Filippi, L., Schillaci, O., Cianni, R., and Bagni, O. (2018). Yttrium-90 resin microspheres and their use in the treatment of intrahepatic cholangiocarcinoma. Future Oncol. 14 (9), 809–818. doi:10.2217/fon-2017-0443
Gallio, E., Richetta, E., Finessi, M., Stasi, M., Pellerito, R. E., Bisi, G., et al. (2016). Calculation of tumour and normal tissue biological effective dose in (90)Y liver radioembolization with different dosimetric methods. Phys. Med. 32 (12), 1738–1744. doi:10.1016/j.ejmp.2016.10.023
Grosser, O. S., Ruf, J., Pethe, A., Kupitz, D., Wissel, H., Benckert, C., et al. (2018). Urinary excretion of Yttrium-90 after radioembolization with Yttrium-90-Labeled resin-based microspheres. Health Phys. 114 (1), 58–63. doi:10.1097/hp.0000000000000734
Gulec, S. A., and McGoron, A. J. (2022). Radiomicrosphere dosimetry: principles and current state of the art. Semin. Nucl. Med. 52 (2), 215–228. doi:10.1053/j.semnuclmed.2021.12.009
Gupta, A., Park, J. Y., Choi, H., Choi, T. H., Chung, Y., Kim, D. H., et al. (2024). Development of alginate-based biodegradable radioactive microspheres labeled with positron emitter through click chemistry reaction: stability and PET imaging study. Mol. Pharm. 21 (10), 5005–5014. doi:10.1021/acs.molpharmaceut.4c00412
Häfeli, U. O., Casillas, S., Dietz, D. W., Pauer, G. J., Rybicki, L. A., Conzone, S. D., et al. (1999). Hepatic tumor radioembolization in a rat model using radioactive rhenium (186Re/188Re) glass microspheres. Int. J. Radiat. Oncol. Biol. Phys. 44 (1), 189–199. doi:10.1016/s0360-3016(98)00554-9
Häfeli, U. O., Pauer, G. J., Unnithan, J., and Prayson, R. A. (2007). Fibrin glue system for adjuvant brachytherapy of brain tumors with 188Re and 186Re-labeled microspheres. Eur. J. Pharm. Biopharm. 65 (3), 282–288. doi:10.1016/j.ejpb.2006.10.016
Häfeli, U. O., Roberts, W. K., Pauer, G. J., Kraeft, S. K., and Macklis, R. M. (2001). Stability of biodegradable radioactive rhenium (Re-186 and Re-188) microspheres after neutron-activation. Appl. Radiat. Isot. 54 (6), 869–879. doi:10.1016/s0969-8043(00)00313-4
Heinrich, M., Blickle, E., Hartrampf, P. E., Hasenauer, N., Kosmala, A., Kerscher, A., et al. (2025). (131)I SPECT/CT provides prognostic information in patients with differentiated thyroid cancer. Eur. J. Nucl. Med. Mol. Imaging 52, 3170–3179. doi:10.1007/s00259-025-07187-1
Jamre, M., Shamsaei, M., Erfani, M., Sadjadi, S., and Ghannadi Maragheh, M. (2018). Preparation and evaluation of (188) re sulfide colloidal nanoparticles loaded biodegradable poly (L-lactic acid) microspheres for radioembolization therapy. J. Label. Comp. Radiopharm. 61 (8), 586–594. doi:10.1002/jlcr.3627
Jamre, M., Shamsaei, M., Ghannadi Maragheh, M., and Sadjadi, S. (2019). Novel (175)Yb-Poly (L-Lactic acid) microspheres for transarterial radioembolization of unrespectable hepatocellular carcinoma. Iran. J. Pharm. Res. 18 (2), 569–578. doi:10.22037/ijpr.2019.1100668
Jiang, Z., Yang, F., and Wang, W. (2024). Applications of Yttrium-90 ((90)Y) in hepatocellular carcinoma. Onco Targets Ther. 17, 149–157. doi:10.2147/ott.S445898
Kawashita, M. (2002). Ceramic microspheres for in situ radiotherapy of cancer. Biomater. Adv. 22 (1), 3–8. doi:10.1016/s0928-4931(02)00105-4
Kawashita, M., Miyaji, F., Kokubo, T., Takaoka, G. H., Inoue, M., Suzuki, Y., et al. (1999). Surface structure and chemical durability of P+-Implanted Y2O3-Al2O3-SiO2 glass for radiotherapy of cancer. J. non-crystalline solids 255 (2), 140–148. doi:10.1016/s0022-3093(99)00377-4
Kühnel, C., Gühne, F., Seifert, P., Freudenberg, R., Freesmeyer, M., and Drescher, R. (2022). Transarterial radioembolization planning and treatment with microspheres containing Holmium-166: determination of renal and intestinal radionuclide elimination, effective half-life, and regulatory aspects. Cancers 15 (1), 68. doi:10.3390/cancers15010068
Larson, S. M., Carrasquillo, J. A., Cheung, N. K., and Press, O. W. (2015). Radioimmunotherapy of human tumours. Nat. Rev. Cancer 15 (6), 347–360. doi:10.1038/nrc3925
Lau, W. Y., Leung, W. T., Ho, S., Leung, N. W., Chan, M., Lin, J., et al. (1994). Treatment of inoperable hepatocellular carcinoma with intrahepatic arterial yttrium-90 microspheres: a phase I and II study. Br. J. Cancer 70 (5), 994–999. doi:10.1038/bjc.1994.436
Li, J., Zhang, X., Zhao, M., Wu, L., Luo, K., Pu, Y., et al. (2025b). Correction to tumor-PH-sensitive PLLA-based microsphere with acid cleavable acetal bonds on the backbone for efficient localized chemotherapy. Biomacromolecules 26 (4), 2759. doi:10.1021/acs.biomac.5c00009
Li, Q., Wang, X., Li, D., Liu, Y., Liu, K., Liang, J., et al. (2024). Y-90 radioembolization and PD-1 inhibitor as neoadjuvant treatment in hepatocellular carcinoma. J. Vis. Exp. 207. doi:10.3791/66407
Li, Y., Cai, H., Xing, Y., Pang, F., and Li, L. (2025a). Preparation of iodine-131 labeled polyvinyl alcohol-collagen microspheres for radioembolization therapy of liver tumors. Sci. Rep. 15 (1), 8830. doi:10.1038/s41598-025-94162-3
Li, Z., Wang, B., Zhang, Z., Wang, B., Xu, Q., Mao, W., et al. (2018). Radionuclide imaging-guided chemo-radioisotope synergistic therapy using a (131)I-Labeled polydopamine multifunctional nanocarrier. Mol. Ther. 26 (5), 1385–1393. doi:10.1016/j.ymthe.2018.02.019
Lim, A. H., Nitchingham, D., Bednarz, J., Bills, M., Lanka, L., Allen, B., et al. (2025). Combined phosphorus-32 implantation and chemotherapy versus chemotherapy alone for locally advanced pancreatic cancer: a propensity-score weighted landmark analysis. Gastrointest. Endosc. doi:10.1016/j.gie.2025.04.054
Liu, Q., Qian, Y., Li, P., Zhang, S., Liu, J., Sun, X., et al. (2018). 131I-Labeled copper sulfide-loaded microspheres to treat hepatic tumors via hepatic artery embolization. Theranostics 8 (3), 785–799. doi:10.7150/thno.21491
Ma, Y., Li, B., Li, L., Duan, L. G., Wei, Y. G., and Chen, X. L. (2012). In vivo distribution of (131)I and (125)I dual-labeled gelatin microspheres after implantation into rabbit liver. Cancer Biother Radiopharm. 27 (4), 267–275. doi:10.1089/cbr.2011.1156
Morsink, C., Klaassen, N., van de Maat, G., Boswinkel, M., Arranja, A., Bruggink, R., et al. (2024). Quantitative CT imaging and radiation-absorbed dose estimations of (166)Ho microspheres: paving the way for clinical application. Eur. Radiol. Exp. 8 (1), 116. doi:10.1186/s41747-024-00511-8
Ni, H. C., Yu, C. Y., Chen, S. J., Chen, L. C., Lin, C. H., Lee, W. C., et al. (2015). Preparation and imaging of rhenium-188 labeled human serum albumin microsphere in orthotopic hepatoma rats. Appl. Radiat. Isot. 99, 117–121. doi:10.1016/j.apradiso.2015.02.020
Nijsen, J. F., Van Steenbergen, M. J., Kooijman, H., Talsma, H., Kroon-Batenburg, L. M., Van De Weert, M., et al. (2001). Characterization of poly(l-lactic acid) microspheres loaded with holmium acetylacetonate. Biomater. Adv. 22 (22), 3073–3081. doi:10.1016/s0142-9612(01)00055-2
Nijsen, J. F. W., Zonnenberg, B. A., Woittiez, J. R. W., Rook, D. W., Woudenberg, S. V., van Rijk, P. P., et al. (1999). Holmium-166 poly lactic acid microspheres applicable for intra-arterial radionuclide therapy of hepatic malignancies: effects of preparation and neutron activation techniques. Eur. J. Nucl. Med. Mol. Imaging 26 (7), 699–704. doi:10.1007/s002590050440
Nuzulia, N. A., Mart, T., Ahmed, I., and Sari, Y. W. (2024). The use of microspheres for cancer embolization therapy: recent advancements and prospective. ACS Biomater. Sci. Eng. 10 (2), 637–656. doi:10.1021/acsbiomaterials.3c00659
O'Neill, E., Kersemans, V., Allen, P. D., Terry, S. Y. A., Torres, J. B., Mosley, M., et al. (2020). Imaging DNA damage repair in vivo after (177)Lu-DOTATATE therapy. J. Nucl. Med. 61 (5), 743–750. doi:10.2967/jnumed.119.232934
Order, S. E., Siegel, J. A., Principato, R., Zeiger, L. E., Johnson, E., Lang, P., et al. (1996). Selective tumor irradiation by infusional brachytherapy in nonresectable pancreatic cancer: a phase I study. Int. J. Radiat. Oncol. Biol. Phys. 36 (5), 1117–1126. doi:10.1016/s0360-3016(96)00484-1
Pang, F., Deng, H., Peng, D., He, J., Zhao, W., Yu, L., et al. (2025). Theranostic radionuclides labeled biodegradable microspheres for the treatment of hepatocellular carcinoma with radioembolization. Radiat. Phys. Chem. 232, 112642. doi:10.1016/j.radphyschem.2025.112642
Pang, F., Li, Y., Zhang, W., Xia, C., He, Q., Li, Z., et al. (2020). Biodegradable (131) iodine-labeled microspheres: potential transarterial radioembolization biomaterial for primary hepatocellular carcinoma treatment. Adv. Healthc. Mater 9 (13), e2000028. doi:10.1002/adhm.202000028
Pourhabib, Z., Ranjbar, H., Bahrami Samani, A., and Shokri, A. A. (2019). Appraisement of 186/188Re-HEDP, a new compositional radiopharmaceutical. J. Radioanalytical Nucl. Chem. 322 (2), 1133–1138. doi:10.1007/s10967-019-06816-y
Rahman, W. Y., Sarmini, E., and Pujiyanto, A. (2020). Analysis distribution of 32P radioisotope in silicone patch using autoradiography scanner. J. Phys. Conf. Ser. 1436 (1), 012020. doi:10.1088/1742-6596/1436/1/012020
Reinders, M. T. M., Smits, M. J. L., van Erpecum, K., de Bruijne, J., Bruijnen, R. C. G., Sprengers, D., et al. (2025). Hepatobiliary scintigraphy and liver function changes in patients with hepatocellular carcinoma treated with (166)Ho-radioembolization: HBS in HCC treated with holmium-166. EJNMMI Res. 15 (1), 2. doi:10.1186/s13550-025-01196-9
Seevinck, P. R., van de Maat, G. H., de Wit, T. C., Vente, M. A., Nijsen, J. F., and Bakker, C. J. (2012). Magnetic resonance imaging-based radiation-absorbed dose estimation of 166Ho microspheres in liver radioembolization. Int. J. Radiat. Oncol. Biol. Phys. 83 (3), e437–e444. doi:10.1016/j.ijrobp.2011.12.085
Son, M. H., Ha, L. N., Bang, M. H., Bae, S., Giang, D. T., Thinh, N. T., et al. (2021). Diagnostic and prognostic value of (99m)Tc-MAA SPECT/CT for treatment planning of (90)Y-resin microsphere radioembolization for hepatocellular carcinoma: comparison with planar image. Sci. Rep. 11 (1), 3207. doi:10.1038/s41598-021-82887-w
Souza, B., Ribeiro, E., da Silva de Barros, A. O., Pijeira, M. S. O., Kenup-Hernandes, H. O., Ricci-Junior, E., et al. (2022). Nanomicelles of radium dichloride [223Ra]RaCl2 Co-Loaded with radioactive gold [198Au]Au nanoparticles for targeted alpha–beta radionuclide therapy of osteosarcoma. Polym. (Basel) 14 (7), 1405. doi:10.3390/polym14071405
Stella, M., Braat, A., Lam, M., de Jong, H., and van Rooij, R. (2020). Quantitative (166)Ho-microspheres SPECT derived from a dual-isotope acquisition with (99m)Tc-colloid is clinically feasible. EJNMMI Phys. 7 (1), 48. doi:10.1186/s40658-020-00317-8
Sun, J., Sun, X., Yin, L., Jin, S., Huang, Q., Dong, Y., et al. (2025). Dual functional radioactive gel-microspheres for combinatorial radioembolization and photothermal therapy of hepatocellular carcinoma. Adv. Healthc. Mater 14 (9), e2401057. doi:10.1002/adhm.202401057
Talebi, A. S., and Rajabi, H. (2022). Outcomes following transarterial radioembolization with (90)Y and nanoparticles loaded resin microspheres. Appl. Radiat. Isot. 188, 110405. doi:10.1016/j.apradiso.2022.110405
Tan, H. Y., Wong, Y. H., Kasbollah, A., Md Shah, M. N., Abdullah, B. J. J., Perkins, A. C., et al. (2022). Development of neutron-activated samarium-153-loaded polystyrene microspheres as a potential Theranostic agent for hepatic radioembolization. Nucl. Med. Commun. 43 (4), 410–422. doi:10.1097/mnm.0000000000001529
Vente, M. A., Zonnenberg, B. A., and Nijsen, J. F. W. (2014). Microspheres for radioembolization of liver malignancies. Expert Rev. Med. Devices 7 (5), 581–583. doi:10.1586/erd.10.48
Wang, J. T., Klippstein, R., Martincic, M., Pach, E., Feldman, R., Šefl, M., et al. (2020). Neutron activated (153)Sm sealed in carbon nanocapsules for in vivo imaging and tumor radiotherapy. ACS Nano 14 (1), 129–141. doi:10.1021/acsnano.9b04898
Wang, L., He, Q., Man, J., Gao, Y., Zhou, G., Si, H., et al. (2025a). Novel bio-carriers for radionuclide delivery in cancer radiotherapy. Coord. Chem. Rev. 533, 216557. doi:10.1016/j.ccr.2025.216557
Wang, Y., Barmin, R., Mottaghy, F. M., Kiessling, F., Lammers, T., and Pallares, R. M. (2025b). Nanoparticles in nuclear medicine: from diagnostics to therapeutics. J. Control Release 383, 113815. doi:10.1016/j.jconrel.2025.113815
Welling, M. M., Duszenko, N., van Meerbeek, M. P., Molenaar, T. J. M., Buckle, T., van Leeuwen, F. W. B., et al. (2023). Microspheres as a carrier system for therapeutic embolization procedures: achievements and advances. J. Clin. Med. 12 (3), 918. doi:10.3390/jcm12030918
Westcott, M. A., Coldwell, D. M., Liu, D. M., and Zikria, J. F. (2016). The development, commercialization, and clinical context of yttrium-90 radiolabeled resin and glass microspheres. Adv. Radiat. Oncol. 1 (4), 351–364. doi:10.1016/j.adro.2016.08.003
Wong, Y. H., Alregib, A. H., Kasbollah, A., Abdullah, B. J. J., and Yeong, C. H. (2025). Neutron-activated theragnostic Doxorubicin- and Samarium-153 loaded microspheres for transarterial chemo-radioembolization of liver cancer. Health Technol. doi:10.1007/s12553-025-00968-6
Wong, Y. H., Kasbollah, A., Abdullah, B. J. J., and Yeong, C. H. (2023). Facile preparation of samarium carbonate-polymethacrylate microspheres as a neutron-activatable radioembolic agent for hepatic radioembolization. Pharmaceutics 15 (3), 877. doi:10.3390/pharmaceutics15030877
Wong, Y. H., Tan, H. Y., Kasbollah, A., Abdullah, B. J. J., Acharya, R. U., and Yeong, C. H. (2020). Neutron-activated biodegradable samarium-153 acetylactonate-poly-L-lactic acid microspheres for intraarterial radioembolization of hepatic tumors. World J. Exp. Med. 10 (2), 10–25. doi:10.5493/wjem.v10.i2.10
Wong, Y. H., Tan, H. Y., Kasbollah, A., Abdullah, B. J. J., and Yeong, C. H. (2019). Preparation and in vitro evaluation of neutron-activated, theranostic Samarium-153-Labeled microspheres for transarterial radioembolization of hepatocellular carcinoma and liver metastasis. Pharmaceutics 11 (11), 596. doi:10.3390/pharmaceutics11110596
Wu, M., Zhang, L., Shi, K., Zhao, D., Yong, W., Yin, L., et al. (2023). Polydopamine-coated radiolabeled microspheres for combinatorial radioembolization and photothermal cancer therapy. ACS Appl. Mater Interfaces 15 (10), 12669–12677. doi:10.1021/acsami.2c19829
Wu, M. R., Shi, K. X., Huang, R. Z., Liu, C. Y., Yin, L. L., Yong, W., et al. (2022b). Facile preparation of 177Lu-microspheres for hepatocellular carcinoma radioisotope therapy. Chin. Chem. Lett. 33 (7), 3492–3496. doi:10.1016/j.cclet.2022.01.007
Wu, X., Ge, L., Shen, G., He, Y., Xu, Z., Li, D., et al. (2022a). (131)I-Labeled silk fibroin microspheres for radioembolic therapy of rat hepatocellular carcinoma. ACS Appl. Mater Interfaces 14 (19), 21848–21859. doi:10.1021/acsami.2c00211
Xiao, L., Li, Y., Geng, R., Chen, L., Yang, P., Li, M., et al. (2023). Polymer composite microspheres loading (177)Lu radionuclide for interventional radioembolization therapy and real-time SPECT imaging of hepatic cancer. Biomater. Res. 27 (1), 110. doi:10.1186/s40824-023-00455-x
Xu, X., Chen, H., He, P., Zhao, Z. W., Gao, X., Liu, C., et al. (2023). 3D hollow porous radio-granular hydrogels for SPECT imaging-guided cancer intravascular brachytherapy. Adv. Funct. Mater. 33 (22), 12. doi:10.1002/adfm.202215110
Yan, Z. P., Lin, G., Zhao, H. Y., and Dong, Y. H. (1993). Yttrium-90 glass microspheres injected via the portal vein. An experimental study. Acta Radiol. 34 (4), 395–398. doi:10.1080/02841859309173266
Yang, S., Mu, C., Liu, T., Pei, P., Shen, W., Zhang, Y., et al. (2023). Radionuclide-labeled microspheres for radio-immunotherapy of hepatocellular carcinoma. Adv. Healthc. Mater 12 (26), e2300944. doi:10.1002/adhm.202300944
Zade, A. A., Rangarajan, V., Purandare, N. C., Shah, S. A., Agrawal, A. R., Kulkarni, S. S., et al. (2013). 90Y microsphere therapy: does 90Y PET/CT imaging obviate the need for 90Y bremsstrahlung SPECT/CT imaging? Nucl. Med. Commun. 34 (11), 1090–1096. doi:10.1097/MNM.0b013e328364aa4b
Zhang, S., Wang, X., Gao, X., Chen, X., Li, L., Li, G., et al. (2025). Radiopharmaceuticals and their applications in medicine. Signal Transduct. Target Ther. 10 (1), 1. doi:10.1038/s41392-024-02041-6
Zhao, Z. W., Chen, Y. L., Liu, H., Tang, H. T., Teng, M. L., Liu, X., et al. (2024). Amidoxime-based radio-microspheres for internal irradiation combined with a checkpoint-blocking nanobody boost antitumor immunity. Nano Today 57, 102383. doi:10.1016/j.nantod.2024.102383
Zhen, Y., Liu, B., Chang, Z., Ren, H., Liu, Z., and Zheng, J. (2019). A pooled analysis of transarterial radioembolization with yttrium-90 microspheres for the treatment of unresectable intrahepatic cholangiocarcinoma. Onco Targets Ther. 12, 4489–4498. doi:10.2147/ott.S202875
Keywords: tumor, radiation, brachytherapy, radioactive microspheres, theranostics
Citation: Jiang X, Chen L and Xu X (2025) Advancements in the investigation of radioactive microspheres for brachytherapy. Front. Bioeng. Biotechnol. 13:1621418. doi: 10.3389/fbioe.2025.1621418
Received: 01 May 2025; Accepted: 08 July 2025;
Published: 16 July 2025.
Edited by:
Shiying Li, Hong Kong Polytechnic University, Hong Kong SAR, ChinaReviewed by:
Shardendu Kumar Mishra, KIET School of Pharmacy (KSOP), IndiaCopyright © 2025 Jiang, Chen and Xu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiao Xu, eGlhb3h1NzIxQHNtdS5lZHUuY24=
†These authors have contributed equally to this work
 Xiaohui Jiang1,2†
Xiaohui Jiang1,2† Xiao Xu
Xiao Xu