Abstract
Backgrounds: Influenza vaccination could decrease the risk of major cardiac events in patients with chronic obstructive pulmonary disease (COPD). However, the effects of the vaccine on decreasing the risk of ventricular arrhythmia (VA) development in such patients remain unclear.
Methods: We retrospectively analyzed the data of 18,658 patients with COPD (≥55 years old) from the National Health Insurance Research Database from January 1, 2001, to December 31, 2012. After a 1:1 propensity score matching by the year of diagnosis, we divided the patients into vaccinated and unvaccinated groups. Time-varying Cox proportional hazards regression was applied to assess the time to event hazards of influenza vaccination exposure.
Results: The risk of VA occurrence was significantly lower in the vaccinated group during influenza season and all seasons [adjusted hazard ratio (aHR): 0.62, 95% CI: 0.41–0.95; aHR: 0.69, 95% CI: 0.44–1.08; and aHR: 0.65, 95% CI: 0.48–0.89, in the influenza season, non-influenza season, and all seasons, respectively]. Among patients with CHA2DS2-VASc scores (conditions and characteristics included congestive heart failure, hypertension, diabetes, stroke, vascular disease, age, and sex) of 2–3, receiving one time and two to three times of influenza vaccination were associated with lower risk of VA occurrence in all seasons (aHR: 0.28, 95% CI: 0.10–0.80; aHR: 0.27, 95% CI: 0.10–0.68, respectively). Among patients without stroke, peripheral vascular disease, and diabetes, a lower risk of VA occurrence after receiving one and two to three times vaccination was observed in all seasons. Among patients with a history of asthma and patients without a history of heart failure, ischemic heart disease, angina hypertension, or renal failure, a significantly lower risk of VA occurrence was observed after the first time of vaccination in all seasons.
Conclusions: Influenza vaccination may be associated with lower risks of VA among patients with COPD aged 55–74. Further investigation is still needed to resolve this clinical question.
Introduction
Chronic obstructive pulmonary disease (COPD) is a worldwide health burden and has risen from the sixth to the third most common cause of death in the past 30 years (1). Studies have revealed that cardiovascular disease is a major cause of death among patients with COPD (2). Because patients with COPD often have similar comorbidities that increase the risk of developing cardiovascular disease, the risks of heart failure, myocardial infarction, and arrhythmia also increase (3, 4). Although supraventricular arrhythmia is the most common form of arrhythmia in patients with COPD arrhythmia (5), the risk of ventricular arrhythmia (VA) is also high (6, 7). Moreover, the risk of sudden death is significantly heightened in patients with COPD (5, 8). In the acute exacerbation of COPD, the most common type of cardiac arrhythmia is premature ventricular contractions (9). Increased QT dispersion, autonomic function, hypoxemia, and hypercapnia were the proposed mechanisms of vulnerability to VA in patients with COPD (7, 10, 11). VA is also a possible risk factor of mortality in patients with COPD during acute exacerbation (12).
Influenza is a common and severe viral infection among patients with COPD. It increases the risk of not only acute respiratory failure but also acute cardiovascular events (13). The risk of myocardial infarction and sudden cardiac death decreases upon influenza vaccination (13–15). However, the association of influenza vaccination and VA among patients with COPD is still unclear. Therefore, our study investigated the association between influenza vaccination and the occurrence of VA in a real-world setting.
Methods
The National Health Insurance (NHI) program established in 1995 provides health insurance coverage for more than 98% of the Taiwanese population (more than 23 million people). The NHI Research Database (NHIRD) is maintained by the Health and Welfare Data Science Center and has been extensively analyzed and validated by earlier studies (16). In Taiwan, the influenza vaccination is provided free of charge for adults aged over 50 years with high-risk comorbidities (i.e., diabetes, chronic liver disease, cirrhosis, cardiovascular disease, or chronic pulmonary disease). The NHIRD research committee and the Joint Institutional Review Board of Taipei Medical University approved our study protocol (TMU-JIRB No. N201905004).
Study Cohort and Study Design
The patients included in the study were diagnosed as having COPD between January 1, 2001, and December 31, 2012. The positive predictive value of the ICD-9-CM codes in COPD diagnosis was previously validated (17). In addition, they had at least two COPD diagnoses as inpatients or outpatients (18) and were aged ≥55 years. Vaccination status was identified by code V048 and/or the use of the vaccine (confirmed by drug codes). A comparison cohort was selected by blinding the outcome using a SAS (Statistical Analysis Software) software package. Each patient with COPD who received vaccination underwent propensity score–based matching for sociodemographic characteristics (age, gender, urbanization level, monthly income); comorbidities (asthma [ICD-9-CM code 493], heart failure [ICD-9-CM code 428], acute myocardial infarction [ICD-9-CM code 410], stroke [ICD-9-CM code 430-438], ischemic heart disease [ICD-9-CM code 414], angina [ICD-9-CM code 413], peripheral vascular disease [ICD-9-CM code 448], hypertension [ICD-9-CM code 405], diabetes [ICD-9-CM code 250], depression [ICD-9-CM code 296.2; 303.4; 296.3; 311], renal failure [ICD-9-CM code 403, 404, 582, 583, 585, 586, 588], chronic liver disease [ICD-9-CM code 456, 571, 572], dementia [ICD-9-CM code 290, 797]); CHA2DS2-VASc score; COPD severity (defined as COPD-related inpatient visits) (19); COPD medications (short-acting beta agonist [SABA], long-acting beta-agonist [LABA], long-acting muscarinic antagonist [LAMA], inhaled corticosteroids [ICS]); and total numbers of COPD medications with those who did not receive vaccinations for comparing VA incidence. After a 1:1 propensity score matching by the year of diagnosis, we divided the patients into vaccinated and unvaccinated groups. Any individual with prior VA in a matched pair was excluded. The cohort entry date (index date) for patients receiving vaccines was defined as the vaccine initiation date. In the matched pairs, the participants who received vaccines and those who did not receive vaccines were given the same index date (vaccine initiation date) for follow-up. All patients were followed up until one of the following occurred: an initial diagnosis of ventricular tachycardia (ICD-9-CM code 427.1), ventricular fibrillation, or ventricular flutter (ICD-9-CM code 427.4, 427.41, or 427.42), loss to follow-up, death, withdrawal from the NHIRD or December 31, 2012 (Figure 1).
Figure 1
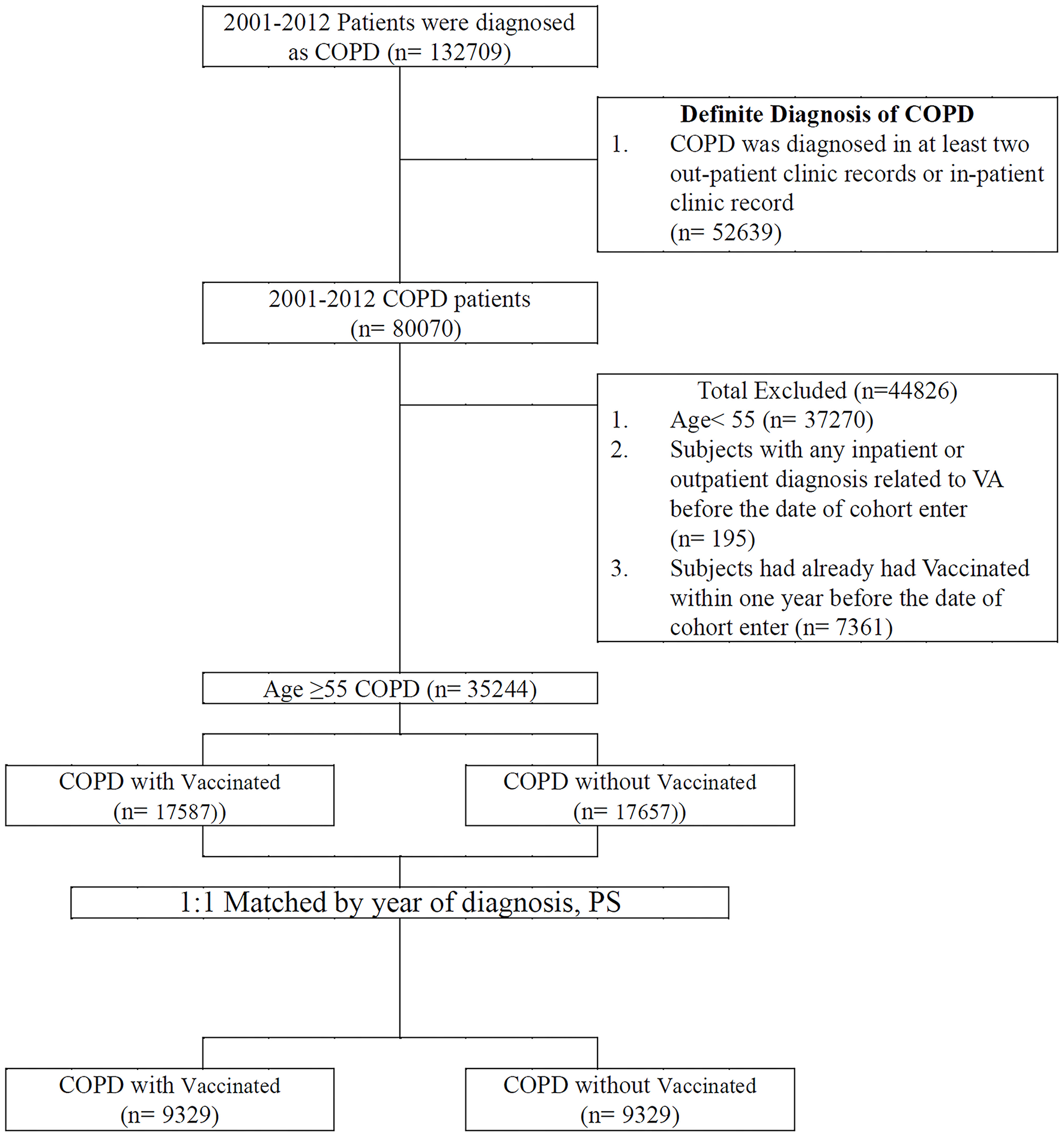
Data selection process.
Potential Confounders
The potential confounders of our cohort were based on sociodemographic characteristics (age, gender, urbanization level, and monthly income), comorbidities (asthma, heart failure, acute myocardial infarction, stroke, ischemic heart disease, angina, peripheral vascular disease, hypertension, diabetes, depression, renal failure, chronic liver disease, dementia, and CHA2DS2-VASc score), COPD severity and treatment (COPD-related inpatient visits, COPD medications, total number of COPD medications), and medication use (aspirin, statin, renin-angiotensin-aldosterone system inhibitor [RAASI], and Class I [sodium channel blocker], Class II [beta blocker], Class III [potassium channel blocker], and Class IV [calcium channel blocker] antiarrhythmic agents).
Statistical Analysis
All baseline patient characteristics mentioned earlier are described in Table 1. Categorical variables are reported as numbers and percentages, and quantitative variables are presented as mean ± SDs. The balance of characteristics was assessed by estimating standardized differences (StDiffs) between the vaccinated group and the unvaccinated group. A time-varying Cox proportional hazard model was used to calculate the hazard ratios (HRs) to determine the risk of VA between the vaccinated and unvaccinated groups (20). The aforementioned confounders were treated as a fixed effect in the Cox model, while influenza vaccination exposure in the follow-up period was the random effect. In the sensitivity analyses, external adjustments clarified the effects of covariates in epidemiological database studies. Thus, in the sensitivity analysis, patients were stratified to estimate the association of age, gender, CHA2DS2-VASc score, COPD-related inpatient visits, asthma, heart failure, stroke, ischemic heart disease, angina, peripheral vascular disease, hypertension, diabetes, renal failure, and chronic liver disease with the incidence of VA in different models. The VA-free survival rate in the vaccinated and unvaccinated patients with COPD was calculated using the Kaplan–Meier method. Influenza season is defined as the period between October and March according to the Taiwan Centers for Disease Control. All analyses were performed using SAS (Version 9.4, SAS, Cary, NC, USA); a two-tailed P-value of < 0.05 was considered significant.
Table 1
| After matching | |||||
|---|---|---|---|---|---|
| COPD without vaccinated | COPD with vaccinated | Standardized difference | |||
| n=(9,329) | n=(9,329) | ||||
| n | % | n | % | ||
| COPD-related inpatient visits | |||||
| 0 | 6,804 | 72.93 | 6,978 | 74.80 | 0.042 |
| 1 | 1,316 | 14.11 | 1,179 | 12.64 | −0.043 |
| ≥2 | 1,09 | 12.96 | 1,172 | 12.56 | −0.012 |
| Age, years (Mean±SD) | 69.82 ± 9.31 | 70.13 ± 8.98 | 0.033 | ||
| 55–64 | 3,459 | 37.08 | 3,368 | 36.10 | −0.020 |
| 65–74 | 3,027 | 32.45 | 3,115 | 33.39 | 0.020 |
| ≥75 | 2,843 | 30.47 | 2,846 | 30.51 | 0.001 |
| Gender | |||||
| Female | 3,953 | 42.37 | 4,096 | 43.91 | 0.031 |
| Male | 5,376 | 57.63 | 5,233 | 56.09 | −0.031 |
| CHA2DS2-VASc score | |||||
| 0 | 1,318 | 14.13 | 1,124 | 12.05 | −0.062 |
| 1 | 1,721 | 18.45 | 1,572 | 16.85 | −0.042 |
| 2–3 | 3,310 | 35.48 | 3,385 | 36.28 | 0.017 |
| ≥4 | 2,980 | 31.94 | 3,248 | 34.82 | 0.061 |
| Comorbidities | |||||
| Asthma | 3,970 | 42.56 | 4,400 | 47.16 | 0.093 |
| Heart failure | 1,087 | 11.65 | 1,136 | 12.18 | 0.016 |
| Acute myocardial infarction | 180 | 1.93 | 194 | 2.08 | 0.011 |
| Stroke | 2,300 | 24.65 | 2,559 | 27.43 | 0.063 |
| Ischemic heart disease | 3,015 | 32.32 | 3,391 | 36.35 | 0.085 |
| Angina | 1,003 | 10.75 | 1,203 | 12.90 | 0.066 |
| Peripheral vascular disease | 940 | 10.08 | 1,051 | 11.27 | 0.039 |
| Hypertension | 5,504 | 59.00 | 5,871 | 62.93 | 0.081 |
| Diabetes | 2,657 | 28.48 | 2,888 | 30.96 | 0.054 |
| Depression | 347 | 3.72 | 447 | 4.79 | 0.053 |
| Renal failure | 1,349 | 14.46 | 1,352 | 14.49 | 0.001 |
| Chronic liver disease | 2,367 | 25.37 | 2,634 | 28.23 | 0.065 |
| Dementia | 600 | 6.43 | 679 | 7.28 | 0.034 |
| Level of urbanization | |||||
| Urban | 6,495 | 69.62 | 6,166 | 66.09 | −0.076 |
| Suburban | 1,969 | 21.11 | 2,015 | 21.60 | 0.012 |
| Rural | 865 | 9.27 | 1,148 | 12.31 | 0.098 |
| Monthly income (NT$) | |||||
| 0 | 1,155 | 12.38 | 981 | 10.52 | −0.059 |
| 1–33,300 | 6,249 | 66.98 | 6,366 | 68.24 | 0.027 |
| ≥33,301 | 1,925 | 20.63 | 1,982 | 21.25 | 0.015 |
| COPD medications | |||||
| SABA | 2,261 | 24.24 | 2,336 | 25.04 | 0.019 |
| SABA+SAMA | 1,913 | 20.51 | 1,962 | 21.03 | 0.013 |
| LABA (Ultra-LABA) | 109 | 1.17 | 125 | 1.34 | 0.015 |
| LAMA | 457 | 4.90 | 577 | 6.19 | 0.056 |
| ICS | 955 | 10.24 | 1,007 | 10.79 | 0.018 |
| ICS+LABA | 1,273 | 13.65 | 1,429 | 15.32 | 0.048 |
| Total COPD medications | |||||
| 0 | 5,484 | 58.78 | 5,377 | 57.64 | −0.023 |
| 1 | 2,014 | 21.59 | 1,974 | 21.16 | −0.010 |
| ≥2 | 1,831 | 19.63 | 1,978 | 21.20 | 0.039 |
| Medication use* | |||||
| Aspirin | 3,103 | 33.26 | 3,853 | 41.30 | 0.167 |
| Statin | 2,006 | 21.50 | 2,640 | 28.30 | 0.158 |
| RAASI | 4,197 | 44.99 | 4,963 | 53.20 | 0.165 |
| Class1 | 194 | 2.08 | 276 | 2.96 | 0.061 |
| Class2 | 2,286 | 24.50 | 2,775 | 29.75 | 0.118 |
| Class3 | 640 | 6.86 | 627 | 6.72 | −0.006 |
| Class4 | 1,451 | 15.55 | 1,692 | 18.14 | 0.069 |
Pooled baseline characteristics balance before and after propensity score matching.
Standardized difference = difference in mean or proportions divided by the standard error; imbalance between groups was defined as an absolute value >0.10 (corresponding to a small effect size).
Propensity score-matched is adjusted for COPD-related inpatient visits, COPD medications, total COPD medications, age, gender, CHA2DS2-VASc score, asthma, heart failure, acute myocardial infarction, stroke, ischemic heart disease, angina, peripheral vascular disease, hypertension, diabetes, depression, renal failure, chronic liver disease, dementia, level of urbanization, monthly income.
Propensity score matching did not adjust these variables.
COPD, chronic obstructive pulmonary disease; SABA, short-acting beta agonist; SAMA, short-acting muscarinic antagonist; LABA, long-acting beta-agonist; LAMA, long-acting muscarinic antagonist; ICS, inhaled corticosteroids; RAASI, renin-angiotensin-aldosterone system inhibitor.
Results
In total, 18,658 patients were enrolled and matched pairs were obtained comprising individuals in the vaccinated and comparison cohorts. Table 1 shows the baseline characteristics of both groups after propensity score matching.
Association Between Influenza Vaccination and VA on Different Age Groups and Genders
Table 2 demonstrates the risk of VA between vaccination and non-vaccination groups stratified by age and gender. We observed a significantly low occurrence of VA during influenza season and all seasons (aHR: 0.62 [95% CI: 0.41–0.95], 0.69 [95% CI: 0.44–1.08], 0.65 [95% CI: 0.48–0.89], in influenza season, non-influenza season, and all seasons, respectively) (Figure 2). In patients with COPD between 55 and 64 years old, a significant lower risk of VA occurrence was observed after receiving vaccination in non-influenza season and all seasons (aHR: 0.22 [95% CI: 0.07–0.68], 0.33 [95% CI: 0.16–0.69], respectively). In patients with ages between 65 and 74 years, a lower risk of VA occurrence in the vaccinated group was observed in all seasons. In patients with age more than 75 years, there was no significant difference in VA occurrence after vaccination in the influenza season, non-influenza season, and all seasons.
Table 2
| All group (n = 18,658) | Unvaccinated (Total follow-up 40796.4 person-years) | Vaccinated (Total follow-up 39432.1 person-years) | Unadjusted HR (95% C.I.) | Adjusted HR† (95% C.I.) | ||
|---|---|---|---|---|---|---|
| No. of patients with VA | Incidence rate (per 105 person-years) (95% C.I.) | No. of patients with VA | Incidence rate (per 105 person-years) (95% C.I.) | |||
| Whole cohort | ||||||
| Influenza season | 60 | 147.1 (109.9, 184.3) | 35 | 88.8 (59.4, 118.2) | 0.59 (0.39, 0.90)* | 0.62 (0.41, 0.95)* |
| Non-influenza season | 46 | 112.8 (80.2, 145.3) | 34 | 86.2 (57.2, 115.2) | 0.71 (0.46, 1.11) | 0.69 (0.44, 1.08) |
| All season | 106 | 259.8 (210.4, 309.3) | 69 | 175.0 (133.7, 216.3) | 0.64 (0.47, 0.87)** | 0.65 (0.48, 0.89)** |
| Age, 55–64a | ||||||
| Influenza season | 15 | 98.5 (48.6, 148.3) | 6 | 44.7 (8.9, 80.4) | 0.42 (0.16, 1.08) | 0.48 (0.18, 1.29) |
| Non-influenza season | 17 | 111.6 (58.5, 164.6) | 4 | 29.8 (0.6, 58.9) | 0.26 (0.09, 0.78)* | 0.22 (0.07, 0.68)** |
| All season | 32 | 210.0 (137.3, 282.8) | 10 | 74.4 (28.3, 120.6) | 0.34 (0.17, 0.69)** | 0.33 (0.16, 0.69)** |
| Age, 65–74b | ||||||
| Influenza season | 25 | 166.4 (101.1, 231.6) | 12 | 79.8 (34.7, 125.0) | 0.48 (0.24, 0.97)* | 0.51 (0.25, 1.03) |
| Non-influenza season | 20 | 133.1 (74.8, 191.4) | 11 | 73.2 (29.9, 116.4) | 0.52 (0.25, 1.08) | 0.48 (0.22, 1.02) |
| All season | 45 | 299.5 (212.0, 386.9) | 23 | 153.0 (90.5, 215.6) | 0.50 (0.30, 0.83)** | 0.49 (0.29, 0.83)** |
| Age, ≥75c | ||||||
| Influenza season | 20 | 189.9 (106.6, 273.1) | 17 | 155.1 (81.3, 228.8) | 0.79 (0.41, 1.51) | 0.82 (0.42, 1.59) |
| Non-influenza season | 9 | 85.4 (29.6, 141.3) | 19 | 173.3 (95.4, 251.2) | 1.87 (0.84, 4.17) | 2.06 (0.91, 4.64) |
| All season | 29 | 275.3 (175.1, 375.5) | 36 | 328.3 (221.1, 435.6) | 1.13 (0.69, 1.84) | 1.20 (0.73, 1.98) |
| Femaled | ||||||
| Influenza season | 27 | 148.8 (92.7, 204.9) | 19 | 108.7 (59.8, 157.6) | 0.71 (0.40, 1.29) | 0.79 (0.43, 1.43) |
| Non-influenza season | 17 | 93.7 (49.1, 138.2) | 16 | 91.5 (46.7, 136.4) | 0.87 (0.44, 1.74) | 0.91 (0.45, 1.84) |
| All season | 44 | 242.5 (170.8, 314.1) | 35 | 200.2 (133.9, 266.5) | 0.78 (0.50, 1.22) | 0.84 (0.53, 1.32) |
| Malee | ||||||
| Influenza season | 33 | 145.7 (96.0, 195.4) | 16 | 72.9 (37.2, 108.6) | 0.49 (0.27, 0.89)* | 0.48 (0.26, 0.88)* |
| Non-influenza season | 29 | 128.0 (81.4, 174.6) | 18 | 82.0 (44.1, 119.9) | 0.62 (0.34, 1.11) | 0.55 (0.30, 1.01) |
| All season | 62 | 273.7 (205.6, 341.9) | 44 | 200.4 (141.2, 259.7) | 0.55 (0.36, 0.83)** | 0.52 (0.34, 0.79)** |
Risk of ventricular arrhythmia among unvaccinated and vaccinated in study cohort.
Total follow-up 15234.5 person-year for unvaccinated and 13436.7 for Vaccinated.
Total follow-up 15027.5 person-year for unvaccinated and 15031.2 for Vaccinated.
Total follow-up 10534.5 person-year for unvaccinated and 10964.2 for Vaccinated.
Total follow-up 18146.1 person-year for unvaccinated and 17481.2 for Vaccinated.
Total follow-up 22650.3 person-year for unvaccinated and 21950.9 for Vaccinated.
p < 0.05;
p < 0.01.
C.I., confidence interval.
HR, hazard ratio.
Main model is adjusted for COPD-related inpatient visits, COPD medications, total COPD medications, age, gender, CHA2DS2-VASc score, asthma, heart failure, acute myocardial infarction, stroke, ischemic heart disease, angina, peripheral vascular disease, hypertension, diabetes, depression, renal failure, chronic liver disease, dementia, level of urbanization, monthly income, antiarrhythmic agents: class1 (sodium channel blocker), class2 (beta blocker), class3 (potassium channel blocker), class4 (calcium channel blocker), aspirin, statin, RAASI.
Figure 2
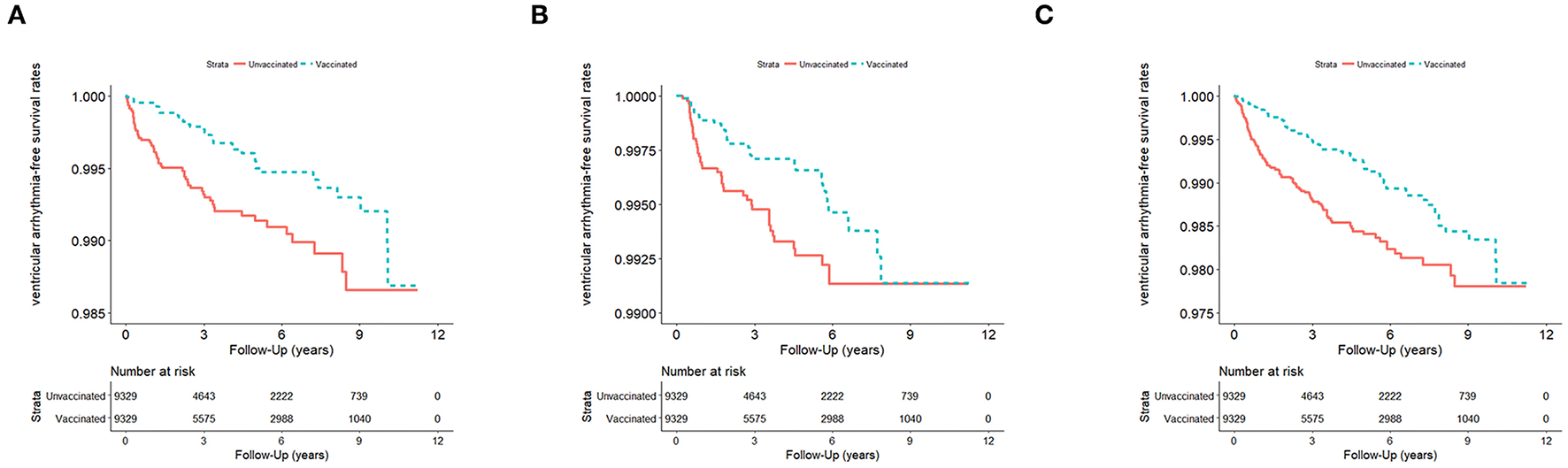
(A) VA events in influenza season (n = 18,658; January 1, 2001–December 31, 2012) in Taiwan, stratified by vaccinated and unvaccinated groups (log rank test, χ2 = 10.686; df = 1; p = 0.001). (B) VA events in non-influenza season (n = 18,658) from January 1, 2001 to December 31, 2012, in Taiwan, stratified by vaccinated and unvaccinated groups (log rank test, χ2 = 4.101; df = 1; p = 0.063). (C) VA events in all seasons (n = 18,658) from January 1, 2001 to December 31, 2012, in Taiwan, stratified by vaccinated and unvaccinated groups (log rank test, χ2 = 14.272; df = 1; p < 0.001). VA, ventricular arrhythmia.
Male patients with COPD had a low occurrence of VA after receiving influenza vaccination in all seasons and influenza season (aHR: 0.52 [95% CI: 0.34–0.79] and 0.48 [95%CI: 0.26–0.88], respectively). Regarding female patients with COPD, no significant reduction in VA occurrence was observed between the vaccinated and unvaccinated groups in all seasons, influenza season, and non-influenza season.
Association Between the Total Number of Influenza Vaccinations and VA
In the main model, there was no significant difference in VA occurrence after receiving one time, two to three, and more than four times influenza vaccination during the influenza season (Table 3). In the subgroup analysis, significant lower risk of VA occurrence was observed among patients without heart failure, ischemic heart disease, angina, peripheral vascular disease, or renal failure after receiving one time of vaccination in influenza season (aHR: 0.37 [95% CI: 0.15–0.94], 0.33 [95% CI: 0.12–0.93], 0.44 [95% CI: 0.20–0.98], 0.46 [95% CI: 0.22–0.97], respectively).
Table 3
| Unvaccinated | Vaccinated | |||
|---|---|---|---|---|
| 1 | 2–3 | ≥4 | ||
| Adjusted HR (95% C.I.) | Adjusted HR (95% C.I.) | Adjusted HR (95% C.I.) | Adjusted HR (95% C.I.) | |
| Unadjusted | 1.00 | 0.53 (0.27, 1.05) | 0.73 (0.42, 1.26) | 0.47 (0.22, 0.98)* |
| Model1† | 1.00 | 0.50 (0.25, 0.98)* | 0.71 (0.41, 1.24) | 0.47 (0.22, 0.99)* |
| Main model‡ | 1.00 | 0.52 (0.27, 1.03) | 0.77 (0.44, 1.33) | 0.54 (0.25, 1.15) |
| Subgroup effects | ||||
| Age, years | ||||
| 55–74 | 1.00 | 0.08 (0.01, 0.60)* | 0.69 (0.34, 1.41) | 0.73 (0.32, 1.67) |
| ≥75 | 1.00 | 1.19 (0.54, 2.65) | 0.87 (0.36, 2.09) | 0.18 (0.02, 1.40) |
| Gender | ||||
| Female | 1.00 | 0.55 (0.21, 1.44) | 1.04 (0.49, 2.19) | 0.75 (0.25, 2.21) |
| Male | 1.00 | 0.51 (0.20, 1.33) | 0.52 (0.22, 1.19) | 0.39 (0.14, 1.13) |
| COPD-related inpatient visits | ||||
| 0 | 1.00 | 0.46 (0.18, 1.18) | 1.10 (0.57, 2.12) | 0.68 (0.26, 1.77) |
| 1 | 1.00 | 0.88 (0.24, 3.26) | 0.23 (0.05, 1.07) | |
| ≥2 | 1.00 | 0.59 (0.12, 2.83) | 0.53 (0.11, 2.56) | 0.95 (0.24, 3.82) |
| CHA2DS2-VASc score | ||||
| 0 | 1.00 | 0.30 (0.07, 1.36) | 0.57 (0.15, 2.16) | |
| 1 | 1.00 | |||
| 2–3 | 1.00 | 0.32 (0.07, 1.39) | 0.33 (0.09, 1.12) | 0.60 (0.20, 1.84) |
| ≥4 | 1.00 | 0.86 (0.38, 1.91) | 1.27 (0.63, 2.56) | 0.18 (0.02, 1.35) |
| Asthma | ||||
| No | 1.00 | 0.80 (0.37, 1.77) | 0.78 (0.35, 1.72) | 0.63 (0.22, 1.81) |
| Yes | 1.00 | 0.23 (0.05, 0.97)* | 0.78 (0.36, 1.68) | 0.50 (0.17, 1.46) |
| Heart failure | ||||
| No | 1.00 | 0.37 (0.15, 0.94)* | 0.72 (0.37, 1.37) | 0.59 (0.26, 1.33) |
| Yes | 1.00 | 0.88 (0.31, 2.45) | 0.93 (0.32, 2.69) | 0.30 (0.04, 2.44) |
| Stroke | ||||
| No | 1.00 | 0.51 (0.21, 1.21) | 0.56 (0.26, 1.21) | 0.57 (0.23, 1.37) |
| Yes | 1.00 | 0.54 (0.18, 1.59) | 1.11 (0.49, 2.51) | 0.43 (0.10, 1.89) |
| Ischemic heart disease | ||||
| No | 1.00 | 0.33 (0.12, 0.93)* | 0.58 (0.27, 1.27) | 0.77 (0.34, 1.77) |
| Yes | 1.00 | 0.78 (0.31, 1.96) | 0.98 (0.44, 2.16) | 0.18 (0.02, 1.40) |
| Angina | ||||
| No | 1.00 | 0.44 (0.20, 0.98)* | 0.73 (0.40, 1.34) | 0.53 (0.24, 1.18) |
| Yes | 1.00 | 0.78 (0.20, 3.10) | 1.04 (0.25, 4.28) | 0.72 (0.08, 6.35) |
| Peripheral vascular disease | ||||
| No | 1.00 | 0.47 (0.22, 1.00)* | 0.57 (0.30, 1.11) | 0.61 (0.29, 1.32) |
| Yes | 1.00 | 0.87 (0.17, 4.42) | 1.38 (0.41, 4.68) | |
| Hypertension | ||||
| No | 1.00 | 0.36 (0.13, 0.95)* | 0.63 (0.18, 2.19) | |
| Yes | 1.00 | 0.80 (0.40, 1.63) | 0.86 (0.44, 1.68) | 0.53 (0.20, 1.38) |
| Diabetes | ||||
| No | 1.00 | 0.52 (0.23, 1.16) | 0.47 (0.22, 1.01) | 0.62 (0.27, 1.42) |
| Yes | 1.00 | 0.53 (0.15, 1.83) | 1.51 (0.65, 3.51) | 0.29 (0.04, 2.26) |
| Renal failure | ||||
| No | 1.00 | 0.46 (0.22, 0.97)* | 0.74 (0.41, 1.32) | 0.50 (0.22, 1.12) |
| Yes | 1.00 | 1.51 (0.26, 8.68) | 1.72 (0.29, 10.08) | 1.03 (0.09, 11.29) |
| Chronic liver disease | ||||
| No | 1.00 | 0.48 (0.22, 1.08) | 0.70 (0.37, 1.34) | 0.69 (0.32, 1.49) |
| Yes | 1.00 | 0.68 (0.19, 2.43) | 0.72 (0.24, 2.13) | |
Sensitivity analysis of adjusted HRs of vaccination in risk reduction of ventricular arrhythmia in influenza season.
p < 0.05; HR, hazard ratio.
Model1 is adjusted for COPD-related inpatient visits, age, gender, CHA2DS2-VASc score, asthma, heart failure, acute myocardial infarction, stroke, ischemic heart disease, angina, peripheral vascular disease, hypertension, diabetes, depression, renal failure, chronic liver disease, dementia, level of urbanization, monthly income.
Main model is adjusted for COPD-related inpatient visits, COPD medications, total COPD medications, age, gender, CHA2DS2-VASc score, asthma, heart failure, acute myocardial infarction, stroke, ischemic heart disease, angina, peripheral vascular disease, hypertension, diabetes, depression, renal failure, chronic liver disease, dementia. level of urbanization, monthly income, antiarrhythmic agents: class1 (sodium channel blocker), class2 (beta blocker), class3 (potassium channel blocker), class4 (calcium channel blocker), aspirin, statin, RAASI.
During non-influenza season, there was no significant difference in VA occurrence after receiving one, two to three, and more than four times of vaccination (Table 4). Among patients with age between 55 and 74 years, a significantly lower risk of VA occurrence was observed after receiving one and two to three times of vaccination (aHR: 0.17 [95% CI: 0.04–0.73], 0.27 [95% CI: 0.10–0.77], respectively).
Table 4
| Unvaccinated | Vaccinated | |||
|---|---|---|---|---|
| 1 | 2–3 | ≥4 | ||
| Adjusted HR (95% C.I.) | Adjusted HR (95% C.I.) | Adjusted HR (95% C.I.) | Adjusted HR (95% C.I.) | |
| Unadjusted | 1.00 | 0.67 (0.35, 1.32) | 0.61 (0.32, 1.19) | 0.89 (0.47, 1.69) |
| Model1† | 1.00 | 0.65 (0.33, 1.26) | 0.59 (0.30, 1.15) | 0.87 (0.46, 1.66) |
| Main model‡ | 1.00 | 0.65 (0.33, 1.26) | 0.60 (0.31, 1.16) | 0.86 (0.45, 1.65) |
| Subgroup effects | ||||
| Age, years | ||||
| 55–74 | 1.00 | 0.17 (0.04, 0.73)* | 0.27 (0.10, 0.77)* | 0.69 (0.33, 1.47) |
| ≥75 | 1.00 | 2.23 (0.87, 5.74) | 2.09 (0.76, 5.76) | 1.59 (0.41, 6.12) |
| Gender | ||||
| Female | 1.00 | 0.81 (0.31, 2.08) | 0.96 (0.37, 2.49) | 1.04 (0.34, 3.22) |
| Male | 1.00 | 0.50 (0.19, 1.33) | 0.41 (0.16, 1.07) | 0.77 (0.34, 1.72) |
| COPD-related inpatient visits | ||||
| 0 | 1.00 | 0.50 (0.21, 1.22) | 0.65 (0.30, 1.44) | 0.67 (0.29, 1.56) |
| 1 | 1.00 | 0.58 (0.09, 3.89) | 0.30 (0.02, 4.19) | |
| ≥2 | 1.00 | 1.29 (0.33, 5.04) | 0.68 (0.14, 3.36) | 2.47 (0.72, 8.45) |
| CHA2DS2-VASc score | ||||
| 0 | 1.00 | 0.33 (0.04, 2.73) | 0.66 (0.14, 3.26) | 1.23 (0.29, 5.13) |
| 1 | 1.00 | |||
| 2–3 | 1.00 | 0.25 (0.06, 1.10) | 0.20 (0.05, 0.87)* | 0.80 (0.31, 2.06) |
| ≥4 | 1.00 | 1.14 (0.48, 2.71) | 1.08 (0.44, 2.69) | 0.81 (0.23, 2.87) |
| Asthma | ||||
| No | 1.00 | 0.97 (0.40, 2.37) | 0.89 (0.35, 2.26) | 1.07 (0.41, 2.78) |
| Yes | 1.00 | 0.43 (0.15, 1.23) | 0.43 (0.16, 1.13) | 0.73 (0.29, 1.83) |
| Heart failure | ||||
| No | 1.00 | 0.41 (0.16, 1.06) | 0.62 (0.30, 1.30) | 0.90 (0.45, 1.78) |
| Yes | 1.00 | 1.62 (0.54, 4.90) | 0.70 (0.14, 3.55) | 0.59 (0.06, 5.45) |
| Stroke | ||||
| No | 1.00 | 0.49 (0.19, 1.27) | 0.56 (0.24, 1.28) | 0.93 (0.43, 2.00) |
| Yes | 1.00 | 0.94 (0.35, 2.50) | 0.67 (0.22, 2.08) | 0.75 (0.21, 2.73) |
| Ischemic heart disease | ||||
| No | 1.00 | 0.73 (0.27, 1.96) | 0.66 (0.25, 1.75) | 1.43 (0.66, 3.09) |
| Yes | 1.00 | 0.63 (0.25, 1.58) | 0.60 (0.24, 1.50) | 0.34 (0.08, 1.47) |
| Angina | ||||
| No | 1.00 | 0.57 (0.26, 1.24) | 0.52 (0.24, 1.11) | 0.89 (0.45, 1.76) |
| Yes | 1.00 | 1.05 (0.25, 4.40) | 1.10 (0.25, 4.80) | 0.72 (0.08, 6.85) |
| Peripheral vascular disease | ||||
| No | 1.00 | 0.46 (0.21, 1.04) | 0.48 (0.22, 1.03) | 0.79 (0.39, 1.60) |
| Yes | 1.00 | 2.49 (0.55, 11.25) | 2.82 (0.55, 14.55) | 1.60 (0.22, 11.48) |
| Hypertension | ||||
| No | 1.00 | 0.62 (0.17, 2.24) | 0.44 (0.10, 1.97) | 1.67 (0.60, 4.66) |
| Yes | 1.00 | 0.65 (0.30, 1.42) | 0.64 (0.30, 1.35) | 0.60 (0.25, 1.46) |
| Diabetes | ||||
| No | 1.00 | 0.59 (0.24, 1.45) | 0.35 (0.12, 1.01) | 0.99 (0.46, 2.16) |
| Yes | 1.00 | 0.67 (0.24, 1.84) | 0.99 (0.40, 2.43) | 0.64 (0.18, 2.25) |
| Renal failure | ||||
| No | 1.00 | 0.52 (0.23, 1.17) | 0.69 (0.34, 1.40) | 0.93 (0.45, 1.90) |
| Yes | 1.00 | 0.90 (0.24, 3.47) | 0.17 (0.02, 1.44) | 0.50 (0.09, 2.89) |
| Chronic liver disease | ||||
| No | 1.00 | 0.73 (0.35, 1.53) | 0.49 (0.22, 1.11) | 0.78 (0.37, 1.65) |
| Yes | 1.00 | 0.46 (0.10, 2.16) | 1.07 (0.32, 3.57) | 1.54 (0.39, 6.08) |
Sensitivity analysis of adjusted HRs of vaccination in risk reduction of ventricular arrhythmia in non-influenza season.
p < 0.05; HR, hazard ratio.
Model1 is adjusted for COPD-related inpatient visits, age, gender, CHA2DS2-VASc score, asthma, heart failure, acute myocardial infartion, stroke, ischemic heart disease, angina, peripheral vascular disease, hypertension, diabetes, depression, renal failure, chronic liver disease, dementia, level of urbanization, monthly income.
Main model is adjusted for COPD-related inpatient visits, COPD medications, total COPD medications, age, gender, CHA2DS2-VASc score, asthma, heart failure, acute myocardial infarction, stroke, ischemic heart disease, angina, peripheral vascular disease, hypertension, diabetes, depression, renal failure, chronic liver disease, dementia, level of urbanization, monthly income, antiarrhythmic agents: class1 (sodium channel blocker), class2 (beta blocker), class3 (potassium channel blocker), class4 (calcium channel blocker), aspirin, statin, RAASI.
During all seasons, a significant lower risk of VA occurrence was observed after receiving one time of vaccination in the main model (aHR: 0.58 [95% CI: 0.36–0.93]) (Table 5). There was no significant difference in VA occurrence after receiving two to three times and more than four times of vaccination. Among patients with age between 55 and 74 years, a significantly lower risk of VA was observed after receiving one and two to three times of vaccination (aHR: 0.13 [95% CI: 0.04–0.40], 0.48 [95% CI: 0.27–0.85], respectively). In patients without stroke, peripheral vascular disease, diabetes, and with CHA2DS2-VASc scores of 2–3, a significantly lower risk of VA occurrence was observed after receiving one time and two to three times of vaccination. In patients with a history of asthma, a significantly lower risk of VA occurrence was observed after the first time of vaccination (aHR: 0.33 [95% CI: 0.14–0.78]). In patients without heart failure, ischemic heart disease, hypertension, angina, and renal failure, significant lower risk of VA occurrence was observed after first time of vaccination (aHR: 0.39 [95% CI: 0.20–0.76], 0.47 [95% CI: 0.23–0.96], 0.26 [95% CI: 0.08–0.86], 0.50 [95% CI: 0.29–0.88], 0.48 [95% CI: 0.28–0.84]).
Table 5
| Unvaccinated | Vaccinated | |||
|---|---|---|---|---|
| 1 | 2–3 | ≥4 | ||
| Adjusted HR (95% C.I.) | Adjusted HR (95% C.I.) | Adjusted HR (95% C.I.) | Adjusted HR (95% C.I.) | |
| Unadjusted | 1.00 | 0.60 (0.37, 0.96)* | 0.68 (0.44, 1.03) | 0.65 (0.40, 1.06) |
| Model1† | 1.00 | 0.57 (0.35, 0.91)* | 0.66 (0.43, 1.01) | 0.65 (0.40, 1.05) |
| Main model‡ | 1.00 | 0.58 (0.36, 0.93)* | 0.69 (0.45, 1.05) | 0.70 (0.43, 1.14) |
| Subgroup effects | ||||
| Age, years | ||||
| 55–74 | 1.00 | 0.13 (0.04, 0.40)*** | 0.48 (0.27, 0.85)* | 0.72 (0.41, 1.25) |
| ≥75 | 1.00 | 1.52 (0.83, 2.76) | 1.22 (0.64, 2.35) | 0.56 (0.19, 1.63) |
| Gender | ||||
| Female | 1.00 | 0.66 (0.34, 1.29) | 1.02 (0.57, 1.84) | 0.87 (0.40, 1.89) |
| Male | 1.00 | 0.51 (0.26, 1.01) | 0.46 (0.25, 0.87)* | 0.59 (0.31, 1.12) |
| COPD-related inpatient visits | ||||
| 0 | 1.00 | 0.48 (0.25, 0.92)* | 0.87 (0.53, 1.44) | 0.69 (0.37, 1.30) |
| 1 | 1.00 | 0.85 (0.30, 2.37) | 0.23 (0.06, 0.79)* | |
| ≥2 | 1.00 | 0.87 (0.32, 2.38) | 0.56 (0.19, 1.69) | 1.65 (0.69, 3.95) |
| CHA2DS2-VASc score | ||||
| 0 | 1.00 | 0.15 (0.02, 1.11) | 0.57 (0.19, 1.69) | 1.12 (0.44, 2.88) |
| 1 | 1.00 | |||
| 2–3 | 1.00 | 0.28 (0.10, 0.80)* | 0.27 (0.10, 0.68)** | 0.71 (0.35, 1.46) |
| ≥4 | 1.00 | 0.97 (0.54, 1.74) | 1.19 (0.68, 2.07) | 0.43 (0.15, 1.21) |
| Asthma | ||||
| No | 1.00 | 0.86 (0.48, 1.54) | 0.81 (0.45, 1.49) | 0.83 (0.41, 1.66) |
| Yes | 1.00 | 0.33 (0.14, 0.78)* | 0.60 (0.33, 1.10) | 0.62 (0.31, 1.23) |
| Heart failure | ||||
| No | 1.00 | 0.39 (0.20, 0.76)** | 0.68 (0.41, 1.10) | 0.75 (0.44, 1.26) |
| Yes | 1.00 | 1.13 (0.54, 2.36) | 0.86 (0.36, 2.05) | 0.43 (0.10, 1.90) |
| Stroke | ||||
| No | 1.00 | 0.50 (0.26, 0.95)* | 0.55 (0.31, 0.97)* | 0.74 (0.42, 1.31) |
| Yes | 1.00 | 0.72 (0.35, 1.46) | 0.93 (0.48, 1.79) | 0.59 (0.23, 1.55) |
| Ischemic heart disease | ||||
| No | 1.00 | 0.47 (0.23, 0.96)* | 0.61 (0.33, 1.11) | 1.03 (0.59, 1.79) |
| Yes | 1.00 | 0.69 (0.36, 1.31) | 0.78 (0.43, 1.42) | 0.26 (0.08, 0.86)* |
| Angina | ||||
| No | 1.00 | 0.50 (0.29, 0.88)* | 0.64 (0.40, 1.02) | 0.70 (0.42, 1.18) |
| Yes | 1.00 | 0.84 (0.32, 2.21) | 0.95 (0.35, 2.56) | 0.66 (0.14, 3.08) |
| Peripheral vascular disease | ||||
| No | 1.00 | 0.47 (0.27, 0.81)** | 0.53 (0.32, 0.87)* | 0.70 (0.42, 1.18) |
| Yes | 1.00 | 1.41 (0.50, 3.94) | 2.12 (0.83, 5.41) | 0.53 (0.11, 2.59) |
| Hypertension | ||||
| No | 1.00 | 0.26 (0.08, 0.86)* | 0.57 (0.25, 1.30) | 1.03 (0.48, 2.21) |
| Yes | 1.00 | 0.73 (0.43, 1.23) | 0.74 (0.45, 1.22) | 0.57 (0.30, 1.09) |
| Diabetes | ||||
| No | 1.00 | 0.55 (0.30, 1.00)* | 0.43 (0.23, 0.79)** | 0.79 (0.45, 1.39) |
| Yes | 1.00 | 0.61 (0.28, 1.33) | 1.21 (0.66, 2.23) | 0.48 (0.17, 1.37) |
| Renal failure | ||||
| No | 1.00 | 0.48 (0.28, 0.84)** | 0.72 (0.46, 1.13) | 0.69 (0.40, 1.17) |
| Yes | 1.00 | 1.11 (0.41, 3.02) | 0.49 (0.14, 1.77) | 0.80 (0.21, 3.02) |
| Chronic liver disease | ||||
| No | 1.00 | 0.60 (0.35, 1.03) | 0.60 (0.36, 1.00)* | 0.74 (0.43, 1.27) |
| Yes | 1.00 | 0.54 (0.20, 1.45) | 1.05 (0.48, 2.31) | 0.59 (0.17, 2.04) |
Sensitivity analysis of adjusted HRs of vaccination in risk reduction of ventricular arrhythmia in all season.
p < 0.05;
p < 0.01;
p < 0.001; HR, hazard ratio.
Model1 is adjusted for COPD-related inpatient visits, age, gender, CHA2DS2-VASc score, asthma, heart failure, acute myocardial infarction, stroke, ischemic heart disease, angina, peripheral vascular disease, hypertension, diabetes, depression, renal failure, chronic liver disease, dementia, level of urbanization, monthly income.
Main model is adjusted for COPD-related inpatient visits, COPD medications, total COPD medications, age, gender, CHA2DS2-VASc score, asthma, heart failure, acute myocardial infarction, stroke, ischemic heart disease, angina, peripheral vascular disease, hypertension, diabetes, depression, renal failure, chronic liver disease, dementia, level of urbanization, monthly income, antiarrhythmic agents: class1 (sodium channel blocker), class2 (beta blocker), class3 (potassium channel blocker), class4 (calcium channel blocker), aspirin, statin, RAASI.
During influenza season, non-influenza season, and all seasons, with more than two times of COPD-related inpatient visits, there was no significant difference of risk of VA occurrence after receiving one, two to three, or more than four times of vaccination. During all seasons, a lower risk of VA occurrence among patients without COPD-related inpatients after the first time of vaccination was observed (aHR: 0.48 [95% CI: 0.25–0.92]). In addition, among patients with one time of COPD-related admission, a lower risk of VA occurrence was associated with more than two times of vaccination (aHR: 0.23 [95% CI: 0.06–0.79]).
Discussion
Major Findings
In this population-based longitudinal cohort study that included 18,658 patients in Taiwan, the following principal findings were revealed: (1) influenza vaccination was associated with a significantly lower risk of potential lethal VA occurrence in patients with COPD, (2) more than one time of vaccination was associated with lower risk of VA occurrence, especially in patients with relative higher CHA2DS2-VASc score, and (3) lower risk of VA occurrence after the first time of vaccination was observed among patients without a history of stroke, ischemic heart disease, peripheral vascular disease, diabetes, and heart failure. After receiving two to three times of vaccination, a lower risk of VA occurrence was observed among patients without these medical histories except heart failure and ischemic heart disease.
To our knowledge, this is the first study to investigate the association between influenza vaccination and the risk of VA in patients with COPD.
Mechanism of VA Development in Patients With COPD
A strong association between COPD and cardiovascular disease has been reported earlier, as they share similar risk factors (2). However, the definite mechanism linking VA and COPD is unclear despite earlier studies demonstrating COPD as an independent risk factor of sudden cardiac death (8). Konecny et al. reported that patients with COPD displayed a high risk of ventricular tachycardia regardless of left ventricular systolic function (6). Moreover, an increasing number of reports have revealed that the severity of COPD could be the leading cause of an increasing burden of VA (21, 22). Moreover, a prolonged QT interval in patients with COPD has been proposed to be a potential contributor to VA development (23). Other possible clinical manifestations, such as hypoxemia (24), pulmonary hypertension, and right ventricular failure, are also possible causes of the increased risk of VA among patients with COPD (25). Overall, patients with COPD are more prone to developing VA compared with healthy ones.
Possible Mechanism of the Association Between Risk of VA and Influenza Vaccination
Earlier studies have revealed that influenza vaccination decreases not only the risk of developing atrial fibrillation (26) but also the risk of primary cardiac arrest in patients without a history of cardiovascular disease (14). In the present study, the risk of developing VA decreased significantly among patients with COPD who underwent vaccination. Among the patients with a concurrent diagnosis of asthma, which indicated the possibility of complex airway and lung parenchymal condition (27), also benefit from influenza vaccination.
A leading cause of VA is an acute coronary syndrome. In a study by Sung et al. (16) influenza vaccination decreases the risk of an acute coronary syndrome admission among elderly patients with COPD. Inflammation plays a critical role in the development of atherosclerotic plaque, thereby triggering acute coronary syndrome (28). During a viral infection of acute influenza, the increased metabolic demand, inflammatory conditions, platelet activation, and procoagulant status increase the risk of acute thrombus formation, subsequently developing into an acute coronary syndrome. Therefore, patients with COPD and ischemic heart disease might become free from VA development by preventing influenza infection upon undergoing vaccination. However, the present study showed that patients without risk of developing VA, such as patients without a medical history of hypertension, heart failure, renal failure, and ischemic heart disease, were associated with a lower risk of VA occurrence after receiving influenza vaccination.
Another possible cause of VA is inflammation of the myocardium caused by influenza infection (29). The incidence of myocarditis after influenza infection could be up to 9% according to a related study (30). Therefore, the risks of both myocarditis and VA occurrence are lowered upon vaccination as it prevents influenza infection.
Future research should focus on the mechanism through which the vaccine protects against VA development in patients with COPD to validate our results.
Different Risks of VA Occurrence on Gender, Age, Comorbidities, and Influenza/Non-Influenza Seasons
Earlier studies have revealed that patients with a mean age of ~65 years had a low risk of major cardiovascular events upon receiving influenza vaccination (15, 31, 32). Our study provided more information; we observed that patients with COPD aged 65–74 years exhibited the greatest benefit from vaccination during influenza season, non-influenza season, and all seasons. In elderly patients (≥75 years), the risk of VA occurrence after the influenza vaccine did not significantly decrease. The elderly patients with COPD and ventricular arrhythmia had a high prevalence of multiple risk factors such as hypertension, coronary artery disease, and diabetes (6). The increased risk of VA development was possible because of increasing comorbidities and fragile health in these patients. In addition, the effectiveness of vaccination might be lowered in elderly patients because of lower immune response to influenza vaccination compared with younger patients (33, 34). In the present study, lower risk of VA occurrence was associated with influenza vaccination in the relative elderly COPD patients (55–74 years) after receiving more than one time of vaccination. Therefore, consecutive vaccination might be important among elderly COPD patients.
In patients with heart failure, peripheral vascular disease, renal failure, and chronic liver disease, the association was not as significant as patients without the above-mentioned comorbidities. The possible explanation is that the mechanisms of VA occurrence are complicated, and the effectiveness of influenza may be diminished while the presence of these comorbidities. However, in previous studies, the overall benefit from influenza vaccination to the patients with these diseases had been demonstrated (35–37). Therefore, annual influenza vaccination was recommended for these patients (38).
In a previous report from McLean et al. (39) the protective effects of the influenza vaccine against different types of viruses may be less notable following repeated vaccinations. However, annual vaccination is still recommended for the prevention and control of influenza infections (38). The fact that vaccine effectiveness in the United States during the 2017–2018 influenza season was only 38% (40), receiving vaccination only once may not be sufficient to prevent contracting the virus. With more than one vaccination, the risk of influenza infection may also decrease, as may the risk of further cardiovascular complications (16, 41), including ventricular arrhythmia.
In Taiwan, the influenza season is between October and March. During this period of significantly lower temperatures, an increased incidence of the acute coronary syndrome was observed (42). Increasing risk of ventricular arrhythmia during wintertime had been reported before (43). Moreover, increasing implantable cardiac defibrillator therapies was also observed during influenza season (44). However, the risk of myocardial infarction could increase even in hot weather (45). The present study demonstrated a significantly decreased risk of VA in the influenza season and all seasons among vaccinated patients, which echoes the results of an earlier study that the cardioprotective effects of the influenza vaccination were sustained beyond influenza season (4). A recent study by Kurihara et al. (46) revealed different pathogenesis of acute coronary syndrome in different temperatures. The predominant cause of acute coronary syndrome during seasons with relatively cold temperatures is plaque rupture, whereas plaque erosion is the major pathology in seasons with hot temperatures (46). Bradykinin 2 receptor-associated pathway and anti-inflammatory response were proposed as the potential mechanisms of the cardioprotective effects of the influenza vaccination (47, 48). Therefore, the decreasing risk of VA among vaccinated patients with COPD might be a consequence of plaque stabilization through all seasons.
An earlier study has reported the difference in the effects of the vaccination by gender in patients with COPD (49). In summary, female patients with COPD displayed more frequent respiratory symptoms, a higher prevalence of anxiety and depression, and better long-term outcomes compared with male patients with COPD (49). An earlier study demonstrated that female patients with COPD had a lower prevalence of ischemic heart disease but a significantly higher prevalence of chronic heart failure than male patients with COPD (50). In the present study, the risk of VA was not significantly different between vaccinated and unvaccinated female patients with COPD after propensity score matching for sociodemographic characteristics, comorbidities, and COPD medications. Future prospective studies that focus on gender differences in the protective effects of influenza vaccination against VA occurrence are necessary for validating this result.
Impact of CHA2DS2-VASc Score and Hospitalization
We also observed that patients with COPD having a CHA2DS2-VASc score of 2–3 exhibited a low risk of VA occurrence after receiving vaccinations one and two to three times. An earlier study revealed that patients with atrial fibrillation having a high CHA2DS2-VASc score displayed a high risk of sudden cardiac death (51). This is because most of the components of the CHA2DS2-VASc score are also associated with an increased risk of VA occurrence. In the present study, we provided more information that could possibly predict the future benefit of vaccination among patients with COPD.
Another finding is that with fewer hospitalizations because of COPD, more benefits could be obtained from influenza vaccination. This result demonstrates that patients with poor pulmonary function or poor response to respiratory medications may not have long-term outcomes despite receiving the influenza vaccination. Earlier studies have demonstrated that influenza vaccination decreased the rate of acute exacerbation and hospitalization but did not reduce mortality rates (52, 53). During the exacerbation of COPD, the incidence of ventricular tachycardia was up to 25% (9). Moreover, acute right heart failure occur during exacerbation (54). The effectiveness of influenza vaccination might be diminished during an acute exacerbation. Another finding is that during the non-influenza season, only patients without any COPD-related inpatient visit had a lower risk of VA occurrence after receiving more than four times vaccination. A possible explanation is that during seasons with higher temperatures, the proportion of severe exacerbation is significantly higher than seasons with lower temperatures (55). Therefore, the importance of maintaining adequate pulmonary function to avoid exacerbation should be emphasized in patients with COPD.
Limitations
The present study had several limitations. First, this is a retrospective observational study. Therefore, the presence of immortal time bias in observational studies could lead to the overestimation of the results (56, 57). In the present study, time-dependent Cox model analysis was performed to minimize immortal time bias (20). However, more future prospective studies are required to validate the findings of the present study. Second, the severity and diagnostic accuracy of COPD according to pulmonary function tests could not be obtained because of the lack of information from ICD-9 coding. However, we demonstrated that patients with few COPD hospitalizations benefit more from vaccinations compared with those with frequent hospitalizations. The readmission rate in patients with COPD is a marker of poor prognosis and is correlated with poor pulmonary function (58). Third, laboratory data, information of body mass index, and smoking status could not be collected because of information limitations from the NHIRD. Therefore, conducting future studies that link these patients to another registry is a possible solution for analyzing these possible cofounders. Fourth, the present study enrolled COPD patients with age more than 55 years because of the policy of free influenza vaccination. A future study is needed to investigate the younger COPD patients. Fifth, the information of rare hereditary or acquired diseases such as congenital heart defect, hypertrophic cardiomyopathy, long QT syndrome, or Brugada syndrome could not be collected from NHIRD. Future studies regarding the effectiveness of vaccination among COPD patients with these diseases should be investigated. Sixth, because of usually short-term prescription, the present study did not analyze the association between antibiotics and VA among patients with COPD. Seventh, the present study did not further evaluate the relationship between ventricular arrhythmia and sudden death. The cause of sudden death among patients with COPD is complicated and may be contributed by different conditions, such as arrhythmia, severe hypoxemia, respiratory failure, heart failure, sepsis, or ischemic heart disease (2, 59). Future study aimed to investigate the mechanism of sudden death in patients with COPD is warranted.
Conclusion
The influenza vaccination may be associated with a lower risk of potential lethal VA occurrence in patients with COPD aged 55–74. Further future research is warranted to resolve this clinical question.
Funding
This work was financially supported by the Higher Education Sprout Project by the Ministry of Education (MOE) in Taiwan and Taipei Medical University-Shuang Ho Hospital (Grant No. 109TMU-SHH-21).
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Statements
Data availability statement
The data analyzed in this study is subject to the following licenses/restrictions: the use of the National Health Insurance Research Database needs to be applied for both IRB authority approval. The use of the database is also restricted to the approved entries. Requests to access these datasets should be directed to Ju-Chi Liu, liumdcv@tmu.edu.
Author contributions
C-CChe, W-RH, K-HC, C-CChi, TY, Y-WW, and J-CL contributed to the study conception and design. C-HL, J-SY, Y-AF, C-CChe, C-CChi, TY, and Y-WW contributed to data acquisition, analysis, and interpretation. The manuscript was drafted by C-CChe and C-HL and critically revised by all other co-authors. All authors read and approved the final version of this manuscript.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1.
MurrayCJLLopezAD. Alternative projections of mortality and disability by cause 1990–2020: Global Burden of Disease Study. Lancet. (1997) 349:1498–504. 10.1016/S0140-6736(96)07492-2
2.
SinDDAnthonisenNRSorianoJBAgustiAG. Mortality in COPD: role of comorbidities. Euro Respir J. (2006) 28:1245. 10.1183/09031936.00133805
3.
AndréSCondeBFragosoEBoléo-ToméJPAreiasVCardosoJ. COPD and cardiovascular disease. Pulmonology. (2019) 25:168–76. 10.1016/j.pulmoe.2018.09.006
4.
JohnstoneJLoebMTeoKKGaoPDyalLLiuLet al. Influenza vaccination and major adverse vascular events in high-risk patients. Circulation. (2012) 126:278–86. 10.1161/CIRCULATIONAHA.111.071100
5.
WilcheskyMErnstPBrophyJMPlattRWSuissaS. Bronchodilator use and the risk of arrhythmia in copd: part 2: reassessment in the larger Quebec cohort. Chest. (2012) 142:305–11. 10.1378/chest.11-1597
6.
KonecnyTSomersKRParkJYJohnAOrbanMDoshiRet al. Chronic obstructive pulmonary disease as a risk factor for ventricular arrhythmias independent of left ventricular function. Heart rhythm. (2018) 15:832–8. 10.1016/j.hrthm.2017.09.042
7.
ShihHTWebbCRConwayWAPetersonETilleyBGoldsteinS. Frequency and significance of cardiac arrhythmias in chronic obstructive lung disease. Chest. (1988) 94:44–8. 10.1378/chest.94.1.44
8.
NarayananKReinierKUy-EvanadoATeodorescuCZhangLChughHet al. Chronic obstructive pulmonary disease and risk of sudden cardiac death. JACC Clin Electrophysiol. (2015) 1:381–7. 10.1016/j.jacep.2015.06.005
9.
RusinowiczTZielonkaTMZycinskaK. Cardiac arrhythmias in patients with exacerbation of COPD. Adv Exp Med Biol. (2017) 1022:53–62. 10.1007/5584_2017_41
10.
SieviNAClarenbachCFCamenGRossiVAvan GestelAJKohlerM. High prevalence of altered cardiac repolarization in patients with COPD. BMC Pulm Med. (2014) 14:55. 10.1186/1471-2466-14-55
11.
WangXJiangZChenBZhouLKongZZuoSet al. Cardiac autonomic function in patients with acute exacerbation of chronic obstructive pulmonary disease with and without ventricular tachycardia. BMC Pulm Med. (2016) 16:124. 10.1186/s12890-016-0287-0
12.
FusoLIncalziRAPistelliRMuzzolonRValenteSPagliariGet al. Predicting mortality of patients hospitalized for acutely exacerbated chronic obstructive pulmonary disease. Am J Med. (1995) 98:272–7. 10.1016/S0002-9343(99)80374-X
13.
BarnesMHeywoodAEMahimboARahmanBNewallATMacintyreCR. Acute myocardial infarction and influenza: a meta-analysis of case-control studies. Heart. (2015) 101:1738–47. 10.1136/heartjnl-2015-307691
14.
SiscovickDSRaghunathanTELinDWeinmannSArbogastPLemaitreRNet al. Influenza vaccination and the risk of primary cardiac arrest. Am J Epidemiol. (2000) 152:674–7. 10.1093/aje/152.7.674
15.
NicholKLNordinJMulloolyJLaskRFillbrandtKIwaneM. Influenza vaccination and reduction in hospitalizations for cardiac disease and stroke among the elderly. N Engl J Med. (2003) 348:1322–32. 10.1056/NEJMoa025028
16.
SungLCChenCIFangYALaiCHHsuYPChengTHet al. Influenza vaccination reduces hospitalization for acute coronary syndrome in elderly patients with chronic obstructive pulmonary disease: a population-based cohort study. Vaccine. (2014) 32:3843–9. 10.1016/j.vaccine.2014.04.064
17.
SuVYYangKYYangYHTsaiYHPerngDWSuWJet al. Use of ICS/LABA combinations or LAMA is associated with a lower risk of acute exacerbation in patients with coexistent COPD and asthma. J Allergy Clin Immunol Pract. (2018) 6:1927–35.e3. 10.1016/j.jaip.2018.01.035
18.
HoTWRuanSYHuangCTTsaiYJLaiFYuCJ. Validity of ICD9-CM codes to diagnose chronic obstructive pulmonary disease from National Health Insurance claim data in Taiwan. Int J Chronic Obstructive Pulmonary Dis. (2018) 13:3055–63. 10.2147/COPD.S174265
19.
VestboJAndersonWCoxsonHOCrimCDawberFEdwardsLet al. Evaluation of COPD longitudinally to identify predictive surrogate end-points (ECLIPSE). Euro Respir J. (2008) 31:869–73. 10.1183/09031936.00111707
20.
AgarwalPMoshierERuMOhriNEnnisRRosenzweigKet al. Immortal time bias in observational studies of time-to-event outcomes: assessing effects of postmastectomy radiation therapy using the national cancer database. Cancer Control. (2018) 25:1073274818789355. 10.1177/1073274818789355
21.
KusunokiYNakamuraTHattoriKMotegiTIshiiTGemmaAet al. Atrial and ventricular arrhythmia-associated factors in stable patients with chronic obstructive pulmonary disease. Respiration. (2016) 91:34–42. 10.1159/000442447
22.
KonecnyTParkJYSomersKRKonecnyDOrbanMSoucekFet al. Relation of chronic obstructive pulmonary disease to atrial and ventricular arrhythmias. Am J Cardiol. (2014) 114:272–7. 10.1016/j.amjcard.2014.04.030
23.
YildizPTükekTAkkayaVSözenABYildizAKorkutFet al. Ventricular arrhythmias in patients with COPD are associated with QT dispersion. Chest. (2002) 122:2055–61. 10.1378/chest.122.6.2055
24.
ShepardJWGarrisonMWGritherDAEvansRSchweitzerPK. Relationship of ventricular ectopy to nocturnal oxygen desaturation in patients with chronic obstructive pulmonary disease. Am J Med. (1985) 78:28–34. 10.1016/0002-9343(85)90457-7
25.
ZangiabadiADe PasqualeCGSajkovD. Pulmonary hypertension and right heart dysfunction in chronic lung disease. BioMed Res Int. (2014) 2014:739674. 10.1155/2014/739674
26.
ChangTYChaoTFLiuCJChenSJChungFPLiaoJNet al. The association between influenza infection, vaccination, and atrial fibrillation: a nationwide case-control study. Heart rhythm. (2016) 13:1189–94. 10.1016/j.hrthm.2016.01.026
27.
DonohueKMHoffmanEABaumhauerHGuoJAhmedFSLovasiGSet al. Asthma and lung structure on computed tomography: the Multi-Ethnic Study of Atherosclerosis Lung Study. J Allergy Clin Immunol. (2013) 131:361–8.e11. 10.1016/j.jaci.2012.11.036
28.
BiasucciLMLeoMDe MariaGL. Local and systemic mechanisms of plaque rupture. Angiology. (2008) 59:73S−6S. 10.1177/0003319708319747
29.
RothbergMBHaesslerSDBrownRB. Complications of viral influenza. Am J Med. (2008) 121:258–64. 10.1016/j.amjmed.2007.10.040
30.
KarjalainenJNieminenMSHeikkiläJ. Influenza A1 myocarditis in conscripts. Acta Med Scand. (1980) 207:27–30. 10.1111/j.0954-6820.1980.tb09670.x
31.
PhrommintikulAKuanprasertSWongcharoenWKanjanavanitRChaiwarithRSukonthasarnA. Influenza vaccination reduces cardiovascular events in patients with acute coronary syndrome. Euro Heart J. (2011) 32:1730–5. 10.1093/eurheartj/ehr004
32.
GurfinkelEPFuenteRLdlMendizOMautnerB. Influenza vaccine pilot study in acute coronary syndromes and planned percutaneous coronary interventions. Circulation. (2002) 105:2143–7. 10.1161/01.CIR.0000016182.85461.F4
33.
GoodwinKViboudCSimonsenL. Antibody response to influenza vaccination in the elderly: a quantitative review. Vaccine. (2006) 24:1159–69. 10.1016/j.vaccine.2005.08.105
34.
WeinbergerBHerndler-BrandstetterDSchwanningerAWeiskopfDGrubeck-LoebensteinB. Biology of immune responses to vaccines in elderly persons. Clin Infect Dis. (2008) 46:1078–84. 10.1086/529197
35.
SongJYCheongHJHaSHHwangISKeeSYJeongHW. Clinical impact of influenza immunization in patients with liver cirrhosis. J Clin Virol. (2007) 39:159–63. 10.1016/j.jcv.2007.04.018
36.
IshigamiJSangYGramsMECoreshJChangAMatsushitaK. Effectiveness of influenza vaccination among older adults across kidney function: pooled analysis of 2005-2006 through 2014-2015 influenza seasons. Am J Kidney Dis. (2020) 75:887–96. 10.1053/j.ajkd.2019.09.008
37.
YedlapatiSHKhanSUTalluriSLoneANKhanMZKhanMSet al. Effects of influenza vaccine on mortality and cardiovascular outcomes in patients with cardiovascular disease: a systematic review and meta-analysis. J Am Heart Assoc. (2021) 10:e019636. 10.1161/JAHA.120.019636
38.
GrohskopfLAAlyanakEBroderKRBlantonLHFryAMJerniganDBet al. Prevention and control of seasonal influenza with vaccines: recommendations of the advisory committee on immunization practices - United States, 2020-21 influenza season. MMWR Recomm Rep. (2020) 69:1–24. 10.15585/mmwr.rr6908a1
39.
McLeanHQThompsonMGSundaramMEMeeceJKMcClureDLFriedrichTCet al. Impact of repeated vaccination on vaccine effectiveness against influenza A(H3N2) and B during 8 seasons. Clin Infect Dis. (2014) 59:1375–85. 10.1093/cid/ciu680
40.
RolfesMAFlanneryBChungJRO'HalloranAGargSBelongiaEAet al. Effects of influenza vaccination in the united states during the 2017-2018 influenza season. Clin Infect Dis. (2019) 69:1845–53. 10.1093/cid/ciaa045
41.
ModinDJørgensenMEGislasonGJensenJSKøberLClaggettBet al. Influenza vaccine in heart failure. Circulation. (2019) 139:575–86. 10.1161/CIRCULATIONAHA.118.036788
42.
LinCCLeePYChenKCLiaoPCHsuJCLiAH. Clinical, demographic, and biochemical characteristics of patients with acute ST-segment elevation myocardial infarction: an analysis of acute coronary syndrome registry data of a single medical center from 2005 to 2016. Acta Cardiol Sin. (2020) 36:1–7. 10.6515/ACS.202001_36(1).20190704D
43.
NguyenJLLadenFLinkMSSchwartzJLuttmann-GibsonHDockeryDW. Weather and triggering of ventricular arrhythmias in patients with implantable cardioverter-defibrillators. J Expo Sci Environ Epidemiol. (2015) 25:175–81. 10.1038/jes.2013.72
44.
SinghSMde SouzaRJKumareswaranR. Increased defibrillator therapies during influenza season in patients without influenza vaccines. J Arrhythmia. (2015) 31:210–4. 10.1016/j.joa.2014.12.006
45.
BhaskaranKHajatSHainesAHerrettEWilkinsonPSmeethL. Effects of ambient temperature on the incidence of myocardial infarction. Heart. (2009) 95:1760–9. 10.1136/hrt.2009.175000
46.
KuriharaOTakanoMYamamotoEYonetsuTKakutaTSoedaTet al. Seasonal variations in the pathogenesis of acute coronary syndromes. J Am Heart Assoc. (2020) 9:e015579. 10.1161/JAHA.119.015579
47.
Bermúdez-FajardoAOviedo-OrtaE. Influenza vaccination promotes stable atherosclerotic plaques in apoE knockout mice. Atherosclerosis. (2011) 217:97–105. 10.1016/j.atherosclerosis.2011.03.019
48.
VeljkovicVGlisicSVeljkovicNBojicTDietrichUPerovicVRet al. Influenza vaccine as prevention for cardiovascular diseases: possible molecular mechanism. Vaccine. (2014) 32:6569–75. 10.1016/j.vaccine.2014.07.007
49.
AryalSDiaz-GuzmanEManninoDM. COPD and gender differences: an update. Transl Res. (2013) 162:208–18. 10.1016/j.trsl.2013.04.003
50.
AlmagroPLópezGarcía FCabreraFJMonteroLMorchónDDíezJet al. Comorbidity and gender-related differences in patients hospitalized for COPD. The ECCO study. Respir Med. (2010) 104:253–9. 10.1016/j.rmed.2009.09.019
51.
KuoLChaoT-FLiuC-JChenS-JTuanT-CLinY-Jet al. Usefulness of the CHA2DS2-VASc score to predict the risk of sudden cardiac death and ventricular arrhythmias in patients with atrial fibrillation. Am J Cardiol. (2018) 122:2049–54. 10.1016/j.amjcard.2018.08.056
52.
KopsaftisZWood-BakerRPooleP. Influenza vaccine for chronic obstructive pulmonary disease (COPD). Cochrane Database Syst Rev. (2018). 10.1002/14651858.CD002733.pub3
53.
MulpuruSLiLYeLHatchetteTAndrewMKAmbroseAet al. Effectiveness of influenza vaccination on hospitalizations and risk factors for severe outcomes in hospitalized patients with COPD. CHEST. (2019) 155:69–78. 10.1016/j.chest.2018.10.044
54.
OzbenBEryukselETanrikuluAMPapilaNOzyigitTCelikelTet al. Acute exacerbation impairs right ventricular function in COPD patients. Hellenic J Cardiol. (2015) 56:324–31.
55.
SoJYZhaoHVoelkerHReedRMSinDMarchettiNet al. Seasonal and regional variations in chronic obstructive pulmonary disease exacerbation rates in adults without cardiovascular risk factors. Ann Am Thorac Soc. (2018) 15:1296–303. 10.1513/AnnalsATS.201801-070OC
56.
SuissaS. Immortal time bias in observational studies of drug effects. Pharmacoepidemiol Drug Safety. (2007) 16:241–9. 10.1002/pds.1357
57.
YadavKLewisRJ. Immortal time bias in observational studies. JAMA. (2021) 325:686–7. 10.1001/jama.2020.9151
58.
Garcia-AymerichJFarreroEFélezMAIzquierdoJMarradesRMAntóJM. Risk factors of readmission to hospital for a COPD exacerbation: a prospective study. Thorax. (2003) 58:100–5. 10.1136/thorax.58.2.100
59.
van den BergMEStrickerBHBrusselleGGLahousseL. Chronic obstructive pulmonary disease and sudden cardiac death: a systematic review. Trends Cardiovasc Med. (2016) 26:606–13. 10.1016/j.tcm.2016.04.001
Summary
Keywords
chronic obstructive pulmonary disease, influenza vaccination, ventricular arrhythmia, ventricular fibrillation, ventricular tachycardia
Citation
Chen C-C, Lin C-H, Hao W-R, Yeh J-S, Chiang K-H, Fang Y-A, Chiu C-C, Yang TY, Wu Y-W and Liu J-C (2021) Influenza Vaccination and the Risk of Ventricular Arrhythmias in Patients With Chronic Obstructive Pulmonary Disease: A Population-Based Longitudinal Study. Front. Cardiovasc. Med. 8:731844. doi: 10.3389/fcvm.2021.731844
Received
28 June 2021
Accepted
15 September 2021
Published
14 October 2021
Volume
8 - 2021
Edited by
Jun-Jun Yeh, Ditmanson Medical Foundation Chia-Yi Christian Hospital, Taiwan
Reviewed by
Victor C. Kok, Asia University, Taiwan; Yu-Feng Wei, E-Da Hospital, Taiwan; Kuang-Ming Liao, Chi Mei Medical Center, Taiwan; Shih-Feng Liu, Kaohsiung Chang Gung Memorial Hospital, Taiwan
Updates
Copyright
© 2021 Chen, Lin, Hao, Yeh, Chiang, Fang, Chiu, Yang, Wu and Liu.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chun-Chih Chiu 17257@s.tmu.edu.twTsung Yeh Yang 15535@s.tmu.edu.twYu-Wei Wu yuwei.wu@tmu.edu.twJu-Chi Liu liumdcv@tmu.edu.tw; liumdcv@ms15.hinet.net
†These authors have contributed equally to this work
This article was submitted to Cardiovascular Epidemiology and Prevention, a section of the journal Frontiers in Cardiovascular Medicine
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.