- 1Department of Medicine, Division of Cardiology, Northwestern University Feinberg School of Medicine, Chicago, IL, USA
- 2Department of Surgery, Division of Vascular Surgery, Northwestern University Feinberg School of Medicine, Chicago, IL, USA
- 3Department of Preventive Medicine, Northwestern University Feinberg School of Medicine, Chicago, IL, USA
- 4Department of Preventive Medicine, Division of Cardiology, Northwestern University Feinberg School of Medicine, Chicago, IL, USA
- 5Department of Medicine, Division of Cardiology, Northwestern University Feinberg School of Medicine, Chicago, IL, USA
Objective: To quantify the association between high-density lipoprotein (HDL) subfractions, efflux capacity, and inflammatory markers at baseline and the effect of supervised exercise on these HDL parameters in patients with peripheral artery disease (PAD).
Methods: The study to improve leg circulation (SILC) was a randomized trial of supervised treadmill exercise, leg resistance training, or control in individuals with PAD. In a post hoc cross-sectional analysis, we quantified the associations between baseline HDL subfraction concentrations (HDL2 and HDL3), HDL-C efflux capacity, and inflammatory markers [C-reactive protein (CRP) and interleukin-6 (IL-6)]. We then examined the effect of supervised exercise on changes in these lipoprotein parameters and inflammatory markers in 88 patients from SILC.
Results: Baseline HDL-C efflux capacity was associated with baseline concentrations of HDL2 (β = 0.008, p = 0.0106), HDL3 (β = 0.013, p < 0.0001), and IL-6 (β = −0.019, p = 0.03). Baseline HDL3 concentration was inversely associated with IL-6 concentration (β = −0.99, p = 0.008). Compared to control, changes in HDL2, HDL3, normalized HDL-C efflux capacity, CRP, or IL-6 were not significantly different at 6 months following the structured exercise intervention.
Conclusion: HDL efflux and HDL3 were inversely associated with IL-6 in PAD patients. Structured exercise was not associated with changes in HDL subfractions, HDL-C efflux capacity, CRP, and IL-6 in PAD patients. Our preliminary findings support the theory that inflammation may adversely affect HDL structure and function; however, further studies are needed to evaluate these findings.
Introduction
Exercise has been observed to alter high-density lipoprotein (HDL) metrics associated with the risk of cardiovascular disease (CVD), including increased HDL subfractions (1) in individuals without known CVD and increased HDL-C efflux capacity in observational studies of athletes (2, 3). Patients with peripheral artery disease (PAD) are at high risk for CVD events, including limb threatening and acute systemic ischemic events. Exercise (4) and physical activity (5) may be associated with reductions in levels of inflammatory markers, including C-reactive protein (CRP) and interleukin-6 (IL-6), which could slow disease progression, functional decline, and reduce risks for cardiovascular outcomes in PAD patients (6–8). Additionally, inflammation may interfere with the protective functions of HDL (i.e., efflux capacity) (9–11). However, little is known about the association among HDL metrics associated with CVD risk, inflammation, and exercise in patients with PAD (12–14).
High-density lipoprotein structure (as measured by subfraction concentration) is associated with CVD risk (15, 16), and measurement of HDL function (as measured by HDL-C efflux capacity) has emerged as a robust marker of prevalent and incident CVD (17). The clinical significance and profile of HDL subfractions in patients with PAD are also not well understood with reports of both increased (18) and decreased (19) HDL3 levels in the setting of reduced HDL2 and HDL-C. The HDL subfractions, HDL2 and HDL3, are particles with unique size and density that may have distinct cholesterol efflux capacities (20), exhibit unique efflux responses to drug therapy (21), and have differential associations with CVD events (22). Thus, structural changes in HDL particles may be paralleled by functional changes with implications for CVD risk (23). A previous meta-analysis of exercise on HDL subfractions in adults without known CVD demonstrated that exercise is associated with increased HDL2 (1). However, the effect of supervised exercise on HDL subfraction concentration and HDL efflux capacity in patients with PAD has not been studied.
The study to improve leg circulation (SILC) was a 6-month randomized trial of supervised treadmill exercise, leg resistance training, or control in individuals with PAD with and without claudication that demonstrated improved physical function and quality of life in response to a supervised exercise intervention compared to control. First, in a post hoc analysis of the SILC study, we quantified the associations among HDL efflux, HDL subfractions, and circulating inflammatory markers at baseline in a cross-sectional analysis. Second, we evaluated the effect of 24 weeks of supervised exercise on changes in HDL efflux, HDL subfractions, and circulating inflammatory markers using changes in 6-min walk as a measure of response to the exercise intervention.
Materials and Methods
Design Overview
The SILC trial enrolled 156 patients with PAD with and without intermittent claudication between April 1, 2004, and August 8, 2008. Participants were recruited from newspaper and radio advertisements (n = 85), non-invasive vascular laboratories at Northwestern Memorial Hospital and other Chicago-area hospitals, and via community mailings and posters. The institutional review boards of the participating hospitals and medical centers approved the protocol. Patients were randomly assigned to supervised treadmill exercise (n = 51), lower extremity resistance training (n = 52), or a control (n = 53) group. Details of the design, patients, outcome definitions, and results have been published (24). Baseline information concerning demographics [age, sex, race, body mass index, ankle brachial index (ABI), and smoking], past medical history (leg symptoms, diabetes, angina, myocardial infarction, heart failure, and cancer), and statin and glitazone use were collected at the time of enrollment in SILC.
Study Interventions
The supervised treadmill exercise intervention consisted of treadmill exercise three times a week for 24 weeks, supervised by an exercise physiologist. The duration and intensity of treadmill exercise was over the course of 24 weeks and/or at near maximal leg symptoms (if present). Participants in the lower extremity resistance training group exercised three times a week for 24 weeks with a certified trainer. They performed three sets of eight repetitions of knee extension, leg press, and leg curl exercises. For each exercise, one repetition maximum was measured at baseline and every 4 weeks, and weights were increased until participants lifted 80% of their one repetition maximum. The control group received 11 nutritional information sessions over 6 months. “Nutritional information sessions were not provided to the supervised exercise groups.”
Leg Symptoms
Leg symptoms were assessed at baseline and follow-up using the San Diego claudication questionnaire (25). Intermittent claudication was defined as exertional calf pain that does not begin at rest, causes the subject to stop walking, and resolves within 10 min of rest (26).
Six-Minute Walk
At baseline and follow-up, 6-min walk performance was assessed using standardized protocols (27). Participants walked up and down a 100-ft hallway for 6 min after instructions to cover as much distance as possible. The distance completed after 6 min was recorded.
Ankle Brachial Index
A handheld Doppler probe (Nicolet Vascular Pocket Dop II; Nicolet Biomedical Inc., Golden, CO, USA) was used to obtain systolic pressures in the right and left brachial, dorsalis pedis, and posterior tibial arteries. Each pressure was measured twice. The ABI was calculated by dividing the mean of the dorsalis pedis and posterior tibial pressures in each leg by the mean of the four brachial pressures (28). Average pressures in the arm with the highest pressure were used when the first brachial pressure was higher than the opposite brachial pressure in both measurement sets and the second brachial pressures differed by 10 mm Hg or higher in the first measurement set (29).
Circulating Inflammatory Markers
Interleukin-6 was measured using an ultrasensitive enzyme-linked immunosorbent assay (R&D Systems, Minneapolis, MN, USA). Concentrations of high-sensitivity CRP were determined using an immunoturbidimetric assay on the Hitachi 911 analyzer (Roche Diagnostics, Indianapolis, IN, USA), using reagents and calibrators from Denka Seiken (Niigata, Japan).
Measurement of HDL-C Efflux Capacity
Two hundred microliters of stored serum (100 μl baseline and 100 μl follow-up) from PAD patients randomized to supervised exercise training and control were assayed for HDL-C efflux capacity.
Cholesterol efflux measurements were performed using the J774 radiolabeled cholesterol assay, which has been extensively used in studies of cholesterol efflux to human serum (30). J774 cells (ATCC, Rockville, MD, USA), derived from a murine macrophage cell line, are plated and radiolabeled with 2 μCi of 3H-cholesterol per milliliter. ATP-binding cassette A1 (ABCA1) is a transmembrane protein involved in reverse cholesterol transport (RCT) by functioning as a cholesterol efflux pump. ABCA1 is upregulated by means of an 18-h incubation with 0.3 mM 8-(4-chlorophenylthio)-cyclic AMP. Subsequently, efflux mediums containing 2.8% apolipoprotein B (apo-B)-depleted serum were added for 4 h. Apo-B-depleted serum was created by mixing 40 parts 20% PEG 6000 (QIAGEN Science, Germantown, MD, USA) with 100 parts serum with gentle mixing, and then incubated at room temperature for 20 min. Apo-B-depleted serum was then obtained by recovery of supernatant following centrifugation (10,000 rpm, 30 min, 4°C). All steps were performed in the presence of the acyl-coenzyme A:cholesterol acyltransferase inhibitor CP113,818 (2 μg/ml). This assay measures efflux mediated by several macrophage-specific RCT pathways, including the ABCA1- and ATP-binding cassette G1- (ABCG1) mediated transport, scavenger receptor B1-mediated transport, and aqueous diffusion (30, 31). Stimulation of J774 cells with cAMP upregulates ABCA1-mediated efflux. Thus in the conditions used here, total release of cholesterol occurred mainly by ABCA1 and passive diffusion.
Liquid scintillation counting was used to quantify the efflux of radioactive cholesterol from the cells. The quantity of radioactive cholesterol incorporated into cellular lipids was calculated by means of isopropanol extraction of control wells not exposed to patient serum. After serial washing with PBS, cell lipids were extracted from control culture dishes with isopropanol for 1 h, then evaporated to dryness under nitrogen gas, and were then reconstituted in chloroform. All assays were performed in triplicate. Percent efflux was calculated by the following formula:
Values were normalized by dividing the efflux capacity of individual patients by the efflux capacity of a serum pool run with each assay.
Lipoprotein and Subfraction Measurement
The vertical auto profile (VAP) test was used to obtain a comprehensive measurement of the lipoprotein cholesterol profile. One hundred forty-two microliters of stored plasma (71 μl at baseline and 71 μl post-exercise) were analyzed from PAD patients randomized to supervised treadmill training (n = 33), strength training (n = 29), and age- and gender-matched controls (n = 26). The VAP test is based on a well-established method of ultracentrifugation for lipoprotein subfraction determination (32). VAP provides cholesterol concentrations of various lipoproteins subclasses, including the HDL subfractions HDL2 and HDL3.
Statistical Analysis
Baseline characteristics were summarized as means and SDs, frequencies, and percentages, as appropriate. Chi-squared tests and one-way analyses of variance were used to compare baseline characteristics of participants across the three groups. Two sample, two-sided t-tests were used to compare changes in outcomes between 6-month follow-up and baseline between each exercise group and the control group, respectively, without adjustments for multiple comparisons. The Shapiro–Wilk test was used to assess for normality of the data.
Relationships between continuous variables of interest were assessed using Pearson correlation coefficients (denoted as r) and multivariable adjusted linear regression models adjusted for age, race, gender, HDL subfractions, inflammatory markers, smoking status, statin, and glitazone.
Two sample, two-sided t-tests were used to compare changes in outcomes between baseline and 6-month follow-up among the treadmill, strength training, and control groups without adjustment for baseline data.
The p value considered statistically significant was p ≤ 0.05. All analyses were performed using the SAS statistical software (version 9.4, SAS Institute Inc., Cary, NC, USA).
Sample Size Power Calculation
Since we used macrophage-to-plasma efflux capacity as in the study by Olchawa et al., sample size estimation was performed using the effect size and SDs of their study. To detect a difference of 2.6% (18.8 vs. 16.2%), we estimated that we would need 22 subjects per group to have an 80% chance of having a statistically significant result at p = 0.05.
Results
Study Participants
One hundred fifty-six PAD patients were enrolled in the SILC study, 68 were excluded because of lack of availability of baseline or follow-up serum sample. Patients were randomly assigned to supervised treadmill exercise (n = 51), lower extremity resistance training (n = 52), or a control (n = 53) group. Among those not included, there was a higher prevalence of heart failure (12 vs. 7%, p = 0.02) and cancer (17 vs. 11%, p = 0.04) compared to the subjects included in the current sub-study. The characteristics of SILC trial participants are shown in Table 1. The mean age of the participants was 70.85 ± 1.72 years. A total of 53.5% of patients were women and 46.5% were black. There were no significant differences in baseline demographics, CV risk factors or disease, severity of PAD, 6-min walk, or HDL-related parameters in any of the study groups. There were also no significant differences in baseline characteristics from study onset to completion following the study interventions.
Association of Baseline HDL Subfractions, Inflammatory Markers, Comorbidities, and Medications with Baseline Cholesterol Efflux Capacity
In all study patients, HDL-C (β = 0.006, p = 0.0001), HLD2 (β = 0.008, p = 0.0105), HDL3 (β = 0.011, p < 0.0001), and IL-6 (β = −0.019, p = 0.0294) but not CRP was associated with baseline HDL-C efflux capacity. However, after adjustment for age, race, gender, current smoking status, IL-6, statin, and glitazone use, only HDL3 (β = 0.0147, p = 0.0014) was associated with baseline cholesterol efflux capacity. Including diabetes, heart failure, coronary artery disease, and cancer into the adjusted model did not change the magnitude or statistical significance of the association between baseline HDL3 with baseline HDL efflux.
Additionally, there was no significant difference in baseline HDL-C efflux capacity in patients receiving statins vs. not receiving statins (mean efflux = 1.18 vs. 1.10, p = 0.61, respectively), or in patients receiving glitazones vs. those not receiving glitazones (mean efflux = 1.13 vs. 1.18, p = 0.21, respectively).
Association of Baseline HDL Subfractions and Inflammatory Markers
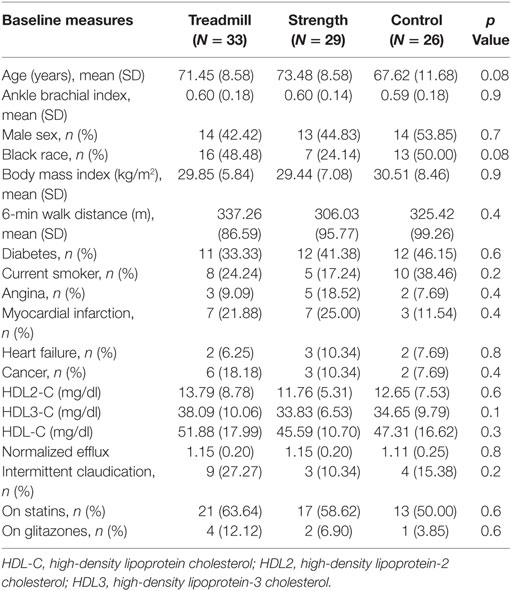
In an unadjusted analysis, we observed an inverse association between baseline IL-6 levels and baseline HDL3 concentration (β = −0.99, p = 0.008). This association was minimally altered (β = −0.94, p = 0.01) after adjustment for age, race, gender, current smoking status, comorbidities, statin, and glitazone use. In addition, among patients with the highest quartile of IL-6, a trend toward an inverse association was observed between baseline IL-6 and baseline efflux capacity (β = −0.1167, p = 0.08) (Figure 1).
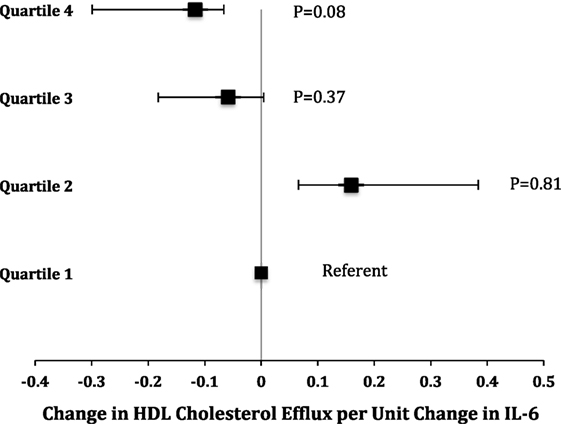
Figure 1. Beta coefficients of linear regression modeling examining the association of baseline cholesterol efflux stratified according to baseline quartiles of interleukin-6 (IL-6) in all study participants.
Association of Exercise with Changes in HDL Subfractions, Cholesterol Efflux Capacity, and Inflammatory Markers
There were no significant changes in HDL2 and HDL3 compared to baseline in the strength trained group [(−0.17 mg/dl, 95% CI: −1.88 to −1.54) and (1.24 mg/dl, 95% CI: −0.85 to 3.33), respectively] and treadmill group [(1.42 mg/dl, 95% CI: −0.18 to 3.03) and (1.42 mg/dl, 95% CI: −0.54 to 3.39), respectively] (Figures 2A,B). Additionally, there was no significant difference in HDL2 and HDL3 in the strength and treadmill groups at 6 months follow compared to control.
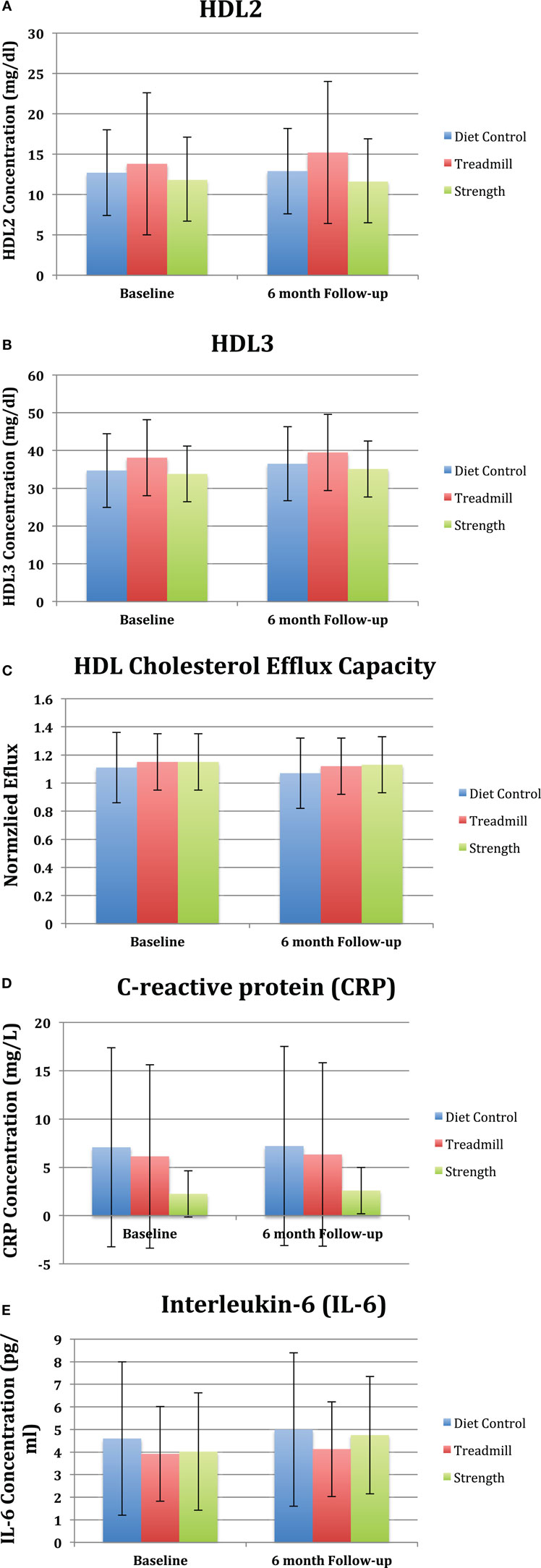
Figure 2. Changes in (A) high-density lipoprotein (HDL) subfractions, (B) cholesterol efflux capacity, and (C) inflammatory markers among peripheral artery disease in study to improve leg circulation patients according to group assignment. All values are average ± SE. (A) p = 0.38 for strength, treadmill, and diet control groups compared to baseline. For differences compared to control at 6-month follow-up: strength (p = 0.7) and treadmill (p = 0.3). (B) p = 0.9 for strength, treadmill, and diet control compared to baseline. For differences compared to control at 6-month follow-up: p = 0.7 for strength and p = 0.8 for treadmill. (C) p = 0.9 for strength, treadmill, and diet control compared to baseline. For differences compared to control at 6-month follow-up, p = 0.7 for strength and p = 0.7 for treadmill. (D) p = 0.99 for strength, treadmill, and diet control compared to baseline. For differences compared to control at 6-month follow-up, p = 0.9 for strength and p = 0.98 for treadmill. (E) p = 0.8 for strength, treadmill, and diet control compared to baseline. For differences compared to control at 6-month follow-up, p = 0.7 for strength and p = 0.8 for treadmill.
No significant changes were observed in normalized cholesterol efflux capacity compared to baseline and control at 6-month follow-up in the strength trained group [(−0.02, 95% CI: −0.1 to 0.07) and (0.03, 95% CI: −0.1 to 0.15; p = 0.7), respectively] and treadmill group [(−0.02, 95% CI: 0.1–0.06) and (0.02, 95% CI: −0.1 to 0.14; p = 0.7), respectively] (Figure 2C). Additionally, no changes were observed in the mean levels of IL-6 or CRP from baseline to follow-up (Figures 2D,E). Lastly, there was no association between changes in HDL-C efflux capacity, HDL2, HDL3, or inflammatory markers (Table 2) among the treadmill, resistance trained, or control groups.
Table 2. Association of changes in high-density lipoprotein (HDL) efflux, HDL subfractions, and circulating inflammatory markers with changes in 6-min walk.
The relationship between changes in 6-min walk distance and changes in cholesterol efflux capacity, HDL2, HDL3, CRP, and IL-6 is shown for individual study participants in Figure 3. As depicted in Figure 3, there is no evidence of a linear relationship between changes in functional capacity as a result of the exercise interventions, HDL-related parameters, and inflammatory mediators.
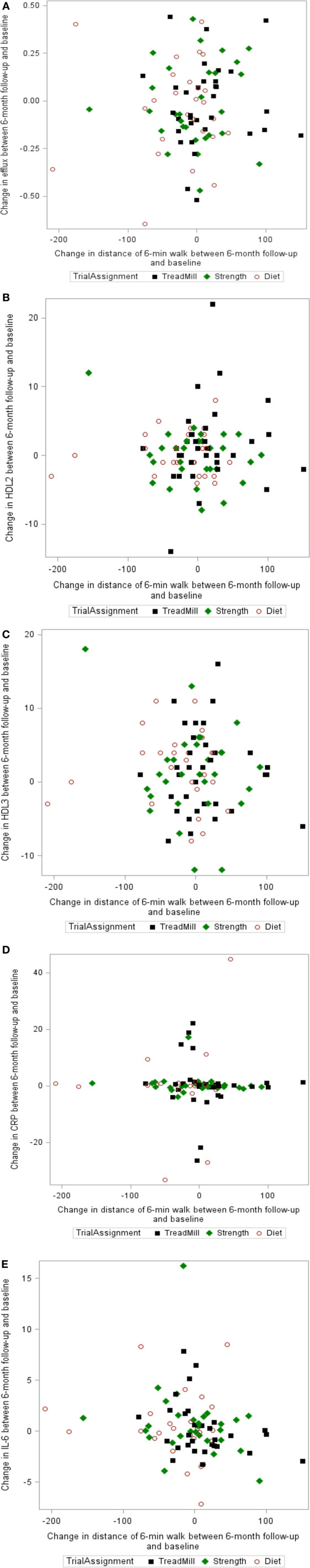
Figure 3. Scatter plots of the relationship between changes in 6-min walk and changes in (A) cholesterol efflux capacity, (B) HDL2, (C) HDL3, (D) C-reactive protein (CRP), and (E) interleukin-6 (IL-6).
Discussion
Our results show that supervised strength and treadmill exercise training are not associated with changes in HDL subfractions, cholesterol efflux capacity, or inflammatory markers in patients with PAD. We observed an association between baseline HDL subfraction concentrations and HDL efflux, an inverse association between baseline IL-6 and HDL efflux, and an inverse association between baseline IL-6 and HDL3 concentration.
The SILC study demonstrated that exercise is associated with beneficial effects in patients with PAD, including improving walking distance, endothelial function, and quality of life (24). However, the mechanisms underlying the beneficial effects of exercise are not well understood in PAD. We studied an assay of HDL-mediated RCT thought to reflect the most relevant pathways involved in atheroprotection (33), and measured HDL subfractions that have been implicated in RCT (34, 35) to explore the biologic mechanisms underlying the favorable effects of exercise. Our results suggest that the beneficial effects of exercise in patients with PAD do not include favorable changes in HDL structure or function as measured by HDL subfraction or efflux, respectively. To our knowledge, this study represents the first investigation of the effect of structured exercise interventions on these HDL-related metrics in patients with PAD.
Supervised exercise did not alter HDL-C, structure, or function as measured in our post hoc analysis of the SILC cohort. A possible explanation for these findings is that a sufficient “exercise dose” may be necessary to increase HDL-C concentration in individuals without known CVD (1, 36, 37), and PAD patients may not exercise at a sufficient intensity to achieve such beneficial changes. Importantly, it is not known if such an exercise threshold exists to favorably remodel HDL subfractions and/or improve HDL efflux in patients with PAD, or even if HDL efflux is modified by exercise in patients with PAD. Koba et al. recently observed an increase in HDL efflux capacity compared to baseline following 6 months of cardiac rehabilitation and intensive lifestyle modification counseling in 57 patients with a recent acute coronary syndrome compared to baseline levels but no significant increase compared to 11 control patients who did not undergo these interventions (38). In a bivariate analysis, they observed that increased HDL efflux was associated with increased HDL-C, apolipoprotein A1, and high-intensity statin use. Interestingly, only patients who achieved a high level of exercise tolerance (defined as a peak VO2 ≥ 19 ml/min/kg) and “complete risk factor control” were observed to have a significant increase in HDL efflux capacity suggesting an exercise dose–response relationship may exist between exercise and efflux. In contrast to the PAD population examined in the current study, the study population of Koba et al. was significantly younger with fewer comorbidities and received a more intensive study intervention that included 6 months of supervised exercise and lifestyle modification counseling. Importantly, HDL-C may not increase with exercise in patients with baseline low levels of HDL-C (39) as is often observed in patients with PAD (40). Furthermore, increased skeletal muscle blood during physical activity and subsequent activation of lipoprotein lipase may be an important mechanism for increasing the concentration of HDL subfractions following exercise, a process that may be significantly diminished in patients with PAD and flow-limiting atherosclerotic lesions in the arterial circulation of their lower extremities (41).
Another possible explanation for our findings may be that patients with severe established atherosclerosis, such as those with PAD, have dysfunctional HDL that is not amenable to improvement via structured exercise or pharmacologic intervention (9, 10, 42). Contrary to other studies of cholesterol efflux capacity in patients with CVD, we did not observe a positive association between HDL efflux and treatment with statin or glitazone medications (30, 38, 43). We did observe, however, a univariate association between baseline IL-6 levels and HDL-C efflux capacity (which was lost after adjustment), and a multivariate association between HDL3 and efflux. PAD patients have elevated levels of IL-6 (44), which may modulate cholesterol efflux to HDL (45–47). It remains unclear if examination of a larger number of inflammatory mediators would have provided additional evidence of an association between inflammation and the HDL-related metrics examined in our study. We also observed that HDL-C efflux in the PAD subjects was elevated compared to the referent pool (i.e., normalized value above 1), and that HDL efflux was refractory to exercise intervention. A normalized efflux value greater than 1 suggests that HDL-C efflux capacity may have been near maximal and therefore not amenable to augmentation in this older cohort with advanced PAD (or that normalization of efflux was performed using an unhealthy referent pool).
Importantly, compared to other studies of HDL efflux and CVD, our study cohort was significantly older with more advanced atherosclerotic burden, and therefore exercise and pharmacologic interventions may be less effective in modifying efflux capacity than they might be in younger individuals.
Our study has several limitations. First, the analyses are post hoc and should be considered exploratory. Second, the small sample size may have contributed to lack of statistical power to detect an effect of exercise on efflux and HDL subfractions; however, our a priori power analysis suggested this was not so. Third, we examined many comparisons, and our analyses did not adjust for multiple comparisons. Our findings regarding baseline HDL subfraction, IL-6, and HDL efflux may be due to chance, and adjustment for multiple comparisons was not possible since most results of this study were not significant. Additionally, a number of factors may have affected the accuracy of this sample size estimate, including that the impact of chronic exercise has not been evaluated in patients with PAD and may be increased or decreased compared to healthy controls, which would either decrease or increase, respectively, the sample size requirements. In addition, Olchawa et al. study did not normalize efflux values relative to a control serum pool run with each assay. Using this normalization technique may theoretically increase the SDs and thus sample size.
Conclusion
In this post hoc analysis of PAD patients from the SILC study, metrics of HDL structure (HDL3) and function (efflux) were inversely associated with a marker of systemic inflammation (IL-6) at baseline; however, the association of IL-6 with HDL efflux was lost after adjustment for comorbidities and HDL3. Supervised exercise did not alter the HDL subfraction profile, improve HDL-C efflux capacity, or modulate levels of CRP and IL-6 in PAD patients from SILC. Future studies of the effect of structured exercise on these HDL-related metrics may be warranted in patients with PAD and elevated inflammatory markers.
Ethics Statement
Northwestern University Feinberg School of Medicine Institutional Review Board approved this study. Patient consent was obtained at the time of the original SILC study for study sample analysis.
Author Contributions
Conceived study, designed experiments, and composed manuscript (MA, JW, and RM); financial support (JW and RM); edited manuscript (MA, ZW, YG, RM, and JW); and performed experiments and analyzed data (MA, ZW, and YG).
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Funding
The authors would like to thank Dr. Neil Stone, MD for his gracious intellectual and financial support of this work. The authors would also like to thank to Atherotech® Diagnostics Lab for their assistance of lipoprotein subfraction analyses.
Abbreviations
HDL2, high-density lipoprotein-2 cholesterol; HDL3, high-density lipoprotein-3 cholesterol.
References
1. Kelley GA, Kelley KS. Aerobic exercise and HDL2-C: a meta-analysis of randomized controlled trials. Atherosclerosis (2006) 184:207–15. doi:10.1016/j.atherosclerosis.2005.04.005
2. Olchawa B, Kingwell BA, Hoang A, Schneider L, Miyazaki O, Nestel P, et al. Physical fitness and reverse cholesterol transport. Arterioscler Thromb Vasc Biol (2004) 24:1087–91. doi:10.1161/01.ATV.0000128124.72935.0f
3. Brites F, Verona J, De Geitere C, Fruchart JC, Castro G, Wikinski R. Enhanced cholesterol efflux promotion in well-trained soccer players. Metabolism (2004) 53:1262–7. doi:10.1016/j.metabol.2004.05.002
4. Tisi PV, Shearman CP. Biochemical and inflammatory changes in the exercising claudicant. Vasc Med (1998) 3:189–98. doi:10.1177/1358836X9800300303
5. Craft LL, Guralnik JM, Ferrucci L, Liu K, Tian L, Criqui MH, et al. Physical activity during daily life and circulating biomarker levels in patients with peripheral arterial disease. Am J Cardiol (2008) 102:1263–8. doi:10.1016/j.amjcard.2008.06.051
6. Vidula H, Tian L, Liu K, Criqui MH, Ferrucci L, Pearce WH, et al. Biomarkers of inflammation and thrombosis as predictors of near-term mortality in patients with peripheral arterial disease: a cohort study. Ann Intern Med (2008) 148:85–93. doi:10.7326/0003-4819-148-2-200801150-00003
7. Tzoulaki I, Murray GD, Lee AJ, Rumley A, Lowe GD, Fowkes FG. C-reactive protein, interleukin-6, and soluble adhesion molecules as predictors of progressive peripheral atherosclerosis in the general population: Edinburgh Artery study. Circulation (2005) 112:976–83. doi:10.1161/CIRCULATIONAHA.104.513085
8. Beckman JA, Preis O, Ridker PM, Gerhard-Herman M. Comparison of usefulness of inflammatory markers in patients with versus without peripheral arterial disease in predicting adverse cardiovascular outcomes (myocardial infarction, stroke, and death). Am J Cardiol (2005) 96:1374–8. doi:10.1016/j.amjcard.2005.07.041
9. de la Llera-Moya M, McGillicuddy FC, Hinkle CC, Byrne M, Joshi MR, Nguyen V, et al. Inflammation modulates human HDL composition and function in vivo. Atherosclerosis (2012) 222:390–4. doi:10.1016/j.atherosclerosis.2012.02.032
10. Mehta NN, Li R, Krishnamoorthy P, Yu Y, Farver W, Rodrigues A, et al. Abnormal lipoprotein particles and cholesterol efflux capacity in patients with psoriasis. Atherosclerosis (2012) 224:218–21. doi:10.1016/j.atherosclerosis.2012.06.068
11. Liao KP, Playford MP, Frits M, Coblyn JS, Iannaccone C, Weinblatt ME, et al. The association between reduction in inflammation and changes in lipoprotein levels and HDL cholesterol efflux capacity in rheumatoid arthritis. J Am Heart Assoc (2015) 4:e001588. doi:10.1161/JAHA.114.001588
12. Mowat BF, Skinner ER, Wilson HM, Leng GC, Fowkes FG, Horrobin D. Alterations in plasma lipids, lipoproteins and high density lipoprotein subfractions in peripheral arterial disease. Atherosclerosis (1997) 131:161–6. doi:10.1016/S0021-9150(97)06097-8
13. Rein P, Saely CH, Silbernagel G, Vonbank A, Mathies R, Drexel H, et al. Systemic inflammation is higher in peripheral artery disease than in stable coronary artery disease. Atherosclerosis (2015) 239:299–303. doi:10.1016/j.atherosclerosis.2015.01.021
14. Brevetti G, Giugliano G, Brevetti L, Hiatt WR. Inflammation in peripheral artery disease. Circulation (2010) 122:1862–75. doi:10.1161/CIRCULATIONAHA.109.918417
15. Salonen JT, Salonen R, Seppanen K, Rauramaa R, Tuomilehto J. HDL, HDL2, and HDL3 subfractions, and the risk of acute myocardial infarction. A prospective population study in eastern Finnish men. Circulation (1991) 84:129–39. doi:10.1161/01.CIR.84.1.129
16. Sich D, Saidi Y, Giral P, Lagrost L, Egloff M, Auer C, et al. Hyperalphalipoproteinemia: characterization of a cardioprotective profile associating increased high-density lipoprotein 2 levels and decreased hepatic lipase activity. Metabolism (1998) 47:965–73. doi:10.1016/S0026-0495(98)90352-3
17. Rohatgi A, Khera A, Berry JD, Givens EG, Ayers CR, Wedin KE, et al. HDL cholesterol efflux capacity and incident cardiovascular events. N Engl J Med (2014) 371(25):2383–93. doi:10.1056/NEJMoa1409065
18. Kasko M, Gaspar L, Dukat A, Gavornik P, Oravec S. High-density lipoprotein profile in newly-diagnosed lower extremity artery disease in Slovak population without diabetes mellitus. Neuro Endocrinol Lett (2014) 35:531–5.
19. Kalofoutis A, Papapanagiotou A, Tzivras M. Clinical significance of plasma HDL subfractions (HDL2, HDL3) in patients with peripheral arterial disease (PAD) in the Greek population. Clin Biochem (1999) 32:149–52. doi:10.1016/S0009-9120(98)00099-X
20. Julia Z, Duchene E, Fournier N, Bellanger N, Chapman MJ, Le Goff W, et al. Postprandial lipemia enhances the capacity of large HDL2 particles to mediate free cholesterol efflux via SR-BI and ABCG1 pathways in type IIB hyperlipidemia. J Lipid Res (2010) 51:3350–8. doi:10.1194/jlr.P009746
21. Catalano G, Julia Z, Frisdal E, Vedie B, Fournier N, Le Goff W, et al. Torcetrapib differentially modulates the biological activities of HDL2 and HDL3 particles in the reverse cholesterol transport pathway. Arterioscler Thromb Vasc Biol (2009) 29:268–75. doi:10.1161/ATVBAHA.108.179416
22. Camont L, Chapman MJ, Kontush A. Biological activities of HDL subpopulations and their relevance to cardiovascular disease. Trends Mol Med (2011) 17:594–603. doi:10.1016/j.molmed.2011.05.013
23. Rosenson RS, Brewer HB Jr, Ansell B, Barter P, Chapman MJ, Heinecke JW, et al. Translation of high-density lipoprotein function into clinical practice: current prospects and future challenges. Circulation (2013) 128:1256–67. doi:10.1161/CIRCULATIONAHA.113.000962
24. McDermott MM, Ades P, Guralnik JM, Dyer A, Ferrucci L, Liu K, et al. Treadmill exercise and resistance training in patients with peripheral arterial disease with and without intermittent claudication: a randomized controlled trial. JAMA (2009) 301:165–74. doi:10.1001/jama.2008.962
25. Criqui MH, Denenberg JO, Bird CE, Fronek A, Klauber MR, Langer RD. The correlation between symptoms and non-invasive test results in patients referred for peripheral arterial disease testing. Vasc Med (1996) 1:65–71. doi:10.1177/1358863X9600100112
26. Rucker-Whitaker C, Greenland P, Liu K, Chan C, Guralnik JM, Criqui MH, et al. Peripheral arterial disease in African Americans: clinical characteristics, leg symptoms, and lower extremity functioning. J Am Geriatr Soc (2004) 52:922–30. doi:10.1111/j.1532-5415.2004.52259.x
27. Montgomery PS, Gardner AW. The clinical utility of a six-minute walk test in peripheral arterial occlusive disease patients. J Am Geriatr Soc (1998) 46:706–11. doi:10.1111/j.1532-5415.1998.tb03804.x
28. McDermott MM, Criqui MH, Liu K, Guralnik JM, Greenland P, Martin GJ, et al. Lower ankle/brachial index, as calculated by averaging the dorsalis pedis and posterior tibial arterial pressures, and association with leg functioning in peripheral arterial disease. J Vasc Surg (2000) 32:1164–71. doi:10.1067/mva.2000.108640
29. Shadman R, Criqui MH, Bundens WP, Fronek A, Denenberg JO, Gamst AC, et al. Subclavian artery stenosis: prevalence, risk factors, and association with cardiovascular diseases. J Am Coll Cardiol (2004) 44:618–23. doi:10.1016/j.jacc.2004.04.044
30. Khera AV, Cuchel M, de la Llera-Moya M, Rodrigues A, Burke MF, Jafri K, et al. Cholesterol efflux capacity, high-density lipoprotein function, and atherosclerosis. N Engl J Med (2011) 364:127–35. doi:10.1056/NEJMoa1001689
31. Rosenson RS, Brewer HB Jr, Davidson WS, Fayad ZA, Fuster V, Goldstein J, et al. Cholesterol efflux and atheroprotection: advancing the concept of reverse cholesterol transport. Circulation (2012) 125:1905–19. doi:10.1161/CIRCULATIONAHA.111.066589
32. Kulkarni KR, Marcovina SM, Krauss RM, Garber DW, Glasscock AM, Segrest JP. Quantification of HDL2 and HDL3 cholesterol by the vertical auto profile-II (VAP-II) methodology. J Lipid Res (1997) 38:2353–64.
33. Cuchel M, Rader DJ. Macrophage reverse cholesterol transport: key to the regression of atherosclerosis? Circulation (2006) 113:2548–55. doi:10.1161/CIRCULATIONAHA.104.475715
34. Linsel-Nitschke P, Jansen H, Aherrarhou Z, Belz S, Mayer B, Lieb W, et al. Macrophage cholesterol efflux correlates with lipoprotein subclass distribution and risk of obstructive coronary artery disease in patients undergoing coronary angiography. Lipids Health Dis (2009) 8:14. doi:10.1186/1476-511X-8-14
35. Tall AR. Cholesterol efflux pathways and other potential mechanisms involved in the athero-protective effect of high density lipoproteins. J Intern Med (2008) 263:256–73. doi:10.1111/j.1365-2796.2007.01898.x
36. King AC, Haskell WL, Young DR, Oka RK, Stefanick ML. Long-term effects of varying intensities and formats of physical activity on participation rates, fitness, and lipoproteins in men and women aged 50 to 65 years. Circulation (1995) 91:2596–604. doi:10.1161/01.CIR.91.10.2596
37. Kokkinos PF, Fernhall B. Physical activity and high density lipoprotein cholesterol levels: what is the relationship? Sports Med (1999) 28:307–14. doi:10.2165/00007256-199928050-00002
38. Koba S, Ayaori M, Uto-Kondo H, Furuyama F, Yokota Y, Tsunoda F, et al. Beneficial effects of exercise-based cardiac rehabilitation on high-density lipoprotein-mediated cholesterol efflux capacity in patients with acute coronary syndrome. J Atheroscler Thromb (2016) 23:865–77. doi:10.5551/jat.34454
39. Couillard C, Despres JP, Lamarche B, Bergeron J, Gagnon J, Leon AS, et al. Effects of endurance exercise training on plasma HDL cholesterol levels depend on levels of triglycerides: evidence from men of the health, risk factors, exercise training and genetics (HERITAGE) family study. Arterioscler Thromb Vasc Biol (2001) 21:1226–32. doi:10.1161/hq0701.092137
40. Bradby GV, Valente AJ, Walton KW. Serum high-density lipoproteins in peripheral vascular disease. Lancet (1978) 2:1271–4. doi:10.1016/S0140-6736(78)92038-X
41. Ruys T, Sturgess I, Shaikh M, Watts GF, Nordestgaard BG, Lewis B. Effects of exercise and fat ingestion on high density lipoprotein production by peripheral tissues. Lancet (1989) 2:1119–22. doi:10.1016/S0140-6736(89)91488-8
42. Roe A, Hillman J, Butts S, Smith M, Rader D, Playford M, et al. Decreased cholesterol efflux capacity and atherogenic lipid profile in young women with PCOS. J Clin Endocrinol Metab (2014) 99:E841–7. doi:10.1210/jc.2013-3918
43. Guerin M, Egger P, Soudant C, Le Goff W, van Tol A, Dupuis R, et al. Dose-dependent action of atorvastatin in type IIB hyperlipidemia: preferential and progressive reduction of atherogenic apoB-containing lipoprotein subclasses (VLDL-2, IDL, small dense LDL) and stimulation of cellular cholesterol efflux. Atherosclerosis (2002) 163:287–96. doi:10.1016/S0021-9150(02)00037-0
44. McDermott MM, Guralnik JM, Corsi A, Albay M, Macchi C, Bandinelli S, et al. Patterns of inflammation associated with peripheral arterial disease: the InCHIANTI study. Am Heart J (2005) 150:276–81. doi:10.1016/j.ahj.2004.09.032
45. Salminen A, Pussinen PJ, Payne JB, Stoner JA, Jauhiainen M, Golub LM, et al. Subantimicrobial-dose doxycycline treatment increases serum cholesterol efflux capacity from macrophages. Inflamm Res (2013) 62:711–20. doi:10.1007/s00011-013-0626-z
46. Robert J, Lehner M, Frank S, Perisa D, von Eckardstein A, Rohrer L. Interleukin 6 stimulates endothelial binding and transport of high-density lipoprotein through induction of endothelial lipase. Arterioscler Thromb Vasc Biol (2013) 33:2699–706. doi:10.1161/ATVBAHA.113.301363
Keywords: exercise, peripheral artery disease, high-density lipoprotein, HDL efflux capacity, HDL subfractions
Citation: Albaghdadi MS, Wang Z, Gao Y, Mutharasan RK and Wilkins J (2017) High-Density Lipoprotein Subfractions and Cholesterol Efflux Capacity Are Not Affected by Supervised Exercise but Are Associated with Baseline Interleukin-6 in Patients with Peripheral Artery Disease. Front. Cardiovasc. Med. 4:9. doi: 10.3389/fcvm.2017.00009
Received: 16 September 2016; Accepted: 15 February 2017;
Published: 02 March 2017
Edited by:
Burak Pamukcu, Istanbul University, TurkeyReviewed by:
Teresa Padro, Barcelona Cardiovascular Research Center (CSIC-ICCC), SpainVasilios Gabriel Athyros, Aristotle University of Thessaloniki, Greece
Copyright: © 2017 Albaghdadi, Wang, Gao, Mutharasan and Wilkins. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: John Wilkins, andpbGtpbnNAbm0ub3Jn
 Mazen S. Albaghdadi
Mazen S. Albaghdadi Zheng Wang
Zheng Wang Ying Gao
Ying Gao R. Kannan Mutharasan1
R. Kannan Mutharasan1