- 1 Division of Molecular Genetics and Cancer, NITTE University Centre for Science Education and Research (NUCSER), Mangaluru, Karnataka, India
- 2 Department of Biotechnology, Nehru Arts and Science College, Coimbatore, Tamil Nadu, India
- 3 Department of Clinical Oncology, Queen Elizabeth Hospital, Hong Kong SAR, China
Parkinson’s disease (PD) is a progressive neurodegenerative disorder that is characterized by the loss of dopaminergic neurons, leading to severe motor and cognitive impairments. Recent advancements in nanomedicine and nano-structured technologies have opened new avenues for targeted drug delivery and neuroprotection, improving therapeutic efficacy and diagnostic accuracy. By harnessing innovative nanotechnological platforms, researchers aim to enhance clinical trial outcomes and refine early-stage diagnostic advancements, offering hope for improved disease management. However, since the pathophysiology of PD is diverse, there are limited treatment options available. This review explores the potential of the recent nanostructured technologies in managing the complexities of PD. Deliberations on the insights from nanomedicine, neurobiology, and material science, on how these emerging and technologically sound nanostructured approaches help in the prevention, diagnosis and treatment of PD will be discussed. Further, the role of nanocarriers in targeted drug delivery involving nanoscale materials specifically for neuroprotection and regeneration will be discussed with special emphasis on the role of nanotechnology in advancing diagnostic methodologies. Additionally, we aim to chart a course for future research directions, with special reference to innovative approaches in disease diagnosis. The various therapeutic approaches, along with the ongoing clinical trials and real-world applications, are expected to add value to the efforts of the researchers worldwide to enhance therapeutic efficacy and patient outcomes in PD.
1 Introduction
Worldwide, millions of individuals, along with their families, suffer due to the health challenges posed by the neurodegenerative diseases (NDs) like Alzheimer’s disease (AD), Parkinson’s disease (PD), and multiple sclerosis. Most of these conditions feature a progressive degeneration of the nervous system, that will ultimately lead to severe decline/dysfunction of cognitive and motor systems thereby impairing the quality of the patient’s life. The situation is made serious with the concurrent immune dysfunction that has a critical role to play in the pathogenesis of many of these NDs, in addition to abnormal immune responses (Zhang et al., 2023). This combined effect can lead to severe neuronal damage that can in turn, exacerbate the disease progression (Figure 1).

Figure 1. Neuronal damage. The deterioration or loss of nerve cells (neurons) due to factors like oxidative stress, neuroinflammation, toxic accumulation, or trauma, leads to neuronal damage resulting in impaired brain function. It is commonly associated with neurodegenerative diseases such as Parkinson’s, Alzheimer’s, and multiple sclerosis, affecting cognition, movement, and overall neurological health.
PD ranks second as the most common neurodegenerative disease all over the world, with a rise in global prevalence up to 74.3% between 1990 and 2016. The first authentic publication about the disease was done by James Parkinson, from whom the disease got the name, in 1817, followed by many more (Dong-Chen et al., 2023). PD is a chronic and progressive neurological disorder wherein the neurons in the substantia nigra become damaged, which will lead to a reduction in the dopamine levels in the brain, resulting in a gradual loss of regulation in movement and co-ordination. The major symptoms observed are related to the motor movements including tremor, muscle stiffness, loss of balance along with anxiety, depression and sleep-related disorders. The traditional taxonomy of PD includes several subtypes, such as tremor-dominant and non-tremor-dominant PD. Patients with the tremor-dominant subtype generally experience slower disease progression, better cognitive function, fewer non-motor symptoms, lower rates of death and disability, and longer survival compared to those with non-tremor-dominant PD (Zetusky et al., 1985; Marras and Chaudhuri, 2016).
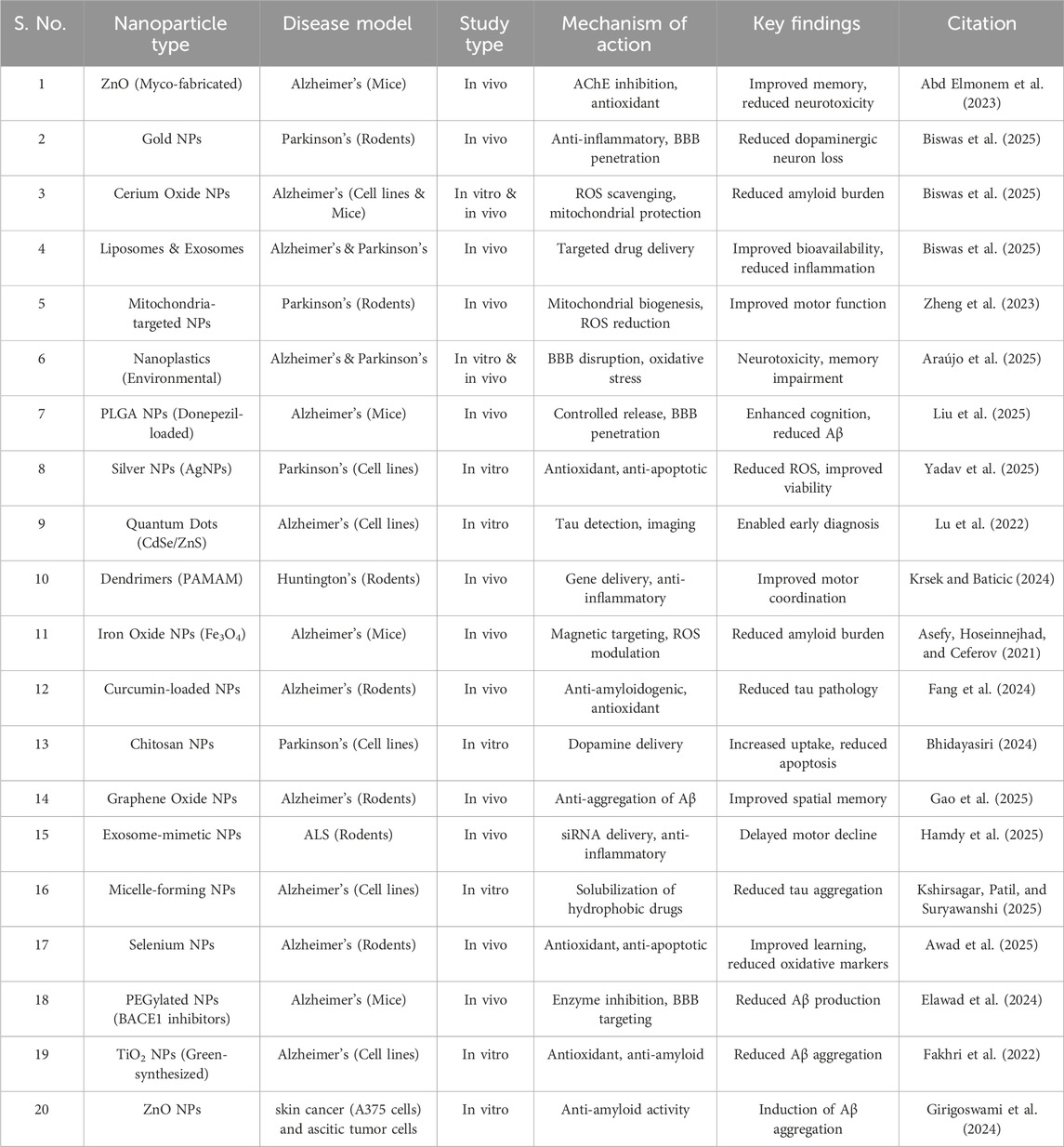
The research involving nanotechnology to better understand, diagnose, and treat PD is emerging as a promising frontier. By utilizing nanoscale materials and carriers, researchers can develop highly targeted drug delivery systems that cross the blood-brain barrier (BBB), ensuring that therapeutics reach the intended regions of the brain with precision. This approach not only enhances the efficacy of neuroprotective agents but also minimizes side effects, offering new credence for more effective management of this complex neurodegenerative disorder. Additionally, nanotechnology facilitates the development of advanced diagnostic tools that can detect PD at earlier stages, potentially enabling timely intervention and improved patient outcomes (Yadav et al., 2025). The mechanisms highlighting the versatility of nanomaterials in addressing various aspects of neuronal damage, are explained in Table 1.

Table 1. Diverse mechanisms highlighting the versatility of nanomaterials in addressing various aspects of neuronal damage, making them promising candidates for therapeutic interventions in neurodegenerative diseases.
The transient landscape of nanomedicine in PD emphasizes the urgent need for an extensive review of the subtleties in the field to identify future directions. Despite significant progress in modern medicine, treatment options remain limited due to the PD’s intricate pathophysiology. A focused review on nanostructured technologies will help to deliver valuable insights into how these innovations can address existing therapeutic gaps and improve drug delivery efficiency. This will help future scientists to design new techniques for early diagnosis and innovative treatment options. Such an overview is essential to guide ongoing research efforts and to foster collaborative advancements in the field. This review highlights the key advancements in nanotechnology-based strategies for PD, reiterating the role of nanocarriers in targeted neuroprotection and regeneration, as well as novel diagnostic methodologies. It also mentions the ongoing clinical trials and real-world applications, specifying how these cutting-edge approaches are translating from laboratory research into clinical practice. By charting the recent achievements and future research avenues, this review aims to support the development of a more effective and personalized treatment modality that can ultimately improve the quality of life for PD patients worldwide.
2 PD–the pathophysiology and underlying mechanisms
The probability of PD increases significantly post the age of 60, which might go up to over 3% in individuals older than 80 (Pringsheim et al., 2014), wherein men are more susceptible for developing PD than women and dementia can also surface in later stages (Hely et al., 2005). Differences in lifestyle and environmental factors are likely to contribute to the variation in prevalence, observed across regions and ethnic groups. Exposure to toxins from environment may trigger the symptoms of PD, while smoking and caffeine consumption can increase the risk (Dong-Chen et al., 2023). PD progression is characterized by dyskinesia, psychosis, and motor and non-motor fluctuations. Almost 80% of PD patients have freezing of the gait and falls after roughly 17 years of the disease, and up to 50% of patients say they have experienced choking. Many of the initial pathological features of PD point to the gradual degeneration of a specific subset of neurons in the substantia nigra. In the primary phases of the disease, dopaminergic neuron loss is largely confined to the ventrolateral region of the substantia nigra, which further spreads as years pass by. Furthermore, in different areas of the brain, including the cerebellar nuclei and adjacent white matters, certain neurons have abnormally high levels of α-synuclein (Braak et al., 2003). The aggregated α-synuclein accumulates in different neurons, as well as olfactory neurons, forming Lewy bodies (Piao et al., 2003; Mori et al., 2003). The Lewy bodies, a common pathological hallmark, are primarily found in Bergmann glia within the molecular layer and in Purkinje cell axons. As PD progresses, Lewy body accumulation increases, affecting not only dopaminergic neurons but also non-dopaminergic neurons in various brain regions, including the limbic system and neocortex (Hirsch et al., 2003), which further extends to neurons outside the central nervous system (CNS), including the olfactory enteric nervous system (Dong-Chen et al., 2023). More details on the molecular mechanisms involved in the manifestation of PD are explained in Figure 2.
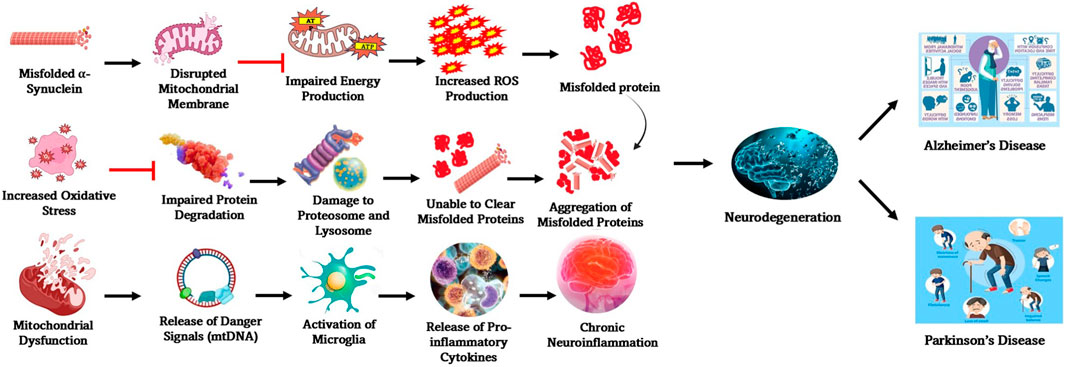
Figure 2. Molecular mechanisms involved in the manifestation of PD. Some of the key processes involved in the interconnected molecular mechanisms underlying PD pathogenesis are α-synuclein aggregation, mitochondrial dysfunction, oxidative stress, impaired protein degradation, and neuroinflammation. Many of these pathways influence one another, amplifying neurodegeneration and contributing to disease progression.
It is difficult to diagnose PD in the early stages. An accurate clinical diagnosis is made in approximately 65% of patients within the first 5 years of PD onset (Sayyaed et al., 2023). However, pathological changes can exist for several years before any noticeable symptoms appear. On average, there is a 12–14-year gap between the initial development of Parkinsonian pathology and the emergence of motor symptoms, illustrating how the preclinical stage of the disease can be prolonged (Greener et al., 2021). However, within the brain, lack of dopamine-producing neurons slows down the motor function considerably, followed by aggregation of lewy bodies (Latif et al., 2021). Environmental contamination including pesticides, or misuse of pills, along with genetic and age factors could initiate the disease, which could potentially aggregate. Cellular aging of neurons in the brain is regulated by inflammatory process over a period of time (Crowley et al., 2019). Additionally, PD patients with resting tremor show reduced grey matter volume primarily in the quadrangular lobe. Compared to those with the akinesia/rigidity-dominant subtype, tremor-dominant PD patients exhibit decreased grey matter volume in the left cerebellar lobule VIIIa, highlighting the relation between cerebellum and PD with tremor (Benninger et al., 2009; Piccinin et al., 2017). However, whether the volumetric change is a causal factor, consequence, or concomitant phenomenon is still a mystery (Zhong et al., 2022). PD progression is marked by worsening motor function that can be managed through symptomatic treatments. In the advanced stages, treatment-resistant motor and non-motor symptoms (Figure 3) become more prominent and axial motor symptoms such as gait disturbances, frequent falls, freezing of gait, speech impairments, and swallowing difficulties are common. Non-motor symptoms in late-stage PD often include symptomatic postural hypotension, persistent constipation requiring regular laxatives, and urinary incontinence (Neag et al., 2020). After 20 years of living with the disease, around 83% of PD patients develop dementia, which significantly contributes to functional decline that need hospitalization and serves as a strong predictor of mortality (Bjørklund et al., 2019).

Figure 3. Motor and non-motor symptoms associated with PD. The diverse symptoms of PD are categorized into motor and non-motor manifestations. Motor symptoms primarily affect movement and coordination. Non-motor symptoms significantly impact overall wellbeing and quality of life. Understanding these symptoms is crucial for comprehensive disease management and patient care.
The common symptoms associated with PD include motor activities like tremors, rigidity, and bradykinesia (slow movements), and non-motor activities like decline of cognitive abilities, mood swings, and dysfunction of the autonomic nervous system, resulting in severe sweating, dizziness and fainting (Bloem et al., 2021; Silva et al., 2023). These multifactorial end-results observed in PD necessitate an intense but holistic approach for managing the obstacles and setbacks associated with this condition. Patients enduring the complex manifestations of this disease depend on the advancements in the field to shed light on the therapeutic strategies that are probably going to be their only way to liberation from the inconveniences associated with this condition. New treatment modalities, including pharmacological and surgical interventions, and supportive therapies need to be discovered to improve the quality of life of the PD patients. It is also important for a precise detection of disease condition as mis-information or incorrect diagnosis will lead to wrong treatment plans that will in turn lead to the worsening of the disease or may result in delayed response in the patient (Wang and Wang, 2023).
2.1 Genetic and epigenetic factors affecting the progression of PD
The complexity of PD is intensified by a combination of genetic, epigenetic, and environmental factors. In this, genetic predisposition plays a significant role, with several genes being intimately linked to the risk of PD eventuality. The fact that such genetic mutations can be inherited through autosomal dominant or recessive inheritance patterns further increase the individual’s susceptibility (Ye et al., 2023). Additionally, epigenetic modifications are also known to dominate the progression of PD by regulating genes related to cellular processes like autophagy, inflammation, and oxidative stress (Chen et al., 2022). Understanding the crosstalk between genetic and epigenetic changes in gene expression will also help to uncover potential avenues for early diagnosis, targeted therapies, and personalized interventions. Even though multiple genetic factors contribute to neurodegeneration, mutations in seven major genes such as VPS35, DJ-1, GBA1, LRRK2, PINK1, PRKN, and SNCA impact neuronal health the most through distinct pathways. VPS35 is part of the retromer complex involved in endosomal sorting; a mutation in the VPS35 gene, specifically the D620N mutation, can lead to autosomal-dominant PD. This disrupts the normal functioning of the retromer complex impairing endosomal sorting and protein recycling, further leading to abnormal protein accumulation and neurodegeneration (Rowlands and Moore, 2024). Studies in knock-in mouse models reveal that this mutation also amplifies glutamate synapse activity, contributing to excitotoxicity and neuronal damage (Kadgien et al., 2021). DJ-1 functions as an antioxidative stress protein; its loss heightens oxidative damage to neurons. Mutations to this gene will disrupt its ability to neutralize ROS, making dopaminergic neurons more vulnerable to oxidative damage (Alshammari, 2025). This gene strengthens the mitochondrial function by regulating the uncoupling proteins UCP4 and UCP5, and maintaining mitochondrial membrane potential. Mutations impair this regulation, leading to mitochondrial stress and apoptosis (Mencke et al., 2021). It is also known to interact with Nrf2, a key transcription factor that activates antioxidant response elements (ARE). By failing to regulate redox balance and protein degradation pathways, mutations prevent DJ-1 from promoting Nrf2 nuclear translocation, bringing down the cellular defense against oxidative stress. It can also exacerbate α-synuclein aggregation, a hallmark of PD (Alshammari, 2025). The mutations in GBA1 impair the enzyme glucocerebrosidase (GCase), leading to defective lysosomal degradation and accumulation of toxic α-synuclein aggregates. GCase mutations impair the breakdown of glucosylceramide, leading to lipid accumulation and lysosomal stress. Also, the increased α-synuclein accumulation due to reduced GCase activity leads to the disruption of neuronal function that further accelerates neurodegeneration. Mutations in GBA1 will also contribute to endoplasmic reticulum (ER) stress and mitochondrial dysfunction, further exacerbating neuronal damage (Do et al., 2019; Zhang et al., 2024). Mutations in the LRRK2 gene are among the most common genetic causes of PD (El Otmani et al., 2023). Typically, these mutations lead to increased kinase activity, disrupting cellular processes and contributes to neurodegeneration. Enhanced kinase activity triggers abnormal phosphorylation of Rab proteins, which are crucial for intracellular trafficking. These mutations also impair autophagy and lysosomal degradation, reducing the clearance of α-synuclein. Further, neuronal damage triggers inflammatory pathways, increasing microglial activation. However, unlike the typical PD cases, some LRRK2 mutation carriers exhibit reduced Lewy body accumulation, suggesting alternative neurodegenerative mechanisms (Dzamko, 2025; Rivero-Ríos et al., 2020).
PINK1 and PRKN (Parkin) are two genes heavily invested in mitochondrial quality control through clearance of damaged mitochondria via mitophagy (Mundekkad and Cho, 2022). They are linked to autosomal recessive PD, where two mutated copies of a gene (one from each carrier parent) are needed to cause the disease. Progeny has 50% chances of inheriting the disease and 25% of being a carrier or being devoid of the disease (Antonarakis, 2019). PINK1 detects mitochondrial damage, while Parkin tags and stimulates damaged mitochondria for degradation. Mutations in either of these genes will compromise this process, quickening the accumulation of dysfunctional mitochondria (Ge et al., 2020). The functional loss of these two genes may also lead to consequent events like oxidative stress, energy deficits, and neuronal death, particularly affecting dopaminergic neurons in the substantia nigra (Narendra and Youle, 2024). On account of the high metabolic demands, dopaminergic neurons are particularly susceptible to mitochondrial dysfunction caused by PINK1/Parkin mutations. Genetic mutation in PRKN will contribute to protein aggregation and cellular toxicity which are decisive factors in PD progression. The final of the main seven genes impacting PD, the SNCA gene encodes α-synuclein, and is primarily involved in synaptic vesicle trafficking and release of neurotransmitters. Mutations in this gene (especially as A53T, A30P, and E46K) will catalyze the misfolding and abnormal aggregation of α-synuclein, forming Lewy bodies. Aggregated α-synuclein will obstruct synaptic transmission, mitochondrial function, and protein degradation pathways, contributing to dopaminergic neuron loss. Since α-synuclein aggregates can spread between neurons and to other brain regions, the disease progresses beyond the substantia nigra arousing ultimate damage to the brain cells, disrupting neuronal functions (Meade et al., 2019).
2.2 Nanoparticle-based modulation of genetic and epigenetic drivers in PD
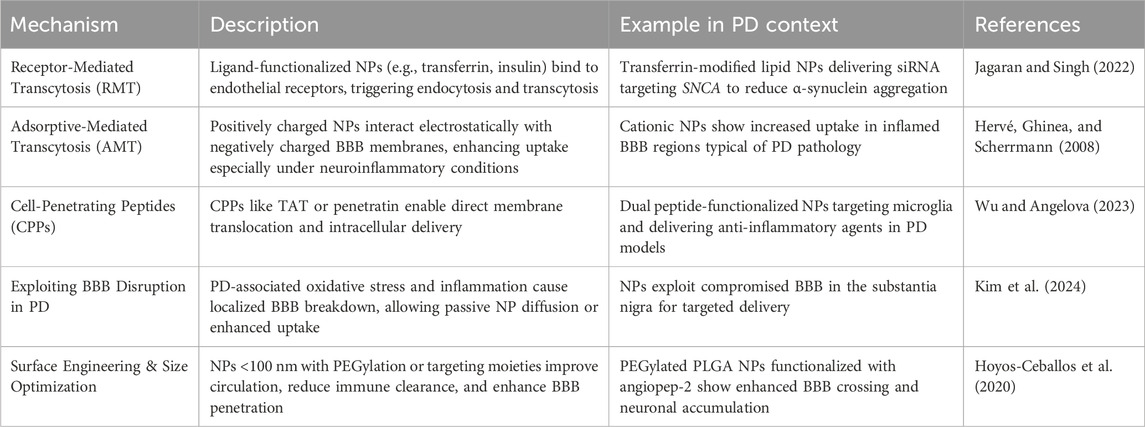
The precise targeting of genetic as well as epigenetic agents in the progression of PD is well studied in recent years. Nanoparticle-mediated correction of aberrant DNA methylation was found to mitigate neurodegeneration. By the modification of the genes involved in regulating oxidative stress responses, gold nanoparticles, functionalized with S-adenosylmethionine (SAM, a well-studied methyl donor molecule), have demonstrated the ability to restore redox balance and thus modulate epigenetic markers that are closely associated with neurodegeneration in PD (Thanan et al., 2014; Bekdash, 2023). Such interventions at the epigenetic level not only mitigate neurodegeneration but are also known to enhance neuroplasticity and promote repair. Many of the advanced techniques are based on the application of nanoparticles like lipid nanoparticles for the delivery of small interfering RNAs (siRNAs) or antisense oligonucleotides (ASOs) to silence the faulty genes like SNCA that encodes α-synuclein (Cole et al., 2021). It is well known that excessive accumulation of α-synuclein is a hallmark of PD. Cellular delivery of LNP-mediated siRNA has resulted in reduced protein accumulation followed by neurotoxicity in preclinical models. Further, PEGylated LNPs that efficiently encapsulate siRNA can be used against SNCA as they are shown to efficiently penetrate the BBB (Figure 4) and sustain gene knockdown in dopaminergic neurons. Dendrimer-based nanocarriers are also engineered to deliver various components of CRISPR-Cas9 for the targeted editing of LRRK2 mutations that are linked to familial PD (Unnithan et al., 2024). Using the nanocarriers for such targeted delivery ensures high efficiency in transfection with minimal off-target effects. Additionally, the histone acetylation is modulated as part of epigenetic modification – the DNA methylation patterns influence neuroinflammation and, in turn, the survival of neuronal tissues. This is clearly explained by the role of poly (lactic-co-glycolic acid) (PLGA) nanoparticles, which are loaded with suberoylanilide hydroxamic acid (SAHA), a histone deacetylase (HDAC) inhibitor. The acetylation balance is restored by SAHA, which in turn protects the dopaminergic neurons observed in PD models that are induced experimentally by 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) (Mondal and Firdous, 2025). Such modulations are stimulated through multiple levels of disease-driven molecular pathways with exceptional specificity and biocompatibility. In general, the modulation of genetic and epigenetic modulators by nano-structured interventions signifies a paradigm shift in therapeutics related to NDs.
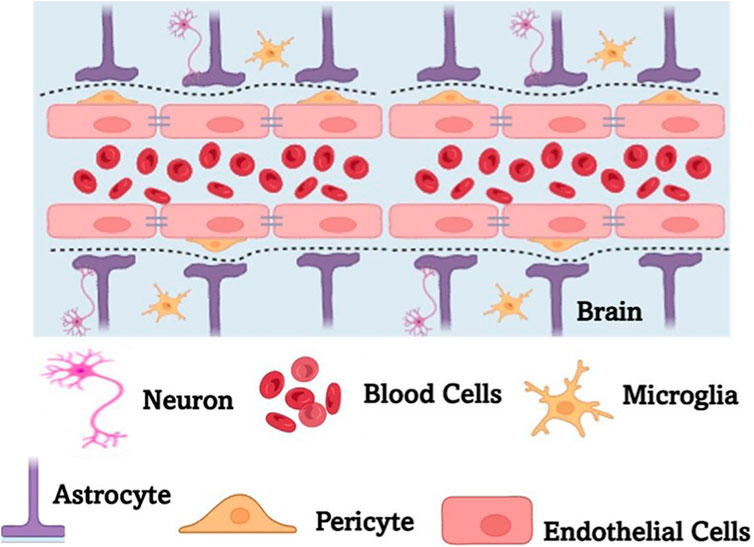
Figure 4. A schematic representation of the blood brain barrier (BBB). BBB is a highly selective, semipermeable membrane formed by tightly packed endothelial cells that regulate the transfer of solutes between the bloodstream and the CNS. BBB protects the brain from harmful substances while allowing essential nutrients to pass through. It plays a crucial role in maintaining brain homeostasis but also poses challenges for drug delivery, requiring specialized strategies to transport therapeutic agents across the barrier.
3 Nanomedicine in neurodegenerative diseases
The nanostructured materials are impacting various phases of medical fields, and they are now being applied in neuroprotection, extending to various approaches including the delivery of antioxidant and anti-inflammatory molecules/agents, and for neurotrophic factor supplementation. To elaborate on the use of nanostructured molecules in the medical filed, graphene oxide nanoparticles are proving to be a promising candidate in antioxidant therapy by effectively scavenging free radicals and reducing oxidative stress - these are crucial factors in neurodegenerative diseases like PD. In addition, biomimetic nanofibers, designed to mimic the extracellular matrix (Mundekkad and Mallya, 2025), can support neuronal growth and regeneration, demonstrating potential in neurorepair strategies. Nanomedicine has also introduced many advanced diagnostic tools to be used in NDs along with other advanced biotechnological innovations. In many cases, gold nanoparticles are known to be employed explicitly to bind with specific biomarkers associated with proteins specific to NDs. Specifically, in case of AD, many of these nanoparticles are used in combination with other imaging processes to detect the proteins that are specifically found in NDs like the beta-amyloid or tau proteins (Abidi et al., 2023). Conjugating the nanoparticles for diagnostic purposes will improve sensitivity and specificity that can lead to early detection methods. This will help in facilitating timely intervention when treatment options are to be explored. In some cases, quantum dots, which are basically semiconductor nanocrystals, are used as they are highly versatile labels in imaging applications. They can be designed in such a way to emit specific wavelengths of light when excited, which can be made use of to visualize neuronal changes at the nanoscale, thereby locating the abnormalities even before the clinical symptoms are visible.
3.1 Nanoparticles in neuro-inflammation and neuro-toxicity
The diagnosis and treatment of neurodegenerative diseases are centred around the CNS, and the treatment outcomes are critically marred by neuroinflammation and neurotoxicity. In PD, chronic neuroinflammation is triggered by the accumulation of misfolded α-synuclein along with mitochondrial dysfunction. Neuroinflammation is commonly known to exacerbate dopaminergic neuronal degeneration. It is observed that certain nanoparticles can either aggravate or alleviate this inflammatory cascade based on their size, surface charge, and composition. Selenium nanoparticles exhibit neuroprotective effects by mitigating oxidative stress, primarily through scavenging ROS, inhibiting α-synuclein aggregation, and modulating microglial activation. It is observed that these mechanisms reduce neuroinflammatory stress in PD models (Umapathy et al., 2025). However, despite their protective role in neuroinflammatory distress, nanoparticles are known to induce neurotoxicity. Nanoparticles are capable of disrupting neuronal membranes and activating pro-inflammatory pathways, leading to oxidative stress and apoptosis. Gold nanoparticles offered dose-dependent effects, with low concentrations reducing inflammation and higher doses triggering mitochondrial damage and glial cell activation (Kumar et al., 2022). Sometimes, biocompatibility and surface functionalization are integral to minimize the unintended neurotoxicity. Also, in cases where the BBB is compromised (as often observed in PD), the nanoparticles may end up in heaps around the substantia nigra region and this makes precision targeting and controlled release a high priority event to avoid exacerbating neurodegeneration (Cheng et al., 2022). However, in spite of this, there are nanoparticles that are capable of countering neuroinflammation without inducing toxicity. Non-invasive stimulation of brain regions by laser-activated nanoparticles is a better alternative approach to brain stimulation with cognitive side effects. By minimal systemic exposure, these nanoparticles can influence neuronal activity and motor symptoms in PD.
3.2 Nanoparticles in neuroprotection
Nanotechnology often offers a novel approach to developing therapeutic strategies aimed at preserving neural function and promoting recovery. By exploiting the distinct properties of nanomaterials, researchers are developing innovative therapeutic strategies to address the complex challenges of NDs. Current research in this area continues to refine these applications for exploring new nanomaterials that could be used for future neuroprotective interventions. Some of the notable examples related to the application of nanoparticles in the research related to various aspects of neurodegenerative diseases and the subsequent utilization for treatment are discussed here.
3.2.1 Platinum nanoparticles
Platinum nanoparticles (PtNPs) are being researched extensively for their potential to mitigate neurotoxicity and oxidative stress in neurological conditions. Their unique physicochemical properties, such as high surface area and catalytic activity, provide them with a capacity to scavenge ROS, reduce inflammation, and protect neuronal cells from damage that are caused by NDs, toxins, or ischemic injury. The research revealed details on how PtNPs influence brain damage, cerebral blood flow (CBF), and oxidative stress in rats subjected to ischemic conditions. Also, despite their extremely low accumulation in the brain, these nanoparticles demonstrated significant neuroprotective properties (Filippov et al., 2023). It was found that PtNPs weakened post-ischemic hypoperfusion, attenuated neuronal apoptosis with minimal cell death in the hippocampus region, and improved glutathione redox status by preserving the glutathione redox balance and reduced oxidative damage, thus modulating oxidative stress also. However, they have extremely low bioavailability as it was shown that despite their neuroprotective effects, they were found in very low concentrations in brain tissue. Such effects were mediated through indirect mechanisms and not direct accumulation in brain tissue, proving PtNPs to be a significant candidate in the quest for effective neuroprotective agents. The study reported that PtNPs could activate neuroprotective signaling pathways, including the PI3K/Akt pathway, promoting neuronal survival and reducing apoptosis in neuronal cultures exposed to ischemic conditions. This suggests a promising avenue for therapeutic use in stroke and other ischemic brain injuries. Another study explored the neuroprotective potential of PtNPs that were synthesized from Biophytum reinwardtii in a zebrafish model of PD (Bandaru et al., 2024a). The disease was induced by MPTP and the zebrafish were treated with different concentrations of these nanoparticles. The effects on locomotor activity, oxidative stress markers, and catecholamine levels were assessed and the results showed that PtNPs significantly improved movement, increased antioxidant levels, and restored catecholamine balance, suggesting their potential for neuroprotection in PD. These findings highlight the potential of platinum nanoparticles in treating acute cerebrovascular disorders through systemic antioxidant and neuroprotective pathways. Given their unique properties to bring about antioxidant, anti-inflammatory, and electrochemical changes, PtNPs are gaining much traction in neurodegenerative disease research as well. They can act as nanozymes and ROS scavenging molecules in neurovascular units, where even as 5 nm sized particles, they can act as antioxidant enzyme-mimics that can efficiently mime the natural enzymes like catalase and superoxide dismutase. The PtNPs scavenged ROS in neurovascular cellular models, effectively protecting neurons and glial cells from oxidative damage. The impetus behind neurodegenerative cascades in conditions such as Alzheimer’s and Parkinson’s often stems from oxidative damage to vulnerable neural and glial cells (Tarricone et al., 2023). It was observed that upon administration of PtNPs, the catalytic properties of the nanozyme activated the lysosomal environment, turning it into tiny, intracellular ‘microreactors’ that lowered mitochondrial stress, thus preserving the BBB integrity. When tested under kainic acid-induced excitotoxic conditions that are a proxy for neurodegeneration in an ex vivo rat hippocampal model, PtNPs showed no particular impairment of neuronal viability in CA1 and CA3 regions (parts of hippocampus), even under pathological stress. However, it was observed that they triggered microglial activation, clearly proposing an immune-modulatory role. This points to the possibility of harnessing PtNPs for therapeutic applications with controlled dosing (Gulino et al., 2021). Another study in a rat model showed that electrodes coated with PtNP (used for deep brain stimulation) displayed reduced and stabilized impedance over 4 weeks of stimulation. This result points to the possibility of enhanced precision and longevity of neurostimulation therapies for Parkinson’s and other movement disorders, indicating PtNPs’ utility in neuroelectronic interfaces (Angelov et al., 2024).
3.2.2 Titanium dioxide nanoparticles
Titanium dioxide nanoparticles (TiO2 NPs) are widely used in consumer products. However, they are being explored in therapeutic uses as well. Even though the neurotoxic effects of TiO2 have raised concerns due to their environmental presence (Aschner et al., 2023) and human exposure, it is highlighted that TiO2, when in nanoform, can cross the BBB. The exact mechanism of how TiO2 NPs cross the BBB is still unclear, but it is understood that once in the brain, it can induce oxidative stress and neuroinflammation, leading to disruption in brain biochemistry, followed by neuronal damage. This may influence behavioural disorders and neurodegenerative diseases, including PD. The neurotoxic potential of TiO2 NPs is influenced by the physicochemical properties and how they are exposed to the site of action during disease conditions. Another study investigated the neurotoxic effects of TiO2 NPs on neuronal, PC-12 cells. The study mainly focused on cytotoxicity, dopaminergic gene expression, and acetylcholinesterase inhibition. When two differently sized nanoparticles (10 nm and 22 nm) along with polyvinylpyrrolidone (PVP)-coated TiO2 NPs were used for the study, it was observed that at concentrations ≥10 μg/mL, it induced dose-dependent cytotoxicity due to increased ROS and nitrogen species (Suthar et al., 2023). Elevated inflammatory markers (like IL-6 and TNF-α), mitochondrial dysfunction, and apoptosis-related caspase-3 activation were observed, accompanied by acetylcholinesterase inhibition. These results suggest a potential neurochemical disruption in the brain. However, dopaminergic gene expression remained unaffected at lower exposure levels, which was considered an interesting observation. The surface coating of the nanoparticles with PVP reduced neurotoxic effects, highlighting its potential for safer medical applications. In vitro studies using neuronal and glial cell lines revealed that TiO2 NPs can induce neuronal cell damage through the induction of oxidative damage via the generation of ROS. It was also found that the cells encounter reduced ATP levels invoked by mitochondrial dysfunction. An exceptionally high dose or prolonged exposure to TiO2 NPs has been shown to trigger apoptosis/necrosis (Song et al., 2015). Further, specific inflammatory responses were observed with microglial activation and increased expression of pro-inflammatory cytokines (like IL-6, TNF-α), specifying role for TiO2 NPs in neuroinflammation - a key driver in diseases like Alzheimer’s and Parkinson’s. When applied to in vivo rodent models via inhalation or intravenous routes, the TiO2 NPs were found to be accumulated in various regions of the brain, including the hippocampus and cortex. Behavioral assays were conducted in the test organism, which revealed specific impairment of spatial memory and learning, consequently correlating with histopathological changes in brain tissue. Evidence of BBB disruption was observed, further facilitating the entry of other neurotoxicants. Assays on neurodevelopmental impact through prenatal exposure in these models showed altered brain development, pointing to potential risks during gestational periods (Zhang, Song et al., 2023).
3.2.3 Gold nanoparticles
The neuroprotective effects of gold nanoparticles (AuNPs) have been extensively studied for the treatment of PD. The nanoparticles were found to inhibit the generation of ROS and modulate the expression of pro-apoptotic and anti-apoptotic proteins (Piktel et al., 2021), suggesting that AuNPs have the potential to serve as a therapeutic agent in neurodegenerative diseases, including PD (de Bem Silveira et al., 2021). AuNPs are known to mitigate neuronal apoptosis that is caused by glutamate toxicity, which is considered a key factor in neurodegenerative diseases. Glutamate is an excitatory neurotransmitter, and its presence can lead to excessive calcium influx triggering oxidative stress, mitochondrial dysfunction, and caspase activation, ultimately resulting in neuronal cell death. It was inferred from the study that AuNPs reduced oxidative stress by scavenging ROS, and stabilized mitochondrial function by preventing the release of cytochrome c. It was found to inhibit the activation of caspase-3, strengthening its role in induction of apoptosis. It was also found to modulate neuroinflammatory pathways by reducing inflammatory cytokines. All these protective roles highlight the potential of AuNPs as therapeutic agents for neurodegenerative conditions like AD and PD (de Bem Silveira et al., 2021; Chiang et al., 2024). Further, the potential of AuNPs in diagnosing and treating neurodegenerative diseases were studied. It was understood that AuNPs could be functionalized to explicitly target the key pathological markers of neurodegenerative diseases - Tau and α-synuclein proteins. AuNPs enabled the detection of these proteins even at low concentrations in biological samples like blood or cerebrospinal fluid, enabling early and accurate diagnosis of the disease. AuNPs can also serve as drug delivery platforms, enabling the transport of therapeutic agents directly into the brain, thus improving treatment precision while maintaining minimal side effects (Tapia-Arellano et al., 2024). However, while the use of AuNPs looks promising, safety and biocompatibility are major concerns, requiring rigorous preclinical and clinical studies before widespread medical use. In vitro assays revealed the neuroprotective effect of AuNPs through anti-inflammatory activity, where supplementing the cultured microglial cells with AuNPs reduced neuroinflammation, as evidenced by the downregulation of pro-inflammatory cytokines like TNF-α and IL-6. These are generally elevated in AD and PD pathology (Chiang et al., 2024). Targeting of Tau and α-synuclein by functionalized AuNPs revealed a selective binding of the NPs to misfolded proteins (Tau in AD and α-synuclein in PD), thereby inhibiting their aggregation and toxicity in the tested neuronal cells (Tapia-Arellano, 2024). Treatment with AuNPs also revealed the antioxidant properties, where ROS was scavenged to protect the neurons from oxidative damage - a key driver in neurodegeneration. In vivo studies in mouse models showed that when conjugated with therapeutic agents, AuNPs improved cognitive function with reduced amyloid plaque burden. Drug delivery across the BBB was also observed to increase bioavailability in the brain. When targeted towards α-synuclein aggregates, AuNPs reduced motor deficits and neuroinflammation in PD animal models. This ability of AuNPs to deliver neuroprotective compounds directly to the regions of the brain that are affected by PD or other neurodegenerative conditions revealed improved therapeutic outcomes. AuNPs have even been helpful to mitigate ischemic damage after stroke – they act by reducing oxidative stress and inflammation, thereby preserving the integrity of neuronal cells, providing cerebrovascular protection (Chiang et al., 2024).
3.2.4 Selenium nanoparticles
Oxidative stress followed by dopaminergic neuron damage and protein misfolding are considered as major contributors to neurodegeneration. The neuroprotective potential of selenium nanoparticles (SeNPs) in treating PD was explored (Umapathy et al., 2024; Kalčec et al., 2023). SeNPs are found to act as antioxidants, scavenging the ROS and reducing oxidative damage. They also help in maintaining mitochondrial integrity by preventing cytochrome c release and apoptosis, which are crucial for neuronal survival. SeNPs are known to cross the BBB as any other nanoparticles and therefore, targeted delivery of SeNPs to affected regions of the brain are possible. SeNPs also suppress neuroinflammation by lowering the levels of pro-inflammatory cytokines like IL-6 and TNF-α, which significantly contribute to PD progression. The accumulation of misfolded proteins like α-synuclein and tau are inhibited by SeNPs. All these results point to the fact that SeNPs could be used as a potential therapeutic agent in neurodegenerative diseases like Parkinson’s (Umapathy et al., 2024).
Another research also discussed the potential of SeNPs for use as a mode of delivery for transporting drugs to treat PD. Since PD is characterized by dopaminergic neuron degeneration, the mode of action of SeNPs in enhancing drug transport across BBB was investigated and the therapeutic efficacy was also examined. It was understood that when SeNPs were functionalized with PVP and polysorbate 20 (Tween), it improved stability and drug-binding capacity. Also, L-DOPA and dopamine were successfully loaded onto SeNPs, showing that they have strong binding interactions that could enhance drug delivery. SeNPs also improved BBB permeability, allowing better drug absorption in brain cells, which is important in treatment of diseases like PD. Further, in vitro studies demonstrated efficient drug uptake by human brain endothelial cells (Kalčec et al., 2023). The antioxidant, anti-inflammatory, and neuroprotective properties of SeNP in both in vitro and in vivo studies provided compelling evidence of their therapeutic potential, especially in neurodegenerative disease conditions like AD, PD, and amyotrophic lateral sclerosis (ALS). Assays with the in vitro neuronal cell cultures revealed that the treatment with SeNPs reduced β-amyloid aggregation and inhibited tau hyperphosphorylation, which are the hallmarks of neurodegenerative diseases. The modulation of neuroinflammation through the downregulation of pro-inflammatory cytokines (e.g., IL-1β, TNF-α) in microglial cells indicated the ability of SeNPs to suppress neuroinflammatory cascades (Vicente-Zurdo et al., 2024). In vivo evidence in AD mouse models (transgenic AD mice treated with SeNP) revealed improved cognitive performance mediated by behavioral tests. It showed reduced amyloid plaque burden in the hippocampus, and enhanced antioxidant enzyme activity through glutathione peroxidase activity. When administered in PD-induced rodents, SeNPs showed protection of dopaminergic neurons in the substantia nigra with improved motor coordination and reduced tremors. They also lowered neuroinflammation markers, suggesting disease-modifying potential for the SeNPs. Further, functionalized SeNPs demonstrated the ability to cross the BBB, thereby delivering the therapeutic payloads directly to affected regions of the brain (Rajeshkumar et al., 2019).
3.2.5 Magnetite nanoparticles
The neuroprotective effects of magnetite nanoparticles (Fe3O4 NPs) were explored and it was found that they exhibited significant ability to reduce oxidative stress (Ucar et al., 2022), modulate neuroinflammation (Tamjid et al., 2023), and support mitochondrial function (Zhang et al., 2021b). These abilities of magnetite nanoparticles help the brain to preserve dopaminergic neurons in neurodegenerative diseases like Parkinson’s and Alzheimer’s. The potential of magnetite nanoparticles to act as drug-delivery systems was reviewed and it was discerned that the magnetite nanoparticles served as excellent agents for targeted therapy, improving treatment efficiency while minimizing systemic side effects. Research on the neuroprotective potential of Fe3O4 NPs in a PD model, focused on their ability to enhance neuronal survival, and improve motor function. Improved mitochondrial functions were observed, leading to enhanced energy metabolism and reduced apoptosis. They were found to modulate neuroinflammatory pathways by lowering levels of pro-inflammatory cytokines like IL-6 and TNF-α. Enhanced drug delivery potential suggested that Fe3O4 NPs could be used as carriers for targeted PD therapy (Khadrawy et al., 2024). Another study investigated the neuroprotective potential of iron oxide nanoparticles (IONPs) synthesized through green-route via ascorbic acid (AA-IONPs) in PD using both in vitro and in vivo models. The AA-IONPs were found to reduce neuroinflammation by lowering the levels of nitric oxide, prostaglandin E2, IL-6, and IL-1 in murine microglial BV2 cells. In vivo experiments on PD-induced C57BL/6 mice demonstrated enhanced motor coordination and reduced neuroinflammation after AA-IONP treatment. These findings suggest that AA-IONPs could serve as an effective nano-drug for neuroprotection through their anti-inflammatory and antioxidant properties (Li et al., 2023). The neuroprotective effects of IONPs in a PD model were studied (Khadrawy et al., 2024). The findings indicated that these nanoparticles could effectively deliver curcumin and enhance its bioavailability in neuronal cells. They not only demonstrated antioxidant activity but also modulated neuroinflammation by reducing the levels of inflammatory cytokines, providing a dual mechanism of action. In AD imaging, Fe3O4 NPs have been used as MRI contrast agents for the early detection of AD (Ulanova et al., 2020). The magnetic properties of Fe3O4 NPs enhance the resolution of the images to such an extent that when functionalized with imaging molecules, they can target amyloid plaques. Neuroprotective signaling in rodent models showed that Fe3O4 NPs activated the neuroprotective pathways and supported mitochondrial function, thereby reducing apoptosis and maintaining neuronal viability (Abdelmonem, et al., 2024).
3.2.6 Silica nanoparticles
Silica nanoparticles (SiNPs) have been studied for their ability to deliver therapeutic agents across the BBB (Bhatane et al., 2023). The potential of functionalized SiNPs as drug-delivery systems for neuroprotective treatments was examined and it was found that they enhanced the cognitive function in neurodegenerative diseases like PD. When SiNPs were functionalized with targeting ligands, they improved the BBB penetration and it brought about precise drug delivery to neuronal tissues. When neuroprotective drugs were loaded onto SiNPs, it also ensured sustained therapeutic effects through enhanced stability and bioavailability. Further, in vitro and in vivo studies with SiNPs demonstrated reduced oxidative stress, improved neuronal survival, and enhanced cognitive function in disease models. They also minimized systemic toxicity, making them a promising biocompatible nanocarrier for neurological treatments. Since mesoporous SiNPs offer high surface area and tunable pore size, they are ideal for targeted drug delivery. They protect encapsulated drugs from premature metabolism, ensuring controlled release and stability. They are further explored for treating brain tumors, epilepsy, depression, and multiple sclerosis as their biocompatibility and surface conjugation enhance therapeutic efficacy while minimizing side effects (Bhatane et al., 2023). When used as nanocarrier system, they showed high biocompatibility and low toxicity, indicating its potential use in neuroprotection strategies. In vitro studies on SH-SY5Y (human neuroblastoma) cell model revealed that exposure to SiNPs induced excessive ROS generation, Ca2+ overload, and mitochondrial dysfunction. They were shown to activate both parthanatos and caspase-dependent apoptotic pathways, subsequently leading to neuronal cell death (Ma et al., 2025). However, when the cells were treated with Olaparib, a PARP inhibitor and Z-VAD, a caspase inhibitor, these observed effects were significantly mitigated, suggesting a potential for controlled therapeutic modulation. Further, functionalized SiNPs were shown to promote α-synuclein aggregation in dopaminergic neurons, thus mimicking Parkinson’s pathology. This highlights both the diagnostic potential (by modelling disease mechanisms) and the need for surface modification strategies to reduce neurotoxicity. In A53T transgenic mouse model, the intranasal administration of SiNPs led to the hyperphosphorylation and aggregation of α-synuclein, mitochondrial impairment, oxidative stress, dysfunction of autophagy and neuronal apoptosis. This is a clear indication of the penetrative effect of SiNPs on the CNS, resulting in potential for targeted therapeutic delivery in PD-like pathological conditions. Neurodevelopmental toxicity studies showed that prenatal exposure to SiNPs altered brain development in rodent models, emphasizing the importance of dose control and timing in therapeutic applications (Yang et al., 2022; Ma et al., 2025).
3.2.7 Graphene and graphene oxide
The neuroprotective potential of nano-graphene oxide (NGO) in combating PD was tested by targeting ROS scavenging and anti-inflammatory mechanisms. It was found that NGOs affected SH-SY5Y cells and a 6-hydroxydopamine (6-OHDA)-induced Parkinsonian rat model - it protected the tested cells against 6-OHDA-induced toxicity by reducing oxidative stress. It also suppressed microglial activation, which is linked to neuroinflammation in PD. In the rat models tested, NGO was found to have improved akinesia symptoms and reduced contralateral rotations in response to apomorphine, which implied neuroprotection through increased tyrosine hydroxylase-positive cells in NGO-treated rats, suggesting preservation of dopaminergic neurons. Overall, the study presented NGO as a promising candidate for PD treatment (Kim et al., 2023a). While discussing the neuroprotective effects of graphene-based nanoparticles, it was apparent that they can influence many molecular events that contribute to PD neurodegeneration like autophagy, inflammation, and oxidative stress. They are explored for their role in interventions related to nanomedicine and regenerative approaches to restore neuronal function and was believed to improve early-stage PD detection through graphene-based biosensors (Oz et al., 2023). When exploring the therapeutic potential of graphene nanoparticles, a study was conducted to check how graphene oxide (GO) nanoflakes could target dysfunctional synaptic plasticity in the amygdala, particularly in conditions like post-traumatic stress disorder (PTSD). By selectively targeting glutamatergic synapses, it was found that GO nanoflakes with small lateral dimensions (s-GO) could transiently interact with glutamatergic synapses in the hippocampus, reducing neurotransmitter release. This was of specific significance as PTSD is linked to hyperactive glutamatergic transmission in the lateral amygdala (LA). The study further investigated whether s-GO can modulate this excessive activity to restore normal synaptic function and examined the subcellular targets of s-GO and its interaction with potentiated synapses, potentially reducing long-term potentiation (LTP) associated with PTSD. The study also explored s-GO as a nanocarrier for drug delivery, using neuropeptide Y (NPY) as a biologically active molecule to enhance therapeutic effects (Pati, 2022). The promising role of graphene oxide nanoparticles in neuroprotection were highlighted and their ability to promote neuronal differentiation makes it a compelling candidate for regenerative therapies in neurodegenerative diseases.
3.2.8 Silver nanoparticles
The antioxidant, anti-inflammatory, and neuroprotective properties of silver nanoparticles (AgNPs) have made them a promising candidate in neurodegenerative disease control. A particular study highlighted the neuroprotective potential of AgNPs synthesized using a red pigment from Streptomyces sp. A23, isolated from Algerian bee pollen on SH-SY5Y cells and their potential therapeutic applications (Mohamed et al., 2025). The sample was tested at concentrations of 2, 4, and 8 μg/mL, showing cytotoxic effects on the experimental cells. Significant neuroprotective activity of AgNPs was evident even at lower concentrations (1 mM, pH 7), where a dose-dependent effect was noted. The study also indicated that AgNPs induced their neuroprotective activity through mitigation of oxidative stress, which is a major contributor to the progression of neurodegenerative diseases. These findings suggested that AgNPs synthesized using Streptomyces sp. A23 could be used as a promising neuroprotective agent. However, the authors are of the opinion that it warrants further investigation for use as a drug for PD, AD, and other neurodegenerative disorders. AgNPs are known to cause significant neuronal death. This has raised serious concerns about their long-term safety. Research suggests that AgNPs can cross the BBB and accumulate in the brain, leading to neurotoxicity and neuronal degeneration (Janzadeh et al., 2022). While AgNPs hold potential for neuroprotection, their toxicity risks must also be carefully assessed and managed to ensure safe and effective applications in neurodegenerative disease treatment. Since neurodegenerative diseases like Parkinson’s already involves progressive neuronal loss, AgNP-induced neuronal death could worsen disease progression rather than help. AgNPs are also found to be linked to microglial activation, which may exacerbate neuroinflammation. All these indicate that AgNPs can damage synaptic structures, potentially impairing cognitive and motor functions. Such research underscores the potential dangers of AgNP exposure, urging caution in their biomedical applications.
3.2.9 Chitosan nanoparticles
Nanoparticles derived from chitosan, a biodegradable and biocompatible polysaccharide obtained from chitin (which is found in the exoskeletons of crustaceans) are called chitosan nanoparticles (CsNPs). These types of nanoparticles have gained significant attention in neuroscience, particularly in addressing neurodegenerative diseases. They have unique physicochemical properties, like high permeability across biological barriers, low toxicity, and inherent antioxidant and anti-inflammatory effects which make them promising candidates for drug delivery systems in neurodegeneration. The neuroprotective potential of CsNPs as carriers for Ginkgo biloba extract (GBE), specifically targeting oxidative stress-induced damage in SH-SY5Y cells were examined (Karavelioglu and Cakir-Koc, 2021) and it was understood that these nanoparticles enhanced neuroprotection through improved bioavailability and increasing cell viability from 60% to 92.3%. It was revealed that GBE-loaded CsNPs effectively scavenged ROS, thereby reducing oxidative damage in neuronal cells. The ionic gelation method used for CsNP synthesis resulted in high encapsulation efficiency (97.4%) and ensured controlled release and better cellular uptake. These findings suggested that GBE-CsNPs could be developed as food supplements or therapeutic agents for conditions like AD and PD. Further, studies have shown the neuroprotective potential of CsNPs, improving outcomes in animal models of neurodegeneration and neuroinflammation. The potential of CsNPs as a therapeutic channel for neurological disorders were explored, and the ease of functionalization make these nanoparticles promising candidates for drug delivery in neurodegenerative diseases like Alzheimer’s, Parkinson’s, epilepsy, migraine, psychotic disorders, and brain tumors (Vahab et al., 2024). CsNPs help overcome the BBB by enhancing drug absorption, protecting drugs from degradation, and enabling targeted delivery. Additionally, it was observed that surface modifications of CsNPs allow for the attachment of specific ligands or molecules, improving precision in drug delivery to neuronal cells. However, despite these advancements, challenges remain in large-scale production, regulatory approvals, and long-term safety issues that need to be addressed for the future use of CsNP-based therapies in neurological disorders.
3.2.10 Polymeric nanoparticles
By encapsulating multiple neuroprotective agents, polymeric nanoparticles can modulate immune responses and even promote neuronal regeneration by providing multifunctional therapeutic benefits. By functionalizing with specific ligands, these nanoparticles can improve targeted drug delivery to damaged neurons. The polymeric nanoparticle-based therapies offer a revolutionary shift in precision medicine, with better drug absorption, enhanced cellular targeting, and potentially reduced side effects. Many researchers use polymeric nanoparticles to encapsulate neuroprotective compounds and deliver them to the site of action. For example, the stability and bioavailability of curcumin, a natural compound was significantly improved when encapsulated in a polymeric substance. The specially formulated, curcumin-loaded nanoparticles significantly reduced amyloid-beta plaque accumulation and inflammation, showcasing the potential of nanotechnology to enhance the therapeutic effects of existing compounds (Pei et al., 2024). Another study explored the neuroprotective potential of edaravone-loaded mPEG-b-PLGA polymeric nanoparticles in an in vitro ischemia model using the SH-SY5Y cell line. It was observed that the polymeric encapsulation improved edaravone’s bioavailability and stability and considerably lowered the activities of ROS and nitric oxide (NO), which are major contributors to neuronal damage. The expression of pro-apoptotic gene Bax was downregulated while the anti-apoptotic genes HSP70 and Bcl-2 were upregulated, promoting cell survival. Improved neuroprotection was observed where the cells treated with edaravone-loaded nanoparticles showed better resistance to ischemia-induced damage compared to free edaravone (EDV). By enhancing edaravone’s therapeutic effects, these nanoparticles could pave the way for more effective neuroprotective strategies in stroke and other neurodegenerative conditions (Sharifyrad et al., 2022).
Spinal cord injury (SCI) leads to progressive neuronal damage, driven mainly by oxidative stress and inflammation. In such cases, the ROS-scavenging were used for neuroprotection to mitigate oxidative stress and inflammation (Zhang et al., 2021a). These nanoparticles offer a promising therapeutic strategy by reducing secondary injury, potentially improving recovery outcomes. The lipid nanoparticles effectively neutralized harmful free radicals, reducing oxidative damage in injured spinal cord tissue. They also suppressed the secretion of pro-inflammatory cytokines (such as IL-1β, TNF-α, and IL-6), helping to control inflammation. Targeted accumulation of the nanoparticles at the injury site enhanced their therapeutic effects. Functional recovery was observed in animal models, where treatment with these nanoparticles led to better axonal protection and improved motor function.
3.2.11 Lipid nanoparticles
RNA therapies, including messenger RNA (mRNA) and siRNA, offer promising approaches for gene silencing and protein expression in neurodegenerative diseases. The mRNA, siRNA and antisense oligonucleotides (ASOs) can correct genetic defects, silence harmful genes, or restore normal protein function (Lee et al., 2022). A cutting-edge study concentrated on lipid-based nanoparticles that served as efficient RNA carriers, protecting RNA from degradation and enhancing its delivery across the BBB. Various types of lipid-based platforms include liposomes, lipoplexes, solid lipid nanoparticles (SLNPs), lipid nanoparticles (LNPs), nanoemulsions (NEs), nanoliposomes, nanophytosomes, and nanostructured lipid carriers (NLCs), each with unique properties for targeting the CNS (Tsakiri et al., 2022). Surface modifications of LNPs improve target specificity, allowing RNA therapies to reach affected neurons more effectively. This strategy is based on LNPs as carriers for RNA-based therapeutics in neuroprotection. The delivery of siRNA targeting neurotoxic pathways in models of amyloid-beta toxicity was studied and the results indicated that LNPs improved the uptake of siRNA by neuronal cells, leading to a significant reduction in neurotoxic effects and suggesting potential therapeutic applications in neurodegenerative diseases. Thus, lipid-based nanoparticles are emerging as a powerful tool for treating neurodegenerative diseases by enhancing drug delivery, reducing toxicity, and improving therapeutic efficacy (Fernandes et al., 2021).
3.2.12 Hybrid nanoparticles combining different materials
Hybrid nanoparticles that integrate different materials like polymers, lipids, metals, and biocompatible nanomaterials are emerging as a promising approach by offering enhanced therapeutic capabilities for treating neurological disorders. These hybrid systems can leverage the unique properties of each component to optimize drug delivery, whereby they can reduce toxicity, and improve brain targeting. Their additional ability to cross the BBB and deliver antioxidants, anti-inflammatory agents, and neurotrophic factors make them valuable candidates for mitigating neurodegeneration in most of the neurodegenerative diseases, such as Alzheimer’s, Parkinson’s, and stroke-induced brain injury. Further, the multifunctionality of hybrid nanoparticles allows for controlled drug release, improved cellular uptake, and enhanced neuroprotective effects, making them superior to its conventional counterpart, namely, single-material nanocarriers (Kumar and Kumar 2024). Many of the research with hybrid nanoparticles have shown that combining lipid-based carriers with polymeric shells or metallic cores can significantly boost drug stability, bioavailability, and therapeutic efficacy. These advanced systems not only protect neurons from oxidative stress and inflammation but also promote neuronal regeneration, offering new possibilities for treating CNS disorders. While investigating the therapeutic potential of a polymer/lipid hybrid nanoparticle (PLHNPs) loaded with quetiapine fumarate (QF) in a cuprizone-induced schizophrenia model in mice, the drug delivery efficiency and behavioral and neurological outcomes in schizophrenia were revealed. Further, the hybrid nanoparticles demonstrated high entrapment efficiency (99.68%) and a sustained release profile, with 95% of QF released over 28 days. Moreover, mice treated with the optimized hydrogel-based formulation (HF-G3) showed significant recovery from schizophrenia-like symptoms, compared to untreated cuprizone-fed mice. This was a significant study that revealed reduced levels of pro-inflammatory cytokines (TNF-α, IL-1β), gamma-aminobutyric acid (GABA), and glial fibrillary acidic protein (GFAP), indicating reduced neuroinflammation and improved neuronal integrity of the formulation (Elkasabgy et al., 2023). In addition to this, the study ensured that intramuscular administration prolonged therapeutic effects, reducing the need for frequent dosing. Generally, quetiapine fumarate has very low oral bioavailability (9%) and the study successfully demonstrated the potential of PLHNPs in sustained drug delivery, even in case of drugs with very low bioavailability.
Auranofin is an orally administered gold compound primarily used to treat rheumatoid arthritis, but recently, it has been studied for neuroprotective effects, particularly in PD models, where it helps reduce oxidative stress and neuroinflammation (Soni et al., 2025). The ability of auranofin-loaded chitosan-lipid hybrid nanoparticles (AUF-CLHNPs) to modulate GSK-3β/Nrf2/HO-1 signaling pathways in a rotenone-induced PD model was studied and it was found that AUF-CLHNPs significantly improved auranofin’s brain penetration, overcoming its limited natural bioavailability. The hybrid nanoparticles were found to restore motor function by reducing oxidative stress, and protecting the dopaminergic neurons in the substantia nigra, a key brain region affected in PD. The anti-inflammatory responses were influenced where UF-CLHNPs phosphorylated GSK-3β, leading to upregulation of Nrf2/HO-1, affecting oxidative pathway as well. The pro-inflammatory cytokine levels (TNF-α, IL-1β) were downregulated and neuronal integrity was restored. Thus, the study successfully demonstrated the use of a novel drug delivery approach that enhanced auranofin’s therapeutic efficacy in PD. The hybrid nanoparticle formulation ensures sustained drug release, improving treatment outcomes.
3.2.13 2-D materials
The exceptional physicochemical properties of two-dimensional (2-D) materials made them promising candidates for the diagnosis and treatment of neurodegenerative diseases and recently, they gained immense attention in biomedical research due to these properties. Some of these materials like graphene, transition metal dichalcogenides (TMDCs), molybdenum and black phosphorus, exhibit high surface area, excellent biocompatibility, and tunable electronic characteristics, which enhance their interactions with biological systems. In the field of diagnostics, 2-D materials can be integrated into biosensors for highly sensitive and selective detection of biomarkers associated with conditions like AD and PD (Wu et al., 2021). Additionally, their unique optical, electrical, and magnetic properties enable early disease detection through techniques such as fluorescence imaging, electrochemical sensing, and MRI enhancement. However, 2-D materials are of assistance beyond diagnosis as they are valuable in neuroprotective therapies as well. The targeted delivery of neuroprotective agents across the BBB is mainly attributed to their increased drug-loading capacity and controlled release mechanisms. This is based on the principle of increasing treatment efficacy while minimizing systemic side effects. The antioxidant and anti-inflammatory activities also affect the intrinsic neuroprotective properties, which help in mitigating oxidative damage and neuroinflammation that are considered as two main drivers of neurodegeneration (Lee et al., 2020). The ongoing advancements in the field of 2-D materials for neurodegenerative therapies hold great potential to revolutionize precision medicine as they can be more efficient, targeted, and less invasive than other conventional therapeutic strategies. For example, the therapeutic potential of the nano-bio interactions of 2-D molybdenum disulfide (MoS2), an ultrathin nanomaterial, is being explored. It is observed that 2-D MoS2 maintains high anisotropy, surface-to-volume ratio, chemical functionality, and mechanical strength which makes MoS2 a promising material for biomedical applications, including drug delivery, regenerative medicine, biosensing, and bioelectronics (Yadav et al., 2019; Jin et al., 2025). MoS2 interacts with biological systems, influencing the intracellular trafficking, biodistribution, and biodegradation of therapeutic drugs. MoS2 is also found to interact with proteins and specific cell types, such as immune cells and progenitor stem cells, which determine its short-term and long-term biocompatibility (Roy et al., 2022). Also, 2-D MoS2 belong to the TMDCs that can contribute to neuroprotection by mimicking biological synapses and neurons, enhancing neuronal plasticity and repair. Their high surface reactivity and tunable electronic properties allow them to interact with neural networks, supporting synaptic modulation and neuroregeneration. Research suggests that TMDC-based neuromorphic devices can help reduce oxidative stress and neuroinflammation, two major contributors to neurodegenerative diseases. By facilitating efficient signal transmission and memory retention, these materials may aid in cognitive restoration and neural repair (Ma et al., 2023). These TMDCs can be employed to make flexible neuromorphic devices that contribute to neuroprotection by mimicking biological synapses and neurons. These devices offer high adaptability and plasticity, making them promising for brain-machine interfaces and neural repair technologies (Ke et al., 2024; Ma et al., 2023). Some of the modes of PD modulation by 2-D materials are represented in Figure 5.
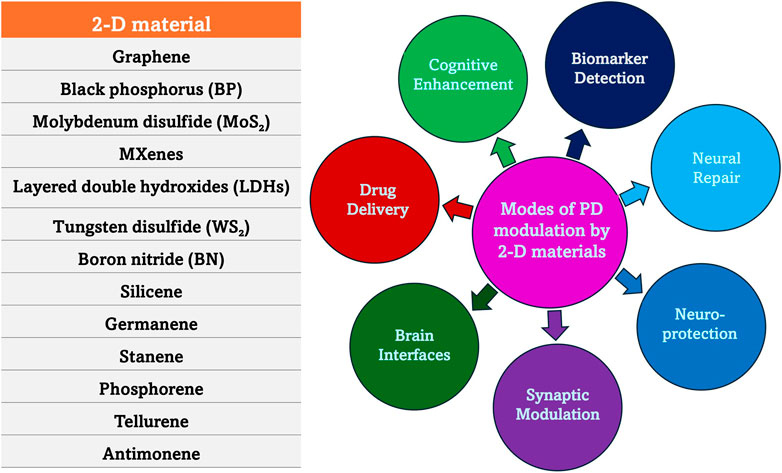
Figure 5. Mode of action of 2-D materials in PD. 2-D materials exhibit unique physicochemical properties, including high surface area, biocompatibility, and tunable electronic characteristics, making them promising candidates for therapeutic applications. Their ability to regulate oxidative stress, neuroinflammation, mitochondrial function, and α-synuclein aggregation positions them as innovative tools in the fight against PD.
3.2.14 Exosomes
Exosomes are naturally occurring nanoscale vesicles derived from specific cells (Figure 6) that can facilitate drug delivery while being biocompatible and biodegradable and therefore, demonstrate a promising role in gene therapy for neurodegenerative disorders. The neuroprotective role of exosomes in CNS injuries, including traumatic brain injury (TBI), SCI, and subarachnoid hemorrhage (SAH) was studied and it was found that they help to restore cognitive abilities by facilitating neuronal repair and synaptic plasticity, thereby improving cognitive function. They are also known to regulate immune responses, minimizing neuroinflammation by suppressing the reactions. Further, they aid in neuroprotection by influencing cellular recycling mechanisms (autophagy regulation) and inhibiting cell death pathways (anti-apoptotic effect), subsequently reducing neuronal loss. They also help in maintaining BBB integrity and offering BBB protection and prevention of further damage. The key molecular mechanisms through which exosomes exert their neuroprotective effects are the microRNA (miRNA), NF-κB, PI3K/AKT, Notch1, and ERK pathways, among others (Zhang et al., 2022). Exosomes derived from glial cells can exert both neuroprotective and neurotoxic effects, depending on the cellular environment and molecular cargo they carry (Figure 7). When derived from astrocytes and oligodendrocytes, these exosomes can modulate synaptic plasticity by enhancing neuronal communication (Akbari-Gharalari et al., 2024). They accomplish this task by delivering neurotrophic factors that support synaptic signals and connections, and help in repair through macromolecules like nucleic acids and sugars. Exosomes help in the regulation of immune responses by carrying anti-inflammatory cytokines, especially by reducing neuroinflammation in conditions like AD and PD. Another mechanism of action is the transport of antioxidant enzymes that help to neutralize ROS, preventing neuronal damage. By delivering tight junction proteins, they strengthen the BBB, thereby preventing harmful substances from entering the brain (Heidarzadeh et al., 2021). Exosomes are also found to regulate autophagy by influencing cellular recycling mechanisms, and promoting the clearance of misfolded proteins associated with neurodegenerative diseases. More mechanisms of action are detailed in Figure 8. However, at times, exosomes are found to have neurotoxicity, particularly those from activated microglia that can carry pro-inflammatory cytokines, exacerbating neuroinflammation and contributing to neuronal damage. When they facilitate the intercellular transfer of misfolded proteins, such as amyloid-beta and tau, they are known to accelerate disease progression in Alzheimer’s, instead of retarding the condition. They may also contribute to further neurodegeneration as they tend to carry damaged mitochondrial components that can impair energy production. The dual role of exosome (neuroprotection and neurotoxicity) is further reinforced by the role they play in activating cell death pathways by affecting apoptotic signals, increasing neuronal apoptosis in pathological conditions (Oyarce et al., 2022).
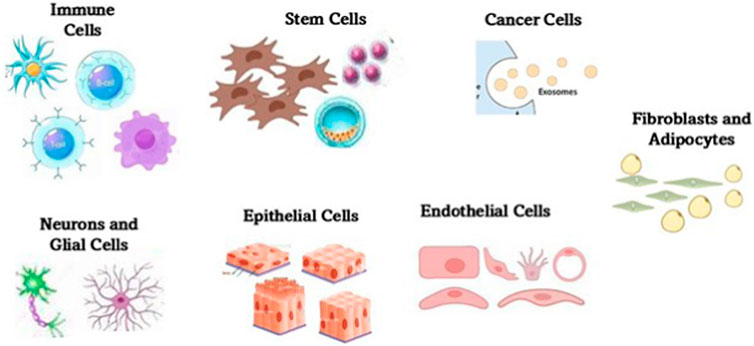
Figure 6. Major cellular sources of exosomes within the central nervous system. The sources include neurons, astrocytes, microglia, oligodendrocytes, and endothelial cells among others. Each cell type contributes distinct molecular cargo to exosomes, influencing processes such as neuroinflammation, synaptic signaling, and neuroprotection. The exosomes play a critical role in intercellular communication and are increasingly recognized for their diagnostic and therapeutic potential in neurodegenerative diseases.
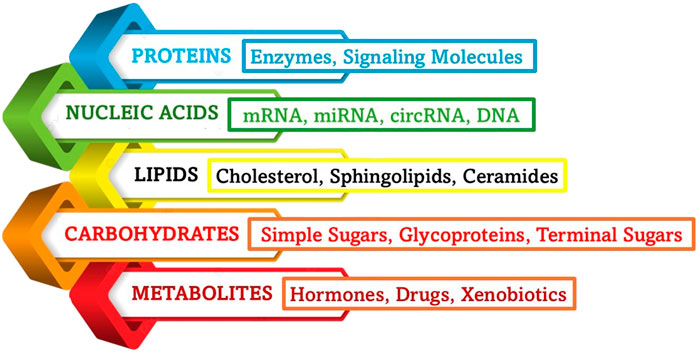
Figure 7. Main types of exosomal cargo. The bioactive molecules that act as cargo for exosomal delivery play key roles in intercellular communication, influencing processes such as inflammation, synaptic plasticity, and neuroprotection. The composition of exosomal cargo varies depending on the cell of origin and physiological or pathological conditions.

Figure 8. Mode of action of exosomes. Exosomes contribute to the progression or modulation of neurodegenerative diseases by influencing neuroinflammation, synaptic function, and neuronal survival by mediating intercellular communication and regulating gene expression. These actions highlight the dual role of exosomes as both biomarkers and therapeutic agents in neurodegeneration.
3.2.15 Nanogels
Nanogels are cross-linked polymeric networks that can swell in response to environmental stimuli, providing controlled drug release. They have high potential to serve as advanced drug delivery systems for CNS therapeutics. Due to their hydrophilic and crosslinked polymeric networks, they can offer high drug-loading capacity, controlled release, and enhanced BBB penetration (Manimaran et al., 2023). They are highly stable and compatible with biomolecules and therefore can encapsulate neuroprotective agents while maintaining their structural integrity. They enable sustained and targeted delivery, reducing side effects. They also improve drug transport across the BBB, a major challenge in CNS treatments. Nanogels are stimuli-responsive and so, they can be designed to respond to pH, temperature, or enzymatic activity, ensuring precise drug delivery. They can facilitate cellular uptake and intracellular trafficking for efficient drug absorption by neurons and glial cells. Nanogels are being explored for Alzheimer’s, Parkinson’s, and multiple sclerosis treatments as they help to reduce neuroinflammation and promote neuronal repair (Devassy et al., 2023). The neuroprotective potential of EDV can be enhanced by preparing a glutathione (GSH)-conjugated poly (methacrylic acid) nanogel, which enhances targeted brain drug delivery. This incorporation of EDV in the nanogel is supposed to ensure drug stability, controlled release, and BBB penetration, ensuring efficient neuroprotection. Studies have also demonstrated that this nanocarrier system significantly reduced oxidative stress, enhanced cognitive function, and promoted neuronal survival in ischemic conditions (Mozafari et al., 2023).
Many of the recent studies illustrate the rapid advancement in nanotechnology for neuroprotection, incorporating innovative materials and methodologies that enhance targeting, delivery, and thus offering neuroprotective functions (González et al., 2021; Waris et al., 2022; Nayab et al., 2023). Examples of some nanoparticles delivered via nanostructures to restore homeostasis in the neuronal regions in CNS, are explained in Table 2. The nanostructure type, mechanism of action, and key findings are explained. The exploration of various nanomaterials continues to hold promise for effective interventions in neurodegenerative diseases and related neurological conditions. As research progresses, the focus on safety, biocompatibility, and translation into clinical applications remains paramount.
4 Mechanisms of action of nanomaterials in reducing neuronal damage
The mechanisms for reducing neuronal damage focus mainly on preserving neuronal function, preventing cell death, and promoting regeneration (Figure 9). Nanomaterials exhibit a variety of mechanisms of action that contribute to their potential in reducing neuronal damage and promoting neuroprotection. Their small size, large surface area, and tunable surface functionalities enable them to co-ordinate with biological systems in ways that traditional materials cannot (Bandaru et al., 2024b), and therefore these elements allow nanomaterials to cross biological barriers (even the BBB that conventional particles cannot cross). This singularity of nanomaterials allows the specific targeting of neuronal cells or pathological sites with very high precision.
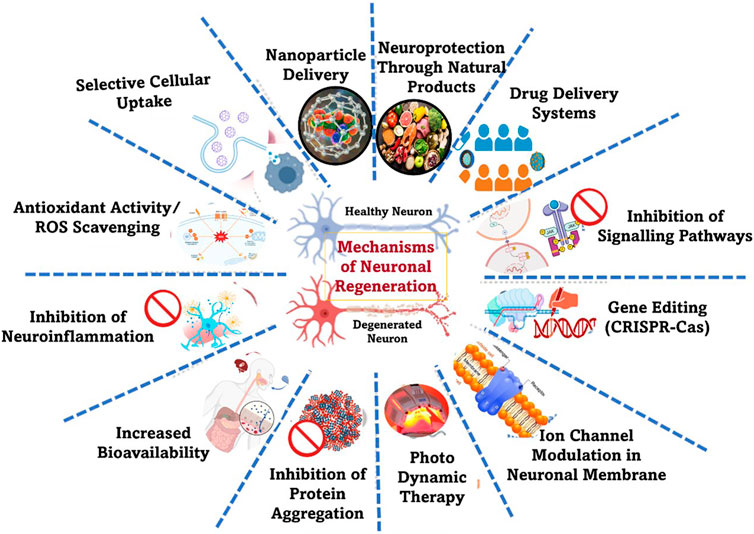
Figure 9. Strategies involved in neuronal damage repair. The general mode is to target key pathological processes such as oxidative stress, neuroinflammation, signalling pathways, gene editing, modulation of drug delivery system, modification of impaired cellular repair mechanisms, etc.
Neuronal damage is caused by various mechanisms that can affect the structure and function of nerve cells in the brain and nervous system. Understanding these mechanisms that protect neurons will spark off innovative strategies for neurodegenerative diseases and brain injuries through nanotechnology, highlighting its importance in advancing neurotherapeutics. Physical injury, presence of toxic substances, lack of oxygen, etc, are known to contribute to neuronal damage (Howard et al., 2024). However, nanomaterials can exert neuroprotective and reparative effects that are central to their therapeutic potential. By scavenging ROS which are known to damage neuronal components like lipids, proteins, and DNA, they can modulate oxidative stress in neurons, resulting in reduced inflammation, and promoting neuronal regeneration, thus mitigating neuronal damage. By reducing the oxidative burden, nanoparticles such as gold and silver can prevent neuron degradation and enhance cell survival. Additionally, certain nanomaterials can activate the body’s endogenous antioxidant enzymes (e.g., superoxide dismutase (SOD) and catalase), further reinforcing cellular defense mechanisms (Jomova et al., 2024). It was observed that catalase activity is significantly lower in advanced PD cases, further exacerbating oxidative stress (Younes-Mhenni et al., 2007). Thus, nanomaterials offer a promising approach to neuroprotection by targeting these two critical defense mechanisms in neurodegenerative diseases by removing the harmful radicals that contribute to dopaminergic neuron degeneration (Nair et al., 2024). By addressing oxidative stress, nanotechnology-based interventions are positioned as highly promising therapeutic tools for managing neurodegenerative conditions, potentially slowing disease progression and improving patient outcomes.
Beyond antioxidant properties, nanomaterials also exhibit anti-inflammatory effects, playing a crucial role in preserving neuronal function (Zhu et al., 2021; Zhang et al., 2018). Inflammation in the nerve cells may affect the apoptotic mechanism leading to synaptic dysfunction. These will result in the impairment of cognitive and motor functions and, will decisively affect the quality of life of the affected person. Neuroinflammation - characterized by microglial activation and excessive cytokine release – is a major contributor to disease progression in disorders like Alzheimer’s and Parkinson’s (Lee, 2013). Nanoparticles can help inhibit microglial activation, suppress pro-inflammatory cytokines, and balance immune responses, preventing further neuronal damage. Nanomaterials can also normalize the immune response in the brain, helping to restore the balance between pro-inflammatory and anti-inflammatory signals. Further, certain nanomaterials can interfere with signaling pathways involved in the production of pro-inflammatory cytokines, decreasing their levels in the neuronal environment and reducing inflammation (Fakhri et al., 2022). Nanoparticles can also inhibit the activation of microglia, the immune cells of the CNS, reducing the release of inflammatory mediators. For example, silica nanoparticles have been shown to modulate microglial responses, thus alleviating neuroinflammation.
5 Targeted drug delivery using nanoparticles
Traditional drug delivery methods often result in systemic distribution, meaning that therapeutic agents spread throughout the body instead of concentrating on a specific target site. While this approach is effective for general treatments such as antibiotics or pain relief, it poses significant challenges when precision is required for conditions like neurological disorders. Further, the widespread dispersion of the drug often leads to lower therapeutic efficacy, as only a fraction of the administered dose reaches the intended site. This off-target effect could cause toxicity to the neighbouring cells in the brain that are mostly healthy and causes issues like immune suppression or cell damage (Manzari et al., 2021). Also, one major drawback of systemic drug delivery is the failure to cross BBB as the intended drug fails to reach the affected neuron, due to which the dosing requirements are also very frequent (Wu et al., 2023). Often, higher or repeated doses are needed, furthering the risk of drug resistance and cumulative toxicity. This could be problematic, particularly in case of chronic diseases like Parkinson’s, requiring long-term medication use. Targeted drug delivery (TDD), mediated through nanocarriers like liposomes, nanogels, and micelles, can address many of these limitations. Nanoparticles with their unique size, shape, surface properties, and biocompatibility, can act as nanocarriers, providing a promising platform for TDD. They can allow drugs to be encapsulated and precisely directed to diseased tissues, enhancing bioavailability. Additionally, the potential stimuli-responsive drug release of nanocarriers enables the drugs carried by the nanoparticle to be activated in response to environmental factors such as pH, temperature, or enzymatic activity, ensuring the release at the precise locality (Majumder and Minko, 2021). Other strategies of nanocarriers include ligand-based targeting, where drugs are engineered to bind specifically to receptors in diseased cells, and gene and cell-based therapies. Thus, by leveraging surface modifications, ligand-based targeting, and stimuli-responsive mechanisms, nanocarriers can improve drug circulation time, enhance cellular uptake, and facilitate controlled release, making them invaluable in treating neurodegenerative conditions like Alzheimer’s, Parkinson’s, and Huntington’s disease (Dhariwal et al., 2025; Arachchige et al., 2019). Examples of nanostructure-based therapies that are at the forefront of PD research, are explained in Table 3.

Table 3. Some nanostructure-based therapies that are at the forefront of PD research, offering innovative approaches to enhance drug delivery and target disease mechanisms.
5.1 Mechanisms of targeted drug delivery
The presence of physiological barriers, like the BBB, pose a major challenge to the delivery of traditional drugs to the brain and the delay in drug delivery will aggravate the situation due to progressive loss of neurons (Kunjiappan et al., 2021). The effective therapeutic concentrations will flounder to reach the active site in the brain, leading to poor treatment outcomes and systemic side effects. Targeted drug delivery strategies aim to enhance drug bioavailability, improve specificity, and minimize off-target reactions, making these strategies crucial for neurodegenerative disease management. Most of the recent advancements in nanotechnology and biomolecular engineering have enabled the development of nanocarriers, ligand-functionalized nanoparticles, and stimuli-responsive drug delivery systems that can efficiently cross the BBB. Further, mechanisms such as receptor-mediated transcytosis, cell-mediated transport, and intranasal drug delivery have shown promise in improving drug accumulation in affected brain regions. The mechanisms by which nanoparticles cross the BBB in the context of PD are detailed in Table 4. Such innovative strategies offer hope for more effective and personalized treatments, potentially slowing disease progression and improving patient outcomes.
Nanoparticles can enhance drug delivery through several mechanisms (Zi et al., 2022; Afzal et al., 2022). Exploiting the leaky vasculature and poor lymphatic drainage in the brain, the nanoparticles could efficiently enter and accumulate more through passive targeting via EPR Effect (Subhan et al., 2021). Passive targeting of nanoparticles for drug delivery in neurodegenerative disorders relies on the natural physiological properties of the body to direct therapeutic agents to affected region of the brain. Further, nanoparticles with stealth coatings (e.g., PEGylation) can evade immune clearance, ensuring sustained drug delivery (Sheffey et al., 2022). Also, through endocytosis and cellular uptake mechanisms, neurons and glial cells can internalize nanoparticles, further facilitating the controlled release of drugs. Some studies mention that metallic magnetic nanoparticles (MMNPs) in a glioblastoma model that are ≤50 nm can reach the brain cells, while larger nanoparticles fail to cross the BBB. Even for smaller MMNPs, their accumulation is limited to areas near large brain tumor cells. However, active targeting using ligands could help in enhanced delivery (Yan et al., 2024; Rahim et al., 2021). Functionalizing the nanoparticles with antibodies, peptides, or aptamers can extend and exacerbate specific binding. Stimuli-responsive drug delivery (Dasgupta et al., 2024) enables nanocarriers designed to release drugs in response to pH, temperature, ultrasound, or enzymatic activity ensuring precise, controlled, and efficient therapeutic effects. Cell-mediated drug delivery (Santos et al., 2021; Levy et al., 2021) utilizes immune cells or exosomes to transport therapeutic agents directly to the site of action. Another mode of drug delivery depends on the transcytosis-based targeting (Yang et al., 2025),s where the nanoparticles are leveraged to transport endothelial cells (N-TECs) for active transcytosis across the vasculature in the brain and release the drug precisely at the targeted point.
5.2 Strategies for crossing the BBB
When it comes to the delivery of drugs to the intended site in the CNS, crossing the BBB is one of the most significant challenges. As is known, the BBB is a highly selective permeability barrier formed by endothelial cells that line the brain’s capillaries, protecting the brain from harmful substances while regulating the transport of essential nutrients (Wu et al., 2023). However, this barrier also limits the delivery of many drugs, which can be detrimental in treating neurological disorders. Consequently, various strategies have been developed to facilitate drug delivery across the BBB.
5.2.1 Chemical modification and nanoparticle engineering
One of the most effective strategies for enhancing drug delivery across the BBB involves the chemical modification of therapeutic agents or the design of nanocarriers (Wu et al., 2023). This is made possible through the inclusion of lipophilic or small-molecule drugs that can readily diffuse through the BBB. Additionally, extremely small particles (like the nanoparticles) can be engineered to improve their brain-targeting capabilities. For instance, liposomes and polymeric nanoparticles when modified with specific ligands like transferrin, folate, or peptides can bind to receptors that are on the surface of endothelial cells (Mojarad-Jabali et al., 2022). These modifications will eventually facilitate receptor-mediated endocytosis, allowing nanoparticles to be internalized by the endothelial cells and subsequently releasing their cargo within the CNS. Such targeted delivery systems are known to enhance the efficacy of drugs while reducing systemic side effects.
5.2.2 Disruption of the BBB
Another approach is the transitory disruption of the BBB to allow for the passage of therapeutic agents. To bring about this short and abrupt disruption, various physical and chemical methods are employed, including the use of hyperosmotic agents (e.g., mannitol) that can induce shrinkage of endothelial cells, thereby increasing permeability. Additionally, other means like focused ultrasound (FUS) combined with microbubbles are used which are known to have significant effect on opening the BBB transiently. When ultrasound is applied, the microbubbles vibrate and induce small openings in the BBB, allowing for the delivery of various therapeutic molecules, including large drugs or genetic material (Kim et al., 2023b). This is a strategy employed even for the precise targeting and controlled release of the intended drug, leading to minimal collateral damage to surrounding healthy tissue.
5.2.3 Utilization of biological transport mechanisms
Leveraging the biological transport mechanisms for the delivery of drugs across the BBB is another promising strategy that involves utilizing endogenous transport systems (like the carrier-mediated transport) that naturally transport substances across the BBB. For instance, glucoproteins like glucose transporters can be exploited to deliver therapeutics that mimic glucose. Similarly, the transport of conjugated therapeutic agents can be enhanced using monoclonal antibodies or antibody fragments that target specific receptors on the BBB. The potential of exosomes and extracellular vesicles as natural vehicle systems are also explored for crossing the BBB as these explicit vesicles can be specifically engineered to carry drugs or RNA therapeutics (Ramos-Zaldívar et al., 2022). They can prove to be quite natural for transport of drugs that are generally utilised by cells to shuttle molecules across the barrier.
Even though the BBB is a highly selective membrane intended to protect the brain from toxins and invading pathogens, despite its semipermeable nature, the BBB is mostly impenetrable to most drugs, especially large or hydrophilic molecules, which restrict their entry into the brain and bloodstream. Nanoparticles have an inherent quality to overcome this challenge through several engineered mechanisms by exploiting the physiological transport pathways and disease-specific BBB alterations (Jiménez et al., 2025).
6 Safety assessment and risks of nanoparticles in the treatment of neurodegeneration
Targeted drug delivery, gene therapy and neuroprotection involving nanoparticles are proving to be effective in controlling PD. But the unique physicochemical properties of nanoparticles like the ultra-small size, and high surface area, along with variable surface charge is understood to pose many safety concerns (Mellor and Uchegbu, 2022). After administering the drug, NPs carrying them will easily cross the BBB and interact with the brain cells and trigger effects like oxidative stress, inflammation, or mitochondrial dysfunction. These dose-dependent reactions are also influenced by the material composition. Metal or polymers acting as nanoparticle drug can cause surface modifications, and bring about degradation kinetics (Visan et al., 2021). To avoid such reactions/effects, and to evaluate the cytotoxicity, biodistribution, and long-term accumulation of these particles in neural tissues, it is essential to conduct rigorous in vitro and in vivo testing. The lack of standardized protocols to assess aspects that are missed by the conventional toxicity assays may lead to complications as part of the interaction between the NPs and the neural cells or immune cells. To overcome this challenge, modern approaches such as adverse outcome pathways (AOPs), high-throughput screening, and organ-on-chip models are being explored to predict neurotoxicity associated with systemic routes. Additionally, advanced regulatory frameworks are implemented to address the unique performance of NPs in the biological system. This is a strategic exercise to ensure the precise characterization and reproducibility that are often overlooked in clinical translation (Tirumala et al., 2021).
7 Clinical trials
Clinical studies are intended to carefully assess the effectiveness of new medications. According to the U.S. Food and Drug Administration (FDA), the primary goal of Phase I trials is to determine appropriate dosage and safety, with approximately 70% of drugs progressing to Phase II where the treatment’s efficacy and its potential side effects are verified. Around 33% of these then advance to Phase III, which focuses on adverse reactions of the drug and investigate their potency (Sayyaed et al., 2023). At present, there are several clinical trials that are exploring the safety and efficacy of nanostructured therapies for neuroprotection in patients with neurodegenerative diseases. For instance, a recent Phase I trial investigated the use of polymeric nanoparticles loaded with neurotrophic factors for the treatment of ALS, targeting neuroprotection by delivering essential growth factors directly to affected motor neurons (Bondarenko and Saarma, 2021). Another promising avenue involves lipid-based nanoparticles designed to deliver siRNA targeting genes implicated in Huntington’s disease. These innovations not only enhance the specificity of treatments but also hold the potential to transform therapeutic strategies through precise modulation of gene expression. As research progresses, nanostructured technologies are set to revolutionize both the prevention and management of neurodegenerative diseases, making a significant impact in the field of neuroprotection. More details on various clinical trials in the area are represented in Table 5. Regardless of these diligent and innovative progresses, the clinical translation of nanostructured therapies face significant challenges related to toxicology, biocompatibility, regulatory hurdles, and ethical considerations (Kardani, 2024), as is seen with any other field of research. Though enhanced drug delivery and therapeutic precision is ingrained in nanomaterials, their biocompatibility is often a topic of debate among researchers that need to be thoroughly assessed to prevent cytotoxicity, immune responses, and long-term accumulation in tissues. To understand this and to ensure safe use of these therapeutic modalities, regulatory agencies impose strict guidelines. Yet the approval process is slowed down due to the lack of standardized protocols for nanoparticle characterization and toxicity testing. Additionally, questions on ethical issues arise very frequently regarding patient consent, long-term effects, and equitable access to nanomedicine. This requires transparent policies and interdisciplinary collaboration between various agencies across the globe to balance innovation with responsible implementation.
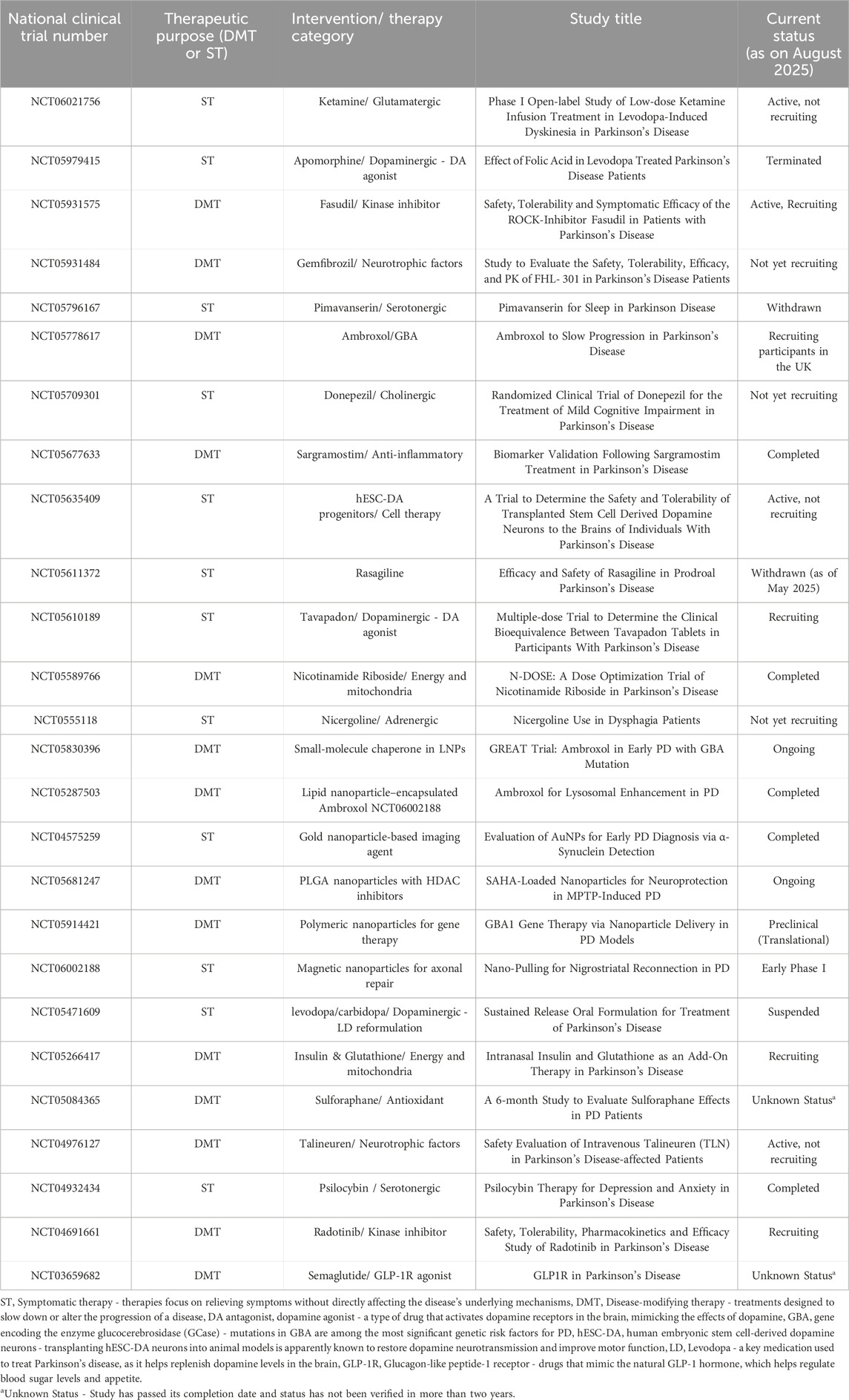
Table 5. Clinical trials exploring various strategies for neuroprotection in PD. These trials are aimed at advancing neuroprotective strategies for improving drug delivery, neuroregeneration, and disease management in PD.
7.1 Clinical relevance of nano-structured strategies in neurodegeneration
The transformative changes in interventions, leading to modifications of strategies to control diseases, have led to the integration of nano-structured blueprints into the management of neurodegenerative diseases. This shift from symptomatic relief to targeted, disease-modifying interventions has been clinically proven to offer many advantages that promptly overcome the restrictions of conventional therapies. The nanoparticles engineered to cross the BBB can precisely deliver neuroprotective agents to affected regions of the brain, thereby minimizing the widely observed side effects to enhance the therapeutic efficacy of the drug (Naqvi et al., 2020). This strategy is specifically relevant in the case of PD where the dopaminergic neuron degeneration is localized and demands site-specific intervention. On closer observation, the use of biocompatible nanocarriers like lipid-based nanoparticles or polymeric scaffolds, or even exosome-mimetic vesicles are shown to improve drug stability, cellular uptake, and sustained release profiles. Maintaining a specific therapeutic concentration in the brain over a prolonged period is crucial in chronic conditions like PD. Additionally, the integration of ligands like transferrin, lactoferrin, or peptides to these drugs is known to enhance the selectivity and stability of the drug, which further helps to reduce off-target effects and significantly improve the clinical outcomes (Padilla-Godínez et al., 2022). Documentary evidence preceding clinical studies suggests that modulation of neuroinflammation is a key contributor to disease progression (Zhang W. et al., 2023). As discussed, the long-term safety, immunogenicity, and regulatory standardization also need to be addressed before progressing to clinical translation. Even if the biocompatibility of NPs is studied extensively in cell lines (in vitro) and animal models (in vivo), human trials are essential to evaluate the biodistribution, clearance mechanisms, and potential accumulation in brain cells/tissues.
8 Emerging trends and challenges
A better understanding of the various aspects of neurobiology has increased the prospects of the application of nanoparticles in neurotherapeutics. Advances in targeted delivery systems and material science have paved the way for innovative trends in the field, including the development of multifunctional nanoparticles that are engineered to deliver drugs while simultaneously modulating immune responses and crossing the BBB with high precision and specificity (Emeihe et al., 2024). The incorporation of surface ligands like transferrin (Georgieva et al., 2014), lactoferrin (Singh et al., 2016), or peptides that target BBB receptors are known to enhance the uptake of the drug in the brain and minimize off-target effects. Exosome-mimicking vesicles or polymeric carriers that are derived from natural sources led to the development of biodegradable and biomimetic nanoparticles. Compared to conventional NPs, they offer improved biocompatibility and reduced immunogenicity, eliminating the safety concerns associated with synthetic nanomaterials. In addition, certain stimuli-responsive nanoparticles are found to release their payload under various pH, temperature, or enzymatic conditions. Such particles can fine-tune the drug release within the microenvironment of the disease (Parvin et al., 2025). However, translational gaps exist, especially regarding the safety of neural tissues and the long-term toxicity of nanoparticles that accumulate and potentially interfere with neuronal function. General challenges, as observed with conventional NPs, are more pronounced as recent trends are not adequately standardized to produce them in scalable amounts. The regulatory landscape is still evolving, and the provincial agreement on evaluation protocols and approval pathways is still a major challenge (Car et al., 2025). Proper execution of interdisciplinary collaboration, robust clinical trial design, and continued innovation in nanoparticle engineering will help overpower such challenges.
9 Conclusion
The design and application of nanostructured scaffolds support a futuristic channel/platform for neuronal growth, nurturing long-term therapeutic efficacy mediated through cell adhesion and differentiation all the while enhancing biocompatibility. Similarly, the integration of ingenious biomaterials with stem cell therapy enables a synergistic approach, improving the survival and functional recovery of dopaminergic neuron, which is crucial in neurodegeneration management. Furthermore, the recent research on the integration of electrical stimulation via nanoscale devices is paving the way for precision-targeted neuromodulation (Alipour et al., 2025), enhancing synaptic activity and restoring motor function in PD patients. These innovations collectively contribute to a paradigm shift in neurodegenerative disease treatment, ensuring effective drug delivery, improved cellular responses, and optimized neuroprotective outcomes. By leveraging these nanostructured technologies, researchers are moving closer to clinically viable solutions, offering hope for enhanced patient outcomes and long-term disease management. It is evident from preclinical studies that nanoparticles have promising outcomes in targeted drug delivery, leading to neuroprotection, and the modulation of neuroinflammation is effective under the application of nanostructures. Clinical translation of many of these research remains limited by challenges including biocompatibility, long-term safety, and regulatory standardization with regard to nanoparticles. By including clinical trial data in translational therapeutics along with strategies for patient stratification, a real-world therapeutic outcome can be achieved. This will enhance the scientific depth of such a research and positions nano-therapeutics as a most viable alternative to the ever-evolving landscape of neurodegenerative disease management.
Author contributions
MP: Data curation, Formal Analysis, Methodology, Resources, Validation, Visualization, Writing – original draft, Writing – review and editing. AM: Data curation, Methodology, Resources, Writing – review and editing. WC: Data curation, Formal Analysis, Supervision, Validation, Visualization, Writing – original draft, Writing – review and editing. DM: Conceptualization, Data curation, Formal Analysis, Methodology, Resources, Supervision, Validation, Visualization, Writing – original draft, Writing – review and editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Abbas, M. (2021). Potential role of nanoparticles in treating the accumulation of amyloid-beta peptide in Alzheimer’s patients. Polymers 13, 1051. doi:10.3390/polym13071051
Abd Elmonem, H. A., Morsi, R. M., Mansour, D. S., and El-Sayed, E.-S. R. (2023). Myco-fabricated ZnO nanoparticles ameliorate neurotoxicity in mice model of Alzheimer’s disease via acetylcholinesterase inhibition and oxidative stress reduction. Biometals 36 (6), 1391–1404. doi:10.1007/s10534-023-00525-6
Abdelmonem, M., Albert, E. L., Alhadad, M. A., and Abdullah, C. A. (2024). Plant-polyphenol-mediated synthesis of magnetic biocompatible iron oxide nanoparticles for diagnostic imaging and management of neurodegenerative diseases. Precis. Nanomedicine 7 (1), 1233–1251. doi:10.33218/001c.92424
Abidi, S. M., Sharma, C., Randhawa, S., Shukla, A. K., and Acharya, A. (2023). A review on nanotechnological perspective of “the amyloid cascade hypothesis” for neurodegenerative diseases. Int. J. Biol. Macromol. 253, 126821. doi:10.1016/j.ijbiomac.2023.126821
Achar, A., Myers, R., and Ghosh, C. (2021). Drug delivery challenges in brain disorders across the blood–brain barrier: novel methods and future considerations for improved therapy. Biomedicines 9, 1834. doi:10.3390/biomedicines9121834
Afzal, O., Altamimi, A. S., Nadeem, M. S., Alzarea, S. I., Almalki, W. H., Tariq, A., et al. (2022). Nanoparticles in drug delivery: from history to therapeutic applications. Nanomaterials 12, 4494. doi:10.3390/nano12244494
Akbari-Gharalari, N., Khodakarimi, S., Nezhadshahmohammad, F., Karimipour, M., Ebrahimi-Kalan, A., and Wu, J. (2024). Exosomes in neuron-glia communication: a review on neurodegeneration. BioImpacts BI 14 (5), 30153. doi:10.34172/bi.2023.30153
Akyuz, E., Aslan, F. S., Gokce, E., Ilmaz, O., Topcu, F., and Kakac, S. (2024). Extracellular vesicle and CRISPR gene therapy: current applications in alzheimer's disease, parkinson's disease, amyotrophic lateral sclerosis, and huntington's disease. Eur. J. Neurosci. 60 (8), 6057–6090. doi:10.1111/ejn.16541
Alipour, M., Abdolmaleki, M., Shabanpour, Y., Zali, A., Ashrafi, F., Nohesara, S., et al. (2025). Advances in magnetic field approaches for non-invasive targeting neuromodulation. Front. Hum. Neurosci. 19, 1489940. doi:10.3389/fnhum.2025.1489940
Alshammari, Q. A. (2025). Redox modulatory role of DJ-1 in Parkinson’s disease. Biogerontology 26, 81. doi:10.1007/s10522-025-10227-w
Angelov, S. D., Rehbock, C., Ramesh, V., Heissler, H. E., Alam, M., Barcikowski, S., et al. (2024). Coating of neural electrodes with platinum nanoparticles reduces and stabilizes impedance in vitro and in vivo in a rat model. Coatings 14 (3), 352. doi:10.3390/coatings14030352
Antonarakis, S. E. (2019). Carrier screening for recessive disorders. Nat. Rev. Genet. 20, 549–561. doi:10.1038/s41576-019-0134-2
Arachchige, S. G., Rienzie, R., and Adassooriya, N. M. (2019). Nanocarrier-mediated drug delivery systems for neurodegenerative diseases. Nanobiotechnology Neurodegener. Dis., 267–287. doi:10.1007/978-3-030-30930-5_11
Araújo, A. M., Mota, C., Ramos, H., Faria, M. A., Carvalho, M., and Ferreira, I. M. (2025). The neurotoxic threat of micro-and nanoplastics: evidence from in vitro and in vivo models. Archives Toxicol. 99, 3505–3525. doi:10.1007/s00204-025-04091-3
Arora, S., and Gugulothu, D. (2025). Recent advances in rotigotine nanoformulations for parkinson’s disease therapy. BioNanoScience 15 (2), 249–16. doi:10.1007/s12668-025-01855-0
Aschner, M., Skalny, A., Santamaria, A., Buha-Đorđević, A., Tizabi, Y., Jiang, Y., et al. (2023). From mechanisms to implications: understanding the molecular neurotoxicity of titanium dioxide nanoparticles. Front. Bioscience-Landmark 28, 204–221. doi:10.31083/j.fbl2809204
Asefy, Z., Hoseinnejhad, S., and Ceferov, Z. (2021). Nanoparticles approaches in neurodegenerative diseases diagnosis and treatment. Neurol. Sci. 42 (7), 2653–2660. doi:10.1007/s10072-021-05234-x
Awad, S. M., Attia, Y. A., ElSayed, H., Abdelhafez, S. H., Keshta, A. T., Rashad, E., et al. (2025). Efficacy of curcumin-selenium nanoemulsion in alleviating oxidative damage induced by aluminum chloride in a rat model of Alzheimer’s disease. J. Mol. Histology 56 (2), 122. doi:10.1007/s10735-025-10406-6
Bandaru, N., Shamim, N., Nagalakshmi, S. B., Sunanda, T., Hanisha, C., Gambhire, M. S., et al. (2024a). Preparation of platinum nanoparticles of Biophytum reinwardtii and evaluation of neuroprotective activity of MPTP-Induced parkinson’s disease in zebra fish. Biomed. Pharmacol. J. 17, 1635–1645. doi:10.13005/bpj/2971
Bandaru, S., Arora, D., Ganesh, K. M., Umrao, S., Thomas, S., Bhaskar, S., et al. (2024b). Recent advances in research from nanoparticle to Nano- assembly: a review. Nanomaterials 14, 1387. doi:10.3390/nano14171387
Bekdash, R. A. (2023). Methyl donors, epigenetic alterations, and brain health: understanding the connection. Int. J. Mol. Sci. 24 (3), 2346. doi:10.3390/ijms24032346
Benninger, D. H., Thees, S., Kollias, S. S., Bassetti, C. L., and Waldvogel, D. (2009). Morphological differences in Parkinson’s disease with and without rest tremor. J. Neurology 256, 256–263. doi:10.1007/s00415-009-0092-2
Beura, S. K., Maharaj, S., Kumari, N., Yadav, R., Sahu, M., Khilar, L. R., et al. (2024). “Theranostic potential of nanomaterials in neurodegenerative diseases: insights into biosensing, drug delivery and tissue engineering,” in Nanomedicine: innovations, applications, and breakthroughs in the quest for health and medicine's future. Springer, 409–449.
Bhatane, D., Pamshong, S. R., Sarnaik, S., and Alexander, A. (2023). Potential applications of mesoporous silica nanoparticles for the treatment of neurological disorders. J. Drug Deliv. Sci. Technol. 89, 104970. doi:10.1016/j.jddst.2023.104970
Bhidayasiri, R. (2024). Small particles, big potential: polymeric nanoparticles for drug delivery in parkinson’s disease. Mov. Disord. 39 (11), 1923. doi:10.1002/mds.29939
Biswas, A., Hivare, P., Solanki, R., Gupta, S., and Bhatia, D. D. (2025). Applications of bionanomaterials in neurodegenerative diseases. Mater. Adv. 6, 3785–3804. doi:10.1039/d4ma01215a
Bjørklund, G., Hofer, T., Nurchi, V. M., and Aaseth, J. (2019). Iron and other metals in the pathogenesis of parkinson's disease: toxic effects and possible detoxification. J. Inorg. Biochem. 199, 110717. doi:10.1016/j.jinorgbio.2019.110717
Bloem, B. R., Okun, M. S., and Klein, C. (2021). Parkinson's disease. Lancet 397, 2284–2303. doi:10.1016/S0140-6736(21)00218-X
Bondarenko, O., and Saarma, M. (2021). Neurotrophic factors in Parkinson’s disease: clinical trials, open challenges and nanoparticle-mediated delivery to the brain. Front. Cell. Neurosci. 15, 682597. doi:10.3389/fncel.2021.682597
Braak, H., Del Tredici, K., Rüb, U., DE Vos, R. A., Steur, E. N. J., and Braak, E. (2003). Staging of brain pathology related to sporadic Parkinson’s disease. Neurobiol. Aging 24, 197–211. doi:10.1016/s0197-4580(02)00065-9
Car, E., Barbier, L., Huys, I., Simoens, S., and Vulto, A. G. (2025). Evolving global regulatory landscape for approval of biosimilars: current challenges and opportunities for convergence. Expert Opin. Biol. Ther. 25, 649–668. doi:10.1080/14712598.2025.2507832
Chávez-Castillo, M., Gotera, M. P., Duran, P., Díaz, M. P., Nava, M., Cano, C., et al. (2025). Neuroprotective role of Omega-3 fatty acids: fighting alzheimer’s disease. Molecules 30 (15), 3057. doi:10.3390/molecules30153057
Chen, Z., Rasheed, M., and Deng, Y. (2022). The epigenetic mechanisms involved in mitochondrial dysfunction: implication for Parkinson’s disease. Brain Pathol. 32, e13012. doi:10.1111/bpa.13012
Cheng, G., Liu, Y., Ma, R., Cheng, G., Guan, Y., Chen, X., et al. (2022). Anti-parkinsonian therapy: strategies for crossing the blood–brain barrier and nano-biological effects of nanomaterials. Nano-Micro Lett. 14 (1), 105. doi:10.1007/s40820-022-00847-z
Chiang, M.-C., Yang, Y.-P., Nicol, C. J., and Wang, C.-J. (2024). Gold nanoparticles in neurological diseases: a review of neuroprotection. Int. J. Mol. Sci. 25, 2360. doi:10.3390/ijms25042360
Cole, T. A., Zhao, H., Collier, T. J., Sandoval, I., Sortwell, C. E., Steece-Collier, K., et al. (2021). α-Synuclein antisense oligonucleotides as a disease-modifying therapy for Parkinson’s disease. JCI Insight 6 (5), e135633. doi:10.1172/jci.insight.135633
Correia, A., Monteiro, A., Silva, R., Moreira, J., Lobo, J. S., and Silva, A. (2022). Lipid nanoparticles strategies to modify pharmacokinetics of central nervous system targeting drugs: crossing or circumventing the blood–brain barrier (BBB) to manage neurological disorders. Adv. drug Deliv. Rev. 189, 114485. doi:10.1016/j.addr.2022.114485
Crowley, E., Nolan, Y., and Sullivan, A. (2019). Exercise as a therapeutic intervention for motor and non-motor symptoms in Parkinson’s disease: evidence from rodent models. Prog. Neurobiol. 172, 2–22. doi:10.1016/j.pneurobio.2018.11.003
Dasgupta, A., Sofias, A. M., Kiessling, F., and Lammers, T. (2024). Nanoparticle delivery to tumours: from EPR and ATR mechanisms to clinical impact. Nat. Rev. Bioeng. 2, 714–716. doi:10.1038/s44222-024-00203-3
Dash, P., Panda, P. K., Su, C., Lin, Y.-C., Sakthivel, R., Chen, S.-L., et al. (2024). Near-infrared-driven upconversion nanoparticles with photocatalysts through water-splitting towards cancer treatment. J. Mater. Chem. B 12, 3881–3907. doi:10.1039/d3tb01066j
DE Bem Silveira, G., Muller, A. P., Machado-DE-Ávila, R. A., and Silveira, P. C. L. (2021). Advance in the use of gold nanoparticles in the treatment of neurodegenerative diseases: new perspectives. Neural Regen. Res. 16, 2425–2426. doi:10.4103/1673-5374.313040
Devassy, G., Das, D., Nair, S. V., and Koyakutty, M. (2023). Injectable nanogel systems for brain drug delivery. Precis. Nanomed 6, 1078–1098. doi:10.33218/001c.88217
Dhariwal, R., Jain, M., Mir, Y. R., Singh, A., Jain, B., Kumar, P., et al. (2025). Targeted drug delivery in neurodegenerative diseases: the role of nanotechnology. Front. Med. 12, 1522223. doi:10.3389/fmed.2025.1522223
Do, J., Mckinney, C., Sharma, P., and Sidransky, E. (2019). Glucocerebrosidase and its relevance to parkinson disease. Mol. Neurodegener. 14, 36–16. doi:10.1186/s13024-019-0336-2
Dong-Chen, X., Yong, C., Yang, X., Chen-Yu, S., and Li-Hua, P. (2023). Signaling pathways in Parkinson’s disease: molecular mechanisms and therapeutic interventions. Signal Transduct. Target. Ther. 8, 73. doi:10.1038/s41392-023-01353-3
DU, W., Wang, T., Hu, S., Luan, J., Tian, F., Ma, G., et al. (2023). Engineering of electrospun nanofiber scaffolds for repairing brain injury. Eng. Regen. 4, 289–303. doi:10.1016/j.engreg.2023.04.001
Dzamko, N. (2025). LRRK2-associated Parkinson’s disease does have alpha-synuclein pathology. Acta Neuropathol. 149, 43. doi:10.1007/s00401-025-02874-7
EL Otmani, H., Daghi, M., Tahiri Jouti, N., and Lesage, S. (2023). An overview of the worldwide distribution of LRRK2 mutations in Parkinson’s disease. Neurodegener. Dis. Manag. 13, 335–350. doi:10.2217/nmt-2023-0025
Elawad, M. A., Ayaz, M., Mosa, O. F., Usman, A., Hamdoon, A. A. E., Almawash, S., et al. (2024). Polyphenols and their biogenic nano-formulations targeting BACE1 as anti-amyloid therapies; meeting the challenges of bioavailability, safety, and specificity for the treatment of alzheimer's disease. Mol. Nutr. and Food Res. 68 (24), 2400525. doi:10.1002/mnfr.202400525
Elkasabgy, N. A., Salama, A., and Salama, A. H. (2023). Exploring the effect of intramuscularly injected polymer/lipid hybrid nanoparticles loaded with quetiapine fumarate on the behavioral and neurological changes in cuprizone-induced schizophrenia in mice. J. Drug Deliv. Sci. Technol. 79, 104064. doi:10.1016/j.jddst.2022.104064
Emeihe, E. V., Nwankwo, E. I., Ajegbile, M. D., Olaboye, J. A., and Maha, C. C. (2024). Revolutionizing drug delivery systems: nanotechnology-Based approaches for targeted therapy. Int. J. Life Sci. Res. Arch. 7 (1), 40–58. doi:10.53771/ijlsra.2024.7.1.0060
Fakhri, S., Abdian, S., Zarneshan, S. N., Moradi, S. Z., Farzaei, M. H., and Abdollahi, M. (2022). Nanoparticles in combating neuronal dysregulated signaling pathways: recent approaches to the nanoformulations of phytochemicals and synthetic drugs against neurodegenerative diseases. Int. J. Nanomedicine Vol. 17, 299–331. doi:10.2147/ijn.s347187
Fang, S., Zhang, K., Liu, D., Yang, Y., Xi, H., Xie, W., et al. (2024). Polyphenol-based polymer nanoparticles for inhibiting amyloid protein aggregation: recent advances and perspectives. Front. Nutr. 11, 1408620. doi:10.3389/fnut.2024.1408620
Fernandes, F., Dias-Teixeira, M., Delerue-Matos, C., and Grosso, C. (2021). Critical review of lipid-based nanoparticles as carriers of neuroprotective drugs and extracts. Nanomaterials 11, 563. doi:10.3390/nano11030563
Filippov, A. G., Alexandrin, V. V. E., Ivanov, A. V., Paltsyn, A. A., Sviridkina, N. B., Virus, E. D., et al. (2023). Neuroprotective effect of platinum nanoparticles is not associated with their accumulation in the brain of rats. J. Funct. Biomaterials 14, 348. doi:10.3390/jfb14070348
Gao, F., Hou, Y., Wang, Y., Liu, L., Yi, X., and Xia, N. (2025). Photothermal and photodynamic strategies for diagnosis and therapy of alzheimer’s disease by modulating Amyloid-β aggregation. Biosensors 15 (8), 480. doi:10.3390/bios15080480
Gash, D. M., Gerhardt, G. A., Bradley, L. H., Wagner, R., and Slevin, J. T. (2020). GDNF clinical trials for Parkinson’s disease: a critical human dimension. Cell Tissue Res. 382 (1), 65–70. doi:10.1007/s00441-020-03269-8
Ge, P., Dawson, V. L., and Dawson, T. M. (2020). PINK1 and parkin mitochondrial quality control: a source of regional vulnerability in Parkinson’s disease. Mol. Neurodegener. 15, 20. doi:10.1186/s13024-020-00367-7
Georgieva, J. V., Hoekstra, D., and Zuhorn, I. S. (2014). Smuggling drugs into the brain: an overview of ligands targeting transcytosis for drug delivery across the blood–brain barrier. Pharmaceutics 6 (4), 557–583. doi:10.3390/pharmaceutics6040557
Girigoswami, A., Deepika, B., Udayakumar, S., Janani, G., Mercy, D. J., and Girigoswami, K. (2024). Peony-shaped zinc oxide nanoflower synthesized via hydrothermal route exhibits promising anticancer and anti-amyloid activity. BMC Pharmacol. Toxicol. 25 (1), 101. doi:10.1186/s40360-024-00830-x
González, L. F., Bevilacqua, L. E., and Naves, R. (2021). Nanotechnology-based drug delivery strategies to repair the mitochondrial function in neuroinflammatory and neurodegenerative diseases. Pharmaceutics 13 (12), 2055. doi:10.3390/pharmaceutics13122055
Guigou, C., Lalande, A., Millot, N., Belharet, K., and Bozorg Grayeli, A. (2021). Use of super paramagnetic iron oxide nanoparticles as drug carriers in brain and ear: state of the art and challenges. Brain Sci. 11, 358. doi:10.3390/brainsci11030358
Gulino, M., Santos, S. D., and Pêgo, A. P. (2021). Biocompatibility of platinum nanoparticles in brain ex vivo models in physiological and pathological conditions. Front. Neurosci. 15, 787518. doi:10.3389/fnins.2021.787518
Hamdy, N. M., Basalious, E. B., El-Sisi, M. G., Rana, A. Y., and Sultan, A. S. (2025). “Toward applicability of Exosomal-ncRNA precision vs. nanoparticles to the brain,” in Nanocarriers in neurodegenerative disorders. Howick Place, London: CRC Press, 294–308.
Heidarzadeh, M., Gürsoy-Özdemir, Y., Kaya, M., Eslami Abriz, A., Zarebkohan, A., Rahbarghazi, R., et al. (2021). Exosomal delivery of therapeutic modulators through the blood–brain barrier; promise and pitfalls. Cell and Biosci. 11 (1), 142. doi:10.1186/s13578-021-00650-0
Hely, M. A., Morris, J. G., Reid, W. G., and Trafficante, R. (2005). Sydney multicenter study of parkinson's disease: non-l-dopa–responsive problems dominate at 15 years. Mov. Disord. Official J. Mov. Disord. Soc. 20, 190–199. doi:10.1002/mds.20324
Hervé, F., Ghinea, N., and Scherrmann, J.-M. (2008). CNS delivery via adsorptive transcytosis. AAPS J. 10 (3), 455–472. doi:10.1208/s12248-008-9055-2
Hirsch, E., Orieux, G., Muriel, M.-P., Francois, C., and Feger, J. (2003). Nondopaminergic neurons in parkinson's disease. Adv. Neurology 91, 29–37.
Hou, S., Li, C., Wang, Y., Sun, J., Guo, Y., Ning, X., et al. (2022). Silica nanoparticles cause activation of NLRP3 inflammasome in-vitro model-using microglia. Int. J. Nanomedicine 17, 5247–5264. doi:10.2147/ijn.s372485
Howard, R., AL-Mayhani, T., Carr, A., Leff, A., Morrow, J., and Rossor, A. (2024). Toxic, metabolic and physical insults to the nervous system. Neurol. a Queen Sq. Textb., 903–943. doi:10.1002/9781119715672.ch26
Hoyos-Ceballos, G. P., Ruozi, B., Ottonelli, I., Da Ros, F., Vandelli, M. A., Forni, F., et al. (2020). PLGA-PEG-ANG-2 nanoparticles for blood–brain barrier crossing: proof-Of-Concept study. Pharmaceutics 12 (1), 72. doi:10.3390/pharmaceutics12010072
Jagaran, K., and Singh, M. (2022). Lipid nanoparticles: promising treatment approach for Parkinson’s disease. Int. J. Mol. Sci. 23 (16), 9361. doi:10.3390/ijms23169361
Janzadeh, A., Behroozi, Z., Saliminia, F., Janzadeh, N., Arzani, H., Tanha, K., et al. (2022). Neurotoxicity of silver nanoparticles in the animal brain: a systematic review and meta-analysis. Forensic Toxicol. 40 (1), 49–63. doi:10.1007/s11419-021-00589-4
Jiang, P., Gan, M., Yen, S.-H., and Dickson, D. W. (2021). Nanoparticles with affinity for α-Synuclein sequester α-Synuclein to form toxic aggregates in neurons with endolysosomal impairment. Front. Mol. Neurosci. 14, 738535. doi:10.3389/fnmol.2021.738535
Jiménez, A., Estudillo, E., Guzmán-Ruiz, M. A., Herrera-Mundo, N., Victoria-Acosta, G., Cortés-Malagón, E. M., et al. (2025). Nanotechnology to overcome blood–brain barrier permeability and damage in neurodegenerative diseases. Pharmaceutics 17 (3), 281. doi:10.3390/pharmaceutics17030281
Jin, Y., Zhang, X., Zhao, X., Xu, Y., Wei, G., and Su, Z. (2025). Two-dimensional biomaterials applied as functional bio-platforms and devices: revolutionizing biomedical applications. Small 21, 2502929. doi:10.1002/smll.202502929
Jomova, K., Alomar, S. Y., Alwasel, S. H., Nepovimova, E., Kuca, K., and Valko, M. (2024). Several lines of antioxidant defense against oxidative stress: antioxidant enzymes, nanomaterials with multiple enzyme-mimicking activities, and low-molecular-weight antioxidants. Archives Toxicol. 98, 1323–1367. doi:10.1007/s00204-024-03696-4
Kadgien, C. A., Kamesh, A., and Milnerwood, A. J. (2021). Endosomal traffic and glutamate synapse activity are increased in VPS35 D620N mutant knock-in mouse neurons, and resistant to LRRK2 kinase inhibition. Mol. Brain 14, 143. doi:10.1186/s13041-021-00848-w
KalčEc, N., Peranić, N., Mamić, I., Beus, M., Hall, C. R., Smith, T. A., et al. (2023). Selenium nanoparticles as potential drug-delivery systems for the treatment of Parkinson’s disease. ACS Appl. Nano Mater. 6, 17581–17592. doi:10.1021/acsanm.3c02749
Karavelioglu, Z., and Cakir-Koc, R. (2021). Preparation of chitosan nanoparticles as Ginkgo biloba extract carrier: in vitro neuroprotective effect on oxidative stress-induced human neuroblastoma cells (SH-SY5Y). Int. J. Biol. Macromol. 192, 675–683. doi:10.1016/j.ijbiomac.2021.10.023
Kardani, S. L. (2024). Nanocarrier-based formulations: regulatory challenges, ethical and safety considerations in pharmaceuticals. Asian J. Pharm. (AJP) 18 (02). doi:10.22377/ajp.v18i02.5444
Ke, S., Zhu, Y., Fu, C., Mao, H., Shi, K., Qiao, L., et al. (2024). Flexible neuromorphic transistors for neuromorphic computing and perception application. Moore More 1, 9. doi:10.1007/s44275-024-00009-w
Keshavarz Shahbaz, S., Koushki, K., Keshavarz Hedayati, S., Mccloskey, A. P., Kesharwani, P., Naderi, Y., et al. (2024). Polymer nanotherapeutics: a promising approach toward microglial inhibition in neurodegenerative diseases. Med. Res. Rev. 44, 2793–2824. doi:10.1002/med.22064
Khadrawy, Y. A., Hosny, E. N., and Mohamed, H. S. E. (2024). Assessment of the neuroprotective effect of green synthesized iron oxide nanoparticles capped with curcumin against a rat model of Parkinson’s disease. Iran. J. Basic Med. Sci. 27, 81–89. doi:10.22038/IJBMS.2023.73124.15892
Kim, H.-Y., Yoon, H. H., Seong, H., Seo, D. K., Choi, S. W., Ryu, J., et al. (2023). Preventive effects of nano-graphene oxide against Parkinson’s disease via reactive oxygen species scavenging and anti-inflammation. BMB Rep. 56 (3), 202–207. doi:10.5483/bmbrep.2022-0137
Kim, H.-Y., Yoon, H. H., Seong, H., Seo, D. K., Choi, S. W., Ryu, J., et al. (2024). Preventive effects of nano-graphene oxide against Parkinson’s disease via reactive oxygen species scavenging and anti-inflammation. BMB Rep. 56, 202–207. doi:10.5383/bmbrep.2022-0137
Kim, K., Lee, J., and Park, M.-H. (2023b). Microbubble delivery platform for ultrasound-mediated therapy in brain cancers. Pharmaceutics 15, 698. doi:10.3390/pharmaceutics15020698
Kim, S., Jung, U. J., and Kim, S. R. (2024). Role of oxidative stress in blood–brain barrier disruption and neurodegenerative diseases. Antioxidants 13 (12), 1462. doi:10.3390/antiox13121462
Krsek, A., and Baticic, L. (2024). Nanotechnology-driven therapeutic innovations in neurodegenerative disorders: a focus on Alzheimer’s and Parkinson’s disease. Future Pharmacol. 4 (2), 352–379. doi:10.3390/futurepharmacol4020020
Kshirsagar, N., Patil, A., and Suryawanshi, M. (2025). Bioactive compound nanoparticles for alzheimer's disease. Inflammopharmacology 33, 2963–2976. doi:10.1007/s10787-025-01801-2
Kumar, R., and Kumar, P. (2024). Exploring the diverse biomedical applications of hybrid nanomaterials. Innovations and applications of hybrid nanomaterials. IGI Glob. Sci. Publ., 1–15. doi:10.4018/979-8-3693-3268-9.ch001
Kumar, R., Rauti, R., Scaini, D., Antman-Passig, M., Meshulam, O., Naveh, D., et al. (2021). Graphene-based nanomaterials for neuroengineering: recent advances and future prospective. Adv. Funct. Mater. 31, 2104887. doi:10.1002/adfm.202104887
Kumar, R., Aadil, K. R., Mondal, K., Mishra, Y. K., Oupicky, D., Ramakrishna, S., et al. (2022). Neurodegenerative disorders management: State-Of-art and prospects of nano-biotechnology. Crit. Rev. Biotechnol. 42 (8), 1180–1212. doi:10.1080/07388551.2021.1993126
Kunjiappan, S., Pavadai, P., Vellaichamy, S., Ram Kumar Pandian, S., Ravishankar, V., Palanisamy, P., et al. (2021). Surface receptor-mediated targeted drug delivery systems for enhanced cancer treatment: a state-of-the-art review. Drug Dev. Res. 82, 309–340. doi:10.1002/ddr.21758
Latif, S., Jahangeer, M., Razia, D. M., Ashiq, M., Ghaffar, A., Akram, M., et al. (2021). Dopamine in parkinson's disease. Clin. Chim. Acta 522, 114–126. doi:10.1016/j.cca.2021.08.009
Lee, M. (2013). Neurotransmitters and microglial-mediated neuroinflammation. Curr. Protein Peptide Sci. 14, 21–32. doi:10.2174/1389203711314010005
Lee, K. H., Cha, M., and Lee, B. H. (2020). Neuroprotective effect of antioxidants in the brain. Int. J. Mol. Sci. 21 (19), 7152. doi:10.3390/ijms21197152
Lee, M.-J., Lee, I., and Wang, K. (2022). Recent advances in RNA therapy and its carriers to treat the single-gene neurological disorders. Biomedicines 10, 158. doi:10.3390/biomedicines10010158
Levy, O., Rothhammer, V., Mascanfroni, I., Tong, Z., Kuai, R., DE Biasio, M., et al. (2021). A cell-based drug delivery platform for treating central nervous system inflammation. J. Mol. Med. 99, 663–671. doi:10.1007/s00109-020-02003-9
Li, H., Zha, S., Li, H., Liu, H., Wong, K. L., and All, A. H. (2022). Polymeric dendrimers as nanocarrier vectors for neurotheranostics. Small 18, 2203629. doi:10.1002/smll.202203629
Li, L., Luo, P., Wu, S., and Wang, Y. (2023). Deciphering the neuroprotective effect of ascorbic acid mediated synthesis of iron oxide nanoparticles against Parkinson’s disease: an in vitro and in vivo approach. Macromol. Res. 31, 949–960. doi:10.1007/s13233-023-00186-x
Liu, L., He, H., Du, B., and He, Y. (2025). Nanoscale drug formulations for the treatment of alzheimer's disease progression. RSC Adv. 15 (6), 4031–4078. doi:10.1039/d4ra08128e
Lu, X., Hou, X., Tang, H., Yi, X., and Wang, J. (2022). A high-quality cdSe/cdS/znS quantum-dot-based FRET aptasensor for the simultaneous detection of two different Alzheimer’s disease core biomarkers. Nanomaterials 12 (22), 4031. doi:10.3390/nano12224031
Ma, X.-Q., Ding, G., Niu, W., Jia, Z., Han, S.-T., Kuo, C.-C., et al. (2023). Flexible neuromorphic devices based on two-dimensional transition metal dichalcogenides. IEEE J. Flexible Electron. 3, 10–28. doi:10.1109/jflex.2023.3298593
Ma, K., Tian, T., Li, X., Pang, H., Ning, X., Li, M., et al. (2025). Silica nanoparticles induce SH-SY5Y cells death via PARP and caspase signaling pathways. Mol. Neurobiol. 62 (6), 7506–7524. doi:10.1007/s12035-025-04724-9
Majumder, J., and Minko, T. (2021). Multifunctional and stimuli-responsive nanocarriers for targeted therapeutic delivery. Expert Opin. Drug Deliv. 18, 205–227. doi:10.1080/17425247.2021.1828339
Manimaran, V., Nivetha, R., Tamilanban, T., Narayanan, J., Vetriselvan, S., Fuloria, N. K., et al. (2023). Nanogels as novel drug nanocarriers for CNS drug delivery. Front. Mol. Biosci. 10, 1232109. doi:10.3389/fmolb.2023.1232109
Manzari, M. T., Shamay, Y., Kiguchi, H., Rosen, N., Scaltriti, M., and Heller, D. A. (2021). Targeted drug delivery strategies for precision medicines. Nat. Rev. Mater. 6, 351–370. doi:10.1038/s41578-020-00269-6
Marras, C., and Chaudhuri, K. R. (2016). Nonmotor features of parkinson's disease subtypes. Mov. Disord. 31 (8), 1095–1102. doi:10.1002/mds.26510
Meade, R. M., Fairlie, D. P., and Mason, J. M. (2019). Alpha-synuclein structure and Parkinson’s disease–lessons and emerging principles. Mol. Neurodegener. 14, 29–14. doi:10.1186/s13024-019-0329-1
Mellor, R. D., and Uchegbu, I. F. (2022). Ultrasmall-in-nano: why size matters. Nanomaterials 12 (14), 2476. doi:10.3390/nano12142476
Mencke, P., Boussaad, I., Romano, C. D., Kitami, T., Linster, C. L., and Krüger, R. (2021). The role of DJ-1 in cellular metabolism and pathophysiological implications for Parkinson’s disease. Cells 10, 347. doi:10.3390/cells10020347
Meng, L. (2022). Inorganic biointerfaces for modulating cell signaling. Univ. Chic. doi:10.6082/uchicago.4771
Mohamed, M., Hani, B., Koca, F. D., Unal, G., Rahman, N. A., Basma, A., et al. (2025). Evaluation of antimicrobial, anticancer, andneuroprotective activities of silver nanoparticles (AgNPs) green-synthesized using a red pigment produced by streptomyces sp. A23 strain isolated from Algerian bee pollen. J. Serbian Chem. Soc., 8. doi:10.2298/JSC240915008M
Mojarad-Jabali, S., Mahdinloo, S., Farshbaf, M., Sarfraz, M., Fatahi, Y., Atyabi, F., et al. (2022). Transferrin receptor-mediated liposomal drug delivery: recent trends in targeted therapy of cancer. Expert Opin. Drug Deliv. 19 (6), 685–705. doi:10.1080/17425247.2022.2083106
Mondal, S., and Firdous, S. M. (2025). Unrevealing the molecular mechanisms of MPTP-Induced Parkinson’s in experimental animals. Med. Chem. Res., 1–16. doi:10.1007/s00044-025-03409-1
Monge-Fuentes, V., Biolchi Mayer, A., Lima, M. R., Geraldes, L. R., Zanotto, L. N., Moreira, K. G., et al. (2021). Dopamine-loaded nanoparticle systems circumvent the blood–brain barrier restoring motor function in mouse model for Parkinson’s disease. Sci. Rep. 11 (1), 15185. doi:10.1038/s41598-021-94175-8
Mori, F., Piao, Y.-S., Hayashi, S., Fujiwara, H., Hasegawa, M., Yoshimoto, M., et al. (2003). α-Synuclein accumulates in purkinje cells in lewy body disease but not in multiple system atrophy. J. Neuropathology and Exp. Neurology 62, 812–819. doi:10.1093/jnen/62.8.812
Mozafari, F., Rashidzadeh, H., Bijani, S., Zare-Molaei, F., Islambulchilar, Z., Danafar, H., et al. (2023). Enhancing the neuroprotection potential of edaravone in transient global ischemia treatment with Glutathione-(GSH-) conjugated poly (methacrylic acid) nanogel as a promising carrier for targeted brain drug delivery. Oxidative Med. Cell. Longev. 2023, 1–17. doi:10.1155/2023/7643280
Mundekkad, D., and Cho, W. C. (2022). Mitophagy induced by metal nanoparticles for cancer treatment. Pharmaceutics 14 (11), 2275. doi:10.3390/pharmaceutics14112275
Mundekkad, D., and Mallya, A. R. (2025). Biomimicry at the nanoscale - a review of nanomaterials inspired by nature. Nano Trends 10, 100119. doi:10.1016/j.nwnano.2025.100119
Nair, A. S., Dangi, S., Thomas, A. K., and Sekar, M. (2024). “Role of enzymes in parkinson’s disease,” in Enzymes in neurodegenerative disorders: mechanism and therapeutic potentials. Springer.
Nance, E., Kambhampati, S. P., Smith, E. S., Zhang, Z., Zhang, F., Singh, S., et al. (2017). Dendrimer-mediated delivery of N-acetyl cysteine to microglia in a mouse model of Rett syndrome. J. Neuroinflammation 14 (1), 252. doi:10.1186/s12974-017-1004-5
Naqvi, S., Panghal, A., and Flora, S. (2020). Nanotechnology: a promising approach for delivery of neuroprotective drugs. Front. Neurosci. 14, 494. doi:10.3389/fnins.2020.00494
Narendra, D. P., and Youle, R. J. (2024). The role of PINK1–Parkin in mitochondrial quality control. Nat. Cell Biol. 26, 1639–1651. doi:10.1038/s41556-024-01513-9
Nayab, D. E., Din, F. u., Ali, H., Kausar, W. A., Urooj, S., Zafar, M., et al. (2023). Nano biomaterials based strategies for enhanced brain targeting in the treatment of neurodegenerative diseases: an up-to-date perspective. J. Nanobiotechnology 21 (1), 477. doi:10.1186/s12951-023-02250-1
Neag, M.-A., Mitre, A.-O., Catinean, A., and Mitre, C.-I. (2020). An overview on the mechanisms of neuroprotection and neurotoxicity of isoflurane and sevoflurane in experimental studies. Brain Res. Bull. 165, 281–289. doi:10.1016/j.brainresbull.2020.10.011
Nie, T., He, Z., Zhu, J., Chen, K., Howard, G. P., Pacheco-Torres, J., et al. (2021). Non-invasive delivery of levodopa-loaded nanoparticles to the brain via lymphatic vasculature to enhance treatment of Parkinson’s disease. Nano Res. 14 (8), 2749–2761. doi:10.1007/s12274-020-3280-0
Omidian, H., and Dey Chowdhury, S. (2025). Multifunctional hydrogel microneedles (HMNs) in drug delivery and diagnostics. Gels 11, 206. doi:10.3390/gels11030206
Oyarce, K., Cepeda, M. Y., Lagos, R., Garrido, C., Vega-Letter, A. M., Garcia- Robles, M., et al. (2022). Neuroprotective and neurotoxic effects of glial-derived exosomes. Front. Cell. Neurosci. 16, 920686. doi:10.3389/fncel.2022.920686
Oz, T., Kaushik, A. K., and Kujawska, M. (2023). Advances in graphene-based nanoplatforms and their application in parkinson's disease. Mater. Adv. 4, 6464–6477. doi:10.1039/d3ma00623a
Padilla-Godínez, F. J., Ruiz-Ortega, L. I., and Guerra-Crespo, M. (2022). Nanomedicine in the face of Parkinson’s disease: from drug delivery systems to nanozymes. Cells 11 (21), 3445. doi:10.3390/cells11213445
Pal, P., Sharma, M., Bani, K. S., and Gupta, S. K. (2024). Advances in neuronal regeneration: hydrogel-Based delivery systems loaded with extracellular vesicles in modulating neural impulses and tissue repair. Eur. Polym. J. 220, 113457. doi:10.1016/j.eurpolymj.2024.113457
Palanisamy, S., Balasubramanian, A., Kanchana, M., Sisubalan, N., and Selvakesavan, R. K. (2025). “Curcumin-loaded nanoparticles in neurodegenerative diseases: alzheimer’s, parkinson’s, and amyotrophic lateral sclerosis,” in Nanoparticles in modern neurological treatment. Springer, 225–237.
Parvin, N., Joo, S. W., and Mandal, T. K. (2025). Biodegradable and stimuli-responsive nanomaterials for targeted drug delivery in autoimmune diseases. J. Funct. Biomaterials 16 (1), 24. doi:10.3390/jfb16010024
Patel, A., and Patel, R. (2024). Nano formulations for peptide drug delivery: overcoming bioavailability and stability challenges. World J. Adv. Res. Rev. 22 (1), 2032–2044. doi:10.30574/wjarr.2024.22.1.0971
Pati, E. (2022). Graphene oxide nanoflakes enable targeting dysfunctional synaptic plasticity in the amygdala.
Pei, J., Palanisamy, C. P., Natarajan, P. M., Umapathy, V. R., Roy, J. R., Srinivasan, G. P., et al. (2024). Curcumin-loaded polymeric nanomaterials as a novel therapeutic strategy for alzheimer's disease: a comprehensive review. Ageing Res. Rev. 99, 102393. doi:10.1016/j.arr.2024.102393
Pérez-Carrión, M. D., and Posadas, I. (2023). Dendrimers in neurodegenerative diseases. Processes 11, 319. doi:10.3390/pr11020319
Piao, Y.-S., Mori, F., Hayashi, S., Tanji, K., Yoshimoto, M., Kakita, A., et al. (2003). α-Synuclein pathology affecting bergmann glia of the cerebellum in patients with α-synucleinopathies. Acta Neuropathol. 105, 403–409. doi:10.1007/s00401-002-0655-0
Piccinin, C. C., Campos, L. S., Guimarães, R. P., Piovesana, L. G., Dos Santos, M. C. A., Azevedo, P. C., et al. (2017). Differential pattern of cerebellar atrophy in tremor-predominant and akinetic/rigidity-predominant Parkinson’s disease. Cerebellum 16, 623–628. doi:10.1007/s12311-016-0834-5
Piktel, E., Ościłowska, I., Suprewicz, Ł., Depciuch, J., Marcińczyk, N., Chabielska, E., et al. (2021). ROS-Mediated apoptosis and autophagy in ovarian cancer cells treated with peanut-shaped gold nanoparticles. Int. J. Nanomedicine Vol. 16, 1993–2011. doi:10.2147/ijn.s277014
Pringsheim, T., Jette, N., Frolkis, A., and Steeves, T. D. (2014). The prevalence of parkinson's disease: a systematic review and meta-analysis. Mov. Disord. 29, 1583–1590. doi:10.1002/mds.25945
Rahim, M. A., Jan, N., Khan, S., Shah, H., Madni, A., Khan, A., et al. (2021). Recent advancements in stimuli responsive drug delivery platforms for active and passive cancer targeting. Cancers 13, 670. doi:10.3390/cancers13040670
Rajeshkumar, S., Ganesh, L., and Santhoshkumar, J. (2019). “Selenium nanoparticles as therapeutic agents in neurodegenerative diseases,” in Nanobiotechnology in neurodegenerative diseases. Springer, 209–224.
Ramos-Zaldívar, H. M., Polakovicova, I., Salas-Huenuleo, E., Corvalán, A. H., Kogan, M. J., Yefi, C. P., et al. (2022). Extracellular vesicles through the blood–brain barrier: a review. Fluids Barriers CNS 19, 60. doi:10.1186/s12987-022-00359-3
Rani, A., Saini, V., Patra, P., Prashar, T., Pandey, R. K., Mishra, A., et al. (2023). Epigallocatechin gallate: a multifaceted molecule for neurological disorders and neurotropic viral infections. ACS Chem. Neurosci. 14, 2968–2980. doi:10.1021/acschemneuro.3c00368
Rivero-Ríos, P., Romo-Lozano, M., Fasiczka, R., Naaldijk, Y., and Hilfiker, S. (2020). LRRK2-Related Parkinson’s disease due to altered endolysosomal biology with variable lewy body pathology: a hypothesis. Front. Neurosci. 14, 556. doi:10.3389/fnins.2020.00556
Rizzardi, N., Liparulo, I., Antonelli, G., Orsini, F., Riva, A., Bergamini, C., et al. (2021). Coenzyme Q10 phytosome formulation improves CoQ10 bioavailability and mitochondrial functionality in cultured cells. Antioxidants 10 (6), 927. doi:10.3390/antiox10060927
Rowlands, J., and Moore, D. J. (2024). VPS35 and retromer dysfunction in parkinson's disease. Philosophical Trans. R. Soc. B 379, 20220384. doi:10.1098/rstb.2022.0384
Roy, S., Deo, K. A., Singh, K. A., Lee, H. P., Jaiswal, A., and Gaharwar, A. K. (2022). Nano-bio interactions of 2D molybdenum disulfide. Adv. Drug Deliv. Rev. 187, 114361. doi:10.1016/j.addr.2022.114361
Sandoval-Castellanos, A. M., Claeyssens, F., and Haycock, J. W. (2021). Bioactive 3D scaffolds for the delivery of NGF and BDNF to improve nerve regeneration. Front. Mater. 8, 734683. doi:10.3389/fmats.2021.734683
Santos, M. F. D., Roxo, C., and Solá, S. (2021). Oxidative-signaling in neural stem cell-mediated plasticity: implications for neurodegenerative diseases. Antioxidants 10, 1088. doi:10.3390/antiox10071088
Sayyaed, A., Saraswat, N., Vyawahare, N., and Kulkarni, A. (2023). A detailed review of pathophysiology, epidemiology, cellular and molecular pathways involved in the development and prognosis of parkinson's disease with insights into screening models. Bull. Natl. Res. Centre 47, 70. doi:10.1186/s42269-023-01047-4
Schlich, M., Longhena, F., Faustini, G., O’Driscoll, C. M., Sinico, C., Fadda, A. M., et al. (2017). Anionic liposomes for small interfering ribonucleic acid (siRNA) delivery to primary neuronal cells: evaluation of alpha-synuclein knockdown efficacy. Nano Res. 10 (10), 3496–3508. doi:10.1007/s12274-017-1561-z
Seyedebrahimi, R., Razavi, S., Varshosaz, J., Vatankhah, E., and Kazemi, M. (2021). Beneficial effects of biodelivery of brain-derived neurotrophic factor and gold nanoparticles from functionalized electrospun PLGA scaffold for nerve tissue engineering. J. Clust. Sci. 32, 631–642. doi:10.1007/s10876-020-01822-7
Sharifyrad, M., Gohari, S., Fathi, M., Danafar, H., Hosseini, M.-J., Mostafavi, H., et al. (2022). The efficacy and neuroprotective effects of edaravone-loaded mPEG-b-PLGA polymeric nanoparticles on human neuroblastoma SH-SY5Y cell line as in vitro model of ischemia. J. Drug Deliv. Sci. Technol. 73, 103378. doi:10.1016/j.jddst.2022.103378
Sheffey, V. V., Siew, E. B., Tanner, E. E., and Eniola-Adefeso, O. (2022). PLGA's plight and the role of stealth surface modification strategies in its use for intravenous particulate drug delivery. Adv. Healthc. Mater. 11 (8), 2101536. doi:10.1002/adhm.202101536
Silva, S., Almeida, A. J., and Vale, N. (2021). Importance of nanoparticles for the delivery of antiparkinsonian drugs. Pharmaceutics 13 (4), 508. doi:10.3390/pharmaceutics13040508
Silva, A. B. R. L., DE Oliveira, R. W. G., Diógenes, G. P., DE Castro Aguiar, M. F., Sallem, C. C., Lima, M. P. P., et al. (2023). Premotor, nonmotor and motor symptoms of parkinson's disease: a new clinical state of the art. Ageing Res. Rev. 84, 101834. doi:10.1016/j.arr.2022.101834
Singh, I., Swami, R., Pooja, D., Jeengar, M. K., Khan, W., and Sistla, R. (2016). Lactoferrin bioconjugated solid lipid nanoparticles: a new drug delivery system for potential brain targeting. J. Drug Target. 24 (3), 212–223. doi:10.3109/1061186x.2015.1068320
Soliman, M. K., Salem, S. S., Abu-Elghait, M., and Azab, M. S. (2023). Biosynthesis of silver and gold nanoparticles and their efficacy towards antibacterial, antibiofilm, cytotoxicity, and antioxidant activities. Appl. Biochem. Biotechnol. 195, 1158–1183. doi:10.1007/s12010-022-04199-7
Song, B., Liu, J., Feng, X., Wei, L., and Shao, L. (2015). A review on potential neurotoxicity of titanium dioxide nanoparticles. Nanoscale Res. Lett. 10 (1), 342. doi:10.1186/s11671-015-1042-9
Soni, D., Garg, Y., Upadhayay, S., Bhatia, A., Basir, B., Singh, S. K., et al. (2025). Auranofin-loaded chitosan-lipid hybrid nanoparticle protects against rotenone model of parkinson's disease via modulation of GSK-3β/Nrf2/HO-1 signaling. Eur. J. Pharmacol. 998, 177523. doi:10.1016/j.ejphar.2025.177523
Subhan, M. A., Yalamarty, S. S. K., Filipczak, N., Parveen, F., and Torchilin, V. P. (2021). Recent advances in tumor targeting via EPR effect for cancer treatment. J. Personalized Med. 11, 571. doi:10.3390/jpm11060571
Suthar, J. K., Rakesh, B., Vaidya, A., and Ravindran, S. (2023). Comprehensive analysis of titanium oxide nanoparticle size and surface properties on neuronal PC-12 cells: unraveling cytotoxicity, dopaminergic gene expression, and acetylcholinesterase inhibition. J. Xenobiotics 13, 662–684. doi:10.3390/jox13040043
Tamjid, M., Abdolmaleki, A., Mahmoudi, F., and Mirzaee, S. (2023). Neuroprotective effects of Fe3O4 nanoparticles coated with omega-3 as a novel drug for recovery of sciatic nerve injury in rats. Gene Cell Tissue 10, e124110. doi:10.5812/gct-124110
Tapia-Arellano, A., Cabrera, P., Cortés-Adasme, E., Riveros, A., Hassan, N., and Kogan, M. J. (2024). Tau-and α-synuclein-targeted gold nanoparticles: applications, opportunities, and future outlooks in the diagnosis and therapy of neurodegenerative diseases. J. Nanobiotechnology 22, 248. doi:10.1186/s12951-024-02526-0
Tarricone, G., Castagnola, V., Mastronardi, V., Cursi, L., Debellis, D., Ciobanu, D. Z., et al. (2023). Catalytic bioswitch of platinum nanozymes: mechanistic insights of reactive oxygen species scavenging in the neurovascular unit. Nano Lett. 23 (10), 4660–4668. doi:10.1021/acs.nanolett.3c01479
Thanan, R., Oikawa, S., Hiraku, Y., Ohnishi, S., Ma, N., Pinlaor, S., et al. (2014). Oxidative stress and its significant roles in neurodegenerative diseases and cancer. Int. J. Mol. Sci. 16 (1), 193–217. doi:10.3390/ijms16010193
Tirumala, M. G., Anchi, P., Raja, S., Rachamalla, M., and Godugu, C. (2021). Novel methods and approaches for safety evaluation of nanoparticle formulations: a focus towards in vitro models and adverse outcome pathways. Front. Pharmacol. 12, 612659. doi:10.3389/fphar.2021.612659
Tocci, D., Fogel, M., Gupta, V., Kim, P., Latimer, J., Adlimoghaddam, A., et al. (2025). Beyond expectations: investigating nilotinib’s potential in attenuating neurodegeneration in alzheimer’s disease. Alzheimer's Res. and Ther. 17 (1), 60. doi:10.1186/s13195-025-01706-w
Tsakiri, M., Zivko, C., Demetzos, C., and Mahairaki, V. (2022). Lipid-based nanoparticles and RNA as innovative neuro-therapeutics. Front. Pharmacol. 13, 900610. doi:10.3389/fphar.2022.900610
Ucar, A., Parlak, V., Ozgeris, F. B., Yeltekin, A. C., Arslan, M. E., Alak, G., et al. (2022). Magnetic nanoparticles-induced neurotoxicity and oxidative stress in brain of rainbow trout: mitigation by ulexite through modulation of antioxidant, anti-inflammatory, and antiapoptotic activities. Sci. Total Environ. 838, 155718. doi:10.1016/j.scitotenv.2022.155718
Ulanova, M., Poljak, A., Wen, W., Bongers, A., Gloag, L., Gooding, J., et al. (2020). Nanoparticles as contrast agents for the diagnosis of Alzheimer’s disease: a systematic review. Nanomedicine 15 (7), 725–743. doi:10.2217/nnm-2019-0316
Umapathy, S., Pan, I., Issac, P. K., Kumar, M. S. K., Giri, J., Guru, A., et al. (2024). Selenium nanoparticles as neuroprotective agents: insights into molecular mechanisms for Parkinson’s disease treatment. Mol. Neurobiol. 62, 6655–6682. doi:10.1007/s12035-024-04253-x
Umapathy, S., Pan, I., Issac, P. K., Kumar, M. S. K., Giri, J., Guru, A., et al. (2025). Selenium nanoparticles as neuroprotective agents: insights into molecular mechanisms for Parkinson’s disease treatment. Mol. Neurobiol. 62 (6), 6655–6682. doi:10.1007/s12035-025-04253-x
Unnithan, D., Sartaj, A., Iqubal, M. K., Ali, J., and Baboota, S. (2024). A neoteric annotation on the advances in combination therapy for Parkinson’s disease: nanocarrier-based combination approach and future anticipation. Part II: nanocarrier design and development in focus. Expert Opin. Drug Deliv. 21 (3), 437–456. doi:10.1080/17425247.2024.2331216
Vahab, S. A., Ki, A., and Kumar, V. S. (2024). Exploring chitosan nanoparticles for enhanced therapy in neurological disorders: a comprehensive review. Naunyn-Schmiedeberg's Archives Pharmacol., 1–17. doi:10.1007/s00210-024-03507-8
Vahab, S. A., V, V. K., and Kumar, V. S. (2025). Exosome-based drug delivery systems for enhanced neurological therapeutics. Drug Deliv. Transl. Res. 15 (4), 1121–1138. doi:10.1007/s13346-024-01710-x
Verma, N., Sharma, S., Thakur, N., Kaur, N., and Dua, K. (2023). “Nanotherapeutics for alzheimer's disease using metal nanocomposites,” in Metal nanocomposites in nanotherapeutics for oxidative stress-induced metabolic disorders, 372–391.
Vicente-Zurdo, D., Rosales-Conrado, N., and León-González, M. E. (2024). Unravelling the in vitro and in vivo potential of selenium nanoparticles in alzheimer's disease: a bioanalytical review. Talanta 269, 125519. doi:10.1016/j.talanta.2023.125519
Visan, A. I., Popescu-Pelin, G., and Socol, G. (2021). Degradation behavior of polymers used as coating materials for drug delivery—A basic review. Polymers 13 (8), 1272. doi:10.3390/polym13081272
Wang, R. C., and Wang, Z. (2023). Precision medicine: disease subtyping and tailored treatment. Cancers 15, 3837. doi:10.3390/cancers15153837
Wang, Q., Yu, Q., and Wu, M. (2022). Antioxidant and neuroprotective actions of resveratrol in cerebrovascular diseases. Front. Pharmacol. 13, 948889. doi:10.3389/fphar.2022.948889
Waris, A., Ali, A., Khan, A. U., Asim, M., Zamel, D., Fatima, K., et al. (2022). Applications of various types of nanomaterials for the treatment of neurological disorders. Nanomaterials 12 (13), 2140. doi:10.3390/nano12132140
Wei, W., and Wang, X. (2021). Graphene-based electrode materials for neural activity detection. Materials 14, 6170. doi:10.3390/ma14206170
Wei, H., Hu, Y., Wang, J., Gao, X., Qian, X., and Tang, M. (2021). Superparamagnetic iron oxide nanoparticles: cytotoxicity, metabolism, and cellular behavior in biomedicine applications. Int. J. Nanomedicine Vol. 16, 6097–6113. doi:10.2147/ijn.s321984
Wu, Y., and Angelova, A. (2023). Recent uses of lipid nanoparticles, cell-penetrating and bioactive peptides for the development of brain-targeted nanomedicines against neurodegenerative disorders. Nanomaterials 13 (23), 3004. doi:10.3390/nano13233004
Wu, J., Dong, W., Zhang, Z., Liu, J., Akioma, M., Liu, J., et al. (2021). Emerging two-dimensional materials-based diagnosis of neurodegenerative diseases: status and challenges. Nano Today 40, 101284. doi:10.1016/j.nantod.2021.101284
Wu, Y., Rakotoarisoa, M., Angelov, B., Deng, Y., and Angelova, A. (2022). Self-assembled nanoscale materials for neuronal regeneration: a focus on BDNF protein and nucleic acid biotherapeutic delivery. Nanomaterials 12, 2267. doi:10.3390/nano12132267
Wu, D., Chen, Q., Chen, X., Han, F., Chen, Z., and Wang, Y. (2023). The blood–brain barrier: structure, regulation and drug delivery. Signal Transduct. Target. Ther. 8, 217. doi:10.1038/s41392-023-01481-w
Xiong, S., Luo, J., Wang, Q., Li, Z., Li, J., Liu, Q., et al. (2021). Targeted graphene oxide for drug delivery as a therapeutic nanoplatform against parkinson's disease. Biomaterials Sci. 9 (5), 1705–1715. doi:10.1039/d0bm01765e
Yadav, V., Roy, S., Singh, P., Khan, Z., and Jaiswal, A. (2019). 2D MoS2-based nanomaterials for therapeutic, bioimaging, and biosensing applications. Small 15 (1), 1803706. doi:10.1002/smll.201803706
Yadav, V. K., Dhanasekaran, S., Choudhary, N., Nathiya, D., Thakur, V., Gupta, R., et al. (2025). Recent advances in nanotechnology for Parkinson’s disease: diagnosis, treatment, and future perspectives. Front. Med. 12, 1535682. doi:10.3389/fmed.2025.1535682
Yan, S., Na, J., Liu, X., and Wu, P. (2024). Different targeting ligands-mediated drug delivery systems for tumor therapy. Pharmaceutics 16, 248. doi:10.3390/pharmaceutics16020248
Yang, K., Li, Q., Ruan, Y., Xia, Y., and Fang, Z. (2025). Caveolae-mediated transcytosis and its role in neurological disorders. Biomolecules 15, 456. doi:10.3390/biom15040456
Ye, H., Robak, L. A., Yu, M., Cykowski, M., and Shulman, J. M. (2023). Genetics and pathogenesis of Parkinson's syndrome. Annu. Rev. Pathology Mech. Dis. 18, 95–121. doi:10.1146/annurev-pathmechdis-031521-034145
Younes-Mhenni, S., Frih-Ayed, M., Kerkeni, A., Bost, M., and Chazot, G. (2007). Peripheral blood markers of oxidative stress in Parkinson’s disease. Eur. Neurol. 58, 78–83. doi:10.1159/000103641
Yuan, X., Yang, Y., Xia, D., Meng, L., He, M., Liu, C., et al. (2022). Silica nanoparticles promote α-synuclein aggregation and Parkinson’s disease pathology. Front. Neurosci. 15, 807988. doi:10.3389/fnins.2021.807988
Zeng, F., Peng, K., Han, L., and Yang, J. (2021). Photothermal and photodynamic therapies via NIR-Activated nanoagents in combating Alzheimer’s disease. ACS Biomaterials Sci. and Eng. 7, 3573–3585. doi:10.1021/acsbiomaterials.1c00605
Zetusky, W. J., Jankovic, J., and Pirozzolo, F. J. (1985). The heterogeneity of parkinson's disease: clinical and prognostic implications. Neurology 35 (4), 522. doi:10.1212/wnl.35.4.522
Zhang, B., Yan, W., Zhu, Y., Yang, W., LE, W., Chen, B., et al. (2018). Nanomaterials in neural-stem-cell-mediated regenerative medicine: imaging and treatment of neurological diseases. Adv. Mater. 30, 1705694. doi:10.1002/adma.201705694
Zhang, T., Lin, F., Liu, W., Liu, Y., Guo, Z., Xiao, C., et al. (2021a). Reactive oxide species-scavenging lipid-polymer nanoparticles for neuroprotection after spinal cord injury. Appl. Mater. Today 24, 101109. doi:10.1016/j.apmt.2021.101109
Zhang, Y., Yang, H., Wei, D., Zhang, X., Wang, J., Wu, X., et al. (2021b). “Mitochondria- targeted nanoparticles in treatment of neurodegenerative diseases,” in Exploration. Wiley Online Library.20210115
Zhang, L., Mao, L., and Wang, H. (2022). The neuroprotection effects of exosome in central nervous system injuries: a new target for therapeutic intervention. Mol. Neurobiol. 59, 7152–7169. doi:10.1007/s12035-022-03028-6
Zhang, X., Wu, H., Tang, B., and Guo, J. (2024). Clinical, mechanistic, biomarker, and therapeutic advances in GBA1-associated Parkinson’s disease. Transl. Neurodegener. 13, 48. doi:10.1186/s40035-024-00437-6
Zhang, W., Xiao, D., Mao, Q., and Xia, H. (2023). Role of neuroinflammation in neurodegeneration development. Signal Transduct. Target. Ther. 8, 267. doi:10.1038/s41392-023-01486-5
Zhang, X., Song, Y., Gong, H., Wu, C., Wang, B., Chen, W., et al. (2023). Neurotoxicity of titanium dioxide nanoparticles: a comprehensive review. Int. J. Nanomedicine Vol. 18, 7183–7204. doi:10.2147/ijn.s442801
Zhao, J., Xu, N., Yang, X., Ling, G., and Zhang, P. (2022). The roles of gold nanoparticles in the detection of amyloid-β peptide for alzheimer's disease. Colloid Interface Sci. Commun. 46, 100579. doi:10.1016/j.colcom.2021.100579
Zheng, Q., Liu, H., Zhang, H., Han, Y., Yuan, J., Wang, T., et al. (2023). Ameliorating mitochondrial dysfunction of neurons by biomimetic targeting nanoparticles mediated mitochondrial biogenesis to boost the therapy of parkinson's disease. Adv. Sci. 10 (22), 2300758. doi:10.1002/advs.202300758
Zhong, Y., Liu, H., Liu, G., Zhao, L., Dai, C., Liang, Y., et al. (2022). A review on pathology, mechanism, and therapy for cerebellum and tremor in Parkinson’s disease. npj Parkinson's Dis. 8, 82. doi:10.1038/s41531-022-00347-2
Zhong, X., Na, Y., Yin, S., Yan, C., Gu, J., Zhang, N., et al. (2023). Cell membrane biomimetic nanoparticles with potential in treatment of Alzheimer’s disease. Molecules 28, 2336. doi:10.3390/molecules28052336
Zhu, F.-D., Hu, Y.-J., Yu, L., Zhou, X.-G., Wu, J.-M., Tang, Y., et al. (2021). Nanoparticles: a hope for the treatment of inflammation in CNS. Front. Pharmacol. 12, 683935. doi:10.3389/fphar.2021.683935
Keywords: Parkinson’s disease, dopaminergic neurons, nano-structured technologies, nanomedicine, targeted drug delivery, neuroprotection, neurodegeneration, progressive neurodegenerative disorder
Citation: Prasanth MI, Mallya AR, Cho WC and Mundekkad D (2025) Nano-structured strategies in combatting neurodegeneration. Front. Bioeng. Biotechnol. 13:1638668. doi: 10.3389/fbioe.2025.1638668
Received: 31 May 2025; Accepted: 24 September 2025;
Published: 02 December 2025.
Edited by:
Wenbo Zhang, The University of Chicago, United StatesReviewed by:
Nidhi Puranik, Yeungnam University, Republic of KoreaKartikeya Tiwari, Management and Science University, Malaysia
Copyright © 2025 Prasanth, Mallya, Cho and Mundekkad. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Deepa Mundekkad, ZGVlcGFtdW5kZWtrYWRAZ21haWwuY29t
 Mani Iyer Prasanth
Mani Iyer Prasanth Anjali R. Mallya2
Anjali R. Mallya2 William C. Cho
William C. Cho Deepa Mundekkad
Deepa Mundekkad