- 1Faculty of Medicine, American University of Beirut Medical Center, Beirut, Lebanon
- 2Division of Pediatric Cardiology, Pediatric Department, American University of Beirut Medical Center, Beirut, Lebanon
- 3Department of Basic Medical Sciences, College of Medicine, QU Health, Qatar University, Doha, Qatar
- 4Biomedical and Pharmaceutical Research Unit, QU Health, Qatar University, Doha, Qatar
- 5Department of Pharmacology and Toxicology, American University of Beirut, Beirut, Lebanon
Coronavirus disease 2019 (COVID-19) is a global pandemic caused by SARS-CoV-2 virus. As of the 30th of September 2020, around 34,000,000 cases have been reported globally. Pediatrics with underlying congenital heart disease represent a small yet a critical proportion of these patients. In general, the majority of infected children experience mild to moderate disease with significant interindividual variability in laboratory and radiographic findings. Nevertheless, in healthy children with COVID-19, cardiac involvement has been documented and is attributed to various causes. Myocarditis, arrhythmias, cardiogenic shock, and serious multisystem inflammatory syndrome in children are all encountered. Since COVID-19 is a recent novel disease and based on previous experience with respiratory infections, children with underlying congenital heart disease should be given special attention. To date, little data is available about COVID-19 presentation, complications, and appropriate treatment in this population. However, variable and inconsistent disease presentation and severity have been observed. This paper discusses COVID-19 course of illness in pediatric population with a special emphasis on the cardiac manifestations of the disease in healthy population and also on the disease course in congenital heart disease patients in particular.
Introduction
Coronaviruses (CoVs) are enveloped single stranded RNA viruses that belong to the Coronaviridae family (1). They are implicated in a wide spectrum of diseases ranging from mild illness such as common cold to more serious life-threatening syndromes such as the Middle East Respiratory Syndrome (MERS) and the Severe Acute Respiratory Syndrome (SARS) (2). Toward the end of 2019, a novel coronavirus, called SARS-CoV-2, led to the unprecedented pandemic of Coronavirus Disease 2019 (COVID-19) (3). At the time of writing this manuscript, the total COVID-19 cases reported are around 34 million, with over 1 million deaths reported.
SARS-CoV-2 infection is not only associated with respiratory symptoms but also with multi-organ manifestations that include cardiac, gastrointestinal, hematologic, renal, and neurologic ones (4–7). Despite the rapid global spread of the pandemic, the disease characteristics in pediatrics with congenital heart disease (CHD) remains largely unclear. This article discusses the clinical course of COVID-19 in pediatric patients along with laboratory and radiologic findings with emphasis on cardiovascular complications and on pediatric patients with CHD.
Congenital Heart Disease
CHD is the most commonly encountered congenital anomaly accounting for around 30% of all congenital defects (8). Its incidence varies greatly among populations with average of 8 per 1,000 annual live births (9). In recent decades, the incidence of CHD increased due to development in screening and detection methods (8, 10). CHD is classified into two main categories: cyanotic and acyanotic heart diseases [Figure 1; (11)]. The acyanotic heart diseases represent the milder types of CHD. They include ventricular septal defects, atrial septal defects, pulmonary stenosis, aortic stenosis among others. On the other hand, the group of cyanotic CHDs usually presents with severe illness early in life. They include Tetralogy of Fallot, hypoplastic right heart, hypoplastic left heart, total anomalous pulmonary venous return among others (11, 12).
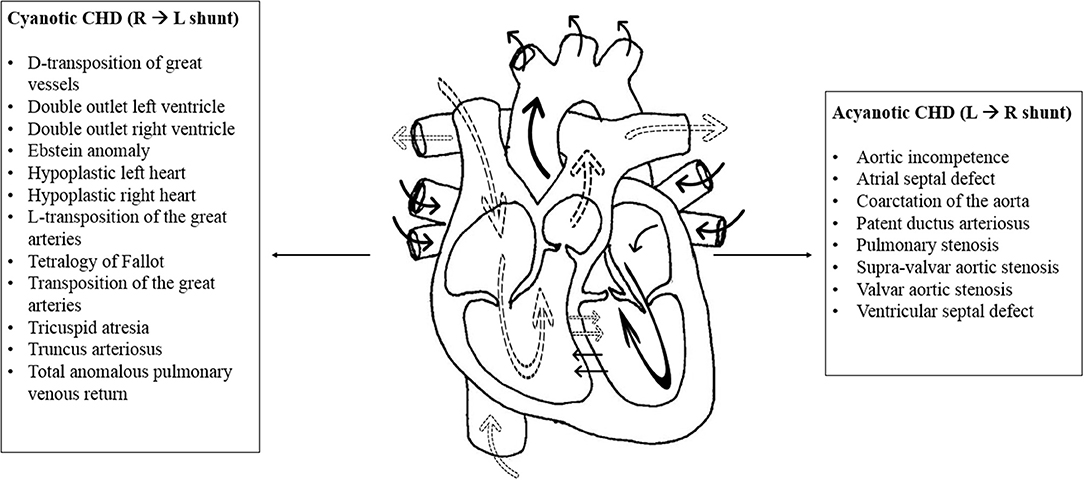
Figure 1. Cyanotic and acyanotic congenital heart diseases. The normal physiology of the heart is represented in the above illustration with the effect of shunts. The dotted arrows represent deoxygenated blood while solid arrows represent oxygenated blood. Shunt is an abnormal communication between the right and left sides of the heart or between the pulmonary and systemic circulations. The cyanotic congenital heart diseases are listed to the left of the diagram. In general, cyanotic lesions cause shunting of deoxygenated blood from the right to the left side of the heart, leading to decreased systemic oxygen saturation and cyanosis. Hence, they present early in life. Acyanotic congenital heart diseases are listed to the right of the diagram. Acyanotic lesions cause left to right shunting of oxygenated blood. They increase blood flow to the pulmonary circulation without affecting oxygen saturation in the systemic blood (11, 12).
With the rapid development in detection methods and surgical techniques, the survival rate of CHD patients significantly improved. It greatly hinges on the type and severity of CHD, where in the milder diseases survival to adulthood reached almost 98% (13). However, these children remain at remarkable risk for increased morbidity and mortality from lower respiratory tract infections (14). For instance, respiratory syncytial virus infection is associated with increased risk of hospitalization, intensive care unit (ICU) admission, and mechanical ventilation requirement in these patients (3). Consequently, it is critical to assess the burden of COVID-19 pandemic on CHD patients. Unfortunately, this remains challenging ought to the low number of reported cases. This article summarizes the available evidence that describes the characteristics of COVID-19 in CHD patients.
SARS-CoV-2 IN Pediatric Patients
In the early phase of the global pandemic, the reported number of infected children was low. As the disease progressed, more cases were reported. Later on, It has been noted that children are less susceptible to COVID-19 and if infected the majority display mild to no symptoms (15). Eventually, attention has drifted toward the pediatric population to explore the disease features and discover their role in spreading the virus. In fact, out of the first 11,791 cases diagnosed in China, only 74 cases were children below 18 years old (16). The first reported pediatric case was on January 20, 2020 (16, 17). Besides, it is estimated that pediatric cases account for around 1–5% of the total reported cases worldwide (18). In the below section we review the clinical features, laboratory and radiologic findings, and treatment of COVID-19 in the pediatric population.
Clinical Features
Reported disease severity of infected children range from asymptomatic to mild, moderate, severe and critically ill cases (Table 1) depicts the clinical features of cases classified with different disease severity. Interestingly, gastrointestinal manifestations could be the initial and even the only symptoms of SARS-CoV-2 infection in children (24). Indeed, China's first critically ill pediatric case displayed only gastrointestinal symptoms in early disease state and then progressed rapidly to acute respiratory distress syndrome, septic shock, and renal failure (24). Most of the infected pediatric patients are asymptomatic, or exhibit mild disease, usually recovering in 2 weeks from the onset of symptoms (17, 19, 23). In concordance, out of 171 cases in a Chinese study around 15% were clinically asymptomatic with negative CT chest findings (25).
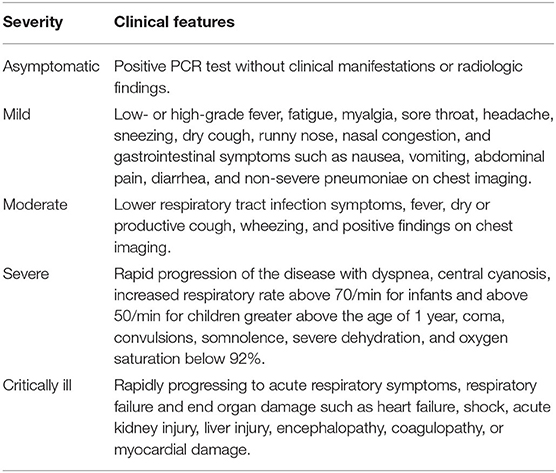
Table 1. This table summarizes the clinical features encountered in pediatric COVID-19 patients according to disease severity and as described by various studies (15, 19–23).
Studies from different countries reporting pediatric cases highlight the mild to moderate disease presentation in this population. Pediatric cases from all ages were reported. In a report by Dong et al., out of 2,135 Chinese children diagnosed with COVID-19 more than 90% were classified in the asymptomatic, mild or moderate disease categories (15). In Turkey, more than 50% of infected children had mild disease (26). Moreover, compared to adults, pediatric cases are less likely to report the common COVID-19 symptoms (25, 27, 28). Among 291 confirmed pediatric cases from the United States, only 56, 54, and 13% reported fever, cough and shortness of breath, respectively (28). Similarly, out of the 171 confirmed pediatric cases in a Chinese study, only 41.5% reported fever (25).
While as mentioned above most cases are mild, severe and critically ill cases were also reported. Of the 171 cases in the Chinese study, 3 patients required pediatric intensive care unit (PICU) admission, and all had underlying medical illness (leukemia on chemotherapy, hydronephrosis and intussusception). Eventually one death was reported for the 10 months old patient with intussusception, at week 4 post-admission, due to multi-organ failure (25). Congruently, among 745 reported cases in the United States, 147 required hospitalization and of which 15 patients required ICU admission (28). Dong el al., reported that of the 2,143 COVID-positive children, around 5% had severe disease while 0.6% were critically ill (21).
Remarkably, the highest hospitalization rate among the pediatrics population was reported in patients aged <12 months, accounting for up to 62% of the total hospitalized COVID-19 positive children (28). Similarly, 32 and 10.6% of the severely ill children were <1 year old (21, 29). In a Turkish study, 80% of PICU admissions were <1 year old (26). The percentage of severely and critically ill patients decreased with age, it is estimated to be 7.3, 4.2, 4.1, and 3% in age groups 1–5 years, 6–10 years, 11–15 years, and 16–17 years, respectively (21).
Fortunately, no evidence of vertical transmission has been detected. All tested neonates (n = 10) born to COVID-19 mothers had negative PCR results, although adverse effects were reported (30). Furthermore, infected neonates tend to show mild to moderate clinical symptoms (31). Only 1 out of 3 neonates had complicated hospital course associated with disseminated intravascular coagulation (DIC) and required non-invasive ventilation, but gradually improved (31). However, this neonate was preterm born at 31 weeks of gestation with neonatal respiratory distress syndrome (31).
Children susceptible to develop severe illness are those with underlying cardiac, respiratory or immunologic diseases such as CHD, asthma or immunodeficiency. 77% (28/37) of hospitalized patients and 100% of the ICU admitted with known information about hospitalization and medical status had underlying medical condition (28).
In summary, children with COVID-19 usually exhibit mild disease with minority requiring hospitalization and PICU admission. Infants have the highest risk of developing severe and critically ill disease. In addition, children with underlying medical condition are more likely to experience complications requiring hospital admission. Remarkably, vertical transmission has not been documented. Infected neonates have mild illness unless complicated by underlying medical problems or by prematurity.
Laboratory Findings
Strangely, the majority of infected children tend to have normal laboratory markers including CRP and liver function enzymes (32, 33). In one study, procalcitonin and CK-MB elevation was reported in 16/20 and 15/20 of infected children, respectively (32). Variability in lymphocyte counts was reported. The majority (around 70%) had normal lymphocyte count (34); however lymphocytopenia was reported in 2 studies accounting for 3 and 3.5% of the population (25, 34). Besides, increase in WBCs counts and leucopenia were both reported (23, 25, 35). Some laboratory markers found to be associated with severe disease including: decreased lymphocyte count and increased procalcitonin, d-dimer, and CK-MB (19, 23). Coinfection with other pathogens was documented. Such pathogens include influenza viruses A and B, respiratory syncytial virus (RSV), cytomegalovirus (CMV) among others (32).
Radiologic Findings
Published data describing the radiographic findings in pediatric patients with COVID-19 is scarce. During routine practice, chest x-ray (CXR) is the preferred modality in children; however, it has low specificity and sensitivity in evaluating lung involvement in confirmed or suspected COVID-19 children (36). In general, children have lower incidence and limited lung involvement compared to adults, as evident on chest imaging (37, 38). Ground glass opacities (GGO) with peripheral distribution in the lower lung fields is the most common reported finding (37, 39–41). Besides, the extent of pulmonary changes on imaging is related to disease severity. Patients with mild disease presentation often exhibit no radiographic changes; however, in one study, GGO were detected in 100% of patients with moderate COVID-19 (23).
More serious changes were seen in patients admitted to the PICU (42). In a study describing eight PICU patients, seven had multiple patch-like shadows, six had GGO, and one had pleural effusion and white lung-like change (42). These changes persisted even after resolution of clinical symptoms. Lesions may also persist in the absence of viral detection on PCR testing (32).
Mixture of findings with heterogenous pattern were reported on chest CT. Among the reported changes are bilateral or unilateral ground glass opacities, patchy ground glass opacities, local and bilateral patchy shadowing, interstitial abnormalities, halo signs, small nodular ground glass opacities, and speckled ground glass opacities, and bronchial pneumonia-like changes (25, 39–41, 43, 44). Rarely, pleural effusion, crazy-paving sign, and lymphadenopathy were reported but were witnessed mainly in severe cases (37, 39, 41). Due to the high radiation associated with CT scan and to the fact that most children experience milder lung involvement than adults, it is suggested that chest CT should not be routinely used unless necessary (45).
Treatment
Most studies and trials targeting COVID-19 treatment were performed on adults. In general, in the pediatric population, treatment is symptomatic and supportive. It aims to provide rest, sufficient caloric intake, and maintaining water and electrolyte balance (19). It includes antipyretics for fever, sedatives in case of seizure, oxygen therapy including mask, nasal catheter, high flow nasal cannula or invasive ventilation, and antibiotics for bacterial superinfection (19, 46). In some cases, antiviral therapies are used as well (47).
Ultimately, vaccination remains the optimal preventive measure that can attenuate the global propagation of COVID-19. Researchers worldwide are working with unpreceded efforts to develop an effective vaccine. Currently, 42 vaccine candidates are being tested in pre-clinical or clinical trials (phases 1–3). Nevertheless, on the 11th of August 2020, the first COVID-19 vaccine has been granted approval. This vaccine stands for the Sputnik V vaccine developed by the Gamaleya Research Institute in Moscow. However, the use of this vaccine has raised a lot of arguments since it has not been tested in phase 3 clinical trials yet (48, 49). Hence, further trials are definitely needed to evaluate the role of any vaccine targeted against SARS-CoV-2.
Cardiac Manifestations of Covid-19 in Pediatric Patients
In previously healthy COVID-19 pediatric patients, Kawasaki-like disease and myocarditis have been the main cardiovascular manifestations of SARS-CoV-2. These manifestations are triggered primarily by the massive immune response mounted against the viral infection (50–55). In fact, elevated levels of inflammatory markers have been noted in patients with COVID-19 associated Kawasaki-like disease or myocarditis (52, 54, 56). Il-6 was found to be inappropriately elevated in two distinct cases of pediatric myocarditis (52, 54). Similarly, CRP, pro-calcitonin and ferritin were elevated in most cases of Kawasaki-like disease and myocarditis (54, 56–58). Kawasaki-like disease may occur following a prior infection of COVID-19 documented by the presence of SARS-CoV-2 IgG antibodies or a known contact with a confirmed case of COVID-19 (58). This suggests the presence of a post-infectious state of immune dysregulation in children previously infected with or exposed to COVID-19 (51, 57).
Besides, compromised lung function may result in compromised cardiac function; this is attributed to numerous COVID-19-induced pulmonary defects denoted by oxidative stress, tissue injury, respiratory failure and ventilation perfusion mismatch (50, 51). Cardiac dysfunction may be possibly caused by direct myocardial damage. SARS-CoV-2 can gain entry to the cardiomyocytes through the ACE2 receptor (59). Subsequently, myocardial infiltration by SARS-CoV-2 and inflammatory cells may result in lethal complications that include fulminant myocarditis and cardiogenic shock (59).
After several cases were reported, the Kawasaki-like disease induced by COVID-19 was defined by the Centers for Disease Control and Prevention (CDC) as the multisystem inflammatory syndrome in children (MIS-C) associated with COVID-19 (60). As of the 15th of July, 342 cases of MIS-C and 6 deaths were declared in 37 American states (60). Eighty-one percent of the cases were aged between 1 and 14 years. Nevertheless, the disease may develop 2–4 weeks after a COVID-19 infection in any patient aged <21 years (60). SARS-CoV-2 infections complicated by MIS-C are often associated with fatal conditions denoted by heart failure, hepatic injury, renal dysfunction and coagulopathies (56).
Furthermore, in a cohort of 58 pediatric patients with MIS-C, 50% of the patients were admitted to the PICU (58). Acute kidney injury was noted in 13 patients, and cardiogenic shock necessitating inotropic agents was experienced by 27 patients (58). Of these 58 patients, 25 were bound to mechanical ventilators (58). Similarly, in a cross-sectional study of 48 patients with severe COVID-19 disease requiring PICU admission, death was witnessed only in two patients (61). Multi-organ dysfunction was the cause of death in both of them. Congruently, this endorses in turn the presence of a state of hyperinflammation in critically ill patients with multi-organ involvement (61). Cases of MIS-C have been reported in further studies as depicted in Table 2. To date, Kawasaki-like manifestations and myocarditis have constituted the key clinical presentations of this syndrome as revealed by most studies.
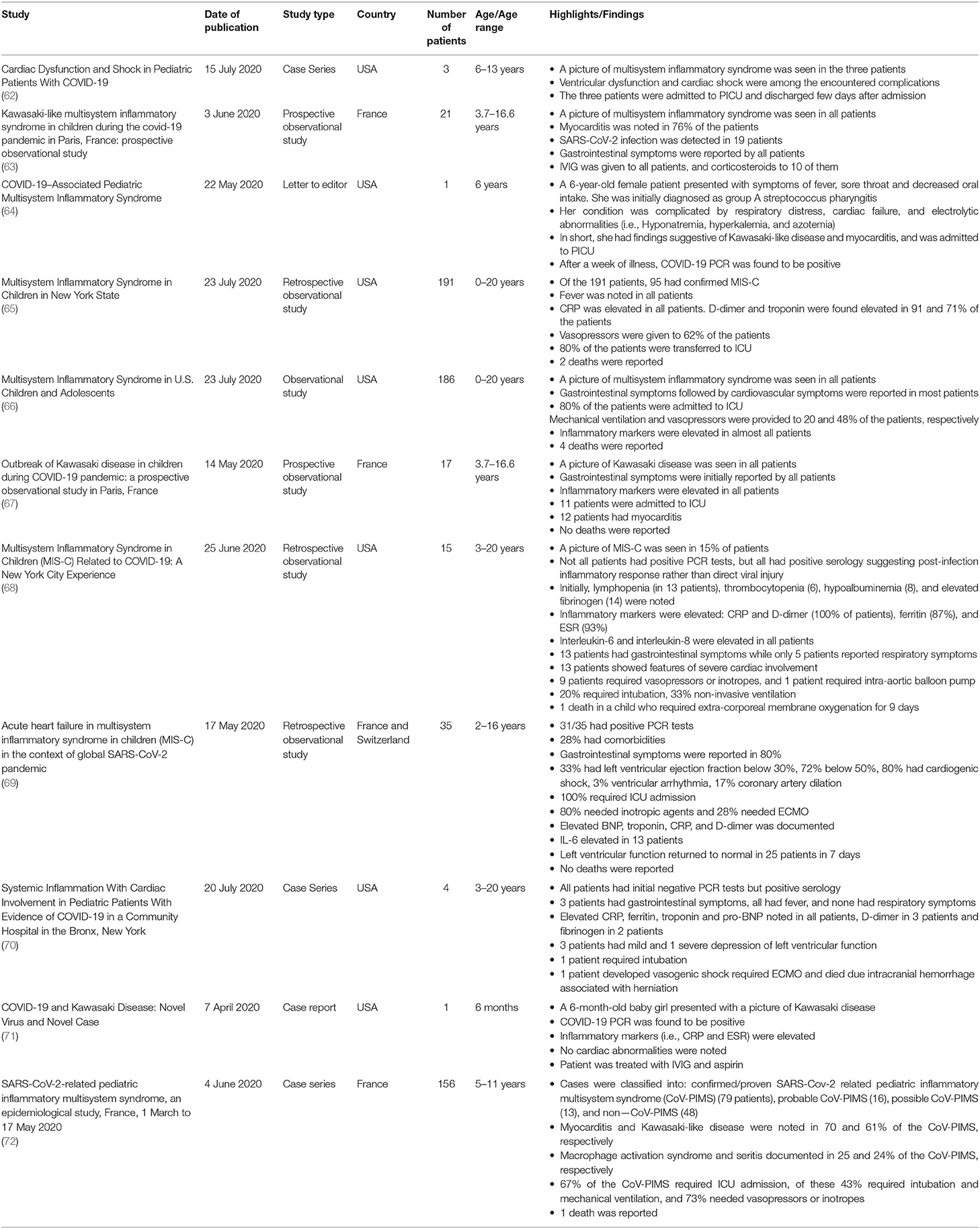
Table 2. This table summarizes the clinical characteristics of children with multisystem inflammatory syndrome reported in a few additional clinical studies.
Finally, despite being less common, arrhythmias were also encountered in children with COVID-19. As compared to adult patients, milder forms of arrhythmias were reported in pediatric patients. In one pediatric study, ventricular tachycardia and ventricular fibrillation were not reported (32). Yet, sinus tachycardia, atrial tachycardias, atrial and ventricular premature beats, bundle branch blocks, and first-degree AV block were the main arrhythmic manifestations of COVID-19 among the studied patients (32).
COVID-19 IN Pediatric CHD Patients
As previously mentioned, children are less prone to acquiring COVID-19. Additionally, as compared to adults, they often exhibit mild disease ranging from flu-like to no symptoms. Nonetheless, children, particularly those with CHD, may develop serious COVID-19 related cardiovascular complications (51, 73). CHD patients are more likely to require ICU admission and artificial respiratory support, especially those with cyanotic defects. In these patients, COVID-19 may lead to worsened hypoxemia and compromised tissue perfusion (51, 73). Additionally, patients with complex CHD complicated by depressed myocardial contractility, pulmonary hypertension, immunodeficiencies (i.e., DiGeorge syndrome) among other comorbid conditions may likely develop severe and critical COVID-19 disease (73, 74). Indeed, as per the British Congenital Cardiac Association (BCCA), CHD patients and particularly those with complex disease (Figure 2) are considered at-risk patients prone to develop severe COVID-19 (75).

Figure 2. Patients with complex CHD who are considered at high-risk of developing severe COVID-19 as per the British Congenital Cardiac Association (BCCA).
Despite the scarcity of available evidence in this area, few studies have reported the complications encountered in SARS-COV-2 positive pediatric cardiac patients. In this context, Lu et al. declared that severe disease was mainly witnessed in children with preexisting life-threatening conditions. Out of 230 patients, two had severe symptoms. One child had a past medical history of surgically treated CHD. The other had complicated kidney disease (22).
Furthermore, in a former Chinese study published on February 2020, Chinese experts have discussed the characteristics of the COVID-19 pandemic along with its diagnosis, treatment and prevention in children aged up to 17 years. They defined CHD as a risk factor for critical SARS-CoV-2 infection (76). This has been likely endorsed by a retrospective study of 25 Chinese children aged between 3 months and 14 years. In this study, serious and complicated COVID-19 was observed only in two patients having a past medical history of surgically treated CHD (77).
Additionally, in a cross-sectional study of 48 participants, Shekerdemian et al. assessed the clinical presentation, characteristics, and outcomes of COVID-19 pediatric patients admitted to ICU in North America. In this study, underlying comorbid conditions were noted in 83% of the patients. A History of CHD was observed in 3 patients (61). Similarly, in a multi-center observation study, data concerning COVID-19 in CHD patients was collected from eight Italian CHD centers (74). Throughout a period of 6 weeks extending from the 21st of February till the 4th of April, a total of 76 SARS-CoV-2 positive CHD patients were reported. Four were children and the remaining 72 were adults aged more than 18 years. The reported cases were likely subdivided into confirmed (9 patients) and suspected COVID-19 cases (67 patients) (74). Cardiovascular complications, such as heart failure, arrhythmias, stroke, myocardial injury, pericardial effusion and pulmonary hypertension were mainly observed in the confirmed cases. Nonetheless, a mild disease course was witnessed in most patients and zero deaths were reported (74).
As deduced from above and just like in healthy individuals, COVID-19 may exhibit distinct clinical courses in CHD patients ranging from no symptoms to critical disease. Nonetheless, COVID-19 manifestations such as chest pain, cyanosis, dyspnea, and palpitation may imitate symptoms of cardiovascular deterioration in these patients (73). Hence, comprehensive evaluation and meticulous care should be offered to any CHD patient presenting with these symptoms that may indicate worsening CHD or new onset SARS-CoV-2 infection. Additionally, the diagnosis of COVID-19 in CHD patients is made primarily through RT-PCR testing of nasopharyngeal samples (51, 59). The detection of suggestive chest CT scan findings plays likely an imperative role in confirming the diagnosis of COVID-19 in suspected cases (51, 59).
In most cases, patients with CHD are managed with supportive measures geared toward fever reduction, symptoms control and oxygen correction. The use of repurposed medicines such as azithromycin, hydroxychloroquine, dexamethasone, remdesivir, and immunotherapies is not part of the standard management (51, 78). Yet, the addition of these medications to the management of CHD patients necessitates careful dosing and thorough monitoring particularly when dealing with cardiotoxic medications (51, 78). Besides, none of the medications that are often used in treating CHD manifestations and complications, and also in maintaining cardiac functions in these patients are shown to affect or exacerbate the clinical course of COVID-19 in this population (73). For instance, the discontinuation of angiotensin converting enzyme inhibitors (ACE-i) and angiotensin II receptor blockers (ARB) in cardiac patients with confirmed or suspected COVID-19 was prohibited by the Heart failure Society of America (HFSA), American College of Cardiology (ACC), American Heart Association (AHA), and the European Society of Cardiology (ESC) (79, 80).
Finally, numerous studies have emphasized the importance of COVID-19 prevention in this population (51, 59, 73, 78, 81). Indeed, effective screening and early detection of SARS-CoV-2 infection in patients with CHD are key for avoiding severe life-threatening manifestations of the disease. Congruently, CHD patients should be educated about the signs and symptoms of COVID-19, and also about the importance of adopting protective measures such as social distancing, repeated hand washing, and effective wearing of face masks and goggles (51, 59, 73, 78, 81).
Limitations
The studies reported in our manuscript have several limitations. First, they are restricted in terms of population size. Most reported studies were from single-centers and had a small number of pateints. Second, no randomized controlled trials were found; the studies were mostly observational, case series and retrospective chart reviews. Hence, conclusive evidence concerning infection rate among various pediatric age groups, genders and those with comorbidities cannot be ascertained.
Similarly, the literaure lacks studies comparing COVID-19 infection in heathy children to those with CHD. In addition, the published data regarding children with multisystem inflammatory syndrome is limited by the fact that patients were only from 3 countries. Seven out of the eleven included studies were from centers across the United States, three were from France and one had patients from both France and Switzerland. Besides, the studies were heterogenous in terms of the assessed laboratory markers. Finally, data describing laboratory values and imagings are scarce and highly variable among infected children with CHD. Despite these limitations, this manuscript was able to describe the spectrum of disease presentation and prognosis in CHD patients with superimposed COVID-19 infection.
Conclusion
Little is known about the SARS-CoV-2 infection in CHD patients. The majority of the published data consist of documentaries, narrative reviews, and letters. Yet, as depicted above, it is now considered that serious clinical symptoms and end-organ complications of COVID-19 may develop in children with CHD or previous history of surgically treated CHD. Ultimately, as the COVID-19 pandemic continues to spread globally at enormous rates, further higher-quality clinical trials with enrolled pediatric CHD patients are required to assess the clinical burdens of COVID-19 in these patients. Similarly, internists, pediatricians, and cardiologists should understand the influence of this pandemic on CHD patients and should provide meticulous care to this at-risk population.
Author Contributions
MA, FB, and AE developed the idea and the review framework. RZ and NY wrote the first draft of the manuscript. AE did the final editing. All authors contributed to corrections and adjustment of subsequent iterations of the manuscript. All authors approve and agree with the content.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. Fehr AR, Perlman S. Coronaviruses: an overview of their replication and pathogenesis. In: Maier HJ, Bickerton E, Britton P, editors. Coronaviruses. Springer (2015). p. 1–23.
2. Cabeça TK, Granato C, Bellei N. Epidemiological and clinical features of human coronavirus infections among different subsets of patients. Influenza Other Respir Viruses. (2013) 7:1040–7. doi: 10.1111/irv.12101
3. Covid C, Team R. Severe outcomes among patients with coronavirus disease 2019 (COVID-19)—United States, February 12–March 16, 2020. MMWR Morb Mortal Wkly Rep. (2020) 69:343–6. doi: 10.15585/mmwr.mm6912e2
4. Filatov A, Sharma P, Hindi F, Espinosa PS. Neurological complications of coronavirus disease (COVID-19): encephalopathy. Cureus. (2020) 12:e7352. doi: 10.7759/cureus.7352
5. Klok F, Kruip M, Van der Meer N, Arbous M, Gommers D, Kant K, et al. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thromb Res. (2020) 191:145–7. doi: 10.1016/j.thromres.2020.04.013
6. Kochi AN, Tagliari AP, Forleo GB, Fassini GM, Tondo C. Cardiac and arrhythmic complications in patients with COVID-19. J Cardiovasc Electrophysiol. (2020) 31:1003–8. doi: 10.1111/jce.14479
7. Pei G, Zhang Z, Peng J, Liu L, Zhang C, Yu C, et al. Renal involvement and early prognosis in patients with COVID-19 pneumonia. J Am Soc Nephrol. (2020) 31:1157–65. doi: 10.1681/ASN.2020030276
8. van der Linde D, Konings EE, Slager MA, Witsenburg M, Helbing WA, Takkenberg JJ, et al. Birth prevalence of congenital heart disease worldwide: a systematic review and meta-analysis. J Am Coll Cardiol. (2011) 58:2241–7. doi: 10.1016/j.jacc.2011.08.025
9. Bernier PL, Stefanescu A, Samoukovic G, Tchervenkov CI. (2010). The challenge of congenital heart disease worldwide: epidemiologic and demographic facts. In: Gaynor JW, Jacobs ML, editors. Seminars in Thoracic and Cardiovascular Surgery: Pediatric Cardiac Surgery Annual. Elsevier (2010). p. 26–34. doi: 10.1053/j.pcsu.2010.02.005
10. Eid AH, Itani Z, Al-Tannir M, Sayegh S, Samaha A. Primary congenital anomalies of the coronary arteries and relation to atherosclerosis: an angiographic study in Lebanon. J Cardiothorac Surg. (2009) 4:58. doi: 10.1186/1749-8090-4-58
11. Hoffman JI, Kaplan S. The incidence of congenital heart disease. J Am Coll Cardiol. (2002) 39:1890–900. doi: 10.1016/S0735-1097(02)01886-7
12. Rohit M, Shrivastava S. Acyanotic and cyanotic congenital heart diseases. Indian J Pediatr. (2018) 85:454–60. doi: 10.1007/s12098-017-2454-6
13. Moons P, Bovijn L, Budts W, Belmans A, Gewillig M. Temporal trends in survival to adulthood among patients born with congenital heart disease from 1970 to 1992 in Belgium. Circulation. (2010) 122:2264–72. doi: 10.1161/CIRCULATIONAHA.110.946343
14. Geskey JM, Cyran SE. Managing the morbidity associated with respiratory viral infections in children with congenital heart disease. Int J Pediatr. (2012) 2012:646780. doi: 10.1155/2012/646780
15. Dong Y, Mo X, Hu Y, Qi X, Jiang F, Jiang Z, et al. Epidemiology of COVID-19 among children in China. Pediatrics. (2020) 145:e20200702. doi: 10.1542/peds.2020-0702
16. Choi S-H, Kim HW, Kang J-M, Kim DH, Cho EY. Epidemiology and clinical features of coronavirus disease 2019 in children. Clin Exp Pediatr. (2020) 63:125. doi: 10.3345/cep.2020.00535
17. Cao Q, Chen Y-C, Chen C-L, Chiu C-H. SARS-CoV-2 infection in children: Transmission dynamics and clinical characteristics. J Formos Med Assoc. (2020) 119:670. doi: 10.1016/j.jfma.2020.02.009
18. Novel CPERE. The epidemiological characteristics of an outbreak of 2019 novel coronavirus diseases (COVID-19) in China. Zhonghua Liu Xing Bing Xue Za Zhi. (2020) 41:145–51. doi: 10.3760/cma.j.issn.0254-6450.2020.02.003
19. Chen Z-M, Fu J-F, Shu Q, Chen Y-H, Hua C-Z, Li F-B, et al. Diagnosis and treatment recommendations for pediatric respiratory infection caused by the 2019 novel coronavirus. World J Pediatr. (2020) 16:240–6. doi: 10.1007/s12519-020-00345-5
20. Cui Y, Tian M, Huang D, Wang X, Huang Y, Fan L, et al. A 55-day-old female infant infected with COVID 19: presenting with pneumonia, liver injury, and heart damage. J Infect Dis. (2020) 221:1775–81. doi: 10.1093/infdis/jiaa113
21. Dong Y, Mo X, Hu Y, Qi X, Jiang F, Jiang Z, et al. Epidemiological characteristics of 2143 pediatric patients with 2019 coronavirus disease in China. Pediatrics. (2020) 58:712–3.
22. Liu W, Zhang Q, Chen J, Xiang R, Song H, Shu S, et al. Detection of Covid-19 in children in early January 2020 in Wuhan, China. N Engl J Med. (2020) 382:1370–1. doi: 10.1056/NEJMc2003717
23. Qiu H, Wu J, Hong L, Luo Y, Song Q, Chen D. Clinical and epidemiological features of 36 children with coronavirus disease 2019 (COVID-19) in Zhejiang, China: an observational cohort study. Lancet Infect Dis. (2020) 20:689–96. doi: 10.1016/S1473-3099(20)30198-5
24. Chen F, Liu Z, Zhang F, Xiong R, Chen Y, Cheng X, et al. Frist case of severe childhood novel coronavirus pneumonia in China. Zhonghua Er Ke Za Zhi. (2020) 58:179–82. doi: 10.3760/cma.j.issn.0578-1310.2020.03.003
25. Lu X, Zhang L, Du H, Zhang J, Li YY, Qu J, et al. SARS-CoV-2 infection in children. N Engl J Med. (2020) 382:1663–5. doi: 10.1056/NEJMc2005073
26. Cascella M, Rajnik M, Cuomo A, Dulebohn SC, Di Napoli R. Features, evaluation and treatment coronavirus (COVID-19). In: Statpearls. StatPearls Publishing (2020).
27. Su L, Ma X, Yu H, Zhang Z, Bian P, Han Y, et al. The different clinical characteristics of corona virus disease cases between children and their families in China–the character of children with COVID-19. Emerg Microb Infect. (2020) 9:707–13. doi: 10.1080/22221751.2020.1744483
28. Team CCR. Coronavirus disease 2019 in children - United States, February 12-April 2, 2020. MMWR Morb Mortal Wkly Rep. (2020) 69:422–6. doi: 10.15585/mmwr.mm6914e4
29. Wang E, Brar K. COVID-19 in children: an epidemiology study from China. J Allergy Clin Immunol. (2020) 8:2118–20. doi: 10.1016/j.jaip.2020.04.024
30. Zhu H, Wang L, Fang C, Peng S, Zhang L, Chang G, et al. Clinical analysis of 10 neonates born to mothers with 2019-nCoV pneumonia. Transl Pediatri. (2020) 9:51. doi: 10.21037/tp.2020.02.06
31. Zeng L, Xia S, Yuan W, Yan K, Xiao F, Shao J, et al. Neonatal early-onset infection with SARS-CoV-2 in 33 neonates born to mothers with COVID-19 in Wuhan, China. JAMA Pediatr. (2020) 174:722–5. doi: 10.1001/jamapediatrics.2020.0878
32. Xia W, Shao J, Guo Y, Peng X, Li Z, Hu D. Clinical and CT features in pediatric patients with COVID-19 infection: different points from adults. Pediatr Pulmonol. (2020) 55:1169–74. doi: 10.1002/ppul.24718
33. Zhang JJ, Dong X, Cao YY, Yuan YD, Yang YB, Yan YQ, et al. Clinical characteristics of 140 patients infected with SARS-CoV-2 in Wuhan, China. Allergy. (2020) 75:1730–41. doi: 10.1111/all.14238
34. Henry BM, Lippi G, Plebani M. Laboratory abnormalities in children with novel coronavirus disease 2019. Clin Chem Lab Med. (2020) 58:1135–8. doi: 10.1515/cclm-2020-0272
35. Wang D, Ju X, Xie F, Lu Y, Li F, Huang H, et al. Clinical analysis of 31 cases of 2019 novel coronavirus infection in children from six provinces (autonomous region) of northern China. Zhonghua Er Ke Za Zhi. (2020) 58:269–74. doi: 10.3760/cma.j.cn112140-20200225-00138
36. Foust AM, Phillips GS, Chu WC, Daltro P, Das KM, Garcia-Peña P, et al. International expert consensus statement on chest imaging in pediatric COVID-19 patient management: imaging findings, imaging study reporting and imaging study recommendations. Radiol Cardiothoracic Imaging. (2020) 2:e200214. doi: 10.1148/ryct.2020200214
37. Chen A, Huang J, Liao Y, Liu Z, Chen D, Yang C, et al. Differences in clinical and imaging presentation of pediatric patients with COVID-19 in comparison with adults. Radiol Cardiothoracic Imaging. (2020) 2:e200117. doi: 10.1148/ryct.2020200117
38. Chen N, Zhou M, Dong X, Qu J, Gong F, Han Y, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. (2020) 395:507–13. doi: 10.1016/S0140-6736(20)30211-7
39. Caro-Dominguez P, Shelmerdine SC, Toso S, Secinaro A, Toma P, Damasio MB, et al. Thoracic imaging of coronavirus disease 2019 (COVID-19) in children: a series of 91 cases. Pediatr Radiol. (2020) 50:1354–68. doi: 10.1007/s00247-020-04747-5
40. Li W, Cui H, Li K, Fang Y, Li S. Chest computed tomography in children with COVID-19 respiratory infection. Pediatr Radiol. (2020) 50:796–9. doi: 10.1007/s00247-020-04656-7
41. Song W, Li J, Zou N, Guan W, Pan J, Xu W. Clinical features of pediatric patients with coronavirus disease (COVID-19). J Clin Virol. (2020) 127:104377. doi: 10.1016/j.jcv.2020.104377
42. Sun D, Li H, Lu X-X, Xiao H, Ren J, Zhang F-R, et al. Clinical features of severe pediatric patients with coronavirus disease 2019 in Wuhan: a single center's observational study. World J Pediatr. (2020) 16:251–9. doi: 10.1007/s12519-020-00354-4
43. Feng K, Yun Y, Wang X, Yang G, Zheng Y, Lin C, et al. Analysis of CT features of 15 children with 2019 novel coronavirus infection. Zhonghua Er Ke Za Zhi. (2020) 58:275–8. doi: 10.3760/cma.j.cn112140-20200210-00071
44. Li Y, Cao J, Zhang X, Liu G, Wu X, Wu B. Chest CT imaging characteristics of COVID-19 pneumonia in preschool children: a retrospective study. BMC Pediatrics. (2020) 20:227. doi: 10.1186/s12887-020-02140-7
45. Duan YN, Zhu YQ, Tang L-L, Qin J. CT features of novel coronavirus pneumonia (COVID-19) in children. Eur Radiol. (2020) 30:4427–33. doi: 10.1007/s00330-020-06860-3
46. Cai J, Xu J, Lin D, Xu L, Qu Z, Zhang Y, et al. A case series of children with 2019 novel coronavirus infection: clinical and epidemiological feature. Clin Infect Dis. (2020) 71:1547–51. doi: 10.1093/cid/ciaa198
47. Sanders JM, Monogue ML, Jodlowski TZ, Cutrell JB. Pharmacologic treatments for coronavirus disease 2019 (COVID-19): a review. JAMA. (2020) 323:1824–36. doi: 10.1001/jama.2020.6019
48. Craven J. COVID-19 Vaccine Tracker. Regulatory Affairs Professionals Society (RAPS) (2020). Available online at: https://www.raps.org/news-and-articles/news-articles/2020/3/covid-19-vaccine-tracker?feed=Regulatory-Focus?utm_source=Facebookandutm_medium=socialandutm_campaign=Regulatory-Focus (accessed August 20, 2020).
49. Wu SC. Progress and concept for COVID-19 vaccine development. Biotechnol J. (2020) 15:e2000147. doi: 10.1002/biot.202000147
50. Akhmerov A, Marban E. COVID-19 and the Heart. Circ Res. (2020) 126:1443–55. doi: 10.1161/CIRCRESAHA.120.317055
51. Dominga Iacobazzi MB, Paolo M, Massimo C. COVID-19, State of the adult and pediatric heart: from myocardial injury to cardiac effect of potential therapeutic intervention. Front Cardiovasc Med. (2020) 7:140. doi: 10.3389/fcvm.2020.00140
52. Giacomet V, Manfredini VA, Meraviglia G, Peri CF, Sala A, Longoni E, et al. Acute inflammation and elevated cardiac markers in a two-month-old infant with severe acute respiratory syndrome coronavirus 2 infection presenting with cardiac symptoms. Pediatr Infect Dis J. (2020) 39:e149–51. doi: 10.1097/INF.0000000000002750
53. Barach P, Lipshultz SE. Rethinking COVID-19 in children: lessons learned from pediatric viral and inflammatory cardiovascular diseases. Prog Pediatr Cardiol. (2020) 57:101233. doi: 10.1016/j.ppedcard.2020.101233
54. Oberweis ML, Codreanu A, Boehm W, Olivier D, Pierron C, Tsobo C, et al. Pediatric life-threatening coronavirus disease 2019 with myocarditis. Pediatr Infect Dis J. (2020) 39:e147–9. doi: 10.1097/INF.0000000000002744
55. Ronconi G, Tete G, Kritas SK, Gallenga CE, Caraffa A, Ross R, et al. SARS-CoV-2, which induces COVID-19, causes kawasaki-like disease in children: role of pro-inflammatory and anti-inflammatory cytokines. J Biol Regul Homeost Agents. (2020) 34:767–73. doi: 10.23812/EDITORIAL-RONCONI-E-59
56. Yasuhara J, Kuno T, Takagi H, Sumitomo N. Clinical characteristics of COVID-19 in children: a systematic review. Pediatr Pulmonol. (2020) 55:2565–75. doi: 10.1002/ppul.24991
57. Waltuch T, Gill P, Zinns LE, Whitney R, Tokarski J, Tsung JW, et al. Features of COVID-19 post-infectious cytokine release syndrome in children presenting to the emergency department. Am J Emerg Med. (2020). doi: 10.1016/j.ajem.2020.05.058. [Epub ahead of print].
58. Whittaker E, Bamford A, Kenny J, Kaforou M, Jones CE, Shah P, et al. Clinical characteristics of 58 children with a pediatric inflammatory multisystem syndrome temporally associated with SARS-CoV-2. JAMA. (2020) 324:259–69. doi: 10.1001/jama.2020.10369
59. Tan W, Aboulhosn J. The cardiovascular burden of coronavirus disease 2019 (COVID-19) with a focus on congenital heart disease. Int J Cardiol. (2020) 309:70–7. doi: 10.1016/j.ijcard.2020.03.063
60. Center for Disease Control and Prevention CDC. Health Department-Reported Cases of Multisystem Inflammatory Syndrome in Children (MIS-C) in the United States. (2020). Available online at: https://www.cdc.gov/mis-c/cases/index.html (accessed July 15, 2020).
61. Shekerdemian LS, Mahmood NR, Wolfe KK, Riggs BJ, Ross CE, McKiernan CA, et al. Characteristics and outcomes of children with coronavirus disease 2019 (covid-19) infection admitted to us and Canadian pediatric intensive care units. JAMA Pediatr. (2020) 174:868–73. doi: 10.1001/jamapediatrics.2020.1948
62. Krittika Joshi DK, Kaplan D, Bakar A, Jennings JF, Hayes DA, Mahajan S, et al. Cardiac dysfunction and shock in pediatric patients with COVID-19. JACC Case Rep. (2020) 2:1267–70. doi: 10.1016/j.jaccas.2020.05.082
63. Toubiana J, Poirault C, Corsia A, Bajolle F, Fourgeaud J, Angoulvant F, et al. Kawasaki-like multisystem inflammatory syndrome in children during the covid-19 pandemic in Paris, France: prospective observational study. BMJ. (2020) 369:m2094. doi: 10.1136/bmj.m2094
64. Deza Leon MP, Redzepi A, McGrath E, Abdel-Haq N, Shawaqfeh A, Sethuraman U, et al. COVID-19-associated pediatric multisystem inflammatory syndrome. J Pediatric Infect Dis Soc. (2020) 9:407–8. doi: 10.1093/jpids/piaa061
65. Dufort EM, Koumans EH, Chow EJ, Rosenthal EM, Muse A, Rowlands J, et al. Multisystem inflammatory syndrome in children in New York State. N Engl J Med. (2020) 383:347–58. doi: 10.1056/NEJMoa2021756
66. Feldstein LR, Rose EB, Horwitz SM, Collins JP, Newhams MM, Son MBF, et al. Multisystem inflammatory syndrome in US children and adolescents. N Engl J Med. (2020) 383:334–46. doi: 10.1056/NEJMoa2021680
67. Julie Toubiana CP, Alice C, Fanny B, Jacques F, Francois A, Agathe D, et al. Outbreak of Kawasaki disease in children during COVID-19 pandemic: a prospective observational study in Paris, France. BMJ. (2020) 369:m2094. doi: 10.1101/2020.05.10.20097394
68. Riollano-Cruz M, Akkoyun E, Briceno-Brito E, Kowalsky S, Posada R, Sordillo EM, et al. Multisystem inflammatory syndrome in children (MIS-C) related to COVID-19: a New York City experience. J Med Virol. (2020) 92:1–10. doi: 10.1002/jmv.26224
69. Belhadjer Z, Meot M, Bajolle F, Khraiche D, Legendre A, Abakka S, et al. Acute heart failure in multisystem inflammatory syndrome in children (MIS-C) in the context of global SARS-CoV-2 pandemic. Circulation. (2020) 142:429–36. doi: 10.1161/CIRCULATIONAHA.120.048360
70. Rogo T, Mathur K, Purswani M. Systemic inflammation with cardiac involvement in pediatric patients with evidence of COVID-19 in a community hospital in the Bronx, NY. J Pediatric Infect Dis Soc. (2020) 9:502–3. doi: 10.1093/jpids/piaa087
71. Jones VG, Mills M, Suarez D, Hogan CA, Yeh D, Segal JB, et al. COVID-19 and Kawasaki disease: novel virus and novel case. Hosp Pediatr. (2020) 10:537–40. doi: 10.1542/hpeds.2020-0123
72. Tanya Rogo KM, Murli P. SARS-CoV-2-related pediatric inflammatory multisystem syndrome, an epidemiological study, France, 1 March to 17 May 2020. J Pediatr Infect Dis Soc. (2020) 25:2001010. doi: 10.2807/1560-7917.ES.2020.25.22.2001010
73. Alsaied T, Aboulhosn JA, Cotts TB, Daniels CJ, Etheridge SP, Feltes TF, et al. Coronavirus disease 2019 (COVID-19) pandemic implications in pediatric and adult congenital heart disease. J Am Heart Assoc. (2020) 9:e017224. doi: 10.1161/JAHA.120.017224
74. Sabatino J, Ferrero P, Chessa M, Bianco F, Ciliberti P, Secinaro A, et al. COVID-19 and congenital heart disease: results from a Nationwide Survey. J Clin Med. (2020) 9:1774. doi: 10.3390/jcm9061774
75. Association BCC. BCCA COVID-19 Guidance for Vulnerable Groups With Congenital Heart Disease. (2020). Available online at: https://www.bcca-uk.org/pages/news_box.asp?NewsID=19495710 (accessed March 18, 2020).
76. Shen K, Yang Y, Wang T, Zhao D, Jiang Y, Jin R, et al. Diagnosis, treatment, and prevention of 2019 novel coronavirus infection in children: experts' consensus statement. World J Pediatr. (2020) 16:223–31. doi: 10.1007/s12519-020-00344-6
77. Zheng F, Liao C, Fan QH, Chen HB, Zhao XG, Xie ZG, et al. Clinical characteristics of children with coronavirus disease 2019 in Hubei, China. Curr Med Sci. (2020) 40:275–80. doi: 10.1007/s11596-020-2172-6
78. Giordano R, Cantinotti M. Congenital heart disease in the era of COVID-19 pandemic. Gen Thorac Cardiovasc Surg. (2020). doi: 10.1007/s11748-020-01417-z. [Epub ahead of print].
79. ACC. HFSA/ACC/AHA Statement Addresses Concerns Re: Using RAAS Antagonists in COVID-19. (2020). Available online at: https://www.acc.org/latest-in-cardiology/articles/2020/03/17/08/59/hfsa-acc-aha-statement-addresses-concerns-re-using-raas-antagonists-in-covid-19 (accessed).
80. ESC. Position Statement of the ESC Council on Hypertension on ACE-Inhibitors and Angiotensin Receptor Blockers (2020).
81. Kocak G, Ergul Y, Nisli K, Hatemi AC, Tutar E, Tokel NK, et al. Evaluation and follow-up of pediatric COVID-19 in terms of cardiac involvement: a scientific statement from the association of turkish pediatric cardiology and pediatric cardiac surgery. Anatol J Cardiol. (2020) 24:13–8. doi: 10.14744/AnatolJCardiol.2020.36559
Keywords: children, congenital heart disease, CHD, COVID-19, coronavirus, pediatric cardiology
Citation: Zareef RO, Younis NK, Bitar F, Eid AH and Arabi M (2020) COVID-19 in Pediatric Patients: A Focus on CHD Patients. Front. Cardiovasc. Med. 7:612460. doi: 10.3389/fcvm.2020.612460
Received: 30 September 2020; Accepted: 05 November 2020;
Published: 27 November 2020.
Edited by:
George W. Booz, University of Mississippi Medical Center School of Dentistry, United StatesReviewed by:
Andriy Yabluchanskiy, University of Oklahoma Health Sciences Center, United StatesStefan Nonchev, Université Grenoble Alpes, France
Copyright © 2020 Zareef, Younis, Bitar, Eid and Arabi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ali H. Eid, YWU4MUBhdWIuZWR1Lmxi; YWxpLmVpZEBxdS5lZHUucWE=; Mariam Arabi, bWE4MUBhdWIuZWR1Lmxi
†These authors have contributed equally to this work
 Rana O. Zareef
Rana O. Zareef Nour K. Younis
Nour K. Younis Fadi Bitar
Fadi Bitar Ali H. Eid
Ali H. Eid Mariam Arabi
Mariam Arabi