- 1Department of Cardiology, The Second Affiliated Hospital of Chongqing Medical University, Chongqing, China
- 2Department of Cardiology, The First Affiliated Hospital of Nanjing Medical University, Nanjing, China
- 3Department of Cardiology, Sir Run Run Shaw Hospital, The First Affiliated Hospital of Zhejiang University, Hangzhou, China
- 4Department of Cardiology and Atrial Fibrillation Center, The First Affiliated Hospital of Zhejiang University, Hangzhou, China
- 5Department of Cardiovascular Medicine, Mayo Clinic, Rochester, MN, United States
Background: Response rates for cardiac resynchronization therapy (CRT) in patients without intrinsic left bundle-branch block (LBBB) morphology are poor.
Objective: We sought to develop a nomogram model to predict response to CRT in patients without intrinsic LBBB.
Methods: We searched electronic health records for patients without intrinsic LBBB who underwent CRT at Mayo Clinic. Logistic regression and Cox proportional hazards regression analysis were performed for the odds of response to CRT and risk of death, respectively. Results were used to develop the nomogram model.
Results: 761 patients without intrinsic LBBB were identified. Six months after CRT, 47.8% of patients demonstrated improvement of left ventricular ejection fraction by more than 5%. The 1-, 3-, and 5-year survival rates were 95.9, 82.4, and 66.70%, respectively. Patients with CRT upgrade from pacemaker [odds ratio (OR), 1.67 (95% CI, 1.05–2.66)] or atrioventricular node (AVN) ablation [OR, 1.69 (95% CI, 1.09–2.64)] had a greater odds of CRT response than those patients who had new implant, or who did not undergo AVN ablation. Patients with right bundle-branch block had a low response rate (39.2%). Patients undergoing AVN ablation had a lower mortality rate than those without ablation [hazard ratio, 0.65 (95% CI, 0.46–0.91)]. Eight clinical variables were automatically selected to build a nomogram model and predict CRT response. The model had an area under the receiver operating characteristic curve of 0.71 (95% CI, 0.63–0.78).
Conclusions: Among patients without intrinsic LBBB undergoing CRT, upgrade from pacemaker and AVN ablation were favorable factors in achieving CRT response and better long-term outcomes.
Introduction
Cardiac resynchronization therapy (CRT) is an effective therapy for patients with heart failure with reduced ejection fraction (HFrEF), left bundle-branch block (LBBB), and/or a wide QRS complex. Randomized clinical trials (RCTs) have shown CRT to be beneficial in improving heart failure (HF) symptoms, left ventricular ejection fraction (LVEF), quality of life, and survival (1–6).
Aside from QRS duration, intrinsic LBBB is generally considered an important determinant of CRT response. The current American College of Cardiology/American Heart Association/Heart Rhythm Society (ACC/AHA/HRS) and European Society of Cardiology guidelines provide the strongest recommendations for the use of CRT in patients with typical LBBB pattern and wide QRS, who benefit most from CRT (7–9). However, nearly 20% to 50% of CRT recipients do not have a good outcome after CRT (10, 11). Conversely, some patients without intrinsic LBBB may have HF improvement (12, 13). These patients usually refers to right bundle-branch block (RBBB), intraventricular conduction delay (IVCD), and predominantly ventricular paced rhythm with non-physiologic depolarization pattern (14, 15). Some post-hoc analyses of landmark RCTs have shown a wide range of CRT response for HFrEF patients without intrinsic LBBB (1, 2, 16–18). The REVERSE (REsynchronization reVErses Remodeling in Systolic Left vEntricular Dysfunction) trial showed that some patients with HFrEF and RBBB derived significant improvement from CRT (18). However, meta-analysis suggested that HFrEF patients without LBBB morphology do not have improvement of mortality or HF hospitalization rates after CRT (19). Therefore, it may be helpful to build a specific and practical predictive model that incorporates variables from a real-word clinical database of CRT recipients without intrinsic LBBB. This study aimed to develop an evidence-based nomogram and use it to predict CRT responders in patients without intrinsic LBBB morphology (20).
Methods
Study Population and Data Source
The study was approved by the Mayo Clinic Institutional Review Board. Between 2002 and 2017, a prospectively designed CRT database collected data from the electronic health record of patients who underwent CRT (pacemaker or implantable cardioverter-defibrillator [ICD]) implantation at Mayo Clinic, Rochester, Minnesota. For this study, we queried the CRT database for the records of patients who underwent successful CRT implantation; for whom preoperative electrocardiography (ECG) did not indicate typical intrinsic LBBB morphology, which was defined as follows: the QRS duration ≥120 ms, QS or rS pattern on V1, Notch/slurred R-wave on I, aVL, V5, V6, and absent Q-wave on V5, V6 (21); and who had available LVEF data at baseline and at 6-month follow-up. Patients who underwent replacement of CRT pulse generator, lead revision or His-Purkinje conduction system pacing were excluded.
Baseline Clinical Data Collection and Preparation
All baseline clinical data and patient demographics were extracted from our database. The missing data points were rechecked through chart review. The key variables from ECG and LVEF were validated by 2 independent investigators (P.-L.X. and J.H.).
Electrocardiography
All available standard 12-lead ECG results before CRT implantation were assessed by chart review. LBBB, RBBB, and IVCD were defined according to the criteria approved by the World Health Organization and 2013ESC guidelines (21, 22).
Echocardiography
All echocardiography parameters were collected by reviewing the echocardiography report. LVEF, left ventricular end-diastolic dimension (LVEDD), and pulmonary artery systolic pressure were collected.
Outcome Measures
The primary endpoint of the study was all-cause death. In addition, we also assessed the benefit of CRT by assessing echocardiographic response at 6-month follow-up. CRT response was defined as an improvement of LVEF by >5% in absolute value at 6-month follow-up after CRT.
Statistical Analysis
Categorical variables were presented as counts and percentages, and continuous variables were reported as mean (SD) or median (interquartile range). Univariable and multivariable logistic regression analysis and Cox proportional hazards regression analysis, respectively, were performed to evaluate the effects of potential predictors on CRT response [reported as odds ratio (OR)] and overall survival [reported as hazard ratio (HR)]. Unadjusted overall survival was estimated with the Kaplan-Meier method, and groups were compared by using the log-rank test. Interaction and stratified analyses were performed for the predictor that had significant interaction with other variables.
For prediction model development and evaluation, we randomly partitioned the study population into training (70%) and validation (30%) sets using statistical software (R version 3.4.3). Backward stepwise selection with the Akaike information criterion was used to select variables for the multivariable logistic regression model. Nomograms were formulated incorporating selected variables to predict the probability of response after CRT implantation using statistical software (rms in R, version 3.4.3; http://www.r-project.org) (20).
The predictive performance of the nomogram included discrimination ability and calibration. The area under the receiver operating characteristic curve was calculated to measure predictive accuracy for individual outcomes. Calibration with 1,000 bootstrap samples was measured by plotting the predicted frequencies against the observed probabilities to determine the prediction capability of the nomogram (23). Statistical analyses were performed with R software, version 3.4.3. Two-sided P-values <0.05 were considered statistically significant.
Results
Baseline Demographics and Clinical Characteristics
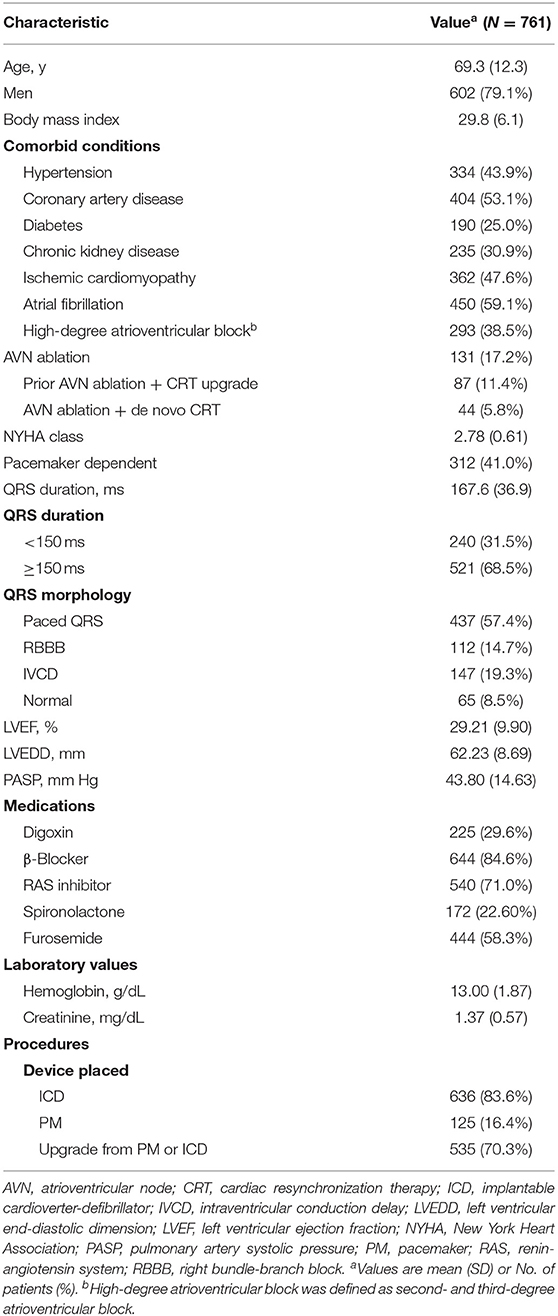
Our search of the database identified 761 patients who received CRT with an ICD (83.6%) or pacemaker (16.4%) and met the inclusion and exclusion criteria from 2002 to 2017. The mean (SD) age was 69 (12) years, and 602 (79%) of the patients were men (Table 1). QRS morphology at baseline was paced in 57.4%, RBBB in 14.7%, and IVCD in 19.3%; 8.5% had normal QRS morphology with atrial fibrillation and were undergoing atrioventricular node (AVN) ablation. A total of 450 patients (59.1%) had atrial fibrillation, of whom 131 (29.1%) had AVN ablation. The mean (SD) LVEF was 29.2% (9.9%). Most of the patients (n = 535, 70.3%) were undergoing CRT upgrade from a pacemaker (n = 281) or ICD (n = 254), and the rest (226, 29.7%) had a de novo CRT implant.
CRT Response and Independent Predictors
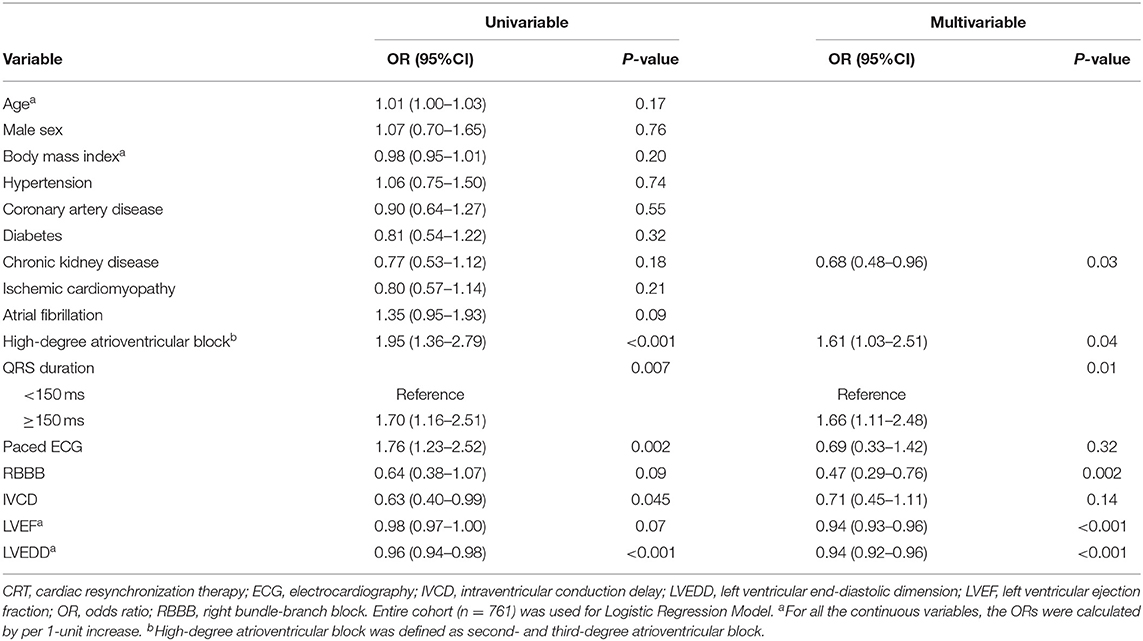
At 6-month follow up, 364 patients (47.8%) had a response to CRT, with an absolute LVEF increase of more than 5%. The rate of CRT response was 50.3% for patients with CRT upgrade, 55.8% for patients undergoing AVN ablation, 39.2% for patients with RBBB, and 36.8% for patients with IVCD. Multivariable logistic regression analysis suggested that preexisting high-degree atrioventricular block (including second- and third-degree atrioventricular block), wider QRS duration, and lower LVEF and a lower LVEDD were associated with greater CRT response, whereas chronic kidney disease (CKD) and RBBB were associated with poor response to CRT (Table 2). Figure 1 depicts the relationship between QRS duration, LVEF, and LVEDD and odds of CRT response. Each was adjusted for all the factors (age, sex, body mass index, hypertension, coronary artery disease, diabetes, CKD, ischemic cardiomyopathy, atrial fibrillation, high-degree atrioventricular block, QRS duration, LVEF, and LVEDD).
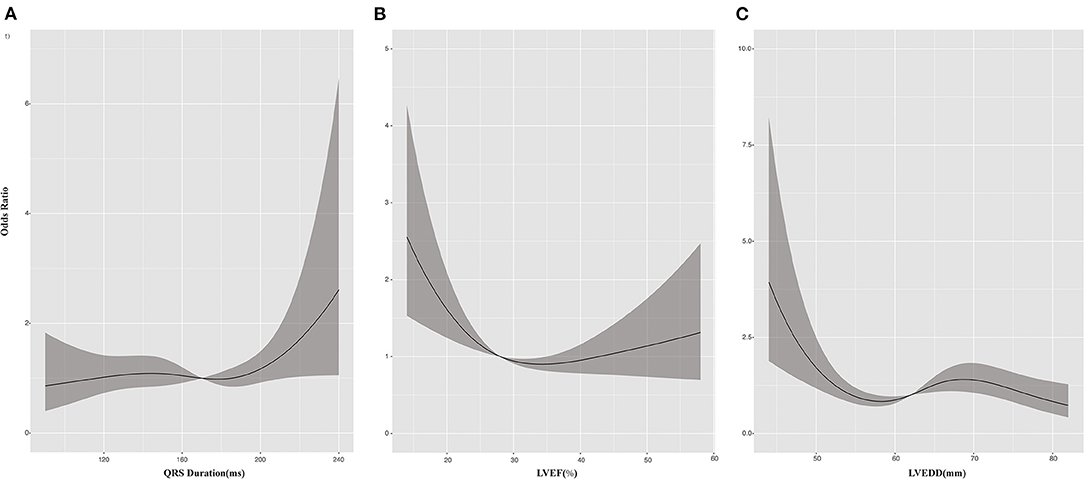
Figure 1. Cubic spline plots for predictors of response to cardiac resynchronization therapy (CRT). Plots show odds ratios (solid lines) and 95% CIs (gray shading) for response to CRT by (A) QRS duration, (B) left ventricular ejection fraction (LVEF), and (C) left ventricular end-diastolic dimension (LVEDD).
Patients who had an upgrade from a pacemaker had a greater CRT response (OR, 1.67; 95% CI, 1.05–2.66), with LVEF improved from 32.4 to 39.7%, whereas those with CRT upgrade from an ICD had no significant benefit (OR, 0.74; 95% CI, 0.48–1.15), with a mean LVEF improvement from 25.9 to 29.2% (Figure 2). AVN ablation was associated with CRT response (OR, 1.69; 95% CI, 1.09–2.64), with mean LVEF improved from 32.3% to 40.1%, especially for patients who had AVN ablation at the time of CRT implant or after CRT (Figure 2).
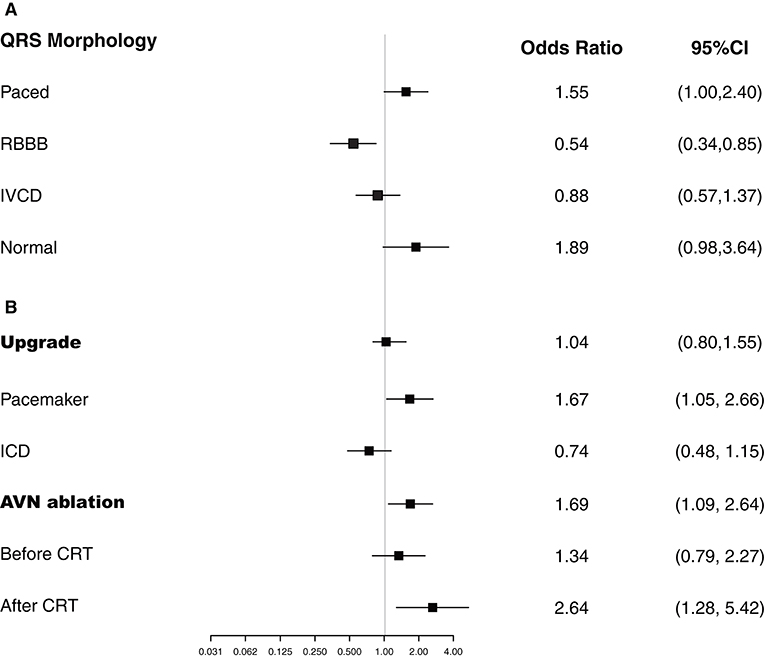
Figure 2. Logistic regression analyses. Plots show the effect of various predictors on response to cardiac resynchronization therapy (CRT). ICD, implantable cardioverter-defibrillator; IVCD, intraventricular conduction delays; RBBB, right bundle branch block.
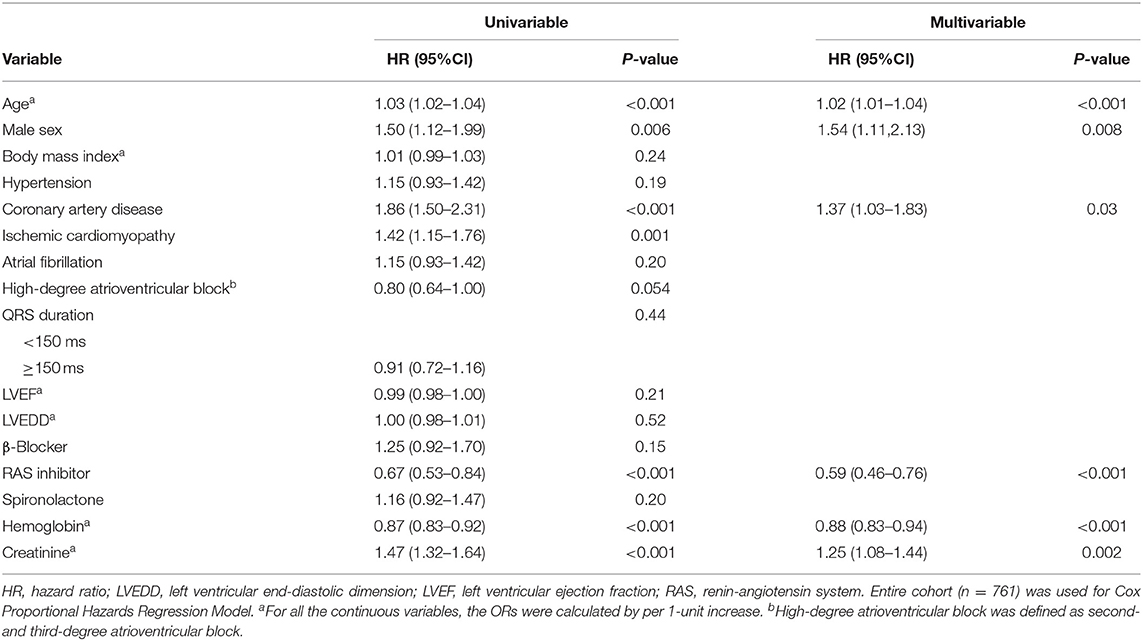
Predictors of Survival
The median follow-up time was 52 (interquartile range, 33–90) months. The 1-, 3-, and 5-year overall survival rates were 95.9, 82.3, and 66.7%, respectively. Multivariable Cox regression analysis suggested that older age, male sex, coronary artery disease, and higher creatinine value were independent predictors of all-cause death (Table 3). Use of a renin-angiotensin system inhibitor and high hemoglobin value were beneficial factors for survival. CRT upgrade had no significant benefit for overall survival (HR, 1.15; 95% CI, 0.87–1.52) compared with de novo implant (Figure 3). AVN ablation was associated with a lower mortality rate (HR, 0.65; 95% CI, 0.46–0.91), especially for those with a higher LVEF (Figure 3).
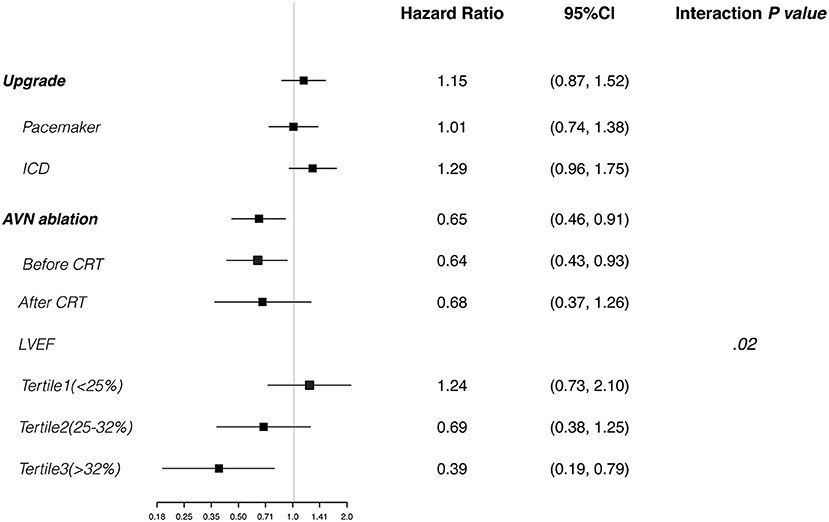
Figure 3. Cox regression analyses. Plots show the effect of various predictors on overall survival (hazard ratio for risk of all-cause death). CRT, cardiac resynchronization therapy; ICD, implantable cardioverter-defibrillator; LVEF, left ventricular ejection fraction.
Patients who were CRT responders had significantly better survival than nonresponders (HR, 0.68; 95% CI, 0.55–0.83; P < 0.001) (Figure 4A). The 5-year overall survival rate was 74.5% for responders and 59.7% for non-responders. After adjustment for the confounding factors, the HR for CRT responders was 0.63 (95% CI, 0.50–0.79; P < 0.001).
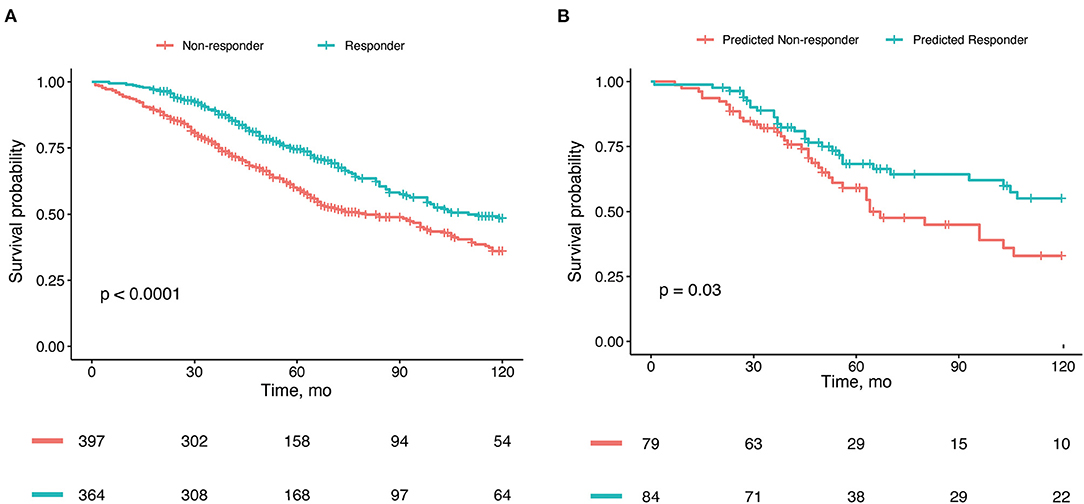
Figure 4. Kaplan-Meier estimates of overall survival. (A) Survival stratified by real response to cardiac resynchronization therapy (CRT) in the whole cohort. (B) Survival stratified by CRT response predicted by our nomogram model in the validation cohort.
CRT Response Nomogram Model
For prediction model development and performance evaluation, 549 participants were randomly assigned to the training cohort, and 212 participants were assigned to the validation cohort. Of the candidate baseline variables listed in Table 1; 8 variables (CKD, QRS >150 ms, high-degree atrioventricular block, RBBB, LVEF, LVEDD, AVN ablation, and ICD upgrade) were automatically selected with backward stepwise selection using the Akaike information criterion method for developing a prediction model for CRT response according to the linear regression model. The nomogram predicting CRT response based on this linear regression model is shown in Figure 5A. The nomogram demonstrated an area under the receiver operating characteristic curve of 0.71 (95% CI, 0.63–0.78) in the validation set (Figure 5B). The calibration plot suggested good consistency between the risk estimation by nomogram and the observed probabilities (Figure 5C). For example, if a patient who had a baseline QRS duration longer than 150 ms (18 points), LVEF of 35% (57.5 points), LVEDD of 60 mm (50 points) without RBBB (17.5 points) and CKD (10 points) underwent pacemaker upgrade (16 points) to CRT and AVN ablation (12.5 points), the patient would have a total of 181.5 points, which equates to a CRT response probability of about 70%.
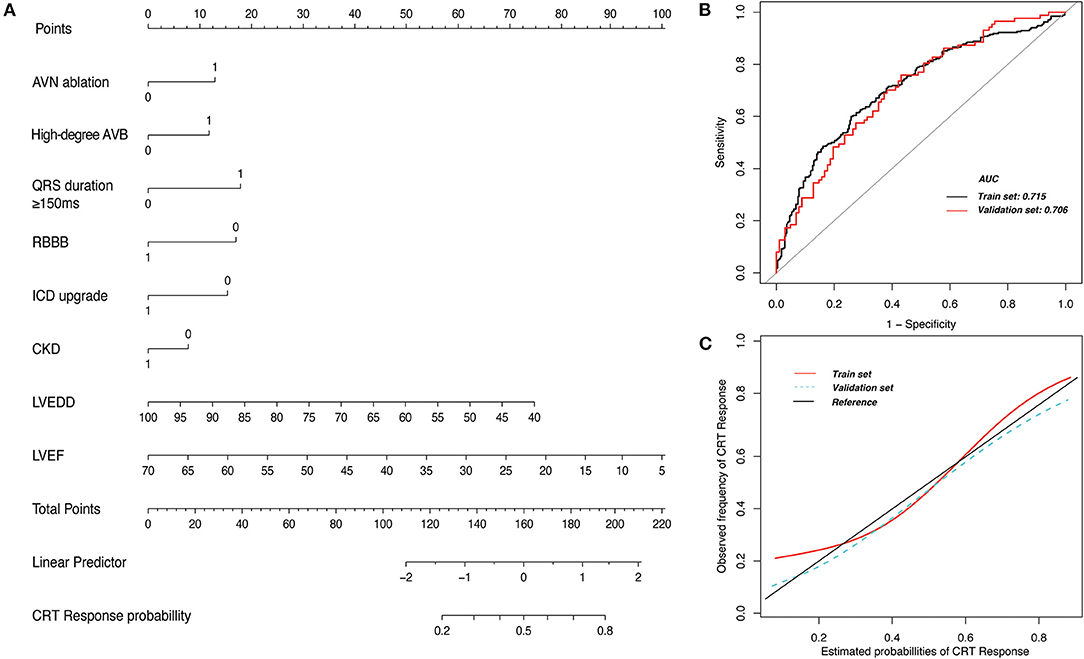
Figure 5. Nomogram prediction model for response to cardiac resynchronization therapy (CRT). (A) The nomogram model includes 8 clinical variables that were scored. The probability of CRT response is predicted by calculating the total points. (B) The area under the receiver operating characteristic curve (AUC) suggests good accuracy for estimating CRT response in the training set and the validation set. (C) The calibration plot suggests good consistency between risk estimation by the nomogram and the observed probabilities in the training set and the validation set. AVB, atrioventricular block; AVN, atrioventricular node; CKD, chronic kidney disease; ICD, implantable cardioverter-defibrillator; LVEDD, left ventricular end-diastolic dimension; LVEF, left ventricular ejection fraction; RBBB, right bundle-branch block. 1 and 0 were allocated for variables where 1 indicated presence of the variable and 0 indicated absence.
The patients who were predicted by our nomogram model to be CRT responders had lower all-cause mortality rates than did those who were predicted to be CRT non-responders in the validation set cohort (HR, 0.54; 95% CI, 0.30–0.96) (Figure 4B).
Discussion
In the current study, we developed a nomogram-based prediction model for CRT response that uses clinical variables in patients without intrinsic LBBB undergoing CRT. The principal findings were that (1) 70% of patients without intrinsic LBBB were undergoing CRT upgrade, and upgrade from a pacemaker (not an ICD), preexisting high-degree atrioventricular block with right ventricular pacing, and a wider QRS complex were associated with a greater CRT response; (2) the patients underwent AVN ablation was associated with greater CRT response and survival; and (3) our nomogram based on clinical features had optimal performance in predicting CRT response in patients without intrinsic LBBB undergoing CRT in our internal validation.
CRT Response and Survival in Patients Without Intrinsic LBBB
The benefit of CRT in patients with LBBB has been proved in multiple RCTs; however, the use of CRT in patients without intrinsic LBBB has been considered to be less favorable. Current guidelines recommend a class IIa (QRS >150 ms) or IIb indication (QRS 120–150 ms) for CRT for patients without intrinsic LBBB (7–9). Post-hoc analysis of the COMPANION and MADIT-CRT studies, however, showed a benefit of CRT-ICD in prolonged PR interval patients who was without intrinsic LBBB (24, 25). Several studies emphasized that these patients without intrinsic LBBB, but with mechanical dyssynchrony, had improved survival after CRT (26, 27). We found that lower LVEF and longer QRS duration were associated with a higher probability of CRT response for patients without intrinsic LBBB, which suggests that QRS duration of 150 ms or longer was an important predictor of CRT response for patients without intrinsic LBBB. A recent prospective study showed that CRT-ICD was associated with better outcomes in patients with IVCD and QRS longer than 150 ms but not in patients with RBBB (28). Other studies have also shown that RBBB was associated with worse outcomes (29, 30), although left ventricular dyssynchrony may be present concomitantly in patients with RBBB (31). Our study confirmed previous findings that neither RBBB nor IVCD was favorable for CRT response. It is not surprising that upgrade from pacemaker (vs. ICD) was associated with better response to CRT. The ICD patients likely had pre-existing cardiomyopathy, whereas the pacemaker patients likely developed cardiomyopathy due to RV pacing (functionally acquired LBBB physiology).
AVN ablation decreases mortality rates in patients with permanent atrial fibrillation (32, 33). CRT at the time of AVN ablation is optimal to ensure biventricular pacing for patients with permanent atrial fibrillation and decreased LVEF (34). Our study patients who underwent AVN ablation had significantly higher CRT response rates than did those without AVN ablation. Because both AVN ablation and upgrade from right ventricular pacing to CRT were favorable factors, the likelihood of CRT response was attributed to the correction of mechanical dyssynchrony and well-controlled ventricular rate, which could be detrimental components of LVEF (13). In agreement with previous studies, age, male sex, coronary artery disease, lower hemoglobin values, and higher creatinine levels predicted poor survival in our study (35).
Nomogram Model to Assess CRT Response
Nomograms have been developed for cancer and cardiovascular diseases to facilitate therapy selection or determine the prognosis (20). Owing to the complexity of CRT response in patients without intrinsic LBBB, a predictive model with representative clinical features is of value. Therefore, we developed a nomogram model with common clinical features for the prediction of CRT response. We found that the nomogram model can leverage clinical variables to assess patients without intrinsic LBBB who may be likely or unlikely to benefit from CRT; these patients, in general, have a low response to CRT. The favorable factors were AVN ablation, preexisting atrioventricular block requiring pacing, wider QRS duration, a less dilated left ventricle, and lower LVEF, whereas RBBB, CKD, and the presence of an ICD were unfavorable factors. The predicted percentage of CRT response can be estimated from these factors. However, because our nomagram model was only based on single-center database, we will consider a prospective study extend to other center.
Limitations
This study had some limitations. All benefits from CRT were relative to those in other participants without LBBB morphology. To minimize the effects of population differences, all results were adjusted for baseline characteristics. Some data also were missing and could not be obtained from the database or chart review. Due to a long-time span in our study, some heterogeneity in the technology of CRT may affect the explanation of these results. In addition, some important variables such as N-terminal pro–brain natriuretic peptide and echocardiography parameters were excluded because of more than 10% missing values. Because this was a retrospective study, patient selection bias may exist. Finally, we only verified the nomogram model with internal validation in our single-center CRT database, and external validation was not performed. A new prospective study is needed to further validate our nomagram model.
Conclusion
Patients who underwent CRT upgrade from a pacemaker and AVN ablation had better CRT response and survival. A nomogram model was developed for the patients without intrinsic LBBB to assess CRT response rate and further facilitate shared decision-making in selecting CRT candidates without intrinsic LBBB.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author/s.
Ethics Statement
The studies involving human participants were reviewed and approved by Mayo Clinic Institutional Review Board. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
All authors listed have made a substantial, direct and intellectual contribution to the work, and approved it for publication.
Funding
Research funding from the National Natural Science Foundation of China (Grant No. 81600215 to P-LX) and Kuanren Talents Program of the second affiliated hospital of Chongqing Medical University (to P-LX).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
AVN, atrioventricular node; CKD, chronic kidney disease; CRT, cardiac resynchronization therapy; HF, heart failure; ICD, implantable cardioverter-defibrillator; LBBB, left bundle-branch block; LVEF, left ventricular ejection fraction; LVEDD, left ventricular end-diastolic dimension; IVCD, intraventricular conduction delay; RBBB, right bundle-branch block.
References
1. Cleland JG, Daubert JC, Erdmann E, Freemantle N, Gras D, Kappenberger L, et al. The effect of cardiac resynchronization on morbidity and mortality in heart failure. N Engl J Med. (2005) 352:1539–49. doi: 10.1056/NEJMoa050496
2. Bristow MR, Saxon LA, Boehmer J, Krueger S, Kass DA, De Marco T, et al. Cardiac-resynchronization therapy with or without an implantable defibrillator in advanced chronic heart failure. N Engl J Med. (2004) 350:2140–50. doi: 10.1056/NEJMoa032423
3. Young JB, Abraham WT, Smith AL, Leon AR, Lieberman R, Wilkoff B, et al. Combined cardiac resynchronization and implantable cardioversion defibrillation in advanced chronic heart failure: the MIRACLE ICD trial. JAMA. (2003) 289:2685–94. doi: 10.1001/jama.289.20.2685
4. Abraham WT, Fisher WG, Smith AL, Delurgio DB, Leon AR, Loh E, et al. Cardiac resynchronization in chronic heart failure. N Engl J Med. (2002) 346:1845–53. doi: 10.1056/NEJMoa013168
5. Auricchio A, Stellbrink C, Sack S, Block M, Vogt J, Bakker P, et al. Long-term clinical effect of hemodynamically optimized cardiac resynchronization therapy in patients with heart failure and ventricular conduction delay. J Am Coll Cardiol. (2002) 39:2026–33. doi: 10.1016/S0735-1097(02)01895-8
6. Cazeau S, Leclercq C, Lavergne T, Walker S, Varma C, Linde C, et al. Effects of multisite biventricular pacing in patients with heart failure and intraventricular conduction delay. N Engl J Med. (2001) 344:873–80. doi: 10.1056/NEJM200103223441202
7. Epstein AE, DiMarco JP, Ellenbogen KA, Estes NA, Freedman RA, Gettes LS, et al. 2012 ACCF/AHA/HRS focused update incorporated into the ACCF/AHA/HRS 2008 guidelines for device-based therapy of cardiac rhythm abnormalities: a report of the american college of cardiology foundation/american heart association task force on practice guidelines and the heart rhythm society. J Am Coll Cardiol. (2013) 61:e6–75. doi: 10.1016/j.jacc.2012.11.007
8. Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE, Drazner MH, et al. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. (2013) 62:e147–239. doi: 10.1016/j.jacc.2013.05.019
9. Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JGF, Coats AJS, et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: the task force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC)Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J. (2016) 37:2129–200. doi: 10.1093/eurheartj/ehw128
10. Feeny AK, Rickard J, Patel D, Toro S, Trulock KM, Park CJ, et al. Machine learning prediction of response to cardiac resynchronization therapy. Circ Arrhythm Electrophysiol. (2019) 12:e007316. doi: 10.1161/CIRCEP.119.007316
11. Vernooy K, van Deursen CJ, Strik M, Prinzen FW. Strategies to improve cardiac resynchronization therapy. Nat Rev Cardiol. (2014) 11:481–93. doi: 10.1038/nrcardio.2014.67
12. Cleland JG, Abraham WT, Linde C, Gold MR, Young JB, Claude Daubert J, et al. An individual patient meta-analysis of five randomized trials assessing the effects of cardiac resynchronization therapy on morbidity and mortality in patients with symptomatic heart failure. Eur Heart J. (2013) 34:3547–56. doi: 10.1093/eurheartj/eht290
13. Stankovic I, Prinz C, Ciarka A, Daraban AM, Mo Y, Aarones M, et al. Long-term outcome after CRT in the presence of mechanical dyssynchrony seen with chronic RV pacing or intrinsic LBBB. JACC Cardiovasc Imaging. (2017) 10:1091–9. doi: 10.1016/j.jcmg.2016.08.015
14. Belkin MN, Upadhyay GA. Does cardiac resynchronization therapy benefit patients with non-left bundle branch block prolonged QRS patterns? Curr Cardiol Rep. (2017) 19:125. doi: 10.1007/s11886-017-0929-8
15. Tanaka H, Hara H, Adelstein EC, Schwartzman D, Saba S, Gorcsan J. Comparative mechanical activation mapping of RV pacing to LBBB by 2D and 3D speckle tracking and association with response to resynchronization therapy. JACC Cardiovasc Imaging. (2010) 3:461–71. doi: 10.1016/j.jcmg.2009.12.014
16. Moss AJ, Hall WJ, Cannom DS, Klein H, Brown MW, Daubert JP, et al. Cardiac-resynchronization therapy for the prevention of heart-failure events. N Engl J Med. (2009) 361:1329–38. doi: 10.1056/NEJMoa0906431
17. Tang AS, Wells GA, Talajic M, Arnold MO, Sheldon R, Connolly S, et al. Cardiac-resynchronization therapy for mild-to-moderate heart failure. N Engl J Med. (2010) 363:2385–95. doi: 10.1056/NEJMoa1009540
18. Linde C, Abraham WT, Gold MR, Sutton MJ, Ghio S, Daubert C, et al. Randomized trial of cardiac resynchronization in mildly symptomatic heart failure patients and in asymptomatic patients with left ventricular dysfunction and previous heart failure symptoms. J Am Coll Cardiol. (2008) 52:1834–43. doi: 10.1016/j.jacc.2008.08.027
19. Cunnington C, Kwok CS, Satchithananda DK, Patwala A, Khan MA, Zaidi A, et al. Cardiac resynchronisation therapy is not associated with a reduction in mortality or heart failure hospitalisation in patients with non-left bundle branch block QRS morphology: meta-analysis of randomised controlled trials. Heart. (2015) 101:1456–62. doi: 10.1136/heartjnl-2014-306811
20. Lei Z, Li J, Wu D, Xia Y, Wang Q, Si A, et al. Nomogram for preoperative estimation of microvascular invasion risk in Hepatitis B virus-related hepatocellular carcinoma within the milan criteria. JAMA Surg. (2016) 151:356–63. doi: 10.1001/jamasurg.2015.4257
21. Brignole M, Auricchio A, Baron-Esquivias G, Bordachar P, Boriani G, Breithardt OA, et al. 2013 ESC Guidelines on cardiac pacing and cardiac resynchronization therapy: the task force on cardiac pacing and resynchronization therapy of the European Society of Cardiology (ESC). developed in collaboration with the european heart rhythm association (EHRA). Eur Heart J. (2013) 34:2281–329. doi: 10.1093/eurheartj/eht150
22. Willems JL, de Medina EOR, Bernard R, Coumel P, Fisch C, Krikler D, et al. Criteria for intraventricular conduction disturbances and pre-excitation world health organizational/international society and federation for cardiology task force Ad Hoc. J Am Coll Cardiol. (1985) 5:1261–75. doi: 10.1016/S0735-1097(85)80335-1
23. Alba AC, Agoritsas T, Walsh M, Hanna S, Iorio A, Devereaux PJ, et al. Discrimination and calibration of clinical prediction models: users' guides to the medical literature. JAMA. (2017) 318:1377–84. doi: 10.1001/jama.2017.12126
24. Lin J, Buhr KA, Kipp R. Effect of pr interval on outcomes following cardiac resynchronization therapy: a secondary analysis of the COMPANION trial. J Cardiovasc Electrophysiol. (2017) 28:185–91. doi: 10.1111/jce.13131
25. Zareba W, Klein H, Cygankiewicz I, Hall WJ, McNitt S, Brown M, et al. Effectiveness of cardiac resynchronization therapy by QRS morphology in the multicenter automatic defibrillator implantation trial-cardiac resynchronization therapy (MADIT-CRT). Circulation. (2011) 123:1061–72. doi: 10.1161/CIRCULATIONAHA.110.960898
26. Kandala J, Upadhyay GA, Altman RK, Parks KA, Orencole M, Mela T, et al. QRS morphology, left ventricular lead location, and clinical outcome in patients receiving cardiac resynchronization therapy. Eur Heart J. (2013) 34:2252–62. doi: 10.1093/eurheartj/eht123
27. Auricchio A, Lumens J, Prinzen FW. Does cardiac resynchronization therapy benefit patients with right bundle branch block: cardiac resynchronization therapy has a role in patients with right bundle branch block. Circ Arrhythm Electrophysiol. (2014) 7:532–42. doi: 10.1161/CIRCEP.113.000628
28. Kawata H, Bao H, Curtis JP, Minges KE, Mitiku T, Birgersdotter-Green U, et al. Cardiac resynchronization defibrillator therapy for nonspecific intraventricular conduction delay versus right bundle branch block. J Am Coll Cardiol. (2019) 73:3082–99. doi: 10.1016/j.jacc.2019.04.025
29. Wokhlu A, Rea RF, Asirvatham SJ, Webster T, Brooke K, Hodge DO, et al. Upgrade and de novo cardiac resynchronization therapy: impact of paced or intrinsic QRS morphology on outcomes and survival. Heart Rhythm. (2009) 6:1439–47. doi: 10.1016/j.hrthm.2009.07.009
30. Bilchick KC, Kamath S, DiMarco JP, Stukenborg GJ. Bundle-branch block morphology and other predictors of outcome after cardiac resynchronization therapy in medicare patients. Circulation. (2010) 122:2022–30. doi: 10.1161/CIRCULATIONAHA.110.956011
31. Leong DP, Hoke U, Delgado V, Auger D, Thijssen J, van Erven L, et al. Predictors of long-term benefit of cardiac resynchronization therapy in patients with right bundle branch block. Eur Heart J. (2012) 33:1934–41. doi: 10.1093/eurheartj/ehr470
32. Dong K, Shen WK, Powell BD, Dong YX, Rea RF, Friedman PA, et al. Atrioventricular nodal ablation predicts survival benefit in patients with atrial fibrillation receiving cardiac resynchronization therapy. Heart Rhythm. (2010) 7:1240–5. doi: 10.1016/j.hrthm.2010.02.011
33. Brignole M, Pokushalov E, Pentimalli F, Palmisano P, Chieffo E, Occhetta E, et al. A randomized controlled trial of atrioventricular junction ablation and cardiac resynchronization therapy in patients with permanent atrial fibrillation and narrow QRS. Eur Heart J. (2018) 39:3999–4008. doi: 10.1093/eurheartj/ehy555
34. Kusumoto FM, Schoenfeld MH, Barrett C, Edgerton JR, Ellenbogen KA, Gold MR, et al. 2018 ACC/AHA/HRS Guideline on the evaluation and management of patients with bradycardia and cardiac conduction delay: a report of the American college of cardiology/American heart association task force on clinical practice guidelines and the heart rhythm society. Circulation. (2019) 140:e382–482. doi: 10.1161/CIR.0000000000000720
Keywords: cardiac resynchronization therapy, left bundle-branch block, atrioventricular node ablation, nomogram, left ventricular ejection fraction (LVEF)
Citation: Xiao P-L, Cai C, Zhang P, Han J, Mulpuru SK, Deshmukh AJ, Yin Y-H and Cha Y-M (2021) Better CRT Response in Patients Who Underwent Atrioventricular Node Ablation or Upgrade From Pacemaker: A Nomogram to Predict CRT Response. Front. Cardiovasc. Med. 8:760195. doi: 10.3389/fcvm.2021.760195
Received: 17 August 2021; Accepted: 01 October 2021;
Published: 01 November 2021.
Edited by:
Matteo Anselmino, University of Turin, ItalyReviewed by:
Elizabeth S. Kaufman, The MetroHealth System, United StatesOsmar Antonio Centurion, National University of Asunción, Paraguay
Copyright © 2021 Xiao, Cai, Zhang, Han, Mulpuru, Deshmukh, Yin and Cha. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yong-Mei Cha, eWNoYUBtYXlvLmVkdQ==
 Pei-Lin Xiao
Pei-Lin Xiao Cheng Cai2
Cheng Cai2 Yue-Hui Yin
Yue-Hui Yin Yong-Mei Cha
Yong-Mei Cha