- 1Cardiac Regeneration and Ageing Lab, Institute of Cardiovascular Sciences, School of Life Science, Shanghai University, Shanghai, China
- 2School of Medicine, Shanghai University, Shanghai, China
Muscle atrophy is a common complication of heart failure. At present, there is no specific treatment to reverse the course of muscle atrophy. Exercise training, due to the safety and easy operation, is a recommended therapy for muscle atrophy induced by heart failure. However, the patients with muscle atrophy are weak in mobility and may not be able to train for a long time. Therefore, it is necessary to explore novel targets of exercise protection for muscle atrophy, so as to improve the quality of life and survival rate of patients with muscular atrophy induced by heart failure. This article aims to review latest studies, summarize the evidence and limitations, and provide a glimpse into the future of exercise for the treatment of muscle atrophy induced by heart failure. We wish to highlight some important findings about the essential roles of exercise sensors in muscle atrophy induced by heart failure, which might be helpful for searching potential therapeutic targets for muscle wasting induced by heart failure.
Introduction
Muscle atrophy induced by various factors such as heart failure, is a neuropathy with motor and sensory disorders and caused by synthesis inferior to degradation (1). The first study to evaluate the link between muscle wasting and chronic heart failure in patients showed that about 20% patients with clinically heart failure presented with muscle wasting, exhibiting reduced exercise capacity and reduced left ventricular ejection fraction (2). In the elder patients with chronic heart failure, about 20% prevalence showed loss of muscle mass and muscle function compared with healthy elderly people (3). The main clinical manifestations of muscle atrophy were chronic progressive distal limb myasthenia and atrophy, hypoesthesia and disappearance of tendon reflex, accompanied by skeletal deformities such as high arch foot and scoliosis (4, 5). Ubiquitin proteasome and autophagy, two most important cellular proteolysis systems, regulate muscle atrophy by controlling protein turnover in muscle (6–8). In the two systems, four major signaling pathways (IGF1-AKT-FOXO, myostatin, NF-κB, and glucocorticoids) coordinate protein synthesis and degradation simultaneously in muscle atrophy (9–12).
At present, there is no effective treatment to reverse the course of muscle atrophy induced by heart failure. In order to maximize the recovery of independent activity, improve the quality of life and reduce the occurrence of disability as far as possible, symptomatic supportive treatments including drug, surgical operation and rehabilitation training are mainly recommended for therapy (13–15). It has been found that ascorbic acid can reduce the expression of peripheral myelin protein 22 (PMP22) and significantly improve muscle atrophy. However, it failed to show significant effectiveness in various randomized controlled trials in children and adults with muscle atrophy (16, 17). Surgery is the main choice to correct foot deformities caused by muscle atrophy, especially in the late stage. However, there is still insufficient evidence for the long-term impact on the functional recovery of patients (18). Rehabilitation treatment plays a major role in the management of muscle atrophy, especially exercise therapy, which can encourage patients to move, improve blood circulation, prevent contracture, strengthen unaffected muscle strength, improve gait, improve walking ability and quality of life (19).
Here, we summarize different exercise patterns, known targets of exercise and provide a glimpse into the future of exercise for the treatment of muscle atrophy induced by heart failure. We wish to highlight some important findings about the essential roles of exercise sensors in muscle atrophy induced by heart failure, which might be helpful for searching potential therapeutic targets.
Different Exercise Patterns for the Treatment of Muscular Atrophy
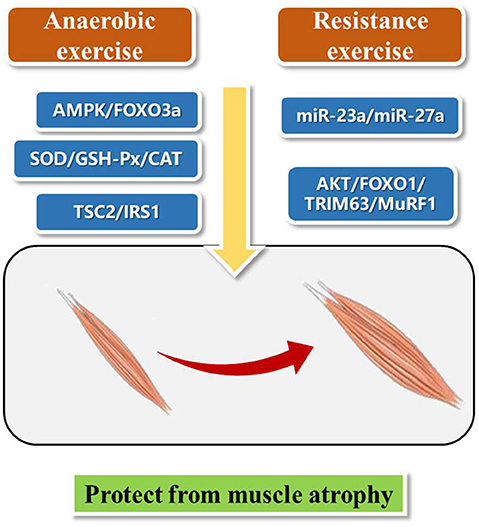
Exercise therapy, leading to increase muscle strength, improve aerobic exercise ability, and protect joints from contracture, is mainly divided into aerobic exercise training and anaerobic exercise training according to whether there is sufficient oxygen supply in the body during exercise (20). At the same time, considering the intensity, duration, frequency, location and activity type, exercise can be divided into endurance and resistance exercise (21). Here, we present aerobic exercise and resistance exercise for the treatment of muscle wasting (Figure 1). We hope to find inspiration from these different exercise patterns and bring new ideas for the future research on exercise protection against muscle atrophy.
Studies have shown that aerobic exercise can improve cardiopulmonary function, muscle strength, and activities of daily living (22, 23). A 24-weeks intermittent training (riding training) clinical trial on patients with muscle atrophy showed that intermittent aerobic exercise can improve muscle strength, motor function and subjective feeling of pain and fatigue of patients with muscle atrophy (24). Eight patients with muscle atrophy underwent treadmill and stretching exercise training for 6 months combined with cardiopulmonary function and proprioception rehabilitation training. The results showed that all participants had increased ankle range of motion and 6-min walking test step length (25). Aerobic exercise improves AMPK and skeletal muscle atrophy (26). Activation of AMPK/FOXO3a signaling pathway leads to the activation of protein degradation system and the loss of skeletal muscle mass. Exercise can inhibit the expression of FOXO3a and its downstream targets, and reduce the activity of protein degradation system, thus promoting the recovery of muscular atrophy (27).
Resistance training is the movement against resistance, such as weightlifting (28). Thirty-two patients with muscle atrophy were randomly divided into resistance training group (18 cases) and control group (14 cases) and a single blind crossover design was used to study a period of 16 weeks. Training group showed significantly increase muscle strength of the left hip flexor muscle, improved hip strength, and no negative effect of exercise (29). Another study showed that resistance training can improve the muscle mass, bone mineral density and peripheral muscle volume of heart transplant patients, so as to prevent and reverse muscle atrophy in heart transplantation patients (30).
Classical Pathways of Exercise Protection for Muscular Atrophy Induced by Heart Failure
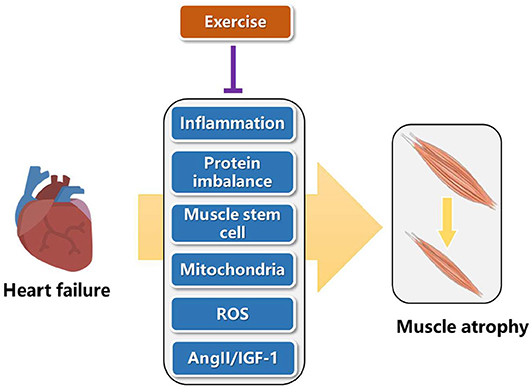
Heart failure can increase inflammation, cause lower protein synthesis than degradation, reduce mitochondrial function, increase reactive oxygen species (ROS), change hormone content such as angiotensin II (AngII) and insulin like growth factor 1 (IGF-1), thus leading to muscle atrophy (31). Here, we present that exercise can reverse the adverse pathways caused by heart failure, so as to alleviate muscle atrophy (Figure 2). In the future, these pathways are good directions to explore novel targets of exercise protection for muscular atrophy.
Chronic inflammation is one of the characteristics of muscle atrophy induced by heart failure (31). Exercise decreases the secretion of inflammatory factors such as TNF-α, IL-6, and C-reactive protein (CRP), which can directly reduce the process of protein hydrolysis and catabolism, reduce the degree of muscle loss, so as to achieve the anti-inflammatory effect of exercise (32, 33).
Muscle atrophy is result from muscle protein loss, caused by more muscle protein decomposition than synthesis. In clinical trials, resistance exercise can increase muscle protein synthesis, reverse skeletal muscle consumption, and increase muscle strength and lean weight mass (34). Exercise can accelerate the activity of amino acid transporter and then quickly and efficiently transport amino acid in plasma into muscle cells through transmembrane transporter, promote muscle protein synthesis, reduce the content of free amino acid in plasma, and upregulate the energy regulation and cell nutrition induction. In addition, exercise promotes muscle protein synthesis, related to the activation of mTOR/P70S6K pathway through regulating the expression of myosin heavy chain (MHC) II, which can improve the protein synthesis of fast muscle in skeletal muscle, and increase the volume and mass of muscle (35). Furthermore, exercise can regulate the negative tension of heart failure patients. Under the intervention of exercise, the appetite of patients is improved, and protein intake is enhanced, which also has a certain role in promoting the maintenance of total muscle protein (36).
Muscle stem cells, also known as satellite cells, mainly function to repair damaged muscle tissue (37). Stem cells are normally in a static state and distributed along muscle fibers. When muscle tissue is damaged, they are awakened and activated and self-proliferate and differentiate into myoblasts. On this basis, myoblasts fuse with each other and gradually become polynuclear muscle fibers, realizing the replacement or repair of damaged muscle tissue, so as to restore or increase the function of muscle tissue (38). Exercise intervention experiment found that after proper aerobic exercise, the number and activity of muscle stem cells were significantly increased, and were not affected by the age of the intervener, which has a positive role in repairing damaged muscle cells and alleviating muscle atrophy (39).
Mitochondria function is impaired by heart failure (40, 41). Exercise can promote the rearrangement of mitochondria in skeletal muscle and promote mitochondria metabolism (42). In addition, exercise can maintain the dynamic balance of mitochondria, improve the quality of mitochondria, increase the number of mitochondria and improve the myosin heavy chain (MHC), leading to the enhancement of muscle strength, the improvement of the body's movement ability and the positive promotion effect (43). Physical exercise could activate autophagy in skeletal muscles accompanying with clearance of damaged cell components and dysfunctional mitochondria, which is crucial for muscle homeostasis (44). Further work extended these observations and demonstrated that exercise-induced autophagy plays an important and previously unrecognized role in muscle metabolism (45). These findings provide a mechanism for explaining the well-known beneficial effects of physical activity in healthy individuals.
Studies have pointed out that heart failure reduces the activity of superoxide dismutase and limits the free radical scavenging system (46). Free radicals may cause structural damage of muscle protein by destroying the normal structure of muscle protein molecule, activate ubiquitin proteasome to recognize the damaged muscle protein, accelerate the degradation of muscle protein and promote muscle atrophy (47). Aerobic exercise can improve the activity of superoxide dismutase (SOD) and reduce the level of free radicals. Four weeks of treadmill exercise in mice enhanced the expression of SOD in gastrocnemius mitochondria, inhibited the chain reaction of free radical production, reduced the production of free radicals, and avoided muscle cell damage caused by imbalance of cell oxidative stress level (48). In clinical human experiments, it is found that proper aerobic exercise can improve the activities of SOD, glutathione peroxidase 1 (GSH-Px) and catalase (CAT), and accelerate the speed and ability of the body to remove free radicals and peroxide products (49).
Heart failure decreased IGF-1 and increased AngII (50, 51). Insulin dominated muscle protein synthesis hormone mainly plays a role in skeletal muscle regeneration and repair, which is mainly reflected in that IGF-1 can inhibit muscle protein decomposition, enhance amino acid transport, accelerate nuclear replication process, promote DNA and RNA production, act on ribosomes, accelerate translation process, and promote muscle protein synthesis. Once the plasma insulin concentration or insulin sensitivity is reduced, it will prevent the body's absorption of amino acids and inhibit the synthesis of muscle protein. Studies have found that appropriate aerobic exercise intervention can improve the expression of TSC2 in muscle cells, inhibit the phosphorylation activity of insulin receptor substrates IRS1-Ser307 and IRS1-Ser636/639, enhance the sensitivity of insulin signal pathway, improve the level and sensitivity of insulin in plasma, strengthen the utilization of glucose in vivo, promote the synthesis of muscle protein, and inhibit muscle atrophy (52). Patients with congestive heart failure elevated AngII serum concentrations and activate the PKD1/HDAC5/TFEB/MuRF1 pathway to induce skeletal muscle wasting (1). MuRF-1, a component of the ubiquitin-proteasome system involved in muscle proteolysis, is increased in the skeletal muscle of patients with heart failure. Exercise training results in reduced MuRF-1 levels, suggesting that it blocks ubiquitin-proteasome system activation (53).
Roles of Microrna in Muscle Atrophy Induced by Heart Failure
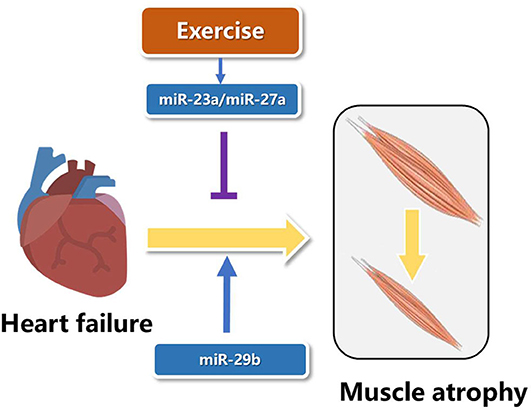
MicroRNAs are short with a length of about 18–25 nucleotides and non-coding RNAs, which function as inhibition of post-transcription of mRNA's translation (54). Two microRNAs from the opposite side of the pre-microRNA name−3p or−5p (55). In recent years, studies have showed that microRNAs play essential roles in the pathogenesis of muscle atrophy induced by heart failure (56). The expression of miR-23a in muscle atrophy was less than controls, and resistance exercise increased the levels of miR-23a and miR-27a. Overexpression of miR-23a/miR-27a attenuated muscle loss, improved grip strength, activated AKT/FOXO1/TRIM63/MuRF1 pathway, reduced myostatin expression and increased the expression of markers of muscle regeneration (57). Although the role of microRNAs in specific types of muscle atrophy has been reported, it is not clear whether there is a common miRNA target in a variety of muscle atrophy. MiR-29b was consistently up-regulated in muscle atrophy induced by various factors, including Angll, aging, denervation, starvation, dexamethasone treatment, and tumor cachexia. AngII and aging are important factors leading to heart failure (58, 59). Overexpression of miR-29b is sufficient to cause muscle atrophy induced by heart failure, and inhibition of miR-29b can prevent and treat muscle atrophy induced by heart failure (Figure 3). Meanwhile, IGF-1 and PI3K were identified as two target genes of miR-29b. MiR-29b mainly induced muscle atrophy by inhibiting IGF1-AKT-PI3K-mTOR pathway (60, 61). Cardiac cachexia is a common complication of heart failure associated with muscle wasting. MiR-29a-3p, miR-29b-3p, miR-210-5p, miR-214, and miR-489 were decreased in muscle wasting during cardiac cachexia (62). In future research, we wish not only to find more microRNAs involved in exercise protection against muscle atrophy induced by heart failure, but also to use these microRNAs in gene therapy and drug development.
The Future Directions of Exercise Protecting Muscle Atrophy Induced by Heart Failure
Muscle atrophy is one of the important characteristics of heart failure. Limb motor dysfunction seriously affects the activities of daily living and quality of life of patients, which is an urgent rehabilitation problem to be solved (63). Inhibition of muscle atrophy is of great significance to maintain the body function of patients, enhance the ability of autonomous exercise, relieve pain and fatigue, improve the quality of life of patients and prolong the survival period. There is no intervention found to totally cure muscle atrophy. Rehabilitation therapy is an important pillar of the treatment of the disease. Exercise therapy can improve the motor function of the upper and lower limbs and improve the quality of life. According to the different clinical symptoms of muscle atrophy, how to select the most appropriate exercise therapy, and how to choose the appropriate orthosis support treatment, in order to achieve symptomatic support treatment and function maintenance, are worth further clinical research in the future. Exercise can alleviate muscle atrophy to a certain extent, and the related mechanism may be related to reducing the inflammatory reaction of patients, promoting the synthesis of muscle protein, increasing the activity of muscle stem cells, improving the functional structure of muscle mitochondria, reducing the degree of fatigue, regulating the level of free radicals and hormones, and improving the quality of life of patients. However, the specific mechanism of exercise intervention in muscular atrophy still needs further study.
Although exercise training is recommended for treating muscle wasting induced by heart failure, the patients with muscle atrophy are weak in mobility and may not be able to train for a long time. Patients with heart failure may have intolerance to exercise. For example, heart failure with preserved ejection fraction (HFpEF) is characterized by exercise intolerance (64, 65). For this part of patients, it is a problem that how to safely and effectively perform exercise therapy. Therefore, it is necessary to explore novel targets of exercise protection for muscle atrophy, so as to improve the quality of life and survival rate of patients with muscular atrophy. Further researches are needed to investigate effective targets and treatment according to exercise in muscle atrophy induced by heart failure. We hope to find inspiration from these different exercise patterns, pathways and microRNAs introduced ahead and bring new ideas for the future research on exercise protection against muscle atrophy. Last but not least, we wish to use these targets in gene therapy and drug development in the future.
Author Contributions
QL wrote the draft and did the figures. JG contributed to the helpful discussion. JX and JD gave the key guidance and edited the draft. All authors read and approved the manuscript.
Funding
This work was supported by the grants from National Natural Science Foundation of China (81722008 and 81911540486 to JX), Innovation Program of Shanghai Municipal Education Commission (2017-01-07-00-09-E00042 to JX), the grant from Science and Technology Commission of Shanghai Municipality (18410722200 and 17010500100 to JX), National Key Research and Development Project (2018YFE0113500 to JX), and the Dawn Program of Shanghai Education Commission (19SG34 to JX).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. Du Bois P, Pablo Tortola C, Lodka D, Kny M, Schmidt F, Song K, et al. Angiotensin II induces skeletal muscle atrophy by activating TFEB-mediated MuRF1 expression. Circ Res. (2015) 117:424–36. doi: 10.1161/CIRCRESAHA.114.305393
2. Fulster S, Tacke M, Sandek A, Ebner N, Tschope C, Doehner W, et al. Muscle wasting in patients with chronic heart failure: results from the studies investigating co-morbidities aggravating heart failure (SICA-HF). Eur Heart J. (2013) 34:512–9. doi: 10.1093/eurheartj/ehs381
3. Suzuki T, Palus S, Springer J. Skeletal muscle wasting in chronic heart failure. ESC Heart Fail. (2018) 5:1099–107. doi: 10.1002/ehf2.12387
4. Burns J, Ryan MM, Ouvrier RA. Evolution of foot and ankle manifestations in children with CMT1A. Muscle Nerve. (2009) 39:158–66. doi: 10.1002/mus.21140
5. Heineke J, Auger-Messier M, Xu J, Sargent M, York A, Welle S, et al. Genetic deletion of myostatin from the heart prevents skeletal muscle atrophy in heart failure. Circulation. (2010) 121:419–25. doi: 10.1161/CIRCULATIONAHA.109.882068
6. Milan G, Romanello V, Pescatore F, Armani A, Paik JH, Frasson L, et al. Regulation of autophagy and the ubiquitin-proteasome system by the FoxO transcriptional network during muscle atrophy. Nat Commun. (2015) 6:6670. doi: 10.1038/ncomms7670
7. Morel J, Palao JC, Castells J, Desgeorges M, Busso T, Molliex S, et al. Regulation of Akt-mTOR, ubiquitin-proteasome and autophagy-lysosome pathways in locomotor and respiratory muscles during experimental sepsis in mice. Sci Rep. (2017) 7:10866. doi: 10.1038/s41598-017-11440-5
8. Odeh M, Tamir-Livne Y, Haas T, Bengal E. P38alpha MAPK coordinates the activities of several metabolic pathways that together induce atrophy of denervated muscles. FEBS J. (2020) 287:73–93. doi: 10.1111/febs.15070
9. Gao Y, Arfat Y, Wang H, Goswami N. Muscle atrophy induced by mechanical unloading: mechanisms and potential countermeasures. Front Physiol. (2018) 9:235. doi: 10.3389/fphys.2018.00235
10. Thoma A, Lightfoot AP. NF-kB and inflammatory cytokine signalling: role in skeletal muscle atrophy. Adv Exp Med Biol. (2018) 1088:267–79. doi: 10.1007/978-981-13-1435-3_12
11. Harish P, Forrest L, Herath S, Dickson G, Malerba A, Popplewell L. Inhibition of myostatin reduces collagen deposition in a mouse model of oculopharyngeal muscular dystrophy (OPMD) with established disease. Front Physiol. (2020) 11:184. doi: 10.3389/fphys.2020.00184
12. Martin AI, Priego T, Lopez-Calderon A. Hormones and muscle atrophy. Adv Exp Med Biol. (2018) 1088:207–33. doi: 10.1007/978-981-13-1435-3_9
13. McGlory C, Gorissen SHM, Kamal M, Bahniwal R, Hector AJ, Baker SK, et al. Omega-3 fatty acid supplementation attenuates skeletal muscle disuse atrophy during two weeks of unilateral leg immobilization in healthy young women. FASEB J. (2019) 33:4586–97. doi: 10.1096/fj.201801857RRR
14. Gandolfi M, Tinazzi M, Magrinelli F, Busselli G, Dimitrova E, Polo N, et al. Four-week trunk-specific exercise program decreases forward trunk flexion in Parkinson's disease: a single-blinded, randomized controlled trial. Parkinsonism Relat Disord. (2019) 64:268–74. doi: 10.1016/j.parkreldis.2019.05.006
15. Bei Y, Shi C, Zhang Z, Xiao J. Advance for cardiovascular health in China. J Cardiovasc Transl Res. (2019) 12:165–70. doi: 10.1007/s12265-018-9852-7
16. Burns J, Ouvrier RA, Yiu EM, Joseph PD, Kornberg AJ, Fahey MC, et al. Ascorbic acid for charcot-marie-tooth disease type 1A in children: a randomised, double-blind, placebo-controlled, safety and efficacy trial. Lancet Neurol. (2009) 8:537–44. doi: 10.1016/S1474-4422(09)70108-5
17. Sereda MW, Nave KA. Animal models of charcot-marie-tooth disease type 1A. Neuromolecular Med. (2006) 8:205–16. doi: 10.1385/NMM:8:1:205
18. Watanabe K. Treatment for patients with charcot-marie-tooth disease: orthopaedic aspects. Brain Nerve. (2016) 68:51–7. doi: 10.11477/mf.1416200345
19. Dhillon RJ, Hasni S. Pathogenesis and management of sarcopenia. Clin Geriatr Med. (2017) 33:17–26. doi: 10.1016/j.cger.2016.08.002
20. Lukacs A, Barkai L. Effect of aerobic and anaerobic exercises on glycemic control in type 1 diabetic youths. World J Diabetes. (2015) 6:534–42. doi: 10.4239/wjd.v6.i3.534
21. Thent ZC, Das S, Henry LJ. Role of exercise in the management of diabetes mellitus: the global scenario. PLoS ONE. (2013) 8:e80436. doi: 10.1371/journal.pone.0080436
22. Lu KD, Cooper DM, Haddad F, Radom-Aizik S. Four months of a school-based exercise program improved aerobic fitness and clinical outcomes in a low-SES population of normal weight and overweight/obese children with asthma. Front Pediatr. (2018) 6:380. doi: 10.3389/fped.2018.00380
23. Gonzales JU, Scheuermann BW. Absence of gender differences in the fatigability of the forearm muscles during intermittent isometric handgrip exercise. J Sports Sci Med. (2007) 6:98–105.
24. El Mhandi L, Millet GY, Calmels P, Richard A, Oullion R, Gautheron V, et al. Benefits of interval-training on fatigue and functional capacities in charcot-marie-tooth disease. Muscle Nerve. (2008) 37:601–10. doi: 10.1002/mus.20959
25. Maggi G, Monti Bragadin M, Padua L, Fiorina E, Bellone E, Grandis M, et al. Outcome measures and rehabilitation treatment in patients affected by charcot-marie-tooth neuropathy: a pilot study. Am J Phys Med Rehabil. (2011) 90:628–37. doi: 10.1097/PHM.0b013e31821f6e32
26. de Lima EA, de Sousa LGO, de STAA, Marshall AG, Zanchi NE, Neto JCR. Aerobic exercise, but not metformin, prevents reduction of muscular performance by AMPk activation in mice on doxorubicin chemotherapy. J Cell Physiol. (2018) 233:9652–62. doi: 10.1002/jcp.26880
27. Fan J, Yang X, Li J, Shu Z, Dai J, Liu X, et al. Spermidine coupled with exercise rescues skeletal muscle atrophy from D-gal-induced aging rats through enhanced autophagy and reduced apoptosis via AMPK-FOXO3a signal pathway. Oncotarget. (2017) 8:17475–90. doi: 10.18632/oncotarget.15728
28. Yoon JR, Ha GC, Kang SJ, Ko KJ. Effects of 12-weeks resistance exercise and interval training on the skeletal muscle area, physical fitness, and mental health in old women. J Exerc Rehabil. (2019) 15:839–47. doi: 10.12965/jer.1938644.322
29. Ramdharry GM, Pollard A, Anderson C, Laura M, Murphy SM, Dudziec M, et al. A pilot study of proximal strength training in charcot-marie-tooth disease. J Peripher Nerv Syst. (2014) 19:328–32. doi: 10.1111/jns.12100
30. Braith RW, Magyari PM, Pierce GL, Edwards DG, Hill JA, White LJ, et al. Effect of resistance exercise on skeletal muscle myopathy in heart transplant recipients. Am J Cardiol. (2005) 95:1192–8. doi: 10.1016/j.amjcard.2005.01.048
31. Lavine KJ, Sierra OL. Skeletal muscle inflammation and atrophy in heart failure. Heart Fail Rev. (2017) 22:179–89. doi: 10.1007/s10741-016-9593-0
32. Toth MJ, Ades PA, Tischler MD, Tracy RP, LeWinter MM. Immune activation is associated with reduced skeletal muscle mass and physical function in chronic heart failure. Int J Cardiol. (2006) 109:179–87. doi: 10.1016/j.ijcard.2005.06.006
33. Cipryan L. The effect of fitness level on cardiac autonomic regulation, IL-6, total antioxidant capacity, and muscle damage responses to a single bout of high-intensity interval training. J Sport Health Sci. (2018) 7:363–71. doi: 10.1016/j.jshs.2016.11.001
34. Gould DW, Lahart I, Carmichael AR, Koutedakis Y, Metsios GS. Cancer cachexia prevention via physical exercise: molecular mechanisms. J Cachexia Sarcopenia Muscle. (2013) 4:111–24. doi: 10.1007/s13539-012-0096-0
35. Engelke S, Koch F, Sciascia Q. Exercise and muscle protein synthesis: not all roads lead to mTORC1. J Physiol. (2016) 594:3179–80. doi: 10.1113/JP272006
36. Li M, Verdijk LB, Sakamoto K, Ely B, van Loon LJ, Musi N. Reduced AMPK-ACC and mTOR signaling in muscle from older men, and effect of resistance exercise. Mech Ageing Dev. (2012) 133:655–64. doi: 10.1016/j.mad.2012.09.001
37. Yin H, Price F, Rudnicki MA. Satellite cells and the muscle stem cell niche. Physiol Rev. (2013) 93:23–67. doi: 10.1152/physrev.00043.2011
38. Dumont NA, Bentzinger CF, Sincennes MC, Rudnicki MA. Satellite cells and skeletal muscle regeneration. Compr Physiol. (2015) 5:1027–59. doi: 10.1002/cphy.c140068
39. Inoue A, Cheng XW, Huang Z, Hu L, Kikuchi R, Jiang H, et al. Exercise restores muscle stem cell mobilization, regenerative capacity and muscle metabolic alterations via adiponectin/AdipoR1 activation in SAMP10 mice. J Cachexia Sarcopenia Muscle. (2017) 8:370–85. doi: 10.1002/jcsm.12166
40. Rosca MG, Tandler B, Hoppel CL. Mitochondria in cardiac hypertrophy and heart failure. J Mol Cell Cardiol. (2013) 55:31–41. doi: 10.1016/j.yjmcc.2012.09.002
41. Baysa A, Fedorov A, Kondratov K, Ruusalepp A, Minasian S, Galagudza M, et al. Release of mitochondrial and nuclear DNA during on-pump heart surgery: kinetics and relation to extracellular vesicles. J Cardiovasc Transl Res. (2019) 12:184–92. doi: 10.1007/s12265-018-9848-3
42. Lundby C, Jacobs RA. Adaptations of skeletal muscle mitochondria to exercise training. Exp Physiol. (2016) 101:17–22. doi: 10.1113/EP085319
43. Grassi B, Majerczak J, Bardi E, Buso A, Comelli M, Chlopicki S, et al. Exercise training in Tgalphaq*44 mice during the progression of chronic heart failure: cardiac vs. peripheral (soleus muscle) impairments to oxidative metabolism. J Appl Physiol. (2017) 123:326–36. doi: 10.1152/japplphysiol.00342.2017
44. Grumati P, Coletto L, Schiavinato A, Castagnaro S, Bertaggia E, Sandri M, et al. Physical exercise stimulates autophagy in normal skeletal muscles but is detrimental for collagen VI-deficient muscles. Autophagy. (2011) 7:1415–23. doi: 10.4161/auto.7.12.17877
45. He C, Bassik MC, Moresi V, Sun K, Wei Y, Zou Z, et al. Exercise-induced BCL2-regulated autophagy is required for muscle glucose homeostasis. Nature. (2012) 481:511–5. doi: 10.1038/nature10758
46. Luo YX, Tang X, An XZ, Xie XM, Chen XF, Zhao X, et al. SIRT4 accelerates Ang II-induced pathological cardiac hypertrophy by inhibiting manganese superoxide dismutase activity. Eur Heart J. (2017) 38:1389–98. doi: 10.1093/eurheartj/ehw138
47. Hyatt H, Deminice R, Yoshihara T, Powers SK. Mitochondrial dysfunction induces muscle atrophy during prolonged inactivity: a review of the causes and effects. Arch Biochem Biophys. (2019) 662:49–60. doi: 10.1016/j.abb.2018.11.005
48. Shibaguchi T, Yamaguchi Y, Miyaji N, Yoshihara T, Naito H, Goto K, et al. Astaxanthin intake attenuates muscle atrophy caused by immobilization in rats. Physiol Rep. (2016) 4:e12885. doi: 10.14814/phy2.12885
49. Li K, Zhu X, Wang Y, Zheng S, Dong G. Effect of aerobic exercise intervention on DDT degradation and oxidative stress in rats. Saudi J Biol Sci. (2017) 24:664–71. doi: 10.1016/j.sjbs.2017.01.040
50. Baan JA, Varga ZV, Leszek P, Kusmierczyk M, Baranyai T, Dux L, et al. Myostatin and IGF-I signaling in end-stage human heart failure: a qRT-PCR study. J Transl Med. (2015) 13:1. doi: 10.1186/s12967-014-0365-0
51. Ames MK, Atkins CE, Pitt B. The renin-angiotensin-aldosterone system and its suppression. J Vet Intern Med. (2019) 33:363–82. doi: 10.1111/jvim.15454
52. Timmer LT, Hoogaars WMH, Jaspers RT. The role of IGF-1 signaling in skeletal muscle atrophy. Adv Exp Med Biol. (2018) 1088:109–37. doi: 10.1007/978-981-13-1435-3_6
53. Gielen S, Sandri M, Kozarez I, Kratzsch J, Teupser D, Thiery J, et al. Exercise training attenuates MuRF-1 expression in the skeletal muscle of patients with chronic heart failure independent of age: the randomized leipzig exercise intervention in chronic heart failure and aging catabolism study. Circulation. (2012) 125:2716–27. doi: 10.1161/CIRCULATIONAHA.111.047381
54. Li Y, Liang Y, Zhu Y, Zhang Y, Bei Y. Noncoding RNAs in cardiac hypertrophy. J Cardiovasc Transl Res. (2018) 11:439–49. doi: 10.1007/s12265-018-9797-x
55. Wang L, Lv Y, Li G, Xiao J. MicroRNAs in heart and circulation during physical exercise. J Sport Health Sci. (2018) 7:433–41. doi: 10.1016/j.jshs.2018.09.008
56. Deng J, Guo M, Li G, Xiao J. Gene therapy for cardiovascular diseases in China: basic research. Gene Ther. (2020) 27:360–9. doi: 10.1038/s41434-020-0148-6
57. Wang B, Zhang C, Zhang A, Cai H, Price SR, Wang XH. MicroRNA-23a and MicroRNA-27a mimic exercise by ameliorating ckd-induced muscle atrophy. J Am Soc Nephrol. (2017) 28:2631–40. doi: 10.1681/ASN.2016111213
58. Patel VB, Zhong JC, Grant MB, Oudit GY. Role of the ACE2/angiotensin 1-7 axis of the renin-angiotensin system in heart failure. Circ Res. (2016) 118:1313–26. doi: 10.1161/CIRCRESAHA.116.307708
59. Triposkiadis F, Xanthopoulos A, Butler J. Cardiovascular aging and heart failure: JACC review topic of the week. J Am Coll Cardiol. (2019) 74:804–13. doi: 10.1016/j.jacc.2019.06.053
60. Li J, Chan MC, Yu Y, Bei Y, Chen P, Zhou Q, et al. miR-29b contributes to multiple types of muscle atrophy. Nat Commun. (2017) 8:15201. doi: 10.1038/ncomms15201
61. Li J, Wang L, Hua X, Tang H, Chen R, Yang T, et al. CRISPR/Cas9-mediated miR-29b editing as a treatment of different types of muscle atrophy in mice. Mol Ther. (2020) 28:1359–72. doi: 10.1016/j.ymthe.2020.03.005
62. Moraes LN, Fernandez GJ, Vechetti-Junior IJ, Freire PP, Souza RWA, Villacis RAR, et al. Integration of miRNA and mRNA expression profiles reveals microRNA-regulated networks during muscle wasting in cardiac cachexia. Sci Rep. (2017) 7:6998. doi: 10.1038/s41598-017-07236-2
63. Yeo D, Kang C, Zhang T, Ji LL. Avenanthramides attenuate inflammation and atrophy in muscle cells. J Sport Health Sci. (2019) 8:189–95. doi: 10.1016/j.jshs.2018.08.002
64. Pandey A, Parashar A, Kumbhani D, Agarwal S, Garg J, Kitzman D, et al. Exercise training in patients with heart failure and preserved ejection fraction: meta-analysis of randomized control trials. Circ Heart Fail. (2015) 8:33–40. doi: 10.1161/CIRCHEARTFAILURE.114.001615
Keywords: exercise, muscle, atrophy, therapy, targets
Citation: Liu Q, Gao J, Deng J and Xiao J (2020) Current Studies and Future Directions of Exercise Therapy for Muscle Atrophy Induced by Heart Failure. Front. Cardiovasc. Med. 7:593429. doi: 10.3389/fcvm.2020.593429
Received: 10 August 2020; Accepted: 01 October 2020;
Published: 23 October 2020.
Edited by:
Chen Liu, First Affiliated Hospital of Sun Yat-sen University, ChinaReviewed by:
Jianqing She, First Affiliated Hospital of Xi'an Jiaotong University, ChinaQingchun Zeng, Southern Medical University, China
Copyright © 2020 Liu, Gao, Deng and Xiao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Junjie Xiao, anVuamlleGlhb0BsaXZlLmNu; Jiali Deng, ZGVuZ2ppYWxpQHNodS5lZHUuY24=
 Qi Liu
Qi Liu Juan Gao
Juan Gao Jiali Deng
Jiali Deng Junjie Xiao
Junjie Xiao