Abstract
This study explores an enhancement to a cell-penetrating peptide (CPP), specifically cyclic deca arginine (cR10), by modifying it with boronic acid to improve the delivery efficiency of ubiquitin (Ub), an essential protein that plays various roles in cellular functions. The hypothesis is that adding boronic acid could boost cellular uptake through glycan-boronic acid interactions. This research assesses how the boronic acid-modified cR10 compares to TAT, a natural CPP derived from the HIV-1 transactivator of transcription, in delivering Ub into cells. Experiments with U2OS cells indicated that the boronic acid-linked cR10Ub cargo achieved a fourfold increase in cellular uptake compared to the TAT-Ub conjugate. The findings from this study could contribute to developing new approaches for enhancing protein delivery methods relevant to biomedical research and therapeutic applications.
Graphical Abstract

Graphical Abstract is made with Biorender.com.
Introduction
The plasma membrane plays essential roles in selective permeability, maintaining osmotic balance, compartmentalization, and facilitating cellular uptake (Dias and Nylandsted, 2021). Small polar molecules, such as ions, amino acids, and sugars, enter cells through specific carriers and channels found in the membrane. Conversely, larger macromolecules like proteins, DNA, and RNA generally cannot enter using this method (Deprey et al., 2019). Consequently, various delivery tools have been devised to improve the cellular uptake of large molecules for essential research and biomedical uses (Fu et al., 2014). Techniques used include mechanical and electrical transfection, chemical and biochemical methods, membrane fusion using synthetic lipids, peptides or proteins, dendrimers, adenovirus-associated viral vectors, and lentiviral vectors. Certain techniques are appropriate for either in vitro or in vivo applications, while others can work for both. Additionally, these delivery methods may utilize viral or nonviral carrier systems (Wang et al., 2023). Nonviral systems have advantages over viral ones, including easier assembly, greater flexibility, lower toxicity, and reduced immunogenicity and insertional risks mutagenesis.
Cell-penetrating peptides (CPPs) have quickly gained popularity in non-viral delivery methods (Jones and Sayers, 2012). The first CPPs were discovered by two independent research teams, featuring a protein transduction domain (PTD) derived from the transactivator of transcription (Tat) linked to HIV-1 (Frankel and Pabo, 1988; Green and Loewenstein, 1988). This domain, consisting of eleven amino acids (YGRKKRRQRRR), allows TAT and its cargo to penetrate cellular membranes. Since then, numerous CPPs have been developed from natural, chimeric, and synthetic sources, typically rich in arginine or lysine, which bestow a positive charge or amphipathicity (Reissmann, 2021). Although widely utilized, the well-known TAT CPP does not always ensure the effective delivery of the desired protein (LaRochelle et al., 2015). In this context, in addition to natural CPP like TAT, cyclic deca arginine (cR10) has been developed to facilitate the delivery of various proteins or other cargos of interest. The utilization of a CPP alone may not suffice in certain situations, prompting the evolution of alternative strategies. For instance, the incorporation of a CPP or a unique peptide co-incubated with the target protein can enhance direct cellular transduction or promote endo-lysosomal escape, as elucidated in the research conducted by the Schneider et al. (2021) and Akishiba et al. (2017) groups. In an alternative approach, the Brik group modified the CPP backbone with specialized molecules, such as an additive, which subsequently enhanced the efficacy of cellular delivery for the protein of interest (Mandal et al., 2021).
In this regard, the Raines et al and the author demonstrated that boronic acid, or its derivative, acts as an effective modification, having been shown to increase the delivery efficacy of CPPs and proteins (Ghosh, 2024; Ghosh and Seitz, 2025). In this instance, the glycan-boronic acid interaction plays a critical role in the internalization process of the CPPs. The advantage of employing boronic acid lies in its capacity to facilitate the formation of next-generation CPPs, as it is susceptible to nucleophilic attack, followed by rapid hydrolysis resulting in alcohol and boric acid. Additionally, boric acid is not genotoxic, and long-term analyses conducted on mice revealed no significant increase in tumor formation. According to the U.S. Environmental Protection Agency, boric acid is characterized by low toxicity, with an oral LD50 of 3,450 mg/kg for male rats and 4,080 mg/kg for female rats. Further potential properties for the discrete role of this boronic moiety have yet to be established. This study aims to modify an artificial CPP, such as cR10, with boronic acid and compare its efficacy in delivering a protein alongside a natural CPP, like TAT (Ghosh, 2024). This endeavor aims to yield the following information: (a) the utility of boronic acid as an additive for protein delivery and (b) the role of natural CPPs in protein delivery (Mandal and Brik, 2022). Such insights may facilitate the exploration of new avenues in this field, ultimately contributing to advancements in the future. In this work, Ubiquitin (Ub) is a protein of considerable interest, representing a globular structure composed of 76 amino acids and displaying evolutionary conservation across all eukaryotic organisms. The covalent attachment of Ub to other proteins, referred to as ubiquitination, constitutes the second most significant form of post-translational modification (PTM) for proteins, thereby regulating essential cellular functions. Notably, ubiquitination is a reversible process; the dysregulation of both ubiquitination and deubiquitination mechanisms has been implicated in various diseases, including cancer and neurodegenerative disorders. Therefore, the efficient delivery of this protein (Ub) is of paramount importance in elucidating this intricate mechanism of action in real time.
Results and discussions
A comparative analysis has been undertaken between the boronic acid CPP) and the well-established Transactivator of Transcription (TAT) CPP to further substantiate their efficacy. Previous studies have indicated that the covalent attachment of the TAT peptide enhances the cellular delivery of various proteins, including both expressed proteins such as Enhanced Green Fluorescent Protein (EGFP), β-galactosidase, RNAse-A, along with synthetic proteins, H2B-associated peptides (Fawell et al., 1994; Hameed et al., 2018; David et al., 2015). The efficacy of boronic acid cyclic deca arginine was compared with its pristine version (Ghosh, 2024; Ghosh and Seitz, 2025). Consequently, the disparity persists in the comparison of the efficacy of natural Cell-Penetrating Peptides (CPPs), such as TAT, with that of cR10B2. This analysis will furnish a comprehensive perspective on the effectiveness of boronic acid-based CPPs and broaden their applicability as delivery vehicles.
The linear TAT peptide (see Table 1) on a Rink amide solid support was manually prepared, employing the standard Fmoc-SPPS technique. Two PEG linkers were coupled as [2-[2-(Fmoc-amino)ethoxy]-ethoxy] acetic acid after the TAT peptide sequence and terminated with Boc-Cys(trt)-OH coupling (Figure 1A). A subsequent resin cleavage and global deprotection followed by purification gave a ∼30% isolated yield of the Cys-TAT peptide. The N-terminus Cys residue was added to the sequence to facilitate the disulfide-linked conjugation with our protein of interest. After purification, the Cys moiety of the synthesized TAT peptide was activated via 5,5′-dithiol-bis-[2-nitropyridine], DTNP. The synthesis of the boronic acid-linked CPP (cR10B2, see Table 1 for the amino acid sequence) was carried out as shown in the schematic in Figure 1B. Briefly, the synthesis was done manually on a Rink amide resin using standard Fmoc-SPPS as per the sequence (r represents D-Arg). To accommodate different functionalities on the sequence, three orthogonally protected amino acid residues were used: Fmoc-Lys(Alloc)-OH, Fmoc-Glu(Oallyl)-OH and Fmoc-Lys(Mtt)-OH. As the sequence was terminated with Boc-Cys(trt)-OH, Pd(PPh3)4/PhSiH3 mediated Alloc and Oallyl group removal followed by lactamization with PyAOP, HOBt, DIEA (1:1:2) for 90 min yielded the cyclized peptide on resin. Upon Mtt group removal with mild TFA/DCM cleavage mixture (2%), the free Lys-side chains were coupled with 4-bromomethyl phenylboronic acid. After that, the resulting peptide was cleaved from the resin, globally deprotected, and purified in ∼13% isolated yield. Like the TAT peptide, cR10B2 contains an N-terminal Cys for further modifications.
TABLE 1
| Peptide/Protein | Sequence |
|---|---|
| CPP: TAT | C-Peg-Peg-Y-G-R-K-K-R-R-Q-R-R-R (linear) |
| CPP: CR10B2 |

|
| Protein: Ub | TAMRA-C-Peg-M→Nle-Q-I-F-V-K-T-L-T-G-K-T-I-T-L-E-V-E-P-S-D-T-I-E- N-V-K-A-K-I-Q-D-K-E-G-I-P-P-D-Q-Q-R-L-I-F-A-G-K-Q-L-E-D-G-R-T-L-S-D-Y-N-I-Q-K-E-S-T-L-H-L-V-L-R-L-R-G-G |
The amino acid sequence of the CPPs and protein (Ub).
FIGURE 1

Synthetic schemes for CPPs: (A) TAT, (B) cR10B2, conjugates: (C) Ub-TAT, (D) Ub-cR10B2.
Ubiquitination, is the second most significant post-translational modification (PTM), regulating essential cellular functions. Understanding this complex mechanism requires efficient delivery of Ub. Therefore, fluorescently labelled Ub (sequence in Table 1) was synthesized manually with an N-terminal Cys to act as the point of CPP attachment on a Rink amide resin, which gave the final isolated yield of TAMRA-C-PEG-Ub ∼23% (Ghosh, 2024; Ghosh and Seitz, 2025).
For preparing Ub-TAT conjugate, DTNP-activated Cys-TAT was reacted with TAMRA-Cys-PEG-Ub dissolved in guanidinium phosphate buffer (pH∼ 7.2) at 37°C for 5−7 min, which generated the hetero disulfide-linked fluorescently labelled Ub-TAT (Figure 1C). For the synthesis of Ub-cR10B2, dithio-bis-[2-nitrobenzoicacid], DTNB-activated Ub was reacted with cR10B2 overnight at 37°C to yield the corresponding conjugate (Figure 1D). The relevant HPLC-MS analysis of CPP-SS-Ub conjugates is available in the SI section.
With the disulfide-linked Ub-TAT and Ub-cR10B2 synthesized, their efficacy in live cell delivery was assessed within U2OS cells, an established osteosarcoma cell line (obtained from ATCC). A cellular uptake analysis of the probes (Ub-TAT and Ub-cR10B2) was conducted at a concentration of 2 μM in a serum-free medium, involving 1 hour of co-incubation at 37°C. Initially, U2OS cells were treated with the probes for 1 hour, followed by washing exclusively with PBS and a heparan sulfate solution in PBS. As the CPPs are positively charged, they can reside on the cell membrane during the co-incubation process, hampering the analysis. Washing with negatively charged heparan sulfate helps in releasing any possible membrane-bound peptides. Subsequently, the cells were rinsed with PBS, culminating in Hoechst staining to visualize viable cell nuclei. Live cell confocal laser scanning microscopy (CLSM) was performed on the treated cells to analyze the delivery outcomes. The CLSM images from Ub-cR10B2 treated cells showed a homogeneous distribution throughout the cytosol and extension towards the nucleus. On the contrary, a negligible delivery was observed for the TAT peptide, where the Ub-TAT probe exhibited mostly punctuate-like distribution within the cytosol (most likely trapped inside endo-lysosomal compartments) (Figures 2A–D). Flow Cytometry data further confirmed that Ub-cR10B2 interacts more strongly with cells compared to Ub-TAT (Figure 2E).
FIGURE 2
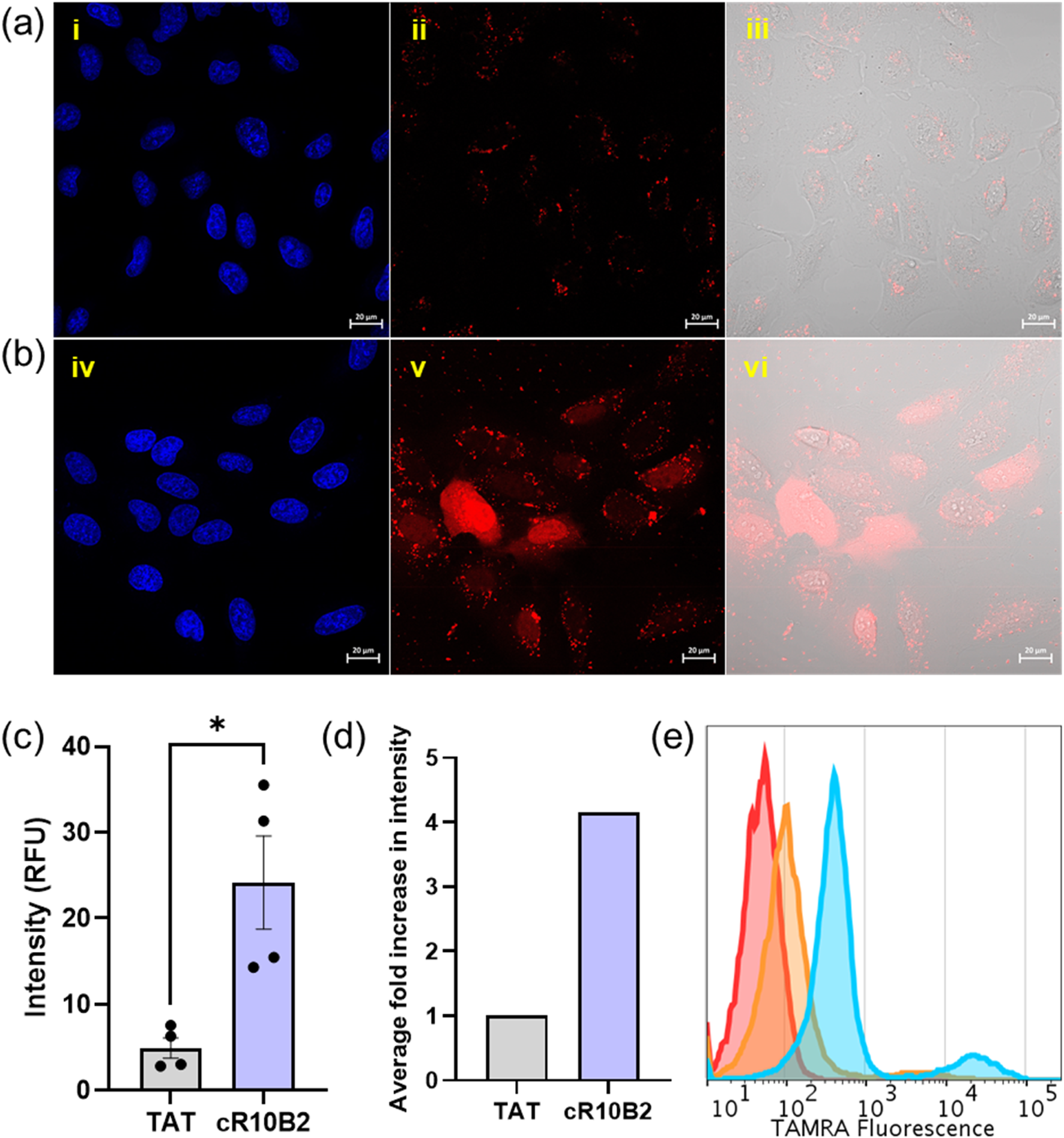
Live cell delivery of the probes; (A) Representative images for the delivery of Ub-TAT in live U2OS cells where (i) Hoechst, (ii) TAMRA, (iii) bright field and TAMRA channels merged; (B) Representative images for the delivery of Ub-cR10B2 in live U2OS cells where (iv) Hoechst, (v) TAMRA, (vi) bright field and TAMRA channels merged; (C) Quantifying nuclear TAMRA intensity for both probes from the four sets of experiments; (D) Fold change in the intracellular delivery of Ub-TAT compared to Ub-cR10B2; (E) flow cytometry data with TAMRA fluorescence for untreated cells (red), Ub-TAT (orange) and Ub-cR10B2 (cyan).
After crossing the plasma membrane, small proteins (smaller than 50–60 kDa) can diffuse freely through the nuclear compartments (Borlido et al., 2009; Luther et al., 2021). The nuclear accumulation/localization of the probes was calculated to explore the efficacy. Notably, the experiment was repeated four times in independent sets and quantified using a Hoechst masking algorithm in ImageJ (FiJi) software (∼300 cells per probe). The quantification shows that the fluorescence intensity for probe Ub-cR10B2 appeared to be four-fold higher than the Ub-TAT probe, which can be visually attributed to the film-like delivery of Ub-cR10B2 conjugate inside live cells.
Conclusion
Utilizing CPPs for protein delivery presents significant opportunities for the targeted delivery of therapeutics directly within cells. Among the various strategies to enhance the efficacy of CPPs, additive-mediated pathways emerge as particularly advantageous. The incorporation of a small molecule into the CPP has the potential to improve efficacy significantly. In this context, boronic acid is an appealing additive due to its susceptibility to nucleophilic attack, followed by rapid hydrolysis resulting in the formation of boric acid, which is non-genotoxic, as extensive studies in mice revealed no significant tumor development. In this study, boronic acid-mediated cyclic deca arginine was employed as a representative of boronic acid-derived CPPs, and its efficacy was evaluated in comparison to well-established CPPs, such as TAT. Due to its broad utility within the human body, Ubiquitin was selected as the target protein of interest for delivery. The delivery efficacy of the Ub-cR10B2 conjugate was observed to be four times greater than that of the Ub-TAT conjugate, as demonstrated by confocal microscopy analysis corroborated by flow cytometry at low micromolar concentrations (specifically, 2 µM of the final protein-CPP conjugate). These findings underscore the potential of boron-based CPPs as a promising class for further exploration into the mechanistic insights surrounding therapeutic proteins in target cells. Future investigations will focus on utilizing various proteins and new classes of CPPs derived from boronic acid or its derivatives.
Statements
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Ethics statement
Ethical approval was not required for the studies on humans in accordance with the local legislation and institutional requirements because only commercially available established cell lines were used.
Author contributions
PG: Conceptualization, Data curation, Formal analysis, Investigation, Supervision, Writing – original draft, Writing – review and editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fchbi.2025.1610127/full#supplementary-material
References
1
Akishiba M. Takeuchi T. Kawaguchi Y. Sakamoto K. Yu H. H. Nakase I. et al (2017). Cytosolic antibody delivery by lipid-sensitive endosomolytic peptide. Nat. Chem.9 (8), 751–761. 10.1038/nchem.2779
2
Borlido J. Zecchini V. Mills I. G. (2009). Nuclear trafficking and functions of endocytic proteins implicated in oncogenesis. Traffic10 (9), 1209–1220. 10.1111/j.1600-0854.2009.00922.x
3
David Y. Vila-Perelló M. Verma S. Muir T. W. (2015). Chemical tagging and customizing of cellular chromatin states using ultrafast trans-splicing inteins. Nat. Chem.7 (5), 394–402. 10.1038/nchem.2224
4
Deprey K. Becker L. Kritzer J. Plückthun A. (2019). Trapped! A critical evaluation of methods for measuring total cellular uptake versus cytosolic localization. Bioconjugate Chem.30 (4), 1006–1027. 10.1021/acs.bioconjchem.9b00112
5
Dias C. Nylandsted J. (2021). Plasma membrane integrity in health and disease: significance and therapeutic potential. Cell Discov.7 (1), 4. 10.1038/s41421-020-00233-2
6
Fawell S. Seery J. Daikh Y. Moore C. Chen L. L. Pepinsky B. et al (1994). Tat-mediated delivery of heterologous proteins into cells. Proc. Natl. Acad. Sci.91 (2), 664–668. 10.1073/pnas.91.2.664
7
Frankel A. D. Pabo C. O. (1988). Cellular uptake of the tat protein from human immunodeficiency virus. Cell55 (6), 1189–1193. 10.1016/0092-8674(88)90263-2
8
Fu A. Tang R. Hardie J. Farkas M. E. Rotello V. M. (2014). Promises and pitfalls of intracellular delivery of proteins. Bioconjugate Chem.25 (9), 1602–1608. 10.1021/bc500320j
9
Ghosh P. (2024). Boronic acid-linked cell-penetrating peptide for protein delivery. ACS omega9 (17), 19051–19056. 10.1021/acsomega.3c09689
10
Ghosh P. Seitz O. (2025). Boronic acid‐linked apo‐zinc finger protein for ubiquitin delivery in live cells. ChemBioChem26, e202401040. 10.1002/cbic.202401040
11
Green M. Loewenstein P. M. (1988). Autonomous functional domains of chemically synthesized human immunodeficiency virus tat trans-activator protein. Cell55 (6), 1179–1188. 10.1016/0092-8674(88)90262-0
12
Hameed D. S. Sapmaz A. Gjonaj L. Merkx R. Ovaa H. (2018). Enhanced delivery of synthetic labelled ubiquitin into live cells by using next‐generation ub–TAT conjugates. ChemBioChem19 (24), 2553–2557. 10.1002/cbic.201800649
13
Jones A. T. Sayers E. J. (2012). Cell entry of cell penetrating peptides: tales of tails wagging dogs. J. Control. Release161 (2), 582–591. 10.1016/j.jconrel.2012.04.003
14
LaRochelle J. R. Cobb G. B. Steinauer A. Rhoades E. Schepartz A. (2015). Fluorescence correlation spectroscopy reveals highly efficient cytosolic delivery of certain penta-arg proteins and stapled peptides. J. Am. Chem. Soc.137 (7), 2536–2541. 10.1021/ja510391n
15
Luther D. C. Jeon T. Goswami R. Nagaraj H. Kim D. Lee Y. W. et al (2021). Protein delivery: if your GFP (or other small protein) is in the cytosol, it will also be in the nucleus. Bioconjugate Chem.32 (5), 891–896. 10.1021/acs.bioconjchem.1c00103
16
Mandal S. Brik A. (2022). Probing the cell delivery of synthetic diubiquitin chains. Chem. Commun.58, 8782–8785. 10.1039/d2cc02476d
17
Mandal S. Mann G. Satish G. Brik A. (2021). Enhanced live‐cell delivery of synthetic proteins assisted by cell‐penetrating peptides fused to DABCYL. Angew. Chem. Int. Ed.60 (13), 7333–7343. 10.1002/anie.202016208
18
Reissmann S. (2021). State of art: cell penetration and cell-penetrating peptides and proteins. Health Educ. Public Health4 (2), 393–410. 10.31488/heph.161
19
Schneider A. F. Kithil M. Cardoso M. C. Lehmann M. Hackenberger C. P. (2021). Cellular uptake of large biomolecules enabled by cell-surface-reactive cell-penetrating peptide additives. Nat. Chem.13 (6), 530–539. 10.1038/s41557-021-00661-x
20
Wang C. Pan C. Yong H. Wang F. Bo T. Zhao Y. et al (2023). Emerging non-viral vectors for gene delivery. J. nanobiotechnology21 (1), 272. 10.1186/s12951-023-02044-5
Summary
Keywords
cell penetrating peptide (CPP), ubiquitin (Ub), boronic acid, live cell activity, microscopy, flow cytometry
Citation
Ghosh P (2025) Boronic acid-modified cell-penetrating peptide exhibits superior performance over natural TAT peptide. Front. Chem. Biol. 4:1610127. doi: 10.3389/fchbi.2025.1610127
Received
11 April 2025
Accepted
28 May 2025
Published
24 June 2025
Volume
4 - 2025
Edited by
Antonello Merlino, University of Naples Federico II, Italy
Reviewed by
Giovanni Gotte, University of Verona, Italy
Gao Li, Minjiang University, China
Updates
Copyright
© 2025 Ghosh.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Pritam Ghosh, ppritamghosh@gmail.com, pritam.ghosh@hu-berlin.de
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.