- Department of Neurology, Affiliated Hospital of Jiujiang University, Jiujiang, China
The rising incidence of neural tumors and neurodegenerative diseases cause significant health, emotional, and financial burdens. Conventional treatments like surgery and chemotherapy often lack effectiveness. However, advancements in nanotechnology, particularly photothermal therapy (PTT), offer new hope. PTT is widely studied for neural tumors and neurodegenerative diseases due to its simplicity, rapid recovery, combined therapeutic potential, and compatibility with imaging techniques. This innovative approach could revolutionize the diagnosis and treatment of neural tumors and neurodegenerative diseases, addressing current limitations and improving outcomes. In this article, we offer a comprehensive overview of the rational design and engineering of various nanomaterials designed specifically for PTT applications in neural tumors and neurodegenerative diseases, including organic platforms such as liposomes, dopamine, etc. and inorganic platforms such as gold nanomaterials, carbon nanomaterials, etc. A comparative analysis of these platforms examines their biocompatibility and potential for biodegradation. It also assesses their manufacturing scalability, cost-effectiveness, regulatory challenges, and ultimate potential for clinical translation. We also update the therapeutic advances of PTT in neural tumors (Glioma, Peripheral nerve sheath tumors, Spinal metastases from in situ tumors and brain metastases) and neurodegenerative diseases (Alzheimer’s disease, Parkinson’s disease, Huntington’s disease), and systematically summarize the mechanisms of PTT application in neural tumors and neurodegenerative diseases. In the end, we provide an in-depth discussion of the advantages and disadvantages of PTT and the perspectives for its application in the above neurological disorders.
1 Introduction
PTT is a novel non-invasive treatment method, whose basic mechanism lies in the fact that with the help of specific photothermal transduction agents (PTAs), it can effectively convert the received photon energy into thermal energy under the irradiation of exogenous light such as near-infrared (NIR) ray, and the effect of this process is mainly reflected in the photothermal ablation of local tissues to exert its therapeutic effect. Currently, there is a high level of interest in research on the application of PTT in oncology (Cheng et al., 2014; Li et al., 2020; Zhao et al., 2021).
Photophysically, a PTA absorbs photon energy upon exposure to specific NIR light. Photon absorption elevates the PTA from its ground singlet state to an excited singlet state. Subsequently, the electronic excitation energy dissipates through vibrational relaxation. The excited PTA then reverts to its ground state via collisions with neighboring molecules. These molecular interactions generate heightened kinetic energy, inducing localized warming (Li et al., 2020). In essence, PTAs undergo lattice vibrations or electronic oscillations after absorbing electromagnetic radiation. Such activity increases the material’s temperature, creating a photothermal effect on the local environment (Wang Y. et al., 2021; Yu et al., 2024).
Different PTAs utilize distinct photothermal conversion mechanisms. Noble metal nanoparticles, like gold and silver, exhibit a unique localized surface plasmon resonance (LSPR) effect. Through LSPR, decaying surface plasmon oscillations radiate energy, converting absorbed light into heat. Semiconductors achieve photothermal conversion via two main pathways. One mechanism is free carrier absorption, analogous to the metallic LSPR effect. The second pathway involves the intrinsic absorption band gap between the material’s valence and conduction bands. For most carbon-based materials, their π bonds are generally weaker than σ bonds. This bond characteristic facilitates electron transitions under illumination, triggering polarization relaxation. Light energy subsequently transfers to crystal lattice vibrations, yielding photothermal conversion (Wang Y. et al., 2021; Yu et al., 2024).
Due to the limitations of PTT monotherapy in treating diseases, PTT is often combined with other nanoplatform-based therapies, such as Chemotherapy (CT), radiotherapy (RT), photodynamic therapy (PDT), Chemodynamic Therapy (CDT), Sonodynamic Therapy (SDT), gene therapy, immunotherapy, etc. to achieve greater synergistic effects (Liu et al., 2019b; Xie et al., 2020; Qiao et al., 2022). PTT is increasingly favored by researchers for its simplicity, short treatment time, rapid recovery, and excellent light-controlled drug release on combination chemotherapy (Cheng et al., 2014; Zhao et al., 2021).
With the growing interest in nanomedicine and its wide application in neurological diseases, this review focuses on the progress of PTT with nanomaterials, which has been rapidly developed in recent years for application in neural tumors and neurodegenerative diseases. We aim to provide a comprehensive overview of current strategies for rational design and engineering modification for multiple nanomaterial platforms, covering both organic (e.g., liposomes, dopamine-based nanomaterials) and inorganic (e.g., gold nanomaterials, carbon-based nanomaterials) materials for specific applications in neurological diseases. In particular, the article compares the organic and inorganic platforms in terms of their biocompatibility and biodegradation, manufacturing scalability and cost-effectiveness, and regulatory challenges and potential for clinical translation. We emphasize how to develop targeted material designs based on the unique pathophysiological features and biological barriers of the nervous system. Subsequently, the article updates its latest research progress in specific diseases, including glioma, peripheral nerve sheath tumor, metastatic neurological tumors, as well as typical neurodegenerative diseases such as Alzheimer’s disease (AD), Parkinson’s disease (PD), Huntington’s disease. We also systematically summarizes the clinical mechanisms by which PTT exerts its efficacy in the treatment of neurological disorders, involving a range of signaling molecules and pathways for apoptosis, etc. By comprehensively analyzing the material strategies in these different diseases, and comparing the strengths, weaknesses and adaptations of various types of nanoplatforms, we seek to provide a broad and in-depth perspective to reveal how material innovations can drive the translational potential of PPT in the treatment of major neurological diseases.
2 Nanomaterials engineered for PTT of neural tumors and neurodegenerative diseases
PTAs commonly used in PTT are broadly classified into organic and inorganic nanomaterials. Among inorganic nanomaterials, gold nanomaterials, carbon nanomaterials, palladium nanosheets, copper sulfide nanoparticles, and some other newly reported materials, such as black phosphorus-based materials, are commonly used; organic nanoparticles include protein-containing near-infrared absorbing conjugated polymers, porphyrins, liposomes, and nanomicelles encapsulated with near-infrared dyes (Cheng et al., 2014; Huang et al., 2021; Overchuk et al., 2023). In the following section, nanomaterials currently used in PTT applied to neural tumors and neurodegenerative diseases will be discussed mainly according to the classification of organic and inorganic materials.
2.1 Neural tumors
2.1.1 Organic nanoplatform
2.1.1.1 Liposome nanoparticles
Liposomes are nanoscale vesicles primarily composed of lipid or phospholipid molecules. Owing to their nanosize, biocompatibility, and biodegradability, liposomes have demonstrated potential in nanomedicine alongside broader applications in cosmetics and food industries (Panahi et al., 2017).
The liposomes’ stability and effective photothermal conversion capabilities also enable simultaneous fluorescence and photoacoustic imaging (PAI) (Zeng et al., 2023). Microfluidics (e.g., lab-on-a-chip platforms) facilitates the precise preparation of liposomes, which was used by Cao et al. to prepare vitexin/indocyanine green (ICG) liposomes, thereby successfully enhancing the poor solubility of vitexin in water, while improving the uniformity and dispersion of the particles. The resulting liposomes promoted combined photothermal and photodynamic effects (Cao X. et al., 2023). Liposomes can also be designed as responsive nanoprobes. Chen et al. designed a Lipo@HRP&ABTS nanoprobe. In which Horseradish peroxidase (HRP) catalyzed the oxidation of ABTS by H2O2 to produce a green derivative with strong near-infrared absorbance. With systemic administration, the nanoprobe detects hydrogen peroxide produced by tumors, enabling in vivo PAI to visualize small (∼2 mm) subcutaneous tumors and gliomas in situ (Chen et al., 2017).
2.1.1.2 Polydopamine nanoparticles
Polydopamine (PDA) is an oxidative self-polymerized form of dopamine under alkaline conditions, originally inspired by the adhesive properties of mussel foot proteins containing catechol and lysine. Developed in nanoparticulate form in 2009, PDA exhibits melanin-like properties, excellent biocompatibility, aqueous solubility, and strong near-infrared absorption for PTT applications (Poinard et al., 2018).
Dube et al. prepared fluorescent poly-levodopamine nanoparticles (FLs) incorporating levodopa, DHI, and DHICA units. Self-assembly yielded diverse species possessing energy gaps from UV-visible to NIR, leading to broad absorption and excitation-dependent emission. The incorporated levodopa unit aids nanoparticle penetration across the blood-brain barrier (BBB). Leveraging the fluorescence and BBB-penetrating capabilities, multifunctional nanoplatforms co-loaded with doxorubicin (DOX)/ICG/peptide, termed FLDIPs, were developed (Dube et al., 2021). PDA also enables synergistic therapeutic approaches. For instance, nanoparticles radiolabeled with 211At and functionalized with a fibroblast activating protein inhibitor (FAPI) impede U87MG cell proliferation via combined photothermal and targeted alpha therapy. Observed cytotoxicity vanished upon omission of either 211At labeling or PTT activation (Li F. et al., 2024). Furthermore, surface engineering has optimized PDA vectors for glioblastoma (GBM). PDA nanoparticles underwent modification with the IL-13Ra2 ligand Pep-1. Resulting particles exhibited strong affinity for U87 cells and enhanced penetration into them. Peptide functionalization was demonstrated to facilitate BBB and cell membrane traversal, enabling efficient nuclear delivery; conversely, unmodified nanoparticles were confined to the cytoplasm (Wu et al., 2023).
2.1.1.3 Peptide nanoparticles
Peptide-based nanomaterials, encompassing cyclic peptides, aromatic dipeptides, amphiphilic peptides, and polypeptides, are engineered into supramolecular nanostructures with adjustable characteristics. Possessing inherent biocompatibility, peptide nanomaterials can also modify photosensitizer behavior via molecular interactions. Their ability to modulate photosensitizer activity allows for precise control over photothermal effects. Consequently, such systems hold significant potential for cancer PTT (Abbas et al., 2017).
Engineered peptide nanoplatforms exhibit enhanced functionality beyond simple drug delivery. There are nanoparticles that enable precision diagnostics, providing features such as dual-modal contrast imaging and efficient photothermal ablation, and incorporate a self-sensing mechanism based on Förster resonance energy transfer (FRET), this allows the therapeutic process to be visualized in situ via corresponding signaling changes, thereby guiding precise PTT (Hu et al., 2018). Other designs promote active tumor targeting and fluorescence imaging (FLI), which facilitates tumor margin detection to guide precision surgery and local PTT (Jia et al., 2019).
Strategies have also improved therapeutic impact and delivery. Dube et al. developed multicomponent ICG-loaded peptide co-assembled nanoparticles. These engineered nanoplatforms exhibited significantly higher collective CT-PDT-PTT efficacy when compared to free, discrete therapeutic fractions (Dube and Panda, 2023). Self-assembled systems, using a phenylalanine peptide core responsive to tumor glutathione conditions, enhanced retention within the tumor. Such constructs achieved significant GBM inhibition in the U87MG-luc model, with an endpoint tumor volume 2.61-fold smaller than that of controls (P < 0.01) (Song W. et al., 2023). Selectivity has been addressed through surface modifications. Zhuge et al. produced indocyanine green-loaded silk fibroin nanoparticles (ICG-SFNPs) and their chitosan-modified variants (ICG-CSF). In vitro experiments, a small number of ICG-CSFNPs were phagocytosed by RAW264.7 macrophages, whereas C6 glioma cells were susceptible to internalization, leading to significant toxicity to these tumor cells after NIR irradiation (ZhuGe et al., 2019).
Specific organic nanomaterials, including dyes and polymers, are applied in PTT for neural tumors. For instance, lipoprotein-mimicking nanoparticles can improve tumor selectivity. Modifying liposomes with ApoE peptides enhances drug delivery and therapeutic efficacy while reducing side effects (Huang et al., 2024).
Novel delivery mechanisms are also under investigation. Doxorubicin-loaded apoptotic bodies are phagocytosed by monocytes/macrophages. Guided by tumor chemokines, these cells infiltrate the glioma, delivering their payload via a “hitchhiking” effect (Liu Y. et al., 2023). Similarly, engineered exosomes provide a biomimetic platform. One design features a microglia-derived exosome shell for targeting and a core with AIE agents. This structure enables mild photothermal therapy (MPTT) and NIR-II fluorescence for glioblastoma therapy (Lin et al., 2023).
Polymer-based nanostructures also show promise. PA1094T nanoparticles combine mild PTT with pyroptosis to augment anti-tumor immunity (Li et al., 2025). Other polymers containing tetraphenylene (TPE) leverage its unique molecular structure. Intramolecular rotation within TPE facilitates heat release, while its twisted conformation promotes strong intramolecular charge transfer in the conjugated copolymer. The interplay of these TPE characteristics enhances photothermal capabilities (Su et al., 2025).
2.1.2 Inorganic nanoplatform
2.1.2.1 Gold nanoparticles
Gold nanoparticles (AuNPs) attract significant research interest owing to their LSPR. LSPR originates from the collective oscillation of conduction electrons when exposed to NIR light, enabling effective conversion of photonic energy into heat. The optical behavior of AuNPs is governed by their morphology and the surrounding dielectric medium. Variations in morphology and medium could influence LSPR characteristics, such as absorption and scattering efficiency, and cause spectral peak shifts under changing surface conditions (Turkmen Koc et al., 2024).
Duan et al. researchers found that gold nanorods (Au NR) in CD-PGEA structures have inherent photoacoustic and X-ray computed tomography imaging capabilities for deep tissue detection, in addition to mediating PTT. The addition of fluorescent quantum dots (QDs) further enhances the sensitivity of both imaging modalities (Duan et al., 2017). The targeting strategy involves combining biotin with gold nanoparticles. This helps the nanoparticles move to the cellular site and promotes their effective binding to tumor cells (He et al., 2021).
Specific morphologies offer distinct benefits. Hollow gold nanoparticles (HGNP), synthesized at higher gold concentrations, exhibit low porosity and absorb at lower wavelengths. HGNP demonstrate resilience against repeated NIR irradiation. Combining HGNP/NIR with DDTC-Cu for PTT achieved significant in vitro cytotoxicity (20%). Such synergy surpassed the outcomes of NIR or the DDTC-Cu/HGNP mixture alone (Liu et al., 2024b). Hu et al. described ARCR, derived from 2D nanomaterials. Possessing large surface areas, ARCR strengthen surface plasmon resonance within the 650–1,350 nm biological window. Tips and small branches on their dendritic structures facilitate rapid heat transfer. Consequently, both PTT and PAI efficiency are significantly improved. Separately, siRNA-loaded AuNSs markedly inhibited polo-like kinase 1 oncogene expression (Hu et al., 2024). MA et al. developed AMMD featuring a complex double-layer porous architecture. Its internal metal-organic framework (MOF) component encapsulates alpelisib. Alpelisib inhibits the PI3K-Akt-mTOR signaling pathway in tumor cells and disrupts osteoclast activity. Following modification with ICG, the AMMD surface displays a strong LSPR effect, indicating potential for PTT and PAI. ICG was specifically selected for use with spherical gold nanoparticles. The choice aimed to prevent light quenching caused by spectral emission overlap with gold nanorods (Ma et al., 2021).
2.1.2.2 Copper nanoparticles
Among emerging nanomaterials for PTT, copper (Cu) nanoparticles have attracted attention due to their biodegradability, moderate cellular tolerance, and surface plasmon resonance near the NIR-I boundary (550–600 nm). Additionally, copper chalcogenides (Cu2-xE; E = S, Se, Te) have been widely investigated for NIR-triggered applications, including PAI and thermal ablation. Uniquely, Cuprous sulfide featuring Cu-deficient stoichiometries (Cu2−xS) demonstrates stoichiometry-dependent LSPR in the NIR, driven by cation vacancy-induced free holes rather than electron oscillations (Tai et al., 2018; Dong et al., 2020).
Unlike the typical LSPR. Hollow copper sulfide nanoparticles (HCuS NPs) exhibit NIR absorption mainly from dd transitions, which is independent of particle size or shape (Tong et al., 2023). Another material, copper-molybdenum sulfide (CMS), contains polyvalent metal ions (Cu, Mo) with strong NIR absorption and suits for antitumor applications (Yao et al., 2022).
Copper-based nanomaterials exhibit significant photothermal activity under NIR laser irradiation. Owing to their nanoscale dimensions, copper sulfide nanoparticles efficiently accumulate in tumor tissues via enhanced permeability and retention effects. Subsequent NIR activation induces tumor ablation, minimizing damage to adjacent healthy tissues (Lan et al., 2021). Beyond the photothermal effect, some materials provide alternative therapeutic mechanisms. Copper sulfide nanoparticles release copper ions within the acidic tumor milieu. The released copper ions generate reactive oxygen species (ROS) via a Fenton-like reaction involving endogenous hydrogen peroxide (Lan et al., 2021). Furthermore, CMS effectively generates cytotoxic superoxide anions (O2⋅−) upon 808 nm laser irradiation (Yao et al., 2022). Advanced platforms enable targeted and combination approaches. For instance, HCuS NPs integrated with pHLIP actively target acidic tumor regions, a capability confirmed through in vivo imaging (Tong et al., 2023). Radiation exposure involving the nanoparticles triggers PTT and immunogenic cell death. The consequent hyperthermia offers further utility: it can melt lauric acid to release the stress granule inhibitor ISRIB. ISRIB subsequently sensitizes cells to PTT (Tong et al., 2023).
2.1.2.3 Carbon nanoparticles
Carbon-based nanomaterials, particularly carbon nanotubes (CNTs), have been extensively explored in PTT due to their favorable physicochemical properties. Structurally composed of one or more concentric layers of carbon atoms in hexagonal arrays, CNTs exhibit mechanical robustness, thermal stability, and efficient NIR photothermal conversion. Multiwalled CNTs are especially effective in tumor ablation via localized hyperthermia. Although their native hydrophobicity hinders aqueous dispersion, functionalization with surfactants, proteins, polysaccharides, or polyethylene glycol (PEG) enhances solubility, biocompatibility, and therapeutic versatility (Liu et al., 2025).
Carbon nanomaterials have unique physical and chemical properties that are valuable for therapeutic purposes. Their high surface area facilitates bulk loading of aromatic drugs. In addition, their surfaces are easily modified by various biofunctional groups, including hydroxyl, epoxide, carboxyl and amino groups. Such functionalization improves their aqueous solubility, stability in physiological solutions, and the enhanced drug-loading capacity (Lu et al., 2014). Graphene nanoparticles also exhibit photothermal anticancer activity in vitro. Such effects include induction of oxidative stress and mitochondrial damage leading to apoptosis and necrosis of tumor cells. Studies have shown that graphene nanoparticles have higher photothermal anticancer efficiency than CNTs due to their smaller size and better dispersion (Markovic et al., 2011). Qian et al. prepared 6–8 nm hollow carbon nanodots (HCCDs) possessing a crystalline core and hydrophilic surface. The HCCDs exhibit strong photoacoustic and photothermal properties, as well as tunable fluorescence. In mice with U87 gliomas, HCCDs specifically concentrated within brain tumors. Their accumulation facilitates dual-mode imaging guidance for PTT, leading to effective anti-tumor results while minimizing damage to normal tissue (Qian et al., 2018).
2.1.2.4 Silicon nanoparticles
Silicon-based nanomaterials constitute a key group of photothermal agents. Their appeal arises from favorable biocompatibility, biodegradability, and abundant availability. Porous silicon nanoparticles, in particular, are widely employed in biomedical applications. Typically produced through electrochemical etching of p-type Si (100) wafers, the nanoparticles possess tunable band gap energies ranging from 1.12 to 2.5 eV. Quantum confinement and an irregular pore distribution account for this tunable property. These structural attributes promote efficient photothermal conversion. The conversion is mediated by electron-hole recombination under laser excitation, highlighting their potential in PTT (Yu et al., 2017).
Atomically thin silicon quantum sheets (Si QS), measuring approximately 14.0 nm in transverse dimension and 1.6 nm in thickness, exhibit high mass extinction coefficients (27.5 L g−1 cm−1) and remarkable photothermal conversion efficiencies (47.2%) at 808 nm, which are reported to outperform other reported 2D mono-elemental materials (Xenes) (Miao et al., 2021). Si QSs have low toxicity and well-balanced stability, and can degrade to non-toxic orthosilicic acid in vivo. Their ultrasmall size and layered structure enable them to cross the blood-brain tumor barrier and efficiently accumulate in glioma tissues through the enhanced penetration and retention effect, without the need for specific targeting modifications (Miao et al., 2021).
Mesoporous silica nanoparticles (MSNs) serve as multifunctional platforms. MSN surfaces possess abundant silanol groups suitable for functionalization, enabling controlled drug delivery (Wang et al., 2014). For instance, Wang et al. developed TsGMSN using MSN technology. TsGMSN incorporates semi-graphitized carbon (sGC) hotspots deposited on inner pore walls. The arrangement ensures direct contact between loaded hydrophobic drugs, like DOX, and the sGC hotspots. Consequently, alterations in pH and NIR radiation can effectively trigger drug release. Locating hydrophobic sGC on a hydrophilic silica wall establishes TsGMSN as an effective therapeutic carrier. Despite moderate sGC content (7.2%) and graphitization degree, a regular distribution of the hotspots provides the nanoparticles with excellent photothermal efficiency (Wang et al., 2014).
2.1.2.5 Iron oxide nanoparticles
Iron oxide nanoparticles, particularly those with a magnetite crystal phase, have been identified as highly efficient for PTT, with magnetite nanocubes and magnetite magnetosomes demonstrating substantial heating in the NIR spectrum. The heating power of these materials can exceed 1–10 kW gFe−1, surpassing typical magnetic hyperthermia thresholds (Van de Walle et al., 2023).
Supramagnetic iron oxide (SPIO) nanoparticles serve as a prominent example. Considered a standard magnetic particle imaging tracer, SPIO is highly regarded for non-radioactivity, extended stability, and biocompatibility, alongside having FDA clinical approval (Huang X. et al., 2023). Furthermore, iron oxide nanoparticles allow integration into sophisticated structures. Wang et al., for example, utilized macrophages for delivering MFe3O4-Cy5.5 nanoparticles. Macrophage-mediated delivery enables multimodal fluorescence, photoacoustic, and magnetic resonance imaging (MRI). The approach provides advantageous probing depth plus a high signal-to-noise ratio. The combination of imaging modalities helps distinguish brain tumors from normal tissue and offers precise guidance during glioma resection (Wang S. et al., 2021).
Some inorganic nanomaterials containing selenium and vanadium are also worth noting. Dai et al. focused on Bi2Se3 nanodisks possessing remarkable ROS scavenging via multienzymatic activity. This characteristic counteracted inflammation typically associated with PTT. Furthermore, these nanodisks exhibited computed tomography capabilities, enabling integrated diagnosis and treatment functions (Dai et al., 2024). Guo et al. prepared TA-VOx nanobelts. The formation involved tannic acid (TA), whose abundant catechol structures provide chelating sites for metal ions, facilitating a self-assembled metal-polyphenol network. Furthermore, TA’s reduction capacity reduces V5+ to V4+ ions, partly enhancing the material’s NIR absorption ability (Guo et al., 2022).
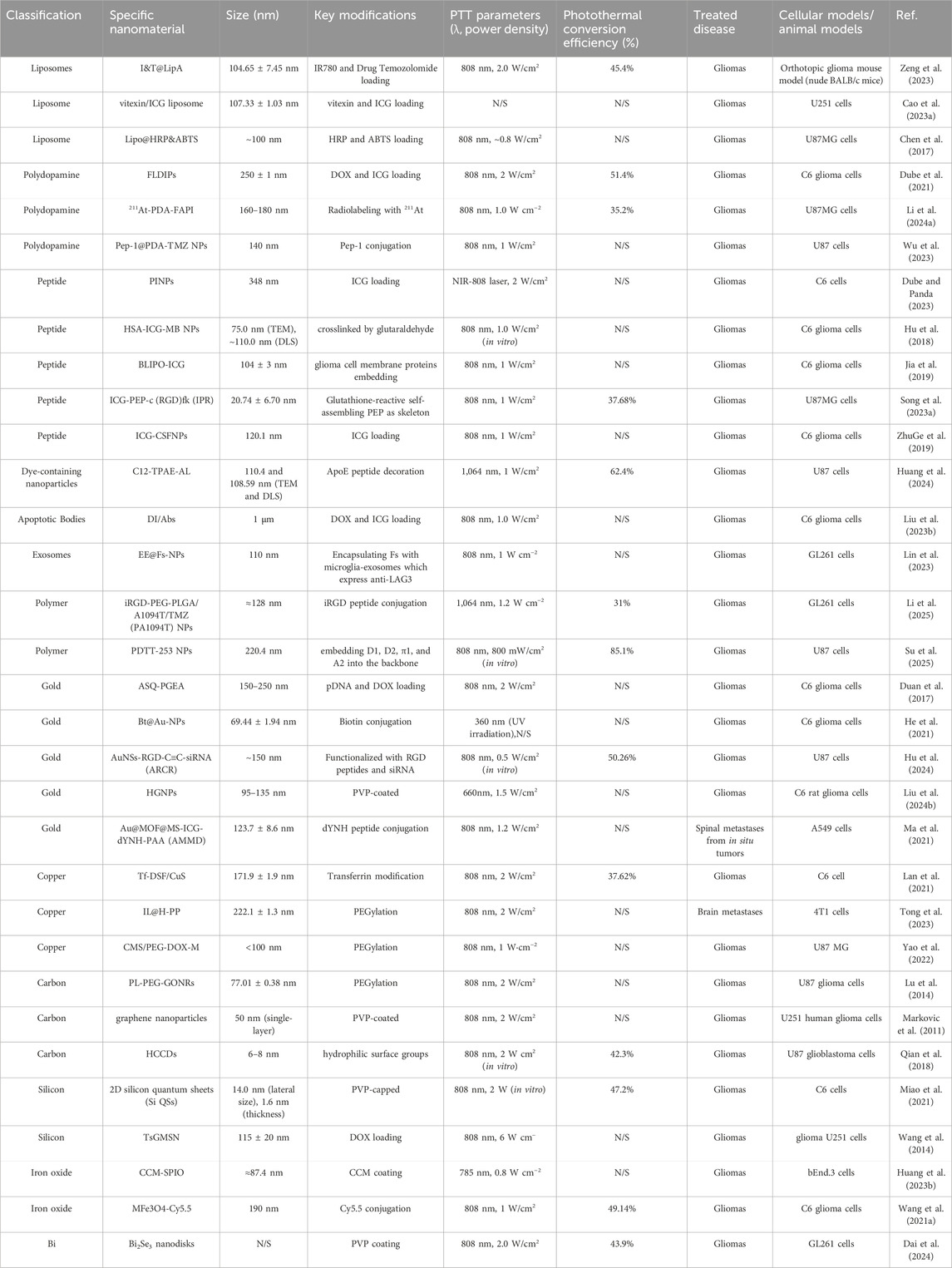
The information involving the classification, parameters and functionalization of the above mentioned nanomaterials for PTT applied to neural tumors is shown in Table 1.
2.2 Neurodegenerative diseases
2.2.1 Organic nanoplatform
2.2.1.1 Polydopamine nanoparticles
PDA NPs have demonstrated multifunctionality relevant to the treatment of neurodegenerative diseases. Chen et al. focused on their promising photothermal conversion capabilities. In addition, they scavenge Aβ-Cu2+-induced intracellular ROS, thereby reducing the relevant cellular damage (Chen et al., 2023). Based on the properties of PDAs, Liu et al. developed PDA-CQDRBC, a composite system combining nitrogen-doped carbon quantum dots (CQDs) with erythrocyte membrane-coated PDAs. Nitrogen doping enhances the absorption/emission and photothermal efficiencies of CQDs, and PDA-CQDRBC exhibits significant Cu2+ binding ability. It retarded Aβ nucleation and inhibited Cu2+-induced Aβ aggregation, which was confirmed by the reduction of thioflavin T fluorescence. Although Cu2+ normally enhances Aβ fibrosis and toxicity, co-incubation with PDA-CQDRBC significantly increased cell viability in a concentration-dependent manner. This protective effect stems from the ability of nanomaterials to resist aggregation and reduce basal ROS levels (Liu et al., 2024a).
2.2.1.2 Other polymeric nanoparticles
Chen et al. developed BDPHPC, which exhibits thermally controlled properties governed by its lowest critical solvation temperature (LCST). Below LCST, it exhibits a hydrophilic, randomly curled state in which π-π stacked, rotationally restricted boron-dipyrrole methylene (BDP) molecules efficiently dissipate absorbed NIR photons in the form of heat. The photothermal effect can be controlled by both concentration and laser power. Above LCST, the BDPHPC transforms into a dehydrated fiber conformation. At this point, the BDP molecule becomes a free-spinning monomer with high photoluminescence, which enhances its specificity as an NIR fluorescent probe against Aβ species. Such a reversible phase transition can burst and temporarily reverse Aβ42 peptide fibrillation (Chen et al., 2022).
2.2.2 Inorganic nanoplatform
2.2.2.1 Carbon nanoparticles
Carbon-based nanomaterials offer a variety of strategies for light-mediated AD therapies targeting Aβ pathology. GO-ThS utilizes the ability of graphene oxide to locally generate heat under NIR laser irradiation, which contributes to selective Aβ protofibril dissociation. GO-ThS also exerts a protective effect against Aβ-related cytotoxicity under NIR light irradiation (Li et al., 2012). Another approach is to employ KD8@N-MCN based on nitrogen-doped mesoporous carbon nanospheres (N-MCN), which can be used as NIR-II photothermite. Their structure is characterized by dispersed sp2 hybridized pore walls that promote light absorption and photothermal conversion. In addition, graphitic nitrogen dopants contribute excess electrons that enhance the light absorption properties. N-MCN uniquely exhibit intrinsic superoxide dismutase (SOD) and catalase activity. The enzymatic function allows them to counteract the oxidative damage associated with AD (Ma et al., 2020). L-CNDs have a unique dual function: they bind Aβ aggregates through hydrophobic interactions and π-π stacking. Simultaneously, L-CNDs have a strong 630 nm absorption and good fluorescence properties. They have minimal toxicity to PC12 and HT22 cells, while reducing Aβ-induced cellular damage (Shao et al., 2025). Yan et al. also developed YCDS-CE6, which employs yellow carbon dots as the NIR response core. Under combined PDT and PTT, YCDS-CE6 effectively inhibited amyloid fibrillation and completely disintegrated mature fibrils. It also inhibited the conformational transition of the protein from α-helical to β-folded structure. In a cellular model, Aβ42 aggregate adhesion was reduced and Aβ42-induced neurotoxicity was attenuated after treatment with YCDS-CE6 (Yan et al., 2022).
2.2.2.2 Molybdenum nanoparticles
Molybdenum-based nanomaterials, encompassing molybdenum disulfide, molybdenum oxides, molybdates, and Mo-based polyoxometalates, have generated significant interest. Tunable valence states and multifunctional properties are key reasons for investigation. High NIR photothermal conversion efficiency, substantial conductivity, large surface area, and favorable biocompatibility promote integration into biomedical applications. Particularly PTT (Liao et al., 2023).
Qi et al. investigated molybdenum disulfide quantum dots (MoS2 QDs). Macrophage membranes were employed for biomimetic modification, aiming to improve QD performance. Exploiting macrophage immunological characteristics potentially enhances evasion of the reticuloendothelial system and prolongs circulation time for the modified QDs. Additionally, the inherent MoS2 material possesses anti-Aβ1-42 aggregation effects. It also acts as an effective nano-enzymatic scavenger of free radicals. Liposomes provided an alternative biomimetic modification for MoS2 QDs, augmenting the material’s anti-aggregation and scavenging capabilities. The Liposome coating effectively reduces recognition and phagocytosis by macrophages, facilitating escape from immune clearance (Qi et al., 2024).
2.2.2.3 Other inorganic nanoparticles
There are other inorganic nanomaterials that are being designed for therapeutic intervention in neurodegenerative diseases. For instance, Zhou et al. developed hollow ruthenium nanoparticles loaded with nerve growth factor (NGF). This design enabled controlled, NIR-triggered delivery and improved BBB permeability. The system inhibited tau hyperphosphorylation and aggregation. In AD mice, it reduced oxidative stress, repaired neurological damage, and improved memory (Zhou et al., 2020).
Black phosphorus (BP), a 2D nanomaterial, demonstrates potent ROS scavenging abilities. A BP-based composite was shown to reduce mitochondrial oxidative stress and α-synuclein accumulation (Cheng et al., 2023). BP also possesses a tunable energy bandgap for broad spectrum absorption. It degrades into biocompatible oxides, ensuring low toxicity (Xiong et al., 2020).
Gold nanorods (GNRs) were employed to construct a dual-function system (GAS) for Aβ aggregation. Without NIR irradiation, GAS serves as a visual detector. Upon NIR exposure, GNR-induced hyperthermia disrupts Aβ protofibrils and activates thermophilic alkaline phosphatase ST0779. The activated system then degrades Aβ, inhibits further aggregation, and reduces Aβ-mediated peroxidase activity, thereby mitigating toxicity (Liu D. et al., 2019). Sudhakar et al. attributed the photothermal effect of Silver Triangular Nanoprisms (AgTNPs) to hyperthermia induced by in-plane dipole resonance. Similarly, NIR-irradiated AgTNPs diminished fibril content and enhanced cell viability (Sudhakar and Mani, 2019).
Prussian blue nanoparticles (PBNPs) exhibit intrinsic enzyme-like activities. One PBNP system, coated with erythrocyte membranes, inhibits and depolymerizes Aβ aggregates via photothermal effects. It also mitigates ROS, eliminates plaques, and repairs memory in mouse models (Li L. et al., 2024). Another was modified with peptides to target Aβ across the BBB (Song X. et al., 2023). In a different approach, a nanosystem combined CQDs, cerium oxide (Ce), and macrophage membranes. The membrane exhibited anti-inflammatory properties, while ultra-small Ce particles grown on it scavenged ROS to mitigate oxidative stress, thereby enhancing the ability of CQDs to inhibit Aβ aggregation (Chi et al., 2024).
2.2.3 Special composite nanoplatform
Composite nanomaterials offer multifaceted therapeutic avenues. Ge et al. developed U-CNCoP, which combines graphitic carbon nitride (g-C3N4) with cobalt phosphide (CoP) cocatalyst. The incorporation of CoP accelerated the segregation and transfer of photogenerated electrons within g-C3N4, thereby enhancing photocatalytic activity. The material exhibited strong anti-oxidative stress mitigation and suppressed Aβ aggregation. In addition, U-CNCoP exerted extensive neuroprotective effects and significantly enhanced cognitive function in an AD mouse model (Ge et al., 2023). Another approach by Ge et al. utilized KLVFFAu-CeO2 (K-CAC) nanocomposites. In this structure, CeO2 provides antioxidant ROS scavenging capacity through its Ce3+/Ce4+ redox cycle, thus preventing cellular damage. Au NRs were used as carriers for adsorption of the Aβ-targeting inhibitory peptide KLVFF. Tip modification of gold nanorods with CeO2 improved the photothermal conversion efficiency. At the same time, the gold nanorod plasma extended the CeO2 photocatalysis into the NIR spectrum via hot electron injection, which enhanced the redox properties of cerium dioxide. K-CAC exhibited enhanced BBB permeability. KLVFF peptide component promotes Aβ targeting, inhibits the aggregation of monomers, and contributes to minimizing the damage to normal tissues (Ge et al., 2022).
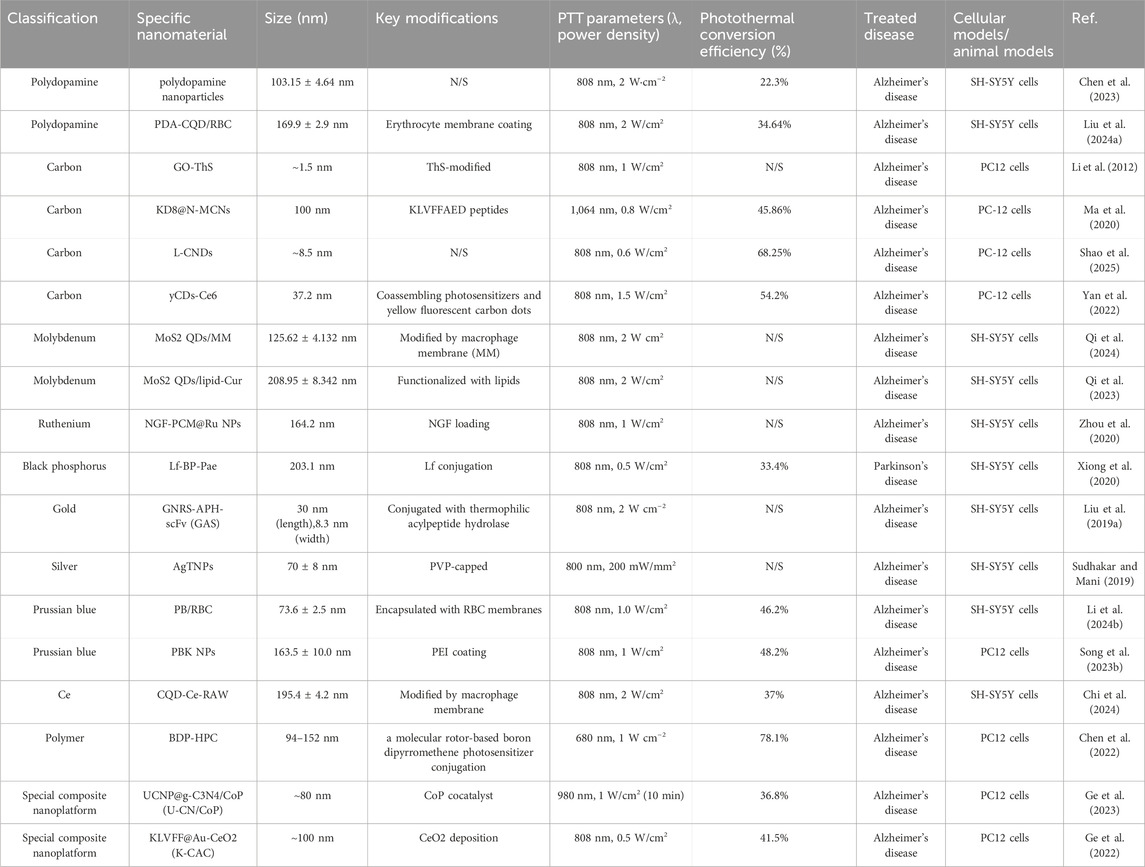
The information involving the classification, parameters and functionalization of the above mentioned nanomaterials for PTT applied to neurodegenerative diseases is shown in Table 2.
2.3 Comparative analysis of organic and inorganic nanoplatforms for neuro-applications
The successful application of photothermal therapy in neurological disorders hinges on the careful selection of nanomaterials. While both organic and inorganic nanoplatforms have shown promise, their inherent differences significantly influence their suitability for specific neuro-applications. This section provides a comparative analysis of these two classes of nanomaterials. We will delve into their biocompatibility and how they interact with biological systems, the scalability and cost-effectiveness of their production, and the regulatory hurdles that must be overcome for clinical use.
2.3.1 Biocompatibility and biodegradation
Organic nanomaterials generally present favorable safety profiles for biomedical applications. Many polymeric nanostructures, for instance, are inherently biodegradable (Bhargav et al., 2020). Specific examples include polycaprolactone, a low-cost, FDA-approved bioresorbable polyester (Chen et al., 2020), and polydopamine, which offers excellent biocompatibility (Dube et al., 2021). Lipid-based nanoparticles and nanogels are also valued for high biodegradability and can be cleared from the body (Bhargav et al., 2020; Vashist et al., 2023). The photosensitizer ICG shows low biotoxicity, binding to plasma proteins for rapid liver excretion (Cao X. et al., 2023). ICG molecules can also decompose during photothermal heating (Hu et al., 2018).
Conversely, the biological fate of inorganic nanomaterials presents challenges. Many are non-degradable and can accumulate, raising toxicity concerns (Lee et al., 2024). The physiological compatibility of single-walled carbon nanotubes remains debated (Sagar and Nair, 2018), while the non-degradable nature of gold nanoparticles prompts concerns about cumulative toxicity (Turkmen Koc et al., 2024). Nevertheless, certain inorganic systems, including zirconium-based MOFs and Bi2Se3 nanodisks, have demonstrated low toxicity (Bybordi et al., 2024; Dai et al., 2024). Furthermore, research indicates cellular degradation of AuNPs is possible, depending on particle size (Dube et al., 2022).
To improve systemic tolerance, nanocarriers are frequently engineered to evade the mononuclear phagocyte system (MPS) (Joseph and Nance, 2022). Surface modification is a key strategy; coating nanostructures with polymers like PEG can mitigate toxicity and limit MPS clearance (Vashist et al., 2023; Turkmen Koc et al., 2024). An advanced technique involves camouflaging nanoprobes with biomimetic membranes, such as those from platelets. This approach boosts biocompatibility and helps the nanostructures elude immune detection (Geng et al., 2020; Huang X. et al., 2023).
2.3.2 Manufacturing scalability and cost-effectiveness
The synthesis of organic photothermal agents has evolved beyond the difficult processes of traditional methods (Lee et al., 2024). Modern strategies like self-assembly offer a simpler route, bypassing complex synthesis and processing stages (Dube and Panda, 2023). Some fluorescent nanoparticles are now fabricated through a straightforward, one-step green method that requires no organic solvents (Dube et al., 2021).
Manufacturing technology shows similar progress. Microfluidics enables precise control over nanoparticle properties, a clear improvement over conventional batch production (Rabiei et al., 2020). This technique allows for precise regulation of liposome size and distribution, yielding high drug-loading efficiency and stability (Cao X. et al., 2023). Cost-effectiveness is improved by using peptide ligands, which are cheaper to produce than antibodies (Rabiei et al., 2020). Additionally, biomimetic nanoprobes derived from abundant platelets hold immense potential for clinical translation (Geng et al., 2020).
While inorganic agents can be costly and difficult to reproduce (Lee et al., 2024), innovative methods now permit scalable, economical production. For example, TA-VOx nanobranches are synthesized via a simple, one-step self-assembly (Guo et al., 2022). Gold nanorose creation has been refined into a fast, green aqueous process (Dube et al., 2022). Scalable techniques for metal nanoparticles include chemical reduction and microwave-assisted synthesis (Singh et al., 2025). Despite such progress, significant financial and technical hurdles still impede the clinical translation of gold nanoparticles, limiting widespread commercial use (Turkmen Koc et al., 2024).
2.3.3 Regulatory hurdles and translational potential
The clinical translation of nanomedicines is impeded by an inadequate systematic exploration of nano-bio interfaces. Upon entering a biological system, nanoparticles acquire a biomolecular corona that profoundly impacts the pharmacokinetic profile, yet research often overlooks how nanocarriers affect vital cellular functions like mitochondrial metabolism (Sagar and Nair, 2018; Bhargav et al., 2020). Progress is further stalled by a lack of uniform methodologies for evaluating toxicity, efficacy, brain uptake, and NIR dosage control (Sagar and Nair, 2018; Bhargav et al., 2020; Joseph and Nance, 2022).
Significant safety considerations also arise. Even targeted nanoformulations exhibit substantial accumulation in the liver, spleen, and kidneys (Rabiei et al., 2020). The long-term in vivo fate of materials like gold nanoparticles remains ambiguous, and non-degradable delivery systems necessitate subsequent surgical removal (Bybordi et al., 2024; Turkmen Koc et al., 2024). For brain therapies, cranial obstruction of NIR light often requires invasive craniotomy (Guo et al., 2022).
Furthermore, preclinical models that fail to recapitulate clinical complexities, such as tumor heterogeneity, can produce misleading outcomes (Bhargav et al., 2020). Testing across multiple animal models, including large animals, is therefore essential before human studies (Joseph and Nance, 2022). Despite these challenges, some nanomedicines have entered early-phase clinical trials for brain cancer (Bhargav et al., 2020). Future advancements depend on designing stimuli-responsive platforms for remote release and personalized systems, like those using patient-specific cancer cell membranes, to address tumor heterogeneity (Rabiei et al., 2020; Huang X. et al., 2023).
3 Applications of PTT in neural tumors and neurodegenerative diseases
3.1 PTT for neural tumors
3.1.1 PTT and glioma
Gliomas are the most common tumors in the nervous system, accounting for nearly half of all brain tumors, with an annual incidence of approximately 30–80 cases per million people (Li T. et al., 2022). A common approach for treating glioma is PTT, which utilizes the penetrating ability of NIR light. In this process, photosensitizers with high photothermal conversion efficiency absorb light and transform it into thermal energy, achieving tumor ablation through heat (Liu D. et al., 2023). To improve precision, nanoparticles integrated with PAI and MRI allow for accurate localization of tumor cells. This capability enables targeted thermal therapy while minimizing side effects (Guo et al., 2018; Tao et al., 2024). Advanced delivery systems, such as photothermally active micromotors, can generate a temperature gradient under continuous illumination. This creates a thermophoretic force that propels the micromotor forward and can trigger dopamine polymerization in the brain, enhancing the regionalization of PTT (Li Z. et al., 2023). These micromotors can also be loaded with chemotherapeutic agents to achieve concurrent anti-tumor effects (Li H. et al., 2023).
While PTT can destroy glioma cells independently, its therapeutic efficacy is constrained by factors like cancer metastasis, patient variability, nanomaterial biotoxicity, and inflammation (Hildebrandt et al., 2002; Cheng et al., 2014; Melamed et al., 2015; Liu Y. et al., 2021; Overchuk et al., 2023). Consequently, research indicates that integrating PTT with other treatments enhances its effectiveness, reduces adverse effects, and improves the overall patient experience (Cheng et al., 2014; Liu et al., 2019b; Xie et al., 2020; Xu and Liang, 2020; Huang et al., 2022; Qiao et al., 2022; Zhang et al., 2022a; Overchuk et al., 2023).
PTT demonstrates significant synergy with chemotherapy through several mechanisms. For instance, mild photothermal effects (40°C–44°C) can increase blood flow, improving drug delivery to the tumor (Cao Y. et al., 2023). Other strategies achieve controlled chemotherapy release. One method uses NIR light to trigger the separation of a gatekeeper molecule, releasing DOX and p53 for concurrent gene therapy (Duan et al., 2017). Another employs a magnetic field to guide nanomaterials, with subsequent DOX release prompted by PTT-induced heat and high tumoral ROS (Jiang et al., 2024). Furthermore, PTT-induced hyperthermia enhances the cellular uptake of nanoparticles, leading to severe lysosomal disruption (Huang et al., 2019). The elevated temperatures also directly increase the cytotoxicity of chemotherapeutic agents (Dong et al., 2016).
A key advantage of PTT is its ability to modulate the tumor microenvironment, thereby sensitizing gliomas to both chemotherapy and radiotherapy. Specifically, PTT can enhance blood flow within tumors and alleviate hypoxia (Li J. et al., 2023). Studies confirm that combining PTT with chemotherapy reduces the expression of hypoxia-inducible factor-1 (Zeng et al., 2023). This approach has been shown to lessen tumor hypoxia and inhibit glioma growth in situ (Zhou et al., 2024). The sensitizing effect also benefits radiotherapy, as experiments reveal that combining PTT with radiation yields a greater destructive impact on cells than radiotherapy alone (Kargar et al., 2018). An innovative strategy combined PTT with hyperbaric oxygen and chemotherapy to overcome temozolomide (TMZ) resistance and increase the chemosensitivity of glioma stem cells (Zeng et al., 2019).
PTT can also be leveraged to initiate a potent anti-tumor immune response, a strategy known as photothermal immunotherapy. The photothermal ablation of cancer cells induces immunogenic cell death (ICD), releasing tumor-associated antigens (TAAs) and other molecular patterns that activate the immune system (Xu and Liang, 2020; Sun et al., 2022). The resulting systemic response can target residual or metastatic tumors and establish immune memory to prevent recurrence (Xu and Liang, 2020; Huang et al., 2021). This process effectively transforms immunologically “cold” tumors into “hot” ones, making them more susceptible to immune checkpoint blockers (Xie et al., 2020; Zhao et al., 2022). Supporting this, one study found that combining PTT with an anti-PD-1 antibody produced the strongest inhibition of tumor regeneration (Nie et al., 2023). Beyond ICD, PTT has also been shown to reprogram tumor-associated macrophages from a tumor-promoting to a tumor-suppressing phenotype, further boosting immunotherapy’s effectiveness (Overchuk et al., 2023).
3.1.2 PTT and peripheral nerve sheath tumors
Peripheral nerve sheath tumors are rare, aggressive sarcomas with a generally poor prognosis. Given that these tumors are resistant to traditional chemotherapy and lack effective targeted therapies, current treatment options remain challenging (Sweeney et al., 2016). PTT can directly eliminate tumors via photothermal ablation. It may also decrease the expression of phosphorylated extracellular regulated protein kinases (ERK). In conjunction with mitogen-extracellular kinase inhibitors, PTT synergistically influences the oncogene Ras signaling pathway. This occurs by impeding ERK activation, thereby promoting cell apoptosis and necrosis (Sweeney et al., 2016). Furthermore, the high temperatures generated during PTT can induce endoplasmic reticulum stress (ERS) in tumor cells. ERS consequently triggers endogenous apoptosis (Gu et al., 2022). Research by Gu et al. involved the innovative application of an NIR-III laser. Photothermal penetration experiments verified its highly efficient photothermal capabilities comparable to conventional NIR-I and NIR-II lasers. Compared to control groups and lower-dose NIR-III treatment (0.5 W cm−2, 5 min), NIR-III application (1 W cm−2, 5 min) resulted in complete tumor eradication (Gu et al., 2022).
3.1.3 PTT and spinal metastases from in situ tumors, and brain metastases
Tumor metastasis presents a significant therapeutic challenge. Spinal metastases are a frequent complication in advanced lung cancer, while brain metastases affect many patients with various primary cancers. These secondary growths cause severe clinical issues and worsen patient prognoses (Hayat et al., 2007; Li et al., 2014; Zakaria et al., 2020; Rahman et al., 2023).
To address this, researchers are developing targeted nanoplatforms. One system, AMMD, uses a peptide to achieve high affinity for tumor cells, improving drug accumulation. It enables a combined photothermal and drug co-delivery strategy for spinal tumors. The specific drugs include phosphoinositide 3-kinase (PI3K) inhibitors and chemotherapeutic agents. The photothermal effect also enhances the drugs’ anti-osteolytic efficacy, reducing microstructural damage to the invaded spine (Ma et al., 2021). In other strategies, PTT can stimulate copper ion release, initiating a Fenton reaction. This elevates ROS levels, oxidizes DNA and proteins, and repolarizes macrophages. Such events trigger the ICD effect, activating anti-tumor immunity and consequently inhibiting tumor development, brain metastasis, and postoperative tumor spread (Tong et al., 2023).
3.2 PTT for neurodegenerative diseases
3.2.1 PTT and Alzheimer’s disease
AD is the most prevalent neurodegenerative condition in older adults, comprising approximately 70% of dementia diagnoses. Projections suggest the number of affected individuals could surpass 100 million by 2050 (Huang D. et al., 2023). Although its complete pathogenesis is not fully understood, two primary pathological events are recognized: the extracellular deposition of Aβ aggregates and the intracellular formation of neurofibrillary tangles (NFTs) (Hamley, 2012; Lv et al., 2019; Sudhakar and Mani, 2019; Liu W. et al., 2021; Zeng et al., 2021). Aβ oligomers trigger both ROS generation and inflammation. They also activate mitochondrial death pathways, leading to tau protein hyperphosphorylation. Hyperphosphorylated tau subsequently detaches from microtubules, aggregates into NFTs, and culminates in neuronal death (Lv et al., 2019).
A central therapeutic goal involves hindering Aβ accumulation and eliminating existing protofibrils (Ling et al., 2021; Zeng et al., 2021). Given that amyloid formation is strongly temperature-dependent, PTT presents a viable approach (Liu W. et al., 2021). Localized heat generated by PTT can disintegrate Aβ aggregates and disrupt the stable physiological conditions necessary for their formation (Zeng et al., 2021). For instance, Song et al. demonstrated that Prussian blue-based nanomaterials under NIR radiation could dissociate Aβ fibrils (Song X. et al., 2023). Ge et al. introduced a CoP co-catalyst whose photothermal properties aid in diminishing Aβ deposition. Concurrently, it improves the photocatalytic efficiency of hydrogen evolution, producing H2 to selectively scavenge detrimental ROS, such as ·OH (Ge et al., 2023).
In addition to amyloid clearance, PTT can enhance BBB permeability during treatment (Zhou et al., 2020; Ge et al., 2022; Qi et al., 2023; Ye et al., 2023; Chi et al., 2024; Liu et al., 2024a). Enhanced permeability creates favorable conditions for drugs and nanocarriers to cross the BBB and reach Aβ protein aggregation sites. A study combining PTT with chemotherapy effectively opened the BBB, increasing substance accumulation within the mouse brain (Qi et al., 2023). Moreover, the photothermal effect can function as a “switch” to control the release of therapeutic drugs (Ling et al., 2021). Research using NGF-PCM@Ru NPs showed that drug release at 42°C was 3.7 times greater than at 37°C (Zhou et al., 2020). These properties suggest a broad potential for PTT in future clinical practice of AD.
3.2.2 PTT and Parkinson’s disease
PD is characterized by dopaminergic neuron death, which depletes striatal dopamine and promotes α-synuclein aggregation (Goedert, 2015). PTT presents a promising strategy by enhancing nanomedicine delivery across the blood-brain barrier and enabling selective drug release via NIR light. This approach boosts the efficacy of medications like minocycline in clearing α-synuclein clumps (Li T. et al., 2021; Cheng et al., 2023). A mild photothermal effect offers synergistic neuroprotection by scavenging surplus ROS, mitigating neuroinflammation, and diminishing pathogenic protein buildup. Such actions help restore striatal dopamine, improve neurotransmitter signaling, and relieve motor impairments in PD mouse models (Xiong et al., 2020; Cheng et al., 2023). NIR irradiation amplified the neuroprotective capacity of BP-MT by facilitating matrine release and accelerating ROS clearance (Cheng et al., 2023).
Notably, minocycline, as part of a synergistic chemical and photothermal treatment platform, shows potential for treating other neurodegenerative conditions like amyotrophic lateral sclerosis and AD. GDY Nanosheets can be loaded with agents like SOD1 or chemical inhibitors targeting Aβ aggregation, allowing for NIR-controlled systemic administration and release (Li T. et al., 2021).
3.2.3 PTT and Huntington’s disease
Huntington’s disease is a severe genetic disorder. It causes psychological instability, along with motor and cognitive decline (Ross and Tabrizi, 2011). The accumulation of large, insoluble protein aggregates is cytotoxic and represents a key therapeutic target. The buildup of protein aggregates can damage Huntington’s proteins essential for normal development (Truant et al., 2008; Arrasate and Finkbeiner, 2012). PTT can address these protein deposits. PTT disrupts existing aggregates through the photothermal treatment of unlabeled polyglutamine (polyQ), the coding trigger for the Huntington’s gene. It can also slow their formation. Furthermore, PTT could eliminate the protection against mutant Huntington’s proteins while producing thermal damage (Nedosekin et al., 2021). Additionally, PTT utilizes optical phenomena associated with the photothermal ablation process. The phenomena enable the visualization of protein aggregates. They also allow monitoring of the ablation process and control over treatment parameters (Nedosekin et al., 2021). High-resolution optical images documented aggregates in living worms before and after laser application. Post-treatment images revealed that some aggregates vanished, while others displayed significant alterations in shape, signifying thermal damage (Nedosekin et al., 2021).
4 Mechanisms of PTT application in neural tumors and neurodegenerative diseases
4.1 PTT initiates heat shock response
When NIR starts PTT of cancer cell regions with the help of PTA, the light-induced heat disrupts the integrity of the cell membrane, causing a large amount of Ca2+ to flow from outside the cell membrane into the cell, triggering chemical damage (Xie et al., 2020). When the tissue temperature exceeds 39°C, some proteins begin to aggregate and denature, and when the temperature rises to 41°C, a heat shock response is initiated, which induces rapid changes in gene expression patterns, of which heat shock proteins (Hsps) are important regulators to mitigate the effects of thermal injury (Jolly and Morimoto, 2000; Didelot et al., 2006; Li et al., 2020; Xie et al., 2020; Ren et al., 2022). Most Hsps are highly cytoprotective and can act as molecular chaperones for other cellular proteins. Upon severe exposure to high temperatures, heat-damaged proteins are sequestered through interactions with molecular chaperones and refolded to their natural state or degraded by the proteasome (Didelot et al., 2006). When cells are exposed to sublethal heat shock, they will temporarily acquire resistance to multiple stress conditions as well as multidrug resistance (Jolly and Morimoto, 2000). There is literature specifically designing photothermal therapies targeting Hsp70 to promote apoptosis in cancer cells (Ali et al., 2016), while SDT shows significant promise in synergistic PTT therapeutic applications due to its ability to downregulate the expression of Hsp90(Cao et al., 2022).
4.2 PTT induces apoptosis
PTT initiates apoptosis through multiple interconnected cellular stress responses. The thermal effects can compromise lysosomal membranes, leading to lysosomal membrane permeability. This allows tissue proteases to escape and activate pro-apoptotic proteins like Bid, which in turn exacerbates mitochondrial damage (Boya and Kroemer, 2008; Cao et al., 2021; Kadkhoda et al., 2022). Research confirms that thermal effects diminish the anti-apoptotic protein Bcl-2 while increasing the pro-apoptotic protein BAX (Liu et al., 2022). As mitochondria are particularly sensitive to heat (Kadkhoda et al., 2022), thermal stress also triggers mitochondrial membrane depolarization. This process results in the generation of ROS (Mocan et al., 2014), which inflict oxidative damage on proteins, lipids, and nucleic acids (Gorrini et al., 2013; Jaque et al., 2014; Ren et al., 2022). Studies on glioma indicate that this oxidative stress damages mitochondria, causing a loss of membrane potential and superoxide production that culminates in caspase-mediated apoptosis (Markovic et al., 2011). Similarly, the high temperatures from PTT can induce ERS. This response impairs the proper folding of proteins, and the resulting accumulation of misfolded proteins can ultimately trigger apoptosis in tumor cells (Gu et al., 2022).
The DNA damage caused by heat or ROS production activates the tumor suppressor p53, a key pro-apoptotic factor (Trachootham et al., 2009; Liou and Storz, 2010; Gorrini et al., 2013). Activated p53 can induce mitochondrial outer-membrane permeabilization (MOMP), which leads to apoptosome formation and initiates the apoptotic cascade (Evan and Vousden, 2001; Chipuk and Green, 2006; Trachootham et al., 2009; Ali et al., 2017; Gousias et al., 2022; Zhao et al., 2022). Experimental results support this, showing elevated p53 expression alongside reduced levels of Bcl-2 following PTT (Yao et al., 2022). Furthermore, p53 activation can deplete cell cycle regulators like p21, promoting G1/M or G2/M arrest and thereby inducing apoptosis (Hildebrandt et al., 2002; Gousias et al., 2022; Yu et al., 2022). Beyond these general pathways, PTT can be directed at specific oncogenic signaling loops. In glioblastoma, for example, the overexpressed oncogene MSH6 forms a feedback circuit with CXCR4 and TGFB1. This loop accelerates gliomagenesis, proliferation, and invasion while providing anti-apoptotic effects. Researchers have identified this specific oncogenic circuit as a potential therapeutic target for PTT in treating this cancer (Chen et al., 2019).
The molecular pathways of apoptosis associated with PTT of neural tumors and neurodegenerative diseases are illustrated in Figure 1.
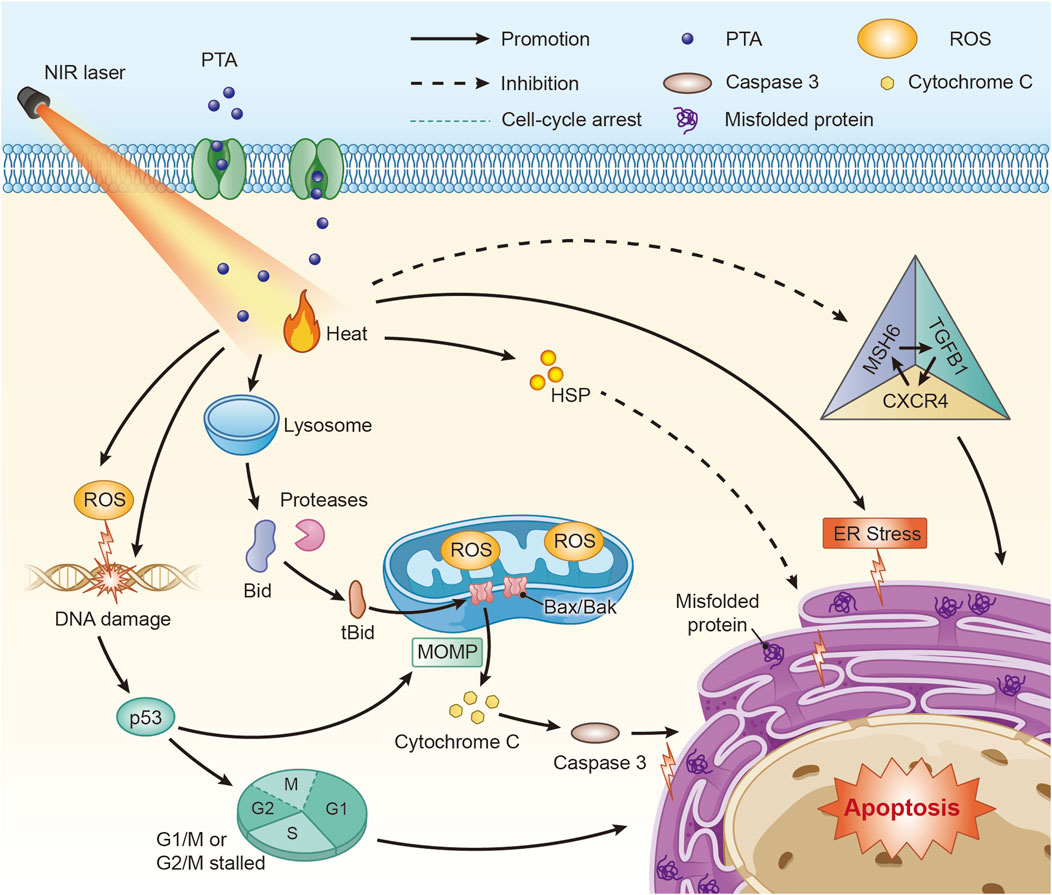
Figure 1. Molecular mechanisms of PTT associated with apoptosis in the treatment of neural tumors and neurodegenerative diseases. PTT can induce apoptosis through various molecular pathways such as p53 and ROS, among which the MSH6-CXCR4-TGFB1 feedback loop is a target used for PTT on GBM(Abbreviations: PTA: photothermal transduction agent, MOMP: mitochondrial outer-membrane permeabilization, ROS: Reactive oxygen species, HSP: heat shock proteins, ER: endoplasmic reticulum.)
4.3 PTT enhances BBB permeability
The BBB is a physiological feature within the cerebral vasculature. It effectively regulates the passage of ions, cells, and molecules between the blood and brain tissue. The BBB also maintains the stable microenvironment crucial for neuronal signaling (Thangudu et al., 2020). However, protective function poses a significant challenge for designing therapies. The barrier’s capacity to prevent foreign substances from entering the brain impedes treatment delivery for brain disorders. Encouragingly, considerable research indicates PTT can enhance BBB permeability via the photothermal effect. This approach shows promise for treating AD (Zhou et al., 2020; Ge et al., 2022; Qi et al., 2023; Ye et al., 2023), PD (Cheng et al., 2023), depression (Zhang et al., 2021), and other neural tumors and neurodegenerative diseases.
4.4 PTT utilizes the photothermal effect to selectively release drugs and promote drug absorption
PTT serves as an external stimulus. It facilitates drug release specifically at the lesion site with temporal and spatial control. Photothermal effects can initiate drug release through various mechanisms, including the disruption of the drug carrier or heat-induced structural changes in sensitive materials. Alternatively, heat-responsive chemical bonds may break, or drug-carrier interactions can be destabilized. Furthermore, adjusting the laser intensity permits regulation of the drug release rate (Rai et al., 2010; Cheng et al., 2014; Liu et al., 2019b; Xie et al., 2020; Zhang et al., 2022a). Mild localized heat (≤43°C), generated by the nanomaterial template, also boosts the fluidity of the cell membrane. This increased fluidity helps nanomaterials penetrate cell or endosomal membranes more readily. Consequently, moderately high temperatures (40°C–43°C) during PTT can enhance cellular uptake. The enhancement results from increased cell membrane permeability (Cheng et al., 2014; Kim et al., 2016).
4.5 PTT improves intra-tumoral blood supply
PTT heating elevates tumor blood flow. It also increases endothelial gaps within the tumor vasculature and improves hemoglobin oxygen saturation (Jaque et al., 2014; Liu et al., 2019b; Overchuk et al., 2023). Elevated blood flow contributes to greater microvascular permeability, which broadens the therapeutic options for cancers resistant to penetration. Enhanced permeability facilitates better drug delivery and diffusion (Cao Y. et al., 2023). As a result, the effectiveness of chemotherapeutic agents against cancer cells is amplified (Dong et al., 2016; Liu et al., 2019b; Xu and Liang, 2020). Increased oxygen saturation within blood vessels can lessen tumor hypoxia. The improvement of the hypoxic microenvironment may counteract tumor drug resistance (Liu et al., 2019b; Xu and Liang, 2020). Improved tumor oxygenation can render the tumor more susceptible to radiotherapy and chemotherapy (Hildebrandt et al., 2002; Jaque et al., 2014; Liu et al., 2019b; Xie et al., 2020).
4.6 PTT utilizes the photothermal effect to promote gene transfection
The photothermal effect can disrupt endosomal membranes, which could promote endosomal escape, aiding cytoplasmic gene delivery in PTT/gene therapy strategies. Normally, cells internalize nucleic acids carried by nanoscale vectors via cytosis. The uptake mechanism produces endosomes that typically fuse with lysosomes. Photothermal facilitation helps therapeutic agents escape from endosomes before the fusion occurs (Kim et al., 2016). Additionally, short double-stranded DNA or RNA can be attached to metal nanoparticles. If the heat generated by PTT is adequate to melt these nucleic acids, it induces their de-hybridization. Consequently, the nucleic acids are released from the nanoparticle surface. The process achieves photothermal-induced gene release and enhances transfection efficiency (Kim and Kim, 2014; Hu et al., 2016; Kim et al., 2016; Xie et al., 2020).
4.7 PTT improves the efficiency of Fenton and Fenton-like reactions
CDT utilizes transition metal ions, including Fe2+, Co2+, Cu+, and Mn2+. These ions facilitate Fenton or Fenton-like reactions. Such processes convert H2O2, often overexpressed in the lesion’s cellular microenvironment, into ROS. The resulting ROS exhibit high toxicity towards cells (Qiao et al., 2022; Zhang et al., 2022a). Additionally, the photothermal effect associated with PTT can boost the efficiency of Fenton and Fenton-like reactions. The enhancement consequently improves the overall therapeutic efficacy of CDT (Pandey et al., 2020; Guo et al., 2022; Zhang et al., 2022a; Cao Y. et al., 2023).
4.8 PTT induces an immune response in the body
PTT ablates cancer cells, triggering ICD. Necrotic tumor cells then discharge various molecules, including damage-associated molecular patterns, TAAs, neoantigens, and pro-inflammatory cytokines. These released substances activate specific anti-tumor immune responses. Such responses involve the activation of immune effector cells and the promotion of T-cell infiltration. Cytokine secretion is also increased. Additionally, acute inflammation results from secreted pro-inflammatory factors and the infiltration of tumor immune cells. The inflammatory state fosters the activation and recruitment of dendritic cells (Xu and Liang, 2020; Zhao et al., 2022; Overchuk et al., 2023). In a similar way, PTT can modulate antiviral immune responses when confronting viruses. The process is achieved through the massive production of inflammatory factors, such as IL-6 and TNF-α during virus inactivation (Bai Y. et al., 2023).
The common mechanisms of PTT in combination with other therapies in the treatment of neural tumors and neurodegenerative disorders are illustrated in Figure 2.
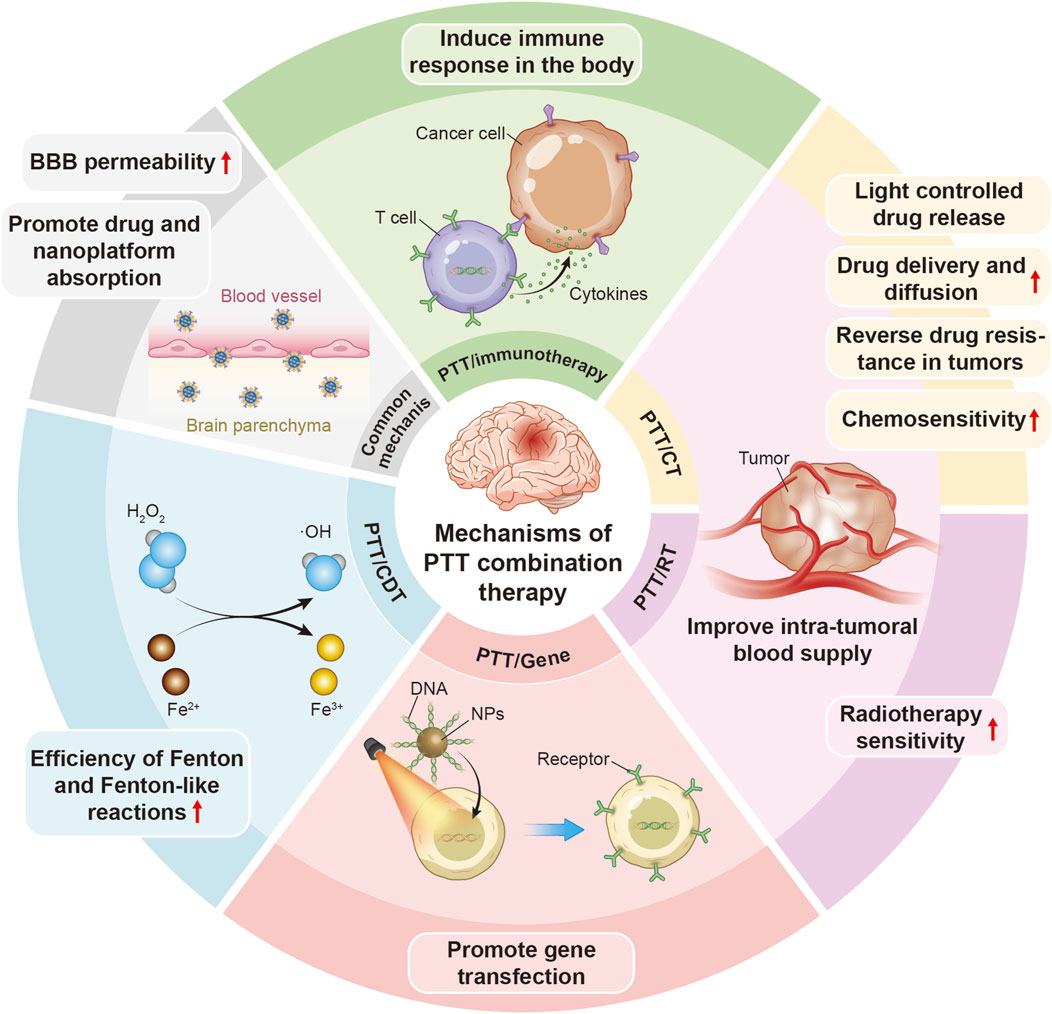
Figure 2. A schematic illustration of common mechanisms of PTT in combination with other therapies in the treatment of neural tumors and neurodegenerative diseases.
5 Advantages and disadvantages of PTT in neural tumors and neurodegenerative diseases
PTT has demonstrated multidimensional advantages in the treatment of nervous system diseases, not only contributing to the innovation of therapeutic strategies, but also greatly enhancing the safety and efficacy of treatment. The synergistic application of PTT and chemotherapy, through targeted delivery of drugs, significantly reduces systemic toxicities and side effects (Wang et al., 2014; Ma et al., 2020; Newland et al., 2022), achieves the precision of treatment and reflects the synergistic principle of “1 + 1>2” (Wang et al., 2014; Lan et al., 2021; Lu et al., 2021; Song Z. et al., 2023), which is also applied in its multimodal combination with PDT (Keyvan Rad et al., 2018), radiotherapy (Kargar et al., 2018; Yin et al., 2022), gene therapy (Hu et al., 2016) and so on. The integration of PTT technology with advanced imaging technologies (e.g., PAI, MRI, FLI) provides real-time, high-precision visualization guidance for surgical operations, which greatly improves the precision and safety of brain tumor surgery (Jin et al., 2017; Hu et al., 2018; Qian et al., 2018; Wang S. et al., 2021; Li Y. et al., 2022; Zhang et al., 2022a).
Further, by stimulating the immune system and triggering innate and adaptive immune responses against tumor cells, PTT not only directly inhibits tumor growth, but also effectively prevents tumor recurrence and thus improves the prognosis of tumors, demonstrating its potential in regulating the body’s immune response (Liu et al., 2019c; Huang et al., 2021; Sun et al., 2022). In addition, PTT facilitates the delivery of therapeutic drugs and platforms for neurological diseases by improving the permeability of the BBB, which covers a wide range from neurological tumors (Cao Y. et al., 2023) to neurodegenerative diseases (e.g., Alzheimer’s disease (Zhou et al., 2020; Ge et al., 2022; Qi et al., 2023; Ye et al., 2023)). Particularly important is that PTT utilizes photothermal response properties to achieve intelligent controlled release of therapeutic drugs, further enhancing the relevance and efficiency of treatment (Agarwal et al., 2011; Wang et al., 2013; Duan et al., 2017; Zhang et al., 2019; Li T. et al., 2021; Ling et al., 2021; Zhang et al., 2021; Wu F. et al., 2022; Manivasagan et al., 2023; Tong et al., 2023).
Although PTT has demonstrated significant advantages in the treatment of nervous system diseases, it still faces multiple challenges that require in-depth investigation and strategy optimization. The first problem is the limitation of NIR light penetration into tissues, which limits the depth of the photothermal effect and the efficiency of drug delivery, thereby affecting the intensity and reliability of the treatment (Lu et al., 2014; Wang et al., 2014). In addition, the heat shock reaction triggered at the initial stage of PTT may enhance the stress adaptation and drug resistance of cancer cells, thus hindering the effectiveness of the treatment (Jolly and Morimoto, 2000). PTT in photothermal ablation causes cellular necrosis, and thus induces inflammation. Inflammation can increase neutrophil levels and form neutrophil extracellular traps, further awakening dormant cancer cells (Liu Y. et al., 2021). In contrast, MPTT induces apoptosis of intact cell membranes, which prevents the inflammation caused by cell necrosis (Liu Y. et al., 2021).
Excessive or continuous heating during PTT may lead to vascular collapse and diminished tumor perfusion (Overchuk et al., 2023). Consequently, treatment protocols require a careful balance of photothermal properties, individual patient differences, and other factors to guarantee both safety and efficacy. Furthermore, PTT can activate autophagy mechanisms. The finding suggests that incorporating anti-autophagy strategies might be essential for improving therapeutic results (Zeng et al., 2022; Li J. et al., 2023). The advancement and use of nanomaterials also necessitate continuous enhancements in biocompatibility, cytotoxicity, and BBB penetration to realize optimal therapeutic performance (Cheng et al., 2014). It is important to recognize that PTT-induced ERS encounters strong resistance in certain malignant peripheral nerve sheath tumor cells, such as STS26T cells. The resistance arises from these tumor cells’ unique endoplasmic reticulum compositions, which permit increased expression of endoplasmic reticulum molecular chaperones and boost the degradation of unfoldable proteins, thereby creating a potent protective effect (Gu et al., 2022). Additionally, the effectiveness of PTT can be episodic and short-lived, especially when addressing protein aggregates in neurodegenerative conditions. This indicates that therapeutic strategies might need to be combined with long-term management programs to overcome the limitations of short-term efficacy and secure lasting therapeutic benefits (Nedosekin et al., 2021).
Nanotherapeutics for the central nervous system pose considerable neurotoxicity and safety risks. Some inorganic materials are directly toxic; silver nanoparticles provoke cerebral oxidative stress and neuroinflammation. Iron oxide nanoparticles can gather in microglia, causing cytotoxicity by releasing iron and generating reactive oxygen species (Singh et al., 2025). Dendrimers may also trigger adverse immune reactions. A particle’s physical properties, like larger dimensions or a positive surface charge, often elicit stronger immune responses (Vashist et al., 2023).
Methods for breaching the BBB could introduce further dangers. Opening the barrier can cause neurobehavioral side effects and allow circulating toxins into the brain (Kaushik et al., 2018; Thangudu et al., 2020). Moreover, the continuous NIR exposure associated with PTT produces excessive heat, which may damage healthy neural tissue. This poses a critical concern in non-cancerous neurological conditions and underscores the need for precise temperature control (Sagar and Nair, 2018; Liu et al., 2020; Zhao et al., 2021; Bai X. et al., 2023; Zeng et al., 2023).
6 Conclusions and perspectives
The convergence of nanotechnology and PTT offers a new therapeutic paradigm for the treatment of neurological diseases characterized by high disability and low efficacy. A unique aspect of this review is the systematic integration of multiple PTT nanomaterial platforms currently applied in neural tumors and neurodegenerative diseases. It covers different types of carriers such as organic systems (e.g., liposomes and peptide-based nanomaterials) and inorganic materials (e.g., gold and copper nanomaterials). The review focuses on analyzing the design principles of these materials for nervous system applications and exploring how they can be engineered to cope with the pathophysiological characteristics and biobarrier issues that are peculiar to nervous system diseases. A key part of this analysis involves comparing the organic and inorganic platforms based on their respective biocompatibility and biodegradation profiles, the scalability of their production, their overall cost-effectiveness, the regulatory obstacles and prospects for clinical translation. By comparing material selection and design strategies in different disease models, we provide a broad and deep perspective, emphasizing the important role of material innovation in enhancing the efficacy of photothermal therapies and expanding their potential applications in neurological disease treatment.
Crucially, the review also presents a comprehensive overview of the multifaceted clinical mechanisms utilized by PTT in these neurological contexts, including direct cell killing pathways (e.g., apoptosis), microenvironmental modulation (e.g., BBB permeability, vascular effects, drug/gene delivery augmentation), chemotherapy augmentation (e.g., Fenton’s response), and stimulation of host immunity.
PTT has demonstrated broad potential for treating nervous system diseases. The technique is minimally invasive, highly specific, and offers spatiotemporal selectivity (Huang and Lovell, 2017). PTT is particularly useful for the photothermal ablation of tumor cells. It is suitable for preoperative preparation, adjuvant surgery, and managing postoperative residual lesions (Gu et al., 2022). Additionally, optimized NIR lasers could provide feedback on the therapeutic effect for tumors. Among these, the NIR-III laser demonstrated higher efficiency than NIR-I in treating malignant peripheral nerve sheath tumors (Gu et al., 2022). For AD, combining PTT with Aβ inhibitors is considered a potentially better treatment strategy. This is because PTT enhances inhibitor efficacy, and light-responsive drug release systems reduce inhibitor side effects (Liu W. et al., 2021). Similar reasoning could address strong resistance induced by ERS. Therefore, it is hypothesized that combining NIR-III laser therapy with ERS inhibitors, like epimedin or growth hormone-releasing peptide, might improve therapeutic efficacy for certain malignant peripheral nerve sheath tumors (Gu et al., 2022).
The precision of PTT is further enhanced by various targeting strategies, such as dYNH-targeted peptides (Ma et al., 2021), RGD-targeted peptides (Huang et al., 2019; Song W. et al., 2023), pH-driven active targeting (Tong et al., 2023), laser targeting (Nedosekin et al., 2021), folate receptor targeting (Keyvan Rad et al., 2018), cellular markers (Zhang et al., 2019), cancer cell membrane (CCM) coating (Huang X. et al., 2023), immunological antibody-targeted photodetection (DeRussy et al., 2014; Li X. et al., 2021; Ren et al., 2021; Manivasagan et al., 2023) and bio-optical detection (Zhang et al., 2022b), which aim to achieve precise interventions for neurological diseases. Researchers are actively working to enhance the therapeutic impact of PTT. Efforts focus on improving the photothermal efficiency of photosensitizers. Specific strategies include modifying materials with hyaluronic acid and PEG (Cao et al., 2022). Others have combined gold nanoclusters with indocyanine green (Zhuo et al., 2022). Optimizing the density of zinc doping is also being explored (Li et al., 2019). Hydrogels have been combined with chitosan in several studies (Ko et al., 2021; Gao et al., 2022; Wu F. et al., 2022; Dong et al., 2023). Even the use of melanin derived from beards has been investigated (Zhang et al., 2021). Further research is anticipated to advance PTAs performance. Identifying chemicals that boost photothermal conversion is also a key goal.
Integrating advanced imaging with PTT is fundamental for improving therapeutic precision. Detailed anatomical information from MRI and computed tomography enables accurate tumor localization (Chen et al., 2019; Caro et al., 2021), while PAI provides real-time tracking of nanoparticle accumulation (Guo et al., 2017).
Finer procedural control is achieved using sophisticated methods. For instance, two-photon photoluminescence microscopy guides laser irradiation at the micro-level (Caro et al., 2021). Additionally, FRET-based nanoprobes offer sensitive, in-situ temperature feedback (Hu et al., 2018). To maximize specificity, activatable agents triggered by tumor biomarkers like elevated H2O2 or acidity confine the photothermal effect, sparing healthy tissue (Chen et al., 2017; Lee et al., 2024).
Repeated laser photothermal therapy may improve the transient nature of PTT, but this approach requires further experimental validation (Nedosekin et al., 2021). Notably, PTT displays potential for easing postoperative complications, such as peripheral nerve adhesions (Zhan et al., 2023). It can also modulate neurons for scientific research purposes (Zhao et al., 2019; Wu X. et al., 2022; Zhuang et al., 2023). Further investigations could examine PTT’s therapeutic impact on postoperative inflammation and residual lesions. Other potential areas include fever, infection, and related complications in diverse neurological disorders. Compared to traditional magnetothermal stimulation, PTT-induced neurostimulation eliminates the need for permanent brain implants or fibers in animals. It is free from size constraints and has a short excursion time. This positions PTT as a new method for controlling animal neuronal activity in research (Wu X. et al., 2022). Moreover, photothermal modulation affects neurons and muscle cells. This indicates potential applicability to other excitable cell types or experimental systems. It also introduces a fresh approach for the clinical management of neurodegenerative diseases and movement disorders. Additionally, it provides a novel strategy for clinical motor rehabilitation training (Zhuang et al., 2023).
It has been suggested in the literature that local heat generated during PTT triggers a heat shock response, thereby stimulating the production of Hsps (Zeng et al., 2021). Acting as molecular chaperones, Hsps regulate protein folding and activity, refolding proteins that have been misfolded or aggregated (Jolly and Morimoto, 2000; Didelot et al., 2006). The biological actions of Hsps could fundamentally reduce abnormal Aβ aggregation. This reduction may consequently mitigate AD. Nevertheless, research examining PTT’s impact on Hsps in AD remains scarce, awaiting further experimental development and investigation (Zeng et al., 2021).
Artificial intelligence, particularly its machine learning (ML) subset, offers a powerful strategy for nanomaterial design. ML provides efficient, controlled fabrication methods, circumventing the slow, resource-intensive nature of traditional synthesis (Tao et al., 2021; Rao et al., 2024). The approach involves two primary functions: prediction and experiment planning. Predictive models forecast nanoparticle characteristics from given synthesis conditions, while experiment planning algorithms intelligently guide the research process by suggesting subsequent experiments to efficiently identify optimal conditions. In parallel, planning algorithms like heuristic genetic algorithms or Bayesian optimization strategically guide research toward optimal parameters (Tao et al., 2021). Automated robotic and microfluidic platforms amplify these computational tools through high-throughput data generation. Integrating ML with such platforms creates closed-loop systems, accelerating optimization and facilitating novel material discovery (Rao et al., 2024).
Author contributions
HZ: Conceptualization, Investigation, Methodology, Project administration, Software, Validation, Visualization, Writing – original draft, Writing – review and editing. WY: Conceptualization, Investigation, Software, Validation, Visualization, Writing – original draft, Writing – review and editing. YS: Project administration, Resources, Supervision, Writing – review and editing. YL: Project administration, Resources, Supervision, Writing – review and editing. XZ: Project administration, Resources, Supervision, Writing – review and editing. JZ: Project administration, Resources, Supervision, Writing – review and editing. ZC: Project administration, Resources, Supervision, Writing – review and editing. XW: Project administration, Resources, Supervision, Writing – review and editing. XY: Funding acquisition, Project administration, Resources, Supervision, Writing – review and editing. BB: Conceptualization, Funding acquisition, Investigation, Methodology, Project administration, Resources, Supervision, Writing – review and editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This study received fundings from National Natural Science Foundation of China (NOs: 81920661 and 82260454) and Development Project of Jiangxi Province (20202BABL206052). The funders were not involved in the study design, collection, analysis, interpretation of data, the writing of this article or the decision to submit it for publication.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Abbas, M., Zou, Q., Li, S., and Yan, X. (2017). Self-assembled Peptide- and protein-based nanomaterials for antitumor photodynamic and photothermal therapy. Adv. Mater 29 (12), 1605021. doi:10.1002/adma.201605021
Agarwal, A., Mackey, M. A., El-Sayed, M. A., and Bellamkonda, R. V. (2011). Remote triggered release of doxorubicin in tumors by synergistic application of thermosensitive liposomes and gold nanorods. ACS Nano 5 (6), 4919–4926. doi:10.1021/nn201010q
Ali, M. R., Ali, H. R., Rankin, C. R., and El-Sayed, M. A. (2016). Targeting heat shock protein 70 using gold nanorods enhances cancer cell apoptosis in low dose plasmonic photothermal therapy. Biomaterials 102, 1–8. doi:10.1016/j.biomaterials.2016.06.017
Ali, M. R., Rahman, M. A., Wu, Y., Han, T., Peng, X., Mackey, M. A., et al. (2017). Efficacy, long-term toxicity, and mechanistic studies of gold nanorods photothermal therapy of cancer in xenograft mice. Proc. Natl. Acad. Sci. U. S. A. 114 (15), E3110-E3118–E3118. doi:10.1073/pnas.1619302114
Arrasate, M., and Finkbeiner, S. (2012). Protein aggregates in huntington's disease. Exp. Neurol. 238 (1), 1–11. doi:10.1016/j.expneurol.2011.12.013
Bai, X., Yang, Y., Zheng, W., Huang, Y., Xu, F., and Bao, Z. (2023a). Synergistic photothermal antibacterial therapy enabled by multifunctional nanomaterials: progress and perspectives. Mater. Chem. Front. 7 (3), 355–380. doi:10.1039/d2qm01141g
Bai, Y., Huang, P., Feng, N., Li, Y., Huang, J., Jin, H., et al. (2023b). Treat the “untreatable” by a photothermal agent: triggering heat and immunological responses for rabies virus inactivation. Adv. Sci. (Weinh) 10 (2), e2205461. doi:10.1002/advs.202205461
Bhargav, A. G., Mondal, S. K., Garcia, C. A., Green, J. J., and Quiñones-Hinojosa, A. (2020). Nanomedicine revisited: next generation therapies for brain cancer. Adv. Ther. 3 (10), 2000118. doi:10.1002/adtp.202000118
Boya, P., and Kroemer, G. (2008). Lysosomal membrane permeabilization in cell death. Oncogene 27 (50), 6434–6451. doi:10.1038/onc.2008.310
Bybordi, S., Safa, P. B., Eslami, S., Alipourpanahi, S., and Irani, M. (2024). Gold/platinum nanorods/temozolomide-UiO-66-NH(2) metal-organic frameworks incorporated to chitosan-grafted polycaprolactone/polycaprolactone core-shell nanofibers for glioblastoma treatment during chemo-photothermal therapy. Int. J. Biol. Macromol. 283 (Pt 4), 137976. doi:10.1016/j.ijbiomac.2024.137976
Cao, M., Luo, X., Wu, K., and He, X. (2021). Targeting lysosomes in human disease: from basic research to clinical applications. Signal Transduct. Target Ther. 6 (1), 379. doi:10.1038/s41392-021-00778-y
Cao, X., Liu, Q., Adu-Frimpong, M., Shi, W., Liu, K., Deng, T., et al. (2023a). Microfluidic generation of near-infrared photothermal Vitexin/ICG liposome with amplified photodynamic therapy. AAPS PharmSciTech 24 (4), 82. doi:10.1208/s12249-023-02539-2
Cao, Y., Jin, L., Zhang, S., Lv, Z., Yin, N., Zhang, H., et al. (2023b). Blood-brain barrier permeable and multi-stimuli responsive nanoplatform for orthotopic glioma inhibition by synergistic enhanced Chemo-/Chemodynamic/Photothermal/Starvation therapy. Eur. J. Pharm. Sci. 180, 106319. doi:10.1016/j.ejps.2022.106319
Cao, Z., Yuan, G., Zeng, L., Bai, L., Liu, X., Wu, M., et al. (2022). Macrophage-targeted sonodynamic/photothermal synergistic therapy for preventing atherosclerotic plaque progression using CuS/TiO(2) heterostructured nanosheets. ACS Nano 16 (7), 10608–10622. doi:10.1021/acsnano.2c02177
Caro, C., Gamez, F., Quaresma, P., Paez-Munoz, J. M., Dominguez, A., Pearson, J. R., et al. (2021). Fe(3)O(4)-Au core-shell nanoparticles as a multimodal platform for in vivo imaging and focused photothermal therapy. Pharmaceutics 13 (3), 416. doi:10.3390/pharmaceutics13030416
Chen, H., Shi, Y., Sun, L., and Ni, S. (2020). Electrospun composite nanofibers with all-trans retinoic acid and MWCNTs-OH against cancer stem cells. Life Sci. 258, 118152. doi:10.1016/j.lfs.2020.118152
Chen, L., Zhou, Z., Zhang, Y., Pan, J., Wang, K., and Wang, H. X. (2022). Near-infrared irradiation controlled thermo-switchable polymeric photosensitizer against beta-amyloid fibrillation. J. Mater Chem. B 10 (25), 4832–4839. doi:10.1039/d2tb00372d
Chen, Q., Liang, C., Sun, X., Chen, J., Yang, Z., Zhao, H., et al. (2017). H(2)O(2)-responsive liposomal nanoprobe for photoacoustic inflammation imaging and tumor theranostics via in vivo chromogenic assay. Proc. Natl. Acad. Sci. U. S. A. 114 (21), 5343–5348. doi:10.1073/pnas.1701976114
Chen, X., Gao, W., Sun, Y., and Dong, X. (2023). Multiple effects of polydopamine nanoparticles on Cu2+-mediated Alzheimer's β-amyloid aggregation. Chin. J. Chem. Eng. 54, 144–152. doi:10.1016/j.cjche.2022.04.007
Chen, Y., Liu, P., Sun, P., Jiang, J., Zhu, Y., Dong, T., et al. (2019). Oncogenic MSH6-CXCR4-TGFB1 feedback loop: a novel therapeutic target of photothermal therapy in glioblastoma multiforme. Theranostics 9 (5), 1453–1473. doi:10.7150/thno.29987
Cheng, G., Li, Z., Liu, Y., Ma, R., Chen, X., Liu, W., et al. (2023). “Swiss Army Knife” black phosphorus-based nanodelivery platform for synergistic antiparkinsonian therapy via remodeling the brain microenvironment. J. Control Release 353, 752–766. doi:10.1016/j.jconrel.2022.12.024
Cheng, L., Wang, C., Feng, L., Yang, K., and Liu, Z. (2014). Functional nanomaterials for phototherapies of cancer. Chem. Rev. 114 (21), 10869–10939. doi:10.1021/cr400532z
Chi, M., Liu, J., Li, L., Zhang, Y., and Xie, M. (2024). In-situ growth of CeO(2) on biofilms: innovative nanoparticles for photothermal therapy and multi-pronged attack on Alzheimer’s disease. Colloids Surf. B Biointerfaces 238, 113887. doi:10.1016/j.colsurfb.2024.113887
Chipuk, J. E., and Green, D. R. (2006). Dissecting p53-dependent apoptosis. Cell Death Differ. 13 (6), 994–1002. doi:10.1038/sj.cdd.4401908
Dai, X., Liu, D., Pan, P., Liang, G., Wang, X., and Chen, W. (2024). Multifunctional two-dimensional Bi(2)Se(3) nanodisks as a non-inflammatory photothermal agent for glioma treatment. J. Colloid Interface Sci. 661, 930–942. doi:10.1016/j.jcis.2024.01.130
DeRussy, B. M., Aylward, M. A., Fan, Z., Ray, P. C., and Tandon, R. (2014). Inhibition of cytomegalovirus infection and photothermolysis of infected cells using bioconjugated gold nanoparticles. Sci. Rep. 4 (1), 5550. doi:10.1038/srep05550
Didelot, C., Schmitt, E., Brunet, M., Maingret, L., Parcellier, A., and Garrido, C. (2006). “Heat shock proteins: endogenous modulators of apoptotic cell death,” in Molecular chaperones in health and disease (Berlin, Heidelberg: Springer), 171–198.
Dong, C., Feng, W., Xu, W., Yu, L., Xiang, H., Chen, Y., et al. (2020). The coppery age: copper (Cu)-Involved nanotheranostics. Adv. Sci. (Weinh) 7 (21), 2001549. doi:10.1002/advs.202001549
Dong, H., Jin, M., Liu, Z., Xiong, H., Qiu, X., Zhang, W., et al. (2016). In vitro and in vivo brain-targeting chemo-photothermal therapy using graphene oxide conjugated with transferrin for gliomas. Lasers Med. Sci. 31 (6), 1123–1131. doi:10.1007/s10103-016-1955-2
Dong, Y., Liu, J., Chen, Y., Zhu, T., Li, Y., Zhang, C., et al. (2023). Photothermal and natural activity-based synergistic antibacterial effects of Ti(3)C(2)T(x) MXene-loaded chitosan hydrogel against methicillin-resistant Staphylococcus aureus. Int. J. Biol. Macromol. 240, 124482. doi:10.1016/j.ijbiomac.2023.124482
Duan, S., Yang, Y., Zhang, C., Zhao, N., and Xu, F. J. (2017). NIR-responsive polycationic gatekeeper-cloaked hetero-nanoparticles for multimodal imaging-guided triple-combination therapy of cancer. Small 13 (9). doi:10.1002/smll.201603133
Dube, T., Kompella, U. B., and Panda, J. J. (2022). Near infrared triggered chemo-PTT-PDT effect mediated by glioma directed twin functional-chimeric peptide-decorated gold nanoroses. J. Photochem Photobiol. B 228, 112407. doi:10.1016/j.jphotobiol.2022.112407
Dube, T., Kumar, N., Bishnoi, M., and Panda, J. J. (2021). Dual blood-brain barrier-glioma targeting peptide-poly(levodopamine) hybrid nanoplatforms as potential near infrared phototheranostic agents in glioblastoma. Bioconjug Chem. 32 (9), 2014–2031. doi:10.1021/acs.bioconjchem.1c00321
Dube, T., and Panda, J. J. (2023). Anti-glioma activity achieved by dual blood-brain barrier/glioma targeting naive chimeric peptides-based Co-Assembled nanophototheranostics. Pharmaceutics 15 (1), 265. doi:10.3390/pharmaceutics15010265
Evan, G. I., and Vousden, K. H. (2001). Proliferation, cell cycle and apoptosis in cancer. Nature 411 (6835), 342–348. doi:10.1038/35077213
Gao, Y., Dong, Y., Yang, S., Mo, A., Zeng, X., Chen, Q., et al. (2022). Size-dependent photothermal antibacterial activity of Ti C T MXene nanosheets against methicillin-resistant Staphylococcus aureus. J. Colloid Interface Sci. 617, 533–541. doi:10.1016/j.jcis.2022.03.032
Ge, K., Li, Z., Wang, A., Bai, Z., Zhang, X., Zheng, X., et al. (2023). An NIR-driven Upconversion/C(3)N(4)/CoP photocatalyst for efficient hydrogen production by inhibiting electron-hole pair recombination for Alzheimer's disease therapy. ACS Nano 17 (3), 2222–2234. doi:10.1021/acsnano.2c08499
Ge, K., Mu, Y., Liu, M., Bai, Z., Liu, Z., Geng, D., et al. (2022). Gold nanorods with spatial separation of CeO(2) deposition for plasmonic-enhanced antioxidant stress and photothermal therapy of Alzheimer's disease. ACS Appl. Mater Interfaces 14 (3), 3662–3674. doi:10.1021/acsami.1c17861
Geng, X., Gao, D., Hu, D., Liu, Q., Liu, C., Yuan, Z., et al. (2020). Active-targeting NIR-II phototheranostics in multiple tumor models using platelet-camouflaged nanoprobes. ACS Appl. Mater Interfaces 12 (50), 55624–55637. doi:10.1021/acsami.0c16872
Goedert, M. (2015). NEURODEGENERATION. Alzheimer's and Parkinson's diseases: the prion concept in relation to assembled Aβ, tau, and α-synuclein. Science 349 (6248), 1255555. doi:10.1126/science.1255555
Gorrini, C., Harris, I. S., and Mak, T. W. (2013). Modulation of oxidative stress as an anticancer strategy. Nat. Rev. Drug Discov. 12 (12), 931–947. doi:10.1038/nrd4002
Gousias, K., Theocharous, T., and Simon, M. (2022). Mechanisms of cell cycle arrest and apoptosis in glioblastoma. Biomedicines 10 (3), 564. doi:10.3390/biomedicines10030564
Gu, Y., Wang, Z., Wei, C., Li, Y., Feng, W., Wang, W., et al. (2022). Photonic hyperthermia of malignant peripheral nerve sheath tumors at the third near-infrared biowindow. Elife 11, e75473. doi:10.7554/eLife.75473
Guo, B., Sheng, Z., Hu, D., Li, A., Xu, S., Manghnani, P. N., et al. (2017). Molecular engineering of conjugated polymers for biocompatible organic nanoparticles with highly efficient photoacoustic and photothermal performance in cancer theranostics. ACS Nano 11 (10), 10124–10134. doi:10.1021/acsnano.7b04685
Guo, B., Sheng, Z., Hu, D., Liu, C., Zheng, H., and Liu, B. (2018). Through scalp and skull NIR-II photothermal therapy of deep orthotopic brain tumors with precise photoacoustic imaging guidance. Adv. Mater 30 (35), e1802591. doi:10.1002/adma.201802591
Guo, Q., Yin, M., Fan, J., Yang, Y., Liu, T., Qian, H., et al. (2022). Peroxidase-mimicking TA-VOx nanobranches for enhanced photothermal/chemodynamic therapy of glioma by inhibiting the expression of HSP60. Mater. and Des. 224, 111366. doi:10.1016/j.matdes.2022.111366
Hamley, I. W. (2012). The amyloid beta peptide: a chemist's perspective. Role in Alzheimer's and fibrillization. Chem. Rev. 112 (10), 5147–5192. doi:10.1021/cr3000994
Hayat, M. J., Howlader, N., Reichman, M. E., and Edwards, B. K. (2007). Cancer statistics, trends, and multiple primary cancer analyses from the surveillance, epidemiology, and end results (SEER) program. Oncologist 12 (1), 20–37. doi:10.1634/theoncologist.12-1-20
He, Y., Gao, Q., Lv, C., and Liu, L. (2021). Improved photothermal therapy of brain cancer cells and photogeneration of reactive oxygen species by biotin conjugated gold photoactive nanoparticles. J. Photochem Photobiol. B 215, 112102. doi:10.1016/j.jphotobiol.2020.112102
Hildebrandt, B., Wust, P., Ahlers, O., Dieing, A., Sreenivasa, G., Kerner, T., et al. (2002). The cellular and molecular basis of hyperthermia. Crit. Rev. Oncol. Hematol. 43 (1), 33–56. doi:10.1016/s1040-8428(01)00179-2
Hu, D., Sheng, Z., Zhu, M., Wang, X., Yan, F., Liu, C., et al. (2018). Forster resonance energy transfer-based dual-modal theranostic nanoprobe for in situ visualization of cancer photothermal therapy. Theranostics 8 (2), 410–422. doi:10.7150/thno.22226
Hu, X., Li, P., Xu, D., Liu, H., Hao, Q., Zhang, M., et al. (2024). Facile Alkyne Assembly-Enabled Functional Au Nanosheets for Photoacoustic Imaging-Guided Photothermal/Gene Therapy of Orthotopic Glioblastoma. J. Am. Chem. Soc. 146 (48), 32965–32978. doi:10.1021/jacs.4c08990
Hu, Y., Zhou, Y., Zhao, N., Liu, F., and Xu, F. J. (2016). Multifunctional pDNA-Conjugated Polycationic Au Nanorod-Coated Fe3 O4 Hierarchical Nanocomposites for Trimodal Imaging and Combined Photothermal/Gene Therapy. Small 12 (18), 2459–2468. doi:10.1002/smll.201600271
Huang, D., Liao, W., Li, J., Chen, T., Wang, X., Zhao, R., et al. (2023a). Alzheimer's disease: status of low-dimensional nanotherapeutic materials. Adv. Funct. Mater. 34 (4), 2302015. doi:10.1002/adfm.202302015
Huang, H., Li, M., Gu, J., Roy, S., Jin, J., Kuang, T., et al. (2024). Bright NIR-II emissive cyanine dye-loaded lipoprotein-mimicking nanoparticles for fluorescence imaging-guided and targeted NIR-II photothermal therapy of subcutaneous glioblastoma. J. Nanobiotechnology 22 (1), 788. doi:10.1186/s12951-024-03074-3
Huang, H., and Lovell, J. F. (2017). Advanced functional nanomaterials for theranostics. Adv. Funct. Mater 27 (2), 1603524. doi:10.1002/adfm.201603524
Huang, H., Yuan, G., Xu, Y., Gao, Y., Mao, Q., Zhang, Y., et al. (2022). Photoacoustic and magnetic resonance imaging-based gene and photothermal therapy using mesoporous nanoagents. Bioact. Mater 9, 157–167. doi:10.1016/j.bioactmat.2021.07.025
Huang, X., Hui, H., Shang, W., Gao, P., Zhou, Y., Pang, W., et al. (2023b). Deep penetrating and sensitive targeted magnetic particle imaging and photothermal therapy of early-stage glioblastoma based on a biomimetic nanoplatform. Adv. Sci. (Weinh) 10 (19), e2300854. doi:10.1002/advs.202300854
Huang, X., Lu, Y., Guo, M., Du, S., and Han, N. (2021). Recent strategies for nano-based PTT combined with immunotherapy: from a biomaterial point of view. Theranostics 11 (15), 7546–7569. doi:10.7150/thno.56482
Huang, X., Wu, J., He, M., Hou, X., Wang, Y., Cai, X., et al. (2019). Combined cancer chemo-photodynamic and photothermal therapy based on ICG/PDA/TPZ-Loaded nanoparticles. Mol. Pharm. 16 (5), 2172–2183. doi:10.1021/acs.molpharmaceut.9b00119
Jaque, D., Martinez Maestro, L., del Rosal, B., Haro-Gonzalez, P., Benayas, A., Plaza, J. L., et al. (2014). Nanoparticles for photothermal therapies. Nanoscale 6 (16), 9494–9530. doi:10.1039/c4nr00708e
Jia, Y., Wang, X., Hu, D., Wang, P., Liu, Q., Zhang, X., et al. (2019). Phototheranostics: active targeting of orthotopic glioma using biomimetic proteolipid nanoparticles. ACS Nano 13 (1), 386–398. doi:10.1021/acsnano.8b06556
Jiang, Z., Zhang, H., Zhang, W., Zhang, Y., Cui, Y., Mei, L., et al. (2024). Smart platelet-based biohybrid delivery system for magnetic-guided targeted delivery and enhanced photothermal-chemo therapy against glioma. Nano Today 56, 102295. doi:10.1016/j.nantod.2024.102295
Jin, H., Zhao, G., Hu, J., Ren, Q., Yang, K., Wan, C., et al. (2017). Melittin-containing hybrid peptide hydrogels for enhanced photothermal therapy of glioblastoma. ACS Appl. Mater Interfaces 9 (31), 25755–25766. doi:10.1021/acsami.7b06431
Jolly, C., and Morimoto, R. I. (2000). Role of the heat shock response and molecular chaperones in oncogenesis and cell death. J. Natl. Cancer Inst. 92 (19), 1564–1572. doi:10.1093/jnci/92.19.1564
Joseph, A., and Nance, E. (2022). Nanotherapeutics and the brain. Annu. Rev. Chem. Biomol. Eng. 13 (13), 325–346. doi:10.1146/annurev-chembioeng-092220-030853
Kadkhoda, J., Tarighatnia, A., Nader, N. D., and Aghanejad, A. (2022). Targeting mitochondria in cancer therapy: insight into photodynamic and photothermal therapies. Life Sci. 307, 120898. doi:10.1016/j.lfs.2022.120898
Kargar, S., Khoei, S., Khoee, S., Shirvalilou, S., and Mahdavi, S. R. (2018). Evaluation of the combined effect of NIR laser and ionizing radiation on cellular damages induced by IUdR-loaded PLGA-coated Nano-graphene oxide. Photodiagnosis Photodyn. Ther. 21, 91–97. doi:10.1016/j.pdpdt.2017.11.007
Kaushik, A., Jayant, R. D., Bhardwaj, V., and Nair, M. (2018). Personalized nanomedicine for CNS diseases. Drug Discov. Today 23 (5), 1007–1015. doi:10.1016/j.drudis.2017.11.010
Keyvan Rad, J., Mahdavian, A. R., Khoei, S., and Shirvalilou, S. (2018). Enhanced photogeneration of reactive oxygen species and targeted photothermal therapy of C6 glioma brain cancer cells by folate-conjugated gold-photoactive polymer nanoparticles. ACS Appl. Mater Interfaces 10 (23), 19483–19493. doi:10.1021/acsami.8b05252
Kim, H., and Kim, W. J. (2014). Photothermally controlled gene delivery by reduced graphene oxide-polyethylenimine nanocomposite. Small 10 (1), 117–126. doi:10.1002/smll.201202636
Kim, J., Kim, J., Jeong, C., and Kim, W. J. (2016). Synergistic nanomedicine by combined gene and photothermal therapy. Adv. Drug Deliv. Rev. 98, 99–112. doi:10.1016/j.addr.2015.12.018
Ko, W. K., Lee, S. J., Kim, S. J., Han, G. H., Han, I. B., Hong, J. B., et al. (2021). Direct injection of hydrogels embedding gold nanoparticles for local therapy after spinal cord injury. Biomacromolecules 22 (7), 2887–2901. doi:10.1021/acs.biomac.1c00281
Lan, Q. H., Du, C. C., Yu, R. J., Zhai, J., Shi, Y., Kou, L., et al. (2021). Disulfiram-loaded copper sulfide nanoparticles for potential anti-glioma therapy. Int. J. Pharm. 607, 120978. doi:10.1016/j.ijpharm.2021.120978
Lee, S., Min, S., Kim, G., and Lee, S. (2024). Recent advances in the design of organic photothermal agents for cancer treatment: a review. Coord. Chem. Rev. 506, 215719. doi:10.1016/j.ccr.2024.215719
Li, F., Ma, H., Luo, H., Shen, G., Su, J., Cai, H., et al. (2024a). 211At-Labeled nanoscale polydopamine decorated with FAPI for synergistic targeted-alpha therapy and photothermal therapy of glioma. ACS Appl. Nano Mater. 7 (7), 6831–6838. doi:10.1021/acsanm.3c05525
Li, H., Zhang, X., Miao, J., Shi, Z., Li, Z., Wen, M., et al. (2023a). Dual drug-loaded calabash-like nanomotor as an active therapeutic for enhanced chemo-photothermal therapy of orthotopic glioblastoma. Chem. Eng. J. 473, 145413. doi:10.1016/j.cej.2023.145413
Li, J., Cai, P., Shalviri, A., Henderson, J. T., He, C., Foltz, W. D., et al. (2014). A multifunctional polymeric nanotheranostic system delivers doxorubicin and imaging agents across the blood-brain barrier targeting brain metastases of breast cancer. ACS Nano 8 (10), 9925–9940. doi:10.1021/nn501069c
Li, J., Liu, X., Tan, L., Cui, Z., Yang, X., Liang, Y., et al. (2019). Zinc-doped Prussian blue enhances photothermal clearance of Staphylococcus aureus and promotes tissue repair in infected wounds. Nat. Commun. 10 (1), 4490. doi:10.1038/s41467-019-12429-6
Li, J., Wang, S., Fontana, F., Tapeinos, C., Shahbazi, M. A., Han, H., et al. (2023b). Nanoparticles-based phototherapy systems for cancer treatment: current status and clinical potential. Bioact. Mater 23, 471–507. doi:10.1016/j.bioactmat.2022.11.013
Li, L., Xiong, Y., Zhang, Y., Yan, Y., Zhao, R., Yang, F., et al. (2024b). Biofilm-camouflaged Prussian blue synergistic mitochondrial mass enhancement for Alzheimer's disease based on Cu(2+) chelation and photothermal therapy. J. Control Release 375, 269–284. doi:10.1016/j.jconrel.2024.09.009
Li, M., Yang, X., Ren, J., Qu, K., and Qu, X. (2012). Using graphene oxide high near-infrared absorbance for photothermal treatment of Alzheimer's disease. Adv. Mater 24 (13), 1722–1728. doi:10.1002/adma.201104864
Li, S., Zeng, F., Zhou, Q., Li, L., Lo, H., Chen, J., et al. (2025). NIR-II photoacoustic imaging-guided chemo-photothermal therapy using PA1094T combined with Anti-CD47 antibody: activating pyroptosis against orthotopic glioblastoma. Adv. Healthc. Mater 14 (3), e2403108. doi:10.1002/adhm.202403108
Li, T., Li, J., Chen, Z., Zhang, S., Li, S., Wageh, S., et al. (2022a). Glioma diagnosis and therapy: current challenges and nanomaterial-based solutions. J. Control Release 352, 338–370. doi:10.1016/j.jconrel.2022.09.065
Li, T., Liu, Y., Bao, W., Luo, J., Gao, L., Chen, X., et al. (2021a). Synergistic photothermal and chemical therapy by smart dual-functional graphdiyne nanosheets for treatment of parkinson's disease. Adv. Ther. 4 (7). doi:10.1002/adtp.202100082
Li, X., Lovell, J. F., Yoon, J., and Chen, X. (2020). Clinical development and potential of photothermal and photodynamic therapies for cancer. Nat. Rev. Clin. Oncol. 17 (11), 657–674. doi:10.1038/s41571-020-0410-2
Li, X., Wu, R., Chen, H., Li, T., Jiang, H., Xu, X., et al. (2021b). Near-infrared light-driven multifunctional tubular micromotors for treatment of atherosclerosis. ACS Appl. Mater Interfaces 13 (26), 30930–30940. doi:10.1021/acsami.1c03600
Li, Y., Pan, Y., Wang, Y., Jiang, Z., Akakuru, O. U., Li, M., et al. (2022b). A D-peptide ligand of neuropeptide Y receptor Y1 serves as nanocarrier traversing of the blood brain barrier and targets glioma. Nano Today 44, 101465. doi:10.1016/j.nantod.2022.101465
Li, Z., Fu, S., Li, H., Chen, B., Xie, D., Fu, D., et al. (2023c). Light-driven micromotor swarm induced in-situ polymerization and synergistic photothermal therapy. Chem. Eng. J. 468, 143393. doi:10.1016/j.cej.2023.143393
Liao, J., Wang, L., Ding, S., Tian, G., Hu, H., Wang, Q., et al. (2023). Molybdenum-based antimicrobial nanomaterials: a comprehensive review. Nano Today 50, 101875. doi:10.1016/j.nantod.2023.101875
Lin, X., Sun, Z., Huang, S., Liu, C., Peng, J., Li, Y., et al. (2023). Engineered microglia-exosomes coated highly twisting AIE photothermal agents to efficiently cross blood-brain-barrier for mild photothermal-immune checkpoint blockade therapy in glioblastoma. Adv. Funct. Mater. 34 (16), 2310237. doi:10.1002/adfm.202310237
Ling, C., Wang, X., and Shen, Y. (2021). Advances in hollow inorganic nanomedicines for photothermal-based therapies. Int. J. Nanomedicine 16, 493–513. doi:10.2147/IJN.S285115
Liou, G. Y., and Storz, P. (2010). Reactive oxygen species in cancer. Free Radic. Res. 44 (5), 479–496. doi:10.3109/10715761003667554
Liu, D., Dai, X., Zhang, W., Zhu, X., Zha, Z., Qian, H., et al. (2023a). Liquid exfoliation of ultrasmall zirconium carbide nanodots as a noninflammatory photothermal agent in the treatment of glioma. Biomaterials 292, 121917. doi:10.1016/j.biomaterials.2022.121917
Liu, D., Li, W., Jiang, X., Bai, S., Liu, J., Liu, X., et al. (2019a). Using near-infrared enhanced thermozyme and scFv dual-conjugated Au nanorods for detection and targeted photothermal treatment of Alzheimer's disease. Theranostics 9 (8), 2268–2281. doi:10.7150/thno.30649
Liu, J., Chi, M., Li, L., Zhang, Y., and Xie, M. (2024a). Erythrocyte membrane coated with nitrogen-doped quantum dots and polydopamine composite nano-system combined with photothermal treatment of Alzheimer's disease. J. Colloid Interface Sci. 663, 856–868. doi:10.1016/j.jcis.2024.02.219
Liu, J., Tagami, T., Ogawa, K., and Ozeki, T. (2024b). Development of hollow gold nanoparticles for photothermal therapy and their cytotoxic effect on a glioma cell line when combined with copper diethyldithiocarbamate. Biol. Pharm. Bull. 47 (1), 272–278. doi:10.1248/bpb.b23-00789
Liu, J. S., Peng, S. J., Li, G. F., Zhao, Y. X., Meng, X. Y., Yu, X. R., et al. (2020). Polydopamine nanoparticles for deep brain ablation via near-infrared irradiation. ACS Biomater. Sci. Eng. 6 (1), 664–672. doi:10.1021/acsbiomaterials.9b01097
Liu, W., Dong, X., Liu, Y., and Sun, Y. (2021a). Photoresponsive materials for intensified modulation of Alzheimer's amyloid-beta protein aggregation: a review. Acta Biomater. 123, 93–109. doi:10.1016/j.actbio.2021.01.018
Liu, Y., Bhattarai, P., Dai, Z., and Chen, X. (2019b). Photothermal therapy and photoacoustic imaging via nanotheranostics in fighting cancer. Chem. Soc. Rev. 48 (7), 2053–2108. doi:10.1039/c8cs00618k
Liu, Y., Chongsathidkiet, P., Crawford, B. M., Odion, R., Dechant, C. A., Kemeny, H. R., et al. (2019c). Plasmonic gold nanostar-mediated photothermal immunotherapy for brain tumor ablation and immunologic memory. Immunotherapy 11 (15), 1293–1302. doi:10.2217/imt-2019-0023
Liu, Y., Hu, D., Gao, D., Gong, P., Zheng, H., Sun, M., et al. (2023b). Engineered apoptotic bodies hitchhiking across the blood-brain barrier achieved a combined photothermal-chemotherapeutic effect against glioma. Theranostics 13 (9), 2966–2978. doi:10.7150/thno.80632
Liu, Y., Li, Q., Gu, M., Lu, D., Xiong, X., Zhang, Z., et al. (2022). A second near-infrared Ru(II) polypyridyl complex for synergistic chemo-photothermal therapy. J. Med. Chem. 65 (3), 2225–2237. doi:10.1021/acs.jmedchem.1c01736
Liu, Y., Yu, B., Dai, X., Zhao, N., and Xu, F. J. (2021b). Biomineralized calcium carbonate nanohybrids for mild photothermal heating-enhanced gene therapy. Biomaterials 274, 120885. doi:10.1016/j.biomaterials.2021.120885
Liu, Z., Huang, Y., Sun, R., Li, N., Xu, F., Zhang, W., et al. (2025). Polyethylene glycolated Shikonin-Functionalized carbon nanotubes as an effective anticancer system combining chemotherapy and photothermal therapy. Langmuir 41 (9), 6236–6246. doi:10.1021/acs.langmuir.4c05257
Lu, L., Wang, K., Lin, C., Yang, W., Duan, Q., Li, K., et al. (2021). Constructing nanocomplexes by multicomponent self-assembly for curing orthotopic glioblastoma with synergistic chemo-photothermal therapy. Biomaterials 279, 121193. doi:10.1016/j.biomaterials.2021.121193
Lu, Y.-J., Lin, C.-W., Yang, H.-W., Lin, K.-J., Wey, S.-P., Sun, C.-L., et al. (2014). Biodistribution of PEGylated graphene oxide nanoribbons and their application in cancer chemo-photothermal therapy. Carbon 74, 83–95. doi:10.1016/j.carbon.2014.03.007
Lv, G., Shen, Y., Zheng, W., Yang, J., Li, C., and Lin, J. (2019). Fluorescence detection and dissociation of Amyloid-β species for the treatment of Alzheimer's disease. Adv. Ther. 2 (9), 1900054. doi:10.1002/adtp.201900054
Ma, M., Gao, N., Li, X., Liu, Z., Pi, Z., Du, X., et al. (2020). A biocompatible second near-infrared nanozyme for spatiotemporal and non-invasive attenuation of amyloid deposition through scalp and skull. ACS Nano 14 (8), 9894–9903. doi:10.1021/acsnano.0c02733
Ma, Y., Chen, L., Li, X., Hu, A., Wang, H., Zhou, H., et al. (2021). Rationally integrating peptide-induced targeting and multimodal therapies in a dual-shell theranostic platform for orthotopic metastatic spinal tumors. Biomaterials 275, 120917. doi:10.1016/j.biomaterials.2021.120917
Manivasagan, P., Khan, F., Rajan Dhatchayeny, D., Park, S., Joe, A., Han, H. W., et al. (2023). Antibody-conjugated and streptomycin-chitosan oligosaccharide-modified gold nanoshells for synergistic chemo-photothermal therapy of drug-resistant bacterial infection. J. Adv. Res. 48, 87–104. doi:10.1016/j.jare.2022.08.009
Markovic, Z. M., Harhaji-Trajkovic, L. M., Todorovic-Markovic, B. M., Kepic, D. P., Arsikin, K. M., Jovanovic, S. P., et al. (2011). In vitro comparison of the photothermal anticancer activity of graphene nanoparticles and carbon nanotubes. Biomaterials 32 (4), 1121–1129. doi:10.1016/j.biomaterials.2010.10.030
Melamed, J. R., Edelstein, R. S., and Day, E. S. (2015). Elucidating the fundamental mechanisms of cell death triggered by photothermal therapy. ACS Nano 9 (1), 6–11. doi:10.1021/acsnano.5b00021
Miao, Z., Hu, D., Gao, D., Fan, L., Ma, Y., Ma, T., et al. (2021). Tiny 2D silicon quantum sheets: a brain photonic nanoagent for orthotopic glioma theranostics. Sci. Bull. (Beijing) 66 (2), 147–157. doi:10.1016/j.scib.2020.09.027
Mocan, T., Matea, C. T., Cojocaru, I., Ilie, I., Tabaran, F. A., Zaharie, F., et al. (2014). Photothermal treatment of human pancreatic cancer using PEGylated multi-walled carbon nanotubes induces apoptosis by triggering mitochondrial membrane depolarization mechanism. J. Cancer 5 (8), 679–688. doi:10.7150/jca.9481
Nedosekin, D. A., Chen, T., Ayyadevara, S., Zharov, V. P., and Shmookler Reis, R. J. (2021). Label-free photothermal disruption of cytotoxic aggregates rescues pathology in a C. elegans model of Huntington's disease. Sci. Rep. 11 (1), 19732. doi:10.1038/s41598-021-98661-x
Newland, B., Starke, J., Bastiancich, C., Goncalves, D. P. N., Bray, L. J., Wang, W., et al. (2022). Well-defined polyethylene glycol microscale hydrogel blocks containing gold nanorods for dual photothermal and chemotherapeutic therapy. Pharmaceutics 14 (3), 551. doi:10.3390/pharmaceutics14030551
Nie, D., Ling, Y., Lv, W., Liu, Q., Deng, S., Shi, J., et al. (2023). In situ attached photothermal immunomodulation-enhanced nanozyme for the inhibition of postoperative malignant glioma recurrence. ACS Nano 17 (14), 13885–13902. doi:10.1021/acsnano.3c03696
Overchuk, M., Weersink, R. A., Wilson, B. C., and Zheng, G. (2023). Photodynamic and photothermal therapies: synergy opportunities for nanomedicine. ACS Nano 17 (9), 7979–8003. doi:10.1021/acsnano.3c00891
Panahi, Y., Farshbaf, M., Mohammadhosseini, M., Mirahadi, M., Khalilov, R., Saghfi, S., et al. (2017). Recent advances on liposomal nanoparticles: synthesis, characterization and biomedical applications. Artif. Cells Nanomed Biotechnol. 45 (4), 788–799. doi:10.1080/21691401.2017.1282496
Pandey, A., Singh, K., Subramanian, S., Korde, A., Singh, R., and Sawant, K. (2020). Heterogeneous surface architectured pH responsive metal-drug Nano-conjugates for mitochondria targeted therapy of glioblastomas: a multimodal intranasal approach. Chem. Eng. J. 394, 124419. doi:10.1016/j.cej.2020.124419
Poinard, B., Neo, S. Z. Y., Yeo, E. L. L., Heng, H. P. S., Neoh, K. G., and Kah, J. C. Y. (2018). Polydopamine nanoparticles enhance drug release for combined photodynamic and photothermal therapy. ACS Appl. Mater Interfaces 10 (25), 21125–21136. doi:10.1021/acsami.8b04799
Qi, X., Li, L., Ye, P., and Xie, M. (2024). Macrophage membrane-modified MoS(2) quantum dots as a nanodrug for combined multi-targeting of Alzheimer's Disease. Adv. Healthc. Mater 13 (6), e2303211. doi:10.1002/adhm.202303211
Qi, X., Ye, P., and Xie, M. (2023). MoS2 quantum dots based on lipid drug delivery system for combined therapy against Alzheimer’s disease. J. Drug Deliv. Sci. Technol. 82, 104324. doi:10.1016/j.jddst.2023.104324
Qian, M., Du, Y., Wang, S., Li, C., Jiang, H., Shi, W., et al. (2018). Highly crystalline multicolor carbon nanodots for dual-modal imaging-guided photothermal therapy of glioma. ACS Appl. Mater Interfaces 10 (4), 4031–4040. doi:10.1021/acsami.7b19716
Qiao, L., Yang, H., Shao, X. X., Yin, Q., Fu, X. J., and Wei, Q. (2022). Research progress on nanoplatforms and nanotherapeutic strategies in treating glioma. Mol. Pharm. 19 (7), 1927–1951. doi:10.1021/acs.molpharmaceut.1c00856
Rabiei, M., Kashanian, S., Samavati, S. S., Jamasb, S., and McInnes, S. J. P. (2020). Active targeting towards and inside the brain based on nanoparticles: a review. Curr. Pharm. Biotechnol. 21 (5), 374–383. doi:10.2174/1389201020666191203094057
Rahman, R., Trippa, L., Lee, E. Q., Arrillaga-Romany, I., Fell, G., Touat, M., et al. (2023). Inaugural results of the individualized screening trial of innovative glioblastoma therapy: a phase II platform trial for newly diagnosed glioblastoma using bayesian adaptive randomization. J. Clin. Oncol. 41 (36), 5524–5535. doi:10.1200/JCO.23.00493
Rai, P., Mallidi, S., Zheng, X., Rahmanzadeh, R., Mir, Y., Elrington, S., et al. (2010). Development and applications of photo-triggered theranostic agents. Adv. Drug Deliv. Rev. 62 (11), 1094–1124. doi:10.1016/j.addr.2010.09.002
Rao, L., Yuan, Y., Shen, X., Yu, G., and Chen, X. (2024). Designing nanotheranostics with machine learning. Nat. Nanotechnol. 19 (12), 1769–1781. doi:10.1038/s41565-024-01753-8
Ren, M., Zhou, J., Song, Z., Mei, H., Zhou, M., Fu, Z. F., et al. (2021). Aptamer and RVG functionalized gold nanorods for targeted photothermal therapy of neurotropic virus infection in the mouse brain. Chem. Eng. J. 411, 128557. doi:10.1016/j.cej.2021.128557
Ren, Y., Yan, Y., and Qi, H. (2022). Photothermal conversion and transfer in photothermal therapy: from macroscale to nanoscale. Adv. Colloid Interface Sci. 308, 102753. doi:10.1016/j.cis.2022.102753
Ross, C. A., and Tabrizi, S. J. (2011). Huntington's disease: from molecular pathogenesis to clinical treatment. Lancet Neurol. 10 (1), 83–98. doi:10.1016/S1474-4422(10)70245-3
Sagar, V., and Nair, M. (2018). Near-infrared biophotonics-based nanodrug release systems and their potential application for neuro-disorders. Expert Opin. Drug Deliv. 15 (2), 137–152. doi:10.1080/17425247.2017.1297794
Shao, X., Li, M., Yan, C., Wang, C., Wang, X., Guan, P., et al. (2025). Photocatalytic, photothermal, and blood-brain barrier-permeable carbon nanodots: a potent multifunctional scavenger for beta-amyloid plaque. Colloids Surf. B Biointerfaces 246, 114380. doi:10.1016/j.colsurfb.2024.114380
Singh, P., Pandit, S., Balusamy, S. R., Madhusudanan, M., Singh, H., Haseef, H. M. A., et al. (2025). Advanced nanomaterials for cancer therapy: gold, silver, and iron oxide nanoparticles in oncological applications. Adv. Healthc. Mater. 14 (4), 2403059. doi:10.1002/adhm.202403059
Song, W., Zhang, X., Song, Y., Fan, K., Shao, F., Long, Y., et al. (2023a). Enhancing photothermal therapy efficacy by in situ self-assembly in glioma. ACS Appl. Mater Interfaces 15 (1), 57–66. doi:10.1021/acsami.2c14413
Song, X., Ding, Q., Wei, W., Zhang, J., Sun, R., Yin, L., et al. (2023b). Peptide-functionalized prussian blue nanomaterial for antioxidant stress and NIR photothermal therapy against Alzheimer's disease. Small 19 (41), e2206959. doi:10.1002/smll.202206959
Song, Z., Zhao, L., Fang, W., Guo, S., Xu, A., Zhan, Z., et al. (2023c). Glioma cell membrane camouflaged cinobufotalin delivery system for combinatorial orthotopic glioblastoma therapy. Nano Res. 16 (8), 11164–11175. doi:10.1007/s12274-023-5807-7
Su, D., Jiang, Z., Xu, Y., Li, J., Qi, Q., Gong, Y., et al. (2025). Molecular design of ternary copolymers with high photothermal performance in the near-infrared window for effective treatment of gliomas in vivo. Acta Biomater. 192, 302–314. doi:10.1016/j.actbio.2024.12.025
Sudhakar, S., and Mani, E. (2019). Rapid dissolution of amyloid beta fibrils by silver nanoplates. Langmuir 35 (21), 6962–6970. doi:10.1021/acs.langmuir.9b00080
Sun, R., Liu, M., Lu, J., Chu, B., Yang, Y., Song, B., et al. (2022). Bacteria loaded with glucose polymer and photosensitive ICG silicon-nanoparticles for glioblastoma photothermal immunotherapy. Nat. Commun. 13 (1), 5127. doi:10.1038/s41467-022-32837-5
Sweeney, E. E., Burga, R. A., Li, C., Zhu, Y., and Fernandes, R. (2016). Photothermal therapy improves the efficacy of a MEK inhibitor in neurofibromatosis type 1-associated malignant peripheral nerve sheath tumors. Sci. Rep. 6 (1), 37035. doi:10.1038/srep37035
Tai, Y. W., Chiu, Y. C., Wu, P. T., Yu, J., Chin, Y. C., Wu, S. P., et al. (2018). Degradable NIR-PTT nanoagents with a potential Cu@Cu(2)O@Polymer structure. ACS Appl. Mater Interfaces 10 (6), 5161–5174. doi:10.1021/acsami.7b15109
Tao, H., Wu, T., Aldeghi, M., Wu, T. C., Aspuru-Guzik, A., and Kumacheva, E. (2021). Nanoparticle synthesis assisted by machine learning. Nat. Rev. Mater. 6 (8), 701–716. doi:10.1038/s41578-021-00337-5
Tao, Q., Yang, S., Wang, S., Yang, Y., Yu, S., Pan, Y., et al. (2024). Neural progenitor cell-mediated magnetic nanoparticles for magnetic resonance imaging and photothermal therapy of glioma. ACS Appl. Bio Mater 7 (7), 4553–4561. doi:10.1021/acsabm.4c00414
Thangudu, S., Cheng, F. Y., and Su, C. H. (2020). Advancements in the blood-brain barrier penetrating nanoplatforms for brain related disease diagnostics and therapeutic applications. Polym. (Basel) 12 (12), 3055. doi:10.3390/polym12123055
Tong, F., Hu, H., Xu, Y., Zhou, Y., Xie, R., Lei, T., et al. (2023). Hollow copper sulfide nanoparticles carrying ISRIB for the sensitized photothermal therapy of breast cancer and brain metastases through inhibiting stress granule formation and reprogramming tumor-associated macrophages. Acta Pharm. Sin. B 13 (8), 3471–3488. doi:10.1016/j.apsb.2022.11.003
Trachootham, D., Alexandre, J., and Huang, P. (2009). Targeting cancer cells by ROS-mediated mechanisms: a radical therapeutic approach? Nat. Rev. Drug Discov. 8 (7), 579–591. doi:10.1038/nrd2803
Truant, R., Atwal, R. S., Desmond, C., Munsie, L., and Tran, T. (2008). Huntington's disease: revisiting the aggregation hypothesis in polyglutamine neurodegenerative diseases. FEBS J. 275 (17), 4252–4262. doi:10.1111/j.1742-4658.2008.06561.x
Turkmen Koc, S. N., Rezaei Benam, S., Aral, I. P., Shahbazi, R., and Ulubayram, K. (2024). Gold nanoparticles-mediated photothermal and photodynamic therapies for cancer. Int. J. Pharm. 655, 124057. doi:10.1016/j.ijpharm.2024.124057
Van de Walle, A., Figuerola, A., Espinosa, A., Abou-Hassan, A., Estrader, M., and Wilhelm, C. (2023). Emergence of magnetic nanoparticles in photothermal and ferroptotic therapies. Mater Horiz. 10 (11), 4757–4775. doi:10.1039/d3mh00831b
Vashist, A., Manickam, P., Raymond, A. D., Arias, A. Y., Kolishetti, N., Vashist, A., et al. (2023). Recent advances in nanotherapeutics for neurological disorders. ACS Appl. Bio Mater 6 (7), 2614–2621. doi:10.1021/acsabm.3c00254
Wang, S., Shen, H., Mao, Q., Tao, Q., Yuan, G., Zeng, L., et al. (2021a). Macrophage-mediated porous magnetic nanoparticles for multimodal imaging and postoperative photothermal therapy of gliomas. ACS Appl. Mater Interfaces 13 (48), 56825–56837. doi:10.1021/acsami.1c12406
Wang, Y., Meng, H. M., and Li, Z. (2021b). Near-infrared inorganic nanomaterial-based nanosystems for photothermal therapy. Nanoscale 13 (19), 8751–8772. doi:10.1039/d1nr00323b
Wang, Y., Wang, K., Yan, X., and Huang, R. (2014). A general strategy for dual-triggered combined tumor therapy based on template semi-graphitized mesoporous silica nanoparticles. Adv. Healthc. Mater 3 (4), 485–489. doi:10.1002/adhm.201300324
Wang, Y., Wang, K., Zhao, J., Liu, X., Bu, J., Yan, X., et al. (2013). Multifunctional mesoporous silica-coated graphene nanosheet used for chemo-photothermal synergistic targeted therapy of glioma. J. Am. Chem. Soc. 135 (12), 4799–4804. doi:10.1021/ja312221g
Wu, F., Zu, Y., Weng, W., Yang, Y., Hu, J., Mao, Y., et al. (2022a). Multifunctional inverse opal film as a responsive drug carrier for spinal cord injury repair. Chem. Eng. J. 436, 135256. doi:10.1016/j.cej.2022.135256
Wu, H., Zhang, T., Liu, Q., Wei, M., Li, Y., Ma, Q., et al. (2023). Polydopamine-based loaded temozolomide nanoparticles conjugated by peptide-1 for glioblastoma chemotherapy and photothermal therapy. Front. Pharmacol. 14, 1081612. doi:10.3389/fphar.2023.1081612
Wu, X., Jiang, Y., Rommelfanger, N. J., Yang, F., Zhou, Q., Yin, R., et al. (2022b). Tether-free photothermal deep-brain stimulation in freely behaving mice via wide-field illumination in the near-infrared-II window. Nat. Biomed. Eng. 6 (6), 754–770. doi:10.1038/s41551-022-00862-w
Xie, Z., Fan, T., An, J., Choi, W., Duo, Y., Ge, Y., et al. (2020). Emerging combination strategies with phototherapy in cancer nanomedicine. Chem. Soc. Rev. 49 (22), 8065–8087. doi:10.1039/d0cs00215a
Xiong, S., Li, Z., Liu, Y., Wang, Q., Luo, J., Chen, X., et al. (2020). Brain-targeted delivery shuttled by black phosphorus nanostructure to treat Parkinson's disease. Biomaterials 260, 120339. doi:10.1016/j.biomaterials.2020.120339
Xu, P., and Liang, F. (2020). Nanomaterial-based tumor photothermal immunotherapy. Int. J. Nanomedicine 15, 9159–9180. doi:10.2147/IJN.S249252
Yan, C., Wang, C., Shao, X., Teng, Y., Chen, P., Hu, X., et al. (2022). Multifunctional carbon-dot-photosensitizer nanoassemblies for inhibiting amyloid aggregates, suppressing microbial infection, and overcoming the blood-brain barrier. ACS Appl. Mater Interfaces 14 (42), 47432–47444. doi:10.1021/acsami.2c14118
Yao, Z., Jiang, X., Yao, H., Wu, Y., Zhang, F., Wang, C., et al. (2022). Efficiently targeted therapy of glioblastoma xenograft via multifunctional biomimetic nanodrugs. Biomater. Res. 26 (1), 71. doi:10.1186/s40824-022-00309-y
Ye, P., Li, L., Qi, X., Chi, M., Liu, J., and Xie, M. (2023). Macrophage membrane-encapsulated nitrogen-doped carbon quantum dot nanosystem for targeted treatment of Alzheimer's disease: regulating metal ion homeostasis and photothermal removal of beta-amyloid. J. Colloid Interface Sci. 650 (Pt B), 1749–1761. doi:10.1016/j.jcis.2023.07.132
Yin, M., Chen, X., Guo, Q., Xiao, L., Gao, P., Zang, D., et al. (2022). Ultrasmall zirconium carbide nanodots for synergistic photothermal-radiotherapy of glioma. Nanoscale 14 (40), 14935–14949. doi:10.1039/d2nr04239h
Yu, X., Fan, S., Zhu, B., El-Hout, S. I., Zhang, J., and Chen, C. (2024). Recent progress on photothermal nanomaterials: design, mechanism, and applications. Green Energy Environ. doi:10.1016/j.gee.2024.09.002
Yu, X., Yang, K., Chen, X., and Li, W. (2017). Black hollow silicon oxide nanoparticles as highly efficient photothermal agents in the second near-infrared window for in vivo cancer therapy. Biomaterials 143, 120–129. doi:10.1016/j.biomaterials.2017.07.037
Yu, Y., Wang, A., Wang, S., Sun, Y., Chu, L., Zhou, L., et al. (2022). Efficacy of temozolomide-conjugated gold nanoparticle photothermal therapy of drug-resistant glioblastoma and its mechanism study. Mol. Pharm. 19 (4), 1219–1229. doi:10.1021/acs.molpharmaceut.2c00083
Zakaria, H. M., Llaniguez, J. T., Telemi, E., Chuang, M., Abouelleil, M., Wilkinson, B., et al. (2020). Sarcopenia predicts overall survival in patients with lung, breast, prostate, or myeloma spine metastases undergoing stereotactic body radiation therapy (SBRT), independent of histology. Neurosurgery 86 (5), 705–716. doi:10.1093/neuros/nyz216
Zeng, F., Peng, K., Han, L., and Yang, J. (2021). Photothermal and photodynamic therapies via NIR-activated nanoagents in combating Alzheimer's disease. ACS Biomater. Sci. Eng. 7 (8), 3573–3585. doi:10.1021/acsbiomaterials.1c00605
Zeng, Q., Ma, X., Song, Y., Chen, Q., Jiao, Q., and Zhou, L. (2022). Targeting regulated cell death in tumor nanomedicines. Theranostics 12 (2), 817–841. doi:10.7150/thno.67932
Zeng, X., Wang, Q., Tan, X., Jia, L., Li, Y., Hu, M., et al. (2019). Mild thermotherapy and hyperbaric oxygen enhance sensitivity of TMZ/PSi nanoparticles via decreasing the stemness in glioma. J. Nanobiotechnology 17 (1), 47. doi:10.1186/s12951-019-0483-1
Zeng, Y., Zhao, L., Li, K., Ma, J., Chen, D., Liu, C., et al. (2023). Aptamer-functionalized nanoplatforms overcoming temozolomide resistance in synergistic chemo/photothermal therapy through alleviating tumor hypoxia. Nano Res. 16 (7), 9859–9872. doi:10.1007/s12274-023-5742-7
Zhan, Y., Zhou, Z., Chen, M., and Gong, X. (2023). Photothermal treatment of polydopamine nanoparticles@Hyaluronic acid methacryloyl hydrogel against peripheral nerve adhesion in a rat model of sciatic nerve. Int. J. Nanomedicine 18, 2777–2793. doi:10.2147/IJN.S410092
Zhang, G., Liu, X., Xie, W., Hong, C., Xu, Y., Zhang, W., et al. (2021). Trash to treasure: a human beard derived photothermal drug delivery platform for depression therapy. Appl. Mater. Today 22, 100891. doi:10.1016/j.apmt.2020.100891
Zhang, L., Liu, Y., Huang, H., Xie, H., Zhang, B., Xia, W., et al. (2022a). Multifunctional nanotheranostics for near infrared optical imaging-guided treatment of brain tumors. Adv. Drug Deliv. Rev. 190, 114536. doi:10.1016/j.addr.2022.114536
Zhang, L., Zhang, D., Tang, H., Zhu, Y., Liu, H., and Yu, R. (2022b). Bacteria wear ICG clothes for rapid detection of intracranial infection in patients after neurosurgery and photothermal antibacterial therapy against Streptococcus mutans. Front. Bioeng. Biotechnol. 10, 932915. doi:10.3389/fbioe.2022.932915
Zhang, X., Liu, J., Yang, X., He, G., Li, B., Qin, J., et al. (2019). CuCo(2)S(4) nanocrystals as a nanoplatform for photothermal therapy of arterial inflammation. Nanoscale 11 (19), 9733–9742. doi:10.1039/c9nr00772e
Zhao, L., Zhang, X., Wang, X., Guan, X., Zhang, W., and Ma, J. (2021). Recent advances in selective photothermal therapy of tumor. J. Nanobiotechnology 19 (1), 335. doi:10.1186/s12951-021-01080-3
Zhao, Q., Wang, J., Yin, C., Zhang, P., Zhang, J., Shi, M., et al. (2019). Near-infrared light-sensitive nano neuro-immune blocker capsule relieves pain and enhances the innate immune response for necrotizing infection. Nano Lett. 19 (9), 5904–5914. doi:10.1021/acs.nanolett.9b01459
Zhao, Y., Liu, X., Liu, X., Yu, J., Bai, X., Wu, X., et al. (2022). Combination of phototherapy with immune checkpoint blockade: theory and practice in cancer. Front. Immunol. 13, 955920. doi:10.3389/fimmu.2022.955920
Zhou, H., Gong, Y., Liu, Y., Huang, A., Zhu, X., Liu, J., et al. (2020). Intelligently thermoresponsive flower-like hollow nano-ruthenium system for sustained release of nerve growth factor to inhibit hyperphosphorylation of tau and neuronal damage for the treatment of Alzheimer's disease. Biomaterials 237, 119822. doi:10.1016/j.biomaterials.2020.119822
Zhou, L., Xiang, H., Liu, S., Chen, H., Yang, Y., Zhang, J., et al. (2024). Folic acid functionalized AQ4N/Gd@PDA nanoplatform with real-time monitoring of hypoxia relief and enhanced synergistic chemo/photothermal therapy in glioma. Int. J. Nanomedicine 19, 3367–3386. doi:10.2147/IJN.S451921
Zhuang, S., He, M., Feng, J., Peng, S., Jiang, H., Li, Y., et al. (2023). Near-infrared photothermal manipulates cellular excitability and animal behavior in Caenorhabditis elegans. Small Methods 7 (11), e2300848. doi:10.1002/smtd.202300848
ZhuGe, D. L., Wang, L. F., Chen, R., Li, X. Z., Huang, Z. W., Yao, Q., et al. (2019). Cross-linked nanoparticles of silk fibroin with proanthocyanidins as a promising vehicle of indocyanine green for photo-thermal therapy of glioma. Artif. Cells Nanomed Biotechnol. 47 (1), 4293–4304. doi:10.1080/21691401.2019.1699819
Zhuo, Y., Zhang, Y., Wang, B., Cheng, S., Yuan, R., Liu, S., et al. (2022). Gold nanocluster and indocyanine green based triple-effective therapy for MRSA infected central nervous system. Appl. Mater. Today 27, 101453. doi:10.1016/j.apmt.2022.101453
Glossary
AD Alzheimer’s disease
BBB blood-brain barrier
BP Black phosphorus
CCM cancer cell membrane
CDT Chemodynamic Therapy
CQDs carbon quantum dots
CT Chemotherapy
DOX doxorubicin
ERK extracellular regulated protein kinases
ERS Endoplasmic reticulum stress
FAPI fibroblast activating protein inhibitor
FLI fluorescence imaging
FLs fluorescent poly-levodopamine nanoparticles
FRET Förster resonance energy transfer
GBM glioblastoma
Hb hemoglobin
HGNP Hollow gold nanoparticles
HRP Horseradish peroxidase
Hsps heat shock proteins
ICD immunogenic cell death
ICG indocyanine green
LCST lowest critical solvation temperature
LSPR localized surface plasmon resonance
ML machine learning
MOF metal-organic framework
MOMP mitochondrial outer-membrane permeabilization
MPTT mild photothermal therapy
MRI magnetic resonance imaging
MSNs Mesoporous silica nanoparticles
NFTs neurofibrillary tangles
NGF nerve growth factor
NIR Near-infrared
PAI photoacoustic imaging
PBNPs Prussian blue nanoparticles
PD Parkinson’s disease
PDA Polydopamine
PDT photodynamic therapy
PEG polyethylene glycol
PTA photothermal transduction agents
PTT photothermal therapy
ROS reactive oxygen species
RT radiotherapy
SDT Sonodynamic Therapy
SOD superoxide dismutase
SPIO Supramagnetic iron oxide
TA tannic acid
TAAs tumor-associated antigens
TMZ temozolomide
TPE main-chain tetraphenylene.
Keywords: photothermal therapy (PTT), glioma, neurodegenerative diseases, combination therapy, blood-brain barrier, heat shock proteins (HSP), organic nanoplatform, inorganic nanoplatform
Citation: Zhu H, Yang W, Suo Y, Liu Y, Zhan X, Zhou J, Chen Z, Wu X, Yin X and Bao B (2025) Nanomaterials engineered for photothermal therapy in neural tumors and neurodegenerative diseases: biomaterial design, clinical mechanisms and applications. Front. Bioeng. Biotechnol. 13:1631627. doi: 10.3389/fbioe.2025.1631627
Received: 20 May 2025; Accepted: 04 July 2025;
Published: 21 July 2025.
Edited by:
Marina Massaro, University of Palermo, ItalyCopyright © 2025 Zhu, Yang, Suo, Liu, Zhan, Zhou, Chen, Wu, Yin and Bao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiaoping Yin, eGlhb3BpbmdidXhpYW9AMTI2LmNvbQ==; Bing Bao, YmFvYmluZzg0MDYwOEAxNjMuY29t
†These authors have contributed equally to this work and share first authorship
 Hanjing Zhu
Hanjing Zhu Wei Yang
Wei Yang Yijun Suo
Yijun Suo Xiaoping Yin
Xiaoping Yin Bing Bao
Bing Bao