- 1Division of Cardiology, Department of Medicine, Emory University School of Medicine, Atlanta, GA, United States
- 2Center for Preventive Cardiology, Knight Cardiovascular Institute, Oregon Health & Science University, Portland, OR, United States
Diabetes is a leading cause of cardiovascular disease and its associated morbidity. While the medical community has had access to numerous glucose lowering therapies over the last decades, it was not until recently that newer agents demonstrated improvement in cardiovascular outcomes. In particular, diabetes care and management of its attendant cardiovascular risk is now being revolutionized with the development and provision of the SGLT-2 inhibitors and GLP1-receptor agonists. Given the exciting data with these new classes of diabetes therapeutics, there is a clear need to improve education and utilization of these evidence-based medications across a wide spectrum of clinicians, including cardiologists. The aim of this review is to familiarize the cardiovascular specialist with the benefits and harms of the most commonly used oral anti- hyperglycemic medications, with an emphasis on SGLT-2 inhibitors and GLP-1 receptor agonists.
Introduction
Eighty percent of individuals with Type 2 diabetes mellitus (DM) will ultimately succumb to death from a cardiovascular cause compared to 30% of the non-diabetic population (1). The adjusted relative risk (RR) for DM compared to non-DM patients is 1.7 for cardiovascular death, 1.8 for myocardial infarction (MI), and are 1.5 for stroke (2). DM confers a high lifetime risk (67% in men and 57% in women) for developing CVD (3). This association is further compounded by the fact that metabolic risk factors for atherosclerotic CVD (ASCVD) are commonly found in patients with diabetes (4).
Unfortunately, the role of glycemic control in prevention of ASCVD and macrovascular disease is complex and has not shown a clear benefit in preventing these outcomes. In the United Kingdom Prospective Diabetes Study (UKPDS) of 5,102 individuals with newly diagnosed DM, intensive glycemic control (treatment to A1C <7%) did not improve macrovascular outcomes as compared to the standard control arm (A1C < 9%) (5). Additionally, meta-analyses of the Action to Control Cardiovascular Risk study (ACCORD), Action in Diabetes and Vascular Disease: Preterax and Diamicron Modified Release Controlled Evaluation trial (ADVANCE), and Veteran Affairs Diabetes Trial (VADT) demonstrated that intensive therapy, compared to conventional therapy, is associated with adverse events, namely hypoglycemia without a significant reduction in cardiovascular events despite a reduction in microvascular complications (6). These findings were likely mediated by an increase in hypoglycemic events in the intensive therapy groups.
Concerns regarding the cardiovascular safety of diabetes medications (e.g., rosiglitazone) prompted the U.S. Food and Drug Administration (FDA) to issue a mandate in 2008 that required dedicated large cardiovascular safety trials as part of all diabetes drug development programs. Specifically, the FDA guidance provided recommendations on how to demonstrate that a new antidiabetic therapy is not associated with an unacceptable increase in cardiovascular risk. After several years of trials of mainly dipeptidyl peptidase-4 inhibitors (DPP-IV) failing to show cardiovascular benefit, the mandate eventually lead to the discovery of the cardiovascular benefits of sodium-glucose cotransporter-2 (SGLT-2) inhibitors and glucagon-like peptide 1 (GLP-1) receptor agonists, particularly in those at highest risk for CVD. These medications demonstrate improvement in cardiovascular outcomes and mortality through mechanisms that likely have little to do with their glucose lowering effects. The aim of this review is to familiarize cardiovascular specialists with the medical management of DM with respect to cardiovascular benefit and risk, with an emphasis on these two new classes of medications.
Pharmacologic Therapy
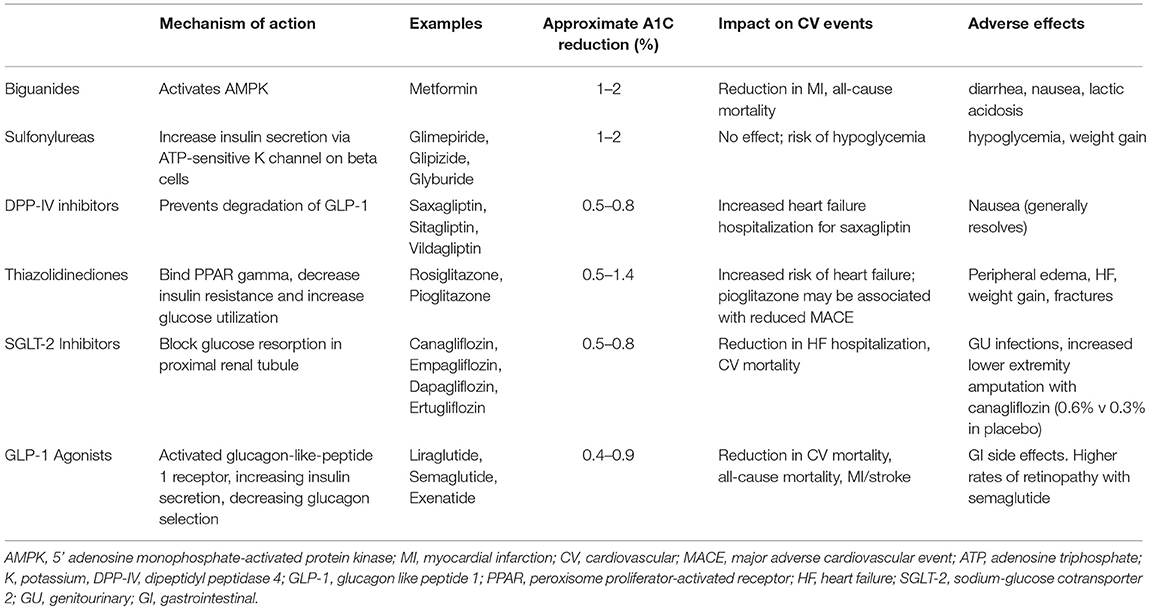
The primary goal for glucose management in patients with type 2 diabetes has long been targeting a hemoglobin A1C of <7.0%. While achievement of optimal hemoglobin A1C levels has been associated with a reduction in microvascular complications, randomized controlled trials have failed to demonstrate a benefit in preventing macrovascular outcomes with this strategy (7, 8). Contemporary trials with new classes of anti-diabetic medications that demonstrate cardiovascular benefit despite only modest glucose lowering challenge this dogma and point to medication class (e.g., mechanism of action) being an important driver of outcomes. The most common classes of diabetes medications, their mechanism of actions, and adverse effects are summarized in Table 1 (9). What follows is a consideration of the various classes of diabetes medications that are associated with either favorable, neutral, or unfavorable cardiovascular outcomes.
Medications With Favorable CV Outcomes
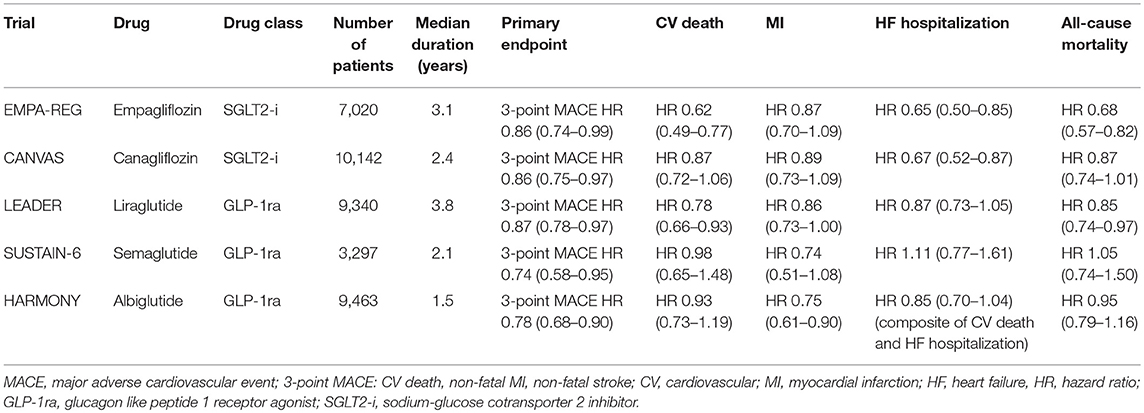
In the absence of contraindications, these drugs should be considered in eligible patients with cardiovascular disease and diabetes given the favorable cardiovascular outcome trial data associated with their use (10) (Table 2).
Biguanides
Metformin is the preferred initial medication for the treatment of type 2 diabetes per current American Diabetes Association (ADA) recommendation (7). This medication exerts its glucose lowering effects by decreasing hepatic gluconeogenesis and increasing insulin sensitivity. In a substudy of the UKPDS that evaluated obese patients, a relative risk reduction of 39% in MI in individuals on metformin was seen as compared to those on insulin or sulfonylureas (8). Additionally, metformin was associated with a 24% reduction in all-cause death (95% CI: 0.65–0.89, P < 0.001) as compared to those not on the medication (11). This evidence supports metformin as first-line therapy for type 2 diabetes given its relative safety and beneficial effects on hemoglobin A1C, weight, and cardiovascular morbidity (12). The mechanism of cardiovascular benefit of these medications is derived mainly from murine models. In murine models of MI, the administration of metformin limits infarct size (13).
Experimentally, this has been found to be due to activation of adenosine monophosphate-activated protein kinase, increased formation of adenosine, and the prevention of opening of the mitochondrial permeability transition pore at reperfusion that contribute to this effect.
Additionally, metformin also attenuates post-infarction cardiac remodeling through mechanisms including the activation of adenosine monophosphate-activated protein kinase and endothelial nitric oxide synthase, and reduced collagen expression (14).
Contraindications for the clinician to be aware of are renal failure (estimated glomerular filtration rate >30 mL/min/1.73 m2) as well as decompensated heart failure given the risk of lactic acidosis (7). Additionally, it is standard of care to discontinue metformin during periods of renal impairment or inpatient heart failure treatment, as well as 24 h prior to and 48 h following contrast exposure (7).
SGLT-2 Inhibitors
The SGLT-2 inhibitors antagonize the sodium-glucose cotransporter 2, located in the proximal tubule of the kidneys. This cotransporter is responsible for 90% of the glucose reabsorption occurring in the kidney (15). Thus, inhibition of this cotransporter leads to glucosuria, which is the predominant anti-hyperglycemic mechanism for these medications.
The first SGLT-2 inhibitor studied in a dedicated cardiovascular outcomes trial was empagliflozin in the Empagliflozin Cardiovascular Outcome Event Trial in Type 2 Diabetes Mellitus Patients- Removing Excess Glucose (EMPA-REG OUTCOME) (16). In EMPA-REG OUTCOME, 7,020 patients were followed over a median follow-up of 3.1 years. This study was a randomized, double-blind trial that compared empagliflozin with placebo in a population with diabetes and known CVD. The primary composite outcome of MI, stroke, and cardiovascular death was reduced by 14% (HR: 0.86 in empagliflozin group, 95% CI: 0.74–0.99, p = 0.04) with a reduction in cardiovascular death of 38% (HR: 0.62, 95% CI: 0.49–0.77, P < 0.001) (Figure 1). Additionally, subjects in the empagliflozin arm demonstrated a 35% reduction in heart failure hospitalization as compared to placebo (HR: 0.65, 95% CI: 0.50–0.85, p = 0.002). Because of the strength of this data, the FDA has included reduction of risk of cardiovascular death in adults with type 2 diabetes and CVD as an indication for empagliflozin (17).
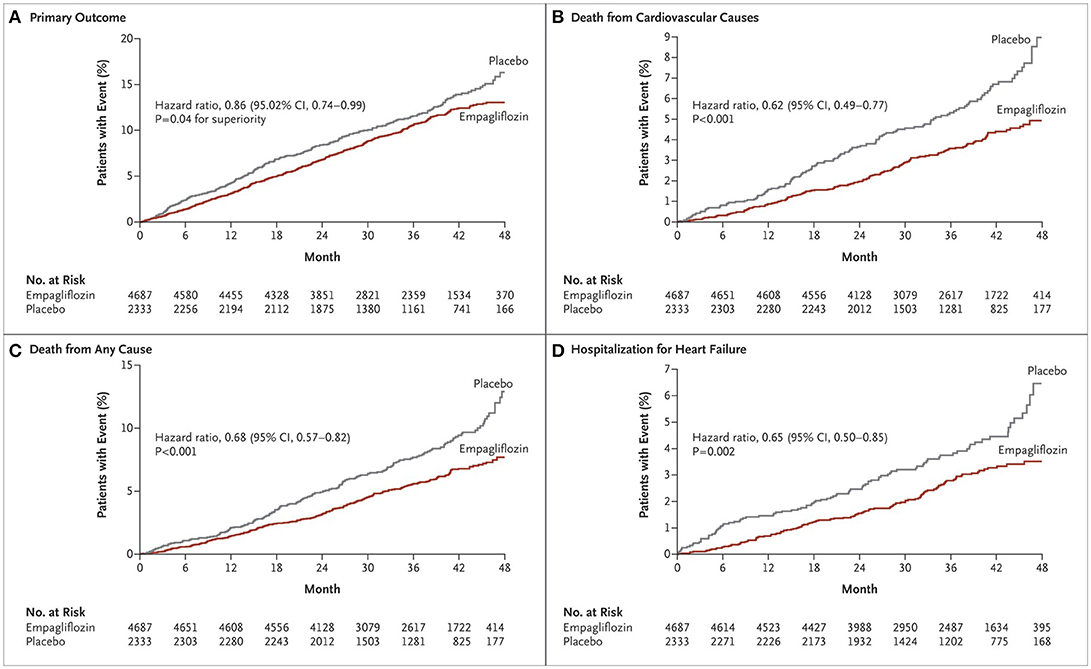
Figure 1. Cardiovascular outcomes and death from any cause in EMPA-REG OUTCOME (16). Cumulative incidence of the primary outcome (death from cardiovascular causes, nonfatal myocardial infarction, or nonfatal stroke) (A); Cumulative incidence of death from cardiovascular causes (B); Kaplan-Meier estimate for death from any cause (C); Cumulative incidence of hospitalization for heart failure (D). Copyright 2015. Massachusetts Medical Society. Reprinted with permission from Massachusetts Medical Society.
Similar findings were replicated in the Canagliflozin Cardiovascular Assessment Study (CANVAS) which investigated another SGLT-2 inhibitor, canagliflozin vs. placebo (18). The trial enrolled 10,142 patients with type 2 DM and high cardiovascular risk with a median follow-up of 3.6 years. Like EMPA-REG OUTCOME, CANVAS demonstrated a significant reduction in the composite outcome of cardiovascular death, MI, or stroke in the canagliflozin group as compared to placebo (HR: 0.86, 95% CI: 0.75–0.97, P < 0.001 for non-inferiority, P = 0.02 for superiority). However, there was not a statistically significant difference in the individual components of cardiovascular death (HR: 0.87, 95% CI: 0.72–1.06), MI (HR: 0.85, 95% CI: 0.69–1.05), or stroke (HR: 0.90, 95% CI: 0.71–1.15). All-cause mortality was also not significantly reduced (HR: 0.87, 95% CI: 0.74–1.01). Heart failure hospitalizations were significantly reduced in the canagliflozin arm by 33% (HR: 0.67, 95% CI: 0.52–0.87). Of note, there was also an increased risk of amputation with canagliflozin (6.3 vs. 3.4 events per 1,000 patient-years; HR: 1.97, 95% CI: 1.41–2.75).
Interestingly, the difference in mortality and cardiovascular endpoints occurred relatively early (within the first few weeks) in these trials. The possible explanations for these observations include osmotic diuresis leading to improved cardiac hemodynamics by reduction in left ventricular afterload, lowering of body weight due to calorie and fluid losses, and lowering of blood pressure (19, 20). Heart failure hospitalizations were reduced in the SGLT-2 inhibitor arm of the CANVAS and EMPA-REG OUTCOME trials, likely due to this osmotic diuretic effect. Another proposed mechanism of cardiovascular benefit may be a shift in fuel energetics to the myocardial cells via increased ketosis (with a rare but serious adverse event of non-hyperglycemic ketoacidosis), which is a preferred substrate for cardiac tissue, as opposed to free fatty acids (21). This improvement in metabolic efficiency is theorized to translate to CVD benefit. Another important finding seen in these studies was the improvement in “hard” renal outcomes. In both canagliflozin and empagliflozin, there was an improvement, as compared to placebo, in the composite outcome of >40% reduction in GFR, need for renal replacement therapy, or death resulting from renal disease (HR: 0.60, 95% CI: 0.47–0.77 for canagliflozin, HR: 0.61, 95% CI: 0.53–0.70 for empagliflozin) (18, 22). These medications were also noted to reduce the progression ofalbuminuria in canagliflozin in CANVAS (HR: 0.73, 95% CI: 0.67–0.79), though the rate of incident albuminuria was not different between placebo and empagliflozin in EMPA- REG. The proposed mechanism for improved renal outcomes is unclear, though it is suspected to be secondary to decreased plasma volume with concomitant reduction in hyperfiltration and intraglomerular pressure (23).
Importantly, a multinational, observational study in 306,156 adults with type 2 DM and only 13% prevalence of established CVD was undertaken in the Comparative Effectiveness of Cardiovascular Outcomes in New Users of SGLT-2 Inhibitors (CVD-REAL) Study (24). This population was in contrast to the study populations of EMPA-REG OUTCOME and CANVAS, who had established CVD or were otherwise at high CV risk. The main medications used in the study were canagliflozin (53%), dapagliflozin (42%), and empagliflozin (5%). SGLT-2 inhibitors were associated with a lower risk of death in individuals with and without CVD (HR: 0.56, 95% CI: 0.44–0.70; HR: 0.56, 95% CI: 0.50–0.63) as well as a reduction in heart failure hospitalizations (HR: 0.72, 95% CI: 0.63–0.82; HR: 0.61, 95% CI: 0.48–0.78). The importance of the mortality benefit noted even in patients without established CVD at baseline cannot be overstated and is a finding that has not been observed with any other class of anti-diabetic medications. Additionally, there was no significant heterogeneity between the effects among the SGLT-2 inhibitor used, indicating the observed benefits are likely a class effect. The Multicenter Trial to Evaluate the Effect of Dapagliflozin on the Incidence of Cardiovascular Events (DECLARE-TIMI 58) is ongoing and is investigating the cardiovascular outcomes of dapagliflozin in patients with established CVD or risk factors for CVD.
There are several important side effects for patients to be aware of prior to initiating treatment with an SGLT-2 inhibitor. An increased rate of genital mycotic infections, volume depletion and dehydration, and increased urinary tract infections are likely secondary to the mechanism of action of this class (glucosuria leading to osmotic diuresis) (25). Additionally, there is a rare but serious, side effect of euglycemic diabetic ketoacidosis as discussed previously. Briefly, this complication is, in part, a result of persistent glycosuria triggering a sequence of metabolic changes that leads to decreased insulin production and increased glucagon secretion, stimulating enhanced ketogenesis (26). This potential adverse event requires vigilance by the clinician as the lack of significant hyperglycemia can lead to delayed or missed diagnosis of diabetic ketoacidosis. Canagliflozin was also associated with a small but statistically significant increase in the risk of bone fractures and amputation. The exact mechanism of the higher rates of amputation with canagliflozin is unknown, though a proposed mechanism is volume depletion leading to circulatory failure in distal peripheral arterial beds (27). This has led to an FDA warning regarding avoiding canagliflozin in individuals at risk for amputation, namely those with a history of prior amputation, peripheral vascular disease, neuropathy, and diabetic foot ulcers (28). These medications are contraindicated in signficant renal disease (GFR < 30 ml/min) and should be used with caution in the elderly and frail given the risk of volume depletion (29).
GLP-1 Agonists
The GLP-1 receptor agonists, liraglutide and semaglutide, have also demonstrated cardiovascular benefits in individuals with diabetes and high-risk for CVD. GLP-1 receptor agonists exert their anti-hyperglycemic effect by potentiating insulin secretion, decreasing postprandial glucagon, delaying gastric emptying, and promoting weight loss (30). GLP-1, along with glucose-dependent insulinoptropic polypeptide (GIP), is responsible for the incretin effect, which is the augmentation of insulin secretion post-prandially after the oral ingestion of glucose (31).
In the Liraglutide Effect and Action in Diabetes: Evaluation of Cardiovascular Outcomes Results-A Long Term Evaluation (LEADER) trial, 9,340 patients with type 2 DM and high-risk for CVD or with known CVD were followed over 3.8 years (32). The primary outcome of CV death, non-fatal MI, or non-fatal stroke occurred in 13.0% of patients in the group randomized to liraglutide as compared to 14.9% in the control arm (HR: 0.87, 95% CI: 0.78–0.97, P < 0.001 for non-inferiority, P = 0.01 for superiority) (Figure 2). Cardiovascular death was significantly reduced in the liraglutide group (4.7 vs. 6.0%, HR: 0.78, 95%CI: 0.66–0.93, P = 0.007). Though the other components of the primary endpoint were numerically in favor of liraglutide, they were not found to be statistically significant (non-fatal MI: HR: 0.88, 95% CI: 0.75–1.03, p = 0.11; non-fatal stroke: HR: 0.89, 95% CI: 0.72–1.11, p = 0.30). Additionally, all-cause mortality was reduced in the liraglutide arm (HR: 0.85, CI: 0.74–0.97, p = 0.02). The liraglutide arm also had an improvement in A1C (−0.40%, 95% CI: −0.45 to −0.34%), weight (−2.3 kg, 95% CI: −2.0 to −2.5 kg), and systolic blood pressure (−1.2 mmHg, 95% CI: −0.5 to −1.9 mmHg). Given the improvement in the primary endpoint seen with liraglutide, the FDA approved its use for reducing the risk of major adverse cardiovascular events (MACE) in individuals with type 2 DM and known CVD in 2017 (33).
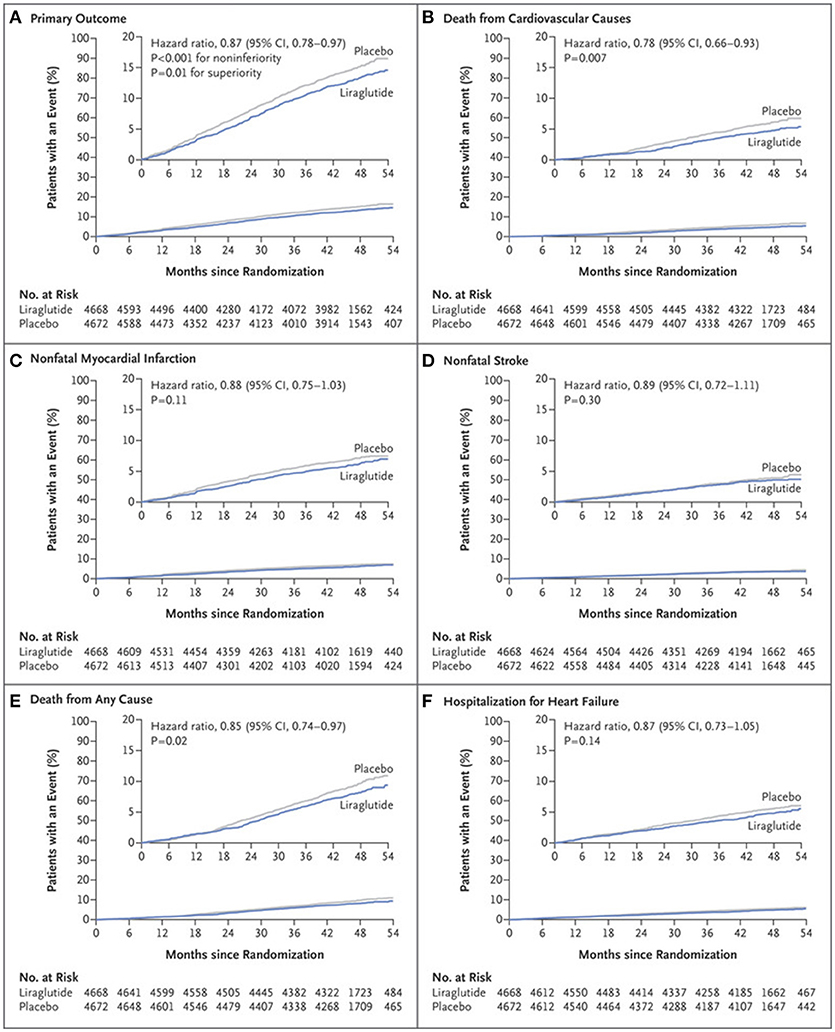
Figure 2. Primary and exploratory outcomes in LEADER trial (32). Cumulative incidence of primary composite outcome (A); Cumulative incidence of death from cardiovascular causes (B); Cumulative incidence of nonfatal myocardial infarction (C); Cumulative incidence of nonfatal stroke (D); Cumulative incidence of death from any cause (E); Cumulative incidence of hospitalization for heart failure (F). Copyright 2016. Massachusetts Medical Society. Reprinted with permission from Massachusetts Medical Society.
In the Semaglutide in Subjects with Type 2 Diabetes (SUSTAIN-6) study, 3,297 patients with CVD or at high risk were randomized to semaglutide, at 0.5 or 1.0 mg, or placebo for 104 weeks (34). A 26% reduction in the primary endpoint of CV death, non-fatal MI, and non-fatal stroke was observed in subjects randomized to semaglutide (HR: 0.74, 95% CI: 0.58–0.95, P < 0.001). However, in contrast to LEADER, CV mortality was not significantly reduced (HR: 0.98, 95% CI: 0.65–1.48, p = 0.92), though non-fatal stroke was improved (HR: 0.61, 95% CI: 0.38–0.99, p = 0.04) along with a non-significant trend to lower rates of MI (HR: 0.74, 95% CI: 0.51–1.08, p = 0.12). Similar to LEADER, as compared to placebo there was an improvement in glycemic control (A1C −0.7 and −1.0% in those receiving 0.5 and 1.0 mg, respectively; p < 0.001 for both groups), body weight (−2.9 kg in those receiving 0.5 mg semaglutide, −4.3 kg in those receiving 1.0 mg; p < 0.001 for both groups), and systolic blood pressure (−1.3 mmHg in the 0.5 mg group, −2.6 mmHg in the 1.0 mg group; p = 0.10 and < 0.001, respectively). Importantly, more patients in the semaglutide group stopped treatment due to adverse events (12.9 vs. 6.7% in placebo), which were namely gastrointestinal complaints. Additionally, there appeared to be a higher rate of retinopathy complications in the semaglutide arm (HR: 1.76, 95%CI: 1.11–2.78, p = 0.02). The mechanism of this is thought due to worsening of pre-existing diabetic retinopathy associated with rapid and large improvements of glycemic control, a phenomenon noted with insulin as well and not thought to be a deleterious effect of semaglutide (35).
Additionally, there is recent evidence of another GLP1 receptor agonists, albiglutide, showing CV benefit. In the Albiglutide and Cardiovascular Outcomes in Patients with Type 2 Diabetes and Cardiovascular Disease (HARMONY) trial, 10,793 patients were followed for a median of 1.8 years (36). This trial demonstrated a reduction in the composite primary outcomes of CV death, MI, or stroke (HR: 0.78, 95% CI: 0.68–0.90, p < 0.0001 for non-inferiority; p = 0.0006 for superiority). In the individual components, there was no difference in CV death or stroke, though there was a significant reduction in MI (HR: 0.75, 95% CI: 0.61–0.90, p = 0.003). There was no difference in heart failure hospitalization or all-cause mortality between albiglutide and placebo.
There are several important considerations within these trials. The reduction in the primary end point was driven by significantly lower CV mortality in LEADER. While there was numerical reduction in MI and stroke rates, this did not reach statistical significance. SUSTAIN- 6 also showed composite CV event reduction with semaglutide, which was driven by a significant reduction in stroke (1.6 vs. 2.7% in placebo; P = 0.04) rather than CV mortality. HARMONY did not show any mortality benefit to albiglutide as compared to placebo, though did show a statistically significant reduction in MI. Neither liraglutide, albiglutide, or semaglutide had a significant effect on HF admissions, suggesting a different mechanism of action than SGLT2 inhibitors. Though not completely understood, it has been suspected that these drugs may have more of an anti-atherothrombotic effect given the end points improved (MI, stroke) as well as the timing of the benefit seen later in the trials (months to years) in contrast to when the benefits were seen with SGLT-2 inhibitors (weeks). However, given the magnitude of the CV mortality improvement seen in LEADER, if an anti-atherogenic mechanism was primarily responsible, a more convincing reduction in MI or stroke would be expected (neither were significantly reduced in LEADER). Other potential effects like blood pressure lowering, weight reduction and avoidance of hypoglycemia may be contributory to improved CV outcomes (37).
There are currently five GLP-1 agonists that are available for clinical use. These agents produce significant improvement in glycemic control in association with modest weight loss. Thus far, only liraglutide 1.2 to 1.8 mg SQ daily has been approved by the FDA with an indication for reducing the risk of cardiovascular events in individuals with T2DM and CVD. Lixisenatide and Exenatide are two other GLP-1 agonists that have also been scrutinized within the context of CVOTs. However, neither of these drugs demonstrated superiority over placebo to reduce the composite endpoint of stroke, MI, and cardiovascular death that was seen in LEADER and SUSTAIN-6 (38, 39).
The most common side effects with GLP-1 agonists are gastrointestinal in nature, namely diarrhea and vomiting. These side effects occur early, but tend to be transient (40). Also given that this class of medications is renally excreted, a GFR < 30 ml/min is a contraindication to this therapy.
Medications With No Effect on CV Outcomes
Insulin and sulfonylureas are two medications that have not demonstrated CV benefit. They should be considered as second- or third-line agents, after having prioritized the medications that have demonstrated improvement in CV outcomes (i.e., metformin, SGLT-2 inhibitors, GLP-1 receptor agonists).
Insulin
Subcutaneous insulin therapy should be considered in patients with: (1) renal or hepatic impairment that precludes the safe use of an oral hypoglycemic, (2) individuals failing to reach their glycemic target on oral hypoglycemics alone, (9) and/or (3) if initiation of treatment is occurring in the inpatient setting (41). Insulin therapy is eventually required by a significant proportion of individuals with type 2 DM given the progressive decrease in insulin production associated with long-standing type 2 DM (42). Generally glucose control is improved by insulin therapy in patients who do not reach their glycemic target on alternative regimens (9). Improved glycemic control has demonstrated improvement in microvascular disease (i.e., retinopathy, nephropathy, neuropathy) (43). Unfortunately, the Outcome Reduction with an Insulin Glargine Intervention (ORIGIN) trial did not show a reduction in macrovascular outcomes when a strategy of early implementation of subcutaneous insulin-based therapies were employed (43). Additionally, the risk of hypoglycemia is a major adverse effect of insulin therapy that can lead to worsened cardiovascular outcomes and death (44).
Sulfonylureas
Sulfonylureas are the oldest class of oral glucose-lowering agents. These medications exert their anti-hyperglycemic effect by increasing endogenous insulin secretion via the ATP- sensitive K channel on beta cells (9). Although the first generation of sulfonylureas exhibited an increase in CV and all-cause mortality, this observation has not been recapitulated with the second and third generation sulfonylureas. The major concern with this class relates to its main side effects of hypoglycemia, an independent contributor to CV death, and weight gain (45). For these reasons, sulfonylureas should be considered second- or third-line agents for the treatment of DM in individuals with cardiovascular disease. In those without CVD, sulfonylureas are considered a second line agent according to the joint American Diabetes Association and European Association for the Study of Diabetes Consensus algorithm (9).
Medications That May Have An Unfavorable Effect on CV Outcomes
Thiazolidinediones
Several oral hypoglycemics should be avoided in those with cardiovascular disease, namely the thiazolidinediones, rosiglitazone, and pioglitazone. Peripheral edema is a noted side effect of this drug class, mediated by increased sodium reabsorption by the renal peroxisome proliferator-activated receptor γ-dependent pathway in the collecting tubules leading to increased plasma volume and subsequent fluid overload (46). Both rosiglitazone and pioglitazone have been associated with an increased risk of heart failure (47, 48). Additionally, rosiglitazone had previously been associated with an increased risk of MI and cardiovascular mortality when evaluated in a 2007 meta-analysis of 42 trials, though this has been a source of contention as other analyses do not suggest safety issues with this agent (49). Nonetheless, the question of increased CV events and mortality lead to the 2008 FDA mandate requiring dedicated safety trials as part of the development programs of new diabetes drugs, as discussed previously. Another thiazolidinedione, pioglitazone, did not show the initial concerning signals that rosiglitazone was thought to show (50). In fact, recent evidence has indicated that pioglitazone may be associated with a reduced risk of MACE, though the risks of heart failure and bone fracture were increased (49). As such, thiazolidinediones should be avoided in those with or at risk of heart failure given the concerns related to sodium and water retention.
Dipeptidyl Peptidase-4 Inhibitors
The safety of DPP-4 inhibitors was evaluated in several CVOTs following the FDA mandate in 2008. In the Saxagliptin Assessment of Vascular Outcomes Recorded in Patients with Diabetes Mellitus-Thrombolysis in Myocardial Infarction 53 (SAVOR-TIMI 53), the Effect of Sitagliptin on Cardiovascular Outcomes in Type 2 Diabetes (TECOS), andthe Examination of Cardiovascular Outcomes with Alogliptin vs. Standard of Care in Patients with Type 2 Diabetes Mellitus and Acute Coronary Syndromes (EXAMINE) trials, saxagliptin, sitagliptin, and alogliptin exhibited similar rates of CVD events as compared to placebo (51–53). However, hospitalization for heart failure in individuals treated with saxagliptin was significantly higher as compared to placebo (3.5 vs. 2.8%; hazard ratio, 1.27; 95% CI: 1.07–1.51; P = 0.007).
Alogliptin and sitagliptin, however, showed a neutral effect on heart failure outcomes. There has not been any cardiovascular benefit observed with this class. In fact, the potential harm related to an increase in heart failure hospitalizations has led to a recommendation away from using these drugs in individuals at risk for CVD, though this is a topic of debate and remains to be further studied.
Future Directions
The above data, in conjunction with the lack of cardiovascular or mortality benefit seen with glycemic control alone, should alter the established paradigm of glycemic control as the pillar of DM treatment. The slavish reliance of targeting a hemoglobin A1C to < 7 has not been fruitful for managing macrovascular risk (54). While managing dysglycemia is important for mitigating microvascular risk, no data demonstrate meaningful improvements in cardiovascular outcomes with aggressive glucose control (4–6, 8). Two new classes of drugs, the SGLT-2 inhibitors and GLP1-receptor agonists, show great promise in transforming the treatment of diabetes by independently improving cardiovascular outcomes, above and beyond what can be achieved with standard of care management. Nonetheless, a great deal remains to be discovered in terms of the mechanisms by which these drugs elicit their positive effects.
While the new diabetes drugs have ushered in an exciting new era of managing cardiovascular risk, additional CVOTs are needed. The landmark studies with the SGLT-2 inhibitors and GLP-1 receptor agonists enrolled only high-risk patients with DM and CVD. The utility and safety of these medications in the general population is an important question that remains to be answered. The impact of these medications is likely to be different when used in in lower risk patients. Additionally, the evaluation of their safety is an important step in determining the risk and benefit calculations clinicians rely on while individualizing treatment. Trials such as DECLARE-TIMI58, VERTIS CV, and SCORED investigating dapagliflozin, ertugliflozin, and sotagliflozin, respectively, are currently under way for the investigation of novel SGLT2-inhibitors. Additionally, PIONEER 6 and REWIND trials investigating semaglutide and albiglutide, respectively, are ongoing as well (55). These medications have shown such a great effect that trials are currently underway to investigate their effects in heart failure populations without overt DM, as in the EMPEROR trial with empagliflozin and the DAPA-HF trial with dapagliflozin. We are at the dawn of a new era in the management of diabetes and cardiovascular risk, and the future is bright!
Author Contributions
All authors listed have made a substantial, direct and intellectual contribution to the work, and approved it for publication.
Funding
MS was supported by NIH K12HD043488.
Conflict of Interest Statement
MS reports advisory activities with the following companies: Regeneron, Novartis, Esperion.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. Cubbon RM, Wheatcroft SB, Grant PJ, Gale CP, Barth JH, Sapsford RJ, et al. Temporal trends in mortality of patients with diabetes mellitus suffering acute myocardial infarction: a comparison of over 3000 patients between 1995 and 2003. Eur Heart J. (2007) 28:540–5. doi: 10.1093/eurheartj/ehl510
2. Centers for Disease Control and Prevention. National Diabetes Statistics Report: Estimates of Diabetes and its Burden in the United States. Atlanta, GA (2014).
3. Lloyd-Jones DM, Leip EP, Larson MG, D'Agostino RB, Beiser A, Wilson PW, et al. Prediction of lifetime risk for cardiovascular disease by risk factor burden at 50 years of age. Circulation (2006) 113:791–8. doi: 10.1161/CIRCULATIONAHA.105.548206
4. Gæde P, Lund-Andersen H, Parving HH, Pedersen O. Effect of a multifactorial intervention on mortality in type 2 diabetes. N Engl J Med. (2008) 358:580–91. doi: 10.1056/NEJMoa0706245
5. Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34). UK Prospective Diabetes Study (UKPDS) Group. Lancet. (1998) 352:854–65.
6. Ray KK, Seshasai SR, Wijesuriya S, Sivakumaran R, Nethercott S, Preiss D, et al. Effect of intensive control of glucose on cardiovascular outcomes and death in patients with diabetes mellitus: a meta-analysis of randomised controlled trials. Lancet (2009) 373:1765–72. doi: 10.1016/S0140-6736(09)60697-8
7. American Diabetes Association. Standards of medical care in diabetes-−2018. Diabetes care. (2018) 41:S1. doi: 10.2337/dc18-Sint01
8. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). UK Prospective Diabetes Study (UKPDS) Group. Lancet (1998) 352:837–53.
9. Nathan DM, Buse JB, Davidson MB, Heine RJ, Holman RR, Sherwin R, Zinman B. Management of hyperglycemia in type 2 diabetes: a consensus algorithm for the initiation and adjustment of therapy: a consensus statement from the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care (2006) 29:1963–72. doi: 10.2337/dc06-9912
10. Sattar N, Petrie MC, Zinman B, Januzzi JL. Novel diabetes drugs and the cardiovascular specialist. J Am Coll Cardiol. (2017) 69:2646–56. doi: 10.1016/j.jacc.2017.04.014
11. Roussel R, Travert F, Pasquet B, Wilson PW, Smith SC Jr, Goto S, et al. Metformin use and mortality among patients with diabetes and atherothrombosis. Arch Intern Med. (2010) 170:1892–9. doi: 10.1001/archinternmed.2010.409
12. Maruthur NM, Tseng E, Hutfless S, Wilson LM, Suarez-Cuervo C, Berger Z, et al. Diabetes medications as monotherapy or metformin-based combination therapy for type 2 diabetes: a systematic review and meta-analysis. Ann Int Med. (2016) 164:740–51. doi: 10.7326/M15-2650
13. Yin M, van der Horst IC, van Melle JP, Qian C, van Gilst WH, Sillje HH, de Boer RA. Metformin improves cardiac function in a nondiabetic rat model of post-MI heart failure. Am J Physiol Heart Circul Physiol. (2011) 301:H459–68. doi: 10.1152/ajpheart.00054.2011
14. Paneni F, Costantino S, Cosentino F. Metformin and left ventricular remodeling after acute myocardial infarction: molecular mechanisms and clinical implications. G Ital Cardiol. (2015) 16:225–31. doi: 10.1714/1848.20186
15. Kalra S. Sodium glucose co-transporter-2 (SGLT2) inhibitors: a review of their basic and clinical pharmacology. Diab Ther. (2014) 5:355–66. doi: 10.1007/s13300-014-0089-4
16. Zinman B, Wanner C, Lachin JM, Fitchett D, Bluhmki E, Hantel S, et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. (2015) 373:2117–28. doi: 10.1056/NEJMoa1504720
17. FDA News Release. FDA Approves Jardiance to Reduce Cardiovascular Death in Adults with Type 2 Diabetes. FDA's Website. Available online at: https://www.fda.gov/newsevents/newsroom/pressannouncements/ucm531517.htm
18. Neal B, Perkovic V, Mahaffey KW, de Zeeuw D, Fulcher G, Erondu N, et al. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med. (2017) 377:644–57. doi: 10.1056/NEJMoa1611925
19. Neeland IJ, McGuire DK, Chilton R, Crowe S, Lund SS, Woerle HJ, et al. Empagliflozin reduces body weight and indices of adipose distribution in patients with type 2 diabetes mellitus. Diab Vasc Dis Res. (2016) 13:119–26. doi: 10.1177/1479164115616901
20. Tikkanen I, Narko K, Zeller C, Green A, Salsali A, Broedl UC et al. Empagliflozin reduces blood pressure in patients with type 2 diabetes and hypertension. Diabetes Care (2015) 38:420–8. doi: 10.2337/dc14-1096
21. Mudaliar S, Alloju S, Henry RR. Can a shift in fuel energetics explain the beneficial cardiorenal outcomes in the EMPA-REG OUTCOME Study? A unifying hypothesis. Diabetes Care (2016) 39:1115–22. doi: 10.2337/dc16-0542
22. Wanner C, Inzucchi SE, Lachin JM, Fitchett D, von Eynatten M, Mattheus M, et al. Empagliflozin and progression of kidney disease in type 2 diabetes. N Engl J Med. (2016) 375:323–34. doi: 10.1056/NEJMoa1515920
23. Cherney DZ, Perkins BA, Soleymanlou N, Maione M, Lai V, Lee A, et al. Renal hemodynamic effect of sodium- glucose cotransporter 2 inhibition in patients with type 1 diabetes mellitus. Circulation (2014) 129:587–97. doi: 10.1161/CIRCULATIONAHA.113.005081
24. Cavender MA, Norhammar A, Birkeland KI, Jørgensen ME, Wilding JP, Khunti K, et al. SGLT-2 Inhibitors and cardiovascular risk. An analysis of CVD-REAL. J Am Coll Cardiol. (2018) 71:2497–506. doi: 10.1016/j.jacc.2018.01.085
25. Trujillo JM, Nuffer WA. Impact of sodium- glucose cotransporter 2 inhibitors on nonglycemic outcomes in patients with type 2 diabetes. Pharmacotherapy (2017) 37:481–91. doi: 10.1002/phar.1903
26. Rosenstock J, Ferrannini E. Euglycemic diabetic ketoacidosis: a predictable, detectable, and preventable safety concern with SGLT2 Inhibitors. Diabetes Care (2015) 38:1638–42. doi: 10.2337/dc15-1380
27. Tanaka A, Node K. Increased amputation risk with canagliflozin treatment: behind the large cardiovascular benefit? Cardiovas Diabetol. (2017) 16:129. doi: 10.1186/s12933-017-0611-x
28. FDA Drug Safety Communication. FDA Confirms Increased Risk of Leg and Foot Amputations With the Diabetes Medicine Canagliflozin (Invokana, Invokamet, Invokamet XR) [news release]. FDA's website. Available online at: https://www.fda.gov/Drugs/DrugSafety/ucm557507.htm
29. Du YF, Ou HY, Beverly EA, Chiu CJ. Achieving glycemic control in elderly patients with type 2 diabetes: a critical comparison of current options. Clin Intervent Aging (2014) 9:1963–80. doi: 10.2147/CIA.S53482
30. Holst JJ, Ørskov C. The incretin approach for diabetes treatment. modulation of islet hormone release by GLP-1 agonism. Diabetes (2004) 53:S197–204. doi: 10.2337/diabetes.53.suppl_3.S197
31. Nauck MA, Kleine N, Orskov C, Holst JJ, Willms B, Creutzfeldt W. Normalization of fasting hyperglycaemia by exogenous glucagon-like peptide 1 (7-36 amide) in type 2 (non- insulin-dependent) diabetic patients. Diabetologia (1993) 36:741–4. doi: 10.1007/BF00401145
32. Marso SP, Daniels GH, Brown-Frandsen K, Kristensen P, Mann JF, Nauck MA, et al. Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med. (2016) 375:311–22. doi: 10.1056/NEJMoa1603827
33. FDA Endocrinologic and Metabolic Drug Advisory Committee. LEADER: Liraglutide Effect and Action in Diabetes: Evaluation of Cardiovascular Outcome Results. Available online at: https://www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/Drugs/EndocrinologicandMetabolicDrugsAdvisoryCommittee/UCM563335.pdf
34. Marso SP, Bain SC, Consoli A, Eliaschewitz FG, Jódar E, Leiter LA, et al. Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med. (2016) 375:1834–44. doi: 10.1056/NEJMoa1607141
35. Vilsboll T, Bain SC, Leiter LA, Lingvay I, Matthews D, Simo R, et al. Semaglutide, reduction in glycated haemoglobin and the risk of diabetic retinopathy. Diab Obes Metabol. (2018) 20:889–897. doi: 10.1111/dom.13172
36. Hernandez AF, Green JB, Janmohamed S, Ralph BD, Christopher BG, Nigel PJ, et al. Albiglutide and cardiovascular outcomes in patients with type 2 diabetes and cardiovascular disease (Harmony Outcomes): a double-blind, randomised placebo-controlled trial. Lancet (2018) 392:1519–29.
37. Shah M, Vella A. Effects of GLP-1 on appetite and weight. Rev Endocr Metabol Disord. (2014) 15:181–7. doi: 10.1007/s11154-014-9289-5
38. Pfeffer MA, Claggett B, Diaz R, Dickstein K, Gerstein HC, Køber LV, et al. Lixisenatide in patients with type 2 diabetes and acute coronary syndrome. N Engl J Med. (2015) 373:2247–57. doi: 10.1056/NEJMoa1509225
39. Holman RR, Bethel MA, Mentz RJ, Thompson VP, Lokhnygina Y, Buse JB, et al. Effects of once-weekly exenatide on cardiovascular outcomes in type 2 diabetes. N Engl J Med. (2017) 377:1228–39. doi: 10.1056/NEJMoa1612917
40. Garber AJ. Long-Acting Glucagon-Like Peptide 1 Receptor Agonists. A review of their efficacy and tolerability. Diabetes Care (2011) 34:S279–84. doi: 10.2337/dc11-s231.
41. Inzucchi SE. Management of hyperglycemia in the hospital setting. N Engl J Med. (2006) 355:1903–11. doi: 10.1056/NEJMcp060094
42. Kahn SE, Haffner SM, Heise MA, Herman WH, Holman RR, Jones NP, et al. Glycemic durability of rosiglitazone, metformin, or glyburide monotherapy. N Engl J Med. (2006) 355:2427–43. doi: 10.1056/NEJMoa066224
43. Investigators OT, Gerstein HC, Bosch J, Dagenais GR, Diaz R, Jung H, et al. Basal insulin and cardiovascular and other outcomes in dysglycemia. N Engl J Med. (2012) 367:319–28. doi: 10.1056/NEJMoa1203858
44. Zoungas S, Patel A, Chalmers J, de Galan BE, Li Q, Billot L, et al. Severe hypoglycemia and risks of vascular events and death. N Engl J Med. (2010) 363:1410–18. doi: 10.1056/NEJMoa1003795
45. Nunes AP, Iglay K, Radican L, Engel SS, Yang J, Doherty MC, et al. Hypoglycaemia seriousness and weight gain as determinants of cardiovascular disease outcomes among sulfonylurea users. Diab Obes Metabol. (2017) 19:1425–35. doi: 10.1111/dom.13000
46. Zhang H, Zhang A, Kohan DE, Nelson RD, Gonzalez FJ, Yang T. Collecting duct- specific deletion of peroxisome proliferator-activated receptor γ blocks thiazolidinedione- induced fluid retention. Proc Nat Acad Sci USA. (2005) 102:9406–11. doi: 10.1073/pnas.0501744102
47. Lincoff AM, Wolski K, Nicholls SJ, Nissen SE. Pioglitazone and risk of cardiovascular events in patients with type 2 diabetes mellitus: a meta-analysis of randomized trials. J Am Med Assoc. (2007) 298:1180–8. doi: 10.1001/jama.298.10.1180
48. Nesto RW, Bell D, Bonow RO, Fonseca V, Grundy SM, Horton ES, et al. Thiazolidinedione use, fluid retention, and congestive heart failure: a consensus statement from the American Heart Association and American Diabetes Association. Circulation (2003) 108:2941–8. doi: 10.1161/01.CIR.0000103683.99399.7E
49. Nissen SE, Wolski K. Effect of rosiglitazone on the risk of myocardial infarction and death from cardiovascular causes. N Engl J Med. (2007) 356:2457–71. doi: 10.1056/NEJMoa072761
50. Liao HW, Saver JL, Wu YL, Chen TH, Lee M, Ovbiagele B. Pioglitazone and cardiovascular outcomes in patients with insulin resistance, pre-diabetes and type 2 diabetes: a systematic review and meta-analysis. BMJ Open (2017) 7:e013927. doi: 10.1136/bmjopen-2016-013927
51. White WB, Bakris GL, Bergenstal RM, Cannon CP, Cushman WC, Fleck P, et al. EXamination of cArdiovascular outcoMes with alogliptIN versus standard of carE in patients with type 2 diabetes mellitus and acute coronary syndrome (EXAMINE): a cardiovascular safety study of the dipeptidyl peptidase 4 inhibitor alogliptin in patients with type 2 diabetes with acute coronary syndrome. Am Heart J. (2011) 162:620–6.e1. doi: 10.1016/j.ahj.2011.08.004
52. Green JB, Bethel MA, Armstrong PW, Buse JB, Engel SS, Garg J, et al. Effect of sitagliptin on cardiovascular outcomes in type 2 diabetes. N Engl J Med. (2015) 373:232–42. doi: 10.1056/NEJMoa1501352
53. Scirica BM, Bhatt DL, Braunwald E, Steg PG, Davidson J, Hirshberg B, et al. Saxagliptin and cardiovascular outcomes in patients with type 2 diabetes mellitus. N Engl J Med. (2013) 369:1317–26. doi: 10.1056/NEJMoa1307684
54. Hemmingsen B, Lund SS, Gluud C, Vaag A, Almdal TP, Hemmingsen C, et al. Targeting intensive glycaemic control versus targeting conventional glycaemic control for type 2 diabetes mellitus. Cochrane Database Syst Rev. (2013):CD008143. doi: 10.1002/14651858.CD008143.pub2
Keywords: diabetes, cardiovascular disease, cardiovascular outcomes trials, SGLT-2 inhibitors, GLP1-receptor agonists
Citation: Dhindsa DS, Sandesara PB and Shapiro MD (2018) The Intersection of Diabetes and Cardiovascular Disease—A Focus on New Therapies. Front. Cardiovasc. Med. 5:160. doi: 10.3389/fcvm.2018.00160
Received: 21 September 2018; Accepted: 18 October 2018;
Published: 13 November 2018.
Edited by:
Sang-Hyun Kim, Seoul Boramae Hospital, South KoreaReviewed by:
Sang-Ho Jo, Hallym University Sacred Heart Hospital, South KoreaHack-Lyoung Kim, SMG-SNU Boramae Medical Center, South Korea
Hyeong-Kyu Park, Soonchunhyang University, South Korea
Copyright © 2018 Dhindsa, Sandesara and Shapiro. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Michael D. Shapiro, c2hhcGlybWlAb2hzdS5lZHU=
 Devinder S. Dhindsa
Devinder S. Dhindsa Pratik B. Sandesara
Pratik B. Sandesara Michael D. Shapiro
Michael D. Shapiro