- 1The Third Department of Orthopedic Surgery, Fuxin Mining General Hospital of Liaoning Health Industry Group, Liaoning, China
- 2Qingdao Film Academy, Qingdao, China
Tendon/ligament (T/L) injuries sustained during motion are highly prevalent and severely impact athletes’ careers and quality of life. Current treatments, including autografts, allografts, and synthetic ligaments, have limitations such as donor site morbidity, immune rejection, and biomechanical mismatch, especially under dynamic loading conditions encountered in motion. 3D bioprinting offers a revolutionary approach for constructing patient-specific T/L grafts. This Mini Review summarizes recent advancements in utilizing 3D bioprinting to fabricate patient-specific grafts for T/L repair, with a particular focus on strategies catering to the functional demands of “in motion” recovery. Key emerging trends in bioink development (balancing mechanical properties with bioactivity), cell selection and optimization, printing strategies (e.g., multi-material hierarchical printing, biomimetic design for complex mechanical loading), and post-printing maturation culture (e.g., multi-modal mechanical stimulation via bioreactors) are discussed. Furthermore, this review highlights critical challenges in the field, including precise matching and long-term maintenance of graft mechanical properties, effective vascularization and innervation, scalable manufacturing and quality control, and hurdles in clinical translation. Finally, this review underscores the immense potential of 3D bioprinting in personalized, functional T/L repair and envisions future research directions, such as the application of smart biomaterials and 4D bioprinting, refined in vitro maturation strategies, and in vivo bioprinting technologies, ultimately aiming to achieve robust tissue functional restoration “in motion.”
1 Introduction
Tendons and ligaments (T/Ls) are crucial connective tissues linking muscles to bones and bones to bones, respectively, essential for joint stability and locomotion (Tachibana et al., 2022). In competitive sports and high-intensity physical activities, T/L injuries such as anterior cruciate ligament (ACL) rupture (Gans et al., 2018), Achilles tendon rupture (Li et al., 2018), and rotator cuff tears are exceedingly common (Doi et al., 2019). These injuries not only cause significant pain and functional impairment but can also terminate athletic careers and substantially diminish quality of life. A specific challenge in repairing these injuries is that the healed tissue must withstand complex and variable dynamic mechanical loads encountered “in motion,” including high stress, high strain rates (Freedman and Mooney, 2019), and sustained fatigue loading, thereby placing stringent demands on the graft’s mechanical properties and biological integration (No et al., 2020).
Current clinical treatments for T/L ruptures primarily involve autografts (Valianatos et al., 2020), allografts (Renshaw et al., 2024), and synthetic grafts (Freedman and Mooney, 2019). Autografts (e.g., hamstring tendon or bone-patellar tendon-bone) eliminate immune rejection risks and possess good biological integration potential (Chen et al., 2025). However, their availability is limited, and harvesting can lead to donor site morbidity (e.g., pain, functional deficit), with graft size and shape often difficult to perfectly match the defect site. Allografts circumvent donor site issues but carry risks of immune rejection, disease transmission (Fishman et al., 2012; Fishman and Grossi, 2014), and potential degradation of biomechanical properties during sterilization and storage, with inflammatory responses potentially affecting long-term stability (Zhang et al., 2022). Synthetic grafts (e.g., polyester materials) can provide initial mechanical strength but are prone to long-term wear and tear, mechanical failure due to mismatch, and chronic inflammation due to suboptimal biocompatibility, lacking biological activity and integration capacity (Rohringer et al., 2023). The inherent limitations of these existing therapeutic modalities underscore the urgent need for novel and effective T/L repair strategies.
In recent years, 3D bioprinting has emerged as a revolutionary technology in tissue engineering and regenerative medicine (Chansoria and Shirwaiker, 2019). By precisely controlling the spatial arrangement of cells, biomaterials, and bioactive factors in three dimensions (Matai et al., 2020), this technology enables the fabrication of tissue/organ substitutes that possess complex anatomical structures and physiological functions (Murphy and Atala, 2014). While other advanced tissue engineering techniques, such as electrospinning, cell sheet engineering, and acellular matrix scaffolds, offer valuable approaches by mimicking specific aspects of native tissues (Xing et al., 2020), 3D bioprinting provides unparalleled advantages. For instance, while electrospinning excels at creating fibrous structures that effectively mimic the extracellular matrix, 3D bioprinting uniquely allows for the achievement of patient-specific macroscopic anatomical structures and precise three-dimensional spatial control over the placement and organization of cells, which is crucial for recreating complex tissue architectures and functions (Yi et al., 2021). Its immense potential in personalized medicine, particularly in manufacturing implants with patient-specific geometries, structures, and biological characteristics, offers new hope for T/L repair. By integrating patient-specific medical imaging data (e.g., MRI, CT), 3D bioprinting can design and manufacture T/L grafts that perfectly match the defect site, thereby promising better restoration of complex functions “in motion” (Wang et al., 2022). This mini review will focus on the latest advancements in 3D bioprinting for constructing patient-specific T/L grafts tailored for “in motion” demands. It will discuss emerging trends in key areas such as biomaterial and bioink innovation, cell source selection and optimization, structural biomimetic design strategies, and post-printing maturation techniques, while also deeply analyzing the major challenges and future prospects in this burgeoning field.
2 Key elements and emerging trends in 3D bioprinting of tendon/ligament grafts
Successful fabrication of 3D bioprinted tendon/ligament grafts hinges on the synergistic optimization of several key factors. Figure 1 illustrates the complete workflow, from patient-specific data acquisition to the culture of a mature construct ready for implantation. Key elements within this process, which have seen considerable progress and debate, include the choice of bioink, determination of the cell source, biomimetic graft design, and post-printing maturation strategies.
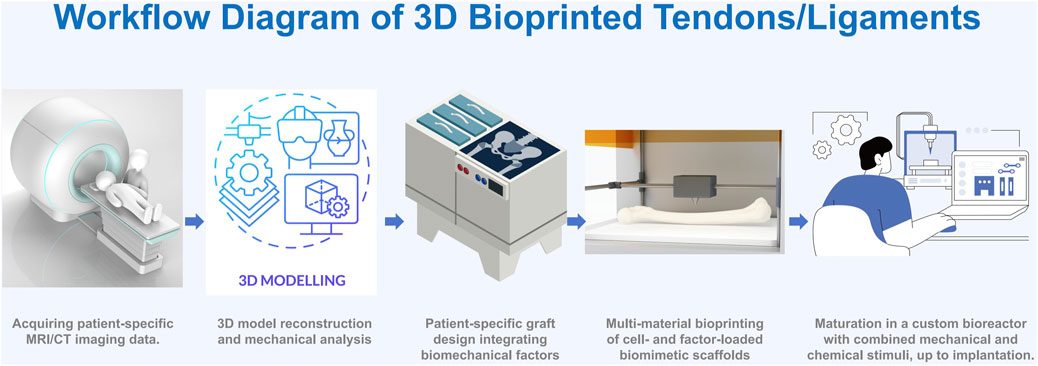
Figure 1. Workflow Diagram of 3D Bioprinted Tendons/Ligaments A schematic illustrating the process from patient imaging (MRI/CT), to 3D modeling and biomechanical analysis, patient-specific graft design, multi-material bioprinting, and maturation in a bioreactor, up to implantation.
2.1 Innovation in bioinks and selection of cell sources
Bioinks play a vital role in 3D bioprinting, as their properties directly influence the feasibility of the printing process and the performance of the final biological structures. In recent years, researchers have made significant progress in developing and optimizing bioinks. For example, studies have shown that the rheological (referring to their flow and deformation properties), physical-mechanical, and biological functionalities of bioinks are key factors affecting their printability and cell viability (Derakhshanfar et al., 2018). Furthermore, the development of multi-component hydrogel bioink systems has opened up new possibilities for enhancing printing accuracy, shape fidelity, and biological functionality (Cui et al., 2020).
In the composition of bioinks, the combination of natural and synthetic materials has been shown to enhance the stability and biocompatibility of bioinks. For example, a composite bioink made from methyl methacrylate-modified xanthan gum and gelatin exhibits excellent shear thinning properties and biocompatibility, which makes it highly suitable for 3D bioprinting with superior printing performance and cell viability (Garcia-Cruz et al., 2021). Moreover, the introduction of nanocomposites offers a new approach to enhancing bioinks by improving their viscosity, printability, and biocompatibility, thus showing significant potential in bone and cartilage tissue engineering (Cai et al., 2022).
In practical applications, the optimization of bioinks involves not only material selection but also adjustments to printing parameters. For instance, studies have shown that by modifying the composition of bioinks and adjusting printing parameters, the shape fidelity and cell viability of printed embryonic stem cells can be significantly enhanced (Ouyang et al., 2016). Furthermore, the microscopic heterogeneity of bioinks has been shown to improve the quality of printed complex structures and cell viability, offering new strategies for high-resolution 3D printing (Maciel et al., 2023).
2.1.1 Natural polymer-based bioinks
Natural polymers such as collagen, gelatin, alginate, silk fibroin, and dECM exhibit excellent biocompatibility, inherent cell recognition, and tissue-specific cues advantageous for T/L repair (Abou Neel et al., 2013; Raftery et al., 2016; Kannan et al., 2025). Research indicates that using natural materials like decellularized extracellular matrix (dECM) and collagen can significantly enhance the biocompatibility of bioinks (Abaci and Guvendiren, 2020), as these materials not only provide the biochemical signals necessary for cell attachment and growth but also mimic the microenvironment of natural tissues. Studies have shown that the rheological, physical-mechanical, and biological functionalities of bioinks are key factors affecting their printability and cell viability. To enhance the printability of bioinks, researchers have developed strategies, including using thermally reversible gelatin networks to temporarily stabilize the bioink (Ouyang et al., 2020). Furthermore, using decellularized matrix-free bioinks can provide a biochemical environment similar to that of natural tissues (Abaci and Guvendiren, 2020). However, significant batch-to-batch variation, difficulty in standardization, immunogenic potential, and relatively weak mechanical properties remain challenges. Despite superior biocompatibility, natural materials (especially dECM) still face challenges such as significant batch-to-batch variation (Hussein et al., 2016), difficulty in standardization, and potential immunogenicity (even after decellularization). Their relatively weak mechanical properties are also a concern (Nakamura et al., 2017). The choice of dECM source (allogeneic, xenogeneic) and optimization of decellularization methods significantly impact the final bioink’s performance and biological effects (Rieder et al., 2016; Kim et al., 2020; de Wit et al., 2023; El-Husseiny et al., 2023), representing a current research focus and point of contention. Some scholars believe that finely tuning dECM composition and degradation products could guide more specific cellular responses and tissue regeneration (Kim and Kim, 2020; Kim et al., 2020). Recent approaches to overcome these include compositing with other biomaterials (e.g., hyaluronic acid, chondroitin sulfate) and refining crosslinking methods (e.g., enzymatic, photo-crosslinking—a method using light to induce material solidification—) to improve mechanical strength and stability (Teixeira et al., 2012).
2.1.2 Synthetic polymer-based bioinks
Synthetic polymers like polycaprolactone (PCL), polylactic acid (PLA), polyglycolic acid (PGA), and their copolymer polylactic-co-glycolic acid (PLGA) see widespread application in tissue engineering contexts that require structural support, owing to their advantageous mechanical properties, tunable degradation rates, and excellent processability. The appropriate mechanical strength and degradation rate are crucial for the application of bioinks; the mechanical strength must be sufficient to support the stability of printed structures, while the degradation rate should match the speed of tissue regeneration. By incorporating components such as nanocellulose and nano-hydroxyapatite, the mechanical properties of bioinks can be significantly enhanced, while maintaining high cell survival rates (Korkeamäki et al., 2025). Improving their inherent bio-inertness and imparting cell adhesiveness often involves combining them with natural hydrogels (such as gelatin or alginate) to create composite bioinks, or modifying their surfaces with cell adhesion peptides like the RGD sequence (Tavafoghi et al., 2020). Emerging synthetic polymers, including certain elastic polyurethanes or poly (glycerol sebacate) (PGS) (Jeffries et al., 2015), are also garnering increased attention due to mechanical properties that more closely mimic natural T/Ls.
Significant challenges and ongoing academic discussions pertain to synthetic polymers. A primary concern is their bio-inertness, which may limit effective cell adhesion and functional expression. Their degradation products, such as acidic byproducts, also pose a risk of triggering local inflammatory responses (da Silva et al., 2018). Academic discussions therefore significantly focus on how to precisely control their degradation rates to align with new tissue formation and how to enhance their biological performance through structural designs like porous architectures and material composites (Zhang et al., 2014). Some researchers propose co-printing with bioactive molecules, for instance, growth factors, as an effective compensatory strategy for addressing the inherent lack of bioactivity in synthetic materials (Longoni et al., 2021).
While synthetic polymers offer tunable mechanical properties crucial for initial load bearing, a key challenge and novel perspective for “in motion” repair lies in designing these bioinks to not only match the dynamic mechanical properties of native T/L tissue but also to adapt over time (Xie et al., 2023). This includes developing synthetic bioinks with programmed viscoelasticity and fatigue resistance that can withstand continuous, complex loading cycles, crucial for robust functional restoration in active scenarios (Van Belleghem et al., 2020). Furthermore, strategies to mitigate their inherent bio-inertness and inflammatory potential under dynamic physiological conditions, such as incorporating mechano-responsive elements or anti-inflammatory agents within their structure, are essential for long-term integration and performance in motion (Shou et al., 2023).
2.1.3 Cell sources and optimization
Tendon stem/progenitor cells (TSPCs), mesenchymal stem cells (MSCs), and induced pluripotent stem cells (iPSCs) represent primary cell sources for constructing T/L grafts, each with distinct advantages and limitations. TSPCs have ideal differentiation potential (Bi et al., 2007); MSCs offer ease of acquisition and immunomodulation but face potential immune rejection (Lohan et al., 2017; Huang et al., 2022); iPSCs provide large-scale autologous cell production but require optimized differentiation protocols (Farkhondeh et al., 2019; Haller et al., 2019). Current research emphasizes enhancing tenogenic differentiation via growth factors, mechanical stimuli, or gene editing (CRISPR/Cas9) (Hsu et al., 2019; Razavi et al., 2024), alongside establishing standardized allogeneic cell banks to manage variability and immunogenic concerns (Hazrati et al., 2022).
Variations in tenogenic differentiation efficiency, immunogenicity (particularly with allogeneic sources), ease of access, and ethical limitations are observed among different cell sources (Subramanian et al., 2017; Oh et al., 2019). Autologous cells, despite being free from immune issues, present limitations in availability, and their quality can be affected by the patient’s age or pathological state. Challenges associated with allogeneic cells, especially MSCs, include potential immune rejection and functional heterogeneity (Jiang and Xu, 2019); consequently, establishing standardized cell banks with good tenogenic potential is recognized as an important developmental direction (Liu et al., 2016). Differing views also exist regarding whether cells should be pre-differentiated towards the T/L lineage before printing, or if an inductive microenvironment should be provided within the printed construct to promote in situ differentiation (Xu et al., 2019; Tu et al., 2023). While optimizing tenogenic differentiation is a foundational goal, a novel and critical perspective for “in motion” T/L repair centers on selecting and engineering cell sources that are intrinsically robust and responsive to the dynamic mechanical cues present during movement (Shojaee et al., 2022). This involves not just achieving basic differentiation but also imbuing cells with enhanced mechanosensing and mechanotransduction capabilities, allowing them to actively participate in the adaptive remodeling of the graft under continuous, variable loads. Future efforts might explore specific genetic or epigenetic modifications, or novel pre-conditioning strategies, to prime cells for optimal survival, integration, and extracellular matrix production that specifically contributes to the dynamic resilience and long-term functional stability required in motion (Lai et al., 2023).
2.1.4 Composite/functionalized bioinks
The development of multi-component hydrogel bioink systems has opened up new possibilities for enhancing printing accuracy, shape fidelity, and biological functionality (Mouser et al., 2020). In the composition of bioinks, the combination of natural and synthetic materials has been shown to enhance the stability and biocompatibility of bioinks. For example, a composite bioink made from methyl methacrylate-modified xanthan gum and gelatin exhibits excellent shear thinning (a property where viscosity decreases under applied shear stress, facilitating smooth extrusion) properties and biocompatibility, which makes it highly suitable for 3D bioprinting with superior printing performance and cell viability (Kozlowska et al., 2020). Moreover, the introduction of nanocomposites offers a new approach to enhancing bioinks by improving their viscosity, printability, and biocompatibility, thus showing significant potential in bone and cartilage tissue engineering (Rodríguez-Padrón et al., 2017). In practical applications, the optimization of bioinks involves not only material selection but also adjustments to printing parameters. For instance, studies have shown that by modifying the composition of bioinks and adjusting printing parameters, the shape fidelity and cell viability of printed embryonic stem cells can be significantly enhanced (Chen et al., 2010). Furthermore, the microscopic heterogeneity of bioinks has been shown to improve the quality of printed complex structures and cell viability, offering new strategies for high-resolution 3D printing (Park et al., 2022). Additionally, using enzyme-induced dynamic degradation methods can gradually release space without affecting cell activity, thereby promoting cell proliferation and the establishment of tissue function (Cianfanelli et al., 2015). The core objective of bioink design is to create a favorable microenvironment for cells. By optimizing the composition and structure of bioinks, it can provide the necessary biochemical and mechanical signals for cell proliferation and differentiation. For instance, using protein-rich bioinks can significantly enhance cell proliferation and responsiveness (Clarke et al., 2017). These methods ensure that the shape of the bioink is maintained and the printing accuracy is maintained during the printing process.
2.2 Structural biomimetic design and post-printing maturation strategies
Native T/L tissue possesses a complex multi-scale, hierarchical, and anisotropic (meaning its properties, like strength or elasticity, vary depending on the direction of measurement) structure, which is fundamental to its superior mechanical properties and physiological functions. 3D bioprinting offers the potential to accurately replicate this complex architecture (Ma et al., 2022; Altunbek et al., 2023).
2.2.1 Multi-scale structural biomimicry
Mimicking the structural features of native tissue at molecular, nano, micro, and macro scales is a crucial goal for ideal T/L grafts (Tachibana et al., 2022). These features encompass the parallel alignment and crimped morphology of collagen fibers, the spindle-shaped and oriented distribution of cells, and the hierarchical assembly from fascicles to tendon bundles to the entire tendon (Laranjeira et al., 2017). Specifically, the tendon-to-bone enthesis displays a pore size gradient from 50 µm in the collagen-rich tendon region to approximately 300 µm in the mineralized fibrocartilage, with the elastic modulus increasing steeply from 200 MPa in tendon to 20 GPa in the bone region, and the osteochondral interface shows a mechanical stiffness increasing from approximately 0.02 MPa (superficial) to 6 MPa (calcified) and up to 15–20 GPa in bone (Khalak et al., 2025). Advanced 3D bioprinting techniques, including multi-nozzle/multi-material printing, microfluidic-assisted bioprinting, near-field direct writing, and hybrid melt-electrowriting, offer powerful tools for constructing T/L scaffolds that possess graded compositions, layered structures, and anisotropic mechanical properties (Altunbek et al., 2023). Computer-aided design (CAD), by integrating patient-specific MRI or CT imaging data, can facilitate patient-specific macroscopic anatomical reconstruction and guide printing paths to control the orientation of internal microstructures, a critical aspect for restoring mechanical transduction “in motion.”
While progress has been achieved in biomimicry at macro and some micro scales, a significant research gap and challenge remain in precisely controlling the orientation of individual cells, alongside the self-assembly and micro-alignment of extracellular matrix (especially collagen fibers) during the printing process (Wang et al., 2021). Such control is essential to achieve functional cell-matrix interactions and effective mechanical transduction, particularly within complex interface regions like the tendon/ligament-bone junction (enthesis).
While significant progress has been achieved in replicating the static multi-scale architecture, a novel perspective for in motion repair focuses on biomimetic designs that ensure structural integrity and dynamic functionality under repetitive and complex physiological loads (Chansoria et al., 2022). This goes beyond mere replication, aiming for architectures that exhibit programmed viscoelasticity, fatigue resistance, and adaptive remodeling capabilities, directly reflecting the demands of natural T/L tissue during continuous movement. Furthermore, the challenge of precisely controlling cell orientation and the self-assembly of extracellular matrix (especially collagen fibers) during printing gains a new dimension when considering the necessity for optimized mechanical transduction and load distribution at tissue interfaces (like the enthesis) during dynamic motion (Pan et al., 2008). These advanced biomimetic designs are paramount for recreating the complex interplay between structure and function essential for robust T/L repair in motion.
2.2.2 Post-printing maturation culture and functionalization
Freshly printed bioconstructs frequently lack the sufficient mechanical strength and biological maturity necessary for direct implantation and withstanding physiological loads, making the post-printing in vitro maturation phase crucial. Bioreactors, through mimicking the in vivo physiological environment and particularly the mechanical stimulus environment (utilizing, for example, uniaxial/multiaxial tensile stretch, perfusion, torsion, or combined mechanical stimuli), can significantly promote cell proliferation, oriented differentiation, and the deposition and remodeling of extracellular matrix, especially Type I collagen (Gensler et al., 2024; Thangadurai et al., 2024). Furthermore, it has been highlighted that without functional vascular networks, the thickness of 3D bioprinted tissue constructs is limited to approximately 1 mm due to diffusional constraints, emphasizing the critical role of promoting angiogenesis, for instance, by encapsulating vascular endothelial growth factor (VEGF) in gelatin micro-particles to achieve controlled delivery for up to 3 weeks and enhance vascularization (Potyondy et al., 2021). This process, in turn, enhances the graft’s mechanical properties, such as elastic modulus, ultimate tensile strength, and toughness, as well as its biological function (Machour et al., 2022). Sequential or targeted addition of growth factors (including TGF-β1/β3, GDF-5/6/7, CTGF, and FGF) to the culture medium has also been demonstrated as an effective method to promote T/L tissue maturation (Zhang et al., 2021).
Determining the optimal parameter combination for mechanical stimulation—encompassing type, frequency, magnitude, duration, loading pattern, and timing of initiation—presents an ongoing challenge, as these parameters are not currently standardized and may vary depending on the cell type used, bioink material, and graft design, thus requiring extensive experimental exploration (Pardo et al., 2022). A further debatable issue is the extent to which in vitro maturation should proceed before implantation. Excessive in vitro maturation carries the risks of increased culture time, cost, and potential contamination, and may also render the graft too “rigid,” thereby limiting its ability to undergo adaptive remodeling within the complex in vivo microenvironment post-implantation (Ng et al., 2020).
While bioreactors significantly promote initial maturation, a novel perspective for in motion repair emphasizes the development of tailored and patient-specific maturation protocols that closely mimic the complex, dynamic, and multi-directional loading conditions encountered during actual movement (Grillner et al., 2020). This pre-conditioning aims to prime the graft for immediate functionality under dynamic physiological loads, not just static strength. Furthermore, the ongoing debate regarding the optimal extent of in vitro maturation before implantation gains new relevance here: the challenge lies in striking a balance that ensures sufficient mechanical competence for early loading (Ménézo et al., 2013), while preserving the graft’s inherent capacity for adaptive remodeling and integration within the dynamic in motion microenvironment post-implantation, ultimately aiming for long-term functional congruence with native tissues during activity.
2.2.3 Emerging and convergent strategies
Beyond the direct biomimicry of structural features and post-printing maturation, the future of T/L tissue engineering may lie in convergent strategies inspired by broader advances in biofabrication. These approaches could address persistent challenges in achieving full biological functionality.
One promising direction is the adoption of hybrid or “bottom-up” assembly strategies. This involves combining 3D printing with self-organizing biological units. For instance, precisely arranged micro-cavities can be printed to guide the self-assembly of cell spheroids into structured tissues, offering enhanced control over local cell density and micro-architecture (Daly and Kelly, 2019). Similarly, integrating organoid technology with bioprinting allows for the creation of more physiologically relevant microenvironments that promote complex cellular interactions and functions (Hu et al., 2025).
Another significant hurdle for engineering large-scale grafts is vascularization. An emerging strategy tackles this by first assembling hundreds of thousands of high-density “organ building blocks” to form a living matrix, followed by printing a perfusable vascular network directly within this construct (Zhang et al., 2020). This method shows potential for rapidly creating patient-specific, vascularized tissues (Ma et al., 2019). Furthermore, novel printing modalities, such as pre-set extrusion bioprinting, are being developed to better control the deposition of multiple materials and cell types simultaneously, which is critical for fabricating heterogeneous structures like the complex tendon-bone junction (Kang et al., 2018). These convergent strategies represent exciting future avenues for creating the next-generation of truly functional T/L grafts.
From an in motion perspective, these approaches offer a novel paradigm for engineering grafts that are not merely structurally sound, but are also inherently designed for dynamic biomechanical integration and adaptive performance within the complex, variable loading environment of active movement (Feller et al., 2017). For instance, hybrid assembly and organoid technologies enable the creation of microenvironments that promote more physiologically relevant cellular interactions and matrix remodeling under dynamic stimuli (Kim and Kim, 2020). Furthermore, advanced vascularization strategies are crucial for sustaining long-term cellular viability and metabolic function under the heightened demands of motion, while novel multi-material printing modalities are key to fabricating interfaces that can efficiently and robustly transmit complex loads encountered in motion (Krishnan et al., 2014). These collective efforts aim to ensure the graft’s resilience, adaptive capacity, and seamless functional restoration throughout a patient’s dynamic life.
3 Discussion
Significant advancements in 3D bioprinting—encompassing bioink development, cell application strategies, structural biomimetic design, and post-printing maturation culture—have created unprecedented opportunities for constructing functional, patient-specific tendon/ligament grafts. This technology holds particular promise for addressing the shortcomings of traditional treatments in restoring complex functions “in motion,” offering new hope for T/L injury repair. However, successfully translating this technology from bench to bedside still faces numerous challenges and unresolved scientific questions.
3.1 Summary of major progress
3D bioprinting allows researchers to control the macro-morphology and micro-architecture of T/L grafts with unprecedented precision, and to integrate cells and bioactive factors, thereby constructing substitutes that more closely resemble native tissues. Bioinks are evolving from single-component to composite and functionalized formulations (Amaral et al., 2021); cell sources are expanding from traditional adult cells to stem cells and genetically engineered cells (Chae et al., 2022); printing strategies are progressing from simple structures to complex biomimetic designs (Loukelis et al., 2024); and in vitro maturation methods are shifting from static culture to functionalization culture under dynamic mechanical stimulation (Hull et al., 2021). These collective advancements are driving 3D bioprinted T/L grafts towards functionalization and personalization (Duan, 2016).
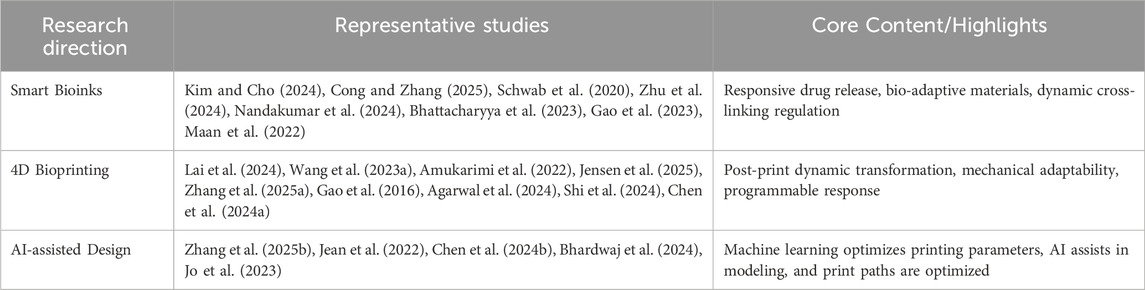
Furthermore, The development of smart bioinks has been particularly rapid, with some studies utilizing them to achieve responsive drug release cued by the in vivo microenvironment (Maan et al., 2022), while others have achieved self-adapting material properties through dynamic crosslinking regulation. Further advancements include the use of smart designs for hybrid bioprinting of scalable and viable tissue constructs and the fabrication of complex tissue scaffolds with in situ homogeneously mixed bioinks using advanced portable biopen technology (Schwab et al., 2020). Alongside smart bioinks, 4D bioprinting is emerging as a transformative strategy, focusing on post-print dynamic transformation, mechanical adaptability (Gao et al., 2016), and programmable responses, including biocompatible composite hydrogels with on-demand swelling-shrinking properties and multimaterial 3D and 4D bioprinting for heterogeneous constructs. Additionally, artificial intelligence (AI) is playing an increasingly pivotal role in AI-assisted design (J et al., 2022), where machine learning optimizes printing parameters, AI assists in modeling, and print paths are optimized, leading to more efficient and precise personalized treatment regimens. For a more comprehensive and extensive list of relevant studies applications, please refer to Table 1.
3.2 Current research gaps
3.2.1 Perfect match and dynamic response of mechanical properties
Achieving an initial mechanical strength, anisotropy, viscoelasticity, and long-term dynamic mechanical behavior (e.g., fatigue resistance, creep, stress relaxation) in printed grafts that closely match native T/L tissue—to withstand complex, sustained, and variable mechanical loads “in motion”—remains a core challenge. This requires breakthroughs not only in materials science but also a deeper understanding of T/L biomechanics and injury repair mechanisms (Freeman and Kelly, 2017; Li et al., 2022).
3.2.2 Vascularization and innervation
For larger-sized 3D bioprinted T/L grafts, the long-term survival and function of internal cells heavily depend on rapid and effective vascularization. Although some strategies have been attempted (e.g., co-culturing endothelial cells, pre-fabricating vascular networks, embedding pro-angiogenic factors), achieving a functional, stable capillary network that eventually anastomoses with the host vascular system remains a formidable challenge (Cui et al., 2016a; Gold et al., 2021). For example, in pre-fabricating vascular networks, approaches include using sacrificial bioinks to print perfusable channels within the main construct, which are then removed after printing is complete to form a vascular network (J et al., 2022), as demonstrated in strategies aiming to create vascularized tissue models. Furthermore, embedding pro-angiogenic factors can involve incorporating biologically inspired smart release systems within 3D bioprinted scaffolds to promote vascularized tissue regeneration (Cui et al., 2016b). Innervation, crucial for proprioception, coordinated movement, and tissue homeostasis, is currently a very nascent area of research in this context (Mörö et al., 2022). Despite the limited current research, potential strategies for achieving functional innervation could include borrowing from nerve tissue engineering to explore co-culturing with neural stem cells, integrating neurotrophic factors into the bioink, or utilizing conductive biomaterials to guide neurite outgrowth and establish functional connections. These promising avenues represent crucial future research hotbeds, as successful innervation would profoundly enhance graft functionality, enabling more natural proprioception, coordinated movement, and overall tissue long-term homeostasis within dynamic “in motion” scenarios.
3.2.3 Interface tissue engineering
The tendon/ligament-bone interface (enthesis) is a transitional zone with a complex graded structure (from tendon/ligament to uncalcified fibrocartilage, calcified fibrocartilage, and then bone) and unique mechanical properties. Its functional regeneration is critical for stable graft anchorage and effective mechanical load transmission. Reconstructing this multi-tissue, multi-phase interface using 3D bioprinting remains extremely challenging (Altunbek et al., 2023; Du et al., 2023).
3.2.4 Immunomodulation and inflammatory response
Even when using autologous cells, the biomaterials themselves, the printing process, and surgical trauma can trigger host immune and inflammatory responses. Modulating the immune microenvironment at the implantation site to suppress destructive inflammation and promote constructive tissue remodeling rather than fibrosis or heterotopic ossification is key to ensuring long-term graft success (Du et al., 2024).
3.3 Different Schools of thought or controversies
3.3.1 “Structure-first” vs “cell/bioactivity-first”
There are differing emphases regarding the core design philosophy for 3D bio-printed grafts. Some scholars emphasize perfectly replicating the multi-scale complex structure of native T/L tissue through precise printing techniques, believing structure is the foundation of function (Altunbek et al., 2023). Others argue that providing the appropriate cell types and a bioactive microenvironment capable of inducing cell differentiation and matrix production is more critical, trusting that cells, under proper guidance, will actively remodel and form a functional structure (Yang et al., 2022b). An ideal strategy likely involves an organic combination of both.
3.3.2 In Vitro maturation vs in vivo remodeling
There are varied opinions on the extent to which 3D bioprinted T/L grafts should be matured in vitro before implantation. Some advocate that grafts should achieve mechanical properties and biological maturity comparable to native tissue through in vitro mechanical stimulation before implantation, ensuring they can withstand early physiological loads (Sun et al., 2020; Yang et al., 2022a). However, excessive or prolonged in vitro maturation not only increases culture costs, time, and potential contamination risks but might also render the graft too “static,” limiting its capacity for adaptive remodeling within the complex in vivo microenvironment post-implantation (Chen et al., 2020). Finding the optimal balance between in vitro pre-maturation and in vivo dynamic remodeling is an important future research direction.
3.4 Challenges faced
3.4.1 Scale-up and standardized production
Most current 3D bioprinting T/L research is still at the laboratory stage. To achieve clinical application, reproducible, cost-effective, and scalable production compliant with Good Manufacturing Practice (GMP) standards must be addressed, including standardized bioink preparation, precise control of the printing process, and quality monitoring (Stanco et al., 2020).
3.4.2 Regulatory approval pathways
The regulatory approval pathway for 3D bioprinted T/L grafts, as novel medical products combining cells, biomaterials, and engineered structures (potentially classified as Advanced Therapy Medicinal Products (ATMPs) or Tissue Engineered Medical Products (TEMPs)), is not yet fully clear or harmonized globally. This requires collaborative efforts from regulatory agencies, researchers, and industry to establish clear evaluation criteria and approval processes (Ying et al., 2018).
3.4.3 Lack of long-term in vivo studies
The vast majority of studies are still limited to in vitro experiments or small animal models, which cannot fully replicate the complex pathophysiology of human T/L injuries or the mechanical loads experienced in motion. There is an urgent need for long-term in vivo functional evaluations in large animal models (such as canines, ovines, porcines, or non-human primates) that more closely mimic human physiological conditions, to validate the safety and efficacy of 3D bioprinted grafts under realistic mechanical loading (Gold et al., 2021).
3.5 Ethical considerations
3.5.1 Ethics of cell sourcing
The rapid advancement of 3D bioprinting technology, particularly for patient-specific grafts, necessitates a robust consideration of various ethical dimensions that extend beyond purely translational challenges. Firstly, the ethics of cell sourcing present significant considerations. While autologous cells offer immune compatibility, their limited availability and variability based on patient age or pathological state are notable (Bell et al., 2017). The increasing reliance on induced pluripotent stem cells (iPSCs) provides a scalable autologous source, yet raises questions concerning the ethical implications of their derivation and manipulation (Carelli et al., 2022), echoing some debates associated with embryonic stem cells. Similarly, the use of allogeneic cell banks, particularly mesenchymal stem cells, while addressing scalability, introduces challenges related to potential immune rejection and the ethical complexities of donor consent, anonymity, and commercialization of biological materials (Parekkadan and Milwid, 2010).
3.5.2 Data privacy and informed consent in personalized therapy
Ensuring data privacy and patient informed consent is paramount in the realm of personalized therapy. The design and fabrication of patient-specific grafts heavily rely on sensitive medical imaging data (e.g., MRI, CT) and potentially genetic information (Chen et al., 2022). Robust frameworks are required to protect this highly personal data from breaches and misuse. Furthermore, given the innovative and experimental nature of 3D bioprinting in clinical applications, comprehensive informed consent processes must clearly articulate the potential risks, benefits, and uncertainties to patients (Hsu and Jiang, 2019), ensuring their understanding and voluntary participation in such advanced therapeutic regimens.
3.5.3 Cost, accessibility, and healthcare equity
The potential cost and accessibility issues associated with such a highly advanced technology pose significant ethical dilemmas regarding healthcare inequalities (Lakdawala et al., 2013). The development and standardized production of 3D bioprinted T/L grafts are likely to be expensive, raising concerns about equitable access for all patients who could benefit. Addressing how to mitigate these cost barriers and ensure that these life-changing therapies do not exacerbate existing disparities in healthcare access is a critical ethical challenge that requires proactive policy development and collaborative efforts among researchers, industry, and healthcare systems (Arasaradnam, 2001).
3.5.4 Regulatory and safety considerations for gene-edited cells
The growing incorporation of gene-edited cells, such as iPSCs modified via CRISPR/Cas9, into 3D bioprinted grafts introduces specific clinical risks that demand robust regulatory oversight (Huo et al., 2022). A primary concern is the potential for off-target effects, where unintended genomic changes could lead to unforeseen consequences, altered cell behavior, immunogenicity by presenting novel antigens, or even tumorigenicity through disruption of regulatory pathways (Tang et al., 2019). Addressing these intricate safety profiles necessitates a stringent regulatory framework. Bodies like the European Medicines Agency and its Committee for Advanced Therapies establish rigorous guidelines for Advanced Therapy Medicinal Products, requiring extensive preclinical safety evaluations (including genome integrity, immunogenicity, and tumorigenic potential), strict manufacturing quality control, and meticulously designed clinical trials with long-term follow-up to ensure safety and efficacy before clinical translation (Vanessa et al., 2021).
4 Potential future developments in the field
Looking ahead, several transformative areas are poised to shape the future of 3D bioprinting for T/L repair, encompassing innovations from smart materials to advanced integration strategies (Figure 2).
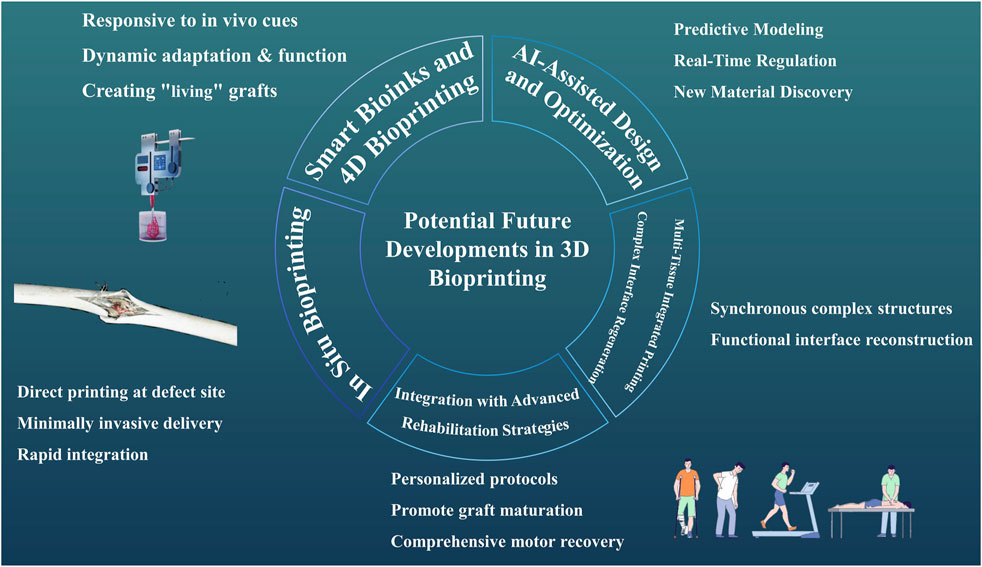
Figure 2. Potential Future Developments in 3D Bioprinting A picture illustrates potential future developments in 3D bioprinting for tendon/ligament (T/L) repair, encompassing smart bioinks and 4D bioprinting, in situ bioprinting, AI-assisted design and optimization, multi-tissue integrated printing, and integration with advanced rehabilitation strategies.
4.1 Smart bioinks and 4D bioprinting
Developing smart bioinks capable of responding to in vivo microenvironmental cues (e.g., pH, temperature, specific enzymes, mechanical signals) by undergoing programmed deformation, releasing bioactive substances, or altering their mechanical properties, combined with 4D bioprinting strategies (i.e., 3D printed structures that change shape or function over time in a pre-programmed manner), promises to create “living” grafts that can better adapt to and participate in the dynamic repair process “in motion” (Maan et al., 2022; Wang et al., 2023a; Abolhassani et al., 2025).
4.2 In Situ bioprinting
Exploring techniques for direct 3D bioprinting at the site of T/L defect in a patient, delivering printing devices minimally invasively to the injury site, and potentially using the patient’s own cells and tissue fluids as part of the printing material This could minimize surgical trauma and promote rapid integration of the graft with host tissues (Macadam et al., 2022; Wang et al., 2023b).
4.3 AI-assisted design and optimization
Utilizing artificial intelligence (AI) and machine learning algorithms, based on extensive experimental data and clinical imaging information, to assist in optimizing bioink formulations, printing parameters, 3D graft structural design, and even predicting graft performance and remodeling processes in vivo, thereby enabling more efficient and precise personalized treatment regimens (Ning et al., 2023; Chen et al., 2024b). Specifically, AI/machine learning can be used not only to optimize printing pathways and parameters but also for: Predictive Modeling: Predicting the mechanical properties and biological functions of the final graft based on bioink composition, cell type, and culture conditions; Real-Time Regulation: Integrating sensors within bioreactors to analyze tissue maturation status in real-time and dynamically adjust mechanical or chemical stimulation protocols; New Material Discovery: Assisting in the design and screening of novel bioink formulations with ideal properties by analyzing vast amounts of literature and experimental data.
4.4 Multi-tissue integrated printing and complex interface regeneration
Developing technologies capable of synchronously printing complex multi-tissue structures containing various cell types (e.g., fibroblasts, chondrocytes, osteoblasts) and different matrix components, to achieve functional reconstruction of complex interfaces like the tendon/ligament-bone junction in a single printing process (Murali and Parameswaran, 2024).
4.5 Integration with advanced rehabilitation strategies
Future research should increasingly focus on combining post-implantation of 3D bio-printed grafts with personalized, biomechanics-informed advanced rehabilitation protocols. By precisely controlling the post-operative mechanical environment, this synergy can promote functional maturation of the graft and comprehensive recovery of the patient’s motor abilities (Daikuara et al., 2021; Rahimnejad et al., 2021).
5 Conclusion
3D bioprinting is poised to revolutionize tendon and ligament (T/L) repair, offering a powerful alternative to traditional grafts by fabricating patient-specific, bioactive, and biomimetic constructs essential for functional recovery “in motion.” Despite significant advances in bioinks, cell integration, and structural design, the field still faces critical challenges, including the need to perfectly match dynamic mechanical properties, ensure long-term viability through vascularization and innervation, and overcome hurdles in scalable manufacturing and clinical translation. Looking forward, emerging technologies like smart materials, 4D bioprinting, and AI-assisted design will be key to surmounting these obstacles. Ultimately, the goal of 3D bioprinting is to shift the treatment paradigm from simple repair to true biological and functional regeneration, enabling patients to fully return to their dynamic lives.
Author contributions
XB: Software, Methodology, Writing – original draft, Investigation, Conceptualization, Data curation, Formal Analysis. YY: Formal Analysis, Conceptualization, Writing – original draft. JC: Conceptualization, Writing – original draft, Visualization. YD: Investigation, Writing – original draft, Software. ML: Writing – original draft, Investigation, Methodology. HY: Funding acquisition, Writing – review and editing, Project administration, Supervision, Conceptualization, Validation.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by the Liaoning Provincial Applied Basic Research Program funded by the Liaoning Provincial Department of Science and Technology (Grant No. 2023JH2/101300099; Project Title: Research and Development of Visualization and Precise Positioning Related Technologies for Posterior Cruciate Ligament Reconstruction Surgical System of the Knee Joint); the Liaoning Provincial Science and Technology Plan Joint Program (Applied Basic Research Project) (Grant No. 2023JH2/101700217; Project Title: Establishment and Clinical Application Research of a Precision System Based on the Research and Development of Knee Arthroscopic Surgical Instruments and Improvement of Surgical Methods); and the Liaojian Group Fuxin Mining General Hospital-level Scientific Research Project (2024) (Grant No. FXKZYY—202301).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Abaci, A., and Guvendiren, M. (2020). Designing decellularized extracellular matrix-based bioinks for 3D bioprinting. Adv. Healthc. Mater. 9, e2000734. doi:10.1002/adhm.202000734
Abolhassani, S., Fattahi, R., Safshekan, F., Saremi, J., and Hasanzadeh, E. (2025). Advances in 4D bioprinting: the next frontier in regenerative medicine and tissue engineering applications. Adv. Healthc. Mater. 14, e2403065. doi:10.1002/adhm.202403065
Abou Neel, E. A., Bozec, L., Knowles, J. C., Syed, O., Mudera, V., Day, R., et al. (2013). Collagen--emerging collagen based therapies hit the patient. Adv. Drug Deliv. Rev. 65, 429–456. doi:10.1016/j.addr.2012.08.010
Agarwal, P., Mathur, V., Kasturi, M., Srinivasan, V., Seetharam, R. N., and S Vasanthan, K. (2024). A futuristic development in 3D printing technique using nanomaterials with a step toward 4D printing. ACS Omega 9, 37445–37458. doi:10.1021/acsomega.4c04123
Altunbek, M., Afghah, F., Koc, B., Yoo, J. J., and Caliskan, O. S. (2023). Design and bioprinting for tissue interfaces. Biofabrication 15, 022002. doi:10.1088/1758-5090/acb73d
Amaral, A. J. R., Mano, J. F., Lavrador, P., and Gaspar, V. M. (2021). Double network laminarin-boronic/alginate dynamic bioink for 3D bioprinting cell-laden constructs. Biofabrication 13, 035045. doi:10.1088/1758-5090/abfd79
Amukarimi, S., Rezvani, Z., Eghtesadi, N., and Mozafari, M. (2022). Smart biomaterials: from 3D printing to 4D bioprinting. Methods (san Diego Calif,) 205, 191–199. doi:10.1016/j.ymeth.2022.07.006
Arasaradnam, R., and Walker, T. (2001). Ethical dilemmas? Clin. Med. Lond. Engl. 1, 515–516. doi:10.7861/clinmedicine.1-6-515b
Bell, G. M., Anderson, A. E., Diboll, J., Reece, R., Eltherington, O., Harry, R. A., et al. (2017). Autologous tolerogenic dendritic cells for rheumatoid and inflammatory arthritis. Ann. Rheum. Dis. 76, 227–234. doi:10.1136/annrheumdis-2015-208456
Bhardwaj, N., Sood, M., and Gill, S. S. (2024). 3D-bioprinting and AI-empowered anatomical structure designing: a review. Curr. Med. Imaging 20, e15734056259274. doi:10.2174/0115734056259274231019061329
Bhattacharyya, A., Ham, H.-W., Sonh, J., Gunbayar, M., Jeffy, R., Nagarajan, R., et al. (2023). 3D bioprinting of complex tissue scaffolds with in situ homogeneously mixed alginate-chitosan-kaolin bioink using advanced portable biopen. Carbohydr. Polym. 317, 121046. doi:10.1016/j.carbpol.2023.121046
Bi, Y., Ehirchiou, D., Kilts, T. M., Inkson, C. A., Embree, M. C., Sonoyama, W., et al. (2007). Identification of tendon stem/progenitor cells and the role of the extracellular matrix in their niche. Nat. Med. 13, 1219–1227. doi:10.1038/nm1630
Cai, Y., Chang, S. Y., Gan, S. W., Ma, S., Lu, W. F., and Yen, C.-C. (2022). Nanocomposite bioinks for 3D bioprinting. Acta Biomater. 151, 45–69. doi:10.1016/j.actbio.2022.08.014
Carelli, V., Hirano, M., Enríquez, J. A., and Chinnery, P. F. (2022). Implications of mitochondrial DNA mutations in human induced pluripotent stem cells. Nat. Rev. Genet. 23, 69–70. doi:10.1038/s41576-021-00430-z
Chae, S., Cho, D.-W., and Choi, Y.-J. (2022). Mechanically and biologically promoted cell-laden constructs generated using tissue-specific bioinks for tendon/ligament tissue engineering applications. Biofabrication 14, 025013. doi:10.1088/1758-5090/ac4fb6
Chansoria, P., and Shirwaiker, R. (2019). Characterizing the process physics of ultrasound-assisted bioprinting. Sci. Rep. 9, 13889. doi:10.1038/s41598-019-50449-w
Chansoria, P., Etter, E. L., and Nguyen, J. (2022). Regenerating dynamic organs using biomimetic patches. Trends Biotechnol. 40, 338–353. doi:10.1016/j.tibtech.2021.07.001
Chen, G., Hou, Z., Gulbranson, D. R., and Thomson, J. A. (2010). Actin-myosin contractility is responsible for the reduced viability of dissociated human embryonic stem cells. Cell Stem Cell 7, 240–248. doi:10.1016/j.stem.2010.06.017
Chen, Y., Tao, J., Wei, X., Huang, Y., Li, Y., Qian, Z., et al. (2020). Noninvasive in vivo 3D bioprinting. Sci. Adv. 6, eaba7406. doi:10.1126/sciadv.aba7406
Chen, H., Gomez, C., Huang, C.-M., and Unberath, M. (2022). Explainable medical imaging AI needs human-centered design: guidelines and evidence from a systematic review. npj Digit. Med. 5, 156. doi:10.1038/s41746-022-00699-2
Chen, A., Wang, W., Mao, Z., He, Y., Chen, S., Liu, G., et al. (2024a). Multimaterial 3D and 4D bioprinting of heterogenous constructs for tissue engineering. Adv. Mater. (deerf. Beach Fla,) 36, e2307686. doi:10.1002/adma.202307686
Chen, H., Zhang, B., and Huang, J. (2024b). Recent advances and applications of artificial intelligence in 3D bioprinting. Biophys. Rev. 5, 031301. doi:10.1063/5.0190208
Chen, K., Zhu, M., Zhang, C., Zhang, J., Zhang, Y., Li, S., et al. (2025). What is the clinical efficacy of combined semitendinosus autograft reconstruction and scar tissue repair for chronic irreparable patellar tendon rupture? Clin. Orthop. doi:10.1097/CORR.0000000000003471
Cianfanelli, V., Fuoco, C., Lorente, M., Salazar, M., Quondamatteo, F., Gherardini, P. F., et al. (2015). AMBRA1 links autophagy to cell proliferation and tumorigenesis by promoting c-myc dephosphorylation and degradation. Nat. Cell Biol. 17, 20–30. doi:10.1038/ncb3072
Clarke, M., Volpe, G., Sheriff, L., Walton, D., Ward, C., Wei, W., et al. (2017). Transcriptional regulation of SPROUTY2 by MYB influences myeloid cell proliferation and stem cell properties by enhancing responsiveness to IL-3. Leukemia 31, 957–966. doi:10.1038/leu.2016.289
Cong, B., and Zhang, H. (2025). Innovative 3D printing technologies and advanced materials revolutionizing orthopedic surgery: current applications and future directions. Front. Bioeng. Biotechnol. 13, 1542179. doi:10.3389/fbioe.2025.1542179
Cui, H., Holmes, B., Zhang, L. G., and Zhu, W. (2016a). Biologically inspired smart release system based on 3D bioprinted perfused scaffold for vascularized tissue regeneration. Adv. Sci. 3, 1600058. doi:10.1002/advs.201600058
Cui, H., Zhu, W., Holmes, B., and Zhang, L. G. (2016b). 3D bioprinting: biologically inspired smart release system based on 3D bioprinted perfused scaffold for vascularized tissue regeneration (Adv. Sci. 8/2016). Adv. Sci. 3, advs.201670043. doi:10.1002/advs.201670043
Cui, X., Li, J., Hartanto, Y., Durham, M., Tang, J., Zhang, H., et al. (2020). Advances in extrusion 3D bioprinting: a focus on multicomponent hydrogel-based bioinks. Adv. Healthc. Mater. 9, e1901648. doi:10.1002/adhm.201901648
da Silva, D., Kaduri, M., Poley, M., Adir, O., Krinsky, N., Shainsky-Roitman, J., et al. (2018). Biocompatibility, biodegradation and excretion of polylactic acid (PLA) in medical implants and theranostic systems. Chem. Eng. J. (lausanne Switz. 1996) 340, 9–14. doi:10.1016/j.cej.2018.01.010
Daikuara, L. Y., Fear, M. W., Yue, Z., Chen, X., Skropeta, D., Wallace, G. G., et al. (2021). 3D bioprinting constructs to facilitate skin regeneration. Adv. Funct. Mater. 32, 2105080. doi:10.1002/adfm.202105080
Daly, A. C., and Kelly, D. J. (2019). Biofabrication of spatially organised tissues by directing the growth of cellular spheroids within 3D printed polymeric microchambers. Biomaterials 197, 194–206. doi:10.1016/j.biomaterials.2018.12.028
de Wit, R. J. J., van Dis, D. J., Bertrand, M. E., Tiemessen, D., Siddiqi, S., Oosterwijk, E., et al. (2023). Scaffold-based tissue engineering: supercritical carbon dioxide as an alternative method for decellularization and sterilization of dense materials. Acta Biomater. 155, 323–332. doi:10.1016/j.actbio.2022.11.028
Derakhshanfar, S., Mbeleck, R., Xu, K., Zhang, X., Zhong, W., and Xing, M. (2018). 3D bioprinting for biomedical devices and tissue engineering: a review of recent trends and advances. Bioact. Mater 3, 144–156. doi:10.1016/j.bioactmat.2017.11.008
Doi, N., Izaki, T., Miyake, S., Shibata, T., Ishimatsu, T., Shibata, Y., et al. (2019). Intraoperative evaluation of blood flow for soft tissues in orthopaedic surgery using indocyanine green fluorescence angiography: a pilot study. Bone Jt. Res. 8, 118–125. doi:10.1302/2046-3758.83.BJR-2018-0151.R1
Du, L., Wu, J., Zhang, H., Huan, Z., Xiao, Y., Wang, Y., et al. (2023). Multicellular bioprinting of biomimetic inks for tendon-to-bone regeneration. Adv. Sci. 10, 2301309. doi:10.1002/advs.202301309
Du, L., Wu, J., Wu, C., and Han, Y. (2024). Immunomodulatory multicellular scaffolds for tendon-to-bone regeneration. Sci. Adv. 10, eadk6610. doi:10.1126/sciadv.adk6610
Duan, B. (2016). State-of-the-art review of 3D bioprinting for cardiovascular tissue engineering. Ann. Biomed. Eng. 45, 195–209. doi:10.1007/s10439-016-1607-5
El-Husseiny, H. M., Mady, E. A., Kaneda, M., Shimada, K., Nakazawa, Y., Usui, T., et al. (2023). Comparison of bovine- and porcine-derived decellularized biomaterials: promising platforms for tissue engineering applications. Pharmaceutics 15, 1906. doi:10.3390/pharmaceutics15071906
Farkhondeh, A., Li, R., Gorshkov, K., Chen, K. G., Might, M., Rodems, S., et al. (2019). Induced pluripotent stem cells for neural drug discovery. Drug Discov. Today 24, 992–999. doi:10.1016/j.drudis.2019.01.007
Feller, L., Khammissa, R. A. G., and Lemmer, J. (2017). Biomechanical cell regulatory networks as complex adaptive systems in relation to cancer. Cancer Cell Int. 17, 16. doi:10.1186/s12935-017-0385-y
Fishman, J. A., and Grossi, P. A. (2014). Donor-derived infection--the challenge for transplant safety. Nat. Rev. Nephrol. 10, 663–672. doi:10.1038/nrneph.2014.159
Fishman, J. A., Greenwald, M. A., and Grossi, P. A. (2012). Transmission of infection with human allografts: essential considerations in donor screening. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 55, 720–727. doi:10.1093/cid/cis519
Freedman, B. R., and Mooney, D. J. (2019). Biomaterials to mimic and heal connective tissues. Adv. Mater. (deerf. Beach Fla,) 31, e1806695. doi:10.1002/adma.201806695
Freeman, F. E., and Kelly, D. J. (2017). Tuning alginate bioink stiffness and composition for controlled growth factor delivery and to spatially direct MSC fate within bioprinted tissues. Sci. Rep. 7, 17042. doi:10.1038/s41598-017-17286-1
Gans, I., Retzky, J. S., Jones, L. C., and Tanaka, M. J. (2018). Epidemiology of recurrent anterior cruciate ligament injuries in national collegiate athletic association sports: the injury surveillance program, 2004-2014. Orthop. J. Sports Med. 6, 2325967118777823. doi:10.1177/2325967118777823
Gao, B., Yang, Q., Zhao, X., Jin, G., Ma, Y., and Xu, F. (2016). 4D bioprinting for biomedical applications. Trends Biotechnol. 34, 746–756. doi:10.1016/j.tibtech.2016.03.004
Gao, Q., Lee, J.-S., Kim, B. S., and Gao, G. (2023). Three-dimensional printing of smart constructs using stimuli-responsive biomaterials: a future direction of precision medicine. Int. J. Bioprinting 9, 638. doi:10.18063/ijb.v9i1.638
Garcia-Cruz, M. R., Postma, A., Frith, J. E., and Meagher, L. (2021). Printability and bio-functionality of a shear thinning methacrylated xanthan-gelatin composite bioink. Biofabrication 13, 035023. doi:10.1088/1758-5090/abec2d
Gensler, M., Malkmus, C., Ockermann, P., Möllmann, M., Hahn, L., Salehi, S., et al. (2024). Perfusable tissue bioprinted into a 3D-printed tailored bioreactor system. Bioeng. (basel Switz.) 11, 68. doi:10.3390/bioengineering11010068
Gold, K. A., Saha, B., Gaharwar, A. K., Cooke, J. P., Jo, J., Pandian, N. K. R., et al. (2021). 3D bioprinted multicellular vascular models. Adv. Healthc. Mater. 10, 2101141. doi:10.1002/adhm.202101141
Grillner, S., Robertson, B., and Kotaleski, J. H. (2020). Basal ganglia-a motion perspective. Compr. Physiol. 10, 1241–1275. doi:10.1002/cphy.c190045
Haller, C., Piccand, J., De Franceschi, F., Ohi, Y., Bhoumik, A., Boss, C., et al. (2019). Macroencapsulated human iPSC-derived pancreatic progenitors protect against STZ-induced hyperglycemia in mice. Stem Cell Rep. 12, 787–800. doi:10.1016/j.stemcr.2019.02.002
Hazrati, A., Malekpour, K., Soudi, S., and Hashemi, S. M. (2022). CRISPR/Cas9-engineered mesenchymal stromal/stem cells and their extracellular vesicles: a new approach to overcoming cell therapy limitations. Biomed. Pharmacother. = Biomed. Pharmacother. 156, 113943. doi:10.1016/j.biopha.2022.113943
Hsu, L., and Jiang, X. (2019). “living” inks for 3D bioprinting. Trends Biotechnol. 37, 795–796. doi:10.1016/j.tibtech.2019.04.014
Hsu, M.-N., Chang, Y.-H., Truong, V. A., Lai, P.-L., Nguyen, T. K. N., and Hu, Y.-C. (2019). CRISPR technologies for stem cell engineering and regenerative medicine. Biotechnol. Adv. 37, 107447. doi:10.1016/j.biotechadv.2019.107447
Hu, Y., Zhu, T., Cui, H., and Cui, H. (2025). Integrating 3D bioprinting and organoids to better recapitulate the complexity of cellular microenvironments for tissue engineering. Adv. Healthc. Mater. 14, e2403762. doi:10.1002/adhm.202403762
Huang, Y., Wu, Q., and Tam, P. K. H. (2022). Immunomodulatory mechanisms of mesenchymal stem cells and their potential clinical applications. Int. J. Mol. Sci. 23, 10023. doi:10.3390/ijms231710023
Hull, S. M., Brunel, L. G., and Heilshorn, S. C. (2021). 3D bioprinting of cell-laden hydrogels for improved biological functionality. Adv. Mater. 34, 2103691. doi:10.1002/adma.202103691
Huo, Y., Xu, Y., Wu, X., Gao, E., Zhan, A., Chen, Y., et al. (2022). Functional trachea reconstruction using 3D-bioprinted native-like tissue architecture based on designable tissue-specific bioinks (adv. Sci. 29/2022). Adv. Sci. 9, 2270184. doi:10.1002/advs.202270184
Hussein, K. H., Park, K.-M., Kang, K.-S., and Woo, H.-M. (2016). Biocompatibility evaluation of tissue-engineered decellularized scaffolds for biomedical application. Mater. Sci. Eng. C Mater. Biol. Appl. 67, 766–778. doi:10.1016/j.msec.2016.05.068
Jean, F., Rachael, P., Ivana, M., Bishara, A., Hubbard, A. E., Celi, L. A., et al. (2022). Clinical artificial intelligence quality improvement: towards continual monitoring and updating of AI algorithms in healthcare. npj Digit. Med. 5 5, 66. doi:10.1038/s41746-022-00611-y
Jeffries, E. M., Allen, R. A., Gao, J., Pesce, M., and Wang, Y. (2015). Highly elastic and suturable electrospun poly(glycerol sebacate) fibrous scaffolds. Acta Biomater. 18, 30–39. doi:10.1016/j.actbio.2015.02.005
Jensen, P. J., Graham, J. P., Busch, T. K., Fitz, O., Jayanadh, S., Pashuck, T. E., et al. (2025). Biocompatible composite hydrogel with on-demand swelling-shrinking properties for 4D bioprinting. Biomater. Sci. 13, 3264–3279. doi:10.1039/d5bm00551e
Jiang, W., and Xu, J. (2019). Immune modulation by mesenchymal stem cells. Cell Prolif. 53, e12712. doi:10.1111/cpr.12712
Jo, Y., Hwang, D. G., Kim, M., Yong, U., and Jang, J. (2023). Bioprinting-assisted tissue assembly to generate organ substitutes at scale. Trends Biotechnol. 41, 93–105. doi:10.1016/j.tibtech.2022.07.001
Kang, D., Ahn, G., Kim, D., Kang, H.-W., Yun, S., Yun, W.-S., et al. (2018). Pre-set extrusion bioprinting for multiscale heterogeneous tissue structure fabrication. Biofabrication 10, 035008. doi:10.1088/1758-5090/aac70b
Kannan, P. R., Sangkert, S., Jiang, C., Li, Y., Zhao, R., Iqbal, M. Z., et al. (2025). Ultrasmall zinc oxide nanoparticle-reinforced chitosan-fucoidan scaffolds for enhanced antibacterial activity and accelerated osteogenesis. Int. J. Biol. Macromol. 310, 143390. doi:10.1016/j.ijbiomac.2025.143390
Khalak, F. A.-H., Decuyper, J. M., Khalak, K. A.-H., Alonso, S. R., Saenz-Del-Burgo, L., and Pedraz Muñoz, J. L. (2025). 3D bioprinting approaches for musculoskeletal interfaces in tissue engineering. Int. J. Pharm. 682, 125939. doi:10.1016/j.ijpharm.2025.125939
Kim, J. J., and Cho, D.-W. (2024). Advanced strategies in 3D bioprinting for vascular tissue engineering and disease modelling using smart bioinks. Virtual Phys. Prototyp. 19, e2395470. doi:10.1080/17452759.2024.2395470
Kim, C.-H., and Kim, T.-H. (2020). Graphene hybrid materials for controlling cellular microenvironments. Mater. (basel Switz.) 13, 4008. doi:10.3390/ma13184008
Kim, B. S., Das, S., Jang, J., and Cho, D.-W. (2020). Decellularized extracellular matrix-based bioinks for engineering tissue- and organ-specific microenvironments. Chem. Rev. 120, 10608–10661. doi:10.1021/acs.chemrev.9b00808
Korkeamäki, J. T., Rashad, A., Ojansivu, M., Karvinen, J., Koivisto, J. T., Syverud, K., et al. (2025). Systematic development and bioprinting of novel nanostructured multi-material bioinks for bone tissue engineering. Biofabrication 17, 025005. doi:10.1088/1758-5090/ada63b
Kozlowska, J., Prus-Walendziak, W., Stachowiak, N., Bajek, A., Kazmierski, L., and Tylkowski, B. (2020). Modification of collagen/gelatin/hydroxyethyl cellulose-based materials by addition of herbal extract-loaded microspheres made from gellan gum and xanthan gum. Mater. (basel Switz.) 13, 3507. doi:10.3390/ma13163507
Krishnan, L., Willett, N. J., and Guldberg, R. E. (2014). Vascularization strategies for bone regeneration. Ann. Biomed. Eng. 42, 432–444. doi:10.1007/s10439-014-0969-9
Lai, H., Liu, Y., Cheng, Y., Shi, L., Wang, R., and Sun, J. (2023). Temperature-triggered adhesive bioelectric electrodes with long-term dynamic stability and reusability. Adv. Sci. Weinh. Baden-wurtt. Ger. 10, e2300793. doi:10.1002/advs.202300793
Lai, J., Liu, Y., Lu, G., Yung, P., Wang, X., Tuan, R. S., et al. (2024). 4D bioprinting of programmed dynamic tissues. Bioact. Mater. 37, 348–377. doi:10.1016/j.bioactmat.2024.03.033
Lakdawala, N., Bercovitch, L., and Grant-Kels, J. M. (2013). A picture is worth a thousand words: ethical dilemmas presented by storing digital photographs in electronic health records. J. Am. Acad. Dermatol. 69, 473–475. doi:10.1016/j.jaad.2013.03.017
Laranjeira, M., Domingues, R. M. A., Reis, R. L., Costa-Almeida, R., and Gomes, M. E. (2017). 3D mimicry of native-tissue-fiber architecture guides tendon-derived cells and adipose stem cells into artificial tendon constructs. Small 13, 1700689. doi:10.1002/smll.201700689
Li, Q., Zhang, Q., Cai, Y., and Hua, Y. (2018). Patients with achilles tendon rupture have a degenerated contralateral achilles tendon: an elastography study. Biomed. Res. Int. 2018, 1–7. doi:10.1155/2018/2367615
Li, M., Chen, L., Feng, W., Yan, L.-T., Wang, C., Jin, Z., et al. (2022). Superstretchable, yet stiff, fatigue-resistant ligament-like elastomers. Nat. Commun. 13, 2279. doi:10.1038/s41467-022-30021-3
Liu, W., Yan, X., Sun, M., Chen, F., Rao, Y., Wei, Q., et al. (2016). Directing the differentiation of parthenogenetic stem cells into tenocytes for tissue-engineered tendon regeneration. Stem Cells Transl. Med. 6, 196–208. doi:10.5966/sctm.2015-0334
Lohan, P., Treacy, O., Griffin, M. D., Ritter, T., and Ryan, A. E. (2017). Anti-donor immune responses elicited by allogeneic mesenchymal stem cells and their extracellular vesicles: are we still learning? Front. Immunol. 8, 1626. doi:10.3389/fimmu.2017.01626
Longoni, A., Li, J., Lindberg, G. C. J., Rnjak-Kovacina, J., Wise, L. M., Hooper, G. J., et al. (2021). Strategies for inclusion of growth factors into 3D printed bone grafts. Essays Biochem. 65, 569–585. doi:10.1042/EBC20200130
Loukelis, K., Chatzinikolaidou, M., Koutsomarkos, N., and Mikos, A. G. (2024). Advances in 3D bioprinting for regenerative medicine applications. Regen. Biomater. 11, rbae033. doi:10.1093/rb/rbae033
Ma, S.-S., Sgm, U., Ll, N., Jh, A., Rl, T., S, D., et al. (2019). Biomanufacturing of organ-specific tissues with high cellular density and embedded vascular channels. Sci. Adv. 5, eaaw2459. doi:10.1126/sciadv.aaw2459
Ma, H., Wang, H., Ma, Z., Luo, Y., Wang, Z., Wang, W., et al. (2022). Multiscale hierarchical architecture-based bioactive scaffolds for versatile tissue engineering. Adv. Healthc. Mater. 11, 2102837. doi:10.1002/adhm.202102837
Maan, Z., Masri, N. Z., and Willerth, S. M. (2022). Smart bioinks for the printing of human tissue models. Biomolecules 12, 141. doi:10.3390/biom12010141
Macadam, A., Chaudry, E., Mctiernan, C. D., Cortes, D., Suuronen, E. J., and Alarcon, E. I. (2022). Development of in situ bioprinting: a mini review. Front. Bioeng. Biotechnol. 10, 940896. doi:10.3389/fbioe.2022.940896
Machour, M., Hen, N., Goldfracht, I., Safina, D., Davidovich-Pinhas, M., Bianco-Peled, H., et al. (2022). Print-and-grow within a novel support material for 3D bioprinting and post-printing tissue growth. Adv. Sci. Weinh. Baden-wurtt. Ger. 9, 2200882. doi:10.1002/advs.202200882
Maciel, B. R., Grimm, A., Oelschlaeger, C., Schepers, U., and Willenbacher, N. (2023). Targeted micro-heterogeneity in bioinks allows for 3D printing of complex constructs with improved resolution and cell viability. Biofabrication 15, 045013. doi:10.1088/1758-5090/acee22
Matai, I., Kaur, G., Seyedsalehi, A., McClinton, A., and Laurencin, C. T. (2020). Progress in 3D bioprinting technology for tissue/organ regenerative engineering. Biomaterials 226, 119536. doi:10.1016/j.biomaterials.2019.119536
Ménézo, Y., Lichtblau, I., and Elder, K. (2013). New insights into human pre-implantation metabolism in vivo and in vitro. J. Assist. Reprod. Genet. 30, 293–303. doi:10.1007/s10815-013-9953-9
Mörö, A., Skottman, H., Puistola, P., Honkamäki, L., Miettinen, S., Oommen, O., et al. (2022). Hyaluronic acid based next generation bioink for 3D bioprinting of human stem cell derived corneal stromal model with innervation. Biofabrication 15, 015020. doi:10.1088/1758-5090/acab34
Mouser, V. H. M., Levato, R., Mensinga, A., Dhert, W. J. A., Gawlitta, D., and Malda, J. (2020). Bio-ink development for three-dimensional bioprinting of hetero-cellular cartilage constructs. Connect. Tissue Res. 61, 137–151. doi:10.1080/03008207.2018.1553960
Murali, A., and Parameswaran, R. (2024). Extrusion 3D printing advances for craniomaxillofacial bone tissue engineering. Polym.-Plast. Technol. Mater. 63, 889–912. doi:10.1080/25740881.2024.2307351
Murphy, S. V., and Atala, A. (2014). 3D bioprinting of tissues and organs. Nat. Biotechnol. 32, 773–785. doi:10.1038/nbt.2958
Nakamura, N., Kimura, T., and Kishida, A. (2017). Overview of the development, applications, and future perspectives of decellularized tissues and organs. ACS Biomater. Sci. Eng. 3, 1236–1244. doi:10.1021/acsbiomaterials.6b00506
Nandakumar, N., Iyyer, S., Mohan, T., Nair, S. V., and Sathy, B. N. (2024). Smart design for hybrid bioprinting of scalable and viable tissue constructs. Tissue Eng. 30, 342–352. doi:10.1089/ten.TEA.2023.0188
Ng, W. L., Chan, A., Ong, Y. S., and Chua, C. K. (2020). Deep learning for fabrication and maturation of 3D bioprinted tissues and organs. Virtual Phys. Prototyp. 15, 340–358. doi:10.1080/17452759.2020.1771741
Ning, H., Joo, S. W., and Zhou, T. (2023). Machine learning boosts three-dimensional bioprinting. Int. J. Bioprinting 9, 739. doi:10.18063/ijb.739
No, Y. J., Castilho, M., Ramaswamy, Y., and Zreiqat, H. (2020). Role of biomaterials and controlled architecture on tendon/ligament repair and regeneration. Adv. Mater. (deerf. Beach Fla,) 32, e1904511. doi:10.1002/adma.201904511
Oh, S.-Y., Kim, H. Y., Choi, Y. M., Park, Y. S., Kim, H. S., Jo, I., et al. (2019). Application of tonsil-derived mesenchymal stem cells in tissue regeneration: concise review. Stem Cells 37, 1252–1260. doi:10.1002/stem.3058
Ouyang, L., Yao, R., Zhao, Y., and Sun, W. (2016). Effect of bioink properties on printability and cell viability for 3D bioplotting of embryonic stem cells. Biofabrication 8, 035020. doi:10.1088/1758-5090/8/3/035020
Ouyang, L., Armstrong, J. P. K., Lin, Y., Wojciechowski, J. P., Lee-Reeves, C., Hachim, D., et al. (2020). Expanding and optimizing 3D bioprinting capabilities using complementary network bioinks. Sci. Adv. 6, eabc5529. doi:10.1126/sciadv.abc5529
Pan, S., Wang, R., Zhou, X., Corvera, J., Kloc, M., Sifers, R., et al. (2008). Extracellular alix regulates integrin-mediated cell adhesions and extracellular matrix assembly. EMBO J. 27, 2077–2090. doi:10.1038/emboj.2008.134
Pardo, A., Rial, R., Domingues, R. M. A., Monteiro, R. F., Teixeira, S. P. B., Gomes, M. E., et al. (2022). Magnetically-assisted 3D bioprinting of anisotropic tissue-mimetic constructs. Adv. Funct. Mater. 32, 2208940. doi:10.1002/adfm.202208940
Parekkadan, B., and Milwid, J. M. (2010). Mesenchymal stem cells as therapeutics. Annu. Rev. Biomed. Eng. 12, 87–117. doi:10.1146/annurev-bioeng-070909-105309
Park, Y.-G., Yun, I., Chung, W. G., Park, W., Lee, D. H., and Park, J.-U. (2022). High-resolution 3D printing for electronics. Adv. Sci. Weinh. Baden-wurtt. Ger. 9, e2104623. doi:10.1002/advs.202104623
Potyondy, T., Uquillas, J. A., Tebon, P. J., Byambaa, B., Hasan, A., Tavafoghi, M., et al. (2021). Recent advances in 3D bioprinting of musculoskeletal tissues. Biofabrication 13, 022001. doi:10.1088/1758-5090/abc8de
Raftery, R. M., Woods, B., Marques, A. L. P., Moreira-Silva, J., Silva, T. H., Cryan, S.-A., et al. (2016). Multifunctional biomaterials from the sea: assessing the effects of chitosan incorporation into collagen scaffolds on mechanical and biological functionality. Acta Biomater. 43, 160–169. doi:10.1016/j.actbio.2016.07.009
Rahimnejad, M., Rezvaninejad, R., França, R., and Rezvaninejad, R. (2021). Biomaterials in bone and mineralized tissue engineering using 3D printing and bioprinting technologies. Biomed. Phys. amp; Eng. Express 7 7, 062001. doi:10.1088/2057-1976/ac21ab
Razavi, Z., Soltani, M., Souri, M., and van Wijnen, A. J. (2024). CRISPR innovations in tissue engineering and gene editing. Life Sci. 358, 123120. doi:10.1016/j.lfs.2024.123120
Renshaw, A., Few, W. E., Desai, B., Godshaw, B., and Jones, D. (2024). Patellar tendon reconstruction using tibialis posterior allograft for treatment of patellar tendon rupture after bone-patellar tendon-bone anterior cruciate ligament reconstruction. Ochsner J. 24, 151–156. doi:10.31486/toj.23.0104
Rieder, E., Steinacher-Nigisch, A., and Weigel, G. (2016). Human immune-cell response towards diverse xenogeneic and allogeneic decellularized biomaterials. Int. J. Surg. Lond. Engl. 36, 347–351. doi:10.1016/j.ijsu.2016.06.042
Rodríguez-Padrón, D., Balu, A. M., Romero, A. A., and Luque, R. (2017). New bio-nanocomposites based on iron oxides and polysaccharides applied to oxidation and alkylation reactions. Beilstein J. Org. Chem. 13, 1982–1993. doi:10.3762/bjoc.13.194
Rohringer, S., Grasl, C., Ehrmann, K., Hager, P., Hahn, C., Specht, S. J., et al. (2023). Biodegradable, self-reinforcing vascular grafts for in situ tissue engineering approaches. Adv. Healthc. Mater. 12, e2300520. doi:10.1002/adhm.202300520
Schwab, A., Levato, R., D’Este, M., Piluso, S., Eglin, D., and Malda, J. (2020). Printability and shape fidelity of bioinks in 3D bioprinting. Chem. Rev. 120, 11028–11055. doi:10.1021/acs.chemrev.0c00084
Shi, Y., Tang, S., Yuan, X., Li, Z., Wen, S., Li, Z., et al. (2024). In situ 4D printing of polyelectrolyte/magnetic composites for sutureless gastric perforation sealing. Adv. Mater. (deerf. Beach Fla,) 36, e2307601. doi:10.1002/adma.202307601
Shojaee, A., Ejeian, F., Parham, A., and Nasr Esfahani, M. H. (2022). Optimizing tenogenic differentiation of equine adipose-derived mesenchymal stem cells (eq-ASC) using TGFB3 along with BMP antagonists. Cell J. 24, 370–379. doi:10.22074/cellj.2022.7892
Shou, Y., Liu, L., Liu, Q., Le, Z., Lee, K. L., Li, H., et al. (2023). Mechano-responsive hydrogel for direct stem cell manufacturing to therapy. Bioact. Mater. 24, 387–400. doi:10.1016/j.bioactmat.2022.12.019
Stanco, D., Bogni, A., Puricelli, L., Boffito, M., Ciardelli, G., Soldati, G., et al. (2020). 3D bioprinting of human adipose-derived stem cells and their tenogenic differentiation in clinical-grade medium. Int. J. Mol. Sci. 21, 8694. doi:10.3390/ijms21228694
Subramanian, G., Stasuk, A., Elsaadany, M., and Yildirim-Ayan, E. (2017). Effect of uniaxial tensile cyclic loading regimes on matrix organization and tenogenic differentiation of adipose-derived stem cells encapsulated within 3D collagen scaffolds. Stem Cells Int. 2017, 1–16. doi:10.1155/2017/6072406
Sun, Y., You, Y., Wang, B., Wu, Q., Jiang, W., and Dai, K. (2020). 3D bioprinting dual-factor releasing and gradient-structured constructs ready to implant for anisotropic cartilage regeneration. Sci. Adv. 6, eaay1422. doi:10.1126/sciadv.aay1422
Tachibana, N., Chijimatsu, R., Okada, H., Oichi, T., Taniguchi, Y., Maenohara, Y., et al. (2022). RSPO2 defines a distinct undifferentiated progenitor in the tendon/ligament and suppresses ectopic ossification. Sci. Adv. 8, eabn2138. doi:10.1126/sciadv.abn2138
Tang, J., Chen, L., and Liu, Y.-G. (2019). Off-target effects and the solution. Nat. Plants 5, 341–342. doi:10.1038/s41477-019-0406-z
Tavafoghi, M., Sheikhi, A., Tutar, R., Jahangiry, J., Baidya, A., Haghniaz, R., et al. (2020). Engineering tough, injectable, naturally derived, bioadhesive composite hydrogels. Adv. Healthc. Mater. 9, e1901722. doi:10.1002/adhm.201901722
Teixeira, L. S. M., Feijen, J., van Blitterswijk, C. A., Dijkstra, P. J., and Karperien, M. (2012). Enzyme-catalyzed crosslinkable hydrogels: emerging strategies for tissue engineering. Biomaterials 33, 1281–1290. doi:10.1016/j.biomaterials.2011.10.067
Thangadurai, M., Srinivasan, S. S., Sekar, M. P., Sethuraman, S., and Sundaramurthi, D. (2024). Emerging perspectives on 3D printed bioreactors for clinical translation of engineered and bioprinted tissue constructs. J. Mater. Chem. B 12, 350–381. doi:10.1039/d3tb01847d
Tu, T., Shi, Y., Zhou, B., Wang, X., Zhang, W., Zhou, G., et al. (2023). Type I collagen and fibromodulin enhance the tenogenic phenotype of hASCs and their potential for tendon regeneration. npj Regen. Med. 8, 67. doi:10.1038/s41536-023-00341-z
Valianatos, P., Papadakou, E., Erginoussakis, D., Kampras, D., Schizas, N., and Kouzoupis, A. (2020). Treatment of chronic patellar tendon rupture with hamstrings tendon autograft. J. Knee Surg. 33, 792–797. doi:10.1055/s-0039-1688499
Van Belleghem, S., Torres, L., Santoro, M., Mahadik, B., Wolfand, A., Kofinas, P., et al. (2020). Hybrid 3D printing of synthetic and cell-laden bioinks for shape retaining soft tissue grafts. Adv. Funct. Mater. 30, 1907145. doi:10.1002/adfm.201907145
Vanessa, A., Seanthel, D. S., Liza, K., Rahmadian, A., Saluja, R., Everest, L., et al. (2021). Assessment of food and drug administration- and european medicines agency-approved systemic oncology therapies and clinically meaningful improvements in quality of life: a systematic review. JAMA Netw. Open 4, e2033004. doi:10.1001/jamanetworkopen.2020.33004
Wang, C., Fang, Y., Sun, W., Xia, J., Xu, Y., Zhou, Z., et al. (2021). Multi-scale hierarchical scaffolds with aligned micro-fibers for promoting cell alignment. Biomed. Mater. 16, 045047. doi:10.1088/1748-605x/ac0a90
Wang, M., Li, W., Hao, J., Gonzales, A., Zhao, Z., Flores, R. S., et al. (2022). Molecularly cleavable bioinks facilitate high-performance digital light processing-based bioprinting of functional volumetric soft tissues. Nat. Commun. 13, 3317. doi:10.1038/s41467-022-31002-2
Wang, H., Bi, S., Lv, X., Qiu, J., Ma, J., Wei, Y., et al. (2023a). Recent advances in engineering bioinks for 3D bioprinting. Adv. Eng. Mater. 25, 2300648. doi:10.1002/adem.202300648
Wang, Y., Peach, C., Vyas, C., Pereira, R. F., Bartolo, P., and Huang, B. (2023b). Robotic in situ bioprinting for cartilage tissue engineering. Int. J. Extreme Manuf. 5, 032004. doi:10.1088/2631-7990/acda67
Xie, W., Wei, X., Kang, H., Jiang, H., Chu, Z., Lin, Y., et al. (2023). Static and dynamic: evolving biomaterial mechanical properties to control cellular mechanotransduction. Adv. Sci. Weinh. Baden-wurtt. Ger. 10, e2204594. doi:10.1002/advs.202204594
Xing, H., Lee, H., Luo, L., and Kyriakides, T. R. (2020). Extracellular matrix-derived biomaterials in engineering cell function. Biotechnol. Adv. 42, 107421. doi:10.1016/j.biotechadv.2019.107421
Xu, T., Lin, J., Hao, Y., Geng, D., Xu, M., Sun, H., et al. (2019). Tenocyte-derived exosomes induce the tenogenic differentiation of mesenchymal stem cells through TGF-β. Cytotechnology 71, 57–65. doi:10.1007/s10616-018-0264-y
Yang, Y., Jia, Y., Yang, Q., and Xu, F. (2022a). Engineering bio-inks for 3D bioprinting cell mechanical microenvironment. Int. J. Bioprinting 9, 632. doi:10.18063/ijb.v9i1.632
Yang, Z., Yi, P., Liu, Z., Zhang, W., Mei, L., Feng, C., et al. (2022b). Stem cell-laden hydrogel-based 3D bioprinting for bone and cartilage tissue engineering. Front. Bioeng. Biotechnol. 10, 865770. doi:10.3389/fbioe.2022.865770
Yi, H.-G., Kim, H., Kwon, J., Choi, Y.-J., Jang, J., and Cho, D.-W. (2021). Application of 3D bioprinting in the prevention and the therapy for human diseases. Signal Transduct. Target. Ther. 6, 177. doi:10.1038/s41392-021-00566-8
Ying, G., Jiang, N., Yu, C., and Zhang, Y. S. (2018). Three-dimensional bioprinting of gelatin methacryloyl (GelMA). Bio-Des. Manuf. 1, 215–224. doi:10.1007/s42242-018-0028-8
Zhang, H., Zhou, L., and Zhang, W. (2014). Control of scaffold degradation in tissue engineering: a review. Tissue Eng. B Rev. 20, 492–502. doi:10.1089/ten.TEB.2013.0452
Zhang, Y., Kumar, P., Lv, S., Xiong, D., Zhao, H., Cai, Z., et al. (2020). Recent advances in 3D bioprinting of vascularized tissues. Mater. Amp; Des. 199, 109398. doi:10.1016/j.matdes.2020.109398
Zhang, J., Rubert, M., Müller, R., and Wehrle, E. (2021). 3D bioprinting of human tissues: biofabrication, bioinks, and bioreactors. Int. J. Mol. Sci. 22, 3971. doi:10.3390/ijms22083971
Zhang, H.-R., Xu, M.-Y., Zhang, L., Zhang, H., Yang, L., Liu, J., et al. (2022). Effects of chemical sterilization and gamma irradiation on the biochemical and biomechanical properties of human tendon allografts in vitro study. Orthop. Surg. 14, 2657–2668. doi:10.1111/os.13465
Zhang, H., Hua, S., He, C., Yin, M., Qin, J., Liu, H., et al. (2025a). Application of 4D-printed magnetoresponsive FOGS hydrogel scaffolds in auricular cartilage regeneration. Adv. Healthc. Mater. 14, e2404488. doi:10.1002/adhm.202404488
Zhang, Z., Zhou, X., Fang, Y., Xiong, Z., and Zhang, T. (2025b). AI-driven 3D bioprinting for regenerative medicine: from bench to bedside. Bioact. Mater. 45, 201–230. doi:10.1016/j.bioactmat.2024.11.021
Keywords: biomechanics, personalized treatment, artificial intelligence, medical-engineering integration, sports injury
Citation: Bai X, Yang Y, Chu J, Deng Y, Li M and Yang H (2025) 3D bioprinting patient-specific grafts for tendon/ligament repair in motion: emerging trends and challenges. Front. Bioeng. Biotechnol. 13:1643430. doi: 10.3389/fbioe.2025.1643430
Received: 08 June 2025; Accepted: 08 August 2025;
Published: 22 August 2025.
Edited by:
Wei Song, Shanghai Sixth People’s Hospital Affiliated to Shanghai Jiao Tong University School of Medicine, ChinaReviewed by:
Xinghao Wang, Capital Medical University, ChinaXi Chen, Zhejiang Chinese Medical University, China
Copyright © 2025 Bai, Yang, Chu, Deng, Li and Yang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Huaiyu Yang, Y2hhcmxpZWdyZXlzb25AeWVhaC5uZXQ=
 Xuejian Bai1
Xuejian Bai1 Huaiyu Yang
Huaiyu Yang