- 1Department of Cardiac Surgery, The First Affiliated Hospital of Sun Yat-sen University, Guangzhou, China
- 2NHC Key Laboratory of Assisted Circulation, Sun Yat-sen University, Guangzhou, China
- 3Department of Pharmacy, Shaoyang University, Shaoyang, China
- 4Department of Hematology Oncology, Shaoyang Central Hospital, Shaoyang, China
Aims: To explore the value of preoperative liver function tests (LFTs) for the prognosis of cardiac surgery patients without liver disease.
Methods: The Medical Information Mart for Intensive Care III (MIMIC-III) database was used to extract the clinical data. Adult cardiac patients (≥18 years) without liver disease in the database were enrolled. The association of LFTs with the time of hospital stay and ICU stay was analyzed with the Spearman correlation. Survival curves were estimated using the Kaplan-Meier method and compared by the log-rank test. Multivariable logistic regression was used to identify LFTs that were independent prognostic factors of mortality.
Results: A total of 2,565 patients were enrolled in this study. Albumin (ALB) was negatively associated with the time of hospital stay and ICU stay, while alanine transaminase (ALT), aspartate aminotransferase (AST), and total bilirubin were positively associated with the time of hospital stay and ICU stay (all p < 0.001). Abnormal ALB, ALT, AST, and total bilirubin were associated with lower 90-day and 4-year survival (all p < 0.001) and could be used as independent risk factors for hospital mortality and 90-day mortality. However, only ALB and total bilirubin were independent risk factors for 4-year mortality.
Conclusion: Preoperative LFT abnormalities were associated with short-term and long-term prognosis of cardiac surgery patients without liver disease.
Introduction
One of the risks of cardiopulmonary bypass (CPB) surgery is to cause postoperative organ dysfunction which may affect the prognosis of patients (1). Approximately 10% of high-risk cardiac surgery patients undergoing CPB can experience liver damage, which directly affects the morbidity and mortality of patients (2). Transient but not permanent abnormal liver function tests (LFTs) after coronary artery bypass graft surgery are possible due to decreased hepatic flow, hypoxia, or pump-induced inflammation (3). Moreover, the severity of liver disease and the degree of abnormality of liver function indicators significantly affect the prognosis of cardiac surgery (4, 5). However, LFTs are very complicated in cardiac surgery patients because not only the operation but also heart disease itself can cause abnormalities.
Abnormal liver function is a common manifestation of heart failure (6, 7) and can be related to a worse prognosis. The detrimental effects of heart failure may be associated with liver congestion secondary to volume and pressure overload, decreased cardiac output, and liver hypoperfusion leading to hypoxic injury (6). Although a variety of cardiohepatic syndromes are recognized by clinicians, there are few investigations of the associations of preoperative LFT abnormalities with short-term and long-term prognosis in cardiac surgery patients.
In this study, we tried to explore the association between preoperative LFTs and the length of hospital and ICU stays, hospital mortality, 90-day mortality, and 4-year mortality of cardiac surgery patients without liver disease.
Methods
Data Source
This study was a retrospective cohort study and data were obtained from a freely accessible critical care database, named Medical Information Mart for Intensive Care III (MIMIC-III). The database consists of clinical data of patients who stayed in the ICU of Beth Israel Deaconess Medical Center between 2001 and 2012 (8). After online training under the Collaborative Institutional Training Initiative (CITI) program of the National Institutes of Health (NIH), the right to access the database and acquire the data was approved by the institutional review boards of the Massachusetts Institute of Technology (Cambridge, MA, USA) (Record ID 41268972). As all the data have been de-identified, informed consent was not required.
Patient Selection
From all patients in the MIMIC-III database, patients were included as follows: (1) patients who underwent on-pump cardiac surgery; (2) those without a history of primary significant liver disease or acute hepatic failure; (3) those older than 18 years; and (4) preoperative routine blood examination was performed within 24 h after admission.
Data Extraction
All clinical data were queried and extracted by using the Structured Query Language (SQL), and pgAdmin4 was used as the administrative platform for PostgreSQL. The extracted data mainly comprised demographics (age and sex), vital signs [diastolic blood pressure (DBP), heart rate (HR), respiratory rate (RR), systolic blood pressure (SBP), percutaneous oxygen saturation (SpO2), and temperature], comorbidities (congestive heart failure, valvular disease, cardiac arrhythmias, hypertension, chronic pulmonary disease, pulmonary circulation disorder, peripheral vascular disease, uncomplicated diabetes, complicated diabetes, liver disease and renal failure), laboratory parameters [peripheral white blood cell count (WBC), serum potassium, serum sodium, platelet count, serum glucose, blood urea nitrogen (BUN), serum creatinine, blood albumin (ALB), blood alanine transaminase (ALT), blood aspartate transaminase (AST), and blood total bilirubin], the Simplified Acute Physiology Score (SAPS) II, the Sequential Organ Failure Assessment (SOFA) score, and the Model for End-Stage Liver Disease (MELD) score. For laboratory events, the initial value measurement after admission and before surgery was used for further analyses. Normal values of ALB were defined as 3.5–5.5 g/dl, ALT as 5–40 U/L, AST as 8–40 U/L, and total bilirubin as 0.3–1.3 mg/dl. Considering the ratio of <1.5% missing data for each variable, we directly omitted them in further analysis.
Outcome Variables
We included the following outcome variables in the study: length of hospital stay, length of ICU stay, hospital mortality, 90-day mortality (post-ICU admission), and 4-year mortality. Among them, hospital mortality was chosen as the primary endpoint of the study, and 4-year mortality was chosen as the secondary endpoint. If a patient was admitted to the ICU multiple times during a single hospitalization, the total ICU hospitalization time was calculated into the length of ICU stay. Because only patients in the CareVue system were followed for at least 4 years, the present study analyzed their 4-year mortality in the CareVue system.
Statistical Analysis
Continuous variables are presented as the median (interquartile range) or mean ± SD and were compared by Mann-Whitney U-test or t-test. Categorical data are presented as numbers with proportions and were analyzed by the χ2 test. The correlation between the length of ICU stay and hospital stay with laboratory parameters was analyzed by using the non-parametric Spearman's rank correlation test. Survival curves were estimated using the Kaplan-Meier method and compared by the log-rank test. Logistic regression was applied for the uni- or multi-variate analyses to identify independent prognostic factors for mortality (hospital, 90-day, and 4-year mortality) for cardiac surgery patients without liver disease. Two different models were used for potential confounders adjusting: Model 1 was adjusted for SOFA score, creatinine, SpO2, hypertension, congestive heart failure, and chronic pulmonary disease. Model 2 was further adjusted for height, SBP, and SAPS II score. p < 0.05 were considered statistically significant.
Results
Baseline Characteristics of the Study Population
A total of 2,565 patients were included in our study, of which 63 patients (2.46%) died in the hospital. The baseline characteristics of the study population are briefly summarized in Table 1, which shows demographics, vital signs, laboratory events, comorbidities, and scores.
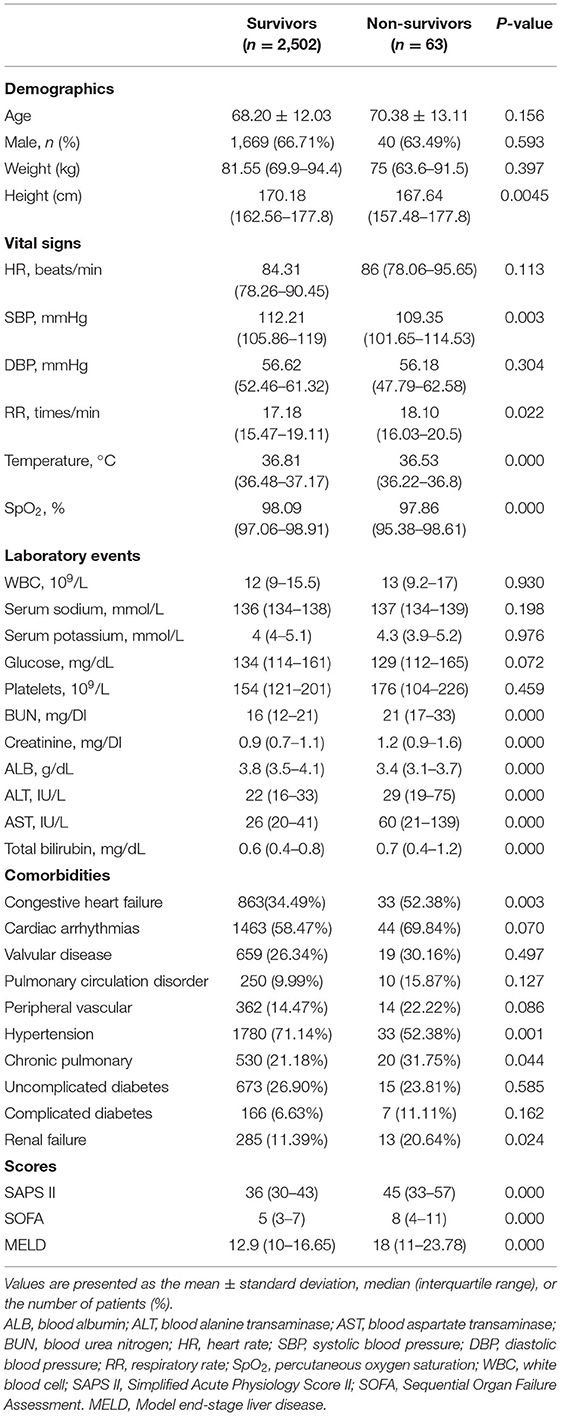
Table 1. Baseline characteristics of the study population with different survival statuses in hospital.
The demographic characteristics of the study population are shown in Table 1. No significant difference was observed in age, sex, or weight between non-survivors and survivors. Non-survivors had much lower ALB values (3.8 vs. 3.4, p = 0.000) and higher ALT (22 vs. 29, p = 0.000), AST (26 vs. 60, p = 0.000), and total bilirubin (0.6 vs. 0.7, p = 0.000) levels (Table 1). Non-survivors tended to have a shorter height, lower SBP, temperature, and SpO2 values, and higher RR, creatinine, BUN, SAPS II scores, and SOFA scores, as well as a history of congestive heart failure, hypertension, chronic pulmonary disease, and renal failure (Table 1).
The Prognostic Significance of the Liver Function Index for Cardiac Surgery Patients Without Liver Disease
We used Spearman's rank correlation test to investigate the association between the liver function index and the length of hospital stay and ICU stay in cardiac surgery patients without liver disease, and the results are briefly summarized in Table 2. ALB was significantly negatively associated with length of hospital stay and ICU stay (hospital stay: Spearman's rho = −0.297, p = 0.000; ICU stay: Spearman's rho = −0.293, p = 0.000). ALT, AST, and total bilirubin were significantly positively associated with length of hospital stay and ICU stay (for ALT, hospital stay: Spearman's rho = 0.082, p = 0.000; ICU stay: Spearman's rho = 0.069, p = 0.001; for AST, hospital stay: Spearman's rho = 0.096, p = 0.000; ICU stay: Spearman's rho = 0.214, p = 0.000; for total bilirubin, hospital stay: Spearman's rho = 0.099, p = 0.000; ICU stay: Spearman's rho = 0.156, p = 0.000).
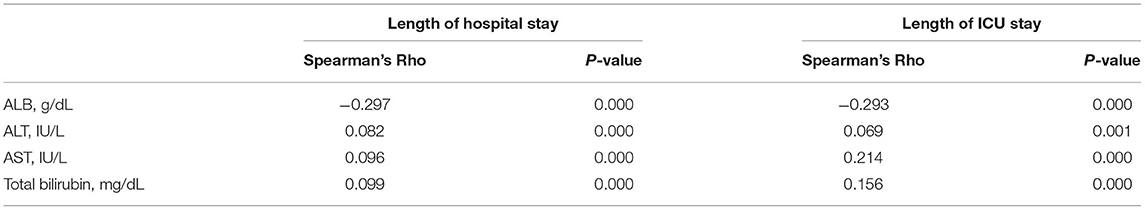
Table 2. The correlation of blood albumin (ALB), alanine transaminase (ALT), aspartate transaminase (AST), and total bilirubin with hospital stay and ICU stay.
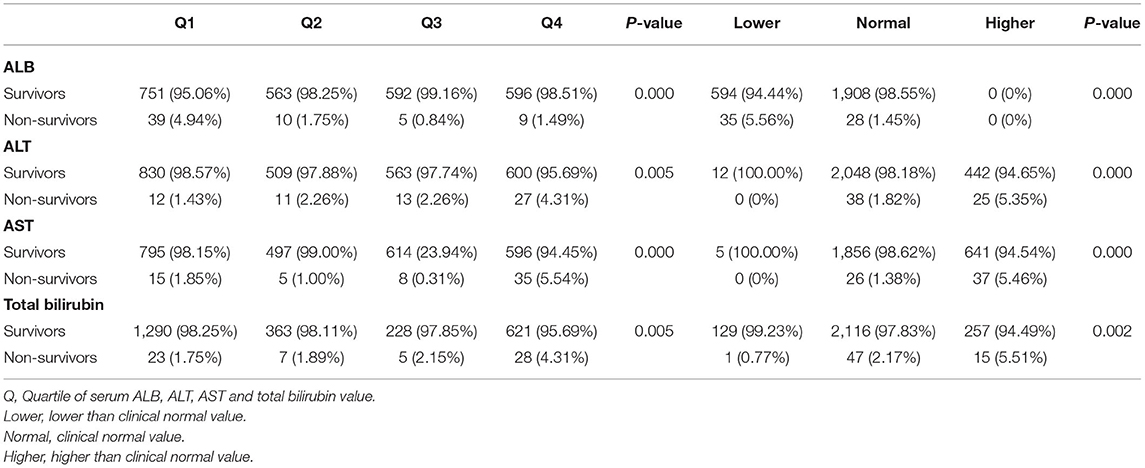
Next, the correlation of the liver function index with hospital mortality of cardiac surgery patients without liver disease was investigated. Quartiles of ALB, ALT, AST, and total bilirubin were significantly correlated with hospital mortality (ALB: p = 0.000; ALT: p = 0.005; AST: p = 0.000; total bilirubin: p = 0.005) (Table 3). For ALB, a higher rate of hospital mortality was observed in the first quartile, while for ALT, AST, and total bilirubin, a higher rate of hospital mortality was observed in the fourth quartile. Similar trends were observed when grouping by clinical normal values. For ALB, a higher rate of hospital mortality was observed in the group with a lower value than normal, while for ALT, AST, and total bilirubin, a higher rate of hospital mortality was observed in the group with a higher value than normal (ALB: p = 0.000; ALT: p = 0.000; AST: p = 0.000; total bilirubin: p = 0.002) (Table 3).
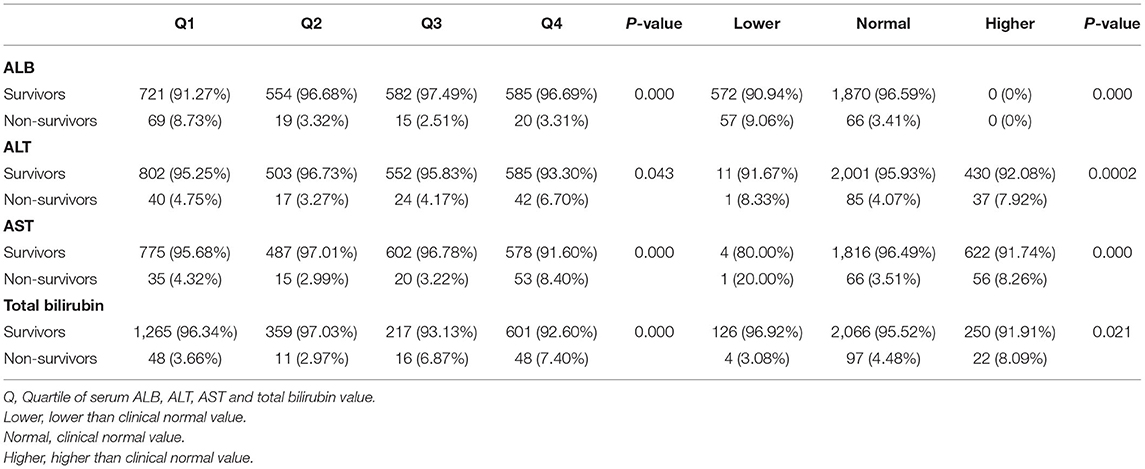
Then, the correlation of the liver function index with a 90-day mortality of cardiac surgery patients without liver disease was investigated. As shown in Table 4, for ALB, a higher rate of 90-day mortality was observed in the first quartile, and for ALT and AST, a higher rate of 90-day mortality was observed in the fourth quartile (ALB: p = 0.000; ALT: p = 0.043; AST: p = 0.000). For total bilirubin, the 90-day mortality of the third and fourth quartiles was higher than that of the first and second quartiles (p = 0.000). Interestingly, grouping according to the clinical normal value revealed different trends than quartile grouping. For ALB and AST, a higher rate of 90-day mortality was observed in the group with lower and higher values than normal, while for total bilirubin, a higher rate of 90-day mortality was observed in the group with a higher value (ALB: p = 0.000; ALT: p = 0.002; AST: p = 0.000; total bilirubin: p = 0.021).
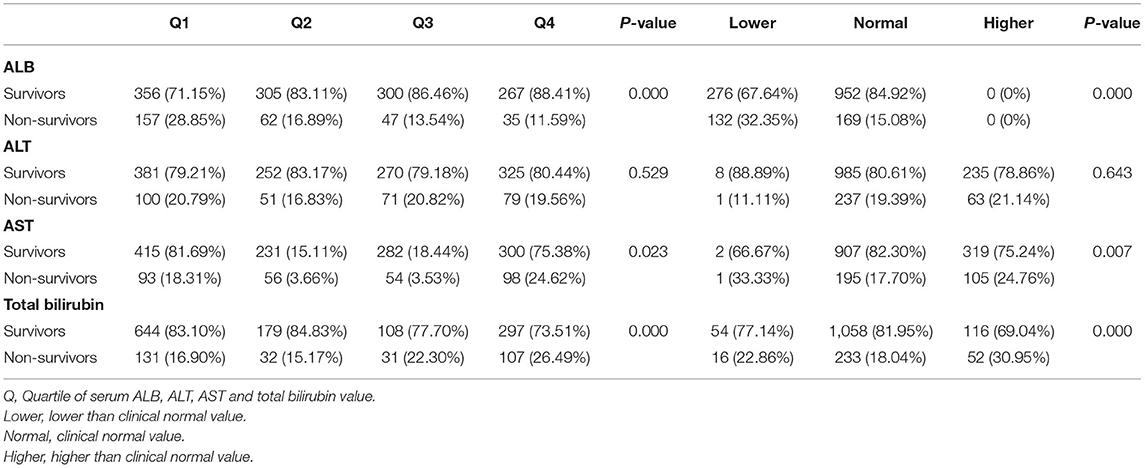
For 4-year mortality, only patients in CareVue who were followed for at least 4 years were analyzed. As shown in Table 5, for ALB, higher 4-year mortality was observed in the first quartile and in the group with lower values than normal (all p = 0.000). For AST, higher 4-year mortality was observed in the first and fourth quartiles (p = 0.023), and in the group with lower and higher values than normal (p = 0.007). For total bilirubin, higher 4-year mortality was observed in the third and fourth quartiles and in the group with lower and higher values than normal (all p = 0.000).
The Kaplan-Meier survival curves comparing patients by liver function index levels are shown in Figure 1. The results showed that patients in the first quartile of ALB and in the fourth quartile of ALT, AST, and total bilirubin had the lowest 90-day survival (all p < 0.05) (Figures 1A–D). The patients with lower ALB, lower and higher ALT/AST, and higher total bilirubin had lower 90-day survival (all p < 0.05) (Figures 1E–H). Moreover, patients in the first quartile of ALB, in the fourth quartile of AST, and in the third and fourth quartiles of total bilirubin had lower 4-year survival (all p < 0.05), but the quartiles of ALT were not significantly different (Figures 2A–D). The patients with lower ALB and lower and higher AST/total bilirubin had lower 4-year survival (all p < 0.05), but such differences in ALT levels were not observed (Figures 2E–H).
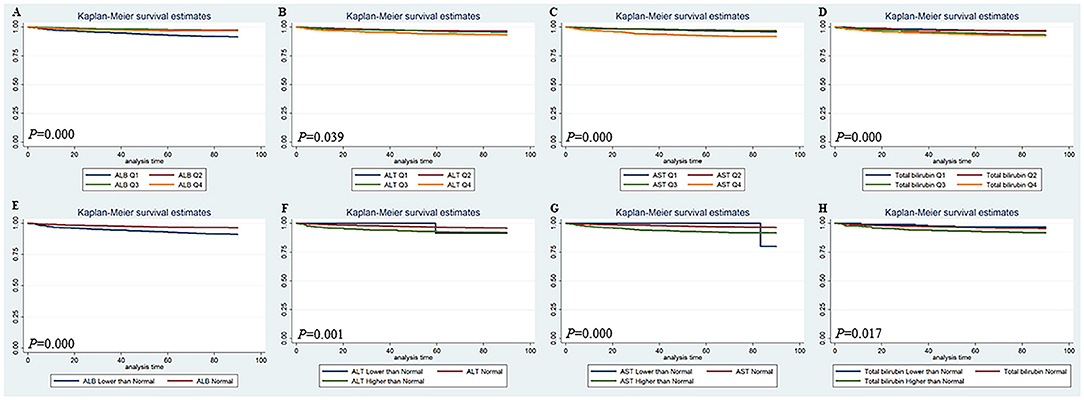
Figure 1. Kaplan-Meier 90-day survival curves comparing patients by liver function index levels. Q, Quartile of serum ALB, ALT, AST and total bilirubin value; Normal, clinical normal value; ALB, blood albumin; ALT, blood alanine transaminase; AST, blood aspartate transaminase.
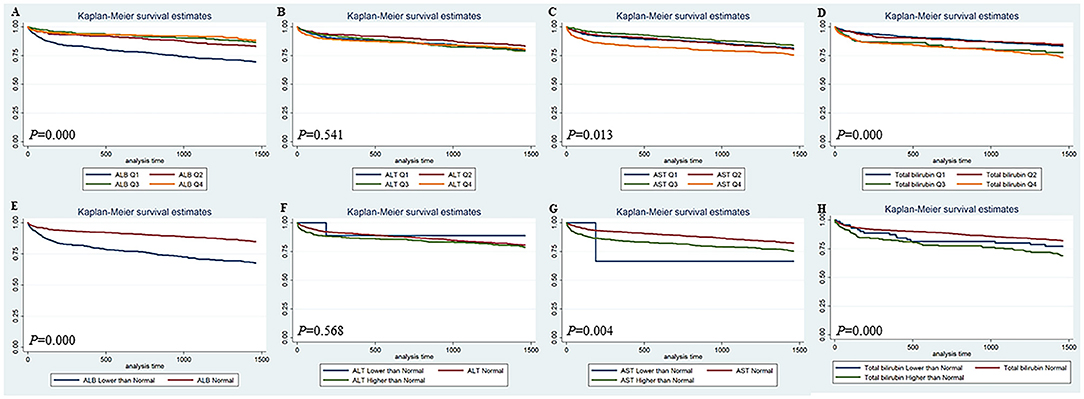
Figure 2. Kaplan-Meier 4-year survival curves comparing patients by liver function index levels. Q, Quartile of serum ALB, ALT, AST and total bilirubin value; Normal, clinical normal value; ALB, blood albumin; ALT, blood alanine transaminase; AST, blood aspartate transaminase.
A univariate logistic regression analysis was performed. As shown in Table 6, ALB was associated with hospital mortality (OR = 0.36, 95% CI = 0.25–0.52, p = 0.000), 90-day mortality (OR = 0.37, 95% CI = 0.28–0.49, p = 0.000), and 4-year mortality (OR = 0.41, 95% CI = 0.33–0.50, p = 0.000). ALT was associated with hospital mortality (OR = 1.00, 95% CI = 1.00–1.01, p = 0.000) and 90-day mortality (OR = 1.00, 95% CI = 1.00–1.01, p = 0.001) but not 4-year mortality (OR = 1.00, 95% CI = 0.99–1.00, p = 0.214). AST was associated with hospital (OR = 1.00, 95% CI = 1.00–1.01, p = 0.000) and 90-day mortality (OR = 1.00, 95% CI = 1.00–1.01, p = 0.001) but not 4-year mortality (OR = 1.00, 95% CI = 0.99–1.00, p = 0.079). Total bilirubin was associated with hospital mortality (OR = 1.49, 95% CI = 1.23–1.81, p = 0.000), 90-day mortality (OR = 1.41, 95% CI = 1.20–1.66, p = 0.000), and 4-year mortality (OR = 1.46, 95% CI = 1.24–1.73, p = 0.000).
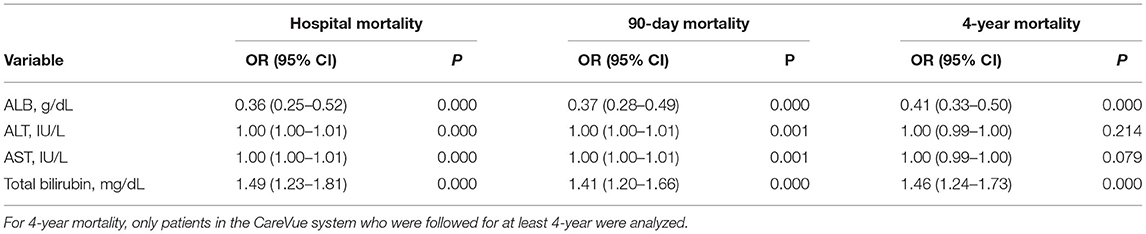
Table 6. Univariate logistic regression analyses for prognosis in cardiac surgery patients without liver disease.
The results of multivariate analysis are shown in Table 7. For multivariate analysis, Model 1 was adjusted for SOFA score, creatinine, SpO2, hypertension, congestive heart failure, and chronic pulmonary disease. Model 2 was further adjusted for height, SBP, and SAPS II score. ALB was associated with hospital mortality (Model 1: OR = 0.48, 95% CI = 0.33–0.70, p = 0.000; Model 2: OR = 0.43, 95% CI = 0.29–0.62, p = 0.000), 90-day mortality (Model 1: OR = 0.46, 95% CI = 0.35–0.61, p = 0.000; Model 2: OR = 0.42, 95% CI = 0.31–0.55, p = 0.000), and 4-year mortality (Model 1: OR = 0.47, 95% CI = 0.37–0.58, p = 0.000; Model 2: OR = 0.48, 95% CI = 0.38–0.59, p = 0.000) in both models. ALT was associated with hospital mortality (Model 1: OR = 1.00, 95% CI = 1.00–1.01, p = 0.003; Model 2: OR = 1.00, 95% CI = 1.00–1.01, p = 0.004) and 90-day mortality (Model 1: OR = 1.00, 95% CI = 1.00–1.01, p = 0.015; Model 2: OR = 1.00, 95% CI = 1.00–1.01, p = 0.008) in both models, but not 4-year mortality (Model 1: OR = 1.00, 95% CI = 0.99–1.19, p = 0.793; Model 2: OR = 1.00, 95% CI = 0.99–1.00, p = 0.647). AST was associated with hospital mortality (Model 1: OR = 1.00, 95% CI = 1.00–1.01, p = 0.002; Model 2: OR = 1.00, 95% CI = 1.00–1.01, p = 0.005) and 90-day mortality (Model 1: OR = 1.00, 95% CI = 1.00–1.01, p = 0.007; Model 2: OR = 1.00, 95% CI = 1.00–1.01, p = 0.008) in both models, but not 4-year mortality (Model 1: OR = 1.00, 95% CI = 0.99–1.00, p = 0.286; Model 2: OR = 1.00, 95% CI = 0.99–1.00, p = 0.304). Total bilirubin was associated with hospital mortality (Model 1: OR = 1.33, 95% CI = 1.07–1.65, p = 0.010; Model 2: OR = 1.45, 95% CI = 1.19–1.78, p = 0.000), 90-day mortality (Model 1: OR = 1.29, 95% CI = 1.07–1.54, p = 0.006; Model 2: OR = 1.40, 95% CI = 1.18–1.65, p = 0.000), and 4-year mortality (Model 1: OR = 1.37, 95% CI = 1.15–1.64, p = 0.001; Model 2: OR = 1.48, 95% CI = 1.23–1.77, p = 0.000) in both models (Table 7).
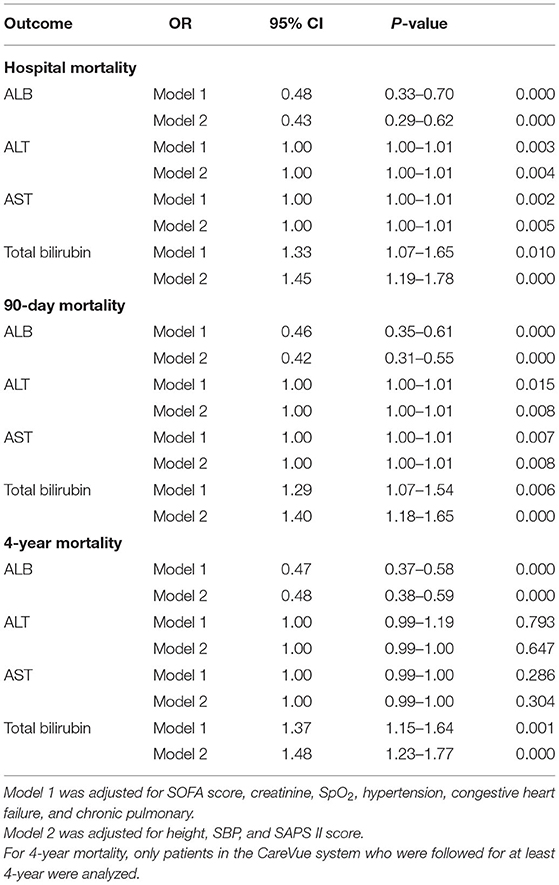
Table 7. Association between ALB, ALT, AST, and total bilirubin with prognosis of cardiac surgery patients without liver disease.
Discussion
There is a complex pathophysiological interaction between the heart and the liver. Thus, pathological changes in one organ system can cause structural and functional abnormalities in the other organ systems (9). In heart failure patients, liver dysfunction is a well-known complication, and it is a risk factor for not only mortality in heart failure patients but also outcomes of cardiac surgery patients (5, 10, 11). However, similar outcome data are lacking in cardiac surgery patients without a history of acute hepatic failure or primary significant liver disease. Our data showed that abnormal ALB, AST, ALT, and total bilirubin were associated with the length of hospital stay and ICU stay, hospital mortality, and 90-day and 4-year mortality in cardiac surgery patients without liver disease (Tables 2–6, Figures 1, 2). Abnormal ALB, AST, ALT, and total bilirubin were independent risk factors for hospital mortality and 90-day mortality, but only ALB and total bilirubin were independent risk factors for 4-year mortality (Table 7). Our investigation suggested that preoperative LFT abnormalities were associated with short-term and long-term prognosis in cardiac surgery patients without liver disease.
Low serum ALB concentration or hypoalbuminemia was considered to be an indicator of prognosis after cardiovascular surgery and might be induced by congestive heart failure or chronic liver insufficiency (12–14). It is well-known that serum ALB can help to maintain the intravascular volume, partially by facilitating the integrity of the vasculature (15). Thus, a lower serum ALB level might result in more tissue edema and reduce the circulating volume by extravasation. In our study, preoperative low serum ALB was significantly associated with short-term and long-term prognosis in cardiac surgery patients without liver disease (Tables 2–6, Figures 1, 2) and could serve as an independent risk factor for mortality (Table 7). We found that the albumin level of the non-survivors was significantly lower than that of survivors although the difference is quite small (3.4 vs. 3.8 mg/dl). This may indicate that, when the albumin level is lower than the normal level, even a slight decrease in albumin level will increase the risk of death of the patient. Thus, preoperative nutritional repletion to correct hypoalbuminemia can benefit cardiac patients before cardiac surgery and avert possible deleterious effects in the postoperative period.
High serum total bilirubin could be induced by haemolysis, decreased liver perfusion, and chronically low cardiac output, or hepatocyte dysfunction due to liver congestion in right-sided heart failure (11, 16, 17). The preoperative level of serum bilirubin was an independent risk factor for cardiac surgery in patients with liver cirrhosis (18). Our results indicated that preoperative high serum total bilirubin could serve as an independent risk factor for short-term and long-term prognosis in cardiac surgery patients without liver disease (Tables 2–7, Figures 1, 2). High serum total bilirubin in cardiac surgery patients without liver disease might be caused by hepatic hypoperfusion and/or hepatic congestion. Under these circumstances, optimizing the treatment of congestive heart failure before surgery can reduce the preoperative serum total bilirubin level. Therefore, the outcome of cardiac surgery could be improved. However, to investigate this problem, more research needs to be done, as a conclusion cannot be drawn from our data.
Alanine transaminase and AST are classic cytoplasmic enzymes and inflammation-associated components that can be immediately released into the bloodstream following the deleterious effect of heart failure on the liver (19, 20). For patients with heart failure, there is a graded relationship between admission transaminase levels and surrogate indicators of in-hospital mortality (21). Furthermore, more significant ALT elevation can be used as an independent predictor of hospital mortality, deterioration of renal function, ICU admission, and longer time of hospital stay (21). Preoperative and postoperative AST/ALT might be an independent predictor of AKI after cardiac surgery (20). Our research is consistent with previous reports. ALT and AST were found to be independent risk factors of hospital mortality and 90-day mortality in cardiac surgery patients without liver disease but not 4-year mortality (Tables 2–7, Figures 1, 2). Notably, increased transaminase levels in patients with significantly increased mortality may be caused by progressive heart pump failure. This hypothesis is supported by the high proportion of positive inotropic and/or vasopressor support in this subgroup of patients and the lack of association between ALT and fatal arrhythmias (21).
Limitations
As a single-center retrospective study, this study has all the limitations of a retrospective observational study. Our findings need to be further confirmed in a larger multicentered cohort or a randomized controlled trial. In addition, the numbers of survivors and non-survivors are quite different, which may cause slight bias in the study.
Conclusion
In summary, we demonstrated that abnormal LFTs were significantly correlated with the length of hospital stay and ICU stay of cardiac surgery patients without liver disease. Abnormal LFTs were associated with higher hospital mortality, 90-day mortality, and 4-year mortality, as well as lower 90-day and 4-year survival. LFTs could serve as an independent predictor of the hospital, 90-day and 4-year mortality in cardiac patients without liver disease.
Data Availability Statement
Publicly available datasets were analyzed in this study. This data can be found at the Medical Information Mart for Intensive Care III (MIMIC-III): https://mimic.mit.edu/ and https://physionet.org/.
Ethics Statement
The studies involving human participants were reviewed and approved by Institutional Review Boards of the Massachusetts Institute of Technology (Cambridge, MA, USA) (Record ID 41268972). Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
Author Contributions
ZW, JY, and JH conceived and designed the study. LS, YA, LinL, LihL, YZ, and JH provided, selected, assembled, analyzed, and interpreted the data. All authors contributed to data analysis, drafting, critically revising the paper, agreed to be accountable for all aspects of the work, and read and confirmed that they meet ICMJE criteria for authorship.
Funding
This study was funded by the National Natural Science Foundation of China (81900294, 81770319, 81570039, and 82070297) and the Natural Science Foundation of Hunan Province, China (2018JJ2369, 2018JJ2362).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We appreciate American Journal Experts (AJE) for their careful English language editing. We apologize to all those researchers whose work could not be cited due to space limitations.
Abbreviations
ALB, blood albumin; ALT, blood alanine transaminase; AST, blood aspartate transaminase; BIDMC, Beth Israel Deaconess Medical Center; CPB, cardiopulmonary bypass; DBP, diastolic blood pressure; CABG, coronary artery bypass grafting; HR, heart rate; ICU, intensive care unit; LFTs, liver function tests; MIT, Massachusetts Institute of Technology; MELD, Model for End-Stage Liver Disease score; MIMIC III, Multiparameter Intelligent Monitoring in Intensive Care III database; RR, respiratory rate; SAPS II, Simplified Acute Physiology Score II; SBP, systolic blood pressure; SOFA, Sequential Organ Failure Assessment score; SpO2, percutaneous oxygen saturation; SQL, Structured Query Language; WBC, white blood cell.
References
1. Kawahira T, Wakita N, Minami H, Sakata M, Kitano I, Shida T. Lymphatic cardiac tamponade after open-heart surgery with liver dysfunction. Jpn J Thorac Cardiovasc Surg. (2003) 51:669–71. doi: 10.1007/s11748-003-0007-6
2. Paparella D, Yau TM, Young E. Cardiopulmonary bypass induced inflammation: pathophysiology and treatment. An update. Eur J Cardiothorac Surg. (2002) 21:232–44. doi: 10.1016/S1010-7940(01)01099-5
3. Sabzi F, Faraji R. Liver function tests following open cardiac surgery. J Cardiovasc Thorac Res. (2015) 7:49–54. doi: 10.15171/jcvtr.2015.11
4. Kohno H, Matsumiya G. Cardiac surgery for patients with liver cirrhosis. Kyobu Geka. (2020) 73:770–4. doi: 10.15106/j_kyobu73_770
5. Garatti A, Daprati A, Cottini M, Russo CF, Dalla Tomba M, Troise G, et al. Cardiac surgery in patients with liver cirrhosis (CASTER) study: early and long-term outcomes. Ann Thorac Surg. (2021) 111:1242–51. doi: 10.1016/j.athoracsur.2020.06.110
6. van Deursen VM, Damman K, Hillege HL, van Beek AP, van Veldhuisen DJ, Voors AA. Abnormal liver function in relation to hemodynamic profile in heart failure patients. J Card Fail. (2010) 16:84–90. doi: 10.1016/j.cardfail.2009.08.002
7. Ambrosy AP, Vaduganathan M, Huffman MD, Khan S, Kwasny MJ, Fought AJ, et al. Clinical course and predictive value of liver function tests in patients hospitalized for worsening heart failure with reduced ejection fraction: an analysis of the EVEREST trial. Eur J Heart Fail. (2012) 14:302–11. doi: 10.1093/eurjhf/hfs007
8. Johnson AE, Pollard TJ, Shen L, Lehman LW, Feng M, Ghassemi M, et al. MIMIC-III, a freely accessible critical care database. Sci Data. (2016) 3:160035. doi: 10.1038/sdata.2016.35
9. Xanthopoulos A, Starling RC, Kitai T, Triposkiadis F. Heart failure and liver disease: cardiohepatic interactions. JACC Heart Fail. (2019) 7:87–97. doi: 10.1016/j.jchf.2018.10.007
10. Lau GT, Tan HC, Kritharides L. Type of liver dysfunction in heart failure and its relation to the severity of tricuspid regurgitation. Am J Cardiol. (2002) 90:1405–9. doi: 10.1016/S0002-9149(02)02886-2
11. Allen LA, Felker GM, Pocock S, McMurray JJ, Pfeffer MA, Swedberg K, et al. Liver function abnormalities and outcome in patients with chronic heart failure: data from the Candesartan in Heart Failure: assessment of Reduction in Mortality and Morbidity (CHARM) program. Eur J Heart Fail. (2009) 11:170–7. doi: 10.1093/eurjhf/hfn031
12. Becker RB, Zimmerman JE, Knaus WA, Wagner DP, Seneff MG, Draper EA, et al. The use of APACHE III to evaluate ICU length of stay, resource use, and mortality after coronary artery by-pass surgery. J Cardiovasc Surg. (1995) 36:1–11.
13. Otaki M. Surgical treatment of patients with cardiac cachexia. An analysis of factors affecting operative mortality. Chest. (1994) 105:1347–51. doi: 10.1378/chest.105.5.1347
14. Knaus WA, Harrell FE Jr, Lynn J, Goldman L, Phillips RS, Connors AF Jr, et al. The SUPPORT prognostic model Objective estimates of survival for seriously ill hospitalized adults Study to understand prognoses and preferences for outcomes and risks of treatments. Ann Intern Med. (1995) 122:191–203. doi: 10.7326/0003-4819-122-3-199502010-00007
15. Dagenais GR, Pogue J, Fox K, Simoons ML, Yusuf S. Angiotensin-converting-enzyme inhibitors in stable vascular disease without left ventricular systolic dysfunction or heart failure: a combined analysis of three trials. Lancet. (2006) 368:581–8. doi: 10.1016/S0140-6736(06)69201-5
16. Reinhartz O, Farrar DJ, Hershon JH, Avery GJ Jr, Haeusslein EA, Hill JD. Importance of preoperative liver function as a predictor of survival in patients supported with Thoratec ventricular assist devices as a bridge to transplantation. J Thorac Cardiovasc Surg. (1998) 116:633–40. doi: 10.1016/S0022-5223(98)70171-0
17. Kavarana MN, Pessin-Minsley MS, Urtecho J, Catanese KA, Flannery M, Oz MC, et al. Right ventricular dysfunction and organ failure in left ventricular assist device recipients: a continuing problem. Ann Thorac Surg. (2002) 73:745–50. doi: 10.1016/S0003-4975(01)03406-3
18. Lin CH, Hsu RB. Cardiac surgery in patients with liver cirrhosis: risk factors for predicting mortality. World J Gastroenterol. (2014) 20:12608–14. doi: 10.3748/wjg.v20.i35.12608
19. Di Tomasso N, Monaco F, Landoni G. Hepatic and renal effects of cardiopulmonary bypass. Best Pract Res Clin Anaesthesiol. (2015) 29:151–61. doi: 10.1016/j.bpa.2015.04.001
20. Gultekin Y, Bolat A, Hatice K, Tekeli Kunt A. Does aspartate aminotransferase to alanine aminotransferase ratio predict acute kidney injury after cardiac surgery? Heart Surg Forum. (2021) 24:e506–e11. doi: 10.1532/hsf.3849
21. Ambrosy AP, Gheorghiade M, Bubenek S, Vinereanu D, Vaduganathan M, Macarie C, et al. The predictive value of transaminases at admission in patients hospitalized for heart failure: findings from the RO-AHFS registry. Eur Heart J Acute Cardiovasc Care. (2013) 2:99–108. doi: 10.1177/2048872612474906
Keywords: preoperative liver function tests, cardiac surgery, short-term prognosis, long-term prognosis, hospital mortality, 90-day mortality
Citation: Shang L, Ao Y, Lv L, Lv L, Zhang Y, Hou J, Yao J and Wu Z (2021) Preoperative Liver Function Test Abnormalities Were Associated With Short-Term and Long-Term Prognosis in Cardiac Surgery Patients Without Liver Disease. Front. Cardiovasc. Med. 8:772430. doi: 10.3389/fcvm.2021.772430
Received: 08 September 2021; Accepted: 04 October 2021;
Published: 01 November 2021.
Edited by:
Robert Jeenchen Chen, The Ohio State University, United StatesReviewed by:
Avishek Samaddar, Alder Hey Children's Hospital, United KingdomMaruti Haranal, National Heart Institute, Malaysia
Bleri Celmeta, Istituto Clinico Sant'Ambrogio, Italy
Copyright © 2021 Shang, Ao, Lv, Lv, Zhang, Hou, Yao and Wu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhongkai Wu, d3V6aGtAbWFpbC5zeXN1LmVkdS5jbg==; Jianping Yao, eWFvamlhbnBAbWFpbC5zeXN1LmVkdS5jbg==; Jian Hou, Y2huaG91akAxNjMuY29t
†These authors have contributed equally to this work
 Liqun Shang
Liqun Shang Yuanhan Ao
Yuanhan Ao Linhua Lv1,2
Linhua Lv1,2 Jian Hou
Jian Hou Zhongkai Wu
Zhongkai Wu